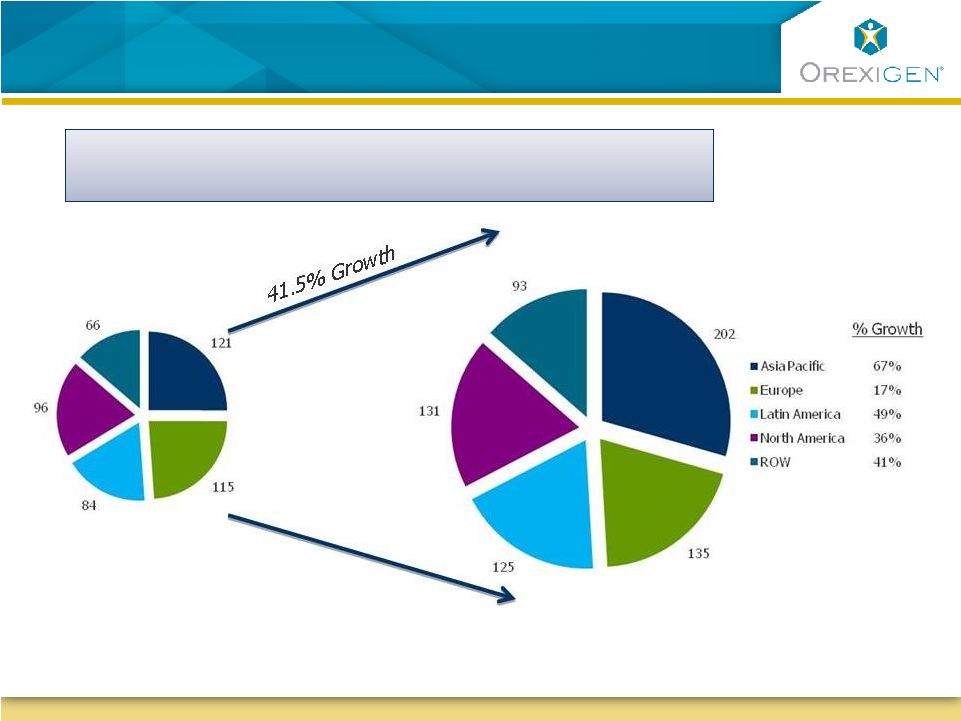
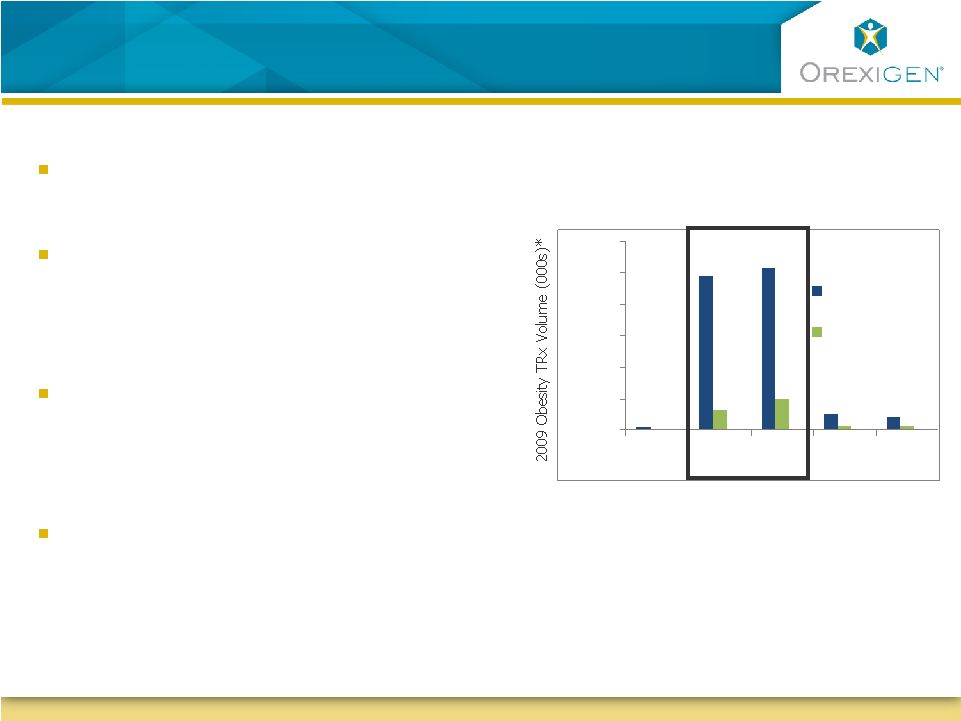
2 Forward-Looking Statements Forward-Looking Statements This presentation contains forward-looking statements about Orexigen Therapeutics, Inc. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed,“ “probability” and similar expressions are intended to identify forward-looking statements. These statements are based on the Company's current beliefs and expectations. These forward-looking statements include statements regarding: a clear and feasible path forward for Contrave ®; the protocol for, and the timing and feasibility of, the Contrave cardiovascular outcomes trial (CVOT); the initiation of enrollment for the CVOT in second quarter of 2012; the expected rate of enrollment of the CVOT; the probability of success of the CVOT; the potential for, and timing of, resubmission and approval of an NDA based on interim results of the CVOT; the prospects for ultimate approval of an NDA for Contrave; the potential to maintain the Company’s existing North American collaboration with Takeda Pharmaceuticals; estimates of the potential market for Contrave; and the sufficiency of the Company’s existing cash to fund the Company’s operations through potential approval of Contrave in 2014. The inclusion of forward-looking statements should not be regarded as a representation by Orexigen that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in Orexigen’s business, including, without limitation: the uncertainty of the FDA approval process, including requirements for additional clinical and non-clinical studies or other commitments prior to the submission and approval of an NDA for Contrave; Orexigen's ability to demonstrate that the risk of major adverse cardiovascular events in overweight and obese subjects treated with Contrave does not adversely affect the product candidate’s benefit-risk profile; the potential for FDA’s planned 2012 public advisory committee meeting on obesity drug development to result in additional NDA approval requirements for Contrave as well as post-approval commitments; Orexigen’s dependence on Takeda Pharmaceuticals for aspects of the development and commercialization of Contrave; reliance on third parties to manufacture and supply Contrave and assist with the conduct of the CVOT; the potential for adverse safety findings relating to Contrave; intense competition in the obesity marketplace and the potential for new products to emerge that provide different or better therapeutic alternatives for obesity and weight loss compared to Contrave; and other risks described in the Company's filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Orexigen undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. Further information regarding these and other risks is included under the heading "Risk Factors" in Orexigen’s Quarterly Report on Form 10-Q, which was filed with the SEC on November 10, 2011 and is available from the SEC's website (www.sec.gov) and on the Company’s website (www.orexigen.com) under the heading "Investor Relations”. All forward-looking statements are qualified in their entirety by this cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. |