
ChromaDex Earnings Presentation Third Quarter 2024 Rob Fried Chief Executive Officer Ozan Pamir Chief Financial Officer Andrew Shao SVP Scientific & Regulatory Affairs Nasdaq: CDXC | October 31, 2024

SAFE HARBOR STATEMENT SAFE HARBOR STATEMENT 2 This presentation and other written or oral statements made from time to time by representatives of ChromaDex contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements reflect the current view about future events. Statements that are not historical in nature, such as 2024 financial outlook, and which may be identified by the use of words like “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “will,” “should,” “could,” “predicts,” “projects,” “continue,” “would” or the negative of these terms and other words of similar meaning, are forward-looking statements. Such statements include, but are not limited to, statements contained in this presentation relating to our expected sales, cash flows, planned investments, and financial performance, business, business strategy, expansion, growth, key drivers (including cost savings and increased investments), products and services we recently offered and their impact on our performance or products and services we may offer in the future and the timing of their development, sales and marketing strategy, and the statements regarding Niagen+. Forward- looking statements are based on management’s current expectations and assumptions regarding our business, the economy and other future conditions and are subject to inherent risks, uncertainties and changes of circumstances that are difficult to predict and may cause actual results to differ materially from those contemplated or expressed. We caution you therefore against relying on any of these forward-looking statements. These risks and uncertainties include those risk factors discussed in Part I, “Item 1A. Risk Factors” of our most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the Securities Exchange Commission (the “Commission”), and in subsequent filings with the Commission. Any forward-looking statements are qualified in their entirety by reference to the factors discussed in these filings with the Commission. Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned. Important factors that could cause actual results to differ materially from those in the forward looking statements include but are not limited to: inflationary conditions and adverse economic conditions; our history of operating losses and need to obtain additional financing; the growth and profitability of our product sales; our ability to maintain and grow sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third- party suppliers; risks of conducting business in China; including unanticipated developments in and risks related to the Company’s ability to secure adequate quantities of pharmaceutical-grade Niagen in a timely manner; the Company’s ability to obtain appropriate contracts and arrangements with U.S. FDA-registered 503B outsourcing facilities required to compound and distribute pharmaceutical-grade Niagen to clinics; the Company’s ability to remain on the U.S. FDA Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Category 1 list; the Company’s ability to maintain and enforce the Company’s existing intellectual property and obtain new patents; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; and the risks and uncertainties associated with our business and financial condition in general. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results. About Non-GAAP Financial Measures ChromaDex’s non-GAAP financial measure, Adjusted EBITDA, is defined as net income (loss) before interest, depreciation, amortization, non-cash share-based compensation costs and severance and restructuring expense. ChromaDex used this non-GAAP measures when evaluating its financial results as well as for internal resource management, planning and forecasting purposes. This non-GAAP measure should not be viewed in isolation from or as a substitute for ChromaDex’s financial results in accordance with GAAP. Reconciliation of this non-GAAP measure to the most directly comparable GAAP measure is attached to this presentation. FDA Disclaimer Statements made in this presentation have not been evaluated by the Food and Drug Administration. ChromaDex products are not intended to diagnose, treat, cure, or prevent any disease. The statements in this presentation are for investor relations and educational purposes only and not intended for consumers or vendors.

3 Q3 2024 & Recent Highlights (1) See slide 11 for the non-GAAP reconciliation In Q3 2024, delivered strong top-line growth, solid bottom-line results and advanced key business objectives. • Total company and Tru Niagen® net sales of $25.6 million and $18.1 million, up 31%, and 4% YoY, respectively. • Total Niagen® ingredient sales, including food-grade and pharmaceutical-grade, reached $6.7 million, up 368% YoY. • Delivered a strong gross margin of 63.5%, up 210 basis points from the prior year quarter. • Sales and marketing expense as a percentage of net sales was 27.5%, an improvement of 350 basis points, compared to 31.0% from the prior year quarter. • Achieved net income of $1.9 million or $0.02 earnings per share, an improvement of $2.8 million and $0.03 YoY. • Underlying business, as measured by Adjusted EBITDA, improved to $2.9 million from $0.5 million in the prior year quarter.(1) • Positive total cash flows of $5.1 million year-to-date, ending with $32.4 million in cash and no debt. • In September 2024, ChromaDex expanded the availability of Niagen+, a product line containing pharmaceutical-grade Niagen®, to an additional 14 leading wellness clinics. These Niagen+ products, including Niagen® IV and and Niagen® Injections, are compounded and distributed by U.S. FDA-registered 503B outsourcing facilities and are available exclusively by prescription at participating wellness clinics. As of October 31, 2024, ChromaDex has expanded Niagen+ availability to over 100 wellness clinics, with further significant expansion expected in the coming weeks. • Adjusted full year 2024 outlook with net sales growth of approximately 15% (previously between 10%-15%) to reflect upside realized from the launch of our Niagen+ product line.

Management Team 4 Rob Fried Chief Executive Officer E-commerce & entertainment industry executive Savoy Pictures, Columbia Pictures, Fried Films, FeeIn, WHN, Healthspan Research Andrew Shao SVP, Global Regulatory & Scientific Affairs Over two decades of global nutrition industry experience at Amway, Herbalife Nutrition, and the Council for Responsible Nutrition Ozan Pamir Chief Financial Officer Over a decade of capital markets and public company experience in the life sciences industry CFA Charterholder Carlos Lopez SVP, General Counsel Over a decade of experience in the dietary supplements industry. Previously served as VP, General Counsel at The Vitamin Shoppe and board member of The Natural Products Association Michiko Kelley Chief Marketing Officer Over two decades of experience in marketing strategy, marketing operation, product management, and leadership at Dexcom and Sony Electronics

The information contained in this documents is confidential, privileged and only for the information of the intended recipient and may not be used, published or redistributed without the prior written consent (2019) Financial Highlights

6 Q3 2024 Net Sales Mix E-Commerce 65% Watson's & Other B2B 24% Food-grade Niagen® 7% Analytical Reference Standards & Services 4% Q3 2023 $19.5 MM E-Commerce 58% Watson's & Other B2B 13% Food-grade Niagen® 23% Pharmaceutical-grade Niagen® 3% Analytical Reference Standards & Services 3% $25.6 MM Q3 2024 • Tru Niagen® net sales totaled 71% of net sales in Q3 2024 compared to 89% in Q3 2023(1) • Niagen®-related net sales increased to 97% of net sales in Q3 2024 compared to 96% in Q3 2023(2) Higher mix of Niagen® Ingredient sales in Q3 2024 compared to the prior year, including strong food-grade Niagen® sales to key partners along with newly launched pharmaceutical-grade Niagen® sales. (1) Tru Niagen® net sales include E-Commerce, Watson’s/Other B2B (2) Niagen®-related sales include Tru Niagen®, food-grade Niagen® and pharmaceutical-grade Niagen®.
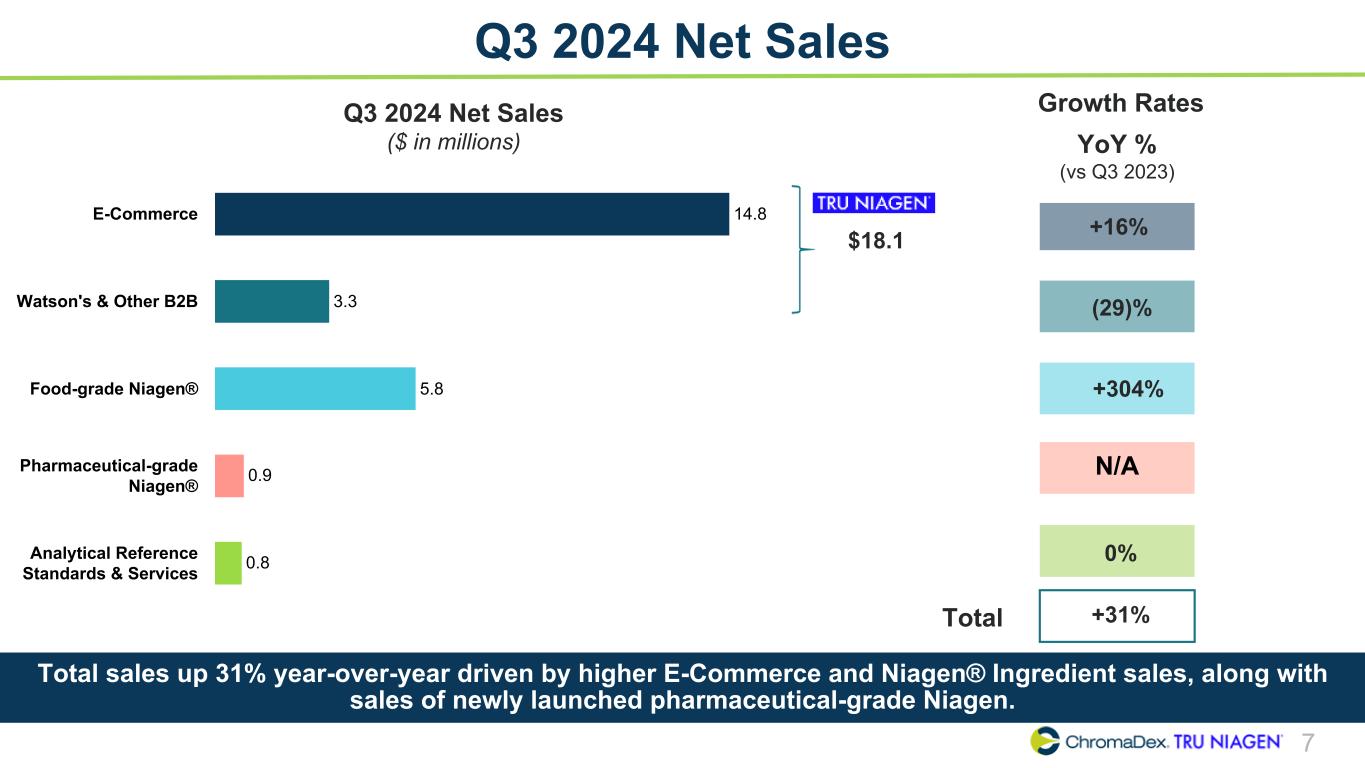
N/A 7 Q3 2024 Net Sales Q3 2024 Net Sales ($ in millions) 14.8 3.3 5.8 0.9 0.8 E-Commerce Watson's & Other B2B Food-grade Niagen® Pharmaceutical-grade Niagen® Analytical Reference Standards & Services $18.1 YoY % (vs Q3 2023) +16% (29)% +304% 0% +31%Total Growth Rates Total sales up 31% year-over-year driven by higher E-Commerce and Niagen® Ingredient sales, along with sales of newly launched pharmaceutical-grade Niagen.

8 Steady growth in total company sales, up 13% year-over-year driven by E-Commerce business and Niagen® ingredient sales. Year-to-Date 2024 Net Sales N/A YTD 2024 Net Sales ($ in millions) Total +13% +7% (4)% +66% +4% (60)% YoY % (vs YTD 2023) $54.1 40.7 13.4 13.0 0.9 2.3 0.2 E-Commerce Watson's & Other B2B Food-grade Niagen® Pharmaceutical-grade Niagen® Analytical Reference Standards & Services Other Ingredients Growth Rates

9 2022 – 2024 YTD Net Sales Summary ($ in millions) 2022 2023 2024 Description Q1 Q2 Q3 Q4 FY Q1 Q2 Q3 Q4 FY Q1 Q2 Q3 E-Commerce 10.9 12.0 11.3 11.1 45.3 12.2 13.0 12.7 13.3 51.2 12.9 13.0 14.8 Watsons 2.6 1.5 2.6 3.3 10.0 3.7 3.0 3.1 3.0 12.8 3.0 3.7 2.0 Other B2B 1.4 1.0 0.7 1.7 4.8 1.7 0.9 1.6 1.3 5.5 1.5 1.9 1.3 Total TRU NIAGEN 14.9 14.5 14.6 16.1 60.1 17.6 16.9 17.4 17.6 69.5 17.4 18.6 18.1 Food-grade NIAGEN 1.1 1.5 1.8 3.9 8.3 3.9 2.5 1.4 2.7 10.5 4.1 3.1 5.8 Pharmaceutical-grade NIAGEN 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.9 Total NIAGEN Ingredient 1.1 1.5 1.8 3.9 8.3 3.9 2.5 1.4 2.7 10.5 4.1 3.1 6.7 Total NIAGEN Related Revenues 16.0 16.0 16.4 20.0 68.4 21.5 19.4 18.8 20.3 80.0 21.5 21.7 24.8 Other Ingredients 0.3 0.0 0.0 0.1 0.4 0.2 0.2 0.0 0.2 0.6 0.0 0.2 0.0 Analytical Reference Standards & Services 0.9 0.7 0.7 0.9 3.2 0.8 0.7 0.7 0.7 2.9 0.7 0.8 0.8 Total Net Sales 17.2 16.7 17.1 21.0 72.0 22.5 20.3 19.5 21.2 83.5 22.2 22.7 25.6 TRU NIAGEN as % of Total Net Sales 87 % 87 % 85 % 77 % 83 % 78 % 83 % 89 % 83 % 83 % 78 % 82 % 71 % NIAGEN Related Revenues as % of Total Net Sales 93 % 95 % 96 % 95 % 95 % 95 % 95 % 96 % 96 % 96 % 97 % 96 % 97 % YOY Growth Rate - Net Sales Total Company 18 % (5) % (1) % 18 % 7 % 31 % 21 % 14 % 1 % 16 % (2) % 12 % 31 % Total NIAGEN Related 18 % (4) % (1) % 20 % 8 % 34 % 21 % 15 % 2 % 17 % — % 12 % 32 % Total TRU NIAGEN 20 % (6) % (1) % 14 % 6 % 18 % 16 % 19 % 9 % 16 % (2) % 10 % 4 %
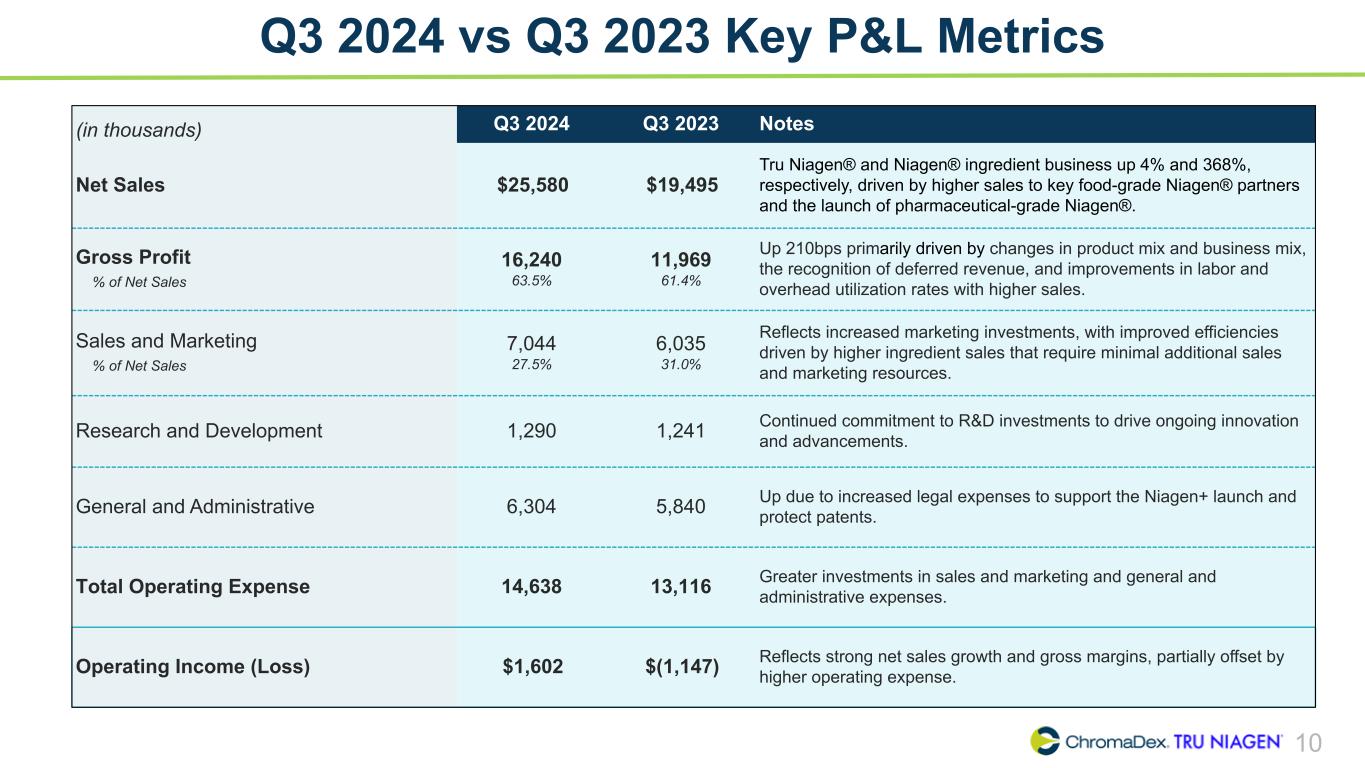
10 Q3 2024 vs Q3 2023 Key P&L Metrics (in thousands) Q3 2024 Q3 2023 Notes Net Sales $25,580 $19,495 Tru Niagen® and Niagen® ingredient business up 4% and 368%, respectively, driven by higher sales to key food-grade Niagen® partners and the launch of pharmaceutical-grade Niagen®. Gross Profit % of Net Sales 16,240 63.5% 11,969 61.4% Up 210bps primarily driven by changes in product mix and business mix, the recognition of deferred revenue, and improvements in labor and overhead utilization rates with higher sales. Sales and Marketing % of Net Sales 7,044 27.5% 6,035 31.0% Reflects increased marketing investments, with improved efficiencies driven by higher ingredient sales that require minimal additional sales and marketing resources. Research and Development 1,290 1,241 Continued commitment to R&D investments to drive ongoing innovation and advancements. General and Administrative 6,304 5,840 Up due to increased legal expenses to support the Niagen+ launch and protect patents. Total Operating Expense 14,638 13,116 Greater investments in sales and marketing and general and administrative expenses. Operating Income (Loss) $1,602 $(1,147) Reflects strong net sales growth and gross margins, partially offset by higher operating expense.

11 Adjusted EBITDA Summary Delivered record-setting Adjusted EBITDA of $2.9 million in Q3 2024 up from $0.5 million in the prior year quarter, driven by improvements in net income slightly offset by lower share-based compensation. ChromaDex Corporation and Subsidiaries Reconciliation of Non-GAAP Finanical Measures (In thousands) Three months ended Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Q1 2024 Q2 2024 Q3 2024 Net income (loss), as reported $ (7,740) $ (6,397) $ (985) $ (1,418) $ (1,902) $ (2,191) $ (959) $ 114 $ (492) $ (15) $ 1,878 Adjustments Interest (income) expense 8 10 5 (26) (66) (125) (188) (282) (239) (241) (276) Depreciation 201 212 235 221 228 232 233 177 178 170 164 Amortization of intangibles 49 50 44 43 41 39 39 39 38 37 38 Amortization of right of use assets 299 169 170 191 171 173 176 157 174 163 164 Share-based compensation 1,888 1,296 1,229 1,326 1,273 1,324 1,117 1,037 984 1,185 735 Severance and restructuring 821 17 181 13 186 766 86 5 27 276 185 Other income - Employee Retention Tax Credit — — (2,085) — — — — — — — — Adjusted EBITDA $ (4,474) $ (4,643) $ (1,206) $ 350 $ (69) $ 218 $ 504 $ 1,247 $ 670 $ 1,575 $ 2,888

12 Q3 2024 Operating Income (Loss) vs Q3 2023 (in millions) -$1.7 MM+$4.4 MM (1.1) 3.7 0.5 0.2 (0.1) (0.1) (0.5) (1.0) 1.6 Q3 2023 Operating Loss Volume Gross Margin Improvement Equity Comp (G+A) Other G+A Severance and Restructuring Legal Sales & Marketing Q3 2024 Operating Income (2.0) (1.0) — 1.0 2.0 3.0 4.0

13 Quarterly Balance Sheet Highlights (in thousands) 12/31/22 3/31/23 6/30/23 9/30/23 12/31/23 3/31/24 6/30/24 9/30/24 Key Drivers (Q4 2023 vs Q3 2024) Cash $20,441 $23,141 $26,406 $26,773 $27,325 $27,565 $27,885 $32,398 Up $5.1 million driven by net income and proceeds from stock option exercises Inventory 14,677 11,908 11,973 12,624 14,525 12,495 11,511 10,544 Down $4.0 million driven by improvements in supply chain management and timing of inventory purchases and sales Trade Receivables 8,482 9,221 6,118 5,601 5,234 6,604 7,818 7,096 Up $1.9 million driven by higher sales and timing of collections Accrued Liabilities 7,337 8,610 8,079 9,193 9,493 10,465 8,621 9,592 Down $0.1 million driven by timing of expenses Accounts Payable 9,679 8,951 10,031 9,198 10,232 7,899 8,105 6,903 Down $3.3 million driven by timing of disbursements Equity $28,672 $28,017 $27,150 $27,308 $28,456 $28,951 $30,718 $34,369 Up $5.9 million driven by net income, share- based compensation and proceeds from stock option exercises Continued growth strengthened overall financial position, resulting in a stronger balance sheet that reflects the health and stability of the business.
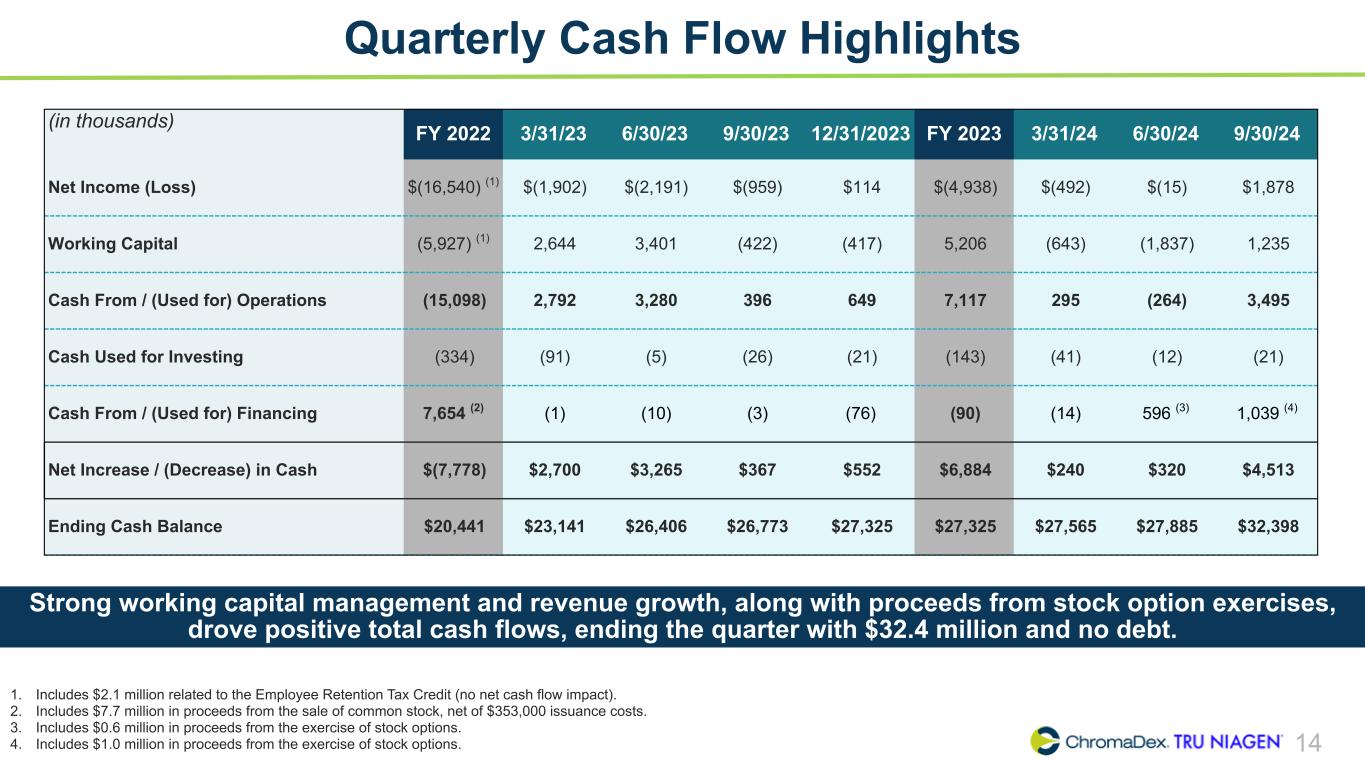
14 Quarterly Cash Flow Highlights (in thousands) FY 2022 3/31/23 6/30/23 9/30/23 12/31/2023 FY 2023 3/31/24 6/30/24 9/30/24 Net Income (Loss) $(16,540) (1) $(1,902) $(2,191) $(959) $114 $(4,938) $(492) $(15) $1,878 Working Capital (5,927) (1) 2,644 3,401 (422) (417) 5,206 (643) (1,837) 1,235 Cash From / (Used for) Operations (15,098) 2,792 3,280 396 649 7,117 295 (264) 3,495 Cash Used for Investing (334) (91) (5) (26) (21) (143) (41) (12) (21) Cash From / (Used for) Financing 7,654 (2) (1) (10) (3) (76) (90) (14) 596 (3) 1,039 (4) Net Increase / (Decrease) in Cash $(7,778) $2,700 $3,265 $367 $552 $6,884 $240 $320 $4,513 Ending Cash Balance $20,441 $23,141 $26,406 $26,773 $27,325 $27,325 $27,565 $27,885 $32,398 1. Includes $2.1 million related to the Employee Retention Tax Credit (no net cash flow impact). 2. Includes $7.7 million in proceeds from the sale of common stock, net of $353,000 issuance costs. 3. Includes $0.6 million in proceeds from the exercise of stock options. 4. Includes $1.0 million in proceeds from the exercise of stock options. Strong working capital management and revenue growth, along with proceeds from stock option exercises, drove positive total cash flows, ending the quarter with $32.4 million and no debt.
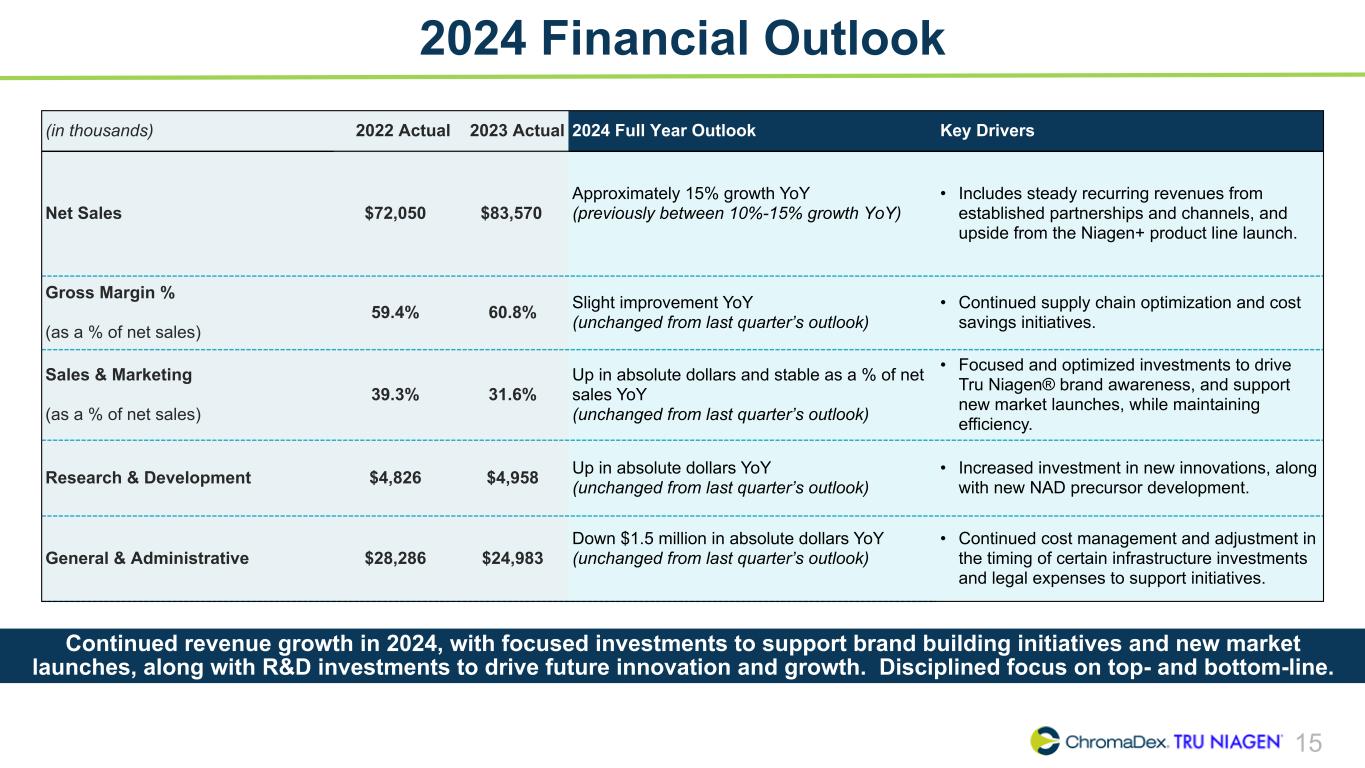
15 2024 Financial Outlook (in thousands) 2022 Actual 2023 Actual 2024 Full Year Outlook Key Drivers Net Sales $72,050 $83,570 Approximately 15% growth YoY (previously between 10%-15% growth YoY) • Includes steady recurring revenues from established partnerships and channels, and upside from the Niagen+ product line launch. Gross Margin % (as a % of net sales) 59.4% 60.8% Slight improvement YoY (unchanged from last quarter’s outlook) • Continued supply chain optimization and cost savings initiatives. Sales & Marketing (as a % of net sales) 39.3% 31.6% Up in absolute dollars and stable as a % of net sales YoY (unchanged from last quarter’s outlook) • Focused and optimized investments to drive Tru Niagen® brand awareness, and support new market launches, while maintaining efficiency. Research & Development $4,826 $4,958 Up in absolute dollars YoY (unchanged from last quarter’s outlook) • Increased investment in new innovations, along with new NAD precursor development. General & Administrative $28,286 $24,983 Down $1.5 million in absolute dollars YoY (unchanged from last quarter’s outlook) • Continued cost management and adjustment in the timing of certain infrastructure investments and legal expenses to support initiatives. Continued revenue growth in 2024, with focused investments to support brand building initiatives and new market launches, along with R&D investments to drive future innovation and growth. Disciplined focus on top- and bottom-line.

The information contained in this documents is confidential, privileged and only for the information of the intended recipient and may not be used, published or redistributed without the prior written consent (2019) 16 The Science

17 Science Continues to Expand (1) There are 101 ongoing, completed, and published clinical studies currently registered on clinicaltrials.gov to investigate the pharmacokinetics and therapeutic effects of NR alone or in combination with other ingredients. 78 of these use NR only. Clinicaltrials.gov also includes two niacin studies and one device monitoring registry for a total of 104 under the search term “nicotinamide riboside.” (As of October 17, 2024) (2) 275+ research collaborations for Niagen® signed with research institutes and universities around the world. More than 90% of the studies are investigator-initiated and were developed to support applications for or receipt of third-party funding. The studies may not have been initiated if investigators were unable to secure funding. • Four new human trials on nicotinamide riboside registered since prior update:(1) ▪ University of Oklahoma (August 2024) • Objective: Investigate whether NR supplementation impacts peripheral blood vessel function, cerebral blood flow, and cognitive function in older adults with peripheral artery disease. • 8 participants will receive 1000mg of NR daily for four weeks. ▪ Cedars-Sinai Medical Center (August 2024) • Objective: Investigate the combined effects of NR and exercise on metabolic and mitochondrial health. • 28 participants will receive 1000 mg of NR daily with an exercise training program for 12 weeks. ▪ Center for Eye Research Australia (September 2024) • Objective: Evaluate the safety and tolerability of NR in preserving vision in individuals diagnosed with macula-off retinal detachment. • 144 participants will receive 2000mg NR daily for four weeks, followed by 1000mg NR daily for 16 weeks after their procedure. ▪ University of Turku (October 2024) • Objective: Investigate whether NAD+-based therapies can enhance insulin sensitivity and brown fat metabolism in adults, potentially reducing obesity and its associated comorbidities like type II diabetes. • 68 participants will receive either a placebo or an NAD+ precursor (NR, nicotinamide, or nicotinamide mononucleotide) for 6 months. • One new research study was signed through ChromaDex External Research Program (CERP®).(2)

18 Scientific Advisory Board Charles Brenner, Ph.D. Alfred E Mann Family Foundation Chair, Department of Diabetes & Cancer Metabolism City of Hope World's Foremost Authority on NAD Metabolism Roger Kornberg, Ph.D. Chairman Professor of Structural Biology Stanford University Nobel Prize Winner, Chemistry, 2006 Rudolph Tanzi, Ph.D. Kennedy Professor of Neurology Harvard University Leading Alzheimer's Researcher, TIME 100 Most Influential 2015 Dr. Bruce German Chairman of Food, Nutrition, & Health University of California, Davis Leader in Food, Nutrition, & Wellness Innovation Professor Sir John Walker, Ph.D. Emeritus Director, MRC Mitochondrial Biology University of Cambridge Nobel Prize Winner, Chemistry, 1997 Brunie H. Felding, Ph.D. Associate Professor of Molecular Medicine Scripps Research Institute Renowned Breast Cancer Researcher focused on NAD+ supplementation Dr. David Katz President of True Health Initiative CEO of Diet ID World renowned physician & preventive medicine expert Dr. Vilhelm (Will) Bohr, M.D., Ph.D., D.Sc. Professor in Genome Instability and Neurodegeneration, Department of Cellular and Molecular Medicine, University of Copenhagen. One of the world’s most published researchers on aging and neurodegenerative disease NOBEL PRIZE WINNERS | CHEMISTRY
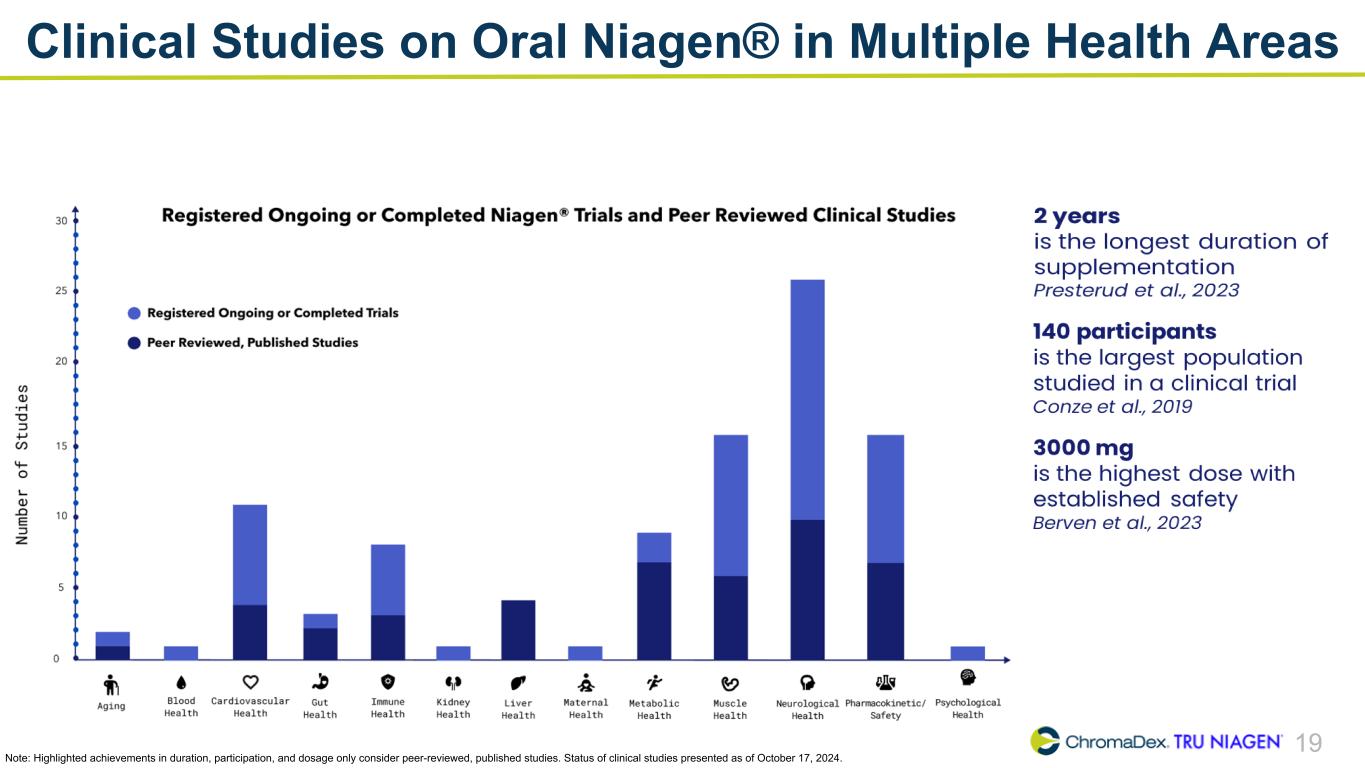
19 Clinical Studies on Oral Niagen® in Multiple Health Areas Note: Highlighted achievements in duration, participation, and dosage only consider peer-reviewed, published studies. Status of clinical studies presented as of October 17, 2024.

The information contained in this documents is confidential, privileged and only for the information of the intended recipient and may not be used, published or redistributed without the prior written consent (2019) Contact Info: Ben Shamsian Lytham Partners T: +1(646) 829-9701 Shamsian@LythamPartners.com www.ChromaDex.com Where to buy Tru Niagen® TruNiagen.com Amazon.com 20



















