UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008
Or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-33438
NEUROGESX, INC.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 94-3307935 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification Number) |
2215 Bridgepointe Parkway, Suite 200
San Mateo, CA 94404
(650) 358-3300
(Address, including zip code, of registrant’s principal executive offices and telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, $0.001 par value
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | |
Large accelerated filer ¨ | | Accelerated filer ¨ |
Non-accelerated filer x (Do not check if a smaller reporting company) | | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates was $21.1 million computed by reference to the last sales price of $3.01 as reported by the NASDAQ Global Market, as of the last business day of the Registrant’s most recently completed second fiscal quarter, June 30, 2008. This calculation does not reflect a determination that certain persons are affiliates of the Registrant for any other purpose.
The number of shares outstanding of the Registrant’s common stock on February 27, 2009 was 17,569,187 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for its 2009 Annual Meeting of Stockholders (the “Proxy Statement”), to be filed with the Securities and Exchange Commission, are incorporated by reference to Part III of this Annual Report on Form 10-K.
NEUROGESX, INC.
FORM 10-K
Year Ended December 31, 2008
INDEX
i
PART I
This report contains forward-looking statements that are based upon current expectations within the meaning of the Private Securities Reform Act of 1995. We intend that such statements be protected by the safe harbor created thereby. Forward-looking statements involve risks and uncertainties and our actual results and the timing of events may differ significantly from those results discussed in the forward-looking statements. Examples of such forward-looking statements include, but are not limited to, statements about or relating to:
| | • | | the timing of the European Commission’s decision on the European Medicines Agency’s, or EMEA, Committee for Medicinal Products for Human Use, or CHMP, opinion, which recommended approval of our marketing authorization application, or MAA, for Qutenza™ (formerly NGX-4010) for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain or the timing of obtaining government sponsored health care reimbursement; |
| | • | | the timing of U.S. Food and Drug Administration’s, or FDA, review of the new drug application, or NDA, for Qutenza that was submitted in October 2008 and the potential receipt of correspondence from the FDA by the assigned Prescription Drug User Fee Act, or PDUFA, date; |
| | • | | the sufficiency of existing resources to fund our operations through at least December 31, 2009; |
| | • | | capital requirements and our needs for additional financing; |
| | • | | potential partners for commercialization of Qutenza or other product candidates in the European Union or the United States; |
| | • | | efforts to expand the scope of indications in which our capsaicin-based product candidates are used, and the timing of potential clinical trials in connection with such expansion; |
| | • | | plans to obtain broader market access for our capsaicin-based product candidates through expansion of approved indications; |
| | • | | the scope and size of research and development efforts and programs, including with respect to development of additional product candidates; |
| | • | | the potential benefits of, and markets for, our product candidates; |
| | • | | losses, costs, expenses, expenditures and cash flows; |
| | • | | potential competitors and competitive products; |
| | • | | our plans for sales, marketing and manufacturing; |
| | • | | future payments under lease obligations and equipment financing lines; |
| | • | | patents and our and others’ intellectual property; and |
| | • | | expected future sources of revenue and capital. |
We undertake no obligation to, and expressly disclaim any obligation to, revise or update the forward-looking statements made herein or the risk factors, whether as a result of new information, future events or otherwise. Forward-looking statements involve risks and uncertainties, which are more fully discussed in the “Risk Factors” section and elsewhere in this Annual Report, including, but not limited to, those risks and uncertainties relating to:
| | • | | difficulties or delays in development, testing, obtaining regulatory approval for, and undertaking production and marketing of our drug candidates; |
| | • | | the CHMP’s positive opinion to the European Commission for approval of Qutenza may not result in approval by the European Commission; |
1
| | • | | unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could slow or prevent product approval or approval for particular indications (including the risk that current and past results of clinical trials or preclinical studies are not indicative of future results of clinical trials, and the difficulties associated with clinical trials for pain indications); |
| | • | | positive results in clinical trials may not be sufficient to obtain FDA or European regulatory approval; |
| | • | | potential for delays in or the inability to complete commercial partnership relationships; |
| | • | | physician or patient reluctance to use Qutenza, if approved, or payer coverage for Qutenza and for the procedure to administer it, which may impact physician utilization of Qutenza; |
| | • | | our inability to obtain additional financing if necessary; |
| | • | | changing standards of care and the introduction of products by competitors or alternative therapies for the treatment of indications we target; |
| | • | | the uncertainty of protection for our intellectual property, through patents, trade secrets or otherwise; and |
| | • | | potential infringement of the intellectual property rights or trade secrets of third parties. |
When used in this Annual Report, unless otherwise indicated, “NeurogesX,” “the Company,” “we,” “our” and “us” refers to NeurogesX, Inc. and its subsidiaries.
Qutenza™ and Neurogesx™ are trademarks we have applied for in the United States and in several other countries. Other service marks, trademarks and trade names referred to in this Annual Report on Form 10-K are the property of their respective owners.
Overview
We are a biopharmaceutical company focused on developing and commercializing novel pain management therapies. We are assembling a portfolio of pain management product candidates based on known chemical entities to develop innovative new therapies which we believe may offer substantial advantages over currently available treatment options. Our initial focus is on the management of chronic peripheral neuropathic pain conditions. Our most advanced product candidate, Qutenza, a dermal patch containing a high concentration of synthetic capsaicin, is designed to manage pain associated with peripheral neuropathic pain conditions. We believe, based on our successful Phase 3 studies that a single 30-or 60-minute application of Qutenza may provide up to 12 weeks of clinically-meaningful pain relief. Moreover, we believe that Qutenza has demonstrated in clinical trials a positive safety and tolerability profile.
We submitted to the FDA an NDA for Qutenza for the management of pain associated with postherpetic neuralgia, or PHN, in October 2008 which was filed by the FDA in December 2008. Our NDA has a PDUFA date of August 16, 2009 at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA. A complete response letter may describe any activities which may be required to gain approval or may indicate that a product candidate is not approvable.
In September 2007 we submitted a marketing authorization application, or MAA, for Qutenza with the European Medicines Agency, or EMEA, under the centralized procedure, seeking approval of Qutenza. On March 19, 2009, the Committee for Medicinal Products for Human Use, or CHMP, issued a positive opinion recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. In conjunction with issuing this recommendation, the CHMP requested us to perform certain clinical evaluations of Qutenza following approval. We are currently awaiting the European Commission’s decision on the CHMP opinion, a
2
process which normally takes approximately 60 to 90 days. If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
Our earlier stage product candidate pipeline consists of:
| | • | | NGX-1998, a non-patch liquid formulation of capsaicin for potential use in neuropathic pain conditions; |
| | • | | NGX-1576, NGX-9674 and NGX-5752, prodrugs of acetaminophen for potential use in acute pain, including traumatic pain, post-surgical pain and fever; and |
| | • | | NGX-6052, an opioid prodrug for potential use in chronic pain indications. |
NGX-1998 has been evaluated in three Phase 1 studies and we are currently evaluating the timing of entering into Phase 2 development. The other product candidates are all in the pre-clinical stage of development.
In response to the generally weak economic conditions which have resulted in a challenging environment for raising capital, we deferred further pre-clinical and clinical development activities for all of our product candidates in order to focus our fiscal and human resources on the prosecution of our MAA and NDA for Qutenza and on continuing support of our strategies for obtaining adequate reimbursement for Qutenza in the United States. We expect to re-initiate some or all of our development programs if additional funds become available. Further, we are currently seeking development partners for our acetaminophen and opioid prodrug product candidates. We hold worldwide commercial rights to all of our product candidates and are actively engaged in discussions with potential commercial partners.
Our Strategy
Our goal is to become a leading biopharmaceutical company focused on the development and commercialization of novel pain management therapies. Key elements of our strategy for achieving this goal include:
Achieve market approval for our Lead Product Candidate, Qutenza in the United States and European Union.In March 2009, in response to our MAA, the CHMP issued a positive opinion, recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults. We are currently awaiting the European Commission’s decision on the CHMP’s, opinion, a process which normally takes approximately 60 to 90 days. In the United States, we submitted an NDA for Qutenza with the FDA in October 2008, for the management of pain due to PHN. Our NDA has been given a PDUFA date of August 16, 2009 at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA.
Obtain an appropriate product reimbursement model for Qutenza.Qutenza is a dermal patch containing a high concentration of capsaicin. Because of the potential for misapplication of the patch, we believe that Qutenza should be administered by a healthcare professional. Our current strategy with regard to reimbursement is to seek Medicare Part B reimbursement for Qutenza which, if successful could provide for reimbursement of both Qutenza and the time incurred by the healthcare professional for the treatment procedure under reimbursement codes that would be issued by the Centers for Medicare and Medicaid Services, or CMS, and the American Medical Association. We are also investigating alternative strategies which may enable the reimbursement of Qutenza under Medicare’s prescription drug benefit (Part D). We believe that obtaining an appropriate reimbursement for Qutenza, in combination with an appropriate sales and distribution model, will be important to the market success of Qutenza.
Maximize the value of Qutenza through an effective commercial launch in the United States and European Union. We have retained exclusive worldwide commercialization rights for Qutenza. In the United States, we are currently evaluating potential marketing collaborations to facilitate the launch of Qutenza pending marketing approval. Our current objective is to launch the United States commercialization efforts of Qutenza through our own sales force and potentially supplement our own efforts through the establishment of a collaboration arrangement which could include co-promotion rights. Outside of the United States, we are actively evaluating potential collaboration partners for the commercialization of Qutenza.
3
Obtain broader market access through expansion of approved indications, including painful diabetic neuropathy, or PDN.We plan to develop our capsaicin-based product candidates to address indications outside of the indications which are currently the subject of marketing applications for Qutenza: PHN in the United States and peripheral neuropathic pain in non-diabetic adults in the European Union. Such development may be carried out through further development of Qutenza or through development of NGX-1998. To date, we have conducted two Phase 3 studies of Qutenza in HIV-distal sensory polyneuropathy, or HIV-DSP, one of which met its primary endpoint. In addition, we have conducted a Phase 2 open-label study of Qutenza in PDN. In the United States, we believe that the FDA requires that we conduct two positive Phase 3 studies for each neuropathic pain indication for which we are seeking approval. Therefore, label expansion of Qutenza in the United States could require at least one additional successful Phase 3 study in HIV-DSP and potentially two successful Phase 3 studies in PDN. In Europe, we believe label expansion may require only one safety and efficacy study in PDN. We currently plan to pursue additional studies in Qutenza, at a minimum in order to address EMEA regulatory requirements. NGX-1998, a liquid formulation which uses the same active ingredient in Qutenza, is also being developed to potentially address indications beyond those currently the subject of the Qutenza NDA and MAA. We are currently evaluating the timing of entering NGX-1998 into Phase 2 development.
Maximize the value of our pre-clinical development programs. Our model is to develop innovative therapies based on known chemical entities, balancing market opportunity with a favorable clinical and regulatory pathway. We are expanding our portfolio of pain management product candidates with the development of an opioid prodrug platform and a series of acetaminophen pro-drug product candidates. We believe these pre-clinical programs present significant opportunities and are currently seeking potential development partnerships to enable their advancement either within NeurogesX or through outlicensing.
Our Product Development Programs
Our current product development programs are focused on candidates in the field of pain with a primary focus on peripheral neuropathic pain. We retain worldwide rights to these product candidates. Our portfolio consists of the following product candidates:
| | | | |
Product Candidate | | Indication | | Phase of Development |
Qutenza | | Peripheral neuropathic pain in non-diabetic adults | | European MAA submitted—positive CHMP opinion recommending approval issued on March 19, 2009. Awaiting the European Commission’s decision on such opinion. |
| | |
| | PHN | | NDA submitted October 2008, PDUFA date August 16, 2009. |
| | |
| | HIV-DSP | | Two Phase 3 trials completed, one with primary endpoint met. |
| | |
| | PDN | | U.S. open-label Phase 2 data. |
| | |
NGX-1998 | | Peripheral neuropathic pain | | IND filed in June 2008. Three Phase 1 studies completed. Currently evaluating the timing of entering Phase 2 development. |
| | |
Acetaminophen Prodrugs (NGX-1576, NGX-9674, NGX-5752) | | Traumatic pain, post-surgical pain and fever | | Preclinical development. |
| | |
Opioid Prodrugs (NGX-6052) | | Acute pain and chronic pain | | Preclinical development. |
4
Neuropathic Pain Conditions
According to Jain PharmaBiotech, 2008, or Jain, chronic neuropathic pain is estimated to effect about 8% of the world population. Jain estimates that there are approximately 6.0 million neuropathic pain sufferers in the United States, who generated over $3.5 billion in neuropathic pain product sales in 2007, which Jain predicts will grow to $8.5 billion in 2017. Jain also estimates that there are 3.0 million neuropathic pain sufferers in Europe. We believe that this projected growth in the market for neuropathic pain medications reflects increasing awareness by the medical community of neuropathic pain diagnosis and treatment options, as well as growth in the elderly population, an increase in the number of people suffering from diabetes and an increase in the life expectancy of people with HIV. Our lead product candidate, Qutenza, is designed to address peripheral neuropathic pain conditions, which affect a majority of patients suffering from neuropathic pain.
Pain results from sensory nerve stimulation often associated with actual or potential tissue damage. Pain is transmitted by specific nerve fibers that carry the pain signal across the nervous system to the brain, where it is recognized as pain. Pain can be acute or chronic. Acute pain is short in duration and tends to be reactive or protective against actual or potential tissue injury. Chronic pain lasts over an extended period of time and often serves no useful purpose. There are two broad categories of chronic pain, inflammatory and neuropathic. Inflammatory pain is associated with tissue damage, often occurring from injury or from inflammatory conditions, such as osteoarthritis or lower back pain. This class of pain is often treated with prescription drugs that act systemically, including opioids, and over-the-counter anti-inflammatory drugs.
Neuropathic pain is a type of chronic pain that results from injury to, or dysfunction of, nerves. The injury can be to the central nervous system, consisting of the brain and spinal cord, or to the peripheral nervous system, consisting of all other nerves. Neuropathic pain can occur in any part of the body and can significantly impair the affected individual’s quality of life. It can result from viruses, as is the case with PHN and HIV, or diseases, such as diabetes. Neuropathic pain can also result from the use of drugs that treat diseases or viruses, such as drugs used to treat HIV or cancer.
Existing Treatments and their Limitations
While there are a number of products currently available for the management of neuropathic pain, we believe that the market is still underserved due to the limitations of current therapies. The primary limitations of current therapies relate to their unwanted systemic side effects, limited efficacy, cumbersome treatment regimens, potential for abuse and drug-drug interaction.
Because patients react to pain and to pain therapies in many ways and because no one therapy offers complete pain relief to all patients without significant side effects, a single standard of care does not exist for the management of neuropathic pain. Initial treatment typically involves one of a few anti-convulsants or anti-depressants. To the extent that the initial therapy does not provide adequate pain relief, the physician may try other anti-convulsants, anti-depressants or opioids alone or in combination, to treat the pain. These systemic treatments are often limited by side effects including dizziness, sedation, confusion, constipation and the potential for drug dependence. Due to these side effects, patient compliance is often poor and physicians often reduce dosing to less than optimal levels which limits the ability of these drugs to reduce pain. For this reason, we believe there is an opportunity for localized, non-systemic analgesics to be used broadly either alone or in combination with other pain medications to reduce the patient’s pain.
To date, one topical product has been approved in the United States and certain European countries for managing peripheral neuropathic pain, specifically to treat PHN. This treatment, a lidocaine patch, should not be worn for more than 12 hours in any 24-hour period. Some patients may require up to two weeks of treatment before experiencing peak pain relief and the patch must continue to be used daily in order to maintain relief. Because of safety issues associated with the use of lidocaine, the labeling for the lidocaine patch states that no more than three patches should be worn simultaneously.
5
Capsaicin-Induced Effects on Peripheral Neuropathic Pain
Peripheral neuropathic pain results from injured or dysfunctional nerve endings that send aberrant pain signals to the brain in the absence of harmful stimuli, inappropriately causing the sensation of pain. We believe capsaicin can desensitize these injured or dysfunctional nerve fibers, reducing their ability to initiate pain signals for a sustained period of time. Capsaicin is a naturally occurring substance that is responsible for making chili peppers hot. Products containing low concentrations of capsaicin, including creams, lotions and patches, have long been sold over-the-counter for the treatment of minor arthritis, back and muscle pain, as well as for other conditions.
Low-concentration capsaicin topical products have not been a viable treatment for chronic peripheral neuropathic pain conditions due in part to poor patient compliance resulting from the treatment of already painful skin with a compound that causes burning sensations, as well as the inconvenience of multiple daily applications. We believe that high-concentration capsaicin cream also does not appear to constitute a viable therapy because application causes significant patient discomfort, creams can allow the capsaicin to disperse beyond the treatment area, and existing creams have not been optimally formulated to allow capsaicin to penetrate the skin. To address the intrinsic limitations of existing capsaicin therapies, we have developed Qutenza and are developing NGX-1998, both product candidates utilizing high-concentration capsaicin.
Our Solution
We are developing novel pain management therapies, beginning with high-concentration capsaicin formulations—Qutenza, a dermal patch and NGX-1998, a dermal liquid formulation, for the management of peripheral neuropathic pain conditions. We believe that these dermal product candidates, if approved by regulatory authorities, may become a standard of care for the management of pain associated with peripheral neuropathic disorders while offering a number of significant advantages over other neuropathic pain management therapies:
| | • | | Non-systemic/localized treatment. Our localized peripheral pain management product candidates are designed to address the origin of the pain signal in the injured or dysfunctional nerves. Unlike most existing pain therapies, our product candidates do not act as general pain suppressants of the entire central nervous system and do not cause a general desensitization to acute pain or other sensations. |
| | • | | Duration of effect. Our dermal product candidates are designed to allow capsaicin to readily penetrate the skin and provide rapid onset of clinically meaningful pain relief that in the case of Qutenza may last for up to 12 weeks from a single application of 60 minutes or less. Our goal for NGX-1998 is to achieve a duration of effect comparable with Qutenza with a shorter application time. We believe these product candidate attributes may address a significant limitation of existing pain therapies, many of which require daily use and gradual increased dosages over time before reaching their peak relief effect. |
| | • | | Compliance. We believe that our dermal product candidates may avoid problems with patient compliance, which can be a significant limitation with currently available treatments, because our product candidates are designed to be administered by a healthcare professional in a single application to provide pain relief for up to 12 weeks. We believe this may address significant limitations of currently available alternatives, such as the Lidoderm patch, which is self-administered and must be applied daily and worn for no more than 12 hours per day, and systemic drugs, which also require daily use and can produce significant side effects. |
| | • | | Safety. Our clinical trials have consistently demonstrated that Qutenza is well tolerated. Treatment-related adverse events have primarily consisted of temporary redness, pain, burning, itching, dryness or swelling at the application site. The application site reactions have generally been short term and well managed with the application of cool compresses, ice or the use of short-acting opioids. To date we have not seen evidence of increased side effects with repeated treatment, including in patients who have received treatments over two or more years. |
6
Our Lead Product Candidate, Qutenza
Qutenza is a non-narcotic analgesic formulated in a dermal patch containing an 8% concentration of synthetic capsaicin. Capsaicin is released from the patch and, with the aid of penetration enhancers, absorbed into the skin during application without significant absorption of capsaicin into the bloodstream. Accordingly, users of Qutenza can avoid the systemic side effects of anti-convulsants, anti-depressants and opioids and the potential for abuse and addiction associated with some of these drugs. Qutenza is administered by a healthcare professional in a non-invasive process that involves pre-treating the painful area with a topical anesthetic for approximately one hour, followed by the application of our patch for 60 minutes, in the case of PHN and potentially less in other indications. Patches are cut to conform to the area to be treated. After the specified application period, the patch is removed and residual capsaicin is removed from the skin with a proprietary cleansing gel. Qutenza has been shown to provide a clinically meaningful reduction in peripheral neuropathic pain for up to 12 weeks in certain of our clinical studies.
In September 2007 we submitted a MAA for Qutenza with the EMEA under the centralized procedure, seeking approval of Qutenza. On March 19, 2009, the CHMP issued a positive opinion recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. In conjunction with issuing this recommendation, the CHMP requested us to perform certain clinical evaluations of Qutenza following approval. We are currently awaiting the European Commission’s decision on the CHMP opinion, a process which normally takes approximately 60 to 90 days. If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
In October 2008, we submitted an NDA for Qutenza with FDA, for the management of pain due to PHN. Our NDA has been given a PDUFA date of August 16, 2009 at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA.
7
Clinical Trials
Qutenza
As the following table illustrates, we have conducted extensive clinical trials in the management of peripheral neuropathic pain. Combined, our completed studies represent over 1,600 subjects treated with Qutenza.
| | | | | | | | |
Indication | | Development
Activity | | Trial
Number | | Number of
Participants | | Status |
| PHN | | Phase 3 | | C117 | | 416 | | Completed; primary endpoint met (p = 0.01) |
| | Phase 3 | | C116 | | 402 | | Completed; primary endpoint met (p = 0.001) |
| | Phase 3 | | C110 | | 155 | | Completed; primary endpoint not met |
| | Open-label safety study | | C118 | | 106* | | Completed |
| | Phase 2/3 | | C108 | | 299 | | Completed; primary endpoint not met |
| | Open-label extension | | C108 | | 206 | | Terminated early |
| | Phase 2 | | C102 | | 44 | | Completed |
| | Open-label extension of C102 | | C106 | | 24 | | Completed |
| | | | |
| HIV-DSP | | Phase 3 | | C119 | | 494 | | Completed; primary endpoint not met |
| | Phase 3 | | C107 | | 307 | | Completed; primary endpoint met (p = 0.0026) |
| | Open-label safety study | | C118 | | 106* | | Completed |
| | Phase 2 | | C109 | | 12 | | Completed |
| | | | |
| PDN, PHN, HIV-DSP | | Open-label tolerability study | | C111 | | 117** | | Completed |
| * | C118 evaluated the safety of applications of Qutenza over a 12 month period. Of the 106 patients enrolled, 52 patients had HIV-DSP and 54 patients had PHN. |
| ** | C111 evaluated the effect of topical anesthetic alternatives on tolerability of Qutenza and included 91 PDN patients, 25 PHN patients and 1 HIV-DSP patient. |
General Trial Design Criteria. Because all of our trials have focused on the treatment of peripheral neuropathic pain, although in different indications, we have generally been able to employ a similar design in each trial. Patients in each of our trials have to be at least 18 years old and have intact, unbroken skin over the painful area to be treated. Patients could be taking doses of other chronic pain medications, but could not be using any topical pain medications on the affected areas. Our blinded trials involve a randomly selected treated group and a control group. The treated patients receive a single application of our Qutenza dermal patch in its standard formulation, containing an 8% concentration of synthetic capsaicin. Our control group patients receive a single application of a low-dose version of our Qutenza dermal patch, containing 0.04% capsaicin. The control groups receive a low-dose capsaicin to ensure that these patients can feel the heat sensation produced by the active ingredient, so that they could not tell that they are receiving the control. All patients receive a topical local anesthetic for one hour prior to application of the patch. The patch is then applied to all patients for a prescribed duration, usually 30 or 60 minutes, although in some of our studies we also tested durations of 90 minutes. The amount of active ingredient delivered to the patient is dependent on the duration of patch application, since the capsaicin is absorbed into the skin over time. In our open-label extension studies, the control group is eliminated and all participants may receive additional single application treatments of Qutenza, typically when pain has returned but not more frequently than once every 12 weeks.
8
General Objectives. The primary objective, or endpoint, of each of our Phase 3 clinical trials has been to assess the percent change in “average pain” from baseline to weeks 2-8, in the case of PHN, or to weeks 2-12, in the case of HIV-DSP. If the primary endpoint is not met, the trial is generally considered to have been unsuccessful. Determining if the primary endpoint has been achieved is based on statistical analysis that has been defined in the protocol, the measurement of which is known as the “p value.” A successful trial is generally based on meeting a p value of less than 0.05, which means that there is a greater than 95% likelihood that the drug was responsible for the difference in effect observed between the treated patients and those receiving a placebo (or in our case, a control patch). The primary method of assessing baseline pain and pain over the course of the study is a Numeric Pain Rating Scale, or NPRS. Eligible subjects had moderate to severe neuropathic pain with a baseline average NPRS score, as measured over a period of one to two weeks prior to treatment, typically of 3 to 9 (with 0 = no pain and 10 = worst possible pain). Secondary efficacy measures included methods of assessing pain other than with NPRS, such as the Patient and Clinical Global Assessments of Change, Gracely Pain Scale, Short-Form McGill Pain Questionnaire, and Brief Pain Inventory, as well as the proportion of “responders,” which are defined as patients who experience a certain minimal threshold of pain relief such as, the percentage of patients who achieve at least a 30% reduction in pain as measured by the NPRS. Each of our studies also assessed safety and tolerability.
General Safety Findings. Our clinical trials have consistently demonstrated that Qutenza is well tolerated. In our Phase 3 trials, over 98% of subjects completed the prescribed duration of patch application, both during the double-blind phase and during the open-label phase of our trials. Treatment-related adverse events have primarily consisted of application-site issues, such as redness, pain, burning, itching, dryness or swelling. Most of these events have been mild to moderate, however, severe application site events have been observed. The application site reactions have been generally short term and managed with the application of cool compresses, ice or the use of short-acting opioids to relieve the treatment-related discomfort. Transient changes in blood pressure have been observed during the treatment procedure and appear to follow treatment-related changes in pain. We have not seen evidence of increased side effects with repeated treatment. In our Phase 3 trials there have been three serious adverse events (totaling less than 1%) related to Qutenza, two related to pain and one case of hypertension. Although in our earlier PHN studies C108 and C110, more cardiac adverse events occurred in subjects treated with Qutenza than subjects receiving the control patch, in the larger subsequently completed Phase 3 PHN studies C116 and C117, no significant difference in the proportion of subjects with cardiac events was observed between the active and control groups. Similarly, in our HIV-DSP Phase 3 studies, we have not observed a difference in the proportion of subjects with cardiac events between active and control groups.
Postherpetic Neuralgia
We have conducted five controlled studies, an open-label extension study, and a one year open-label repeat dose safety study evaluating the effect of Qutenza in subjects with PHN. Overall, over 900 subjects have received Qutenza in these studies with over 1,300 Qutenza treatments being administered. In October 2008, we submitted an NDA for PHN to the FDA. This submission was filed by the FDA in December 2008 and our NDA was given a PDUFA date of August 16, 2009, at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA.
Background on PHN. PHN is a painful condition affecting sensory nerve fibers. It is a complication of shingles, a second outbreak of the varicella-zoster virus, which initially causes chickenpox. Following an initial infection, some of the virus can remain dormant in nerve cells. Years later, age, illness, stress, medications or other factors that are not well understood can lead to reactivation of the virus. The rash and blisters associated with shingles usually heal within about six weeks, but some people continue to experience pain for years thereafter. This pain is known as postherpetic neuralgia, or PHN. PHN may occur in almost any area, but is especially common on the torso.
Potential Market. According to the Centers for Disease Control, or CDC, there are approximately 1.0 million cases of shingles in the United States each year, and approximately one in five shingles sufferers go on to develop PHN. The likelihood of developing PHN from shingles increases with age, with approximately
9
25% of people over 55, 50% of people over 60, and 75% of people over 70 estimated to eventually develop PHN after contracting shingles. Estimates as to the number of people suffering from PHN in the United States range from under 200,000 to 500,000. According to Jain, there were approximately 500,000 people in the United States living with PHN and, according to The Mattson Jack Group in 2007, there were approximately 333,000 people in the United Kingdom, France, Germany, Italy and Spain, combined, living with PHN.
Clinical Trial Results
C117 Phase 3 Clinical Trial.This trial was a randomized, double-blind, controlled, multicenter trial performed at 61 sites in the United States and Canada. Inclusion criteria included pain for at least six months following resolution of shingles. We enrolled 416 subjects and randomly assigned them to receive a 60-minute application of Qutenza (n= 212) or control (n=204) patches, according to a 1:1 allocation scheme. Based on the results from previous PHN studies, randomization was stratified by gender and by cardiovascular risk to balance the treatment groups.
The study met its primary endpoint, showing a reduction in “average pain” from baseline to weeks 2-8 for the Qutenza treated group over the control group. The Qutenza group demonstrated a 32.0% decrease in pain score, a result that was statistically significant in comparison to the 24.4% decrease in the control group (p=0.0108). The superiority of Qutenza treatment was also demonstrated for the secondary assessment period of weeks 2-12, in which the group treated with Qutenza demonstrated a 32.3% decrease in pain, while the control group decreased by 25.0% (p=0.0172). In a week by week comparison, Qutenza subjects achieved statistically significant mean reductions in NPRS scores by week 2 (p=0.039) and at every subsequent week through week 12.
C116 Phase 3 Clinical Trial. This trial was a randomized, double-blind, controlled, multicenter trial performed at 52 sites in the United States. Inclusion criteria included pain for at least six months following resolution of shingles. We enrolled 402 subjects and randomly assigned them to receive a 60-minute application of Qutenza (n = 206) or control (n = 196) patches, according to a 1:1 allocation scheme. Based on the results from the previous PHN studies, randomization was stratified by gender and by cardiovascular risk to ensure balance between the treatment groups.
The study met its primary endpoint of showing a reduction in “average pain” from baseline to weeks 2-8 for the Qutenza treated group over the control group. The Qutenza group demonstrated a 29.6% decrease in pain score, a result that was statistically significant in comparison to the 19.9% decrease in the control group (p = 0.001). The superiority of Qutenza treatment was also demonstrated for the secondary assessment period of weeks 2-12, in which the group treated with Qutenza demonstrated a 29.9% decrease in pain, while the control group decreased by 20.4% (p = 0.0016). In a week-by-week comparison, Qutenza subjects achieved statistically significant mean reductions in NPRS scores as early as week 1 (p = 0.0438) and at every subsequent week through week 12.
Additional studies in PHN.
C118 Phase 2 Clinical Trial—Safety. This study was a multicenter, open-label, one year safety study of Qutenza for the treatment of peripheral neuropathic pain in patients with PHN or HIV-DSP. The primary objective of this study was to assess the safety of up to four repeated applications of Qutenza for the treatment of PHN and HIV-DSP. The study enrolled a total of 106 patients (54 PHN and 52 HIV-DSP). Qutenza treatments were generally well tolerated with greater than 98% of the subjects completing the prescribed duration of treatment. We believe the study demonstrated that Qutenza was not associated with increasing toxicity following multiple treatment cycles. Further, clinical assessments suggested no impairment of protective nerve function (such as the ability to feel pressure or heat) over the one year study period.
C110 Phase 3 Clinical Trial.In 2004, we completed C110, a Phase 3 trial of 155 PHN patients. Unlike our C117 and C116 trials and our earlier Phase 2 trials in PHN, this study only required subjects to have had pain for at least three months following resolution of their shingles rash, rather than six months. Subjects treated with
10
Qutenza experienced a mean percent decrease in pain scores from baseline of approximately 37% compared to an approximately 30% decrease in the control group. As a result of this higher than anticipated control group response, this study did not meet its primary endpoint. In a week-by-week comparison, an improvement over time was observed in the control group, suggesting a spontaneous improvement may have occurred and contributed to the unexpectedly high control group response. Spontaneous improvement of PHN during the first three to six months has been reported in scientific literature. An analysis not specified in the protocol was performed evaluating subjects with PHN for at least six months. This analysis demonstrated significantly greater reductions in pain in Qutenza treated patients compared to control. Based on the results of this study, we revised the inclusion criteria for our subsequent PHN clinical trials to include subjects with pain for at least six months post-shingles resolution.
C108 Phase 2/3 Clinical Trial. In 2004, we completed C108, a 299 patient randomized, double-blind, 12-week, controlled, dose-finding study of Qutenza for PHN. The primary objective of this study was to assess the efficacy, safety, and tolerability of Qutenza administered at three different dose levels (30-, 60- and 90-minutes) for the treatment of PHN. Pain scores during weeks 2–8 following 30-, 60- and 90-minute Qutenza treatments were similar, with patients’ pain scores declining approximately 25% to 28%. This study did not meet its primary endpoint. Compared with the control group pain scores, only the 90-minute dose group demonstrated a mean percent pain score decrease from baseline that reached statistical significance (p = 0.044). In study C108, a gender imbalance was noted between the individual dose groups with more males being assigned to the 60-minute Qutenza group than in the other treatment groups. To adjust for this imbalance, a gender-stratified analysis not specified in the protocol was performed. The results of this analysis demonstrated significant reductions in pain in both the 90- and 60-minute Qutenza dose groups (p <0.05) suggesting that the primary analysis of this study was confounded by the imbalance in gender in the 60-minute dose group. Based on the results of this study, we modified our clinical trial analysis plans in subsequent PHN clinical trials to include a gender-stratification analysis that accounts for potential gender imbalances in treatment groups.
The C108 study’s open-label extension phase was terminated prior to completion after the data from the double-blind portion of the study were unblinded. During the open-label extension, subjects could receive up to three Qutenza treatments no more frequently than every 12 weeks. Of the 299 subjects, 206 (69%) received one or more open-label Qutenza treatments. Treatment was generally well tolerated. There were no observed safety concerns with subjects receiving up to four Qutenza treatments.
C102 Phase 2 Clinical Trial. In C102, a Phase 2 trial, we demonstrated that a single 60-minute treatment with Qutenza was feasible in subjects with PHN, appeared to be well tolerated and was associated with a reduction in PHN pain over a 28-day period. C106, an open-label extension of C102, suggested that a single 60-minute Qutenza treatment is associated with a reduction in PHN pain over a 12-week period and that treatment appeared to be well-tolerated when administered up to four times over the course of one year.
Painful HIV-Distal Sensory Polyneuropathy
We have conducted two controlled studies, an open-label extension study and an open-label long-term safety study evaluating the effect of Qutenza in subjects with HIV-DSP. Overall, 632 subjects have received Qutenza in these studies with over 1,000 Qutenza treatments being administered.
Background on HIV-DSP. HIV-DSP is caused primarily by three factors: direct activation of cells known as sensory neurons by the HIV virus, the immune system’s fight against the infection and the drugs administered to treat HIV. Painful HIV-DSP is characterized by significant pain in the feet and hands.
Potential Market. According to Frost & Sullivan, neuropathic pain is a common neurological complication of antiretroviral treatments of HIV and affects approximately 15% of the HIV infected community. According to the CDC, in 2005 the estimated number of AIDS diagnosis in the United States and dependent areas was 984,155. There are currently no specific treatments approved in the United States or Europe for HIV-DSP.
11
Estimate as to the number of people with HIV-DSP in the United States vary from under 200,000 to nearly 300,000. According to The Mattson Jack Group, 2007, there are approximately 270,000 and 136,000 people with HIV-DSP in the United States and in the United Kingdom, France, Germany, Italy and Spain, combined, respectively.
Clinical Trial Results
C119 Phase 3 Clinical Trial.In February 2008, we completed a randomized, double-blind, controlled trial performed at 77 sites in the United States, Canada, Australia and the United Kingdom. Inclusion criteria included pain due to HIV-DSP or neurotoxic antiretroviral drug exposure for at least two months and average NPRS scores during the screening period of three to nine, inclusive. The primary objective of this study was to assess efficacy, safety, and tolerability of two doses of Qutenza, 30- and 60-minute applications, over the 12-week study period. The primary efficacy assessment was the change in “average pain” from baseline in weeks 2-12. We enrolled 494 subjects and randomly assigned them to receive either a 30 minute or 60 minute application of Qutenza or control patches according to a 2:1 allocation scheme for each dose.
Study C119 did not meet its primary endpoint. Overall there was a 29.5% reduction in pain in the Qutenza treatment group, a result that was consistent with what we have observed in other Qutenza studies. The control group reported a 24.6% reduction in pain from baseline, a control group response greater than we observed in our prior HIV-DSP Phase 3 study. The p-value for this comparison was p=0.1. For the individual dose groups, the 30-minute Qutenza group achieved a 26.1% reduction in pain from baseline compared to the 30-minute control group, which reported a 19.1% reduction in pain. The p-value for this comparison was p=0.1. The 60-minute dose group reported a 32.8% reduction in pain from baseline, however, the 60-minute control group reported a 30.1% reduction in pain (p=0.5).
C107 Phase 3 Clinical Trial. In 2005, we completed a randomized, double-blind, controlled, dose finding study of Qutenza for the treatment of HIV-DSP performed at 32 sites in the United States. The primary objective of this study was to assess efficacy, safety, and tolerability of Qutenza. Efficacy was measured in terms of change in “average pain” from baseline to weeks 2-12. The study consisted of a 12-week randomized, double-blind, controlled phase and a 40-week open-label extension. Three different dose levels (30-, 60- and 90-minute applications) were evaluated together, and then each dose level was evaluated individually. The study also provided information about the efficacy, safety, and tolerability of repeated treatment with Qutenza over one year. A total of 307 subjects were enrolled at 32 clinical sites in the United States, divided approximately equally among the 30-, 60- and 90-minute dose levels, with three patients treated with Qutenza for every one subject treated with the control.
The results of the study demonstrated that in the aggregate, across all active treatment groups, Qutenza significantly reduced pain in subjects with HIV-DSP compared to the control group. Subjects treated with Qutenza demonstrated a mean reduction in pain score from baseline of 22.8% that was statistically greater than the 10.7% decrease in the control group (p = 0.0026).
Among the individual dose groups, the 90-minute Qutenza group demonstrated a mean reduction in pain of 24.7% that was significantly greater than the decrease of 10.7% reported by the control group (p = 0.005). The 60-minute Qutenza group also demonstrated a greater reduction in pain scores of 15.8%, but the difference from control was not statistically significant. The 30-minute Qutenza group had a mean percent decrease from baseline of 27.7%, which was similar to the pain reduction reported for the 90-minute Qutenza group (p=0.0007). The effect of treatment was maintained for up to 12 weeks, with the Qutenza group demonstrating significantly greater pain reduction compared to the control group during the second week and at each subsequent week through week 12. Among several secondary measures of pain relief, all three doses showed meaningful improvement compared to control. This study demonstrated that treatment with Qutenza was generally well tolerated. A single Qutenza treatment provided a stable reduction in pain over a 12-week period. Although the pre-specified statistical testing of the primary analysis stopped after the 60-minute dose was found not to have
12
reached statistical significance, the data from all the active dosing groups combined from this study, including evaluation of secondary endpoints, support the conclusion that all the Qutenza doses tested (30-, 60-, and 90-minute) provided pain relief in subjects with HIV-DSP. Repeated treatments for up to one year in an open-label efficacy study appeared to have been equally efficacious, generally well tolerated and without cumulative toxicity.
C118 Phase 2 Clinical Trial—Safety. This study was a multicenter, open-label trial of Qutenza for the treatment of peripheral neuropathic pain in patients with HIV-DSP or PHN. The primary objective of this study was to assess the safety of up to four repeated applications of Qutenza for the treatment of HIV-DSP and PHN. The study enrolled a total of 106 patients (54 HIV-DSP and 54 PHN). Qutenza treatments were generally well tolerated with greater than 98% of subjects completing the prescribed duration of treatment. We believe the study demonstrated that Qutenza was not associated with increasing toxicity following multiple treatment cycles. Further, clinical assessments suggested no impairment of protective nerve function (such as the ability to feel pressure or heat) over the one year study period.
Prior Clinical Experience. In 2003, we completed C109, an open-label pilot study of high-concentration capsaicin patches in the treatment of HIV-DSP. The primary objective of this study was to obtain preliminary information on the efficacy, safety, and tolerability of Qutenza in subjects with HIV-DSP. This Phase 2, multicenter, open-label trial enrolled 12 subjects at three clinical sites in the United States. Subjects received a single 60-minute treatment with Qutenza and were followed for 12 weeks. This study demonstrated that treatment with Qutenza was feasible and was generally well-tolerated. The study also provided preliminary evidence of efficacy indicating that Qutenza could reduce pain associated with HIV-DSP for 12 weeks after treatment.
Painful Diabetic Neuropathy
Background on PDN. PDN is caused by injury to the sensory nerves, which arises from the toxic effects of some glucose metabolites and damage to blood vessels associated with nerves. The condition causes progressive pain or loss of feeling in the toes, feet, legs, hands and arms. Like HIV-DSP, PDN is typically first felt as pain in the feet and hands.
Potential Market. The CDC estimates that 20.8 million people in the United States suffered from diabetes, of which it is estimated by Jain that 6.0 million suffered from some form of neuropathy. The number of PDN sufferers in the United States is currently estimated by Jain to be 3.0 million. According to The Mattson Jack Group, there are approximately 2.85 million people with PDN in the United Kingdom, France, Germany, Italy and Spain, combined.
Clinical Trial Results
Phase 2a Clinical Trial Description. In 2004, we completed C111, a randomized, open-label multicenter evaluation of the tolerability of treatment with Qutenza in conjunction with pre-patch topical application of one of three lidocaine 4%-based local anesthetic products. The study enrolled 25 subjects with PHN, 91 subjects with PDN and 1 subject with HIV-DSP. Tolerability of the procedure was similar among all topical anesthetics tested. Preliminary efficacy data were obtained for the PHN group and the PDN group. PHN subjects experienced a 27.7% reduction in pain over weeks 2 through 12; pain was reduced by 31.4% in PDN subjects. Our experience in C111 suggests that neuropathies of the feet, such as PDN and HIV-DSP, regardless of the underlying disease, may respond similarly to treatment with Qutenza.
13
NGX-1998—Liquid High Concentration Topical Capsaicin
We are developing a liquid formulation of the same active ingredient in Qutenza for the treatment of peripheral neuropathic pain, as well as potentially for other chronic pain syndromes. NGX-1998 is intended to provide:
| | • | | Rapid Delivery: Deliver capsaicin more rapidly into the skin than Qutenza, without allowing significant amounts to enter the bloodstream, thereby potentially shortening the treatment procedure without impacting the safety or efficacy profile. By reducing treatment time, a larger base of physicians may be willing to administer NGX-1998. |
| | • | | Improved Comfort: Reduce treatment-related discomfort. We believe that the rapid delivery of capsaicin may reduce the need for pre-treatment with a local anesthetic, as the extremely rapid skin delivery of capsaicin could quickly inhibit the activity of nerve fibers. |
| | • | | Expanded Indications: The liquid formulation can be applied to many places on the body that pose a challenge for a patch formulation, expanding the potential indications to include such pain syndromes as arthritis, vulvodynia and oral mucositis. |
An IND was filed for NGX-1998 in June 2008, under which we have conducted one Phase 1 study to evaluate potential control formulations for future controlled clinical studies. In addition, NGX-1998 has been evaluated in two Phase 1 clinical studies under an exploratory IND. In one study, we tested multiple liquid capsaicin formulations in order to identify those with the highest surrogate efficacy and tolerability characteristics. A second study involving 30 healthy volunteers evaluated the effect of NGX-1998 on epidermal nerve fiber density, which we believe may be a surrogate measure of efficacy. Each volunteer was treated with NGX-1998 for 5, 15 and 25 minutes as well as a 60-minute application of Qutenza. Epidermal nerve fiber density was measured by punch biopsy seven days after treatment for the treated areas and for one placebo treated area from each volunteer that was taken as a control. In addition, the study evaluated treatment related discomfort at each of the treatment times. Results of the study indicate that each of the three NGX-1998 treatment times produced comparable nerve fiber density reduction as that observed following a 60-minute application of Qutenza and all groups showed a statistically significant reduction in nerve fiber density compared to control. The study also indicated a significant reduction in treatment related discomfort between all of the NGX-1998 treatment groups and Qutenza.
We are currently evaluating the timing of entering this product candidate into Phase 2 development.
Acetaminophen Prodrug Candidates—Preclinical Program
Acetaminophen, first approved by the FDA for marketing in the United States in 1955, is the most widely used drug for pain relief and the reduction of fever in the United States and is currently available in numerous pharmaceutical products. Acetaminophen is often perceived to be safer than other non-steroidal anti-inflammatory drugs, or NSAIDs. Although acetaminophen is used widely in over the counter and some oral prescription products, two major limitations exist regarding its use. First, due to the intrinsic low solubility of acetaminophen, its ability to be used in injected or infused formulations is significantly impacted, limiting non-orally administered usage of acetaminophen (e.g. for post-operative pain). Second, is acetaminophen-induced liver toxicity, which is the most common cause of acute liver failure in the United States, prompting label requirements which were enacted by the FDA in 2006 regarding the potential for liver damage. We believe that we have addressed these two limitations through the discovery of novel prodrugs of acetaminophen.
Acetaminophen in injectable or intravenous formulations are available in the European Union and are marketed by Bristol-Myers Squib under the tradename Perfalgan®. Such acetaminophen formulations require a 15-minute, 100 mL infusion as these formulations provide only 10 mg of acetaminophen per mL due to acetaminophen’s intrinsic low solubility. Despite this inconvenient dosing requirement, Perfalgan has become the market-leading injectable analgesic in the European Union. In the United States, this same formulation has been licensed by and is under development by Cadence Pharmaceuticals, under the trade name Acetavance®.
14
To address the low water solubility of acetaminophen, we have designed NGX-9674 and NGX-5752 which are novel prodrugs of acetaminophen. Preliminary data from our pre-clinical studies indicates that these prodrugs are approximately 10 times more soluble in water than acetaminophen.
To address safety concerns regarding acetaminophen use, specifically the potential for liver damage, we have developed NGX-1576, a novel prodrug which couples acetaminophen to a molecule known to prevent acetaminophen induced-liver toxicity. We have evaluated NGX-1576 in preclinical studies, including in animal models. One such study in mice indicated that certain dose levels of NGX-1576 produce less liver-toxicity than equivalent doses of acetaminophen with pharmacokinetic data also showing equivalent blood levels between NGX-1576 and acetaminophen treated mice.
NGX-6052 Opioid Analgesic Prodrug Preclinical Program
Opioid analgesics, a mainstay of pain management, are used to manage pain associated with a wide variety of acute and chronic conditions. However, despite their effectiveness and widespread use, pain management with opioids presents many well-recognized challenges. Acute adverse effects associated with opioid administration include nausea, vomiting, itching and the potential for respiratory depression. In addition, constipation and bowel dysfunction are commonly experienced. Actions in the central nervous system induce sedation, dizziness and cognitive impairment. Finally, another highly visible issue is the potential for opioid abuse. Many companies have developed or are developing formulations in an attempt to alleviate the adverse side effects or the abuse potential of commonly used opioid analgesics.
NeurogesX has designed and synthesized prodrugs of a number of commonly prescribed opioid analgesics. We believe that our novel compounds, which are prodrugs of existing well known and widely used opioids, may have inherent abuse resistance, the potential for decreased gastro-intestinal side effects for oral dosage forms, and facilitate bolus dosing while potentially limiting peak systemic exposure from these injected or infused compounds. Our most advanced compound, NGX-6052, has been evaluatedin vitro andin vivo proof-of-concept studies.
Manufacturing
We do not own facilities for the manufacture of any products or product candidates. We utilize contract manufacturers to produce clinical supplies of the active ingredient in Qutenza, synthetic capsaicin, as well as the Qutenza dermal patch, the associated cleansing gel and the fully assembled Qutenza treatment kit. Although we intend to continue to rely on contract manufacturers to produce our products for both clinical and commercial supplies, we oversee the production of the Qutenza treatment kit and each of its components.
There are multiple primary raw material components of our synthetic capsaicin, all but one is generally available from more than one supplier. We currently obtain our supplies of synthetic capsaicin from Formosa Laboratories in Taiwan, who obtains the raw materials from qualified suppliers. While Formosa is our sole supplier currently, other potential suppliers exist and we may qualify a second source of supply after initial market approval.
We have only one supplier for our clinical and commercial supply of Qutenza. We have engaged LTS Lohmann Therapie-Systeme AG, or LTS, in Germany as the exclusive manufacturer to formulate the active ingredient from a powder into a dermal patch. Under the terms of our clinical supply, development and license agreement and our commercial supply and license agreement with LTS, we are obligated to purchase all of our Qutenza clinical and commercial supply requirements from LTS. The terms of our clinical supply, development and license agreement with LTS will remain in effect, subject to bi-annual renewals at our election, the current renewal period expires in June 2010, until we obtain regulatory approval for, and begin to commercialize Qutenza in a particular territory, at which time, we will obtain our supply of Qutenza for that territory under the commercial supply and license agreement. The commercial supply and license agreement establishes standard commercial terms on which product will be supplied, as well as continuing the licenses initially granted in the
15
clinical supply agreement. The term of the commercial supply and license agreement expires 10 years from the delivery of product first ordered under the agreement and automatically renews for additional two year terms, unless terminated by either party with two years prior written notice.
For our clinical and commercial supply of our cleansing gel, we have engaged Contract Pharmaceuticals Limited as the manufacturer. While we currently have only one supplier for the cleansing gel, we believe there are a number of potential suppliers and intend to evaluate the need for qualifying a second source of supply if we commercialize Qutenza.
In Europe, we have engaged Grenzach Produktions GmbH, or Grenzach, to prepare the commercial product package containing the Qutenza patch and cleansing gel. Grenzach will also assemble the Qutenza treatment kit (containing components such as nitrile gloves, gauze, package insert), including sourcing all component parts, packaging and preparation for distribution. In the United States, we intend to engage at least one company to carry out the treatment kit assembly process, and we believe there are numerous potential candidates to fill this role.
If we obtain FDA approval, or approval outside the United States, for our product candidates, including Qutenza, we plan to rely on contract manufacturers to produce sufficient quantities for large scale commercialization. These contract manufacturers will be subject to extensive governmental regulation, including initial site inspections to gain market approval and periodic ongoing inspections. Regulatory authorities in the markets that we intend to serve require that drugs be manufactured, packaged and labeled in conformity with current Good Manufacturing Practices, or cGMPs. In this regard, we plan to engage only contract manufacturers who have the capability to manufacture drug products in compliance with cGMPs in bulk quantities for commercialization.
Sales, Marketing and Distribution
If Qutenza receives marketing approval from the FDA, we currently plan to build our own U.S. sales force to market Qutenza directly to approximately 5,000 pain centers and 10,000 physicians in the United States who prescribe a majority of neuropathic pain medications. We anticipate that we would initially launch Qutenza on a limited basis, targeting major population centers in the United States with high incidence rates of PHN. We plan to fund expansion of our sales efforts for Qutenza outside of the initially targeted markets if additional financial resources become available. We are also evaluating the potential to launch Qutenza in the United States under marketing or commercial partnerships with other companies, which may enable us to launch Qutenza more broadly in the United States.
For the European Union, we are currently in discussions for a potential commercial partnership. Outside of the United States and the European Union, and subject to marketing approval in the relevant countries, we intend to engage sales, marketing and distribution partners.
Competition
If Qutenza receives marketing approval, it will compete against, and may be used in combination with, well-established products currently used both on and off-label in our target indications. The most directly competitive currently marketed products in the United States are Lidoderm, an FDA-approved 5% lidocaine topical patch for the treatment of PHN marketed by Endo Pharmaceuticals and Lyrica, an oral anti-convulsant, marketed by Pfizer for use in the treatment of PHN. In addition to these branded drugs, the FDA has approved gabapentin (Neurontin) for use in the treatment of PHN. Pfizer has also received FDA approval of Lyrica for the treatment of PDN, fibromyalgia and epilepsy indications. The FDA has approved Cymbalta from Eli Lilly for use in the treatment of PDN, general anxiety disorder and depression.
By the time we are able to commercialize a product candidate, the competition and potential competition may be greater and more direct. There are many other companies working to develop new drugs and other
16
therapies to treat pain in general and neuropathic pain in particular, including GlaxoSmithKline, Abbott Laboratories, Depomed, Inc., Newron Pharmaceuticals S.p.A., Novartis AG, UCB S.A. and Eli Lilly. Some of the compounds in development by such companies are already marketed for other indications, such as depression and epilepsy. Other companies are focusing on new compounds or reformulations of existing compounds such as sustained release gabapentin.
We expect to compete on, among other things, the safety and efficacy of our products. Competing successfully will depend on our continued ability to attract and retain skilled and experienced personnel, to identify, secure the rights to and develop pharmaceutical products and compounds and to exploit these products and compounds commercially before others are able to develop competitive products. In addition, our ability to compete may be affected because insurers and other third-party payors in some cases seek to encourage the use of generic products making branded products less attractive, from a cost perspective, to buyers.
Patents and Proprietary Rights
Our success will depend in part on our ability to protect Qutenza and future products and product candidates by obtaining and maintaining a strong proprietary position both in the United States and in other countries. To develop and maintain our proprietary position, we will rely on patent protection, regulatory protection, trade secrets, know-how, continuing technological innovations and licensing opportunities.
The commercial success, if any, of Qutenza depends, in part, on a device patent granted in the United States, certain European Union countries and Hong Kong. The device patent covers the use of a high-concentration-capsaicin dermal patch for the treatment of neuropathic pain. We exclusively license these patents, as well as a related patent granted in Canada, from the University of California. We do not currently own, and do not have rights under this license agreement to any issued patents that cover Qutenza outside of the United States, Canada, certain European Union countries and Hong Kong.
We license a method patent granted in the United States from the University of California concerning the use of high-concentration capsaicin delivery for the treatment of neuropathic pain. Two of the three inventors named in the method patent did not assign their patent rights to the University of California. As a result, our rights under this patent are non-exclusive. Anesiva, a company also focused on the development and commercialization of treatments for pain, has licensed the right to use the technology under the method patent from one of the non-assigning inventors. There can be no assurances that other entities will not similarly obtain rights to use the technology under the method patent. If other entities license the right to use this patent, we may face more products competitive with Qutenza and our business will suffer.
Under the terms of our license agreement with the University of California, we will be required to pay royalties on net sales of the licensed product up to a maximum of $1.0 million per annum as well as a percentage of upfront and milestone payments resulting from the sublicense of our rights under the agreement. We are also required to make three annual cash payments commencing in 2008 with an aggregate amount of approximately $12,000.
We currently license the rights from LTS to three pending U.S. patent applications, patents granted in certain countries of Europe and pending patent applications in Europe, Canada and other foreign countries, each filed and prosecuted by LTS. These patent applications seek to cover a microreservoir patch, which includes the type of patch used in Qutenza. We license the rights to these patents and patent applications under a January 2007 exclusive commercial supply and license agreement with LTS, which is subject to certain purchase and other obligations. We will be required to pay LTS royalties on net sales of the licensed product in addition to a one-time payment of €100,000 if Qutenza is approved for marketing in certain territories.
We have also filed several patent applications in the United States and certain other countries including the European Union, relating to kits, methods and formulations to remove residual capsaicin left on the skin after a
17
topical application of capsaicin, as well as a number of patent applications which deal with differing formulations and delivery models of capsaicin at varying concentrations, including an application that supports our NGX-1998 program. We have also filed patent applications related to our acetaminophen prodrugs and opioid prodrug platform which contain both composition and method claims. We have also licensed an issued patent which we believe to be relevant to our opioid prodrug platform.
The patent positions of pharmaceutical companies like us are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, we do not know whether any of the products or product candidates we acquire or license will result in the issuance of patents or, if any patents are issued, whether they will provide significant proprietary protection or will be circumvented or challenged and found to be unenforceable or invalid. For example, one or more of the inventors named in the method patent which we have licensed from the University of California, may assert a claim of inventorship rights to the device patent, also licensed from the University of California, which could result in our loss of exclusive use of this patent. Although we do not believe these individuals are co-inventors, there can be no assurance that we would prevail if such a claim were asserted. The absence of exclusive rights to utilize such patent exposes us to a greater risk of direct competition and could materially harm our business. In addition, other parties may own patent rights that might be infringed by our products or other activities. For example, in 2005 and again in 2007, Winston Laboratories informed us of their U.S. patent related to ciscapsaicin, and suggested that our synthetic capsaicin formulation could infringe this patent. We responded by denying any infringement. We believe that our products, if commercialized, will not infringe the Winston patent, which is due to expire in 2009, but may be extended under certain circumstances. In limited instances, patent applications in the United States and certain other jurisdictions are maintained in secrecy until patents issue, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention or in opposition proceedings in a foreign patent office, any of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that a court of competent jurisdiction would hold the patents, if issued, valid. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using such technology. To the extent prudent, we intend to bring litigation against third parties that we believe are infringing our patents. We also rely on trade secret protection for our confidential and proprietary information. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology or that we can meaningfully protect our trade secrets. However, we believe that the substantial costs and resources required to develop technological innovations will help us protect our products.
It is our policy to require our employees, consultants, contractors, or scientific and other advisors, to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. These agreements provide that all inventions related to our business that are conceived by the individual during the course of our relationship, shall be our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Government Regulation
United States
Regulation by governmental authorities in the United States and other countries is a significant factor in the development, manufacture and marketing of pharmaceuticals. All of our product candidates require regulatory approval by governmental agencies prior to commercialization. In particular, our products candidates are subject
18
to rigorous preclinical testing and clinical trials and other premarketing approval requirements by the FDA and regulatory authorities in other countries. Various federal, state and foreign statutes and regulations govern or affect the manufacturing, safety, labeling, storage, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. When and if regulatory approval is obtained for any of our product candidates, the approval may be limited in scope, which may significantly limit the indicated uses for which our product candidates may be marketed, promoted and advertised. Additionally, approval may be conditioned upon our agreement to conduct further studies, which could either delay our planned product launch and/or significantly increase our costs in order to comply with these commitments. Further, approved pharmaceuticals and manufacturers are subject to ongoing review and discovery of previously unknown problems that may result in restrictions on the manufacture, sale or use of approved pharmaceuticals or in their withdrawal from the market.
Preclinical Studies
Before testing any compounds with potential therapeutic value in human subjects in the United States, stringent governmental requirements for preclinical data must be satisfied. Preclinical testing includes both in vitro and in vivo laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Preclinical testing results obtained from these studies, including tests in several animal species, are submitted to the FDA as part of an investigational new drug application, or IND, and are reviewed by the FDA prior to the commencement of human clinical trials. These preclinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers.
Clinical Trials
If a company wants to conduct clinical trials in the United States to test a new drug in humans, an IND must be prepared and submitted with the FDA. The IND becomes effective, if not rejected or put on clinical hold by the FDA, within 30 days of filing the application. In addition, an Institutional Review Board must review and approve the trial protocol and monitor the trial on an ongoing basis. The FDA may, at any time during the 30-day review period or at any time thereafter, impose a clinical hold on proposed or ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization and then only under terms authorized by the FDA. The IND application process can result in substantial delay and expense.
Clinical Trial Phases
Clinical trials typically are conducted in three sequential phases, Phases 1, 2 and 3, with Phase 4 trials potentially conducted after marketing approval. These phases may be compressed, may overlap or may be omitted in some circumstances.
| | • | | Phase 1 clinical trials. After an IND becomes effective, Phase 1 human clinical trials can begin. These trials evaluate a drug’s safety profile and the range of safe dosages that can be administered to healthy volunteers or patients, including the maximum tolerated dose that can be given to a trial subject. Phase 1 trials also determine how a drug is absorbed, distributed, metabolized and excreted by the body, and duration of its action. |
| | • | | Phase 2 clinical trials. Phase 2 clinical trials are generally designed to establish the optimal dose, to evaluate the potential effectiveness of the drug in patients who have the target disease or condition and to further ascertain the safety of the drug at the dosage given in a larger patient population. |
| | • | | Phase 3 clinical trials. In Phase 3 clinical trials, the drug is usually tested in a controlled, randomized trial comparing the investigational new drug to a control (which may be an approved form of therapy) in an expanded and well-defined patient population and at multiple clinical sites. The goal of these trials is to obtain definitive statistical evidence of safety and effectiveness of the investigational new drug regime as compared to control in defined patient populations with a given disease and stage of illness. |
19
Additionally, the Food and Drug Administration Amendments Act of 2007, or FDAAA, requires that all controlled clinical trials conducted for our drug candidates be included in a clinical trials registry database that is available and accessible to the public through the internet. If we fail to properly participate in the clinical trial database registry we would be subject to significant civil monetary penalties.
Manufacturing Process Development
In order to gain marketing approval, a product candidate’s manufacturing process must be evaluated through a lengthy and detailed review process to ensure that it can be consistently manufactured to meet predetermined specifications. A robust manufacturing process must be developed and validated for both the active ingredient and the formulated product candidate which ensures that the product can be reproducibly manufactured at the intended commercial scale. Appropriate specifications must be developed and approved to ensure that the quality and safety of the product can be assured. Analytical methods used for quality control testing must be developed and validated to ensure each lot of product meets the approved specifications. Stability testing of active ingredient and drug product must be performed to provide evidence that the product remains stable over time and that a shelf life for the product can be established. The FDA reviews the adequacy of the manufacturing process, specifications, quality control testing and stability during the NDA application process. In addition, an inspection of the manufacturing site is performed to ensure the adequacy of the manufacturing facility to meet both the technical manufacturing requirements and compliance to current good manufacturing practices or cGMP regulations.
New Drug Application
After completion of clinical trials, if there is substantial evidence that the drug is both safe and effective, an NDA is prepared and submitted for the FDA to review. The NDA must contain all of the essential information on the drug gathered to that date, including data from preclinical studies and clinical trials, and the content and format of an NDA must conform with all FDA regulations and guidelines. In addition, the FDA generally requires successful completion of at least two adequate and well-controlled Phase 3 clinical trials to gain marketing approval for an indication. Accordingly, the preparation and submission of an NDA is an expensive and major undertaking.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information from the sponsor rather than accepting an NDA for filing. In such an event, the NDA must be submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. By law, the FDA has 180 days in which to review the NDA and respond to the applicant. Under the goals and policies agreed to by the FDA under PDUFA IV, the FDA has 10 months from the submission date in which to complete its initial review of a standard NDA and respond to the applicant and six months from the submission date for a priority NDA. The FDA may meet its PDUFA requirements by completing its initial review within the specified time-frames 90% of the time. Additionally, the FDA does not always meet the PDUFA goal dates for standard or priority NDAs. The review process is often significantly extended as a result of FDA requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation as to whether the application should be approved and the scope of any approval. The FDA is not bound by the recommendation, but gives great weight to it. Upon completion of the FDA’s review of the NDA, it issues either an approval letter or a complete response letter. A complete response letter may describe any activities which may be required to gain approval or may indicate that a product candidate is not approvable.
The Hatch-Waxman Act
Under the Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, newly approved drugs may benefit from a statutory period of non-patent data exclusivity in the United
20
States. The Hatch-Waxman Act provides 5 years of data exclusivity to the first applicant to gain approval of an NDA under Section 505(b) of the Food, Drug and Cosmetic Act for a new chemical entity. A drug qualifies as a new chemical entity if the FDA has not previously approved any other drug containing the same active ingredient. Hatch-Waxman provides data exclusivity by prohibiting abbreviated new drug applications, or ANDAs, and 505(b)(2) applications, which are marketing applications where the applicant does not own or have a legal right of reference to all the data required for approval, to be submitted by another company for another version of such drug during the exclusivity period. Protection under Hatch-Waxman will not prevent the filing or approval of a full NDA under Section 505(b)(1) for the same active ingredient, although the applicant would be required to conduct its own adequate and well-controlled clinical trials to demonstrate safety and effectiveness. Our NDA for Qutenza was filed as a 505(b)(2) application as our application referenced certain publicly available pre-clinical data regarding capsaicin. Therefore, if another product containing the same active ingredient as Qutenza is approved before Qutenza, then our potential approval could be delayed by 5 years. However, in such event, we believe we can petition the FDA to modify our application to be under 505(b)(1). While such petition would require the FDA’s approval, and there can be no assurance that such petition would be granted by the FDA, we believe that reference to third party information may be removed from our application and that our proprietary pre-clinical data would be sufficient to support an NDA.
The Hatch-Waxman Act also provides three years of data exclusivity for the approval of supplemental NDAs for new indications, dosages or strengths of an existing drug if new clinical investigations are essential to the approval. This three-year exclusivity covers only the changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient.
The Hatch-Waxman Act also permits a patent extension term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent extension cannot extend the remaining term of a patent beyond a total of 14 years. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and it must be applied for prior to expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for patent term extension. We are considering applying for a patent term extension for one of our current patents associated with Qutenza.
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting an NDA. If the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product that has orphan drug designation subsequently receives FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for up to seven years after receiving FDA approval.
We were granted orphan status for the use of capsaicin to treat painful HIV-associated neuropathy in May 2003. When appropriate, we intend to seek orphan status for additional indications and products. We cannot predict the ultimate impact, if any, of orphan status on the timing or likelihood of FDA approval on any of our potential products.
Fast Track Designation and Priority Review
The FDA’s fast track program is intended to facilitate the development and expedite the review of drugs that are intended for the treatment of a serious or life-threatening condition for which there is no effective treatment
21
and which demonstrate the potential to address unmet medical needs for their condition. We were granted fast track designation of Qutenza for treatment of HIV-DSP in July 2004. When appropriate, we intend to seek additional fast track designations for our products, although we did not seek such designation for our NDA for Qutenza in PHN. We cannot predict the ultimate impact, if any, of the fast track process on the timing or likelihood of FDA approval on any of our potential products.
In some cases, after an NDA has been accepted for review by the FDA, the FDA may designate a product for priority review. A product is eligible for priority review, or review within a targeted six-month time frame from the time an NDA is accepted for filing, if the product provides a significant improvement compared to marketed products in the treatment, diagnosis or prevention of a disease. A fast-track designated product generally meets the FDA’s criteria for priority review. We cannot guarantee any of our products will receive a priority review designation, or if a priority designation is received, that review or approval will be faster than conventional FDA procedures.
If we seek approval for HIV-DSP from the FDA, we intend to seek and we believe that we may be granted priority review for Qutenza in the treatment of HIV-DSP as there are currently no approved drugs for the treatment of this disease. However, there can be no assurance that we will be granted priority review.
Other Regulatory Requirements
Any products we manufacture or distribute under FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP, regulations which impose procedural and documentation requirements upon us and each third party manufacturer we utilize.
The FDA closely regulates the marketing and promotion of drugs. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturers from communicating on the subject of off-label use.
The FDA’s policies may change and additional government regulations may be enacted which could prevent or delay regulatory approval of Qutenza and our future product candidates or approval of new indications for our future products. We cannot predict the likelihood, nature or extent of adverse governmental regulations that might arise from future legislative or administrative action, either in the United States or abroad.
European Union
Clinical Trials
In common with the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. The regulatory controls on clinical research in the European Union are now largely harmonized following the implementation of the Clinical Trials Directive 2001/20/EC, or CTD. Compliance with the national implementations of the CTD has been mandatory from May 1, 2004. However, variations in the member state regimes continue to exist, particularly in the small number of member states that have yet to implement the CTD fully. Clinical trials must be separately authorized in each European Union member state where they are conducted.
22
All member states currently require regulatory and independent ethics committee approval of interventional clinical trials, as well as informed consent and other measures to protect the interest of human subjects. European regulators and ethics committees also require the submission of adverse event reports during a study and a copy of the final study report. Procedures exist to suspend studies if necessary to protect the safety of subjects.
Marketing Authorization
In the European Union, approval of new medicinal products can be obtained through the mutual recognition procedure or the centralized procedure. The mutual recognition procedure entails initial assessment by the national authorities of a single member state and subsequent review by national authorities in other member states based on the initial assessment. The centralized procedure entails submission of a single MAA to the EMEA leading to an approval that is valid in all European Union member states. Our MAA has been accepted for review under the centralized procedure.
Under the centralized procedure, an MAA is submitted to the EMEA. Two European Union member states are appointed to conduct an initial evaluation of each MAA. In the case of our MAA for Qutenza, Portugal and Hungary were appointed for the initial evaluation. These countries each prepare an assessment report, which are then used as the basis of a scientific opinion of the CHMP. Before the opinion is issued, there is an opportunity for the applicant to respond to questions and, in most cases, to make a presentation to the CHMP. If this opinion is favorable, it is sent to the European Commission, which drafts a decision. After consulting with the member states, the European Commission adopts a decision and grants a marketing authorization, which is valid throughout the European Union and confers the same rights and obligations in each of the member states as a marketing authorization granted by that member state.
In September 2007 we submitted a MAA for Qutenza with the EMEA under the centralized procedure, seeking approval of Qutenza. On March 19, 2009, the CHMP issued a positive opinion recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. In conjunction with issuing this recommendation, the CHMP requested us to perform certain clinical evaluations of Qutenza following approval. We are currently awaiting the European Commission’s decision on the CHMP opinion, a process which normally takes approximately 60 to 90 days. If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
Data Exclusivity
For complete and independent applications for new active substances submitted after November 20, 2005, European Union law provides a data exclusivity period of eight years from initial authorization of the reference product during which generic drug manufacturers cannot file abridged applications. This is followed by an additional two years data exclusivity during which generic applications may be submitted, reviewed and approved but during which generic drug manufacturers cannot place their product on the market. The ten year marketing protection may be extended by one year if a new therapeutic indication is granted during the first eight years since the initial marketing authorization, and, if it represents a significant clinical benefit in comparison to existing therapies. These periods of exclusivity do not preclude a court challenge by a competitor attempting to abridge the data and place a generic product on the market at an earlier time.
Other Regulatory Requirements
The holder of a marketing authorization is subject to ongoing regulatory obligations including record keeping requirements and adverse event reporting, manufacturing compliance with cGMP, and compliance with rules and regulations governing advertising and promotion. While the legal responsibility and liability of a marketing authorization holder, or MAH, cannot be delegated, the MAH can delegate the performance of related tasks to third parties, provided that this delegation is appropriately documented.
23
We may hold marketing authorizations for our products in our own name, or appoint an affiliate or a collaboration partner to hold the marketing authorization on our behalf. Any failure by an MAH to comply with ongoing regulatory obligations may result in regulatory action against the MAH and its approvals and ultimately threaten our ability to commercialize our products.
Approvals Outside of the United States and the European Union
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products. Whether or not FDA approval or European marketing authorization has been obtained, approval of a product by the comparable regulatory authorities of other foreign countries must still be obtained prior to manufacturing or marketing the product in those countries. The approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval or a European marketing authorization. We offer no assurance that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
Third-Party Reimbursement and Pricing Controls
General. In the United States and elsewhere, patients’ access to pharmaceutical products depends in significant part on the coverage and reimbursement of a product or service by third party payors, such as government programs, private insurance plans and employers. Third party payors increasingly are challenging the medical necessity of and prices charged for medical products and services. It will be time consuming and expensive for us to go through the process of seeking reimbursement from Medicare, Medicaid and private payors. We may be unable to achieve reimbursement from some payors because they may not consider our products to be “reasonable and necessary” or cost-effective. Furthermore, it is possible that even if payors are willing to reimburse for our products, the reimbursement levels may not be sufficient to allow us to sell our products on a competitive and profitable basis.
In many foreign countries, particularly the countries in the European Union, the pricing of prescription drugs is subject to direct governmental control and is influenced by drug reimbursement programs that employ a variety of price control mechanisms. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from country to country. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to restrict the entry of new products, as exemplified by the National Institute for Clinical Excellence in the United Kingdom which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert a commercial pressure on pricing within a country.
In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to implement means by which the government can negotiate lower drug prices for Medicare and Medicaid beneficiaries. While we cannot predict whether such legislative bills will become law, their enactment could have a material adverse effect on our business, financial condition and results of operations.
Medicare. Subject to obtaining required marketing approvals, we plan to market Qutenza for use in the treatment of PHN. We expect that in the United States a majority of the patients who are treated with Qutenza for this indication will be Medicare beneficiaries. CMS is the agency within the Department of Health and Human Services that administers both the Medicare and Medicaid programs. Because Qutenza is a dermal patch which
24
we expect will require administration by a healthcare professional, two elements of Medicare reimbursement may be relevant to Qutenza: the availability of reimbursement for services to administer Qutenza and the availability of reimbursement for Qutenza itself.
CMS has the authority not to cover particular products or services if it determines that they are not “reasonable and necessary” for the treatment of Medicare beneficiaries. CMS may make a national coverage determination, or NCD, for a product, which establishes on a nationwide basis the indications that will be covered, and any restrictions or limitations. However, for most new drugs that are eligible for payment, CMS does not create an NCD. We currently do not anticipate seeking an NCD for Qutenza. However, CMS or a third party may request an NCD independent of us. If such request is made, we can not assure you that such NCD will contain favorable coverage terms.
If there is no NCD, the local Medicare contractors that are responsible for administering the program on a state or regional basis have the discretion to deny coverage and reimbursement for the drug or issue a local coverage determination, or LCD. These LCDs can include both coverage criteria for the drug and frequency limits for the administration of the drug. The local contractors in different areas of the country may determine that Qutenza should be treated like most patches and may deny coverage under Part B or, even if they allow coverage, may establish varying coverage criteria and frequency limits for Qutenza. Furthermore, overturning restrictive LCDs in the various regions can be a time-consuming and expensive process.
We are currently evaluating various methods of gaining the most advantageous reimbursement scenario for Qutenza. We currently anticipate that we will seek reimbursement under Medicare Part B. As mentioned above, if Medicare coverage for Qutenza is available, CMS may determine to reimburse through one of two avenues: Part B coverage for physician-administered drugs or Part D coverage for outpatient prescription drugs. Under Part B coverage, Medicare reimburses physicians for purchasing and administering drugs that meet the following statutory requirements:
| | • | | The product is reasonable and necessary; |
| | • | | The product is not usually self-administered; |
| | • | | The product is administered in conjunction with a physician’s service; and |
| | • | | The administering physician bills Medicare directly for the product. |
Currently, topical products are considered “usually self-administered;” therefore, coverage under Part B would require a specific determination that Qutenza differs from most topical products and should therefore be covered under Part B. There can be no guarantee that we will obtain such a determination. For reasons discussed below, failure to obtain such a determination could materially and adversely affect our revenue.
Medicare payment for physician services related to the administration of Qutenza, if any, will be determined according to a prospectively set payment rate, linked to a procedure code established by the American Medical Association. These codes, called Current Procedural Terminology, or CPT, describe the procedure performed. We believe that existing CPT codes are inadequate for our use and that a specific code for Qutenza administration may be required. We currently plan to apply for a specific CPT code. At launch local Medicare contractors will require claims to be submitted with an existing miscellaneous CPT code until such time as we are granted a specific CPT code. Use of miscellaneous codes causes claims processing delays and may lead to lower payments to physicians. An alternative to seeking a specific CPT code and the use of miscellaneous codes in the interim, we may pursue reimbursement of physician services associated with a Qutenza treatment procedure utilizing existing office visit codes, such as evaluation and management services codes, which have established reimbursement amounts.
Under Medicare Part B, reimbursement for Qutenza is currently limited to 106% of the manufacturer’s average sales price (as defined by statute and regulation). CMS has been considering other changes to Medicare
25
reimbursement that could result in lower payments for physician-administered drugs, and Congress may also consider legislation that would mandate lower reimbursement levels. A reduction in reimbursement levels could materially and adversely affect our revenue.
CMS may determine that Qutenza does not qualify for Part B coverage and should instead be covered under the Part D outpatient prescription drug benefit. Unlike Part B, Part D reimburses only for the drug itself and does not provide reimbursement for the physician’s administration services. Even though a product is reimbursed under Part D, local contractors may permit physicians to bill under Part B for their administration services. Physicians may not consider Qutenza as attractive a treatment option if it is reimbursed under Part D instead of Part B. In addition, under Part D, there are multiple types of plans and numerous plan sponsors, each with its own formulary and product access requirements. While CMS evaluates Part D plans’ proposed formularies for potentially discriminatory practices, the plans have considerable discretion in establishing formularies, establishing tiered co-pay structures and placing prior authorization and other restrictions on the utilization of specific products. Moreover, Part D plan sponsors are permitted and encouraged to negotiate rebates with manufacturers. Revenue for Qutenza will be substantially affected by its formulary status on Part D plans and the rebates that Part D plan sponsors are able to negotiate.
Medicaid. Most State Medicaid programs have established preferred drug lists, or PDLs, and the process, criteria and timeframe for obtaining placement on the PDL varies from State to State. A federal law establishes a minimum rebate, currently 15.1%, that a manufacturer must pay for Medicaid utilization of a brand-name product, and many States have established supplemental rebate programs as a condition for including a drug product on a PDL. Submitting a PDL application to each State will be a time-consuming and expensive process, and it is not clear how many or which State programs will accept the applications. Review times for these applications can vary from weeks to 14 months or more.
Private Insurance Reimbursement. Commercial insurers usually offer two types of benefits: medical benefits and pharmacy benefits. In most private insurance plans, physician-administered drugs are provided under the medical benefit. If private insurers decide to cover Qutenza, they will reimburse for the drug and its administration in a variety of ways, depending on the insurance plan’s policies, employer and benefit manager input and contracts with their physician network. Like Medicare and Medicaid, commercial insurers have the authority to place coverage and utilization limits on physician-administered drugs. Private insurers tend to adopt reimbursement methodologies for a product similar to those adopted by Medicare. Revenue for Qutenza may be materially and adversely affected if private payors make unfavorable reimbursement decisions or delay making favorable reimbursement decisions.
Employees
As of December 31, 2008, we had 42 employees, of which 26 work in research and development, 14 work in general and administrative and 2 work in sales and marketing. None of our employees are represented by a labor union or are the subject of a collective bargaining agreement.
Facilities
We lease approximately 26,386 square feet of space in our headquarters in San Mateo, California under a lease that expires in July 2012. We have no laboratory, research or manufacturing facilities.
Form of Organization
We were incorporated in California as Advanced Analgesics, Inc. on May 28, 1998 and changed our name to NeurogesX, Inc. in September 2000. In February 2007, we reincorporated into Delaware.
26
Available Information
We file electronically with the Securities and Exchange Commission, or SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. The public may read or copy any materials we file with the SEC at the SEC’s Public Reference Room at 450 Fifth Street, NW, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov.
You may obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports on the day of filing with the SEC on our website on the World Wide Web at http://www.neurogesx.com or by contacting our corporate offices by calling 650-358-3300. Information contained on our website is not part of this report or any other report filed with the SEC.
Our future operating results may vary substantially from anticipated results due to a number of factors, many of which are beyond our control. The following discussion highlights some of these factors and the possible impact of these factors on future results of operations. You should carefully consider these factors before making an investment decision. If any of the following factors actually occur, our business, financial condition or results of operations could be harmed. In that case, the price of our common stock could decline, and you could experience losses on your investment.
Risks Related to our Business
Our success depends on our ability to obtain U.S. regulatory approval for our lead product candidate, Qutenza.
Our success depends substantially on obtaining regulatory approval for our most advanced product candidate, Qutenza, a dermal patch containing a high concentration of synthetic capsaicin. Regulatory approval in the United States requires the completion of extensive non-clinical and clinical evaluation of a product candidate to demonstrate substantial evidence of safety and efficacy of the product candidate, as well as development of manufacturing processes which demonstrate the ability to reliably and consistently produce the product candidate under current Good Manufacturing Practice regulations. Each of these elements of our Qutenza development program include certain judgments of applicable regulatory requirements of what will ultimately be deemed acceptable to the regulatory authority including the FDA’s evaluation of additional data, such as a “responder” analysis and other secondary endpoints when evaluating whether our product can be approved. The FDA in reviewing our application will evaluate all components of that program including conducting audits of clinical sites used in our clinical trials and inspecting our manufacturing sites to ensure compliance with regulatory requirements. There can be no assurance that any or all aspects of our development program or our manufacturing processes will satisfy the regulatory requirements for approval, the failure of which to do so would significantly delay or even prevent approval of our product candidate and seriously harm our ability to generate revenue. Qutenza has been evaluated in three completed Phase 3 clinical trials for the management of pain associated with PHN, one of which did not meet its primary endpoint, and two completed Phase 3 clinical trials for the management of pain associated with HIV-DSP, one of which did not meet its primary endpoint. The FDA generally requires successful completion of at least two adequate and well-controlled Phase 3 clinical trials for each indication for which we seek marketing approval before submission of an NDA. Although our analyses of two Phase 3 studies in PHN indicated that their primary endpoints were met, the FDA may not agree with our analyses and may require that we complete additional studies or perform other activities to support an approval of the PHN indication. We may not have adequate financial or other resources to pursue this product candidate
27
through regulatory approval or through commercialization. If we do not receive marketing approval from the FDA we will not be able to commercialize Qutenza in the United States. Further, if the FDA delays approval as a result of requirements to conduct additional clinical studies, commercialization of Qutenza could be significantly delayed. Significant delay or the inability to commercialize Qutenza in the United States would significantly harm our business and as a result we may be unable to become profitable or continue our operations, or, even if we are able to commercialize in the United States, there can be no assurance that we can become profitable. As a consequence of any of these factors, our stock price would be adversely affected. We have filed an NDA with the FDA for Qutenza for PHN. We are continuing to evaluate whether to seek approval for Qutenza in HIV-DSP and whether additional studies would be necessary to achieve such an approval. If we decide to seek approval in HIV-DSP, we would do so no sooner than after the FDA has completed its review of the PHN NDA submission, which we anticipate may occur by the PDUFA date of August 16, 2009, but could be significantly longer. Further, we may decide not to conduct further Qutenza studies in HIV-DSP and ultimately may not seek approval of Qutenza in the HIV-DSP indication, in which case our potential revenues could be negatively impacted.
We may not be successful in obtaining European regulatory approval for Qutenza.
On March 19, 2009, we received a positive opinion recommending the approval of our MAA from the CHMP for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. We are currently awaiting the European Commission’s decision on the CHMP opinion, a process which normally takes approximately 60 to 90 days. Although generally the recommendations of the CHMP are adopted by the European Union at large, there can be no assurance that that will be the case with Qutenza. A decision by the European Union member states to not adopt the recommendation of the CHMP would cause us to not be able to commercialize Qutenza in Europe and consequently, our ability to generate revenue would be significantly harmed.
If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
We will require substantial additional funding and may be unable to raise capital when needed.
We had cash, cash equivalents and short-term investments totaling $24.5 million at December 31, 2008 and for the year then ended, we used cash of $27.3 million in operating activities. Although we have deferred clinical programs and reduced our planned spending for 2009 until such time as additional resources are attained, we expect our negative cash flows from operations to continue beyond potential regulatory approval and product launch of Qutenza and there can be no assurance that we will ever achieve positive cash flows from operations. We believe, based on our current operating plan that our cash, cash equivalents and short-term investments will be sufficient to fund our operations through at least December 31, 2009. However, our planned activities beyond 2009 including the establishment of a sales and marketing organization to launch Qutenza, if approved, in the United States along with continuing our development programs for Qutenza, NGX-1998 and our other development programs will require substantial additional funding. There can be no assurance that additional funding will be available on terms that are acceptable, or at all.
We have no manufacturing capabilities and depend on other parties for our manufacturing operations. If these manufacturers fail to meet our requirements and strict regulatory requirements, our product development efforts may be negatively affected, we may not be able to obtain regulatory approval for our product candidates and our commercialization efforts may be materially harmed.
We currently depend on three contract manufacturers as single source suppliers for the components of our Qutenza product candidate: synthetic capsaicin, the dermal patch and the associated cleansing gel. We have entered into long term commercial supply agreements for these components. In addition we anticipate entering into long-term agreements for the assembly of the Qutenza treatment kits in the United States and the European Union. If our relationship with any of these manufacturers is terminated, or if any manufacturer is unable to produce required quantities on a timely basis or at all, our operations would be delayed and our business harmed.
28
Our reliance on contract manufacturers exposes us to additional risks, including:
| | • | | failure of our current and future manufacturers to comply with strictly-enforced regulatory requirements; |
| | • | | failure of our current and future manufacturers to complete the development and scale-up of the manufacturing process including adequately analyzing and documenting the source and chemical make-up of ingredients that make up our product candidate and the ability to reliably and consistently produce the product candidate under current Good Manufacturing Practice regulations; |
| | • | | failure to manufacture to our specifications, or to deliver sufficient quantities in a timely manner; |
| | • | | the possibility that we may terminate a contract manufacturer and need to engage a replacement; |
| | • | | the possibility that our current and future manufacturers may not be able to manufacture our product candidates and products without infringing the intellectual property rights of others; |
| | • | | the possibility that our current and future manufacturers may not have adequate intellectual property rights to provide for exclusivity and prevent competition; and |
| | • | | insufficiency of intellectual property rights to any improvements in the manufacturing processes or new manufacturing processes for our products. |
Any of these factors could result in significant delay or suspension of our clinical trials, regulatory submissions, receipt of required approvals or commercialization of our products and harm our business.
Before a new drug can be marketed in the United States, three consecutive manufacturing runs have to be completed and those manufacturing runs need to be validated to ensure that manufacturing processes are reliable. Required validation of the manufacturing processes of our third party suppliers to secure FDA approval for Qutenza has not yet been completed. While our plans contemplate having these process validations complete in 2009, if validation of our third party supplier manufacturing processes is delayed or if our suppliers fail to complete the requisite validation batches, even if all other aspects of our NDA are approvable by the FDA, commercial sales in the United States may need to be delayed until validation can be successfully completed, if at all.
In addition, because our third party manufacturers operate outside of the United States, and many of the raw materials and the labor that are used to manufacture our product candidates are based in foreign countries, we may experience currency exchange rate risks, even though, in some instances our contracts are denominated in U.S. dollars. We do not currently engage in forward contracts to hedge this currency risk and as a result, may suffer adverse financial consequences as a result of this currency risk.
Further, materials used by these entities to manufacture our product candidates originate outside the United States, including certain materials which may be sourced from China and India. The FDA has increased its diligence with regard to foreign sourced materials and manufacturing processes which may result in increased costs of maintaining foreign manufacturing and could lengthen or delay the regulatory review process required to gain approval for our product candidates and could potentially prevent approval of our product candidates.
Our product candidates may never achieve market acceptance even if we obtain regulatory approvals.
Even if we receive regulatory approvals for the commercial sale of our product candidates, the commercial success of these product candidates will depend on, among other things, acceptance by physicians and patients. Market acceptance of, and demand for, any product that we develop and commercialize will depend on many factors, including:
| | • | | our ability to provide acceptable evidence of safety and efficacy; |
| | • | | our ability to obtain adequate pricing and sufficient insurance coverage and reimbursement; |
29
| | • | | availability, relative cost and relative efficacy and safety of alternative and competing treatments; |
| | • | | the effectiveness of our or our collaborators’ sales, marketing and distribution strategy; |
| | • | | publicity concerning our products or competing products and treatments; and |
| | • | | our ability to produce product in commercial quantities sufficient to meet demand. |
If our product candidates fail to gain market acceptance, we may be unable to earn sufficient revenue to continue our business.
If physicians are not adequately reimbursed for their time and services in administering Qutenza, it is likely that they will not prescribe Qutenza.
Because many people suffering from PHN are elderly, in order for Qutenza to be economically viable for this indication in the United States, we will need Medicare coverage for Qutenza, if Qutenza is approved by the FDA for marketing. Medicare policymakers or local contractors that process claims for Medicare may determine that Qutenza is not “reasonable and necessary” for Medicare beneficiaries or is reasonable and necessary only under limited circumstances. If Medicare policymakers or a significant portion of contractors determine that Qutenza is not reasonable and necessary for and deny or significantly limit reimbursement for Qutenza, our business would be harmed, not only because Medicare beneficiaries represent a substantial portion of our target market, but also because Medicare’s coverage decisions would likely affect the determination of many state Medicaid programs and private payors.
Even if Qutenza is covered by Medicare, we cannot determine whether that coverage will be primarily under Medicare Part B or Medicare Part D. Although products administered by a physician, as we expect Qutenza will be, are ordinarily covered by Medicare Part B, which also reimburses the physician for services in administering the product, Medicare Part B does not currently provide reimbursement for the use of topical patches in the treatment of peripheral neuropathic pain. Obtaining coverage for Qutenza and its related administration under Part B is important to our future success, and there is a possibility that our efforts to achieve such a change in a policy will not be successful or if successful, will likely take one or more years to achieve. Any delay in achieving reimbursement under Part B will have a negative impact on our ability to generate revenues.
Part D may provide reimbursement for Qutenza, but we do not view Part D coverage as being as favorable as Part B coverage, because each Part D plan establishes its own formulary and may or may not decide to include Qutenza, or if it does, may seek to negotiate significantly lower prices in order to include the product in their formularies. Additionally, Part D does not include reimbursement for the physician’s administration of the product, although such services may be covered under existing evaluation and management codes available for reimbursement of office visits. Patient preparation and Qutenza application time is significant and may take two hours or longer, which significantly impacts a physician’s ability to see other patients and, consequently, the physician’s revenue. If physicians are not adequately reimbursed for their time and services in administering Qutenza, it is likely that they will not prescribe Qutenza, which would significantly impair our ability to obtain revenues.
We also will need to obtain favorable coverage and reimbursement decisions for Qutenza from private insurers, including managed care organizations. We expect that private insurers will consider the efficacy, cost-effectiveness and safety of Qutenza in determining whether to provide reimbursement for Qutenza and at what level. Obtaining these coverage and reimbursement decisions will be a time consuming process requiring substantial resources and we may not receive adequate reimbursement of Qutenza from private insurers.
We expect to experience pricing pressures in connection with the sale of Qutenza, if approved, and our potential future products, due to the trend toward programs and legislation aimed at reducing healthcare costs, as well as the increasing influence of managed care organizations. In many foreign countries, particularly the
30
countries of the European Union, the pricing of prescription drugs is subject to direct governmental control and is influenced by drug reimbursement programs that employ a variety of price control mechanisms. In these countries, pricing negotiations with governmental authorities or reimbursement programs can take 6 to 12 months or longer after the receipt of regulatory marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional studies, such as a study to evaluate the cost-effectiveness of Qutenza compared to other currently available therapies. If reimbursement for Qutenza is unavailable, delayed or limited in scope or amount or if pricing is set at unsatisfactory levels, our business would be materially harmed.
We must enter into an agreement with, and depend upon, one or more partners to assist us in commercializing our lead product candidate, Qutenza, in Europe.
Because of our limited financial and other resources, we must actively seek and enter into a collaboration with one or more European partners to assist us in our planned European Qutenza launch, if marketing approval is granted. Any collaboration agreement we enter into may contain unfavorable terms, for example, with respect to product candidates covered, control over decisions and responsibilities, termination rights, payment, and other significant terms. Our ability to receive any significant revenue from our product candidates covered by the collaboration agreement will be dependent on the efforts of our collaboration partner and may result in lower levels of income to us than if we marketed our product candidates entirely on our own. The collaboration partner may not fulfill its obligations or commercialize our product candidates as quickly as we would like. We could also become involved in disputes with our partner, which could lead to delays in or termination of our commercialization programs and time-consuming and expensive litigation or arbitration. If a collaboration partner terminates or breaches its agreement with us, or otherwise fails to complete its obligations in a timely manner, the chances of successfully developing or commercializing our product candidates would be materially and adversely affected.
Additionally, depending upon the collaboration partner that we choose, other companies that might otherwise be interested in developing products with us could be less inclined to do so because of our relationship with the collaboration partner. If our ability to work with present or future strategic partners or collaborators is adversely affected as a result of our collaboration agreement, our business prospects may be limited and our financial condition may be adversely affected. There can be no assurance that we will be able to enter into a collaboration for commercialization in Europe, or that if we do, it is on a time frame and on economic terms that are favorable to us. Further, although the CHMP has issued a positive opinion recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults, there can be no assurance that the European Commission will support the recommendation and accordingly, our efforts to complete a European commercial partnership may be delayed or we may be unable to complete a European commercial partnership.
If we are unable to establish a sales, marketing and distribution infrastructure or enter into collaborations with partners to perform these functions, we will not be successful in commercializing our product candidates.
In order to commercialize any of our product candidates successfully, we must either acquire or internally develop a capable sales, marketing and distribution infrastructure or enter into collaborations with partners to perform these services for us. The acquisition or development of a capable sales, marketing and distribution infrastructure will require substantial resources, which may divert the attention of our management and key personnel and negatively impact our product development efforts. We intend to enter into partnering or other distribution arrangements for commercialization outside the United States. While we currently intend to develop a direct sales and marketing organization in the United States for Qutenza, because we believe that we can best serve our target customers with a focused, specialty sales force, we are currently evaluating the potential for commercializing Qutenza in the United States with a collaboration partner. If we enter into an agreement with a
31
collaboration partner in the United States, such a collaboration may negatively impact our ability to seek additional strategic relationships and/or may negatively impact the value of your investment in us. Factors that may inhibit our efforts to develop an internal sales, marketing and distribution infrastructure include:
| | • | | lack of available financial resources; |
| | • | | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| | • | | the inability of sales personnel to obtain access to or convince adequate numbers of physicians to prescribe our products; |
| | • | | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| | • | | unforeseen costs and expenses associated with creating a sales and marketing organization. |
We also may not be able to enter into collaborations on acceptable terms, if at all, and we may face competition in our search for partners with whom we may collaborate. If we are not able to build a sales, marketing and distribution infrastructure or collaborate with a partner to perform these functions, we may be unable to commercialize our product candidates successfully, which would adversely affect our business and financial condition.
Even if our product candidates receive regulatory approval, they will be subject to ongoing regulatory requirements, including possible commitments by us to perform post market authorization activities, and may face regulatory or enforcement action.
Any product candidate for which we receive regulatory approval, together with our third-party manufacturing facilities and processes, post-approval clinical data, and advertising and promotional activities for the product, will be subject to significant review and ongoing and changing regulation by the FDA, the EMEA and other regulatory agencies. Failure to comply with regulatory requirements may subject us to administrative and judicially-imposed sanctions. These may include warning letters, adverse publicity, civil and criminal penalties, injunctions, product seizures or detention, product recalls, total or partial suspension of production, and refusal to approve pending product marketing applications.
Even if we receive regulatory approval to market a particular product candidate, the approval could be conditioned on us conducting additional costly post-approval studies or could limit the indicated uses included in our labeling. For example, the positive opinion of the CHMP recommending approval of Qutenza in the European Union, if adopted by the European Union, requires us to conduct certain post authorization commitments including ongoing evaluations of safety of Qutenza’s use in the labeled indications as well as clinical evaluation in patients with PDN, although the necessary timing of clinical evaluations in PDN has not yet been determined. These studies may prove to be expensive and difficult to complete and the results of these studies may identify safety, efficacy or other issues related to Qutenza use that could be harmful to us. Moreover, the product may later be found to cause adverse effects that limit or prevent its widespread use, force us to withdraw it from the market or impede or delay our ability to obtain regulatory approvals in additional countries.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, health care providers, pharmaceutical companies or others selling our products. If we cannot successfully defend ourselves against these claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| | • | | decreased demand for our product candidates; |
| | • | | impairment of our business reputation; |
32
| | • | | withdrawal of clinical trial participants; |
| | • | | costs of related litigation; |
| | • | | substantial monetary awards to patients or other claimants; |
| | • | | the inability to commercialize our product candidates. |
Although we currently have product liability insurance coverage for our clinical trials with limits that we believe are customary and adequate to provide us with coverage for foreseeable risks associated with our product candidate development efforts, our insurance coverage may not reimburse us or may be insufficient to reimburse us for the actual expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for our product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing.
We face substantial competition which may result in others discovering, developing or commercializing products before or more successfully than us.
If Qutenza receives marketing approval, it will compete against more established products marketed by large pharmaceutical companies with far greater name recognition and resources than we have. Qutenza will also compete with medications that are potentially prescribed for off-label use. The most directly-competitive currently-marketed products in the United States are Lidoderm, an FDA-approved 5% lidocaine topical patch for the treatment of PHN marketed by Endo Pharmaceuticals, and Lyrica, an oral anti-convulsant, marketed by Pfizer for use in the treatment of PHN. In addition to these branded drugs, the FDA has approved gabapentin (Neurontin) for use in the treatment of PHN. Gabapentin is marketed by Pfizer and multiple generic manufacturers, and is the most widely-prescribed drug in the United States for treatment of neuropathic pain. Pfizer has also received FDA approval of Lyrica for the treatment of PDN, fibromyalgia, epilepsy and general anxiety disorder. The FDA has approved Cymbalta from Eli Lilly for use in the treatment of PDN, general anxiety disorder, depression and fibromyalgia.
Prior to any market launch, competition may become stronger and more direct and products in development, including products that we are unaware of, may compete with Qutenza. There are many other companies working to develop new drugs and other therapies to treat pain in general and neuropathic pain in particular, including GlaxoSmithKline, Newron Pharmaceuticals S.p.A, Depomed Inc., Novartis AG, UCB S.A, Pfizer and Eli Lilly. Many of the compounds in development by such companies are already marketed for other indications, such as anti-depressants or anti-seizure drugs. In addition, physicians employ other interventional procedures, such as nerve stimulation or nerve blocks, to treat patients with difficult to treat neuropathic pain conditions. Furthermore, new developments, including the development of other drug technologies and methods of preventing the incidence of disease, such as vaccines, occur in the biopharmaceutical industry at a rapid pace. Any of these developments may render our product candidates obsolete or noncompetitive.
Many of our potential competitors, either alone or together with their partners, have substantially greater financial resources, research and development programs, clinical trial and regulatory experience, expertise in prosecution of intellectual property rights, and manufacturing, distribution and sales and marketing capabilities than we do. As a result of these factors, our competitors may:
| | • | | develop product candidates and market products that are less expensive, safer, more effective or involve more convenient treatment procedures than our future products; |
| | • | | commercialize competing products before we can launch any of our product candidates; |
33
| | • | | initiate or withstand substantial price competition more successfully than we can; |
| | • | | have greater success in recruiting skilled scientific workers from the limited pool of available talent; |
| | • | | more effectively negotiate third-party licenses and strategic alliances; and |
| | • | | take advantage of acquisition or other opportunities more readily than we can. |
The life sciences industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change we may be unable to compete effectively.
We may not be able to obtain Hatch-Waxman Act data exclusivity or equivalent regulatory data exclusivity protection in other jurisdictions for Qutenza.
We intend to rely, in part, on Hatch-Waxman exclusivity for the commercialization of Qutenza in the United States. The Hatch-Waxman Act provides 5 years of data exclusivity to the first applicant to gain approval of an NDA under Section 505(b) of the Food, Drug and Cosmetic Act for a new chemical entity. A drug qualifies as a new chemical entity if the FDA has not previously approved any other drug containing the same active ingredient. Hatch-Waxman provides data exclusivity by prohibiting abbreviated new drug applications, or ANDAs, and 505(b)(2) applications, which are marketing applications where the applicant does not own or have a legal right of reference to all the data required for approval, to be submitted by another company for another version of such drug during the exclusivity period. Protection under Hatch-Waxman will not prevent the filing or approval of a full NDA under Section 505(b)(1) for the same active ingredient, although the applicant would be required to conduct its own adequate and well-controlled clinical trials to demonstrate safety and effectiveness. Our NDA for Qutenza was filed as a 505(b)(2) application as our application referenced certain publicly available preclinical data regarding capsaicin. Therefore, if another product containing the same active ingredient as Qutenza is approved before Qutenza, then our potential approval could be delayed by 5 years. However, in such event, we believe we can petition the FDA to modify our application to be under Section 505(b)(1). Such petition would require the FDA’s approval, and there can be no assurance that such petition would be granted by the FDA. While we believe that the FDA has not approved another product containing the active ingredient of Qutenza, a highly pure synthetic capsaicin, there can be no assurance that a competing product containing a synthetic capsaicin will not achieve approval before Qutenza or that Qutenza will be able to qualify for the five-year Hatch-Waxman exclusivity.
We are aware of a company that may file an NDA for a product candidate that contains a low concentration of a closely related compound to capsaicin. While we believe that this product, should it be approved by the FDA, may not preclude the granting of data exclusivity under Hatch-Waxman to Qutenza, we can make no assurance to such belief. If we are unable to achieve data exclusivity, our revenues could be significantly harmed.
There can be no assurance that European authorities will grant data exclusivity to Qutenza. Even if European data exclusivity is granted for Qutenza, that may not protect us from direct competition. Given the well-established use of capsaicin as a pain reliever, a competitor with a generic version of Qutenza may be able to obtain approval of their product during Qutenza’s period of data exclusivity, by submitting an MAA with a less than full package of preclinical and clinical data.
Our “fast track” designation for development of Qutenza for treatment of painful HIV-associated neuropathy may not actually lead to a faster development or regulatory review or approval process.
A product intended for the treatment of a serious or life-threatening condition that demonstrates the potential to address unmet medical needs for such a condition may be submitted to the FDA for “fast track” designation. Although we received fast track designation from the FDA for Qutenza for the treatment of HIV-DSP, there is no assurance that, if we decide to file an NDA for Qutenza in HIV-DSP, that we will experience a faster development process, review or approval, compared to conventional FDA standards, or that
34
the product will be approved at all. Further, we anticipate the FDA will, as is the case with other indications, require two successful Phase 3 studies in HIV-DSP to support an approval for that indication, and therefore, we may never seek approval for HIV-DSP or we will likely need to conduct one or more additional Phase 3 studies prior to submission of an NDA for HIV-DSP. The FDA may also withdraw our fast track designation if it believes that the designation is no longer supported by data from our clinical development program.
We may not be able to obtain or maintain orphan drug exclusivity for Qutenza.
The FDA granted us orphan drug status with regard to Qutenza for the treatment of HIV-DSP. In addition we may obtain orphan drug status for other indications, including PHN. If a product that has orphan drug designation subsequently receives FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity—that is, for seven years, the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances. We may be unable to obtain orphan drug designations for any additional product candidates or exclusivity for any of our product candidates, or our potential competitors may obtain orphan drug exclusivity for capsaicin-based products competitive with our product candidates before we do, in which case we may be excluded from that market for the exclusivity period. In addition, orphan drug designation previously granted may be withdrawn under certain circumstances. Even if we obtain orphan drug exclusivity for any of our product candidates, we may not be able to maintain it if a competitive product based on the same active compound is shown to be clinically superior to our product. Although obtaining FDA approval to market a product with orphan exclusivity can be advantageous, there can be no assurance that it would provide us with a significant commercial advantage.
Delays in the commencement or completion of clinical testing could result in increased costs to us and delay our ability to generate revenues.
We do not know whether future clinical trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be disrupted for a variety of reasons, including difficulties in:
| | • | | availability of financial resources; |
| | • | | addressing issues raised by the FDA or European health authorities regarding safety, design, scope and objectives of future clinical studies; |
| | • | | recruiting and enrolling patients to participate in a clinical trial; |
| | • | | obtaining regulatory approval to commence a clinical trial; |
| | • | | reaching agreement on acceptable terms with prospective clinical research organizations and trial sites; |
| | • | | manufacturing sufficient quantities of a product candidate; and |
| | • | | obtaining institutional review board approval to conduct a clinical trial at a prospective site. |
A clinical trial may also be suspended or terminated by us, the FDA or other regulatory authorities due to a number of factors, including:
| | • | | failure to conduct the clinical trial in accordance with regulatory requirements or in accordance with our clinical protocols; |
| | • | | inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; |
| | • | | unforeseen safety issues; or |
| | • | | inadequate patient enrollment or lack of adequate funding to continue the clinical trial. |
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes, which could impact the cost, timing or successful completion of a clinical trial. If we experience delays in the commencement or completion of our clinical trials, the commercial prospects
35
for our product candidates and our ability to generate product revenues will be harmed. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also lead to the denial of regulatory approval of a product candidate.
Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which could prevent or delay regulatory approval and commercialization.
Before obtaining regulatory approvals for the commercial sale of Qutenza or any other product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that the product is both safe and effective for use in each target indication. Clinical trial results from the study of neuropathic pain are inherently difficult to predict. The primary measure of pain is subjective patient feedback, which can be influenced by factors outside of our control, and can vary widely from day to day for a particular patient, and from patient to patient and site to site within a clinical study. The results we have obtained in completed clinical trials may not be predictive of results from our ongoing or future trials. Additionally, we may suffer significant setbacks in advanced clinical trials, even after promising results in earlier studies.
Some of our trial results have been negatively affected by factors that had not been fully anticipated prior to our examination of the trial results. For example, as is the case in our most recent Phase 3 study in HIV-DSP, we have from time to time observed a significant “placebo effect” within our control groups—a phenomenon in which a sham treatment or, in the case of our studies, a low-dose capsaicin treatment that we believed would not be effective, results in a beneficial effect Although we design our clinical study protocols to address known factors that may negatively affect our study results, there can be no assurance that our protocol designs will be adequate or that factors that we may or may not be aware of or anticipate, will not have a negative effect on the results of our clinical trials, which could significantly disrupt our efforts to obtain regulatory approvals and commercialize our product candidates. Furthermore, once a study has commenced, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable safety risk to patients. In our completed Phase 3 trials there have been three serious adverse events (totaling less than 1%) related to Qutenza, two related to pain and one case of hypertension. In our PHN studies C108 and C110, more cardiac adverse events occurred in subjects treated with Qutenza than subjects receiving the control patch. Evaluation of these adverse events did not indicate that they were treatment related. In our most recent PHN studies, C116 and C117, a similar number of subjects in the Qutenza and control groups had cardiac events. However, future late stage clinical trials in other indications or in a larger patient population could reveal more frequent, more severe or additional side effects that were not seen or deemed unrelated in earlier studies, any of which could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities stopping further development of or denying approval of our product candidates. Based on results at any stage of clinical trials, we may decide to repeat or redesign a trial, modify our regulatory strategy or even discontinue development of one or more of our product candidates.
A number of companies in the biotechnology and pharmaceutical industries have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials. If our product candidates are not shown to be both safe and effective in clinical trials, the resulting delays in developing other compounds and conducting associated preclinical testing and clinical trials, as well as the potential need for additional financing, would have a material adverse effect on our business, financial condition and results of operations.
We rely on third parties to conduct our non-clinical studies and clinical trials. If these third parties do not perform as contractually required or otherwise expected, we may not be able to obtain regulatory approval for our product candidates.
We do not currently conduct non-clinical studies and clinical trials on our own, and instead rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to assist us with our non-clinical and clinical trials. We are also required to comply with regulations
36
and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. If these third parties do not successfully carry out their duties to us or regulatory obligations or meet expected deadlines, if the third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our non-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our product candidates.
Even though certain of our clinical trials for Qutenza in treatment of PHN and HIV-DSP have met their primary endpoints, certain other studies in these indications have not met their primary endpoints and, our clinical trials for other indications, if we decide to conduct them, may not succeed, which would adversely impact our long term success.
We have not prepared for or conducted any Qutenza clinical trials for indications other than PHN, HIV-DSP and PDN. We have conducted one Phase 2 clinical trial for the use of Qutenza for the management of PDN. PDN represents a much larger market opportunity than either PHN or HIV-DSP, and unless we successfully complete required clinical trials and obtain regulatory approvals for the use of Qutenza for PDN patients, we will be unable to market Qutenza for this indication in the United States and the European Union and possibly in other countries. If this occurs, our long term ability to succeed will be significantly and negatively impacted. We believe that to market Qutenza in the United States for future indications, including PDN, we will have to conduct two successful Phase 3 trials for those indications, and that for PDN in particular, we may be required to perform additional safety studies. We are evaluating our development programs related to PDN in Qutenza and may decide not to conduct studies in PDN beyond those required by the post-marketing requirements associated with our MAA. If we decide not to conduct the required number of Phase 3 studies in PDN that meet their primary endpoint, we will not gain approval for Qutenza in this indication, which will significantly harm our ability to potentially generate revenue.
Results of clinical trials of Qutenza for patients with PHN or HIV-DSP do not necessarily predict the results of clinical trials involving other indications. If we decide to conduct additional clinical studies with Qutenza in PDN or other indications, those studies may fail to show desired safety and efficacy for management of pain associated with PDN and other indications, despite results from earlier clinical trials involving PDN, PHN and/or HIV-DSP. Any failure or significant delay in completing clinical trials for Qutenza with PDN and other indications, or in receiving regulatory approval involving such indications, may significantly harm our business.
We have limited experience in regulatory affairs.
We have limited experience in preparing, submitting and prosecuting regulatory filings including NDAs, MAAs and other applications necessary to gain regulatory approvals. Moreover, some of our product candidates are based on novel applications of therapies that have not been extensively tested in humans, and the regulatory requirements governing these types of products may be less well defined or more rigorous than for conventional products. As a result of these factors, in comparison to our competitors, we may require more time and incur greater costs to obtain regulatory approvals of products that we develop, license or acquire.
We depend on our key personnel. If we are not able to retain them, our business will suffer.
We are highly dependent on the principal members of our management and scientific staff. The competition for skilled personnel among biopharmaceutical companies in the San Francisco Bay Area is intense and the employment services of our scientific, management and other executive officers are terminable at-will. If we lose one or more of these key employees, our ability to implement and execute our business strategy successfully could be seriously harmed. Replacing key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize products successfully. We do not carry key man life insurance on any of our key personnel.
37
Risks Related to Our Finances and Capital Requirements
We have incurred operating losses in each year since inception and expect to continue to incur substantial and increasing losses for the foreseeable future.
We have not generated any revenue to date and we have incurred operating and net losses each year since our inception in 1998. Our net loss for the year ended December 31, 2008 was approximately $26.0 million. As of December 31, 2008 we had an accumulated deficit of approximately $190.1 million. We had cash, cash equivalents and short-term investments totaling $24.5 million at December 31, 2008 and for the year ended December 31, 2008, we used cash of $27.3 million in operating activities. We expect to continue to incur losses for several years, as we seek regulatory approvals for and commercialize Qutenza, and continue other research and development activities. If Qutenza does not gain regulatory approval or does not achieve market acceptance, we will not generate any revenue. We cannot assure you that we will be profitable even if we commercialize Qutenza. If we fail to achieve and maintain profitability, or if we are unable to fund our continuing losses, you could lose all or part of your investment.
If we do not raise additional capital, we may be forced to further delay; reduce or eliminate our development programs or commercialization efforts.
We have deferred further clinical development of Qutenza, NGX-1998 and our other development programs until such time as additional capital is available. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
| | • | | the costs and timing of regulatory approval; |
| | • | | the costs of establishing or contracting for sales and marketing capabilities; |
| | • | | the need to conduct additional clinical trials; |
| | • | | the rate of progress and cost of our clinical trials and other development activities; |
| | • | | the effect of competing technological and market developments; |
| | • | | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| | • | | the extent to which we acquire or in-license new products, technologies or businesses. |
We intend to seek additional funding through strategic alliances, debt facilities or other financing vehicles which may include the public or private sales of our equity securities. There can be no assurance, however, that additional funding will be available on reasonable terms, if at all. If adequate funds are not available, we may be required to further delay, reduce the scope of, or eliminate one or more of our planned development, commercialization or expansion activities.
Raising additional funds by issuing securities or through licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital by issuing equity securities, our existing stockholders’ ownership will be diluted. Any debt financing we enter into may involve covenants that restrict our operations. These restrictive covenants may include limitations on additional borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens, pay dividends, redeem our stock or make investments. In addition, if we raise additional funds through licensing arrangements, it may be necessary to relinquish potentially valuable rights to our potential products or proprietary technologies, or grant licenses on terms that are not favorable to us.
38
Risks Related to our Intellectual Property
The commercial success, if any, of Qutenza depends, in part, on the rights we have under certain patents.
The commercial success, if any, of Qutenza depends, in part, on a device patent granted in the United States and device patents granted in Canada, Hong Kong and certain countries of Europe concerning the use of a dermal patch for high-concentration capsaicin delivery for the treatment of neuropathic pain. We exclusively license these patents from the University of California. We do not currently own, and do not have rights under this license to any issued patents that cover Qutenza outside Europe, Hong Kong, Canada and the United States. One or more of the inventors named in the method patent described below may assert a claim of inventorship rights to such patent, which may result in our loss of exclusive use of this patent. Although we do not believe these individuals are co-inventors, there can be no assurance that we would prevail if such a claim were asserted. The absence of exclusive rights to utilize such patent exposes us to a greater risk of direct competition and could materially harm our business.
In addition to other patents and patent applications which have been licensed under our agreements with third party manufacturers, including the issued patents and pending applications licensed under our commercial supply agreement for Qutenza, we also license a method patent granted in the United States from the University of California concerning the delivery of high-concentration capsaicin for the treatment of neuropathic pain. Two of the three inventors named in the method patent did not assign their patent rights to the University of California. As a result, our rights under this patent are non-exclusive. Anesiva, a company focused on the development and commercialization of treatments for pain, including injection or infiltration of capsaicin for post-surgical pain, osteoarthritis or interdigital neuroma, has licensed from one of the non-assigning inventors the right to use the technology under the method patent. There can be no assurances that other entities will not similarly obtain rights to use the technology under the method patent. If other entities license the right to use this patent, we may face more products competitive with Qutenza and our business will suffer.
If we are unable to maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
Our commercial success will depend, in part, on obtaining and maintaining patent protection, trade secret protection and regulatory protection (such as Hatch-Waxman protection or orphan drug designation) of our proprietary technology and information as well as successfully defending against third-party challenges to our proprietary technology and information. We will be able to protect our proprietary technology and information from use by third parties only to the extent that valid and enforceable patents, trade secrets or regulatory protection cover them and we have exclusive rights to utilize them.
Our commercial success will continue to depend in part on the patent rights we own, the patent rights we have licensed, the patent rights of our collaborators and suppliers and the patent rights we may obtain related to future products we may market. Our success also depends on our and our licensors’, collaborators’ and suppliers’ ability to maintain these patent rights against third-party challenges to their validity, scope or enforceability. Further, we do not fully control the patent prosecution of our licensed patent applications. There is a risk that our licensors will not devote the same resources or attention to the prosecution of the licensed patent applications as we would if we controlled the prosecution of the patent applications, and the resulting patent protection, if any, may not be as strong or comprehensive as if we had prosecuted the applications ourselves.
The patent positions of biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
| | • | | we or our licensors might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
39
| | • | | we or our licensors might not have been the first to file patent applications for these inventions; |
| | • | | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| | • | | it is possible that none of our pending patent applications or the pending patent applications of our licensors will result in issued patents; |
| | • | | our issued patents and the issued patents of our licensors may not provide a basis for commercially viable products, or may not provide us with any competitive advantages, or may be challenged and invalidated by third parties; |
| | • | | we may not develop additional proprietary technologies or product candidates that are patentable; or |
| | • | | the patents of others may have an adverse effect on our business. |
We also rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. While we seek to protect confidential information, in part, by confidentiality agreements with our employees, consultants, contractors, or scientific and other advisors, they may unintentionally or willfully disclose our information to competitors. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets.
If we are not able to defend the patent or trade secret protection position of our technologies and product candidates, then we will not be able to exclude competitors from developing or marketing competing products, and we may not generate enough revenue from product sales to justify the cost of development of our product candidates and to achieve or maintain profitability.
If we are sued for infringing intellectual property rights of other parties, such litigation will be costly and time consuming, and an unfavorable outcome would have a significant adverse effect on our business.
Although we believe that we would have valid defenses to allegations that our current product candidates, production methods and other activities infringe the valid and enforceable intellectual property rights of any third parties of which we are aware, we cannot be certain that a third party will not challenge our position in the future. Other parties may own patent rights that might be infringed by our products or other activities. For example, in June 2005, Winston Laboratories sent us a letter informing us of their U.S. patent related tocis-capsaicin, and suggested that our synthetic capsaicin formulation could infringe this patent. We responded in August 2005 by denying any infringement. In 2007, Winston reiterated its claim and offered to discuss a license to its patent. We responded by denying infringement. We believe that our products, if commercialized, will not infringe the Winston patent, which is due to expire in 2009, but may be extended under certain circumstances. There has been, and we believe that there will continue to be, significant litigation and demands for licenses in our industry regarding patent and other intellectual property rights. Our competitors or other patent holders may assert that our products and the methods we employ are covered by their patents. These parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages or possibly prevent us from commercializing our product candidates. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be non-exclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect
40
of our business operations if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
The cost to us of any litigation or other proceedings relating to intellectual property rights, even if resolved in our favor, could be substantial. Some of our potential competitors may be better able to sustain the costs of complex patent litigation because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to continue our operations. Should third parties file patent applications, or be issued patents claiming technology also claimed by us in pending applications, we may be required to participate in interference proceedings in the U.S. Patent and Trademark Office to determine priority of invention which could result in substantial costs to us or an adverse decision as to the priority of our inventions. An unfavorable outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties. There is no guarantee that any prevailing party would offer us a license or that we could acquire any license made available to us on commercially acceptable terms.
If we fail to comply with our obligations in the agreements under which we license development or commercialization rights to products or technology from third parties, we could lose license rights that are important to our business.
We are a party to a number of technology licenses that are important to our business and expect to enter into additional licenses in the future. For example, we hold licenses from The University of California and LTS Lohmann Therapie-Systeme AG under patents and patent applications relating to Qutenza, our lead product candidate. These licenses impose various commercialization, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with these obligations, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license, including Qutenza.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to an Investment in our Common Stock
We expect that our stock price will fluctuate significantly, and you may not be able to resell your shares at or above your investment price.
The stock market has experienced significant volatility, particularly with respect to pharmaceutical, biotechnology and other life sciences company stocks. The volatility of pharmaceutical, biotechnology and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. Factors that could cause volatility in the market price of our common stock include, but are not limited to:
| | • | | potential inability to preserve or raise sufficient capital to maintain our operations; |
| | • | | delays in the process of seeking or our ability to obtain regulatory approvals; |
41
| | • | | delay in entering, or termination of, strategic partnership relationships; |
| | • | | general economic conditions and slow or negative growth of our expected markets; |
| | • | | third-party healthcare reimbursement policies or determinations; |
| | • | | failure or delays in entering additional product candidates into clinical trials or in commencing additional clinical trials for current product candidates; |
| | • | | results from and any delays related to the clinical trials for our product candidates; |
| | • | | our ability to develop and market new and enhanced product candidates on a timely basis; |
| | • | | announcements by us or our collaborators or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships or capital commitments; |
| | • | | issuance of new or changed securities analysts’ reports or recommendations for our stock or the discontinuation of one or more securities analysts’ research coverage for our stock; |
| | • | | actual or anticipated quarterly variations in our results of operations or those of our collaborators or competitors; |
| | • | | developments or disputes concerning our intellectual property or other proprietary rights; |
| | • | | commencement of, or our involvement in, litigation; |
| | • | | changes in governmental regulations or in the status of our regulatory approvals; |
| | • | | market conditions in the life sciences sector; and |
| | • | | any major change in our board or management. |
If we fail to meet the requirements for continued listing on the NASDAQ Global Market and do not meet initial listing requirements to transfer to the NASDAQ Capital Market, our common stock could be delisted from trading, which would adversely affect the liquidity of our common stock and our ability to raise additional capital.
Our common stock is currently listed on the NASDAQ Global Market and to maintain our listing, we must meet certain financial requirements in accordance with the rules of the NASDAQ Stock Market LLC, or Nasdaq, including, but not limited to, the requirement to maintain a minimum closing bid price of at least $1.00 per share for our common stock and certain other quantitative standards. Although we have maintained our listing status on the NASDAQ Global Market since our initial listing on May 2, 2007, and despite Nasdaq suspending minimum bid price and certain other requirements until July 20, 2009, there can be no assurance that we will maintain our listing on this market in the future if our bid price deteriorates or if we fail to meet other requirements. If we are delisted from the NASDAQ Global Market, we can apply to be listed on the NASDAQ Capital Market or other exchanges, which generally have lower standards for listing. Any potential delisting of our common stock would adversely affect the liquidity of our common stock and our ability to raise additional capital.
We will continue to incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could affect our operating results.
As a public company, we will continue to incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting requirements. We also have incurred and will incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act, as well as new rules implemented by the Securities and Exchange Commission and the NASDAQ Stock Market LLC. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly.
42
If the ownership of our common stock continues to be highly concentrated, it may prevent you and other stockholders from influencing significant corporate decisions and may result in conflicts of interest that could cause our stock price to decline.
Our executive officers, directors and their affiliates and significant stockholders beneficially own or control approximately 63% of the outstanding shares of our common stock as of December 31, 2008 (after giving effect to the exercise of all of their outstanding vested options and warrants exercisable within 60 days of such date). Accordingly, these executive officers, directors and their affiliates and significant stockholders acting as a group, will have substantial influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transactions. These stockholders may also delay or prevent a change of control of us, even if such a change of control would benefit our other stockholders. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Future sales of shares by existing stockholders could cause our stock price to decline.
The market price of our common stock could decline as a result of sales by our existing stockholders, including sales by our executive officers, of shares of common stock in the market, or the perception that these sales could occur. These sales might also make it more difficult for us to sell equity securities at a time and price that we deem appropriate.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include a classified board of directors, a prohibition on actions by written consent of our stockholders and the ability of our board of directors to issue preferred stock without stockholder approval. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Recent events in the credit markets have, and will continue to, impact our investment returns.
Recent events in the credit markets have caused a liquidity crisis in most credit facilities including mortgage-backed securities and auction-rate securities. In response to these events we have undertaken a continual evaluation of our investment portfolio to protect principal balances and maintain liquidity of our investments. This evaluation resulted in our initially converting, over time, all of our investment holdings into U.S. Treasury securities. Subsequently, as the government has issued guarantees of certain corporate debt, we have broadened our investment holdings to include only those corporate securities that are fully backed by the United States Government. As a result of the credit crisis and our shift in investments to U.S. Treasury securities, we anticipate that our investment returns will be below historical levels. These lower returns will likely continue for some time and we cannot predict when market conditions will improve and when higher yielding investment options may be available to us.
43
We have never paid dividends on our capital stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, have contractual restrictions against paying cash dividends and currently intend to retain our future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be an investors’ sole source of gain for the foreseeable future.
| Item 1B. | Unresolved Staff Comments |
There are no unresolved staff comments regarding any of our periodic or current reports.
We lease approximately 26,386 square feet of office space located at 2215 Bridgepointe Parkway, Suite 200, San Mateo, California until 2012. We believe that these facilities are suitable and adequate for our current needs.
We are not a party to any material legal proceedings.
| Item 4. | Submission of Matters to a Vote of Security Holders |
There were no matters submitted to a vote of the security holders during the fourth quarter of 2008.
44
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is quoted on the NASDAQ Global Market under the symbol “NGSX,” and has been quoted on such market since our initial public offering on May 1, 2007. Prior to such date, there was no public market for our common stock. The following table sets forth the high and low sales price per share of our common stock as reported on the NASDAQ Global Market for the periods indicated.
| | | | | | |
| | | Sale Price |
| | | High | | Low |
Fiscal 2007: | | | | | | |
Second Quarter | | $ | 10.99 | | $ | 7.20 |
Third Quarter | | $ | 9.86 | | $ | 6.06 |
Fourth Quarter | | $ | 9.04 | | $ | 5.75 |
| | |
Fiscal 2008: | | | | | | |
First Quarter | | $ | 6.96 | | $ | 2.18 |
Second Quarter | | $ | 4.00 | | $ | 2.52 |
Third Quarter | | $ | 3.63 | | $ | 1.85 |
Fourth Quarter | | $ | 2.70 | | $ | 0.81 |
On February 27, 2009, the last reported sale price for our common stock on the NASDAQ Global Market was $1.21 per share. We currently expect to retain future earnings, if any, for use in the operation and expansion of our business and have not paid and do not in the foreseeable future anticipate paying any cash dividends. As of February 27, 2009 there were 61 holders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company, or DTC. All of the shares of our common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC, and are therefore considered to be held of record by Cede & Co. as one stockholder.
During the quarter and year ended December 31, 2008, there was no employee stock repurchase activity.
As of December 31, 2008, approximately 785 shares of common stock held by employees and service providers remain subject to repurchase by us.
The information regarding the securities authorized for issuance under our equity compensation plans is incorporated by reference from Item 12 of this Annual Report on Form 10-K.
45
Comparison of Historical Cumulative Total Return (*) Among NeurogesX, Inc., the NASDAQ Composite Index and the NASDAQ Biotechnology Index
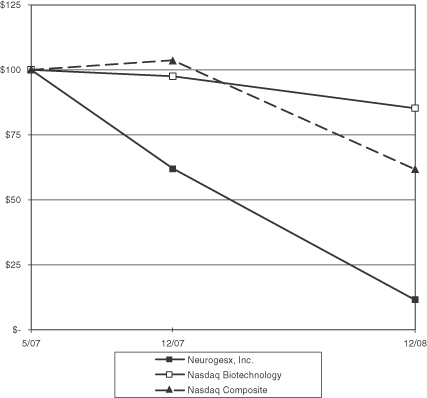
| | | | | | | | | |
| | | | | Cumulative Total
Return as of |
| | | 5/2/07 | | 12/31/07 | | 12/31/08 |
NeurogesX, Inc. | | $ | 100.00 | | $ | 62.05 | | $ | 11.41 |
NASDAQ Composite Index | | $ | 100.00 | | $ | 103.69 | | $ | 61.65 |
NASDAQ Biotechnology Index | | $ | 100.00 | | $ | 97.52 | | $ | 85.21 |
| (*) | The above graph shows the cumulative total stockholder return of an investment of $100 in cash on May 2, 2007, the date the Company’s Stock began to trade on the NASDAQ Global Market, through December 31, 2008 for: (i) the Company’s Common Stock; (ii) the NASDAQ Composite Index; and (iii) the NASDAQ Biotechnology Index. All values assume reinvestment of the full amount of all dividends. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns. |
The information contained under this caption “Comparison of Historical Cumulative Total Return(*) Among NeurogesX, Inc., the NASDAQ Composite Index and the NASDAQ Biotechnology Index” shall not be deemed to be soliciting material or to be filed with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference into such filing.
46
| Item 6. | Selected Financial Data |
The following selected financial data should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Item 8, “Financial Statements and Supplemental Data” of this Form 10-K.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | Period from
May 28, 1998
(inception) to
December 31,
2008 | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | | |
| | | (in thousands except share and per share data) | | | | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Research and development | | $ | 16,104 | | | $ | 25,321 | | | $ | 20,919 | | | $ | 11,847 | | | $ | 16,492 | | | $ | 111,492 | |
General and administrative | | | 10,182 | | | | 7,455 | | | | 6,110 | | | | 1,715 | | | | 5,113 | | | | 39,055 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 26,286 | | | | 32,776 | | | | 27,029 | | | | 13,562 | | | | 21,605 | | | | 150,547 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (26,286 | ) | | | (32,776 | ) | | | (27,029 | ) | | | (13,562 | ) | | | (21,605 | ) | | | (150,547 | ) |
Interest income (expense), net | | | 265 | | | | 454 | | | | 156 | | | | 393 | | | | 262 | | | | 2,254 | |
Other income (expense), net | | | (14 | ) | | | 366 | | | | (3,272 | ) | | | 58 | | | | — | | | | (2,947 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss before cumulative effect of change in accounting principle | | | (26,035 | ) | | | (31,956 | ) | | | (30,145 | ) | | | (13,111 | ) | | | (21,343 | ) | | | (151,240 | ) |
Cumulative effect of change in accounting principle | | | — | | | | — | | | | — | | | | (32 | ) | | | — | | | | (32 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | (26,035 | ) | | | (31,956 | ) | | | (30,145 | ) | | | (13,143 | ) | | | (21,343 | ) | | | (151,272 | ) |
Accretion of redeemable convertible preferred stock | | | — | | | | (4,626 | ) | | | (11,293 | ) | | | (8,269 | ) | | | (6,782 | ) | | | (38,872 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (26,035 | ) | | $ | (36,582 | ) | | $ | (41,438 | ) | | $ | (21,412 | ) | | $ | (28,125 | ) | | $ | (190,144 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted net loss per share attributable to common stockholders(1) | | $ | (1.49 | ) | | $ | (4.06 | ) | | $ | (116.20 | ) | | $ | (70.56 | ) | | $ | (97.76 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Weighted average number of shares used to compute basic and diluted net loss per share attributable to common stockholders(1) | | | 17,519,415 | | | | 9,017,627 | | | | 356,600 | | | | 303,476 | | | | 287,702 | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2004 | |
| | | (in thousands) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents and short-term investments | | $ | 24,506 | | | $ | 52,851 | | | $ | 13,902 | | | $ | 12,050 | | | $ | 19,205 | |
Working capital | | | 19,109 | | | | 44,139 | | | | 578 | | | | 8,840 | | | | 17,356 | |
Restricted cash | | | 200 | | | | 240 | | | | — | | | | — | | | | 500 | |
Total assets | | | 25,590 | | | | 54,185 | | | | 14,818 | | | | 12,722 | | | | 20,629 | |
Preferred stock warrant liability | | | — | | | | — | | | | 7,549 | | | | 736 | | | | — | |
Notes payable—non-current portion | | | 191 | | | | 3,024 | | | | 6,737 | | | | — | | | | — | |
Redeemable convertible preferred stock | | | — | | | | — | | | | 116,164 | | | | 93,690 | | | | 80,766 | |
Deficit accumulated during the development stage | | | (190,144 | ) | | | (164,109 | ) | | | (127,527 | ) | | | (86,089 | ) | | | (64,677 | ) |
Total stockholders’ equity (deficit) | | | 19,274 | | | | 41,361 | | | | (122,066 | ) | | | (84,580 | ) | | | (62,897 | ) |
| (1) | See Note 2 of the notes to the consolidated financial statements for an explanation of the method used to calculate the net loss per share attributable to common stockholders and the number of shares used in the computation of the per share amounts. |
47
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This discussion and analysis should be read in conjunction with our financial statements and accompanying notes included elsewhere in this report. Operating results are not necessarily indicative of results that may occur in future periods.
Overview
We are a biopharmaceutical company focused on developing and commercializing novel pain management therapies. We are assembling a portfolio of pain management product candidates based on known chemical entities to develop innovative new therapies which we believe may offer substantial advantages over currently available treatment options. Our initial focus is on the management of chronic peripheral neuropathic pain conditions. Our most advanced product candidate, Qutenza, a dermal patch containing a high concentration of synthetic capsaicin, is designed to manage pain associated with peripheral neuropathic pain conditions. We believe, based on our successful Phase 3 studies that a single 30- or 60-minute application of Qutenza may provide up to 12 weeks of clinically-meaningful pain relief. Moreover, we believe that Qutenza has demonstrated in clinical trials a positive safety and tolerability profile.
We submitted to the FDA an NDA for Qutenza for the management of pain associated with PHN in October 2008 which was filed by the FDA in December 2008. Our NDA has a PDUFA date of August 16, 2009 at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA. A complete response letter may describe any activities which may be required to gain approval or may indicate that a product candidate is not approvable.
In September 2007 we submitted a MAA for Qutenza with the EMEA under the centralized procedure, seeking approval of Qutenza. On March 19, 2009, the CHMP issued a positive opinion recommending the approval of our MAA for Qutenza for the treatment of peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. In conjunction with issuing this recommendation, the CHMP requested us to perform certain clinical evaluations of Qutenza following approval. We are currently awaiting the European Commission’s decision on the CHMP opinion, a process which normally takes approximately 60 to 90 days. If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
Our earlier stage product candidate pipeline consists of:
| | • | | NGX-1998, a non-patch liquid formulation of capsaicin for potential use in neuropathic pain conditions; |
| | • | | NGX-1576, NGX-9674 and NGX-5752, prodrugs of acetaminophen for potential use in acute pain including traumatic pain, post-surgical pain and fever; and |
| | • | | NGX-6052, an opioid prodrug for potential use in chronic pain indications. |
NGX-1998 has been evaluated in three Phase 1 studies and is currently being considered for entry into Phase 2 evaluation. The other product candidates are all in the pre-clinical stage of development.
In response to the generally weak economic conditions which have resulted in a challenging environment for raising capital, we deferred further pre-clinical and clinical development activities for all of our product candidates in order to focus our fiscal and human resources on the prosecution of our MAA and NDA for Qutenza and on continuing support of our strategies for obtaining adequate reimbursement for Qutenza in the United States. We expect to re-initiate some or all of our development programs if additional funds become available, including the studies associated with MAA approval and the continuation of the NGX-1998 clinical
48
program into Phase 2. Further, we are currently seeking development partners for our acetaminophen and opioid prodrug product candidates. We hold worldwide commercial rights to all of our product candidates and are actively engaged in discussions with potential commercial partners.
We were incorporated in 1998 as Advanced Analgesics, Inc., and commenced operations in 2000 as NeurogesX, Inc. From inception through 2001 our primary activities were related to formulation development and preclinical studies of our lead product candidate, Qutenza. Since 2002, our focus has expanded to include clinical development of Qutenza, establishing sources of supply and manufacturing processes for Qutenza and, more recently, regulatory activities including those related to our MAA, which was submitted in September 2007 and for which a recommendation for approval by the CHMP was issued on March 19, 2009, and our NDA which was submitted to the FDA in October 2008 and filed by the FDA in December 2008. Our focus has also expanded to include preparation for potential commercialization of Qutenza should marketing approval be attained and seeking commercial partners for Qutenza. Additionally, we have begun development programs for NGX-1998, a liquid formulation of the same active ingredient used in Qutenza, as well as limited preclinical development of certain prodrug product candidates.
We are a development stage company. To date, we have not generated any revenues and have funded our operations primarily by selling equity securities and establishing debt facilities. We have incurred significant losses since our inception. As of December 31, 2008, we had a deficit accumulated during the development stage of approximately $190.1 million, of which approximately $38.9 million represents non-cash charges for the accretion of redeemable convertible preferred stock. We had cash, cash equivalents and short-term investments totaling $24.5 million at December 31, 2008 and for the year then ended, we used cash of $27.3 million in operating activities. We expect to continue to incur annual operating losses over the next several years and those losses may increase as we continue our efforts to gain marketing approval for Qutenza in the United States and the European Union, prepare for potential commercialization if Qutenza is approved for marketing, and resume development of our product candidates.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and related disclosures. Actual results could differ from those estimates.
While our significant accounting policies are described in more detail in Note 2 of Notes to Consolidated Financial Statements included elsewhere in this report, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
Research and Development
We expense research and development costs as incurred. Research and development expenses include:
| | • | | personnel and personnel related costs; |
| | • | | costs associated with pre-clinical and clinical development activities, such as clinical trials, including amounts paid to clinical research organizations and clinical investigators; |
| | • | | product and manufacturing costs such as process development, clinical product supply costs and the cost of commercially saleable product that is manufactured prior to market approval for that product candidate; |
| | • | | internal and external costs associated with our regulatory compliance and quality assurance functions including the costs of outside consultants and contractors that assist in the process of submitting and maintaining regulatory filings; and |
| | • | | overhead costs including allocated facility and related expenses. |
49
Clinical Trials
We accrue and expense costs for clinical trial activities performed by third parties, including clinical research organizations and clinical investigators, based upon estimates made, as of the reporting date, of the work completed through the reporting date as a percentage of the total work contracted for under our agreements with such third parties. We make our estimates at the end of each reporting period after discussion with internal personnel and outside service providers and thorough evaluation as to progress or stage of completion of trials or services, as of the end of each reporting period. On a periodic basis we adjust our estimated clinical trial costs to reflect actual expenses incurred to date. Due to the nature of the estimation of clinical trial costs, we can not assure you that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of clinical trials.
Stock-Based Compensation
Effective January 1, 2006, we adopted the provisions of SFAS No. 123R,Share-Based Payments. In March 2005, the Securities and Exchange Commission, or SEC, issued Staff Accounting Bulletin, or SAB, No. 107 relating to SFAS No. 123R. We have applied the provisions of SAB No. 107, for which the simplified method of determining the expected term of an option has been extended by SAB No. 110, in our adoption of SFAS No. 123R. Under SFAS No. 123R, stock-based awards, including stock options, are recorded at fair value as of the grant date and recognized to expense over the employee’s requisite service period (generally the vesting period), which we have elected to amortize on a straight-line basis. Because non-cash stock compensation expense is based on awards ultimately expected to vest, it has been reduced by an estimate for future forfeitures. SFAS No. 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We adopted the provisions of SFAS No. 123R using the prospective transition method. Under the prospective transition method, beginning January 1, 2006, compensation cost recognized includes: compensation cost for all share-based payments granted prior to, but not yet vested as of December 31, 2005, based on the intrinsic value in accordance with the provisions of APB No. 25; and compensation cost for all share-based payments granted subsequent to December 31, 2005, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123R. All awards granted, modified, or settled after the date of adoption are accounted for using the measurement, recognition, and attribution provisions of SFAS No. 123R. We have elected to use the Black-Scholes option valuation model to estimate the fair value of stock options and significant judgment is required on the part of management in determining the proper assumptions used in this model. The assumptions used in the Black-Scholes option valuation model include the risk free interest rate, expected term, expected volatility and dividend yield. We base our assumptions on historical data where available. However, these assumptions consist of estimates of future market conditions, which are inherently uncertain, and therefore subject to management’s judgment. See Note 9 of Notes to Consolidated Financial Statements included elsewhere in this report for further detail.
Estimation of Fair Value of Warrants to Purchase Redeemable Convertible Preferred Stock
We accounted for warrants to purchase redeemable convertible preferred stock pursuant to the FASB Staff Position, No. 150-5,Issuers Accounting under Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that are Redeemable, or FSP No. 150-5, which required us to classify these warrants as current liabilities and to adjust the value of these warrants to their fair value at the end of each reporting period. We estimated the fair value of these warrants at the respective balance sheet dates using the Black-Scholes option valuation model, based on the estimated market value of the underlying redeemable convertible preferred stock at the valuation measurement date, the remaining contractual term of the warrant, risk-free interest rates and expected dividends on and expected volatility of the price of the underlying redeemable convertible preferred stock. These estimates, especially the market value of the underlying redeemable convertible preferred stock and the expected volatility, are highly judgmental. At the time of adoption of FSP No. 150-5, in 2005, we recorded $32,000 for the cumulative effect of this change in accounting principle to reflect the cumulative change in estimated fair value of these warrants as of that date. We recorded $58,000 of other income for the decrease in fair value for the remainder of 2005 and $3.3 million of other expense for the year ended December 31, 2006 to reflect increases in the estimated fair value of all preferred stock warrants.
50
Upon the closing of our initial public offering, or IPO, on May 7, 2007, all outstanding warrants to purchase shares of preferred stock were converted to warrants to purchase shares of our common stock and, as a result, are no longer subject to FSP No. 150-5. The then-current aggregate fair value of these warrants of approximately $426,000 was reclassified from liabilities to additional paid-in capital, a component of stockholders’ equity (deficit), in the second quarter of 2007 and we have ceased to record any further periodic fair value adjustments. We recorded $360,000 of other income for the year ended December 31, 2007 to reflect decreases in the estimated fair value of all preferred stock warrants.
Results of Operations
Our research and development expenses consist of internal and external costs. Our internal costs are primarily employee salaries and benefits, contract employee expense, non-cash stock compensation expense, allocated facility and other overhead costs. Our external costs are primarily expenses related to the development of product candidates including formulation development, manufacturing process development, non-clinical studies, clinical trial costs, such as the cost of clinical research organizations and clinical investigators, and costs associated with preparation and filing of regulatory submissions.
Since our inception, Qutenza has accounted for in excess of 90% of our external research and development expenses, although Qutenza has been declining as a percentage of our external research and development expenses in recent years due to the completion of clinical studies in Qutenza and the relative increase in spending related primarily to our NGX-1998 development program. Specifically, in the years ended December 31, 2008, 2007 and 2006, our external research and development costs totaled $6.9 million, $16.7 million and $14.6 million, respectively, and of these amounts 84%, 92% and 94%, respectively, were incurred in programs related to Qutenza. We commence tracking the separate, external costs of a project when we determine that a project has a reasonable chance of entering clinical development. We use our internal research and development resources across several projects and many resources are not attributable to specific projects. Accordingly, we do not account for our internal research and development costs on a project basis. However, over time, we believe that our internal costs are expended on our development projects generally in proportion to our external development costs for such project relative to total external development costs.
The process of conducting preclinical testing and clinical trials necessary to obtain FDA approvals is costly and time consuming. The probability of success for each product candidate and clinical trial may be affected by a variety of factors, including, among others, patient enrollment, manufacturing capabilities, successful clinical results, our funding, and competitive and commercial viability. As a result of these and other factors, we are unable to determine the duration and completion costs of current or future clinical stages of our product candidates or when or to what extent we will generate revenues from commercialization and sale of any of our product candidates. Currently we are primarily focused on completing the development, through regulatory approval, of our lead product candidate, Qutenza, for patients with PHN in the United States and for patients with peripheral neuropathic pain conditions in the European Union. Our MAA which was filed with the EMEA, received, in March 2009, a positive opinion recommending the approval for the use of Qutenza for peripheral neuropathic pain in non-diabetic adults either alone or in combination with other medicinal products for pain. We are currently awaiting the European Commission’s decision on the CHMP opinion, a process which normally takes approximately 60 to 90 days. We also submitted an NDA in the United States for Qutenza for the management of pain associated with PHN in 2008. Our NDA has been given a PDUFA date of August 16, 2009 at which time we would anticipate receiving either an approval letter or a complete response letter from the FDA. We anticipate that our overall research and development expenses, excluding non-cash stock-based compensation expense will be consistent or possibly less than that experienced in recent quarters and will remain at those levels until we attain additional funding to support further development activities in Qutenza, NGX-1998 and our other development programs.
Our general and administrative expenses consist primarily of salaries and benefits, professional fees related to our administrative, finance, human resource, legal and information technology functions, marketing expenses, costs associated with our status as a public company and patent costs. In addition, general and administrative expenses include allocated facility, basic operational and support costs and insurance costs. We anticipate that
51
our general and administrative expenses will increase in absolute dollars and also as a percentage of total expenses over the next several years if we obtain additional capital. These increases are likely to be attributable to increasing marketing activities in anticipation of and upon receipt of, required regulatory approvals, the costs of hiring and deploying a sales force in the United States to support a commercial launch of our product should we achieve FDA approval, and the costs of being a public company, as well as the need to add additional personnel in all of the key functional areas that support growth of our general operations, including accounting and finance, legal and human resources.
Comparison of Years Ended December 31, 2008 and 2007
| | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Increase
(Decrease) | | | %
Increase
(Decrease) | |
| | | 2008 | | | 2007 | | | |
| | | (in thousands, except percentages) | |
Research and development expenses | | $ | (16,104 | ) | | $ | (25,321 | ) | | $ | (9,217 | ) | | (36 | %) |
General and administrative expenses | | | (10,182 | ) | | | (7,455 | ) | | | 2,727 | | | 37 | % |
Interest income | | | 1,057 | | | | 1,673 | | | | (616 | ) | | (37 | %) |
Interest expense | | | (792 | ) | | | (1,219 | ) | | | (427 | ) | | (35 | %) |
Other income (expense), net | | | (14 | ) | | | 366 | | | | (380 | ) | | (104 | %) |
Research and Development expenses. Research and development expenses decreased approximately $9.2 million, or 36%, to $16.1 million in the year ended December 31, 2008 from $25.3 million for the same period in 2007. The year over year change was attributable to, in part, a decision to reduce our non-clinical and clinical development expenses related both to potential label expansion of Qutenza as well as our other product candidate programs. This was due to our desire to preserve cash resources to support our regulatory submissions processes in both the United States and the European Union and to devote resources to certain pre-commercialization activities. Specifically, our research and development expenses declined as a result of a $7.8 million decrease in clinical study related costs associated with Qutenza. During 2007, we were conducting two Phase 3 clinical trials, whereas we completed our most recent Phase 3 clinical trial in the first half of 2008. Also contributing to the year over year change was a $0.9 million decrease related to manufacturing costs in support of Qutenza. In 2007, our manufacturing costs were primarily attributed to manufacturing development activities including validation of our active pharmaceutical ingredient, or API, manufacturing processes to support our MAA submission in the third quarter of 2007 and manufacturing costs associated with support of our two Phase 3 clinical trials, whereas in 2008 there was a lower level of activity in these areas. Additionally, there was a $0.3 million decrease in external regulatory expenses resulting from the recognition of our MAA filing fees in the third quarter of 2007. Additional decreases included a $0.3 million reduction in spending related to NGX-1998, which was primarily driven by the completion of IND-enabling non-clinical toxicology studies in 2007, a $0.2 reduction in external quality assurance costs as a result of a lower level of clinical trial activity in 2008 and a $0.1 million reduction in non-cash stock based compensation expense. These decreases were partially offset by a $0.7 million increase in spending related to our manufacturing support infrastructure and increased staffing within the regulatory and quality assurance functions to support our regulatory submissions and prepare for potential commercial launch of our lead product candidate if marketing approval is attained.
General and Administrative expenses. General and administrative expenses increased approximately $2.7 million, or 37%, to $10.2 million in the year ended December 31, 2008 from $7.5 million for the same period in 2007. The year over year change was due to a $1.2 million increase in spending for pre-commercialization activities such as medical education, marketing materials development and work in support of our pricing and reimbursement strategies. Also contributing to the year over year change was a $0.8 million increase in general and administrative and marketing employee related expenses as a result of an increase in staffing in support of being a public company and in support of pre-commercialization activities and a $0.4 million increase in allocated overhead costs related to the move of our corporate headquarters to San Mateo, California in October 2007. Other increases were due to the costs of being a public company, including a $0.4 million increase in
52
directors and officers’ insurance expense, professional fees and other public company costs. These increases were partially offset by a $0.1 million decrease in non-cash stock based compensation expense.
Interest income. Interest income decreased approximately $0.6 million, or 37%, to $1.1 million in 2008 from $1.7 million in 2007. This decrease was primarily attributable to a decrease in the rate of return on our invested assets as rates declined in the market, in general, and as we moved our invested assets to investments of lower relative risk in light of events in the credit markets. The decrease in our interest income was somewhat limited due to the fact that we had a higher average investment balance during 2008 as compared to 2007.
Interest expense.Interest expense decreased approximately $0.4 million, or 35%, to $0.8 million in 2008 from $1.2 million in 2007. This decrease was related to the reduction in the outstanding principal balance of notes payable as a result of the scheduled repayment of principal on those notes payable.
Other income (expense), net. Other income (expense), net, decreased approximately $0.4 million to less than $0.1 million in expense in 2008 from $0.4 million in income in 2007. The other income amount of $0.4 million in the 2007 period was attributable to a decline in the fair value of our preferred stock warrant liability. Due to the conversion of all outstanding shares of preferred stock into common stock in connection with our IPO in the second quarter of 2007, these warrants became exercisable for common stock, were reclassified to stockholders’ equity and are no longer required to be recorded at fair value at each reporting date. We performed a final remeasurement in the period ended June 30, 2007 and have ceased to record any further periodic fair value adjustments.
Comparison of Years Ended December 31, 2007 and 2006
| | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Increase
(Decrease) | | %
Increase
(Decrease) | |
| | | 2007 | | | 2006 | | | |
| | | (in thousands, except percentages) | |
Research and development expenses | | $ | (25,321 | ) | | $ | (20,919 | ) | | $ | 4,402 | | 21 | % |
General and administrative expenses | | | (7,455 | ) | | | (6,110 | ) | | | 1,345 | | 22 | % |
Interest income | | | 1,673 | | | | 719 | | | | 954 | | 133 | % |
Interest expense | | | (1,219 | ) | | | (563 | ) | | | 656 | | 117 | % |
Other income (expense), net | | | 366 | | | | (3,272 | ) | | | 3,638 | | 111 | % |
Research and Development expenses. Research and development expenses increased approximately $4.4 million, or 21%, to $25.3 million in the year ended December 31, 2007 from $20.9 million for the same period in 2006. The year over year change was primarily attributable to a $1.8 million increase in clinical and manufacturing related costs associated with Qutenza. The increase in clinical costs was due to the size and scope of our Phase 3 studies conducted in 2007 compared to those conducted in 2006. The increase in manufacturing costs was primarily related to the development activities that were performed in support of our MAA filing in the third quarter of 2007 and preparation for an NDA filing in 2008. Also contributing to the year over year change was a $1.8 million increase in regulatory and quality assurance expenses related to an increase in staffing and consulting in support of the MAA filing, filing fees payable to the EMEA and preparation for an NDA filing in 2008, a $0.8 million increase in development work related to new product areas including conducting toxicology work in support of our planned IND filing for NGX-1998 as well as research in support of our new product initiatives and a $0.3 million increase related to allocated facilities charges in connection with the move of our corporate headquarters to San Mateo, California in 2007. Non-cash stock based compensation expense contributed $0.2 million to the increase in research and development expenses. These increases were partially offset by a $0.6 million decrease in nonclinical costs associated with Qutenza as the majority of our nonclinical activities for our lead product candidate were completed in 2006.
General and Administrative expenses. General and administrative expenses increased approximately $1.3 million, or 22%, to $7.5 million in the year ended December 31, 2007 from $6.1 million for the same period in 2006. The year over year change was due to a $1.1 million increase in marketing expenses related to consulting
53
and analysis in support of our pricing and reimbursement strategies, and other pre-commercialization market research and other pre-launch activities as well as costs associated with our efforts to build out our commercial infrastructure. Additional increases included a $1.0 million increase in general and administrative employee related expenses and board compensation expense resulting from infrastructure development and the initiation of our board compensation program upon completion of our IPO, respectively. In addition, as a result of our IPO and costs attendant to being a public company, there was a $1.6 million increase in professional and corporate fees, including legal and accounting fees, public company directors and officers insurance, consulting fees and costs associated with the move of our corporate headquarters to San Mateo, California in 2007. These increases were partially offset by a $2.3 million decrease in non-cash stock based compensation expense primarily due to the cessation of variable accounting for stock-based awards upon the forgiveness of certain notes payable in January 2007.
Interest income. Interest income increased approximately $1.0 million, or 133%, to $1.7 million in the year ended December 31, 2007 from $0.7 million for the same period in 2006. The year over year change was primarily attributable to an increase in invested assets, due to both the completion of our IPO on May 7, 2007 as well as the exercise of preferred stock warrants in the first quarter of 2007. Also contributing to the year over year change in interest income, although to a lesser extent, was an increase in the rate of return on invested assets.
Interest expense. Interest expense increased approximately $0.7 million, or 117%, to $1.2 million in the year ended December 31, 2007. The increase was related to our borrowing a total of approximately $10.0 million under certain notes payable in July and September 2006. The notes were outstanding for the entire year ended December 31, 2007 which resulted in higher interest expense in 2007 compared to 2006.
Other income (expense), net. Other income (expense), net, increased approximately $3.6 million, or 111%, to $0.4 million in income in the year ended December 31, 2007 from $3.3 million in expense in the same period in 2006. The other expense of $3.3 million in 2006 was primarily attributable to the increase in fair value of our preferred stock warrant liability. The other income of $0.4 million in 2007 was primarily attributable to a subsequent decline in the fair value of our preferred stock warrant liability in 2007. Due to the conversion of all outstanding shares of preferred stock into common stock in connection with our IPO in the second quarter of 2007, these warrants became exercisable for common stock and are no longer required to be recorded at fair value at each reporting date. We performed a final remeasurement in the period ended June 30, 2007 and have ceased to record any further periodic fair value adjustments.
Accretion of Redeemable Convertible Preferred Stock
Our redeemable convertible preferred stock outstanding prior to our initial public offering was redeemable at the request of the holders on or after June 30, 2008. We accreted the carrying value of the preferred stock issuances to the redemption amount using the effective interest method through periodic charges to additional paid-in capital. Upon completion of our initial public offering on May 1, 2007, our preferred stock converted to common stock and the carrying value of our preferred stock was reclassified to common stock and additional paid-in capital.
Liquidity and Capital Resources
Since our inception through December 31, 2008, we have financed our operations primarily through private placements and a public offering of our equity securities and, to a lesser extent, through debt facilities. Through December 31, 2008, we have received approximately $158.7 million from the sale of our equity securities, net of issuance costs. On May 7, 2007, we completed an initial public offering of our common stock which resulted in net cash proceeds, after deducting total expenses including underwriting discounts and commissions and other-offering related expenses, of approximately $38.1 million. On December 28, 2007 we completed the first closing
54
of a private placement of our common stock and warrants resulting in net cash proceeds of $21.5 million and on January 3, 2008, we completed the second and final closing of this private placement of our common stock and warrants resulting in net cash proceeds of $2.3 million.
As of December 31, 2008, we had approximately $24.5 million in cash, cash equivalents and short-term investments and working capital of $19.1 million. Our cash and investment balances are typically held in a variety of interest bearing instruments including corporate bonds, commercial paper, money market funds and obligations of U.S. government agencies. Cash in excess of immediate operational requirements is invested in accordance with our investment policy primarily with a view to liquidity and capital preservation. Further, to reduce portfolio risk, our investment policy specifies a concentration limit of 10% in any one issuer or group of issuers of corporate bonds or commercial paper at the time of purchase. Recent events in the credit markets have caused a liquidity crisis in most credit facilities including mortgage-backed securities and auction-rate securities. We do not hold any auction rate securities or mortgage-backed securities. In response to a broad based tightening of credit and worsening economic environment in the fourth quarter, and to protect the principal balances and maintain liquidity of our investments, we have, over time, converted all of our investment holdings into U.S. Treasury or money market funds consisting of only U.S. Treasury securities as of December 31, 2008. Our sales of investments in other than U.S. Treasury securities were generally conducted at or near cost, however certain individual investments were sold at either a gain or loss, that were not material individually or in the aggregate. While we have resumed investing in commercial paper in 2009, these investments have been limited to securities that are guaranteed in full by the Federal Deposit Insurance Corporation through the Temporary Liquidity Guarantee Program.
Net cash used in operating activities was approximately $27.2 million, $28.7 million and $22.3 million in 2008, 2007 and 2006, respectively. Net cash used in each of these periods was primarily a result of external research and development expenses, internal personnel costs associated with our research and development programs and infrastructure costs supporting our research and development activities. Included in net cash used in operating activities are net changes in assets and liabilities affecting cash, including significant reductions in accounts payable and accrued expenses in 2008 due to a generally lower level of research and development activity in 2008, the timing of research and development activities and the timing of our payments to our suppliers, vendors and employees.
Net cash provided by investing activities was approximately $7.8 million in 2008 and net cash used in investing activities was approximately $19.2 million and $0.1 million in 2007 and 2006, respectively. Investing activities consisted primarily of the purchase, sale and maturity of marketable securities and to a lesser extent, the purchase of capital equipment. The net cash provided by investing activities in 2008 reflected maturities and sales of marketable securities in excess of the amount purchased during this period as we continued our efforts to reduce the relative risk of our investment portfolio. Changes in restricted cash resulted from the establishment in 2007 and subsequent reduction in 2008 related to required collateral on our line of credit in connection with our operating lease in San Mateo, California. Net cash used in investing activities was significantly higher in 2007 compared to 2006 due to the investment of net proceeds from financing activities. Purchases of property and equipment increased in 2007 due to our relocating our corporate headquarters in September 2007. We expect that property and equipment expenditures may increase if our NDA for Qutenza is approved by the FDA. Such increase is expected to relate to the capital needs for infrastructure to support commercial operations.
Net cash used in financing activities was approximately $1.6 million in 2008 and net cash provided by financing activities was approximately $67.4 million and $24.3 million in 2007 and 2006, respectively. Financing activities consisted primarily of the sales of our common stock and warrants and the exercise of stock options in 2008, offset by principal repayments on our venture loan financing arrangements. In 2007, financing activities consisted primarily of our IPO, a private placement of our common stock and warrants and the exercise of warrants underlying our preferred stock, partially offset by principal repayments on our venture loan financing arrangements. In 2006, financing activities consisted of the sale of equity securities including common and preferred stock and the exercise of options and warrants underlying these securities, as well as proceeds received from our venture loan financing arrangements, partially offset by principal repayments on such loans.
55
Future minimum payments under all noncancelable lease obligations and payments under our venture loan agreement are as follows as of December 31, 2008 (in thousands):
| | | | | | |
Year Ended December 31, | | Operating
Leases | | Notes
Payable |
2009 | | $ | 457 | | $ | 3,095 |
2010 | | | 581 | | | 193 |
2011 | | | 634 | | | — |
2012 | | | 391 | | | — |
2013 | | | 15 | | | — |
| | | | | | |
| | $ | 2,078 | | | 3,288 |
| | | | | | |
Less: amounts representing interest | | | 183 |
| | | | | | |
| | | | | $ | 3,105 |
| | | | | | |
We enter into contracts in the normal course of business with clinical research organizations and clinical investigators, for third party manufacturing and formulation development, and increasingly with organizations that are supporting our pre-commercialization activities, among others. These contracts generally provide for termination with notice, and therefore we believe that our noncancelable obligations under these agreements are not material.
In October 2000 and as amended, we licensed certain patents from the University of California for high-concentration capsaicin for neuropathic pain. Under the terms of this license agreement, we are required to pay royalties on net sales of the licensed product up to a maximum of $1,000,000 per annum as well as a percentage of upfront and milestone payments resulting from sublicense of our rights under the agreement.
In January 2007, we entered into a Commercial Supply and License Agreement with LTS Lohman Therapie-Systeme AG, or LTS, to manufacture commercial and clinical supply of Qutenza, which is currently being evaluated for marketing approval in both the United States and European Union. Under the terms of the agreement, we are required to pay a transfer price for product purchased under the agreement as well as a royalty on net sales of product purchased under such agreement. Additionally, upon first market approval of Qutenza, we are required to make a one time milestone payment of €100,000.
Our future capital uses and requirements depend on numerous forward-looking factors. These factors include but are not limited to the following:
| | • | | the costs and timing of seeking regulatory approvals; |
| | • | | the conduct of manufacturing activities including process development and manufacture of clinical product supply and potentially commercial product supply; |
| | • | | the scope and cost of pre-commercial activities; |
| | • | | our ability to establish and maintain strategic collaborations, including licensing and other arrangements that we have or may establish; |
| | • | | the costs of establishing sales and marketing infrastructure, distribution capabilities and potentially a sales force; |
| | • | | the costs and timing of any post-approval regulatory commitments; |
| | • | | the progress of our development programs including the number, size and scope of clinical trials and non-clinical development; |
| | • | | the success of the commercialization of our products; |
| | • | | the costs involved in enforcing or defending patent claims or other intellectual property rights; and |
| | • | | the extent to which we acquire or invest in other products, technologies and businesses. |
56
At December 31, 2008, we had approximately $24.5 million in cash, cash equivalents and short-term investments and the balance of our notes payable totaled approximately $3.1 million. During the three months ended December 31, 2008, we used a total of approximately $5.3 million in operating activities and approximately $1.0 million in the repayment of notes payable. We currently anticipate that our cash uses will generally remain at these levels or less until such time as we are able to secure additional funding to increase our investment in pre-commercialization activities and advance our development programs. As a result, we anticipate that our existing cash and investments will be sufficient to meet our projected operating requirements through at least December 31, 2009. Additionally, should we achieve market approval in the United States and additional resources become available, we expect that our cash uses will increase to support additional pre-launch activities as well as launch related activities.
To date, we have incurred recurring net losses and negative cash flows from operations. Until we can generate significant cash from our operations, if ever, we expect to continue to fund our operations with existing cash resources generated from the proceeds of offerings of our equity securities as well as potentially through strategic collaboration agreements, debt financing or the sale of other equity securities. However, we may not be successful in obtaining collaboration agreements, or in receiving milestone or royalty payments under those agreements. In addition, we cannot be sure that our existing cash and investment resources will be adequate or that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or our stockholders. Having insufficient funds may require us to further delay, scale back or eliminate some or all of our development programs, relinquish some or even all rights to product candidates at an earlier stage of development or negotiate less favorable terms than we would otherwise choose. Failure to obtain adequate financing also may adversely affect the launch of our product candidates, if approved for marketing, or our ability to continue in business. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
Off-balance Sheet Arrangements
Since inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Recently Issued Accounting Standards
Not Yet Adopted
In December 2007, the Emerging Issues Task Force, or EITF, issued Issue 07-1,Accounting for Collaborative Arrangements, or EITF 07-1, which applies to collaborative arrangements where no separate legal entity exists and in which the parties are active participants and are exposed to significant risks and rewards that depend on the success of the activity. This issue, among other things, requires certain income statement presentation of transactions with third parties and of payments between parties to the collaborative arrangement, along with disclosure about the nature and purpose of the arrangement. The provisions of EITF 07-1 are effective for fiscal years beginning on or after December 15, 2008. We are currently evaluating the impact of the adoption of EITF 07-1 on our consolidated financial statements.
Adopted in 2008
In June 2007, the EITF issued Issue No. 07-3,Accounting for Nonrefundable Advance Payments for Goods or Services To Be Used in Future Research and Development Activities, or EITF 07-3, which concluded that nonrefundable advance payments for goods or services to be received in the future for use in research and development activities should be deferred and capitalized. The capitalized amounts should be expensed as the related goods are delivered or services are performed. Such capitalized amounts should be charged to expense if
57
expectations change such that the goods or services will not be delivered. The provisions of EITF 07-3 are effective for new contracts entered into during fiscal years beginning after December 15, 2007. The consensus may not be applied to earlier periods and early adoption is not permitted. We adopted EITF 07-3 on January 1, 2008. The adoption of EITF 07-3 did not have a material impact on our financial position and results of operations.
We adopted the provisions of the Financial Accounting Standards Board, or FASB, Statement of Financial Accounting Standards, or SFAS, No. 157,Fair Value Measurements, or SFAS No. 157, effective January 1, 2008. SFAS No. 157 defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined under SFAS No. 157 as the exchange price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The adoption of this statement did not have a material impact on our consolidated results of operations and financial condition.
Effective January 1, 2008, we adopted SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities, or SFAS No. 159. SFAS No. 159 allows an entity the irrevocable option to elect fair value for the initial and subsequent measurement for specified financial assets and financial liabilities. We did not elect the fair value option under this Statement.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risks |
Interest Rate and Market Risk
Our exposure to market risk is confined to our cash, cash equivalents and short term investments which have maturities of less than one year. The goal of our investment policy is primarily liquidity and capital preservation while attempting to maximize the income we receive without assuming significant risk. To achieve these objectives our investment policy allows us to maintain a portfolio of cash equivalents and short term investments in a variety of securities, including U.S. government agencies, corporate bonds, commercial paper and money market funds. Further, to reduce portfolio risk through diversification, our investment policy specifies a concentration limit of 10% in any one issuer or group of issuers of corporate bonds or commercial paper at the time of purchase. Our cash and investments as of December 31, 2008 consisted of U.S. Treasury securities and money market funds.
At December 31, 2008, we did not hold any auction rate securities, which have experienced liquidity problems due to failed auctions. The fair value and liquidity of our investments has not been materially impacted by the general events in the credit market. We believe that none of our investments have been impaired during the recent sub-prime mortgage market crisis, although there can be no assurance that there won’t be any future impairment of our investments if the sub-prime market crisis spreads to other sectors of the economy or if credit markets deteriorate further.
58
If interest rates rise, the market value of our investments may decline, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. A hypothetical increase in interest rates by 25 basis points would not result in a material decrease in the fair value of our net investment position. Additionally, as our debt facilities bear interest at fixed rates, we are not subject to market risk with respect to this debt.
Foreign Currency Exchange Rate Risk
We have recorded a liability related to filing fees associated with our MAA filing, which occurred in the three months ended September 30, 2007, that will be payable upon either our withdrawal of the MAA or upon the European Commission’s final decision regarding our MAA. This obligation, which is payable in Euros, creates exposure to changes in that exchange rate. However, the risks related to foreign currency exchange rates are not expected to be material to our consolidated financial position or results of operations.
Our third party manufacturers including the manufacturer of our active ingredient, trans-capsaicin, the manufacturer of Qutenza and the manufacturer of our cleansing gel, are foreign manufacturers. As a result, we may experience changes in product supply costs as a result of changes in exchange rates between the U.S. dollar and the local currency where the manufacturing activities occur.
59
| Item 8. | Financial Statements and Supplementary Data |
NEUROGESX, INC.
(A Development Stage Company)
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
60
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
NeurogesX, Inc.
We have audited the accompanying consolidated balance sheets of NeurogesX, Inc. (a development stage company) as of December 31, 2008 and 2007, and the related consolidated statements of operations, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2008 and for the period from May 28, 1998 (inception) to December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of NeurogesX, Inc. (a development stage company) at December 31, 2008 and 2007, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2008 and for the period from May 28, 1998 (inception) to December 31, 2008, in conformity with U.S. generally accepted accounting principles.
Palo Alto, California
March 20, 2009
61
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
| Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 10,435 | | | $ | 31,478 | |
Short-term investments | | | 14,071 | | | | 21,373 | |
Prepaid expenses and other current assets | | | 412 | | | | 585 | |
Restricted cash | | | 40 | | | | — | |
| | | | | | | | |
Total current assets | | | 24,958 | | | | 53,436 | |
Property and equipment, net | | | 468 | | | | 453 | |
Restricted cash | | | 160 | | | | 240 | |
Other assets | | | 4 | | | | 56 | |
| | | | | | | | |
Total assets | | $ | 25,590 | | | $ | 54,185 | |
| | | | | | | | |
| Liabilities and Stockholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 370 | | | $ | 1,712 | |
Accrued compensation | | | 1,039 | | | | 680 | |
Accrued research and development | | | 1,067 | | | | 1,198 | |
Other accrued expenses | | | 540 | | | | 1,848 | |
Notes payable—current portion | | | 2,833 | | | | 3,859 | |
| | | | | | | | |
Total current liabilities | | | 5,849 | | | | 9,297 | |
| | |
Non-current liabilities: | | | | | | | | |
Notes payable—non-current portion | | | 191 | | | | 3,024 | |
Deferred rent | | | 276 | | | | 156 | |
Accrued research and development—non-current | | | — | | | | 347 | |
| | | | | | | | |
Total non-current liabilities | | | 467 | | | | 3,527 | |
| | |
Commitments and contingencies (Note 7) | | | | | | | | |
| | |
Stockholders’ equity: | | | | | | | | |
Common stock, $0.001 par value;
100,000,000 shares authorized at December 31, 2008 and 2007; 17,568,402 and 17,096,806 shares issued and outstanding at December 31, 2008, and 2007, respectively | | | 18 | | | | 17 | |
Additional paid-in capital | | | 209,370 | | | | 205,417 | |
Deferred stock-based compensation | | | (2 | ) | | | (15 | ) |
Accumulated other comprehensive income | | | 32 | | | | 51 | |
Deficit accumulated during the development stage | | | (190,144 | ) | | | (164,109 | ) |
| | | | | | | | �� |
Total stockholders’ equity | | | 19,274 | | | | 41,361 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 25,590 | | | $ | 54,185 | |
| | | | | | | | |
See accompanying notes.
62
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Period from
May 28, 1998
(inception) to
December 31,
2008 | |
| | | 2008 | | | 2007 | | | 2006 | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development(1) | | $ | 16,104 | | | $ | 25,321 | | | $ | 20,919 | | | $ | 111,492 | |
General and administrative(2) | | | 10,182 | | | | 7,455 | | | | 6,110 | | | | 39,055 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 26,286 | | | | 32,776 | | | | 27,029 | | | | 150,547 | |
| | | | | | | | | | | | | | | | |
Loss from operations | | | (26,286 | ) | | | (32,776 | ) | | | (27,029 | ) | | | (150,547 | ) |
Interest income | | | 1,057 | | | | 1,673 | | | | 719 | | | | 4,972 | |
Interest expense | | | (792 | ) | | | (1,219 | ) | | | (563 | ) | | | (2,718 | ) |
Other income (expense), net | | | (14 | ) | | | 366 | | | | (3,272 | ) | | | (2,947 | ) |
| | | | | | | | | | | | | | | | |
Net loss before cumulative effect of change in accounting principle | | | (26,035 | ) | | | (31,956 | ) | | | (30,145 | ) | | | (151,240 | ) |
Cumulative effect of change in accounting principle | | | — | | | | — | | | | — | | | | (32 | ) |
| | | | | | | | | | | | | | | | |
Net loss | | | (26,035 | ) | | | (31,956 | ) | | | (30,145 | ) | | | (151,272 | ) |
Accretion of redeemable convertible preferred stock | | | — | | | | (4,626 | ) | | | (11,293 | ) | | | (38,872 | ) |
| | | | | | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (26,035 | ) | | $ | (36,582 | ) | | $ | (41,438 | ) | | $ | (190,144 | ) |
| | | | | | | | | | | | | | | | |
Basic and diluted net loss per share attributable to common stockholders | | $ | (1.49 | ) | | $ | (4.06 | ) | | $ | (116.20 | ) | | | | |
| | | | | | | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share attributable to common stockholders | | | 17,519,415 | | | | 9,017,627 | | | | 356,600 | | | | | |
| | | | | | | | | | | | | | | | |
Non-cash stock-based compensation expense
included in operating expenses: | | | | | | | | | | | | | | | | |
| | | | |
(1) Research and development | | $ | 745 | | | $ | 893 | | | $ | 644 | | | | | |
(2) General and administrative | | | 755 | | | | 869 | | | | 3,199 | | | | | |
| | | | | | | | | | | | | | | | |
| | $ | 1,500 | | | $ | 1,762 | | | $ | 3,843 | | | | | |
| | | | | | | | | | | | | | | | |
See accompanying notes.
63
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | Deferred
Stock-Based
Compensation | | | Accumulated
Other
Comprehensive
Income (Loss) | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | | Amount | | | | | |
Issuance of common stock to founders for cash at $0.015 per share in July 1998 | | 199,994 | | | $ | — | | $ | 3 | | $ | — | | | $ | — | | $ | — | | | $ | 3 | |
Issuance of common stock for cash and services at $0.90 per share in June 2000 | | 20,000 | | | | — | | | 19 | | | — | | | | — | | | — | | | | 19 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | — | | | | — | | | (378 | ) | | | (378 | ) |
Net loss from May 28, 1998 (inception) to December 31, 2000 | | — | | | | — | | | — | | | — | | | | — | | | (1,115 | ) | | | (1,115 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2000 | | 219,994 | | | | — | | | 22 | | | — | | | | — | | | (1,493 | ) | | | (1,471 | ) |
Issuance of common stock for cash at $0.90 per share in April 2001 | | 3,833 | | | | — | | | 4 | | | — | | | | — | | | — | | | | 4 | |
Issuance of warrants in connection with loan in June 2001 | | — | | | | — | | | 14 | | | — | | | | — | | | — | | | | 14 | |
Compensation expense relating to stock options granted to consultants | | — | | | | — | | | 1 | | | — | | | | — | | | — | | | | 1 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | — | | | | — | | | (793 | ) | | | (793 | ) |
Net loss | | — | | | | — | | | — | | | — | | | | — | | | (6,225 | ) | | | (6,225 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2001 | | 223,827 | | | | — | | | 41 | | | | | | | — | | | (8,511 | ) | | | (8,470 | ) |
Issuance of common stock to employees upon exercise of stock options at $0.90-$1.20 per share for cash | | 4,213 | | | | — | | | 4 | | | — | | | | — | | | — | | | | 4 | |
Issuance of common stock upon payment of note receivable at $0.90 per share | | 6,666 | | | | — | | | 6 | | | — | | | | — | | | — | | | | 6 | |
Issuance of warrants in connection with loan in May 2002 | | — | | | | — | | | 12 | | | — | | | | — | | | — | | | | 12 | |
Deferred stock-based compensation relating to variable accounting of stock options and restricted common stock | | — | | | | — | | | 4 | | | (4 | ) | | | — | | | — | | | | — | |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | | — | | | 75 | | | — | | | | — | | | — | | | | 75 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | — | | | | — | | | (3,234 | ) | | | (3,234 | ) |
Net loss | | — | | | | — | | | — | | | — | | | | — | | | (7,908 | ) | | | (7,908 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2002 | | 234,706 | | | | — | | | 142 | | | (4 | ) | | | — | | | (19,653 | ) | | | (19,515 | ) |
Issuance of common stock upon exercise of stock options for cash at $0.90-$1.20 per share | | 3,679 | | | | — | | | 4 | | | — | | | | — | | | — | | | | 4 | |
Reclassification of unvested common stock at $1.20 per share | | (379 | ) | | | — | | | — | | | — | | | | — | | | — | | | | — | |
Issuance of common stock upon payment of note receivable | | 19,998 | | | | — | | | 25 | | | — | | | | — | | | — | | | | 25 | |
Deferred stock-based compensation relating to variable accounting of stock options and restricted common stock | | — | | | | — | | | 29 | | | (29 | ) | | | — | | | — | | | | — | |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | | — | | | 188 | | | — | | | | — | | | — | | | | 188 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | — | | | | — | | | (3,498 | ) | | | (3,498 | ) |
Net loss | | — | | | | — | | | — | | | — | | | | — | | | (13,400 | ) | | | (13,400 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2003 (carried forward) | | 258,004 | | | $ | — | | $ | 388 | | $ | (33 | ) | | $ | — | | $ | (36,551 | ) | | $ | (36,196 | ) |
64
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | | Deferred
Stock-Based
Compensation | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | | Amount | | | | | |
Balances at December 31, 2003 (brought forward) | | 258,004 | | | $ | — | | $ | 388 | | | $ | (33 | ) | | $ | — | | | $ | (36,551 | ) | | $ | (36,196 | ) |
Issuance of common stock upon exercise of stock options for cash at $0.90-$6.00 per share | | 8,217 | | | | — | | | 14 | | | | — | | | | — | | | | — | | | | 14 | |
Issuance of common stock at $1.20 per share upon vesting of early exercised options | | 245 | | | | — | | | — | | | | — | | | | — | | | | (1 | ) | | | (1 | ) |
Issuance of common stock upon payment of notes receivable at $0.90-$1.20 per share | | 37,333 | | | | — | | | 37 | | | | — | | | | — | | | | — | | | | 37 | |
Shares repurchased upon rescission of stock option exercise at $2.25 per share | | (3,333 | ) | | | — | | | (8 | ) | | | — | | | | — | | | | — | | | | (8 | ) |
Deferred stock-based compensation related to variable accounting of stock options and restricted common stock | | — | | | | — | | | 100 | | | | (100 | ) | | | — | | | | — | | | | — | |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | | — | | | 1,401 | | | | — | | | | — | | | | — | | | | 1,401 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | | — | | | | — | | | | (6,782 | ) | | | (6,782 | ) |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain/(loss) on investments | | — | | | | — | | | — | | | | — | | | | (19 | ) | | | — | | | | (19 | ) |
Net loss | | — | | | | — | | | — | | | | — | | | | — | | | | (21,343 | ) | | | (21,343 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | (21,362 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2004 | | 300,466 | | | | — | | | 1,932 | | | | (133 | ) | | | (19 | ) | | | (64,677 | ) | | | (62,897 | ) |
Issuance of common stock upon exercise of stock options for cash at $1.95-$2.55 per share | | 8,533 | | | | — | | | 19 | | | | — | | | | — | | | | — | | | | 19 | |
Issuance of common stock at $0.90 per share upon vesting of early exercised stock options | | 66 | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Reclassification of unvested common stock at $2.25 per share | | (3,333 | ) | | | — | | | (7 | ) | | | — | | | | — | | | | — | | | | (7 | ) |
Registration cost of additional option pool shares | | — | | | | — | | | (1 | ) | | | — | | | | — | | | | — | | | | (1 | ) |
Issuance of common stock upon payment of note receivable at $0.90 per share | | 13,333 | | | | — | | | 20 | | | | — | | | | — | | | | — | | | | 20 | |
Deferred stock-based compensation related to variable accounting of stock options and restricted common stock | | — | | | | — | | | (107 | ) | | | 107 | | | | — | | | | — | | | | — | |
Deferred stock-based compensation related to stock options granted below re-assessed fair value of common stock | | — | | | | — | | | 67 | | | | (67 | ) | | | — | | | | — | | | | — | |
Reversal of deferred stock-based compensation in connection with employee terminations | | — | | | | — | | | (8 | ) | | | 8 | | | | — | | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | | — | | | — | | | | 6 | | | | — | | | | — | | | | 6 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | | — | | | | — | | | | (8,269 | ) | | | (8,269 | ) |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | | — | | | (326 | ) | | | — | | | | — | | | | — | | | | (326 | ) |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain/(loss) on investments | | — | | | | — | | | — | | | | — | | | | 18 | | | | — | | | | 18 | |
Net loss | | — | | | | — | | | — | | | | — | | | | — | | | | (13,143 | ) | | | (13,143 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | (13,125 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2005 (carried forward) | | 319,065 | | | $ | — | | $ | 1,589 | | | $ | (79 | ) | | $ | (1 | ) | | $ | (86,089 | ) | | $ | (84,580 | ) |
65
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | | Deferred
Stock-Based
Compensation | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | | Amount | | | | | |
Balances at December 31, 2005 (brought forward) | | 319,065 | | | $ | — | | $ | 1,589 | | | $ | (79 | ) | | | (1 | ) | | | (86,089 | ) | | | (84,580 | ) |
Issuance of common stock upon exercise of stock options for cash at $1.95-$4.05 per share | | 25,483 | | | | — | | | 57 | | | | — | | | | — | | | | — | | | | 57 | |
Registration cost of additional option pool shares | | — | | | | — | | | (1 | ) | | | — | | | | — | | | | — | | | | (1 | ) |
Issuance of common stock at $1.20-$3.00 per share upon vesting of early exercised stock options | | 1,942 | | | | — | | | 4 | | | | — | | | | — | | | | — | | | | 4 | |
Reclassification of unvested common stock at $3.00 per share | | (3,512 | ) | | | — | | | (11 | ) | | | — | | | | — | | | | — | | | | (11 | ) |
Issuance of common stock upon payment of notes receivable at $0.90-$1.20 per share | | 43,331 | | | | 1 | | | 58 | | | | — | | | | — | | | | — | | | | 59 | |
Deferred stock-based compensation related to variable accounting of stock options and restricted common stock | | — | | | | — | | | (14 | ) | | | 14 | | | | — | | | | — | | | | — | |
Reversal of deferred stock-based compensation in connection with employee terminations | | — | | | | — | | | (8 | ) | | | 8 | | | | — | | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | | — | | | — | | | | 12 | | | | — | | | | — | | | | 12 | |
Accretion of redeemable convertible preferred stock | | — | | | | — | | | — | | | | — | | | | — | | | | (11,293 | ) | | | (11,293 | ) |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | | — | | | 3,516 | | | | — | | | | — | | | | — | | | | 3,516 | |
Stock-based compensation expense under SFAS No. 123(R) | | — | | | | — | | | 315 | | | | — | | | | — | | | | — | | | | 315 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain/(loss) on investments | | — | | | | — | | | — | | | | — | | | | 1 | | | | — | | | | 1 | |
Net loss | | — | | | | — | | | — | | | | — | | | | — | | | | (30,145 | ) | | | (30,145 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | (30,144 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2006 (carried forward) | | 386,309 | | | $ | 1 | | $ | 5,505 | | | $ | (45 | ) | | $ | — | | | $ | (127,527 | ) | | $ | (122,066 | ) |
66
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | | Deferred
Stock-Based
Compensation | | | Accumulated
Other
Comprehensive
Income (Loss) | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | Amount | | | | | |
Balances at December 31, 2006 (brought forward) | | 386,309 | | $ | 1 | | $ | 5,505 | | | $ | (45 | ) | | $ | — | | $ | (127,527 | ) | | $ | (122,066 | ) |
Issuance of common stock upon exercise of stock options for cash at $0.90—$3.75 per share | | 27,476 | | | — | | | 65 | | | | — | | | | — | | | — | | | | 65 | |
Issuance of common stock at $1.20-$3.75 per share upon vesting of early exercised stock options | | 9,834 | | | — | | | 18 | | | | — | | | | — | | | — | | | | 18 | |
Issuance of common stock under the Employee Stock Purchase Plan | | 15,367 | | | — | | | 90 | | | | — | | | | — | | | — | | | | 90 | |
Issuance of common stock in an initial public offering for cash at $11.00 per share, net of $5,914 issuance costs | | 4,000,000 | | | 4 | | | 38,081 | | | | — | | | | — | | | — | | | | 38,085 | |
Issuance of common stock for cash at $6.18 per share, net of $1,100 issuance costs | | 3,638,741 | | | 3 | | | 17,973 | | | | — | | | | — | | | — | | | | 17,976 | |
Issuance of warrants to purchase common stock to investors at $0.125 per share including fair value | | — | | | — | | | 3,547 | | | | — | | | | — | | | — | | | | 3,547 | |
Conversion of preferred stock to common stock in connection with initial public offering in May 2007 | | 8,722,013 | | | 9 | | | 138,115 | | | | — | | | | — | | | — | | | | 138,124 | |
Issuance of common stock upon forgiveness of notes receivable at $0.90—$2.25 per share | | 263,733 | | | — | | | 379 | | | | — | | | | — | | | — | | | | 379 | |
Issuance of common stock to consultant for service | | 33,333 | | | — | | | 291 | | | | — | | | | — | | | — | | | | 291 | |
Deferred stock-based compensation related to variable accounting of stock options and restricted common stock | | — | | | — | | | (12 | ) | | | 12 | | | | — | | | — | | | | — | |
Reversal of deferred stock-based compensation in connection with employee terminations | | — | | | — | | | (9 | ) | | | 9 | | | | — | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | — | | | — | | | | 9 | | | | — | | | — | | | | 9 | |
Accretion of redeemable convertible preferred stock | | — | | | — | | | — | | | | — | | | | — | | | (4,626 | ) | | | (4,626 | ) |
Compensation expense relating to stock options granted to consultants and variable accounting of stock options and restricted common stock | | — | | | — | | | (88 | ) | | | — | | | | — | | | — | | | | (88 | ) |
Stock-based compensation expense under SFAS No.123(R) | | — | | | — | | | 1,462 | | | | — | | | | — | | | — | | | | 1,462 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain/(loss) on investments | | — | | | — | | | — | | | | — | | | | 51 | | | — | | | | 51 | |
Net loss | | — | | | — | | | — | | | | — | | | | — | | | (31,956 | ) | | | (31,956 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (31,905 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2007 (carried forward) | | 17,096,806 | | $ | 17 | | $ | 205,417 | | | $ | (15 | ) | | $ | 51 | | $ | (164,109 | ) | | $ | 41,361 | |
67
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)—(Continued)
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
Paid-in
Capital | | | Deferred
Stock-Based
Compensation | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Deficit
Accumulated
During the
Development
Stage | | | Total
Stockholders’
Equity
(Deficit) | |
| | | Shares | | Amount | | | | | |
Balances at December 31, 2007 (brought forward) | | 17,096,806 | | $ | 17 | | $ | 205,417 | | | $ | (15 | ) | | $ | 51 | | | $ | (164,109 | ) | | $ | 41,361 | |
Issuance of common stock upon exercise of stock options for cash at $0.90—$3.75 per share | | 36,706 | | | — | | | 86 | | | | — | | | | — | | | | — | | | | 86 | |
Issuance of common stock at $2.25—$3.75 per share upon vesting of early exercised stock options | | 1,503 | | | — | | | 4 | | | | — | | | | — | | | | — | | | | 4 | |
Issuance of common stock under the Employee Stock Purchase Plan | | 51,217 | | | — | | | 94 | | | | — | | | | — | | | | — | | | | 94 | |
Issuance of common stock for cash at $6.18 per share, net of $100 issuance costs | | 382,170 | | | 1 | | | 1,847 | | | | — | | | | — | | | | — | | | | 1,848 | |
Issuance of warrants to purchase common stock to investors at $0.125 per share including fair value | | — | | | — | | | 434 | | | | — | | | | — | | | | — | | | | 434 | |
Reversal of deferred stock-based compensation in connection with employee terminations | | — | | | — | | | (4 | ) | | | 4 | | | | — | | | | — | | | | — | |
Amortization of deferred stock-based compensation | | — | | | — | | | — | | | | 9 | | | | — | | | | — | | | | 9 | |
Compensation expense relating to stock options granted to consultants | | — | | | — | | | 2 | | | | — | | | | — | | | | — | | | | 2 | |
Stock-based compensation expense under SFAS No. 123(R) | | — | | | — | | | 1,490 | | | | — | | | | — | | | | — | | | | 1,490 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | |
Unrealized gain/(loss) on investments | | — | | | — | | | — | | | | — | | | | (19 | ) | | | — | | | | (19 | ) |
Net loss | | — | | | — | | | — | | | | — | | | | — | | | | (26,035 | ) | | | (26,035 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | (26,054 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2008 | | 17,568,402 | | $ | 18 | | $ | 209,370 | | | $ | (2 | ) | | $ | 32 | | | $ | (190,144 | ) | | $ | 19,274 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
68
NEUROGESX, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF CASH FLOWS
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Period from
May 28, 1998
(inception) to
December 31,
2008 | |
| | | 2008 | | | 2007 | | | 2006 | | |
| | | (in thousands) | | | | |
Operating activities | | | | | | | | | | | | | | | | |
Net loss | | $ | (26,035 | ) | | $ | (31,956 | ) | | $ | (30,145 | ) | | $ | (151,272 | ) |
Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | | | | | | | | | |
Depreciation and amortization | | | 181 | | | | 89 | | | | 260 | | | | 1,546 | |
Amortization of debt issuance costs | | | 198 | | | | 198 | | | | 78 | | | | 492 | |
Amortization/accretion of investment premiums/(discounts),net | | | (651 | ) | | | (778 | ) | | | (47 | ) | | | (1,088 | ) |
Stock-based compensation | | | 1,500 | | | | 1,762 | | | | 3,843 | | | | 8,455 | |
Loss on sales of short-term investments | | | 8 | | | | — | | | | — | | | | 8 | |
(Gain)/loss on disposal of fixed assets | | | 5 | | | | (6 | ) | | | — | | | | 87 | |
Revaluation of preferred stock warrant liability | | | — | | | | (360 | ) | | | 3,274 | | | | 2,888 | |
Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | |
Prepaid expenses and other current assets | | | 173 | | | | 74 | | | | (257 | ) | | | (412 | ) |
Other assets | | | 10 | | | | — | | | | (106 | ) | | | (106 | ) |
Accounts payable | | | (1,342 | ) | | | 37 | | | | (5 | ) | | | 370 | |
Accrued compensation | | | 359 | | | | 481 | | | | 2 | | | | 1,039 | |
Accrued research and development | | | (478 | ) | | | 412 | | | | 424 | | | | 1,067 | |
Deferred rent | | | 144 | | | | 156 | | | | — | | | | 300 | |
Other accrued expenses | | | (1,326 | ) | | | 1,237 | | | | 358 | | | | 528 | |
| | | | | | | | | | | | | | | | |
Net cash used in operating activities | | | (27,254 | ) | | | (28,654 | ) | | | (22,321 | ) | | | (136,098 | ) |
| | | | | | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | | | | | |
Purchases of short-term investments | | | (64,363 | ) | | | (53,050 | ) | | | (4,949 | ) | | | (187,243 | ) |
Proceeds from maturities of short-term investments | | | 39,360 | | | | 34,500 | | | | 5,023 | | | | 141,354 | |
Proceeds from sales of short-term investments | | | 32,929 | | | | — | | | | — | | | | 32,929 | |
Change in restricted cash | | | 40 | | | | (240 | ) | | | — | | | | (200 | ) |
Proceeds from disposal of property and equipment | | | 2 | | | | 6 | | | | — | | | | 8 | |
Purchases of property and equipment | | | (203 | ) | | | (383 | ) | | | (141 | ) | | | (2,109 | ) |
| | | | | | | | | | | | | | | | |
Net cash provided by/(used in) investing activities | | | 7,765 | | | | (19,167 | ) | | | (67 | ) | | | (15,261 | ) |
| | | | | | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | | | | | |
Proceeds from notes payable | | | — | | | | — | | | | 10,000 | | | | 11,092 | |
Repayment of notes payable | | | (4,015 | ) | | | (2,446 | ) | | | (434 | ) | | | (7,988 | ) |
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs | | | — | | | | 10,083 | | | | 14,585 | | | | 96,187 | |
Proceeds from issuance of warrants | | | 14 | | | | 136 | | | | — | | | | 150 | |
Proceeds from issuance of common stock | | | 2,447 | | | | 59,618 | | | | 114 | | | | 62,353 | |
| | | | | | | | | | | | | | | | |
Net cash provided by/(used in) financing activities | | | (1,554 | ) | | | 67,391 | | | | 24,265 | | | | 161,794 | |
| | | | | | | | | | | | | | | | |
Net increase/(decrease) in cash and cash equivalents | | | (21,043 | ) | | | 19,570 | | | | 1,877 | | | | 10,435 | |
Cash and cash equivalents, beginning of period | | | 31,478 | | | | 11,908 | | | | 10,031 | | | | — | |
| | | | | | | | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 10,435 | | | $ | 31,478 | | | $ | 11,908 | | | $ | 10,435 | |
| | | | | | | | | | | | | | | | |
Supplemental cash flow information | | | | | | | | | | | | | | | | |
Cash paid for interest | | $ | 634 | | | $ | 950 | | | $ | 467 | | | $ | 2,157 | |
| | | | | | | | | | | | | | | | |
Noncash investing and financing activities | | | | | | | | | | | | | | | | |
Accretion of redeemable convertible preferred stock | | $ | — | | | $ | 4,626 | | | $ | 11,293 | | | $ | 38,872 | |
| | | | | | | | | | | | | | | | |
Warrants issued in connection with preferred stock or debt financings | | $ | — | | | $ | — | | | $ | 3,539 | | | $ | 4,301 | |
| | | | | | | | | | | | | | | | |
Issuance of common stock to a consultant for services rendered in connection with preferred stock financing | | $ | — | | | $ | (69 | ) | | $ | 334 | | | $ | 291 | |
| | | | | | | | | | | | | | | | |
Deferred stock compensation related to variable accounting of stock options and restricted common stock | | $ | — | | | $ | 21 | | | $ | 14 | | | $ | 275 | |
| | | | | | | | | | | | | | | | |
Conversion of preferred stock into common stock upon IPO | | $ | — | | | $ | 138,124 | | | $ | — | | | $ | 138,124 | |
| | | | | | | | | | | | | | | | |
See accompanying notes.
69
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Nature of Operation and Basis of Preparation
NeurogesX, Inc. (the “Company”) is a biopharmaceutical company focused on developing and commercializing novel pain management therapies. The Company is assembling a portfolio of pain management product candidates based on known chemical entities to develop innovative new therapies which the Company believes may offer substantial advantages over currently available treatment options. The Company’s initial focus is on the management of chronic peripheral neuropathic pain conditions. The Company’s most advanced product candidate, Qutenza (formerly NGX-4010), a dermal patch containing a high concentration of synthetic capsaicin, is designed to manage pain associated with peripheral neuropathic pain conditions.
The Company submitted to the Food and Drug Administration (“FDA”) a new drug application (“NDA”) for Qutenza for the management of pain associated with postherpetic neuralgia (“PHN”) in October 2008, which was accepted for filing by the FDA in December 2008. The Company’s NDA has a Prescription Drug User Fee Act (“PDUFA”) date of August 16, 2009 at which time the Company anticipates receiving either an approval letter or a complete response letter from the FDA. A complete response letter may describe any activities which may be required to gain approval or may indicate that a product candidate is not approvable.
In September 2007 the Company submitted a marketing authorization application (“MAA”) for Qutenza with the European Medicines Agency (“EMEA”) under the centralized procedure, seeking approval of Qutenza. On March 19, 2009, the Committee for Medicinal Products for Human Use (“CHMP”) issued a positive opinion recommending the approval of the Company’s MAA for Qutenza for the treatment of peripheral neuropathic in non-diabetic adults either alone or in combination with other medicinal products for pain. The Company is currently awaiting the European Commission’s decision on the CHMP’s opinion, a process which normally takes approximately 60 to 90 days. If the European Commission issues a marketing authorization, in most European Union member states product pricing for government sponsored health-care reimbursement must be negotiated or for hospital-based products, product pricing may be established directly with hospitals. We believe this process can take months or substantially longer to complete, if at all.
The Company was incorporated in California as Advanced Analgesics, Inc. on May 28, 1998 and changed its name to NeurogesX, Inc. in September 2000. In February 2007, the Company reincorporated into Delaware. The Company is located in San Mateo, California. Since its inception, the Company has devoted substantially all of its efforts to the development of Qutenza and other potential products, establishing its offices, recruiting personnel, performing business and financial planning, and raising capital. Accordingly, the Company is considered to be in the development stage. The Company has not generated any revenue to date and has incurred operating and net losses each year since inception in 1998.
In 2008, in response to the generally weak economic conditions which the Company believes has resulted in a challenging environment for raising capital, the Company has deferred further pre-clinical and clinical development activities for all of its product candidates in order to focus its fiscal and human resources on the prosecution of its MAA and NDA for Qutenza and on continuing support of its strategies for obtaining adequate reimbursement for Qutenza in the United States. The Company expects to re-initiate some or all of its development programs if additional funds become available. Further, the Company is currently seeking development partners for its acetaminophen and opioid prodrug product candidates. The Company holds worldwide commercial rights to all of its product candidates and is actively engaged in discussions with potential commercial partners.
70
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The Company had cash, cash equivalents and short-term investments totaling $24.5 million at December 31, 2008, which the Company believes is sufficient to fund its operations through at least December 31, 2009. The Company intends to seek additional funding through strategic alliances, debt facilities or other financing vehicles which may include the public or private sales of its equity securities.
Principles of Consolidation
The accompanying financial statements include the accounts of the Company and its wholly-owned subsidiary, NeurogesX UK Limited, which was incorporated as of June 1, 2004. NeurogesX UK Limited was established for the purposes of conducting clinical trials in the UK and marketing approval submission. The subsidiary has no assets other than the initial formation capital totaling one Pound Sterling.
Reverse Stock Split
On April 13, 2007, the Company effected a 1-for-15 reverse split of its common stock. All common stock share and per share amounts have been retroactively restated to reflect the reverse stock split in the accompanying consolidated financial statements and notes for all periods presented.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
| 2. | Summary of Significant Accounting Policies |
Cash and Cash Equivalents and Short Term Investments
The Company invests its available cash balances in bank deposits, money market funds, U.S. government securities and other investment grade debt securities that have strong credit ratings. The Company considers all highly liquid investments with an original maturity of three months or less at the time of purchase to be cash equivalents.
The Company accounts for its investments in marketable securities in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 115,Accounting for Certain Investments in Debt and Equity Securities. Management determines the appropriate classification of securities at the time of purchase. To date, all marketable securities have been classified as available-for-sale, and are carried at fair value as determined based on quoted market prices or other observable market inputs with unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) in stockholders’ equity. The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company has classified all investments as short term.
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary for available-for-sale securities, if any, are included in other income/(expense), net and have not been significant to date. Realized gains and losses are computed on a specific identification basis. Interest and dividends are included in interest income.
71
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Concentrations of Credit Risk and Financial Instruments
The Company invests cash that is not currently being used for operational purposes in accordance with its investment policy. The policy allows for the purchase of low risk debt securities issued by U.S. government agencies and highly rated corporations subject to concentration limits of 10% of any one issuer or group of issuers at the time of purchase, with the exception of debt securities issued by U.S. government agencies where the Company is not subject to any concentration limit. The maturities of these securities on a weighted-average basis may be no longer than 12 months. The Company believes that it has established guidelines for investment of its excess cash that maintains safety and liquidity through its policies on diversification and investment maturity.
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash and cash equivalents and short term investments, available for sale investment securities in high-credit quality corporate debt, and debt securities issued by the U.S. government and government-sponsored enterprises. The carrying amounts of borrowings under the Company’s debt facilities approximate fair value based on the current interest rates for similar borrowing arrangements.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is determined using the straight-line method and recorded over the estimated useful lives of the respective assets, generally three to five years. Leasehold improvements are amortized over the life of the lease or the useful economic life, whichever is shorter.
Research and Development
The Company expenses research and development costs as incurred. Research and development expenses include personnel and personnel related costs, costs associated with pre-clinical and clinical development activities, such as clinical trials, including amounts paid to clinical research organizations and clinical investigators, product and manufacturing costs such as process development, clinical product supply costs and the cost of commercially saleable product that is manufactured prior to market approval for that product candidate, internal and external costs associated with our regulatory compliance and quality assurance functions including the costs of outside consultants and contractors that assist in the process of submitting and maintaining regulatory filings and overhead costs including allocated facility and related expenses.
Clinical Trials
The Company accrues and expenses costs for clinical trial activities performed by third parties, including clinical research organizations and clinical investigators, based upon estimates of the work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines these estimates through discussion with internal personnel and outside service providers as to progress or stage of completion of trials or services pursuant to contracts with numerous clinical trial centers and clinical research organizations and the agreed upon fee to be paid for such services.
Stock-Based Compensation
Effective January 1, 2006, the Company adopted the provisions of SFAS No. 123R,Share-Based Payments. In March 2005, the Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin (“SAB”) No. 107 relating to SFAS No. 123R. The Company has applied the provisions of SAB No. 107, for which the simplified method of determining the expected term of an option has been extended by SAB No. 110, in its adoption of SFAS No. 123R. Under SFAS No. 123R, stock-based awards, including stock options, are recorded
72
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
at fair value as of the grant date and recognized to expense over the employee’s requisite service period (generally the vesting period) which the Company has elected to amortize on a straight-line basis. Because non-cash stock compensation expense is based on awards ultimately expected to vest, it has been reduced by an estimate for future forfeitures. SFAS No. 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company adopted the provisions of SFAS No. 123R using the prospective transition method. Under the prospective transition method, beginning January 1, 2006, compensation cost recognized includes: (a) compensation cost for all share-based payments granted prior to, but not yet vested as of December 31, 2005, based on the intrinsic value in accordance with the provisions of APB No. 25, and (b) compensation cost for all share-based payments granted subsequent to December 31, 2005, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123R. All awards granted, modified, or settled after the date of adoption are accounted for using the measurement, recognition, and attribution provisions of SFAS No. 123R. The Company has elected to use the Black-Scholes option valuation model to estimate the fair value of stock options.
At December 31, 2008, the Company had three share-based compensation plans, which are described in Note 9.
Interest Expense
Interest expense is comprised of interest relating to the Company’s notes payable, the amortization of the debt premium which represents the initial fair value of warrants to purchase preferred stock issued in connection with notes payable (see Note 6), and the amortization of debt issuance costs recorded as “Prepaid expenses and other current assets” and “Other assets” on the balance sheet.
Income taxes
The Company utilizes the liability method of accounting for income taxes as required by SFAS No. 109,Accounting for Income Taxes (“SFAS No. 109”) and interpreted by FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109 (“FIN No. 48”). Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Uncertain tax positions are evaluated in accordance with FIN No. 48 and if appropriate, the amount of unrecognized tax benefits are recorded within deferred tax assets, as further described in Note 11. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Currently, there is no provision for income taxes as the Company has incurred operating losses to date.
Comprehensive Loss
SFAS No. 130,Reporting Comprehensive Income, requires components of other comprehensive income, including gains and losses on available-for-sale investments, to be included as part of total comprehensive income (loss). The Company displays comprehensive loss and its components as part of the statement of stockholders’ equity (deficit). Comprehensive loss consists of net loss and unrealized gains and losses on available-for-sale investments for all periods presented. The fluctuation in accumulated other comprehensive income represents the net change in fair value for invested assets as a result of changes in interest rates and other factors affecting fair value and as a result of sales of investments prior to their maturities. The cumulative effect of these periodic fluctuations is reflected as other comprehensive income on the Company’s balance sheet.
73
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period less the weighted average unvested common shares subject to repurchase and without consideration of common stock equivalents. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common share and share equivalents outstanding for the period, less the weighted average unvested common shares subject to repurchase. For purposes of this calculation, warrants and options to purchase common stock, as well as preferred stock prior to the initial public offering (“IPO”), are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share as their effect is anti-dilutive.
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands except share and per share data) | |
Numerator: | | | | | | | | | | | | |
Net loss | | $ | (26,035 | ) | | $ | (31,956 | ) | | $ | (30,145 | ) |
Accretion of redeemable convertible preferred stock | | | — | | | | (4,626 | ) | | | (11,293 | ) |
| | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (26,035 | ) | | $ | (36,582 | ) | | $ | (41,438 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Weighted-average common shares outstanding | | | 17,520,778 | | | | 9,020,726 | | | | 360,217 | |
Less: Weighted-average unvested common shares subject to repurchase | | | (1,363 | ) | | | (3,099 | ) | | | (3,617 | ) |
| | | | | | | | | | | | |
Denominator for basic and diluted net loss per share attributable to common stockholders | | | 17,519,415 | | | | 9,017,627 | | | | 356,600 | |
| | | | | | | | | | | | |
Basic and diluted net loss per share attributable to common stockholders | | $ | (1.49 | ) | | $ | (4.06 | ) | | $ | (116.20 | ) |
| | | | | | | | | | | | |
| |
| | | December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Historical outstanding securities not included in diluted net loss per share attributable to common stockholders calculation: | | | | | | | | | | | | |
Redeemable convertible preferred stock | | | — | | | | — | | | | 7,825,731 | |
Options to purchase common stock | | | 1,392,560 | | | | 1,132,876 | | | | 817,234 | |
Warrants outstanding | | | 1,265,846 | | | | 1,151,195 | | | | 955,855 | |
Common stock subject to repurchase | | | 785 | | | | 2,289 | | | | 4,575 | |
| | | | | | | | | | | | |
| | | 2,659,191 | | | | 2,286,360 | | | | 9,603,395 | |
| | | | | | | | | | | | |
Preferred Stock Warrant Liability
Effective July 1, 2005, the Company adopted the provisions of FASB Staff Position (“FSP”) No. 150-5,Issuer’s Accounting under Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that are Redeemable, an interpretation of SFAS No. 150,Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity. Pursuant to FSP No. 150-5, freestanding warrants for shares that are either puttable or warrants for shares that are redeemable are classified as liabilities on the balance sheet
74
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
at fair value. At the end of each reporting period, changes in fair value during the period are recorded as a component of other income or expense. Prior to July 1, 2005, the Company accounted for warrants for the purchase of preferred stock under EITF Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services.
For the year ended December 31, 2006, the Company recorded approximately $3,274,000 of other expense for the increase in fair value of all preferred stock warrants. In January and February 2007, the Company issued a total of 13,444,450 shares of Series C2 preferred stock at $0.75 per share upon exercise of warrants resulting in aggregate net cash proceeds of approximately $10,083,000. As a result of this transaction, the Company recognized approximately $59,000 as other income related to the change in fair value of the preferred stock warrant liability on the date of the transaction and reclassified approximately $6,763,000 from preferred stock warrant liability to preferred stock. In May 2007, with the completion of the Company’s initial public offering at which time the preferred stock warrant liabilities were reclassified to stockholders’ equity (deficit) when the warrants were converted to common stock warrants, the Company ceased to adjust the preferred stock warrant liabilities for changes in fair value. The Company performed a final remeasurement to determine the fair value of such financial instruments immediately prior to their conversion. The resulting fair value of $426,000 was reclassified to additional paid-in capital. The Company recorded approximately $360,000 reflected as other income for the decrease in estimated fair value of all preferred stock warrants in the year ended December 31, 2007.
Recently Issued Accounting Standards
Not Yet Adopted
In December 2007, the EITF issued EITF Issue 07-1,Accounting for Collaborative Arrangements(“EITF 07-1”), which applies to collaborative arrangements where no separate legal entity exists and in which the parties are active participants and are exposed to significant risks and rewards that depend on the success of the activity. This issue, among other things, requires certain income statement presentation of transactions with third parties and of payments between parties to the collaborative arrangement, along with disclosure about the nature and purpose of the arrangement. The provisions of EITF 07-1 are effective for fiscal years beginning on or after December 15, 2008. The Company is currently evaluating the impact of the adoption of EITF 07-1 on its consolidated financial statements.
Adopted in 2008
The Company adopted the provisions of the Financial Accounting Standards Board (“FASB”), Statement No. 157,Fair Value Measurements (“SFAS No. 157”), effective January 1, 2008. SFAS No. 157 defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined under SFAS No. 157 as the exchange price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
75
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The adoption of this statement did not have a material impact on the Company’s consolidated results of operations and financial condition.
SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities (“SFAS No. 159”) became effective for the Company on January 1, 2008, which allows an entity the irrevocable option to elect fair value for the initial and subsequent measurement for specified financial assets and financial liabilities. The Company did not elect the fair value option under this Statement.
| 3. | Cash and Cash Equivalents and Short Term Investments |
The following are summaries of cash, cash equivalents and short term investments (in thousands):
| | | | | | | | | | | | | |
| | | Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | | Estimated
Fair Value |
As of December 31, 2008: | | | | | | | | | | | | | |
Cash and money market funds | | $ | 9,933 | | $ | — | | $ | — | | | $ | 9,933 |
U.S. Treasury securities | | | 14,541 | | | 32 | | | — | | | | 14,573 |
| | | | | | | | | | | | | |
| | $ | 24,474 | | $ | 32 | | $ | — | | | $ | 24,506 |
| | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | | | $ | 10,435 |
Short-term investments | | | | | | | | | | | | | 14,071 |
| | | | | | | | | | | | | |
| | | | | | | | | | | | $ | 24,506 |
| | | | | | | | | | | | | |
As of December 31, 2007: | | | | | | | | | | | | | |
Cash and money market funds | | $ | 31,478 | | $ | — | | $ | — | | | $ | 31,478 |
Commercial paper | | | 10,839 | | | 47 | | | — | | | | 10,886 |
Corporate debt securities | | | 5,751 | | | 1 | | | (1 | ) | | | 5,751 |
Asset-backed securities | | | 4,732 | | | 4 | | | — | | | | 4,736 |
| | | | | | | | | | | | | |
| | $ | 52,800 | | $ | 52 | | $ | (1 | ) | | $ | 52,851 |
| | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | | | $ | 31,478 |
Short-term investments | | | | | | | | | | | | | 21,373 |
| | | | | | | | | | | | | |
| | | | | | | | | | | | $ | 52,851 |
| | | | | | | | | | | | | |
At December 31, 2008 and 2007, the contractual maturities of investments held were less than one year. During the year ended December 31, 2008, the Company received approximately $32,929,000 in gross proceeds from the sales of certain short-term investments in commercial paper, corporate debt and asset-backed securities. As a result of these sales, during the year ended December 31, 2008, the Company recognized a loss, consisting of both realized gains and realized losses, of approximately $8,000, which was recorded in other income. The Company did not sell any of its investments prior to maturity during the year ended December 31, 2007.
76
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 4. | Fair Value Measurements |
The Company adopted the provisions of SFAS No. 157 effective January 1, 2008. SFAS No. 157 defines fair value, establishes a framework for measuring fair value under generally accepted accounting principles and enhances disclosures about fair value measurements. Fair value is defined under SFAS No. 157 as the exchange price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
In accordance with SFAS No. 157, the following table represents the Company’s financial assets (cash equivalents and short-term investments) measured at fair value on a recurring basis and their level within the fair value hierarchy as of December 31, 2008 (in thousands):
| | | | | | | | | | | | |
| | | Fair Value Measurements
at December 31, 2008 Using | | Total |
| | | Quoted Prices
in Active
Markets for
Identical Assets
(Level 1) | | Significant
Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | |
Components of cash equivalents and short-term investments measured at fair value: | | | | | | | | | | | | |
Money market funds | | $ | 9,496 | | $ | — | | $ | — | | $ | 9,496 |
U.S. Treasury securities | | | 14,573 | | | — | | | — | | | 14,573 |
| | | | | | | | | | | | |
Total financial assets measured at fair value | | $ | 24,069 | | $ | — | | $ | — | | | 24,069 |
| | | | | | | | | | | | |
Components of cash and cash equivalents not measured at fair value: | | | | | | | | | | | | |
Operating cash | | | | | | | | | | | | 437 |
| | | | | | | | | | | | |
Total cash and cash equivalents and short-term investments | | | | | | | | | | | $ | 24,506 |
| | | | | | | | | | | | |
77
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Property and equipment consists of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2007 | |
Software | | $ | 103 | | | $ | 79 | |
Leasehold improvements | | | 275 | | | | 257 | |
Office furniture and equipment | | | 288 | | | | 270 | |
Research equipment | | | 39 | | | | 39 | |
Computer equipment | | | 499 | | | | 374 | |
| | | | | | | | |
| | | 1,204 | | | | 1,019 | |
Less: accumulated depreciation and amortization | | | (736 | ) | | | (566 | ) |
| | | | | | | | |
Property and equipment, net | | $ | 468 | | | $ | 453 | |
| | | | | | | | |
Depreciation expense was $181,000, $89,000 and $242,000 for the years ended December 31, 2008, 2007 and 2006, respectively and $1,546,000 for the period from May 28, 1998 (inception) to December 31, 2008.
In July 2006, the Company entered into a venture loan agreement with two venture finance institutions for an aggregate note payable amount of $10,000,000, of which $5,000,000 was drawn in July 2006 and the remaining $5,000,000 was drawn in September 2006. These notes bear interest at 12.21% and 11.75%, respectively. The loan is collateralized by a first priority security interest in the tangible and intangible assets of the Company, excluding intellectual property. These notes require interest only repayment for the period from initial borrowing to October 2006 and July 2007, respectively. Principal and interest repayment on the notes commenced in November 2006 and August 2007, respectively, for 30 months. As of December 31, 2008, outstanding principal under these notes was $3,104,546. A debt premium related to the initial fair value of warrants to purchase 840,000 shares of Series C2 preferred stock issued in connection with the loan agreement of $469,000 was recorded. Upon closing of the Company’s IPO, warrants to purchase 840,000 shares of the Company’s preferred stock were converted into warrants to purchase 56,000 shares of the Company’s common stock. The initial fair value of $469,000 is being amortized to interest expense over the term of the notes. The Company recognized $156,000, $156,000 and $78,000 for the years ended December 31, 2008, 2007 and 2006, respectively, as interest expense. In connection with the notes payable, the Company is restricted from paying cash dividends or distributions on any equity with the exception of dividends payable solely in capital stock.
Interest expense, including expense associated with the valuation of warrants, recognized in connection with all loans was $792,000, $1,219,000 and $563,000 for the years ended December 31, 2008, 2007 and 2006, respectively and $2,718,000 for the period from May 28, 1998 (inception) to December 31, 2008.
78
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Principal payments due over the remaining term for notes payable are as follows (in thousands):
| | | | |
Years Ending December 31, | | | |
2009 | | $ | 2,914 | |
2010 | | | 191 | |
| | | | |
Total principal payments due in future periods | | | 3,105 | |
Less: amounts representing unamortized debt premium associated with warrants to purchase common stock | | | (80 | ) |
| | | | |
| | $ | 3,025 | |
| | | | |
| 7. | Commitments and Contingencies |
Operating Leases
The Company leases its office facility and certain office equipment under operating leases. The Company relocated its corporate headquarters in September, 2007 and entered into a sublease agreement for its new facility. The facility, consisting of approximately 26,386 square feet of office space, is in San Mateo, California. The term of the lease commenced on September 14, 2007 and expires on July 31, 2012. The terms of the sublease include base rent of approximately $2.3 million payable over the sublease term, a period of free rent and rent escalation, which the Company is accounting for on a straight line basis over the lease term. The terms of the sublease also include a tenant improvement allowance of approximately $106,000, which when received will be recorded to deferred rent and amortized over the remaining term of the lease. As of December 31, 2008, the Company has recorded a total of $300,000 in deferred rent, of which $276,000 is classified as a non-current liability. The Company’s obligation under the sublease is secured by a letter of credit in the amount of $240,000, and in accordance with the provisions of the sublease agreement, the Company can reduce the letter of credit, if certain conditions are met, by $40,000 on each of the first three anniversaries of the lease commencement date. As a result of having met these conditions, the Company reduced the letter of credit to $200,000 at December 31, 2008, of which the full amount is secured by a certificate of deposit that is included in the Company’s balance sheet at December 31, 2008 as restricted cash.
The Company records rent expense on a straight line basis over the lease term and has recorded approximately $489,000, $335,000 and $210,000 for the years ended December 31, 2008, 2007 and 2006, respectively and approximately $2,743,000 for the period from May 28, 1998 (inception) to December 31, 2008 for its operating and equipment leases.
Future minimum payments under all noncancelable operating lease obligations are as follows as of December 31, 2008 (in thousands):
| | | |
Year End December 31, | | |
2009 | | $ | 457 |
2010 | | | 581 |
2011 | | | 634 |
2012 | | | 391 |
2013 | | | 15 |
| | | |
Total minimum lease payments | | $ | 2,078 |
| | | |
79
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
2006 Acquisition Bonus Plan
In November 2006, and subsequently as amended, the board of directors approved the 2006 Acquisition Bonus Plan which provides for bonus payments to certain members of management in the event of a change of control. The applicable provisions of the plan provide that an aggregate 1% of the value of a merger transaction will be paid to certain members of management.
Guarantees and Indemnifications
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and generally provide for various indemnifications including indemnification from claims resulting from clinical trial activities and intellectual property matters. The Company’s liability under these agreements is unknown because it involves the potential for future claims that may be made against the Company, but have not yet been made. To date, the Company has not received any claims under its various indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
The Company, as permitted under Delaware law and in accordance with its bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The Company may terminate the indemnification agreements with its officers and directors upon 90 days written notice, but termination will not affect claims for indemnification relating to events occurring prior to the effective date of termination. The maximum amount of potential future indemnification is unlimited. The Company believes the fair value of these indemnification agreements is minimal. Accordingly, the Company has not recorded any liabilities for these agreements as of December 31, 2008.
In October 2000 and as amended, the Company licensed certain patents from the University of California for high-concentration capsaicin for neuropathic pain. Under the terms of the agreement, the Company will be required to pay royalties on net sales of the licensed product up to a maximum of $1,000,000 per annum as well as a percentage of upfront and milestone payments resulting from sublicense of the Company’s rights under the agreement. During 2008, the Company paid $4,000 to the University of California under the license agreement.
In January 2007, the Company entered into a Commercial Supply and License Agreement (“LTS Agreement”) with LTS Lohman Therapie-Systeme AG (“LTS”) to manufacture commercial and clinical supply of Qutenza, the Company’s lead product candidate. Under the terms of the agreement, the Company is required to pay a transfer price for product purchased under the agreement as well as a royalty on net sales of product purchased under the LTS Agreement. Additionally, upon first market approval of Qutenza, the Company is required to make a one time milestone payment of €100,000.
9. Stockholders’ Equity
Common Stock
The Company completed its IPO and sold 4,000,000 shares of common stock at $11.00 per share on May 7, 2007. Gross proceeds from the offering totaled approximately $44,000,000. The net offering proceeds to the Company, after deducting expenses of approximately $5,914,000, totaled $38,086,000. Upon closing of the IPO, all of the outstanding shares of the Company’s redeemable convertible preferred stock converted to 8,722,013 shares of the Company’s common stock.
80
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
On December 28, 2007, the Company completed the first closing of a private placement in which the Company sold 3,638,741 shares of common stock at $6.18 per share and warrants at $0.125 per share to purchase an additional 1,091,622 shares of its common stock. The stock and warrants were offered solely to accredited investors. On January 3, 2008, the Company completed the second closing of the private placement in which the Company issued 382,170 shares of common stock at $6.18 per share and warrants at $0.125 per share to purchase an additional 114,651 shares of its common stock. The warrants from both closings have a term of five years, contain a net-exercise provision, and have an exercise price of $8.034 per share. The fair value of the warrants issued in the first closing was approximately $3,411,000 and the fair value of the warrants issued in the second closing was approximately $420,000. The fair value of the warrants issued in both closings was allocated from the net proceeds of the financing and was recorded in additional paid-in capital. The net cash proceeds to the Company from both closings, after deducting expenses of approximately $1,195,000 totaled $23,805,000.
In connection with the Series C2 preferred stock financing and the exercise of warrants associated with the Series C2 preferred stock offering, the Company committed to issue to a consultant a total of 33,333 shares of common stock from November 2005 through February 2007. These shares were issued in June 2007 and the aggregate non-cash value of approximately $291,000 was recorded in additional paid-in capital.
2000 Stock Incentive Plan
The Company’s 2000 Stock Incentive Plan (the “2000 Plan”) provides for the grant of incentive and nonstatutory stock options by the board of directors to employees, officers, directors, and consultants of the Company. Incentive stock options may be granted with exercise prices not less than estimated fair value, and nonstatutory stock options may be granted with an exercise price of not less than 85% of the estimated fair value of the common stock on the date of grant, as determined by the board of directors. Options granted under the 2000 Plan expire no later than 10 years from the date of grant. Options granted and shares underlying stock purchase rights issued under the 2000 Plan vest over periods determined by the board of directors, generally over four to six years. Unvested shares of common stock purchased under stock purchase rights are subject to a repurchase option by the Company upon termination of the purchaser’s employment or services. The repurchase right lapses over a period of time as determined by the board of directors. At December 31, 2008 there were no stock purchase rights outstanding subject to a repurchase right by the Company. For certain options, vesting accelerates upon the achievement of specified milestones. The 2000 Plan terminates automatically 10 years after its adoption by the board of directors.
The 2000 Plan allows for the early exercise of stock options prior to vesting. The Company has issued an aggregate of 11,945 shares of common stock pursuant to the early exercise of stock options, which are not deemed to be issued until those shares vest. As of December 31, 2008, there were 785 early exercised shares unvested, issued and subject to the Company’s right to repurchase at the original issuance price. The amounts received in exchange for these shares have been recorded as a liability for early exercise of stock options in the accompanying balance sheets and will continue to be reclassified into equity as the shares vest.
2007 Stock Plan
The Company’s 2007 Stock Plan provides for the grant of incentive stock options by the board of directors to employees and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to its employees, directors and consultants. The board of directors has the authority to determine the terms of the awards, including exercise price, the number of shares subject to each such award, the exercisability of the awards and consideration payable upon exercise.
81
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Incentive stock options may be granted with exercise prices at least equal to the fair market value of the Company’s common stock on the date of grant. The term of an incentive stock option granted under this plan may not exceed ten years, except that with respect to any participant who owns 10% of the voting power of all classes of the Company’s outstanding stock as of the grant date, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the date of grant.
Upon adoption of the plan, the Company reserved 1,333,333 shares of its common stock for issuance under the 2007 Stock Plan. Any shares returned to the 2000 Stock Incentive Plan as a result of termination of options or the repurchase of shares issued under the 2000 Stock Incentive Plan are added to the 2007 Stock Plan. In addition, the 2007 Stock Plan provides for annual increases in the number of shares available for issuance thereunder on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year, equal to the lesser of:
| | • | | 5% of the outstanding shares of the Company’s common stock on the last day of the immediately preceding fiscal year; |
| | • | | such other amount as the Company’s board of directors may determine. |
The Company’s 2007 Stock Plan also provides for the automatic grant of non-statutory options to the Company’s non-employee directors. Each non-employee director that is newly appointed to the board of directors after the Company’s IPO will receive an initial option to purchase 13,333 shares upon such appointment. Additionally, non-employee directors who have been directors for at least twelve months will receive a subsequent option to purchase 5,000 shares immediately following each annual meeting of the Company’s stockholders.
On January 15, 2009, the board of directors approved options to purchase a total of 256,892 shares of the Company’s common stock to the Company’s executive officers. These stock options were granted to officers in lieu of full cash payment for their performance under the Company’s 2008 bonus plan and were immediately exercisable as of the date of grant. Also on January 15, 2009, the board of directors approved options to purchase 220,000 shares of the Company’s common stock to the Company’s executive officers. These options are exercisable over four years with vesting acceleration features based on attainment of certain corporate milestones. On February 13, 2009, the board of directors approved options to purchase a total of 170,250 shares of the Company’s common stock to certain of its employees. The options are also exercisable over four years with vesting acceleration features based on attainment of certain corporate milestones.
2007 Employee Stock Purchase Plan
Under the Company’s 2007 Employee Stock Purchase Plan (the “Purchase Plan”), eligible employees can participate and purchase common stock semi-annually through accumulated payroll deductions. The Purchase Plan is administered by the Company’s board of directors or a committee appointed by the Company’s board of directors. Under the Purchase Plan eligible employees may purchase stock at 85% of the lower of the fair market value of a share of Common Stock on the offering date or the exercise date. The Purchase Plan provides for consecutive, overlapping twelve-month offering periods generally starting on the first trading day on or after May 15 and November 15 of each year. There are two 6-month purchase periods in each offering period. Eligible employees may contribute up to 15% of their eligible compensation which includes a participant’s straight time gross earnings, commissions, overtime and shift premium, exclusive of payments for incentive compensation,
82
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
bonuses and other compensation. A participant may purchase a maximum of 1,333 shares of common stock per purchase period. If the fair market value of the Company’s common stock at the end of a purchase period is less than the fair market value at the beginning of the offering period, participants will be withdrawn from the then current offering period following the purchase of shares on the purchase date and automatically will be re-enrolled in a new offering period.
The Purchase Plan was effective upon the completion of the Company’s IPO, at which time a total of 333,333 shares of the Company’s common stock were made available for sale. Annual increases in the number of shares available for issuance will be made on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year. The annual increases will be equal to the lesser of: (a) 2% of the outstanding shares of the Company’s common stock on the first day of the fiscal year; (b) 533,333 shares; or (c) such other amount as may be determined by the board of directors. In the years ended December 31, 2008 and 2007, 51,217 and 15,367 shares, respectively, were issued and 608,731 shares were reserved for future issuance under the Purchase Plan as of December 31, 2008.
83
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes stock option activity under both the 2000 Stock Incentive and 2007 Stock plans:
| | | | | | | | | |
| | | Options Outstanding |
| | | Shares Available
for Grant | | | Number of Shares | | | Weighted-Average
Exercise Price |
Balance at inception (May 28, 1998) | | — | | | — | | | | — |
Shares authorized | | 109,333 | | | — | | | | — |
Options granted | | (38,661 | ) | | 38,661 | | | $ | 0.90 |
Options canceled | | 333 | | | (333 | ) | | $ | 0.90 |
| | | | | | | | | |
Balance at December 31, 2000 | | 71,005 | | | 38,328 | | | $ | 0.90 |
Options granted | | (32,770 | ) | | 32,770 | | | $ | 0.90 |
Options canceled | | 4,998 | | | (4,998 | ) | | $ | 0.90 |
| | | | | | | | | |
Balance at December 31, 2001 | | 43,233 | | | 66,100 | | | $ | 0.90 |
Shares authorized | | 80,000 | | | — | | | | — |
Options granted | | (115,392 | ) | | 115,392 | | | $ | 1.20 |
Options exercised | | — | | | (4,162 | ) | | $ | 1.00 |
Options canceled | | 12,118 | | | (12,118 | ) | | $ | 1.05 |
| | | | | | | | | |
Balance at December 31, 2002 | | 19,959 | | | 165,212 | | | $ | 1.10 |
Shares authorized | | 133,334 | | | — | | | | — |
Options granted | | (114,930 | ) | | 114,930 | | | $ | 1.35 |
Options exercised | | — | | | (3,249 | ) | | $ | 1.20 |
Options canceled | | 2,199 | | | (2,199 | ) | | $ | 1.20 |
| | | | | | | | | |
Balance at December 31, 2003 | | 40,562 | | | 274,694 | | | $ | 1.20 |
Shares authorized | | 433,333 | | | — | | | | — |
Options granted | | (378,812 | ) | | 378,812 | | | $ | 2.85 |
Options exercised | | — | | | (5,083 | ) | | $ | 1.06 |
Options canceled | | 105,046 | | | (105,046 | ) | | $ | 2.70 |
| | | | | | | | | |
Balance at December 31, 2004 | | 200,129 | | | 543,377 | | | $ | 2.06 |
Shares authorized | | 158,333 | | | — | | | | — |
Options granted | | (214,820 | ) | | 214,820 | | | $ | 2.25 |
Options exercised | | — | | | (18,599 | ) | | $ | 1.28 |
Options canceled | | 84,728 | | | (84,728 | ) | | $ | 2.33 |
| | | | | | | | | |
Balance at December 31, 2005 | | 228,370 | | | 654,870 | | | $ | 2.11 |
Shares authorized | | 166,667 | | | — | | | | — |
Options granted | | (285,186 | ) | | 285,186 | | | $ | 3.82 |
Options exercised | | — | | | (67,244 | ) | | $ | 1.45 |
Options canceled | | 55,578 | | | (55,578 | ) | | $ | 2.65 |
| | | | | | | | | |
Balance at December 31, 2006 | | 165,429 | | | 817,234 | | | $ | 2.72 |
Shares authorized | | 1,333,333 | | | — | | | | — |
Options granted | | (526,407 | ) | | 526,407 | | | $ | 9.06 |
Options exercised | | — | | | (161,193 | ) | | $ | 1.73 |
Options canceled | | 47,283 | | | (47,283 | ) | | $ | 5.83 |
| | | | | | | | | |
Balance at December 31, 2007 | | 1,019,638 | | | 1,135,165 | | | $ | 5.69 |
Additional shares authorized | | 854,954 | | | — | | | | — |
Options granted | | (486,750 | ) | | 486,750 | | | $ | 4.37 |
Options exercised | | — | | | (38,210 | ) | | $ | 2.39 |
Options canceled | | 190,360 | | | (190,360 | ) | | $ | 5.79 |
| | | | | | | | | |
Balance at December 31, 2008 | | 1,578,202 | | | 1,393,345 | | | $ | 5.31 |
| | | | | | | | | |
84
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Stock-Based Compensation Expense under SFAS No. 123R
On January 1, 2006, the Company adopted the provisions of SFAS No. 123R,Share-Based Payment. SFAS No. 123R establishes accounting for stock-based awards made to employees and directors. Accordingly, stock-based compensation expense is measured at grant date, based on the fair value of the award, and is recognized as expense over the remaining requisite service period. Total stock-based compensation accounted for under SFAS No. 123R of $1,490,000, $1,462,000 and $315,000 was recorded during the years ended December 31, 2008, 2007 and 2006, respectively.
The fair value for the Company’s employee stock options was estimated at the date of grant using the Black-Scholes valuation model with the following average assumptions:
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Expected volatility | | 70 | % | | 72 | % | | 77 | % |
Expected term (years) | | 6.0 | | | 6.0 | | | 6.0 | |
Risk-free interest rate | | 3.0 | % | | 4.2 | % | | 4.7 | % |
Dividend yield | | 0 | % | | 0 | % | | 0 | % |
The Company’s computation of expected volatility for the years ended December 31, 2008, 2007 and 2006, respectively, is based on an average of the historical volatility of a peer-group of similar companies. The Company’s computation of expected term in the years ended December 31, 2008, 2007 and 2006, respectively, utilizes the simplified method in accordance with SAB No. 107, as further extended by SAB No. 110. The risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. The Company recognizes stock-based compensation expense for the fair values of these awards on a straight-line basis over the requisite service period of each of these awards. Stock compensation expense relating to options with acceleration of vesting dependent upon the achievement of milestones is recognized over a period which is the shorter of 1) the Company’s evaluation of the probability of achievement of each respective milestone and the related estimated date of achievement or 2) the otherwise stated time-based vesting period.
The following table represents stock option activity for the year ended December 31, 2008:
| | | | | | | | | | | |
| | | Number of
Shares | | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contract Life | | Aggregate
Intrinsic
Value |
Outstanding options at beginning of period | | 1,135,165 | | | $ | 5.69 | | | | | |
Granted | | 486,750 | | | | 4.37 | | | | | |
Exercised | | (38,210 | ) | | | 2.39 | | | | | |
Forfeited | | (166,557 | ) | | | 5.80 | | | | | |
Expired | | (23,803 | ) | | | 5.68 | | | | | |
| | | | | | | | | | | |
Outstanding options at end of period | | 1,393,545 | | | $ | 5.31 | | 7.48 | | $ | — |
| | | | | | | | | | | |
Options expected to vest at end of period | | 1,335,908 | | | $ | 5.31 | | 7.32 | | $ | — |
| | | | | | | | | | | |
Exercisable options at end of period | | 767,048 | | | $ | 5.19 | | 6.41 | | $ | — |
| | | | | | | | | | | |
85
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2008, 2007 and 2006, was $2.81, $6.96 and $5.02 per share, respectively. Less than $1,000 of intrinsic value for stock options outstanding or exercisable existed at December 31, 2008. Total intrinsic value of options exercised for the years ended December 31, 2008, 2007 and 2006 was $45,000, $157,000 and $42,000, respectively. Cash received from stock option exercises and employee stock purchases under its employee stock purchase plan for the year ended December 31, 2008 was approximately $180,000. Because of the Company’s net operating losses, the Company did not realize any tax benefits for the tax deductions from share-based payment arrangements during the years ended December 31, 2008 and 2007. As of December 31, 2008, the total compensation cost related to stock-based awards granted under SFAS No. 123R to employees and directors but not yet amortized was approximately $2,471,000 million, net of estimated forfeitures. These costs, adjusted for changes in estimated forfeiture rates from time to time, will be amortized over the next five years.
The Company accounts for its Purchase Plan as a compensatory plan and recorded compensation expense of approximately $118,000 and $74,000 for the years ended December 31, 2008 and 2007, respectively. The Company accounts for the Purchase Plan in accordance with SFAS 123(R) and FASB Technical Bulletin No. 97-1,“Accounting under Statement 123 for Certain Employee Stock Purchase Plans with a Look-Back Option.” The estimated fair value of shares granted under the Purchase Plan was determined at the date of grant using the Black-Scholes pricing model with the following assumptions:
| | | | |
| | | Year Ended December 31, |
| | | 2008 | | 2007 |
Expected dividend yield | | 0% | | 0% |
Expected volatility | | 44 – 83% | | 44 – 68% |
Expected life (in years) | | 0.5 to 1.0 | | 0.5 to 1.0 |
Risk-free interest rate | | 0.8 – 5.0% | | 3.6 – 5.0% |
Warrants to Purchase Common Stock
In May 2007, the Company completed its IPO as a result of which all of the existing shares of the Company’s preferred stock were converted to common stock. At the time of the completion of the IPO, the Company had outstanding warrants to purchase 33,600 shares of its Series A preferred stock, 20,000 shares of its Series B preferred stock, and 840,000 shares of its Series C2 preferred stock. Upon closing of the Company’s IPO, warrants to purchase 893,600 shares of the Company’s preferred stock converted to warrants to purchase 59,573 shares of the Company’s common stock. Of these warrants to purchase 59,573 shares of the Company’s stock, warrants to purchase 56,000 shares of the Company’s common stock automatically exercise in the event of an acquisition of the Company. The Company performed a final remeasurement to determine the fair value of such financial instruments immediately prior to the conversion. The resulting fair value of $426,000 was reclassified to additional paid-in capital during the year ended December 31, 2007.
In connection with the private placement of common stock in December 2007 and January 2008, the Company issued the investors warrants to purchase 1,206,273 share of common stock at $8.034 per share. The warrants became exercisable immediately upon issuance and expire five years from issuance. The fair value of the warrants to purchase 1,091,622 shares of common stock issued in conjunction with the first closing of the private placement in December 2007 was approximately $3,411,000 and was determined using the Black-Scholes method with the following assumptions: expected volatility of 67%, a dividend yield of 0%, a risk-free interest rate of 3.52%, and an expected life of five years. The fair value of the warrants to purchase 114,651 shares of common stock issued in conjunction with the second closing of the private placement in January 2008 was
86
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
approximately $420,000 and was determined using the Black-Scholes method with the following assumptions: expected volatility of 67%, a dividend yield of 0%, a risk-free interest rate of 3.26%, and an expected life of five years. The fair value of the warrants issued was allocated from the proceeds of the financing.
Restricted Stock Purchases
In 2000, prior to its adoption of the 2000 Plan, the Company issued 400,000 shares of its common stock to founders and employees of the Company under restricted stock purchase agreements of which 196,000 shares were for cash and 204,000 shares were in exchange for promissory notes. Under the terms of the restricted stock purchase agreements, shares purchased generally vested over a three-year period. Upon termination of employment or services, unvested shares were subject to repurchase by the Company at the original issuance price. As of December 31, 2007, all shares had vested and were no longer subject to repurchase. The promissory notes bore interest at a rate of 6.1% and were repayable in four equal installments at the end of each year after the date of notes or within 30 days following termination of employment. The amount payable to the Company relating to these notes was $126,000 at December 31, 2006. Due to the extension of the repayment of these notes as authorized by the Company’s Board of Directors, in accordance with EITF No. 00-23,Issues Related to Accounting for Stock Compensation under APB Opinion No. 25 and FASB Interpretation No. 44, these notes were treated as non-recourse in nature and were subject to variable accounting. The shares of common stock relating to the note receivables amounts outstanding were not deemed to be issued until the promissory notes were repaid and therefore are excluded from the shares of common stock outstanding at each balance sheet date and the net loss per share computation for each respective period. Stock compensation expense relating to variable accounting for these promissory notes was $1,617,000 and $2,217,000 for the year ended December 31, 2006 and for the period from May 28, 1998 (inception) to December 31, 2008, respectively. There was no compensation expense recorded in the years ended December 31, 2007 or 2008 relating to variable accounting for these promissory notes. In January 2007, the Company’s board of directors forgave notes receivable of $175,000, including accrued interest, from current and certain former officers of the Company and as a result, no future stock compensation expense relating to variable accounting for these promissory notes will be recorded.
Notes Receivable from Stockholders
In April 2002, February 2003, April 2003 and March 2004, 96,662, 2,000, 83,333 and 6,666 shares of common stock, respectively, were issued to employees of the Company upon exercise of stock options in exchange for non-recourse promissory notes. These notes bore interest at a rate of 6.0%–6.1% compounded semi-annually, were repayable in five equal installments at the end of each year after the date of notes or within 30 days following termination of employment. The underlying shares generally vested over a four-year period. Unvested shares, which amounted to 278 at December 31, 2007, were subject to repurchase by the Company at the original issue price, however there are no unvested shares at December 31, 2008 and consequently no shares subject to repurchase by the Company. As the interest rate on each note is not deemed to represent a market rate at the time the option shares vest, in accordance with EITF No. 00-23, each award was subject to variable accounting until the award was vested or forfeited. The shares of common stock relating to the note receivables amounts outstanding were not deemed to be issued until the promissory note was repaid and therefore are excluded from the shares of common stock outstanding at each balance sheet date and the earnings per share computation for each respective period. Shares excluded total 130,884 as of December 31, 2006. No shares were excluded as of December 31, 2007 as a result of the forgiveness of these promissory notes by the Company’s board of directors. As a result of the forgiveness of these promissory notes, the Company recorded $204,000 in compensation cost in the year ended December 31, 2007. Shares of common stock issued upon payment of note receivable was 43,331 shares during the year ended 2006. Stock compensation expense relating to variable
87
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
accounting for these promissory notes, which excludes the compensation cost in association with the forgiveness of such notes, was less than $1,000, $6,000 and $1,587,000, for the years ended December 31, 2008, 2007 and 2006, respectively and $2,231,000 for the period from May 28, 1998 (inception) to December 31, 2008.
Rescission of Stock Option Exercises
During 2004, the Company allowed the rescission of two stock option exercises by an executive and a director for a total of 23,333 shares of common stock. In accordance with EITF No. 00-23 and EITF Topic D-93, the rescissions of the exercises were treated as though each award was regranted on the respective dates of the rescissions and thus the awards were subject to variable accounting. Additional stock compensation associated with the rescission based on EITF Topic D-93 was not material. Stock compensation expense relating to variable accounting for these options was $(139,000) and $245,000 for the years ended December 31, 2007 and 2006 and $166,000 for the period from May 28, 1998 (inception) to December 31, 2008. No compensation expense was recorded in 2008 as the stock options subject to variable accounting discussed above were fully vested by December 31, 2007.
| 10. | Redeemable Convertible Preferred Stock and Preferred Stock Warrant Liability |
The carrying value of the Company’s Series A, Series B, Series C, and Series C2 preferred stock was increased by periodic accretion, using the effective interest method, so that the carrying amount would equal the redemption value at the redemption date. Upon completion of the Company’s IPO in May 2007, all of the existing shares of the Company’s preferred stock were converted to 8,722,013 shares of common stock and therefore all periodic accretion ceased immediately prior to the IPO. The Company recorded $4,626,000, $11,293,000 and $38,872,000 relating to accretion for the years ended December 31, 2007, 2006 and for the period from May 28, 1998 (inception) to December 31, 2007, respectively.
In connection with the Company’s Series C2 preferred stock financing, warrants to purchase 13,444,450 shares of Series C2 preferred stock at a purchase price of $0.75 per share were issued to investors. The initial fair value of these warrants of $3,070,000 was recognized as a liability and allocated from the proceeds of the preferred stock financing thus reducing the carrying value of the preferred stock. In January and February 2007, these warrants were exercised, resulting in aggregate net cash proceeds of approximately $10,083,000. As a result of this transaction, the Company recognized approximately $59,000 as other income related to the change in fair value of the warrant liability on the date of the transaction and reclassified approximately $6,763,000 from preferred stock warrant liability to preferred stock.
The Company has valued its warrants to purchase its preferred stock using the Black-Scholes valuation method. The assumptions used in valuing these warrants are presented in the table below. There were no warrants that were subject to fair value remeasurement after of all the warrants to purchase preferred stock converted to warrants to purchase common stock as a result of the Company’s IPO, therefore assumptions used in valuing these warrants are presented only for the periods in which there were periodic remeasurements:
| | | | |
| | | Period from
January 1, 2007
to May 7, 2007 | | Twelve Months
Ended
December 31,
2006 |
Expected dividend yield | | — | | — |
Expected volatility | | 59 – 74% | | 54 – 77% |
Expected life | | 1.6 – 6.8 | | 1.8 – 7.0 |
Risk-free interest rate | | 4.6 – 4.9% | | 4.6 – 5.2% |
88
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
For the year ended December 31, 2007, the Company recorded approximately $360,000, reflected as other income for the decrease in fair value of all preferred stock warrants and has ceased to record any further periodic fair value adjustments. For the year ended December 31, 2006, the Company recorded approximately $3,274,000, reflected as other expense for the increase in fair value of all preferred stock warrants.
The reconciliation of income tax expense (benefit) computed at the statutory federal income tax rate of 35% to amounts included in the statements of operations is as follows (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | | | 2006 | |
Tax at federal statutory rate | | $ | (9,112 | ) | | $ | (11,185 | ) | | $ | (10,551 | ) |
Meals & entertainment | | | 8 | | | | 8 | | | | 12 | |
Stock compensation expense | | | 357 | | | | 477 | | | | 851 | |
Amortization of warrant cost associated with debt issuances | | | 54 | | | | 54 | | | | 27 | |
Warrant revaluation expenses/(gain) | | | — | | | | (126 | ) | | | 1,149 | |
Change in valuation allowance | | | (8,693 | ) | | | (10,772 | ) | | | (8,512 | ) |
| | | | | | | | | | | | |
Total | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | |
Deferred tax assets and liabilities reflect the net tax effects of net operating loss and tax credit carryovers and the temporary differences between the carrying amounts of assets and liabilities for financial reporting and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows (in thousands):
| | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2007 | |
Accrued expenses and other current assets | | $ | 362 | | | $ | 1,393 | |
Net operating loss carryforward | | | 37,621 | | | | 35,001 | |
Capitalized research | | | 8,650 | | | | 10,504 | |
Research and development and other credits | | | 2,002 | | | | 4,372 | |
Basis difference in fixed assets | | | 167 | | | | 139 | |
Stock options | | | 990 | | | | 797 | |
| | | | | | | | |
Total deferred tax assets | | | 49,792 | | | | 52,206 | |
Valuation allowance | | | (49,792 | ) | | | (52,206 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance decreased by $2,414,000 during 2008 and increased by $12,369,000 and $11,079,000 during 2007 and 2006, respectively. Our deferred tax assets reflect an estimate of the potential limitation of our utilization of our net operating loss carryforwards and research and development and other credits.
As of December 31, 2008, the Company had net operating loss carryforwards for federal income tax purposes of approximately $96,433,000 which will begin to expire in the year 2020 and federal research and development tax credits of approximately $613,000 which will begin to expire in the year 2020.
89
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
As of December 31, 2008, the Company had net operating loss carryforwards for state income tax purposes of approximately $67,348,000 which will begin to expire in the year 2010 and state research and development tax credits of approximately $3,798,000 which have no expiration date.
Utilization of the net operating losses may be subject to substantial annual limitation due to federal and state ownership limitations. The annual limitation could result in the expiration of the net operating losses before utilization.
In June 2006, the FASB issued FIN No. 48, which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with SFAS No. 109. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 also provides guidance on de-recognition of tax benefits, classification on the balance sheet, interest and penalties, accounting in interim periods, disclosure and transition. The Company adopted FIN No. 48 effective January 1, 2007. In accordance with FIN No. 48, paragraph 19, the Company has decided to classify interest and penalties as a component of tax expense. As a result of the implementation of FIN No. 48, the Company recorded $2,087,000 in unrecognized tax benefits as a reduction to deferred tax assets, all of which are currently offset by a full valuation allowance that had no effect on the beginning balance of accumulated deficit.
The Company had unrecognized tax benefits of $1,628,000 and $2,832,000 as of December 31, 2008 and December 31, 2007, respectively, all of which are offset by a full valuation allowance. These unrecognized tax benefits, if recognized, would not affect the effective tax rate for the periods presented. There was no interest or penalties accrued at the adoption date or for any period presented.
The Company files income tax returns in the U.S. federal and California state tax jurisdictions. The tax years 2002 to 2008 remain open to examination by the U.S. and California state tax authorities.
A reconciliation of the change in the unrecognized tax benefit balance from January 1, 2007 to December 31, 2008 is as follows:
| | | | |
(In thousands) | | Federal and
State Tax | |
Balance as of January 1, 2007 | | $ | 2,087 | |
Additions for tax positions related to current year | | | 745 | |
Additions for tax positions related to prior years | | | — | |
| | | | |
Balance at December 31, 2007 | | | 2,832 | |
Additions for tax positions related to current year | | | 103 | |
Reductions for tax positions related to prior years | | | (1,307 | ) |
| | | | |
Balance at December 31, 2008 | | $ | 1,628 | |
| | | | |
90
NEUROGESX, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| 13. | Unaudited Quarterly Information |
Certain unaudited quarterly financial information for the years ended December 31, 2008 and 2007 is presented below:
| | | | | | | | | | | | | | | | |
| | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | |
| | | (In thousands, except per share amounts) | |
2008 | | | | |
Net Loss | | $ | (7,990 | ) | | $ | (6,944 | ) | | $ | (6,313 | ) | | $ | (4,788 | ) |
Net loss attributable to common stockholders | | $ | (7,990 | ) | | $ | (6,944 | ) | | $ | (6,313 | ) | | $ | (4,788 | ) |
Basic and diluted net loss per share attributable to common stockholders | | $ | (0.46 | ) | | $ | (0.40 | ) | | $ | (0.36 | ) | | $ | (0.27 | ) |
| | | | |
2007 | | | | | | | | | | | | | | | | |
Net Loss | | $ | (7,919 | ) | | $ | (6,957 | ) | | $ | (9,191 | ) | | $ | (7,889 | ) |
Net loss attributable to common stockholders | | $ | (11,356 | ) | | $ | (8,145 | ) | | $ | (9,191 | ) | | $ | (7,889 | ) |
Basic and diluted net loss per share attributable to common stockholders | | $ | (17.25 | ) | | $ | (0.99 | ) | | $ | (0.68 | ) | | $ | (0.58 | ) |
91
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
| Item 9A(T). | Controls and Procedures |
| | (a) | Evaluation of disclosure controls and procedures |
Our management evaluated, with the participation and under the supervision of our Chief Executive Officer and our Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded, subject to the limitations described below, that our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission, or SEC, rules and forms, and that such information is accumulated and communicated to management as appropriate to allow timely decisions regarding required disclosures.
| | (b) | Internal control over financial reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. We maintain a system of internal controls that is designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Management assessed our internal control over financial reporting as of December 31, 2008, the end of our last fiscal year. Management based its assessment on criteria established in “Internal Control—Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies and our overall control environment.
Based on our assessment, management has concluded that our internal control over financial reporting was effective as of December 31, 2008 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC that permit us to provide only management’s report in this annual report.
There have not been any changes in our internal controls over financial reporting (as such item is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during our fiscal quarter ended December 31, 2008 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
The information contained under this caption “Internal control over financial reporting” shall not be deemed to be filed with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference into such filing.
92
| | (c) | Limitations on the Effectiveness of Controls |
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the controls are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Accordingly, our controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our control system are met.
| Item 9B. | Other Information |
On October 6, 2008, we executed an amended exclusive license agreement that amends an exclusive license agreement originally entered into in November 2000, with the University of California for high-concentration capsaicin for neuropathic pain. Under the terms of the agreement, we will be required to pay royalties on net sales of the licensed product up to a maximum of $1,000,000 per annum as well as a percentage of upfront and milestone payments resulting from sublicense of our rights under the agreement.
93
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required to be disclosed under this Item, other than as set forth below, is incorporated by reference from our Proxy Statement for our 2009 Annual Meeting of Stockholders where it appears under the heading “Directors and Executive Officers.”
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our officers and directors, and persons who beneficially own more than ten percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. Such officers, directors and ten-percent stockholders are also required by SEC rules to furnish us with copies of all forms that they file pursuant to Section 16(a). Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during fiscal 2008, our executive officers, directors and ten-percent stockholders complied with all applicable filing requirements.
Code of Ethics
We have adopted a Code of Ethics that applies to all directors, officers and employees of the Company. We publicize the Code of Ethics through posting the policy on our website, http://www.neurogesx.com. We will disclose on our website any waivers of, or amendments to, our Code of Ethics.
| Item 11. | Executive Compensation |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Executive Compensation and other Matters.”
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this Item regarding security ownership of certain beneficial owners and management is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Security Ownership of Certain Beneficial Owners and Management.”
The following table summarizes the securities authorized for issuance under our equity compensation plans as of December 31, 2008:
| | | | | | | |
Plan Category | | Number of
Securities to
be Issued
Upon
Exercise of
Outstanding
Options,
Warrants
and Rights | | Weighted
Average
Exercise
Price of
Outstanding
Options,
Warrants
and Rights | | Number of
Securities
Remaining
Available for
Future
Issuance
Under Equity
Compensation
Plans(1) |
Equity compensation plans approved by stockholders | | 1,393,345 | | $ | 5.31 | | 2,187,000 |
Equity compensation plans not approved by stockholders | | 1,265,846 | | $ | 8.18 | | — |
| | | | | | | |
Total | | 2,659,191 | | $ | 6.68 | | 2,187,000 |
| | | | | | | |
| (1) | The number of authorized shares under the 2007 Stock Plan automatically increases on January 1 of each year by a number of shares equal to the lesser of (i) 1,333,333 shares, (ii) 5.0% of the outstanding shares on the last day of the immediately preceding fiscal year, or (iii) an amount determined by the Board of Directors. The number of authorized shares under the 2007 Employee Stock Purchase Plan automatically increases on January 1 of each year by a number of shares equal to the lesser of (i) 533,333 shares, (ii) 2.0% of the outstanding shares on such date, or (iii) an amount determined by the Board of Directors. |
94
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Certain Business Relationships and Related Party Transactions.”
| Item 14. | Principal Accounting Fees and Services |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Principal Accountant Fees and Services.”
95
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| | (a) | The following documents are filed as part of this Form 10-K: |
| | (1) | Financial Statements (included in Part II of this report): |
| | • | | Report of Independent Registered Public Accounting Firm |
| | • | | Consolidated Balance Sheets |
| | • | | Consolidated Statements of Operations |
| | • | | Consolidated Statements of Stockholders’ Equity (Deficit) |
| | • | | Consolidated Statements of Cash Flows |
| | • | | Notes to Consolidated Financial Statements |
| | (2) | Financial Statement Schedules: |
None—All financial statement schedules are omitted because the information is inapplicable or presented in the notes to the financial statements.
| | |
Exhibit
Number | | Exhibit Title |
| 3.1(1) | | Amended and Restated Certificate of Incorporation. |
| |
| 3.2(1) | | Amended and Restated Bylaws. |
| |
| 4.1(1) | | Specimen Common Stock Certificate. |
| |
| 4.2(1) | | Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of November 14, 2005. |
| |
| 4.3(2) | | Amendment No. 1 to the Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of December 28, 2007. |
| |
| 4.4(1) | | Warrant to Purchase Series A Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of December 14, 2000. |
| |
| 4.5(1) | | Warrant to Purchase Series B Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of May 1, 2002. |
| |
| 4.6(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company II LLC, dated as of July 7, 2006. |
| |
| 4.7(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company III LLC, dated as of July 7, 2006. |
| |
| 4.8(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Oxford Finance Corporation, dated as of July 7, 2006. |
| |
| 4.9(1) | | Form of First Warrant to Purchase Series C2 Preferred Stock. |
| |
| 4.10(1) | | Form of Second Warrant to Purchase Series C2 Preferred Stock. |
| |
| 4.11(2) | | Registration Rights Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. |
96
| | |
Exhibit
Number | | Exhibit Title |
| 4.12(2) | | Form of Warrant to Purchase Common Stock. |
| |
| 10.1(1) | | 2007 Stock Plan. |
| |
| 10.2(1) | | 2007 Employee Stock Purchase Plan. |
| |
| 10.3(1) | | Form of Indemnification Agreement entered into between NeurogesX, Inc. and each of its directors and officers. |
| |
| 10.4 | | Exclusive License Agreement between NeurogesX, Inc. and The Regents of the University of California, executed as of October 6, 2008. |
| |
| 10.5(1)† | | Clinical Supply, Development and License Agreement between NeurogesX, Inc. and LTS Lohmann Therapie-Systeme AG, dated as of January 15, 2004. |
| |
| 10.6(4)† | | Manufacturing and Supply Agreement, effective as of August 19, 2008 between NeurogesX, Inc. and Formosa Laboratories, Inc. |
| |
| 10.7† | | Commercial Supply and License Agreement with Lohmann Therapie-Systeme AG and NeuorgesX, Inc., effective as of January 2007. |
| |
| 10.8 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Anthony DiTonno, effective as of December 31, 2008. |
| |
| 10.9 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Stephen Ghiglieri, effective as of December 31, 2008. |
| |
| 10.10 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Keith Bley, effective as of December 31, 2008. |
| |
| 10.11 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Michael Markels, effective as of December 31, 2008. |
| |
| 10.12 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Jeffrey Tobias, effective as of December 31, 2008. |
| |
| 10.13 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Russell Kawahata, effective as of December 31, 2008. |
| |
| 10.14 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Susan Rinne, effective as of December 31, 2008. |
| |
| 10.15(3) | | Sublease between NeurogesX, Inc. and Oracle USA, Inc., dated September 6, 2007. |
| |
| 10.16(2) | | Securities Purchase Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. |
| |
| 21.1(1) | | List of Subsidiaries. |
| |
| 23.1 | | Consent of Independent Registered Public Accounting Firm. |
| |
| 24.1 | | Power of Attorney (see page 99). |
| |
| 31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1 | | Certifications of the Principal Executive Officer and the Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 1350). |
97
| (1) | Incorporated by reference from our registration statement on Form S-1, registration number 333-140501, declared effective by the Securities and Exchange Commission on May 1, 2007. |
| (2) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 28, 2007. |
| (3) | Incorporated by reference from our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2007. |
| (4) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 22, 2008. |
| † | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted from this filing and have been filed separately with the Securities and Exchange Commission. |
The exhibits listed under Item 14(a)(3) hereof are filed as part of this Form 10-K other than Exhibit 32.1 which shall be deemed furnished.
| | (c) | Financial Statement Schedules |
All financial statement schedules are omitted because the information is inapplicable or presented in the notes to the financial statements.
98
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| NEUROGESX, INC. |
| |
By: | | /s/ ANTHONY A. DITONNO |
| | Anthony A. DiTonno President, Chief Executive Officer and Director |
Dated: March 26, 2009
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Anthony A. DiTonno and Stephen F. Ghiglieri, and each of them, his true and lawful attorneys-in-fact, each with full power of substitution, for him in any and all capacities, to sign any amendments to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact or their substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/s/ ANTHONY A. DITONNO Anthony A. DiTonno | | President, Chief Executive Officer
and Director (Principal Executive
Officer) | | March 26, 2009 |
| | |
/s/ STEPHEN F. GHIGLIERI Stephen F. Ghiglieri | | Chief Financial Officer (Principal
Financial and Accounting
Executive) | | March 26, 2009 |
| | |
/s/ JEAN-JACQUES BIENAIMÉ Jean-Jacques Bienaimé | | Chairman of the Board of
Directors | | March 26, 2009 |
| | |
/s/ NEIL M. KURTZ Neil M. Kurtz | | Director | | March 26, 2009 |
| | |
/s/ ROBERT T. NELSEN Robert T. Nelsen | | Director | | March 26, 2009 |
| | |
/s/ BRUCE A. PEACOCK Bruce A. Peacock | | Director | | March 26, 2009 |
99
EXHIBIT INDEX
| | |
Exhibit Number | | Exhibit Title |
3.1(1) | | Amended and Restated Certificate of Incorporation. |
| |
3.2(1) | | Amended and Restated Bylaws. |
| |
4.1(1) | | Specimen Common Stock Certificate. |
| |
4.2(1) | | Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of November 14, 2005. |
| |
4.3(2) | | Amendment No. 1 to the Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of December 28, 2007. |
| |
4.4(1) | | Warrant to Purchase Series A Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of December 14, 2000. |
| |
4.5(1) | | Warrant to Purchase Series B Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of May 1, 2002. |
| |
4.6(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company II LLC, dated as of July 7, 2006. |
| |
4.7(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company III LLC, dated as of July 7, 2006. |
| |
4.8(1) | | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Oxford Finance Corporation, dated as of July 7, 2006. |
| |
4.9(1) | | Form of First Warrant to Purchase Series C2 Preferred Stock. |
| |
4.10(1) | | Form of Second Warrant to Purchase Series C2 Preferred Stock. |
| |
4.11(2) | | Registration Rights Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. |
| |
4.12(2) | | Form of Warrant to Purchase Common Stock. |
| |
10.1(1) | | 2007 Stock Plan. |
| |
10.2(1) | | 2007 Employee Stock Purchase Plan. |
| |
10.3(1) | | Form of Indemnification Agreement entered into between NeurogesX, Inc. and each of its directors and officers. |
| |
10.4 | | Exclusive License Agreement between NeurogesX, Inc. and The Regents of the University of California, executed as of October 6, 2008. |
| |
10.5(1)† | | Clinical Supply, Development and License Agreement between NeurogesX, Inc. and LTS Lohmann Therapie-Systeme AG, dated as of January 15, 2004. |
| |
10.6(4)† | | Manufacturing and Supply Agreement, effective as of August 19, 2008 between NeurogesX, Inc. and Formosa Laboratories, Inc. |
| |
10.7† | | Commercial Supply and License Agreement with Lohmann Therapie-Systeme AG and NeuorgesX, Inc., effective as of January 2007. |
| |
10.8 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Anthony DiTonno, effective as of December 31, 2008. |
| |
10.9 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Stephen Ghiglieri, effective as of December 31, 2008. |
100
EXHIBIT INDEX
| | |
Exhibit Number | | Exhibit Title |
10.10 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Keith Bley, effective as of December 31, 2008. |
| |
10.11 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Michael Markels, effective as of December 31, 2008. |
| |
10.12 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Jeffrey Tobias, effective as of December 31, 2008. |
| |
10.13 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Russell Kawahata, effective as of December 31, 2008. |
| |
10.14 | | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Susan Rinne, effective as of December 31, 2008. |
| |
10.15(3) | | Sublease between NeurogesX, Inc. and Oracle USA, Inc., dated September 6, 2007. |
| |
10.16(2) | | Securities Purchase Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. |
| |
21.1(1) | | List of Subsidiaries. |
| |
23.1 | | Consent of Independent Registered Public Accounting Firm. |
| |
24.1 | | Power of Attorney (see page 99). |
| |
31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
32.1 | | Certifications of the Principal Executive Officer and the Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 1350). |
| (1) | Incorporated by reference from our registration statement on Form S-1, registration number 333-140501, declared effective by the Securities and Exchange Commission on May 1, 2007. |
| (2) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 28, 2007. |
| (3) | Incorporated by reference from our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2007. |
| (4) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 22, 2008. |
| † | Confidential treatment has been requested for portions of this exhibit. These portions have been omitted from this filing and have been filed separately with the Securities and Exchange Commission. |
101
