QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| (Mark One) | |
| ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2007 |
OR |
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to |
Commission file number: 001-33204
OBAGI MEDICAL PRODUCTS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | | 22-3904668 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
310 Golden Shore, Long Beach, CA 90802 |
(Address of principal executive offices) |
(562) 628-1007
(Registrant's telephone number, including area code)
Not applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
Title of class
| | Name of each exchange on which registered
|
|---|
| Common Stock, par value $0.001 per share | | Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
Not applicable
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yeso Noý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yeso Noý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesý Noo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer, as defined in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer ý |
Non-accelerated filer o
(Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yeso Noý
Based on the closing price of $17.72 of the registrant's Common Stock on the Nasdaq Global Market on June 29, 2007 (the last business day of the registrant's most recently completed second fiscal quarter), the aggregate market value of the voting common equity held by non-affiliates of the registrant on that date was approximately $104,496,878.
As of February 25, 2008, there were 22,649,120 shares of the Registrant's common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference from the definitive proxy statement for the Annual Meeting of Stockholders to be held on June 7, 2008.
OBAGI MEDICAL PRODUCTS, INC.
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
| |
| | PAGE
|
|---|
|
|
PART I |
|
|
Item 1. |
|
Business |
|
3 |
Item 1A. |
|
Risk Factors |
|
30 |
Item 1B. |
|
Unresolved SEC Staff Comments |
|
47 |
Item 2. |
|
Properties |
|
47 |
Item 3. |
|
Legal Proceedings |
|
47 |
Item 4. |
|
Submission of Matters to a Vote of Security Holders |
|
47 |
|
|
PART II |
|
|
Item 5. |
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
|
48 |
Item 6. |
|
Selected Financial Data |
|
50 |
Item 7. |
|
Management's Discussion and Analysis of Financial Condition and Results of Operations |
|
51 |
Item 7A. |
|
Quantitative and Qualitative Disclosures About Market Risk |
|
69 |
Item 8. |
|
Financial Statements and Supplementary Data |
|
69 |
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
69 |
Item 9A. |
|
Controls and Procedures |
|
69 |
Item 9B. |
|
Other Information |
|
70 |
|
|
PART III |
|
|
Item 10. |
|
Directors, Executive Officers and Corporate Governance |
|
70 |
Item 11. |
|
Executive Compensation |
|
70 |
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
70 |
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
70 |
Item 14. |
|
Principal Accountant Fees and Services |
|
70 |
|
|
PART IV |
|
|
Item 15. |
|
Exhibits and Financial Statement Schedules |
|
71 |
2
Forward-looking statements
We have made forward-looking statements in this Annual Report on Form 10-K, including the sections entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations and Business," that are based on our management's beliefs and assumptions and on information currently available to our management. Forward-looking statements include the information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, the effects of future regulation and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by the use of forward-looking terminology such as the words "believe," "expect," "anticipate," "intend," "plan," "estimate" or similar expressions.
Forward-looking statements involve risks, uncertainties and assumptions. Actual results may differ materially from those expressed in the forward-looking statements. We do not have any intention to update forward-looking statements after this Annual Report of Form 10-K is filed. You should understand that many important factors, in addition to those discussed elsewhere in this Annual Report on Form 10-K, could cause our results to differ materially from those expressed in the forward-looking statements.
PART I
ITEM 1: BUSINESS
Corporate information
Dr. Zein Obagi founded WorldWide Product Distribution, Inc. in 1988. OMP Acquisition Corporation was formed as a California corporation in October 1997 to purchase substantially all of the assets and to assume the accounts payable and related operating liabilities of WorldWide Product Distribution, Inc. and subsequently changed its name to Obagi Medical Products, Inc. in December 1997. OMP, Inc. was incorporated in Delaware in November 2000 and, in January 2001, Obagi Medical Products, Inc. was merged into OMP, Inc., with OMP, Inc. as the surviving corporation. In December 2004, the stockholders of OMP, Inc. exchanged their shares of OMP, Inc. for an equal number of shares in a newly formed holding company incorporated in Delaware, Obagi Medical Products, Inc., which became the parent holding company for all existing operations.
Our principal executive offices are located at 310 Golden Shore, Long Beach, California 90802 and our telephone number is (562) 628-1007. Our website address is http://www.obagi.com. Copies of our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our other reports filed with the Securities and Exchange Commission ("SEC"), can be obtained, free of charge as soon as reasonably practicable after such material is electronically filed with, or furnished to the SEC, by calling Ina McGuinness at Integrated Corporate Relations at (310) 954-1100, through the SEC's website by clicking the SEC Filings link from the Investor Relations page on our website at www.obagi.com or directly from the SEC's website at www.sec.gov. Our website and the information contained therein or connected thereto are not intended to be incorporated into this Annual Report on Form 10-K.
Overview
We are a specialty pharmaceutical company focused on the aesthetic and therapeutic skin health markets. We develop and commercialize prescription-based, topical skin health systems that enable physicians to treat a range of skin conditions, including pre-mature aging, photo-damage, hyperpigmentation (irregular or patchy discoloration of the skin), acne and soft tissue deficits, such as fine lines and wrinkles. Our products are designed to improve the underlying health of patients' skin, and our clinical studies have demonstrated that the use of our Obagi Nu-Derm System results in skin
3
that looks and acts younger and healthier. We focus our research and development activities on improving the efficacy of established prescription and over-the-counter ("OTC"), therapeutic agents by enhancing the penetration of these agents across the skin barrier using proprietary technologies collectively known as Penetrating Therapeutics. Through our own domestic sales force and foreign distribution partners, we market and sell physician-dispensed skin care systems directly to plastic surgeons, dermatologists and other physicians who are focused on aesthetic and therapeutic skin care. We are the market leader in the growing physician-dispensed skin care channel, according to an independent 2007 study by Kline & Co., an independent market research firm. Our net sales have grown from approximately $35.6 million in 2001 to approximately $78.0 million in 2006 to $102.6 million for the year ended December 31, 2007.
We currently market and sell a range of systems and related products for the enhancement of skin health. Our leading product line is our Obagi Nu-Derm System. This system was launched in 1988, and since that time, we have made substantial enhancements to the system through the application of our Penetrating Therapeutics technology. We believe that our Obagi Nu-Derm System is the only clinically proven, prescription-based topical skin health system on the market that has been shown to enhance the skin's overall health by correcting photo- damage using drugs that, by definition, work at the cellular level, resulting in a reduction of the visible signs of aging.
In 2004, we launched the Obagi-C Rx System, which we believe is the only prescription-based system that reduces the early effects of sun damage and evens skin tones through the use of Vitamin C serum combined with 4% hydroquinone. We are the sole licensee of certain Avon patents relating to this technology. In 2005, we launched Professional-C, a series of high potency antioxidant Vitamin C serums that help to counteract the effects of ultraviolet radiation and other environmental influences. Professional-C represents an improved product line with more effective skin barrier penetration replacing our Vitamin C serum offerings marketed under the Cffectives and Obagi-C brands that we introduced in 2000.
In July 2006, we launched Obagi Condition and Enhance targeted for use with Botox (Botox is a registered trademark of Allergan, Inc.). This system provides adjunctive therapy following Botox injections, and is designed to enhance aesthetic outcomes and improve overall patient satisfaction.
In October 2006, we introduced the first product in the Obagi ELASTIderm™ ("ELASTIderm") product line, ELASTIderm Eye Cream, a nighttime eye cream for treatment of skin laxity around the eye. In February 2007, we launched a second product in the ELASTIderm line for daytime use, ELASTIderm Eye Gel, to use along with our ELASTIderm Eye Cream, as an eye therapy system. ELASTIderm products are formulated with a patent pending bi-mineral complex, which is clinically shown to help the body's own natural ability to increase epidermal thickness, augment hypodermal fat and increase elastin levels.
In February 2007, we launched Obagi CLENZIderm M.D.™ ("CLENZIderm") for normal to oily skin, a system of products for acne treatment, featuring a novel formulation of Benzoyl Peroxide ("BPO"). Our clinical studies have shown that CLENZIderm M.D. penetrates more readily into the skin follicle than current creams or gels because it is in solution form. We believe that our solution-based acne system is a more effective treatment for acne because a greater amount of the active ingredient, BPO, will penetrate the hair follicle to more rapidly kill P. acne bacteria. In July 2007, we launched CLENZIderm M.D. for normal to dry skin.
In addition, we offer tretinoin, a generic equivalent to Retin-A, which has been among the most widely used acne treatments for approximately 25 years. We currently distribute a Food and Drug Administration ("FDA"), approved generic equivalent in the physician-dispensed skin care channel under an exclusive license agreement with Triax Pharmaceuticals, LLC ("Triax"). We also sell the Obagi Blue Peel which has been cited by Kline & Co. as one of the most well known brands for use in physician-strength facial peel procedures. While the Obagi Blue Peel products are not dispensed for
4
daily home use in a system and are therefore not a significant source of our revenue, they are used to aid the physician in skin peeling activities. Acceptance and awareness of the Obagi Blue Peel among physicians give it an intrinsic value as a marketing tool in driving new account growth.
We believe that our products have the potential to be used in a number of applications and procedures beyond their current use. For example, our Obagi Condition and Enhance Systems may complement and enhance patient outcomes in commonly performed cosmetic procedures, such as Botox injections, as well as improve wound appearance and reduce the post-inflammatory hyperpigmentation that typically follows laser therapy and basal cell carcinoma excisions, and we are conducting clinical studies to evaluate the adjunctive use of our systems before and after these types of procedures. We expanded this initiative in 2005, and are working with physicians and physicians' associations to evaluate the use of our Obagi Nu-Derm System in enhancing skin healing in ablative and non-ablative laser procedures. In 2005 and 2006, we conducted a study of physician and patient evaluations of skin quality on patients using our Obagi Nu-Derm System in conjunction with other cosmetic facial procedures. Based on the results of this clinical use study of over 2,600 patients, we launched the Obagi Condition and Enhance Systems. These systems are positioned under the Obagi Condition and Enhance brand, with an initial focus on use in conjunction with Botox injections. We are using this data to evaluate possible individual clinical studies on the use of Obagi Condition and Enhance with specific procedures. We plan to continue to build clinical support for the benefits of our systems in conditioning the skin and enhancing the outcomes of the most commonly performed cosmetic procedures, such as chemical peels, dermabrasion, and laser resurfacing, as well as the aesthetic and wound healing aspects of surgical procedures such as basal cell carcinoma excisions.
We are engaged in an active development program using our Penetrating Therapeutics technology to enhance the efficacy of established FDA-approved and OTC active ingredients. Positive findings from completed pilot studies of these new systems may not be duplicated in the larger studies that we are currently completing, or the incidence of side effects in these larger studies may force us to reformulate our products. We will continue to seek additional market opportunities where we believe we can improve the effectiveness of existing products through the application of our Penetrating Therapeutics technology to address conditions such as acne, skin elasticity, rosacea, fungal infections, dermatitis, psoriasis, hair loss and hair removal.
We also advance our development objectives through product and license agreements with third parties. These agreements may include patent and technology licenses, product licenses and new product collaboration agreements. For example, we have developed and are continuing to develop products for the regeneration of elasticity in skin which are covered by claims contained in a patent application which we license from JR Chem LLC ("JR"). Our initial focus was centered on products applied around the eyes, and we will continue to focus on products applied on the neck and on the back of the hands, where the break down in skin elasticity is most visible in aging skin. The first products to result from our collaboration with JR are the products in our ELASTIderm product line. In addition, in December 2005, we entered into a product supply and collaboration agreement with Triax for the supply of certain of its tretinoin products and to provide Obagi-branded tretinoin products in various concentrations.
In the United States, we sell our systems and related products directly to physicians through our internal sales force, which as of December 31, 2007 consisted of 131 sales, marketing and education specialists, including 100 direct sales representatives and managers. Physicians dispense our products in-office, directly to their patients, a distribution method commonly referred to as the "physician-dispensed" channel. We believe that the physician-dispensed distribution model ultimately results in higher patient satisfaction because it is better suited to the provision of system-based skin care than traditional drug distribution channels. Our physician customer base consists primarily of plastic surgeons and dermatologists, but also includes an increasing number of physicians from other practice
5
areas, such as internal medicine and obstetrics and gynecology ("OBGYN"), who are adding skin care to their practices.
As of December 31, 2007, we sold our products to approximately 5,200 accounts in the United States, which we believe represented over 8,000 individual practicing physicians. Based on a 2007 study by Kline & Co., we are the leading skin health company in the physician-dispensing channel, with an estimated 26.4% market share and nearly three times the market share of the next largest competitor. Outside the United States, we utilize distribution partners for the sale of our systems and related products. As of December 31, 2007, we currently have 13 distribution partners who sell our products through their own dedicated sales forces in approximately 36 countries.
We also compete in the Japanese retail skin care markets through a strategic licensing agreement with Rohto Pharmaceutical Co., Ltd. ("Rohto"). Rohto is a Japanese pharmaceutical manufacturer and distributor. Under our agreement, Rohto is licensed to manufacture and sell a series of OTC products under the Obagi brand name, including Obagi-C (a Vitamin C based topical serum in various concentrations), in the Japanese drug store channel and we receive a royalty based upon sales of Obagi branded products in Japan by Rohto. We have other licensing arrangements in Japan to market and sell OTC product systems under the Obagi brand, both for in-office use in facial procedures, as well as for sale as a take-home product kit in the spa channel. Our net licensing revenue from skin health systems and products in Japan was approximately $4.7 million for the year ended December 31, 2007.
Background
Skin damage and disorders
The skin is the largest organ in the body, consisting primarily of two layers: the epidermis, a thin outer layer; and the dermis, a relatively thick inner layer. The epidermis is comprised of specialized cells such as keratinocytes and melanocytes. Keratinocytes are formed in the epidermis and travel to the skin's surface and are exfoliated, or shed off, as they die in a maturation cycle which normally takes approximately six weeks. Buildup of excess keratinocytes can result in rough, thick or dry skin. Melanocytes produce melanin, the pigment that determines skin color and protects the body from ultraviolet radiation. The dermis is comprised largely of connective tissue fibers made of collagen and elastin. Collagen is a tough, fibrous protein that helps give skin its strength and resiliency. Elastin is a tissue that helps maintain healthy skin tension and gives skin its shape, but does not readily regenerate post-puberty and degrades over time. As the elastin degrades, skin tone and elasticity become diminished, resulting in loose, sagging skin.
The health and appearance of a person's skin is impacted by a variety of intrinsic and extrinsic factors, including pre-mature aging, photo-damage, hormones, stress, pollutants, diets and skin diseases. These factors cause newly created cells to be damaged which leads to an increase in the skin cell maturation cycle. The result is that skin cells are disorganized and pigment cell activity is increased. The damaged epidermal cells cause a wide variety of conditions such as mottled pigmentation (varied pigment density across the skin), melasma (skin discoloration often caused by hormonal changes such as those from pregnancy), age spots, fine lines and dry thickened sallow skin. In the dermis of extrinsic or intrinsic aged skin, the amount of new collagen and elastin produced decreases, resulting in fibers that do not support the structure and the dermis becoming thinner. As a result deep lines, wrinkles and sagging skin make the appearance of skin significantly worse. Skin health is also impacted by diseases such as acne, rosacea, dermatitis, and psoriasis. Imbalanced production of skin oils such as sebum encourages accelerated growth of microbes in the skin such as Propionibacterium acnes ("P. acne"). The skin can also become host to viral or fungal infections.
While these conditions and diseases are not life-threatening, they are readily apparent, sometimes disfiguring, usually chronic, and can be debilitating in terms of a person's self image and confidence. As
6
a result, people are often highly motivated to seek treatment programs to restore the look and feel of their skin.
The skin care market
In 2005, the global skin care market was estimated to be $36.2 billion, of which over 62% were facial skin care products, according to Global Industry Analysts, Inc., a market research firm. Additionally, the independent research firm, Kalorama Information, estimates that from 2005 to 2010, over 70 million people in the United States alone will receive cosmetic facial procedures for which they will pay over $60 billion. We believe this reflects a growing desire and acceptance among the aging population to seek aesthetic facial products and procedures from their physicians. A key driver of this trend is the aging of the "baby boomer" segment of the U.S. population. In addition, life expectancy in the United States has extended in recent years, leading to a further increase in the average age of the country's population. Because healthcare needs, including the treatment of skin disorders, tend to increase with age, we expect the demand for dermatologic products to continue to increase over time. In particular, women tend to demonstrate a higher motivation than men to improve their personal appearances. The number of women between the ages of 35 and 65, the primary users of our products, was estimated by the U.S. Census Bureau to have grown 30% between 1990 and 2006. With this segment's strong desire to reduce the signs of pre-mature aging, we expect the aging female population to continue to increase the market opportunity for skin care products.
Consumer demand for physician-dispensed skin care products and procedures has been steadily growing. We believe this growth is due to consumers realizing that many non-prescription consumer cosmetic products are unable to fully meet their needs. Consumers have increasingly turned to their physicians for products and simple in-office procedures that can provide better results than consumer cosmetics. For example, physician-directed cosmetic products and commonly performed cosmetic procedures such as Botox injections, laser hair removal and microdermabrasion (a cosmetic procedure that removes the outermost layer of the skin to promote skin rejuvenation), have experienced substantial growth as consumers learn that they can achieve positive cosmetic results with minimally invasive techniques. According to the American Society of Plastic Surgeons ("ASPS"), the number of minimally-invasive cosmetic office procedures performed increased 85% from approximately 4.9 million in 2002 to approximately 9.1 million in 2006. This increase was led by facial procedures such as Botox injections, up 264% in 2006 compared to 2002, and injectable fillers, increased 69% in 2006 compared to 2004.
Beyond anti-aging and aesthetic treatments, there is significant market demand for effective treatments of skin diseases such as acne, rosacea, psoriasis, and eczema (dermatitis). While a number of therapies and treatments exist for such diseases, most treatments consist of either topical applications with efficacy that is limited by their inability to cross the skin barrier effectively, or systemic (oral) applications that carry significant potential side effects.
According to Kline & Co., in 2007, there were approximately 45,000 physicians practicing dermatology and plastic surgery in the United States. Based on our experience with physicians who have opened accounts with us, we believe a growing number of general practice, family practice, and OBGYN physicians are continuing to dedicate resources in their practices to skin care. We believe that these physicians are responding to the rapid increase in consumer demand for non-invasive skin care treatments. Furthermore, many of these physicians are dispensing prescription and non-prescription skin care products directly to their patients. According to a 2007 Kline & Co. study, a combined number of approximately 11,200 of the physicians practicing dermatology and plastic surgery dispensed skin care products directly to their patients.
Outside the United States, the physician-dispensed skin care market varies by country due to cultural differences and regulatory variations. Cultural desires for skin with lighter and more even
7
pigmentation have created large and growing aesthetic skin care demand across the Pacific Rim countries, particularly Japan, China and Korea. European and certain South American countries such as Brazil also present large skin care markets due to the complementary growth in cosmetic procedures and willingness on the part of their consumers to spend discretionary income on aesthetic enhancements. We believe that the growth in major international markets will also be driven by cultural desires to lessen the appearance of skin darkening caused by exposure to sun, aging populations and a heightened awareness and acceptance of physician-dispensed products and procedures. Additionally, while physician dispensing is common in most countries, certain countries prohibit or limit the types of products that can by dispensed from the physician office, requiring physicians to either partner with a retail pharmacy or drug store, or to simply forgo dispensing.
Limitations of traditional products and procedures
Most of the cosmetic skin care products and procedures available today are designed to mask the effects of aging and skin disorders, rather than treat the underlying health of the skin. As a result of using these cosmetic skin care products, consumers may see temporary skin surface improvements, but underlying skin restoration often does not occur. We believe that the limitations of traditional products and procedures result primarily from the following causes:
- •
- The outer layer of human skin is a highly effective protective barrier against the entry of foreign particles into the body. The active agents in many competing topical products and procedures lack the ability to effectively penetrate the skin barrier, reducing their ability to improve the health of the skin at the stratum corneum, epidermis and dermis level. The most commonly used skin care products are cosmetics by definition under the U.S. Food, Drug and Cosmetic Act ("FDCA"), consisting largely of surface covers and moisturizers, which only add water to the cells on the surface of the skin, providing superficial and temporary improvement in the appearance of the skin. Moisturizers are not capable of causing the skin to generate healthy new cells to replace older, damaged ones that make up the epidermis.
- •
- Most traditional approaches to skin care are not comprehensive programs designed to integrate complementary products. As a result, individual products, even those that are widely used by consumers (such as facial soaps or sunscreens) are not generally designed to work together, and therefore may cause unintended side effects or reduced effectiveness when used in combination. Furthermore, the range of skin types in any given patient population is highly varied and different skin types respond differently to treatment, yet few products are capable of treating the specific needs of the individual patient's overall skin health.
The Obagi Medical Products approach
We believe the effects of aging and skin disorders are best addressed not at the surface of the skin but at a deeper level, where the skin's natural regeneration processes occur. Our Obagi Nu-Derm and Obagi-C Rx Systems improve the overall health of the skin by improving cellular processes such as collagen and elastin production, keratinocyte clearing, and melanocyte regulation, using drugs that, by definition, work at the cellular level. With improved underlying skin health, we believe a patient's skin shows fewer signs of aging, is less susceptible to disease, and is better able to combat exposure to the elements. We have developed skin care systems that we believe address the limitations of traditional skin care products and procedures, including the following:
- •
- Our Obagi Nu-Derm and Obagi-C Rx Systems are drug-based systems designed to penetrate below the skin's surface to correct damage in all layers of the skin (the stratum corneum, the epidermis and the dermis) and accelerate cellular turnover. We have demonstrated in clinical studies that by enhancing the penetration of the intended active ingredient (tretinoin), more of this drug gets to the targeted tissue, improving patient outcomes. The increased penetration of a
8
system of active ingredients triggers a therapeutic cascade that (i) pushes fresher cells to the surface faster, resulting in smoother skin, reduced wrinkles and increased tolerance, (ii) corrects current hyperpigmentation (including freckles and age spots) and prevents the appearance of new hyperpigmentation, (iii) promotes more uniform cells at the deepest layer for better skin structure and balanced, even skin tone, (iv) helps stimulate collagen and elastin for firmer, more resilient skin, and (v) helps increase natural hydration and circulation for supple, healthy-looking skin.
- •
- To achieve improved skin health, our Penetrating Therapeutics technology integrates proprietary formulations of existing prescription and non-prescription skin care products into treatment programs specially designed and physician-tailored to address the unique needs of each patient's skin. The individual products within our systems are formulated to work synergistically using our proprietary Penetrating Therapeutics technology in formulations that enhance the stability and efficacy of what are often otherwise unstable molecules. When this system of products is applied within a physician directed protocol tailored to the patient's skin health needs, overall penetration of the active ingredient to the appropriate layer of skin is achieved, resulting in greater efficacy and improved patient outcomes.
Our business strategy
Our objective is to become the leading specialty pharmaceutical company dedicated to developing and commercializing systems that enable physicians to improve skin health at the cellular level. Key elements of our strategy include:
- •
- Leveraging the strength of our physician-dispensed marketing and distribution channel to increase market share and introduce new Obagi products. We believe that our market-leading position in the physician-dispensed skin care channel presents us with the opportunity to increase the market share of our existing products and to launch a range of new Obagi Systems and products. We have built long-term relationships with skin health professionals based on the success of our products during the 20 years since the first Obagi Systems and products were launched. We will continue our sales and marketing efforts aimed at helping physicians understand how our systems' products can meet growing patient demand for effective skin care treatments, thereby generating additional sources of revenue for physician practices. Furthermore, we believe that our systems' product offerings and our experienced sales force uniquely position us to benefit from growth in the number of physicians who dispense skin care products directly to their patients. According to Kline & Co., in 2007 only about 25% of the approximately 45,000 physicians practicing plastic surgery and dermatology in the United States dispense professional skin care products. According to the results of a study we sponsored by Wirthlin Worldwide Company, an additional 40% of non-dispensing surveyed plastic surgeons and dermotologists indicated that they are considering dispensing skin care products. Based on our experience with physicians who have opened accounts with us, we believe a growing number of general practice, family practice, and OBGYN physicians are also dedicating resources in their practices to skin care. We believe that these physicians are responding to the rapid increase in consumer demand for non-invasive skin care treatments. Furthermore, many of these physicians are already dispensing prescription and non-prescription skin care products directly to their patients.
- •
- Continuing to develop and market new indications for Obagi Systems. We believe a significant opportunity exists to use current Obagi Systems as non-invasive adjunctive therapies to improve certain current skin care procedures, resulting in overall better patient outcomes and satisfaction. Many cosmetic procedures are limited in their ability to provide healthier skin and an overall enhanced aesthetic outcome. For example, Botox injections for reducing wrinkles have no effect on the skin's color, hyperpigmentation, age spots, acne or the overall health of the skin. We believe patients who are treated with our Obagi Systems following Botox injections will achieve
9
greater overall aesthetic outcomes and satisfaction. We conducted a clinical study to evaluate the adjunctive use of our systems before and after these types of procedures, and based on outcomes for over 2,600 patients, we launched the Obagi Condition and Enhance Systems, which have an initial focus on use in conjunction with Botox injections. We plan to continue to build clinical support for the benefits of our systems in conditioning the skin and enhancing the outcomes of the most commonly performed cosmetic procedures, such as chemical peels, dermabrasion and laser resurfacing.
- •
- Creating additional clinically proven Obagi Systems that increase the efficacy of commonly prescribed dermatological agents in addressing new areas of skin disease. We focus our research and development efforts on increasing the ability of FDA-allowed skin agents to penetrate the skin barrier, thereby increasing the effectiveness of such agents within the Generally Recognized As Safe, ("GRAS"), OTC or Drug Efficacy Study Indication ("DESI"), classification as defined by the FDA. This approach accelerates the commercialization timeline and avoids the lengthy clinical development processes typically required to obtain new drug approvals. We intend to further differentiate our products and systems by supporting them with randomized and comparative clinical studies conducted by leading experts in the markets in which we are developing products. Supporting this strategy we initiated an aggregate of 18 clinical studies in 2005 and 2006 in the areas of acne and elasticity, and we initiated eight clinical studies in 2007 in the areas of acne and aesthetics.
- •
- Establishing new strategic collaborations and relationships. We intend to continue accessing new and complementary products through in-licensing, strategic collaborations and strategic acquisitions. We also intend to explore new product distribution partnerships in high growth channels such as the spa and salon channel, in which manufacturers' sales of skin care products are estimated at over $508 million in 2007, according to Kline & Co. We plan to target new products and channels, which will expand the Obagi brand and Obagi System concept but will not compete directly with the physician-dispensed skin care channel or products. We believe that skin health professionals will be receptive to new products we introduce under the Obagi brand name, and that the brand credibility that exists among skin health professionals will allow for more rapid trial and acceptance. This belief is supported by a 2003 study we sponsored by Wirthlin Worldwide Company of 1,000 medical professionals (primarily consisting of plastic surgeons, dermatologists, and medical skin care professionals), which found that the Obagi brand had the highest total awareness, at 97%, among leading physician-dispensed brands.
- •
- Continuing to expand intellectual property protection. Our intellectual property protection is based on a combination of issued patents, patent applications, licensed patents, licensed product methods and technologies and trade secrets. As of December 31, 2007, we were the sole licensee of four patents, and have filed 39 U.S. provisional and non-provisional patent applications since the beginning of 2004. We will continue to pursue additional invention and method patents as we find new applications and improvements to our existing intellectual property. We also pursue an aggressive trademark registration policy as a means to increase brand recognition and product differentiation in the market.
Our Obagi Systems and Related Products
We currently market and sell our systems and related products to physicians for the treatment of age-related skin disorders, incorporating a range of individual prescription and non-prescription therapeutic agents, as well as cosmetic ingredients. The individual components of each system have been formulated to complement one another, enhancing the effectiveness of the system as a whole and allowing the physician to tailor the treatment program to the specific needs of the patient. The design
10
of our systems is proprietary to us, and we are the sole licensee of U.S. patents and have patent applications for the composition of certain of the products.
System and related products
| | Segment/product category
| | Description
| | Applications
| | Launch date
|
|---|
Obagi Nu-Derm System |
|
Skin Health/Nu-Derm |
|
Comprehensive system of six products including prescription and OTC drugs |
|
Fine lines, wrinkles, acne, photo -damage, hyperpigmentation, melasma, laxity, skin sallowness |
|
1988 |
Obagi Condition and Enhance Systems |
|
Skin Health/Nu-Derm |
|
Line extension of Nu-Derm designed for use after non-surgical cosmetic procedures |
|
Enhances patient outcomes and patient satisfaction |
|
2006 |
Obagi-C Rx System |
|
Skin Health/Vitamin C |
|
Highly stable Vitamin C serum with 4% hydroquinone system; prescription-based |
|
Fine lines, wrinkles, hyperpigmentation, skin sallowness |
|
2004 |
Professional-C and Cffectives |
|
Skin Health/Vitamin C |
|
Highly stable Vitamin C serums; non-prescription |
|
Antioxidant protection, fine lines, wrinkles, hyperpigmentation |
|
2005 |
ELASTIderm System |
|
Skin Health/Skin Laxity |
|
System of skin health products built around a novel formulation of a mineral complex; non-prescription |
|
Increase elasticity and skin tone of eyes, face, neckline and chest |
|
2006 |
CLENZIderm M.D. Systems |
|
Skin Health/Other |
|
Systems for acne treatment built around a novel formulation of Benzoyl Peroxide, or BPO |
|
Acne |
|
2007 |
Tretinoin |
|
Skin Health/Other |
|
Generic equivalent of Retin-A available in the United States through an exclusive license |
|
Acne |
|
2002 |
Obagi Blue Peel System |
|
Skin Health/Other |
|
Topical system to aid in the application of TCA (trichloroacetic acid) chemical peels |
|
Fine lines, wrinkles, hyperpigmentation |
|
1988 |
- (1)
- Professional-C includes improved formulations and higher concentrations of Vitamin C, and replaces our Cffectives and Obagi-C brands that were first introduced in 2000.
11
Obagi Nu-Derm System
Our Obagi Nu-Derm System consists of a combination of six prescription and OTC drugs and adjunctive cosmetic skin care products to treat visible skin conditions such as photo-damage and hyperpigmentation resulting from extrinsic damage and intrinsic changes to the skin. The Obagi Nu-Derm cosmetic skin care products include cleansers and exfoliating creams. Three of these products contain the drug hydroquinone in a 4% prescription concentration, which acts as a bleaching agent that is designed to correct skin pigmentation problems by normalizing the production of new melanin in the epidermis. Physicians may also prescribe the drug tretinoin as a complement to the system, in various concentrations, depending on the physician's judgment of patient need. We believe that the use of these prescription drugs, the ability of the drugs to penetrate the skin's surface and the order of application distinguishes our Obagi Nu-Derm System from other commonly prescribed regimens. While we have designed our Obagi Nu-Derm System to include products that patients can use in a systematic treatment regimen, we also make the component products available for individual sale. We believe that physicians who dispense the Obagi Nu-Derm System generally encourage their patients to use the component products together in a systematic treatment regimen. However, we also believe that some patients elect to use the products that make up the system individually. Products that are used individually at times include the sun screen products and Obagi Nu-Derm Clear, which physicians may dispense on occasion to address localized pigmentation problems. Side effects from use of the products may include redness, mild to moderate irritation and/or excessive flaking or sloughing of the outer layers of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use.
In 2003, we sponsored a 301-subject, six-month, randomized, controlled, two-center, clinical use study that was independently designed and conducted by Thomas J. Stephens & Associates to compare the efficacy, side effects and tolerability of our Obagi Nu-Derm System with other commonly prescribed regimens of tretinoin, hydroquinone and OTC moisturizer. One of our minority stockholders assisted in the preparation of the study. Improvements in perioral (around the mouth) and periocular (around the eyes) fine wrinkles, facial mottled hyperpigmentation, clarity, sallowness, laxity (the appearance of loose, sagging and/or excess skin), and tactile roughness were measured. After 24 weeks of treatment, the mean changes observed with our Obagi Nu-Derm System were consistently larger than, and statistically superior to, the changes produced with the other treatment regimens. Of particular note were the changes in the perioral fine wrinkles, mottled pigmentation and laxity. While treatment with all regimens was generally well tolerated, there was a higher level of both objective and subjective irritation with the Obagi Nu-Derm System that largely resolved by the end of the study.
The Obagi Nu-Derm System, including Obagi Condition and Enhance Systems, accounted for approximately 68% and 61% of our consolidated net sales for the years ended December 31, 2006 and 2007, respectively. We sell our Obagi Nu-Derm System primarily at a single-price. Although volume of sales for each individual product within the system varies, we believe the majority of our sales of each component product is due to the fact that they are sold as part of the system.
Obagi-C Rx System
The Obagi-C Rx System consists of a combination of four prescription and OTC drugs and adjunctive cosmetic skin care products to treat skin conditions resulting from sun damage and the oxidative damage of free radicals. The central ingredients in the system are 4% hydroquinone, a prescription drug, and Vitamin C. This combination distinguishes Obagi-C Rx from other Vitamin C based products available in the physician office. Two Obagi-C Rx System products contain this concentration of hydroquinone, which is designed to correct skin pigmentation problems by normalizing the production of new melanin in the epidermis. The Obagi-C Rx System includes cosmetic skin care cleansers and exfoliating lotions. When combined in a system, we believe hydroquinone, Vitamin C and a sunscreen provide correction of, and protection against, premature skin aging. As with the Obagi
12
Nu-Derm System, the products that make up the Obagi-C Rx System are generally used together in a coordinated regimen by the majority of patients. Side effects from use of these products may include redness and/or mild to moderate irritation of the treated skin. Side effects generally resolve after the first 10 days of use, or in cases with certain sensitive individuals, in a few days upon discontinuance of use. We sponsored an in vitro study at the University of California, Irvine in September 2003 to evaluate the percutaneous absorption and bioavailability of our patented 10% Vitamin C and 4% hydroquinone combination product compared with the leading Vitamin C competitor, SkinCeuticals 20 Vitamin C. Our product demonstrated, with statistical significance, more Vitamin C in all layers of the skin than SkinCeuticals 20 Vitamin C serum.
Obagi Professional-C
The Obagi Professional-C products are a complete line of proprietary, non-prescription products, which consist of Vitamin C serums used to reduce the appearance of damage to the skin caused by ultraviolet radiation and other environmental influences. Vitamin C (L-ascorbic acid) acts as a potent antioxidant. The Obagi Professional-C line consists of a 5% serum for the area around the eyes and 10%, 15% and 20% serums for the face, neck and chest. Professional-C represents an expanded line that replaced our 5% and 10% Vitamin C serum offerings marketed under the Cffectives brand domestically, and Obagi-C brand internationally, as introduced in 2000. Obagi Professional-C products are sold individually and are used on their own, or in combination with other Obagi system products. These products are classified as cosmetics and side effects are not generally associated with their use, however certain sensitive individuals may experience mild irritation of the skin where product is applied. In addition, the product has been shown in studies we sponsored at the University of California, Irvine in September 2003 and July 2005 to penetrate all levels of skin better than SkinCeuticals 20 Vitamin C serum.
Obagi ELASTIderm
Our ELASTIderm product line consists of products for the treatment of skin laxity featuring novel mineral complexes which may help the body's own natural ability to increase epidermal thickness, augment hypodermal fat and increase elastin levels by supplying increased local concentrations of natural mineral actives to the relevant tissue, thereby improving the elasticity and skin tone around the eyes. We believe this is the first clinically proven skin health system to aid in the regeneration of elastin, which we believe is a new and novel topical application for anti-aging. A nine-week treatment, randomized, double-blind clinical study was conducted with 33 subjects to determine the safety and efficacy of our novel under eye elastin regeneration product. The primary endpoints were appearance in under eye skin elasticity, fine line wrinkles and moisturization. A visible improvement in the appearance of fine lines and wrinkles was observed throughout the study, based on photographic evidence. In addition, coarse wrinkles decreased at all time points and reached statistical significance at nine weeks. Statistically significant increases in elasticity were observed at all time points during the study and as early as two weeks following treatment. We believe that a more rapid improvement in elasticity is essential to earning customer compliance and repurchase. Based on clinically significant improvements in measured collagen and elastin in skin treated over the eight week period, we launched a nighttime eye cream product under the Obagi ELASTIderm brand name in mid-October 2006. In February 2007, we launched a daytime eye gel which is the second product under the Obagi ELASTIderm brand.
CLENZIderm M.D.—Acne
We have developed a system of products for acne treatment featuring a novel formulation of BPO which our clinical studies show to penetrate more readily into the skin follicle than current creams or gels because it is in solution form. We believe that our solution-based acne system will be a more effective treatment for acne because a greater amount of the active ingredient, BPO, will penetrate the
13
hair follicle to act on P. acne bacteria. We conducted several clinical studies that demonstrated that our novel BPO alone achieved greater intrafollicular and skin surface bactericidal activity (or kill) against P. acnes than both a prescription generic BPO formulation and a prescription BPO/antibiotic combination product. We believe these studies show the efficacy of our novel BPO formulations in reducing P. acnes and clearing visible acne lesions on the skin. Additionally, in mid-November 2006, we conducted under the CLENZIderm M.D. brand name, a controlled experience trial with select dermatologists who currently prescribe the existing Obagi systems. Based on the results of that trial, we began a full commercial launch of the CLENZIderm M.D. for normal to oily skin in February 2007 and for normal to dry skin in July 2007.
Tretinoin
Tretinoin creams and related adjunctive acne care products are used for the topical treatment of acne in the United States. Tretinoin, the active ingredient in the prescription acne drug Retin-A, is a Vitamin A derivative and has been the primary prescription acne therapy for approximately 25 years. Topical tretinoin normalizes the growth rate of skin cells, disrupting the onset of acne. We offer FDA-approved formulations of tretinoin through an exclusive license in the physician-dispensed skin care channel. Our Tretinoin cream line is available in concentrations of 0.1%, 0.05% and 0.025%. These products are sold individually and are used by doctors as a single therapy, in combination with Obagi Nu-Derm, or in combination with other acne therapies including but not limited to salicylic acid and clindamycin. Side effects include excessively red, edematous, blistered, or crusted skin for certain sensitive individuals. If these effects occur, the medication should either be discontinued until the integrity of the skin is restored, or the medication should be adjusted to a level the patient can tolerate. To date, generally adverse effects have been reversible upon discontinuation of therapy.
Obagi Blue Peel
Obagi Blue Peel is a topical system to aid in the application of trichloroacetic acid ("TCA"), chemical peels used to smooth the surface of skin, improve skin tone and color, diminish wrinkles and shrink pore sizes. Chemical peels are an in-office procedure performed either by a physician or a member of a physician's staff, depending on the skin depth of the peel. During the procedure, acidic solutions are combined in our delivery system and applied to the face to remove the thin surface layers of aged and damaged skin. After removal, the body will naturally replace the removed skin layers with new, healthy skin cells. The Obagi Blue Peel provides for an even application and slows the penetration of the solution into the skin, allowing physicians to more accurately monitor the peel. This produces a more uniform and consistent application, which reduces the risk of complications. We believe that the Obagi Blue Peel is especially effective as a complementary treatment to our Obagi Nu-Derm System. The Obagi Blue Peel products have no known side effects in and of themselves. Patients receiving blue peel products as part of an acid peel procedure can expect to experience side effects that are associated with such procedures.
Expanded Applications for Existing Products
We believe that many of our products have applications in areas beyond their current uses. For example, we are conducting studies to evaluate the adjunctive use of our systems with commonly performed cosmetic procedures such as laser therapy, Botox injections and basal cell carcinoma excisions. In July 2006, we launched our Obagi Condition and Enhance System, for use primarily with Botox injections.
Nu-Derm
Market opportunity. According to the ASPS, the number of minimally-invasive cosmetic office procedures performed increased 85% from approximately 4.9 million in 2002 to approximately
14
9.1 million in 2006. This increase was led by facial procedures such as Botox, up 264% in 2006 compared to 2002, and injectable fillers increased 69% in 2006 compared to 2004.
Our Obagi Nu-Derm System restores skin to a healthier state and helps regulate skin cell functions and improve circulation. This improves the skin's ability to constantly renew itself, repair damage, and act as an effective barrier. It also restores the skin to an active and tolerant state so that it responds better to the trauma of surgical procedures, and is less likely to exhibit an undesirable post-procedure response. Clinicians who use our products have reported decreased recovery periods and reduction in post-inflammatory hyperpigmentation. Another potential advantage of pre-treating patients undergoing laser resurfacing with our Obagi Nu-Derm System is that priming collagen might enhance the response to laser resurfacing, thereby improving cosmetic results. Further, our Obagi Nu-Derm System, when used as adjunctive therapy following Botox injections, has the possibility of enhanced aesthetic outcomes and improved overall patient satisfaction as suggested to us by several dermatologists and plastic surgeons and supported by our physician use study.
Clinical development. In 2005 and 2006, we conducted a large, multi-center, physician use study supporting independent clinical research on our systems. The study entailed physician and patient evaluations of skin quality on patients using our Obagi Nu-Derm System in conjunction with other cosmetic facial procedures. Based on the results of this clinical use study of over 2,600 patients, we launched the Obagi Condition and Enhance Systems. The results indicate enhanced patient outcomes and satisfaction are achieved with combined use of our Obagi Nu-Derm System and several commonly performed cosmetic procedures including Botox and facial laser resurfacing. We plan to continue to build clinical support for the benefits of our systems in conditioning the skin and enhancing the outcomes of the most commonly performed cosmetic procedures, such as chemical peels, dermabrasion and laser resurfacing.
Product line
| | Description
| | Applications
| | Launch date
|
|---|
| Obagi Condition and Enhance for Botox | | Line extension of Nu-Derm System designed for use after facial injections | | Enhances patient outcomes and satisfaction for Botox and other injectible procedures | | 2006 |
Obagi Condition and Enhance for Non-Surgical Cosmetic Procedures |
|
Line extension of Nu-Derm System designed for use with non-surgical cosmetic procedures such as Botox, fillers and lasers |
|
Enhances patient outcomes and patient satisfaction |
|
2007 |
Obagi Condition and Enhance for Surgical Cosmetic Procedures |
|
Line extension of Nu-Derm System designed for use before and after surgical cosmetic procedures |
|
Enhances patient outcomes and patient satisfaction |
|
2007 |
Obagi Condition and Enhance for Botox
Market opportunity. According to ASPS, there were approximately 4.1 million Botox injections performed in 2006. Botox is a non-surgical, physician administered cosmetic treatment that can temporarily reduce moderate to severe frown lines between eye brows (glabellar lines associated with corrugator and/or procerus muscle activity) for up to four months. In order to maintain the results, a patient needs to be injected every three to four months. We are planning to conduct a 12-week multi-center, randomized, single-blind, post-market study comparing the aesthetic outcome of the application of Botox alone to the application of Botox with our post treatment Obagi Condition and Enhance System. The primary endpoints will be additive improvements in periorbital and periocular fine wrinkles, facial mottled hyperpigmentation, skin clarity, sallowness, laxity, tactile roughness and overall patient satisfaction. We initiated this study in 2007 for completion in 2008.
15
Obagi Condition and Enhance for Laser
Market opportunity. We are evaluating and sponsoring studies to evaluate the use of Obagi Nu-Derm products in aiding skin healing in non-ablative and ablative laser procedures, which are procedures using lasers that work primarily by removing the top layer of the skin, while the subsequent layer is heated. These initiatives are based on anecdotal evidence, supplied by physicians who are experienced with our Obagi Nu-Derm System, that indicates the possibility of improved recovery and healing time when our Obagi Nu-Derm System is used with ablative laser procedures. We decided not to pursue the joint experince trial with Syneron in favor of conducting a more formal study with IPL. We are in the final phase of a multi center clinical trial looking at our Condition & Enhance system in conjunction with IPL laser versus the IPL plus a placebo system. This trial is being conducted by two leading physicians in the area of aesthetics and lasers However, there can be no assurance that these studies will be completed or the results will be positive.
Obagi Condition and Enhance for Basal Cell Carcinoma
Market opportunity. According to the American Society for Dermatologic Surgery, approximately 1.7 million procedures to treat skin cancer were performed in the United States in 2005. Basal cell carcinoma ("BCC"), is the most common skin cancer. Electrodessication ("EDC"), and curettage is a commonly employed treatment in which superficial skin cancers are scraped with a curette followed by electrocautery of the site in three successive cycles. Electrodessication and curettage usually leaves a scar and hypopigmented, firm, and demarcated skin. We have initiated a multi-center, randomized, single-blind, controlled study comparing the efficacy of pre-treatment and post-treatment with our Obagi Nu-Derm System with a commonly prescribed regimen of gentle cleanser and emollient on skin healing. The primary endpoints will include improvement in scar appearance, scar formation and hypopigmentation, firmness and skin demarcations.
Clinical development. A nine-week, multi-center, randomized, investigator-blinded, controlled study was conducted in 51 subjects, undergoing Electrodessication and curettage EDC for biopsy-proven superficial truncal basal cell carcinoma to evaluate scar cosmesis outcomes with the Obagi Nu-Derm System versus a commonly prescribed regimen of a gentle cleanser and emollient. Patients were randomly assigned to receive twice-daily treatment with either the Obagi Nu-Derm System or gentle cleanser and emollient. The assigned treatment was applied to the wound site for three weeks before and three weeks after EDC (post-EDC therapy began at least two weeks after EDC, once patients had³ 75% re-epithelialization). Wound cosmesis was evaluated at the end of treatment using a modified version of the validated Beausang scale. At the end of treatment, 72% of the wound sites treated with the Obagi-Nu-Derm System were determined to be a treatment success (i.e. excellent or good wound appearance) according to the investigator global assessment, the primary end-point of the study, versus 63% of the wounds treated with the gentle cleanser and emollient. One-hundred percent (100%) of the wounds treated with the Obagi Nu-Derm System showed no or mild distortion (wound contraction) versus 93% with the gentle cleanser and emollient. Photographs of the post-treatment wound sites were evaluated for treatment success, based on the study's primary endpoint, by an independent panel of masked expert dermatologists and plastic surgeons. The expert panel scored 36% of the Obagi Nu-Derm System treated wounds as a success versus 16% for the gentle cleanser and emollient.
Based on the results of this study, further research on the potential benefits of pre- and post treatment with the Obagi Nu-Derm system to improve wound cosmesis in a range of surgical procedures is planned.
16
New Products and Product Lines
ELASTIderm—Anti-Aging
Market opportunity. Elastin is a protein found in the dermis which allows the skin to resume its normal shape after stretching. As skin ages, it loses its elasticity and begins to sag, particularly around the eyes, on the neck and on the hands. According to Kline & Co., in 2007, the eye, hand and neck skin care market in the spa and salon channel was estimated at over $178 million per year, comprised of products that are primarily designed to improve skin appearance through added moisturization, or building collagen in the skin. We conducted a soft-launch of an eye cream product under the Obagi ELASTIderm brand name in mid-October 2006, limited to our aesthetic sales force and targeted for existing accounts. In February 2007, we announced the formal launch of an ELASTIderm Eye Therapy, including both the eye cream and a new eye gel product. In January 2008, we announced the launch of ELASTIderm Décolletage System, a system designed to treat mottled hyperpigmentation, including age spots and freckles, while reducing the appearance of fine lines and wrinkles on the chest and neck area.
We are also evaluating the use of future ELASTIderm products on other areas of the body which may help the body's own natural ability to increase epidermal thickness, augment hypodermal fat and increase elastin levels by supplying increased local concentrations of natural mineral actives to the relevant tissue, thereby improving the elasticity and skin tone. These areas may include the hands, full face, arms and other areas of the body. However, there can be no assurance that additional products will be successfully developed in the future. The level of commercial acceptance of the recently introduced products cannot yet be determined.
Clinical development. A nine-week treatment, single blind clinical study was conducted with 33 subjects to determine the efficacy of our ELASTIderm product which contains a bi-mineral complex known as "copper zinc malonate." The primary endpoints were appearance in under eye skin elasticity (Cutometer® Evaluation), fine line wrinkles (Skin Replica Analysis) and moisturization (Corneometer® Evaluation). Twice daily application of this novel bi-mineral complex resulted in significant improvements in elasticity as early as week two. Improvements in elasticity increased with each successive visit, with a peak 46% improvement relative to baseline at week nine. P values in each week of the study ranged from .01 to ..001, and were considered statistically significant.
A significant reduction in the number of coarse periorbital (skin around the eyes) wrinkles was also evident, in objective measurements of wrinkles using soft silicone impressions, which showed a maximal reduction of 16% (p = 0.05) at week nine. Subjective feedback further showed a 214% increase, from baseline, in the number of subjects that considered their periorbital wrinkles to be not perceptible or mild to barely noticeable at week nine. Moreover, standardized digital photography demonstrated visible improvements in the appearance of periorbital fine lines and wrinkles in as little as two weeks. Our clinical results provide objective and subjective support that our novel bi-mineral complex was effective at improving photodamaged (sun damaged) periorbital skin elasticity and fine lines and wrinkles. Previously mentioned quantitative measurements of elasticity were supported by subjective feedback that demonstrated a 75% increase, from baseline, in the number of subjects that considered the lax/loose appearance of their periorbital skin to be non-existent or mild to barely noticeable at week nine.
In 2007, we initiated a 24-week single-centerd, investigator-blinded, randomized study to evaluate the efficacy and tolerability of the ELASTIderm Décolletage System (Wrinkle Reducing Lotion and Skin Lightening Complex). Key efficacy variables being evaluated are lentigines, mottled hyperpigmentation, fine and coarse wrinkling, tactile roughness, crepiness and laxity. A variety of photographic techniques are being used to quantitatively and qualitatively evaluate the reduction in photo-damage and hyperpigmentation in the décolletage (chest and neck) area. In addition, elasticity is being measured by quantitative methods.
17
CLENZIderm M.D.—Acne
Market opportunity. Acne is an inflammatory disease of the skin that results from a blockage of follicles or pores, as a consequence of accumulating dead skin cells and sebum, together which create a perfect medium for bacteria. The bacterium, P. acne, is a gram positive anaerobic bacterium that colonizes the sebaceous follicles and is implicated in the pathogenesis of acne. The prescription topical anti-acne market primarily includes BPO as a single active ingredient, or monotherapy product, BPO combination products (with antibiotics), topical retinoids, such as Retin-A, and topical antibiotics. Kalorama projects that the global market for prescription acne and rosacea drugs, including BPO, retinoids, and antibiotics, will increase 6% from $1.6 billion to $1.7 billion over the five years from 2006 to 2011.
Current formulations of BPO are emulsions, comprised of large, highly insoluble particles which do not pass easily into the skin follicle to treat the underlying causes of acne. As a result, the efficacy of existing formulations of currently marketed BPO products is limited. We have developed proprietary technologies that we believe will increase the ability of BPO to penetrate the epidermis and compromised sebaceous follicle.
Obagi CLENZIderm M.D. We have developed a system of products for acne treatment featuring a novel formulation of BPO which our clinical studies show to penetrate more readily into the skin follicle than current creams or gels because it is in solution form. We believe that our solution-based acne system will be a more effective treatment for acne because a greater amount of the active ingredient, BPO, will penetrate the hair follicle to act on P. acne bacteria.
Clinical development. In 2007, we conducted several clinical studies with our CLENZIderm M.D. Systems (Normal to Dry and Normal to Oily) versus the leading prescription BPO/antibiotic combination products.
We completed a 4-week multi-centered, randomized investigator-blinded study of CLENZIderm M.D. (Normal to Oily) versus a leading prescription BPO/antibiotic product was conducted. The primary endpoints for this study were the reduction in non-inflammatory and inflammatory lesions and tolerability with monotherapy using the System's BPO Serum Gel alone versus the combination product. The study showed that when compared to twice-daily treatment with a leading prescription BPO/clindamycin combination product, twice-daily monotherapy with Obagi's solubilized Serum Gel resulted in a greater and more rapid reduction in the number of non-inflammatory and inflammatory lesions (42% versus. 28% and 70% versus. 61%, respectively) at week 4.
We also completed a 4-week multi-centered, randomized investigator-blinded study of CLENZIderm M.D. (Normal-to-Dry Skin) versus a leading prescription BPO/antibiotic product was conducted. The primary endpoints for this study were the reduction in non-inflammatory and inflammatory lesions and tolerability with once daily use of the System versus the prescription BPO/antibiotic. In this study, the investigators found that, compared to the combination product, the once-daily use of CLENZIderm M.D. System for (Normal-to-Dry) skin provided comparable improvements by reducing the non-inflammatory count by a mean of 16% and reducing the inflammatory lesion count by a mean of 46% (versus 36% and 49%, respectively, with BPO/clindamycin) at week 4.
Although statistical significance was not achieved in these studies, the CLENZIderm M.D. Systems have consistently demonstrated clinically significant reductions in both non-inflammatory and non-inflammatory lesions when compared to leading prescription BPO/antibiotic combinations products. Obagi's CLENZIderm M.D. Systems were well tolerated by patients. Mean levels of erythema, dryness and itching were mild or less than mild; patients consistently reported high levels of patient satisfaction.
18
We are conducting a multi-center, randomized, investigator-blinded study in 140 patients with mild to moderately severe acne. In this study the reduction in non-inflammatory and inflammatory lesions and tolerability are being evaluated over a 10-week treatment period.
Areas of future growth
We believe that our Penetrating Therapeutics technology may be applicable in other skin health areas. We are currently screening therapeutic candidates in related areas such as rosacea, fungal infections, dermatitis/seborrhea, hair growth and topical hair removal. According to Kalorama, in 2007 these therapeutic areas represented markets ranging from $600 million to over $3.1 billion in size.
Our product development strategy is to enhance the efficacy of established, widely prescribed, FDA-approved dermatology products (both prescription and OTC) by developing mechanisms that significantly enhance the penetration of these agents across the skin's protective barrier into the deeper layers of the skin, where therapeutic benefits are realized. We describe this enabling technology as Penetrating Therapeutics, in which the individual chemical characteristics of both active and inactive agents are addressed and then combined in a systematic manner based on their pharmacokinetic properties.
We seek to demonstrate through clinical studies the improved efficacy of various topical agents when used as part of our systems. We are conducting and plan to initiate numerous clinical studies to demonstrate the advantages of our products in both blinded, randomized controlled studies and direct comparative studies that will provide both clinically and statistically significant results. We will work with leading researchers and clinicians in their respective fields of applications and markets. We intend to use these results in marketing our products.
Sales and Marketing
Domestic. We believe we are the market leader in the physician-dispensed skin care channel with a 26.4% share and nearly three times the market share of the next largest competitor, according to a 2007 study by Kline & Co. As of December 31, 2007, we had approximately 5,200 active accounts in the United States. Each account has at least one licensed physician on-site and we estimate that there are approximately 8,000 physicians in total practicing under these active accounts. This includes physicians on-site at a small but rapidly growing number of medical spas.
The U.S. market accounted for 82% and 85% of our net sales for the years ended December 31, 2006 and 2007. In the United States, we focus our sales and marketing efforts primarily toward plastic surgeons, dermatologists and other physicians with an aesthetic skin care focus using our direct sales force. As of December 31, 2007, we had 131 sales, marketing and education specialists, including 100 dedicated sales representatives and managers. We believe that we have sufficient sales representation to enable us to effectively target the plastic surgeons, dermatologists and aesthetic skin care physicians who dispense skin care products in the United States. According to a 2007 Kline & Co. study, there were approximately 11,200 of these physicians in the United States.
In addition to effective systems, we also offer turn-key practice building programs and patient events that help the physicians grow their practices. These resources help each physician practice improve their recurring patient visits and revenue streams.
Our marketing efforts have a dual focus. First, we market directly to physicians in an attempt to both create treatment awareness and encourage the use of our products by both new and existing physician clients. To support this effort, we use medical journal advertising, direct mail, sales aids, video, CD and slide demonstrations, physician-to-physician speaker presentations, trade shows, reminder items, educational and training support, internet resources, and telemarketing. Second, we attempt to create and then build upon the relationships with our physician customers by marketing our products to their patients through their medical practices. To support this effort, we provide patient
19
education booklets and videos, funding for cooperative advertising, training and direct assistance in patient seminars and other programs, continuous product education and direct to consumer advertising and public relations programs for patient referrals.
In addition, our research and development staff is designing and coordinating clinical studies to support the application of our existing product lines in new markets and to enhance our current marketing efforts. We have conducted numerous studies with the Department of Dermatology at the University of California, Irvine and Education and Research Foundation, Lynchburg, VA. In addition, we are currently in discussions with leading dermatologists associated with numerous reputable institutions, to coordinate additional clinical studies for new product applications and formulations. We believe evidence from our clinical studies may validate the performance of our existing products and provide a platform for the development of new products and product applications.
International. International markets accounted for 18% and 15% of our net sales for the years ended December 31, 2006 and 2007, respectively. We address international markets through 13 international distribution partners that have sales and marketing activities in approximately 36 countries outside of the United States, and two trademark and know-how license agreements for the drug store and aesthetic spa channel of Japan. We target distribution partners who are capable and willing to mirror our sales and distribution model in the United States and who have an established business and reputation in the physician channel. Much like our business model in the United States, these distributors address their territories through direct sales representatives selling to physicians, or through alternative distribution channels, depending on regulatory requirements and industry practices. The sale of skin health and restoration products through physician offices is not as widely established internationally as it is in the United States. For example, some of our more successful international distribution partners include our partners in Southeast Asia, the Middle East, the Philippines and North America (excluding the United States). In these territories, our partners utilize Obagi-branded medical centers to employ trained physicians for patient sales, as well as a training center to provide product and sales training to regional physicians and their staff. Separately, some of our distribution partners, such as our partners for Mexico and Canada, leverage their existing complimentary product offerings to gain rapid account penetration for Obagi Systems in their territories. International sales are diversified, and our largest individual medical channel distribution partner purchased approximately $2.5 million from us during the year ended December 31, 2007.
We intend to continue expanding our international presence by entering into strategic relationships in key locations such as Asia, Europe and South America. We believe that there is potential for significant sales growth of our products in international markets due to cultural emphasis on overall skin health and appearance and the continued development and acceptance of surgical and non-surgical cosmetic procedures throughout many countries of the world.
Licensing. Where the physician-dispensed skin care channel is underdeveloped we will look for alternative models to build a presence and brand awareness for our products. For example, in Japan we have pursued three separate distribution channels for our brand and product concepts. In 2002, we launched our first formal long-term relationship in Japan by entering into a 10-year trademark and know-how license agreement with Rohto to market and sell our Obagi-developed products in Japan. Rohto is a Japanese pharmaceutical manufacturer and distributor. Under our agreement, Rohto is licensed to manufacture and sell a series of OTC products under the Obagi brand name, including Obagi-C (a vitamin C based topical serum in various concentrations) in the Japanese drug store channel. Rohto's Obagi branded products achieved retail annual sales of approximately $68 million in 2007 through approximately 5,000 high-end drug stores. We have additional licensing arrangements in Japan as well to market and sell OTC product systems under the Obagi brand, both for in-office use in facial procedures, as well as for sale as a take-home product kit. Separately, in early 2004, we entered into a distribution agreement with Koken Ltd., granting Koken exclusive rights to distribute certain prescription based Obagi Systems in the physician-dispensed channel. Our strategic partners in Japan
20
have engaged in aggressive direct to consumer advertising, which we believe has raised consumer demand in Japan which creates greater brand awareness in the physician channel to the benefit of our core prescription lines. During the year ended December 31, 2007, these separate distribution channels in Japan generated approximately $4.7 million in net sales for us.
We will continue to look for credible partners to address new geographies, and to evaluate alternative channel opportunities in other countries to drive brand awareness and accelerate overall market penetration.
Manufacturing
We believe our manufacturing processes are a competitive advantage, which we have developed through years of experience formulating skin care products. We maintain manufacturing scalability and flexibility by maintaining manufacturing with five qualified contract manufacturers. For all of our proprietary product concepts, we also own the related manufacturing processes, methods and formulations. In the fourth quarter of 2005, we invested in our own manufacturing facility in order to develop the ability to manage and protect the manufacturing process with respect to certain of our new product pipeline concepts. Currently, we plan to use this facility solely for the purpose of smaller scale product manufacturing in connection with new product technologies and smaller market introduction quantities. We currently perform part of the manufacturing process for the CLENZIderm system in this facility. This facility will allow us to protect intellectual property related to our products in the initial stages unless and until we believe we can transfer such know-how to third-party manufacturers with full protection of related intellectual property.
We use only FDA compliant manufacturers who specialize in the manufacture of prescription and OTC pharmaceutical and/or cosmetic products. These parties manufacture products pursuant to our specifications. All of these manufacturers are required by law and by our manufacturing standards to comply with current Good Manufacturing Practice ("cGMP"). We pre-qualify and continually monitor our manufacturers for quality and compliance. We also require documentation of compliance and quality from those manufacturers that we act as representative for in connection with the promotion and sale of their products. For most of our key products, we have two or more qualified manufacturers. Certain products, including some of our sun protection products, are supplied by a single source. We have one supplier for our Healthy Skin Protection and Sun Block sunscreen products. That supply arrangement can be terminated at any time, and there is no guarantee that we could replace the aesthetic qualities or exact formulation of those sun screen products.
In addition, since physicians can and in many cases do write a prescription for tretinoin to be fulfilled by a pharmacy in conjunction with our Obagi Nu-Derm System, we also purchase tretinoin directly from Triax in order to provide physicians the option of dispensing tretinoin directly from the office along with the Obagi products. These products are currently purchased under a five-year product supply agreement, entered into in December 2005, which also provides Obagi with the right to develop Obagi branded product offerings.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
21
We have pursued an aggressive trademark registration policy as a means to achieve brand recognition and product differentiation in the market. We own various U.S. and foreign trademark registrations and applications and common law marks. In connection with developing new products and product applications, we have filed 39 U.S. provisional and non-provisional patent applications since 2004. We do not own any issued patents with claims covering any of our Obagi Nu-Derm products.
We have also licensed certain patent applications owned by JR covering our ELASTIderm and CLENZIderm systems. We have relied on services provided by JR in the development of new products to address acne and skin elasticity products, under a five-year contract that expires in 2010. We also have licensed the rights to four patents owned by Avon Products, Inc. ("Avon"), one of which expires in 2008 while the remaining three expire between 2013 and 2018, covering methods and formulations for stabilizing Vitamin C in a serum for facial skin benefits, which is in our Obagi-C Rx C-Clarifying serum, Professional-C 5% serum, Professional-C 10% serum, Professional-C 15% serum and Professional-C 20% serum. We entered into the license with Avon in June 2003, for an initial three-year term, and the license is renewed year to year thereafter, at our option, through the life of the last patent to expire.
We have acquired rights to market, distribute, sell and, in some cases, make products pursuant to license agreements with third parties. Such agreements contain provisions which require us to meet requirements under these agreements, such as payment or royalty obligations, in order to maintain the rights granted under the agreements.
Below is a table that includes the patents we license that are material to our business as of December 31, 2007:
Patent title
| | Country in which patent is issued
| | Licensor
| | Expiration date of patent/license
|
|---|
| Cosmetic Preparation Incorporating Stabilized Ascorbic Acid | | United States | | Avon | | January 8, 2008 |
Use of Ascorbic Acid to Reduce Irritation of Topically Applied Active Ingredients |
|
United States |
|
Avon |
|
May 14, 2013 |
Ascorbic Acid Compositions for Reducing Irritation of Topically Applied Active Ingredients |
|
United States |
|
Avon |
|
April 26, 2013 |
Stable Ascorbic Acid Preparation for Topical Use |
|
United States |
|
Avon |
|
September 10, 2018 |
Certain Material Agreements
Triax
Under a product supply agreement with Triax, entered into on December 8, 2005, we have exclusive rights to sell certain tretinoin products in 0.1%, 0.05% and 0.025% concentrations in the physician-dispensed skin care channel in the United States (including all territories of the United States). If we do not purchase a minimum of 100,000 units of products each year in any combination of concentrations, we will lose our exclusive selling rights in the United States. During the years ended December 31, 2007 and 2006, we met the minimum purchase requirements. Under the terms of the agreement, we are required to pay Triax Pharmaceuticals a fixed price for the products, subject to volume discounts we may receive if we purchase a certain number of products and those products are sold to our customers within 90 days. These volume discounts are based on calendar year performance and applied to cumulative quarterly purchases. The initial term of our agreement is five years, with an automatic renewal for a successive five year term unless written notice is provided by either party at least 120 days before the end of the current term. Each party has the right to terminate the agreement upon 30 days' prior written notice in the case of material breach and failure to take action to cure such a breach within such 30 day period. Each party also has the option to terminate the agreement by written notice if the other party ceases to carry on its business or becomes the subject of any
22
proceeding under state or federal law for the relief of debtors or otherwise becomes insolvent, bankrupt or makes an assignment for the benefit of creditors, or upon the appointment of a receiver for the other party or the reorganization of the other party for the benefit of creditors.
Jose Ramirez and JR
Pursuant to a consultant services and confidentiality agreement with Jose Ramirez and JR, we have hired JR to perform research and development activities including product formulation, product development and regulatory work, as detailed in various statements of work. Under the terms of the agreement, JR must assign or license to us all rights, title and interests to any and all inventions made during JR's engagement with us or during the one year period thereafter if such inventions involve or are related to our actual or anticipated research or development, or incorporate or are based on any of our confidential information or ideas. We agreed to pay JR a minimum fee of $100,000 per year for five years plus reasonable and customary expenses incurred at our request in connection with the provision of such services, commencing on January 1, 2005, for this product formulation, product development and regulatory work. We also agreed to pay a tiered royalty for successful commercialization of products developed or identified by JR based on annual net sales, with a maximum royalty paid per product, capped at $5.0 million per year. The tiered royalty payable for each new product will be 3% on annual net sales from $0 to $50 million; 4% on annual net sales from $50 million to $75 million; and 5% on annual net sales above $75 million.
We have a right of first refusal for the exclusive license of any and all of JR's inventions related to skin healthcare that are developed or reduced to practice by JR during the term of the agreement, but not in connection with certain service activities documented in the agreement or certain various statements of work, that are not assignable to us under the terms of the agreement. We would pay a royalty fee for any such exclusive licenses.
The initial term of the agreement is five years, starting in the beginning of 2005, and may be extended for up to two, one-year renewable terms with the mutual written agreement of each party within 60 days prior to the expiration of the then-current term. We have the right to terminate the agreement at any time in our sole discretion with 30 days' notice by exercising an option to buy-out JR's remaining service obligation for the then-current term. In our sole discretion, we may pay for the buy-out option with our stock or a combination of stock and cash at a fair market valuation. We have the right to terminate the agreement, without triggering the buy-out option, upon 30 days' notice for any violation by JR of this agreement, unless JR is able to cure such violation within such 30 day period. We also have the right to immediately terminate the agreement, without triggering the buy-out option, if JR is convicted of a felony or other crime involving material harm to our standing or reputation, for JR's nonfeasance or willful misconduct, for conduct that brings us into public disgrace or disrepute, or for continuous inattentiveness to JR's duties after written notice of the same. If we exercise our right to terminate the agreement, all of our obligations to JR, except for royalty obligations, shall cease immediately.
To date, pursuant to this agreement, we have licensed six pending patent applications that cover novel methods and formulations for the treatment of acne, which we are using as the basis for our new acne system marketed under the CLENZIderm M.D. brand, including the first product launched in February 2007. We have also licensed three pending patent applications dealing with composition of matter, wound healing and method of anti-aging treatment, which we are using as the basis for our new ELASTIderm System, including the first product launched in October 2006 and the second product launched in February 2007.
Avon
Under a license agreement with Avon, dated June 26, 2003, we have an exclusive worldwide license to manufacture and sell skin care products containing an ascorbic acid component directly to physicians
23
and medical spas. Under the terms of the agreement, we also have a non-exclusive license to manufacture and sell such products directly to drug stores outside of the United States. We have agreed to pay Avon a non-refundable license issue fee of $400,000, payable in three installments. Additionally, we pay a non-refundable annual renewal fee of $100,000. The original term of the agreement expired in 2006 and we have the option to renew the agreement annually until the last of the licensed patents expires on September 10, 2018. Each party, at its option, may terminate the agreement upon 45 days' prior written notice in the case of default in the performance of any obligation under the agreement by the other party and failure to take action to cure such a default within such 45 day period. If we become bankrupt or insolvent, or file a petition for bankruptcy, or if our business is placed in the hands of a receiver, assignee or trustee from which we cannot extract ourselves within 120 days, or if we are liquidated or substantially all of our assets or shares are sold, exchanged or transferred, or in the event of a merger or consolidation to which we are a party and to which Avon reasonably objects, the agreement shall immediately terminate without notice.
Competition
The market for skin health and restoration is highly competitive with many established manufacturers, suppliers and distributors engaged in all phases of the business. We believe that we face strong competition. Competitive factors in our market include:
- •
- product efficacy and uniqueness;
- •
- brand awareness and recognition;
- •
- product quality, reliability of performance and convenience of use;
- •
- cost-effectiveness;
- •
- breadth of product offerings;
- •
- sales and marketing capabilities and methods of distribution;
- •
- resources devoted to product education and technical support; and
- •
- speed of introducing new competitive products and existing product upgrades.
We face and will continue to face intense competition. A number of our competitors have greater research and development and marketing capabilities and greater financial resources than we do. These competitors may have developed, or could in the future develop, new technologies that compete with our products or render our products obsolete. We are also likely to encounter increased competition as we enter new markets and as we attempt to further penetrate existing markets. Some of our competitors have in the past and may in the future compete by lowering prices on their products. We may respond by lowering our prices, exiting the market or competing by investing in the development of new, improved products.
We believe our direct competitors in the physician-dispensed skin care channel include BioMedic from La-Roche Posay, TNS from SkinMedica, Inc., Kinerase from Valeant Pharmaceuticals International, various products from SkinCeuticals, a division of L'Oreal S.A., M.D. Forté and PREVAGE from Allergan, Inc., Vitamin C and various products from IS Clinical, and Neova from PhotoMedex, Inc.
We believe our indirect competitors which generally sell skin care products directly to consumers consist of large cosmetic companies, including but not limited to The Estee Lauder Companies Inc., Helene Curtis Industries, Inc., L'Oreal S.A., Matrix Essentials, Inc., a division of L'Oreal, Procter & Gamble Company, Neutrogena, a division of Johnson & Johnson, Revlon, Inc. and Unilever N.V.
Our acne products compete with Triaz from Medicis Pharmaceutical Corporation, Benzaclin from Dermik Laboratories, Inc., Brevoxyl and Duac from Stiefel Laboratories Inc., Benzac from Galderma
24
Laboratories, L.P. and ZoDerm from Bradley Pharmaceuticals, Inc. Our acne products also indirectly compete with OTC anti-acne products.
Our elasticity products have demonstrated clinical evidence of aiding the restoration of elasticity and mature elastin in aged skin. We currently know of no other products which have clinical evidence to support this claim. However, there are several products which claim to enhance elastin.
Our products also compete with current and future medical devices, such as lasers, which are or will be positioned for a variety of skin enhancements such as facial rejuvenation, dermal thickening, acne and other uses. However, we believe, when used as a pre/post treatment, our Obagi Systems are complementary to many of these procedures.
Government Regulation
Federal, state and local governmental authorities in the United States and other countries regulate, among other things, the testing, production, distribution, advertising promotion and sale of prescription and OTC drugs and cosmetics. In the United States, the FDA, acting under the FDCA and other federal statutes and Agency implementing regulations, regulates products primarily on the basis of their intended use, as determined by the labeling claims made for the product.
FDA regulation of cosmetics
The FDCA defines cosmetics as products and their components intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance. Cosmetic products are not subject to FDA pre-market approval authority, although the FDA can take enforcement action for marketed cosmetic products that are adulterated or misbranded, including violations of product safety requirements, use and quantity of ingredients, labeling and promotion and methods of manufacture. Additionally, the FDA monitors compliance of cosmetic products through random inspections of cosmetic manufacturers and distributors. The labeling of cosmetic products is subject to the requirements of the FDCA, the Fair Packaging and Labeling Act and other FDA regulations.
We believe that many of our products in the Obagi Nu-Derm, Obagi-C Rx, Obagi Professional-C, ELASTIderm, CLENZIderm M.D. and other product lines, as labeled and intended for use, fall within the FDA definition of cosmetics and therefore do not require pre-market review and approval. Cosmetics may be sold both over the counter and through a physician's office, subject to state laws governing the commercial practices of physicians.
FDA regulation of drug products
The FDCA defines drugs as products intended to cure, mitigate, treat or prevent a disease or to affect the structure or any function of the human body. Drug products are subject to more comprehensive safety and effectiveness requirements of the FDCA and its implementing regulations. In general, products falling within the FDCA's definition of "new drugs" require pre-marketing clearance by the FDA. Products falling within the FDCA's definition of "drugs" that are not "new drugs" and that are generally recognized as "safe and effective" for the indication for which they are being marketed generally do not require pre-market review and approval from the FDA. Such drug products are commonly commercialized under the FDA Compliance Policy Guide entitled Marketed New Drugs Without Approved New Drug Applications or Abbreviated New Drug Applications and are subject to compliance with FDA regulations concerning manufacture, labeling, distribution, and recordkeeping for drug products.
We market a number of sunscreen products that are regulated by the FDA as OTC drug products subject to an FDA final monograph but not requiring FDA pre-market review or approval. We also market a number of products containing 4% hydroquinone that we believe are not "new drug"
25
products and that are generally recognized as safe and effective for the indications for which they are being marketed. We also market a number of products containing 4% hydroquinone that are currently not subject to FDA pre-market approval. In August 2006, the FDA issued a proposed rule that stated that OTC skin bleaching products containing hydroquinone and currently marketed outside the physician channel at 2% concentrations or less, were not generally recognized as safe and effective, were misbranded, and are new drugs within the meaning of the FDCA. The FDA proposed that because of the carcinogenic and ochronosis potential of hydroquinone, its use in skin bleaching drug products should be restricted to prescription use only, and users of such products should be closely monitored under medical supervision. The FDA also withdrew a tentative proposed monograph that concluded that hydroquinone products were generally recognized as safe and effective and stated its intent to require New Drug Application approval for continued marketing of prescription hydroquinone products at the time of publication of the final rule. The FDA is presently reviewing public comments received in response to the August 2006 Proposed Rule. Finally, our tretinoin prescription drug product offerings are licensed from and supplied by Triax, which holds FDA approval of Abbreviated New Drug Applications ("ANDAs"), for those products.
FDA regulation of "new drug" products
The steps required before a "new drug" may be marketed in the United States include (i) pre-clinical laboratory and animal testing, (ii) submission to the FDA of an Investigational New Drug ("IND"), application which must become effective before clinical trials may commence, (iii) adequate and well-controlled clinical trials to establish the safety and efficacy of the drug, (iv) submission to the FDA of a New Drug Application ("NDA"), and (v) FDA approval of the NDA prior to any commercial sale or shipment of the drug. An Abbreviated New Drug Application ("ANDA"), is required to be submitted and approved when the drug product intended for commercialization is bioequivalent to an already approved NDA product; an ANDA application does not need to repeat the clinical trials that were required for approval of the NDA, or reference listed drug. In addition to obtaining FDA approval for each product, each domestic drug-manufacturing establishment must be registered with the FDA. Drug product manufacturing establishments located in California also must be licensed by the State of California in compliance with separate regulatory requirements.
Pre-clinical testing is generally conducted on laboratory animals to evaluate the potential safety and the efficacy of a drug. The results of these studies are submitted to the FDA as a part of an IND, which must be approved before clinical trials in humans can begin. Typically, clinical evaluation involves a time consuming and costly three-phase process. In Phase I, clinical trials are conducted with a small number of subjects to determine the early safety profile, the pattern of drug distribution and metabolism. In Phase II, clinical trials are conducted with groups of patients afflicted with a specific disease to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In Phase III, large-scale, multi-center, comparative trials are conducted with patients afflicted with a target disease to provide sufficient data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical trials and may, at its discretion, re-evaluate, alter, suspend or terminate the testing based upon the data that have been accumulated to that point and its assessment of the risk/benefit ratio to the patient.
The OTC monograph system
While FDA approval is generally required before a new drug product may be marketed in the U.S., many OTC drugs are exempt from the FDA's pre-marketing approval requirements. In 1972, the FDA instituted the ongoing OTC Drug Review to evaluate the safety and effectiveness of OTC drug ingredients in the market. Through this process, the FDA issues monographs for therapeutic product categories that set forth the specific active ingredients, dosages, strengths, indications for use, warnings
26
and labeling statements for OTC drug ingredients that the FDA will consider generally recognized as safe and effective for OTC use and therefore not subject to pre-market approval.
OTC drug ingredients are classified by the FDA in one of three categories: Category I ingredients which are deemed generally recognized as "safe and effective and not misbranded" for OTC use; Category II ingredients which are deemed "not generally recognized as safe and effective or would result in misbranding" for OTC use; and Category III ingredients which are those for which "available data is insufficient" to classify them in Category I or II. Based upon the results of ongoing studies, the FDA may reclassify all Category III ingredients as Category I or Category II ingredients.
For most categories of OTC drugs not yet subject to a final monograph, the FDA usually permits such drugs to continue to be marketed until a final monograph becomes effective, unless the drug will pose a potential health hazard to consumers. The FDA's policy also generally applies to prescription drugs containing the same active ingredients as a marketed OTC product for the same or similar uses as the OTC product.
Drugs subject to final monographs, as well as drugs that are subject only to proposed monographs, are subject to various FDA regulations concerning, for example, manufacturing in accordance with cGMPs, general and specific labeling requirements and prohibitions against promotion for conditions other than those stated in the labeling. Drug manufacturing facilities are subject to FDA inspection, and failure to comply with applicable regulatory requirements may lead to administrative or judicially imposed penalties. State agencies also may require registration, licensure and inspection of manufacturing or distribution facilities and may require reporting of promotional activities.
The active ingredient in Tolereen, hydrocortisone, the active ingredient in Bacitracin, bacitracin zinc and the active ingredient in Sunblock, zinc oxide, are currently classified by the FDA as Category I ingredients. We currently market these products and must do so in accordance with FDA's final monographs for their respective therapeutic categories.
FDA enforcement discretion for marketed unapproved drug products
The Obagi Nu-Derm Clear, Blender and Sunfader products and the Obagi-C Rx C-Clarifying Serum and C-Night Therapy products contain the active ingredient hydroquinone at a 4% concentration and are marketed as prescription drugs under the FDA Compliance Policy Guide ("CPG"), entitled Marketed New Drugs Without Approved New Drug Applications or Abbreviated New Drug Applications. These hydroquinone products must be administered under the supervision of a physician but are not subject to prior FDA approval when formulated and labeled in accordance with guidelines for prescriber information under direction of a physician. 4% Hydroquinone is known as a DESI II drug, as its active ingredient was marketed prior to FDA's 1962 institution of drug effectiveness requirements. To date, the FDA has not required NDA approval for the continued marketing of hydroquinone. In its August 2006 proposed rule, regarding OTC hydroquinone, the FDA indicated its intent to require NDA approval for prescription 4% hydroquinone products. See "Risks Related to Regulatory Matters," and "FDA Regulation of Drug Products." If the FDA does change the status of 4% hydroquinone to require an NDA, and if the FDA does not allow us to continue to market our products while we attempt to comply with such new requirement as imposed, or if the FDA does not approve an NDA filed by us or a third party for 4% hydroquinone drug product, then we will no longer be able to rely on the CPG to market our Obagi Nu-Derm and Obagi-C Rx Systems containing hydroquinone. FDA approval of a 4% hydroquinone drug product under an NDA filed by another party may adversely affect our ability to continue to market our products under the CPG.
FDA regulation of drug manufacturing
We and the third-party manufacturers on which we rely for the manufacture of our products are subject to requirements that drugs be manufactured, packaged and labeled in conformity with cGMPs. To comply with cGMPs, manufacturers must continue to spend time, money and effort to meet
27
requirements relating to personnel, facilities, equipment, production and process, labeling and packaging, quality control, recordkeeping and other requirements. The FDA and state agencies periodically inspect drug manufacturing facilities to evaluate compliance with cGMPs.
FDA Regulation of Our Products and Product Candidates
The following table summarizes the current status of the active ingredients in, FDA pre-marketing approval (if any) required for, FDA regulatory category of, and launch date for our material products and product candidates:
Product
| | Main Functioning or Active Ingredients(1)
| | FDA Pre-Marketing Approval Required
| | Product Status(2)
| | Date Launched
|
|---|
| Obagi Nu-Derm Gentle Cleanser(3) | | Mild Cleansers | | No | | Cosmetic | | 1988 |
| Obagi Nu-Derm Foaming Gel(3) | | Mild Cleansers | | No | | Cosmetic | | 1988 |
| Obagi Nu-Derm Toner(3) | | Hamamelis Virginiana (Witch Hazel) Distillate | | No | | Cosmetic | | 1988 |
| Obagi Nu-Derm Clear(3) | | Hydroquinone 4% | | No | | DESI II | | 1988 |
| Obagi Nu-Derm Exfoderm(3) | | Phytic Acid | | No | | OTC | | 1988 |
| Obagi Nu-Derm Exfoderm Forte(3) | | Glycolic Acid, Lactic Acid | | No | | Cosmetic | | 1988 |
| Obagi Nu-Derm Blender(3) | | Hydroquinone 4% | | No | | DESI II | | 1988 |
| Obagi Nu-Derm Sun Block SPF 32(3) | | zinc oxide 18.5% | | No | | OTC | | 2004 |
| Obagi Nu-Derm HSP SPF 35(3) | | Octyl Methoxycinnamate 7.5%, Zinc Oxide 9.0%; | | No | | OTC | | 2002 |
| Obagi Nu-Derm Sunfader | | Hydroquinone 4% Octyl Methoxycinnamate 7.5%, Oxybenzone 5.5% | | No | | DESI II | | 1984 |
| Obagi Nu-Derm Eye Cream | | Mild Moisturizers | | No | | Cosmetic | | 1984 |
| Obagi-C Rx C-Cleansing Gel | | Mild Cleansers | | No | | Cosmetic | | 2004 |
| Obagi-C Rx C-Exfoliating Day Lotion | | Glycolic Acid | | No | | OTC | | 2004 |
| Obagi-C Rx C-Clarifying Serum | | Hydroquinone 4% | | No | | DESI II | | 2004 |
| Obagi-C Rx C-Therapy Night Cream | | Hydroquinone 4% | | No | | DESI II | | 2004 |
| Obagi-C Rx C-Sunguard SPF 30 | | Octyl Methoxycinnamate 7.5%, Zinc Oxide 9.0% | | No | | OTC | | 2004 |
| Obagi Professional-C Serum | | L Ascorbic Acid (Vitamin C) 5% to 20% concentrations | | No | | Cosmetic | | 2005 |
| Obagi Nu-Derm Tretinoin | | Tretinoin 0.025% to 0.1% | | Yes | | ANDA | | 2006(4) |
| Obagi CLENZIderm M.D. Daily Care Foaming Cleanser | | Salicylic Acid 2% | | No | | OTC | | February 2007 |
| Obagi CLENZIderm M.D. Pore Therapy | | Salicylic Acid 2% | | No | | OTC | | February 2007 |
| Obagi CLENZIderm M.D. Serum Gel | | Benzoyl Peroxide 5% | | No | | OTC | | February 2007 |
| Obagi ELASTIderm Eye Cream | | Mineral complexes | | No | | Cosmetic | | 2006 |
| Obagi ELASTIderm Eye Gel | | Mineral complexes | | No | | Cosmetic | | February 2007 |
| Obagi CLENZIderm M.D. Therapeutic Lotion | | Benzoyl Peroxide 5% | | No | | OTC | | July 2007 |
| Obagi CLENZIderm M.D. Therapeutic | | Glyceryn 20% Dimethieme 1% | | No | | OTC | | July 2007 |
| Obagi CLENZIderm M.D. Daily Care Cream Cleanser | | N/A | | No | | Cosmetic | | July 2007 |
- (1)
- By definition, products classified as cosmetics do not have active ingredients. For cosmetics, the main functioning ingredient is listed.
28
- (2)
- Product Status Definitions:
OTC, or Over-the-Counter, products are defined as products that are considered drugs by the FDA, but that do not require physician prescription or oversight.
DESI II products are defined as products that are considered drugs that require physician prescription, but are not subject to FDA pre-marketing approval as they are generally recognized as safe and effective for their intended uses and are commercialized under an FDA Compliance Policy Guide for marketed unapproved drugs, which includes Drug Efficacy Study Indication, or DESI drugs. ANDA products are defined as drugs that have received FDA approval under an abbreviated new drug application; our only ANDA product requires physician prescription.
Cosmetic products are defined as products which are not considered drugs by the FDA, are not allowed to make drug claims, and do not require FDA pre-marketing approval or physician prescription or oversight.
- (3)
- These products are also included in our Obagi Condition and Enhance System, which was launched in 2006.
- (4)
- Obagi Nu-Derm branded Tretinoin is manufactured by Triax under its ANDAs, and was launched under the Obagi brand in 2006, however, we have been selling tretinoin supplied by Triax under the Spear brand since 2003.
Federal regulation of advertising and promotion
The FDA regulates the advertisement of prescription drug products. The U.S. Federal Trade Commission ("FTC"), and state authorities regulate the advertising of OTC drugs and cosmetics, as well as exercise general authority to prevent unfair or deceptive trade practices.
In addition to FDA restrictions on marketing of prescription products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn are used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services, reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer.
Also, as part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. This practice is overseen by the FDA, state and other governmental authorities under the Prescription Drug Marketing Act and regulations that include requirements concerning record keeping and control procedures.
29
Certain states, including California, have also recently begun regulating the promotion of prescription drug products and require compliance with annual certification and disclosure requirements regarding our policies for drug promotion and the amount of money we spend per prescribing physician on drug promotion. Compliance with changing federal and state laws and regulations on prescription product promotion requires a great deal of time and effort.
Other government regulation
We and our suppliers or third-party manufacturers may also be subject to regulations under other federal, state, and local laws, including the Occupational Safety and Health Act, the Environmental Protection Act, the Clean Air Act and import, export and customs regulations as well as the laws and regulations of other countries.
Employees
As of December 31, 2007, we had 182 employees, all of whom were located in the United States. Our employees include 131 in sales and marketing, 24 in product development, manufacturing and distribution and 27 in administrative functions. Our employees are all non-unionized, and we believe our relations with our employees are good.
ITEM 1A: RISK FACTORS
An investment in our common stock involves a high degree of risk. You should consider carefully the following risks and other information contained in this Annual Report on Form 10-K before you decide whether to buy our common stock. If any of the events contemplated by the following discussion of risks should occur, our business, results of operations and financial condition could suffer significantly. As a result, the market price of our common stock could decline, and you may lose all or part of the money you paid to buy our common stock.
Risks related to our business
Our revenues and financial results depend significantly on sales of our Obagi Nu-Derm System. If we are unable to manufacture or sell our Obagi Nu-Derm System in sufficient quantities and in a timely manner, or maintain physician and/or patient acceptance of our Obagi Nu-Derm System, our business will be materially and adversely impacted.
To date, substantially all of our revenues have resulted from sales of our principal product line, our Obagi Nu-Derm System and related products. Our Obagi Nu-Derm System and related products accounted for approximately 61%, 68% and 72% of our net sales for the year ended December 31, 2007, 2006 and 2005, respectively. Although we have introduced new products such as Obagi-C Rx, ELASTIderm and CLENZIderm, and we intend to introduce additional products, we still expect sales of our Obagi Nu-Derm System and related products to account for a significant majority of our sales for the foreseeable future. Because our business is highly dependent on our Obagi Nu-Derm System and related products, factors adversely affecting the pricing of, or demand for, these products could have a material and adverse effect on our business. Our Obagi Nu-Derm Systems also experience seasonality. We believe this is due to variability in patient compliance that relates to several factors such as a tendency to travel and/or engage in other disruptive activities during the summer months. Additionally, our commercial success depends in large part on our ability to sustain market acceptance of our Obagi Nu-Derm System. If existing users of our products determine that our products do not satisfy their requirements, or if our competitors develop a product that is perceived by patients or physicians to better satisfy their respective requirements, sales of our Obagi Nu-Derm System and related products may decline, and our total net sales may correspondingly decline. We cannot assure you that we will be able to continue to manufacture these products in commercial quantities at
30
acceptable costs. Our inability to do so would adversely affect our operating results and cause our business to suffer.
We face intense competition, in some cases from companies that have significantly greater resources than we do, which could limit our ability to generate sales.
The market for aesthetic and therapeutic skin health products is highly competitive and we expect the intensity of competition to increase in the future. We also expect to encounter increased competition as we enter new markets and as we attempt to penetrate existing markets with new products. We may not be able to compete effectively in these markets, we may face significant pricing pressure from our competitors and we may lose market share to our competitors. Our principal competitors are large, well-established companies in the fields of pharmaceuticals, medical devices, cosmetics and health care. Our direct competitors include La-Roche Posay, SkinMedica, Inc., Valeant Pharmaceuticals International, SkinCeuticals, a division of L'Oreal S.A., Allergan, Inc., IS Clinical, and PhotoMedex, Inc.
We believe our indirect competitors, who generally sell skin care products directly to consumers, consist of large cosmetic companies, including but not limited to, The Estee Lauder Companies Inc., Helene Curtis Industries, Inc., L'Oreal S.A., Matrix Essentials, Inc., a division of L'Oreal S.A, Procter & Gamble Company, Neutrogena, a division of Johnson & Johnson, Revlon, Inc. and Unilever N.V. We also face competition from medical device companies offering products used to enhance the skin's appearance to physicians, such as Candela Corp., CoolTouch Corporation, Cynosure, Inc., Lumenis Ltd., Reliant Technologies, Inc., Syneron Medical Ltd. and Thermage, Inc.
We may not be able to successfully expand the use of our current product lines or develop new products.
We are working to improve, extend and reformulate many of our existing products. Continued market acceptance of our products will depend on our ability to successfully develop additional applications of our existing products. The development of additional applications will require significant commitments of personnel and financial resources and we cannot assure you that they will be successful. If the attempted extensions of our product lines are not commercially successful, our business will be adversely affected.
We are also developing new product lines by applying our Penetrating Therapeutics technology to new agents. We also have acquired rights to technologies described in certain patent applications relating to additional methods and formulations. New products, in various stages of development, include other applications for our acne and skin elasticity products and other new systems. These development activities, as well as clinical studies, which must be completed before these products can be marketed and sold, will require significant commitments of personnel and financial resources. We cannot assure you that we will be able to develop new products or technologies in a timely manner, or at all. Delays in the development or testing processes will cause a corresponding delay in revenue generation from those products. Regardless of whether such new products or technologies are ever released to the market, the expense of such processes, which may be considerable, will have already been incurred and we may not be able to recover such expenses.
We reevaluate our development efforts regularly to assess whether our efforts to develop a particular new product or technology are progressing at a rate that justifies our continued expenditures. On the basis of these reevaluations, we have abandoned in the past, and may abandon in the future, our efforts on a particular product or technology. New products that we develop may not be successfully commercialized. If we fail to take a product or technology from the development stage to market on a timely basis, we may incur significant expenses without a near-term financial return or any financial return.
31
Our failure to successfully in-license or acquire additional products and technologies would impair our ability to grow.
We intend to in-license, acquire, develop and market new products and technologies. Because we have limited internal research capabilities, our business model depends in part on our ability to license patents, products and/or technologies from third parties. The success of this strategy also depends upon our ability and the ability of our third-party formulators to formulate products under such licenses, as well as our ability to manufacture, market and sell such licensed products.
We may not be able to successfully identify any new products to in-license, acquire or internally develop. Moreover, negotiating and implementing an economically viable acquisition is a lengthy and complex process. Other companies, including those with substantially greater financial, marketing and sales resources, may compete with us for the acquisition of products. We may not be able to acquire or in-license the rights to such products on terms that we find acceptable, or at all. As a result, our ability to grow our business or increase our profits could be adversely impacted.
Our marketed products and our products under development could be rendered obsolete by technological or medical advances.
Our marketed products and our products under development may be rendered obsolete or uneconomical by the development of medical advances to treat the conditions that our products are designed to address. The treatment of skin conditions and the enhancement of the appearance of skin, which is what all of our products target, are the subjects of active research and development by many potential competitors, including major pharmaceutical companies, such as Johnson & Johnson and Galderma, specialized biotechnology firms, such as Allergan and Medicis, universities and other research institutions. Competitive advances may also include the potential development of new laser or radio frequency therapies being developed by manufacturers such as Candela, Syneron and Thermage, aimed at treating hyperpigmentation and photo-damaged skin. While we intend to expand our technological capabilities to remain competitive, research and development by others may render our technology or products obsolete or noncompetitive or result in treatments superior to any therapy we develop, as our competitors may develop and patent products which are better than ours, which could harm our competitive position.
If we lose key personnel or are unable to attract and retain other qualified personnel, we may be unable to execute our business plan and our business would be materially adversely affected.
As of December 31, 2007, we had 182 employees. Our success depends on our continued ability to attract, retain and motivate highly qualified management, business development, sales and marketing, product development and other personnel. In the future we may not be able to recruit and retain qualified personnel, particularly for senior sales and marketing and research and product development positions due to intense competition for personnel among businesses like ours, and the failure to do so could have a significant negative impact on our future product sales and business results. Our success depends in large part on the efforts and abilities of Steven Carlson, our Chief Executive Officer, Stephen Garcia, our Chief Financial Officer, and David Goldstein, our Executive Vice President of Sales, as well as other members of our senior management and our scientific and technical personnel. We may not be able to retain the services of our officers. In addition, we do not have "key person" insurance policies on any of our executive officers that would compensate us for the loss of their services. If we lose the services of one or more of these individuals, finding a replacement could be difficult, may take an extended period of time and could significantly impede the achievement of our business objectives. This may have a material adverse effect on our results of operations and financial condition.
32
To sustain our continued growth, we will need to increase the size of our organization, and we may encounter difficulties managing our growth, which could adversely affect our results of operations.
If we are able to successfully develop additional products and extend the use of our current products, we may experience growth in the number of our employees and the scope of our operations. To the extent that we acquire and launch additional products, the resulting growth and expansion of our sales force will place a significant demand on our financial, managerial and operational resources. Since many of the new products or systems we are working on may involve new technologies or entering new markets, we may not be able to accurately forecast the number of employees required and the timing of their hire or the associated cost. The extent of any expansion we may experience will be driven largely by the success of our new products and systems. As a result, management's ability to project the size of any such expansion and its cost to the company is limited by the following uncertainties: (i) we will not have previously sold any of the new products and technologies and the ultimate success of these new products and technologies is unknown; (ii) we will be entering new markets; and (iii) the costs associated with any expansion will be partially driven by factors that may not be fully in our control (e.g., timing of hire, market salary rates). As of December 31, 2007, subject to these uncertainties, we believe that our current business plan may require us to hire between 30 and 40 new employees within the next 12 months at an incremental cost of between $2.6 million and $3.5 million. Due to the uncertainty surrounding the new product lines, this estimate may prove to be incorrect, and our costs could be significantly higher. Our success will also depend on the ability of our executive officers and senior management to continue to implement and improve our operational, information management and financial control systems, particularly in light of our status as a public company subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, and to expand, train and manage our employee base. Our inability to manage growth effectively could cause our operating costs to grow even faster than we are currently anticipating and adversely affect our results of operations.
Because we have limited research and development capabilities, we will be dependent on third parties to perform research and development for us.
We have limited internal research and development capabilities and currently outsource all of our product research and development to third-party research labs. In particular, we have licensed technologies that may issue as patents under certain patent applications filed by JR, and have relied heavily on services provided by JR in the development of new products to address acne and skin elasticity. We have received sufficient support from our third-party research labs to drive our current new product development, and we expect to continue to rely on third parties to research and develop new products.
There are a limited number of third-party research and development companies that specialize or have the expertise required to achieve our product development objectives. As a result, it may be difficult for us to engage research and development labs and personnel for our anticipated future needs. If we are unable to arrange for third-party research and development of our products, or to do so on commercially reasonable terms, we may not be able to develop new products or expand the application of our existing products.
Reliance on third-party research and development labs entails risks to which we would not be subject if we performed the research and development ourselves. These risks include reliance on the third party for maintaining the confidentiality of the proprietary information relating to the product being developed and for maintaining quality assurance, the possibility of breach of the research and development agreement by the third party, and the possibility of termination or non-renewal of the agreement by the third party.
33
Dependence upon third parties for the research and development of our future products may limit our ability to commercialize and deliver products on a timely and competitive basis.
Because we currently have limited commercial manufacturing capabilities, we will continue to be dependent on third parties to manufacture products for us for some time.
We have limited commercial manufacturing experience and currently outsource all of our non-Benzoyl Peroxide product manufacturing to third-party contract manufacturers. Although we have received sufficient material from our manufacturers to meet our current needs, we do not have long-term contracts with most of these third parties. Triax is our sole supplier and manufacturer of tretinoin pursuant to a contract that has an initial termination in 2010. The termination of that agreement or any loss of services under that agreement would be difficult for us to replace. We expect to continue to rely on third parties to produce materials required for clinical trials and for the commercial production of our products.
There are a limited number of third-party manufacturers that operate under the FDA's current cGMP, regulations and that have the necessary expertise and capacity to manufacture our products. As a result, it may be difficult for us to locate manufacturers for our anticipated future needs. If we are unable to arrange for third-party manufacturing of our products, or to do so on commercially reasonable terms, we may not be able to complete development of, market and sell our new products.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the third party for regulatory compliance and quality assurance, the possibility of breach of the manufacturing agreement by the third party, and the possibility of termination or non-renewal of the agreement by the third party.
We have developed a manufacturing facility dedicated to the production of our CLENZIderm M.D. Serum Gel. We may expand the capabilities of that manufacturing site and may in the future elect to manufacture certain new products developed or certain existing products without the assistance of third parties. However, in order to make that election, we will need to invest substantial additional funds and recruit qualified personnel in order to operate our development manufacturing facility on a commercial basis. There can be no assurance that we will successfully manufacture our own products, and if we are not able to make or obtain adequate supplies of our products, it will be more difficult for us to maintain and grow sales of CLENZIderm M.D., or launch other new products and compete effectively.
Dependence upon third parties for the manufacture of our products may reduce our profit margins, or the sale of our products, and may limit our ability to develop and deliver products on a timely and competitive basis.
Our growth may suffer if an economic downturn in any of our major markets inhibits people from spending their disposable income on aesthetic and skin health products.
Our growth depends significantly on continued economic growth in the markets where we sell our products. Because many treatments in which our products are used are considered cosmetic in nature, they are typically paid directly by the patient out of disposable income and are not subject to reimbursement by third-party payers such as health insurance organizations. As a result, an economic downturn in any of our major markets, such as North America, the Pacific Rim and the Middle East, could have an adverse effect on the sales and profitability of our products.
34
Our products may cause undesirable side effects that could limit their use, require their removal from the market or prevent further development.
The most common side effects associated with our therapeutic products are temporary redness, stinging, burning sensation, skin peeling, flaking, acne flare-ups and photo-sensitivity normally experienced within approximately the first ten weeks of use. While these side effects generally are not severe, they may limit the use of our products, particularly if physicians or patients perceive that the risks or discomfort outweigh the benefits or if they perceive that the side effects of competitive products are less significant.
Undesirable side effects caused by our products could interrupt, delay or halt our development programs, including clinical trials, and could result in adverse regulatory action by the FDA or other regulatory authorities. More severe side effects associated with our products may be observed in the future. Even if we are able to complete the development of a new product and obtain any required regulatory approval, undesirable side effects could prevent us from achieving or maintaining market acceptance of the product or could substantially increase the costs and expenses of commercializing the product. Negative publicity concerning our products, whether accurate or inaccurate, could also reduce market or regulatory acceptance of our products, which could result in decreased product demand, removal from the market or an increased number of product liability claims, whether or not such claims have merit.
The FDA has issued a proposed rule which cites evidence that an active ingredient contained in some of our Obagi Nu-Derm and Obagi-C Rx Systems may have negative side effects.
In August 2006, the FDA issued a proposed rule which cites some evidence that hydroquinone may be a carcinogen, if orally administered, and may be related to a skin condition called ochronosis, which results in the darkening and thickening of the skin, and the appearance of small bumps and grayish-brown spots. Hydroquinone is an active ingredient contained in our Obagi Nu-Derm Clear, Obagi Nu-Derm Blender and Obagi Nu-Derm Sunfader products, which are part of our Obagi Nu-Derm System, and in our Obagi-C Rx C-Clarifying Serum and Obagi-C Rx C-Night Therapy products, which are part of our Obagi-C Rx System. The FDA also concluded that it could not rule out the potential carcinogenic risk from topically applied hydroquinone. After further review of the evidence cited in the notice, or information provided in the public comments on the proposed rule, or if additional evidence of cancer or ochronosis, or other side effects associated with any of our products were to be reported to or observed by the FDA or other regulatory authorities, we could be required to suspend the marketing of our products that contain hydroquinone, conduct additional safety tests and potentially cease the sale of affected products, which would harm our business. In addition, patients who experience side effects from our products may bring product liability claims against us.
All of our products which contain hydroquinone are prescription-based. The FDA is considering regulating all hydroquinone products, including prescription-based hydroquinone products, as new drugs, and may conclude that the continued use of prescription-based hydroquinone products will require the submission and approval of an NDA. If we are required to submit an NDA for our prescription-based hydroquinone products, we believe that the FDA may allow us to continue to market these products while we are preparing, submitting and waiting for approval of such NDA. However, there can be no assurance that we will be able to continue to market our products during the NDA process, and we may be required to suspend marketing of our prescription-based hydroquinone products until such time as an NDA is approved. In addition, there can be no assurance that any NDA we submit for our prescription-based hydroquinone products will be approved. If we are required to suspend or cease marketing of our prescription-based hydroquinone products, it would adversely affect our business.
35
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
Our business exposes us to the risk of product liability claims that are inherent to the development, clinical testing and marketing of aesthetic and skin health products. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of those products. Although we maintain general liability and product liability insurance in an amount that we believe is reasonably adequate to insulate us from potential claims, this insurance may not fully cover potential liabilities. In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercial production and sale of our products, which could adversely affect our business.
We are subject to risks associated with doing business internationally.
Our international sales currently depend upon the marketing efforts of and sales by certain distributors and licensees, particularly Rohto Pharmaceuticals, a licensee of certain of our trademarks and products for the retail drug store channel in Japan, from whom we receive royalties that accounted for approximately 4% and 4% of our net sales and approximately 5% and 5% of our gross margin for the years ended December 31, 2007 and 2006, respectively. Because incremental costs associated with this agreement are minimal, a material decline in licensing revenues from or termination of this agreement would have a material adverse effect on our net income. While no other international distribution or license partner accounted for more than 4% of our net sales for both of the years ended December 31, 2007 and 2006, our business is subject to certain risks inherent in international business, many of which are beyond our control. These risks include:
- •
- adverse changes in tariff and trade protection measures;
- •
- unexpected changes in foreign regulatory requirements;
- •
- potentially negative consequences from changes in tax laws;
- •
- the potential business failure of one or more of our distribution partners;
- •
- changing economic conditions in countries where our products are sold or manufactured;
- •
- exchange rate risks;
- •
- potential political unrest and hostilities;
- •
- differing degrees of protection for intellectual property; and
- •
- difficulties in coordinating foreign distribution.
Any of these factors could adversely affect our business, financial condition and results of operations. We cannot assure you that we can successfully manage these risks or avoid their effects.
Potential business combinations could require significant management attention and prove difficult to integrate with our business, which could distract our management, disrupt our business, dilute stockholder value and adversely affect our operating results.
If we become aware of potential business combination candidates that are complementary to our business, we may decide to combine with such businesses or acquire their assets in the future. Business combinations generally involve a number of additional difficulties and risks to our business, including:
- •
- failure to integrate management information systems, personnel, research and development and marketing, operations, sales and support;
36
- •
- disruption of our ongoing business and diversion of management's attention from other business matters;
- •
- potential loss of the acquired company's customers;
- •
- failure to develop further the acquired company's technology successfully;
- •
- unanticipated costs and liabilities; and
- •
- other accounting consequences.
In addition, we may not realize benefits from any business combination we may undertake in the future. If we fail to successfully integrate such businesses, or the technologies associated with such business combinations into our company, the revenue and operating results of the combined company could be adversely affected. Any integration process would require significant time and resources, and we may not be able to manage the process successfully. If our customers are uncertain about our ability to operate on a combined basis, they could delay or cancel orders for our products. We may not successfully evaluate or utilize the acquired technology or accurately forecast the financial impact of a combination, including accounting charges or volatility in the stock price of the combined entity. If we fail to successfully integrate other companies with which we may combine in the future, our business could be adversely affected.
Risks related to regulatory matters
Our ability to commercially distribute our products and our business may be significantly harmed if the regulatory environment governing our products changes, if the FDA takes enforcement action against us or our competitors marketing similar products, or if a third party obtains FDA approval of an NDA for 4% hydroquinone for the same uses for which we market our hydroquinone products.
The FDA and comparable agencies of other countries regulate our products. In the United States, FDA regulations govern, among other things, the activities that we perform, including product development, product testing, product labeling, product storage, manufacturing, advertising, promotion, product sales, reporting of certain product adverse events and failures, and distribution. Individual state regulations may also govern drug product manufacturing, distribution, advertising and promotion.
In addition, the Obagi Nu-Derm and Obagi-C Rx Systems contain products that include 4% hydroquinone as an active ingredient and are marketed in the United States without an FDA-approved marketing application. We believe that these products are not currently subject to FDA pre-market approval. In August 2006, the FDA issued a proposed rule that, if adopted in its current form, would establish that OTC skin bleaching drug products, such as hydroquinone, are not generally recognized as safe and effective and are misbranded, and could seek to require NDAs for new products using skin bleaching drug products, including prescription skin bleaching drug products. The FDA has indicated that upon adoption of the final rule it intends to consider all skin bleaching drug products, whether currently marketed on a prescription or OTC basis, to be new drugs requiring an approved NDA for continued marketing. This may require us to withdraw the Obagi Nu-Derm and Obagi-C Rx Systems until required clinical trials are performed and new drug approvals are obtained, in effect foreclosing us from selling the Obagi Nu-Derm and Obagi-C Rx Systems. If we are required to seek new drug approval for these products, our attention and resources will be dedicated to the process of obtaining new drug approval, which may be time-consuming and expensive. In addition, we may not successfully obtain such approval or may be delayed in obtaining such approval if one of our competitors obtains approval and nonpatent exclusivity for the same uses for which we seek approval. Other manufacturers of 4% prescription hydroquinone include SkinMedica, Inc., Taro Pharmaceuticals Inc. and Stiefel Laboratories, Inc. If we are unable to obtain such approval, we would be prohibited from selling the Obagi Nu-Derm and Obagi-C Rx Systems, which would have a material adverse impact on our business.
37
Finally, as discussed above, the August 2006 proposed rule cites some evidence that hydroquinone may be a carcinogen and may be related to ochronosis. If new studies are published that corroborate such evidence, or if based on further review of the current evidence, the FDA determines that such potential health risks warrant a ban on the sale of 4% hydroquinone, such determinations would have a material adverse effect on our sale of the Obagi Nu-Derm and Obagi-C Rx Systems. The FDA's proposed rulemaking itself, and the concerns expressed therein relating to the use of hydroquinone, could have an adverse impact on the sales of our Obagi Nu-Derm and Obagi-C Rx Systems. Certain of our competitors are attempting to use the FDA's proposed rulemaking in order to convince physicians and patients not to use our products containing hydroquinone. To date, these marketing efforts by our competitors have not had a negative impact on our sales levels. However, we cannot provide assurance that such marketing efforts will not have a negative impact on our sales levels in the future.
FDA and the FTC, regulations limit the type of marketing claims we can make about our products. If the FDA determines that any of our marketing claims are false or misleading, or suggest a clinical benefit that is not supported in the studies we have done, we may be required to cease making the challenged marketing claims, issue corrective communications, pay fines, or stop selling products until the incorrect claims have been corrected. FDA or FTC enforcement actions regarding promotional claims, including warning letters, would also divert management attention and create public relations issues for our customers and opportunities for our competitors.
We are also subject to review, periodic inspection and marketing surveillance by the FDA and comparable state agencies to determine our compliance with regulatory requirements. Our manufacturing processes, any clinical trials that we perform, our distribution and our promotional activities are subject to ongoing regulatory obligations. In addition, state agencies also may require registration, licensure and inspection of manufacturing or distribution facilities and may require reporting of promotional activities. If the FDA or any state or comparable agency finds that we have failed to comply with these requirements or later discovers previously unknown problems with our products, including unanticipated adverse events of unanticipated severity or frequency, or our manufacturer or manufacturing processes, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, including
- •
- fines, injunctions and civil penalties;
- •
- recall or seizure of our products;
- •
- restrictions on our products manufacturing processes, or distribution systems, including operating restrictions, partial suspension or total shutdown of production;
- •
- denial of requests for approvals of product candidates;
- •
- withdrawal of approvals already granted;
- •
- disgorgement of profits; and
- •
- criminal prosecution.
Any of these enforcement actions could affect our ability to commercially distribute our products in the United States and may also harm our ability to conduct the clinical trials necessary to support the marketing, clearance or approval of these products and could materially and adversely affect our business.
New regulations could prohibit physicians from dispensing our products directly.
In our primary market, the United States, we market our products and systems directly to physicians to dispense in their offices. Most of the products and systems we sell are dispensed by physicians directly to their patients in their offices, although some patients choose to have prescriptions
38
for our products filled by pharmacies instead of the treating physician. In the event state regulations change to limit or prohibit the ability of physicians to dispense our products directly to patients in their offices or change to limit or prevent our ability to distribute products, patients may be required to purchase our products in pharmacies, as opposed to directly from their physicians. If patients are unable to purchase our products directly from physicians, it could result in patients purchasing less of our product than they otherwise would, which would harm our business.
Failure to obtain regulatory approvals in foreign jurisdictions would prevent us from marketing our products internationally.
We market our products outside of the United States. In order to market our products in many non-U.S. jurisdictions we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. In others, we do not have to obtain prior regulatory approval but do have to comply with other regulatory restrictions on the manufacture, marketing and sale of our products. We may be unable to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. The approval procedure varies among countries and can involve additional testing and data review. The time required to obtain approval in non-U.S. jurisdictions may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. If we get approval by the FDA, that does not ensure approval by regulatory agencies in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. The failure to obtain these approvals could harm our business.
If any of our third-party manufacturers do not operate in accordance with current Good Manufacturing Practices, we could be subject to FDA enforcement actions, including the seizure of our products and the halt of our production.
Third-party manufacturers that we currently rely on or will rely on in the future must continuously adhere to the cGMPs set forth in the FDA's regulations and guidance documents. In complying with cGMPs, we and our third-party manufacturers must expend significant time, money and effort in development, testing, production, record keeping and quality control to assure that our products meet applicable specifications and other regulatory requirements. The failure to comply with these specifications and other requirements could result in an FDA enforcement action, including the seizure of products and shutting down of production. Our third-party manufacturers may also be subject to comparable or more stringent regulations of foreign regulatory authorities. If our third-party manufacturers are unable to comply with cGMPs and applicable foreign regulatory requirements, our ability to develop, produce and sell our products could be impaired.
Risks related to intellectual property
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely primarily on maintaining the confidentiality of our trade secrets and the protection of trade secret laws, as well as a combination of patent, copyright, and trademark (including common law trademark) laws, and nondisclosure, confidentiality and other contractual restrictions, to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our trade secrets may be misappropriated by current or former employees, contractors, or parties with whom we partner, or may be inadvertently disclosed or obtained by breach of a confidentiality agreement. We do not own any issued patents with claims covering any of our Obagi Nu-Derm products. We have recently applied for several patents both in the United States
39
and abroad. These patent applications may not issue as patents at all, or the applications may not issue as a patent in a form that will be advantageous to us or may issue and be subsequently successfully challenged by others and invalidated or rendered unenforceable. Both the patent application process and the process of managing patent disputes can be time-consuming and expensive. Competitors may be able to design around our patents or develop products that provide outcomes comparable to ours even without misappropriating our trade secrets. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. Further, the parties with whom we enter into confidentiality and intellectual property assignment agreements could dispute the ownership of intellectual property developed under these agreements. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
In order to protect or enforce our intellectual property rights, we may initiate litigation. In addition, others may initiate litigation related to intellectual property against us. Companies against whom we might initiate litigation or who might initiate litigation against us may be better able to sustain the costs of litigation because they have substantially greater resources. We may become subject to interference proceedings conducted in patent and trademark offices to determine the priority of inventions. There are numerous issued and pending patents in the skin care product field. The validity and breadth of such patents may involve complex legal and factual questions for which important legal principles may remain unresolved. If third parties file oppositions to our patent applications in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of our filed foreign patent applications. We also have worked with consultants in developing our intellectual property portfolio. To the extent any of these consultants are involved in litigation involving intellectual property related to us, we may also become a party to such actions or otherwise be adversely affected by virtue of our relationships with the consultants. For example, we recently learned that JR, a consultant who helped us develop CLENZIderm and has granted us a license under certain patent applications that cover CLENZIderm, is involved in a lawsuit in which the plaintiff claims that the owner of JR misappropriated the plaintiff's trade secrets. Based upon our review of certain documents filed in connection with this lawsuit, it appears that the plaintiff may believe that JR may have utilized the plaintiff's trade secrets in developing CLENZIderm and filing the patent applications that cover CLENZIderm. To date, no claims have been asserted against us by the plaintiff, but if the plaintiff is successful in pursuing its claims against JR, it could result in a material adverse effect on our CLENZIderm business.
Litigation may be necessary for us to assert or defend against infringement claims, enforce our issued and licensed patents, protect our trade secrets or know-how or determine the enforceability, scope and validity of the proprietary rights of others. Our involvement in intellectual property claims and litigation could:
- •
- divert existing management, scientific and financial resources;
- •
- subject us to significant liabilities;
- •
- result in a ruling that allows our competitors to market competitive products without obtaining a license from us;
40
- •
- require us to enter into royalty or licensing agreements, which may not be available on terms acceptable to us, if at all; or
- •
- force us to discontinue selling or modify our products, or to develop new products.
If any of these events occur, our business will be materially and adversely affected.
We and our manufacturers and suppliers license certain technologies and patents from third parties. If these licenses are breached, terminated or disputed, our ability to commercialize products dependent on these technologies and patents may be compromised.
We have licensed four patents, including patents related to our Vitamin C serums, from Avon. We entered into the license in June 2003, for an initial three-year term, and the license is renewed year to year thereafter, at our option through the life of the last patent to expire (which will be in 2018). These licensed patents contain claims that cover our Obagi-C Rx C-Clarifying serum, Professional-C 5% serum, Professional-C 10% serum, Professional-C 15% serum and Professional-C 20% serum. If one or more of our licenses with Avon or licenses we have with other parties terminate, if we violate the terms of our licenses or otherwise lose our rights to these patents, we may be unable to continue developing and selling our products that are covered by claims in the patents we license. Our licensors or others may dispute the scope of our rights under any of these licenses. The licensors under these licenses may breach the terms of their respective agreements or fail to prevent infringement of the licensed patents by third parties. Loss of any of these licenses for any reason could materially and adversely affect our financial condition and operating results.
Further, we purchase products from manufacturers and suppliers who have licensed patent rights to use and sell these products from third-party licensors, and if any dispute arises as to these licensed rights, the third-party licensors may bring legal actions against us, our respective licensees, suppliers, customers or collaborators, and claim damages and seek to enjoin the manufacturing and marketing of such products.
In addition, if we determine that our products do not incorporate the patented technology that we have licensed from third parties, or that one or more of the patents that we have licensed are not valid, we may dispute our obligation to pay royalties to our licensors. Any dispute with a licensor could be complex, expensive and time-consuming and an outcome adverse to us could materially and adversely affect our business and impair our ability to commercialize our patent-licensed products.
Risks related to our capital requirements and finances
If we fail to generate sufficient cash flow from our operations, we will be unable to continue to develop and commercialize new products.
We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, and our commercialization, clinical trials, research and development and manufacturing activities. We believe that our net cash provided by operating activities and existing cash and cash equivalents will be sufficient to fund our operations for the foreseeable future. However, our present and future funding requirements will depend on many factors, including, among other things:
- •
- the level of research and development investment required to maintain and improve our competitive position;
- •
- the success of our product sales and related collections;
- •
- our need or decision to acquire or license complementary businesses, products or technologies or acquire complementary businesses;
- •
- costs relating to the expansion of the sales force, management and operational support;
41
- •
- competing technological and market developments; and
- •
- costs relating to changes in regulatory policies or laws that affect our operations.
As a result of these factors, we may need to raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms when needed, if at all. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
Our quarterly operating results are variable, which may cause our stock price to decline.
Our quarterly results of operations have varied in the past and are likely to vary significantly in the future due to a number of factors, many of which are outside of our control, including:
- •
- demand for and market acceptance of our products;
- •
- the development of new competitive products by others;
- •
- changes in regulatory classifications of our products;
- •
- changes in physician or patient acceptance of the use of physician-dispensed products;
- •
- changes in treatment practices of physicians who currently prescribe our products;
- •
- reduced demand for our products during the summer months due to variability of patient compliance resulting from travel and other disruptive activities, particularly during July and August;
- •
- delays between our expenditures to acquire new product lines or businesses and the generation of revenues from those acquired products or businesses;
- •
- the timing, release and competitiveness of our products;
- •
- increases in the cost of raw materials used to manufacture our products;
- •
- the mix of products that we sell during any time period;
- •
- increased price competition; and
- •
- adverse changes in the level of economic activity in the United States and other major regions in which we do business.
Due to the factors summarized above, we do not believe that period-to-period comparisons of our results of operations are necessarily meaningful and should not necessarily be relied upon to predict future results of operations. It is also possible that in future periods, our results of operations will not meet the expectations of investors or analysts, or any published reports or analyses regarding our company. In that event, the price of our common stock could decline, perhaps substantially. For example, due to the reduced demand for our products during the summer months discussed above, our results of operations for the third quarter have historically been lower than our results of operations for the second quarter. We do not expect this will change in the future.
Changes in, or interpretations of, accounting rules and regulations, such as expensing of stock options, could result in unfavorable accounting charges or require us to change our compensation policies.
Changes to, or interpretations of, accounting methods or policies in the future may require us to reclassify, restate or otherwise change or revise our financial statements, including those contained in
42
this Annual Report on Form 10-K. For example, the Financial Accounting Standards Board, or FASB, has adopted an accounting pronouncement requiring the recording of expense for the fair value of stock options granted. Beginning January 1, 2006, we have adopted Statement of Financial Accounting Standards ("SFAS"), or SFAS No. 123R, Share-Based Payment, which required us to change our accounting policy to record compensation expense for the fair value of stock options granted, and as a result, our operating expenses have increased. We rely on stock options to motivate current employees and attract new employees. As a result of the requirement to expense stock options, we may choose to reduce our reliance on stock options as a recruitment or motivation tool. If we reduce our use of stock options, it may be more difficult for us to attract and retain qualified employees. However, if we do not reduce our reliance on stock options, our operating expenses may continue to increase.
Impairment of our significant intangible assets may reduce our profitability.
The costs of our goodwill, acquired product rights, distribution rights, and trademark are recorded as intangible assets and all, except for goodwill, are amortized over the period that we expect to benefit from the assets. As of December 31, 2007, acquired net intangible assets and goodwill comprised approximately 17% of our total assets. We evaluate periodically the recoverability and the amortization period of our intangible assets. Some factors we consider important in assessing whether or not impairment exists include performance relative to expected historical or projected future operating results, significant changes in the manner of our use of the assets or the strategy for our overall business, and significant negative industry or economic trends. These factors, assumptions, and changes in them could result in an impairment of our long-lived assets. Any impairment of our intangible assets may reduce our profitability and have a material adverse effect on our results of operations and financial condition.
Fluctuations in demand for our products could create inventory maintenance uncertainties and could adversely affect our business.
As a result of customer buying patterns, a substantial portion of our revenues has been recognized in the last month of each quarter and the last month of the year. We schedule our inventory purchases to meet anticipated customer demand. As a result, relatively small delays in the receipt of manufactured products by us could result in revenues being deferred or lost. Our operating expenses are based upon anticipated sales levels, and a high percentage of our operating expenses are relatively fixed in the short term. Consequently, variations in the timing of sales could cause significant fluctuations in operating results from period to period and may result in unanticipated periodic earnings shortfalls or losses.
If we overestimate demand, we may be required to write off inventories and increase our reserves for product returns. If we underestimate demand, we may not have sufficient inventory of products to ship to our customers. Our products have expiration dates that range from 24 to 36 months from the date of manufacture. We establish reserves for potentially excess, dated or otherwise impaired inventories. We may not be able to accurately estimate the reserve requirement that will be needed in the future. Although our estimates are reviewed quarterly for reasonableness, our product return, rebate or chargeback activity could differ significantly from our estimates. Judgment is required in estimating these reserves and we rely on data from third parties, including, but not limited to, distributor forecasts and independent market research reports. The actual amounts could be different from our estimates, and differences are accounted for in the period in which they become known. If we determine that the actual amounts exceed our reserve amounts, we will record a charge to earnings to approximate the difference. A material reduction in earnings resulting from a charge could have a material adverse effect on our net income, results of operations and financial condition.
43
The agreements governing our credit facilities impose restrictions on our business that may limit our business opportunities and hinder our ability to execute our business strategy.
Our senior secured credit facility contains, and other agreements we may enter into in the future may contain, covenants imposing significant restrictions on our business. These restrictions may affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities as they arise. These covenants place restrictions on our ability to, among other things, incur additional debt, create liens, make investments, enter into transactions with affiliates, sell assets, guarantee debt, declare or pay dividends, redeem common stock or make other distributions to stockholders, and consolidate or merge.
Under the terms of our senior secured credit facilities, if Stonington Capital Appreciation 1994 Fund, L.P., or Stonington, ceases to own at least 20% of our outstanding common stock, it would be considered an event of default. If Stonington's ownership of our outstanding common stock falls under 20% on a fully diluted basis, we would be in default under our credit facilities, which could result in us having to pay off the remainder of the debt from cash reserves or operating cash flow. We may not have sufficient cash or cash flow to repay such indebtedness.
Our ability to comply with these covenants may be affected by events beyond our control, including prevailing economic, financial, and industry conditions. An event of default under our debt agreements would permit our lenders to declare all amounts borrowed from them to be due and payable, together with accrued and unpaid interest. If we were unable to repay debt to our senior lenders, these lenders could proceed against the collateral securing that debt.
We will be subject to the requirements of Section 404 of the Sarbanes-Oxley Act. If we are unable to comply with Section 404 in a timely manner it may affect the reliability of our internal control over financial reporting.
As of December 31, 2007, we are subject to the requirements of Section 404 of the Sarbanes-Oxley Act and the Securities and Exchange Commission rules and regulations require an annual management report on our internal controls over financial reporting, including, among other matters, management's assessment of the effectiveness of our internal control over financial reporting, and an attestation report by our independent registered public accounting firm addressing the effectiveness of our internal control over financial reporting. Although our independent registered public accounting firm did not identify material weaknesses in our internal controls in connection with their audit of our financial statements as of and for the year ended December 31, 2007, we cannot assure you that we will maintain an effective system of internal controls in the future. If we are not able to adequately comply with the requirements of Section 404 we may be subject to sanctions or investigation by regulatory authorities, including the SEC or the Nasdaq National Market. Moreover, if we are unable to assert that our internal control over financial reporting is effective in any future period (or if our independent registered public accounting firm are unable to express an opinion on the effectiveness of our internal controls), we could lose investor confidence in the accuracy and completeness of our financial reports, which may have an a material adverse effect on our Company.
We incur costs as a result of being a public company.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. We incur costs associated with our public company reporting requirements. We also are incurring costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002. We expect these rules and regulations to continue to increase our legal and financial compliance costs and to make some activities more time-consuming and costly. While we have taken steps to improve our financial accounting organization and processes to date, we may still need to make additional changes, including adding staff in the areas of internal control and
44
internal audit. We may also need to adopt and implement additional policies and procedures to further strengthen our financial reporting capability. However, the process of designing and implementing an effective financial reporting system is a continuous effort that will require us to anticipate and react to changes in our business and the economic and regulatory environments. All of this will also require us to expend significant resources. We also expect these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. We are currently evaluating these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Our Board of Directors can issue preferred stock without stockholder approval of the terms of such stock.
Our amended and restated certificate of incorporation authorizes our Board of Directors, without stockholder approval, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges, and restrictions granted to or imposed upon the preferred stock, including voting rights, dividend rights, conversion rights, terms of redemption, liquidation preference, sinking fund terms, subscription rights, and the number of shares constituting any series or the designation of a series. Our Board of Directors can issue preferred stock with voting and conversion rights that could adversely affect the voting power of the holders of common stock, without stockholder approval. As of December 31, 2007, no shares of preferred stock were outstanding and we have no present plan to issue any shares of preferred stock.
Risks related to owning our stock
We expect that the price of our common stock will fluctuate substantially.
The market price for our common stock will be affected by a number of factors, including:
- •
- changes in earnings estimates, investors' perceptions, recommendations by securities analysts or our failure to achieve analysts' earnings estimates;
- •
- quarterly variations in our or our competitors' results of operations;
- •
- the announcement of new products or service enhancements by us or our competitors;
- •
- announcements related to litigation;
- •
- developments in our industry; and
- •
- general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors.
The interests of our controlling stockholder may conflict with the interests of our other stockholders.
As of December 31, 2007, Stonington beneficially owned approximately 20.9% of the outstanding shares of our voting capital stock, without giving effect to the exercise of outstanding options. As a result, Stonington has significant influence over the outcome of matters requiring stockholder approval, including:
- •
- the election and removal of our directors; and
- •
- the approval of mergers, consolidations or the sale of all or substantially all of our assets.
Currently, John A. Bartholdson and Albert J. Fitzgibbons III, both of whom serve on our Board of Directors, are employees of Stonington and serve on the board of directors of Stonington's general partner. This concentration of stock ownership could limit the ability of our other stockholders to influence corporate matters and could have the effect of delaying, deferring or preventing a change in
45
control of the company, or impeding a merger or consolidation, takeover or other business combination or a sale of all or substantially all of our assets. In addition, the significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors' perception that conflicts of interest may exist or arise.
Future sales of our common stock, or the perception in the public markets that these sales may occur, could depress our stock price.
Sales of substantial amounts of our common stock in the public market, or the perception in the public markets that these sales may occur, could cause the market price of our common stock to decline. This could also impair our ability to raise additional capital through the sale of our equity securities. As of December 31, 2007, we had 22,643,564 shares of our common stock outstanding. In addition, we had outstanding options to purchase a total of 1,215,633 shares under our 2000 Stock Option/Stock Issuance Plan and 2005 Stock Incentive Plan of which 559,050 were vested. In February 2007, we filed a Form S-8 registration statement to register all the shares of common stock issuable under our equity incentive plans, including the 2005 Stock Incentive Plan.
Each of Stonington, the holder of approximately 4,722,285 shares of our common stock as of December 31, 2007 and the Zein and Samar Obagi Family Trust, the holder of approximately 2,138,767 shares of our common stock as of December 31, 2007, has rights to demand the registration of their shares or include their shares in registration statements that we may file on our behalf or on behalf of other stockholders.
The partnership through which Stonington holds its shares terminated in March 2007. Although Stonington may return the investment in us through a distribution of shares to the fund's investors, Stonington has also exited its investments in publicly traded companies through a combination of sales under Rule 144, registered secondary offerings, and the distribution of shares to the fund's investors. By exercising its registration rights and selling a large number of shares, Stonington could cause the price of our common stock to decline, which could impede our ability to make acquisitions through the issuance of additional shares of our common stock. Furthermore, if we file a registration statement to offer additional shares of our common stock and have to include shares held by Stonington or other holders of registration rights, it could impair our ability to raise needed capital by depressing the price at which we could sell our common stock.
Provisions in our charter documents and Delaware law could discourage a takeover you may consider favorable or could cause current management to become entrenched and difficult to replace.
Provisions in our certificate of incorporation and our bylaws, as well as Delaware law could make it more difficult for other companies to acquire us, even if doing so would benefit our stockholders. Our certificate of incorporation and bylaws contain the following provisions, among others, which may inhibit an acquisition of our company by a third party:
- •
- advance notification procedures for matters to be brought before stockholder meetings;
- •
- a limitation on who may call stockholder meetings;
- •
- a prohibition on stockholder action by written consent; and
- •
- the ability of our Board of Directors to issue up to 10 million shares of preferred stock without a stockholder vote.
We are also subject to provisions of Delaware law that prohibit us from engaging in any business combination with any "interested stockholder," meaning generally that a stockholder who beneficially owns more than 15% of our stock cannot acquire us for a period of three years from the date this person became an interested stockholder unless various conditions are met, such as approval of the
46
transaction by our Board of Directors. Any of these restrictions could have the effect of delaying or preventing a change in control.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
We currently anticipate that we will retain all future earnings, if any, to finance the growth and development of our business and do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will depend upon our financial condition, capital requirements, earnings and other factors deemed relevant by our Board of Directors. Further, under our Credit Facility with Merrill Lynch Capital, OMP, Inc. (our operating subsidiary) is prohibited from making certain distributions, including payment of dividends, except in certain circumstances.
ITEM 1B: UNRESOLVED SEC STAFF COMMENTS
None.
ITEM 2: PROPERTIES
As of December 31, 2007, we leased the following facilities:
Function
| | Location
| | Square feet
| | Lease expiration
|
|---|
| Headquarters | | Long Beach, CA | | 17,320 | | July 2008 |
| Distribution Center | | Carson, CA | | 27,161 | | October 2008 |
| Manufacturing | | Milford, CT | | 4,000 | | November 2008 |
| Marketing & Training Center | | Beverly Hills, CA | | 2,100 | | July 2011 |
We are currently negotiating a new lease for our headquarters with a new landlord in Long Beach, CA. We believe our facilities are adequate for their intended use and are sufficient for our current level of operations.
Our skin health and licensing activities are initiated and coordinated from our Long Beach headquarters. Our skin health development efforts are based at our Long Beach headquarters, with development and manufacturing facilities in Milford Connecticut. The lease for our manufacturing facility expires in November 2008. Our skin health training center for employees is also located at our Long Beach headquarters. Our cGMP compliance training for distribution personnel and sales training for customer service representatives is provided in Long Beach. In January 2007, we opened a marketing and training center in Beverly Hills to train physicians and aestheticians in the U.S. and internationally. This facility is leased from an affiliate of Dr. Obagi.
Our skin health distribution center is strategically located for quick, convenient and reliable distribution with access to all major carriers in Southern California.
ITEM 3: LEGAL PROCEEDINGS
From time to time, we are involved in litigation and other legal matters in the normal course of business. Management does not believe that the outcome of any of these matters will have a material adverse effect on our consolidated financial position, results of operations or cash flows.
ITEM 4: SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
47
PART II
ITEM 5: MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is quoted on the Nasdaq Global Market under the symbol "OMPI" and has been quoted since our initial public offering on December 13, 2006. The initial public offering price of our common stock on December 14, 2006 was $11.00 per share. No sales of our common stock took place prior to December 14, 2006. The following table sets forth for the fiscal periods indicated the high and low sale prices for our common stock as reported by the Nasdaq:
| | High
| | Low
|
|---|
| Fiscal 2006 | | | | | | |
| | Fourth Quarter(1) | | $ | 11.40 | | $ | 9.40 |
| Fiscal 2007 | | | | | | |
| | First Quarter | | $ | 15.82 | | $ | 8.80 |
| | Second Quarter | | | 19.42 | | | 12.48 |
| | Third Quarter | | | 19.75 | | | 15.15 |
| | Fourth Quarter | | | 24.96 | | | 16.67 |
- (1)
- Beginning December 14, 2006, the first day our stock was listed on Nasdaq.
Holders
The approximate number of stockholders of record of our common stock was 3,005 as of February 15, 2008. Because many of the shares of our common stock are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial owners represented by these shareholders of record.
Dividends
During February 2005, we declared and paid a $3.60 per share common stock dividend, totaling approximately $63.1 million, in cash. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with certain covenants under our credit facilities, which restrict or limit our ability to declare or pay dividends, and will depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our board of directors may deem relevant.
48
Securities authorized for issuance under equity compensation plans
The following table sets forth information regarding outstanding options and rights and shares reserved for future issuance under our equity compensation plans as of December 31, 2007:
Plan Category
| | Number of securities to be issued upon exercise of outstanding options, warrants and rights
| | Weighted-average exercise price of outstanding options, warrants and rights
| | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))(1)
|
|---|
| | (a)
| | (b)
| | (c)
|
|---|
| Equity compensation plans approved by security holders | | 1,215,633 | | $ | 11.21 | | 1,213,095 |
| Equity compensation plans not approved by security holders | | — | | | — | | — |
| | |
| |
| |
|
| Total | | 1,215,633 | | $ | 11.21 | | 1,213,095 |
| | |
| |
| |
|
- (1)
- The 2005 Plan provides that the number of shares authorized for issuance will be cumulatively increased on January 1, 2007 and on each January 1 thereafter for nine years by the least of 500,000 shares, 3% of our outstanding common stock as of the preceding December 31 and a number of shares determined by our board of directors or compensation committee.
Performance Graph
The following information is not deemed to be "soliciting material" or to be "filed" with the SEC or subject to the liabilities of Section 18 of the Exchange Act, and the report shall not be deemed to be incorporated by reference into any prior or subsequent filing by the Company under the Securities Act or the Exchange Act.
The Performance Graph below represents a comparison of the total return of our common stock, the NASDAQ Market Index and the SIC—Pharmaceutical Preparations for the period between December 14, 2006, and December 31, 2007. The graph assumes $100 was invested on December 14, 2006, and dividends are reinvested for the fiscal year ended December 31, 2007.
49
COMPARISON OF CUMULATIVE TOTAL RETURN
AMOUNG OBAGI MEDICAL PRODUCTS, INC.,
NASDAQ MARKET INDEX AND SIC CODE INDEX
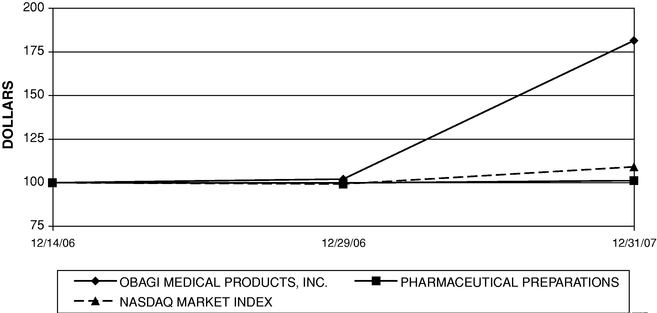
Use of Proceeds from sale of registered securities
We have used all of the remaining proceeds for normal operating purposes from our initial public offering during the year ended December 31, 2007.
ITEM 6: SELECTED FINANCIAL DATA
The selected consolidated financial data set forth below are derived from our consolidated financial statements. The consolidated statements of income data for the years ended December 31, 2005, 2006, and 2007, and consolidated balance sheet data as of December 31, 2006 and 2007, are derived from our audited consolidated financial statements for such years and as of such dates, which are included elsewhere in this Annual Report on Form 10-K. The consolidated statements of income data for the year ended December 31, 2003 and 2004, and consolidated balance sheet data as of December 31, 2005 and 2004 are derived from our audited consolidated financial statements for such years and as of such dates, which are not included in this Annual Report on Form 10-K. The consolidated balance sheet data as of December 31, 2003 are derived from our unaudited consolidated financial statements as of such date, which are not included in this Annual Report on Form 10-K. The unaudited consolidated financial statements include, in the opinion of management, all adjustments, consisting only of normal, recurring adjustments, that management considers necessary for a fair statement of the results of those periods.
50
The information in the following table should be read together with "Management's discussion and analysis of financial condition and results of operations" and our consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K.
| | Year Ended December 31,
|
|---|
| | 2003
| | 2004
| | 2005
| | 2006
| | 2007
|
|---|
| Consolidated statements of income data: | | | | | | | | | | | | | | | |
| Net sales | | $ | 49,261 | | $ | 56,256 | | $ | 64,941 | | $ | 77,996 | | $ | 102,648 |
| Income from operations | | | 18,030 | | | 21,395 | | | 21,084 | | | 17,747 | | | 27,702 |
| Income from operations attributable to common shares(1) | | | | | | | | | | | | | | | |
| | Basic | | $ | 1.11 | | $ | 1.34 | | $ | 1.20 | | $ | 0.99 | | $ | 1.26 |
| | |
| |
| |
| |
| |
|
| | Diluted | | $ | 1.03 | | $ | 1.24 | | $ | 1.19 | | $ | 0.98 | | $ | 1.25 |
| | |
| |
| |
| |
| |
|
| | December 31,
|
|---|
| | 2003
| | 2004
| | 2005
| | 2006
| | 2007
|
|---|
| | (unaudited)
| |
| |
| |
| |
|
|---|
| Consolidated balance sheet data: | | | | | | | | | | | | | | | |
| Total assets | | $ | 35,468 | | $ | 48,704 | | $ | 45,947 | | $ | 52,961 | | $ | 59,763 |
| Long-term debt less current portion | | | 44 | | | 30 | | | 63,195 | | | 23,052 | | | 42 |
| Redeemable preferred stock | | | 22,175 | | | 22,511 | | | — | | | — | | | — |
| Cash dividends declared and paid | | | — | | | — | | | 63,088 | | | — | | | — |
| | Per share(1) | | | — | | | — | | | 3.60 | | | — | | | — |
- (1)
- On November 27, 2006, the Board of Directors of the Company approved an amended and restated Certificate of Incorporation and amended and restated Bylaws in preparation for a contemplated initial public offering. This amended and restated Certificate of Incorporation was approved by the stockholders on November 28, 2006, and was filed by the Secretary of State of the State of Delaware prior to the effectiveness of the Company's Registration Statement on Form S-1. All share and per share amounts included in our financial statements have been adjusted to reflect this 1-for-1.2 reverse stock split for all periods presented.
ITEM 7: MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review the "Risk Factors" section of this Annual Report on Form 10-K for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a specialty pharmaceutical company focused on the aesthetic and therapeutic skin health markets. We develop and commercialize prescription-based, topical skin health systems that enable physicians to treat a range of skin conditions, including pre-mature aging, photo-damage, skin laxity, hyperpigmentation, acne and soft tissue deficits, such as fine lines and wrinkles.
Current products. Our primary product is the Obagi Nu-Derm System, which we believe is the only clinically proven, prescription-based, topical skin health system on the market that has been shown
51
to enhance the skin's overall health by correcting photo-damage at the cellular level, resulting in a reduction of the visible signs of aging. The primary active ingredients in this system are 4% hydroquinone and OTC skin care agents. In April 2004, we introduced the Obagi-C Rx System consisting of a combination of prescription and OTC drugs and adjunctive cosmetic skin care products to treat skin conditions resulting from sun damage and the oxidative damage of free radicals. The central ingredients in this system are 4% hydroquinone and Vitamin C. In October 2005, we launched the Obagi Professional-C products, a complete line of proprietary, non-prescription products, which consists of Vitamin C serums used to reduce the appearance of damage to the skin caused by ultraviolet radiation and other environmental influences. In July 2006, we launched our Obagi Condition and Enhance System, for use in conjunction with commonly performed cosmetic procedures including Botox ® injections. In October 2006, we launched our first product in the ELASTIderm™ product line, an eye cream for improving the elasticity and skin tone around the eyes. We introduced the CLENZIderm M.D.™ system and a second product in the ELASTIderm™ system to address acne and skin elasticity, respectively, based on positive interim clinical results, in February 2007. In July 2007, we launched our second system in the CLENZIderm M.D. line, CLENZIderm M.D. System II, that is specifically formulated for normal to dry skin. In August 2007, we launched two new Nu-Derm Condition and Enhance Systems. One is designed specifically for use with non-surgical procedures while the other has been developed for use with surgical procedures. We also market tretinoin, used for the topical treatment of acne in the United States, and Obagi Blue Peel products, used to aid the physician in the application of skin peeling actives.
Future products. We focus our research and new product development activities on improving the efficacy of established prescription and OTC therapeutic agents by enhancing the penetration of these agents across the skin barrier using our proprietary technologies collectively known as Penetrating Therapeutics. There can be no assurance that we will be able to introduce any additional systems using these technologies.
U.S. distribution. We market all of our products through our direct sales force in the United States primarily to plastic surgeons, dermatologists and other physicians who are focused on aesthetic skin care.
Aesthetic skin care. As of December 31, 2007, we sold our products to over 5,200 physician-dispensing accounts in the United States, with no single customer accounting for more than 5% of our net sales. Our current products are not eligible for reimbursement by Medicare or other third-party payors. We generated U.S. net sales of $87.0 million and $63.9 million during the years ended December 31, 2007 and 2006, respectively.
International distribution. We market our products internationally through 13 international distribution partners that have sales and marketing activities in 36 countries outside of the United States. Much like our business model in the United States, these distributors sell our products through direct sales representatives to physicians, or through alternative distribution channels depending on regulatory requirements and industry practices. We generated international net sales of $11.0 million and $10.0 million during the years ended December 31, 2007 and 2006, respectively.
Licensing. We market our products in the Japanese retail markets through a trademark and know-how license agreement with Rohto. Under our agreement, Rohto is licensed to manufacture and sell a series of OTC products under the Obagi brand name in the Japanese drug store channel, and we receive a royalty based upon sales of Obagi branded products in Japan by Rohto. Rohto's Obagi branded products are sold through approximately 5,000 high-end drug stores. We have other licensing arrangements in Japan to market and sell OTC product systems under the Obagi brand, both for in-office use in facial procedures, as well as for sale as a take-home product kit in the spa channel. We receive royalties based upon these arrangements. We generated licensing revenue of $4.7 million and $4.1 million during the years ended December 31, 2007 and 2006, respectively.
52
Sales growth. Our total net sales have grown from $78.0 million for the year ended December 31, 2006 to $102.6 million for the year ended December 31, 2007. Our sales growth has been driven primarily by:
- •
- Our professional marketing efforts to physicians. Our professional sales force targets physicians who are providing aesthetic treatments and educates them on how to best provide our products to their patients. Our professional sales force also provides education to physicians, aestheticians, other staff and patients about the benefits of promoting skin health. We have increased our sales force from 96 employees as of January 1, 2006 to 118 employees as of December 31, 2007. We will continue to invest in expanding our sales force as warranted by the success of new product offerings and continued core product growth.
- •
- Strong growth in the aesthetic skin care market. In 2005, the global skin care market was estimated to be $36.2 billion, of which over 62% were facial skin care products, according to Global Industry Analysts, Inc., a market research firm. Additionally, the independent research firm, Kalorama Information, estimates that from 2005 to 2010, over 70 million people in the United States will receive cosmetic facial procedures for which they will pay over $60 billion. We believe this reflects a growing desire and acceptance among the aging population to seek aesthetic facial products and procedures from their physicians. With our leading position in the physician-dispensed channel, the clinically proven aesthetic benefits of our products and the potential of our systems to enhance or complement many other facial procedures, we believe we are well positioned to meet this growing patient demand.
- •
- Conducting clinical studies on our products. We have completed 37 clinical studies since 2003 to demonstrate the efficacy of our products. We believe that these clinical studies provide our products added scientific credibility in the physician-dispensed market. We are currently conducting four clinical studies on new and existing products and plan on initiating six additional clinical studies by December 31, 2008.
- •
- International. We have formal distribution agreements with 13 distribution partners covering over 50 countries and a trademark and product know-how license agreement in the OTC market of Japan. Currently, those distributors have sales activities in approximately 36 countries, with growth in 2006 and 2007 coming primarily from the Middle East, Far East, Europe, and North America (excluding the United States).
Results of operations. We commenced operations in 1997, and as of December 31, 2007, we had an accumulated deficit of $6.0 million. The accumulated deficit arose when we paid a $63.1 million common stock dividend in February 2005. At December 31, 2004, we had accumulated earnings of $20.4 million. We reported net income of $15.2 million and $6.1 million for the years ended December 31, 2007 and December 31, 2006, respectively.
Seasonality. Sales of our products have historically been higher between September and March. We believe this is due to increased product use and patient compliance during these months. We believe this increased usage and compliance relates to several factors such as higher patient tendencies toward daily compliance inversely proportionate to their tendency to travel and/or engage in other disruptive activities during summer months. Patient travel and other disruptive activities that affect compliance are at their peak during July and August.
Economy. Many treatments in which our products are used are considered cosmetic in nature, are typically paid for by the patient out of disposable income and are not subject to reimbursement by third-party payers such as health insurance organizations. As a result, we believe that our future sales growth may to some extent be influenced by the economic conditions within the geographic markets we sell our products. Historically, we have not been able to quantify with any degree of certitude the impact, if any, economic conditions have had on our sales. We believe, however, that the aesthetic nature of our products, the desire to maintain a healthy and youthful appearance and the demographics
53
of the patients that use our products should limit the negative impact on our sales assuming any such economic downturn is not severe.
Future growth. We believe that our future growth will be driven by increased direct sales coverage, ongoing marketing efforts to create increased awareness of the Obagi brand and the benefits of skin health and new product offerings. We plan to continue to invest significant resources on the commercialization of new applications of our current products, the continuing development of our pipeline products and the in-licensing or acquisition of new product opportunities. Our on-going profitability is primarily dependent upon the continued success of our current product offerings.
Critical accounting policies and use of estimates
Our discussion and analysis of our financial condition and results of operations is based upon our financial statements which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, sales and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On a periodic basis, we evaluate our estimates, including those related to revenue recognition, sales return reserve, accounts receivable, inventory and goodwill and other intangible assets. We use authoritative pronouncements, historical experience and other assumptions as the basis for making estimates. By their nature, these estimates are subject to an inherent degree of uncertainty. As a result, we cannot assure you that actual results will not differ significantly from estimated results. Our significant accounting policies are further described in Note 2 to our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue recognition. We recognize revenues in accordance with Securities and Exchange Commission ("SEC") Staff Accounting Bulletin ("SAB") No. 104 ("SAB No. 104"),Revenue Recognition in Financial Statements, which provides guidance on the recognition, presentation and disclosure of revenue in financial statements filed with the SEC. SAB No. 104 outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure related to revenue recognition policies. We recognize revenue when (i) persuasive evidence of an arrangement exists, (ii) shipment of products has occurred or services have been rendered, (iii) the sales price charged is fixed or determinable and (iv) collection is reasonably assured. Our shipment terms are FOB shipping point as outlined in our sales agreements.
Our domestic sales agreements do not provide for rights of return or price protection. However, we may approve returns on a case-by-case basis at our discretion. Certain international distribution agreements do provide for rights of return and price protection. Generally, such return rights are for a period of not more than 90 days after the products have been shipped to the distributors. In accordance with SFAS No. 48,Revenue Recognition When Right of Return Exists, we continuously monitor and track product returns and record a provision for the estimated future amount of such future returns, based on historical experience and any notification we receive of pending returns. We do not grant any warranty provisions on our products. We provide for discounts and allowances based on historical experience at the time revenue is recognized as a reduction to revenue. To date such provisions have approximated management's estimates. Other than our provision for future returns, the only estimates that we have to make regarding revenue recognition pertain to the collectability of the resulting receivable, both of which are discussed below.
We grant price protection rights to certain international distributors. Such price protection rights require us to pay the distributor if there is a reduction in the list price of our products. Price protection payments would be required for the distributor's inventory on-hand or in-transit on the date of the price reduction, for a period not to exceed 90 days prior to the date of the price reduction. We have
54
not recorded a liability in connection with such price protection rights as we have never reduced the list prices of our products.
In September 2002, we entered into a licensing agreement with Rohto (see Note 8 to our financial statements), a large Japan-based company that specializes in the distribution and marketing of OTC medical oriented products in the drug store and retail channels. In January 2006, we entered into a licensing agreement with Tokyo Beauty Center (see Note 8), a diversified Japanese consumer products and services company, which also owns and operates a large chain of aesthetic spas in Japan. Royalty revenue is recognized as earned and is based upon a predetermined rate within the respective licensing agreement.
Sales returns and allowances. When we sell our products, we reduce the amount of revenue recognized from such sales by an estimate of future product returns and other sales allowances. Sales allowances include cash discounts, rebates and sales incentives relating to products sold in the current period. Factors that are considered in our estimates regarding sales returns include the historical rate of returns and current market conditions. We maintain a return policy that allows our customers to return product within a specified period after shipment of the product has occurred. Factors that are considered in our estimates regarding sales allowances include quality of product and recent promotional activity. If actual future experience for product returns and other sales allowances exceed the estimates we made at the time of sale, our financial position, results of operations and cash flow would be negatively impacted. To date, such provisions have approximated management's estimates.
Accounts receivable. We perform periodic credit evaluations of our customers and adjust credit limits based upon payment history and the customer's current creditworthiness, as determined by our review of current credit information. We monitor collections and payments from our customers and maintain an allowance for doubtful accounts based upon our historical experience and any specific customer collection issues that we have identified. Receivables are charged to the allowance for doubtful accounts when an account is deemed to be uncollectible, taking into consideration the financial condition of the customer and the value of any collateral. Recoveries of receivables previously charged off as uncollectible are credited to the allowance. If the financial condition of our customers deteriorates, resulting in an impairment of their ability to make payments and this exceeds our estimates of the balance sheet date, we would need to charge additional receivables to our allowances, and our financial position, results of operation and cash flow would be negatively impacted. Our credit losses have historically been within our expectations and the allowance established.
Inventory. We state our inventories at the lower of cost or market, computed at actual cost on a first-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Inventory reserves are established when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. Inventory reserves are measured as the difference between the cost of inventory and estimated market value. Inventory reserves are charged to cost of sales and establish a lower cost basis for the inventory. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to changing technology, new product offerings and customer demand levels. Unfavorable changes in market conditions may result in a need for additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins. To date, actual reserve requirements approximated management's estimates.
Goodwill and other intangible assets. Effective January 1, 2002, we adopted SFAS No. 142 ("SFAS No. 142"),Goodwill and Other Intangible Assets, which requires, among other things, the use of a nonamortization approach for purchased goodwill and certain intangibles. Under a nonamortization approach, goodwill and intangibles having an indefinite life are not amortized, but instead will be reviewed for impairment at least annually or if an event occurs or circumstances indicate the carrying amount may be impaired. Events or circumstances which could indicate an impairment include a significant change in the business climate, economic and industry trends, legal factors, negative
55
operating performance indicators, significant competition, changes in our strategy or disposition of a reporting unit or a portion thereof. Goodwill impairment testing is performed at the reporting unit level.
SFAS No. 142 requires that goodwill be tested for impairment using a two-step process. The first step of the goodwill impairment test, used to identify potential impairment, compares the fair value of a reporting unit with its carrying amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test must be performed to measure the amount of impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of reporting unit goodwill with the carrying amount of that goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. If the carrying amount of the reporting unit goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess.
Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to such reporting units, assignment of goodwill to such reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated using a discounted cash flow methodology. This requires significant judgments including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth for our business, the useful life over which cash flows will occur, and determination of our weighted average cost of capital. Changes in these estimates and assumptions could materially affect the determination of fair value and/or goodwill impairment for each reporting unit.
We have selected September 30 as the date on which we will perform our annual goodwill impairment test. Based on our analyses, no impairment charges were recognized for the years ended December 31, 2007, 2006 and 2005.
Other intangible assets consist of trademarks, distribution rights, covenants not-to-compete, patents, customer lists, and proprietary formulations. Other intangible assets are amortized over the expected period of benefit using the straight-line method over the following lives: trademarks (20 years); distribution rights (ten years); covenants not-to-compete (seven years); and other intangible assets (three to 17 years). Any unfavorable changes in market conditions or our product lines may result in the impairment of goodwill or an intangible asset, which could adversely impact our net income.
Leases. We account for leases under the provisions of SFAS No. 13,Accounting for Leases, and subsequent amendments, which require that our leases be evaluated and classified as either operating leases or capital leases for financial reporting purposes. Minimum base rents for our operating leases, which generally have scheduled rent increases over the term of the lease, are recorded on a straight-line basis over the lease term. The initial lease term includes the period from when we are given access and control over the lease property, whether or not rent payments are due under the terms of the lease.
For leases with renewal periods at our option, we generally consider the lease term to consist of the initial lease term, as exercise of the renewal options as determined at lease inception are not considered to be reasonably assured of exercise. However, if failure to exercise a renewal option imposes an economic penalty of sufficient magnitude to us, then the renewal, at inception, is reasonably assured and will be included in the determination of the appropriate lease term.
In certain instances, we disburse cash for leasehold improvements, furnishings, fixtures and equipment to renovate leased premises. If costs are paid directly by the landlord or reimbursed to us by the landlord, we record a deferred rent liability and amortize the deferred rent liability over the lease
56
term as a reduction to rent expense. In other instances, we may expend cash for landlord additions that we make to lease premises that are reimbursed to us by the landlord. Based on the specifics of the leased space and the lease agreement, during the renovation period, amounts paid will be recorded as prepaid rent and any landlord reimbursement will be recorded as an offset to prepaid rent.
Stock-based compensation. We account for stock-based compensation in accordance with the provisions of SFAS No. 123R ("SFAS No. 123R"),Share-Based Payment. In connection with our January 2006 adoption of SFAS No. 123R, we adopted the modified prospective transition method in the first quarter of fiscal 2006, and as a result, did not retroactively adjust results from prior periods. Under this transition method, stock-based compensation is recognized for: (i) expense related to the remaining unvested portion of all stock option awards granted during the one year period preceding our initial public offering, based on the grant date fair value estimated in accordance with the original provisions of SFAS No. 123; and (ii) expense related to all stock option awards granted on or subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123R. In accordance with SAB No. 107, the remaining unvested options we issued prior to the one year period preceding our initial public offering, with the exception of the October 2005 grants, are not included in the SFAS No. 123R option pool as they were valued using the minimum value method. As a result, unless subsequently modified, repurchased or cancelled, such unvested options will not be included in stock-based compensation. Determining the appropriate fair-value model and calculating the fair value of stock-based awards at the date of grant using any valuation model requires judgment. We use the Black-Scholes option-pricing model, which requires the input of highly subjective assumptions. These assumptions include estimating the length of time employees will retain their stock options before exercising them ("expected term"), the estimated volatility of our common stock price over the expected term and the number of options that will ultimately not complete their vesting requirements ("forfeitures"). We estimated our options' expected terms in accordance with SAB No. 107 and SAB No. 110, using our best estimate of the period of time from the grant date that we expect the options to remain outstanding. If we determine another method to estimate expected volatility or expected term was more reasonable than our current methods, or if another method for calculating these input assumptions is prescribed by authoritative guidance, the fair value calculated for stock-based awards could change significantly. Higher volatility and expected terms result in a proportional increase to stock-based compensation determined at the date of grant. The expected dividend rate and expected risk-free rate of return are not as significant to the calculation of fair value.
As a result of adopting SFAS No. 123R, the impact to our Consolidated Statement of Income for the year ended December 31, 2006 on income before income taxes and net income was $305,000 and $195,000, and did not have a material effect on basic and diluted earnings per share, respectively. For the year ended December 31, 2007, the impact on income before income taxes and net income was $1,557,000 and $930,000 and had a $0.04 and a $0.04 impact on basic and dilutive earnings per share, respectively. In addition, prior to the adoption of SFAS No. 123R, we presented the tax benefit resulting from the exercise of stock options as operating cash inflows in our Consolidated Statements of Cash Flows. Upon the adoption of SFAS No. 123R, the excess tax benefits for those options are classified as financing cash inflows.
Prior to our common stock being actively traded, our board of directors, the members of which we believe have extensive business, finance and investment experience, were required to estimate the fair value of our common stock at the time of each option grant for purposes of determining stock-based compensation expense for the periods presented herein. In response to that requirement, our board of directors appointed members of the compensation committee to analyze our stock value and recommend common stock valuation estimates. For economic reasons, we initially chose to value our options using a model that employed a market based approach based principally upon applying appropriate valuation multiples to the EBITDA and revenue and not to obtain a contemporaneous valuation by an independent third party. As a privately held company, we believed that the members of
57
our compensation committee had the requisite experience and expertise to determine the fair value of the options. If a valuation model using different input variables and assumptions had been used, our common stock valuation may have been different.
If we had made different assumptions and estimates, the amount of our recognized and to be recognized stock-based compensation expense, net income and net income per share amounts could have been materially different. We believe that we have used reasonable methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Practice Guide,Valuation of Privately-Held-Company Equity Securities Issued as Compensation, to determine the fair market value of our common stock.
Uncertainty in Income Taxes. Effective January 1, 2007, we began to measure and record tax contingency accruals in accordance with FIN 48 ("FIN 48"),Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109. The expanded disclosure requirements of FIN 48 are presented in Note 6 to the Consolidated Condensed Financial Statements in Part I, Item I. FIN 48 prescribes a threshold for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Only tax positions meeting the more-likely-than-not recognition threshold at the effective date may be recognized or continue to be recognized upon adoption of this interpretation. FIN 48 also provides guidance on accounting for derecognition, interest and penalties, and classification and disclosure of matters related to uncertainty in income taxes. Prior to January 1, 2007, we recorded accruals for tax contingencies and related interest when it was probable that a liability had been incurred and the amount of the contingency could be reasonably estimated based on specific events such as an audit or inquiry by a taxing authority. As a result of the implementation of FIN 48, the Company recognized approximately a $0.1 million increase in the liability for unrecognized tax benefits, which was accounted for as an increase to the January 1, 2007 accumulated deficit balance.
Results of operations
The year ended December 31, 2007 compared the year ended December 31, 2006
Net sales. The following table compares net sales by product line and certain selected products for the year ended December 31, 2007 and 2006:
| | Year ended December 31,
| |
|---|
| | 2007
| | 2006
| | Change
| |
|---|
| | (in thousands)
| |
| |
|---|
| Net Sales by Product Category: | | | | | | | | | |
| | Skin Health | | | | | | | | | |
| | | Nu-Derm | | $ | 62,184 | | $ | 53,836 | | 16 | % |
| | | Vitamin C | | | 11,438 | | | 9,986 | | 15 | |
| | | Skin laxity | | | 8,356 | | | 2,278 | | 267 | |
| | | Therapeutic | | | 5,753 | | | 180 | | 3097 | |
| | | Other | | | 10,261 | | | 7,660 | | 34 | |
| | |
| |
| |
| |
| | | | Total | | | 97,992 | | | 73,940 | | 33 | |
| | | Licensing fees | | | 4,656 | | | 4,056 | | 15 | |
| | |
| |
| |
| |
| Total net sales | | $ | 102,648 | | $ | 77,996 | | 32 | % |
| | |
| |
| |
| |
| United States | | | 85 | % | | 82 | % | | |
| International | | | 15 | % | | 18 | % | | |
Net sales increased by $24.6 million, or 32%, to $102.6 million during the year ended December 31, 2007, as compared to $78.0 million during the year ended December 31, 2006. During the year ended December 31, 2007, we experienced Nu-Derm sales growth of $8.3 million. In addition, we saw sales growth of $1.4 million and $2.6 million in the Vitamin C and Other categories, respectively. The two product categories formally launched in 2007, Skin laxity and Therapeutic,
58
increased $6.1 million and $5.6 million, respectively. Licensing fees increased $0.6 million during the year ended December 31, 2007. Our sales growth was comprised of $23.1 million of growth in the United States and $1.5 million in growth from international markets. U.S. growth was fueled by the overall growth in the skin care market, the recent launch of two new product lines and the expansion of our sales force. Over 83% of the product category sales growth experienced was attributable to unit volume growth. The Nu-Derm product category growth was largely driven by the expansion of our sales force, the promotional and educational efforts of our professional sales force and the launch of Nu-Derm Condition and Enhance for Botox® in July 2006 and the launch of two Condition and Enhance Systems in August 2007, which on a combined basis contributed $9.1 million. The growth in the Vitamin C category was primarily driven by the strong sales performance by the Obagi-C Rx product line. The Skin laxity product category growth was due to a full year of sales of our ELASTIderm Eye Cream product during the year ended December 31, 2007 as compared to only 3 months during the year ended December 31, 2006 and the February 2007 launch of the second product in the category, ELASTIderm Eye Gel. The Therapeutic product category growth is attributable to the official launch of our CLENZIderm™ product line in February 2007 and the launch of the second CLENZIderm system in July 2007. The Other product category growth was driven by the continued success of our system approach concept launched in early 2005. The system approach is designed to educate physicians on the benefits of prescribing the complete Nu-Derm system as opposed to partial systems. International sales growth was primarily in the Skin laxity, Vitamin C and Therapeutic product lines and primarily came from four regions, $0.5 million from the Europe and Other region, $0.2 million from North America excluding the United States, $0.1 million from the Middle East, and $0.1 million from the Far East. Our licensing fees increased $0.6 million primarily due to fees received under our agreement with Rohto.
Gross margin percentage. Our gross margin percentage decreased to 82.4%, for the year ended December 31, 2007 compared to 82.7% for the year ended December 31, 2006, primarily as a result of: (i) the $5.6 million increase in Therapeutic sales, which solely consist of our CLENZIderm product line that has a lower gross margin compared to our other product line offerings; (ii) start up manufacturing costs for the BPO serum gel burden on the initial units produced and sold; and (iii) a decline in our Vitamin C margin due to a reserve for obsolete inventories arising from product reformulation of approximately $0.1 million. This was partially offset by improved margins on products in our Other product line due to new contract volume purchasing discounts with the manufacturer. Although we anticipate gaining efficiency in our manufacturing processes for CLENZIderm, we believe our margins will remain slightly lower in the near future as we ramp up the sales volume of our CLENZIderm product line. Cost of sales also consists of outbound shipping and handling, work order scrap, licensing and royalty fees related to licensed intellectual property, depreciation and amortization attributable to products sold, and an inventory reserve for shrinkage and write-downs.
Selling, general and administrative. Selling, general and administrative expense consists primarily of salaries and other personnel-related costs, professional fees, insurance costs, stock based compensation, depreciation and amortization not attributable to products sold, warehousing costs, advertising, travel expense and other selling expenses. Selling, general and administrative expenses increased $10.5 million to $51.4 million during the year ended December 31, 2007, as compared to $40.9 million for the year ended December 31, 2006. This increase is primarily due to the following: (i) a $5.5 million increase due to the hiring of additional employees, largely direct sales and administrative support personnel, during the year ended December 31, 2007, as compared to the year ended December 31, 2006; (ii) a $1.3 million increase in expenses for physician training, practice building and patient acquisition; (iii) $1.2 million increase primarily consisting of market research targeting physicians and patients and for product development; (iv) a $1.1 million increase in non-cash compensation expense due to the adoption of SFAS No. 123R on January 1, 2006; (v) a $1.1 million increase due to expenses related to the secondary offering completed in October 2007; (vi) a $0.7 million increase in insurance; (vii) a $0.5 million increase in volume driven activities; (viii) a $0.5 million increase in operational and
59
manufacturing support activities; (ix) a $0.2 million increase in depreciation and amortization; and (x) a $0.1 million increase in advertising costs; offset by, (i) a $1.0 million decrease in expenses relating to operating as a public company; (ii) a $0.4 million decrease in management fees; and (iii) a $0.3 million decrease in professional fees. As a percentage of net sales, selling, general and administrative expenses in the year ended December 31, 2007 were 50% as compared to 52% in the year ended December 31, 2006. We expect to incur additional operating costs, such as professional fees and insurance costs, related to the growth of our business. However, we believe selling, general and administrative expenses will decrease as a percentage of net sales.
Research and development. Research and development expenses decreased $0.4 million to $5.5 million for the year ended December 31, 2007 as compared to $5.9 million for the year ended December 31, 2006. This decrease is primarily due to a one-time payment of $0.4 million to Dr. Zein Obagi, or Dr. Obagi, one of our principal stockholders and one of our former officers and directors, under a non compete agreement during the year ended December 31, 2006. There were no such payments made in 2007. As a percentage of net sales, research and development costs in the year ended December 31, 2007 were 5% as compared to 8% in the year ended December 31, 2006.
Interest income and Interest expense. Interest expense was $2.4 million during the year ended December 31, 2007, as compared to $8.2 million for the year ended December 31, 2006. The decline is primarily attributable to the prepayment of $35.0 million in debt with the net cash proceeds received in our initial public offering completed in December 2006. During the year ended December 31, 2007 we made $23.5 million in prepayments, $7.0 million of which came from the net proceeds received in our secondary public offering completed in October 2007, in addition to our regularly scheduled payments of $0.8 million and an excess cash payment of $1.3 million. The additional prepayments resulted in a complete paydown of our outstanding debt. Interest income increased $177,000 to $190,000 during the year ended December 31, 2007, as compared to $13,000 for the year ended December 31, 2006. The increase is primarily due to an increase in our average cash balance to $8.6 million during the year ended December 31, 2007, compared to $4.1 million during the year ended December 31, 2006.
Income taxes. Income tax expense increased $6.8 million to $10.3 million for year ended December 31, 2007, as compared to $3.5 million for the year ended December 31, 2006. Our effective tax rate increased 4% to 40% for the year ended December 31, 2007, compared to 36% for the year ended December 31, 2006. The increase is primarily due to the decrease in the tax benefit, as a percentage of net income, derived from the research and development credit and certain costs incurred during the secondary offering completed in October 2007 not being deductible for tax purposes during the year ended December 31, 2007.
60
The year ended December 31, 2006 compared the year ended December 31, 2005
Net sales. The following table compares net sales by product line and certain selected products for the year ended December 31, 2006 and 2005:
| | Year ended December 31,
| |
|---|
| | 2006
| | 2005
| | Change
| |
|---|
| | (in thousands)
| |
| |
|---|
| Net Sales by Product Category: | | | | | | | | | |
| | Skin Health | | | | | | | | | |
| | | Nu-Derm | | $ | 53,836 | | $ | 47,115 | | 14 | % |
| | | Vitamin C | | | 9,986 | | | 8,570 | | 17 | |
| | | Skin laxity | | | 2,278 | | | — | | — | |
| | | Therapeutic | | | 180 | | | — | | — | |
| | | Other | | | 7,660 | | | 5,854 | | 31 | |
| | |
| |
| |
| |
| | | | Total | | | 73,940 | | | 61,539 | | 20 | |
| | | Licensing fees | | | 4,056 | | | 3,402 | | 19 | |
| | |
| |
| |
| |
| Total net sales | | $ | 77,996 | | $ | 64,941 | | 20 | % |
| | |
| |
| |
| |
| United States | | | 82 | % | | 81 | % | | |
| International | | | 18 | % | | 19 | % | | |
Net sales increased by $13.1 million, or 20%, to $78.0 million during the year ended December 31, 2006, as compared to $64.9 million during the year ended December 31, 2005. During the year ended December 31, 2006, we experienced Nu-Derm sales growth of $6.7 million, which was largely driven by the launch of our Nu-Derm Condition and Enhance System for Botox ® in July 2006. In addition, we saw a combined sales growth of $3.2 million in the Vitamin C and Other product categories, new sales of $2.3 million from the launch of the our ELASTIderm™ product within the Skin laxity product category, $0.7 million of growth in licensing fees and $0.2 million from the soft launch of the CLENZIderm™ product line in October 2006, within the Therapeutic product category. This sales growth was comprised of $11.1 million of growth in the United States and $2.0 million in growth from international markets. U.S. growth was fueled by the overall growth in the skin care market. Over 79% of the product category sales growth experienced was attributable to unit volume growth. The Nu-Derm product category growth was largely driven by the expansion of our sales force, the promotional and educational efforts of our professional sales force and the launch of Nu-Derm Condition and Enhance for Botox, which contributed $4.9 million. The growth in the Vitamin C category was primarily due to the successful launch of a new Vitamin C line during 2005 and early 2006, and a strong sales performance by the Obagi-C Rx. The skin laxity product category growth was driven by the launch of the first product within the ELASTIderm product line in the latter half of October 2006. The Other product category growth was driven by the launch of a new product and a system approach concept launched in early 2005. The system approach is designed to educate physicians on the benefits of prescribing the complete Nu-Derm system as opposed to partial systems. International sales growth was primarily in the Nu-Derm product line and primarily came from four regions, $0.6 million from the Middle East, $0.3 million from North America excluding the United States, $0.3 million from the Far East, and $0.1 million from the Europe and Other region. Our licensing fees increased $0.7 million primarily due to fees received under our agreement with Tokyo Beauty Centers Group, Inc.
Gross margin percentage. Our gross margin percentage increased to 82.7%, for the year ended December 31, 2006 compared to 82.2% for the year ended December 31, 2005, primarily as a result of: (i) the $1.4 million increase in Vitamin C sales, which have an improved gross margin over the prior year; and (ii) improved margins on products in our Other product line due to new contract volume purchasing discounts with the manufacturer. We launched a new vitamin C line in October 2005 and
61
January 2006 to replace third-party-produced Vitamin C with our own branded product, which has significantly higher margins. The Nu-Derm product line gross margin percentage for the year ended December 31, 2006 declined slightly when compared to the year ended December 31, 2005, primarily due to an increase in practice building initiatives. During the introduction of the first product in the ELASTIderm product line, the Company used pricing promotions as part of its launch strategy to drive ELASTIderm into its existing customer base. These pricing promotions slightly reduced overall margins. Our cost of sales includes the cost of our finished goods, which includes product and packaging materials purchased from third party vendors and bulk and fill purchased from independent manufacturers. Cost of sales also consists of outbound shipping and handling, work order scrap, licensing and royalty fees related to licensed intellectual property, depreciation and amortization attributable to products sold, and an inventory reserve for shrinkage and write-downs.
Selling, general and administrative. Selling, general and administrative expense consists primarily of salaries and other personnel-related costs, professional fees, insurance costs, stock based compensation, depreciation and amortization not attributable to products sold, warehousing costs, advertising, travel expense and other selling expenses. Selling, general and administrative expenses increased $11.9 million to $40.9 million during the year ended December 31, 2006, as compared to $29.0 million for the year ended December 31, 2005. This increase is primarily due to the following: (i) a $4.4 million increase due to the hiring of additional employees, largely direct sales and administrative support personnel, during the year ended December 31, 2006, as compared to the year ended December 31, 2005; (ii) a $2.9 million increase in marketing and sales expenses related to our acne and elasticity product lines; (iii) a $2.3 million increase in market research and promotion activity aimed towards targeting physicians and patients; (iv) a $1.8 million increase in expenses related to our initial public offering; (v) a $0.4 million increase in depreciation and amortization; (vi) a $0.4 million increase in legal fees; (vii) a $0.3 million increase in new indication sales research and promotions; and (viii) a $0.3 million increase in non-cash compensation expense due to the adoption of SFAS No. 123R on January 1, 2006; offset by a $0.9 million decline in advertising costs. As a percentage of net sales, selling, general and administrative expenses in the year ended December 31, 2006 were 52% as compared to 45% in the year ended December 31, 2005. We expect to incur additional operating costs, such as professional fees and insurance costs, related to the growth of our business and our operations as a public company. However, we believe selling, general and administrative expenses will decrease as a percentage of net sales.
Research and development. Research and development expenses increased $2.6 million to $5.9 million for the year ended December 31, 2006 as compared to $3.3 million for the year ended December 31, 2005. This increase is primarily due to the following: (i) a $3.0 million increase in development costs related to our acne and elasticity product lines and future product pipeline development; (ii) a one-time payment of $0.4 million to Dr. Zein Obagi, or Dr. Obagi, one of our principal stockholders and one of our former officers and directors, under a non compete agreement; and (iii) a $0.1 million increase due to the employment of additional employees during the year ended December 31, 2006, as compared to the year ended December 31, 2005, offset by a $0.9 million decline in research and development expenses related to our existing products. As a percentage of net sales, research and development costs in the year ended December 31, 2006 were 8% as compared to 5% in the year ended December 31, 2005.
Interest income and Interest expense. Interest expense was $8.2 million during the year ended December 31, 2006, as compared to $6.1 million for the year ended December 31, 2005. Approximately $1.3 million of the increase is due to the write down in debt issuance costs resulting from a prepayment of $35.0 million in debt with the net cash proceeds received in our initial public offering. The remaining increase is due to the fact that we only recorded eleven months of interest for the facility for the year ended December 31, 2005, as we obtained a $70.0 million Credit Agreement on January 28, 2005, compared to twelve months of interest being recorded for the year ended December 31, 2006.
62
Interest income decreased $48,000 to $13,000 during the year ended December 31, 2006, as compared to $61,000 for the year ended December 31, 2005. The decrease is primarily due to a decline in our average cash balance to $4.1 million during the year ended December 31, 2006, compared to $5.4 million during the year ended December 31, 2005.
Income taxes. Income tax expense decreased $2.5 million to $3.5 million for year ended December 31, 2006, as compared to $6.0 million for the year ended December 31, 2005. Our effective tax rate declined 4% to 36% for the year ended December 31, 2006, compared to 40% for the year ended December 31, 2005. The decline is primarily due to the impact of the research and development credit recorded during the year ended December 31, 2006.
Liquidity and capital resources
Management assesses our liquidity by our ability to generate cash to fund our operations. Significant factors in the management of liquidity are: funds generated by operations; levels of accounts receivable, inventories, accounts payable and capital expenditures; funds required for acquisitions; adequate credit facilities; and financial flexibility to attract long-term capital on satisfactory terms. As of December 31, 2007, our accumulated deficit was $6.0 million. We currently invest our cash and cash equivalents in large money market funds consisting of debt instruments of the U.S. government, its agencies and high-quality corporate issuers. Historically, we have generated cash from operations in excess of working capital requirements and through private and public sales of common stock. We had $10.0 million available on our revolving line of credit as of December 31, 2007 and December 31, 2006. In December 2006, pursuant to the third amendment of our Credit Facility entered into in November 2006, we made a prepayment of $35.0 million on the outstanding balance of the term loans with our net proceeds received from the completion of our initial public offering on December 19, 2006. During the year ended December 31, 2007, we also made a mandatory $1.3 million excess cash payment, $0.8 million in principal payments and $23.5 million in optional principal repayments. Approximately $9.2 million of the net proceeds generated from our secondary public offering on October 12, 2007 was utilized to pay down our outstanding balance of our term loans and our revolving line of credit. As of December 31, 2007, we had completely paid down the outstanding debt under the facility. Borrowings under the facility are subject to certain financial and operating covenants that include, among other provisions, maintaining a maximum Debt to EBITDA Ratio and minimum EBITDA levels and restrictions on our ability to pay dividends. Under the terms of the facility, if Stonington ceases to own at least 20% of our outstanding common stock, it would be considered an event of default. Certain covenants also limit annual capital expenditures and use of proceeds from the issuance of debt and equity securities. We were in compliance with all financial and non-financial covenants as of December 31, 2007 and 2006.
On May 29, 2007, we entered into a Settlement and Consent Agreement and Mutual Release, or the Agreement, with the estate of Austin T. McNamara, the former Chairman of our Board of Directors, President and Chief Executive Officer, the McNamara Family Irrevocable Trust dated December 17, 2004, the McNamara Family Trust dated December 27, 2004 or collectively, the Trusts, Lucy B. McNamara, individually and as trustee of the Trusts and as executor of the estate of Austin T. McNamara, and Lighthouse Venture Group, LLC, a limited liability company that was controlled by Mr. McNamara or collectively, the McNamara Parties. Pursuant to the Agreement, we and the McNamara Parties have released each other from all claims we may have against each other, except for claims related to potential taxes and related charges, should they arise, with respect to the past exercise of certain stock options by the Trusts. No payments were made by the parties to each other in connection with the settlement of the claims. The settlement includes the release of claims by the McNamara Parties that we were obligated to purchase the 1,875,001 shares of our common stock owned by the Trusts and all claims related to Mr. McNamara's employment with us and the termination of his employment. In connection with the release, we have written off a net receivable of
63
approximately $0.1 million for a claimed compensation overpayment to a McNamara Party. The McNamara Parties also sold all of their Obagi stock held at the time of the settlement to an institutional investor in a privately negotiated transaction.
As of December 31, 2007 and 2006, we had approximately $14.1 million and $11.3 million, respectively, of cash and cash equivalents and working capital of $34.2 million and $21.9 million, respectively, which includes a current deferred tax asset of $1.8 million and $1.0 million as of December 31, 2007 and 2006, respectively, related primarily to the future benefit of our net operating losses for tax purposes. We anticipate our selling and marketing expenses to increase as we seek to: (i) expand our professional sales force; (ii) increase our efforts towards physician training and patient awareness; (iii) support the launch of new products or expanded application of existing products; and (iv) expand our distribution to new physician specialties. We anticipate that our administrative expenses will increase to support our current growth plans and compliance with our obligations as a public company.
Our research and development expenses will likely increase as a result of our current plans to (i) research and develop new products and (ii) expand the application of current products. We believe that our cash outflows related to acquiring products and entering into licensing agreements may increase as we seek to expand our product portfolio. Additionally, if sales of our current products continue to increase and/or we increase the number of products in our portfolio, we may find it necessary to consider alternative manufacturing relationships, which may include the acquisition of our own manufacturing facilities or additional capital expenditures for facilities and equipment of third-party manufacturers that are dedicated to our products.
We continually evaluate new opportunities for therapeutic systems or products and, if and when appropriate, intend to pursue such opportunities through the acquisitions of companies, products or technologies and our own development activities. Our ability to execute on such opportunities in some circumstances may be dependent, in part, upon our ability to raise additional capital on commercially reasonable terms. There can be no assurance that funds from these sources will be available when needed or on terms favorable to us or our stockholders. If additional funds are raised by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock.
On November 27, 2006, our board of directors approved an amended and restated certificate of incorporation and amended and restated bylaws in preparation for our initial public offering. This amended and restated certificate of incorporation was approved by the stockholders on November 28, 2006 and was filed with the Secretary of State of the State of Delaware in December 2006. The changes included: (i) increasing our total authorized shares to 110,000,000 (100,000,000 common and 10,000,000 preferred); (ii) modifying the classes, term and number of the members of the board of directors; and (iii) implementing other changes deemed necessary for a public reporting company. We also revised our compensation committee and nominating committee charters and adopted new policies to follow current best practices for public companies.
We have maintained a positive operating cash flow in each year since 2001, and we believe that the net cash provided by operating activities and existing cash and equivalents will provide us with sufficient resources to meet our expected working capital requirements, debt service and other cash needs for the foreseeable future.
Inflation
Although at reduced levels in recent years, inflation continues to apply upward pressure on the cost of goods and services that we use. The competitive environments in many markets substantially limit our ability to fully recover these higher costs through increased selling prices. We continually seek
64
to mitigate the adverse effects of inflation through cost containment and improved productivity and manufacturing processes.
Foreign currency fluctuations
Approximately 5% of our net sales in 2007 were derived from operations outside the United States and were denominated in Japanese Yen. None of our international cost structure is denominated in currencies other than the U.S. dollar. As a result, we are subject to fluctuations in sales and earnings reported in U.S. dollars due to changing currency exchange rates. We do not believe, however, that we currently have significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies.
Cash flow
Year ended December 31, 2007. For the year ended December 31, 2007, net cash provided by operating activities was $14.3 million. The primary sources of cash were $15.2 million in net income, including the effect of: (i) adjusting for non-cash items; (ii) a net increase in accounts payable through timing of payment for operational, promotional and inventory purchases; (iii) a decrease of inventory through an improvement in our inventory turn ratio from 2.0 to 2.8; offset by (i) an increase in accounts receivable from high sales volumes and an increase in days sales outstanding, or DSO, from 44 days to 59 days; and (ii) an increase in prepaid and other current assets.
Net cash used in investing activities was $1.0 million for the year ended December 31, 2007. This was primarily through capital invested in information technology equipment and software upgrades. In addition, we invested in intellectual property, primarily patents, relating to both our new product line offerings and our core product line offerings. We anticipate spending approximately $5.0 million for total capital expenditures in 2008. The increase in capital expenditures anticipated in 2008 is due to moving our corporate offices to a new location and planned investments in a new enterprise resource planning software system.
Net cash used in financing activities was $10.5 million for the year ended December 31, 2007. This was primarily due to the complete paydown of our outstanding debt of $25.7 million in 2007. The paydown of our debt was offset by the net proceeds received, net of underwriter commissions and offering costs, from our sale of 800,000 shares of our common stock in our secondary public offering on October 12, 2007, of $14.3 million.
Year ended December 31, 2006. For the year ended December 31, 2006, net cash provided by operating activities was $12.9 million. The primary sources of cash were $6.1 million in net income, including the effect of: (i) adjusting for non-cash items; (ii) a decrease of accounts receivable through an improvement of days sales outstanding, or DSO, from 56 days to 44 days (The higher DSO in the fourth quarter of 2005 was due to the significant sales volumes and related accounts receivable experienced in the fourth quarter; (iii) an increase in prepaid assets through the occurrence of funded promotional initiatives to take place during Q1 2007; (iv) an increase of inventory through added stocking levels of the Nu-Derm System in anticipation of peak seasonal sales between the months of January through March, stocking levels of the new Nu-Derm Condition and Enhance Systems, and for new products in our CLENZIderm M.D. and ELASTIderm product lines scheduled for launch in October 2006 and February 2007 (our inventory turn ratio decreased from 3.4 to 2.0); and (v) a net increase in accounts payable and current liabilities through increased costs associated with our initial public offering and timing of payment for operational and inventory purchases.
Net cash used in investing activities was $2.2 million for the year ended December 31, 2006. This was primarily through capital invested in a marketing and training center located within a building owned by Dr. Obagi, capital invested in our new manufacturing facility, and the cost of general leasehold improvements, information technology equipment and software upgrades.
65
Net cash used in financing activities was $2.8 million for the year ended December 31, 2006. This was primarily due to scheduled principal payments of $5.8 million and a prepayment of $35.0 million made under the Credit Agreement. The payments were offset by the proceeds received, net of underwriter commissions and offering costs, from our sale of 4,000,000 shares of our common stock in our initial public offering on December 19, 2006, of $38.1 million.
Off-balance sheet arrangements
We do not have any off-balance sheet arrangements.
Commitments and contractual obligations
Our major outstanding contractual obligations relate to operating leases and capital leases. These contractual obligations as of December 31, 2007 are as follows:
| | Payments due by period
|
|---|
Contractual Obligations
| | Total
| | Less than 1 year
| | 1-3 years
| | 3-5 years
| | More than 5 years
|
|---|
| | (in thousands)
|
|---|
| Capital Lease Obligations | | $ | 100 | | $ | 56 | | $ | 44 | | $ | — | | $ | — |
| Operating Lease Obligations | | | 1,630 | | | 1,081 | | | 549 | | | — | | | — |
| Dr. Obagi Services Agreement Obligation | | | 2,095 | | | 670 | | | 1,140 | | | 285 | | | — |
| Other Services Agreement Obligation | | | 200 | | | 100 | | | 100 | | | — | | | — |
| | |
| |
| |
| |
| |
|
| | Total | | $ | 4,024 | | $ | 1,907 | | $ | 1,832 | | $ | 285 | | $ | — |
| | |
| |
| |
| |
| |
|
Capital leases. We lease certain office equipment for use in our operations which are classified under capital leases. Our payment obligations under capital leases represent both principle and interest at implied fixed rates. The leases expire through July 2010. As of December 31, 2007, we amended one of our leases to expire in December 2010.
Operating leases. We lease our corporate offices in Long Beach, California and distribution center in Carson, California under separate leases expiring through October 2008. We are currently negotiating a new lease for our headquarters with a new landlord in Long Beach, CA. We lease our manufacturing facility in Milford, Connecticut under a lease expiring in November 2008. In addition, we lease automobiles for our sales representatives under 36-month contracts.
Services agreement. On July 18, 2005, we entered into a consulting agreement with a third party. We agreed to pay this third party a minimum fee of $100,000 per year for five years plus reasonable and customary expenses incurred at our request in connection with the provision of such services, commencing on January 1, 2005, for product formulation, product development and regulatory work. This agreement can be extended for up to two, one-year renewal terms. We agreed to pay a tiered royalty for successful commercialization of products developed or identified by the third party based on annual net sales, with a maximum royalty paid per product, capped at $5.0 million per year. Tiered royalty payable for each new product will be 3% on annual net sales from $0 to $50 million; 4% on annual net sales from $50 million to $75 million; and 5% on annual net sales above $75 million. See "Certain Material Agreements—Jose Ramirez and JR Chem" for additional information.
In June 2006, we leased our Marketing and Training Center in Beverly Hills, California from an affiliate of Dr. Obagi. We prepaid rent due under this five year lease expiring in July 2011. We have one five year extension. Since we have prepaid all amounts owing under this lease, no amounts from this lease were included in the table above.
In June 2006, we entered into a services agreement with Zein E. Obagi, MD Inc. ("Obagi, Inc."), Dr. Obagi, Samar Obagi, the Zein and Samar Obagi Family Trust and Skin Health Properties, Inc. We
66
have agreed to pay Obagi, Inc. an annual retainer of $570,000 for advising and formulating services and the marketing and support services. In addition, the Company has agreed to pay Obagi, Inc. an annual fee of $200,000 for the first two years of the agreement for the development of Proderm products. At the end of the two years, the Company has an option to continue to sell Proderm products, in which case the Company will pay Obagi, Inc. an annual royalty payment of the greater of $200,000, or 5%, of the Company's net Proderm revenues. The Company will also pay Obagi, Inc. royalty fees for developing other products identified in the agreement equal to 5% of the Company's net revenues from sales of those products. In addition, the Company has agreed to reimburse up to 50% of all invoiced commercially reasonable marketing design and development expenses associated with the opening of the property in Beverly Hills, not to exceed $100,000. Unless otherwise terminated in accordance with its terms, the agreement's initial term is five years, and it may be renewed for additional terms upon the mutual consent of the parties upon six months' written notice prior to the end of the initial term.
Recent accounting pronouncements
New accounting standards
In December 2007, the Financial Accounting Standards Board ("FASB") issued SFAS No. 141(revised 2007) ("SFAS No. 141(R)"),Business Combinations, which requires us to record fair value estimates of contingent consideration and certain other potential liabilities during the original purchase price allocation, expense acquisition costs as incurred and does not permit certain restructuring activities previously allowed under Emerging Issues Task Force Issue ("EITF") No. 95-3 to be recorded as a component of purchase accounting. This statement is effective as of the beginning of an entity's first fiscal year beginning after December 15, 2008. We will adopt the provisions of this standard beginning January 1, 2009. The provisions of SFAS No. 141(R) will only impact us if we are party to a business combination after the standard has been adopted.
In December 2007, the FASB issued SFAS No. 160 ("SFAS No. 160"),Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51, which causes noncontrolling interests in subsidiaries to be included in the equity section of the balance sheet. This statement is effective as of the beginning of an entity's first fiscal year beginning after December 15, 2008. We will adopt the provisions of this standard beginning January 1, 2009. We are currently evaluating the effects, if any, that SFAS No. 160 may have on our financial position, results of operations and cash flows.
In June 2007, the FASB issued EITF Issue No. 07-03 ("EITF 07-03"),Accounting for Non-Refundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities. EITF 07-03 provides guidance on whether non-refundable advance payments for goods that will be used or services that will be performed in future research and development activities should be accounted for as research and development costs or deferred and capitalized until the goods have been delivered or the related services have been rendered. EITF 07-03 is effective for fiscal years beginning after December 15, 2007. We have not yet determined the impact that the adoption of EITF 07-03 will have on our consolidated financial position, results of operations or cash flows.
In February 2007, the FASB issued SFAS No. 159 ("SFAS No. 159"),The Fair Value Option for Financial Assets and Financial Liabilities, to permit all entities to choose to elect, at specified election dates, to measure eligible financial instruments at fair value. An entity shall report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date, and recognize upfront costs and fees related to those items in earnings as incurred and not deferred. SFAS No. 159 applies to fiscal years beginning after November 15, 2007, with early adoption permitted for an entity that has also elected to apply the provisions of SFAS No.157 ("SFAS No. 157"),Fair Value Measurements. An entity is prohibited from retrospectively applying SFAS No. 159, unless it chooses early adoption. SFAS No. 159 also applies to eligible items existing at
67
November 15, 2007 (or early adoption date). We will adopt the provisions of SFAS No. 159 on January 1, 2008. We do not expect the adoption of SFAS No. 159 to have an impact on our consolidated financial position, results of operations or cash flows.
In December 2006, the FASB issued FASB Staff Position ("FSP") EITF 00-19-2 ("FSP EITF 00-19-2"),Accounting for Registration Payment Arrangements, which specifies that the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement should be separately recognized and measured in accordance with FASB Statement No. 5,Accounting for Contingencies. Adoption of FSP EITF 00-19-02 is required for fiscal years beginning after December 15, 2006. The adoption of FSP EITF 00-19-2 as of January 1, 2007 did not have an impact on our financial position, results of operations or cash flows.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements. This new standard provides guidance for using fair value to measure assets and liabilities. Under SFAS No. 157, fair value refers to the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants in the market in which the reporting entity transacts. In this standard, the FASB clarifies the principle that fair value should be based on the assumptions market participants would use when pricing the asset or liability. In support of this principle, SFAS No. 157 establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. The fair value hierarchy gives the highest priority to quoted prices in active markets and the lowest priority to unobservable data, for example, the reporting entity's own data. Under the standard, fair value measurements would be separately disclosed by level within the fair value hierarchy. The provisions of SFAS No. 157 are effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. However, FSP No. 157-2, Effective Date of FASB Statement No. 157, was issued in February 2008 which defers the effective date of SFAS No. 157 for nonfinancial assets and liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years. We have not yet determined the impact that the adoption of SFAS No. 157 will have on our consolidated financial position, results of operations or cash flows.
In July 2006, the FASB issued FIN 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, which prescribes accounting for and disclosure of uncertainty in tax positions. This interpretation defines the criteria that must be met for the benefits of a tax position to be recognized in the financial statements and the measurement of tax benefits recognized. We adopted the provisions of FIN 48 as of January 1, 2007, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings.
In February 2006, the EITF issued EITF No. 06-3 ("EITF No. 06-3"),How Sales Taxes Collected from Customers and Remitted to Governmental Authorities Should Be Presented in the Income Statement (That Is, Gross Versus Net Presentation). EITF 06-3 requires disclosures surrounding a company's accounting policy (i.e., gross or net presentation) regarding presentation of taxes within the scope of EITF No. 06-3. If taxes included in gross revenues are significant, a company should disclose the amount of such taxes for each period for which an income statement is presented. EITF 06-3 is effective for the first annual or interim reporting period beginning after December 15, 2006. The disclosures are required for annual and interim financial statements for each period for which an income statement is presented. We began applying the provisions of EITF 06-3 as of January 1, 2007. As we present such taxes on a net basis in our consolidated Statements of Income, the adoption of EITF No. 06-3 did not have an impact on our consolidated Statements of Income.
68
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We invest our excess cash primarily in U.S. government securities and investment-grade marketable debt securities of financial institutions and corporations. Except for the interest rate cap required by our Credit Agreement, we do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions in any material fashion. Accordingly, we believe that, while the instruments we hold are subject to changes in the financial standing of the issuer of such securities, we are not subject to any material risks arising from changes in interest rates, foreign currency exchange rates, commodity prices, equity prices or other market changes that affect market risk sensitive instruments.
Although substantially all of our sales and purchases are denominated in U.S. dollars, future fluctuations in the value of the U.S. dollar may affect the price competitiveness of our products outside the U.S. We do not believe, however, that we currently have significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies.
Interest rate risk
Our interest income and expense is more sensitive to fluctuations in the general level of U.S. prime rate and LIBOR interest rates than to changes in rates in other markets. Changes in U.S. LIBOR interest rates affect the interest earned on our cash and cash equivalents. At December 31, 2007, we had approximately $14.1 million of cash and cash equivalents. If the interest rates on our cash and cash equivalents were to increase or decrease by 1% for the year, annual interest income would increase or decrease by approximately $0.1 million.
We purchased a LIBOR interest rate cap agreement as an economic hedge against our Credit Agreement borrowings. The interest rate cap is 6.0% on a decreasing notional amount starting at $35.0 million decreasing to approximately $34.0 million. This agreement expires on January 1, 2008. We reflected the cap on the consolidated balance sheet at its fair market value and any change in fair value is reported in interest expense. As we paid the remaining balance of our Credit Agreement borrowings upon the consummation of our Secondary Offering completed on October 12, 2007, the fair value of the interest rate cap was $0 as of December 31, 2007.
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item is incorporated herein by reference to the financial statements set forth in Item 15(a) of Part IV of this report.
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
Not applicable.
ITEM 9A: CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of the end of the period covered by this report, the Company carried out an evaluation, under the supervision and with the participation of its management, including the Chief Executive Officer and the Chief Financial Officer, of the effectiveness of the design and operation of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended). Based upon that evaluation, the Company's Chief Executive Officer and Chief Financial Officer have concluded that the Company's disclosure controls and procedures are effective to provide reasonable assurance that information is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such
69
information is accumulated and communicated to the Company's management, including the Chief Executive Officer and the Chief Financial Officer as appropriate, to allow timely decisions regarding required disclosure.
Management's Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15(d)-15(f) of the Securities Exchange Act of 1934, as amended). Under the supervision and with the participation of the Company's management, including the Chief Executive Officer and the Chief Financial Officer, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting based upon the framework inInternal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, the Company's management concluded that its internal control over financial reporting was effective as of December 31, 2007.
The effectiveness of the Company's internal control over financial reporting as of December 31, 2007, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their Report of Independent Registered Public Accounting Firm under Part IV, Item 15 of this Form 10-K.
Changes in Internal Control Over Financial Reporting
There has been no change in the Company's internal control over financial reporting during the Company's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
ITEM 9B: OTHER INFORMATION
None.
PART III
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is incorporated into this Annual Report on Form 10-K by reference to the definitive proxy statement for our 2008 Annual Meeting of Stockholders (the "Proxy Statement").
ITEM 11: EXECUTIVE COMPENSATION
The information required by this item is incorporated into this Annual Report on Form 10-K by reference to the Proxy Statement.
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated into this Annual Report on Form 10-K by reference to the Proxy Statement.
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated into this Annual Report on Form 10-K by reference to the Proxy Statement.
ITEM 14: PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated into this Annual Report on Form 10-K by reference to the Proxy Statement.
70
PART IV
ITEM 15: EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)1.Consolidated Financial Statements and Supplementary Data:
The following financial statements are included as a separate section of this report commencing on page F-1:
| Report of Independent Registered Public Accounting Firm | | F-1 |
| Consolidated Balance Sheets as of December 31, 2007 and 2006 | | F-2 |
| Consolidated Statements of Income for the years ended December 31, 2007, 2006, and 2005 | | F-3 |
| Consolidated Statements of Stockholders' Equity and Comprehensive Income (Loss) for the years ended December 31, 2007, 2006, and 2005 | | F-4 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2007, 2006, and 2005 | | F-5 |
| Notes to Consolidated Financial Statements | | F-6 |
| Quarterly Data (unaudited) | | F-42 |
(a)2.Financial Statement Schedules:
Schedule II—Valuation and Qualifying Accounts
All other schedules have been omitted for the reason that the required information is presented in financial statements or notes thereto, the amounts involved are not significant or the schedules are not applicable.
| | Balance at Beginning of Period
| | Charged to Cost and Expense
| | Charged to Contra-Revenue
| | Deductions
| | Balance at End of Period
|
|---|
| | (in thousands)
|
|---|
| Year ended December 31, 2005 | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts and sales return reserve | | $ | 392 | | $ | 344 | | $ | (18 | ) | $ | (217 | ) | $ | 501 |
| Reserve for inventories | | | 316 | | | 339 | | | — | | | (218 | ) | | 437 |
| Year ended December 31, 2006 | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts and sales return reserve | | $ | 501 | | $ | 180 | | $ | 142 | | $ | (172 | ) | $ | 651 |
| Reserve for inventories | | | 437 | | | 81 | | | — | | | (242 | ) | | 276 |
| Year ended December 31, 2007 | | | | | | | | | | | | | | | |
| Allowance for doubtful accounts and sales return reserve | | $ | 651 | | $ | 285 | | $ | 214 | | $ | (305 | ) | $ | 845 |
| Reserve for inventories | | | 276 | | | 303 | | | — | | | (110 | ) | | 469 |
71
(a)3.Exhibits:
Exhibit
| | Exhibit title
| |
|
|---|
| 3.1 | | Amended and Restated Certificate of Incorporation of the Company. | | (1) |
| 3.2 | | Second Amended and Restated Bylaws of the Company. | | (5) |
| 4.1 | | Specimen Stock Certificate. | | (1) |
| 4.2 | | Investor's Rights Agreement, by and between the Company and Austin T. McNamara, dated April 1, 2002. | | (1) |
| 4.3 | | Investor's Rights Agreement, by and among the Company, Mandarin Partners LLC and the Zein & Samar Obagi Family Trust dated December 2, 1997, as amended and assigned. | | (1) |
| 10.1 | | OMP, Inc. 2000 Stock Option/Stock Issuance Plan and forms of award agreements.** | | (1) |
| 10.2 | | Amended and Restated Obagi Medical Products, Inc. 2005 Stock Incentive Plan and forms of award agreements.** | | (1) |
| 10.3 | | Catalina Landing Amended and Restated Office Lease, by and between the Company and AC-Catalina Landing, LLC, dated May 9, 2003, as amended. | | (1) |
| 10.4 | | Standard Sublease, by and between the Company and Marlog Cargo USA, Inc., dated July 26, 2004. | | (1) |
| 10.5 | | Lease Agreement between the Company and D'Amato Investments, LLC, dated December 1, 2005. | | (1) |
| 10.6 | | Distribution Agreement, by and between the Company and Cellogique Corporation, dated November 10, 2005, as amended. | | (1) |
| 10.7 | | Know-How and Trademark License Agreement, by and between the Company and Rohto Pharmaceutical Co, Ltd., dated September 13, 2002, as amended.+ | | (1) |
| 10.8 | | Agreement by and between the Company and Dr. Zein E. Obagi, Inc, dated as of June 29, 2006. | | (1) |
| 10.9 | | Separation and Release Agreement between the Company and Zein Obagi, M.D., dated June 29, 2006. | | (1) |
| 10.10 | | Retail Lease Agreement by and between Skin Health Properties, Inc. as Landlord and OMP, Inc. as Tenant, dated as of June 29, 2006. | | (1) |
| 10.11 | | Letter Agreement between the Company and Skin Health Properties, Inc., dated June 29, 2006. | | (1) |
| 10.12 | | Employment Agreement, by and between the Company and Austin T. McNamara, dated September 1, 2001.** | | (1) |
| 10.13 | | Employment Agreement, by and between the Company and Steven R. Carlson, dated March 1, 2005.** | | (1) |
| 10.14 | | Form of Severance Agreement.** | | (1) |
| 10.15 | | Distributorship Agreement between the Company and CNO Chinese Obagi Corporation, dated September 23, 1997. | | (1) |
| 10.16 | | Distributorship Agreement the Company and between VNO Vietnamese Obagi Corporation, dated September 23, 1997. | | (1) |
| 10.17 | | Management Services Agreement, by and between the Company and Mandarin Management Partners, Inc, dated December 2, 1997, assigned to Lighthouse Venture Group, as amended. | | (1) |
72
| 10.18 | | Credit Agreement, by and between the Company and Merrill Lynch Capital, dated January 28, 2005, as amended. | | (1) |
| 10.19 | | Product Supply Agreement, by and between the Company and Triax Pharmaceuticals, LLC, dated December 8, 2005.+ | | (1) |
| 10.20 | | Consultant Services and Confidentiality Agreement, dated July 18, 2005, by and among the Company, Jose Ramirez, and JR Chem LLC.+ | | (1) |
| 10.21 | | Form of indemnification agreement. | | (1) |
| 10.22 | | Management Services Agreement, dated December 18, 2002, between Stonington Partners, Inc. and the Company. | | (1) |
| 10.23 | | Patent License Agreement by and between the Company and Avon Products, Inc., dated June 26, 2003.+ | | (1) |
| 10.24 | | Letter Agreement re Repurchase of Series A Preferred Stock among the Company, Stonington Capital Appreciation 1994 Fund LP and the Zein and Samar Obagi Family Trust, dated January 25, 2005. | | (1) |
| 10.25 | | Financial Advisory Services Agreement, by and between the Company and Stonington Partners, Inc., dated November 19, 2004. | | (1) |
| 10.26 | | Non-Employee Director Compensation Policy, adopted November 14, 2006.** | | (1) |
| 10.27 | | Form of Subordinated Promissory Note tendered to McNamara Family Trust, dated November 17, 2006. | | (1) |
| 10.28 | | Form of Subordinated Promissory Note tendered to McNamara Family Irrevocable Trust, dated November 17, 2006. | | (1) |
| 10.29 | | Subordination Agreement, by and between Merrill Lynch Capital, McNamara Family Irrevocable Trust Under Agreement Dated December 17, 2004, McNamara Family Trust, OMP, Inc. and the Company, dated June 2, 2005. | | (1) |
| 10.30 | | Employment Agreement by and between the Company and Curtis Cluff, dated November 30, 2001.** | | (1) |
| 10.31 | | Employment Agreement by and between the Company and David Goldstein, dated August 10, 1999.** | | (1) |
| 10.32 | | Employment Agreement by and between the Company and Stephen Garcia, dated June 13, 2003.** | | (1) |
| 10.33 | | Employment Agreement by and between the Company and Judith Hattendorf, dated August 23, 2000.** | | (1) |
| 10.34 | | Settlement and Consent Agreement and Mutual Release, dated May 29, 2007. | | (2) |
| 10.35 | | Form of Amendment to Employee Agreement. | | (3) |
| 10.36 | | Separation Agreement and General Release between the Company and Curtis A. Cluff dated September 7, 2007. | | (4) |
| 10.37 | | Credit Facility Fee Letter, dated January 28, 2008. | | * |
| 21.1 | | Subsidiaries of the Company. | | (1) |
| 23.1 | | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | | * |
| 31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | * |
73
| 31.2 | | Certification of Principal Accounting Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | | * |
| 32.1 | | Certification of Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | * |
| 32.2 | | Certification of Principal Accounting Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | | * |
- *
- Filed herewith
- +
- Material has been omitted pursuant to a request for confidential treatment and such material has been filed separately with the SEC.
- **
- Management contracts or compensatory plans and arrangements required to be filed pursuant to Item 601(b)(10)(ii)(A) or (iii) of Regulation S-K.
- (1)
- Incorporated herein by reference to the Company's Registration of Form S-1/A (Registration No. 333-137272, previously filed with the Commission.
- (2)
- Incorporated herein by reference to the exhibits filed with the Company's Form 10-Q for the quarter ended June 30, 2007, previously filed with the Commission.
- (3)
- Incorporated herein by reference to the exhibits filed with the Company's Form 8-K filed with the Commission on August 7, 2007.
- (4)
- Incorporated herein by reference to the exhibits filed with the Company's Form 8-K filed with the Commission on September 11, 2007.
- (5)
- Incorporated herein by reference to the exhibits filed with the Company's Form 8-K filed with the Commission on February 29, 2008.
74
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | OBAGI MEDICAL PRODUCTS, INC. |
Date: March 4, 2008 |
|
By: |
/s/ STEVEN R. CARLSON
Steven R. Carlson
Chief Executive Officer
(Principal Executive Officer) |
Date: March 4, 2008 |
|
By: |
/s/ STEPHEN A. GARCIA
Stephen A. Garcia
Chief Financial Officer
(Principal Financial Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
| | Title
| | Date
|
|---|
|
|
|
|
|
/s/ STEVEN R. CARLSON
Steven R. Carlson |
|
Chief Executive Officer, President and Director (Principal Executive Officer) |
|
March 4, 2008 |
/s/ STEPHEN A. GARCIA
Stephen A. Garcia |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 4, 2008 |
/s/ ALBERT J. FITZGIBBONS III
Albert J. Fitzgibbons III |
|
Chairman of the Board of Directors |
|
March 4, 2008 |
/s/ JOHN A. BARTHOLDSON
John A. Bartholdson |
|
Director |
|
March 4, 2008 |
/s/ JOHN H. DUERDEN
John H. Duerden |
|
Director |
|
March 4, 2008 |
/s/ EDWARD A. GRANT
Edward A. Grant |
|
Director |
|
March 4, 2008 |
/s/ ALBERT F. HUMMEL
Albert F. Hummel |
|
Director |
|
March 4, 2008 |
/s/ RONALD P. BADIE
Ronald P. Badie |
|
Director |
|
March 4, 2008 |
75
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Obagi Medical Products, Inc.
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of Obagi Medical Products, Inc. and its subsidiaries at December 31, 2007 and 2006, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control Over Financial Reporting, appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company's internal control over financial reporting based on our audits (which was an integrated audit in 2007). We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in the notes to the consolidated financial statements, the Company changed the manner in which it accounts for uncertain tax positions as of January 1, 2007 (Note 6), and share-based compensation as of January 1, 2006 (Note 2).
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| /s/ PRICEWATERHOUSECOOPERS LLP |
Los Angeles, California |
| February 27, 2008 |
F-1
Obagi Medical Products, Inc.
Consolidated Balance Sheets
(Dollars in thousands, except share and per share amounts)
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| Assets | | | | | | | |
| Current assets | | | | | | | |
| | Cash and cash equivalents | | $ | 14,054 | | $ | 11,298 | |
| | Accounts receivable, net of allowance for doubtful accounts and sales returns of $845 and $651 as of December 31, 2007 and 2006, respectively | | | 17,370 | | | 10,049 | |
| | Accounts receivable from related parties | | | 982 | | | 1,501 | |
| | Inventories, net | | | 6,047 | | | 6,266 | |
| | Deferred income taxes | | | 1,841 | | | 1,000 | |
| | Prepaid expenses and other current assets | | | 3,382 | | | 2,895 | |
| | Income taxes receivable | | | 559 | | | 905 | |
| | |
| |
| |
| | | Total current assets | | | 44,235 | | | 33,914 | |
| Property and equipment, net | | | 2,759 | | | 3,749 | |
| Goodwill | | | 4,629 | | | 4,629 | |
| Intangible assets, net | | | 5,760 | | | 6,308 | |
| Deferred income taxes | | | 1,179 | | | 1,971 | |
| Other assets | | | 1,201 | | | 2,390 | |
| | |
| |
| |
| | | Total assets | | $ | 59,763 | | $ | 52,961 | |
| | |
| |
| |
| Liabilities and Stockholders' Equity | | | | | | | |
| Current liabilities | | | | | | | |
| | Accounts payable | | $ | 6,664 | | $ | 5,512 | |
| | Current portion of long-term debt | | | 54 | | | 2,729 | |
| | Accrued liabilities | | | 3,139 | | | 3,598 | |
| | Amounts due to related parties | | | 135 | | | 152 | |
| | |
| |
| |
| | | Total current liabilities | | | 9,992 | | | 11,991 | |
| Long-term debt | | | 42 | | | 23,052 | |
| | |
| |
| |
| | | Total liabilities | | | 10,034 | | | 35,043 | |
| | |
| |
| |
| Commitments and contingencies (Note 10) | | | | | | | |
| Stockholders' equity | | | | | | | |
| | Common stock, $.001 par value; 100,000,000 shares authorized, 22,653,349 and 21,804,689 shares issued and 22,643,564 and 21,801,961 shares outstanding at December 31, 2007 and 2006, respectively | | | 23 | | | 22 | |
| | Additional paid-in capital | | | 55,805 | | | 39,109 | |
| | Accumulated deficit | | | (6,031 | ) | | (21,144 | ) |
| | Stockholder receivable | | | — | | | (23 | ) |
| | Accumulated other comprehensive loss | | | (68 | ) | | (46 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 49,729 | | | 17,918 | |
| | |
| |
| |
| | | Total liabilities and stockholders' equity | | $ | 59,763 | | $ | 52,961 | |
| | |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-2
Obagi Medical Products, Inc.
Consolidated Statements of Income
(Dollars in thousands, except share and per share amounts)
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Net sales | | $ | 102,648 | | $ | 77,996 | | $ | 64,941 | |
| Cost of sales | | | 18,049 | | | 13,467 | | | 11,572 | |
| | |
| |
| |
| |
| | | Gross profit | | | 84,599 | | | 64,529 | | | 53,369 | |
| Selling, general and administrative expenses | | | 51,400 | | | 40,863 | | | 29,023 | |
| Research and development expenses | | | 5,497 | | | 5,919 | | | 3,262 | |
| | |
| |
| |
| |
| | | Income from operations | | | 27,702 | | | 17,747 | | | 21,084 | |
| Interest income | | | 190 | | | 13 | | | 61 | |
| Interest expense | | | (2,414 | ) | | (8,186 | ) | | (6,146 | ) |
| | |
| |
| |
| |
| | | Income before provision for income taxes | | | 25,478 | | | 9,574 | | | 14,999 | |
| Provision for income taxes | | | 10,275 | | | 3,458 | | | 6,043 | |
| | |
| |
| |
| |
| | | Net income | | | 15,203 | | | 6,116 | | | 8,956 | |
| Dividends on mandatorily redeemable preferred stock | | | — | | | — | | | (140 | ) |
| | |
| |
| |
| |
| | | Net income attributable to common stockholders | | $ | 15,203 | | $ | 6,116 | | $ | 8,816 | |
| | |
| |
| |
| |
| Net income attributable to common shares | | | | | | | | | | |
| | Basic | | $ | 0.69 | | $ | 0.34 | | $ | 0.50 | |
| | |
| |
| |
| |
| | Diluted | | $ | 0.69 | | $ | 0.34 | | $ | 0.50 | |
| | |
| |
| |
| |
| Weighted average common shares outstanding | | | | | | | | | | |
| | Basic | | | 21,989,121 | | | 17,996,158 | | | 17,522,611 | |
| | Diluted | | | 22,100,737 | | | 18,071,048 | | | 17,765,716 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Obagi Medical Products, Inc.
Consolidated Statements of Stockholders' Equity (Deficit) and Comprehensive Income
(Dollars in thousands, except share and per share amounts)
| | Common Stock
| |
| |
| | Treasury Stock
| |
| |
| |
| |
|---|
| | Additional Paid-In Capital
| | Accumulated Earnings (Deficit)
| | Stockholder Receivable
| | Accumulated Other Comprehensive Income (Loss)
| |
| |
|---|
| | Shares
| | Amount
| | Shares
| | Amount
| | Total
| |
|---|
| Balances, as of December 31, 2004 | | 17,701,346 | | $ | 18 | | $ | 4,989 | | $ | 20,445 | | (1,513,024 | ) | $ | (5,447 | ) | $ | — | | $ | 78 | | $ | 20,083 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Translation adjustment, net of tax effect ($3) | | — | | | — | | | — | | | — | | — | | | — | | | — | | | (97 | ) | | (97 | ) |
| | Net income for the year ended December 31, 2005 | | — | | | — | | | — | | | 8,956 | | — | | | — | | | — | | | — | | | 8,956 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | 8,859 | |
| Issuance of common stock upon exercise of stock options | | — | | | — | | | (80 | ) | | (1,994 | ) | 1,336,136 | | | 4,810 | | | — | | | — | | | 2,736 | |
| Tax benefit from exercise of stock options | | — | | | — | | | 3,652 | | | — | | — | | | — | | | — | | | — | | | 3,652 | |
| Preferred stock dividends accrued ($11 and $5 per share for Series A and Series B, respectively) | | — | | | — | | | — | | | (140 | ) | — | | | — | | | — | | | — | | | (140 | ) |
| Common stock dividends paid ($3.60 per share) | | — | | | — | | | (8,561 | ) | | (54,527 | ) | — | | | — | | | — | | | — | | | (63,088 | ) |
| Conversion of redeemable preferred stock into common stock | | 86,237 | | | — | | | 863 | | | — | | 176,888 | | | 637 | | | — | | | — | | | 1,500 | |
| Issuance of common stock for services received | | 5,000 | | | — | | | 37 | | | — | | — | | | — | | | — | | | — | | | 37 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balances, as of December 31, 2005 | | 17,792,583 | | | 18 | | | 900 | | | (27,260 | ) | — | | | — | | | — | | | (19 | ) | | (26,361 | ) |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Translation adjustment, net of tax effect ($17 benefit) | | — | | | — | | | — | | | — | | — | | | — | | | — | | | (27 | ) | | (27 | ) |
| | Net income for the year ended December 31, 2006 | | — | | | — | | | — | | | 6,116 | | — | | | — | | | — | | | — | | | 6,116 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | 6,089 | |
| Issuance of common stock upon exercise of incentive stock options | | 6,600 | | | — | | | 9 | | | — | | — | | | — | | | — | | | — | | | 9 | |
| From exercise of stock options | | — | | | — | | | (222 | ) | | — | | — | | | — | | | — | | | — | | | (222 | ) |
| Stock compensation expense | | — | | | — | | | 305 | | | — | | — | | | — | | | — | | | — | | | 305 | |
| Issuance of common stock in initial public offering, net of offering costs | | 4,000,000 | | | 4 | | | 38,094 | | | — | | — | | | — | | | — | | | — | | | 38,098 | |
| Issuance of common stock in exchange for stockholder receivable | | 2,778 | | | — | | | 23 | | | — | | — | | | — | | | (23 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balances, as of December 31, 2006 | | 21,801,961 | | | 22 | | | 39,109 | | | (21,144 | ) | — | | | — | | | (23 | ) | | (46 | ) | | 17,918 | |
| Cumulative effect of adoption of FIN 48 | | — | | | — | | | — | | | (90 | ) | — | | | — | | | — | | | — | | | (90 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balances, as of January 1, 2007, as adjusted | | 21,801,961 | | | 22 | | | 39,109 | | | (21,234 | ) | — | | | — | | | (23 | ) | | (46 | ) | | 17,828 | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Translation adjustment, net of tax effect ($14 benefit) | | — | | | — | | | — | | | — | | — | | | — | | | — | | | (22 | ) | | (22 | ) |
| | Net income for the year ended December 31, 2007 | | — | | | — | | | — | | | 15,203 | | — | | | — | | | — | | | — | | | 15,203 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | 15,181 | |
| Stock compensation expense | | — | | | — | | | 1,557 | | | — | | — | | | — | | | — | | | — | | | 1,557 | |
| Payment received on stockholder receivable | | — | | | — | | | — | | | — | | — | | | — | | | 23 | | | — | | | 23 | |
| Issuance of common stock in public offering, net of offering costs | | 800,000 | | | 1 | | | 15,027 | | | — | | — | | | — | | | — | | | — | | | 15,028 | |
| Issuance of vested restricted stock | | 9,869 | | | — | | | — | | | — | | — | | | — | | | — | | | — | | | — | |
| Tax benefit from exercise of stock options | | — | | | — | | | 10 | | | — | | — | | | — | | | — | | | — | | | 10 | |
| Issuance of common stock upon exercise of incentive stock options | | 31,734 | | | — | | | 102 | | | — | | — | | | — | | | — | | | — | | | 102 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balances, as of December 31, 2007 | | 22,643,564 | | $ | 23 | | $ | 55,805 | | $ | (6,031 | ) | — | | $ | — | | $ | — | | $ | (68 | ) | | 49,729 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Obagi Medical Products, Inc.
Consolidated Statements of Cash Flows
(Dollars in thousands, except share and per share amounts)
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Cash flows from operating activities | | | | | | | | | | |
| Net income | | $ | 15,203 | | $ | 6,116 | | $ | 8,956 | |
| Adjustments to reconcile net income to net cash provided by operating activities | | | | | | | | | | |
| | Depreciation and amortization | | | 2,788 | | | 2,758 | | | 2,090 | |
| | Write down of debt issuance costs | | | 781 | | | 1,282 | | | — | |
| | Loss on disposal of property and equipment | | | 1 | | | 72 | | | — | |
| | Provision for doubtful accounts | | | 194 | | | 150 | | | 110 | |
| | Deferred income taxes | | | (49 | ) | | (450 | ) | | 487 | |
| | Issuance of common shares to outside directors | | | — | | | — | | | 37 | |
| | Stock compensation expense | | | 1,557 | | | 305 | | | — | |
| | Tax benefit (expense) from exercise of stock options | | | — | | | (222 | ) | | 3,652 | |
| | Changes in operating assets and liabilities | | | | | | | | | | |
| | | Accounts receivable | | | (7,515 | ) | | 905 | | | (3,393 | ) |
| | | Accounts receivable from related parties | | | 519 | | | (356 | ) | | (264 | ) |
| | | Income taxes receivable | | | 346 | | | 1,945 | | | (2,850 | ) |
| | | Inventories | | | 219 | | | (3,158 | ) | | (296 | ) |
| | | Prepaid expenses and other current assets | | | (487 | ) | | (876 | ) | | (807 | ) |
| | | Other assets | | | 153 | | | 875 | | | (1,929 | ) |
| | | Accounts payable | | | 1,151 | | | 3,187 | | | (949 | ) |
| | | Accrued liabilities | | | (549 | ) | | 438 | | | 655 | |
| | | Amounts due to related parties | | | (17 | ) | | (53 | ) | | (63 | ) |
| | |
| |
| |
| |
| | | | Net cash provided by operating activities | | | 14,295 | | | 12,918 | | | 5,436 | |
| | |
| |
| |
| |
| Cash flows from investing activities | | | | | | | | | | |
| Purchases of property and equipment | | | (603 | ) | | (1,818 | ) | | (2,040 | ) |
| Purchase of other intangible assets | | | (391 | ) | | (366 | ) | | (264 | ) |
| | |
| |
| |
| |
| | | | Net cash used in investing activities | | | (994 | ) | | (2,184 | ) | | (2,304 | ) |
| | |
| |
| |
| |
| Cash flows from financing activities | | | | | | | | | | |
| Principal payments on term loans | | | (25,651 | ) | | (40,849 | ) | | (3,500 | ) |
| Principal payments on capital lease obligations | | | (35 | ) | | (35 | ) | | (13 | ) |
| Proceeds from the issuance of common stock, net of underwriting discounts and deferred offering costs | | | 15,028 | | | 38,098 | | | — | |
| Proceeds from issuance of debt | | | — | | | — | | | 70,000 | |
| Proceeds from exercise of stock options | | | 102 | | | 9 | | | 2,736 | |
| Payment received for stockholder receivable | | | 23 | | | — | | | — | |
| Tax benefit (expense) from exercise of stock options | | | 10 | | | — | | | — | |
| Dividends to common stockholders | | | — | | | — | | | (63,088 | ) |
| Dividends to preferred stockholders | | | — | | | — | | | (9,329 | ) |
| Redemption of redeemable preferred stock | | | — | | | — | | | (11,822 | ) |
| Debt issuance costs | | | — | | | — | | | (2,886 | ) |
| | |
| |
| |
| |
| | | | Net cash used in financing activities | | | (10,523 | ) | | (2,777 | ) | | (17,902 | ) |
| | |
| |
| |
| |
| Effect of exchange rate changes on cash and cash equivalents | | | (22 | ) | | (26 | ) | | (97 | ) |
| | |
| |
| |
| |
| | | | Net increase (decrease) in cash and cash equivalents | | | 2,756 | | | 7,931 | | | (14,867 | ) |
| | |
| |
| |
| |
| Cash and cash equivalents at beginning of year | | | 11,298 | | | 3,367 | | | 18,234 | |
| Cash and cash equivalents at end of year | | $ | 14,054 | | $ | 11,298 | | $ | 3,367 | |
| Supplemental disclosure of cash flow information: | | | | | | | | | | |
| | Cash paid during the year for: | | | | | | | | | | |
| | | Interest, net of amount capitalized | | $ | 1,235 | | $ | 5,822 | | $ | 5,146 | |
| | |
| |
| |
| |
| | | Income taxes, net of refunds | | $ | 9,878 | | $ | 2,135 | | $ | 4,858 | |
| | |
| |
| |
| |
| Non cash investing and financing activities | | | | | | | | | | |
| | Capital lease obligations incurred | | $ | — | | $ | 47 | | $ | 68 | |
| | Accrual of dividends on preferred stock | | $ | — | | $ | — | | $ | 26 | |
| | Interest rate cap fair value adjustment | | $ | (3 | ) | $ | (16 | ) | $ | — | |
During the year ended December 31, 2005, the holder of the Series B preferred stock converted 9,065 shares of Series B preferred stock, plus related cumulative and unpaid dividends, into 315,750 shares of common stock.
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements
(Dollars in thousands, except share and per share amounts)
Note 1: The Company
Obagi Medical Products, Inc. (the "Company") is a specialty pharmaceutical company focused on the aesthetic and skin health markets. The Company develops and commercializes prescription-based, topical skin health systems. The Company is incorporated under the laws of the state of Delaware. The Company markets its products through its own sales force throughout the United States, and through 13 distribution partners in 36 other countries in regions including North America, Europe, the Far East, the Middle East, Central America, and South America. The Company also licenses certain non-prescription product concepts under the Obagi trademark to a large Japanese based pharmaceutical company for sale through consumer distribution channels in Japan.
Reverse Stock Split
On November 27, 2006, the Board of Directors approved, and on November 28, 2006, the stockholders approved, a 1-for-1.2 reverse stock split of the Company's common stock to become effective upon the filing of a Certificate of Amendment with the Secretary of State of the state of Delaware. The filing of the Certificate of Amendment was a condition precedent to the Company's request that its Registration Statement on Form S-1 be declared effective. The Company's Registration Statement on Form S-1 was declared effective on December 13, 2006. All share and per share amounts included in the Company's financial statements have been adjusted to reflect this reverse stock split for all periods presented.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
On November 27, 2006, the Board of Directors of the Company approved an amended and restated Certificate of Incorporation and an amended and restated Bylaws in preparation for a contemplated initial public offering. This amended and restated Certificate of Incorporation was approved by the stockholders on November 28, 2006 and was filed by the Secretary of State of the State of Delaware prior to the effectiveness of the Company's Registration Statement on Form S-1. The changes included: (i) total authorized shares increased to 110,000,000 (100,000,000 common and 10,000,000 preferred); (ii) the classes, term and number of the members of the board of directors were modified; and (iii) other changes deemed necessary as for a public reporting company.
Initial Public Offering
On December 13, 2006, the Company completed its initial public offering of 5,350,000 shares of common stock at a price of $11.00 per share. The offering consisted of 4,000,000 newly issued shares sold by the Company and 1,350,000 shares sold by selling stockholders. The offering generated gross proceeds of approximately $44,000 and net proceeds of $38,098.
Secondary Public Offering
On October 17, 2007, the Company completed a registered public offering of 7,245,000 shares of common stock at a price of $20.00 per share. The offering consisted of 800,000 newly issued shares sold by the Company and 6,445,000 shares sold by selling stockholders. The offering generated gross proceeds of approximately $16,000 and net proceeds of $14,287 after all offering expenses.
F-6
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies
Principles of consolidation
The consolidated financial statements include the accounts of Obagi Medical Products, Inc. and its wholly owned subsidiary, OMP, Inc. The consolidated financial statements of OMP, Inc. include the accounts of its wholly owned subsidiaries and its majority owned subsidiary, Obagi Singapore. All intercompany accounts and transactions have been eliminated in consolidation.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current year's presentation.
Cash and cash equivalents
Cash equivalents include demand deposits and short-term investments with a maturity of three months or less when purchased.
The Company maintains its cash deposits at numerous banks located throughout the United States, which at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk. At December 31, 2007 and 2006, cash on deposit was in excess of the federally insured limit of $100.
Accounts receivable
The Company performs periodic credit evaluations of the financial condition of its customers, monitors collections and payments from customers, and generally does not require collateral. Receivables are generally due within 30 days. The Company provides for the possible inability to collect accounts receivable by recording an allowance for doubtful accounts. The Company writes off an account when it is considered to be uncollectible. The Company estimates its allowance for doubtful accounts based on historical experience, aging of accounts receivable, and information regarding the creditworthiness of its customers. The Company estimates its allowance for doubtful accounts pursuant to sales returns based on the Company's historical rate of returns and current market conditions. To date, losses have been within the range of management's expectations.
Concentrations of credit risk, with respect to trade receivables, are limited due to the large number of customers comprising the Company's customer base and their dispersion across different geographic regions. As of December 31, 2007 and 2006, no single customer represented more than 10% of the net accounts receivable balance.
F-7
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
Inventories
Inventories consist of raw materials and finished goods purchased from third parties and are valued at the lower of cost or market. Cost is determined by the first-in, first-out method.
Inventory reserves are established when conditions indicate that the selling price could be less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand and reductions in selling prices. Inventory reserves are measured as the difference between cost of inventory and the estimated net realizable value. Inventory reserves are charged to cost of sales. The Company's estimated inventory reserve is provided for in the consolidated financial statements and actual reserve requirements approximated management's estimates.
Impairment of long-lived assets
The Company periodically assesses potential impairments of its long-lived assets in accordance with the provisions of Statement of Financial Accounting Standards ("SFAS") No. 144,Accounting for the Impairment or Disposal of Long-lived Assets. An impairment review is performed whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Factors considered by the Company include, but are not limited to, significant underperformance relative to expected historical or projected future operating results; significant changes in the manner of use of the acquired assets or the strategy for the overall business; and significant negative industry or economic trends. When the carrying value of a long-lived asset may not be recoverable based upon the existence of one or more of the above indicators of impairment, the Company will assess the recoverability of the assets by estimating the future undiscounted operating cash flows expected to result from the use of the asset and its eventual disposition. If the sum of the expected future undiscounted cash flows and eventual disposition is less than the carrying amount of the asset, the Company recognizes an impairment loss. An impairment loss is reflected as the amount by which the carrying amount of the asset exceeds the fair value of the asset, based on the fair market value if available, or discounted cash flows, if not. To date, the Company has not recognized an impairment charge related to the write-down of long-lived assets.
Property and equipment
Property and equipment are stated at cost and are depreciated using the straight-line method over their estimated useful lives, as follows:
| Furniture and fixtures | | 3-5 years |
| Computer software and equipment | | 3 years |
| Lab and office equipment | | 3 years |
| Leasehold improvements | | Lesser of useful life or term of lease |
| Capital lease (office equipment) | | Lesser of useful life or term of lease |
Property and equipment are stated at cost, less accumulated depreciation and amortization. Expenditures for major renewals and betterments are capitalized, while minor replacements, maintenance and repairs, which do not extend the assets useful lives, are charged to operations as
F-8
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
incurred. Upon sale or disposition, the cost and related accumulated depreciation is removed from the accounts and any gain or loss is included in operations.
Goodwill and other intangibles
SFAS No. 142 ("SFAS No. 142"),Goodwill and Other Intangible Assets, requires the Company to test goodwill for impairment on an annual basis and more frequently if there is reason to suspect that the value has been diminished or impaired, with any corresponding write-downs recognized as necessary.
SFAS No. 142 requires that goodwill be tested for impairment using a two-step process. The first step of the goodwill impairment test used to identify potential impairment compares the fair value of a reporting unit with its carrying amount including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test must be performed to measure the amount of impairment loss, if any. The second step of the impairment test compares the implied fair value of reporting unit goodwill with the carrying amount of that goodwill. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. If the carrying amount of the reporting unit goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess.
Application of the goodwill impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units, assignment of goodwill to reporting units, and determination of the fair value of each reporting unit. The fair value of each reporting unit is estimated using a discounted cash flow methodology. This requires significant judgments including estimation of future cash flows, which is dependent on internal forecasts, estimation of the long-term rate of growth of the Company's business, the useful life over which cash flows will occur, and determination of the Company's weighted average cost of capital. Changes in these estimates and assumptions could materially affect the determination of fair value and/or goodwill impairment for each reporting unit.
The Company has selected September 30 as the date on which it will perform its annual goodwill impairment test. Based on the Company's analysis, no impairment charges were recognized for the years ended December 31, 2007, 2006, and 2005.
Other intangible assets consist of trademarks, distribution rights, covenants not-to-compete, patents, customer lists, and proprietary formulations. Other intangible assets are amortized over the expected period of benefit using the straight-line method over the following lives: trademarks (twenty years); distribution rights (ten years); covenants not-to-compete (seven years); other intangible assets (three to seventeen years).
F-9
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
Intangible assets
At December 31, 2007 and 2006, the carrying amounts and accumulated amortization of intangible assets is as follows:
| | December 31, 2007
| | December 31, 2006
|
|---|
| | Gross Amount
| | Accumulated Amortization
| | Net Book Value
| | Gross Amount
| | Accumulated Amortization
| | Net Book Value
|
|---|
| Trademarks | | $ | 7,343 | | $ | (3,668 | ) | $ | 3,675 | | $ | 7,308 | | $ | (3,305 | ) | $ | 4,003 |
| Distribution rights | | | 1,082 | | | (876 | ) | | 206 | | | 1,082 | | | (768 | ) | | 314 |
| Covenant not-to-compete | | | 931 | | | (931 | ) | | — | | | 931 | | | (798 | ) | | 133 |
| Licenses | | | 1,875 | | | (1,107 | ) | | 768 | | | 1,775 | | | (901 | ) | | 874 |
| Other intangible assets | | | 3,327 | | | (2,216 | ) | | 1,111 | | | 3,071 | | | (2,087 | ) | | 984 |
| | |
| |
| |
| |
| |
| |
|
| | | $ | 14,558 | | $ | (8,798 | ) | $ | 5,760 | | $ | 14,167 | | $ | (7,859 | ) | $ | 6,308 |
| | |
| |
| |
| |
| |
| |
|
Amortization expense related to all intangible assets, including certain amounts reflected in cost of sales, for the years ended December 31, 2007, 2006, and 2005 was $939, $941, and $1,078, respectively.
Future estimated aggregate amortization expense for the next five years and thereafter is as follows:
| Years ending December 31, | | | |
| 2008 | | $ | 814 |
| 2009 | | | 770 |
| 2010 | | | 739 |
| 2011 | | | 663 |
| 2012 | | | 637 |
| Thereafter | | | 2,137 |
| | |
|
| | | $ | 5,760 |
| | |
|
Debt issuance costs
Direct costs incurred in connection with indebtedness agreements are capitalized as incurred and amortized on the effective interest method over the term of the related indebtedness. Debt issuance costs are included in other assets and represent fees and other costs incurred in connection with the Senior Secured Credit Facility (the "Facility") entered into on January 28, 2005. In connection with this transaction, the Company incurred $2,886 in costs during fiscal year 2005.
Pursuant to the Third Amendment of the Senior Secured Credit Facility, the Company made a repayment of $35,000 in debt with the net cash proceeds received in the initial public offering consummated on December 13, 2006. As a result, the Company recorded a write down of debt issuance costs of $1,282 during the year ended December 31, 2006. During the year ended December 31, 2007, the Company paid the remaining balance outstanding under the Facility (see Note 4 for further discussion of paydowns made during the year). In conjunction with the paydowns on the Facility, the Company wrote off $781 of debt issuance costs during the year ended December 31, 2007. The write
F-10
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
downs of debt issuance costs are included as a component of interest expense. At December 31, 2007 and 2006, the Company had debt issuance costs of $211 and $1,254, respectively, net of accumulated amortization, of $1,721, and $1,459, respectively. The remaining balance of debt issuance costs are applicable to the Company's line of credit.
Derivative financial instruments
In 2001, the Company adopted SFAS No. 133 ("SFAS No. 133"),Accounting for Derivative Instruments and Hedging Activities and its related amendments. As a result of the adoption of SFAS No. 133, the Company recognizes derivative financial instruments, such as its interest rate cap contract, as either an asset or a liability in the consolidated financial statements at fair value.
The accounting for changes in the fair value (i.e., unrealized gains or losses) of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and further, on the type of hedging relationship. Derivatives that are not hedges must be adjusted to fair value through current earnings.
The Company enters into interest rate caps to manage its exposure to changes in interest rates. Interest rate caps also allow the Company to raise funds at floating rates and effectively cap the interest rate.
Income taxes
Income taxes are determined using an annual effective tax rate, which generally differs from the United States federal statutory rate, primarily because of state taxes and research and development tax credits. The Company recognizes deferred tax assets and liabilities for temporary differences between the financial reporting basis and the tax basis of the Company's assets and liabilities, along with net operating loss and credit carryforwards.
The effect on deferred taxes of a change in tax rates is recognized in income in the period that includes the enactment date. Income tax benefits credited to stockholders' equity (deficit) relate to tax benefits associated with amounts that are deductible for income purposes but do not impact net income. These benefits are principally generated from employee exercises of non-qualified stock options.
Fair values of financial instruments
The following methods and assumptions were used by the Company in estimating the fair value of its financial instruments for disclosure purposes:
Long-term debt: The carrying value of the Company's debt approximates fair value. The carrying value of the Company's long-term debt approximates fair value because the interest rate adjusts to market interest rates.
Interest rate cap agreement: The fair value of the interest rate cap agreement, as previously disclosed, is the amount at which it could be settled.
F-11
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
Leases
The Company accounts for leases under the provisions of SFAS No. 13,Accounting for Leases, and subsequent amendments, which require that the Company's leases be evaluated and classified as either operating leases or capital leases for financial reporting purposes. Minimum base rents for the Company's operating leases, which generally have scheduled rent increases over the term of the lease, are recorded on a straight-line basis over the lease term. The initial lease term includes the period from when the Company is given access and control over the leased property, whether or not rent payments are due under the terms of the lease.
For leases with renewal periods at the Company's option, the Company generally considers the lease term to comprise only of the initial lease term as exercise of the renewal options are not considered to be reasonably assured of exercise as determined at lease inception. However, if failure to exercise a renewal option imposes an economic penalty of sufficient magnitude to the Company, then the renewal, at inception, is reasonably assured and will be included in the determination of the appropriate lease term.
In certain instances, the Company disburses cash for leasehold improvements, furnishings, fixtures and equipment to renovate leased premises. If costs are paid directly by the landlord or reimbursed to the Company by the landlord, the Company records a deferred rent liability and amortizes the deferred rent liability over the lease term as a reduction to rent expense. In other instances, the Company may expend cash for landlord additions that the Company makes to lease premises that are reimbursed to the Company by the landlord. Based on the specifics of the leased space and the lease agreement, during the renovation period, amounts paid will be recorded as prepaid rent and any landlord reimbursement will be recorded as an offset to prepaid rent.
Treasury stock
The Company records treasury stock under the cost method whereby the entire cost of the acquired stock is recorded as treasury stock. The Company records reissuances of treasury stock at the fair value of the underlying transactions. Differences between the fair value of the issuance and the cost basis of the treasury stock is recorded as either an adjustment to additional paid-in capital if the transaction results in a gain or accumulated earnings (deficit) if the transaction results in a loss.
Revenue recognition
The Company recognizes revenue in accordance with Securities and Exchange Commission ("SEC") Staff Accounting Bulletin ("SAB") No. 104 ("SAB No. 104"),Revenue Recognition in Financial Statements, which provides guidance on the recognition, presentation and disclosure of revenue in financial statements filed with the SEC. SAB No. 104 outlines the basic criteria that must be met to recognize revenue and provides guidance for disclosure related to revenue recognition policies. In general, the Company recognizes revenue when (i) persuasive evidence of an arrangement exists, (ii) shipment of products has occurred, (iii) the sales price charged is fixed or determinable, and (iv) collection is reasonably assured. The Company's shipment terms are FOB shipping point as outlined in its sales agreements.
F-12
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
The Company's domestic sales agreements do not provide for rights of return or price protection. However, the Company may approve returns on a case-by-case basis at its discretion. Based on the Company's historical experience, such returns generally approximate 1.9% of the Company's total gross sales. Certain international distribution agreements do provide for rights of return and price protection. Generally, such return rights are for a period of not more than 90 days after shipment. In accordance with SFAS No. 48,Revenue Recognition When Right of Return Exists, the Company continuously monitors and tracks product returns and records a provision for the estimated future amount of such future returns, based on historical experience and any notification received of pending returns. The allowance for future sales returns as of December 31, 2007 and 2006 was $538, and $324, respectively, and is recorded as a reduction to revenue. The Company does not grant any warranty provisions on its products. The Company provides for discounts and allowances based on historical experience at the time revenue is recognized as a reduction to revenue. To date, actual provisions have approximated management's estimates.
The Company grants price protection rights to certain international distributors. Such price protection rights require the Company to pay the distributor if there is a reduction in the list price of the Company's products. Price protection payments would be required for the distributor's inventory on-hand or in-transit on the date of the price reduction, for a period not to exceed 90 days prior to the date of the price reduction. The Company has not recorded a liability in connection with such price protection rights as the Company has never reduced the list prices of its products.
In September 2002, the Company entered into a licensing agreement (as discussed in the royalty licensee agreements footnote, Note 8) with an international distributor that specializes in the distribution and marketing of over-the-counter medical oriented products in the drug store and retail channels. In January 2006, the Company entered into a licensing agreement with a diversified Japanese consumer products and services company, which also owns and operates a large chain of aesthetic spas in Japan. The Company recognizes royalty revenues related to the licensing agreements based on the respective distributor's sale of related products. These royalty revenues are recognized as earned and are based upon a predetermined rate within the respective licensing agreement.
Included in revenues are fees charged to customers for shipping and handling. Such revenues amounted to $2,430, $1,831, and $1,220 for the years ended December 31, 2007, 2006, and 2005, respectively. Shipping and handling costs incurred in a sales transaction to ship products to customers are included as a component of cost of sales.
Advertising costs
Advertising costs are expensed the first time the advertising takes place. Advertising and promotion costs incurred for the years ended December 31, 2007, 2006, and 2005 were approximately, $344, $251, and $1,143, respectively. Advertising costs are recorded as a component of selling, general and administrative expenses.
Foreign currency translation
The financial statements of subsidiaries outside the United States are measured using the local currency as the functional currency. Assets and liabilities of these subsidiaries are translated at the rates
F-13
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
of exchange at the balance sheet date. Revenue and expense items are translated at average monthly rates of exchange. The resultant translation adjustments are recorded in comprehensive income (loss), a separate component of stockholders' equity (deficit). Such adjustments amounted to an unrealized loss of $22 and $27 for the years ended December 31, 2007 and 2006, respectively and an unrealized gain of $5 for the year ended December 31, 2005. During the year ended December 31, 2005, the Company disposed of certain of its wholly owned, non-operating, subsidiaries outside of the United States. As a result, the comprehensive income related to foreign currency translation was recognized as a gain in the accompanying consolidated financial statements. The total gain recognized was $111, $102 of which related to foreign currency translation during the year ended December 31, 2005.
Research and development
Research and development activities have historically focused on improving the efficacy of existing pharmaceutical ingredients by enhancing penetration. These activities primarily consist of formulation, chemistry and analytical manufacturing controls and stability work. Research and development expenses include, among other things, wages and benefits, raw materials and supplies, facilities, outside professionals and professional service provider fees. Clinical trials and certain support functions in preparing protocols, reports and other regulatory documents are performed by scientific consultants and third-party contract research organizations. Research and development costs are expensed as incurred.
Stock-based compensation
Prior to the January 1, 2006 adoption of Financial Accounting Standards Board ("FASB") Statement No. 123 (revised 2004) ("SFAS No. 123R"),Share-Based Payment, the Company accounted for grants of options to employees to purchase its common stock using the intrinsic value method in accordance with Accounting Principles Board Opinion No. 25 ("APB No. 25"),Accounting for Stock Issued to Employees, and FASB Interpretation No. 44,Accounting for Certain Transactions Involving Stock Compensation. As permitted by SFAS No. 123 ("SFAS No. 123"),Accounting for Stock-Based Compensation, and as amended by SFAS No. 148 ("SFAS No. 148"),Accounting for Stock-Based Compensation-Transition and Disclosure, the Company had chosen to continue to account for such option grants under APB No. 25 and provide the expanded disclosures specified in SFAS No. 123, as amended by SFAS No. 148.
Effective January 1, 2006, the Company adopted SFAS No. 123R using the modified prospective transition method, and as a result, did not retroactively adjust results from prior periods. Under this transition method, stock-based compensation was recognized for: (i) expense related to the remaining unvested portion of all stock option awards granted during the one year period preceding the Company's initial public offering, based on the grant date fair value estimated in accordance with the original provisions of SFAS No. 123 and (ii) expense related to all stock option awards granted on or subsequent to January 1, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS No. 123R. In accordance with Staff Accounting Bulletin No. 107 ("SAB No. 107"),Share-Based Payment, the remaining unvested options issued by the Company prior to the one year period preceding the initial public offering, with the exception of the October 2005 grants, are not included in the SFAS No. 123R option pool as such options were valued using the minimum value
F-14
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
method. As a result, unless subsequently modified, repurchased or cancelled, such unvested options will not be included in stock-based compensation. The Company applies the Black-Scholes valuation model in determining the fair value of share-based payments to employees. The resulting compensation expense is recognized on a straight-line basis over the requisite service period, which equals the option vesting term of three years.
Upon adoption of SFAS No. 123R, the Company utilized the "long form" method for calculating the tax effects of stock-based compensation pursuant to SFAS No. 123R, paragraph 81. Under the "long form" method, the Company determined the beginning balance of the additional paid-in capital pool ("APIC pool") related to the tax effects of the employee stock-based compensation "as if" the Company had adopted the recognition provisions of SFAS No. 123 since its effective date of January 1, 1995.
Compensation expense is recognized only for those options expected to vest, with forfeitures estimated based on the Company's historical experience and future expectations. Prior to the adoption of SFAS No. 123R, the effect of forfeitures on the pro forma expense amounts was recognized as the forfeitures occurred.
As a result of adopting SFAS No. 123R, the impact on the Consolidated Statement of Income for the year ended December 31, 2006 on income before income taxes and net income was $305 and $195, and did not have a material effect on basic and diluted earnings per share, respectively. For the year ended December 31, 2007, the impact on income before income taxes and net income was $1,557 and $930 and had a $0.04 and a $0.04 impact on basic and dilutive earnings per share, respectively. In addition, prior to the adoption of SFAS No. 123R, the Company presented the tax benefit resulting from the exercise of stock options as operating cash inflows in the Consolidated Statements of Cash Flows. Upon the adoption of SFAS No. 123R, the excess tax benefits for those options are classified as financing cash inflows.
Had compensation expense for the Company's option grants been determined based on their fair value at the grant date for awards consistent with the provisions of SFAS No. 123, the Company's net
F-15
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
income per common share for the year ended December 31, 2005 would have been decreased to the adjusted pro forma amounts indicated below:
| | Year Ended December 31, 2005
| |
|---|
| Net income attributed to common shares | | | | |
| | As reported | | $ | 8,816 | |
| | Equity-based employee compensation cost, net of related tax effects, that would have been included in the determination of net income attributed to common shares if the fair value method had been applied | | | (166 | ) |
| | |
| |
| | Adjusted | | $ | 8,650 | |
| | |
| |
| Basic earnings per common share: | | | | |
| | As reported | | $ | 0.50 | |
| | Equity-based employee compensation cost, net of related tax effects, that would have been included in the determination of net income attributed to common shares if the fair value method had been applied | | | (0.01 | ) |
| | |
| |
| | Adjusted | | $ | 0.49 | |
| | |
| |
| Diluted earnings per share: | | | | |
| | As reported | | $ | 0.50 | |
| | Equity-based employee compensation cost, net of related tax effects, that would have been included in the determination of net income attributed to common shares if the fair value method had been applied | | | (0.01 | ) |
| | |
| |
| | Adjusted | | $ | 0.49 | |
| | |
| |
The fair value of each option granted to employees and directors is estimated using the Black-Scholes option-pricing model with the following weighted-average assumptions for the years ended December 31, 2007, 2006, and 2005:
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Expected stock price volatility | | 32.9 | % | 33.5 | % | 16.9 | % |
| Expected dividend yield | | 0.0 | % | 0.0 | % | 0.0 | % |
| Risk-free interest rate | | 4.2 | % | 4.6 | % | 4.3 | % |
| Expected life of options | | 6.0 years | | 6.0 years | | 6.0 years | |
Expected stock price volatility is based on a combined average expected stock price volatility of publicly traded peer companies deemed to be similar entities whose share or option prices are publicly available. For fiscal years 2007 and 2006 the Company based its expected stock price volatility on the combined average stock price volatility of five publicly traded peer companies, and for fiscal year 2005,
F-16
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
the Company based its expected stock price volatility on the combined average stock price volatility of three publicly traded peer companies. Until such time that the Company has enough historical data, it will continue to rely on peer companies' volatility and will ensure that the selected peer companies are still appropriate. The risk-free interest rate is based on the United States Treasury yield curve in effect at the time of grant with an equivalent remaining term. The Company has paid dividends only once in the past and does not currently plan to pay dividends in the near future. The Company has applied the provisions of SAB No. 107 and SAB No. 110, in electing the simplified method for determining the expected life of options granted subsequent to the date of the shareholder transaction.
Prior to the one-year period preceding the anticipated initial public offering, with the exception of the October 2005 grants, the Company used the minimum value method to determine fair value of option grants.
The aggregate intrinsic value for options outstanding at December 31, 2007 was $8,467. This amount changes based on the estimated fair market value of the Company's stock. Total intrinsic value of options exercised for the year ended December 31, 2007 was $438. As of December 31, 2007, total unrecognized stock-based compensation expense related to nonvested stock options was approximately $2,742, which is expected to be recognized over a weighted average period of approximately 2.16 years. Aggregate intrinsic value represents the total pretax intrinsic value (the difference between the Company's estimated stock price on December 31, 2007 and the exercise price, multiplied by the number of the in-the-money options) that would have been received by the option holders had all the option holders exercised their options on December 31, 2007.
During the year ended December 31, 2005, the Company issued stock options to certain employees. Given the absence of an active market for the Company's common stock, the board of directors was required to estimate the fair value of its common stock at the time of each option grant for purposes of determining stock-based compensation expense. The board of directors granted employees stock options during 2005 at exercise prices ranging from $8.40 per share in March 2005 to $14.40 per share in October 2005. As the exercise price was determined to be equal to or greater than the fair value of the underlying common stock on the date of each grant, the Company did not recognize stock-based compensation expense for the options granted in 2005.
In connection with the preparation of the consolidated financial statements, the Company reassessed the fair value of its employee stock options granted during 2005. In making this reassessment, the Company considered the guidance provided in American Institute of Certified Public Accountants Technical Practice Aid,Valuation of Privately-Held Company Equity Securities Issued as Compensation. Based on this reassessment, the Company concluded that the fair value of the Company's stock at March 2005 and October 2005 was equal to or greater than the fair value of the underlying common stock on the date of each grant. The Company did not recognize any additional compensation expense as the exercise prices of the stock options were at or above the fair value of the common stock on the date of grant based on the Company's reassessed fair value.
F-17
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
Earnings per common share ("EPS")
The Company accounts for earnings per share in accordance with SFAS No. 128,Earnings per Share. Basic earnings per share are computed by dividing net income by the weighted-average number of common shares outstanding. Diluted earnings per common share is computed similar to basic earnings per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Common share equivalents are excluded from the computation if their effect is anti-dilutive. The Company's common share equivalents consist of stock options issued under the Company's Stock Option Plan as well as mandatory redeemable convertible preferred stock.
Under the treasury stock method, the assumed proceeds calculation includes: (a) the actual proceeds to-be-received from the employee upon exercise, (b) the average unrecognized compensation cost during the period and (c) any tax benefits that will be credited upon exercise to additional paid-in capital. The Company determines whether our windfall pool of available excess tax benefits is sufficient to absorb the shortfall. If so, the effect of the hypothetical deferred tax asset write-off reduces the assumed proceeds in the treasury stock calculation. If there is no pool of available excess tax benefits, or if the amount of the pool is insufficient to absorb the entire hypothetical deficient tax deduction, the amount of the deficiency that is charged to income tax expense is not considered to be a reduction of the assumed proceeds. Currently, the Company has determined that we have sufficient windfall pool available.
Basic and diluted earnings per common share were calculated using the following units for the years ended December 31, 2007, 2006, and 2005:
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Weighted average shares outstanding—basic | | 21,989,121 | | 17,996,158 | | 17,522,611 |
| | Effect of dilutive stock options | | 111,616 | | 74,890 | | 90,275 |
| | Effect of dilutive mandatorily redeemable convertible preferred stock | | — | | — | | 152,830 |
| | |
| |
| |
|
| Weighted average shares outstanding—diluted | | 22,100,737 | | 18,071,048 | | 17,765,716 |
| | |
| |
| |
|
For the years ended December 31, 2007, 2006 and 2005, diluted earnings per share does not include the impact of common stock options then outstanding of 172,015, 970,823 and 807,991, respectively, as the effect of their inclusion would be anti-dilutive.
New accounting pronouncements
In December 2007, FASB issued SFAS No. 141(revised 2007) ("SFAS No. 141(R)"),Business Combinations, which amongst other things, requires the Company to record fair value estimates of contingent consideration and certain other potential liabilities during the original purchase price allocation, expense acquisition costs as incurred and does not permit certain restructuring activities previously allowed under Emerging Issues Task Force Issue ("EITF") No. 95-3,Recognition of Liabilities
F-18
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
in Connection with a Purchase Business Combination, to be recorded as a component of purchase accounting. This statement is effective as of the beginning of an entity's first fiscal year beginning after December 15, 2008. The Company will adopt the provisions of this standard beginning January 1, 2009. The provisions of SFAS No. 141(R) will only impact the Company if it is party to a business combination after the standard has been adopted.
In December 2007, the FASB issued SFAS No. 160 ("SFAS No. 160"),Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51, which causes noncontrolling interests in subsidiaries to be included in the equity section of the balance sheet. This statement is effective as of the beginning of an entity's first fiscal year beginning after December 15, 2008. The Company will adopt the provisions of this standard beginning January 1, 2009. The Company is currently evaluating the effects, if any, that SFAS No.160 may have on its financial position, results of operations and cash flows.
In June 2007, the FASB issued EITF Issue No. 07-03 ("EITF 07-03"),Accounting for Non-Refundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities. EITF 07-03 provides guidance on whether non-refundable advance payments for goods that will be used or services that will be performed in future research and development activities should be accounted for as research and development costs or deferred and capitalized until the goods have been delivered or the related services have been rendered. EITF 07-03 is effective for fiscal years beginning after December 15, 2007. The Company has not yet determined the impact that the adoption of EITF 07-03 will have on its consolidated financial position, results of operations or cash flows.
In February 2007, the FASB issued SFAS No. 159 ("SFAS No. 159"),The Fair Value Option for Financial Assets and Financial Liabilities, to permit all entities to choose to elect, at specified election dates, to measure eligible financial instruments at fair value. An entity shall report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date, and recognize upfront costs and fees related to those items in earnings as incurred and not deferred. SFAS No. 159 applies to fiscal years beginning after November 15, 2007, with early adoption permitted for an entity that has also elected to apply the provisions of SFAS No.157 ("SFAS No. 157"),Fair Value Measurements. An entity is prohibited from retrospectively applying SFAS No. 159, unless it chooses early adoption. SFAS No. 159 also applies to eligible items existing at November 15, 2007 (or early adoption date). The Company will adopt the provisions of SFAS No. 159 on January 1, 2008. The Company does not expect the adoption of SFAS No. 159 to have an impact on its consolidated financial position, results of operations or cash flows.
In December 2006, the FASB issued FASB Staff Position ("FSP") EITF 00-19-2 ("FSP EITF 00-19-2"),Accounting for Registration Payment Arrangements, which specifies that the contingent obligation to make future payments or otherwise transfer consideration under a registration payment arrangement should be separately recognized and measured in accordance with FASB Statement No. 5,Accounting for Contingencies. Adoption of FSP EITF 00-19-02 is required for fiscal years beginning after December 15, 2006. The adoption of FSP EITF 00-19-2 as of January 1, 2007 did not have an impact on the Company's financial position, results of operations or cash flows.
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements. This new standard provides guidance for using fair value to measure assets and liabilities. Under SFAS No. 157, fair value
F-19
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 2: Summary of significant accounting policies (Continued)
refers to the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants in the market in which the reporting entity transacts. In this standard, the FASB clarifies the principle that fair value should be based on the assumptions market participants would use when pricing the asset or liability. In support of this principle, SFAS No. 157 establishes a fair value hierarchy that prioritizes the information used to develop those assumptions. The fair value hierarchy gives the highest priority to quoted prices in active markets and the lowest priority to unobservable data, for example, the reporting entity's own data. Under the standard, fair value measurements would be separately disclosed by level within the fair value hierarchy. The provisions of SFAS No. 157 are effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. However, FSP No. 157-2, Effective Date of FASB Statement No. 157, was issued in February 2008 which defers the effective date of SFAS No. 157 for nonfinancial assets and liabilities, except for items that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years. The Company has not yet determined the impact that the adoption of SFAS No. 157 will have on its consolidated financial position, results of operations or cash flows.
In July 2006, the FASB issued FASB Interpretation No. 48 ("FIN 48"),Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, which prescribes accounting for and disclosure of uncertainty in tax positions. This interpretation defines the criteria that must be met for the benefits of a tax position to be recognized in the financial statements and the measurement of tax benefits recognized. The Company adopted the provisions of FIN 48 as of January 1, 2007, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings. See Note 6 for further discussion.
In February 2006, the EITF issued EITF No. 06-3 ("EITF No. 06-3"),How Sales Taxes Collected from Customers and Remitted to Governmental Authorities Should Be Presented in the Income Statement (That Is, Gross Versus Net Presentation). EITF 06-3 requires disclosures surrounding a company's accounting policy (i.e., gross or net presentation) regarding presentation of taxes within the scope of EITF No. 06-3. If taxes included in gross revenues are significant, a company should disclose the amount of such taxes for each period for which an income statement is presented. EITF 06-3 is effective for the first annual or interim reporting period beginning after December 15, 2006. The disclosures are required for annual and interim financial statements for each period for which an income statement is presented. The Company began applying the provisions of EITF 06-3 as of January 1, 2007. As the Company presents such taxes on a net basis in its consolidated Statements of Income, the adoption of EITF No. 06-3 did not have an impact on the Company's consolidated Statements of Income.
F-20
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 3: Composition of certain financial statement captions
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| Inventories, net | | | | | | | |
| | Raw materials | | $ | 2,610 | | $ | 3,251 | |
| | Finished goods | | | 3,906 | | | 3,291 | |
| | |
| |
| |
| | | | 6,516 | | | 6,542 | |
| | Less reserve for inventories | | | (469 | ) | | (276 | ) |
| | |
| |
| |
| | | $ | 6,047 | | $ | 6,266 | |
| | |
| |
| |
General and administrative expense capitalized into inventory during the years ended December 31, 2007 and 2006 was $554 and $150, respectively. As of December 31, 2007 and 2006, general and administrative expenses included in inventory was $209 and $150, respectively.
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| Property and equipment | | | | | | | |
| | Furniture and fixtures | | $ | 776 | | $ | 776 | |
| | Computer software and equipment | | | 2,906 | | | 2,443 | |
| | Lab and office equipment | | | 543 | | | 494 | |
| | Leasehold improvements | | | 3,107 | | | 3,111 | |
| | Capital lease (office equipment) | | | 261 | | | 256 | |
| | Construction in progress | | | 157 | | | 73 | |
| | |
| |
| |
| | | | 7,750 | | | 7,153 | |
| | Less accumulated depreciation and amortization | | | (4,991 | ) | | (3,404 | ) |
| | |
| |
| |
| | | $ | 2,759 | | $ | 3,749 | |
| | |
| |
| |
Depreciation expense for the years ended December 31, 2007, 2006, and 2005 was $1,587, $968, and $402, respectively. At December 31, 2007 and 2006, accumulated depreciation for fixed assets under capital lease was $193 and $154, respectively. Interest costs that were capitalized into construction in progress and other property and equipment totaled $0 and $157 for the years ended December 31, 2007 and 2006, respectively.
F-21
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 3: Composition of certain financial statement captions (Continued)
During the years ended December 31, 2007 and 2006, the Company recorded a loss on disposal of fixed assets of $1 and $72, respectively. The loss is reported as a component of selling, general and administrative expenses.
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Accrued liabilities | | | | | | |
| | Salaries and related benefits | | $ | 2,035 | | $ | 2,535 |
| | Other | | | 1,104 | | | 1,063 |
| | |
| |
|
| | | $ | 3,139 | | $ | 3,598 |
| | |
| |
|
Note 4: Line of credit, notes payable and capital lease obligations
Notes payable and capital lease obligations consist of the following at December 31, 2007 and 2006:
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| Senior Secured Credit Facility | | | | | | | |
| | Term A | | $ | — | | $ | 5,556 | |
| | Term B | | | — | | | 20,095 | |
| Capital lease obligations | | | 96 | | | 130 | |
| | |
| |
| |
| | | | 96 | | | 25,781 | |
| Less current maturities | | | 54 | | | (2,729 | ) |
| | |
| |
| |
| | | $ | 42 | | $ | 23,052 | |
| | |
| |
| |
On January 28, 2005, the Company entered into an $80,000 Senior Secured Credit Facility. The Facility is structured with two term loans and a revolving line of credit. The term loans include: (i) $20,000 in principal borrowings under Senior Secured Term Loan A (the "Term Loan A") maturing January 28, 2010; and (ii) $50,000 in principal borrowings under Senior Secured Term Loan B (the "Term Loan B") maturing January 1, 2011. The Senior Secured Revolving Credit Facility (the "Revolver") provides availability up to $10,000 for working capital needs. Borrowings under this Facility are collateralized by a first lien on all of the assets of the Company. The Company pays an unused line fee of 0.5% monthly on the unused balance of the Revolver. There was no outstanding balance on the Revolver as of December 31, 2007 and 2006. The availability of the Revolver is based upon a multiple of 3.25 times the Company's EBITDA less outstanding debt. As of December 31, 2007 and 2006, the availability of the Revolver was $10,000.
The Facility contains certain financial and non-financial covenants. Such non-financial covenants include certain limitations on annual capital expenditures and use of proceeds from the issuance of debt and equity securities, and prohibit the Company from making dividend distributions. The financial covenants contained in the agreement include, among other provisions, maintaining a minimum EBITDA, a maximum Debt to EBITDA Ratio and a minimum Fixed Charge Ratio. On September 6,
F-22
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 4: Line of credit, notes payable and capital lease obligations (Continued)
2006, the Company entered into the Second Amendment to the Senior Secured Credit Facility. Pursuant to the Amendment, the calculation of EBITDA and Total Debt used in the covenant calculations were modified. On November 13, 2006, the Company entered into the Third Amendment to the Senior Secured Credit Facility. Pursuant to the Amendment, certain terms were modified, some of which took effect upon the completion of the initial public offering, to change the Company's (i) ability to make payments to subordinated debt, (ii) use of proceeds from an initial public offering, (iii) calculation of Total Debt used in the covenant calculations, (iv) negative covenants and (v) financial reporting requirements. In addition, the mandatory principal prepayment was modified to equal 25% to 50% of Consolidated Excess Cash Flow.
As of December 31, 2007 and 2006, the maximum Debt to EBITDA requirement was 1.50 and 2.50, respectively, and the minimum Fixed Charge Ratio was 1.25 and 1.20, respectively. The Company was in compliance with all financial and non-financial covenants as of December 31, 2007 and 2006.
In accordance with the Third Amendment of the Senior Secured Credit Facility, the Company made a repayment of $35,000 in debt with the net cash proceeds received in the initial public offering consummated on December 13, 2006. As of December 31, 2006, the Company was subject to a $1,313 mandatory principal prepayment which was paid within the terms of the Facility on April 2, 2007. In addition, during the year ended December 31, 2007, the Company made regularly scheduled payments of $805 and optional principal prepayments of $23,533. Approximately $9,172 of the net cash proceeds received in the secondary public offering were utilized to paydown the outstanding balance of the term loans and the revolving credit facility. The optional prepayments resulted in the complete paydown of Term Loan A and Term Loan B on October 17, 2007. The Company did not incur any prepayment penalties or premiums upon making the optional prepayments.
Loans under the Facility bear variable interest based on a margin, at the Company's option, over prime rate or LIBOR. The margins applicable to portions of amounts borrowed vary depending on the Company's consolidated Debt to EBITDA Ratio. As of October 17, 2007, the date of complete paydown, applicable margins were 1.75% and 2.00% for prime rate borrowings and 3.25% and 3.50% for LIBOR borrowings for Term Loan A and Term Loan B, respectively. As of December 31, 2006, the applicable margins were 2.50% and 2.75% for prime rate borrowings and 4.00% and 4.25% for LIBOR borrowings, for Term Loan A and Term Loan B, respectively. As of October 17, 2007, Term Loan A was bearing interest at a LIBOR based rate of 8.38% and Term Loan B was bearing interest at a LIBOR based rate of 8.63%. As of December 31, 2006, Term Loan A was bearing interest at a LIBOR based rate of 9.35% and Term Loan B was bearing interest at a LIBOR based rate of 9.60%. The weighted-average interest rate for borrowings under this Facility was 8.96% and 9.15% as of October 17, 2007 and December 31, 2006, respectively. Interest expense incurred attributable to this Facility for the years ended December 31, 2007, 2006 and 2005 was $1,179, $5,749 and $5,435, respectively.
F-23
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 4: Line of credit, notes payable and capital lease obligations (Continued)
The total annual maturities of capital lease obligations as of December 31, 2007 are as follows:
| | Capital Leases
| |
|---|
| Years ending December 31, | | | | |
| 2008 | | $ | 56 | |
| 2009 | | | 25 | |
| 2010 | | | 19 | |
| | |
| |
| | | | 100 | |
| Less amount representing future interest cost | | | (4 | ) |
| | |
| |
| | | $ | 96 | |
| | |
| |
Interest rate cap
In March 2005, the Company entered into a LIBOR interest rate cap agreement as an economic hedge against Facility borrowings. The interest rate is capped at 6.0% on a decreasing notional amount starting at $35,000 decreasing to approximately $34,038. The agreement expires on January 1, 2008. The interest rate cap is recognized on the Consolidated Balance Sheet at its fair market value and any change in fair value is reported in interest expense. As of December 31, 2007, 2006 and 2005, the fair value of the interest rate cap was approximately $0, $3 and $19, respectively.
Letter of credit agreements
On May 18, 2006, the Company provided an irrevocable standby letter of credit to the Florida Department of Health in the amount of $100, which may be drawn upon to satisfy any unpaid administrative fines or penalties. This letter of credit expired on May 17, 2007 and was renewed for a period of one year.
On May 23, 2006, the Company provided an irrevocable standby letter of credit to the California State Board of Pharmacy in the amount of $100, to serve as a security device for the performance of the Company of its obligations under the Business and Professions Code. This letter of credit expired on May 22, 2007. A new irrevocable standby letter of credit was executed in February 2008 (see Note 15).
Note 5: Common stock
Common stock
In 2003, the Company repurchased 2,090,524 shares of its common stock from several of its existing stockholders at a price of $3.60 per share. The Company recorded the repurchases as treasury stock utilizing the cost method since the Company intended to reissue such shares in the future. During 2005 and 2004, the Company reissued treasury shares as a result of the exercise of stock options and the conversion of Series B mandatorily redeemable preferred stock into common stock.
In December 2005, the Company recorded compensation expense of $37 related to the issuance of 5,000 shares of common stock to two new board members for consideration of these individuals joining
F-24
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 5: Common stock (Continued)
the board. The compensation expense and related additional paid-in-capital was recorded at the fair market value at the time of grant. The Company determined the fair market value of its common stock after board of director and management consideration of a number of objective and subjective factors.
Note 6: Income taxes
The current and deferred provision for federal, state and foreign income taxes consists of the following:
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Current | | | | | | | | | |
| Federal | | $ | 8,378 | | $ | 3,123 | | $ | 4,358 |
| State | | | 1,946 | | | 785 | | | 1,198 |
| | |
| |
| |
|
| | | | 10,324 | | | 3,908 | | | 5,556 |
| | |
| |
| |
|
| Deferred | | | | | | | | | |
| Federal | | | (104 | ) | | (313 | ) | | 384 |
| State | | | 55 | | | (137 | ) | | 103 |
| | |
| |
| |
|
| | | | (49 | ) | | (450 | ) | | 487 |
| | |
| |
| |
|
| | | $ | 10,275 | | $ | 3,458 | | $ | 6,043 |
| | |
| |
| |
|
The provision for income taxes differs from the amount that would result from applying the federal statutory rate to pre-tax income for the years ended December 31 as follows:
| | Year Ended December 31,
| |
|---|
| | 2007
| | 2006
| | 2005
| |
|---|
| Federal statutory income tax rate | | 35.0 | % | 35.0 | % | 35.0 | % |
| State income tax, net of federal benefit | | 5.1 | % | 4.4 | % | 5.7 | % |
| Research and development credit | | (1.3 | )% | (3.4 | )% | — | |
| Other | | 1.5 | % | 0.1 | % | (0.4 | )% |
| | |
| |
| |
| |
| | | 40.3 | % | 36.1 | % | 40.3 | % |
| | |
| |
| |
| |
F-25
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 6: Income taxes (Continued)
The significant components of the net deferred tax assets and liabilities at December 31 are as follows:
| | December 31,
| |
|---|
| | 2007
| | 2006
| |
|---|
| Deferred assets | | | | | | | |
| | Financial statement reserves | | $ | 781 | | $ | 375 | |
| | State taxes | | | 219 | | | — | |
| | Depreciation and amortization | | | 536 | | | 652 | |
| | Trademark expenses | | | 179 | | | 200 | |
| | Compensation expense | | | 947 | | | 719 | |
| | Prepaid royalty | | | 489 | | | 492 | |
| | Debt issuance costs | | | 5 | | | 606 | |
| | Foreign operating loss | | | 57 | | | 56 | |
| | Other | | | 136 | | | 147 | |
| | |
| |
| |
| | | | 3,349 | | | 3,247 | |
| Deferred liabilities | | | | | | | |
| | Prepaid expenses | | | (272 | ) | | (180 | ) |
| | State taxes | | | — | | | (40 | ) |
| | |
| |
| |
| | | | (272 | ) | | (220 | ) |
| Net deferred assets | | | 3,077 | | | 3,027 | |
| Less valuation allowance | | | (57 | ) | | (56 | ) |
| | |
| |
| |
| | | $ | 3,020 | | $ | 2,971 | |
| | |
| |
| |
The Company recorded a deferred tax asset of $57 and $56 related to the net operating loss carryover of its majority owned subsidiary, Obagi Singapore, as of December 31, 2007 and 2006, respectively. A full valuation allowance was provided for the loss carryover as the Company believes that it is more likely than not that this deferred tax asset will not be realized.
The Company adopted the provisions of FIN 48 on January 1, 2007. As a result of the implementation of FIN 48, the Company recognized approximately a $90 increase in the liability for unrecognized tax benefits, which was accounted for as an increase to the January 1, 2007 accumulated deficit balance. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Balance as of January 1, 2007 | | $ | 111 |
| Additions based on tax positions related to the current year | | | 50 |
| | |
|
| Balance as of December 31, 2007 | | $ | 161 |
| | |
|
Any change in the above unrecognized tax benefits will impact the effective tax rate.
The Company does not believe there will be any material changes in its unrecognized tax benefits within the next 12 months.
F-26
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 6: Income taxes (Continued)
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. During the years ended December 31, 2007 and 2006, the Company recognized approximately $11 and $29 in interest and penalties. As of the adoption date of January 1, 2007 and as of December 31, 2007, accrued interest related to uncertain tax positions was $29 and $40, respectively.
The tax years 2003-2006 remain open to examination by the major taxing jurisdictions to which we are subject.
Note 7: Concentrations of credit risk and significant customers
For the years ended December 31, 2007, 2006, and 2005, no single customer accounted for 5% or more of the Company's net revenues.
The Company currently has limited internal manufacturing capabilities and outsources most of its product manufacturing. Additionally, the Company does not have long term contracts with most of these third-party manufacturers. Although there are a limited number of third-party manufacturers, management believes that other suppliers could provide similar services on comparable terms. A change in suppliers, however, could cause a delay in manufacturing and a possible loss of sales, which would affect operating results adversely.
The Company currently has limited internal research and development capabilities and primarily outsources its product research and development to third-party research labs. Although there are a limited number of third-party research labs, management believes that other labs could provide similar services on comparable terms. A change in providers, however, could cause a delay in the Company's ability to develop and deliver products on a timely and competitive basis, which could affect operating results adversely.
Note 8: Royalty licensee agreements
All of the Company's products are marketed under the name "Obagi" and other marketed brands. The Company owns the registered trademark and trade name "Obagi." In December 2002, the Company entered into a new license agreement, replacing a previous agreement, with Dr. Obagi, the Company's former medical director and board member and one of its current principal stockholders (refer to Note 9). As part of the new agreement, Dr. Obagi is to receive an annual payment in an amount equal to $200 plus a royalty of 5% of the net sales of two nonprescription product line concepts, if and to the extent, that such lines are developed, marketed and sold. The payments and royalties for any year are not to exceed $500. For the years ended December 31, 2007, 2006, and 2005, no sales of such nonprescription product lines were made; accordingly, no royalty payments were required. Additionally, the annual payment is expensed as incurred and costs have been included as a component of selling, general and administrative expenses. In addition, according to the new agreement, the Company must provide Dr. Obagi with the ability to purchase products in connection with his own private practice, at a discount equal to the maximum discount that the Company provides to independent third-party customers. As of December 31, 2007, 2006 and 2005, the Company did not owe Dr. Obagi any amounts due under the agreement. As of December 2, 2005, this licensing agreement had expired.
F-27
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 8: Royalty licensee agreements (Continued)
Pursuant to the Patent License Agreement dated June 26, 2003 with a third party, the Company has licensed four interlocking method and formulation patents in exchange for a non-refundable fee in the amount of $200 for the first year, and an additional $100 payable on each of the first and second anniversaries of this agreement. The agreement also calls for the Company to pay royalties in the amount of (i) 5% of the net sales of products sold in a territory covered by the licensed patents and (ii) 3.5% of net sales of products manufactured in a territory covered by the licensed patents and sold in a territory not covered by the licensed patents. Such royalties are to be paid on a quarterly basis. For the years ended December 31, 2007, 2006, and 2005, related royalties of $297, $179 and $122 have been included as a component of cost of sales, respectively. The initial term of this agreement is three years and is renewable annually at the option of the Company provided the Company makes an annual non-refundable renewal fee in the amount of $100. The Patent License Agreement may terminate at the licensor's option upon merger or acquisition of the Company and may be cancelled by either party upon default.
In January 2006, the Company entered into an Option and Product License Agreement with a third party to license certain technology, in exchange for a non-refundable fee in the amount of $250, with an additional $250 payable within 15 days of confirming successful clinical results. The non-refundable fee of $250 may either be offset against future royalties payable under the agreement or offset against future purchases of inventory. For the first product developed under this agreement, an additional license payment of $250 is payable upon the product becoming commercially available for distribution with another $250 due in the subsequent quarter to that event. For each additional product developed, an additional license payment of $250 is due up to a maximum $1,000 for all products developed. The agreement also calls for the Company to pay royalties in the amount of (i) 8% of the net sales of products sold in a territory covered by the licensed patents, and (ii) the Company shall pay a minimum annual payment equal of $1,000 in year one, for all subsequent years the Company is required to pay the greater of $500, or 90%, of the prior year royalty payments. Such royalties are to be paid on a quarterly basis. In addition to the royalties, the Company shall also pay one time milestone payments based upon achieving certain levels of net sales. As of December 31, 2007, there have been no commercially viable products developed under this agreement. The term of this agreement is for the life of the patent. The patent application for the licensed technology is currently pending. The Option and Product License Agreement may terminate at the licensor's option upon the Company's failure to meet commercially reasonable development benchmarks. Pursuant to this agreement, the initial payment of $250 is included in long term Other assets as of December 31, 2007 and 2006. Royalty payments will be expensed as incurred. As of December 31, 2007, no other payments have been made.
In March 2004, as part of an agreement with a third-party international licensor, the Company received an ongoing, non-exclusive right to market and sell any and all products marketed under the "Obagi" brand containing a specific range of Kinetin concentration, limited to existing channels of trade in Japan. Under the terms of the license agreement with the same party, the Company's license rights are valid until the last of the related patents expire in December 2014. In exchange for this right, the Company paid, on April 2, 2004, an initial payment of $1,250, net of cash receipts in the amount of $250 from a sublicensing arrangement with an international distributor in Japan. In addition, the Company will pay royalties up to an additional maximum amount of $500 based on future sales in Japan of such skincare products. Pursuant to this agreement, the Company recorded the initial net
F-28
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 8: Royalty licensee agreements (Continued)
payment as an intangible asset and is amortizing such costs on a straight line basis over the license period. During the years ended December 31, 2007, 2006 and 2005, the Company recognized $106, $106 and $106 in amortization expense, respectively. Such amounts are included as a component of cost of sales. Royalty payments are expensed as incurred and have amounted to $39, $70 and $43 for the years ended December 31, 2007, 2006, and 2005, respectively.
Note 9: Related-party transactions
Dr. Zein Obagi, M.D.
On December 17, 2002, the Company entered into an amended and restated employment agreement with Zein Obagi, M.D., the Company's former executive medical director and board member and one of its current principal stockholders. The employment agreement was effective until December 2, 2005 or upon triggering of the termination provisions of the agreement. Unless either party notified the other in writing, the term of the agreement was automatically extended by successive one-year terms. Under the agreement, Dr. Obagi was entitled to a base salary of $330 per year subject to an increase as determined by the Company's compensation committee. In addition, Dr. Obagi was entitled to an incentive bonus of up to $225 as determined by the Company's compensation committee. The compensation committee based its determination on Dr. Obagi's success of specific activities within his control and other Company-wide measures. The Company gave Dr. Obagi notice of termination in 2005 as required by the agreement. Subsequently, the agreement was extended until June 30, 2006.
Under the agreement, if Dr. Obagi was terminated for cause, he would receive no severance. If Dr. Obagi was terminated without cause upon 30 days' prior written notice, then he would have been entitled to his then existing base salary for the entire period remaining on the term of his employment agreement and certain rights with respect to his stock. If Dr. Obagi terminated his employment upon 90 days' prior written notice, he would have been entitled to 90 days' salary.
Dr. Obagi is also a prominent physician in the medical community and was granted the right to purchase products from the Company at a discount equal to the maximum discount offered to the Company's unrelated customers. In addition to his primary medical practice in Beverly Hills, California, Dr. Obagi is also a 75% owner of two other dermatology clinics, the Chinese Obagi Corporation and Vietnamese Obagi Corporation (the "Clinics"). The Clinics are located in Southern California and cater to the local Chinese and Vietnamese communities. Other than the common ownership interest by Dr. Obagi, the Company is otherwise unrelated to these two corporations.
In 2005, the Company began negotiations with an affiliate of Dr. Obagi to lease space for a marketing and training center (the "Center"). The space is located in a property owned by the affiliate of Dr. Obagi. In conjunction with these negotiations which were ongoing as of December 31, 2005, the Company began renovations on the space. On June 29, 2006, the Company entered into a lease agreement and a letter agreement with this affiliate, making an advance to this affiliate on future rent. As of December 31, 2007, amounts included in Prepaid expenses and other current assets and Other assets totaled $184 and $475, respectively. As of December 31, 2006, amounts included in Prepaid expenses and other current assets and Other assets totaled $184 and $659, respectively. As of December 31, 2007 and 2006, there was no balance in construction in progress related to the Center.
F-29
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
2006 Services Agreement. On June 29, 2006, the Company entered into an agreement with Zein E. Obagi, MD Inc. ("Obagi, Inc."), Dr. Obagi, Samar Obagi, the Zein and Samar Obagi Family Trust and Skin Health Properties, Inc ("SHP, Inc."). The agreement provides that Dr. Obagi (and his affiliates that entered into the agreement, including Obagi, Inc.) and/or SHP, Inc. (an entity controlled by Dr. Obagi and his affiliates) will promote and provide services to support the marketing of the Company's products, including oversight of the property the Company is leasing in Beverly Hills, California. Additionally, Dr. Obagi (and his affiliates that entered into the agreement) will be available to advise and assist the Company in the formulation and clinical testing of new products on a retainer basis, and he will also provide training and/or education seminars on a fee basis and participate in at least one clinical study per year. The Company has agreed to pay Obagi, Inc. an annual retainer of $570 for the advising and formulating services and the marketing and support services described above, as well as for Dr. Obagi's agreement to chair an annual Obagi Skin Health Alumni Symposium and up to two clinical advisory meetings per year. In addition, the Company has agreed to pay Obagi, Inc. an annual fee of $200 for the first two years of the agreement for the development of Proderm products, a line of skincare products. At the end of the two years, the Company has an option to continue to sell Proderm products, in which case the Company will pay Obagi, Inc. an annual royalty payment of the greater of $200, or 5%, of the Company's net Proderm revenues. During the years ended December 31, 2007 and 2006, the Company recorded the $200 annual payment as research and development expense as the research of new products in the Proderm line is still in progress. The Company will also pay Obagi, Inc. royalty fees for developing other products identified in the agreement equal to 5% of the Company's net revenues from sales of those products. As there were no products commercialized under the agreement, the Company did not pay any royalties pursuant to the agreement during the years ended December 31, 2007 and 2006. The Company has agreed to pay additional fees and expenses for training and educational services under the agreement. In addition, the Company has agreed to reimburse up to 50% of all invoiced commercially reasonable marketing design and development expenses associated with the opening of the property in Beverly Hills, not to exceed $100. As of December 31, 2007 and 2006, the Company recorded a payable to Dr. Obagi for $80 and $80, respectively, related to reimbursable expenses for the opening of the Center.
The Company has also agreed to indemnify Dr. Obagi and his affiliates for any claims against the Company's products, or any claims arising out of the Company's acts or omissions or any breach of warranties given by the Company contained in the agreement.
Under the agreement, the Company has been granted a perpetual, royalty-free, non-exclusive license to all accounts, customer lists and other customer information and data (subject to federal and state privacy laws) regarding Obagi, Inc.'s patients, as well as a non-exclusive license to use and reproduce the marketing materials produced by Obagi, Inc. and/or SHP, Inc. The Company granted to Obagi, Inc. and/or SHP, Inc. a limited, non-exclusive, irrevocable license for the use of certain of the Company's trademarks, as well as a non-exclusive license to use and reproduce the marketing materials designated by the Company from time to time for the promotion and marketing services being provided under the agreement.
Under the agreement, the maximum discount that the Company provides to independent third-party physicians in the United States will apply to all products distributed by the Company that are supplied by the Company to Obagi, Inc. and/or SHP, Inc. in connection with the promotion and
F-30
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
marketing services under the agreement, as well as those supplied to Obagi, Inc. in connection with Dr. Obagi's practice within the United States.
Unless otherwise terminated in accordance with its terms, the agreement's initial term is five years, and it may be renewed for additional terms upon the mutual consent of the parties upon six months' written notice prior to the end of the initial term.
2006 Separation and release agreement. In connection with the 2006 Services Agreement with Dr. Obagi described above, the Company entered into a separation and release agreement, which contains non-competition provisions, with Dr. Obagi effective as of June 29, 2006. Under the agreement, Dr. Obagi agreed not to directly or indirectly compete with the Company for a five year period. In connection with the agreement, the Company paid Dr. Obagi $368.
2006 Lease agreement and letter agreement. In connection with the 2006 Services Agreement with Dr. Obagi described above, the Company entered into a lease agreement for the Beverly Hills property described above and a letter agreement with SHP, Inc. as landlord dated June 29, 2006. The lease has a term of five years beginning August 1, 2006 and can be extended or terminated earlier under the terms of the lease. The base rent under the lease is $87 per year, and will be raised at a rate of 3.5% per year thereafter. During the years ended December 31, 2007 and 2006, the Company recorded $184 and $77, respectively, in rent expense related to the lease agreement.
The Company also entered into a letter agreement in connection with the lease with SHP, Inc., dated June 29, 2006, that relates to leasehold improvements and prepayment of rent. Under the letter agreement, SHP, Inc. acknowledges that the Company has paid $2,197 in respect of leasehold improvements and prepayment of rent under the lease, and the Company will not be required to pay any additional amounts.
Cellogique Corporation
Dr. Obagi is also a 70% beneficial shareholder in Cellogique Corporation ("Cellogique"), one of the Company's largest international distribution partners. On November 10, 2005, the Company entered into a new Distribution Agreement with Cellogique. The agreement granted Cellogique the exclusive right to promote, market, sell, distribute and sub-distribute certain specified products to customers within the Middle East. The agreement includes discounts off of the distributors' base price based on volume purchases, and certain advertising and promotional activities. Such discounts may at times exceed 58% of the distributors' base price listing. The agreement is for a term of 12 years effective January 1, 2006. Prior to the new agreement, the agreement had the option to renew in perpetuity.
Prior to the November 2005 agreement, Cellogique was under a Product Distribution Agreement, which had a term of 30 months and was renewable each two years thereafter. Under this superseded agreement, Cellogique received product discounts off of United States doctor list prices based on volume purchases. The agreement granted Cellogique the exclusive right to promote, market, sell, distribute and sub-distribute certain specified products to customers within the Middle East.
F-31
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
Total sales made to Dr. Obagi, Cellogique, and the Clinics, and the related cost of sales for the years ended December 31, 2007, 2006 and 2005 are included in the Company's consolidated statements of operations and are as follows:
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Sales, net of discounts | | $ | 3,182 | | $ | 3,214 | | $ | 2,569 |
| Costs of sales | | | 600 | | | 569 | | | 551 |
Austin T. McNamara
On September 1, 2001, the Company entered into an employment agreement with Austin T. McNamara, former chairman of the board, president and chief executive officer. The agreement was for an initial period of three years and automatically renewed for successive one-year periods. Under the agreement, Mr. McNamara was entitled to a base salary of not less than $500 per year. In addition, Mr. McNamara was entitled to an annual bonus as determined by the compensation committee, with a target of 100% of his base salary based on the achievement of performance-based milestones.
If Mr. McNamara was terminated without cause or due to change in control, then he would have been entitled to either 12 months of his base salary or any unpaid base salary for the entire remaining term of his employment agreement, whichever is greater. In addition, all of his unvested options would have immediately vested in full. Change of control was defined to occur if any institution, other than Stonington Capital Appreciation 1994 Fund, L.P. ("Stonington Fund"), a principal stockholder, or its affiliates, acquired greater than 50% of the Company's voting stock, or greater than 30% of the Company's voting stock and Stonington Fund did not own at least 30% of the stock. Mr. McNamara is also subject to a confidentiality covenant, a covenant not to solicit any employee to leave the Company's employment during the term of the agreement and one year thereafter, and a covenant not-to-compete with the Company during the same time period.
On December 2, 1997, the Company entered into a Management Services Agreement with Mandarin Management Partners, Inc. ("Mandarin Partners"). The agreement provided for Mandarin Partners to provide management consulting and business advisory services to the Company. As compensation for those services, the Company paid Mandarin Partners, and then Lighthouse Venture Group ("LVG"), to whom the agreement was assigned on December 31, 2001; a monthly fee equal to two percent of the Company's adjusted gross sales. Under an oral agreement between LVG and the Company, LVG paid 50% of Mr. McNamara's salary and bonus through December 2005, and to the extent the Company's fees to LVG exceeded the amounts paid by LVG to Mr. McNamara for his salary and bonus, LVG remitted the additional amounts back to the Company, except for amounts to cover expenses. For the year ended December 31, 2005, the Company made $300 in payments to LVG, which are included in selling, general and administrative expenses. The oral and written agreements with LVG terminated as of December 31, 2005. LVG is controlled by Mr. McNamara.
In April 2002, the Company entered into an Investor's Rights Agreement with its former Chairman and Chief Executive Officer, Austin McNamara. Subsequently, trusts established by Mr. McNamara, the McNamara Family Irrevocable Trust dated December 17, 2004 and the McNamara Family Trust dated December 27, 2004 (the "Trusts"), became the owners of the 1,875,001 shares of the Company's
F-32
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
common stock owned by Mr. McNamara and parties to the Investor's Rights Agreement. Mr. McNamara resigned as a member of the Company's board of directors and as an employee in May 2006 and died in December 2006. The Investor's Rights Agreement contained a repurchase obligation under which the Company was required to repurchase the shares of the Company held by the Trusts upon the exercise of a repurchase right. Subsequent to Mr. McNamara's resignation, the Trusts exercised this repurchase right. Pursuant to procedures set forth in the Investor's Rights Agreement, three valuation firms were retained to prepare valuations of the stock held by the trusts and the total purchase price was to have been $28,201.
Any obligation the Company would have had to repurchase the trusts' shares was to have been subordinated to its Credit Agreement while the Credit Agreement is in place. The Investor's Rights Agreement contains subordination provisions and the Trusts signed an additional subordination agreement in connection with the Facility pursuant to which they agreed to be subordinated to the loans under the Facility. On May 18, 2006, the trusts' exercised the repurchase right contained in the Investor's Rights Agreement to require the Company to repurchase the shares owned by the trusts. On November 17, 2006, the Company tendered promissory notes in the aggregate principal amount of $28,201 and a cash payment of $1,500 as partial prepayment of the notes, to the Trusts in order to close on the Company's repurchase of the shares held by the Trusts pursuant to the terms of the Investor's Rights Agreement. The Trusts refused to accept the Company's tender of these payments and refused to tender their shares and close on the repurchase of the shares they hold. As a result, the Company believed the Trusts were in material breach of their obligations under the Investor's Rights Agreement. Based upon this material breach by the Trusts, as well as for other reasons, the Company believed it was no longer obligated to repurchase the shares held by the Trusts pursuant to the Investor's Rights Agreement and the right of the Trusts to require the Company to repurchase the shares had expired and could no longer be enforced against the Company.
The Trusts had taken the position that they did not breach their obligations under the Investor's Rights Agreement and that instead the Company had breached its obligation to repurchase the Trusts' shares under the Investor's Rights Agreement.
On May 29, 2007, the Company entered into a Settlement and Consent Agreement and Mutual Release (the "Agreement") with the estate of Austin T. McNamara, the Trusts, Lucy B. McNamara, individually and as trustee of the Trusts and as executor of the estate of Austin T. McNamara, and LVG.
Pursuant to the Agreement, the Company and the McNamara Parties have released each other from all claims they may have against each other, except for claims related to potential taxes and related charges, should they arise, with respect to the past exercise of certain stock options by the Trusts. No payments were made by the parties to each other in connection with the settlement of the claims.
The settlement includes the release of claims by the McNamara Parties, as described above, that the Company was obligated to purchase the 1,875,001 shares of the Company's common stock then owned by the Trusts and all claims related to Mr. McNamara's employment with the Company and the termination of his employment. In connection with the release, the Company has written off a net receivable of approximately $143 for a claimed compensation overpayment to a McNamara Party.
F-33
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
The McNamara Parties also sold all of their ownership in the Company's stock to an institutional investor in a privately negotiated transaction. As a condition to that transaction, the purchaser of the shares signed the lock-up agreement executed by other Company stockholders in connection with the Company's initial public offering. This lock-up agreement expired on June 12, 2007.
Receivables due from related parties as of at December 31, 2007 and 2006 are as follows:
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Due from Dr. Obagi | | $ | 500 | | $ | 560 |
| Due from Cellogique | | | 424 | | | 646 |
| Due from China Obagi Corporation | | | 58 | | | 27 |
| Due from LVG | | | — | | | 268 |
| | |
| |
|
| | | $ | 982 | | $ | 1,501 |
| | |
| |
|
Stonington Capital Appreciation 1994 Fund, L.P.
In December 2002, the Company entered into a management services agreement ("Services Agreement") with Stonington Partners, Inc. ("Stonington"), an affiliate of Stonington Fund, our majority stockholder. The agreement calls for Stonington to provide management with consulting and business advisory services. As compensation for these services, the Company pays Stonington an annual fee equal to 1.5% of its capital invested in the Company plus expenses for services provided in the 2003 and 2002 fiscal years, and is renewable annually thereafter. This agreement terminated upon the completion of the initial public offering. In addition, the Company, at times, reimburses board members affiliated with Stonington, for expenses related to travel to and from board meetings.
For the years ended December 31, 2006 and 2005, management fees totaled approximately $400 and $400, respectively, and are included as a component of selling, general and administrative expenses. In addition, the Company paid an affiliate of Stonington two installments of $500, in December 2004 and January 2005, for consulting services associated with obtaining the $80,000 Facility. These payments were capitalized as debt issuance costs and are included in Other assets in the accompanying Consolidated Balance Sheets.
Amounts payable to Dr. Obagi for any bonus, annual payment or royalties and interest, and to other stockholders and their affiliates for management fees and other services as of December 31, 2007 and 2006 are reflected in the accompanying Consolidated Balance Sheets as amounts due to related parties as follows:
| | December 31,
|
|---|
| | 2007
| | 2006
|
|---|
| Dr. Obagi | | $ | 118 | | $ | 137 |
| Stonington | | | 17 | | | 15 |
| | |
| |
|
| | | $ | 135 | | $ | 152 |
| | |
| |
|
F-34
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 9: Related-party transactions (Continued)
During February 2005, the Company repurchased all of its outstanding Series A mandatorily redeemable preferred stock for approximately $11,822 and paid the related accrued dividends of approximately $9,329. Dr. Obagi and Stonington received approximately $3,288 and $17,863 for 18,375 and 99,840 shares of Series A preferred stock and the related accrued dividends, respectively.
During February 2005, the Company declared and paid a $3.60 per share common stock dividend, totaling approximately $63,088, in cash. A substantial portion of the common stock dividend was paid to Stonington, Dr. Obagi, and Mr. McNamara for approximately $38,924, $15,142, and $6,750, respectively.
In February 2005, one officer of the Company issued a note for $15 and LVG issued notes for $1,225 and $1,400 to the Company to exercise their vested options. These full-recourse notes were repaid in full later in February 2005.
Note 10: Commitments and contingencies
Lease commitments
The Company leases some of its facilities and equipment under operating leases. Some of the leases require payment of property taxes and include rent escalation clauses. Future minimum operating lease commitments under non-cancelable leases are as follows as of December 31, 2007:
| | Operating Leases
|
|---|
| Years ending December 31, | | | |
| 2008 | | $ | 1,081 |
| 2009 | | | 433 |
| 2010 | | | 116 |
| | |
|
| Total minimum payments required | | $ | 1,630 |
| | |
|
Rent expense for the years ended December 31, 2007, 2006, and 2005 was approximately $906, $639, and $494, respectively.
Indemnifications
The Company is a party to a variety of agreements entered into in the ordinary course of business pursuant to which it may be obligated to indemnify the other parties for certain liabilities that arise out of or relate to the subject matter of the agreements. Some of the agreements entered into by the Company require it to indemnify the other party against losses due to property damage including environmental contamination, personal injury, failure to comply with applicable laws, the Company's negligence or willful misconduct, or breach of representations and warranties and covenants.
The Company provides for indemnification of directors, officers and other persons in accordance with limited liability agreements, certificates of incorporation, bylaws, articles of association or similar organizational documents, as the case may be. The Company maintains directors' and officers' insurance which should enable the Company to recover a portion of any future amounts paid.
F-35
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 10: Commitments and contingencies (Continued)
While the Company's future obligations under certain agreements may contain limitations on liability for indemnification, other agreements do not contain such limitations and under such agreements it is not possible to predict the maximum potential amount of future payments due to the conditional nature of the Company's obligations and the unique facts and circumstances involved in each particular agreement. Historically, no payments have been made under any of these indemnities.
On September 1, 2006, the Company entered into indemnification agreements with each of its directors and executive officers that provide them with rights to indemnification and expense advancement to the fullest extent permitted under the Delaware General Corporation Law.
Employment agreements
The Company has entered into employment agreements with certain of its management employees which require annual gross salary payments which range from $400 to $500 per annum. The employment agreements range from a period of three to five years and include a provision for annual bonuses based on specific performance criteria. In the event that such key management employees are terminated without cause, the Company is contractually obligated to pay from 18 months up to the remaining balance due on the employment contracts. As of December 31, 2007, the Company's future minimum annual payments under such agreements is $67 for the year ended December 31, 2008.
Severance agreements
On September 1, 2006, the Company entered into severance agreements with four of our executive officers. The severance agreements provide that if one of these officers employment with us is terminated within 12 months following a change of control without cause or by the officer with good reason, the officer will be entitled, upon execution of a release in a form acceptable to us, an amount equal to 100% of the officer's then current annual base salary, payable over 12 consecutive months.
Litigation
On March 8, 2006, Austin McNamara, the former chairman of the board, president and chief executive officer of the Company, filed a charge of discrimination against us with the California Department of Fair Employment and Housing ("DFEH"). Mr. McNamara died in December 2006. Mr. McNamara alleged that the Company demoted, harassed and otherwise discriminated against him due to his purported physical disability and medical condition. Mr. McNamara requested an immediate right-to-sue notice. On March 20, 2006, the DFEH closed its case. The DFEH did not conduct an investigation or make a determination on the merits of the complaint. Mr. McNamara's estate had the right to file a discrimination lawsuit in a state court action within one year of the DFEH's letter closing its case. While Mr. McNamara did not allege a specific amount of damages, the remedies available under California law include compensatory damages, as well as attorneys' fees. Mr. McNamara also threatened to file a wage claim with the California Labor Commissioner, though the Company is not aware of either Mr. McNamara or his estate actually filing the claim. Mr. McNamara alleged that the Company did not pay all wages, bonuses, and severance owed to him. Mr. McNamara served as the Company's chief executive officer from September 2001 until July 2005, and as the Company's president from September 2001 until March 2005. He also served on the Company's board of directors and as chairman from September 2001 until May 2006.
F-36
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 10: Commitments and contingencies (Continued)
On May 29, 2007, the Company entered into the Settlement Agreement with the McNamara Parties. Pursuant to the Settlement Agreement, the Company and the McNamara Parties have released each other from all claims they may have against each other, except for claims related to potential taxes and related charges, should they arise, with respect to the past exercise of certain stock options by the Trusts. No payments were made by the parties to each other in connection with the settlement of the claims.
From time to time, the Company is involved in litigation and other legal matters in the normal course of business. Management does not believe that the outcome of any current matters will have a material adverse effect on the Company's consolidated financial position, results of operations or cash flows.
Note 11: Stock options
The 1997 Stock Option/Stock Issuance Plan (the "Plan") provided for the grant of options to purchase common stock to employees, consultants, and directors. The Plan included incentive stock options ("ISOs") and nonqualified stock options ("NSOs"). The maximum number of shares of common stock that were allowed to be issued over the term of the Plan was not to exceed 750,000 shares of the Company's common stock. In March 2001, the Board of Directors amended the Plan to discontinue further option grants under the Plan. At December 31, 2005, due to the aforementioned discontinuance of further option grants under the Plan, 501,225 shares are no longer available for the granting of additional options.
Under the option grant program, the exercise price per share for ISOs shall be no less than 100% of the fair market value per share as determined by the Board of Directors on the grant date. The exercise price per share for NSOs shall be no less than 85% of the fair market value per share as determined by the Board of Directors on the grant date. For ISOs and NSOs, the exercise price per share shall be no less than 110% of the fair market value per share as determined by the Board of Directors on the grant date for an individual who, at the time of grant, owns stock representing more than 10% of the total combined voting power of all classes of stock of the Company. The right to exercise ISOs and NSOs vests at a rate in accordance with the individual stock option agreements. The vesting period of options granted under this plan typically ranges from one to four years. Options expire within a period of not more than ten years from the grant date. ISOs granted to an employee, who at the time of grant owns stock representing more than 10% of the total combined voting power of all classes of stock of the Company, expire within a period of not more than five years from the grant date.
In November 2000, the Company adopted the 2000 Stock Option/Stock Issuance Plan (the "2000 Plan"). The terms of the 2000 Plan are consistent with those of the 1997 Stock Option/Stock Issuance Plan, except that the maximum number of shares of common stock that may be issued over the term of the 2000 Plan shall not exceed 375,000 shares of the Company's common stock. In addition, the maximum number of shares of common stock issued to any person under the 2000 Plan in any calendar year shall not exceed 150,000 shares. In September 2001, the Board of Directors amended the 2000 Plan to increase the maximum number of shares of common stock that may be issued over the term of the 2000 Plan to 2,083,334 shares. The vesting period of options granted under this plan typically ranges from one to five years. Options expire within a period of not more than ten years from the
F-37
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 11: Stock options (Continued)
grant date. In November 2005, the Board of Directors amended the Plan to discontinue further option grants under the Plan. At December 31, 2006 and 2007, due to the aforementioned discontinuance of further option grants under the Plan, 151,795 shares are no longer available for the granting of additional options.
In November 2005, the Company adopted the 2005 Stock Incentive Plan (the "2005 Plan"). The 2005 Plan provides for the grant of ISOs and NSOs to employees, consultants and directors. On November 27, 2006, the Company's Board of Directors amended the Company's 2005 Plan. The amendments to the 2005 Plan included: (i) increasing the number of authorized shares to 1,500,000; (ii) establishing an evergreen clause that replenishes the 2005 Plan each year with a share amount equal to the lesser of 500,000 or 3% of outstanding shares; (iii) and other administrative provisions. The amended 2005 Plan was approved by the stockholders of the Company on November 28, 2006. On January 9, 2007, the Board of Directors replenished the 2005 Plan with 500,000 shares available for granting of additional options.
Pursuant to the 2005 Plan, the exercise price per share for both ISOs and NSOs shall not be less than 100% of the fair market value per share of the Company's common stock on the grant date. The exercise price per share for both ISOs and NSOs may not be less than 110% of the fair market value of the Company's common stock on the grant date for an individual who, at the time of grant, owns stock representing more than 10% of the total combined voting power of all classes of the Company's stock. The right to exercise the ISOs and the NSOs vests at a rate in accordance with the individual stock option agreements. The vesting period of the options granted under this plan typically ranges from one to three years. Options expire within a period of not more than ten years from the grant date. ISOs granted to an employee, who at the time of grant owns stock representing more than 10% of the total combined voting power of all classes of stock of the Company, expire within a period of not more than five years from the grant date.
A total of 3,583,334 shares have been authorized for issuance under the 2000 Plan and the 2005 Plan (the "Plans"). Of the shares that have been authorized for issuance, 1,414,266 and 1,382,532 shares have been issued for options which have been exercised as of December 31, 2007 and 2006, respectively, and 1,215,633 and 1,285,116 shares have been reserved for options that are outstanding as of December 31, 2007 and 2006, respectively. At December 31, 2007 and 2006, there were 1,213,095 and 747,272 shares available for granting of additional options, respectively.
During the year ended December 31, 2005, the Company's board of directors granted 514,593 options with exercise prices ranging from $8.40 to $14.40, which was equal to or greater than the fair value of the underlying common stock on the date of each grant.
During the year ended December 31, 2006, the Company's board of directors granted stock options to the Company's management and employees to purchase a total of 750,000 shares of the Company's common stock upon the consummation of an initial public offering. The stock options were granted at the initial public offering price of $11.00, which was the fair market value on the date of grant.
During the year ended December 31, 2007, the Company's board of directors granted 123,000 options with exercise prices ranging from $11.85 to $20.34, which was equal to or greater than the fair value of the underlying common stock on the date of each grant.
F-38
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 11: Stock options (Continued)
A summary of the option activity under the Plans is as follows:
| | Shares
| | Weighted Average Exercise Price Per Share
|
|---|
| Outstanding at December 31, 2004 | | 1,382,253 | | $ | 2.10 |
| Granted | | 514,593 | | | 9.72 |
| Exercised | | (1,336,137 | ) | | 2.05 |
| Canceled | | (2,325 | ) | | 6.07 |
| | |
| |
|
| Outstanding at December 31, 2005 | | 558,384 | | $ | 9.22 |
| Granted | | 750,000 | | | 11.00 |
| Exercised | | (9,378 | ) | | 3.40 |
| Canceled | | (13,890 | ) | | 9.12 |
| | |
| |
|
| Outstanding at December 31, 2006 | | 1,285,116 | | $ | 10.31 |
| Granted | | 123,000 | | | 18.22 |
| Exercised | | (31,734 | ) | | 3.21 |
| Canceled | | (160,749 | ) | | 10.91 |
| | |
| |
|
| Outstanding at December 31, 2007 | | 1,215,633 | | $ | 11.21 |
| | |
| |
|
| Exercisable at December 31, 2005 | | 44,953 | | $ | 7.41 |
| | |
| |
|
| Exercisable at December 31, 2006 | | 214,277 | | $ | 9.05 |
| | |
| |
|
| Exercisable at December 31, 2007 | | 559,050 | | $ | 10.13 |
| | |
| |
|
A summary of options outstanding and exercisable as of December 31, 2007 are as follows:
| | Options Outstanding
| | Options Exercisable
|
|---|
Range of Exercise Price Per Share
| | Number of Shares Outstanding at December 31, 2007
| | Weighted Average Per Share Exercise Price
| | Weighted Average Remaining Life (Years)
| | Number of Shares Exercisable at December 31, 2007
| | Weighted Average Exercise Price
|
|---|
| $1.20 to $1.29 | | 1,650 | | $ | 1.29 | | 2.62 | | 1,650 | | $ | 1.29 |
| $8.40 to $10.00 | | 282,918 | | $ | 8.42 | | 7.11 | | 213,473 | | $ | 8.43 |
| $10.80 to $14.40 | | 830,065 | | $ | 11.20 | | 8.74 | | 343,927 | | $ | 11.22 |
| $16.40 to $20.40 | | 101,000 | | $ | 19.32 | | 9.79 | | — | | $ | — |
| | |
| | | | | | |
| | | |
| | | 1,215,633 | | $ | 11.21 | | 8.44 | | 559,050 | | $ | 10.13 |
| | |
| | | | | | |
| | | |
On December 13, 2006, the Company issued 2,728 shares of restricted common stock under the 2005 Plan to one of its outside directors. During the year ended December 31, 2007, the Company issued a total of 16,926 shares of restricted common stock under the 2005 Plan to seven outside directors. The resulting compensation expense from the restricted stock grants is recognized on a straight-line basis over the requisite service period, which equals the restricted stock vesting term of one year. The Company determined the fair market value of the restricted stock granted on December 13, 2006, based upon the initial public offering price of our stock, which was $11.00. The fair market value of the restricted stock granted during the year ended December 31, 2007 ranged from
F-39
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 11: Stock options (Continued)
$13.11 to $17.36, which was based upon the fair value of the Company's common stock on the date of each grant.
Note 12: Segments
SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information, requires that the Company disclose certain information about its operating segments where operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and in assessing performance. Generally, financial information is required to be reported on the basis that is used internally for evaluating segment performance and deciding how to allocate resources to segments.
The Company operates its business on the basis of two reportable segments (i) skin health and (ii) licensing. The skin health segment produces a broad range of topical skin health systems and products that enable physicians to treat a range of skin conditions, including pre-mature aging, photo-damage, hyperpigmentation, acne and soft tissue deficits, such as fine lines and wrinkles. The licensing segment includes revenues generated from licensing arrangements with international distributors that specialize in the distribution and marketing of over-the-counter medical oriented products in the drug store, retail and aesthetic spa channels.
Management evaluates its segments on a revenue and gross profit basis, which is presented below. The United States information is presented separately as the Company's headquarters reside in the United States, and United States sales represented 84.8%, 82.0%, and 81.4% of total consolidated net sales for the years ended December 31, 2007, 2006 and 2005, respectively. No other country or single customer generates over 10% of total Company consolidated net sales.
The Company occasionally utilizes promotions to enhance sales. The Company previously classified sales under these promotions in the Other product line. The Company currently classifies these promotional activities under the product line being promoted by the promotion. Prior periods have been reclassified to conform to the current presentation.
F-40
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 12: Segments (Continued)
All of the Company's long-lived assets are located in the United States. The Company does not disaggregate assets on a segment basis for internal management reporting and, therefore, such information is not presented.
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Net sales by segment | | | | | | | | | |
| | Skin health | | $ | 97,992 | | $ | 73,940 | | $ | 61,539 |
| | Licensing | | | 4,656 | | | 4,056 | | | 3,402 |
| | |
| |
| |
|
| | Net sales | | $ | 102,648 | | $ | 77,996 | | $ | 64,941 |
| | |
| |
| |
|
| Gross profit by segment | | | | | | | | | |
| | Skin health | | $ | 80,089 | | $ | 60,650 | | $ | 50,117 |
| | Licensing | | | 4,510 | | | 3,879 | | | 3,252 |
| | |
| |
| |
|
| | Gross profit | | $ | 84,599 | | $ | 64,529 | | $ | 53,369 |
| | |
| |
| |
|
| Geographic information | | | | | | | | | |
| | United States | | $ | 87,040 | | $ | 63,929 | | $ | 52,883 |
| | International | | | 15,608 | | | 14,067 | | | 12,058 |
| | |
| |
| |
|
| | Net sales | | $ | 102,648 | | $ | 77,996 | | $ | 64,941 |
| | |
| |
| |
|
| | Year Ended December 31,
|
|---|
| | 2007
| | 2006
| | 2005
|
|---|
| Net sales by product line | | | | | | | | | |
| | Skin health | | | | | | | | | |
| | | Nu-Derm | | $ | 62,184 | | $ | 53,836 | | $ | 47,115 |
| | | Vitamin C | | | 11,438 | | | 9,986 | | | 8,570 |
| | | Skin laxity | | | 8,356 | | | 2,278 | | | — |
| | | Therapeutic | | | 5,753 | | | 180 | | | — |
| | | Other | | | 10,261 | | | 7,660 | | | 5,854 |
| | |
| |
| |
|
| | | | Total | | | 97,992 | | | 73,940 | | | 61,539 |
| | Licensing | | | 4,656 | | | 4,056 | | | 3,402 |
| | |
| |
| |
|
| Total net sales | | $ | 102,648 | | $ | 77,996 | | $ | 64,941 |
| | |
| |
| |
|
Note 13: 401(k) plan
On February 1, 1999, the Company established an employee savings and retirement plan and a 401(k) defined contribution retirement plan, covering substantially all full-time employees. The Company matches 25% of employee contributions up to 6% of employee compensation. The Company may also make, at the discretion of the board of directors, additional contributions subject to statutory limits. Beginning April 2006, the Company matches 100% of employee contributions up to 2% of employee compensation. The Company contributed approximately $213, $125, and $42 for the years
F-41
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 13: 401(k) plan (Continued)
ended December 31, 2007, 2006, and 2005, respectively. Administrative expenses paid on behalf of the plan were nominal for each of the respective years.
Note 14: Quarterly Financial Data (Unaudited)
Quarterly financial data for the years ended December 31, 2007 and 2006 are as follows:
| | Quarter Ended
|
|---|
| | March 31, 2007
| | June 30, 2007
| | September 30, 2007
| | December 31, 2007
|
|---|
| Net sales | | $ | 23,046 | | $ | 26,067 | | $ | 26,281 | | $ | 27,254 |
| Gross profit | | $ | 19,085 | | $ | 21,507 | | $ | 21,677 | | $ | 22,330 |
| Net income | | $ | 2,926 | | $ | 3,936 | | $ | 3,862 | | $ | 4,479 |
| Net income attributable to common shares | | | | | | | | | | | | |
| | Basic | | $ | 0.13 | | $ | 0.18 | | $ | 0.18 | | $ | 0.20 |
| | |
| |
| |
| |
|
| | Diluted | | $ | 0.13 | | $ | 0.18 | | $ | 0.18 | | $ | 0.20 |
| | |
| |
| |
| |
|
| | Quarter Ended
|
|---|
| | March 31, 2006
| | June 30, 2006
| | September 30, 2006
| | December 31, 2006
|
|---|
| Net sales | | $ | 17,204 | | $ | 18,722 | | $ | 19,105 | | $ | 22,965 |
| Gross profit | | $ | 14,432 | | $ | 15,705 | | $ | 15,762 | | $ | 18,630 |
| Net income | | $ | 1,496 | | $ | 1,078 | | $ | 1,006 | | $ | 2,536 |
| Net income attributable to common shares | | | | | | | | | | | | |
| | Basic | | $ | 0.08 | | $ | 0.06 | | $ | 0.06 | | $ | 0.14 |
| | |
| |
| |
| |
|
| | Diluted | | $ | 0.08 | | $ | 0.06 | | $ | 0.06 | | $ | 0.14 |
| | |
| |
| |
| |
|
Note 15: Subsequent events
On January 9, 2008, the Company entered into an Assignment Agreement with ZSO, LP, an affiliate of Dr. Obagi, whereby the Company assigned its rights under a construction contract associated with the Company's leased space for its marketing and training center.
On January 9, 2008, the Company entered into an Indemnification Agreement with Dr. Obagi, and his affiliates, Zein E. Obagi, M.D., Inc., Samar Obagi, the Zein and Samar Obagi Family Trust, SHP, Inc., and ZSO, LP, placing in escrow approximately $340 in exchange for an indemnification for any liability arising from the underlying construction contract.
On February 26, 2008, the Company provided a new irrevocable standby letter of credit to the California State Board of Pharmacy in the amount of $100, to serve as a security device for the performance of the Company of its obligations under the Business and Professions Code. This letter of credit automatically renews annually unless written notice is provided by the Company at least 30 days before each expiry date to the California State Board of Pharmacy.
F-42
Obagi Medical Products, Inc.
Notes to Consolidated Financial Statements (Continued)
(Dollars in thousands, except share and per share amounts)
Note 15: Subsequent events (Continued)
Pursuant to the 2005 Plan, on February 27, 2008, the Board of Directors replenished the 2005 Plan with 500,000 shares, for a total of 1,713,095 shares available for granting of additional options.
On February 27, 2008, the Company entered into an indemnification agreement with one of its directors that provide him with rights to indemnification and expense advancement to the fullest extent permitted under the Delaware General Corporation Law.
On February 27, 2008, the Board of Directors amended and restated Article V, Section 5.9, and Article VIII, Sections 8.3, 8.4 and 8.5 of the Amended and Restated Bylaws of the Company to permit the issuance of shares of the Company's capital stock in uncertificated form as required by Nasdaq Rule 4350(l). The amendments to the Amended and Restated Bylaws will permit direct or "book-entry" registration of shares of the Company's capital stock and thereby facilitate the Company's eligibility to participate in a direct registration system.
On February 27, 2008, the Company granted to its officers 45,000 shares of restricted stock units and options for the purchase of 28,000 shares of its common stock. The Company also granted options for the purchase of 134,500 shares of its common stock to employees other than officers of the Company.
F-43
QuickLinks
OBAGI MEDICAL PRODUCTS, INC. ANNUAL REPORT ON FORM 10-K TABLE OF CONTENTSForward-looking statementsPART IPART IICOMPARISON OF CUMULATIVE TOTAL RETURN AMOUNG OBAGI MEDICAL PRODUCTS, INC., NASDAQ MARKET INDEX AND SIC CODE INDEXPART IIIPART IVSIGNATURESReport of Independent Registered Public Accounting FirmObagi Medical Products, Inc. Consolidated Balance Sheets (Dollars in thousands, except share and per share amounts)Obagi Medical Products, Inc. Consolidated Statements of Income (Dollars in thousands, except share and per share amounts)Obagi Medical Products, Inc. Consolidated Statements of Stockholders' Equity (Deficit) and Comprehensive Income (Dollars in thousands, except share and per share amounts)Obagi Medical Products, Inc. Consolidated Statements of Cash Flows (Dollars in thousands, except share and per share amounts)Obagi Medical Products, Inc. Notes to Consolidated Financial Statements (Dollars in thousands, except share and per share amounts)
