
Q3 2022 Business Update November 8, 2022 NASDAQ:OCGN

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, which are based on the beliefs and assumptions of Ocugen, Inc. and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks, and uncertainties that may cause actual events or results to differ materially from our current expectations. These and other risks and uncertainties are more fully described in our periodic filings with the Securities and Exchange Commission (SEC), including the risk factors described in the section entitled “Risk Factors” in the quarterly and annual reports that we file with the SEC. Forward-looking statements that we make in this presentation speak only as of the date of this presentation. Except as required by law, we assume no obligation to update forward-looking statements contained in this presentation whether as a result of new information, future events, or otherwise, after the date of this presentation.
Q3 2022 Accomplishments —Progress Toward Long-Term Strategy OCU500 Licensing Agreement for a Mucosal COVID-19 Vaccine • Acquired rights to Develop, Manufacture and Commercialize in U.S., Europe, and Japan • An important addition to Ocugen’s COVID-19 vaccine portfolio • Represents a potential universal booster, regardless of previous COVID-19 vaccination Modifier Gene Therapy Platform • Introduction of OCU410ST to potentially treat Stargardt disease • Provided a deeper dive at 2022 American Academy of Ophthalmology Meeting and Ocugen R&D Day 3
OCU500: Mucosal Vaccine • Potential to generate rapid local immunity in the nose, mouth, upper airways, and lungs -where SARS- CoV-2 enters and infects the body • Generates neutralizing IgG, mucosal IgA, and T cell responses to help reduce transmission rate • Mucosal immunity has been demonstrated as a potential way to prevent infection and spread, thus limiting the origin of new variants 4 Other features include: • Non-invasive • Needle-free administration • Potential for increased compliance • Scalable manufacturing • Stored and shipped at standard refrigerated conditions • Potential to develop multi-strain and variant specific versions
COVAXIN™(BBV152) Enrollment completed for Phase 2/3 immuno-bridging and broadening clinical trial • No safety concerns identified to date • Topline data expected in early 2023 • Successfully completed demonstration batch as part of the technology transfer 5
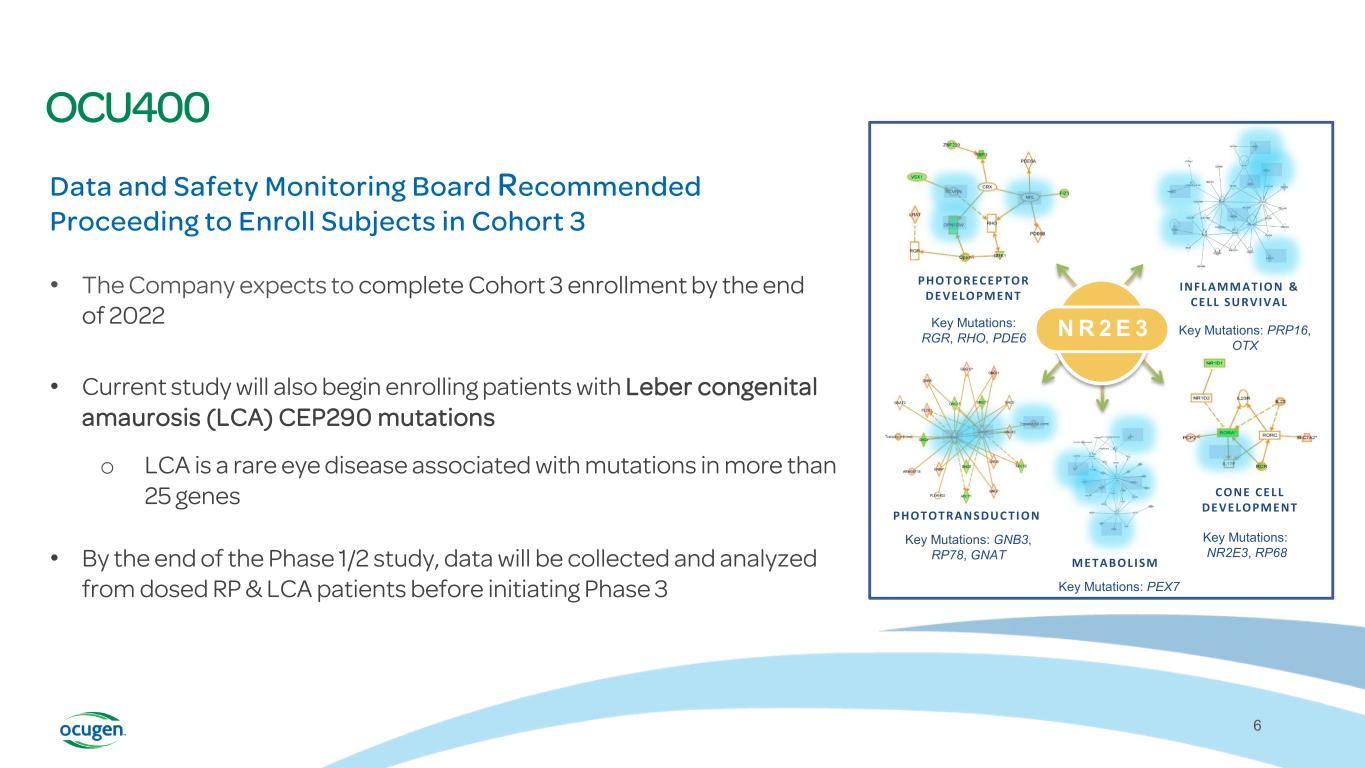
OCU400 Data and Safety Monitoring Board Recommended Proceeding to Enroll Subjects in Cohort 3 • The Company expects to complete Cohort 3 enrollment by the end of 2022 • Current study will also begin enrolling patients with Leber congenital amaurosis (LCA) CEP290 mutations o LCA is a rare eye disease associated with mutations in more than 25 genes • By the end of the Phase 1/2 study, data will be collected and analyzed from dosed RP & LCA patients before initiating Phase 3 6 INFLAMMATION & CELL SURVIVAL PHOTOTRAN SDUC TI ON METABOLISM PHOTORECEPTOR DEVELOPMENT CONE CELL DEVELOPMENT Key Mutations: RGR, RHO, PDE6 Key Mutations: PRP16, OTX Key Mutations: GNB3, RP78, GNAT Key Mutations: PEX7 Key Mutations: NR2E3, RP68 N R 2 E 3

OCU410 & OCU410ST Utilizes AAV delivery platform for the retinal delivery of the RORA (RAR Related Orphan Receptor A) gene OCU410 • Being developed for the treatment of dry AMD • Successfully completed cGMP manufacturing in support of clinical trials • Currently conducting IND-enabling toxicology studies • Planning to file IND application in Q2 2023 to initiate Phase 1/2 clinical trial OCU410ST • Being developed to potentially treat Stargardt disease, an orphan disease • Planning to file IND application in Q2 2023 to initiate Phase 1/2 clinical trial 7
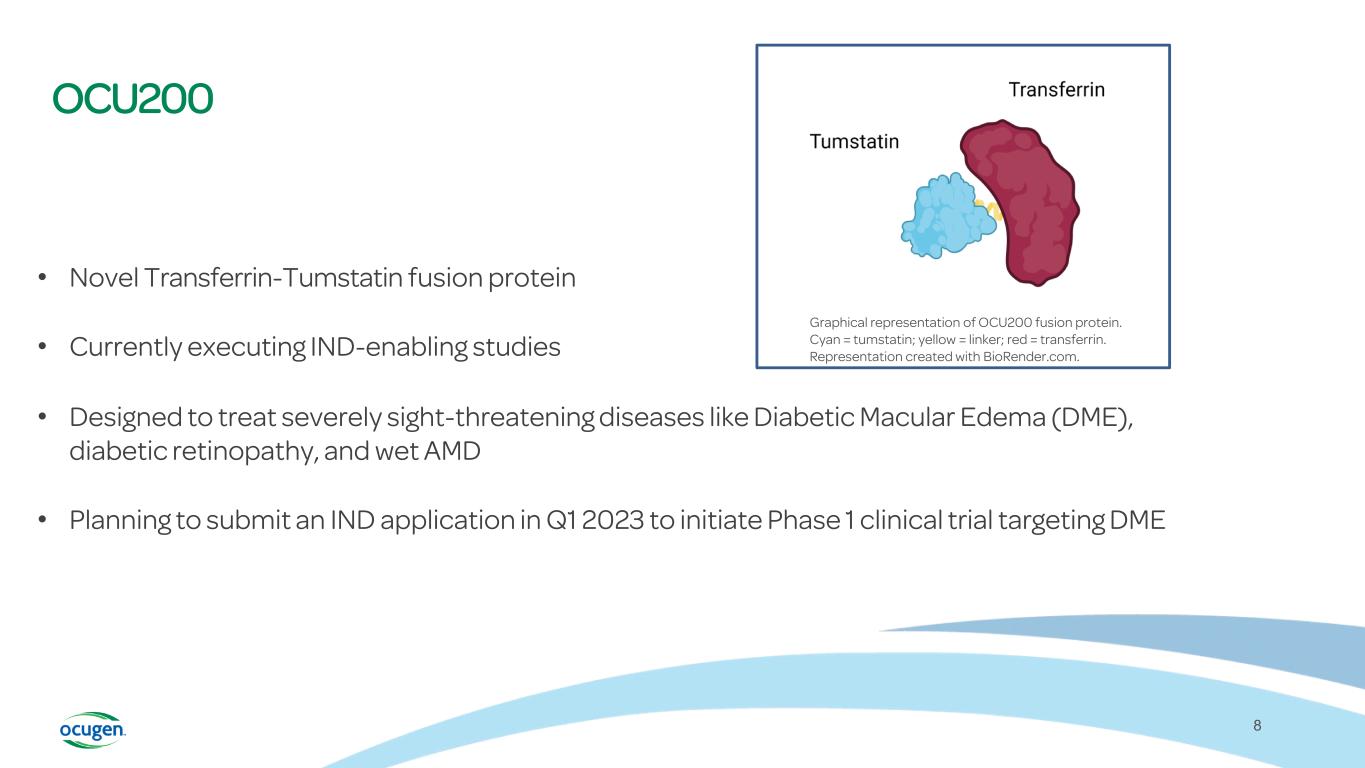
OCU200 • Novel Transferrin-Tumstatin fusion protein • Currently executing IND-enabling studies • Designed to treat severely sight-threatening diseases like Diabetic Macular Edema (DME), diabetic retinopathy, and wet AMD • Planning to submit an IND application in Q1 2023 to initiate Phase 1 clinical trial targeting DME 8 Graphical representation of OCU200 fusion protein. Cyan = tumstatin; yellow = linker; red = transferrin. Representation created with BioRender.com.

NeoCart® - Ocugen’s Expansion into Regenerative Cell Therapy • NeoCart® shows potential to accelerate healing and reduce pain, rebuilding damaged knee cartilage and limiting the progression towards osteoarthritis • Working with the FDA to finalize the Phase 3 clinical trial protocol necessary to advance development of NeoCart® • Building our own manufacturing suites to prepare for the study 9

We’re Here t o Make an Impact Through 10 Courageous Innovation

Financial Update 11
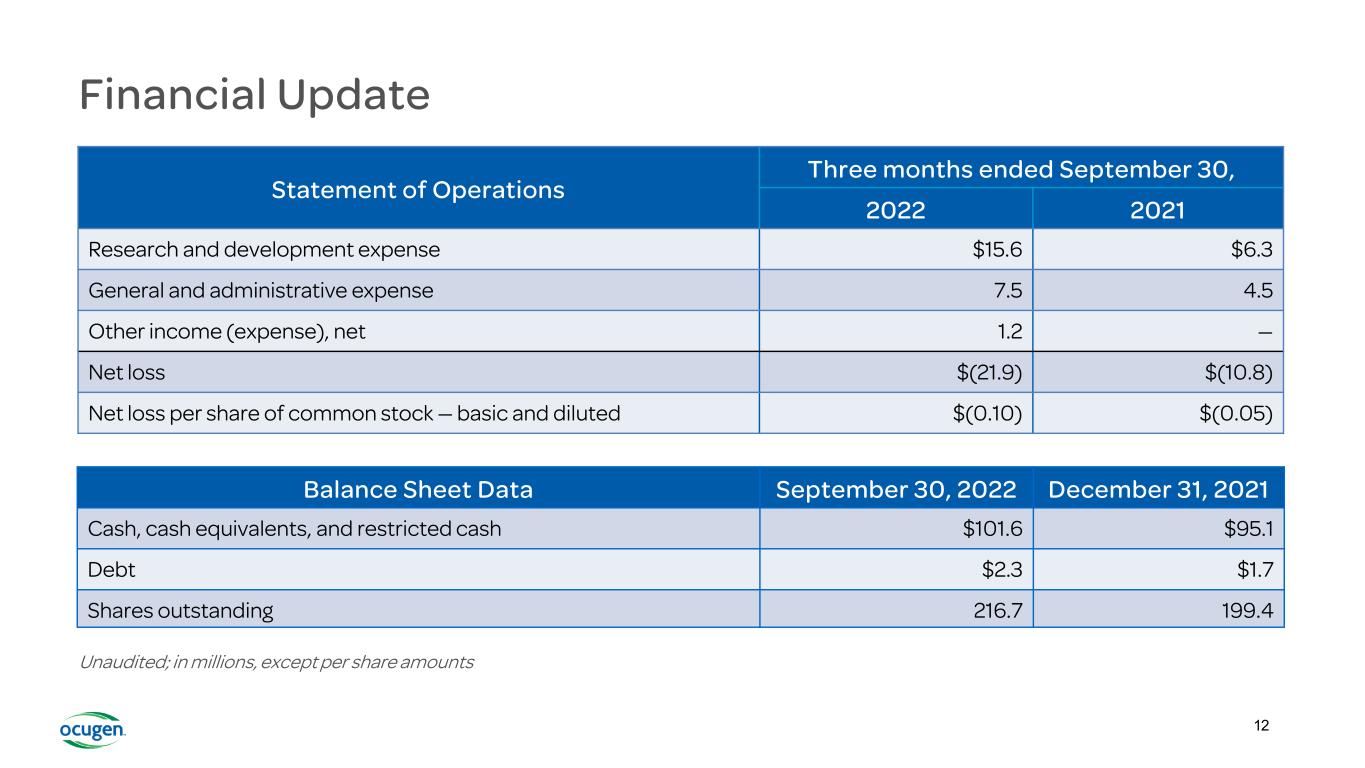
Financial Update Statement of Operations Three months ended September 30, 2022 2021 Research and development expense $15.6 $6.3 General and administrative expense 7.5 4.5 Other income (expense), net 1.2 — Net loss $(21.9) $(10.8) Net loss per share of common stock — basic and diluted $(0.10) $(0.05) Balance Sheet Data September 30, 2022 December 31, 2021 Cash, cash equivalents, and restricted cash $101.6 $95.1 Debt $2.3 $1.7 Shares outstanding 216.7 199.4 Unaudited; in millions, except per share amounts 12

Questions & Answers 13

November 8, 2022 NASDAQ:OCGN Thank you! 14













