Exhibit 99.2
Study Update: OCU400 Phase 1/2 for RP and LCA A PHASE 1/2 STUDY TO ASSESS THE SAFETY AND EFFICACY OF OCU400 FOR RETINITIS PIGMENTOSA ASSOCIATED WITH NR2E3 AND RHO MUTATIONS AND LEBER CONGENITAL AMAUROSIS WITH MUTATION(S) IN CEP290 GENE

2 Forward Looking Statements This presentation contains forward - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 , which are subject to risks and uncertainties, including, but not limited to, statements regarding qualitative assessments of ava ilable data, potential benefits, expectations for ongoing clinical trial results, and anticipated timing of clinical trial updates a nd regulatory interactions. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimat es, ” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertain ty of future events or outcomes to identify these forward - looking statements. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations, in cluding, but not limited to, the risks that preliminary, interim and top - line clinical trial results may not be indicative of, and may di ffer from, final clinical data; that unfavorable new clinical trial data may emerge in the Phase 1/2 clinical trial or through further a nal yses of existing clinical trial data; that earlier non - clinical and clinical data and testing of may not be predictive of the results or success of later clinical trials; and that that clinical trial data are subject to differing interpretations and assessments, including by regulatory authorities. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Ex change Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual rep ort s that we file with the SEC. Any forward - looking statements that we make in this presentation speak only as of the date of this presentation. Except as required by law, we assume no obligation to update forward - looking statements contained in this presentation whether as a result of new information, future events, or otherwise, after the date of this presentation.
OCU400: A Novel Gene - Agnostic Modifier Gene Therapy for RP and LCA FDA granted expanded Orphan Drug Designations for all retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA) mutations Despite its high prevalence, RP and LCA patients have limited treatment options • US: RP & LCA affect 110,000 and 15,000 people, respectively • Worldwide: conditions affect approximately 1.6M people Current approved and in - development gene therapies focus on individual gene • More than 125 mutated genes associated with RP and LCA • Developing a single therapy to treat each mutation is not feasible and a daunting task OCU400 addresses shortcomings of current gene therapy approaches • Broad - spectrum, gene - agnostic approach to genetically diverse inherited retinal diseases • Consists of human Nuclear Hormone Receptor gene, NR2E3 , with potential to preserve/improve/restore retina function • Being developed as one - time, curative therapy with a single sub - retinal injection, using NR2E3 Dose escalation and recruitment of RP patients completed • High dose established as Maximum Tolerable Dose (MTD) • Continue to enroll patients with LCA and Pediatric patients • Intend to initiate a Phase 3 trial near the end of 2023/early 2024* * Depends on FDA alignment on Phase 3 clinical development plan 1
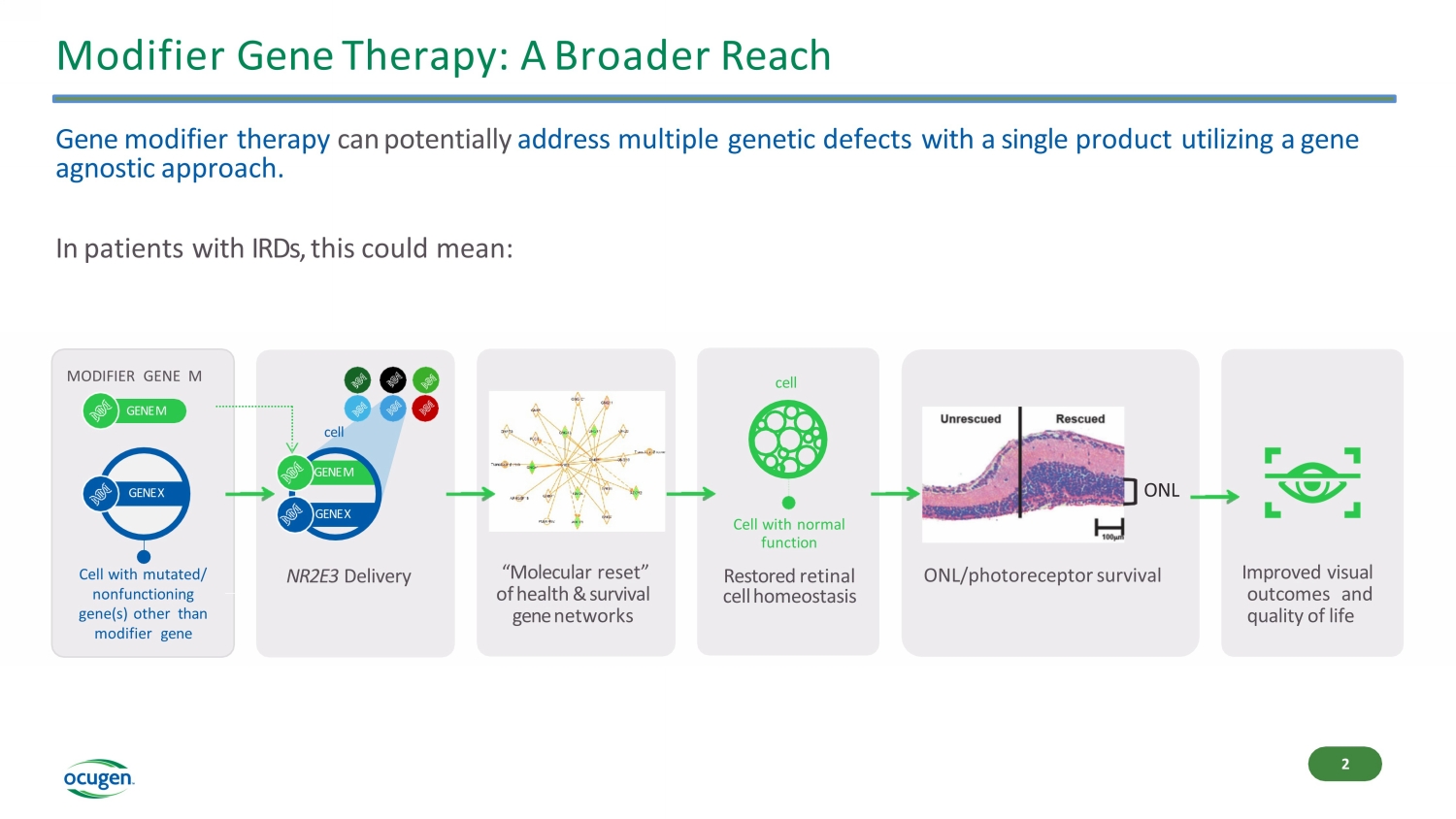
Modifier Gene Therapy: A Broader Reach Gene modifier therapy can potentially address multiple genetic defects with a single product utilizing a gene agnostic approach. In patients with IRDs, this could mean: Improved visual outcomes and quality of life GENE X GENE M cell NR2E3 Delivery “Molecular reset” of health & survival gene networks cell Cell with normal function Restored retinal cell homeostasis ONL ONL/photoreceptor survival GENE X Cell with mutated/ nonfunctioning gene(s) other than modifier gene MODIFIER GENE M GENE M 2
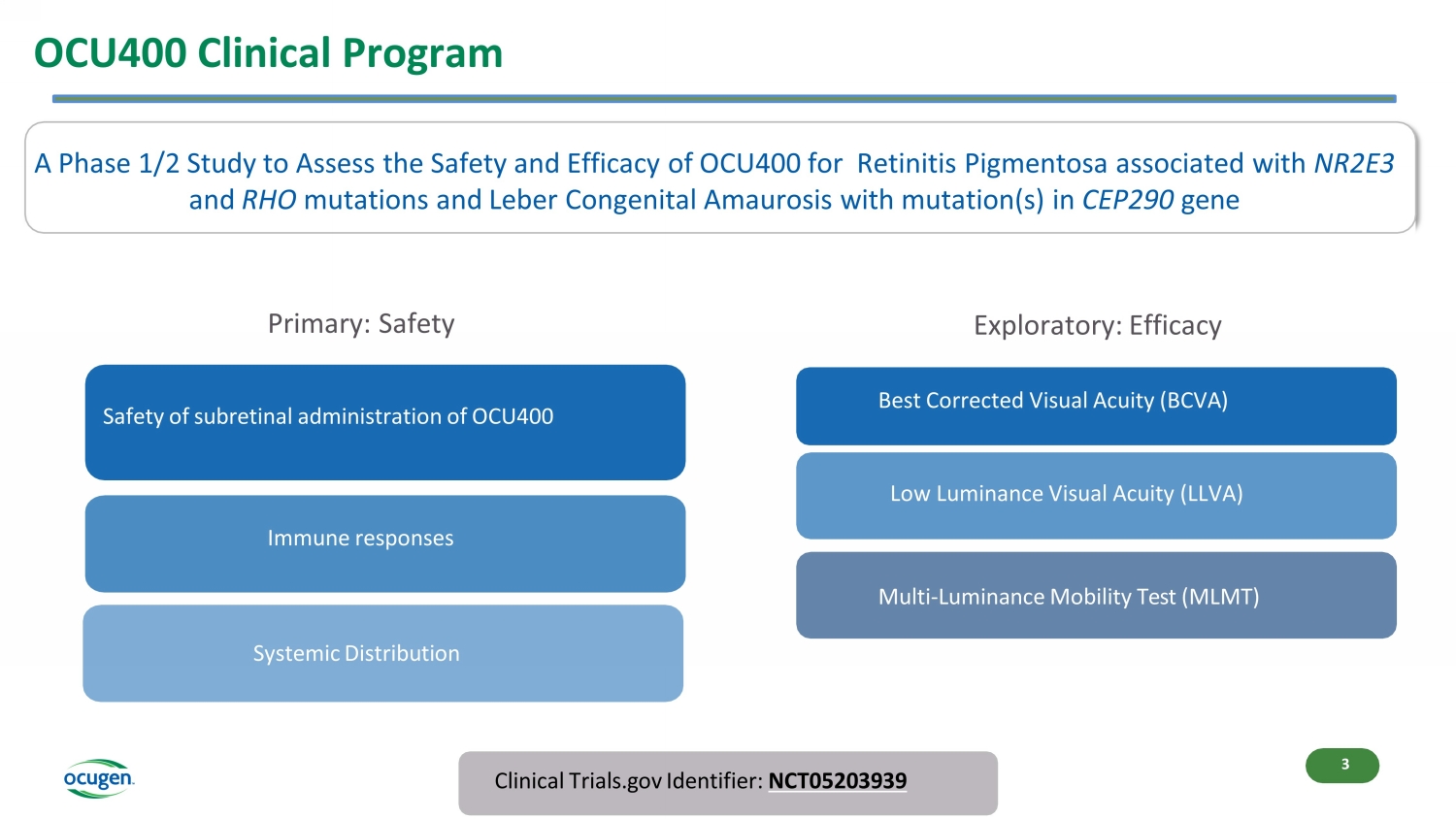
OCU400 Clinical Program A Phase 1/2 Study to Assess the Safety and Efficacy of OCU400 for Retinitis Pigmentosa associated with NR2E3 and RHO mutations and Leber Congenital Amaurosis with mutation(s) in CEP290 gene Clinical Trials.gov Identifier: NCT05203939 Safety of subretinal administration of OCU400 Immune responses Systemic Distribution Best Corrected Visual Acuity (BCVA) Multi - Luminance Mobility Test (MLMT) Low Luminance Visual Acuity (LLVA) Primary: Safety Exploratory: Efficacy 3
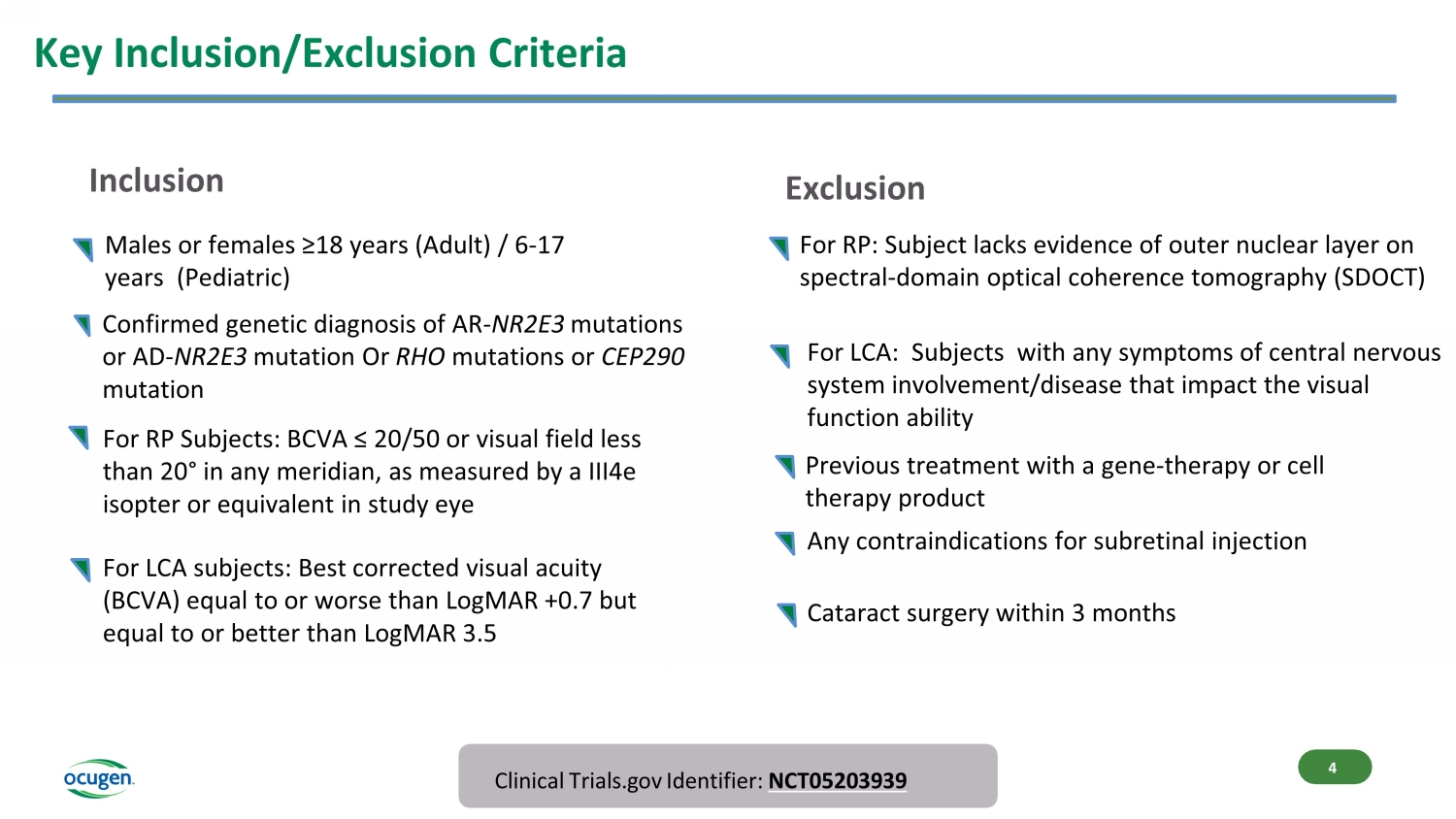
Key Inclusion/Exclusion Criteria Clinical Trials.gov Identifier: NCT05203939 For RP Subjects: BCVA ≤ 20/50 or visual field less than 20 ° in any meridian, as measured by a III4e isopter or equivalent in study eye Inclusion Exclusion 4 Confirmed genetic diagnosis of AR - NR2E3 mutations or AD - NR2E3 mutation Or RHO mutations or CEP290 mutation Males or females ≥18 years (Adult) / 6 - 17 years (Pediatric) For RP: Subject lacks evidence of outer nuclear layer on spectral - domain optical coherence tomography (SDOCT) Previous treatment with a gene - therapy or cell therapy product Any contraindications for subretinal injection Cataract surgery within 3 months For LCA subjects: Best corrected visual acuity (BCVA) equal to or worse than LogMAR +0.7 but equal to or better than LogMAR 3.5 For LCA: Subjects with any symptoms of central nervous system involvement/disease that impact the visual function ability

Enrollment Status 5 Adult RP AR NR2E3 n=5 AD NR2E3 n=3 AD RHO n=10 AR=AutosomalRecessive,AD=AutosomalDominant COMPLETED Adult LCA ARCEP290 n=3 Pediatric RP/LCA AR CEP290, AD RHO, AR/ADNR2E3 n=3 ENROLLING 5 • Subjects (N=18) with ages ranging 18 - 77 years enrolled in this study to date • Consists of 10 RHO subjects, 5 Autosomal Recessive NR2E3 and 3 Autosomal Dominant NR2E3 subjects • Sub - retinal injection was mostly around the central region (and few subjects with central temporal, superior - temporal regions)
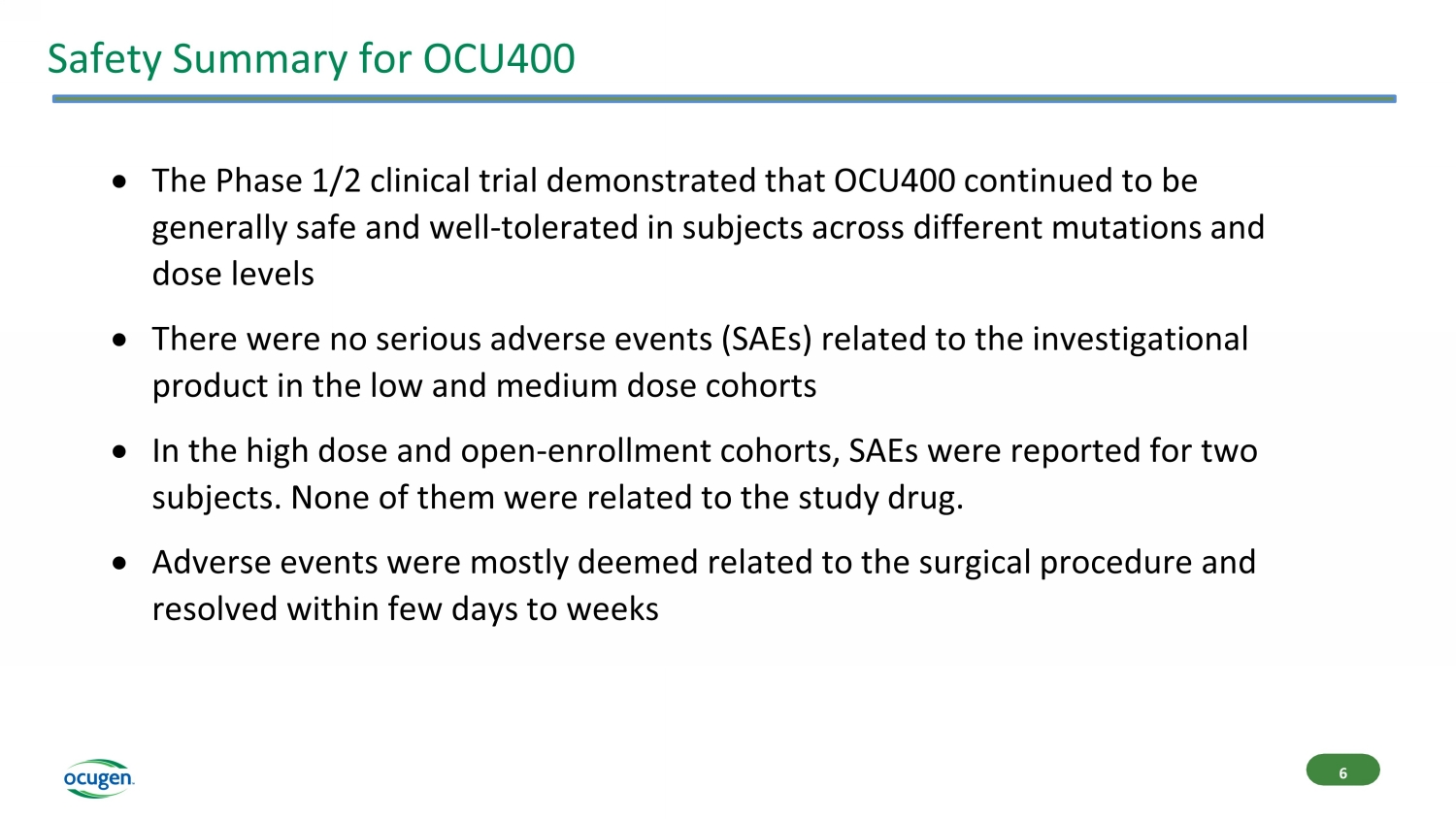
Safety Summary for OCU400 The Phase 1/2 clinical trial demonstrated that OCU400 continued to be generally safe and well - tolerated in subjects across different mutations and dose levels There were no serious adverse events (SAEs) related to the investigational product in the low and medium dose cohorts In the high dose and open - enrollment cohorts, SAEs were reported for two subjects. None of them were related to the study drug. Adverse events were mostly deemed related to the surgical procedure and resolved within few days to weeks 6
RP and LCA - Unmet need and Treatment Benefit Target 7 • IRDs, such as RP and LCA, are a group of heterogenous genetic disorders that affect the retina, the light - sensitive tissue at the back of the eye • They often lead to a gradual loss of vision over time and can ultimately result in blindness • Stabilization of vision is crucial for patients with RP and LCA due to the progressive and degenerative nature of these diseases • Preservation of remaining vision, slowing disease progression, or improving the vision can significantly impact patients’ quality of life. Such outcomes not only can enhance the quality of life for affected individuals but also provide hope that future treatments could ultimately lead to vision restoration. • Comprehensive care, early diagnosis, and access to emerging therapies are essential components of a strategy to stabilize vision in RP and LCA patients
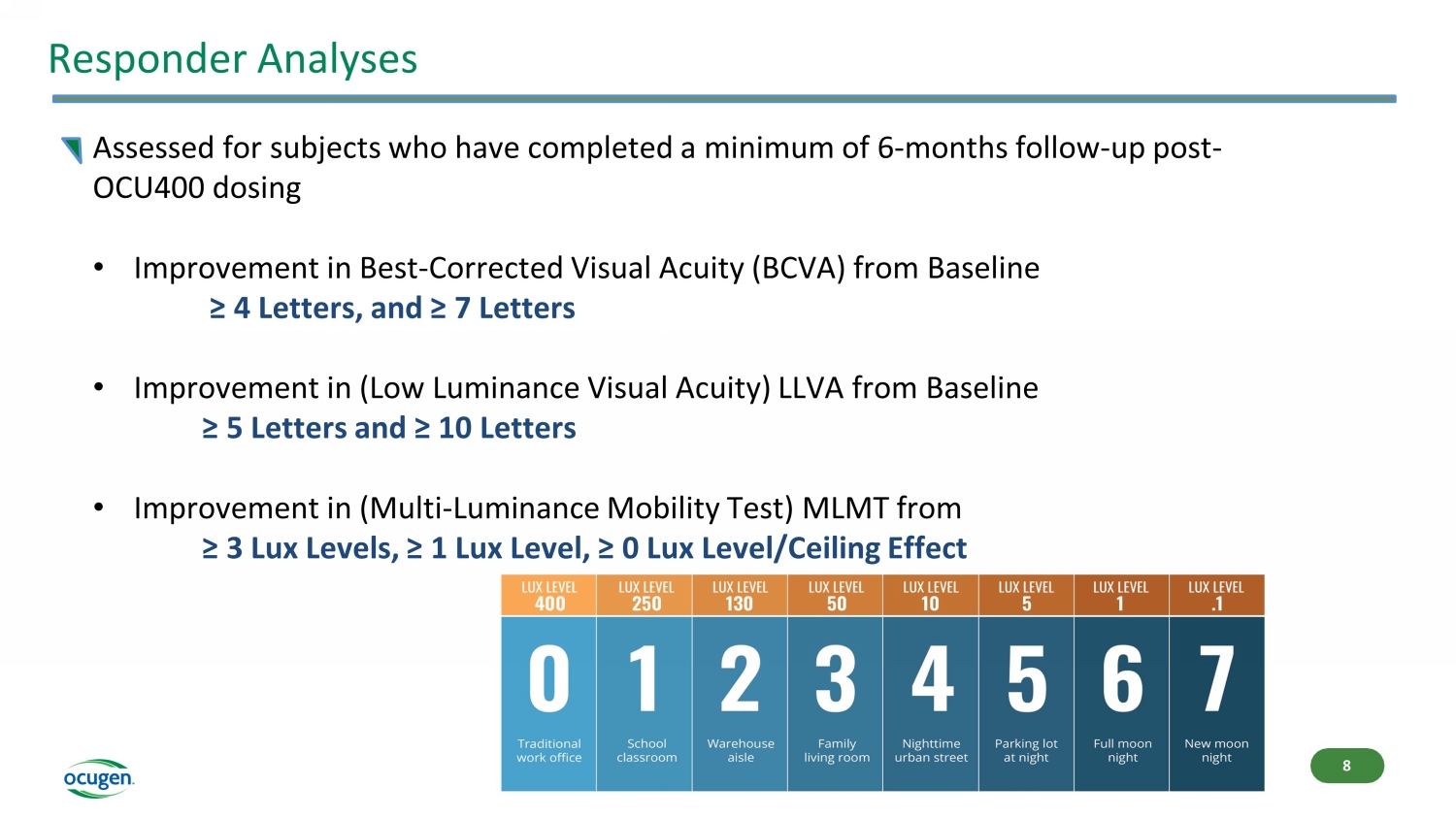
Responder Analyses Assessed for subjects who have completed a minimum of 6 - months follow - up post - OCU400 dosing • Improvement in Best - Corrected Visual Acuity (BCVA) from Baseline ≥ 4 Letters, and ≥ 7 Letters • Improvement in (Low Luminance Visual Acuity) LLVA from Baseline ≥ 5 Letters and ≥ 10 Letters • Improvement in (Multi - Luminance Mobility Test) MLMT from ≥ 3 Lux Levels, ≥ 1 Lux Level, ≥ 0 Lux Level/Ceiling Effect 8
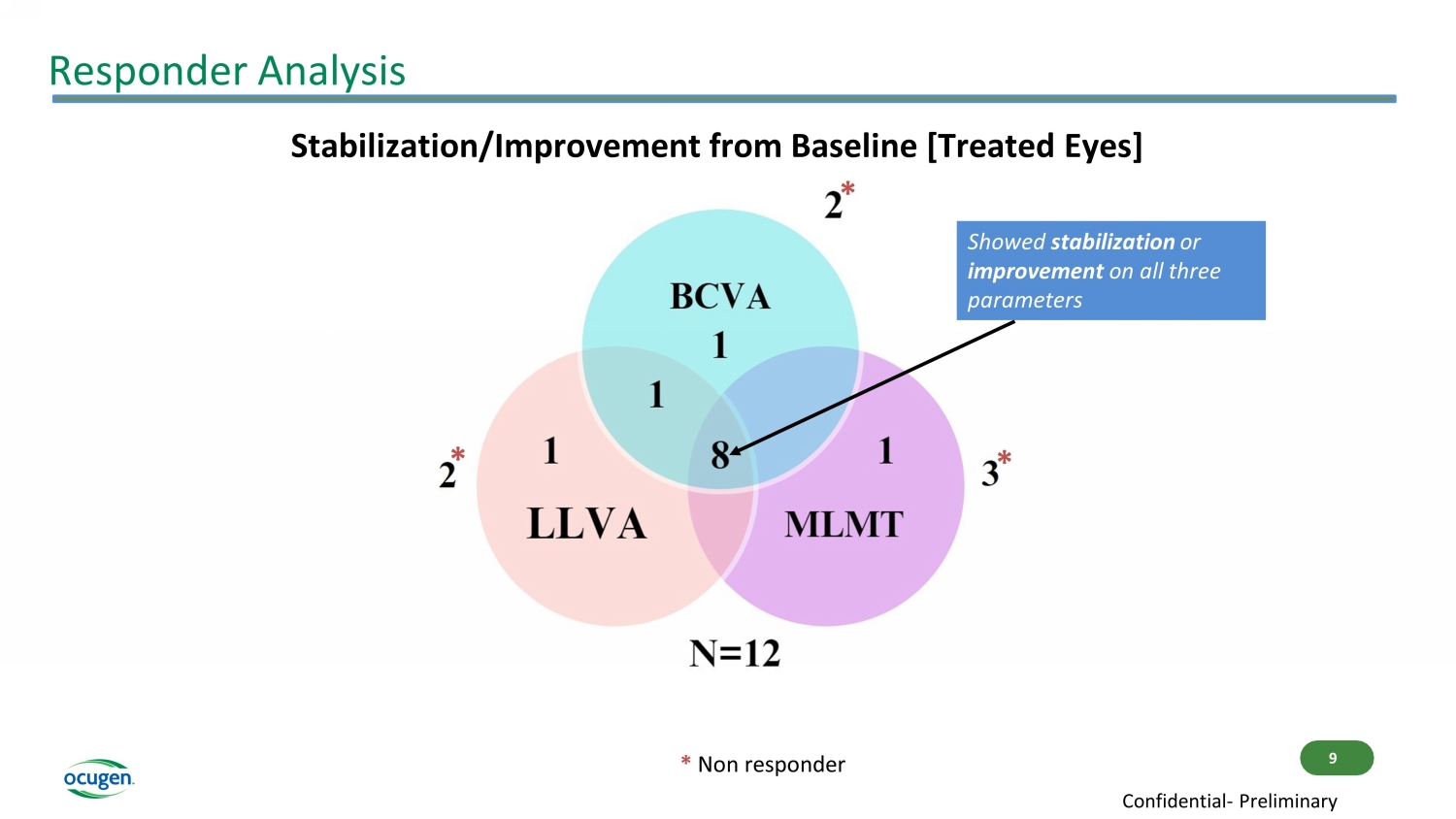
Responder Analysis Stabilization/Improvement from Baseline [Treated Eyes] * * * * Non responder 9 Confidential - Preliminary Showed stabilization or improvement on all three parameters
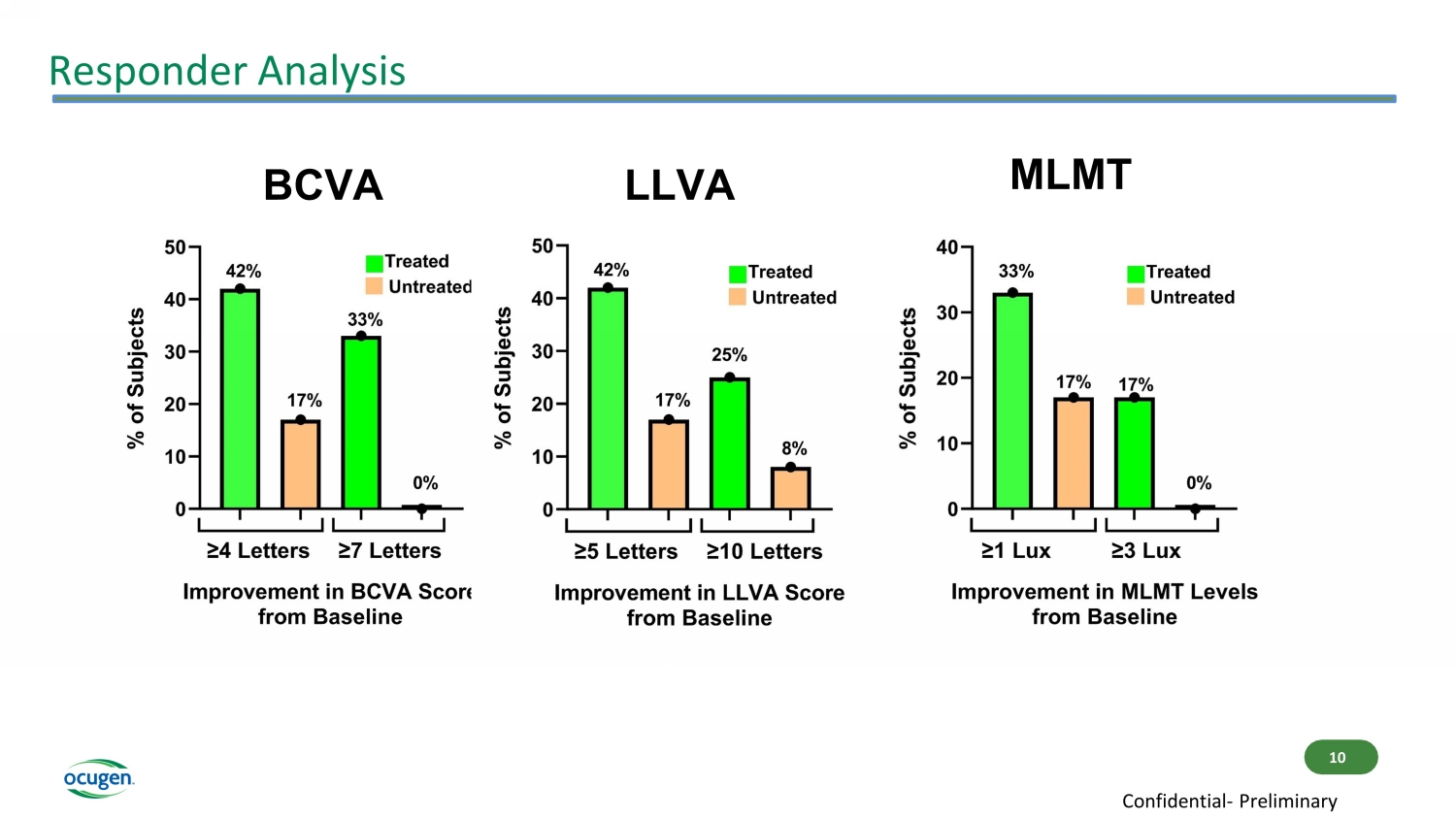
Responder Analysis BCVA LLVA MLMT 10 Confidential - Preliminary
Conclusions • OCU400 continues to demonstrate favorable safety and tolerability profile of OCU400 investigational drug product in Phase 1/2 study • Clinical study update suggests continued positive trends in Best - Corrected Visual Acuity (BCVA) and Multi - Luminance Mobility Testing (MLMT), as well as positive trends in Low - Luminance Visual Acuity (LLVA) among treated eyes • 83% (10/12) of subjects demonstrated preservation or improvement in the treated eye either on BCVA or LLVA or MLMT scores from baseline • 75% (9/12) of subjects demonstrated stabilization or improvement in treated eyes in MLMT scores from baseline • 86% (6/7) of RHO mutation subjects experienced either stabilization or increase in MLMT scores from baseline, among which 29% (2/7) demonstrated 3 Lux luminance level improvement • Treatment effect in RHO patients supports the gene - agnostic mechanism of action of OCU400 11

Key Takeaways The Phase 1/2 clinical trial demonstrated that OCU400 continued to be generally safe and well - tolerated in subjects across different mutations and dose levels Our study supports the gene - agnostic mechanism of action of the drug and demonstrates positive trends for key efficacy outcome measures like Best - Corrected Visual Acuity, Low - Luminance Visual Acuity, and Multi - Luminance Mobility testing parameters 12

Acknowledgements We would like to thank the study investigators, study participants, site coordinators, and research teams. 13