
Long-Term Outlook Shankar Musunuri, PhD, MBA Chairman of the Board, CEO & Co-founder R&D Day November 1, 2022

Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are based on the beliefs and assumptions of Ocugen, Inc. and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC. In addition, this presentation contains estimates, projections and other information concerning market, industry and other data. We obtained this data from our own internal estimates and research and from academic and industry research, publications, surveys, and studies conducted by third parties, including governmental agencies. These data involve a number of assumptions and limitations, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed in our filings with the SEC. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. While we believe such information is generally reliable, we have not independently verified any third-party information. Forward-looking statements that we make in this presentation are based on a combination of facts and factors currently known to us and speak only as of the date of this presentation. Except as required by law, we assume no obligation to update forward-looking statements contained in this presentation whether as a result of new information, future events, or otherwise, after the date of this presentation.

We’re Here to Make an Impact Through Courageous Innovation 3 Mission: Developing cutting-edge innovations for people facing serious disease and conditions with a commitment to ensuring global market access Pioneering modifier gene therapy for inherited retinal diseases, as well as larger blindness diseases with unmet need Innovating a novel biologic to treat eye diseases that can lead to vision loss for millions of people Developing vaccines to provide choice to Americans in the fight against COVID-19 Pursuing Regenerative Cell Therapy to treat serious conditions like articular cartilage lesions

4 Pipeline Overview Asset/Program Indication Current Status Vaccines COVAXIN™ (BBV152) SARS-CoV-2 virus COVID-19 • EUA for adults in Mexico; EUA for 5 to 18-year-olds submitted • Recruitment completed for U.S. Phase 2/3 Immuno-bridging and broadening clinical trial OCU500 Mucosal vaccine COVID-19 • License secured from Washington University • Phase 1/2 pending FDA discussions Cell therapies (Regenerative Medicine) NeoCart® (Autologous chondrocyte-derived neocartilage) Treatment of Articular Cartilage Defects in the Knee U.S. Regenerative Medicine Advanced Therapy (RMAT) designation; Phase 3 clinical trial under development and subject to finalization with FDA Gene therapies OCU400 ** AAV-hNR2E3 Gene mutation-associated retinal degeneration* NR2E3 Mutation (RP) Phase 1/2 RHO Mutation (RP) Phase 1/2 CEP290 Mutation (LCA) Phase 1/2 OCU410 AAV-hRORA Dry Age-Related Macular Degeneration (Dry AMD)** IND planned for Q2 OCU410ST AAV-hRORA Stargardt (orphan disease) IND planned for Q2 Biologicals OCU200 Transferrin – Tumstatin Diabetic Macular Edema IND planned for Q1 Diabetic Retinopathy IND enabling Wet Age-Related Macular Degeneration (Wet AMD) IND enabling *No approved therapies exist https://www.aao.org/eye-health/diseases/retinitis-pigmentosa-treatment | https://www.aao.org/eye-health/diseases/amd-treatment **ORPHAN DRUG DESIGNATION in the US; Broad ORPHAN MEDICINAL PRODUCT DESIGNATION by the EC for the treatment of retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA)

Corporate Executive Summary Ocugen has an exciting and unique portfolio spanning ocular gene therapies, a novel biologic, an orthopedic regenerative cell therapy, and COVID-19 vaccines. We believe the modifier gene therapy platform assets (OCU400 and OCU410) are the most significant drivers of value. We believe each asset has the potential to be significant if clinical data and commercial assumptions are positive— more conservative estimates still offer a meaningful valuation upside. Ocugen will need to carefully manage available capital in the near-term to maximize value for the ocular gene therapies. Additional capital raises and partnerships will be required to extend the runway and accelerate the portfolio. Investments through business development in capability building and portfolio diversification will be important to enable the current portfolio and scale the portfolio in the longer-term. 1 2 3 4 5
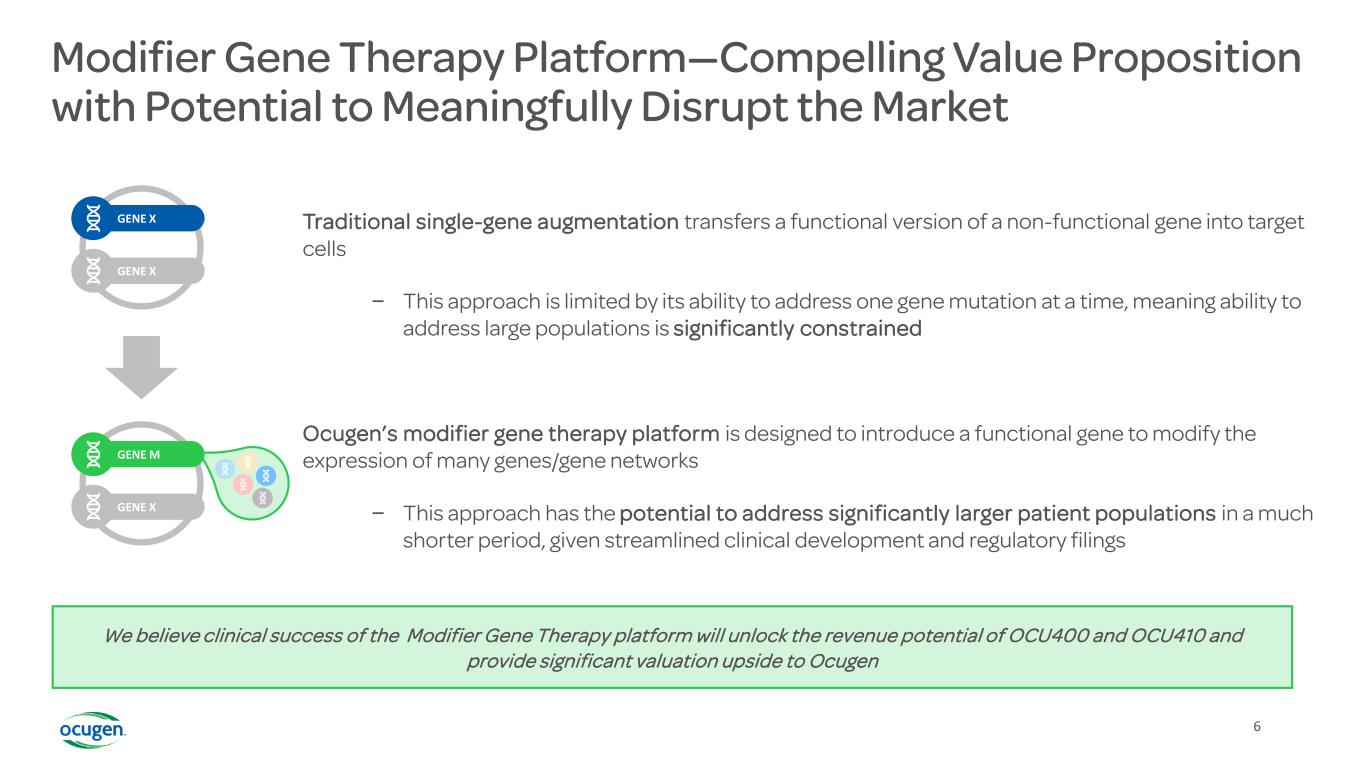
Modifier Gene Therapy Platform—Compelling Value Proposition with Potential to Meaningfully Disrupt the Market Traditional single-gene augmentation transfers a functional version of a non-functional gene into target cells – This approach is limited by its ability to address one gene mutation at a time, meaning ability to address large populations is significantly constrained Ocugen’s modifier gene therapy platform is designed to introduce a functional gene to modify the expression of many genes/gene networks – This approach has the potential to address significantly larger patient populations in a much shorter period, given streamlined clinical development and regulatory filings We believe clinical success of the Modifier Gene Therapy platform will unlock the revenue potential of OCU400 and OCU410 and provide significant valuation upside to Ocugen GENE X GENE X GENE M GENE X 6

Potential Market Opportunity for Ocugen Gene Therapies— Revenue Potential of up to ~$40B in 2032 # Doses (in Thousands)* OCU400 6.0 18.6 19.8 25.5 29.4 34.1 39.5 OCU410 - 11.7 24.1 32.1 50.2 71.5 79.3 Forecasted Net-Revenue (OCU400, OCU410) Key Assumptions – 90% Ability-To-Pay (adjusting for affordability, including uninsured patient population) – 25% standard GTN discount (as observed for high-priced GTs) $0 $5 $10 $15 $20 $25 2026 2027 2028 2029 2030 2031 2032 Bi llio ns ~$23B ~$17B Launch in Broad Spectrum (US) Launch in Late dAMD (US) Launch in Late dAMD (EU) Launch in Moderate dAMD and Mild dAMD (EU) Launch in Moderate dAMD and Mild dAMD (US) Launch in Mutation Specific (US)* Launch in Broad Spectrum (EU) *Not risk-adjusted ** Disease prevalence: U.S./EU/UK RP/LCA > 250,000. U.S./EU/UK dAMD (Geographic Atrophy) > 2 million . 7

Key Potential Milestones for Portfolio Assets C UR RE N T PO RT FO LI O M IIL ES TO N ES CA PI TA L AV AI LA BL E 2022 2023 2024 2025 2026 2027 OCU500 (mucosal) Ph I/II (Potential EUA Path) COVAXIN Ph 3 Safety US BLA Filing US BLA FilingNeoCart® Phase III BLA Filing BLA FilingOCU400 Ph I/II Global Ph III Global Ph IIIOCU410* Ph I/II Phase III (DME)OCU200 Ph I Phase II US US MX Launch “EXTEND THE RUNWAY” In the near-term: Efficiently manage capital, including seeking USG support for COVID-19 vaccines development “RAISE & ACCELERATE” In the medium-term “SCALE THE PORTFOLIO” In the longer-termST RA TE GI C IM PE RA TI VE S 6-mth reviewCOVAXIN Immuno. Ph 2/3 OCU500 Booster BLA Filing 8

9 Ocugen™ Vision Fully integrated, patient-centric biotech company focused on vaccines in support of public health and gene and cell therapies targeting unmet medical needs through Courageous Innovation








