
Acquisition of Audentes Establishing a leading position in gene therapy Naoki Okamura Representative Director, Corporate Executive Vice President, Chief Strategy Officer and Chief Financial Officer Astellas Pharma Inc. December 3, 2019 Exhibit 99.3

This document contains “forward-looking statements” relating to the acquisition of Audentes by Astellas. Such forward-looking statements include, but are not limited to, the ability of Audentes and Astellas to complete the transactions contemplated by the merger agreement, including the parties’ ability to satisfy the conditions to the consummation of the offer contemplated thereby and the other conditions set forth in the merger agreement, statements about the expected timetable for completing the transaction, Astellas’ and Audentes’ beliefs and expectations and statements about the benefits sought to be achieved in Astellas’ proposed acquisition of Audentes, the potential effects of the acquisition on both Astellas and Audentes, the possibility of any termination of the merger agreement, as well as the expected benefits and success of Audentes’ product candidates, the timing and nature of regulatory filings for Audentes’ product candidates, the timing of Audentes’ presentation of non-clinical data and the timing and nature of Audentes’ preclinical studies, clinical trials and manufacturing activities. In some cases, forward-looking statements may be identified by terminology such as “believe,” “may,” “will,” “should”, “predict”, “goal”, “strategy”, “potentially,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect,” “seek” and similar expressions and variations thereof. These words are intended to identify forward-looking statements. Astellas and Audentes have based these forward-looking statements on current expectations and projections about future events and trends that they believe may affect the financial condition, results of operations, business strategy, short-term and long-term business operations and objectives and financial needs of Astellas and Audentes, but there can be no guarantee that such expectations and projections will prove accurate in the future. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Actual results may differ materially from current expectations because of risks associated with uncertainties as to the timing of the offer and the subsequent merger; uncertainties as to how many of Audentes’ stockholders will tender their shares in the offer; the risk that competing offers or acquisition proposals will be made; the possibility that various conditions to the consummation of the merger and the offer contemplated thereby may not be satisfied or waived; the effects of disruption from the transactions contemplated by the merger agreement on Audentes’ business and the fact that the announcement and pendency of the transactions may make it more difficult to establish or maintain relationships with employees, suppliers and other business partners; and the risk that stockholder litigation in connection with the offer or the merger may result in significant costs of defense, indemnification and liability. Moreover, Astellas and Audentes operate in very competitive and rapidly changing environments, and new risks emerge from time to time. Although Astellas and Audentes believe that the expectations reflected in such forward-looking statements are reasonable, they cannot guarantee future events, results, actions, levels of activity, performance or achievements, business and market conditions, the timing and results of biotechnology development and potential regulatory approval and whether the conditions to the closing of the proposed transaction are satisfied on the expected timetable or at all. Forward-looking statements are also subject to risks and uncertainties pertaining to the business of Audentes, including those set forth in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Audentes’ Annual Report on Form 10-K for the year ended December 31, 2018, and Quarterly Report on Form 10-Q for the quarter ended September 30, 2019, which are on file with the SEC and available on the SEC’s website at www.sec.gov. In addition to the risks described above and in Audentes’ other filings with the SEC, other unknown or unpredictable factors could also affect Audentes’ results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information contained in this document is provided only as of the date hereof, and no party undertakes any obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof, except as required by law. The tender offer for the outstanding shares of common stock of Audentes has not yet commenced. This communication is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Audentes common stock, nor is it a substitute for the tender offer materials that Astellas and its acquisition subsidiary will file with the SEC upon commencement of the tender offer. At the time the tender offer is commenced, Astellas will file a tender offer statement on Schedule TO with the SEC, and thereafter Audentes will file a solicitation/recommendation statement on Schedule 14D-9 with respect to the offer. THE TENDER OFFER STATEMENT (INCLUDING AN OFFER TO PURCHASE, A RELATED LETTER OF TRANSMITTAL AND OTHER OFFER DOCUMENTS) AND THE SOLICITATION / RECOMMENDATION STATEMENT WILL CONTAIN IMPORTANT INFORMATION THAT SHOULD BE READ CAREFULLY AND CONSIDERED BY AUDENTES’ STOCKHOLDERS BEFORE ANY DECISION IS MADE WITH RESPECT TO THE TENDER OFFER. Both the tender offer statement and the solicitation/recommendation statement will be mailed to Audentes’ stockholders free of charge. A free copy of the tender offer statement and the solicitation/recommendation statement will also be made available to all stockholders of Audentes by contacting Audentes at ir@audentestx.com or by phone at (415) 818-1033. In addition, the tender offer statement, the related letter of transmittal and certain other tender offer documents and the solicitation/recommendation statement (and all other documents filed with the SEC) will be available at no charge on the SEC’s website: www.sec.gov, upon filing with the SEC. In addition to these documents, Audentes files annual, quarterly and current reports and other information with the SEC. These filings with the SEC are also available to the public for free at the SEC’s website at www.sec.gov. In addition, the solicitation/recommendation statement and the other documents filed by the Audentes with the SEC are available to all stockholders of Audentes free of charge at http://investors.audentestx.com/sec-filings. AUDENTES’ STOCKHOLDERS ARE ADVISED TO READ THE SCHEDULE TO AND THE SCHEDULE 14D-9 CAREFULLY, AS EACH MAY BE AMENDED OR SUPPLEMENTED FROM TIME TO TIME, AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC WHEN THEY BECOME AVAILABLE BEFORE THEY MAKE ANY DECISION WITH RESPECT TO THE TENDER OFFER, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES THERETO, AS WELL AS IMPORTANT INFORMATION THAT HOLDERS OF SHARES OF AUDENTES’ COMMON STOCK SHOULD CONSIDER BEFORE MAKING ANY DECISION REGARDING TENDERING THEIR SHARES. Important Additional Information Cautionary Notice Regarding Forward-Looking Statements

Agenda I Transaction Summary II III Overview of Audentes Strategic Rationale
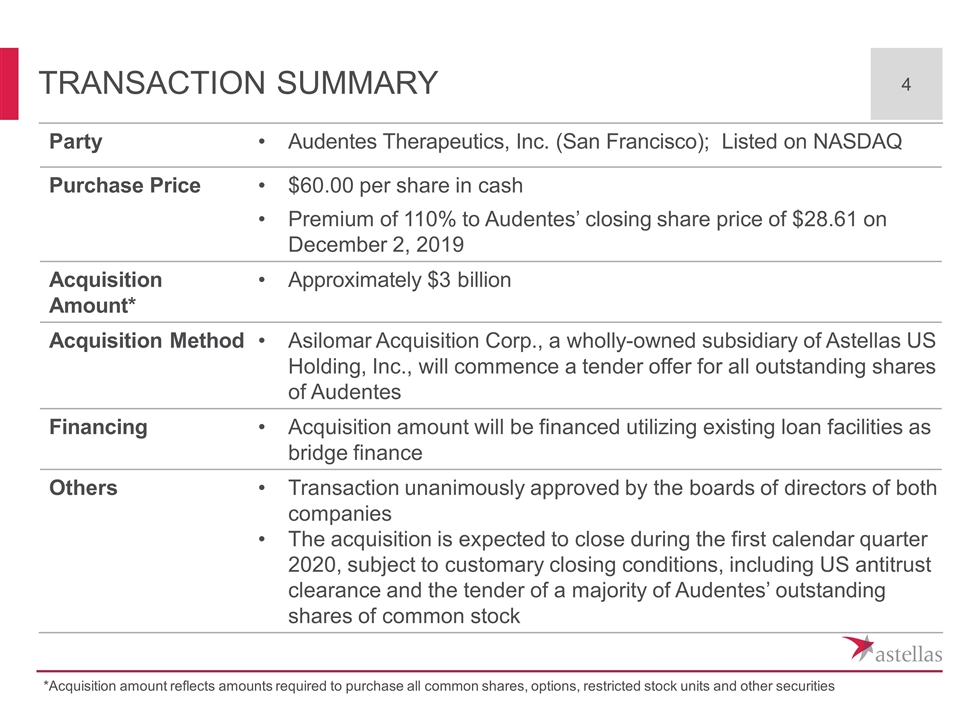
Transaction Summary Party Audentes Therapeutics, Inc. (San Francisco); Listed on NASDAQ Purchase Price $60.00 per share in cash Premium of 110% to Audentes’ closing share price of $28.61 on December 2, 2019 Acquisition Amount* Approximately $3 billion Acquisition Method Asilomar Acquisition Corp., a wholly-owned subsidiary of Astellas US Holding, Inc., will commence a tender offer for all outstanding shares of Audentes Financing Acquisition amount will be financed utilizing existing loan facilities as bridge finance Others Transaction unanimously approved by the boards of directors of both companies The acquisition is expected to close during the first calendar quarter 2020, subject to customary closing conditions, including US antitrust clearance and the tender of a majority of Audentes’ outstanding shares of common stock *Acquisition amount reflects amounts required to purchase all common shares, options, restricted stock units and other securities

Agenda I Transaction Summary II III Overview of Audentes Strategic Rationale

Company Overview Audentes Therapeutics, Inc. Headquartered in San Francisco, California Founded in 2012; IPO on NASDAQ in July 2016 Clinical-stage AAV-based gene therapy company focusing on rare neuromuscular diseases Experienced management Of total 270 employees, 220 engage in R&D and manufacturing AAV: Adeno-Associated Virus
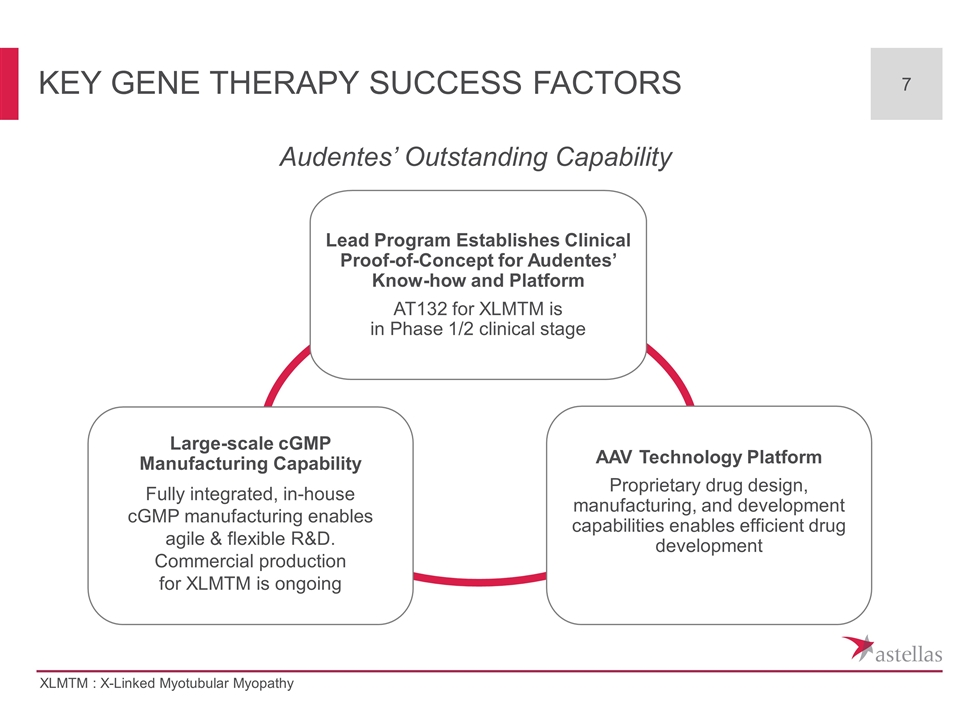
Key Gene Therapy Success Factors Lead Program Establishes Clinical Proof-of-Concept for Audentes’ Know-how and Platform AT132 for XLMTM is in Phase 1/2 clinical stage AAV Technology Platform Proprietary drug design, manufacturing, and development capabilities enables efficient drug development Audentes’ Outstanding Capability Large-scale cGMP Manufacturing Capability Fully integrated, in-house cGMP manufacturing enables agile & flexible R&D. Commercial production for XLMTM is ongoing XLMTM : X-Linked Myotubular Myopathy
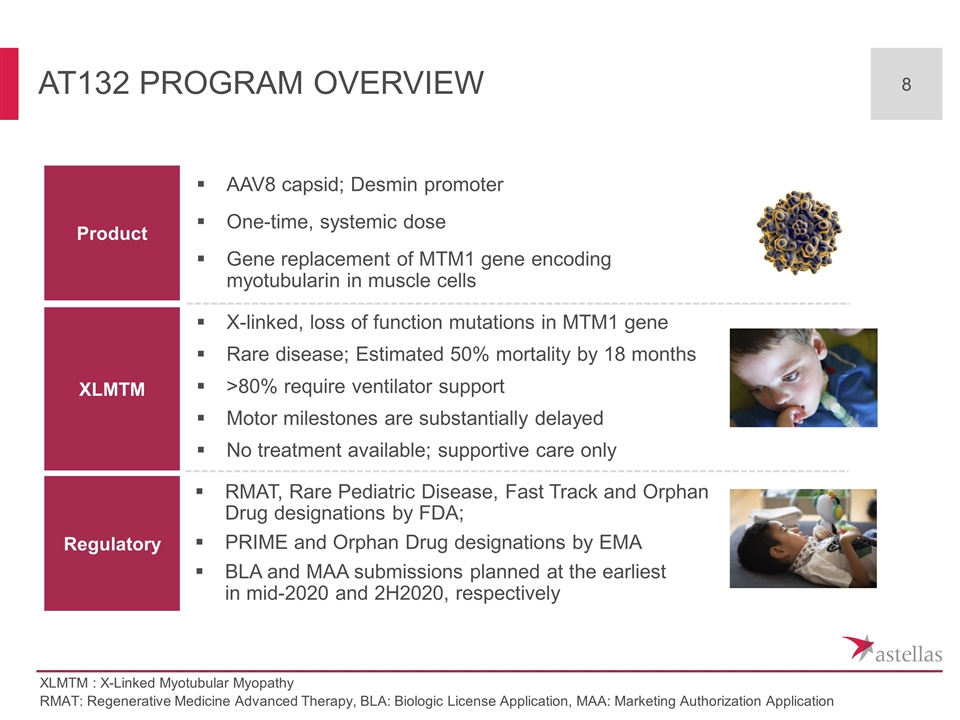
AT132 Program overview RMAT: Regenerative Medicine Advanced Therapy, BLA: Biologic License Application, MAA: Marketing Authorization Application XLMTM : X-Linked Myotubular Myopathy Product XLMTM Regulatory AAV8 capsid; Desmin promoter One-time, systemic dose Gene replacement of MTM1 gene encoding myotubularin in muscle cells X-linked, loss of function mutations in MTM1 gene Rare disease; Estimated 50% mortality by 18 months >80% require ventilator support Motor milestones are substantially delayed No treatment available; supportive care only RMAT, Rare Pediatric Disease, Fast Track and Orphan Drug designations by FDA; PRIME and Orphan Drug designations by EMA BLA and MAA submissions planned at the earliest in mid-2020 and 2H2020, respectively
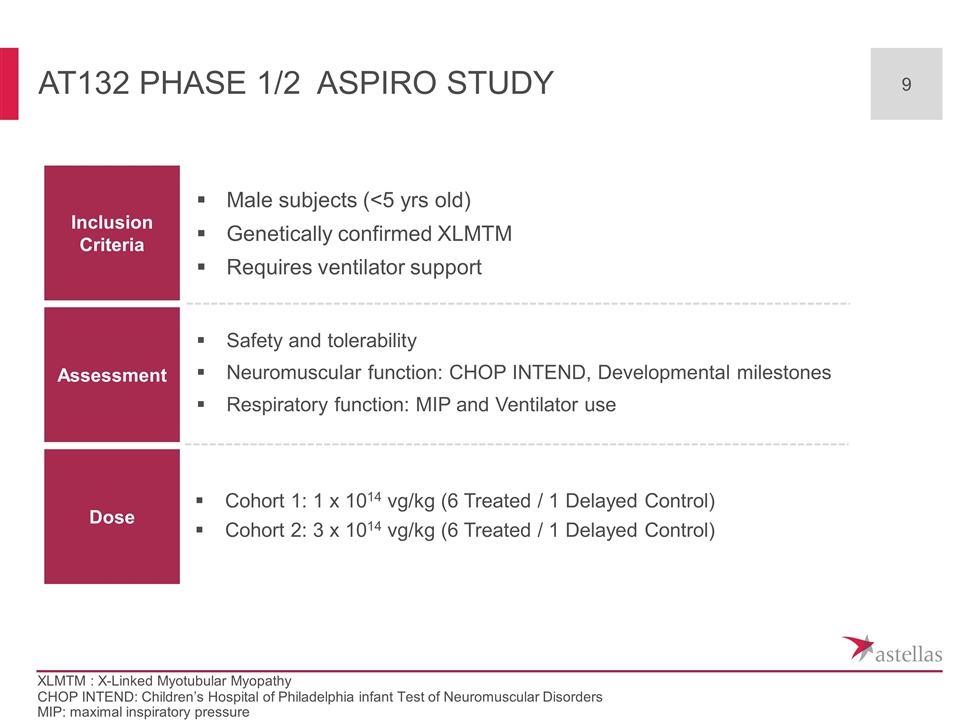
AT132 phase 1/2 ASPIRO Study Inclusion Criteria Assessment Dose Male subjects (<5 yrs old) Genetically confirmed XLMTM Requires ventilator support Safety and tolerability Neuromuscular function: CHOP INTEND, Developmental milestones Respiratory function: MIP and Ventilator use Cohort 1: 1 x 1014 vg/kg (6 Treated / 1 Delayed Control) Cohort 2: 3 x 1014 vg/kg (6 Treated / 1 Delayed Control) CHOP INTEND: Children’s Hospital of Philadelphia infant Test of Neuromuscular Disorders MIP: maximal inspiratory pressure XLMTM : X-Linked Myotubular Myopathy
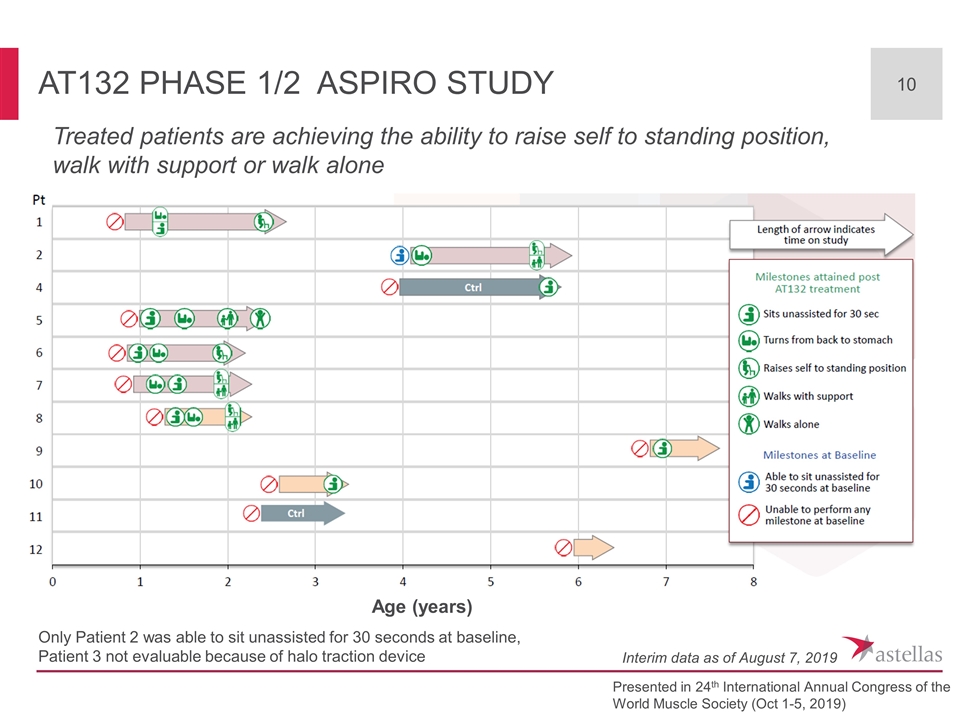
AT132 Phase 1/2 ASPIRO Study Presented in 24th International Annual Congress of the World Muscle Society (Oct 1-5, 2019) Age (years) Only Patient 2 was able to sit unassisted for 30 seconds at baseline, Patient 3 not evaluable because of halo traction device Treated patients are achieving the ability to raise self to standing position, walk with support or walk alone Interim data as of August 7, 2019
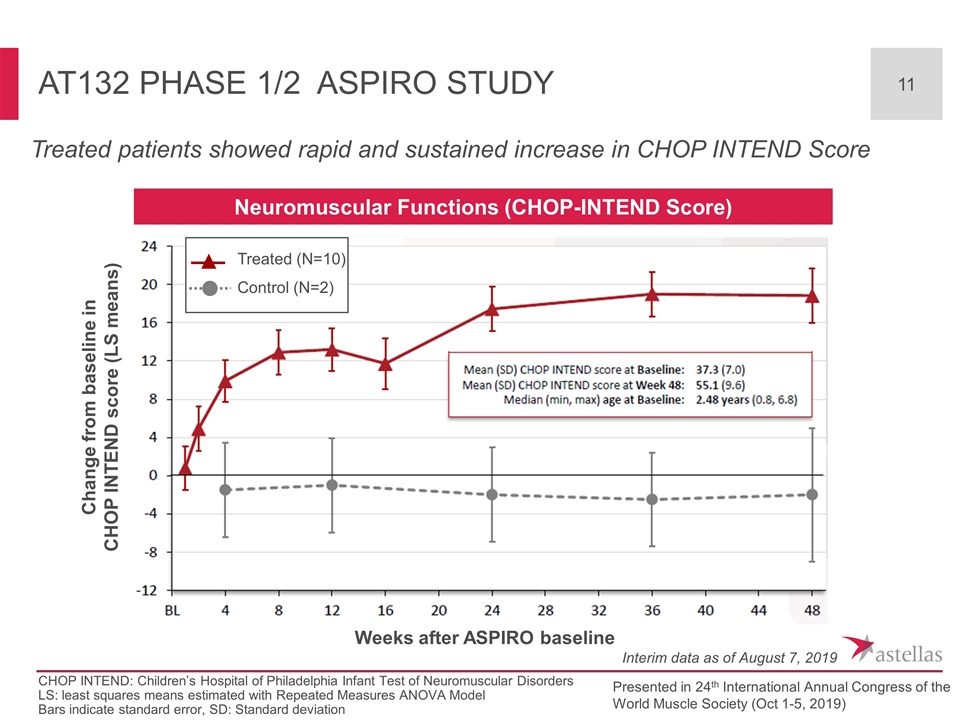
AT132 Phase 1/2 ASPIRO Study Treated patients showed rapid and sustained increase in CHOP INTEND Score CHOP INTEND: Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders LS: least squares means estimated with Repeated Measures ANOVA Model Bars indicate standard error, SD: Standard deviation Weeks after ASPIRO baseline Change from baseline in CHOP INTEND score (LS means) Interim data as of August 7, 2019 Presented in 24th International Annual Congress of the World Muscle Society (Oct 1-5, 2019) Neuromuscular Functions (CHOP-INTEND Score) Treated (N=10) Control (N=2)
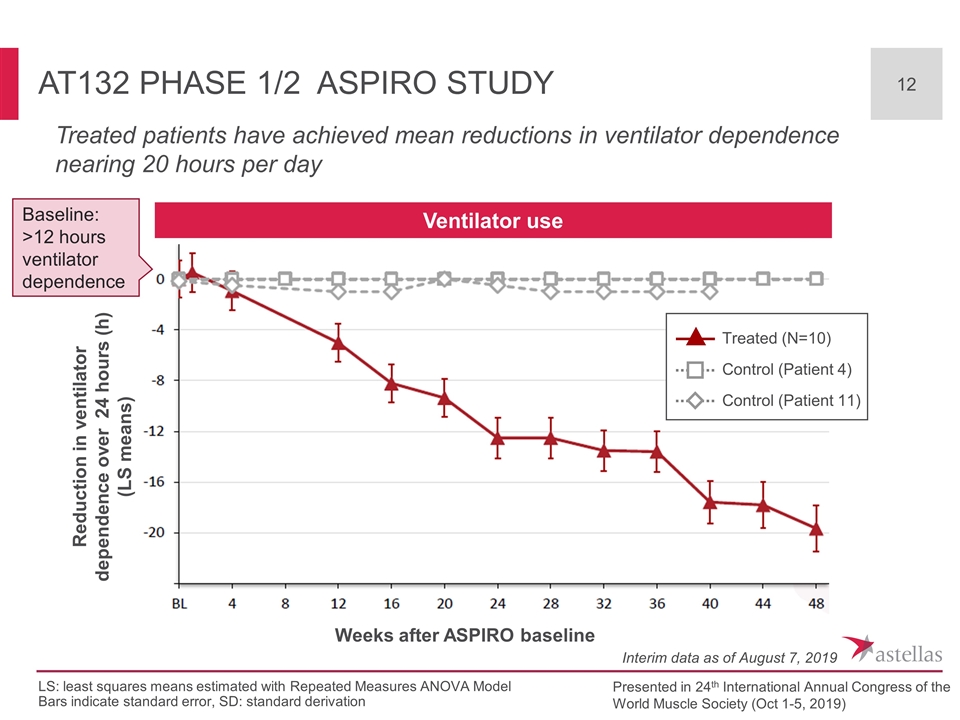
AT132 Phase 1/2 ASPIRO Study Treated (N=10) Control (Patient 4) Control (Patient 11) Ventilator use Treated patients have achieved mean reductions in ventilator dependence nearing 20 hours per day Weeks after ASPIRO baseline Presented in 24th International Annual Congress of the World Muscle Society (Oct 1-5, 2019) LS: least squares means estimated with Repeated Measures ANOVA Model Bars indicate standard error, SD: standard derivation Interim data as of August 7, 2019 Baseline: >12 hours ventilator dependence Reduction in ventilator dependence over 24 hours (h) (LS means)
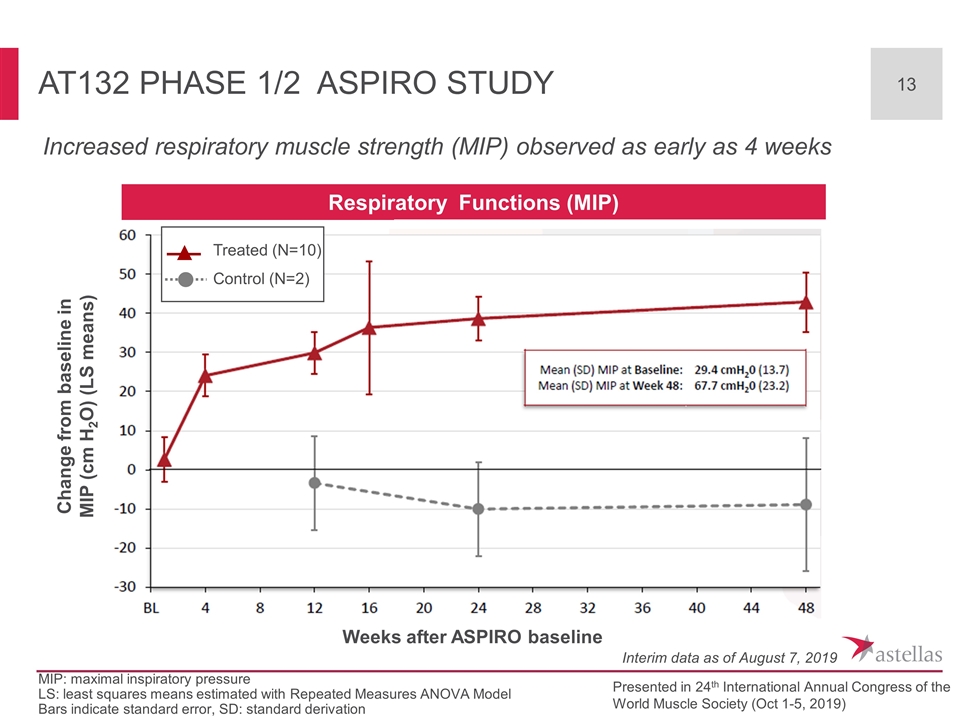
AT132 Phase 1/2 ASPIRO Study Increased respiratory muscle strength (MIP) observed as early as 4 weeks Respiratory Functions (MIP) Change from baseline in MIP (cm H2O) (LS means) Weeks after ASPIRO baseline Interim data as of August 7, 2019 Presented in 24th International Annual Congress of the World Muscle Society (Oct 1-5, 2019) MIP: maximal inspiratory pressure LS: least squares means estimated with Repeated Measures ANOVA Model Bars indicate standard error, SD: standard derivation Treated (N=10) Control (N=2)
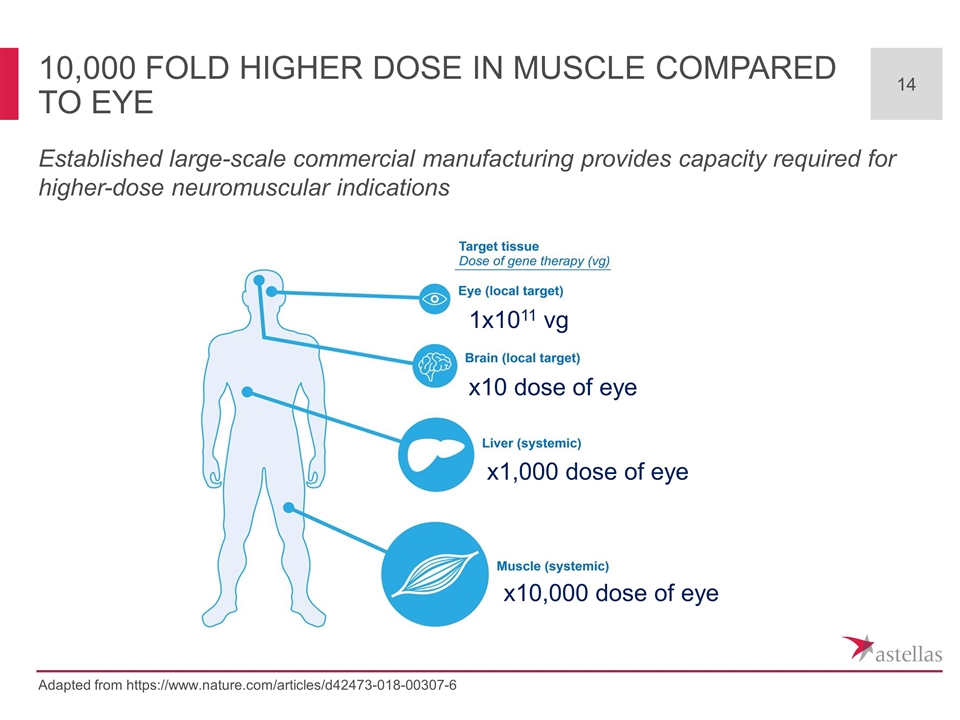
10,000 fold higher dose in Muscle compared to Eye Adapted from https://www.nature.com/articles/d42473-018-00307-6 x10,000 dose of eye x1,000 dose of eye x10 dose of eye 1x1011 vg Established large-scale commercial manufacturing provides capacity required for higher-dose neuromuscular indications
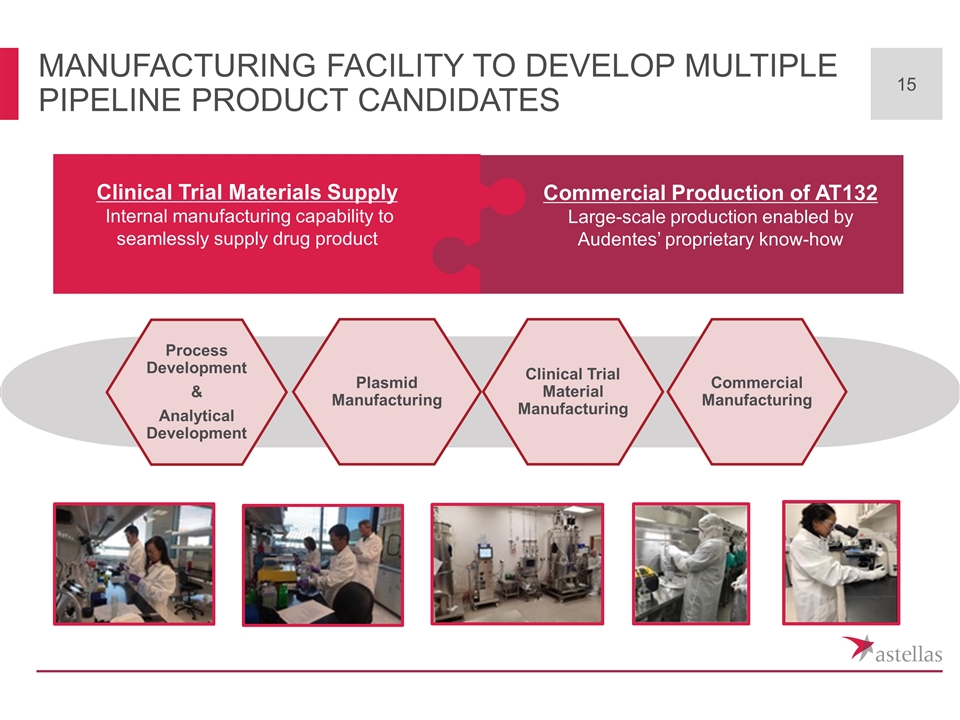
Manufacturing Facility to develop multiple Pipeline Product candidates Plasmid Manufacturing Clinical Trial Material Manufacturing Process Development & Analytical Development Commercial Manufacturing Clinical Trial Materials Supply Internal manufacturing capability to seamlessly supply drug product Commercial Production of AT132 Large-scale production enabled by Audentes’ proprietary know-how
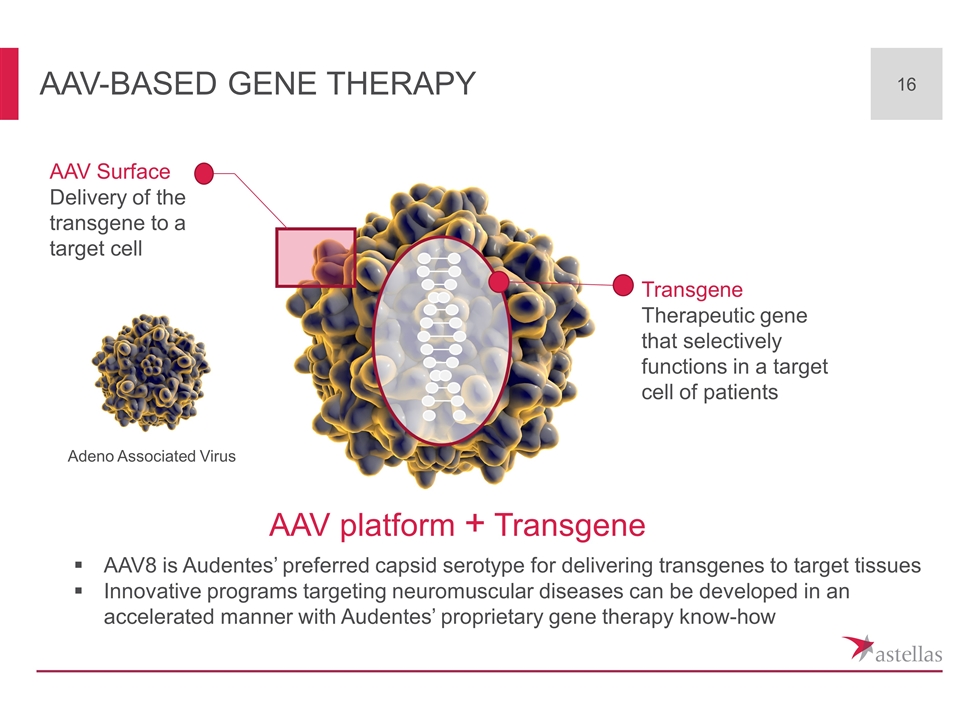
AAV-based gene therapy AAV Surface Delivery of the transgene to a target cell Transgene Therapeutic gene that selectively functions in a target cell of patients AAV8 is Audentes’ preferred capsid serotype for delivering transgenes to target tissues Innovative programs targeting neuromuscular diseases can be developed in an accelerated manner with Audentes’ proprietary gene therapy know-how AAV platform + Transgene Adeno Associated Virus
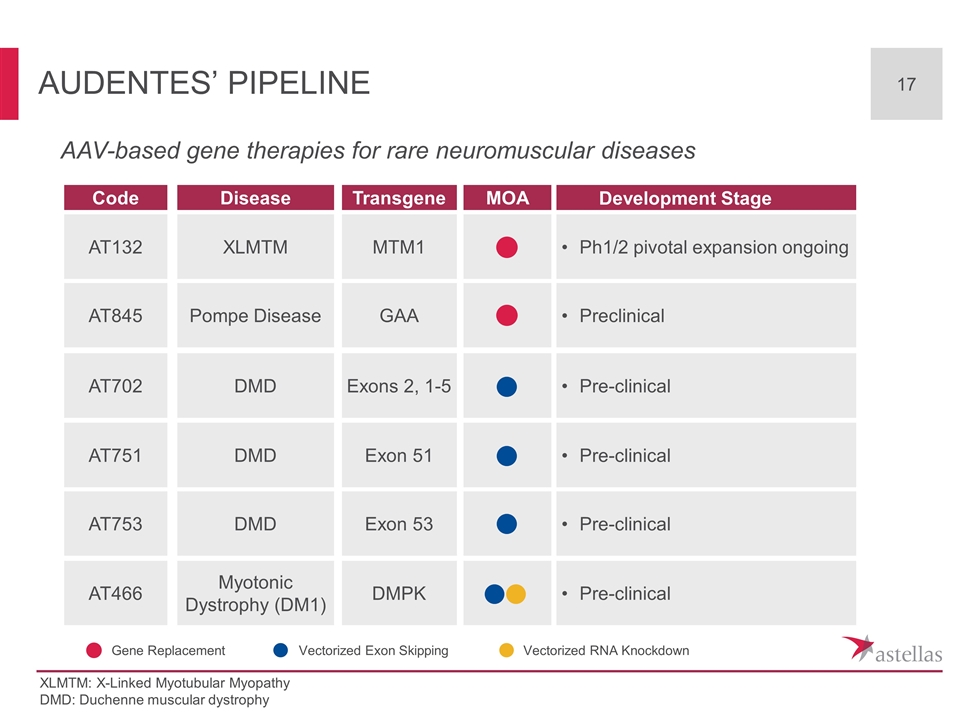
AAV-based gene therapies for rare neuromuscular diseases Audentes’ Pipeline MTM1 GAA Exons 2, 1-5 Exon 51 Exon 53 DMPK Transgene AT132 AT845 AT702 AT751 AT753 AT466 Code XLMTM Pompe Disease DMD DMD DMD Myotonic Dystrophy (DM1) Disease Ph1/2 pivotal expansion ongoing Preclinical Pre-clinical Pre-clinical Pre-clinical Pre-clinical Development Stage MOA Gene Replacement Vectorized Exon Skipping Vectorized RNA Knockdown XLMTM: X-Linked Myotubular Myopathy DMD: Duchenne muscular dystrophy

Agenda I Transaction Summary II III Overview of Audentes Strategic Rationale
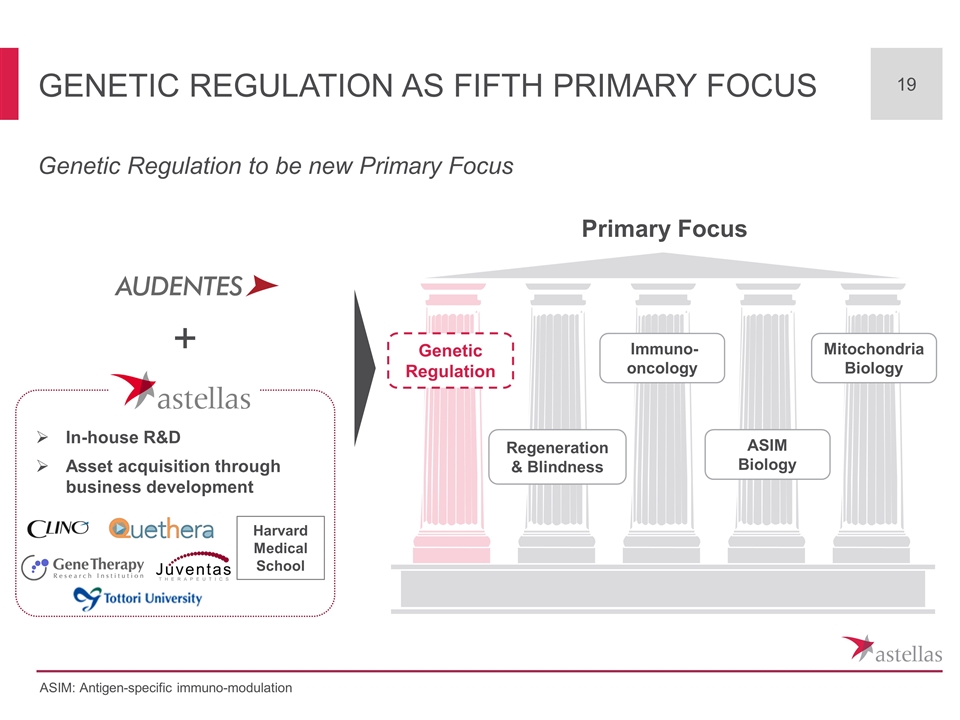
Genetic regulation as fifth Primary Focus Genetic Regulation to be new Primary Focus ASIM: Antigen-specific immuno-modulation In-house R&D Asset acquisition through business development + Mitochondria Biology Regeneration & Blindness Immuno-oncology ASIM Biology Genetic Regulation Primary Focus Harvard Medical School
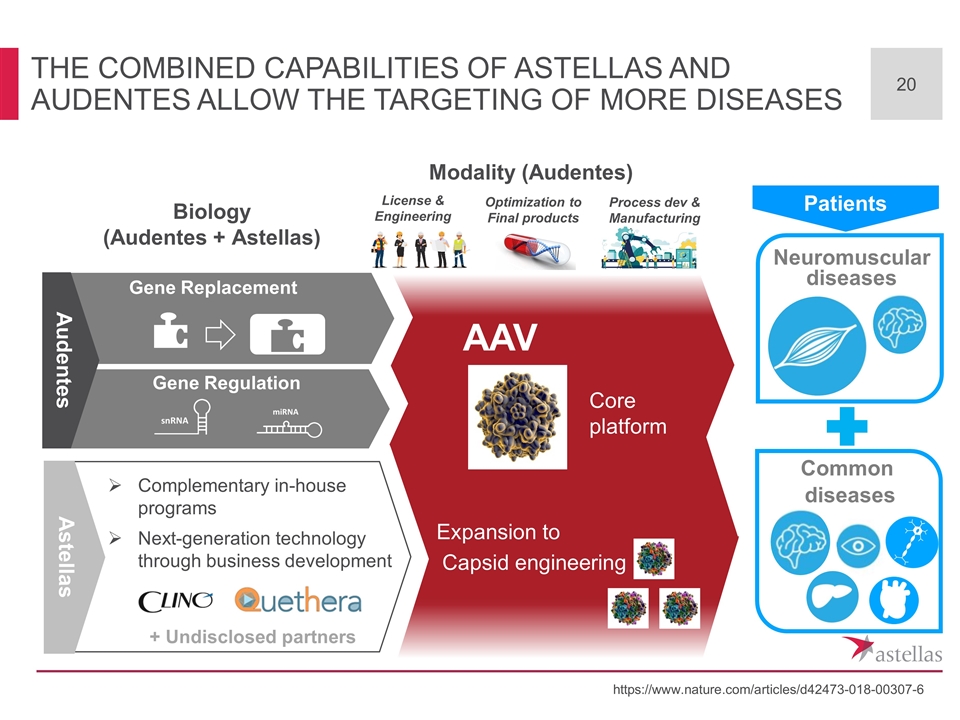
The Combined capabilities of Astellas and Audentes Allow the targeting of more diseases Gene Replacement https://www.nature.com/articles/d42473-018-00307-6 Neuromuscular diseases Biology (Audentes + Astellas) Common diseases Patients AAV Modality (Audentes) Core platform Complementary in-house programs Next-generation technology through business development Astellas Capsid engineering Expansion to + Undisclosed partners Gene Replacement Audentes Gene Regulation Optimization to Final products License & Engineering Process dev & Manufacturing

Acquisition of Audentes: summary Transforms Genetic Regulation Primary Focus into a new growth area for Astellas, building on a complementary technology platform and capabilities in gene therapy to swiftly bring products to patients Develop innovative gene therapies that address the unmet medical needs of patients Acquisition of Audentes is a major step to establishing a leading position in gene therapy +

On the Forefront of Healthcare Change





















