Exhibit 99.1

NASDAQ:CNAT
December 3, 2013
Conatus
Pharmaceuticals
Piper Jaffrey Healthcare Conference

Caution on Forward-Looking Statements
Special Note Regarding Forward-Looking Statements
This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These known risks and uncertainties are described in detail in our filings made with the Securities and Exchange Commission from time to time. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements.
All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and we undertake no obligation to revise or update this presentation to reflect events or circumstances after the date hereof.
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
2

Conatus Pharmaceuticals
San Diego-Based Biotech (NASDAQ:CNAT)
Focused on extending and improving the lives of patients with liver disease Lead compound: Emricasan
First-in-class, orally active pan-caspase protease inhibitor
Dual mechanism of action applies to entire spectrum of liver disease
In Phase 2b development for rare, catastrophic liver failure
High unmet medical need; attractive market potential
Filings planned for US and EU; ROW likely to be partnered
Potential to pursue larger disease populations
Extensive expertise in caspase inhibition and strong IP position Well-funded to key inflection points
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
3
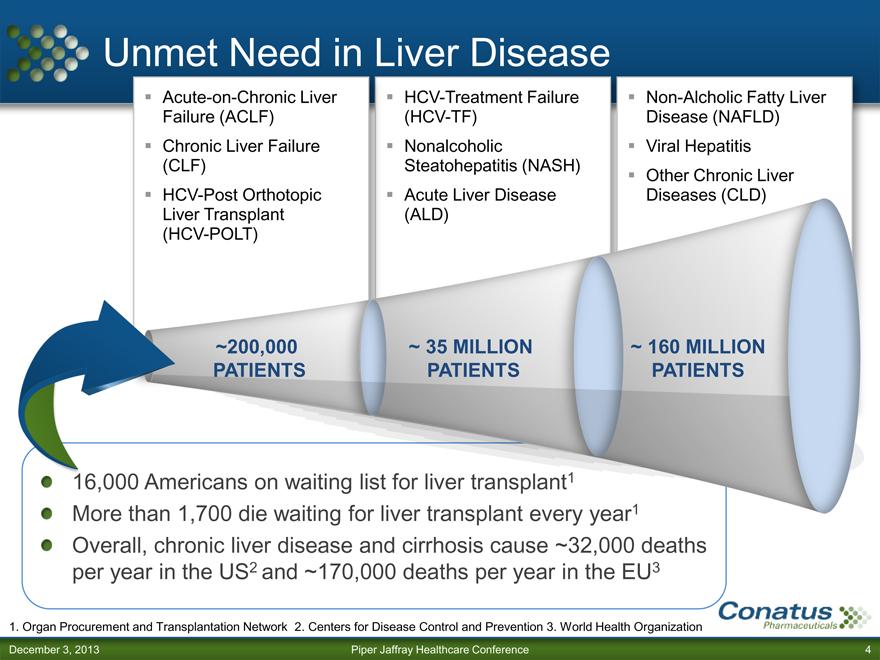
Unmet Need in Liver Disease
Acute-on-Chronic Liver Failure (ACLF) Chronic Liver Failure (CLF) HCV-Post Orthotopic Liver Transplant (HCV-POLT)
HCV-Treatment Failure (HCV-TF) Nonalcoholic Steatohepatitis (NASH) Acute Liver Disease (ALD)
Non-Alcholic Fatty Liver Disease (NAFLD) Viral Hepatitis Other Chronic Liver Diseases (CLD)
~200,000 PATIENTS
~ 35 MILLION
PATIENTS
~ 160 MILLION PATIENTS
16,000 Americans on waiting list for liver transplant1 More than 1,700 die waiting for liver transplant every year 1
Overall, chronic liver disease and cirrhosis cause ~32,000 deaths per year in the US2 and ~170,000 deaths per year in the EU3
1. Organ Procurement and Transplantation Network 2. Centers for Disease Control and Prevention 3. World Health Organization
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
4
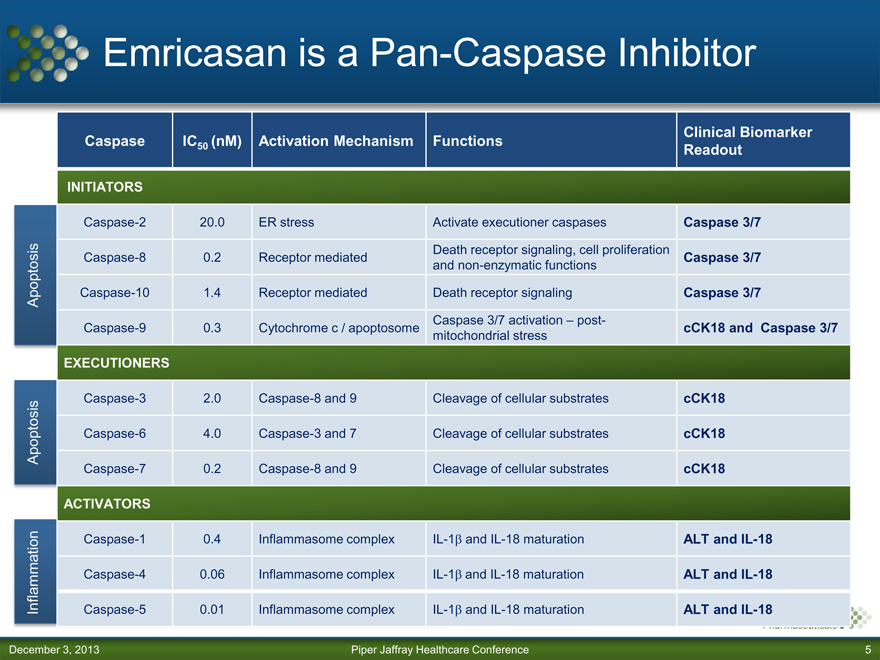
Emricasan is a Pan-Caspase Inhibitor
Caspase IC (nM) Activation Mechanism Functions Clinical Biomarker
50 Readout
INITIATORS
Caspase-2 20.0 ER stress Activate executioner caspases Caspase 3/7
Death receptor signaling, cell proliferation
Caspase-8 0.2 Receptor mediated Caspase 3/7
and non-enzymatic functions
Apoptosis Caspase-10 1.4 Receptor mediated Death receptor signaling Caspase 3/7
Caspase 3/7 activation – post-
Caspase-9 0.3 Cytochrome c / apoptosome cCK18 and Caspase 3/7
mitochondrial stress
EXECUTIONERS
Caspase-3 2.0 Caspase-8 and 9 Cleavage of cellular substrates cCK18
Apoptosis Caspase-6 4.0 Caspase-3 and 7 Cleavage of cellular substrates cCK18
Caspase-7 0.2 Caspase-8 and 9 Cleavage of cellular substrates cCK18
ACTIVATORS
Caspase-1 0.4 Inflammasome complex IL-1 and IL-18 maturation ALT and IL-18
Caspase-4 0.06 Inflammasome complex IL-1 and IL-18 maturation ALT and IL-18
Inflammation Caspase-5 0.01 Inflammasome complex IL-1 and IL-18 maturation ALT and IL-18
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
5
Two Key Biomarkers of Liver Disease
Alanine aminotransferase (ALT) and cleaved Cytokeratin 18 (cCK18) implicated in liver disease severity and progression
ALT: Clinically important and validated biomarker
Liver disease causes ALT release into blood, resulting in elevated levels Routinely used as a marker of generalized liver damage and inflammation
cCK18: Mechanism-specific biomarker of apoptosis and caspase activity
Apoptosis results in release of cCK18 into the blood stream
Independent studies have demonstrated clinical utility of cCK18 as a marker in ACLF, CLF, HCV-POLT and other indications
Conatus
Pharmaceuticals
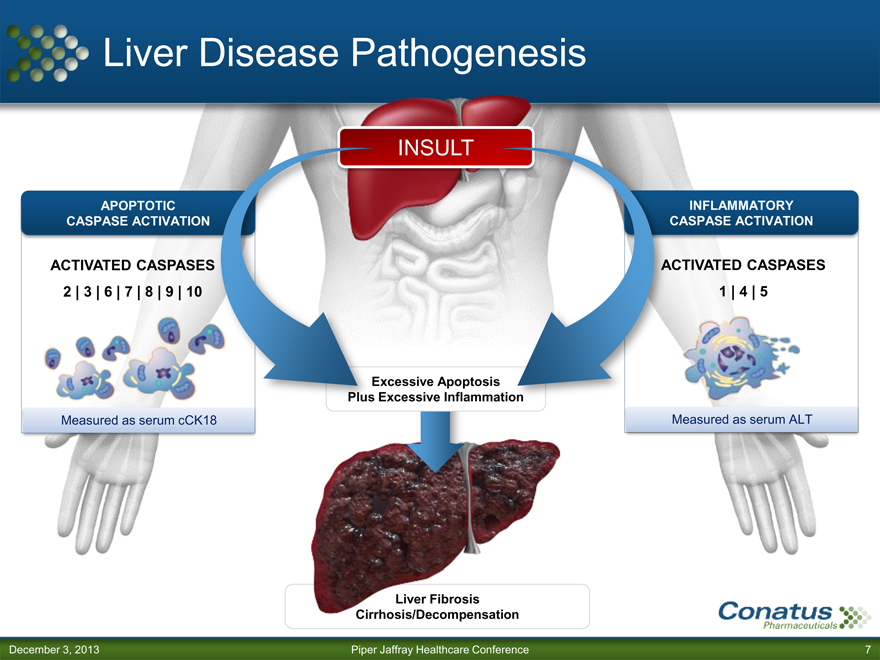
Liver Disease Pathogenesis
APOPTOTIC CASPASE ACTIVATION
ACTIVATED CASPASES
2 | 3 | 6 | 7 | 8 | 9 | 10
Measured as serum cCK18
INSULT
Excessive Apoptosis Plus Excessive Inflammation
Liver Fibrosis Cirrhosis/Decompensation
INFLAMMATORY CASPASE ACTIVATION
ACTIVATED CASPASES
1 | 4 | 5
Measured as serum ALT
December 3, 2013
Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
7
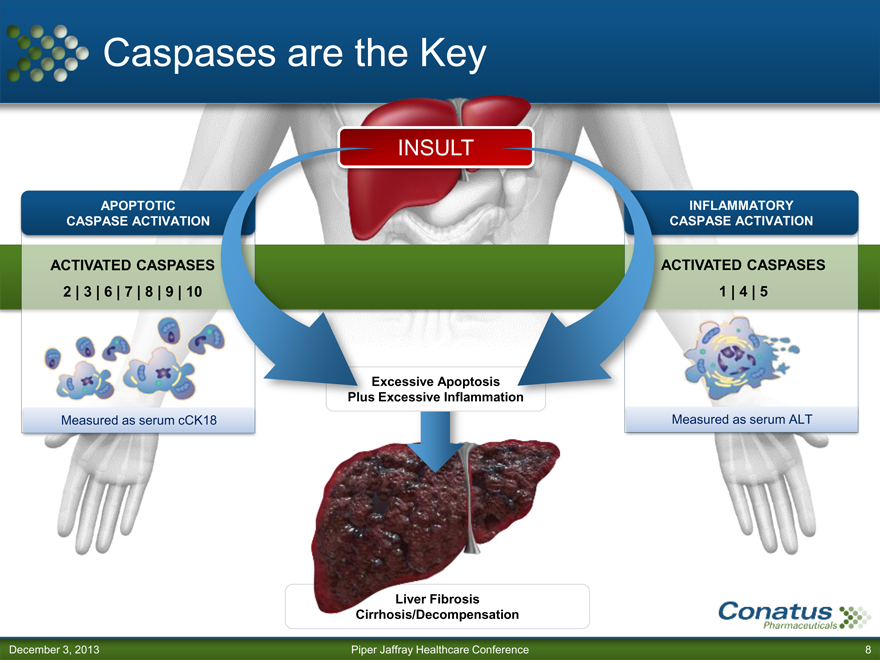
Caspases are the Key
APOPTOTIC CASPASE ACTIVATION
ACTIVATED CASPASES
2 | 3 | 6 | 7 | 8 | 9 | 10
Measured as serum cCK18
INSULT
Excessive Apoptosis
Plus Excessive Inflammation
Liver Fibrosis Cirrhosis/Decompensation
INFLAMMATORY CASPASE ACTIVATION
ACTIVATED CASPASES
1 | 4 | 5
Measured as serum ALT
December 3, 2013
Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
8
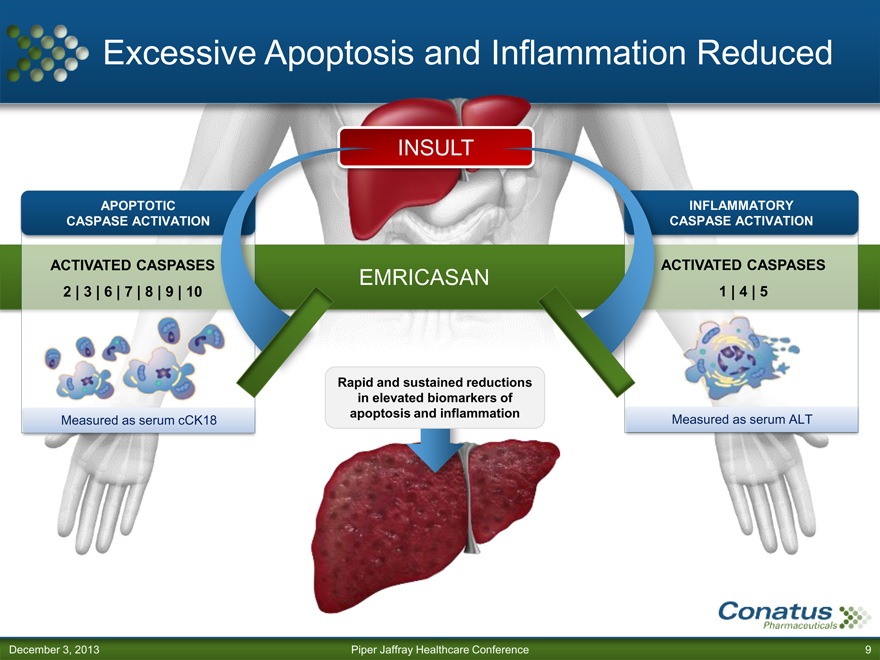
Excessive Apoptosis and Inflammation Reduced
APOPTOTIC CASPASE ACTIVATION
ACTIVATED CASPASES
2 | 3 | 6 | 7 | 8 | 9 | 10
Measured as serum cCK18
INSULT
EMRICASAN
Rapid and sustained reductions in elevated biomarkers of apoptosis and inflammation
INFLAMMATORY CASPASE ACTIVATION
ACTIVATED CASPASES
1 | 4 | 5
Measured as serum ALT
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
9

Pre-Clinical Data
Conatus
Pharmaceuticals
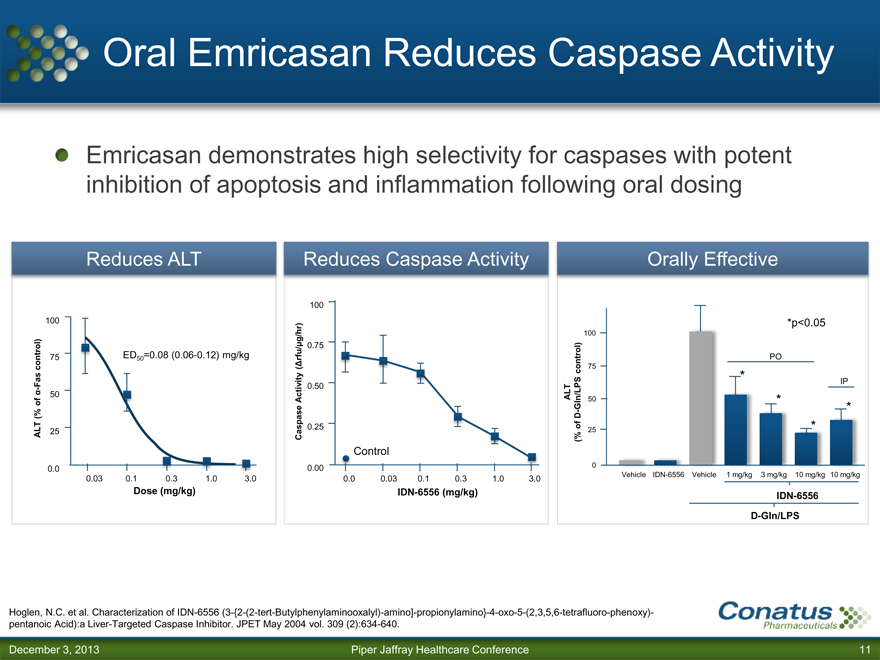
Oral Emricasan Reduces Caspase Activity
Emricasan demonstrates high selectivity for caspases with potent inhibition of apoptosis and inflammation following oral dosing
Reduces ALT Reduces Caspase Activity Orally Effective
100
100 *p<0.05
g/hr) 100
0.75
75 ED50 =0.08 (0.06-0.12) mg/kg rfu/ PO
control) ( control) 75
Fas 0. * IP
- 50
50
of Activity ALT Gln/LPS 50 * *
(% D-
ALT 25 Caspase 0.25 of 25 *
(%
Control
0.0 0.00 0
0.03 0.1 0.3 1.0 3.0 0.0 0.03 0.1 0.3 1.0 3.0 Vehicle IDN-6556 Vehicle 1 mg/kg 3 mg/kg 10 mg/kg 10 mg/kg
Dose (mg/kg) IDN-6556 (mg/kg) IDN-6556
D-Gln/LPS
Hoglen, N.C. et al. Characterization of IDN-6556 (3-{2-(2-tert-Butylphenylaminooxalyl)-amino]-propionylamino}-4-oxo-5-(2,3,5,6-tetrafluoro-phenoxy)-pentanoic Acid):a Liver-Targeted Caspase Inhibitor. JPET May 2004 vol. 309 (2):634-640.
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
11
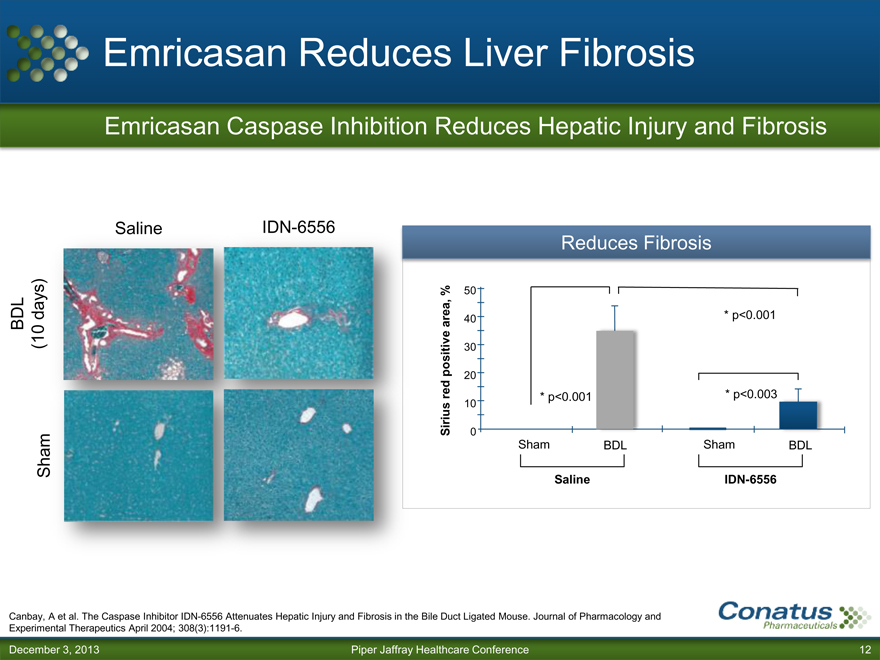
Emricasan Reduces Liver Fibrosis
Emricasan Caspase Inhibition Reduces Hepatic Injury and Fibrosis
BDL Sham (10 days)
Saline
IDN-6556
Reduces Fibrosis
% 50
area, 40 * p<0.001
positive 30
20
red * p<0.001 * p<0.003
10
Sirius 0
Sham BDL Sham BDL
Saline IDN-6556
Canbay, A et al. The Caspase Inhibitor IDN-6556 Attenuates Hepatic Injury and Fibrosis in the Bile Duct Ligated Mouse. Journal of Pharmacology and
Experimental Therapeutics April 2004; 308(3):1191-6.
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
12

Clinical Experience
Conatus
Pharmaceuticals
Emricasan Clinical Experience to Date
Studied 500+ patients
Six Phase 1 trials conducted in the US, EU and Asia
Predominately healthy volunteers
Four randomized, placebo-controlled Phase 2 trials conducted in the US and EU
Included patients with liver diseases due to a variety of causes
Emphasis on HCV infection
Clinically relevant results
Reproducible reductions in elevated biomarkers of inflammation and apoptosis
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
14
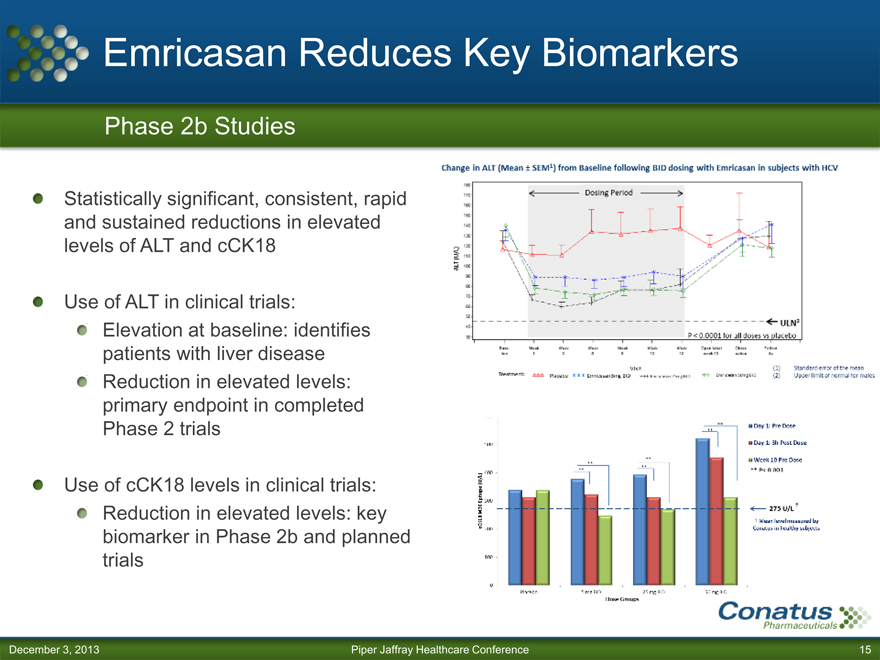
Emricasan Reduces Key Biomarkers
Phase 2b Studies
Statistically significant, consistent, rapid and sustained reductions in elevated levels of ALT and cCK18
Use of ALT in clinical trials:
Elevation at baseline: identifies patients with liver disease Reduction in elevated levels: primary endpoint in completed Phase 2 trials
Use of cCK18 levels in clinical trials:
Reduction in elevated levels: key biomarker in Phase 2b and planned trials
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
15

Clinical Programs
Conatus
Pharmaceuticals
Acute-on-Chronic Liver Failure (ACLF)
Rapid Deterioration of Liver Function
Follows acute event in patients with underlying cirrhosis
~45% likely to die, progress to next organ failure, or require transplant within 28 days
Current intervention involves treatment of underlying cause of acute event and support for failing organs
Liver transplantation may be required to improve survival and QOL
~150,000 patients in US and EU
NEED: Prevent catastrophic organ failure
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
17
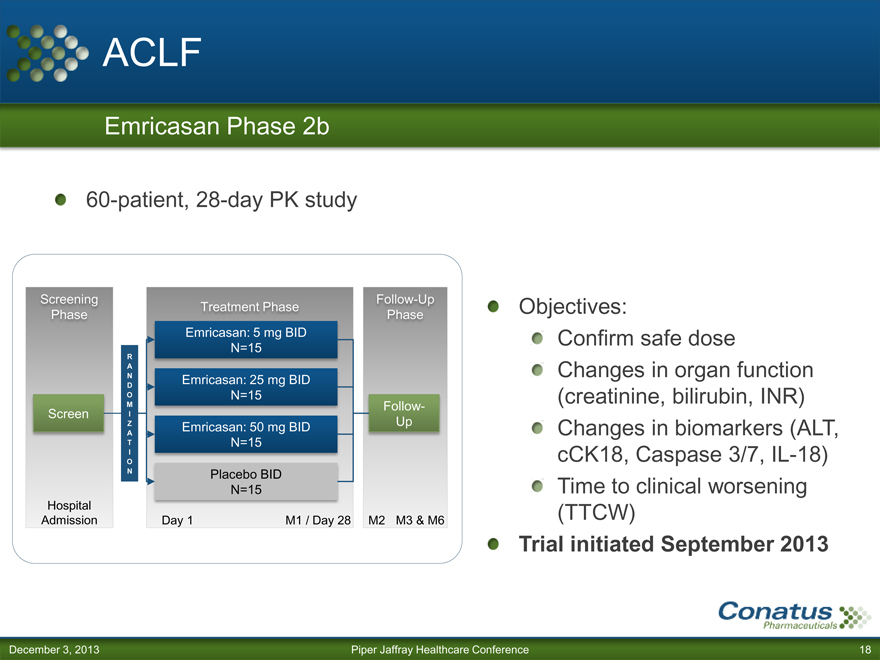
ACLF
Emricasan Phase 2b
60-patient, 28-day PK study
Screening Follow-Up
Treatment Phase
Phase Phase
Emricasan: 5 mg BID
N=15
R
A
N E
mricasan: 25 mg BID
D
O N=15
M Follow-
Screen I
Z Emricasan: 50 mg BID Up
A
T N=15
I
O
N Placebo BID
N=15
Hospital
Admission Day 1 M1 / Day 28 M2 M3 & M6
Objectives: Confirm safe dose
Changes in organ function (creatinine, bilirubin, INR)
Changes in biomarkers (ALT, cCK18, Caspase 3/7, IL-18) Time to clinical worsening (TTCW)
Trial initiated September 2013
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
18

ACLF Phase 2b: Goals
Identify a safe dose for Phase 3 studies
Understand the PK and PD in this population
Support for End of Phase Two/Scientific Advice regulatory meetings
Provide exploratory information on efficacy
Relationship between reductions in biomarkers and improvement in functional parameters and time to clinical worsening Data to support dosing in patients with mild to severe hepatic impairment
Understand the complexities of the patient population to inform Phase 3
Clinical event rate
Regional variation in co-morbidities and concomitant medications Challenges of conducting studies in the population Heterogeneity of population based on disease etiology
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
19
Severe Renal Impairment
Organ Failure Progression
Rationale
Renal impairment is a frequent complication in both ACLF and CLF
Kidney usually next organ to fail after liver (Hepatorenal syndrome)
ACLF Phase 2b study expected to provide data in mild to moderate renal impairment, but not severe
Data in severe renal failure needed
NEED: Broaden ACLF and CLF treatment populations
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
20
Severe Renal Impairment
Emricasan Phase 1b Study
Goals
Evaluate PK in this population Evaluate safety and tolerability Generate data to address:
Potential to broaden eligibility in future studies
Any need for dose adjustment in this patient population
Logistics
2 Phase 1 units in US
8 patients per group with 8 matching controls (N=16)
Open label study
Single 50 mg dose
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
21
Chronic Liver Failure (CLF)
Late-stage Liver Disease
Rationale for Chronic Liver Failure/Chronic cirrhosis study
Include patients with late-stage liver failure
Different etiologies
Both listed and not suitable for listing for transplant
Ideal would be 1 study with both populations
Number of subjects ~100
Severe renal failure data can allow sicker patients into the study
Could potentially use the study to support ACLF MAA/NDA
~10,000 patients in US and EU
NEED: Provide more time to obtain liver transplant
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
22
CLF – A Separate and Distinct Indication
CLF patients may be compensated or decompensated with different prognosis
At risk of developing ACLF
Not as critically ill as the ACLF population
Different study endpoints may be applicable
Example: transplant is a good outcome for emricasan in CLF, but not in ACLF
May require different dosing durations
ACLF – get patient through the acute crisis period
CLF – keep patients as healthy as possible prior to liver transplantation
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
23
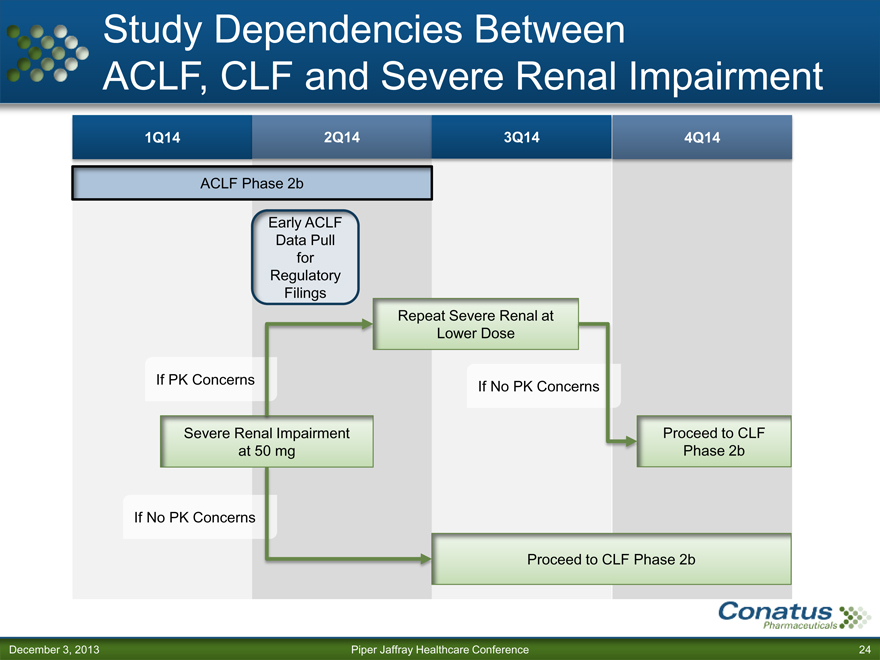
Study Dependencies Between
ACLF, CLF and Severe Renal Impairment
1Q14 2Q14 3Q14 4Q14
ACLF Phase 2b
Early ACLF
Data Pull
for
Regulatory
Filings
Repeat Severe Renal at
Lower Dose
If PK Concerns If No PK Concerns
Severe Renal Impairment Proceed to CLF
at 50 mg Phase 2b
If No PK Concerns
Proceed to CLF Phase 2b
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
24
HCV-Post Orthotopic Liver Transplant (HCV-POLT)
Rapid Progression of Fibrosis
Significant risk of accelerated fibrosis in transplanted livers of patients with HCV
Residual HCV immediately infects new liver
~55% likely to progress at least 1 stage on Ishak Fibrosis Score within 2 years
Current treatments focus on underlying HCV infection
Even with newer HCV antivirals, number of transplants needed is likely to significantly exceed available organs
~50,000 patients in US and EU
STATUS
Delayed Phase 2b/3 trial: Data suggesting potential curative therapy with new HCV antivirals pose recruitment challenges
Will continually evaluate future clinical trial options
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
25

Larger Market Opportunity
Conatus
Pharmaceuticals
Nonalcoholic Steatohepatitis (NASH)
An Emerging Opportunity
Preclinical studies suggest a therapeutic opportunity for emricasan in NASH
Compelling preclinical data in models of NASH and NAFLD
NASH study in patients with fibrosis is a challenge:
Fibrosis progression rate not characterized
Unlike HCV-POLT, longitudinal data patient databases are not yet available Some insight may come from ongoing studies (i.e. Gilead Phase 2 studies)
Potential for emricasan as fast follower to more advanced programs
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
27
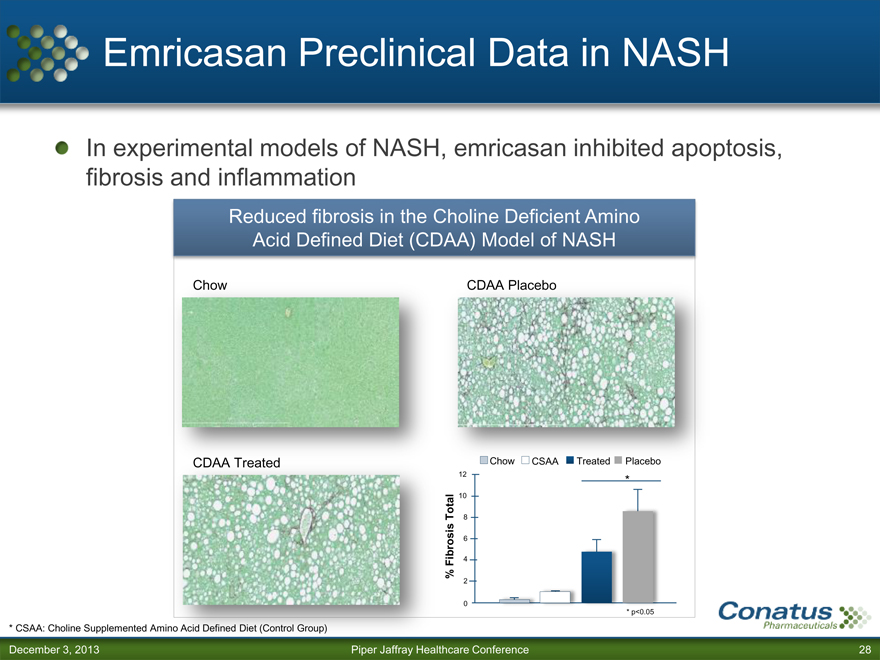
Emricasan Preclinical Data in NASH
In experimental models of NASH, emricasan inhibited apoptosis, fibrosis and inflammation
Reduced fibrosis in the Choline Deficient Amino Acid Defined Diet (CDAA) Model of NASH
Chow CDAA Placebo
CDAA Treated Chow CSAA Treated Placebo
12 *
10
Total 8
6
Fibrosis 4
% 2
0
* p<0.05
* CSAA: Choline Supplemented Amino Acid Defined Diet (Control Group)
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
28
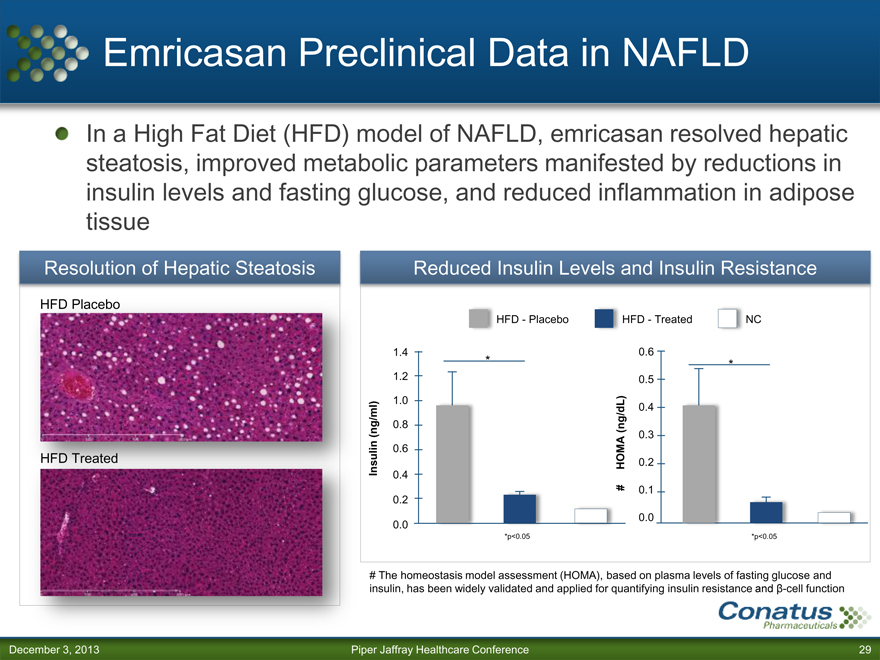
Emricasan Preclinical Data in NAFLD
In a High Fat Diet (HFD) model of NAFLD, emricasan resolved hepatic steatosis, improved metabolic parameters manifested by reductions in insulin levels and fasting glucose, and reduced inflammation in adipose tissue
Resolution of Hepatic Steatosis
HFD Placebo
HFD Treated
Reduced Insulin Levels and Insulin Resistance
HFD—Placebo HFD—Treated NC
1.4 0.6
* *
1.2 0.5
1.0 0.4
(ng/ml) 0.8 (ng/dL)
0.3
0.6
Insulin HOMA 0.2
0.4
# 0.1
0.2
0.0
0.0
*p<0.05 *p<0.05
# The homeostasis model assessment (HOMA), based on plasma levels of fasting glucose and insulin, has been widely validated and applied for quantifying insulin resistance and ß-cell function
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
29
NASH Program
Characterize the effect of emricasan on cCK18 and ALT in patients with fatty liver disease
cCK18 becoming an accepted clinical biomarker in NAFLD and NASH 28 days of dosing in approximately 40 patients with carefully defined inclusion criteria Study initiation targeted for 1H14
Use data to explore whether activity is similar to that seen in HCV population
If so, use the dose response rationale for HCV for NASH program
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
30
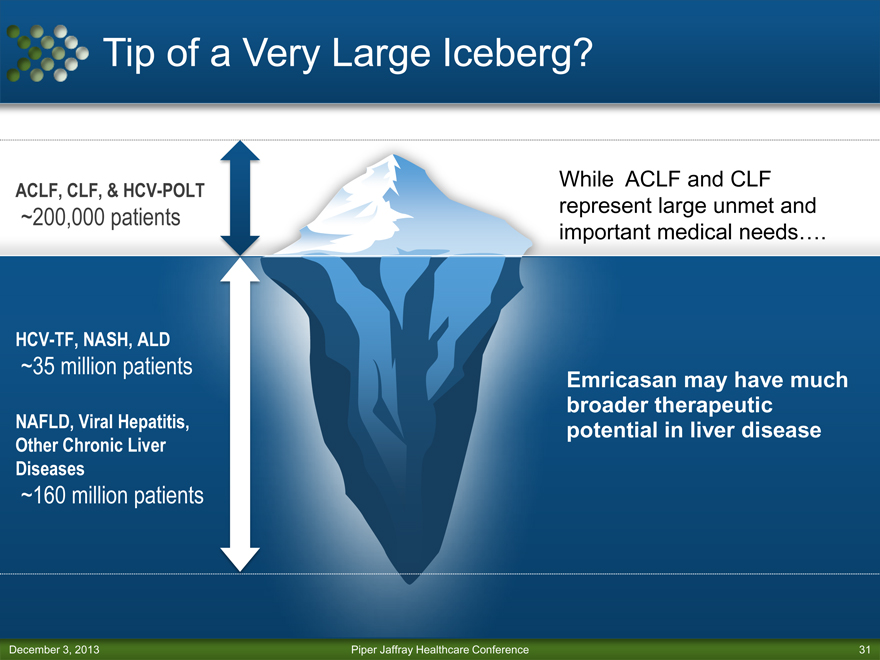
Tip of a Very Large Iceberg?
ACLF, CLF, & HCV-POLT
~200,000 patients
HCV-TF, NASH, ALD
~35 million patients
NAFLD, Viral Hepatitis, Other Chronic Liver Diseases
~160 million patients
While ACLF and CLF represent large unmet and important medical needs….
Emricasan may have much broader therapeutic potential in liver disease
December 3, 2013 Piper Jaffray Healthcare Conference
31

Conatus
Pharmaceuticals
Summary
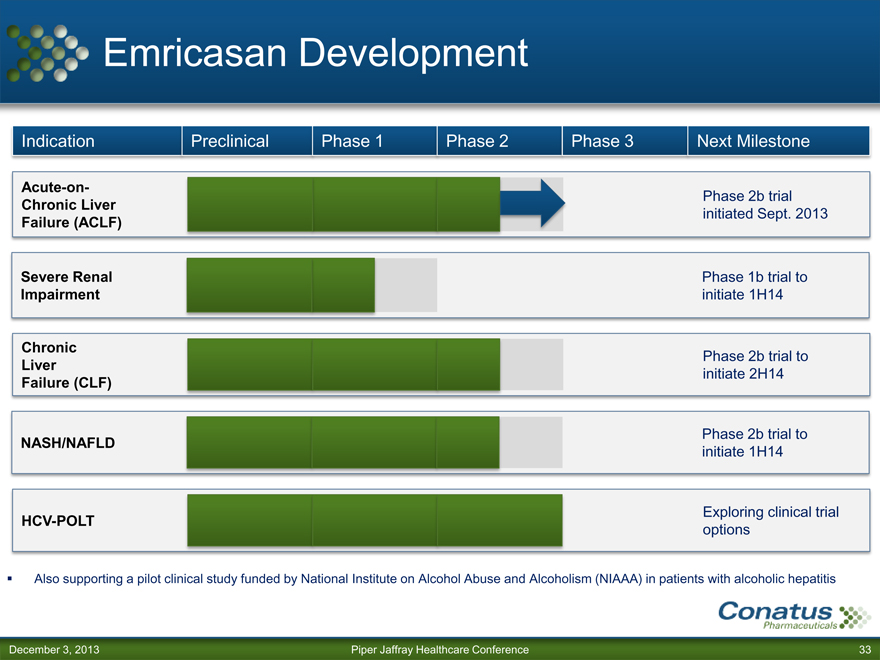
Emricasan Development
Indication Preclinical Phase 1 Phase 2 Phase 3 Next Milestone
Acute-on- Phase 2b trial
Chronic Liver
Failure (ACLF) initiated Sept. 2013
Severe Renal Phase 1b trial to
Impairment initiate 1H14
Chronic Phase 2b trial to
Liver initiate 2H14
Failure (CLF)
Phase 2b trial to
NASH/NAFLD initiate 1H14
Exploring clinical trial
HCV-POLT options
Also supporting a pilot clinical study funded by National Institute on Alcohol Abuse and Alcoholism (NIAAA) in patients with alcoholic hepatitis
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
33

Financials
Balance Sheet as of September 30, 2013
Cash, cash equivalents and short-term investments
$59,591,948
Note payable $ 1,000,000
Accounts payable & accrued compensation $ 1,155,610
Stockholders’ equity $ 58,086,150
Common shares outstanding at October 31, 2013: 15,619,879
Planned use of IPO Proceeds
$4.7M—ACLF Phase 2b study $4.5M—CLF Phase 2b study
Remainder to fund the further clinical development of emricasan and for working capital and general corporate purposes
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
34

Executive Management Team
Steven J. Mento, PhD
Co-founder, President &
Chief Executive Officer
Charles J. Cashion
Co-founder, SVP Finance &
Chief Financial Officer
Alfred P. Spada, PhD
Co-founder, SVP R&D & Chief Scientific Officer
Gary C. Burgess, MD
SVP Clinical Research & Chief Medical Officer
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
35

Value Proposition
Emricasan is first-in-class, oral pan-caspase protease inhibitor
Caspases play central role in underlying mechanism of disease in liver Key biomarkers demonstrate clinical effect
Clinical programs in areas of high unmet medical need, attractive market potential
Near-term milestones; US and EU filings planned
Potential to treat across entire spectrum of liver disease
Conatus has extensive experience in caspase inhibition and strong IP position
Successful IPO; well-funded to near-term milestones
December 3, 2013 Piper Jaffray Healthcare Conference
Conatus
Pharmaceuticals
36

THANK YOU!
www.conatuspharma.com
Conatus
Pharmaceuticals
NASDAQ:CNAT




































