Exhibit 99.2
Investor Presentation February 2, 2016 TE CONNECTIVITY ACQUIRES CREGANNA MEDICAL

Forward-Looking Statements and Non-GAAP Measures 2 Forward-Looking Statements This presentation contains certain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based on management’s current expectations and are subject to risks, uncertainty and changes in circumstances, which may cause actual results, performance, financial condition or achievements to differ materially from anticipated results, performance, financial condition or achievements. All statements contained herein that are not clearly historical in nature are forward-looking and the words “anticipate,” “believe,” “expect,” “estimate,” “plan,” and similar expressions are generally intended to identify forward-looking statements. We have no intention and are under no obligation to update or alter (and expressly disclaim any such intention or obligation to do so) our forward-looking statements whether as a result of new information, future events or otherwise, except to the extent required by law. The forward-looking statements in this presentation include statements addressing our future financial condition and operating results; our ability to fund and consummate the transaction, including the receipt of regulatory approvals; and our ability to realize projected financial impacts of and to integrate the acquisition. Examples of factors that could cause actual results to differ materially from those described in the forward-looking statements include, among others, business, economic, competitive and regulatory risks, such as conditions affecting demand for products, particularly in the automotive, medical device and data and devices industries; competition and pricing pressure; fluctuations in foreign currency exchange rates and commodity prices; natural disasters and political, economic and military instability in countries in which we operate; developments in the credit markets; future goodwill impairment; compliance with current and future environmental and other laws and regulations; the possible effects on us of changes in tax laws, tax treaties and other legislation; the risk that the transaction may not be consummated; the risk that a regulatory approval that may be required for the transaction is not obtained or is obtained subject to conditions that are not anticipated; the risk that Creganna Medical’s operations will not be successfully integrated into ours; and the risk that revenue opportunities, cost savings and other anticipated synergies from the transaction may not be fully realized or may take longer to realize than expected. More detailed information about these and other factors is set forth in TE Connectivity Ltd.’s Annual Report on Form 10-K for the fiscal year ended Sept. 25, 2015 as well as in our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other reports filed by us with the U.S. Securities and Exchange Commission. Non-GAAP Measures Where we have used non-GAAP financial measures, reconciliations to the most comparable GAAP measure are provided, along with a disclosure on the usefulness of the non-GAAP measure, in this presentation.
$170 BILLION Our Connected World TE is the World Leader in Connectivity & Sensor Solutions CONNECTIVITY AND SENSOR MARKET 12+ TE ANNUAL REVENUE Aerospace & Defense Appliances Commercial Transportation Automotive Data & Devices Industrial Equipment Subsea Communications Energy Oil & Gas BILLION Our highly engineered solutions are key enablers of the connected world Medical 3

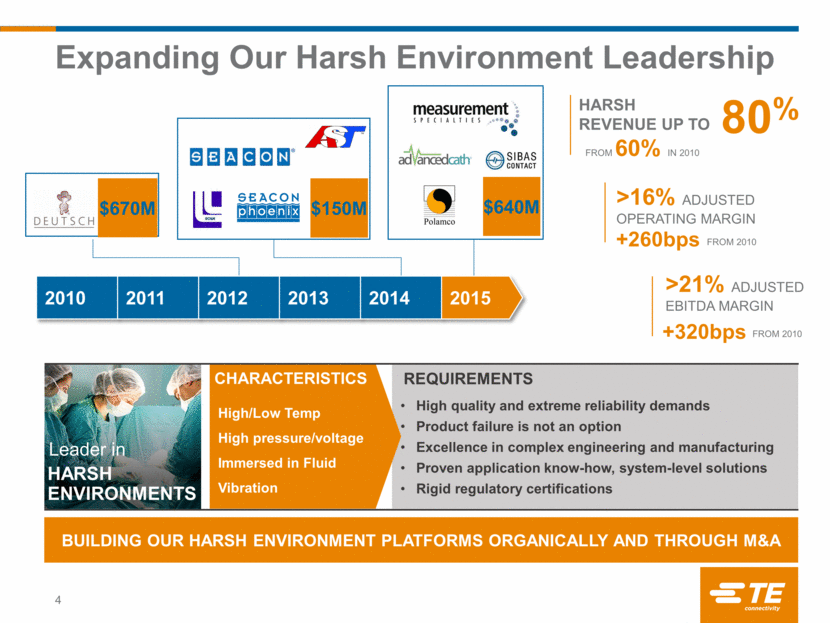
Expanding Our Harsh Environment Leadership 4 High quality and extreme reliability demands Product failure is not an option Excellence in complex engineering and manufacturing Proven application know-how, system-level solutions Rigid regulatory certifications BUILDING OUR HARSH ENVIRONMENT PLATFORMS ORGANICALLY AND THROUGH M&A High/Low Temp High pressure/voltage Immersed in Fluid Vibration HARSH ENVIRONMENTS Leader in CHARACTERISTICS REQUIREMENTS $670M $150M 2010 2011 2012 2013 2014 2015 80% HARSH REVENUE UP TO >16% Adjusted Operating Margin +260bps >21% Adjusted EBITDA Margin +320bps From 2010 From 2010 From 60% in 2010 $640M
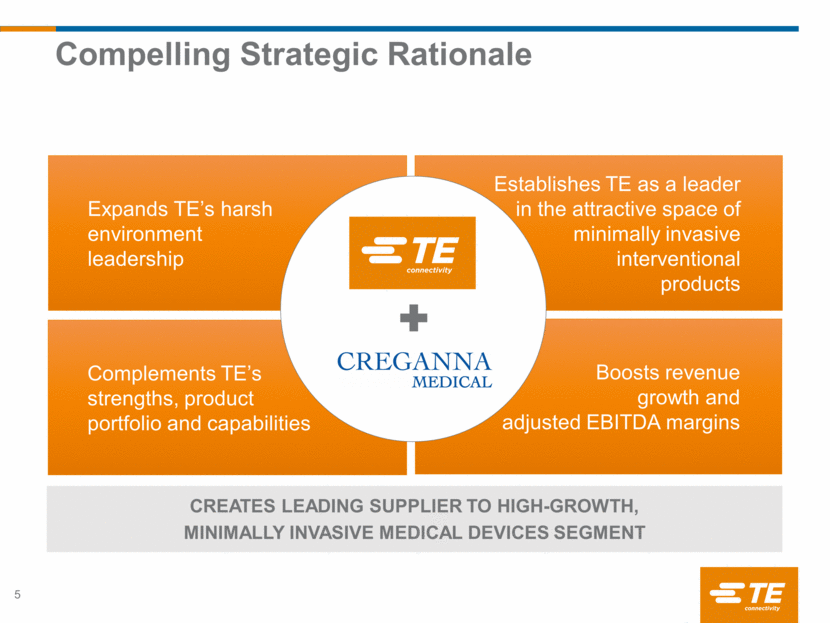
Expands TE’s harsh environment leadership Complements TE’s strengths, product portfolio and capabilities Establishes TE as a leader in the attractive space of minimally invasive interventional products Boosts revenue growth and adjusted EBITDA margins Compelling Strategic Rationale 5 CREATES LEADING SUPPLIER TO HIGH-GROWTH, MINIMALLY INVASIVE MEDICAL DEVICES SEGMENT
Favorable demographics with aging population Increasing demand for minimally invasive procedures Focus on reducing hospital stay and related costs Expected market growth rates of 7% Secular growth trends of healthcare spending & outsourcing Increased access to healthcare in emerging markets OEMs relying more on strategic suppliers with broad capabilities Requires scale and financial stability Broad product portfolio and ability to be a one-stop-shop Harsh environment applications requiring highly engineered solutions Reputation for superior product quality and reliability Global manufacturing footprint and processes Attractiveness of the $3B Interventional Market FAVORABLE MARKET DYNAMICS ATTRACTIVE GROWTH RATES SUPPLY CHAIN CONSOLIDATION 6 LEVERAGES TE STRENGTHS HIGH GROWTH MARKET WITH MULTIPLE SECULAR DRIVERS
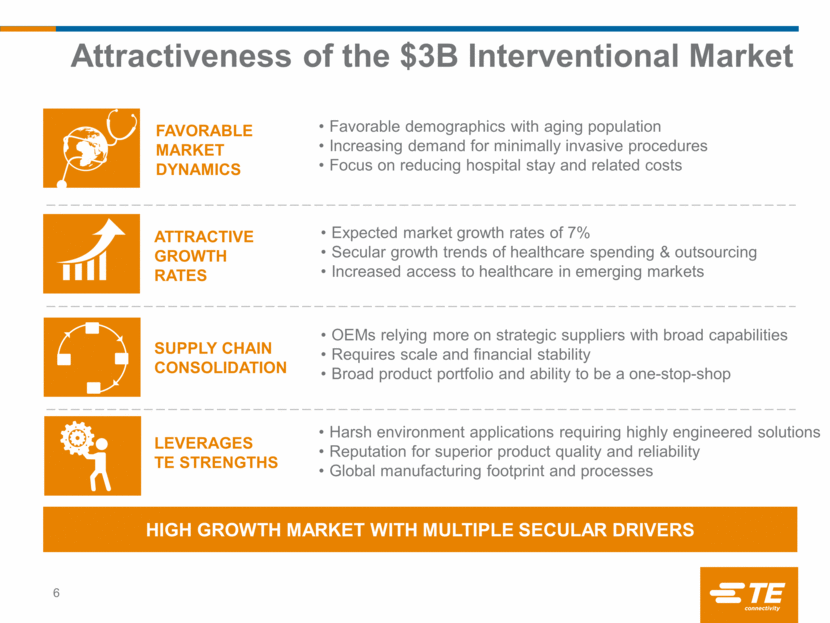
Creganna Medical Overview 7 Leading designer and manufacturer of minimally invasive Interventional devices ~$250M FY 2015 revenue with high single-digit growth; 24% EBITDA margins Strategic supplier to the Top Interventional OEMs Global customer base: ~40% of revenue outside the U.S. Industry-leading product design and development team of ~225 engineers Global manufacturing footprint: 6 facilities around the globe Stroke Treatment Heart/Stent replacement Heart Valve replacement Heart arrhythmia treatment Aneurism treatment Components, sub-assemblies, full-device design & manufacturing World-class processes for quality and regulatory management Experienced team with demonstrated track record of above-market growth and margin improvement 7% CAGR BROAD PRODUCT PORTFOLIO AND MARKET-SPECIFIC CAPABILITIES FAST- GROWING MARKET Attractive asset in a ADDRESSES SEVERAL ADVANCED AND FAST-GROWING THERAPY AREAS
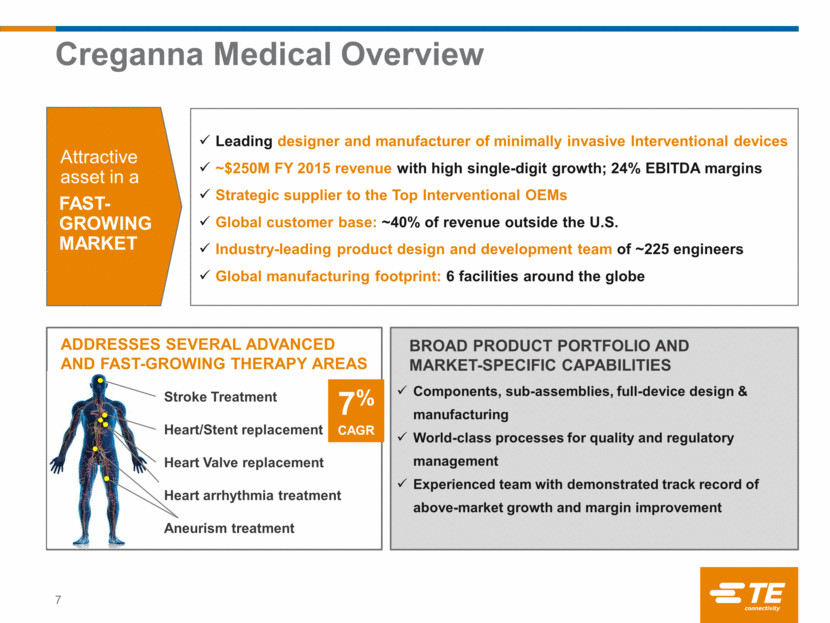
BRINGS COMPLEMENTARY STRENGTHS ENABLES TE TO ADDRESS > 90% OF THE CONTENT IN INTERVENTIONAL DEVICES TE Becomes the Broadest Solution Provider 8 Well positioned to serve the leading medical device OEMs driving advanced Interventional therapies Creganna strength TE strength Laser-cut Metal hypotubes Wire & coil Medical balloons Braided shafts Handles and deployment system Heat shrink tube Fine wire and connectors Sensors Full device design and manufacturing TE ADDRESSABLE CONTENT PER AVERAGE INTERVENTIONAL DEVICE EXPANDS FROM $10 TO $100
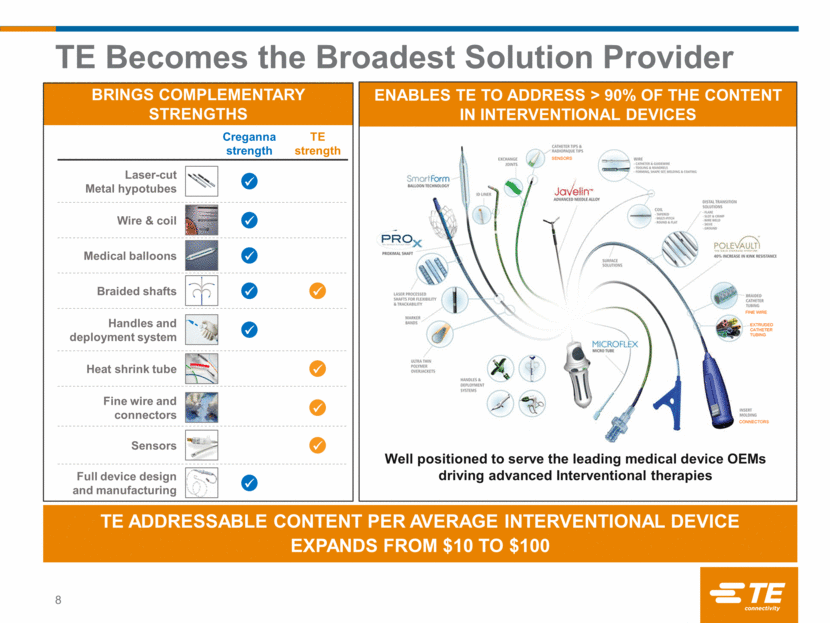
Transaction highlights 9 Transaction Value Financial Impact Timing Revenue and Cost Synergies Capital $895M purchase price Approximately 12x expected FY16 EBITDA post run rate synergies Cross-selling revenue synergy opportunities Significant cost savings through application of TEOA & tax synergies Expected to be accretive to adjusted EPS by $0.03 in first full fiscal year Accretive to revenue growth and Adjusted EBITDA margin Funding acquisition with cash on hand and debt Anticipate completing share repurchase with BNS proceeds in Q3 Expect to continue to return ~2/3 of FCF to shareholders over time Deal subject to normal closing conditions Expect to close in the third quarter of fiscal year 2016
Summary 10 Furthers TE’s strategy to expand our leadership in attractive harsh environment applications Establishes TE as a leading supplier in a fast-growing segment of the medical device market Leverages TE’s strengths in engineering, manufacturing and portfolio breadth Acquisition will be accretive to sales growth rates, adjusted EBITDA margins and Adjusted EPS

Appendix 11

Non-GAAP Measures 12 ”Adjusted Operating Income”, “Adjusted Operating Margin,” “Adjusted Earnings Per Share,” “Adjusted EBITDA,” “Adjusted EBITDA Margin,” “EBITDA,” and “Free Cash Flow” are non-GAAP measures and should not be considered replacements for results in accordance with accounting principles generally accepted in the U.S. (“GAAP”). These non-GAAP measures may not be comparable to similarly-titled measures reported by other companies. The primary limitation of these measures is that they exclude the financial impact of items that would otherwise either increase or decrease our reported results. This limitation is best addressed by using these non-GAAP measures in combination with the most directly comparable GAAP measures in order to better understand the amounts, character and impact of any increase or decrease in reported amounts. The following provides additional information regarding these non-GAAP measures: Adjusted Operating Income – represents operating income (the most comparable GAAP measure) before special items including charges or income related to restructuring and other charges, acquisition related charges, impairment charges, and other income or charges, if any. We utilize Adjusted Operating Income to assess segment level core operating performance and to provide insight to management in evaluating segment operating plan execution and underlying market conditions. It also is a significant component in our incentive compensation plans. Adjusted Operating Income is a useful measure for investors because it provides insight into our underlying operating results, trends, and the comparability of these results between periods. Adjusted Operating Margin – represents operating margin (the most comparable GAAP measure) before special items including charges or income related to restructuring and other charges, acquisition related charges, impairment charges, and other income or charges, if any. We present Adjusted Operating Margin before special items to give investors a perspective on the underlying business results. This measure should be considered in conjunction with operating margin calculated using our GAAP results in order to understand the amounts, character and impact of adjustments to operating margin. Adjusted Earnings Per Share – represents diluted earnings per share from continuing operations attributable to TE Connectivity Ltd. (the most comparable GAAP measure) before special items, including charges or income related to restructuring and other charges, acquisition related charges, impairment charges, tax sharing income related to certain proposed adjustments to prior period tax returns and other tax items, certain significant special tax items, other income or charges, if any, and, if applicable, the related tax effects. We present Adjusted Earnings Per Share because we believe that it is appropriate for investors to consider results excluding these items in addition to results in accordance with GAAP. We believe such a measure provides a picture of our results that is more comparable among periods since it excludes the impact of special items, which may recur, but tend to be irregular as to timing, thereby making comparisons between periods more difficult. It also is a significant component in our incentive compensation plans. The forecasted Adjusted EPS accretion amount that is set forth in this presentation is a non-GAAP measure and should not be considered a replacement for GAAP results. With regard to this forward-looking forecasted non-GAAP financial measure, reconciliation to the applicable forward-looking forecasted GAAP financial measure is not provided because it is not available without unreasonable effort.
Non-GAAP Measures, continued Adjusted EBITDA and Adjusted EBITDA Margin - represent net income and net income as a percentage of net sales (the most comparable GAAP measures) before interest expense, interest income, income taxes, depreciation, and amortization, as adjusted for net other income, income from discontinued operations, and special items including charges or income related to restructuring and other charges, acquisition related charges, impairment charges, and other income or charges, if any. We present Adjusted EBITDA and Adjusted EBITDA Margin to give investors a perspective in assessing our operating performance, trends, and the comparability of our results between periods. The forecasted EBITDA amount for Creganna Medical that is set forth in this presentation is a non-GAAP measure and should not be considered a replacement for GAAP results. EBITDA represents net income (the most comparable GAAP measures) before interest expense, interest income, income taxes, depreciation, and amortization. With regard to the forward-looking financial measure of the forecasted EBITDA for Creganna Medical, reconciliation to the applicable forward-looking forecasted GAAP financial measure is not provided because it is not available without unreasonable effort. Free Cash Flow (FCF) – is a useful measure of our ability to generate cash. The difference between net cash provided by continuing operating activities (the most comparable GAAP measure) and Free Cash Flow consists mainly of significant cash outflows and inflows that we believe are useful to identify. We believe Free Cash Flow provides useful information to investors as it provides insight into the primary cash flow metric used by management to monitor and evaluate cash flows generated from our operations. Free Cash Flow is defined as net cash provided by continuing operating activities excluding voluntary pension contributions and the cash impact of special items, if any, minus net capital expenditures. Net capital expenditures consist of capital expenditures less proceeds from the sale of property, plant, and equipment. These items are subtracted because they represent long-term commitments. Voluntary pension contributions are excluded from the GAAP measure because this activity is driven by economic financing decisions rather than operating activity. Certain special items, including net payments related to pre-separation tax matters, also are considered by management in evaluating Free Cash Flow. Free Cash Flow subtracts certain cash items that are ultimately within management’s and the Board of Directors’ discretion to direct and may imply that there is less or more cash available for our programs than the most comparable GAAP measure indicates. It should not be inferred that the entire Free Cash Flow amount is available for future discretionary expenditures, as our definition of Free Cash Flow does not consider certain non-discretionary expenditures, such as debt payments. In addition, we may have other discretionary expenditures, such as discretionary dividends, share repurchases, and business acquisitions, that are not considered in the calculation of Free Cash Flow. 13
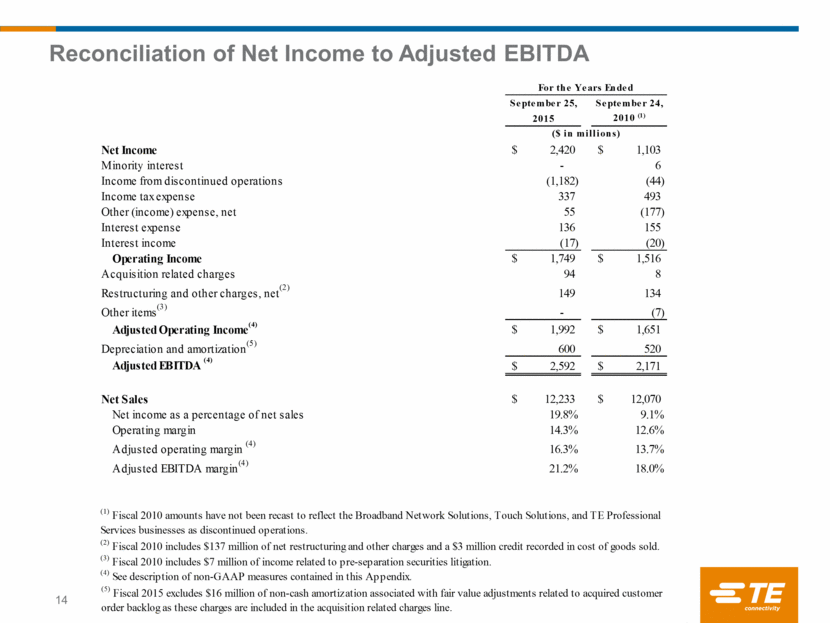
Reconciliation of Net Income to Adjusted EBITDA 14 September 25, September 24, 2015 2010 (1) Net Income 2,420 $ 1,103 $ Minority interest - 6 Income from discontinued operations (1,182) (44) Income tax expense 337 493 Other (income) expense, net 55 (177) Interest expense 136 155 Interest income (17) (20) Operating Income 1,749 $ 1,516 $ Acquisition related charges 94 8 Restructuring and other charges, net (2) 149 134 Other items (3) - (7) Adjusted Operating Income (4) 1,992 $ 1,651 $ Depreciation and amortization (5) 600 520 Adjusted EBITDA (4) 2,592 $ 2,171 $ Net Sales 12,233 $ 12,070 $ Net income as a percentage of net sales 19.8% 9.1% Operating margin 14.3% 12.6% Adjusted operating margin (4) 16.3% 13.7% Adjusted EBITDA margin (4) 21.2% 18.0% (5) Fiscal 2015 excludes $16 million of non-cash amortization associated with fair value adjustments related to acquired customer order backlog as these charges are included in the acquisition related charges line. (1) Fiscal 2010 amounts have not been recast to reflect the Broadband Network Solutions, Touch Solutions, and TE Professional Services businesses as discontinued operations. For the Years Ended ($ in millions) (2) Fiscal 2010 includes $137 million of net restructuring and other charges and a $3 million credit recorded in cost of goods sold. (3) Fiscal 2010 includes $7 million of income related to pre-separation securities litigation. (4) See description of non-GAAP measures contained in this Appendix.