Exhibit 99.1
| Corporate Presentation October 9, 2012 © 2012 NuPathe Inc. All rights reserved. |
| Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. All remarks and information that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, remarks and information relating to: the Company’s ability to satisfy the closing conditions and complete its recently announced financing; the sufficiency of the proceeds to be received upon closing of the financing, together with recently announced cost-containment measures, to fund operations into the fourth quarter of 2013; the Company’s interpretation of the FDA’s complete response letter (CRL); the timing and prospects for FDA approval of Zecuity (also referred to as NP101 and migraine patch); the therapeutic benefits, pricing, reimbursement and market opportunity for Zecuity; partnering and commercialization plans for the Company’s product candidates; duration and scope of IP protection; and other and all other remarks and information relating to the Company’s projections, expectations, beliefs, future performance or plans or objectives for future operations (including assumptions underlying or relating to any of the foregoing). Forward-looking statements are based on the Company’s current expectations, plans and beliefs and are subject to a number of risks, uncertainties, assumptions and other factors that could cause actual results to differ materially and adversely from those indicated by such statements including, among others: the Company’s ability to satisfy the closing conditions and complete the financing; the Company’s ability to continue operations until the closing of the financing; the adequacy of activities undertaken to address the issues contained in the CRL; the extent to which the FDA may request or require additional information, studies or redesign of NP101; physician and patient acceptance of the Company’s product candidates; safety or other risks that could require the Company to abandon or delay development of, or preclude or limit approval of, the Company’s product candidates; varying interpretation of study and market data; risks and uncertainties relating to intellectual property; and the other factors discussed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2011 under the caption “Risk Factors” and elsewhere in such report, which is available at on the Company’s website at www.nupathe.com in the “Investor Relations – SEC Filings” section. As a result, you are cautioned not to place undue reliance on any forward-looking statements. While the Company may update certain forward-looking statements from time to time, it specifically disclaims any obligation to do so, whether as a result of new information, future developments or otherwise. |
| ZecuityTM: substantial near-term commercial opportunity The first migraine patch Delivers sumatriptan, the #1 Rx migraine treatment Uniquely designed to overcome migraine-related nausea & vomiting Impacts half of all migraine sufferers PDUFA date Jan 17, 2013 Focused on gaining FDA approval, securing commercial partners, and select pre-launch activities Financing & cost containment measures expected to provide cash runway into 4Q13 NuPathe – Innovative Neuroscience Solutions |
| Applied during migraine Rapidly delivers sumatriptan Bypasses GI tract Simple to use, single use Multiple application sites Strong intellectual property protection until April 2027 Zecuity – First and Only Migraine Patch |
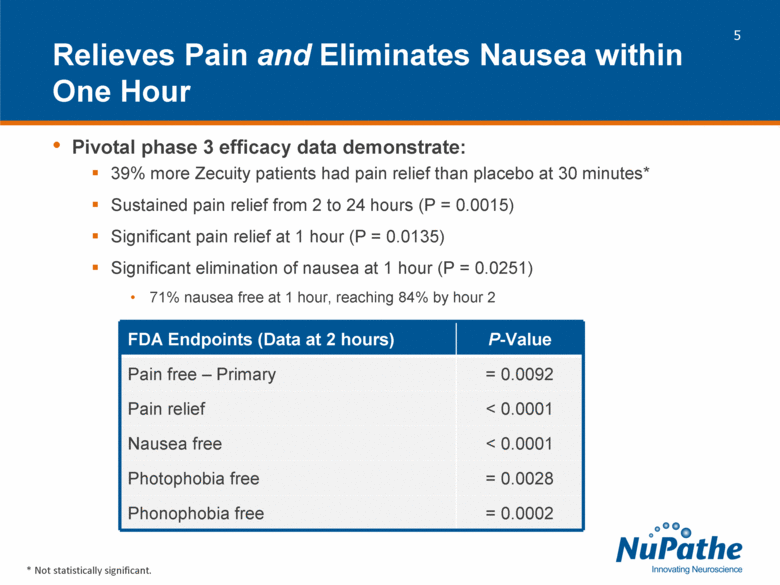
| Pivotal phase 3 efficacy data demonstrate: 39% more Zecuity patients had pain relief than placebo at 30 minutes* Sustained pain relief from 2 to 24 hours (P = 0.0015) Significant pain relief at 1 hour (P = 0.0135) Significant elimination of nausea at 1 hour (P = 0.0251) 71% nausea free at 1 hour, reaching 84% by hour 2 Relieves Pain and Eliminates Nausea within One Hour * Not statistically significant. FDA Endpoints (Data at 2 hours) P-Value Pain free – Primary = 0.0092 Pain relief < 0.0001 Nausea free < 0.0001 Photophobia free = 0.0028 Phonophobia free = 0.0002 |
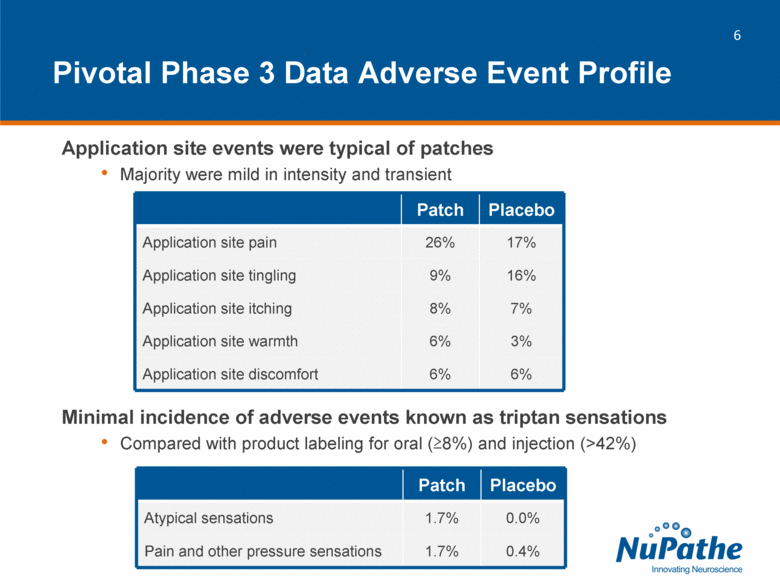
| Pivotal Phase 3 Data Adverse Event Profile Application site events were typical of patches Majority were mild in intensity and transient Minimal incidence of adverse events known as triptan sensations Compared with product labeling for oral (>8%) and injection (>42%) Patch Placebo Application site pain 26% 17% Application site tingling 9% 16% Application site itching 8% 7% Application site warmth 6% 3% Application site discomfort 6% 6% Patch Placebo Atypical sensations 1.7% 0.0% Pain and other pressure sensations 1.7% 0.4% |
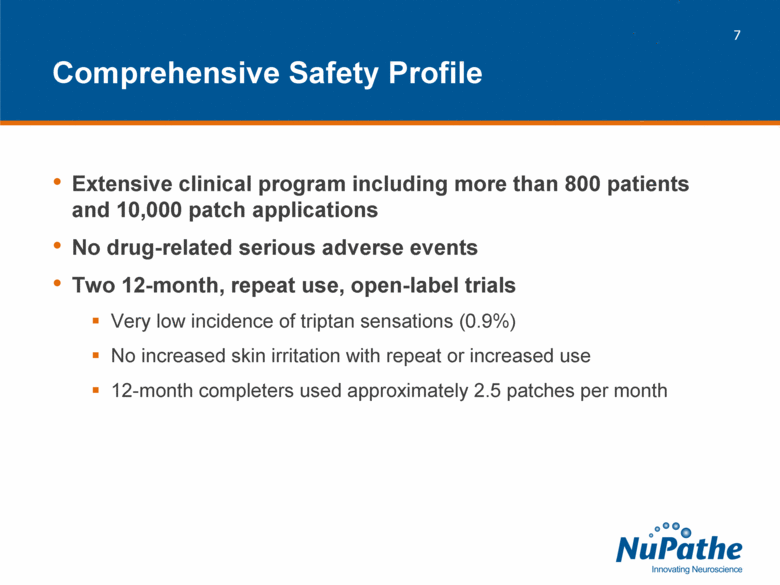
| Extensive clinical program including more than 800 patients and 10,000 patch applications No drug-related serious adverse events Two 12-month, repeat use, open-label trials Very low incidence of triptan sensations (0.9%) No increased skin irritation with repeat or increased use 12-month completers used approximately 2.5 patches per month Comprehensive Safety Profile |
| Key CRL Issue Action Taken Skin events of concern Implemented device enhancement Prevents patch from activating when misapplied Packaging Usability Uniformity Containment Modified secondary packaging Usability study demonstrated all patients correctly applied patch during a migraine Additional data supports consistent uniformity and containment Repeat BE study Successfully completed Required new in vitro method for product release Developed and validated new in vitro method Justification of waiver for CA study Demonstrated that sumatriptan not passively delivered even in presence of penetration enhancers in two laboratory dermal studies Zecuity NDA Resubmitted PDUFA Jan 17, 2013 |
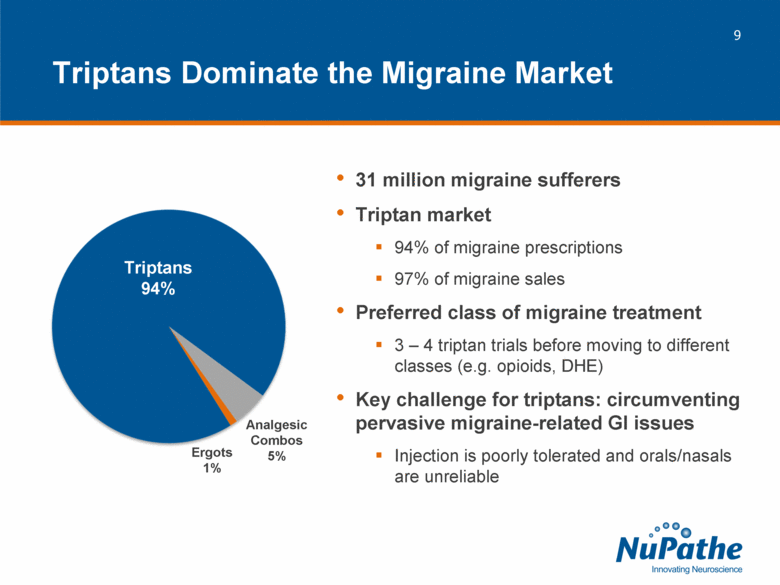
| 31 million migraine sufferers Triptan market 94% of migraine prescriptions 97% of migraine sales Preferred class of migraine treatment 3 – 4 triptan trials before moving to different classes (e.g. opioids, DHE) Key challenge for triptans: circumventing pervasive migraine-related GI issues Injection is poorly tolerated and orals/nasals are unreliable Triptans Dominate the Migraine Market Triptans 94% Analgesic Combos 5% Ergots 1% |
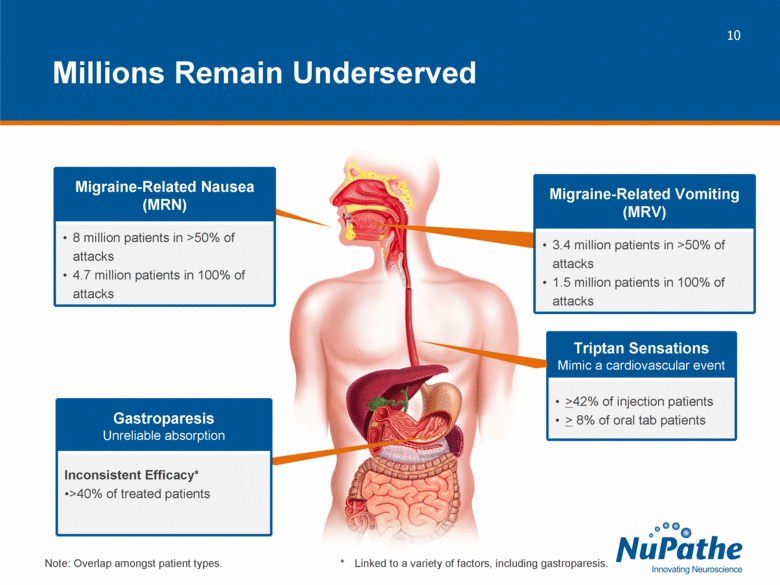
| Triptan Sensations Mimic a cardiovascular event >42% of injection patients > 8% of oral tab patients Millions Remain Underserved * Linked to a variety of factors, including gastroparesis. Gastroparesis Unreliable absorption Inconsistent Efficacy* >40% of treated patients Migraine-Related Nausea (MRN) 8 million patients in >50% of attacks 4.7 million patients in 100% of attacks Migraine-Related Vomiting (MRV) 3.4 million patients in >50% of attacks 1.5 million patients in 100% of attacks Note: Overlap amongst patient types. |
| Frequent nausea patients are less satisfied with their migraine medications Frequent nausea patients drive direct medical costs for migraine 5.4 times as many emergency/urgent care visits 8.2 times higher overnight hospital stay costs Presence of nausea predicts poor response to oral medications in treating headache pain Headache pain relief with patch not compromised by presence of nausea* MRN Drives Tremendous Disease Burden * Ad hoc data analysis of Phase 3 pivotal study ( P <0.0001 ). |
| Insurance is >90% commercial private pay Payors see value in a patch for migraine Acknowledge barrier of GI issues Recognize cost of poorly controlled migraine patients Accept patch as a unique solution Payors indicate broad formulary access Majority Tier 3; opportunities to contract for Tier 2 Non-orals priced up to $90 per unit (WAC) Annual gross revenue per patient using 2 patches/month $80/patch = $1,900 $100/patch = $2,400 Broad Formulary Coverage Expected |
| Physicians rate the patch superior to oral, nasal, and injectable triptans for MRN patients on key attributes including: Ability to use early in a migraine attack despite GI issues Frequency of triptan sensations and overall tolerability Elimination of nausea at 1 and at 2 hours 77% of MRN patients will ask their physician about the patch Quantitative Research Confirms the Market Opportunity for the Patch Physicians (n=409) Priority segments Heightened concern about MRN High interest in prescribing a migraine patch Patients (n=400) Priority segments Actively seeking new treatments Highest % of migraines with nausea |
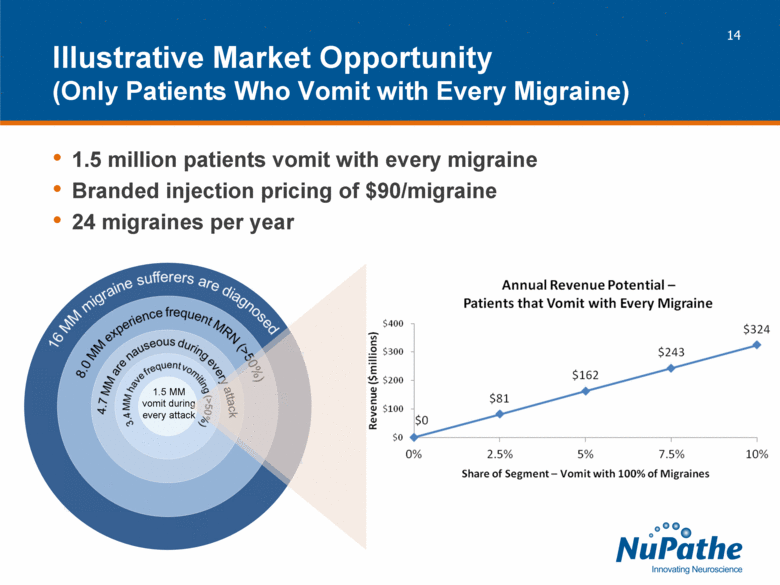
| 1.5 million patients vomit with every migraine Branded injection pricing of $90/migraine 24 migraines per year Illustrative Market Opportunity (Only Patients Who Vomit with Every Migraine) 1.5 MM vomit during every attack |
| Secure commercial partnerships Patch is a versatile asset within a portfolio Migraine co-morbidity rates include: sleep disorders (52%), depression (44%), arthritis (26%), GERD (25%) Specialists (~10K) drive 1/3rd of triptan prescriptions Headache specialists and neurologists Primary care drives the balance of the market ~30K PCPs account for 1/3rd of the market Significant commercial opportunity in ROW Commercialization Strategy 15 |
| NP202: 90-day risperidone biodegradable implant to treat schizophrenia and bipolar disorder Designed to address significant patient non-compliance Continuous delivery reduces psychotic breaks and improves patient functionality1 Seeking global development & commercial partner NP201: 60-day ropinirole biodegradable implant to treat the signs and symptoms of Parkinson’s disease Designed to address the challenges of “on and off” time due to intermittent dosing and reducing drug-related adverse events Evidence suggests continuous dopaminergic stimulation delays disease progression and improves treatment2 Seeking partner for continued development Pipeline 16 |
| Financing & cost containment measures expected to provide cash runway into 4Q13 $28 million financing expected to close in Oct/Nov Restructured $7.5 million of outstanding debt Headcount reduction and reduced spend on pipeline and commercialization activities Focused on achievement of key milestones Zecuity approval Securing commercial partnerships Select pre-launch activities Financial 17 |
| ZecuityTM: substantial near-term commercial opportunity The first migraine patch Delivers sumatriptan, the #1 Rx migraine treatment Uniquely designed to overcome migraine-related nausea & vomiting Impacts half of all migraine sufferers PDUFA date Jan 17, 2013 Focused on gaining FDA approval, securing commercial partners, and select pre-launch activities Financing & cost containment measures expected to provide cash runway into 4Q13 NuPathe – Innovative Neuroscience Solutions 18 |
| References Slide 9: 1. Lipton, R. Prevalence and Burden of Migraine in the United States: Data from the American Migraine Study II. Headache 2001 41:646-657. and US Census data (2010) 2. Data from IMS MAT June 2011. Triptans include Treximet. Analgesic combos = analgesic combos + Cambia 3. NuPathe proprietary market research. Slides 10: 1. Lipton, R. Data presented at the 53rd Annual Scientific Meeting of the American Headache Society (AHS), June 2011. 2.Silberstein, S. Migraine symptoms: results of a survey of self-reported migraineurs. Headache 1995;35:387-396. 3. US prescribing information Imitrex tablets and Imitrex injection. 4. Ferrari et al. Oral triptans (serotonin 5-HT1B/1D agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 2001; 358: 1668–75. Slide 11: 1. Lipton et al. Data presented at the 53rd Annual Scientific Meeting of the American Headache Society (AHS), June 2011. 2. Buse et al. Data presented at the 53rd Annual Scientific Meeting of the American Headache Society (AHS), June 2011. 3. Lipton et al. Data presented at the Academy of Managed Care Pharmacy’s 2011 Educational Conference, October 2011. 4.Diener et al. Predicting the response to sumatriptan, the sumatriptan naratriptan aggregate database. Neurology 2004;63:520–524. 5. Diener et al. Identification of negative predictors of pain-free response to triptans: analysis of the eletriptan database. Cephalalgia 2007, 28, 35–40. Slides 14: 1. Lipton, R. Data presented at the 53rd Annual Scientific Meeting of the American Headache Society (AHS), June 2011. 2.Silberstein, S. Migraine symptoms: results of a survey of self-reported migraineurs. Headache 1995;35:387-396. Slide 15: 1. The Migraine Market: US Core Therapeutic Report, National Health and Wellness Survey (2006). 2. Data from IMS MAT June 2011. Triptans include Treximet. Analgesic combos = analgesic combos + Cambia. Slide 16: 2. Thieda P. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatric Services 2003: 54;508-516. 1. Stocchi F 2009 (The therapeutic concept of continuous dopaminergic stimulation (CDS) in the treatment of Parkinson’s disease. Parkinsonism Related Disorders. 2009 Dec;15 Suppl 3:S68-71). 19 |