Exhibit 99.1

NASDAQ CNAT Company Update Striving to improve human health April 2, 2019

2 April 2, 2019 | Company Update Forward - looking Statements This presentation contains forward - looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products are forward - looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. These known risks and uncertainties are described in detail in our filings made with the Securities and Exchange Commission from time to time. Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as predictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements. All forward - looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof.

3 April 2, 2019 | Company Update Conatus at a Glance Lead in - licensed compound emricasan has the potential to modify liver disease outcome Novartis partnership fully funds any further emricasan development Lead internally developed compound CTS - 2090 targeted for clinical testing 1H20 Capital at least sufficient to fund emricasan commitments and CTS - 2090 IND - enabling studies Two ongoing Phase 2b NASH cirrhosis trials reading out in mid - 2019
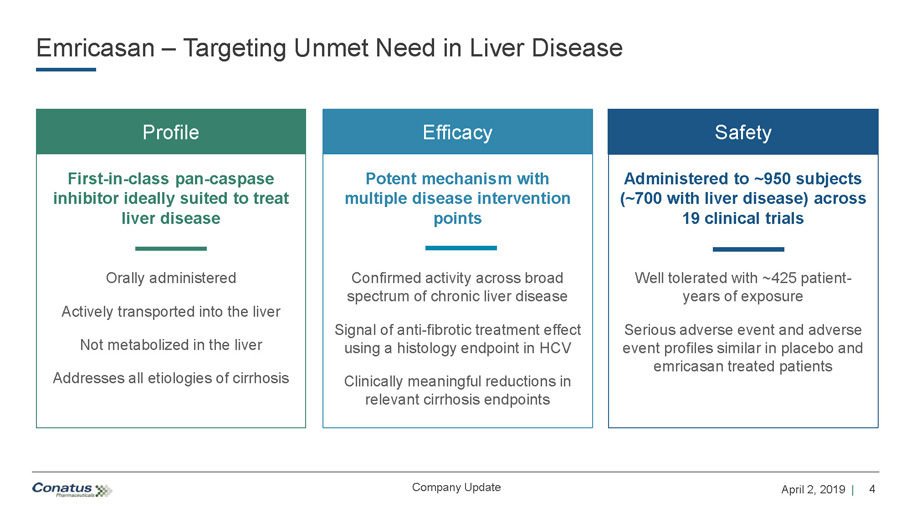
4 April 2, 2019 | Company Update Emricasan – Targeting Unmet Need in Liver Disease Profile Orally administered Actively transported into the liver Not metabolized in the liver Addresses all etiologies of cirrhosis First - in - class pan - caspase inhibitor ideally suited to treat liver disease Efficacy Potent mechanism with multiple disease intervention points Confirmed activity across broad spectrum of chronic liver disease Signal of anti - fibrotic treatment effect using a histology endpoint in HCV Clinically meaningful reductions in relevant cirrhosis endpoints Safety Administered to ~950 subjects (~700 with liver disease) across 19 clinical trials Well tolerated with ~425 patient - years of exposure Serious adverse event and adverse event profiles similar in placebo and emricasan treated patients

5 April 2, 2019 | Company Update Phase 2b ENCORE - PH Trial Extension 24 - week treatment/placebo continuation Following for liver function and clinical outcomes In NASH Cirrhosis Patients with Severe Portal Hypertension EmricasaN , a Caspase inhibitOR , for Evaluation in Portal Hypertension Top - line HVPG results reported 4Q18 Extension results expected mid - 19 Design ~240 patients at ~70 sites across US and EU. Randomized 1:1:1:1 to receive placebo or emricasan at 5 mg, 25 mg, or 50 mg BID NASH cirrhosis and severe portal hypertension (HVPG ≥12 mmHg) Double - blind, placebo - controlled Primary Endpoint Change from baseline to Week 24 in mean HVPG in each dosing group compared with placebo Strong basis for HVPG as a surrogate endpoint in patients with severe portal hypertension
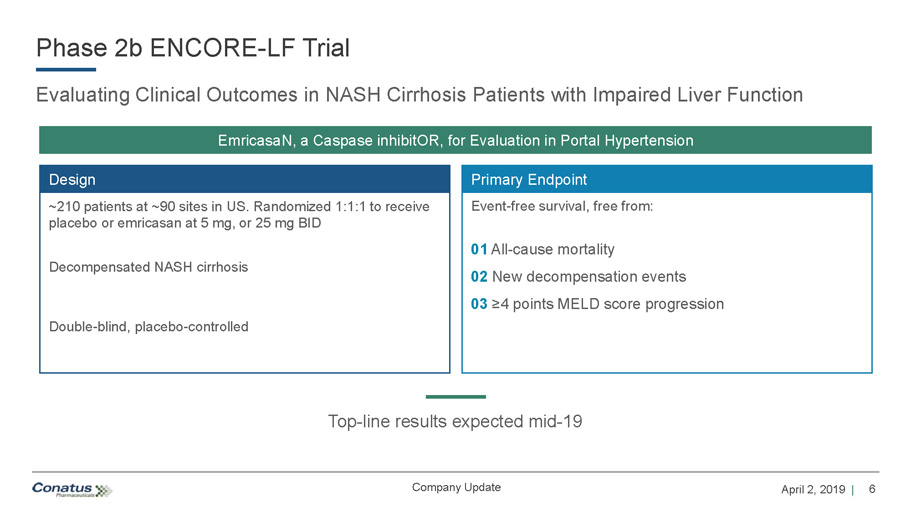
6 April 2, 2019 | Company Update Phase 2b ENCORE - LF Trial Evaluating Clinical Outcomes in NASH Cirrhosis Patients with Impaired Liver Function Primary Endpoint Event - free survival, free from: 01 All - cause mortality 02 New decompensation events 03 ≥4 points MELD score progression Top - line results expected mid - 19 Design ~210 patients at ~90 sites in US. Randomized 1:1:1 to receive placebo or emricasan at 5 mg, or 25 mg BID Decompensated NASH cirrhosis Double - blind, placebo - controlled EmricasaN , a Caspase inhibitOR , for Evaluation in Portal Hypertension

7 April 2, 2019 | Company Update Phase 2b Trials Define Distinct Paths to NASH Cirrhosis Market Ongoing P2b Trial Endpoint Clinical Benefit Market ENCORE - PH Extension Compensated or early decompensated cirrhosis with severe portal hypertension Liver Function Clinical Outcomes Improve portal hypertension and improve liver function Cirrhosis w/ Severe PH ENCORE - LF Decompensated cirrhosis Event - free Survival Prevent decompensation event and improve liver function Decompensated Cirrhosis Potential Accelerated and Regular Approval Opportunities
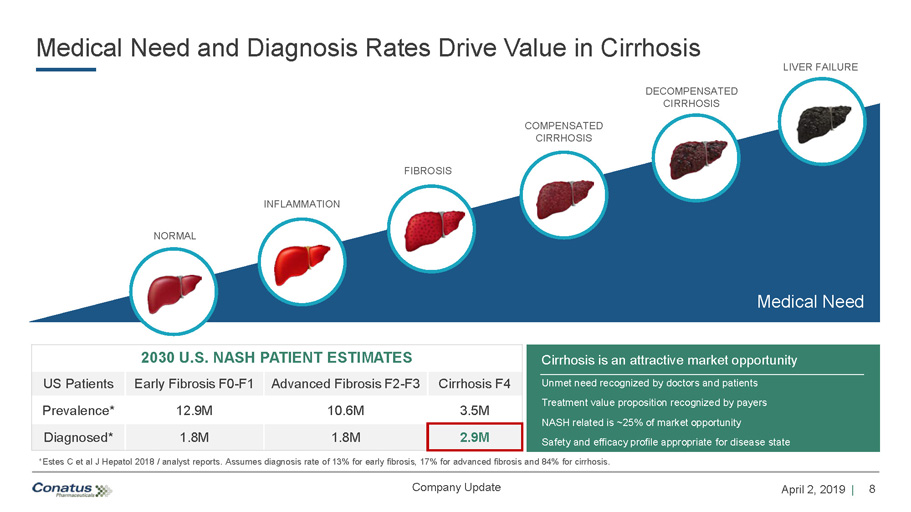
8 April 2, 2019 | Company Update Medical Need and Diagnosis Rates Drive Value in Cirrhosis *Estes C et al J Hepatol 2018 / analyst reports. Assumes diagnosis rate of 13% for early fibrosis, 17% for advanced fibrosis and 84% for cirrhosis. 2030 U.S. NASH PATIENT ESTIMATES US Patients Early Fibrosis F0 - F1 Advanced Fibrosis F2 - F3 Cirrhosis F4 Prevalence* 12.9M 10.6M 3.5M Diagnosed* 1.8M 1.8M 2.9M Cirrhosis is an attractive market opportunity Unmet need recognized by doctors and patients Treatment value proposition recognized by payers NASH related is ~25% of market opportunity Safety and efficacy profile appropriate for disease state NORMAL INFLAMMATION FIBROSIS COMPENSATED CIRRHOSIS DECOMPENSATED CIRRHOSIS Medical Need LIVER FAILURE

CTS - 2090 Inflammasome Diseases

10 April 2, 2019 | Company Update IL - 1 β Blocking Agents – Set the Stage for Next Generation Products • Multiple approved biologic agents that block IL - 1 β – Canakinumab ( Ilaris ® ) – Anakinra (Kineret ® ) – Rilonacept ( Arcalyst ® ) • Global sales of over $700 million in 2018 • Approved rare disease indications include Cryopyrin - Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome, Muckle - Wells, Twin Anemia Polycythemia Sequence, Hyper - IgD Syndrome, Familial Mediterranean Fever

11 April 2, 2019 | Company Update IL - 1 b - Driven by Multiple Inflammasomes Solberger G, et al. Innate Immun. 2014, p115 - 125.
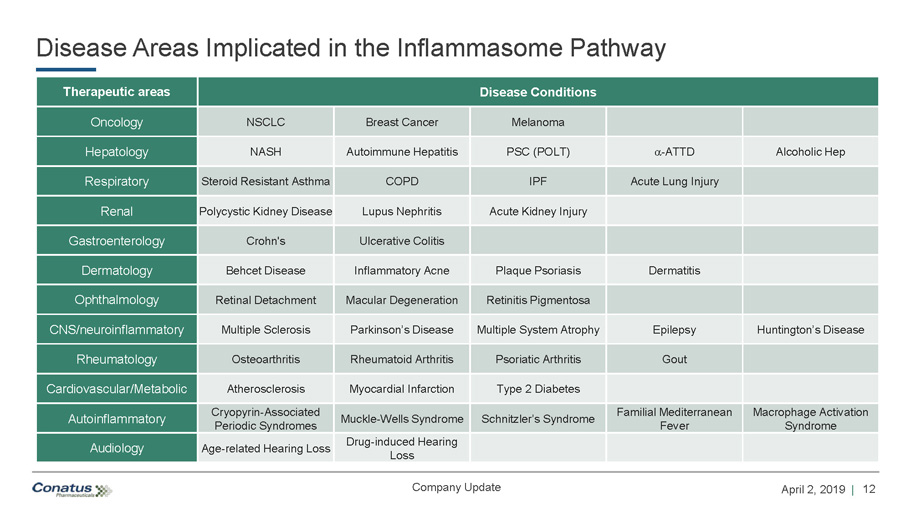
12 April 2, 2019 | Company Update Disease Areas Implicated in the Inflammasome Pathway Therapeutic areas Disease Conditions Oncology NSCLC Breast Cancer Melanoma Hepatology NASH Autoimmune Hepatitis PSC (POLT) a - ATTD Alcoholic Hep Respiratory Steroid Resistant Asthma COPD IPF Acute Lung Injury Renal Polycystic Kidney Disease Lupus Nephritis Acute Kidney Injury Gastroenterology Crohn's Ulcerative Colitis Dermatology Behcet Disease Inflammatory Acne Plaque Psoriasis Dermatitis Ophthalmology Retinal Detachment Macular Degeneration Retinitis Pigmentosa CNS/neuroinflammatory Multiple Sclerosis Parkinson’s Disease Multiple System Atrophy Epilepsy Huntington’s Disease Rheumatology Osteoarthritis Rheumatoid Arthritis Psoriatic Arthritis Gout Cardiovascular/Metabolic Atherosclerosis Myocardial Infarction Type 2 Diabetes Autoinflammatory Cryopyrin - Associated Periodic Syndromes Muckle - Wells Syndrome Schnitzler’s Syndrome Familial Mediterranean Fever Macrophage Activation Syndrome Audiology Age - related Hearing Loss Drug - induced Hearing Loss
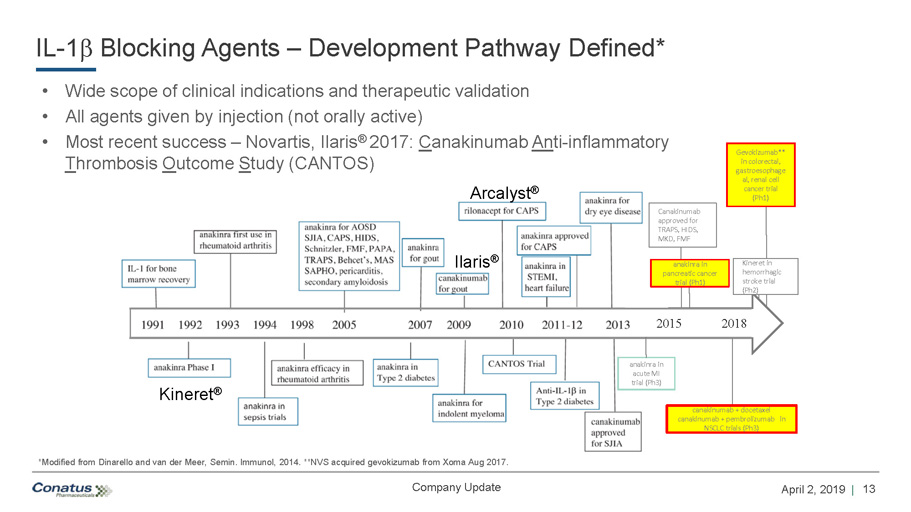
13 April 2, 2019 | Company Update IL - 1 b Blocking Agents – Development Pathway Defined* • Wide scope of clinical indications and therapeutic validation • All agents given by injection (not orally active) • Most recent success – Novartis, Ilaris ® 2017: C anakinumab An ti - inflammatory T hrombosis O utcome S tudy (CANTOS) Confidential Kineret ® Ilaris ® Arcalyst ® 2015 2018 anakinra in pancreatic cancer trial (Ph1) canakinumab + docetaxel canakinumab + pembrolizumab in NSCLC trials (Ph3) Canakinumab approved for TRAPS, HIDS, MKD, FMF anakinra in acute MI trial (Ph3) Gevokizumab** in colorectal, gastroesophage al, renal cell cancer trial (Ph1) Kineret in hemorrhagic stroke trial (Ph2) *Modified from Dinarello and van der Meer, Semin . Immunol, 2014. **NVS acquired gevokizumab from Xoma Aug 2017.

14 April 2, 2019 | Company Update The Future of IL - 1 β Blocking May Be in Cancer

15 April 2, 2019 | Company Update Inflammasome Companies: Investor and Pharma Interest • Jecure Therapeutics raised a $30mm Series A from Versant in 2017. Acquired by Genentech in 2018. • Inflazome closed a $17mm Series A in 2016 from Novartis Venture Fund and others. Recently closed a $46mm Series B to move compounds to POC. Investors included Forbion , Longitude, Novartis Venture Fund, and Fountain Healthcare Partners. • IFM TRE raised a $31mm Series A in 2018 from Atlas, Abingworth , and BMS. Acquisition by Novartis announced April 2019 for $310M upfront (Phase 1) and up to $1.6B total.

16 April 2, 2019 | Company Update Caspase 1 Inhibitors (Novartis) (Amgen) ( Regeneron ) Caspase 1 Inhibitors Caspase 1 Inhibitors Target Inflammasome Pathway at Optimal Intervention Point We believe a caspase 1 selective small molecule inhibitor can be a safe and efficacious agent for intervention in the inflammasome pathway Modified from Mangan M et al. Nature Reviews Drug Discovery, 2018 p. 588 - 606.
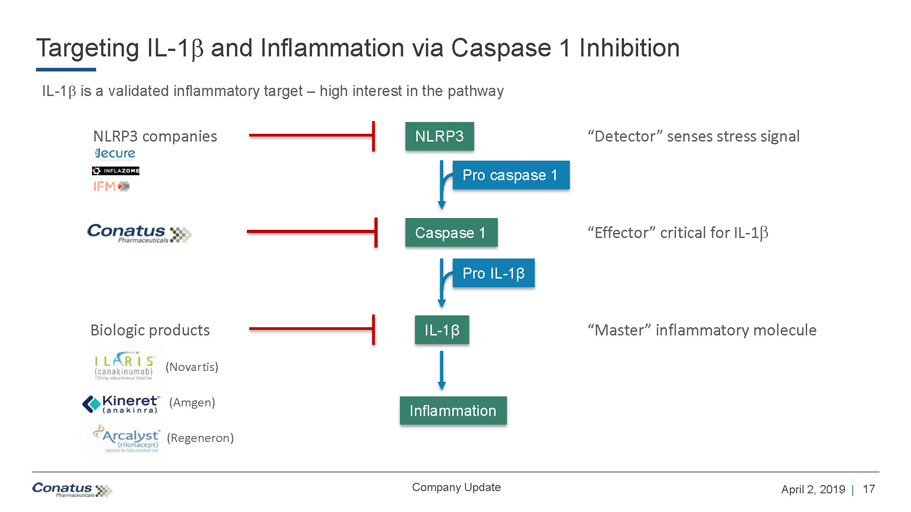
17 April 2, 2019 | Company Update Targeting IL - 1 b and Inflammation via Caspase 1 Inhibition IL - 1 b is a validated inflammatory target – high interest in the pathway NLRP3 Caspase 1 IL - 1 β Pro caspase 1 Pro IL - 1 β Inflammation NLRP3 companies Biologic products (Novartis) (Amgen) (Regeneron) “Detector” senses stress signal “Master” inflammatory molecule “Effector” critical for IL - 1 b

18 April 2, 2019 | Company Update Caspase - 1 Inhibition Improves Survival Better than Blocking IL - 1 β , IL - 18 or Both • Constitutively active NLRP3 (FCAS model) results in chronic activation of caspase 1 – Leads to production of IL - 1 β and IL - 18 – Leads to pyroptotic cell death via gasdermin D cleavage • Caspase - 1 KO showed improved survival over IL - 1 β KO , IL - 18 KO or both Mimics a caspase 1 inhibitor Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL - 1, IL - 18, and cell death in NLRP3 inflammasomopathies . J Clin Invest 2013;123:4695 - 4705.
19 April 2, 2019 | Company Update Conatus Caspase 1 Inhibitor Portfolio • Designed highly selective caspase 1 inhibitors – Incorporated novel chemistry to remove activity against apoptotic caspases – Maintained sub - nanomolar activity against caspase 1 – Inhibits downstream IL - 1 b (a clinically validated target) • Demonstrated functional activity and selectivity in cellular models – High potency in cell models of NLRP3 driven inflammation – Little to no activity in cellular models of apoptosis • Demonstration of functional activity and selectivity in animal models – Orally active in animal model of NLRP3 driven inflammation – Potency similar to NLRP3 reference standard (MCC950) – Not active in a standard animal model of apoptosis

20 April 2, 2019 | Company Update Conatus’ Lead Caspase 1 Inhibitor: CTS - 2090 • Potency – Sub - nanomolar IC 50 against human caspase - 1 – Sub - micromolar IC 50 in THP 1 cells • Selectivity – Broad panel of receptors and enzymes, >100,000 fold selectivity over caspase 1 – No anti - apoptotic activity in cells at 10 m M – enzyme selectivity reflected in cell - based functional assay – No anti - apoptotic activity in vivo at 10mg/kg – cellular functional selectivity translates in vivo • Oral Bioavailability – Good oral bioavailability – ~10 - fold higher than benchmark pan - caspase inhibitor in rodents 1 • Tissue Distribution – Not specific for liver – AUC liver ~ AUC plasma. • In vivo activity – Pathway inhibition in mouse model of LPS/urate crystal - induced IL - 1 b production – potency similar to MCC950 1 Hoglen NC. Discovery of a First in Class Apoptotic Caspase Inhibitor Emricasan PF 03491390 IDN 6556. CRC Press 2009:211 - 224.

21 April 2, 2019 | Company Update Caspase Selectivity Profile Validated in Cell - based Functional Assays • CTS - 2090 is a potent inhibitor of LPS - stimulated NLRP3 driven IL - 1 b production in THP 1 cells • IC 50 value similar to that of the potent pan - caspase inhibitor, IDN - 7314 • CTS - 2090 does not protect Jurkat cells from a - Fas – induced apoptosis (0% protection at 10 m M) • Pan - caspase inhibitor IDN - 7314 is protective with IC 50 ~ 50nM (greater than 200 - fold window of selectivity) • Cellular profile confirms mechanistic potency and functional selectivity of CTS - 2090 10 -9 10 -8 10 -7 10 -6 10 -5 10 -4 0 2000 4000 6000 Inhibitor (M) [ h I L - 1 b ] ( p g / m l ) IDN-7314 CTS-2090 I D N - 7 3 1 4 Z - V A D ( 5 0 u M ) C T S - 2 0 8 6 C T S - 2 0 8 7 C T S - 2 0 8 8 C T S - 2 0 8 9 C T S - 2 0 9 0 C T S - 2 0 9 1 C T S - 2 0 9 2 C T S - 2 0 9 3 C T S - 2 0 9 4 C T S - 2 0 9 5 0 25 50 75 100 125 % c e l l s u r v i v a l 10 uM CTS - 2090: IC 50 ~ 350nM in THP 1 cell assay IDN - 7314: IC 50 ~ 240nM in THP 1 cell assay CTS - 2090 is inactive at 10 m M in Jurkat cell assay IDN - 7314: IC 50 ~ 50nM in Jurkat cell assay
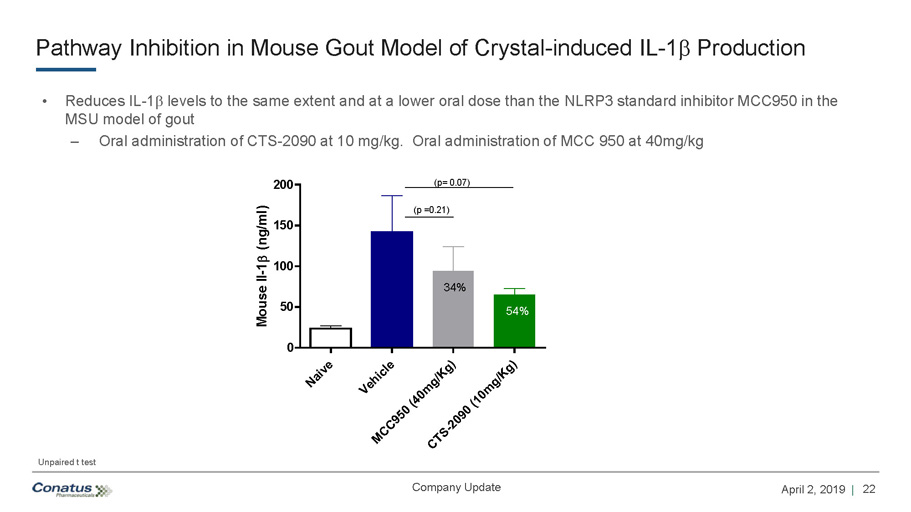
22 April 2, 2019 | Company Update Pathway Inhibition in Mouse Gout Model of Crystal - induced IL - 1 b Production • Reduces IL - 1 b levels to the same extent and at a lower oral dose than the NLRP3 standard inhibitor MCC950 in the MSU model of gout – Oral administration of CTS - 2090 at 10 mg/kg. Oral administration of MCC 950 at 40mg/kg Naive Vehicle MCC950 (40mg/Kg)CTS-2090 (10mg/Kg) 0 50 100 150 200 Mouse Il-1 (ng/ml) 34% 54% (p= 0.07) (p =0.21) Unpaired t test
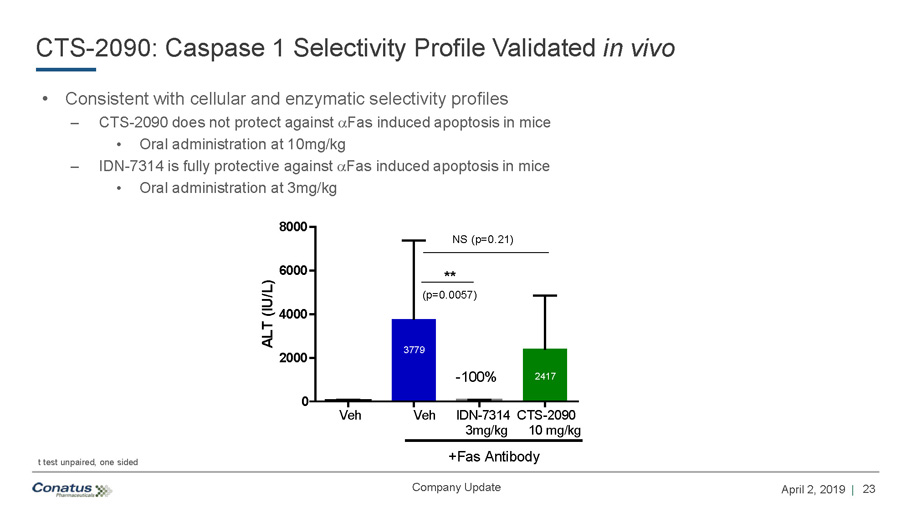
23 April 2, 2019 | Company Update CTS - 2090: Caspase 1 Selectivity Profile Validated in vivo • Consistent with cellular and enzymatic selectivity profiles – CTS - 2090 does not protect against a Fas induced apoptosis in mice • Oral administration at 10mg/kg – IDN - 7314 is fully protective against a Fas induced apoptosis in mice • Oral administration at 3mg/kg 0 2000 4000 6000 8000 ALT (IU/L) Veh Veh IDN-7314 CTS-2090 3mg/kg 10 mg/kg -100% NS (p=0.21) +Fas Antibody ** (p=0.0057) 3779 2417 t test unpaired, one sided

24 April 2, 2019 | Company Update Autoinflammatory Diseases as Initial Clinical Target for CTS - 2090 • Single - gene mutations that activate inflammasomes and result in IL - 1 β production • Spectrum of diseases with variable organ system involvement, severity and age of onset – Cryopyrin - associated periodic syndromes (CAPS) • Familial cold urticaria syndrome or familial cold autoinflammatory syndrome (FCAS) • Muckle - Wells syndrome (MWS) • Neonatal - onset multisystemic inflammatory disease (NOMID)/chronic infantile neurological cutaneous articular syndrome (CINCA) – Familial Mediterranean Fever (FMF) – Hyper - IgD syndrome – TRAPS (TNF receptor - associated periodic syndrome) • Canakinumab, rilonacept and anakinra (anti - IL - 1 β therapies) proven to be highly efficacious but are injectable, expensive and have differing durations of action

25 April 2, 2019 | Company Update Overview of CTS - 2090 Development Plan PK, Safety, Tolerability Phase 1, part A Single - dose PK Phase 1, part B Multiple - dose PK Phase 2a Multiple - dose/endotoxin Phase 2 PoC Autoinflammatory Diseases Other Potential Indications • Gout flares • Oncology • IBD/Crohn’s Dose - finding Proof of concept

26 April 2, 2019 | Company Update Highlights of Conatus Approach • Like inflammasome intervention, caspase 1 inhibition is a small molecule strategy to block the formation of IL - 1 b • Additional benefits include IL - 18 inhibition, gasdermin D inhibition, and a decrease in inflammation driven cell death (pyroptosis) vs. IL - 1 b blocking agents • Multiple routes of administration possible (oral, inhaled, topical, ophthalmology, etc.) • Conatus has developed a portfolio of novel, potent, orally bioavailable, highly selective inhibitors of caspase 1 – Demonstrated functional activity and selectivity in cellular and animal models • Lead compound CTS - 2090 selected to advance toward clinical development – IND - enabling studies in progress, projecting initial clinical trial 1H20

27 April 2, 2019 | Company Update Program (Indication) Preclinical Phase 1 Phase 2 Milestone *Emricasan ENCORE - PH (NASH Cirrhosis) Phase 2b extension data expected mid - 19 *Emricasan ENCORE - LF (NASH Cirrhosis) Phase 2b top - line data expected mid - 19 CTS - 2090 Caspase - 1 Inhibitor (Inflammasome Diseases) Initial clinical trial expected to begin 1H20 Two Phase 2b NASH cirrhosis clinical trials with lead partnered program and one independent preclinical program provide multiple paths forward Expanded Pipeline Addressing Underserved Chronic Disease Markets 263 patients at 75 sites in the US and EU ~210 patients at ~90 sites in the US Liver Function, Clinical Endpoint Clinical Endpoint *Emricasan is partnered with Novartis
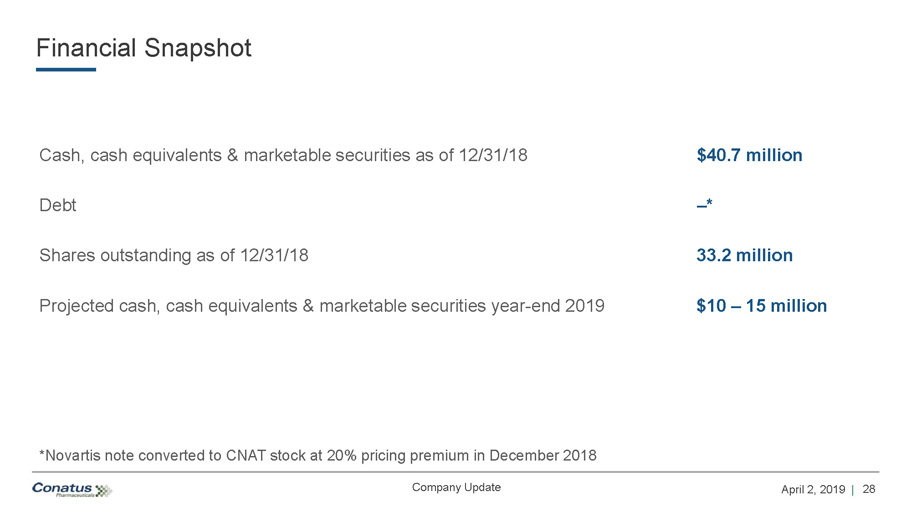
28 April 2, 2019 | Company Update Financial Snapshot Cash, cash equivalents & marketable securities as of 12/31/18 $40.7 million Debt – * Shares outstanding as of 12/31/18 33.2 million Projected cash, cash equivalents & marketable securities year - end 2019 $10 – 15 million *Novartis note converted to CNAT stock at 20% pricing premium in December 2018

29 April 2, 2019 | Company Update Conatus at a Glance Lead in - licensed compound emricasan has the potential to modify liver disease outcome Novartis partnership fully funds any further emricasan development Lead internally developed compound CTS - 2090 targeted for clinical testing 1H20 Capital at least sufficient to fund emricasan commitments and CTS - 2090 IND - enabling studies Two ongoing Phase 2b NASH cirrhosis trials reading out in mid - 2019

NASDAQ CNAT Company Update Striving to improve human health April 2, 2019





























