Property and equipment are recorded on the basis of cost. For financial statement purposes, property, plant and equipment are depreciated using the straight-line method over their estimated useful lives.
Expenditures for repair and maintenance which do not materially extend the useful lives of property and equipment are charged to operations. When property or equipment is sold or otherwise disposed of, the cost and related accumulated depreciation are removed from the respective accounts with the resulting gain or loss reflected in operations. Management periodically reviews the carrying value of its property and equipment for impairment in accordance with the guidance for impairment of long lived assets.
Accrued expenses consisted of the following as of September 30, 2015 and December 31, 2014:
During the nine months ended September 30, 2014, the Company issued an aggregate of 45,232 shares of its common stock in settlement of outstanding accounts payable and accrued expenses. In connection with the issuance, the Company incurred $105,737 loss in settlement of debt.
During the nine months ended September 30, 2015, the Company issued an aggregate of 20,819 shares of its common stock in settlement of outstanding accounts payable. In connection with the issuance, the Company incurred $86,763 gain in settlement of debt.
During the nine months ended September 30, 2015, the Company incurred a gain of $50,638 in relief of accounts payable.
During the nine months ended September 30, 2015, the Company issued an aggregate of 24,353 shares of its common stock in settlement of accumulative outstanding accounts payable due to Guarantors of the Company of $961,124. In connection with the issuance, the Company incurred a $791,024 gain in settlement of debt.
During the nine months ended September 30, 2015, the Company settled an outstanding subordinated debt, related accrued interest and accounts payable due to the guarantor by issuing a five year, non-interest bearing note payable. (See Note 7). In connection with the note issuance, the Company settled $624,737 of outstanding guarantor fees.
On October 23, 2014, the Company, entered into a common stock purchase agreement (the “Purchase Agreement”) with Magna Equities II, LLC, a New York limited liability company (the “Investor”). The Purchase Agreement provides that, upon the terms and subject to the conditions set forth therein, the Investor is committed to purchase up to $3,000,000 (the “Total Commitment”) worth of the Company’s common stock, $0.001 par value (the “Shares”), over the 24-month term of the Purchase Agreement.
From time to time over the term of the Purchase Agreement, commencing on the trading day immediately following the date on which the initial registration statement was declared effective by the Securities and Exchange Commission (the “Commission”), the Company may provide the Investor with a draw down notice to purchase a specified dollar amount of Shares, with each draw down subject to certain limitations. The Company may not deliver any Draw Down Notice to the Investor if the Initial Purchase Price with respect to the Shares subject to such Draw Down Notice is less than $2.50 as of the date the applicable Draw Down Notice is received by the Investor (the “Draw Down Exercise Date”).
In 2014, the Company paid to the Investor as a commitment fee for entering into the Purchase Agreement equal an aggregate of to 12,000 shares of the Company’s common stock.
During the nine months ended September 30, 2015, the Company issued an aggregate of 87,812 shares of its common stock in exchange for $521,538 under the Purchase Agreement. (See Note 10)
Notes payable were comprised of the following as of September 30, 2015 and December 31, 2014:
On October 25, 2010, the Company entered into a Loan Agreement with Seaside National Bank and Trust for a $980,000 loan at 4.25% per annum interest that was used to refinance the Company’s loan with Bank of America. The obligation is guaranteed by certain shareholders of the Company. The Company renewed the loan with Seaside National Bank and Trust during the first quarter of 2014 to extend the maturity date to December 23, 2015.
At September 30, 2015 and December 31, 2014, the Company has two outstanding notes payable with interest at 8% per annum due at maturity. The two notes, $61,150 and $323,822, are payable in one balloon payment upon the date the Noteholder provides written demand, however the Company is not obligated to make payments until the Northstar (or successor) Loan is paid off.
During the nine months ended September 30, 2015, the Company entered into Securities Purchase Agreements with Asher Enterprises, Inc. (“Asher”) or affiliates, for the sale of 8% convertible notes in aggregate principal amount of $180,000 (the “Asher Notes”). The Company incurred legal fees in the amount of $15,000 which were deducted from the proceeds of the notes.
These embedded derivatives included certain conversion features and reset provision. The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date of Asher Notes and to fair value as of each subsequent reporting date, which at September 30, 2015 was $72,522. At the inception of the Asher Notes, the Company determined the aggregate fair value of $211,575 of the embedded derivatives.
During the nine months ended September 30, 2015, $151,000 of notes plus accrued interest that were outstanding at December 31, 2014, and $114,000 of notes plus accrued interest that were issued during 2015, were converted into shares of the Company’s common stock (see Note 10).
The remaining aggregate Asher Notes unconverted principle balance as of September 30, 2015 was $66,000.
During the nine months ended September 30, 2015, the Company entered into Securities Purchase Agreements with Daniel James Management (“Daniel”) for the sale of 9.5% convertible note in aggregate principal amount of $100,000 (the “Daniel Notes”).
During the nine months ended September 30, 2015, $75,000 of notes plus accrued interest that were outstanding at December 31, 2014, and $25,000 of notes plus accrued interest that were issued during 2015, were converted into shares of the Company’s common stock (see Note 10).
The remaining aggregate Daniel Notes unconverted principle balance as of September 30, 2015 was $75,000.
During the nine months ended September 30, 2015, the Company entered into Securities Purchase Agreements with Fourth Man, LLC. (“Fourth Man”), for the sale of a 9.5% convertible notes in the aggregate principal amount of $100,000 (the “Notes”).
The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date of Fourth Man Notes and to fair value as of each subsequent reporting date which at September 30, 2015 was $108,731. At the inception of the Fourth Man Notes, the Company determined the aggregate fair value of $198,855 of the embedded derivatives.
During the nine months ended September 30, 2015, $75,000 of notes plus accrued interest that were outstanding at December 31, 2014, and $25,000 of notes plus accrued interest that were issued during 2015, were converted into shares of the Company’s common stock (see Note 10).
The remaining aggregate Fourth Man, LLC Notes unconverted principle balance as of September 30, 2015 was $75,000.
On August 6, 2015, the Company amended the securities purchase agreement with Magna amending the maturity date of its convertible notes payable with Magna from August 7, 2015 to November 7, 2015. This amendment also changes to conversion price of the notes from $0.01035 to the lesser of a) $0.006 or b) a 40% discount from the lowest trading price in the five trading days prior to conversion.
The Company has identified the embedded derivatives related to the Magna Notes. These embedded derivatives included certain conversion features and reset provision. The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date of Magna Notes and to fair value as of each subsequent reporting date which at September 30, 2015 was $83,787.
During the nine months ended September 30, 2015, $140,405 of notes that were outstanding at December 31, 2014, plus accrued interest, were converted into shares of the Company’s common stock (see Note 10). The remaining aggregate Magna notes unconverted principle balance as of September 30, 2015 was $64,595.
At December 31, 2014, a related party to one of the Company’s former officers provided notes in aggregate of $1,500,000. As of December 31, 2014, these notes were not considered as related party. The notes range from 4.75% to 8% per annum and are due upon payoff of the Northstar note payable described in Note 8.
On June 1, 2015, the Company issued an amended and restated promissory note of $1,697,762 in settlement of the $1,500,000 outstanding subordinated debt (See Note 12), related accrued interest of $373,469 and accumulated and unpaid guarantor fees of $624,737 (See Note 5).
The note is unsecured and non-interest bearing with four semi-annual payments of $75,000 beginning on December 31, 2015 with the remaining unpaid balance due June 1, 2020.
The Company imputed an interest rate of 5% and discounted the promissory note accordingly. The imputed debt discount of $368,615 is amortized to interest expense using the effective interest method.
In connection with the settlement, the Company recorded a gain on settlement of debt of $1,169,058.
For the three and nine months ended September 30, 2015, the Company amortized $21,396 and $28,141 of debt discounts to current period operations as interest expense, respectively.
The initial fair value of the embedded debt derivative of $591,360 was allocated as a debt discount up to the proceeds of the notes ($363,964) with the remainder ($227,396) charged to current period operations as interest expense. For the three and nine months ended September 30, 2015, the Company amortized an aggregate of $207,196 and $632,825 of debt discounts to current period operations as interest expense, respectively.
As of September 30, 2015 and December 31, 2014, the Company’s officers and directors have provided advances in the aggregate of $155,363 and $148,759, respectively, for working capital purposes. The advances are unsecured, due on demand and non-interest bearing.
On February 29, 2012, a note issued to BlueCrest Master Fund Limited was assigned to Northstar Biotechnology Group, LLC (“Northstar”), owned partly by certain directors and existing shareholders of the Company, including Dr. William P. Murphy Jr., Dr. Samuel Ahn and Charles Hart. At the date of the assignment, the principal amount of the BlueCrest note was $544,267.
On March 30, 2012, the Company and Northstar agreed to extend until May 1, 2012 the initial payment date for any and all required monthly under the Note, such that the first of the four monthly payments required under the Note will be due and payable on May, 2012 and all subsequent payments will be due on a monthly basis thereafter commencing on June 1, 2012, and to waive any and all defaults and/or events of default under the Note with respect to such payments. The Company did not make the required payment, and as a result, was in default of the revised agreement The Company renegotiated the terms of the Note and Northstar agreed to suspend the requirement of principal payments by the Company and allow payment of interest-only in common stock.
In addition, the Company granted Northstar a perpetual license on products as described for resale, relicensing and commercialization outside the United States. In connection with the granted license, Northstar shall pay the Company a royalty of up to 8% on revenues generated.
Effective October 1, 2012, the effective interest rate was 12.85% per annum. The parties agreed, as of February 28, 2013, to reduce the interest rate to 7% per annum.
In connection with the consideration paid, Northstar waived, from the effective date through the earlier of termination or expiration of the agreement, satisfaction of the obligations as described in the forbearance agreement.
In 2012, 5,000,000 shares of Series A Convertible Preferred Stock were approved to be issued, which was subsequently increased to 20,000,000 shares of preferred stock as Series A Convertible Preferred Stock. In addition, the Company is obligated to issue additional preferred stock equal in lieu of payment of cash of accrued and unpaid interest on each six month anniversary of the effective date (October 1, 2012). In lieu of the initial two payments in preferred stock, the parties have determined to modify the voting rights of the Series A Convertible Preferred Stock from 20 votes per share on matters to be voted on by the common stock holders to 25 votes per share on matters to be voted on by the common stock holders and all prior and subsequent payments of interest will be in common stock. The Company is required to issue additional shares of its common stock (as amended), in lieu of cash, each six month anniversary of the effective date for any accrued and unpaid interest.
As described above, during the year ended December 31, 2013, the Company issued the 5,000,000 shares of Series A Convertible Preferred Stock and the 10,000 of common stock described above in exchange for the $210,000 as payment towards outstanding principle of the debt. In addition, the Company issued 15,000,000 shares of Series A Convertible Preferred Stock as a penalty in settlement of the terms of the forbearance agreement. The fair value of the Preferred Stock of $274,050 was included in interest expense for the year ended December 31, 2013.
On September 30, 2013, the Company issued 8,772 shares of its common stock as payment of $100,000 towards cash advances.
On December 24, 2013, the Company issued 3,916 shares of its common stock as payment of accrued interest through June 30, 2013 of $85,447.
On April 2, 2014, the Company issued 275 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2014 per the forbearance agreement.
On September 17, 2014, limited waiver and forbearance agreement entered into on October 1, 2012 to provide that the perpetual license on products as described for resale, relicensing and commercialization outside the United States was amended as such to condition upon NorthStar providing certain financing, which financing the Company, in its sole discretion, could decline and retain the license.
On October 3, 2014, the Company issued 515 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,705 due October 1, 2014 per the forbearance agreement.
On April 3, 2015, the Company issued 1,363 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2015 per the forbearance agreement.
As of September 30, 2015 and December 31, 2014, the principle of this note was $362,000.
Notes payable, Dr. Murphy
At September 30, 2015 and December 31, 2014, the Company has outstanding notes payable to Dr. Murphy with interest at 8% per annum due at maturity in aggregate $465,240. Of the outstanding balance, certain subordinated notes totaling $100,000 and $140,000 were previously due on November 30, 2012 and June 4, 2011 respectively, and are unsecured. The Company is not obligated to make payment until Northstar (or successor) Loan is paid off.
Notes payable, Mr. Tomas
In 2013, the Company issued a promissory note payable for previous advances and accrued compensation. The promissory note bears interest of 5% per annum and due on demand. During the nine months ended September 30, 2015, the Company paid off $79,104 of the outstanding promissory note. The principle outstanding balance of this note as of September 30, 2015 is $252,250.
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
On August 1, 2013, the Company issued a $375,000 promissory note due on demand in settlement of accrued compensation. The promissory note bears interest of 5% per annum and is due on demand. The principle outstanding balance of this note as of September 30, 2015 is $375,000.
On July 1, 2014, the Company issued a $500,000 promissory note in settlement of accrued compensation. The promissory note bears interest of 5% per annum and was due on January 1, 2015. The principle outstanding balance of this note as of September 30, 2015 is $500,000.
Notes payable, Ms. Comella
On July 1, 2014, the Company issued a $300,000 promissory note in settlement of accrued compensation. The promissory note bears interest of 5% per annum and due on January 1, 2015. During the nine months ended September 30, 2015, the Company paid off $11,693 of the outstanding promissory note. The principle outstanding balance of this note as of September 30, 2015 is $287,772.
Transactions with Pavilion
During the three and nine months ended September 30, 2015, the Company purchased $69,973 and $242,271 lab kits, respectively, from Pavillion, Inc., a related party, whose owner is related to an officer of the Company.
NOTE 9 — DERIVATIVE LIABILITIES
Reset warrants
On October 1, 2012, in connection with the forbearance agreement with Northstar as discussed in Note 8 above, the Company issued an aggregate of 15,000 common stock purchase warrants to purchase the Company’s common stock with an exercise price of $0.014 per share for ten years with anti-dilutive (reset) provisions.
The Company has identified embedded derivatives related to the issued warrants. These embedded derivatives included certain and anti-dilutive (reset) provisions. The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date and to fair value as of each subsequent reporting date.
At September 30, 2015, the fair value of the reset provision related to the embedded derivative liability of $23,751 was determined using the Binomial Option Pricing model with the following assumptions: dividend yield: 0%; volatility: 136.32%; risk free rate: 1.74%; and expected life: 7.00 years. The Company recorded a gain on change in derivative liabilities of $58,760 and $126,168 during the three and nine months ended September 30, 2015, respectively.
Convertible notes
In 2014 and the nine months ended September 30, 2015, the Company issued convertible notes (see Note 7 above).
These notes are convertible into common stock, at holders’ option, at a discount to the market price of the Company’s common stock. The Company has identified the embedded derivatives related to these notes relating to certain anti-dilutive (reset) provisions. These embedded derivatives included certain conversion features. The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date of these notes and to fair value as of each subsequent reporting date.
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
The fair value of the embedded derivatives at September 30, 2015, in the amount of $375,577, was determined using the Binomial Option Pricing Model based on the following assumptions: (1) dividend yield of 0%; (2) expected volatility of 136.32%, (3) weighted average risk-free interest rate of 0.01 to 0.33%, (4) expected lives of 0.25 to 0.92 years, and (5) estimated fair value of the Company’s common stock of $0.0019 per share. The Company recorded a gain on change in derivative liabilities of $315,924 and $134,784 during the three and nine months ended September 30, 2015, respectively.
Based upon ASC 840-15-25 (EITF Issue 00-19, paragraph 11) the Company has adopted a sequencing approach regarding the application of ASC 815-40 to its outstanding convertible notes. Pursuant to the sequencing approach, the Company evaluates its contracts based upon earliest issuance date.
NOTE 10 — STOCKHOLDERS’ EQUITY
On October 12, 2015, the Company filed an amendment to its Articles of Incorporation and effected a 1-for-1,000 reverse stock split of its issued and outstanding shares of common stock, $0.001 par value, effective November 19, 2015. The Financial Industry Regulatory Authority (“FINRA”) declared the ex-dividend date for the dividend date as November 4, 2015. All per share amounts and number of shares in the condensed financial statements and related notes have been retroactively restated to reflect the reverse stock split as if it had occurred on the first day of the first period presented resulting in the transfer of $580,852 from common stock to additional paid in capital at December 31, 2014.
During the nine months ended September 30, 2015, the Company issued an aggregate of 20,819 shares of its common stock in the amount of $152,777 for the settlement of outstanding accounts payable and accrued expenses. In connection with the issuance of the shares the Company recognized a gain on settlement of accounts payable and accrued expenses in the amount of $86,763 (see Note 5).
During the nine months ended September 30, 2015, the Company issued 6,650 shares of common stock in settlement of litigation. In connection with the issuances, the Company recognized a loss in the amount of $59,850, which is included in the marketing, general and administration expense in the Statement of Operations (see Note 12).
On April 3, 2015, the Company issued 1,363 shares of its common stock in lieu of payment in cash of accrued and unpaid interest of $12,635 due April 1, 2015 per the forbearance agreement on Northstar note (See Note 8).
During the nine months ended September 30, 2015, the Company issued an aggregate of 247,524 shares of its common stock for the conversion of $623,237 of notes payable and related accrued interest. Upon conversion of the notes, the Company recorded an adjustment to the derivative liability in the amount of $672,350 (see Note 13).
During the nine months ended September 30, 2015, the Company purchased 37,852 shares of the Company’s common stock in the open market at an average cost of $4.17 per share.
During the nine months ended September 30, 2015, the Company issued an aggregate of 87,812 shares of common stock in exchange for $521,538 under the stock purchase agreement with Magna Equities II, LLC (see Note 6), and issued an aggregate of 7,852 shares of common stock in exchange for $61,270. In connection with the stock sale, the Company issued an aggregate of 1,443,656 warrants to purchase the Company’s common stock (see Note 11).
During the nine months ended September 30, 2015, the Company issued an aggregate of 24,353 shares of its common stock in settlement of accumulative outstanding accounts payable due to Guarantors of the Company of $961,125. In connection with the issuance, the Company incurred a $791,024 gain in settlement of debt.
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
NOTE 11 — STOCK OPTIONS AND WARRANTS
Stock Options
In December 1999, the Board of Directors and shareholders adopted the 1999 Officers and Employees Stock Option Plan, or the Employee Plan, and the 1999 Directors and Consultants Stock Option Plan, or the Director Plan. The Employee Plan and the Director Plan are collectively referred to herein as the Plans. The Plans are administered by the Board of Directors and the Compensation Committee.
The objectives of the Plans include attracting and retaining key personnel by encouraging stock ownership in the Company by such persons. In February 2010, the Directors & Consultants Plan was amended to extend the termination date of the Plan to December 1, 2011.
On April 1, 2013, the Board of Directors approved, subject to shareholder approval, the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan”. The 2013 Omnibus Plan reserves up to fifty thousand shares of common stock for issuance. On August 4, 2014, the Board of Directors approved to set the reserve to one hundred thousand shares of common stock for issuance and to close the 1999 Officers and Employees Stock Option Plan. On February 2, 2015, at the annual meeting of shareholders, the majority of shareholders approved the 2013 Omnibus Equity Compensation Plan. On November 2, 2015, the Board of Directors approved the increase of the reserve under the 2013 Omnibus Plan to (pre-split) five hundred million shares of common stock for issuance.
A summary of options at September 30, 2015 and activity during the nine months then ended is presented below:
| | | Shares | | | Weighted- Average Exercise Price | | | Weighted- Average Remaining Contractual Term (in years) | |
| | | | | | | | | | | | |
| Options outstanding at January 1, 2014 | | | 23,921 | | | $ | 150.00 | | | 9.0 | |
| Granted | | | 43,148 | | | $ | 23.00 | | | 10.0 | |
| Exercised | | | — | | | $ | | | | | |
| Forfeited/Expired | | | (136 | ) | | $ | 5,200.00 | | | | |
| Options outstanding at December 31, 2014 | | | 66,933 | | | $ | 56.00 | | | 8.9 | |
| Granted | | | 7,100 | | | $ | 11.16 | | | 10.0 | |
| Exercised | | | — | | | | | | | | |
| Forfeited/Expired | | | (229 | ) | | $ | 5,112.48 | | | | |
| Options outstanding at September 30, 2015 | | | 73,804 | | | $ | 36.32 | | | 8.3 | |
| Options exercisable at September 30, 2015 | | | 44,468 | | | $ | 46.41 | | | 8.3 | |
| Available for grant at September 30, 2015 | | | 89,400 | | | | | | | | |
The following information applies to options outstanding and exercisable at September 30, 2015:
| | | | Options Outstanding | | | Options Exercisable | |
| | | | Shares | | | Weighted- Average Remaining Contractual Term | | | Weighted- Average Exercise Price | | | Shares | | | Weighted- Average Exercise Price | |
| | | | | | | | | | | | | | | | | |
| $ | 0.00 – $20.00 | | | | 52,690 | | | 8.2 | | | $ | 16.73 | | | | 34,490 | | | $ | 16.38 | |
| $ | 20.01 – $30.00 | | | | 19,849 | | | 8.8 | | | $ | 26.96 | | | | 8,713 | | | $ | 26.94 | |
| $ | 30.01 – $100.00 | | | | 300 | | | 5.9 | | | $ | 70.00 | | | | 300 | | | $ | 70.00 | |
| $ | >100.00 | | | | 965 | | | 4.3 | | | $ | 1,243.27 | | | | 965 | | | $ | 1,243.27 | |
| | | | | | 73,804 | | | 8.3 | | | $ | 36.32 | | | | 44,468 | | | $ | 46.41 | |
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
On February 2, 2015, the Company issued an aggregate 7,000 options to purchase the Company’s common stock at $11.16 per share to members of the Board of Directors, vesting immediately and exercisable over 10 years. The aggregate fair value of $121,735, determined using the Black Scholes option pricing model with the following assumptions: Dividend yield: 0%; Volatility: 142.65% and Risk free rate: 1.68%.
On August 24, 2015, the Company issued 100 options to purchase the Company’s common stock at $5.31 per share to a consultant, vesting immediately and exercisable over 4 years. The aggregate fair value of $347, determined using the Black Scholes option pricing model with the following assumptions: Dividend yield: 0%; Volatility: 129.01% and Risk free rate: 1.39%.
The fair value of all options vesting during the three and nine months ended September 30, 2015 of $73,520 and $345,761, respectively, was charged to current period operations. The fair value of all options vesting during the three and nine months ended September 30, 2014 of $164,247 and $407,616, respectively, was charged to period operations.
Warrants
A summary of common stock purchase warrants at September 30, 2015 and activity during the period then ended is presented below:
| | | Shares | | | Weighted- Average Exercise Price | | | Weighted- Average Remaining Contractual Term (in years) | |
| | | | | | | | | | | | | |
| Outstanding at January 1, 2014 | | | 118,134 | | | $ | 220.00 | | | | 6.3 | |
| Issued | | | 57,582 | | | $ | 20.00 | | | | 8.2 | |
| Exercised | | | (11,918 | ) | | $ | 10.00 | | | | | |
| Expired | | | (13,178 | ) | | $ | 80.00 | | | | | |
| Outstanding at December 31, 2014 | | | 150,620 | | | $ | 10.00 | | | | 6.6 | |
| Issued | | | 1,444 | | | $ | 11.27 | | | | 10.0 | |
| Exercised | | | | | | $ | | | | | | |
| Expired | | | (13,325 | ) | | $ | 24.00 | | | | | |
| Outstanding at September 30, 2015 | | | 138,739 | | | $ | 182.90 | | | | 6.5 | |
| Exercisable at September 30, 2015 | | | 133,194 | | | $ | 100.27 | | | | 6.6 | |
The following information applies to common stock purchase warrants outstanding and exercisable at September 30, 2015:
| | | | Warrants Outstanding | | | Warrants Exercisable | |
| | | | Shares | | | Weighted- Average Remaining Contractual Term | | | Weighted- Average Exercise Price | | | Shares | | | Weighted- Average Exercise Price | |
| | | | | | | | | | | | | | | | | |
| $ | 0.01 – $20.00 | | | | 94,108 | | | | 7.0 | | | $ | 15.54 | | | | 94,108 | | | $ | 15.54 | |
| $ | 20.01 – $30.00 | | | | 29,743 | | | | 6.3 | | | $ | 24.52 | | | | 27,743 | | | $ | 24.72 | |
| $ | 30.01 – $50.00 | | | | 6,253 | | | | 3.4 | | | $ | 48.36 | | | | 4,253 | | | $ | 48.49 | |
| $ | 50.01 – $60.00 | | | | 543 | | | | 2.2 | | | $ | 60.00 | | | | 543 | | | $ | 60.00 | |
| $ | >60.00 | | | | 8,092 | | | | 4.4 | | | $ | 2,823.67 | | | | 6,547 | | | $ | 1,675.28 | |
| | | | | | 138,739 | | | | 6.5 | | | $ | 182.90 | | | | 133,194 | | | $ | 100.27 | |
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
In conjunction with the authorized issuance of common stock, the Company granted 1,444 common stock purchase warrants during the nine months ended September 30, 2015. The warrants are exercisable at an exercise price of approximately $11.27 per share for ten years.
NOTE 12 — COMMITMENTS AND CONTINGENCIES
Litigation
On March 19 2015, the Company settled a prospective dispute with a third party over the use of proprietary information through the issuance of 6,650 shares of common stock. (See Note 10)
On November 10, 2014, the Company was served with a lawsuit by an alleged assignee and a guarantor to a Loan Guarantee, Payment and Security Agreement. These parties claim breach of that Agreement and damages of approximately $2.3 Million plus interest. The assignor and assignee also sued the Company’s directors and two past directors and an affiliate shareholder for breach of fiduciary duty, claiming damages as alleged creditors arising out of these parties’ alleged participation in Northstar Biotech Group, LLC, a secured creditor of the Company. On June 1, 2015, the Company settled the lawsuit. As part of the settlement, the Company recorded a gain of $1,169,058. The Company provided a note to the original guarantor in the amount of $1,697,762, to be paid over a five year period with a 0% interest rate.
The Company is subject to other legal proceedings that arise in the ordinary course of business. In the opinion of management, as of September 30, 2015, the amount of ultimate liability with respect to such matters, if any, in excess of applicable insurance coverage, is not likely to have a material impact on the Company’s business, financial position, results of operations or liquidity. However, as the outcome of litigation and other claims is difficult to predict significant changes in the estimated exposures could exist.
NOTE 13 — FAIR VALUE MEASUREMENT
The Company adopted the provisions of Accounting Standards Codification subtopic 825-10, Financial Instruments (“ASC 825-10”) on January 1, 2008. ASC 825-10 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. ASC 825-10 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825-10 establishes three levels of inputs that may be used to measure fair value:
Level 1 – Quoted prices in active markets for identical assets or liabilities.
Level 2 – Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities.
All items required to be recorded or measured on a recurring basis are based upon level 3 inputs.
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes, the level in the fair value hierarchy within which the fair value measurement is disclosed and is determined based on the lowest level input that is significant to the fair value measurement.
Upon adoption of ASC 825-10, there was no cumulative effect adjustment to beginning retained earnings and no impact on the financial statements.
The carrying value of the Company’s cash and cash equivalents, accounts receivable, accounts payable, short-term borrowings (including convertible notes payable), and other current assets and liabilities approximate fair value because of their short-term maturity.
As of September 30, 2015 or December 31, 2014, the Company did not have any items that would be classified as level 1 or 2 disclosures.
The Company recognizes its derivative liabilities as level 3 and values its derivatives using the methods discussed in notes 7 and 9. While the Company believes that its valuation methods are appropriate and consistent with other market participants, it recognizes that the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values using the methods discussed in Notes 7 and 9 are that of volatility and market price of the underlying common stock of the Company.
As of September 30, 2015 and December 31, 2014, the Company did not have any derivative instruments that were designated as hedges.
The derivative liability as of September 30, 2015, in the amount of $399,328 has a level 3 classification.
The following table provides a summary of changes in fair value of the Company’s Level 3 financial liabilities as of September 30, 2015:
| | | Warrant Liability | | | Debt Derivative | |
| Balance, December 31, 2013 | | | 146,855 | | | $ | 256,956 | |
| Total (gains) losses | | | | | | | | |
| Initial fair value of debt derivative at note issuance | | | — | | | | 1,443,708 | |
| Initial fair value of derivative relating to reset warrants | | | — | | | | — | |
| Mark-to-market at December 31, 2014: | | | 3,065 | | | | (429,018 | ) |
| Transfers out of Level 3 upon increase in authorized shares | | | — | | | | — | |
| Transfers out of Level 3 upon conversion and settlement of notes | | | — | | | | (680,295 | ) |
| Balance, December 31, 2014 | | $ | 149,920 | | | $ | 591,351 | |
| Total (gains) losses | | | | | | | | |
| Initial fair value of debt derivative at note issuance | | | — | | | | 591,360 | |
| Mark-to-market at September 30, 2015: | | | (126,169 | ) | | | (134,784 | ) |
| Transfers out of Level 3 upon conversion or payoff of notes payable | | | — | | | | (672,350 | ) |
| Balance, September 30, 2015 | | $ | 23,751 | | | $ | 375,577 | |
| Net gain for the period included in earnings relating to the liabilities held at September 30, 2015 | | $ | 126,169 | | | $ | 134,784 | |
U.S. STEM CELL, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
SEPTEMBER 30, 2015
(unaudited)
Fluctuations in the Company’s stock price are a primary driver for the changes in the derivative valuations during each reporting period. The Company’s stock price decreased approximately 82% from December 31, 2014 to September 30, 2015. As the stock price decreases for each of the related derivative instruments, the value to the holder of the instrument generally decreases.. Additionally, stock price volatility is one of the significant unobservable inputs used in the fair value measurement of each of the Company’s derivative instruments.
The estimated fair value of these liabilities is sensitive to changes in the Company’s expected volatility. Increases in expected volatility would generally result in a higher fair value measurement.
NOTE 14 — SUBSEQUENT EVENTS
Subsequent stock issuances
In October and November 2015, the Company issued an aggregate of 252,157 shares of its common stock in settlement of $136,095 of outstanding convertible notes payable and $7,499 accrued interest; 14,848 shares of its common stock in settlement of services of approximately $65,015. In addition, the Company issued an aggregate 38,678 shares of its common stock in settlement of $100,000 principal and $12,705 accrued interest on Northstar debt. The Company also issued an aggregate of 274,211 shares of its common stock in settlement of $471,000 of outstanding notes payable and advances to related parties.
Subsequent financing
On October 1, 2015, the Company entered into a Securities Purchase Agreement with Magna Equities, II, LLC for the sale of 12% convertible note in the principal amount of $160,000 (the “Note”).
The Note bears interest at the rate of 12% per annum. All interest and principal must be repaid on August 1, 2016. The Note is convertible into common stock, at Magna Equities, II, LLC’s option, at a $0.72 per share or if a registration statement registering the underlying shares of common stock is not filed within 70 days, 45% of the lowest trading price during five consecutive trading days prior to conversion with anti-dilutive provisions.
On October 7, 2015, the Company entered into a Securities Purchase Agreement with Fourth Man, LLC., for the sale of a 9.5% convertible note in the principal amount of $25,000 (the “Note”).
The Note bears interest at the rate of 9.5% per annum. All interest and principal must be repaid on October 6, 2016. The Note is convertible into common stock, at Fourth Man’s option, at a 47% discount to the lowest daily closing trading price of the common stock during the 10 trading day period prior to conversion. In the event the Company prepays the Note in full, the Company is required to pay off all principal at 150%, interest and any other amounts.
The Company amended its Articles of Incorporation to change its name to U.S. Stem Cell, Inc. and to implement a reverse stock split in the ratio of 1 share for every 1,000 shares of common stock. This amendment was approved and filed of record by the Florida Secretary of State on October 12, 2015, effective on October 19, 2015. FINRA has declared the Company’s 1-for-1,000 reverse stock split market effective as of November 4, 2015. The number of authorized shares of common and preferred stock remained unchanged. All fractional shares will be rounded up. In addition, the ticker symbol will be BHRTD for 20 business days on November 4th 2015 and will change to USRM (OTC Markets) on December 2, 2015.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Unless otherwise indicated, references in this Quarterly Report on Form 10-Q to “we,” “us,” and “our” are to the Company, unless the context requires otherwise. The following discussion and analysis by our management of our financial condition and results of operations should be read in conjunction with our unaudited condensed interim financial statements and the accompanying related notes included in this quarterly report and our audited financial statements and related notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our Annual Report on Form 10-K for the year ended December 31, 2014 filed with the Securities and Exchange Commission.
Cautionary Statement Regarding Forward-Looking Statements
This report may contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act, and we intend that such forward-looking statements be subject to the safe harbors created thereby. These forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Any such forward-looking statements would be contained principally in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Risk Factors.” Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities and the effects of regulation. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “hopes,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions.
Forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We discuss many of these risks in greater detail in “Risk Factors.” Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management’s beliefs and assumptions only as of the date of this report. You should read this report and the documents that we reference in this report and have filed as exhibits to the report completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
Additional information concerning these and other risks and uncertainties is contained in our filings with the Securities and Exchange Commission, including the section entitled “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2014.
Unless otherwise indicated or the context otherwise requires, all references in this Form 10-Q to “we,” “us,” “our,” “our company,” “U. S. Stem Cell, Inc. (f.k.a. Bioheart)” or the “Company” refer to U.S. Stem Cell, Inc. (f.k.a. Bioheart, Inc.) and its subsidiaries.
Our Ability to Continue as a Going Concern
Our independent registered public accounting firm has issued its report dated March 16, 2015, in connection with the audit of our annual financial statements as of December 31, 2014, that included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern and Note 2 to the unaudited financial statements for the period ended September 30, 2015 also describes the existence of conditions that raise substantial doubt about our ability to continue as a going concern.
Overview
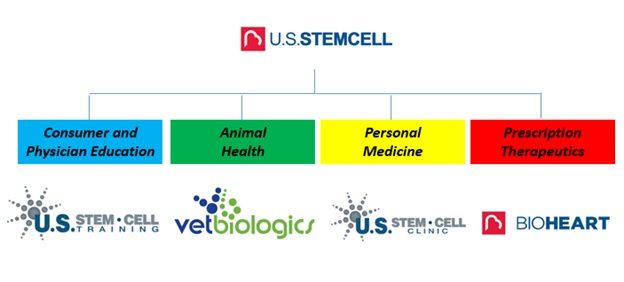
We are an emerging enterprise in the regenerative medicine/cellular therapy industry. We are focused on the discovery, development and commercialization of cell based therapeutics that prevent, treat or cure disease by repairing and replacing damaged or aged tissue, cells and organs and restoring their normal function. Our business includes the development of proprietary cell therapy products as well as revenue generating physician and patient based regenerative medicine / cell therapy training services, cell collection and cell storage services, the sale of cell collection and treatment kits for humans and animals, and the operation of a cell therapy clinic.
US Stem Cell Training, (“SCT”), an operating division of our company, is a content developer of regenerative medicine/cell therapy informational and training materials for physicians and patients. SCT also provides in-person and online training courses which are delivered through in-person presentations at SCT’s state of the art facilities and globally at university, hospital and physician’s office locations as well as through online webinars. Additionally, SCT provides hands-on clinical application training for physicians and health care professionals interested in providing regenerative medicine / cell therapy procedures.
Vetbiologics, (“VBI”), an operating division of our company, is a veterinary regenerative medicine company committed to providing veterinarians with the ability to deliver the highest quality regenerative medicine therapies to dogs, cats and horses. VBI provides veterinarians with extensive regenerative medicine capabilities including the ability to isolate regenerative stem cells from a patient’s own adipose (fat) tissue directly on-site within their own clinic or stall-side.
US Stem Cell Clinic, LLC, (“SCC”), a partially owned investment of our company, is a physician run regenerative medicine/cell therapy clinic providing cellular treatments for patients afflicted with neurological, autoimmune, orthopedic and degenerative diseases. SCC is operating in compliance with the FDA 1271s which allow for same day medical procedures to be considered the practice of medicine. We isolate stem cells from bone marrow and adipose tissue and also utilize platelet rich plasma.
U.S. Stem Cell, Inc.’s comprehensive map of products and services:
All living complex organisms start as a single cell that replicates, differentiates (matures) and perpetuates in an adult organism through its lifetime. Cellular therapy is the process that uses cells to prevent, treat or cure disease, or regenerate damaged or aged tissue. To date, the most common type of cell therapy has been the replacement of mature, functioning cells such as through blood and platelet transfusions. Since the 1970s, first bone marrow and then blood and umbilical cord-derived stem cells have been used to restore bone marrow, as well as blood and immune system cells damaged by the chemotherapy and radiation that are used to treat many cancers. These types of cell therapies are standard of practice world-wide and are typically reimbursed by insurance.
Within the field of cell therapy, research and development using stem cells to treat a host of diseases and conditions has greatly expanded. Stem cells (in either embryonic or adult forms) are primitive and undifferentiated cells that have the unique ability to transform into or otherwise affect many different cells, such as white blood cells, nerve cells or heart muscle cells. Our cell therapy development efforts are focused on the use of adult stem cells; those cells which are found in the muscle, fat tissue and peripheral blood.
There are two general classes of cell therapies: Patient Specific Cell Therapies ("PSCTs") and Off-the-Shelf Cell Therapies ("OSCTs"). In PSCTs, cells collected from a person (“donor”) are transplanted, with or without modification, to a patient (“recipient”). In cases where the donor and the recipient are the same individual, these procedures are referred to as “autologous”. In cases in which the donor and the recipient are not the same individual, these procedures are referred to as “allogeneic.” Autologous cells offer a low likelihood of rejection by the patient and we believe the long-term benefits of these PSCTs can best be achieved with an autologous product. In the case of OSCT, donor cells are expanded many fold in tissue culture, and large banks of cells are frozen in individual aliquots that may result in treatments for as many as 10,000 people from a single donor tissue. By definition, OSCTs are always allogeneic in nature.
Various adult stem cell therapies are in clinical development for an array of human diseases, including autoimmune, oncologic, neurologic and orthopedic, among other indications. While no assurances can be given regarding future medical developments, we believe that the field of cell therapy holds the promise to better the human experience and minimize or ameliorate the pain and suffering from many common diseases and/or from the process of aging.
According to Robin R. Young’s Stem Cell Summit Executive Summary-Analysis and Market Forecasts 2014-2024, the United States stem cell therapy market is estimated to grow from an estimated $237 million in 2013 to more than $5.7 billion in 2020.
Specific to cellular therapy, we are focused on the discovery, development and commercialization of autologous cellular therapies for the treatment of chronic and acute heart damage as well as vascular and autoimmune diseases.
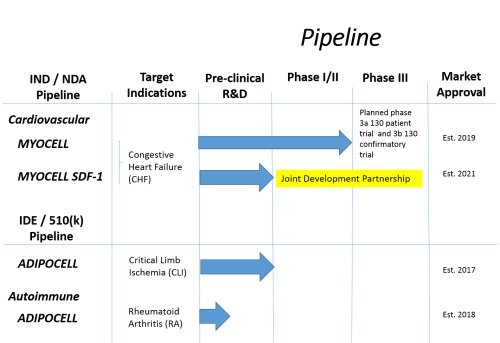
In our pipeline, we have multiple product candidates for the treatment of heart damage, including MyoCell and Myocell SDF-1. MyoCell and MyoCell SDF-1 are autologous muscle-derived cellular therapies designed to populate regions of scar tissue within a patient’s heart with new living cells for the purpose of improving cardiac function in chronic heart failure patients.
MyoCell SDF-1 is intended to be an improvement to MyoCell. MyoCell SDF-1 is similar to MyoCell but the myoblast cells to be injected for use in MyoCell SDF-1 are modified prior to injection by an adenovirus vector or non-viral vector so that they will release extra quantities of the SDF-1 protein, which expresses angiogenic factors.
AdipoCell is a patient-derived cell therapy proposed for the treatment of lower limb ischemia and potentially, among other autoimmune diseases, rheumatoid arthritis. We hope to demonstrate that these product candidates are safe and effective complements to existing therapies for chronic and acute heart damage.
Our Clinical Development Pipeline Chart:
Our mission is to advance to market novel regenerative medicine and cellular therapy products that substantially benefit humankind. Our business strategy is, to the extent possible, finance our clinical development pipeline through revenue (cash in-flows) generated through the marketing and sales of unique educational and training services, animal health products and personalized cellular therapeutic treatments.
A fundamental shift in venture capital investment strategies where, management believes, financial sponsorship is now directed toward commercial or near commercial enterprises has required us to adapt our mission combining immediate revenue generating opportunities with longer-term development programs. Accordingly, we have developed a multifaceted portfolio of revenue generating products and services in our US Stem Cell Training, Vetbiologics, and US Stem Cell Clinic, operating divisions that will, if successful, financially support its clinical development programs. Our goal is to maximize shareholder value through the generation of short-term profits that increase cash in-flows and decrease the need for venture financings – a modern biotechnology company development strategy.
Today, our company is a combination of opportunistic business enterprises. We estimate that the products and services we offer through US Stem Cell Training, Vetbiologics, and US Stem Cell Clinics has the potential, although we cannot provide assurances as to if and when it will be accomplished, to drive up to $100 million dollars in cumulative peak annual revenues. What we are establishing is a foundation of value in the products and services we are and plan to sell from US Stem Cell Training, Vetbiologics, and US Stem Cell clinics. Our strategy is to expand the revenues generated from each of these operating divisions and to reinvest the profits we generate into our clinical development pipeline.
On January 29th, 2015 we announced an update and diversification of our clinical development pipeline. Our cardiovascular and vascular product candidates have been streamlined, putting, we believe, our best opportunities at the forefront of our efforts. The MYOCELL and MYOCELL SDF-1 candidates will, in our opinion, advance forward in the treatment of chronic heart failure (CHF). We are in active prospective partnering discussion for the MYOCELL SDF-1 program. Partnering, we contend, will enhance our capabilities, reduce our development cost through cost sharing and potentially accelerate our time to approval and commercialization. We will apply our ADIPOCELL technology to the treatment of critical limb ischemia. Additionally, we have expanded and diversified our clinical development pipeline to include autoimmune disease, specifically applying ADIPOCELL to the treatment of Rheumatoid Arthritis (RA). We believe that updating and diversifying our clinical development programs increases the probability of our success, brings operational and fiscal clarity to our company, and will ultimately enhance shareholder value.
We will continue to evaluate and act upon opportunities to increase our top line revenue position and that correspondingly increase cash in-flows. These opportunities include but are not limited to the development and marketing of new products and services, mergers and acquisitions, joint ventures, licensing deals and more.
Further, if the opportunity presents itself whereby we can raise additional capital at a reasonable fair market value, our management will do so. Accordingly, we plan to continue in our efforts to restructure, equitize or eliminate legacy balance sheet issues that are obstacles to market capitalization appreciation and capital fund raising.
Following the quarter ended September 30, 2015, we amended our Articles of Incorporation to change our name to U.S. Stem Cell, Inc. and to implement a reverse stock split in the ratio of 1 share for every 1,000 shares of common stock. This amendment was approved and filed of record by the Florida Secretary of State on October 12, 2015, effective on October 19, 2015. FINRA has declared our 1-for-1,000 reverse stock split market effective as of November 4, 2015. The number of authorized shares of common and preferred stock remained unchanged. All fractional shares will be rounded up. The trading symbol after December 2, 2015 will be USRM (OTC Markets).
Results of Operations Overview
We are a research and development company and our MyoCell product candidate has not received regulatory approval or generated any material revenues and is not expected to until 2016, if ever. We have generated substantial net losses and negative cash flow from operations since inception and anticipate incurring significant net losses and negative cash flows from operations for the foreseeable future as we continue clinical trials, undertake new clinical trials, apply for regulatory approvals, make capital expenditures, add information systems and personnel, make payments pursuant to our license agreements upon our achievement of certain milestones, continue development of additional product candidates using our technology, establish sales and marketing capabilities and incur the additional cost of operating as a public company.
Three Months Ended September 30, 2015 as compared to the Three Months Ended September 30, 2014
Revenues
We recognized revenues of $557,612 for the three months ended September 30, 2015, generated from the sales of kits and equipment, services, MyoCath Catheters, AdipoCell, and laboratory services. We recognized revenues of $579,536 for the three months ended September 30, 2014 from the sale of MyoCath catheters, AdipoCell, physician training, patient studies and laboratory services. The differential in revenue reflected a moderate decrease based on the products and services provided.
Cost of Sales
Cost of sales consists of the costs associated with the production of MyoCath, laboratory supplies necessary for laboratory services, production of AdipoCell systems and materials, physician course materials, test kits and clinic supplies required for patient studies.
Cost of sales was $243,940 and $455,603 in the three month periods ended September 30, 2015 and 2014, respectively. Associated gross margins were $313,672 (56.3%) and $123,933 (21.4%) for the three months periods ended September 30, 2015 and 2014, respectively. During the three months ended September 30, 2014, we recognized expense related to employee bonuses that directly affected the 2014 margins.
Research and Development
Our research and development expenses consist of costs incurred in identifying, developing and testing our products and services. Research and development expenses were $12,764 in the three month period ended September 30, 2015, an increase of $4,183 from the research and development expenses of $8,581 in the three month period ended September 30, 2014. Our increase is proportionate to our increase in management focus on research and development and its corresponding ongoing costs. The timing and amount of our planned research and development expenditures is dependent on our ability to obtain additional financing.
Marketing, General and Administrative
Our marketing, general and administrative costs were $563,676 for the three month period ended September 30, 2015 compared to $1,580,325 for the three month period ended September 30, 2014, a decrease of $1,016,649. The decrease in costs primarily due bonuses paid in 2014, of $500,000, reduction of stock based compensation of $51,918 reduction in service providers and professional fees of $299,087 and reduction in commission expense of $49,242.
Our marketing, general and administrative expenses primarily consist of the costs associated with our general management and product and service marketing programs, including, but not limited to, salaries and related expenses for executive, administrative and marketing personnel, rent, insurance, legal and accounting fees, consulting fees, travel and entertainment expenses, conference costs and other clinical marketing and trade program expenses.
Gain on settlement of debt
During the three months ended September 30, 2015, we incurred a gain of $58,873 in open accounts payable during the current period as compared to a net aggregate gain of $85,229 for the same period last year.
Gain (loss) on change in fair value of derivative liabilities
During 2014 and 2015, we issued convertible promissory notes with an embedded derivative, all requiring us to fair value the derivatives each reporting period and mark to market as a non-cash adjustment to our current period operations. This resulted in a gain of $374,684 and $239,296 on change in fair value of derivative liabilities for the three months ended September 30, 2015 and 2014, respectively.
Interest Expense
Interest expense during the three months ended September 30, 2015 was $312,576 compared to $331,026 three months ended September 30, 2014. Interest expense primarily consists of interest incurred on the principal amount of the Northstar loan, our former Bank of America loan, the Seaside National Bank loan, accrued fees and interest payable to the Guarantors, and the amortization of debt discounts and non-cash interest incurred relating to our issued convertible notes payable. The debt discounts amortization and non-cash interest incurred during the three months ended September 30, 2015 and 2014 was $246,289 and $246,602, respectively.
Nine Months Ended September 30, 2015 as compared to the Nine Months Ended September 30, 2014
Revenues
We recognized revenues of $1,612,476 for the nine months ended September 30, 2015, generated from the sales of kits and equipment, services, MyoCath Catheters, AdipoCell, and laboratory services. We recognize revenues of $1,563,864 for the nine months ended September 30, 2014 from the sale of MyoCath catheters, AdipoCell, physician training, patient studies and laboratory services. The increase in revenue resulted from the growth of the sale of our products and services.
Cost of Sales
Cost of sales consists of the costs associated with the production of MyoCath, laboratory supplies necessary for laboratory services, production of AdipoCell systems and materials, physician course materials, test kits and clinic supplies required for patient studies.
Cost of sales was $868,742 and $782,487 in the nine month periods ended September 30, 2015 and 2014, respectively. Associated gross margins were $743,734 (46.1%) and $781,377 (50.0%) for the nine months periods ended September 30, 2015 and 2014, respectively. During the nine months ended September 30, 2014, we recognized expense related to employee bonuses that directly affected the 2014 margins.
Research and Development
Our research and development expenses consist of costs incurred in identifying, developing and testing our products and services. Research and development expenses were $39,696 in the nine month period ended September 30, 2015, an increase of $5,780 from the research and development expenses of $33,916 in the nine month period ended September 30, 2014. Our increase is proportionate to our increase in management focus on research and development and its corresponding ongoing costs. The timing and amount of our planned research and development expenditures is dependent on our ability to obtain additional financing.
Marketing, General and Administrative
Our marketing, general and administrative costs were $2,252,058 for the nine month period ended September 30, 2015 compared to $3,289,419 for the nine month period ended September 30, 2014, a decrease of $1,037,361. The decrease in costs primarily due bonuses paid in 2014 of $500,000, reduction of stock based compensation of $104,736, reduction in service providers and professional fees of $327,695 and reduction in commission expense of $136,635.
Our marketing, general and administrative expenses primarily consist of the costs associated with our general management and product and service marketing programs, including, but not limited to, salaries and related expenses for executive, administrative and marketing personnel, rent, insurance, legal and accounting fees, consulting fees, travel and entertainment expenses, conference costs and other clinical marketing and trade program expenses.
Gain on settlement of debt
During the nine months ended September 30, 2015, we settled $1,585,862 in guarantor fees, accrued interest of $373,469 and an outstanding note payable of $1,500,000 for a net gain of $1,960,082. In addition, we incurred a gain of $137,401 in open accounts payable. In the nine months ended September 30, 2014, we settled outstanding debt and related accrued interest for a net gain of $81,568, licensing fees settled for a gain of $2,122,130 and a gain on settlement of accounts payable of $68,585.
Gain (loss) on change in fair value of derivative liabilities
During 2014 and 2015, we issued convertible promissory notes with an embedded derivative, all requiring us to fair value the derivatives each reporting period and mark to market as a non-cash adjustment to our current period operations. This resulted in a gain of $260,953 and $61,339 on change in fair value of derivative liabilities for the nine months ended September 30, 2015 and 2014, respectively.
Interest Expense
Interest expense during the nine months ended September 30, 2015 was $1,126,668 compared to $1,035,118 nine months ended September 30, 2014. Interest expense primarily consists of interest incurred on the principal amount of the Northstar loan, our former Bank of America loan, the Seaside National Bank loan, accrued fees and interest payable to the Guarantors, the amortization of debt discounts and non-cash interest incurred relating to our issued convertible notes payable. The debt discounts amortization and non-cash interest incurred during the nine months ended September 30, 2015 and 2014 was $860,221 and $729,093, respectively.
Stock-Based Compensation
Stock-based compensation reflects our recognition as an expense of the value of stock options and other equity instruments issued to our employees and non-employees over the vesting period of the options and other equity instruments. We have granted to our employees options to purchase shares of common stock at exercise prices equal to the fair market value of the underlying shares of common stock at the time of each grant, as determined by our Board of Directors, with input from management.
The Company follows Accounting Standards Codification subtopic 718-10. Compensation (“ASC 718-10”) which requires that all share-based payments to both employee and non-employees be recognized in the income statement based on their fair values.
In awarding our common stock, our Board of Directors considered a number of factors, including, but not limited to:
| ● | our financial position and historical financial performance; |
| ● | arm’s length sales of our common stock; |
| ● | the development status of our product candidates; |
| ● | the business risks we face; |
| ● | vesting restrictions imposed upon the equity awards; and |
| ● | an evaluation and benchmark of our competitors; and |
| ● | prospects of a liquidity event. |
In April 1, 2013, the Board of Directors authorized, (and approved by our shareholders on February 2, 2015), the establishment of the Bioheart 2013 Omnibus Equity Compensation Plan, or the “2013 Omnibus Plan”. The 2013 Omnibus Plan reserves up to fifty thousand shares of common stock for issuance, subsequently increased to 100,000 on November 3, 2014. We currently have 89,400 available to future issuances.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. While our critical accounting policies are described in Note 1 to our financial statements appearing elsewhere in this report, we believe the following policies are important to understanding and evaluating our reported financial results:
Revenue Recognition
We recognize revenue in accordance with Accounting Standards Codification subtopic 605-10, Revenue Recognition (“ASC 605-10”) which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the selling price is fixed and determinable; and (4) collectability is reasonably assured. Determination of criteria (3) and (4) are based on management’s judgments regarding the fixed nature of the selling prices of the products delivered and the collectability of those amounts. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments are provided for in the same period the related sales are recorded.
At the time of each transaction, management assesses whether the fee associated with the transaction is fixed or determinable and whether or not collection is reasonably assured. The assessment of whether the fee is fixed or determinable is based upon the payment terms of the transaction. Collectability is assessed based on a number of factors, including past transaction history with the client and the creditworthiness of the client.
Revenues for test kits and equipment sold are not recorded until test kits are delivered. We have revenue sharing arrangements for the sale of goods whereby the Company is the primary obligor, sets pricing with the customers and bears all associated credit risks with the customers. Sales under revenue share arrangements are recorded as gross sales and any portion shared with third parties under such arrangements are classified as selling expense due to the nature of the marketing activities performed by the third party. Revenues from trainings are not recorded until the completion of the training. Any cash received as a deposit for trainings are recorded by the company as a liability.
Patent treatments and laboratory services revenue are recognized when those services have been completed or satisfied.
Revenues for bank sales are accounted for as Multiple-Element Arrangements under ASC 605-10 which incorporates Accounting Standards Codification subtopic 605-25, Multiple-Element Arrangements (“ASC 605-25”). ASC 605-25 addresses accounting for arrangements that may involve the delivery or performance of multiple products, services and/or rights to use assets.
Research and Development Activities
We account for research and development costs in accordance with Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and development costs are expensed when the contracted work has been performed or as milestone results have been achieved as defined under the applicable agreement. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred.
Derivative financial instruments
Accounting Standards Codification subtopic 815-40, Derivatives and Hedging, Contracts in Entity’s own Equity (“ASC 815-40”) became effective for the Company on October 1, 2009. The Company has identified the embedded derivatives related to the issued Notes and anti-dilutive warrants. These embedded derivatives included in our debt contain certain conversion features and reset provision. The accounting treatment of derivative financial instruments requires that the Company record fair value of the derivatives as of the inception date of Asher Notes and to fair value as of each subsequent reporting date.
Inflation
Our opinion is that inflation has not had, and is not expected to have, a material effect on our operations.
Climate Change
Our opinion is that neither climate change, nor governmental regulations related to climate change, have had, or are expected to have, any material effect on our operations.
Concentrations of Credit Risk
As of September 30, 2015, five customers represented 15%, 5%, 7%, 15% and 14% respectively for an aggregate of 56% of the Company’s accounts receivable. As of December 31, 2014, two customers represented 38% and 17%, for an aggregate of 55%, of the Company’s accounts receivable.
Liquidity and Capital Resources
In the nine months ended September 30, 2015, we continued to finance our considerable operational cash needs with cash generated from financing activities.
Operating Activities
Net cash used in operating activities was $789,462 in the nine month period ended September 30, 2015 as compared to $747,184 of cash used in the nine month period ended in September 30, 2014.
Our use of cash for operations in the nine months ended September 30, 2015 reflected a net loss generated during the period of $282,982, adjusted for non-cash items such as stock-based compensation of $341,629, depreciation of $3,947, amortization of debt discounts of $612,811, and non-cash interest expense of $227,396, net with gain on change in fair value of derivative liabilities of $260,953, net gain on settlement of debt of $2,097,483 and income from investments of $23,934. In addition we had a net decrease in operating assets of $1,739 and an increase in accrued expenses of $272,322, in accounts payable of $324,026 and deferred revenue of $32,170.
Investing Activities
Net cash used in investing activities was $168,558 for the nine months ended September 30, 2015 were to acquire office equipment of $894, additional investment of $10,000 and purchase of treasury stock of $157,664 as compared to $8,121 of cash used for the purchase of equipment for the same period of 2014.
Buy-Back Program
On January 13, 2015, we issued a press release announcing that our Board of Directors approved a share repurchase program authorizing us to repurchase outstanding common stock when beneficially prudent for our company and our shareholders. As of September 30, 2015, we have purchased an aggregate of 37,852 shares of our common stock pursuant to our share repurchase program. The share repurchase program authorizing us to repurchase outstanding common stock by our company and our directors has continued through the final quarter of 2015.
Financing Activities
Net cash provided by financing activities was an aggregate of $987,004 in the nine month period ended September 30, 2015 as compared to $755,670 in the nine month period ended in September 30, 2014. In the nine month period ended September 30, 2015 we sold, in private placements, shares of common stock and common stock purchase warrants for aggregate net cash proceeds of $582,808 and received proceeds from issuance of note payable of $541,410 net with repayments of notes payable of $143,818 and received $6,604 related party advances.
Existing Capital Resources and Future Capital Requirements
Our MyoCell product candidate has not received regulatory approval or generated any material revenues. We do not expect to generate any material revenues or cash from sales of our MyoCell product candidate until commercialization of MyoCell, if ever. We have generated substantial net losses and negative cash flow from operations since inception and anticipate incurring significant net losses and negative cash flows from operations for the foreseeable future. Historically, we have relied on proceeds from the sale of our common stock and our incurrence of debt to provide the funds necessary to conduct our research and development activities and to meet our other cash needs.
At September 30, 2015, we had cash and cash equivalents totaling $65,658. However our working capital deficit as of such date was approximately $7.5 million. Our independent registered public accounting firm has issued its report dated March 16, 2015 in connection with the audit of our financial statements as of December 31, 2014 that included an explanatory paragraph describing the existence of conditions that raise substantial doubt about our ability to continue as a going concern and Note 2 of our unaudited financial statement for the quarter ended September 30, 2015 addresses the issue of our ability to continue as a going concern.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Not required under Regulation S-K for “smaller reporting companies.”
Item 4. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures, which are designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our CEO and Chief Accounting Officer, as appropriate, to allow timely decisions regarding required disclosure.
Based on their evaluation, as of September 30, 2015, our management has concluded that our disclosure controls and procedures (as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) are effective to provide reasonable assurance that information required to be disclosed by us in reports that we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, summarized, processed and reported within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management as appropriate to allow timely decisions regarding required disclosure.
Changes In Internal Control Over Financial Reporting
There were no changes in the Company’s internal controls over financial reporting during the most recently completed fiscal quarter that have materially affected or are reasonably likely to materially affect the Company’s internal control over financial reporting.
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
On November 10, 2014, the Company was served with a lawsuit by an alleged assignee and a guarantor to a Loan Guarantee, Payment and Security Agreement. These parties claim breach of that Agreement and damages of approximately $2.3 Million plus interest. The assignor and assignee also sued the Company’s directors and two past directors and an affiliate shareholder for breach of fiduciary duty, claiming damages as alleged creditors arising out of these parties’ alleged participation in Northstar Biotech Group, LLC, a secured creditor of the Company. On June 1, 2015, the Company settled the lawsuit.
Apart from the above, our Company is not involved in any material litigation pursuant to Item 103 of Regulation S-K. However, the biotechnology and medical device industries have been characterized by extensive litigation regarding patents and other intellectual property rights. In addition, from time to time, we may become involved in litigation relating to claims arising from the ordinary course of our business.
Not required under Regulation S-K for “smaller reporting companies.”
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
During the nine months ended September 30, 2015, the Company sold an aggregate of 7,852 shares of the Company’s common stock and common stock purchase warrants to purchase 1,444 shares of the Company’s common stock for aggregate gross cash proceeds of $61,270. The warrants are (i) exercisable solely for cash at an exercise prices of approximately $11.27 per share, (ii) non-transferable for six months following issuance and (iii) exercisable, in whole or in part, at any time during the period commencing on the date that is six months and one day following the date of issuance and ending on the tenth year anniversary of the date of issuance.
The issuance of such shares of our common stock was effected in reliance on the exemptions for sales of securities not involving a public offering, as set forth in Rule 506 promulgated under the Securities Act of 1933, as mended (the “Securities Act”) and in Section 4(2) of the Securities Act, based on the following: the investors confirmed to us that they were “accredited investors,” as defined in Rule 501 of Regulation D promulgated under the Securities Act and had such background, education and experience in financial and business matters as to be able to evaluate the merits and risks of an investment in the securities; (b) there was no public offering or general solicitation with respect to the offering; (c) the investors were provided with certain disclosure materials and all other information requested with respect to our company; (d) the investors acknowledged that all securities being purchased were “restricted securities” for purposes of the Securities Act, and agreed to transfer such securities only in a transaction registered under the Securities Act or exempt from registration under the Securities Act; and (e) a legend was placed on the certificates representing each such security stating that it was restricted and could only be transferred if subsequent registered under the Securities Act or transferred in a transaction exempt from registration under the Securities Act.
Item 3. Defaults Upon Senior Securities
There were no defaults upon senior securities during the period ended September 30, 2015.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None
| Exhibit No. | | Exhibit Description |
| 3.1(6) | | Amended and Restated Articles of Incorporation of the registrant, as amended |
| 3.2(9) | | Articles of Amendment to the Articles of Incorporation of the registrant |
| 3.3(37) | | Articles of Amendment to the Articles of Incorporation of the registrant |
| 3.3(8) | | Amended and Restated Bylaws |
| 4.1(5) | | Loan and Security Agreement, dated as of May 31, 2007 by and between BlueCrest Capital Finance, L.P. and the registrant |