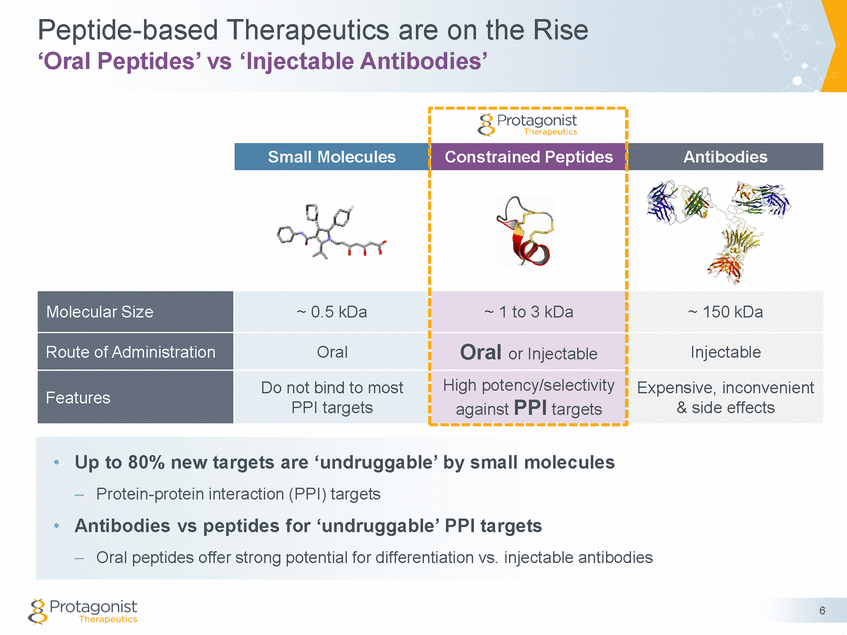
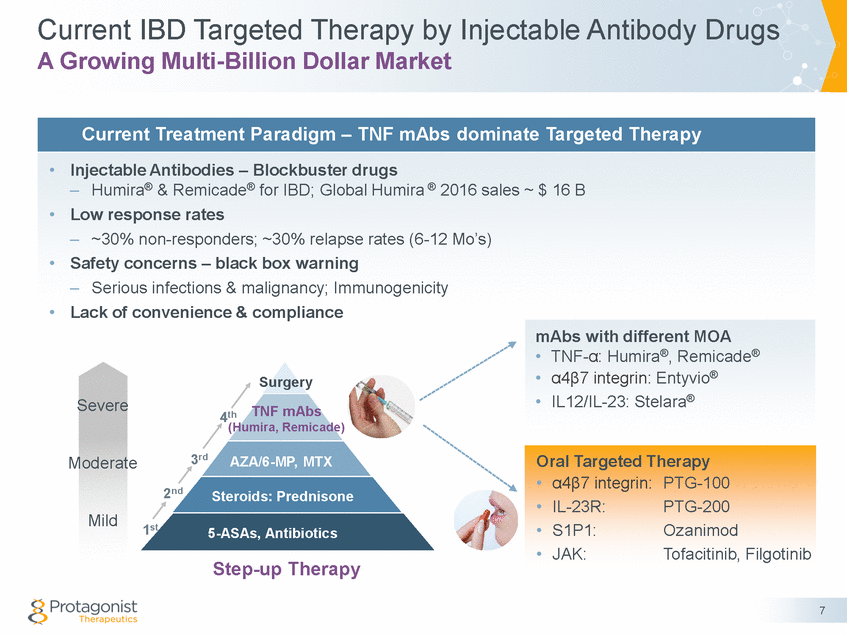
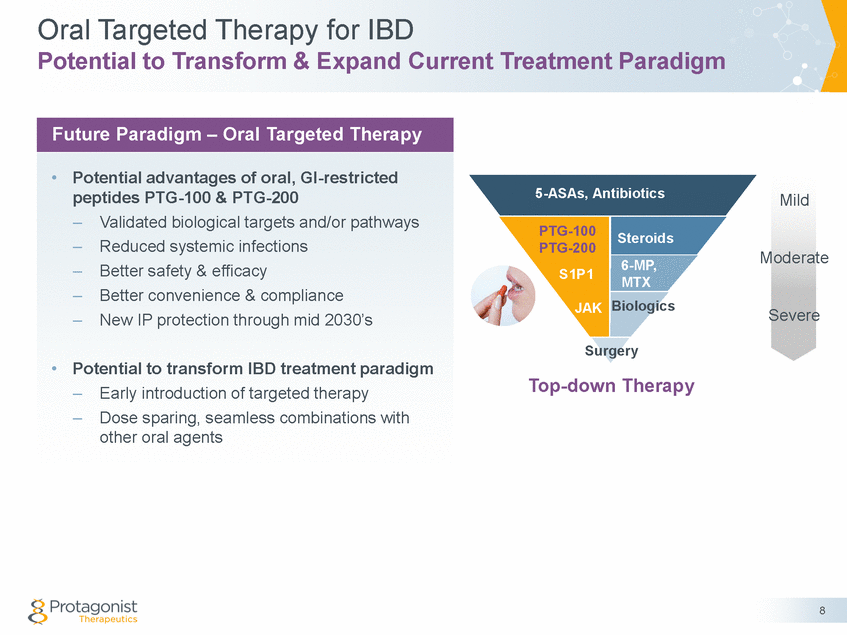
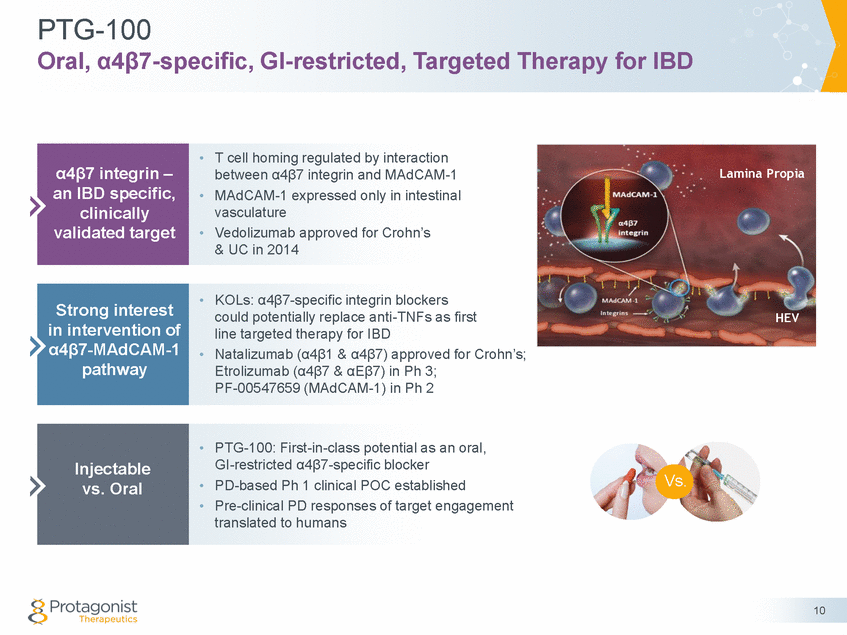
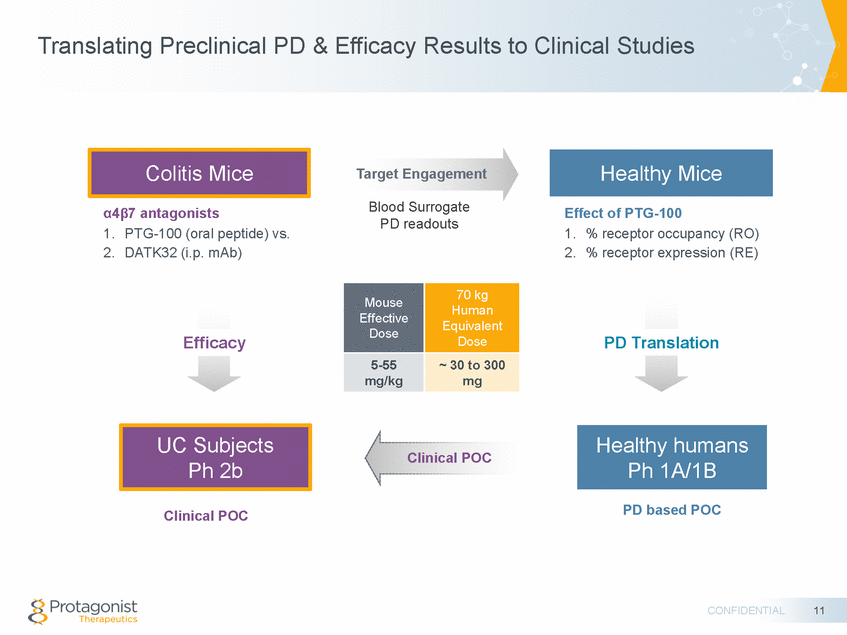
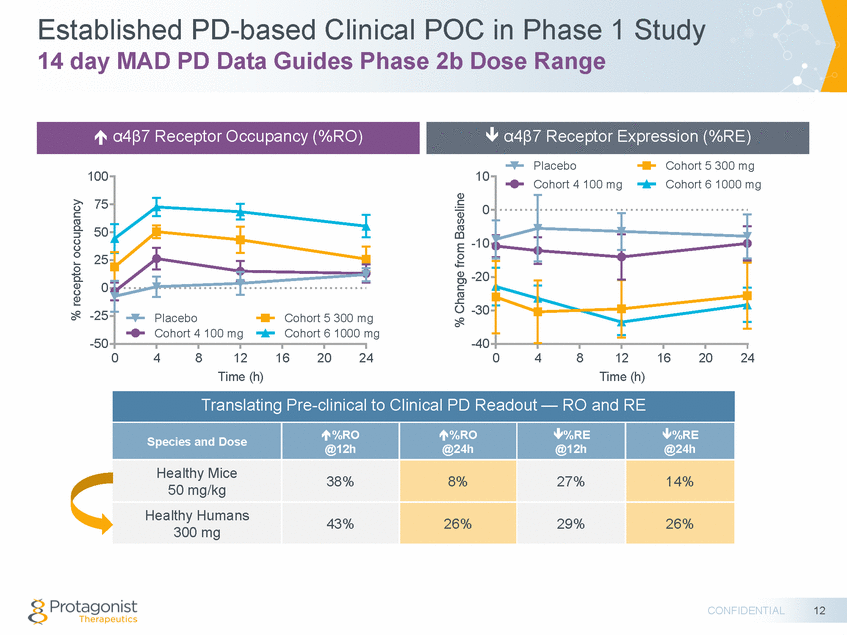
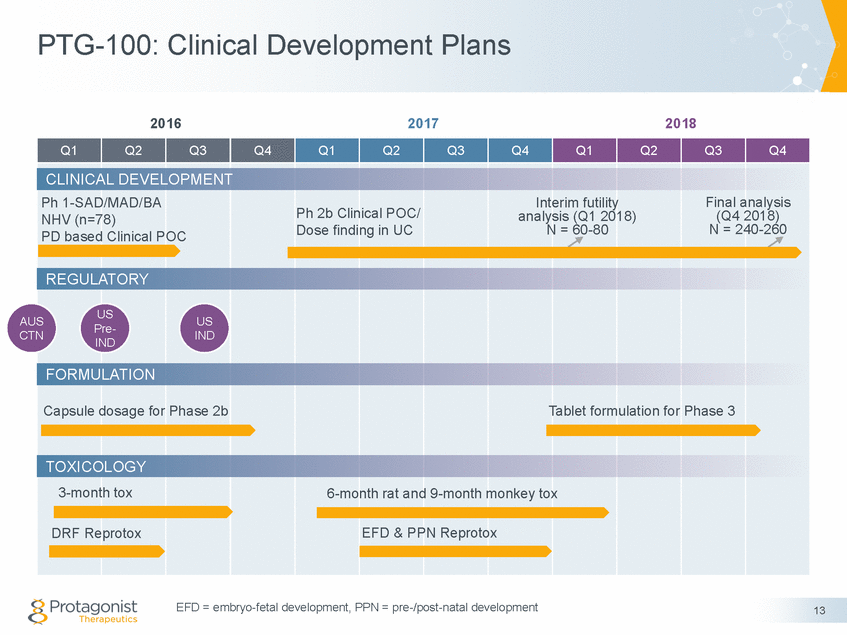
Forward Looking Statements This presentation contains forward-looking statements of Protagonist Therapeutics, Inc. (Protagonist). All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, our capital resources, business strategy, prospective products, potential market for our products, availability of funding, enrollment in our clinical trials, clinical trial results, product approvals and regulatory pathways, timing and likelihood of success, plans, objectives and opinions of management regarding future operations, future results of current and anticipated products and our potential receipt of milestone payments and royalties under our Exclusive License and Collaboration Agreement with Janssen Biotech, Inc., are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Protagonist discusses many of these risks and uncertainties in detail under the heading “Risk Factors” contained in our most recent periodic reports on Forms 10-K and 10-Q, which are filed with the Securities and Exchange Commission. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). They are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or Protagonist or any director, employee, agent or advisor of Protagonist. This presentation does not purport to be all inclusive or to contain all the information you may desire. 2