UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
x QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended: September 30, 2009
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________ to _____________
Commission File Number: 000-52807
| CHINA BIOLOGIC PRODUCTS, INC. |
| (Exact name of registrant as specified in its charter) |
| | Delaware | | 75-2308816 | |
| | (State or other jurisdiction of incorporation | | (I.R.S. Employer Identification No.) | |
| | or organization) | | | |
| No. 14 East Hushan Road |
| Tai’an City, Shandong 271000 |
| People’s Republic of China |
| (Address of principal executive offices) |
| (+86) 538-620-2306 |
| (Registrant’s telephone number, including area code) |
| |
| |
| (Former name, former address and former fiscal year, if changed since last report) |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o |
| Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The number of shares outstanding of each of the issuer’s classes of common stock, as of November 12, 2009 is as follows:
| Class of Securities | | Shares Outstanding |
| Common Stock, $0.0001 par value | | 22,650,442 |

Quarterly Report on FORM 10-Q
Three and Nine Months Ended September 30, 2009
TABLE OF CONTENTS
PART I
FINANCIAL INFORMATION
| Item 1. | Financial Statements | 3 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 39 |
| Item 3. | Quantitative and Qualitative Disclosures About Market Risk | 57 |
| Item 4. | Controls and Procedures | 57 |
PART II
OTHER INFORMATION
| Item 1. | Legal Proceedings | 59 |
| Item 1A. | Risk Factors | 59 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 59 |
| Item 3. | Defaults Upon Senior Securities | 59 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 59 |
| Item 5. | Other Information | 59 |
| Item 6. | Exhibits | 59 |
PART I
FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS.
CHINA BIOLOGIC PRODUCTS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Contents | | Page(s) |
| Consolidated Balance Sheets (unaudited) | | 3 |
| Consolidated Statements of Operations and Other Comprehensive Income (Loss) (unaudited) | | 4 |
| Consolidated Statements of Changes in Equity (unaudited) | | 5 |
| Consolidated Statements of Cash Flows (unaudited) | | 6 |
| Notes to the Consolidated Financial Statements (unaudited) | | 7 |
2
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
AS OF SEPTEMBER 30, 2009 AND DECEMBER 31, 2008
ASSETS
| | | September 30, | | | December 31, | |
| | | 2009 | | | 2008 | |
| | | (Unaudited) | | | | |
| CURRENT ASSETS: | | | | | | |
| Cash and cash equivalents | $ | 50,348.133 | | $ | 8,814,616 | |
Accounts receivable, net of allowance for doubtful accounts of $1,393,567 and
$1,268,052 as of September 30, 2009 and December 31, 2008, respectively |
|
1,473,942 |
|
|
313,087 |
|
| Accounts receivable - related party | | 41,430 | | | - | |
| Dividend receivable | | - | | | 147,256 | |
| Other receivables | | 926,581 | | | 356,957 | |
| Inventories | | 33,218,618 | | | 14,949,196 | |
| Prepayments and deferred expense | | 1,582,566 | | | 614,704 | |
| Total current assets | | 87,591,270 | | | 25,195,816 | |
PLANT AND EQUIPMENT, net |
|
27,849,832 |
|
|
19,299,364 |
|
OTHER ASSETS: |
|
|
|
|
|
|
| Investment in unconsolidated affiliate | | 6,277,894 | | | 6,533,977 | |
| Refundable deposit for potential acquisition | | - | | | 14,181,800 | |
| Prepayments - non-current | | 4,870,735 | | | 955,874 | |
| Intangible assets, net | | 21,152,417 | | | 1,002,561 | |
| Goodwill | | 12,425,589 | | | - | |
| Total other assets | | 44,726,635 | | | 22,674,212 | |
Total assets |
$ |
160,167,737 |
|
$ |
67,169,392 |
|
| LIABILITIES AND EQUITY | |
| | | | | | | |
| CURRENT LIABILITIES: | | | | | | |
| Accounts payable | $ | 3,610,470 | | $ | 2,481,889 | |
| Notes payable | | - | | | 29,340 | |
| Short term loans - bank | | 10,782,450 | | | - | |
| Short term loans - holder of noncontrolling interest | | 4,425,777 | | | 773,277 | |
| Other payables and accrued liabilities | | 17,118,218 | | | 3,962,931 | |
| Other payable - related parties | | 3,086,940 | | | - | |
| Accrued interest - holder of noncontrolling interest | | 1,319,556 | | | - | |
| Distribution payable to holder of noncontrolling interest | | 759,319 | | | 3,252,354 | |
| Customer deposits | | 7,751,013 | | | 1,091,792 | |
| Taxes payable | | 5,913,231 | | | 4,060,010 | |
| Long term loan - bank, current maturities | | 3,374,100 | | | - | |
| Investment payable | | 2,625,405 | | | 3,275,501 | |
| Total current liabilities | | 60,766,479 | | | 18,927,094 | |
OTHER LIABILITIES: |
|
|
|
|
|
|
| Non-current other payable - land use right | | 324,121 | | | 325,390 | |
| Notes payable, net of discount of $9,508,965 as of September 30, 2009 | | 45,175 | | | - | |
| Long term loan - bank, net of current maturities | | - | | | 5,868,000 | |
| Derivative liability - conversion option | | 12,784,873 | | | - | |
| Fair value of derivative instruments | | 7,943,174 | | | - | |
| Total other liabilities | | 21,097,343 | | | 6,193,390 | |
Total liabilities |
|
81,863,822 |
|
|
25,120,484 |
|
COMMITMENTS AND CONTINGENCIES |
|
- |
|
|
- |
|
EQUITY: |
|
|
|
|
|
|
Common stock, $0.0001 par value, 100,000,000 shares authorized, 22,650,442
and 21,434,942 shares issued and outstanding at September 30, 2009 and
December 31, 2008, respectively |
|
2,265 |
|
|
2,143 |
|
| Paid-in-capital | | 19,191,623 | | | 10,700,032 | |
| Statutory reserves | | 13,413,353 | | | 6,989,801 | |
| Retained earnings | | 13,074,618 | | | 15,392,253 | |
| Accumulated other comprehensive income | | 5,102,487 | | | 4,752,885 | |
| Total shareholders' equity | | 50,784,346 | | | 37,837,114 | |
NONCONTROLLING INTEREST |
|
27,519,569 |
|
|
4,211,794 |
|
Total equity |
|
78,303,915 |
|
|
42,048,908 |
|
Total liabilities and equity |
$ |
160,167,737 |
|
$ |
67,169,392 |
|
The accompanying notes are an integral part of these consolidated financial statements.
-3-
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME (LOSS)
FOR THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(Unaudited)
| | | Three months ended | | | Nine months ended | |
| | | September 30, | | | September 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| | | | | | | | | | | | | |
| REVENUES | | | | | | | | | | | | |
| Revenues | $ | 26,871,259 | | $ | 13,799,915 | | $ | 80,861,353 | | $ | 33,574,764 | |
| Revenues – related party | | 168,480 | | | - | | | 508,529 | | | - | |
| Total revenues | | 27,039,739 | | | 13,799,915 | | | 81,369,882 | | | 33,574,764 | |
COST OF REVENUES |
|
|
|
|
|
|
|
|
|
|
|
|
| Cost of revenues | | 6,942,948 | | | 4,138,077 | | | 22,283,881 | | | 9,725,103 | |
| Cost of revenues – related party | | 17,953 | | | - | | | 53,715 | | | - | |
| Total cost of revenues | | 6,960,901 | | | 4,138,077 | | | 22,337,596 | | | 9,725,103 | |
GROSS PROFIT |
|
20,078,838 |
|
|
9,661,838 |
|
|
59,032,286 |
|
|
23,849,661 |
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
|
|
|
| Selling expenses | | 619,467 | | | 780,246 | | | 2,313,577 | | | 1,785,340 | |
| General and administrative expenses | | 5,169,137 | | | 1,634,233 | | | 14,996,846 | | | 5,756,087 | |
| Research and development expenses | | 262,500 | | | 201,037 | | | 1,098,083 | | | 664,652 | |
| Total operating expenses | | 6,051,104 | | | 2,615,516 | | | 18,408,506 | | | 8,206,079 | |
INCOME FROM OPERATIONS |
|
14,027,734 |
|
|
7,046,322 |
|
|
40,623,780 |
|
|
15,643,582 |
|
OTHER EXPENSES (INCOME): |
|
|
|
|
|
|
|
|
|
|
|
|
| Equity in loss (income) of unconsolidated affiliate | | (31,051 | ) | | - | | | 19,092 | | | - | |
| Change in fair value of derivative liabilities | | 13,242,333 | | | - | | | 14,931,088 | | | - | |
| Interest expense (income), net | | 724,771 | | | (21,713 | ) | | 1,979,538 | | | (7,531 | ) |
| Other expense (income), net | | 337,645 | �� | | 57,815 | | | 372,955 | | | 110,267 | |
| Total other expenses, net | | 14,273,698 | | | 36,102 | | | 17,302,673 | | | 102,736 | |
INCOME (LOSS) BEFORE PROVISION FOR INCOME TAXES AND NONCONTROLLING INTEREST |
|
(245,964 |
) |
|
7,010,220 |
|
|
23,321,107 |
|
|
15,540,846 |
|
PROVISION FOR INCOME TAXES |
|
2,535,023 |
|
|
1,572,816 |
|
|
7,547,318 |
|
|
4,437,141 |
|
NET INCOME (LOSS) BEFORE NONCONTROLLING INTEREST |
|
(2,780,987 |
) |
|
5,437,404 |
|
|
15,773,789 |
|
|
11,103,705 |
|
Less: Net income attributable to noncontrolling interest |
|
3,412,582 |
|
|
958,858 |
|
|
10,738,295 |
|
|
2,323,205 |
|
NET INCOME (LOSS) ATTRIBUTABLE TO CONTROLLING INTEREST |
|
(6,193,569 |
) |
|
4,478,546 |
|
|
5,035,494 |
|
|
8,780,500 |
|
OTHER COMPREHENSIVE INCOME (LOSS): |
|
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation adjustments | | (62,767 | ) | | 121,814 | | | 349,602 | | | 1,992,939 | |
COMPREHENSIVE INCOME (LOSS) |
$ |
(6,256,336 |
) |
$ |
4,600,360 |
|
$ |
5,385,096 |
|
$ |
10,773,439 |
|
BASIC EARNINGS PER SHARE: |
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average number of shares | | 21,632,793 | | | 21,434,942 | | | 21,504,002 | | | 21,434,942 | |
| Earnings (loss) per share | $ | (0.29 | ) | $ | 0.21 | | $ | 0.23 | | $ | 0.41 | |
DILUTED EARNINGS PER SHARE: |
|
|
|
|
|
|
|
|
|
|
|
|
| Weighted average number of shares | | 21,632,793 | | | 21,504,629 | | | 21,767,086 | | | 21,713,170 | |
| Earnings (loss) per share | $ | (0.29 | ) | $ | 0.21 | | $ | 0.23 | | $ | 0.40 | |
The accompanying notes are an integral part of these consolidated financial statements.
-4-
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
| | | China Biologic Products. Inc.'s shareholders | | | | | | | |
| | | | | | | | | | | | Retained earnings | | | Accumulated other | | | | | | | |
| | | Common stock | | | Additional | | | Statutory | | | | | | comprehensive | | | Noncontrolling | | | | |
| | | Shares | | | Par value | | | paid in capital | | | reserves | | | Unrestricted | | | income | | | interest | | | Total | |
| BALANCE, December 31, 2007 | | 21,434,942 | | $ | 2,143 | | $ | 9,388,305 | | $ | 3,934,703 | | $ | 6,461,680 | | $ | 2,608,794 | | $ | 3,885,892 | | $ | 26,281,517 | |
Stock based compensation |
|
|
|
|
|
|
|
1,283,801 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,283,801 |
|
| Net income | | | | | | | | | | | | | | 8,780,500 | | | | | | 2,323,205 | | | 11,103,705 | |
| Dividend declared to noncontrolling interest shareholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,640,960 |
) |
|
(1,640,960 |
) |
| Adjustment to statutory reserve | | | | | | | | | | | 1,962,866 | | | (1,962,866 | ) | | | | | | | | - | |
| Foreign currency translation adjustments | | | | | | | | | | | | | | | | | 1,992,939 | | | | | | 1,992,939 | |
BALANCE, September 30, 2008 (unaudited) |
|
21,434,942 |
|
$ |
2,143 |
|
$ |
10,672,106 |
|
$ |
5,897,569 |
|
$ |
13,279,314 |
|
$ |
4,601,733 |
|
$ |
4,568,137 |
|
$ |
39,021,002 |
|
Stock based compensation |
|
|
|
|
|
|
|
27,926 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
27,926 |
|
| Net income | | | | | | | | | | | | | | 3,205,171 | | | | | | 980,636 | | | 4,185,807 | |
| Dividend declared to noncontrolling interest shareholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,341,085 |
) |
|
(1,341,085 |
) |
| Adjustment to statutory reserve | | | | | | | | | | | 1,092,232 | | | (1,092,232 | ) | | | | | | | | - | |
| Foreign currency translation adjustments | | | | | | | | | | | | | | | | | 151,152 | | | 4,106 | | | 1,55,258 | |
BALANCE, December 31, 2008 |
|
21,434,942 |
|
$ |
2,143 |
|
$ |
10,700,032 |
|
$ |
6,989,801 |
|
$ |
15,392,253 |
|
$ |
4,752,885 |
|
$ |
4,211,794 |
|
$ |
42,048,908 |
|
Cumulative effect of reclassification of warrants |
|
|
|
|
|
|
|
(738,449 |
) |
|
|
|
|
(929,577 |
) |
|
|
|
|
|
|
|
(1,668,026 |
) |
| Stock based compensation | | | | | | | | 62,281 | | | | | | | | | | | | | | | 62,281 | |
| Warrants exercised | | 1,215,500 | | | 122 | | | 9,167,759 | | | | | | | | | | | | | | | 9,167,881 | |
| Net income | | | | | | | | | | | | | | 5,035,494 | | | | | | 10,738,295 | | | 15,773,789 | |
| Dividend declared to noncontrolling interest shareholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(8,955,392 |
) |
|
(8,955,392 |
) |
| Noncontrolling interest acquired from acquisition |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
21,525,059 |
|
|
21,525,059 |
|
| Adjustment to statutory reserve | | | | | | | | | | | 6,423,552 | | | (6,423,552 | ) | | | | | | | | - | |
| Foreign currency translation adjustments | | | | | | | | | | | | | | | | | 349,602 | | | (187 | ) | | 349,415 | |
BALANCE, September 30, 2009 (unaudited) |
|
22,650,442 |
|
$ |
2,265 |
|
$ |
19,191,623 |
|
$ |
13,413,353 |
|
$ |
13,074,618 |
|
$ |
5,102,487 |
|
$ |
27,519,569 |
|
$ |
78,303,915 |
|
The accompanying notes are an integral part of these consolidated financial statements.
-5-
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2009 AND 2008
(Unaudited)
| | | 2009 | | | 2008 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | |
| Net income attributable to controlling interest | $ | 5,035,494 | | $ | 8,780,500 | |
| Net income attributable to noncontrolling interest | | 10,738,295 | | | 2,323,205 | |
| Consolidated net income | | 15,773,789 | | | 11,103,705 | |
| Adjustments to reconcile net income to cash provided by operating activities: | | | | | | |
| Depreciation | | 2,158,206 | | | 914,575 | |
| Amortization | | 2,654,269 | | | 80,753 | |
| Loss on disposal of equipment | | 114,246 | | | 73,310 | |
| Recovery of bad debt previously reserved | | (9,621 | ) | | - | |
| Allowance for bad debt - accounts receivables | | 90,442 | | | - | |
| Allowance for bad debt - other receivables and prepayments | | 659,788 | | | - | |
| Stock based compensation | | 62,281 | | | 1,283,801 | |
| Change in fair value of warrant liabilities | | 14,931,088 | | | - | |
| Amortization of deferred note issuance cost | | 110,938 | | | - | |
| Amortization of discount on convertible notes | | 45,175 | | | - | |
| Equity in loss of unconsolidated affiliate | | 19,092 | | | - | |
| Change in operating assets and liabilities: | | | | | | |
| Notes receivable | | - | | | 43,011 | |
| Accounts receivable | | (1,306,293 | ) | | (353,412 | ) |
| Accounts receivable - related party | | 378,308 | | | - | |
| Other receivables | | (485,641 | ) | | 15,251 | |
| Inventories | | (9,729,616 | ) | | (3,206,654 | ) |
| Prepayments and deferred expenses | | (511,819 | ) | | (355,012 | ) |
| Accounts payable | | (149,764 | ) | | 72,681 | |
| Other payables and accrued liabilities | | 4,236,622 | | | 1,267,099 | |
| Accrued interest - holder of noncontrolling interest | | 1,319,555 | | | - | |
| Customer deposits | | 4,154,255 | | | 383,703 | |
| Taxes payable | | 942,929 | | | 3,477,543 | |
| Contingent liability | | - | | | (108,430 | ) |
| Net cash provided by operating activities | | 35,458,229 | | | 14,691,924 | |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
| Cash acquired through acquisition | | 11,945,303 | | | - | |
| Proceeds from dividend receivable | | 147,256 | | | - | |
| Payments made for acquisition | | (10,373,854 | ) | | - | |
| Payments made for unconsolidated affiliate | | (3,224,980 | ) | | - | |
| Purchase of plant and equipment | | (2,323,903 | ) | | (3,154,996 | ) |
| Additions to intangible assets | | (1,374,146 | ) | | (9,620 | ) |
| Proceeds from sale of equipment | | 513 | | | 53,078 | |
| Advances for potential acquisition | | - | | | (1,463,000 | ) |
| Advances on non-current assets | | (855,298 | ) | | (160,256 | ) |
| Net cash used in investing activities | | (6,059,109 | ) | | (4,734,794 | ) |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
| Change in restricted cash | | - | | | (338,353 | ) |
| Payments on notes payable | | (29,318 | ) | | - | |
| Proceeds from warrants conversion | | 3,455,059 | | | - | |
| Proceeds from issuance of convertible notes | | 8,967,516 | | | - | |
| Repayments of former shareholders loan in acquiring company | | (2,782,278 | ) | | - | |
| Proceeds from short term loans - bank | | 13,515,598 | | | - | |
| Payments on short term loans - bank | | (2,814,528 | ) | | (716,850 | ) |
| Payments on long term loan - bank | | (5,863,600 | ) | | - | |
| Dividends paid to noncontrolling interest shareholders | | (2,293,888 | ) | | (286,740 | ) |
| Net cash provided by (used in) financing activities | | 12,095,925 | | | (1,341,943 | ) |
EFFECTS OF EXCHANGE RATE CHANGE IN CASH |
|
38,472 |
|
|
598,736 |
|
INCREASE IN CASH |
|
41,533,517 |
|
|
9,213,923 |
|
| CASH and CASH EQUIVALENTS, beginning of period | | 8,814,616 | | | 5,010,033 | |
CASH and CASH EQUIVALENTS, end of period |
$ |
50,348,133 |
|
$ |
14,223,956 |
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION |
|
|
|
|
|
|
| Income taxes paid | $ | 7,525,262 | | $ | 1,830,589 | |
| Interest paid (net of capitalized interest) | $ | 911,846 | | $ | 47,197 | |
| Non-cash investing and financing activities: | | | | | | |
| Reclassification of warrant liability to paid-in capital upon warrants conversion | $ | 5,712,822 | | $ | - | |
| Dividend paid by offsetting accounts receivable-related party | $ | 943,907 | | $ | - | |
| Dividend paid in exchange of holder of noncontrolling interest loan | $ | 3,737,283 | | $ | - | |
| Dividend paid by offsetting loan due from holder of noncontrolling interest | $ | 4,470,995 | | $ | - | |
| Net assets acquired with prepayments made in prior periods | $ | 14,248,548 | | $ | - | |
| Net assets acquired with unpaid investment | $ | 2,849,710 | | $ | - | |
| Land use right acquired with prepayments made in prior periods | $ | 131,931 | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements.
-6-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Note 1 – Organization background and principal activities
Principal Activities and Reorganization
China Biologic Products, Inc. (the “Company” or “CBP”) was originally incorporated in 1992 under the laws of the state of Texas. After it completed the acquisition with Logic Express Limited, it converted to a Delaware corporation. The Company through its direct and indirect subsidiaries is principally engaged in the research, development, commercialization, manufacture and sale of human blood products to customers in the People’s Republic of China (the “PRC”) and to some extent in India.
Current Development
Dalin Acquisition and Entrustment Agreement
Logic Express Ltd. (“Logic Express”), CBP’s wholly owned subsidiary, through Logic Holdings (Hong Kong) Ltd. (“Logic Holdings”) completed the acquisition of 90% interest in Guiyang Dalin Biologic Technologies Co. Ltd. (“Dalin”), previously known as Chongqing Dalin Biologic Technologies Co. Ltd., in April 2009 upon payment of 90% of the total purchase price of approximately RMB 194,400,000 ($28,479,600). The Company is obligated to pay the remaining 10% of the purchase price, RMB 19,440,000 (approximately $2,847,960), on or before April 9, 2010, the one-year anniversary of the local Administration for Industry and Commerce’s approval of the equity transfer. Guiyang Qianfeng Biological Products Co., Ltd. (“Qianfeng”), Dalin’s 54% owned subsidiary, is one of the largest plasma-based biopharmaceutical companies in China and is the only manufacturer currently operating in Guizhou Province. Qianfeng is in compliance with Good Manufacturing Practices, or GMP, standards, and has been approved by the PRC’s State Food and Drug Administration or the SFDA to produce six types of plasma-based products including Human Albumin, Human Immunoglobulin, Human Intravenous Immunoglobulin, Human Hepatitis B Immunoglobulin, Human Tetanus Immunoglobulin and Human Rabies Immune Globulin.
In accordance with the terms of the equity transfer agreement, Logic Holdings effectively became a 90% shareholder in Dalin, including the right to receive its pro rata share of the profits on January 1, 2009.
On April 6, 2009, Logic Express entered into an equity transfer and entrustment agreement, or Entrustment Agreement, among Logic Express, Shandong Taibang Biological Products Co. Ltd (“Shandong Taibang”), and the Shandong Institute of Biological Products (“the Shandong Institute”), the holder of the minority interests in Shandong Taibang, pursuant to which, Logic Express agreed to permit Shandong Taibang and the Shandong Institute to participate in the indirect purchase of Qianfeng’s equity interests. Under the terms of the Entrustment Agreement, Shandong Taibang agreed to contribute 18% or RMB 35,000,000 (approximately $5,116,184) of the Dalin purchase price and the Shandong Institute agreed to contribute 12.86% or RMB 25,000,000 (approximately $3,654,917) of the Dalin purchase price. Logic Express is obligated to repay to Shandong Taibang and the Shandong Institute their respective investment amounts on or before April 6th, 2010, along with their pro rata share, based on their percentage of the Dalin purchase price contributed, of any distribution on the indirect equity investment in Qianfeng payable to Logic Express during 2009. Logic Express has agreed that if these investment amounts are not repaid within five days of the payment due date, then Logic Express is obligated to pay Shandong Taibang and the Shandong Institute liquidated damages equal to 0.03% of the overdue portion of the amount due until such time as it is paid. Logic Express has also agreed to pledge 30% of its ownership in Shandong Taibang to the Shandong Institute as security for nonpayment. If failure to repay continues for longer than three months after the payment due date, then the Shandong Institute will be entitled to any rights associated with the pledged interests, including but not limited to rights of disposition and profit distribution, until such time as the investment amount has been repaid. Logic Express also provided a guarantee that Shandong Taibang and the Shandong Institute will receive no less than a 6% return based on their original investment amount.
-7-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Huitian Acquisition
Shandong Taibang purchased a 35% interest in Xi’an Huitian Blood Products Co. Ltd (“Huitian”) at a purchase price of RMB 44,000,000 (approximately $6,446,000) on October 10, 2008 and paid the final installment on July 16, 2009. Huitian is a manufacturer of plasma-based biopharmaceutical products in Shaanxi Province and is one of only 32 such manufacturers in China who are government approved. Huitian is in compliance with GMP standards and it is also approved by the SFDA for the production of Human Albumin, Human Immunoglobulin, Human Immunoglobulin for Intravenous Injection, and Human Hepatitis B Immunoglobulin products.
Formation of Logic Holding
On December 12, 2008, the Company established Logic Holding, the Company’s wholly-owned Hong Kong subsidiary of Logic Express, for the purpose of being a holding company for the majority interest in Dalin.
Note 2 – Summary of significant accounting policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The Company’s functional currency is the Chinese Renminbi (“RMB”); however, the Company’s reporting currency is the United States Dollar (“USD”); therefore, the accompanying consolidated financial statements have been translated and presented in USD. All material inter-company transactions and balances have been eliminated in the consolidation.
While management has included all normal recurring adjustments considered necessary to give a fair presentation of the operating results for the periods presented, interim results are not necessarily indicative of results for a full year. The information included in this Form 10-Q should be read in conjunction with information included in the 2008 annual report filed on Form 10-K.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. For example, management estimates the fair value of stock based compensation as well as potential losses on outstanding receivables. Management believes that the estimates utilized in preparing its financial statements are reasonable and prudent. Actual results could differ from these estimates.
Foreign Currency Translation
The reporting currency of the Company is the US dollar. The Company’s functional currency is the Chinese Renminbi (“RMB”), also the local currency of the Company’s principal operating subsidiaries. Results of operations and cash flows are translated at average exchange rates during the period. Assets and liabilities are translated at the unified exchange rate as quoted by the People’s Bank of China at the end of the period. Translation adjustments resulting from this process are included in accumulated other comprehensive income in the statements of changes in equity. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
In accordance with Financial Accounting Standards Board’s (FASB) accounting standard, cash flows from the Company's operations is calculated based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
The consolidated balance sheet amounts, with the exception of equity at September 30, 2009 and December 31, 2008 were translated at RMB 6.82 to $1.00 and RMB 6.82 to $1.00, respectively. The equity accounts were stated at their historical rate. The average translation rates applied to consolidated statements of income and cash flow for the nine months ended September 30, 2009 and 2008 were RMB 6.82 and RMB 6.97 to $1.00, respectively.
-8-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Revenue Recognition
The Company recognizes revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable, which are generally considered to be met upon delivery and acceptance of products at the customer site. Sales are presented net of any discounts given to customers. As a policy, the Company does not accept any product returns and based on the Company’s records, product returns, if any, are immaterial. Sales revenue represents the invoiced value of goods, net of a value-added tax (“VAT”). All products produced by the Company and sold in the PRC are subject to a Chinese VAT at a rate of 6% of the gross sales price or at a rate approved by the Chinese local government. Products distributed by Shandong Medical and plasma raw material inter-company sales from Puding Plasma Company to Qianfeng are subjected to a 17% VAT.
Shipping and Handling
Shipping and handling costs related to costs of goods sold are included in selling expenses and totaled $82,786 and $12,468 for the three months ended September 30, 2009 and 2008, respectively. For the nine months ended September 30, 2009 and 2008, shipping and handling costs totaled $206,577 and $34,004, respectively.
Financial Instruments
On January 1, 2008, the Company adopted FASB’s accounting standard related to fair value measurements and began recording financial assets and liabilities subject to recurring fair value measurement at the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. These fair value principles prioritize valuation inputs across three broad levels. Receivables, payables, short and long term loans, and derivative liabilities qualify as financial instruments. Management concluded the carrying values of the receivables, payables and short term loans approximate their fair values because of the short period of time between the origination of such instruments and their expected realization, and if applicable, their stated rates of interest are equivalent to interest rates currently available. The fair values of the long term debt and derivative liabilities are measured pursuant to the three levels defined by the FASB’s accounting standard as follow:
- Level 1: inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
- Level 2: inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
- Level 3: inputs to the valuation methodology are unobservable and significant to the fair value.
As required by FASB’s accounting standard, financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. Depending on the product and the terms of the transaction, the fair value of the derivative liabilities were modeled using a series of techniques, including closed-form analytic formula, such as the Black-Scholes Option Pricing Model, which does not entail material subjectivity because the methodology employed does not necessitate significant judgment, and the pricing inputs are observed from actively quoted markets. Derivative liabilities related to warrants issued by the Company and the liability related to derivative instruments (including the conversion option) embedded in the Company’s Senior Secured Convertible Notes are carried at fair value, with changes in the fair value charged or credited to income. The fair values are determined using the Black-Scholes Model or a binomial model, defined in FASB’s accounting standard related to fair value measurements as level 2 inputs.
| | | Carrying Value as of | | | Fair Value Measurements at September 30, 2009 | |
| | | September 30, 2009 | | | using Fair Value Hierarchy | |
| | | | | | Level 1 | | | Level 2 | | | Level 3 | |
| Derivative liabilities-Conversion option | $ | 12,784,873 | | $ | - | | $ | 12,784,873 | | $ | - | |
| Warrants liabilities | $ | 7,943,174 | | $ | - | | $ | 7,943,174 | | $ | - | |
-9-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The Company did not identify any other assets or liabilities that are required to be presented on the balance sheet at fair value in accordance with FASB’s accounting standard.
Concentration Risks
The Company's operations are carried out in the PRC and are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. Accordingly, the Company's business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC economy. The Company's results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company maintains balances at financial institutions which, from time to time, may exceed Federal Deposit Insurance Corporation insured limits for the banks located in the United States or may exceed Hong Kong Deposit Protection Board insured limits for the banks located in Hong Kong. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. Total cash in banks as of September 30, 2009 and December 31, 2008 amounted to $49,853,355 and $8,689,414, respectively, $4,866,254 and $47,865 of which are covered by insurance, respectively. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts.
The Company’s major product, human albumin: - 20%/10ml, 20%/25ml and 20%/50ml, and 10%/10ml, 10%/25ml and 10%/50ml, accounted for 42.7% and 58.3% of total revenues, for the three months ended September 30, 2009 and 2008, respectively. 48.7% and 58.0% of total revenues, for the nine months ended September 30, 2009 and 2008, respectively. If the market demands for human albumin cannot be sustained in the future or if the price of human albumin decreases, it would adversely affect the Company’s operating results.
All of the Company’s customers are located in the PRC and India. As of September 30, 2009 and 2008, the Company had no significant concentration of credit risk, except for the amounts due from related parties. There were no customers that individually comprised 10% or more of the revenue during the three and nine months ended September 30, 2009 and 2008. No individual customer represented more than 10% of trade receivables at September 30, 2009 and December 31, 2008. The Company performs ongoing credit evaluations of its customers’ financial condition and, generally, requires no collateral from its customers.
There were no vendors that individually comprised 10% or more of the purchase during the three and nine months ended September 30, 2009. No individual vendors represented more than 10% of accounts payables at September 30, 2009 and December 31, 2008. The Company’s top three vendors comprised 32.7% and 41.5% of the Company’s purchases for the three and nine months ended September 30, 2008, respectively.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC, Hong Kong and the United States. The Company considers all highly liquid investments with original maturities of three months or less at the time of purchase to be cash equivalents.
Accounts Receivable
During the normal course of business, the Company extends unsecured credit to its customers. Management reviews its accounts receivable on a regular basis to determine if the allowance for doubtful accounts is adequate. An estimate for doubtful accounts is made when collection of the full amount is no longer probable. Account balances are written-off after management has exhausted all efforts of collection.
-10-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Inventories
Inventories are stated at the lower of cost or market using the weighted average method. The cost of finished goods included direct costs of raw materials as well as direct labor used in production. Indirect production costs such as utilities and indirect labor related to production such as assembling, shipping and handling for raw material costs are also included in the cost of inventories.
The Company reviews its inventory periodically for possible obsolete goods and cost in excess of net realizable value to determine if any reserves are necessary. As of September 30, 2009 and December 31, 2008, the Company has determined that no reserve is necessary.
Plant and Equipment
Plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets with 5% residual value.
Estimated useful lives of the assets are as follows:
| | Estimated Useful Life |
| Buildings and improvement | 30 | years |
| Machinery and equipment | 10 | years |
| Furniture, fixtures and office equipment | 5-10 | years |
Construction in progress represents the costs incurred in connection with the construction of buildings, new additions, and capitalized interest incurred in connection with the Company’s plant facilities. In accordance with the provisions of FASB’s accounting standard related to capitalization of interest, interest incurred on borrowings is capitalized to the extent that borrowings do not exceed construction in progress. The credit is a reduction of interest expense. No depreciation is provided for construction in progress until such time as the assets are completed and placed into service. Maintenance, repairs and minor renewals are charged directly to expenses as incurred. Major additions and betterment to property and equipment are capitalized.
The Company periodically evaluates the carrying value of long-lived assets in accordance with FASB’s accounting standard related to accounting for impairment and disposal of long-lived assets. When estimated cash flows generated by those assets are less than the carrying amounts of the asset, the Company recognizes an impairment loss. Based on its review, the Company believes that, as of September 30, 2009 and December 31, 2008, there were no impairments of its long-lived assets.
Investment in Unconsolidated Affiliate
Equity method investments are recorded at original cost and adjusted to recognize the Company’s proportionate share of the investee’s net income or losses and additional contributions made and distributions received. The Company recognizes a loss if it is determined that other than temporary decline in the value of the investment exists. Subsidiaries in which the Company has the ability to exercise significant influence, but does not have a controlling interest is accounted for using the equity method. Significant influence is generally considered to exist when the Company has an ownership interest in the voting stock between 20% and 50%, and other factors, such as representation on the Board of Directors, voting rights and the impact of commercial arrangements, are considered in determining whether the equity method of accounting is appropriate. The Company accounts for investments with ownership less than 20% using cost method.
Intangible Assets
Intangible assets are stated at cost (estimated fair value upon contribution or acquisition), less accumulated amortization. Amortization expense is recognized on the straight-line basis over the estimated useful lives of the assets as follows:
| Intangible assets | | Estimated useful lives |
| Land use rights | | 50 | years |
| Permits and licenses | | 5-10 | years |
| Blood donor network | | 10 | years |
| Software | | 3.8 | years |
| Good Manufacturing Practice certificate | | 5-10 | years |
| Long-term customer-relationship intangible assets | | 4 | years |
-11-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
All land in the PRC is owned by the government; however, the government grants “land use rights.” The Company has obtained rights to use various parcels of land for 50 years. The Company amortizes the cost of the land use rights over their useful life using the straight-line method.
Other intangible assets represent permits, licenses, blood donor network, software, Good Manufacturing Practice (“GMP”) certificate and long-term customer-relationship intangible assets. The Company amortized the cost of these intangible assets over their useful life using the straight-line method.
Intangible assets of the Company are reviewed at least annually or more often if circumstances dictate, to determine whether their carrying value has become impaired. The Company considers assets to be impaired if the carrying value exceeds the future projected cash flows from related operations. The Company also re-evaluates the years of amortization to determine whether subsequent events and circumstances warrant revised estimates of useful lives. As of September 30, 2009, the Company expects these assets to be fully recoverable.
Revenues
The Company’s revenues are primarily derived from the manufacture and sale of human blood products. The Company’s revenues by significant types of product for the three months and nine months ended September 30, 2009 and 2008 are as follows:
| | | Three months ended | | | Nine months ended | |
| | | September 30, | | | September 30, | |
| | | (Unaudited) | | | (Unaudited) | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
Human Albumin – 20%/10% in 10ml,
25ml and 50ml |
$ |
11,556,199 |
|
$ |
8,049,853 |
|
$ |
39,649,829 |
|
$ |
19,463,172 |
|
| Human Hepatitis B Immunoglobulin | | 1,317,786 | | | 1,321,667 | | | 2,611,945 | | | 2,909,204 | |
| Human Immunoglobulin for Intravenous | | 10,251,433 | | | 2,705,502 | | | 29,787,712 | | | 6,427,129 | |
| Human Rabies Immunoglobulin | | 1,348,213 | | | 883,242 | | | 3,903,464 | | | 2,526,634 | |
| Human Tetanus Immunoglobulin | | 812,201 | | | 472,769 | | | 2,184,811 | | | 1,337,690 | |
| Human Immunoglobulin | | 1,426,667 | | | - | | | 1,962,607 | | | - | |
| Others | | 327,240 | | | 366,882 | | | 1,269,514 | | | 910,935 | |
| Totals | $ | 27,039,739 | | $ | 13,799,915 | | $ | 81,369,882 | | $ | 33,574,764 | |
The Company is engaged in sale of human blood products to customers in China and India. The amount sold in India was less than 10% of total sales for the three and nine months ended September 30, 2009.
Research and Development Costs
Research and development costs are expensed as incurred.
Retirement and Other Post Retirement Benefits
Contributions to retirement schemes (which are defined contribution plans) are charged to the statement of operations as and when the related employee service is provided.
Product Liability
The Company’s products are covered by product liability insurance of approximately $2,934,000 (RMB 20,000,000). As of September 30, 2009 and December 31, 2008, no claim on the insurance policy was filed. However, there are two pre-existing potential claims against Qianfeng’s products, which are still in the court proceedings as explained in the legal proceeding section below.
-12-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Government Grants
The Company’s subsidiary, Shandong Taibang, is entitled to receive grants from the Tai’an municipal government due to its operation in the high and new technology business sector. For the three and nine months ended September 30, 2009 and 2008, no non-refundable grants were received from the Tai’an municipal government. Grants received from the Tai’an municipal government can be used for enterprise development and technology innovation purposes.
Income Taxes
The Company reports income taxes pursuant to FASB’s accounting standard for income taxes. Under the asset and liability method of accounting for income taxes as required by this accounting standard, deferred income tax liabilities and assets are determined based on the temporary differences between the financial statement and tax basis of assets and liabilities using tax rates expected to be in effect during the years in which the basis differences reverse. A valuation allowance is recorded when it is more likely than not that some of the deferred tax assets will not be realized. FASB’s accounting standard for accounting for uncertainty in income taxes requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. As of January 1, 2007, income tax positions must meet a more-likely-than-not recognition threshold to be recognized. A tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. Provision for income taxes consist of taxes currently due plus deferred taxes. Since the Company had no operations within the United States there is no provision for US taxes and there are no deferred tax amounts at September 30, 2009 and 2008.
Deferred tax is accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences, and deferred tax assets are recognized to the extent that it is probably that taxable profit will be available against which deductible temporary differences can be utilized.
Deferred tax is calculated using tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the income statement, except when it is related to items credited or charged directly to equity, in which case the deferred tax is also dealt with in equity.
Deferred tax assets and liabilities are offset when they related to income taxes levied by the same taxation authority and the Company intends to settle its current tax assets and liabilities on a net basis.
Value Added Tax
Enterprises or individuals, who sell products, engage in repair and maintenance or import and export goods in the PRC are subject to a VAT in accordance with Chinese laws. The VAT rate applicable to the Company is 6% of the gross sales price. Products distributed by Shandong Medical and plasma raw material inter-company sales from Puding Plasma Company to Qianfeng are subjected to a 17% VAT. No credit is available for VAT paid on purchases.
Stock-based Compensation
The Company accounts and reports stock-based compensation pursuant to FASB’s accounting standard related to accounting for stock-based compensation which defines a fair-value-based method of accounting for stock based employee compensation and transactions in which an entity issues its equity instruments to acquire goods and services from non-employees. Stock compensation for stock granted to non-employees has been determined in accordance with this standard as the fair value of the consideration received or the fair value of equity instruments issued, whichever is more reliably measured.
-13-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Noncontrolling Interest
Effective January 1, 2009, the Company adopted FASB’s accounting standard regarding non-controlling interest in consolidated financial statements. Certain provisions of this statement are required to be adopted retrospectively for all periods presented. Such provisions include a requirement that the carrying value of noncontrolling interests (previously referred to as minority interests) be removed from the mezzanine section of the balance sheet and reclassified as equity.
Further, as a result of adoption this accounting standard, net income attributable to noncontrolling interests is now excluded from the determination of consolidated net income.
Recently Issued Accounting Pronouncements
In January 2009, the Financial Accounting Standards Board issued an accounting standard which amended the impairment model by removing its exclusive reliance on “market participant” estimates of future cash flows used in determining fair value. Changing the cash flows used to analyze other-than-temporary impairment from the “market participant” view to a holder’s estimate of whether there has been a “probable” adverse change in estimated cash flows allows companies to apply reasonable judgment in assessing whether an other-than-temporary impairment has occurred. The adoption of this accounting standard did not have a material impact on the Company’s consolidated financial statements because all of the investments in debt securities are classified as trading securities.
In April 2009, the Financial Accounting Standards Board issued an accounting standard that makes the other-than-temporary impairments guidance more operational and improves the presentation of other-than-temporary impairments in the financial statements. This standard replaced the existing requirement that the entity’s management assert it has both the intent and ability to hold an impaired debt security until recovery with a requirement that management assert it does not have the intent to sell the security, and it is more likely than not it will not have to sell the security before recovery of its cost basis. This standard provides increased disclosure about the credit and noncredit components of impaired debt securities that are not expected to be sold and also requires increased and more frequent disclosures regarding expected cash flows, credit losses, and an aging of securities with unrealized losses. Although this standard does not result in a change in the carrying amount of debt securities, it does require that the portion of an other-than-temporary impairment not related to a credit loss for a held-to-maturity security be recognized in a new category of other comprehensive income and be amortized over the remaining life of the debt security as an increase in the carrying value of the security. The Company adopted this accounting standard, but it did not have a material impact on its consolidated financial statements.
In April 2009, the Financial Accounting Standards Board issued an accounting standard that requires disclosures about fair value of financial instruments not measured on the balance sheet at fair value in interim financial statements as well as in annual financial statements. Prior to this accounting standard, fair values for these assets and liabilities were only disclosed annually. This standard applies to all financial instruments within its scope and requires all entities to disclose the method(s) and significant assumptions used to estimate the fair value of financial instruments. This standard does not require disclosures for earlier periods presented for comparative purposes at initial adoption, but in periods after the initial adoption, this standard requires comparative disclosures only for periods ending after initial adoption. The Company adopted this accounting standard, but it did not have a material impact on the disclosures related to its consolidated financial statements.
In June 2009, the Financial Accounting Standards Board issued an accounting standard amending the accounting and disclosure requirements for transfers of financial assets. This accounting standard requires greater transparency and additional disclosures for transfers of financial assets and the entity’s continuing involvement with them and changes the requirements for derecognizing financial assets. In addition, it eliminates the concept of a qualifying special-purpose entity (“QSPE”). This accounting standard is effective for financial statements issued for fiscal years beginning after November 15, 2009, and the Company does not expect this standard to have a material effect on its consolidated financial statements.
-14-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
In June 2009, the Financial Accounting Standards Board also issued an accounting standard amending the accounting and disclosure requirements for the consolidation of variable interest entities (“VIEs”). The elimination of the concept of a QSPE, as discussed above, removes the exception from applying the consolidation guidance within this accounting standard. Further, this accounting standard requires a company to perform a qualitative analysis when determining whether or not it must consolidate a VIE. It also requires a company to continuously reassess whether it must consolidate a VIE. Additionally, it requires enhanced disclosures about a company’s involvement with VIEs and any significant change in risk exposure due to that involvement, as well as how its involvement with VIEs impacts the company’s financial statements. Finally, a company will be required to disclose significant judgments and assumptions used to determine whether or not to consolidate a VIE. This accounting standard is effective for financial statements issued for fiscal years beginning after November 15, 2009, and the Company does not expect this standard to have a material effect on its consolidated financial statements.
In June 2009, the Financial Accounting Standards Board issued an accounting standard which establishes the FASB Accounting Standards Codification™ (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. The Codification is effective for interim and annual periods ending after September 15, 2009, and as of the effective date, all existing accounting standard documents will be superseded. The Codification is effective for the Company in the third quarter of 2009, and accordingly, the Company’s Quarterly Report on Form 10-Q for the quarter ending September 30, 2009 and all current and subsequent public filings will reference the Codification as the sole source of authoritative literature.
In August 2009, the Financial Accounting Standards Board issued an Accounting Standards Update (“ASU”) regarding measuring liabilities at fair value. This ASU provides additional guidance clarifying the measurement of liabilities at fair value in circumstances in which a quoted price in an active market for the identical liability is not available; under those circumstances, a reporting entity is required to measure fair value using one or more of valuation techniques, as defined. This ASU is effective for the first reporting period, including interim periods, beginning after the issuance of this ASU. The Company is currently evaluating the impact of this ASU on its consolidated financial statements.
In October 2009, the Financial Accounting Standards Board issued an ASU regarding accounting for own-share lending arrangements in contemplation of convertible debt issuance or other financing. This ASU requires that at the date of issuance of the shares in a share-lending arrangement entered into in contemplation of a convertible debt offering or other financing, the shares issued shall be measured at fair value and be recognized as an issuance cost, with an offset to additional paid-in capital. Further, loaned shares are excluded from basic and diluted earnings per share unless default of the share-lending arrangement occurs, at which time the loaned shares would be included in the basic and diluted earnings-per-share calculation. This ASU is effective for fiscal years beginning on or after December 15, 2009, and interim periods within those fiscal years for arrangements outstanding as of the beginning of those fiscal years. The Company is currently evaluating the impact of this ASU on its consolidated financial statements.
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation. These reclassifications have no effect on net income or cash flows.
Note 3 – Related party transactions
The material related party transactions undertaken by the Company with related parties as of September 30, 2009 and December 31, 2008 are presented as follows:
-15-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
| | | | | | September 30, 2009 | | | December 31, 2008 | |
| Assets | | Purpose | | | (unaudited) | | | | |
| Accounts receivable – related party(1) | | Processing fees | | $ | 41,430 | | $ | - | |
Liabilities |
|
Purpose |
|
| September 30, 2009
(unaudited) |
|
|
December 31, 2008 |
|
| Short term loans – holder of noncontrolling interest(2) | | Loan | | $ | 758,277 | | $ | 773,277 | |
| Short term loans – holder of noncontrolling interest(3) | | Loan | | | 3,667,500 | | | 773,277 | |
| Total | | | | $ | 4,425,777 | | $ | 773,277 | |
Accrued interest – holder of noncontrolling interest(3) |
|
Interest payable |
| $
|
1,319,556 |
| $
|
- |
|
Other payable – related parties(4) |
|
Loan |
| $
|
2,122,772 |
| $
|
- |
|
| Other payable – related parties(5) | | Contribution | | | 964,168 | | | - | |
| Total | | | | $ | 3,086,940 | | $ | - | |
(1) Qianfeng provides processing services for Guizhou Eakan, one of the Qianfeng’s non-controlling shareholders. The total processing services income amounted to $168,480 and $508,529 for the three and nine months period ended September 30, 2009, respectively. As of September 30, 2009, Guizhou Eakan owes Qianfeng processing fees in an amount of $41,430. This balance has been paid in cash at the end of October 2009.
(2) As of September 30, 2009 and December 31, 2008, the Company borrowed an aggregate of $758,277 and $773,277, respectively, from its noncontrolling interest shareholder, Shandong Institute, for working capital purposes. The Company is required to repay the loan in cash due by December 2010, with an annual interest rate of 6%.
(3) On April 6, 2009, Logic Express entered into an equity transfer and entrustment agreement, or Entrustment Agreement, among Logic Express, Shandong Taibang, and the Shandong Institute of Biological Products, or the Shandong Institute, the holder of the noncontrolling interests in Shandong Taibang, pursuant to which, Logic Express agreed to permit Shandong Taibang and the Shandong Institute to participate in the indirect purchase of Qianfeng's equity interests. Under the terms of the Entrustment Agreement, Shandong Institute agreed to contribute 12.86% or $3,667,500 (RMB 25,000,000) of the Dalin purchase price. Logic express is obligated to repay to the Shandong Institute their investment amount on or before April 6th, 2010, along with their pro rata share, based on their percentage of the Dalin purchase price contributed, of any distribution on the indirect equity investment in Qianfeng payable to Logic Express during 2009. The accrued interest – holder of noncontrolling interest amounted to $1,319,556 represents the pro rata share of equity investment income pursuant of Entrustment Agreement for the nine month period ended September 30, 2009.
(4) Qianfeng has payables to Guizhou Eakan Investing Corp. in the amount of approximately $2,122,772 (RMB14, 470,160). Guizhou Eakan Investing Corp. is one of the shareholders of Guizhou Eakan, one of the Qianfeng’s minority shareholders. The Company borrowed the amount for working capital purposes. The balance is due on demand in the form of cash.
(5) Qianfeng has payables to Guizhou Jie’an, a holder of noncontrolling interest, in amount of approximately $964,168 (RMB 6,569,840). In 2007, Qianfeng received additional contributions from Guizhou Jie’an in the amount of $962,853 to maintain Jie’an ownership interest in the Company at 9%. However, due to legal dispute among Shareholders over Raising Additional Capital as stated in legal proceeding section, commitment and contingent liabilities, the money may be returned to Jie’an.
Note 4 – Accounts receivable
Trade accounts receivable consist of the following:
-16-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Trade accounts receivable | $ | 2,867,509 | | $ | 1,581,139 | |
| Less: Allowance for doubtful accounts | | (1,393,567 | ) | | (1,268,052 | ) |
| Total | $ | 1,473,942 | | $ | 313,087 | |
The activity in the allowance for doubtful accounts for trade accounts receivable for the nine months ended September 30, 2009 and the year ended December 31, 2008 is as follows:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Beginning allowance for doubtful accounts | $ | 1,268,052 | | $ | 1,238,772 | |
| Additional charged to bad debt expense | | 90,442 | | | - | |
| Recovery of amount previously reserved | | (9,621 | ) | | (56,462 | ) |
| Write-off charged against the allowance | | - | | | - | |
| Foreign currency translation adjustment | | 44,694 | | | 85,742 | |
| Ending allowance for doubtful accounts | $ | 1,393,567 | | $ | 1,268,052 | |
Note 5 – Inventories
Inventories consisted of the following:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Raw materials | $ | 17,329,704 | | $ | 7,043,349 | |
| Work-in-process | | 7,046,910 | | | 4,801,768 | |
| Finished goods | | 8,842,004 | | | 3,104,079 | |
| Total | $ | 33,218,618 | | $ | 14,949,196 | |
Note 6 – Prepayments and deferred expense
Prepayments and deferred expense represent partial payments for deposits on material purchases, prepaid leases and prepayment for insurance expenses and amounted to $1,582,566 and $614,704 as of September 30, 2009 and December 31, 2008, respectively.
Long term prepayments represent partial payments or deposits on plant and equipment and intangible assets purchases and amounted to $4,870,735 and $955,874 as of September 30, 2009 and December 31, 2008, respectively.
Note 7 – Plant and equipment, net
Plant and equipment consist of the following:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Buildings and improvements | $ | 12,728,183 | | $ | 5,809,724 | |
| Machinery and equipment | | 23,464,542 | | | 12,308,174 | |
| Furniture, fixtures, office equipment and vehicle | | 3,527,426 | | | 1,501,946 | |
| Total depreciable assets | | 39,720,151 | | | 19,619,844 | |
| Accumulated depreciation | | (13,402,281 | ) | | (3,099,259 | ) |
| Plant and equipment, net | | 26,317,870 | | | 16,520,585 | |
| Construction in progress | | 1,531,962 | | | 2,778,779 | |
| Total | $ | 27,849,832 | | $ | 19,299,364 | |
-17-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Depreciation expense for the three months ended September 30, 2009 and 2008 amounted to $568,581 and $334,821, respectively. Depreciation expense for the nine months ended September 30, 2009 and 2008 amounted to $2,158,206 and $914,575, respectively. No interest was capitalized into construction in progress in either of the three and nine months ended September 30, 2009 and 2008.
Note 8 – Investment in unconsolidated affiliate
On October 10, 2008, Shandong Taibang entered into an Equity Transfer Agreement (the "Huitian Agreement") with Mr. Fan Qingchun (the "Transferor"), a PRC citizen holding 35% of the equity interest in Huitian, a PRC limited liability company. Pursuant to the Huitian Agreement, the Transferor agrees to sell to Shandong Taibang, and Shandong Taibang agrees to purchase from the Transferor, 35% equity interest in Huitian for an aggregate purchase price of $6,502,901 (or RMB 44,327,890) including interest of $48,101 (RMB 327,890). Huitian is one of the 32 government approved plasma-based product producers in China, and it is in compliance with Good Manufacturing Practices (“GMP”) standards. It is also approved by the PRC’s State Food and Drug Administration (“SFDA”) to produce four types of plasma-based products.
Logic Express also entered into an investment entrustment agreement (the "Investment Agreement") with the minority shareholder in Shandong Taibang, Shandong Institute, pursuant to which Logic Express agrees to provide the investment amount for the acquisition and the Shandong Institute agree to entrust Shandong Taibang to acquire the 35% equity interest of Huitian in its name. In exchange Logic Express is also obligated to pay Shandong Taibang approximately $17,604 (or RMB120,000) per year as consideration for Shandong Taibang's performance under this agreement. Under the Investment Agreement, after the acquisition, Logic Express will be in charge of Huitian's daily operation and management, will bear the costs, expenses, liabilities and losses incurred in its operation, and will enjoy its profits. Shandong Taibang will perform relevant tasks according to Logic Express's instruction, and will not exercise any management right over Huitian or derive any financial return from Huitian. Logic Express agreed to indemnify Shandong Taibang for any loss in connection with the investment and pledged its equity interest in Shandong Taibang as collateral against such losses.
Summarized unaudited financial information of Huitian is as follows:
| | | September 30, 2009 | | | December 31, 2008 | |
| Current assets | $ | 9,813,186 | | $ | 8,039,180 | |
| Non-current assets | | 8,906,123 | | | 10,145,248 | |
| Total assets | | 18,719,309 | | | 18,184,428 | |
| Current liabilities | | 3,380,402 | | | 2,747,573 | |
| Non-current liabilities | | - | | | - | |
| Shareholders' equity | | 15,338,907 | | | 15,436,855 | |
| Total liabilities and shareholders' equity | $ | 18,719,309 | | $ | 18,184,428 | |
The portion of the difference between the cost of an investment and the amount of underlying equity in net assets of Huitian that is recognized as goodwill shall not be amortized, but instead should continue to be reviewed for impairment in accordance with FASB’s accounting standard.
Summarized unaudited financial information of Huitian is as follows:
| | | Nine months ended | |
| | | September 30, 2009 | |
| Net sales | $ | 4,321,473 | |
| Gross profit | | 1,782,297 | |
| Income before taxes | | 74,174 | |
| Net loss | | (54,550 | ) |
| Company’s share of net loss | $ | (19,092 | ) |
-18-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The rollforward of investment in Huitian in the balance sheet is shown below:
| | | Huitian - 35% | |
| | | Ownership | |
| December 31, 2007 | $ | - | |
| Investment made | | 6,502,902 | |
| Net income from 2008 | | 175,231 | |
| Dividend declared | | (147,256 | ) |
| Foreign currency translation gain | | 3,100 | |
| December 31, 2008 | | 6,533,977 | |
| Net loss from the nine months ended September 30, 2009 | | (19,092 | ) |
| Dividend declared | | (236,815 | ) |
| Foreign currency translation loss | | (176 | ) |
| September 30, 2009 (unaudited) | $ | 6,277,894 | |
Note 9 – Intangible assets, net
Intangible assets consisted of the following:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Land use rights | $ | 3,346,354 | | $ | 848,982 | |
| Permits and licenses | | 11,367,820 | | | 389,709 | |
| Blood donor network | | 2,347 | | | 22,885 | |
| Software | | 124,156 | | | 40,758 | |
| GMP certificate | | 2,327,885 | | | - | |
| Long-term customer-relationship | | 6,941,170 | | | - | |
| Totals | | 24,109,732 | | | 1,302,334 | |
| Accumulated amortization | | (2,957,315 | ) | | (299,773 | ) |
| Intangible assets, net | $ | 21,152,417 | | $ | 1,002,561 | |
Total amortization expense for the three months ended September 30, 2009 and 2008 amounted to $950,021 and $27,561 respectively. Amortization expense for the nine months ended September 30, 2009 and 2008 amounted to $2,654,269 and $80,753, respectively.
The amortization expense related to purchased and other intangible assets due to the consolidation of Dalin is $793,278 for the three months ended September 30, 2009. The amortization expense related to purchased and other intangible assets due to the consolidation of Dalin is $2,379,185 for the nine months ended September 30, 2009.
Amortization expense for intangible assets for the next five fiscal years is as follows:
| | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2013 | | | Thereafter | |
| Amortization expense | $ | 838,577 | | $ | 3,352,322 | | $ | 3,352,070 | | $ | 3,345,523 | | $ | 1,582,855 | | $ | 8,708,291 | |
Note 10 – Debt
Short term loans and current maturities of long term loan
Short term loans represent renewable loans due to various banks which are normally due within one year.
-19-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The Company’s bank loans consisted of the following:
| | | | | | Annual | | | September 30, | | | December 31, | |
| Loans | | Due by | | | interest rates | | | 2009 | | | 2008 | |
| | | | | | | | | (Unaudited) | | | | |
| Short term loans: | | | | | | | | | | | | |
Short term bank loan, un-secured | | June 1, 2010 | | | 5.40% | | $ | 4,401,000 | | $ | - | |
Short term bank loan, un-secured | | January 7, 2010 | | | 5.31% | | | 5,868,000 | | | - | |
Short term loan, un-secured | | On demand | | | 0.00% | | | 73,350 | | | - | |
Short term loan, secured by raw material(1) | | February 16, 2010 | | | 5.84% | | | 440,100 | | | - | |
Subtotal | | | | | | | | 10,782,450 | | | - | |
| | | | | | | | | | | | | |
| Long term bank loan – current maturities: |
|
|
|
|
|
|
|
|
|
|
| |
Long term loan, secured by building, machinery and equipment(2) |
| December 25, 2009 |
|
| 7.76% |
|
| 440,100 |
|
| - | |
Long term loan, secured by building, machinery and equipment(2) |
| April 25, 2010 |
|
| 7.76% |
|
| 1,467,000 |
|
| - | |
Long term loan, secured by building, machinery and equipment(2) | | June 25, 2010 | |
| 7.76% |
|
| 1,467,000 |
|
| - | |
Subtotal | | | | | | | | 3,374,100 | | | - | |
Long term bank loan, secured by buildings and land use rights | | August 3, 2010 | | | 7.02% | | | - | | | 5,868,000 | |
Total |
|
|
|
|
|
| $ | 14,156,550 |
| $ | 5,868,000 | |
| | |
| (1) | The interest rate for this short term loan is adjustable quarterly at 1.10 times of the prevailing rate as published by Bank of China. As of September 30, 2009, the interest rate is fixed at 5.84% per annum. |
| | |
| (2) | The interest rate is adjustable monthly at 1.15 times of the prevailing rate as published by Bank of China. As of September 30, 2009, the interest rate is fixed at 7.76% per annum. The long term loan is comprised of $440,100, $1,467,000, and $1,467,000 with respective due date on December 25, 2009, on April 25, 2010 and on June 25, 2010, respectively. |
Interest expense totaling $849,304 and $15,128 was incurred during the three months ended September 30, 2009 and 2008, respectively.
Interest expense totaling $2,414,118 and $59,800 was incurred during the nine months ended September 30, 2009 and 2008, respectively.
The above loans are secured by Shandong Taibang’s land use rights and buildings located in Taian, Shandong Province, PRC and Qianfeng’s buildings and machinery and equipment located in Guiyang, Guizhou Province, PRC, with carrying net values as follows:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Buildings in Taian, Shandong | $ | 1,238,010 | | $ | 1,417,138 | |
| Land use rights in Taian, Shandong | | 433,793 | | | 195,691 | |
| Buildings in Guiyang, Guizhou | | 1,298,572 | | | - | |
| Machinery and equipment in Guiyang, Guizhou | | 9,108,351 | | | - | |
| Total | $ | 12,078,726 | | $ | 1,612,829 | |
-20-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Other payables and accruals
Other payables and accruals consist of the following:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Other payables(1) | $ | 10,290,251 | | $ | 319,699 | |
| Payable to Sansui Finance department for pending investment on plasma stations(2) |
| 1,175,067 |
|
| - |
|
| Accruals for salaries and welfare | | 912,248 | | | 830,388 | |
| Accruals for RTO expenses | | 245,657 | | | 245,657 | |
| Accruals for selling commission and promotion fee | | 1,710,073 | | | 1,508,102 | |
| Other Payable - Government Grant | | 143,488 | | | 114,148 | |
| Other payable - Deposit received | | 207,705 | | | 283,826 | |
| Other payable - Funds | | 1,981,482 | | | 627,157 | |
| Accrued interest | | - | | | 33,954 | |
| Others(3) | | 452,247 | | | - | |
Total | $ | 17,118,218 | | $ | 3,962,931 | |
| (1) | The other payables mainly comprise of deposits by potential strategic investors with the amount of $7,465,640. As of September 30, 2009, Qianfeng has received in an aggregate amount of $7,465,640 from potential private strategic investors in connection with subscribing shares from Qianfeng pursuant to Equity Purchase Agreement. The registration of the new investors as Qianfeng’s shareholders and the related increase in registered capital of Qianfeng with the Administration for Industry and Commerce (“AIC”) is in incomplete due to share holders dispute as disclosed in below legal proceedings section below. |
| | |
| (2) | In early 2007, Qianfeng submitted RMB 8,010,000 (approximately $1,175,067) to the finance department of Sansui County, in China’s Qiandongnan Autonomous Region, for acquiring the Sansui Plasma Collection Station from the local government, which action was legitimized and fully endorsed by relevant provincial government authorities. However, the finance department refused to implement the provincial government’s authorization and has returned the funds to Qianfeng. As of September 30, 2009, Qianfeng has set aside the full amount as a payable to Sansui County, pending the outcome of provincial government’s administrative review as disclosed in the legal proceedings note below. |
| | |
| (3) | Others mainly comprise of the contingent liability due to the pending, outcome of the preceding of Qianfeng’s Guarantee to a Third Party as disclosed in below legal proceedings section below, Qianfeng provisioned a loss contingency reserve during its third quarter of 2009 for approximately $451,006 (RMB 3,074,342) to cover its share of the enforcement of this judgment. |
Other payable - land use rights
In July 2003, Shandong Taibang obtained certain land use rights from the Tai’an municipal government. Shandong Taibang is required to make payments totaling approximately $20,369 (RMB 138,848) per year to the local state-owned entity, for the 50-year life of the rights or until Biological Institute completes its privatization process. The Company recorded “land use rights” equal to “other payable – land use rights” totaling $ 324,121 and $325,390 as of September 30, 2009 and December 31, 2008, respectively, determined using present value of annual payments over 50 years.
Note 11 – Convertible Notes
-21-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| $9,554,140, 3.8% Senior Secured Convertible Notes, due June 5, 2011 | $ | 9,554,140 |
| $ | - |
|
| Less: unamortized discount | | (9,508,965 | ) | | - | |
| Notes payables, net | | 45,175 | | | | |
| Accrued interest | | - | | | | |
| Total | $ | 45,175 | | $ | - | |
On June 5, 2009, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with certain accredited investors (collectively, the “Investors”), pursuant to which the Company agreed to issue to the Investors, 3.8% Senior Secured Convertible Notes in the aggregate principal amount of $9,554,140 (the “Notes”) and warrants (the “Warrants” and together with the Notes, the “Subscribed Securities”) to purchase up to 1,194,268 shares of common stock of the Company (the “Warrant Shares” and together with the Conversion Shares, the “Underlying Securities”). The transaction closed on June 10, 2009. Other than with respect to this transaction, none of the Investors have had a material relationship with the Company or any of the Company’s officers, directors or affiliates or any associate of any such officer or director.
The Notes accrue interest at 3.8% per annum (the “Interest Rate”), from the closing until repayment, whether on maturity on June 5, 2011, by acceleration or otherwise. Interest on the Notes is due and payable in cash semi-annually on September 30 and March 31 of each year, commencing September 30, 2009, but the Company has the option to pay the interest due through the issuance of its common stock at a conversion price of $4.00 per share. If the Company defaults in the payment of the principal of or interest on the Notes when due, then upon the Investors’ election, the Company is obligated to either (a) redeem all or a portion of the Notes pursuant to the redemption rights discussed below or (b) pay interest on such defaulted amount at a rate equal to the Interest Rate plus 2.0% . The Notes are convertible at any time before maturity into shares of our common stock at a conversion price of $4.00 per share, subject to certain adjustments as specified in the Notes.
The Company’s obligations under the Notes are secured by the pledge by Siu Ling Chan, our board chair and a principal shareholder, of 3,000,000 shares of common stock held by her, pursuant to the terms of a Guarantee and Pledge Agreement among the Company, the investors and Ms. Chan. To induce Ms. Chan to enter into the Guarantee and Pledge Agreement with the Investors, the Company has agreed to indemnify her for all damages, liabilities, losses and expenses of any kind (“losses”), which may be sustained or suffered by her, arising out of or in connection with any enforcement action instituted by the Investors pursuant to the Guarantee and Pledge Agreement. The Company’s indemnification obligation is limited to losses that arise as the result of any negligent or unlawful conduct of the Company that is caused unilaterally by the Company and is beyond Ms. Chan’s control in her capacity as a director of the Company, and will not exceed the fair market value of the pledged shares as of the closing of the transaction.
The Warrants have a term of 3 years, an exercise price of $4.80 per share, subject to adjustments as provided in the Warrants, from time to time pursuant to anti-dilution and other customary provisions, and are exercisable by the Investors at any time after the date on which their related Notes are converted, except that if any of the Notes is converted in part, the Investors may only exercise a corresponding portion of the related Warrant.
The Company has granted the Investors demand and piggy-back registration rights with respect to the Underlying Securities, pursuant to a registration rights agreement among the Company and the Investors.
The Company paid its placement agent a cash fee of 6.1% of the proceeds received in connection with the issuance of the Notes and also issued to the placement agent a 3-year warrant to purchase 93,750 shares of the Company’s common stock at an exercise price of $6.00 per share, expiring after 3 years. The aggregate $870,417 fees paid to the placement agent, including the fair value of the warrant issued to them was deferred and is being amortized over the life of the Notes.
The Company allocated $6,552,504 of the proceeds received to the fair value of the derivative instruments embedded in the Notes (including the conversion option) and $3,826,897 to the fair value of the Warrants issued to the Investors. As a result, the Company recognized an initial charge to income of $825,261 for the amount by which the fair value of these liabilities exceeded the face amount of the Notes for the three and nine months ended September 30, 2009. The Notes are being accreted to their redemption value over the period to maturity, using an effective interest method.
-22-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The fair values of the embedded derivatives and the warrants issued to the Investors and the placement agents were determined using a binomial model, based on the market price of the Company’s common stock, volatility estimated at 130% based on a review of the historic volatility of the Company’s common stock, an expected dividend yield of zero, the remaining life of the instruments and risk-free rates of return of 1.11% - 1.88% .
For the three and nine months ended September 30, 2009, the Company recorded a loss of $10,813,201 and $9,656,224, respectively, related to the change in the fair value of the embedded derivative instruments in the Notes and the warrants.
Note 12 - Earnings (loss) per share
Basic earnings/(loss) per share is computed by dividing net income by the weighted average number of common shares outstanding during the period. Diluted earnings/(loss) per share is calculated by dividing net income by the weighted average number of common shares outstanding and dilutive potential common shares outstanding during the period.
Earnings (loss) per share is as follows for the three months ended September 30,
| | | 2009 | | | 2008 | |
| | | (unaudited) | | | (unaudited) | |
| Net income (loss) attributable to controlling interest for earnings (loss) per share | $ | (6,193,569 | ) | $ | 4,478,546 | |
Weighted average shares used in basic computation |
|
21,632,793 |
|
|
21,434,942 |
|
| Diluted effect of warrants and options | | - | | | 69,687 | |
| Weighted average shares used in diluted computation | | 21,632,793 | | | 21,504,629 | |
| Earnings (loss) per share: | | | | | | |
| Basic | $ | (0.29 | ) | $ | 0.21 | |
| Diluted | $ | (0.29 | ) | $ | 0.21 | |
For the three months ended September 30, 2009, all of the warrants, stock options and conversion options were excluded in the calculation of diluted earnings per share because of their anti-dilutive nature.
For the three months ended September 30, 2008, the average stock price was greater than the exercise prices of the 1,284,000 warrants which resulted in additional weighted average common stock equivalents of 69,687. However, 937,500 options were excluded from the calculation because of their anti-dilutive nature.
Earning per share is as follows for the nine months ended September 30,
| | | 2009 | | | 2008 | |
| | | (unaudited) | | | (unaudited) | |
| Net income attributable to controlling interest for earnings per share | $ | 5,035,494 | | $ | 8,780,500 | |
Weighted average shares used in basic computation |
|
21,504,002 |
|
|
21,434,942 |
|
| Diluted effect of warrants and options | | 263,084 | | | 278,228 | |
| Weighted average shares used in diluted computation | | 21,767,086 | | | 21,713,170 | |
| Earnings per share: | | | | | | |
| Basic | $ | 0.23 | | $ | 0.41 | |
| Diluted | $ | 0.23 | | $ | 0.40 | |
-23-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
For the nine months ended September 30, 2009, the average stock price was greater than the exercise prices of the 1,284,000 warrants which resulted in additional weighted average common stock equivalents of 263,084. However, 4,644,053 warrants, stock options and conversion options were excluded in the calculation of diluted earnings per share because of their anti-dilutive nature.
For the nine months ended September 30, 2008, the average stock price was greater than the exercise prices of the 1,284,000 warrants which resulted in additional weighted average common stock equivalents of 278,228. However, 937,500 options were excluded from the calculation because of their anti-dilutive nature.
Note 13 – Taxes
Income taxes
The Company is governed by the Income Tax Law of the People’s Republic of China (PRC) concerning Foreign Investment Enterprises and Foreign Enterprises and various local income tax laws (the Income Tax Laws). Under the Income Tax Laws, foreign investment enterprises (FIE) generally are subject to an income tax at an effective rate of 33% (30% state income taxes plus 3% local income taxes) on income as reported in their statutory financial statements after appropriate tax adjustments unless the enterprise is located in specially designated regions of cities for which more favorable effective tax rates apply. Upon approval by the PRC tax authorities, FIEs scheduled to operate for a period of 10 years or more and engaged in manufacturing and production may be exempt from income taxes for two years, commencing with their first profitable year of operations, after taking into account any losses brought forward from prior years, and thereafter with a 50% exemption for the next three years.
In 2002, the Company became a Sino-foreign joint venture. In 2003, the Company was granted by the state government for benefit of income tax exemption in first 2 years from January 2003 to December 2004 and 50% exemption for the third to fifth years from January 2005 to December 2007.
Beginning January 1, 2008, the new Enterprise Income Tax (“EIT”) law replaced the laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”).
The key changes are:
| a. | The new standard EIT rate of 25% will replace the 33% rate currently applicable to both DES and FIEs, except for High Tech companies who pays at a reduced rate of 15%; and |
| | |
| b. | Companies established before March 16, 2007 will continue to enjoy tax holiday treatment approved by local government for a grace period of the next 5 years or until the tax holiday term is completed, whichever is sooner. |
The Company’s subsidiary, Shandong Taibang, was established before March 16, 2007 and is, therefore, qualified to continue enjoying the reduced tax rate as described above.
Starting from January 1, 2008, Shandong Taibang became subject to 25% income tax rate according to the newly issued Income Tax Laws of PRC. According to PRC’s central government policy, certain new technology or high technology companies will enjoy preferential tax treatment of 15%, instead of 25%. On February 12, 2009, Shandong Taibang received the new technology or high technology certification from Shandong provincial government. The Certification allows the Company to receive the 15% preferential income tax rate, for a period of three years starting from January 1, 2008.
Starting from January 1, 2008, all dividends paid to foreign parents are subject to a 10% income tax. As a result, Logic Express recorded $334,877 and $0 income tax expense for the nine months ended September 30, 2009 and 2008, respectively, for dividends Shandong Taibang paid to its foreign parent, Logic Express. Logic Holdings recorded $374,073 and $0 income tax expense for the nine months ended September 30, 2009 and 2008, respectively, for dividends Dalin paid to its foreign parent, Logic Holdings.
-24-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The following table reconciles the U.S. statutory rates to the Company’s effective tax rate for the three months and the nine months ended September 30, 2009 and September 30, 2008:
| | | For the three months | | | For the nine months | |
| | | ended September 30, | | | ended September 30, | |
| | | 2009 | | | 2008 | | | 2009 | | | 2008 | |
| U.S. statutory rates | | 34.0 | % | | 35.0 | % | | 34.0 | % | | 35.0 | % |
| Foreign income | | (34.0 | ) | | (35.0 | ) | | (34.0 | ) | | (35.0 | ) |
| China tax rates | | 25.0 | | | 25.0 | | | 25.0 | | | 25.0 | |
| China income tax exemption | | (10.0 | ) | | (10.0 | ) | | (10.0 | ) | | (10.0 | ) |
| Other items(1) | | (15.0 | ) | | 7.4 | | | 17.4 | | | 13.6 | |
| Effective income tax rates | | 0.0 | % | | 22.4 | % | | 32.4 | % | | 28.6 | % |
(1)The (15.0)% represents the $13.2 million derivative mark-to-market expense as disclosed in below note 16 and the $1.7 million expenses incurred by CBP, Logic Express and Logic Holding that are not deductible in PRC for the three months ended September 30, 2009, which caused a loss of $245,964 before provision for income taxes and non-controlling interest. Therefore, we have incurred a zero effective tax rate for the three months ended September 30, 2009 due to a loss before provision for income tax. The 7.4% represents the $0.2 million expenses incurred by CBP, Logic Express that are not deductible in PRC for the three months ended September 30, 2008.
The 17.4% represents the $14.9 million derivative mark-to-market expense as disclosed in below note 16 and the $5.0 million expenses incurred by CBP, Logic Express and Logic Holding that are not deductible in PRC for the nine months ended September 30, 2009. The 13.6% represents the $2.5 million expenses incurred by CBP, Logic Express that are not deductible in PRC for the nine months ended September 30, 2008.
The estimated tax savings due to the tax exemption for the three months ending September 30, 2009 and 2008 amounted to $1,514,862 and $563,374, respectively. The net effect on earnings per share if the income tax had been applied would decrease basic earnings per share for the three months ended September 30, 2009 and 2008 by $0.07 and $0.03, respectively. The net effect on earnings per share if the income tax had been applied would decrease diluted earnings per share for the three months ended September 30, 2009 and 2008 by $0.07 and $0.03, respectively.
The estimated tax savings due to the tax exemption for the nine months ending September 30, 2009 and 2008 amounted to $4,506,389 and $1,358,863, respectively. The net effect on earnings per share if the income tax had been applied would decrease basic earnings per share for the nine months ended September 30, 2009 and 2008 by $0.21 and $0.06, respectively. The net effect on earnings per share if the income tax had been applied would decrease diluted earnings per share for the nine months ended September 30, 2009 and 2008 by $0.21 and $0.06, respectively.
CBP was incorporated in the United States and has incurred net operating losses for income tax purposes for the period ending September 30, 2009. The estimated net operating loss carry forwards for United States income taxes amounted to $4,344,584 and $2,996,886 as of September 30, 2009 and December 31, 2008, respectively, which may be available to reduce future years’ taxable income. These carry forwards will expire, if not utilized, from 2026 through 2029. Management believes that the realization of the benefits from these losses appears uncertain due to the Company’s limited operating history and continuing losses for United States income tax purposes. Accordingly, the Company has provided a 100% valuation allowance on the deferred tax asset benefit to reduce the asset to zero. Management reviews this valuation allowance periodically and makes adjustments as warranted. The following table represents the rollforward of the deferred tax valuation allowance:
| | | For the nine months ended | | | For the year ended | |
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (unaudited) | | | | |
| Balance as of beginning of period | $ | 1,018,941 | | $ | 640,318 | |
| Increase | | 458,217 | | | 378,623 | |
| Balance as of end of period | $ | 1,477,158 | | $ | 1,018,941 | |
-25-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The Company has cumulative undistributed earnings of foreign subsidiaries of approximately $35 million as of September 30, 2009, which is included in consolidated retained earnings and will continue to be indefinitely reinvested in international operations. Accordingly, no provision has been made for U.S. deferred taxes related to future repatriation of these earnings, nor is it practicable to estimate the amount of income taxes that would have to be provided if we concluded that such earnings will be remitted in the future.
Value added tax
VAT on sales amounted to $1,981,600 and $903,665 for the three months ended September 30, 2009 and 2008, respectively. VAT on sales amounted to $5,834,961 and $2,192,304 for the nine months ended September 30, 2009 and 2008, respectively. Sales are recorded net of VAT collected and paid as the Company acts as an agent for the government. VAT taxes are not impacted by the income tax holiday.
Taxes payable consisted of the following:
| | | September 30, 2009 | | | December 31, 2008 | |
| | | (Unaudited) | | | | |
| VAT tax payable | $ | 690,247 | | $ | 331,505 | |
| Income tax payable | | 5,018,767 | | | 3,630,878 | |
| Other miscellaneous tax payable | | 204,217 | | | 97,627 | |
| | $ | 5,913,231 | | $ | 4,060,010 | |
Note 14 – Commitments and contingent liabilities
Capital and lease commitments
The Company’s 82.76% owned subsidiary, He Ze Plasma Company, entered into a lease agreement on January 13, 2005, with the Yun Cheng Lan Tian Transportation Company in Yun Cheng County, Shandong Province, to lease land use rights for a period of 10 years. The annual lease amount is approximately $1,751 (RMB 12,000) with no early termination penalty. The Company has the right of first refusal to renew the lease after the ten year lease term.
The Company’s 82.76% owned subsidiary, Qi He Plasma Company, entered into a lease agreement on April 26, 2007, with the Zhang Bo Shi Village in Qi He County, Shandong Province, to lease land use rights for a period of 50 years. The annual lease amount is approximately $4,566 (RMB 31,144) with no early termination penalty.
The Company’s 82.76% owned subsidiary, Zhang Qiu Plasma Company, leased land use right and the use of building and equipment for a period of 10 year from January 1, 2007 with annual lease payment of $43,977 (RMB300,000). The lease was terminated in March 2008. The Company entered into a lease agreement on April 1, 2008, with the Zhang Qiu Red Cross Blood Center, to lease land use rights and the use building and equipment for a period of 10 years. The annual lease payment is approximately $1,466 (RMB 10,000) with no early termination penalty.
The Company’s 48.6% indirectly owned subsidiary, Qianfeng, entered into a lease agreement on June 1, 2006 with a group of individuals in an area located next to its production facility, to lease and use the space for processing industrial waste for 10 years. The annual lease amount is approximately $1,530 (RMB 10,438).
The Company’s indirectly owned subsidiary, Huang Ping Plasma Company, entered into a lease with Huang Ping County Finance Department on April 28, 2007, Guizhou Province, to lease land use rights and use a building and equipment for a period of 3 years. The annual lease payment is approximately $10,261 (RMB 70,000).
The Company’s indirectly owned subsidiary, Pu Ding Plasma Company, entered into a lease with Pu Ping County Health Department, Guizhou Province on March 31, 2007, to lease land use rights and use a building and equipment for a period of 3 years. The annual lease payment is approximately $21,989 (RMB 150,000).
-26-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The Company’s indirectly owned subsidiary, Na Yong Plasma Company, entered into a lease with Na Yong County Health Department, Guizhou Province on March 31, 2007, to lease land use rights and use a building and equipment for a period of 3 years. The annual lease payment is approximately $21,989 (RMB 150,000).
The Company’s indirectly owned subsidiary, Wei Ning Plasma Company, entered into a lease with Wei Ning County Health Department, Guizhou Province on April 9, 2007, to lease land use rights and use building and equipment for a period of 3 years. The annual lease payment is approximately $11,727 (RMB 80,000).
The Company recognizes lease expense on a straight line basis over the term of the lease in accordance to FASB’s accounting standard related to leases. Total capital and operating lease commitments outstanding for the fiscal year ended December 31:
| | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2013 | | | Thereafter | |
| Property and equipment | $ | 239,278 | | $ | - | | $ | - | | $ | - | | $ | - | | $ | - | |
| Lease | | 73,393 | | | 26,622 | | | 9,327 | | | 9,327 | | | 9,327 | | | 205,172 | |
| Total | $ | 312,671 | | $ | 26,622 | | $ | 9,327 | | $ | 9,327 | | $ | 9,327 | | $ | 205,172 | |
For the three months ended September 30, 2009 and 2008, total rent expense amounted to $44,395 and $1,451, respectively.
For the nine months ended September 30, 2009 and 2008, total rent expense amounted to $102,721 and $19,507, respectively.
Legal proceedings
Transfer of Equity Interests
Mr. Zu Ying Du was one of the original equity holders in our operating subsidiary, Shandong Taibang. Pursuant to a joint venture agreement, among the original equity holders, Mr. Du was obligated to make a capital contribution of RMB 20 million (or approximately $2.6 million) for a 25% interest in Shandong Taibang. Mr. Du made this contribution using funds borrowed from the Beijing Chen Da Technology Investment Company, or Beijing Chen Da. Mr. Du failed to repay Beijing Chen Da for his loan of the capital contribution amount. Mr. Du disputes that the money was due and owing. A Beijing court found that Beijing Chen Da had given money to Mr. Du but found that the loan agreement failed to comply with Chinese law. A notice was issued on July 5, 2004 by the Shenzhen Public Security Bureau Economic Crime Investigation Unit requesting a stay of the Beijing action pending their investigation into money laundering relating to the 20 million RMB loan to Zu Ying Du.
Subsequently, Beijing Chen Da entered into an equity transfer agreement with Mr. Du, pursuant to which Mr. Du's 25% equity interest in Shandong Taibang was transferred to Beijing Chen Da as repayment of the RMB 20 million debt. This agreement was signed by Mr. Du's brother who held a power of attorney from Mr. Du. Mr. Du disputes the legitimacy of this transfer and has argued that his brother, Du Hai Shan, exceeded the scope of the power of attorney. Mr. Du sued his brother in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
Missile Engineering, another original equity holder wholly controlled by Mr. Du, was obligated to contribute RMB 32.8 million (or $4.2 million) for a 41% interest in Shandong Taibang by means of cash, equipment and patent technology. It was obligated to obtain new drug certificate and production license of its patent technology from the government within a stipulated period in order to be recognized as a valid capital contribution, or in the alternative, make a cash payment. The patent technology was valued as RMB 26.4 million (or approximately $3.4 million). However, Missile Engineering failed to obtain the new drug certificate and production license within the stipulated period. Mr. Du also disputes whether the period for obtaining the certificate and license had expired. Pursuant to a stockholders resolution on September 26, 2004, Missile Engineering agreed to sell its 41% interest in Shandong Taibang to Up-Wing and Up-Wing agreed to take up the obligation of Missile Engineering to pay the RMB 26.4 million in cash. Missile Engineering disputes this transaction and sued the brother of Mr. Du in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
-27-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
In June 10, 2005, Beijing Chen Da also sold its equity interest in Shandong Taibang to Up-Wing Investments Limited, or Up-Wing, pursuant to a share transfer agreement, which became effective on September 2, 2005, upon approval by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. In March 2006, Up-Wing sold its equity interests in Shandong Taibang to Logic Express, our subsidiary.
In 2006, Missile Engineering applied for arbitration before the China International Economic and Trade Arbitration Commission, or CIETAC, to challenge the effectiveness of the transfer to Up-Wing Investments Limited, of the equity interests in Shandong Taibang formerly owned by Missile Engineering. The equity transfer had been approved by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. Missile Engineering later voluntarily withdrew this application and instead applied for administrative reconsideration of the equity transfer, but this application was rejected by the Ministry of Commerce in 2007. Missile Engineering applied with the District Court of Lixia District, Jinan City, Shandong province requesting revocation of Shandong COFTEC's approval of the equity transfer to Up-wing by Missile Engineering. Missile Engineering later voluntarily withdrew the action. In April 2007, Logic Express initiated an arbitration proceeding before the Shandong Tai'an Arbitration Committee, to establish that Logic Express is the lawful shareholder of Shandong Taibang. The parties to that proceeding were Logic Express Ltd. and Shandong Taibang Biological Products Co., Ltd. The Arbitration Committee's decision on September 6, 2007 confirmed that Logic Express had legitimate ownership as a result of the transfer of Shandong Taibang. Up-Wing started an action in the Intermediate Court of Tai'an City, Shandong province requesting the court to establish that Up-Wing is the lawful shareholder of Shandong Taibang. The Intermediate Court of Tai'an City, Shandong province on December 20, 2007 rejected the application on the basis that the same matter had been tried by the arbitration panel.
Up-Wing filed a defamation case in the District Court of Hi-technology and Industry Development District, Tai'an City, Shandong province claiming defamation against Mr. Du and the 21st Century Economic Report Newspaper. Judgment in favor of Up-Wing was rendered on July 22, 2008 ordering the newspaper and Mr. Du to publish an apology to Up-Wing.
Mr. Du and Missile Engineering filed two actions in the Intermediate Court of Wuhan City, Hubei province, against the following defendants, Du Hai Shan, his brother, Beijing Chen Da and Logic Express. Mr. Du and Missile Engineering have requested that the Wuhan Intermediate Court to restore the equity interests originally held by the plaintiffs, 25% equity interest held by Mr. Du and 41% equity interest held by Missile Engineering. The Wuhan Intermediate Court issued a preliminary order attaching 66% of the equity of Shandong Taibang pending the outcome of the case. However, on September 25, 2009, the Higher People’s Court of Hubei overruled the Wuhan Intermediate Court’s acceptance of jurisdiction over the case and ruled that the Tai’an Intermediate Court in Shandong Province, where the Company is located, had the proper jurisdiction over the parties’ dispute. As a result, the attached 66% of the equity of Shandong Taibang were released. The court ruled that while the plaintiffs had the right to bring a lawsuit for the validity of the share transfer agreement because they did not attend the previous arbitration hearing and never reached an arbitration agreement regarding their dispute, the Tai’an Intermediate Court has the proper jurisdiction over the dispute pursuant to the prior agreement of the parties. Although the plaintiffs may decide bring their suit to Tai’an, Management believes that the possibility of the plaintiffs prevailing in Tai’an is slim in light of the historical Tai’an proceedings in connection with the dispute: a Tai’an arbitration panel has already confirmed that the share transfer agreements are effective, including the share transfer agreement between the plaintiffs and the Company, and the Tai’an Intermediate Court has already confirmed the legal force of the arbitration award. Failure to resolve these disputes in our favor may adversely affect our business and operations.
-28-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Bobai County Collection Station
In January 2007, the Company's PRC subsidiary, Shandong Taibang, advanced $413,697 (RMB3.0 million) to Feng Lin, the 20% minority shareholder in Fang Cheng Plasma Company, the Company's majority owned subsidiary, for the purpose of establishing or acquiring a plasma collection station. Mr. Lin and Shandong Taibang intended to establish the Bobai Kangan Plasma Collection Co., Ltd. (“Bobai”) in Bobai County, Guangxi and on January 18, 2007, Shandong Taibang signed a letter of intent to acquire the assets of the Bobai Plasma Collection Station, which was co-owned by Mr. Lin and Mr. Keliang Huang. However, in January 2007, Hua Lan Biological Engineering Co., Ltd. (“Hua Lan”) filed suit in the District Court of Hong Qi District, Xin Xiang City, Henan Province, alleging that Feng Lin, Keliang Huang and Shandong Taibang established and/or sought to operate the Bobai Plasma Collection Station using a permit for collecting and supplying human plasma in Bobai County, that was originally granted to Hua Lan by the government of the Guangxi region, without Hua Lan's permission. The establishment and registration of Bobai was never realized as a result of this law suit. On January 29, 2007, on Hua Lan's motion, the District Court entered an order to freeze funds in the amount of approximately $386,100 (RMB3,000,000) held by the defendants in the case, including approximately $65,750 (RMB500,000) in funds held in Shandong Taibang's bank account in Tai'an City. A hearing was held on June 25, 2007 and judgment was entered against the defendants along with a $226,780 (RMB1,700,000) joint financial judgment. The Company appealed the District Court judgment to the Henan Province High Court. In November 2007, the High Court affirmed the judgment against the three defendants and increased the amount of the joint financial judgment to approximately $405,954 (RMB3,000,000).
In January 2008, Hua Lan enforced the judgment granted by the High Court to freeze the Company's bank accounts. Shandong Taibang has filed a separate action against Hua Lan before the Tai'an City District Court to seek recovery of any losses in connection with Hua Lan's claim and to request that the Tai'an City District Court preserve Hua Lan's property or freeze up to approximately $411,300 (RMB 3 million) of Hua Lan's assets to secure the return of such funds to the Company. The intermediate court in Tai'an City accepted the application on February 14, 2008 but the matter is still pending. Pending the outcome of the proceedings, Shandong Taibang increased its loss contingency reserve during its fourth quarter of 2007 from approximately $75,593 (RMB566,667) to $133,400 (RMB1,000,000) to cover its share of the enforcement of this judgment. During the fourth quarter of 2008, full amount of the judgment, including Feng Lin and Keliang Huang's portions of the judgment and the related fees, approximately $456,222 (RMB 3,109,900) has been withdrawn from Shandong Taibang's account. The Company recorded Feng Lin and Keliang Huang's portion of the judgment, approximately $304,143 (RMB2,073,234), as receivable as a result of the withdraw. As of December 31, 2008, the Company determined that it is unlikely that the Company will be able to recover such receivable from those two individuals and wrote off the receivable as bad debt expense.
In light of the foregoing, it is unlikely that the Company's planned acquisition of the assets of Bobai will go forward.
Dispute among Qianfeng Shareholders over Raising Additional Capital
On May 28, 2007, a 91% majority of Qianfeng's shareholders approved a plan to raise additional capital from private strategic investors through the issuance of an additional 20,000,000 shares of Qianfeng equity interests at RMB 2.80 per share. The plan required all existing Qianfeng shareholders to waive their rights of first refusal to subscribe for the additional shares. The remaining 9% minority holder of Qianfeng's shares, the Guizhou Jie'an Company, or Jie'an, did not support the plan and did not agree to waive its right of first refusal. On May 29, 2007, the majority shareholders caused Qianfeng to sign an Equity Purchase Agreement with certain investors, pursuant to which the investors agreed to invest an aggregate of RMB 50,960,000 (approximately $7,475,832) in exchange for 18,200,000 shares, or 21.4%, of Qianfeng's equity interests. At the same time, Jie'an also subscribed for 1,800,000 shares, representing its 9% pro rata share of the 20,000,000 shares being offered. The proceeds from all parties were received by Qianfeng in accordance with the agreement.
In June 2007, Jie'an brought suit in the High Court of Guizhou province, China, against Qianfeng and the three other original Qianfeng shareholders, alleging the illegality of the Equity Purchase Agreement. In its complaint, Jie'an alleged that it had a right to acquire the shares waived by the original Qianfeng shareholders and offered to the investors in connection with the Equity Purchase Agreement. On September 12, 2008, the Guizhou High Court ruled against Jie'an and sustained the Equity Purchase Agreement, but on November 2008, Jie'an appealed the Guizhou High Court judgment to the People's Supreme Court in Beijing. On May 13, 2009, the People's Supreme Court sustained the original ruling and denied the rights of first refusal of Jie'an over the additional shares waived by the original Qianfeng's shareholders. The registration of the new investors as Qianfeng's shareholders and the related increase in registered capital of Qianfeng with the Administration for Industry and Commerce is still pending. Due to the flaws of the Equity Purchase Agreement and the pre-conditions of the agreement no longer valid, the Company is evaluating the possibility of voiding the agreement to maintain the original share structure. If the Company is unable to void the agreement, Dalin's interests in Qianfeng may be reduced to approximately 41.3% .
-29-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Dispute over Qianfeng Technical Consulting Agreement
In 1997, Qianfeng entered into a Technical Cooperation Agreement with Sin Kyung Ye, or Sin, a Korean individual, to provide certain fractionation equipment and transfer processing know-how to Qianfeng. In August 2004, Sin filed a law suit against Qianfeng with the Intermediate Court in Guiyang City, China, alleging non-payment of RMB 100,000 (approximately, $14,670) for his fractionation equipment and RMB 5,000,000 (approximately, $733,500) for the transfer of his technological know-how. The Intermediate Court ruled in favor of Sin and found that Qianfeng owed Sin RMB 10,376,160 (approximately, $ 1,522,183), but Qianfeng appealed the Intermediate Court ruling to the Guizhou High Court. The Guizhou High Court agreed in part with Qianfeng's grounds for appeal and reduced the amount of know-how transfer fee to RMB 1,970,413 (approximately, $289,060). In May 2007, Sin appealed the Guizhou High Court's decision to the People's Supreme Court in Beijing. The People's Supreme Court heard in April 2008, but had not issued its decision as of November 13, 2009.
Qianfeng Product Liability Claims
In January 2008, Qianfeng, along with two local hospitals and a local blood center, was sued in the Zhuhui District Court in Hengyang, Hunan province, China, by a resident of Hunan province, for RMB 1,749,358 (approximately, $256,631) in damages, in connection with his alleged HIV contamination via blood transfusion during the plaintiff's treatment following an April 2006 traffic accident. The Zhuhui District Court awarded the plaintiff RMB 200,000 (approximately, $29,340), but found that the defendants were not responsible for his HIV contamination. All parties appealed to the Zhuhui Middle Court. On December 4, 2008, the Zhuhui Middle Court remanded the case to the lower court for retrial, on grounds that the HIV contamination could not be directly linked to the plaintiff's treatment by the hospitals or to Qianfeng's products. There have been no further developments on this case as of November 13, 2009.
Administration Interference
Qianfeng is party to an administrative proceeding against the government of the Qiandongnan Autonomous Region, or the Qiandongnan Authorities, in Guizhou Province, China, in connection with the ownership of three of Qianfeng's eight plasma stations in Guizhou Province. Qianfeng was authorized to acquire a total of eight plasma stations in Guizhou Province based on several national and provincial administrative authorizations issued by the PRC State Council and the Guizhou Ministry of Health between 2006 and 2007, but to date, the governmental authorizations have not been fully implemented by the Qiandongnan Authorities. In early 2007, Qianfeng submitted RMB 8,010,000 (approximately $1,173,465) to the local finance department of Sansui County, Qiandongnan, for acquiring the Sansui Plasma Collection Station (“Sansui”), but the local finance department refused to honor the purchase and returned the full consideration to Qianfeng. Furthermore, subsequent local rulings published by the Qiandongnan Authorities February 28, 2008 appear to authorize another private company to acquire the Sansui and two other stations, the Zhengyuan Plasma Collection Station and the Shibing Plasma Collection Station. In December 2008 Qianfeng filed an administrative review application with the People's Government of Guizhou Province, or the Guizhou Provincial Government, but the Guizhou Provincial Government has delayed making a final decision pending further review of regulations regarding administrative authorizations. Qianfeng has received verbal notification from staff in the Guizhou Provincial Government that the Qiandongnan Authorities have withdrawn the local rulings authorizing acquisition of the three plasma stations, but management has not received any written confirmation of such withdrawal. As a result, Qianfeng has maintained its application with the Guizhou Provincial Government for a formal administrative ruling on its right to acquire all eight plasma stations in Guizhou Province. In addition, Qianfeng has set aside the purchase price payable for Sansui pending the outcome of the administrative review.
-30-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Dispute over Raw Plasma Supply Agreement with Xintai
On March 10, 2009, Henan Xintai Medicine Company (previously known as Henan Zhongtai Medicine, “Xintai”) brought suit against Shandong Taibang and its two wholly-owned plasma collecting subsidiaries in Shandong for breach of a raw plasma supply agreement. The suit was subsequently withdrawn by Xintain on May 31, 2009. The agreement, signed by Shandong Taibang and Xintai on October 10, 2006, requires the two subsidiaries to provide to Xintai 40 metric tons of raw plasma per year from 2007 to 2009. The subsidiaries provided more than 34 metric tons of plasma to Xintai during 2007 in accordance with the agreement. On October 31, 2007, PRC State Department published the Regulation on Plasma Collection Stations. The Company believes the agreement is invalid because it violates the clause 43 of the new Regulation, which prohibits plasma collecting stations from providing raw plasma to manufacturer other than their direct parent. To comply with the Regulation, the subsidiaries ceased supplying plasma to Xintai in late 2007. On March 12, 2009, Shandong Taibang filed a suit in the Shandong Taian Middle Court against Xintai seeing damages of RMB50,000 (approximately, $7,335) for the plasma already supplied to Xintai during 2007. On June 29, 2009, Xintai re-filed the suit in Shandong Taian Middle Court against Taibang and the two subsidiaries seeking compensation of RMB6,000,000 (approximately, $880,200) for contract breach and demanding that Taibang and the subsidiaries continue to honor the agreement. On October 20, 2009, the Taian Middle Court combined and heard the two suits, and the Company is awaiting the Court’s ruling.
Qianfeng’s Guarantee to a Third Party
In 2007, as a condition to purchase Huang Ping Plasma Station, Qianfeng entered into an agreement with Guizhou Zhongxin Investment Company (“Zhongxin”) in which Qianfeng agreed to repay Zhongxin’s debt out of Qianfeng’s payables to Zhongxin arising from plasma purchased from Zhongxin. In the same agreement, Qianfeng also guaranteed to the Huang Ping County Hospital (“Huang Ping Hospital”), which was the co-owner with Zhongxin of the Huang Ping Plasma Station, for the amount of RMB3,000,000 (approximately, $440,100) of debt that Zhongxin owed to Huang Ping Hospital. On June 1, 2009, Huang Ping Hospital brought suit, in Huang Ping Country People’s Court of Guizhou Province, against Zhongxin for non-payment of its payables and debt due to Huang Ping Hospital and Qianfeng as the guarantor. On November 2, 2009, the court ruled in favor of the plantiff and Qianfeng will need to repay the RMB3,000,000 debt to Huang Ping Hospital on behalf of Zhongxin as the guarantor. The Equity Transfer Agreement pursuant to which we acquired a 90% interest in Dalin, Qianfeng’s majority shareholder, provides that the sellers shall be responsible, in accordance with their equity proportion in Qianfeng, for damages incurred by Qianfeng from Zhongxin’s debt and shall repay Qianfeng the sellers’ proportionate share of payments made by Qianfeng to creditors in connection with Zhongxin’s debt within 10 days after payment by Qianfeng.
Note 15 – Warrants and options
Warrants
On July 18, 2006, the Company entered into a securities purchase agreement with certain accredited investors and completed the sale of 2,200,000 shares of common stock and 1,070,000 warrants with an exercise price of $2.8425 per share (“2006 Warrants”). The warrants have a 5-year term and are callable by the Company if the shares trade at 160% of the exercise price for 15 consecutive trading days. On July 28, 2006, the Company also issued 214,000 warrants with an exercise price at $2.8425 (“Placement Agent Warrant”) to Lane Capital Markets, LLC, the placement agent. These warrants have a 5-year term and are non-callable.
Effective January 1, 2009, 1,284,000 warrants previously treated as equity pursuant to the derivative treatment exemption are no longer afforded equity treatment because the strike price of the warrants is denominated in US dollar, a currency other than the Company’s functional currency, the Chinese Renminbi. As a result, the warrants are not considered indexed to the Company’s own stock, and as such, all future changes in the fair value of these warrants will be recognized currently in earnings until such time as the warrants are exercised or expired.
As such, effective January 1, 2009, the Company reclassified the original fair value of these warrants of $738,449 from equity to a liability, as if these warrants were treated as a derivative liability since their date of issue in July 2006. On January 1, 2009, the Company reclassified from additional paid-in capital, as a cumulative effect adjustment, $929,577 from beginning retained earnings and $1,668,026 to a long-term derivative liability to recognize the fair value of such warrants on such date.
-31-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
In September 2009, as authorized by the board of directors, the Company redeemed all of its outstanding 2006 Warrants with an exercise price of $2.8425 per share, in connection with the above-mentioned Securities Purchase Agreement dated July 18, 2006. In addition, there were 145,500 shares of Placement Agent Warrants were converted into common stock. As a result, as of September 30, 2009, there were 1,215,500 warrants converted into the Company’s common stock. The fair value of these warrants at the conversion date totaled $5,712,822 was transferred to equity. In addition, the fair value of the remaining 68,500 common stock purchase warrants was $404,807 as of September 30, 2009. As such the Company recognized a loss of $2,429,132 and $4,449,603, respectively, from the change in fair value of these warrants for three and nine months ended September 30, 2009.
On June 5, 2009, the Company entered into a securities purchase agreement with certain accredited investors pursuant to which the Company issued 3.8% Senior Secured Convertible Notes in the aggregate principal amount of $9,554,140 and Warrants to purchase up to 1,194,268 shares of common stock of the Company. The Warrants have a term of 3 years, an exercise price of $4.80 per share, as adjusted from time to time pursuant to anti-dilution and other customary provisions, and are exercisable by the Investors at any time after the date on which their related Notes are converted, except that if any of the Notes is converted in part, the Investors may only exercise a corresponding portion of the related Warrant. The Company also issued to the placement agents 93,750 Warrants to purchase common stock at an exercise price of $6.00 per share, expiring after 3 years.
These common stock purchase warrants were not issued with the intent of effectively hedging any future cash flow, fair value of any asset, liability or any net investment in a foreign operation. The warrants do not qualify for hedge accounting, and as such, all future changes in the fair value of these warrants will be recognized currently in earnings until such time as the warrants are exercised or expire. These common stock purchase warrants do not trade in an active securities market, and as such, the Company estimated the fair value of these warrants using the Black-Scholes option pricing model, based on the market price of the Company’s common stock, volatility estimated at 130% based on a review of the historic volatility of the Company’s common stock, an expected dividend yield of zero, the remaining life of the warrants and risk-free rates of return of 1.11% - 1.88% .
Historical volatility was computed using daily pricing observations for recent periods that correspond to the term of the warrants. The Company believes this method produces an estimate that is representative of our expectations of future volatility over the expected term of these warrants. The Company has no reason to believe future volatility over the expected remaining life of these warrants likely to differ materially from historical volatility. The expected life is based on the remaining term of the warrants. The risk-free interest rates used are based on the yield on U.S. Treasury securities with a similar according to the remaining term as the warrants.
The summary of warrant activity is as follows:
| | | | | | Weighted | | | Average | |
| | | Warrants | | | Average | | | Remaining | |
| | | Outstanding | | | Exercise Price | | | Contractual Life | |
| December 31, 2007 | | 1,284,000 | | $ | 2.84 | | | 3.55 | |
| Granted | | - | | | - | | | - | |
| Forfeited | | - | | | - | | | - | |
| Exercised | | - | | | - | | | - | |
| September30, 2008 (unaudited) | | 1,284,000 | | $ | 2.84 | | | 2.80 | |
| Granted | | - | | | - | | | - | |
| Forfeited | | - | | | - | | | - | |
| Exercised | | - | | | - | | | - | |
| December 31, 2008 | | 1,284,000 | | $ | 2.84 | | | 2.55 | |
| Granted | | 1,288,018 | | | 4.89 | | | 2.70 | |
| Forfeited | | - | | | - | | | - | |
| Exercised | | (1,215,500 | ) | | 2.84 | | | 1.81 | |
| September 30, 2009 (unaudited) | | 1,356,518 | | $ | 4.78 | | | 2.67 | |
Options
On May 9, 2008, the Company adopted the 2008 Equity Incentive Plan, which provides up to 5,000,000 shares of Company’s Common Stock to be made available to employees and directors at various prices as established by the Board of Directors of the Company. On May 9, 2008, the Company granted options to purchase an aggregate of 937,500 shares of the Company’s common stock under the 2008 Plan to certain directors and employees, pursuant to stock option agreements between the Company and each of these directors or employees. The options have an exercise price of $4.00 per share, will vest immediately and will expire on June 1, 2018. On July 24, 2008, the Company granted options to purchase an aggregate of 60,000 shares of the Company’s common stock under the 2008 plan to its three independent directors. These options have an exercise price of $4.00 per share and 30,000 shares were vested on January 24, 2009 and the remaining 30,000 shares were vested on July 24, 2009, with the expiration date of July 24, 2018. As of September 30, 2009, there were 4,002,500 shares available under the plan.
-32-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The fair value of each option granted on May 9, 2008 and July 24, 2008 are estimated on the date of grant using the Black-Scholes option pricing model with the following assumptions:
| Granted on | | May 9, 2008 | | | July 24, 2008 | |
| Expected dividend yield | | 0% | | | 0% | |
| Risk-free interest rate | | 3.56% | | | 3.56% | |
| Expected life (in years) | | 5 | | | 5 | |
| Weighted average expected volatility | | 59.4% | | | 81.2% | |
The volatility of the Company’s common stock was estimated by management based on the historical volatility of the Company’s common stock, the risk free interest rate was based on Treasury Constant Maturity Rates published by the U.S. Federal Reserve for periods applicable to the estimated life of the options, and the expected dividend yield was based on the Company’s current and expected dividend policy. The value of the options was based on the Company’s common stock price on the date the options were granted. Because the Company does not have a history of employee stock options, the Company utilized the simplified method to estimate the life of the options which is the same as assuming that the options are exercised at the mid-point between the vesting date and expiration date. For the nine months ended September 30, 2009 and 2008, the Company expensed $62,281 and $1,283,801 in compensation expense. The options are accounted for as equity under FASB’s accounting standard related to derivative instruments and hedging activities. The options activity is as follows:
| | | | | | | | | Weighted | | | Average | | | | |
| | | | | | | | | Average | | | Remaining | | | Aggregate | |
| | | Options | | | Options | | | Exercise | | | Contractual | | | Intrinsic | |
| | | Outstanding | | | Exercisable | | | Price | | | Life | | | Value | |
| December 31, 2007 | | - | | | - | | $ | - | | | - | | $ | - | |
| Granted | | 997,500 | | | 937,500 | | | 4.00 | | | 10.00 | | | - | |
| Forfeited | | - | | | - | | | - | | | - | | | - | |
| Exercised | | - | | | - | | | - | | | - | | | - | |
| September 30, 2008 (unaudited) | | 997,500 | | | 937,500 | | $ | 4.00 | | | 9.68 | | $ | - | |
| Granted | | - | | | - | | | - | | | - | | | - | |
| Forfeited | | - | | | - | | | - | | | - | | | - | |
| Exercised | | - | | | - | | | - | | | - | | | - | |
| December 31, 2008 | | 997,500 | | | 937,500 | | $ | 4.00 | | | 9.43 | | $ | - | |
| Granted | | - | | | 60,000 | | | 4.00 | | | 9.06 | | | - | |
| Forfeited | | - | | | - | | | - | | | - | | | - | |
| Exercised | | - | | | - | | | - | | | - | | | - | |
| September 30, 2009 (unaudited) | | 997,500 | | | 997,500 | | $ | 4.00 | | | 8.68 | | $ | - | |
Note 16 – Change in fair value of derivative liabilities
Loss (gain) on change in fair value of derivative liabilities for the three and nine months ended September 30, 2009 comprised as following:
-33-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
| | | Three months ended | | | Nine months ended | |
| | | September 30, 2009 | | | September 30, 2009 | |
| Change in fair value of derivative liabilities of: | | (Unaudited) | | | (Unaudited) | |
| Conversion option of convertible notes | $ | 6,988,311 | | $ | 6,232,369 | |
| Warrants attached to convertible notes | | 3,554,340 | | | 3,181,749 | |
| Warrants issued to placement agent | | 270,550 | | | 242,106 | |
| Subtotal | | 10,813,201 | | | 9,656,224 | |
| Warrants issued with prior placements | | 2,429,132 | | | 4,449,603 | |
| Initial charge to income from convertible notes | | - | | | 825,261 | |
| Total | $ | 13,242,333 | | $ | 14,931,088 | |
Note 17 – Interest expense (income), net
Interest expense (income), net for the three months ended September 30, 2009 and 2008 comprised as following:
| | | 2009 | | | 2008 | |
| Interest expense (income), net | | (Unaudited) | | | (Unaudited) | |
| Interest expense – bank and other loans | $ | 713,364 | | $ | 15,128 | |
| Interest expense – convertible notes | | 135,940 | | | - | |
| Interest income | | (124,533 | ) | | (36,841 | ) |
| Total | $ | 724,771 | | $ | (21,713 | ) |
Interest expense (income), net for the nine months ended September 30, 2009 and 2008 comprised as following:
| | | 2009 | | | 2008 | |
| Interest expense (income), net | | (Unaudited) | | | (Unaudited) | |
| Interest expense – bank and other loans | $ | 2,257,000 | | $ | 59,800 | |
| Interest expense – convertible notes | | 157,118 | | | - | |
| Interest income | | (434,580 | ) | | (67,331 | ) |
| Total | $ | 1,979,538 | | $ | (7,531 | ) |
Note 18 – Statutory reserves
In accordance with the “Law of the PRC on Joint Ventures Using Chinese and Foreign Investment” and the Company’s Articles of Association, appropriations from net profit should be made to the Reserve Fund and the Enterprise Expansion Fund, after offsetting accumulated losses from prior years, and before profit distributions to the investors. The percentages to be appropriated to the Reserve Fund and the Enterprise Expansion Fund are determined by the Board of Directors of the Company.
Reserve fund
10% of the net income determined in accordance with PRC accounting rules and regulations are transferred to a statutory surplus reserve fund until such reserve balance reaches 50% of the Company’s registered capital. As of September 30, 2009, approximately $5 million still needs to be transferred to statutory reserve. The transfer to this reserve must be made before distribution of any dividend to shareholders. The surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing stockholders in proportion to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance after such issue is not less than 25% of the registered capital.
Enterprise expansion fund
The enterprise fund may be used to acquire plant and equipment or to increase the working capital to expend on production and operation of the business. The Company’s policy is to transfer 5% of the Shandong Taibang’s net income to this fund determined in accordance with the Company’s policy.
-34-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Note 19 – Retirement benefit plans
Regulations in the PRC require the Company to contribute to a defined contribution retirement plan for the benefit of all permanent employees. All permanent employees are entitled to an annual pension equal to their basic salaries at retirement. The PRC government is responsible for the benefit liability to these retired employees. The Company is required to make contributions to the state retirement plan at 20% of the monthly base salaries of the current employees. For the three months ended September 30, 2009 and 2008, the Company made pension contributions in the amount of $145,089 and $100,702, respectively. For the nine months ended September 30, 2009 and 2008, the Company made pension contributions in the amount of $487,459 and $274,347, respectively.
Note 20 - Noncontrolling interest and distribution
The roll forward of noncontrolling interest in the balance sheet is shown below:
| | | Fang Cheng | | | Shandong | | | Guizhou | | | Guiyang | | | Guiyang | | | | |
| | | Plasma Co. | | | Taibang | | | Renyuan | | | Qianfeng | | | Dalin | | | | |
| | | Minority | | | Minority | | | Minority | | | Minority | | | Minority | | | Total | |
| | | Owner | | | Owner | | | Owners | | | Owners | | | Owner | | | Noncontrolling | |
| | | (20%) | | | (17.24%) | | | (75%) | | | (46%) | | | (10%) | | | interest | |
| December 31, 2007 | $ | 82,994 | | $ | 3,802,898 | | $ | - | | $ | - | | $ | - | | $ | 3,885,892 | |
| Net income(loss) | | (83,938 | ) | | 3,387,779 | | | - | | | - | | | - | | | 3,303,841 | |
| Foreign currency translation gain/(loss) |
| 944
|
|
| 3,162
|
|
| -
|
|
| -
|
|
| -
|
|
| 4,106
|
|
| Dividend declared | | - | | | (2,982,045 | ) | | - | | | - | | | - | | | (2,982,045 | ) |
| December 31, 2008 | $ | - | | $ | 4,211,794 | | $ | - | | $ | - | | $ | - | | $ | 4,211,794 | |
Dalin acquisition |
|
- |
|
|
- |
|
|
2,444,304 |
|
|
17,317,066 |
|
|
1,763,689 |
|
|
21,525,059 |
|
Net income(loss) |
|
(4,372 |
) |
|
3,729,183 |
|
|
(84, 745 |
) |
|
6,308,851 |
|
|
789,378 |
|
|
10,738,295 |
|
| Foreign currency translation gain/(loss) |
| - |
|
| (187 | ) |
| - |
|
| - | |
| - |
|
| (187 | ) |
| Dividend declared | | - | | | (1,212,834 | ) | | - | | | (7,327,205 | ) | | (415,353 | ) | | (8,955,392 | ) |
September 30, 2009
(unaudited) |
$ |
(4,372 |
) |
$ |
6,727,956 |
|
$ |
2,359,559 |
|
$ |
16,298,712 |
|
$ |
2,137,714 |
|
$ |
27,519,569 |
|
Dividends declared are split pro rata between the shareholders according to their ownership interest. The payment of the dividends may occur at different times to the shareholders resulting in distributions which do not appear to be reflective of the minority ownership percentages. As of September 30, 2009, minority shareholders owned 17.24% of the Shandong Taibang, 10% of Dalin and 46% of Qianfeng. The table below shows the minority shareholder and dividends outstanding.
| | | Shandong | | | Guiyang | | | Guiyang | | | | |
| | | Taibang | | | Qianfeng | | | Dalin | | | Total | |
| | | Noncontrolling | | | Noncontrolling | | | Noncontrolling | | | Noncontrolling | |
| | | shareholder | | | shareholder | | | shareholder | | | shareholder | |
| Distribution payable, December 31, 2007 | $ | 506,626 | | $ | - | | $ | - | | $ | 506,626 | |
| Dividend declared | | 2,982,045 | | | - | | | - | | | 2,982,045 | |
| Dividend paid | | (288,300 | ) | | - | | | - | | | (288,300 | ) |
| Foreign currency translation adjustments | | 51,983 | | | - | | | - | | | 51,983 | |
| Distribution payable, December 31, 2008 | $ | 3,252,354 | | | | | | | | $ | 3,252,354 | |
| Dividend declared | | 1,212,834 | | | 7,327,205 | | | 415,353 | | | 8,955,392 | |
| Dividend paid | | (3,720,649 | ) | | (7,330,671 | ) | | (415,353 | ) | | (11,466,673 | ) |
| Foreign currency translation adjustments | | 14,780 | | | 3,466 | | | - | | | 18,246 | |
| Distribution payable, September 30, 2009 (unaudited) | $ | 759,319 | | $ | - | | $ | - | | $ | 759,319 | |
-35-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
Note 21 – Business combinations
On September 26, 2008, Logic Express (“Party B”) entered into an equity transfer agreement with Dalin, a PRC limited liability company, and Fan Shaowen, Chen Aimin, Chen Aiguo and Yang Gang, the shareholders of Dalin (collectively “Party A”), relating to the purchase of an aggregate 90% equity interest in Dalin, for a total purchase price of RMB194,400,000 (approximately $28,479,600), due in four installments. The parties agreed that (i) if Logic will have paid 90% of the purchase price of approximately $25,632,000 (or RMB 174,960,000) on or before April 7, 2009, then Logic will be entitled to its share of Dalin’s portion of the profit generated by Qianfeng starting from January 1, 2009, and (ii) if Logic fails to pay the said amount, the profit generated by Qianfeng from January 1, 2009 until the day of payment of said amount will be shared by Party A and Party B (i.e., Logic will be entitled to its share of Dalin's portion of the profit generated by Qianfeng calculated according to the proportion of the purchase price paid by it, and Party A will be entitled to the rest of Dalin’s portion of the profit generated by Qianfeng). The Company timely initiated the third installment payment to achieve 90% of the purchase price on April 7, 2009, in accordance with the instructions provided by the Dalin shareholders, which was subsequently paid on April 8 and April 14, 2009. The transaction was deemed by the Party A that Party B fulfilled its obligations under the agreement. As a result, Logic Holdings, the Hong Kong subsidiary, is now entitled to all the rights and privileges of a 90% shareholder in Dalin, including the right to receive its pro rata share of the profits generated by Dalin's 54% majority-owned operating subsidiary, Qianfeng Biological Products Co., Ltd., or Qianfeng, as of January 1, 2009, subject to a possible dilution to as low as 41.3%, if a dissenting Qianfeng shareholder prevails in a pre-existing suit to obtain additional equity interests in Qianfeng. The Company is obligated to pay the fourth and final installment, representing the remaining 10% of the Dalin purchase price, on or before April 9, 2010, the one-year anniversary of the local Administration for Industry and Commerce's approval of the equity transfer. On January 16, 2009, Qianfeng's shareholders, including Dalin, elected four members to its seven member Board of Directors that were nominated by Dalin, and on January 17, 2009, the Qianfeng's Board of Directors elected a new management team consisting of all Logic Express' and Dalin's appointees, including a new CEO, Executive Senior Vice President, CFO and Director of Sales. The operational control of Qianfeng passed to the Company effective January 1, 2009. According to the Equity Transfer Agreement, as amended, the Company can exercise the shareholder’s rights, as well as taking over all the corporate seals and license, of Dalin upon the payment of the second installment. The Company paid the second installment according to the agreement on December 14, 2008. However, under the section 1-2.2 of the Equity Transfer Agreement, as amended on December 12, 2008, the Company is entitled to the profit generated from Dalin’s main operating subsidiary, Qianfeng, after January 1, 2009. As a result, the Equity Transfer Agreement became effective on January 1, 2009. Therefore, the Company believes that January 1, 2009, the date on which the Company legally obtained control, acquired the assets, assumed the liabilities and became entitled to Dalin's share of the profit generated by Qianfeng as the acquisition date for the accounting purpose according to the FASB’s accounting standard related to business combination. The results of Dalin's and its subsidiaries' operations from January 1, 2009 through September 30, 2009 are included in the Company's Consolidated Statements of Operations and Comprehensive Income
Effective January 1, 2009, the Company adopted FASB’s accounting standard related to business combination which required acquisition method of accounting to be used for all business combinations and for an acquirer to be identified for each business combination. This accounting standard requires an acquirer to recognize the assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions. It also requires the acquirer in a business combination achieved in stages (sometimes referred to as a step acquisition) to recognize the identifiable assets and liabilities, as well as the noncontrolling interest in the acquiree, at the full amounts of their fair values (or other amounts determined in accordance with the standard).
The Company’s acquisition of Dalin was accounted for in accordance with this standard and the Company has allocated the purchase price of Dalin based upon the fair value of the net assets acquired and liabilities assumed and the fair value of the noncontrolling interest measured at the acquisition date. The Company estimated the fair values of the assets acquired and liabilities assumed at the acquisition date in accordance with the business combination standard issued by FASB and, except for cash and cash equivalents, fair value was estimated using level 3 inputs under FASB’s accounting standard related to fair value measurements. Level 3 inputs for the nonfinancial assets included a valuation report (prepared by a third party appraisal firm) that primarily utilized a combination of Income approach, cost approach and Market approach valuation techniques. Level 3 inputs for other assets and liabilities included present value techniques applied to after-tax income, expected after-tax cash flows and estimated selling prices (less costs of disposal and profit allowance) for inventories. In accordance with FASB’s accounting standard related to goodwill and other intangible assets, indefinite lived intangibles and goodwill are not being amortized.
-36-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
The following table summarizes the net book value and the fair value of the assets acquired and liabilities assumed at the date of acquisition, which represents the purchase price allocation at the date of the acquisition of Dalin based on valuation report which was prepared by a third party appraisal firm:
| | | Net Book Value | | | Fair Value | |
| Current assets | $ | 26,883,246 | | $ | 26,883,246 | |
| Property, plant and equipment, net | | 6,060,024 | | | 8,098,959 | |
| Intangibles | | 1,729,112 | | | 21,471,408 | |
| Other non-current assets | | 3,449,162 | | | 3,449,162 | |
| Goodwill | | - | | | 12,425,589 | |
| Total assets | | 38,121,544 | | | 72,328,364 | |
| Total liabilities | | (21,911,373 | ) | | (21,911,373 | ) |
| Net assets | $ | 16,210,171 | | $ | 50,416,991 | |
The Company determined the $50.4 million fair value of the acquired assets of Dalin based on an evaluation by an independent appraisal and the final asset evaluation by the management. The excess of the cost of an acquired entity over the net of the amounts assigned to assets acquired and liabilities assumed shall be recognized as goodwill. As a result, the $12.4 million of goodwill was due to the acquisition purchase price over the fair value of the assets acquired. During the three months ended September 30, 2009, the Company did not record any impairment charge from write-downs of purchased intangible assets since the Company do not identify any trends caused a reduction in expected future cash flows.
The following table presents the details of the fair value purchased intangible assets acquired through business combinations as of January 1, 2009:
| | | Useful life | | | | |
| | | (in years) | | | Fair Value | |
| Plasma collection permits | | 10 | | $ | 10,891,092 | |
| Land use rights | | 40 | | | 1,285,968 | |
| Long-term customer-relationship intangible assets | | 4 | | | 6,955,384 | |
| GMP certificate | | 5.8 | | | 2,332,652 | |
| Software | | 3.8 | | | 6,312 | |
| Total | | | | $ | 21,471,408 | |
In addition, the Company determined the $21.5 million fair value of the noncontrolling interest of Dalin based on an evaluation by an independent third party appraisal firm. Level 3 inputs for noncontrolling interest included considering average control premium in relevant Merger and Acquisition premium.
Pro Forma
The following unaudited pro forma condensed income statement for the three and nine months ended September 30, 2008 were prepared under generally accepted accounting principles as if the acquisition of Dalin has occurred on January 1, 2008. The pro forma information may not be indicative of the results that actually would have occurred if the acquisition had been in effect from and on the date indicated.
-37-
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2009
(Unaudited)
| | | Three months ended | | | Nine months ended | |
| | | September, 2008 | | | September, 2008 | |
| Revenues | $ | 19,306,855 | | $ | 61,653,874 | |
| Cost of revenues | | 4,647,386 | | | 18,252,493 | |
| Gross profit | | 14,659,469 | | | 43,401,381 | |
| Operating expenses | | 3,480,736 | | | 12,423,749 | |
| Other expenses, net | | 25,920 | | | 168,702 | |
| Provision for Income taxes | | 1,932,007 | | | 6,698,344 | |
| Net income before noncontrolling interest | | 9,220,806 | | | 24,110,586 | |
| Less: net income attributable to noncontrolling interest | | 2,327,527 | | | 7,823,067 | |
| Net income attributable to controlling interest | $ | 6,893,279 | | $ | 16,287,519 | |
| Basic - earning per share | | | | | | |
| Weighted average number of shares | | 21,434,942 | | | 21,434,942 | |
| Earnings per share | $ | 0.32 | | $ | 0.76 | |
| Diluted - earning per share | | | | | | |
| Weighted average number of shares | | 21,504,629 | | | 21,713,170 | |
| Earnings per share | $ | 0.32 | | $ | 0.75 | |
Note 22 – Subsequent Events
The Company has performed an evaluation of subsequent events through November 13, 2009, which is the date the financial statements were issued.
-38-
| ITEM 2. | MANAGEMENT’S DISCUSSIONANDANALYSISOFFINANCIALCONDITIONANDRESULTS OF OPERATIONS |
Special Note Regarding Forward Looking Statements
This Quarterly Report on Form 10-Q, including the following “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements include, among others, those concerning our expected financial performance and strategic and operational plans, as well as all assumptions, expectations, predictions, intentions or beliefs about future events. You are cautioned that any such forward-looking statements are not guarantees of future performance and that a number of risks and uncertainties could cause actual results of the Company to differ materially from those anticipated, expressed or implied in the forward-looking statements. The words “believe,” “expect,” “anticipate,” “project,” “targets,” “optimistic,” “intend,” “aim,” “will” or similar expressions are intended to identify forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Risks and uncertainties that could cause actual results to differ materially from those anticipated include risks related to, among others: our potential inability to raise additional capital that is necessary to fund our operations and our expansion, including our intended acquisitions; the possibility that third parties hold proprietary rights that preclude us from marketing our products; the emergence of additional competing technologies; changes in domestic and foreign laws, regulations and taxes; changes in economic conditions; uncertainties related to China’s legal system and economic, political and social events in China; a general economic downturn; a downturn in the securities markets; and Securities and Exchange Commission regulations which affect trading in the securities of “penny stocks.” Additional disclosures regarding factors that could cause our results and performance to differ from results or performance anticipated by this Report are discussed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2008 at Item 1A. “Risk Factors.”
Readers are urged to carefully review and consider the various disclosures made by us in this Report and our other filings with the SEC. These reports attempt to advise interested parties of the risks and factors that may affect our business, financial condition and results of operations and prospects. The forward-looking statements made in this Report speak only as of the date hereof and we disclaim any obligation to provide updates, revisions or amendments to any forward-looking statements to reflect changes in our expectations or future events, except as required by law.
Use of Terms
Except as otherwise indicated by the context, all references in this report to (i) “China Biologic,” the “Company,” “we,” “us,” or “our,” are references to the combined business of China Biologic Products, Inc., a Delaware corporation, and its direct and indirect subsidiaries; (ii) “Logic Express” are to our wholly owned subsidiary Logic Express Limited, a BVI company; (iii) “Logic Holding” are to our wholly-owned subsidiary Logic Holding (Hong Kong) Limited, a Hong Kong company; (iv) “Shandong Taibang” are to our subsidiary Shandong Taibang Biological Products Co. Ltd., a sino-foreign joint venture incorporated in China; (v) “Dalin” are to our majority owned subsidiary, Chongqing Dalin Biologic Technologies Co., Ltd., a PRC limited company; (vi) “Qianfeng” are to our majority owned subsidiary Qianfeng Biological Products Co., Ltd., a PRC company; (vii) “Huitian” are to our minority owned subsidiary Xi’an Huitian Blood Products Co., Ltd., a PRC company; (viii) “Securities Act” are to the Securities Act of 1933, as amended; (ix) “Exchange Act” are to the Securities Exchange Act of 1934, as amended; (x) “RMB” are to Renminbi, the legal currency of China; (xi) “U.S. dollar,” “$” and “US$” are to the legal currency of the United States; (xii) “China” and “PRC” are to the People’s Republic of China; (xiii) “BVI” are to the British Virgin Islands; and (xiv) “Hong Kong” are to the Hong Kong Special Administrative Region of China.
Overview of Our Business
We are a biopharmaceutical company and through our indirect Chinese subsidiaries, Shandong Taibang and Qianfeng, we are principally engaged in the research, development, manufacturing and sales of plasma-based pharmaceutical products in China. Shandong Taibang operates from our manufacturing facility located in Tai’an City, Shandong Province and Qianfeng operates in Guizhou Province. Our minority owned subsidiary, Huitian, operates from facilities in Xi’an Province. The plasma-based biopharmaceutical manufacturing industry in China is highly regulated by both the provincial and central governments. Accordingly, the manufacturing process of our products is strictly monitored from the initial collection of plasma from human donors to finished products. Our principal products include our approved human albumin and immunoglobulin products.
- 39 -
We are approved to sell 10% and 20% human albumin in10ml, 25ml and 50ml. Human albumin is our top-selling product. Sales of these human albumin products represented approximately 44.6% and 58.3% of our total revenues, respectively, for the three months ended September 30, 2009 and 2008. Human albumin is principally used to increase blood volume while immunoglobulin is used for certain disease preventions and cures. Our approved human albumin and immunoglobulin products use human plasma as the basic raw material. Albumin has been used for almost 50 years to treat critically ill patients by replacing lost fluid and maintaining adequate blood volume and pressure. All of our products are prescription medicines administered in the form of injections.
We sell our products to customers in the PRC, mainly hospitals and inoculation centers. Our sales have historically been made on the basis of short-term arrangements and our largest customers have changed over the years. For the three months ended September 30, 2009 and 2008, our top 5 customers accounted for approximately 17.7% and 23.3%, respectively, of our total revenue. For the nine months ended September 30, 2009 and 2008, our largest customer accounted for approximately 4.8% and 6.9%, of our revenue, respectively. As we continue to diversify our geographic presence, customer base and product mix, we expect that our largest customers will continue to change from year to year.
We operate and manage our business as a single segment. We do not account for the results of our operations on a geographic or other basis.
All our business has been conducted in Renminbi, the official currency of China. Renminbi is still not a free floating currency. The value of Renminbi is subject to changes in the Chinese government’s policies and depends to a large extent on China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of Renminbi to U.S. dollars has generally been stable, and Renminbi has appreciated against the U.S. dollar since July 2005.
The following chart reflects our corporate organizational structure:
- 40 -
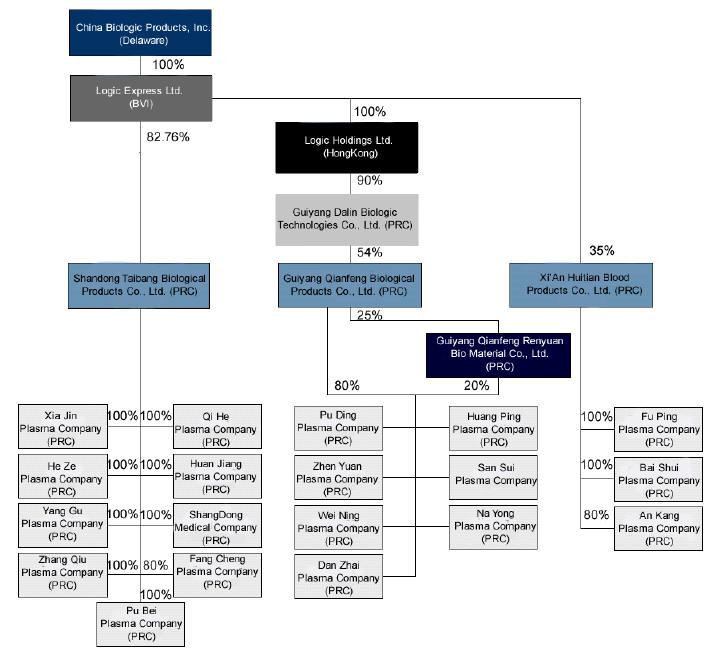
Third Quarter Financial Performance Highlights
We continued to experience strong demand for our products and services during the third quarter of 2009, which resulted in growth in our revenue and net income. The following are some financial highlights for the three months ended September 30, 2009:
- Revenue: Revenue increased $13,239,824, or 95.9%, to $27,039,739 for the three months ended September 30, 2009, from $13,799,915 for the same period in 2008.
- Income from operations: Income from operations increased $6,981,412, or 99.1%, to $14,027,734 for the three months ended September 30, 2009, from $7,046,322 for the same period in 2008.
- Net income: Net income decreased $10,672,115, or 238.3%, to a net loss of $6,193,569 for the three months ended September 30, 2009, from a net income of $4,478,546 for the same period in 2008, as a result of the $13.2 million non-cash charge of fair value of derivative liability.
- Fully diluted net income per share: Fully diluted net loss per share was $0.29 for the three months ended September 30, 2009, as compared to the net income of $0.21 for the same period in 2008.
- 41 -
Recent Developments
Dalin Acquisition and Entrustment Agreement
We acquired a 90% interest in Dalin in January 2009 and completed the transaction in April 2009 upon our payment of 90% of the purchase price. We are obligated to pay the remaining 10% of the purchase price, RMB 19,440,000 (approximately $2,847,960), on or before April 9, 2010, the one-year anniversary of the Dalin equity transfer’s approval by the local Administration for Industry and Commerce. Qianfeng is one of the largest plasma-based biopharmaceutical companies in China and is the only manufacturer currently operating in Guizhou Province. With a population of 39 million, Guizhou Province has historically produced the highest volumes of plasma collection in China, because a higher proportion of its population has been willing to engage in the collection process. Guizhou Province has a total of 19 plasma collection stations in operation, collecting approximately 1,200 metric tons of plasma supply each year. Qianfeng owns seven of these plasma collection stations, of which six are currently in operation and collecting approximately 250 metric tons of plasma per year, with an annual capacity of 400 metric tons. We intend to employ more advanced collection techniques at these stations to improve yields and generate additional plasma supply. Qianfeng is in compliance with Good Manufacturing Practices, or GMP, standards, and has been approved by the PRC’s State Food and Drug Administration or the SFDA to produce six types of plasma-based products including Human Albumin, Human Immunoglobulin, Human Intravenous Immunoglobulin, Human Hepatitis B Immunoglobulin, Human Tetanus Immunoglobulin and Human Rabies Immune Globulin. Based on publicly available data published by the PRC government, we believe that Qianfeng currently controls approximately 9.5% of the market for plasma-based biopharmaceutical products in China.
On April 6, 2009, Logic Express entered into an equity transfer and entrustment agreement, or Entrustment Agreement, among Logic Express, Shandong Taibang, and the Shandong Institute of Biological Products, or the Shandong Institute, the holder of the minority interests in Shandong Taibang, pursuant to which, Logic Express agreed to permit Shandong Taibang and the Shandong Institute to participate in the indirect purchase of Qianfeng’s equity interests. Under the terms of the Entrustment Agreement, Shandong Taibang agreed to contribute 18% or RMB 35,000,000 (approximately $5,116,184) of the Dalin purchase price and the Shandong Institute agreed to contribute 12.86% or RMB 25,000,000 (approximately $3,654,917) of the Dalin purchase price. Logic Express is obligated to repay to Shandong Taibang and the Shandong Institute their respective investment amounts on or before April 6, 2010, along with their pro rata share, based on their percentage of the Dalin purchase price contributed, of any distribution on the indirect equity investment in Qianfeng payable to Logic Express during 2009. Logic Express has agreed that if these investment amounts are not repaid within five days of the payment due date, then Logic Express is obligated to pay Shandong Taibang and the Shandong Institute liquidated damages equal to 0.03% of the overdue portion of the amount due until such time as it is paid. Logic Express has also agreed to pledge 30% of its ownership in Shandong Taibang to the Shandong Institute as security for nonpayment. If failure to repay continues for longer than 3 months after the payment due date, then the Shandong Institute will be entitled to any rights associated with the pledged interests, including but not limited to rights of disposition and profit distribution, until such time as the investment amount has been repaid. Logic Express also provided a guarantee that Shandong Taibang and the Shandong Institute will receive no less than a 6% return based on their original investment amount.
In July 2009, Dalin relocated from Chongqing, Sichuan to Guiyang, Guizhou to be in the same campus of Qianfeng to ease the administrative operations. The name of Dalin was also changed to Guiyang Dalin Biological Technology Company to reflect the relocation.
Huitian Acquisition
We acquired a 35% interest in Huitian in October 2008 and completed the transaction in July 2009 upon our final payment of the purchase price. Huitian is a manufacturer of plasma-based biopharmaceutical products in Shaanxi Province and is one of only 32 such manufacturers in China who are government approved. Shaanxi Province, which has a population of 37 million, has had a historically high collection volume with approximately ten plasma collection stations in operation, collecting approximately 300 metric tons of plasma each year. Only four of the collection stations in Shaanxi Province are government approved and three of these are owned by Huitian. Huitian produces about 80 metric tons of plasma-based products per year and has 200 tons of annual production capacity. Huitian believes that it currently controls approximately 1.2% of the market for plasma-based biopharmaceutical products in China, which management believes to be a strong indicator of long-term growth. Huitian is in compliance with GMP standards and it is also approved by the SFDA for the production of Human Albumin, Human Immunoglobulin, Human Immunoglobulin for Intravenous Injection, and Human Hepatitis B Immunoglobulin products.
- 42 -
Principal Factors Affecting Our Financial Condition
The following are key factors that affect our financial condition and results of operations and we believe them to be important to the understanding of our business:
Global Financial Crisis
The ongoing downturn in global financial markets has is expected to slow down China’s GDP growth, and may have an adverse effect on the demand for our products which are still not affordable to many PRC patients. If the current global economic slowdown continues and a depressed economic environment make our products less affordable to more patients or result in an overall decreased demand for our products, such reductions and disruptions could have a material adverse effect on our projected total sales increase and deteriorate our profit margins.
We believe that due to the rate of attrition of non-compliant companies in the wake of increased governmental regulations imposed on our industry, we have not yet seen a decline in the demand for our products. However, we can give no assurance that this demand for our products will continue.
Raw Material Prices
The primary raw material used in the production of our albumin and immunoglobulin products is human plasma. These products are still not affordable to many PRC patients. As China’s economy grows, we expect more Chinese people will become consumers of medical treatments and procedures, including procedures requiring the use of human plasma, resulting in increased demand for human plasma. Collection of human plasma in China is regulated and until 2006, only licensed Plasmapheresis stations owned and operated by the government could collect human plasma. Each collection station was only allowed to supply plasma to the one manufacturer that has signed the “Quality Responsibility” statement with such collection station. The price of human plasma is negotiated on an annual basis and is determined by a number of factors including, but not limited to, the cost of operating the collection stations, the nutritional supplement fee awarded to the donors for each donation, and the anticipated volume of total plasma donated. However, in March 2006, the Ministry of Health promulgated certain “Measures on Reforming Plasma Collection Stations,” whereby the ownership and management of PRC plasma stations are required to be transferred to plasma-based biopharmaceutical companies while the regulatory supervision and administrative control remain with the State. Plasma stations that did not complete their reform by December 31, 2006, risked revocation of their license to collect plasma.
In December 2006, we acquired five of the six then existing plasma stations in Shandong. On January 1, 2007, we obtained the permit to operate these stations. These acquisitions have allowed us to have direct influence on the operation of these collection stations and secure a stable source of plasma supply for production. In April 2007, we acquired two plasma stations in Guangxi Province, and we obtained permit to operate them.
Our recent acquisitions have led to an increase in our plasma supply and did not result in any material differences in our cost structure. Due to current market conditions, we have generally been able to pass substantially all cost increases in recent years on to our customers.
Prices of and Demand for Our Products
In recent years, due to increased regulatory restrictions and market demand, we have been able to increase the selling price of most of our key products. The demand for our products is largely affected by the general economic conditions in China. Currently, our products are still not affordable to many patients. As China’s economy grows, we expect more Chinese people will become consumers of medical treatments and procedures, including procedures requiring human plasma. A significant improvement in the economic environment in China will likely improve consumer income which in turn would make our products more affordable and consequently increase the demand for our products. We have been able to expand our product range and markets by introducing new products required by customers. We believe that our technical expertise is important in introducing products that are in demand.
- 43 -
Production Capacity
Our sales volume could be limited by our annual production capacity. As we grow our business in the future, our ability to fulfill additional and larger orders will depend on our ability to increase our production capacity. Our plan to expand our production capacity will depend on, inter alia, the availability of capital to meet our needs of expansion or upgrading of production lines, and the availability of stable plasma supply.
As of September 2009, with the operations in Shandong Taibang and Guiyang Qianfeng, our production capacity was 1,100 metric tons per annum. We estimate that the production capacity of our major competitors ranges from 300 metric tons to 1,000 metric tons per annum. Due to the shortage of raw material supply, our current production capacity should be sufficient for next couple years.
Competition
We are subject to intense competition. There are both local and overseas pharmaceutical enterprises that are engaged in the manufacture and sale of potential substitute or similar biopharmaceutical products as our products in the PRC. These competitors may have more capital, better research and development resources, manufacturing and marketing capability and experience than we do. In our industry, we compete based upon product quality, product cost, ability to produce a diverse range of products and logistical capabilities.
We believe that we have strengthened our position in the marketplace with our recent acquisition of a 90% equity interest in Dalin and its 54% majority-owned operating subsidiary, Qianfeng and a 35% equity interest in Huitian, a Xi’an -based biopharmaceutical company.
Our profitability may be adversely affected if (i) competition intensifies; (ii) competitors drastically reduce prices; or (iii) competitors develop new products or product substitutes with comparable medicinal applications or therapeutic effects which are more effective or less costly than those produced by us.
Taxation
United States andBritish Virgin Islands
We are subject to United States tax at a tax rate of 34%. No provision for income taxes in the United States has been made as we have no income taxable in the United States. Our subsidiary, Logic Express, was incorporated in the BVI and under the current laws of the BVI, is not subject to income taxes.
China
Before the implementation of the New EIT Law described below, Foreign Invested Enterprises, or FIEs, established in the PRC were generally subject to an enterprise income tax, or EIT, rate of 33.0%, which includes a 30.0% state income tax and a 3.0% local income tax.
On March 16, 2007, the National People’s Congress of China passed a new Enterprise Income Tax Law, or the New EIT Law, and on December 6, 2007, the State Council of China passed the Implementing Rules for the New EIT Law, or the Implementing Rules, which took effect on January 1, 2008. The New EIT Law and Implementing Rules impose a unified EIT rate of 25.0% on all domestic-invested enterprises and FIEs, unless they qualify under certain limited exceptions. Therefore, nearly all FIEs are subject to the new tax rate alongside other domestic businesses rather than benefiting from the old FIE tax laws, and its associated preferential tax treatments, beginning January 1, 2008.
Despite these changes, the New EIT Law gives foreign-invested enterprises, or FIEs, established before March 16, 2007, or Old FIEs, such as our 82.76% owned subsidiary Shandong Taibang, a five-year grandfather period during which they can continue to enjoy their existing preferential tax treatment. During this five-year grandfather period, the Old FIEs which enjoyed tax rates lower than 25% under the original EIT law shall gradually increase their EIT rate by 2% per year until the tax rate reaches 25%. In addition, the Old FIEs that are eligible for the “two-year exemption and three-year half reduction” or “five-year exemption and five-year half-reduction” under the original EIT law, can continue to enjoy their preference until these holidays expire. The discontinuation of any such special or preferential tax treatment or other incentives would have an adverse effect on any organization’s business, fiscal condition and current operations in China.
- 44 -
As a sino-foreign joint venture company, Shandong Taibang has been granted a preferential tax holiday by the Tax Bureau of the PRC as of 2003. Accordingly, Shandong Taibang is entitled to tax concessions from 2003 whereby the profit for the first two financial years beginning with the first profit-making year is exempt from income tax in the PRC, and the profit for each of the subsequent three financial years is taxed at 50% of the prevailing state income tax rate. Local income tax of 3% is exempted for five years starting from the first profit-making year. Shandong Taibang will be allowed the benefits of tax holidays under the grandfather treatment over a five-year transition period, and the applicable income rate will be 25% after the tax holiday. According to the PRC’s central government policy, new or high technology companies will enjoy preferential tax treatment of 15%, instead of 25%. On February 12, 2009, Shandong Taibang received the new technology or high technology certification from Shandong provincial government. The Certification allows Shandong Taibang to receive the 15% preferential income tax rate, for a period of three years starting from January 1, 2008.
In addition to the changes to the current tax structure, under the New EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a resident enterprise and will normally be subject to an EIT of 25.0% on its global income. The Implementing Rules define the term “de facto management bodies” as “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise.
On April 22, 2009, the State Administration of Taxation issued the Notice Concerning Relevant Issues Regarding Cognizance of Chinese Investment Controlled Enterprises Incorporated Offshore as Resident Enterprises pursuant to Criteria of de facto Management Bodies, or the Notice, further interpreting the application of the New EIT Law and its implementation with respect to non-Chinese enterprise or group controlled offshore entities. Pursuant to the Notice, an enterprise incorporated in an offshore jurisdiction and controlled by a Chinese enterprise or group will be classified as a “non-domestically incorporated resident enterprise” if (i) its senior management in charge of daily operation reside or perform their duties mainly in China; (ii) its financial or personnel decisions are made or approved by bodies or persons in China; (iii) its substantial properties, accounting books, corporate chops, board and shareholder minutes are kept in China; and (iv) ½ of directors with voting rights or senior management often reside in China. Such resident enterprise would be subject to an EIT rate of 25% on its worldwide income and must pay a withholding tax at a rate of 10% when paying dividends to its non-PRC shareholders. However, it remains unclear as to whether the Notice is applicable to an offshore enterprise incorporated by a Chinese natural person. Nor are detailed measures on imposition of tax from non-domestically incorporated resident enterprises available. Therefore, it is unclear how tax authorities will determine tax residency based on the facts of each case.
However, we may be deemed to be a resident enterprise by Chinese tax authorities. If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a number of unfavorable PRC tax consequences could follow. First, we may be subject to the enterprise income tax at a rate of 25% on our worldwide taxable income as well as PRC enterprise income tax reporting obligations. In our case, this would mean that income such as interest on financing proceeds and non-China source income would be subject to PRC enterprise income tax at a rate of 25%. Second, although under the New EIT Law and its Implementing Rules dividends paid to us from our PRC subsidiaries would qualify as “tax-exempt income,” we cannot guarantee that such dividends will not be subject to a 10% withholding tax, as the PRC foreign exchange control authorities, which enforce the withholding tax, have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises for PRC enterprise income tax purposes. Finally, it is possible that future guidance issued with respect to the new “resident enterprise” classification could result in a situation in which a 10% withholding tax is imposed on dividends we pay to our non-PRC stockholders and with respect to gains derived by our non-PRC stockholders from transferring our shares. We are actively monitoring the possibility of “resident enterprise” treatment and are evaluating appropriate organizational changes to avoid this treatment, to the extent possible.
Results of Operations
Comparison of Three Months Ended September 30, 2009 and 2008
The following tables set forth key components of our results of operations for the periods indicated, both in dollars and as a percentage of sales revenue.
- 45 -
(All amounts, other than percentages of revenue, in U.S. Dollars)
| | | Three Months Ended September 30, | | | Period-over-period | |
| | | 2009 | | | 2008 | | | Increase (Decrease) | |
| | | | | | % of | | | | | | % of | | | | | | | |
| | | Amount | | | Revenue | | | Amount | | | Revenue | | | Amount | | | % | |
| Revenue | $ | 27,039,739 | | | 100.0% | | $ | 13,799,915 | | | 100.0% | | $ | 13,239,824 | | | 95.9% | |
| Costs of revenue | | 6,960,901 | | | 25.7% | | | 4,138,077 | | | 30.0% | | | 2,822,824 | | | 68.2% | |
| Gross profit | | 20,078,838 | | | 74.3% | | | 9,661,838 | | | 70.0% | | | 10,417,000 | | | 107.8% | |
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Selling expenses | | 619,467 | | | 2.3% | | | 780,246 | | | 5.7% | | | (160,779 | ) | | (20.6% | ) |
| General and administrative expenses | | 5,169,137 | | | 19.1% | | | 1,634,233 | | | 11.8% | | | 3,534,904 | | | 216.3% | |
| Research and development expenses | | 262,500 | | | 1.0% | | | 201,037 | | | 1.5% | | | 61,463 | | | 30.6% | |
| Total operating expenses | | 6,051,104 | | | 22.4% | | | 2,615,516 | | | 19.0% | | | 3,435,588 | | | 131.4% | |
| Income from operations | | 14,027,734 | | | 51.9% | | | 7,046,322 | | | 51.1% | | | 6,981,412 | | | 99.1% | |
Other Expense (Income): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in loss (income) of
unconsolidated affiliate |
|
(31,051 |
) |
|
(0.1% |
) |
|
- |
|
|
- |
|
|
(31,051 |
) |
|
100% |
|
Change in fair value of warrant
liabilities |
|
13,242,333 |
|
|
49.0% |
|
|
- |
|
|
- |
|
|
13,242,333 |
|
|
100% |
|
| Interest expense (income), net | | 724,771 | | | 2.7% | | | (21,713 | ) | | (0.2% | ) | | 746,484 | | | 3438.0% | |
| Other expense (income), net | | 337,645 | | | 1.2% | | | 57,815 | | | 0.4% | | | 279,830 | | | 484.0% | |
| Total Other Expense | | 14,273,698 | | | 52.8% | | | 36,102 | | | 0.3% | | | 14,237,596 | | | 39437.1% | |
(Loss)Income before taxes and non-
controlling interest |
|
(245,964 |
) |
|
(0.9% |
) |
|
7,010,220 |
|
|
50.8% |
|
|
(7,256,184 |
) |
|
(103.5% |
) |
| Provision for income taxes | | 2,535,023 | | | 9.4% | | | 1,572,816 | | | 11.4% | | | 962,207 | | | 61.2% | |
| Non-controlling interest | | 3,412,582 | | | 12.6% | | | 958,858 | | | 6.9% | | | 2,453,724 | | | 255.9% | |
Net (loss) income attributable to
controlling interest |
$ |
(6,193,569 |
) |
|
(22.9% |
) |
$ |
4,478,546 |
|
|
32.5% |
|
$ |
(10,672,115 |
) |
|
(238.3% |
) |
Revenues
Our revenues are derived primarily from the sales of our human albumin and immunoglobulin products. Our revenues increased 95.9%, or $13,239,824 period of 2008. The increase in revenues during the third quarter of 2009 is primarily attributable to a general increase in the price of plasma-based products, but was partially offset by a decrease in our sales volume for one of our products. Among the factors that contributed to the growth in revenue, foreign exchange translation accounted for 0.3% of the increase. Our third quarter financial results also benefited from the consolidation of Dalin, which we acquired in the first quarter of 2009. Dalin contributed approximately $10.0 million in revenue, which accounted for approximately 37.1% of our total revenue for the third quarter of 2009. All of our approved products, except human immunoglobulin for intravenous injection, recorded price increases ranging from 3.4% to 118.8% . For the quarter ended September 30, 2009, the average price for our approved human albumin products, which contributed 42.7% to our total revenues, increased 3.4%, the average price for our approved human immunoglobulin for intravenous injection products, which contributed 37.9% to our revenues, decreased 2.5%, the average price for our approved human tetanus immunoglobulin products, which contributed 3.0% to our revenue, increased 11.5%, the average price for our approved human rabies immunoglobulin products, which contributed 5.0% to our revenue, increase 40.6%, and the average price for our approved human hepatitis B immunoglobulin products, which contributed 4.9%, increased 118.8% as compared to the same period in 2008. On July 1, 2008, the SFDA implemented the new 90-day quarantine period on plasma raw material. This new measure further tightens the raw material that is available for production, and has adversely impacted the already short supply of plasma-based products. As a result, during the third quarter of 2009, the supply of plasma-based products remained very tight industry-wide.
The continuing price increase of our products since 2008 was primarily attributable to the government’s stringent control on the quality standard of the plasma-based production industry, which resulted in a shortage in the supply of finished products. We were able to adjust our production plan to take advantage of the limited market supply of plasma resources to realize higher profit margins. In addition, there is a shortage in the market supply for human albumin products which has increased the value of our products in the market place. The plasma-based industry has been immune from the impact of the on-going global financial crisis as the demand for our products has out-paced supply. As a result, our selling price, cost of revenue and operating expenses during the third quarter of 2009 were not impacted by the global financial turmoil. With the acquisition of Dalin, and its operating subsidiary Qianfeng, we are better situated to serve our existing and new customers with expanded production capacity and market coverage. Our management expects that our revenue growth will remain strong for the remainder of 2009.
- 46 -
Cost of Revenues
Our cost of sales increased $2,822,824, or 68.2%, to $6,960,901 for the quarter ended September 30, 2009, from $4,138,077 during the same period in 2008. This increase was partly due to a 0.3% increase in foreign exchange translation, in addition to an actual 68.0% increase in cost of revenues. The increase in cost of revenues is primarily due to our consolidation of Dalin and the increase in the cost of raw materials during the 2009 period. Cost of revenues as a percentage of sales revenue was 25.7% for the quarter ended September 30, 2009, as compared to 30.0% during the same period in 2008. The decrease in cost of revenues as a percentage of sales revenue is primarily due to the sales increase in higher gross margin products, which was partially offset by the increase in raw material costs in connection with the expansion of our donor base during the 2009 period.
Gross Profit
Gross profit increased by $10,417,000, or 107.8%, to $20,078,838 for the quarter ended September 30, 2009, from $9,661,838 for the same period in 2008. As a percentage of sales revenue, our gross profit margin increased by 107.8% to 74.3% for the quarter ended September 30, 2009, from 70.0% for the same period in 2008. The increase in our gross profit as a percentage of revenues is due primarily to the general price increase and increase in sales of higher margin products, which partially offset by the increase in our raw material costs.
Operating Expenses
Our total operating expenses increased by $3,435,588, or 131.4%, to $6,051,104 for the quarter ended September 30, 2009, from $2,615,516 for the same period in 2008. The increase was primarily attributable to the 216.3% increase in our general and administrative expenses during the 2009 period. Asa percentage of sales revenue, total operating expenses increased by 3.4% to 22.4% .
Selling Expenses. For the quarter ended September 30, 2009, our selling expenses decreased to $619,467, from $780,246 for the quarter ended September 30, 2008, a decrease of $160,779, or 20.6% . As a percentage of sales, our selling expenses for the quarter ended September 30, 2009 decreased by 3.4%, to 2.3%, from 5.7% for third quarter 2008. The decrease in selling expenses is due primarily to reduction in marketing and promotion activities that initiated in 2008, which was offset by the selling expenses arose from consolidation of Dalin as well as more efforts spent on broadening new hospital customers. The decrease in selling expenses as a percentage of sales is due to our expanded sales following the Dalin acquisition.
General and Administrative Expenses. For the three months ended September 30, 2009, our general and administrative expenses increased to $5,169,137, from $1,634,233 for the quarter ended September 30, 2008, a $3,534,904, or 216.3% increase. General and administrative expenses as a percentage of sales increased by 7.3% to 19.1% for the third quarter of 2009, from 11.8% for the same period in 2008. The dollar increase was mainly due to the consolidation of Dalin in 2009 and an increase of $1.5 million in our personnel-related costs and extra $0.8 million of depreciation and amortization expenses in connection with our acquisition of Dalin as result of fair value adjustments, as well as additional professional service charge related to the acquisition of Dalin. Non-cash employee compensation for the quarter ended September 30, 2009 decreased to $7,314, from $20,613 for the same period in 2008, primarily as a result of grants to employees, consultants and directors made under our 2008 Equity Incentive Plan during the third quarter of 2008. The $7,314 compensation expense, which was included in the General and Administrative Expenses, represents the amortization of the compensation expense related to the grant of options to the independent directors.
Research and Development Expenses. For the quarter ended September 30, 2009 and 2008, our research and development expenses were $262,500 and $201,037, respectively, an increase of $61,463 or 30.6% . As a percentage of revenues, our research and development expenses for the quarter ended September 30, 2009 and 2008 were 1.0% and 1.5%, respectively. The dollar increase was due primarily to the consolidation of Dalin and increased costs from continuing clinical trial on new products.
- 47 -
Change in Fair Value of Derivative Liability
Prior to January 1, 2009, the warrants issued in 2006 were accounted for as equity instruments. Because the strike price of the warrants is denominated in USD and the Company’s functional currency is the RMB, the warrants are now classified as a derivative liability carried at fair value, with periodic changes in the fair value charged or credited to income each period. Similarly, the embedded derivatives (including the conversion option) in our senior secured convertible notes and warrants that were issued in June 2009 are classified as derivative liabilities carried at fair value. For the three months ended September 30, 2009 and 2008, the Company recognized a loss on the change in the fair value of derivative liabilities of $13,242,333 and $0, respectively. The recognized loss on the change in the fair value is the direct result of the Company’s stock price increase from $4.03 to $7.52 as of June 30, 2009 and September 30, 2009, respectively. Future changes in the market price of our common stock could cause the fair value of these derivative financial instruments to change significantly in future periods.
Interest Expense (income), net
Our net interest expense increased $746,484 to $724,771 for the quarter ended September 30, 2009, from interest income of $21,713 for the same period in 2008. The increase in interest expense is primarily due to the financing related to the acquisition of Dalin.
Income before Taxes and Non-Controlling Interest
Income before taxes and non-controlling interest for the three months ended September 30, 2009 and 2008 was a loss of $245,964 and income of $7,010,220, respectively, a decrease of $7,256,184, or 103.5% . Income before taxes and non-controlling interest as a percentage of revenues was a loss of 0.9% and an income of 50.8% for the three months ended September 30, 2009 and 2008, respectively. The decrease is due directly to the non-cash charge of change in fair value of derivative liabilities of $13.2 million and the $0.7 million interest expenses arose from financing Dalin acquisition.
Provision for Income Taxes
Our provision for income taxes increased $962,207, or 61.2%, to $2,535,023 for the quarter ended September 30, 2009, from $1,572,816 for the same period in 2008. The increase in provision for income taxes is mainly due to the consolidation of Dalin, which was offset by the decrease of Shandong Taibang’s provision for income taxes as Shandong Taibang accrued its 2008 taxes at 25% before it was granted with preferential 15% tax rate for the 2008 tax year in early 2009.
Non-controlling interest
Non-controlling interest increased $2,453,724, or 255.9%, to $3,412,582 for the quarter ended September 30, 2009, from $958,858 for the same period in 2008.
Net Income attributable to controlling interest
As a result of the factors described above, net loss attributable to controlling interest decreased $10,672,115, or 238.3%, to $6,193,569 during the three months ended September 30, 2009, from the net income of $4,478,546 for the same period in 2008.
Comparison of Nine Months Ended September 30, 2009 and 2008
The following tables set forth key components of our results of operations for the periods indicated, both in dollars and as a percentage of sales revenue.
- 48 -
(All amounts, other than percentages of revenue, in U.S. Dollars)
| | | Nine Months Ended September 30, | | | Period-over-period | |
| | | 2009 | | | 2008 | | | Increase (Decrease) | |
| | | | | | % of | | | | | | % of | | | | | | | |
| | | Amount | | | Revenue | | | Amount | | | Revenue | | | Amount | | | % | |
| Revenue | $ | 81,369,882 | | | 100.0% | | $ | 33,574,764 | | | 100.0% | | $ | 47,795,118 | | | 142.4% | |
| Costs of revenue | | 22,337,596 | | | 27.5% | | | 9,725,103 | | | 29.0% | | | 12,612,493 | | | 129.7% | |
| Gross profit | | 59,032,286 | | | 72.5% | | | 23,849,661 | | | 71.0% | | | 35,182,625 | | | 147.5% | |
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Selling expenses | | 2,313,577 | | | 2.8% | | | 1,785,340 | | | 5.3% | | | 528,237 | | | 29.6% | |
| General and administrative expenses | | 14,996,846 | | | 18.4% | | | 5,756,087 | | | 17.1% | | | 9,240,759 | | | 160.5% | |
| Research and development expenses | | 1,098,083 | | | 1.3% | | | 664,652 | | | 2.0% | | | 433,431 | | | 65.2% | |
| Total operating expenses | | 18,408,506 | | | 22.6% | | | 8,206,079 | | | 24.4% | | | 10,202,427 | | | 124.3% | |
Income from operations |
|
40,623,780 |
|
|
49.9% |
|
|
15,643,582 |
|
|
46.6% |
|
|
24,980,198 |
|
|
159.7% |
|
Other Expense (Income): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in loss (income) of
unconsolidated affiliate |
|
19,092 |
|
|
0.0% |
|
|
- |
|
|
- |
|
|
19,092 |
|
|
100.0% |
|
Change in fair value of warrant
liabilities |
|
14,931,088 |
|
|
18.3% |
|
|
- |
|
|
- |
|
|
14,931,088 |
|
|
100.0% |
|
| Interest expense (income), net | | 1,979,538 | | | 2.4% | | | (7,531 | ) | | (0.0% | ) | | 1,987,069 | | | 26385.2% | |
| Other expense (income), net | | 372,955 | | | 0.5% | | | 110,267 | | | 0.3% | | | 262,688 | | | 238.2% | |
| Total Other Expense | | 17,302,673 | | | 21.3% | | | 102,736 | | | 0.3% | | | 17,199,937 | | | 16741.9% | |
Income before taxes and non- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| controlling interest | | 23,321,107 | | | 28.7% | | | 15,540,846 | | | 46.3% | | | 7,780,261 | | | 50.1% | |
Provision for income taxes |
|
7,547,318 |
|
|
9.3% |
|
|
4,437,141 |
|
|
13.2% |
|
|
3,110,177 |
|
|
70.1% |
|
| Non-controlling interest | | 10,738,295 | | | 13.2% | | | 2,323,205 | | | 6.9% | | | 8,415,090 | | | 362.2% | |
Net income |
$ |
5,035,494 |
|
|
6.2% |
|
$ |
8,780,500 |
|
|
26.2% |
|
$ |
(3,745,006 |
) |
|
(42.7% |
) |
Revenues
Our revenues are derived primarily from the sales of our human albumin and immunoglobulin products. Our revenues increased 142.4%, or $47,795,118, to $81,369,882 for the nine months ended September 30, 2009, compared to revenues of $33,574,764 for the same period of 2008. The increase in revenues during the nine months of 2009 is primarily attributable to a general increase in the price of plasma based products, but was partially offset by a decrease in our sales volume for one of our products. Among the factors that contributed to the growth in revenue, foreign exchange translation accounted for 5.3% of the increase. Our financial results for the nine months ended September 30, 2009 also benefited from the consolidation of Dalin, which we acquired in the first quarter of 2009. Dalin contributed approximately $33.8 million in revenue, which accounted for approximately 41.5% of our total revenue for the nine months ended September 30, 2009. All of our approved products, except human hepatitis B immunoglobulin, recorded price increases ranging from 3.4% to 43.8% .. For the nine months ended September 30, 2009, the average price for our approved human albumin products, which contributed 48.7% to our total revenues, increased 3.4%, the average price for our approved human immunoglobulin for intravenous injection products, which contributed 36.6% to our revenues, increased 8.8%, the average price for our approved human tetanus immunoglobulin products, which contributed 2.7% to our revenue, increased 12.2%, the average price for our approved human rabies immunoglobulin products, which contributed 4.8% to our revenues, increased 43.8%, and the average price for our approved human hepatitis B immunoglobulin products, which contributed 3.2% to our revenues, decreased 5.4% as compared to the same period in 2008. On July 1, 2008, the SFDA implemented the new 90-day quarantine period on plasma raw material. This new measure further tightens the raw material that is available for production, and has adversely impacted the already short supply of plasma-based products. As a result, during the nine months ended September 30, 2009, the supply of plasma-based products remained very tight industry-wide.
- 49 -
The continuing price increase of our products since 2008 was primarily attributable to the government’s stringent control on the quality standard of the plasma-based production industry, which resulted in a shortage in the supply of finished products. We were able to adjust our production plan to take advantage of the limited market supply of plasma resources to realize higher profit margins. In addition, there is a shortage in the market supply for human albumin products which has increased the value of our products in the market place. The plasma-based industry has been immune from the impact of the on-going global financial crisis as the demand for our products has out-paced supply. As a result, our selling price, cost of revenue and operating expenses during the nine months of 2009 were not impacted by the global financial turmoil. With the acquisition of Dalin, and its operating subsidiary Qianfeng, we are better situated to serve our existing and new customers with expanded production capacity and market coverage. Our management expects that our revenue growth will remain strong for the remainder of 2009.
Cost of Revenues
Our cost of sales increased $12,612,493, or 129.7%, to $22,337,596 for the nine months ended September 30, 2009, from $9,725,103 during the same period in 2008. This increase was partly due to a 5.0% increase in foreign exchange translation, in addition to an actual 124.7% increase in cost of revenues. The increase in cost of revenues is primarily due to our consolidation of Dalin and the increase in the cost of raw material costs in connection with the expansion of our donor base during the 2009 period. Cost of revenues as a percentage of sales revenue for the nine months ended September 30, 2009 and 2008 were 27.5% and 29.0%, respectively. The decrease in cost of revenue as a percentage of revenue is mainly due to the general price increases in our products.
Gross Profit
Gross profit increased by $35,182,625, or 147.5%, to $59,032,286 for the nine months ended September 30, 2009, from $23,849,661 for the same period in 2008. As a percentage of sales revenue, our gross profit margin increased by 1.5% to 72.5% for the nine months ended September 30, 2009, from 71.0% for the same period in 2008. The amount increase in our gross profit is due primarily to the consolidation of Dalin.
Operating Expenses
Our total operating expenses increased by $10,202,427, or 124.3%, to $18,408,506 for the nine months ended September 30, 2009, from $8,206,079 for the same period in 2008. The increase was primarily attributable to the 160.5% increase in our general and administrative expenses during the 2009 period, as well as the 29.6% increase in selling expense, and the 65.2% increase in Research and development expenses. As a percentage of sales revenue, total operating expenses decreased by 1.8% to 22.6% for the nine months ended September 30, 2009, from 24.4% for the same period in 2008.
Selling Expenses. For the nine months ended September 30, 2009, our selling expenses increased to $2,313,577, from $1,785,340 for the same period in 2008, an increase of $528,237, or 29.6% . Our selling expenses as a percentage of sales for the nine months ended September 30, 2009 decreased by 2.5%, to 2.8%, from 5.3% for the same period in 2008. The increase in selling expenses is due primarily to our consolidation of Dalin’s selling activities as well as more efforts spent on broadening new hospital customers. The decrease in selling expenses as a percentage of sales is due to our expanded sales following the Dalin acquisition.
General and Administrative Expenses. For the nine months ended September 30, 2009, our general and administrative expenses increased to $14,996,846, from $5,756,087 for same period in 2008, a $9,240,759, or 160.5% increase. General and administrative expenses as a percentage of sales increased by 1.3% to 18.4% for the nine months ended September 30, 2009, from 17.1% for the same period in 2008. The dollar increase was mainly due to an increase in our personnel-related costs and extra depreciation and amortization expenses in connection with our acquisition of Dalin resulting from fair value adjustments, as well as additional professional service charge related to the acquisition of Dalin. Non-cash employee compensation for the nine months ended September 30, 2009 decreased to $62,281, from $1,283,801 for the same period in 2008, primarily as a result of grants to employees, consultants and directors made under our 2008 Equity Incentive Plan during our third quarter of 2008. The $62,281 compensation expense, which was included in the General and Administrative Expenses, represents the amortization of the compensation expense related to the grant of options to the independent directors.
Research and Development Expenses. For the nine months ended September 30, 2009 and 2008, our research and development expenses were $1,098,083 and $664,652, respectively, an increase of $433,431 or 65.2% .
- 50 -
As a percentage of revenues, our research and development expenses for the nine months ended September 30, 2009 and 2008 was 1.3% and 2.0%, respectively. The dollar increase was due primarily to the consolidation of Dalin and increased costs from continuing clinical trial on new products, while the decrease in the percentage of revenues is due to the increase in revenue from consolidation of Dalin.
Change in Fair Value of Derivative Liabilities
Prior to January 1, 2009, the warrants issued in 2006 were accounted for as equity instruments. Because the strike price of the warrants is denominated in USD and the Company’s functional currency is the RMB, the warrants are now classified as a derivative liability carried at fair value, with periodic changes in the fair value charged or credited to income each period. Similarly, the embedded derivatives (including the conversion option) in our senior secured convertible notes and warrants that were issued in June 2009 are classified as derivative liabilities carried at fair value. For the nine months ended September 30, 2009 and 2008, the Company recognized a loss on the change in the fair value of derivative liabilities of $14,931,088 and $0, respectively. The recognized loss on the change in the fair value is the direct result of the Company’s stock price increase from $2.00 to $7.52 as of December 31, 2008 and September 30, 2009, respectively. Future changes in the market price of our common stock could cause the fair value of these derivative financial instruments to change significantly in future periods.
Interest Expense (income), net
Our net interest expense increased $1,987,069 to $1,979,538 for the nine months ended September 30, 2009, from interest income of $7,531 for the same period in 2008. The increase in interest expense is primarily due to the financing related to the acquisition of Dalin.
Income before Taxes and Non-Controlling Interest
Income before taxes and non-controlling interest for the nine months ended September 30, 2009 and 2008 was $23,321,107 and $15,540,846, respectively, an increase of $7,780,261, or 50.1% . Income before taxes and non-controlling interest as a percentage of revenues was 28.7% and 46.3% for the nine months ended September 30, 2009 and 2008, respectively. The increase is due directly to an increase in the selling prices of our products and the consolidation of Dalin, which was offset by the non-cash change in fair value of derivative liabilities of $14,931,088.
Provision for Income Taxes
Our provision for income taxes increased $3,110,177, or 70.1 %, to $7,547,318 for the nine months ended September 30, 2009, from $4,437,141 for the same period in 2008. The increase in provision for income taxes is mainly due to the consolidation of Dalin, which was offset by the decrease of Shandong Taibang’s provision for income taxes as Shandong Taibang accrued its 2008 taxes at 25% before it was granted with preferential 15% tax rate for the 2008 tax year in early 2009. Our effective tax rate for the quarter ended September 30, 2009 and 2008 was 32.4% and 28.6%, respectively.
Non-controlling interest
Non-controlling interest increased $8,415,090 or 362.2%, to $10,738,295 for the nine months ended September 30, 2009, from $2,323,205 for the same period in 2008.
Net Income attributable to controlling interest
As a result of the factors described above, net income attributable to controlling interest decreased $3,745,006, or 42.7%, to $5,035,494 during the nine months ended September 30, 2009, from $8,780,500 for the same period in 2008.
Liquidity and Capital Resources
As of September 30, 2009, we had cash and cash equivalents of $50,348,133, primarily consisting of cash on hand and demand deposits.To date, we have financed our operations primarily through cash flows from operations, augmented by short-term bank borrowings and equity contributions by our stockholders.
- 51 -
With the bank credit facilities that are available to us and other financing activities, we expect that cash on hand, funds generated from our operations and funds generated from companies that we may acquire in the future will be sufficient to satisfy our current and future commitments for at least the next twelve months. We do not believe that we have any significant short term liquidity problems.
The following table provides detailed information about our net cash flow for all financial statement periods presented in this report.
Cash Flow
(All amounts in U.S. dollars)
| | | Nine Months Ended September 30, | |
| | | 2009 | | | 2008 | |
| Net cash provided by (used in) operating activities | $ | 35,458,229 | | $ | 14,691,924 | |
| Net cash used in investing activities | | (6,059,109 | ) | | (4,734,794 | ) |
| Net cash provided by (used in) financing activities | | 12,095,925 | | | (1,341,943 | ) |
| Effect of exchange rate change on cash | | 38,472 | | | 598,736 | |
| Net increase (decrease) in cash and cash equivalents | | 41,533,517 | | | 9,213,923 | |
| Cash and cash equivalents, beginning | | 8,814,616 | | | 5,010,033 | |
| Cash and cash equivalents, ending | | 50,348,133 | | | 14,223,956 | |
Operating activities
Net cash provided by operating activities was $35,458,229 for the nine months ended September 30, 2009, as compared to $14,691,924 net cash provided by operating activities for the same period in 2008. The increase in net cash provided by operating activities was mainly due to the increase in operating income of $24,980,198 to $40,623,780 for the nine months ended September 30, 2009, as compared to $15,643,582 in the same period of 2008. For the nine months ended September 30, 2009, the net income attributed to non-controlling interest of $10,738,295, the warrants and derivative MTM charge of $14,931,088 and other non-cash activities of $5,904,816 brought the operating income to a net income of $5,035,494. The increase in customer deposit provided $4,154,255 in net cash. The increase in other payables and accrued liabilities provided $4,236,622 in net cash, which was offset by an increase in inventory of $9,729,616.
Investing activities
Our use of cash for investing activities is primarily for the acquisition of property, plant and equipment and intangibles. Net cash used by investing activities for the nine months ended September 30, 2009 was $6,059,109, as compared to $4,734,794 net cash used in investing activities during the same period of 2008. The cash used for acquiring additional plant and equipment and intangible assets is $3,698,049. The cash used for the payment of Dalin acquisition were offset by the acquisition of Dalin’s existing cash,while payment for Huitian investment used $3,224,980 of the cash.
Financing activities
Net cash provided by financing activities for the nine months ended September 30, 2009 was $12,095,925 as compared to $1,341,943 used in financing activities in the same period of 2008. The increase of the cash provided by financing activities was mainly attributable to the proceeds from issuance of convertible note of $8,967,516, proceeds from warrants exercised of $3,455,059 and bank loans of $13,515,598, which was offset by shareholder loan repayments of $2,782,278, a non-controlling shareholder dividend payment of $2,293,888, a short term bank loan payment of $2,814,528 and a long term bank loan payment of $5,863,600.
Loan Facilities
On June 25, 2007, Qianfeng entered into a long term loan agreement with the Guiyang City, Yunyan sub-branch of the Agricultural Bank of China, pursuant to which the bank loaned Qianfeng RMB23,000,000 (approximately $3,374,100) for the purchase of raw materials. The loan bears an interest rate of 1.15 times the prevailing interest rate published by the Bank of China and is secured by Qianfeng’s machinery and equipment. Approximately $440,100 of the total loan amount matured on December 25, 2009, and the remaining $2,934,000 balance of the loan is scheduled to mature in two equal lump sums on April 25, 2010 and June 25, 2010, respectively.
- 52 -
On January 8, 2009, Shandong Taibang entered into a short term loan agreement with the Taishan Sub-Branch of the Bank Of China, pursuant to which the bank loaned Shandong Taibang RMB40 million (approximately $5,868,000). The Loan has an annual interest rate of 5.31% on all outstanding principal and is due and payable in full on January 7, 2010. Shandong Taibang is obligated under the loan agreement to pay the interest quarterly and repay the principal along with any remaining unpaid interest in full on the maturity date. Shandong Taibang may not prepay the loan without the bank’s prior written consent, which should be solicited no later than 30 days before such prepayment, and any such prepayment will be subject to a penalty equal to 0.0005% of the principal. If the loan is not paid in full by the maturity date, the interest rate will be increased to 6.90%, until full payment is made. In addition, if the loan is used for any other purpose than to fund the purchase of raw materials, the bank will have the right to increase the interest rate to 8.23% on the misused portion of the loan, effective as of the date such funds are misused, until the misused portion is repaid in full. Furthermore, if the quarterly interest payments required under the loan agreement are not timely made during the term, the bank will have the right to increase the interest rate to 6.90%, and if any such quarterly interest payments are outstanding after the maturity date and are not timely made thereafter, then the bank will have the right to charge an interest rate of 8.23% . Shandong Taibang currently plans to use the loan to fund the purchase of raw materials. In November 2009, with the consent from the bank, the company repaid RMB 20 million (approximately $2,939,000) of the loan amount prior to its maturity.
On February 17, 2009, Qianfeng entered into a short term loan agreement with the Guiyang City, Yunyan sub-branch of the Agricultural Bank of China, pursuant to which the bank loaned Qianfeng RMB3,000,000 (approximately $440,100) for use as plasma purchase. This loan bears an interest rate of 1.1 times the prevailing Bank of China interest rate and is secured by Qianfeng’s main fractionation facility located in Guizhou, China. The loan matures on February 16, 2010.
On May 31, 2009, Taibang entered into two unsecured short term loan agreements, for the amount of RMB20,000,000 (approximately $2,980,000) each, with the Taishan sub-branch of the Communication Bank of China to replace the RMB 40,000,000 (approximately $5,860,000) long term loan originally dated October 28, 2008. Pursuant to the agreements, these loans are for the working capital purpose and mature on June 1, 2010. Both loans bear a fixed interest rate of 5.4% with the interest payable quarterly. As of September 30, 2009, the loan has an outstanding balance of RMB 30 million (approximately $4,401,000). In November 2009, with the consent from the bank, the company further reduced the loan by RMB 20 million (approximately $2,939,000).
Obligations under Material Contracts
On September 26, 2008, Logic Express entered into an equity transfer agreement with Dalin, and Fan Shaowen, Chen Aimin, Chen Aiguo and Yang Gang, the shareholders of Dalin, relating to the purchase of an aggregate 90% equity interest in Dalin, for a total purchase price of RMB194,400,000 (approximately, $28,479,600), due in four installments. On April 14, 2009, we paid the third installment towards the acquisition which represented our payment of 90% of the purchase price in the aggregate, and in accordance with terms of the equity transfer agreement, Logic Holdings is now entitled to all the rights and privileges of a 90% shareholder in Dalin, including the right to receive its pro rata share of the profits generated by Dalin’s 54% majority-owned operating subsidiary, Qianfeng, as of January 1, 2009, subject to a possible dilution to as low as 41.3%, if a dissenting Qianfeng shareholder prevails in a pre-existing suit to obtain additional equity interests in Qianfeng. We are obligated to pay the fourth and final installment, representing the remaining 10% of the purchase price, on or before April 9, 2010, the one-year anniversary of the local Administration for Industry and Commerce’s approval of the equity transfer.
On April 6, 2009, Logic Express entered into an equity transfer and entrustment agreement, or Entrustment Agreement, among Logic Express, Shandong Taibang, and the Shandong Institute of Biological Products, or the Shandong Institute, the holder of the minority interests in Shandong Taibang, pursuant to which Logic Express agreed to permit Shandong Taibang and the Shandong Institute to participate in the indirect purchase of Qianfeng’s equity interests. Under the terms of the Entrustment Agreement, Shandong Taibang agreed to contribute 18%, or RMB35,000,000 (approximately, $5,116,184), of the purchase price for Dalin and the Shandong Institute agreed to contribute 12.86%, or RMB25,000,000 (approximately, $3,654,917), of the purchase price. Logic Express is obligated to repay to Shandong Taibang and the Shandong Institute their respective investment amounts on or before April 6th, 2010, along with their pro rata share, based on their percentage of the purchase price contributed, of any distribution on the indirect equity investment in Qianfeng payable to Logic Express during 2009. Logic Express has agreed that if these investment amounts are not repaid within 5 days of the payment due date, then Logic Express is obligated to pay Shandong Taibang and the Shandong Institute liquidated damages equal to 0.03% of the overdue portion of the amount due until such time as it is paid. Logic Express has also agreed to pledge 30% of its ownership in Shandong Taibang to the Shandong Institute as security for nonpayment. If failure to repay continues for longer than 3 months after the payment due date, then the Shandong Institute will be entitled to any rights associated with the pledged interests, including but not limited to rights of disposition and profit distribution, until such time as the investment amount has been repaid. Logic Express also provided a guarantee that Shandong Taibang and the Shandong Institute will receive no less than a 6% return based on their original investment amount.
- 53 -
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial conditions and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management's current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements.
Fair Value of Financial Instruments
On January 1, 2008, the Company adopted FASB’s accounting standard related to fair value measurements and began recording financial assets and liabilities subject to recurring fair value measurement at the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. These fair value principles prioritize valuation inputs across three broad levels. The Company considers the carrying amount of cash, receivables, payables including accrued liabilities and short term loans to approximate their fair values because of the short period of time between the origination of such instruments and their expected realization and if applicable, their stated rates of interest are equivalent to interest rates currently available. The fair values are measured pursuant to the three levels defined by the FASB’s accounting standard as follow:
- Level 1: inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
- Level 2: inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
- Level 3: inputs to the valuation methodology are unobservable and significant to the fair value.
Revenue Recognition
We recognize revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable, which are generally considered to be met upon delivery and acceptance of products at the customer site. Sales are presented net of any discounts given to customers. As a policy, we do not accept any product returns and based on our records, product returns, if any, are immaterial. Sales revenue represents the invoiced value of goods, net of a value-added tax, or VAT. All products produced by us and sold in the PRC are subject to a Chinese VAT at a rate of 6% of the gross sales price or at a rate approved by the Chinese local government. Products distributed by Shandong Medical are subjected to a 17% VAT.
Inventories
Due to its unique nature, our principal raw material, human blood plasma is subject to various quality and safety control issues which include, but are not limited to, contaminations and blood born diseases. In addition, limitations of current technology pose biological hazards inherent in plasma that have yet to be discovered, which could result in a widespread epidemic due to blood infusion. In the event that human plasma is discovered to contain pathogens or infectious agents or other bio-hazards, we would be required to write down our inventory to net realizable value. We determine the net realizable value of our inventories on the basis of anticipated sales proceeds less estimated selling expenses. At each balance sheet date, we evaluate inventories that may be worth less than current carrying amounts. Total inventories amounted to $33.2 million as of September 30, 2009. In order to ensure that the growing demand for our products is met, as well as the 90-day quarantine period requirement on plasma raw material implemented by the PRC government, we have been gradually increasing our inventory level of raw materials. We strictly follow the production processes required by government regulations resulting in the relatively high level of work-in-progress customary to our industry.
- 54 -
Impairment of Long-Lived Assets
We review periodically the carrying amounts of long-lived assets including property, plant and equipment, and intangible assets with finite useful lives, to assess whether they are impaired. We evaluate these assets for impairment whenever events or changes in circumstances indicate that their carrying amounts may not be recoverable such as a change of business plan, technical obsolescence, or a period of continuous losses. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. In determining estimates of future cash flows, significant judgment in terms of projection of future cash flows and assumptions is required.
Use of Estimates
The preparation of consolidated financial statements in accordance with US GAAP requires us to make a number of estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. On an ongoing basis, we review our estimates and assumptions, including those related to the recoverability of the carrying amount and the estimated useful lives of long-lived assets, valuation allowances for accounts receivable and realizable values for inventories. Changes in facts and circumstances may result in revised estimates.
Contingencies
In the normal course of business, we are subject to contingencies, including, legal proceedings and claims arising out of the business that relate to a wide range of matters, including among others, product liability. We recognize a liability for such contingency if we determine that it is probable that a loss has occurred and a reasonable estimate of the loss can be made. We may consider many factors in making these assessments, including past history and the specifics of each matter. As we have not become aware of any product liability claim since operations commenced, we have not recognized a liability for any product liability claims.
Recent Accounting Pronouncements
Effective January 1, 2009, the Company adopted FASB’s accounting standard related to business combination which required acquisition method of accounting to be used for all business combinations and for an acquirer to be identified for each business combination. This accounting standard requires an acquirer to recognize the assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions. It also requires the acquirer in a business combination achieved in stages (sometimes referred to as a step acquisition) to recognize the identifiable assets and liabilities, as well as the non-controlling interest in the acquiree, at the full amounts of their fair values (or other amounts determined in accordance with the standard)
Effective January 1, 2009, the Company adopted FASB’s accounting standard regarding non-controlling interest in consolidated financial statements. Certain provisions of this statement are required to be adopted retrospectively for all periods presented. Such provisions include a requirement that the carrying value of non-controlling interests (previously referred to as minority interests) be removed from the mezzanine section of the balance sheet and reclassified as equity. Further, as a result of adoption this accounting standard, net income attributable to non-controlling interests is now excluded from the determination of consolidated net income. In addition, foreign currency translation adjustment is allocated between controlling and non-controlling interests.
- 55 -
In January 2009, the Financial Accounting Standards Board issued an accounting standard which amended the impairment model by removing its exclusive reliance on “market participant” estimates of future cash flows used in determining fair value. Changing the cash flows used to analyze other-than-temporary impairment from the “market participant” view to a holder’s estimate of whether there has been a “probable” adverse change in estimated cash flows allows companies to apply reasonable judgment in assessing whether another-than-temporary impairment has occurred. The adoption of this accounting standard did not have a material impact on the Company’s consolidated financial statements because all of the investments in debt securities are classified as trading securities.
In April 2009, the Financial Accounting Standards Board issued an accounting standard that makes the other-than-temporary impairments guidance more operational and improves the presentation of other-than-temporary impairments in the financial statements. This standard replaced the existing requirement that the entity’s management assert it has both the intent and ability to hold an impaired debt security until recovery with a requirement that management assert it does not have the intent to sell the security, and it is more likely than not it will not have to sell the security before recovery of its cost basis. This standard provides increased disclosure about the credit and noncredit components of impaired debt securities that are not expected to be sold and also requires increased and more frequent disclosures regarding expected cash flows, credit losses, and an aging of securities with unrealized losses. Although this standard does not result in a change in the carrying amount of debt securities, it does require that the portion of an other-than-temporary impairment not related to a credit loss for a held-to-maturity security be recognized in a new category of other comprehensive income and be amortized over the remaining life of the debt security as an increase in the carrying value of the security. The Company adopted this accounting standard, but it did not have a material impact on its consolidated financial statements.
In April 2009, the Financial Accounting Standards Board issued an accounting standard that requires disclosures about fair value of financial instruments not measured on the balance sheet at fair value in interim financial statements as well as in annual financial statements. Prior to this accounting standard, fair values for these assets and liabilities were only disclosed annually. This standard applies to all financial instruments within its scope and requires all entities to disclose the method(s) and significant assumptions used to estimate the fair value of financial instruments. This standard does not require disclosures for earlier periods presented for comparative purposes at initial adoption, but in periods after the initial adoption, this standard requires comparative disclosures only for periods ending after initial adoption. The Company adopted this accounting standard, but it did not have a material impact on the disclosures related to its consolidated financial statements.
In June 2009, the Financial Accounting Standards Board issued an accounting standard amending the accounting and disclosure requirements for transfers of financial assets. This accounting standard requires greater transparency and additional disclosures for transfers of financial assets and the entity’s continuing involvement with them and changes the requirements for derecognizing financial assets. In addition, it eliminates the concept of a qualifying special-purpose entity (“QSPE”). This accounting standard is effective for financial statements issued for fiscal years beginning after November 15, 2009, and the Company does not expect this standard to have a material effect on its consolidated financial statements.
In June 2009, the Financial Accounting Standards Board also issued an accounting standard amending the accounting and disclosure requirements for the consolidation of variable interest entities (“VIEs”). The elimination of the concept of a QSPE, as discussed above, removes the exception from applying the consolidation guidance within this accounting standard. Further, this accounting standard requires a company to perform a qualitative analysis when determining whether or not it must consolidate a VIE. It also requires a company to continuously reassess whether it must consolidate a VIE. Additionally, it requires enhanced disclosures about a company’s involvement with VIEs and any significant change in risk exposure due to that involvement, as well as how its involvement with VIEs impacts the company’s financial statements. Finally, a company will be required to disclose significant judgments and assumptions used to determine whether or not to consolidate a VIE. This accounting standard is effective for financial statements issued for fiscal years beginning after November 15, 2009, and the Company does not expect this standard to have a material effect on its consolidated financial statements.
- 56 -
In June 2009, the Financial Accounting Standards Board issued an accounting standard which establishes the FASB Accounting Standards Codification™ (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. The Codification is effective for interim and annual periods ending after September 15, 2009, and as of the effective date, all existing accounting standard documents will be superseded. The Codification is effective for the Company in the third quarter of 2009, and accordingly, the Company’s Quarterly Report on Form 10-Q for the quarter ending September 30, 2009 and all current and subsequent public filings will reference the Codification as the sole source of authoritative literature.
In August 2009, the Financial Accounting Standards Board issued an Accounting Standards Update (“ASU”) regarding measuring liabilities at fair value. This ASU provides additional guidance clarifying the measurement of liabilities at fair value in circumstances in which a quoted price in an active market for the identical liability is not available; under those circumstances, a reporting entity is required to measure fair value using one or more of valuation techniques, as defined. This ASU is effective for the first reporting period, including interim periods, beginning after the issuance of this ASU. The Company is currently evaluating the impact of this ASU on its consolidated financial statements.
In October 2009, the Financial Accounting Standards Board issued an ASU regarding accounting for own-share lending arrangements in contemplation of convertible debt issuance or other financing. This ASU requires that at the date of issuance of the shares in a share-lending arrangement entered into in contemplation of a convertible debt offering or other financing, the shares issued shall be measured at fair value and be recognized as an issuance cost, with an offset to additional paid-in capital. Further, loaned shares are excluded from basic and diluted earnings per share unless default of the share-lending arrangement occurs, at which time the loaned shares would be included in the basic and diluted earnings-per-share calculation. This ASU is effective for fiscal years beginning on or after December 15, 2009, and interim periods within those fiscal years for arrangements outstanding as of the beginning of those fiscal years. The Company is currently evaluating the impact of this ASU on its consolidated financial statements.
Seasonality of our Sales
Our operating results and operating cash flows historically have not been subject to seasonal variations. This pattern may change, however, as a result of new market opportunities or new product introductions.
Inflation
Inflation does not materially affect our business or the results of our operations.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our investors.
ITEM 3.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not Applicable.
ITEM 4.
CONTROL AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act). Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
- 57 -
As required by Rule 13a-15(e), our management has carried out an evaluation, with the participation and under the supervision of our Chief Executive Officer, Mr. Chao Ming Zhao and our Chief Financial Officer, Mr. Y. Tristan Kuo, of the effectiveness of the design and operation of our disclosure controls and procedures, as of September 30, 2009. Based upon, and as of the date of this evaluation, Messrs. Zhao and Kuo, determined that, because of the material weaknesses described in Item 9A. “Controls and Procedures” on our annual report on Form 10-K for the year ended December 31, 2008, which we are still in the process of remediating, as of September 30, 2009, our disclosure controls and procedures were not effective. Investors are directed to Item 9A of our annual report on Form 10-K for the year ended December 31, 2008 for the description of these weaknesses.
Remediation Measures for Material Weaknesses
Management believes that the material weaknesses identified in our annual report on Form 10-K for the year ended December 31, 2008 were the direct result of our changes in both junior and senior accounting personnel during the 2008 period and our lack of audit committee oversight during a part of that period. We began to remediate the material weaknesses described and plan to continue implementing the measures described below in our ongoing efforts to address these internal control deficiencies as soon as practicable.
We believe that the material weaknesses identified above were the direct result of our recent expansion toward the year end of 2008. We have taken the measures below and plan to continue taking measures to remediate these material weaknesses as soon as practicable:
| (1) | We have hired two additional financial reporting and accounting personnel with relevant accounting experience, skills and knowledge in the preparation of financial statements under the requirements of US GAAP and financial reporting disclosure under the requirement of SEC rules. |
| | |
| (2) | We have developed a rigorous process for collecting and reviewing information required for the preparation of the financial statements including footnotes. The Company has engaged the public accounting firm of Ernst & Young to assist management in developing this process. |
When implemented, management believes that these proposed controls will operate effectively for a sufficient period of time to reduce to a less than reasonably possible likelihood the possibility of a material misstatement and remediate the material weakness reported by our independent registered public accounting firm.
Our management does not believe that these material weaknesses had a material effect on our financial condition or results of operations or caused our financial statements as of and for the year ended December 31, 2008 and for the nine months ended September 30, 2009, to contain a material misstatement.
We do not believe that the costs of remediation for the above material weaknesses will have a material effect on our financial position, cash flows, or results of operations and we expect to implement these corrective measures by the end of fiscal year 2009.
Changes in Internal Controls over Financial Reporting
We regularly review our system of internal control over financial reporting and make changes to our processes and systems to improve controls and increase efficiency, while ensuring that we maintain an effective internal control environment. Changes may include such activities as implementing new, more efficient systems, consolidating activities, and migrating processes.
Except as described above, there were no changes in our internal controls over financial reporting during the third quarter of fiscal 2009 that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
- 58 -
PART II
OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these, or other matters, may arise from time to time that may harm our business.
Except as disclosed Amendment No. 1 to our registration statement on Form S-1 (File No. 333-160774) filed with the Securities and Exchange Commission, on November 9, 2009, we are currently not aware of any such legal proceedings or claims that we believe will have a material adverse affect on our business, financial condition or operating results. Investors are directed to Part II, Item 1 of such report for the description of these proceedings. There has been no material developments to these proceedings as of the date of this report.
ITEM 1A. RISK FACTORS.
Not Applicable.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
We have not sold any equity securities during the quarter ended September 30, 2009 that was not previously disclosed in a current report on Form 8-K filed during that period.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES.
None.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
No matters were submitted to our security holders during the quarter ended September 30, 2009 that were not reported in a current report on Form 8-K filed during that period.
ITEM 5. OTHER INFORMATION.
We have no information to include that was required to be but was not disclosed in a report on Form 8-K during the period covered by this report. There have been no material changes to the procedures by which security holders may recommend nominees to our board of directors.
ITEM 6. EXHIBITS.
The following exhibits are filed as part of this report or incorporated by reference:
- 59 -
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: November 13, 2009 | CHINA BIOLOGIC PRODUCTS, INC. |
| | | |
| | By: | /s/ Chao Ming Zhao |
| | | Chao Ming Zhao, Chief Executive Officer |
| | | (Principal Executive Officer) |
| | | |
| | By: | /s/ Y. Tristan Kuo |
| | | Y. Tristan Kuo, Chief Financial Officer |
| | | (Principal Financial Officer and Accounting |
| | | Officer) |
- 60 -

