
4/29/2024 [**] Certain identified information has been excluded from this exhibit because it is (i) not material and (ii) is the type of information that the registrant treats as private or confidential. Double asterisks denote omission. [**] [**] [**] [**] [**] [**] [**] [**] [**]

Modification P00011 makes the following changes to the contract: 1. Reduces the annual not to exceed (NTE) amount for option years 5 through 9, 2. Reduces the minimum annual order quantity from [**] of ACAM2000 in Option Years 5 through 9; 3. Increases the total quantity of [**] from [**] and amends the option period in which [**] is provided; 4. Increases the quantity of [**] for Option Year 5 from [**]; and 5. Revises the Performance Work Statement to add [**] to the requirement at no additional cost to the Government. ALL OTHER TERMS AND CONDITIONS REMAIN THE SAME. Details of changes: Option Year 5, NTE amount reduced from [**] Option Year 6, NTE amount reduced from [**] Option Year 7, NTE amount reduced from [**] Option Year 8, NTE amount reduced from [**] Option Year 9, NTE amount reduced from [**] Table 1: Overall Price Summary, amended as follows (changes made by Modification P00011 are shown in red): Item CLIN X001 – Doses CLIN X002 – Diluent Replacement CLIN X003 – Syringe Replacement CLIN X004 – Re-labeling [**] [**] ACAM Testing Grand Total Contract Year ACAM Doses Extended Price Diluent Vials Extended Price Transfer Syringes Extended Price Target Expired Doses/SNS Expired Doses/SNS 000X Base [**] [**] [**] 100X Option Year 1 [**] [**] [**] [**] 200X Option Year 2 [**] [**] [**] [**] 300X Option Year 3 [**] [**] [**] 400X Option Year 4 [**] [**] [**] [**] [**] [**] [**] [**] 500X Option Year 5 [**] [**] [**] [**] [**] [**] [**] [**] [**] 600X Option Year 6 [**] [**] [**] [**] [**] [**] [**] [**] 700X Option Year 7 [**] [**] [**] [**] [**] [**] [**] [**] [**] 800X Option Year 8 [**] [**] [**] [**] [**] [**] [**] [**] 900X Option Year 9 [**] [**] [**] [**] [**] [**] [**] [**] [**] Overall [**] [**] [**] [**] [**] [**] [**] [**] [**] [**] Note: [**] Timing for [**] and ACAM testing will be determined annually, following the exercise of the associated annual procurement (CLIN X001). Table 2: Task 1 – ACAM2000 Vaccine Manufacture and Provision to the SNS, amended as follows (changes made by Modification P00011 are shown in red): 0001 (Base) 1001 (Option) 2001 (Option) 3001 (Option) 4001 (Option) Contract Year 08/15/19-09/30/19 10/01/19-09/30/20 10/01/20-09/30/21 10/01/21-10/30/22 10/31/22-09/30/23 Target Exercise- By Date 8/15/2019 12/1/2019 12/1/2020 12/1/2021 12/1/2022 Delivery Target 12/30/2019 12/30/2020 12/30/2021 12/30/2022 12/30/2023 [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**]Doses [**] [**] [**] [**] [**] Page 2 of 10
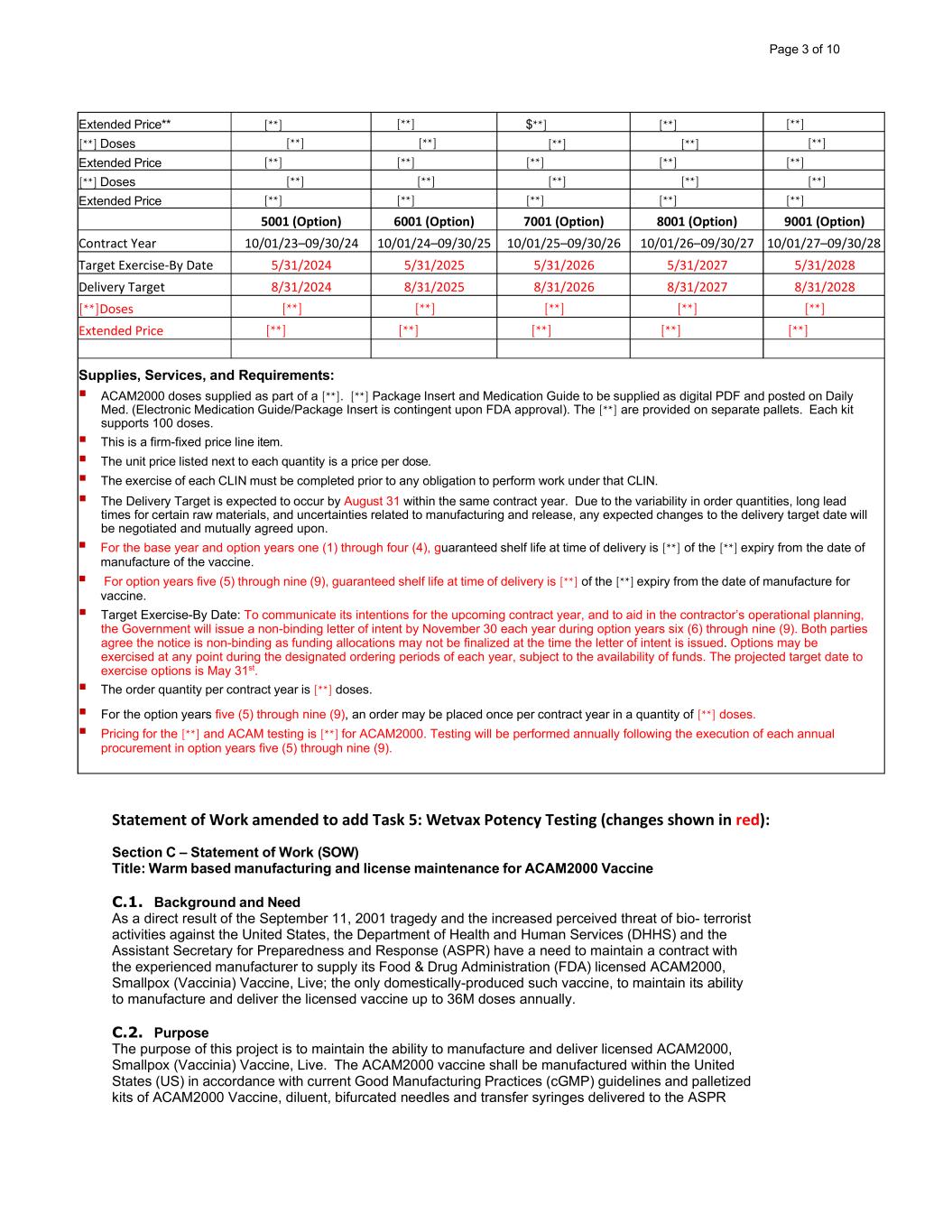
Extended Price** [**] [**] $**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] [**] Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] 5001 (Option) 6001 (Option) 7001 (Option) 8001 (Option) 9001 (Option) Contract Year 10/01/23–09/30/24 10/01/24–09/30/25 10/01/25–09/30/26 10/01/26–09/30/27 10/01/27–09/30/28 Target Exercise-By Date 5/31/2024 5/31/2025 5/31/2026 5/31/2027 5/31/2028 Delivery Target 8/31/2024 8/31/2025 8/31/2026 8/31/2027 8/31/2028 [**]Doses [**] [**] [**] [**] [**] Extended Price [**] [**] [**] [**] [**] Supplies, Services, and Requirements: ACAM2000 doses supplied as part of a [**]. [**] Package Insert and Medication Guide to be supplied as digital PDF and posted on Daily Med. (Electronic Medication Guide/Package Insert is contingent upon FDA approval). The [**] are provided on separate pallets. Each kit supports 100 doses. This is a firm-fixed price line item. The unit price listed next to each quantity is a price per dose. The exercise of each CLIN must be completed prior to any obligation to perform work under that CLIN. The Delivery Target is expected to occur by August 31 within the same contract year. Due to the variability in order quantities, long lead times for certain raw materials, and uncertainties related to manufacturing and release, any expected changes to the delivery target date will be negotiated and mutually agreed upon. For the base year and option years one (1) through four (4), guaranteed shelf life at time of delivery is [**] of the [**] expiry from the date of manufacture of the vaccine. For option years five (5) through nine (9), guaranteed shelf life at time of delivery is [**] of the [**] expiry from the date of manufacture for vaccine. Target Exercise-By Date: To communicate its intentions for the upcoming contract year, and to aid in the contractor’s operational planning, the Government will issue a non-binding letter of intent by November 30 each year during option years six (6) through nine (9). Both parties agree the notice is non-binding as funding allocations may not be finalized at the time the letter of intent is issued. Options may be exercised at any point during the designated ordering periods of each year, subject to the availability of funds. The projected target date to exercise options is May 31st. The order quantity per contract year is [**] doses. For the option years five (5) through nine (9), an order may be placed once per contract year in a quantity of [**] doses. Pricing for the [**] and ACAM testing is [**] for ACAM2000. Testing will be performed annually following the execution of each annual procurement in option years five (5) through nine (9). Statement of Work amended to add Task 5: Wetvax Potency Testing (changes shown in red): Section C – Statement of Work (SOW) Title: Warm based manufacturing and license maintenance for ACAM2000 Vaccine C.1. Background and Need As a direct result of the September 11, 2001 tragedy and the increased perceived threat of bio- terrorist activities against the United States, the Department of Health and Human Services (DHHS) and the Assistant Secretary for Preparedness and Response (ASPR) have a need to maintain a contract with the experienced manufacturer to supply its Food & Drug Administration (FDA) licensed ACAM2000, Smallpox (Vaccinia) Vaccine, Live; the only domestically-produced such vaccine, to maintain its ability to manufacture and deliver the licensed vaccine up to 36M doses annually. C.2. Purpose The purpose of this project is to maintain the ability to manufacture and deliver licensed ACAM2000, Smallpox (Vaccinia) Vaccine, Live. The ACAM2000 vaccine shall be manufactured within the United States (US) in accordance with current Good Manufacturing Practices (cGMP) guidelines and palletized kits of ACAM2000 Vaccine, diluent, bifurcated needles and transfer syringes delivered to the ASPR Page 3 of 10

Strategic National Stockpile (SNS). C.3. Scope of Work The Contractor, as an independent organization and not as an agent of the Government, shall furnish all labor, materials, supplies, facilities, equipment, transportation and travel necessary to manufacture and deliver listed doses of licensed ACAM2000 vaccine, in accordance with C.10. The contractor shall deliver an equivalent quantity of ancillaries [**] for use in the administration of the ACAM2000 vaccine. The contractor shall perform [**] provided by the ASPR/SNS. The contractor shall comply with the following objectives: (Task 1) Manufacture and deliver, Food & Drug Administration (FDA) licensed ACAM2000 under Biologic License Application (BLA) Submission Tracking Number (STN) 125158 from approved facilities with the timeframe and quantities in accordance with Task 1. (Task 1) Maintain the agency-approved Stability testing structure which assesses the long- term safety and efficacy of the ACAM2000 Lyophilized Drug Product at the Strategic National Stockpile and supports [**] of shelf life ([**]) from the time of product manufacture. (Task 1) Perform [**] on ACAM2000. (Task 2) Replace expiring ACAM2000 diluent as related to new doses supplied under this contract (Task 3) Replace expiring transfer syringes as related to new doses supplied under this contract (Task 4) Relabel vaccine to [**] shelf-life (Task 5) Perform [**] provide by ASPR. (C.5.) Provide quarterly reports and update meetings by the 30th day following the end of the quarter. C.4 Task 1: Manufacturing and Delivery of ACAM2000 Vaccine The Contractor shall manufacture and deliver, in accordance with this Task 1, licensed ACAM2000, including ancillary components which shall be delivered to the SNS annually, and produced entirely in the US in accordance with this Statement of Work. The product shall meet all requirements as specified in the approved FDA license and any approved supplements or amendments thereto. Ancillary components include [**] One vial of [**] will be provided for USP for each [**] The vaccine and [**] will be labeled and packaged as outlined in the BLA and subsequently approved FDA license. Manufacture of Bulk Vaccine The contractor shall manufacture the cell cultures required for the domestic production of ACAM2000 at its US bulk vaccine manufacturing facility at the 500L bioreactor scale. The contractor shall perform all Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) on any equipment required to produce the vaccine. The domestic manufacturing of the ACAM2000 vaccine in Vero cells grown on micro carrier beads at the 500 Liter scale shall be in accordance with the approved ACAM2000 BLA on file with the FDA. Vaccine Fill and Finish The Contractor shall maintain a validated filling, lyophilization, labeling and packaging of ACAM2000 at its US fill and finish facility and shall perform all IQ, OQ, and PQ on any additional equipment required at that facility and associated process development. Requirements for Packaging & Delivery The products shall be delivered as follows: ACAM2000, (Smallpox (Vaccinia) Vaccine, Live), shall be provided in a multiple dose [**]. Each vial shall contain [**] after reconstitution with [**]. Each vaccine box shall Page 4 of 10

contain [**]. Each case shall contain [**]. For the base and option years one (1) through four (4), the guaranteed shelf life at time of delivery is [**] of the [**]expiry date from the date of manufacture of the vaccine. For option years five (5) through nine (5), the guaranteed shelf life at time of delivery is [**] of the [**] expiry from the date of manufacture for the vaccine. ACAM2000 Diluent shall be provided as [**] Each diluent box shall contain 50 vials. Each case shall contain [**]. The diluent shall have a [**] shelf life. The diluent should have [**] of shelf life remaining at the time of delivery. Bifurcated needles shall be supplied in boxes containing [**] each. Each case shall contain [**] bifurcated needles with an expiry date of [**] from date of manufacture. Transfer Syringes shall be provided as [**]. Each box shall contain [**] per case. Transfer syringes have an expiry date of [**] from the date of manufacture. [**] Complete, unopened cases of [**] will be provided. The additional quantity required to provide complete cases (rounded up quantity in excess of [**] requirements) will be provided at no additional cost to the Government). The provided kit summary should note both the Emergent lot number and the manufacturer’s lot number for needles and syringes. FDA-approved ACAM2000 Package Insert and Medication Guides shall be provided per the license as a compact disk (CD). Emergent will work with the FDA to phase-out the CDs and provide a suitable electronic solution. The contractor shall notify the Contracting Officer’s Representative (COR) when the Package Insert or Medication Guide are revised and will ship the current PDF version with each delivery order. Boxes, cases and shelf cartons shall not contain mixed lot numbers. All pallets are to have the identical TiHi stack pattern except for the last final pallet per lot number. All ACAM2000 vaccine product shall be delivered on standard 48" by 40" pallet, not to exceed 60” in height, stretch wrapped and secured to pallet for safe transport. Prior to an ACAM2000 vaccine and ancillary delivery, the Kit Component Inventory Summary Sheet which provides the ACAM2000 Kit [**] Inventory Summary, shall be provided outlining the products in each delivery; lot numbers of each item; number of full and partial boxes, cases and pallets; and the total quantities of each items in vials or each. The contractor shall provide Certificates of Analysis and Certificates of Conformance for the vaccine at least 3 days prior to shipment arriving at the SNS facility. The contractor shall also provide Certificates of Analysis and Vendor Certificate of Manufacturing (or Conformance) for the diluent prior to shipment arriving at the SNS facility. Driver information for each delivery truck shall be provided as soon as available to the SNS site. After the delivery, a documented review of the temperature data from the vaccine and diluent shipments will be provided. SNS Locations Delivery location shall be in accordance with United States Government (USG) instructions provided one (1) month prior to the shipment and are specific exclusively to Continental US (CONUS) locations. Locations outside CONUS may incur additional shipping/validation charges which will result in a contract modification to be performed prior to shipment. No more than[**] annually. Vaccine relabeling shall occur at current inventory locations unless otherwise agreed upon. Delivery shall also include ancillary items for reconstitution of each vial of vaccine (diluent, transfer syringe) as well as a bifurcated needle for administration of each dose of vaccine. Packaging, labeling and delivery shall be done according to the methodology and specifications outlined in the current BLA filed with the FDA and as outlined in Section C.6, C.8. Section D, Section E. Page 5 of 10

Warranties A warranty shall be provided for vaccines, diluent, transfer syringes, and bifurcated needles delivered to the USG during this 10-year contract. Vaccine will have [**] of shelf-life remaining at time of delivery for the base and option years one (1) through four (4). Vaccine will have [**] of shelf-life remaining at time of delivery for option years five (5) through nine (9). Diluent will have [**] of shelf-life remaining at time of delivery. The transfer syringes should have [ **] of shelf life remaining at the time of delivery. Transfer Syringes and Needles are commercial products. In the event of supplier and/or delivery issues that result in shelf life not meeting minimum requirements, the parties will develop suitable alternatives in delivery and pricing. For ACAM2000 delivered during this contract, if it is determined that the ACAM2000 vials delivered do not meet the shelf life standard of [**] expiry from the date of manufacture (with a minimum of [**] of shelf life remaining at the time of delivery) and that USG has met its storage and handling obligations for such products pursuant to a separate Quality Agreement, then the Contractor will provide an equitable remedy based on the remaining shelf life of the non-conforming product(s), which will include one of the following: (a) replacement product (only during the contract period), (b) a credit against future purchases under the contract, or (c) reimbursement. Historical Lots- Testing Program and Quarantine Doses manufactured and distributed prior to 2018 had labeled expiry periods of less than the current 16- year expiry. As these lots approach their labeled expiry dates, Emergent will coordinate with SNS and the SNS sites to re-label impacted lots with [**]. Once lots have exceeded the FDA approved [**] expiry, they will be quarantined by SNS and will not be distributed for any use. Given the commitment for a long-term contract to replace the stockpile, Emergent will agree to test representative lots of quarantined product on an annual basis and provide the data as For Information Only (FIO) to SNS until replenished stockpile inventory levels meet the desired threat assessment level. This information will be provided as soon as it is available and following the Government placing its annual optional CLIN order for at least 9 million ACAM2000 doses. Under this Vaccine Testing Program, Emergent understands that, in the event of an emergency, the SNS would be responsible for seeking an appropriate regulatory mechanism to deploy for use quarantined product. The contractor is permitted to follow its own procedures for stability testing of historical lots. In the event of an unexpected or atypical result, the contractor may conduct an investigation to rule out laboratory error. Upon confirmation of the results and testing, results shall be shared with the SNS; Up to seven (7) historical lots shall be tested; Testing would continue only with the concurrent exercise of the option for product delivery; Testing would only continue until the stockpile reaches the threat assessment level ([**] doses); Based on the lots tested and the proposed delivery schedule, the maximum that lots would be tested is anticipated to be no more than [**] from the date of manufacture; The product expiration will remain at [**]. This testing will not be used to support any further extension to the product expiry dating. Under this agreement, the product maintained in the SNS stockpile beyond the shelf life of [**] is no longer considered to be under our license. Emergent is not making any representation or guarantees that the testing will support extensions to the shelf life and the testing data is not being provided to the SNS in support of expiry extensions. As a result, Emergent will also no longer be performing any activities beyond the submission of the data to the SNS. The testing data is not being provided to the SNS in support of expiry extensions, and any discussions with the FDA regarding the use of the stockpile in an emergency event or any use of this product will be captured under a different regulatory mechanism outside of Emergent’ s license and will be the responsibility of the SNS. Page 6 of 10

The following table provides a summary detailing the strategy for managing expiring ACAM2000 vaccine: Table 6: Strategy for managing expiring ACAM2000 vaccine Task 2: Diluent Replacement The contractor shall replace expiring ACAM2000 diluent related this contract (see Task 2 table). Government to provide forecast annually to confirm the quantity to be delivered Options may be exercised after the base year is awarded through year 10 at the quantities also outlined herein. If the quantity of diluent requiring replacement changes from the quantity outlined in Section C.6, both parties shall agree to the change. Note timing listed in C.6 are approximations as replacement timing is dependent of the manufacturing date of shipped quantities. The contractor shall deliver [**]. The diluent shall be packaged in [**] vials per case. The diluent shall have a [**] shelf life. At the time of delivery to the SNS the diluent should have [**] of shelf life remaining. Task 3: Syringe Replacement Transfer Syringe quantities will be in increments of a full case box (divisible by [**]). Replacement of transfer syringes sold prior to this contract are outside the scope of this proposal and can be quoted separately upon request. Task 4: ACAM2000 Vaccine Limited Re-Labeling Activities The ACAM2000 vaccine currently has an expiration date of [**] Emergent will re-label vaccine lots located at the Strategic National Stockpile (SNS) for lots produced by the Rockville MD facility which have less than an expiry of [**] as they approach their current labeled expiration. (See table below). Quantities are estimates and will be verified prior to relabeling efforts. Task 5: [**] Category Proposed Strategy Older lots with [**] since date of manufacture All lots can be managed as having [**] per STN 125158/203 (approved 15 Dec 2017). These lots will be re- labeled with [**] expiry per the schedule in Section C below. Once these lots exceed [**] the lots will be quarantined by SNS, per below. Older lots that have [**] since date of manufacture These lots have, or will have, exceeded the FDA approved expiry period and will be quarantined by SNS. The lots will not be distributed by ASPR for any purpose (vaccination, research, etc.) except under an approved appropriate regulatory mechanism. Once SNS has achieved its inventory goal for ACAM2000 vaccine, all quarantined expired doses should be destroyed. New lots with [**] expiry* *It is expected the U.S. Government will deploy in date vaccine first and quarantined vaccine would only be deployed after all in date product is depleted (and with the appropriate regulatory oversight). All newly manufactured lots under this contract will be labeled with [**] expiry. Therefore, none of these lots will expire during the [**] period of this contract. When the lots do expire in the future, the expired materials should be destroyed. Page 7 of 10

[**] Table 7: Re-labeling of Rockville MD Manufactured Lots with [**] expiry Page 8 of 10 [**]

C.5. Reporting Requirements (Emergent) The contractor shall submit a Quarterly Progress Report, which shall include the information listed below that is applicable for the performance period during the quarter being reported. The contractor shall provide the Contracting Officer’s Representative (COR) with one electronic copy of the Quarterly Progress Report via e-mail. Any attachments to the report shall be submitted in Microsoft Word, Adobe Acrobat, or similar files. The contractor shall meet with the COR quarterly to discuss the Quarterly Progress Report. The contractor shall submit the Quarterly Reports and schedule the meetings by the 30th day following the end of the quarter. The following shall be included in the quarterly report. 1. Quarterly Reporting Requirements Manufacturing and Delivery of ACAM2000® Kits a. Procurement and Production b. Quality Control Testing and Potency c. Quality Manufacturing Deviations (major) 2. FDA inspections, consultation results or recommendations and any files to the FDA concerning the ACAM2000 BLA 3. Security Assessment 4. Stability Program Assessment (Provided Annually) 5. Overall Project Assessment a. Delivery Summary b. Projected Deliveries for next reporting period c. Plan vs. Actual and Specific problems to address C.6. Reporting Requirements (SNS) SNS shall provide the following information to Emergent at the frequency described below: 1. Doses delivered from SNS. This number is needed for safety reporting (ANNUALLY, October) 2. Destruction of expired lots of ACAM Vaccine. (ANNUALLY for record retention) 3. ACAM Vaccine lots in inventory, number of vials, and quantity in quarantine. (ANNUALLY for over-labelling planning) 4. ACAM Diluent lots in inventory, number of vials. (ANNUALLY) 5. Note: Updated inventory on specific lots may be required as part of investigations throughout the contract. 6. The Contractor shall prepare and submit the following report: Report Type Description Format Due Date Monthly Status Report The contractor shall report on contractor’s compliance with the requirements of FAR 52.223-99 Ensuring Adequate COVID-19 Safety Protocols for Federal Contractors (OCT 2021) (Deviation) Email to the Contracting Officer and COR Monthly, no later than the 5th calendar day of the month following the previous monthly period of performance. C.7. Delivery Notifications Emergent shall notify the COR of the total quantity of product(s) and pallet count that will be delivered utilizing the Kit Component Inventory Summary Sheet, which provides the ACAM2000 Kit [**] Inventory Summary to the SNS at least five (5) business days prior to each delivery. C.8. Quality Inspections Site Visits/Audits: The Government shall perform annual site visits/security audits as deemed necessary by the Government throughout the period of performance of the contract. Quality: The Government reserves the right to visit the contractor’s site for purposes of Page 9 of 10

assessing quality on an annual basis or as deemed necessary by the Government throughout the period of performance of the contract. Notice: The Government will provide 2 weeks advance notice prior to the Contractor of all site visits and audits. The notice will include a statement concerning the intended scope of the audit and a list of the required documents or access to personnel. Note: Facilities with live vaccine is in use (core production/testing areas) require vaccinations or waivers prior to entry. All audits will be conducted between normal business hours i.e., 8 a.m. through 4 p.m., Monday through Friday. Report to be provided by the Government as to any observations associated with site visits/audits. The government reserves the right to inspect any contractor or subcontractor facility used for the manufacture, packaging, storage, transportation or any other handling of products ordered as a result of this contract without prior notice. These inspections do not replace any required inspections conducted by the FDA but are in addition to such inspections. The contractor will be required to respond to any finding’s resultant from these inspections with remediation plans or an explanation of why no remediation is required. Page 10 of 10









