Exhibit 99.1

1 COMPANY OVERVIEW Dinesh V. Patel, PhD | President & CEO April 2022

Forward - looking Statements 2 This presentation and the accompanying oral presentation contain forward - looking statements made pursuant to the safe harbor pro visions of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this pre sentation, including statements regarding our future results of operations and financial position, business strategy, product candidates, capital res ources, potential markets for our product candidates, enrollment in our clinical trials, any potential impact on our business related to COVID - 19, our pot ential receipt of milestone payments and royalties under our Collaboration Agreement with Janssen Biotech, Inc., are forward - looking statements. In some ca ses, you can identify forward - looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “will” or the negative of these terms or other similar expressions. The forward - looking statements made in this presentation involve known and unknown risks, uncertainties and other important fact ors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievemen ts expressed or implied by the forward - looking statements. These forward - looking statements are subject to risks and uncertainties, including those discuss ed in Protagonist’s filings with the Securities and Exchange Commission, including in the “Risk Factors” and “Management’s Discussion and Analysi s o f Financial Condition and Results of Operations” sections of most recently filed periodic reports on Form 10 - K and Form 10 - Q and subsequent filings an d in the documents incorporated by reference therein. Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as pre dictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or othe rwi se. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by Federal law to investigational use, and no representation is made as to their s afe ty or effectiveness for the purposes for which they are being investigated. The trademarks included herein are the property of the owners thereof and are us ed for reference purposes only. Such use should not be construed as an endorsement of such products. Nothing contained in this presentation i s, or should be construed as, a recommendation, promise or representation by the presenter or Protagonist or any director, employee, agent or ad visor of Protagonist. This presentation does not purport to be all inclusive or to contain all the information you may desire.
Protagonist Therapeutics Discovery and Development of Novel Peptide Therapeutics in Rare and Common Diseases 3 • Proprietary Peptide Technology Platform for de novo discovery and optimization of first - in - class and best - in - class medicines – Applied to develop agonists and antagonists of difficult targets such as cytokines, cytokine receptors, integrin, transmembrane solute transporters, GPCRs, and ion channels. – Injectables and oral drug candidates are designed to maximize systemic drug levels, or to minimize systemic drug levels in an orally - delivered GI - restricted approach. • Co - Development partnership portfolio earned $112.5 Million in milestone payments to date; potential up to $850 Million future milestones – Granted Janssen Pharmaceutical Companies of Johnson & Johnson an exclusive worldwide license to research, develop and commercialize oral IL - 23 receptor antagonists – PN - 235 □ FRONTIER 1, a Phase 2b multicenter, randomized, placebo controlled, dose - ranging study in moderate - to - severe plaque psoriasis, commenced in early 2022 • Significant opportunities to unlock and capture value within 12 - 24 months Robust, advancing peptide therapeutic candidate portfolio addressing multiple indications with multi - billion dollar market potential • Rusfertide , an investigational, injectable hepcidin mimetic □ REVIVE Phase 2 proof - of - concept clinical trial for polycythemia vera (PV) □ PACIFIC Phase 2 study in PV subjects with high hematocrit levels □ Completed Phase 2a study for hereditary hemochromatosis □ Initiated patient screening for Phase 3 VERIFY, a global, randomized, placebo - controlled trial evaluating the efficacy and safety of a once weekly, subcutaneously self - administered dose of rusfertide . • PN - 943 , an investigational orally delivered, gut - restricted alpha - 4 - beta - 7 integrin specific antagonist peptide □ IDEAL Phase 2 study in adults with moderate to severe active ulcerative colitis.
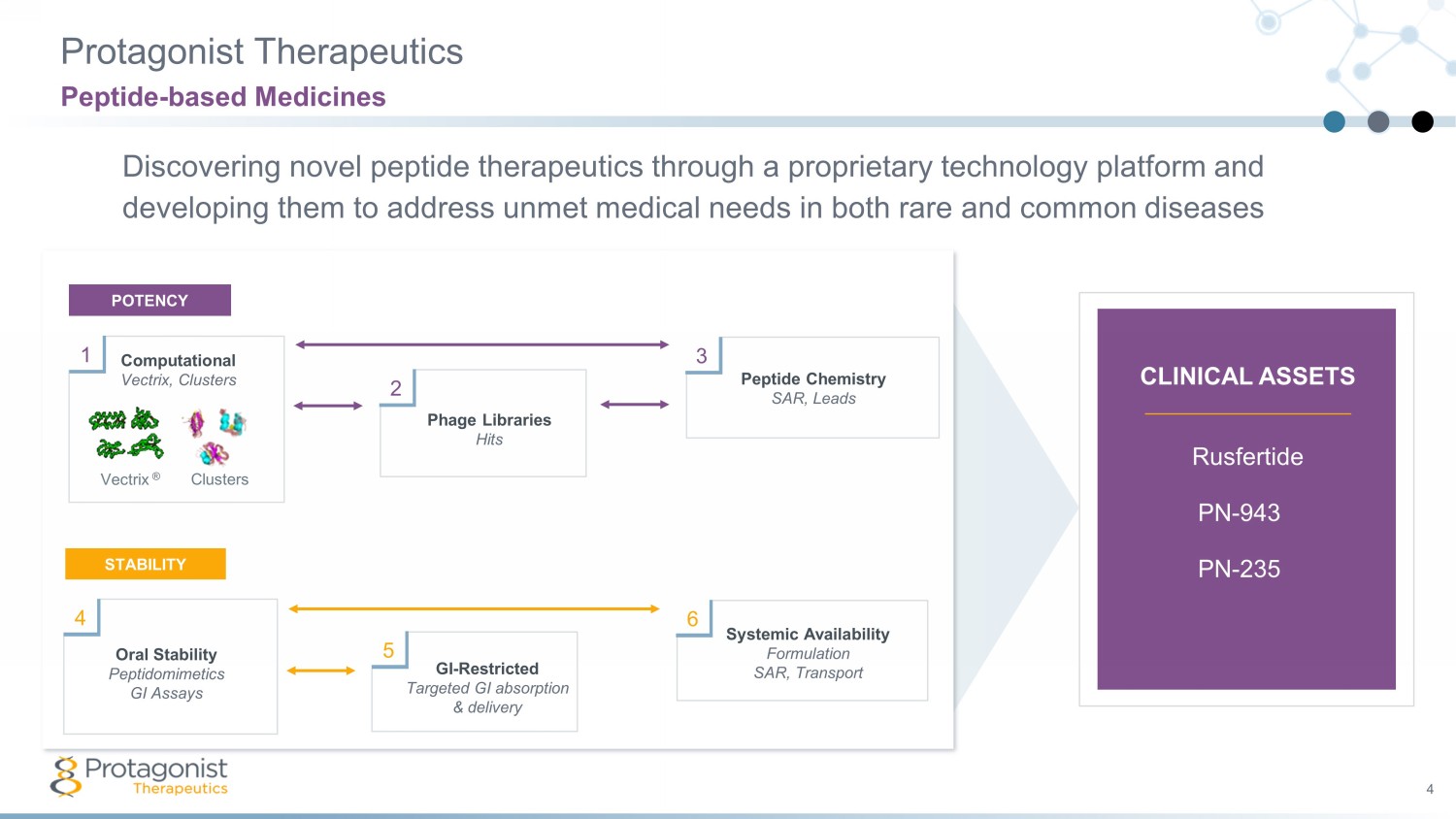
Protagonist Therapeutics 4 Discovering novel peptide therapeutics through a proprietary technology platform and developing them to address unmet medical needs in both rare and common diseases Peptide - based Medicines Rusfertide PN - 943 PN - 235 Peptide Chemistry SAR, Leads Phage Libraries Hits Computational Vectrix, Clusters Vectrix ® Clusters 1 2 3 POTENCY Oral Stability Peptidomimetics GI Assays GI - Restricted Targeted GI absorption & delivery Systemic Availability Formulation SAR, Transport 4 5 6 STABILITY CLINICAL ASSETS

PROGRAMS CANDIDATE STUDY PHASE 1 PHASE 2 Key Milestones HEMATOLOGY & BLOOD DISORDERS Hepcidin Mimetic rusfertide (PTG - 300) s.c. VERIFY 300 - 11 PV Ph3 • Sites activated, screening underway REVIVE 300 - 04 • Enrollment complete • Updates at 2022 medical conferences PACIFIC 300 - 08 • All patients resumed dosing 300 - 06 • Clinical PoC established INFLAMMATORY & IMMUNOMODULATORY DISEASES Oral GI Restricted a4b7 - Integrin Antagonist PN - 943 IDEAL • 150 patient study • Enrollment complete • Topline data by mid - May 2022 Oral IL - 23R Antagonist PN - 235 FRONTIER 1 Plaque Psoriasis Ph2 • 240 patient study initiated Q1 2022 IBD Ph2 • Initiation in 2H 2022 Product Portfolio 4 Addressing Unmet Needs in Multiple Indications with Multi - billion Dollar Market Potential PV Ph2 PoC Ulcerative Colitis (UC) Ph2 PoC PV Ph2 in Patients with Elevated Hematocrit Hereditary Hemochromatosis (HH) Ph2 PoC Polycythemia Vera (PV) Ph3 initiation Plaque Psoriasis Ph2b PoC initiation

5 Rusfertide (PTG - 300): Hepcidin Mimetic To Potentially Address Unmet Needs in Both Rare & Common Diseases
Polycythemia Vera 7 Disease Background Myeloproliferative neoplasm characterized by excessive production of red blood cells (RBCs) • Characterized by Janus Kinase 2 (JAK2) mutation Treatment goal is to control hematocrit level <45% • Elevated hematocrit is a hallmark of the disease • Maintaining hematocrit <45% is critical to minimizing thrombosis, CV events, and death Serious chronic condition • Rare disease: ~100,000 diagnosed patients in USA • Diagnosed commonly in individuals 50 - 70 years of age • Median survival ~20 years • Thrombotic and cardiovascular risks; may progress to myelofibrosis or leukemia
Rusfertide for Polycythemia Vera 8 Overview of Current Status and Recent Progress • Orphan Drug designation, Fast Track status, and Breakthrough Therapy designation • Phase 2 REVIVE Study: − Clinical update presented at ASH 2021 − Enrollment complete; post - hold re - enrollment rate of 87% • Phase 3 VERIFY Study: − Both FDA and CHMP (EU) have approved the Phase 3 VERIFY study design − Two clinical studies, REVIVE and VERIFY, are required for both the US (NDA) and EU (MAA) submissions − FDA has signed off on presented toxicity plan, nonclinical DMPK, clinical pharmacology plan, and Chemistry, Manufacturing and Controls plan. • Major agreements now in place for US NDA: − Non - clinical studies including two - year rat carcinogenicity study − Nonclinical and clinical pharmacology studies − Chemistry, Manufacturing and Controls plan Continued Progress Underway

Polycythemia Vera 9 Current Treatment Options 1. MPN Landmark Survey 2017, Trinity Primary Research 2019 • Treatment goal is to maintain HCT ≤ 45% • HCT control may be erratic with up and down excursions from 45% • Can lead to iron deficiency • Recommended when HCT cannot be controlled, or in high - risk patients • Potential long - term side effects • Some patients reluctant to use chemotherapeutic agents 1 • Jakafi ( ruxolitinib ) approved for HU resistant/intolerant patients • ~5,300 patients/ yr treated** • ~25% develop intolerance or resistance • Potential side effects include cytopenia CHRONIC TREATMENT OVER ~20 YEARS Hydroxyurea +/ - Phlebotomy Jakafi Phlebotomy *Newly FDA approved, undergoing U.S. commercial launch; uncertain place in the therapeutic paradigm at present **Represents patients treated with Jakafi; uncertain of Besremi population due to recent approval Besremi * ( ropeginterferon alfa - 2b - njft injection)
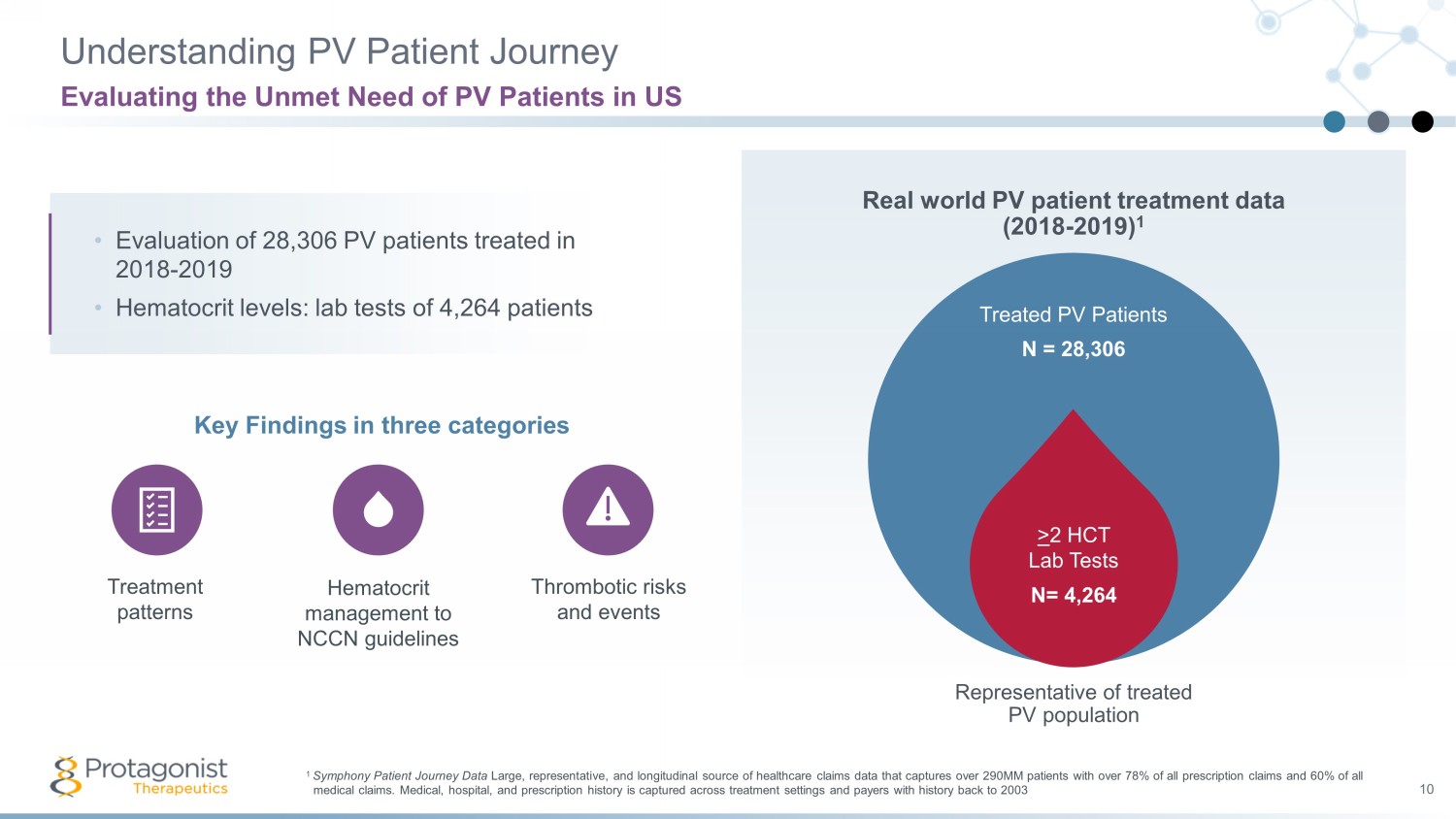
Understanding PV Patient Journey 10 Evaluating the Unmet Need of PV Patients in US 1 Symphony Patient Journey Data Large, representative, and longitudinal source of healthcare claims data that captures over 290MM patients with over 78% of a ll prescription claims and 60% of all medical claims. Medical, hospital, and prescription history is captured across treatment settings and payers with history bac k t o 2003 Treated PV Patients N = 28,306 Representative of treated PV population > 2 HCT Lab Tests N= 4,264 • Evaluation of 28,306 PV patients treated in 2018 - 2019 • Hematocrit levels: lab tests of 4,264 patients Key Findings in three categories Treatment patterns Hematocrit management to NCCN guidelines Thrombotic risks and events Real world PV patient treatment data (2018 - 2019) 1
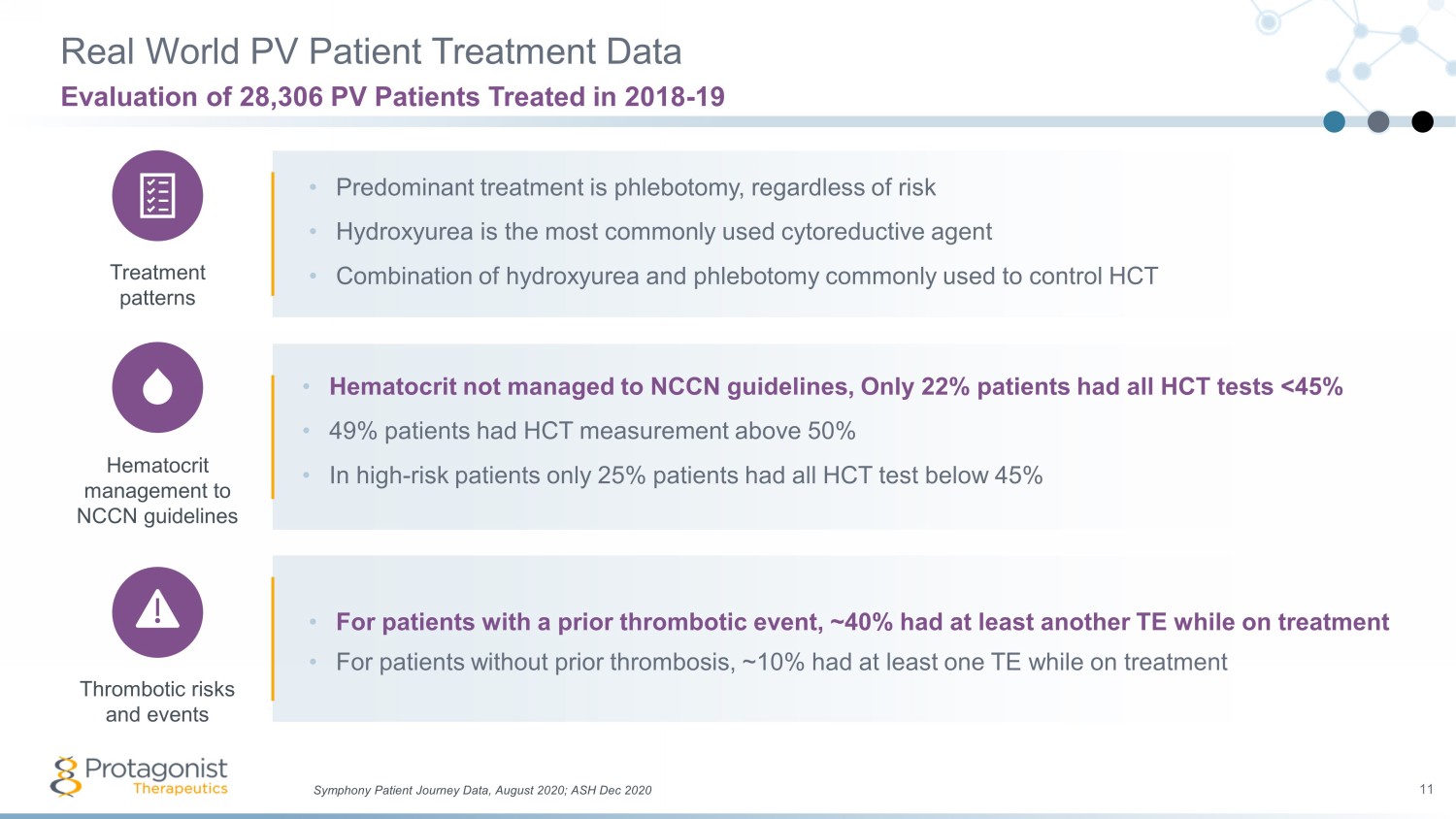
Real World PV Patient Treatment Data 11 Evaluation of 28,306 PV Patients Treated in 2018 - 19 • Predominant treatment is phlebotomy, regardless of risk • Hydroxyurea is the most commonly used cytoreductive agent • Combination of hydroxyurea and phlebotomy commonly used to control HCT • Hematocrit not managed to NCCN guidelines, Only 22% patients had all HCT tests <45% • 49% patients had HCT measurement above 50% • In high - risk patients only 25% patients had all HCT test below 45% • For patients with a prior thrombotic event, ~40% had at least another TE while on treatment • For patients without prior thrombosis, ~10% had at least one TE while on treatment Symphony Patient Journey Data, August 2020; ASH Dec 2020 Treatment patterns Hematocrit management to NCCN guidelines Thrombotic risks and events
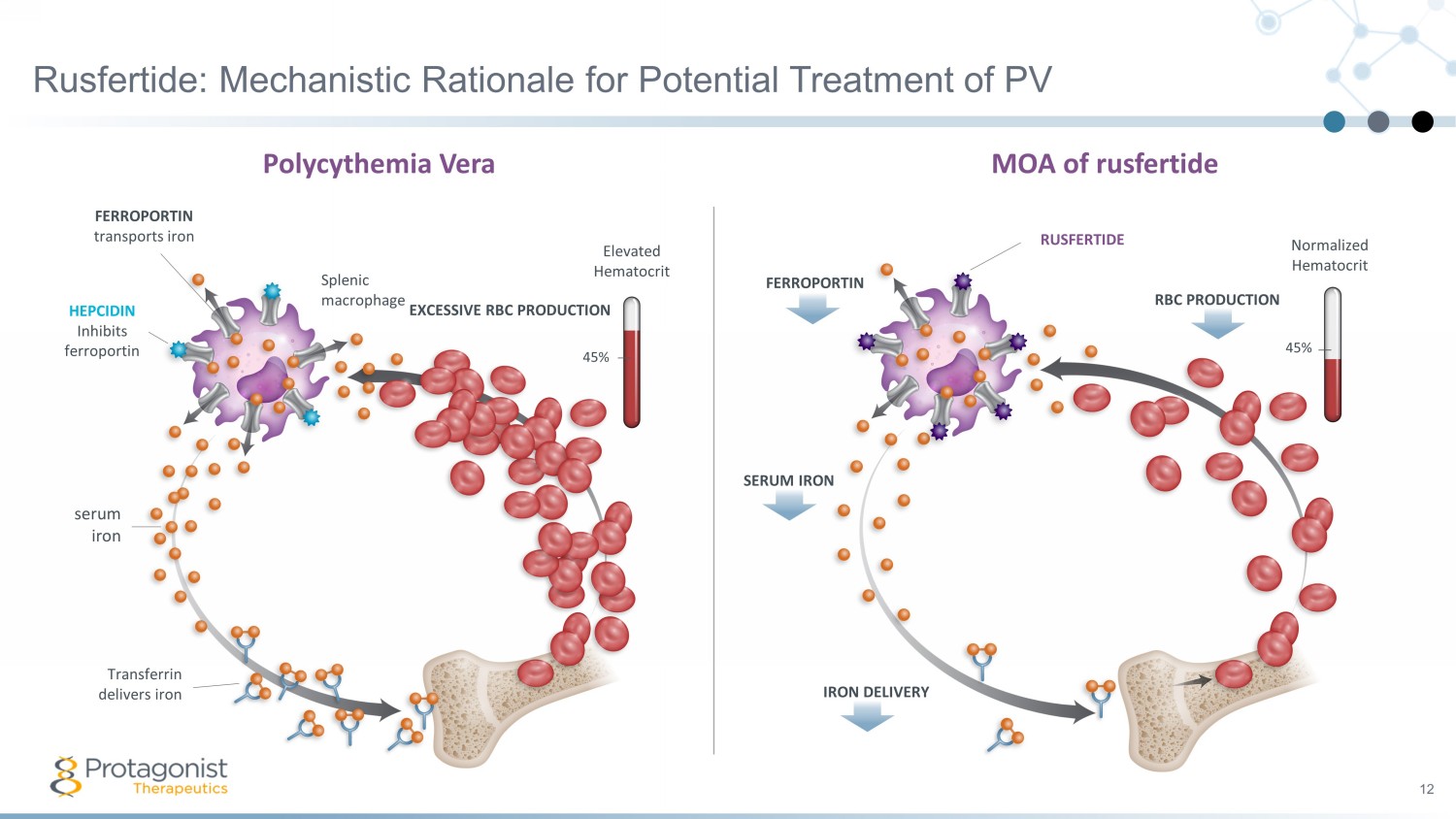
FERROPORTIN Normalized Hematocrit 45% SERUM IRON IRON DELIVERY MOA of rusfertide RBC PRODUCTION RUSFERTIDE Rusfertide: Mechanistic Rationale for Potential Treatment of PV 12 FERROPORTIN transports iron HEPCIDIN Inhibits ferroportin serum iron Transferrin delivers iron Splenic macrophage EXCESSIVE RBC PRODUCTION 45% Elevated Hematocrit Polycythemia Vera
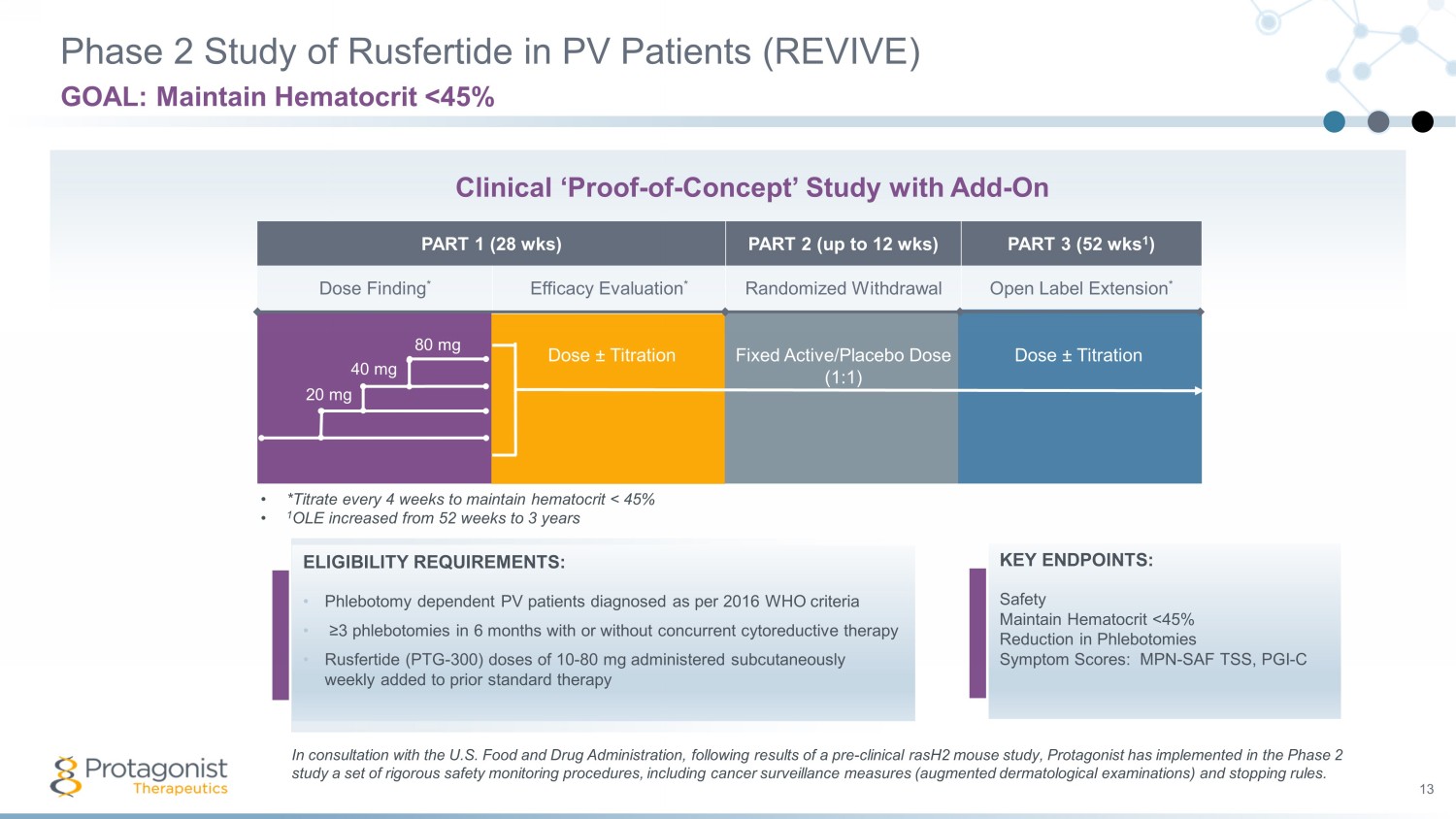
PART 1 (28 wks) PART 2 (up to 12 wks) PART 3 (52 wks 1 ) Dose Finding * Efficacy Evaluation * Randomized Withdrawal Open Label Extension * Phase 2 Study of Rusfertide in PV Patients (REVIVE) 13 GOAL: Maintain Hematocrit <45% ELIGIBILITY REQUIREMENTS: • Phlebotomy dependent PV patients diagnosed as per 2016 WHO criteria • ≥3 phlebotomies in 6 months with or without concurrent cytoreductive therapy • Rusfertide (PTG - 300) doses of 10 - 80 mg administered subcutaneously weekly added to prior standard therapy Dose ± Titration Fixed Active/Placebo Dose (1:1) Dose ± Titration • *Titrate every 4 weeks to maintain hematocrit < 45% • 1 OLE increased from 52 weeks to 3 years 20 mg 40 mg 80 mg Clinical ‘Proof - of - Concept’ Study with Add - On KEY ENDPOINTS: Safety Maintain Hematocrit <45% Reduction in Phlebotomies Symptom Scores: MPN - SAF TSS, PGI - C In consultation with the U.S. Food and Drug Administration, following results of a pre - clinical rasH2 mouse study, Protagonist h as implemented in the Phase 2 study a set of rigorous safety monitoring procedures, including cancer surveillance measures (augmented dermatological examin ati ons) and stopping rules.
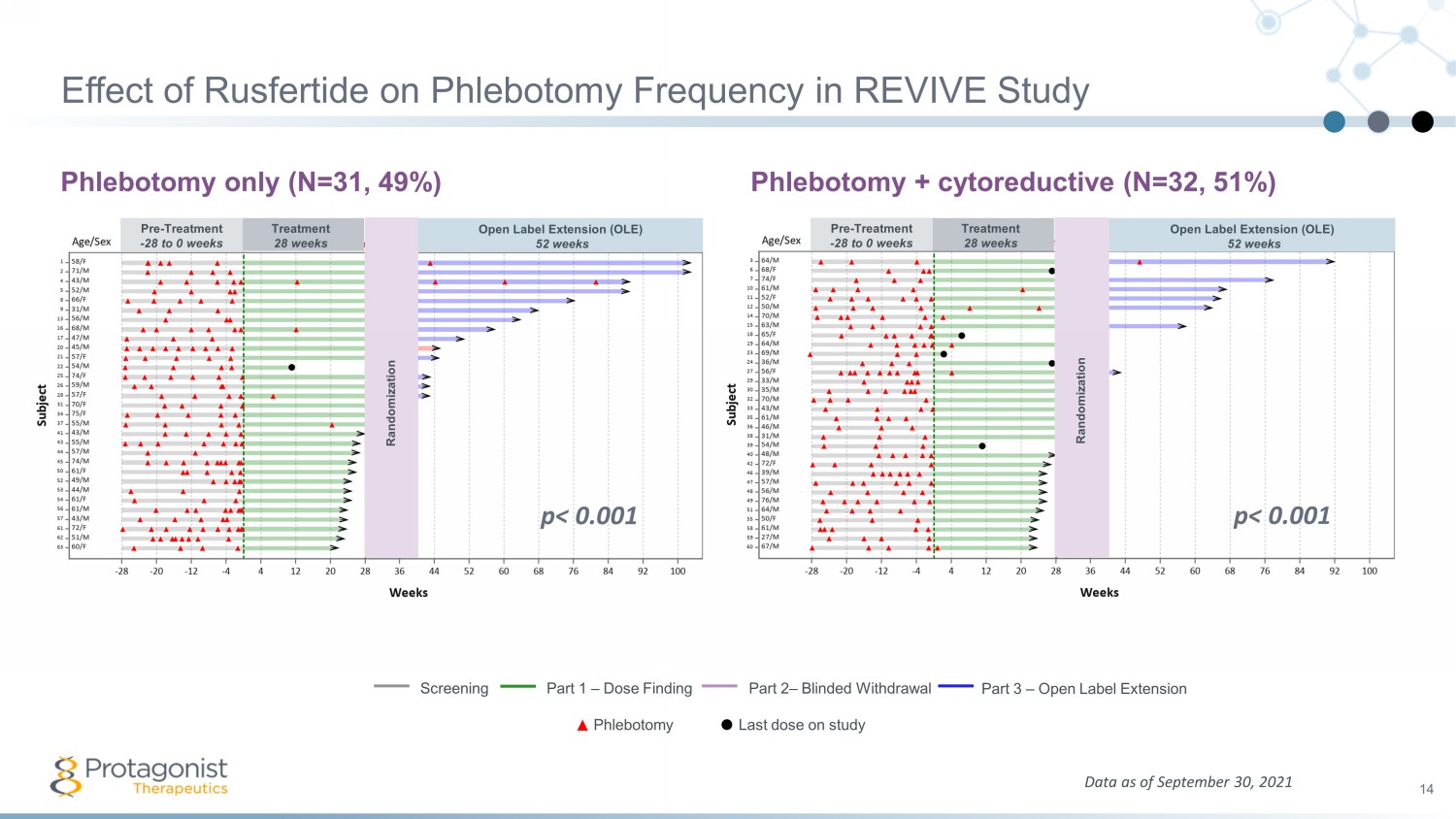
Effect of Rusfertide on Phlebotomy Frequency in REVIVE Study Phlebotomy only (N=31, 49%) Phlebotomy + cytoreductive (N=32, 51%) 14 Data as of September 30, 2021 Screening Part 1 – Dose Finding Part 2 – Blinded Withdrawal Part 3 – Open Label Extension Phlebotomy Last dose on study Pre - Treatment - 28 to 0 weeks Treatment 28 weeks Open Label Extension (OLE) 52 weeks Randomization Pre - Treatment - 28 to 0 weeks Treatment 28 weeks Open Label Extension (OLE) 52 weeks Randomization p< 0.001 p< 0.001
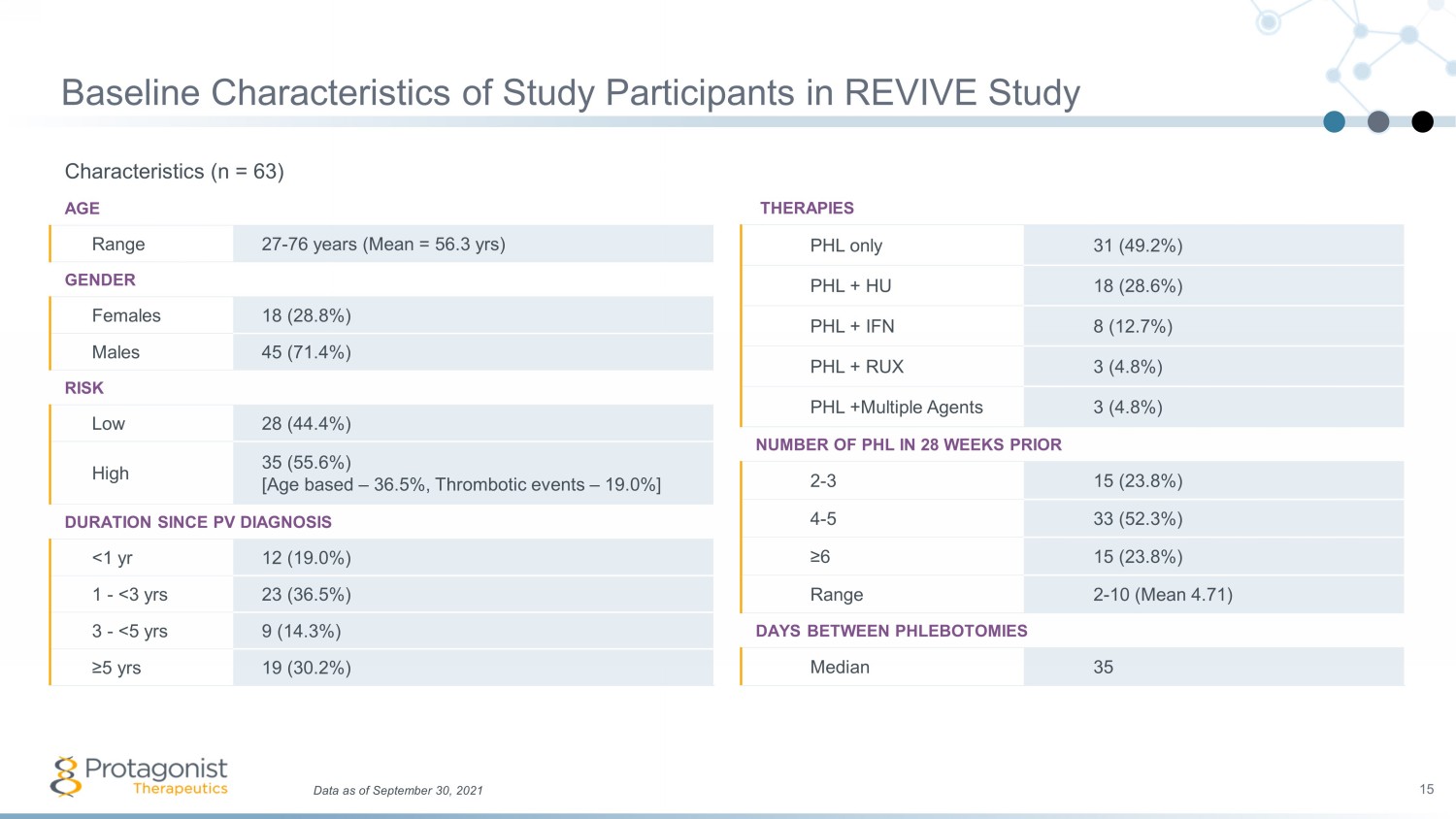
Baseline Characteristics of Study Participants in REVIVE Study Characteristics (n = 63) AGE Range 27 - 76 years (Mean = 56.3 yrs ) GENDER Females 18 (28.8%) Males 45 (71.4%) RISK Low 28 (44.4%) High 35 (55.6%) [Age based – 36.5%, Thrombotic events – 19.0%] DURATION SINCE PV DIAGNOSIS <1 yr 12 (19.0%) 1 - <3 yrs 23 (36.5%) 3 - <5 yrs 9 (14.3%) ≥5 yrs 19 (30.2%) 15 Data as of September 30, 2021 THERAPIES PHL only 31 (49.2%) PHL + HU 18 (28.6%) PHL + IFN 8 (12.7%) PHL + RUX 3 (4.8%) PHL +Multiple Agents 3 (4.8%) NUMBER OF PHL IN 28 WEEKS PRIOR 2 - 3 15 (23.8%) 4 - 5 33 (52.3%) ≥6 15 (23.8%) Range 2 - 10 (Mean 4.71) DAYS BETWEEN PHLEBOTOMIES Median 35
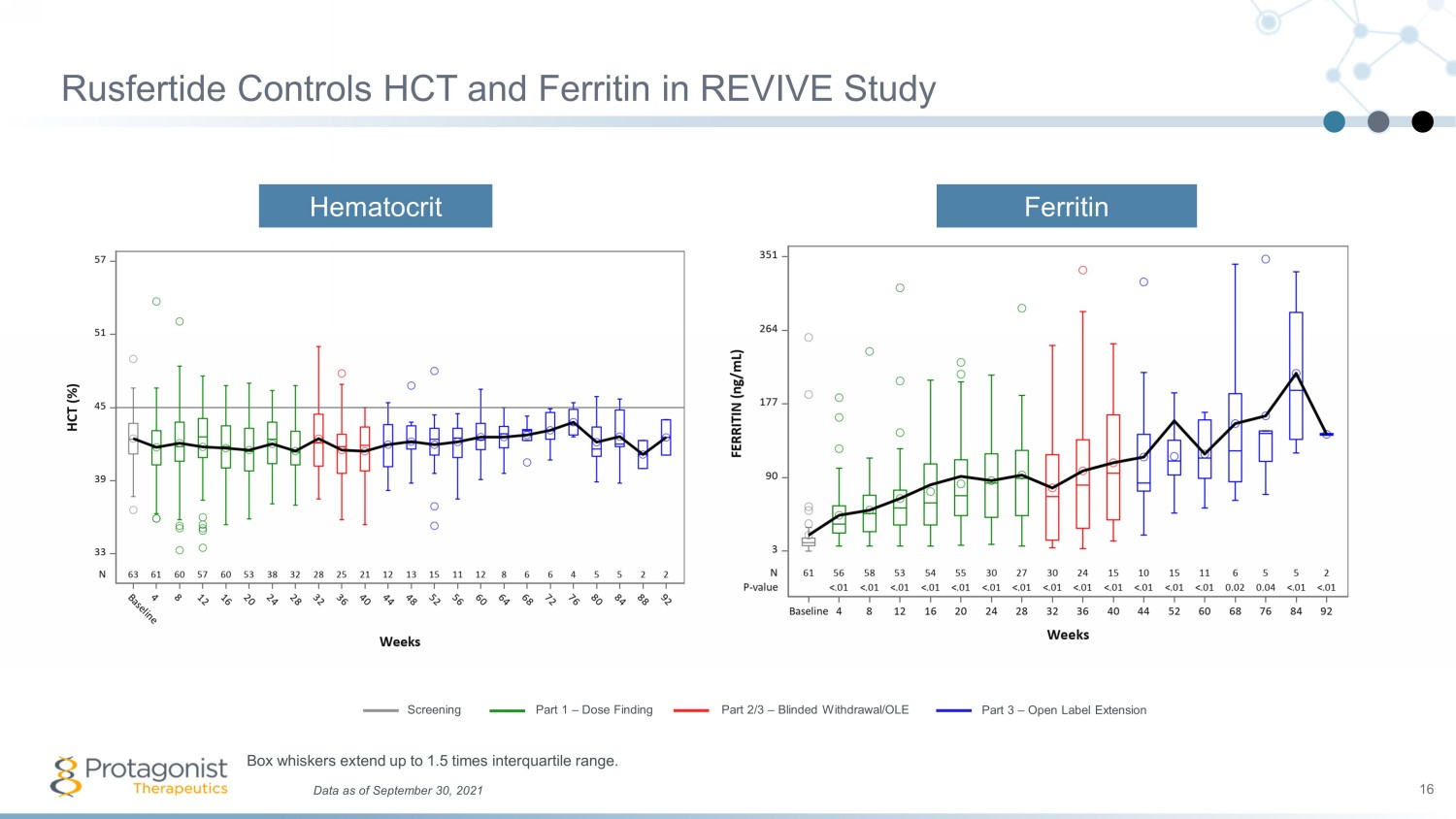
Rusfertide Controls HCT and Ferritin in REVIVE Study 16 Hematocrit Ferritin Data as of September 30, 2021 Box whiskers extend up to 1.5 times interquartile range. Screening Part 1 – Dose Finding Part 2/3 – Blinded Withdrawal/OLE Part 3 – Open Label Extension
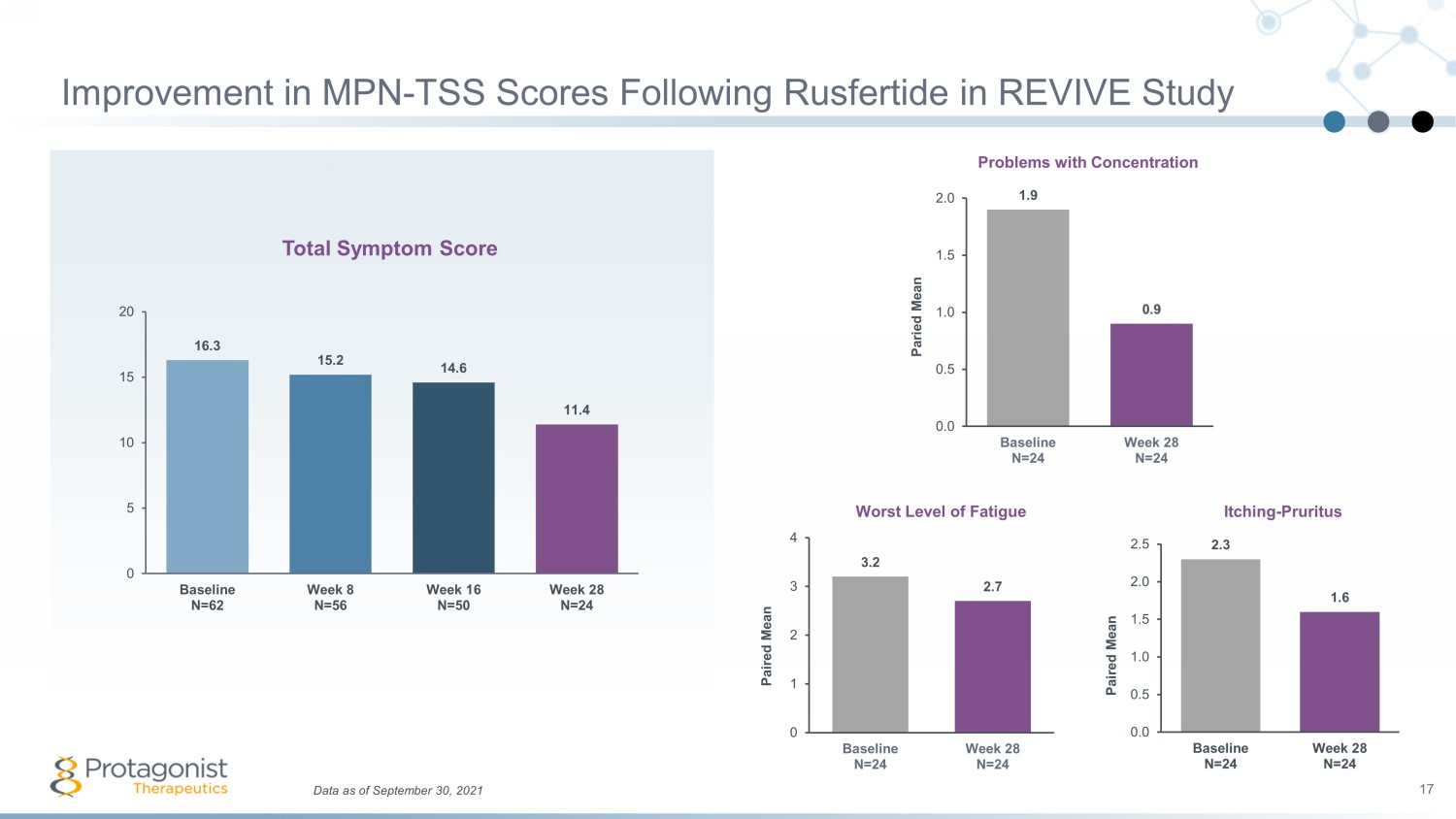
Data as of September 30, 2021 17 Improvement in MPN - TSS Scores Following Rusfertide in REVIVE Study 1.9 0.9 0.0 0.5 1.0 1.5 2.0 Baseline N=24 Week 28 N=24 Paried Mean Total Symptom Score Worst Level of Fatigue Problems with Concentration Itching - Pruritus 3.2 2.7 0 1 2 3 4 Baseline N=24 Week 28 N=24 Paired Mean 2.3 1.6 0.0 0.5 1.0 1.5 2.0 2.5 Baseline N=24 Week 28 N=24 Paired Mean 16.3 15.2 14.6 11.4 0 5 10 15 20 Baseline N=62 Week 8 N=56 Week 16 N=50 Week 28 N=24
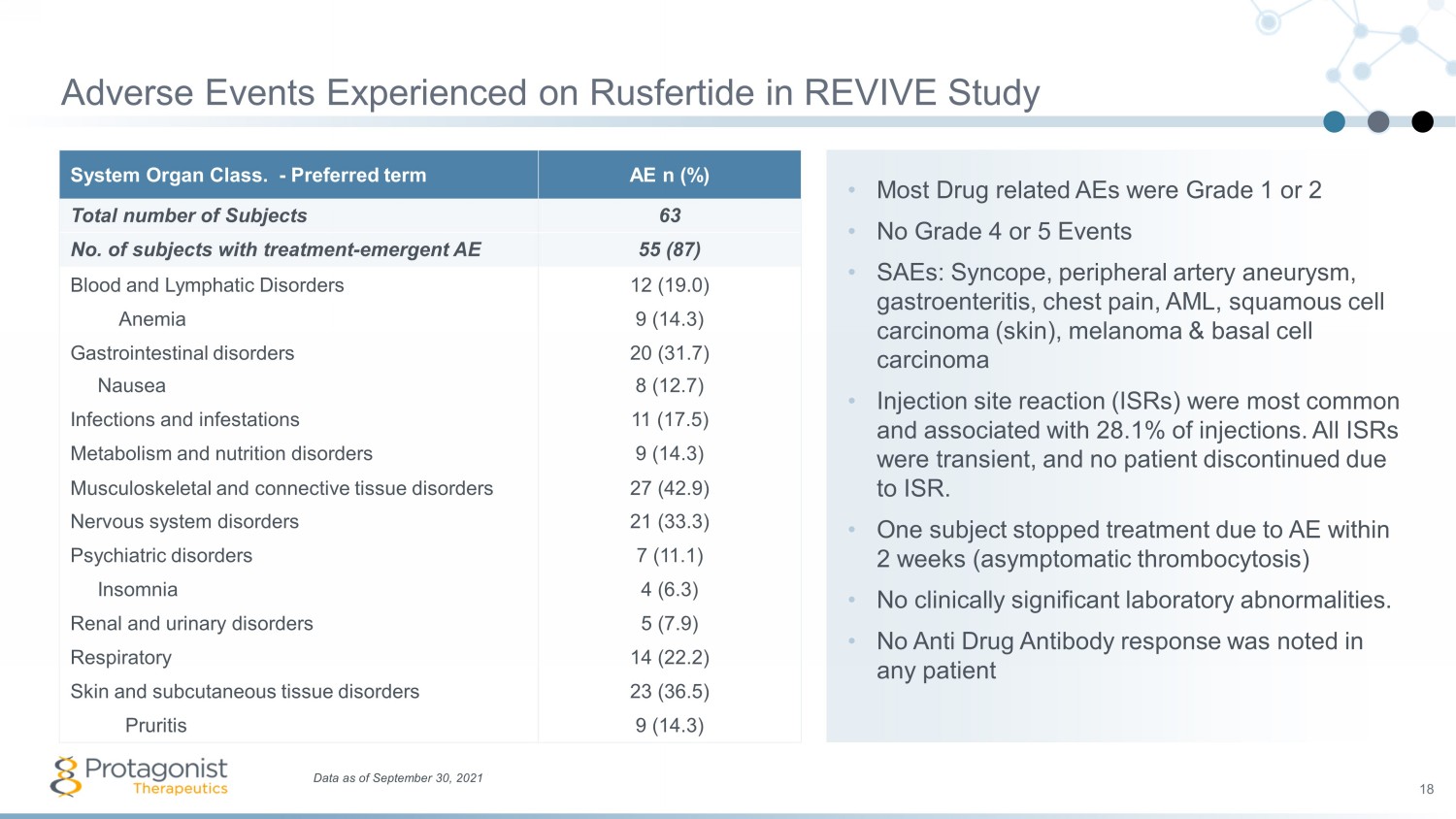
• Most Drug related AEs were Grade 1 or 2 • No Grade 4 or 5 Events • SAEs: Syncope, peripheral artery aneurysm, gastroenteritis, chest pain, AML, squamous cell carcinoma (skin), melanoma & basal cell carcinoma • Injection site reaction (ISRs) were most common and associated with 28.1% of injections. All ISRs were transient, and no patient discontinued due to ISR. • One subject stopped treatment due to AE within 2 weeks (asymptomatic thrombocytosis) • No clinically significant laboratory abnormalities. • No Anti Drug Antibody response was noted in any patient System Organ Class. - Preferred term AE n (%) Total number of Subjects 63 No. of subjects with treatment - emergent AE 55 (87) Blood and Lymphatic Disorders 12 (19.0) Anemia 9 (14.3) Gastrointestinal disorders 20 (31.7) Nausea 8 (12.7) Infections and infestations 11 (17.5) Metabolism and nutrition disorders 9 (14.3) Musculoskeletal and connective tissue disorders 27 (42.9) Nervous system disorders 21 (33.3) Psychiatric disorders 7 (11.1) Insomnia 4 (6.3) Renal and urinary disorders 5 (7.9) Respiratory 14 (22.2) Skin and subcutaneous tissue disorders 23 (36.5) Pruritis 9 (14.3) Data as of September 30, 2021 18 Adverse Events Experienced on Rusfertide in REVIVE Study

19 Select SAEs in Further Context 1 • A total of 168 patients have been treated with rusfertide to date: beta - thalassemia, hereditary hemochromatosis (HH), and polycythemia vera (PV) − Of these, 7 subjects with 8 observed cancer cases, inclusive of cases confirmed as pre - existing − Of the 89 PV patients, 5 subjects with 6 events observed during re - enrollment skin exams − One confirmed pre - existing − One with 2 dermatologic events − Of the 3 remaining subjects: events observed only in patients on other therapeutic agents with known carcinogenicity risks (e .g. , hydroxyurea, ruxolitinib ) − Other confounding variables include disease progression, advanced age (>60 years), Skin Type 1 2 and cumulative sun exposure − Malignancies include AML, squamous cell carcinoma (skin), melanoma & basal cell carcinoma as described at ASH 2021 • All patients with treatment emergent skin cancers had prior episodes of skin cancer. • Phase 2 REVIVE study’s stopping rules are aligned to background rates of cancer in PV patients; observed cases at no point reached that threshold. − Phase 3 VERIFY study stopping rules are the same 1. FDA granted Breakthrough Therapy designation to rusfertide in PV in 2021; the Agency has notified the Company of its intent to rescind BTD based on observed malignancies, in follow - up to the FDA clinical hold imposed on September 16, 2021. The Company has submitted a response and requested a meeting. Orphan Drug and Fa st Track designations remain in place. Protagonist anticipates no changes to its development plans for rusfertide , including no anticipated changes to timelines for the Phase 3 VERIFY study in PV. 2. Per the Fitzpatrick Scale. See, for example: Gupta, V. and Sharma, V.K., 2019. Skin typing: Fitzpatrick grading and others. Clinics in Dermatology , 37 (5), pp.430 - 436. Data as of April 12, 2022
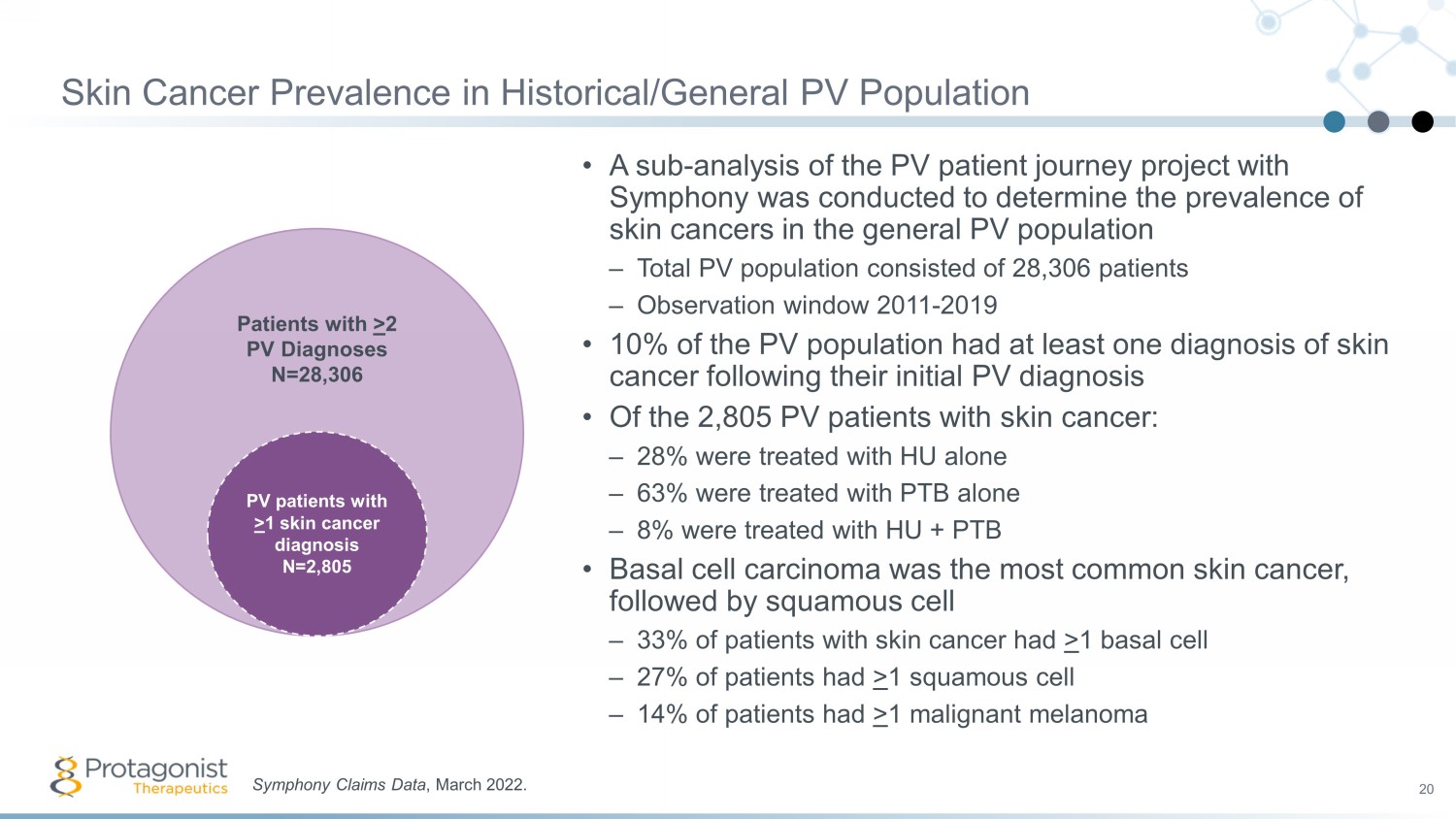
Skin Cancer Prevalence in Historical/General PV Population 20 • A sub - analysis of the PV patient journey project with Symphony was conducted to determine the prevalence of skin cancers in the general PV population – Total PV population consisted of 28,306 patients – Observation window 2011 - 2019 • 10% of the PV population had at least one diagnosis of skin cancer following their initial PV diagnosis • Of the 2,805 PV patients with skin cancer: – 28% were treated with HU alone – 63% were treated with PTB alone – 8% were treated with HU + PTB • Basal cell carcinoma was the most common skin cancer, followed by squamous cell – 33% of patients with skin cancer had > 1 basal cell – 27% of patients had > 1 squamous cell – 14% of patients had > 1 malignant melanoma Symphony Claims Data , March 2022. Patients with > 2 PV Diagnoses N=28,306 PV patients with > 1 skin cancer diagnosis N=2,805
Conclusions from Phase 2 REVIVE Study 21 Rusfertide therapy resulted in rapid, sustained and durable hematocrit control without clinically meaningful changes in white blood cell and platelet counts Rusfertide demonstrated similar efficacy in all categories of patients, independent of the PV patient risk category or concurrent therapy with hydroxyurea, interferon or ruxolitinib Subjects have been treated up to 1.5 years with subjects remaining essentially phlebotomy - free Benefits from rusfertide treatment were noted in patient reported outcomes as assessed by MPN - SAF Total Symptom Score Results to date suggest that rusfertide is well tolerated; the most common adverse events were grade 1 or 2

PACIFIC: Open - Label Phase 2 PV Study in Subjects with high hematocrit (>48%) Full overview available at https://clinicaltrials.gov/ct2/show/NCT04767802 22 Patient met the WHO criteria for PV diagnosis and had a baseline hematocrit (HCT) >48%, and a history of ≥3 HCT values >48% in the prior year prior All adult males or females with both high - risk and low - risk criteria treated with phlebotomy alone or with concurrent cytoreductive therapy were enrolled Initial dose: (40 mg SQ twice weekly). After hematocrit was <45%, dosing regimen was adjusted individually. Clinical endpoint is proportion of subject with a hematocrit <45% by Week 16 and safety follow up for 1 year Maintenance dose: Once each subject’s HCT decreased (< 45%) for 2 consecutive visits, physicians choice to adjust dosing regimen to maintain HCT < 45%
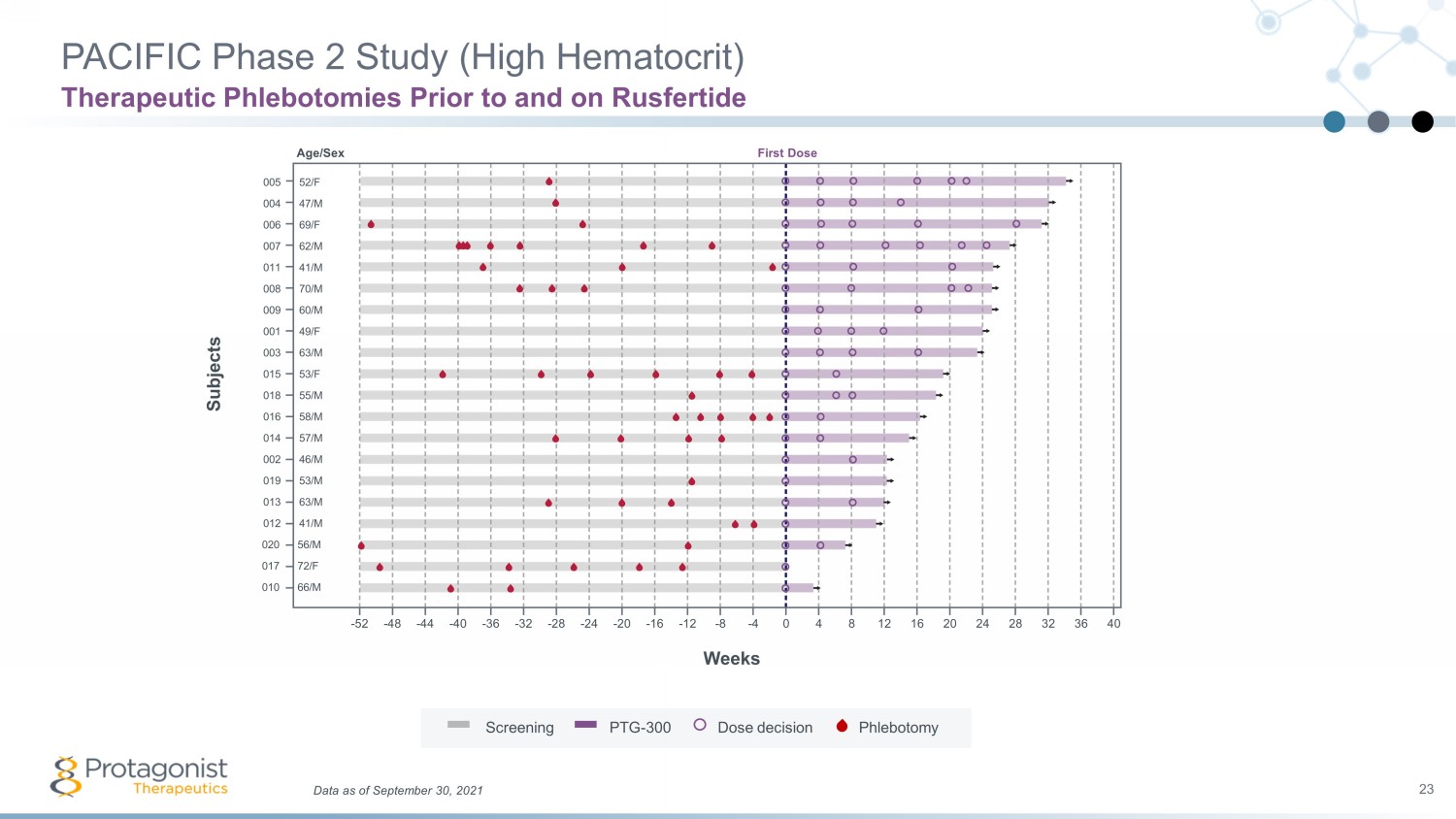
005 004 006 007 011 009 001 003 015 018 016 014 002 019 013 012 008 020 017 010 Weeks Subjects Data as of September 30, 2021 23 PACIFIC Phase 2 Study (High Hematocrit) Therapeutic Phlebotomies Prior to and on Rusfertide 52/F 47/M 69/F 62/M 41/M 60/M 49/F 63/M 53/F 55/M 58/M 57/M 46/M 53/M 63/M 41/M 70/M 56/M 72/F 66/M - 52 - 40 - 20 - 12 0 20 24 28 32 36 40 - 16 - 24 - 48 16 12 8 4 - 4 - 8 - 28 - 32 - 36 - 44 Screening PTG - 300 Dose decision Phlebotomy Age/Sex First Dose
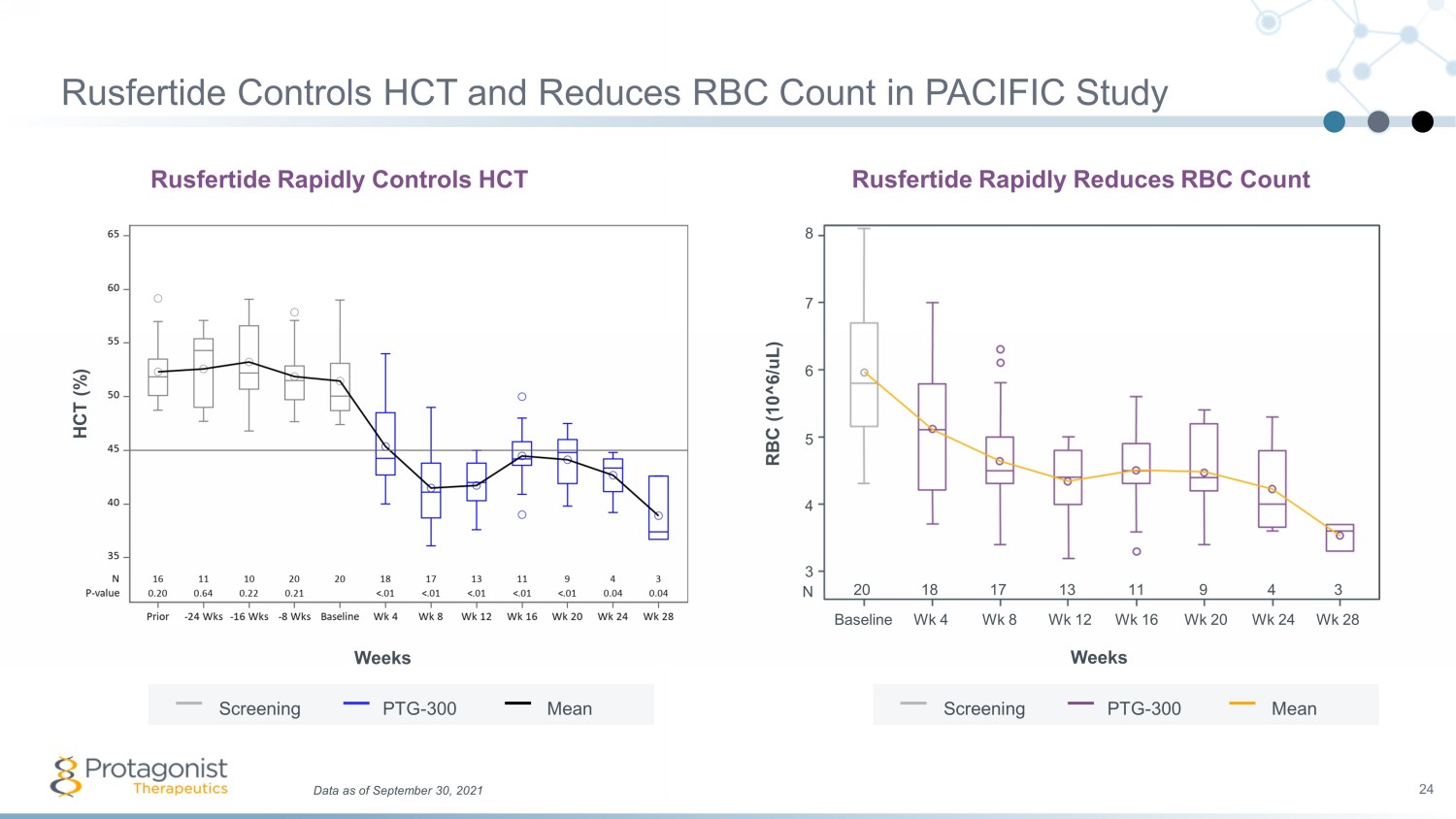
Data as of September 30, 2021 24 Rusfertide Controls HCT and Reduces RBC Count in PACIFIC Study Rusfertide Rapidly Reduces RBC Count RBC (10^6/ uL ) Weeks 8 7 6 5 4 3 Wk 12 Baseline Wk 4 Wk 8 Wk 16 Wk 20 Wk 24 Wk 28 20 18 17 13 11 9 4 3 N Screening PTG - 300 Mean Rusfertide Rapidly Controls HCT Weeks HCT (%) Screening PTG - 300 Mean
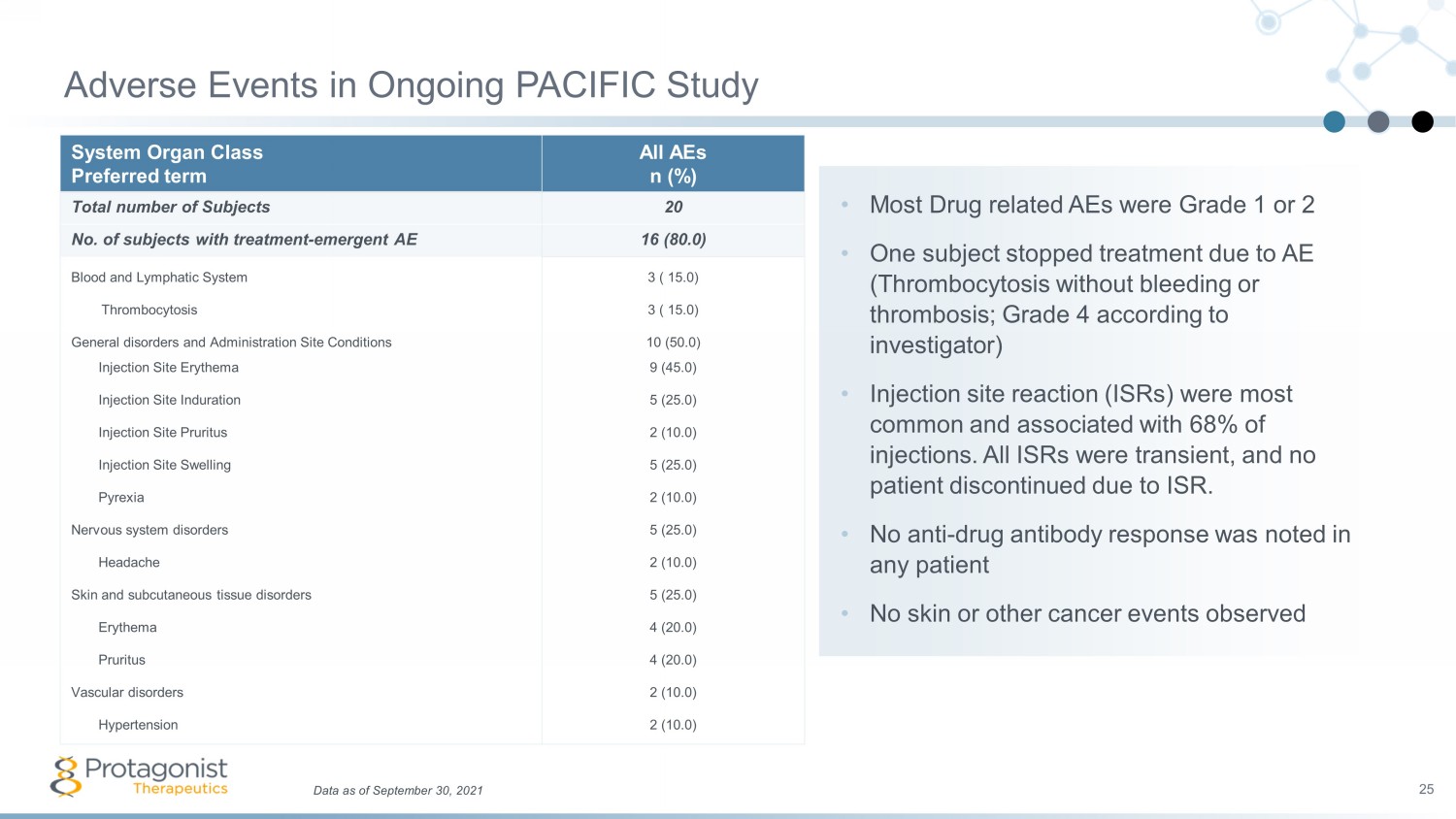
• Most Drug related AEs were Grade 1 or 2 • One subject stopped treatment due to AE (Thrombocytosis without bleeding or thrombosis; Grade 4 according to investigator) • Injection site reaction (ISRs) were most common and associated with 68% of injections. All ISRs were transient, and no patient discontinued due to ISR. • No anti - drug antibody response was noted in any patient • No skin or other cancer events observed System Organ Class Preferred term All AEs n (%) Total number of Subjects 20 No. of subjects with treatment - emergent AE 16 (80.0) Blood and Lymphatic System 3 ( 15.0) Thrombocytosis 3 ( 15.0) General disorders and Administration Site Conditions 10 (50.0) Injection Site Erythema 9 (45.0) Injection Site Induration 5 (25.0) Injection Site Pruritus 2 (10.0) Injection Site Swelling 5 (25.0) Pyrexia 2 (10.0) Nervous system disorders 5 (25.0) Headache 2 (10.0) Skin and subcutaneous tissue disorders 5 (25.0) Erythema 4 (20.0) Pruritus 4 (20.0) Vascular disorders 2 (10.0) Hypertension 2 (10.0) Adverse Events in Ongoing PACIFIC Study 25 Data as of September 30, 2021

Conclusions from PACIFIC High Hematocrit Study 26 Rusfertide induction therapy with twice weekly dosing is effective at rapidly achieving target hematocrit below 45% without phlebotomy in all PV patients. Rapid hematocrit control: <45% in 4 - 6 weeks, without a clinically meaningful change in platelets or WBC count. Patients transition to once weekly maintenance dose and stable for up to week 36 (to - date) after achieving hematocrit control in ~4 weeks Taken together, use of rusfertide in erythrocytic PV patients represents a potential therapeutic direction for patients unwilling or unable to utilize the current standard of care.
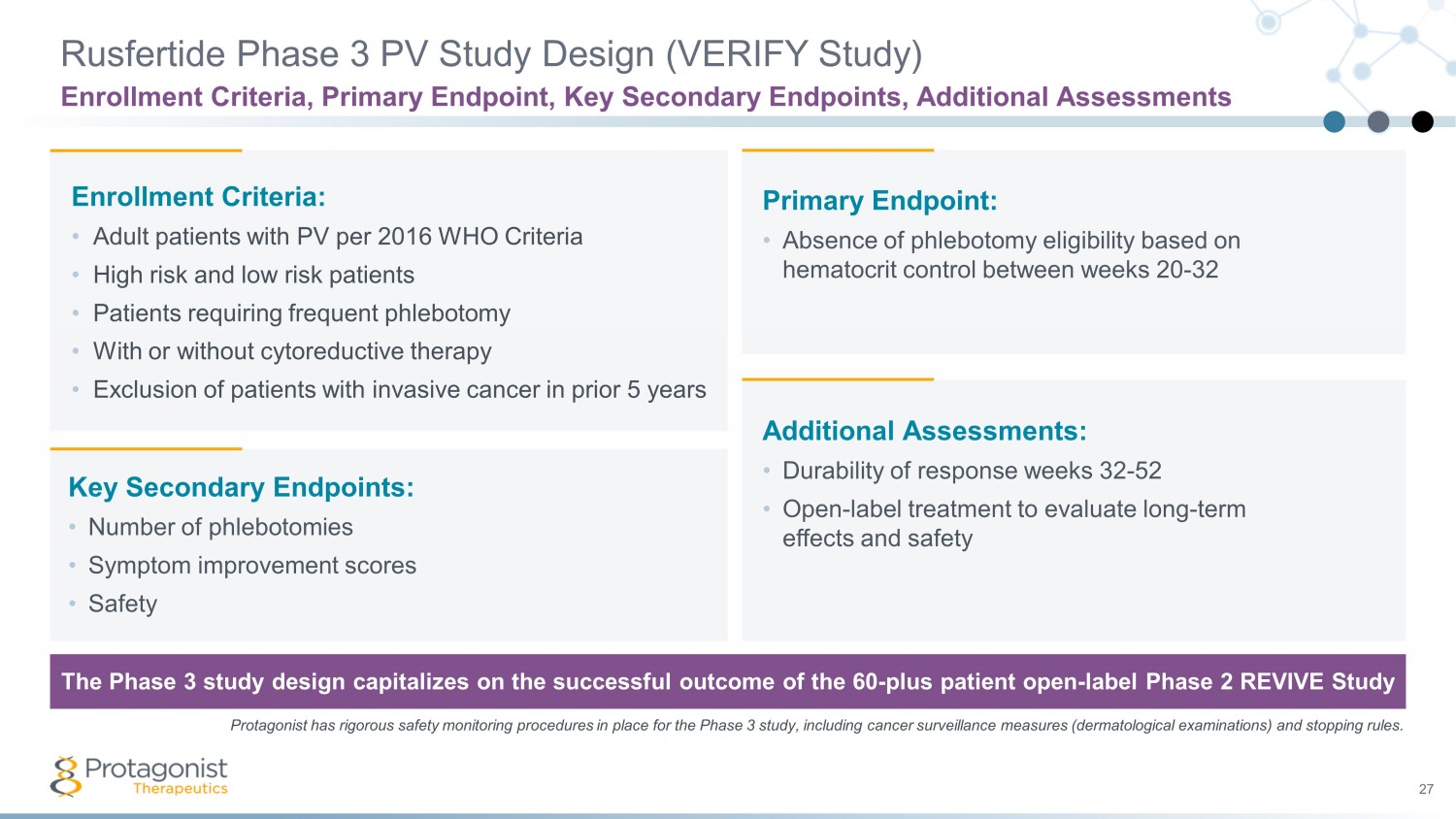
27 Enrollment Criteria: • Adult patients with PV per 2016 WHO Criteria • High risk and low risk patients • Patients requiring frequent phlebotomy • With or without cytoreductive therapy • Exclusion of patients with invasive cancer in prior 5 years Primary Endpoint: • Absence of phlebotomy eligibility based on hematocrit control between weeks 20 - 32 Key Secondary Endpoints: • Number of phlebotomies • Symptom improvement scores • Safety Additional Assessments: • Durability of response weeks 32 - 52 • Open - label treatment to evaluate long - term effects and safety The Phase 3 study design capitalizes on the successful outcome of the 60 - plus patient open - label Phase 2 REVIVE Study Rusfertide Phase 3 PV Study Design (VERIFY Study) Enrollment Criteria, Primary Endpoint, Key Secondary Endpoints, Additional Assessments Protagonist has rigorous safety monitoring procedures in place for the Phase 3 study, including cancer surveillance measures (de rmatological examinations) and stopping rules.
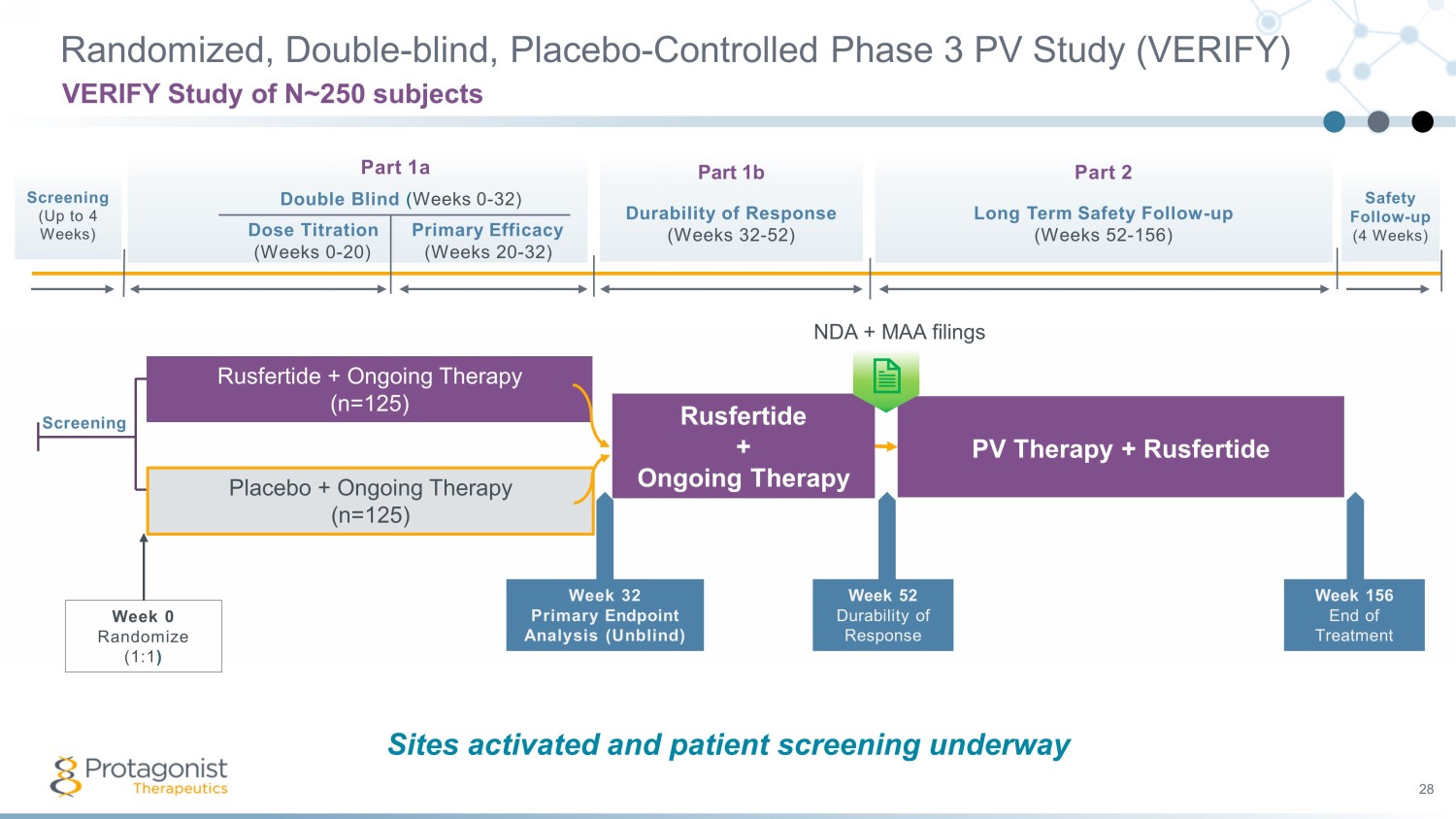
Part 1a Double Blind ( Weeks 0 - 32) Dose Titration Primary Efficacy (Weeks 0 - 20) (Weeks 20 - 32) Part 1b Durability of Response (Weeks 32 - 52) Part 2 Long Term Safety Follow - up ( Weeks 52 - 156) Screening (Up to 4 Weeks) Randomized, Double - blind, Placebo - Controlled Phase 3 PV Study (VERIFY) 28 VERIFY Study of N~250 subjects Safety Follow - up (4 Weeks) Screening Week 32 Primary Endpoint Analysis (Unblind) Placebo + Ongoing Therapy (n= 125 ) Week 0 Randomize (1:1 ) Rusfertide + Ongoing Therapy (n= 125 ) Rusfertide + Ongoing Therapy PV Therapy + Rusfertide Week 156 End of Treatment Week 52 Durability of Response NDA + MAA filings The Phase 3 study design capitalizes on the successful outcome of the 60 - plus patient open - label Phase 2 REVIVE Study Sites activated and patient screening underway
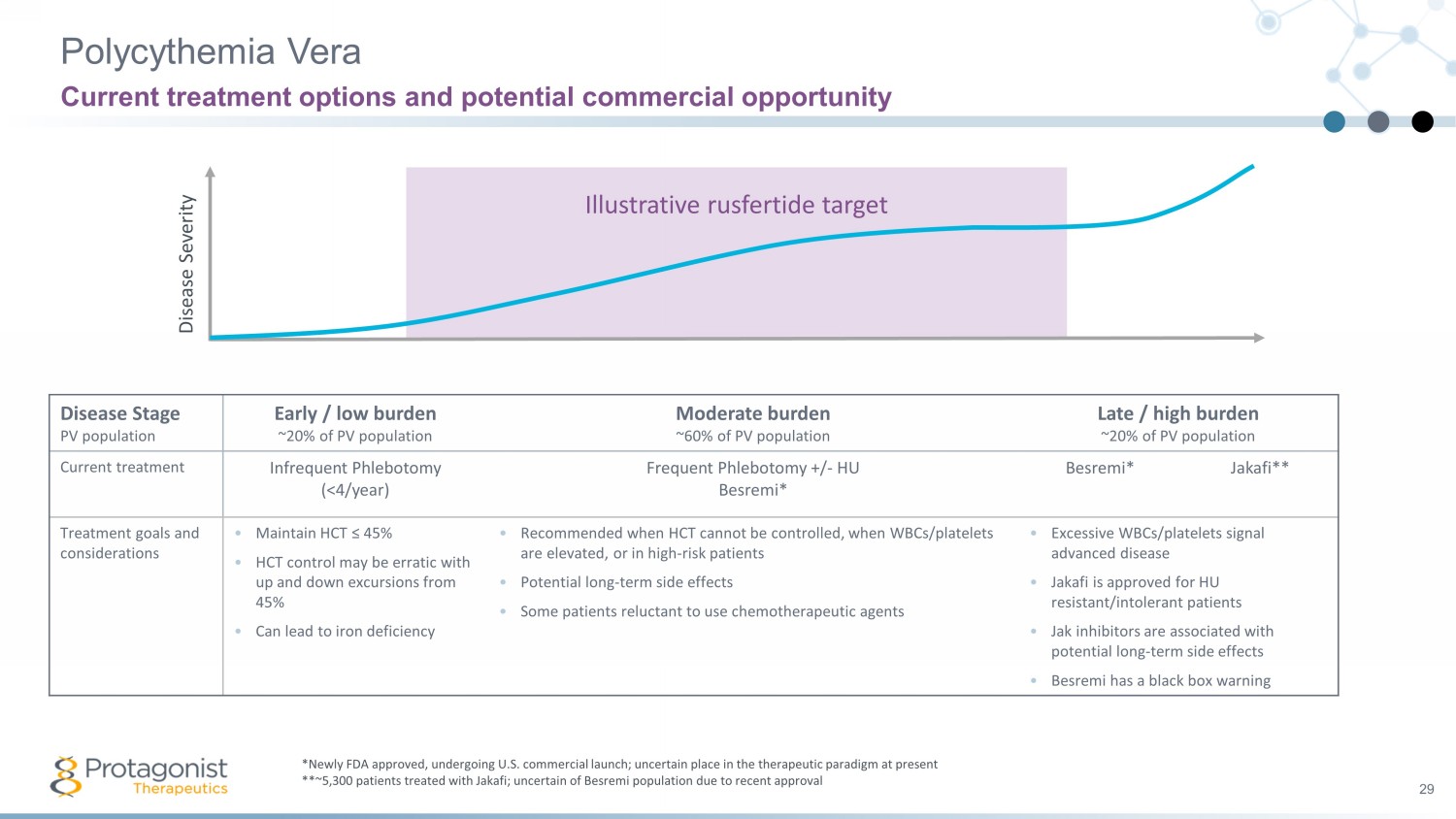
Illustrative rusfertide target Polycythemia Vera 29 Current treatment options and potential commercial opportunity *Newly FDA approved, undergoing U.S. commercial launch; uncertain place in the therapeutic paradigm at present **~5,300 patients treated with Jakafi; uncertain of Besremi population due to recent approval Disease Severity Disease Stage PV population Early / low burden ~20% of PV population Moderate burden ~60% of PV population Late / high burden ~20% of PV population Current treatment Infrequent Phlebotomy (<4/year) Frequent Phlebotomy +/ - HU Besremi * Besremi * Jakafi** Treatment goals and considerations • Maintain HCT ≤ 45% • HCT control may be erratic with up and down excursions from 45% • Can lead to iron deficiency • Recommended when HCT cannot be controlled, when WBCs/platelets are elevated, or in high - risk patients • Potential long - term side effects • Some patients reluctant to use chemotherapeutic agents • Excessive WBCs/platelets signal advanced disease • Jakafi is approved for HU resistant/intolerant patients • Jak inhibitors are associated with potential long - term side effects • Besremi has a black box warning
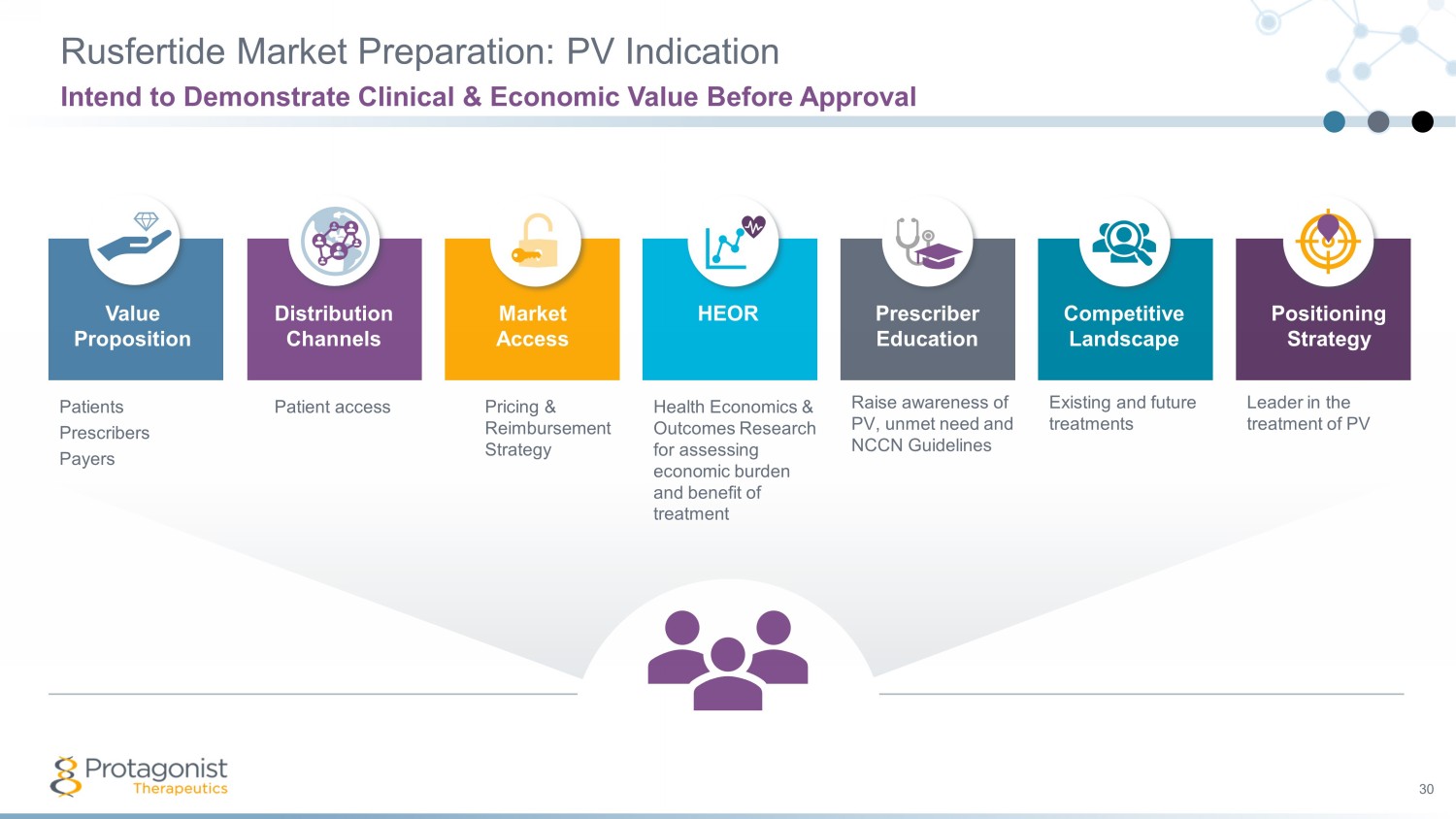
Rusfertide Market Preparation: PV Indication Intend to Demonstrate Clinical & Economic Value Before Approval 30 Value Proposition Market Access HEOR Competitive Landscape Positioning Strategy Prescriber Education Distribution Channels Patients Prescribers Payers Patient access Pricing & Reimbursement Strategy Health Economics & Outcomes Research for assessing economic burden and benefit of treatment Raise awareness of PV, unmet need and NCCN Guidelines Leader in the treatment of PV Existing and future treatments

Hereditary Hemochromatosis (HH) 31 Disease Prevalence and Treatment If untreated, iron overload can cause hepatomegaly, diabetes mellitus, skin hyperpigmentation, cardiomyopathy, diastolic dysfunction, heart failure, cirrhosis, etc . Iron overload disease Unmet medical need in specific subpopulations Phlebotomy is the only therapeutic option; no approved drugs Source: Porter JL, Rawla P. Hemochromatosis. (Updated 2020 Jun 18], https:// www.ncbi.nlm.nih.gov /books/NBK430862 / Excessive iron accumulation in heart, liver, pancreas, skin, joint tissues HH is predominately due to genetic mutation, leading to a deficiency of hepcidin in the body Rusfertide, if approved, could serve as a hormone replacement therapy
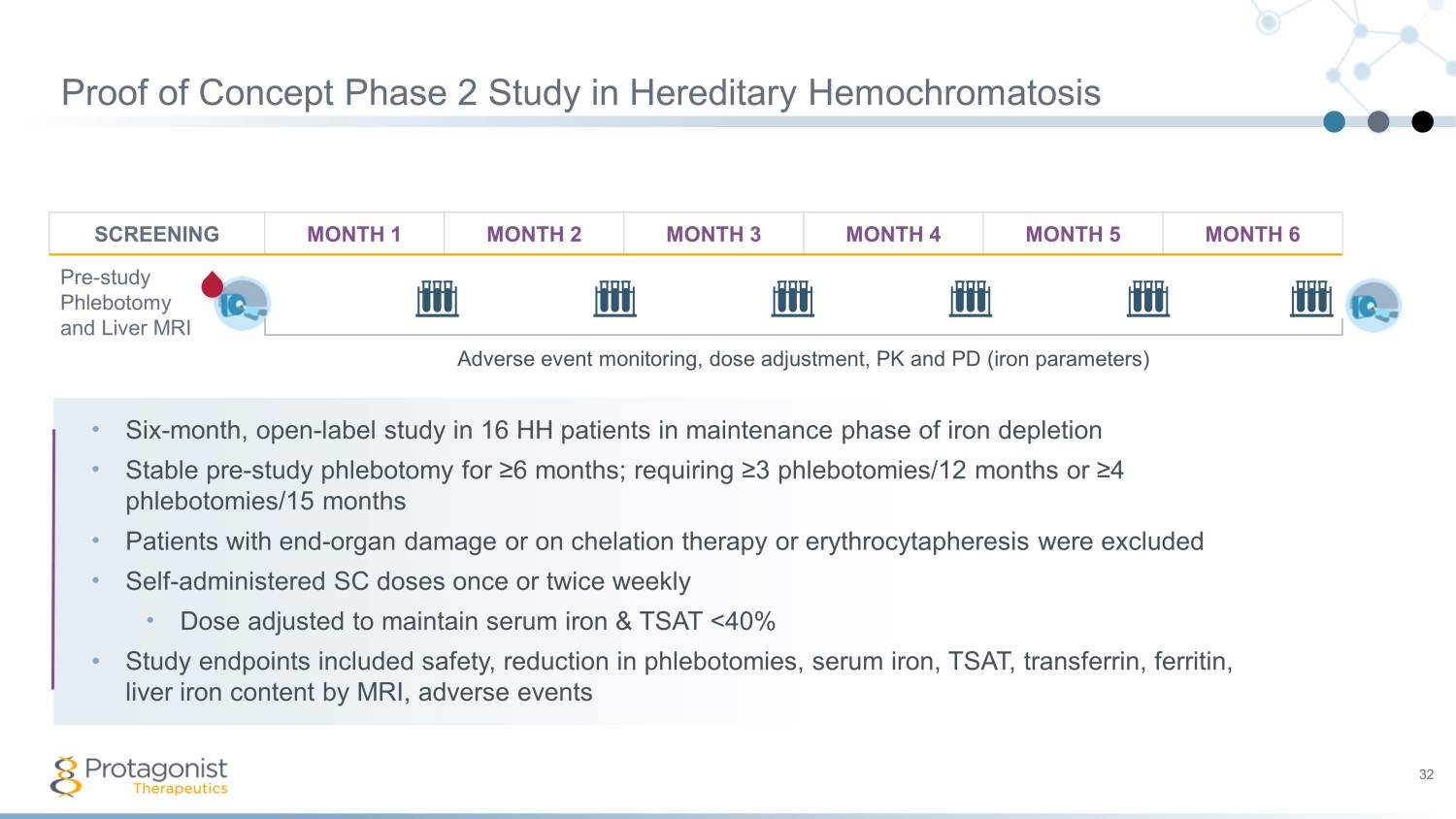
Proof of Concept Phase 2 Study in Hereditary Hemochromatosis 32 SCREENING MONTH 1 MONTH 2 MONTH 3 MONTH 4 MONTH 5 MONTH 6 Pre - study Phlebotomy and Liver MRI • Six - month, open - label study in 16 HH patients in maintenance phase of iron depletion • Stable pre - study phlebotomy for ≥6 months; requiring ≥3 phlebotomies/12 months or ≥4 phlebotomies/15 months • P atients with end - organ damage or on chelation therapy or erythrocytapheresis were excluded • Self - administered SC doses once or twice weekly • Dose adjusted to maintain serum iron & TSAT <40% • Study endpoints included safety, reduction in phlebotomies, serum iron, TSAT, transferrin, ferritin, liver i ron c ontent by MRI, adverse e vents Adverse event monitoring, dose adjustment, PK and PD (iron parameters)
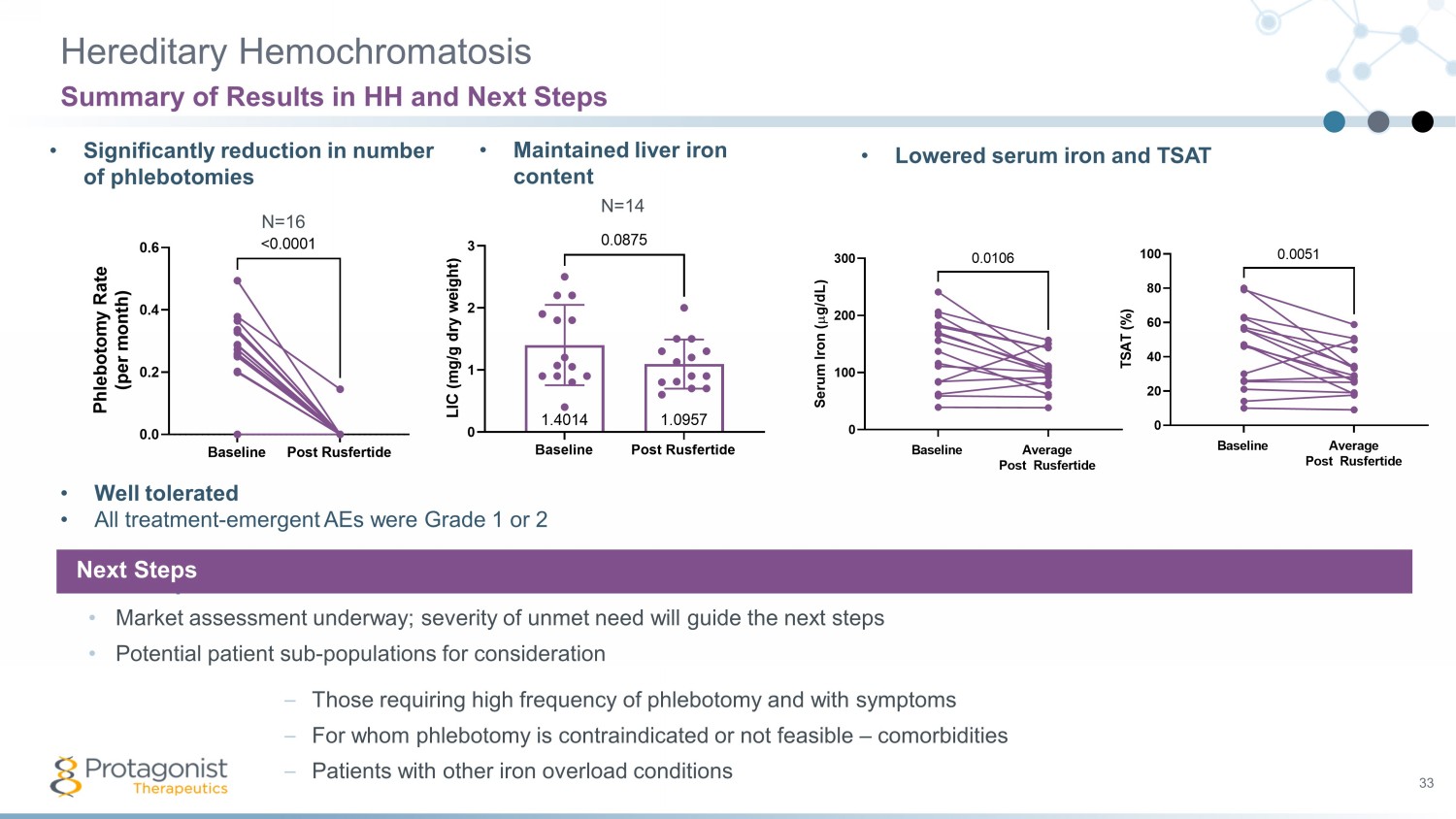
Hereditary Hemochromatosis 33 Summary of Results in HH and Next Steps • Significantly reduction in number of phlebotomies • Maintained liver iron content • Lowered serum iron and TSAT • Well tolerated • All treatment - emergent AEs were Grade 1 or 2 Next Steps • Market assessment underway; severity of unmet need will guide the next steps • Potential patient sub - populations for consideration – Those requiring high frequency of phlebotomy and with symptoms – For whom phlebotomy is contraindicated or not feasible – comorbidities – Patients with other iron overload conditions Next Steps N=16 Baseline Post Rusfertide 0.0 0.2 0.4 0.6 P h l e b o t o m y R a t e ( p e r m o n t h ) <0.0001 N=14 Baseline Post Rusfertide 0 1 2 3 1.09571.4014 L I C ( m g / g d r y w e i g h t ) 0.0875

PN - 943 and IL - 23 Receptor Antagonists Oral Targeted Investigational Therapies for IBD and non - IBD indications 34
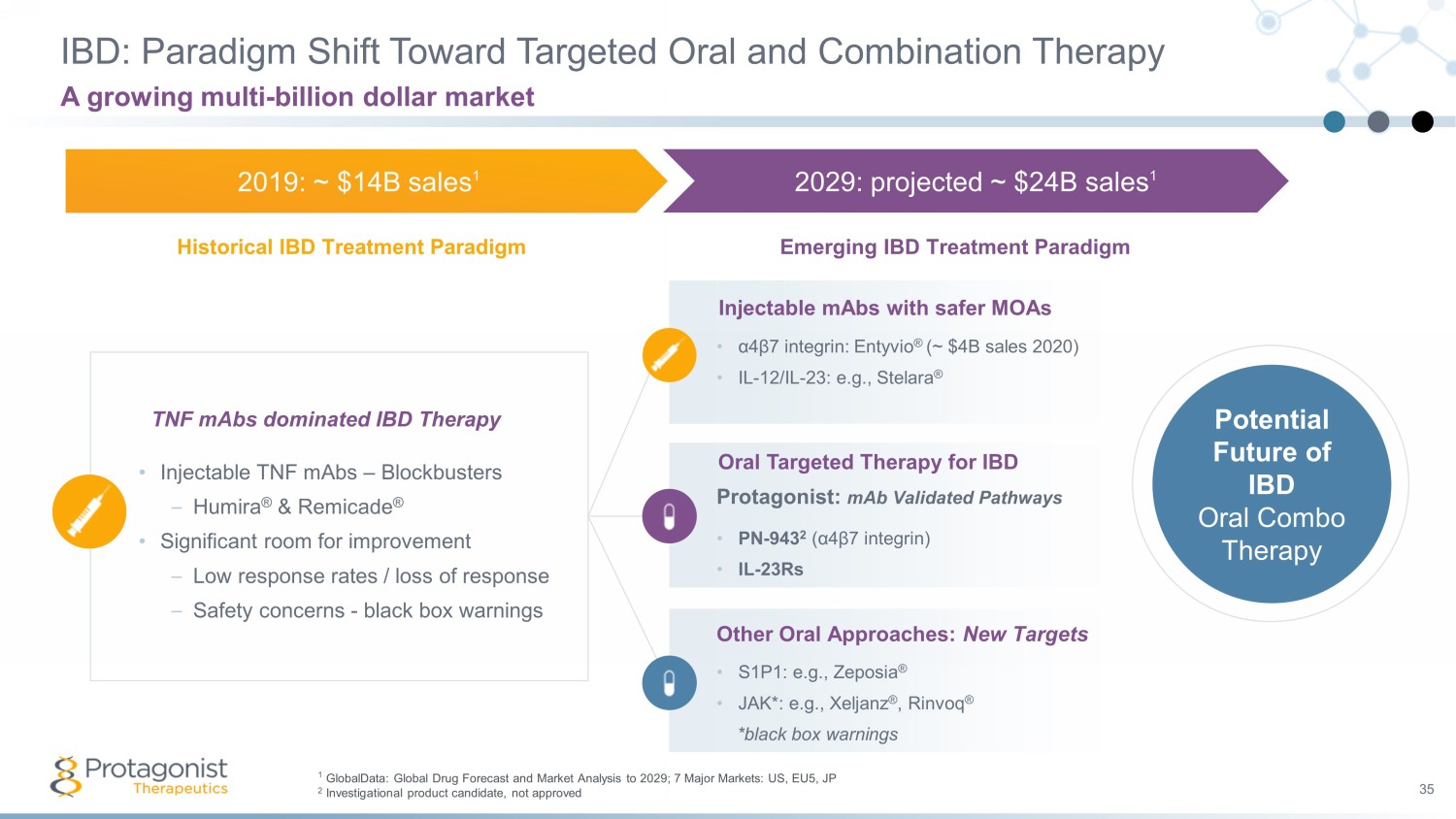
IBD: Paradigm Shift Toward Targeted Oral and Combination Therapy 35 A growing multi - billion dollar market • Injectable TNF mAbs – Blockbusters – Humira ® & Remicade ® • Significant room for improvement – Low response rates / loss of response – Safety concerns - black box warnings TNF mAbs dominated IBD Therapy 2019: ~ $14B sales 1 2029: projected ~ $24B sales 1 Historical IBD Treatment Paradigm Emerging IBD Treatment Paradigm • α 4 β 7 integrin: Entyvio ® (~ $4B sales 2020) • IL - 12/IL - 23: e.g., Stelara ® Injectable mAbs with safer MOAs Protagonist: mAb Validated Pathways • PN - 943 2 ( α 4 β 7 integrin) • IL - 23Rs Oral Targeted Therapy for IBD • S1P1: e.g., Zeposia ® • JAK*: e.g., Xeljanz ® , Rinvoq ® *black box warnings Other Oral Approaches: New Targets Potential Future of IBD Oral Combo Therapy 1 GlobalData: Global Drug Forecast and Market Analysis to 2029; 7 Major Markets: US, EU5, JP 2 Investigational product candidate, not approved

Oral, Gut - Restricted, a 4 b 7 - Integrin Peptide Antagonists: PN - 943 36 Fully Owned and Validated Asset and Approach Vs. Briskin M, et al Am J Pathol. 1997;151:97 - 110 Clinically Validated, IBD Specific Target • T cell homing regulated by α4β7 integrin and MAdCAM - 1 interaction • MAdCAM - 1 expressed only in GI vasculature • Entyvio (Vedolizumab) approved for Crohn’s & UC – ~$5B fiscal 2021 sales • Superior efficacy for Entyvio vs. Humira in 52 wk Ph3B VARSITY study PN - 943: Validated, Gut - restricted Approach • First - in - class potential as an oral, GI - restricted α4β7 - specific antagonist • PN - 943 is ~3x more potent in numerous pre - clinical studies & Ph1 NHV study vs 1 st generation candidate PTG - 100 (DDW, 2019) 1 – PTG - 100 showed signals of clinical efficacy in Ph2a UC trial (Gastroenterology , 2021 ) 2 • PN - 943 global Ph2 150 patient study in UC patients in progress • Topline data readout anticipated by mid - May 2022 1 Mattheakis, L., Tang, T., Venkataraman, S., Rao, N., Wang, L., Zhao, L., ... & Liu, D. Y. (2019). The oral α4β7 integrin specific antagonist PN - 10943 is more effective than PTG - 100 in multiple preclinical studies. Gastroenterology , 156 (Suppl 6), S80 - S81. 2 Sandborn, W. J., Mattheakis, L. C., Modi, N. B., Pugatch , D., Bressler, B., Lee, S., ... & Gupta, S. (2021). PTG - 100, an oral α4β7 antagonist peptide: preclinical development and phase 1 and 2a studies in ulcerative colitis. Gastroenterology , 161 (6), 1853 - 1864.
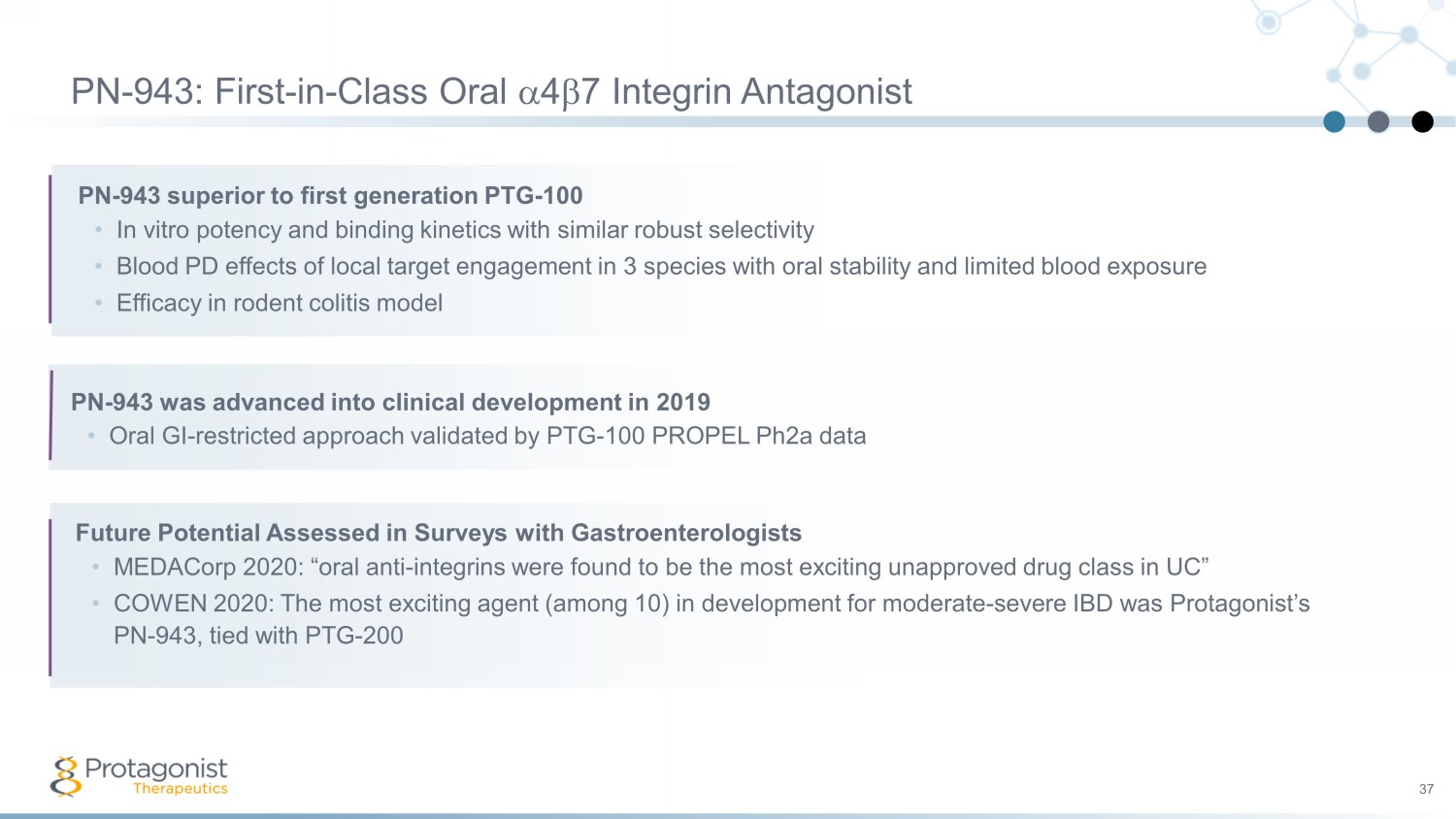
37 PN - 943 superior to first generation PTG - 100 • In vitro potency and binding kinetics with similar robust selectivity • Blood PD effects of local target engagement in 3 species with oral stability and limited blood exposure • Efficacy in rodent colitis model PN - 943: First - in - Class Oral a 4 b 7 Integrin Antagonist PN - 943 was advanced into clinical development in 2019 • Oral GI - restricted approach validated by PTG - 100 PROPEL Ph2a data Future Potential Assessed in Surveys with Gastroenterologists • MEDACorp 2020: “oral anti - integrins were found to be the most exciting unapproved drug class in UC” • COWEN 2020: The most exciting agent (among 10) in development for moderate - severe IBD was Protagonist’s PN - 943, tied with PTG - 200

PN - 943 vs. PTG - 100: Blood %RO based Clinical Proof - of - Concept Ph1 NHV Single Ascending Dose Study Ph2A Study UC PTG - 100 900 mg Histologic Remission 44% (7/16) Clinical Remission* 16% (3/19) *based on blinded endoscopic re - reads * Clinical remission: SFS ≤1, RBS = 0, ESS ≤1 Established 74% blood RO in healthy subjects as a translational benchmark Ph1 SAD 100 mg 300 mg 1000 mg 0 20 40 60 80 100 M a x i m u m % R O 83% 74% 94% 74% Ph1 MAD, Day 14 PN - 943 vs. PTG - 100 Ph1 NHV study • Higher effect on blood %RO confirms ~3x superiority of PN - 943 vs. PTG - 100 – PN - 943 300mg blood %RO > PTG - 100 1000 mg blood %RO effect • Saturable target engagement at 1000 mg QD dose Ph 1 Study NHVs PTG - 100 1000 mg % Blood RO 74% PTG - 100 Ph2 UC PoC PTG - 100 PN - 943 PN - 943 vs PTG - 100 Ph1 Data 38 Modi, N. B., Cheng, X., Mattheakis, L., Hwang, C. C., Nawabi , R., Liu, D., & Gupta, S. (2021). Single - and Multiple - Dose Pharmacokinetics and Pharmacodynamics of PN - 943, a Gastrointestinal - Restricted Oral Peptide Antagonist of α4β7, in Healthy Volunteers. Clinical pharmacology in drug development .
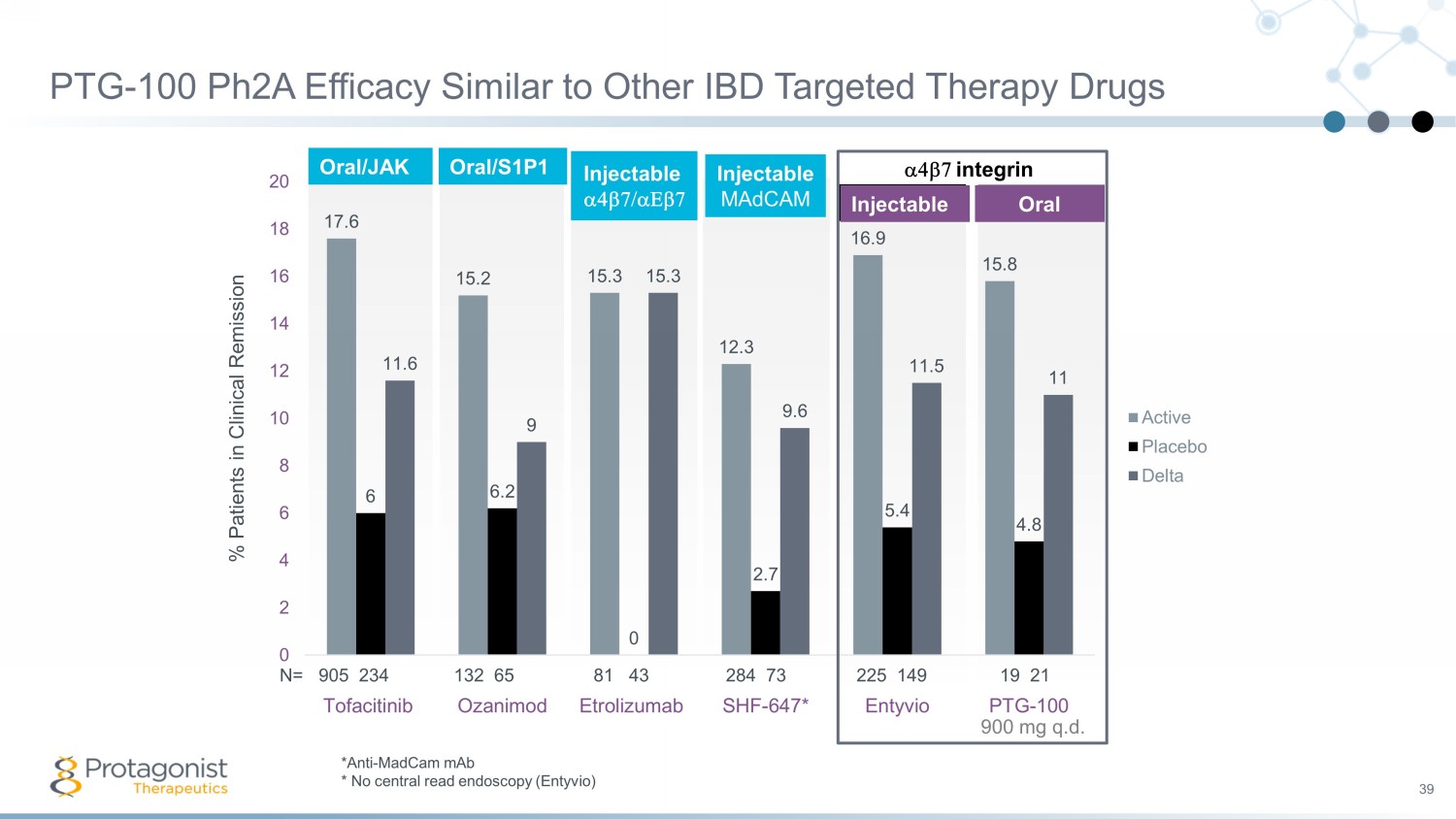
PTG - 100 Ph2A Efficacy Similar to Other IBD Targeted Therapy Drugs 39 Oral/S1P1 17.6 15.2 15.3 12.3 16.9 15.8 6 6.2 0 2.7 5.4 4.8 11.6 9 15.3 9.6 11.5 11 0 2 4 6 8 10 12 14 16 18 20 Tofacitinib Ozanimod Etrolizumab SHF-647* Entyvio PTG-100 % Patients in Clinical Remission Active Placebo Delta a4b7 integrin N= 905 234 132 65 81 43 284 73 225 149 19 21 Injectable Oral Injectable MAdCAM 900 mg q.d . Oral/JAK Injectable a4b7/aEb7 *Anti - MadCam mAb * No central read endoscopy ( Entyvio )
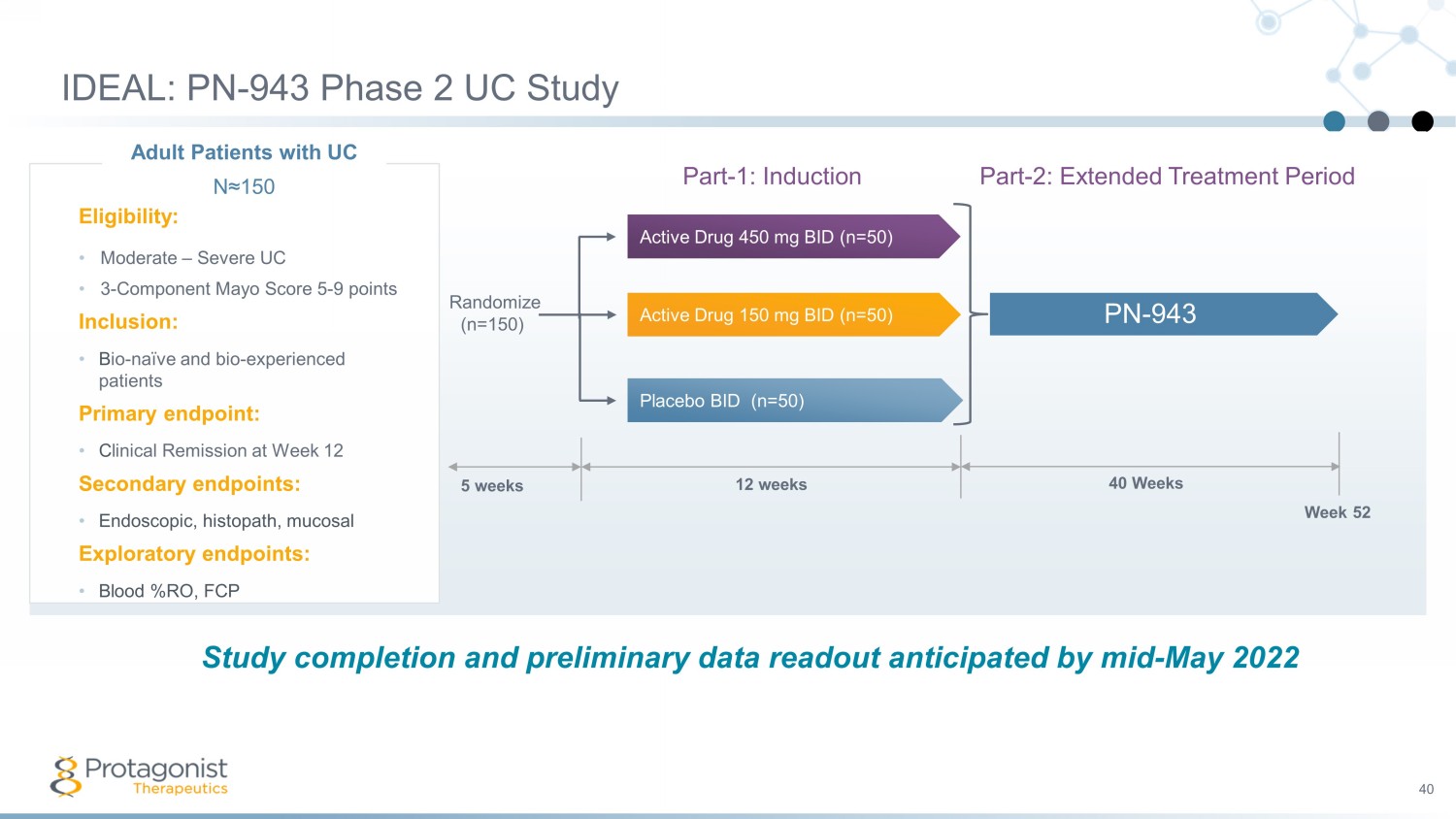
IDEAL: PN - 943 Phase 2 UC Study 40 Part - 1: Induction Part - 2: Extended Treatment Period 5 weeks 12 weeks 40 Weeks Week 52 Randomize (n=150) Placebo BID (n=50) Active Drug 450 mg BID (n=50) Active Drug 150 mg BID (n=50) PN - 943 Adult Patients with UC N≈150 Eligibility: • Moderate – Severe UC • 3 - Component Mayo Score 5 - 9 points Inclusion: • B io - naïve and bio - experienced patients Primary endpoint: • C linical Remission at Week 12 Secondary endpoints: • Endoscopic, histopath , mucosal Exploratory endpoints: • Blood %RO, FCP Study completion and preliminary data readout anticipated by mid - May 2022
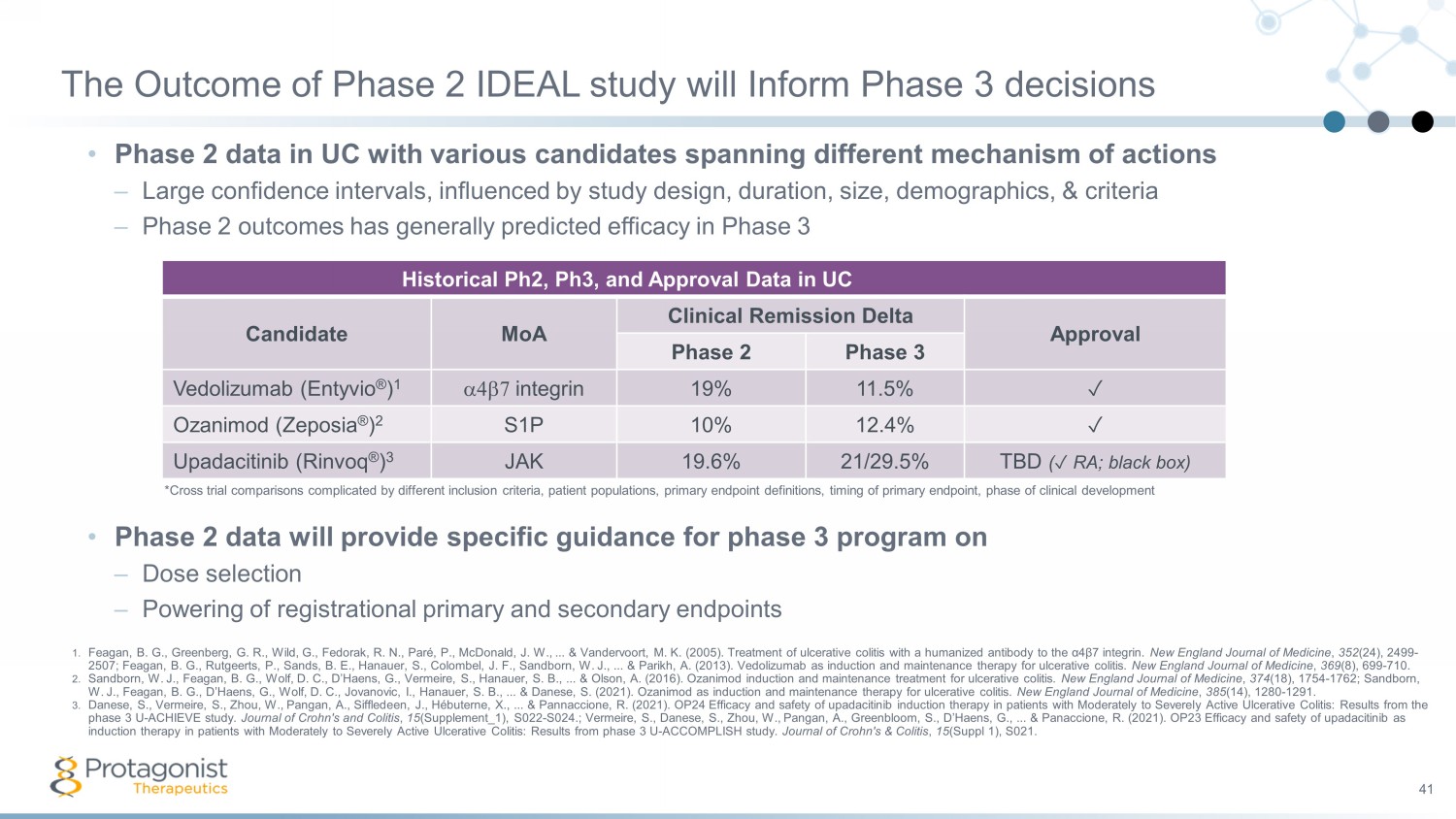
The Outcome of Phase 2 IDEAL study will Inform Phase 3 decisions • Phase 2 data in UC with various candidates spanning different mechanism of actions – Large confidence intervals, influenced by study design, duration, size, demographics, & criteria – Phase 2 outcomes has generally predicted efficacy in Phase 3 41 Translating H Historical Ph2, Ph3, and Approval Data in UC 2 ➞ Phase 3 ➞ Approval* Candidate MoA Clinical Remission Delta Approval Phase 2 Phase 3 Vedolizumab (Entyvio ® ) 1 a4b7 integrin 19% 11.5% ✓ Ozanimod ( Zeposia ® ) 2 S1P 10% 12.4% ✓ Upadacitinib ( Rinvoq ® ) 3 JAK 19.6% 21/29.5% TBD ( ✓ RA; black box) 1. Feagan , B. G., Greenberg, G. R., Wild, G., Fedorak , R. N., Paré , P., McDonald, J. W., ... & Vandervoort , M. K. (2005). Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. New England Journal of Medicine , 352 (24), 2499 - 2507; Feagan , B. G., Rutgeerts , P., Sands, B. E., Hanauer , S., Colombel , J. F., Sandborn , W. J., ... & Parikh, A. (2013). Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine , 369 (8), 699 - 710. 2. Sandborn , W. J., Feagan , B. G., Wolf, D. C., D’Haens , G., Vermeire , S., Hanauer , S. B., ... & Olson, A. (2016). Ozanimod induction and maintenance treatment for ulcerative colitis. New England Journal of Medicine , 374 (18), 1754 - 1762; Sandborn , W. J., Feagan , B. G., D’Haens , G., Wolf, D. C., Jovanovic, I., Hanauer , S. B., ... & Danese , S. (2021). Ozanimod as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine , 385 (14), 1280 - 1291. 3. Danese , S., Vermeire , S., Zhou, W., Pangan , A., Siffledeen , J., Hébuterne , X., ... & Pannaccione , R. (2021). OP24 Efficacy and safety of upadacitinib induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from the phase 3 U - ACHIEVE study. Journal of Crohn's and Colitis , 15 (Supplement_1), S022 - S024. ; Vermeire , S., Danese , S., Zhou, W., Pangan , A., Greenbloom , S., D’Haens , G., ... & Panaccione , R. (2021). OP23 Efficacy and safety of upadacitinib as induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from phase 3 U - ACCOMPLISH study. Journal of Crohn's & Colitis , 15 (Suppl 1), S021. • Phase 2 data will provide specific guidance for phase 3 program on – Dose selection – Powering of registrational primary and secondary endpoints *Cross trial comparisons complicated by different inclusion criteria, patient populations, primary endpoint definitions, timi ng of primary endpoint, phase of clinical development
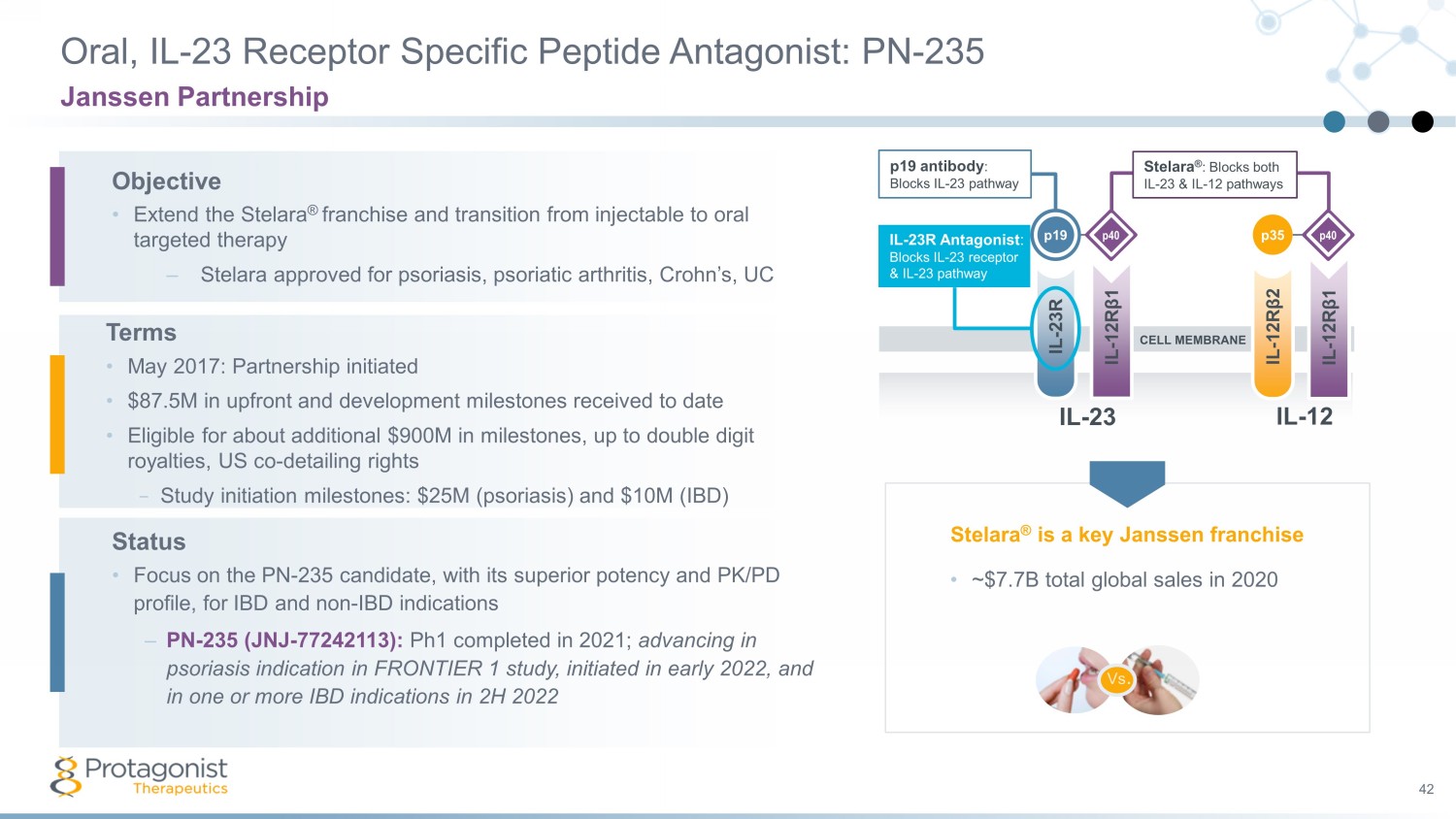
Oral, IL - 23 Receptor Specific Peptide Antagonist: PN - 235 42 Janssen Partnership IL - 23R IL - 12R β 1 IL - 12R β 2 IL - 12R β 1 Stelara ® : Blocks both IL - 23 & IL - 12 pathways IL - 23R Antagonist : Blocks IL - 23 receptor & IL - 23 pathway p19 antibody : Blocks IL - 23 pathway p40 p40 p19 p35 CELL MEMBRANE IL - 23 IL - 12 Objective • Extend the Stelara ® franchise and transition from injectable to oral targeted therapy – Stelara approved for psoriasis, psoriatic arthritis, Crohn’s, UC Terms • May 2017: Partnership initiated • $87.5M in upfront and development milestones received to date • Eligible for about additional $900M in milestones, up to double digit royalties, US co - detailing rights − Study initiation milestones: $25M (psoriasis) and $10M (IBD) Status • Focus on the PN - 235 candidate, with its superior potency and PK/PD profile, for IBD and non - IBD indications – PN - 235 (JNJ - 77242113): Ph1 completed in 2021; advancing in psoriasis indication in FRONTIER 1 study, initiated in early 2022, and in one or more IBD indications in 2H 2022 Vs . • ~$7.7B total global sales in 2020 Stelara ® is a key Janssen franchise

Janssen FRONTIER 1 Phase 2b Plaque Psoriasis ( PsO ) Study 43 PN - 943 Adult Patients with PP N≈240 Eligibility: • Moderate – Severe PP Inclusion: • BSA > 10% • PASI > 12 Primary endpoint: • PASI > 75 at Week 16 Screening Treatment Safety Follow - up (4 Weeks) (Up to 4 Weeks)* (Weeks 0 - 16) Dose 1 QD Safety Follow - up Dose 2 QD Dose 3 QD Dose 1 BID Dose 3 BID Placebo Week 0 Randomize Week 16 Primary Endpoint Week 156 End of Treatment Randomize Qualified for $25M milestone payment when third patient was dosed

44 44 PROTAGONIST THERAPEUTICS Protagonist Team and Financials

Protagonist Team 45 Experience & Expertise in Drug Discovery, Clinical Development, and Commercialization Dinesh Patel, PhD President & CEO David Liu, PhD Chief R&D Strategy Officer Samuel Saks, MD Clinical Development Advisor Suneel Gupta, PhD Chief Development Officer Asif Ali (announced) Chief Financial Officer Tracy Woody EVP, Commercial Strategy Matthew Gosling, JD EVP, General Counsel Mohammad Masjedizadeh, PhD EVP, Chief Technical Officer Scott Plevy , MD EVP & Therapeutic Head, Gastroenterology Ashok Bhandari, PhD EVP, Chief Drug Discovery & Preclinical Development Officer Paula O’Connor, MD S VP, Clinical Development Abha Bommireddi, MS SVP, Program Management Carter King, MBA SVP, Business Development Nishit Modi. PhD, MBA SVP, Clinical Pharmacology Sarita Khanna, PhD SVP, Biometrics
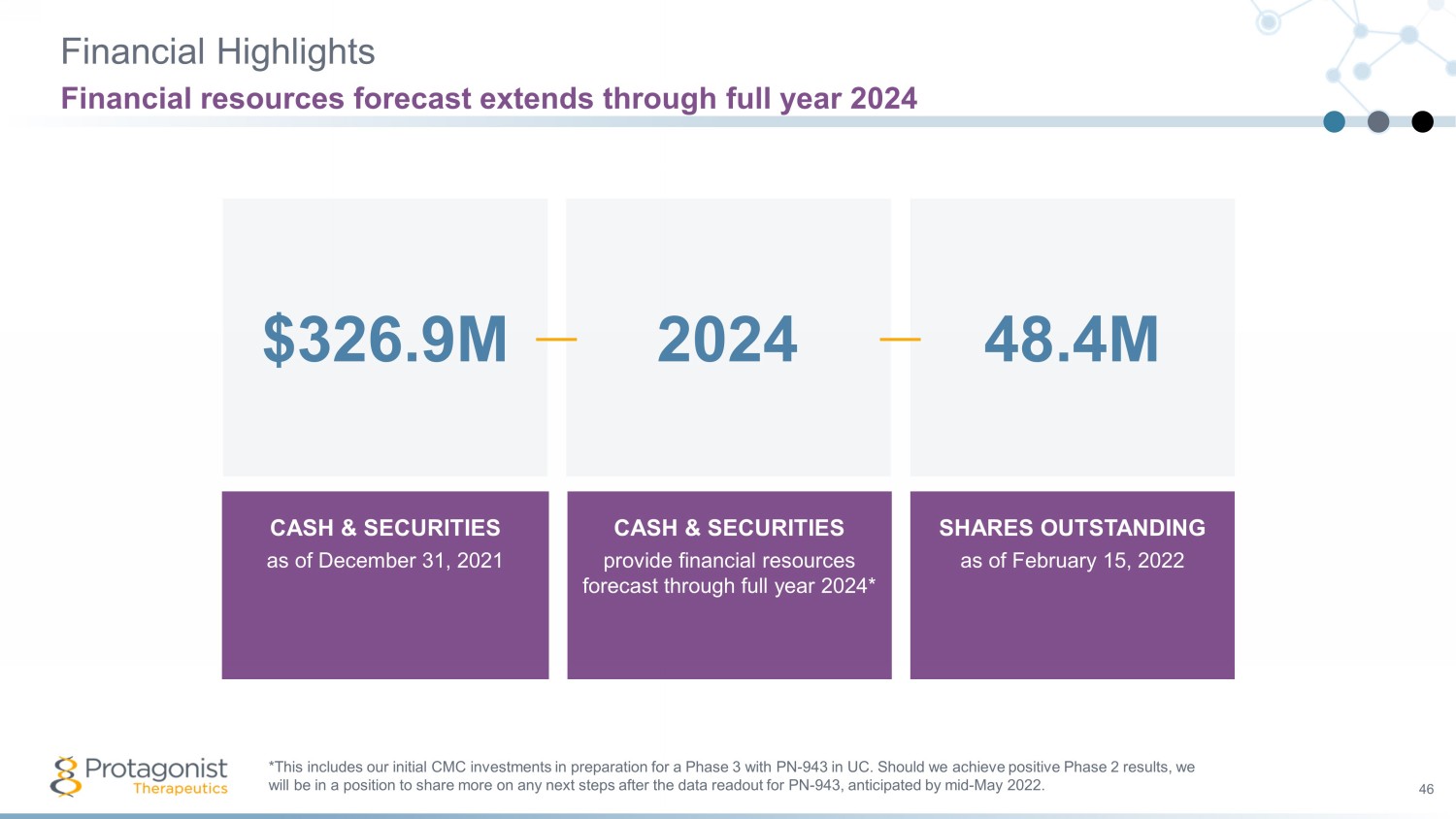
Financial Highlights 46 Financial resources forecast extends through full year 2024 CASH & SECURITIES a s of December 31, 2021 CASH & SECURITIES provide financial resources forecast through full year 2024* SHARES OUTSTANDING as of February 15, 2022 2024 48.4M $326.9M *This includes our initial CMC investments in preparation for a Phase 3 with PN - 943 in UC. Should we achieve positive Phase 2 re sults, we will be in a position to share more on any next steps after the data readout for PN - 943, anticipated by mid - May 2022.

47 Products Anticipated Events of 2022 2023 Q1 Q2 Q3 Q4 Rusfertide • VERIFY: Ph3 250 patient PV study initiation • REVIVE: Ph2 PV study (300 - 04) continuation • Ph3 enrollment completion (1H) • Ph2 randomization results (Q1) • 2 nd indication: HH market opportunity and next steps PN - 943 • Ph3 UC study initiation* * assuming positive Ph2 data PN - 235 • Ph2 initiation – Plaque psoriasis • $25M milestone • Ph2 initiation in IBD (2H) • $10M milestone • Ph2 plaque psoriasis study results Discovery Pre - Clinical • Nomination of oral hepcidin mimetic candidate • Nomination of new development candidate – new target for new indication(s) Upcoming Catalysts in 2022 and Beyond Significant opportunities to unlock and capture value in the 12 - 24 months ahead ASH EHA 1 2 3 4 5 6 7 8 • IDEAL: Ph2 150 patient UC study topline results ( first half of Q2 )

Thank you 48















































