Exhibit 10.18
| September 24, 2012 | Page 1 of 13 |
CLINICAL TRIAL AGREEMENT
THIS CLINICAL TRIAL AGREEIVIENT ("Agreement") is made by and between Johns Hopkins University, having a School of Medicine with an Office of Research Administration located at 733 North Broadway, Suite 117, Baltimore, Maryland 21205 ("Site") and Actinium Pharmaceuticals, Inc, with an address of 501 Fifth Avenue, rt Floor, New York, NY 10017 ("Actinium"). This Agreement is entered into as of the last signature (the "Effective Date").
This Agreement provides for the conduct of a clinical investigation using a proprietary drug which is not, at this time, cleared for human use by the Food and Drug Administration, Any use of this drug must be pursuant to an Investigational New Drug Exemption (IND 10807) held by Actinium. Aptiv Solutions, Inc., a Delaware corporation having a principal place of business at 1925 Isaac Newton Square, Suite 100, Reston, VA 20190 ("Ante) has been engaged by Actinium to oversee and manage the Study.
In consideration of the mutual covenants and conditions set forth in this Agreement and for good and valuable consideration, the sufficiency of which is hereby acknowledged, the parties hereby agree as follows:
| 1. | Definitions. When used in this Agreement, the listed terms shall have the following meanings: | |
| a. | "Study" means the conduct of human research using the Study Drug manufactured by Actinium on Qualified Subjects at the Site pursuant to the protocol which has been reviewed and approved by the IRH and the FDA prior to the commencement of the Study. | |
| b. | "Protocol" means the details of that certain clinical Study to be performed pursuant to this Agreement entitled A Phase I/11 Study of Low Dose Cytarabine and Actinium-255 Hum 195 in Older Patients with Untreated Acute Myeloid Leukemia, The Protocol, including any amendments is hereby incorporated by reference. and made part of this AgreeMent. | |
| c. | "MB" means the institutional review board of Site. | |
| d. | "Study Drug" means the compound known as Actinium-255 Hum 195. | |
| e. | "Study Data" means all of the data collected and records compiled during the Study relating to the conduct of the Study and/or the Study Drug. Study Data includes without limitation, all records prepared by the Investigator and all clinical research assistants, all Case Report Farms, all Screening records, all records on Qualified Subjects, Completed Subjects, Withdrawn Subjects, Uncompleted Subjects and Non-Qualified Subjects, all Informed Consent forms, all adverse/unexpected/serious or other reportable events, and all modifications, adjustments, suggestions for improvement of the Study Drug. | |
| f. | "Potential Subject" is a patient or individual who could possibly participate in the Study. | |
| g. | "Screening" is the process of identifying Potential Subjects and of conducting the examinations and tests necessary to select Qualified Subjects for the Study. | |
| h. | "Qualified Subject" is a subject who, on inclusion in the treatment phase of the Study, has met all of the inclusion criteria and none of the exclusion criteria in the Protocol and has given his/her written Informed Consent to participate in the Study. | |
| i. | "Completed Subject" is a Qualified Subject who has completed the Study and met the minimum attendance and compliance standards in the Protocol for evaluation of the safety and effectiveness of the Study Drug. | |
| j. | "Withdrawn Subject" is a Qualified Subject who has been withdrawn from the Study because of treatment failure or adverse event, but who otherwise met the Protocol entry requirements. "Uncompleted Subject" is a Qualified Subject who was initially included in the Study but who failed to complete the Study satisfactorily because of insufficient clinic attendance, poor compliance, voluntary withdrawal, and loss to fol low-up or other Protocol violations. | |
| 1. | "Non-Qualified Subject" is a subject who has not met the inclusion criteria as defined in the Protocol. | |
| m. | Informed Consent Form" means the written form agreed upon by Actinium and the Site in conformance with all applicable FDA regulations and guidances, and approved by the IRB for use in this Study. | |
| September 24, 2012 | Page 2 of 13 |
| n. | "Case Report Form (CRF)" means the report in the Actinuim format which is completed by the Investigator or his/her authorized designee documenting the use of the Study Drug in subjects. | |
| o. | "FDA" means the Food and Drug Administration of the United States Department of Health and Human Services, and any successor government agency. | |
| p. | "Investigator's Brochure" is a document describing the Study Drug, which is provided to the Investigator prior to the start of the Study. | |
| q. | "investigator" means Richard i,. Wahl , M.D., employee of Site. | |
| 2. | Compliance with laws and procedures. | |
| a. | All parties shall conduct the Study in accordance with all applicable laws, regulations and guidances, as each of the foregoing may be amended than time to time. Without limiting the foregoing, the parties expressly agree to comply with 21 CFR 312 - Investigational New Drug Application, 21 CFR 50 - Protection of Human Subjects, and 21 CFR 56 — Institutional Review Boards. | |
| b. | The Site agrees to comply with the terms of this Agreement and all IRB and FDA procedures and applicable decisions for the Study. | |
| c. | Actinium and Aptly shall comply with all applicable laws and regulations regarding subject data privacy. In addition, Actinium and Aptly will review and approve the Informed Consent and Authorization documents (collectively, the "Authorization Documents") relating to the use and disclosure of indivhtually identifiable health information of subjects enrolled in the Study ("Health Information"), including receipt and use of Health Information by Actinium and Aptiv. Actinium and Aptly agree, and Actinium and Aptiv wilt require that any party to whom Actinium and Aptly discloses Health Information ("Recipient") agrees, to use and disclose the Health Information only as permitted in the Authorization Documents and in accordance with all applicable laws and regulations. The Authorization Documents will not authorize Actinium and Aptov or any Recipient to use Health Information to recruit research subjects to additional studies, to advertise additional studies or products or to perform marketing or marketing research. | |
| 3. | Scope of Study | |
| a. | This Study is governed by this Agreement. All parties agree that no Study Drug shall be used on a subject until the IRB and the FDA have both approved the Study. After the FDA and IRB approval, the Protocol may only be amended when I) there Is written agreement between the Site, Actinium, and the Investigator to amend the Protocol, and 2) any and all such amendments have been reviewed and apprOved by the IRB and if applicable the FDA. No Protocol amendments shall be implemented until receipt of the IRB and FDA written approval. Nothing in this paragraph shall limit the Investigator's ability to act under 21 CFR 312.50, 312.60, 312.62, 312.64 (Subpart D). | |
| b. | The Site agrees to strictly comply with all IRB procedures and policies which govern the review, approval and conduct of this Study. | |
| c. | The parties agree that Screening for Qualified Subjects shall begin within thirty (30) days of receipt of the following: I) written approval of the Study and the Informed Consent by the IRB, 2) notification by Aptiv or Actinium that the FDA has granted the Investigational New Drug Application for the Study Drug, and 3) completion of Initiation Visit of the Site by Aptiv and Actinium for Study participation. The goal of the Study is to enroll up to ten ( 10) Qualified Subjects per year. The Site agrees to use reasonable efforts to complete subject enrollment as soon as practical, after commencement of Screening at each dose group. | |
| 4. | Responsibilities of Actinium | |
| a. | Actinium represents and warrants that it has the authority to enter into this Agreement on its own behalf. | |
| September 24, 2012 | Page 3 of 13 |
| b. | Actinium agrees to provide to the Site and the Investigator the information necessary to properly eontluet the Study, including without limitation, the Protocol, the Investigator's Brochure and data of any prior investigations of the Study Drug. Actinium agrees to provide any new information related to the safety and efficacy of the Study Drug as such information becomes available during the course of the Study. Actinium advises the Site and the Investigator that the effectiveness and safety in humans of the Study Drug have not been fully investigated. | |
| c. | Actinium shall provide, free of charge, the necessary quantity of the Study Drug. Actinium or Aptiv shall ship the Study Drug only to the Site. | |
| d. | Aptly shall monitor the Study and shall require evidence that IRB review and approval are obtained. | |
| e. | Actinium agrees that Institution, its affiliates and all Study team members shall have the sole authority over the clinical care of the Study subjects and nothing in this Agreement shall prevent institution or Investigator from taking any action which is, in the reasonable medical judgment of the Study team members, In the Study subject's best interest. Any time Actinium or Aptly becomes aware of a significant Study subject safety issue it will communicate such information to Institution. Actinium further agrees to promptly report to Institution the results of any monitoring reports that could affect the safety of Study participants, influence the conduct of the Study, alter the instil Minitel Review Board ("MB") approval to continue the Study, and/or affect the willingness of Study Subjects to continue in the Study. During the Study and after its completion, Actinium shall promptly report to Institution and the Investigator any Study results that could directly affect the safety or medical care of Study Subjects. | |
| 5. | Responsibilities of Site | |
| a. | The Site warrants and represents that Investigator is an employee of the Site, and is sufficiently qualified by training and experience to conduct the Study using the Study Drug. A true and complete copy of the Investigator's current curriculum vitae is attached as Exhibit B and made part of this Agreement. | |
| b. | The Site warrants that the investigator has never been involved in any investigation or research at the Site which was terminated by the FDA, National Institutes of Health (NIH) or any sponsor for non-compliance. | |
| c. | The Site warrants and represents that Investigator has not been disbarred under Section 306 of the Federal Food, Drug and Cosmetic Act, or any other section of said act or its successor, and further, that the Investigator will not use in any capacity, the services of any individual or entity which has been so disbarred, in any aspect of this Study. The Site agrees to promptly notify Actinium if the Investigator or any individual or entity involved in this Study is the subject of a disbarment proceeding or becomes disbarred. | |
| d. | In addition to and Without limiting the obligations of Section 2a above, the Site agrees to conduct the Study in strict accordance with this Agreement, the Protocol, all associated documentation provided by A ptiv (e.g. CRF, . CRP Completion guidelines, User Manuals, and Regulatory Binder documentation), applicable regulations, and all conditions of approval imposed by the reviewing IRB or FDA. The Site shall permit the use of the Study Drug only on Qualified Subjects under Investigator's personal supervision only for the purpose of the Study. The Site shall not supply the Study Drug to any other person or entity not authorized under FDA regulation to receive it, nor to any person for any purpose other than the Study. The Site shall not modify or alter the Study Drug. The Site shall maintain proper eontrol of all Study Drug inventory and return of unused quantities of Study Drug as required by regulation and directed by Actinium. | |
| The Site agrees that the Investigator will supervise or perform all testing of the Study Drug involving human subjects. | ||
| e. | The Site agrees to maintain all records and make all reports as required by regulation, the Study, the IRB and this Agreement. | |
| f. | The Site agrees to use reasonable efforts, on a diligent and continuous basis, to recruit Qualified Subjects, to prepare true and accurate Case Report Forms, to make all required reports, to complete the Study within the time limits set forth in this Agreement, and to perform all long-term follow-up examinations, visits and data collection as required by the Study and/or regulation from time to time. in addition, all CRF and Study Data shall he promptly submitted to Aptly upon written request. This provision shalt survive termination or expiration of this Agreement. | |
| September 24, 2012 | Page 4 of 13 |
| g. | The Site shall exclusively use the Informed Consent. The Site agrees that the IRB approved consent form must be provided to and acknowledged by Actinium prior to use. The Site agrees that the Investigator shall not conduct any screening procedures, enroll any Potential Stibject nor use the Study Drug on any Potential Subject who has net given written consent by signing and dating the specified informed Consent form, The Site agrees that Investigator shall ensure that all the requirements for obtaining informed consent are met. | |
| h. | The Site agrees that Investigator will follow good medical practice and exercise the customary standard of care practiced in his professional specialty. | |
| i. | The Site ensures that Investigator will provide sufficient accurate financial disclosure information to allow Actinium or Aptly to submit a complete and accurate certification or disclosure statement as required under 21 CFR part 54, as it may be amended from time to time. Further, the Site agrees that the Investigator shall promptly update this financial disclosure information If any relevant changes occur during the course of the.Study and for one (1) year fallowing completion of the study. The Site also agrees that Investigator will promptly update this financial disclosure information upon request by Aptiv. The Site understands that thiS information shall be submitted in any marketing application involving the Study Drug, This provision shall survive termination, or expiration of this Agreement. | |
| j. | The Site agrees to provide sufficient resources to the IRB to enable the 1R13 to operate as required by law, regulation and its own procedures. | |
| k. | During the Study, and subject to the terms of this Agreement, Site agrees to use reasonable efforts to cause the Investigator to conduct the Study pursuant to the Protocol and to provide to Investigator reasonable access to all Site facilities, staff and resources which the Investigator determines necessary or desirable to the conduct of the Study. All such Site facilities, staff and resources used in the Study are subject to the supervision or the Investigator. | |
| l. | The Site agrees to provide the facilities necessary to the conduct of the Study, and to notify Actinium and Aptly promptly of any failure of the Investigator, the Site or the ERB itself, to follow any of the established protocols for the Study. | |
| m. | The Site agrees to allow Aptiv and Actinium reasonable supervised access to the study site and to Facilities and staff as reasonably needed to conduct long-term follow-tip ofStudy subjects, at Apt1V's expense. The Site wilt ensure that the Investigator will be available, during mutually agreed upon regular business hours, to meet with a study monitor to review the status of the Study and discuss any pending issues, Aptiv will provide no less than five (5) days advance notice of monitoring visits and will use all reasonable efforts to coordinate the scheduling of the visits with the Investigator and Study Coordinator. | |
| n. | The Site agrees to allow Actinium and Aptly reasonable supervised access to Study Data, including without limitation, patient records (subject to patient consent), and Case Report Forms, as necessary for completion of the Study, long-term follow-up, and compliance efforts, at Actinium's expense. | |
| o. | The Site warrants and represents that it will not use in the Study, in any capacity whatsoever, whether as employee, consultant, cold-Motor or agent, the services of any individual or entity who has been disbarred under Section 306 of the Federal Food, Drug and Cosmetic Act, or any other section of said act or its successor. The Site agrees to promptly notify Aptly if any individual or entity involVed in this study is the subject or a disbarment proceeding or becomes disbarred. | |
| p. | In the event the Investigator becomes unable to complete the Protocol for any reason, Site will, to the extent possible, propose a substitute Investigator with qualifications and experience at least equal to or greater than those of the Investigator for Actinium's approval, which approval shall not be unreasonably withheld, In the event Actinium and 'Site ago upon a substitute Investigator, tins Agreement shall continue in full force and effect. If Actinium and She are unable to agree on a substitute Investigator, this Agreement may be terminated in accordance with the provisions of this Agreement. | |
| q. | Subparagraphs 5,1-51) shall survive termination or expiration of this Agreement. | |
| r. | The Site and Investigator agree to notify ANN and Actinium as soon as possible of an adverse, serious or unexpected event, or any deviation in the Protocol permitted by 21 CFR 312.60(a)(2). The Investigator shall complete all reports when and in the manner required by 21 CFR 312.62 and 312.64. The Investigator shall make all other reports as required by 21 CFR 312,62 and 312,64. |
| September 24, 2012 | Page 5 of 13 |
| s. | Tice Site agrees to cooperate with any study monitor designated by Aptly to monitor this Study. The Site agrees to cooperate. with authorized FDA employees conducting an audit or inspection, in the manner required by 21 CFR 312.68. The Investigator shall promptly notify Actinium of any request for an audit of the Study by the Site, the FDA or any other governmental agency. As required by law and Site's policy, if any inspection occurs, the Site will provide Actinium and Aptly with copies of all auditor (including FDA and 1RB) materials, correspondence, statements, forms and records that are received by the investigator or the Site. Site shall promptly implement any necessary corrective action. This provision shall survive termination or expiration of this Agreement. | |
| t. | "the Site warrants that its investigator has made all disclosures required regarding conflict of interest in connection with this Study. | |
| u. | The Site hereby assures Actinium that the Study will be reviewed and approved by its MB before any Study Drug is tested on a human subject, and further, that said 1RB is functioning in compliance with the applicable regulations and all times. The Site shall provide, upon request, evidence of In approvals related to this Study in a timely manner filing or request front Actinium or Aptly, whichever is the case. This provision shall survive termination or expiration of this Agreement. | |
| 6. | Payment | |
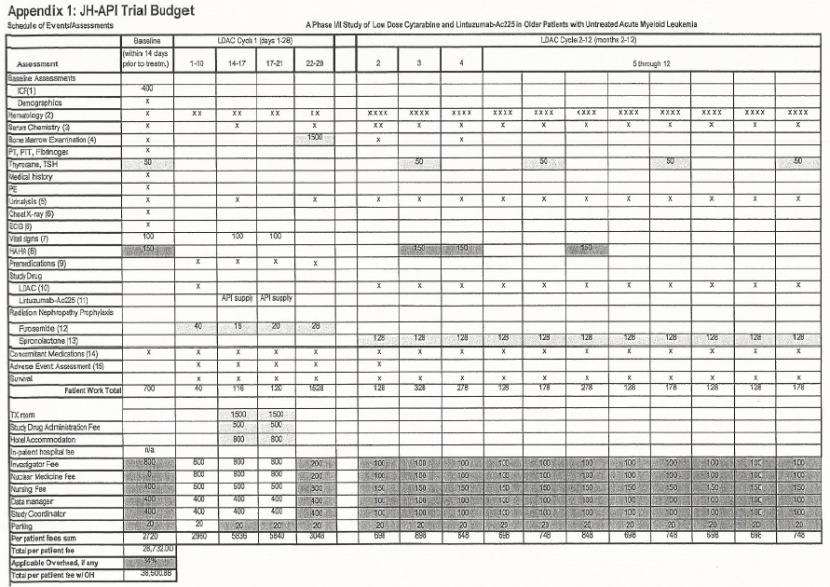
| a. | Actinium will pay to Site, subject to the terms and conditions of this Agreement the sum of $38,500,88 for each Completed Subject of the Study, as set Ruth in the Site Budget and Payment schedule, attached to and made part of this Agreement as Appendix 1, In no event shall payments to the Site exceed $38,500.88 for each Completed Subject up to a maximum of sixty (60) Completed Subjects. All payments are gross in US Dollars; all approved invoices are net thirty (30) days. | |
Payment of all sums due hereunder shall be made by check payable to Site as follows: | ||
| Payable to: | Johns Hopkins University | |
| Tax ID No: | 52-0595110 | |
| Address: | Johns Hopkins University Central Lockbox | |
| Bank of America | ||
| 12529 Collections Center Drive | ||
| Chicago, IL 60693 | ||
| Checks must also include the following: | ||
| IPN: | 13031596 | |
| Investigator: | Richard L. Wahl, M.D. | |
| b. | Any additional payments must be approved in advanCe by Actinium in writing. | ||
| e. | The Site agrees to be responsible for invoicing Actinium in accordance with the Budget and Payment Schedule (Exhibit C). | ||
| d. | Any equipment (except for the Study Drug) purchased by the Site as part of the Protocol shall be owned by the Site, shall be physically located at Site, and shall remain the property of Site following completion of the Study. | ||
| e. | In no event is Actinium required to make any payment for any costs incurred with respect to Non-Qualified Subjects entered into the treatment phase of the Study or for any Subject who has not given written Informed Consent to participate in the Study. | ||
| f. | Nothing contained herein shall be construed as requiring She, the Investigator or any Site research staff to work on any project or process which is prohibited by law or by any international treaty to which the United States of America is a party, or which may be harmful or detrimental to public health, patient safety or good clinical care or which may be considered to be immoral. No payment is subject to submission of favorable clinical results or evaluations. | ||
| September 24, 2012 | Page 6 of 13 |
| g. | Notwithstanding the foregoing, in no event shall any payment be made under this Agreement which is contrary to 42 LiSC 1320a-7b, as it or any successor law may be in effect from time to time. In accordance with the statute, in no event shall the Investigator or any member of his immediate family, receive any payment, loyalty, form of compensation, or remuneration of any nature, sort or description, for any use of all or any portion of the Study Drug by any hospital, clinic or other Site where he works. In no event shall any request for reimbursement or payment under any private or public health insurance carrier he made which is contrary to law. | ||
| 7. | Publications | ||
| (a) Actinium acknowledge that the Site and the. Investigator are free to publish, present, or use any results arising out of this Study for their own instructional, research, or publication objectives, provided that such publication does not disclose any of Actinium's Proprietary Information, as defined in this Agreement. Site agrees to submit the draft of any proposed publication to Actinium at least thirty (30) days prior to submission for publication, presentation, or use, and agrees, at the request of Actinium, to withhold any such submission for an additional period, not to exceed ninety (90) days to allow Actinium to the patent applications or to take any other action designed to protect its patent rights. | |||
| (b) The parties recognize that because this is a multi-center Study, the Site and the Investigator agree that the first publication of the results of the Study shall be made in conjunction with the presentation of a joint, multi-center publication of the Study results, with the investigators froM all sites contributing data, analyses, and comments. Actinium shall provide all Investigators access to the combined results and data provided by all sites performing the study. However, if no multi-site publication is submitted within twelve (12) months of the completion of the Study from all sites, or a multi-center publication is published in a shorter time the Site and the InVestigator shall be free to publish the Study results from their Site individually, subject to Actinium's rights under Section 7(a) of this Agreement. | |||
| 8. | Confidential information | ||
| a. The parties acknowledge that as part of the scientific collaboration between Actinium, Aptiv and the Site in connection with the Study, Aptly or Actinium may find it necessary to disclose certain confidential and proprietary information and trade secrets of Actinium and/or Aptiv. Such confidential and proprietary information includes, without limitation, the Protocol, all intellectual property contained ie the Study Drug, the design and manufacturing procesSes utilized to produce and test the Study Drug, the identity of Actinium's suppliers, data concerning scientific discoveries made by Actinium and/or Aptiv; Actinium's manufacturing strategies and processes; Actinium's marketing plans; data from Actinium's evaluations in animals and humans; Actinium's strategy kw or status of regulatory approval; or Actinium's forecasts of sales and sales data, and any other information which by its nature would be considered confidential (hereafter referred to collectively as "Actinium Confidential Information"), Such Actinium Confidential Information shall remain the confidential and proprietary properly of Actinium and shall he disclosed to Site's employees, affiliates or agents on a "need to know" basis, and who are bound by similar obligations to protect the Actinium Confidential Information from unauthorized disclosure. | |||
| b. The Site may find it necessary to disclose certain confidential and proprietary information and trade secrets of Site to Aptiv Such confidential and proprietary information includes, any data, records or other information disclosed to Apliv, or its designee, (hereinafter collectively, "Site Confidential Information"). Such Site Confidential information shall remain the confidential and proprietary property of Site and shall be disclosed to A ptiv's or its designees, employees, affiliates or agents on a "need to know" basis. | |||
| c. The Site and Actinium shall have joint ownership plait Confidential Data that is generated by this Study. Confidential Data shall include all Study results, which includes information entered onto patient case report forms and patient medical records. Actinium shall have the sole tight to use such Confidential Data for all commercial purposes, and Sites use shall be thrilled to those instances dealing with patient care and treatment, academic uses, and publication. Actinium shall have sole ownership of the original copies of all patient case report forms; however, Site shall have the right to retain one copy of each forth for documentation purposes. Notwithstanding anything to the contrary herein, Site may use study results in developing Sponsor Inventions or Other Inventions (as those toms are defined) and it will not be deemed a commercial use pursuant to this Section 8. | |||
| September 24, 2012 | Page 7 of 13 |
| Subject to the terms and conditions of the Agreement, each party hereby agrees that during the term of the Study Agreeinent and for a period of three (3) years thereafter, neither party shall (i) publicly divulge, disseminate, publish or otherwise disclose any of the other party's confidential information without prior written consent; (ii) limit access to each party's confidential information to those of the other party's, co-workers and staff who are involved in the Study and have a need for such confidential information in connection with the conduct of the Study, and (iii) cause the return to the other party, as the case may be, any and all documents, drawings, sketches, designs, products or samples containing confidential information, together with any copies thereof, promptly. upon termination by this Agreement or upon the other party's request therefore, provided that such obligations undertaken by the said party shall remain in force for five (5) years after completion of the Study with respect to the Chemical Manufacturing and Control Section, Toxicity Studies or Performance Studies. | |||
| d. | Notwithstanding the foregoing, the obligations of confidentiality and nondisclosure shall not apply to the following information: | ||
| (1) | Information that was in the public, domain prior to the date of disclosure to the receiving party coming into possession thereof, or becomes part of the public domain by publication or otherwise through no fault or unauthorized act or omission on the part of the receiving party; | ||
| (2) | Information that is disclosed to the receiving party by a third party legally entitled to disclose such information, as demonstrated by competent evidence; | ||
| (3) | Information that was rightfully in the possession of or already known to the receiving party as demonstrated by prior written records or other reliable evidence; | ||
| (4) | Information that is independently developed by the receiving party without reference to any confidential information, as demonstrated by competent evidence; or | ||
(5) | Information that is required to be disclosed to a government authority or by order of a court of competent jurisdiction, provided that (a) such disclosure is subject to all applicable governmental or judicial protection available for like material; (b) reasonable advance notice is given to the disclosing party; and (c) the receiving party take all reasonable steps to limit the scope of such disclosure. | ||
| The terms of this Agreement supersede any previous non-disclosure agreements or any other preliminary representations or understandings that have been entered into by the parties to this Agreement with regard to the subject Study. The terms or this Agreement will be treated as confidential; however, the existence ()lithe Agreement and Study will not be confidential. The Site may maintain one archival copy of all Proprietaty Information for the purpose of demonstrating its compliance with Its obligations hereunder. | |||
| e. | Site, in accordance with its policies and procedures, may post the Protocol on its internal database (referred to as "FYI") and share the Protocol, or portions thereof, as 'tummy i) to comply with applicable laws and regulations; ii) for internal patient care billing audits with Site's affiliates, and iii) to provide information to third party payors as necessary, in connection with the processing or payment all claim submitted in relation to a Study subject, Site shall also be allowed to post a synopsis of the Protocol on its recruitment website. | ||
| In addition, the parties agree that the Investigator may disclose the title of the Study on his curriculum vitae and grant application(s), | |||
| 9. | Intellectual Property | ||
The parties further acknowledge and agree that Actinium is the owner or authorized licensee of the Study Drug. Neither Site nor Investigator shall obtain any license to make, have made, sell, distribute, rent, lease, or otherwise transfer or use the Study Drug or Actinium Confidential Information, or their derivatives. Actinium Confidential Information is licensed for use only on and in combination with the Study Drug, and may not be used on or with third party products without Aptiv's prior express written permission. This Agreement grants no implied rights.
| September 24, 2012 | Page 8 of 13 |
Aptly on behalf of Actinium, hereby grants to Site a non-exclusive royally free license to the Intellectual Property for Site's own internal nonprofit research and education related purposes.
It is expressly agreed that neither Actinium nor Site transfer by operation of this Agreement to the other party hereto any patent right, copyright, or other proprietary right that either party owns or controls, except as specifically set forth herein.
Site agrees that any inventions, discoveries, or Improvements arising out of work performed hereunder that are dependent on Actinium's patent claims or are expressly anticipated by the protocol (hereinafter "Actinium's Inventions") shall bo assigned to Act:Minim and shall be-promptly disclosed by Site to Actinium.
All other inventions developed under this Agreement ("Other Inventions") that are developed solely by Site shall be owned by Site, All Other Inventions developed by one or more employees of both Actinium and She under this Agreement shall he ownedjointly by Actinium and Site Site shall grant Actinium no option to negotiate to obtain an exclusive, royalty bearing, worldwide license, including the right to sublicense, to make, have made, use, and sell products incorporating such sole Other Inventions or Institution's rights to jointly owned Other Inventions. Actinium's option may be exercised at any time during a period of one hundred and eighty (180) days (the "Option Period") after the written submission to Actiunium of each such Invention by notice in writing from Actinium to Site. Upon Actinium's exercise of its option with regard to any particular Invention, Site and Actinium will negotiate in good faith in an attempt to reach a license agreement satisfactory to both parties (the "Negotiation Period"). Unless extended by the written mutual consent of the parties, the Option Period and the Negotiation Period shall not exceed ten (10) months in the aggregate. Upon the expiration (lithe unexcreised option or the NegOtialion Period, Site shall have no further obligation to Actinium under this Agreement with regard to specific Other Inventions tinder consideration.
| 10. | Indemnification | ||
| a. | The Site shall, to the extent authorized by applicable law, indemnify, defend and hold harmless Actinium and Aptiv, their agents and employees (collectively the "Indemnitees") from any and all liabilities, claims, actions, or suits (collectively "Claims") resulting from the negligence or wrongful acts or omissions of the Site, the Investigator, their agents or employees pertaining to the activities of this Study and/or this Agreement, provided, however, that: | ||
| (i) | the Site shall not indemnify, defend and hold harmless the indemnitees from Claims arising out of the negligence or wrongful acts or omissions of the Indemnitees; | ||
| (ii) | the Site is promptly, and in any event within thirty (30) days after an I Wein ni tee's receipt of notice of any complaint, claim or injury relating to any loss subject to this indemnification, notified In writing of any such complaint, claim or injury; | ||
| (iii) | the Site has sole control over the detense and settlement army such claim or suit, including the right to select defense counsel and to direct the defense or settlement of any such claim or suit, provided that Site shall not admit fault or liability on behalf of any Indemnitee in the defense and settlement of such claim or suit; and | ||
| (iv) | the Indemnitees reasonably cooperate with the Site and its legal representatives in the investigation and defense of any claims or suits covered under this Section 12(b), | ||
| b. | Actinium indemnification. | ||
| Actinium and Aptiv shall indemnify, defend and hold harmless the Site, Investigator, The Johns Hopkins Hospital, The Johns Hopkins Bayview Medical Center, and/or other affiliated and cooperating hospitals and institutions, as well as its trustees, directors, ()Moors, medical and professional staff, affiliates, employees, students, the members of the Institutional Review Boards, and other holding academic appointments within those institutions and agents and their respective successors, heirs and assigns (collectively the "Site Indemnitees"), against any liability, damage, loss or expense (including reasonable attorneys' fees and expenses of litigation) incurred by or imposed upon the Site Indemnitees or any one of them in connection with any third party claims, suits, actions, demands or judgments that arise from Site Indemnities participation in and/or performance of the subject Study | |||
| September 24, 2012 | Page 9 of 13 |
| Actinium's indemnification shall not apply to any liability, damage, loss or expense attributable to (i) the negligent activities, reckless misconduct or intentional misconduct of the Site Indemnitees; (ii) (iii) failure of the Site I ndemnitees to adhere to the terms of the Protocol for the Study or follow all prior written instructions provided by Actinium or Aptly, (iv) actions of the Site Indemnitees in violation of applicable laws or regulations , or (v) material breach of this Agreement by the Site Indemnitees. | |||
| This obligation to indemnify is subject to the Site Indemnitees giving Actinium prompt notice of any claim, suit or demand and full control of any defense and settlements of such claim, suit or demand. The Site inderrinitees will also notify Actinium promptly in the event any one of them becomes aware of any potential claim, or likelihood of any potential claim of indemnification rights under this Section, Site Indemnitees will cooperate fully, at Actinium's expense, in the defense or settlement orally claim or action. | |||
| This Paragraph shall survive expiration or termination of this Agreement. | |||
| c. | Study-Related Injury. | ||
| Subject to Section 10(b), above, Actinium agrees to reimburse the Site, other accredited medical care providers, or Study participants (as appropriate) for all reasonable costs incurred for the care and treatment of any illness or injury to a Subject resulting from his or her participation in the Study and that is not covered by the participant's medical insurance. | |||
| 11. | Insurance | ||
| The Site and Actinium shall, at its sole cost and expense, procure and maintain commercial general liability insurance or equivalent self insurance in amounts not less than V million per incident and $5 million annual aggregate with tweet to the Study. | |||
| 12. | Term and Termination | ||
Unless earlier terminated in accordance with its terms, this Agreement shall commence on the date when it is signed by all parties, (the "Effective Date", and shall continue in full force and effect until two (2) years after the Study has been completed.
| a. | This Agreement shall be terminated immediately in the event that: I) the authorization and IND issued by the FDA is withdrawn, or 2) the approval of the IRE is withdrawn. | ||
| b. | Except as otherwise provided in this section, any party may terminate this Agreement upon sixty (60) days prior written notice in the event of any material breach by another party of any material term or condition hereof; provided such breach is not cured within said sixty (60) day notice period. | ||
| c. | Any party may terminate or suspend this Study immediately for the safety of Subjects, pursuant to applicable regulations. In such case, the patty terminating or suspending the study will provide prompt written notice to the other party. | ||
| d. | Any party may terminate this Agreement upon written notice immediately in the event a party engages in criminal, unprofessional or fraudulent conduct. | ||
| e. | AO)/ may terminate this Agreement upon sixty (60) days prior written notice in the event that: I) the Protocol is suspended by the 1R.13; 2) the Principal Investigator is unable to complete the Study and a substitute Principal Investigator cannot be agreed upon, or 3) if circumstances reasonably beyond Site's control preclude the Site from continuing the Study, and such suspension of the Study exceeds sixty (60) consecutive days or ninety (90) days in the aggregate In any year during the term (or renewal) of this Agreement. | ||
| September 24, 2012 | Page 10 of 13 |
| f. | Aptly may terminate this Agreement at any time upon one (1) month prior written notice to the Site. In such case, Aptly will provide funding of expenses actually incurred under the Study prior to the date of said notice or prior to ii patient's completion or the Study if said completion is in the best interest of the patient as reasonably determined by the Investigator. Site may terminate this Agreement at any time upon ninety (90) days prior written notice to Aptly. | ||
| g. | Any provision of this Agreement, which provides continuous enforcement or operation thereof after the termination hereof, shall survive the termination of this agreement. | ||
| 13. | Effect of Termination | ||
| Except as otherwise provided herein, termination of this Agreement shall not be construed to release either party from any obligation hereunder which has matured prior to the date of said termination. Upon termination of this Agreement, Site shall promptly return to Aptly the Study Drug, Study Data, including without limitation, all CRF and Actinium Confidential Information at Aptiv's expense. | |||
| 14. | Translation Services | ||
| The Site shall provide the consent form applicable to the Study in written Form translated to the appropriate language to any non-English speaking minorities included in the Study. Any cost incurred by the Site for the development of a translated informed consent from the English original will be agreed upon in advance and reimbursed by Aptiv. The Site shall present Aptly with an invoice For translation services which Aptiv shall reimburse to the Site within thirty (30) days of receipt. The parties acknowledge that said translation costs are not included in the Study budget set out in Appendix 13 and are not included in the Total Cost of the Study. | |||
| 15. | Communications | ||
| All medical/scientific and other communications, reports and notices shall be delivered by hand, by facsimile, by secure electronic means or sent by first class mail postage prepaid and addressed as follows: | |||
| If to Aptiv: | Anthony Apieella | |
278 l lalfway Pond Rd Plymouth, MA 02360 Phone: 805-791-5305 Email: Anthorly.Apicella@aptivsolut ions,com | ||
| If to Actinium: | Dragon Ode, COO/CMO 501 Fifth Avenue, 314 Floor New York, NY 10017 | |
| If to Site: | Michael B. Amey | |
Associate Dean, Research Administration Office of Research Administration The Johns Hopkins University School of Medicine 735 North Broadway, Suite 117 |
| September 24, 2012 | Page 11 of 13 |
Baltimore, Maryland 21205 Phone: 410-955-1566 Fax: 410-502-6004 Email: intuncylahmi.cdu | |
| With a copy to Investigator: | Richard Wahl, M.D. |
601 North Caroline Street JI-TOC #3223 Baltimore, MD 21287 Phone: 410-614-3764 Fax: 443-287-2933 Email: nyillAgliml.edu | |
| With a copy to: | John Crandall |
601 North Caroline Street JHOC ff4230 Baltimore, MD 21287 Phone: 410-502-2186 Fax: 410-614-9979 Email: jcrandalghtni.edu | |
| If to Accounts Receivable: | Lee Ann Comeau |
Research Department of Radiology The Johns Hopkins University Park East Rini/306F 600 N. Wolfe Street Baltimore MD 21287 (tel) 410-614-9173 (fax) 410-614-6150 Icoutcal@liani.edu |
| 16. | Use of Names |
Except as otherwise required by law, each party agrees not to use directly or by implication the names of the other party, nor any of the other party's affiliates or contractors, nor any abbreviations thereof, or of any staff member, faculty member, student, or employee of the other pad), in connection with any products, publicity, promotion, financing, advertising, or other public disclosure without the prior written permission of the other party or individual whose name or employee's name is to be used.
| 17. | General Provisions |
| a. | All rights and remedies hereunder are exclusive and not cumulative. |
| b. | This Agreement may be amended only by written agreement signed by all parties. |
| September 24, 2012 | Page 12 of 13 |
| c. | It is expressly agreed by the parties hereto that the Site, the Investigator and Aptiv are independent contractors and nothing in this Agreement is intended to create an employer relationship, joint venture, or partnerships between the parties. No party has the authority to bind any other. |
| d. | This Agreement, including all exhibits, constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all proposal, negotiations and other communications between the parties, whether written or oral, with respect to the subject matter hereof. |
| e. | If any provisions of this Agreement shall be held to be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions or this Agreement shall not be impaired thereby, and the party against whom the holding is made, shalt be entitled to substitute a similar provision that preserves the benefit of the bargain. |
| f. | The failure of any party to insist on strict performance of any provision of this Agreement or exercise any right hereunder will not constitute a waiver of that provision or right. |
| g. | This Agreement may be executed in any number of counterparts, each of which shall be deemed an original as against the party whose signature appears thereon, but all of which taken together shall constitute but one and the same instrument. |
| h. | Each party hereto agrees to execute, acknowledge and deliver such further instruments and do all such further acts as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement. |
| i. | The paragraph headings contained in this Agreement are for reference purposes only and shall not in any way affect the meaning or interpretation of this Agreement. |
This Agreement shall be governed by and construed in accordance with the laws of the State of Maryland (excepting any conflict of laws provisions which would serve to defeat application of Maryland substantive law), Each of the Parties hereto agrees to venue in and submits to the exclusive jurisdiction of the state and/or federal courts located within the State of Maryland for any Shit, hearing or other legal proceeding of every nature, kind and description whatsoever in the event of any dispute or controversy arising hereunder or relating hereto, or in the event any ruling, finding or other legal determination is required or desired hereunder.
IN WITNESS WHEREOF, the parties intending to be legally bound have caused this Agreement to be executed by their duly authorized representatives or, in the case of the Investigator, have duly executed this Agreement, on the dates stated beneath their names:
SITE Accepted by: THE JOHNS HOPKINS UNIVERSITY | |||
| By: | /s/ Michael B. Atney | ||
Michael B. Atney Associate Dean for Research Administration | |||
| Date: | 9/25/12 | ||
| September 24, 2012 | Page 13 of 13 |
ACTINIUM PHARMACEUTICALS, INC. Accepted by: | |||
| Name: | DRAGAN CICIC | ||
| Title: | COOICMO | ||
| Dated: | 9/26/2012 | ||
Read and Agreed to abide by the terms contained herein, but not as a party hereto :
INVESTtGATOR:
| /s/ Richard L | |||
| Name: | Richard L, Wahl, MD | ||
| Investigator: | |||
| Dated: | 9-26-12 | ||