UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
Filed by the Registrant ☒ Filed by a Party other than the Registrant ☐
Check the appropriate box:
| | |
| ☐ | | Preliminary Proxy Statement |
| |
| ☐ | | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| ☒ | | Definitive Proxy Statement |
| |
| ☐ | | Definitive Additional Materials |
| |
| ☐ | | Soliciting Material Pursuant to §240.14a-12 |
TETRAPHASE PHARMACEUTICALS, INC.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| | | | |
| |
| ☒ | | No fee required. |
| |
| ☐ | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | |
| | (1) | | Title of each class of securities to which transaction applies: |
| | (2) | | Aggregate number of securities to which transaction applies: |
| | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | (4) | | Proposed maximum aggregate value of transaction: |
| | (5) | | Total fee paid: |
| |
| ☐ | | Fee paid previously with preliminary materials: |
| |
| ☐ | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| | |
| | (1) | | Amount previously paid: |
| | (2) | | Form, Schedule or Registration Statement No.: |
| | (3) | | Filing Party: |
| | (4) | | Date Filed: |
TETRAPHASE PHARMACEUTICALS, INC.
480 ARSENAL WAY
WATERTOWN, MASSACHUSETTS 02472
(617)715-3600
NOTICE OF 2018 ANNUAL MEETING OF
STOCKHOLDERS
To Be Held On May 30, 2018
To our Stockholders:
NOTICE IS HEREBY GIVEN that the annual meeting of stockholders of Tetraphase Pharmaceuticals, Inc. will be held on Wednesday, May 30, 2018 at 10:00 a.m., Eastern Time, at the offices of Wilmer Cutler Pickering Hale and Dorr LLP, 60 State Street, Boston, Massachusetts 02109. At the meeting, stockholders will consider and vote on the following matters:
| | 1. | To elect Jeffrey Chodakewitz, Gerri Henwood and Guy Macdonald as class II directors, each to serve for a three-year term expiring at the 2021 annual meeting of stockholders; |
| | 2. | To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2018; |
| | 3. | To approve, on anon-binding advisory basis, the compensation of our named executive officers; and |
| | 4. | To transact any other business that may properly come before the annual meeting or any adjournment thereof. |
Stockholders of record at the close of business on April 6, 2018 are entitled to vote at the meeting.
We have elected to provide access to our proxy materials over the Internet under the Securities and Exchange Commission’s “notice and access” rules. We believe that providing our proxy materials over the Internet expedites stockholders’ receipt of proxy materials, lowers costs and reduces the environmental impact of our annual meeting.
We encourage all stockholders to attend the annual meeting in person. Whether or not you plan to attend the annual meeting in person, we encourage you to read this proxy statement and submit your proxy or voting instructions as soon as possible. Please review the instructions on each of your voting options described in the proxy statement.
Thank you for your ongoing support and continued interest in Tetraphase Pharmaceuticals, Inc.
By Order of the Board of Directors,

Guy Macdonald
President and Chief Executive Officer
Watertown, Massachusetts
April 20, 2018
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting of Stockholders to be Held on May 30, 2018:This proxy statement and our 2017 Annual Report to Stockholders are available at http://ir.tphase.com. These documents are also available to any stockholder who wishes to receive a paper copy by calling(866) 648-8133 or emailing paper@investorelections.com.
TABLE OF CONTENTS
i

480 ARSENAL WAY
WATERTOWN, MASSACHUSETTS 02472
(617)715-3600
PROXY STATEMENT
2018 ANNUAL MEETING OF STOCKHOLDERS
To Be Held on May 30, 2018
This proxy statement and the enclosed proxy card are being furnished in connection with the solicitation of proxies by the board of directors of Tetraphase Pharmaceuticals, Inc. for use at the 2018 annual meeting of stockholders of Tetraphase Pharmaceuticals, Inc. to be held on Wednesday, May 30, 2018 at 10:00 a.m., Eastern Time, at the offices of Wilmer Cutler Pickering Hale and Dorr LLP, 60 State Street, Boston, Massachusetts 02109, and at any adjournment thereof. Except where the context otherwise requires, references to “Tetraphase Pharmaceuticals,” “Tetraphase,” “we,” “us,” “our” and similar terms refer to Tetraphase Pharmaceuticals, Inc. and its consolidated subsidiaries. References to our website are inactive textual references only and the contents of our website should not be deemed to be incorporated by reference into this proxy statement.
This proxy statement summarizes information about the proposals to be considered at the meeting and other information you may find useful in determining how to vote. We are making this proxy statement, the related proxy card and our annual report to stockholders for the fiscal year ended December 31, 2017 available to stockholders for the first time on or about April 20, 2018.
A copy of our Annual Report on Form10-K for the fiscal year ended December 31, 2017, as filed with the Securities and Exchange Commission, or SEC, except for exhibits, will be furnished without charge to any stockholder upon written request to Tetraphase Pharmaceuticals, Inc., 480 Arsenal Way, Watertown, Massachusetts 02472, Attention: Investor Relations. Exhibits will be provided upon written request and payment of an appropriate processing fee. This proxy statement and our Annual Report on Form10-K for the fiscal year ended December 31, 2017 are also available on the SEC’s website atwww.sec.gov.
1
QUESTIONS AND ANSWERS ABOUT THE ANNUAL MEETING AND VOTING
| Q. | Why did I receive these proxy materials? |
| A. | Our board of directors has made these materials available to you on the Internet in connection with the solicitation of proxies for use at our 2018 annual meeting of stockholders to be held at the offices of Wilmer Cutler Pickering Hale and Dorr LLP, 60 State Street, Boston, Massachusetts, 02109 on Wednesday, May 30, 2018 at 10:00 a.m., Eastern Time. As a holder of record of common stock as of the close of business on April 6, 2018, you are invited to attend the annual meeting and are requested to vote on the items of business described in this proxy statement. This proxy statement includes information that we are required to provide to you under SEC rules and that is designed to assist you in voting your shares. |
| Q. | Why did I receive a notice in the mail regarding the Internet availability of proxy materials instead of a full set of proxy materials? |
| A. | In accordance with the SEC rules, we may furnish proxy materials, including this proxy statement and our annual report, to our stockholders by providing access to such documents on the Internet instead of mailing printed copies. |
| Q. | What is the purpose of the annual meeting? |
| A. | At the annual meeting, stockholders will consider and vote on the following matters: |
| | 1. | To elect Jeffrey Chodakewitz, Gerri Henwood and Guy Macdonald as class II directors, each to serve for a three-year term expiring at the 2021 annual meeting of stockholders (proposal 1); |
| | 2. | To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2018 (proposal 2); |
| | 3. | To approve, on anon-binding advisory basis, the compensation of our named executive officers (proposal 3); and |
| | 4. | To transact any other business that may properly come before the annual meeting or any adjournment thereof. |
| Q. | Who can vote at the annual meeting? |
| A. | To be entitled to vote, you must have been a stockholder of record at the close of business on April 6, 2018, the record date for our annual meeting. There were 51,629,987 shares of our common stock outstanding and entitled to vote at the annual meeting as of the record date. |
| Q. | How many votes do I have? |
| A. | Each share of our common stock that you own as of the record date will entitle you to one vote on each matter considered at the annual meeting. |
| A. | If you are the “record holder” of your shares, meaning that your shares are registered in your name in the records of our transfer agent, American Stock Transfer & Trust Company, you may vote your shares at the meeting in person or by proxy as follows: |
| | (1) | Over the Internet: To vote over the Internet, please go to the following website:www.proxydocs.com/ttph, and follow the instructions at that site for submitting your proxy electronically. If you vote over the Internet, you do not need to complete and mail your proxy card or vote your proxy by telephone. |
2
| | (2) | By Telephone: To vote by telephone, please call (866)416-3857, and follow the instructions provided on the proxy card. If you vote by telephone, you do not need to complete and mail your proxy card or vote your proxy over the Internet. |
| | (3) | By Mail: To vote by mail, you must mark, sign and date the proxy card and then mail the proxy card in accordance with the instructions on the proxy card. If you vote by mail, you do not need to vote over the Internet or by telephone. If you return your proxy card but do not specify how you want your shares voted on any particular matter, they will be voted in accordance with the recommendations of our board of directors. |
| | (4) | In Person at the Meeting: If you attend the annual meeting, you may deliver your completed proxy card in person or you may vote by completing a ballot, which we will provide to you at the meeting. |
If your shares are held in “street name,” meaning they are held for your account by an intermediary, such as a broker, then you are deemed to be the beneficial owner of your shares and the broker that actually holds the shares for you is the record holder and is required to vote the shares it holds on your behalf according to your instructions. The proxy materials, as well as voting and revocation instructions, should have been forwarded to you by the broker that holds your shares. In order to vote your shares, you will need to follow the instructions that your broker provides you. Many brokers solicit voting instructions over the Internet or by telephone.
If you do not give instructions to your broker, your broker will still be able to vote your shares with respect to certain “discretionary” items. The ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm (proposal 2) is considered a discretionary item. Accordingly, your broker may vote your shares in its discretion with respect to that matter even if you do not give instructions.
All of the other matters being put to a vote are“non-discretionary” items. Accordingly, your broker may not vote your shares with respect to these other matters. A “brokernon-vote” occurs when shares held by a broker are not voted with respect to a particular proposal because the broker does not have or did not exercise discretionary authority to vote on the matter and has not received voting instructions from its clients.
Regardless of whether your shares are held in street name, you are welcome to attend the meeting. You may not vote shares held in street name in person at the meeting, however, unless you obtain a proxy, executed in your favor, from the holder of record (i.e., your broker).
| A. | If your shares are registered directly in your name, you may revoke your proxy and change your vote at any time before the vote is taken at the annual meeting. To do so, you must do one of the following: |
| | (1) | Vote over the Internet or by telephone as instructed above. Only your latest Internet or telephone vote is counted. |
| | (2) | Sign and return a new proxy card. Only your latest dated proxy card will be counted. |
| | (3) | Attend the annual meeting and vote in person as instructed above. Attending the annual meeting will not alone revoke your Internet vote, telephone vote or proxy card submitted by mail, as the case may be. |
| | (4) | Give our corporate secretary written notice before or at the meeting that you want to revoke your proxy. |
If your shares are held in “street name,” you may submit new voting instructions with a later date by contacting your broker.
3
| Q. | How many shares must be represented to have a quorum and hold the annual meeting? |
| A. | A majority of our shares of common stock outstanding at the record date must be present in person or represented by proxy to hold the annual meeting. This is called a quorum. For purposes of determining whether a quorum exists, we count as present any shares that are voted over the Internet, by telephone or by submitting a proxy card or that are represented in person at the meeting. Further, for purposes of establishing a quorum, we will count as present shares that a stockholder holds even if the stockholder votes to abstain or only votes on one of the proposals. In addition, we will count as present shares held in “street name” by brokers who indicate on their proxies that they do not have authority to vote those shares. If a quorum is not present, we expect to adjourn the annual meeting until we obtain a quorum. |
| Q. | What vote is required to approve each matter and how are votes counted? |
| A. | Proposal 1—Election of Class II Directors |
A nominee will be elected as a Class II director at the annual meeting if the nominee receives a plurality of the votes cast “for” the applicable seat on the board of directors. You may vote FOR all of the nominees, to WITHHOLD your vote from all of the nominees or WITHHOLD your vote from any one or more of the nominees. Votes that are withheld will not be included in the vote tally for the election of the directors.
Proposal 2—Ratification of the Appointment of Independent Registered Public Accounting Firm
The affirmative vote of the holders of shares of common stock representing a majority of the votes cast on the matter is required to ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2018.
Proposal 3—Advisory Vote on Executive Compensation
The affirmative vote of the holders of shares of common stock representing a majority of the votes cast on the matter is required to approve, on anon-binding advisory basis, the compensation of our named executive officers, as described in this proxy statement.
This advisory vote on executive compensation is not binding on our board of directors. However, our board of directors and the compensation committee of our board of directors will take into account the result of the vote when making future decisions regarding executive compensation.
Shares which abstain from voting and “brokernon-votes” with respect to a matter will not be counted as votes in favor of such matter, and will also not be counted as shares voting on such matter. Accordingly, abstentions and “brokernon-votes” will have no effect on the voting on the proposals referenced above.
| Q. | Who will count the vote? |
| A. | The votes will be counted, tabulated and certified by Mediant Communications LLC. |
| Q. | How does the board of directors recommend that I vote on the proposals? |
| A. | Our board of directors recommends that you vote: |
FOR the election of the three nominees to serve as class II directors, each for a three year term;
FOR the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2018; and
FORthe approval, on anon-binding, advisory basis, of the compensation of our named executive officers.
| Q. | Are there other matters to be voted on at the annual meeting? |
| A. | We do not know of any matters that may come before the annual meeting other than the matters noted above. If any other matters are properly presented at the annual meeting, the persons named in the accompanying proxy intend to vote, or otherwise act, in accordance with their judgment on the matter. |
4
| Q. | Where can I find the voting results? |
| A. | We plan to announce preliminary voting results at the annual meeting and will report final voting results in a Current Report on Form8-K filed with the SEC within four business days following the conclusion of our annual meeting. |
| Q. | What are the costs of soliciting these proxies? |
| A. | We will bear the cost of soliciting proxies. In addition to solicitation by mail, our directors, officers and employees may solicit proxies by telephone,e-mail, facsimile and in person without additional compensation. We may reimburse brokers or persons holding stock in their names, or in the names of their nominees, for their expenses in sending proxies and proxy material to beneficial owners. We have also hired a proxy solicitor who may also solicit proxies from shareholders by telephone,e-mail, facsimile and in person and whose fees we will reimburse. |
Stockholders Sharing the Same Address
Some brokers and other nominee record holders may be “householding” our proxy materials. This means a single notice and, if applicable, the proxy materials, will be delivered to multiple stockholders sharing an address unless contrary instructions have been received. We will promptly deliver a separate copy of the notice and, if applicable, the proxy materials, to you if you call or write us at our principal executive offices, 480 Arsenal Way, Watertown, Massachusetts 02472, Attn: Investor Relations, telephone:(617) 715-3600. In the future, if you want to receive separate copies of the proxy materials, or if you are receiving multiple copies and would like to receive only one copy per household, you should contact your broker, or you may contact us at the above address and telephone number.
5
OWNERSHIP OF OUR COMMON STOCK
Unless otherwise provided below, the following table sets forth information regarding beneficial ownership of our common stock as of March 1, 2018 by:
| | • | | each person, or group of affiliated persons, known to us to be the beneficial owner of 5% or more of the outstanding shares of our common stock; |
| | • | | each of our current directors; |
| | • | | our principal executive officer and our other executive officers who served during the year ended December 31, 2017, named in the Summary Compensation table below, whom, collectively, we refer to as our named executive officers; and |
| | • | | all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with SEC rules. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares of common stock issuable upon the exercise of stock options that are immediately exercisable or exercisable within 60 days after March 1, 2018. Except as otherwise indicated, all of the shares reflected in the table are shares of common stock and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose.
The column entitled “Percentage of Shares Beneficially Owned” is based on a total of 51,629,987 shares of our common stock outstanding as of March 1, 2018. Except as otherwise indicated in the footnotes below, the address of the beneficial owner is c/o Tetraphase Pharmaceuticals, Inc., 480 Arsenal Way, Watertown, MA 02472.
| | | | | | | | | | | | | | | | |
Name of Beneficial Owner | | Number of
Shares
Beneficially
Owned | | | Common Stock
Underlying
Options
Exercisable
Within 60 Days | | | Total Securities
Beneficially
Owned | | | Percentage of
Shares
Beneficially
Owned | |
BlackRock, Inc.(1) | | | 4,044,340 | | | | 0 | | | | 4,044,340 | | | | 7.8 | % |
Millennium Management LLC(2) | | | 2,677,995 | | | | 0 | | | | 2,677,995 | | | | 5.2 | % |
| | | | |
Named Executive Officers and Directors | | | | | | | | | | | | | | | | |
Guy Macdonald | | | 129,006 | | | | 899,449 | | | | 1,028,455 | | | | 2.0 | % |
Jacques Dumas, Ph.D. | | | 0 | | | | 159,688 | | | | 159,688 | | | | | * |
Patrick T. Horn, M.D., Ph.D. | | | 17,155 | | | | 0 | | | | 17,155 | | | | | * |
Maria Stahl.(3) | | | 17,274 | | | | 181,875 | | | | 199,149 | | | | | * |
Kamalam Unninayar | | | 0 | | | | 0 | | | | 0 | | | | | * |
Christopher Watt | | | 19,114 | | | | 70,303 | | | | 89,417 | | | | | * |
L. Patrick Gage, Ph.D.(4) | | | 42,705 | | | | 78,919 | | | | 121,624 | | | | | * |
Garen Bohlin | | | 0 | | | | 91,940 | | | | 91,940 | | | | | * |
Jeffrey Chodakewitz | | | 0 | | | | 49,375 | | | | 49,375 | | | | | * |
John Freund, M.D.(5) | | | 300,650 | | | | 59,375 | | | | 360,025 | | | | | * |
Gerri Henwood | | | 0 | | | | 39,375 | | | | 39,375 | | | | | * |
Nancy Wysenski | | | 0 | | | | 49,375 | | | | 49,375 | | | | | * |
| | | | | | | | | | | | | | | | |
All current executive officers and directors as a group
(12 persons) | | | 525,904 | | | | 1,679,674 | | | | 2,205,578 | | | | 4.1 | % |
| | | | | | | | | | | | | | | | |
| * | Represents beneficial ownership of less than 1% of our outstanding stock. |
6
| (1) | BlackRock, Inc. (“Blackrock”) reports that it is a parent holding company or control person and has the sole voting power of 3,985,817 shares of common stock and sole dispositive power of 4,044,340 shares of common stock, but also notes that various persons have the right to receive or the power to direct the receipt of dividends from, or the proceeds from the sale of, the company’s shares and that no one person’s interest in the company’s shares is more than 5% of the total outstanding shares of the company. Blackrock’s address is 55 East 52nd Street, New York, NY 10055. This information is based on a Schedule 13G/A filed by Blackrock with the SEC on February 1, 2018. |
| (2) | Millennium Management LLC (“Millennium Management”), Millennium Group Management LLC (“Millennium Group Management”) and Israel Englander (“Mr. Englander”) have shared voting power of 2,677,995 shares of common stock and shared dispositive power of 2,677,995 shares of common stock. Millennium Management is the general partner of the managing member of Integrated Core Strategies (US) LLC and Integrated Assets II LLC. Integrated Core Strategies (US) LLC beneficially owns 1,501,603 shares of common stock. Integrated Assets II LLC beneficially owns 1,176,392 shares of common stock. Mr. Englander controls the managing members of Millennium Group Management. The address for each of the entities and Mr. Englander is 666 Fifth Avenue, New York, NY 10103. This information is based on a Schedule 13G/A filed by Millennium Management with the SEC on January 8, 2018. |
| (3) | Consists of 2,500 shares of common stock held by a trust for the benefit of Ms. Stahl’s spouse, 2,500 shares of common stock held by a trust for the benefit of Ms. Stahl, 12,274 shares of common stock held directly by Ms. Stahl and 181,875 shares of common stock issuable upon the exercise of options exercisable within 60 days after March 1, 2018. |
| (4) | Consists of 38,880 shares of common stock held directly by Dr. Gage, 3,825 shares of common stock held by Dr. Gage’s spouse and 78,919 shares of common stock issuable upon the exercise of options exercisable within 60 days after March 1, 2018. |
| (5) | Consists of 290,146 shares of common stock held by Skyline Venture Partners Qualified Purchaser Fund IV, L.P.; 4,492 shares of common stock held by Skyline Venture Management IV, LLC; 6,012 shares of common stock held by the John G. Freund as Trustee of the John G. Freund Revocable Trust u/a/d 6/26/01; and 59,375 shares of common stock issuable upon the exercise of options exercisable within 60 days after March 1, 2018. Dr. Freund is a Managing Member of Skyline Venture Management IV, LLC, which is the sole general partner of Skyline Venture Partners Qualified Purchaser Fund IV, L.P., and as such Dr. Freund may be deemed to share voting and dispositive power with respect to all shares held by Skyline Venture Partners Qualified Purchaser Fund IV, L.P. Dr. Freund disclaims beneficial ownership of such shares except to the extent of any pecuniary interest therein. |
7
PROPOSAL 1
ELECTION OF DIRECTORS
Directors and Nominees for Directors
Our board of directors is divided into three classes, with members of each class holding office for staggered three-year terms. There are currently three class II directors (Jeffrey Chodakewitz, Gerri Henwood and Guy Macdonald), whose terms expire at this annual meeting of stockholders; two class III directors (Garen Bohlin and John Freund), whose terms expire at the 2019 annual meeting; and two class I directors (L. Patrick Gage and Nancy Wysenski), whose terms expire at the 2020 annual meeting (in all cases subject to the election and qualification of their successors or to their earlier death, resignation or removal). We have no contractual obligations regarding the election of our directors.
Our board of directors, on the recommendation of our nominating and corporate governance committee, has nominated Jeffrey Chodakewitz, Gerri Henwood and Guy Macdonald for election as class II directors at the annual meeting to hold office until the 2021 annual meeting of stockholders and until his or her successor is elected and qualified. Each of the nominees is presently a director, and each has indicated a willingness to continue to serve as director, if elected. If a nominee becomes unable or unwilling to serve, however, the proxies may be voted for substitute nominees selected by our board of directors. Unless authority to do so is withheld, shares represented by executed proxies will be voted for the election of the three class II nominees named below.
Below are the names, ages and certain other information for each member of the board, including the nominees for election as class II directors. Information with respect to the number of shares of common stock beneficially owned by each director as of March 1, 2018 appears under the heading “Ownership of Our Common Stock.” There are no familial relationships among any of our directors, nominees for director and executive officers. In addition to the detailed information presented below for each of our directors, we also believe that each of our directors is qualified to serve on our board and has the integrity, business acumen, knowledge and industry experience, diligence, freedom from conflicts of interest and the ability to act in the interests of our stockholders.
Class I Directors
L. Patrick Gage, Ph.D.,age 75, has served as a member of our board of directors and as Chairman of our board of directors since December 2011. Since July 2002, Dr. Gage has served as a consultant to the biopharmaceutical industry. From 1998 to 2002, Dr. Gage served as President of Wyeth Research (now part of Pfizer, Inc.). Prior to joining Wyeth Research, he served in various positions at Genetics Institute, Inc., a biotechnology company, from 1989 to 1998, first as head of Research and Development, then as Chief Operating Officer and eventually as President. From 1971 to 1989, Dr. Gage served in various positions in research management withHoffmann-La Roche Inc., a pharmaceutical company, most recently serving as Vice President responsible for U.S. drug discovery. Dr. Gage has served on the board of directors of Cytokinetics, Incorporated, a publicly traded biopharmaceuticals company, since November 2009 and as Chairman of its board of directors since March 2010. Dr. Gage also currently serves in an advisory role to other private companies and organizations. Previously he served on the board of directors of PDL BioPharma, Inc., a publicly traded biotechnology company, from 2003 through 2008, as the Chairman of its board of directors in 2007, and as its Interim Chief Executive Officer from 2007 to 2008. Dr. Gage currently serves on the board of directors of twonon-profit organizations, the Marine Biological Laboratories and the Wistar Institute. Dr. Gage received an S.B. in physics from the Massachusetts Institute of Technology and a Ph.D. in biophysics from the University of Chicago. We believe that Dr. Gage’s extensive industry and board experience as well as his independence allows him to serve as an effective Chairman of our board of directors and to be a key contributor to our board of directors.
Nancy Wysenski,age 60, has served as a member our board of directors since March 2014. From December 2009 through June 2012, Ms. Wysenski served as the Executive Vice President and Chief Commercial Officer of
8
Vertex Pharmaceuticals Incorporated, a publicly traded pharmaceutical company. Prior to joining Vertex, Ms. Wysenski held the position of Chief Operating Officer of Endo Pharmaceuticals, a1,200-person specialty pharmaceutical company, where she led sales, marketing, commercial operations, supply chain management, human resources and various business development initiatives. Prior to her time at Endo, Ms. Wysenski participated in the establishment of EMD Pharmaceuticals, Inc., where she held various leadership positions, including the role of President and Chief Executive Officer from 2001 to 2006 and Vice President of Commercial from 1999 to 2001. From 1984 to 1998, Ms. Wysenski held several sales-focused roles at major pharmaceutical companies, including Vice President of Field Sales for Astra Merck, Inc. Ms. Wysenski serves as a director of Alkermes plc, a publicly traded biopharmaceutical companies. She is a founder of the Research Triangle Park Chapter of the Healthcare Businesswomen’s Association and served on the Nominating Committee and National Advisory Board of the Healthcare Businesswomen’s Association. Ms. Wysenski received a B.S.N. in Nursing from Kent State University and an M.B.A. from Baldwin-Wallace College. We believe that Ms. Wysenski’s experience, leadership skills and knowledge of the life sciences industry allows her to provide valuable insight to our board with respect to the launch and commercialization of pharmaceutical products.
Class II Director Nominees to be elected at the annual meeting
Jeffrey A. Chodakewitz, M.D.,age 62, has served as a member of our board of directors since June 2014. Since April 2018, Dr. Chodakewitz has served as Executive Vice President, Clinical Medicine and External Innovation of Vertex Pharmaceuticals Incorporated. From October 2014 to March 2018, Dr. Chodakewitz has served as Executive Vice President, Global Medicines Development and Medical Affairs, and Chief Medical Officer of Vertex. From January 2014 to October 2014, Dr. Chodakewitz served as Senior Vice President and Chief Medical Officer of Vertex. Dr. Chodakewitz oversees all global clinical development programs, medical affairs and other related functions. Prior to joining Vertex, Dr. Chodakewitz spent more than 20 years at Merck & Co., Inc., where he held a variety of roles including Vice President of Clinical Research—Infectious Diseases & Vaccines, Vice President of Clinical Pharmacology/Early Stage Development, Senior Vice President of Late Stage Development, and Senior Vice President of Global Scientific Strategy (Infectious Diseases, Respiratory/Immunology). Prior to his tenure at Merck, he served as the Director of the HIV Outpatient Clinic at the Veterans Administration Medical Center in West Haven, Connecticut, and held various academic positions at Yale University and New York University Schools of Medicine. Dr. Chodakewitz is a Diplomate of the National Board of Medical Examiners, the American Board of Internal Medicine (both Internal Medicine and Infectious Diseases), and is a member of the Infectious Disease Society of America (IDSA) and the American Society for Clinical Pharmacology & Therapeutics (ASCPT). He received a B.S. in Biochemistry from Yale University, cum laude, and an M.D. from the Yale University School of Medicine. We believe that Dr. Chodakewitz’s scientific, medical and business background allows him to be a key contributor to our board of directors.
Gerri Henwood,age 65, has served as a member of our board of directors since April 2015. Since 2008, Ms. Henwood has served as President and Chief Executive Officer and a director of Recro Pharma, Inc., a publicly traded specialty pharmaceutical company developing acute care products, includingnon-opioid therapeutics for the treatment of acute pain. From 2006 to 2013, Ms. Henwood served as the President of Malvern Consulting Group, or MCG. She is theco-founder of Auxilium Pharmaceuticals, Inc. and served as its President, Chief Executive Officer and director from 1999 to 2006. Prior to founding Auxilium, in 1985, Ms. Henwood founded, and was President and Chief Executive Officer of, a publicly traded contract research organization, IBAH, Inc., which was acquired by Omnicare, Inc. Prior to founding IBAH, Inc., Ms. Henwood began her career with Smith Kline & French, now part of GlaxoSmithKline plc, in the pharmaceutical management program. She held many positions there, including the position of head of Regulatory and Medical Affairs for the U.S. business and the position of Group Director—Marketing in the International Pharmaceutical Division. Ms. Henwood holds a B.S. in Biology from Neumann University. We believe Ms. Henwood’s expertise in product commercialization, clinical development and regulatory approval processes allows her to be a key contributor to our board of directors.
Guy Macdonald,age 59, has served as our President and Chief Executive Officer and a member of our board of directors since January 2008. From August 2003 until January 2008, Mr. Macdonald served as
9
Executive Vice President, Operations, of Idenix Pharmaceuticals, Inc., a biopharmaceutical company. Prior to joining Idenix, he served in various positions at Merck & Co., Inc., a pharmaceutical company, from 1981 to 2003, most recently serving as the Vice President for Anti-Infective and Hospital Products. Mr. Macdonald currently serves as chairman of the board of Scynexis, Inc., a publicly traded biotechnology company. Mr. Macdonald received an Honours Degree in biochemistry from Dundee University in Dundee, Scotland. We believe Mr. Macdonald’s qualifications to serve on our board of directors include his extensive experience in the healthcare industry as well as his extensive knowledge of our company and our business through service as our President and Chief Executive Officer.
Class III Directors
Garen Bohlin,age 70, has served as a member of our board of directors since July 2010. Since May 2012, Mr. Bohlin has served on the board of directors and as a consultant to multiple life sciences companies. From January 2010 until April 2012, he served as Executive Vice President of Constellation Pharmaceuticals, Inc., a biopharmaceutical company. Prior to joining Constellation, Mr. Bohlin served as Chief Operating Officer of Sirtris Pharmaceuticals, Inc., a biotechnology company, from 2006 to December 2009. Mr. Bohlin was the founding Chief Executive Officer of Syntonix Pharmaceuticals, Inc., a biopharmaceutical company, from 1999 through December 2005. Earlier in his career, he held multiple executive positions at Genetics Institute, Inc., a biotechnology company, and was a partner at Arthur Andersen & Co., a public accounting and consulting organization. Mr. Bohlin currently serves on the board of directors of Collegium Pharmaceutical, Inc., Karyopharm Therapeutics, Inc. and Proteon Therapeutics, Inc., all publicly traded biotechnology companies. He also served on the board of directors for Acusphere, Inc., a specialty pharmaceutical company that was publicly traded company, from 2005 to 2014, SpringLeaf Therapeutics, Inc., a private biotechnology company, from 2010 to 2013 and Precision Dermatology, Inc., a private dermatology company from 2012 to 2014. Mr. Bohlin received his B.S. in accounting and finance from The University of Illinois. We believe that Mr. Bohlin’s industry and board experience, including his audit committee experience, with both publicly traded and privately held companies makes him a key contributor to our board of directors.
John Freund, M.D.,age 64, has served as a member of our board of directors since October 2012. Dr. Freundco-founded Skyline Ventures in 1997 and has served as a managing director at Skyline since its founding. Prior to joining Skyline, Dr. Freund served as managing director in the private equity group of Chancellor Capital Management, a private capital investment firm. In 1995, heco-founded Intuitive Surgical, Inc. a medical device company, and served on its board of directors until 2000. From 1988 to 1994, Dr. Freund served in various positions at Acuson Corporation, a maker of ultrasound equipment that is now part of Siemens, most recently as Executive Vice President. Prior to joining Acuson, Dr. Freund was a general partner of Morgan Stanley Venture Partners from 1987 to 1988. From 1982 to 1988, Dr. Freund was at Morgan Stanley & Co., an investment banking company, where heco-founded the Healthcare Group in the Corporate Finance Department in 1983. He has served on the board of directors of Collegium Pharmaceutical, Inc., a publicly traded biotechnology company, since 2014, and Proteon Therapeutics, Inc., a publicly traded biotechnology company, since 2014. Dr. Freund also serves on the board of directors of six U.S. registered investment funds managed by Capital Research and Management. He also previously served on the board of directors of a number of publicly traded companies, including XenoPort, Inc., a biopharmaceutical company, where he served as chairman of the board; Map Pharmaceuticals, Inc. a biopharmaceutical company; Hansen Medical, Inc., a medical device company; Mako Surgical Corp., a medical device company; and Concert Pharmaceuticals, Inc., a biopharmaceutical company. Dr. Freund is a member of the Advisory Board for the Harvard Business School Healthcare Initiative. Dr. Freund received a B.A. in history from Harvard College, an M.D. from Harvard Medical School, and an M.B.A. from Harvard Business School. We believe that Dr. Freund’s extensive investment experience, his experience as an executive and his service on the board of directors of numerous public and privately held companies allows him to be a key contributor to our board of directors.
OUR BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR” THE ELECTION OF JEFFREY CHODAKEWITZ, GERRI HENWOOD AND GUY MACDONALD TO SERVE AS CLASS II DIRECTORS.
10
DIRECTOR COMPENSATION
Under our director compensation program, we pay ournon-employee directors cash retainers. We do not pay any compensation to our president and chief executive officer in connection with his service on our board of directors. The compensation that we pay to our president and chief executive officer is discussed elsewhere in this proxy statement. Eachnon-employee director receives a cash retainer for service on the board of directors and for service on each committee on which the director is a member. The chairmen of the board and of each committee receive higher retainers for such service. These fees are payable quarterly in arrears. The fees paid tonon-employee directors for service on the board of directors and for service on each committee of the board of directors on which the director is a member are as follows:
| | | | | | | | |
| | | Member
Annual Fee | | | Chairman
Annual Fee | |
Board of Directors | | $ | 35,000 | | | $ | 70,000 | |
Audit Committee | | | 7,500 | | | | 15,000 | |
Compensation Committee | | | 7,500 | | | | 15,000 | |
Nominating and Corporate Governance Committee | | | 5,000 | | | | 7,500 | |
In addition, under our director compensation program, eachnon-employee director that is elected to our board of directors receives an option to purchase 25,000 shares of our common stock, which option vests in equal quarterly installments over a three-year period measured from the date of grant, subject to thenon-employee director’s continued service as a director, and becoming exercisable in full upon a change in control of our company. Further, on the date of the first board meeting held after each annual meeting of stockholders, eachnon-employee director that has served on our board of directors for at least six months receives an option to purchase 12,500 shares of our common stock. Each of these options vests in equal quarterly installments over aone-year period measured from the date of grant, subject to thenon-employee director’s continued service as a director, and becomes exercisable in full upon a change in control of our company. The exercise price of these options equals the fair market value of our common stock on the date of grant.
This program is intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our stockholders.
We reimburse ournon-employee directors for reasonable travel andout-of-pocket expenses incurred in connection with attending board of director and committee meetings. The following table sets forth information regarding compensation earned by ournon-employee directors during the year ended December 31, 2017.
Director Compensation for 2017
| | | | | | | | | | | | |
Name | | Fees Earned or Paid
in Cash ($) | | | Stock Option Awards
($)(1)(2) | | | Total ($) | |
L. Patrick Gage, Ph.D.(3) | | $ | 81,250 | | | $ | 61,358 | | | $ | 142,608 | |
Garen Bohlin(4) | | | 50,000 | | | | 61,358 | | | | 111,358 | |
Jeffrey Chodakewitz, M.D.(5) | | | 42,500 | | | | 61,358 | | | | 103,858 | |
John Freund , M.D.(6) | | | 47,500 | | | | 61,358 | | | | 108,858 | |
Gerri Henwood(7) | | | 42,500 | | | | 61,358 | | | | 103,858 | |
Nancy Wysenski(8) | | | 50,000 | | | | 61,358 | | | | 111,358 | |
| (1) | The amounts in the Stock Option Awards column reflect the grant date fair value of stock option awards granted during 2017 under our stock incentive plans, in accordance with Financial Accounting Standards Codification Topic 718, Compensation-Stock Compensation, or FASB ASC Topic 718. There can be no |
11
| | assurance that FASB ASC Topic 718 amounts will reflect actual amounts realized. Refer to Note 7, “Stock-Based Compensation”, in the Notes to Consolidated Financial Statements included in the Annual Report on Form10-K for 2017 filed with the SEC on March 6, 2018 for the relevant assumptions used to determine the valuation of our option awards. |
| (2) | The number of shares underlying stock option awards granted to ournon-employee directors in 2017 and the grant date fair value of such stock options as determined in accordance with FASB ASC Topic 718 are: |
| | | | | | | | | | | | |
Director | | Grant Date | | | Number of Shares
Underlying Stock
Option Grants in 2017 | | | Grant Date Fair
Value of Stock Option
Grants in 2017 ($) | |
Dr. Gage | | | 5/31/2017 | | | | 12,500 | | | $ | 61,358 | |
Mr. Bohlin | | | 5/31/2017 | | | | 12,500 | | | | 61,358 | |
Dr. Chodakewitz | | | 5/31/2017 | | | | 12,500 | | | | 61,358 | |
Dr. Freund | | | 5/31/2017 | | | | 12,500 | | | | 61,358 | |
Ms. Henwood | | | 5/31/2017 | | | | 12,500 | | | | 61,358 | |
Ms. Wysenski | | | 5/31/2017 | | | | 12,500 | | | | 61,358 | |
| (3) | At December 31, 2017, Dr. Gage held stock options to purchase 82,044 shares of our common stock. |
| (4) | At December 31, 2017, Mr. Bohlin held stock options to purchase 95,065 shares of our common stock. |
| (5) | At December 31, 2017, Dr. Chodakewitz held stock options to purchase 52,500 shares of our common stock. |
| (6) | At December 31, 2017, Dr. Freund held stock options to purchase 62,500 shares of our common stock. |
| (7) | At December 31, 2017, Ms. Henwood held stock options to purchase 42,500 shares of our common stock. |
| (8) | At December 31, 2017, Ms. Wysenski held stock options to purchase 52,500 shares of our common stock. |
12
CORPORATE GOVERNANCE
General
We believe that good corporate governance is important to ensure that our company is managed for the long-term benefit of our stockholders. We periodically review our corporate governance policies and practices and compare them to those suggested by various authorities in corporate governance and the practices of other public companies. As a result, we have adopted policies and procedures that we believe are in the best interests of our company and our stockholders.
Corporate Governance Guidelines
Our corporate governance guidelines assist our board of directors in the exercise of its duties and responsibilities and to serve the best interests of our company and our stockholders. These guidelines, which provide a framework for the conduct of our board’s business, provide that:
| | • | | the principal responsibility of the directors is to oversee our management; |
| | • | | a majority of the members of the board shall be independent directors, unless otherwise permitted by NASDAQ rules; |
| | • | | the independent directors meet at least twice a year and at other times at the request of any independent director; |
| | • | | directors have full and free access to management and, as necessary and appropriate, independent advisors; and |
| | • | | at least annually, the nominating and corporate governance committee oversees a self-evaluation by the board to assess the effectiveness of the board and its committees. |
Code of Business Conduct and Ethics
We have also adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of the code is posted on the “Investors—Corporate Governance” section of our website, which is located at www.tphase.com. If we make any substantive amendments to, or grant any waivers from, the code of business conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a Current Report on Form8-K to be filed with the SEC.
Determination of Independence
Rule 5605 of the NASDAQ Listing Rules requires a majority of a listed company’s board of directors to be comprised of independent directors. In addition, the NASDAQ Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent, that audit committee members also satisfy independence criteria set forth in Rule10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and that compensation committee members also satisfy independence criteria set forth in Rule10C-1 under the Exchange Act.
Under Rule 5605(a)(2) of the NASDAQ Listing Rules, a director will only qualify as an “independent director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule10A-3 of the Exchange Act, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries.
13
In addition, in affirmatively determining the independence of any director who will serve on a company’s compensation committee, Rule10C-1 under the Exchange Act requires that a company’s board of directors consider all factors specifically relevant to determining whether a director has a relationship to such company which is material to that director’s ability to be independent from management in connection with the duties of a compensation committee member, including, but not limited to: (i) the source of compensation of the director, including any consulting advisory or other compensatory fee paid by such company to the director; and (ii) whether the director is affiliated with the company or any of its subsidiaries or affiliates.
Our board of directors undertook a review of the composition of our board of directors and its committees and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Mr. Macdonald, is an “independent director” as defined under Rule 5605(a)(2) of the NASDAQ Listing Rules. Our board of directors also determined that Garen Bohlin, John Freund and L. Patrick Gage, who comprise our audit committee, Jeffrey Chodakewitz, Gerri Henwood and Nancy Wysenski, who comprise our compensation committee and John Freund and L. Patrick Gage, who comprise our nominating and corporate governance committee, satisfy the independence standards for such committees established by the SEC and the NASDAQ Listing Rules, as applicable. In making such determinations, our board of directors considered the relationships that each suchnon-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by eachnon-employee director.
Board Self-Assessment; Director Candidates: Criteria and Diversity
Our board performs an annual self-assessment. During this self-assessment, the board considers many factors, including, but not limited to, the expertise of existing board members and the potential expertise of directors we may need as we transition from a clinical development stage company to becoming a commercial enterprise. The assessment of this potential expertise consists of a review of the business expertise of our current directors as well as the potential of evaluating new director candidates.
The process followed by our nominating and corporate governance committee to identify and evaluate director candidates includes requests to board members and others for recommendations, meetings from time to time to evaluate biographical information and background material relating to potential candidates and interviews of selected candidates by members of the committee and our board. The nominating and corporate governance committee has from time to time engaged independent director search firms to assist in identifying and evaluating potential nominees for election to our board of directors.
In considering whether to recommend to our board of directors any particular candidate for inclusion in the board’s slate of recommended director nominees, including candidates recommended by stockholders, the nominating and corporate governance committee of our board applies the criteria set forth in our Corporate Governance Guidelines. These criteria include the candidate’s integrity, business acumen, knowledge of our business and industry, experience in one or more areas relevant to our business and strategy, diligence, conflicts of interest and the ability to act in the interests of all stockholders.
Our nominating and corporate governance committee does not have a policy (formal or informal) with respect to diversity, but believes that our board, taken as a whole, should embody a diverse set of skills, experiences and backgrounds. In this regard, the committee also takes into consideration the diversity (with respect to gender, race and national origin) of our board members. The committee does not make any particular weighting of diversity or any other characteristic in evaluating nominees and directors.
Stockholders may recommend individuals to our nominating and corporate governance committee for consideration as potential director candidates by submitting their names, together with appropriate biographical information and background materials and a statement as to whether the stockholder or group of stockholders
14
making the recommendation has beneficially owned more than 5% of our common stock for at least a year as of the date such recommendation is made, to Nominating and Corporate Governance Committee, c/o Corporate Secretary, 480 Arsenal Way, Watertown, Massachusetts 02472. Assuming that appropriate biographical and background material has been provided on a timely basis, the committee will evaluate stockholder-recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others. If the board determines to nominate a stockholder-recommended candidate and recommends his or her election, then his or her name will be included in our proxy card for the next annual meeting.
Stockholders also have the right under our bylaws to directly nominate director candidates, without any action or recommendation on the part of the committee or our board, by following the procedures set forth under “Stockholder Proposals for the 2019 Annual Meeting.”
Communication from Stockholders
The board will give appropriate attention to written communications that are submitted by stockholders, and will respond if and as appropriate. The chairman of the board of directors is primarily responsible for monitoring communications from stockholders and for providing copies or summaries to the other directors as he considers appropriate.
Communications are forwarded to all directors if they relate to important substantive matters and include suggestions or comments that the chairman of the board considers to be important for the directors to know. In general, communications relating to corporate governance and corporate strategy are more likely to be forwarded than communications relating to ordinary business affairs, personal grievances and matters as to which we receive repetitive or duplicative communications.
Board and Committee Meetings
Our board of directors held 24 meetings during 2017. During 2017, each of the directors then in office attended at least 75% of the aggregate of all meetings of the board of directors and all meetings of the committees of the board of directors on which such director then served. Continuing directors and nominees for election as directors in a given year are required to attend the annual meeting of stockholders, barring significant commitments or special circumstances. All directors then in office attended the 2017 annual meeting of stockholders.
We have established an audit committee, a compensation committee and a nominating and corporate governance committee. Each of these committees operates under a charter that has been approved by our board of directors. A copy of each charter can be found under the “Investors—Corporate Governance” section of our website, which is located at www.tphase.com.
Audit Committee
The current members of our audit committee are Garen Bohlin, John Freund and L. Patrick Gage. Garen Bohlin is the chair of the audit committee. Our board of directors has determined that Mr. Bohlin qualifies as an audit committee financial expert within the meaning of SEC regulations. Our audit committee assists our board of directors in its oversight of our accounting and financial reporting process and the audits and quarterly reviews of our financial statements. We currently do not have an internal audit function. The audit committee held seven meetings during the 2017 fiscal year. The audit committee’s responsibilities include:
| | • | | appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm; |
| | • | | overseeing the work of our independent registered public accounting firm, including through the receipt and consideration of reports from such firm; |
15
| | • | | reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| | • | | monitoring our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics; |
| | • | | overseeing an internal audit function, should we have one in the future; |
| | • | | discussing our risk management policies; |
| | • | | establishing policies regarding hiring employees from our independent registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns; |
| | • | | meeting independently with our internal finance staff, our independent registered public accounting firm and management; |
| | • | | reviewing and approving or ratifying any related person transactions; and |
| | • | | preparing the audit committee report required by SEC rules. |
All audit services to be provided to us and allnon-audit services, other than de minimisnon-audit services, to be provided to us by our independent registered public accounting firm must be approved in advance by our audit committee.
Compensation Committee
The current members of our compensation committee are Jeffrey Chodakewitz, Gerri Henwood and Nancy Wysenski. Nancy Wysenski is the current chair of the compensation committee. Our compensation committee assists our board of directors in the discharge of its responsibilities relating to the compensation of our executive officers. The compensation committee held eight meetings and acted by written consent once during the 2017 fiscal year. The compensation committee’s responsibilities include:
| | • | | reviewing and approving, or making recommendations to our board of directors with respect to, the compensation of our chief executive officer and other executive officers; |
| | • | | overseeing the evaluation of our senior executives; |
| | • | | reviewing and making recommendations to our board of directors with respect to our incentive-compensation and equity-based compensation plans; |
| | • | | overseeing and administering our equity-based plans; |
| | • | | reviewing and making recommendations to our board of directors with respect to director compensation; |
| | • | | reviewing and discussing annually with management our executive compensation disclosure; and |
| | • | | preparing the compensation committee report required by SEC rules. |
Nominating and Corporate Governance Committee
The current members of our nominating and corporate governance committee are John Freund and L. Patrick Gage. L. Patrick Gage is the chair of the nominating and corporate governance committee. The nominating and corporate governance committee held two meetings during the 2017 fiscal year. The nominating and corporate governance committee’s responsibilities include:
| | • | | identifying individuals qualified to become members of our board of directors; |
| | • | | recommending to our board of directors the persons to be nominated for election as directors and to each of our board’s committees; |
16
| | • | | developing and recommending to our board of directors corporate governance principles; and |
| | • | | overseeing an annual evaluation of our board of directors. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves, or in the past has served, as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of our board of directors or our compensation committee. None of the members of our compensation committee is an officer or employee of our company, nor have they ever been an officer or employee of our company.
Board Leadership Structure
Our board has chosen to separate the role of our chief executive officer and the role of chairman of our board. We believe that this separation is appropriate since our chief executive officer is responsible for the strategic direction of our company, while the chairman of our board is responsible for overseeing the function of the board and for providing guidance to our chief executive officer as needed.
Our board of directors is currently chaired by L. Patrick Gage, an independent director, who possesses anin-depth knowledge of our issues, opportunities and challenges. We believe he is the person best positioned to ensure our board of directors’ time and attention is focused on the most critical matters. Our board of directors believes Dr. Gage is a decisive leader who commands accountability and enhances our ability to communicate our message and strategy clearly and consistently to stockholders, employees and strategic partners.
Oversight of Risk
Our board of directors has responsibility for the oversight of the company’s risk management processes and, either as a whole or through its committees, regularly discusses with management our major risk exposures, the potential impact of these risks on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board to understand the company’s risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, strategic and reputational risk.
The audit committee reviews information regarding liquidity and operations, and oversees our management of financial risks. Periodically, the audit committee reviews our policies with respect to risk assessment, risk management, loss prevention and regulatory compliance. Oversight by the audit committee includes direct communication with our external auditors, and discussions with management regarding significant risk exposures and the actions management has taken to limit, monitor or control such exposures. The compensation committee is responsible for assessing whether any of our compensation policies or programs has the potential to encourage excessive risk-taking. The nominating and corporate governance committee manages risks associated with the independence of the board, corporate disclosure practices, and potential conflicts of interest. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire board is regularly informed through committee reports about such risks. Matters of significant strategic risk are considered by our board as a whole.
Certain Relationships and Related Party Transactions
We have not been a party to any transactions since January 1, 2017 in which any of our directors, executive officers or beneficial owners of more than 5% of our voting securities, or affiliates or immediate family members of any of our directors, executive officers or beneficial owners of more than 5% of our voting securities, had or will have a direct or indirect material interest.
17
Policies and Procedures for Related Person Transactions
Our board of directors has adopted a written related person transaction policy setting forth the procedures for the review and approval or ratification of related person transactions. This policy covers any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, the amount involved exceeds $120,000, and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person.
Our related person transaction policy contains exceptions for any transaction or interest that is not considered a related person transaction under SEC rules as in effect from time to time. In addition, the policy provides that an interest arising solely from a related person’s position as an executive officer of another entity that is a participant in a transaction with us will not be subject to the policy if each of the following conditions is met:
| | • | | the related person and all other related persons own in the aggregate less than a 10% equity interest in such entity; |
| | • | | the related person and his or her immediate family members are not involved in the negotiation of the terms of the transaction with us and do not receive any special benefits as a result of the transaction; and |
| | • | | the amount involved in the transaction equals less than the greater of $200,000 or 5% of the annual gross revenue of the company receiving payment under the transaction. |
The policy provides that any related person transaction proposed to be entered into by us must be reported to our chief financial officer and will be reviewed and approved by our audit committee in accordance with the terms of the policy, prior to effectiveness or consummation of the transaction whenever practicable. The policy provides that if our chief financial officer determines that advance approval of a related person transaction is not practicable under the circumstances, our audit committee will review and, in its discretion, may ratify the related person transaction at the next meeting of the audit committee. The policy also provides that alternatively, our chief financial officer may present a related person transaction arising in the time period between meetings of the audit committee to the chair of the audit committee, who will review and may approve the related person transaction, subject to ratification by the audit committee at the next meeting of the audit committee.
In addition, the policy provides that any related person transaction previously approved by the audit committee or otherwise already existing that is ongoing in nature will be reviewed by the audit committee annually to ensure that such related person transaction has been conducted in accordance with the previous approval granted by the audit committee, if any, and that all required disclosures regarding the related person transaction are made.
A related person transaction reviewed under this policy will be considered approved or ratified if it is authorized by the audit committee in accordance with the standards set forth in the policy after full disclosure of the related person’s interests in the transaction. As appropriate for the circumstances, the policy provides that the audit committee will review and consider:
| | • | | the related person’s interest in the related person transaction; |
| | • | | the approximate dollar value of the amount involved in the related person transaction; |
| | • | | the approximate dollar value of the amount of the related person’s interest in the transaction without regard to the amount of any profit or loss; |
| | • | | whether the transaction was undertaken in the ordinary course of business of our company; |
18
| | • | | whether the transaction with the related person is proposed to be, or was, entered into on terms no less favorable to us than the terms that could have been reached with an unrelated third party; and |
| | • | | any other information regarding the related person transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction. |
The policy provides that the audit committee will review all relevant information available to it about the related person transaction. The policy provides that the audit committee may approve or ratify the related person transaction only if the audit committee determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, our best interests. The policy provides that the audit committee may, in its sole discretion, impose such conditions as it deems appropriate on us or the related person in connection with approval of the related person transaction.
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
This section discusses the material elements of our executive compensation policies and decisions and the most important factors relevant to an analysis of these policies and decisions. It provides qualitative information regarding the manner and context in which compensation is awarded to and earned by our executive officers named in the “Summary Compensation Table” below, or our “named executive officers,” and is intended to place in perspective the data presented in the following tables and the corresponding narrative. Our named executive officers for 2017 are:
| | • | | Guy Macdonald, our president and chief executive officer; |
| | • | | Jacques Dumas, our chief scientific officer; |
| | • | | Patrick Horn, our former chief medical officer; |
| | • | | Maria Stahl, our senior vice president, general counsel; |
| | • | | Kamalam Unninayar, our former chief financial officer, and |
| | • | | Christopher Watt, our senior vice president, finance. |
Mr. Watt served as our principal financial and accounting officer until immediately after the filing of our Quarterly Report on Form10-Q for the period ended September 30, 2017 which was filed with the SEC on November 1, 2017. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation on March 16, 2018. Dr. Horn ceased to serve as our chief medical officer on December 29, 2017.
Executive Summary
Our Business
We are a clinical-stage biopharmaceutical company using our proprietary chemistry technology to create novel antibiotics for serious and life-threatening multidrug-resistant infections. We are developing our lead product candidate, eravacycline, a fully synthetic tetracycline derivative, as an intravenous, or IV, and oral antibiotic for use as a first-line empiric monotherapy for the treatment of multidrug-resistant infections, including multidrug-resistant Gram-negative infections.
19
2017 and Early 2018 Corporate Performance
During 2017, we made significant progress in the continued clinical development of IV eravacycline, the continued development of oral eravacycline, our pipeline programs and in the completion of regulatory filings for IV eravacycline for the treatment of complicated intra-abdominal infections, or cIAI. Some of the key highlights for 2017 are as follows:
IGNITE4 Phase III Clinical Data Readout: In July 2017, we announced positivetop-line data from our IGNITE4 phase 3 clinical trial where eravacycline met the primary endpoint of statisticalnon-inferiority of clinical response. Prior to IGNITE 4, we conducted IGNITE1, a phase 3 clinical trial of twice daily IV eravacycline for the treatment of cIAI. In IGNITE1, eravacycline met the primary endpoint of statisticalnon-inferiority of clinical response.
Regulatory Filings for cIAI Indication: In August 2017, we filed a marketing authorization application with the European Medicines Agency based on the results of IGNITE1. In December 2017, we filed a new drug application with the U.S. Food and Drug Administration, or FDA, based on the results of IGNITE1 and IGNITE4. Both of these regulatory applications are currently under review by the respective regulatory authority.
During 2017, we were also developing eravacycline for the treatment of complicated urinary tract infections, or cUTI. In January 2017, we initiated IGNITE3, a phase 3 clinical trial evaluating the efficacy and safety of once-daily IV eravacycline compared to ertapenem, the control therapy in this trial, for the treatment of cUTI and in September 2017, we completed enrollment in IGNITE3.
Unfortunately, in February 2018 we announced that our IGNITE3 clinical trial did not meet the co-primary efficacy endpoints of statisticalnon-inferiority of clinical response. We are disappointed that the IGNITE3 trial did not achieve itsco-primary endpoints, but this outcome does not affect our belief that eravacycline can serve as an important new antibiotic for patients with serious cIAI, particularly those caused bydifficult-to-treat Gram-negative bacteria, and resistant mixed infections. Positive data from both the IGNITE1 and IGNITE4 phase 3 clinical trials, which evaluated eravacycline in cIAI, support this belief with both studies having met their primary endpoints. There were no safety concerns in IGNITE 3 that would change our plans in the cIAI indication and we are actively preparing for the commercialization of eravacycline as a treatment in cIAI in both the U.S and in Europe, subject to regulatory approvals.
Impact of 2017 and Early 2018 Corporate Performance on 2017 and 2018 Compensation
Our 2017 corporate goals did not include any milestones related to eravacycline for the treatment of cUTI or the outcome of our phase 3 IGNITE3 clinical trial. The compensation committee evaluated our 2017 corporate performance in early January 2018 and established a performance rating based on the extent to which our 2017 corporate goals were achieved, as further described below under “2017 Corporate Goals.”
In early January 2018, the compensation committee also established our 2018 corporate goals for purposes of our short and long-term compensation arrangements, several of which relate to eravacycline for the treatment of cUTI. As a result of the subsequent February 2018 announcement regarding the result of the outcome of our phase 3 IGNITE3 clinical trial, several of these goals may now be unachievable.
Overview of Our Executive Compensation Program
We have designed our executive compensation program to motivate our management team to create long-term value for our stockholders through the achievement of strategic business objectives, while effectively managing the risks and challenges inherent in a clinical stage biopharmaceutical company. Specifically, our executive compensation program is designed to promote the achievement of key strategic objectives by linking executives’ short- and long-term cash and equity incentives to the achievement of measurable corporate and
20
individual performance goals. Due to our stage of development, our corporate goals for 2017 were primarily focused on the clinical development of eravacycline for the treatment of cIAI. Before our February 2018 IGNITE3 announcement, our 2018 corporate goals included the achievement of regulatory approval of eravacycline and subsequent commercialization efforts for the treatment of cIAI and other milestones related to eravacycline for the treatment of cUTI.
Our executive compensation programs are designed to be competitive with our peer group to enable us to attract, motivate, reward, and retain outstanding talent. Our compensation programs are based on the following key principles:
| | • | | Linkage of pay with performance and the achievement of our strategic goals. |
| | • | | Alignment of our executives’ interests with those of our stockholders through equity compensation, a significant portion of which will vest based upon achievement of performance goals that are aligned with our business plan. |
| | • | | Overall compensation that is competitive in the industry in which we compete for executive talent. Compensation opportunities should be competitive with clinical stage biotechnology companies of similar size and comparable stage of development, but also should be designed to be flexible enough to attract talent as needed from larger biopharmaceutical companies as we move toward becoming a commercial enterprise. |
| | • | | Recognition of individual contributions, teamwork and corporate performance. |
Compensation Governance Highlights
| | | | | | |
| | | What we do | | | | What we do not do |
| ☒ | | Design executive compensation program to align pay with performance | | ☒ | | No excessive change in control or severance payments |
| ☒ | | Grant equity awards with performance-based vesting | | ☒ | | No repricing of underwater stock options without stockholder approval |
| ☒ | | Prohibit hedging and pledging by executive officers and directors | | ☒ | | No taxgross-ups |
| ☒ | | Conduct an annualsay-on-pay vote | | ☒ | | No perquisites |
| ☒ | | Seek input from, listen to and respond to stockholders | | ☒ | | No guaranteed bonuses |
2017Say-on-Pay Vote
We conducted our secondsay-on-pay vote at our 2017 annual meeting of our stockholders. Approximately 97% of the votes cast on thesay-on-pay proposal supported the proposal, showing the positive results of our prior stockholder outreach and changes to our named executive officers’ compensation based on stockholder feedback. The compensation committee has considered the results of our 2016 and 2017say-on-pay advisory votes in the context of our overall compensation philosophy, policies and decisions. Our compensation committee believes the result of our 2017say-on-pay vote endorsed the compensation philosophy and decisions we made for 2017. After discussing the result of the vote in 2017 and taking into account changes made to our compensation program in 2017 as a result of the 2016 vote onsay-on-pay, our compensation committee decided to maintain a consistent approach to compensation decisions for 2018. In particular:
| | • | | In 2017 we adopted a performance-based equity program. As further described below, in 2017, approximately 25% of each named executive officer’s annual long-term equity incentive award was granted in the form of restricted stock units (RSUs) that are only eligible to vest if we achieve certain clinical development and regulatory milestones that are related to our business strategy. In 2018, the |
21
| | percentage of each named executive officer’s annual long-term equity incentive award that was granted in the form of performance-based RSUs was increased to approximately 30%. |
| | • | | We continue to provide descriptive detail in this compensation discussion and analysis, our rationale and process for selecting specific corporate and individual goals, how we determined target awards for our named executive officers and the accomplishments related to each goal. |
| | • | | Our stockholders also emphasized the importance of aligning executive pay with corporate performance. We believe it is important to align executive compensation with corporate performance and we continue to monitor the alignment of our chief executive officer’s total compensation to our total shareholder return, or TSR. The chart below shows our TSR over the past two years with the total reported compensation of Mr. Macdonald for the last three fiscal years, as disclosed in the Summary Compensation Table. TSR is the return on our stock measured from December 31 to December 31 of each relevant period. The share price of our common stock increased approximately 56% from the end of fiscal year 2016 to the end of fiscal year 2017. During the same time period, Mr. Macdonald’s reported compensation decreased 20%. The apparent discrepancy between the decrease in Mr. Macdonald’s compensation compared to the increase in share price for the same year is a function of using the grant date value of stock options (that is calculated for accounting purposes and related to the price of our common stock on the grant date which was January 31, 2017) to calculate Mr. Mcdonald’s compensation, even though the value, if any, that he will ultimately realize will depend on the price of our common stock on the date on which his options are exercised. Despite this apparent discrepancy, our compensation committee believes that there is strong alignment between TSR and Mr. Macdonald’s compensation. |

Overview of Our Philosophy and Procedures for Determining Executive Compensation
Role of the Compensation Committee
The compensation committee has primary responsibility for designing, implementing and maintaining a compensation program for our executive officers, including our named executive officers. The responsibilities of the compensation committee are set forth in detail in the compensation committee charter, which can be found on our website at www.tphase.com under the caption “Investors—Corporate Governance—Committee Charters.” In particular, the compensation committee annually reviews the base salaries, cash incentives and equity compensation of our named executive officers and periodically reviews other elements of our compensation program.
22
Our General Practice
Compensation decisions are based primarily on the following:
| | • | | Annual Performance Reviews—Our chief executive officer conducts and presents an assessment of our corporate performance and the performance reviews of the other named executive officers to the compensation committee after the end of each fiscal year. In reviewing and determining the compensation of each named executive officer, the compensation committee also considers individual factors, such as: potential for future contributions to our growth, industry experience and retention concerns. |
| | • | | Peer and Industry Data—The compensation committee considers peer and industry data provided by its compensation consultant, W.T. Haigh & Company, as a reference in setting base salaries and target cash compensation, determining appropriate levels and mix of equity compensation and determining the type and portion of compensation tied to performance goals. |
| | • | | Chief Executive Officer Recommendations—The compensation committee seeks input from our chief executive officer for setting the salary and target cash compensation levels for the other executive officers, and also for purposes of setting annual performance metrics and target incentive amounts for awards granted to the other executive officers. |
To achieve the objectives described above, the compensation committee evaluates our compensation program with the goal of setting compensation at levels that are based on each executive’s level of experience, performance and responsibility and that are competitive with those of other companies in our industry that compete with us for executive talent. The compensation committee seeks to ensure that our executive compensation program contains an appropriate amount of compensation for each of our executive officers that is “at risk” and subject to the achievement of critical business objectives.
Role of the Compensation Consultant
To help evaluate the appropriate levels of compensation with respect to each component of our compensation program, the compensation committee annually reviews the compensation level of our executive officers, including our named executive officers, against market information, including publicly available compensation levels of individuals in comparable positions of a peer group of companies. The compensation committee has the authority to retain compensation consultants and other outside advisors to assist in the evaluation of executive officer compensation. During 2017, the compensation committee again retained W.T. Haigh & Company, an independent executive compensation consulting firm, to provide assistance in evaluating and developing our executive compensation program. W.T. Haigh & Company provides consulting activities on behalf of the compensation committee and does not provide consulting or additional services for Tetraphase management. The compensation committee has determined that no conflicts of interest exist with respect to W.T. Haigh & Company’s work.
W.T. Haigh & Company provides the committee with compensation survey data for purposes of comparing each compensation component within our executive compensation program—namely base salary, cash performance incentives, equity awards and benefits—to a group of other publicly traded companies engaged in the discovery and development of drug products, which were selected generally based primarily on the similarity of their stage of development, market capitalization and headcount. W.T. Haigh & Company also generally provides the committee with the following:
| | • | | assistance interpreting various sets of compensation data; |
| | • | | recommendations regarding our compensation policies in general, compensation packages for each of our executive officers and the competitiveness and effectiveness of our executive officer compensation levels; and |
| | • | | assistance in the selection of our peer group companies. |
23
In gathering competitive market compensation data, W.T. Haigh & Company generally utilizes two primary sources:
| | • | | published compensation surveys for biotechnology and pharmaceutical companies; and |
| | • | | proxy information of selected peer group companies. |
In 2017, W.T. Haigh & Company utilized information from the Radford Global Life Sciences survey and comparable executive compensation information published in publicly available proxy statements for a peer group of companies of similar size and market capitalization in the biotechnology industry to develop a competitive analysis report. In addition, W.T. Haigh & Company considered the overall economic environment and trends within the biopharmaceutical industry when making observations and recommendations. W.T. Haigh & Company presented its findings and observations in a written report to the compensation committee prior to the compensation committee making any determination regarding the 2017 compensation of our executive officers.
Procedures for Establishing 2017 Compensation
Peer Group
The compensation committee reviews and approves the list of peer companies each year. When determining the 2017 peer group used for purposes of determining 2017 compensation, our compensation committee considered biopharmaceutical companies that are of similar size to the Company in terms of market capitalization, research and development spend, stage of development and number of employees. At the time our 2017 peer group was selected in September 2016, our year to date market capitalization was at the 20th percentile of the peer group, our research and development spend was at the 75th percentile of the peer group, our revenue was at the 47th percentile of the peer group and our number of employees was at the 40th percentile of our peer group. Our compensation committee also considered the geographic locations of the peer companies to the extent relevant when considering companies with which we compete for executive talent.
Aerie Pharmaceuticals Inc.
Agenus, Inc.
Akebia Therapeutics, Inc.
Biocryst Pharmaceuticals, Inc.
Chimerix, Inc.
Cempra, Inc.
Concert Pharmaceuticals, Inc.
Contrafect Corporation
Dicerna Pharmceuticals, Inc.
Enanta Pharmaceuticals, Inc.
Epizyme, Inc.
Esperion Therapeutics, Inc.
Genocea Biosciences, Inc.
Karyopharm Therapeutics, Inc.
Oncomed Pharmaceuticals, Inc.
Paratek Pharmaceuticals, Inc.
PTC Therapeutics, Inc.
Regulus Therapeutics Inc.
Revance Therapeutics Inc.
Trevena Inc.
Zafgen Inc.
Philosophy and Process Regarding Compensation Elements and Total Compensation
Our compensation committee reviews market practices and compensation data for our peer companies’ comparably-situated executives when making decisions about compensating our executive officers. For 2017, the compensation committee established total compensation targets for our executive officers after considering information from the 2016 Radford Global Life Sciences Executive Compensation Survey (covering approximately 100 public companies with headcounts between 50 and 150 employees) and the proxy information of the 2017 peer group comprised of the 21 companies listed above.
The compensation committee regularly analyzes how changes in any element of each executive officer’s compensation could impact other elements. Such analysis has become a key component in the compensation committee’s review of an executive’s compensation as the analysis allows the compensation committee to consider an executive’s overall compensation rather than only one or two specific components.
24
When reviewing and analyzing the amount of each major component and the total compensation opportunity for each of our executive officers, our compensation committee reviews each component at the 25th, 50th and 75th percentiles of our peer companies’ comparably-situated executives for guidance. Our compensation committee reviews these pay levels as reference points in its overall decision making and as indicative of the level of compensation necessary to attract, retain and motivate our executive officers. Our compensation committee sets the actual amount of each element of compensation and the total compensation opportunity of each executive officer based in part on its review of peer group data and in part on the other factors discussed above and below.
Determining appropriate base salaries is critical to the compensation program because other elements of our compensation are affected by changes in base salary. For example, payments under our annual cash performance incentive plan are targeted and paid out as a percentage of base salary. Adjustments to the base salary in any year are made based on comparisons to the peer group and survey data noted above and evaluation of the executive’s level of responsibility, experience and performance.
Our executives are eligible to participate in our annual cash performance incentive plan, which is an annual variable cash incentive plan offered to all our employees. The payout for our chief executive officer is based primarily upon achievement ofpre-determined corporate goals, and for our other executives is based upon achievement of bothpre-determined corporate goals and individual goals. Mr. Macdonald does not have individual goals. Our executives are also eligible to participate in long-term incentives through stock option grants and grants of performance-based restricted stock units, which we believe helps to retain our executives and align their interests with those of our stockholders.
Process for Annual Performance-Based Incentive Compensation
In the beginning of each fiscal year, corporate goals for the year are presented by the chief executive officer. These goals are weighted by the chief executive officer based on relative importance to Tetraphase’s success and business strategy. The corporate goals are then presented to the compensation committee, which actively engages in the process of refining the objectives and their respective weightings for review and recommendation to the board of directors. The corporate goals and their respective weightings are finalized and approved by the board of directors. In addition, at the beginning of each fiscal year, the chief executive officer establishes individual goals for each other executive officer. The chief executive officer’s incentive awards are generally based on achievement of the corporate goals due to the unique nature of his position.
At the end of each fiscal year, the extent to which corporate and individual goals (for officers other than the chief executive officer) are achieved is used in determining annual cash incentive payments earned for that year and is considered in determining equity awards for our executives during the following fiscal year. In the first quarter of each fiscal year, the compensation committee evaluates the company’s actual performance for the prior year against thepre-determined corporate objectives to determine the amount of funding for the total cash incentive pool percentage for all employees, including our named executive officers.
Upon completion of the fiscal year, the chief executive officer evaluates the performance of each executive officer (other than himself) and assigns a proposed rating to such officer based upon his or her achievement of the corporate and individual goals. The chief executive officer presents a summary recommendation to the compensation committee of the performance evaluations and ratings along with compensation recommendations for the other executive officers. The compensation committee reviews these recommended evaluations and ratings based on performance against the corporate goals and individual goals for each executive other than the chief executive officer), as further described below, and decides whether to approve or adjust the recommendations for individual executives made by the chief executive officer. In determining the actual success of the executive’s performance in any year, including 2017, the compensation committee considers the difficulty of attaining the corporate and individual objectives, whether there were any extenuating circumstances or factors that needed to be considered and whether the stated objectives were actually met.
25
In addition, the compensation committee meets in executive session to discuss and review the compensation of the chief executive officer and his performance over the past year compared to the previously approved goals for the corresponding year and compares his compensation to third party compensation data prepared by W.T. Haigh & Company. For 2017, the board of directors also discussed, reviewed and approved Mr. Macdonald’s 2017 performance and his compensation for 2017.
No executive officer, including our chief executive officer, recommends or determines any element or component of his or her own pay package or total compensation amount.
Process for Long-Term Performance-Based Equity Incentive Compensation
Beginning in 2017 and in response to feedback from our stockholders, the compensation committee established a long-term performance-based equity compensation program. The program is comprised of performance-based restricted stock units that may be earned upon the achievement of certain milestones (clinical, regulatory or other) selected by the compensation committee, and may vest on the third anniversary of the grant date, subject to the recipient’s continued employment through the vesting date. The compensation committee must certify that thesepre-determined milestones have in fact been met prior to a determination being made that such milestones have been achieved. In January 2018, the compensation committee certified that three of the five milestones had been achieved under our long-term performance-based equity compensation program that covers the years 2017 to 2020.
Elements of Executive Direct Total Compensation
We strive to recognize the efforts involved in managing our business by compensating our named executive officers for the demands and risks associated with our business through three elements that are designed to reward performance in a simple and straightforward manner—base salaries, annual performance-based cash incentives and long-term equity awards. During our previous stockholder outreach, our key stockholders expressed support for the elements of our executive compensation program, including our continued use of stock options as one portion of long-term equity awards. However, several stockholders also recommended granting a portion of long-term equity awards with performance-based vesting. As reflected in the chart below, we responded to the feedback from our stockholders by including performance-based vesting for a portion of our long-term incentive equity awards.
The table below summarizes the purpose and key characteristics of each of our compensation elements.
| | | | |
| Element | | Purpose | | Key Characteristics |
| Base Salaries | | Provides a fixed level of compensation for performing the essentialday-to-day elements of the job; gives executives a degree of certainty in light of having a majority of their compensation at risk. | | Fixed compensation that is reviewed annually and adjusted if and when appropriate; reflects each named executive officer’s performance, experience, skills, level of responsibility and the breadth, scope and complexity of the position as well as the competitive marketplace for executive talent specific to our industry. |
26
| | | | |
| Element | | Purpose | | Key Characteristics |
| Annual Incentive Program | | Motivates executive officers to achieve corporate and individual business goals annually, which we believe increase stockholder value, while providing flexibility to respond to opportunities and changing market conditions. | | Annual cash incentive based on corporate and individual performance compared topre-established goals. Our chief executive officer’s incentive is based entirely on corporate goals. Corporate goals focus on overarching objectives for Tetraphase, while individual objectives represent key performance expectations at the departmental or individual level. Corporate goals are aligned with our business strategy and weighted by relative importance. |
| | |
| Long-Term Equity Incentives (Stock Options) | | Motivates executive officers to achieve our business objectives by tying incentives to the appreciation of our common stock over the long term. | | Stock options have an exercise price equal to or greater than the fair market value on the date of grant vesting over four years. The ultimate value realized, if any, depends on the appreciation of our common stock price and if our stock price does not appreciate, there is no value realized by our executive officers. In determining the aggregate size of equity grants in any given year, the compensation committee (or our board of directors in the case of our chief executive officer) generally considers the same factors described above under “Base Salaries” with respect to performance during the prior fiscal year, as well as the criticality of the executive to the long-term achievement of corporate goals. The compensation committee also considers the impact of dilution by reviewing overall share utilization and usage. |
| | |
| Long-Term Equity Incentives (RSUs) | | Motivates executive officers to achieve our corporate objectives by tying compensation to the performance of our common stock over the long term and/or the achievement of business, clinical development and regulatory goals over the long term; motivates our executive officers to remain with Tetraphase by mitigating swings in incentive values during periods when market volatility weighs on our stock price. | | Restricted stock unit awards may vest based on continued service over a specified period of time and/or achievement of performance goals; the ultimate value realized varies with our common stock price. For 2017 approximately 25% of our executive officers’ annual grants were performance-based restricted stock unit awards vesting upon achievement of clinical development and regulatory milestones and continued employment through the end of the three-year vesting period. This percentage increased to approximately 30% for our executive officers’ 2018 annual grants |
Our 2017 Mix of Target Direct Compensation: Our compensation is structured to ensure that a significant portion of the total compensation opportunity for our named executive officers is directly related to our performance and other factors that directly and indirectly influence stockholder value. For 2017, our fixed compensation (as set forth in the darker blue in the table below) versus targeted variable compensation (as set
27
forth in the lighter blue in the table below) was structured as set forth below for our president and chief executive officer and other named executive officers. The actual ratio of fixed compensation to variable compensation will fluctuate year over year as a result of the price of our common stock on the date of any stock option grants or the date the value of any performance-based RSU is measured. This fluctuation is the same as that discussed above related to TSR.
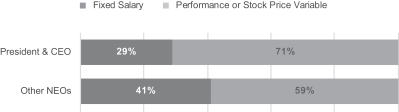
We also believe our executive compensation should be structured to appropriately balance cash compensation with equity-based compensation. For our CEO in 2017, the Performance Share Unit (PSUs) component comprised approximately 16% of the total target compensation. For the other NEOs, the PSU component does not include Ms. Unninayar as she was not granted any PSUs during 2017.
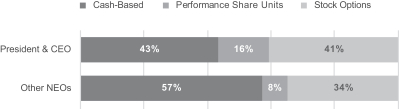
Review of Management’s Actual Performance for 2017 Compared toPre-Determined Goals
In the first quarter of each fiscal year, the compensation committee evaluates the company’s actual performance for the prior year against thepre-determined corporate goals to determine the amount of funding for the total cash incentive pool for all employees, as discussed more fully below, and to evaluate and determine the level of performance during the prior fiscal year for purposes of determining the amount of long-term equity incentive grants to officers and employees at the beginning of the subsequent fiscal year. The compensation committee also considers any significant corporate events or other significant accomplishments that were not contemplated at the beginning of the performance period in determining the extent to which the strategic goals were satisfied, such as the circumstances surrounding the feasibility of a goal being achieved. Likewise, the Compensation Committee also has the discretion to modify or otherwise change the corporate performance and strategic objectives during the applicable performance year to take into account circumstances then existing, but it did not do so during 2017.
In the first quarter of the 2017 performance year, upon recommendation by our chief executive officer and the compensation committee, the board established the corporate goals set forth in the chart below for evaluating corporate performance during the 2017 fiscal year that were used for purposes of determining annual cash incentive awards for 2017 and the equity incentive awards granted in January 2018. For 2017, the compensation committee evaluated in January 2018 each corporate performance goal adopted for the 2017 performance year, established a percentage rating for each goal based on the extent to which the goal was achieved and then determined an overall corporate rating based on the cumulative weightings of the ratings for all the goals. The determination of the corporate rating, while based primarily on the numerical rating for each goal and the relative weight assigned to each goal, also reflects the compensation committee’s qualitative assessment of performance. For the 2017 fiscal year, the compensation committee concluded based upon its evaluation of the achievement of these corporate performance goals as further set forth in the chart below, that
28
the overall corporate rating for the fiscal year was 135%. The compensation committee determined that the bonus pool and level of equity awards granted in the first quarter of 2018 should be aligned with the overall corporate rating.
Other than our chief executive officer, a blending of the achievement of individual and corporate goals determined actual bonus payouts. The chief executive officer’s bonus payout is primarily based on corporate performance. However, for 2017, the board of directors, upon the recommendation of the compensation committee, awarded Mr. Macdonald a cash bonus award greater than that based solely on the corporate performance. This increased award was due to primarily to Mr. Macdonald’s strong leadership in overseeing the completion of several strategic goals in the face of personnel challenges, including completing a capital raise without a chief financial officer and ensuring the company’s new drug application was timely filed with the FDA.
2017 Corporate Goals
For 2017, the following represents a summary of our five corporate performance categories and goals, achievements with respect to each category, respective relative weightings assigned to each category and actual ratings determined based on performance during 2017. Achievement of these strategic corporate goals is inherently less objectively measurable than with respect to quantitative goals and requires the compensation committee’s judgment as to the levels of achievement.
| | | | | | | | |
Corporate
Category | | 2017 Goals | | 2017 Achievements | | Relative
Weighting | | 2017
Achievement
Rating |
| Clinical Advancement of IV Eravacycline Program | | The goals associated with this category focused on the submission of regulatory filings in the United States and Europe for IV eravacycline and the completion of IGNITE4, our phase 3 clinical trial for the treatment of complicated intra-abdominal infections using eravacycline. | | The compensation committee determined that we exceeded these goals by the positive clinical results of IGNITE4 and the filing of an MAA with the EMA earlier than expected and an NDA with the FDA for the treatment of complicated intra-abdominal infections using IV eravacycline. The compensation committee also determined that we met nearly all the other goals in this category earlier than anticipated. | | 50% | | 85% |
| Clinical Advancement of Oral Eravacycline Program | | The goals associated with this category focused on obtaining data from several phase 1 clinical studies using the existing formulation. | | The compensation committee determined that the studies and the data readouts were completed on time. | | 15% | | 15% |
| Commercial Preparation | | The goals associated with this category primarily focused on developing our commercial and medical affairs infrastructure in preparation for the regulatory filing of eravacycline in the United States. | | The compensation committee determined that we met these goals. | | 5% | | 5% |
29
| | | | | | | | |
Corporate
Category | | 2017 Goals | | 2017 Achievements | | Relative
Weighting | | 2017
Achievement
Rating |
| Progress of Early Stage Clinical Programs | | The goals associated with this category focused on advancing our pipeline of preclinical candidates,TP-6076 andTP-271. | | The compensation committee determined that we met most of the goals in this category in light of our initiating a phase 1 multiple-ascending dose clinical trial ofTP-6076 and initiating phase 1 clinical trials involving IV and oralTP-271. There was a one quarter delay in the oralTP-271 study and for this reason the achievement rating was only 10%. | | 15% | | 10% |
General
Operations | | The goals associated with this category related principally to our general operations, potential capital raises and efficiently managing our finances. | | The compensation committee determined that we exceeded the goals in this category as a result of the capital raised during 2017. | | 15% | | 20% |
For 2017, the compensation committee evaluated in January 2018 each corporate performance goal, established a percentage rating for each goal based on the extent to which the goal was achieved and then determined an overall corporate rating based on the cumulative weightings of the ratings for all the goals. The determination of the corporate rating, while based primarily on the numerical rating for each goal and the relative weight assigned to each goal, also reflects the compensation committee’s qualitative assessment of performance.
The compensation committee recognized several key accomplishments during 2017 as further described above, including positive results of IGNITE4, a phase 3 clinical trial using eravacycline to treat patients with complicated intra-abdominal infections; the filing of a marketing authorization application with the European Medicines Agency earlier than expected and the filing of a new drug application with the FDA, both for the use of IV eravacycline to treat patients with cIAI; and the early completion of the enrollment of IGNITE3, a phase 3 clinical trial using eravacycline to treat patients with complication urinary tract infections. The compensation committee reviewed its assessment of the company’s achievement of 2017 corporate goals with the board of directors.
2017 Executive Compensation of Our Named Executive Officers
Base Salary
With respect to base salaries, in January 2017 and January 2018, respectively, the compensation committee (and the board of directors with respect to Mr. Macdonald) determined that base salaries for our named executive officers would be increased as a result of individual performance and data provided by W.T. Haigh & Company (including benchmark data from the Radford Global Life Sciences survey) to the compensation committee regarding the annual base salaries of similar positions of companies in our peer group for each of 2017 and 2018. The table below sets forth such increases.
Consistent with our overall compensation philosophy, Mr. Macdonald’s 8.3% increase from 2016 to 2017 and 5.7% increase from 2017 to 2018 resulted from the fact that his base salary was well below the 50th percentile of our peer companies. The compensation committee, and subsequently the board, determined to increase his salary such that it is commensurate with Mr. Macdonald’s experience, performance and effective leadership during 2017 in his role as chief executive officer.
30
| | | | | | | | | | | | | | |
Named Executive Officer | | Title | | 2016 Base
Salary | | | 2017 Base
Salary | | | 2018 Base
Salary | |
Guy Macdonald | | President and Chief Executive Officer | | $ | 489,250 | | | $ | 530,000 | | | $ | 560,000 | |
Jacques Dumas, Ph.D. | | Chief Scientific Officer | | $ | 360,500 | | | $ | 371,300 | | | $ | 384,300 | |
Patrick T. Horn, M.D., Ph.D.(1) | | Former Chief Medical Officer | | $ | 394,461 | | | $ | 406,300 | | | | — | |
Maria Stahl | | Senior Vice President, General Counsel | | $ | 340,000 | | | $ | 350,200 | | | $ | 364,200 | |
Kamalam Unninayar(2) | | Former Chief Financial Officer | | | — | | | $ | 340,000 | | | $ | 340,000 | |
Christopher Watt(3) | | Senior Vice President, Finance | | $ | 291,500 | | | $ | 303,200 | | | $ | 303,200 | |
| (1) | Dr. Horn ceased to serve as our chief medical officer on December 29, 2017. |
| (2) | Ms. Unninayar joined the company in October 2017 and ceased to serve as our chief financial officer upon her resignation on March 16, 2018. |
| (3) | Mr. Watt served as our principal financial and accounting officer until immediately after the filing of our Quarterly Report on Form10-Q for the three months ended September 30, 2017 which was filed with the SEC on November 1, 2017. As of the date of this proxy statement, he is serving as our principal financial and accounting officer following Ms. Unninayar’s resignation. |
Cash Incentive Performance for 2017
According to the compensation bonus plan approved by the compensation committee and the board of directors, the overall corporate rating for 2017 was 135%. The compensation committee determined that the bonus pool should be aligned with the overall corporate rating as discussed previously. The compensation committee or the board of directors can modify an individual’s bonus after evaluating his or her level of general achievement against his or her individualpre-determined goals (for all executive officers other than Mr. Macdonald) and the corporate goals.
Each named executive officer has a target cash performance bonus amount based on a percentage of his or her salary. This target is determined by the compensation committee annually based upon a review of the peer and industry data provided by W.T. Haigh & Company to the compensation committee. In reviewing current bonus targets for the named executive officers compared to data provided, the compensation committee determined that bonus targets would remain the same for 2017 as 2016 for all the named executive officers other than Mr. Macdonald, whose bonus target was increased to 55% from 50% (based on market data provided by the compensation committee’s consultant that indicated the increase was appropriate to remain competitive) and Ms. Stahl, whose bonus target was increased to 40% from 35% (based on a combination of peer company market data and to be aligned with the other named executive officers).
Mr. Macdonald’s annual cash incentive performance award is generally based on corporate performance. Although the corporate rating for 2017 was 135% (which would have resulted in a payment of $357,750), the board of directors determined that amount should be increased to recognize the strategic direction and individual contribution Mr. Macdonald provided during 2017 given the achievements of (1) a capital raise in July; (2) regulatory filings with the EMA and the FDA for the use of IV eravacycline to treat patients with cIAI; (3) the positive clinical results of IGNITE4; (4) the completed enrollment of IGNITE3; and (5) strategic business development initiatives. In particular, the NDA regulatory filing was critical to the company’s ongoing success and transition from a clinical stage company to a commercialization organization. Moreover, the completed enrollment of IGNITE3 occurred several quarters prior to expectations. For these reasons, Mr. Macdonald was awarded a bonus of $384,250, representing 145% of his target.
31
During 2017, Dr. Dumas led the company’s chemistry, manufacturing and controls, or CMC, group and their varied efforts to complete the necessary manufacturing and validation work in preparation for the regulatory filings completed by the company during 2017 with the EMA and the FDA, respectively. As a result of these and other individual achievements and the achievement of corporate goals during 2017, the compensation committee determined that Dr. Dumas had earned a cash performance incentive of 135%, equal to the overall corporate rating.
Dr. Horn was ineligible for a cash bonus award for his achievements during 2017 because he ceased to serve as the company’s chief medical officer on December 29, 2017. The company’s bonus program is explicit that an executive must be employed by the company at the time that any cash bonus is paid in order to be eligible to receive such cash bonus.
Due to Ms. Stahl’s achievement of a successful resolution of the class action filed against the company, continued management of the company’s patent portfolio, contribution in the financing of July 2017 and other individual goals and the achievement of corporate goals associated with general operations during 2017, the compensation committee determined that Ms. Stahl had earned a cash performance incentive of 150%, higher than the overall corporate rating.
Ms. Unninayar joined the company in October 2017 and ceased to serve as our chief financial officer upon her resignation on March 16, 2018. Due to her limited time with the company during 2017, she was not eligible for a cash bonus for 2017 performance.
While Mr. Watt achieved certain of the corporate goals associated with general operations, he did not achieve all of his individual goals and therefore the compensation committee determined that Mr. Watt had earned a cash performance incentive of 85%, below the overall corporate rating.
The targeted cash performance incentive awards set in January 2017, along with actual amounts paid in 2018 for performance in 2017, for our named executive officers is set forth in the following table:
| | | | | | | | | | | | | | |
Named Executive Officer | | Title | | 2017 Targeted
Cash
Performance
Incentive As a
Percentage of
Base Salary | | | Actual
Performance
Incentive as a
Percentage of
Base Salary for
2017 | | | Actual Cash
Performance
Incentive
Paid for
2017 | |
Guy Macdonald | | President and Chief Executive Officer | | | 50 | % | | | 72 | % | | $ | 384,250 | |
Jacques Dumas, Ph.D. | | Chief Scientific Officer | | | 40 | % | | | 54 | % | | $ | 200,500 | |
Patrick T. Horn M.D., Ph.D. | | Former Chief Medical Officer | | | 40 | % | | | — | | | | $ — | |
Maria Stahl | | Senior Vice President and General Counsel | | | 35 | % | | | 52 | % | | $ | 183,700 | |
Kamalam Unninayar | | Former Chief Financial Officer | | | 40 | % | | | — | | | | $ — | |
Christopher Watt | | Senior Vice President, Finance | | | 30 | % | | | 26 | % | | $ | 77,400 | |
The cash incentive awards set forth in the table above were paid to the named executive officers in February 2018.
32
2017 Equity Awards
In January 2017 our named executive officers received long-term equity incentive awards as part of the annual review process. After reviewing the achievement of corporate goals for the prior fiscal year, as well as individual performance goals for our executive officers other than Mr. Macdonald, the compensation committee granted equity awards to Drs. Dumas and Horn, Ms. Stahl, Mr. Watt and Mr. Macdonald. Ms. Unninayar joined the company in October 2017 and therefore was not eligible for any equity grant for performance in 2016, but was granted a new hire equity award in the form of stock options. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018 and therefore no shares underlying her equity grants vested.
2017 Performance Based Equity Awards
In January 2017 our board of directors, based on a recommendation from the compensation committee, approved a long-term performance based restricted stock unit program. Under the program, performance-based restricted stock units may be earned upon the achievement of various clinical and regulatory milestones, and if earned, will vest on January 31, 2020 subject to continued employment through that date. We do not disclose the milestones during the performance period because it would be competitively harmful to do so during the performance period. Approximately 25% of our named executive officer’s equity compensation granted in January 2017 based upon an evaluation of 2016 performance was in the form of performance-based RSUs. The performance-based RSUs were granted as a portion of each person’s total equity compensation, as opposed to being granted in addition to each person’s regular total equity compensation.
Of the performance based restricted stock units granted in 2017, in January 2018 the compensation committee certified that three of the 2017 performance metrics had been met and therefore earned. These performance metrics related to the results of IGNITE4 data, the filing of a marketing authorization application with the European Medicines Agency and the filing of a new drug application with the United States Food and Drug Administration. Combined, these milestones represent 60% of the overall 2017 performance based restricted stock units, which will not vest until January 31, 2020, subject to continued employment through that date.
2017 Time-Based Equity Awards
Approximately 75% of the equity award granted in January 2017 to our named executive officers other than Ms. Unninayar constituted stock option awards subject to time-based vesting. Ms Unninayar was granted a stock option award upon commencing employment with us. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018 and therefore no shares underlying this stock option award vested.
Set forth in the table below are the equity awards granted to the named executive officers in January 2017 based upon an assessment of 2016 performance by the compensation committee (and the board of directors, in the case of Mr. Macdonald) and Ms. Unninayar’snew-hire grant. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018 and therefore no shares underlying her equity grants vested.
| | | | | | | | | | |
Named Executive Officer | | Title | | Stock
Options
Grant Date
Dollar Value
Granted
in 2017 ($) | | | Performance-
Based
Restricted
Stock Units
Grant Date
Dollar Value
Granted in
2017 ($) | |
Guy Macdonald | | President and Chief Executive Officer | | $ | 861,390 | | | $ | 287,250 | |
Jacques Dumas, Ph.D. | | Chief Scientific Officer | | | 387,520 | | | | 114,900 | |
Patrick T. Horn, M.D., Ph.D. | | Former Chief Medical Officer | | | 387,520 | | | | 114,900 | |
Maria Stahl | | Senior Vice President, General Counsel | | | 318,320 | | | | 95,750 | |
Kamalam Unninayar | | Former Chief Financial Officer | | | 861,460 | | | | — | |
Christopher Watt | | Senior Vice President, Finance | | | 166,080 | | | | 57,450 | |
33
2018 Equity Awards
Similar to 2017, all of our named executive officers were granted long-term equity incentive awards in January 2018. After reviewing the achievement of the corporate goals for 2017 discussed above, as well as individual performance goals for our executive officers other than Mr. Macdonald, the compensation committee (and the board of directors in the case of Mr. Macdonald) granted performance based RSUs and stock option awards that are subject to time-based vesting to Mr. Macdonald, Dr. Dumas, Ms. Stahl and Mr. Watt, and solely performance based RSUs to Ms. Unninayar. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018 and therefore no shares underlying her performance based RSUs grant will vest.
Under our 2018 long-term performance based RSU program, performance-based RSUs may be earned upon the achievement of various clinical, regulatory and commercial milestones, and if earned, will vest on January 31, 2021 subject to continued employment through that date. Approximately 30% of each of our named executive officer’s equity compensation granted in January 2018 (other than Ms. Unninayar) was in the form of performance-based RSUs, an increase from 25% of the equity incentive awards granted as performance-based awards in January 2017. The grants of performance-based RSUs are forward-looking in nature. The remaining 70% of the equity incentive awards were granted as stock option awards subject to time-based vesting that vest over four years in equal quarterly installments following grant.
Set forth in the table below are the equity awards granted to the named executive officers in January 2018 and based, in part, upon an assessment of 2017 performance by the compensation committee (and the board of directors, in the case of Mr. Macdonald). Dr. Horn ceased to serve as our chief medical officer on December 29, 2017. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018 and therefore no shares underlying her equity grants vested. Moreover, all of the equity grants set forth below are presently“out-of-the-money” as the grant price was $6.24 per share and the last reported sale price on The NASDAQ Global Select Market was $3.08 on April 6, 2018, the record date.
| | | | | | | | | | |
Named Executive Officer | | Title | | Stock
Options
Grant Date
Dollar Value
Granted in 2018
for
2017
Performance ($) | | | Performance-
Based
Restricted
Stock Units
Grant Date
Dollar Value
Granted in
2018 ($) | |
Guy Macdonald | | President and Chief Executive Officer | | $ | 1,443,120 | | | $ | 634,000 | |
Jacques Dumas, Ph.D. | | Chief Scientific Officer | | | 662,704 | | | | 249,600 | |
Patrick T. Horn, M.D., Ph.D. | | Former Chief Medical Officer | | | — | | | | — | |
Maria Stahl | | Senior Vice President, General Counsel | | | 544,364 | | | | 199,680 | |
Kamalam Unninayar | | Former Chief Financial Officer | | | — | | | | 249,600 | |
Christopher Watt | | Senior Vice President, Finance | | | 236,680 | | | | — | |
Employment Agreements, Severance and Change in Control Arrangements
We have entered into employment offer letters with each of Mr. Macdonald, Dr. Dumas, Messes. Stahl and Unninayar and Mr. Watt, and prior to his termination, we had entered into an offer letter with Dr. Horn, pursuant to which such executive officer is (or was) employed “at will,” meaning he or she or we may terminate the employment arrangement at any time. Such offer letters confirm the named executive officers’ titles, compensation arrangements, eligibility for benefits made available to employees generally and also provide for certain benefits upon termination of employment under specified conditions. Dr. Horn’s offer letter provided the same benefits upon termination and a change in control as our other named executive officers (other than Mr. Macdonald), but no payments were made under his offer letter upon termination of his service on December 29, 2017. Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018. No payments were made under her offer letter upon termination of her services on March 16, 2018.
34
Benefits Provided Upon Termination Without Cause
Under the terms of the offer letters we have entered into with each of Mr. Macdonald, Dr. Dumas, Messes. Stahl and Unninayar and Mr. Watt, if such executive’s employment is terminated by us without cause, subject to the executive’s signing a separation agreement that will include a general release of potential claims against us:
| | • | | he or she will be entitled to continue to receive his or her monthly base salary for a period of 12 months (6 months in the case of Mr. Watt) and we will continue to provide medical, dental and vision benefits (to the extent that he or she was receiving them at the time of termination) for 12 months (6 months in the case of Mr. Watt). |
Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018. No benefits were provided to her under her offer letter upon termination of her services on March 16, 2018.
Benefits Provided Upon a Change in Control
We have designed ourchange-in-control policies to provide income continuity after achange-in-control of the company that results in the executive being separated from the company. Our policy in the case ofchange-in-control benefits has been to structure these as “double trigger” benefits. In other words, thechange-in-control does not itself trigger benefits; rather, benefits are paid only if the employment of the executive is terminated or the executive terminates his or her employment for good reason during a specified period after thechange-in-control. We believe a “double trigger” benefit maximizes shareholder value because it prevents an unintended windfall to executives in the event of a friendlychange-in-control, while still providing them appropriate incentives to cooperate in negotiating anychange-in-control in which they believe they may lose their jobs. Under the terms of their respective employment arrangements, if, within one year following a change in control, each of our named executive officer’s employment is terminated by us or the succeeding company, as applicable, without cause or he or she terminates his or her employment for good reason (as defined in the applicable offer letter), subject to the executive’s signing a separation agreement that will include a general release of potential claims against us:
| | • | | in the case of Mr. Macdonald, (1) he will be entitled to continue to receive his monthly base salary for a period of 18 months, (2) he will be entitled to receive a lump sum payment equal to 150% of his target bonus at the time he ceases to be employed by the company or the succeeding company, as applicable, and (3) the company or the succeeding company, as applicable, will continue to provide medical and dental benefits (to the extent that he was receiving them at the time he ceased to be employed by the company) for eighteen months; |
| | • | | in the case of each of Dr. Dumas, Messes. Stahl and Unninayar and Mr. Watt, (1) he or she will be entitled to continue to receive his or her monthly base salary for a period of 12 months, (2) he or she will be entitled to receive a lump sum payment equal to 100% of his or her target bonus at the time he or she ceases to be employed by the company or the succeeding company, as applicable, and (3) the company or the succeeding company, as applicable, will continue to provide medical and dental benefits (to the extent that he or she was receiving them at the time he or she ceased to be employed by the company) for 12 months; and |
| | • | | in the case of all executive officers immediate vesting and exercisability of all stock option awards. |
Ms. Unninayar ceased to serve as our chief financial officer upon her resignation in March 2018. She is no longer eligible to receive benefits under her offer letter upon a change in control.
Other Agreements
We have also entered intonon-competition,non-solicitation andnon-disclosure agreements with each of our named executive officers. Under thenon-competition,non-solicitation andnon-disclosure agreements, each
35
named executive officer has agreed (i) not to compete with us during his or her employment and for a period of one year after the termination of his or her employment, (ii) not to solicit our employees during his or her employment and for a period of one year after the termination of his or her employment, (iii) to protect our confidential and proprietary information, and (iv) to assign to us related intellectual property developed during the course of his or her employment.
Benefits
We maintain benefits that are provided to all employees, including health, dental and vision insurance, life and disability insurance and a 401(k) plan. All eligible and participating employees receive a 401(k) match of fifty percent (50%) onpre-tax contributions, up to the first six percent (6%) of eligible compensation. Executive officers are eligible to participate in all of our employee benefit plans, in each case on the same basis as other employees.
We also provide all employees, including executive officers, with a flexible spending account plan, an employee stock purchase plan and paid time off benefits including, vacation, sick time and holidays. We do not offer or provide any additional perquisites (other than those noted here) to the chief executive officer or any other officer of the company.
Insider Trading Policy Prohibitions and Hedging Policy
Our company maintains an Insider Trading Policy that prohibits our officers, directors and employees from, among other things, engaging in speculative transactions in our securities, including by way of the purchase or sale of “put” or “call” options or other derivative securities directly linked to our equity; short sales of our equity; the use of our equity as a pledge or as collateral in a margin account; and trading in straddles, equity swaps, or other hedging transactions directly linked to our equity, even if such persons do not possess material,non-public information.
Tax Considerations
The Internal Revenue Service, pursuant to Section 162(m) of the Internal Revenue Code of 1986, as amended, generally disallows a tax deduction for compensation in excess of $1.0 million paid to our chief executive officer and to each other officer (other than our chief executive officer and our chief financial officer) whose compensation is required to be reported to our stockholders pursuant to the Exchange Act by reason of being among the three most highly paid executive officers. The Tax Cuts and Jobs Act of 2017 expanded the scope of Section 162(m) to cover our chief financial officer for taxable years beginning after December 31, 2017, and eliminated certain exceptions from the deduction limitation under Section 162(m), including the exception for qualified performance-based compensation. We periodically review the potential consequences of Section 162(m) on the compensation granted to our named executive officers. However, the compensation committee may, in its judgment, authorize compensation payments that may not be deductible as a result of Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent and are in the best interests of our stockholders.
Narrative Disclosure of our Compensation Policies and Practices as They Relate to Risk Management
Our compensation committee does not believe that any risks arising from our employee compensation policies and practices are reasonably likely to have a material adverse effect on our company. In addition, we do not believe that the mix and design of the components of our executive compensation program encourage management to assume excessive risk. Our compensation committee believes that any such risks are mitigated by:
| | • | | the multiple elements of our compensation packages, including base salary, annual bonus programs, equity awards that vest over multiple years and are intended to motivate employees to take a long-term view of our business, and performance-based equity awards that vest only if certain clinical development, regulatory and commercial milestones are achieved; |
36
| | • | | the structure of our annual cash bonus program that is based on a number of different performance measures (including goals related to our drug candidates and related clinical programs, our discovery program and objectives relating to our general operations of the company, such as budget control and financial reporting) and, generally, on both individual and corporate goals to avoid employees placing undue emphasis on any particular performance metric at the expense of other aspects of our business; |
| | • | | the use of individual performance targets that we believe are reasonable and should not require undue risk-taking to achieve; |
| | • | | goals being set appropriately to avoid targets that, if not achieved, result in a large percentage loss of compensation; |
| | • | | annual cash performance incentive awards for all employees are capped at two hundred percent (200%) of target amount; and |
| | • | | multi-year vesting of our equity awards, which we believe encourages executives to take a long-term view of our business. |
Summary
The compensation committee believes that our compensation programs are designed and administered in a manner consistent with its compensation philosophy and objectives. We continually monitor these programs in recognition of the dynamic marketplace in which we compete for talent. We intend to continue to emphasizepay-for-performance and equity-based incentive programs that reward executives for actual results and that are consistent with shareholder interests.
Compensation Committee Report
The compensation committee has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of RegulationS-K with our management. Based on this review and discussion, the compensation committee recommended to our board of directors that the Compensation Discussion and Analysis be included in this proxy statement.
By the compensation committee of the board of directors,
Nancy J. Wysenski, Chair
Jeffrey A. Chodakewitz, M.D.
Gerri Henwood
Executive Officers
The following table lists the positions, names and ages of our executive officers as of April 10, 2018:
| | | | |
Guy Macdonald | | 59 | | President and Chief Executive Officer, Director |
Jacques Dumas, Ph.D. | | 52 | | Chief Scientific Officer |
Larry Edwards | | 46 | | Chief Operating Officer |
Maria Stahl | | 47 | | Senior Vice President and General Counsel |
Larry Tsai, M.D. | | 46 | | Chief Medical Officer |
Christopher Watt | | 53 | | Senior Vice President, Finance |
Guy Macdonald is a continuing member of our board of directors. See “Proposal One—Election of Directors” for more information about Mr. Macdonald.
37
Jacques Dumas, Ph.D. has served as our Chief Scientific Officer since July 2015. Prior to joining Tetraphase, Dr. Dumas served as Vice-President, Idenix for Merck& Co., a publicly traded pharmaceutical company, from January 2015 to July 2015. Prior to his position at Merck, Dr. Dumas served as Executive Vice President, Chief Scientific Officer of Idenix Pharmaceuticals, Inc., a publicly traded biotechnology company until its acquisition by Merck & Co., from January 2014 to August 2014. Dr. Dumas served as Vice President and Head of Strategy, Infection Innovative Medicines at AstraZeneca PLC from December 2010 to January 2014 where Dr. Dumas was responsible for disease area strategy and external collaborations on behalf of AstraZeneca’s small molecule infectious diseases unit. Prior to that, Dr. Dumas held several positions at AstraZeneca, including Associate Director, Medicinal Chemistry and Emerging Product Director, Oncology and Infection Therapeutic Area. Prior to joining AstraZeneca, Dr. Dumas held several positions at Bayer Health Care Pharmaceuticals, Inc., a subsidiary of Bayer AG, from 1995 to 2007. Dr. Dumas received his Ph.D. from Paris VI University in Organic Chemistry.
Larry Edwards has served as our Chief Operating Officer since March 2018. From December 2016 to February 2018, Mr. Edwards served as our Senior Vice President, Chief Commercial Officer and from January 2016 to December 2016 as our Vice President, Commercial Operations. He also served as our Vice President, Marketing from July 2015 to January 2016. Prior to joining Tetraphase, from April 2014 to June 2015, Mr. Edwards served as Senior Director, Marketing at Cubist Pharmaceuticals, Inc., a publicly traded biopharmaceutical company acquired by Merck & Co. in January 2015. Mr. Edwards previously served in various roles at Merck & Co., a publicly traded pharmaceutical company, from 1999 to April 2014, most recently serving as Global Marketing Director, Clostridium Difficile and New Infectious Disease Products. Mr. Edwards received a B.S. from Ohio University.
Maria Stahl has served as our Senior Vice President and General Counsel since March 2015. Prior to joining Tetraphase, Ms. Stahl served as Senior Vice President, General Counsel of Idenix Pharmaceuticals, Inc., a publicly traded biotechnology company until its acquisition by Merck & Co., from October 2010 to August 2014. Ms. Stahl received a B.A. from Providence College and a J.D. from Yale Law School.
Larry Tsai, M.D. has served as our Chief Medical Officer since December 2017 and prior to that he served as our President, Clinical Development from June 2015 to December 2017 and as our Senior Medical Director from April 2014 to June 2015. Prior to joining Tetraphase, Dr. Tsai served as Vice President of Research of Aeris Therapeutics, Inc., a biotechnology company, from January 2011 to November 2013 and was practicing medicine from November 2013 to April 2014. Dr. Tsai is a part-time instructor in medicine at the Harvard Medical School and is also a practicing physician at Bringham and Women’s Hospital in Boston, Massachusetts. Dr. Tsai received a B.S. from Stanford University and an M.D. from Harvard Medical School. He is also board certified in critical care medicine, internal medicine and pulmonary disease.
Christopher Watt has served as our Senior Vice President, Finance since January 2017. He previously served as our Vice President, Finance from July 2015 to January 2017. Prior to joining Tetraphase, Mr. Watt spent ten years at Biogen, Inc., a publicly traded pharmaceutical company, most recently serving as Senior Director, Global Commercial Finance. From 2009 to 2011, Mr. Watt served as the Finance Director for Biogen’s UK/Ireland affiliate and from 2006 to 2009 as Director of Business Planning, International. Prior to Biogen, Mr. Watt spent fifteen years in various financial roles at InterSystems Corporation, Putnam Investments, Procter and Gamble and Shawmut Bank. Mr. Watt received a B.A. from Colby College and an M.B.A. from the University of Michigan.
There are no family relationships between or among any of our executive officers.
38
Summary Compensation Table
The following table sets forth information regarding compensation earned by our named executive officers for the years ended December 31, 2017, 2016 and 2015.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position | | Year | | | Salary
($) | | | Bonus
($) | | | Stock
Awards
($)(1) | | | Option
Awards
($)(1) | | | Non-Equity
Incentive Plan
Compensation
($)(2) | | | All Other
Compensation
($)(3) | | | Total
($) | |
Guy Macdonald, President and Chief
Executive Officer | |
| 2017
2016 2015 |
| | $
| 530,000
489,250 475,000 |
| | $
| 26,500
— — |
| | $
| 287,250
— — |
| | $
| 861,390
1,856,880 4,404,000 |
| | $
| 357,750
225,000 125,000 |
| | $
| 10,422
9,847 8,962 |
| | $
| 2,073,312
2,580,977 5,012,962 |
|
| | | | | | | | |
Jacques Dumas Ph.D.,(4) Chief Scientific Officer | |
| 2017
2016 2015 |
| |
| 371,300
360,500 161,538 |
| |
$ | —
— 30,000 |
| |
| 114,900
296,450 — |
| |
| 387,520
618,960 3,519,500 |
| |
| 200,500
137,000 34,636 |
| |
| 9,342
11,058 4,650 |
| |
| 1,083,562
1,423,968 3,750,324 |
|
| | | | | | | | |
Patrick T. Horn M.D., Ph.D.,(5) Former Chief Medical Officer | |
| 2017
2016 2015 |
| |
| 406,300
394,461 384,840 |
| |
| —
— — |
| |
| 114,900
296,450 — |
| |
| 387,520
618,960 2,202,000 |
| |
| —
134,100 80,816 |
| |
| 6,242
3,564 1,554 |
| |
| 914,962
1,447,535 2,669,210 |
|
| | | | | | | | |
Maria Stahl,(6) Senior Vice President, General Counsel | |
| 2017
2016 2015 |
| |
| 350,200
340,000 275,423 |
| |
| —
— — |
| |
| 95,750
254,100 — |
| |
| 318,320
495,168 2,662,500 |
| |
| 183,700
107,100 57,529 |
| |
| 8,910
9,464 7,538 |
| |
| 956,880
1,205,832 3,002,990 |
|
| | | | | | | | |
Kamalam Unninayar(7). Former Chief Financial Officer | | | 2017 | | | | 71,923 | | | | — | | | | — | | | | 861,460 | | | | — | | | | 1,808 | | | | 935,191 | |
| | | | | | | | |
Christopher Watt,(8) Senior Vice President, Finance | |
| 2017
2016 2015 |
| |
| 303,200
291,500 124,346 |
| |
| —
— — |
| |
| 57,450
105,875 109,340 |
| |
| 166,080
154,740 1,451,785 |
| |
| 77,400
78,700 21,600 |
| |
| 9,342
8,520 314 |
| |
| 613,472
639,335 1,707,385 |
|
| (1) | The assumptions we used in valuing equity awards are described in Note 7, “Stock-based Compensation,” to our audited financial statements included in our Annual Report on Form10-K for the fiscal year ended December 31, 2017. With respect to stock awards, amounts reported reflect the grant date fair value which was determined to be equal to the fair market value of the underlying shares on the date of grant. With respect to option awards, amounts reported reflect the aggregate grant date fair value as calculated in accordance with ASC 718 for the indicated year in connection with options we granted in the indicated year, adjusted to disregard the effects of any estimate of forfeitures related to service-based vesting. |
| (2) | This amount consists of cash bonuses paid to our named executive officers under our annual performance-based incentive plan for performance in the year indicated. With respect to Mr. Macdonald’s cash bonus for 2017, our board of directors determined in their discretion to pay him a cash bonus in excess of his target as more fully discussed in “Compensation Discussion and Analysis.” This discretionary amount is set forth in the “Bonus” column. |
| (3) | Represents the value of perquisites and other personal benefits which include company-paid premiums for group term life insurance, long term disability and, with respect to 2015 only, a company match to executives’ 401(k) contributions, other than Dr. Horn and Mr. Watt. |
| (4) | Dr. Dumas joined the company in July 2015. |
| (5) | Dr. Horn ceased to serve as our chief medical officer on December 29, 2017. |
| (6) | Ms. Stahl joined the company in March 2015. |
| (7) | Ms. Unninayar joined the company in October 2017 and resigned as our chief financial officer on March 16, 2018. |
| (8) | Mr. Watt joined the company in July 2015. Mr. Watt served as our principal financial and accounting officer until immediately after the filing of our Quarterly Report on Form10-Q for the three months ended September 30, 2017, which was filed with the SEC on November 1, 2017. As of the date of this proxy statement, Mr. Watt is serving as our principal financial and accounting officer following Ms. Unninayar’s resignation in March 2018. |
39
Grants of Plan-Based Awards
The following table shows information concerning each grant of an award made to our named executive officers during 2017 under any plan, contract, authorization or arrangement pursuant to which cash, securities, similar instruments or other property may be received.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Estimated Future Payouts
UnderNon-Equity
Incentive Plan Awards(1) | | | Estimated Future Payouts
Under Equity
Incentive Plan Awards | | | All
Other
Stock
Awards:
Number
of
Shares
of
Stock or
Units
(#)(2)(3)
(i) | | | All Other
Option
Awards:
Number of
Securities
Underlying
Options
(#)(3)
(j) | | | Exercise
or
Base
Price
of
Option
Awards
($/Sh)(4)
(k) | | | Grant
Date
Fair
Value
of
Stock
and
Option
Awards
($)(5)
(l) | |
Name (a) | | Grant
Date
(b) | | | Threshold
($)
(c) | | | Target
($)
(d) | | | Maximum
($)
(e) | | | Threshold
(#)
(f) | | | Target
(#)
(g) | | | Maximum
(#)
(h) | | | | | |
Guy Macdonald | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Annual Cash | | | 1/31/2017 | | | | — | | | $ | 265,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock Option Award | | | 1/31/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 300,000 | (6) | | $ | 3.83 | | | $ | 861,390 | |
PRSU Award | | | 1/31/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 75,000 | (7) | | | — | | | | — | | | | 287,250 | |
| | | | | | | | | | | |
Jacques Dumas, Ph.D. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Annual Cash | | | 1/30/2017 | | | | — | | | $ | 148,520 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock Option Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 140,000 | (6) | | | 3.69 | | | | 387,520 | |
PRSU Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 30,000 | (7) | | | — | | | | — | | | | 114,900 | |
| | | | | | | | | | | |
Patrick T. Horn, M.D., Ph.D. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Annual Cash | | | 1/30/2017 | | | | — | | | $ | 162,520 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock Option Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 140,000 | (6) | | | 3.69 | | | | 387,520 | |
PRSU Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 30,000 | (7) | | | — | | | | — | | | | 114,900 | |
| | | | | | | | | | | |
Maria Stahl | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Annual Cash | | | 1/30/2017 | | | | — | | | $ | 122,570 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock Option Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 115,000 | (6) | | | 3.69 | | | | 318,320 | |
PRSU Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 25,000 | (7) | | | — | | | | — | | | | 95,750 | |
| | | | | | | | | | | |
Kamalam Unninayar | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Stock Option Award | | | 11/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 190,000 | (8) | | | 6.07 | | | | 861,460 | |
| | | | | | | | | | | |
Christopher Watt | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Annual Cash | | | 1/30/2017 | | | | — | | | $ | 90,960 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock Option Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 60,000 | (6) | | | 3.69 | | | | 166,080 | |
PRSU Award | | | 1/30/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 15,000 | (7) | | | — | | | | — | | | | 57,450 | |
| (1) | Consists of potential cash payments under our annual performance-based incentive bonus plan for executives for 2016. Actual cash bonus amounts awarded in January 2018 for 2017 performance are set forth in the Summary Compensation Table above under the column entitled“Non-Equity Incentive Plan Compensation” for 2017. |
| (2) | Consists of stock awards for executives under our annual performance-based incentive bonus plan for executives. For such stock awards, the grant date fair value, in accordance with FASB ASC Topic 718, is set forth in the Summary Compensation Table under the column “Stock Awards” for 2017. |
| (3) | No set maximum exists for equity incentive plan awards. Actual equity incentive plan awards are made at the discretion of our compensation committee or, in the case of awards to our chief executive officer, at the discretion of either our compensation committee or our board of directors based upon the compensation committee’s recommendation. |
| (4) | The exercise price of a share of our common stock on a particular date for purposes of granting stock options is determined as the closing price as reported on The NASDAQ Global Select Market on the date of grant. |
| (5) | Amounts reported reflect the aggregate grant date fair value as calculated in accordance with ASC 718. These amounts do not represent the actual amounts paid or realized by the named executive officer during 2017. The assumptions we used in valuing equity awards are described in Note 7, “Stock-based Compensation,” to our audited financial statements included in our Annual Report on Form10-K for the fiscal year ended December 31, 2017. |
| (6) | Granted pursuant to our 2013 Stock Incentive Plan. This option becomes exercisable, so long as the executive continues to be employed with us, as to approximately 6.25% of the shares at the end of each successive three-month period following the grant date. |
| (7) | Granted pursuant to our 2013 Stock Incentive Plan. These performance-based restricted stock units may be earned upon the achievement of various clinical and regulatory milestones and, if earned, vest on January 31, 2020, subject to continued employment. |
| (8) | Granted as an inducement grant pursuant to Nasdaq Listing Rule 5635(c)(4). This option would have become exercisable, as to 25% of the shares on theone-year anniversary of the grant date and as to an additional 6.25% of the shares at the end of each successive three-month period thereafter. Ms. Unninayar resigned in March 2018 and, therefore, none of the shares underlying this award vested or will vest. |
40
Outstanding Equity Awards at FiscalYear-End
The following table shows information regarding unexercised stock options and other equity awards held by our named executive officers as of December 31, 2017.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Option Awards | | | Stock Awards | |
Name | | Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable | | | Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable | | | Option
Exercise
Price
($) | | | Option
Expiration
Date | | | Number of
Shares or
Units that
Have Not
Vested (#) | | | Market Value
of Shares or
Units that
Have Not
Vested ($)(1) | | | Equity
Incentive
Plan
Awards:
Number of
Unearned
Shares or
Other
Rights
that Have
Not
Vested (#) | | | Equity
Incentive
Plan
Awards:
Market or
Payout
Value of
Unearned
Shares or
Other
Rights that
Have Not
Vested ($) | |
Guy Macdonald | | | 56,250 | (2) | | | 243,750 | | | $ | 3.83 | | | | 1/30/2027 | | | | | | | | | | | | 75,000 | (13) | | $ | 472,500 | |
| | | 131,250 | (3) | | | 168,750 | | | $ | 8.47 | | | | 1/5/2026 | | | | | | | | | | | | | | | | | |
| | | 137,500 | (4) | | | 62,500 | | | $ | 39.94 | | | | 1/6/2025 | | | | | | | | | | | | | | | | | |
| | | 150,000 | (5) | | | 10,000 | | | $ | 14.99 | | | | 1/7/2024 | | | | | | | | | | | | | | | | | |
| | | 180,000 | (6) | | | — | | | $ | 7.94 | | | | 5/14/2023 | | | | | | | | | | | | | | | | | |
| | | 12,534 | (6) | | | — | | | $ | 2.03 | | | | 6/5/2022 | | | | | | | | | | | | | | | | | |
| | | 57,331 | (6) | | | — | | | $ | 2.03 | | | | 9/27/2020 | | | | | | | | | | | | | | | | | |
| | | 20,000 | (6) | | | — | | | $ | 0.87 | | | | 9/10/2019 | | | | | | | | | | | | | | | | | |
| | | 25,834 | (6) | | | — | | | $ | 5.80 | | | | 8/7/2018 | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Jacques Dumas, Ph.D. | | | — | (2) | | | 113,750 | | | $ | 3.69 | | | | 1/30/2027 | | | | 11,666 | (12) | | $ | 73,496 | | | | 30,000 | (13) | | $ | 189,000 | |
| | | 43,750 | (3) | | | 56,250 | | | $ | 8.47 | | | | 1/5/2026 | | | | | | | | | | | | | | | | | |
| | | 70,313 | (7) | | | 54,687 | | | $ | 48.83 | | | | 7/12/2025 | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Patrick Horn, M.D., Ph.D(8). | | | 26,250 | | | | — | | | $ | 3.69 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 43,750 | | | | — | | | $ | 8.47 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 68,750 | | | | — | | | $ | 39.94 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 93,750 | | | | — | | | $ | 14.99 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 135,000 | | | | — | | | $ | 7.94 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 44,126 | | | | — | | | $ | 2.03 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | 47,689 | | | | — | | | $ | 2.03 | | | | 3/29/2018 | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Maria Stahl | | | 21,563 | (2) | | | 93,437 | | | $ | 3.69 | | | | 1/30/2027 | | | | 10,000 | (12) | | $ | 63,000 | | | | 25,000 | (13) | | $ | 157,500 | |
| | | 35,000 | (3) | | | 45,000 | | | $ | 8.47 | | | | 1/5/2026 | | | | | | | | | | | | | | | | | |
| | | 85,938 | (9) | | | 39,062 | | | $ | 38.50 | | | | 3/3/2025 | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Kamalam Unninayer | | | — | (10) | | | 190,000 | | | $ | 6.07 | | | | 11/14/2027 | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Christopher Watt | | | 11,250 | (2) | | | 48,750 | | | $ | 3.69 | | | | 1/30/2027 | | | | 4,166 | (12) | | $ | 26,245 | | | | 15,000 | (13) | | $ | 94,500 | |
| | | 10,938 | (3) | | | 14,062 | | | $ | 8.47 | | | | 1/5/2026 | | | | | | | | | | | | | | | | | |
| | | 28,125 | (11) | | | 21,875 | | | $ | 50.49 | | | | 7/14/2025 | | | | | | | | | | | | | | | | | |
| (1) | Based on the closing price of the stock on December 29, 2017 of $6.30. |
| (2) | This option vested as to 6.25% of the shares on April 30, 2017 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until January 31, 2021. |
| (3) | This option vested as to 6.25% of the shares on April 6, 2016 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until January 6, 2020. |
| (4) | This option vested as to 6.25% of the shares on April 7, 2015 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until January 7, 2019. |
| (5) | This option vested as to 6.25% of the shares on April 8, 2014 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until January 8, 2018. This option fully vested on January 8, 2018. |
| (6) | This option was fully vested as of December 31, 2016. |
| (7) | This option vested as to 25% of the shares on July 13, 2016 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until July 13, 2019. |
| (8) | Dr. Horn ceased to serve as our chief medical officer on December 29, 2017. His options stopped vesting on such date and his exercisable options expire three months after December 29, 2017. Dr. Horn has also forfeited any and all other stock awards previously granted to him that had not vested as of December 29, 2017. |
41
| (9) | This option vested as to 25% of the shares on March 4, 2016 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until March 4, 2019. |
| (10) | This option was scheduled to vest as to 25% of the shares on November 15, 2018 and as to 6.25% of the shares at the end of each successive three-month period thereafter until November 15, 2021. Ms. Unninayar resigned in March 2018 and, therefore, none of the shares underlying this award vested or will vest. |
| (11) | This option vested as to 25% of the shares on July 15, 2016 and is scheduled to vest as to 6.25% of the shares at the end of each successive three-month period thereafter until July 15, 2019. |
| (12) | These restricted stock units vest in January 2019. |
| (13) | These performance based stock units vest in January 2020 but only if specific regulatory and clinical milestones are achieved, subject to the named executive officer’s continued employment. |
Option Exercises and Stock Vested
The following table sets forth certain information regarding the exercise of stock options and the vesting of stock awards during 2017 for each of our named executive officers:
| | | | | | | | | | | | | | | | |
| | | Option Awards | | | Stock Awards | |
| | | Number of
Shares
Acquired Upon
Exercise (#) | | | Value Realized
Upon Exercise
($)(1) | | | Number of
Shares
Acquired on
Vesting (#) | | | Value Realized
on Vesting
($)(1) | |
Guy Macdonald | | | 30,378 | | | $ | 46,478 | | | | — | | | | — | |
Jacques Dumas, Ph.D. | | | 26,250 | | | | 59,850 | | | | 11,667 | | | | 51,218 | (2) |
Patrick T. Horn, M.D., Ph.D. | | | 10,000 | | | | 52,600 | | | | 11,667 | | | | 51,218 | (2) |
Maria Stahl | | | — | | | | — | | | | 10,000 | | | | 43,900 | (2) |
Kamalam Unninayar | | | — | | | | — | | | | — | | | | — | |
Christopher Watt | | | — | | | | — | | | | 4,167 | | | | 18,293 | (2) |
| (1) | Value realized represents the difference between the closing price per share of our common stock on each date of exercise and the exercise price per share, multiplied by the number of shares acquired on exercise. |
| (2) | The value realized on vesting is determined by multiplying the number of shares of stock vesting by the market value of the underlying shares as reported by the NASDAQ Capital Market on the vesting date, or $4.39. |
Potential Payments Upon Termination or Change in Control
Potential payments made to our named executive officers in the instance of a termination without cause or a termination for good reason or in the case of change in control benefits upon a “double trigger” are discussed in greater detail under “Compensation Discussion and Analysis—Severance and Change in Control Benefits”.
The table below sets forth the potential payments to our named executive officers assuming a termination event occurred as of December 31, 2017. Dr. Horn resigned as our chief medical officer effective December 29, 2017 and therefore would not be eligible for any termination payments.
42
POTENTIAL TERMINATION PAYMENTS
| | | | | | | | | | | | | | | | |
| | | Salary(1) | | | Acceleration
of
Vesting of
Equity
Awards(2) | | | Other
Payments(3) | | | Total | |
Guy Macdonald | | $ | 530,000 | | | $ | — | | | $ | 43,579 | | | $ | 573,579 | |
Jacques Dumas, Ph.D. | | | 371,300 | | | | — | | | | 43,579 | | | | 414,879 | |
Patrick T. Horn, M.D., Ph.D. | | | — | | | | — | | | | — | | | | — | |
Maria Stahl | | | 350,200 | | | | — | | | | 43,579 | | | | 393,779 | |
Kamalam Unninayar | | | 340,000 | | | | — | | | | 43,579 | | | | 383,579 | |
Christopher Watt | | | 151,600 | | | | — | | | | 21,790 | | | | 173,390 | |
| (1) | This amount represents a sum equivalent to one times the executive’s base salary at the time of termination, other than for Mr. Watt which is a sum equivalent to one half times his base salary at the time of termination. |
| (2) | No equity awards vest and become immediately exercisable in full upon a termination event. |
| (3) | Represents amounts related to continued medical, dental and vision benefits for such officer and his or her eligible dependents for up to 12 months, other than for Mr. Watt which amounts relate to continued medical, dental and vision benefits for him and his eligible dependents for up to 6 months. |
The table below sets forth the potential payments to our named executive officers assuming a change in control event occurred as of December 31, 2017. Dr. Horn resigned as our chief medical officer effective December 29, 2017 and therefore would not be eligible for any termination payments. Upon a change in control, our named executive officers receive the amounts he or she would have been entitled to if terminated without cause and also additional payments as set forth below.
POTENTIAL CHANGE IN CONTROL PAYMENTS
| | | | | | | | | | | | | | | | | | | | |
| | | Salary(1) | | | Acceleration
of
Vesting of
Equity
Awards(2) | | | Bonus
Payments(3) | | | Other
Payments(4) | | | Total | |
Guy Macdonald | | $ | 795,000 | | | $ | 602,063 | | | $ | 397,500 | | | $ | 65,369 | | | $ | 1,859,932 | |
Jacques Dumas, Ph.D. | | | 371,300 | | | | 296,888 | | | | 148,520 | | | | 43,579 | | | | 860,287 | |
Patrick T. Horn, M.D., Ph.D. | | | — | | | | — | | | | — | | | | — | | | | — | |
Maria Stahl | | | 350,200 | | | | 243,873 | | | | 122,570 | | | | 43,579 | | | | 760,222 | |
Kamalam Unninayar | | | 340,000 | | | | 43,700 | | | | 136,000 | | | | 43,579 | | | | 563,279 | |
Christopher Watt | | | 303,200 | | | | 127,499 | | | | 90,960 | | | | 43,579 | | | | 565,238 | |
| (1) | This amount represents a sum equivalent to one times the executive’s base salary at the time of termination following a change in control for each of the executive officers other than Mr. Macdonald. Mr. Macdonald will receive an additional six months’ of base salary at the time of termination following a change in control. |
| (2) | The amount representing the acceleration of vesting of stock option awards is equal to the number of options multiplied by the difference between the exercise price of such option and the closing stock price of our common stock on December 29, 2017 ($6.30) as reported by The NASDAQ Global Select Market. |
| (3) | This amount represents a lump sum payment equivalent to one times the executive’s target bonus (one andone-half times in the case of Mr. Macdonald). |
| (4) | Represents amounts related to continued medical, dental and vision benefits for such officer and his or her eligible dependents for up to 12 months other than for Mr. Macdonald. Mr. Macdonald will receive an additional six months’ of continued medical, dental and vision benefits at the time of termination following a change in control. |
43
Equity Compensation Plan Information
The following table contains information about our equity compensation plans as of December 31, 2017. In addition, from time to time, we grant “inducement grants” pursuant to Nasdaq Listing Rule 5635(c)(4).
Equity Compensation Plan Information
| | | | | | | | | | | | |
Plan Category | | Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights | | | Weighted-average
exercise price of
outstanding options,
warrants and rights | | | Number of securities
remaining available for
future issuance under
equity compensation
plans (excluding
securities reflected in
column (a)) | |
| | | (a) | | | (b) | | | (c) | |
Equity compensation plans approved by security holders | | | 5,786,828 | (1) | | $ | 11.59 | | | | 1,028,889 | (2) |
Equity compensation plans not approved by security holders | | | 490,000 | (3) | | | 29.78 | | | | — | |
| | | | | | | | | | | | |
Total | | | 6,276,828 | | | $ | 13.01 | | | | 1,028,889 | |
| (1) | Consists of (i) 313,386 shares of our common stock issuable under our 2006 stock incentive plan, (ii) 5,194,408 shares of our common stock issuable under our 2013 stock incentive plan and (iii) 282,034 shares of restricted stock issuable under our 2013 stock incentive plan. |
| (2) | Consists of (i) 890,011 shares of our common stock available for future issuance under our 2013 stock incentive plan; and (ii) 138,878 shares of our common stock available for future issuance under our 2014 employee stock purchase plan. |
| (3) | Consists of stock options issued as inducement grants as of December 31, 2017. These stock options are generally subject to the same terms and conditions as those awarded pursuant to the plans approved by our stockholders. |
PAY RATIO DISCLOSURE
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of RegulationS-K (the “Rule”), we are required to provide to our shareholders specified disclosure regarding the relationship of CEO total compensation to the total compensation of our median employee, referred to as “pay ratio” disclosure. The pay ratio included in this section is our reasonable estimate calculated in a manner consistent with the Rule and applicable guidance.
For fiscal 2017, the median of the annual total compensation of all employees of the company (other than the CEO) was $198,734 and the annual total compensation of our CEO, as reported in the Summary Compensation Table included in this Proxy Statement, was $2,073,576. As a result, the ratio of the annual total compensation of the CEO to the median of the annual total compensation of all employees was 10.4 to 1.
The Rule provides significant flexibility in how companies identify the median employee, and each company may use a different methodology and make different assumptions particular to that company. As a result, in considering the pay ratio disclosure, shareholders should keep in mind that the Rule was not designed to facilitate comparisons of pay ratios among different companies, even companies within the same industry, but rather to allow shareholders to better understand and assess each particular company’s compensation practices and pay ratio disclosures.
44
Set forth below is a description of the methodology, including any material assumptions, adjustments and estimates, we used to identify our median employee for purposes of the Rule.
First, we determined our total population of employees, including all full-time, part-time and temporary employees, as of December 8, 2017. None of our employees are located outside of the U.S. To identify our “median employee” from our employee population as determined above, we compared the sum of each employee’s 2017 annualized base salary and 2017 target annual incentive opportunity. This compensation measure was consistently applied to all employees included in the calculation.
AUDIT COMMITTEE REPORT
The following is the report of the audit committee with respect to our audited consolidated financial statements for the year ended December 31, 2017.
The audit committee has reviewed our audited consolidated financial statements for the fiscal year ended December 31, 2017 and discussed them with the company’s management and our independent registered public accounting firm for the year ended December 31, 2017, Ernst & Young LLP.
The audit committee has also received from, and discussed with, Ernst & Young LLP various communications that Ernst & Young LLP is required to provide to the audit committee, including the matters to be discussed as required by Auditing Standards No. 1301, Communications with Audit Committees, as adopted by the Public Company Accounting Oversight Board, or PCAOB, and all other communications required under the PCAOB.
In addition, Ernst & Young LLP provided the audit committee with the written disclosures and the letter required by applicable requirements of the PCAOB regarding our independent registered public accounting firm’s communications with the audit committee concerning independence, and the audit committee has discussed with the company’s independent registered public accounting firm their independence.
Based on the review and discussions referred to above, the audit committee recommended to the board of directors that the audited consolidated financial statements be included in the company’s Annual Report onForm 10-K for the year ended December 31, 2017.
By the audit committee of the board of directors,
Garen Bohlin, Chair
L. Patrick Gage, Ph.D.
John Freund, M.D.
45
PROPOSAL 2
RATIFICATION OF THE APPOINTMENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Our audit committee has appointed the firm of Ernst & Young LLP, an independent registered public accounting firm, as our independent auditors for the fiscal year ending December 31, 2018. Ernst & Young LLP has served as our independent registered public accounting firm since 2007. Our engagement partner rotates every five years, with the most recent rotation beginning in 2016. Although stockholder approval of our audit committee’s appointment of Ernst & Young LLP is not required by law, our board believes that it is advisable to give stockholders an opportunity to ratify this appointment. If this proposal is not approved at the annual meeting, our audit committee will reconsider its appointment of Ernst & Young LLP. Representatives of Ernst & Young LLP are expected to be present at the annual meeting and will have the opportunity to make a statement, if they desire to do so, and will be available to respond to appropriate questions from our stockholders.
Principal Accountant Fees and Services
Ernst & Young LLP audited our financial statements for the year ended December 31, 2017. The board of directors has appointed Ernst & Young LLP to serve as our independent registered public accounting firm for the fiscal year ending December 31, 2018.
The following table summarizes the fees of Ernst & Young LLP billed or expected to be billed to us for each of the last two fiscal years.
| | | | | | | | |
Fee Category | | 2017 | | | 2016 | |
Audit Fees(1) | | $ | 874,000 | | | $ | 705,179 | |
Audit-Related Fees(2) | | | 36,000 | | | | — | |
Tax Fees(3) | | | 69,500 | | | | 58,704 | |
All Other Fees(4) | | | 16,500 | | | | — | |
| | | | | | | | |
Total Fees | | $ | 996,000 | | | $ | 763,883 | |
| (1) | “Audit Fees” consist of fees for the audit of our annual financial statements, the review of our interim financial statements included in our quarterly reports on Form10-Q, a public offering of our common stock which was completed in August 2017, and consultations on miscellaneous SEC filings and other professional services provided in connection with regulatory filings or engagements. |
| (2) | “Audit-Related Fees” consists of fees billed by an independent registered public accounting firm for assurance and related services that are reasonably related to the performance of the audit or review of our consolidated financial statements. |
| (3) | “Tax Fees” consist of fees for tax compliance, advice and tax services, including fees for tax preparation. |
| (4) | “All Other Fees” consists of fees billed for products and services, other than those described above under Audit Fees and Tax fees. We incurred no such fees in 2016. |
All such accountant services and fees werepre-approved by our audit committee in accordance with the “Audit CommitteePre-Approval Policies and Procedures” described below.
Audit CommitteePre-Approval Policies and Procedures
Our audit committee has adopted procedures requiring thepre-approval of all audit andnon-audit (including tax) services that are to be performed by our independent registered public accounting firm in order to assure that these services do not impair the auditor’s independence. These procedures generally approve the performance of specific services subject to a cost limit for all such services. This general approval is to be reviewed, and if necessary modified, at least annually. Management must obtain the specific prior approval of the audit committee
46
for each engagement of the independent registered public accounting firm to perform any other audit ornon-audit services. The audit committee does not delegate its responsibility to approve services performed by the independent registered public accounting firm to any member of management.
The standard applied by the audit committee in determining whether to grant approval of any type ofnon-audit service, or of any specific engagement to perform anon-audit service, is whether the services to be performed, the compensation to be paid therefore and other related factors are consistent with the independent registered public accounting firm’s independence under guidelines of the SEC and applicable professional standards. Relevant considerations include whether the work product is likely to be subject to, or implicated in, audit procedures during the audit of our financial statements, whether the independent registered public accounting firm would be functioning in the role of management or in an advocacy role, whether the independent registered public accounting firm’s performance of the service would enhance our ability to manage or control risk or improve audit quality, whether such performance would increase efficiency because of the independent registered public accounting firm’s familiarity with our business, personnel, culture, systems, risk profile and other factors, and whether the amount of fees involved, or thenon-audit services portion of the total fees payable to the independent registered public accounting firm in the period would tend to reduce the independent registered public accounting firm’s ability to exercise independent judgment in performing the audit.
All of the services rendered by Ernst & Young LLP with respect to the 2017 and 2016 fiscal years werepre-approved by the audit committee in accordance with this policy.
OUR BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT THE STOCKHOLDERS VOTE “FOR” THE RATIFICATION OF THE APPOINTMENT OF ERNST & YOUNG LLP AS OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM FOR THE FISCAL YEAR ENDING DECEMBER 31, 2018.
47
PROPOSAL 3
ADVISORY VOTE ON EXECUTIVE COMPENSATION
We are providing our stockholders the opportunity to vote to approve, on an advisory,non-binding basis, the compensation of our named executive officers as disclosed in this proxy statement in accordance with the SEC’s rules. This proposal, which is commonly referred to as“say-on-pay,” is required by the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, which added Section 14A to the Exchange Act. Section 14A of the Exchange Act also requires that stockholders have the opportunity, at least once every six years, to cast anon-binding advisory vote with respect to whether future executive compensation advisory votes will be held every one, two or three years, which is commonly referred to as“say-on-frequency”. The stockholders voted “every year” at our 2016 annual meeting of stockholders, which was ratified by our board of directors. As such, the next advisory(non-binding) vote regardingsay-on- frequency will be at our 2022 annual meeting of stockholders.
Our executive compensation program is designed to attract, motivate, and retain our executive officers, who are critical to our success. Under these programs, our named executive officers are rewarded for the achievement of our near-term and longer-term financial and strategic goals and for driving corporate financial performance and stability. The programs contain elements of cash and equity-based compensation and are designed to align the interests of our executives with those of our stockholders as well as promote the achievement of the company’s strategic and financial performance measures by linking executive compensation to the achievement of measurable corporate and individual performance goals. Our equity incentive program is the primary compensation vehicle aligning our named executive officers’ compensation to the long-term performance of the company in addition to creating an ownership culture that helps unify the interests of our executives and stockholders. The equity incentive program encourages a long-term focus by our executives by using four-year minimum vesting requirements for stock options, one to three year vesting requirements for restricted stock units and performance-based restricted stock units that do not vest unless certainpre-defined milestones are met. Our board of directors and the compensation committee regularly review the compensation programs for our named executive officers and undertake a comprehensive annual review to ensure that our compensation policies and programs align with current market practices and the interests of our stockholders.
The “Executive Compensation” section of this proxy statement, including “Compensation Discussion and Analysis,” describes in detail our executive compensation programs and the decisions made by the compensation committee and the board of directors with respect to the fiscal year ended December 31, 2017. Highlights of our executive compensation program include the following:
| | • | | our executives are eligible to participate in long-term incentives through stock option grants and performance-based restricted stock units, with the potential to benefit only if shareholder value is increased as a result of increases in our stock price from the dates of such stock option grants and only upon the achievement ofpre-defined strategic milestones; and |
| | • | | our executives are eligible to participate in our annual cash performance incentive plan but only receive payouts whenpre-determined individual and corporate goals are met. |
As we describe in the “Compensation Discussion and Analysis” section of this proxy statement, our executive compensation program embodies apay-for-performance philosophy that supports our business strategy and aligns the interests of our executives with our stockholders. Our board believes this link between compensation and the achievement of our near- and long-term business goals is imperative. At the same time, we believe our program does not encourage excessive risk-taking by management.
48
Our board of directors is asking stockholders to approve anon-binding advisory vote on the following resolution:
RESOLVED, that the compensation paid to the named executive officers of Tetraphase Pharmaceuticals, Inc., as disclosed pursuant to the compensation disclosure rules of the Securities and Exchange Commission, including the compensation discussion and analysis, the compensation tables and any related material disclosed in this proxy statement, is hereby approved.
As an advisory vote, this proposal is not binding. The outcome of this advisory vote will not overrule any decision by the company or our board of directors (or any committee thereof), create or imply any change to the fiduciary duties of the company or our board of directors (or any committee thereof), or create or imply any additional fiduciary duties for the company or our board of directors (or any committee thereof). However, our compensation committee and board of directors value the opinions expressed by our stockholders in their vote on this proposal and will consider the outcome of the vote when making future compensation decisions for named executive officers.
OUR BOARD OF DIRECTORS RECOMMENDS THAT THE STOCKHOLDERS VOTE TO APPROVE, ON AN ADVISORY BASIS, THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS BY VOTING “FOR” PROPOSAL 3.
49
OTHER INFORMATION
Other Matters
Our board of directors does not know of any other matters which may come before the meeting. However, if any other matters are properly presented to the meeting, it is the intention of the persons named in the proxy card to vote, or otherwise act, in accordance with their judgment on such matters.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act requires our directors and officers and holders of more than 10% of our common stock to file with the SEC initial reports of ownership of our common stock and other equity securities on a Form 3 and reports of changes in such ownership on a Form 4 or Form 5. Directors and officers and holders of 10% of our common stock are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. To our knowledge, based solely on a review of our records and representations made by our directors and officers regarding their filing obligations, all Section 16(a) filing requirements were satisfied with respect to 2017.
Stockholder Proposals for the 2019 Annual Meeting
Proposals of stockholders intended to be presented at our annual meeting of stockholders to be held in 2019 must be received by us no later than December 21, 2018, which is 120 days prior to the first anniversary of the mailing date of this proxy, in order to be included in our proxy statement and form of proxy relating to that meeting, unless the date of the 2019 annual meeting of stockholders is changed by more than 30 days from the anniversary of our 2019 annual meeting, in which case the deadline for such proposals will be a reasonable time before we begin to print and send our proxy materials. These proposals must comply with the requirements as to form and substance established by the SEC for such proposals in order to be included in the proxy statement.
In addition, our bylaws establish an advance notice procedure for nominations for election to our board of directors and other matters that stockholders wish to present for action at an annual meeting other than those to be included in our proxy statement. In general, notice must be received at our principal executive offices not less than 90 calendar days before nor more than 120 calendar days before theone-year anniversary of the previous year’s annual meeting of stockholders. Therefore, to be presented at our 2019 annual meeting of stockholders, such a proposal must be received by us no earlier than January 30, 2019 and no later than March 1, 2019. However, if the date of the annual meeting is more than 20 days earlier or more than 60 days later than such anniversary date, notice must be received not later than the close of business 120 calendar days prior to such annual meeting and no later than the close of business on the later of 90 days prior to such annual meeting and 10 days following the day on which notice of the date of such annual meeting was mailed or public announcement of the date of such annual meeting was first made. If the stockholder fails to give notice by these dates, then the persons named as proxies in the proxies solicited by the board of directors for the 2019 annual meeting may exercise discretionary voting power regarding any such proposal. Stockholders are advised to review our bylaws which also specify requirements as to the form and content of a stockholder’s notice.
Stockholders also have the right under our bylaws to nominate director candidates directly, without any action or recommendation on the part of the nominating and corporate governance committee or the board of directors, by following the procedures set forth in our bylaws, including advance notice requirements. Candidates nominated by stockholders in accordance with the procedures set forth in our bylaws will not be included in our proxy card for the next annual meeting.
Any proposals, notices or information about proposed director candidates should be sent to:
Tetraphase Pharmaceuticals, Inc.,
480 Arsenal Way
Watertown, Massachusetts 02472
Attention: Chair of the Nominating and Corporate Governance Committee
50

ANNUAL MEETING OF TETRAPHASE PHARMACEUTICALS, INC.
| | |
Date: | | Wednesday, May 30, 2018 |
Time: | | 10:00 A.M. (Eastern Time) |
Place: | | Wilmer Cutler Pickering Hale and Dorr LLP 60 State Street, Boston, Massachusetts 02109 |
Please make your marks like this: ☒ Use dark black pencil or pen only
The Board of Directors Recommends a VoteFOR all the nominees listed in proposal 1 and for proposals 2 and 3.
| | | | |
1: | | To elect Jeffrey Chodakewitz, Gerri Henwood and Guy Macdonald as class II directors, each to serve for a three-year term expiring at the 2021 annual meeting of stockholders. |
| | | | |
Vote For All Nominees | | Withhold Vote From All Nominees | | Vote For All Except |
| ☐ | | ☐ | | ☐ |
| | |
| INSTRUCTIONS:To withhold authority to vote for any nominee, mark the “Exception” box and write the number(s) in the space provided to the right. | | |
| | | | | | | | | | |
| | | | | For | | Against | | Abstain | | |
2: | | To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2018. | | ☐ | | ☐ | | ☐ | | |
| | | | | For | | Against | | Abstain | | |
3: | | To approve, on a non-binding advisory basis, the compensation of our named executive officers. | | ☐ | | ☐ | | ☐ | | |
| | | | | |
| | To attend the meeting and vote your shares in person, please mark this box. | | | | ☐ | | | | |
| | | | | |
| | Authorized Signatures - This section must be completed for your Instructions to be executed. | | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| | Please Sign Here | | | | Please Date Above | | |
| | | | | | | | |
| | Please Sign Here | | | | Please Date Above | | |
| | Please sign exactly as your name(s) appears on your stock certificate. If held in joint tenancy, all persons should sign. Trustees, administrators, etc., should include title and authority. Corporations should provide full name of corporation and title of authorized officer signing the proxy. |
|
 Please separate carefully at the perforation and return just this portion in the envelope provided. Please separate carefully at the perforation and return just this portion in the envelope provided.  |

Annual Meeting of Tetraphase Pharmaceuticals, Inc.
to be held on Wednesday, May 30, 2018
for Holders as of April 6, 2018
This proxy is being solicited on behalf of the Board of Directors
| | | | | | | | | | |
| | | | VOTE BY: | | | | |
| |  INTERNET INTERNET | | | | | |  TELEPHONE TELEPHONE |
| | | | | | | | | | |
Go To | | | | | | | | Call |
www.proxypush.com/ttph | | | | | | | | 866-416-3857 |
| | | | | | | | | | |
| • | | Cast your vote online. | | | | OR | | • | | Use any touch-tone telephone. |
• | | Have your Proxy Card/Voting Instructions Form ready. | | | |  MAIL MAIL
| | •
•
| | Have your Proxy Card/Voting Instruction Form ready. Follow the simple recorded instructions. |
• | | View Meeting Documents. | | | | | | | |
| | | | | | | | |
| | OR | | • | | Mark, sign and date your Proxy Card/Voting Instruction Form. | | |
| | | | • | | Detach your Proxy Card/Voting Instruction Form. | | |
| | | | • | | Return your Proxy Card/Voting Instruction Form in the | | |
| | | | | | postage-paid envelope provided. | | |
The undersigned hereby appoints Guy Macdonald and Maria Stahl, and each of them, as the true and lawful attorneys of the undersigned, with full power of substitution and revocation, and authorizes them, and each of them, to vote all the shares of capital stock of Tetraphase Pharmaceuticals, Inc. which the undersigned is entitled to vote at said meeting and any adjournment thereof upon the matters specified and upon such other matters as may be properly brought before the meeting or any adjournment thereof, conferring authority upon such true and lawful attorneys to vote in their discretion on such other matters as may properly come before the meeting and revoking any proxy heretofore given.
THE SHARES REPRESENTED BY THIS PROXY WILL BE VOTED AS DIRECTED OR, IF NO DIRECTION IS GIVEN, SHARES WILL BE VOTED FOR THE ELECTION OF THE DIRECTORS IN ITEM 1, FOR THE PROPOSALS IN ITEMS 2 AND 3 AND IN THE DISCRETION OF THE PROXYHOLDERS ON ANY OTHER MATTER THAT PROPERLY COMES BEFORE THE MEETING.
All votes must be received by 5:00 P.M., Eastern Time, May 29, 2018.
| | | | | | | | | | | | | | |
| | | | | | | | | | PROXY TABULATOR FOR TETRAPHASE PHARMACEUTICALS, INC. c/o MEDIANT COMMUNICATIONS P.O. BOX 8016 CARY, NC 27512-9903 |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |






 Please separate carefully at the perforation and return just this portion in the envelope provided.
Please separate carefully at the perforation and return just this portion in the envelope provided. 
 INTERNET
INTERNET TELEPHONE
TELEPHONE MAIL
MAIL