UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
☑ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
Commission file number: 1-33818
RESHAPE LIFESCIENCES INC.
(Exact name of registrant as specified in its charter)
| |
Delaware | 48-1293684 |
(State or other jurisdiction of incorporation) | (IRS Employer Identification No.) |
1001 Calle Amanecer, San Clemente, California 92673
(Address of principal executive offices, including zip code)
(949) 429-6680
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of Class | | Name of Exchange on which Registered |
Common stock, $0.01 par value per share | | The NASDAQ Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ◻ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ◻ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ◻
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ◻
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ◻
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| |
Large accelerated filer ◻ | Accelerated filer ◻ |
Non-accelerated filer ◻ (Do not check if a smaller reporting company) | Smaller reporting company ☑ |
| Emerging growth company ◻ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ◻
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ◻ No ☑
At June 30, 2017, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant, based upon the closing price of a share of the registrant’s common stock as reported by the NASDAQ Capital Market on that date was $36,362,152.
As of February 28, 2018, 30,957,113 shares of the registrant’s Common Stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant’s Definitive Proxy Statement, which will be filed with the Commission pursuant to Regulation 14A in connection with the registrant’s 2018 Annual Meeting of Stockholders, to be held June 6, 2018 (the Proxy Statement), are incorporated by reference into Part III of this report. Except with respect to information specifically incorporated by reference in this report, the Proxy Statement is not deemed to be filed as a part hereof.
RESHAPE LIFESCIENCES INC.
FORM 10-K
TABLE OF CONTENTS
Registered Trademarks and Trademark Applications: In the United States we have registered trademarks for vBLOC®, ENTEROMEDICS®, MAESTRO®, RESHAPE®, RESHAPE DUO®, and RESHAPE MEDICAL®, RESHAPE® DUAL BALLOON each registered with the United States Patent and Trademark Office, and trademark applications for vBLOC POWER TO CHOOSE, RESHAPE vBLOC , vBLOC ACHIEVE, RESHAPE VEST, and VBLOC POWER TO CHOOSE AND DESIGN. In addition, some or all of the marks vBLOC, ENTEROMEDICS, MAESTRO, MAESTRO SYSTEM ORCHESTRATING OBESITY SOLUTIONS, vBLOC POWER TO CHOOSE, vBLOC POWER TO CHOOSE AND DESIGN, RESHAPE, RESHAPE DUO, RESHAPE MEDICAL and RESHAPE LIFESCIENCES are the subject of either a trademark registration or application for registration in Australia, Brazil, Canada, China, the European Community, India, Kuwait, Mexico, Saudi Arabia, Switzerland and the United Arab Emirates. We believe that we have common law trademark rights to RESHAPE VEST. This Annual Report on Form 10-K contains other trade names and trademarks and service marks of ReShape Lifesciences and of other companies.
PART I.
ITEM 1. BUSINESS
This Annual Report on Form 10-K contains forward-looking statements. These forward-looking statements are based on our current expectations about our business and industry. In some cases, these statements may be identified by terminology such as “may,” “will,” “should,” “expects,” “could,” “intends,” “might,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue,” or the negative of such terms and other comparable terminology. These statements involve known and unknown risks and uncertainties that may cause our results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Factors that may cause or contribute to such differences include, among others, those discussed in this report in Item 1A “Risk Factors.” Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this report.
Our Company
Our vision is to be recognized as a leading medical technology company focused on the design, development and commercialization of transformative technology to treat obesity and metabolic diseases. Our growing and differentiated product portfolio is utilized by bariatric surgeons, general surgeons, and gastroenterologists. We believe obesity is a global epidemic and that the majority of patients need treatment options that are anatomy friendly and provide for weight loss and comorbidity improvements along with long-term, ongoing obesity support and prevention.
Corporate Background
We were incorporated in Minnesota in December 2002 as two separate legal entities, Alpha Medical, Inc. and Beta Medical, Inc., both of which were owned 100% by a common stockholder. In October 2003, the two entities were combined and we changed our name to EnteroMedics Inc. In 2004 we reincorporated in Delaware. In October 2017, we changed our company name to ReShape Lifesciences Inc.
On January 14, 2015, the vBloc® System, our initial product, which we now refer to as ReShape vBloc, received U.S. Food and Drug Administration (FDA) approval for vBloc Therapy, delivered via the ReShape vBloc, for the treatment of adult patients with obesity who have a Body Mass Index (BMI) of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, and who have tried to lose weight in a supervised weight management program and failed within the past five years. vBloc Therapy is delivered via a pacemaker-like device that helps patients feel full and eat less by intermittently blocking hunger signals on the vagus nerve. Our therapy limits the expansion of the stomach, helps control hunger sensations between meals, reduces the frequency and intensity of stomach contractions and produces a feeling of early and prolonged fullness. We believe the ReShape vBloc offers obese patients a minimally-invasive treatment that can result in significant, durable and sustained weight loss. We believe that our ReShape vBloc allows bariatric surgeons to offer a new option to obese patients who are concerned about the risks and complications associated with currently available anatomy-altering, restrictive or malabsorptive surgical procedures.
In 2015 we began a controlled commercial launch of the ReShape vBloc at select surgical centers in the United States and had our first commercial sales. During 2015, we initiated a controlled expansion of our commercial operations and started the process of building a sales force. In January 2016, we hired new executives to oversee this expansion. Throughout 2016, our sales force called directly on key opinion leaders and bariatric surgeons at commercially-driven surgical centers that met our certification criteria. Additionally, beginning in 2016, through a distribution agreement with Academy Medical, LLC, U.S. Department of Veterans Affairs (VA) medical facilities began to offer the ReShape vBloc as a treatment option for veterans, at little to no cost to veterans in accordance with their veteran healthcare benefits. Our goal for the ReShape vBloc remains broad coverage and reimbursement for vBloc Therapy. We believe that the most significant barrier to adoption for patients who want vBloc Therapy has been cost and lack of payer coverage.
On May 22, 2017, we acquired the Gastric Vest System™, which we now refer to as the ReShape Vest, through our acquisition of BarioSurg. The ReShape Vest System is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients. The device wraps around the stomach, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy. The acquisition was completed under the terms of a merger agreement
pursuant to which BarioSurg became a wholly-owned subsidiary of our company. The aggregate merger consideration we paid for all of the outstanding shares of capital stock and outstanding options of BarioSurg was: (i) 1.38 million shares of our common stock, (ii) 1.0 million shares of our newly created conditional convertible preferred stock, which shares converted into 5.0 million shares of our common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) $2 million in cash.
On October 2, 2017, we acquired ReShape Medical, Inc., a privately-held medical technology company that develops, manufactures and markets the ReShape® Integrated Dual Balloon, which now we refer to as the ReShape Balloon, an FDA-approved, minimally invasive intragastric balloon designed to treat obesity patients with a BMI between 30 and 40, with at least one related comorbidity. The aggregate merger consideration we paid for all of the outstanding shares of capital stock and securities convertible into shares of capital stock of ReShape Medical was: (i) approximately 2.4 million shares of our common stock, (ii) 187,772 shares of newly created series C convertible preferred stock, which shares became convertible into approximately 18.8 million shares of common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) approximately $5 million in cash, which amount, together with ReShape Medical’s cash on-hand, was used to pay ReShape Medical’s outstanding senior secured indebtedness and certain transaction expenses of ReShape Medical.
The ReShape Balloon provides a new option for individuals who have not succeeded at diet and exercise alone, and do not want or do not qualify for bariatric surgery. Two connected balloons are placed into the stomach during a short, outpatient endoscopic procedure. The balloons remain in the stomach for six months and are then removed endoscopically. During balloon treatment, and for six more months following removal of the balloons, the patient receives access to nutritional counseling and access to exclusive tools to help them achieve their weight loss goals. The ReShape Balloon was approved by the FDA in July of 2015 and has had CE-marking in Europe since 2011.
On October 23, 2017 we changed our company name from EnteroMedics Inc. to ReShape Lifesciences Inc. (NASDAQ: RSLS) in recognition of our expansion and growth in developing and commercializing transformative technologies to address the continuum of care for obesity and its associated health conditions. The ReShape brand name is strong and well-established in the marketplace and we expect this to not only help our other products succeed, but we also believe it will accelerate growth in our industry overall. In December, 2017, we rebranded the three products under the ReShape Lifesciences brand. Our portfolio of transformative technologies, designed to help patients lose weight and live a healthier life, includes two FDA-approved devices, ReShape™ vBloc (formerly vBloc) and ReShape™ Balloon, as well as the investigational ReShape™ Vest (formerly Gastric Vest System).
In 2015, our first year of commercial activity, we sold 24 ReShape vBloc units for $292,000 in revenue and in 2016, we sold 62 ReShape vBloc units for $787,000 in revenue. In 2017, our total revenues were $1.3 million, $718,000 from 2017 fourth quarter revenue resulting from the acquisition of ReShape Medical, $250,000 from service revenue and $319,000 from the sale of 28 ReShape vBloc units. We have incurred and expect to continue to incur significant sales, marketing, clinical, and R&D expenses prior to recording sufficient revenue to offset these expenses. Additionally, our selling, general and administrative expenses have continued, as we build the infrastructure necessary to support our expanding commercial sales, operate as a public company and develop our intellectual property portfolio. For these reasons, we expect to continue to incur operating losses for the next several years. We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on cash investments.
Our Market
The Obesity and Metabolic Disease Epidemic
Obesity is a disease that has been increasing at an alarming rate with significant medical repercussions and associated economic costs. Since 1980, the worldwide obesity rate has more than doubled, with about 13% of the world’s adult population now being obese. The World Health Organization (WHO) currently estimates that as many as 600 million people worldwide are obese and more than 1.9 billion adults are overweight. Being overweight or obese is also the fifth leading risk for global deaths, with approximately 3.4 million adults dying each year as a result.
According to the World Health Organization, there are over 70 progressive obesity-related diseases and disorders associated with obesity, which are also known as comorbidities, including Type 2 diabetes, hypertension, infertility and certain cancers. Worldwide, 44% of the diabetes burden, 23% of the heart disease burden and between 7% and 41% of certain cancer burdens are attributable to overweight and obesity.
We believe that this epidemic will continue to grow worldwide given dietary trends in developed nations that favor highly processed sugars, larger meals and fattier foods, as well as increasingly sedentary lifestyles. Despite the growing obesity rate, increasing public interest in the obesity epidemic and significant medical repercussions and economic costs associated with obesity, there continues to be a significant unmet need for effective treatments.
The United States Market
Obesity has been identified by the U.S. Surgeon General as the fastest growing cause of disease and death in the United States, and according to a 2014 McKinsey Report is the leading cause of preventable death in the U.S. Currently, the Center for Disease Control (CDC) estimates that 35.7% of U.S. adults (or approximately 73 million people) are obese, having a BMI of 30 or higher. BMI is calculated by dividing a person’s weight in kilograms by the square of their height in meters. It is estimated that if obesity rates stay consistent, 51% of the U.S. population will be obese by 2030. According to data from the U.S. Department of Health and Human Services, almost 80% of adults with a BMI above 30 have comorbidity, and almost 40% have two or more of these comorbidities. According to The Obesity Society and the CDC, obesity is associated with many significant weight-related comorbidities including Type 2 diabetes, high blood-pressure, sleep apnea, certain cancers, high cholesterol, coronary artery disease, osteoarthritis and stroke. According to the American Cancer Society, 572,000 Americans die of cancer each year, over one-third of which are linked to excess body weight, poor nutrition and/or physical inactivity. Over 75% of hypertension cases are directly linked to obesity, and approximately two-thirds of U.S. adults with Type 2 diabetes are overweight or have obesity.
Currently, medical costs associated with obesity in the U.S. are estimated to be up to $210 billion per year and nearly 21% of medical costs in the U.S. can be attributed to obesity. An estimated approximately $1.5 billion was spent in 2015 alone in the U.S. on approximately 200,000 bariatric surgical procedures to treat obesity. By 2025, it is estimated that up to $3.8 billion will be spent in the U.S. on approximately 800,000 bariatric surgical procedures to treat obesity. Researchers estimate that if obesity trends continue, obesity related medical costs could rise by another $44-$66 billion each year in the U.S. by 2030. The per person medical costs paid by third-party payers for people who are obese were $2,741 per year, or 42% higher than those of people who are normal weight and the average cost to employers is $6,627 to $8,067 per year per obese employee (BMI of 35 to 40 and higher).
Current Treatment Options and Their Limitations
We believe existing bariatric surgery and endoscopic procedural options for the treatment of obesity have seen limited adoption to date, with approximately 1% of the obese population qualifying for treatment actually seeking treatment, due to patient concerns and potential side effects including permanently altered anatomy and morbidity.
The principal treatment alternatives available today for obesity include:
| · | | Behavioral modification. Behavioral modification, which includes diet and exercise, is an important component in the treatment of obesity; however, most obese patients find it difficult to achieve and maintain significant weight loss with a regimen of diet and exercise alone. |
| · | | Pharmaceutical therapy. Pharmaceutical therapies often represent a first option in the treatment of obese patients but carry significant safety risks and may present troublesome side effects and compliance issues. |
| · | | Bariatric Surgery and Endoscopic Procedures. In more severe cases of obesity, patients may pursue more aggressive surgical treatment options, such as gastric banding, sleeve gastrectomy and gastric bypass. These procedures promote weight loss by surgically restricting the stomach’s capacity and outlet size. While largely effective, these procedures generally result in major lifestyle changes, including dietary restrictions and food intolerances, and they may present substantial side effects and carry short- and long-term safety and side effect risks that have limited their adoption. |
Our Competition
The market for obesity treatments is competitive, subject to technological change and significantly affected by new product development. Our primary competition in the obesity treatment market is currently from bariatric surgical procedures and from endoscopic procedures. We believe we are the first company having neuroblocking therapy for the treatment of obesity. There are currently no other FDA-approved neuromodulation or neuroblocking therapies for the
treatment of obesity, but in the future we expect other new stimulation systems and neurotechnology devices to come on the market.
Our ReShape vBloc and our ReShape Balloon compete, and we expect that our ReShape Vest System will compete, with surgical obesity procedures, including gastric bypass, gastric balloons, gastric banding, sleeve gastrectomy and the endoscopic sleeve. These current surgical procedures are performed in less than 1% of all eligible obese patients today. Current manufacturers of gastric balloon and banding products that are approved in the United States include Apollo Endosurgery Inc. (Lap-Band, ORBERA Intragastric Balloon System, and OverStitch Endoscopic Suturing System) and Obalon Therapeutics, Inc. (Obalon Balloon System).
In June 2016, Aspire Bariatrics, Inc. received FDA approval for the Aspire Assist® System, an endoscopic alternative to weight loss surgery for people with moderate to severe obesity. We are also aware that GI Dynamics, Inc. has received approvals in various international countries to sell its EndoBarrier Gastrointestinal Liner.
We also compete against the manufacturers of pharmaceuticals that are directed at treating obesity and the 99% of obese patients eligible for surgery that are not willing to pursue a surgical option. We are aware of a number of drugs that are approved for long-term treatment of obesity in the United States: Orlistat, marketed by Roche as Xenical and GlaxoSmithKline as Alli, Belviq marketed by Arena Pharmaceuticals, Inc., Qsymia, marketed by VIVUS, Inc. and Contrave, marketed by Orexigen Therapeutics, Inc. In addition, we are aware of a pivotal trial for GELESIS100 that is being conducted by Gelesis, Inc.
In addition to competition from surgical obesity procedures, we compete with several private early-stage companies developing neurostimulation devices for application to the gastric region and related nerves for the treatment of obesity. Further, we know of two intragastric balloon companies either in clinical trials or working toward clinical trials in the US: Spatz3 Adjustable Balloon and Allurion Technology’s Elipse Balloon. These companies may prove to be significant competitors, particularly through collaborative arrangements with large and established companies. They also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
In addition, there are many larger potential competitors experimenting in the field of neurostimulation to treat various diseases and disorders. For example, Medtronic plc, which develops deep brain stimulators and spinal cord stimulators, acquired TransNeuronix, which sought to treat obesity by stimulating the smooth muscle of the stomach wall and nearby tissue. St. Jude Medical, Inc., through its acquisition of Advanced Neuromodulation Systems, is developing spinal cord stimulators. LivaNova PLC is developing vagus nerve stimulators to modulate epileptic seizures and other neurological disorders. Boston Scientific Corporation, through its Advanced Bionics division, is developing neurostimulation devices such as spinal cord stimulators and cochlear implants. Ethicon-Endo Surgery acquired LivaNova PLC’s patents and patent applications pertaining to vagus nerve stimulation for the treatment of obesity and two related comorbidities, diabetes and hypertension, in overweight patients.
We believe that the principal competitive factors in our market include:
| · | | acceptance by healthcare professionals, patients and payers; |
| · | | published rates of safety and efficacy; |
| · | | reliability and high quality performance; |
| · | | effectiveness at controlling comorbidities such as diabetes and hypertension; |
| · | | invasiveness and the inherent reversibility of the procedure or device; |
| · | | cost and average selling price of products and relative rates of reimbursement; |
| · | | effective marketing, education, sales and distribution; |
| · | | regulatory and reimbursement expertise; |
| · | | technological leadership and superiority; and |
| · | | speed of product innovation and time to market. |
Many of our competitors are larger than we are and are either publicly-traded or are divisions of publicly-traded companies, and they enjoy several competitive advantages over us, including:
| · | | significantly greater name recognition; |
| · | | established relations with healthcare professionals, customers and third-party payers; |
| · | | established distribution networks; |
| · | | greater experience in research and development, manufacturing, preclinical testing, clinical trials, obtaining regulatory approvals, obtaining reimbursement and marketing approved products; and |
| · | | greater financial and human resources. |
As a result, we cannot assure you that we will be able to compete effectively against these companies or their products.
Market Opportunity
Given the limitations of behavioral modification, pharmaceutical therapy and traditional bariatric surgical approaches, we believe there is a substantial need for patient-friendly, safer, effective and durable solutions that:
| · | | preserves normal anatomy; |
| · | | are “non-punitive” in that they support continued ingestion and digestion of foods and micronutrients such as vitamins and minerals found in a typical, healthy diet while allowing the user to modify his or her eating behavior appropriately without inducing punitive physical restrictions that physically force a limitation of food intake; |
| · | | minimize undesirable side-effects; |
| · | | minimize the risks of re-operations, malnutrition and mortality; and |
| · | | reduce the natural hunger drive of patients. |
Our Strategic Focus
Develop and Commercialize a Differentiated Portfolio of Products/Therapies
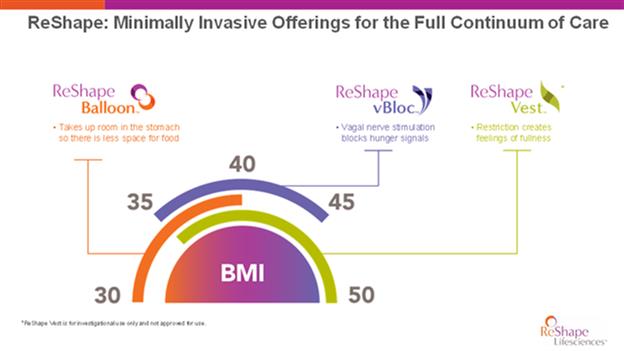
An overarching strategy for our company is to develop and commercialize a product portfolio that is differentiated from our competition by offering transformative technologies to bariatric surgeons and gastroenterologists that consists of a selection of patient friendly, non-anatomy-changing alternatives to traditional bariatric surgery. With ReShape vBloc, the ReShape Balloon, and the ReShape Vest (if approved for commercial use), we believe we will have three compelling and differentiated medical devices, two of which are currently FDA approved. We believe that we are well positioned for the existing market and can serve more of the overweight and obese population with our solutions and thereby help expand the addressable market for obesity.
Obtain Broad Coverage and Reimbursement
We are working to obtain coverage for our products from insurance carriers, local coverage entities and self-insured plans, including Integrated Delivery Networks (IDNs) and Medicare Administrative Contractors (MACs). Initial coverage for ReShape vBloc will likely occur in self-contained healthcare systems that operate as IDNs, as these systems are able to evaluate risk-benefit ratios in a closed environment. For example, in the first quarter of 2016, we announced that the Winthrop Hospital System in New York, a significant IDN in the northeast, would cover our therapy for their employees. Other similar arrangements are in active discussion.
While payers are not our direct customers, their coverage and reimbursement policies influence patient and physician selection of obesity treatment. Our commercialization is coverage-centric, focused on payer and employer engagement, in order to obtain support for ReShape vBloc and our ReShape Balloon. We plan to establish a market price for the ReShape vBloc in the United States that is competitive with other available weight loss surgical procedures and comparable to other active implantable devices such as implantable cardioverter defibrillators, neurostimulation devices for chronic pain and depression, and cochlear implant systems.
CMS issued a national coverage determination for several specific types of bariatric surgery in 2006, which we view as positive potential precedent and guidance factors that CMS might use in deciding to cover our vBloc Therapy. Although Medicare policies are often emulated or adopted by other third-party payers, other governmental and private insurance coverage currently varies by carrier and geographic location.
Drive the Adoption of Our Portfolio through Obesity Therapy Experts and Patient Ambassadors
Our clinical development strategy is to collaborate closely with regulatory bodies, obesity therapy experts and others involved in the obesity management process, patients and their advocates and scientific experts. We have established credible and open relationships with obesity therapy experts and have identified ReShape vBloc and ReShape Balloon patient ambassadors and we believe these individuals will be important in promoting patient awareness and gaining widespread adoption of the ReShape vBloc, the ReShape Balloon, and the ReShape Vest System.
Expand and Protect Our Intellectual Property Position
We believe that our issued patents and our patent applications encompass a broad platform of neuromodulation therapies, including vagal blocking and combination therapy focused on obesity, diabetes, hypertension and other gastrointestinal disorders. We also have broad patent coverage and pending patent applications for our ReShape Balloon and our ReShape Vest products. We intend to continue to pursue further intellectual property protection through U.S. and foreign patent applications.
Leverage our vBloc Technology for Other Disease States
We intend to continue to conduct research and development for other potential applications for our vBloc Therapy and believe we have a broad technology platform that will support the development of additional clinical applications and therapies for other metabolic and gastrointestinal disorders in addition to obesity.
Alternative Weight Loss Solutions
If we are able to commercialize the ReShape Vest, we believe we will be able to offer three distinct approaches that may be selected by the physician, depending on the severity of the patient’s BMI or related co-morbidities. Together, the ReShape Vest, ReShape vBloc and the ReShape Balloon provide a minimally-invasive continuum of care for bariatric patients and their providers.
Concentrate Our Resources on the U.S. Market while Achieving Measured International Expansion
We intend to devote our near-term efforts toward our commercialization in the United States. We intend to explore select international markets to commercialize the ReShape vBloc and the ReShape Balloon as our resources permit, using direct, dealer and distributor sales models as the targeted market best dictates. With the ReShape Vest we intend on collecting data in our clinical trials sufficient to obtain future CE Mark approval and subsequent country approvals.
Our Product Portfolio
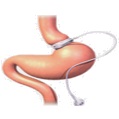
ReShape vBloc
ReShape vBloc, our initial product, uses vBloc Therapy to block the gastrointestinal effects of the vagus nerve using high-frequency, low-energy electrical impulses to intermittently interrupt naturally occurring neural impulses on the vagus nerve between the brain and the digestive system. Our therapy controls hunger sensations between meals, limits the expansion of the stomach and reduces the frequency and intensity of stomach contractions, leading to earlier fullness. The resulting physiologic effects of vBloc Therapy produce a feeling of early and prolonged fullness following smaller meal portions. By intermittently blocking the vagus nerve and allowing it to return to full function between therapeutic episodes, our therapy limits the body’s natural tendency to circumvent the therapy, which can result in long-term weight loss.
Benefits. We have designed ReShape vBloc to address a significant market opportunity that we believe exists for a patient-friendly, safe, effective, less-invasive and durable therapy that is intended to address the underlying causes of hunger and obesity. Our ReShape vBloc offers each of the following benefits, which we believe could lead to the adoption of vBloc Therapy as the surgical therapy of choice for obesity and its comorbidities:
| · | | Preserves Normal Anatomy. The ReShape vBloc is designed to deliver therapy that blocks the neural signals that influence a patient’s hunger and sense of fullness without altering digestive system anatomy. |
Accordingly, patients should experience fewer and less severe side effects compared to treatments that incorporate anatomical alterations. |
| · | | Allows Continued Ingestion and Digestion of Foods Found in a Typical, Healthy Diet. Because our therapy leaves the digestive anatomy unaltered, patients are able to maintain a more consistent nutritional balance compared to conventional surgical approaches, thus allowing them to effect positive changes in their eating behavior in a non-forced and potentially more consistent way. |
| · | | May be Implanted on an Outpatient Basis and Adjusted Non-Invasively. The ReShape vBloc is designed to be laparoscopically implanted within a 60-90 minute procedure, allowing patients to leave the hospital or clinic on the same day. The implantable system is designed to be turned off and left in place for patients who reach their target weight. When desired, the follow-up physician can simply and non-invasively turn the therapy back on. Alternatively, the implantable system can be removed in a laparoscopic procedure. |
| · | | Offers Favorable Safety Profile. We have designed our clinical trials to demonstrate the safety of the ReShape vBloc. In our clinical trials to date, including the ReCharge trial, we have not observed any mortality related to our device or any unanticipated adverse device effects. We have also not observed any long-term problematic clinical side effects in any patients, including in those patients who have been using vBloc Therapy for more than one year. |
| · | | Targets Multiple Factors that Contribute to Hunger and Obesity. We designed vBloc Therapy to target the digestive, metabolic and information transmission functions of the vagus nerve and to affect the perception of hunger and fullness, which together contribute to obesity and its metabolic consequences. |
ReShape vBloc, Implantation Procedure and Usage.
ReShape vBloc. Our ReShape vBloc delivers vBloc Therapy via two small electrodes that are laparoscopically implanted and placed in contact with the trunks of the vagus nerve just above the junction between the esophagus and the stomach, near the diaphragm.
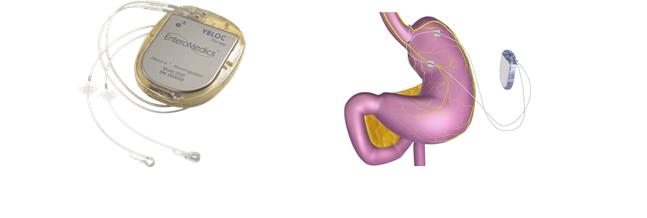
The major components of ReShape vBloc include:
| · | | Neuroregulator. The neuroregulator, a pacemaker-like device, is an implanted device that controls the delivery of vBloc Therapy to the vagus nerve. It is surgically implanted just below, and parallel to, the skin, typically on the side of the body over the ribs. |
| · | | Lead System. Proprietary leads are powered by the neuroregulator and deliver electrical pulses to the vagus nerve via the electrodes. The leads and electrodes are similar to those used in traditional cardiac rhythm management products. |
| · | | Mobile Charger. The mobile charger is an electronic device worn by the patient externally while recharging the device. It connects to the transmit coil and provides information on the battery status of the neuroregulator and the mobile charger. |
| · | | Transmit Coil. The transmit coil is positioned for short periods of time on top of the skin over the implanted neuroregulator to deliver radiofrequency battery charging and therapy programming information across the skin into the device. |
| · | | Clinician Programmer. The clinician programmer connects to the mobile charger to enable clinicians to customize therapy settings as necessary and retrieve reports stored in system components. The reports include patient use and system performance information used to manage therapy. The clinician programmer incorporates our proprietary software and is operated with a commercially available laptop computer. |
Implantation Procedure. ReShape vBloc is implanted by a laparoscopically trained surgeon using a procedure that is typically performed within 60-90 minutes. During the procedure, the surgeon laparoscopically implants the electrodes in contact with the vagal nerve trunks and then connects the lead wires to the neuroregulator, which is subcutaneously implanted. The implantation procedure and usage of the ReShape vBloc carry some risks, such as the risks generally associated with laparoscopic procedures as well as the possibility of device malfunction. Adverse events related to the therapy, device or procedure may include, but are not limited to: transient pain at the implant site, heartburn, constipation, nausea, depression, diarrhea, infection, organ or nerve damage, surgical explant or revision, device movement, device malfunction and allergic reaction to the implant.
Usage of ReShape vBloc. The physician activates ReShape vBloc after implantation. vBloc Therapy is then delivered intermittently through the neuroregulator each day as scheduled (recommended during the patient’s waking hours when food is consumed) through the neuroregulator. The scheduled delivery of the intermittent pulses blocking the vagus nerve is customized for each patient’s weight loss and overall treatment objectives.
The physician is able to download reports to monitor patient use and system performance information. This information is particularly useful to physicians to ensure that patients are properly using the system. Although usage of our ReShape vBloc generally proceeds without complications, as part of the therapy or intentional weight loss, patients in our clinical trials have observed side-effects such as transient pain at the implant site, heartburn, bloating, dysphagia, eructation, cramps, diarrhea, nausea, constipation, and excessive feelings of fullness, especially after meals. In addition, patient noncompliance with properly charging ReShape vBloc may render vBloc Therapy less effective in achieving long-term loss.
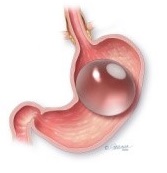
The ReShape Balloon
The ReShape Balloon technology, which we acquired in October 2017 in connection with our acquisition of ReShape Medical, is a non-surgical, removable, dual weight loss balloon technology that is approved for people with a body mass index between 30 and 40 with one or more related comorbid conditions who have failed previous attempts to lose weight through diet and exercise. Our ReShape Balloon adds a lower-cost option to our portfolio of products, allowing access to additional patients within the obesity market. This expansion further reinforces our strategy and commitment to the entire continuum of care in obesity.


Benefits: The ReShape Balloon is a non-invasive weight loss solution ideal for patients who have failed at diet and exercise, and who are not indicated for or are afraid of surgery. The ReShape Balloon offers the following benefits:
| · | | Satiety: The ReShape Balloon has more potential fill volume to aid in patients’ weight loss than any other product on the market. The larger fill volume takes up more room in the stomach, so that patients eat less and feel full longer. |
| · | | Patient Comfort: Unlike other balloons, we believe that our device differentiates itself with two interconnected balloons designed to better fit the natural contour of the stomach, thereby increasing the level of patient comfort. |
| · | | Designed for Safety: The ReShape Balloon is the only intragastric balloon designed to mitigate the potential risk of migration. The dual balloon design allows for one balloon to remain inflated and in the stomach, in the unlikely event the other balloon deflates. Other single balloons can deflate and risk migrating. The ReShape Balloon is inserted through the mouth - endoscopically - during a 20-minute outpatient procedure - with no incisions or scars. After six months, the balloon is removed endoscopically, in a procedure similar to the insertion procedure |
| · | | Customized Aftercare: For the six months the balloon is in and for six months after the balloon removal, patients obtain monthly customized coaching focused on changing behaviors and relationships with food. |
The ReShape Balloon was approved by the FDA in July 2015, and to date, more than 4,000 patients have been treated with this technology. The ReShape Balloon also has received CE Mark approval, but due to limited capital resources, ReShape Medical had not focused on penetrating European markets. The ReShape Balloon was made available to three areas in the Middle East in 2017: Kuwait, Qatar and UAE. Further expansion opportunities will be evaluated based on market opportunity and resources to manage expansion.
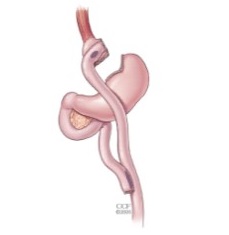
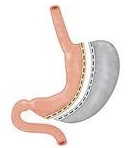
The ReShape Vest
The ReShape Vest, which we acquired in May 2017 in connection with our acquisition of BarioSurg, is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients with a BMI of at least 35. This device is designed to restrict the intake of food and provide the feeling of fullness without cutting or permanently removing portions of the stomach, or bypassing, any portion of the gastrointestinal tract. The implantation of the device mimics a traditional weight-loss surgery without permanently altering the anatomy and may not require vitamin supplementation.
In a small pilot study conducted outside the U.S., at 12 months ReShape Vest patients demonstrated a mean percent excess weight loss (%EWL) of 85% and a mean percent total body weight loss (%TBWL) of 30.2%, an average drop in HbA1c (Hemoglobin A1c) of 2.1 points, an average decrease of systolic blood pressure of 13mmHg, an average waist circumference reduction of 38 centimeters, or approximately 15 inches, and an average increase in HDL “good cholesterol” of 29 mg/dl.
Benefits. The ReShape Vest, if approved for sale, would allow us to offer an additional weight loss solution that emulates the effect of conventional weight loss surgery through a procedure that is minimally invasive. The ReShape Vest System potentially offers the following benefits:
| · | | Minimizes Changes to Normal Anatomy. The ReShape Vest System emulates the effects of conventional weight-loss surgery without stapling, cutting or removing any portion of the stomach. |
| · | | Minimally Invasive Procedure. Unlike conventional weight loss surgery, which typically is performed in a hospital setting under general anesthesia and requires a hospital stay of up to four days, the ReShape Vest System is inserted laparoscopically in an outpatient procedure. |
| · | | Removable/Reversible. The ReShape Vest System is designed to be removed laparoscopically, permitting the removal of the device at a later time, if that is desired. |
| · | | Allows Continued Ingestion and Digestion of Foods Found in a Typical, Healthy Diet. Because the ReShape Vest System also leaves the digestive anatomy largely unaltered, patients are able to maintain a more consistent nutritional balance compared to conventional surgical approaches, thus allowing them to effect positive changes in their eating behavior in a non-forced and potentially more consistent way. |
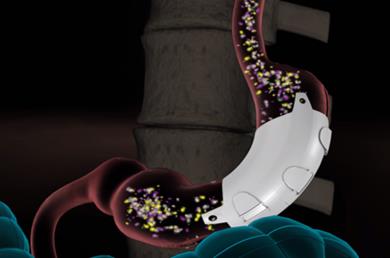
Implantation Procedure. The ReShape Vest is a thin, implantable-grade silicone device that wraps around the stomach, as shown below. The device wraps around the stomach after it has been rearranged into a banana-like shape using sutures, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy. By decreasing the cross-sectional area of the stomach, food travels faster through the stomach, resulting in faster gastric emptying. The smaller amount of food in the stomach coupled with restriction is intended to stimulate the stretch receptors along the stomach, which send signals to the brain that the patient should stop eating.
Clinical Data – vBloc Therapy
We have conducted a series of clinical trials to date, which have shown that vBloc Therapy offers physicians a programmable method to selectively and reversibly block the vagus nerve resulting in clinically and statistically significant EWL.
We have not observed any mortality related to our device or any unanticipated adverse device effects in any of our completed or ongoing studies. Reported events include those associated with laparoscopic surgery or any implantable electronic device. The effects of vBloc Therapy include changes in appetite, and, in some patients, effects that may be expected with decreased intra-abdominal vagus nerve activity, such as temporary abdominal discomfort and short episodes of belching, bloating, cramping or nausea.
Findings from our clinical trials have resulted in publication in numerous peer-reviewed journals, including The Journal of the American Medical Association, Journal of Obesity, Obesity Surgery, Surgery for Obesity and Related Diseases, Journal of Diabetes and Obesity, Surgery and Journal of Neural Engineering, and data have been presented at several scientific sessions including the American Society for Metabolic and Bariatric Surgery, International Federation for Surgery of Obesity and Metabolic Disorders, the Obesity Surgery Society of Australia & New Zealand and The Obesity Society.
We obtained European CE Mark approval for our ReShape vBloc in 2011 for the treatment of obesity. The CE Mark approval for ReShape vBloc was expanded in 2014 to also include use for the management of Type 2 diabetes in obese patients. Additionally, the final ReShape vBloc components were previously listed on the Australian Register of Therapeutic Goods by the Therapeutic Goods Administration. We believe that, the costs and resources required to successfully commercialize ReShape vBloc internationally are currently beyond our capability and, as result, we made the decision in late 2017 to temporarily abandon CE-marking of ReShape vBloc. Accordingly, we will continue to devote our near-term efforts toward mounting a successful system launch in the United States. We intend to explore select international markets to commercialize ReShape vBloc as our resources permit, using direct, dealer and distributor sales models as the targeted market best dictates.
To date, we have not observed any mortality related to ReShape vBloc or any unanticipated adverse device effects in our human clinical trials. We have also not observed any long-term problematic clinical side effects in any patients. In addition, data from our VBLOC-DM2 ENABLE trial outside the United States demonstrate that vBloc Therapy may hold promise in improving obesity-related comorbidities such as diabetes and hypertension. We are conducting, or plan to conduct, further studies in each of these comorbidities to assess vBloc Therapy’s potential in addressing multiple indications.
Below is a more detailed description of our past and ongoing vBloc clinical studies:
ReCharge Trial
In October 2010, we received an unconditional Investigational Device Exemption (IDE) approval from the FDA to conduct a randomized, double-blind, sham-controlled, multicenter pivotal clinical trial, called the ReCharge trial, testing the effectiveness and safety of vBloc Therapy utilizing our second generation ReShape vBloc. Enrollment and implantation in the ReCharge trial was completed in December 2011 in 239 randomized patients (233 implanted) at 10 centers. All patients in the trial received an implanted device and were randomized in a 2:1 allocation to treatment or control groups. The control group received a non-functional device during the trial period. All patients were expected to participate in a standard weight management counseling program. The primary endpoints of efficacy and safety were evaluated at 12 months. The ReCharge trial met its primary safety endpoint with a 3.7% serious adverse event rate, significantly lower than the threshold of 15% (p<0.0001). The safety profile at 12 months was further supported by positive cardiovascular signals including a 5.5 mmHg drop in systolic blood pressure, a 2.8 mmHg drop in diastolic blood pressure and a 3.6 bpm drop in average heart rate.
Although the trial did not meet its predefined co-primary efficacy endpoints, it did demonstrate in the ITT population (n=239) a clinically meaningful and statistically significant EWL of 24.4% (approximately 10% TBL) for vBloc Therapy-treated patients, with 52.5% of patients achieving at least 20% EWL. In the per protocol population, the trial demonstrated an EWL of 26.3% for vBloc Therapy-treated patients, with 56.8% of patients achieving at least 20% EWL. As a result of the positive safety and efficacy profile of vBloc Therapy, we used the data from the ReCharge trial to support a PMA application for the ReShape vBloc, which was submitted to the FDA in June 2013 and was accepted for review and filing in July 2013. An Advisory Panel meeting was held on June 17, 2014 to review our PMA application for approval of the ReShape vBloc. The Advisory Panel voted 8 to 1 “in favor” that the ReShape vBloc is safe when used as designed and voted 4 to 5 “against” on the issue of a reasonable assurance of efficacy. The final vote, on whether the relative benefits outweighed the relative risk, was 6 to 2 “in favor,” with 1 abstention. We received FDA approval on January 14, 2015 for vBloc Therapy, delivered via the ReShape vBloc, for the treatment of adult patients with obesity who have a BMI of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, and who have tried to lose weight in a supervised weight management program and failed within the past five years.
Further analysis of the 12 month data show that in the primary analysis (ITT) population (n=239), vBloc Therapy-treated patients achieved a 24.4% average EWL (approximately 10% TBL) compared to 15.9% for sham control patients. This 8.5% difference demonstrated statistical superiority over sham control (p=0.002), but not super-superiority at the pre-specified 10% margin (p=0.705). In total, 52.5% of vBloc Therapy-treated patients had 20% or more EWL compared to 32.5% in the control group (p=0.004), and 38.3% of vBloc Therapy-treated patients had 25% or more EWL compared to 23.4% in the sham control group (p=0.02). While the respective co-primary endpoint targets of 55% and 45% were not met, the endpoint targets were within the 95% confidence intervals for the observed rates and therefore the observed rates were not significantly lower than these pre-specified rates. These efficacy data demonstrate vBloc Therapy’s positive effect on weight loss.
In the per protocol group, which included only those patients who received therapy per the trial design (n=211), the vBloc Therapy-treated patients had a 26.3% average EWL (approximately 10% TBL) compared to 17.3% for the sham control group (p=0.003). In total, 56.8% of vBloc Therapy-treated patients achieved at least 20% EWL, which was above the predefined threshold of 55% compared to 35.4% in the sham control group (p=0.004). 41.8% of vBloc Therapy-treated patients also achieved at least 25% EWL in this population, which is slightly less than the predefined threshold of 45%, compared to 26.2% in the sham control group (p=0.03).
Additionally, two-thirds of vBloc Therapy-treated patients achieved at least 5% TBL at 12 months. According to the CDC, 5% TBL can have significant health benefits on obesity related risk factors, or comorbidities, including
reduction in blood pressure, improvements in Type 2 diabetes and reductions in triglycerides and cholesterol. Further analysis of our data at 12 months showed a meaningful impact on these comorbidities as noted in the below table showing the improvements seen at 10% TBL, the average weight loss in vBloc Therapy-treated patients.
| | |
Risk Factor | | 10% TBL |
Systolic BP (mmHg) | | (9) |
Diastolic BP (mmHg) | | (6) |
Heart Rate (bpm) | | (6) |
Total Cholesterol (mg/dL) | | (15) |
LDL (mg/dL) | | (9) |
Triglycerides (mg/dL) | | (41) |
HDL (mg/dL) | | 3 |
Waist Circumference (inches) | | (7) |
HbA1c (%) | | (0.5) |
Approximately 93% of patients reached the 12 month assessment in the trial, consistent with a rigorously executed trial. vBloc Therapy-treated patients maintained their weight loss at 18 months and 24 months with an EWL of 23.5% and 21.1%, respectively. The trial’s positive safety profile also continued throughout this reported time period.
VBLOC-DM2 ENABLE Trial
Enrollment of the VBLOC-DM2 ENABLE trial began in 2008. The VBLOC-DM2 ENABLE trial is designed to evaluate the efficacy and safety of vBloc Therapy on obese subjects as well as its effect on glucose regulation in approximately 30 patients who are using the ReShape vBloc. The trial is an international, open-label, prospective, multi-center study. At each designated trial endpoint the efficacy of vBloc Therapy is evaluated by measuring average percentage EWL, HbA1c (blood sugar), FPG (fasting plasma glucose), blood pressure, calorie intake, appetite and other endpoints at one week, one month, three, six, 12 and 18 months and longer. The following results were reported at 12 month intervals.
| · | | Percent EWL (from implant, Company updated interim data): |
| | | | |
Visit (post-device activation) | | % EWL | | N |
12 Months | | (24.5) | | 26 |
24 Months | | (22.7) | | 22 |
36 Months | | (24.3) | | 18 |
| · | | HbA1c change in percentage points (Baseline HbA1c = 7.8 + 0.2%) (Company updated interim data): |
| | | | |
| | % HbA1c | | |
Visit (post-device activation) | | change | | N |
12 Months | | (1) | | 26 |
24 Months | | (0.5) | | 24 |
36 Months | | (0.6) | | 17 |
| · | | Fasting Plasma Glucose change (Baseline 151.4 + 6.5 mg/dl average) (Company updated interim data): |
| | | | |
| | Glucose | | |
| | change | | |
Visit (post-device activation) | | (mg/dl) | | N |
12 Months | | (27.6) | | 25 |
24 Months | | (20.3) | | 24 |
36 Months | | (24) | | 17 |
| · | | Change in mean arterial pressure (MAP) in hypertensive patients (baseline 99.5 mmHg) (Company updated interim data): |
| | | | |
| | MAP | | |
| | change | | |
Visit (post-device activation) | | (mmHg) | | N |
12 Months | | (7.8) | | 14 |
24 Months | | (7.5) | | 12 |
36 Months | | (7.3) | | 10 |
To date, no deaths related to our device or unanticipated adverse device effects have been reported during the VBLOC-DM2 ENABLE trial and the safety profile is similar to that seen in the other vBloc trials.
Caloric Intake Sub-study: A sub-study, conducted as part of the VBLOC-DM2 ENABLE trial, evaluated 12-month satiety and calorie intake in 10 patients with Type 2 diabetes mellitus enrolled in the trial. Follow-up measures among patients enrolled in the sub-study included EWL, 7-day diet records assessed by a nutritionist, calorie calculations and visual analogue scale (VAS) questions to assess satiety by 7-day or 24-hour recall at the following time periods: baseline, 4 and 12 weeks and 6 and 12 months post device initiation. A validated program, Food Works™, was used to determine calorie and nutrition content. Results include:
| · | | Mean EWL for the sub-study was 33+5% (p<0.001) at 12 months; |
| · | | Calorie intake decreased by 45% (p<0.001), 48% (p<0.001), 38% (p<0.001) and 30% (p=0.02), at 4 and 12 weeks, 6 months and 12 months, respectively, from a baseline of 2,062 kcal/day; and |
| · | | VAS recall data, using a repeated measures analysis, documented fullness at the beginning of meals (p=0.005), less food consumption (p=0.02) and less hunger at the beginning of meal (p=0.03) corroborating the reduction in caloric intake. |
ReNew Trial
The ReNew Trial is a Post Approval Study required by the FDA as a condition of approval. ReNew is a five-year, multi-center trial to evaluate the long-term safety and efficacy of the Maestro Rechargeable System in treating obesity in 200 patients at 10 to 15 sites. The ReNew trial contains both randomized and observational cohorts. The first implantation of the ReNew trial was in August 2017 and we expect enrollment for the ReNew trial to continue throughout 2018, 2019 and 2020.
Kaiser Diabetes Trial
On April 26, 2017, we entered into a Clinical Trial Agreement with Southern California Permanente Medical Group (“Southern”), a division of Kaiser Permanente, with an effective date of June 1, 2017. Under the agreement, we are sponsoring an investigator-initiated three-year, 60 patient study with Southern to study vBloc Therapy as a treatment for Type 2 diabetic patients with obesity. As sponsor of the study, we are obligated to pay Southern approximately $3.4 million over three years to fund the study. This study is expected to have patient enrollment to continue through 2018.
All clinical data generated during the study will be disclosed to us and may be used for any purpose stated in the informed consent form or otherwise in compliance with applicable law. We will have the right to publish, present or use any final results arising out of the study. We believe that results of the study will aid in our efforts to obtain insurance reimbursement from payers.
vBloc Now Registry
In June 2017, we launched our vBloc Now program. The vBloc Now program provides qualified patients battling obesity the opportunity to receive vBloc Therapy, including the device, procedure, and vBloc Achieve follow up program, at an affordable price in exchange for sharing detailed health data with us. The program is available for a limited time, will reduce patient total out-of-pocket costs, and compete with leading covered bariatric surgery procedures as well as other low-cost weight loss devices
In addition, the vBloc Now program provides us with additional commercial data concerning vBloc Therapy in order to enhance our case with third-party payers that the ReShape vBloc can have a clinically meaningful level of effectiveness in reducing the incidence of diabetes and other comorbidities in certain patients. We will collect real-world outcome data in 125 patients from select vBloc institutes in the vBloc Now program. While we do not expect to recognize any revenues in conjunction with the vBloc Now program, we anticipate that vBloc Now program expenses will be offset by a reduction in marketing and advertising expenses and will not increase the Company’s overall operating expenses.
Clinical Data – ReShape Balloon
REDUCE Trial
The REDUCE study, which was ReShape Medical’s U.S. prospective randomized pivotal trial, demonstrated that patients who underwent the ReShape Non-Surgical Weight Loss Procedure lost 2.3 times more excess weight at six months compared to control patients treated with diet and exercise alone, and 55% of patients treated with the ReShape Balloons lost at least 25% of their excess weight. Additionally, there were significant and sustained improvements in co-morbidities and strong patient satisfaction, along with maintenance of two-thirds of the weight loss, through twelve months of study follow up.
In 2009, ReShape Medical received an unconditional IDE approval from the FDA to conduct the REDUCE trial. The REDUCE trial was ReShape Medical’s U.S. prospective, sham-controlled, double blinded, randomized, multicenter pivotal trial, testing the effectiveness and safety of the DUO® Integrated Dual Balloon System. Enrollment and insertion in the REDUCE Trial was completed in February 2014. There were 326 patients enrolled (187 treatment; 139 control) and, of those, 265 insertions were completed (187 treatment and 78 control). The control group received 24 weeks of diet and exercise counselling. After 24 weeks of counselling control subjects either exited the trial or, if willing and eligible, were treated with the DUO device. Patients who underwent the ReShape Non-Surgical Weight Loss Procedure using the DUO® Integrated Dual Balloon System lost 2.3 times more excess weight at six months compared to control patients treated with diet and exercise alone. Fifty-five percent (55%) of patients treated with the ReShape Balloons lost at least 25% of their excess weight. Additionally, there were significant and sustained improvements in co-morbidities, quality of life and strong patient satisfaction, along with maintenance of two-thirds of the weight loss, through 12 months of study follow up.
The following co-morbidity results were reported:
| | | | |
Mean Systolic Blood Pressure Levels in Study Subjects During Study Follow-Up |
| | | | |
Visit Interval | | Systolic Blood Pressure
Mean (SD) | | N |
Baseline | | 130.4 (13.9) | | 187 |
Week 12 | | -8.2 (14.2) | | 173 |
Week 24 | | -8.3 (15.6) | | 169 |
Week 36 | | -9.3 (16.2) | | 123 |
Week 48 | | -6.6 (15.8) | | 136 |
| | | | |
| | | | |
Mean Systolic Blood Pressure Levels During Study Follow-Up in Subjects with Established Hypertension at Baseline |
| | | | |
Visit Interval | | Systolic Blood Pressure
Mean (SD) | | N |
Baseline | | 136.5 (15.8) | | 54 |
Week 12 | | -11.9 (13.9) | | 48 |
Week 24 | | -12.9 (19.0) | | 48 |
Week 36 | | -14.5 (16.7) | | 34 |
Week 48 | | -11.1 (18.6) | | 39 |
| | | | |
Mean Fasting Insulin Levels in Study Subjects During Study Follow-Up |
| | | | |
Visit Interval | | Fasting Insulin
Mean (SD) | | N |
Baseline | | 17.84 (19.88) | | 185 |
Week 12 | | -4.76 (20.84) | | 170 |
Week 24 | | -3.80 (22.20) | | 167 |
Week 36 | | -0.70 (22.46) | | 118 |
Week 48 | | -1.05 (21.47) | | 130 |
| | | | |
Mean Hemoglobin A1c Levels in Study Subjects During Study Follow-Up |
| | | | |
Visit Interval | | Fasting Insulin
Mean (SD) | | N |
Baseline | | 5.66 (0.69) | | 187 |
Week 12 | | -0.13 (0.35) | | 171 |
Week 24 | | -0.22 (0.35) | | 168 |
Week 36 | | -0.26 (0.42) | | 120 |
Week 48 | | -0.19 (0.34) | | 133 |
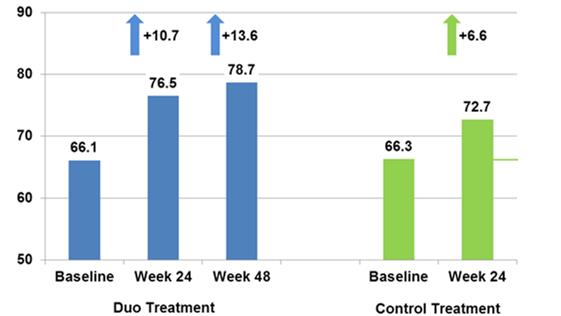
The total Impact of Weight on Quality of Life-Lite (IWQoL) scores improved for both groups, with a greater improvement seen at Weeks 24 and 48 for treatment subjects compared with control subjects. These changes are both statistically and clinically significant, including the larger response in treatment subjects compared with control subjects.
These clinically significant findings are depicted in the following chart:
IWQoL-Lite Scores at Baseline and Follow-Up
The overall safety profile of the ReShape Duo Integrated Dual Balloon System in the REDUCE Pivotal Trial was favorable. There were no unanticipated adverse device effects, no deaths, no intestinal obstructions and no gastric perforations. Procedural risk was consistent with low risk endoscopic interventions. Accommodative symptoms occurred, generally diminished or resolved within the first week of treatment. There was a low rate of device- or procedure-related SAEs (7.5%). The gastric ulceration rate was substantially reduced by a minor design modification to the device.
REDUCE PAS
The REDUCE PAS is a Post Approval Study Required by the FDA as a condition of approval. REDUCE PAS is a 48-month open-label, single arm study to demonstrate safety and efficacy of the ReShape Dual Balloon System in 250 patients in 15 trial sites. The first insertion was September 2016 and enrollment is expected to continue through 2018, completing early 2019.
Clinical Data – ReShape Vest
ReShape Vest ENDURE Trial
The ReShape Vest was studied internationally in the ENDURE trial, which was a non-randomized, single center pilot designed to evaluate the safety and efficacy of the ReShape Vest. Of the 17 patients enrolled, 14 have completed their 12-month follow-up visit. Results from these 14 patients show that the ReShape Vest demonstrated a mean excess weight loss (%EWL) of 85.5% compared to approximately 75% and 65% for gastric bypass and sleeve gastrectomy. The patients also experienced an average HgA1c decrease of 2.1%, and an average waist circumference reduction of 38 cm, or 15 inches. The ReShape Vest will continue to be studied in upcoming trials in the US and internationally.
Our Research and Development
Current R&D Focus
We have an experienced research and development team, including clinical, regulatory affairs and quality assurance, comprised of scientists, electrical engineers, software engineers and mechanical engineers with significant clinical knowledge and expertise. Our research and development efforts are focused in the following major areas:
| · | | supporting the current ReShape vBloc and ReShape Balloon; |
| · | | testing and developing the ReShape Vest; |
| · | | developing the next-generation ReShape vBloc and ReShape Balloon; |
| · | | identifying the effect of vagal blocking on nerve and organ function; and |
| · | | investigating the ReShape vBloc and ReShape Vest platforms for the treatment of gastrointestinal disorders and comorbidities in addition to obesity. |
We have spent a significant portion of our capital resources on research and development. Our research and development expenses were $5.8 million in 2017, $5.1 million in 2016, $8.1 million in 2015 and $11.0 million in 2014. Having obtained FDA approval in January 2015, our main focus has been on commercialization efforts, resulting in decreases in spending on research and development in each of 2015 and 2016 compared to 2014, when we were still working through the FDA approval process.
Other Diseases and Disorders
We believe that our vBloc Therapy and ReShape Vest may have the potential, if validated through appropriate clinical studies, to treat a number of additional gastrointestinal disorders or comorbidities frequently associated with obesity, including the following:
| · | | Type 2 Diabetes. Type 2 diabetes is an escalating global health epidemic often related to obesity that affects nearly 200 million people worldwide, 50 million in the United States alone. Those with diabetes are susceptible to cardiovascular morbidity and mortality, and up to two out of three people with diabetes have high blood pressure. We believe that vBloc Therapy has significant potential in treating metabolic syndrome (diabetes with high blood pressure). We have launched an international feasibility trial, VBLOC-DM2 ENABLE, to further explore the efficacy of vBloc Therapy in this patient population and have reported preliminary findings in the “Our Clinical Experience” section above. vBloc Therapy for patients with Type 2 diabetes will continue to be studied primarily in our Kaiser Diabetes Trial. |
| · | | Hypertension. Blood pressure normally rises and falls throughout the day. When it consistently stays too high for too long, it is called hypertension. Globally, nearly one billion people have high blood pressure (hypertension); of these, two-thirds are in developing countries. About one in three American adults has high blood pressure or hypertension. Hypertension is one of the most important causes of premature death worldwide and the problem is growing; in 2025, an estimated 1.56 billion adults will be living with hypertension. Hypertension kills nearly 8 million people every year worldwide. We believe that vBloc Therapy may improve mean systolic and diastolic blood pressure in hypertensive patients. We completed a subgroup analysis of patients from an earlier clinical trial and have included an evaluation of the blood pressure effects of vBloc Therapy in our international feasibility trial, VBLOC-DM2 ENABLE, to further explore the efficacy of vBloc Therapy in this patient population and have reported preliminary findings in the “Our Clinical Experience” section above. |
| · | | Pancreatitis. Primary and recurrent cases of acute pancreatitis are estimated to number from 150,000 to 200,000 annually, resulting in approximately 80,000 hospital admissions each year in the United States. In animal studies, we have shown that vBloc Therapy suppresses pancreatic exocrine secretion, suggesting its potential efficacy in treating pancreatitis. |
| · | | Other Gastrointestinal Disorders. We believe that vBloc Therapy may have potential in a number of other gastrointestinal disorders, including irritable bowel syndrome and inflammatory bowel disease. |
None of the above conditions were included in our ReShape vBloc PMA application that was approved by the FDA on January 14, 2015, nor are they approved for sale internationally. Additional approvals will be required to market the ReShape vBloc or ReShape Vest for these indications in the United States or internationally.
Our Intellectual Property
Our success will depend in part on our ability to obtain and defend patent protection for our products and processes, to preserve our trade secrets and to operate without infringing or violating the proprietary rights of third parties. We own numerous U.S. and foreign patents, and have numerous patent applications pending, most of which pertain to treating gastrointestinal disorders and we believe provide us with broad intellectual property protection covering electrically-induced vagal blocking and methods for treating obesity. Assuming timely payment of maintenance fees as they become due, many of these patents will expire in 2023. Our acquisition of the ReShape Vest included four U.S. patents, one pending U.S. patent application, four foreign patents, and five pending foreign patent applications. The patents we acquired related to the ReShape Vest will expire between 2028 and 2034. We have also received or applied for patents in Europe, Australia, China, India and Japan. These applications primarily pertain to our vagal blocking technology and its application to obesity as well as other gastrointestinal disorders. The applications that we acquired related to the ReShape Vest primarily pertain to methods of gastric restriction for treating obesity. Our acquisition of the ReShape Balloon included broad coverage for multi-balloon gastric implants and methods for its placement and retrieval. Patent coverage also includes methods of manufacturing and additional therapy applications. There are 35 patents granted in the US, Europe, Canada, and Japan with additional U.S. and international patent applications pending. The key patents we acquired in connection with our acquisition of ReShape Medical will expire between 2027 and 2030.
We also register the trademarks and trade names through which we conduct our business. In the United States we have registered trademarks for vBLOC®, ENTEROMEDICS®, MAESTRO®, RESHAPE®, RESHAPE DUO®, and RESHAPE MEDICAL®, RESHAPE® DUAL BALLOON each registered with the United States Patent and Trademark Office, and trademark applications for vBLOC POWER TO CHOOSE, RESHAPE vBLOC , vBLOC ACHIEVE, RESHAPE VEST, and VBLOC POWER TO CHOOSE AND DESIGN. In addition, some or all of the marks vBLOC, ENTEROMEDICS, MAESTRO, MAESTRO SYSTEM ORCHESTRATING OBESITY SOLUTIONS, vBLOC POWER TO CHOOSE, vBLOC POWER TO CHOOSE AND DESIGN, RESHAPE, RESHAPE DUO, RESHAPE MEDICAL and RESHAPE LIFESCIENCES are the subject of either a trademark registration or application for registration in Australia, Brazil, Canada, China, the European Community, India, Kuwait, Mexico, Saudi Arabia, Switzerland and the United Arab Emirates. We believe that we have common law trademark rights to RESHAPE VEST.
In addition to our patents, we rely on confidentiality and proprietary information agreements to protect our trade secrets and proprietary knowledge. These confidentiality and proprietary information agreements generally provide that all confidential information developed or made known to individuals by us during the course of their relationship with us is to be kept confidential and not disclosed to third parties, except in specific circumstances. The agreements also provide for ownership of inventions conceived during the course of such agreements. If our proprietary information is shared or our confidentiality agreements are breached, we may not have adequate remedies, or our trade secrets may otherwise become known to or independently developed by competitors.
Sales and Distribution
We started the process of building a sales force and a controlled expansion of our operations and hired three new executives in January 2016 to oversee this expansion. Throughout 2015, 2016, and 2017 our sales force called directly on bariatric surgeons and gastroenterologists at commercially-driven bariatric centers of excellence that met our certification criteria. Additionally, in 2016, through a distribution agreement with Academy Medical, VA medical facilities now offer the ReShape vBloc as a treatment option to veteran healthcare benefits. We intend to continue to build on these efforts in 2018 through self-pay patient and veteran focused direct-to-patient marketing and key opinion leader and center specific partnering.
In 2017, we acquired ReShape Medical and trained the existing vBloc sales team on the ReShape Balloon. The three vBloc sales specialists combined with the 10 existing ReShape Balloon sales team member, resulting in a sales
force of 13 with two area directors and a VP of Sales. Additionally, one VP sales executive is now responsible for the US VA business and international sales. In 2018, we expect to continue to increase our direct sales organization.
Our sales representatives are supported by field clinical experts who are responsible for training, technical support, and other support services at various implant centers. Our sales representatives with the assistance of three field-based marketing specialists implement consumer marketing programs and provide surgical centers and implanting surgeons with educational patient materials.
We market directly to patients but sell ReShape vBloc and our ReShape Balloon to select surgical centers throughout the United States that have patients that would like to treat obesity and its comorbidities. The surgical centers then sell our product to the patients and implant. In 2015, 2016, and 2017, almost all the patients that purchased ReShape vBloc or the ReShape Balloon paid for the therapy themselves and did not receive reimbursement from an insurance provider, with the exception of veterans who received the ReShape vBloc through Veteran Administration Hospitals and, as we announced in December 2017, the employees of a major telecom company who subscribe to CarePlus supplemental insurance who received full reimbursement for the ReShape Balloon procedure.
We plan to build on these efforts in 2018 with self-pay and veteran focused direct-to-patient marketing, key opinion leader and center-specific partnering, and a multi-faceted reimbursement strategy.
In January 2018, we launched a limited time and scope pilot program selling private-labeled meal replacements and nutritional products that are manufactured and fulfilled by third-party vendors. This program is primarily designed to serve individuals who may have interest in, but do not qualify for treatment with our ReShape vBloc or ReShape Balloon devices. This program includes a customized nutrition program designed by, and weekly support from, a registered dietician. We will evaluate this pilot program in order to determine whether to continue it on a long-term basis.
On July 25, 2017, we entered into a Collaboration Agreement with Galvani Bioelectronics Limited (“Galvani”). Under the Collaboration Agreement, we will modify our ReShape vBloc for use in pre-clinical research by Galvani. We will receive payments for our development work and supply under this agreement. We will retain all rights, title, and ownership in the intellectual property for the new device, which will be licensed to Galvani. Galvani has been granted a right of first negotiation for the potential exclusive or non-exclusive supply by us of the developed device, exercisable at Galvani’s election. We believe that this collaboration is an example of opportunities that may exist to leverage the company’s intellectual property portfolio and custom development services to provide third party sales and licensing opportunities. Galvani is a joint venture between GlaxoSmithKline and Verily Life Sciences (an Alphabet company) that was established in 2016 to enable the research, development and commercialization of bioelectronic medicines
Our Manufacturers and Suppliers
We have designed and developed all of the elements of ReShape vBloc, except for the clinician programmer hardware, which uses a commercially available laptop computer. We use third parties to manufacture ReShape vBloc to minimize our capital investment, help control costs and take advantage of the expertise these third parties have in the large-scale production of medical devices. We do not currently plan to manufacture ReShape vBloc ourselves. We have designed and developed all of the elements of the recently acquired ReShape Balloon system and manufacture it in-house, with the exception of accessories in the form of a guidewire, pump and tubing, which are all commercially available but private-labeled for the Company.
To date, all of the materials and components of our products, as well as any related outside services, are procured from qualified suppliers and contract manufacturers in accordance with our proprietary specifications. All of our key manufacturers and suppliers have experience working with commercial implantable device systems, are ISO certified and are regularly audited by various regulatory agencies including the FDA. Our key manufacturers and suppliers have a demonstrated record of compliance with international regulatory requirements.
Given that we rely on third-party manufacturers and suppliers for the production of our products, our ability to increase production going forward will depend upon the experience, certification levels and large scale production capabilities of our suppliers and manufacturers. Qualified suppliers and contract manufacturers have been and will continue to be selected to supply products on a commercial scale according to our proprietary specifications. Because we manufacture the majority of the ReShape Balloon in-house, our ability to increase production will depend upon our
ability to hire and retain additional qualified personnel as well as the ability of our suppliers to meet increased demand. We have modestly increased our inventory levels to support commercial forecasts as we expand our implanting centers and intend to continue to increase our inventory levels as we determine necessary. Our FDA approval process required us to name and obtain approval for the suppliers of key components of ReShape vBloc and ReShape Balloon.
Many of our parts are custom designed and require custom tooling and, as a result, we may not be able to quickly qualify and establish additional or replacement suppliers for the components of our products. Any new approvals of vendors required by the FDA or other regulatory agencies in other international markets for our products as a result of the need to qualify or obtain alternate vendors for any of our components would delay our ability to sell and market our products and could have a material adverse effect on our business.
We believe that our current manufacturing and supply arrangements will be adequate to continue our ongoing commercial sales and our ongoing and planned clinical trials. In order to produce our products in the quantities we anticipate to meet future market demand, we will need our manufacturers and suppliers to increase, or scale up, manufacturing production and supply arrangements by a significant factor over the current level of production. There are technical challenges to scaling up manufacturing capacity and developing commercial-scale manufacturing facilities that may require the investment of substantial additional funds by our manufacturers and suppliers and hiring and retaining additional management and technical personnel who have the necessary experience. If our manufacturers or suppliers are unable to do so, we may not be able to meet the requirements to expand the launch of the product in the United States or launch the product internationally or to meet future demand, if at all. We may also represent only a small portion of our suppliers’ or manufacturers’ business and if they become capacity constrained they may choose to allocate their available resources to other customers that represent a larger portion of their business. We currently anticipate that we will continue to rely on third-party manufacturers and suppliers for the production of ReShape vBloc as we expand our commercial launch. If we are unable to obtain a sufficient supply of our product, our revenue, business and financial prospects would be adversely affected.
Government Regulations
Device Classification and Regulations
United States
Our ReShape vBloc, ReShape Balloon and our proposed ReShape Vest are regulated by the FDA as medical devices under the Federal Food, Drug, and Cosmetic Act (FFDCA) and the regulations promulgated under the FFDCA. Pursuant to the FFDCA, the FDA regulates the research, design, testing, manufacture, safety, labeling, storage, record keeping, advertising, sales and distribution, post-market adverse event reporting, production and advertising and promotion of medical devices in the United States. Noncompliance with applicable requirements can result in warning letters, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the government to grant premarket approval for devices and criminal prosecution.
Medical devices in the United States are classified into one of three classes, Class I, II or III, on the basis of the amount of risk and the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I, low risk, devices are subject to general controls (e.g., labeling and adherence to good manufacturing practices). Class II, intermediate risk, devices are subject to general controls and to special controls (e.g., performance standards, and premarket notification). Generally, Class III devices are those which must receive premarket approval by the FDA to ensure their safety and effectiveness (e.g., life-sustaining, life-supporting and implantable devices, or new devices which have not been found substantially equivalent to legally marketed devices), and require clinical testing to ensure safety and effectiveness and FDA approval prior to marketing and distribution. The FDA also has the authority to require clinical testing of Class II devices. In both the United States and certain international markets, there have been a number of legislative and regulatory initiatives and changes, such as the Modernization Act, which could and have altered the healthcare system in ways that could impact our ability to sell our medical devices profitably.
The FFDCA provides two basic review procedures for medical devices. Certain products may qualify for a submission authorized by Section 510(k) of the FFDCA, where the manufacturer submits to the FDA a premarket notification of the manufacturer’s intention to commence marketing the product. The manufacturer must, among other things, establish that the product to be marketed is substantially equivalent to another legally marketed product. Marketing may commence when the FDA issues a letter finding substantial equivalence. If a medical device does not
qualify for the 510(k) procedure, the manufacturer must file a premarket approval (PMA) application with the FDA. This procedure requires more extensive pre-filing clinical and preclinical testing than the 510(k) procedure and involves a significantly longer FDA review process. A PMA is required to establish the safety and effectiveness of the device and a key component of a PMA submission is the pivotal clinical trial data, as discussed in more detail below.
Premarket Approval
Our ReShape vBloc and our ReShape Balloon are medical devices that required PMAs from the FDA to market in the United States. The FDA approved ReShape vBloc in January of 2015 and the ReShape Balloon in July of 2015 with post-approval conditions intended to ensure the safety and effectiveness of the devices. Failure to comply with the conditions of approval can result in material adverse enforcement action, including the loss or withdrawal of the approvals. Even after approval of the PMAs, new PMAs or supplemental PMAs will be required for significant modifications to the manufacturing process, labeling, use and design of a device that is approved through the premarket approval process. Premarket approval supplements often require submission of the same type of information as a PMA except that the supplement is limited to information needed to support any changes from the device covered by the original PMA. In addition, holders of an approved PMA are required to submit annual reports to the FDA that include relevant information on the continued use of the device.
The ReShape Vest will likely be considered a Class III Long Term Implantable (LTI) product by the FDA requiring the premarket approval (PMA) path. A pivotal trial for the ReShape Vest will likely include approximately 250 implanted patients monitored up to three years. Other implantable devices for the treatment of obesity relied on 12 month endpoints for the PMA submission with annual follow-up visits up to five years and we expect the pivotal trial for the ReShape Vest to be similar. A US pivotal trial requires FDA Investigational Device Exemption (IDE) submission and approval. We expect to submit our IDE application to the FDA in the second quarter of 2018 and expect the first U.S. PMA implants of the ReShape Vest to take place in the third quarter of 2018. Our goal is to obtain PMA approval by the end of 2021. We intend to initiate a CE Mark trial in the European Union of 65 patients with a 12-month weight loss and safety endpoint with a minimum 24-month follow up. We expect the first EU implants will start in the second quarter of 2018. Our goal is to obtain CE mark approval by the second quarter of 2020.
Clinical Trials
A clinical trial is almost always required to support a PMA. Clinical trials for a “significant risk” device such as ours require submission to the FDA of an application for an IDE for clinical studies to be conducted within the United States. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. Clinical trials for a significant risk device in the United States may begin once the IDE application is approved by the FDA and by the Institutional Review Boards (IRBs) overseeing the clinical trial at the various investigational sites.
Clinical trials require extensive recordkeeping and detailed reporting requirements. Our clinical trials must be conducted under the oversight of an IRB for each participating clinical trial site and in accordance with applicable regulations and policies including, but not limited to, the FDA’s good clinical practice requirements. We, the trial Data Safety Monitoring Board, the FDA or the IRB for each site at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Pervasive and Continuing U.S. Regulation
Numerous regulatory requirements apply. These include:
| · | | Quality System Regulation, which requires manufacturers to follow design, testing, control, documentation, complaint handling and other quality assurance procedures during the design and manufacturing processes; |
| · | | regulations which govern product labels and labeling, prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling and promotional activities; |
| · | | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; |
| · | | notices of correction or removal and recall regulations; |
| · | | periodic reporting of progress related to clinical trials, post approval studies required as conditions of PMA approval and relevant changes to information contained within the PMA approval; and |
| · | | reporting of transfers of value and payments to physicians and teaching hospitals. |
Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, some promotional activities for FDA-regulated products have resulted in enforcement actions brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act, competitors and others can initiate litigation relating to advertising claims.
Compliance with regulatory requirements is enforced through periodic facility inspections by the FDA, which may be unannounced. Because we rely on contract manufacturing sites and service providers, these additional sites are also subject to these FDA inspections. Failure to comply with applicable regulatory requirements can result in enforcement action, which may include any of the following sanctions:
| · | | warning letters or untitled letters; |
| · | | fines, injunction and civil penalties; |
| · | | recall or seizure of our products; |
| · | | customer notification, or orders for repair, replacement or refund; |
| · | | operating restrictions, partial suspension or total shutdown of production or clinical trials; |
| · | | refusing our request for premarket approval of new products; |
| · | | withdrawing premarket approvals that are already granted; and |
International Regulations
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ. The primary regulatory environment in Europe is that of the European Economic Community (EEC), which consists of 28 European Union (EU) member states encompassing nearly all the major countries in Europe. Additional countries that are not part of the EU, but are part of the European Economic Area (EEA), and other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the EEC with respect to medical devices. The EEC has adopted Directive 90/385/EEC as amended by 2007/47/EC for active implantable medical devices and numerous standards that govern and harmonize the national laws and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices that are marketed in member states. Medical devices that comply with the requirements of the national law of the member state in which their Notified Body is located will be entitled to bear CE marking, indicating that the device conforms to applicable regulatory requirements, and, accordingly, can be commercially marketed within the EEA and other countries that recognize this mark for regulatory purposes.
We obtained European CE Mark approval for our ReShape vBloc in 2011 for the treatment of obesity. The CE Mark approval for our ReShape vBloc was expanded in 2014 to also include use for the management of Type 2 diabetes in obese patients. The method of assessing conformity with applicable regulatory requirements varies depending on the
class of the device, but for our ReShape vBloc (which is considered an Active Implantable Medical Device (AIMD) in Australia and the EEA, and falls into Class III within the United States), the method involved a combination of self-assessment and issuance of declaration of conformity by the manufacturer of the safety and performance of the device, and a third-party assessment by a Notified Body of the design of the device and of our quality system. A Notified Body is a private commercial entity that is designated by the national government of a member state as being competent to make independent judgments about whether a product complies with applicable regulatory requirements. The assessment included, among other things, a clinical evaluation of the conformity of the device with applicable regulatory requirements. We use DEKRA Certification B.V. (formerly known as KEMA Quality) in the Netherlands as the Notified Body for our CE marking approval process. We believe that the costs and resources required to successfully commercialize ReShape vBloc internationally are currently beyond our capability and as result, we made decisions in 2016 and late 2017, respectively, to temporarily abandon the AIMD and CE-marking of ReShape vBloc.
Continued compliance with CE marking requirements is enforced through periodic facility inspections by the Notified Body, which may be unannounced. Because we rely on contract manufacturing sites and service providers, these additional sites may also be subject to these Notified Body inspections.
We are also subject to the regulatory frameworks for medical devices in certain countries in the Middle East where we sell our products, including our sales of the ReShape Balloon in Kuwait, Qatar and the UAE. These medical device laws vary from country to country, but include approval and registration requirements. In addition, certain of these countries require that the registered distributor of a product be wholly owned by nationals of that particular country. Therefore, we rely on our distributors on those countries to comply with the applicable regulatory requirements.
Patient Privacy Laws
United States and various international laws have been evolving to protect the confidentiality of certain patient health information, including patient medical records. These laws restrict the use and disclosure of certain patient health information. Enforcement actions, including financial penalties, related to patient privacy issues are globally increasing. The management of patient data may have an impact on certain clinical research activities and product design considerations.
Employees
As of December 31, 2017, we had 83 employees. All of these employees are located in the United States.
From time to time we also employ independent contractors, consultants and temporary employees to support our operations. None of our employees are subject to collective bargaining agreements. We have never experienced a work stoppage and believe that our relations with our employees are good.
Executive Officers
The following table sets forth information regarding our executive officers, including their ages, as of March 15, 2018:
| | | | |
Name | | Age | | Position |
Dan W. Gladney | | 65 | | President and Chief Executive Officer |
Scott P. Youngstrom | | 58 | | Chief Financial Officer and Senior Vice President, Finance |
Dan W. Gladney has served as our President and Chief Executive Officer since November 16, 2015 and as Chairman of our board of directors since October 14, 2016. Mr. Gladney joined the Company on November 2, 2015 as President-Elect and a member of the board of directors. Prior to joining us, Mr. Gladney served as Chairman and Chief Executive Officer of Lanx, Inc., a medical device company focused on developing and commercializing innovative devices for spinal surgery. Prior to his time at Lanx, Inc., Mr. Gladney was a Healthcare Operating Partner at Norwest Equity Partners (NEP) from 2008 until 2010, where he was responsible for strategic planning, business growth and corporate governance for NEP portfolio companies and executing new investment opportunities for the firm. Prior to joining NEP, Mr. Gladney served as President and Chief Executive Officer of several medical device companies, including Heart Leaflet Technologies and Acist Medical Systems, both of which were acquired by The Bracco Group. He also served as Chairman, Chief Executive Officer and President of Compex Technologies, a publicly traded
orthopedic and health and wellness electro therapy company, from 2002 until 2006. Mr. Gladney currently serves on the board of directors of Aria CV, Inc. and has been a member of a number of other private and public company boards. After the sale of Lanx, he acted as a private investor and small business consultant.
Scott P. Youngstrom is our Senior Vice President, Finance and has served as our Chief Financial Officer since October 3, 2016. Mr. Youngstrom has over 25 years of strategic financial and operational experience in a variety of medical device companies, most recently having served as Chief Financial Officer and Vice President, Finance at Galil Medical, a leading developer of cryotherapy technology. Prior to Galil Medical, from 2009-2014, Mr. Youngstrom served as Vice President, Chief Operating Officer, and Chief Financial Officer at DGIMED Ortho, Inc., a developer of orthopedic medical devices. Mr. Youngstrom has previously served as Chief Financial Officer and Vice President, Finance with Anulex Technologies, Enpath Medical, Compex Technologies, Acist Medical Systems, and Cardiotronics.
Our Corporate Information
We were incorporated in Minnesota in December 2002 as two separate legal entities, Alpha Medical, Inc. and Beta Medical, Inc., both of which were owned 100% by a common stockholder. In October 2003, the two entities were combined and we changed our name to EnteroMedics Inc. In 2004 we reincorporated in Delaware. On October 23, 2017 we changed the company name from EnteroMedics Inc. to ReShape Lifesciences Inc. (NASDAQ: RSLS).
We file reports and other information with the Securities and Exchange Commission (SEC) including annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy or information statements. Those reports and statements as well as all amendments to those documents filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (1) are available at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549, (2) may be obtained by sending an electronic message to the SEC at publicinfo@sec.gov or by sending a fax to the SEC at 1-202-777-1027, (3) are available at the SEC’s internet site (http://www.sec.gov), which contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC and (4) are available free of charge through our website as soon as reasonably practicable after electronic filing with, or furnishing to, the SEC. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Our principal executive offices are located at 1001 Calle Amanecer, San Clemente, California 92673, and our telephone number is (949) 429-6680. Our website address is www.reshapelifesciences.com. The information on, or that may be accessed through, our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered a part of this Annual Report on Form 10-K.
ITEM 1A. RISK FACTORS
Risks Related to Our Recently Completed Acquisition of ReShape Medical and BarioSurg
Our acquisitions of ReShape Medical in October 2017 and BarioSurg in May 2017 could adversely affect our operations, financial results and financial condition.
In October 2017, we acquired ReShape Medical, Inc., a privately-held medical technology company that develops, manufactures and markets the ReShape® Dual Weight Loss Balloon (the “ReShape Balloon”), an FDA-approved, minimally invasive intragastric balloon designed to treat obesity patients with a body mass index (BMI) between 30 and 40, with one or more related comorbid conditions. In May 2017, we acquired BarioSurg, Inc., a privately held medical device company that developed the proprietary, minimally invasive and reversible device, the Gastric Vest, which we now refer to as the ReShape Vest, to treat obesity and related comorbidities. In addition, we may pursue additional acquisitions of other companies or product lines. A successful acquisition depends on our ability to identify, negotiate, complete and integrate such acquisition and to obtain any necessary financing. With respect to our acquisitions of ReShape Medical and BarioSurg and any future acquisitions, we may experience:
| · | | difficulties in integrating the acquired businesses and their respective personnel and products into our existing business; |
| · | | difficulties in integrating commercial organizations; difficulties or delays in realizing the anticipated benefits of the acquisition; |
| · | | diversion of our management’s time and attention from other business concerns; challenges due to limited or no direct prior experience in new markets or countries we may enter; inability to successfully develop new products and services on a timely basis that address our new market opportunities post-acquisition; |
| · | | inability to compete effectively against companies already serving the broader market opportunities expected to be available to us post-acquisition; |
| · | | unanticipated costs and other contingent liabilities; and any unforeseen compliance risks and accompanying financial and reputational exposure or loss not uncovered in the due diligence process and which are imputed to our company, such as compliance with federal laws and regulations, the advertising and promotion regulations under the federal Food, Drug and Cosmetic Act, the Anti-kickback Statute, the False Claims Act, the Physician Sunshine Payments Act and other applicable laws. |
We have invested, and expect to continue to invest, significant cash and other resources in connection with our acquisition of ReShape Medical and BarioSurg and our development of the ReShape Balloon and the ReShape Vest. The consideration we paid to acquire ReShape Medical and BarioSurg included approximately $5 million and $2 million in cash, respectively, and our efforts to continue the development of, and to successfully commercialize, the acquired products and technologies will require significant cash expenditures. There can be no assurance that we will be successful in our efforts. Should we be unable to obtain adequate financing or generate sufficient revenue in the future, our business, results of operations, liquidity and financial condition could be materially and adversely harmed.
The U.S. Food and Drug Administration (FDA) has published an announcement to alert health care providers of unanticipated deaths involving the ReShape Balloon, which could harm our business.
The FDA has published an announcement to alert health care providers of five reports of unanticipated deaths that occurred within one month of the placement of an intragastric balloon, one of which involved the ReShape Balloon. The announcement indicated that the root cause or incidence of patient death in these cases had not been found and the FDA was not able to definitively attribute the deaths to the balloon devices or their respective insertion procedures. The announcement also indicated that the FDA had received an additional report of a death related to potential complications associated with an esophageal perforation related to the ReShape Balloon. If these adverse events occur more frequently or other serious adverse effects are detected in liquid-filled intragastric balloons, the ReShape Balloon product may be subject to adverse FDA action or additional communications from the FDA, which could harm our business. In addition, we believe that the FDA announcement has negatively impacted, and may continue to negatively impact, our sales of the ReShape Balloon, which could have an adverse effect on our business, results of operations, liquidity and financial condition.
If we do not achieve the contemplated benefits of our acquisitions of ReShape Medical and BarioSurg, our business and financial condition may be materially impaired.
We may not achieve the desired benefits from our acquisitions of ReShape Medical and BarioSurg. For any of the reasons described above and elsewhere in this report and even if we are able to successfully operate ReShape Medical and BarioSurg within our company, we may not be able to realize the revenue and other growth that we anticipate from the acquisitions in the time frame that we currently expect, and the costs of achieving these benefits may be higher than what we currently expect, because of a number of risks, including, but not limited to:
| · | | the possibility that the acquisitions may not further our business strategy as we expected; |
| · | | the possibility that the revenue from our ReShape Balloon may be lower than we expected; |
| · | | the possibility that we may not be able to obtain the required regulatory approvals for the ReShape Vest; and the possibility that we may not be able to commercialize the ReShape Vest. |
As a result of these risks, we may not achieve the anticipated strategic and financial benefits of the ReShape Medical and BarioSurg acquisitions.
Our proposed ReShape Vest product is in the early stages of development. If the development of this product is not successfully completed or any required regulatory approvals are not obtained, the ReShape Vest may not be commercialized and our business prospects may suffer.
The ReShape Vest that we acquired as part of acquisition of BarioSurg is in the early stages of development and has not yet reached the clinical trial stage. Our ability to market the ReShape Vest in the United States and abroad depends upon our ability to demonstrate the safety, and in the case of the United States, efficacy, of the product with clinical data to support our requests for regulatory approval. The ReShape Vest may not be found to be safe and, where required, effective in clinical trials and may not ultimately be approved for marketing by U.S. or foreign regulatory authorities, which would have a negative impact on our net sales.
There is no assurance that we will be successful in achieving the desired results in our anticipated clinical trials for the ReShape Vest or, if we do, that the FDA or other regulatory agencies will approve the product for sale without the need for additional clinical trial data to demonstrate safety and efficacy. We continually evaluate the potential financial benefits and costs of clinical trials and the products being evaluated in them. If we determine that the costs associated with obtaining regulatory approval of a product exceed the potential financial benefits of that product or if the projected development timeline is inconsistent with our investment horizon, we may choose to stop a clinical trial and/or the development of a product.
Our Board of Directors and executive officers are able to influence matters requiring stockholder approval and could discourage the purchase of our outstanding shares at a premium.
As of December 31, 2017, Dr. Raj Nihalani, the founder and former Chief Executive Officer of BarioSurg and our Chief Technology Officer, beneficially owned approximately 15.1% of our outstanding common stock. Under a voting agreement entered into in connection with our acquisition of BarioSurg, Dr. Nihalani agreed to vote all shares of our common stock he owns in accordance with the recommendation of our Board of Directors and granted an irrevocable proxy to our Board of Directors to vote his shares in accordance with the terms of the voting agreement. In addition, as of December 31, 2017, HealthCor Partners Fund II, L.P. (“HealthCor”) beneficially owned approximately 8.5% of our outstanding common stock. Michael Y. Mashaal, M.D., a member of our Board of Directors, is managing director of the investment manager of HealthCor and therefore may be deemed to beneficially own the shares held by HealthCor. In connection with our acquisition of ReShape Medical, we entered into a voting and standstill agreement with HealthCor and each other former ReShape Medical stockholder who holds at least 5% of our outstanding common stock (on an as-converted basis) after the acquisition pursuant to which such stockholders agreed to (i) vote all shares of our common stock in the same manner as and in the same proportion as the votes cast on the matter by the holders of our voting securities entitled to vote on the matter, unless such requirement is waived by our Board of Directors, and (ii) certain customary standstill provisions pursuant to which such stockholders will refrain from various actions that might relate to the acquisition of control of our company, such as making proposals to acquire our company or launching a proxy context.
Collectively, our directors and executive officers as a group beneficially own approximately 25% of our outstanding common stock. As a result of Dr. Nihalani’s and Dr. Mashaal’s share ownership and the voting agreements described above, our Board of Directors and executive officers are able to influence matters requiring stockholder approval, such as the election of directors and approval of significant corporate transactions. The interests of our Board of Directors and executive officers may differ from the interests of our other stockholders. For example, our Board of Directors and executive officers could oppose a third party offer to acquire us that the other stockholders might consider attractive. In such case and in similar situations, our other stockholders may disagree with our Board of Directors and executive officers as to whether the action opposed or supported by our Board of Directors and executive officers is in the best interest of our stockholders.
The shares of series C convertible preferred stock issued in connection with our acquisition of ReShape Medical have certain rights and preferences senior to our common stock.
In connection with the ReShape Medical merger, we issued 187,772 shares of newly created non-voting series C convertible preferred stock, which shares became convertible into 18,777,200 shares of voting common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules. On December 19, 2017, the date of stockholder approval, 82,384 shares of series C convertible preferred stock automatically converted into 8,238,400 shares of common stock. The remaining 95,388 shares of series C convertible preferred stock are
convertible at the option of their holders into approximately 9.5 million shares of common stock, provided that the former ReShape Medical holders will not be permitted to convert their shares of series C convertible preferred stock into shares of common stock to the extent such conversion would cause them to holder more than 49.0% of our outstanding voting securities at the time of any such conversion. In general, the series C convertible preferred stock is entitled to receive dividends (on an as-if-converted-to-common stock basis) actually paid on shares of common stock when, as and if such dividends are paid on shares of common stock. No other dividends will be paid on shares of series C convertible preferred stock. While the series C convertible preferred stock generally does not have voting rights, as long as any shares of series C convertible preferred stock remain outstanding, we cannot, without the affirmative vote of holders of a majority of the then-outstanding shares of series C convertible preferred stock, (a) alter or change adversely the powers, preferences or rights given to the series C convertible preferred stock (including by the designation, authorization, or issuance of any shares of preferred stock that purports to have equal rights with, or be senior in rights or preferences to, the series C convertible preferred stock), (b) alter or amend the series C convertible preferred stock certificate of designation, (c) amend our certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of series C convertible preferred stock, (d) increase the number of authorized shares of series C convertible preferred stock or (e) enter into any agreement with respect to any of the foregoing. The series C convertible preferred stock has a liquidation preference of $274.8774 per share, or $2.748774 per underlying share of common stock. Holders of the series C convertible preferred stock have the right to convert their shares into shares of common stock instead of receiving the liquidation preference.
Risks Related to Our Business and Industry
If we are unable to either substantially improve our operating results or obtain additional financing in the future, we will be unable to continue as a going concern.
Our independent registered public accounting firm’s report on our December 31, 2017 audited financial statements includes an explanatory paragraph referring to our ability to continue as a going concern. The proceeds from any future financings that we may be able to obtain, if any, together with our other available cash, may not be sufficient to fund our operating expenses, capital expenditures and other cash requirements. Should we be unable to obtain adequate financing or generate sufficient revenue in the future, our business, result of operations, liquidity and financial condition would be materially and adversely harmed, and we would be unable to continue as a going concern. These events and circumstances could have a material adverse effect on our ability to raise additional capital and on the market value of our common stock and the warrants offered hereby. Moreover, should we experience a cash shortage that requires us to curtail or cease our operations, or should we be unable to continue as a going concern, you could lose all or part of your investments in our securities.
We currently are not generating revenue from operations that is significant relative to our level of operating expenses, and we do not anticipate generating revenue sufficient to offset operating costs in the short-term to mid-term. We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on investments. Our history of operating losses, limited cash resources and lack of certainty regarding obtaining significant third-party reimbursement for our products or timing thereof, raise substantial doubt about our ability to continue as a going concern absent a strengthening of our cash position. As of December 31, 2017, we had $10.2 million of cash and cash equivalents to fund our operations through early 2018. On April 2, 2018 the Company announced that it had entered into a securities purchase agreement with an institutional investor providing for the purchase and sale in a registered direct offering of shares of series D convertible preferred stock and a warrant to purchase shares of common stock for a purchase price of $6.0 million. The transaction is expected to close on or about April 4, 2018 and the Company expects to receive net proceeds of approximately $5.25 million after deducting placement agent fees and other offering expenses.
Our anticipated operations include plans to (i) integrate the sales and operations of our company with the newly acquired ReShape Medical in order to expand sales of both the ReShape Balloon and ReShape vBloc as well as to obtain cost savings synergies, (ii) expand the controlled commercial launch of ReShape vBloc Therapy, delivered via ReShape vBloc, (iii) continue development of the ReShape Vest, (vi) seek opportunities to leverage our intellectual property portfolio and custom development services to provide third party sales and licensing opportunities, and (v) explore and capitalize on synergistic opportunities to expand our portfolio and offer future minimally invasive treatments and therapies in the obesity continuum of care. We believe that we have the flexibility to manage the growth of our expenditures and operations depending on the amount of available cash flows, which could include reducing expenditures for marketing, clinical and product development activities. However, we will ultimately need to achieve
sufficient revenues from product sales and obtain additional debt or equity financing in addition to the proceeds from this offering to support our operations.
If we are unsuccessful in our pursuit of various funding options, we will be unable to continue as a going concern.
Management is currently pursuing various funding options, including seeking additional equity financing, to continue the development of, and to successfully commercialize, the ReShape vBloc, the ReShape Balloon and the ReShape Vest. There can be no assurance that we will be successful in our efforts. Should we be unable to obtain adequate financing or generate sufficient revenue in the future, our business, result of operations, liquidity and financial condition would be materially and adversely harmed, and we would be unable to continue as a going concern. Additionally, there can be no assurance that, assuming we are able to strengthen our cash position, we will achieve sufficient revenue or profitable operations to continue as a going concern.
We are a medical device company with a limited history of operations and sales, and we cannot assure you that we will ever generate substantial revenue or be profitable.
We are a medical device company with a limited operating history upon which you can evaluate our business. We received FDA approval to sell our vBloc System, which we now refer to as ReShape vBloc, in the United States on January 14, 2015 and we have had commercial sales within the United States since 2015. We have also completed the regulatory process required to sell our ReShape vBloc in Australia, the European Economic Area and other countries that recognize the European CE Mark, and have not generated revenue from commercial sales outside of the United States since 2012 and then only on a limited basis in Australia and the Middle East. Because we believe that the costs and resources required to successfully commercialize ReShape vBloc internationally are currently beyond our capability, we made the decision in late 2017 to temporarily abandon CE-marking of ReShape vBloc. Similarly, we discontinued the regulatory processes required to sell ReShape vBloc in Australia in 2016.
We have been engaged in research and development and clinical trials since our inception in 2002 and have invested substantially all of our time and resources in developing our vBloc Therapy, which we have begun to commercialize in the form of our ReShape vBloc. The success of our business will depend on our ability to establish a sales force, make sales and control costs, as well as our ability to obtain additional regulatory approvals needed to market new versions of our ReShape vBloc or ReShape Balloon or regulatory approvals needed to market our ReShape Vest and any other products we may develop in the future, all of which we may be unable to do. If we are unable to successfully market our ReShape vBloc and ReShape Balloon for their indicated use or develop and commercialize the ReShape Vest, we may never become profitable and may have to cease operations as a result. Our lack of a significant operating history also limits your ability to make a comparative evaluation of us, our products and our prospects.
We have incurred losses since inception and we anticipate that we will continue to incur losses for the foreseeable future.
We have incurred losses in each year since our formation in 2002. Our net loss applicable to common stockholders for the fiscal years ended December 31, 2017, 2016 and 2015 was $33.8 million, $23.4 million and $25.5 million, respectively. We have funded our operations to date principally from the sale of securities and the issuance of indebtedness. Development of a new medical device, including conducting clinical trials and seeking regulatory approvals, is a long, expensive and uncertain process. Although we have received the regulatory approval required to sell our ReShape vBloc in the United States and have the approvals required for sales in the European Economic Area and other countries that recognize the European CE Mark, we have only generated limited revenue from commercial sales in the United States and have not generated revenue from commercial sales outside of the United States since 2012 and then only on a limited basis in Australia and the Middle East. Because we believe that the costs and resources required to successfully commercialize ReShape vBloc internationally are currently beyond our capability, we made the decision in late 2017 to temporarily abandon CE-marking of ReShape vBloc. Similarly, we discontinued the regulatory processes required to sell ReShape vBloc in Australia in 2016.
We expect to incur significant sales and marketing expenses prior to recording sufficient revenue to offset these expenses. We expect our general and administrative expenses to increase as we continue to add the infrastructure necessary to support our initial commercial sales, market the ReShape Balloon, develop the ReShape Vest, operate as a public company and develop our intellectual property portfolio. For these reasons, we expect to continue to incur significant operating losses for the next several years. These losses, among other things, have had and will continue to
have an adverse effect on our stockholders’ equity and working capital. Because of the numerous risks and uncertainties associated with developing new medical devices, we are unable to predict the extent of any future losses or when we will become profitable, if ever.
We will need substantial additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or liquidate some or all of our assets.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts on the development and commercialization of our products and on research and development, including conducting current and future clinical trials for our ReShape vBloc, ReShape Balloon, ReShape Vest (if approved for sale) and subsequent versions of our products. Cash used in operations was $24.6 million, $20.7 million, and $22.6 million for the fiscal years ended December 31, 2017, 2016 and 2015, respectively. We expect that our cash used in operations will continue to be significant in the upcoming years, and that we will need to raise additional capital to commercialize our ReShape vBloc in the United States, the European Economic Area, other countries that recognize the European CE Mark and other international markets, to explore other indications for the ReShape vBloc and ReShape Balloon, to develop the ReShape Vest, to continue our research and development programs, and to fund our ongoing operations.
Our future funding requirements will depend on many factors, including:
| · | | the cost and timing of establishing sales, marketing and distribution capabilities; |
| · | | the cost of establishing clinical and commercial supplies of our ReShape vBloc, ReShape Balloon, ReShape Vest and any products that we may develop; the rate of market acceptance of our ReShape vBloc and vBloc Therapy, ReShape Balloon, ReShape Vest and any other product candidates; |
| · | | the rate of market acceptance of our ReShape vBloc and vBloc Therapy, ReShape Balloon, ReShape Vest (if approved for sale) and any other product candidates; |
| · | | the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights; |
| · | | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights; |
| · | | the effect of competing products and market developments; |
| · | | the cost of explanting clinical devices; |
| · | | the terms and timing of any collaborative, licensing or other arrangements that we may establish; |
| · | | any revenue generated by sales of our ReShape vBloc, ReShape Balloon, ReShape Vest (if approved for sale) or our future products; |
| · | | the scope, rate of progress, results and cost of any clinical trials and other research and development activities; |
| · | | the cost and timing of obtaining any further required regulatory approvals; and |
| · | | the extent to which we invest in products and technologies, although we currently have no commitments or agreements relating to these types of transactions. |
Until the time, if ever, when we can generate a sufficient amount of product revenue, we expect to finance our future cash needs through public or private equity offerings, debt financings or corporate collaboration, licensing arrangements and grants, as well as through interest income earned on cash balances.
Additional capital may not be available on terms favorable to us, or at all. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may involve restrictive covenants or additional security interests in our assets. Any additional debt or equity financing that we complete may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or products, or grant licenses on terms that are not favorable to us. If we are unable to raise adequate funds, we may have to delay, reduce the scope of, or eliminate some or all of, our development programs or liquidate some or all of our assets.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives.
As a public company, we incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act), as well as rules subsequently implemented by the SEC and NASDAQ have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations result in increased legal and financial compliance costs and will make some activities more time-consuming and costly.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure. In particular, we are required to perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Specifically, we have identified a material weakness in our internal controls over financial reporting as of December 31, 2017 related to our purchase accounting related to our acquisition or BarioSurg. In our allocation of the BarioSurg purchase price, we failed to record a $7.6 million deferred income tax liability related to the indefinite-lived intangible asset, In- Process Research and Development, with an offsetting increase in Goodwill in our condensed consolidated balance sheets. We do not believe that these balance sheet misstatements related to non-cash items as of June 30, 2017 and September 30, 2017 were material to our financial statements on those dates taken as a whole. However, the omission of this deferred tax liability led to what we have concluded to be a material weakness in internal control over financial reporting. Specifically, because the unrecorded deferred tax liability relates to an indefinite-lived intangible asset, we cannot offset the deferred tax liability with available deferred tax assets when determining our net deferred tax position under generally accepted accounting principles. As a result, when the unrecorded deferred tax liability required remeasurement due to the December 22, 2017 enactment of the Tax Cuts and Jobs Act, this in turn required recognition of a $2.3 million income tax benefit in our consolidated statement of operations for the quarter and year ended December 31, 2017.
We have incurred and continue to expect to incur significant expense and devote substantial management effort toward ensuring compliance with Section 404. Moreover, if we do not comply with the requirements of Section 404, or if we identify additional deficiencies in our internal controls that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by NASDAQ, the SEC or other regulatory authorities, which would entail expenditure of additional financial and management resources.
We face significant uncertainty in the industry due to government healthcare reform.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. The Patient Protection and Affordable Care Act, as amended, (the Affordable Care Act) as well as any future healthcare reform legislation, may have a significant impact on our business. The impact of the Affordable Care Act on the health care industry is extensive and includes, among other things, the federal government assuming a larger role in the health care system, expanding healthcare coverage of United States citizens and mandating basic healthcare benefits. The Affordable Care Act contains many provisions designed to generate the revenues necessary to fund the coverage expansions and to reduce costs of Medicare and Medicaid, including imposing a 2.3% excise tax on domestic sales of many medical devices by manufacturers that began in 2013. Although a moratorium was placed on the medical device excise tax through 2019, if Congress does not pass legislation further extending or otherwise eliminating the excise tax, our sales and selling, general and administrative expenses may be adversely effected in the future.
Congress is considering legislation to replace or repeal elements or all of the Affordable Care Act. In addition, there have been recent public announcements by members of Congress and the new presidential administration regarding
their plans to repeal and replace the Affordable Care Act. Further, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Affordable Care Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Affordable Care Act that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. At this time, it is not clear whether the Affordable Care Act will be repealed in whole or in part, and, if it is repealed, whether it will be replaced in whole or in part by another plan and what impact those changes will have on coverage and reimbursement for healthcare items and services covered by plans that were authorized by the Affordable Care Act. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and also indirectly affect the amounts that private payers are willing to pay. In addition, any healthcare reforms enacted in the future may, like the Affordable Care Act, be phased in over a number of years but, if enacted, could reduce our revenue, increase our costs, or require us to revise the ways in which we conduct business or put us at risk for loss of business. In addition, our results of operations, financial position and cash flows could be materially adversely affected by changes under the Affordable Care Act and changes under any federal or state legislation adopted in the future.
We are subject, directly or indirectly, to United States federal and state healthcare fraud and abuse and false claims laws and regulations. Prosecutions under such laws have increased in recent years and we may become subject to such litigation. If we are unable to, or have not fully complied with such laws, we could face substantial penalties.
Our operations are directly, or indirectly through customers, subject to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and federal False Claims Act. These laws may impact, among other things, our sales, marketing and education programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad and, despite a series of narrow safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim to, or the knowing use of false statements to obtain payment from the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of medical device, pharmaceutical and healthcare companies to have to defend a False Claim Act action. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Various states have also enacted laws modeled after the federal False Claims Act.
We are unable to predict whether we could be subject to actions under any of these laws, or the impact of such actions. If we are found to be in violation of any of the laws described above or other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare reimbursement programs and the curtailment or restructuring of our operations.
We operate in a highly competitive industry that is subject to rapid change. If our competitors are able to develop and market products that are safer or more effective than our products, our commercial opportunities will be reduced or eliminated.
The health care industry is highly competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. The obesity treatment market in which we operate has grown significantly in recent years and is expected to continue to expand as technology continues to evolve and
awareness of the need to treat the obesity epidemic grows. Although we are not aware of any competitors in the neuroblocking market, we face potential competition from pharmaceutical and surgical obesity treatments. Many of our competitors in the obesity treatment field have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly if they pursue competing solutions through collaborative arrangements with large and established companies, such as Allergan, Apollo Endosurgery, Boston Scientific, LivaNova PLC, Johnson & Johnson, Medtronic or St. Jude Medical. Our competitors may develop and patent processes or products earlier than us, obtain regulatory approvals for competing products more rapidly than we are able to and develop more effective, safer and less expensive products or technologies that would render our products non-competitive or obsolete.
Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches, service interruptions, or data corruption could significantly disrupt our operations and adversely affect our business and operating results.
We currently rely on information technology and telephone networks and systems, including the Internet, to process and transmit sensitive electronic information and will rely on such systems to manage or support a variety of business processes and activities, including sales, billing, customer service, procurement and supply chain, manufacturing, and distribution. We use enterprise information technology systems to record, process, and summarize financial information and results of operations for internal reporting purposes and to comply with regulatory financial reporting, legal, and tax requirements.
Our information technology systems, some of which are managed by third-parties, may be susceptible to damage, disruptions or shutdowns due to computer viruses, attacks by computer hackers, failures during the process of upgrading or replacing software, databases or components thereof, power outages, hardware failures, telecommunication failures, user errors or catastrophic events. We are not aware of any breaches of our information technology infrastructure. Despite the precautionary measures we have taken to prevent breakdowns in our information technology and telephone systems, if our systems suffer severe damage, disruption or shutdown and we are unable to effectively resolve the issues in a timely manner, our business and operating results may suffer.
Selling products for human consumption involves inherent legal and other risks, including product contamination, spoilage, tampering, allergens or other adulteration, which could lead to product liability or other claims.
In January 2018, we launched a limited time and scope pilot program selling private-labeled meal replacements and nutritional products that are manufactured and fulfilled by third-party vendors. This program is primarily designed to serve individuals who may have interest in, but do not qualify for, treatment with our ReShape vBloc or ReShape Balloon devices. This program includes a customized nutrition program designed by, and weekly support from, a registered dietitian. We rely on our third-party vendors to ensure that the products they manufacture and sell comply with applicable regulatory, health and safety, and other legal requirements. We also rely on our third-party vendors to ensure that the products are safe for human consumption and that any nutritional advice is appropriate under the circumstances. However, any product liability or other claims related to our meal replacement and nutritional products or services could significantly damage our reputation and consumer confidence in our products and could materially and adversely affect our business.
Risks Associated with Development and Commercialization of the ReShape vBloc, ReShape Balloon and ReShape Vest
Our efforts to commercialize our ReShape vBloc, ReShape Balloon and ReShape Vest may not succeed or may encounter delays which could significantly harm our ability to generate revenue.
Our ability to generate revenue will depend upon the successful commercialization of our ReShape vBloc, ReShape Balloon and our ReShape Vest (if approved for sale). Our efforts to commercialize these products may not succeed for a number of reasons, including:
| · | | we may not be able to obtain the regulatory approvals required for our ReShape Vest or for any future modifications to our ReShape vBloc or ReShape Balloon; |
| · | | our products may not be accepted in the marketplace by physicians, patients and third-party payers; |
| · | | sales of our ReShape Balloon may be negatively impacted by the FDA announcement to alert health care providers of unanticipated deaths involving the ReShape Balloon; |
| · | | the price of our products, associated costs of the surgical procedure and treatment and the availability of sufficient third-party reimbursement for the system implantation and follow-up procedures; |
| · | | appropriate reimbursement and/or coding options may not exist to enable billing for the system implantation and follow-up procedures; |
| · | | we may not be able to sell our products at a price that allows us to meet the revenue targets necessary to generate enough revenue for profitability; |
| · | | the frequency and severity of any side effects of our products; |
| · | | physicians and potential patients may not be aware of the perceived effectiveness and sustainability of the results of our products; |
| · | | we, or the investigators of our products, may not be able to have information on the outcome of the trials published in medical journals; |
| · | | the availability and perceived advantages and disadvantages of alternative treatments; |
| · | | any rapid technological change may make our products obsolete; |
| · | | we may not be able to have our products manufactured in commercial quantities or at an acceptable cost; |
| · | | we may not have adequate financial or other resources to complete the development and commercialization of our products or to develop sales and marketing capabilities for our products; and |
| · | | we may be sued for infringement of intellectual property rights and could be enjoined from manufacturing or selling our products. |
Besides requiring physician adoption, market acceptance of our products will depend on successfully communicating the benefits of our products to three additional constituencies involved in deciding whether to treat a particular patient using our products: (1) the potential patients themselves; (2) institutions such as hospitals, where the procedure would be performed and opinion leaders in these institutions; and (3) third-party payers, such as private healthcare insurers and governmental payers, such as Medicare and Medicaid in the United States, which would ultimately bear most of the costs of the various providers and equipment involved in our vBloc Therapy, ReShape Balloon and ReShape Vest (if approved for sale). Marketing to each of these constituencies requires a different marketing approach, and we must convince each of these groups of the efficacy and utility of our products to be successful.
If our products, or any other therapy or products for other gastrointestinal diseases and disorders that we may develop, do not achieve an adequate level of acceptance by the relevant constituencies, we may not generate significant product revenue and may not become profitable.
We may not be able to obtain required regulatory approvals for our ReShape Vest in a cost-effective manner or at all, which could adversely affect our business and operating results.
The production and marketing of our ReShape Vest and our ongoing research and development, preclinical testing and clinical trial activities are subject to extensive regulation and review by numerous governmental authorities both in the United States and abroad. U.S. and foreign regulations applicable to medical devices are wide-ranging and govern, among other things, the development, testing, marketing and premarket review of new medical devices, in addition to regulating manufacturing practices, reporting, advertising, exporting, labeling and record keeping procedures. We are
required to obtain regulatory approval before we can market our ReShape Vest in the United States and certain foreign countries. The regulatory process will require significant time, effort and expenditures to bring products to market, and it is possible that our ReShape Vest will not be approved for sale. Even if regulatory approval of our ReShape Vest is granted, it may not be granted within the timeframe that we expect, which could have an adverse effect on our operating results and financial condition. Even after our ReShape Vest is approved by the FDA, we may have ongoing responsibilities under FDA regulations, non-compliance of which could result in the subsequent withdrawal of such approvals, or such approvals could be withdrawn due to the occurrence of unforeseen problems following initial approval. We also are subject to medical device reporting regulations that require us to report to the FDA if any of our products causes or contributes to a death or serious injury or if a malfunction were it to occur might cause or contribute to a death or serious injury. Any failure to obtain regulatory approvals on a timely basis or the subsequent withdrawal of such approvals could prevent us from successfully marketing our products, which could adversely affect our business and operating results.
While we previously had received approval from the regulatory body of the European Economic Area to market our ReShape vBloc for the treatment of obesity, that approval will lapse March 31, 2018 and we have not received, and may never receive, approval from the regulatory bodies of any other foreign countries.
We do not have the necessary regulatory approvals to market our ReShape vBloc in any foreign market other than the European Economic Area for which we received CE Mark approval for our ReShape vBloc in March 2011 for the treatment of obesity and other countries which accept these regulatory approvals. The CE Mark approval for our ReShape vBloc was expanded in 2014 to also include use for the management of Type 2 diabetes in obese patients. This CE Mark approval lapses March 31, 2018. Additionally, the ReShape vBloc was previously listed on the Australian Register of Therapeutic Goods (ARTG). We commenced commercialization of our ReShape vBloc product in Australia and the Middle East in 2012, but have not generated revenue from commercial sales outside of the United States since 2012 as we focused our resources on the U.S. regulatory approval process and commercialization of ReShape vBloc in the United States and we do not know when, or if, we will have the resources to commercialize our ReShape vBloc internationally.
In order to market our ReShape vBloc outside of the United States, we will need to establish and comply with the numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries may differ from that required to obtain FDA approval. The regulatory approval process in other countries may also include all of the risks detailed below.
Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. While the ReShape vBloc was previously listed on the ARTG and has European CE Marking until March 31, 2018 we cannot assure you when, or if, we will be able to restart sales in, Australia or the Middle East, commence sales or obtain approval to market our ReShape vBloc product in other countries outside the United States.
Because vBloc Therapy represents a novel way to effect weight loss in the treatment of obesity, and because there is a large population of obese patients who might be eligible for treatment, it is possible that other regulatory bodies will review an application for approval of our ReShape vBloc with greater scrutiny, which could cause that process to be lengthier and more involved than that for products without such characteristics. Such regulatory bodies can delay, limit or deny approval of our ReShape vBloc for many reasons, including our inability to demonstrate safety or effectiveness to their satisfaction, insufficient or inadequate data from our clinical trials, the facilities of our third-party manufacturers or suppliers may not meet applicable requirements; and changes in the regulatory bodies’ approval policies, expectations with regard to the type or amount of scientific data required or adoption of new regulations may require additional data or additional clinical studies.
We have limited data and experience regarding the safety and efficacy of the ReShape vBloc. Any long-term data that is generated may not be positive or consistent with our limited short-term data, which would affect market acceptance of these products.
Because our technology is relatively new in the treatment of obesity, we have performed clinical trials only with limited patient populations. The long-term effects of using the ReShape vBloc in a large number of patients have not
been studied and the results of short-term clinical use of the ReShape vBloc do not necessarily predict long-term clinical benefits or reveal long-term adverse effects.
Clinical trials conducted with the ReShape vBloc have involved procedures performed by physicians who are very technically proficient. Consequently, both short and long-term results reported in these studies may be significantly more favorable than typical results achieved by physicians, which could negatively impact market acceptance of the ReShape vBloc and materially harm our business.
We may be unable to complete our current clinical trials or any additional clinical trials, or we may experience significant delays in completing those clinical trials, which could impact market acceptance of our ReShape vBloc and ReShape Balloon products and impair our financial position.
We continue to evaluate the vBloc Therapy in human clinical trials, including the EMPOWER trial, the ReCharge trial and the ReNew trial. We continue to evaluate the ReShape Balloon product in the ReShape Post Approval Study. Conducting a clinical trial, which involves screening, assessing, testing, treating and monitoring patients at several sites across the country and possibly internationally, and coordinating with patients and clinical institutions, is a complex and uncertain process.
The completion of our ongoing and future clinical trials, could be delayed, suspended or terminated for several reasons, including:
| · | | ongoing discussions with regulatory authorities regarding the scope or design of our preclinical results or clinical trial or requests for supplemental information with respect to our preclinical results or clinical trial results; |
| · | | our failure or inability to conduct the clinical trials in accordance with regulatory requirements; |
| · | | sites currently participating in the trial may drop out of the trial, which may require us to engage new sites or petition the FDA for an expansion of the number of sites that are permitted to be involved in the trial; |
| · | | patients may not remain in or complete, clinical trials at the rates we expect; |
| · | | patients may experience serious adverse events or side effects during the trial, which, whether or not related to our product, could cause the FDA or other regulatory authorities to place the clinical trial on hold; |
| · | | clinical investigators may not perform our clinical trials on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices; |
| · | | we may be unable to obtain a sufficient supply of ReShape vBloc or ReShape Balloon product necessary for the timely conduct of the clinical trials; and |
| · | | we may not have the financial resources necessary to fund the clinical trials on a timely basis. |
Although we believe that we have adequate personnel and procedures in place to manage the clinical trial process, the complexity of managing this process while also commercializing our products and fulfilling our disclosure and other obligations to our stockholders, lenders, regulators and other constituents could result in our inadvertently taking actions outside the clinical trial process, which could adversely impact the trial. As is always the case, if the FDA ultimately determined that such actions materially violated the protocol for the trial, the FDA could suspend, terminate or reject the results of the clinical trial and require us to repeat the process.
If our clinical trials are delayed, it will take us longer to ultimately commercialize a product and generate revenue or the delay could result in our being unable to do so. Moreover, our development costs will increase if we have material delays in our clinical trials or if we need to perform more or larger clinical trials than planned.
We depend on clinical investigators and clinical sites to enroll patients in our clinical trials, and on other third parties to manage the trials and to perform related data collection and analysis, and, as a result, we may face costs and delays that are outside of our control.
We rely on clinical investigators and clinical sites to enroll patients in our clinical trials and other third parties to manage the trials and to perform related data collection and analysis. However, we may not be able to control the amount and timing of resources that clinical sites may devote to our clinical trials. If these clinical investigators and clinical sites fail to enroll a sufficient number of patients in our clinical trials, ensure compliance by patients with clinical protocols or comply with regulatory requirements, we will be unable to complete these trials, which could prevent us from obtaining or maintaining regulatory approvals for our product. Our agreements with clinical investigators and clinical trial sites for clinical testing place substantial responsibilities on these parties and, if these parties fail to perform as expected, our trials could be delayed or terminated. If these clinical investigators, clinical sites or other third parties do not carry out their contractual duties or obligations or fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated, or the clinical data may be rejected by the FDA, adversely affecting our ability to successfully commercialize our product.
Modifications to the ReShape vBloc or ReShape Balloon may require additional approval from regulatory authorities, which may not be obtained or may delay our commercialization efforts.
The FDA and our European Notified Body require medical device companies to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance; however, some of these regulatory authorities can review a company’s decision. Any modifications to an approved device that could significantly affect its safety or efficacy, or that would constitute a major change in its intended use could require additional clinical studies and separate regulatory applications. Product changes or revisions will require all the regulatory steps and associated risks discussed above possibly including testing, regulatory filings and clinical study. We may not be able to obtain approval of supplemental regulatory approvals for product modifications, new indications for our product or new products. Delays in obtaining future clearances would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our commercialization efforts and future growth.
Our neuroblocking therapy for the treatment of obesity is a unique form of treatment. Physicians may not widely adopt our ReShape vBloc and vBloc Therapy unless they determine, based on experience, long-term clinical data and published peer reviewed journal articles, that vBloc Therapy provides a safe and effective alternative to other existing treatments for obesity.
We believe we are the first and only company currently pursuing neuroblocking therapy for the treatment of obesity. Physicians tend to be slow to change their medical treatment practices because of the time and skill required to learn a new procedure, the perceived liability risks arising from the use of new products and procedures, and the uncertainty of third-party coverage and reimbursement. Physicians may not widely adopt our ReShape vBloc and vBloc Therapy unless they determine, based on experience, long-term clinical data and published peer reviewed journal articles, that the use of our vBloc Therapy provides a safe and effective alternative to other existing treatments for obesity, including pharmaceutical solutions and bariatric surgical procedures.
We cannot provide any assurance that the data collected from our current and planned clinical trials will be sufficient to demonstrate that our vBloc Therapy is an attractive alternative to other obesity treatment procedures. We rely on experienced and highly trained surgeons to perform the procedures in our clinical trials and both short-and long-term results reported in our clinical trials may be significantly more favorable than typical results of practicing physicians, which could negatively impact rates of adoption of our ReShape vBloc and vBloc Therapy. We believe that published peer-reviewed journal articles and recommendations and support by influential physicians regarding our ReShape vBloc and vBloc Therapy will be important for market acceptance and adoption, and we cannot assure you that we will receive these recommendations and support, or that supportive articles will be published.
If we fail to obtain adequate coding, coverage or payment levels for our product by governmental healthcare programs and other third-party payers, there may be no commercially viable markets for our ReShape vBloc or other products we may develop or our target markets may be much smaller than expected.
Healthcare providers generally rely on third-party payers, including governmental payers, such as Medicare and Medicaid in the United States, as well as private healthcare insurers, to adequately cover and reimburse the cost of medical devices. Importantly, third-party payers are increasingly challenging the price of medical products and services and instituting cost containment measures to control or significantly influence the purchase of medical products and services. We expect that third-party payers will continue to attempt to contain or reduce the costs of healthcare by challenging the prices charged for healthcare products and services. If reimbursement for our ReShape vBloc and the related surgery and facility costs is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, market acceptance of our ReShape vBloc will be impaired and our future revenue, if any, would be adversely affected. As such, even though we have obtained FDA approval for our ReShape vBloc and began to market it in 2015, the availability and level of third-party coverage and reimbursement could substantially affect our ability to successfully commercialize our ReShape vBloc and other products we may develop.
The efficacy, safety, ease of use and cost-effectiveness of our ReShape vBloc and of any competing products will, in part, determine the availability and level of coverage and payment. In particular, we expect that securing coding, coverage and payment for our ReShape vBloc will be more difficult if healthcare providers and obese individuals do not consider the percentage of EWL from a pre-implementation baseline that our clinical trials have demonstrated to be clinically meaningful, whether or not regulatory agencies consider the improvement of patients treated in clinical trials to have been clinically meaningful.
In some international markets, pricing of medical devices is subject to government control. In the United States and international markets, we expect that both government and third-party payers will continue to attempt to contain or reduce the costs of healthcare by challenging the prices charged for healthcare products and services. If payment for our ReShape vBloc and the related surgery and facility costs is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, market acceptance of our ReShape vBloc will be impaired and our future revenue, if any, would be adversely affected.
We cannot predict the likelihood or pace of any significant regulatory or legislative action in any of these areas, nor can we predict whether or in what form healthcare legislation being formulated by various governments will be passed. We also cannot predict with precision what effect such governmental measures would have if they were ultimately enacted into law. However, in general, we believe that such legislative activity will likely continue. If adopted, such measures can be expected to have an impact on our business.
If we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated product problems, our ReShape vBloc or ReShape Balloon could be subject to restrictions or withdrawal from the market.
Completion of our clinical trials and continued commercialization of our ReShape vBloc and ReShape Balloon will require access to manufacturing facilities that meet applicable regulatory standards to manufacture a sufficient supply of our products. We rely solely on third parties to manufacture and assemble our ReShape vBloc product and do not currently plan to manufacture or assemble our ReShape vBloc product ourselves in the future. While we currently manufacture the ReShape Balloon in our own facility, for both ReShape vBloc and ReShape Balloon products, we rely on outside suppliers and service providers to deliver materials and services that comply with standards set by the FDA and other regulators.
Any product for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data and promotional activities for such product, will be subject to continual review and periodic inspections by our European Notified Body and the FDA and other regulatory bodies. In particular we and our manufacturers and suppliers are required to comply with ISO requirements, Good Manufacturing Practices, which for medical devices is called the Quality System Regulation (QSR), and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain marketing approval. The FDA enforces the QSR through inspections, which may be unannounced, and the CE system enforces its certification through inspections and audits as well. Our quality system has received certification of compliance to the requirements of ISO 13485:2003 and will have to continue to successfully complete such inspections to maintain regulatory approvals for sales outside of the United States. Failure by us or one of our
manufacturers or suppliers to comply with statutes and regulations administered by the FDA, CE authorities and other regulatory bodies, or failure to adequately respond to any observations, could result in enforcement actions against us or our manufacturers or suppliers, including, restrictions on our product or manufacturing processes, withdrawal of the product from the market, voluntary or mandatory recall, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties.
If any of these actions were to occur it would harm our reputation and cause our product sales to suffer. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with applicable regulatory requirements. If the FDA or any other regulatory body finds their compliance status to be unsatisfactory, our commercialization efforts could be delayed, which would harm our business and our results of operations.
Additionally, if the FDA determines that our promotional materials, training or other activities constitute promotion of an unapproved use, we could be subject to significant liability, the FDA could request that we cease, correct or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
We are subject to medical device reporting regulations that require us to report to the FDA, Competent Authorities or other governmental authorities in other countries if our products cause or contribute to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar governmental authorities in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacturing. A government mandated, or voluntary, recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall would divert managerial and financial resources and could harm our reputation with customers. There can be no assurance that there will not be product recalls in the future or that such recalls would not have a material adverse effect on our business. Once the product is approved and implanted in a large number of patients, infrequently occurring adverse events may appear that were not observed in the clinical trials. This could cause health authorities in countries where the product is available to take regulatory action, including marketing suspension and recall.
We recently received two Form 483s from the FDA. This could lead to a warning letter, which could have a material adverse effect on our business.
In December 2017, following an inspection of our St. Paul, Minnesota facility, the FDA issued us a Form 483. The Form 483 identified seven observations, including (i) that procedures for corrective and preventive actions have not been adequately established, (ii) certain product components that do not conform to specifications were not adequately controlled, (iii) certain processes have not been adequately validated according to established procedures, (iv) our risk analysis for certain procedures is incomplete, (v) two instances where we did not report adverse patient events to the FDA, (vi) procedures to ensure that all purchased or received products and services conform to specified requirements have not been adequately established, and (vii) quality audits were not performed at defined intervals to determine whether the quality system activities and results comply with quality system procedures.
In February 2018, following an inspection of our San Clemente, California facility, the FDA issued us another Form 483, which identified two observations. The first observation relates to inconsistencies in our documentation of the resolution of certain self-identified corrective actions. The second observation relates to multiple instances of ReShape Medical, both before and after our acquisition of the company, reporting adverse patient events related to implanted ReShape Balloons to the FDA after the required 30 day deadline, all of which were either submitted to the FDA shortly after the deadline or were caused by technical issues with the FDA submission portal.
Following our receipt of each Form 483, we agreed to take corrective and preventive actions to fully address the FDA observations. The outcome of these matters is presently uncertain. We cannot assure you that the FDA will conclude that our corrective and preventive actions are adequate to address the observations. If the FDA were to find that we failed to comply with applicable regulations, the FDA could institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as fines and civil money penalties against us or our officers; delays in clearing or approving, or refusal to clear or approve, our products; withdrawal or suspension of approval of our
products; product recall or seizure; interruption of production; operating restrictions; injunctions; or criminal prosecution.
We may not be successful in our efforts to utilize our vBloc Therapy to treat comorbidities associated with obesity and other gastrointestinal diseases and disorders.
As part of our long-term business strategy, we plan to research the application of our vBloc Therapy to treat comorbidities associated with obesity and other gastrointestinal diseases and disorders. Research to identify new target applications requires substantial technical, financial and human resources, whether or not any new applications for our vBloc Therapy are ultimately identified. We may be unable to identify or pursue other applications of our technology. Even if we identify potential new applications for our vBloc Therapy, investigating the safety and efficacy of our therapy requires extensive clinical testing, which is expensive and time-consuming. If we terminate a clinical trial in which we have invested significant resources, our prospects will suffer, as we will have expended resources on a program that will not provide a return on our investment and missed the opportunity to allocate those resources to potentially more productive uses. We will also need to obtain regulatory approval for these new applications, as well as achieve market acceptance and an acceptable level of reimbursement.
We depend on a limited number of manufacturers and suppliers of various critical components for our products. The loss of any of these manufacturer or supplier relationships could prevent or delay commercialization of our products.
We rely entirely on third parties to manufacture our ReShape vBloc product and to supply us with all of the critical components of our products, including our leads, implantable batteries, neuroregulators, transmit coils and controllers. Additionally, we rely on third party suppliers to provide the materials necessary for us to manufacture the ReShape Balloon. If any of our existing suppliers were unable or unwilling to meet our demand for product components, or if the components or finished products that they supply do not meet quality and other specifications, completion of our clinical trials or commercialization of our product could be delayed. Alternatively, if we have to switch to a replacement manufacturer or replacement supplier for any of our product components, we may face additional regulatory delays, and the manufacture and delivery of our products could be interrupted for an extended period of time, which could delay completion of our clinical trials or commercialization of our products.
If our device manufacturers or our suppliers are unable to provide an adequate supply of our products, our growth could be limited and our business could be harmed.
In order to produce our products in the quantities that we anticipate will be required to meet anticipated market demand, we will need our manufacturers to increase, or scale-up, the production process by a significant factor over our current level of production. There are technical challenges to scaling-up manufacturing capacity and developing commercial-scale manufacturing facilities that may require the investment of substantial additional funds by us or our manufacturers and hiring and retaining additional management and technical personnel who have the necessary manufacturing experience. If we or our manufacturers are unable to do so, we may not be able to meet future demand, if any. We may also represent only a small portion of our supplier’s or manufacturer’s business and if they become capacity constrained they may choose to allocate their available resources to other customers that represent a larger portion of their business. We currently anticipate that we will continue to rely on third-party manufacturers and suppliers for the production of our products. If we are unable to obtain a sufficient supply of our products or the materials necessary to manufacture our products, our revenue, business and financial prospects would be adversely affected.
If we are unable to establish sales and marketing capabilities or enter into and maintain arrangements with third parties to market and sell our ReShape vBloc and ReShape Balloon products, our business may be harmed.
We have limited experience as a company in sales, marketing and distribution of our product and began the process of developing a sales and marketing organization in 2015 and have continued its development in 2016, 2017 and into 2018. We market our products in the United States through a direct sales force supported by field technical managers who provide training, technical and other support services to our customers. We have begun to develop the necessary sales and marketing infrastructure in order to commercialize our product, but developing a sales force is expensive and time consuming and we may be unable to develop an effective sales and marketing organization on a timely basis, if at all, or maintain our current sales and marketing capabilities, either of which would delay or prevent us from generating enough revenue to become profitable. Our sales force will be competing with the experienced and well-funded marketing and sales organizations of our more established competitors. If we are unable to establish and maintain
our own sales and marketing capabilities, we will need to contract with third parties to market and sell our product. In this event, our profit margins would likely be lower than if we performed these functions ourselves. In addition, we would necessarily be relying on the skills and efforts of others for the successful marketing of our product. If we are unable to establish and maintain effective sales and marketing capabilities, independently or with others, we may not be able to generate product revenue and may not become profitable.
When we have sufficient resources to commercialize our ReShape vBloc internationally, we intend to use direct, dealer and distributor sales models as the targeted geography best dictates. With the acquisition of ReShape Medical in October 2017, we have distributor agreements to sell the ReShape Balloon in the Middle East.
To generate sales and launch the commercialization of our product in other geographic regions we may need to identify and enter into other third-party distributor agreements. There is no assurance that we can do so on economically acceptable terms or that if we do so, that a third-party distributor will be successful in selling our product.
The commercialization of our products in countries outside the United States will expose our business to certain risks associated with international operations.
When we have sufficient resources to do so, we intend to commercialize our products in the European Economic Area, Australia and the Middle East and other international markets in which we obtain necessary regulatory approvals. Conducting international operations will subject us to unique risks, including:
| · | | unfamiliar legal requirements with which we would need to comply; |
| · | | fluctuations in currency exchange rates; |
| · | | potentially adverse tax consequences, including the complexities of foreign value added tax systems and restrictions on the repatriation of earnings; |
| · | | increased financial accounting and reporting burdens and complexities; and |
| · | | reduced or varied protection for intellectual property rights in some countries. |
The occurrence of any one of these risks could negatively affect our business and results of operations generally. Additionally, operating in international markets requires significant management attention. We cannot be certain that investments required to establish operations in other countries will produce desired levels of revenues or profitability.
We may be unable to attract and retain management and other personnel we need to succeed.
Our success depends on the services of our senior management and other key employees. The loss of the services of one or more of our officers or key employees could delay or prevent the successful completion of our clinical trials and the commercialization of our ReShape vBloc and ReShape Balloon and the development of our ReShape Vest. Our continued growth will require hiring a number of qualified clinical, scientific, commercial and administrative personnel. Accordingly, recruiting and retaining such personnel in the future will be critical to our success. There is intense competition from other companies and research and academic institutions for qualified personnel in the areas of our activities. If we fail to identify, attract, retain and motivate these highly skilled personnel, we may be unable to continue our development and commercialization activities.
We may be unable to manage our growth effectively.
Our business strategy entails significant future growth. For example, we will have to expand existing operations in order to commercialize our ReShape vBloc, ReShape Balloon and develop our ReShape Vest, conduct additional clinical trials, increase our contract manufacturing capabilities, hire and train new personnel to handle the marketing and sales of our product, assist patients and healthcare providers in obtaining reimbursement for the use of our product and create and develop new applications for our technology. This growth may place significant strain on our management and financial and operational resources. Successful growth is also dependent upon our ability to implement appropriate financial and management controls, systems and procedures. Our ability to effectively manage growth depends on our success in
attracting and retaining highly qualified personnel, for which the competition may be intense. If we fail to manage these challenges effectively, our business could be harmed.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to obtain adequate product liability insurance.
Our business exposes us to a risk of product liability claims that is inherent in the testing, manufacturing and marketing of medical devices. The medical device industry has historically been subject to extensive litigation over product liability claims. We may be subject to product liability claims if our products cause, or appear to have caused, an injury. Claims may be made by consumers, healthcare providers, third-party strategic collaborators or others selling our products.
We have product liability insurance, which covers the use of our ReShape vBloc and vBloc Therapy and ReShape Balloon in our clinical trials and any commercial sales, in an amount we believe is appropriate. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, the coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost and on acceptable terms for an adequate coverage amount, or otherwise to protect against potential product liability claims, we could be exposed to significant liabilities, which may harm our business. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations. These liabilities could prevent or interfere with our product commercialization efforts. Defending a suit, regardless of merit, could be costly, could divert management attention and might result in adverse publicity, which could result in the withdrawal of, or inability to recruit, clinical trial volunteers or result in reduced acceptance of our products in the market.
We may be subject to product liability claims even if it appears that the claimed injury is due to the actions of others. For example, we rely on the expertise of surgeons and other associated medical personnel to perform the medical procedure to implant and remove our products and to perform the related therapy. If these medical personnel are not properly trained or are negligent, the therapeutic effect of our products may be diminished or the patient may suffer critical injury, which may subject us to liability. In addition, an injury that is caused by the negligence of one of our suppliers in supplying us with a defective component that injures a patient could be the basis for a claim against us. A product liability claim, regardless of its merit or eventual outcome, could result in decreased demand for our products; injury to our reputation; diversion of management’s attention; withdrawal of clinical trial participants; significant costs of related litigation; substantial monetary awards to patients; product recalls or market withdrawals; loss of revenue; and the inability to commercialize our products under development.
Risks Related to Intellectual Property
If we are unable to obtain or maintain intellectual property rights relating to our technology and neuroblocking therapy, the commercial value of our technology and any future products will be adversely affected and our competitive position will be harmed.
Our commercial success depends in part on our ability to obtain protection in the United States and other countries for our ReShape vBloc and vBloc Therapy, ReShape Balloon and ReShape Vest by establishing and maintaining intellectual property rights relating to or incorporated into our technology and products. We own numerous U.S. and foreign patents and have numerous patent applications pending, most of which pertain to treating gastrointestinal disorders and the treatment of obesity. We have also received or applied for patents in Europe, Australia, China, India, Japan, Israel and Canada. In addition, we are the exclusive licensee of three U.S. patents owned by the Mayo Foundation for Medical Education and Research, which are unrelated to our vBloc Therapy. Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that will provide us any competitive advantage. We expect to incur substantial costs in obtaining patents and, if necessary, defending our proprietary rights. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. We do not know whether we will obtain the patent protection we seek, or that the protection we do obtain will be found valid and enforceable if challenged. If we fail to obtain adequate protection of our intellectual property, or if any protection we obtain is reduced or eliminated, others could use our intellectual property without compensating us, resulting in harm to our business. We may also determine that it is in our best interests to voluntarily challenge a third-party’s products or patents in litigation or administrative proceedings, including patent interferences, re-examinations or under more recently promulgated Inter Partes Review proceedings, depending on when
the patent application was filed. In the event that we seek to enforce any of our owned or exclusively licensed patents against an infringing party, it is likely that the party defending the claim will seek to invalidate the patents we assert, which, if successful could result in the loss of the entire patent or the relevant portion of our patent, which would not be limited to any particular party. Any litigation to enforce or defend our patent rights, even if we were to prevail, could be costly and time-consuming and could divert the attention of our management and key personnel from our business operations. Even if we were to prevail in any litigation, we cannot assure you that we can obtain an injunction that prevents our competitors from practicing our patented technology. Our competitors may independently develop similar or alternative technologies or products without infringing any of our patent or other intellectual property rights, or may design around our proprietary technologies.
We cannot assure you that we will obtain any patent protection that we seek, that any protection we do obtain will be found valid and enforceable if challenged or that it will confer any significant commercial advantage. U.S. patents and patent applications may also be subject to interference proceedings and U.S. patents may be subject to re-examination proceedings in the U.S. Patent and Trademark Office (USPTO), or under more recently promulgated Inter Partes Review proceedings, depending on when the patent application was filed, and foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices, which proceedings could result in either loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of, the patent or patent application. In addition, such interference, re-examination and opposition proceedings may be costly. Moreover, the U.S. patent laws have recently changed with the adoption of the America Invents Act (AIA), possibly making it easier to challenge patents. Some of our technology was, and continues to be, developed in conjunction with third parties, and thus there is a risk that such third parties may claim rights in our intellectual property. Thus, any patents that we own or license from others may provide limited or no protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties, may not result in patents being issued. If issued, they may not provide us with proprietary protection or competitive advantages against competitors with similar technology.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may result in loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States, particularly in the field of medical products and procedures.
Many of our competitors have significant resources and incentives to apply for and obtain intellectual property rights that could limit or prevent our ability to commercialize our current or future products in the United States or abroad.
Many of our competitors who have significant resources and have made substantial investments in competing technologies may seek to apply for and obtain patents that will prevent, limit or interfere with our ability to make, use or sell our products either in the United States or in international markets. Our current or future U.S. or foreign patents may be challenged, circumvented by competitors or others or may be found to be invalid, unenforceable or insufficient. In most cases in the United States patent applications are published 18 months after filing the application, or corresponding applications are published in other countries, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make the inventions covered by each of our pending patent applications, or that we were the first to file patent applications for such inventions.
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely on our unpatented proprietary technology, trade secrets, processes and know-how. We generally seek to protect this information by confidentiality agreements with our employees, consultants, scientific advisors and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently developed by competitors. To the extent that our employees, consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Intellectual property litigation is a common tactic in the medical device industry to gain competitive advantage. If we become subject to a lawsuit, we may be required to expend significant financial and other resources and our management’s attention may be diverted from our business.
There has been a history of frequent and extensive litigation regarding patent and other intellectual property rights in the medical device industry, and companies in the medical device industry have employed intellectual property litigation to gain a competitive advantage. Accordingly, we may become subject to patent infringement claims or litigation in a court of law, or interference proceedings declared by the USPTO to determine the priority of inventions or an opposition to a patent grant in a foreign jurisdiction. We may also become subject to claims or litigation seeking payment of royalties based on sales of our product in connection with licensing or similar joint development arrangements with third parties or in connection with claims of patent infringement.
Specifically, on April 20, 2017, Fulfillium, Inc. filed a complaint in the United States District Court for the District of Delaware accusing ReShape Medical, our wholly owned subsidiary, of trade secret misappropriation and patent infringement of U.S. Patent Nos. 9,445,930 and 9,456,915, which we are currently in the process of defending. The defense and prosecution of intellectual property suits, USPTO interference proceedings, reexamination proceedings, or under more recently promulgated Inter Partes Review proceedings, depending on when the patent application was filed, or opposition proceedings and related legal and administrative proceedings, are both costly and time consuming and could result in substantial uncertainty to us. Litigation or regulatory proceedings may also be necessary to enforce patent or other intellectual property rights of ours or to determine the scope and validity of other parties’ proprietary rights. Any litigation, opposition or interference proceedings, with or without merit, may result in substantial expense to us, cause significant strain on our financial resources, divert the attention of our technical and management personnel and harm our reputation. We may not have the financial resources to defend our patents from infringement or claims of invalidity. An adverse determination in any litigation could subject us to significant liabilities to third parties, require us to seek licenses from or pay royalties to third parties or prevent us from manufacturing, selling or using our proposed products, any of which could have a material adverse effect on our business and prospects.
Our vBloc Therapy or ReShape vBloc, ReShape Balloon or ReShape Vest may infringe or be claimed to infringe patents that we do not own or license, including patents that may issue in the future based on patent applications of which we are currently aware, as well as applications of which we are unaware. For example, we are aware of other companies that are investigating neurostimulation, including neuroblocking, and of patents and published patent applications held by companies in those fields. While we believe that none of such patents and patent applications are applicable to our products and technologies under development, third parties who own or control these patents and patent applications in the United States and abroad could bring claims against us that would cause us to incur substantial expenses and, if such claims are successfully asserted against us, they could cause us to pay substantial damages, could result in an injunction preventing us from selling, manufacturing or using our proposed products and would divert management’s attention. Because patent applications in many countries such as the United States are maintained under conditions of confidentiality and can take many years to issue, there may be applications now pending of which we are unaware and which may later result in issued patents that our products infringe. If a patent infringement suit were brought against us, we could be forced to stop our ongoing or planned clinical trials, or delay or abandon commercialization of the product that is subject of the suit.
As a result of patent infringement claims, or to avoid potential claims, we may choose or be required to seek a license from a third-party and be required to pay license fees or royalties, or both. A license may not be available at all or on commercially reasonable terms, and we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct additional clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time-consuming and expensive. Even if we were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be forced to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms. This could harm our business significantly.
Risks Relating to Ownership of Our Common Stock
The trading price of our common stock has been volatile and is likely to be volatile in the future.
The trading price of our common stock has been highly volatile. Further, our common stock has a limited trading history. The market price for our common stock will be affected by a number of factors, including:
| · | | the denial or delay of regulatory clearances or approvals of our product or receipt of regulatory approval of competing products; |
| · | | our ability to accomplish clinical, regulatory and other product development milestones and to do so in accordance with the timing estimates we have publicly announced; |
| · | | changes in policies affecting third-party coverage and reimbursement in the United States and other countries; |
| · | | changes in government regulations and standards affecting the medical device industry and our product; |
| · | | ability of our products to achieve market success; |
| · | | the performance of third-party contract manufacturers and component suppliers; |
| · | | our ability to develop sales and marketing capabilities; |
| · | | actual or anticipated variations in our results of operations or those of our competitors; |
| · | | announcements of new products, technological innovations or product advancements by us or our competitors; |
| · | | developments with respect to patents and other intellectual property rights; |
| · | | sales of common stock or other securities by us or our stockholders in the future; |
| · | | additions or departures of key scientific or management personnel; |
| · | | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| · | | the trading volume of our common stock; |
| · | | changes in earnings estimates or recommendations by securities analysts, failure to obtain or maintain analyst coverage of our common stock or our failure to achieve analyst earnings estimates; |
| · | | public statements by analysts or clinicians regarding their perceptions of our clinical results or the effectiveness of our products; |
| · | | decreases in market valuations of medical device companies; and |
| · | | general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors. |
The stock prices of many companies in the medical device industry have experienced wide fluctuations that have often been unrelated to the operating performance of these companies. Following periods of volatility in the market price of a company’s securities, securities class action litigation often has been initiated against a company. If class action litigation is initiated against us, we may incur substantial costs and our management’s attention may be diverted from our operations, which could significantly harm our business.
Our inability to comply with the listing requirements of the NASDAQ Stock Market could result in our common stock being delisted, which could affect its market price and liquidity and reduce our ability to raise capital.
We are required to meet certain qualitative and financial tests (including a minimum closing bid price of $1.00 per share for our common stock) to maintain the listing of our common stock on the NASDAQ Stock Market. If we do not maintain compliance with the continued listing requirements for the NASDAQ Stock Market within specified periods and subject to permitted extensions, our common stock may be recommended for delisting (subject to any appeal we would file). If our common stock were delisted, it could be more difficult to buy or sell our common stock and to obtain accurate quotations, and the price of our stock could suffer a material decline. Delisting would also impair our ability to raise capital.
Sales of a substantial number of shares of our common stock in the public market by existing stockholders, or the perception that they may occur, could cause our stock price to decline.
Sales of substantial amounts of our common stock by us or by our stockholders, announcements of the proposed sales of substantial amounts of our common stock or the perception that substantial sales may be made, could cause the market price of our common stock to decline. We may issue additional shares of our common stock in follow-on offerings to raise additional capital, upon the exercise of options or warrants, or in connection with acquisitions or corporate alliances. We also plan to issue additional shares to our employees, directors or consultants in connection with their services to us. All of the currently outstanding shares of our common stock are freely tradable under federal and state securities laws, except for shares held by our directors, officers and certain greater than five percent stockholders and shares received by the former ReShape Medical equity holders in connection with the merger, which may be subject to holding period, volume and other limitations under Rule 144. Due to these factors, sales of a substantial number of shares of our common stock in the public market could occur at any time and could reduce the market price of our common stock.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
The shares of common stock issued to the former ReShape Medical equity holders in connection with the merger were issued in a transaction intended to be exempt from registration under the Securities Act of 1933. Therefore, those shares are not currently freely tradable under the federal securities laws (further described in Note 4 to the financial statements). We may also issue additional registered or unregistered shares of our common stock in connection with acquisitions or corporate alliances. If our stockholders sell substantial amounts of our common stock in the public market upon the expiration of any statutory holding period under Rule 144, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital for general corporate purposes, in the future we may offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may be lower than the current price per share of our common stock. In addition, investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in prior offerings.
In addition, in connection with the ReShape Medical merger, we issued 187,772 shares of newly created non-voting series C convertible preferred stock, which shares became convertible into 18,777,200 shares of voting common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules. On December 19, 2017, the date of stockholder approval, 82,384 shares of series C convertible preferred stock automatically converted into 8,238,400 shares of common stock. The remaining 95,388 shares of series C convertible preferred stock are convertible at the option of their holders into approximately 9.5 million shares of common stock, provided that the former ReShape Medical holders will not be permitted to convert their shares of series C convertible preferred stock into
shares of common stock to the extent such conversion would cause them to holder more than 49.0% of our outstanding voting securities at the time of any such conversion.
Our organizational documents and Delaware law make a takeover of our company more difficult, which may prevent certain changes in control and limit the market price of our common stock.
Our certificate of incorporation and bylaws and Section 203 of the Delaware General Corporation Law contain provisions that may have the effect of deterring or delaying attempts by our stockholders to remove or replace management, engage in proxy contests and effect changes in control. These provisions include:
| · | | the ability of our board of directors to create and issue preferred stock without stockholder approval, which could be used to implement anti-takeover devices; |
| · | | the authority for our board of directors to issue without stockholder approval up to the number of shares of common stock authorized in our certificate of incorporation, that, if issued, would dilute the ownership of our stockholders; |
| · | | the advance notice requirement for director nominations or for proposals that can be acted upon at stockholder meetings; |
| · | | a classified and staggered board of directors, which may make it more difficult for a person who acquires control of a majority of our outstanding voting stock to replace all or a majority of our directors; |
| · | | the prohibition on actions by written consent of our stockholders; |
| · | | the limitation on who may call a special meeting of stockholders; |
| · | | the prohibition on stockholders accumulating their votes for the election of directors; and |
| · | | the ability of stockholders to amend our bylaws only upon receiving a majority of the votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
In addition, as a Delaware corporation, we are subject to Delaware law, including Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless certain specific requirements are met as set forth in Section 203. These provisions, alone or together, could have the effect of deterring or delaying changes in incumbent management, proxy contests or changes in control.
These provisions also could discourage proxy contests and make it more difficult for you and other stockholders to elect directors and take other corporate actions. The existence of these provisions could limit the price that investors might be willing to pay in the future for shares of our common stock. Some provisions in our certificate of incorporation and bylaws may deter third parties from acquiring us, which may limit the market price of our common stock.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our common stock.
We have never paid dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The payment of dividends on our common stock will depend on our earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 2. PROPERTIES
We lease approximately 28,388 square feet of lab and office space in St. Paul, Minnesota. The original lease agreement began October 1, 2008 and was set to expire September 30, 2015. On August 25, 2015 we entered into an amendment extending the term of the lease for three years until September 30, 2018.
In connection with our acquisition of BarioSurg in May 2017, we acquired approximately 1,949 square feet of office space in Lake Forest, California under an operating lease that expires September 30, 2018.
In connection with our acquisition of ReShape Medical in October 2017, we acquired (i) approximately 14,479 square feet of office/warehouse space in San Clemente, California under an operating lease that expires June 30, 2022 and (ii) approximately 8,000 square feet of office and manufacturing space in San Clemente, California under an operating lease that expires October 31, 2019.
ITEM 3. LEGAL PROCEEDINGS
On February 28, 2017, the Company received a class action and derivative complaint filed on February 24, 2017 in U. S. District Court for the District of Delaware by Vinh Du, one of the Company’s shareholders. The complaint names as defendants ReShape Lifesciences, the board of directors and four members of our senior management, namely, Scott Youngstrom, Nick Ansari, Peter DeLange and Paul Hickey, and contains a purported class action claim for breach of fiduciary duty against the board of directors and derivative claims for breach of fiduciary duty against the board of directors and unjust enrichment against our senior management. The allegations in the complaint relate to the increase in the number of shares authorized for grant under our Second Amended and Restated 2003 Stock Incentive Plan (the “Plan”), which was approved by our shareholders at the Special Meeting of Shareholders held on December 12, 2016 (the “Special Meeting”), and to our subsequent grant of stock options on February 8, 2017, to the Company’s Directors and senior management to purchase an aggregate of 1,093,450 shares of our common stock (the “Option Grants”). In the complaint, the plaintiff contends that (i) the number of shares authorized for grant under the Plan, as adjusted by the board of directors after the Special Meeting for the subsequent recapitalization of the Company, resulted from an alleged breach of fiduciary duties by the board of directors, and (ii) our senior management was allegedly unjustly enriched by the subsequent Option Grants. The plaintiff seeks relief in the form of an order rescinding the Plan as approved by the shareholders at the Special Meeting, an order cancelling the Option Grants, and an award to plaintiff for his costs, including fees and disbursements of attorneys, experts and accountants. On April 17, 2017, we filed a motion to dismiss the complaint based on the plaintiff’s failure to satisfy Delaware’s demand requirement for a derivative action and failure to state a valid claim. The court denied the motion to dismiss on November 30, 2017. Discovery is on-going. We believe the allegations in the complaint are without merit, and intend to defend the action vigorously.
On April 20, 2017, Fulfillium, Inc. filed a Complaint against the Company in the United States District Court for the District of Delaware, which alleged misappropriation of trade secrets and infringement of two United States Patents. On July 28, 2017, ReShape Medical moved to dismiss both the misappropriation of trade secret claim and the claims of patent infringement, and to transfer the litigation to the United States District Court for the Central District of California. On October 10, 2017, Fulfillium filed a motion to amend its Complaint to add SV Health Investors, LLC as a co-defendant; that motion is fully briefed and pending. On October 16, 2017, the Court granted ReShape Medical’s motion to dismiss the trade secret and willful infringement claims. The Court also ordered the case transferred to the United States District Court for the Central District of California. On November 20, 2017, Fulfillium filed an Amended Complaint, which abandoned certain trade secret claims, and expanded upon allegations regarding other of its trade secret claims and claims for willful infringement. On December 6, 2017, ReShape Medical moved to dismiss those amended claims and for reconsideration of denial of its prior motion to dismiss certain patent infringement claims; both motions were denied on February 7, 2018. On February 5, 2018, Fulfillium filed a second motion to further amend its Complaint to add a claim of infringement of another Fulfillium patent. ReShape Medical opposed that motion. On February 21, 2018, ReShape Medical filed its Answer and Counterclaims to the Amended Complaint, including counterclaims for declarations of non-infringement, invalidity, unenforceability and/or co-inventorship of the asserted Fulfillium patents and state-law tort claims against Fulfillium and its founder personally. Fulfillium and its founder have not yet responded to those counterclaims. At the initial case management conference held on March 19, 2018, the Court
ordered that Fulfillium’s motions to amend be stricken and ordered Fulfillium to file a consolidated motion to amend. The Court encouraged the parties to confer to narrow the disputes for the Court’s consideration, and indicated that Fulfillium need not respond to ReShape Medical’s counterclaims until the scope of the amended complaint was decided. The Court assigned key dates for the litigation, including close of fact discovery on September 15, 2018, last day for filing motions on September 19, 2018, pretrial conference on November 19, 2018 and first day of trial on December 4, 2018. The Company intends to vigorously defend itself against Fulfillium, Inc.’s claims and to vigorously pursue its counterclaims.
We currently are unable to estimate the losses or range of losses for these two matters where there is a reasonable possibility of a loss or it is probable that a loss may have ben incurred.
Except as disclosed in the foregoing paragraphs, the Company is not currently a party to any litigation and the Company is not aware of any pending or threatened litigation against it that could have a material adverse effect on the Company’s business, operating results or financial condition. The medical device industry in which the Company operates is characterized by frequent claims and litigation, including claims regarding patent and other intellectual property rights as well as improper hiring practices. As a result, the Company may be involved in various legal proceedings from time to time.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market For Our Common Stock
Our common stock has been traded on the NASDAQ Stock Market since our initial public offering (IPO) on November 15, 2007, initially under the symbol “ETRM”and, after our name change on October 23, 2017, under the symbol “RSLS.” Prior to November 15, 2007, there was no public market for our common stock. Our stock was traded on the NASDAQ Global Market from its initial listing at the time of our IPO until January 21, 2010. Subsequently, in anticipation of not curing our deficiencies with the continued listing requirements of the NASDAQ Global Market, we requested and were approved to transfer to the NASDAQ Capital Market, effective January 22, 2010.
As of February 28, 2018, there were approximately 63 holders of record of our common stock and 30,957,113 shares of common stock outstanding. No dividends have been paid on our common stock to date, and we do not anticipate paying any dividends in the foreseeable future.
The following table sets forth the high and low sales prices of our common stock as quoted on the NASDAQ Stock Market for the periods indicated. These prices have been adjusted to reflect the 1-for-70 reverse split of our common stock that was effected after trading on December 27, 2016 and the 1-for-15 reverse split of our common stock that was effected after trading on January 6, 2016.
Price Range of Common Stock
| | | | | | |
| | Price Range |
| | High | | Low |
Fiscal 2016 | | | | | | |
First Quarter | | $ | 157.50 | | $ | 57.40 |
Second Quarter | | $ | 86.80 | | $ | 18.90 |
Third Quarter | | $ | 31.50 | | $ | 7.70 |
Fourth Quarter | | $ | 9.80 | | $ | 1.95 |
Fiscal 2017 | | | | | | |
First Quarter | | $ | 30.41 | | $ | 1.75 |
Second Quarter | | $ | 6.48 | | $ | 4.00 |
Third Quarter | | $ | 5.20 | | $ | 1.60 |
Fourth Quarter | | $ | 2.60 | | $ | 1.23 |
The closing price for our common stock as reported by the NASDAQ Stock Market on March 29, 2018 was $1.45 per share.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by this Item regarding equity compensation plans is incorporated by reference to the information set forth in Part III, Item 12 of this Annual Report on Form 10-K.
Unregistered Sales of Equity Securities
Other than as previously reported in our Current Reports on Form 8-K filed on May 23, 2017 and October 3, 2017, as amended, during the period covered by this report, we did not sell any securities which were not registered under the Securities Act of 1933, as amended.
Uses of Proceeds from Sale of Registered Securities
None.
Dividend Policy
We have never paid cash dividends on our common stock. The board of directors presently intends to retain all earnings for use in our business and does not anticipate paying cash dividends in the foreseeable future. We do not have a dividend reinvestment plan or a direct stock purchase plan.
Issuer Purchases of Equity Securities
None.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Except for the historical information contained herein, the matters discussed in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere in this Form 10-K are forward-looking statements that involve risks and uncertainties. The factors listed in Item 1A “Risk Factors,” as well as any cautionary language in this Form 10-K, provide examples of risks, uncertainties and events that may cause our actual results to differ materially from those projected. Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this report.
Overview
We are a medical technology company focused on the design, development and commercialization of transformative technology to treat obesity and metabolic diseases. Our growing and differentiated product portfolio is utilized by bariatric surgeons, general surgeons, and gastroenterologists. We believe obesity is a global epidemic and that the majority of patients need treatment options that are anatomy friendly and provide for weight loss and comorbidity improvements along with long-term, ongoing obesity support and prevention. We were incorporated in Minnesota on December 19, 2002 as “EnteroMedics Inc.” and later reincorporated in Delaware on July 22, 2004. In October 2017 we changed our name to “ReShape Lifesciences Inc.”
On January 14, 2015, the vBloc® System, our initial product, which we now refer to as ReShape vBloc, received U.S. Food and Drug Administration (FDA) approval for vBloc Therapy, delivered via the ReShape vBloc, for the treatment of adult patients with obesity who have a Body Mass Index (BMI) of at least 40 to 45 kg/m2, or a BMI of at least 35 to 39.9 kg/m2 with a related health condition such as high blood pressure or high cholesterol levels, and who have tried to lose weight in a supervised weight management program and failed within the past five years vBloc Therapy is delivered via a pacemaker-like device that helps patients feel full and eat less by intermittently blocking hunger signals on the vagus nerve. Our therapy limits the expansion of the stomach, helps control hunger sensations between meals, reduces the frequency and intensity of stomach contractions and produces a feeling of early and prolonged fullness. In 2015 we began a controlled commercial launch of the ReShape vBloc at select surgical centers in the United States and had our first commercial sales. During 2015, we initiated a controlled expansion of our commercial operations and began building a sales force, which, in 2015 and 2016, called directly on key opinion leaders and bariatric surgeons at commercially-driven surgical centers that met our certification criteria. Additionally, beginning in 2016, through a distribution agreement with Academy Medical, LLC, U.S. Department of Veterans Affairs (VA) medical facilities began to offer the ReShape vBloc as a treatment option for veterans, at little to no cost to veterans in accordance with their veteran healthcare benefits. Our goal for the ReShape vBloc remains broad coverage and reimbursement for vBloc Therapy. We believe that the most significant barrier to adoption for patients who want vBloc Therapy has been cost and lack of payer coverage.
On May 22, 2017, we acquired the Gastric Vest System™, which we now refer to as the ReShape Vest, through our acquisition of BarioSurg. The ReShape Vest System is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in morbidly obese patients. The device wraps around the stomach, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy. The acquisition was completed under the terms of a merger agreement pursuant to which BarioSurg became a wholly-owned subsidiary of our company. The aggregate merger consideration we paid for all of the outstanding shares of capital stock and outstanding options of BarioSurg was: (i) 1.38 million shares of our common stock, (ii) 1.0 million shares of our newly created conditional convertible preferred stock, which shares converted into 5.0 million shares of our common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) $2 million in cash.
On October 2, 2017, we acquired ReShape Medical, Inc., a privately-held medical technology company that develops, manufactures and markets the ReShape® Integrated Dual Balloon, which now we refer to as the ReShape Balloon, an FDA-approved, minimally invasive intragastric balloon designed to treat obesity patients with a BMI between 30 and 40, with at least one related comorbidity. The aggregate merger consideration we paid for all of the outstanding shares of capital stock and securities convertible into shares of capital stock of ReShape Medical was: (i) approximately 2.4 million shares of our common stock, (ii) 187,772 shares of newly created series C convertible preferred stock, which shares became convertible into approximately 18.8 million shares of common stock upon the post-closing approval of our stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) approximately
$5 million in cash. The ReShape Balloon provides a new option for individuals who have not succeeded at diet and exercise alone, and do not want or do not qualify for bariatric surgery. Two connected balloons are placed into the stomach during a short, outpatient endoscopic procedure. The balloons remain in the stomach for six months and are then removed endoscopically. During balloon treatment, and for six more months following removal of the balloons, the patient receives access to nutritional counseling and access to exclusive tools to help them achieve their weight loss goals. The ReShape Balloon was approved by the FDA in July of 2015 and has had CE-marking in Europe since 2011.
We have a limited operating history and our ReShape vBloc and ReShape Balloon products only recently received U.S. Food and Drug Administration (FDA) approval to sell our product in the United States. Since our formation and until the 2017 acquisitions of BarioSurg and ReShape Medical, we have devoted substantially all of our resources to the development and commercialization of the vBloc System. With the addition of the ReShape Balloon product from ReShape Medical and if we are able to commercialize the ReShape Vest acquired from BarioSurg, we believe we will be able to offer three distinct approaches to treating obesity that may be selected by the physician, depending on the severity of the patient’s BMI or related co-morbidities. Together, we believe the ReShape Vest, ReShape vBloc and the ReShape Balloon provide a minimally-invasive continuum of care for bariatric patients and their providers.
The following financing transactions occurred in 2017 to fund the Company’s operations and acquisitions:
| · | | On January 23, 2017, we closed an underwritten public offering consisting of units of common stock, convertible preferred stock and warrants to purchase common stock. Gross proceeds of the offering were $19.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.5 million. |
| · | | During 2017, common stock warrants for 559,670 shares of common stock were exercised by warrant holders with proceeds to the Company of $3.3 million. |
| · | | On August 16, 2017, we closed an underwritten public offering consisting of units of Series B Convertible Preferred Stock and warrants to purchase common stock. Gross proceeds of the offering were $20.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.0 million. |
We currently are not generating revenue from operations that is significant relative to our level of operating expenses, and we do not anticipate generating revenue sufficient to offset operating costs in the short-term to mid-term. We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on investments. Our history of operating losses, limited cash resources and lack of certainty regarding obtaining significant third-party reimbursement for ReShape vBloc or timing thereof, raise substantial doubt about our ability to continue as a going concern absent a strengthening of our cash position. As of December 31, 2017, we had $10.2 million of cash and cash equivalents to fund our operations through early 2018. Our anticipated operations include plans to (i) integrate the sales and operations of our company with the newly acquired ReShape Medical in order to expand sales of both the ReShape Balloon and ReShape vBloc as well as to obtain cost savings synergies, (ii) expand the controlled commercial launch of ReShape vBloc Therapy, delivered via ReShape vBloc, (iii) continue development of the ReShape Vest, (vi) seek opportunities to leverage our intellectual property portfolio and custom development services to provide third party sales and licensing opportunities, and (v) explore and capitalize on synergistic opportunities to expand our portfolio and offer future minimally invasive treatments and therapies in the obesity continuum of care. We believe that we have the flexibility to manage the growth of our expenditures and operations depending on the amount of available cash flows, which could include reducing expenditures for marketing, clinical and product development activities. However, we will ultimately need to achieve sufficient revenues from product sales and obtain additional debt or equity financing in addition to the proceeds from this offering to support our operations.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported expenses during the reporting periods. We evaluate our
estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements included in Item 8 of this Annual Report on Form 10-K, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, title or risk of loss has passed, the selling price is fixed or determinable and collection is reasonably assured. Products are sold through direct sales or medical device distributors. ReShape vBloc revenue is recognized upon sale to a bariatric center or a medical device distributor when no right of return or price protection exists. ReShape Balloon revenue is typically recognized when title is passed, usually at point of shipment. A provision for returns reducing revenues is recorded only if product sales provide for a right of return. No provision for returns was recorded for the years ended December 31, 2015 and December 31, 2016, as vBloc product sales recorded did not provide for rights of return. A provision for returns of $39,000 was recorded in the fourth quarter of 2017 to estimate potential returns of the ReShape Balloon product.
Revenues for services are recognized when earned, subject to limitations, if any, related to multiple deliverables. We evaluate each element in these multiple-element arrangements to determine whether they represent a separate unit of accounting and recognize each element as the services are performed or as product is shipped.
Inventory
We account for inventory at the lower of cost or market and record any long-term inventory as other assets in the consolidated balance sheets. We establish inventory reserves for obsolescence based upon projected sales and for defect based upon specific identification of defective or unsalable units. As of December 31, 2017 and 2016, respectively, there was $1.0 million and 676,000 of long-term inventory included as other assets.
Goodwill and Other Intangible Assets
We evaluate long-lived assets, including finite-lived intangible assets, for impairment by comparison of the carrying amounts to future net undiscounted cash flows expected to be generated by such assets when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Should an impairment exist, the impairment loss would be measured based on the excess carrying value of the asset over the asset’s fair value or estimates of future discounted cash flows.
For goodwill and indefinite-lived intangible assets, in-process research and development, we review for impairment annually and upon the occurrence of certain events as required by Accounting Standards Codification (“ASC”) Topic 350, “Intangibles — Goodwill and Other.” Goodwill and indefinite-lived intangible assets are tested at least annually for impairment and more frequently if events or changes in circumstances indicate that the asset might be impaired. We review goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. If we are able to determine that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, we would conclude that goodwill is not impaired. If the carrying amount of a reporting unit is zero or negative, the second step of the impairment test is performed to measure the amount of impairment loss, if any, when it is more likely than not that a goodwill impairment exists. We did not record any losses for impairment during the year ended December 31, 2017.
Stock-Based Compensation
We account for share-based payments using the fair value method, which requires compensation expense to be recognized using a fair-value-based method for costs related to all share-based payments including stock options. Companies are required to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. Calculating stock-based compensation expense requires the input of highly subjective assumptions, which represent our best estimates and involve inherent uncertainties and the application of management’s judgment. Estimates of stock-based compensation expenses are significant to our consolidated financial statements, but these expenses are
based on the Black-Scholes pricing model and will never result in the payment of cash by us. All option grants are expensed on a straight-line basis over the vesting period.
The application of share-based payment principles may be subject to further interpretation and refinement over time. There are significant differences among option valuation models, and this may result in a lack of comparability with other companies that use different models, methods and assumptions. If factors change and we employ different assumptions in the application of share-based payment accounting in future periods, or if we decide to use a different valuation model, the compensation expense that we record in the future may differ significantly from what we have recorded in the current period and could materially affect our operating loss, net loss and net loss per share.
The fair value method is applied to all share-based payment awards issued to employees and where appropriate, nonemployees, unless another source of literature applies.
Net Operating Losses and Tax Credit Carryforwards
At December 31, 2017, we had federal gross net operating loss carryforwards of approximately $253.8 million. These net operating loss carryforwards will expire in varying amounts from 2022 through 2037, if not utilized. The Internal Revenue Code (IRC) imposes restrictions on the utilization of various carryforward tax attributes in the event of a change in ownership of the Company, as defined by IRC Section 382. In addition, IRC Section 382 may limit our tax credits and our built-in items of deduction, including capitalized start-up costs and research and development costs. During 2011, we completed an IRC Section 382 review and the results of this review indicate ownership changes have occurred which would cause a limitation on the utilization of carryforward attributes. Our gross net operating loss carryforwards, start-up costs and research and development credits are all subject to limitation. Under these tax provisions, the limitation is applied first to any built-in losses, then to any net operating losses and then to any general business credits. It is likely that ownership changes have occurred since we completed our IRC Section 382 review in 2011 and could result in further limitations on the utilization of carryforward attributes. A valuation allowance has been established to reserve for the potential benefits of the remaining carryforwards and tax credits in our consolidated financial statements to reflect the uncertainty of future taxable income required to utilize available tax loss carryforwards and other deferred tax assets. We have applied the valuation allowance to our net deferred tax assets, however, an extreme limitation under Section 382 could result in impact to our effective tax rate due to the presence of deferred tax liabilities from recent acquisitions.
Financial Overview
Revenue
We received FDA approval on January 14, 2015 for vBloc Therapy. In 2015 we began a controlled commercial launch at select surgical centers in the United States and had our first commercial sales. During 2015, we initiated a controlled expansion of our commercial operations and started the process of building a sales force. Our direct sales force is supported by field clinical engineers who provide training, technical and other support services to our customers. Throughout 2016, our sales force called directly on key opinion leaders and bariatric surgeons at commercially-driven surgical centers that met our certification criteria. Additionally, in 2016, through a distribution agreement with Academy Medical, VA medical facilities began offering ReShape vBloc as a treatment option to veterans using their veteran healthcare benefits. With the acquisition of ReShape Medical and the ReShape Balloon in October 2017, we began the process of integrating our sales forces and offering both ReShape vBloc and the ReShape Balloon to bariatric surgeons, general surgeons and gastroenterologists. In 2017, we also began offering the ReShape Balloon product to customers in certain Middle East countries via third party distribution agreements. Also in 2017, we provided certain custom development services based on our intellectual property portfolio. In 2018 we also intend to build on our previous efforts with self-pay and veteran patient focused direct-to-patient marketing, key opinion leader and center specific partnering, and a multi-faceted reimbursement strategy and to continue to pursue ReShape Balloon sales opportunities, particularly in the Middle East and Canada.
In 2015, our first year of commercial activity, we sold 24 ReShape vBloc units for $292,000 in revenue and in 2016, we sold 62 ReShape vBloc units for $787,000 in revenue. In 2017, our total revenues were $1.3 million, $718,000 from 2017 fourth quarter revenue from ReShape Balloon sales resulting from the acquisition of ReShape Medical, $250,000 from service revenue and $319,000 from the sale of 29 ReShape vBloc units.
Both ReShape vBloc and the ReShape Balloon remain relatively new products in the United States and internationally and it is difficult to predict the amount of revenue they, or the investigational-stage ReShape Vest, will generate going forward. In any event, such revenue will only modestly reduce our continued losses resulting from our research and development and other activities.
Service and Other Revenue
Service revenue is comprised of custom development services provided to third parties based on our intellectual property portfolio. Other revenue includes amounts billed to customers for shipping.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses consist primarily of compensation for executive, finance, market development and administrative personnel, including stock-based compensation. Other significant expenses include costs associated with attending medical conferences and trade shows, professional fees for legal services, including legal services associated with our efforts to obtain and maintain broad protection for the intellectual property related to our products, reimbursement development, including costs associated with the vBloc Now program, and accounting services, cash management fees, consulting fees and travel expenses.
Research and Development Expenses
Our research and development expenses primarily consist of engineering, product development, quality assurance and clinical and regulatory expenses, incurred in the development of our three products, ReShape vBloc, ReShape Vest and the ReShape Balloon. Research and development expenses also include employee compensation, including stock-based compensation, consulting services, outside services, materials, clinical trial expenses, including supplies, devices, explants and revisions, depreciation and travel. We expense research and development costs as they are incurred.
Results of Operations
Comparison of the Years Ended December 31, 2017 and 2016
Product Sales Revenue. Revenues from product sales totaled $1.0 million for the year end December 31, 2017 compared with $787,000 for the year ended December 31, 2016. The increase was driven by $692,000 fourth quarter 2017 ReShape Balloon product sales subsequent to the October 2, 2017 acquisition of ReShape Medical. The increase due to ReShape Balloon product sales was partially offset by a $467,000 decline in sales of ReShape vBloc. During 2017, 28 units of ReShape vBloc product were sold compared with 62 during 2016. The decline in ReShape vBloc sales was driven primarily by the Company’s 2017 focus on the vBloc Now program, which provides qualified patients battling obesity the opportunity to receive vBloc Therapy, including the device, procedure, and follow up program at an affordable price in exchange for sharing detailed health data with the Company. The Company intends to use the data to enhance its case with third-party payers that vBloc Reshape can provide a clinically meaningful level of weight loss while also providing a positive impact on diabetes and other comorbidities in certain patients.
Service and Other Revenue. During the 2017 third quarter, the Company provided certain custom development services totaling $250,000 based on its intellectual property portfolio that had been requested and contracted for by a third party. Other revenue includes amounts billed customers for shipping ReShape Balloon product.
Cost of Goods Sold. Cost of goods sold was $765,000 for the year ended December 31, 2017 compared to $431,000 for the year ended December 31, 2016. The $334,000 increase was primarily driven by fourth quarter sales of the ReShape Balloon product. Gross margin for product sales was 24.4% for 2017 compared with 45.2% for 2016. The decrease in gross margin percentage reflects fourth quarter 2017 sales of lower margin ReShape Balloon product compared with ReShape vBloc product.
Cost of Service and Other Revenue. Cost of service and other revenue reflects the cost of custom development services provided by the Company as well as the cost of shipping revenues.
Selling, General and Administrative Expenses. Selling, general and administrative expenses were $26.0 million for the year ended December 31, 2017, compared to $18.0 million for the year ended December 31, 2016. The increase
of $8.0 million was primarily driven by $4.0 million of fourth quarter operating 2017 expenses from ReShape Medical, which included approximately $645,000 of non-cash intangible amortization expenses, $1.1 million of acquisition and integration expenses related to the 2017 acquisitions of BarioSurg and ReShape Medical, $2.1 million of increased stock compensation expenses, $812,000 of expenses related to the 68 ReShape vBloc units implanted in conjunction with the vBloc Now program, $584,000 increase in severance costs driven by the fourth quarter 2017 reduction in executive and administration positions after the ReShape Medical acquisition and a $480,000 increase in other professional fees. These increases were partially offset by a $676,000 decrease in advertising and marketing expenses and $552,000 of other payroll related expenses.
Research and Development Expenses. Research and development expenses were $5.8 million for the year ended December 31, 2017 compared with $5.2 million for the year ended December 31, 2016, an increase of 11.7 percent. The increase of $606,000 was driven by $832,000 of expenses related to research and development of the ReShape Vest, acquired with the acquisition of BarioSurg on May 22, 2017, and approximately $283,000 of fourth quarter 2017 research and development expenses from ReShape Medical, which was acquired October 2, 2017. These increases were partially offset by decreases of approximately $509,000 due to reduced payroll-related and supply expenses, primarily related to the ReShape vBloc product.
Interest Expense. Interest expense was $4,000 for the year ended December 31, 2017 compared with $4.1 million for the year ended December 31, 2016. The decrease of $4.1 million from 2016 was driven by the absence of any remaining balances of convertible notes, which were all paid off by December 31, 2016.
Change in Value of Warrant Liability. The change in the value of the common stock warrant liability for our Series A Warrants and Note Warrants increased $283,000 during the year ended December 31, 2017, primarily the result of the marking to market of the Series A Warrants and the Note Warrants for 48,272 common shares as of the date of their exercise. The 2017 exercises of these warrants occurred during the first two quarters of 2017, generally at points in time when the Company’s stock price had increased from $2.00 at December 31, 2016 to levels higher than the exercise price of the warrants. The value of the common stock warrant liability for our Series A and Note Warrants decreased $3.5 million during the year ended December 31, 2016. The fair market value of the warrant liability is calculated using the Black-Scholes valuation model, and was primarily driven by the reduction in the Company’s stock price during 2016, from $136.50 at December 31, 2015 to $2.00 at December 31, 2016.
Comparison of the Years Ended December 31, 2016 and 2015
Sales. Sales were $787,000 for the year ended December 31, 2016 compared with $292,000 for the year ended December 31, 2016. The increase of $495,000 is the result of the continued controlled commercial launch of ReShape vBloc at select surgical centers in the United States which resulted in sales of 62 units during 2016 versus 24 units during 2015. We received FDA approval to sell ReShape vBloc on January 14, 2015.
Cost of Goods Sold. Cost of goods sold were $431,000 for the year ended December 31, 2016 compared to $125,000 for the year ended December 31, 2015. The increase was driven primarily by the 158% increase in the number of units sold in 2016 over 2015. Gross margin percentage for 2016 was 45.2% versus 57.2% for the prior year. The decline in gross margin percentage was primarily due to higher supply chain costs in 2016 than in 2015.
Selling, General and Administrative Expenses. Selling, general and administrative expenses were $18.0 million for the year ended December 31, 2016, compared to $19.9 million for the year ended December 31, 2015. The decrease of $1.9 million, or 9.6%, was primarily due to a $3.4 million decrease in payroll-related expenses, partially offset by a $1.1 million increase in professional services expenses and a $366,000 increase in severance expenses. The increase in professional services expenses were the result of increasing commercialization efforts as we continued the controlled commercial launch of ReShape vBloc at select surgical centers in the United States.
Research and Development Expenses. Research and development expenses were $5.2 million for the year ended December 31, 2016, compared to $8.1 million for the year ended December 31, 2015. The decrease of $3.0 million or 36.5%, was primarily due to decreases of $2.0 million, $436,000 and $119,000 in payroll-related expenses, supply expenses and professional services expenses, respectively. The decreases are the result of a continued shift away from a research and development focus toward commercialization of ReShape vBloc.
Interest Expense. Interest expense was $4.1 million for the year ended December 31, 2016, compared to $939,000 for the year ended December 31, 2015. The increase of $3.2 million was driven by interest costs from the three Note closings that occurred on November 9, 2015, January 11, 2016 and May 2, 2016, and increased interest costs due to conversions of remaining amounts due under the Notes into common shares by holders of the Notes during the year ended December 31, 2016, and, as a result, accelerations of “make whole” interest amounts due under the Notes. Additionally, $277,000 in debt issuance costs were expensed during the quarter ended June 30, 2016.
Change in Value of Warrant Liability. The value of the common stock warrant liability increased $217,000 for the year ended December 31, 2016, compared to the year ended December 31, 2015. Common stock warrant liabilities were recorded during the year ended December 31, 2015 for the Series A Warrants on July 8, 2015 and for the Note Warrants issued on November 9, 2015. In addition, Note Warrants were issued on January 11, 2016 and May 2, 2016. The decline in the value of the warrants was driven by the decrease in the Company’s stock price, which declined throughout 2016, from $136.50 per share on December 31, 2015 to $2.00 on December 31, 2016.
Warrants Expense. In August, 2017, we issued warrants to former holders of the senior amortizing convertible notes issued in November, 2015 as consideration for the waiver by each of the former note holders of their right to participate in future securities offerings by the Company, which resulted in $4.4 million of warrants expense for the year ended December 31, 2017.
Income tax benefit. The revaluation of our deferred income taxes upon enactment of the Tax Cuts and Jobs Act on December 22, 2017 resulted in $2.3 million of income tax benefit for the year ended December 31, 2017.
Liquidity and Capital Resources
As of December 31, 2017, we had $10.2 million in cash bank deposits. While we had no short-term money market funds or other investments at December 31, 2017, we periodically invest in short-term money market funds that are not considered to be bank deposits and are not insured or guaranteed by the Federal Deposit Insurance Company or other government agency. These money market funds seek to preserve the value of the investment at $1.00 per share; however, it is possible to lose money investing in these funds. Periodically, we invest cash in excess of immediate requirements in accordance with our investment policy, primarily with a view to liquidity and capital preservation. At times, such deposits may be in excess of insured limits. We have not experienced any losses on our deposits of cash and cash equivalents.
We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on investments. As of December 31, 2017, we had $10.2 million of cash and cash equivalents to fund operations into early 2018. The following financing transactions occurred in 2015, 2016, 2017 and early 2018 to fund the Company’s operations:
April 2018 (see Note 19, “Subsequent Events,” to the Consolidated Financial Statements on Form 10-K for the Year Ended December 31, 2017)
| · | | On April 2, 2018 the Company announced that it had entered into a securities purchase agreement with an institutional investor providing for the purchase and sale in a registered direct offering of shares of series D convertible preferred stock and a warrant to purchase shares of common stock for a purchase price of $6.0 million. The transaction is expected to close on or about April 4, 2018 and the Company expects to receive net proceeds of approximately $5.25 million after deducting placement agent fees and other offering expenses. |
2015, 2016 and 2017
| · | | On July 8, 2015, we closed a public offering of units consisting of common stock and the Series A Warrants. Gross proceeds of the offering were $16.0 million, prior to deducting offering expenses of approximately $1.4 million |
| · | | On November 4, 2015 we entered into a securities purchase agreement (the Purchase Agreement) with institutional investors to issue up to $25.0 million of senior amortizing convertible notes (the Notes) and Note |
Warrants, in three separate closings. $1.5 million of the Notes was funded at the first closing on November 9, 2015 (the First Closing). |
| · | | An additional $11.0 million of the Notes was funded at the second closing on January 11, 2016 (the Second Closing). |
| · | | An additional $6.25 million of the Notes was funded at the third closing on May 2, 2016 (the Third Closing). |
| · | | On January 23, 2017, we closed an underwritten public offering consisting of units of common stock, convertible preferred stock and warrants to purchase common stock. Gross proceeds of the offering were $19.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.5 million. |
| · | | During 2017, common stock warrants for 559,670 shares of common stock were exercised by warrant holders with proceeds to the Company of $3.3 million. |
| · | | On August 16, 2017, we closed an underwritten public offering consisting of units of Series B Convertible Preferred Stock and warrants to purchase common stock. Gross proceeds of the offering were $20.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.0 million. |
Our anticipated operations include plans to (i) integrate the sales and operations of the Company with the newly acquired ReShape Medical in order to expand sales of both the ReShape Balloon and ReShape vBloc as well as to obtain cost savings synergies, (ii) expand the controlled commercial launch of vBloc Therapy, delivered via the ReShape vBloc System, (iii) continue development of the ReShapec Vest, (vi) seek opportunities to leverage our intellectual property portfolio and custom development services to provide third party sales and licensing opportunities, and (v) explore and capitalize on synergistic opportunities to expand our portfolio and offer future minimally invasive treatments and therapies in the obesity continuum of care. The Company believes that it has the flexibility to manage the growth of its expenditures and operations depending on the amount of available cash flows, which could include reducing expenditures for marketing, clinical and product development activities. However, we will ultimately need to achieve sufficient revenues from product sales and obtain additional debt or equity financing to support its operations.
Management is currently pursuing various funding options, including seeking additional equity or debt financing approximately one or two times per year as well as a strategic merger or other transaction to obtain additional funding or expand its product line to continue the development of, and to successfully commercialize, the ReShape Balloon, the ReShape vBloc System and the ReShape Vest. While the acquisition of ReShape Medical does provide incremental revenues to the Company, the cost to further develop and commercialize the ReShape Balloon is expected to significantly exceed revenues for the foreseeable future. While there can be no assurance that the Company will be successful in its efforts, the Company has a long history of raising equity financing to fund its development activities. Should the Company be unable to obtain adequate financing in the near term, the Company’s business, result of operations, liquidity and financial condition would be materially and negatively affected, and the Company would be unable to continue as a going concern. Additionally, there can be no assurance that, assuming the Company is able to strengthen its cash position, it will achieve sufficient revenue or profitable operations to continue as a going concern.
Sales Agreement—July 2015
On July 8, 2015, we closed a public offering, where we sold 30,476 units at an aggregate price of $525.00 per unit, for gross proceeds of $16.0 million, before deducting estimated offering expenses of approximately $1.4 million, of which $532,000 was assigned to the warrants issued with each unit sold. Each unit consisted of: (A)(i) one share of common stock or (ii) one pre-funded Series C warrant to purchase one share of common stock at an exercise price equal to $525.00 per share (Series C Warrant); and (B) one Series A warrant to purchase one share of common stock at an exercise price equal initially to $630.00 per share (Series A Warrant). Each purchaser of a unit could elect to receive a Series C Warrant in lieu of a share of common stock. No Series C Warrants were issued.
The Series A Warrants are exercisable for a period of 42 months from the closing date of the public offering. The exercise price and number of shares of common stock issuable on the exercise of the Series A Warrants are subject to adjustment upon the issuance of any shares of common stock or securities convertible into shares of common stock
below the then-existing exercise price, with certain exceptions, and in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction. The holder of the Series A Warrant does not have the right to exercise any portion of the Series A Warrant if the holder, together with its affiliates, would, subject to certain limited exceptions, beneficially own in excess of 9.99% of our common stock outstanding immediately after the exercise or 4.99% as may be elected by the purchaser.
The exercise price of the Series A Warrants was reduced to $168.00 per share on November 9, 2015 as a result of the issuance of the Notes and was further reduced to $67.90 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Series A Warrants and was further reduced at various times during the year ended December 31, 2016 as a result of installment and acceleration payments made on the Notes. As of December 31, 2016, the exercise price of the Series A Warrants was $2.80 per share and on January 20, 2017, the 16th trading day following the Second Reverse Stock Split, the exercise price of the Series A Warrants was adjusted to $2.18 per share, per the terms of the Series A Warrants.
Senior Amortizing Convertible Notes
On November 4, 2015, we entered into the Purchase Agreement to issue and sell to four institutional investors 7% senior amortizing convertible notes due 2017 in three separate closings. The Notes were initially convertible into shares of our common stock at a price equal to $304.50 per share with an aggregate principal amount of $25.0 million. Each Note was sold with a Note Warrant with an exercise price of $325.50 per share. We issued and sold Notes and Note Warrants for aggregate total proceeds of $12.5 million in the First Closing and Second Closing. Subsequent to the Second Closing, we entered into the First Amendment, which provided that the scheduled third closing would be divided into two separate closings, issued and sold Notes and Note Warrants for aggregate total proceeds of $6.25 million in the Third Closing. After the Third Closing, we entered into the Second Amendment, which set a deadline of December 30, 2016 for the final closing and provided the consent of the holders of the Notes to we reduce the conversion price of the Notes from time to time in order to incentivize the holders of the Notes to convert their Notes into shares of our common stock. As the final closing did not occur prior to the December 30, 2016 deadline, the remaining $6.25 million of Notes was not funded. Additionally, after entering into the Second Amendment, we reduced the conversion price of the Notes frequently in order to incentivize the holders of the Notes to convert all of the outstanding amounts outstanding under the Notes. As of December 31, 2016, all of the Notes were fully repaid.
During the year ended December 31, 2016, $18.7 million of aggregate principal amount of Notes were converted by holders of the Notes into approximately 2,632,000 shares of the Company’s common stock.
Description of the Notes
The Notes were payable in monthly installments, accrued interest at a rate of 7.0% per annum from the date of issuance and had a maturity date 24 months after the First Closing. The Notes were repayable, at the Company’s election, in either cash or shares of our common stock at a discount to the then-current market price. The Notes were also convertible from time to time, at the election of the holders, into shares of our common stock at an initial conversion price of $304.50 per share. The conversion price was adjusted to $76.30 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Notes. The Notes also allowed us to reduce the conversion price from time-to-time, upon the holders’ consent, which was provided in the Second Amendment.
The holder of each Note has the right to convert any portion of such Note unless the holder, together with its affiliates, beneficially owned in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to the conversion, as such percentage ownership was determined in accordance with the terms of the Notes. The holders were also able to increase or decrease such percentage to any other percentage, but in no event above 9.99%, provided that any increase of such percentage would not be effective until 61 days after providing us notice.
The First Closing occurred on November 9, 2015. At the First Closing, we issued and sold Notes with an aggregate principal amount of $1.5 million, along with Note Warrants exercisable for 1,679 shares. During the quarter ended September 30, 2016, all remaining principal and interest amounts outstanding under the Notes issued at the First Closing were paid off via conversions to common shares.
The Second Closing occurred on January 11, 2016 after we received approval of the offering by the Company’s stockholders and the satisfaction of certain customary closing conditions. At the Second Closing, we issued and sold
Notes with an aggregate principal amount of $11.0 million, along with Note Warrants exercisable for 12,312 shares. The fair value of Note Warrants issued on January 11, 2016 was determined to be $515,000 using a Black-Scholes valuation model and the following assumptions: (1) dividend yield of 0%; (2) expected volatility of 85.90%; (3) weighted average risk –free interest rate of 1.58%; and (4) expected life of 5.0 years. During the year and quarter ended December 31, 2016, all remaining principal and interest amounts outstanding under Notes issued at the Second Closing were paid off via conversions to common shares.
The Third Closing occurred on May 2, 2016 after we entered into the First Amendment and satisfied certain closing conditions. At the Third Closing, we issued and sold Notes with an aggregate principal amount of $6.25 million, along with Note Warrants exercisable for 6,995 shares. The fair value of the Note Warrants issued on May 2, 2016 was determined to be $150,195 using a Black-Scholes valuation model and the following assumptions: (1) dividend yield of 0%; (2) expected volatility of 89.28%; (3) weighted average risk –free interest rate of 1.32%; and (4) expected life of 5.0 years. During the quarter and year ended December 31, 2016, all remaining principal and interest amounts outstanding under Notes issued at the Third Closing were paid off via conversions to common shares.
The following table summarizes the installment amounts and additional conversions by the holders of the Notes through December 31, 2016:
First Closing:
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | Interest | | Total | | Shares | |
Installment amount at December 31, 2015 | | $ | 65,217 | | $ | 23,651 | | $ | 88,868 | | 814 | |
Holder conversions during the quarter ended December 31, 2015 | | | 18,261 | | | 2,375 | | | 20,636 | | 189 | |
Total installments and conversions, December 31, 2015 | | | 83,478 | | | 26,026 | | | 109,504 | | 1,003 | |
Installment amount at February 29, 2016 | | | 65,217 | | | 23,681 | | | 88,898 | | 1,314 | |
Installment amount at March 31, 2016 | | | 65,217 | | | 14,827 | | | 80,044 | | 1,271 | |
Holder conversions during the quarter ended March 31, 2016 | | | 104,784 | | | 12,762 | | | 117,546 | | 1,524 | |
Total installments and conversions, March 31, 2016 | | | 318,696 | | | 77,296 | | | 395,992 | | 5,112 | |
Installment amount at April 30, 2016 | | | 65,217 | | | 13,853 | | | 79,070 | | 1,454 | |
Installment amount at May 31, 2016 | | | 65,217 | | | 13,082 | | | 78,299 | | 2,121 | |
Installment amount at June 30, 2016 | | | 54,217 | | | 11,275 | | | 65,492 | | 3,590 | |
Holder conversions during the quarter ended June 30, 2016 | | | 1,627 | | | 174 | | | 1,801 | | 29 | |
Total installments and conversions, June 30, 2016 | | | 504,974 | | | 115,680 | | | 620,654 | | 12,306 | |
Installment amount at July 31, 2016 | | | 65,217 | | | 10,148 | | | 75,365 | | 5,521 | |
Installment amount at August 31, 2016 | | | 46,957 | | | 5,830 | | | 52,787 | | 4,593 | |
Holder conversions during the quarter ended September 30, 2016 | | | 882,852 | | | 78,634 | | | 961,486 | | 72,528 | |
Total installments and conversions, September 30, 2016 and December 31, 2016 | | $ | 1,500,000 | | $ | 210,292 | | $ | 1,710,292 | | 94,948 | |
Second Closing:
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | | Interest | | | Total | | Shares | |
| | | | | | | | | | | | |
Installment amount at March 2, 2016 | | $ | 404,762 | | $ | 149,300 | | $ | 554,062 | | * | |
Holder conversions during the quarter ended March 31, 2016 | | | 987,000 | | | 124,050 | | | 1,111,050 | | 14,974 | |
Total installments and conversions, March 31, 2016 | | | 1,391,762 | | | 273,350 | | | 1,665,112 | | 14,974 | |
Installment amount at April 29, 2016 | | | 404,762 | | | 149,497 | | | 554,259 | | 10,190 | |
Installment amount at May 31, 2016 | | | 291,428 | | | 86,518 | | | 377,946 | | 10,238 | |
Installment amount at June 30, 2016 | | | 404,762 | | | 82,913 | | | 487,675 | | 22,842 | |
Holder conversions during the quarter ended June 30, 2016 | | | 25,373 | | | 2,995 | | | 28,368 | | 414 | |
Total installments and conversions, June 30, 2016 | | | 2,518,087 | | | 595,273 | | | 3,113,360 | | 58,658 | |
Installment amount at July 31, 2016 | | | 213,429 | | | 47,457 | | | 260,886 | | 19,113 | |
Installment amount at August 31, 2016 | | | 631,429 | | | 116,511 | | | 747,940 | | 64,810 | |
Installment amount at September 30, 2016 | | | 404,762 | | | 45,846 | | | 450,608 | | 51,698 | |
Holder conversions during the quarter ended September 30, 2016 | | | 4,868,679 | | | 418,847 | | | 5,287,526 | | 418,253 | |
Total installments and conversions, September 30, 2016 | | | 8,636,386 | | | 1,223,934 | | | 9,860,320 | | 612,532 | |
Installment amount at Oct 31, 2016 | | | 340,000 | | | 24,738 | | | 364,738 | | 70,665 | |
Installment amount at Nov 30, 2016 | | | 291,429 | | | 27,528 | | | 318,957 | | 81,952 | |
Installment amount at December 31, 2016 | | | 156,867 | | | 11,425 | | | 168,292 | | 57,453 | |
Holder conversions during the quarter ended December 31, 2016 | | | 1,575,318 | | | 122,624 | | | 1,697,942 | | 450,385 | |
Total installments and conversions, December 31, 2016 | | $ | 11,000,000 | | $ | 1,410,249 | | $ | 12,410,249 | | 1,272,987 | |
Third Closing:
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | Interest | | Total | | Shares | |
| | | | | | | | | | | | |
Installment amount at June 30, 2016 | | $ | 212,158 | | $ | 90,659 | | $ | 302,817 | | 16,600 | |
Holder conversions during the quarter ended June 30, 2016 | | | — | | | — | | | — | | — | |
Total installments and conversions, June 30, 2016 | | | 212,158 | | | 90,659 | | | 302,817 | | 16,600 | |
Installment amount at July 31, 2016 | | | 147,368 | | | 32,374 | | | 179,742 | | 13,168 | |
Cash Payment – July 31, 2016 installment | | | 42,105 | | | 6,107 | | | 48,212 | | * | |
Installment amount at August 31, 2016 | | | 336,842 | | | 62,059 | | | 398,901 | | 34,684 | |
Installment amount at September, 2016 | | | 263,158 | | | 41,822 | | | 304,980 | | 34,523 | |
Holder conversions during the quarter ended September 30, 2016 | | | 1,915,698 | | | 175,092 | | | 2,090,790 | | 155,272 | |
Total installments and conversions, September 30, 2016 | | | 2,917,329 | | | 408,113 | | | 3,325,442 | | 254,247 | |
Installment amount at Oct 31, 2016 | | | 221,053 | | | 35,004 | | | 256,057 | | 48,192 | |
Installment amount at Nov 30, 2016 | | | 221,053 | | | 31,259 | | | 252,312 | | 64,828 | |
Installment amount at December 31, 2016 | | | 221,053 | | | 14,526 | | | 235,579 | | 81,872 | |
Holder conversions during the quarter ended December 31, 2016 | | | 2,669,512 | | | 170,359 | | | 2,839,871 | | 816,707 | |
Total installments and conversions, December 31, 2016 | | $ | 6,250,000 | | $ | 659,261 | | $ | 6,909,261 | | 1,265,846 | |
* Cash payments
Description of the Note Warrants
Each Note Warrant was exercisable immediately and for a period of 60 months from the date of the issuance of the Warrant. The Note Warrants entitled the holders of the Note Warrants to purchase, in aggregate, 27,982 shares of our common stock upon the completion of the Third Closing, subject to certain adjustments. The Note Warrants were
initially exercisable at an exercise price equal to $325.50, subject to adjustment on the eighteen month anniversary of issuance, and certain other adjustments. The exercise price and number of shares of common stock issuable on the exercise of the Note Warrants were subject to adjustment upon the issuance of any shares of common stock or securities convertible into shares of common stock below the then-existing exercise price, with certain exceptions, and in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction. The holder of each Note Warrant did not have the right to exercise any portion of such Note Warrant if the holder, together with its affiliates, beneficially owns in excess of 4.99% of the number of shares of the Company’s common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Note Warrants. However, any holder could increase or decrease such percentage to any other percentage, but in no event above 9.99%, provided that any increase of such percentage would not be effective until 61 days after providing us notice.
The exercise price of the Note Warrants issued November 9, 2015 was reduced to $76.30 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Note Warrants. Per the terms of the Note Warrants, the exercise price of each of the Note Warrants issued January 11, 2016 and May 2, 2016 remained $325.50 until January 20, 2017, the 16th trading day following the Second Reverse Stock Split, at which point the exercise price of all of the Note Warrants was adjusted to $2.18 per share. All remaining Note Warrants were exercised during the first quarter of 2017.
Net Cash Used in Operating Activities
Net cash used in operating activities was $24.6 million, $20.7 million and $22.6 million for the years ended December 31, 2017, 2016 and 2015, respectively. Net cash used in operating activities primarily reflects the net loss for those periods, less noncash expenses for stock-based compensation, depreciation and amortization, provision for doubtful accounts, change in value of warrant liability, and partially offset by changes in operating assets and liabilities. The increase in net cash used in operations is primarily due to incremental cash needed for the operations of BarioSurg and ReShape Medical.
Net Cash Used in Investing Activities
Net cash used in investing activities was $6.4 million, $14,000 and $39,000 for the years ended December 31, 2017, 2016 and 2015, respectively. In 2017, $6.2 million of cash, net of cash acquired, was used as part of the consideration paid to acquire BarioSurg and ReShape Medical. Additionally, net cash used in investing activities is primarily related to the purchase of property and equipment.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $37.8 million, $16.1 million and $19.0 million for the years ended December 31, 2017, 2016 and 2015, respectively. For the year ended December 31, 2017, net cash provided by financing activities was primarily the result of approximately $37.4 million of gross proceeds of from the issuance of common stock, convertible preferred stock and warrants in January and August of 2017, less issuance costs of approximately $2.9 million along with $3.3 million of proceeds from warrants exercised.
For the year ended December 31, 2016, net cash provided by financing activities was primarily the result of gross proceeds from the Second Closing and Third Closing of the Notes, which totaled $17.3 million, less cash principal payments on Notes of $447,000 and debt issuance costs of $750,000.
For the year ended December 31, 2015, net cash provided by financing activities was primarily the result of gross proceeds of $16.0 million from the July 8, 2015 public offering, $6.7 million from issuances of common stock and warrants and $1.5 million in gross proceeds from the issuance of the Notes on November 9, 2015. These increases were offset by $1.7 million in financing costs, $477,000 of debt issuance costs and principal repayments of $3.0 million on our long-term debt.
Operating Capital and Capital Expenditure Requirements
We received FDA approval on January 14, 2015 for vBloc Therapy, delivered via the vBloc System, and began a controlled commercial launch at select bariatric centers of excellence in the United States. In 2015, our first year of
commercial activity, we sold 24 ReShape vBloc units for $292,000 in revenue and in 2016, we sold 62 ReShape vBloc units for $787,000 in revenue. In 2017, our total revenues were $1.3 million, $718,000 from 2017 fourth quarter revenue resulting from the acquisition of ReShape Medical, $250,000 from service revenue and $319,000 from the sale of 28 ReShape vBloc units. We have incurred and expect to continue to incur significant sales, marketing, clinical, and R&D expenses prior to recording sufficient revenue to offset these expenses. Additionally, our selling, general and administrative expenses have continued, as we build the infrastructure necessary to support our expanding commercial sales, operate as a public company and develop our intellectual property portfolio. For these reasons, we expect to continue to incur operating losses for the next several years. We have financed our operations to date principally through the sale of equity securities, debt financing and interest earned on cash investments.
Our anticipated operations include plans to (i) integrate the sales and operations of the Company with the newly acquired ReShape Medical in order to expand sales of both the ReShape Balloon and ReShape vBloc as well as to obtain cost savings synergies, (ii) expand the controlled commercial launch of vBloc Therapy, delivered via the ReShape vBloc System, (iii) continue development of the ReShape Vest, (vi) seek opportunities to leverage our intellectual property portfolio and custom development services to provide third party sales and licensing opportunities, and (v) explore and capitalize on synergistic opportunities to expand our portfolio and offer future minimally invasive treatments and therapies in the obesity continuum of care. The Company believes that it has the flexibility to manage the growth of its expenditures and operations depending on the amount of available cash flows, which could include reducing expenditures for marketing, clinical and product development activities. However, we will ultimately need to achieve sufficient revenues from product sales and obtain additional debt or equity financing to support its operations.
We believe that we have the flexibility to manage the growth of our expenditures and operations depending on the amount of our cash flows. However, we will ultimately need to achieve sufficient revenues from product sales and/or obtain additional debt or equity financing in order to support our operations. Obtaining funds through the warrant holders’ exercise of outstanding common stock warrants or the sale of additional equity and debt securities may result in dilution to our stockholders. If we raise additional funds through the issuance of debt securities, these securities could have rights senior to those of our common stock and could contain covenants that would restrict our operations. The sale of additional equity may require us to obtain approval from our stockholders to increase the number of shares of common stock we have authorized under our certificate of incorporation. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, if at all. If we are unable to obtain additional financing, we may be required to reduce the scope of, delay, or eliminate some or all of, our planned research, development and commercialization activities, which could materially harm our business. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to products or proprietary technologies, or grant licenses on terms that are not favorable.
Our forecast of the period of time through which our financial resources will be adequate to support our operations, the costs to complete development of products and the cost to commercialize our products are forward-looking statements and involve risks and uncertainties, and actual results could vary materially and negatively as a result of a number of factors, including the factors discussed in Part I, Item 1A, Risk Factors, of our Annual Report on Form 10-K. We have based these estimates on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect.
Management is currently pursuing various funding options, including seeking additional equity or debt financing approximately one or two times per year as well as a strategic merger or other transaction to obtain additional funding or expand its product line to continue the development of, and to successfully commercialize, the ReShape Balloon, the ReShape vBloc System and the ReShape Vest. While the acquisition of ReShape Medical does provide incremental revenues to the Company, the cost to further develop and commercialize the ReShape Balloon is expected to significantly exceed revenues for the foreseeable future. While there can be no assurance that the Company will be successful in its efforts, the Company has a long history of raising equity financing to fund its development activities. Should the Company be unable to obtain adequate financing in the near term, the Company’s business, result of operations, liquidity and financial condition would be materially and negatively affected, and the Company would be unable to continue as a going concern. Additionally, there can be no assurance that, assuming the Company is able to strengthen its cash position, it will achieve sufficient revenue or profitable operations to continue as a going concern.
Because of the numerous risks and uncertainties associated with the development of medical devices, such as our vBloc System, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to
complete the development of the products and successfully deliver a commercial product to the market. Our future capital requirements will depend on many factors, including, but not limited to, the following:
| · | | the cost and timing of establishing sales, marketing and distribution capabilities; |
| · | | the cost of establishing clinical and commercial supplies of our ReShape vBloc, ReShape Balloon, ReShape Vest and any products that we may develop; |
| · | | the rate of market acceptance of our ReShape vBloc Therapy, ReShape Balloon, ReShape Vest and any other product candidates; |
| · | | the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights; |
| · | | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights; |
| · | | the effect of competing products and market developments; |
| · | | the cost of explanting clinical devices; |
| · | | the terms and timing of any collaborative, licensing or other arrangements that we may establish; |
| · | | any revenue generated by sales of our ReShape vBloc, ReShape Balloon, ReShape Vest or our future products; |
| · | | the scope, rate of progress, results and cost of our clinical trials and other research and development activities; |
| · | | the cost and timing of obtaining any further required regulatory approvals; and |
| · | | the extent to which we invest in products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions. |
Contractual Obligations
On August 25, 2015, we entered into an amendment extending the term of the operating lease for our St. Paul, Minnesota location for three years until September 30, 2018, with monthly base rent ranging from $18,925 to $20,345.
In connection with our acquisition of BarioSurg in May 2017, we assumed an operating lease for office space in Lake Forest, California with monthly base rent of $2,818 that expires September 30, 2018.
In connection with our acquisition of ReShape Medical in October 2017, we assumed (i) an operating lease for office/warehouse space in San Clemente, California with monthly base rent ranging from $24,614 to $27,510 that expires June 30, 2022 and (ii) an operating lease for office and manufacturing space in San Clemente, California with monthly base rent of $10,877 that expires October 31, 2019.
The following table summarizes our contractual obligations as of December 31, 2017 and the effect those obligations are expected to have on our financial condition and liquidity position in future periods:
| | | | | | | | | | | | | | | |
| | Payments Due By Period |
| | | | Less Than 1 | | | | | | More than |
Contractual Obligations | | Total | | Year | | 1-3 Years | | 3-5 Years | | 5 Years |
Operating lease | | $ | 1,865,049 | | $ | 633,695 | | $ | 738,344 | | $ | 493,010 | | $ | — |
Total contractual cash obligations | | $ | 1,865,049 | | $ | 633,695 | | $ | 738,344 | | $ | 493,010 | | $ | — |
The table above reflects only payment obligations that are fixed and determinable based on our current agreements.
Off-balance-sheet Arrangements
Since our inception, we have not engaged in any off-balance-sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities as defined by rules enacted by the SEC and FASB, and accordingly, no such arrangements are likely to have a current or future effect on our financial position, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Recent Accounting Pronouncements
In April 2015, FASB issued Simplifying the Presentation of Debt Issuance Costs, (Accounting Standards Update No. 2015-03 (ASU 2015-03)), which changes the presentation of debt issuance costs in the financial statements. Under ASU 2015-03, an entity presents such costs in the balance sheet as a direct deduction from the recognized debt liability rather than as an asset. Amortization of the costs is reported as interest expense. This guidance will be effective for interim and annual reporting periods beginning after December 15, 2015. We have evaluated the impact of adopting ASU 2015-03 and do not believe the new guidance will have a material effect on our financial position, results of operations or cash flows.
In May 2014, FASB issued Revenue from Contracts with Customers, Topic 606 (Accounting Standards Update No. 2014-09 (ASU 2014-09)), which outlines a single comprehensive revenue model for entities to use in accounting for revenue arising from contracts with customers. The guidance supersedes most current revenue recognition guidance, including industry-specific guidance, and requires a company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services. ASU 2014-09 is effective for fiscal years and interim periods within those years beginning after December 15, 2017. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented (full retrospective method), or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (modified retrospective method). In 2016, the FASB issued further guidance that offers narrow scope improvements and clarifies certain implementation issues related to revenue recognition, including principal versus agent considerations, the identification of performance obligations and licensing. These additional updates have the same effective date as the new revenue guidance. The new standard and its related amendments are collectively known as “ASC 606.”
We adopted ASC 606 using the modified retrospective method as of January 1, 2018. This approach was applied to all contracts not completed as of January 1, 2018. In addition to the enhanced footnote disclosures related to customer contracts, we anticipate that the most significant impact of the new standard will relate to the timing of revenue recognition for product sales with conditional rebates. No other significant changes to the accounting for revenue are expected.
We have completed our quantitative assessment and the impact of adoption of ASC 606 did not have a material impact on the Company’s consolidated financial position, results of operations, equity or cash flows. As part of completing this assessment, we updated and enhanced our internal controls over financial reporting.
In February 2016 FASB issued Accounting Standards Update No. 2016-02 Leases (Topic 842) that changes the recognition of lease assets and lease liabilities by lessees for those leases classified as operating lease. The amendments in this Update are effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years for a public business entity. Early adoption is permitted. Management is evaluating the standard's impact on the consolidated financial statements.
In March 2016, FASB issued Improvements to Employee Share-Based Payment Accounting, (Accounting Standards Update No. 2016-09 (ASU 2016-09)), which is intended to simplify several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, the estimation of forfeitures, shares withheld for taxes and classification of shares withheld for taxes on the statement of cash flows. As part of the adoption of this guidance the Company has elected to account for forfeitures of share-based awards as they occur. The Company prospectively adopted ASU 2016-09 as required on January 1, 2017 and the adoption did not have a material effect on its consolidated financial statements.
In January 2017 FASB issued Accounting Standards Update No. 2017-04 Intangibles—Goodwill and Other (Topic 350) Simplifying the Test for Goodwill Impairment. Under the amendments in this update an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. The amendments in this Update are required for public business entities in fiscal years beginning after December 15, 2019. The adoption of this standard is not expected to have a material impact to the Company’s consolidated financial statements.
In July 2017, FASB issued ASU 2017-11, Earnings Per Share (Topic 260); Distinguishing Liabilities from Equity (Topic 480); Derivatives and Hedging (Topic 815): (Part I) Accounting for Certain Financial Instruments with Down Round Features, (Part II) Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception. The amendments in this update are intended to simplify the accounting for certain equity-linked financial instruments and embedded features with down round features that result in the strike price being reduced on the basis of the pricing of future equity offerings. Under the new guidance, a down round feature will no longer need to be considered when determining whether certain financial instruments or embedded features should be classified as liabilities or equity instruments. That is, a down round feature will no longer preclude equity classification when assessing whether an instrument or embedded feature is indexed to an entity's own stock. In addition, the amendments clarify existing disclosure requirements for equity-classified instruments. These amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. We early adopted the applicable amendments in the third quarter of 2017 on a retrospective basis, which permitted the Company to classify the warrants issued along with its Series B Convertible Preferred Stock in August 2017 (Note 11) containing such down round provisions to equity instruments to stockholders’ equity.
In August 2014, FASB issued Disclosure of Uncertainties About an Entity’s Ability to Continue as a Going Concern, (Accounting Standards Update No. 2014-15 (ASU 2014-15)), which provides a framework for entities to evaluate going concern issues as well as potential related disclosures. This guidance became effective and the Company adopted it for the year ended December 31, 2016. See Note 3, Liquidity and Management’s Plans.
Various other accounting standards and interpretations have been issued with 2017 effective dates and effective dates subsequent to December 31, 2017. We have evaluated the recently issued accounting pronouncements that are currently effective or will be effective in 2017 and believe that none of them have had or will have a material effect on our financial position, results of operations or cash flows.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Our exposure to market risk is confined to our cash and cash equivalents. As of December 31, 2017 we had $10.2 million in cash and cash equivalents. The goals of our investment policy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, we may maintain a portfolio of cash equivalents and investments in a variety of securities of high credit quality. The securities in our investment portfolio, if any, are not leveraged, are classified as either available for sale or held-to-maturity and are, due to their very short-term nature, subject to minimal interest rate risk. We currently do not hedge interest rate exposure. Because of the short-term maturities of our cash equivalents, we do not believe that an increase in market rates would have any material negative impact on interest income recognized in our statement of operations. We have no investments denominated in foreign currencies and therefore our investments are not subject to foreign currency exchange risk.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
ReShape Lifesciences Inc.
San Clemente, California
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of ReShape Lifesciences Inc. and subsidiaries (the "Company") as of December 31, 2017 and 2016, the related consolidated statements of operations, stockholders' equity, and cash flows, for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company does not expect to generate sufficient operating revenue to sustain its operations in the near-term, and will require additional debt or equity financing, which raises substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, MN
April 2, 2018
We have served as the Company's auditor since 2006.
RESHAPE LIFESCIENCES INC.
Consolidated Balance Sheets
| | | | | | |
| | December 31, | | December 31, |
| | 2017 | | 2016 |
ASSETS | | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 10,163,208 | | $ | 3,310,787 |
Accounts receivable (net of allowance for bad debts of $155,872 and $20,000 at December 31, 2017 and 2016, respectively) | | | 488,613 | | | 143,692 |
Inventory | | | 2,817,112 | | | 1,789,578 |
Prepaid expenses and other current assets | | | 467,783 | | | 476,624 |
Total current assets | | | 13,936,716 | | | 5,720,681 |
Property and equipment, net | | | 438,621 | | | 200,720 |
Goodwill | | | 27,186,620 | | | — |
Other intangible assets, net | | | 46,152,577 | | | — |
Other assets | | | 990,015 | | | 1,119,405 |
Total assets | | $ | 88,704,549 | | $ | 7,040,806 |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | |
Current liabilities: | | | | | | |
Accounts payable | | $ | 1,088,271 | | $ | 1,311,706 |
Accrued expenses | | | 5,955,518 | | | 2,751,415 |
Total current liabilities | | | 7,043,789 | | | 4,063,121 |
| | | | | | |
Deferred income taxes | | | 5,292,291 | | | — |
Common stock warrant liability | | | 1,600 | | | 39,119 |
Total liabilities | | | 12,337,680 | | | 4,102,240 |
Commitments and contingencies (Note 16) | | | | | | |
Stockholders’ equity: | | | | | | |
Preferred stock, 5,000,000 shares authorized: | | | | | | |
Series A convertible preferred stock, $0.01 par value; zero shares outstanding at December 31, 2017 and 2016; 12,531 and zero shares issued at December 31, 2017 and 2016, respectively | | | — | | | — |
Conditional convertible preferred stock, $0.01 par value; 1,000,181 and zero shares issued and zero outstanding at December 31, 2017 and 2016 | | | — | | | — |
Series B convertible preferred stock, $0.01 par value; 20,000 shares issued and 6,055 and zero shares outstanding at December 31, 2017 and December 31, 2016, respectively | | | 61 | | | — |
Series C convertible preferred stock, $0.01 par value; 187,772 shares issued and 95,388 and zero shares outstanding at December 31, 2017 and December 31, 2016, respectively | | | 954 | | | — |
Common stock, $0.01 par value; 275,000,000 and 300,000,000 shares authorized at December 31, 2017 and 2016, respectively; 30,957,113 and 2,736,621 shares issued and outstanding at December 31, 2017 and December 31, 2016, respectively | | | 309,571 | | | 27,366 |
Additional paid-in capital | | | 410,815,637 | | | 303,852,582 |
Accumulated deficit | | | (334,759,354) | | | (300,941,382) |
Total stockholders’ equity | | | 76,366,869 | | | 2,938,566 |
Total liabilities and stockholders’ equity | | $ | 88,704,549 | | $ | 7,040,806 |
See accompanying notes to consolidated financial statements.
RESHAPE LIFESCIENCES INC.
Consolidated Statements of Operations
| | | | | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Product sales | | $ | 1,011,377 | | $ | 786,660 | | $ | 292,000 | |
Service and other revenue | | | 275,777 | | | — | | | — | |
Total revenue | | | 1,287,154 | | | 786,660 | | | 292,000 | |
| | | | | | | | | | |
Cost of goods sold | | | 764,805 | | | 431,476 | | | 125,047 | |
Cost of service and other revenue | | | 171,581 | | | — | | | — | |
Total cost of revenue | | | 936,386 | | | 431,476 | | | 125,047 | |
Gross profit | | | 350,768 | | | 355,184 | | | 166,953 | |
Operating expenses: | | | | | | | | | | |
Selling, general and administrative | | | 25,983,547 | | | 17,981,525 | | | 19,892,424 | |
Research and development | | | 5,775,098 | | | 5,169,286 | | | 8,141,323 | |
Total operating expenses | | | 31,758,645 | | | 23,150,811 | | | 28,033,747 | |
Operating loss | | | (31,407,877) | | | (22,795,627) | | | (27,866,794) | |
Other income (expense): | | | | | | | | | | |
Interest income | | | 1,066 | | | 5,837 | | | 1,819 | |
Interest expense | | | (3,874) | | | (4,104,003) | | | (939,182) | |
Warrants expense | | | (4,438,149) | | | — | | | — | |
Change in value of warrant liability | | | (283,097) | | | 3,512,816 | | | 3,295,536 | |
Other, net | | | (652) | | | 20,133 | | | 9,874 | |
Income (loss) before income taxes | | | (36,132,583) | | | (23,360,844) | | | (25,498,747) | |
| | | | | | | | | | |
Income tax benefit | | | 2,314,611 | | | — | | | — | |
Net loss | | $ | (33,817,972) | | $ | (23,360,844) | | $ | (25,498,747) | |
Net loss per share—basic and diluted | | $ | (3.07) | | $ | (37.53) | | $ | (298.97) | |
Shares used to compute basic and diluted net loss per share | | | 11,022,299 | | | 622,431 | | | 85,290 | |
See accompanying notes to consolidated financial statements.
RESHAPE LIFESCIENCES INC.
Consolidated Statements of Stockholders’ Equity
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Additional | | | | | Total |
| | Preferred Stock | | Common Stock | | Paid-in | | Accumulated | | Stockholders’ |
| | Shares | | Amount | | Shares | | Amount | | Capital | | Deficit | | Equity |
Balance December 31, 2014 | | — | | | — | | 66,332 | | $ | 663 | | $ | 258,745,523 | | $ | (252,081,791) | | $ | 6,664,395 |
Net loss | | — | | | — | | — | | | — | | | — | | | (25,498,747) | | | (25,498,747) |
Employee stock-based compensation expense | | — | | | — | | — | | | — | | | 6,974,489 | | | — | | | 6,974,489 |
Nonemployee stock-based compensation expense | | — | | | — | | — | | | — | | | (34,712) | | | — | | | (34,712) |
Issuance of common stock through “at-the-market” equity offerings in 2015 for cash from $1,162.00 to $1,589.00 per share, net of financing costs of $259,560 | | — | | | — | | 4,604 | | | 46 | | | 6,392,326 | | | — | | | 6,392,372 |
Issuance of common stock, net of warrants to purchase approximately 301,905 shares of common stock valued at $6,003,932, in registered public offering in July 2015 for cash at an aggregate price of $525.00 per unit, net of financing costs of $929,920 | | — | | | — | | 30,476 | | | 305 | | | 9,065,843 | | | — | | | 9,066,148 |
Issuance of common stock for payments made in shares on convertible notes payable | | — | | | — | | 1,003 | | | 10 | | | 109,494 | | | — | | | 109,504 |
Balance December 31, 2015 | | — | | | — | | 102,415 | | $ | 1,024 | | $ | 281,252,963 | | $ | (277,580,538) | | $ | 3,673,449 |
Net loss | | — | | | — | | — | | | — | | | — | | | (23,360,844) | | | (23,360,844) |
Employee stock-based compensation expense | | — | | | — | | — | | | — | | | 2,327,402 | | | — | | | 2,327,402 |
Nonemployee stock-based compensation expense | | — | | | — | | — | | | — | | | 3,535 | | | — | | | 3,535 |
Common stock financing costs | | — | | | — | | — | | | — | | | (28,000) | | | — | | | (28,000) |
Exercise of 1,428 warrants in 2016 for cash at $3.50 per share | | — | | | — | | 1,428 | | | 14 | | | 4,986 | | | — | | | 5,000 |
Issuance of common stock for payments made in shares on convertible notes payable | | — | | | — | | 2,632,778 | | | 26,328 | | | 20,291,696 | | | — | | | 20,318,024 |
Balance December 31, 2016 | | — | | | — | | 2,736,621 | | | 27,366 | | | 303,852,582 | | | (300,941,382) | | | 2,938,566 |
Net loss | | — | | | — | | — | | | — | | | — | | | (33,817,972) | | | (33,817,972) |
Employee stock-based compensation expense | | — | | | — | | — | | | — | | | 4,252,567 | | | — | | | 4,252,567 |
Nonemployee stock-based compensation expense | | — | | | — | | — | | | — | | | 184,230 | | | — | | | 184,230 |
Issuance of common stock, series A convertible preferred stock and warrants to purchase approximately 2,359,894 shares of common stock, in underwritten public offering in January 2017 for cash at an aggregate price of $5.31 per unit, net of financing costs of $2,505,244 | | 12,531 | | | 125 | | 1,218,107 | | | 12,181 | | | 16,481,598 | | | — | | | 16,493,904 |
Issuance of common stock and conditional convertible preferred stock related to May 2017 acquisition of BarioSurg, Inc. | | 1,000,181 | | | 10,002 | | 1,380,684 | | | 13,807 | | | 26,235,154 | | | | | | 26,258,963 |
Issuance of series B convertible preferred stock and warrants to purchase 8,700,000 shares of common stock in firm commitment underwritten public offering in August 2017 for cash at an aggregate price of $1,000.00 per unit, net of financing costs of $2,019,761 | | 20,000 | | | 200 | | — | | | — | | | 17,980,039 | | | — | | | 17,980,239 |
Issuance of warrants to former holders of convertible notes to purchase 2,575,000 shares of common stock valued at $4,438,149 | | — | | | — | | — | | | — | | | 4,438,149 | | | — | | | 4,438,149 |
Issuance of common stock and series C convertible preferred stock related to October 2017 acquisition of ReShape Medical, Inc. | | 187,772 | | | 1,878 | | 2,356,729 | | | 23,567 | | | 33,957,912 | | | | | | 33,983,357 |
Conversions of convertible preferred stock into common stock | | (1,119,041) | | | (11,190) | | 22,665,302 | | | 226,653 | | | (215,463) | | | — | | | — |
Exercise of warrants into 599,670 shares of common stock for cash | | — | | | — | | 599,670 | | | 5,997 | | | 3,648,869 | | | — | | | 3,654,866 |
Balance December 31, 2017 | | 101,443 | | $ | 1,015 | | 30,957,113 | | $ | 309,571 | | $ | 410,815,637 | | $ | (334,759,354) | | $ | 76,366,869 |
See accompanying notes to consolidated financial statements.
RESHAPE LIFESCIENCES INC.
Consolidated Statements of Cash Flows
| | | | | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Cash flows from operating activities: | | | | | | | | | | |
Net loss | | $ | (33,817,972) | | $ | (23,360,844) | | $ | (25,498,747) | |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | |
Depreciation | | | 205,513 | | | 139,576 | | | 189,491 | |
Provision for doubtful accounts | | | 12,666 | | | 20,000 | | | — | |
Deferred income taxes | | | (2,314,611) | | | — | | | — | |
Stock-based compensation | | | 4,436,797 | | | 2,330,937 | | | 6,939,777 | |
Warrants issued to former holders of convertible notes | | | 4,438,149 | | | — | | | — | |
Amortization of commitment fees, debt issuance costs and original issue discount | | | — | | | 1,836,340 | | | 825,735 | |
Amortization of intangible assets | | | 719,950 | | | — | | | — | |
Change in value of warrant liability | | | 283,097 | | | (3,512,816) | | | (3,295,536) | |
Change in operating assets and liabilities: | | | | | | | | | | |
Accounts receivable | | | 28,103 | | | (105,625) | | | (55,116) | |
Inventory | | | 68,441 | | | (103,254) | | | (705,805) | |
Prepaid expenses and other current assets | | | 182,584 | | | 354,732 | | | (409,822) | |
Other assets | | | 212,274 | | | (600,634) | | | 349,709 | |
Accounts payable | | | (223,435) | | | 1,139,656 | | | (222,636) | |
Accrued expenses | | | 1,180,236 | | | (891,060) | | | (235,351) | |
Accrued interest payable | | | — | | | 2,097,199 | | | (487,739) | |
Net cash used in operating activities | | | (24,588,208) | | | (20,655,793) | | | (22,606,040) | |
Cash flows from investing activities: | | | | | | | | | | |
Acquisitions, net of cash acquired | | | (6,230,567) | | | — | | | — | |
Purchases of property and equipment | | | (137,123) | | | (14,000) | | | (38,915) | |
Net cash used in investing activities | | | (6,367,690) | | | (14,000) | | | (38,915) | |
Cash flows from financing activities: | | | | | | | | | | |
Proceeds from warrants exercised | | | 3,334,176 | | | 5,000 | | | — | |
Proceeds from sale of common stock and warrants for purchase of common stock (January 2017) | | | 6,468,148 | | | — | | | 22,651,932 | |
Proceeds from sale of convertible preferred stock (January 2017) | | | 12,531,000 | | | | | | | |
Proceeds from sale of convertible preferred stock (August 2017) | | | 20,000,000 | | | | | | | |
Common stock financing costs | | | (2,505,244) | | | (28,000) | | | (1,721,794) | |
Preferred stock financing costs | | | (2,019,761) | | | | | | | |
Proceeds from convertible notes payable | | | — | | | 17,250,000 | | | 1,500,000 | |
Repayments on convertible notes payable | | | — | | | (446,867) | | | — | |
Repayments on notes payable | | | — | | | — | | | (3,000,000) | |
Debt issuance costs | | | — | | | (726,793) | | | (477,110) | |
Net cash provided by financing activities | | | 37,808,319 | | | 16,053,340 | | | 18,953,028 | |
Net increase in cash and cash equivalents | | | 6,852,421 | | | (4,616,453) | | | (3,691,927) | |
Cash and cash equivalents: | | | | | | | | | | |
Beginning of period | | | 3,310,787 | | | 7,927,240 | | | 11,619,167 | |
End of period | | $ | 10,163,208 | | $ | 3,310,787 | | $ | 7,927,240 | |
Supplemental disclosure: | | | | | | | | | | |
Cash paid for interest | | $ | 3,874 | | $ | 155,407 | | $ | 601,185 | |
Noncash investing and financing activities: | | | | | | | | | | |
Issuance of convertible preferred shares and common shares for acquisitions | | $ | 60,242,320 | | $ | — | | $ | — | |
Conversion of convertible notes and interest payable | | $ | — | | $ | — | | $ | 109,504 | |
See accompanying notes to consolidated financial statements.
ReShape Lifesciences Inc.
Notes to Consolidated Financial Statements
(1)Description of the Business; Risks and Uncertainties of the Business
Description of Business
ReShape Lifesciences Inc. (the Company) is focused on the design, development and commercialization of transformative technology to treat obesity and metabolic diseases. The Company was incorporated in the state of Minnesota on December 19, 2002, originally as two separate legal entities, Alpha Medical, Inc. and Beta Medical, Inc., both of which were owned 100% by a common stockholder. Effective October 1, 2003, the two entities were combined and the combined entity changed its name to EnteroMedics Inc. The Company reincorporated in Delaware on July 22, 2004. The Company has devoted substantially all of its resources to recruiting personnel, developing its product technology, obtaining patents to protect its intellectual property, commercialization activities and raising capital and has recently commenced commercial operations in the United States deriving revenues from its primary business activity in 2015. On May 22, 2017, the Company acquired BarioSurg, Inc. (BarioSurg), a company developing the Gastric Vest System and on October 2, 2017 it acquired ReShape Medical, Inc. (ReShape Medical), a company that develops, manufactures and markets a minimally invasive intragastric balloon resigned to treat certain obesity patients. ReShape Medical LLC became a wholly-owned subsidiary of the Company on October 2, 2017 and its balance sheet and statement operations for the period October 2, 2017 through December 31, 2017 are included with the Company’s 2017 consolidated financial statements. Subsequent to the acquisition of ReShape Medical, the Company relocated its headquarters from St. Paul, Minnesota to San Clemente, California.
EnteroMedics Europe Sárl (EnteroMedics Europe), a wholly owned subsidiary of the Company, was formed in January 2006. EnteroMedics Europe is a Swiss entity established as a means to conduct clinical trials in Switzerland. Upon establishment there were 20 shares of EnteroMedics Europe issued and outstanding with a par value of 1,000 Swiss Francs. The Company purchased 100% of the shares and then issued one share to a fiduciary agent. The one share is the property of the Company and is held by the fiduciary in a fiduciary capacity under terms of the Fiduciary Agreement. The functional currency of EnteroMedics Europe has been determined to be the U.S. Dollar.
On October 23, 2017, the Company announced that it had changed its name from “EnteroMedics Inc.” to “ReShape Lifesciences Inc.” effective October 23, 2017. In addition, in connection with the name change, the Company’s ticker symbol was changed to “RSLS” which became effective at the start of trading on October 23, 2017, and the Company’s common stock continued to trade on The NASDAQ Capital Market.
During 2016, the Company’s board of directors and stockholders approved two reverse stock splits (collectively, the Reverse Stock Splits). Neither reverse stock split changed the par value of the Company’s common stock or the number of preferred shares authorized by the Company’s certificate of incorporation. The first reverse stock split was a 1-for-15 reverse split (the First Reverse Stock Split) of the Company’s outstanding common stock that became effective after trading on January 6, 2016. The First Reverse Stock Split also decreased the number of shares of common stock authorized by the Company’s certificate of incorporation proportionately, and proportional adjustments were also made to the Company’s outstanding stock options and warrants and the number of shares authorized under the Company’s Amended and Restated 2003 Stock Incentive Plan . In connection with the First Reverse Stock Split, an amendment to the Company’s certificate of incorporation was also approved to increase the number of shares of the Company’s common stock authorized for issuance to 150 million shares, effective immediately after the First Reverse Stock Split on January 6, 2016.
The second reverse stock split was a 1-for-70 reverse split (the Second Reverse Stock Split) of the Company’s outstanding common stock that became effective after trading on December 27, 2016 pursuant to the Company’s Sixth Amended and Restated Certificate of Incorporation. In connection with the Second Reverse Stock Split, proportional adjustments were also made to the Company’s outstanding stock options and warrants. Additionally, in connection with the Second Reverse Stock Split, a second amendment was approved to increase the number of shares of the Company’s common stock authorized for issuance to 300 million shares, effective after the Second Reverse Stock Split on December 27, 2016.
All share and per-share amounts have been retroactively adjusted to reflect the Reverse Stock Splits for all periods presented.
On October 26, 2017, the Company filed a Certificate of Amendment to its Sixth Amended and Restated Certificate of Incorporation to reduce the number of authorized shares of the Company’s common stock from 300 million to 275 million.
Risks and Uncertainties
The Company is focused on the design, development and commercialization of transformative technology to treat obesity and metabolic diseases and its growing and differentiated product portfolio is utilized by bariatric surgeons, general surgeons, and gastroenterologists. We believe obesity is a global epidemic and that the majority of patients need treatment options that are anatomy friendly and provide for weight loss and comorbidity improvements along with long-term, ongoing obesity support and prevention.
We have a limited operating history and the Company’s products require approval from the U.S. Food and Drug Administration (FDA) or corresponding foreign regulatory agencies prior to commercial sales. On January 14, 2015, the vBloc® System, our initial product, which we now refer to as ReShape vBloc, received U.S. Food and Drug Administration (FDA) approval for vBloc Therapy, delivered via the ReShape vBloc. vBloc Therapy is delivered via a pacemaker-like device that helps patients feel full and eat less by intermittently blocking hunger signals on the vagus nerve. Our therapy limits the expansion of the stomach, helps control hunger sensations between meals, reduces the frequency and intensity of stomach contractions and produces a feeling of early and prolonged fullness.
On May 22, 2017 the Company acquired the Gastric Vest System (ReShape Vest) through the acquisition of BarioSurg, Inc. The ReShape Vest is an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in obese and morbidly obese patients. The ReShape Vest wraps around the stomach after it has been rearranged into a banana-like shape using sutures, emulating the effect of conventional weight-loss surgery, and is intended to enable gastric volume reduction without permanently changing patient anatomy.
On October 2, 2017 the Company acquired ReShape Medical, a privately-held medical technology company that develops, manufactures and markets the ReShape® Dual Weight Loss Balloon (the ReShape Balloon), an FDA-approved, minimally invasive intragastric balloon designed to treat obesity patients with a body mass index (BMI) between 30 and 40, with one or more related comorbid conditions. The acquisition of and results of operations for ReShape Medical for the period October 2, 2017 through December 31, 2017 are included in these consolidated financial statements.
The medical device industry is characterized by frequent and extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often difficult to predict, and the outcome may be uncertain until the court has entered final judgment and all appeals are exhausted. The Company’s competitors may assert that its products or the use of the Company’s products are covered by U.S. or foreign patents held by them.
The Company’s activities are subject to significant risks and uncertainties, including the ability to obtain additional financing, and there can be no assurance that the Company will be successful in obtaining additional financing on favorable terms, or at all. If adequate funds are not available, the Company may have to further reduce its cost structure until financing is obtained and/or delay development or commercialization of products or license to third parties the rights to commercialize products or technologies that the Company would otherwise seek to commercialize.
(2)Summary of Significant Accounting Policies
Basis of Presentation
The Company has prepared the accompanying consolidated financial statements in conformity with accounting principles generally accepted in the United States of America. The Company’s fiscal year ends on December 31.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All intercompany transactions and accounts have been eliminated in consolidation.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents. Cash and cash equivalents are primarily deposited in demand and money market accounts. At times, such deposits may be in excess of insured limits. Investments in money market funds are not considered to be bank deposits and are not insured or guaranteed by the federal deposit insurance company or other government agency. These money market funds seek to preserve the value of the investment at $1.00 per share; however, it is possible to lose money investing in these funds. The Company has not experienced any losses on its deposits of cash and cash equivalents.
Fair Value of Financial Instruments
Carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable and accrued liabilities approximate fair value due to their short maturities. The Company’s common stock warrants are required to be reported at fair value and the Company elected to report its senior amortizing convertible notes at fair value. The fair values of common stock warrants and investments in debt and equity securities, if any, are disclosed in Note 6. The 2015 and 2016 fair values of the Company’s senior amortizing convertible notes is disclosed in Notes 6 and 10.
Common Stock Warrant Liability
The common stock warrants that were issued in connection with the July 8, 2015 public offering (the Series A Warrants) and the common stock warrants issued in connection with the November 9, 2015, January 11, 2016 and May 2, 2016 senior amortizing convertible notes (the Note Warrants) are classified as a liability in the consolidated balance sheets, as the common stock warrants issued provide for certain anti-dilution protections in the event shares of common stock or securities convertible into shares of common stock are issued below the then-existing exercise price. The fair value of these common stock warrants is re-measured at each financial reporting period and immediately before exercise, with any changes in fair value being recognized as a component of other income (expense) in the consolidated statements of operations.
Cash and Cash Equivalents
The Company considers highly liquid investments generally with maturities of 90 days or less when purchased to be cash equivalents. Cash equivalents are stated at cost, which approximates market value. The Company’s cash equivalents are primarily in money market funds and certificates of deposit. The Company deposits its cash and cash equivalents in high-quality credit institutions.
Short-Term Investments
The Company considers all investments with maturities greater than three months and less than one year at the time of purchase as short-term investments and classifies them as either available for sale or held to maturity. The Company also considers certain investments with maturities greater than one year but which are also held for liquidity purposes and are available for sale as short-term investments.
Available-for-sale securities are carried at fair value based on quoted market prices, with the unrealized gains and losses included in other comprehensive income within stockholders’ equity in the consolidated balance sheets. Realized
gains and losses and declines in value judged to be other than temporary on available-for-sale securities are included in interest and other income. Interest and dividends on securities classified as available for sale are included in interest income. The cost of securities sold is based on the specific identification method.
Short-term investments in debt securities which the Company has the positive intent and ability to hold to maturity are reported at cost, adjusted for premiums and discounts that are recognized in interest income, using the interest method, over the period to maturity. Unrealized losses on held-to-maturity securities reflecting a decline in value determined to be other than temporary are charged to income.
Inventory
The Company accounts for inventory at the lower of cost or market and records any long-term inventory as other assets in the consolidated balance sheets. The Company establishes inventory reserves for obsolescence based upon projected sales and for defect based upon specific identification of defective or unsalable units.
Property and Equipment, Net
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of five to seven years for furniture and equipment and three to five years for computer hardware and software. Leasehold improvements are amortized on a straight-line basis over the lesser of their useful life or the term of the lease. Upon retirement or sale, the cost and related accumulated depreciation or amortization are removed from the consolidated balance sheets and the resulting gain or loss is reflected in the consolidated statements of operations. Repairs and maintenance are expensed as incurred.
Impairment of Long-Lived Assets, Intangible Assets and Goodwill
The Company evaluates its long-lived assets, including its finite-lived intangible assets, for impairment by comparison of the carrying amounts to future net undiscounted cash flows expected to be generated by such assets when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Should an impairment exist, the impairment loss would be measured based on the excess carrying value of the asset over the asset’s fair value or estimates of future discounted cash flows.
For goodwill and indefinite-lived intangible assets, in-process research and development, the Company reviews for impairment annually and upon the occurrence of certain events as required by Accounting Standards Codification (“ASC”) Topic 350, “Intangibles — Goodwill and Other.” Goodwill and indefinite-lived intangible assets are tested at least annually for impairment and more frequently if events or changes in circumstances indicate that the asset might be impaired. The Company reviews goodwill for impairment by first assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the two-step goodwill impairment test. If the Company is able to determine that it is not more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company would conclude that goodwill is not impaired. If the carrying amount of a reporting unit is zero or negative, the second step of the impairment test is performed to measure the amount of impairment loss, if any, when it is more likely than not that a goodwill impairment exists. The Company did not record any losses for impairment during the year ended December 31, 2017.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance for deferred income tax assets is recorded when it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The Company has provided a full valuation allowance against the net deferred tax assets, excluding deferred tax liabilities for indefinite-lived intangible assets, as of December 31, 2017 and 2016 (see Note 12).
The Company’s policy is to classify interest and penalties related to income taxes as income tax expense in the consolidated statements of operations.
Medical Device Excise Tax
On January 14, 2015, the Company received FDA approval for vBloc Therapy, delivered via the vBloc Rechargeable System, and starting in the second quarter of 2015 revenues were generated from sales in the United States. As a result, the Company is now required to pay a quarterly medical device tax under the Affordable Care Act, which imposes a 2.3% excise tax on the sale of certain medical devices, including ReShape vBloc and the ReShape Balloon, by device manufactures, producers or importers (the Medical Device Tax). The Medical Device Tax was effective on sales of devices made after December 31, 2012. The Company records the Medical Device Tax as an operating expense in the consolidated statements of operations, which totaled $1,363 for 2015. A moratorium was placed on the Medical Device Tax for 2016 and 2017 and, consequently, the Company was not required to pay the Medical Device Tax in 2016 or 2017. In January 2018, the moratorium was extended to 2018 and 2019.
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a company during a period from transactions and other events and circumstances excluding transactions resulting from distributions to owners. There was no difference from reported net loss for the years ended December 31, 2017, 2016 and 2015.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, title or risk of loss has passed, the selling price is fixed or determinable and collection is reasonably assured. Products are sold through direct sales or medical device distributors. ReShape vBloc revenue is recognized upon sale to a bariatric center or a medical device distributor when no right of return or price protection exists. ReShape balloon revenue is typically recognized when title is passed, usually at point of shipment. A provision for returns reducing revenues is recorded only if product sales provide for a right of return. No provision for returns was recorded for the years ended December 31, 2015 and December 31, 2016, as vBloc product sales recorded did not provide for rights of return. A provision for returns of $39,000 was recorded in the fourth quarter of 2017 to estimate potential returns of ReShape balloon product.
Revenues for services are recognized when earned, subject to limitations, if any, related to multiple deliverables. We evaluate each element in these multiple-element arrangements to determine whether they represent a separate unit of accounting and recognize each element as the services are performed or as product is shipped.
Shipping and Handling
The Company records amounts invoiced to its customers for outbound freight and shipping as other revenue and the related expense as cost of goods sold.
Research and Development Expenses
Research and development expenses are charged to expense as incurred. Research and development expenses include, but are not limited to, product development, clinical trial expenses, including supplies, devices, explants and revisions, quality assurance, regulatory expenses, payroll and other personnel expenses, materials and consulting costs.
Patent Costs
Costs associated with the submission of a patent application are expensed as incurred given the uncertainty of the patents resulting in probable future economic benefits to the Company. Patent-related legal expenses included in general and administrative costs were $305,000, $269,000, and $200,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
Stock-Based Compensation
The fair value method is applied to all share-based payment awards issued to employees and where appropriate, nonemployees, unless another source of literature applies. When determining the measurement date of a nonemployee’s share-based payment award, the Company measures the stock options at fair value and remeasures such stock options to the current fair value until the performance date has been reached. All option grants are expensed on a straight-line basis over the vesting period.
Net Loss Per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is based on the weighted-average common shares outstanding during the period plus dilutive potential common shares calculated using the treasury stock method. Such potentially dilutive shares are excluded when the effect would be to reduce a net loss per share. The Company’s potential dilutive shares, which include outstanding common stock options and warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be anti-dilutive.
The following table sets forth the computation of basic and diluted net loss per share for the years ended December 31, 2017, 2016 and 2015:
| | | | | | | | | | |
| | Year Ended | |
| | December 31, | |
| | 2017 | | 2016 | | 2015 | |
Numerator: | | | | | | | | | | |
Net loss | | $ | (33,817,972) | | $ | (23,360,844) | | $ | (25,498,747) | |
Denominator for basic and diluted net loss per share: | | | | | | | | | | |
Weighted-average common shares outstanding | | | 11,022,299 | | | 622,431 | | | 85,290 | |
Net loss per share—basic and diluted | | $ | (3.07) | | $ | (37.53) | | $ | (298.97) | |
The following table sets forth the potential shares of common stock that are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented:
| | | | | |
| | December 31, | |
| | 2017 | | 2016 | |
Stock options outstanding | | 3,830,447 | | 19,840 | |
Common shares underlying convertible preferred stock | | 12,172,725 | | — | |
Warrants to purchase common stock | | 14,308,337 | | 55,044 | |
Segment Reporting
Operating segments are defined as components of an enterprise for which discrete financial information is available that is evaluated regularly by the chief operating decision maker (“CODM”) in deciding how to allocate resources and in assessing performance. Our CODM is the Chief Executive Officer.
Under the provisions of ASC 280, Segment Reporting, we have determined that we have one operating segment related to the design, development and commericialization of transformative technology to treat obesity and metabolic diseases. The CODM evaluates operating performance and allocates resources on a total portfolio basis.
Recently Issued Accounting Standards
In May 2014, FASB issued Revenue from Contracts with Customers, Topic 606 (Accounting Standards Update No. 2014-09 (ASU 2014-09)), which outlines a single comprehensive revenue model for entities to use in accounting for revenue arising from contracts with customers. The guidance supersedes most current revenue recognition guidance, including industry-specific guidance, and requires a company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services. ASU 2014-09 is effective for fiscal years and interim periods within those years beginning after December 15, 2017. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented (full
retrospective method), or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (modified retrospective method). In 2016, the FASB issued further guidance that offers narrow scope improvements and clarifies certain implementation issues related to revenue recognition, including principal versus agent considerations, the identification of performance obligations and licensing. These additional updates have the same effective date as the new revenue guidance. The new standard and its related amendments are collectively known as “ASC 606.”
We adopted ASC 606 using the modified retrospective method as of January 1, 2018. This approach was applied to all contracts not completed as of January 1, 2018. In addition to the enhanced footnote disclosures related to customer contracts, we anticipate that the most significant impact of the new standard will relate to the timing of revenue recognition for product sales with conditional rebates. No other significant changes to the accounting for revenue are expected.
We have completed our quantitative assessment and the impact of adoption of ASC 606 did not have a material impact on the Company’s consolidated financial position, results of operations, equity or cash flows. As part of completing this assessment, we updated and enhanced our internal controls over financial reporting.
In February 2016 FASB issued Accounting Standards Update No. 2016-02 Leases (Topic 842) that changes the recognition of lease assets and lease liabilities by lessees for those leases classified as operating lease. The amendments in this Update are effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years for a public business entity. Early adoption is permitted. Management is evaluating the standard's impact on the consolidated financial statements.
In March 2016, FASB issued Improvements to Employee Share-Based Payment Accounting, (Accounting Standards Update No. 2016-09 (ASU 2016-09)), which is intended to simplify several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, the estimation of forfeitures, shares withheld for taxes and classification of shares withheld for taxes on the statement of cash flows. As part of the adoption of this guidance the Company has elected to account for forfeitures of share-based awards as they occur. The Company prospectively adopted ASU 2016-09 as required on January 1, 2017 and the adoption did not have a material effect on its consolidated financial statements.
In January 2017 FASB issued Accounting Standards Update No. 2017-04 Intangibles—Goodwill and Other (Topic 350) Simplifying the Test for Goodwill Impairment. Under the amendments in this update an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. Additionally, an entity should consider income tax effects from any tax deductible goodwill on the carrying amount of the reporting unit when measuring the goodwill impairment loss, if applicable. The amendments in this Update are required for public business entities in fiscal years beginning after December 15, 2019. The adoption of this standard is not expected to have a material impact to the Company’s consolidated financial statements.
In July 2017, FASB issued ASU 2017-11, Earnings Per Share (Topic 260); Distinguishing Liabilities from Equity (Topic 480); Derivatives and Hedging (Topic 815): (Part I) Accounting for Certain Financial Instruments with Down Round Features, (Part II) Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception. The amendments in this update are intended to simplify the accounting for certain equity-linked financial instruments and embedded features with down round features that result in the strike price being reduced on the basis of the pricing of future equity offerings. Under the new guidance, a down round feature will no longer need to be considered when determining whether certain financial instruments or embedded features should be classified as liabilities or equity instruments. That is, a down round feature will no longer preclude equity classification when assessing whether an instrument or embedded feature is indexed to an entity's own stock. In addition, the amendments clarify existing disclosure requirements for equity-classified instruments. These amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2018, with early adoption permitted. We early adopted the applicable amendments in the third quarter of 2017 on a retrospective basis, which permitted the Company to classify the warrants issued along with its Series B Convertible Preferred Stock in August 2017 (Note 11) containing such down round provisions to equity instruments to stockholders’ equity.
Various other accounting standards and interpretations have been issued with 2017 effective dates and effective dates subsequent to December 31, 2017. The Company has evaluated the recently issued accounting pronouncements that are currently effective or will be effective in 2018 and believe that none of them have had or will have a material effect on the Company’s financial position, results of operations or cash flows.
(3)Liquidity and Management’s Plans
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company currently is not generating revenue from operations that is significant relative to its level of operating expenses, and does not anticipate generating revenue sufficient to offset operating costs in the short-term to mid-term. The Company has financed its operations to date principally through the sale of equity securities, debt financing and interest earned on investments. The Company’s history of operating losses, limited cash resources and lack of certainty regarding obtaining significant third-party reimbursement for its products, raise substantial doubt about our ability to continue as a going concern absent a strengthening of our cash position. As of December 31, 2017, the Company had $10.2 million of cash and cash equivalents to fund its operations into early 2018.
The following financing transactions occurred in 2015, 2016 and 2017 to fund the Company’s operations and acquisitions:
| · | | On July 8, 2015, the Company closed a public offering of units consisting of common stock and the Series A Warrants. Gross proceeds of the offering were $16.0 million, prior to deducting offering expenses of approximately $1.4 million |
| · | | On November 4, 2015 the Company entered into a securities purchase agreement (the Purchase Agreement) with institutional investors to issue up to $25.0 million of senior amortizing convertible notes (the Notes) and Note Warrants, in three separate closings. $1.5 million of the Notes was funded at the first closing on November 9, 2015 (the First Closing). |
| · | | An additional $11.0 million of the Notes was funded at the second closing on January 11, 2016 (the Second Closing). |
| · | | An additional $6.25 million of the Notes was funded at the third closing on May 2, 2016 (the Third Closing). |
| · | | On January 23, 2017, the Company closed an underwritten public offering consisting of units of common stock, convertible preferred stock and warrants to purchase common stock. Gross proceeds of the offering were $19.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.5 million. |
| · | | During 2017, common stock warrants for 559,670 shares of common stock were exercised by warrant holders with proceeds to the Company of $3.3 million. |
| · | | On August 16, 2017, the Company closed an underwritten public offering consisting of units of Series B Convertible Preferred Stock and warrants to purchase common stock. Gross proceeds of the offering were $20.0 million, prior to deducting underwriting discounts and commissions and offering expenses of approximately $2.0 million. |
| · | | On April 2, 2018 the Company announced that it had entered into a securities purchase agreement with an institutional investor providing for the purchase and sale in a registered direct offering of shares of series D convertible preferred stock and a warrant to purchase shares of common stock for a purchase price of $6.0 million. The transaction is expected to close on or about April 4, 2018 and the Company expects to receive net proceeds of approximately $5.25 million after deducting placement agent fees and other offering expenses. |
The Company’s anticipated operations include plans to (i) integrate the sales and operations of the Company with the newly acquired ReShape Medical in order to expand sales of both the ReShape Balloon and ReShape vBloc as well as to obtain cost savings synergies, (ii) expand the controlled commercial launch of vBloc Therapy, delivered via the ReShape vBloc System, (iii) continue development of the ReShape Vest, (vi) seek opportunities to leverage the Company’s intellectual property portfolio and custom development services to provide third party sales and licensing opportunities, and (v) explore and capitalize on synergistic opportunities to expand our portfolio and offer future minimally invasive treatments and therapies in the obesity continuum of care. The Company believes that it has the flexibility to manage the growth of its expenditures and operations depending on the amount of available cash flows, which could include reducing expenditures for marketing, clinical and product development activities. However, the Company will ultimately need to achieve sufficient revenues from product sales and obtain additional debt or equity financing to support its operations.
Management is currently pursuing various funding options, including seeking additional equity or debt financing approximately one or two times per year as well as a strategic merger or other transaction to obtain additional funding or expand its product line to continue the development of, and to successfully commercialize, the ReShape Balloon, the ReShape vBloc System and the ReShape Vest. While the acquisition of ReShape Medical does provide incremental revenues to the Company, the cost to further develop and commercialize the ReShape Balloon is expected to significantly exceed revenues for the foreseeable future. While there can be no assurance that the Company will be successful in its efforts, the Company has a long history of raising equity financing to fund its development activities. Should the Company be unable to obtain adequate financing in the near term, the Company’s business, result of operations, liquidity and financial condition would be materially and negatively affected, and the Company would be unable to continue as a going concern. Additionally, there can be no assurance that, assuming the Company is able to strengthen its cash position, it will achieve sufficient revenue or profitable operations to continue as a going concern. The consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
(4)Acquisitions
BarioSurg, Inc.
On May 22, 2017, the Company entered into an Agreement and Plan of Merger (the "Merger Agreement") to acquire all of the ownership interests of BarioSurg, Inc. ("BarioSurg"), a company developing the Gastric Vest System (which we now refer to as the “ReShape Vest”), an investigational, minimally invasive, laparoscopically implanted medical device being studied for weight loss in obese and morbidly obese patients.
The consideration paid by the Company for all of the outstanding shares of capital stock and outstanding options of BarioSurg consisted of: (i) 1.38 million shares of common stock, par value $0.01 per share, of the Company ("Company Common Stock"), (ii) 1.0 million shares of newly created conditional convertible preferred stock, par value $0.01 per share, of the Company ("Company Preferred Stock"), which shares converted into 5.0 million shares of Company Common Stock on October 25, 2017 upon the post-closing approval of the Company's stockholders in accordance with the NASDAQ Stock Market Rules, and (iii) $2.0 million in cash. At the closing of the Merger, 100,018 shares of Company Preferred Stock were deposited with an escrow agent to fund-post closing indemnification obligations of BarioSurg’s former stockholders. The total consideration paid by the Company, preliminarily valued at $28.3 million, includes: (a) $2.0 million in cash paid from our existing cash balances and (b) $26.3 million from the issuance of Company Common Stock and Company Preferred Stock. The preliminary valuation of the Company Common Stock and Company Preferred Stock took into account (i) the conversion ratio of the Company Preferred Stock, (ii) the closing prices of our common stock on the NASDAQ Stock Market on the date the transaction was announced, and (iii) a 19% discount for lack of marketability related to the shares issued in the transaction.
The purchase price consideration of $28.3 million does not include expenses of $454,000 for legal, accounting, audit, valuation and other services that were incurred from the May 22, 2017 acquisition date through December 31, 2017 as part of the transaction and were expensed as incurred.
The transaction was accounted for as a business combination and the following table summarizes the estimated fair values of the assets acquired and liabilities assumed as a result of the BarioSurg acquisition. The excess of the cost of the acquisition over the fair value of assets acquired was recorded as goodwill. The assessment of fair value and the
determination of deferred tax assets aquired is preliminary, and is based on information that was available at the time the consolidated financial statements were prepared. Accordingly, the allocation of purchase price to intangible assets and to deferred tax assets and liabilities to the acquired intangible assets is preliminary and, therefore, subject to adjustment in future periods.
| | | |
Cash | | $ | 151,280 |
Property and equipment | | | 3,000 |
Goodwill | | | 14,004,573 |
In Process Research & Development | | | 20,720,939 |
Trademarks/tradenames | | | 1,090,363 |
Covenant not to compete | | | 75,884 |
Other assets | | | 5,826 |
Current liabilities assumed | | | (186,000) |
Deferred income tax liability | | | (7,606,902) |
Net assets acquired | | $ | 28,258,963 |
We believe that the amount of goodwill relative to identifiable intangible assets relates to several factors including (i) potential synergies related to market opportunities for multiple product offerings, (ii) future technology, and (iii) initial relationships and awareness of the Gastric Vest.
In-process research and development (“IPR&D”) consists of the Gastric Vest, which has not yet been clinically tested in the United States and has not yet been approved by the FDA. Acquired IPR&D assets are initially recognized at fair value and are classified as indefinite-lived assets until the successful completion or abandonment of the associated research and development efforts. The value assigned to IPR&D was determined by estimating the net cash flows from the Gastric Vest development project and discounting the net cash flows to their present value. During the development period, this asset will not be amortized as charges to earnings; instead, this asset will be subject to periodic impairment testing. Upon successful completion of the development process for the acquired IPR&D, the asset would then be considered a finite-lived intangible asset and amortization will commence. Trademarks/tradenames were valued using the relief from royalty method and are being amortized over a 10-year period. The covenant not to compete was valued using the comparative business valuation method-income approach and is being amortized over a three-year period. The values of these intangible assets are considered Level 3 measurements.
In the fourth quarter of 2017, the Company determined that a deferred tax liability of $7.6 million related to the IPR&D asset acquired in the BarioSurg acquisition, along with an equal amount of additional goodwill, should have been included in the Company’s initial purchase price allocation and reported on its balance sheet as of June 30, 2017 and September 30, 2017. The Company has corrected this error as of December 31, 2017 and believes the effect of the error, which had no impact on previously reported quarterly net loss, is immaterial to the previously filed quarterly financial statements as of June 30 and September 30, 2017. The deferred tax liability was remeasured as of December 22, 2017, with the enactment of the Tax Cuts and Jobs Act, which resulted in a $2.3 million income tax benefit being recognized in the consolidated statement of operations for the year ended December 31, 2017 (see also Note 12).
The results of this acquisition, zero revenues and a $832,000 loss for the 2017 year-to-date period through December 31, 2017, are included in our consolidated operations beginning May 22, 2017.
At a Special Meeting of Shareholders on October 25, 2017, Company shareholders approved the conversion of 1,000,181 shares of conditional convertible preferred stock held by the former shareholders of BarioSurg, Inc. into 5,000,905 shares of common stock.
Unaudited Pro Forma Information-BarioSurg Acquisition
The following unaudited pro forma financial information presents our combined results of operations as if the acquisition of BarioSurg and the related issuance of Company common stock had occurred on January 1, 2016. Pro forma information reflects adjustments that give effect to pro forma events that are directly attributable to the acquisition, factually supportable and expected to have a continuing impact on the combined results following the acquisition. In addition, the unaudited pro forma financial information do not purport to be indicative of the results that
would have actually been obtained if the acquisition had occurred as of January 1, 2016 or that may be obtained in the future, and do not reflect future synergies, integration costs, or other such costs or savings.
| | | | | | |
| | Year Ended December 31, |
| | 2017 | | 2016 |
Revenues | | $ | 1,287,154 | | $ | 786,660 |
Net loss | | $ | (33,760,443) | | $ | (23,996,256) |
Net loss per share—basic and diluted | | $ | (2.92) | | $ | (11.98) |
The unaudited pro forma results include adjustments due to increases in amortization expense and acquisition related costs. The per share unaudited pro forma results also reflect adjustment of weighted average common shares outstanding to reflect the assumed issuance of 1.38 million shares of Company Common Stock as of January 1, 2016.
ReShape Medical, Inc.
On October 2, 2017 the Company acquired ReShape Medical, Inc., a privately-held medical technology company that develops, manufactures and markets the ReShape® Dual Weight Loss Balloon, an FDA and CE marked approved, minimally invasive intragastric balloon designed to treat obesity patients with a body mass index (BMI) between 30 and 40, with one or more related comorbid conditions.
The consideration paid by the Company for ReShape Medical consisted of: (i) 2,356,729 shares of Company Common Stock, par value $0.01 per share, (ii) 187,772 shares of series C convertible preferred stock (which became convertible into 18,777,200 shares of common stock upon the December 19, 2017 approval of the Company’s stockholders under NASDAQ rules, of which 8.3 million shares of common stock were automatically converted on that date), and (iii) approximately $5.0 million in cash, which amount was immediately used to pay ReShape Medical’s outstanding senior secured indebtedness and certain transaction expenses of ReShape Medical.
The total consideration paid by the Company, preliminarily valued at $39.0 million, includes: (a) $5.0 million in cash paid from our existing cash balances and (b) $34.0 million from the issuance of the common stock and the series C convertible preferred stock. The preliminary valuation of the common stock and series C preferred stock took into account (i) the conversion ratio of the series C convertible preferred stock, (ii) the closing price of our common stock on the NASDAQ Stock Market on the date the transaction was announced, and (iii) a 20% discount for lack of marketability related to the shares issued in the transaction. The common stock and series C convertible preferred stock issued to the the former ReShape Medical equity holders in connection with the transaction, including any common stock acquired via conversion of series C convertible preferred stock to common stock, are subject to six month holding period, volume and other limitations under Rule 144.
The purchase price consideration of $39.0 million does not include expenses of $736,000 for legal, accounting, audit, valuation and other services that were incurred from the October 2, 2017 acquisition date through December 31, 2017 as part of the transaction and were expensed as incurred.
The transaction was accounted for as a business combination and the following table summarizes the estimated fair values of the assets acquired and liabilities assumed as a result of the ReShape Medical acquisition. The excess of the cost of the acquisition over the fair value of assets acquired was recorded as goodwill. The assessment of fair value and the determination of deferred tax assets acquired is preliminary and is based on information that was available at the
time the consolidated condensed financial statements were prepared. Accordingly, the allocation of purchase price to the intangible assets and to deferred tax assets and liabilities is preliminary and subject to adjustment in future periods.
| | | |
Cash | | $ | 617,229 |
Accounts receivable | | | 385,690 |
Inventory | | | 1,095,975 |
Prepaid expenses and other current assets | | | 173,743 |
Property and equipment | | | 303,291 |
Goodwill | | | 13,182,047 |
Developed technology | | | 18,451,360 |
Trademarks/tradenames | | | 2,780,448 |
Customer relationships | | | 3,753,533 |
Other assets | | | 77,058 |
Current liabilities assumed | | | (1,837,941) |
Net assets acquired | | $ | 38,982,433 |
We believe that the amount of goodwill relative to identifiable intangible assets relates to several factors including (i) potential synergies related to market opportunities for multiple product offerings, (ii) future technology, and (iii) intact workforce.
Developed technology consists of the ReShape® Dual Weight Loss Balloon (the ReShape Balloon), an FDA-approved, minimally invasive intragastric balloon designed to treat obesity patients with a body mass index (BMI) between 30 and 40, with one or more related comorbid conditions. The acquired developed technology assets are valued by estimating cash flows using an income approach and they are amortized over a 12-year period, consistent with the remaining lives of the technology’s key patents. Trademarks/tradenames are valued using the relief from royalty method and are being amortized over a 10-year period. Customer relationships are valued using the with-and-without method under the income approach and are being amortized over 5 years. The values of these intangible assets are considered Level 3 measurements.
The results of this acquisition for the period beginning with the October 2, 2017 acquisition date through December 31, 2017 are included in our 2017 statement of operations and include $718,000 of revenue and a $4.1 million loss.
At a Special Meeting of the Stockholders on December 19, 2017, Company stockholders approved the issuance of up to 18,777,200 shares of common stock upon the conversion of 187,772 shares of series C convertible preferred stock issued to the former equity holders of ReShape Medical. On that date, 82,384 shares of series C convertible preferred stock automatically converted into 8,238,400 shares of common stock and an additional 10,000 shares of series C convertible preferred stock were optionally converted into 1,000,000 shares of common stock.
Unaudited Pro Forma Information – ReShape Medical Acquisition
The following unaudited pro forma financial information presents our combined results of operations as if the acquisition of ReShape Medical and the related issuance of company common stock had occurred on January 1, 2016. Pro forma information reflects adjustments that give effect to pro forma events that are directly attributable to the acquisition, factually supportable and expected to have a continuing impact on the combined results following the acquisition. In addition, the unaudited pro forma financial information do not purport to be indicative of the results that
would have actually been obtained if the acquisition had occurred as of January 1, 2016 or that may be obtained in the future, and do not reflect future synergies, integration costs, or other such costs or savings.
| | | | | | |
| | Year Ended December 31, |
| | 2017 | | 2016 |
Revenues | | $ | 3,874,908 | | $ | 6,745,933 |
Net loss | | $ | (46,871,794) | | $ | (43,195,299) |
Net loss per share—basic and diluted | | $ | (3.66) | | $ | (14.50) |
The unaudited pro forma results include adjustments due to increases in amortization expense and acquisition related costs. The per share unaudited pro forma results also reflect adjustment of weighted average common shares outstanding to reflect the assumed issuance of 2.36 million shares of the Company’s common stock as of January 1, 2016.
(5) Goodwill and Other Intangible Assets
Allocations of purchase prices related to the acquisitions of BarioSurg and ReShape Medical during the year ended December 31, 2017 resulted in the recording of $27.2 million of goodwill and $46.9 million of other intangible assets as discussed in Note 4.
The following table summarizes the activity of intangible assets, excluding goodwill for the year ended December 31, 2017:
| | | | | | | | | | | | | | |
| | Useful Life (years) | | Gross Carrying Amount | | Accumulated Amortization | | Impairment | | Net Book Value |
In Process Research & Development | | indefinite | | $ | 20,720,939 | | $ | — | | $ | — | | $ | 20,720,939 |
Trademarks/Tradenames | | 10 | | | 3,870,811 | | | (133,116) | | | — | | | 3,737,695 |
Covenant not to compete | | 3 | | | 75,884 | | | (14,755) | | | — | | | 61,129 |
Developed technology | | 12 | | | 18,451,360 | | | (384,402) | | | — | | | 18,066,958 |
Customer relationships | | 5 | | | 3,753,533 | | | (187,677) | | | — | | | 3,565,856 |
Total | | | | $ | 46,872,527 | | $ | (719,950) | | $ | — | | $ | 46,152,577 |
The following table summarizes the expected amortization of intangible assets as of December 31, 2017:
| | | |
Year ending December 31, | | | |
2018 | | $ | 2,700,696 |
2019 | | | 2,700,696 |
2020 | | | 2,685,940 |
2021 | | | 2,675,401 |
2022 | | | 2,487,722 |
Thereafter | | | 12,181,183 |
| | $ | 25,431,638 |
(6)Fair Value Measurements
Fair value of financial assets and liabilities is defined as the price that would be received to sell an asset or transfer a liability in an orderly transaction between market participants at the measurement date. A fair value hierarchy has been established that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described below:
| · | | Level 1—Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
| · | | Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active or model-derived valuations for which all significant inputs are observable, either directly or indirectly. |
| · | | Level 3—Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable. |
The Company did not hold any short-term investments classified as available for sale or held to maturity as of December 31, 2017 and 2016.
The fair value of each of the Company’s common stock warrant liability is calculated using a Black-Scholes valuation model and is classified as Level 2 in the fair value hierarchy. The fair values are presented below along with valuation assumptions:
| | | | | | | | | | |
| | | Series A Warrants | | | |
| | December 31, 2017 | | December 31, 2016 | | | |
Risk-free interest rates | | | 1.76 | % | | 1.20 | % | | | |
Expected life | | | 12 | months | | 24 | months | | | |
Expected dividends | | | — | % | | — | % | | | |
Expected volatility | | | 193.28 | % | | 122.03 | % | | | |
Fair value | | $ | 1,600 | | $ | 36,000 | | | | |
| | | | | | | | | | |
| | December 31, 2016 | |
| | November 2015 Note Warrants | | January 2016 Note Warrants | | May 2016 Note Warrants | |
Risk-free interest rates | | | 1.47 | % | | 1.93 | % | | 1.93 | % |
Expected life | | | 46 | months | | 48 | months | | 52 | months |
Expected dividends | | | — | % | | — | % | | — | % |
Expected volatility | | | 102.29 | % | | 108.57 | % | | 106.37 | % |
Fair value | | $ | 449 | | $ | 1,633 | | $ | 1,037 | |
During the year ended December 31, 2016 all the amounts outstanding under the Notes were paid off via conversions into shares of common stock.
(7)Inventory
The Company accounts for inventory at the lower of cost or market and records any long-term inventory as other assets in the consolidated balance sheets. There was approximately $1.0 million and $676,000 of long-term inventory, primarily consisting of raw materials, as of December 31, 2017 and 2016, respectively.
Current inventory consists of the following as of:
| | | | | | |
| | December 31, |
| | December 31, | | December 31, |
| | 2017 | | 2016 |
Raw materials | | $ | 707,919 | | $ | 335,606 |
Work-in-process | | | 1,494,278 | | | 1,437,957 |
Finished goods | | | 614,915 | | | 16,015 |
Inventory | | $ | 2,817,112 | | $ | 1,789,578 |
(8)Property and Equipment
Property and equipment consists of the following as of:
| | | | | | |
| | December 31, |
| | 2017 | | 2016 |
Furniture and equipment | | $ | 2,393,644 | | $ | 2,302,878 |
Computer hardware and software | | | 887,059 | | | 596,292 |
Leasehold improvements | | | 123,542 | | | 62,651 |
| | | 3,404,245 | | | 2,961,821 |
Less accumulated depreciation and amortization | | | (2,965,624) | | | (2,761,101) |
Property and equipment, net | | $ | 438,621 | | $ | 200,720 |
(9)Accrued Expenses
Accrued expenses consist of the following:
| | | | | | |
| | December 31, |
| | 2017 | | 2016 |
Professional service related expenses | | $ | 2,643,342 | | $ | 1,858,912 |
Payroll related expenses | | | 2,480,219 | | | 507,327 |
Other expenses | | | 831,957 | | | 385,176 |
Accrued expenses | | $ | 5,955,518 | | $ | 2,751,415 |
(10)Senior Amortizing Convertible Notes
On November 4, 2015, the Company entered into the Purchase Agreement to issue and sell to four institutional investors 7% senior amortizing convertible notes due 2017 in three separate closings. The Notes were initially convertible into shares of the Company’s common stock at a price equal to $304.50 per share with an aggregate principal amount of $25.0 million. Each Note was sold with Note Warrant with an exercise price of $325.50 per share. The Company issued and sold Notes and Note Warrants for aggregate total proceeds of $12.5 million in the First Closing and Second Closing and after entering into the First Amendment, which provided that the scheduled third closing would be split into two separate closings, issued and sold Notes and Note Warrants for aggregate total proceeds of $6.25 million in the Third Closing. After the Third Closing, the Company entered into the Second Amendment, which set a deadline of December 30, 2016 for the final closing and provided the consent of the holders of the Notes to the Company reducing the conversion price of the Notes from time to time in order to incentivize the holders of the Notes to convert their Notes into shares of the Company’s common stock. As the final closing did not occur prior to the December 30, 2016 deadline, the remaining $6.25 million of Notes was not funded. Additionally, after entering into the Second Amendment, the Company reduced the conversion price of the Notes frequently in order to incentivize the holders of the Notes to convert all of the outstanding amounts outstanding under the Notes. As of December 31, 2016, all of the Notes were fully repaid.
During the year ended December 31, 2016, $18.7 million of aggregate principal amount of Notes were converted by holders of the Notes into approximately 2,632,000 shares of the Company’s common stock.
Description of the Notes
The Notes were payable in monthly installments, accrued interest at a rate of 7.0% per annum from the date of issuance and had a maturity date 24 months after the First Closing. The Notes were repayable, at the Company’s election, in either cash or shares of the Company’s common stock at a discount to the then-current market price. The Notes were also convertible from time to time, at the election of the holders, into shares of the Company’s common stock at an initial conversion price of $304.50 per share. The conversion price was adjusted to $76.30 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Notes. The Notes also allowed the Company to reduce the conversion price from time-to-time, upon the holders’ consent, which was provided for in the Second Amendment.
The holder of each Note had the right to convert any portion of such Note unless the holder, together with its affiliates, beneficially owned in excess of 4.99% of the number of shares of the Company’s common stock outstanding
immediately after giving effect to the conversion, as such percentage ownership was determined in accordance with the terms of the Notes. The holders were also able to increase or decrease such percentage to any other percentage, but in no event above 9.99%, provided that any increase of such percentage would not be effective until 61 days after providing notice to the Company.
The Company determined that the conversion feature in the Notes requires bifurcation and liability classification and measurement, at fair value, and requires evaluation at each reporting period. Under Accounting Standards Codification (ASC) 825, Financial Instruments, the FASB provides an alternative to bifurcation and companies may instead elect fair value measurement for the entire instrument, including the debt and conversion feature. The Company elected the fair value alternative in order to simplify its accounting and reporting of the Notes upon issuance. The fair value of the Note Warrants was recorded as a discount to the Notes and amortized to interest expense following the effective interest rate method over the term of the Notes.
The First Closing occurred on November 9, 2015. At the First Closing, the Company issued and sold Notes with an aggregate principal amount of $1.5 million, along with Note Warrants exercisable for 1,679 shares. During the quarter ended September 30, 2016, all remaining principal and interest amounts outstanding under the Notes issued at the First Closing were paid off via conversions to common shares.
The Second Closing occurred on January 11, 2016 after the Company received approval of the offering by the Company’s stockholders and the satisfaction of certain customary closing conditions. At the Second Closing, the Company issued and sold Notes with an aggregate principal amount of $11.0 million, along with Note Warrants exercisable for 12,312 shares. The fair value of Note Warrants issued on January 11, 2016 was determined to be $515,000 using a Black-Scholes valuation model. During the quarter ended December 31, 2016, all remaining principal and interest amounts outstanding under Notes issued at the Second Closing were paid off via conversions to common shares.
The Third Closing occurred on May 2, 2016 after the Company entered into the First Amendment and satisfied certain closing conditions. At the Third Closing, the Company issued and sold Notes with an aggregate principal amount of $6.25 million, along with Note Warrants exercisable for 6,995 shares. The fair value of the Note Warrants issued on May 2, 2016 was determined to be $150,195 using a Black-Scholes valuation model. During the quarter ended December 31, 2016, all remaining principal and interest amounts outstanding under Notes issued at the Third Closing were paid off via conversions to common shares.
On December 31, 2015, the fair value of the outstanding Notes was determined to be $1.3 million using a Binomial Lattice model.
The following table summarizes the installment amounts and additional conversions by the holders of the Notes through December 31, 2016:
First Closing:
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | Interest | | Total | | Shares | |
Installment amount at December 31, 2015 | | $ | 65,217 | | $ | 23,651 | | $ | 88,868 | | 814 | |
Holder conversions during the quarter ended December 31, 2015 | | | 18,261 | | | 2,375 | | | 20,636 | | 189 | |
Total installments and conversions, December 31, 2015 | | | 83,478 | | | 26,026 | | | 109,504 | | 1,003 | |
Installment amount at February 29, 2016 | | | 65,217 | | | 23,681 | | | 88,898 | | 1,314 | |
Installment amount at March 31, 2016 | | | 65,217 | | | 14,827 | | | 80,044 | | 1,271 | |
Holder conversions during the quarter ended March 31, 2016 | | | 104,784 | | | 12,762 | | | 117,546 | | 1,524 | |
Total installments and conversions, March 31, 2016 | | | 318,696 | | | 77,296 | | | 395,992 | | 5,112 | |
Installment amount at April 30, 2016 | | | 65,217 | | | 13,853 | | | 79,070 | | 1,454 | |
Installment amount at May 31, 2016 | | | 65,217 | | | 13,082 | | | 78,299 | | 2,121 | |
Installment amount at June 30, 2016 | | | 54,217 | | | 11,275 | | | 65,492 | | 3,590 | |
Holder conversions during the quarter ended June 30, 2016 | | | 1,627 | | | 174 | | | 1,801 | | 29 | |
Total installments and conversions, June 30, 2016 | | | 504,974 | | | 115,680 | | | 620,654 | | 12,306 | |
Installment amount at July 31, 2016 | | | 65,217 | | | 10,148 | | | 75,365 | | 5,521 | |
Installment amount at August 31, 2016 | | | 46,957 | | | 5,830 | | | 52,787 | | 4,593 | |
Holder conversions during the quarter ended September 30, 2016 | | | 882,852 | | | 78,634 | | | 961,486 | | 72,528 | |
Total installments and conversions, September 30, 2016 and December 31, 2016 | | $ | 1,500,000 | | $ | 210,292 | | $ | 1,710,292 | | 94,948 | |
Second Closing:
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | Interest | | Total | | Shares | |
| | | | | | | | | | | | |
Installment amount at March 2, 2016 | | $ | 404,762 | | $ | 149,300 | | $ | 554,062 | | * | |
Holder conversions during the quarter ended March 31, 2016 | | | 987,000 | | | 124,050 | | | 1,111,050 | | 14,974 | |
Total installments and conversions, March 31, 2016 | | | 1,391,762 | | | 273,350 | | | 1,665,112 | | 14,974 | |
Installment amount at April 29, 2016 | | | 404,762 | | | 149,497 | | | 554,259 | | 10,190 | |
Installment amount at May 31, 2016 | | | 291,428 | | | 86,518 | | | 377,946 | | 10,238 | |
Installment amount at June 30, 2016 | | | 404,762 | | | 82,913 | | | 487,675 | | 22,842 | |
Holder conversions during the quarter ended June 30, 2016 | | | 25,373 | | | 2,995 | | | 28,368 | | 414 | |
Total installments and conversions, June 30, 2016 | | | 2,518,087 | | | 595,273 | | | 3,113,360 | | 58,658 | |
Installment amount at July 31, 2016 | | | 213,429 | | | 47,457 | | | 260,886 | | 19,113 | |
Installment amount at August 31, 2016 | | | 631,429 | | | 116,511 | | | 747,940 | | 64,810 | |
Installment amount at September 30, 2016 | | | 404,762 | | | 45,846 | | | 450,608 | | 51,698 | |
Holder conversions during the quarter ended September 30, 2016 | | | 4,868,679 | | | 418,847 | | | 5,287,526 | | 418,253 | |
Total installments and conversions, September 30, 2016 | | | 8,636,386 | | | 1,223,934 | | | 9,860,320 | | 612,532 | |
Installment amount at Oct 31, 2016 | | | 340,000 | | | 24,738 | | | 364,738 | | 70,665 | |
Installment amount at Nov 30, 2016 | | | 291,429 | | | 27,528 | | | 318,957 | | 81,952 | |
Installment amount at December 31, 2016 | | | 156,867 | | | 11,425 | | | 168,292 | | 57,453 | |
Holder conversions during the quarter ended December 31, 2016 | | | 1,575,318 | | | 122,624 | | | 1,697,942 | | 450,385 | |
Total installments and conversions, December 31, 2016 | | $ | 11,000,000 | | $ | 1,410,249 | | $ | 12,410,249 | | 1,272,987 | |
Third Closing
| | | | | | | | | | | | |
| | | | | | | | | | | Common | |
| | Principal | | Interest | | Total | | Shares | |
| | | | | | | | | | | | |
Installment amount at June 30, 2016 | | $ | 212,158 | | $ | 90,659 | | $ | 302,817 | | 16,600 | |
Holder conversions during the quarter ended June 30, 2016 | | | — | | | — | | | — | | — | |
Total installments and conversions, June 30, 2016 | | | 212,158 | | | 90,659 | | | 302,817 | | 16,600 | |
Installment amount at July 31, 2016 | | | 147,368 | | | 32,374 | | | 179,742 | | 13,168 | |
Cash Payment – July 31, 2016 installment | | | 42,105 | | | 6,107 | | | 48,212 | | * | |
Installment amount at August 31, 2016 | | | 336,842 | | | 62,059 | | | 398,901 | | 34,684 | |
Installment amount at September, 2016 | | | 263,158 | | | 41,822 | | | 304,980 | | 34,523 | |
Holder conversions during the quarter ended September 30, 2016 | | | 1,915,698 | | | 175,092 | | | 2,090,790 | | 155,272 | |
Total installments and conversions, September 30, 2016 | | | 2,917,329 | | | 408,113 | | | 3,325,442 | | 254,247 | |
Installment amount at Oct 31, 2016 | | | 221,053 | | | 35,004 | | | 256,057 | | 48,192 | |
Installment amount at Nov 30, 2016 | | | 221,053 | | | 31,259 | | | 252,312 | | 64,828 | |
Installment amount at December 31, 2016 | | | 221,053 | | | 14,526 | | | 235,579 | | 81,872 | |
Holder conversions during the quarter ended December 31, 2016 | | | 2,669,512 | | | 170,359 | | | 2,839,871 | | 816,707 | |
Total installments and conversions, December 31, 2016 | | $ | 6,250,000 | | $ | 659,261 | | $ | 6,909,261 | | 1,265,846 | |
* Cash payments
Description of the Note Warrants
Each Note Warrant was exercisable immediately and for a period of 60 months from the date of the issuance of the Note Warrant. After completion of the Third Closing, the Note Warrants entitle their holders to purchase, in aggregate, 27,982 shares of the Company’s common stock. The Note Warrants were initially exercisable at an exercise price equal to $325.50, subject to adjustment on the eighteen month anniversary of issuance, and certain other adjustments. The exercise price and number of shares of common stock issuable on the exercise of the Note Warrants was subject to adjustment upon the issuance of any shares of common stock or securities convertible into shares of common stock below the then-existing exercise price, with certain exceptions. Additionally, the exercise price and number of shares of common stock issuable upon the exercise of the Note Warrants were subject to adjustment in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction.
The exercise price of the Note Warrants issued November 9, 2015 was reduced to $76.30 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Note Warrants. Per the terms of the Note Warrants, the exercise price of each of the Note Warrants issued January 11, 2016 and May 2, 2016 remained $325.50 until January 20, 2017, the 16th trading day following the Second Reverse Stock Split, at which point the price of all of the Note Warrants was adjusted to $2.18 per share. All remaining Note Warrants were exercised during the first quarter of 2017.
(11)Stock Sales
January 2017 Issuance of Common Stock, Convertible Preferred Stock and Warrants
On January 23, 2017, the Company closed an underwritten public offering consisting of units of common stock, convertible preferred stock and warrants to purchase common stock. Gross proceeds of the offering were $19.0 million, prior to deducting underwriting discounts and commissions and offering expenses of $2.5 million.
The offering was comprised of Class A Units, priced at a public offering price of $5.31 per unit, with each unit consisting of one share of common stock and one five-year warrant (each, a "2017 Warrant") to purchase one share of common stock with an exercise price of $5.84 per share, and Class B Units, priced at a public offering price of $1,000 per unit, with each unit comprised of one share of Series A Preferred Stock (the Preferred Stock), which was convertible into 188 shares of common stock, and 2017 Warrants to purchase 188 shares of common stock. The conversion price of the Preferred Stock issued in the transaction as well as the exercise price of the 2017 Warrants are fixed priced and do
not contain any variable pricing features nor any price based anti-dilutive features apart from customary adjustments for splits and reverse splits of common stock and both have been recorded within Shareholders’ Equity in the condensed consolidated balance sheet. The Preferred Stock included a beneficial ownership limitation of 4.99%, but had no dividend preference (except to extent dividends are also paid on the common stock), liquidation preference or other preferences over common stock. The securities comprising the units were issued separately in the offering.
A total of 1,218,107 shares of common stock, 12,531 shares of Preferred Stock convertible into 2,359,894 shares of common stock, and 2017 Warrants to purchase 3,577,994 shares of common stock were issued in the offering including the underwriters’ exercise of their over-allotment option to purchase 466,695 shares of common stock and 2017 Warrants to purchase an additional 466,695 shares of common stock.
On January 23 and January 24, 2017 all shares of Preferred Stock issued in conjunction with the offering were converted by their holders into 2,359,894 shares of common stock.
August 2017 Issuance of Convertible Preferred Stock and Warrants
On August 16, 2017, the Company closed a firm commitment underwritten public offering of 20,000 units consisting of one share of Series B Convertible Preferred Stock, par value $0.01 per share (the “Series B Preferred Stock”), which is convertible into 435 shares of Common Stock, at a conversion price of $2.30 per share, and one seven-year warrant to purchase 435 shares of Common Stock at an exercise price of $2.30 per share, at a public offering price of $1,000 per unit.
The net proceeds received by the Company from the sale of the units was approximately $18.0 million, after deducting approximately $2.0 million of underwriting discounts and offering expenses.
The Series B Preferred Stock was determined to not be mandatorily redeemable under ASC 480. Additionally, the Company identified two embedded features within the Series B Preferred Stock: (1) optional conversion by the holder, and (2) redemption in the event of a fundamental change and the Company determined that neither of these embedded features required bifurcation under ASC 815. Since the Series B Preferred Stock is only redeemable in an ordinary liquidation, upon the occurrence of a fundamental transaction which is solely within the Company’s control, or in circumstances when all common shareholders are entitled to receive the same form of consideration, the Series B Preferred Stock is presented within permanent equity. The Series B Preferred Stock contains an anti-dilution feature that requires the Company to adjust the conversion price in the event of future stock sales at a lower unit price. In the event anti-dilution provision is triggered, the Company is required to evaluate whether a contingent beneficial conversion feature has been met and, if so, evaluate and account for any value attributable to the contingent beneficial conversion feature.
The warrants issued with the Series B Preferred Stock were also classified in stockholders’ equity as they are both indexed to the Company’s own stock and meet the scope exception in ASC 815-10-15-74(a) and, accordingly, do not require derivative liability accounting pursuant to ASC 815. The terms of the warrants contain an anti-dilution feature that, if triggered, require the Company to evaluate and account for the value attributable to the reduced warrant exercise price.
As of December 31, 2017, 13,945 of the 20,000 shares of the Series B Preferred Stock issued in the offering had been converted into 6,066,075 shares of Common Stock.
On August 16, 2017, the Company also issued warrants to purchase an aggregate of 2,575,000 shares of Common Stock to certain parties (each, a “Holder”) to the Securities Purchase Agreement (as amended, the “Purchase Agreement”), dated November 4, 2015, between the Company and the other parties named therein, as consideration for the waiver by each of the Holders of their right to participate in future securities offerings by the Company, which rights were granted pursuant to the Purchase Agreement. These warrants are in substantially the same form, and on the same terms as, the Warrants issued pursuant to the Offering. Because the Company received no additional consideration or future rights related to the warrants issued to the Holders, the Black Scholes value of the warrants was recorded as $4.4 million of expense as of the August 16, 2017 issuance date. The Black Scholes value was estimated using a risk-free interest rate of 2.03%, an expected life of 7.0 years, expected dividends of zero and expected volatility of 112.03%.
Sales Agreement—July 2015
On July 8, 2015, the Company closed a public offering, where it sold 30,476 units at an aggregate price of $525.00 per unit, for gross proceeds of $16.0 million before deducting estimated offering expenses of approximately $1.4 million, of which $532,000 was assigned to the warrants issued with each unit sold and was recognized immediately as interest expense in the consolidated statements of operations as the warrants are exercisable upon issuance. Each unit consisted of: (A)(i) one share of common stock or (ii) one pre-funded Series C warrant to purchase one share of common stock at an exercise price equal to $525.00 per share (Series C Warrant); and (B) one Series A Warrant with an exercise price initially equal to $630.00 per share (Series A Warrant). Each purchaser of a unit could elect to receive a Series C Warrant in lieu of a share of common stock. No Series C Warrants were issued.
The Series A Warrants are exercisable for a period of 42 months from the closing date of the public offering. The exercise price and number of shares of common stock issuable on the exercise of the Series A Warrants are subject to adjustment upon the issuance of any shares of common stock or securities convertible into shares of common stock below the then-existing exercise price, with certain exceptions, and in the event of any stock split, reverse stock split, recapitalization, reorganization or similar transaction. The holder of the Series A Warrant does not have the right to exercise any portion of the Series A Warrant if the holder, together with its affiliates, would, subject to certain limited exceptions, beneficially own in excess of 9.99% of the Company’s common stock outstanding immediately after the exercise or 4.99% as may be elected by the purchaser.
The exercise price of the Series A Warrants issued July 8, 2015 was reduced to $168.00 per share on November 9, 2015 as a result of the issuance of the Notes and was further reduced to $67.90 per share on January 29, 2016, the 16th trading day following the First Reverse Stock Split, per the terms of the Series A Warrants and was further reduced at various times during the year ended December 31, 2016 as a result of installment and acceleration payments made on the Notes. As of December 31, 2016, the exercise price of the warrants was $2.80 per share and on January 20, 2017, the 16th trading day following the Second Reverse Stock Split, the exercise price of the Series A Warrants was adjusted to $2.18 per share, per the terms of the Series A Warrants.
(12)Income Taxes
On December 22, 2017, H.R. 1, originally known as the Tax Cuts and Jobs Act ("2017 Tax Act"), was enacted into law in the United States, resulting in significant changes from previous tax law. The 2017 Tax Act reduces the federal corporate income tax rate to 21% effective January 1, 2018. The rate change resulted in a reduction of our net deferred tax assets, before application of the valuation allowance, of $37.1 million. The Company recorded a $2.3 million tax benefit in the fourth quarter of 2017 related to the remeasurement of the deferred tax liability for the indefinite-lived intangible asset related to the BarioSurg acquisition in May 2017 (see Note 4).
In December 2017, the SEC staff issued Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the Tax Cuts and Jobs Act (“SAB 118”), which allows for the recording of provisional amounts during a measurement period not to extend beyond one year of the enactment date since the 2017 Tax Act was passed late in the fourth quarter of 2017 and ongoing guidance from the Internal Revenue Service and Treasury is expected over the next 12 months. As a result of the 2017 Tax Act, NOLs generated in taxable years ending after December 31, 2017 have an indefinite carryforward period. The Company continues to evaluate the extent that deductible temporary differences are expected to reverse and generate an indefinite-lived NOL and the related impact on the valuation allowance. The accounting for the valuation allowance is, therefore, considered to be incomplete due to the forthcoming guidance and the Company’s ongoing analysis of final year-end data and tax positions. In accordance with SAB 118, a provisional tax benefit of $2.3 million was recorded, and the Company expects to complete its analysis within the measurement period.
The Company has incurred net operating losses (NOLs) since inception. The Company has not reflected any benefit of such net operating loss carryforwards in the accompanying consolidated financial statements.
Income tax expense (benefit) for the three years ended December 31 was as follows:
| | | | | | | | |
| 2017 | | 2016 | | 2015 |
Deferred: | | | | | | | | |
Federal | $ | (2,538,611) | | $ | — | | $ | — |
State | | 224,000 | | | — | | | — |
Total income tax expense (benefit) | $ | (2,314,611) | | $ | — | | $ | — |
The income tax expense benefit differed from the amount computed by applying the U.S. federal income tax rate of 34% to income before income taxes as a result of the following:
| | | | | | | | | |
| | 2017 | | | 2016 | | | 2015 | |
Computed "expected" tax benefit | | 34.0 | % | | 34.0 | % | | 34.0 | % |
Other permanent adjustments | | (5.5) | % | | 3.1 | % | | 1.6 | % |
Research and development credit | | 0.7 | % | | 0.6 | % | | 0.9 | % |
Change in Federal tax rate | | (102.5) | % | | — | % | | — | % |
Federal valuation allowance | | 79.7 | % | | (37.7) | % | | (36.5) | % |
| | 6.4 | % | | — | % | | — | % |
The tax effect of temporary differences that give rise to significant portions of the deferred tax assets as of December 31 is presented below:
| | | | | | |
| | 2017 | | 2016 |
Deferred tax assets (liabilities): | | | | | | |
Start-up costs | | $ | 4,166,000 | | $ | 6,662,000 |
Capitalized research and development costs | | | 12,494,000 | | | 23,012,000 |
Reserves and accruals | | | 7,642,000 | | | 8,933,000 |
Property and equipment | | | 46,000 | | | 83,000 |
Research and development credit | | | 4,387,000 | | | 2,198,000 |
Net operating loss carryforwards | | | 63,794,000 | | | 41,657,000 |
Total gross deferred tax assets | | | 92,529,000 | | | 82,545,000 |
Valuation allowance | | | (86,033,000) | | | (82,545,000) |
Deferred tax assets | | | 6,496,000 | | | — |
Intangible assets | | | (11,788,000) | | | — |
Total gross deferred tax liabilities | | | (11,788,000) | | | — |
Net deferred tax liability | | $ | (5,292,000) | | $ | — |
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during periods in which those temporary differences become deductible. In addition, certain limitations imposed under the Internal Revenue Code (IRC) could further limit the Company’s realization of these deferred tax assets in the event of changes in ownership of the Company, as defined by IRC Section 382. Based on the level of historical taxable losses, projections of future taxable income (losses) over the periods in which the deferred tax assets can be realized, and consideration of the “more likely than not standard” required by ASC 740, management currently believes the Company will not realize the benefits of these deductible differences, except to the extent of reversing taxable temporary differences. Accordingly, the Company has provided a valuation allowance against the net deferred tax assets, excluding deferred tax liabilities for indefinite-lived intangible assets, as of December 31, 2017 and 2016.
The Company’s ability to utilize its net operating loss carryforwards, tax credits, and built-in items of deduction, including capitalized start-up costs and research and development costs, may be substantially limited due to ownership changes that may have occurred or that could occur in the future. These ownership changes may limit the amount of net operating loss carryforwards, credits and built-in items of deduction that can be utilized annually to offset future taxable income. In general, an ownership change, as defined in IRC Section 382, results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50% of the outstanding stock of a company by certain stockholders or public groups. During 2011, the Company completed an IRC Section 382 review
and the results of the review indicated that ownership changes had occurred. While the Company has not completed an IRC Section 382 review since 2011, it believes that it is likely that additional ownership changes have occurred since then. Since the Company has experienced an ownership change, utilization of carryforward attributes are subject to an annual limitation, which is determined by first multiplying the value of the Company’s common stock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any such limitation may result in the expiration of a significant portion of the carryforward attributes before utilization and the permanent loss of built-in items of deduction. Any carryforward attributes that expire prior to utilization or permanent loss of built-in items of deduction as a result of such limitations will be removed from deferred tax assets with a corresponding adjustment to the valuation allowance. Due to the existence of the valuation allowance, it is not expected that any possible limitation will have an impact on the results of operations of the Company.
As of December 31, 2017, the Company has generated or acquired U.S. federal net operating loss carryforwards of approximately $253.8 million. Of the total federal net operating loss, $48.0 million will expire unused as a result of the 2011 Section 382 limitation. The federal net operating loss carryforwards expire in the years 2022 through 2037. The Company’s research and development credit carryforwards, if not used, begin to expire in 2024.
Net operating loss carryforwards of the Company are subject to review and possible adjustment by the taxing authorities. With certain exceptions (e.g. the net operating loss carryforwards), the Company is no longer subject to U.S. federal, state or local examinations by tax authorities for years prior to 2014. There are no tax examinations currently in progress.
(13)Stock Options
The Company has adopted the Second Amended and Restated 2003 Stock Incentive Plan (the Plan) that includes both incentive stock options and nonqualified stock options to be granted to employees, officers, consultants, independent contractors, directors and affiliates of the Company. At December 31, 2017 and 2016, according to the Plan 40,000,000 shares were authorized and reserved. Pursuant to the terms of the Plan, the shares authorized under the Plan were not adjusted automatically as part of the Second Reverse Stock Split. Instead, pursuant to the terms of the Plan, the board of directors exercised its power to adjust the number of shares authorized under the Plan as it determines is necessary after a stock split or other similar event to prevent dilution or enlargement of the benefits intended to be made available under the Plan. On February 8, 2017, pursuant to the terms of the Plan, the board of directors adjusted the number of shares authorized under the Plan to 3,000,000 shares as a result of the recapitalization of the Company consisting of the Second Reverse Stock Split and the public offering of the Company’s stock which closed on January 23, 2017. Pursuant to the terms of the Plan, the board of directors is required to adjust the number of shares authorized under the Plan as it determines necessary after a recapitalization or other similar corporate transaction to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Plan.
On December 19, 2017, the Company’s stockholders approved an increase in the number of common shares reserved under the Plan by 5,500,000 shares to a total of 8,500,000 authorized shares.
The board of directors establishes the terms and conditions of all stock option grants, subject to the Plan and applicable provisions of the IRC. Incentive stock options must be granted at an exercise price not less than the fair market value of the common stock on the grant date. The options granted to participants owning more than 10% of the Company’s outstanding voting stock must be granted at an exercise price not less than 110% of fair market value of the common stock on the grant date. The options expire on the date determined by the board of directors, but may not extend more than 10 years from the grant date, while incentive stock options granted to participants owning more than 10% of the Company’s outstanding voting stock expire five years from the grant date. The vesting period for employees is generally over four years. The vesting period for nonemployees is determined based on the services being provided.
Stock option activity for the Plan is as follows:
| | | | | | | | | | |
| | | | Outstanding Options | | | |
| | | | | | | | | | |
| | Shares | | | | | | | Aggregate |
| | Available For | | Number of | | Weighted-Average | | Intrinsic |
| | Grant | | Shares (1) | | Exercise Price (1) | | Value |
Balance, December 31, 2014 | | 6,740 | | 12,051 | | $ | 2,597.00 | | $ | 519,546 |
Shares reserved | | — | | — | | | — | | | |
Options granted | | (3,238) | | 3,238 | | | 1,104.00 | | | |
Options exercised | | — | | — | | | — | | | |
Options cancelled | | 735 | | (735) | | | 1,942.00 | | | |
| | | | | | | | | | |
Balance, December 31, 2015 | | 4,237 | | 14,554 | | | 2,298.00 | | $ | — |
Shares reserved (1) | | 2,976,486 | | — | | | | | | |
Options granted | | (2,174) | | 2,174 | | | 45.35 | | | |
Options exercised | | — | | — | | | | | | |
Options cancelled | | 9,694 | | (9,694) | | | 2,134.47 | | | |
| | | | | | | | | | |
Balance, December 31, 2016 | | 2,988,243 | | 7,034 | | | 1,824.87 | | $ | — |
Shares reserved (2) | | 5,500,000 | | — | | | — | | | |
Options granted | | (2,686,371) | | 2,686,371 | | | 4.26 | | | |
Options exercised | | — | | — | | | — | | | |
Options cancelled | | 162,650 | | (162,650) | | | 16.63 | | | |
| | | | | | | | | | |
Balance, December 31, 2017 | | 5,964,522 | | 2,530,755 | | $ | 8.68 | | $ | — |
| (1) | | Reflects the board of directors’ February 8, 2017 adjustment of number of shares reserved under the Plan from 40,000,000 to 3,000,000. |
| (2) | | Reflects approval by the Company’s stockholders to increase the number of shares reserved under the Plan by 5,500,000 on December 19, 2017. |
On June 27, 2016 the Company completed an option exchange offer to its employees whereby certain outstanding options to purchase shares of the Company’s common stock were tendered by employees in exchange for new options with the exercise price to be set at the then current market price of the Company’s common stock. Options to purchase 6,424 shares of the Company’s common stock, which included all the options eligible for exchange, were tendered by employees and cancelled by the Company. On the same date, options to purchase 1,083 shares of the Company’s common stock were issued with an exercise price of $23.28 per share, which was the Company’s closing stock price on June 27, 2016. Because the fair value of the tendered options immediately before the exchange approximated the fair value of the new options granted, no additional compensation expense was recognized.
In addition to the stock options granted pursuant to the Plan, the Company from time to time grants options to individuals as an inducement to accepting positions as employees (Inducement Grants). These Inducement Grants are made at the discretion of the board of directors and are issued outside of the Plan. Inducement Grants are summarized below:
| | | | | | | | | | |
| | | | Outstanding Options | | | |
| | | | | | | | | | |
| | Shares | | | | | | | Aggregate |
| | Available For | | Number of | | Weighted-Average | | Intrinsic |
| | Grant | | Shares | | Exercise Price | | Value |
Balance, December 31, 2014 | | — | | — | | | — | | | |
Shares reserved | | 7,380 | | — | | | | | | |
Options granted | | (7,380) | | 7,380 | | $ | 262.50 | | | |
Options exercised | | — | | — | | | | | | |
Options cancelled | | — | | — | | | — | | | |
| | | | | | | | | | |
Balance, December 31, 2015 | | — | | 7,380 | | | 262.50 | | $ | — |
Shares reserved | | 5,426 | | | | | | | | |
Options granted | | (5,426) | | 5,426 | | | 94.05 | | | |
Options exercised | | — | | — | | | | | | |
Options cancelled | | — | | — | | | | | | |
| | | | | | | | | | |
Balance, December 31, 2016 | | — | | 12,806 | | | 191.12 | | $ | — |
Shares reserved | | 1,379,000 | | — | | | — | | | |
Options granted | | (1,379,000) | | 1,379,000 | | | 2.04 | | | |
Options exercised | | — | | — | | | — | | | |
Options cancelled | | — | | (92,114) | | | 4.18 | | | |
| | | | | | | | | | |
Balance, December 31, 2017 | | — | | 1,299,692 | | $ | 3.75 | | $ | — |
Each of the Inducement Grants will vest as follows: 25% of the shares will vest as of one year from the date of the officer’s employment agreement, and the remaining 75% of the shares will then vest in equal 2.0833% installments each month thereafter for 36 months. The options awarded as Inducement Grants were not eligible for the option exchange program
The options outstanding, vested and currently exercisable for the Plan and Inducement Grants are set forth by exercise price at December 31, 2017 in the following table:
| | | | | | | | | | | | | | | |
| | Outstanding Options and Expected to Vest | | Options Exercisable and Vested |
| | | | Weighted-Average | | | | | | | | | | | |
| | Number of | | Remaining | | Aggregate | | | | | | | Aggregate |
Exercise | | Shares | | Contractual Life | | Intrinsic | | Number of | | Weighted-Average | | Intrinsic |
Price | | Outstanding | | (Years) | | Value | | Options | | Exercise Price | | Value |
$1.64 to $1.96 | | 310,600 | | 9.8 | | $ | — | | — | | | n/a | | $ | — |
$2.00 to $2.05 | | 2,381,321 | | 9.8 | | | — | | — | | | n/a | | | — |
$4.28 to $7.12 | | 1,122,117 | | 9.2 | | | — | | 526,896 | | $ | 7.07 | | | — |
$11.99 to $96.60 | | 4,172 | | 8.1 | | | — | | 3,284 | | | 79.20 | | | — |
$241.50 to $924.00 | | 7,706 | | 7.8 | | | — | | 4,321 | | | 271.43 | | | — |
$1,165.50 and over | | 4,531 | | 5.1 | | | — | | 4,527 | | | 2,537.71 | | | — |
| | 3,830,447 | | 9.6 | | $ | — | | 539,028 | | $ | 30.88 | | $ | — |
Stock-Based Compensation for Nonemployees
Stock-based compensation expenses related to stock options granted to nonemployees is recognized as the stock options are earned. The Company believes that the fair value of the stock options is more reliably measurable than the fair value of the services received. The fair value of the stock options granted is calculated at each reporting date, using the Black-Scholes option-pricing model, until the award vests or there is a substantial disincentive for the nonemployee
not to perform the required services. The fair value for the years ended December 31, 2017 and 2015 was calculated using the following assumptions, defined below:
| | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Risk-free interest rates | | 2.34%–2.38% | | N/A | | 0.02%–2.10% | |
Expected life | | 9.38 years–10.00 years | | N/A | | 0.08 years–8.51 years | |
Expected dividends | | 0% | | N/A | | 0% | |
Expected volatility | | 114.94%–131.24% | | N/A | | 37.36%–132.01% | |
Stock-based compensation expense charged to operations on options granted to nonemployees for the years ended December 31, 2017, 2016 and 2015 was $184,000, $4,000 and $(35,000), respectively.
Employee Stock-Based Awards
Compensation cost for employee stock-based awards is based on the estimated grant-date fair value and is recognized over the vesting period of the applicable award on a straight-line basis. The weighted average estimated fair value of the employee stock options granted for the years ended December 31, 2017, 2016 and 2015 was $2.82, $60.00, and $426.30, per share, respectively.
The Company uses the Black-Scholes pricing model to determine the fair value of stock options. The determination of the fair value of stock-based payment awards on the date of grant is affected by the Company’s stock price as well as assumptions regarding a number of complex and subjective variables. These variables include the Company’s expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rates and expected dividends. The estimated grant-date fair values of the employee stock options were calculated using the Black-Scholes valuation model, based on the following assumptions for the years ended December 31, 2017, 2016 and 2015:
| | | | | | | |
| | Year Ended December 31, | |
| | 2017 | | 2016 | | 2015 | |
Risk-free interest rates | | 1.94%–2.19% | | 0.87%–1.64% | | 1.49%–1.80% | |
Expected life | | 6.25 years | | 4.0 years–6.25 years | | 5.50 years–6.25 years | |
Expected dividends | | 0% | | 0% | | 0% | |
Expected volatility | | 116.49%–119.54% | | 88.43%–114.38% | | 83.36%–111.77% | |
Expected Life. The expected life is based on the “simplified” method described in the SEC Staff Accounting Bulletin, Topic 14: Share-Based Payment.
Volatility. The expected volatility was based on the Company’s historical volatility.
Risk-Free Interest Rate. The risk-free rate is based on the daily yield curve rate from the U.S. Treasury with remaining terms similar to the expected term on the options.
Dividend Yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future, and, therefore, used an expected dividend yield of zero in the valuation model.
Forfeitures. The Company is required to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods. If the Company’s actual forfeiture rate is materially different from its estimate, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
As of December 31, 2017 there was approximately $7.6 million of total unrecognized compensation costs, net of estimated forfeitures, related to unvested stock option awards ($7.5 million of which relate to employee awards), which are expected to be recognized over a weighted-average period of 3.0 years.
There were no stock option exercises for the years ended December 31, 2017, 2016 and 2015.
(14)Warrants
Stock warrant activity is as follows:
| | | | | |
| | | | Weighted |
| | | | Average |
| | Common | | Exercise |
| | Shares | | Price |
Balance, December 31, 2014 | | 23,044 | | $ | 1,929.20 |
Granted (1) | | 32,155 | | | 116.20 |
Exercised | | — | | | — |
Cancelled | | (324) | | | 2,299.50 |
Balance, December 31, 2015 | | 54,875 | | | 1,929.20 |
Granted (1) | | 19,308 | | | 325.50 |
Exercised | | (1,428) | | | 3.50 |
Cancelled | | (17,706) | | | 2,257.50 |
Balance, December 31, 2016 | | 55,049 | | | 238.90 |
Granted (1) | | 14,852,994 | | | 3.15 |
Exercised | | (599,670) | | | 5.56 |
Cancelled | | (36) | | | 238.90 |
Balance, December 31, 2017 | | 14,308,337 | | $ | 3.51 |
| (1) | | See Notes 11 and 14 for discussions relating to the issuance of warrants in 2017, 2016 and 2015. |
At December 31, 2017, 2016 and 2015, the weighted-average remaining contractual life of outstanding warrants was 6.08, 2.76 and 2.18 years, respectively. All of the warrants outstanding are currently exercisable at the option of the holder into the equivalent number of shares of common stock.
(15)Related Party Transactions
Consulting Agreement—Anthony Jansz
The Company entered into a consulting agreement with Anthony Jansz, who is a former member of the board of directors, for the period from June 1, 2011 through April 30, 2015. In exchange for consulting services provided, Mr. Jansz was entitled to receive consulting fees and options to purchase 16,663 shares of common stock at a weighted average exercise price of $29.78. Total stock-based compensation expense recorded was approximately $600 for the year ended December 31, 2015. Due to a failure to meet certain performance conditions, 471 shares of the options granted to Mr. Jansz did not vest. In addition to the option grants, the Company paid Mr. Jansz approximately $75,000 in fees and expenses for consulting services provided during the year ended December 31, 2015. No consulting fees or expenses were paid to Mr. Jansz in 2016 or 2017.
Consulting Agreement—Jon Tremmel
Effective August 10, 2015, the Company entered into a one year consulting agreement with Jon Tremmel & Associates, LLC, which is wholly-owned by Jon Tremmel, a member of the board of directors. In exchange for consulting services provided, Mr. Tremmel was entitled to receive consulting fees and an option to purchase 16,666 shares of common stock at $3.45 per share. Total stock-based compensation expense recorded was approximately $3,000 and $13,000 for the years ended December 31, 2016 and 2015, respectively. In addition to the option grant, the Company paid Mr. Tremmel approximately $50,000 in fees and expenses for consulting services provided during the year ended December 31, 2015. No consulting fees or expenses were paid to Mr. Tremmel in 2016 or 2017.
(16)Commitments and Contingencies
Operating Lease
The Company rents its office, warehouse and laboratory facilities in St. Paul, Minnesota under an operating lease, which was originally set to expire on September 30, 2015. On August 25, 2015, the Company entered into an amendment extending the term of the operating lease for three years until September 30, 2018, with monthly base rent ranging from $18,925 to $20,345. Total rent expense recognized for the years ended December 31, 2017, 2016 and 2015, respectively, was $357,000, $229,000 and $262,000. Facility related expenses are included as general and administrative costs on the consolidated statements of operations.
In connection with our acquisition of BarioSurg in May 2017, we assumed an operating lease for office space in Lake Forest, California with monthly base rent of $2,818 that expires September 30, 2018.
In connection with our acquisition of ReShape Medical in October 2017, we assumed (i) an operating lease for office/warehouse space in San Clemente, California with monthly base rent ranging from $24,614 to $27,510 that expires June 30, 2022 and (ii) an operating lease for office and manufacturing space in San Clemente, California with monthly base rent of $10,877 that expires October 31, 2019.
The following is a schedule of total future minimum lease payments due as of December 31, 2017:
| | | |
Year ending December 31, | | | |
2018 | | $ | 633,695 |
2019 | | | 419,082 |
2020 | | | 319,262 |
2021 | | | 327,949 |
2022 | | | 165,061 |
| | $ | 1,865,049 |
Product Liability Claims
The Company is exposed to product liability claims that are inherent in the testing, production, marketing and sale of medical devices. Management believes any losses that may occur from these matters are adequately covered by insurance, and the ultimate outcome of these matters will not have a material effect on the Company’s financial position or results of operations. The Company is not currently a party to any product liability litigation and is not aware of any pending or threatened litigation that could have a material adverse effect on the Company’s business, operating results or financial condition.
Clinical Trials
The Company is evaluating the vBloc System in human clinical trials, including the EMPOWER trial and ReCharge trial. Both of these clinical trials require patients to be followed out to 60 months. The Company is required to pay for patient follow up visits only to the extent they occur. In the event a patient does not attend a follow up visit, the Company has no financial obligation. The Company is also required to pay for explants or revisions, including potential conversions of ReCharge control devices to active devices, should a patient request or be required to have one during the course of the clinical trials. The Company has no financial obligation unless an explant, revision or conversion is requested or required. Clinical trial costs are expensed as incurred.
Litigation
On February 28, 2017, the Company received a class action and derivative complaint filed on February 24, 2017 in U. S. District Court for the District of Delaware by Vinh Du, one of the Company’s shareholders. The complaint names as defendants ReShape Lifesciences, the board of directors and four members of our senior management, namely, Scott Youngstrom, Nick Ansari, Peter DeLange and Paul Hickey, and contains a purported class action claim for breach of fiduciary duty against the board of directors and derivative claims for breach of fiduciary duty against the board of directors and unjust enrichment against our senior management. The allegations in the complaint relate to the increase in the number of shares authorized for grant under our Second Amended and Restated 2003 Stock Incentive Plan (the
“Plan”), which was approved by our shareholders at the Special Meeting of Shareholders held on December 12, 2016 (the “Special Meeting”), and to our subsequent grant of stock options on February 8, 2017, to the Company’s Directors and senior management to purchase an aggregate of 1,093,450 shares of our common stock (the “Option Grants”). In the complaint, the plaintiff contends that (i) the number of shares authorized for grant under the Plan, as adjusted by the board of directors after the Special Meeting for the subsequent recapitalization of the Company, resulted from an alleged breach of fiduciary duties by the board of directors, and (ii) our senior management was allegedly unjustly enriched by the subsequent Option Grants. The plaintiff seeks relief in the form of an order rescinding the Plan as approved by the shareholders at the Special Meeting, an order cancelling the Option Grants, and an award to plaintiff for his costs, including fees and disbursements of attorneys, experts and accountants. On April 17, 2017, we filed a motion to dismiss the complaint based on the plaintiff’s failure to satisfy Delaware’s demand requirement for a derivative action and failure to state a valid claim. The court denied the motion to dismiss on November 30, 2017. Discovery is on-going. We believe the allegations in the complaint are without merit, and intend to defend the action vigorously.
On April 20, 2017, Fulfillium, Inc. filed a Complaint against the Company in the United States District Court for the District of Delaware, which alleged misappropriation of trade secrets and infringement of two United States Patents. On July 28, 2017, ReShape Medical moved to dismiss both the misappropriation of trade secret claim and the claims of patent infringement, and to transfer the litigation to the United States District Court for the Central District of California. On October 10, 2017, Fulfillium filed a motion to amend its Complaint to add SV Health Investors, LLC as a co-defendant; that motion is fully briefed and pending. On October 16, 2017, the Court granted ReShape Medical’s motion to dismiss the trade secret and willful infringement claims. The Court also ordered the case transferred to the United States District Court for the Central District of California. On November 20, 2017, Fulfillium filed an Amended Complaint, which abandoned certain trade secret claims, and expanded upon allegations regarding other of its trade secret claims and claims for willful infringement. On December 6, 2017, ReShape Medical moved to dismiss those amended claims and for reconsideration of denial of its prior motion to dismiss certain patent infringement claims; both motions were denied on February 7, 2018. On February 5, 2018, Fulfillium filed a second motion to further amend its Complaint to add a claim of infringement of another Fulfillium patent. ReShape Medical opposed that motion. On February 21, 2018, ReShape Medical filed its Answer and Counterclaims to the Amended Complaint, including counterclaims for declarations of non-infringement, invalidity, unenforceability and/or co-inventorship of the asserted Fulfillium patents and state-law tort claims against Fulfillium and its founder personally. Fulfillium and its founder have not yet responded to those counterclaims. At the initial case management conference held on March 19, 2018, the Court ordered that Fulfillium’s motions to amend be stricken and ordered Fulfillium to file a consolidated motion to amend. The Court encouraged the parties to confer to narrow the disputes for the Court’s consideration, and indicated that Fulfillium need not respond to ReShape Medical’s counterclaims until the scope of the amended complaint was decided. The Court assigned key dates for the litigation, including close of fact discovery on September 15, 2018, last day for filing motions on September 19, 2018, pretrial conference on November 19, 2018 and first day of trial on December 4, 2018. The Company intends to vigorously defend itself against Fulfillium, Inc.’s claims and to vigorously pursue its counterclaims.
We currently are unable to estimate the losses or range of losses for these two matters where there is a reasonable possibility of a loss or it is probable that a loss may have ben incurred.
Except as disclosed in the foregoing paragraphs, the Company is not currently a party to any litigation and the Company is not aware of any pending or threatened litigation against it that could have a material adverse effect on the Company’s business, operating results or financial condition. The medical device industry in which the Company operates is characterized by frequent claims and litigation, including claims regarding patent and other intellectual property rights as well as improper hiring practices. As a result, the Company may be involved in various legal proceedings from time to time.
(17)Retirement Plan
The Company has a 401(k) profit-sharing plan that provides retirement benefits to employees. Eligible employees may contribute a percentage of their annual compensation, subject to Internal Revenue Service limitations. The Company’s matching is at the discretion of the Company’s board of directors. For the years ended December 31, 2017, 2016, and 2015, the Company did not provide any matching of employees’ contributions.
(18)Quarterly Data (unaudited)
The following table represents certain unaudited quarterly information for each of the eight quarters in the period ended December 31, 2016. In management’s opinion, this information has been prepared on the same basis as the audited consolidated financial statements and includes all the adjustments necessary to fairly state the unaudited quarterly results of operations (in thousands, except per share data).
| | | | | | | | | | | | |
| | First Quarter | | Second Quarter (1) | | Third Quarter (1)(2) | | Fourth Quarter (1)(3)(4) |
2017: | | | | | | | | | | | | |
Revenue | | $ | 40 | | $ | 93 | | $ | 360 | | $ | 794 |
Net loss | | $ | (7,367) | | $ | (6,840) | | $ | (9,994) | | $ | (9,617) |
Basic and diluted net loss per share | | $ | (1.27) | | $ | (0.91) | | $ | (1.06) | | $ | (0.45) |
2016: | | | | | | | | | | | | |
Revenue | | $ | 72 | | $ | 276 | | $ | 297 | | $ | 142 |
Net loss | | $ | (7,409) | | $ | (4,995) | | $ | (6,522) | | $ | (4,434) |
Basic and diluted net loss per share | | $ | (66.14) | | $ | (33.96) | | $ | (11.77) | | $ | (2.65) |
| (1) | | The 2017 second, third and fourth quarters contain net losses of $116,000, $327,000 and $389,000, respectively, related to the inclusion of the operations of BarioSurg after its acquisition May 22, 2017. |
| (2) | | The 2017 third quarter net loss includes $4.4 million of expense related to warrants issued to purchase 2,575,000 shares of common stock to former holders of the Company’s convertible notes as consideration for their waiver of their right to participate in future securities offerings by the Company. |
| (3) | | The 2017 fourth quarter contains revenues of $718,000 and net loss of $4.1 million related to the inclusion of the operations of ReShape Medical after its October 2, 2017 acquisition. |
| (4) | | The 2017 fourth quarter net loss includes the effect of a $2.3 million tax benefit related to the remeasurement of a deferred tax liability due to the December enactment of the Tax Cuts and Jobs Act by Congress. |
(19) Subsequent Events
On April 2, 2018 the Company announced that it had entered into a securities purchase agreement with an institutional investor providing for the purchase and sale in a registered direct offering of shares of series D convertible preferred stock and a warrant to purchase shares of common stock for a purchase price of $6.0 million.
The shares of series D convertible preferred stock will be convertible into an aggregate of 8.0 million shares of common stock at a conversion price of $0.75 per share and the warrants will have a one-year term (or, if later, eight months after the requisite stockholder approval is obtained) and be exercisable for 35 million shares of common stock at an exercise price of $0.75 per share. The Company expects to receive net proceeds of approximately $5.25 million after deducting placement agent fees and other offering expenses. If the requisite stockholder approval is obtained, and if the warrants are exercised in full, the Company would receive an additional $26.25 million in gross proceeds based on the initial warrant exercise price of $0.75 per share. The Company intends to use the net proceeds from the registered direct offering to continue its commercialization efforts, for clinical and product development activities, and for other working capital and general corporate purposes. The closing of the offering is expected to take place on or about April 4, 2018, subject to the satisfaction or waiver of customary closing conditions.
The Company intends to ask its stockholders at its 2018 annual meeting for the requisite approval for the conversion or exercise of the securities described above into shares of common stock exceeding 19.99% of the company’s currently outstanding common stock for purposes of the NASDAQ Stock Market Rules and has entered into voting agreements with stockholders representing a majority of the company’s outstanding common stock pursuant to which those stockholders have agreed to vote in favor of that proposal.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), that are intended to ensure that information that would be required to be disclosed in Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer and the Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
We carried out an evaluation, under the supervision, and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2017. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of December 31, 2017 due to the material weakness in internal control over financial reporting related to the design of its internal control over accounting for acquisition-related deferred income taxes as described below.
Notwithstanding the material weakness in our internal control over financial reporting, we have concluded that the consolidated financial statements and other financial information included in the Original Filing, fairly present in all material respects our financial condition, results of operations and cash flows as of, and for, the periods presented.
Management’s Report on Internal Control Over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) promulgated under the Exchange Act as a process, designed by, or under the supervision of the Company’s principal executive and principal financial officers and effected by the Company’s Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions and disposition of assets; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures are made only in accordance with management and Board authorizations; and providing reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Management excluded from its assessment the internal control over financial reporting at ReShape Medical, which was acquired on October 2, 2017 and whose assets constituted 48% of consolidated assets and 56% of consolidated revenues as of and for the year ended December 31, 2017. This exclusion was in accordance with Securities and Exchange Commission guidance that an assessment of a recently acquired business may be omitted in management’s report on internal control over financial reporting in the year of acquisition.
Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with policies or procedures may deteriorate. Management, with
the participation of the Company’s principal executive and principal financial officers, conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2017 based on the framework and criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. This evaluation included review of the documentation of controls, evaluation of the design effectiveness of controls, testing of the operating effectiveness of controls and a conclusion on this evaluation. Based on the foregoing, management concluded that the Company’s internal control over financial reporting was not effective as of December 31, 2017 for the reasons described below.
Management has identified a material weakness in the design of its internal control over financial reporting related to accounting for acquisition-related deferred income taxes. We have developed a remediation plan for this material weakness, which is described below under “Remediation Activities.”
In connection with the completion of the Company’s year-end audit procedures and the preparation of its Annual Report on Form 10-K, management determined an error existed in purchase accounting related to its acquisition of BarioSurg, Inc. that were reflected in the Company’s unaudited condensed consolidated balance sheets as of June 30, 2017 and September 30, 2017 . Specifically, in its allocation of the BarioSurg, Inc. purchase price, the Company failed to record a $7.6 million deferred income tax liability related to the indefinite-lived intangible asset, In-Process Research and Development, with an offsetting increase in Goodwill in its condensed consolidated balance sheets. The Company does not believe that these balance sheet misstatements as of June 30, 2017 and September 30, 2017 were material to its financial statements on those dates taken as a whole. However, the omission of this deferred tax liability led to what we have concluded to be a material weakness in internal control over financial reporting. Specifically, because the unrecorded deferred tax liability relates to an indefinite-lived intangible asset, the Company cannot offset the deferred tax liability with available deferred tax assets when determining its net deferred tax position under generally accepted accounting principles. As a result, when the unrecorded deferred tax liability required remeasurement due to the December 22, 2017 enactment of the Tax Cuts and Jobs Act, this in turn required recognition of a $2.3 million income tax benefit in our consolidated statement of operations for the quarter and year ended December 31, 2017. While the Company utilizes the assistance of an external income tax specialist to prepare its annual tax provision, management has concluded there to be a material weakness in the design of the Company’s income tax controls in that the specialist was not adequately engaged to assist in the determination of deferred taxes associated with material transactions, such as the business acquisitions occurring in 2017.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to permanent exemption rules of the Dodd-Frank Wall Street Reform and Consumer Protection Act that permit the Company to provide only management’s report in this annual report.
Changes in Internal Control Over Financial Reporting
In conjunction with the acquisitions of BarioSurg and ReShape Medical during the year ended December 31, 2017, the Company implemented new controls over the initial valuation of goodwill and other intangible assets as well as the evaluation of those assets for impairment.
Material Weakness Remediation Activities
The Company, the Audit Committee and the Company’s Board of Directors are committed to maintaining a strong internal control environment, and are currently evaluating remediation efforts that will be designed to enhance our control environment. We expect that the remediation efforts will include engaging its external income tax service provider to specifically analyze deferred income tax attributes of acquisitions and other significant transactions and to perform such analysis as promptly as possible after such transactions. Once the remediation plan is finalized and implemented, the identified material weaknesses in internal control over financial reporting will be considered fully addressed when the relevant internal controls have been in operation for a sufficient period of time for our management to conclude that the material weaknesses have been fully remediated and our internal control over financial reporting is effective. The Company will work to design, implement and rigorously test these new controls in order to make these final determinations.
Other than these items and the material weakness noted above, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that occurred during the year
ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III.
Certain information required by Part III is omitted from this report, and is incorporated by reference to our Definitive Proxy Statement to be filed with the SEC pursuant to Regulation 14A (the Proxy Statement) in connection with our 2018 Annual Meeting of Stockholders.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item concerning our directors and executive officers is hereby incorporated by reference to the sections of our Proxy Statement under the headings “Nominees,” “Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Board Meetings and Committees—Audit Committee.”
We have adopted a code of business conduct and ethics, which applies to all directors and employees, including executive officers, including, without limitation, our principal executive officer, principal financial officer, principal accounting officer and persons performing similar functions. A copy of this code of business conduct and ethics is available on our website at www.reshapelifesciences.com (under “Investors,” “Corporate Governance”) and we intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding any waivers from or amendments to any provision of the code of business conduct and ethics by disclosing such information on the same website.
In addition, we intend to promptly disclose (1) the nature of any amendment to our code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our code of business conduct and ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item is hereby incorporated by reference to the sections of our Proxy Statement entitled “Director Compensation,” “Executive Compensation,” “Compensation Committee Interlocks and Insider Participation” and “Compensation Committee Report.”
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
(a) Equity Compensation Plans
The following table sets forth information as of December 31, 2017 with respect to our equity compensation plans:
| | | | | | | | |
| | | | | | | Number of Securities | |
| | Number of | | Weighted- | | Remaining Available | |
| | Securities to be | | Average | | for Future Issuance | |
| | Issued Upon | | Exercise Price | | Under Equity | |
| | Exercise of | | of Outstanding | | Compensation Plans | |
| | Outstanding | | Options, | | (Excluding Securities | |
| | Options, Warrants | | Warrants and | | Reflected in Second | |
Plan Category | | and Rights | | Rights | | Column) | |
Equity compensation plans approved by security holders | | 2,530,755 | (1) | $ | 8.68 | | 5,964,522 | (2) |
Equity compensation plans not approved by security holders | | 1,299,692 | (3) | | 3.75 | | — | |
Total | | 3,830,447 | | $ | 7.01 | | 5,964,522 | |
(1)Consists of options awarded under the Amended and Restated 2003 Stock Incentive Plan, which was amended and restated as the Second Amended and Restated 2003 Stock Incentive Plan (the “Plan) on December 12, 2016.
(2)Represents the maximum number of shares of common stock available to be awarded under the Plan as of December 31, 2017 adjusted to reflect: (i) the Company’s board of directors’ February 8, 2017 action to adjust the number of shares reserved under the Plan from 40,000,000 to 3,000,000 in connection with the Company’s recapitalization and (ii) approval by the Company’s stockholders to increase the number of common shares reserved under the Plan by 5,500,000 on December 19, 2017
(3)Consists of the inducement grants awarded in 2015, 2016 and 2017 to newly hired executives and other employees.
(b) Security Ownership
The information required by this Item is hereby incorporated by reference to the section of our Proxy Statement entitled “Security Ownership of Certain Beneficial Owners and Management.”
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is hereby incorporated by reference to the section of our Proxy Statement entitled “Certain Relationships and Related Transactions, and Director Independence.”
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item is hereby incorporated by reference to the section of our Proxy Statement entitled “Principal Accountant Fees and Services” and “Administration of Engagement of Independent Auditor.”
PART IV.
ITEM 15. EXHIBITS, FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULES
(a) Financial Statements and Schedules: Consolidated Financial Statements for the three years ended December 31, 2017 are included in Part II, Item 8 of this Annual Report on Form 10-K. All schedules are omitted because they are not applicable or the required information is shown in the consolidated financial statements or notes thereto.
(b) Exhibits: The list of exhibits on the Exhibit Index on page 108 of this report is incorporated herein by reference.
ITEM 16. FORM 10-K SUMMARY
Not applicable
EXHIBIT INDEX
| | |
Exhibit
Number | | Description of Document |
1.1 | | Underwriting Agreement, dated August 11, 2017, among the Company and Ladenburg Thalmann & Co. Inc., as representative of the underwriters named therein (incorporated by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 16, 2017). |
2.1 | | Agreement and Plan of Merger, dated as of May 22, 2017, by and among EnteroMedics Inc., BarioSurg, Inc., Acorn Subsidiary Inc., Acorn Subsidiary Holdings LLC and the Stockholder Representative (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 23, 2017). |
2.2 | | Agreement and Plan of Merger, dated as of October 2, 2017, by and among the Company, ReShape Medical, Inc., Nixon Subsidiary Inc., Nixon Subsidiary Holdings LLC and the ReShape Holder Committee (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 3, 2017). |
3.1 | | Sixth Amended and Restated Certificate of Incorporation of the Company. (Incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on December 28, 2016 (File No. 1-33818)). |
3.2 | | Form of Certificate of Designation of Series A Preferred Stock. (Incorporated herein by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-1 filed on January 11, 2017 (File No. 333-213704)). |
3.3 | | Form of Series A Preferred Stock Certificate. (Incorporated herein by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-1 filed on January 11, 2017 (File No. 333-213704)). |
3.4 | | Amended and Restated Bylaws of the Company, as currently in effect. (Incorporated herein by reference to Exhibit 3.4 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 filed on July 6, 2007 (File No. 333-143265)). |
3.5 | | Certificate of Designation of Conditional Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K/A filed with the Securities and Exchange Commission on August 1, 2017). |
3.6 | | Certificate of Designation of Series B Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 16, 2017). |
3.7 | | Certificate of Designation of Series C Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 3, 2017). |
3.8 | | Certificate of Amendment to Sixth Amended and Restated Certificate of Incorporation of the Company, dated October 20, 2017 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 23, 2017). |
3.9 | | Certificate of Amendment to Sixth Amended and Restated Certificate of Incorporation of the Company, dated October 26, 2017 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 30, 2017). |
10.1 | | Form of Warrant to purchase stock under Loan and Security Agreement, dated April 16, 2012, between the Company and Silicon Valley Bank. (Incorporated herein by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on May 10, 2012 (File No. 1-33818)). |
10.2 | | Securities Purchase Agreement, dated as of February 22, 2013, by and between Craig-Hallum Capital Group LLC and the Company. (Incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on February 22, 2013 (File No. 1-33818)). |
10.3 | | Form of Common Stock Warrant, dated as of February 22, 2013, by and between the Company and several accredited investors. (Incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on February 22, 2013 (File No. 1-33818)). |
10.4 | | Sales Agreement, dated as of June 13, 2014, by and between Cowen and Company, LLC and the Company. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 13, 2014 (File No. 1-33818)). |
10.5 | | Amendment No. 1 to the Sales Agreement, dated as of August 25, 2015, by and between Cowen and Company, LLC and the Company. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 1, 2015 (File No. 1-33818)). |
10.6 | | Underwriting Agreement, dated as of July 7, 2015, by and between Canaccord Genuity Inc. and the Company. (Incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on July 7, 2015 (File No. 1-33818)). |
10.7 | | Form of Series A Warrant, dated as of July 8, 2015, by and between the Company and several accredited investors. (Incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on July 7, 2015 (File No. 1-33818)). |
10.8 | | Form of Series C Warrant, dated as of July 8, 2015, by and between the Company and several accredited investors. (Incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on July 7, 2015 (File No. 1-33818)). |
10.9 | | Form of Securities Purchase Agreement. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 5, 2015 (File No. 1-33818)). |
10.10 | | Form of Amendment No. 1 to the Securities Purchase Agreement dated November 4, 2015, between the Company and the buyers listed therein. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 6, 2016 (File No. 1-33818)). |
10.11 | | Form of Amendment No. 2 to the Securities Purchase Agreement dated November 4, 2015, as amended by Amendment No.1 thereto dated May 2, 2016, among the Company and the buyers listed therein. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 15, 2016 (File No. 1-33818)). |
10.12 | | Form of Senior Convertible Note. (Incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on November 5, 2015 (File No. 1-33818)). |
10.13 | | Form of Warrant. (Incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on November 5, 2015 (File No. 1-33818)). |
10.14 | | Form of Underwriting Agreement. (Incorporated herein by reference to Exhibit 1.1 to the Company’s Registration Statement on Form S-1 filed on January 11, 2017 (File No. 333-213704)). |
10.15 | | Form of Warrant to purchase shares of Common Stock. (Incorporated herein by reference to Exhibit 4.3 to the Company’s Registration Statement on Form S-1 filed on January 11, 2017 (File No. 333-213704)). |
10.16 | | Warrant Agency Agreement, by and between the Company and Wells Fargo Bank, National Association, dated January 20, 2017. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 24, 2017 (File No. 1-33818)). |
10.17† | | Second Amended and Restated 2003 Stock Incentive Plan. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 14, 2017 (File No. 1-33818)). |
10.18† | | Standard form of Incentive Stock Option Agreement pursuant to the Amended and Restated 2003 Stock Incentive Plan. (Incorporated herein by reference to Exhibit 10.13 to the Company’s Registration Statement on Form S-1 filed on May 25, 2007 (File No. 333-143265)). |
10.19† | | Standard form of Non-Incentive Stock Option Agreement pursuant to the Amended and Restated 2003 Stock Incentive Plan. (Incorporated herein by reference to Exhibit 10.14 to the Company’s Registration Statement on Form S-1 filed on May 25, 2007 (File No. 333-143265)). |
10.20† | | Form of Non-Incentive Stock Option Agreement for the new options granted October 29, 2010 pursuant to the option exchange program. (Incorporated herein by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q filed on November 8, 2010 (File No. 1-33818)). |
10.21† | | Standard form of Restricted Stock Agreement. (Incorporated herein by reference to Exhibit 10.15 to the Company’s Registration Statement on Form S-1 filed on May 25, 2007 (File No. 333-143265)). |
10.22† | | Form of Indemnification Agreement entered into by and between the Company and each of its executive officers and directors. (Incorporated herein by reference to Exhibit 10.17 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 filed on July 6, 2007 (File No. 333-143265)). |
10.23† | | Inducement Option Plan. (Incorporated herein by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on January 22, 2016 (File No. 1-33818)). |
10.24† | | Form of Non-Incentive Stock Option Agreement pursuant to the Inducement Option Plan. (Incorporated herein by reference to Exhibit 10.33 to the Company’s Annual Report on Form 10-K filed on March 28, 2016 (File No. 1-33818)). |
10.25† | | Form of Non-Incentive Stock Option Agreement for New Options granted June 27, 2016 pursuant to the option exchange offer. (Incorporated herein by reference to Exhibit (d)(6) to the Company’s Tender Offer Statement under Section 14(d)(1) on Schedule TO filed on May 27, 2016). |
10.26† | | Executive Employment Agreement, dated October 28, 2015, by and between the Company and Dan W. Gladney. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 2, 2015 (File No. 1-33818)). |
10.27† | | Form of Non-Incentive Stock Option Agreement for non-plan executive inducement option grants. (Incorporated herein by reference to Exhibit 10.47 to the Company’s Annual Report on Form 10-K filed on March 28, 2016 (File No. 1-33818)). |
10.28† | | Executive Employment Agreement, dated January 19, 2016, by and between the Company and Naqeeb “Nick” Ansari. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 22, 2016 (File No. 1-33818)). |
10.29† | | Executive Employment Agreement, dated January 18, 2016, by and between the Company and Peter DeLange. (Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on January 22, 2016 (File No. 1-33818)). |
10.30† | | Executive Employment Agreement, dated January 22, 2016, by and between the Company and Paul Hickey. (Incorporated herein by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on January 22, 2016 (File No. 1-33818)). |
10.31† | | Executive Employment Agreement, dated October 3, 2016, by and between the Company and Scott Youngstrom. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 6, 2016 (File No. 1-33818)). |
10.32† | | Management Incentive Plan. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 12, 2008 (File No. 1-33818)). |
10.33† | | Amendments to the Management Incentive Plan described in Item 5.02(e). (Incorporated herein by reference to Item 5.02(e) of the Company’s Current Report on Form 8-K filed on May 10, 2016 (File No. 1-33818)). |
10.34† | | Amendments to the Management Incentive Plan described in Item 5.02(e). (Incorporated herein by reference to Item 5.02(e) of the Company’s Current Report on Form 8-K filed on September 20, 2016 (File No. 1-33818)). |
10.35 | | Lease Agreement, effective October 1, 2008, by and between the Company and Roseville Properties Management Company. (Incorporated herein by reference to Exhibit 10.23 to the Company’s Annual Report on Form 10-K filed on March 12, 2009 (File No. 1-33818)). |
10.36 | | First Amendment to Lease Agreement, entered into August 25, 2015, by and between the Company and Roseville Properties Management Company. (Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on September 1, 2015 (File No. 1-33818)). |
10.37 | | Exclusive Federal Government Business Channel Sales Agreement, effective April 25, 2016, by and between the Company and Academy Medical, LLC, as amended July 26, 2016. (Incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on August 12, 2016 (File No. 1-33818)). |
10.38* | | Lease agreement, entered into January 20, 2017, by and between ReShape Medical, Inc. and San Clemente Holdings, LLC. |
10.39* | | Lease agreement, entered into March 28, 2008 and as amended, by and between ReShape Medical, Inc. and Richard G. Henderson. |
10.40* | | Consulting Agreement, entered into March 1, 2017, by and between ReShape Medical, Inc. and Human Capital SAL. |
10.41* | | International Distributorship Agreement, entered into May 24, 2017, by and between ReShape Medical, Inc. and Al Danah Medical Company. |
10.42* | | International Distributorship Agreement, entered into May 26, 2017, by and between ReShape Medical, Inc. and Al Zahrawi Medical Supplies LLC. |
10.43* | | International Distributorship Agreement, entered into May 11, 2017, by and between ReShape Medical, Inc. and Shifli Gulf for Trading Drugs & Equipment and Devices Company. |
10.44* | | International Distributorship Agreement, entered into July 31, 2017, by and between ReShape Medical, Inc. and Dar Al Zahrawi Medical LLC. |
10.45* | | Royalty Agreement, entered into December 18, 2006 and as amended, by and between Abdominis, Inc. and Intersect Partners, LLC. |
10.46* | | Royalty Agreement, entered into December 18, 2006, by and between Abdominis, Inc. and John Alverdy, M.D. |
10.47 | | Clinical Trial Agreement by and between EnteroMedics Inc. and Southern California Permanente Medical Group effective as of June 1, 2017 (Incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on May 15, 2017 (File No. 1-33818)). |
10.48 | | Voting Agreement and Irrevocable Proxy, dated as of May 22, 2017, by and between EnteroMedics Inc. and Dr. Raj Nihalani (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 23, 2017) |
10.49† | | Executive Employment Agreement, dated as of May 22, 2017, by and between EnteroMedics Inc. and Dr. Raj Nihalani (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 23, 2017) |
10.50† | | Non-Competition and Non-Solicitation Agreement, dated as of May 22, 2017, by and between EnteroMedics Inc. and Dr. Raj Nihalani (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 23, 2017) |
10.51 | | Form of Warrant, dated August 16, 2017 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 16, 2017). |
10.52 | | Warrant Agency Agreement, by and between the Company and Wells Fargo Bank, National Association, dated August 16, 2017 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 16, 2017). |
10.53 | | Form of Voting and Standstill Agreement between the Company and certain ReShape Medical, Inc. Holders (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 3, 2017). |
10.54† | | 2017 Employment Inducement Incentive Award Plan (Incorporated herein by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2017 (File No. 1-33818)). |
10.55† | | Form of Stock Option Grant Notice and Stock Option Agreement under 2017 Employment Inducement Incentive Award Plan (Incorporated herein by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2017 (File No. 1-33818)). |
10.56† | | Form of Stock Option Grant Notice and Stock Option Agreement under Second Amended and Restated 2003 Stock Incentive Plan (Incorporated herein by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed on November 14, 2017 (File No. 1-33818)). |
10.57† | | Second Amended and Restated 2003 Stock Incentive Plan, as amended on December 19, 2017 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 22, 2017) |
14.1 | | Code of Conduct and Ethics of the Company. (Incorporated herein by reference to Exhibit 14.1 to the Company’s Registration Statement on Form S-1 filed on May 25, 2007 (File No. 333-143265)). |
23.1* | | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm. |
24.1* | | Power of Attorney (included on signature page to this Form 10-K). |
31.1* | | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2* | | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1* | | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
32.2* | | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101* | | Financial statements from the Annual Report on Form 10-K of the Company for the year ended December 31, 2017, formatted in Extensible Business Reporting Language: (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Stockholders’ Equity; (iv) the Consolidated Statements of Cash Flows and (v) the Notes to Consolidated Financial Statements. |
* Filed herewith.
† Indicates management contract or compensation plan or agreement.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| | RESHAPE LIFESCIENCES INC. |
| | |
| By: | /S/ DAN W. GLADNEY |
| | Dan W. Gladney |
| | Chairman, President and Chief Executive Officer |
Dated: April 2, 2018
POWERS OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dan W. Gladney and Scott P. Youngstrom, and each of them, as his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them or their substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/S/ DAN W. GLADNEY | | Chairman of the Board, President, and Chief Executive Officer (principal executive officer) | | April 2, 2018 |
Dan W. Gladney |
| | | | |
/S/ SCOTT P. YOUNGSTROM | | Senior Vice President and Chief Financial Officer (principal financial and accounting officer) | | April 2, 2018 |
Scott P. Youngstrom | |
| | | | |
/S/ GARY D. BLACKFORD | | Director | | April 2, 2018 |
Gary D. Blackford | | |
| | | | |
/S/ MICHAEL Y. MASHAAL, M.D. | | Director | | April 2, 2018 |
Michael Y. Mashaal, M.D. | |
| | | | |
/S/ BOBBY I. GRIFFIN | | Director | | April 2, 2018 |
Bobby I. Griffin | |
| | | | |
/S/ LORI C. MCDOUGAL | | Director | | April 2, 2018 |
Lori McDougal | |
| | | | |
/S/ JON T. TREMMEL | | Director | | April 2, 2018 |
Jon T. Tremmel | |