
Proprietary and Confidential. © 2014 Concert Pharmaceuticals, Inc. All Rights Reserved. Product Innovation. Patient Benefit. April 2017

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management and expected market growth, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward- looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make as a result of various important factors, including the factors discussed in the "Risk Factors" section of our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission and in other filings that we make with the Securities and Exchange Commission. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. 2

This communication is being made in respect of the proposed asset sale with Vertex. The proposed asset sale and the asset purchase agreement will be submitted to the shareholders of the Company for their consideration and approval. In connection with the proposed asset sale, the Company will file a proxy statement with the SEC. This communication does not constitute a solicitation of any vote or proxy from any shareholder of the Company. Investors are urged to read the proxy statement carefully and in its entirety when it becomes available and any other relevant documents or materials filed or to be filed with the SEC or incorporated by reference in the proxy statement, because they will contain important information about the proposed asset sale. The definitive proxy statement will be mailed to the Company’s shareholders. In addition, the proxy statement and other documents will be available free of charge at the SEC’s internet website, www.sec.gov. When available, the proxy statement and other pertinent documents may also be obtained free of charge at the Investors section the Company’s website, www.concertpharma.com, or by directing a written request to Concert Pharmaceuticals, Inc., Attn: Corporate Communications and Investor Relations, in writing, at 99 Hayden Ave, #500, Lexington, MA 02421. The Company and its directors, executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in connection with the proposed asset sale. Information about the Company’s directors and executive officers is included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2016 filed with the SEC on March 6, 2017. Additional information regarding these persons and their interests in the transaction will be included in the proxy statement relating to the proposed asset sale when it is filed with the SEC. These documents can be obtained free of charge from the sources indicated above. Additional Information about the Transaction and Where to Find It 3

Corporate Highlights CTP-656, proprietary next-generation treatment for cystic fibrosis – Recent asset purchase agreement with Vertex Pharmaceuticals realizes significant value for CTP-656 Pro forma cash expected to fund operations into 2021 Multiple efficacy studies in 2017 – CTP-543, proprietary JAK 1/2 inhibitor for moderate-to-severe alopecia areata, entering Phase 2 – CTP-656 U.S. Phase 2 study in gating mutations underway – AVP-786, Phase 3 trial for Alzheimer’s agitation expected to complete Q3 2018 (partnered with Avanir/Otsuka) 4

Robust Clinical Pipeline 5 Phase I Phase 2 Product Candidate Lead Indication(s) Worldwide Rights AVP-786 Deuterated dextromethorphan CTP-730 Deuterated apremilast Inflammatory Diseases JZP-386 Deuterated sodium oxybate CTP-543 Deuterated ruxolitinib Phase 3 CTP-656* Deuterated ivacaftor Multiple Neurologic/Psychiatric Indications Alzheimer’s Agitation Cystic Fibrosis Alopecia Areata Narcolepsy Market *Subject to closing of the CTP-656 Asset Purchase Agreement with Vertex Pharmaceuticals

CTP-656: Next Generation Treatment for Cystic Fibrosis Cystic fibrosis is a genetic disease affecting ~75,000 patients worldwide Average life expectancy has doubled in last two decades CTP-656 (deuterated analog of ivacaftor*) is a potentiator which improves CFTR activity – Potential to improve upon efficacy of Kalydeco® (ivacaftor) – Phase 2 U.S. trial underway Entered asset purchase agreement with Vertex Pharmaceuticals March 2017 Treatment for Cystic Fibrosis Undergoing Major Advances 6 Source: cff.org * Ivacaftor is marketed by Vertex Pharmaceuticals, Inc. under the brand name Kalydeco®
CTP-656: Asset Purchase Agreement Up to $250 million agreement with Vertex Pharmaceuticals – $160 million upfront payment with $90 million in milestone potential – Vertex to acquire all rights to CTP-656 Vertex is a pioneer in CF with a broad pipeline and the capability to efficiently advance CTP-656 – Potential for combination products benefiting the larger CF patient population Closing of transaction subject to Concert shareholder approval and HSR clearance Upon closing, cash projected into 2021 – Enable advancement of CTP-543 into pivotal testing – Strengthen proprietary pipeline – Potential to realize AVP-786 royalties 7
CTP-656: U.S. Phase 2 Trial Ongoing Phase 2 trial in patients with gating mutations initiated December 2016 – 30-40 patients – 4 week treatment duration – 3 doses of CTP-656 – Placebo and Kalydeco comparators – Key endpoints: sweat chloride and FEV1 Trial continuing as planned Phase 2 topline data expected Q4 2017* 8*Pending transfer of program to Vertex
CTP-543: Potential First Oral Treatment for Alopecia Areata Target indication: moderate-to-severe alopecia areata – Common autoimmune disorder causing partial or widespread loss of hair on the scalp and/or body CTP-543: deuterated ruxolitinib – Ruxolitinib: potent, selective JAK1/2 inhibitor approved for myelofibrosis, polycythemia vera Clinical proof-of-concept demonstrated with ruxolitinib in alopecia areata Phase 2a trial planned 9 Opportunity to address important unmet medical need source. derm101.com
Alopecia Areata: A Devastating and Poorly Treated Autoimmune Disease Up to 650,000 patients affected with alopecia areata in the U.S. at any given time* Chronic condition affecting women, men and children of all ages Often leads to psychological consequences, including anxiety and depression No FDA-approved treatment options FDA selected alopecia areata as area of focus under its 2016-2017 Patient- Focused Drug Development Initiative *Fricke M. Clinical, Cosmetic and Investigational Dermatology, 2015. 10
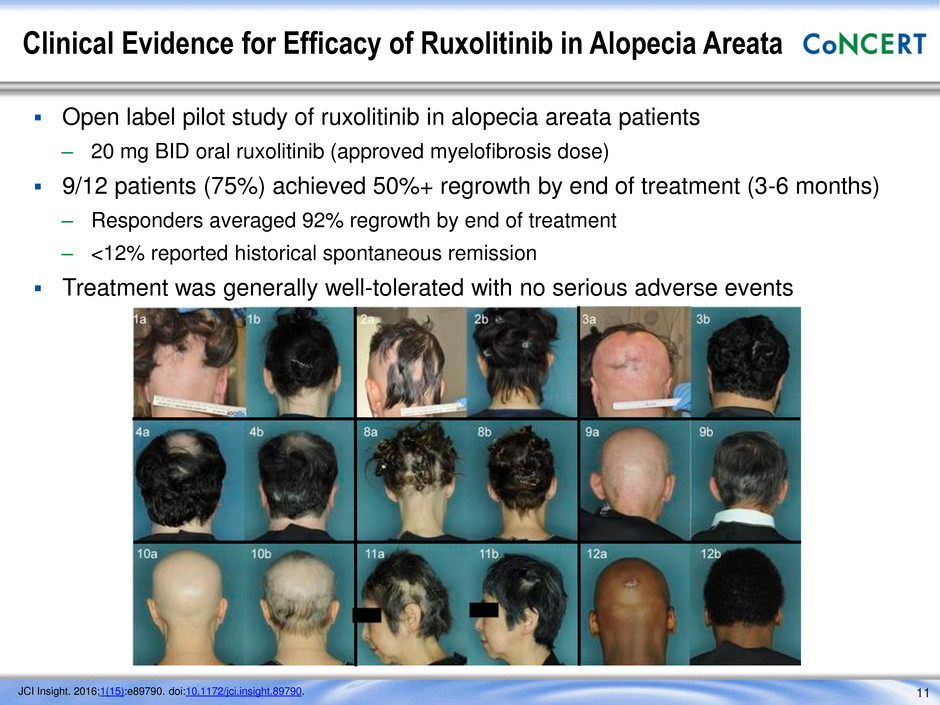
Clinical Evidence for Efficacy of Ruxolitinib in Alopecia Areata 11 Open label pilot study of ruxolitinib in alopecia areata patients – 20 mg BID oral ruxolitinib (approved myelofibrosis dose) 9/12 patients (75%) achieved 50%+ regrowth by end of treatment (3-6 months) – Responders averaged 92% regrowth by end of treatment – <12% reported historical spontaneous remission Treatment was generally well-tolerated with no serious adverse events JCI Insight. 2016;1(15):e89790. doi:10.1172/jci.insight.89790.
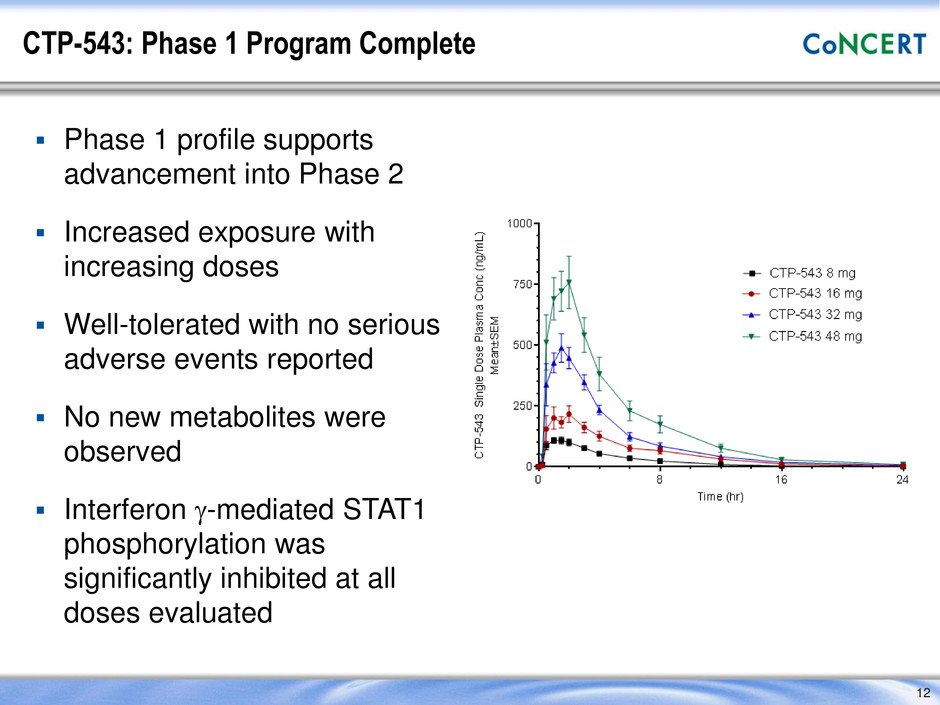
CTP-543: Phase 1 Program Complete Phase 1 profile supports advancement into Phase 2 Increased exposure with increasing doses Well-tolerated with no serious adverse events reported No new metabolites were observed Interferon g-mediated STAT1 phosphorylation was significantly inhibited at all doses evaluated 12

CTP-543: Potential First FDA-Approved Oral Treatment for Alopecia Areata Phase 2a trial planned – Double-blind, placebo-controlled, dose-ranging – Approximately 100 adults with moderate-to- severe alopecia areata At least 50% hair loss as measured by Severity of Alopecia Tool (SALT) – 12 month dosing with primary efficacy analysis at week 24 Primary Endpoint: 50% relative reduction in SALT between week 24 and baseline Topline data expected Q1 2018 13

AVP-786: Potential First-in-Class Treatment for Agitation in Alzheimer’s Disease Estimated 5.3M Americans have Alzheimer’s disease; approximately 50% of patients experience agitation – No currently approved therapies Phase 3 trials underway for blockbuster indication – Expected completion: Q3 2018 Otsuka (Avanir) responsible for development and commercialization – $170M upfront/milestone potential; $8M achieved to date – $5M milestone on acceptance of NDA – Mid-single to low-double digit royalties 14
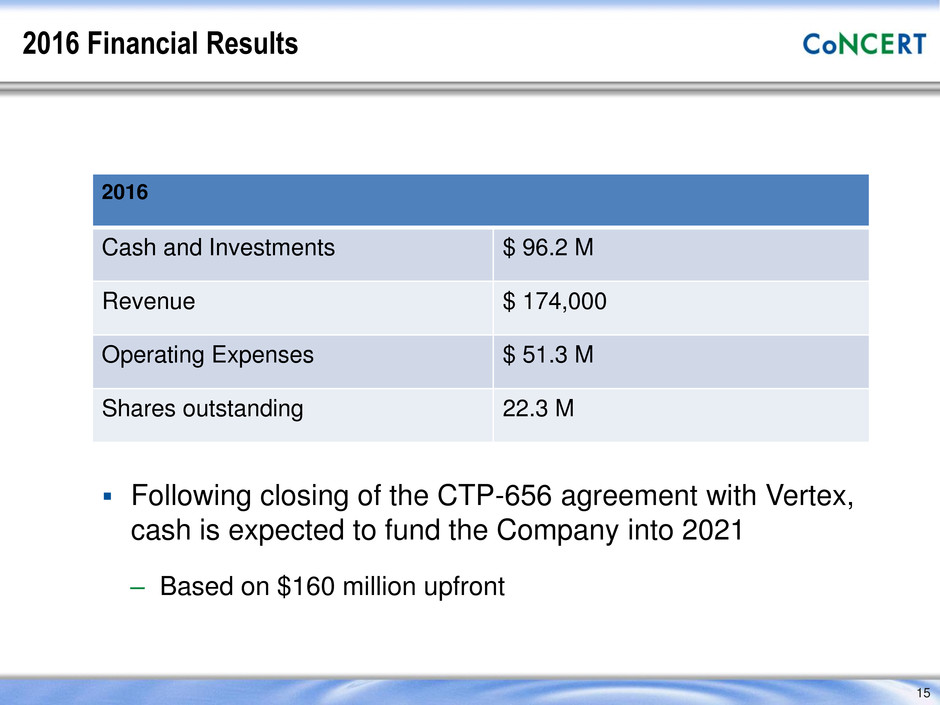
2016 Financial Results 2016 Cash and Investments $ 96.2 M Revenue $ 174,000 Operating Expenses $ 51.3 M Shares outstanding 22.3 M 15 Following closing of the CTP-656 agreement with Vertex, cash is expected to fund the Company into 2021 – Based on $160 million upfront

2017 Multiple Potential Value Creating Events 16 CTP-656 – Closing of asset purchase agreement with Vertex – Report US Phase 2 monotherapy top-line efficacy data (Q4 2017)* CTP-543 Report Phase 1 PD top-line data (Q1 2017) Report Phase 1 crossover data (Q1 2017) – Initiate Phase 2a trial (2017) – Report Phase 2a topline results (Q1 2018) Upcoming Development Milestones for Proprietary Compounds *Pending transfer of program to Vertex

Proprietary and Confidential. © 2014 Concert Pharmaceuticals, Inc. All Rights Reserved. NASDAQ: CNCE www.concertpharma.com For additional information contact: Justine Koenigsberg ir@concertpharma.com
















