Table of Contents
As filed with the Securities and Exchange Commission on May 24, 2012
Registration Statement File No. 333-180694
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
HYPERION THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 61-1512713 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
601 Gateway Boulevard, Suite 200
South San Francisco, California 94080
(650) 745-7802
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Donald J. Santel
Chief Executive Officer
Hyperion Therapeutics, Inc.
601 Gateway Boulevard, Suite 200
South San Francisco, California 94080
(650) 745-7802
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Laura A. Berezin Jon Layman Hogan Lovells US LLP 525 University Avenue Palo Alto, CA 94301 (650) 463-4000 | Jeffrey S. Farrow Chief Financial Officer Hyperion Therapeutics, Inc. 601 Gateway Boulevard, Suite 200 (650) 745-7802 | Mark B. Weeks Brett D. White Cooley LLP 3175 Hanover Street Palo Alto, CA 94304 (650) 843-5000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is declared effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY 24, 2012
PROSPECTUS
Shares

Common Stock
Hyperion Therapeutics, Inc. is offering shares of common stock. This is our initial public offering, and no public market currently exists for our common stock. We anticipate that the initial public offering price will be between $ and $ per share.
We have applied to list our common stock on The NASDAQ Global Market under the symbol “HPTX.”
Investing in our common stock involves risks. See “Risk Factors” beginning on page 10.
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 and will be subject to reduced public company reporting requirements.
| Per Share | Total | |||||||
Initial public offering price | $ | $ | ||||||
Underwriting discounts and commissions | $ | $ | ||||||
Proceeds, before expenses, to us | $ | $ | ||||||
We have granted the underwriters an option for 30 days from the date of this prospectus to purchase up toadditional shares of our common stock at the initial public offering price, less underwriting discounts and commissions, to cover over-allotments.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock on or about , 2012.
Leerink Swann | Cowen and Company |
Joint Book-Running Managers
| Needham & Company |
The date of this prospectus is , 2012.
Table of Contents
| Page | ||||
| 1 | ||||
| 10 | ||||
| 42 | ||||
| 43 | ||||
| 43 | ||||
| 44 | ||||
| 47 | ||||
| 50 | ||||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 52 | |||
| 74 | ||||
| 107 | ||||
| 116 | ||||
| 131 | ||||
| 138 | ||||
| 143 | ||||
| 148 | ||||
Material U.S. Federal Income and Estate Tax Consequences to Non-U.S. Holders | 151 | |||
| 155 | ||||
| 160 | ||||
| 160 | ||||
| 160 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not authorized anyone to provide you with information that is different. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock.
Until and including , 2012, 25 days after the date of this prospectus, all dealers that buy, sell or trade our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
For investors outside of the United States: neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
In this prospectus, unless otherwise stated or the context otherwise indicates, references to “Hyperion,” “we,” “us,” “our” and similar references refer to Hyperion Therapeutics, Inc. and our wholly-owned subsidiary. The names Hyperion Therapeutics, Inc. TM and RavictiTM are our trademarks. BUPHENYL® and AMMONUL® are registered trademarks of Ucyclyd Pharma, Inc., a wholly owned subsidiary of Medicis Pharmaceutical Corporation. All other trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
i
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. Before you decide to invest in our common stock, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes appearing at the end of this prospectus.
Our Company
We are a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat disorders in the areas of orphan diseases and hepatology. We are developing Ravicti™ (glycerol phenylbutyrate) to treat the most prevalent urea cycle disorders, or UCD, and hepatic encephalopathy, or HE. UCD and HE are generally characterized by elevated levels of ammonia in the bloodstream. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. Ravicti is designed to lower ammonia in the blood. On December 23, 2011, we submitted an NDA for Ravicti for the chronic management of UCD in patients aged 6 years and above based on data from our pivotal Phase III trial in adult patients and the results of two Phase II trials, one in adults and one in pediatric patients aged 6 through 17 years. The U.S. Food and Drug Administration, or FDA, accepted the NDA for review in February 2012. Under the Prescription Drug User Fee Act, or PDUFA, the FDA is currently due to notify us regarding Ravicti’s approval status by October 23, 2012, unless that action date is extended by the FDA. In April 2012, we submitted data from the switchover portion of a clinical trial in UCD patients aged 29 days through 5 years and a revised draft package insert requesting approval of Ravicti to include this patient population. We currently expect to commercially launch Ravicti in the first half of 2013.
UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or protein transporters that constitute the urea cycle, which in a healthy individual removes ammonia through the conversion of ammonia to urea. We believe UCD occur in approximately 1 in 10,000 births in the United States. Ravicti was granted orphan drug designation by the FDA for the maintenance treatment of patients with UCD. Orphan drug designation is given to a drug candidate intended to treat a rare disease or condition, which affects fewer than 200,000 individuals in the United States.
Currently, the only branded therapy approved by the FDA for chronic management of the most prevalent UCD is BUPHENYL® (sodium phenylbutyrate) Tablets and Powder, which is currently commercialized by Ucyclyd Pharma, Inc., or Ucyclyd, a wholly owned subsidiary of Medicis Pharmaceutical Corporation. We believe BUPHENYL use is limited due to the combination of high pill burden or large quantity of powder that must be taken, frequency of dosing (3-6 times per day), the unpleasant taste and smell, and tolerability issues. In addition, the sodium content of the maximum daily dose of BUPHENYL exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure. Ravicti uses the same vehicle for ammonia removal as BUPHENYL but requires a much smaller volume of drug. For example, approximately 1 tablespoon of Ravicti liquid is equivalent to the FDA-approved maximum daily dose of 40 tablets of BUPHENYL. Furthermore, Ravicti is nearly tasteless and odorless and does not contain any sodium. Significantly elevated ammonia levels with corresponding neurological symptoms are known as hyperammonemic, or HA, crises. We believe that Ravicti may reduce HA crises as compared to BUPHENYL and, if approved, will offer benefits that enhance tolerability and increase compliance in support of improved disease management.
In March 2012, pursuant to an asset purchase agreement, or purchase agreement, with Ucyclyd we purchased all of the worldwide rights to Ravicti for an upfront payment of $6.0 million, future payments based upon the achievement of regulatory milestones in indications other than UCD, sales milestones, and mid to high single digit royalties on global net sales of Ravicti. Pursuant to an amended and restated collaboration agreement,
1
Table of Contents
or restated collaboration agreement, with Ucyclyd, also entered into in March 2012, we have an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL® (sodium phenylacetate and sodium benzoate) injection 10%/10%, the only adjunctive therapy currently FDA-approved for the treatment of HA crises in patients with the most prevalent UCD, for an upfront payment of $22.0 million, plus subsequent milestone and royalty payments. To fund this upfront payment, we may choose to draw on a loan commitment from Ucyclyd, which loan would be payable over eight quarters. We will be permitted to exercise this option for a period of 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. If we exercise our option, Ucyclyd has a time-limited option to retain AMMONUL for a purchase price of $32.0 million. If Ucyclyd exercises its option under the restated collaboration agreement and retains AMMONUL, the upfront purchase price for BUPHENYL will be $19.0 million resulting in a net payment from Ucyclyd to us of $13.0 million upon close of the transaction.
To expand the commercial potential of Ravicti, we have completed a Phase II trial assessing the safety and efficacy of Ravicti for the treatment of episodic HE. The FDA has also granted orphan drug designation for Ravicti for this indication. HE is a serious but potentially reversible neurological disorder that can occur in patients with liver scarring, known as cirrhosis, or acute liver failure. HE is believed to occur when the brain is exposed to gut-derived toxins that are normally removed from the blood by a healthy liver. Episodic HE can be diagnosed clinically through a set of signs and symptoms. Similar to UCD patients who may experience HA crises, patients with episodic HE often experience periods in which their symptoms worsen, referred to as HE events, that are manifested by symptoms ranging from disorientation to coma, and frequently require hospitalization. Our HE development program is targeting patients with episodic HE and is designed to determine whether treatment with Ravicti will decrease the number of HE events. The Phase II trial met its primary endpoint, which was to demonstrate that the proportion of patients experiencing an HE event was significantly lower on Ravicti versus placebo, both administered in addition to standard of care, including lactulose and/or rifaximin. We believe that ammonia plays a central role in HE and that Ravicti, if approved, could be beneficial in managing this disease. Moreover, given its mechanism of action of removing ammonia from the body, Ravicti could be complementary to currently approved agents, such as rifaximin, that may limit the local production of ammonia.
Ravicti Clinical Development
We have completed two Phase II trials and one pivotal Phase III trial in which we evaluated the non-inferiority of Ravicti as compared to BUPHENYL in controlling blood ammonia levels in adult and pediatric patients with UCD. We successfully demonstrated non-inferiority in each of these trials and a pooled analysis of the data from these trials demonstrated statistically significant lower ammonia levels in patients on Ravicti as compared to BUPHENYL. A non-inferiority trial compares a test drug to an established treatment with the goal of showing that any difference in the performance of the test drug is small enough to support a conclusion that the test drug is not inferior to the established treatment, and that the test drug is, therefore, also effective. In our trial, non-inferiority of Ravicti would be demonstrated if the upper 95% confidence interval of ammonia on Ravicti would not be more than 25% higher than that seen on BUPHENYL. A 95% confidence interval means that if the trial was run multiple times, 95% of the time, ammonia levels on Ravicti were not more than 25% higher than that seen on BUPHENYL. We believe the ammonia control provided by Ravicti is responsible for improved executive function seen in UCD patients aged 6 through 17 years after 12 months of treatment with Ravicti. In the 12-month safety extension to our pivotal Phase III trial, patients on Ravicti have experienced fewer HA crises than they reported having experienced in the prior year while on BUPHENYL. In addition, in our Phase II trials, 34 of 36 patients expressed a preference for Ravicti over BUPHENYL. Forty-one of the forty-four patients in our pivotal Phase III trial who had been treated chronically with BUPHENYL before trial enrollment agreed to continue treatment and monthly monitoring with Ravicti beyond the initial four-week treatment period. Sixty-seven of sixty-nine patients who completed 12 months of treatment with Ravicti elected to enroll in an expanded access protocol to continue receiving Ravicti.
2
Table of Contents
We are currently conducting a fourth clinical trial in UCD patients aged 29 days through 5 years designed to demonstrate the safety and efficacy of Ravicti in this patient population. The efficacy portion of this trial is complete, and a complete study report was submitted to the FDA in April 2012 as part of an update to our NDA. We expect the results of the 12-month safety extension portion of the trial to be available by the second quarter of 2013. As part of the April 2012 update, we submitted a revised draft package insert requesting the approval of Ravicti for all UCD patients down to 29 days of age. If the FDA classifies this submission as a major amendment, the PDUFA action date will likely be delayed.
Our HE clinical program comprises two trials which have enrolled patients with cirrhosis. The Phase II clinical trial design was similar to that used to evaluate rifaximin, the only therapy approved by the FDA for episodic HE within the last 30 years. The Phase II trial was a multinational randomized, placebo-controlled, double-blind study of Ravicti versus placebo in 178 patients with episodic HE receiving standard of care including lactulose and/or rifaximin. The Phase II trial met its primary endpoint: the proportion of patients experiencing at least one HE event was significantly lower on Ravicti versus placebo (21.1% vs. 36.4%, p = 0.0214). Patients receiving Ravicti also experienced fewer total HE events in the course of the study versus placebo (35 vs. 57; p = 0.0354). There were trends favoring Ravicti in numbers of patients hospitalized for HE events, total HE-related hospitalizations and total hospital days for HE-related admissions, suggesting a potentially important pharmacoeconomic benefit to the treatment of HE with Ravicti.
Among patients on lactulose only or no therapy at study entry, a population similar to that enrolled in the rifaximin pivotal study, Ravicti significantly reduced the proportion of patients experiencing at least one HE event versus placebo (10.0% vs. 32.2%; p = 0.0031) as well as the proportion of patients who experienced the more severe West Haven grade³2 events versus placebo (5% vs. 25.4 %; p = 0.001). In this subgroup, there was an 82% reduction in the risk of experiencing a grade³2 HE event on Ravicti as compared with placebo (p = 0.0073). Based upon the positive results of our Phase II trial in HE we are planning to request an end of Phase II meeting with the FDA for the fourth quarter of 2012, which is critical for evaluating our Phase III clinical options with respect to Ravicti in this indication.
Our Business Strategy
Our strategy is to commercialize a product portfolio, including Ravicti, for the treatment of UCD and to develop Ravicti for the treatment of HE and other indications. The key elements of our strategy are to:
| • | obtain FDA approval of Ravicti; |
| • | commercialize Ravicti and improve patient care in UCD; |
| • | market BUPHENYL and AMMONUL for patients ineligible for Ravicti; |
| • | develop Ravicti for the treatment of HE; and |
| • | expand Ravicti into additional indications and acquire additional products and product candidates. |
Risk Factors Associated with Our Business
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our common stock. In particular, our risks include, but are not limited to, the following:
| • | we depend substantially on the success of our only product candidate, Ravicti, and we may not obtain regulatory approval of Ravicti for the treatment of UCD or we may be unable to successfully commercialize it; |
3
Table of Contents
| • | regulatory approval could be substantially delayed if the pediatric data we have submitted and intend to submit does not satisfy the FDA or if the FDA requires additional time or studies to assess the safety and efficacy of Ravicti; |
| • | the patient population suffering from UCD is small and has not been established with precision; |
| • | we currently have no source of revenue and may never become profitable; |
| • | we may need to obtain additional financing to fund our operations; |
| • | if we choose to draw on a loan commitment from Ucyclyd to fund the purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we might be unable to service the loan due to a lack of cash flow, which could result in default; |
| • | termination of the restated collaboration agreement with Ucyclyd prior to our purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL would result in our losing rights to these products; and |
| • | if we cannot successfully defend our intellectual property, additional competitors could enter the market, including with generic versions of our products, and sales of affected products may decline materially. |
Our Corporate Information
We were incorporated under the laws of the State of Delaware in November 2006. Our principal executive offices are located at 601 Gateway Boulevard, Suite 200, South San Francisco, CA 94080, and our telephone number is (650) 745-7802. Our website address is www.hyperiontx.com. The information contained on, or that can be accessed through, our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
4
Table of Contents
THE OFFERING
Common stock to be offered by us | shares |
Common stock to be outstanding after
this offering | shares |
Over-allotment option | We have granted the underwriters an option for 30 days from the date of this prospectus to purchase up to additional shares of common stock to cover over-allotments. |
Use of proceeds | We expect to use the net proceeds from this offering to: fund clinical development, regulatory approval, post-marketing studies and, if approved, the commercial launch of Ravicti for UCD; to fund license payments to Brusilow Enterprises, LLC; and for general corporate purposes. See “Use of Proceeds” on page 43. |
Proposed NASDAQ Global Market
symbol | HPTX |
Risk factors | You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
The number of shares of common stock outstanding immediately after this offering is based on shares of common stock outstanding as of March 31, 2012. This number excludes:
| • | 7,718,537 shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2012 under our 2006 Equity Incentive Plan having a weighted average exercise price of $0.39 per share; |
| • | 1,810 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2012 having a weighted average exercise price of $294.13 per share, which warrants are expected to remain outstanding upon completion of this offering; and |
| • | shares of common stock (which includes the 631,904 shares reserved for issuance under our 2006 Equity Incentive Plan as of March 31, 2012) reserved for future issuance under our 2012 Omnibus Incentive Plan, which will become effective immediately upon the effectiveness of this registration statement, as well as any future increases in the number of shares of common stock reserved for issuance under this plan. |
Unless otherwise indicated, all information in this prospectus assumes or gives effect to:
| • | a 2-for-359 reverse stock split of our common stock effected June 29, 2009; |
| • | a -for- reverse stock split of our common stock and convertible preferred stock to be effected prior to the completion of this offering; |
| • | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
5
Table of Contents
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of warrants outstanding as of March 31, 2012 that we issued in connection with a bridge loan financing in April 2011, or the April 2011 warrants, and in May 2011, or the May 2011 warrants, into shares of our common stock, at an exercise price of $0.67 per share, and which will expire upon completion of this offering if not exercised; |
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of warrants that we issued in connection with a bridge loan financing in October 2011, or the October 2011 warrants, in November 2011, or the November 2011 warrants, and in February 2012, or the February 2012 warrants, into shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants, at an exercise price equal to $1.58 per share, and which will expire upon completion of this offering if not exercised; |
| • | the automatic conversion of the principal and accrued interest outstanding under our $17.5 million in aggregate principal amount of convertible promissory notes, or the April 2011 notes, $8,285 in aggregate principal amount of convertible promissory notes, or the May 2011 notes, $7.5 million in aggregate principal amount of convertible promissory notes, or the October 2011 notes, and $3,551 in aggregate principal amount of convertible promissory notes, or the November 2011 notes, and $7.5 million in aggregate principal amount of convertible promissory notes, or the February 2012 notes, into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012; |
| • | the filing of our amended and restated certificate of incorporation, which will occur immediately prior to the completion of this offering; and |
| • | no exercise of the underwriters’ over-allotment option. |
Because the number of shares that will be issued upon exercise of the April 2011 warrants, the May 2011 warrants, the October 2011 warrants, the November 2011 warrants and the February 2012 warrants and upon the conversion of the April 2011 notes, the May 2011 notes, the October 2011 notes, the November 2011 notes and the February 2012 notes depends upon the actual initial public offering price per share in this offering and, in the case of the notes, the closing date of this offering, the actual number of shares issuable upon such exercise and conversion may differ from the respective number of shares set forth above. We collectively refer to the April 2011 warrants, the May 2011 warrants, the October 2011 warrants, the November 2011 warrants and the February 2012 warrants as the “bridge warrants,” and we collectively refer to the April 2011 notes, the May 2011 notes, the October 2011 notes, the November 2011 notes and the February 2012 notes as the “bridge notes.”
A $1.00 increase in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would decrease the number of shares of our common stock issued upon exercise of the bridge warrants and upon conversion of the bridge notes (and therefore the number of shares to be outstanding after this offering) by shares, assuming that the closing date of this offering (and therefore the conversion date of the bridge notes) is , 2012. A $1.00 decrease in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase the number of shares of our common stock issued upon exercise of the bridge warrants and upon conversion of the bridge notes (and therefore the number of shares to be outstanding after this offering) by shares, assuming that the closing date of this offering (and therefore the conversion date of the bridge notes) is , 2012. To the extent the closing date of this offering occurs after ,
6
Table of Contents
2012, the bridge notes will continue to accrue interest at a rate of 6% per annum and additional shares of our common stock will be issued upon conversion of this additional accrued interest. Likewise, if the closing date occurs prior to , 2012, fewer shares will be issued upon conversion of the bridge notes.
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table summarizes our consolidated financial data. We have derived the following consolidated statements of operations data for the years ended December 31, 2009, 2010 and 2011, and the consolidated balance sheet data as of December 31, 2011 from our audited consolidated financial statements, included elsewhere in this prospectus. The consolidated statements of operations data for the three months ended March 31, 2011 and 2012 and the consolidated balance sheet data as of March 31, 2012 are derived from our unaudited consolidated financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. The following summary consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus.
| (in thousands, except share and per share amounts) | Year Ended December 31, | Three Months Ended March 31, | ||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||||||
Revenue | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
Cost of revenue | — | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Gross profit | — | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | 11,030 | 23,111 | 17,236 | 4,300 | 8,902 | |||||||||||||||
General and administrative | 1,909 | 2,693 | 8,162 | 1,361 | 2,077 | |||||||||||||||
Selling and marketing | 462 | 797 | 761 | 250 | 246 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total operating expenses | 13,401 | 26,601 | 26,159 | 5,911 | 11,225 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Loss from operations | (13,401 | ) | (26,601 | ) | (26,159 | ) | (5,911 | ) | (11,225 | ) | ||||||||||
Interest income | 39 | 43 | 28 | 4 | 4 | |||||||||||||||
Interest expense | (763 | ) | (1 | ) | (2,554 | ) | — | (1,040 | ) | |||||||||||
Other income (expense), net | 525 | 1,106 | (731 | ) | — | 375 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss | $ | (13,600 | ) | $ | (25,453 | ) | $ | (29,416 | ) | (5,907 | ) | (11,886 | ) | |||||||
Accretion of Series B preferred stock to redemption value | (78 | ) | — | — | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss attributable to common stockholders | $ | (13,678 | ) | $ | (25,453 | ) | $ | (29,416 | ) | $ | (5,907 | ) | $ | (11,886 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss per share attributable to common stockholders — basic and diluted(1) | $ | (15.24 | ) | $ | (10.13 | ) | $ | (10.29 | ) | $ | (2.07 | ) | $ | (4.16 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Weighted average shares of common stock outstanding used in computing net loss per share attributable to common stockholders — basic and diluted(1) | 897,239 | 2,512,320 | 2,858,251 | 2,858,251 | 2,858,251 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Pro forma net loss per share attributable to common stockholders — basic and diluted (unaudited)(1) | $ | $ | ||||||||||||||||||
|
|
|
| |||||||||||||||||
Weighted average shares of common stock outstanding used in computing the pro forma net loss per share attributable to common stockholders — basic and diluted(1) | ||||||||||||||||||||
|
|
|
| |||||||||||||||||
7
Table of Contents
| (1) | See Note 15 to our consolidated financial statements for an explanation of the method used to calculate basic and diluted net loss per share of common stock, the unaudited pro forma basic and diluted net loss per share of common stock and the weighted average number of shares used in computation of the per share amounts. |
| (in thousands) | As of March 31, 2012 | |||||||
| Actual | Pro Forma | Pro Forma as Adjusted | ||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||
Consolidated Balance Sheet Data: | ||||||||
| Cash and cash equivalents | $ | 3,736 | ||||||
| Working capital (deficit) | (32,889 | ) | ||||||
| Total assets | 5,014 | |||||||
| Convertible notes payable | 30,736 | |||||||
| Warrants liability | 3,464 | |||||||
| Convertible preferred stock | 58,326 | |||||||
| Total stockholders’ equity (deficit) | (93,864 | ) | ||||||
The unaudited pro forma column in the balance sheet data above gives effect to the following transactions and adjustments as if they had occurred as of March 31, 2012:
| (1) | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
| (2) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the April 2011 warrants and the May 2011 warrants into shares of our common stock, at an exercise price of $0.67 per share, and which will expire upon completion of this offering if not exercised; |
| (3) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants into shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants, at an exercise price of $1.58 per share, and which will expire upon completion of this offering if not exercised; |
| (4) | the automatic conversion of the bridge notes and accrued interest, into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012; |
| (5) | the reclassification of the bridge notes liability to common stock and additional paid-in-capital in connection with the conversion based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus; and |
| (6) | the reclassification of the bridge warrants liability to common stock and additional paid-in-capital in connection with the exercise based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus. |
8
Table of Contents
The unaudited pro forma as adjusted column in the balance sheet data above gives further effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale of the shares in this offering had occurred as of March 31, 2012.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders’ equity (deficit) by approximately $ million, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
9
Table of Contents
An investment in our common stock involves a high degree of risk. We operate in a dynamic and rapidly changing industry that involves numerous risks and uncertainties. The risks and uncertainties described below are not the only ones we face. Other risks and uncertainties, including those that we do not currently consider material, may impair our business. If any of the risks discussed below actually occur, our business, financial condition, operating results or cash flows could be materially adversely affected. This could cause the trading price of our common stock to decline, and you may lose all or part of your investment.
Risks Related to Development, Commercialization and Regulatory Approval
We depend substantially on the success of our only product candidate, Ravicti, and we may not obtain regulatory approval of Ravicti for the treatment of UCD or we may be unable to successfully commercialize it.
We have invested a significant portion of our efforts and financial resources in the development of Ravicti, which is currently our only product candidate. As a result, our business is substantially dependent on our ability to complete the development of, obtain regulatory approval for, and successfully commercialize Ravicti in a timely manner. The process to develop, obtain regulatory approval for and commercialize Ravicti is long, complex and costly.
The FDA has substantial discretion in the approval process and may form the opinion, after review of our data, that the NDA is insufficient to allow approval of Ravicti. The FDA may require that we conduct additional clinical, nonclinical, manufacturing validation or drug product quality studies and submit those data before it will consider or reconsider the NDA. Depending on the extent of these or any other studies, approval of any applications that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve the NDA. If any of these outcomes occur, we may not receive approval for Ravicti.
Even if we obtain FDA approval for Ravicti for the treatment of UCD, the approval might contain significant limitations related to use restrictions for certain age groups, warnings, precautions or contraindications, or may be subject to significant post-marketing studies or risk mitigation requirements. If we are unable to successfully commercialize Ravicti, we may not be able to earn sufficient revenues to continue our business.
Regulatory approval in UCD could be substantially delayed if the pediatric data we have submitted and intend to submit does not satisfy the FDA or if the FDA requires additional time or studies to assess the safety and efficacy of Ravicti.
In December 2011, we submitted an NDA for Ravicti for the chronic management of UCD in patients aged 6 years and above. Under PDUFA, the FDA is due to notify us regarding Ravicti’s approval status by October 23, 2012. The FDA is not under a binding obligation to respond to us by the PDUFA action date. The FDA does not always meet the PDUFA action date, and even when the FDA does, approval often requires more than one review cycle. If the FDA determines that additional data are required to support approval of Ravicti, it will issue a complete response letter outlining the deficiencies that must be addressed before the FDA will consider approval of the NDA. If the FDA issues a complete response letter to the NDA for Ravicti, approval of Ravicti to treat UCD will likely be delayed and may be denied completely.
In our pre-NDA meeting, the FDA expressed concern that pediatric patients constitute an important population of UCD patients, and indicated it may require a further evaluation of safety and dosing in certain pediatric UCD patients despite the legal exemption under the Pediatric Research Equity Act that orphan drugs such as Ravicti have from generally applicable pediatric testing requirements. We have submitted data which we believe demonstrate that the maximum concentration of phenylacetic acid, or PAA, in blood plasma in UCD
10
Table of Contents
patients aged 6 years and above treated with Ravicti has been significantly below the toxic range and similar to those observed by BUPHENYL. However, these data may not be sufficient to satisfy the FDA, particularly because the FDA expressed concerns specifically about PAA toxicity in pediatric patients.
We are currently conducting a clinical trial in UCD patients aged 29 days through 5 years designed to demonstrate the safety and efficacy in this patient population. The efficacy portion of this trial is complete and a complete study report was submitted to the FDA in April 2012; however, the data from the 12-month safety extension portion of the study will not be available until the second quarter of 2013. As part of the April update to the FDA, we submitted a revised draft package insert requesting approval of Ravicti for UCD patients down to 29 days of age. If the FDA classifies this submission as a major amendment, the PDUFA action date will likely be delayed.
Although we have entered into a Special Protocol Assessment agreement with the FDA relating to our pivotal Phase III trial of Ravicti, this agreement does not guarantee any particular outcome with respect to regulatory review of the pivotal trial or with respect to regulatory approval of Ravicti.
The protocol for our pivotal Phase III trial of Ravicti to treat UCD in adult patients was reviewed and agreed upon by the FDA under a Special Protocol Assessment agreement, or SPA, which allows for FDA evaluation of whether a clinical trial protocol could form the primary basis of an efficacy claim in support of an NDA. The SPA is an agreement that a Phase III trial’s design, clinical endpoints, patient population and statistical analyses are sufficient to support the efficacy claim. Agreement on an SPA is not a guarantee of approval, and there is no assurance that the design of, or data collected from, the trial will be adequate to obtain the requisite regulatory approval. In addition, the NDA currently requests approval of Ravicti in UCD patients aged 6 years and above; however, the SPA covers UCD in adult patients only. Further, the SPA is not binding on the FDA if public health concerns unrecognized at the time the SPA was entered into become evident or other new scientific concerns regarding product safety or efficacy arise. In addition, upon written agreement of both parties, the SPA may be changed, and the FDA retains significant latitude and discretion in interpreting the terms of an SPA and any resulting trial data. As a result, we do not know how the FDA will interpret the parties’ respective commitments under the SPA, how it will interpret the data and results from the pivotal Phase III trial, whether the FDA will require that we conduct or complete one or more additional clinical trials to support potential approval, including the completion of our ongoing clinical trial of Ravicti in pediatric patients aged 29 days through 5 years, or whether Ravicti will receive any regulatory approvals.
In June 2011, we completed a preclinical carcinogenicity study of Ravicti in rats, the results of which may delay or prevent approval of Ravicti.
In June 2011, we completed a 24-month carcinogenicity study of Ravicti in rats. The data from this study showed an increased rate of seven different tumor types in rats. While we do not have evidence that individuals who have taken the active ingredient in Ravicti have an increased rate of cancer, the FDA may view these data as posing concerns with respect to the long term safety of Ravicti. The FDA may request that we conduct additional nonclinical studies. If we are unable to explain these data to the satisfaction of the FDA, the approval of Ravicti may be delayed or denied.
The patient population suffering from UCD is small and has not been established with precision. If the actual number of patients is smaller than we estimate, if we are unable to convert patients from BUPHENYL to Ravicti or if any FDA approval is limited to adults only, our revenue and ability to achieve profitability may be adversely affected.
We estimate that the number of individuals in the United States with UCD is approximately 2,100, of which approximately 1,100 are currently diagnosed and approximately 425 are treated with BUPHENYL, and 90 are treated with Ravicti. Of these, we estimate that approximately 60% are children and 40% are adults. Our estimate
11
Table of Contents
of the size of the patient population is based on published studies as well as internal analyses. If the results of these studies or our analysis of them do not accurately reflect the number of patients with UCD, our assessment of the market may be inaccurate, making it difficult or impossible for us to meet our revenue goals, or to obtain and maintain profitability. In addition, if any FDA approval is limited to adult UCD patients, then the potential market for Ravicti will be smaller than we anticipate, our potential revenues will be limited and it will be more difficult to achieve profitability. Also, if we are unable to successfully convert patients from BUPHENYL to Ravicti, it will be more difficult to achieve profitability.
The number of patients in the United States who might be prescribed Ravicti if it is approved could be significantly less than the 515 currently estimated to be on Ravicti or BUPHENYL. Since Ravicti, BUPHENYL and AMMONUL target diseases with small patient populations, the per-patient drug pricing must be high in order to recover our development and manufacturing costs, fund adequate patient support programs and achieve profitability. We may be unable to maintain or obtain sufficient sales volume at a price high enough to justify our product development efforts and manufacturing expenses.
To obtain regulatory approval to market Ravicti in indications other than UCD, including HE, costly and lengthy nonclinical studies and clinical trials may be required, and the results of the studies and trials are highly uncertain.
As part of the regulatory approval process, we must conduct, at our own expense, nonclinical studies in the laboratory and in animals and clinical trials on humans for each indication that we intend to pursue. We expect the number of nonclinical studies and clinical trials that the regulatory authorities will require will vary depending on the disease or condition the drug is being developed to address and regulations applicable to the particular drug. Generally, the number and size of clinical trials required for approval varies based on the nature of the disease and size of the expected patient population that may be treated with a drug. We may need to perform multiple nonclinical studies using various doses and formulations before we can begin clinical trials, which could result in delays in our ability to market Ravicti for any additional indications, including HE. Furthermore, even if we obtain favorable results in nonclinical studies, the results in humans may be significantly different. After we have conducted nonclinical studies, we must demonstrate that our drug products are safe and efficacious for use in the targeted human patients in order to receive regulatory approval for commercial sale.
Serious adverse events or other safety risks could require us to abandon development and preclude or limit approval of Ravicti to treat UCD or HE.
We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants or if preliminary data demonstrate that the product is unlikely to receive regulatory approval or unlikely to be successfully commercialized. In addition, regulatory agencies, institutional review boards or data safety monitoring boards may at any time order the temporary or permanent discontinuation of our clinical trials or request that we cease using investigators in the clinical trials if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, or that they present an unacceptable safety risk to participants. If we elect or are forced to suspend or terminate a clinical trial of Ravicti to treat UCD or HE, the commercial prospects for Ravicti will be harmed and our ability to generate product revenues from Ravicti may be delayed or eliminated.
Even though we have received orphan drug designation, we may not receive orphan drug exclusivity for Ravicti.
As part of our business strategy, we have obtained orphan drug designation in the United States for glyceryl tri (4 phenylbutyrate), brand name Ravicti, for the maintenance treatment of patients with UCD and for the intermittent or chronic treatment of patients with cirrhosis and any grade of HE. In the United States, the company that first obtains FDA approval for a designated orphan drug for the specified rare disease or condition
12
Table of Contents
receives orphan drug marketing exclusivity for that drug for a period of seven years. This orphan drug exclusivity prevents the FDA from approving another application, including a full NDA, to market the same drug for the same orphan indication, except in very limited circumstances, including when the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active chemical entity and is intended for the same use as the drug in question. To obtain orphan drug exclusivity for a drug that shares the same active chemical entity as an already orphan designated drug, it must be demonstrated to the FDA that the drug is safer or more effective than the approved orphan designated drug, or that it makes a major contribution to patient care. In addition, a designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
In our case, Ravicti contains the same active chemical entity as BUPHENYL, which is approved for the treatment of UCD, the intended use for Ravicti. Ravicti was granted orphan designation for UCD based upon a potential safety benefit over BUPHENYL because of the absence of sodium. We will not receive orphan drug exclusivity in UCD unless the FDA in reviewing the NDA concludes that Ravicti is safer or more effective than BUPHENYL or makes a major contribution to patient care. Even if we obtain orphan drug exclusivity for Ravicti, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition.
Approval of Ravicti may require FDA approval of a companion diagnostic test, which would substantially delay FDA approval of Ravicti for UCD.
Our proposed labeling for Ravicti includes dose adjustment based on levels of urinary phenylacetylglutamine, or PAGN. Our plan is for the urinary PAGN testing to be available only as a Laboratory Developed Test that is commercialized by a laboratory certified under the Clinical Laboratory Improvement Amendments without approval or clearance from the FDA. Approval of all Laboratory Developed Tests is required by the State of New York prior to testing patient samples from that state. A test for urinary PAGN may be considered a companion diagnostic test by the FDA. We have not discussed our PAGN-based dosing adjustment labeling strategy with the FDA and do not know whether the FDA will accept a Laboratory Developed Test or instead will consider the test a companion diagnostic and therefore require a Premarket Approval Application, a filing through thede novo reclassification process, or 510(k) clearance for a urinary PAGN test, prior to approving Ravicti. If FDA approval or clearance of a urinary PAGN test is required, any approval and launch of Ravicti could be delayed and additional costs would be required for us to reach agreement with a clinical laboratory or a third-partyin vitro diagnostic test manufacturer to seek and obtain premarket approval,de novo reclassification, or premarket clearance from the FDA. The State of New York approval process, and the FDA premarket review process if required, can be lengthy and would require submission of clinical study data.
Our potential purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL could be hampered or prevented by regulatory action as well as by government or private litigation.
We are subject to antitrust review if we exercise our option to purchase Ucyclyd’s worldwide rights for BUPHENYL and AMMONUL, including, if the necessary jurisdictional thresholds are met at that time, review under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, or the HSR Act. Even if the planned purchase is approved, the terms and conditions of the approval that is granted, if accepted by the parties, may impose requirements, limitations, and additional costs and place restrictions on the conduct of our business. There is no assurance that we will receive the necessary approvals under the HSR Act or that any other conditions, terms, obligations, or restrictions sought to be imposed, and if accepted, would not have a material
13
Table of Contents
adverse effect on us. If the government challenges the purchase under the HSR Act and the challenge cannot be resolved by consent decree, our restated collaboration agreement with Ucyclyd will automatically terminate and we would not have any rights to BUPHENYL or AMMONUL. In addition, whether or not HSR filings are required to purchase Ucyclyd’s worldwide rights for BUPHENYL and AMMONUL, federal antitrust regulators could, before or after the purchase, take any action under the antitrust laws that they consider necessary or desirable in the public interest, including seeking to enjoin the purchase or to seek the divestiture of assets or the imposition of licensing obligations on us. Private parties as well as State Attorneys General and foreign antitrust regulators may also bring legal actions under the antitrust laws under some circumstances, the outcome of which could have a material adverse effect on us.
Even if the FDA approves Ravicti in the United States, we may never obtain approval for or commercialize Ravicti outside of the United States, which would limit our ability to realize its full market potential.
In order to market Ravicti outside of the United States, we must comply with regulatory requirements of, and obtain required regulatory approvals in, other countries. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and obtaining regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could require additional nonclinical studies or clinical trials, which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of Ravicti in those countries. We do not have any products approved for sale in any jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our products will be harmed.
If we obtain approval to commercialize Ravicti outside of the United States and continue to maintain the existing Ucyclyd distribution agreements for BUPHENYL and AMMONUL outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If Ravicti is approved for commercialization outside the United States, we will likely enter into agreements with third parties to market Ravicti outside the United States. In addition, if we purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we will assume Ucyclyd’s rights and obligations under its existing agreements for distribution of these drugs outside the United States, including Ucyclyd’s obligation to provide Swedish Orphan AB with a right of first refusal for the distribution of Ravicti and other newly developed products for urea cycle disorders on terms and conditions reasonably satisfactory to us. We expect that we will be subject to additional risks related to entering into or maintaining these international business relationships, including:
| • | different regulatory requirements for drug approvals in foreign countries; |
| �� | differing United States and foreign drug import and export rules; |
| • | reduced protection for intellectual property rights in foreign countries; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | different reimbursement systems; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
14
Table of Contents
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign taxes, including withholding of payroll taxes; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
| • | potential liability resulting from development work conducted by these distributors; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters. |
Even if we obtain regulatory approval of Ravicti and purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we will continue to face extensive development and regulatory requirements.
Even if a drug is FDA-approved, regulatory authorities may still impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-marketing studies. Furthermore, any new legislation addressing drug safety issues could result in delays or increased costs to assure compliance.
BUPHENYL and AMMONUL are, and if Ravicti is approved, Ravicti will be, subject to ongoing regulatory requirements for labeling, packaging, storage, advertising, promotion, sampling, record-keeping and submission of safety and other post-market information, including both federal and state requirements in the United States. In addition, manufacturers and manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP. As such, we and our contract manufacturers are subject to continual review and periodic inspections to assess compliance with cGMP. Accordingly, we and others with whom we work must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have FDA approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or disagrees with the promotion, marketing, or labeling of a product, a regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may:
| • | issue warning letters; |
| • | impose civil or criminal penalties; |
| • | suspend regulatory approval; |
| • | suspend any of our ongoing clinical trials; |
15
Table of Contents
| • | refuse to approve pending applications or supplements to approved applications submitted by us; |
| • | impose restrictions on our operations, including closing our contract manufacturers’ facilities; or |
| • | seize or detain products or require a product recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenues from Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected. Additionally, if we are unable to generate revenues from the sale of Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd, our potential for achieving profitability will be diminished and the capital necessary to fund our operations will be increased.
If third-party manufacturers fail to comply with manufacturing regulations, our financial results and financial condition will be adversely affected.
Before they can begin commercial manufacture of Ravicti, BUPHENYL or AMMONUL, contract manufacturers must obtain regulatory approval of their manufacturing facilities, processes and quality systems. In addition, pharmaceutical manufacturing facilities are continuously subject to inspection by the FDA and foreign regulatory authorities, before and after product approval. Due to the complexity of the processes used to manufacture pharmaceutical products and product candidates, any potential third-party manufacturer may be unable to continue to pass or initially pass federal, state or international regulatory inspections in a cost effective manner.
If a third-party manufacturer with whom we contract is unable to comply with manufacturing regulations, we may be subject to fines, unanticipated compliance expenses, recall or seizure of our products, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or civil prosecution. These possible sanctions would adversely affect our financial results and financial condition.
If our competitors are able to develop and market products that are preferred over Ravicti, BUPHENYL or AMMONUL, our commercial opportunity will be reduced or eliminated.
We face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions, which may in the future develop products to treat UCD or HE. During the lifetime of the United States patents covering Ravicti, and for any longer period of market exclusivity granted by the FDA for Ravicti, Ucyclyd and its affiliates are contractually prohibited from developing or commercializing new products, anywhere in the world, for the treatment of UCD or HE that are chemically similar to Ravicti, except for products delivered parenterally for the treatment of HE. In countries outside the United States, this contractual restriction will continue, on a country-by-basis, for the lifetime of patents covering Ravicti in each such country and for any longer period of regulatory exclusivity granted for Ravicti in each such country. Since this restriction only applies to specific indications and to products that are chemically similar to Ravicti, it may not prevent Ucyclyd or its affiliates from developing and commercializing products that compete with Ravicti. Moreover, products approved for indications other than UCD and HE may compete with Ravicti if physicians prescribe such products off-label for UCD or HE. Ucyclyd may develop and commercialize such products and, under the purchase agreement, we granted Ucyclyd a time-limited option to acquire the right to use and reference certain Ravicti data for the development and commercialization of products (other than Ravicti) for the treatment of a specific indication that we are not pursuing. Furthermore, unless and until we purchase Ucyclyd’s worldwide rights to BUPHENYL, Ucyclyd is allowed to continue to market and sell BUPHENYL, and its sales of BUPHENYL will continue to compete with our sales of Ravicti for UCD.
16
Table of Contents
In addition to competition from BUPHENYL, in November 2011 Ampolgen Pharmaceuticals, LLC received FDA approval for a generic version of sodium phenylbutrate tablets which may compete with Ravicti and BUPHENYL in treating UCD. We are also aware that Orphan Europe is conducting a clinical trial of carglumic acid to treat some of the UCD enzyme deficiencies for which we expect Ravicti to be approved. Carglumic acid is approved to treat HA crises resulting from a different rare disorder than UCD and is sold under the name Carbaglu. If the results of this trial are successful and Orphan Europe is able to complete development and obtain approval of Carbaglu to treat additional UCD enzyme deficiencies, we would face competition from this compound. In addition, if we complete development, obtain regulatory approval and commercialize Ravicti to treat HE, we will face competition from Salix Pharmaceuticals, Inc., the manufacturer of rifaximin, as well as generic manufacturers of lactulose. In addition to currently marketed treatments for HE, Ocera Therapeutics, Inc. has conducted two Phase II trials of one of their compounds to treat mild HE and is conducting a Phase II trial of a second compound delivered intravenously to patients with cirrhosis in which they are assessing ammonia control versus placebo. In addition, researchers are continually learning more about UCD and HE, and new discoveries may lead to new therapies. As a result, Ravicti, BUPHENYL and AMMONUL may be rendered less competitive, or even obsolete, at any time. Other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects, are more convenient or are less expensive than Ravicti, BUPHENYL and AMMONUL. We expect that our ability to compete effectively will depend upon, among other things, our ability to:
| • | successfully and rapidly complete clinical trials and obtain all requisite regulatory approvals in a timely and cost-effective manner; |
| • | maintain patent protection for Ravicti and otherwise prevent the introduction of generics of Ravicti, BUPHENYL and AMMONUL; |
| • | attract and retain key personnel; |
| • | build an adequate sales and marketing infrastructure; |
| • | obtain adequate reimbursement from third-party payors; and |
��
| • | maintain positive relationships with patient advocacy groups. |
The commercial success of Ravicti will depend upon the degree of market acceptance among physicians, patients, patient advocacy groups, health care payors and the medical community.
Ravicti may not gain market acceptance among physicians, patients, patient advocacy groups, health care payors and the medical community. The degree of market acceptance of Ravicti will depend on a number of factors, including:
| • | the effectiveness of Ravicti as compared with BUPHENYL; |
| • | the prevalence and severity of any side effects; |
| • | potential advantages over BUPHENYL or any generic versions of BUPHENYL; |
| • | the market price and patient out-of-pocket costs of Ravicti relative to BUPHENYL and other UCD treatment options, including any generics; |
| • | relative convenience and ease of administration; |
17
Table of Contents
| • | willingness by patients to stop using BUPHENYL and adopt Ravicti; |
| • | restriction on healthcare provider prescribing of and patient access to Ravicti due to a Risk Evaluation Mitigation Strategy, or REMS; |
| • | the strength of our marketing and distribution organizations; |
| • | the quality of our relationship with patient advocacy groups; and |
| • | sufficient third-party coverage or reimbursement. |
If we fail to achieve market acceptance of Ravicti in the United States, our revenue will be more limited and it will be more difficult to achieve profitability.
If we fail to obtain and sustain an adequate level of reimbursement for our products by third-party payors, sales would be adversely affected.
The course of treatment for UCD patients is and will continue to be expensive. We expect UCD patients to need treatment throughout their lifetimes. We expect that most families of patients will not be capable of paying for this treatment themselves. There will be no commercially viable market for Ravicti without reimbursement from third-party payors. Additionally, even if there is a commercially viable market, if the level of reimbursement is below our expectations, our revenue and gross margins will be adversely affected.
Third-party payors, such as government or private health care insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, drugs. Reimbursement rates from private health insurance companies vary depending on the company, the insurance plan and other factors. A current trend in the United States health care industry is toward cost containment. Large public and private payors, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and reimbursement levels for, particular treatments. Such third-party payors, including Medicare, are questioning the coverage of, and challenging the prices charged for medical products and services, and many third-party payors limit coverage of or reimbursement for newly approved health care products. In particular, third-party payors may limit the covered indications. Cost-control initiatives could decrease the price we might establish for products, which could result in product revenues being lower than anticipated. If the prices for our products decrease or if governmental and other third-party payors do not provide adequate coverage and reimbursement levels, our revenue and prospects for profitability will suffer. Reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis.
Reimbursement in the European Union must be negotiated on a country-by-country basis and in many countries the product cannot be commercially launched until reimbursement is approved. The negotiation process in some countries can exceed 12 months.
If Ravicti is approved to treat HE in the future, the cost of Ravicti to treat UCD may decline significantly, which could materially affect our UCD revenues.
Given the relative differences in the size of the affected patient population, the number of requests third-party payors receive to reimburse drugs for the treatment of HE is significantly greater than the number of requests for UCD. As a result, we will likely experience greater pricing pressure if Ravicti is approved by the FDA to treat HE than if it is only approved to treat UCD. We do not currently have a plan to differentiate the formulation of Ravicti for UCD and HE, nor can we guarantee success if we attempt to differentiate the formulations for UCD and HE. We expect the required dosing volume to be similar for UCD and HE, if Ravicti
18
Table of Contents
is approved for both indications. If Ravicti is approved by the FDA for HE after FDA approval and launch of the drug for UCD, we will need to significantly decrease the price for Ravicti from that established with respect to UCD in order to gain third-party reimbursement for broad use in HE patients. This would result in a significant decrease in revenues generated by the UCD patient population. We believe the Ravicti revenue potential for HE is much larger than for UCD; however, if the market for Ravicti in HE is significantly smaller than we anticipate, or if we are unsuccessful in any commercial launch of Ravicti for the treatment of HE, total Ravicti revenues may decrease significantly and we may be unable to achieve or maintain profitability. If the Ravicti price is decreased with the introduction of the drug for HE, we may need to decrease our UCD specialty pharmacy and patient support service offerings. This may result in lower UCD revenues due to fewer UCD patients electing to begin use of Ravicti and/or remain compliant.
If we are unable to establish a direct sales force in the United States, our business may be harmed.
We currently do not have an established sales organization. If Ravicti is approved by the FDA for commercial sale, we intend to market Ravicti directly to physicians in the United States through our own sales force. We will need to incur significant additional expenses and commit significant additional management resources to establish and train a sales force to market and sell Ravicti, and BUPHENYL and AMMONUL if we purchase Ucyclyd’s worldwide rights to those products. We may not be able to successfully establish these capabilities despite these additional expenditures. We will also have to compete with other pharmaceutical and life sciences companies to recruit, hire, train and retain sales and marketing personnel. In the event we are unable to successfully market and promote Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd, our business may be harmed.
If we fail to establish an effective distribution process utilizing specialty pharmacies our business may be adversely affected.
We do not currently have the infrastructure necessary for distributing pharmaceutical UCD products to patients. We intend to contract with a third-party logistics company to warehouse these products and distribute them to specialty pharmacies. A specialty pharmacy is a pharmacy that specializes in the dispensing of medications for complex or chronic conditions which require a high level of patient education and ongoing management. This distribution network will require significant coordination with our sales and marketing and finance organizations. Failure to secure contracts with a logistics company and specialty pharmacies could negatively impact the distribution of our UCD products, and failure to coordinate financial systems could negatively impact our ability to accurately report product revenue. If we are unable to effectively establish and manage the distribution process, the commercial launch and sales of our UCD products will be delayed or severely compromised and our results of operations may be harmed.
In addition, the use of specialty pharmacies involves certain risks, including, but not limited to, risks that these specialty pharmacies will:
| • | not provide us with accurate or timely information regarding their inventories, the number of patients who are using our UCD products, or complaints regarding those drugs; |
| • | not effectively sell or support our UCD products; |
| • | reduce their efforts or discontinue to sell or support our UCD products; |
| • | not devote the resources necessary to sell our UCD products in the volumes and within the time frames that we expect; |
| • | not comply with any requirements imposed on pharmacies through a REMS; |
19
Table of Contents
| • | be unable to satisfy financial obligations to us or others; or |
| • | cease operations. |
Any such failure may result in decreased product sales and lower product revenue, which would harm our business.
If we are found in violation of federal or state “fraud and abuse” laws, we may be required to pay a penalty and/or be suspended from participation in federal or state health care programs, which may adversely affect our business, financial condition and results of operation.
In the United States, we are subject to various federal and state health care “fraud and abuse” laws, including anti-kickback laws, false claims laws and other laws intended to reduce fraud and abuse in federal and state health care programs. The federal Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce the referral of business, including the purchase, order or prescription of a particular drug for which payment may be made under a federal health care program, such as Medicare or Medicaid. Under federal government regulations, some arrangements, known as safe harbors, are deemed not to violate the federal Anti-Kickback Statute. Although we seek to structure our business arrangements in compliance with all applicable requirements, these laws are broadly written, and it is often difficult to determine precisely how the law will be applied in specific circumstances. Accordingly, it is possible that our practices may be challenged under the federal Anti-Kickback Statute. False claims laws prohibit anyone from knowingly and willfully presenting or causing to be presented for payment to third-party payors, including government payors, claims for reimbursed drugs or services that are false or fraudulent, claims for items or services that were not provided as claimed, or claims for medically unnecessary items or services. Cases have been brought under false claims laws alleging that off-label promotion of pharmaceutical products or the provision of kickbacks has resulted in the submission of false claims to governmental health care programs. Under the Health Insurance Portability and Accountability Act of 1996, we are prohibited from knowingly and willfully executing a scheme to defraud any health care benefit program, including private payors, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and/or exclusion or suspension from federal and state health care programs such as Medicare and Medicaid and debarment from contracting with the U.S. government. In addition, private individuals have the ability to bring actions on behalf of the government under the federal False Claims Act as well as under the false claims laws of several states.
Many states have adopted laws similar to the federal anti-kickback statute, some of which apply to the referral of patients for health care services reimbursed by any source, not just governmental payors. In addition, California and a few other states have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America, or PhRMA, Code on Interactions with Healthcare Professionals. In addition, several states impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement we could be subject to penalties.
Neither the government nor the courts have provided definitive guidance on the application of fraud and abuse laws to our business. Law enforcement authorities are increasingly focused on enforcing these laws, and it is possible that some of our practices may be challenged under these laws. While we believe we have structured our business arrangements to comply with these laws, it is possible that the government could allege violations
20
Table of Contents
of, or convict us of violating, these laws. If we are found in violation of one of these laws, we could be required to pay a penalty and could be suspended or excluded from participation in federal or state health care programs, and our business, financial condition and results of operations may be adversely affected.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and may affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-marketing activities and affect our ability to profitably sell our products for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly by establishing Medicare Part D and introduced a new reimbursement methodology based on average sales prices for physician-administered drugs under Medicare Part B. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class under the new Part D program. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement rate that we receive for any of our approved products. While the Medicare Modernization Act only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively PPACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. PPACA increased manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate amount for both branded and generic drugs and revised the definition of “average manufacturer price,” or AMP, which may also increase the amount of Medicaid drug rebates manufacturers are required to pay to states. The legislation also expanded Medicaid drug rebates, which previously had been payable only on fee-for-service utilization, to Medicaid managed care utilization, and created an alternative rebate formula for certain new formulations of certain existing products that is intended to increase the rebates due on those drugs. The Centers for Medicare and Medicaid Services, which administers the Medicaid Drug Rebate Program, also has proposed to expand Medicaid rebates to the utilization that occurs in the territories of the United States, such as Puerto Rico and the Virgin Islands. Also effective in 2010, the new law expanded the types of entities eligible to receive discounted 340B pricing, although, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. Further, beginning in 2011, PPACA imposed a significant annual fee on companies that manufacture or import branded prescription drug products and requires manufacturers to provide a 50% discount off the negotiated price of prescriptions filled by beneficiaries in the Medicare Part D coverage gap, referred to as the “donut hole”. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners. For example, beginning in 2013 pharmaceutical companies will be required to track and report to the federal government certain payments made to physicians and teaching hospitals in the preceding year. We will not know the full effects of PPACA until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of PPACA, the new law appears likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
21
Table of Contents
Legislative and regulatory proposals have been introduced at both the state and federal level to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the United States Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. Furthermore, the concerns raised by patients, patient advocacy groups and congressional representatives about the recent pricing of orphan drugs, could result in changes to the Orphan Drug Act or limitations in the approval pathway or pricing and reimbursement of orphan drugs.
Risks Related to Our Financial Position and Need for Additional Capital
We currently have no source of revenue and may never become profitable.
We are a development stage biopharmaceutical company with a limited operating history. Our ability to generate revenue and become profitable depends upon our ability to successfully complete the development of Ravicti for the chronic management of UCD and obtain the necessary regulatory approvals for Ravicti. We have generated no revenue in the last three years. Even if we receive regulatory approval for Ravicti and purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we do not know when our UCD products will generate revenue for us, if at all. Our ability to generate product revenue depends on a number of factors, including our ability to:
| • | successfully complete clinical and nonclinical development, and receive FDA approval, for Ravicti for the chronic management of UCD; |
| • | purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL; |
| • | set an acceptable price for our products; |
| • | obtain commercial quantities of our UCD products at acceptable cost levels; |
| • | obtain adequate reimbursement from third-party payors; |
| • | successfully market and sell our UCD products in the United States; |
| • | delay the introduction of generic versions of our UCD products; |
| • | maintain our licenses or sublicenses to intellectual property rights to Ravicti; and |
| • | maintain existing distribution agreements for BUPHENYL and AMMONUL outside the United States. |
In addition, because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses, or when, or if, we will be able to achieve or maintain profitability. For example, if the FDA requires us to complete the 12-month safety portion of the study in pediatric patients aged 29 days through 5 years and present the data before the FDA will consider approving the NDA for Ravicti in any patients, our ability to generate revenue may be substantially delayed. In addition, our expenses could increase beyond expectations if we are required by the FDA to perform studies in addition to those that we currently anticipate. Even if Ravicti is approved for commercial sale and we purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we anticipate incurring significant costs associated with the commercial launch of these products.
22
Table of Contents
Even if we are able to generate revenues from the sale of our products, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
We have incurred net losses since inception and anticipate that we will continue to incur net losses for the foreseeable future.
We have incurred losses in each year since our inception on November 1, 2006. Our losses were $13.6 million in 2009, $25.5 million in 2010, $29.4 million in 2011 and $11.9 million for the three months ended March 31, 2012. As of March 31, 2012, we had a deficit accumulated during the development stage of $118.6 million. We have devoted most of our financial resources to research and development, including our nonclinical development activities and clinical trials. To date, we have financed our operations primarily through the sale of equity securities and debt. Ravicti will require the completion of regulatory review, significant marketing efforts and substantial investment before it can provide us with any revenue. We expect our research and development expenses to continue to be significant in connection with our ongoing and planned clinical trials for Ravicti and any other clinical trials or nonclinical testing that we may initiate. In addition, we expect to incur increased sales and marketing expenses. As a result, we expect to continue to incur significant and increasing operating losses and negative cash flows for the foreseeable future. These losses have had and will continue to have an adverse effect on our stockholders’ deficit and working capital.
We may need to obtain additional financing to fund our operations.
We may need to obtain additional financing to fund our future operations, including the development and commercialization of Ravicti, the purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL pursuant to the restated collaboration agreement, and supporting sales and marketing activities related to Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd. We would likely need to obtain additional financing to conduct a Phase III trial in HE, for additional studies for the approval of Ravicti in UCD if requested by the FDA, and for development of any additional product candidates we might acquire. Moreover, our fixed expenses such as rent, license payments, interest expense and other contractual commitments are substantial and are expected to increase in the future.
Our future funding requirements will depend on many factors, including, but not limited to:
| • | our ability to successfully commercialize Ravicti for the treatment of UCD, and BUPHENYL and AMMONUL if purchased from Ucyclyd; |
| • | the amount of sales and other revenues from products that we may commercialize, if any, including the selling prices for such products and the availability of adequate third-party reimbursement; |
| • | selling and marketing costs associated with our UCD products, including the cost and timing of expanding our marketing and sales capabilities and establishing a network of specialty pharmacies; |
| • | the progress, timing, scope and costs of our nonclinical studies and clinical trials, including the ability to timely enroll patients in our planned and potential future clinical trials; |
| • | the time and cost necessary to obtain regulatory approvals and the costs of post-marketing studies that may be required by regulatory authorities; |
| • | the costs of obtaining clinical and commercial supplies of Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd; |
23
Table of Contents
| • | payments of milestones and royalties to third parties, including Ucyclyd; |
| • | cash requirements of any future acquisitions of product candidates; |
| • | the time and cost necessary to respond to technological and market developments; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| • | any changes made to, or new developments in, our restated collaboration agreement with Ucyclyd or any new collaborative, licensing and other commercial relationships that we may establish. |
Until we can generate a sufficient amount of revenue, we expect to finance future cash needs through public or private equity offerings or debt financings. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available, we may be required to delay or reduce the scope of or eliminate one or more of our research or development programs or our commercialization efforts. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time.
We believe that our current cash and cash equivalents, the net proceeds from this offering, as well as potential payments from Ucyclyd beginning January 1, 2013 if Ravicti is not approved by the FDA prior to that, will be sufficient to fund our operations through the commercial launch of Ravicti in UCD, assuming commercialization occurs in the first half of 2013. We have based this estimate on a number of assumptions that may prove to be wrong, and changing circumstances beyond our control may cause us to consume capital more rapidly than we currently anticipate. For example, if the FDA requires us to complete the 12-month safety portion of the study in pediatric patients aged 29 days through 5 years and present the data before the FDA will consider approving the NDA for Ravicti in any patients, our ability to generate revenue may be substantially delayed. Pursuant to the restated collaboration agreement, if the Ravicti NDA for UCD is not approved by January 1, 2013, then Ucyclyd is obligated to make monthly payments of $0.5 million to us until the earliest of (1) FDA approval of the Ravicti NDA for UCD, (2) June 30, 2013, and (3) our written notification of our decision not to purchase BUPHENYL and AMMONUL. Our inability to obtain additional funding when we need it could seriously harm our business.
We might be unable to service our potential loan from Ucyclyd due to a lack of cash flow and might be subject to default.
Under the terms of our restated collaboration agreement, we have an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed upfront purchase price, with additional payments for regulatory milestones, net sales milestones and royalties. If we exercise this option, Ucyclyd has a time-limited right to retain ownership of AMMONUL by paying us a predefined price. If Ucyclyd exercises its right to retain AMMONUL, then the upfront purchase price for Ucyclyd’s worldwide rights to BUPHENYL will be offset against the amount due to us from Ucyclyd, resulting in a net payment to us of $13.0 million upon closing of our purchase of BUPHENYL. If Ucyclyd does not exercise its right to retain AMMONUL, we will owe Ucyclyd a payment of $22.0 million upon closing of our purchase of BUPHENYL and AMMONUL. To fund this upfront purchase price, we may draw on a loan commitment from Ucyclyd. The loan, which would be repayable in eight quarterly payments, would be secured by the BUPHENYL and AMMONUL assets and carry a 9% annual interest rate. Any default under the loan security agreement and resulting foreclosure would have a material adverse effect on our financial condition and our ability to continue our operations. For example, if we do not make the required payments when due, if we breach the note or the security agreement related to the note or if we become bankrupt, Ucyclyd could elect to declare all amounts outstanding to be immediately due and payable. Even if we were able to repay the full amount due in cash, any such repayment could leave us with little
24
Table of Contents
or no working capital for our business. If we are unable to repay the full amount due, Ucyclyd would have a first claim on our assets pledged under the loan security agreement and we could lose our rights to BUPHENYL and AMMONUL. If Ucyclyd should attempt to foreclose on the collateral, it is possible that there would be no assets remaining after repayment in full of such secured indebtedness.
We may sell additional equity or debt securities to fund our operations, which may result in dilution to our stockholders and impose restrictions on our business.
In order to raise additional funds to support our operations, we may sell additional equity or debt securities, which would result in dilution to all of our stockholders or impose restrictive covenants that adversely impact our business. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we are unable to expand our operations or otherwise capitalize on our business opportunities, our business, financial condition and results of operations could be materially adversely affected.
Our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2011. We have no current source of revenue to sustain our present activities, and we do not expect to generate revenue until, and unless, we receive regulatory approval of and successfully commercialize Ravicti, or purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL. Accordingly, our ability to continue as a going concern will require us to obtain additional financing to fund our operations. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
Risks Related to Our Reliance on Third Parties
We have no manufacturing capacity and anticipate continued reliance on third-party manufacturers for the development and commercialization of our products.
We do not currently operate manufacturing facilities for clinical or commercial production of Ravicti, BUPHENYL or AMMONUL. We have no experience in drug formulation, and we lack the resources and the capabilities to manufacture Ravicti, BUPHENYL or AMMONUL on a clinical or commercial scale. We do not intend to develop facilities for the manufacture of products for clinical trials or commercial purposes in the foreseeable future. We rely on third-party manufacturers to produce bulk drug substance and drug products required for our clinical trials. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of our drug product candidates if and when approved for marketing by the applicable regulatory authorities. We have clinical supplies of Ravicti manufactured for us by two drug substance suppliers, Helsinn Chemicals SA, or Helsinn, and DSM Fine Chemicals Austria Nfg GmbH, or DSM, on a purchase order basis. We have included both Helsinn and DSM as suppliers of drug substance in the Ravicti NDA. However, neither of our contract manufacturers has completed process validation for the drug substance manufacturing process. If neither contract manufacturers are approved by the FDA, our commercial supply of drug substance will be significant delayed and may result in significant additional costs. We purchase finished Ravicti drug product from Lyne Laboratories, Inc., or Lyne, on a purchase order basis in accordance with a clinical supply agreement. We do not have an agreement in place for, and we have not identified, a secondary fill/finish supplier. If we need to identify an additional fill/finish manufacturer,
25
Table of Contents
we would not be able to do so without significant delay and likely significant additional cost. We have not secured commercial supply agreements with any contract manufacturers and can give no assurance that we will enter commercial supply agreements with any contract manufacturers on favorable terms or at all.
Our contract manufacturers’ failure to achieve and maintain high manufacturing standards, in accordance with applicable regulatory requirements, or the incidence of manufacturing errors, could result in patient injury or death, product shortages, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously harm our business. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. For example, we recently discovered a contaminated lot of Ravicti, which we believe was caused by a failure in a filtration step by one of our third-party drug substance manufacturers. As a result, we have a limited commercial supply of Ravicti, and we will need to manufacture another lot, which could cause a delay in the commercial launch of Ravicti.
Our existing manufacturers and any future contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business. In the event of a natural disaster, business failure, strike or other difficulty, we may be unable to replace a third-party manufacturer in a timely manner and the production of our UCD products would be interrupted, resulting in delays and additional costs.
In addition, because our contract manufacturers of the bulk drug substance are located outside of the United States, we may face difficulties in importing our UCD products into the United States as a result of, among other things, FDA import inspections, incomplete or inaccurate import documentation or defective packaging.
Some of the intellectual property necessary for the commercialization of our UCD products is or will be licensed from third parties, which will require us to pay milestones and royalties.
Ucyclyd has granted us a license to use some of the technology developed by Ucyclyd in connection with the manufacturing of Ravicti. The purchase agreement under which we purchased the worldwide rights to Ravicti further obligates us to pay Ucyclyd regulatory and sales milestone payments relating to Ravicti, as well as royalties on the net sales of Ravicti. If we purchase BUPHENYL and AMMONUL under the restated collaboration agreement with Ucyclyd, we will also receive a license to use some of the manufacturing technology developed by Ucyclyd in connection with the manufacturing of these products. The restated collaboration agreement will obligate us to pay Ucyclyd regulatory and sales milestone payments, as well as royalties on net sales of these products.
We may become obligated to make a milestone or royalty payments when we do not have the cash on hand to make these payments, or have budgeted cash for our development efforts. This could cause us to delay our development efforts, curtail our operations, scale back our commercialization and marketing efforts or seek additional capital to meet these obligations, which could be on terms unfavorable to us. Additionally, if we fail to make a required payment to Ucyclyd and do not cure the failure with the required time period, Ucyclyd may be able to terminate our license to use its manufacturing technology for our UCD products.
We also license intellectual property necessary for commercialization of Ravicti from Brusilow Enterprises, LLC, or Brusilow. Brusilow may be entitled to terminate our license if we breach that agreement or do not meet specified diligence obligations in our development and commercialization of Ravicti and do not cure the failure within the required time period. If our license from Brusilow is terminated, it may be difficult or impossible for us to commercialize Ravicti.
26
Table of Contents
Termination of the restated collaboration agreement prior to our purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL would result in our losing rights to these products.
If the restated collaboration agreement terminates before closing of our purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we would lose our rights to these products and would be unable to generate any revenue from these products. The restated collaboration agreement will automatically terminate if any of the following events occur:
| • | we fail to exercise the option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL during the required time period; |
| • | after we exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we are unable to resolve a challenge to our purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL from the Federal Trade Commission or Antitrust Division of the Department of Justice; or |
| • | after we exercise the option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, we do not consummate the purchase within the required time period. |
Although we anticipate exercising our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL in the future, we have no control over Ucyclyd’s conduct of the BUPHENYL and AMMONUL business in the intervening time period.
Under the restated collaboration agreement, we will be permitted to exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL for a period of 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. Between now and the time that we can exercise our option, Ucyclyd has full control over the commercialization of BUPHENYL and AMMONUL, and we are entirely dependent on Ucyclyd to preserve the value of the businesses related to these products. If the value of the BUPHENYL and AMMONUL businesses decreases significantly, we may decide not to exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, in which case we would be unable to generate any revenue from these products.
Any collaboration arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our current and potential future product candidates.
We may seek collaboration arrangements with pharmaceutical or biotechnology companies for the development or commercialization of our current and potential future product candidates. We may enter into these arrangements on a selective basis depending on the merits of retaining commercialization rights for ourselves as compared to entering into selective collaboration arrangements with leading pharmaceutical or biotechnology companies for each product candidate, both in the United States and internationally. We will face, to the extent that we decide to enter into collaboration agreements, significant competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements should we so chose to enter into such arrangements. The terms of any collaborations or other arrangements that we may establish may not be favorable to us.
Any future collaborations that we enter into may not be successful. The success of our collaboration arrangements will depend heavily on the efforts and activities of our collaborators. Collaborators generally have significant discretion in determining the efforts and resources that they will apply to these collaborations.
Disagreements between parties to a collaboration arrangement regarding clinical development and commercialization matters, can lead to delays in the development process or commercializing the applicable
27
Table of Contents
product candidate and, in some cases, termination of the collaboration arrangement. These disagreements can be difficult to resolve if neither of the parties has final decision making authority.
Collaborations with pharmaceutical or biotechnology companies and other third parties often are terminated or allowed to expire by the other party. Any such termination or expiration would adversely affect us financially and could harm our business reputation.
We currently depend on third parties to conduct some of the operations of our clinical trials, and depend on Ucyclyd to supply BUPHENYL for our clinical uses in connection with the development of, and application for regulatory approval of, Ravicti.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to oversee some of the operations of our clinical trials and to perform data collection and analysis. As a result, we may face additional delays outside of our control if these parties do not perform their obligations in a timely fashion or in accordance with regulatory requirements. If these third parties do not successfully carry out their contractual duties or obligations and meet expected deadlines, if they need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our financial results and the commercial prospects for Ravicti or our other potential product candidates could be harmed, our costs could increase and our ability to obtain regulatory approval and commence product sales could be delayed.
Ucyclyd currently supplies us with BUPHENYL under a clinical supply agreement effective as of January 31, 2008 and amended on March 22, 2012, for our clinical activities in connection with the development of and regulatory approval for Ravicti. This contractual obligation for Ucyclyd to supply us with BUPHENYL will continue in effect through the period of our option to purchase Ucyclyd’s worldwide rights to BUPHENYL under the restated collaboration agreement and through closing of the purchase, or if we elect not to exercise the option then the clinical supply ends upon expiration of the 90-day option period. If Ucyclyd does not successfully carry out its contractual obligations and meet our requirements for clinical supply of BUPHENYL, then our development and clinical activities with respect to Ravicti may be compromised.
Risks Related to Our Intellectual Property
We may not be able to protect our proprietary technology in the marketplace.
Where appropriate, we seek patent protection for certain aspects of our technology. Patent protection may not be available for some of the products or technology we are developing. If we must spend significant time and money protecting or enforcing our patents, designing around patents held by others or licensing, potentially for large fees, patents or other proprietary rights held by others, our business and financial prospects may be harmed. We may not develop additional proprietary products which are patentable.
The patent positions of pharmaceutical products are complex and uncertain. The scope and extent of patent protection for Ravicti and our future products and product candidates are particularly uncertain. Publication of information related to Ravicti and our future products and product candidates may prevent us from obtaining or enforcing patents relating to these products and product candidates, including without limitation composition-of-matter patents, which are generally believed to offer the strongest patent protection.
We have licensed patents in the United States and in certain foreign jurisdictions related to Ravicti, including U.S. Patent 5,968,979, which covers the composition of matter of Ravicti, which we license from Brusilow. Our Brusilow license may be terminated if we do not comply with the terms of the applicable license. Patents that we own or license do not ensure the protection of our intellectual property for a number of reasons, including without limitation the following:
| • | our patents may not be broad or strong enough to prevent competition from other products including identical or similar products; |
28
Table of Contents
| • | U.S. Patent 5,968,979 covering Ravicti composition of matter expires February 7, 2015, unless its term is successfully extended; |
| • | upon expiration of U.S. Patent 5,968,979, we do not at this time own or control a granted U.S. Patent that prevents generic entry into the United States market for Ravicti; |
| • | we may be required to disclaim part of the term of one or more patents; |
| • | there may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim; |
| • | there may be prior art of which we are aware, which we do not believe affects the validity or enforceability of a patent claim, but which, nonetheless ultimately may be found to affect the validity or enforceability of a patent claim; |
| • | there may be other patents existing in the patent landscape for Ravicti that will affect our freedom to operate; |
| • | if our patents are challenged, a court could determine that they are not valid or enforceable; |
| • | a court could determine that a competitor’s technology or product does not infringe our patents; and |
| • | our patents could irretrievably lapse due to failure to pay fees or otherwise comply with regulations, or could be subject to compulsory licensing. |
As a result of our purchase of the worldwide rights to Ravicti, we also own several pending patent applications in the United States and in foreign jurisdictions relating to methods of using, administering, and adjusting the dosage of Ravicti. These applications do not ensure the protection of our intellectual property. Additionally, these pending applications may not issue or may issue with claims significantly narrower than we currently seek. Unless and until our pending applications issue, their protective scope is impossible to determine, and even after issuance their protective scope may be limited. For example, we may not have developed a method for determining dosing for Ravicti before others developed identical, similar methods or distinct methods, in which case we may not receive a granted patent or any granted patent may not cover potential competition.
If we encounter delays in our development or clinical trials, the period of time during which we could market our products under patent protection would be reduced.
Additional competitors could enter the market, including with generic versions of our products, and sales of affected products may decline materially.
The Ravicti composition of matter patent expires in the United States in 2015. Based on current projections, we expect to receive an extension of this patent under the Drug Price Competition and Patent Term Restoration Act, or Hatch-Waxman Amendments, which we expect to extend this patent coverage for approximately an additional three years.
We own a first set of pending patent applications in the United States, Europe, Japan, and Canada, and a second set of pending patent applications in the United States and internationally pursuant to the Patent Cooperation Treaty, or PCT. These applications are directed to methods of using, administering, and adjusting the effective dosage of Ravicti. If granted, these applications could extend market protection until 2029 to 2032; however, there is a significant risk that these applications will not issue timely, or that they may not issue at all. In particular, claims directed to dosing and dose adjustment may be substantially less likely to issue in light of
29
Table of Contents
the recent Supreme Court decision inMayo Collaborative Services v. Prometheus Laboratories, Inc. InMayo, the Court held that claims directed to methods of determining whether to adjust drug dosing levels based on drug metabolite levels in the blood were not patent eligible because they were directed to a law of nature. This decision may have wide-ranging implications on the validity and scope of pharmaceutical method claims, although its full impact will not be known for many years.
Ravicti holds orphan drug designation for UCD; however, we cannot guarantee that orphan drug exclusivity, and the associated seven years of market exclusivity, will be granted.
Under the Hatch-Waxman Act, a pharmaceutical manufacturer may file an abbreviated new drug application, or ANDA, seeking approval of a generic copy of an approved innovator product. Under the Hatch-Waxman Act, a manufacturer may also submit an NDA under section 505(b)(2) that references the FDA’s prior approval of the innovator product. A 505(b)(2) NDA product may be for a new or improved version of the original innovator product.
Hatch-Waxman also provides for certain periods of regulatory exclusivity, which preclude FDA approval (or in some circumstances, FDA filing and reviewing) of an ANDA or 505(b)(2) NDA. These include, subject to certain exceptions, the period during which an FDA-approved drug is subject to orphan drug exclusivity. In addition to the benefits of regulatory exclusivity, an innovator NDA holder may have patents claiming the active ingredient, product formulation or an approved use of the drug, which would be listed with the product in the FDA publication, “Approved Drug Products with Therapeutic Equivalence Evaluations,” known as the “Orange Book.” If there are patents listed in the Orange Book, a generic or 505(b)(2) applicant that seeks to market its product before expiration of the patents must include in the ANDA what is known as a “Paragraph IV certification,” challenging the validity or enforceability of, or claiming non-infringement of, the listed patent or patents. Notice of the certification must be given to the innovator, too, and if within 45 days of receiving notice the innovator sues to protect its patents, approval of the ANDA is stayed for 30 months, or as lengthened or shortened by the court.
We anticipate that, if approved, Ravicti will qualify for a three-year period of exclusivity, based on the fact that data from clinical trials with the product will be necessary to obtain approval. That exclusivity would mean that, even in the absence of any patent protection, FDA could not grant final approval to an ANDA for a generic version of Ravicti until three years after approval of Ravicti. It would not delay a generic competitor submitting an ANDA, or the FDA reviewing it, or granting it “tentative approval.” The exclusivity would also prohibit FDA from approving a 505(b)(2) NDA that references FDA’s approval of Ravicti or includes the same active ingredient and uses as Ravicti.
Accordingly, competitors could file ANDAs for generic versions of Ravicti, or 505(b)(2) NDAs that reference Ravicti, immediately after approval of an NDA for Ravicti, and if there are patents listed for Ravicti in the Orange Book, those ANDAs and 505(b)(2) NDAs would be required to include a certification as to each listed patent indicating whether the ANDA applicant does or does not intend to challenge the patent. We cannot predict whether Ravicti will be approved or, if approved, whether it will be granted any regulatory exclusivity, or the scope of that exclusivity. We also cannot predict whether any patents issuing from our pending patent applications will be eligible for listing in the Orange Book, how any generic competitor would address such patents, whether we would sue on any such patents, or the outcome of any such suit.
The composition of matter patent and orphan drug exclusivity for BUPHENYL have expired. Because BUPHENYL has no regulatory exclusivity or listed patents, a competitor could at any time submit an ANDA for a generic version of BUPHENYL and request immediate approval. We are aware of one ANDA for BUPHENYL tablets which was approved in the fourth quarter of 2011. The ANDA process is a confidential one, so there may be other BUPHENYL ANDAs pending.
We own a first set of patent applications in the United States, Europe, Japan, and Canada and a second set of patent applications in the United States and internationally pursuant to the PCT. The applications directed to
30
Table of Contents
methods of using, administering, and adjusting the dosage of BUPHENYL. If granted, these applications could extend market protection until 2029 to 2032; however, there is a significant risk that these applications will not issue timely, or that they may not issue at all. In particular, claims directed to dosing and dose adjustment may be substantially less likely to issue in light of the recent Supreme Court decision inMayo. This decision may have wide-ranging implications on the validity and scope of pharmaceutical method claims, although its full impact will not be known for many years. Moreover, even if granted these applications may not provide protection sufficient to protect against the use of generic forms of BUPHENYL.
In the absence of any additional patent protection or even if U.S. Patents issued from our pending patent applications, a competitor may seek and obtain FDA approval for, and subsequently sell, a generic version of BUPHENYL. For example, in November 2011, FDA approved a generic version of BUPHENYL tablets. Such a generic product may be priced at a discount to our branded BUPHENYL and Ravicti, and physicians, patients, or payors may decide that this less expensive alternative is preferable to either of our drugs. If this occurs, our UCD product sales could be materially reduced, but we would nevertheless be required to make royalty payments to Ucyclyd and Brusilow at the same royalty rates.
Although AMMONUL also has no patents listed in the Orange Book, it was the subject of orphan drug exclusivity that expired in February 2012, which means that the FDA can approve a generic version of AMMONUL at any time.
We may not be successful in securing or maintaining proprietary patent protection for products we currently market or for products and technologies we develop or license. Moreover, if any patents that are granted and listed in the Orange Book are successfully challenged by way of a Paragraph IV certification and subsequent litigation, the affected product could more immediately face generic competition and its sales would likely decline materially. Should sales decline, we may have to write off a portion or all of the intangible assets associated with the affected product and our results of operations and cash flows could be materially and adversely affected.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to life sciences. This could make it difficult for us to stop the infringement of our in-licensed patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. For example, there could be issued patents of which we are not aware that our products infringe. There also could be patents that we believe we do not infringe, but that we may
31
Table of Contents
ultimately be found to infringe. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes.
Third parties may assert that we are employing their proprietary technology without authorization. If a court held that any third-party patents are valid, enforceable and cover our products or their use, the holders of any of these patents may be able to block our ability to commercialize our products unless we obtained a license under the applicable patents, or until the patents expire. We may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost or on reasonable terms. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary know-how and technological advances, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. Failure to obtain or maintain trade secret protection could enable competitors to use our proprietary information to develop products that compete with our products or cause additional, material adverse effects upon our competitive business position.
Any lawsuits relating to infringement of intellectual property rights necessary to defend ourselves or enforce our rights will be costly and time consuming.
Our ability to defend our intellectual property may require us to initiate litigation to enforce our rights or defend our activities in response to alleged infringement of a third party. In addition, we may be sued by others who hold intellectual property rights who claim that their issued patents are infringed by Ravicti or any future products, including BUPHENYL or AMMONUL, or product candidates. These lawsuits can be very time consuming and costly. There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries generally.
In addition, our patents and patent applications, or those of our licensors, could face other challenges, such as interference proceedings, opposition proceedings, and re-examination proceedings. Any of these challenges, if successful, could result in the invalidation of, or in a narrowing of the scope of, any of our patents and patent applications subject to challenge. Any of these challenges, regardless of their success, would likely be time consuming and expensive to defend and resolve and would divert our management’s time and attention.
Risks Related to Our Business Operations and Industry
We depend upon our key personnel and our ability to attract and retain employees.
Our future growth and success depend on our ability to recruit, retain, manage and motivate our employees. The loss of the services of any member of our senior management or the inability to hire or retain experienced management personnel could adversely affect our ability to execute our business plan and harm our operating results.
32
Table of Contents
Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. In particular, the loss of one or more of our senior executive officers could be detrimental to us if we cannot recruit suitable replacements in a timely manner. We do not currently carry “key person” insurance on the lives of members of senior management. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense competition, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Failure to build our finance infrastructure and improve our accounting systems and controls could impair our ability to comply with the financial reporting and internal controls requirements for publicly traded companies.
As a public company, we will operate in an increasingly challenging regulatory environment which requires us to comply with the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, and the related rules and regulations of the Securities and Exchange Commission, or SEC, expanded disclosures, accelerated reporting requirements and more complex accounting rules. Company responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. We will be required to disclose material changes made in our internal controls and procedures on a quarterly basis. However, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until the later of the year following our first annual report required to be filed with the SEC or the date we are no longer an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, because we are taking advantage of the exemptions contained in the JOBS Act.
To build this infrastructure, we will need to hire additional accounting personnel and improve our accounting systems, disclosure policies, procedures and controls. We are currently in the process of:
| • | initiating our plans to upgrade our computer systems, including hardware and software; |
| • | establishing written policies and procedures; and |
| • | enhancing internal controls and our financial statement review process. |
If we are unsuccessful in building an appropriate accounting infrastructure, we may not be able to prepare and disclose, in a timely manner, our financial statements and other required disclosures, or comply with existing or new reporting requirements. If we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed and investors could lose confidence in our reported financial information.
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
We are a small company with 14 employees as of March 31, 2012. In order to commercialize our products, we will need to substantially increase our operations, including expanding our employee base of managerial, operational and financial personnel. Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
| • | manage our clinical trials and the regulatory process effectively; |
| • | manage the manufacturing of products for commercial and clinical use; |
33
Table of Contents
| • | integrate current and additional management, administrative, financial and sales and marketing personnel; |
| • | hire new personnel necessary to effectively commercialize product candidates we license; |
| • | develop our administrative, accounting and management information systems and controls; and |
| • | hire and train additional qualified personnel. |
Product candidates that we may acquire in the future may be intended for patient populations that are significantly larger than those for UCD and HE. In order to continue development and marketing of these products, if approved, we would need to significantly expand our operations. Our staff, financial resources, systems, procedures or controls may be inadequate to support our operations and our management may be unable to manage successfully future market opportunities or our relationships with customers and other third parties.
If we engage in acquisitions, we will incur a variety of costs and we may never realize the anticipated benefits of such acquisitions.
We may attempt to acquire businesses, technologies, services, products or product candidates that we believe are a strategic fit with our business. We have no present agreement regarding any material acquisitions other than the restated collaboration agreement, under which we have an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL. However, if we do undertake any acquisitions, the process of integrating an acquired business, technology, service, products or product candidates into our business may result in unforeseen operating difficulties and expenditures, including diversion of resources and management’s attention from our core business. In addition, we may fail to retain key executives and employees of the companies we acquire, which may reduce the value of the acquisition or give rise to additional integration costs. Future acquisitions could result in additional issuances of equity securities that would dilute the ownership of existing stockholders. Future acquisitions could also result in the incurrence of debt, contingent liabilities or the amortization of expenses related to other intangible assets, any of which could adversely affect our operating results. In addition, we may fail to realize the anticipated benefits of any acquisition.
Our business is affected by macroeconomic conditions.
Various macroeconomic factors could adversely affect our business and the results of our operations and financial condition, including changes in inflation, interest rates and foreign currency exchange rates and overall economic conditions and uncertainties, including those resulting from the current and future conditions in the global financial markets. For instance, if inflation or other factors were to significantly increase our business costs, it may not be feasible to pass through price increases to patients. Interest rates, the liquidity of the credit markets and the volatility of the capital markets could also affect the value of our investments and our ability to liquidate our investments in order to fund our operations.
Interest rates and the ability to access credit markets could also adversely affect the ability of patients and distributors to purchase, pay for and effectively distribute our products. Similarly, these macroeconomic factors could affect the ability of our contract manufacturers, sole-source or single-source suppliers to remain in business or otherwise manufacture or supply product. Failure by any of them to remain a going concern could affect our ability to manufacture products.
If product liability lawsuits are successfully brought against us, we will incur substantial liabilities and may be required to limit the commercialization of Ravicti or other products.
We face potential product liability exposure related to the testing of our product candidates in human clinical trials, and we may face exposure to claims by an even greater number of persons if we begin marketing
34
Table of Contents
and distributing our products commercially. In the future, an individual may bring a liability claim against us alleging that one of our products or product candidates caused an injury. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for our products; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs of related litigation; |
| • | substantial monetary awards to patients and others; |
| • | loss of revenues; and |
| • | the inability to commercialize our products. |
In addition, while we continue to take what we believe are appropriate precautions, we may be unable to avoid significant liability if any product liability lawsuit is brought against us.
If product liability lawsuits are successfully brought against us, our insurance may be inadequate.
We are exposed to the potential product liability risks inherent in the testing, manufacturing and marketing of human pharmaceuticals. We plan to maintain insurance against product liability lawsuits for commercial sale of Ravicti, if Ravicti is approved for sale, and for BUPHENYL and AMMONUL if we purchase Ucyclyd’s worldwide rights to those products. We currently maintain insurance for the clinical trials of Ravicti. Biopharmaceutical companies must balance the cost of insurance with the level of coverage based on estimates of potential liability. Historically, the potential liability associated with product liability lawsuits for pharmaceutical products has been unpredictable. Although we believe that our current insurance is a reasonable estimate of our potential liability and represents a commercially reasonable balancing of the level of coverage as compared to the cost of the insurance, we may be subject to claims in connection with our clinical trials and commercial use of Ravicti, BUPHENYL and AMMONUL, for which our insurance coverage may not be adequate.
The product liability insurance we will need to obtain in connection with the commercial sales of our product candidates if and when they receive regulatory approval may be unavailable in meaningful amounts or at a reasonable cost. If we are the subject of a successful product liability claim that exceeds the limits of any insurance coverage we obtain, we may incur substantial charges that would adversely affect our earnings and require the commitment of capital resources that might otherwise be available for the development and commercial launch of our product programs.
Business interruptions could delay us in the process of developing our products and could disrupt our sales.
Our headquarters is located in the San Francisco Bay Area, near known earthquake fault zones and is vulnerable to significant damage from earthquakes. We are also vulnerable to other types of natural disasters and other events that could disrupt our operations. We do not carry insurance for earthquakes or other natural disasters and we may not carry sufficient business interruption insurance to compensate us for losses that may occur. Any losses or damages we incur could have a material adverse effect on our business operations.
35
Table of Contents
Risks Related to this Offering and Ownership of Our Common Stock
The market price of our common stock may be highly volatile, and you may not be able to resell your shares at or above the initial public offering price.
Prior to this offering, there has not been a public market for our common stock. If an active trading market for our common stock does not develop following this offering, you may not be able to sell your shares quickly or at the market price. The initial public offering price for the shares will be determined by negotiations between us and representatives of the underwriters and may not be indicative of prices that will prevail in the trading market.
The trading price of our common stock is likely to be volatile. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| • | announcements of regulatory approval or a complete response letter to Ravicti, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| • | whether we exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, and any associated delays or difficulties in completing the purchase or otherwise acquiring such rights, including as a result of antitrust review of the transaction; |
| • | announcements of therapeutic innovations or new products by us or our competitors; |
| • | adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| • | changes or developments in laws or regulations applicable to Ravicti and the products that we may acquire under our restated collaboration agreement with Ucyclyd; |
| • | any adverse changes to our relationship with Ucyclyd or other licensors, manufacturers or suppliers; |
| • | the success of our testing and clinical trials; |
| • | the success of our efforts to acquire or license additional product candidates; |
| • | any intellectual property infringement actions in which we may become involved; |
| • | announcements concerning our competitors or the pharmaceutical industry in general; |
| • | achievement of expected product sales and profitability; |
| • | manufacture, supply or distribution shortages; |
| • | actual or anticipated fluctuations in our operating results; |
| • | changes in financial estimates or recommendations by securities analysts; |
| • | trading volume of our common stock; |
| • | sales of our common stock by us, our executive officers and directors or our stockholders in the future; |
| • | general economic and market conditions and overall fluctuations in the United States equity markets; |
| • | changes in accounting principles; and |
| • | the loss of any of our key scientific or management personnel. |
36
Table of Contents
In addition, the stock market in general, and The NASDAQ Stock Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. Further, the current decline in the financial markets and related factors beyond our control, including the credit and mortgage crisis in the United States and worldwide, may cause our stock price to decline rapidly and unexpectedly.
We may be subject to securities litigation, which is expensive and could divert management attention.
Our share price may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
Our principal stockholders, executive officers and directors own a significant percentage of our common stock and will be able to exert a significant control over matters submitted to our stockholders for approval.
After this offering, our officers and directors, and stockholders who own more than 5% of our outstanding common stock before this offering will, in the aggregate, beneficially own approximately % of our common stock (after giving effect to the conversion of all outstanding shares of our convertible preferred stock, the conversion of the principal and accrued interest outstanding under our convertible promissory notes and the net exercise of the warrants issued in connection with our bridge loan financings but assuming no exercise of the underwriters’ over-allotment option, no exercise of outstanding options and no exercise of outstanding warrants other than those issued in connection with our bridge loan financings). As a result, these stockholders, if they acted together, could significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these stockholders may not always coincide with our interests or the interests of other stockholders.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
The assumed initial public offering price is substantially higher than the net tangible book value per share of our common stock. Investors purchasing common stock in this offering will pay a price per share that substantially exceeds the net tangible book value of our common stock. As a result, investors purchasing common stock in this offering will incur immediate dilution of $ per share, based on the assumed initial public offering price of $ per share, and our pro forma net tangible book value as of March 31, 2012. Further, investors purchasing common stock in this offering will contribute approximately % of the total amount invested by stockholders since our inception through this offering, but will own only approximately % of the shares of common stock outstanding.
This dilution is due to our investors who purchased shares prior to this offering having paid substantially less than the price offered to the public in this offering when they purchased their shares, and to the exercise of stock options granted to our employees. In addition, as of March 31, 2012, options to purchase 7,718,537 shares of our common stock at a weighted average exercise price of $0.39 per share were outstanding. The exercise of these options would result in additional dilution. As a result of this dilution, investors purchasing stock in this offering may receive significantly less than the purchase price paid in this offering in the event of liquidation. For more information, please refer to the section of this prospectus entitled “Dilution.”
37
Table of Contents
Sales of a substantial number of shares of our common stock in the public market by our existing stockholders could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock. Substantially all of our existing stockholders are subject to lock-up agreements with the underwriters of this offering that restrict the stockholders’ ability to transfer shares of our common stock for at least 180 days from the date of this prospectus. The lock-up agreements limit the number of shares of common stock that may be sold immediately following the public offering. Subject to limitations, approximately 42,976,200 shares will become eligible for sale upon expiration of the lockup period, as calculated and described in more detail in the section entitled “Shares Eligible for Future Sale.” In addition, shares issued or issuable upon exercise of options and warrants vested as of the expiration of the lock-up period will be eligible for sale at that time. Sales of stock by these stockholders could have a material adverse effect on the trading price of our common stock.
Some of the holders of our securities are entitled to rights with respect to the registration of their shares under the Securities Act of 1933, as amended, or the Securities Act, subject to the 180-day lock-up arrangement described above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by our affiliates as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
Our management will have broad discretion in the use of the net proceeds from this offering and may allocate the net proceeds from this offering in ways that you and other stockholders may not approve.
Our management will have broad discretion in the use of the net proceeds, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure of our management to use these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our stock, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Because we do not intend to declare cash dividends on our shares of common stock in the foreseeable future, stockholders must rely on appreciation of the value of our common stock for any return on their investment.
We have never declared or paid cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. As a result, only appreciation of the price of our common stock, if any, will provide a return to investors in this offering.
38
Table of Contents
Our ability to use our net operating loss carryforwards may be limited.
As of December 31, 2011, we had net operating losses of approximately $75.0 million and $95.0 million for both U.S. federal and California income tax purposes, respectively, which begin to expire in 2026 for U.S. federal income tax purposes and 2016 for California income tax purposes. If we experience an “ownership change” for purposes Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, we may be subject to annual limits on our ability to utilize net operating loss carryforwards. An ownership change is, as a general matter, triggered by sales or acquisitions of our stock in excess of 50% on a cumulative basis during a three-year period by persons owning 5% or more of our total equity value. We are not currently subject to any annual limits on our ability to utilize net operating loss carryforwards. Our deferred tax assets have been fully offset by a valuation allowance as of December 31, 2011.
The requirements associated with being a public company will require significant company resources and management attention.
Following this offering, we will become subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or Exchange Act, the Sarbanes-Oxley Act, the listing requirements of the securities exchange on which our common stock is traded, and other applicable securities rules and regulations. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition and maintain effective disclosure controls and procedures and internal control over financial reporting. In addition, subsequent rules implemented by the Securities and Exchange Commission, or SEC, and The NASDAQ Stock Market may also impose various additional requirements on public companies. As a result, we will incur additional legal, accounting and other expenses that we did not incur as a nonpublic company, particularly after we are no longer an “emerging growth company” as defined in the JOBS Act. Further, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy. We have made, and will continue to make, changes to our corporate governance standards, disclosure controls and financial reporting and accounting systems to meet our reporting obligations. However, the measures we take may not be sufficient to satisfy our obligations as a public company, which could subject us to delisting of our common stock, fines, sanctions and other regulatory action and potentially civil litigation.
The recently enacted JOBS Act will allow us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our company and adversely affect the market price of our common stock.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies” including:
| • | the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| • | the “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Act and some of the disclosure requirements of the Dodd-Frank Act relating to compensation of its chief executive officer; |
39
Table of Contents
| • | the requirement to provide detailed compensation discussion and analysis in proxy statements and reports filed under the Securities Exchange Act of 1934, and instead provide a reduced level of disclosure concerning executive compensation; and |
| • | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
We may take advantage of these exemptions until we are no longer an “emerging growth company.” We would cease to be an “emerging growth company” upon the earliest of: (i) the first fiscal year following the fifth anniversary of this offering; (ii) the first fiscal year after our annual gross revenues are $1 billion or more; (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt securities; or (iv) as of the end of any fiscal year in which the market value of our common stock held by non-affiliates exceeded $700 million as of the end of the second quarter of that fiscal year.
Although we are still evaluating the JOBS Act, we currently intend to take advantage of some, but not all, of the reduced regulatory and reporting requirements that will be available to us so long as we qualify as an “emerging growth company.” For example, we have irrevocably elected not to take advantage of the extension of time to comply with new or revised financial accounting standards available under Section 102(b) of the JOBS Act. Our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an “emerging growth company,” which may increase the risk that weaknesses or deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an “emerging growth company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate our company.We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our restated certificate of incorporation and our bylaws that will become effective following the closing of this offering, as well as provisions of the Delaware General Corporation Law, or DGCL, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions include:
| • | authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| • | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| • | limiting the removal of directors by the stockholders; |
| • | eliminating the ability of stockholders to call a special meeting of stockholders; and |
| • | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
40
Table of Contents
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. In addition, we are subject to Section 203 of the DGCL, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder, unless such transactions are approved by our board of directors. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders.
41
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this prospectus constitute forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” “intends” or “continue,” or the negative of these terms or other comparable terminology.
Forward-looking statements include, but are not limited to, statements about:
| • | FDA approval of, or other action with respect to, Ravicti; |
| • | the commercial launch and future sales of Ravicti or any other future products or product candidates; |
| • | our ability to achieve premium pricing for Ravicti; |
| • | our plans with respect to the purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL; |
| • | our expectations regarding the commercial supply of our UCD products; |
| • | third-party payor reimbursement for Ravicti, BUPHENYL and AMMONUL; |
| • | our estimates regarding anticipated capital requirements and our needs for additional financing; |
| • | the UCD or HE patient market size and market adoption of Ravicti by physicians and patients; |
| • | the timing, cost or other aspects of the commercial launch of Ravicti; |
| • | the timing or cost of a Phase III trial in HE; |
| • | the development and approval of the use of Ravicti for additional indications or in combination therapy; and |
| • | our expectations regarding licensing, acquisitions and strategic operations. |
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
42
Table of Contents
We expect to receive approximately $ million in net proceeds from the sale of shares of common stock offered by us in this offering (approximately $ million if the underwriters exercise their over-allotment option in full), based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for:
| • | completing the clinical development of Ravicti for UCD, including the long-term safety portion of our trial in UCD patients under 6 years of age, regulatory approval and post-marketing studies, currently estimated to be $ ; |
| • | commercial launch of Ravicti for UCD, including payroll and related costs, marketing and promotional costs, and manufacturing of commercial supplies, currently estimated at $ ; |
| • | license payments under our license agreement with Brusilow of up to $ over the next 12 months; and |
| • | the remainder for general corporate purposes. |
The amounts set forth above are estimates, and we cannot be certain that actual costs will not vary from these estimates. Our management has significant flexibility and broad discretion in applying the net proceeds received in this offering. We may also use a portion of the net proceeds for the licensing or acquisition of, or development of, additional product candidates other than BUPHENYL and AMMONUL. However, we have no present agreement regarding any material acquisitions. Pending use of the net proceeds, we intend to invest in interest-bearing, investment-grade securities.
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after the deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming that the assumed initial public offering price remains the same, and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We do not expect that a change in the offering price or the number of shares by these amounts would have a material effect on our uses of the proceeds from this offering, although it may accelerate the time at which we will need to seek additional capital.
We have never declared or paid any cash dividends on our capital stock and do not anticipate paying any cash dividends in the foreseeable future. Payment of cash dividends, if any, in the future will be at the discretion of our board of directors and will depend on then-existing conditions, including our financial condition, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
43
Table of Contents
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2012:
| • | on an actual basis; |
| • | on a pro forma basis to give effect to the following transactions and adjustments as if they had occurred on March 31, 2012: |
| (1) | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
| (2) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the April 2011 warrants and the May 2011 warrants into shares of our common stock, at an exercise price of $0.67 per share, which will expire upon completion of this offering if not exercised; |
| (3) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants, into shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants, at an exercise price equal to $1.58 per share, and which will expire upon completion of this offering if not exercised; |
| (4) | the automatic conversion of the bridge notes into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012; |
| (5) | the reclassification of the bridge notes liability to common stock and additional paid-in-capital in connection with the conversion based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus; and |
| (6) | the reclassification of the bridge warrants liability to common stock and additional paid-in-capital in connection with the exercise based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus. |
| • | on a pro forma as adjusted basis to give further effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale of the shares in this offering has occurred on March 31, 2012. |
44
Table of Contents
Because the number of shares that will be issued upon net exercise of the bridge warrants and conversion of the bridge notes depends upon the actual initial public offering price per share in this offering and, in the case of the bridge notes, the closing date of this offering, the actual number of shares issuable upon such exercise and conversion may differ from the respective number of shares set forth above. You should read this table in conjunction with the sections titled “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| (in thousands, except share and per share data) | March 31, 2012 | |||||||
| Actual | Pro Forma | Pro Forma as Adjusted | ||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||
Cash and cash equivalents | $ | 3,736 | ||||||
|
|
|
| |||||
Convertible notes payable | $ | 30,736 | ||||||
Warrants liability | 3,464 | |||||||
|
|
|
| |||||
Convertible preferred stock, par value $0.0001: 66,000,000 shares authorized, 40,045,749 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 58,326 | |||||||
|
|
|
| |||||
Stockholders’ equity (deficit): | ||||||||
Common stock, par value $0.0001: 80,000,000 shares authorized, 2,858,251 shares issued and outstanding, actual; 100,000,000 shares authorized, shares issued and outstanding, pro forma; 100,000,000 shares authorized, shares issued and outstanding, pro forma as adjusted | — | |||||||
Preferred stock, $0.0001 par value; no shares authorized, issued and outstanding, actual; 10,000,000 shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted | — | |||||||
Additional paid-in capital | 24,756 | |||||||
Deficit accumulated during the development stage | (118,620 | ) | ||||||
|
|
|
| |||||
Total stockholders’ equity (deficit) | (93,864 | ) | ||||||
|
|
|
| |||||
Total capitalization | $ | (1,338 | ) | |||||
|
|
|
| |||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $ million, assuming that the assumed initial public offering price, the midpoint of the price range set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
45
Table of Contents
The outstanding share information above excludes:
| • | 7,718,537 shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2012 under our 2006 Equity Incentive Plan having a weighted average exercise price of $0.39 per share; |
| • | 1,810 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2012 having a weighted average exercise price of $294.13 per share, which warrants are expected to remain outstanding upon completion of this offering; and |
| • | shares of common stock (which includes 631,904 shares reserved for issuance under our 2006 Equity Incentive Plan as of March 31, 2012) reserved for future issuance under our 2012 Omnibus Incentive Plan, which will become effective immediately upon the execution and delivery of the underwriting agreement for this offering, as well as any future increases in the number of shares of common stock reserved for issuance under this plan. |
46
Table of Contents
If you invest in our common stock, you will experience immediate and substantial dilution to the extent of the difference between the assumed initial public offering price of our common stock and the pro forma as adjusted net tangible book value (deficit) per share of our common stock immediately after the offering.
Our historical net tangible book value (deficit) per share is determined by dividing our total tangible assets, less total liabilities and convertible preferred stock, by the actual number of outstanding shares of our common stock. The historical net tangible book value (deficit) of our common stock as of March 31, 2012 was $(93.9) million, or $(32.84) per share. The pro forma net tangible book value (deficit) of our common stock as of March 31, 2012 was $ million, or $ per share. The pro forma net tangible book value (deficit) per share gives effect to:
| (1) | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
| (2) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the April 2011 warrants and the May 2011 warrants into shares of our common stock, at an exercise price equal to $0.67 per share, and which will expire upon completion of this offering if not exercised; |
| (3) | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants, into shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the warrants, at an exercise price equal to $1.58 per share, and which will expire upon completion of this offering if not exercised; |
| (4) | the automatic conversion of the bridge notes into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, based on the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012; |
| (5) | the reclassification of the bridge notes liability to common stock and additional paid-in-capital in connection with the conversion based on the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus; and |
| (6) | the reclassification of the bridge warrants liability to common stock and additional paid-in-capital in connection with the exercise based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus. |
Because the number of shares that will be issued upon exercise of the bridge warrants and conversion of the bridge notes depends upon the actual initial public offering price per share in this offering and, in the case of the bridge notes, the closing date of this offering, the actual number of shares issuable upon such exercise and conversion may differ from the respective number of shares set forth above. See “Prospectus Summary — The Offering.”
47
Table of Contents
The pro forma as adjusted net tangible book value (deficit) of our common stock as of March 31, 2012 was $ million, or $ per share. The pro forma as adjusted net tangible book value (deficit) gives effect to (1) the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, and (2) the pro forma transactions and other adjustments described above. The difference between the initial public offering price and the pro forma as adjusted net tangible book value (deficit) per share represents an immediate dilution of $ per share to new investors purchasing common stock in this offering.
The following table illustrates this dilution on a per share basis to new investors:
Assumed initial public offering price per share | $ | |||||||
|
| |||||||
Historical net tangible book value (deficit) per share as of March 31, 2012 | $ | (32.84 | ) | |||||
|
| |||||||
Pro forma increase in net tangible book value (deficit) per share attributable to the pro forma transactions and other adjustments described above | ||||||||
|
| |||||||
Pro forma net tangible book value (deficit) before this offering | ||||||||
Pro forma increase in net tangible book value (deficit) per share attributable to new investors | ||||||||
|
| |||||||
Pro forma as adjusted net tangible book value (deficit) per share after this offering | ||||||||
|
| |||||||
Dilution per share to new investors purchasing common stock in this offering | $ | |||||||
|
|
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value (deficit) by approximately $ million, or approximately $ per share, and the dilution per share to new investors purchasing common stock in this offering by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase of 1.0 million shares in the number of shares offered by us would increase our pro forma as adjusted net tangible book value (deficit) by approximately $ million, or $ per share, and the dilution per share to new investors purchasing common stock in this offering would be $ per share, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, a decrease of 1.0 million shares in the number of shares offered by us would decrease our pro forma as adjusted net tangible book value (deficit) by approximately $ million, or $ per share, and the dilution per share to new investors purchasing common stock in this offering would be $ per share, assuming that the assumed initial public offering price remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
If the underwriters’ over-allotment option to purchase additional shares from us is exercised in full, and based on the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, the pro forma as adjusted net tangible book value (deficit) per share after this offering would be $ per share, the increase in pro forma as adjusted net tangible book value (deficit) to existing stockholders would be $ per share and the dilution to new investors purchasing shares in this offering would be $ per share.
48
Table of Contents
The table below summarizes as of March 31, 2012, on the pro forma basis described above, the number of shares of common stock we issued and sold, the total consideration we received and the average price per share (1) paid by our existing stockholders and (2) to be paid by new investors purchasing our common stock in this offering at the assumed initial public offering price of $ per share, before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||
| (in thousands) | ||||||||||||||||
Existing stockholders | % | % | ||||||||||||||
New investors | ||||||||||||||||
|
|
|
|
|
|
| ||||||||||
Total | 100 | % | $ | 100 | % | |||||||||||
|
|
|
|
|
|
| ||||||||||
The number of common stock outstanding immediately after this offering is based on shares of common stock outstanding as of March 31, 2012 and giving effect to the pro forma transactions described above. This number excludes:
| • | 7,718,537 shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2012 under our 2006 Equity Incentive Plan having a weighted average exercise price of $0.39 per share; |
| • | 1,810 shares of common stock issuable upon the exercise of warrants outstanding as of December 31, 2011 having a weighted average exercise price of $294.13 per share, which warrants are expected to remain outstanding upon completion of this offering; and |
| • | shares of common stock (which includes the 631,904 shares reserved for issuance under our 2006 Equity Incentive Plan as of March 31, 2012) reserved for future issuance under our 2012 Omnibus Incentive Plan, which will become effective immediately upon the execution and delivery of the underwriting agreement for this offering, as well as any future increases in the number of shares of common stock reserved for issuance under this plan. |
Effective upon the closing of this offering, an aggregate of shares of our common stock will be reserved for future issuance under our equity benefit plans, and the number of reserved shares will also be subject to automatic annual increases in accordance with the terms of the plans. To the extent that new options are granted under our equity benefit plans, there will be further dilution to investors purchasing common stock in this offering.
49
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated financial data together with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our consolidated financial statements and the accompanying notes appearing at the end of this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2009, 2010 and 2011 and the consolidated balance sheet data as of December 31, 2010 and 2011 from our audited consolidated financial statements appearing in this prospectus. We have derived the consolidated statements of operations data for the years ended December 31, 2007 and 2008 and the consolidated balance sheet data as of December 31, 2007, 2008 and 2009 from our audited consolidated financial statements not included in this prospectus. The selected consolidated statements of operations data for the three months ended March 31, 2011 and 2012 and the selected consolidated balance sheet data as of March 31, 2012 are derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and include, in our opinion, all adjustments, consisting only of normal recurring adjustments, necessary for the fair statement of the financial information in those statements. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period.
| (in thousands, except share and per share amounts) | Year Ended December 31, | Three Months Ended March 31, (unaudited) | ||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||||||||||||||
Revenue | $ | 242 | $ | 44 | $ | — | $ | — | $ | — | $ | — | — | |||||||||||||||
Cost of revenue | 10 | — | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Gross profit | 232 | 44 | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Research and development | 4,407 | 14,452 | 11,030 | 23,111 | 17,236 | 4,300 | 8,902 | |||||||||||||||||||||
General and administrative | 2,764 | 3,469 | 1,909 | 2,693 | 8,162 | 1,361 | 2,077 | |||||||||||||||||||||
Selling and marketing | 2,058 | 2,997 | 462 | 797 | 761 | 250 | 246 | |||||||||||||||||||||
Impairment of development and promotion rights acquisition cost | — | 7,059 | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Total operating expenses | 9,229 | 27,977 | 13,401 | 26,601 | 26,159 | 5,911 | 11,225 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Loss from operations | (8,997 | ) | (27,933 | ) | (13,401 | ) | (26,601 | ) | (26,159 | ) | (5,911 | ) | (11,225 | ) | ||||||||||||||
Interest income | 220 | 111 | 39 | 43 | 28 | 4 | 4 | |||||||||||||||||||||
Interest expense | (249 | ) | (1,677 | ) | (763 | ) | (1 | ) | (2,554 | ) | — | (1,040 | ) | |||||||||||||||
Other income (expense), net | (12 | ) | 400 | 525 | 1,106 | (731 | ) | 375 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss | $ | (9,038 | ) | $ | (29,099 | ) | $ | (13,600 | ) | $ | (25,453 | ) | $ | (29,416 | ) | (5,907 | ) | (11,886 | ) | |||||||||
Accretion of Series B preferred stock to redemption value | (7 | ) | (29 | ) | (78 | ) | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss attributable to common stockholders | $ | (9,045 | ) | $ | (29,128 | ) | $ | (13,678 | ) | $ | (25,453 | ) | $ | (29,416 | ) | (5,907 | ) | (11,886 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss per share attributable to common stockholders — basic and diluted(1) | $ | (2,813.48 | ) | $ | (8,191.26 | ) | $ | (15.24 | ) | $ | (10.13 | ) | $ | (10.29 | ) | $ | (2.07 | ) | $ | (4.16 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Weighted average shares of common stock outstanding used in computing net loss per share attributable to common stockholders — basic and diluted(1) | 3,215 | 3,556 | 897,239 | 2,512,320 | 2,858,251 | 2,858,251 | 2,858,251 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Pro forma net loss per share attributable to common stockholders — basic and diluted (unaudited)(1) | $ | $ | ||||||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||||||
Weighted average shares of common stock outstanding used in computing the pro forma net loss per share attributable to common stockholders — basic and diluted(1) | ||||||||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||||||
| (1) | See Note 15 to our consolidated financial statements for an explanation of the method used to calculate basic and diluted net loss per share of common stock, the unaudited pro forma basic and diluted net loss per share of common stock and the weighted average number of shares used in computation of the per share amounts. |
50
Table of Contents
| (in thousands) | As of December 31, | As of March 31, 2012 | ||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | ||||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||||||||||
Cash and cash equivalents | $ | 14,566 | $ | 1,089 | $ | 10,073 | $ | 6,579 | $ | 7,018 | $ | 3,736 | ||||||||||||
Working capital (deficit) | 14,857 | (12,848 | ) | 6,713 | 3,650 | (21,282 | ) | (32,889 | ) | |||||||||||||||
Total assets | 25,340 | 1,756 | 11,171 | 7,387 | 8,142 | 5,014 | ||||||||||||||||||
Long-term debt | 9,444 | 3,889 | — | — | — | — | ||||||||||||||||||
Warrants liability | 400 | — | — | — | 2,574 | 3,464 | ||||||||||||||||||
Convertible preferred stock | 21,827 | 21,856 | 36,265 | 58,326 | 58,326 | 58,326 | ||||||||||||||||||
Total stockholders’ deficit | (9,148 | ) | (38,125 | ) | (29,162 | ) | (54,176 | ) | (82,104 | ) | (93,864 | ) | ||||||||||||
51
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and other parts of the prospectus contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat disorders in the areas of orphan diseases and hepatology. We are developing our product candidate, Ravicti, to treat two different diseases in which blood ammonia is elevated: the most prevalent urea cycle disorders, or UCD, and hepatic encephalopathy, or HE. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or protein transporters that constitute the urea cycle, which in a healthy individual removes ammonia through the conversion of ammonia to urea. HE may develop in some patients with liver scarring, known as cirrhosis, or acute liver failure and is a chronic disease which fluctuates in severity and may lead to serious neurological damage. On December 23, 2011, we submitted a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for Ravicti for the chronic management of UCD in patients aged 6 years and above. The FDA accepted the NDA for review in February 2012. Under the Prescription Drug User Fee Act, or PDUFA, the FDA is due to notify us regarding Ravicti’s approval status by October 23, 2012, unless that action date is extended by the FDA. In April 2012, we submitted data from the switchover portion of a clinical trial in UCD patients aged 29 days through 5 years and a revised draft package insert requesting approval of Ravicti to include this patient population. We currently expect to commercially launch Ravicti in the first half of 2013. In May 2012, our Phase II HE trial data was unblinded and the trial met its primary endpoint, which was to demonstrate that the proportion of patients experiencing an HE event was significantly lower on Ravicti versus placebo, both administered in addition to a standard of care, including lactulose and/or rifaximin.
Pursuant to an asset purchase agreement, or purchase agreement, with Ucyclyd Pharma, Inc., or Ucyclyd, a wholly owned subsidiary of Medicis Pharmaceutical Corporation, we purchased the worldwide rights to Ravicti in March 2012 for an upfront payment of $6.0 million, future payments based upon the achievement of regulatory milestones in indications other than UCD, sales milestones, and mid to high single digit royalties on global net sales of Ravicti. Pursuant to an amended and restated collaboration agreement, or restated collaboration agreement, with Ucyclyd entered into on March 2012, we have an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL® (sodium phenylacetate and sodium benzoate) injection 10%/10%, the only adjunctive therapy currently FDA-approved for the treatment of HA crises in UCD patients, for an upfront payment of $22.0 million, plus subsequent milestone and royalty payments. We will be permitted to exercise this option for a period of 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. To fund this upfront payment, we may draw on a loan commitment from Ucyclyd, which loan would be payable over eight quarters. If we exercise our option, Ucyclyd has a time-limited option to retain AMMONUL at a purchase price of $32.0 million. If Ucyclyd exercises its option and retains AMMONUL, the upfront purchase price for Ucyclyd’s worldwide rights to BUPHENYL will be $19.0 million resulting in a net payment from Ucyclyd to us of $13.0 million upon close of the transaction.
We are a development stage company and have incurred net losses since our inception. As of March 31, 2012, we had a deficit accumulated during the development stage of $118.6 million. We recorded net losses of $13.6 million, $25.5 million, $29.4 million and $11.9 million in the years ended December 31, 2009, 2010 and 2011, and the three months ended March 31, 2012, respectively. We anticipate that a substantial portion of our capital resources and efforts in the foreseeable future will be focused on completing the development and obtaining regulatory approval of Ravicti, and preparing for potential commercialization of Ravicti, and
52
Table of Contents
BUPHENYL and AMMONUL if purchased from Ucyclyd. We expect to incur significant and increasing operating losses and negative cash flows in the near future as we continue to conduct clinical trials, seek regulatory approval of Ravicti in UCD and HE, expand our organization, prepare for the potential commercial launch of Ravicti if approved by the FDA, and purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain AMMONUL. In addition, any future acquisitions of products or product candidates may require additional capital and personnel.
To date, substantially all of our operations have been funded through the private placement of equity securities and convertible debt. Through March 31, 2012, we have raised net cash proceeds of approximately $66.1 million from the sales of convertible preferred stock, and $15.3 million from the issuance of convertible notes which subsequently converted into shares of convertible preferred stock. Additionally, during 2011 and during the first quarter of 2012 we issued approximately $25.0 million and $7.5 million, respectively, in convertible notes.
In May 2012, we completed a Phase II trial of Ravicti in HE which met its primary endpoint. We expect our research and development expenses to increase if we initiate a Phase III trial of Ravicti in HE or if the FDA requires us to do additional studies for the approval of Ravicti in UCD. If we obtain marketing approval for Ravicti in UCD, we will likely incur significant commercial, sales, marketing and outsourced manufacturing expenses. Additionally, upon completion of this offering, we expect to incur additional expenses associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future.
Financial Overview
Revenue
We have generated no revenue from the sale of any products in the last three years, and we do not expect to generate any revenue unless or until we obtain marketing approval of and commercialize Ravicti, or exercise the option to purchase Ucyclyd’s worldwide rights to and commercialize BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain AMMONUL.
For the period from inception to March 31, 2012, we have only generated limited revenue from the promotion of BUPHENYL and AMMONUL during 2007 and 2008, and earned no revenue during 2006 and 2009 through March 31, 2012.
Research and Development Expenses
Since our inception, we have focused on our clinical development programs. We recognize research and development expenses as they are incurred. Our research and development expenses consist primarily of:
| • | salaries and related expenses for personnel, including expenses related to stock options or other stock-based compensation granted to personnel in development functions; |
| • | fees paid to clinical consultants, clinical trial sites and vendors, including clinical research organizations, or CROs, in conjunction with implementing and monitoring our clinical trials and acquiring and evaluating clinical trial data, including all related fees, such as for investigator grants, patient screening fees, laboratory work and statistical compilation and analysis; |
| • | other consulting fees paid to third parties; |
| • | expenses related to production of clinical supplies, including fees paid to contract manufacturers; |
| • | expenses related to license fees and milestone payments under in-licensing agreements; |
| • | expenses related to compliance with drug development regulatory requirements in the United States, the European Union and other foreign jurisdictions; and |
| • | depreciation and other allocated expenses. |
53
Table of Contents
We expense both internal and external research and development expenses as they are incurred. We did not begin tracking our research and development expenses on a program-by-program basis until January 1, 2010. We have been developing Ravicti in both UCD and HE in parallel, and we typically use our employees, consultants and infrastructure resources across our two programs. Thus, some of our research and development expenses are not attributable to an individual program, but rather are allocated across our two clinical stage programs and these costs are included in unallocated costs as detailed below. Allocated expenses include salaries, stock-based compensation and related benefit expenses for our employees, consulting fees and fees paid to clinical suppliers. The following table shows our research and development expenses for the years ended December 31, 2010 and 2011 and the three months ended March 31, 2011 and 2012:
| Years Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| (in thousands) | 2010 | 2011 | 2011 | 2012 | ||||||||||||
| (unaudited) | ||||||||||||||||
UCD Program | $ | 12,859 | $ | 7,900 | $ | 1,592 | $ | 1,104 | ||||||||
HE Program | 4,892 | 5,162 | 1,456 | 873 | ||||||||||||
Unallocated | 5,360 | 4,174 | 1,252 | 6,925 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 23,111 | $ | 17,236 | $ | 4,300 | $ | 8,902 | ||||||||
|
|
|
|
|
|
|
| |||||||||
We expect our research and development expenses to increase if we initiate our Phase III trial of Ravicti for the treatment of patients with episodic HE or if the FDA requires us to do additional studies for the approval of Ravicti for UCD. Due to the inherently unpredictable nature of product development, we are currently unable to estimate the expenses we will incur in the continued development of Ravicti.
Our research and development expenditures are subject to numerous uncertainties in timing and cost to completion. Development timelines, the probability of success and development expenses can differ materially from expectations. Clinical trials in orphan diseases, such as UCD and HE, may be difficult to enroll given the small number of patients with these diseases. Completion of clinical trials may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| • | the number of trials required for approval; |
| • | the number of sites included in the trials; |
| • | the length of time required to enroll suitable patients; |
| • | the number of patients that participate in the trials; |
| • | the drop-out or discontinuation rates of patients; |
| • | the duration of patient follow-up; |
| • | the number and complexity of analyses and tests performed during the trial; |
| • | the phase of development of the product candidate; and |
| • | the efficacy and safety profile of the product candidate. |
Our expenses related to clinical trials are based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and contract research organizations that conduct and manage clinical trials on
54
Table of Contents
our behalf. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis.
As a result of the uncertainties discussed above, we are unable to determine with certainty the duration and completion costs of our Ravicti development programs or when and to what extent we will receive revenue from the commercialization and sale of Ravicti.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and stock based compensation for employees in administration, finance and business development. Other significant expenses include allocated facilities expenses and professional fees for accounting and legal services, including legal services associated with obtaining and maintaining patents.
We expect that our general and administrative expenses will increase with the continued development of, and if approved, the commercialization of Ravicti and as we begin to operate as a public company after the completion of this offering. We expect these increases will likely include increased expenses for insurance, expenses related to the hiring of additional personnel and payments to outside consultants, lawyers and accountants.
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of salaries and benefits for employees in the marketing, commercial and sales functions. Other significant expenses include professional and consulting fees related to these functions. We expect to incur increased sales and marketing expenses in connection with the commercialization of Ravicti, and BUPHENYL and AMMONUL if purchased from Ucyclyd.
Interest Income
Interest income consists of interest earned on our cash and cash equivalents.
Interest Expense
Interest expense consists primarily of non-cash interest costs related to our borrowings.
Other Income (Expense), net
Other income (expense), net in 2009 and 2010 consists primarily of the change in the fair value of the freestanding financial instrument associated with the Series C-2 convertible preferred stock. In June 2009, we entered into a tranched Series C-2 convertible preferred stock transaction. In connection with the initial closing in June 2009, we agreed to issue to the purchasers and the purchasers agreed to purchase additional shares of the Series C-2 convertible preferred stock at a future date. We determined that the liability to issue additional Series C-2 convertible preferred stock at a future date was a freestanding financial instrument that should be accounted for as a liability based upon the guidance of Accounting Standard Codification, or ASC, Topic 480-10,Distinguishing Liabilities from Equity. Accordingly, we recorded a liability related to this instrument at the time of the initial close in 2009 and remeasured the liability at each reporting period with the corresponding gain or loss from the adjustment recorded as other income (expense), net. The liability expired when the second tranche of Series C-2 convertible preferred stock was issued in April 2010.
55
Table of Contents
In 2011 and the three months ended March 31, 2012, other income (expense), net consists primarily of the changes in the fair value of the common and preferred stock warrants liability and call option liability associated with the issuance of approximately $32.5 million of convertible notes. Under ASC 815,Derivatives and Hedging and ASC 480, we account for the common stock warrants and preferred stock warrants issued in 2011 and 2012, or the common stock warrants and preferred stock warrants, respectively, at fair value and recorded as liabilities on the date of each issuance. The fair value was determined and subsequently remeasured using the Black-Scholes option-pricing model on each reporting date.
Income Taxes
Since inception, we have only generated revenues in the U.S. and have not generated revenues outside the U.S. The only revenues generated in the U.S. have been from commissions for promotion services in 2007 and 2008 through the Ucyclyd collaboration agreement related to the sales of BUPHENYL and AMMONUL for UCD. We have incurred net losses and have not recorded any U.S. federal or state income tax benefits for the losses as they have been offset by valuation allowances.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the revenues and expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. Significant estimates include assumptions used in the determination of the fair value measurement of certain financial assets and liabilities at the fair value, including convertible notes payable, common stock warrants, preferred stock warrants and call option liability, and research and development expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are more fully described in Note 2 to our consolidated financial statements included elsewhere in this prospectus. The following accounting policies are important in fully understanding and evaluating our reported financial results.
Preclinical and Clinical Trial Accruals
As part of the process of preparing consolidated financial statements, we are required to estimate accrued expenses. We base our expenses related to clinical trials on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
56
Table of Contents
Warrants and Other Derivative Liabilities
We account for our warrants and other derivative financial instruments as either equity or liabilities based upon the characteristics and provisions of each instrument. We record warrants classified as equity as additional paid-in capital on the consolidated balance sheet and make no further adjustments to their valuation. We record warrants classified as derivative liabilities and other derivative financial instruments, such as call option liability recorded in connection with convertible notes and preferred stock liability recorded in connection with Series C-2 convertible preferred stock, that require separate accounting as liabilities on our consolidated balance sheets at their fair value on the date of issuance and remeasure them on each subsequent balance sheet, with fair value changes recognized as increases or reductions to other income (expense), net in the consolidated statements of operations. We estimate the fair value of these liabilities using option pricing models and assumptions that are based on the individual characteristics of the warrants or instruments on the valuation date, as well as assumptions for future financings, expected volatility, expected life, yield, and risk-free interest rate.
We account for our warrants for shares of convertible preferred stock that are contingently redeemable as liabilities. We will continue to adjust the liability for changes in fair value of these warrants until the earlier of: (i) exercise of warrants; (ii) expiration of warrants; (iii) a change of control of the company; or (iv) the closing of our initial public offering.
We account for our warrants for shares of common stock as liabilities in accordance with accounting guidance for derivatives. The accounting guidance provides a two-step model to be applied in determining whether a financial instrument is indexed to an entity’s own stock that would qualify the financial instruments for a scope exception. This scope exception specifies that a contract that would otherwise meet the definition of a derivative financial instrument would not be considered as such if the contract is both (i) indexed to the entity’s own stock and (ii) classified in the stockholders’ deficit section of the balance sheet. We determined that our common stock warrants issued with convertible notes in 2011 are ineligible for equity classification and we will continue to adjust the liability for changes in fair value until the earlier of: (i) exercise of warrants; (ii) expiration of warrants; (iii) a change of control of the company; (iv) occurrence of a qualified or non-qualified financing as defined in the applicable agreements; (v) the maturity of convertible notes; or (vi) the closing of our initial public offering.
Stock-Based Compensation
We recognize as compensation expense the fair value of stock options and other stock-based compensation issued to employees over the requisite service periods, which are typically the vesting periods. We record equity instruments issued to non-employees at their fair value, periodically revalue them as the equity instruments vest and recognize expense over the related service period.
Stock-based compensation has not been a significant expense to date. In future periods, we expect our stock-based compensation expense to increase as we issue additional stock-based awards in order to attract and retain employees and non-employee consultants.
57
Table of Contents
Stock-based compensation expense includes stock options granted to employees and non-employees and has been reported in our consolidated statements of operations as follows:
| Years Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| (in thousands) | 2009 | 2010 | 2011 | 2011 | 2012 | |||||||||||||||
(unaudited) | ||||||||||||||||||||
Research and development | $ | 84 | $ | 61 | $ | 137 | $ | 16 | $ | 37 | ||||||||||
General and administrative | 211 | 110 | 182 | 28 | 34 | |||||||||||||||
Sales and marketing | 18 | 14 | 26 | 3 | 7 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | $ | 313 | $ | 185 | $ | 345 | $ | 47 | $ | 78 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Significant Factors, Assumptions and Methodologies Used in Determining Fair Value
We calculate the fair value of stock-based compensation awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including stock price volatility and the expected term of stock options. As a private company, we do not have sufficient history to estimate the volatility of our common stock price or the expected term of our options. We calculate expected volatility based on reported data for a selected group of similar publicly traded companies, or guideline peer group, for which the historical information is available. We will continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants. The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. We determine the average expected term of stock options according to the “simplified method” as described in Staff Accounting Bulletin 110, which is the mid-point between the vesting date and the end of the contractual term. We determine the risk-free interest rate by reference to implied yields available from five-year and seven-year U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. We estimate forfeitures based on our historical analysis of actual stock option forfeitures. The assumptions used in the Black-Scholes option-pricing model for the years ended December 31, 2009, 2010 and 2011 are set forth in Note 11 of our consolidated financial statements included elsewhere in this prospectus.
There is a high degree of subjectivity involved when using option-pricing models to estimate stock-based compensation. Currently, there is not a market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of employee stock-based awards is determined using an option-pricing model, this value may not be indicative of the fair value observed in a market transaction between a willing buyer and willing seller. If factors change and we employ different assumptions when valuing our options, the compensation expense that we record in the future may differ significantly from what we have historically reported.
Information regarding equity instruments issued since January 1, 2011 is summarized as follows:
Date of Transaction | Equity Type | Number of Shares Underlying Options or Warrants Granted | Exercise Price Per Share | Management's Fair Value Per Share Estimate | ||||||||||
April 1, 2011 | Common Stock Warrants | (1 | ) | $ | 0.67 | (2 | ) | |||||||
April 15, 2011 | Common Stock Options | 2,484,477 | $ | 0.67 | $ | 0.52 | (3) | |||||||
October 26, 2011 | Preferred Stock Warrants | (1 | ) | $ | 1.58 | (2 | ) | |||||||
February 8, 2012 | Preferred Stock Warrants | (1 | ) | $ | 1.58 | (2 | ) | |||||||
58
Table of Contents
| (1) | The number of shares to be issued is calculated based upon 30% of the principal amount of the notes divided by either: |
| (i) | the price per share paid by a new investor in a preferred stock qualified financing; |
| (ii) | in the event the notes have been converted into shares of Series C-2 preferred stock, the series C-2 preferred original issue price of $1.58 per share; |
| (iii) | the price per share paid by a new investor for equity securities in a non-qualified financing; or |
| (iv) | a price per share of $1.58 in the event of an initial public offering. |
| (2) | The fair value of warrants in the aggregate was determined by using an income approach by first estimating the equity value of our company, then allocating the value to our various securities using the option pricing method. The option pricing method was applied in various scenarios based on the potential liquidity alternatives available to us. See Note 7 to our consolidated financial statements included elsewhere in this prospectus. |
| (3) | We reassessed the fair value of our common stock subsequent to the grant date of these awards. |
The table above does not include the grant of 2,760,950 stock options at an exercise price of $1.20 per share, which equals the fair value of common stock as determined by our board of directors on April 16, 2012 or the granting of 462,686 warrants for common stock at an exercise price of $0.67 per share on April 19, 2012 as part of our loan and security agreement entered into with Silicon Valley Bank and Leader Lending LLC - Series B.
The intrinsic value of all outstanding options as December 31, 2011 was $3.7 million based on the estimated fair value for our common stock of $0.83 per share at December 31, 2011.
There were no stock option grants to our employees during the three months ended March 31, 2012. The intrinsic value of all outstanding options as of March 31, 2012 was $6.6 million based on estimated fair value for our common stock of $1.20 per share at March 31, 2012.
All options granted by our board of directors on the date noted above were intended to be exercisable at the fair value of our stock based on information known at that time. For the purposes of recording stock-based compensation expense, we reviewed the historical pattern of our common stock values, and subsequently reassessed the fair value of our stock for financial reporting purposes during the year ended December 31, 2011 and the three months ended March 31, 2012.
The fair values of the common stock underlying our stock-based awards were estimated on each grant date by our board of directors, with input from management. The majority of our directors are not employees and have significant experience in the pharmaceutical and biotechnology industries. We believe that our board of directors has the relevant experience and expertise to determine a fair value of our common stock on each respective grant date. Given the absence of a public trading market of our common stock, and in accordance with the American Institute of Certified Public Accountants Practice Guide,Valuation of Privately-Held-Company Equity Securities Issued as Compensation, our board of directors exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of our common stock including:
| • | valuations performed by unrelated third party specialists; |
| • | prices for our convertible preferred stock sold to outside investors in arm’s-length transactions; |
| • | rights, preferences and privileges of our convertible preferred stock relative to those of our common stock; |
59
Table of Contents
| • | actual operating and financial performance; |
| • | status of our collaboration with Ucyclyd; |
| • | hiring of key personnel and the experience of our management; |
| • | status of research and development efforts, including the clinical trial results for Ravicti in UCD and HE; |
| • | risks inherent in the development of our products and services; |
| • | likelihood of achieving a liquidity event, such as an initial public offering or a sale of our company given prevailing market conditions and the nature and history of our business; |
| • | market values of transactions of similar pharmaceutical and biotechnology companies; |
| • | illiquidity of stock-based awards involving securities in a private company; |
| • | industry information such as market size and growth; and |
| • | macroeconomic conditions. |
Our board of directors considered common stock valuations performed as of February 28, 2011, April 1, 2011, October 31, 2011, December 31, 2011 and March 1, 2012 in determining or confirming the grant date fair value of common stock. Using these valuations, and the other factors described above, we made the following estimates of fair value of our common stock.
Valuation Date | Fair Value Per Share | |||
February 28, 2011 | $ | 0.67 | ||
April 1, 2011 | $ | 0.52 | ||
October 31, 2011 | $ | 0.66 | ||
December 31, 2011 | $ | 0.83 | ||
March 1, 2012 | $ | 1.20 | ||
In valuing our common stock, the board of directors determined the equity value of our company by utilizing the income approach. The income approach is based on the premise that the value of a business is the present value of a company’s future earning capacity. The application of this approach involves estimating the free cash flows for the business, calculating a terminal value, and then discounting the cash flows and terminal value back to a present value at an appropriate discount rate. We utilized a market approach for calculating the terminal value within the income approach, by applying market multiples of comparable publicly traded companies in our industry or similar lines of business.
We prepared a financial forecast for each valuation which was used to estimate the free cash flows of the business. These cash flows were discounted at a rate which was calculated using inputs from comparable publicly traded companies also in the business of developing and commercializing treatments which require regulatory approval. In selecting the comparable publicly traded companies in our industry or similar lines of business, we considered a variety of factors including: line of business (specifically, companies operating in the business of developing and commercializing therapies for regulatory approval); therapy type (specifically, companies which develop therapies to treat gastrointestinal, digestive, and liver conditions); size; addressable markets; and geographic location.
We captured the risk relating to the regulatory approval of Ravicti in the discount rate utilized within the income approach. Specifically, we applied an additional risk premium to the discount rate to capture risk related
60
Table of Contents
to our company’s size, the fact that we are in the clinical stage of development, and the risks related to raising the required capital needed to bring Ravicti to market.
After determining an equity value utilizing the income approach, we then allocated the fair value of our company to each of our classes of stock using either the Option Pricing Method, or OPM, or the Probability Weighted Expected Return Method, or PWERM. The OPM treats common stock and convertible preferred stock as call options on an enterprise value, with exercise prices based on the value thresholds at which the allocation among the various holders of a company’s securities changes. Under this method, the common stock has value only if the funds available for distribution to the stockholders exceed the value of the liquidation preferences of our preferred stock at the time of a liquidity event such as a merger, sale or initial public offering, assuming the enterprise has funds available to make a liquidation preference meaningful and collectible by the stockholders. The common stock is modeled to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the convertible preferred stock is liquidated. The OPM uses the Black-Scholes option-pricing model to price the call option. This model defines the securities’ fair values as functions of the current fair value of a company and uses assumptions such as the anticipated timing of a liquidity event and the estimated volatility of the equity securities. A discount for lack of marketability was applied to reflect the increased risk arising from the inability to readily sell the shares.
The PWERM involves a forward-looking analysis of the possible future outcomes of the enterprise. The future outcomes considered under the PWERM included non-IPO market based outcomes as well as IPO scenarios. In the non-IPO scenarios, a large portion of the equity value is allocated to the convertible preferred stock to incorporate the aggregate liquidation preferences. In the IPO scenarios, the equity value is allocated pro rata among the shares of common stock and each series of convertible preferred stock, which causes the common stock to have a higher relative value per share than under the non-IPO scenario. The fair value of the enterprise determined using the IPO and non-IPO scenarios would be weighted according to the board of directors’ estimate of the probability of each scenario.
Discussion of Specific Valuation Inputs
Over time, a combination of factors caused changes in the fair value of our common stock. The following summarizes the changes in value from January 2011 to March 2012 and the major factors that caused each change.
January 2011 through April 2011: As of January 2011, we continued to make progress with Ravicti for patients for the treatment of UCD. During the period from January to February 2011, we held discussions with investment banks regarding our prospects for an IPO. In these discussions, we gained additional understanding of the financial markets. We utilized this information when applying a PWERM allocation method using multiple sale scenarios, as well as an IPO scenario. Each of these scenarios is based on a combination of the expected timing of future financing or liquidity events and the progress achieved in our clinical studies. As a result of the developments in our business and applying the common stock valuation methodology described above, we estimated the fair value of our common stock to be $0.67 per share as of February 28, 2011.
During the period between February 2011 and April 2011 we continued to make progress with Ravicti and we raised $17.5 million in convertible notes from our existing investors, which addressed our short-term liquidity needs. Also during the period, our collaboration partner Ucyclyd disagreed with our filing approach of the NDA. Based on these factors, specifically relating to the additional funding and reassessed IPO timeline, our common stock valuation methodology was simplified into two scenarios — a remaining private company scenario and an IPO scenario. As discussed above, we utilized an income approach to value our equity for each of the scenarios. For option grants in April 2011, the board of directors deemed the fair value of the common stock to be $0.67 per share. However, for purposes of computing the related stock-based compensation expense, we reassessed the fair value of our common stock at $0.52 per share utilizing a retrospective valuation.
61
Table of Contents
May 2011 through October 2011:In June 2011, we filed a demand for arbitration before the American Arbitration Association for a determination of our rights and obligations and those of Ucyclyd under a collaboration agreement between the parties. In our demand for arbitration, we requested a judgment regarding the rights of the parties in connection with the development activities relating to Ravicti, including our rights relating to the submission of an NDA to the FDA for Ravicti for the treatment of UCD. In September 2011, an arbitration date was established for January 2012 to adjudicate the matter. In August 2011, the last patient completed the 12 months of follow-up in the long-term safety extension portion of our Phase II, fixed-sequence, open-label study of the safety and tolerability of Ravicti when compared to BUPHENYL in children aged 6 through 17 years with UCD. In September 2011, the last patient completed the 12 months of follow-up in the long-term safety extension portion of our pivotal Phase III, open-label study in adults of Ravicti for the long-term treatment of UCD. In October 2011, we completed the enrollment for a Phase II, randomized, double-blind, placebo-controlled study of the safety and efficacy of Ravicti for maintaining remission in subjects with HE. Also in October 2011, we raised an additional $7.5 million of convertible notes from our existing investors. We utilized an income approach to value our equity and continued to use two scenarios in our common stock valuation methodology — a remaining private company scenario at 50% probability and an IPO scenario at 50% probability. As a result of these factors, we estimated the fair value of our common stock to be $0.66 per share as of October 31, 2011. No options were granted between May 2011 and October 2011.
November 2011 through December 2011:In December 2011, the last patient completed the switch-over, open-label study of the safety, pharmacokinetics, and efficacy of Ravicti, which is followed by a long-term safety extension portion in pediatric patients with UCD under 6 years of age. In December 2011, we also submitted an NDA to the FDA for UCD. During this period, we made significant progress in negotiating a revised agreement with Ucyclyd related to Ravicti, BUPHENYL and AMMONUL. We utilized an income approach to value our equity and continued to use two scenarios in our common stock valuation methodology — a remaining private company scenario at 50% probability and an IPO scenario at 50% probability. As a result of business developments and applying our common stock valuation methodology, we estimated the fair value of our common stock to be $0.83 per share as of December 31, 2011. No options were granted during the period from November to December 2011.
January 2012 through March 2012:In January 2012, we agreed on key terms with Ucyclyd related to our interpretation of the collaboration agreement with them. In February 2012, we received notice of our NDA acceptance by the FDA for UCD. In March 2012, the last patient enrolled completed the study for a Phase II, randomized, double-blind, placebo-controlled study of the safety and efficacy of Ravicti for subjects with overt HE. Additionally, during this period, we started and completed the enrollment of the long-term treatment portion of the open-label study of the safety, pharmacokinetics, and efficacy of Ravicti in patients aged 29 days through 5 years. In March 2012, we entered into a revised collaboration agreement with Ucyclyd. During the period, we re-engaged in discussions with investment banks regarding a potential IPO. We utilized an income approach to value our equity and continued to use two scenarios in our common stock valuation methodology — a remaining private company scenario at 30% probability and an IPO scenario at 70% probability. As a result of business developments and applying our common stock valuation methodology, we estimated the fair value of our common stock to be $1.20 per share as of March 1, 2012.
Results of Operations
Comparison of the Three Months Ended March 31, 2011 and 2012
| Three Months Ended March 31, | Increase/ (Decrease) | % Increase/ (Decrease) | ||||||||||||||
| (in thousands, except for percentages) | 2011 | 2012 | ||||||||||||||
| (unaudited) | ||||||||||||||||
Research and development | $ | 4,300 | $ | 8,902 | $ | 4,602 | 107 | % | ||||||||
General and administrative | 1,361 | 2,077 | 716 | 53 | % | |||||||||||
Selling and marketing | 250 | 246 | (4 | ) | (0 | ) | ||||||||||
Interest income | 4 | 4 | — | — | ||||||||||||
Interest expense | — | (1,040 | ) | (1,040 | ) | 100 | ||||||||||
Other income (expense), net | — | 375 | 375 | 100 | ||||||||||||
62
Table of Contents
Research and Development Expenses
Research and development expenses increased by $4.6 million, or 107%, to $8.9 million for the three months ended March 31, 2012 from $4.3 million for the three months ended March 31, 2011. The increase in research and development expenses in the first quarter of 2012 as compared to the first quarter of 2011 was primarily due to $5.7 million incurred in connection with the purchase agreement with Ucyclyd as discussed in Note 3 to our consolidated financial statements. The increase was offset in part by lower clinical development costs of $1.0 million and lower professional and consulting fees of $0.2 million due to lower costs in our HE Phase II trial as patient enrollment was largely completed in the fourth quarter of 2011 and as a result of completing the long term safety extension trial in adults with UCD in 2011.
For the period from inception to March 31, 2012, research and development expenses amounted to $79.1 million. Research and development expenses comprise primarily clinical and pre-clinical development costs of $42.6 million, payroll related costs of $12.5 million, professional consulting costs of $7.1 million, the expenses incurred for the purchase of Ravicti of $5.7 million, and regulatory related costs of $3.3 million.
General and Administrative Expenses
General and administrative expenses increased by $0.7 million, or 53%, to $2.1 million for the three months ended March 31, 2012 from $1.4 million for the three months ended March 31, 2011. The increase in the first quarter of 2012 was primarily due to an increase in consulting costs in preparation for our Form S-1 registration statement filing and also due to legal fees related to our amended agreements with Ucyclyd.
For the period from inception to March 31, 2012, general and administrative expenses amounted to $21.2 million. General and administrative expenses comprise primarily payroll related expenses of $7.5 million, professional and consulting costs of $11.5 million and office and rent related costs of $1.1 million.
Selling and Marketing Expenses
Selling and marketing expenses did not significantly change for the first quarter of 2012 compared to the same period in 2011.
For the period from inception to March 31, 2012, selling and marketing expenses amounted to $7.3 million. Selling and marketing expenses comprise primarily payroll related expenses of $3.8 million, professional and consulting costs of $1.3 million, and marketing related costs of $1.5 million.
Interest Income
Interest income consists of interest earned on our cash and cash equivalents. There was no change in the first quarter of 2012 compared to the same period in 2011.
For the period from inception to March 31, 2012, interest income amounted to $0.4 million which consists of interest earned on our cash and cash equivalents.
Interest Expense
We recognized $1.0 million in interest expense for the three months ended March 31, 2012 compared to none in the same period in 2011. The main components of our interest expense are $0.7 million interest expense incurred relating to our 2011 convertible notes and $0.3 million amortization of our debt discount.
63
Table of Contents
For the period from inception to March 31, 2012, interest expense amounted to $6.3 million primarily related to our interest expense on our convertible notes payable and our loan and security agreement entered into in 2007.
Other Income (Expense), net
Other income of $0.4 million primarily relates to the change in fair values of our common and preferred stock warrants and call option liability associated with our convertible notes. During the three months ended March 31, 2012, we recorded a change in fair value of $0.5 million of expense and $0.2 million of income related to the common stock warrants and the preferred stock warrants, respectively. Additionally, we recorded $0.7 million to other income relating to the change in the fair value of call option liability upon issuance of convertible notes in February 2012. The call option related to our convertible notes is more fully described in Note 6 to our consolidated financial statements included elsewhere in this prospectus.
For the period from inception to March 31, 2012, other income amounted to $1.7 million, consisting primarily of the change in the fair value of the preferred stock liability associated with the Series C-2 convertible preferred stock.
Comparison of the Year Ended December 31, 2010 and the Year Ended December 31, 2011
| Years Ended December 31, | Increase/ (Decrease) | % Increase/ (Decrease) | ||||||||||||||
| (in thousands, except for percentages) | 2010 | 2011 | ||||||||||||||
Research and development | $ | 23,111 | $ | 17,236 | $ | (5,875 | ) | (25 | )% | |||||||
General and administrative | 2,693 | 8,162 | 5,469 | 203 | ||||||||||||
Selling and marketing | 797 | 761 | (36 | ) | (5 | ) | ||||||||||
Interest income | 43 | 28 | (15 | ) | (35 | ) | ||||||||||
Interest expense | 1 | 2,554 | 2,553 | NM | ||||||||||||
Other income (expense), net | 1,106 | (731 | ) | (1,837 | ) | NM | ||||||||||
Research and Development Expenses
Research and development expenses decreased by $5.9 million, or 25%, to $17.2 million for the year ended December 31, 2011 from $23.1 million for the year ended December 31, 2010.
The decrease in research and development expenses in 2011 as compared to 2010 was primarily due to:
| • | lower clinical development expenses by $4.9 million primarily as a result of partial year (approximately nine-months) of expenses incurred related to our pivotal Phase III trial in UCD compared to full year of expenses incurred in 2010. Additionally, we initiated and completed a heart rhythm safety trial in Ravicti for UCD in 2010 with no corresponding trial in 2011; |
| • | lower manufacturing expenses of $1.3 million primarily as a result of manufacturing of Ravicti for our clinical trials that occurred in 2010 without similar expenses in 2011; |
| • | lower professional, consulting expenses and travel related expenses, which decreased by $0.4 million as a result of the completion of our pivotal Phase III trial in UCD in 2011; |
| • | lower preclinical related expenses of $0.6 million as a result of fewer ongoing preclinical studies in 2011 as compared to 2010. |
64
Table of Contents
The decrease was partially offset by:
| • | higher clinical regulatory related expenses, which increased by $1.1 million in 2011 as a result of the higher expenses associated with the filing and submission of the NDA for Ravicti in UCD patients aged 6 years and above; and |
| • | the receipt of a U.S. therapeutic discovery project grant of $0.2 million, which decreased 2010 research and development expenses. There was no similar grant in 2011. |
General and Administrative Expenses
General and administrative expenses increased by $5.5 million, or 203%, to $8.2 million for the year ended December 31, 2011 from $2.7 million for the year ended December 31, 2010. The increase in 2011 was primarily due to an increase in professional and consulting costs in preparation for a potential financing and also due to legal fees incurred in relation to our arbitration with Ucyclyd.
Selling and Marketing Expenses
Selling and marketing expenses did not significantly change from 2010 to 2011.
Interest Income
Interest income consists of interest earned on our cash and cash equivalents. The decrease in interest income in 2011 to $28,000 from $43,000 in 2010 was primarily due to lower aggregate interest rates in 2011 as compared to 2010.
Interest Expense
Interest expense increased to approximately $2.6 million for the year ended December 31, 2011 from $1,000 for the year ended December 31, 2010. The increase in interest expense in 2011 was the result of a $1.0 million interest expense incurred relating to our convertible notes and a $1.6 million amortization of our debt discount.
Other Income (Expense), net
Other income (expense), net, decreased by $1.8 million to $0.7 million of expense for the year ended December 31, 2011 from $1.1 million of income for the year ended December 31, 2010. In 2011, the component of other income (expense), net primarily relates to the change in fair values related to common and preferred stock warrants and call option liability associated with our convertible notes. During the year-ended December 31, 2011, we recorded $0.9 million and $0.2 million in other expense to reflect the change in fair value of the common stock warrants and the preferred stock warrants, respectively. Also during the year ended December 31, 2011, we recorded $0.4 million in other income to reflect the change in the fair value of the call options related to our convertible notes. The call options are more fully described in Note 6 to our consolidated financial statements included elsewhere in this prospectus.
In 2010, other income (expense), net, relates to the re-measurement of the preferred stock liability in April 2010 upon the issuance of the second tranche of the Series C-2 preferred stock.
Comparison of the Year Ended December 31, 2009 and the Year Ended December 31, 2010
| (in thousands, except for percentages) | Years Ended December 31, | Increase/ (Decrease) | % Increase/ (Decrease) | |||||||||||||
| 2009 | 2010 | |||||||||||||||
Research and development | $ | 11,030 | $ | 23,111 | $ | 12,081 | 110 | % | ||||||||
General and administrative | 1,909 | 2,693 | 784 | 41 | ||||||||||||
Selling and marketing | 462 | 797 | 335 | 73 | ||||||||||||
Interest income | 39 | 43 | 4 | 10 | ||||||||||||
Interest expense | 763 | 1 | 762 | 99 | ||||||||||||
Other income (expense), net | 525 | 1,106 | 581 | 111 | ||||||||||||
65
Table of Contents
Research and Development Expenses
Research and development expenses increased by $12.1 million, or 110%, to $23.1 million for the year ended December 31, 2010 from $11.0 million for the year ended December 31, 2009.
The increase in research and development expenses in 2010 as compared to 2009 was primarily due to:
| • | higher clinical development expenses, which increased by $9.4 million primarily as a result of: a full year of expenses incurred related to our pivotal Phase III trial in UCD and our Phase II clinical trial in HE as compared to a partial year for these trials in 2009, and initiation and completion of a heart rhythm safety trial in UCD in 2010 with no corresponding trial in 2009; |
| • | higher manufacturing expenses, which increased by $1.4 million primarily as a result of manufacturing of Ravicti for our clinical trials that occurred in 2010 without similar expenses in 2009; |
| • | higher professional and consulting expenses, which increased by $1.0 million as a result of our greater clinical development efforts in 2010; and |
| • | higher clinical regulatory related expenses, which increased by $0.9 million as a result of the greater expenses incurred in 2010 for the preparation for the planned submission of the NDA for Ravicti in UCD patients aged 6 years and above. |
The increase was partially offset by:
| • | lower preclinical related expenses of $0.4 million as a result of fewer ongoing preclinical studies in 2010 as compared to 2009. |
| • | the receipt of a U.S. therapeutic discovery project grant totaling $0.2 million, which was offset against 2010 research and development expenses. |
General and Administrative Expenses
General and administrative expenses increased by $0.8 million, or 41%, to $2.7 million for the year ended December 31, 2010 from $1.9 million for the year ended December 31, 2009. The increase in 2010 was primarily due to an increase in professional and consulting costs of $0.5 million as well as an increase in salary and related expenses of $0.2 million related to hiring two employees in finance and administration to address our infrastructure needs.
Selling and Marketing Expenses
Selling and marketing expenses increased by $0.3 million, or 73%, to $0.8 million for the year ended December 31, 2010 from $0.5 million for the year ended December 31, 2009. The net increase in 2010 is primarily due to increased consulting expenses and marketing research expenses of $0.4 million offset by a $0.1 million decrease in salaries.
Interest Income
The increase in interest income in 2010 was primarily due to the higher aggregate cash and cash equivalents in 2010 as compared to 2009.
Interest Expense
Interest expense decreased by $0.8 million, or 99%, to $1,000 for the year ended December 31, 2010 from $0.8 million for the year ended December 31, 2009. The decrease was due to the conversion of the convertible
66
Table of Contents
notes issued in 2008 and 2009 to Series C-1 convertible preferred stock in June 2009 and the repayment of outstanding bank loans in July 2009.
Other Income (Expense), net
Other income (expense), net increased by $0.6 million, or 111%, to $1.1 million for the year ended December 31, 2010 from $0.5 million for the year ended December 31, 2009. The increase in other income (expense), net, was due to the re-measurement of preferred stock liability in April 2010 upon issuance of the second tranche of the Series C-2 preferred stock, which resulted in an increase in non-cash income for the year ended December 31, 2010.
Liquidity and Capital Resources
Since our inception in November 2006, we have funded our operations primarily through proceeds from the sale of convertible preferred stock, bank debt and the issuance of convertible debt. We have not generated any revenue from the sale of any products in the last three years. We have incurred losses and generated negative cash flows from operations since inception. As of December 31, 2011 and March 31, 2012, our principal sources of liquidity were our cash and cash equivalents, which totaled $7.0 million and $3.7 million, respectively.
From inception through March 31, 2012, we have received net proceeds of $66.1 million from the sale of convertible preferred stock and $32.5 million from the issuance of convertible debt that has not converted into preferred stock as of March 31, 2012.
In April 2011, we entered into a bridge loan financing, or the April 2011 bridge financing, in which we issued $17.5 million in aggregate principal amount of convertible promissory notes in April 2011, or the April 2011 notes, and $8,285 in aggregate principal amount of convertible promissory notes in May 2011, or the May 2011 notes. The April 2011 notes and May 2011 notes bear interest at 6% per annum and will automatically convert into shares of our common stock immediately prior to the closing of this offering. For additional information, see Note 6 to our consolidated financial statements appearing elsewhere in this prospectus.
In October 2011, we entered into a bridge loan financing, or the October 2011 bridge financing, in which we issued $7.5 million in aggregate principal amount of convertible promissory notes in October 2011, or the October 2011 notes, $3,551 in aggregate principal amount of convertible promissory notes in November 2011, or the November 2011 notes, and $7.5 million in aggregate principal amount of convertible promissory notes in February 2012, or the February 2012 notes. The October 2011 notes, November 2011 and February 2012 notes bear interest at 6% per annum and will automatically convert into shares of our common stock immediately prior to the closing of this offering. For additional information, see Note 6 to our consolidated financial statements appearing elsewhere in this prospectus.
In April 2012, we entered into a $10.0 million loan and security agreement, or the loan agreement with Silicon Valley Bank and Leader Lending, LLC - Series B, or the lenders. The loan carries an interest rate of 8.88%, with interest only payments for the period of 9 months from May 1, 2012. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from February 1, 2013. In connection with the loan agreement, we granted a security interest in all of our assets, except intellectual property. Our obligations to the lender include restrictions on borrowing, asset transfers, placing liens or security interest on our assets including our intellectual property, mergers and acquisitions and distributions to stockholders. The loan agreement also requires us to provide the lenders monthly financials and compliance certificate within 30 days of each month end, annual audited financials within 180 days of each fiscal year-end and annual approved financial projections. We issued warrants to the lenders to purchase a total of 462,686 shares of common stock with an exercise price of $0.67 per share. The loan agreement requires immediate repayment of amounts outstanding
67
Table of Contents
upon an event of default, as defined in the loan agreement, which includes events such as a payment default, a covenant default or the occurrence of a material adverse change, as defined in the loan agreement.
Pursuant to the terms of the loan agreement, once we raise at least $30.0 million from the sale of equity securities or subordinated debt, the lenders also agreed to grant us a one-time single loan in the amount of $2.5 million, or the bank term loan. The lender’s obligation to lend terminates on the earlier of (i) an event of default or (ii) September 30, 2012. The principal amount outstanding for the bank term loan accrues interest at a per annum rate equal to the greater of (i) 8.88% and (ii) the Treasury Rate, as defined in the loan agreement, on the date the loan is funded plus 8.50%, with interest only payments for the period of 9 months from the date the loan is funded. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from the date the loan is funded.
Our recurring operating losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our consolidated financial statements as of and for the year ended December 31, 2011 with respect to this uncertainty. We have no current source of revenue to sustain our present activities, and we do not expect to generate revenue until, and unless, the FDA or other regulatory authorities approve Ravicti for the treatment of UCD or until we exercise our option to purchase Ucylcyd’s worldwide rights to BUPHENYL and AMMONUL and begin commercializing them. Accordingly, our ability to continue as a going concern will require us to obtain additional financing to fund our operations.
Cash Flows
The following table sets forth the major sources and uses of cash for the periods set forth below (in thousands)
| Year Ended December 31 | Three Months Ended March 31 | |||||||||||||||||||
| (In thousands) | 2009 | 2010 | 2011 | 2011 | 2012 | |||||||||||||||
| (unaudited) | ||||||||||||||||||||
Net cash (used in) provided by: | ||||||||||||||||||||
Operating activities | $ | (11,540 | ) | $ | (25,889 | ) | $ | (24,531 | ) | $ | (3,838 | ) | $ | (10,706 | ) | |||||
Investing activities | (4 | ) | (40 | ) | (12 | ) | (2 | ) | (128 | ) | ||||||||||
Financing activities | 20,528 | 22,435 | 24,982 | — | 7,552 | |||||||||||||||
Net increase (decrease) in cash and cash equivalents | $ | 8,984 | $ | (3,494 | ) | $ | 439 | $ | (3,840 | ) | $ | (3,282 | ) | |||||||
Net cash used in operating activities was $11.5 million, $25.9 million and $24.5 million for the years ended December 31, 2009, 2010 and 2011, respectively, and $3.8 million and $10.7 million for the three months ended March 31, 2011 and 2012, respectively. The primary use of cash was to fund our operations related to the development of Ravicti in UCD and HE in each of these years and for the three months ended March 31, 2011 and 2012. The lower net cash used in operating activities in 2009 was primarily due to reduced development expenses, lower sales and marketing and general and administrative expenses resulting from our reduction in workforce in June 2008. The increase in 2010 was due to increased development expenses primarily related to our UCD clinical trials. The slight decrease in 2011 was primarily due to a decrease in development expenses as we completed certain UCD clinical trials during 2011. The increase in operating activities for the three months ended March 31, 2012 was due mainly to the expense incurred for the purchase of Ravicti of $5.7 million.
Net cash used in investing activities amounted to approximately $4,000, $40,000, $12,000 and $2,000 for the years ended December 31, 2009, 2010 and 2011 and for the three months ended March 31, 2011, respectively, which consisted mainly of property and equipment purchases. For the three months ended March 31, 2012, net cash used in investing activities amounted to approximately $0.1 million, consisting mainly of the purchase of the option
68
Table of Contents
to purchase rights to BUPHENYL and AMMONUL of $0.3 million partially offset by a decrease in restricted cash of $0.2 million.
Net cash provided by financing activities amounted to $20.5 million, $22.4 million and $25.0 million for 2009, 2010 and 2011, respectively, and none and $7.6 million for the three months ended March 31, 2011 and 2012, respectively. The net cash provided by financing activities in 2009 consisted primarily of $22.1 million in net proceeds from the issuance of convertible preferred stock, and $5.0 million in proceeds from the issuance of convertible notes, partially offset by principal payments of $6.6 million under a loan and security agreement entered into in 2007. The net cash provided by financing activities in 2010 consisted primarily of net proceeds from the issuance of convertible preferred stock in the amount of $22.4 million. The net cash provided by financing activities in 2011 consisted primarily of net proceeds from the issuance of the April 2011 notes and October 2011 notes in the amount of $25.0 million. The net cash provided by financing activities for the three months ended March 31, 2012, related to the issuance of the February 2012 Notes in the amount of $7.5 million.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of March 31, 2012 (in thousands):
| Payments Due By Period | ||||||||||||||||||||
Contractual Obligations | Total | Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | |||||||||||||||
Principal obligations on the convertibles notes(1) | $ | 32,486 | $ | 32,486 | $ | — | $ | — | $ | — | ||||||||||
Interest obligations on the convertibles notes(1) | 2,776 | 2,776 | — | — | — | |||||||||||||||
Operating Leases(2) | 362 | 190 | 173 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total(3) | $ | 35,624 | $ | 35,452 | $ | 173 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
The following table summarizes our contractual obligations and commitments as of December 31, 2011 (in thousands):
| Payments Due By Period | ||||||||||||||||||||
Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
Principal obligations on the convertible notes(1) | $ | 24,982 | $ | 24,982 | $ | — | $ | — | $ | — | ||||||||||
Interest obligations on the convertible notes(1) | 2,372 | 2,372 | — | — | — | |||||||||||||||
Operating leases(2) | 443 | 270 | 173 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total(3) | $ | 27,797 | $ | 27,624 | $ | 173 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| (1) | The principal and accrued interest under the convertible notes will convert into common stock upon the completion of our initial public offering. |
| (2) | Operating lease obligations consist primarily of lease payments for our South San Francisco facility. |
| (3) | This table does not include (a) any milestone payments, which may become payable to third parties under license agreements, as the timing and likelihood of such payments are not known, and (b) any royalty payments to third parties as the amounts, timing and likelihood of such payments are not known. |
Amended Collaboration Agreement with Ucyclyd
On March 22, 2012, we entered into a purchase agreement with Ucyclyd under which we purchased the worldwide rights to Ravicti and restated collaboration agreement under which Ucyclyd granted us an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed price at a future defined date,
69
Table of Contents
plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended.
Under the purchase agreement, we purchased all of the worldwide rights to Ravicti for an initial upfront payment of $6.0 million. We will also pay tiered mid to high single digit royalties on global net sales of Ravicti and may owe regulatory milestones of up to $15.8 million related to approval of Ravicti in HE, regulatory milestones of up to $7.3 million per indication for approval of Ravicti in indications other than UCD or HE, and net sales milestones of up to $38.8 million if Ravicti is approved for use in indications other than UCD (such as HE) and all annual sales targets are reached. In addition, the intellectual property license agreement executed between Ucyclyd and Brusilow Enterprises, LLC, or Brusilow, and the research agreement executed between Ucyclyd and Dr. Marshall L. Summar, or Summar, were assigned to us, and we have assumed the royalty obligation under the Brusilow agreement for sales of Ravicti in any indication, and the royalty obligations under the Summar agreement on sales of Ravicti to treat HE. We will also pay Brusilow an annual license extension fee to keep the Brusilow license in effect. The extension fee is payable until our first commercial sale of Ravicti following FDA approval. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Under the terms of the restated collaboration agreement, we have an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain AMMONUL. We will be permitted to exercise this option for 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. The upfront purchase price for AMMONUL and BUPHENYL is $22.0 million, which we may fund by drawing on a loan commitment from Ucyclyd. The loan commitment would be payable in eight quarterly payments and would bear interest at a rate of 9% per annum, and would be secured by the BUPHENYL and AMMONUL assets. If the Ravicti NDA for UCD is not approved by January 1, 2013, then Ucyclyd is obligated to make monthly payments of $0.5 million to us until the earliest of (1) FDA approval of the Ravicti NDA for UCD, (2) June 30, 2013 and (3) our written notification of our decision not to purchase BUPHENYL and AMMONUL.
If we exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, then Ucyclyd has the time-limited right to elect to retain all rights to AMMONUL for a purchase price of $32.0 million. If Ucyclyd exercises this option, Ucyclyd will pay us a net payment of $13.0 million on closing of the purchase transaction, which reflects the purchase price for BUPHENYL being set-off against Ucyclyd’s retention payment for AMMONUL. If Ucyclyd retains rights to AMMONUL, subject to certain terms and conditions, we retain a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
April 2011 Convertible Notes Payable
In connection with the April 2011 bridge financing, we entered into a convertible note and warrant purchase agreement, or the April 2011 note agreement, with existing investors pursuant to which we issued the April 2011 notes and the May 2011 notes raising $17.5 million. The principal and the interest under the April 2011 notes and the May 2011 notes are automatically convertible into common stock immediately prior to the close of our initial public offering, at a conversion price equal to our initial public offering price. The April 2011 notes and the May 2011 notes accrue interest at a rate of 6% per annum. For additional information, see Note 6 to our consolidated financial statements appearing elsewhere in this prospectus.
October 2011 and February 2012 Convertible Notes Payable
In October 2011, we entered into a convertible note and warrant purchase agreement, or the October 2011 note agreement, with existing investors pursuant to which we issued the October 2011 notes and the November
70
Table of Contents
2011 notes raising $15.0 million and the February 2012 notes raising $7.5 million. The principal and the interest under the October 2011 notes, the November 2011 notes and the February 2012 notes are automatically convertible into common stock immediately prior to the close of our initial public offering, at a conversion price equal to our initial public offering price. The October 2011 notes, the November 2011 notes and the February 2012 notes accrue interest at a rate of 6% per annum. For additional information, see Note 6 to our consolidated financial statements appearing elsewhere in this prospectus.
Common Stock Warrants Liability
Pursuant to our April 2011 note agreement, we issued warrants to purchase shares of our common stock at an exercise price of $0.67 per share in April 2011, or the April 2011 warrants, and in May 2011, or the May 2011 warrants. Each of the April 2011 warrants and the May 2011 warrants contain a customary net issuance feature. The April 2011 warrants and the May 2011 warrants will expire if unexercised prior to the close of our initial public offering. For additional information, see Note 7 to our consolidated financial statements appearing elsewhere in this prospectus.
Preferred Stock Warrants Liability
Pursuant to our October 2011 note agreement, we issued warrants to purchase shares of our preferred stock in October 2011, or the October 2011 warrants, in November 2011, or the November 2011 warrants, and February 2012, or the February 2012 warrants, at exercise prices dependent upon the occurrence of certain events. Each of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants contain a customary net issuance feature. The October 2011 warrants, the November 2011 warrants and the February 2012 warrants will expire if unexercised prior to the close of our initial public offering. For additional information, see Note 7 to our consolidated financial statements appearing elsewhere in this prospectus.
Future Funding Requirements
We will likely need to obtain additional financing to fund our future operations, including the development, approval and commercialization of Ravicti in UCD and supporting sales and marketing activities related to BUPHENYL and AMMONUL (if not retained by Ucyclyd), a potential Phase III trial in HE, as well as the development of any additional product candidates we might acquire or develop on our own. Our future funding requirements will depend on many factors, including, but not limited to:
| • | our ability to successfully commercialize Ravicti for the treatment of UCD, and Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL if purchased from Ucyclyd; |
| • | the amount of sales and other revenues from products that we may commercialize, if any, including the selling prices for such products and the availability of adequate third-party reimbursement; |
| • | selling and marketing costs associated with our UCD products, including the cost and timing of expanding our marketing and sales capabilities and establishing a network of specialty pharmacies; |
| • | the progress, timing, scope and costs of our nonclinical studies and clinical trials, including the ability to timely enroll patients in our planned and potential future clinical trials; |
| • | the time and cost necessary to obtain regulatory approvals and the costs of post-marketing studies that may be required by regulatory authorities; |
| • | the costs of obtaining clinical and commercial supplies of Ravicti, and BUPHENYL and AMMONUL if we purchase these products from Ucyclyd; |
71
Table of Contents
| • | payments of milestones and royalties to third parties, including Ucyclyd; |
| • | cash requirements of any future acquisitions of product candidates; |
| • | the time and cost necessary to respond to technological and market developments; |
| • | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| • | any changes made to, or new developments in, our restated collaboration agreement with Ucyclyd or any new collaborative, licensing and other commercial relationships that we may establish. |
We have not generated any revenue from the sale of any products in the last three years. We do not know when, or if, we will generate any revenue. We do not expect to generate any revenue unless or until we obtain marketing approval of, and commercialize, Ravicti, or if we purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL (if not retained by Ucyclyd). We expect our continuing operating losses to result in increases in cash used in operations over the next several years.
We believe that our current cash and cash equivalents, together with the net proceeds from this offering, as well as potential payments from Ucyclyd beginning January 1, 2013 if Ravicti is not approved by the FDA prior to that, will be sufficient to fund our operations through commercial launch of Ravicti in UCD, assuming commercialization occurs in the first half of 2013. We may raise additional funds within this period of time through collaborations and public or private debt or equity financings.
We have based these estimates on a number of assumptions that may prove to be wrong, and changing circumstances beyond our control may cause us to consume capital more rapidly than we currently anticipate. For example, our ongoing clinical trial of Ravicti in pediatric patients aged 29 days through 5 years may encounter technical or other difficulties that could increase our development costs more than we currently expect or if the FDA requires us to conduct additional clinical trials prior to approving Ravicti. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
Additional financing may not be available when we need it or may not be available on terms that are favorable to us. We may seek to raise additional capital through a combination of private and public equity offerings and debt financings. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends.
If adequate funds are not available to us on a timely basis, or at all, we may be required to terminate or delay clinical trials or other development activities for Ravicti, or delay our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize Ravicti, BUPHENYL and AMMONUL, if we obtain marketing approval. We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable.
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have
72
Table of Contents
been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts.
Quantitative and Qualitative Disclosure About Market Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. We had cash and cash equivalents of $6.6 million, $7.0 million and $3.7 million at December 31, 2010 and 2011 and March 31, 2012, respectively. Given the short-term nature of our cash equivalents, we believe that our interest rate risk is not significant to our consolidated financial statements. Our April and October 2011 notes carry a fixed interest rate and, as such, are not subject to interest rate risk. We do not have any foreign currency or other derivative financial instruments.
Jumpstart Our Business Startups Act of 2012
The JOBS Act permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
Recent Accounting Pronouncements
Effective January 1, 2012, we adopted the Financial Accounting Standards Board, or FASB, Accounting Standards Update, or ASU, No. 2011-04,“Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and International Financial Reporting Standards (“IFRS”),” issued in May 2011. This pronouncement was issued to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and IFRS. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements particularly for Level 3 fair value measurements. This pronouncement is effective for reporting periods beginning on or after December 15, 2011. The new guidance will require prospective application. The adoption of this accounting standard update required expanded disclosure only and did not have an impact on our consolidated financial position, results of operations or cash flows.
In December 2011, FASB issued ASU No. 2011-11,“Balance Sheet (Topic 210).” This update provides enhanced disclosure requirements regarding the nature of an entity’s right of offset related to arrangements associated with its financial instruments and derivative instruments. The new guidance requires the disclosure of the gross amounts subject to rights of set-off, the amounts offset in accordance with the accounting standards followed, and the related net exposure. This pronouncement is effective for financial reporting period beginning on or after January 1, 2013 and full retrospective application is required. We do not expect that the adoption of this ASU will have any material impact on our consolidated financial statements.
73
Table of Contents
Overview
We are a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat disorders in the areas of orphan diseases and hepatology. Our product candidate, Ravicti™ (glycerol phenylbutyrate), is designed to lower ammonia in the blood. Ammonia is produced in the intestine after a person eats protein and is normally detoxified in the liver by conversion to urea. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. We are developing Ravicti to treat two different diseases in which blood ammonia is elevated: the most prevalent urea cycle disorders, or UCD, and hepatic encephalopathy, or HE. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or protein transporters that constitute the urea cycle, which in a healthy individual removes ammonia through the conversion of ammonia to urea. HE may develop in some patients with liver scarring, known as cirrhosis, or acute liver failure and is a chronic disease which fluctuates in severity and may lead to serious neurological damage. On December 23, 2011, we submitted a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for Ravicti for the chronic management of UCD in patients aged 6 years and above. The FDA accepted the NDA for review in February 2012. Under the Prescription Drug User Fee Act, or PDUFA, the FDA is due to notify us regarding Ravicti’s approval status by October 23, 2012, unless that action date is extended by the FDA. In April 2012, we submitted data from the switchover portion of a clinical trial in UCD patients aged 29 days through 5 years and a revised draft package insert requesting approval of Ravicti to include this patient population. We currently expect to commercially launch Ravicti in the first half of 2013.
Ravicti was granted orphan drug designation by the FDA for the maintenance treatment of patients with UCD. Orphan drug designation is given to a drug candidate intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States. We have also been granted fast track designation by the FDA for this indication.
We believe UCD occur in approximately 1 in 10,000 births in the United States. We estimate there are approximately 2,100 cases of UCD in the United States of which approximately 1,100 have been diagnosed. However, we estimate only 425 patients are currently treated with BUPHENYL® (sodium phenylbutyrate) Tablets and Powder, which is the only branded therapy approved by the FDA for chronic management of the most prevalent UCD. Additionally, there are approximately 90 patients currently on Ravicti in our ongoing long-term safety clinical trials.
We believe BUPHENYL use is limited due to the combination of high pill burden or large quantity of powder that must be taken, frequency of dosing (3-6 times per day), the unpleasant taste and smell, and tolerability issues. In addition, the sodium content of the maximum daily dose of BUPHENYL exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure.
Significantly elevated ammonia levels with corresponding neurological symptoms are known as hyperammonemic, or HA, crises. We believe that Ravicti may reduce HA crises as compared to BUPHENYL, and, if approved, will offer benefits that enhance tolerability and increase compliance in support of improved disease management. Four clinical trials have shown ammonia control with Ravicti to be non-inferior and directionally favorable to BUPHENYL. Ravicti is nearly tasteless and odorless and does not contain any sodium. Ravicti uses the same vehicle for ammonia removal as BUPHENYL but requires a much smaller volume of drug. For example, approximately 1 tablespoon of Ravicti liquid is equivalent to the FDA-approved maximum daily dose of 40 tablets of BUPHENYL. The smaller volume of drug required for Ravicti is due to the differences in physical and chemical properties between Ravicti and BUPHENYL. Based on our market research with physicians and patient preference data from our clinical trials, we anticipate that most BUPHENYL patients for whom Ravicti is indicated under the FDA-approved label will transition to Ravicti if the drug is approved.
74
Table of Contents
In November 2010, we announced the successful completion of a pivotal Phase III trial for Ravicti in the treatment of UCD in adults in accordance with a Special Protocol Assessment agreement, or SPA, with the FDA. A SPA is an agreement with the FDA that a Phase III trial’s design, clinical endpoints, patient population and statistical analyses are acceptable for FDA approval. This four-week, multi-center, randomized, double-blind, placebo-controlled, cross-over study met its primary endpoint of demonstrating the non-inferiority of Ravicti to BUPHENYL in controlling blood ammonia in adults aged 18 years and above with UCD. An open-label pediatric Phase II trial in 11 UCD patients aged 6 through 17 years that compared Ravicti to BUPHENYL also demonstrated the non-inferiority of Ravicti to BUPHENYL in ammonia control. We are currently conducting a clinical trial in UCD patients aged 29 days through 5 years designed to demonstrate the safety and efficacy in this patient population. The efficacy portion of this trial is complete and a complete study report was submitted to the FDA in April 2012; however, the results of the 12-month safety extension portion of the study will not be available until the second quarter of 2013. As part of the April update to the FDA, we submitted a revised draft package insert requesting approval of Ravicti for patients aged down to 29 days. If the FDA classifies this submission as a major amendment, the PDUFA action date will likely be delayed.
We originally obtained rights to develop Ravicti from Ucyclyd Pharma, Inc., or Ucyclyd, a wholly owned subsidiary of Medicis Pharmaceutical Corporation in 2007 pursuant to a collaboration agreement. Under the terms of the original collaboration agreement, we obtained: limited research and development rights to Ravicti for UCD, HE and other indications; the right to promote BUPHENYL and AMMONUL in the United States for UCD, which right was later terminated in a subsequent amendment to the collaboration agreement; and certain future rights to purchase the worldwide rights to BUPHENYL, AMMONUL and Ravicti. In March 2012, pursuant to an asset purchase agreement, or purchase agreement, with Ucyclyd we purchased all of the worldwide rights to Ravicti for an upfront payment of $6.0 million, future payments based upon the achievement of regulatory milestones in indications other than UCD, sales milestones, and mid to high single digit royalties on global net sales of Ravicti. In addition, we simultaneously entered into an amended and restated collaboration agreement, or restated collaboration agreement, with Ucyclyd pursuant to which we have an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL® (sodium phenylacetate and sodium benzoate) injection 10%/10%, the only adjunctive therapy currently FDA-approved for the treatment of HA crises in patients with the most prevalent UCD, for an upfront payment of $22.0 million, plus subsequent milestone and royalty payments. We will be permitted to exercise this option for a period of 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. To fund this upfront payment, we may draw on a loan commitment from Ucyclyd, which loan would be payable over eight quarters. If we exercise our option, Ucyclyd has a time-limited option to retain AMMONUL at a purchase price of $32.0 million. If Ucyclyd exercises its option and retains AMMONUL, the upfront purchase price for Ucyclyd’s worldwide rights to BUPHENYL will be $19.0 million resulting in a net payment from Ucyclyd to us of $13.0 million upon close of the transaction.
To expand the commercial potential of Ravicti we have completed a Phase II trial assessing the safety and efficacy of Ravicti in the treatment of episodic HE. Episodic HE can be diagnosed clinically through a set of signs and symptoms. The Phase II trial met its primary endpoint, which was to demonstrate that the proportion of patients experiencing an HE event was significantly lower on Ravicti versus placebo, both administered in addition to standard of care, including lactulose and/or refaximin. The FDA has also granted orphan drug designation for Ravicti for this indication. HE is a serious but potentially reversible neurological disorder that can occur in patients with cirrhosis or acute liver failure. It comprises a spectrum of neuropsychiatric abnormalities and motor disturbances that are associated with varying degrees of disability, ranging from subtle to lethal. HE is believed to occur when the brain is exposed to gut-derived toxins that are normally removed from the blood by a healthy liver. We believe that ammonia plays a central role in this disease, and the most commonly utilized therapies for the treatment of HE are believed to act by reducing ammonia. Published epidemiological data suggest that there are approximately 140,000 patients in the United States who have episodic HE. We believe Ravicti, if approved, would treat episodic HE through a systemic reduction of ammonia.
75
Table of Contents
Business Strategy
Our strategy is to commercialize a product portfolio, including Ravicti, for the treatment of UCD and to develop Ravicti for the treatment of HE and other indications. The key elements of our strategy are to:
| • | Obtain FDA approval of Ravicti. We intend to seek marketing approval for Ravicti for the chronic management of UCD in patients down to 29 days of age. On December 23, 2011, we submitted an NDA to the FDA for approval of Ravicti for UCD patients 6 years and above based on data from our Phase II and III clinical trials that demonstrated non-inferiority and directionally favorable ammonia control as compared to BUPHENYL. In April 2012, we submitted a revised draft package insert to the FDA requesting approval of Ravicti for UCD patients down to 29 days of age based on data submitted in the NDA and data from our ongoing clinical trial in patients aged 29 days through 5 years. |
| • | Commercialize Ravicti and improve patient care in UCD.Subject to obtaining FDA approval of Ravicti for the treatment of UCD, we intend to establish sales, marketing, and reimbursement functions to commercialize Ravicti in the United States. A cornerstone of our strategy will be to facilitate the rapid transition of patients from BUPHENYL to Ravicti with a small, scientifically-focused sales force of approximately 10 representatives and a network of specialty pharmacies. By distributing directly through specialty pharmacies, we intend to provide patients with access to enhanced services that assist in overcoming challenges in healthcare delivery and in financing treatment posed by therapies that are necessarily expensive. |
| • | Market BUPHENYL and AMMONUL for patients ineligible for Ravicti. We anticipate that we will exercise our option to purchase all of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL during the applicable option period. We intend to market BUPHENYL for use by UCD patients who do not transition to Ravicti or who otherwise may be ineligible to use Ravicti. If Ucyclyd does not retain AMMONUL, we also intend to sell AMMONUL for the treatment of HA crises in UCD patients. Outside the United States we intend to assume Ucyclyd’s existing distribution agreements for BUPHENYL and for AMMONUL. If Ucyclyd exercises its option to retain AMMONUL, we will not have rights to sell AMMONUL in the United States or any other territory. |
| • | Develop Ravicti for the treatment of HE.Based on the positive results of our Phase II trial assessing the safety and efficacy of Ravicti in the treatment of episodic HE, we are planning to request an end of Phase II meeting with the FDA for the fourth quarter of 2012, which is critical for evaluating our Phase III clinical options with respect to Ravicti in this indication. |
| • | Expand Ravicti into additional indications and acquire additional products and product candidates. We may explore the use of Ravicti in indications other than UCD and HE. We intend to continue to identify and may license or acquire products or product candidates being developed for orphan diseases and hepatology. |
UCD & HE: Diseases Related to Elevated Ammonia Levels
UCD and HE are generally characterized by elevated levels of ammonia in the bloodstream. Ammonia is a potent neurotoxin, primarily produced in the intestine as a byproduct of protein metabolism. Individuals with a healthy liver remove ammonia by converting it to urea, which is excreted in urine. In both UCD and HE, the liver’s ability to remove ammonia is diminished. UCD patients have a genetic disability, and individuals with HE have an acquired disability related to a decline in liver function that occurs in patients with more severe cases of cirrhosis.
In both UCD and HE patients, ammonia can build up to toxic levels which can lead to severe medical complications, including death. Both UCD and HE fluctuate in severity, and patients may experience crises which typically require hospitalization and may result in irreversible neurological damage.
76
Table of Contents
UCD
Background
UCD are inherited genetic diseases caused by a deficiency of one of the enzymes or protein transporters that constitute the urea cycle. The urea cycle involves a series of biochemical steps in which ammonia, a potent neurotoxin, is converted to urea, which is excreted in the urine. If left untreated, UCD can cause HA crises which may result in irreversible brain damage, coma or death. UCD symptoms may first occur at any age depending on the severity of the disorder, with more severe defects presenting earlier in life.
Diagnosis and Prevalence
UCD are diagnosed either through newborn screening or when symptoms present. Current newborn screening can only detect three of the UCD subtypes and does not detect the most prevalent subtype. Thus, screening is believed to identify only approximately one-third of newborns with UCD. Initial UCD symptoms range from catastrophic illness with coma occurring within a few days of birth to milder symptoms such as difficulty sleeping, headache, nausea, vomiting, disorientation and seizures, particularly in patients who present later in life. Because these symptoms are common to a number of ailments, physicians often do not consider the possibility of UCD and therefore may not measure levels of blood ammonia. As a result, the most mildly affected patients can go undiagnosed for decades despite having symptoms. Because many cases of UCD remain undiagnosed and because infants born with severe UCD often die without a definitive diagnosis, the exact incidence and prevalence of UCD are unknown and, we believe, likely underestimated.
We believe UCD occur in approximately 1 in 10,000 births in the United States. We estimate that there are approximately 2,100 individuals in the United States that suffer from UCD. We estimate that only half, or approximately 1,100 patients with UCD in the United States, have been diagnosed. Based on demographic data for those patients enrolled in the National Institute of Health sponsored UCD consortium, or UCDC, longitudinal study, we estimate that in the United States 28% of the diagnosed patient population is under 6 years of age, 32% is aged 6 through 17 years and 40% is 18 years of age or older.
Current Treatment Options for UCD
Management of UCD involves decreasing ammonia production through reduction of protein in the diet, amino acid supplementation, the use of dietary supplements such as arginine and citrulline, and the use of ammonia lowering agents, including sodium benzoate and BUPHENYL. We believe that patients with mild to moderate UCD are typically treated with dietary management and that patients with more severe UCD are generally treated with BUPHENYL but are often noncompliant. Liver transplantation is an option reserved for the most severely affected patients, typically those who present very early in life. Because liver transplantation is technically difficult in newborns, a company called Cytonet GmbH & Co. is developing a therapy for severely affected newborns, which involves the infusion of human liver cells with the aim of prolonging crisis-free survival until the patients are old enough to undergo a liver transplantation.
BUPHENYL, approved by the FDA in 1996, is the only branded therapy currently FDA-approved for the chronic management of the most prevalent UCD. It is available in powder and tablet forms. A generic of the tablet form of BUPHENYL was approved by the FDA in November 2011. Similar to Ravicti, BUPHENYL removes ammonia from the bloodstream and patients take the drug for the balance of their life to help maintain control of their blood ammonia. BUPHENYL is also available for the treatment of UCD in select countries throughout Europe, the Middle East, and the Asia-Pacific Region. In Europe and the Middle East the product is sold under the brand name AMMONAPS®.
When UCD are not well controlled, HA crises may occur. In these acute situations, AMMONUL is often administered intravenously, and dialysis is sometimes used. AMMONUL is currently the only FDA-approved adjunctive therapy for the treatment of HA crises in patients with the most prevalent UCD. Currently, AMMONUL is not approved for use outside the United States, but is being prescribed by physicians in parts of Europe.
77
Table of Contents
Limitations of Treatment Options for UCD
We believe that approximately 425 of the estimated 1,100 patients currently diagnosed with UCD are treated with BUPHENYL, approximately 90 patients are currently taking Ravicti in one of our clinical trials and the remainder go untreated or elect to manage their disease through protein restriction and/or the use of dietary supplements. Although BUPHENYL is an effective treatment and in many cases is lifesaving, it has important limitations including a high pill burden or large quantity of powder that must be taken, unpleasant taste and smell, and frequent dosing (3-6 times per day), which make compliance for many UCD patients difficult. The amount of BUPHENYL prescribed is based on a patient’s weight or body surface. The maximum daily dose of 20 grams requires patients to consume either 40 vitamin-sized uncoated tablets or 6.9 teaspoons of powder mixed with liquid or food. Approximately 30% of all patients on BUPHENYL are taking or have taken medications to treat gastrointestinal side effects. The powder form of BUPHENYL is often mixed with food, which can result in food aversion. Due to palatability issues, gastrointestinal side effects associated with BUPHENYL and symptoms of their disease, we believe that up to 45% of pediatric patients have or have had a feeding tube. In addition, the sodium content of the maximum daily dose of BUPHENYL exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure.
Despite the life-threatening nature of UCD and the irreversible brain damage that can occur if a patient’s disease becomes uncontrolled and an acute crisis occurs, non-compliance is common. For example, in the pivotal study for AMMONUL, which was a 25-year, open-label, uncontrolled investigator-sponsored study in 299 patients with UCD, approximately 10% of HA crises were attributed to non-compliance with BUPHENYL. In addition, approximately 22% of HA crises reported by patients on BUPHENYL in the year before their enrollment in our pivotal study were attributed to non-compliance.
Many patients with mild to moderate disease manage their condition through protein restriction alone and risk long-term complications if the underlying disease is not well-controlled. Common neurological manifestations of patients with poorly controlled mild to moderate disease include hyperactive behavior, self-injurious behavior, stroke-like episodes, behavioral problems, cognitive dysfunction, and psychiatric symptoms. Recent clinical research suggests that even mildly symptomatic patients demonstrate cognitive deficits. Even mild to moderately affected patients risk an HA crisis if their disease is poorly controlled. According to data gathered by the UCDC, approximately 40% of patients not taking BUPHENYL who enrolled in the consortium sponsored longitudinal study had reported at least one acute crisis prior to enrollment.
Ravicti for the Treatment of UCD
Ravicti is being developed for the chronic management of patients with UCD and is intended for oral administration. Both Ravicti and BUPHENYL function as systemic ammonia lowering agents and provide an alternate pathway to the urea cycle for removing ammonia from the bloodstream. Both BUPHENYL and Ravicti release the active ingredient, phenylbutyrate, or PBA, which is converted to phenylacetic acid, or PAA. PAA facilitates the removal of ammonia when it is converted to phenylacetylglutamine, or PAGN, which is excreted in urine and replaces urea as a vehicle for ridding the body of ammonia. Due to its physical and chemical properties, Ravicti contains the same quantity of the active ingredient as BUPHENYL in a much smaller drug volume and has a longer half-life.
Key Advantages of Ravicti
Our analysis of data from our Phase II and Phase III trials evaluated the non-inferiority of Ravicti as compared to BUPHENYL in controlling blood ammonia levels in adult and pediatric UCD patients. A non-inferiority trial compares a test drug to an established treatment with the goal of showing that any difference in the performance of the test drug is small enough to support a conclusion that the test drug is not inferior to the established treatment, and that the test drug is, therefore, also effective. We successfully demonstrated non-inferiority in each of our Phase II and Phase III trials. We believe Ravicti provides incremental benefits in
78
Table of Contents
part due to its slow release profile, which appears to provide better late afternoon and nighttime control of ammonia levels. The following summarizes the expected key advantages of Ravicti as compared to BUPHENYL:
| • | Ammonia control: Four clinical trials have shown that blood ammonia is lower on Ravicti and statistically as good as, or non-inferior, to BUPHENYL. A pooled analysis of the data from the Phase II and Phase III trials included in the NDA was performed in which all ammonia values obtained over 24 hours on Ravicti from all clinical trials were combined and compared with similar values obtained on BUPHENYL. This analysis demonstrated that blood ammonia is significantly lower after treatment with Ravicti as compared to BUPHENYL. We believe the ammonia control provided by Ravicti is responsible for improved executive function seen in UCD patients aged 6 through 17 years after 12 months of treatment with Ravicti. |
| • | Improved palatability to drive compliance:Ravicti is a nearly odorless and tasteless liquid and requires a smaller daily drug volume (e.g., approximately 1 tablespoon contains the same amount of PBA as 40 tablets of BUPHENYL), all of which we believe makes Ravicti easier and more palatable to swallow. Approximately 10% of patients enrolled in our studies were receiving BUPHENYL through a gastrostomy tube, or G-tube. |
| • | Safety:In the 12-month safety extension to our pivotal Phase III trial, patients on Ravicti experienced approximately 40% fewer HA crises than they reported having experienced in the prior year while on BUPHENYL. In addition, unlike Ravicti, which contains no sodium, the sodium content of the maximum daily dose of BUPHENYL exceeds the FDA’s recommended daily allowance, which may lead to high blood pressure. |
| • | Patient preference. In our Phase II trial in patients down to 29 days of age, 34 of 36 patients expressed a preference for Ravicti over BUPHENYL. Forty of the forty-four patients in our pivotal Phase III trial agreed to continue 12 months of treatment and monthly monitoring with Ravicti beyond the initial four-week treatment period. Sixty-seven of sixty-nine patients who completed 12 months of treatment with Ravicti elected to enroll in an expanded access protocol to continue receiving Ravicti. |
Registration Plan
In November 2010, we successfully completed our pivotal Phase III trial in adults under an SPA with the FDA. On December 23, 2011, we submitted an NDA for Ravicti for the chronic management of UCD patients aged 6 years and above. We included data from our Phase II and Phase III trials in patients aged 6 years and above as well as safety data from 69 UCD patients with 12 months of treatment on Ravicti and the results of two nonclinical carcinogenicity studies in the NDA submission. We continue to gather data from our ongoing continued access protocol designed to provide more experience with Ravicti treatment. We are also currently conducting a clinical trial in UCD patients aged 29 days through 5 years designed to demonstrate the safety and efficacy of Ravicti in this age group. The efficacy portion of this trial is complete and a complete study report was submitted to the FDA in April 2012; however, the data from the 12-month safety extension portion of the study will not be available until the second quarter of 2013. As part of the April update to the FDA, we submitted a revised draft package insert requesting approval of Ravicti for UCD patients down to 29 days of age.
We hold FDA orphan drug designation for the use of Ravicti in treating UCD. Ravicti was granted orphan designation for UCD based upon a potential safety benefit over BUPHENYL because of the absence of sodium. We will not receive orphan drug exclusivity in UCD unless the FDA, in reviewing the NDA, concludes that Ravicti is safer or more effective than BUPHENYL or makes a major contribution to patient care.
We also received fast track designation for the NDA for Ravicti. Fast track status is intended to expedite or facilitate the process for reviewing new drugs and biological products that are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition.
79
Table of Contents
Ravicti Clinical Development in UCD Patients
Our Phase II and Phase III trials were designed to demonstrate the safety and efficacy of Ravicti as compared to BUPHENYL in adult and pediatric UCD patients. Our objective in these trials was to demonstrate the non-inferiority of Ravicti as compared to BUPHENYL with respect to ammonia control. In each study, patients were enrolled on their prescribed dose of BUPHENYL and then switched to an amount of Ravicti that delivered the same amount of PBA. Ammonia control on each drug was assessed by measuring blood ammonia levels over 24 hours in a monitored clinical setting, where both diet and drug dosing were tightly controlled. All three studies demonstrated the non-inferiority of Ravicti as compared to BUPHENYL. Ravicti was well tolerated, and its safety profile was comparable to that of BUPHENYL in all of these trials.
Pivotal Phase III Trial.We conducted a pivotal Phase III trial of Ravicti in adult patients 18 years of age or older with UCD. The four-week, multi-center, randomized, double-blind, placebo-controlled cross-over study was designed to evaluate the non-inferiority of Ravicti to BUPHENYL. The study was conducted under an SPA granted by the FDA in June 2009. The primary efficacy measure was blood ammonia control, assessed as 24-hour area under the curve on days 14 and 28, the last day of each two-week treatment period. The primary efficacy measure of 24-hour area under the curve was intended to assess comparative ammonia exposure on the two drugs. Subjects were administered a BUPHENYL dose equivalent to their prescribed dose before study enrollment or a dose of Ravicti which delivered the same amount of PBA. The double-blind design required that all patients receive active or placebo BUPHENYL tablets or powder, as well as either active or placebo Ravicti, throughout the study. The drugs were administered three times a day with meals, and diet was strictly controlled.
In accordance with the SPA, our pivotal Phase III trial was designed as a non-inferiority trial comparing the ratio of mean ammonia values obtained from treatment with Ravicti against BUPHENYL. A non-inferiority trial compares a test drug to an established treatment with the goal of showing that any difference in the performance of the test drug is small enough to support a conclusion that the test drug is not inferior to the established treatment, and that the test drug is, therefore, also effective. In a non-inferiority trial, the permitted difference between the performance of the test drug and the established treatment is defined in advance. This is referred to as the non-inferiority margin. If the trial results show that the test drug performed at least as well as the established treatment within the non-inferiority margin, the test drug is determined to have been shown non-inferior to the established treatment in the trial and the non-inferiority objective, or endpoint, for the trial will be considered to have been met. The non-inferiority margin agreed upon with the FDA was 1.25 which is a conventional margin applied to similar studies. Accordingly, non-inferiority of Ravicti would be demonstrated if the upper 95% confidence interval of ammonia on Ravicti was not more than 25% higher than that seen on BUPHENYL. A 95% confidence interval means that if the trial was run multiple times, 95% of the time, ammonia levels on Ravicti would not be more than 25% higher than that seen on BUPHENYL.
The study enrolled 46 adults at 19 sites in North America. Of the 46 adults enrolled, 45 subjects received at least one dose of study drug and 44 subjects completed the study and are included in the primary efficacy analysis. Subjects were required to be on a stable dose of BUPHENYL before enrollment.
This trial met its primary endpoint of demonstrating the non-inferiority of Ravicti to BUPHENYL. Ravicti was generally well tolerated. Twenty-three subjects reported at least one adverse event during BUPHENYL treatment and 27 subjects reported at least one adverse event during Ravicti treatment. The most common adverse events reported during BUPHENYL treatment were dizziness, headache, nausea, diarrhea and abdominal pain or discomfort. During Ravicti treatment, the most common adverse events reported were diarrhea, flatulence, headache, vomiting, fatigue, decreased appetite and abdominal pain or discomfort. There was one serious adverse event, gastroenteritis, during treatment with Ravicti which was deemed not to be drug related. No deaths occurred during the study, and no clinically significant lab or electrocardiogram changes were observed for either treatment. One patient experienced an HA crisis during BUPHENYL treatment. In addition, one subject withdrew early from the study during BUPHENYL treatment because of high ammonia and headache. We had no HA crises or subject withdrawals from the study during dosing with Ravicti.
80
Table of Contents
All subjects completing the study were eligible to enter a 12-month, open-label safety study. Forty of the forty-four patients in the pivotal Phase III trial agreed to continue treatment and monthly monitoring with Ravicti.
Phase II Adult Trial. We completed a Phase II trial of Ravicti in adult patients aged 18 years or older with UCD. This trial was an open-label, switchover study of the safety, tolerability, pharmacokinetic profile, and ammonia control of Ravicti compared to BUPHENYL. The study was conducted at four centers in the United States and enrolled 13 adult UCD patients, 10 of whom completed the trial. Subjects were required to be on a stable dose of BUPHENYL before enrollment. Upon enrollment, all subjects received BUPHENYL for seven days and were then admitted to a monitored clinical setting for overnight observation and 24-hour pharmacokinetic and ammonia measurements and urine collection. Subjects were then switched over to Ravicti, stayed on the Ravicti dose for seven days and were then re-admitted to the monitored clinical setting for repeated pharmacokinetic and ammonia measurements, and urine collection. Ravicti was well tolerated and exhibited a similar safety profile to BUPHENYL. There were two serious adverse events related to HA crises; both occurred during BUPHENYL treatment.
Phase II Pediatric Trial Aged 6 through 17 years. We completed a second Phase II trial at five centers in North America in UCD patients aged 6 through 17 years. This trial included two phases: a two-week, open-label, switchover comparison of the safety, tolerability, pharmacokinetic characteristics and ammonia control of Ravicti compared to BUPHENYL, and a 12-month safety extension. The switchover phase enrolled 11 UCD patients all of whom completed the study and enrolled in the extension phase. The extension portion of the trial enrolled an additional 6 patients for a total of 17 patients. Subjects were required to be on a stable dose of BUPHENYL before enrollment. Upon enrollment in the switchover phase, all subjects received BUPHENYL for seven days and were then admitted to a monitored clinical setting for overnight observation and 24-hour pharmacokinetic and ammonia measurements and urine collection. Subjects were then switched over to Ravicti. Subjects stayed on the Ravicti dose for seven days and were then re-admitted to the monitored clinical setting for repeated pharmacokinetic, ammonia and urine collection. Ravicti was well tolerated and exhibited a safety profile similar to BUPHENYL.
Efficacy Results of Phase II and Phase III Trials in Patients Aged 6 Years and Above. The non-inferiority endpoint was achieved in the pivotal Phase III trial and in the two Phase II clinical trials. The non-inferiority endpoint was prospectively defined in the pivotal Phase III trial and in the Phase II pediatric trial in patients aged 6 years and above, and the same non-inferiority analysis was conducted on a post hoc, retrospective basis in the Phase II adult trial.
81
Table of Contents
During these Phase II and Phase III trials, Ravicti demonstrated a differentiated rate of gastrointestinal absorption and pharmacokinetic profile as compared with BUPHENYL. This was demonstrated by PBA entering the circulation more slowly when administered as Ravicti than as BUPHENYL. We believe that this slow release profile of Ravicti as compared with BUPHENYL explains the lower ammonia levels observed on Ravicti over late afternoon and nighttime hours.
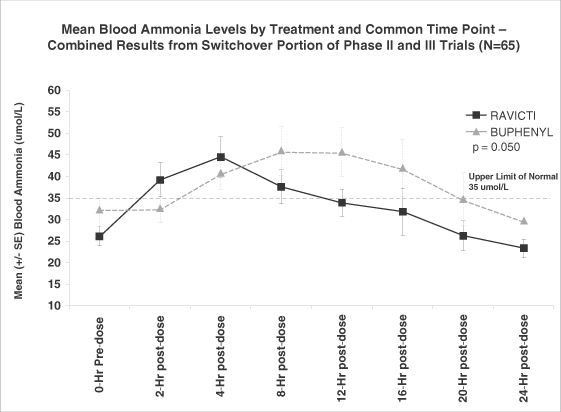
82
Table of Contents
Open-Label Studies.We enrolled 77 adult and pediatric UCD patients in our two 12-month open-label safety studies, 69 of whom completed the studies. We also currently have approximately 70 patients enrolled in our continued access protocol designed to provide more experience with Ravicti treatment. As depicted in the chart below, Ravicti continues to demonstrate a durable effect on ammonia control, with mean fasting ammonia values well below the upper limit of normal.
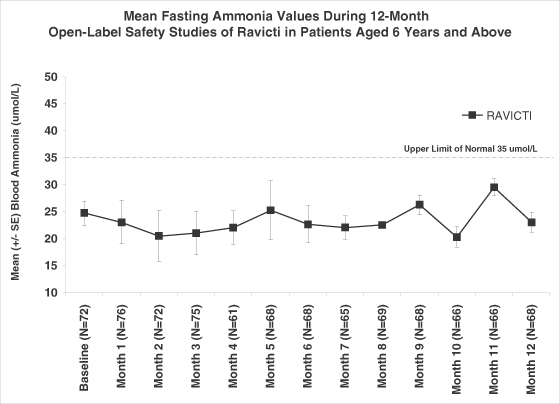
We have also seen a decrease in the rate and severity of HA crises versus what patients in these studies reported they experienced in the year before trial enrollment, when all patients were on BUPHENYL in an uncontrolled environment. During one year of treatment with Ravicti in our clinical trials, patients aged 6 years and above experienced approximately 40% fewer HA crises versus the prior 12 months and their peak ammonia values during crises were lower. Neuropsychological evaluations at baseline and after 12 months of treatment with Ravicti also show evidence of clinically significant improvements in executive function among pediatric patients aged 6 through 17 years, including behavioral regulation (e.g., flexibility, inhibitory control) and metacognitive skills (e.g., goal setting, planning, self-monitoring).
Pharmacokinetic Differences in PAA Production Between Adult and Pediatric UCD Patients Receiving Ravicti.Data from our clinical studies and mathematical modelling to predict pharmacokinetics indicates that metabolism and elimination of Ravicti and BUPHENYL varies with body surface area. In particular, exposure to both drugs’ active moiety, PAA, tends to be higher among pediatric patients versus adults. High levels of PAA have been associated with reversible toxicity in previously published studies involving cancer patients who received intravenously infused PAA. No relationship has been observed so far between adverse events and PAA levels in our clinical studies of Ravicti in UCD patients, and PAA exposure among UCD patients administered Ravicti has been below the range associated with toxicity in these previously published studies.
Pediatric Study Under 6 Years.In response to concerns raised by the FDA during our pre-NDA meeting that data from our Phase II trial in pediatric patients aged 6 to 17 years showed a higher level of PAA than that seen in adult UCD patients receiving Ravicti and that pediatric patients receiving Ravicti had higher PAA levels than
83
Table of Contents
pediatric patients receiving BUPHENYL, we accelerated the evaluation of the safety, pharmacokinetics, and ammonia control of Ravicti in pediatric patients under 6 years of age. We initiated a study in 15 pediatric UCD patients aged 29 days through 5 years in the second half of 2011. The protocol is generally similar in design to the study for pediatric patients aged 6 through 17 years described above, in that it consists of an open-label BUPHENYL to Ravicti switchover comparison of the safety, pharmacokinetics and ammonia control during treatment with the two drugs followed by a 12-month open-label extension study. Similar to the findings in prior studies among the 15 patients who enrolled in and completed the switchover part of the study, ammonia tended to be lower on Ravicti as compared with BUPHENYL. We have completed pharmacokinetic analyses and findings to date suggest that PAA levels continue to reflect age related differences in body surface area and are similar for Ravicti and BUPHENYL.
Pooled Analysis of Pediatric Data.In a post-hoc analysis of pooled ammonia data from the two pediatric studies encompassing the age range of 29 days up to 18 years of age, total daily ammonia exposure was significantly lower during treatment with Ravicti as compared with BUPHENYL as depicted in the figure below.
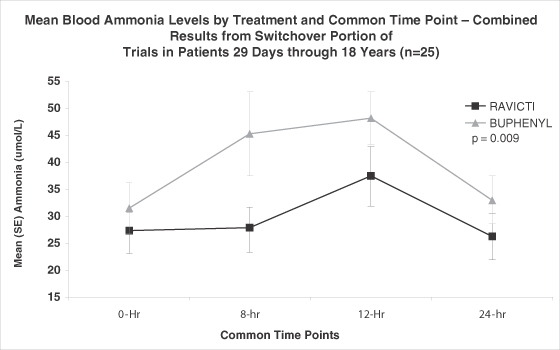
Ravicti Nonclinical Development
As part of the development program for Ravicti for treatment of UCD, we conducted nonclinical genotoxicity and carcinogenicity studies to assess the tumorigenic potential of Ravicti in animals and to assess the relevant risk in humans. In a 24-month carcinogenicity study in male and female rats, seven different tumor types occurred at an incidence suggestive of a relationship to Ravicti administration. In July 2011, we convened an expert panel of oncologists, industry and former FDA toxicologists, and human and veterinary pathologists to review the results of the study and provide guidance on the human relevance and potential risks associated with the findings. The expert panel reviewed the data from the study in detail, as well as additional relevant published data. The members of the panel determined unanimously that the results of the rat study were not predictive of human risk. We have not seen any incidence of cancer to date in any of our clinical trials of Ravicti in UCD patients, and we are not aware of any reported cases of cancer in patients taking BUPHENYL. Liver cancer was identified in three patients in our HE study, two of whom had a predisposing history of hepatitis C and one of whom had cirrhosis of unknown cause. A white paper summarizing the outcome of the expert panel review was
84
Table of Contents
submitted to the FDA along with the carcinogencity studies results as part of the Ravicti NDA for UCD. While we expect the white paper will be reviewed as part of the NDA review, we have not had any additional discussions with the FDA regarding the white paper or received a formal or informal response. If we are unable to explain these data to the satisfaction of the FDA, the FDA may request that we conduct additional nonclinical studies and the approval of Ravicti may be delayed or denied.
HE
Background
HE is a serious but potentially reversible neurological disorder that can occur in patients with cirrhosis or acute liver failure. HE is believed to occur when the brain is exposed to gut-derived toxins that are normally removed from the blood by a healthy liver. While a variety of gut-derived toxins may contribute to HE, we believe that ammonia plays a central role in this disease. The spectrum of symptoms which constitute HE is very similar to that for UCD, including neuropsychiatric abnormalities and motor disturbances that are associated with varying degrees of disability ranging from subtle to lethal. The manifestations of HE vary over time. Similar to UCD patients who may experience HA crises, patients with episodic HE often experience periods where their symptoms worsen, or HE events. HE events are manifested by symptoms ranging from disorientation to coma, and frequently require hospitalization. Our HE development program is targeting patients with episodic HE who have experienced past HE events and is designed to determine whether treatment with Ravicti will decrease the number of HE events.
Diagnosis and Prevalence
Symptoms in patients with episodic HE can range from subtle changes in personality to overt disorientation and impaired consciousness that can progress to coma or death, if untreated. Published epidemiological data suggest that there are approximately one million patients in the United States with cirrhosis of whom an estimated 140,000 have clinically recognizable episodic HE. HE is diagnosed based on the presence of compatible signs and symptoms in a patient with cirrhosis in whom other causes of brain dysfunction have been excluded. In contrast to patients with episodic HE in whom the manifestations are recognizable clinically, patients with minimal HE exhibit normal mental and neurological status upon clinical examination and need standardized neurological testing to establish a diagnosis.
The West Haven criteria, a widely used approach, grade the severity of episodic HE based upon a clinical assessment of a patient’s mental status, behavior, short term memory, alteration of consciousness and neuromuscular function. The scale for episodic HE ranges from Grade 1 to 4. Stable patients with Grade 1 or 2 HE are typically ambulatory and can usually be managed as outpatients. By contrast, Grade 3 and 4 patients are hospitalized and often require intensive support. Prevention of HE events is therefore important both from the standpoint of patient well-being and health care costs.
Current Therapies and Limitations
The most commonly utilized agents for the treatment of HE are poorly or non-absorbable sugars, such as lactulose or lactitol, and rifaximin, a poorly absorbed non-systematic oral antibiotic manufactured by Salix Pharmaceuticals, Ltd. These agents are believed to limit the local production of ammonia in the intestine. Other products currently in early development include Ocera Pharmaceutical’s AST-120, a non-specific adsorbent believed to bind putative toxins in the intestine, and OCR-002, which is believed to lower ammonia. BUPHENYL is not an appropriate treatment for most HE patients given the FDA warning regarding the use of the drug in patients with sodium retention and edema which is common for patients with HE.
Abdominal cramping, diarrhea and flatulence are common side effects with lactulose, making the drug difficult for many patients to tolerate. Moreover, a published review of clinical trials involving lactulose and lactitol in the treatment of HE concluded that those agents failed to demonstrate a statistically significant benefit.
85
Table of Contents
Rifaximin 550 mg tablets were FDA approved in March 2010 for the reduction in risk of overt HE recurrence in patients 18 years of age or older. Although rifaximin represents the current standard of care, approximately 20% of patients experienced breakthrough HE events while taking rifaximin over a period of six months in a pivotal study. We believe, the treatment of HE remains a major unmet medical need.
Ravicti for the Treatment of HE
Rationale for Ravicti Treatment in HE
HE is widely assumed by clinicians to involve the systemic accumulation of ammonia resulting from impaired liver function. Therefore, we believe Ravicti, which lowers ammonia systemically, can be beneficial in managing this disease. Moreover, given its mechanism of action of removing ammonia from the body, Ravicti could potentially be complementary to currently approved agents that limit the local production of ammonia.
Ravicti Clinical Development in HE Patients
Our HE clinical program comprises two trials which have enrolled patients with cirrhosis. Based on the positive results of our recently completed Phase II trial, we are planning to request an end of Phase II meeting with the FDA for the fourth quarter of 2012, which is critical for evaluating our Phase III clinical options with respect to Ravicti for HE. Our Phase II clinical trial design was similar to that used to evaluate rifaximin, the only therapy approved by the FDA for episodic HE within the last 30 years. The rifaximin trial, conducted by Salix Pharmaceuticals, or Salix, was a single pivotal Phase III trial which enrolled 299 patients. The primary endpoint was time to the first HE event with the key secondary endpoint of time to hospitalization.
Phase I Trial. Ucyclyd conducted a Phase I safety and pharmacokinetic study in healthy adults and adults with cirrhosis. The trial was an open-label, single and multiple dose study of Ravicti in 24 cirrhotic and 8 healthy subjects. Ravicti was generally well tolerated. There were no serious adverse events or withdrawals due to adverse events. The most common individual adverse events were increased body temperature and decreased platelet count. While patients with the most severely impaired liver function tended to metabolize Ravicti somewhat more slowly than healthy adults, even these patients were able to effectively metabolize Ravicti and thereby utilize the drug for waste removal.
Phase IITrial. We have recently completed a Phase II trial of patients with episodic HE. Part A of this study involved an open-label dose escalation to assess the safety and pharmacokinetics of Ravicti in 15 patients with cirrhosis and HE. We assessed doses of 6mL and 9mL taken twice per day. The 6mL dose lowered mean fasting ammonia levels to below the average upper limit of normal and exhibited superior tolerability compared to the 9mL dose, which showed little incremental ammonia effect. The 6mL dose was therefore selected as the dose for Part B of the study.
Part B was a multi-center, randomized, double-blind trial of Ravicti versus placebo in patients with episodic HE. This study shared many of the essential features of Salix Pharmaceutical’s pivotal trial for rifaximin in HE. In the Salix pivotal study rifaximin reduced the risk of occurrence of an HE event and risk of hospitalization due to an HE event. We followed the general design of the Salix study, recruited similar patients and applied similar definitions of HE events as the primary outcome measure. However the duration of our study was 4 months in contrast to Salix’s study which was 6 months. In order to be included in our study, patients must have had at least two HE events in the previous six months. The primary efficacy measure was similar to the rifaximin trial and was defined as the proportion of patients that exhibited at least one HE event while on the study. Secondary measures included total HE events during the study, pharmacokinetics, hospitalizations due to HE, subjects with one or more symptomatic days, and impact on severe HE and minimal HE, which involves mild neurological impairment as detected by standardized testing.
Patients were allowed to continue standard of care therapy (including lactulose and/or rifaximin) while enrolled in the trial. Unlike the rifaximin pivotal trial which required that patients exit after experiencing their first HE event, our trial allowed patients to remain in the trial at the physician’s discretion after experiencing an
86
Table of Contents
HE event. Patients’ standard of care therapy also could be modified by their physician during the trial after experiencing their first HE event. For example, a physician could introduce rifaximin to patients not taking rifaximin at study entry.
The trial enrolled 178 patients: 88 patients from 28 sites in the US, 50 patients from 9 sites in Russia, and 40 patients from 7 sites in the Ukraine. The ex-US sites were included both to facilitate enrollment and diversify the background standard of care. All sites were pre-qualified and continuously monitored by us throughout the study to ensure adherence to the protocol and Good Clinical Practices.
The trial met its primary endpoint: the proportion of patients experiencing at least one HE event was significantly lower on Ravicti versus placebo (21.1% vs. 36.4%, p = 0.0214). Patients receiving Ravicti also experienced fewer total HE events in the course of the study versus placebo (35 vs. 57; p = 0.0354). In addition, fewer patients on Ravicti experienced one or more symptomatic days versus placebo (13 vs. 27; p = 0.0148). While not statistically significant, there were trends favoring Ravicti in numbers of patients hospitalized for HE events, total HE-related hospitalizations and total hospital days for HE-related admissions, suggesting a potentially important pharmacoeconomic benefit to the treatment of HE with Ravicti. Some of the trial data are summarized in the following table:
| Ravicti n=90 | Placebo n=88 | % Reduction | p Value | |||||||||||||
Primary Efficacy Endpoint: | ||||||||||||||||
Proportion of patients with at least one HE event — Number of subjects (%) | 19 (21.1% | ) | 32 (36.4% | ) | 42% | 0.0214 | ||||||||||
Secondary Endpoints: | ||||||||||||||||
Total HE events | 35 | 57 | 37% | 0.0354 | ||||||||||||
Subjects with a symptomatic day | 13 | 27 | 52% | 0.0148 | ||||||||||||
Total HE hospitalizations | 13 | 25 | 48% | 0.064 | ||||||||||||
We are continuing to analyze data related to the secondary endpoints from this study, including a secondary endpoint related to minimal HE which was not met.
87
Table of Contents
The population most similar to that enrolled in the rifaximin pivotal trial was the subgroup of patients on no therapy or lactulose only at study entry. In this population, Ravicti significantly reduced the proportion of patients experiencing at least one HE event versus placebo (10% vs. 32.2%; p = 0.0031) as well as the proportion of patients who experienced the more severe West Haven grade> 2 events versus placebo (5% vs. 25.4%; p = 0.001), and the total number of HE events on study (7 vs. 31; p = 0.0002). Among these patients, there was also a highly significant, 82%, reduction in the risk of experiencing a grade 2 HE event on Ravicti as compared with placebo (p = 0.0073).
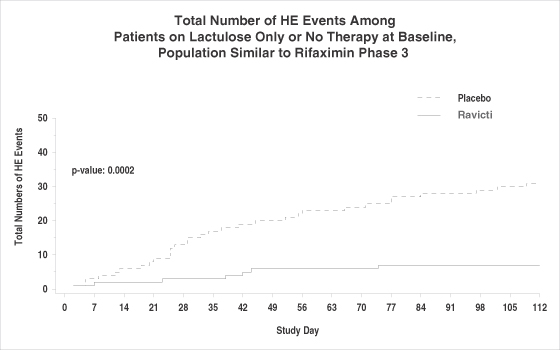
Among patients who received rifaximin at any time during the study (n=69), those receiving Ravicti as well experienced fewer total HE events, fewer patients hospitalized for HE events and fewer total HE hospitalizations. While the differences were not statistically significant, these trends may suggest a possible benefit of Ravicti in patients who have experienced HE events while taking rifaximin. However, the proportion of patients taking rifaximin at study entry that experienced at least one HE event while on study was similar for Ravicti and placebo (43.3% vs. 44.8%; non-significant). A summary of the results of patients on rifaximin at any time during the study is as follows:
| Ravicti + Rifaximin (n = 31) | Rifaximin + Placebo (n = 38) | |||||||
Subjects with HE Events | 13 | 22 | ||||||
Total HE Events | 28 | 42 | ||||||
Total HE hospitalizations | 11 | 20 | ||||||
% of HE hospitalizations < 5 days | 14 | % | 30 | % | ||||
Annualized hospitalization rate (hosp/pt/yr) | 1.74 | 3.21 | ||||||
The rate of adverse events was similar on Ravicti versus placebo. There were three deaths in the study, two on Ravicti and one on placebo, all of which were judged by the clinical investigators to be unrelated to the study drug. There were 20 serious adverse events, or SAEs, on Ravicti, of which one was deemed possibly related to Ravicti by blinded assessment at the time of the study, and 12 SAEs on placebo, of which four were deemed possibly related to the placebo by blinded assessment at the time of the study. We believe the higher number of
88
Table of Contents
SAEs in the Ravicti group reflects the greater number of Child-Pugh C patients (i.e., the most severely ill patients) randomized to Ravicti versus placebo (21 vs. 8).
We are currently conducting additional analyses of the study results and planning for an end of Phase II meeting with the FDA in the fourth quarter of 2012 to discuss these findings and gain the agency’s input on our plans for a Phase III trial in HE. We plan to submit the results of the trial for consideration for presentation at the Liver Meeting of the American Association for the Study of Liver Diseases.
Sales, Marketing and Distribution
The two current branded products FDA-approved for the most prevalent UCD, BUPHENYL and AMMONUL, are not currently promoted in the United States by a sales force, and market education and support efforts are limited. If Ravicti is approved by the FDA, we plan to establish sales, marketing, and reimbursement functions, in the United States, consistent with those maintained by other companies to support orphan medications marketed to small patient populations. We plan to hire approximately 10 representatives to reach the specialists involved in treating the majority of patients with UCD. We intend to distribute our UCD product portfolio through a limited network of specialty pharmacies with a single dedicated call center responsible for interfacing with patients, physicians and payors. We believe this strategy will be critical to our commercial success as it supports a case managed approach to getting patients on treatment quickly and supporting long-term compliance.
A cornerstone of our strategy will be to facilitate the rapid transition of patients approved under FDA-labeling from BUPHENYL to Ravicti. Based on our market research with physicians and patient preference data from our clinical trials, we anticipate that most BUPHENYL patients will rapidly transition to Ravicti if it is approved by the FDA for commercial sale. If Ravicti is approved, and we purchase Ucyclyd’s worldwide rights to BUPHENYL, we will continue to sell BUPHENYL for any patients who are not included in the FDA-approved Ravicti label or who may prefer BUPHENYL. If we purchase Ucyclyd’s worldwide rights to AMMONUL, we will also sell AMMONUL for the treatment of HA crises in UCD patients.
As part of the NDA submission for Ravicti for the treatment of UCD, we proposed a Risk Evaluation and Mitigation Strategy, or REMS, to address concerns raised by the FDA in our pre-NDA meeting regarding differences seen in the pharmacokinetic profile of Ravicti between adults and children and potential use of Ravicti in children below the age of 6 years prior to its approval for use in this age group. The proposed REMS program is intended to support informed dosing and treatment decisions between patients and their healthcare providers by educating them on the safe use of Ravicti and to limit access to Ravicti only to patients aged 6 years and over until such time as the Ravicti label is expanded to include this patient population. If required, the REMS will be administered though our single dedicated call center which will enable us to maintain control over distribution and facilitate education of patients and healthcare providers. The proposed REMS may not be sufficient to address the FDA’s concern regarding the potential safety risks of Ravicti in pediatric patients. The specific elements of any required REMS will be negotiated with FDA during the NDA review process.
Once the transition of patients from BUPHENYL to Ravicti is underway, we will devote increasing resources to expanding the number of diagnosed and treated patients through ongoing market education. Our sales and marketing organization will be structured for flexibility in anticipation of additional products.
Outside the United States we will assume Ucyclyd’s existing distribution agreements for BUPHENYL and AMMONUL, if we acquire those products.
Third-Party Reimbursement
Sales of pharmaceutical products depend in significant part on the availability of coverage and adequate reimbursement by third-party payors, such as state and federal governments, including Medicare and Medicaid,
89
Table of Contents
managed care providers, and private insurance plans. Decisions regarding the extent of coverage and amount of reimbursement to be provided for Ravicti and BUPHENYL will be made on a plan by plan, and in some cases, patient by patient, basis. Particularly given the rarity of UCD, we anticipate that securing coverage and appropriate reimbursement from third-party payors will require targeted education. To that end, we plan to establish a dedicated group of reimbursement experts focused on ensuring that clinically qualified patients have affordable access to therapy.
Within the Medicare program, as a self-administered drug, Ravicti would be, and BUPHENYL is, reimbursed under the expanded prescription drug benefit, known as Medicare Part D. This program is a voluntary Medicare benefit administered by private plans that operate under contracts with the federal government. These Part D plans negotiate discounts with drug manufacturers, which are passed on to each of the plan’s enrollees. Historically, Part D beneficiaries have been exposed to significant out-of-pocket costs after they surpass an annual coverage limit and until they reach a catastrophic coverage threshold. However, changes made by recent legislation will reduce this patient coverage gap, known as the donut hole, by transitioning patient responsibility in that coverage range from 100% in 2010 to only 25% in 2020. To help achieve this reduction, beginning in 2011, pharmaceutical manufacturers are required to pay quarterly discounts of 50% off the negotiated price of branded drugs issued to Medicare Part D patients in the donut hole.
An ongoing trend has been for third-party payors, including the United States government, to apply downward pressure on the reimbursement of pharmaceutical products. Also, the trend towards managed health care in the United States and the concurrent growth of organizations such as health maintenance organizations may result in lower reimbursement for pharmaceutical products. We expect that these trends will continue as these payors implement various proposals or regulatory policies, including various provisions of the recent health reform legislation that affects reimbursement of these products. There are currently, and we expect that there will continue to be, a number of federal and state proposals to implement controls on reimbursement and pricing, directly and indirectly.
Research and Development
We are conducting development activities to expand the commercial potential of Ravicti. We sponsor and conduct clinical research activities with investigators and institutions to measure key clinical outcomes that can influence market adoption of Ravicti.
In the years ended December 31, 2009, 2010, and 2011, and for the three months ended March 31, 2012, we incurred $11.0 million, $23.1 million, $17.2 million and $8.9 million, respectively, of research and development expense.
Ucyclyd Asset Purchase Agreement and Amended and Restated Collaboration Agreement
On March 22, 2012, we entered into a purchase agreement with Ucyclyd under which we purchased the worldwide rights to Ravicti and a restated collaboration agreement under which Ucyclyd granted us an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed price at a future defined date, plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended.
Asset Purchase Agreement.Under the purchase agreement, we purchased all of the worldwide rights to Ravicti for an initial upfront payment of $6.0 million. We will also pay tiered mid to high single digit royalties on global net sales of Ravicti and may owe regulatory milestones of up to $15.8 million related to approval of Ravicti in HE, regulatory milestones of up to $7.3 million per indication for approval of Ravicti in indications other than UCD or HE, and net sales milestones of up to $38.8 million if Ravicti is approved for use in
90
Table of Contents
indications other than UCD (such as HE) and all annual sales targets are reached. In addition, the intellectual property license agreement executed between Ucyclyd and Brusilow Enterprises, LLC, or Brusilow, and the research agreement executed between Ucyclyd and Dr. Marshall L. Summar, or Summar, were assigned to us, and we have assumed the royalty obligation under the Brusilow agreement for sales of Ravicti in any indication, and the royalty obligations under the Summar agreement on sales of Ravicti to treat HE. We will also pay Brusilow an annual license extension fee to keep the Brusilow license in effect, which extension fee is payable until our first commercial sale of Ravicti following FDA approval. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Subject to Ucyclyd’s right to commercialize BUPHENYL and AMMONUL for UCD for as long as it owns these products, the purchase agreement prohibits Ucyclyd from developing or commercializing any product for the treatment of UCD or HE that comprises, incorporates or contains glycerol phenylbutyrate, sodium phenylbutyrate or any other active pharmaceutical ingredient that converts to PAA. This restriction is in force until the later of (a) the expiration of the last patent covering Ravicti in the United States, or (b) the expiry of any other market exclusivity granted by the FDA for Ravicti. Thereafter, the restriction will remain in force on a country-by-country basis until the later of (a) the expiration of the last patent covering Ravicti in the applicable country, or (b) the expiration of any other market exclusivity granted for Ravicti by the governing regulatory agency in the applicable country. This restriction does not prevent Ucyclyd from developing or commercializing BUPHENYL or AMMONUL for indications other than UCD or HE, and Ucyclyd will retain the right to develop or commercialize the active ingredient(s) of BUPHENYL or AMMONUL for indications other than UCD or HE even if we purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL pursuant to the restated collaboration agreement.
As part of our purchase of the worldwide rights to Ravicti, and in return for the payment of the royalties described above, we received a license to Ucyclyd’s manufacturing technology for use with respect to Ravicti. In addition, concurrent with our purchase of Ravicti, Ucyclyd granted us a royalty-bearing license to any developed Ucyclyd formulation technology that may be useful to our efforts with respect to Ravicti in UCD and HE, and we received a right to use and reference certain Ucyclyd-owned data relating to BUPHENYL and AMMONUL.
Under the terms of the purchase agreement, Ucyclyd has an option to purchase the right to use and reference any and all data we control with respect to Ravicti and any other product that comprises, incorporates or contains glycerol phenylbutyrate, sodium phenylbutyrate or any other active pharmaceutical ingredient that converts to PAA. If exercised, Ucyclyd’s right to use and reference our data is limited to use only for development of products (other than Ravicti) for the treatment of a specific indication that we are not currently pursuing, and other new indications that are requested by Ucyclyd and as approved by us, with our right to withhold such approval subject to certain terms and conditions. If Ucyclyd exercises this option and we approve a new indication, we are obligated to disclose any relevant patents and discuss in good faith a nonexclusive, field-limited license to such patents to be entered on commercially reasonable terms and conditions. This option is exercisable any time until our acquisition of BUPHENYL and AMMONUL (subject to Ucyclyd’s right to retain AMMONUL) or the expiry of our option period. If Ucyclyd exercises the option, Ucyclyd will pay us a one-time up-front payment, and may owe us an additional regulatory milestone payment. In addition, Ucyclyd will pay us a mid single digit royalty on net sales of products for any new indications (excluding the specific indication mentioned above), not to exceed an aggregate dollar value.
The purchase agreement cannot be terminated by either party. However, we will have a license to certain Ucyclyd manufacturing technology, and Ucyclyd may have a license to certain of our technology, and the party granting a license will be permitted to terminate the license if the other party fails to comply with any payment obligations relating to the license and does not cure such failure within a defined time period. The license with Brusilow that was assigned to us may be terminated for any uncured breach, including of payment obligations and if we do not meet certain diligence obligations in our development and commercialization of Ravicti.
91
Table of Contents
Amended and Restated Collaboration Agreement. Under the terms of the restated collaboration agreement, we have an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain AMMONUL. We will be permitted to exercise this option for 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. The upfront purchase price for AMMONUL and BUPHENYL is $22.0 million, which we may fund by drawing on a loan commitment from Ucyclyd. The loan would be payable in eight quarterly payments and would bear interest at a rate of 9% per annum, and would be secured by the BUPHENYL and AMMONUL assets. If the Ravicti NDA for UCD is not approved by January 1, 2013, then Ucyclyd is obligated to make monthly payments of $0.5 million to us until the earliest of (1) FDA approval of the Ravicti NDA for UCD, (2) June 30, 2013 and (3) our written notification of our decision not to purchase BUPHENYL and AMMONUL.
If we exercise our option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, then Ucyclyd has the time-limited right to elect to retain all rights to AMMONUL for a purchase price of $32.0 million. If Ucyclyd exercises this option, Ucyclyd will pay us a net payment of $13.0 million on closing of the purchase transaction, which reflects the purchase price for BUPHENYL being set-off against Ucyclyd’s retention payment for AMMONUL. If Ucyclyd retains rights to AMMONUL, subject to certain terms and conditions, we retain a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
If we acquire BUPHENYL, we will pay Ucyclyd royalties on any net sales in the United States of BUPHENYL to UCD patients outside of the FDA-approved labeled age range for Ravicti. The royalties on BUPHENYL net sales will be payable at the Ravicti royalty rate then in effect pursuant to the purchase agreement. If we purchase Ucyclyd’s worldwide rights to AMMONUL, AMMONUL net sales will be included in the calculation of commercial milestone payments due to Ucyclyd under the purchase agreement, and we will pay regulatory milestones of up to $11.5 million based on the development of AMMONUL for HE and up to $2.0 million for each additional indication that we decide to pursue. We will also pay Ucyclyd low double digit royalties on global net sales of AMMONUL in all indications, including UCD, if we obtain regulatory approval for an indication other than UCD. If we purchase Ucyclyd’s worldwide rights to AMMONUL and BUPHENYL, upon closing of the purchase transaction we will assume Ucyclyd’s rights in the purchased products subject to Ucyclyd’s current obligations to certain third-party distributors and licensees. The restated collaboration agreement provides that royalty obligations will continue, without adjustment, even if generic versions of these products are introduced and sold in the relevant country.
The restated collaboration agreement will expire if we fail to exercise our purchase option or if we exercise our purchase option but fail to pay the initial purchase price or otherwise fail to consummate the purchase within the required time period. In addition, our ability to consummate the purchase transaction contemplated under the restated collaboration agreement may require that we obtain clearance from the Federal Trade Commission, or FTC, or Department of Justice pursuant to the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, or the HSR Act. If the FTC or the Antitrust Division of the Department of Justice, or the Antitrust Division, were to challenge the transaction and we were unable to resolve the challenge through a consent decree, either party could terminate the restated collaboration agreement and we would lose our rights to BUPHENYL and AMMONUL. Following our purchase of these rights, the restated collaboration agreement cannot be terminated by either party. However, we will have a license to specified Ucyclyd manufacturing technology, and Ucyclyd will be permitted to terminate this license if we fail to comply with any payment obligations relating to the license and we fail to cure this failure within a defined time period.
Manufacturing
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on third-party manufacturers to produce bulk drug substance and drug products required for our clinical trials. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to
92
Table of Contents
manufacture commercial quantities of our drug product candidates if and when we receive approval for marketing by the applicable regulatory authorities.
We have clinical supplies of Ravicti manufactured for us by two alternate drug substance suppliers, Helsinn and DSM on a purchase order basis. We have included both Helsinn and DSM as suppliers of drug substance in the Ravicti NDA. If either or both Helsinn and DSM are approved by the FDA, we believe our commercial requirements of drug substance can be satisfied without significant delay or material additional costs. We purchase finished Ravicti drug product from Lyne on a purchase order basis in accordance with a clinical supply agreement. We do not have an agreement in place for and we have not identified a secondary fill/finish supplier. We do not have a long-term commercial supply arrangement in place with any of our contract manufacturers. If we need to identify an additional fill/finish manufacturer, we would not be able to do so without significant delay and likely significant additional cost.
Prior to our acquisition of the worldwide rights to Ravicti from Ucyclyd, Ucyclyd owned all Ravicti manufacturing technology developed by Helsinn, other than generally applicable confidential know-how. Pursuant to the purchase agreement with Ucyclyd, Ucyclyd continues to own all Ravicti manufacturing technology developed as of August 23, 2007, and we own all Ravicti manufacturing technology developed after that date. We have a license to the Ravicti manufacturing technology owned by Ucyclyd.
If we purchase BUPHENYL and AMMONUL under the restated collaboration agreement with Ucyclyd, we will assume all of Ucyclyd’s rights and obligations under its manufacturing agreements for these products.
Our third-party manufacturers, their facilities and all lots of drug substance and drug products used in our clinical trials are required to be in compliance with current Good Manufacturing Practices, or cGMP. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements and FDA satisfaction before any product is approved and we can manufacture commercial products. Our third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. These actions could have a material impact on the availability of our products. Contract manufacturers often encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel. For example, we recently discovered a contaminated lot of Ravicti, which we believe was caused by a failure in a filtration step by one of our third-party drug substance manufacturers. As a result, we have a limited commercial supply of Ravicti, and we have to manufacture another lot, which could cause a delay in the commercial launch of Ravicti.
Competition
We face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions, among others, which may in the future develop products to treat UCD or HE. Our commercial opportunity may be reduced significantly if our competitors develop and commercialize products that are safer, more effective, more convenient, have fewer side effects or are less expensive than Ravicti, or BUPHENYL and AMMONUL. Public announcements regarding the development of competing drugs could adversely affect the commercial potential of Ravicti.
Currently, there is no cure for UCD. Management of UCD involves decreasing ammonia levels through reduction of protein in the diet, amino acid supplementation and the use of ammonia lowering agents, including sodium benzoate and BUPHENYL. Liver transplantation is an option reserved for the most severely affected
93
Table of Contents
patients, typically those who present very early in life. If a curative treatment for UCD is developed, Ravicti and BUPHENYL may become obsolete for that indication.
BUPHENYL is the only branded therapy currently FDA-approved for the chronic management of patients with the most prevalent UCD. We are aware of one generic sodium phenylbutyrate tablet product manufactured by Ampolgen Pharmaceuticals, LLC, which received FDA approval in November 2011 under an abbreviated new drug application, or ANDA. We are aware that other companies, including Forte BV and Navinta LLC, are developing taste masking technologies for sodium phenylbutyrate. We do not know whether these technologies will be introduced to the market and if so, the timing or success of such introduction. In addition, Orphan Europe is conducting a clinical trial of carglumic acid to treat some of the UCD enzyme deficiencies for which we expect Ravicti to be approved. Carglumic acid is approved to treat HA crises resulting from a different rare disorder than UCD and is sold under the name Carbaglu. If the results of this trial are successful and Orphan Europe is able to complete development and obtain approval of Carbaglu to treat additional UCD enzyme deficiencies, we would face competition from this compound. AMMONUL is the only FDA-approved adjunctive therapy for HA crises in patients with the most prevalent UCD.
Currently, there is no cure for HE other than liver transplantation, which is limited by donor availability and patient eligibility. Although lactulose has been commonly used, rifaximin is the only FDA-approved therapy for reduction in risk of episodic HE recurrence. In addition to currently marketed treatments for HE, Ocera Therapeutics, Inc. has conducted two Phase II trials of one of their compounds to treat mild HE and is conducting a Phase II trial of a second compound measuring ammonia control versus placebo in patients with cirrhosis. To be commercially viable in HE, we must demonstrate Ravicti is at least as safe and effective as competitive products or can be used safely in combination. If a curative treatment for HE is developed other than liver transplantation, Ravicti may become obsolete for that indication.
Intellectual Property
We intend to seek patent protection in the United States and internationally for our products and product candidates. Our policy is to pursue, maintain and defend patent rights developed internally and to protect the technology, inventions and improvements that are commercially important to the development of our business. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of the existing patents upon which our product candidates rely or any patents granted to us in the future will be commercially useful in protecting our technology. We also rely on trade secrets to protect our product candidates. Our commercial success also depends in part on our non-infringement of the patents or proprietary rights of third parties. For a more comprehensive discussion of the risks related to our intellectual property, please see “Risk Factors — Risks Related to Our Intellectual Property.”
Our success depends in part on our ability to:
| • | obtain and maintain proprietary and marketing exclusivity rights for Ravicti, and BUPHENYL and AMMONUL if we purchase those products; |
| • | preserve trade secrets; |
| • | prevent third parties from infringing upon the proprietary rights; and |
| • | operate our business without infringing the patents and proprietary rights of third parties, both in the United States and internationally. |
The anticipated market protection for Ravicti includes orphan drug exclusivity and issued patents.
Ravicti was granted orphan drug designation for the maintenance treatment of UCD and for the intermittent or chronic treatment of patients with cirrhosis and any grade HE. If we are awarded orphan drug exclusivity for
94
Table of Contents
each indication, we would receive seven years of orphan exclusivity from the date of the FDA approval for each indication, which we believe would help protect our competitive position in the market. Whether Ravicti will receive orphan exclusivity for the UCD indication will depend on whether the FDA concludes that Ravicti is safer than BUPHENYL, which shares the same active substance as Ravicti, and comparable in terms of effectiveness. Should the FDA determine that the safety of Ravicti in treating UCD is not sufficiently better, or that the product does not have comparable effectiveness, Ravicti may not be granted orphan exclusivity.
We have licensed the rights to the Ravicti composition of matter patents from Brusilow, which have been issued in the United States, Canada, and the primary countries of the European Union. Upon the expiration of the Ravicti composition of matter patents, our license agreement with and our license payment obligations to Brusilow will terminate and we will have a fully paid, royalty-free, sublicensable license. We will continue to have payment obligations to Brusilow as part of an amendment to the license in 2007. The United States composition of matter patent will expire in 2015, without taking into account the patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Amendments. Based on current projections, we expect to receive an extension under the Hatch-Waxman Amendments, which would extend this patent coverage for approximately an additional three years to 2018. We also expect to receive three-year Hatch-Waxman exclusivity based on the submission of new and essential clinical trials conducted or sponsored by us in support of the new product. This exclusivity would prevent the FDA from giving final approval to any generic forms of Ravicti for a period of three years from the anniversary date of the approval of Ravicti. It would also prohibit the agency from approving any 505(b)(2) NDAs that seek to reference the agency’s approval of Ravicti for a period of three years. This three year period would run in parallel with any award of orphan drug exclusivity.
We own pending patent applications in the United States, Europe, Japan and Canada directed to methods of using, administering, and adjusting the dosage of drugs, including Ravicti and BUPHENYL, which operate via the active chemical entity PAA. Any patents issuing from these applications would expire in 2029. We also own pending patent applications in the United States and internationally pursuant to the Patent Cooperation Treaty that incorporate fasting ammonia level measurements into methods of treating and determining dosage for UCD patients, which if issued would expire in 2032. If granted, one or more of these pending patent applications could provide an additional layer of market protection to 2029 to 2032. However, there is a significant risk that these applications will not issue, or that they may issue with substantially narrower claims than those that are currently sought.
The orphan drug exclusivity and composition of matter patent for BUPHENYL have expired. Should a patent from the pending patent applications described above be issued, market protection for this drug could extend from 2029 to 2032. However, as discussed above, there is a significant risk that these applications will not issue or will issue with substantially narrower claims than are currently being sought.
We also protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Regulatory Matters
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and related regulations. Drugs are also subject to other federal, state and local statutes and regulations. Failure to comply with the applicable United States regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an Institutional Review Board, or IRB, of a clinical
95
Table of Contents
hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising and promotion of our products.
The FDA’s policies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of Ravicti or any future product candidates or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Approval
The process required by the FDA before drugs may be marketed in the United States generally involves the following:
| • | nonclinical laboratory and animal tests; |
| • | submission of an Investigational New Drug, or IND, application which must become effective before clinical trials may begin; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; |
| • | pre-approval inspection of manufacturing facilities and clinical trial sites; and |
| • | FDA approval of an NDA which must occur before a drug can be marketed or sold. |
The testing and approval process requires substantial time and financial resources, and we cannot be certain that any new approvals for our product candidates will be granted on a timely basis if at all.
Our planned clinical trials for our product candidates may not begin or be completed on schedule, if at all. Clinical trials can be delayed for a variety of reasons, including delays in:
| • | obtaining regulatory approval to commence a study; |
| • | reaching agreement with third-party clinical trial sites and their subsequent performance in conducting accurate and reliable studies on a timely basis; |
| • | obtaining institutional review board approval to conduct a study at a prospective site; |
| • | recruiting patients to participate in a study; and |
| • | supply of the drug. |
Prior to commencing the first clinical trial, an initial IND application must be submitted to the FDA. The IND application automatically becomes effective 30 days after receipt by the FDA unless the FDA within the
96
Table of Contents
30-day time period raises concerns or questions about the conduct of the clinical trial. In such case, the IND application sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. A separate submission to the existing IND application must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each study subject. Regulatory authorities, an IRB, a data safety monitoring board or the sponsor, may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk.
For purposes of NDA approval, human clinical trials are typically conducted in phases that may overlap:
| • | Phase I — the drug is initially given to healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. These studies may also gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacologic effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials. |
| • | Phase II — studies are conducted in a limited number of patients in the target population to identify possible adverse effects and safety risks, to determine the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase III clinical trials. |
| • | Phase III — when Phase II evaluations demonstrate that a dosage range of the product appears effective and has an acceptable safety profile, and provide sufficient information for the design of Phase III studies, Phase III trials are undertaken to provide statistically significant evidence of clinical efficacy and to further test for safety in an expanded patient population at multiple clinical study sites. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to further evaluate dosage, effectiveness and safety, to establish the overall benefit-risk relationship of the investigational drug, and to provide an adequate basis for product approval by the FDA. |
| • | Phase IV — post-marketing studies, or Phase IV clinical trials, may be conducted after initial marketing approval. These studies may be required by the FDA as a condition of approval and are used to gain additional experience from the treatment of patients in the intended therapeutic indication. The FDA also now has express statutory authority to require post-market clinical studies to address safety issues. |
All of these trials must be conducted in accordance with good clinical practice requirements in order for the data to be considered reliable for regulatory purposes.
Typically, if a drug product is intended to treat a chronic disease, as is the case with Ravicti, safety and efficacy data must be gathered over an extended period of time, which can range from six months to three years or more. Government regulation may delay or prevent marketing of product candidates or new drugs for a considerable period of time and impose costly procedures upon our activities. We cannot be certain that the FDA or any other regulatory agency will grant approvals for Ravicti or any future product candidates on a timely basis, if at all. Success in early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval.
The NDA Approval Process
In order to obtain approval to market a drug in the United States, a marketing application must be submitted to the FDA that provides data establishing to the FDA’s satisfaction the safety and effectiveness of the investigational
97
Table of Contents
drug for the proposed indication. Each NDA submission requires a substantial user fee payment unless a waiver or exemption applies. The application includes all relevant data available from pertinent nonclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, or from a number of alternative sources, including studies initiated by investigators.
The FDA will initially review the NDA for completeness before it accepts it for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Based on pivotal Phase III trial results submitted in an NDA, upon the request of an applicant, the FDA may grant a priority review designation to a product, which sets the target date for FDA action on the application at six months, rather than the standard ten months. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a significant improvement compared to marketed products or offers a therapy where no satisfactory alternative therapy exists. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
After the FDA completes its initial review of an NDA, it will communicate to the sponsor that the drug will either be approved, or it will issue a complete response letter to communicate that the NDA will not be approved in its current form and inform the sponsor of changes that must be made or additional clinical, nonclinical or manufacturing data that must be received before the application can be approved, with no implication regarding the ultimate approvability of the application.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical sites to assure compliance with good clinical practices (GCPs). If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the NDA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase IV studies may be made a condition to be satisfied for continuing drug approval. The results of
98
Table of Contents
Phase IV studies can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA now has express statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
In June 2011, we completed a 24-month carcinogenicity study of Ravicti in rats. The data from this study showed an increased rate of seven different tumor types in rats. While we do not have evidence that individuals who have taken the active ingredient in Ravicti have an increased rate of cancer, the FDA may view these data as posing concerns with respect to the long term safety of Ravicti. The FDA may request that we conduct additional nonclinical studies.
We submitted the NDA for Ravicti for UCD in patients aged 6 years and above in December 2011 based primarily on data from already completed clinical trials, including the pivotal Phase III trial in adult patients with UCD. The FDA may determine that these data are not adequate to support approval, or are adequate only to support a limited approval.
In a pre-NDA meeting held on December 7, 2010, the FDA said that pediatric patients constitute an important patient population for Ravicti, and expressed concern that data from our Phase II trial in pediatric patients showed a higher level of PAA than that seen in adult UCD patients receiving Ravicti and that pediatric patients receiving Ravicti had higher PAA levels than pediatric patients receiving BUPHENYL. The FDA indicated that this raises a potential safety concern because PAA has been reported in the scientific and medical literature to be associated with central nervous system toxicity (also referred to as neurotoxicity) in studies evaluating PAA in the treatment of cancer. Because of this concern, the FDA stated that a clinical trial establishing dosing and safety in pediatric patients under the age of 6 years was needed. The FDA also said that approving Ravicti without this pediatric data would raise concerns, because the drug might be used in pediatric patients even if not FDA-approved for such use. The FDA clarified that this issue would not prevent the FDA from accepting the NDA submission for Ravicti for filing, but that during the NDA review, the FDA would likely consider the issue of safety and dosing data for Ravicti in a pediatric population under 6 years of age, and would seek a better understanding of PAA levels in patients aged 6 through 17 years.
Based on our pre-NDA meeting, we accelerated the initiation of a switchover clinical trial evaluating the safety and efficacy of Ravicti in a pediatric population aged 29 days through 5 years. However, as the data from the 12-month safety extension portion of the study will not be available until the second quarter of 2013, we submitted the NDA for Ravicti for UCD in adult and pediatric patients down to age 6 years without the data from this trial. For patients aged 6 through 17 years, we provided the FDA additional information on PAA levels associated with Ravicti. We believe these data show that Ravicti is safe and effective for patients aged 6 through 17 years.
In April 2012, we submitted data from the switchover portion of our clinical trial in UCD patients aged 29 days through 5 years and a revised draft package insert requesting approval of Ravicti to include this patient population. The FDA may disagree, and limit approval to an adult population, a sub-segment of the pediatric population or not approve the NDA at all. Any approval could be withdrawn if required post-marketing trials or analyses do not meet the FDA requirements, which could materially harm the commercial prospects for Ravicti.
The FDA also has authority to require a REMS from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks. A sponsor may also voluntarily propose a REMS as part of the NDA submission. The need for a REMS is determined as part of the review of the NDA. Based on statutory standards, elements of a REMS may include “dear doctor letters,” a medication guide, more elaborate targeted educational programs, and in some cases restrictions on distribution. These elements are negotiated as part of the NDA approval, and in some cases if consensus is not obtained until after the PDUFA review cycle, the approval date may be delayed. Once adopted, REMS are subject to periodic assessment and modification.
For the NDA for Ravicti, which we filed in December 2011, we proposed a REMS program (i) to support informed dosing and treatment decisions between patients and their healthcare providers by educating them on
99
Table of Contents
the safe use of Ravicti, and (ii) to limit access to Ravicti only to patients six years of age and over until such time as the Ravicti label is expanded to include UCD patients under the age of six. We believe that a REMS program will assist in the FDA’s benefit to risk assessment regarding off-label use in patients not approved under the final labeling; however, FDA may still have serious concerns that preclude them from approving Ravicti for any age group until additional data are available. Although we believe an appropriate REMS for Ravicti will not be unduly burdensome, there are circumstances in which a REMS can contain restrictions or requirements that negatively affect the commercial viability of a product.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of onerous risk management plans, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delay in obtaining, or failure to obtain, regulatory approval for Ravicti, or obtaining approval but for significantly limited use, would harm our business. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Fast Track Designation
We have received fast track designation for Ravicti for the treatment of UCD. Fast track status is intended to expedite or facilitate the process for reviewing new drugs and biological products that are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast track designation, which must be requested by the applicant, provides access to various programs, including more frequent meetings and correspondence with the FDA regarding product development, approval based on a surrogate endpoint, and rolling review, under which completed sections of an application may be submitted for review serially, rather than waiting until the entire application is completed, as is the normal practice. The FDA may revoke a fast track designation if the designation is no longer supported by the emerging data or the development program is no longer being pursued. Fast track status may not provide us with a material commercial advantage.
Companion Diagnostic Review and Approval
Our proposed labeling for Ravicti includes dose adjustment based on levels of urinary phenylacetylglutamine, or PAGN. Our plan is for the urinary PAGN testing to be available only as a Laboratory Developed Test that is commercialized by a laboratory certified under the Clinical Laboratory Improvement Amendments without approval or clearance from the FDA. Approval of all Laboratory Developed Tests is required by the State of New York prior to testing patient samples from that state. A test for urinary PAGN may be considered a companion diagnostic test by the FDA. We have not discussed our PAGN-based dosing adjustment labeling strategy with the FDA and do not know whether the FDA will accept a Laboratory Developed Test or instead will consider the test a companion diagnostic and therefore require a Premarket Approval Application, a filing through thede novo reclassification process, or 510(k) clearance for a urinary PAGN test prior to approving Ravicti. If FDA approval or clearance of a urinary PAGN test is required, any approval and launch of Ravicti could be delayed and additional costs would be required for us to reach agreement with a clinical laboratory or a third-partyin vitro diagnostic test manufacturer to seek and obtain premarket approval,de novo reclassification, or premarket clearance from the FDA. The State of New York approval process, and the FDA premarket review process if required, can be lengthy and would require submission of clinical study data.
Hatch-Waxman
Under the Hatch-Waxman Amendments, a portion of a product’s patent term that was lost during clinical development and application review by the FDA may be restored. The Hatch-Waxman Amendments also provide
100
Table of Contents
for a statutory protection, known as non-patent exclusivity, against the FDA’s acceptance or approval of certain competitor applications.
Patent term restoration can compensate for time lost during product development and the regulatory review process by returning up to five years of patent life for a patent that covers a new product or its use. This period is generally one-half the time between the effective date of an IND (falling after issuance of the patent) and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application, provided the sponsor acted with diligence. Patent term restorations, however, cannot extend the remaining term of a patent beyond a total of 14 years. The application for patent term extension is subject to approval by the United States Patent and Trademark Office, or PTO, in conjunction with the FDA. It takes at least six months to obtain approval of the application for patent term extension.
A patent term extension is only available when the FDA approves a drug product for the first time. We believe the active ingredient in Ravicti (glycerol phenylbutyrate) is a new active ingredient not previously approved by the FDA. However, we cannot be certain that the PTO and the FDA will agree with our analysis or will grant a patent term extension.
If, as would be the case of Ravicti, NDA approval is received for a new active ingredient and new dosage form, based on the submission to the FDA of new clinical investigations conducted or by or for the NDA sponsor, then the Hatch-Waxman Amendments prohibit the FDA from making effective the approval of an ANDA for a generic version of such drug, or a 505(b)(2) NDA that relies on our approval, for a period of three years from the date of the NDA approval for Ravicti.
FDA Post-Approval Requirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping and reporting of adverse experiences with the drug. Drug manufacturers are required to register their facilities with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality and purity characteristics that it purports to have. Certain states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, fail to approve any NDA or other application, require us to recall a drug from distribution, shut down manufacturing operations or withdraw approval of the NDA for that drug. Noncompliance with cGMP or other requirements can result in issuance of warning letters, civil and criminal penalties, seizures and injunctive action.
Labeling, Marketing and Promotion
The FDA closely regulates the labeling, marketing and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a drug that are consistent with FDA approval, and the company is allowed to actively market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of drugs.
101
Table of Contents
Orphan Designation
Ravicti received orphan designation for maintenance treatment of patients with deficiencies in enzymes of the urea cycle, and a separate designation for intermittent or chronic management of patients with cirrhosis and episodic HE of any grade. Under the Orphan Drug Act, the FDA may grant orphan designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan designation must be requested before submitting an NDA. Generally, if a drug that receives orphan designation is approved for the orphan indication, it receives orphan drug exclusivity, which for seven years prohibits the FDA approving another product with the same active chemical entity for the same indication. Orphan exclusivity will not bar approval of another product under certain circumstances, including if the new drug is shown to be clinically superior to the approved product on the basis of greater efficacy or safety, or providing a major contribution to patient care. After the FDA grants orphan designation, the identity of the applicant, as well as the name of the therapeutic agent and its designated orphan use, are disclosed publicly by the FDA.
Ravicti shares the same active chemical entity as BUPHENYL, which was previously granted orphan designation and awarded orphan exclusivity that has since expired. Ravicti was granted orphan designation for UCD based upon a potential safety benefit over BUPHENYL because of the absence of sodium. Whether Ravicti will receive orphan exclusivity will be determined upon approval, if any, and will be based on whether the FDA concludes that Ravicti is, in fact, safer than BUPHENYL and comparable in terms of effectiveness. Should the FDA determine that safety of Ravicti is not sufficiently better, despite the removal of sodium, or that the product does not have comparable effectiveness, Ravicti may not be granted orphan exclusivity.
Orphan designation for Ravicti for HE was granted based on data demonstrating that this disease affects fewer than 200,000 patients in the United States. Because orphan designation was granted solely on the basis of the number of patients at the time of designation, we do not believe there would be any basis to deny market exclusivity if and when Ravicti for HE is approved by the FDA.
Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product that has orphan designation is the first such product to receive FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that subsequent to approval the FDA may not approve any other applications to market a drug with the same active moiety for the same disease, except in limited circumstances, for seven years. During orphan exclusivity, the FDA may only permit additional companies to market a drug with the same active chemical entity for the designated condition if such companies can demonstrate clinical superiority, or if the company with the orphan drug exclusivity is not able to meet market demand. More than one product may also be approved by the FDA for the same orphan indication or disease as long as the products contain different active ingredients. As a result, even though Ravicti has received orphan designation, the FDA can still approve other drugs that have a different active chemical entity for use in treating the same indication or disease covered by Ravicti, which could create a more competitive market for us.
Pediatric Research Equity Act
The Pediatric Research Equity Act, or PREA, amended the FDCA to authorize the FDA to require certain research into drugs used in pediatric patients. The intent of PREA is to compel sponsors whose drugs have pediatric applicability to study those drugs in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The Secretary of Health and Human Services may defer or waive these requirements under specified circumstances. By its terms, PREA does not apply to any drug for an indication for which orphan designation has been granted, unless the FDA issues regulations saying otherwise. Because the FDA has not issued any such regulations, submission of a pediatric assessment is not required for an application to market a product for an orphan-designated indication.
102
Table of Contents
In its pre-NDA pre-meeting written response to us, the FDA acknowledged that Ravicti, as an orphan designated drug, is exempt from PREA. However, the FDA also stated its view that the evaluation of safety and dosing of Ravicti in pediatric UCD patients was necessary, based on the number of pediatric UCD patients that the FDA expects will be prescribed this drug.
Should the FDA determine during the NDA review period that there is not substantial evidence to support pediatric use, we believe that the FDA is obliged to consider approving Ravicti for use in adults. We believe there is a strong public health need for access to Ravicti for use in adults, as the FDA recognized by designating Ravicti as both an orphan drug and a fast track product. We believe that the FDCA limits the FDA’s ability to deny approval of Ravicti for adults only based on concerns regarding the product’s use in pediatric populations.
In our view, if the FDA withholds approval of Ravicti for use in adults with UCD based on the FDA’s concerns regarding the use of Ravicti in pediatric populations, the agency would be eliminating the orphan drug exception in PREA. In effect, the agency would be mandating pediatric studies for a product for which Congress has explicitly stated that none is required. We believe PREA therefore limits the FDA’s ability to require pediatric data for Ravicti and the agency’s ability to deny approval of Ravicti for use in adults because of a lack of pediatric data. The FDA may not agree with these points, and may insist that greater pediatric data support Ravicti, whether or not PREA applies.
Anti-Kickback and False Claims Laws
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing and scientific/educational grant programs must comply with the Medicare-Medicaid Anti-Fraud and Abuse Act, as amended, (the “Anti-Kickback Statute”), the False Claims Act, as amended, the privacy regulations promulgated under the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
As noted above, in the United States, we are subject to complex laws and regulations pertaining to healthcare “fraud and abuse,” including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act, and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to five years in prison, criminal fines, administrative civil money penalties, and exclusion from participation in federal healthcare programs. In addition, many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to the referral of patients for healthcare services reimbursed by any insurer, not just federal healthcare programs such as Medicare and Medicaid. Due to the breadth of these federal and state anti-kickback laws, the absence of guidance in the form of regulations or court decisions, and the potential for additional legal or regulatory change in this area, it is possible that our future sales and marketing practices and/or our future relationships with physicians might be challenged under anti-kickback laws, which could harm us. Because we intend to commercialize products that could be reimbursed under a federal healthcare program and other governmental healthcare programs, we plan to develop a comprehensive compliance program that establishes
103
Table of Contents
internal controls to facilitate adherence to the rules and program requirements to which we will or may become subject.
The federal False Claims Act prohibits anyone from knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Although we would not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state, and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the federal False Claims Act in connection with their off-label promotion of drugs. Penalties for a False Claims Act violation include three times the actual damages sustained by the government, plus mandatory civil penalties of between $5,500 and $11,000 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. If the government were to allege that we were, or convict us of, violating these false claims laws, we could be subject to a substantial fine and may suffer a decline in our stock price. In addition, private individuals have the ability to bring actions under the federal False Claims Act and certain states have enacted laws modeled after the federal False Claims Act.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, as discussed below, beginning in 2013, a similar federal requirement will require manufacturers to track and report to the federal government certain payments made to physicians and teaching hospitals made in the previous calendar year. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
Patient Protection and Affordable Health Care Act
In March 2010, the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively PPACA, was enacted, which includes measures that have or will significantly change the way health care is financed by both governmental and private insurers. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Effective in 2010, PPACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic agents from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. PPACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization as of 2010 and by expanding the population potentially eligible for Medicaid drug benefits, to be phased-in by 2014. The Centers for Medicare and Medicaid Services, or CMS, have proposed to expand Medicaid rebate liability |
104
Table of Contents
to the territories of the United States as well. In addition, PPACA provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. The implementation of this requirement by the CMS may also provide for the public availability of pharmacy acquisition of cost data, which could negatively impact our sales. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. Effective in 2010, PPACA expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. |
| • | Effective in 2011, PPACA imposed a requirement on manufacturers of branded drugs and biologic agents to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., “donut hole”). |
| • | Effective in 2011, PPACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. |
| • | Effective in 2012, PPACA will require pharmaceutical manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers will be required to report this information beginning in 2013. |
| • | As of 2010, a new Patient-Centered Outcomes Research Institute was established pursuant to PPACA to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | PPACA created the Independent Payment Advisory Board which, beginning in 2014, will have authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. |
| • | PPACA established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
Many of the details regarding the implementation of PPACA are yet to be determined, and at this time, it remains unclear the full effect that PPACA would have on our business.
Antitrust
We are also subject to antitrust review of our planned acquisition of BUPHENYL and potentially AMMONUL, including, if the necessary jurisdictional thresholds are met at that time, review under the HSR Act.
105
Table of Contents
Under the HSR Act, and the related rules and regulations that have been issued by the FTC, certain transactions, potentially including those in which those products will be acquired, may not be completed until specified information and documentary material has been furnished for review by the FTC and the Antitrust Division by both us and Ucyclyd and specified waiting periods have been satisfied. If triggered, the HSR Act would provide for an initial 30-calendar day waiting period after both parties submit their filings. If the 30th calendar day of the initial waiting period is not a business day, the initial waiting period is extended until 11:59 PM Eastern of the next business day. If, before expiration or early termination of the initial 30-calendar day waiting period, either the FTC or the Antitrust Division issues a request for additional information and documentary material from the parties, the waiting period will be extended for an additional period of 30-calendar days following the date of both parties’ substantial compliance with that request. Only one extension of the waiting period pursuant to a request for additional information is authorized by the HSR Act. After that time, the waiting period may be extended only by court order or with the parties’ consent. The FTC or Antitrust Division may terminate the additional 30-calendar day waiting period before its expiration. In practice, complying with a request for additional information or documentary material may take a significant period of time. In addition, if the FTC or Antitrust Division were to challenge our purchase and we were unable to resolve the challenge through a consent decree, Ucyclyd could terminate the restated collaboration agreement and we would lose our rights to BUPHENYL and AMMONUL.
Whether or not HSR filings are required, at any time before or after the acquisition of Ravicti, BUPHENYL and potentially AMMONUL, the FTC or the Antitrust Division could take any action under the antitrust laws that it either considers necessary or desirable in the public interest, including seeking to enjoin the acquisition or to seek the divestiture of certain assets or the imposition of certain licensing obligations on us or any of our subsidiaries or affiliates. Private parties as well as State Attorneys General and foreign antitrust regulators may also bring legal actions under the antitrust laws under certain circumstances.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Employees
We had 14 full-time employees as of March 31, 2012. None of our employees are represented by any collective bargaining unit. We believe that we maintain good relations with our employees.
Property and Facilities
Our headquarters is currently located in South San Francisco, California, and consists of approximately 8,167 square feet of leased office space under a lease that expires on August 31, 2013. We may require additional space and facilities as our business expands.
Legal Proceedings
We are not currently subject to any material legal proceedings.
106
Table of Contents
Directors and Executive Officers
The following table sets forth our directors and executive officers, their ages and the positions they held as of May 15, 2012.
Name | Age | Position | ||
Directors | ||||
James I. Healy, M.D., Ph.D.(1) | 47 | Chairman | ||
Gaurav Aggarwal, M.D. | 39 | Director | ||
David W. Gryska(1)(2)(3)(4) | 56 | Director | ||
Bo Jesper Hansen, M.D., Ph.D.(1)(2)(3) | 52 | Director | ||
Robert Hopfner, Ph.D. | 39 | Director | ||
Jake R. Nunn(2) | 41 | Director | ||
Bijan Salehizadeh, M.D. | 39 | Director | ||
Lota S. Zoth(2)(3) | 52 | Director | ||
Executive Officers | ||||
Donald J. Santel | 51 | Chief Executive Officer, President and Director | ||
Bruce F. Scharschmidt, M.D. | 66 | Chief Medical Officer and Senior Vice President | ||
Jeffrey S. Farrow | 50 | Chief Financial Officer and Secretary | ||
Klara A. Dickinson | 45 | Senior Vice President, Regulatory Affairs and Compliance | ||
Christine A. Nash | 39 | Senior Vice President and Chief Commercial Officer | ||
| (1) | Member of Nominating and Corporate Governance Committee. |
| (2) | Member of Audit Committee. Upon completion of this offering Mr. Nunn will no longer be a member of the audit committee. |
| (3) | Member of Compensation Committee. |
| (4) | Lead Director. |
The following includes a brief biography for each of our directors and executive officers, with each director biography including information regarding the experiences, qualifications, attributes or skills that caused our board of directors to determine that each member of our board of directors should serve as a director as of the date of this prospectus. There are no family relationships among any of our directors or executive officers.
Directors
James I. Healy, M.D., Ph.D. has been a member of our board of directors since 2006 and Chairman of our board of directors since July 2009. Dr. Healy has been a General Partner of Sofinnova Ventures, a venture capital firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of Amarin Corporation plc, Anthera Pharmaceuticals, Inc., InterMune, Inc., and several private companies. Previously, he served as a board member of CoTherix, Inc. and Movetis NV and several private companies. Dr. Healy holds an M.D. and a Ph.D. in Immunology from the Stanford School of Medicine and holds a B.A. in molecular biology and a B.A. in Scandinavian Studies from the University of California at Berkeley. Dr. Healy’s experience in the pharmaceutical industries and investing in life sciences companies, as well as his medical and scientific background, provide him with the qualifications and skills to serve as a director.
107
Table of Contents
Gaurav Aggarwal, M.D. joined our board of directors in June 2009. Dr. Aggarwal has been a Partner of Panorama Capital, LLC, a venture capital fund, since October 2010 and a member of the General Partner of Panorama Capital, L.P., a venture capital fund, since August 2006. Dr. Aggarwal focuses on life sciences investments. Dr. Aggarwal was an associate with JPMorgan Partners from March 2004 until August 2006. Prior to joining JPMorgan Partners, Dr. Aggarwal was employed by KBL Healthcare Ventures, most recently as Vice President, where he also focused on venture capital investments in biopharmaceutical and medical device companies and by the venture capital group at Wasserstein Perella & Co. Dr. Aggarwal currently serves on and has previously served on the board of directors of several private companies. Dr. Aggarwal received his M.D. from Columbia University, College of Physicians & Surgeons and his B.S. in Agricultural Economics from Cornell University. Dr. Aggarwal’s investment experience and medical background provides him with the qualifications and skills to serve as a director.
David W. Gryska has been a member of our board of directors since November 2010. Since May 11, 2012, Mr. Gryska has served as Chief Operating Officer of Myrexis Inc., a biotechnology company, From December 2006 to October 2010, Mr. Gryska served as Senior Vice President and Chief Financial Officer of Celgene Corporation, a biopharmaceutical company. From October 2004 to December 2006, he was a principal at Strategic Consulting Group, where he provided strategic consulting to early-stage biotechnology companies. Prior to that time, Mr. Gryska held positions at Scios, Inc. and Cardiac Pathways Corporation and served as a partner at Ernst & Young LLP. Mr. Gryska currently serves as a member of the board of directors of Seattle Genetics, Inc. Mr. Gryska holds an M.B.A. from Golden Gate University and a B.A. in accounting and finance from Loyola University. Mr. Gryska is also a Certified Public Accountant. Mr. Gryska’s experience as Chief Financial Officer at Celgene, Scios, and Cardiac Pathways provided him valuable and relevant experience as a senior financial executive at life sciences and biotechnology companies dealing with financings, mergers, acquisitions and global expansion and other strategic transactions and provides him with the qualifications and skills to serve as a director. Additionally, Mr. Gryska has extensive knowledge of accounting principles and financial reporting rules and regulations, tax compliance and oversight of the financial reporting processes, which assists Mr. Gryska in fulfilling his duties as Lead Director and as a member of our Audit Committee.
Bo Jesper Hansen, M.D., Ph.D. has been a member of our board of directors since April 2011. Since January 2010, Dr. Hansen has served as chairman of the board of Swedish Orphan Biovitrum AB, a Swedish specialty pharmaceutical company focusing on rare diseases with unmet medical needs. Previously, Dr. Hansen held various positions in Swedish Orphan International AB from 1993 and was President and Chief Executive Officer of Swedish Orphan International Group of Companies from 1998 until the merger with Biovitrum in 2010. Prior to joining Swedish Orphan International AB, Dr. Hansen worked as a medical advisor for several of the largest pharmaceutical companies throughout the world, including Synthélabo, Pfizer, Inc., Pharmacia Corporation and Yamanouchi Pharmaceutical Co. Ltd. Dr. Hansen also founded the Scandinavian Medical Research. Dr. Hansen is the chairman of the board of directors of Topotarget A/S and is a member of the boards of directors of two private companies. Dr. Hansen received an M.D. and a Ph.D. from the University of Copenhagen. Dr. Hansen’s experience includes international marketing and contract negotiations, extensive knowledge within regulatory, pharmacovigilance, medical marketing and business development and he has a strong network and close collaborations in the pharmaceutical industry in general and in the orphan drug area in particular, all of which provides him with the qualifications and skills to serve as director.
Robert Hopfner, Ph.D. has been a member of our board of directors since December 2010. Dr. Hopfner has served as an Investment Partner at Bay City Capital, a venture capital firm, since August 2002. Before joining Bay City Capital, Dr. Hopfner worked as an associate in DuPont Pharmaceuticals’ Business Development & Strategic Planning group and as an analyst at Ag-West Biotech, a Western Canadian seed-stage biotech venture capital firm. He is a member of the board of directors of a private company. Dr. Hopfner holds a Ph.D. in Pharmacology and a B.S. in Pharmacy from the University of Saskatchewan and an M.B.A. with specializations in Entrepreneurship, Finance and Strategy from the University of Chicago Booth School of Business. Dr. Hopfner’s experience in the venture capital industry and in investing in life sciences companies, as well as his medical background, provides him with the qualifications and skills to serve as director.
Jake R. Nunnhas been a member of our board of directors since April 2009. Mr. Nunn joined New Enterprise Associates, Inc., a venture capital firm, in 2006 as a Partner, where he focuses on later-stage specialty
108
Table of Contents
pharmaceuticals, biotechnology and medical device investments. From January 2001 to June 2006, Mr. Nunn served as a Partner and an analyst for the MPM BioEquities Fund, a public life sciences fund at MPM Capital, L.P., a private equity firm, where he specialized in life sciences investing. Previously, Mr. Nunn was a healthcare research analyst and portfolio manager at Franklin Templeton Investments and an investment banker with Alex. Brown & Sons. Mr. Nunn is currently on the boards of directors at Transcept Pharmaceuticals, Inc. and three private companies. Mr. Nunn received an M.B.A. from the Stanford University Graduate School of Business and an A.B. in Economics from Dartmouth College. Mr. Nunn holds the Chartered Financial Analyst designation, and is a member of the CFA Society of San Francisco. Mr. Nunn’s experience in investing in life sciences, later-stage specialty pharmaceuticals, biotechnology and medical device investments, as well as his business and educational background, provides him with the qualifications and skills to serve as director.
Bijan Salehizadeh, M.D. has been a member of our board of directors since August 2007. Dr. Salehizadeh currently serves as co-founder and Managing Director at NaviMed Capital Advisors LLC, a private investment firm focused on growth venture capital healthcare investments. Dr. Salehizadeh also serves as an advisor to Highland Capital Partners LLC, a venture capital firm. From September 2004 to August 2011, Dr. Salehizadeh was an investment professional at Highland Capital Partners, where he most recently served as General Partner. Dr. Salehizadeh focused on healthcare investments, primarily in medical products, healthcare services, and biopharmaceutical companies. Prior to this, Dr. Salehizadeh served in various positions at Medtronic and HealthCentral. Dr. Salehizadeh currently serves on the board of directors of several private healthcare companies. Dr. Salehizadeh received an M.D. and an M.S. in Health Policy from Columbia University, an M.B.A. from Harvard Business School and an A.B. in Molecular Biology from Princeton University. Our board believes that Dr. Salehizadeh’s experience in investing in healthcare and life sciences companies, as well as his medical background, provides him with the qualifications and skills to serve as a director.
Lota S. Zothhas been a member of our board of directors since April 2008. Ms. Zoth served as Senior Vice President and Chief Financial Officer of MedImmune, Inc. from April 2004 to July 2007 and also served as its Controller and Principal Accounting Officer. Prior to joining MedImmune in 2002, Ms. Zoth served as Senior Vice President, Corporate Controller and Principal Accounting Officer at PSINet Inc., Vice President, Corporate Controller and Chief Accounting Officer at Sodexho Marriott Services, Inc., Marriott International and PepsiCo, Inc. Ms. Zoth also served as an auditor at Ernst & Young, LLP and is a Certified Public Accountant. Ms. Zoth is a member of the boards of directors of Orexigen Therapeutics, Inc. and two private companies. Ms. Zoth received a B.B.A. in accounting from Texas Tech University. Ms. Zoth’s experience as Chief Financial Officer, Controller and Principal Accounting Officer provided her valuable and relevant experience as a senior financial executive at life sciences and biotechnology companies dealing with financings, mergers, acquisitions and global expansion and other strategic transactions and provides her with the qualifications and skills to serve as a director.
Executive Officers
Donald J. Santel has served as our Chief Executive Officer since June 2008. Mr. Santel has been a member of our board of directors since March 2007. Previously, Mr. Santel was a member of the board of directors and the Chief Executive Officer of CoTherix, Inc., a private biopharmaceutical company he co-founded in 2000, where he was responsible for the oversight of all aspects of the business and led the sale of the company to Actelion in January 2007. Prior to joining CoTherix, Mr. Santel was employed by or consultant to several medical device companies, including Reflow, Inc., Cardiac Pathways Corporation and Medtronic, Inc. Mr. Santel is currently on the board of directors and the audit and compensation committees of Anthera Pharmaceuticals, Inc. and previously served as a director of ChemGenex Pharmaceuticals, Inc. Mr. Santel holds an M.S. in electrical engineering from the University of Minnesota and a B.S.E. in biomedical engineering from Purdue University.
Bruce F. Scharschmidt, M.D. has served as our Chief Medical Officer and Senior Vice President since April 2008. From April 2006 to April 2008, Dr. Scharschmidt served as Vice President of Scientific Affairs for NOVARTIS Vaccines, a division of NOVARTIS, a healthcare products company, where Dr. Scharschmidt was
109
Table of Contents
responsible for developing the clinical strategy for early-stage vaccines. From August 1996 to April 2006, Dr. Scharschmidt held senior positions at Chiron Corporation, including VP and head of clinical development for vaccines and therapeutics and, most recently, as Vice President of Scientific Affairs in the Corporate Group, where he was involved in the strategic direction and management of key research and development programs including vaccines, therapeutics and blood testing. Before joining Chiron, Dr. Scharschmidt was Chief of Gastroenterology and Professor of Medicine at the University of California San Francisco. Dr. Scharschmidt received both his M.D. and undergraduate degree from Northwestern University as part of a six-year Honors Program in Medical Education. Dr. Scharschmidt completed his training in Internal Medicine and Gastroenterology at the University of California, San Francisco.
Jeffrey S. Farrow has served as our Chief Financial Officer since July 2010 and served as our Vice President, Finance from February 2010 to June 2010. From May 2008 to December 2009, Mr. Farrow was Vice President, Finance at Evotec AG, a drug discovery and development company, where Mr. Farrow was responsible for Evotec’s corporate treasury function and compliance with the Sarbanes Oxley Act, as well as overseeing the finance and general & administrative functions of the company’s Renovis subsidiary. From January 2004 to May 2008, Mr. Farrow held various positions, with the most recent being Vice President, Finance and Chief Accounting Officer, at Renovis, Inc., a drug discovery and development company, which was acquired by Evotec AG in May 2008. While at Renovis Mr. Farrow was a key member of the management team responsible for the merger with Evotec, as well as Renovis’ initial public offering and secondary offering. Previously, Mr. Farrow held various positions over his seven years in the audit practice of KPMG LLP and was most recently a Senior Manager. Mr. Farrow holds a B.A. in Business Administration with a concentration in Corporate Finance from California State University at Fullerton.
Klara A. Dickinson has served as our Senior Vice President, Regulatory Affairs and Compliance since October 2007. Previously, Ms. Dickinson spent three years with CoTherix, Inc. and was most recently Vice President, Regulatory Affairs and Healthcare Compliance Officer from January 2004 to January 2007. In that role, Ms. Dickinson led the filing of the NDA and label negotiations for the company’s initial product, Ventavis® (iloprost) Inhalation Solution. Prior to CoTherix, Inc., Ms. Dickinson held various positions at biopharmaceutical companies Scios, Inc. and DEY Laboratories, a subsidiary of Mylan, Inc. Ms. Dickinson holds a B.S. in Biology from the College of Great Falls in Montana and is certified by the Regulatory Affairs Certification Board.
Christine A. Nash has served as our Senior Vice President and Chief Commercial Officer since May 2012. From August 2008 until May 2012 she served as Vice President, Strategic Marketing and Corporate Business Development and she joined us in August 2007 as Senior Director, Marketing. From October 2004 to February 2007, Ms. Nash held various positions at CoTherix, Inc., including most recently as Director of Marketing. As Director of Marketing, Ms. Nash led all marketing and product support aspects for the launch of the company’s initial product, Ventavis® (iloprost) Inhalation Solution. Ms. Nash’s previous experience includes business development and product planning and management roles with Genesoft Pharmaceuticals Inc., Oncology Therapeutics Network, Eli Lilly and Company, and Imana, Inc. Ms. Nash holds an M.B.A and a B.A. with Honors in Public Policy, both from Stanford University.
Director Independence
Under the listing requirements and rules of The NASDAQ Stock Market, or Nasdaq, independent directors must compose a majority of a listed company’s board of directors within a one year period following the completion of this offering. In addition, applicable Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating committees must be independent within the meaning of applicable Nasdaq rules. Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Our board of directors has undertaken a review of the independence of each director and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent
110
Table of Contents
judgment in carrying out his or her responsibilities. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director. As a result of this review, our board of directors determined that Dr. Hansen, Mr. Gryska and Ms. Zoth qualify as “independent” directors within the meaning of the Nasdaq rules. Although Nasdaq rules require that a majority of the board of directors and each member of our audit compensation and nominating committees must be independent, under special phase-in rules applicable to new public companies, we will have until one year from the effective date of our initial public offering to comply with these independence requirements. As required under applicable Nasdaq rules, we anticipate that our independent directors will meet in regularly scheduled executive sessions at which only independent directors are present.
Board Composition and Election of Directors
Our board of directors currently consists of nine directors. In accordance with our amended and restated certificate of incorporation, to be effective upon the closing of this offering, our board of directors may establish from time to time by resolution the authorized number of directors. Currently, ten directors are authorized. Following this offering, our amended and restated certificate of incorporation will provide for a classified board of directors consisting of three classes of directors. We will have three directors in each class, each serving a staggered three-year term. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. After the completion of this offering, our directors will be divided among the three classes as follows:
| • | the Class I directors will be Drs. Aggarwal, Hopfner and Salehizadeh, and their terms will expire at the annual meeting of stockholders to be held in 2013; |
| • | the Class II directors will be Dr. Healy, Mr. Nunn and Ms. Zoth, and their terms will expire at the annual meeting of stockholders to be held in 2014; and |
| • | the Class III directors will be Messrs. Gryska and Santel and Dr. Hansen, and their terms will expire at the annual meeting of stockholders to be held in 2015. |
The classification of the board of directors may have the effect of delaying or preventing changes in control of our company. We expect that additional directorships resulting from an increase in the number of directors, if any, will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors.
Committees of the Board of Directors
Our board of directors has an audit committee, a compensation committee and a nominating and corporate governance committee. The composition and responsibilities of each committee are described below. Members serve on these committees until their resignation or until otherwise determined by our board.
Audit Committee
The members of our audit committee following the offering will be Dr. Hansen, Mr. Gryska and Ms. Zoth. Ms. Zoth serves as chair of the audit committee. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and Nasdaq and which will be available on our website prior to the completion of this offering at www.hyperiontx.com. The inclusion of our website address here and elsewhere in this prospectus does not include or incorporate by reference the information on our website into this prospectus.
111
Table of Contents
Our board of directors has determined that all members of our audit committee who will continue to be on the audit committee following our initial public offering are independent as independence is currently defined in Rule 5605(a)(2) of the Nasdaq listing standards and Rule 10A-3 under the Exchange Act.
In addition, our board of directors has determined that each member of the audit committee is financially literate and that Ms. Zoth qualifies as an “audit committee financial expert” as defined in applicable SEC rules. In making this determination, our board has considered the formal education and nature and scope of her previous experience, coupled with past and present service on various audit committees.
The responsibilities of our audit committee include, among other things:
| • | reviewing our annual and quarterly financial statements and reports, discussing the statements and reports with our independent registered public accounting firm and management and recommending to the board whether to include the financial statements in the annual reports filed with the SEC; |
| • | discussing the type of information to be disclosed and the type of presentation to be made regarding financial information and earnings guidance to analysts and ratings agencies; |
| • | overseeing our disclosure controls and procedures, including internal controls over our financial reporting, and reviewing and discussing our management’s periodic review of the effectiveness of our internal control over financial reporting; |
| • | reviewing with our independent registered public accounting firm and management significant issues that arise regarding accounting principles and financial statement presentation, matters concerning the scope, adequacy and effectiveness of our financial controls, effects of alternative accounting principles generally accepted in the United States of America, methods on our financial statements and any correspondence or reports that raise issues with or could have a material effect on our financial statements; |
| • | retaining, appointing, setting compensation of and evaluating the performance, independence, internal quality control procedures and qualifications of our independent auditors; |
| • | reviewing and approving in advance the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services; |
| • | reviewing with our independent registered public accounting firm the planning and staffing of the audit, including the rotation requirements and other independence rules; |
| • | reviewing and, if acceptable, approving any related person transactions and establishing and reviewing our code of business conduct and ethics; |
| • | overseeing and discussing with management our policies with respect to risk assessment and risk management, and our significant financial and operational risk exposures; |
| • | setting policies for our hiring of employees or former employees of our independent registered public accounting firm; and |
| • | reviewing and assessing the adequacy of our audit committee charter periodically. |
Compensation Committee
The members of our compensation committee are Mr. Gryska, Dr. Hansen and Ms. Zoth. Mr. Gryska serves as chair of the compensation committee. All members of our Compensation Committee are independent as
112
Table of Contents
independence is currently defined in Section 5605(a)(2) of the Nasdaq listing standards and qualify as outside directors under Section 162(m) of the Code. The compensation committee operates under a written charter that satisfies the applicable standards of Nasdaq and which will be available on our website prior to the completion of this offering at www.hyperiontx.com.
The responsibilities of our compensation committee include, among other things:
| • | approving the compensation and other terms of employment of our chief executive officer, which are then reviewed and ratified by our board of directors; |
| • | approving or recommending to our board of directors the compensation and other terms of employment of our executive officers, other than the chief executive officer; |
| • | approving annually the corporate goals and objectives relevant to the compensation of our chief executive officer and assessing at least annually our chief executive officer’s performance against these goals and objectives; |
| • | reviewing annually our compensation strategy, including base salary, incentive compensation and equity-based grants, as well as adoption, modification or termination of this compensation; |
| • | evaluating at least annually and recommending to our board of directors the type and amount of compensation to be paid or awarded to non-employee board members; |
| • | reviewing the competitiveness of our executive compensation programs and evaluating the effectiveness of our compensation policy and strategy in achieving expected benefits to us; |
| • | approving the terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for our executive officers; |
| • | reviewing at least annually the adequacy of our compensation committee charter; and |
| • | reviewing and evaluating, at least annually, the performance of the compensation committee. |
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Drs. Healy and Hansen and Mr. Gryska. Dr. Healy serves as chair of this committee. Currently, our board of directors has determined that Mr. Gryska and Dr. Hansen are independent as independence is currently defined in Section 5605(a)(2) of the Nasdaq listing standards. We expect that membership of this committee will be changed to comply with independence requirements prior to the end of the phase-in period permitted by Nasdaq. The nominating and corporate governance committee operates under a written charter that satisfies the applicable standards of Nasdaq and which will be available on our website prior to the completion of this offering atwww.hyperiontx.com.
The responsibilities of our nominating and corporate governance committee include, among other things:
| • | identifying, considering and nominating candidates to serve on our board of directors; |
| • | developing and recommending the minimum qualifications for service on our board of directors; |
| • | overseeing the evaluation of the board and management on an annual basis; |
| • | considering nominations by stockholders of candidates for election to the board of directors; |
113
Table of Contents
| • | reviewing annually the independence of the non-employee directors and members of the independent committees of the board; |
| • | developing and recommending to our board of directors a set of corporate governance guidelines, and reviewing and recommending to our board of directors any changes to such principles; |
| • | developing and recommending to our board of directors a code of business conduct and ethics, and reviewing and recommending to our board of directors any changes to the code; and |
| • | reviewing the adequacy of its charter, our corporate governance guidelines and our code of business conduct and ethics on an annual basis. |
Compensation Committee Interlocks and Insider Participation
Our compensation committee currently consists of Mr. Gryska, Dr. Hansen and Ms. Zoth. No member of our compensation committee has ever been an officer or employee of ours. None of our executive officers serves, or has served during the last three years, as a member of the board of directors or compensation committee of any other entity that has one or more of its officers serving on our board of directors or compensation committee.
Board Leadership Structure and Role in Risk Oversight
Board Leadership Structure
The positions of our chairman of the board and chief executive officer are separated. Separating these positions allows our chief executive officer to focus on our day-to-day business, while allowing the chairman of the board to lead the board of directors in its fundamental role of providing advice to, and independent oversight of, management. Our board of directors recognizes the time, effort and energy that the chief executive officer must devote to his position in the current business environment, as well as the commitment required to serve as our chairman, particularly as the board of directors’ oversight responsibilities continue to grow. Our board of directors also believes that this structure ensures a greater role for the independent directors in the oversight of our company and active participation of the independent directors in setting agendas and establishing priorities and procedures for the work of our board of directors.
Although the amended and restated bylaws that will be in effect upon the closing of this offering will not require that our chairman and chief executive officer positions be separate, our board of directors believes that having separate positions is the appropriate leadership structure for us at this time and demonstrates our commitment to good corporate governance. The board recognizes that depending on the circumstances, other leadership models, such as combining the role of chairman with the role of chief executive officer, might be appropriate. Accordingly, the board may periodically review its leadership structure. Our board of directors believes its administration of its risk oversight function has not affected its leadership structure.
Our chairman of the board is Dr. Healy. Because Dr. Healy is not independent, we have designated Mr. Gryska as our lead director. The board of directors believe our leadership structure is appropriately balanced by the designation of a lead director role. The lead director is selected from among our independent directors. The lead director’s duties include: (i) presiding at all meetings of the board of directors at which our chairman is not present, including executive sessions of the independent directors; (ii) serving as liaison between management and the independent directors; (iii) calling meetings of the independent directors; (iv) consulting with the chairman in planning and setting schedules and agendas for board meetings to be held during the year; and (v) performing such other functions as the board may direct.
Our non-employee directors meet from time to time, but not less than twice per year, in executive sessions without any members of management present. In addition, the independent directors shall meet alone in
114
Table of Contents
executive session at no less than two regular meetings of the board each year. Additional executive sessions of the independent directors may be called at any time by the lead director, and shall be called by the lead director at the request of a majority of the independent directors. The purpose of these executive sessions is to promote open and candid discussion among the non-employee directors.
Risk Oversight
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. We face a number of risks, including those described under the caption “Risk Factors” contained elsewhere in this prospectus. Our board of directors is actively involved in oversight of risks that could affect us. This oversight is conducted primarily through committees of the board of directors, as disclosed in the descriptions of each of the committees above, but the full board of directors has retained responsibility for general oversight of risks. The board of directors considers that our leadership structure facilitates the board’s oversight of risk management and communication with management, because the board of directors has named a lead director with defined responsibilities including participation in planning meeting agendas. The lead director and each of the other directors are encouraged to raise matters at any time for board and committee meetings. Additionally, our board of directors satisfies this responsibility through full reports by each committee chair regarding the committee’s considerations and actions, as well as through regular reports directly from officers responsible for oversight of particular risks within our company. Our board of directors believes that full and open communication between management and the board of directors is essential for effective risk management and oversight.
Code of Business Conduct and Ethics
We adopted a code of business conduct and ethics that applies to all of our employees, officers and directors including those officers responsible for financial reporting. Upon the completion of this offering, the code of business conduct and ethics will be available on our website atwww.hyperiontx.com. We intend to disclose future amendments to the code or any waivers of its requirements on our website to the extent permitted by the applicable rules and exchange requirements.
115
Table of Contents
Summary Compensation Table
The following table sets forth information for the year ended December 31, 2011 regarding compensation awarded to or earned by our named executive officers.
Name and Principal Position | Year | Salary ($) | Option Awards(1) ($) | Non-Equity Incentive Plan Compensation(2) ($) | Total ($) | |||||||||||||||
Donald J. Santel | 2011 | 412,000 | 94,631 | 173,040 | 679,671 | |||||||||||||||
Chief Executive Officer and President | ||||||||||||||||||||
Jeffrey S. Farrow | 2011 | 272,750 | 126,383 | 85,388 | 484,521 | |||||||||||||||
Chief Financial Officer | ||||||||||||||||||||
Bruce F. Scharschmidt, M.D. | 2011 | 336,518 | 62,343 | 105,351 | 504,212 | |||||||||||||||
Chief Medical Officer and Senior Vice President | ||||||||||||||||||||
Klara A. Dickinson | 2011 | 309,300 | 53,527 | 96,830 | 459,657 | |||||||||||||||
Senior Vice President, Regulatory Affairs and Compliance | ||||||||||||||||||||
Christine A. Nash | 2011 | 252,000 | 77,729 | 78,892 | 408,621 | |||||||||||||||
Senior Vice President and Chief Commercial Officer | ||||||||||||||||||||
| (1) | Amounts reflect the grant date fair value of option awards granted in 2011 in accordance with ASC 718. For information regarding assumptions underlying the valuation of equity awards, see Note 11 to our consolidated financial statements and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Critical Accounting Policies — Stock-Based Compensation included elsewhere in this prospectus. These amounts do not correspond to the actual value that will be recognized by the named executive officers. Each grant vests in equal monthly installments over four years beginning on April 15, 2011. |
| (2) | Amounts represent amounts earned in 2011, which were paid during 2012, under our bonus program assuming the full achievement of performance goals and other factors deemed relevant by our board of directors and compensation committee. Our 2011 company objectives were related to development and regulatory milestones and achieving financial objectives. For 2011, our chief executive officer’s annual performance bonus was determined solely based on attainment of company objectives, which our board of directors and compensation committee determined was appropriate given our chief executive officer’s responsibility for the overall direction and success of our business. The 2011 annual performance bonuses for each of the other named executive officers was based 75% on the achievement of company objectives and 25% on individual performance. For 2011, the compensation committee determined that the company’s objectives had been exceeded and that in combination with each named executive officer’s individual performance, each named executive officer, other than Mr. Santel, was entitled to approximately 104% of his or her target bonus. The compensation committee recommended that Mr. Santel receive 105% of his target bonus, which our board of directors approved. |
116
Table of Contents
Grants of Plan-Based Awards
The following table provides information concerning grants of plan based awards to each of our named executive officers during 2011.
Name | Grant Date | Estimated Possible Payouts Under Non-Equity Incentive Plan Awards(1) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards(2) ($/Sh) | Grant Date Fair Value of Stock and Option Awards(3) ($) | |||||||||||||||
| Target ($) | ||||||||||||||||||||
Donald J. Santel | 4/15/2011 | 164,800 | — | — | — | |||||||||||||||
| 4/15/2011 | — | 242,933 | 0.67 | 94,631 | ||||||||||||||||
Jeffrey S. Farrow | 4/15/2011 | 81,825 | — | — | — | |||||||||||||||
| 4/15/2011 | — | 323,893 | 0.67 | 126,383 | ||||||||||||||||
Bruce F. Scharschmidt, M.D. | 4/15/2011 | 100,955 | — | — | — | |||||||||||||||
| 4/15/2011 | — | 159,771 | 0.67 | 62,343 | ||||||||||||||||
Klara A. Dickinson | 4/15/2011 | 92,790 | — | — | — | |||||||||||||||
| 4/15/2011 | — | 137,179 | 0.67 | 53,527 | ||||||||||||||||
Christine A. Nash | 4/15/2011 | 75,600 | — | — | — | |||||||||||||||
| 4/15/2011 | — | 199,202 | 0.67 | 77,729 | ||||||||||||||||
| (1) | Amounts represent amounts payable under our bonus program assuming the full achievement of performance goals and other factors deemed relevant by our board of directors and compensation committee. Actual amounts paid are set forth under the heading “Executive Compensation — Summary Compensation Table.” |
| (2) | Amounts represent the fair value of our common stock as determined in good faith by our board of directors on the date of grant. |
| (3) | Reflects the grant date fair value of each award computed in accordance with ASC 718. These amounts do not correspond to the actual value that will be recognized by the named executive officers. The assumptions used in the valuation of these awards are consistent with the valuation methodologies specified in Note 11 to our consolidated financial statements appearing elsewhere in this prospectus. |
117
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding equity awards held by the named executive officers that were outstanding as of December 31, 2011.
| Option Awards | ||||||||||||||||
Name | Number of Securities Underlying Unexercised Options Exercisable (#) | Number of Securities Underlying Unexercised Options Unexercisable (#) | Option Exercise Price ($/Sh) | Option Expiration Date | ||||||||||||
Donald J. Santel | 1,030 | — | 53.85 | 9/3/2017 | ||||||||||||
| 1,366,666 | — | 0.21 | 11/2/2019 | |||||||||||||
| 1,987,180 | — | 0.21 | 11/2/2019 | |||||||||||||
| 40,488 | 202,445 | (1) | 0.67 | 4/14/2021 | ||||||||||||
Jeffrey S. Farrow | 71,875 | 78,125 | (2) | 0.21 | 4/14/2020 | |||||||||||
| 71,875 | 78,125 | (2) | 0.21 | 6/29/2020 | ||||||||||||
| 53,982 | 269,911 | (1) | 0.67 | 4/14/2021 | ||||||||||||
Bruce F. Scharschmidt, M.D. | 167 | — | 53.85 | 4/21/2018 | ||||||||||||
| 1,047 | 122 | (2) | 53.85 | 4/30/2018 | ||||||||||||
| 199,875 | 67,125 | (1) | 0.21 | 11/2/2019 | ||||||||||||
| 26,628 | 133,143 | (1) | 0.67 | 4/14/2021 | ||||||||||||
Klara A. Dickinson | 1,030 | — | 53.85 | 8/30/2017 | ||||||||||||
| 167 | — | 53.85 | 4/21/2018 | |||||||||||||
| 427,000 | 61,000 | (1) | 0.21 | 11/2/2019 | ||||||||||||
| 22,863 | 114,316 | (1) | 0.67 | 4/14/2021 | ||||||||||||
Christine A. Nash | 557 | — | 53.85 | 8/30/2017 | ||||||||||||
| 111 | — | 53.85 | 4/21/2018 | |||||||||||||
| 299,250 | 42,750 | (1) | 0.21 | 11/2/2019 | ||||||||||||
| 33,200 | 166,002 | (1) | 0.67 | 4/14/2021 | ||||||||||||
| (1) | These options vest over four years in equal monthly installments. |
| (2) | These options vest 25% on the one year anniversary of the vesting commencement date and then the remainder vest over three years in equal monthly installments. |
Option Exercises and Stock Vested
No options were exercised by our named executive officers during the fiscal year ended December 31, 2011.
Pension Benefits
We did not maintain any plan providing for payments or other benefits at, following, or in connection with retirement, during the fiscal year ended December 31, 2011.
Nonqualified Deferred Compensation
We did not maintain any deferred compensation plans for any named executive officer for the year ended December 31, 2011.
118
Table of Contents
Employment, Change of Control and Severance Agreements
Employment Agreement and Offer Letter Agreements. We have offer letter agreements with all of our named executive officers other than Mr. Santel with whom we have entered into an employment agreement. These agreements were designed to be part of a competitive compensation package and keep our executive officers focused on our business goals and objectives. The agreements provide for base salaries, incentive compensation benefits and, in some cases, change of control and severance benefits and each component reflects the scope of each named executive officer’s anticipated responsibilities, the individual experience they bring to the company, the compensation committee and board members’ experiences and knowledge in compensating similarly situated individuals at other companies and reference to survey data.
Donald J. Santel.In April 2012, we entered into an employment agreement with Mr. Santel, which provides for an annual base salary of $430,000. Mr. Santel is also eligible for a discretionary bonus of up to 40% of his base salary, payable at the discretion of our board of directors or compensation committee. In the event that Mr. Santel’s employment is terminated without “cause” or if he terminates his employment for “good reason,” each as defined in the employment agreement, Mr. Santel will be entitled to receive the following severance benefits, subject to executing a general release of claims in favor of us:
| • | payments equal to 18 months of his base salary at the highest annualized rate in effect at any time on or before his termination date payable in substantially equal installments in accordance with our normal payroll policies, less applicable withholdings, with such installments to commence on the first payroll period following the 60th day after the date of his termination of employment; |
| • | a lump sum payment equal to 1.5 times Mr. Santel’s target bonus for the year in which the termination occurred, payable on the first payroll period following the 60th day after the date of his termination of employment; |
| • | eighteen months of acceleration of any unvested equity awards; and |
| • | if elected by Mr. Santel, payment or reimbursement of COBRA premiums through the earlier of 18 months from his termination date, the date Mr. Santel and his covered dependents, if any, become eligible for group health insurance through another employer, or the date Mr. Santel becomes ineligible for COBRA coverage. |
In addition, at Mr. Santel’s election, we will either pay a lump sum amount of $15,000 for outplacement assistance, tax planning, educational assistance, or similar transition support, or provide the same or similar services through a professional outplacement firm selected by us.
If there is a “qualifying termination,” as defined in the employment agreement, of Mr. Santel within 12 months of a change of control of the Company, Mr. Santel will be entitled to receive the following severance benefits, subject to executing a general release of claims in favor of us:
| • | payments equal to 24 months of his base salary at the highest annualized rate in effect at any time on or before his termination date payable in substantially equal installments in accordance with our normal payroll policies, less applicable withholdings, with such installments to commence on the first payroll period following the 60th day after the date of his termination of employment; |
| • | a lump sum payment equal to two times Mr. Santel’s target bonus for the year in which the termination occurred, payable on the first payroll period following the 60th day after the date of his termination of employment; |
| • | full acceleration of any unvested equity awards; and |
119
Table of Contents
| • | if elected by Mr. Santel, payment or reimbursement of COBRA premiums through the earlier of 18 months from his termination date, the date Mr. Santel and his covered dependents, if any, become eligible for group health insurance through another employer, or the date Mr. Santel becomes ineligible for COBRA coverage. |
In addition, at Mr. Santel’s election, we will either pay a lump sum amount of $15,000 for outplacement assistance, tax planning, educational assistance, or similar transition support, or provide the same or similar services through a professional outplacement firm selected by us. Mr. Santel’s employment agreement also provides that in the event any payment or distribution, by any person who acquires ownership or effective control or ownership of a substantial portion of our assets within the meaning of section 280G of the Internal Revenue Code of 1986, as amended, or the Code, would be subject to the excise tax imposed by section 4999 of the Code or any interest or penalties with respect to such excise tax, or the excise tax, Mr. Santel shall be entitled to receive an additional payment in an amount equal to the full tax gross up.
Jeffrey S. Farrow. On November 12, 2009, we entered into an offer letter agreement with Mr. Farrow for the position of vice president, finance. Mr. Farrow has subsequently been promoted to chief financial officer. Mr. Farrow’s offer letter agreement provides for an initial base salary of $200,000 and an option to purchase 150,000 shares of our common stock. Mr. Farrow is also eligible for a target bonus of 30% of his annual base salary based upon our performance. Mr. Farrow is eligible to participate in our employee benefit plans to the extent he is eligible for those plans, on the same terms as other similarly-situated executive officers.
Bruce F. Scharschmidt. On March 14, 2008, we entered into an offer letter agreement with Dr. Scharschmidt for the position of senior vice president and chief medical officer. Dr. Scharschmidt’s offer letter agreement provides for an initial base salary of $300,000 and an option to purchase 210,000 shares of our common stock. Dr. Scharschmidt is also eligible for a target bonus of 30% of his annual base salary based upon our performance. Dr. Scharschmidt is eligible to participate in our employee benefit plans to the extent he is eligible for those plans, on the same terms as other similarly-situated executive officers.
Klara A. Dickinson. On September 7, 2007, we entered into an offer letter agreement with Ms. Dickinson for the position of senior vice president, regulatory and compliance. Ms. Dickinson’s offer letter agreement provides for an initial base salary of $275,000, a one-time sign-on bonus of $25,000 and an option to purchase 185,000 shares of our common stock. Ms. Dickinson is also eligible for a target bonus of 30% of her annual base salary based upon our performance. Ms. Dickinson is eligible to participate in our employee benefit plans to the extent she is eligible for those plans, on the same terms as other similarly-situated executive officers.
Christine A. Nash. On September 7, 2007, we entered into an offer letter agreement with Ms. Nash for the position of senior director, marketing. Ms. Nash has subsequently been promoted to senior vice president and chief commercial officer. Ms. Nash’s offer letter agreement provides for an initial base salary of $175,000, a one-time sign-on bonus of $15,000 and an option to purchase 100,000 shares of our common stock. Ms. Nash is also eligible for a target bonus of 30% of her annual base salary based upon our performance. Ms. Nash is eligible to participate in our employee benefit plans to the extent she is eligible for those plans, on the same terms as other similarly-situated executive officers.
Change of Control and Severance Agreements. We have entered into executive change of control and severance agreements, or the severance agreements, with each of our named executive officers other than Mr. Santel. These severance agreements provide that in the event the executive’s employment is terminated without “cause” or if he or she terminates his or her employment for “good reason,” as each is defined in the severance agreements, at any time, the executive will be entitled to receive the following severance benefits, subject to executing a general release of claims in favor of us:
| • | payments equal to 12 months of the executive’s base salary as of the date of the executive’s termination payable in substantially equal installments in accordance with our normal payroll policies, less applicable withholdings, with such installments to commence on the first payroll period following the 60th day after the date of the executive’s termination of employment; |
120
Table of Contents
| • | a lump sum payment equal to the executive’s target bonus for the year in which the termination occurred, payable on the first payroll period following the 60th day after the date of the executive’s termination of employment; |
| • | twelve months of acceleration of any unvested equity awards; and |
| • | if elected by the executive, payment or reimbursement of COBRA premiums through the earlier of 18 months from the executive’s termination date or the date the executive and his or her covered dependents, if any, become eligible for group health insurance through another employer. |
In addition, at the executive’s election, we will either pay a lump sum amount of $15,000 for outplacement assistance, tax planning, educational assistance, or similar transition support, or provide the same or similar services through a professional outplacement firm selected by us.
If within 12 months following a change of control of the company, the executive’s employment is terminated without cause or he or she terminates his or her employment for good reason the executive will be entitled to receive the following severance benefits, subject to executing a general release of claims in favor of us:
| • | payments equal to 12 months of the executive’s base salary as of the date of the executive’s termination payable in substantially equal installments in accordance with our normal payroll policies, less applicable withholdings, with such installments to commence on the first payroll period following the 60th day after the date of the executive’s termination of employment; |
| • | a lump sum payment equal to the executive’s target bonus for the year in which the termination occurred, payable on the first payroll period following the 60th day after the date of the executive’s termination of employment; |
| • | full acceleration of any unvested equity awards; and |
| • | if elected by the executive, payment or reimbursement of COBRA premiums through the earlier of 18 months from the executive’s termination date or the date the executive and his or her covered dependents, if any, become eligible for group health insurance through another employer. |
In addition, at the executive’s election we will either pay a lump sum amount of $15,000 for outplacement assistance, tax planning, educational assistance, or similar transition support, or provide the same or similar services through a professional outplacement firm selected by us. The severance agreements also provide that in the event that the severance and other benefits provided for or otherwise payable to the executive constitute “parachute payments” within the meaning of Section 280G of the Code, then the executive’s severance benefits under the severance agreement will be reduced so that none of the payments constitute excess parachute payments for purposes of Section 280G of the Code.
121
Table of Contents
Potential Payments Upon Termination or Change of Control
As discussed under the caption “Employment, Change of Control and Severance Agreements” above, in April 2012 we entered into employment, change of control and severance agreements with Messrs. Santel and Farrow, Dr. Scharschmidt, Ms. Dickinson and Ms. Nash. Assuming these agreements were in effect on December 31, 2011, we have summarized and quantified the estimated payments under these agreements, assuming a termination event occurred on December 31, 2011, below.
Name | Benefit | Upon Termination Without Cause or For Good Reason – No Change of Control ($) | Upon Termination Without Cause – Change of Control ($) | |||||||
Donald J. Santel | Salary Continuation(1) | 618,000 | 824,000 | |||||||
| Lump Sum Payment(2) | 247,200 | 329,600 | ||||||||
| Option Acceleration(3) | ||||||||||
| Continued Healthcare(4) | 8,043 | 8,043 | ||||||||
| Transition Support(5) | 15,000 | 15,000 | ||||||||
Jeffrey S. Farrow | Salary Continuation(1) | 272,750 | 272,750 | |||||||
| Lump Sum Payment(2) | 81,825 | 81,825 | ||||||||
| Option Acceleration(3) | ||||||||||
| Continued Healthcare(4) | 8,043 | 8,043 | ||||||||
| Transition Support(5) | 15,000 | 15,000 | ||||||||
Bruce F. Scharschmidt, M.D. | Salary Continuation(1) | 336,518 | 336,518 | |||||||
| Lump Sum Payment(2) | 100,955 | 100,955 | ||||||||
| Option Acceleration(3) | ||||||||||
| Continued Healthcare(4) | 18,399 | 18,399 | ||||||||
| Transition Support(5) | 15,000 | 15,000 | ||||||||
Klara A. Dickinson | Salary Continuation(1) | 309,300 | 309,300 | |||||||
| Lump Sum Payment(2) | 92,790 | 92,790 | ||||||||
| Option Acceleration(3) | ||||||||||
| Continued Healthcare(4) | 14,801 | 14,801 | ||||||||
| Transition Support(5) | 15,000 | 15,000 | ||||||||
Christine A. Nash | Salary Continuation(1) | 252,000 | 252,000 | |||||||
| Lump Sum Payment(2) | 75,600 | 75,600 | ||||||||
| Option Acceleration(3) | ||||||||||
| Continued Healthcare(4) | — | — | ||||||||
| Transition Support(5) | 15,000 | 15,000 | ||||||||
| (1) | Amounts represent 12 months of base salary in effect as of December 31, 2011 for each named executive, except for Mr. Santel. For Mr. Santel, amounts represent 18 months of base salary in effect as of December 31, 2011 payable upon termination without cause or for good reason and 24 months of base salary payable upon a qualifying termination within 12 months of a change of control. |
| (2) | Amounts represent the eligible bonus for the year ended December 31, 2011 for each named executive officer, except for Mr. Santel. For Mr. Santel, amounts represent 1.5 times his eligible bonus payable upon termination without cause or for good reason and two times his eligible bonus payable upon a qualifying termination within 12 months of a change of control. |
| (3) | For each named executive officer except Mr. Santel, amounts represent the value of 12 months of acceleration of any unvested option upon termination without cause or for good reason, and the value of full acceleration of any option upon termination without cause or for good reason within 12 months in connection with a change of control. For Mr. Santel, amounts represent the value of 18 months of acceleration of any unvested option upon termination without cause or for good reason, and the value of full acceleration of any option upon a qualifying termination within 12 months of a change of control. The value of the option acceleration is calculated based on an assumed initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, with respect to unvested option shares subject to acceleration minus the exercise price of these unvested option shares. |
122
Table of Contents
| (4) | Amounts represent 18 months of continued health benefits for each named executive officer. |
| (5) | Amounts represent the right to receive, at the election of each named executive officer, either a lump sum amount of $15,000 for transition support, or the services of a professional outplacement firm selected by us. |
Other Benefits
Our named executive officers are eligible to participate in all of our employee benefit plans, such as medical, dental, vision, group life, short and long-term disability, and our 401(k) plan, in each case on the same basis as other employees, subject to applicable laws. We also provide vacation and other paid holidays to all employees, including our named executive officers.
We believe these benefits are important to attracting and retaining experienced executives. Like many private companies, we do not currently provide perquisites to our executive officers, given our attention to the cost-benefit tradeoff of such benefits, and the board of directors’ knowledge of the benefit offerings at other private companies.
Tax and Accounting Considerations
Section 162(m) of the Code generally disallows a tax deduction for compensation in excess of $1.0 million paid to our chief executive officer and our three other most highly paid executive officers other than our chief financial officer. Qualifying performance-based compensation is not subject to the deduction limitation if specified requirements are met. We generally intend to structure the performance-based portion of our executive compensation, when feasible, to comply with exemptions in Section 162(m) so that the compensation remains tax deductible to us. However, our board of directors may, in its judgment, authorize compensation payments that do not comply with the exemptions in Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent.
The compensation committee also takes into account whether components of our compensation program may be subject to the penalty tax associated with Section 409A of the Code, and aims to structure the elements of compensation to be compliant with or exempt from Section 409A to avoid such potential adverse tax consequences.
In addition, we account for equity compensation paid to our employees in accordance with FASB ASC Topic 718, or ASC 718, which requires us to estimate and record an expense over the service period of the award. Our cash compensation is recorded as an expense at the time the obligation is accrued. The accounting impact of our compensation programs is one of many factors that are considered in determining the size and structure of our programs.
Equity Benefit Plans
2012 Omnibus Incentive Plan
Prior to the completion of this offering, our board of directors will adopt, and our stockholders are expected to approve, our 2012 Plan, for the purpose of attracting and retaining non-employee directors, executive officers and other key employees and service providers, including officers, employees and service providers of our subsidiaries and affiliates, and to stimulate their efforts toward our continued success, long-term growth and profitability. The 2012 Plan provides for the grant of stock options, stock appreciation rights, restricted stock, unrestricted stock, stock units, dividend equivalent rights, other equity-based awards and cash bonus awards. We have reserved shares of common stock for issuance pursuant to the 2012 Plan, subject to certain adjustments set forth in the plan. In addition, all shares of common stock remaining available for issuance under our 2006 Plan as of the completion of this offering will become available for issuance under the 2012 Plan. As of
123
Table of Contents
March 31, 2012, this number was 631,904. In addition, effective January 1, 2013, the number of shares of common stock available for issuance under the 2012 Plan shall automatically increase annually by 4% of the total number of issued and outstanding shares of our common stock as of December 31 of the immediately preceding year. This summary is qualified in its entirety by the detailed provisions of the 2012 Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Section 162(m) of the Code limits publicly held companies to an annual deduction for U.S. federal income tax purposes of $1,000,000 for compensation paid to each of their chief executive officer and their three highest compensated executive officers (other than the chief executive officer or the chief financial officer) determined at the end of each year, referred to as covered employees. However, performance-based compensation is excluded from this limitation. The 2012 Plan is designed to permit the compensation committee to grant awards that qualify as performance-based for purposes of satisfying the conditions of Section 162(m), but it is not required under the 2012 Plan that awards qualify for this exception.
Administration of the 2012 Plan. The 2012 Plan will be administered by our compensation committee, and our compensation committee will determine all terms of awards under the plan. Each member of our compensation committee that administers the plan will be both a “non-employee director” within the meaning of Rule 16b-3 of the Exchange Act, and an “outside director” within the meaning of Section 162(m) of the Code. Our compensation committee will also determine who will receive awards under the plan, the type of award and its terms and conditions and the number of shares of our common stock subject to the award, if the award is equity-based. Our compensation committee will also interpret the provisions of the plan. During any period of time in which we do not have a compensation committee, the plan will be administered by our board of directors or another committee appointed by our board of directors. References below to the compensation committee include a reference to the board of directors or another committee appointed by the board of directors for those periods in which the board of directors or such other committee appointed by the board of directors is acting.
Eligibility. All of our employees and the employees of our subsidiaries and affiliates are eligible to receive awards under the 2012 Plan. In addition, our non-employee directors and consultants and advisors who perform services for us and our subsidiaries and affiliates may receive awards under the 2012 Plan, other than incentive stock options.
Share Authorization. As stated above, we have reserved shares of common stock for issuance under the 2012 Plan, in addition to all shares of common stock that remain available for issuance under the 2006 Plan as of the completion of this offering. In connection with stock splits, dividends, recapitalizations and certain other events, our board will make proportionate adjustments that it deems appropriate in the aggregate number of shares of common stock that may be issued under the 2012 Plan and the terms of outstanding awards. If any shares of stock covered by an award granted under the 2012 Plan are not purchased or are forfeited or expire, or if an award otherwise terminates without delivery of any shares of stock subject thereto, or is settled in cash in lieu of shares of stock, then the number of shares of stock counted against the aggregate number of shares of stock available under the 2012 Plan with respect to such award shall again be available for making awards under the plan. In addition, the number of shares of common stock available for issuance under the 2012 Plan will be increased by any shares of common stock used to pay the exercise price, to satisfy tax withholding obligations, or purchased by us with proceeds from option exercises.
During any time that the transition period under Section 162(m) of the Code has expired or does not apply, the maximum number of shares of common stock subject to options or stock appreciation rights that can be issued under the 2012 Plan to any person is in any single calendar year. The maximum number of shares of common stock that can be issued under the 2012 Plan to any person other than pursuant to an option or stock appreciation right is in any single calendar year. The maximum amount that may be earned as an annual incentive award or other cash award in any calendar year by any one person is and the maximum amount that may be earned as a performance award or other cash award in respect of a performance period by any one person is .
124
Table of Contents
Options. The 2012 Plan authorizes our compensation committee to grant incentive stock options (under Section 421 of the Code) and options that do not qualify as incentive stock options, or nonstatutory stock options. Any or all of the shares of stock available for issuance under the 2012 Plan at the time of this offering shall be available for issuance pursuant to incentive stock options. The exercise price of each option will be determined by the compensation committee, provided that the price will be equal to at least the fair market value of the shares of common stock on the date on which the option is granted. If we were to grant incentive stock options to any 10% stockholder, the exercise price may not be less than 110% of the fair market value of our shares of common stock on the date of grant.
The term of an option cannot exceed 10 years from the date of grant. If we were to grant incentive stock options to any 10% stockholder, the term cannot exceed five years from the date of grant. The compensation committee determines at what time or times each option may be exercised and the period of time, if any, after retirement, death, disability or termination of employment during which options may be exercised. Options may be made exercisable in installments. The exercisability of options may be accelerated by the compensation committee. The exercise price of an option may not be amended or modified after the grant of the option, and an option may not be surrendered in consideration of or exchanged for a grant of a new option having an exercise price below that of the option which was surrendered or exchanged without stockholder approval.
The aggregate fair market value, determined at the time of grant, of our common stock with respect to incentive stock options that are exercisable for the first time by an optionee during any calendar year under all of our stock plans may not exceed $100,000. Options or portions thereof that exceed such limit will generally be treated as nonstatutory stock options.
Stock Awards. The 2012 Plan also provides for the grant of stock awards (which includes restricted stock and stock units). A stock award is an award of shares of common stock that may be subject to restrictions on transferability and other restrictions as our compensation committee determines in its sole discretion on the date of grant. The restrictions, if any, may lapse over a specified period of time or through the satisfaction of conditions, in installments or otherwise, as our compensation committee may determine. A participant who receives a restricted stock award will have all of the rights of a stockholder as to those shares, including, without limitation, the right to vote and the right to receive dividends or distributions on the shares, except that the board of directors may require any dividends to be reinvested in shares. During the period, if any, when stock awards are non-transferable or forfeitable, a participant is prohibited from selling, transferring, assigning, pledging or otherwise encumbering or disposing of his or her award shares.
Stock Appreciation Rights. The 2012 Plan authorizes our compensation committee to grant stock appreciation rights that provide the recipient with the right to receive, upon exercise of the stock appreciation right, cash, shares of common stock or a combination of the two. The amount that the recipient will receive upon exercise of the stock appreciation right generally will equal the excess of the fair market value of our common stock on the date of exercise over the shares’ fair market value on the date of grant. Stock appreciation rights will become exercisable in accordance with terms determined by our compensation committee. Stock appreciation rights may be granted in tandem with an option grant or independently from an option grant. The term of a stock appreciation right cannot exceed 10 years from the date of grant.
Stock Units. The 2012 Plan also authorizes our compensation committee to grant stock units. Stock units represent the participant’s right to receive a compensation amount, based on the value of the shares of common stock, if vesting criteria established by the compensation committee are met. If the vesting criteria are met, stock units will be paid in cash, shares of common stock or a combination thereof.
Bonuses. Cash performance bonuses payable under the 2012 Plan may be based on the attainment of performance goals that are established by the compensation committee and relate to one or more performance criteria described in the plan. Cash performance bonuses, for which there is no minimum payout, must be based
125
Table of Contents
upon objectively determinable bonus formulas established in accordance with the plan, as determined by the compensation committee.
Dividend Equivalents. Our compensation committee may grant dividend equivalents in connection with the grant of any equity-based award. Dividend equivalents may be paid currently or may be deemed to be reinvested in additional shares of stock, which may thereafter accrue additional equivalents, and may be payable in cash, shares of common stock or a combination of the two. Our compensation committee will determine the terms of any dividend equivalents.
Performance awards. The 2012 Plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid to a covered executive officer imposed by Section 162(m) of the Code. To help assure that the compensation attributable to performance-based awards will so qualify, our compensation committee can structure such awards so that stock or cash will be issued or paid pursuant to such award only after the achievement of certain pre-established performance goals during a designated performance period.
The performance goals that may be selected are one or more of the following: (1) net earnings or net income; (2) operating earnings; (3) pretax earnings; (4) earnings per share of stock; (5) stock price, including growth measures and total stockholder return; (6) earnings before interest and taxes; (7) earnings before interest, taxes, depreciation and/or amortization; (8) sales or revenue growth, whether in general, by type of product or service, or by type of customer; (9) gross or operating margins; (10) return measures, including return on assets, capital, investment, equity, sales or revenue; (11) cash flow, including operating cash flow, free cash flow, cash flow return on equity and cash flow return on investment; (12) productivity ratios; (13) expense targets; (14) market share; (15) financial ratios as provided in credit agreements of our company and its subsidiaries; (16) working capital targets; (17) completion of acquisitions of business or companies; (18) completion of divestitures and asset sales; (19) revenues under management; (20) funds from operations; (21) successful implementation of clinical trials, including components thereof and (22) any combination of any of the foregoing business criteria.
The performance goals may be based on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Awards that are intended to qualify as performance-based compensation may not be adjusted upward. The plan administrator shall retain the discretion to adjust performance-based awards downward, either on a formula or discretionary basis, or any combination as the compensation committee determines. The performance goals may differ from participant to participant and from award to award.
Other Equity-Based Awards. Our compensation committee may grant other types of equity-based awards under the 2012 Plan. Other equity-based awards are payable in cash, shares of common stock or other equity, or a combination thereof, and may be restricted or unrestricted, as determined by our compensation committee. The terms and conditions that apply to other equity-based awards are determined by the compensation committee.
Change in Control. If we experience a change in control in which equity-based awards that are not exercised prior to the change in control will not be assumed or continued by the surviving entity, unless otherwise provided in an award agreement: (i) all restricted shares will vest, and all stock units will vest and the underlying shares will be delivered immediately before the change in control, and (ii) at the board of directors’ discretion either all options and stock appreciation rights will become exercisable 15 days before the change in control and terminate upon the consummation of the change in control, or all options, stock appreciation rights, restricted shares and stock units will be cashed out before the change in control. In the case of performance shares and performance units, however, if more than half of the performance period has lapsed, the performance shares will be converted based on actual performance to date. If less than half of the performance period has
126
Table of Contents
lapsed, or if actual performance is not determinable, the performance shares and performance units will be converted assuming target performance has been achieved.
Amendment; Termination. Our board of directors may amend or terminate the 2012 Plan at any time; provided that no amendment may adversely impair the benefits of participants with outstanding awards. Our stockholders must approve any amendment if such approval is required under applicable law or Nasdaq regulations. Unless terminated sooner by our board of directors or extended with stockholder approval, the 2012 Plan will terminate on the tenth anniversary of the adoption of the plan.
2006 Equity Incentive Plan
General. In December 2006, our board of directors and our stockholders adopted our 2006 Plan, which was subsequently amended on June 25, 2009, June 30, 2010 and April 15, 2011. The 2006 Plan is administered by our board of directors. Our board of directors has determined not to grant any additional awards under the 2006 Plan after the completion of this offering. However, the 2006 Plan will continue to govern the terms and conditions of the outstanding awards granted under the 2006 Plan which, as of the date of this prospectus, constitute all of our outstanding stock options.
Share Reserve. As of March 31, 2012, a total of 8,823,187 shares of our common stock had been authorized for issuance under the 2006 Plan. As of March 31, 2012, options to purchase a total of 7,718,537 shares of our common stock were issued and outstanding, a total of 472,746 shares of our common stock had been issued upon the exercise of options or pursuant to other awards granted under the 2006 Plan and 631,904 shares remained available for future grant. Such remaining share balance will become available for issuance under the 2012 Plan upon completion of this offering.
Types of Awards. Our 2006 Plan provides for the grant of nonstatutory stock options, restricted stock, restricted stock units, and stock appreciation rights to our employees, directors and consultants. Our 2006 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Code, to employees of such company or a “parent corporation” or “subsidiary corporation” thereof (as such terms are defined in Sections 424(e) and (f) of the Code). Our board of directors administers the 2006 Plan. The administrator has the authority to determine the terms and conditions of the awards granted under the 2006 Plan.
Our 2006 Plan does not allow for the transfer of awards other than by will or the laws of descent and distribution and only the recipient of an award may exercise such award during his or her lifetime, unless the board of directors provides for additional transfer terms as permitted by Section 260.140.41(d) of Title 10 of the California Code of Regulations at the time of grant.
Corporate Transaction. Our 2006 Plan provides that in the event of our merger with or into another corporation, or a sale of all or substantially all of our assets, the successor corporation or its parent or subsidiary will assume or substitute for each outstanding award. If the outstanding awards are not assumed or substituted, the vesting of such awards will accelerate in full prior to the consummation of the transaction.
401(k) Retirement Plan
We maintain a defined contribution employee retirement plan for our employees. Our 401(k) plan is intended to qualify as a tax-qualified plan under Section 401 of the Code so that contributions to our 401(k) plan and income earned on such contributions are not taxable to participants until withdrawn or distributed from the 401(k) plan. Our 401(k) plan provides that each participant may contribute up to 100% of his or her pre-tax compensation, up to a statutory limit of $16,500 for 2011 and $17,000 for 2012. Participants who are at least 50 years old can also make “catch-up” contributions, which in 2011 and 2012 may be up to an additional $5,500 above the statutory limit. Under our 401(k) plan, each employee is fully vested in his or her deferred salary
127
Table of Contents
contributions. Employee contributions are held and invested by the plan’s trustee. Our 401(k) plan also permits us to make discretionary contributions and matching contributions, subject to established limits and a vesting schedule. To date, we have not made any discretionary or matching contributions to the plan on behalf of participating employees.
Non-Employee Director Compensation
Cash and Equity Compensation
In April 2012, our board of directors approved a non-employee director compensation policy, which will be effective for all non-employee directors upon the effective date of the registration statement for this offering. Each non-employee director will receive an annual base retainer of $30,000. In addition, our non-employee directors will receive the following cash compensation for board services, as applicable:
| • | the chairman of the board of directors and the lead director will each receive an additional annual retainer of $30,000 and $15,000, respectively; |
| • | each member of our audit, compensation and nominating and corporate governance committees, other than the chairperson, will receive an additional annual retainer of $7,500, $7,500 and $4,000, respectively; and |
| • | each chairperson of our audit, compensation and nominating and corporate governance committees will receive an additional annual retainer of $15,000, $10,000 and $7,750, respectively. |
All amounts will be paid in quarterly installments. We will also reimburse each of our directors for their travel expenses incurred in connection with their attendance at board of directors and committee meetings.
In addition, newly appointed non-employee directors will receive a one-time initial award of options to purchase 20,000 shares of our common stock which will vest monthly over a four-year period subject to the director’s continued service on the board of directors. Thereafter, each non-employee director will receive an annual award of options to purchase 12,000 shares of our common stock, which will vest on the one-year anniversary of the date of grant, subject to the director’s continued service on the board of directors.
Director Compensation Table
The following table sets forth information concerning compensation accrued or paid to our independent, non-employee directors during the year ended December 31, 2011 for their service on our board of directors. Directors who are also our employees receive no additional compensation for their services as directors and are not set forth in the table below.
Name | Fees Earned or Paid in Cash ($) | Option Awards(3)(4) ($) | Total ($) | |||||||||
Gaurav Aggarwal, M.D.(1) | — | 28,288 | 28,288 | |||||||||
David W. Gryska | 35,000 | 27,963 | 62,963 | |||||||||
Bo Jesper Hansen, M.D., Ph.D.(2) | 30,362 | 28,288 | 58,650 | |||||||||
James I. Healy, M.D., Ph.D.(1) | — | 28,288 | 28,288 | |||||||||
Robert Hopfner, Ph.D.(1) | — | 28,288 | 28,288 | |||||||||
Jake R. Nunn(1) | — | 28,288 | 28,288 | |||||||||
Bijan Salehizadeh, M.D.(1) | — | 28,288 | 28,288 | |||||||||
Lota S. Zoth | 35,000 | — | 35,000 | |||||||||
128
Table of Contents
| (1) | These directors are affiliated with our investors and as such receive no additional cash compensation for their services as directors during 2011. |
| (2) | Dr. Hansen joined our board of directors on April 15, 2011. |
| (3) | On April 15, 2011, the board of directors granted options to purchase 72,200 shares of our common stock to each director listed above with the exception of Ms. Zoth, who received a grant of options to purchase 72,200 shares of our common stock upon her appointment to the board of directors. These options vest 25% on the one year anniversary of the vesting commencement date and the remainder vest over the next three years in equal monthly installments. The following table provides the total number of options outstanding for each director as of December 31, 2011: |
Name | Options Outstanding (#) | |||
Gaurav Aggarwal, M.D. | 72,200 | |||
David W. Gryska | 72,200 | |||
Bo Jesper Hansen, M.D., Ph.D. | 72,200 | |||
James I. Healy, M.D., Ph.D. | 72,200 | |||
Robert Hopfner, Ph.D. | 72,200 | |||
Jake R. Nunn | 72,200 | |||
Bijan Salehizadeh, M.D. | 72,200 | |||
Lota S. Zoth | 72,541 | |||
| (4) | Amounts reflect the grant date fair value of option awards granted in 2011 in accordance with ASC 718. For information regarding assumptions underlying the valuation of equity awards, see Note 11 to our consolidated financial statements and the discussion under “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Critical Accounting Policies — Stock-Based Compensation” included elsewhere in this prospectus. These amounts do not correspond to the actual value that will be recognized by the named executive officers. |
Limitation of Liability and Indemnification Agreements
Our amended and restated certificate of incorporation and amended and restated bylaws, each to become effective upon the closing of this offering, will provide that we will limit the liability of our directors and officers, and may indemnify other of our employees and other agents, to the maximum extent permitted by the Delaware General Corporation Law, or DGCL. The DGCL provides that directors and officers of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| • | breach of their duty of loyalty to the corporation or its stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payment of dividends or redemption of shares; or |
| • | transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under federal securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
We have entered into separate indemnification agreements with our directors and officers in addition to the indemnification provided for in our amended and restated certificate of incorporation and amended and restated bylaws. These indemnification agreements provide, among other things, that we will indemnify our directors and
129
Table of Contents
officers for certain expenses, including damages, judgments, fines, penalties, settlements and costs and attorneys’ fees and disbursements, incurred by a director in any claim, action or proceeding arising in his or her capacity as a director or officer of our company or in connection with service at our request for another corporation or entity. The indemnification agreements also provide for procedures that will apply in the event that a director or officer makes a claim for indemnification.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
130
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
We describe below transactions and series of similar transactions, since January 1, 2009, to which we were a party or will be a party, in which:
| • | the amounts involved exceeded or will exceed $120,000; and |
| • | any of our directors, executive officers, holders of more than 5% of our capital stock or any member of their immediate family had or will have a direct or indirect material interest, other than compensation arrangements with directors and executive officers, which are described where required under “Executive Compensation — Employment, Change of Control and Severance Agreements” and “Executive Compensation — Non-Employee Director Compensation.” |
Bridge Financing
April 2011 Bridge Financing
On April 1, 2011, we entered into a bridge loan financing, or the April 2011 bridge financing, in which we issued (i) the April 2011 notes and the May 2011 notes to existing investors identified in the table below for an aggregate principal amount of $17.5 million and (ii) the April 2011 warrants and the May 2011 warrants to purchase shares of our common stock at an exercise price of $0.67 per share, subject to adjustments upon the occurrence of certain events, in an amount to be calculated based on 30% of the principal amount of the notes. The April 2011 notes and the May 2011 notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding April 2011 notes and May 2011 notes, or the requisite consent, under certain circumstances, which demand may not be made earlier than December 31, 2012, or (ii) an event of default as described each of the notes. The April 2011 notes and the May 2011 notes cannot be prepaid, except on demand by the holders of such notes.
Immediately prior to the closing of this offering, the April 2011 notes and the May 2011 notes will automatically convert into shares of common stock at a conversion price equal to the initial public offering price, based on the assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012.
The April 2011 warrants and the May 2011 warrants are not currently exercisable but in connection with this offering will become exercisable by their terms for an aggregate of 3,318,704 shares of common stock at an exercise price of approximately $0.67 immediately prior to the closing of this offering. The April 2011 warrants and the May 2011 warrants terminate if they are not exercised prior to the closing of this offering. Each April 2011 warrants and May 2011 warrants contain a customary net issuance feature, which allows the warrant holder to pay the exercise price of the warrant by forfeiting a portion of the exercised warrant shares with a value equal to the aggregate exercise price. The April 2011 warrants and the May 2011 warrants will automatically net exercise and terminate immediately prior to closing of the initial public offering.
The following table summarizes the participation in the April 2011 bridge financing by holders of more than 5% of our capital stock and their affiliated entities:
Name | Aggregate Loan Amount ($) | Aggregate Shares of Common Stock Issuable Upon Exercise of April 2011 warrants and May 2011 warrants(1) | ||||||
Funds affiliated with Bay City Capital | 3,318,989 | 630,187 | (2) | |||||
Funds affiliated with Panorama Capital | 2,212,659 | 420,125 | (3) | |||||
Funds affiliated with New Enterprise Associates | 4,018,596 | 763,024 | (4) | |||||
Funds affiliated with Highland Capital Partners | 3,470,447 | 658,944 | (5) | |||||
Funds affiliated with Sofinnova Ventures | 4,018,596 | 763,024 | (6) | |||||
131
Table of Contents
| (1) | Represents shares of common stock issuable upon exercise of the April 2011 warrants and May 2011 warrants immediately prior to the closing of this offering. Does not represent amounts issuable pursuant to the net exercise provisions or amounts issuable in connection with events other than this offering. |
| (2) | Includes a note held by Bay City Capital Fund V, L.P. with a principal amount of $3,256,924 and a note held by Bay City Capital Fund V Co-Investment Fund, L.P. with a principal amount of $62,065. Bay City Capital Management V LLC is the general partner of each of Bay City Capital Fund V, L.P. and Bay City Capital Fund V Co-Investment Fund, L.P. Dr. Robert Hopfner, a member of our board of directors, is a member of Bay City Capital Management V LLC. |
| (3) | Consists of a note held by Panorama Capital, L.P. with a principal amount of $2,212,659. Dr. Gaurav Aggarwal, a member of our board of directors, is an employee of Panorama Capital, LLC and a member of Panorama Capital Management, LLC. Panorama Capital Management, LLC is the general partner of Panorama Capital, L.P. |
| (4) | Consists of a note held by New Enterprise Associates 12, Limited Partnership with a principal amount of $4,018,596. Jake R. Nunn, a member of our board of directors, is a partner of New Enterprise Associates 12, Limited Partnership. |
| (5) | Includes a note held by Highland Capital Partners VII Limited Partnership with a principal amount of $2,133,628, a note held by Highland Capital Partners VII-B Limited Partnership with a principal amount of $517,020, a note held by Highland Capital Partners VII-C Limited Partnership with a principal amount of $752,944 and a note held by Highland Entrepreneurs’ Fund VII Limited Partnership with a principal amount of $66,856. Collectively, Highland Capital Partners VII Limited Partnership, Highland Capital Partners VII-B Limited Partnership, Highland Capital Partners VII-C Limited Partnership and Highland Entrepreneurs’ Fund VII Limited Partnership are referred to herein as the Highland Investing Entities. Highland Management Partners VII Limited Partnership is the general partner of the Highland Investing Entities. Highland Management Partners VII, LLC is the general partner of Highland Management Partners VII Limited Partnership. |
| (6) | Consists of a note held by Sofinnova Venture Partners VII, L.P. with a principal amount of $4,018,596. Sofinnova Management VII, L.L.C., is the general partner of Sofinnova Ventures VII, L.P. Dr. James I. Healy, a member of our board of directors, is a managing member of Sofinnova Management VII, L.L.C. |
October 2011 Bridge Financing
On October 26, 2011, we entered into a bridge loan financing, or the October 2011 bridge financing, in which we issued (i) the October 2011 notes, the November 2011 notes and the February 2012 notes to certain existing investors identified in the table below for an aggregate principal amount of $15.0 million and (ii) the October 2011 warrants, the November 2011 warrants and the February 2012 warrants to purchase shares of our Series C-2 convertible preferred stock at an exercise price of $1.58 per share, subject to adjustments upon the occurrence of certain events, in an amount to be calculated based on 30% of the principal amount of the October 2011 notes, the November 2011 notes and the February 2012 notes. The October 2011 notes, the November 2011 notes and the February 2012 notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding October 2011 notes, November 2011 notes and February 2012 notes, or the requisite consent, under certain circumstances, which demand may not be made earlier than December 31, 2012, or (ii) an event of default as described in each of the notes. The October 2011 notes, the November 2011 notes and the February 2012 notes cannot be prepaid, except on demand by the holders of such notes.
Immediately prior to the closing of this offering, the October 2011 notes, the November 2011 notes and the February 2012 notes will automatically convert into shares of common stock at a conversion price equal to the initial public offering price, based on the assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012.
132
Table of Contents
The October 2011 warrants, the November 2011 warrants and the February 2012 warrants are not currently exercisable but in connection with this offering will become exercisable by their terms for an aggregate of approximately 2,849,440 shares of Series C-2 convertible preferred stock at an exercise price of $1.58 immediately prior to the closing of this offering and the conversion of the preferred stock into common stock. The October 2011 warrants, the November 2011 warrants and the February 2012 warrants terminate if they are not exercised prior to the closing of this offering. Each of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants contain a customary net issuance feature, which allows the warrant holder to pay the exercise price of the warrant by forfeiting a portion of the exercised warrant shares with a value equal to the aggregate exercise price. The October 2011 warrants, the November 2011 warrants and the February 2012 warrants will automatically net exercise and terminate immediately prior to closing of the initial public offering.
The following table summarizes the participation in the October 2011 bridge financing by holders of more than 5% of our capital stock and their affiliated entities:
Name | Aggregate Loan Amount ($) | Aggregate Shares of Common Stock Issuable Upon Conversion of Series C-2 Convertible Preferred Stock Issuable Upon Exercise of October 2011 warrants, November 2011 warrants and February 2012 warrants(1) | ||||||
Funds affiliated with Bay City Capital | 2,849,692 | 541,080 | (2) | |||||
Funds affiliated with Panorama Capital | 1,899,794 | 360,720 | (3) | |||||
Funds affiliated with New Enterprise Associates | 3,450,376 | 655,134 | (4) | |||||
Funds affiliated with Highland Capital Partners | 2,979,734 | 565,768 | (5) | |||||
Funds affiliated with Sofinnova Ventures | 3,450,376 | 655,134 | (6) | |||||
| (1) | Represents shares of common stock issuable upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the October 2011 warrants, the November 2011 warrants and the February 2012 warrants immediately prior to the closing of this offering. Does not represent amounts issuable pursuant to the net exercise provisions or amounts issuable in connection with events other than this offering. |
| (2) | Includes two notes held by Bay City Capital Fund V, L.P. each with a principal amount of $1,398,201 and two notes held by Bay City Capital Fund V Co-Investment Fund, L.P. each with a principal amount of $26,645. Bay City Capital Management V LLC is the general partner of each of Bay City Capital Fund V, L.P. and Bay City Capital Fund V Co-Investment Fund, L.P. Dr. Robert Hopfner, a member of our board of directors, is a member of Bay City Capital Management V LLC. |
| (3) | Consists of two notes held by Panorama Capital, L.P. each with a principal amount of $949,897. Dr. Gaurav Aggarwal, a member of our board of directors, is an employee of Panorama Capital, LLC and a member of Panorama Capital Management, LLC. Panorama Capital Management, LLC is the general partner of Panorama Capital, L.P. |
| (4) | Consists of two notes held by New Enterprise Associates 12, Limited Partnership each with a principal amount of $1,725,188. Jake R. Nunn, a member of our board of directors, is a partner of New Enterprise Associates 12, L.P. |
| (5) | Includes two notes held by Highland Capital Partners VII Limited Partnership each with a principal amount of $915,969, two notes held by Highland Capital Partners VII-B Limited Partnership each with a principal amount of $221,957, two notes held by Highland Capital Partners VII-C Limited Partnership each with a principal amount of $323,240 and two notes held by Highland Entrepreneurs’ Fund VII Limited Partnership each with a principal amount of $28,701. Collectively, Highland Capital Partners VII Limited Partnership, Highland Capital Partners VII-B Limited Partnership, Highland Capital Partners VII-C Limited Partnership and Highland Entrepreneurs’ Fund VII Limited Partnership are referred to herein as the Highland Investing Entities. Highland Management Partners VII Limited Partnership is the general partner of the Highland Investing Entities. Highland Management Partners VII, LLC is the general partner of Highland Management Partners VII Limited Partnership. |
133
Table of Contents
| (6) | Consists of two notes held by Sofinnova Venture Partners VII, L.P. each with a principal amount of $1,725,188. Sofinnova Management VII, L.L.C., is the general partner of Sofinnova Ventures VII, L.P. Dr. James I. Healy, a member of our board of directors, is a managing member of Sofinnova Management VII, L.L.C. |
Ucyclyd Asset Purchase Agreement and Amended and Restated Collaboration Agreement
On June 29, 2009, we issued to Ucyclyd approximately 1.7 million shares of our common stock, which at the time of issuance represented 5% of our outstanding shares on a fully-diluted basis, in exchange for the restructuring of the royalty and milestone payments under a prior collaboration agreement. Pursuant to the common stock purchase agreement, Ucyclyd was entitled to additional shares of common stock in the event we sold additional shares of Series C-2 preferred stock. In connection with a second closing of the sale of Series C-2 preferred stock, in April 2010, we issued an additional 0.7 million shares to Ucyclyd. We have no further obligation to provide additional shares to Ucyclyd.
On March 22, 2012, we entered into a purchase agreement with Ucyclyd under which we purchased the worldwide rights to Ravicti and a restated collaboration agreement, under which Ucyclyd granted us an option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL at a fixed price at a future defined date, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended.
The restated collaboration agreement is described in more detail under “Business – Ucyclyd Asset Purchase Agreement and Amended and Restated Collaboration Agreement.”
Preferred Stock Financings
Issuance of Series C-1 and Series C-2 Convertible Preferred Stock
During June, July and August 2009 and April 2010, we issued and sold in a series of closings, an aggregate of 11,647,769 shares of our Series C-1 convertible preferred stock in exchange for convertible debt and accrued interest at a price per share of $1.33 and 28,397,980 shares of our Series C-2 convertible preferred stock in exchange for cash at a price per share of $1.58, for gross proceeds of $60.4 million. In connection with the Series C-1 and C-2 financing, the convertible notes that had been issued in seven installments pursuant to a convertible note purchase agreement that we entered into in August 2008 were converted into shares of Series C-1 convertible preferred stock. Convertible notes issued in the first six installments were converted at a conversion discount rate of approximately 1.2 for 1. Convertible notes issued in the seventh installment were converted at a 1 for 1 ratio.
134
Table of Contents
The table below sets forth the number of shares of Series C-1 and C-2 convertible preferred stock purchased by our directors, executive officers and 5% stockholders and their affiliates. Each share of preferred stock in the table below will convert into one share of our common stock upon completion of this offering.
Participants | Series C-1 Convertible Preferred Stock (#) | Aggregate Purchase Price of Series C-1 Convertible Preferred Stock ($) | Series C-2 Convertible Preferred Stock (#) | Aggregate Purchase Price of Series C-2 Convertible Preferred Stock ($) | ||||||||||||
5% Stockholders: | ||||||||||||||||
Funds affiliated with Bay City Capital(1) | — | — | 7,594,937 | 12,000,000 | ||||||||||||
Funds affiliated with Panorama Capital(2) | — | — | 5,063,292 | 8,000,000 | ||||||||||||
Funds affiliated with New Enterprise Associates(3) | 3,722,117 | 4,953,376 | 5,473,751 | 8,648,527 | ||||||||||||
Funds affiliated with Highland Capital Partners | 3,722,114 | 4,953,376 | 4,219,409 | 6,666,666 | ||||||||||||
Funds affiliated with Sofinnova Ventures(4) | 3,722,117 | 4,953,376 | 5,473,751 | 8,648,527 | ||||||||||||
Executive Officers: | ||||||||||||||||
Klara A. Dickinson | 1,697 | 2,235 | — | — | ||||||||||||
Christine A. Nash | 593 | 782 | — | — | ||||||||||||
| (1) | Dr. Robert Hopfner, one of our directors, is a member of Bay City Capital Management V LLC. |
| (2) | Dr. Gaurav Aggarwal, one of our directors, is an employee of Panorama Capital, LLC and a member of Panorama Capital Management, LLC. |
| (3) | Jake R. Nunn, one of our directors, is a partner of New Enterprise Associates, Inc. |
| (4) | Dr. James I. Healy, one of our directors, is a managing member of Sofinnova Management VII, L.L.C. |
Conversion of Series B Convertible Preferred Stock
In June 2009, in connection with our Series C-1 and C-2 convertible preferred stock financing, 11,471,597 shares of our Series B convertible preferred stock were converted into shares of our common stock. The Series B convertible preferred stock was issued in 2007 in exchange for convertible debt, accrued interest and cash for gross proceeds of $20.0 million.
The table below sets forth the number of shares of common stock received in the conversion of the Series B convertible preferred stock by our directors, executive officers and 5% stockholders and their affiliates.
Participant | Series B Convertible Preferred Stock Converted (#) | Shares of Common Stock Issued Upon Conversion of Series B Convertible Preferred Stock | ||||||
5% Stockholders: | ||||||||
Funds affiliated with New Enterprise Associates(1) | 3,675,627 | 20,477 | ||||||
Funds affiliated with Highland Capital Partners | 3,675,627 | 20,474 | ||||||
Funds affiliated with Sofinnova Ventures(2) | 3,675,627 | 20,477 | ||||||
Executive Officers: | ||||||||
Klara A. Dickinson | 5,715 | 31 | ||||||
Christine A. Nash | 2,000 | 11 | ||||||
| (1) | Jake R. Nunn, one of our directors, is a partner of New Enterprise Associates, Inc. |
| (2) | Dr. James I. Healy, one of our directors, is a managing member of Sofinnova Management VII, L.L.C. |
135
Table of Contents
Conversion of Series A Convertible Preferred Stock
In June 2009, in connection with our Series C-1 and C-2 convertible preferred stock financing, 2,000,000 shares of Series A convertible preferred stock were converted into shares of our common stock. The Series A convertible preferred stock was issued in December 2006 in exchange for cash for gross proceeds of $2.0 million.
The table below sets forth the number of shares of common stock received in the conversion of the Series A convertible preferred stock by our directors, executive officers and 5% stockholders and their affiliates.
Participant | Series A Convertible Preferred Stock Converted (#) | Shares of Common Stock Issued Upon Conversion of Series A Convertible Preferred Stock | ||||||
5% Stockholders: | ||||||||
Funds affiliated with New Enterprise Associates(1) | 625,000 | 3,481 | ||||||
Funds affiliated with Highland Capital Partners | 625,000 | 3,479 | ||||||
Funds affiliated with Sofinnova Ventures(2) | 625,000 | 3,481 | ||||||
| (1) | Jake R. Nunn, one of our directors, is a partner of New Enterprise Associates, Inc. |
| (2) | Dr. James I. Healy, one of our directors, is a managing member of Sofinnova Management VII, L.L.C. |
Investor Rights Agreement
We are party to a second amended and restated investor rights agreement, or the amended investor rights agreement, dated June 2009, with the holders of our preferred stock, certain holders of our common stock and certain holders of options to purchase our capital stock. The amended investor rights agreement provides that the holders of common stock issuable upon conversion of our convertible preferred stock have the right to demand that we file a registration statement or request that their shares of common stock be covered by a registration statement that we are otherwise filing. In addition to the registration rights, the amended investor rights agreement provides for certain information rights and rights of first refusal. The provisions of the amended investor rights agreement will terminate upon the date ten years following the closing of this offering, other than certain rights related to information and inspection rights and certain covenants of the company which will terminate upon the completion of this offering. The registration rights are described in more detail under “Description of Capital Stock — Registration Rights.”
Voting Agreement
We have entered into an amended and restated voting agreement with certain holders of our common stock and certain holders of our convertible preferred stock. Pursuant to the amended and restated voting agreement, holders of our preferred stock have agreed to vote such that: one director be a designee of Sofinnova Venture Partners VII, L.P., who is currently Dr. James I. Healy; one director be a designee of Panorama Capital, L.P., who is currently Dr. Gaurav Aggarwal; one director be a designee of Bay City Capital Fund V, LP, who is currently Dr. Robert Hopfner; one director be a designee of New Enterprise Associates 12, Limited Partnership, who is currently Jake R. Nunn; and one director be a designee of Highland Capital Partners, who is currently Dr. Bijan Salehizadeh. The provisions of the amended and restated voting agreement will terminate upon the completion of this offering.
Other Transactions
We have entered into various employment related agreements and compensatory arrangements with our directors and executive officers that, among other things, provide for compensatory and certain severance and change of control benefits. For a description of these agreements and arrangements, see the sections entitled “Executive Compensation — Employment, Change of Control and Severance Agreements” and “Executive Compensation — Non-Employee Director Compensation.”
136
Table of Contents
We have entered into indemnification agreements with each of our current directors and officers. See “Executive Compensation — Limitation of Liability and Indemnification Agreements.”
Policies and Procedures for Related Person Transactions
In April 2012, our board of directors adopted a written related person transaction policy that will be in effect upon the closing of this offering. Accordingly, following this offering, all future related person transactions will be reviewed and approved by our audit committee (or any other committee of the board consisting of independent directors) or our board of directors. This review will cover any material transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, and a related person had or will have a direct or indirect material interest, including, purchases of goods or services by or from the related party or entities in which the related person party has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. A “related person” is any person who is or was one of our executive officers, directors or director nominees or is a holder of more than 5% of our common stock, or their immediate family members or any entity owned or controlled by any of the foregoing persons.
All of the transactions described above were entered into prior to the adoption of this policy and were approved by our board of directors.
137
Table of Contents
The following table sets forth certain information known to us regarding beneficial ownership of our common stock as of March 31, 2012, and as adjusted to reflect the sale of the shares of common stock in this offering and the conversion of all outstanding shares of our convertible preferred stock by:
| • | our named executive officers; |
| • | our directors; |
| • | all of our executive officers and directors as a group; and |
| • | each person known by us to be the beneficial owner of more than 5% of any class of our voting securities. |
We have based our calculation of beneficial ownership prior to the offering on 42,976,200 shares of common stock outstanding on March 31, 2012, which assumes the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock. We have based our calculation of beneficial ownership after the offering on shares of our common stock outstanding immediately after the completion of this offering, which gives effect to the issuance of shares of common stock in this offering and the automatic conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock and assumes:
| • | the exercise, on a net issuance basis, of the bridge warrants into shares of our common stock immediately prior to the closing of this offering, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus; and |
| • | the automatic conversion of the principal and accrued interest outstanding under the bridge notes immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012. |
The actual numbers of shares issued upon exercise of the bridge warrants and upon the conversion of the bridge notes is based on the assumptions set forth above and will likely differ from the numbers appearing in this discussion and the following table and footnotes. See “Prospectus Summary — The Offering.” Ownership information assumes no exercise of the underwriters’ over-allotment option.
Information with respect to beneficial ownership has been furnished to us by each, director, executive officer or 5% or more stockholder, as the case may be. Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he or she possesses sole or shared voting or investment power of that security, and includes options and warrants that are currently exercisable within 60 days of March 31, 2012. Options to purchase shares of our common stock that are exercisable within 60 days of March 31, 2012, are deemed to be beneficially owned by the persons holding these options for the purpose of computing percentage ownership of that person, but are not treated as outstanding for the purpose of computing any other persons’ ownership percentage. Unless otherwise indicated, to our knowledge, each stockholder possesses sole voting and investment power over the shares listed, except for shares owned jointly with that person’s spouse.
138
Table of Contents
Unless otherwise indicated, the address for each of the stockholders in the table below is c/o Hyperion Therapeutics, Inc., 601 Gateway Boulevard, Suite 200, South San Francisco, CA 94080.
Name and Address of Beneficial Owner | Shares of Common Stock Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||||||
| Before Offering | After Offering | Before Offering | After Offering | |||||||||
Named Executive Officers and Directors: | ||||||||||||
Donald J. Santel(1) | 3,482,574 | 7.5 | % | |||||||||
Jeffrey S. Farrow(2) | 262,721 | 0.6 | % | |||||||||
Bruce F. Scharschmidt, M.D.(3) | 530,419 | 1.2 | % | |||||||||
Klara A. Dickinson(4) | 517,910 | 1.1 | % | |||||||||
Christine A. Nash(5) | 390,097 | 0.8 | % | |||||||||
James I. Healy, M.D., Ph.D.(6) | 9,292,027 | 21.6 | % | |||||||||
Gaurav Aggarwal, M.D.(7) | 72,200 | * | ||||||||||
David W. Gryska(8) | 72,200 | * | ||||||||||
Bo Jesper Hansen, M.D., Ph.D.(9) | 72,200 | * | ||||||||||
Robert Hopfner, Ph.D.(10) | 72,200 | * | ||||||||||
Jake R. Nunn(11) | 72,200 | * | ||||||||||
Bijan Salehizadeh, M.D.(12) | 72,200 | * | ||||||||||
Lota S. Zoth(13) | 72,541 | * | ||||||||||
All executive officers and directors as a group (13 persons) | 15,910,059 | 32.2 | % | |||||||||
5% Stockholders | ||||||||||||
Entities affiliated with Bay City Capital(14) | 7,594,937 | 17.6 | % | |||||||||
Entities affiliated with Highland Capital Partners(15) | 7,965,476 | 18.5 | % | |||||||||
Panorama Capital, L.P.(16) | 5,063,292 | 11.7 | % | |||||||||
New Enterprise Associates 12, Limited Partnership(17) | 9,219,827 | 21.4 | % | |||||||||
Sofinnova Venture Partners VII, L.P.(18) | 9,219,827 | 21.4 | % | |||||||||
Ucyclyd Pharma, Inc.(19) | 2,380,333 | 5.5 | % | |||||||||
| * | Represents beneficial ownership of less than one percent of our outstanding common stock. |
| (1) | Consists of (a) 3,420,670 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012 and (b) 61,904 shares of common stock held by the Donald J. Santel and Kelly L. McGinnis, Trust UA 12/19/08 FBO Margaret Cate Santel. |
| (2) | Consists solely of 262,721 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012. |
| (3) | Consists of (a) 230,000 shares of common stock held by Bruce Frederick Scharschmidt and Peggy Sue Crawford Family Trust dated October 9, 2001 and (b) 300,419 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012. |
| (4) | Consists of (a) 1,728 shares of common stock and (b) 516,182 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012. |
| (5) | Consists of (a) 604 shares of common stock and (b) 389,493 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012. |
| (6) | Consists of (a) 72,200 shares of common stock held by Dr. Healy and (b) 9,219,827 shares held by Sofinnova Venture Partners VII, L.P. Dr. Healy is a managing member of Sofinnova Management VII, L.L.C., the general partner of Sofinnova Venture Partners VII, L.P., and may be considered to have beneficial ownership of Sofinnova Venture Partners VII, L.P.’s interest in us. Dr. Healy disclaims beneficial ownership of all shares held by Sofinnova Venture Partners VII, L.P., except to the extent of his pecuniary interest therein. In addition, the number of shares beneficially owned by Dr. Healy after the offering include those set forth in footnote 18 below. |
| (7) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
139
Table of Contents
| (8) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
| (9) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
| (10) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
| (11) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
| (12) | Consists solely of 72,200 shares of common stock issuable upon exercise of stock options within 60 days of March 31, 2012. |
| (13) | Consists solely of 72,541 shares of common stock issuable upon the exercise of stock options within 60 days of March 31, 2012. |
| (14) | Consists of (a) 142,025 shares held by Bay City Capital Fund V Co-Investment Fund, L.P., or BCC Co-Investment, and (b) 7,452,912 shares held by Bay City Capital Fund V, L.P, or BCC V. In addition, the number of shares beneficially owned after the offering, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, includes (a) shares of common stock issuable upon conversion of an April 2011 note held by BCC Co-Investment, and shares of common stock issuable upon conversion of an April 2011 note held by BCC V, (b) shares of common stock issuable upon the net exercise of an April 2011 warrant held by BCC Co-Investment, and shares of common stock issuable upon net exercise of an April 2011 warrant held by BCC V, (c) shares of common stock issuable upon conversion of an October 2011 note held by BCC Co-Investment, and shares of common stock issuable upon conversion of an October 2011 note held by BCC V, (d) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant held by BCC Co-Investment, and shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon net exercise of an October 2011 warrant held by BCC V, (e) shares of common stock issuable upon conversion of a February 2012 note held by BCC Co-Investment, and shares of common stock issuable upon conversion of a February 2012 note held by BCC V, and (f) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant held by BCC Co-Investment, and shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon net exercise of a February 2012 warrant held by BCC V. The voting and dispositive decisions with respect to the shares held by BCC Co-Investment and BCC V are made by the following members of the investment committee of its general partner, Bay City Capital Management V LLC: Fred Craves, Carl Goldfischer, Lionel Carnot, Jeanne Cunicelli, William Gerber, Douglas Given and Dayton Misfeldt, each of whom disclaims beneficial ownership of such shares, except to the extent of his or her actual pecuniary interest therein. The address for the funds affiliated with Bay City Capital is 750 Battery St., Suite 400, San Francisco, CA 94111. |
| (15) | Consists of (a) 4,897,179 shares held by Highland Capital Partners VII Limited Partnership, or HCP VII, (b) 1,186,680 shares held by Highland Capital Partners VII-B Limited Partnership, or HCP VII-B, (c) 1,728,182 shares held by Highland Capital Partners VII-C Limited Partnership, or HCP VII_C, and (d) 153,435 shares held by Highland Entrepreneurs’ Fund VII Limited Partnership, or HEF VII. In addition, the number of shares beneficially owned after the offering, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, includes (a) shares of common stock issuable upon conversion of an April 2011 note held by HCP VII, shares of common stock issuable upon conversion of an April 2011 note held by HCP VII-B, shares of common stock issuable upon conversion of an April 2011 note held by HCP VII-C and shares of common stock issuable upon conversion of an April 2011 note held by HEF VII, (b) shares of common stock issuable upon the net exercise of an April 2011 warrant held by HCP VII, shares of common stock issuable upon the net exercise of an April 2011 warrant held by HCP VII-B, shares of common stock issuable upon the net exercise of an April 2011 warrant held by HCP VII-C, and shares of common stock issuable upon net exercise of an April 2011 warrant held |
140
Table of Contents
| by HEF VII, (c) shares of common stock issuable upon conversion of an October 2011 note held by HCP VII, shares of common stock issuable upon conversion of an October 2011 note held by HCP VII-B, shares of common stock issuable upon conversion of an October 2011 note held by HCP VII-C and shares of common stock issuable upon conversion of an October 2011 note held by HEF VII, (d) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant held by HCP VII, shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant held by HCP VII-B, shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant held by HCP VII-C, and shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon net exercise of an October 2011 warrant held by HEF VII, (e) shares of common stock issuable upon conversion of a February 2012 note held by HCP VII, shares of common stock issuable upon conversion of a February 2012 note held by HCP VII-B, shares of common stock issuable upon conversion of a February 2012 note held by HCP VII-C and shares of common stock issuable upon conversion of a February 2012 note held by HEF VII, and (f) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant held by HCP VII, shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant held by HCP VII-B, shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant held by HCP VII-C, and shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon net exercise of a February 2012 warrant held by HEF VII. Collectively, HCP VII, HCP VII-B, HCP VII-C and HEF VII are referred to herein as the Highland Investing Entities. |
Highland Management Partners VII Limited Partnership is the general partner of the Highland Investing Entities. Highland Management Partners VII, LLC is the general partner of Highland Management Partners VII Limited Partnership. The voting and dispositive decisions with respect to the shares held by the Highland Investing Entities are made by the following members, who are also managers, of Highland Management Partners VII, LLC, as the general partner of the general partner of each of the Highland Investing Entities: Robert F. Higgins, Paul A. Maeder, Daniel J. Nova, Sean M. Dalton, Robert J. Davis, Fergal J. Mullen and Corey M. Mulloy, each of whom disclaims beneficial ownership of such shares, except to the extent of each such member’s actual pecuniary interest therein. Each of Highland Management Partners VII Limited Partnership and Highland Management Partners VII, LLC disclaims beneficial ownership of the shares of the Highland Investing Entities, except to the extent of each such entity’s actual pecuniary interest therein. The address for the Highland Investing Entities and their related general partners and managing members is One Broadway, 16th Floor, Cambridge, MA 02142.
| (16) | Consists solely of 5,063,292 shares held by Panorama Capital, L.P. In addition, the number of shares beneficially owned after the offering, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, includes (a) shares of common stock issuable upon conversion of an April 2011 note, (b) shares of common stock issuable upon the net exercise of an April 2011 warrant, (c) shares of common stock issuable upon conversion of an October 2011 note, (d) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant, (e) shares of common stock issuable upon conversion of a February 2012 note, and (f) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant, all of which are held by Panorama Capital, L.P. The voting and dispositive decisions with respect to the shares held by Panorama Capital, L.P., are made by the following Managing Members of its general partner, Panorama Capital Management, LLC: Christopher J. Albinson, Rodney A. Ferguson, Shahan D. Soghikian, and Damion Wicker, each of whom disclaims beneficial ownership of such shares, except to the extent of his or her actual pecuniary interest therein. The address for the funds affiliated with Panorama Capital is 2440 Sand Hill Road, Suite 302, Menlo Park, CA 94025. |
| (17) | Consists solely of 9,219,827 shares held by New Enterprise Associates 12, Limited Partnership. In addition, the number of shares beneficially owned after the offering, assuming an initial public offering price of |
141
Table of Contents
| $ per share, the mid-point of the price range set forth on the cover page of this prospectus, includes (a) shares of common stock issuable upon conversion of an April 2011 note, (b) shares of common stock issuable upon the net exercise of an April 2011 warrant, (c) shares of common stock issuable upon conversion of an October 2011 note, (d) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant, (e) shares of common stock issuable upon conversion of a February 2012 note, and (f) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant, all of which are held by New Enterprise Associates 12, Limited Partnership. NEA Partners 12, Limited Partnership is the general partner of New Enterprise Associates 12, Limited Partnership. NEA 12 GP, LLC is the general partner of NEA Partners 12, Limited Partnership. The voting and dispositive decisions with respect to the shares held by New Enterprise Associates 12, Limited Partnership, are made by NEA Partners 12, Limited Partnership, NEA 12 GP, LLC and the following members of NEA 12 GP, LLC as the general partner of the general partner of New Enterprise Associates 12, Limited Partnership: M. James Barrett, Peter J. Barris, Forest Baskett, Ryan D. Drant, Patrick J. Kerins, Krishna Kolluri, C. Richard Kramlich, Charles W. Newhall III, Mark W. Perry and Scott D. Sandell, each of whom disclaims beneficial ownership of such shares, except to the extent of his or her actual pecuniary interest therein. Each of NEA 12 GP, LLC and NEA Partners 12, Limited Partnership disclaims beneficial ownership of such shares except to the extent of its actual pecuniary interest therein. The address for the funds affiliated with New Enterprise Associates is 1954 Greenspring Drive, Suite 600, Timonium, MD 21093. |
| (18) | Consists solely of 9,219,827 shares held by Sofinnova Venture Partners VII, L.P. In addition, the number of shares beneficially owned after the offering, assuming an initial public offering price of $ per share, the mid-point of the price range set forth on the cover page of this prospectus, includes (a) shares of common stock issuable upon conversion of an April 2011 note, (b) shares of common stock issuable upon the net exercise of an April 2011 warrant, (c) shares of common stock issuable upon conversion of an October 2011 note, (d) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of an October 2011 warrant, (e) shares of common stock issuable upon conversion of a February 2012 note, and (f) shares of common stock upon conversion of Series C-2 convertible preferred stock issuable upon the net exercise of a February 2012 warrant, all of which are held by Sofinnova Venture Partners VII, L.P. The voting and dispositive decisions with respect to the shares held by Sofinnova Venture Partners VII, L.P., are made by the following managing members of its general partner, Sofinnova Management VII, L.L.C.: Dr. James I. Healy, Dr. Michael F. Powell and Eric P. Buatois, each of whom disclaims beneficial ownership of such shares, except to the extent of his or her actual pecuniary interest therein. The address for the funds affiliated with Sofinnova Venture Partners VII, L.P., Sofinnova Management VII, L.L.C., and its managing members, is 2800 Sand Hill Road, Suite 150, Menlo Park, CA 94025. |
| (19) | The address for Ucyclyd Pharma, Inc. is 7720 North Dobson Road, Scottsdale, AZ 85256. |
142
Table of Contents
Upon the closing of this offering, our amended and restated certificate of incorporation will authorize us to issue up to 100,000,000 shares of common stock, $0.0001 par value, and 10,000,000 shares of preferred stock, $0.0001 par value. As of March 31, 2012, after giving effect to the adjustments described below, there were outstanding:
| • | shares of common stock outstanding held by approximately 29 stockholders; |
| • | 7,718,537 shares of common stock subject to outstanding options; and |
| • | 1,810 shares of common stock issuable upon the exercise of outstanding warrants that are expected to remain outstanding upon completion of this offering. |
The number of shares of our common stock outstanding as of March 31, 2012 as shown above assumes:
| • | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the April 2011 warrants and May 2011 warrants into shares of our common stock, at an exercise price of $0.67 per share, and which will expire upon completion of this offering if not exercised; |
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the October 2011 warrants, the November 2011 warrants, and the February 2012 warrants, into shares of our common stock upon conversion of the Series C-2 convertible preferred stock issuable upon exercise of such warrants, at an exercise price equal to $1.58 per share, and which will expire upon completion of this offering if not exercised; and |
| • | the automatic conversion of the principal and accrued interest outstanding under the April 2011 notes, the May 2011 notes, the October 2011 notes, the November 2011 notes and the February 2012 notes, into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012. |
The following description of our capital stock is not complete and is subject to and qualified in its entirety by our amended and restated certificate of incorporation and amended and restated bylaws and by the provisions of applicable Delaware law. Copies of these documents will be filed with the SEC as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of the common stock, preferred stock and warrants reflect changes to our capital structure that will occur immediately upon completion of this offering.
Common Stock
Voting Rights. Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders. The affirmative vote of holders of at least 66% of the voting power of all of the then outstanding shares of capital stock, voting as a single class, will be required to amend certain provisions of our certificate of incorporation, including provisions relating to amending our bylaws, the classified board, the size of the board, removal of directors, director liability, vacancies on the board, special meetings, stockholder notices, actions by written consent and exclusive jurisdiction.
Dividends. Subject to preferences that may be applicable to any outstanding preferred stock, holders of our common stock are entitled to receive ratably any dividends that may be declared by the board of directors out of funds legally available for that purpose.
143
Table of Contents
Liquidation. In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any outstanding preferred stock.
Rights and Preferences. Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
Fully Paid and Nonassessable. All outstanding shares of common stock are fully paid and non-assessable, and the shares of common stock to be issued upon the closing of this offering will be fully paid and non-assessable.
Preferred Stock
Upon the completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the number, rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, and sinking fund terms, and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control or other corporate action. Upon completion of this offering, no shares of preferred stock will be outstanding and we have no present plan to issue any shares of preferred stock.
Warrants
Comerica Bank Warrant
Pursuant to our loan and security agreement dated October 2, 2007 with Comerica Bank, we issued a warrant to purchase 1,671 shares of our common stock at an exercise price of $314.12 per share, or the Comerica Warrant. The Comerica Warrant may be exercised in whole or in part at the option of Comerica Bank at any time prior to expiration on October 2, 2017.
Keelin Reeds Partners Warrant
Pursuant to our letter agreement dated March 6, 2007 with Keelin Reeds Partners, in exchange for services, we issued a warrant to purchase 139 shares of our common stock at an exercise price of $53.85 per share, or the Keelin Reeds Warrant. The Keelin Reeds Warrant contains a net exercise feature and may be exercised in whole or in part at the option of Keelin Reeds Partners at any time prior to expiration on December 13, 2012. The Keelin Reeds Warrant also contains a market stand-off agreement restricting the sale, disposal of, transfer, short sale, grant of option for the purchase of, or entry into any hedging or similar transaction for a period of time to be specified by the managing underwriters (not to exceed 180 days) following the effective date of our registration statement in connection with our initial public offering.
Silicon Valley Bank and Leader Equity Warrants
Pursuant to our loan and security agreement dated April 19, 2012 with Silicon Valley Bank and Leader Lending, LLC, we issued warrants to purchase 231,343 shares of our common stock at an exercise price of $0.67 per share to each of Silicon Valley Bank and Leader Equity, LLC, or the SVB Warrants. The SVB Warrants
144
Table of Contents
contain a cashless exercise feature and may be exercised in whole or in part at the option of Silicon Valley Bank or Leader Equity, LLC, respectively, at any time prior to expiration on April 18, 2022.
Registration Rights
Holders of 46,290,604 shares of preferred stock, common stock, and common stock and preferred stock issuable upon exercise of warrants, have the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing, as described below.
Demand Registration Rights
At any time after 180 days after the closing of this offering, the holders of a majority of the shares having demand registration rights may request that we register all or a portion of their common stock for sale under the Securities Act. We will effect the registration as requested, unless in the good faith judgment of our board of directors, such registration would be seriously detrimental to the company and its stockholders and should be delayed. In addition, when we are eligible for the use of Form S-3, or any successor form, holders of a majority of the shares having demand registration rights may make unlimited requests that we register all or a portion of their common stock for sale under the Securities Act on Form S-3, or any successor form, so long as the aggregate price to the public in connection with any such offering is at least $1.0 million.
Incidental Registration Rights
In addition, if at any time after this offering we register any shares of our common stock, the holders of all shares having registration rights are entitled to notice of the registration and to include all or a portion of their common stock in the registration.
Other Provisions
In the event that any registration in which the holders of registrable shares participate pursuant to the registration rights agreement is an underwritten public offering, the number of registrable shares to be included may, in specified circumstances, be limited due to market conditions.
We will pay all registration expenses, other than underwriting discounts and selling commissions, and the reasonable fees and expenses of a single special counsel for the selling stockholders, related to any demand or piggyback registration. The registration rights agreement contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them. The demand, piggyback and Form S-3 registration rights described above will expire, with respect to any particular stockholder, five years after our initial public offering or when that stockholder can sell all of its shares under Rule 144 of the Securities Act.
Anti-Takeover Provisions
Certificate of Incorporation and Bylaw to be in Effect Upon the Completion of this Offering
Our amended and restated certificate of incorporation and amended and restated bylaws, each to become effective immediately prior to the completion of this offering, will include a number of provisions that may deter or impede hostile takeovers or changes of control or management. These provisions include:
| • | Issuance of undesignated preferred stock. After the filing of our amended and restated certificate of incorporation, our board of directors will have the authority, without further action by the stockholders, |
145
Table of Contents
to issue up to 10,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by the board of directors. The existence of authorized but unissued shares of preferred stock enables our board of directors to make it more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. |
| • | Classified board. Our amended and restated certificate of incorporation provides for a classified board of directors consisting of three classes of directors, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. This provision may have the effect of delaying a change in control of the board. |
| • | Board of directors vacancies. Our amended and restated certificate of incorporation and amended and restated bylaws authorize only our board of directors to fill vacant directorships. In addition, the number of directors constituting our board of directors may be set only by resolution adopted by a majority vote of our entire board of directors. These provisions prevent a stockholder from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with its own nominees. |
| • | Stockholder action; special meetings of stockholders. Our amended and restated certificate of incorporation provides that our stockholders may not take action by written consent, but may only take action at annual or special meetings of our stockholders. Stockholders will not be permitted to cumulate their votes for the election of directors. Our amended and restated certificate of incorporation further provides that special meetings of our stockholders may be called only by the chairman of our board of directors or by a majority of our board of directors. |
| • | Advance notice requirements for stockholder proposals and director nominations. Our amended and restated bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election as directors at our annual meeting of stockholders. Our bylaws also specify certain requirements as to the form and content of a stockholder’s notice. These provisions may make it more difficult for our stockholders to bring matters before our annual meeting of stockholders or to nominate directors at our annual meeting of stockholders. |
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of us. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, these provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they may also reduce fluctuations in the market price of our shares that could result from actual or rumored takeover attempts.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in a business combination with any interested stockholder for a period of three years following the date the person became an interested stockholder, with the following exceptions:
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested holder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the |
146
Table of Contents
time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (a) by persons who are directors and also officers and (b) pursuant to employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the entity’s or person’s affiliates and associates, beneficially owns, or is an affiliate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Choice of Forum
Our amended and restated certificate of incorporation will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty owed by any director, officer or employee to us or our stockholders, any action asserting a claim against us arising pursuant to the Delaware General Corporation Law or any action asserting a claim against us that is governed by the internal affairs doctrine. However, several lawsuits involving other companies have been brought challenging the validity of choice of forum provisions in certificates of incorporation, and it is possible that a court could rule that such provision is inapplicable or unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is .
147
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options and warrants, or the anticipation of these sales, could adversely affect prevailing market prices from time to time and could impair our ability to raise equity capital in the future. Furthermore, since only a limited number of shares will be available for sale shortly after this offering because of certain contractual and legal restrictions on resale described below, sales of substantial amounts of our common stock in the public market after the restrictions lapse could adversely affect the prevailing market price and our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of March 31, 2012, upon the closing of this offering, shares of common stock will be outstanding. The number of shares outstanding upon completion of this offering assumes:
| • | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 40,045,749 shares of common stock upon completion of this offering; |
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, of the April 2011 warrants and the May 2011 warrants into shares of our common stock, at an exercise price equal to $0.67 per share, and which will expire upon completion of this offering if not exercised; |
| • | the exercise, on a net issuance basis based on an assumed initial public offering price of $ per share, of the October 2011 warrants, the November 2011 notes, and the February 2011 notes outstanding as of March 31, 2012 which are exercisable for shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the warrants, at an exercise price equal to $1.58 per share, and which will expire upon completion of this offering if not exercised; |
| • | the automatic conversion of the bridge notes into shares of common stock immediately prior to the closing of this offering at a conversion price equal to the initial public offering price, based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and assuming the conversion occurs on , 2012; |
| • | no exercise of the underwriters’ over-allotment option. |
| • | no exercise of outstanding options or warrants, other than the bridge warrants. |
However, because the number of shares of common stock that will be issued upon exercise of the bridge warrants and upon the conversion of the bridge notes depends upon the actual initial public offering price per share in this offering and the closing date of this offering, the actual number of shares issuable upon such conversion will likely be different from the amount we have assumed for purposes of this discussion. See “Prospectus Summary — The Offering.”
All of the shares sold in this offering will be freely tradable unless purchased by our affiliates. The remaining shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements as described below. Following the expiration of the lock-up period, all shares will be eligible for resale in compliance with Rule 144 or Rule 701 to the extent these shares have been released from any repurchase option that we may hold.
148
Table of Contents
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, any person who is not our affiliate and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction. In addition, under Rule 144, any person who is not an affiliate of ours and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available.
Beginning 90 days after the date of this prospectus, a person who is our affiliate or who was our affiliate at any time during the preceding three months and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of shares within any three-month period that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; and |
| • | The average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities Pursuant to Rule 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Upon expiration of the 180-day lock-up period described above, approximately 42,976,200 shares of our common stock will be eligible for sale under Rule 144. We cannot estimate the number of shares of our common stock that our existing stockholders will elect to sell under Rule 144.
Rule 701
In general, under Rule 701, any of an issuer’s employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Act of 1933, as amended, or the Securities Act, is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
Lock-up Agreements
We, along with our directors and executive officers and all of our other security holders have agreed with the underwriters that, for a period of 180 days following the date of this prospectus, we or they will not offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our common stock (including any shares issued in this offering or other issuer-directed shares), or any options or warrants to purchase any shares of our common stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of our common stock, whether now owned or later acquired, owned directly or with respect to which we or they have beneficial ownership within the rules and regulations of the SEC, subject to specified exceptions. The underwriters may, in their sole discretion, at any time without prior notice, release all or any portion of the shares from the restrictions in any such agreement.
149
Table of Contents
The 180-day lock-up period described in the preceding paragraph will be extended if:
| • | during the last 17 days of the 180-day lock-up period, we release earnings results or announce material news or a material event; or |
| • | prior to the expiration of the 180-day lock-up period, we announce that we will release earnings results during the 15-day period following the last day of the 180-day period, |
in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the date of release of the earnings results or the announcement of the material news or material event, unless such extension is waived, in writing, by Leerink Swann LLC and Cowen and Company, LLC on behalf of the underwriters.
Registration Rights
Holders of 46,290,604 shares of our preferred stock, common stock, and common stock and preferred stock issuable upon exercise of warrants, have the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. See “Description of Capital Stock — Registration Rights.” Except for shares purchased by affiliates, registration of their shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon effectiveness of the registration statement, subject to the expiration of the lock-up period and to the extent these shares have been released from any repurchase option that we may hold.
Equity Incentive Plans
We intend to file one or more registration statements on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issuable under our 2006 Plan and 2012 Plan. We expect to file the registration statement covering shares offered pursuant to our stock plans shortly after the date of this prospectus, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144. Our equity incentive plans are described in more detail under “Executive Compensation — Equity Benefit Plans.”
150
Table of Contents
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSEQUENCES
TO NON-U.S. HOLDERS
The following summary describes the material U.S. federal income and estate tax consequences of the acquisition, ownership and disposition of our common stock acquired in this offering by Non-U.S. Holders (as defined below). This discussion does not address all aspects of U.S. federal income and estate taxes and does not deal with foreign, state and local consequences that may be relevant to Non-U.S. Holders in light of their particular circumstances, nor does it address U.S. federal tax consequences other than income and estate taxes. Special rules different from those described below may apply to certain Non-U.S. Holders that are subject to special treatment under the Internal Revenue Code of 1986, as amended, or the Code, such as financial institutions, insurance companies, tax-exempt organizations, broker-dealers and traders in securities, U.S. expatriates, “controlled foreign corporations,” “passive foreign investment companies,” corporations that accumulate earnings to avoid U.S. federal income tax, persons that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or integrated investment or other risk reduction strategy, partnerships and other pass-through entities, and investors in such pass-through entities. Such Non-U.S. Holders are urged to consult their own tax advisors to determine the U.S. federal, state, local and other tax consequences that may be relevant to them. Furthermore, the discussion below is based upon the provisions of the Code, and Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively, so as to result in U.S. federal income and estate tax consequences different from those discussed below. We have not requested a ruling from the U.S. Internal Revenue Service, or IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions. This discussion assumes that the Non-U.S. Holder holds our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment).
The following discussion is for general information only and is not tax advice. Persons considering the purchase of our common stock pursuant to this offering should consult their own tax advisors concerning the U.S. federal income and estate tax consequences of acquiring, owning and disposing of our common stock in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction, including any state, local or foreign tax consequences.
For the purposes of this discussion, a “Non-U.S. Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock that is neither a U.S. Holder, a partnership (or other entity treated as a partnership for U.S. federal income tax purposes regardless of its place of organization or formation), nor an entity that is treated as a disregarded entity for U.S. federal income tax purposes (regardless of its place of organization or formation). A “U.S. Holder” means a beneficial owner of our common stock that is for U.S. federal income tax purposes (a) an individual who is a citizen or resident of the United States, (b) a corporation or other entity treated as a corporation created or organized in or under the laws of the United States, any state thereof or the District of Columbia, (c) an estate the income of which is subject to U.S. federal income taxation regardless of its source or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Distributions
Subject to the discussion below, distributions, if any, made on our common stock to a Non-U.S. Holder of our common stock to the extent made out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles) generally will constitute dividends for U.S. tax purposes and will be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. To obtain a reduced rate of withholding under a treaty, a Non-U.S. Holder generally will be required to provide us with a properly executed IRS Form W-8BEN, or other appropriate form, certifying the Non-U.S.
151
Table of Contents
Holder’s entitlement to benefits under that treaty. In the case of a Non-U.S. Holder that is an entity, Treasury Regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a Non-U.S. Holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If a Non-U.S. Holder is eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, the Non-U.S. Holder should contact its tax advisor regarding the possibility of obtaining a refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS.
We generally are not required to withhold tax on dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment that such holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that the dividends are so connected, is furnished to us (or, if stock is held through a financial institution or other agent, to such agent). In general, such effectively connected dividends will be subject to U.S. federal income tax, on a net income basis at the regular graduated rates, unless a specific treaty exemption applies. A corporate Non-U.S. Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected earnings and profits, subject to certain adjustments.
To the extent distributions on our common stock, if any, exceed our current and accumulated earnings and profits, they will constitute a non-taxable return of capital and will first reduce the Non-U.S. Holder’s adjusted basis in our common stock, but not below zero, and then will be treated as gain and taxed in the same manner as gain realized from a sale or other disposition of common stock as described in the next section.
Gain on Disposition of Our Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless (a) the gain is effectively connected with a trade or business of such holder in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment that such holder maintains in the United States), (b) the Non-U.S. Holder is a nonresident alien individual and is present in the United States for 183 or more days in the taxable year of the disposition and certain other conditions are met, or (c) we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) at any time within the shorter of the five-year period preceding such disposition or such holder’s holding period. In general, we would be a United States real property holding corporation if interests in U.S. real estate comprised (by fair market value) at least half of our business assets. We believe that we are not, and do not anticipate becoming, a United States real property holding corporation. Even if we are treated as a United States real property holding corporation, gain realized by a Non-U.S. Holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the Non-U.S. Holder owned, directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (i) the five-year period preceding the disposition or (ii) the holder’s holding period and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market.
If you are a Non-U.S. Holder described in (a) above, you will be required to pay tax on the net gain derived from the sale at regular graduated U.S. federal income tax rates, unless a specific treaty exemption applies, and corporate Non-U.S. Holders described in (a) above may be subject to the additional branch profits tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. If you are an individual Non-U.S. Holder described in (b) above, you will be required to pay a flat 30% tax on the gain derived from the sale, which gain may be offset by U.S. source capital losses (even though you are not considered a resident of the United States).
152
Table of Contents
Information Reporting Requirements and Backup Withholding
Generally, we must report information to the IRS with respect to any dividends we pay on our common stock including the amount of any such dividends, the name and address of the recipient, and the amount, if any, of tax withheld. A similar report is sent to the holder to whom any such dividends are paid. Pursuant to tax treaties or certain other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence.
Dividends paid by us (or our paying agents) to a Non-U.S. Holder may also be subject to U.S. backup withholding. U.S. backup withholding generally will not apply to a Non-U.S. Holder who provides a properly executed IRS Form W-8BEN or otherwise establishes an exemption. The current backup withholding rate is 28%.
Under current U.S. federal income tax law, U.S. information reporting and backup withholding requirements generally will apply to the proceeds of a disposition of our common stock effected by or through a U.S. office of any broker, U.S. or foreign, except that information reporting and such requirements may be avoided if the holder provides a properly executed IRS Form W-8BEN or otherwise meets documentary evidence requirements for establishing Non-U.S. Holder status or otherwise establishes an exemption. Generally, U.S. information reporting and backup withholding requirements will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through a non-U.S. office of a non-U.S. broker. Information reporting and backup withholding requirements may, however, apply to a payment of disposition proceeds if the broker has actual knowledge, or reason to know, that the holder is, in fact, a U.S. person. For information reporting purposes, certain brokers with substantial U.S. ownership or operations will generally be treated in a manner similar to U.S. brokers.
Backup withholding is not an additional tax. A holder subject to backup withholding should contact the holder’s tax advisor regarding the possibility of obtaining a refund or a tax credit and any associated requirements to provide information to the IRS or other relevant tax authority.
Legislation Affecting Taxation of Our Common Stock Held by or Through Foreign Entities
On February 8, 2012, the United States Treasury Department issued proposed regulations relating to the Foreign Account Tax Compliance Act or “FATCA,” which was enacted in March of 2010. As a general matter, FATCA imposes a 30% withholding tax on dividends on, and gross proceeds from the sale or other disposition of, our common stock if paid to a foreign entity (whether such foreign entity is the beneficial owner or an intermediary) unless (i) if the foreign entity is a “foreign financial institution,” the foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the foreign entity is not a “foreign financial institution,” the foreign entity identifies certain of its U.S. investors, or (iii) the foreign entity is otherwise excepted under FATCA. Under the proposed regulations, withholding is required (i) with respect to dividends on our common stock beginning on January 1, 2014, and (ii) with respect to gross proceeds from a sale or other disposition of our common stock that occurs on or after January 1, 2015.
Notwithstanding the foregoing, the proposed regulations will not be effective until issued in final form. There can be no assurance either as to when final regulations relating to FATCA will be issued or as to the particular form that those final regulations might take. If withholding is required under FATCA on a payment related to our common stock, investors that otherwise would not be subject to withholding (or that otherwise would be entitled to a reduced rate of withholding) on such payment generally will be subject to FATCA withholding and, even if an exception or reduction continues to apply, will be required to seek a refund or credit from the IRS to obtain the benefit of such exemption or reduction. We will not pay any additional amounts in respect of amounts withheld under FATCA. Prospective investors should consult their own tax advisors regarding the effect of FATCA in their particular circumstances.
153
Table of Contents
Federal Estate Tax
An individual Non-U.S. Holder who is treated as the owner of, or has made certain lifetime transfers of, an interest in our common stock will be required to include the value thereof in his or her gross estate for U.S. federal estate tax purposes, and may be subject to U.S. federal estate tax unless an applicable estate tax treaty provides otherwise, even though such individual was not a citizen or resident of the United States at the time of his or her death.
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAW.
154
Table of Contents
Subject to the terms and conditions set forth in an underwriting agreement dated the date of this prospectus among us and the underwriters named below, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares of common stock listed next to its name in the following table. Leerink Swann LLC and Cowen and Company, LLC are acting as joint book-running managers for the offering and as representatives of the underwriters.
Name | Number of Shares | |
Leerink Swann LLC | ||
Cowen and Company, LLC | ||
Needham & Company, LLC | ||
| ||
Total | ||
|
The underwriters are committed to purchase all the shares of common stock offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are not obligated to purchase the shares of our common stock covered by the underwriters’ over-allotment option described below.
The underwriters are offering our shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer our shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering of our shares, the public offering price and other selling terms may be changed by the representatives.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Without Option | With Option | ||||||||||
Public offering price | $ | $ | $ | |||||||||
Underwriting discounts and commissions | $ | $ | $ | |||||||||
Proceeds, before expenses, to us | $ | $ | $ | |||||||||
The total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $ million and are payable by us.
Over-Allotment Option
We have granted the underwriters an option to purchase up to additional shares of our common stock at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover sales of shares of common stock by the underwriters in excess of the total number of shares set forth in the table above. If any shares are purchased
155
Table of Contents
pursuant to this over-allotment option, the underwriters will purchase the additional shares in approximately the same proportion as shown in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Initial Public Offering Pricing
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between us and the representatives. Among the factors considered in these negotiations are:
| • | the prospects for our company and the industry in which we operate; |
| • | our past and present financial and operating performance; |
| • | financial and operating information and market valuations of publicly traded companies engaged in activities similar to ours; |
| • | the prevailing conditions of U.S. securities markets at the time of this offering; and |
| • | other factors deemed relevant. |
The estimated initial public offering price range set forth on the cover of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Lock-Up Agreements
We, our officers and directors and holders of all of our outstanding stock, options and warrants have entered into lock-up agreements with the underwriters. Under these agreements, we and these other individuals have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, our common stock, during a period ending 180 days after the date of this prospectus, without first obtaining the written consent of Leerink Swann LLC and Cowen and Company, LLC. Specifically, we and these other individuals have agreed not to:
| • | offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our common stock (including any shares issued in this offering or other issuer-directed shares), or any options or warrants to purchase any shares of our common stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of our common stock; or |
| • | engage in any hedging or other transactions, including, without limitation, any short sale or any purchase, sale or grant of any right (including without limitation any put or call option) with respect to any of the shares of our common stock or with respect to any security that includes, relates to, or derives any significant part of its value from the individual’s shares of our common stock; |
whether any such transaction described above is to be settled by delivery of common stock or other securities, in cash or otherwise.
The restrictions described above, applicable to us, do not apply to:
| • | the sale of shares of common stock to the underwriters pursuant to the underwriting agreement; |
| • | the issuance by us of shares of our common stock upon the exercise of an option or warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing or that is described in this prospectus; |
156
Table of Contents
| • | the grant by us of stock options or other stock-based awards, or the issuance of shares of our common stock upon exercise thereof, to eligible participants pursuant to employee benefit or equity incentive plans described in this prospectus, provided that, prior to the grant of any such stock options or other stock-based awards that vest within the restricted period, each recipient of such grant shall sign and deliver a lock-up agreement agreeing to be subject to the restrictions on transfer described above; and |
| • | the filing by us of a registration statement on Form S-8 or any successor form thereto with respect to the registration of securities to be offered under any employee benefit or equity incentive plans described in this prospectus. |
The restrictions described above, applicable to our officers and directors and holders of all of our outstanding stock, options and warrants, do not apply to:
| • | transactions by security holders relating to shares of our common stock or other securities acquired in open market transactions after the completion of this offering, provided that no public reports, including but not limited to reports pursuant to Rule 144 of the Securities Act, or pursuant to Section 16 of the Exchange Act, are required or are voluntarily made in connection with subsequent sales of our common stock or other securities acquired in such open market transactions; |
| • | the exercise of any option or warrant to purchase shares of our common stock or the conversion of a convertible promissory note outstanding on the date of the underwriting agreement of which the representatives have been advised in writing or that is described in this prospectus, provided that the underlying shares of our common stock issued upon exercise remain subject to the restrictions imposed by the lock-up agreement; |
| • | transfers or contributions by security holders of shares of our common stock or any security convertible into common stock as a bona fide gift, in connection with estate planning or upon death by will or intestate succession; |
| • | transfers or distributions by security holders of shares of our common stock or any security convertible into common stock to limited partners, general partners, limited liability company members, stockholders, affiliates or any wholly owned subsidiary of the security holder; |
| • | transfers or contributions by security holders of shares of our common stock or any security convertible into common stock to any trust for the direct or indirect benefit of the security holder or the immediate family of the security holder; or |
| • | transfers by operation of law, including a merger or a qualified domestic order; |
provided that in the case of each of the preceding four types of transactions, each transferee or distributee signs and delivers a lock-up agreement agreeing to be subject to the restrictions on transfer described above and no filing under Section 16(a) of the Exchange Act, reporting a reduction in beneficial ownership of shares of common stock, is required or is voluntarily made during the restricted period.
The 180-day restricted period is subject to extension if (1) during the last 17 days of the restricted period we issue an earnings release or material news or a material event relating to us occurs or (2) prior to the expiration of the restricted period, we announce that we will release earnings results during the 15-day period beginning on the last day of the restricted period, in which case the restrictions imposed in the lock-up agreements will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event. In addition, if the underwriters agree to release any party from the restrictions set forth in the lock-up agreement with such party prior to the expiration of the restricted period, all other parties subject to the lock-up agreement shall be entitled to a proportionate release of their shares from the
157
Table of Contents
lock-up agreement restrictions, except that the underwriters may, in their sole discretion, release employees from such restrictions without triggering the release of any other shares of our common stock so long as such shares released for any such employee amount to less than $100,000 worth of common stock.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
The NASDAQ Global Market Listing
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “HPTX.”
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our common stock, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase common stock in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued at any time without notice.
158
Table of Contents
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Notice to Non-U.S. Investors
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive, each of which we refer to as a relevant member state, with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state, or the relevant implementation date, an offer of securities described in this prospectus may not be made to the public in that relevant member state other than:
| • | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| • | to any legal entity that has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| • | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of representatives for any such offer; or |
| • | in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive; |
provided that no such offer of securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares of common stock in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe for the shares, as the same may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions.
159
Table of Contents
The validity of the shares of common stock to be issued in this offering will be passed upon for us by Hogan Lovells US LLP, Palo Alto, California. Certain legal matters relating to this offering will be passed upon for the underwriters by Cooley LLP, Palo Alto, California.
The consolidated financial statements as of December 31, 2010 and 2011, and for each of the three years in the period ended December 31, 2011 and, cumulatively, for the period from November 1, 2006 (date of inception) to December 31, 2011, included in this prospectus have been so included in reliance on the report (which contains an explanatory paragraph relating to our ability to continue as a going concern as described in Note 1 to the consolidated financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of our common stock offered in this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the accompanying exhibits and schedules. Some items included in the registration statement are omitted from this prospectus in accordance with the rules and regulations of the SEC. For further information with respect to us and the common stock offered in this prospectus, we refer you to the registration statement and the accompanying exhibits and schedules. Statements contained in this prospectus as to the contents of any contract, agreement or any other document are summaries of the material terms of these contract, agreement or other document. With respect to each of these contracts, agreements or other documents filed as an exhibit to the registration statement, reference is made to such exhibit for a more complete description of the matter involved. A copy of the registration statement, and the accompanying exhibits and schedules, may be inspected without charge and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. The SEC maintains a web site that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the SEC’s website is http://www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act, and we will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at http://www.hyperiontx.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
160
Table of Contents
(A development stage company)
December 31, 2009, 2010 and 2011, and
for the Cumulative Period from November 1, 2006 (Date of Inception) to March 31, 2012
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit | F-5 | |||
| F-7 | ||||
| F-8 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Hyperion Therapeutics, Inc.
(A development stage company)
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of convertible preferred stock and stockholders’ deficit and of cash flows present fairly, in all material respects, the financial position of Hyperion Therapeutics, Inc. and its subsidiary (a development stage company) (the “Company”) at December 31, 2010 and December 31, 2011 and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011 and, cumulatively, for the period from November 1, 2006 (date of inception) to December 31, 2011 (the cumulative information, other than the statement of convertible preferred stock and stockholders’ deficit, is not presented herein), in conformity with accounting principles generally accepted in the United States of America. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has incurred losses from operations and negative cash flows from operations since its inception that raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. These consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
San Jose, California
April 13, 2012
F-2
Table of Contents
(A development stage company)
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| December 31, | March 31, | Pro forma stockholders’ deficit as of March 31, 2012 | ||||||||||||||
| 2010 | 2011 | 2012 | ||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
Assets | ||||||||||||||||
Current assets | ||||||||||||||||
Cash and cash equivalents | $ | 6,579 | $ | 7,018 | $ | 3,736 | ||||||||||
Prepaid expenses and other current assets | 244 | 741 | 313 | |||||||||||||
Restricted cash | — | 305 | 150 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total current assets | 6,823 | 8,064 | 4,199 | |||||||||||||
Property and equipment, net | 81 | 19 | 15 | |||||||||||||
Restricted cash | 326 | 25 | 25 | |||||||||||||
Deferred offering costs | — | — | 419 | |||||||||||||
Other non-current assets | 157 | 34 | 356 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total assets | $ | 7,387 | $ | 8,142 | $ | 5,014 | ||||||||||
|
|
|
|
|
| |||||||||||
Liabilities, Convertible Preferred Stock and Stockholders’ Deficit | ||||||||||||||||
Current liabilities | ||||||||||||||||
Accounts payable | $ | 684 | $ | 1,887 | $ | 2,528 | ||||||||||
Accrued liabilities | 2,489 | 3,310 | 3,824 | |||||||||||||
Call option liability | — | 737 | — | |||||||||||||
Convertible notes payable | — | 23,412 | 30,736 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total current liabilities | 3,173 | 29,346 | 37,088 | |||||||||||||
Warrants liability | — | 2,574 | 3,464 | |||||||||||||
Other non-current liabilities | 64 | — | — | |||||||||||||
|
|
|
|
|
| |||||||||||
Total liabilities | 3,237 | 31,920 | 40,552 | |||||||||||||
|
|
|
|
|
| |||||||||||
Commitments and contingencies (Note 8) | ||||||||||||||||
Convertible preferred stock, par value $0.0001 — 41,000,000 at December 31, 2010 and 66,000,000 shares authorized at December 31, 2011 and March 31, 2012 (unaudited), respectively; 40,045,749 shares issued and outstanding at December 31, 2010 and 2011 and March 31, 2012 (unaudited), and shares issued and outstanding at March 31, 2012 pro forma (unaudited) (Aggregate liquidation preference of $63,272 at December 31, 2011 and March 31, 2012 unaudited) | 58,326 | 58,326 | 58,326 | |||||||||||||
|
|
|
|
|
| |||||||||||
Stockholders’ deficit | ||||||||||||||||
Common stock, par value $0.0001 — 57,000,000 at December 31, 2010 and 80,000,000 shares authorized at December 31, 2010 and 2011 and March 31, 2012 (unaudited), respectively; 2,858,251 shares issued and outstanding at December 31, 2010 and 2011 and 2,930,451 shares issued and outstanding at March 31, 2012 (unaudited); and shares issued and outstanding at March 31, 2012 pro forma (unaudited) | — | — | — | |||||||||||||
Additional paid-in capital | 23,142 | 24,630 | 24,756 | |||||||||||||
Deficit accumulated during the development stage | (77,318 | ) | (106,734 | ) | (118,620 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total stockholders’ deficit | (54,176 | ) | (82,104 | ) | (93,864 | ) | $ | |||||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities, convertible preferred stock and stockholders’ deficit | $ | 7,387 | $ | 8,142 | $ | 5,014 | ||||||||||
|
|
|
|
|
| |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
(A development stage company)
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Year Ended December 31, | Three Months Ended March 31, | Cumulative Period from November 1, 2006 (Date of Inception) to March 31, 2012 | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
Revenue | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 286 | ||||||||||||
Cost of revenue | — | — | — | — | — | 10 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Gross profit | — | — | — | — | — | 276 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Operating expenses | ||||||||||||||||||||||||
Research and development | 11,030 | 23,111 | 17,236 | 4,300 | 8,902 | 79,137 | ||||||||||||||||||
General and administrative | 1,909 | 2,693 | 8,162 | 1,361 | 2,077 | 21,204 | ||||||||||||||||||
Selling and marketing | 462 | 797 | 761 | 250 | 246 | 7,322 | ||||||||||||||||||
Impairment of development and promotion rights acquisition cost | — | — | — | — | — | 7,059 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses | 13,401 | 26,601 | 26,159 | 5,911 | 11,225 | 114,722 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Loss from operations | (13,401 | ) | (26,601 | ) | (26,159 | ) | (5,911 | ) | (11,225 | ) | (114,446 | ) | ||||||||||||
Interest income | 39 | 43 | 28 | 4 | 4 | 446 | ||||||||||||||||||
Interest expense | (763 | ) | (1 | ) | (2,554 | ) | — | (1,040 | ) | (6,282 | ) | |||||||||||||
Other income (expense), net | 525 | 1,106 | (731 | ) | — | 375 | 1,662 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net loss | (13,600 | ) | (25,453 | ) | $ | (29,416 | ) | (5,907 | ) | (11,886 | ) | (118,620 | ) | |||||||||||
Accretion of Series B preferred stock to redemption value | (78 | ) | — | — | — | — | (114 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net loss attributable to common stockholders | $ | (13,678 | ) | $ | (25,453 | ) | $ | (29,416 | ) | $ | (5,907 | ) | $ | (11,886 | ) | $ | (118,734 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net loss per share attributable to common stockholders: | ||||||||||||||||||||||||
Basic and diluted | $ | (15.24 | ) | $ | (10.13 | ) | $ | (10.29 | ) | $ | (2.07 | ) | $ | (4.16 | ) | |||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Pro forma basic and diluted (unaudited) (Note 15) | $ | $ | ||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||
Weighted average number of shares used to compute net loss per share of common stock: | ||||||||||||||||||||||||
Basic and diluted | 897,239 | 2,512,320 | 2,858,251 | 2,858,251 | 2,858,251 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Pro forma basic and diluted (unaudited) (Note 15) | $ | $ | ||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
(A development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit
Period From November 1, 2006 (Date of Inception) to March 31, 2012
(In thousands, except share and per share amounts)
| Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Deficit Accumulated during the Development Stage | Total Stockholders’ Deficit | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
Balances as of November 1, 2006 (Date of Inception) | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
Issuance of common stock in December 2006 at $1.80 per share | — | — | 139 | — | — | — | — | |||||||||||||||||||||||
Issuance of restricted common stock in December 2006 at $1.80 per share | — | — | 2,646 | — | 5 | — | 5 | |||||||||||||||||||||||
Issuance of Series A convertible preferred stock in December 2006 at $1.00 per share, net of issuance costs of $63 | 2,000,000 | 1,936 | — | — | — | — | — | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (128 | ) | (128 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2006 | 2,000,000 | 1,936 | 2,785 | — | 5 | (128 | ) | (123 | ) | |||||||||||||||||||||
Issuance of Series B redeemable convertible preferred stock in August 2007 at $1.75 per share for cash and conversion of notes at $1.40 per share, net of issuance costs of $117 | 11,471,597 | 19,884 | — | — | — | — | — | |||||||||||||||||||||||
Accretion to redemption value of redeemable convertible preferred stock | — | 7 | — | — | (7 | ) | — | (7 | ) | |||||||||||||||||||||
Repurchase of common stock at $1.80 per share | — | — | (139 | ) | — | — | — | — | ||||||||||||||||||||||
Exercise of stock options at $17.95 per share | — | — | 918 | — | 16 | — | 16 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 4 | — | 4 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (9,038 | ) | (9,038 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2007 | 13,471,597 | 21,827 | 3,564 | — | 18 | (9,166 | ) | (9,148 | ) | |||||||||||||||||||||
Accretion to redemption value of redeemable convertible preferred stock | — | 29 | — | — | (29 | ) | — | (29 | ) | |||||||||||||||||||||
Issuance of common stock warrant in connection with services | — | — | — | — | 5 | — | 5 | |||||||||||||||||||||||
Repurchase of common stock at $17.95 per share | — | — | (313 | ) | — | (6 | ) | — | (6 | ) | ||||||||||||||||||||
Exercise of stock options at $17.95 and $53.85 per share | — | — | 433 | — | 16 | — | 16 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 137 | — | 137 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (29,099 | ) | (29,099 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2008 | 13,471,597 | $ | 21,856 | 3,684 | — | $ | 141 | $ | (38,265 | ) | $ | (38,124 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit
Period From November 1, 2006 (Date of Inception) to March 31, 2012
(In thousands, except share and per share amounts)
| Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Deficit Accumulated during the Development Stage | Total Stockholders’ Deficit | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
Balances as of December 31, 2008 | 13,471,597 | $ | 21,856 | 3,684 | $ | — | $ | 141 | $ | (38,265 | ) | $ | (38,124 | ) | ||||||||||||||||
Accretion to redemption value of Series B redeemable convertible preferred stock | — | 78 | — | — | (78 | ) | — | (78 | ) | |||||||||||||||||||||
Recapitalization (Note 9) | (13,471,597 | ) | (21,934 | ) | 75,039 | — | 21,934 | — | 21,934 | |||||||||||||||||||||
Issuance of Series C-1 convertible preferred stock in June 2009 in connection with conversion of notes payable and accrued interest at $1.33 per share | 11,647,769 | 15,501 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of Series C-2 convertible preferred stock in June 2009 at $1.58 per share for cash, net of issuance costs of $305 and net of preferred stock liability of $1,369 | 14,201,455 | 20,764 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock in June 2009 at $0.21 per share in connection with the collaboration agreement (Note 3) | — | — | 1,670,261 | — | 351 | — | 351 | |||||||||||||||||||||||
Exercise of stock options at $0.21 per share | — | — | 201,904 | — | 42 | — | 42 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 313 | — | 313 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (13,600 | ) | (13,600 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2009 | 25,849,224 | 36,265 | 1,950,888 | — | 22,703 | (51,865 | ) | (29,162 | ) | |||||||||||||||||||||
Issuance of Series C-2 convertible preferred stock in April 2010 at $1.58 per share for cash, net of issuance costs of $8 and value of preferred stock liability of $361 | 14,196,525 | 22,061 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock in April 2010 at $0.30 per share in connection with the collaboration agreement (Note 3) | — | — | 710,072 | — | 213 | — | 213 | |||||||||||||||||||||||
Exercise of stock options at $0.21 per share | — | — | 197,291 | — | 41 | — | 41 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 185 | — | 185 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (25,453 | ) | (25,453 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2010 | 40,045,749 | 58,326 | 2,858,251 | — | 23,142 | (77,318 | ) | (54,176 | ) | |||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 345 | — | 345 | |||||||||||||||||||||||
Gain on extinguishment of debt (Note 6) | — | — | — | — | 1,143 | — | 1,143 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (29,416 | ) | (29,416 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of December 31, 2011 | 40,045,749 | 58,326 | 2,858,251 | — | 24,630 | (106,734 | ) | (82,104 | ) | |||||||||||||||||||||
Stock-based compensation expense (unaudited) | — | — | — | — | 78 | — | 78 | |||||||||||||||||||||||
Exercise of stock options at $0.67 per share (unaudited) | — | — | 72,200 | — | 48 | — | 48 | |||||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | (11,886 | ) | (11,886 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balances as of March 31, 2012 (unaudited) | 40,045,749 | $ | 58,326 | 2,930,451 | $ | — | $ | 24,756 | $ | (118,620 | ) | $ | (93,864 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
(A development stage company)
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | Three Months Ended March 31, | Cumulative Period From November 1, 2006 (Date of Inception) to March 31, 2012 | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
Cash flows from operating activities | ||||||||||||||||||||||||
Net loss | $ | (13,600 | ) | $ | (25,453 | ) | $ | (29,416 | ) | $ | (5,907 | ) | $ | (11,886 | ) | $ | (118,620 | ) | ||||||
Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||||||||||||||
Depreciation and amortization | 156 | 96 | 69 | 18 | 5 | 496 | ||||||||||||||||||
Amortization of debt discount | — | — | 1,549 | — | 368 | 1,917 | ||||||||||||||||||
Conversion of accrued interest to convertible preferred stock | 514 | — | — | — | — | 514 | ||||||||||||||||||
Remeasurement of warrants liability | — | — | 1,072 | — | 343 | 1,011 | ||||||||||||||||||
Remeasurement of call option liability and preferred stock liability | (625 | ) | (1,105 | ) | (351 | ) | — | (737 | ) | (2,818 | ) | |||||||||||||
Stock-based compensation expense | 313 | 185 | 345 | 47 | 78 | 1,062 | ||||||||||||||||||
Issuance of common stock in connection with collaboration agreement | 351 | 213 | — | — | — | 564 | ||||||||||||||||||
Acquisition of development and promotion rights | — | — | — | — | — | (10,000 | ) | |||||||||||||||||
Amortization of debt issuance costs | — | — | 13 | — | 20 | 437 | ||||||||||||||||||
Amortization of development and promotion rights acquisition cost | — | — | — | — | — | 2,941 | ||||||||||||||||||
Impairment of development and promotion rights acquisition cost | — | — | — | — | — | 7,059 | ||||||||||||||||||
Other | (1 | ) | — | — | — | — | 4 | |||||||||||||||||
Changes in assets and liabilities | ||||||||||||||||||||||||
Prepaid expenses and other current assets | (581 | ) | 365 | (510 | ) | (35 | ) | 408 | (346 | ) | ||||||||||||||
Other non-current assets | — | (132 | ) | 123 | 31 | (39 | ) | (73 | ) | |||||||||||||||
Accounts payable | 703 | (761 | ) | 1,203 | 739 | 326 | 2,213 | |||||||||||||||||
Accrued liabilities and other non-current liabilities | 1,230 | 703 | 1,372 | 1,269 | 408 | 4,332 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net cash used in operating activities | (11,540 | ) | (25,889 | ) | (24,531 | ) | (3,838 | ) | (10,706 | ) | (109,307 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Cash flows from investing activities | ||||||||||||||||||||||||
Acquisition of property and equipment | (4 | ) | (15 | ) | (8 | ) | (2 | ) | — | (318 | ) | |||||||||||||
Option to purchase Buphenyl and Ammonul (Note 3) | (283 | ) | (283 | ) | ||||||||||||||||||||
Change in restricted cash | — | (25 | ) | (4 | ) | — | 155 | (174 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net cash used in investing activities | (4 | ) | (40 | ) | (12 | ) | (2 | ) | (128 | ) | (775 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Cash flows from financing activities | ||||||||||||||||||||||||
Proceeds from issuance of common stock, net of repurchases | 42 | 41 | — | — | 48 | 169 | ||||||||||||||||||
Proceeds from issuance of convertible preferred stock, net | 22,133 | 22,423 | — | — | — | 66,074 | ||||||||||||||||||
Proceeds from issuance of convertible notes payable | 4,996 | — | 24,982 | — | 7,504 | 47,774 | ||||||||||||||||||
Proceeds from issuance of notes payable | — | — | — | — | — | 10,000 | ||||||||||||||||||
Principal payments under notes payable | (6,556 | ) | — | — | — | — | (10,000 | ) | ||||||||||||||||
Principal payments under capital lease obligations | (87 | ) | (29 | ) | — | — | — | (193 | ) | |||||||||||||||
Repurchase of common stock | — | — | — | — | — | (6 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net cash provided by financing activities | 20,528 | 22,435 | 24,982 | — | 7,552 | 113,818 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net increase (decrease) in cash and cash equivalents | 8,984 | (3,494 | ) | 439 | (3,840 | ) | (3,282 | ) | 3,736 | |||||||||||||||
Cash and cash equivalents, beginning of period | 1,089 | 10,073 | 6,579 | 6,579 | 7,018 | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Cash and cash equivalents, end of period | $ | 10,073 | $ | 6,579 | $ | 7,018 | $ | 2,739 | $ | 3,736 | $ | 3,736 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Supplemental cash flow information | ||||||||||||||||||||||||
Cash paid for interest | $ | 566 | $ | 1 | $ | — | $ | — | $ | — | $ | 1,792 | ||||||||||||
Supplemental disclosure of noncash investing and financing activities | ||||||||||||||||||||||||
Warrants issued in connection with notes payable | — | — | 1,502 | — | 547 | 2,454 | ||||||||||||||||||
Issuance of call option related to convertible notes payable | — | — | 1,707 | — | — | 1,707 | ||||||||||||||||||
Gain on extinguishment of debt | — | — | 1,143 | — | — | 1,143 | ||||||||||||||||||
Conversion of promissory notes to Series B redeemable convertible preferred stock | — | — | — | — | — | 301 | ||||||||||||||||||
Accretion to redemption value of Series B redeemable convertible preferred stock | 78 | — | — | — | — | 114 | ||||||||||||||||||
Purchase of property and equipment under capital leases | — | — | — | — | — | 193 | ||||||||||||||||||
Conversion of notes payable and accrued interest to Series C-1 convertible preferred stock | 15,501 | — | — | — | — | 15,501 | ||||||||||||||||||
Conversion of Series A and Series B redeemable convertible preferred stock to common stock | 21,934 | — | — | — | — | 21,934 | ||||||||||||||||||
Preferred stock liability related to the second tranche of Series C-2 preferred stock | — | 361 | — | — | — | 361 | ||||||||||||||||||
Accrued deferred offering costs | — | — | — | — | 419 | 419 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-7
Table of Contents
(A development stage company)
Notes to Consolidated Financial Statements
| 1. | Formation and Business of the Company |
Hyperion Therapeutics, Inc. (the “Company”) was incorporated in the state of Delaware on November 1, 2006. The Company’s activities since inception have consisted primarily of raising capital, negotiating a promotion and drug development collaboration agreement, establishing a management team and performing drug development activities. Accordingly, the Company is considered to be in the development stage.
The Company is a biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat disorders in the areas of orphan diseases and hepatology. The Company is developing Ravicti™ (glycerol phenylbutyrate) to treat the most prevalent urea cycle disorders (“UCD”) and hepatic encephalopathy (“HE”). UCD and HE are generally characterized by elevated levels of ammonia in the bloodstream. Elevated levels of ammonia are potentially toxic and can lead to severe medical complications which may include death. The Company’s product candidate, Ravicti, is designed to lower ammonia in the blood. UCD are inherited rare genetic diseases caused by a deficiency of one or more enzymes or transporters that constitute the urea cycle, which in a healthy individual removes ammonia through conversion of ammonia to urea. HE is a serious but potentially reversible neurological disorder that can occur in patients with liver scarring, known as cirrhosis, or acute liver failure. On December 23, 2011, the Company submitted a New Drug Application (“NDA”) to the U.S. Food and Drug Administration (the “FDA”) for Ravicti for the chronic treatment of UCD in patients aged 6 years and above. The FDA accepted the NDA for review in February 2012.
Hyperion Therapeutics Limited was formed in January 2008 as a private limited company under the Companies Act 1985 for England and Wales and is wholly owned by the Company. There has been no activity in Hyperion Therapeutics Limited for the last three fiscal years.
The consolidated financial statements have been prepared on a going concern basis that contemplates the realization of assets and discharge of liabilities in their normal course of business. Since inception, the Company has incurred recurring net operating losses and negative cash flows from operations. During the year ended December 31, 2011 and the three months ended March 31, 2012 (unaudited), the Company incurred a net loss of $29.4 million and $11.9 million, respectively, and used $24.5 million and $10.7 million of cash in operations, respectively. At December 31, 2011 and March 31, 2012 (unaudited), the Company had a deficit accumulated during the development stage of $106.7 million and $118.6 million, respectively, and a working capital deficit of $21.3 million and $32.9 million, respectively. The Company expects to incur increased research and development expenses if the Company initiates a Phase III trial of Ravicti for the treatment of patients with episodic HE or if the FDA requires the Company to do additional studies for the approval of Ravicti for UCD. In addition, the Company expects to incur sales and marketing expenses if Ravicti is approved for UCD. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with respect to these matters include utilization of a substantial portion of the Company’s capital resources and efforts in completing the development and obtaining regulatory approval of Ravicti in UCD and HE, expanding the Company’s organization, and preparing for potential commercialization of Ravicti, if approved by the FDA. The Company needs to raise additional funds through equity or debt financing and, when and if necessary, to reduce discretionary spending. Although management has been successful in raising additional funding in the past, most recently in April 2012 (Note 17), there can be no assurance that they will be successful or that any needed financing will be available in the future at terms acceptable to the Company. Failure to achieve these plans may result in the Company not being able to achieve its business objectives. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-8
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation and the Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of the accompanying consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Basis of Consolidation
The consolidated financial statements include the accounts of the Company and Hyperion Therapeutics Limited. All intercompany balances and transactions, if any, have been eliminated for purposes of consolidation.
Segment Reporting
The Company operates as one operating segment and uses one measurement of profitability to manage its business. The Company’s only revenue since its inception was from Ucyclyd Pharma, Inc. (“Ucyclyd”), which is located in the United States. All long-lived assets are maintained in the United States.
Unaudited Interim Financial Information
The accompanying unaudited interim consolidated balance sheet as of March 31, 2012, the consolidated statement of operations and consolidated statement of cash flows for the three months ended March 31, 2012 and 2011 and consolidated statement of convertible preferred stock and stockholders’ deficit for the three months ended March 31, 2012 are unaudited. The unaudited interim consolidated financial statements have been prepared in accordance with generally accepted accounting principles for interim financial information and on a basis consistent with the annual consolidated financial statements, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to state fairly the Company’s financial position as of March 31, 2012 and its results of operations and cash flows for the three months ended March 31, 2012 and 2011. The financial data and other financial information disclosed in these notes to the financial statements related to the three month periods are also unaudited. The results of operations for the three months ended March 31, 2012 are not necessarily indicative of the results to be expected for the year ending December 31, 2012 or for any other future annual or interim period.
Unaudited Pro Forma Stockholders’ Deficit
The March 31, 2012 unaudited pro forma stockholders’ deficit has been prepared assuming immediately upon completion of the Company’s initial public offering: (i) the automatic conversion of all outstanding shares of preferred stock into shares of common stock; (ii) the automatic conversion of the convertible notes into shares of common stock and the related reclassification of the convertible notes payable to common stock and additional paid-in-capital; and (iii) the net exercise of warrants to purchase shares of common stock and preferred stock, assuming an initial public offering price of $ per share, that will expire upon the completion of the Company’s initial public offering, if not exercised, and the related reclassification of the warrants liability to common stock and additional paid-in-capital. The unaudited pro forma stockholders’ deficit does not assume any proceeds from the proposed initial public offering.
F-9
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
2009 Recapitalization
In June 2009, the Board of Directors of the Company approved a reverse stock split of the Company’s common stock. As a result, the Company’s common stock, stock options and common stock warrants were adjusted at a ratio of 2-for-359, effective June 29, 2009. All share and per share data referenced throughout the consolidated financial statements have been retroactively adjusted to reflect this reverse stock split (Note 9).
Cash and Cash Equivalents
Cash and cash equivalents are stated at cost, which approximates fair value. The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents include money market accounts and various deposit accounts.
Restricted Cash
Restricted cash as of December 31, 2011 consisted of a certificate of deposit of $0.3 million in connection with the office facility operating lease, included in the current assets of the consolidated balance sheets and a certificate of deposit of $25,000 related to a security deposit, reflected in non-current assets within consolidated balance sheets. As of December 31, 2010, the restricted cash balance comprised a certificate of deposit of $0.3 million and $25,000 security deposit, presented in non-current assets within consolidated balance sheets.
Restricted cash of March 31, 2012 (unaudited) included a deposit of $0.2 million in connection with the Company’s loan and security agreement (Note 17), which is included in the current assets of the consolidated balance sheets and $25,000 security deposit presented in non-current assets within the consolidated balance sheets.
Risks and Uncertainties
The Company’s future results of operations involve a number of risks and uncertainties. Factors that could affect the Company’s future operating results and cause actual results to vary materially from expectations include, but are not limited to, uncertainty of results of clinical trials and reaching milestones, uncertainty of regulatory approval of Ravicti in UCD and HE, uncertainty of the ability to complete the purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL under the amended and restated collaboration agreement with Ucyclyd (the “restated collaboration agreement”) (Note 3), uncertainty of market acceptance of any Company products, competition from branded and generic products and larger companies, securing and protecting of proprietary and marketing exclusivity rights, and dependence on key individuals and sole source suppliers.
Products developed by the Company require approvals from the FDA or other international regulatory agencies prior to commercial sales. There can be no assurance that Ravicti or any future product candidates will receive the necessary approvals. If the Company is denied approval, approval is delayed or the Company is unable to maintain approval, it could have a materially adverse impact on the Company’s business and its consolidated financial statements.
Concentration of Credit Risk
The Company’s cash and cash equivalents are maintained with financial institutions located in the United States. Deposits in these institutions may exceed the amount of insurance provided on such deposits. The
F-10
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Company has not recognized any losses from credit risks during the periods presented and management does not believe that the Company is exposed to significant credit risk from its cash or cash equivalents.
Fair Value of Financial Instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid for to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. The carrying amounts of the Company’s financial instruments, including cash equivalents, restricted cash, accounts payable and accrued liabilities, approximate fair value due to their short maturities. The carrying amounts of the preferred stock liability, the common stock warrants liability, the preferred stock warrants liability and the call option liability represents its estimated fair value. See Note 4, Fair Value Measurements, regarding the fair value of the Company’s convertible notes payable.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. All property and equipment is depreciated on a straight-line basis over the following estimated useful lives:
Computer and office equipment | 3 – 5 years | |||
Software | 3 years |
Leasehold improvements are amortized over the lesser of their useful life or the term of the applicable lease. Upon sale or retirement of assets, the cost and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in operations. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
The Company reviews its property, equipment and long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset class may not be recoverable. Recoverability is measured by comparing the carrying amount to the future net undiscounted cash flows that the assets are expected to generate. If such assets are considered impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value determined using projected discounted future net cash flows arising from the assets.
Preclinical and Clinical Trial Accruals
The Company’s clinical trial accruals are based on estimates of patient enrollment and related costs at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions and clinical research organizations that conduct and manage clinical trials on the Company’s behalf. The Company accrues expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, the Company modifies the estimates of accrued expenses accordingly. To date, the Company has had no significant adjustments to accrued preclinical and clinical trial expenses.
F-11
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Warrants Liability
The Company accounts for its warrants and other derivative financial instruments as either equity or liabilities based upon the characteristics and provisions of each instrument. Warrants classified as equity are recorded as additional paid-in capital on the consolidated balance sheet and no further adjustments to their valuation are made. Warrants classified as derivative liabilities and other derivative financial instruments that require separate accounting as liabilities are recorded on the Company’s consolidated balance sheet at their fair value on the date of issuance and are revalued on each subsequent balance sheet, with fair value changes recognized as increases or reductions to other income (expense), net in the consolidated statements of operations. The Company estimates the fair value of these liabilities using option pricing models and assumptions that are based on the individual characteristics of the warrants or instruments on the valuation date, as well as assumptions for future financings, expected volatility, expected life, yield, and risk-free interest rate.
The Company accounts for its warrants for shares of convertible preferred stock issued with the convertible notes in 2011 that are contingently redeemable as liabilities. The Company will continue to adjust the liability for changes in fair value of these warrants until the earlier of: (i) exercise of warrants; (ii) expiration of warrants; (iii) a change of control of the Company; or (iv) the consummation of the Company’s initial public offering.
The Company accounts for its warrants for shares of common stock as liabilities in accordance with accounting guidance for derivatives. The accounting guidance provides a two-step model to be applied in determining whether a financial instrument is indexed to an entity’s own stock that would qualify such financial instruments for a scope exception. This scope exception specifies that a contract that would otherwise meet the definition of a derivative financial instrument would not be considered as such if the contract is both (i) indexed to the entity’s own stock and (ii) classified in the stockholders’ deficit section of the balance sheet. The Company determined that its warrants for shares of common stock issued with convertible notes in 2011 are ineligible for equity classification and will continue to adjust the liability for changes in fair value until the earlier of the: (i) exercise of warrants; (ii) expiration of warrants; (iii) a change of control of the Company; (iv) occurrence of a qualified or non-qualified financing as defined in the agreement; (v) maturity of convertible notes; or (vi) the consummation of the Company’s initial public offering.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605,Revenue Recognition,when the following criteria have been met: persuasive evidence of an arrangement exists; delivery has occurred and risk of loss has passed; and the seller’s price to the buyer is fixed or determinable and collectability is reasonably assured. The Company’s only source of revenue has been commissions for promotion services that were generated through the collaboration agreement with Ucyclyd related to the sales of BUPHENYL and AMMONUL for UCD. These promotion services were terminated effective June 2008 (Note 3). The Company considered the guidance in ASC 605-50,Revenue Recognition, Customer Payments and Incentives, to determine the appropriate classification of the promotion commission.
Cost of Revenue
Cost of revenue was related to royalty payments to a third party in connection with the commissions received from Ucyclyd related to sales of BUPHENYL and AMMONUL under the prior collaboration agreement with Ucyclyd (the “collaboration agreement”) (Note 3).
F-12
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Research and Development Expenses
Costs related to research and development of products are charged to expense as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, clinical trial supplies, fees for clinical trial services, consulting costs and allocated overhead, including rent, equipment, depreciation and utilities.
Stock-Based Compensation
The Company accounts for stock-based employee compensation arrangements in accordance with provisions of ASC 718,Compensation — Stock Compensation. ASC 718 requires the recognition of compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The Company calculates the fair value of stock options using the Black-Scholes method and expenses using the straight-line attribution approach.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company accounts for uncertain tax positions in accordance with ASC 740-10,Accounting for Uncertainty in Income Taxes. The Company assesses all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and is measured at the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and the Company will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate. The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Comprehensive Loss
For all periods presented the comprehensive loss was equal to the net loss; therefore, a separate statement of comprehensive loss is not included in the accompanying consolidated financial statements.
Net Loss per Share of Common Stock
Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration for potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss attributable to
F-13
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
common stockholders by the weighted-average number of common shares and potentially dilutive securities outstanding for the period determined using the treasury-stock and if-converted methods. For purposes of the diluted net loss per share calculation, convertible preferred stock, convertible notes payable, stock options and common and preferred stock warrants are considered to be potentially dilutive securities but are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive and therefore, basic and diluted net loss per share were the same for all periods presented.
Unaudited Pro Forma Net Loss per Share
The unaudited pro forma basic and diluted net loss per share reflects the conversion of all outstanding shares of convertible preferred stock, convertible notes payable, common and preferred stock warrants and stock options, as if the conversion had occurred at the earlier of the beginning of the period or the date of issuance if later. Potential common shares of convertible notes payable were calculated based on the “if-converted” method. Under the “if-converted” method, when computing the dilutive effect of the convertible notes payable, the numerator is adjusted to add back the amount of interest expense related to the amortization of debt discount, debt issuance costs and fair value change on call options liability. The dilutive effect of warrants and stock options were measured using the treasury method. Under the treasury method, the common and preferred stock warrants and the stock options are assumed exercised and common shares are issued at the beginning of the period (or at time of issuance), the proceeds from the exercise is assumed used to purchase common stock at the average market price.
The unaudited pro forma basic and diluted net loss per share amounts do not give effect to the issuance of shares from the planned initial public offering nor do they give effect to potential dilutive securities where the impact would be anti-dilutive.
Recent Accounting Pronouncements
Effective January 1, 2012, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2011-04,“Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and International Financial Reporting Standards (“IFRS”),” issued in May 2011. This pronouncement was issued to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and IFRS. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements particularly for Level 3 fair value measurements. This pronouncement is effective for reporting periods beginning on or after December 15, 2011. The new guidance will require prospective application. The adoption of this accounting standard update required expanded disclosure only and did not have an impact on the Company’s consolidated financial position, results of operations or cash flows.
In December 2011, FASB issued ASU No. 2011-11,“Balance Sheet (Topic 210).” This update provides enhanced disclosure requirements regarding the nature of an entity’s right of offset related to arrangements associated with its financial instruments and derivative instruments. The new guidance requires the disclosure of the gross amounts subject to rights of set-off, the amounts offset in accordance with the accounting standards followed, and the related net exposure. This pronouncement is effective for financial reporting period beginning on or after January 1, 2013 and full retrospective application is required. The Company does not expect that the adoption of this ASU will have a material impact on its consolidated financial statements.
F-14
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 3. | Collaboration Agreement with Ucyclyd Pharma, Inc. |
In August 2007, the Company executed the collaboration agreement with Ucyclyd. Under the terms of the collaboration agreement, the Company obtained: (1) the research and development rights to Ravicti for UCD, HE and other indications (the “Development Products”), (2) the right to promote BUPHENYL and AMMONUL in the United States, and (3) certain future rights to purchase the worldwide rights to develop and commercialize BUPHENYL, AMMONUL and Ravicti (the “Purchase Transaction”).
Under the collaboration agreement, the Company is responsible for the continued clinical development of Development Products and to establish a sales force to promote the approved drugs BUPHENYL and AMMONUL for UCD in the United States. Upon approval of Ravicti for UCD, the Company is obligated to acquire the worldwide commercialization rights of Ravicti, BUPHENYL and AMMONUL. In the event this approval is received, the purchase price for these worldwide commercialization rights would be $25.0 million. Upon a determination that no scientific or medical basis exists to file an NDA for Ravicti for either UCD or HE, the Company has the right, but not the obligation, to acquire the worldwide commercialization rights of Ravicti, BUPHENYL and AMMONUL. Upon such a determination, the purchase price for these worldwide commercialization rights would be based on net sales of BUPHENYL and AMMONUL. In addition to customary termination rights for uncured breaches, Ucyclyd has the right to terminate the amended collaboration agreement for certain events such as if the Company fails to obtain FDA acceptance of the filing of the NDA for Ravicti for treatment of UCD by 54 months from October 2007, unless the failure to obtain acceptance of the filing of the NDA is excused due to a determination that there is no longer a reasonable scientific or medical basis, subject to the rights of Ucyclyd and Brusilow Enterprises, LLC, a licensor, to contest the Company’s determination that no such basis exists. Ucyclyd also has the right to terminate the collaboration agreement if, after the Company’s purchase rights or obligations arise as set forth above, the Company does not consummate the purchase within the required time period. In addition, the Company’s ability to consummate the purchase transaction will require the Company to obtain clearance from the Federal Trade Commission (“FTC”) or Department of Justice pursuant to the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended. If the FTC or the Antitrust Division of the Department of Justice were to challenge the transaction and the Company were unable to resolve the challenge through a consent decree, either party could terminate the collaboration agreement. Finally, prior to the closing of the purchase, Ucyclyd can terminate the collaboration agreement in the event the Company or its investors fail to provide written assurance to Ucyclyd upon their request that the Company have, or will have within 30 days, sufficient cash reserves or immediately available lines of credit to meet six months of operating expenses.
Prior to November 2008, when the Company entered into the first amendment (the “first amendment”) to the collaboration agreement, Ucyclyd paid a quarterly commission to the Company for promotion services in the U.S. for BUPHENYL and AMMONUL. The commission was based on a portion of net sales. Under the collaboration agreement, Ucyclyd had responsibility for manufacturing, shipping, billing and collecting for U.S. sales of BUPHENYL and AMMONUL. The Company did not purchase product from Ucyclyd for resale.
The Company paid a $10.0 million nonrefundable upfront fee to Ucyclyd to acquire the development and promotional rights and licenses under the collaboration agreement. The Company intended to recognize the $10.0 million over a period through the expected purchase option exercise date, which was approximately 51 months. The collaboration agreement provides for the payment for contingent development milestones, cumulative sales milestones and sales royalties related to the Development Products. The collaboration agreement also contains a number of covenants.
F-15
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
In November 2008, the Company entered into the first amendment to the collaboration agreement, which terminated the Company’s rights to co-promote BUPHENYL and any and all development rights granted to the Company with respect to AMMONUL under the first amendment. The Company has no further rights, and Ucyclyd has no further obligations to the Company, with respect to AMMONUL, except in the event of a Purchase Transaction, the Company will obtain the applicable commercialization rights to AMMONUL. The Company continued focusing its efforts on developing Ravicti. The Company assessed the impact of the first amendment on the accounting treatment of the unamortized portion of the nonrefundable upfront fee. The Company performed an impairment analysis under ASC 360-10,Impairment or Disposal of Long-Lived Assets, and determined that the entire asset was impaired. As a result of this determination, the Company recorded an impairment charge of $7.1 million in 2008.
In June 2009, the Company entered into the second amendment (the “second amendment”) to the collaboration agreement. In connection with the second amendment, the Company issued 1,670,261 shares of common stock to Ucyclyd in exchange for restructuring third party distribution agreements, and royalty and milestone payments and arranging seller financing. The Company recorded the fair value of these shares of $0.4 million as a research and development expense. In addition, pursuant to the second amendment, Ucyclyd agreed to provide seller financing in the event that the Company acquires the commercial rights with respect to Ravicti, BUPHENYL and AMMONUL. The issuance of common stock in consideration for the restructuring of the royalty and milestone payments was valued using the fair value of the Company’s common stock on the date of the issuance of the shares and was recorded as a research and development expense.
In October 2009, the Company entered into the third amendment (the “third amendment”) to the collaboration agreement. Under the terms of the third amendment, the Company relieved Ucyclyd of the obligation to file a drug master file with the FDA and other regulatory authorities in connection with the manufacture of Ravicti.
In April 2010, in accordance with the second amendment, the Company issued 710,072 additional shares of common stock to Ucyclyd. The Company valued the shares based upon the fair value of the Company’s common stock on the date of issuance of the shares and recorded the fair value of $0.2 million as a research and development expense.
In March 2012, the Company entered into the purchase agreement with Ucyclyd under which the Company purchased the worldwide rights to Ravicti and the restated collaboration agreement under which Ucyclyd granted the Company an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL at a fixed price at a future defined date, plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended. The entry into the purchase agreement and the restated collaboration agreement resolves the dispute that two parties had with respect to their rights under the prior collaboration agreement.
Under the purchase agreement, the Company made a payment of $6.0 million of which (i) $5.7 million was allocated to the worldwide rights to Ravicti and (ii) $0.3 million was allocated to the option to purchase rights to BUPHENYL and AMMONUL, based on their relative fair values. The allocated amount to the rights to Ravicti of $5.7 million was recorded to research and development expense in the consolidated statements of operations for the period ended March 31, 2012 due to the uncertainty of an alternative future use. The allocated amount to the option to purchase rights to BUPHENYL and AMMONUL in the amount of $0.3 million is included within other non-current assets and will be evaluated for potential impairment until exercised, at which time it will be added to the option exercise price.
F-16
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
The Company will also pay tiered mid to high single digit royalties on global net sales of Ravicti and may owe regulatory milestones of up to $15.8 million related to approval of Ravicti in HE, regulatory milestones of up to $7.3 million per indication for approval of Ravicti in indications other than UCD or HE, and net sales milestones of up to $38.8 million if Ravicti is approved for use in indications other than UCD (such as HE) and all annual sales targets are reached.
In addition, the intellectual property license agreements executed between Ucyclyd and Dr. Marshall L. Summar, or Summar, and Ucyclyd and Brusilow Enterprises, LLC, or Brusilow, were assigned to the Company, and the Company has assumed the royalty and milestone obligations under the Brusilow agreement for sales of Ravicti in any indication and the royalty obligations under the Summar agreement on sales of Ravicti to treat HE. The Company will also pay Brusilow an annual license extension fee to keep the Brusilow license in effect, which extension fee is payable until the Company’s first commercial sale of Ravicti following FDA approval. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Under the terms of the restated collaboration agreement, the Company has an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain rights to AMMONUL. The Company will be permitted to exercise this option for 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. The upfront purchase price for AMMONUL and BUPHENYL is $22.0 million, which the Company may fund by drawing on a loan commitment from Ucyclyd. The loan would be payable in eight quarterly payments and would bear interest at a rate of 9% per year, and would be secured by the BUPHENYL and AMMONUL assets. If the Ravicti NDA for UCD is not approved by January 1, 2013, then Ucyclyd is obligated to make monthly payments of $0.5 million to the Company until the earliest of (1) FDA approval of the Ravicti NDA for UCD, (2) June 30, 2013 and (3) the Company’s written notification of the decision not to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL.
If the Company exercises its option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, then Ucyclyd has the time-limited right to elect to retain all rights to AMMONUL for a purchase price of $32.0 million. If Ucyclyd exercises this option, Ucyclyd will pay the Company a net payment of $13.0 million on closing of the purchase transaction, which reflects the purchase price for Ucyclyd’s worldwide rights to BUPHENYL being set-off against Ucyclyd’s retention payment for AMMONUL. If Ucyclyd retains rights to AMMONUL, subject to certain terms and conditions, the Company retains a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
| 4. | Fair Value Measurements |
The Company follows ASC 820-10, “Fair Value Measurements and Disclosures,” which among other things, defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, a three-tier fair value hierarchy has been established, which prioritizes the inputs used in measuring fair value as follows:
| • | Level 1 — Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date. |
F-17
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| • | Level 2 — Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life. |
| • | Level 3 — Inputs reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model. |
As of December 31, 2010, the Company had no assets or liabilities carried at fair value on a recurring basis. The following table presents the Company’s fair value hierarchy for assets and liabilities measured at fair value on a recurring basis as of December 31, 2011 and March 31, 2012 (in thousands):
| December 31, 2011 | ||||||||||||
| Quoted prices in Active Markets for Identical Items (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||||
Liabilities: | ||||||||||||
Common stock warrants liability | $ | — | $ | — | $ | 1,978 | ||||||
Preferred stock warrants liability | — | — | 596 | |||||||||
Call option liability | — | — | 737 | |||||||||
|
|
|
|
|
| |||||||
| $ | — | $ | — | $ | 3,311 | |||||||
|
|
|
|
|
| |||||||
| March 31, 2012 (unaudited) | ||||||||||||
| Quoted prices in Active Markets for Identical Items (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | ||||||||||
Liabilities: | ||||||||||||
Common stock warrants liability | $ | — | $ | — | $ | 2,487 | ||||||
Preferred stock warrants liability | — | — | 977 | |||||||||
|
|
|
|
|
| |||||||
| $ | — | $ | — | $ | 3,464 | |||||||
|
|
|
|
|
| |||||||
Upon issuance of the common and preferred stock warrants liability and the call option liability, the Company estimates the fair value and subsequent remeasurement using the Black-Scholes option-pricing model at each reporting date, using the following inputs: the risk-free interest rates; the expected dividend rates; the remaining expected life of the warrants and the call options; and the expected volatility of the price of the underlying common stock. The estimates are based, in part, on subjective assumptions and could differ materially in the future.
F-18
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
The following table presents the changes in the fair values of level 3 liabilities (in thousands):
| 2009 Preferred Stock Liability | April 2011 Common Stock Warrants Liability | October 2011 Preferred Stock Warrants Liability | April 2011 Call Option Liability | October 2011 Call Option Liability | ||||||||||||||||
Fair value at January 1, 2009 | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
Recognition of fair value at the issuance date (Note 9) | 1,369 | — | — | — | — | |||||||||||||||
Change in fair value recorded in other income (expense), net | (625 | ) | — | — | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Fair value at December 31, 2009 | 744 | — | — | — | — | |||||||||||||||
Change in fair value recorded in other income (expense), net | (1,105 | ) | — | — | — | — | ||||||||||||||
Fair value of preferred stock liability at the issuance date of the second tranche of Series C-2 preferred stock (Note 9) | 361 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Fair value at December 31, 2010 | — | — | — | — | — | |||||||||||||||
Recognition of fair value at the date of issuance (Notes 6 and 7) | — | 1,089 | 413 | 869 | 838 | |||||||||||||||
Change in fair value recorded in other income (expense), net | — | 889 | 183 | (250 | ) | (101 | ) | |||||||||||||
Change in fair value recorded in additional paid-in-capital (Note 6) | — | — | — | (619 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Fair value at December 31, 2011 | $ | — | $ | 1,978 | $ | 596 | $ | — | $ | 737 | ||||||||||
Recognition of fair value at the date of issuance of second closing in February 2012 (Notes 6 and 7) (unaudited) | — | — | 547 | — | — | |||||||||||||||
Change in fair value recorded in other income (expense), net (unaudited) | — | 509 | (166 | ) | — | (737 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Fair value at March 31, 2012 (unaudited) | $ | — | $ | 2,487 | $ | 977 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
The following table presents the carrying value and estimated fair value of the Company’s convertible notes as of March 31, 2012:
| March 31, 2012 | ||||||||
| Carrying Value | Estimated Fair Value | |||||||
| (unaudited) | ||||||||
April 2011 Notes | $ | 16,981 | $ | 16,736 | ||||
October 2011 Notes | 13,755 | 13,814 | ||||||
|
|
|
| |||||
Total | $ | 30,736 | $ | 30,550 | ||||
|
|
|
| |||||
The fair values of the convertible notes were determined by using an income approach to determine the value of the Company, then allocating the Company’s value to its various securities using the Option Pricing Method. The Option Pricing Method was applied in various scenarios based on the potential liquidity alternatives available to the Company. These scenarios were probability weighted and included a potential initial public offering, as well as remaining private throughout 2011 and 2012. The Convertible Notes are classified within Level 3 of the hierarchy of fair value measurements.
F-19
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 5. | Accrued Liabilities |
The following table represents the components of accrued liabilities (in thousands):
| December 31, | March 31, | |||||||||||
| 2010 | 2011 | 2012 | ||||||||||
| (unaudited) | ||||||||||||
Preclinical and clinical trial expenses | $ | 1,484 | $ | 1,489 | $ | 1,564 | ||||||
Payroll and related expenses | 777 | 1,235 | 602 | |||||||||
Interest payable | — | 392 | 1,064 | |||||||||
Deferred rent, current portion | 39 | 7 | — | |||||||||
Other | 189 | 187 | 594 | |||||||||
|
|
|
|
|
| |||||||
| $ | 2,489 | $ | 3,310 | $ | 3,824 | |||||||
|
|
|
|
|
| |||||||
| 6. | Notes Payable |
2007 Notes Payable
In October 2007, the Company entered into a Loan and Security Agreement for borrowings of up to $15.0 million collateralized by substantially all of the Company’s assets. In connection with the Loan and Security Agreement, the Company issued warrants for 300,000 shares of the Company’s Series B redeemable convertible preferred stock (Note 9). The Company borrowed $10.0 million in October 2007. Amounts borrowed were payable over 36 months. Interest on outstanding borrowings was fixed at 9.71% per annum. Interest only was payable through October 2008. Equal payments of loan principal commenced in November 2008.
In 2008, the Company failed to meet certain minimum cash reserve requirements and, accordingly, defaulted on the loan covenants. Subsequently, in August 2008, the Loan and Security Agreement was amended. Pursuant to the amended agreement, the lender agreed not to exercise any remedies it had against the Company as a result of the default until the earlier of October 31, 2008 (or January 31, 2009 if certain specified clinical development activities continue) or any further event of default. In addition, under the amended agreement, the Company was required to make two principal payments of $1.0 million each on August 6, 2008 and October 1, 2008, and the interest rate on the loan was increased from 9.71% to 10.71%. All remaining payments were due in accordance with the original agreement terms. The remaining $5.0 million under the original Loan and Security Agreement was no longer available to the Company.
In November 2008, the Company entered into a second amendment to the Loan and Security Agreement. Pursuant to the second amendment, the lender agreed not to exercise any remedies it had against the Company as a result of the default until the earlier of March 31, 2009, if certain specified clinical development activities continued, or any further event of default. Under the second amendment, the Company was required to make one principal payment of $1.0 million in November 2008, and the interest rate on the loan was increased from 10.71% to 12.00%. All remaining payments were due in accordance with the original agreement terms.
In accordance with ASC 470, the Company accounted for the initial amendment to the Loan and Security Agreement as a modification and the second amendment was accounted for as an extinguishment of the old loan and the entering into a new loan agreement with the lender. As such, all issuance costs and penalties associated with the loan prior to November 2008 were recorded immediately in the consolidated statement of operations.
F-20
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
In July 2009, the Company prepaid the outstanding principal amount and the accrued interest. Accordingly, the Company paid a prepayment penalty of $0.1 million, which was included under other income (expense), net in the consolidated statement of operations.
2008 Convertible Notes Payable
In 2008, the Company entered into a convertible note agreement with certain investors. The notes had an interest rate of 6% per annum and were convertible upon the completion of a new preferred stock financing in the amount of not less than $25.0 million, including the convertible notes outstanding on that date. The Company drew down $10.0 million and $5.0 million in 2008 and 2009, respectively. In June 2009, the outstanding convertible notes and accrued interest payable in the amount of $15.5 million in the aggregate were converted into 11,647,769 shares of Series C-1 convertible preferred stock (Note 9).
April 2011 Convertible Notes Payable
In April 2011, the Company entered into a convertible notes financing (the “April 2011 convertible notes financing”), in which it issued an aggregate principal amount of $17.5 million of convertible notes in an initial closing in April and an aggregate principal amount of $8,285 of convertible notes in subsequent initial closings in May 2011 (collectively, the “April 2011 Notes”) pursuant to the Convertible Note and Warrant Purchase Agreement dated April 1, 2011 (the “April 2011 Purchase Agreement”). The April 2011 Purchase Agreement permits the Company to issue up to an aggregate principal amount of $35.0 million.
The April 2011 Notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding April 2011 Notes under certain circumstances, which demand may not be made earlier than December 31, 2012, as amended or (ii) an event of default. The April 2011 Notes cannot be prepaid, except on demand by the holders of the April 2011 Notes, as described above. The principal and the interest under the April 2011 notes are automatically convertible (i) into securities that are sold in an issuance of preferred stock generating gross proceeds of at least $30.0 million, referred to herein as a qualified financing, at the lowest price at which such securities are sold to certain new investors in the qualified financing, (ii) into Series C-2 convertible preferred stock upon the occurrence of certain change of control events, unless the holders of 66% of the principal amount of the then-outstanding April 2011 Notes notify the Company of their election to accelerate the unpaid principal and interest of the April 2011 Notes in connection with the change of control event, or (iii) into common stock immediately prior to the consummation of an initial public offering, at a conversion price equal to the initial public offering price. In addition, holders of 66% of the principal amount of the then-outstanding April 2011 Notes have the option to convert the April 2011 Notes (i) in the event that the Company consummates an equity financing that is not a “qualified financing,” as described above, prior to the maturity of the April 2011 Notes, into the equity securities issued in the equity financing, or (ii) upon maturity of the April 2011 Notes, if the April 2011 Notes have not been previously converted, into shares of the Company’s Series C-2 convertible preferred stock.
April 2011 Call Option Liability
The April 2011 Purchase Agreement also provides that so long as there has not been a qualified financing, change of control or initial public offering, on or before June 30, 2011, or in the event that the April 2011 Notes issued in an initial closing or subsequent initial closing have not been previously converted into common or
F-21
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
preferred stock as set forth in the April 2011 Purchase Agreement, upon the election and approval of the holders of 66% of the principal amount of the then-outstanding April 2011 Notes, the Company will issue (i) notes with an aggregate principal amount of up to $7.5 million in the event that none of the subsequent closing notes have been issued or (ii) up to an aggregate principal amount of up to $10.5 million in the event all or a portion of the subsequent closing notes have been issued. The additional note amount was determined to be a call option (“April 2011 Call Option”) that was recorded at its fair value of $0.9 million as a debt discount that has been amortized to interest expense over the term of the April 2011 Notes. The fair value of the April 2011 Call Option was determined using the Black-Scholes option-pricing model on the date of the issuance using the following assumptions: expected life of 7 months, risk free interest rate of 0.27%, dividend yield of 0% and expected volatility of 50%. During the year ended December 31, 2011, the Company recorded $0.3 million in other income (expense), net to reflect the change in the fair value of the April 2011 Call Option, and $0.6 million in the gain on the extinguishment of debt to reflect the termination of the April 2011 Call Option in October 2011.
Amendment to the April 2011 Convertible Notes Payable
In October 2011, the Company substantially amended the April 2011 Purchase Agreement to extend the term of the April 2011 Notes from January 31, 2012 to December 31, 2012, and to terminate the April 2011 Call Option. As a result, the transaction was accounted for as an extinguishment of debt in the amount of $1.1 million, which includes $0.6 million on termination of the April 2011 Call Option, calculated as the excess of the carrying amount of the notes, including accrued interest, over the fair value of the amended notes. In accordance with ASC 470-50-40-2, the Company reflected the gain on extinguishment of debt resulting from this related party transaction as a capital contribution and credited this amount to additional paid-in capital within the statement of stockholders’ deficit.
October 2011 Convertible Notes Payable
In October 2011, the Company entered into a convertible notes financing (the “October 2011 convertible notes financing”), in which it issued an aggregate principal amount of $7.5 million of convertible notes in an initial closing in October and an aggregate principal amount of $3,551 of convertible notes in a subsequent initial closing in November 2011 and aggregate amount of $7.5 million of convertible notes in the second closing in February 2012 (collectively, the “October 2011 Notes”) pursuant to the Convertible Note and Warrant Purchase Agreement dated October 26, 2011 (the “October 2011 Purchase Agreement”). The October 2011 Purchase Agreement permitted the Company to issue up to an aggregate principal amount of $15.0 million.
The October 2011 Notes accrue interest at a rate of 6% per annum and have a maturity date of the earliest of (i) demand by the holders of 66% of the principal amount of the then-outstanding October 2011 Notes under certain circumstances, which demand may not be made earlier than December 31, 2012, or (ii) an event of default. The October 2011 Notes cannot be prepaid, except on demand by the holders of the October 2011 Notes, as described above. The principal and the interest under the October 2011 Notes are automatically convertible (a) into securities that are sold in an issuance of preferred stock generating gross proceeds of at least $40.0 million, referred to herein as a qualified financing, equal to the quotient of (i) the outstanding principal amount plus unpaid accrued interest divided by (ii) the price per share paid by the investors purchasing new preferred stock in the qualified financing; (b) upon the occurrence of certain change in control events, into new series of preferred stock equal to the quotient of (i) the outstanding principal amount plus accrued interest divided by (ii) the Series C-2 original issue price, or (c) into common stock immediately prior to the consummation of an initial public offering, at a conversion price equal to the initial public offering price. In addition, holders of 66%
F-22
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
of the principal amount of the then-outstanding October 2011 Notes have the option to convert the October 2011 Notes (i) in the event that the Company consummates an equity financing that is not a “qualified financing,” as described above, prior to the maturity of the October 2011 Notes, into the equity securities issued in the equity financing, or (ii) upon maturity of the October 2011 Notes, if the October 2011 Notes have not been previously converted, into shares of the Company’s Series C-2 convertible preferred stock.
October 2011 Call Option Liability
The October 2011 Purchase Agreement also provides that so long as there has not been a qualified financing, change of control or initial public offering, on or before June 30, 2012, or in the event that the October 2011 Notes issued in the initial closing or subsequent initial closings have not been previously converted into common or preferred stock as set forth in the agreement, upon the election and approval of the holders of 66% of the principal amount of the then-outstanding October 2011 Notes, the Company will issue (i) notes with an aggregate principal amount of $7.5 million or (ii) up to $7.5 million of notes in the event all or a portion of the subsequent initial closing notes have been issued. The additional note amount was determined to be a call option (“October 2011 call option”) that was recorded as its fair value of $0.8 million as a debt discount that has been amortized to interest expense over the term of the October 2011 notes. The fair value of October 2011 call option was determined using the Black-Scholes option-pricing model on the date of issuance using the following assumptions: expected life of 8 months, risk free interest rate of 0.12%, dividend yield of 0% and expected volatility of 50%. During the year ended December 31, 2011, the Company recorded $0.1 million in other income (expense), net to reflect the change in the fair value of the October 2011 Call Option. The Company determined the fair value of the October 2011 Call Option at December 31, 2011 to be $0.7 million, using the Black-Scholes option-pricing model with the following assumptions: expected life 6 months, risk-interest rate of 0.12%, dividend yield of 0% and expected volatility of 50%.
For the three months ended March 31, 2012 (unaudited), the Company recorded $0.7 million to other income (expense), net in its consolidated statements of operations upon issuance of the second closing of the October 2011 Notes in February 2012 for $7.5 million.
As of December 31, 2011, the carrying values of the April 2011 Notes and the October 2011 Notes are $17.0 million (based on the estimated fair value of April 2011 Notes after the amendment) and $6.4 million, respectively, totaling to $23.4 million. As of March 31, 2012 (unaudited), the carrying values of the April 2011 Notes and October 2011 Notes are $17.0 million and $13.7, respectively, totaling $30.7 million
During the year ended December 31, 2011, the Company recorded amortization for the debt discount of $1.6 million related to the April 2011 Notes and October 2011 Notes. During the three months ended March 31, 2012 (unaudited), the Company recorded amortization for debt discount of $0.4 million related to the October 2011 Notes.
In addition, the Company determined that the April 2011 Notes and the October 2011 Notes have contingent beneficial conversion features related to the conversion options described above. Upon the occurrence of the contingent event underlying those conversion options, the Company may recognize a charge based on the difference, if any, between the adjusted conversion price and the fair market value of common stock at the original issuance date. This charge, if any, will impact net income (loss) attributable to common stockholders and basic and diluted net income (loss) per share attributable to common stockholders.
F-23
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 7. | Warrants |
October 2007 Common Stock Warrants
In connection with the Loan and Security Agreement entered into in October 2007 (Note 6), the Company issued warrants to purchase 300,000 shares of Series B convertible preferred stock. The warrants were exercisable at $1.75 per share and expire in October 2017. Using the Black-Scholes option pricing model, the fair value of the warrants was $0.4 million and was recorded as debt issuance cost, which was amortized to interest expense over the term of the loan facility.
In June 2009, as part of the recapitalization (Note 9), the October 2007 warrants to purchase Series B redeemable convertible preferred stock were converted into 1,671 warrants to purchase shares of common stock at an exercise price of $314.12 (the “October 2007 common stock warrants”). The October 2007 common stock warrants were outstanding as of December 31, 2011.
April 2008 Common Stock Warrants
In exchange for services received, the Company issued a warrant to purchase 25,000 shares of common stock at an exercise price of $0.30 per share in April 2008 (the “April 2008 common stock warrants”). The fair value of the warrant was $4,500 which was determined using the Black-Scholes option pricing model with the following assumptions: volatility of 71%; risk-free rate of 2.84%; and exercise price of $0.30 and an expected term of five years. As part of the reverse stock split (Note 9), the warrant to purchase 25,000 shares of common stock converted to a warrant to purchase 139 shares of common stock at an exercise price of $53.85. The April 2008 common stock warrants were outstanding as of December 31, 2011.
April 2011 Common Stock Warrants
In connection with the April 2011 convertible notes financing (Note 6), the Company issued warrants to purchase shares of the Company’s common stock in an initial closing in April 2011 and in subsequent initial closings in May 2011 (collectively, the “April 2011 common stock warrants”) both at an exercise price of $0.67 per share and subject to adjustments upon the occurrence of certain events, including but not limited to a capital reorganization, reclassification or subdivision of common shares. The number of shares of common stock are calculated based on 30% of the principal amount of the April 2011 Notes divided by either (i) in the event that the holder’s notes have been converted into shares of new preferred stock, the price per share paid by a new investor in a qualified financing, (ii) in the event that the holder’s April 2011 Notes have been converted into shares of Series C-2 preferred stock, the Series C-2 original issue price of $1.58, (iii) in the event that the holder’s April 2011 Notes have been converted into equity securities in a non-qualified financing, the price paid per share by an investor in a non-qualified financing, or (iv) a price of $1.58 in the event of an initial public offering. The April 2011 common stock warrants are exercisable until April 2021. The April 2011 Warrants will automatically net exercise and terminate immediately prior to the closing of the initial public offering.
F-24
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
October 2011 Preferred Stock Warrants
In connection with the October 2011 convertible notes financing (Note 6), the Company issued warrants to purchase shares of the Company’s preferred stock, in an initial closing in October 2011, and in a subsequent initial closing in November 2011 and in the second closing in February 2012 (collectively, the “October 2011 preferred stock warrants”) both at exercise prices subject to adjustments upon the occurrence of certain events, including but not limited to a capital reorganization, reclassification or subdivision of common shares. The number of shares of preferred stock are calculated based on 30% of the principal amount of the October 2011 Notes divided by either: (i) the price per share paid by the investors for the new preferred stock in the qualified financing; (ii) Series C-2 preferred stock original price at $1.58; (iii) price per share paid by the investors for equity securities in the nonqualified financing, or (iv) a price of $1.58 in the event of an initial public offering. These October 2011 preferred stock warrants are exercisable until October 25, 2018. The additional preferred stock warrants issued in February 2012 are exercisable until February 7, 2019. The October 2011 Warrants will automatically net exercise and terminate immediately prior to the closing of the initial public offering.
Common and Preferred Stock Warrants Fair Value Measurements
Under ASC 815 and ASC 480-10, the Company accounts for the April 2011 common stock warrants and the October 2011 preferred stock warrants, respectively, at fair value and recorded as liabilities on the date of the issuance. On the date of the issuance and in subsequent remeasurement, the Company determined the fair value of the April and October 2011 warrants by allocating the Company equity value using the Black-Scholes option-pricing model at each reporting date. The Company’s equity value was allocated among the various convertible debt and equity classes expected to be outstanding at the liquidity events based on the rights and preferences of each class.
The fair value of the April 2011 common stock warrants as of the date of issuance was determined to be $1.1 million, recorded as a debt discount and amortized to interest expense over the term of the April 2011 Notes. The fair value was determined using the following assumptions: expected life of 2 years; risk free interest rate of 0.80%; and expected volatility of 70%.
The fair value of the October 2011 preferred stock warrants as of the date of issuance date was determined to be $0.4 million, recorded as a debt discount and amortized to interest expense over the term of the October 2011 Notes. The fair value was determined using the following assumptions: expected life of 1.50 years; risk free interest rate of 0.12%; and the expected volatility of 70%. The fair value of the preferred stock warrants issued in February 2012 in connection with the second closing of the October 2011 Notes was determined to be $0.5 million, recorded as a debt discount and amortized to interest expense over the term of the October 2011 Notes. The fair value was determined using the following assumptions: expected life of 1 year; risk free interest rate of 0.20%; and expected volatility of 70%.
During the year ended December 31, 2011, the Company recorded $0.9 million and $0.2 million in other income (expense), net to reflect the change in fair value of the April 2011 common stock warrants and October 2011 preferred stock warrants, respectively. At December 31, 2011, the fair values of the common and preferred stock warrants was determined to be $2.0 million and $0.6 million respectively, using the following assumptions: expected life of 1.50 years; risk free interest rate of 0.12%; and expected volatility of 70%.
For the three months ended March 31, 2012 (unaudited), the Company recorded $0.5 million and $0.2 million in other income (expense), net to reflect the change in fair value of the April 2011 common stock warrants and October 2011 preferred stock warrants, respectively. As of March 31, 2012 (unaudited), the fair
F-25
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
values of the common stock warrants and preferred stock warrants was determined to be $2.5 million and $1.0 million, respectively, using the following assumptions: expected life of 10 months; risk free interest rate of 0.23%; and expected volatility of 70%.
The following table summarizes the outstanding warrants and the corresponding exercise price as of December 31, 2010 and 2011 and March 31, 2012:
| Number of Shares Outstanding | Per Share Exercise Price | |||||||||||||||
| December 31, | March 31, | |||||||||||||||
| 2010 | 2011 | 2012 | ||||||||||||||
| (unaudited) | ||||||||||||||||
October 2007 common stock warrants | 1,671 | 1,671 | 1,671 | $ | 314.12 | |||||||||||
April 2008 common stock warrants | 139 | 139 | 139 | $ | 53.85 | |||||||||||
The above table does not include the April 2011 common stock warrants or the October 2011 preferred stock warrants.
| 8. | Commitments and Contingencies |
Operating Leases
The Company leases its office facility under an operating lease, which expires in August 2013. As of December 31, 2010 and 2011, the lease is collateralized by a certificate of deposit for $0.3 million (Note 2). The total monthly base rent is approximately $20,000. Under the terms of the lease, the Company is responsible for taxes, insurance and maintenance expense. The Company recognizes rent expense on a straight-line basis over the lease period. In addition, the Company also leases certain office equipment under an operating lease, which expires in December 2013.
Aggregate total future minimum lease payments under operating facility and equipment leases as of December 31, 2011 were as follows (in thousands):
Years Ending December 31, | ||||
2012 | $ | 270 | ||
2013 | 173 | |||
|
| |||
Total | $ | 443 | ||
|
|
Rent expense including maintenance fees was $0.4 million for the years ended December 31, 2009, 2010, 2011, respectively, and $1.7 million, cumulatively, for the period from November 1, 2006 (date of inception) to December 31, 2011. For the three months ended March 31, 2011 and 2012 (unaudited), rent expense including maintenance fees was $90,000 and $70,000, respectively, and $1.8 million, cumulatively, for the period from November 1, 2006 (date of inception) to March 31, 2012 (unaudited).
In May 2010, the Company subleased a portion of its office facility to a third party. The sublease expired in January 2012. Minimum monthly lease payments were approximately $6,000 in addition to subtenant’s share of monthly common maintenance expenses. Amounts collected under this sublease are offset against rental expense.
F-26
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Contingencies
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. Further, the Company may be subject to certain contingent liabilities that arise in the ordinary course of its business activities. The Company accrues a liability for such matters when it is probable that future expenditures will be made and such expenditures can be reasonably estimated.
In accordance with the Company’s amended and restated Certificate of Incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that may enable it to recover a portion of any amounts paid for future claims.
The Company is contingently committed for development milestone payments as well as sales-related milestone payments and royalties relating to potential future product sales under the amended collaboration agreement and Asset Purchase Agreement with Ucyclyd (Note 3). The amount, timing and likelihood of these payments are unknown as they are dependent on the occurrence of future events that may or may not occur, including approval by the FDA of Ravicti and the closing of the purchase of Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL under the purchase agreement and the restated collaboration agreement.
| 9. | Convertible Preferred Stock |
In October 2011, the Company amended its Certificate of Incorporation to increase the total number shares which the Company is authorized to issue up to 146,000,000 shares, 80,000,000 shares of which are common stock and 66,000,000 shares of which are preferred stock.
At December 31, 2011 and March 31, 2012 (unaudited), preferred stock consisted of the following:
Series | Shares Authorized | Issued and Outstanding | Carrying Value (in thousands) | Liquidation Preference Per Share | ||||||||||||
C-1 | 11,647,769 | 11,647,769 | $ | 15,501 | $ | 1.58 | ||||||||||
C-2 | 53,673,645 | 28,397,980 | 42,825 | $ | 1.58 | |||||||||||
Nondesignated | 678,586 | — | — | |||||||||||||
|
|
|
|
|
| |||||||||||
| 66,000,000 | 40,045,749 | $ | 58,326 | |||||||||||||
|
|
|
|
|
| |||||||||||
At December 31, 2010, preferred stock consisted of the following:
Series | Shares Authorized | Issued and Outstanding | Carrying Value (in thousands) | Liquidation Preference Per Share | ||||||||||||
C-1 | 11,647,769 | 11,647,769 | $ | 15,501 | $ | 1.58 | ||||||||||
C-2 | 28,773,136 | 28,397,980 | 42,825 | $ | 1.58 | |||||||||||
Nondesignated | 579,095 | — | — | |||||||||||||
|
|
|
|
|
| |||||||||||
| 41,000,000 | 40,045,749 | $ | 58,326 | |||||||||||||
|
|
|
|
|
| |||||||||||
F-27
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
2009 Recapitalization
In June 2009, the Company engaged in a recapitalization pursuant to which shares of Series A convertible preferred stock and Series B redeemable convertible preferred stock converted to common stock at a 1-to-1 ratio and warrants to purchase Series B redeemable convertible preferred stock were converted to warrants to purchase common stock at a 1-to-1 ratio. Immediately after the recapitalization, the Company effected a reverse stock split of its common stock at a ratio of 2-for-359. In addition, the convertible notes payable along with accrued interest converted to Series C-1 convertible preferred stock at a discounted per share price of $1.33 per share (Note 6). The details of the conversion are as follows:
Original Preferred Stock Series | Number of Shares | Preferred Stock Series Converted to | Number of Shares of Common Stock Received Upon Conversion | |||||||||
Series A | 2,000,000 | Common stock | 2,000,000 | |||||||||
Series B | 11,471,597 | Common stock | 11,471,597 | |||||||||
|
|
|
| |||||||||
Total | 13,471,597 | 13,471,597 | ||||||||||
|
|
|
| |||||||||
Original Warrants for Preferred Stock Series | Number of Warrants | Preferred Stock Warrants Converted to | Number of Common Stock Warrants Received Upon Conversion | |||||||||
Series B | 300,000 | Common stock | 300,000 | |||||||||
Common Stock Shares/ Warrants/Stock Options | Number of Shares Before Reverse Stock Spilt | Reverse Stock Split Ratio | Number of Shares of Common Stock/ Warrants/ Stock Options After Reverse Stock Split | |||||||
Common stock | 14,133,735 | 2/359 | 78,723 | |||||||
Common stock warrants | 325,000 | 2/359 | 1,810 | |||||||
Outstanding stock options | 1,132,877 | 2/359 | 6,456 | |||||||
All share and per share data referenced throughout the consolidated financial statements have been retroactively adjusted to reflect the 2-for-359 reverse stock split of the Company’s common stock.
The rights, preferences and privileges of the convertible preferred stockholders are:
Dividends
The holders of preferred stock are entitled to receive noncumulative annual dividends at the rate of 8% of the issue price when, and as if declared by the Board of Directors, out of any assets legally available for payment thereof, prior and in preference to any declaration or payment of any dividend on the common stock of the Company. No dividends have been declared or paid since inception.
F-28
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Voting
The holders of each share of preferred stock are entitled to voting rights equal to the number of shares of common stock into which each share of preferred stock could be converted into, at the record date, for a vote or consent of stockholders, except as otherwise required by law.
The holders of Series C-1 convertible preferred stock were entitled to elect three directors. The holders of Series C-2 convertible preferred stock were entitled to elect two directors. In addition, the holders of preferred stock acting as a single class were entitled to elect two directors.
As long as any shares of preferred stock remains outstanding, the Company must obtain approval from holders of at least 66% of the outstanding preferred stock in order to increase or decrease the authorized number of shares of preferred or common stock, increase or decrease the authorized number of members of the Board of Directors, change the Company’s principal line of business, declare dividends, or make any distribution on any shares of common stock or preferred stock, create a subsidiary, acquire or merge with or into another entity or acquire all or substantially all of the assets or any material tangible or intangible assets or interests of another entity, issue any capital stock of the Company (other than pursuant to an equity plan, in an amount no greater than 5,804,254 shares or if approved by the Board of Directors, including a majority of the directors designated by the holders of preferred stock), take any action intended to result in a liquidation event or enter into any agreement regarding a liquidation event, incur indebtedness in excess of $1.0 million (unless approved by the Board of Directors, including a majority of the directors designated by the holders of preferred stock), amend the existing equity plan or approve a new equity incentive plan so as to increase the shares reserved for issuance with all equity incentive plans to more than 5,804,254 shares, authorize or issue a new class or series of stock or any other securities convertible into equity securities on par or senior to the preferred stock, redeem or repurchase any outstanding shares of the Company’s capital stock or rights to acquire capital stock except with respect to stock repurchased upon termination or an employee, officer, director or consultant to which the Company has the option to repurchase such shares at cost upon the occurrence of certain event, enter into or modify any agreement transaction or arrangement with any of its officers, directors, employees or affiliates, except for customary compensation or benefit arrangements as approved by the Board and enter into any voluntary dissolution, liquidation, or winding up of the Company or any reclassification or recapitalization of the outstanding capital stock of the Company.
Further, the Company must obtain approval of at least 77% of the holders of preferred stock in order to amend, restate or waive any provisions of the Certificate of Incorporation or the bylaws of the Company in a manner that alters or changes the designations, powers, preferences or rights of any series of preferred stock in an adverse and disproportionate manner relative to any other series of preferred stock, or that amends or waives the special mandatory conversion provision of the Certificate of Incorporation.
Liquidation Rights
Upon liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary, or any acquisition or asset transfer, before any payment to holders of common stock, the holders of preferred stock, on a pari passu basis, are entitled to be paid out of the assets of the Company legally available for distribution for each share of preferred stock held equal to the original issue price plus all declared and unpaid dividends on the preferred stock. If upon any liquidation, dissolution, or winding up the Company, the available assets to be distributed to preferred stock holders are insufficient to make payment in full, then all of the assets will be distributed ratably among the holders of preferred stock in proportion to the full amounts to which they would
F-29
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
otherwise be respectively entitled. These liquidation features cause the Company’s convertible preferred stock to be classified as mezzanine capital rather than as a component of stockholders’ deficit.
Amounts available for distribution in excess of the liquidation preference amounts will be distributed to the holders of the preferred stock and common stock pro rata based on the number of shares held by each, assuming conversion of all such preferred stock into common stock, until holders of preferred stock have received an amount three times (including liquidation preference) their respective original issue price, subject to adjustments, plus all declared and unpaid dividends. Thereafter, the remaining assets available for distribution will be distributed to the holders of common stock pro rata based on the number of shares held by each.
Conversion
Each share of preferred stock, at the option of the holder, is convertible into the number of fully paid and nonassessable shares of common stock, which results from multiplying the conversion rate then in effect by the number of shares being converted.
Each share of preferred stock is automatically converted into shares of common stock at its then effective conversion rate at any time upon the affirmative election of the holders of at least 66% of the outstanding shares of preferred stock voting as a single class on an as-converted to common stock basis, or immediately prior to the closing of a firm commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended covering the offer and sale of common stock to the public at a price per share of not less than three times the original issue price of the Series C-2 preferred stock (as adjusted for any stock splits, dividends, recapitalizations and the like after the filing date thereof) and with aggregate gross cash proceeds of not less than $35.0 million. The conversion ratio for Series C-1 and Series C-2 preferred stock was 1-to-1 as of December 31, 2011 and is subject to adjustment for dilutive issuances.
Redemption
The preferred stock is not redeemable.
June 2009 Preferred Stock Liability
In June 2009, the Company entered into a tranched Series C-2 convertible preferred stock transaction. In connection with the initial closing in June 2009, the Company agreed to issue to the purchasers and the purchasers agreed to purchase from the Company an aggregate of 14,196,525 shares of Series C-2 convertible preferred stock at a purchase price of $1.58 per share in a subsequent sale of Series C-2 preferred stock. The Company determined that the liability to issue Series C-2 convertible preferred stock shares at a future date was a freestanding instrument and should be accounted as a liability under ASC 480.The fair value of this freestanding instrument was determined using the Black-Scholes option pricing model on the date of the issuance of the first tranche and was recorded as a liability in the amount of $1.4 million. The Company used the following assumptions: expected life of 1 year, risk-free interest rate of 0.56% and expected volatility of 45%.
At December 31, 2009, the fair value of the freestanding instrument was remeasured at $0.7 million using the following assumptions: expected life of 0.5 years, risk-free interest rate of 0.20% and expected volatility of 45%. As a result, the Company recorded $0.6 million to other income (expense), net in its consolidated statement of operations.
F-30
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
April 2010 Preferred Stock Liability
In April 2010, upon the issuance of the second tranche of the Series C-2 convertible preferred stock, the fair value of the freestanding instrument was re-measured using the following assumptions: expected life of 0.25 years, risk-free interest rate of 0.16% and expected volatility of 45%. Accordingly, in 2010, the Company recorded $1.1 million to other income (expense), net in its consolidated statement of operations. The fair value of preferred stock liability in the amount of $0.4 million was offset against the proceeds received from the issuance of preferred stock on the date of issuance of the second tranche of Series C-2 convertible preferred stock.
| 10. | Common Stock |
As of December 31, 2011, the Company’s Certificate of Incorporation, as amended, authorized the Company to issue up to 146,000,000 shares, 80,000,000 shares of which are common stock at $0.0001 par value, and 66,000,000 shares of which are preferred stock at $0.0001 par value. Common stockholders are entitled to dividends when and if declared by the Board of Directors subject to prior rights of the preferred stockholders. The holder of each share of common stock is entitled to one vote.
At December 31, 2011, the Company had reserved common stock for future issuances as follows:
Conversion of convertible preferred stock | 40,045,749 | |||
Issuance of options under stock plan | 631,904 | |||
Issuance upon exercise of options under stock plan | 7,790,737 | |||
Issuance upon exercise of October 2007 and April 2008 common stock warrants | 1,810 | |||
|
| |||
Total | 48,470,200 | |||
|
|
At March 31, 2012 (unaudited), the Company had reserved common stock for future issuances as follows:
Conversion of convertible preferred stock | 40,045,749 | |||
Issuance of options under stock plan | 631,904 | |||
Issuance upon exercise of options under stock plan | 7,718,537 | |||
Issuance upon exercise of October 2007 and April 2008 common stock warrants | 1,810 | |||
|
| |||
Total | 48,398,000 | |||
|
|
The above table does not include potential issuance of common stock related to the 2011 convertible notes or the common stock warrants and preferred stock warrants.
Restricted Stock
During 2006, the Company issued to its founders 2,645 shares of restricted common stock subject to repurchase. There were no shares subject to repurchase as of December 31, 2010 and 2011.
| 11. | Stock Option Plan |
In December 2006, the Company adopted the 2006 Equity Incentive Plan (the “2006 Plan”), under which 2,785 shares of the Company’s common stock had been originally reserved for issuance to employees, directors
F-31
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
and consultants. The 2006 Plan provides for the grant of the following stock awards: incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards and stock appreciation rights. Incentive stock options may be granted only to Company employees, which include officers and directors of the Company. Nonstatutory stock options and stock purchase rights may be granted to employees and consultants. Stock awards other than incentive stock options may be granted to employees, directors and consultants.
The Board of Directors has the authority to determine to whom options will be granted, the number of options, the term and the exercise price. The exercise price of the stock option shall not be less than 100% of the fair market value of the common stock subject to the option on the date the option is granted. For individuals holding more than 10% of the voting rights of all classes of stock, the exercise price of an option will not be less than 110% of fair market value and the option is not exercisable after the expiration of five years from the date of the grant. Options granted to an employee who is not an officer, director or consultant shall provide for vesting of the total number of shares of common stock at a rate of at least 20% per year over five years from the date the option was granted, subject to reasonable conditions such as continued employment, at any time or during any period established by the Company. The contractual term of each option is ten years.
As of December 31, 2010 and 2011, and March 31, 2012 (unaudited), the Company had reserved 5,990,809, 8,823,187 and 8,823,187 shares of common stock, respectively, for issuance under the 2006 Plan.
Activity under the Plan is as follows:
| Number of Shares Available for Grant | Outstanding Options | |||||||||||
| Number of Shares Underlying Outstanding Options | Weighted-Average Exercise Price Per Share | |||||||||||
Shares reserved at inception of the Plan | 2,785 | — | $ | — | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2006 | 2,785 | — | — | |||||||||
Additional shares authorized | 20,404 | — | — | |||||||||
Options granted | (1,253 | ) | 1,253 | 17.95 | ||||||||
Options exercised | — | (918 | ) | 17.95 | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2007 | 21,936 | 335 | 17.95 | |||||||||
Options granted | (19,177 | ) | 19,177 | 53.85 | ||||||||
Options exercised | — | (433 | ) | 35.90 | ||||||||
Options cancelled | 12,623 | (12,623 | ) | 53.85 | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2008 | 15,382 | 6,456 | 53.19 | |||||||||
Additional shares authorized | 5,832,170 | — | — | |||||||||
Options granted | (5,441,950 | ) | 5,441,950 | 0.21 | ||||||||
Options exercised | — | (201,904 | ) | 0.21 | ||||||||
Options cancelled | 155 | (155 | ) | 53.85 | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2009 | 405,757 | 5,246,347 | 0.27 | |||||||||
Additional shares authorized | 135,450 | — | — | |||||||||
Options granted | (540,000 | ) | 540,000 | 0.21 | ||||||||
Options exercised | — | (197,291 | ) | 0.21 | ||||||||
Options cancelled | 153,237 | (153,237 | ) | 0.39 | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2010 | 154,444 | 5,435,819 | 0.27 | |||||||||
Additional shares authorized | 2,832,378 | — | — | |||||||||
Options granted | (2,484,477 | ) | 2,484,477 | 0.67 | ||||||||
Options cancelled | 129,559 | (129,559 | ) | 0.46 | ||||||||
|
|
|
| |||||||||
Balances at December 31, 2011 | 631,904 | 7,790,737 | 0.39 | |||||||||
Options exercised (unaudited) | — | (72,200 | ) | 0.67 | ||||||||
|
|
|
| |||||||||
Balances at March 31, 2012 (unaudited) | 631,904 | 7,718,537 | $ | 0.39 | ||||||||
|
|
|
| |||||||||
F-32
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
The aggregate intrinsic value of options exercised under the Plan was $0 for the year ended December 31, 2009 and $26,000 for the year ended December 31, 2010. There were no options exercised during 2011. The aggregate intrinsic value of options exercised for the three months ended March 31, 2012 (unaudited) was $38,000. Intrinsic value is calculated as the difference between the exercise price of the underlying options and the fair value of the common stock for the options that had exercise prices that were lower than the fair value per share of the common stock on the date of exercise.
At December 31, 2010, there were 5,435,819 options outstanding of which 3,615,200 were exercisable at a weighted-average exercise price of $0.27 per share.
Aggregate options outstanding and vested and exercisable by exercise price at December 31, 2011 are as follows (dollars in thousands except per share values):
Options Outstanding | Options Vested and Exercisable | |||||||||||||||||||||||
Exercise | Number Outstanding | Aggregate Intrinsic Value | Weighted Average Remaining Life (in Years) | Number Outstanding | Aggregate Intrinsic Value | Exercise Price | ||||||||||||||||||
$ 0.21 | 5,370,046 | $ | 3,329 | 7.9 | 4,878,021 | $ | 3,024 | $ | 0.21 | |||||||||||||||
$ 0.67 | 2,414,918 | 386 | 9.3 | 344,570 | 55 | $ | 0.67 | |||||||||||||||||
$53.85 | 5,773 | — | 5.8 | 5,533 | — | $ | 53.75 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
| 7,790,737 | $ | 3,715 | 8.3 | 5,228,124 | $ | 3,079 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
The intrinsic values of outstanding, vested and exercisable options were determined by multiplying the number of shares by the difference in exercise price of the options and the fair value of the common stock as of December 31, 2011 of $0.83 per share.
Aggregate options outstanding and vested and exercisable by exercise price at March 31, 2012 (unaudited) are as follows (dollars in thousands except per share values):
Options Outstanding | Options Vested and Exercisable | |||||||||||||||||||||||
Exercise Price | Number Outstanding | Aggregate Intrinsic Value | Weighted Average Remaining Life (in Years) | Number Outstanding | Aggregate Intrinsic Value | Exercise Price | ||||||||||||||||||
$ 0.21 | 5,370,046 | $ | 5,316 | 7.6 | 5,020,908 | $ | 4,971 | $ | 0.21 | |||||||||||||||
$ 0.67 | 2,342,718 | 1,242 | 9.0 | 479,679 | 254 | $ | 0.67 | |||||||||||||||||
$53.85 | 5,773 | — | 5.6 | 5,661 | — | $ | 53.75 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
| 7,718,537 | $ | 6,558 | 8.0 | 5,506,248 | $ | 5,225 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||||||
The intrinsic values of outstanding, vested and exercisable options were determined by multiplying the number of shares by the difference in exercise price of the options and the fair value of the common stock as of March 31, 2012 of $1.20 per share.
Stock-Based Compensation Associated with Awards to Employees
During the years ended December 31, 2009, 2010 and 2011, the Company granted stock options to employees to purchase 5,441,950, 540,000 and 2,484,477 shares of common stock, respectively, under the 2006
F-33
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Plan with a weighted-average grant date fair value of $0.11, $0.23 and $0.39 per share, respectively. Stock-based compensation expense recognized during the years ended December 31, 2009, 2010, 2011 and for the period from November 1, 2006 (date of inception) to December 31, 2011, includes compensation expense for stock-based awards granted to employees based on the grant date fair value estimated in accordance with the provisions of ASC 718 of $0.3 million, $0.2 million, $0.3 million and $1.0 million, respectively. As of December 31, 2011, there was a total unrecognized compensation cost of $0.8 million related to unvested stock-based awards granted under the 2006 Plan. These amounts are expected to be recognized over a period of approximately 2.87 years.
The Company did not grant stock options to employees to purchase shares of common stock for the three months ended March 31, 2011 and 2012. Stock-based compensation expense recognized for the three months ended March 31, 2011 and 2012 (unaudited) and for the period from November 1, 2006 (date of inception) to March 31, 2012 (unaudited) was $47,000, $78,000 and $1.0 million, respectively. As of March 31, 2012 (unaudited), there was a total unrecognized compensation cost of $0.7 million related to unvested stock-based award granted under the 2006 Plan. These amounts are expected to be recognized over a period of approximately 2.73 years.
The Company estimates the fair value of stock options using the Black-Scholes option valuation model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards. The fair value of the employee stock options was estimated using the following weighted-average assumptions:
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
Expected volatility | 56 | % | 60 | % | 62 | % | ||||||
Risk-free interest rate | 3.41 | % | 2.34 | % | 2.48 | % | ||||||
Dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||||||
Expected term (in years) | 5.62 | 5.86 | 6.01 | |||||||||
Determining Fair Value of Stock Options
The fair value of each grant of stock options was determined by the Company using the methods and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Expected Term — The expected term of stock options represents the weighted average period the stock options are expected to be outstanding. For option grants that are considered to be “plain vanilla”, the Company has opted to use the simplified method for estimating the expected term as provided by the Securities and Exchange Commission. The simplified method calculates the expected term as the average time-to-vesting and the contractual life of the options. For other option grants, the expected term is derived from the Company’s historical data on employee exercises and post-vesting employment termination behavior taking into account the contractual life of the award.
Expected Volatility — The expected stock price volatility assumption was determined by examining the historical volatilities of a group of industry peers, as the Company did not have any trading history for the Company’s common stock. The Company will continue to analyze the historical stock price volatility and expected term assumptions as more historical data for the Company’s common stock becomes available
Risk-Free Interest Rate — The risk free rate assumption is based on the U.S. Treasury instruments the terms of which were consistent with the expected term of the Company’s stock options.
F-34
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
Expected Dividend — The expected dividend assumption is based on the Company’s history and expectation of dividend payouts.
Forfeiture Rate — ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience.
Fair Value of Common Stock — The fair value of the shares of common stock underlying the stock options has historically been the responsibility of and determined by the Company’s board of directors. Because there has been no public market for the Company’s common stock, the board of directors determined fair value of common stock at the time of grant of the option by considering a number of objective and subjective factors including independent third-party valuations of the Company’s common stock, sales of convertible preferred stock to unrelated third parties, operating and financial performance, the lack of liquidity of capital stock and general and industry specific economic outlook, amongst other factors. The fair value of the underlying common stock will be determined by the Company’s board of directors until such time as the Company’s common stock is listed on an established exchange or national market system.
Total stock-based compensation expense related to options granted to employees was allocated as follows (in thousands):
| Year Ended December 31, | Three Months Ended March 31, | Cumulative Period from November 1, 2006 (Date of Inception) to March 31, | ||||||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | 2012 | |||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
Research and development | $ | 84 | $ | 61 | $ | 137 | $ | 16 | $ | 37 | $ | 367 | ||||||||||||
General and administrative | 211 | 110 | 182 | 28 | 34 | 603 | ||||||||||||||||||
Sales and marketing | 18 | 14 | 26 | 3 | 7 | 75 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total | $ | 313 | $ | 185 | $ | 345 | $ | 47 | $ | 78 | $ | 1,045 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Allocations to research and development, general and administrative and sales, and marketing expense are based upon the department to which the associated employee reported. No related tax benefits of the stock-based compensation expense have been recognized.
| 12. | Reduction in Force |
In June 2008, the Company announced an initiative to reduce employee headcount from 37 to 11 employees. This reduction represented approximately 70% of the total workforce and was completed in June 2008. The total cash payments and expenses incurred in connection with this reduction in workforce was approximately $1.6 million of which $0.4 million was included in research and development, $1.0 million was included in general and administrative, and $0.2 million was included in selling and marketing expenses in the consolidated statement of operations. All amounts were paid during the year ended December 31, 2008.
F-35
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 13. | Income Taxes |
Due to the ongoing operating losses and the inability to recognize any income tax benefit, there is no provision for income taxes in any period presented in these consolidated financial statements. Since inception, the Company has only generated pretax losses in the U.S. and has not generated any pretax income or loss outside the U.S.
The reconciliation of income tax expense (benefit) computed at the statutory federal income tax rate of 34% to amounts included in the consolidated statements of operations is as follows:
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
Statutory rate | 34.0 | % | 34.0 | % | 34.0 | % | ||||||
State tax | 4.9 | % | 6.5 | % | 5.4 | % | ||||||
Tax credits | 0.1 | % | 2.5 | % | 24.8 | % | ||||||
Stock options | (0.7) | % | (0.2) | % | (0.3) | % | ||||||
Valuation allowance | (40.6) | % | (44.3) | % | (60.1) | % | ||||||
Other | 2.3 | % | 1.5 | % | (3.8) | % | ||||||
|
|
|
|
|
| |||||||
| 0.0 | % | 0.0 | % | (0.0) | % | |||||||
|
|
|
|
|
| |||||||
Deferred tax assets and liabilities reflect the net tax effects of net operating loss and tax credit carryovers and the temporary differences between the carrying amounts of assets and liabilities for financial reporting and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2010 | 2011 | |||||||
Deferred tax assets | ||||||||
Fixed assets, capitalized intangibles and other assets | $ | 3,478 | $ | 3,213 | ||||
Net operating loss | 27,503 | 31,011 | ||||||
Tax credits | 1,714 | 15,858 | ||||||
Other | 544 | 845 | ||||||
|
|
|
| |||||
Total | 33,239 | 50,927 | ||||||
Valuation allowance | (33,239 | ) | (50,927 | ) | ||||
|
|
|
| |||||
Net deferred tax assets | $ | — | $ | — | ||||
|
|
|
| |||||
Due to the Company’s lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance at December 31, 2010 and 2011. The increase in the valuation allowance on the deferred tax assets was $5.5 million, $11.3 million and $17.7 million for December 31, 2009, 2010 and 2011, respectively.
At December 31, 2011, the Company had net operating loss carryforwards of approximately $75.0 million and $95.0 million available to reduce future taxable income, if any, for both federal and California state income tax purposes, respectively. The net operating loss carryforwards will begin to expire in 2026 for federal and 2016 for state purposes and valuation allowances have been reserved, where necessary. If the Company experiences an “ownership change” for purposes Section 382 of the Internal Revenue Code of 1986, as amended, it may be
F-36
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
subject to annual limits on its ability to utilize net operating loss carryforwards. An ownership change is, as a general matter, triggered by sales or acquisitions of our stock in excess of 50% on a cumulative basis during a three-year period by persons owning 5% or more of our total equity value. The Company is not currently subject to any annual limits on its ability to utilize net operating loss carryforwards. The Company’s deferred tax assets have been fully offset by a valuation allowance as of December 31, 2011. The Company also had federal and state research and development credit carryforwards of approximately $15.4 million and $0.6 million respectively, at December 31, 2011. The federal credits will expire starting in 2027, if not utilized. The California credits have no expiration date.
The Company was granted orphan drug designation in 2009 by the FDA for its products currently under development. The orphan drug designation allows the Company to claim increased federal tax credits for its research and development activities. During 2011, the Company made claims for 2009 and 2010 Orphan Drug Credits, resulting in additional federal credits of approximately $8.5 million. The future tax benefits of such claims have been included in deferred taxes. The Company will also claim the Orphan Drug Credit with its 2011 tax return.
A reconciliation of the beginning and ending balances of the unrecognized tax benefits during the years ended December 31, 2009, 2010 and 2011 is as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2009 | 2010 | 2011 | ||||||||||
Balance at the beginning of the year | $ | 212 | $ | 355 | $ | 625 | ||||||
Additions based on prior period tax positions | — | — | 2,839 | |||||||||
Additions based on current period tax positions | 143 | 270 | 1,893 | |||||||||
|
|
|
|
|
| |||||||
Balance at the end of the year | $ | 355 | $ | 625 | $ | 5,357 | ||||||
|
|
|
|
|
| |||||||
The entire amount of the unrecognized tax benefits would not impact the Company’s effective tax rate if recognized.
There was no interest or penalties accrued at January 1, 2009 and December 31, 2011. The Company’s policy is to recognize any related interest or penalties in income tax expense. The material jurisdiction in which the Company is subject to potential examination by tax authorities for tax years ended 2006 through the current period include the U.S. and California. The Company is not currently under income tax examinations by any tax authorities.
| 14. | Defined Contribution Plan |
The Company sponsors a defined contribution plan under Section 401(k) of the Internal Revenue Code covering substantially all full-time U.S. employees. Employee contributions are voluntary and are determined on an individual basis subject to the maximum allowable under federal tax regulations. Participants are always fully vested in their contributions. The Company has not made any employer contributions.
F-37
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
| 15. | Net loss per share and Unaudited Pro Forma per Share of Common Stock |
The following table sets forth the computation of basic and diluted net loss per share of common stock and unaudited pro forma basic and diluted net loss per share of common stock for the periods indicated (in thousands except per share amounts):
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
Historical net loss per share | ||||||||||||||||||||
Numerator: | ||||||||||||||||||||
Net loss | $ | (13,600 | ) | $ | (25,453 | ) | $ | (29,416 | ) | $ | (5,907 | ) | $ | (11,886 | ) | |||||
Accretion of Series B redeemable convertible preferred stock | (78 | ) | — | — | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss attributable to common stockholders | $ | (13,678 | ) | $ | (25,453 | ) | $ | (29,416 | ) | $ | (5,907 | ) | $ | (11,886 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Denominator: | ||||||||||||||||||||
Weighted-average number of common shares used in calculating net loss per share — basic and diluted outstanding | 897,239 | 2,512,320 | 2,858,251 | 2,858,251 | 2,858,251 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss per share attributable to common stockholders — basic and diluted | $ | (15.24 | ) | $ | (10.13 | ) | $ | (10.29 | ) | $ | (2.07 | ) | $ | (4.16 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Pro Forma net loss per share | ||||||||||||||||||||
Numerator for pro forma calculation: | ||||||||||||||||||||
Net loss attributable to common stockholders | $ | (29,416 | ) | $ | (11,886 | ) | ||||||||||||||
Add: | ||||||||||||||||||||
Interest expense on convertible notes payable | ||||||||||||||||||||
Amortization of debt discount on debt | ||||||||||||||||||||
Amortization of debt issuance costs | ||||||||||||||||||||
Changes in fair value of call options liability | ||||||||||||||||||||
|
|
|
| |||||||||||||||||
Net loss attributable to common stockholders for pro forma calculation | $ | $ | ||||||||||||||||||
|
|
|
| |||||||||||||||||
Denominator for pro forma calculation: | ||||||||||||||||||||
Weighted-average number of common shares used to compute basic and diluted net loss per share | ||||||||||||||||||||
Pro forma adjustments to reflect assumed conversion of convertible notes payable (unaudited) | ||||||||||||||||||||
Pro forma adjustments to reflect assumed conversion of convertible preferred stock (unaudited) | ||||||||||||||||||||
|
|
|
| |||||||||||||||||
Weighted-average common shares outstanding used to compute pro forma basic and diluted net loss per share (unaudited) | ||||||||||||||||||||
|
|
|
| |||||||||||||||||
Pro forma basic and diluted net loss per share (unaudited) | $ | $ | ||||||||||||||||||
|
|
|
| |||||||||||||||||
F-38
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
As the Company incurred net losses for all of the periods presented, the following outstanding potentially dilutive securities were excluded from the computation of diluted net loss per share, as the effect of including them would have been antidilutive:
| As of December 31, | As of March 31, | |||||||||||||||||||
| 2009 | 2010 | 2011 | 2011 | 2012 | ||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||
Convertible preferred stock | 25,849,224 | 40,045,749 | 40,045,749 | 40,045,749 | 40,045,749 | |||||||||||||||
Stock options | 5,246,357 | 5,435,819 | 7,790,737 | 5,435,819 | 7,718,537 | |||||||||||||||
October 2007 and April 2008 common stock warrants | 1,810 | 1,810 | 1,810 | 1,810 | 1,810 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | 31,097,391 | 45,483,378 | 47,838,296 | 45,483,378 | 47,766,096 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| 16. | Subsequent Events |
On February 8, 2012, the Company received the proceeds associated with the additional financing related to the October 2011 convertible notes in the aggregate amount of $7.5 million.
On March 22, 2012, the Company entered into the purchase agreement with Ucyclyd under which the Company purchased the worldwide rights to Ravicti and the restated collaboration agreement under which Ucyclyd granted the Company an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL at a fixed price at a future defined date, plus subsequent milestone and royalty payments, subject to Ucyclyd’s right to retain AMMONUL for a predefined price. The restated collaboration agreement superseded the collaboration agreement with Ucyclyd, dated August 23, 2007, as amended.
Under the purchase agreement, the Company purchased all of the worldwide rights to Ravicti for an initial up-front payment of $6.0 million. The Company will also pay tiered mid to high single digit royalties on global net sales of Ravicti and may owe regulatory milestones of up to $15.8 million related to approval of Ravicti in HE, regulatory milestones of up to $7.3 million per indication for approval of Ravicti in indications other than UCD or HE, and net sales milestones of up to $38.8 million if Ravicti is approved for use in indications other than UCD (such as HE) and all annual sales targets are reached. In addition, the intellectual property license agreements executed between Ucyclyd and Summar, and Ucyclyd and Brusilow, were assigned to the Company, and the Company has assumed the royalty and milestone obligations under the Brusilow agreement for sales of Ravicti in any indication and the royalty obligations under the Summar agreement on sales of Ravicti to treat HE. The Company will also pay Brusilow an annual license extension fee to keep the Brusilow license in effect, which extension fee is payable until the Company’s first commercial sale of Ravicti following FDA approval. The Brusilow and Summar agreements provide that royalty obligations will continue, without adjustment, even if generic versions of the licensed products are introduced and sold in the relevant country.
Under the terms of the restated collaboration agreement, the Company has an option to purchase all of Ucyclyd’s worldwide rights in BUPHENYL and AMMONUL, subject to Ucyclyd’s option to retain rights to AMMONUL. The Company will be permitted to exercise this option for 90 days beginning on the earlier of the date of the approval of Ravicti for the treatment of UCD and June 30, 2013, but in no event earlier than January 1, 2013. The upfront purchase price for AMMONUL and BUPHENYL is $22.0 million, which the Company may fund by drawing on a loan commitment from Ucyclyd. The loan would be payable in eight quarterly payments and would bear interest at a rate of 9% per year, and would be secured by the BUPHENYL
F-39
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
and AMMONUL assets. If the Ravicti NDA for UCD is not approved by January 1, 2013, then Ucyclyd is obligated to make monthly payments of $0.5 million to the Company until the earliest of (1) FDA approval of the Ravicti NDA for UCD, (2) June 30, 2013 and (3) the Company’s written notification of the decision not to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL.
If the Company exercises its option to purchase Ucyclyd’s worldwide rights to BUPHENYL and AMMONUL, then Ucyclyd has the time-limited right to elect to retain all rights to AMMONUL for a purchase price of $32.0 million. If Ucyclyd exercises this option, Ucyclyd will pay the Company a net payment of $13.0 million on closing of the purchase transaction, which reflects the purchase price for Ucyclyd’s worldwide rights to BUPHENYL being set-off against Ucyclyd’s retention payment for AMMONUL. If Ucyclyd retains rights to AMMONUL, subject to certain terms and conditions, the Company retains a right of first negotiation should Ucyclyd later decide to sell, exclusively license, or otherwise transfer the AMMONUL assets to a third party.
On April 6, 2012, the board of directors of the Company adopted, subject to the approval of the Company’s stockholders, the 2012 Omnibus Incentive Plan (the “2012 Plan”) and the Amended and Restated Certificate of Incorporation. The 2012 Plan provides for the grant of stock options, stock appreciation rights, restricted stock, unrestricted stock, stock units, dividend equivalent rights, other equity-based awards and cash bonus awards.
For the issuance of the annual consolidated financial statements as of December 31, 2011 and the year then ended, the Company has evaluated subsequent events through April 13, 2012, the date the consolidated financial statements were issued.
| 17. | Subsequent Events (unaudited) |
On April 16, 2012, the board of directors of the Company amended the 2006 Plan to increase the number of shares available for grant by 2,760,950 shares and also approved the grant of 2,760,950 stock options under the Plan at an exercise price of $1.20.
In April 2012, the Company entered into a $10.0 million loan and security agreement (the “Loan Agreement”) with Silicon Valley Bank and Leader Lending, LLC - Series B (the “Lenders”). The loan carries an interest rate of 8.88%, with interest only payments for the period of 9 months from May 1, 2012. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from February 1, 2013. In connection with the Loan Agreement, the Company granted a security interest in all of its assets, except intellectual property. The Company’s obligations to the Lenders include restrictions on borrowing, asset transfers, placing liens or security interest on its assets including the Company’s intellectual property, mergers and acquisitions and distributions to stockholders. The Loan Agreement also requires the Company to provide the Lenders monthly financials and compliance certificate within 30 days of each month end, annual audited financials within 180 days of each fiscal year-end and annual approved financial projections. The Company issued warrants to the Lenders to purchase a total of 462,686 shares of common stock with an exercise price of $0.67 per share. The Loan Agreement requires immediate repayment of amounts outstanding upon an event of default, as defined in the Loan Agreement, which includes events such as a payment default, a covenant default or the occurrence of a material adverse change, as defined in the Loan Agreement.
Pursuant to the terms of the Loan Agreement, once the Company raises at least $30.0 million from the sale of equity securities or subordinated debt, the Lenders also agreed to grant the Company a one-time single loan in the amount of $2.5 million (the “Bank Term Loan”). The Lender’s obligation to lend terminates on the earlier of
F-40
Table of Contents
Hyperion Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
(i) an event of default or (ii) September 30, 2012. The principal amount outstanding for the Bank Term Loan accrues interest at a per annum rate equal to the greater of (i) 8.88% and (ii) the Treasury Rate, as defined in the Loan Agreement, on the date the loan is funded plus 8.50%, with interest only payments for the period of 9 months from the date the loan is funded. The loan is then payable in equal monthly principal payments plus interest over a period of 27 months from the date the loan is funded.
For the issuance of the unaudited interim consolidated financial statements as of March 31, 2012, and the three months then ended, the Company has evaluated subsequent events through May 24, 2012, the date the unaudited interim consolidated financial statements were issued.
F-41
Table of Contents
Shares

Hyperion Therapeutics, Inc.
Common Stock
PROSPECTUS
Leerink Swann Cowen and Company
Needham & Company
, 2012
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the fees and expenses, other than underwriting discounts and commissions, payable in connection with the registration of the common stock hereunder. All amounts are estimates except the SEC registration fee, the FINRA filing fee and The NASDAQ Global Market listing fee.
| Amount | ||||
SEC registration fee | $ | 6,590 | ||
FINRA filing fee | 6,250 | |||
The NASDAQ Global Market listing fee | 125,000 | |||
Accountants’ fees and expenses | * | |||
Legal fees and expenses | * | |||
Blue Sky fees and expenses | * | |||
Transfer Agent’s fees and expenses | * | |||
Printing and engraving expenses | * | |||
Miscellaneous | * | |||
|
| |||
Total | $ | * | ||
|
| |||
| * | To be filed by amendment. |
Item 14. Indemnification of Directors and Officers.
Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, provides that a Delaware corporation, in its certificate of incorporation, may limit the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| • | Transaction from which the director derived an improper personal benefit; |
| • | Act or omission not in good faith or that involved intentional misconduct or a knowing violation of law; |
| • | Unlawful payment of dividends or redemption of shares; or |
| • | Breach of the director’s duty of loyalty to the corporation or its stockholders. |
Section 145(a) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) because that person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise. The indemnity may include against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, so long as the person acted in good faith and in a manner he or she reasonably believed was in or not opposed to the corporation’s best interests, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the DGCL provides, in general, that a Delaware corporation may indemnify any person who was or is a party, or is threatened to be made a party, to any threatened, pending or completed action or suit by or in the right of the corporation to obtain a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director,
II-1
Table of Contents
officer, employee or agent of another corporation or other enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action, so long as the person acted in good faith and in a manner the person reasonably believed was in or not opposed to the corporation’s best interests, except that no indemnification shall be permitted without judicial approval if a court has determined that the person is to be liable to the corporation with respect to such claim. Section 145(c) of the DGCL provides that if a present or former director or officer has been successful in defense of any action referred to in Sections 145(a) and (b) of the DGCL, the corporation must indemnify such officer or director against the expenses (including attorneys’ fees) he or she actually and reasonably incurred in connection with such action.
Section 145(g) of the DGCL provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or other enterprise against any liability asserted against and incurred by such person, in any such capacity, or arising out of his or her status as such, whether or not the corporation could indemnify the person against such liability under Section 145 of the DGCL.
Our restated certificate of incorporation and our bylaws, each of which will become effective upon the closing of this offering, each provide for the indemnification of our directors and officers to the fullest extent permitted under the DGCL.
We have entered into indemnification agreements with our directors and executive officers. These indemnification agreements may require us, among other things, to indemnify each such director and executive officer for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by him in any action or proceeding arising out of his service as one of our directors or executive officers.
We intend to purchase and maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
We have entered into an underwriting agreement, which provides for indemnification by the underwriters of us, our officers and directors, for certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, or the Securities Act.
Item 15.Recent Sales of Unregistered Securities.
The following list sets forth information regarding all securities sold by us since April 1, 2009:
Issuances of Capital Stock
(1) During June, July and August 2009 and April 2010, we issued and sold in a series of closings to 17 accredited investors, an aggregate of 11,647,769 shares of our Series C-1 convertible preferred stock in exchange for convertible debt and accrued interest at a price per share of $1.33 and 28,397,980 shares of our Series C-2 convertible preferred stock in exchange for cash at a price per share of $1.58, for gross proceeds of $60.4 million. Each share of Series C-1 and Series C-2 convertible preferred stock will convert into one share of our common stock upon completion of this offering.
(2) In June 2009, 11,471,597 shares of Series B convertible preferred stock were converted into shares of our common stock. After the conversion into common stock, all of the shares of common stock were recapitalized in a reverse stock split whereby the holders of common stock received two shares of common stock for every 359 shares owned.
II-2
Table of Contents
(3) In June 2009, 2,000,000 shares of Series A convertible preferred stock were converted into shares of our common stock. After the conversion into common stock, all of the shares of common stock were recapitalized in a reverse stock split whereby the holders of common stock received two shares of common stock for every 359 shares owned.
(4) Between June 2009 and April 2010, we issued Ucyclyd Pharma, Inc. an aggregate of 2,380,333 shares of our common stock as consideration for the restructuring of the royalty and milestone payments under our amended collaboration agreement.
Convertible Note Financings and Warrants
(5) Between April 2009 and June 2009, we issued convertible promissory notes to nine accredited investors for an aggregate principal amount of $3.2 million. On June 29, 2009, the notes were exchanged for shares of our Series C-1 convertible preferred stock.
(6) Between April 2011 and May 2011, in connection with a bridge loan financing, we issued convertible promissory notes to 14 accredited investors for an aggregate principal amount of $17.5 million. The convertible promissory notes accrue interest at a rate equal to 6% per year, and have a maturity date of December 31, 2012, unless converted prior thereto. Upon completion of this offering, these convertible promissory notes will convert into shares of our common stock at a conversion price equal to the initial public offering price.
(7) Between April 2011 and May 2011, in connection with a bridge loan financing, we granted warrants to purchase approximately 3,318,704 shares of our common stock to 14 accredited investors at an exercise price of $0.67 per share. The warrants will automatically net exercise and terminate immediately prior to consummation of our initial public offering.
(8) Between October 2011 and February 2012, in connection with a bridge loan financing, we issued convertible promissory notes to 14 accredited investors for an aggregate principal amount of $15.0 million. The convertible promissory notes accrue interest at a rate equal to 6% per year, and have a maturity date of December 31, 2012, unless converted prior thereto. Upon completion of this offering, these convertible promissory notes will convert into shares of our common stock at a conversion price equal to the initial public offering price.
(9) Between October 2011 and February 2012, in connection with a bridge loan financing, we granted warrants to purchase approximately 2,849,440 shares of our common stock upon conversion of Series C-2 convertible preferred stock issuable upon exercise of the warrants to 14 accredited investors at an exercise price of $1.58 per share. The warrants will automatically net exercise and terminate immediately prior to consummation of our initial public offering.
(10) On April 19, 2012, in connection with a loan and security agreement with Silicon Valley Bank and Leader Lending, LLC - Series B, we granted warrants to purchase 231,343 shares of our common stock at an exercise price of $0.67 per share to each of Silicon Valley Bank and Leader Equity, LLC, or the SVB Warrants. The SVB Warrants contain a cashless exercise feature and may be exercised in whole or in part at the option of Silicon Valley Bank or Leader Equity, LLC, respectively, at any time prior to expiration on April 18, 2022.
Stock Option Grants
(11) Between April 1, 2009 and May 23, 2012, we have granted stock options to purchase an aggregate of 11,227,377 shares of our common stock with exercise prices ranging from $0.21 to $1.20 per share, to our employees and directors pursuant our 2006 Plan.
Securities Act Exemptions
We deemed the offers, sales and issuances of the securities described in paragraphs (1) through (10) above to be exempt from registration under the Securities Act, in reliance on Section 4(2) of the Securities Act, including
II-3
Table of Contents
Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options described in paragraph (11), except to the extent described above as exempt pursuant to Section 4(2) of the Securities Act, to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16.Exhibits and Financial Statement Schedules.
(a)Exhibits
The exhibits to the registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
(b) Financial Statements Schedules:
No financial statement schedules are provided, because the information called for is not required or is shown either in the financial statements or the notes thereto.
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) The registrant will provide to the underwriters at the closing as specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(2) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in
II-4
Table of Contents
the form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(3) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco, State of California, on this 24 day of May, 2012.
| Hyperion Therapeutics, Inc. | ||
By | /S/ DONALD J. SANTEL | |
| Donald J. Santel | ||
| Chief Executive Officer and President | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
Signature | Title | Date | ||
/S/ DONALD J. SANTEL Donald J. Santel | Chief Executive Officer, President and Director (Principal Executive Officer) | May 24, 2012 | ||
/S/ JEFFREY S. FARROW Jeffrey S. Farrow | Chief Financial Officer (Principal Financial and Accounting Officer) | May 24, 2012 | ||
/S/ JAMES I. HEALY* James I. Healy, M.D., Ph.D. | Chairman of the Board | May 24, 2012 | ||
/S/ GAURAV AGGARWAL* Gaurav Aggarwal, M.D. | Director | May 24, 2012 | ||
/S/ DAVID W. GRYSKA* David W. Gryska | Director | May 24, 2012 | ||
/S/ BO JESPER HANSEN* Bo Jesper Hansen, M.D., Ph.D. | Director | May 24, 2012 | ||
/S/ ROBERT HOPFNER* Robert Hopfner | Director | May 24, 2012 | ||
/S/ JAKE R. NUNN* Jake R. Nunn | Director | May 24, 2012 | ||
/S/ BIJAN SALEHIZADEH* Bijan Salehizadeh, M.D. | Director | May 24, 2012 | ||
/S/ LOTA S. ZOTH* Lota S. Zoth | Director | May 24, 2012 | ||
| * | Pursuant to Power of Attorney |
By: | /s/ DONALD J. SANTEL | |
| Donald J. Santel | ||
| Attorney-in-Fact | ||
II-6
Table of Contents
EXHIBIT INDEX
| Exhibit No. | Description | |
| 1.1 * | Form of Underwriting Agreement. | |
| 2.1 †^ | Asset Purchase Agreement by and between the Company and Ucyclyd Pharma, Inc. (“Ucyclyd”), a wholly owned subsidiary of Medicis Pharmaceutical Corporation, dated March 22, 2012. | |
| 3.1 # | Amended and Restated Certificate of Incorporation of the Company, currently in effect. | |
| 3.2 | Amended and Restated Certificate of Incorporation of the Company, to be in effect upon the closing of this offering. | |
| 3.3 # | Amended and Restated Bylaws of the Company, currently in effect. | |
| 3.4 | Amended and Restated Bylaws of the Company, to be in effect upon the closing of this offering. | |
| 4.1* | Specimen Common Stock Certificate of the Company. | |
| 4.2* | Warrant issued pursuant to the Loan and Security Agreement by and between the Company and Comerica Bank, dated October 2, 2007. | |
| 4.3 # | Warrant to Purchase Common Stock issued to Keelin Reeds, dated December 14, 2007. | |
| 4.4 # | Form of Warrant to Purchase Common Stock issued pursuant to the Convertible Note and Warrant Purchase Agreement by and among the Company and the purchasers named therein, dated April 1, 2011 (the “April 2011 Purchase Agreement”). | |
| 4.5 # | Form of Warrant to Purchase Preferred Stock issued pursuant to the Convertible Note and Warrant Purchase Agreement by and among the Company and the purchasers named therein, dated October 26, 2011 (the “October 2011 Purchase Agreement”). | |
| 4.6 # | Form of Convertible Unsecured Promissory Note issued pursuant to the April 2011 Purchase Agreement. | |
| 4.7 # | Form of Convertible Unsecured Promissory Note issued pursuant to the October 2011 Purchase Agreement. | |
| 4.8 | Form of Secured Promissory Note issued pursuant to the Loan and Security Agreement by and among the Company, Silicon Valley Bank and the Lenders listed therein, dated April 19, 2012 (the “SVB Loan and Security Agreement”). | |
| 4.9 | Form of Warrant to Purchase Stock issued pursuant to the SVB Loan and Security Agreement. | |
| 5.1* | Opinion of Hogan Lovells US LLP. | |
| 10.1 # | Second Amended and Restated Investor Rights Agreement by and among the Company and the investors named therein, dated June 29, 2009. | |
| 10.2 # | The April 2011 Purchase Agreement. | |
| 10.3 | Restated Omnibus Amendment to Convertible Note and Warrant Purchase Agreement dated April 1, 2011, Convertible Unsecured Promissory Notes dated April 1, 2011, May 2, 2011, May 4, 2011 and May 10, 2011 and Warrants to Purchase Shares of Common Stock dated April 1, 2011, May 2, 2011, May 4, 2011 and May 10, 2011, dated April 6, 2012 by and among the purchasers named therein. | |
| 10.4 # | The October 2011 Purchase Agreement. | |
| 10.5 # | Form of Indemnification Agreement by and between the Company and each of its directors. | |
| 10.6 +# | Employment Agreement by and between the Company and Donald J. Santel, dated April 9, 2012. | |
| 10.7 +# | Offer Letter Agreement by and between the Company and Jeffrey Farrow, dated November 12, 2009. | |
| 10.8 +# | Offer Letter Agreement by and between the Company and Bruce F. Scharschmidt, M.D., dated March 14, 2008. | |
| 10.9 +# | Offer Letter Agreement by and between the Company and Klara A. Dickinson, dated September 7, 2007. | |
| 10.10 +# | Offer Letter Agreement by and between the Company and Christine A. Nash, dated September 7, 2007. | |
Table of Contents
Exhibit | Description | |
| 10.11 +# | Form of Executive Change of Control and Severance Agreement by and among the Company and certain officers. | |
| 10.12 +# | 2006 Equity Incentive Plan, as amended. | |
| 10.13 +# | 2006 Equity Incentive Plan Amendment, dated April 15, 2011. | |
| 10.14 +# | Form of Option Agreement under the 2006 Equity Incentive Plan. | |
| 10.15 + | 2012 Omnibus Incentive Plan. | |
| 10.16 + | Form of Incentive Stock Option Agreement under the 2012 Omnibus Incentive Plan. | |
| 10.17 + | Form of Nonstatutory Option Agreement under the 2012 Omnibus Incentive Plan. | |
| 10.18 # | Office Lease by and between the Company and Gateway Center, LLC, dated September 6, 2007. | |
| 10.19 # | First Amendment to Office Lease by and between the Company and Gateway Center, LLC, dated October 31, 2011. | |
| 10.20 † | Amended and Restated Collaboration Agreement by and between the Company and Ucyclyd, dated March 22, 2012. | |
| 10.21 † | License Agreement by and among Saul Brusilow, M.D. (“Brusilow”), Brusilow Enterprises, Inc. (“Brusilow Enterprises”) and Medicis Pharmaceutical Corporation (“Medicis”) dated April 16, 1999. | |
| 10.22 † | Settlement Agreement and First Amendment to License Agreement by and among Brusilow, Brusilow Enterprises, Medicis and Ucyclyd, dated August 21, 2007. | |
| 10.23 † | Agreement by and between Dr. Marshall L. Summar and Medicis, dated April 1, 2002. | |
| 10.24 | The SVB Loan and Security Agreement. | |
| 21.1 # | Subsidiaries of the Company. | |
| 23.1 | Consent of PricewaterhouseCoopers LLP. | |
| 23.2* | Consent of Hogan Lovells US LLP (included in Exhibit 5.1). | |
| 24.1 # | Power of Attorney (included on signature page to this registration statement). | |
| * | To be filed by subsequent amendment. |
| # | Previously filed. |
| + | Indicates a management contract or compensatory plan. |
| † | Registrant has requested confidential treatment for certain portions of this agreement. This exhibit omits the information subject to this confidentiality request. The omitted portions have been filed separately with the SEC. |
| ^ | Schedules and similar attachments omitted pursuant to Item 601(b)(2) will be furnished to the SEC supplementally upon request. |