
Our Path Forward Robert G. Kramer President & Chief Executive Officer 40th Annual J.P. Morgan Healthcare Conference January 10, 2022
This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including, without limitation, our financial guidance and related projections and statements regarding our ability to meet such projections in the anticipated timeframe, if at all, statements regarding our long-term growth opportunities, growth goals, vision, M&A and investment opportunities, future performance and meeting milestones in our R&D portfolio, the timing of our final 2021 financial results, future revenue levels and the sources of such revenues, capital expenditures, ACAM 2000 vaccine deliveries, the impact of a generic marketplace on NARCAN Nasal Spray, future NARCAN Nasal Spray sales and supporting a generic naloxone partner, gross margin, the timing of early-stage vaccine programs and approval of AV7909, other future regulatory submissions, progress of the CHIKV VLP Phase 3 clinical trial and efficacy of the product candidate, initiating a modest relaunch of our Travel Health vaccines, cultivating additional support of OUS governments, increasing network utilization, future manufacturing and productivity and any other statements containing the words “will,” “believes,” “expects,” “anticipates,” “intends,” “plans,” “targets,” “forecasts,” “estimates” and similar expressions in conjunction with, among other things, discussions of the Company’s outlook, financial performance or financial condition, financial and operation goals, strategic goals, growth strategy, product sales, government development or procurement contracts or awards, government appropriations, manufacturing capabilities, and the timing of certain regulatory approvals or expenditures are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs, and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statements speak only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events, or circumstances. There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including the availability of U.S. government funding for procurement of AV7909 and/or BioThrax or ACAM2000 and our other U.S. government procurement and development contracts, the timing of completion of our submission of the application for and our ability to secure licensure of AV7909 from the FDA within the anticipated timeframe, if at all, our ability to perform under our contracts with the U.S. government, including the timing of and specifications relating to deliveries, whether we will realize the full benefit of our investments in additional manufacturing and quality control systems, our ability to meet our commitments to continued quality and manufacturing compliance at our manufacturing facilities and the potential impact on our ability to continue production of bulk drug substance for Johnson & Johnson’s COVID-19 vaccine, our ability to provide CDMO services for the development and/or manufacture of product candidates of our customers at required levels and on required timelines, our ability and the ability of our contractors and suppliers to maintain compliance with Current Good Manufacturing Practices and other regulatory obligations, our ability to obtain and maintain regulatory approvals for our product candidates and the timing of any such approvals, changes to U.S. government priorities for the SNS, our ability to negotiate additional U.S. government procurement or follow-on contracts for our public health threat products that have expired or will be expiring, the negotiation of further commitments or contracts related to the collaboration and deployment of capacity toward future commercial manufacturing under our CDMO contracts, our ability to develop a safe and effective treatment for COVID-19 and obtain emergency use authorization or approval of such treatment from the FDA, our ability to comply with the operating and financial covenants required by our senior secured credit facilities and our 3.875% Senior Unsecured Notes due 2028, procurement by U.S. government entities under regulatory exemptions prior to approval by the FDA and corresponding procurement by government entities outside of the United States under regulatory exemptions prior to approval by the corresponding regulatory authorities in the applicable country, the full impact of COVID-19 disease on our markets, operations and employees as well as those of our customers and suppliers, the impact on our revenues from and duration of declines in sales of our vaccine products that target travelers due to the reduction of international travel caused by the COVID-19 pandemic, our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria, the success of our commercialization, marketing and manufacturing capabilities and strategy, and the accuracy of our estimates regarding future revenues, expenses and capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement as well as the risk factors identified in our periodic reports filed with the Securities and Exchange Commission when evaluating our forward-looking statements. Trademarks BioThrax® (Anthrax Vaccine Adsorbed), RSDL® (Reactive Skin Decontamination Lotion Kit), BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F and G)-(Equine)), Anthrasil® (Anthrax Immune Globulin Intravenous (Human)), VIGIV (Vaccinia Immune Globulin Intravenous (Human)), Trobigard® (atropine sulfate, obidoxime chloride), ACAM2000® (Smallpox (Vaccinia) Vaccine, Live), Vivotif® (Typhoid Vaccine Live Oral Ty21a), Vaxchora® (Cholera Vaccine, Live, Oral), NARCAN® (naloxone HCI) Nasal Spray and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners. Safe Harbor Statement/Trademarks 240th Annual J.P. Morgan Healthcare Conference

This presentation contains two financial measures Adjusted EBITDA (Earnings Before Interest, Taxes, and Depreciation and Amortization) and Adjusted EBITDA Margin, both of which are considered “non-GAAP” financial measures under applicable Securities and Exchange Commission rules and regulations. These non-GAAP financial measures should be considered supplemental to and not a substitute for financial information prepared in accordance with generally accepted accounting principles. The Company’s definition of these non-GAAP measures may differ from similarly titled measures used by others. Adjusted EBITDA reflects net income excluding the impact of depreciation, amortization, interest expense and income taxes, excluding specified items that can be highly variable and the non-cash impact of certain accounting adjustments. Adjusted EBITDA Margin is defined as Adjusted EBITDA divided by total revenues. The Company views these non-GAAP financial measures as a means to facilitate management’s financial and operational decision-making, including evaluation of the Company’s historical operating results and comparison to competitors’ operating results. These non-GAAP financial measures reflect an additional way of viewing aspects of the Company’s operations that, when viewed with GAAP results and the reconciliations to the corresponding GAAP financial measure may provide a more complete understanding of factors and trends affecting the Company’s business. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial measures exclude the effect of items that will increase or decrease the Company’s reported results of operations, management strongly encourages investors to review the Company’s consolidated financial statements and publicly filed reports in their entirety. For additional information on the non-GAAP financial measures noted here, please refer to the reconciliation tables provide in the Appendix to this presentation as well as the associated press release which can be found on the Company’s website at www.emergentbiosolutions.com. Non-GAAP Financial Measures 340th Annual J.P. Morgan Healthcare Conference

What We’re Going to Cover Today 440th Annual J.P. Morgan Healthcare Conference The Company • Our Vision • Introduction Business Performance • Government/Medical Countermeasures (MCM) Products Business • Commercial Products Business • Research & Development (R&D) • Contract Development & Manufacturing (CDMO) Services Business Financials • 2021 Preliminary • 2022 Guidance AGENDA Key Takeaways

PROPRIETARY AND CONFIDENTIAL 540th Annual J.P. Morgan Healthcare Conference Who We Are Our Vision | Introduction
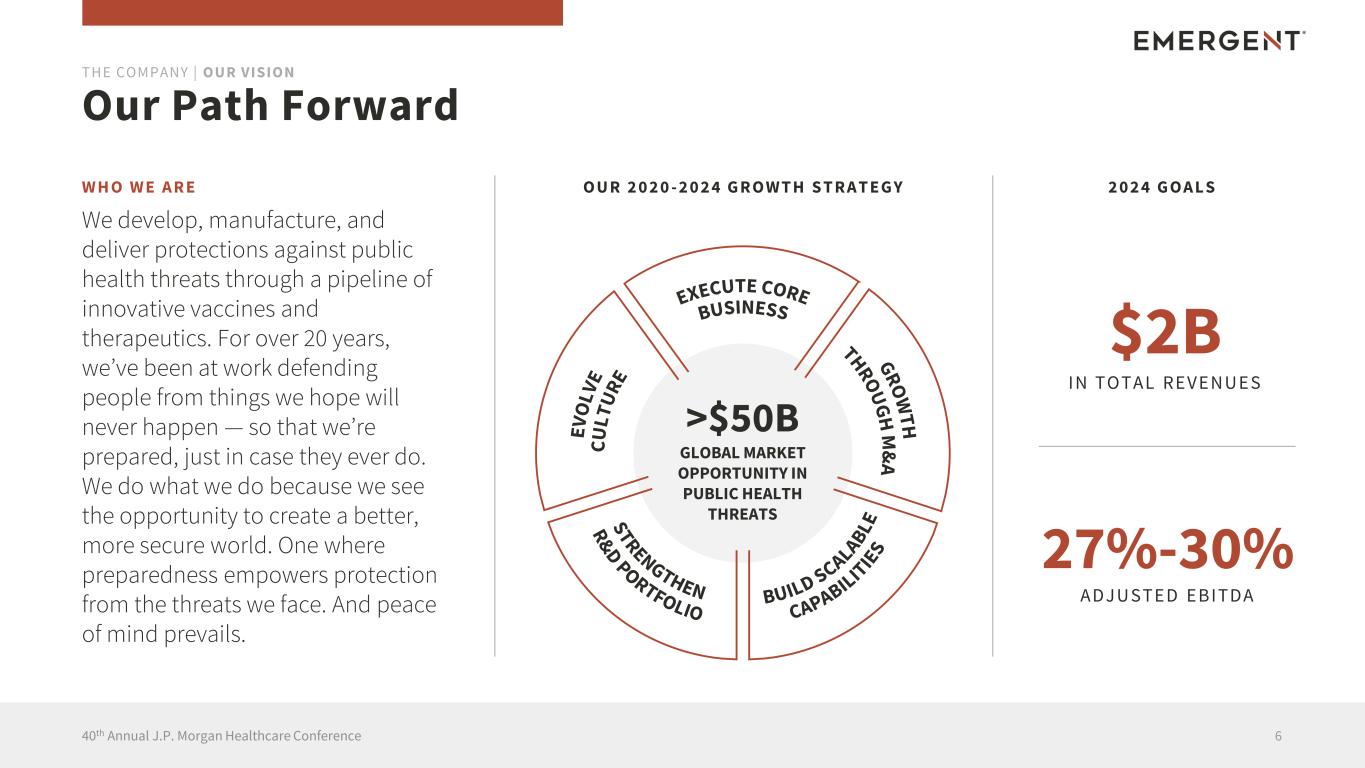
W H O W E A R E We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 20 years, we’ve been at work defending people from things we hope will never happen — so that we’re prepared, just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. Our Path Forward 640th Annual J.P. Morgan Healthcare Conference THE COMPANY | OUR VISION O U R 2 0 2 0 - 2 0 2 4 G R O W T H S T R A T E G Y >$50B GLOBAL MARKET OPPORTUNITY IN PUBLIC HEALTH THREATS 2 0 2 4 G O A L S $2B IN TOTAL REVENU ES 27%-30% ADJU STED EBITDA
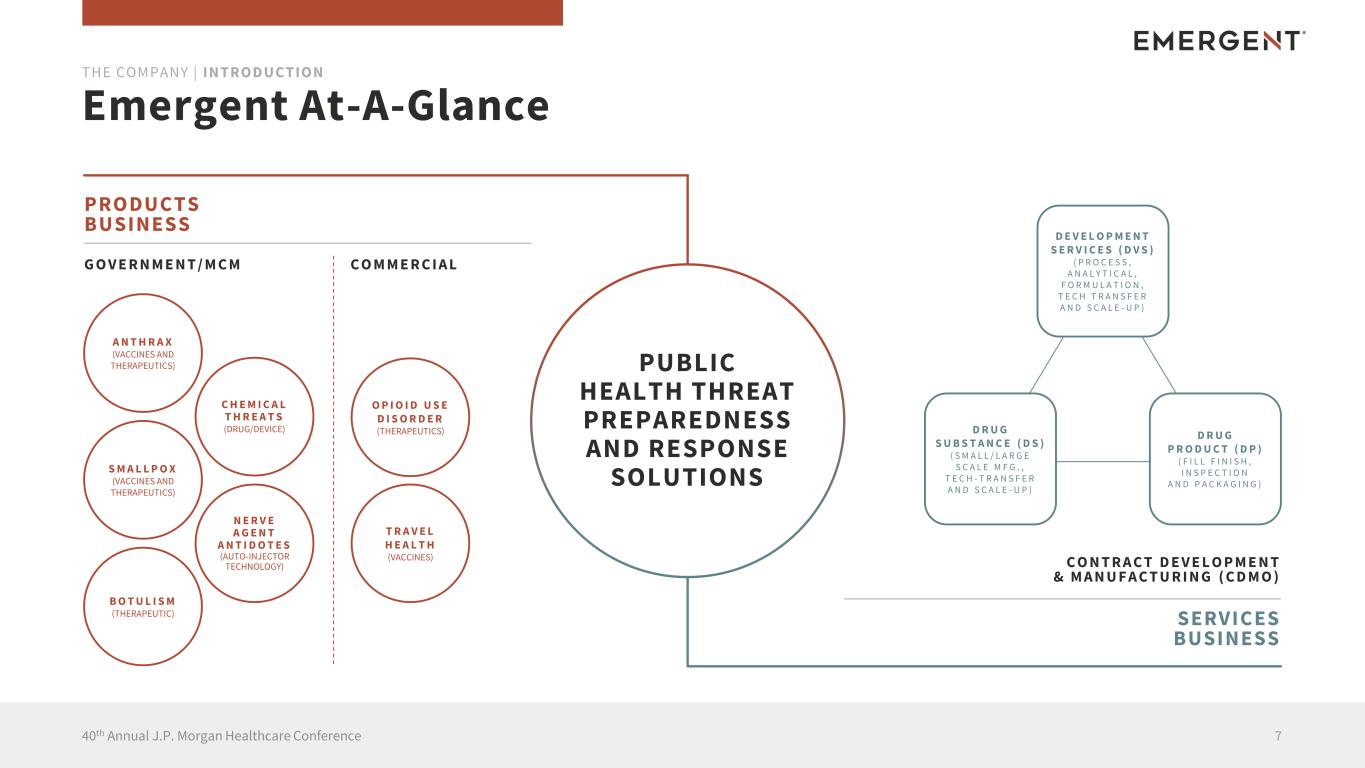
Emergent At-A-Glance 740th Annual J.P. Morgan Healthcare Conference THE COMPANY | INTRODUCTION PUBLIC HEALTH THREAT PREPAREDNESS AND RESPONSE SOLUTIONS PRODUCTS BUSINESS G O V E R N M E N T / M C M C O M M E R C I A L C O N T R A C T D E V E L O P M E N T & M A N U F A C T U R I N G ( C D M O ) SERVICES BUSINESS A N T H R A X (VACCINES AND THERAPEUTICS) S M A L L P O X (VACCINES AND THERAPEUTICS) B O T U L I S M (THERAPEUTIC) C H E M I C A L T H R E A T S (DRUG/DEVICE) N E R V E A G E N T A N T I D O T E S (AUTO-INJECTOR TECHNOLOGY) O P I O I D U S E D I S O R D E R (THERAPEUTICS) T R A V E L H E A L T H (VACCINES) D E V E L O P M E N T S E R V I C E S ( D V S ) ( P R O C E S S , A N A L Y T I C A L , F O R M U L A T I O N , T E C H T R A N S F E R A N D S C A L E - U P ) D R U G S U B S T A N C E ( D S ) ( S M A L L / L A R G E S C A L E M F G . , T E C H - T R A N S F E R A N D S C A L E - U P ) D R U G P R O D U C T ( D P ) ( F I L L F I N I S H , I N S P E C T I O N A N D P A C K A G I N G )

PROPRIETARY AND CONFIDENTIAL40th Annual J.P. Morgan Healthcare Conference Business Performance Government/MCM Products Business | Commercial Products Business | Research & Development | CDMO Services Business 8

940th Annual J.P. Morgan Healthcare Conference BUSINESS PERFORMANCE | GOVERNMENT/MEDICAL COUNTERMEASURES L O N G - T E R M G R O W T H O P P O R T U N I T I E S • Continue to support product requirements of the US Strategic National Stockpile (SNS) • Continue to support active use needs of multiple US government agencies • Further cultivate and support preparedness requirements of OUS governments 2 0 2 1 A C C O M P L I S H M E N T S • Secured key contract wins for ACAM2000 and AV7909 • Realized consistent contribution from OUS markets • Secured Belgian Health Authority approval for Trobigard Auto-injector MCM Products Contribute to Public Health Threat Preparedness and Response for Governments Worldwide G O V E R N M E N T /M C M P R O D U C T S M A R K E T D Y N A M I C • BioThrax® • AV79091 • Anthrasil® • Raxibacumab • ACAM2000® • VIGIV • BAT® • RSDL® • Trobigard® Auto-injector1 • US Government • Non-US Government (OUS) • Stockpiling • Active Use (Military) • Long-Term Procurement Contracts with Firm Fixed Pricing • Funded R&D Through Multi-Year Contracts and Grants 1. AV7909 is not approved by the FDA or any other health regulatory authority, and Trobigard is not approved by the FDA; both are procured by authorized government agencies under special circumstances.

Opioid Use Disorder and Travel Health Franchises Provide Opportunity to Impact Patients and Customers 1040th Annual J.P. Morgan Healthcare Conference BUSINESS PERFORMANCE | COMMERCIAL L O N G - T E R M G R O W T H O P P O R T U N I T I E S • Continue to sell branded NARCAN Nasal Spray • Initiate modest relaunch of Travel Health vaccines Vivotif and Vaxchora into select channels 2 0 2 1 A C C O M P L I S H M E N T S • Continued progress of awareness, access, and affordability initiatives for NARCAN Nasal Spray • Licensed Sandoz AG to launch an authorized generic version of NARCAN Nasal Spray C O M M E R C I A L P R O D U C T S K E Y C U S T O M E R S • NARCAN® Nasal Spray • Vaxchora® • Vivotif® • US Retail Pharmacy Consumers • US Public Interest Customers • Canadian Public Health Organizations • US/EU Travelers

Diverse R&D Portfolio Offers Potential for Expanded Impact to Global Public Health 1140th Annual J.P. Morgan Healthcare Conference BUSINESS PERFORMANCE | RESEARCH & DEVELOPMENT P R O G R A M E X T E R N A L P A R T N E R C U R R E N T S T A T U S AV7909 (Anthrax Vaccine Adsorbed (AVA), Adjuvanted) BARDA PHASE 3 CHIKV VLP (Chikungunya virus VLP vaccine) NA PHASE 3 COVID-HIG (Human polyclonal hyperimmune with antibodies to SARS-CoV-2) DoD/NIAID PHASE 1, 3 UniFlu (Universal influenza vaccine) NA PHASE 1 CGRD-001 (pralidoxime chloride/atropine) DoD PRECLINICAL AP-003 (naloxone multidose nasal spray) NA PRECLINICAL S E L E C T L I S T O F R & D P R O G R A M S L O N G - T E R M G R O W T H O P P O R T U N I T I E S • Initiate clinical trials for one or more early-stage programs • Complete submission to FDA of the AV7909 BLA • Submit one or more regulatory license applications for drug/device and auto-injector based programs • Successfully complete Phase 3 CHIKV VLP trial 2 0 2 1 A C C O M P L I S H M E N T S • Advanced key late-stage and early-stage candidates and successfully positioned for continued progress in 2022 - Initiated rolling submission to the FDA of the AV7909 BLA - Initiated pivotal Phase 3 study for CHIKV VLP - Initiated Phase 1 study for UniFlu - Participated in NIAID-sponsored Phase 3 study using COVID-HIG

Biologics CDMO Services Remain Well-Positioned to Support Needs of Global Pharma/Biotech Innovators 1240th Annual J.P. Morgan Healthcare Conference BUSINESS PERFORMANCE | CDMO SERVICES BUSINESS L O N G - T E R M G R O W T H O P P O R T U N I T I E S • Increase network utilization • Drive a higher mix of drug substance manufacturing • Realize scale efficiencies and improve productivity • Pursue select investments in new capacity/capability informed by continued strong industry demand 2 0 2 1 A C C O M P L I S H M E N T S • Secured ~$415M of new business across all three service offerings (DVS+DS+DP), ending the year with ~60 customers • Significantly expanded service capabilities and contribution of Winnipeg site • Implemented state-of-the-art Aseptic Filling Technology (added 3 new aseptic filling lines to the CDMO network) N E T W O R K O F S I T E S S U P P O R T I N G T H E C D M O S E R V I C E S B U S I N E S S B A Y V I E W C A M D E N G A I T H E R S B U R G R O C K V I L L E W I N N I P E G T E C H N O L O G IE S • Viral • Mammalian • Bacterial • Non-viral • Viral • Mammalian • Bacterial • Viral • Plasma • Lotion • Complex formulation C A P A B IL IT IE S DEVELOPMENT SERVICES (DVS) ● ● DRUG SUBSTANCE (DS) ● ● DRUG PRODUCT (DP) ● ● ●

120M+ Dose Equivalents of COVID-19 Vaccine Released for Global Distribution Bayview Facility Represents Significant Contributor to Potential Future Growth and Impact 1340th Annual J.P. Morgan Healthcare Conference BUSINESS PERFORMANCE | BAYVIEW SITE UPDATE 2 0 2 1 A C C O M P L I S H M E N T S • Completed comprehensive facility enhancements in response to FDA inspection findings • Resumed production in August • Received GMP compliant status from certain health regulatory authorities L O N G - T E R M G R O W T H O P P O R T U N I T I E S • Global supply chain partner for J&J • Increase utilization of existing Drug Substance capacity

PROPRIETARY AND CONFIDENTIAL 1440th Annual J.P. Morgan Healthcare Conference Financials 2021 Preliminary | 2022 Guidance

2017 2018 2019 2020 2021E 2022G 2024G T O T A L R E V E N U E S 1 , 2 ( $ M ) 1540th Annual J.P. Morgan Healthcare Conference FINANCIALS | 2021 PRELIMINARY & 2022 GUIDANCE 1. 2021E (preliminary and unaudited) and 2022G (guidance) reflect the ranges provided in the press release issued by the Company on January 9, 2022. 2. AV7909 is not approved by the FDA or any other health regulatory authority, and Trobigard is not approved by the FDA; both are procured by authorized government agencies under special circumstances. 3. See the Appendix for a definition of non-GAAP terms and reconciliation tables. A D J U S T E D E B I T D A M A R G I N ( %) 1 , 3 $561 $782 $1,106 $1,555 $1,770 - $1,790 $1,400 - $1,500 Financial Performance Reflects Track Record of Diversified Profitable Revenue Growth 2017 2018 2019 2020 2021E 2022G x 20% - 23% 28% - 29% 41% 26% 32% 2024 TARGET $2,000 2024 TARGET 27% - 30%25% Product Sales | CDMO Services | Contracts & Grants

PROPRIETARY AND CONFIDENTIAL 1640th Annual J.P. Morgan Healthcare Conference Key Takeaways
Business on track to achieve 2024 goals Summary 1740th Annual J.P. Morgan Healthcare Conference KEY TAKEAWAYS New operating structure focused on customers and markets Strong manufacturing network with capacity for growth Continued focus on M&A to drive diversified profitable revenue growth Broad R&D portfolio offers additional drivers of growth 1 billion TO PROTECT AND ENHANCE LIVES BY 2030 WHERE OUR PATH FORWARD IS HEADED

1840th Annual J.P. Morgan Healthcare Conference Appendix

Reconciliation of Net Income to Adjusted EBITDA 2022G and 2021E-2017 (unaudited) 1940th Annual J.P. Morgan Healthcare Conference APPENDIX * Includes interest income of $0.5M in 2022G, $0.6M in 2021E and $1.1M in 2020. Full Year Guidance 2022G 2021E 2020 2019 2018 2017 Net Income $85.0 - $130.0 $260.0 - $280.0 $305.1 $54.5 $62.7 $82.6 Adjustments: + Depreciation & amortization 125.0 127.0 114.5 110.7 61.3 40.8 COGS; SG&A; R&D + Income taxes 34.0 - 49.0 75.0 - 80.0 102.1 22.9 18.8 36.0 Income Taxes + Total interest expense, net* 33.0 34.0 30.2 36.1 8.3 4.8 Other Expense + Changes in fair value of contingent consideration 1.0 3.0 31.7 24.8 3.1 7.8 COGS + Impairment of IPR&D intangible asset -- -- 29.0 12.0 -- -- R&D + Exit and disposal costs -- -- 17.2 -- 0.4 1.5 COGS; SG&A; Other Income + Acquisition-related costs (transaction & integration) 2.0 1.0 0.6 12.6 27.3 5.6 SG&A + Impact of purchase accounting on inventory step-up -- -- -- 6.1 18.4 2.6 COGS Total adjustments $195.0 - $210.0 $240.0 - $245.0 $325.3 $225.2 $137.6 $99.1 Adjusted EBITDA $280.0 - $340.0 $500.0 - $525.0 $630.4 $279.7 $200.3 $181.7 ($ in millions) Twelve Months Ended December 31, Source

Virology • Alphavirus, vector-borne, three genotypes • Enveloped RNA virus • Acute febrile illness with symptoms including fever, fatigue, and incapacitating joint pain • Many patients develop chronic arthritis and arthralgia which may persist for years The Chikungunya Virus (CHIKV) 2040th Annual J.P. Morgan Healthcare Conference APPENDIX Ecology • Transmitted by day-biting Aedes mosquitos • Distribution - Urban and suburban areas throughout tropics/subtropics - Currently established in more than 100 countries and territories - Mosquito vector distribution is predicted to continue to expand in the coming decades Epidemiology • Re-emergence in 2006 • Spread globally by 2013 • More than 7000 chikungunya cases in Europe and US since 2014 • Unpredictable, large outbreaks of acute febrile disease
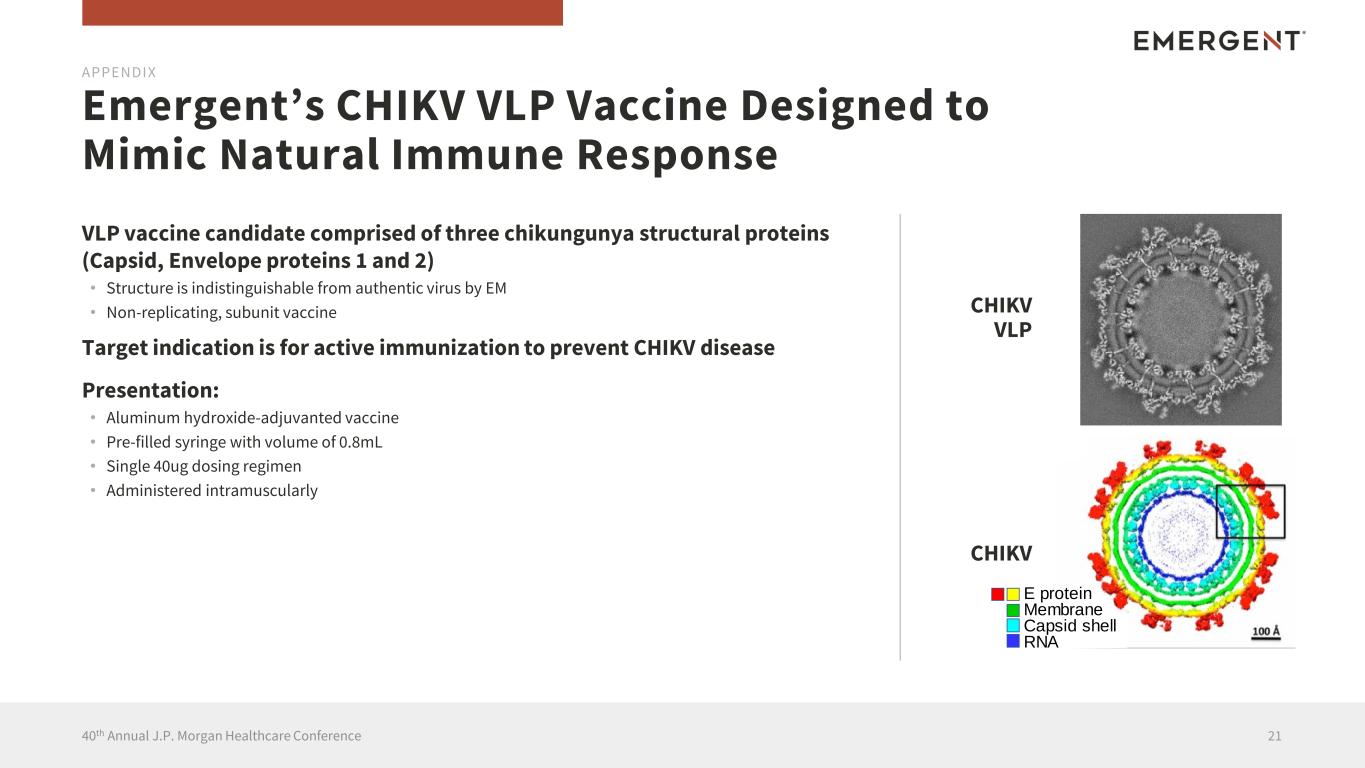
VLP vaccine candidate comprised of three chikungunya structural proteins (Capsid, Envelope proteins 1 and 2) • Structure is indistinguishable from authentic virus by EM • Non-replicating, subunit vaccine Target indication is for active immunization to prevent CHIKV disease Presentation: • Aluminum hydroxide-adjuvanted vaccine • Pre-filled syringe with volume of 0.8mL • Single 40ug dosing regimen • Administered intramuscularly Emergent’s CHIKV VLP Vaccine Designed to Mimic Natural Immune Response 2140th Annual J.P. Morgan Healthcare Conference CHIKV CHIKV VLP E protein Membrane Capsid shell RNA CHI VLP CHI APPENDIX

www.emergentbiosolutions.com





















