 To Engage in Business Combination with
To Engage in Business Combination with
KBL Healthcare Acquisition Corp. III (NYSE Amex: KHA) Investor Presentation PRWT Services, Inc. May 8, 2009
www.kblhealthcare.com www.prwt.com
 2 Interests of the Parties in the Merger KBL HEALTHCARE ACQUISTION CORP. III (“KBL”) AND PRWT SERVICES, INC. (“PRWT”) ARE HOLDING PRESENTATIONS FOR CERTAIN OF KBL’S STOCKHOLDERS, AS WELL AS
2 Interests of the Parties in the Merger KBL HEALTHCARE ACQUISTION CORP. III (“KBL”) AND PRWT SERVICES, INC. (“PRWT”) ARE HOLDING PRESENTATIONS FOR CERTAIN OF KBL’S STOCKHOLDERS, AS WELL AS
OTHER PERSONS WHO MIGHT BE INTERESTED IN PURCHASING KBL SECURITIES, REGARDING THE BUSINESS COMBINATION BETWEEN KBL AND PRWT, AS DESCRIBED IN
THE PRELIMINARY PROXY STATEMENT/PROSPECTUS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION ON APRIL 22, 2009, AS SAME IS AMENDED FROM TIME TO
TIME. SUCH PRELIMINARY PROXY STATEMENT/PROSPECTUS, INCLUDING SOME OR ALL OF THE EXHIBITS ATTACHED THERETO, AND THIS PRESENTATION WILL BE
DISTRIBUTED TO PARTICIPANTS AT SUCH PRESENTATIONS. CITIGROUP GLOBAL MARKETS, INC. (“CITIGROUP”), JEFFERIES & COMPANY, INC. (“JEFFERIES”) AND EARLYBIRDCAPITAL, INC. (“EBC”), EACH AN UNDERWRITER OF KBL’S
INITIAL PUBLIC OFFERING (“IPO”) CONSUMMATED IN JULY 2007, ARE ASSISTING KBL IN THESE EFFORTS WITHOUT CHARGE, OTHER THAN THE REIMBURSEMENT OF THEIR
OUT-OF-POCKET EXPENSES. ADDITIONALLY, THE UNDERWRITERS DEFERRED $4,140,000 OF THE COMMISSIONS OWED TO THEM IN CONNECTION WITH THE IPO UNTIL THE
CLOSING OF KBL’S BUSINESS COMBINATION. FURTHER, KBL HEALTHCARE MANAGEMENT, INC. (“KHMI”), AN AFFILIATE OF CERTAIN OF THE EXECUTIVE OFFICERS AND
DIRECTORS OF KBL, HAS ENTERED INTO A GENERAL ADVISORY AGREEMENT WITH PRWT, WHICH WILL BECOME EFFECTIVE UPON CONSUMMATION OF THE BUSINESS
COMBINATION BETWEEN KBL AND PRWT, UNDER WHICH KHMI WOULD BE PAID A FEE OF $250,000 PER YEAR IN CONNECTION WITH SERVICES TO BE RENDERED TO PRWT,
AND CERTAIN OF PRWT’S OFFICERS WILL ENTER INTO NEW EMPLOYMENT AGREEMENTS TO BE EFFECTIVE UPON CONSUMMATION OF THE BUSINESS COMBINATION. KBL AND ITS DIRECTORS AND EXECUTIVE OFFICERS, PRWT AND ITS STOCKHOLDERS, DIRECTORS AND EXECUTIVE OFFICERS, AND THEIR RESPECTIVE AFFILIATES, MAY
ENTER INTO ARRANGEMENTS TO PURCHASE SHARES OF COMMON STOCK AND/OR WARRANTS OF KBL IN OPEN MARKET OR PRIVATELY NEGOTIATED TRANSACTIONS PRIOR
TO THE CONSUMMATION OF THE BUSINESS COMBINATION. KBL AND ITS DIRECTORS AND EXECUTIVE OFFICERS, PRWT AND ITS STOCKHOLDERS, DIRECTORS AND EXECUTIVE OFFICERS, AND EACH OF CITIGROUP, JEFFERIES AND
EBC MAY BE DEEMED TO BE PARTICIPANTS IN THE SOLICITATION OF PROXIES FOR THE SPECIAL MEETING OF KBL STOCKHOLDERS TO BE HELD TO APPROVE THE MERGER. STOCKHOLDERS OF KBL AND OTHER INTERESTED PERSONS ARE ADVISED TO READ KBL’S AND PRWT’S PRELIMINARY PROXY STATEMENT/PROSPECTUS AND, WHEN
AVAILABLE, KBL’S AND PRWT’S DEFINITIVE PROXY STATEMENT/PROSPECTUS IN CONNECTION WITH KBL’S SOLICITATION OF PROXIES FOR THE SPECIAL MEETING BECAUSE
THESE PROXY STATEMENT/PROSPECTUSES WILL CONTAIN IMPORTANT INFORMATION. SUCH PERSONS CAN ALSO READ KBL’S FINAL PROSPECTUS, DATED JULY 19, 2007,
FOR A DESCRIPTION OF THE SECURITY HOLDINGS OF THE KBL OFFICERS AND DIRECTORS AND OF THE UNDERWRITERS AND THEIR RESPECTIVE INTERESTS IN THE
SUCCESSFUL CONSUMMATION OF THIS BUSINESS COMBINATION. THE DEFINITIVE PROXY STATEMENT/PROSPECTUS WILL BE MAILED TO KBL STOCKHOLDERS AS OF THE
RECORD DATE TO VOTE ON THE ACQUISITION. STOCKHOLDERS WILL ALSO BE ABLE TO OBTAIN A COPY OF THE DEFINITIVE PROXY STATEMENT/PROSPECTUS, WITHOUT
CHARGE, BY DIRECTING A REQUEST TO: KBL HEALTHCARE ACQUISTION CORP. IIII, 380 LEXINGTON AVENUE, 31ST FLOOR, NEW YORK, NEW YORK 10168. THE DEFINITIVE
PROXY STATEMENT/PROSPECTUS CAN ALSO BE OBTAINED, WITHOUT CHARGE, AT THE SECURITIES AND EXCHANGE COMMISSION’S INTERNET SITE (HTTP://WWW.SEC.GOV).
 3 Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS REGARDING KBL’S
3 Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS REGARDING KBL’S
AND/OR PRWT’S OR THEIR RESPECTIVE MANAGEMENT’S EXPECTATIONS, HOPES, BELIEFS, INTENTIONS OR STRATEGIES REGARDING THE FUTURE. IN ADDITION, ANY
STATEMENTS THAT REFER TO PROJECTIONS, FORECASTS OR OTHER CHARACTERIZATIONS OF FUTURE EVENTS OR CIRCUMSTANCES, INCLUDING ANY UNDERLYING
ASSUMPTIONS, ARE FORWARD-LOOKING STATEMENTS. THE WORDS “ANTICIPATE,” “BELIEVE,” “CONTINUE,” “COULD,” “ESTIMATE,” “EXPECT,” “INTEND,” “MAY,” “MIGHT,” “PLAN,”
“POSSIBLE,” “POTENTIAL,” “PREDICT,” “SHOULD,” “WOULD” AND SIMILAR EXPRESSIONS MAY IDENTIFY FORWARD-LOOKING STATEMENTS, BUT THE ABSENCE OF THESE
WORDS DOES NOT MEAN THAT A STATEMENT IS NOT FORWARD-LOOKING. FORWARD-LOOKING STATEMENTS MAY INCLUDE, FOR EXAMPLE, STATEMENTS ABOUT OUR:
ABILITY TO COMPLETE A COMBINATION WITH ONE OR MORE TARGET BUSINESSES; SUCCESS IN RETAINING OR RECRUITING, OR CHANGES REQUIRED IN, OUR OFFICERS,
KEY EMPLOYEES OR DIRECTORS FOLLOWING A BUSINESS COMBINATION; OUR MANAGEMENT TEAM’S ALLOCATION OF THEIR TIME TO OTHER BUSINESSES AND
POTENTIALLY HAVING CONFLICTS OF INTEREST WITH OUR BUSINESS OR IN APPROVING A BUSINESS COMBINATION; POTENTIAL INABILITY TO OBTAIN ADDITIONAL
FINANCING TO COMPLETE A BUSINESS COMBINATION; AN INABILITY TO SATISFY THE CONDITIONS TO THE BUSINESS COMBINATION; LIMITED POOL OF PROSPECTIVE
TARGET BUSINESSES; POTENTIAL CHANGE IN CONTROL IF WE ACQUIRE ONE OR MORE TARGET BUSINESSES FOR STOCK; PUBLIC SECURITIES’ LIMITED LIQUIDITY AND
TRADING; FAILURE TO LIST OR DELISTING OF OUR SECURITIES FROM THE NYSE AMEX STOCK EXCHANGE OR AN INABILITY TO HAVE OUR SECURITIES LISTED ON THE
NASDAQ STOCK EXCHANGE FOLLOWING A BUSINESS COMBINATION; USE OF PROCEEDS NOT IN TRUST OR AVAILABLE TO US FROM INTEREST INCOME ON THE TRUST
ACCOUNT BALANCE; OUR FINANCIAL PERFORMANCE FOLLOWING THE BUSINESS COMBINATION; PRWT’S INABILITY TO ACHIEVE ITS BUSINESS OBJECTIVES OR IMPLEMENT
ITS STRATEGIES OR MAINTAIN ITS MINORITY COMPANY CERTIFCATIONS; OR AN INABILITY TO SECURE CONTRACTS CURRENTLY BEING NEGOTIATED OR TO BE NEGOTIATED
IN THE FUTURE. THE FORWARD-LOOKING STATEMENTS CONTAINED IN THIS PRESENTATION ARE BASED ON OUR CURRENT EXPECTATIONS AND BELIEFS CONCERNING FUTURE
DEVELOPMENTS AND THEIR POTENTIAL EFFECTS ON US. THERE CAN BE NO ASSURANCE THAT FUTURE DEVELOPMENTS AFFECTING US WILL BE THOSE THAT WE HAVE
ANTICIPATED. THESE FORWARD-LOOKING STATEMENTS INVOLVE A NUMBER OF RISKS, UNCERTAINTIES (SOME OF WHICH ARE BEYOND OUR CONTROL) OR OTHER
ASSUMPTIONS THAT MAY CAUSE ACTUAL RESULTS OR PERFORMANCE TO BE MATERIALLY DIFFERENT FROM THOSE EXPRESSED OR IMPLIED BY THESE FORWARD-
LOOKING STATEMENTS. THESE RISKS AND UNCERTAINTIES INCLUDE, BUT ARE NOT LIMITED TO, THOSE FACTORS DESCRIBED UNDER THE HEADING “RISK FACTORS.”
SHOULD ONE OR MORE OF THESE RISKS OR UNCERTAINTIES MATERIALIZE, OR SHOULD ANY OF OUR ASSUMPTIONS PROVE INCORRECT, ACTUAL RESULTS MAY VARY IN
MATERIAL RESPECTS FROM THOSE PROJECTED IN THESE FORWARD-LOOKING STATEMENTS. WE UNDERTAKE NO OBLIGATION TO UPDATE OR REVISE ANY FORWARD-
LOOKING STATEMENTS, WHETHER AS A RESULT OF NEW INFORMATION, FUTURE EVENTS OR OTHERWISE, EXCEPT AS MAY BE REQUIRED UNDER APPLICABLE SECURITIES
LAWS AND/OR IF AND WHEN MANAGEMENT KNOWS OR HAS A REASONABLE BASIS ON WHICH TO CONCLUDE THAT PREVIOUSLY DISCLOSED PROJECTIONS ARE NO LONGER
REASONABLY ATTAINABLE. THIS CURRENT REPORT AND THE EXHIBITS HERETO INCLUDES CERTAIN FINANCIAL INFORMATION NOT DERIVED IN ACCORDANCE WITH GENERALLY ACCEPTED
ACCOUNTING PRINCIPLES ("GAAP"). KBL AND PRWT BELIEVE THAT THE PRESENTATION OF THESE NON-GAAP MEASURES PROVIDE INFORMATION THAT IS USEFUL TO
INVESTORS. WE HAVE INCLUDED A RECONCILIATION OF THIS INFORMATION TO THE MOST COMPARABLE GAAP MEASURES WHERE APPLICABLE.
 Executive Summary Transaction Consideration Management
Executive Summary Transaction Consideration Management
and Board PRWT and KBL (NYSE Amex: KHA) entered into a definitive merger agreement on March 13, 2009 The combined company will be named PRWT and will apply for listing on the NASDAQ Expected closing no later than July 2009 Approximately $142.3 million upfront consideration at closing: 11.95 million shares (~$93.8 million valued at $7.85 estimated cash held in trust per share) $3.5 million cash to current stockholders of PRWT Assumption of $45.0 million of PRWT’s net debt Up to 8 million additional shares issuable upon the achievement of EBITDA targets and/or
occurrence of certain events PRWT shareholders entered into long-term lock-up agreement (up to 3 years) Current executives remain in place Management has long-standing business relationships in all of its operating industries Nine person board appointed by PRWT and KBL Company
Overview Rapidly-growing, minority-controlled pharmaceutical services company with legacy businesses in
facilities management and business processing services (BPS) Founded in 1988, headquartered in Philadelphia, national footprint, 1,300+ employees Over $240 million revenue currently contracted for 2009 4
 Investment Highlights 5 $1 billion potential opportunity based on existing pharmaceutical platform State-of-the-art pharmaceutical facility with significant under-utilized capacity, strong operating leverage and
Investment Highlights 5 $1 billion potential opportunity based on existing pharmaceutical platform State-of-the-art pharmaceutical facility with significant under-utilized capacity, strong operating leverage and
contracts with industry leaders, including Merck and Sigma-Aldrich Fine Chemicals Leading minority-controlled supplier positioned to capture significant portion of $100 billion annual corporate
diversity spend Recurring revenue base under long-term contracts with a combined backlog of approximately $870 million;
additional >$500 million under negotiation Scalable platform to deliver rapid sales and EBITDA growth Proven and incentivized management team with average 35+ years experience and unique ability to access
new opportunities Demonstrated commitment to transaction via $2 million purchase of KBL warrants by PRWT (May 6, 2009)
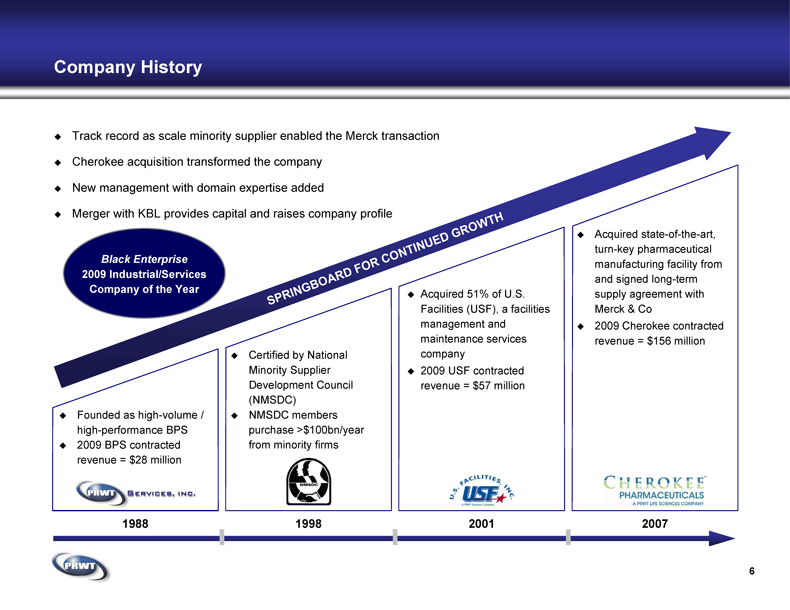 Company History Certified by National
Company History Certified by National
Minority Supplier
Development Council
(NMSDC) NMSDC members
purchase >$100bn/year
from minority firms Acquired 51% of U.S.
Facilities (USF), a facilities
management and
maintenance services
company 2009 USF contracted
revenue = $57 million 1998 2007 Founded as high-volume /
high-performance BPS 2009 BPS contracted
revenue = $28 million 6 Acquired state-of-the-art,
turn-key pharmaceutical
manufacturing facility from
and signed long-term
supply agreement with
Merck & Co 2009 Cherokee contracted
revenue = $156 million 2001 1988 Track record as scale minority supplier enabled the Merck transaction Cherokee acquisition transformed the company New management with domain expertise added Merger with KBL provides capital and raises company profile Black Enterprise
2009 Industrial/Services
Company of the Year
 7 Cherokee Pharmaceuticals Cherokee Pharmaceuticals facility in Riverside, PA. 340 Acres 105 Buildings 400 Employees
7 Cherokee Pharmaceuticals Cherokee Pharmaceuticals facility in Riverside, PA. 340 Acres 105 Buildings 400 Employees
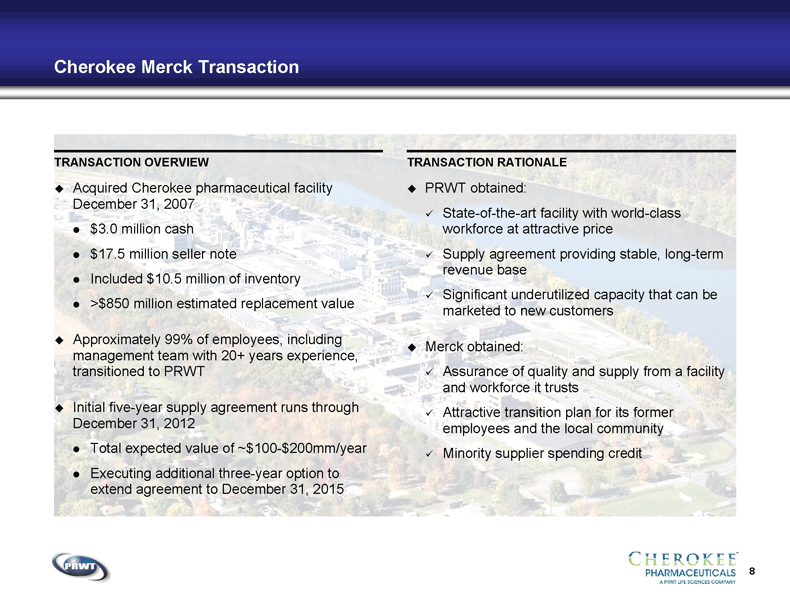 8 Cherokee Merck Transaction TRANSACTION OVERVIEW TRANSACTION RATIONALE Acquired Cherokee pharmaceutical facility
8 Cherokee Merck Transaction TRANSACTION OVERVIEW TRANSACTION RATIONALE Acquired Cherokee pharmaceutical facility
December 31, 2007 $3.0 million cash $17.5 million seller note Included $10.5 million of inventory >$850 million estimated replacement value Approximately 99% of employees, including
management team with 20+ years experience,
transitioned to PRWT Initial five-year supply agreement runs through
December 31, 2012 Total expected value of ~$100-$200mm/year Executing additional three-year option to
extend agreement to December 31, 2015 PRWT obtained: State-of-the-art facility with world-class
workforce at attractive price Supply agreement providing stable, long-term
revenue base Significant underutilized capacity that can be
marketed to new customers Merck obtained: Assurance of quality and supply from a facility
and workforce it trusts Attractive transition plan for its former
employees and the local community Minority supplier spending credit
 9 Cherokee Business Model Substantial revenue growth opportunity Estimated current capacity >$600 million per year, only ~25% utilized Total potential opportunity >$1 billion Significant near-term growth locked in via existing and pending contracts Target 20%+ EBITDA margins Base business and initial supply agreement cover most fixed costs and provide reasonable margin New business expected to be highly profitable due to minimal incremental staff and fixed costs required
9 Cherokee Business Model Substantial revenue growth opportunity Estimated current capacity >$600 million per year, only ~25% utilized Total potential opportunity >$1 billion Significant near-term growth locked in via existing and pending contracts Target 20%+ EBITDA margins Base business and initial supply agreement cover most fixed costs and provide reasonable margin New business expected to be highly profitable due to minimal incremental staff and fixed costs required
= high operating leverage High EBITDA to net income efficiency Attractive interest rates on long-term debt facility Tax advantages include 178.25 acre Keystone Opportunity Zone (“KOZ”) – PA tax free through 2020 Depreciation expenses modest due to low initial basis & maintenance capital expenditure requirements
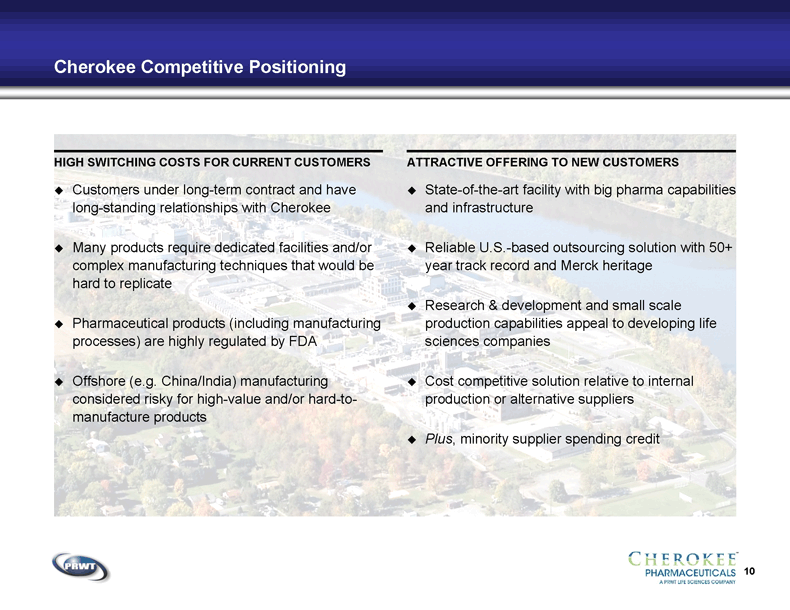 10 Cherokee Competitive Positioning Customers under long-term contract and have
10 Cherokee Competitive Positioning Customers under long-term contract and have
long-standing relationships with Cherokee Many products require dedicated facilities and/or
complex manufacturing techniques that would be
hard to replicate Pharmaceutical products (including manufacturing
processes) are highly regulated by FDA Offshore (e.g. China/India) manufacturing
considered risky for high-value and/or hard-to-
manufacture products State-of-the-art facility with big pharma capabilities
and infrastructure Reliable U.S.-based outsourcing solution with 50+
year track record and Merck heritage Research & development and small scale
production capabilities appeal to developing life
sciences companies Cost competitive solution relative to internal
production or alternative suppliers Plus, minority supplier spending credit HIGH SWITCHING COSTS FOR CURRENT CUSTOMERS ATTRACTIVE OFFERING TO NEW CUSTOMERS
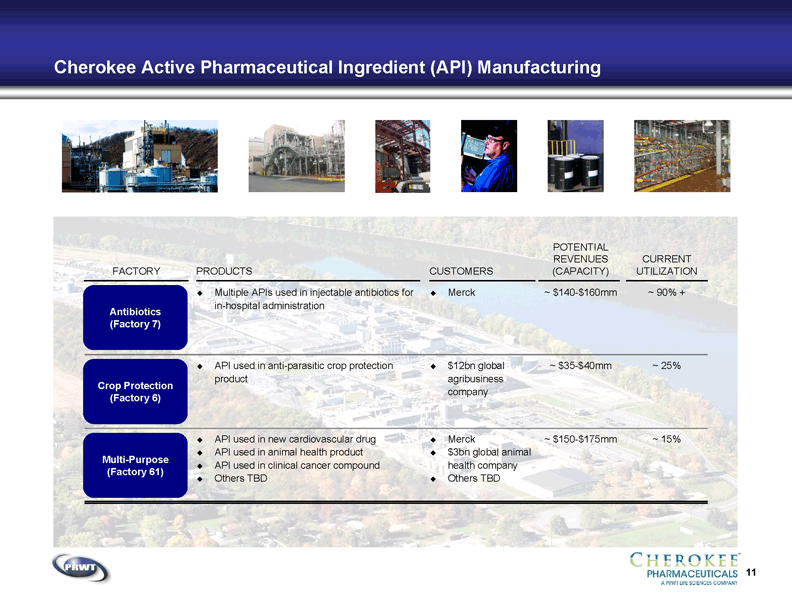 11 Cherokee Active Pharmaceutical Ingredient (API) Manufacturing PRODUCTS Multiple APIs used in injectable antibiotics for
11 Cherokee Active Pharmaceutical Ingredient (API) Manufacturing PRODUCTS Multiple APIs used in injectable antibiotics for
in-hospital administration API used in anti-parasitic crop protection
product API used in new cardiovascular drug API used in animal health product API used in clinical cancer compound Others TBD CUSTOMERS $12bn global
agribusiness
company Merck $3bn global animal
health company Others TBD Merck CURRENT
UTILIZATION ~ 90% + ~ 25% ~ 15% Antibiotics
(Factory 7) Crop Protection
(Factory 6) Multi-Purpose
(Factory 61) FACTORY POTENTIAL
REVENUES
(CAPACITY) ~ $35-$40mm ~ $140-$160mm ~ $150-$175mm
 12 Cherokee Additional Offerings Specialty Chemical
12 Cherokee Additional Offerings Specialty Chemical
Warehouse Fermentation Facility Distribution Laboratory Services Fermentor Fermentation Specialty
Chemical
Distribution Laboratory and
Development
Services OFFERING PRODUCTS/SERVICES Ingredients for bio-pesticides, bio-fungicides,
oil & bio-fuels, food additives & nutraceuticals Procurement, warehousing and distribution of
specialty chemicals, flavors & fragrances 10 warehouses, 105,000 sq. ft., sophisticated
testing and handling capabilities Product release testing Process development and control Microbiological analysis Investigational services CUSTOMERS Launching 2Q 2009
in partnership with
Sigma-Aldrich Fine
Chemicals Servicing existing
API customers Initial contract with
a bio-tech company Initial contracts with
bio-tech and
agriculture
companies (pilots) CURRENT
UTILIZATION < 1% 0% (internal use
only) < 1% POTENTIAL
REVENUES
(CAPACITY) ~ $50mm ~ $200-$250mm ~ $50mm
 13 Cherokee Future Possibilities Expand Current Facility Ample land, infrastructure,
13 Cherokee Future Possibilities Expand Current Facility Ample land, infrastructure,
management and staff to support
new operations at site Would reside within KOZ and be
PA tax-free Acquire Additional Facilities Broaden from API to finished
dosage forms, packaging, etc. Merck-like transactions with other
pharmaceutical companies that
have similar rationales for
divesting manufacturing facilities Offer Cherokee-Labeled Products Authorized generics from current
or new customers Other in-licensed products
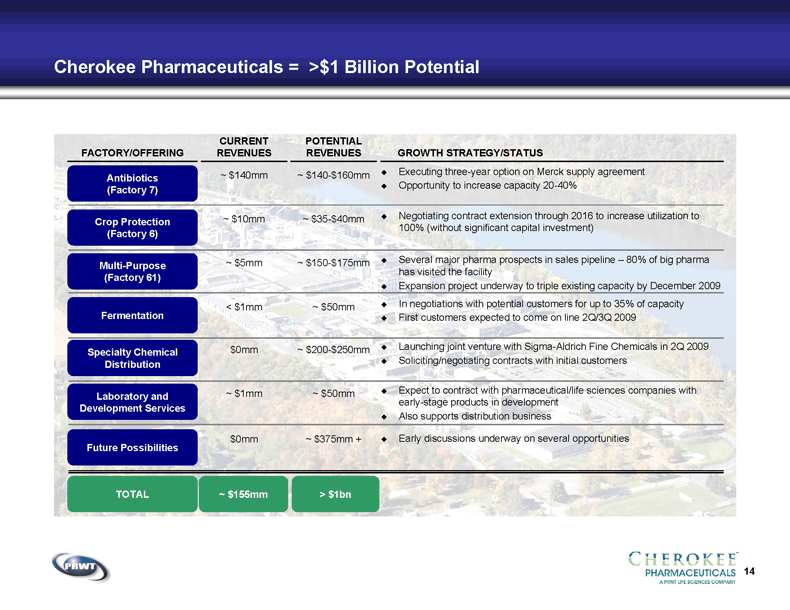 14 Cherokee Pharmaceuticals = >$1 Billion Potential ~ $155mm TOTAL Antibiotics
14 Cherokee Pharmaceuticals = >$1 Billion Potential ~ $155mm TOTAL Antibiotics
(Factory 7) FACTORY/OFFERING Crop Protection
(Factory 6) Multi-Purpose
(Factory 61) Fermentation Specialty Chemical
Distribution Laboratory and
Development Services Future Possibilities CURRENT
REVENUES ~ $10mm ~ $5mm < $1mm $0mm ~ $1mm ~ $140mm $0mm POTENTIAL
REVENUES ~ $35-$40mm ~ $150-$175mm ~ $50mm ~ $200-$250mm ~ $140-$160mm ~ $50mm ~ $375mm + > $1bn GROWTH STRATEGY/STATUS Executing three-year option on Merck supply agreement Opportunity to increase capacity 20-40% Negotiating contract extension through 2016 to increase utilization to
100% (without significant capital investment) Several major pharma prospects in sales pipeline – 80% of big pharma
has visited the facility Expansion project underway to triple existing capacity by December 2009 In negotiations with potential customers for up to 35% of capacity First customers expected to come on line 2Q/3Q 2009 Launching joint venture with Sigma-Aldrich Fine Chemicals in 2Q 2009 Soliciting/negotiating contracts with initial customers Expect to contract with pharmaceutical/life sciences companies with
early-stage products in development Also supports distribution business Early discussions underway on several opportunities
 15 Legacy Businesses Provides professional facilities management and maintenance
15 Legacy Businesses Provides professional facilities management and maintenance
services focusing on complex and highly secure projects Provides high volume/high performance services, including
mailroom, lockbox, document & payment processing, customer
care services and technical support services Stable base businesses with reasonable growth and profitability Generate cash flows that can be re-invested in Cherokee or elsewhere Facilitate access to new opportunities Merck initially approached PRWT through facilities management 20+ year operating history, track record of service excellence, demonstrated ability to handle large-scale projects and
existing minority certification were some of the key factors in convincing Merck to sell plant to PRWT Combination of Cherokee, USF and PRWT Services capabilities offers unique opportunity for cross-selling USF and PRWT Services recently won large contract to manage all North American facilities for global pharmaceutical
company in partnership with Jones Lang Lasalle Targeting additional life sciences companies for facilities management and business processing services contracts
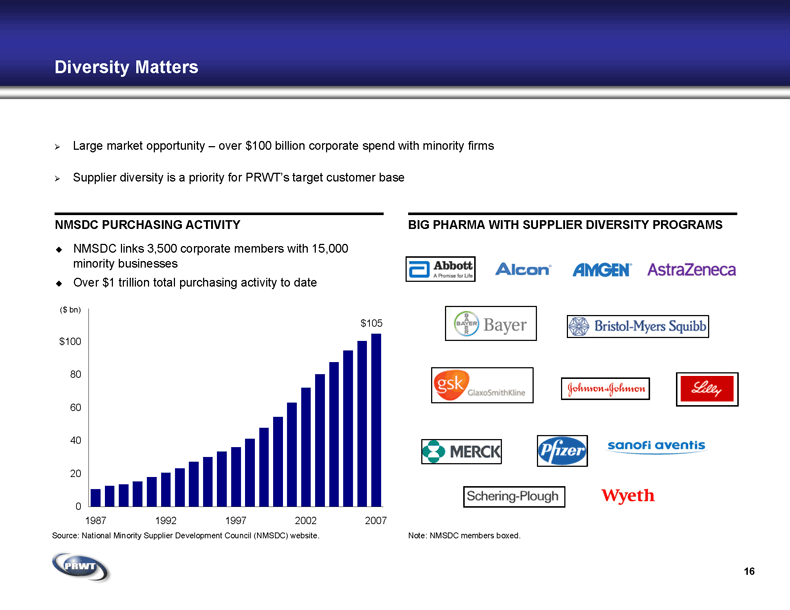 16 Diversity Matters BIG PHARMA WITH SUPPLIER DIVERSITY PROGRAMS NMSDC links 3,500 corporate members with 15,000
16 Diversity Matters BIG PHARMA WITH SUPPLIER DIVERSITY PROGRAMS NMSDC links 3,500 corporate members with 15,000
minority businesses Over $1 trillion total purchasing activity to date NMSDC PURCHASING ACTIVITY Large market opportunity – over $100 billion corporate spend with minority firms Supplier diversity is a priority for PRWT’s target customer base Note: NMSDC members boxed. Source: National Minority Supplier Development Council (NMSDC) website. ($ bn)
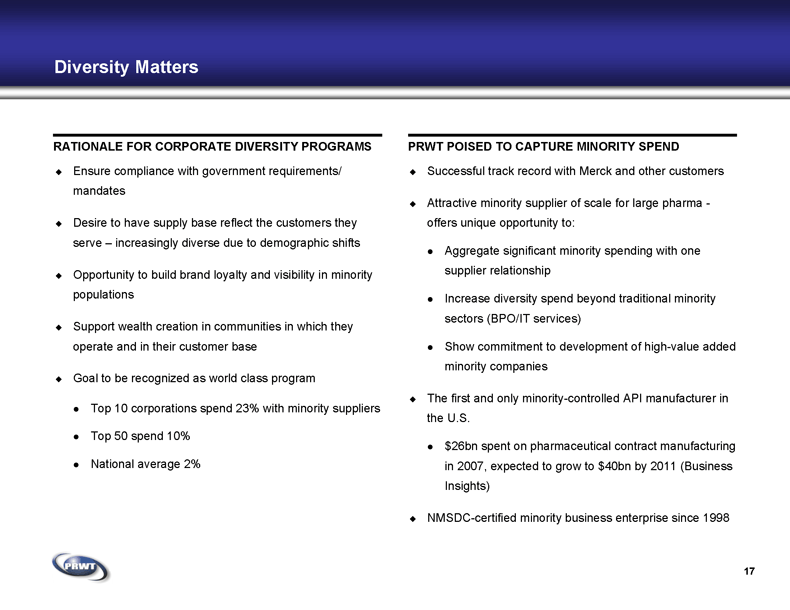 Diversity Matters RATIONALE FOR CORPORATE DIVERSITY PROGRAMS PRWT POISED TO CAPTURE MINORITY SPEND Successful track record with Merck and other customers Attractive minority supplier of scale for large pharma -
Diversity Matters RATIONALE FOR CORPORATE DIVERSITY PROGRAMS PRWT POISED TO CAPTURE MINORITY SPEND Successful track record with Merck and other customers Attractive minority supplier of scale for large pharma -
offers unique opportunity to: Aggregate significant minority spending with one
supplier relationship Increase diversity spend beyond traditional minority
sectors (BPO/IT services) Show commitment to development of high-value added
minority companies The first and only minority-controlled API manufacturer in
the U.S. $26bn spent on pharmaceutical contract manufacturing in 2007, expected to grow to $40bn by 2011 (Business Insights) NMSDC-certified minority business enterprise since 1998 Ensure compliance with government requirements/
mandates Desire to have supply base reflect the customers they
serve – increasingly diverse due to demographic shifts Opportunity to build brand loyalty and visibility in minority
populations Support wealth creation in communities in which they
operate and in their customer base Goal to be recognized as world class program Top 10 corporations spend 23% with minority suppliers Top 50 spend 10% National average 2% 17
 18 Management Willie Johnson, Founder/Chairman 18 years Cmmr, PA Office of Social
18 Management Willie Johnson, Founder/Chairman 18 years Cmmr, PA Office of Social
Services Former owner/CEO, Fidelity Systems Jerry Johnson, Vice Chairman Chairman, Radnor Trust Holdings Former EVP, Safeguard Scientifics (NYSE:
SFE) Harold Epps, President & CEO President since Nov 2007;
CEO since Oct 2008 Former VP, Quadrant-EPP Murvin Lackey, President, Cherokee Distribution Former VP, GlaxoSmithKline Formerly held senior positions at AMOCO,
Digital Equipment Corp. Mark Schweiker, President, PRWT Services President & CEO, Greater Philadelphia
Chamber of Commerce Former PA Governor & Lieutenant Gov. George Burrell, EVP & General Counsel Former CEO, Innovation Philadelphia Former Secretary of External Affairs for the
City of Philadelphia John McCarey, EVP/CFO Former CFO, Lockheed Martin IMS Former corporate SVP of finance and EVP,
ACS Government Solutions John Elliot, President, Cherokee Pharma President of Cherokee since 2008 Prior 25-year career at GlaxoSmithKline, most
recently as Senior Vice President James Dobrowolski, President,
U.S. Facilities Former President, Halifax Technical
Services Skip Lee, EVP, Business Development Former SVP, TSS; Former SVP (M&A),
ACS; Former Senior positions at Lockheed
Martin IMS Note: Chairman Willie Johnson and Vice Chairman Jerry Johnson are not related. Mark Schweiker expected to begin in July 2009.
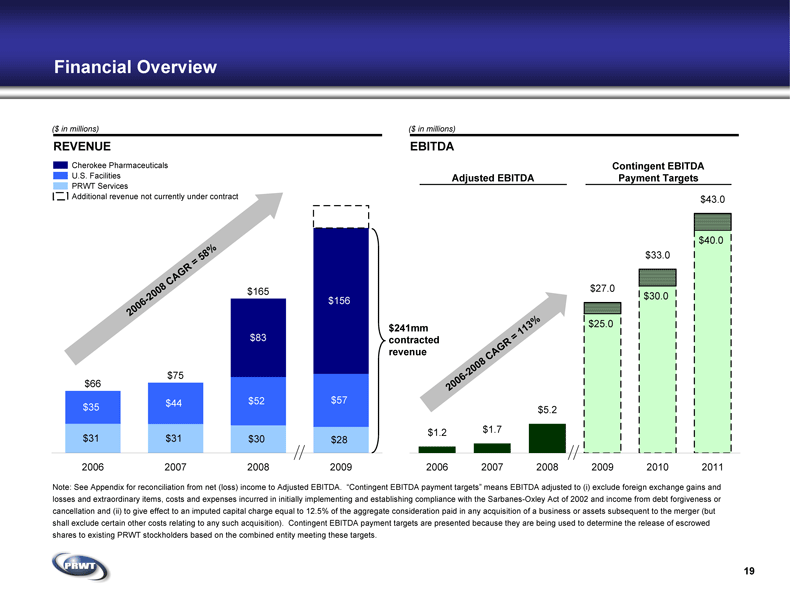 Financial Overview REVENUE Cherokee Pharmaceuticals U.S. Facilities PRWT Services EBITDA ($ in millions) ($ in millions) Contingent EBITDA
Financial Overview REVENUE Cherokee Pharmaceuticals U.S. Facilities PRWT Services EBITDA ($ in millions) ($ in millions) Contingent EBITDA
Payment Targets Adjusted EBITDA Note: See Appendix for reconciliation from net (loss) income to Adjusted EBITDA. “Contingent EBITDA payment targets” means EBITDA adjusted to (i) exclude foreign exchange gains and
losses and extraordinary items, costs and expenses incurred in initially implementing and establishing compliance with the Sarbanes-Oxley Act of 2002 and income from debt forgiveness or
cancellation and (ii) to give effect to an imputed capital charge equal to 12.5% of the aggregate consideration paid in any acquisition of a business or assets subsequent to the merger (but
shall exclude certain other costs relating to any such acquisition). Contingent EBITDA payment targets are presented because they are being used to determine the release of escrowed
shares to existing PRWT stockholders based on the combined entity meeting these targets. 19 // // $241mm
contracted
revenue Additional revenue not currently under contract $31 $31 $30 $28 $35 $44 $52 $57 $156 $83 $165 $75 $66 2006 2007 2008 2009 $25.0 $30.0 $40.0 $43.0 $33.0 $27.0 $5.2 $1.7 $1.2 2006 2007 2008 2009 2010 2011
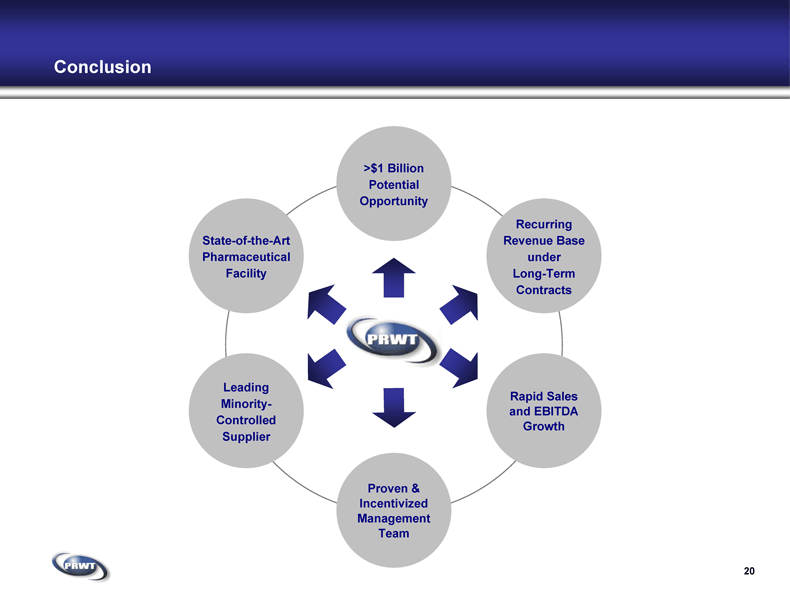 Conclusion 20 State-of-the-Art
Conclusion 20 State-of-the-Art
Pharmaceutical
Facility >$1 Billion
Potential
Opportunity Recurring
Revenue Base
under
Long-Term
Contracts Rapid Sales
and EBITDA
Growth Leading
Minority-
Controlled
Supplier Proven &
Incentivized
Management
Team
 21 Appendix PRWT Services, Inc.
21 Appendix PRWT Services, Inc.
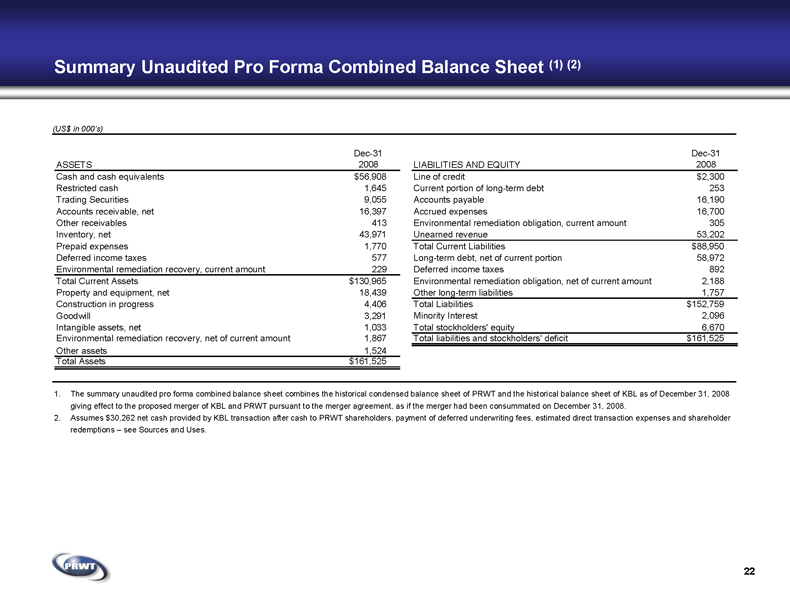 22 Summary Unaudited Pro Forma Combined Balance Sheet (1) (2) (US$ in 000’s) 1. The summary unaudited pro forma combined balance sheet combines the historical condensed balance sheet of PRWT and the historical balance sheet of KBL as of December 31, 2008
22 Summary Unaudited Pro Forma Combined Balance Sheet (1) (2) (US$ in 000’s) 1. The summary unaudited pro forma combined balance sheet combines the historical condensed balance sheet of PRWT and the historical balance sheet of KBL as of December 31, 2008
giving effect to the proposed merger of KBL and PRWT pursuant to the merger agreement, as if the merger had been consummated on December 31, 2008. 2. Assumes $30,262 net cash provided by KBL transaction after cash to PRWT shareholders, payment of deferred underwriting fees, estimated direct transaction expenses and shareholder
redemptions – see Sources and Uses. ASSETS Dec-31 2008 Cash and cash equivalents $56,908 Restricted cash 1,645 Trading Securities 9,055 Accounts receivable, net 16,397 Other receivables 413 Inventory, net 43,971 Prepaid expenses 1,770 Deferred income taxes 577 Environmental remediation recovery, current amount 229 Total Current Assets $130,965 Property and equipment, net 18,439 Construction in progress 4,406 Goodwill 3,291 Intangible assets, net 1,033 Environmental remediation recovery, net of current amount 1,867 Other assets 1,524 Total Assets $161,525 LIABILITIES AND EQUITY Dec-31 2008 Line of credit $2,300 Current portion of long-term debt 253 Accounts payable 16,190 Accrued expenses 16,700 Environmental remediation obligation, current amount 305 Unearned revenue 53,202 Total Current Liabilities $88,950 Long-term debt, net of current portion 58,972 Deferred income taxes 892 Environmental remediation obligation, net of current amount 2,188 Other long-term liabilities 1,757 Total Liabilities $152,759 Minority Interest 2,096 Total stockholders' equity 6,670 Total liabilities and stockholders' deficit $161,525
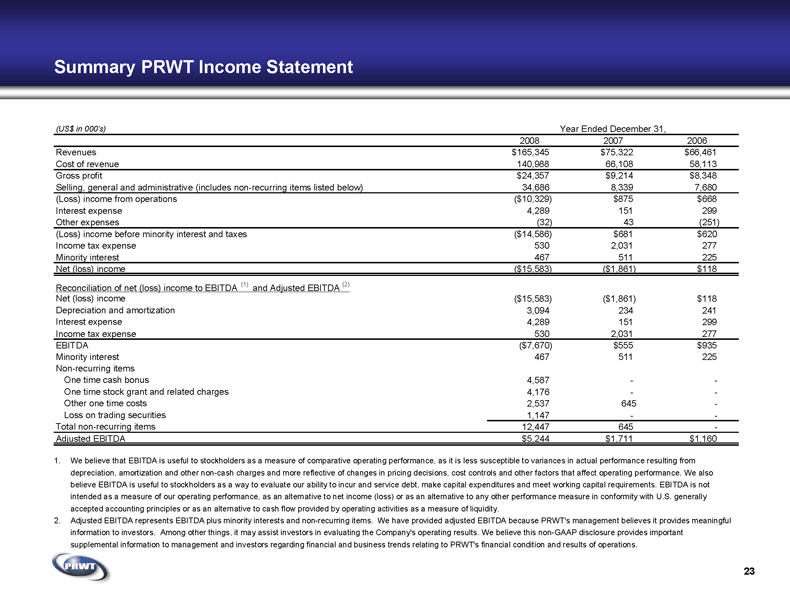 23 Summary PRWT Income Statement 1. We believe that EBITDA is useful to stockholders as a measure of comparative operating performance, as it is less susceptible to variances in actual performance resulting from
23 Summary PRWT Income Statement 1. We believe that EBITDA is useful to stockholders as a measure of comparative operating performance, as it is less susceptible to variances in actual performance resulting from
depreciation, amortization and other non-cash charges and more reflective of changes in pricing decisions, cost controls and other factors that affect operating performance. We also
believe EBITDA is useful to stockholders as a way to evaluate our ability to incur and service debt, make capital expenditures and meet working capital requirements. EBITDA is not
intended as a measure of our operating performance, as an alternative to net income (loss) or as an alternative to any other performance measure in conformity with U.S. generally
accepted accounting principles or as an alternative to cash flow provided by operating activities as a measure of liquidity. 2. Adjusted EBITDA represents EBITDA plus minority interests and non-recurring items. We have provided adjusted EBITDA because PRWT's management believes it provides meaningful
information to investors. Among other things, it may assist investors in evaluating the Company's operating results. We believe this non-GAAP disclosure provides important
supplemental information to management and investors regarding financial and business trends relating to PRWT's financial condition and results of operations. (US$ in 000's) Year Ended December 31, 2008 2007 2006 Revenues $165,345 $75,322 $66,461 Cost of revenue 140,988 66,108 58,113 Gross profit $24,357 $9,214 $8,348 Selling, general and administrative (includes non-recurring items listed below) 34,686 8,339 7,680 (Loss) income from operations ($10,329) $875 $668 Interest expense 4,289 151 299 Other expenses (32) 43 (251) (Loss) income before minority interest and taxes ($14,586) $681 $620 Income tax expense 530 2,031 277 Minority interest 467 511 225 Net (loss) income ($15,583) ($1,861) $118 Reconciliation of net (loss) income to EBITDA (1) and Adjusted EBITDA (2) Net (loss) income ($15,583) ($1,861) $118 Depreciation and amortization 3,094 234 241 Interest expense 4,289 151 299 Income tax expense 530 2,031 277 EBITDA ($7,670) $555 $935 Minority interest 467 511 225 Non-recurring items One time cash bonus 4,587 - - One time stock grant and related charges 4,176 - - Other one time costs 2,537 645 - Loss on trading securities 1,147 - - Total non-recurring items 12,447 645 - Adjusted EBITDA $5,244 $1,711 $1,160
 24 Summary PRWT Cash Flow Statement (US$ in 000's) Year Ended December 31, 2008 2007 2006 Cash flows from operating activities Net (loss) income ($15,583) ($1,861) $118 Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activties: Depreciation and amortization 3,094 234 241 Bad debt expense 68 60 182 Deferred income taxes (16) 763 (54) Share-based compensation 2,868 - - Minority interest expense 467 511 225 Unrealized loss on warrants - 457 - Non-cash interest expense 3,653 - - Environmental obligation and recovery 222 76 - Short term investments (9,055) - - Changes in assets and liabilities 3,430 27,146 2,118 Net cash (used in) provided by operating activities ($10,851) $27,389 $2,831 Cash flows from investing activities Acquisition of assets - (4,660) - Due from affiliates (139) (51) - (Issuance) repayment of shareholder loans (181) (468) 26 Purchase of property and equipment and construction in process (12,820) (457) (151) Net cash used in investing activities ($13,140) ($5,636) ($124) Cash flows from financing activities Repayments of long-term debt (231) (504) (837) Borrowings of long-term debt 41,514 - - Proceeds (repayments) of line of credit 126 1,174 (1,942) Deposited relating to letters of credit (947) - - Dividends paid to minority interest (260) - - Redemption of outstanding warrants (4,000) - - Redemption of preferred stock - (2,000) - Purchase of treasury stock (5,660) (763) (116) Net cash provided by (used in) financing activities $30,542 ($2,093) ($2,895) Net increase (decrease) in cash and cash equivalents 6,551 19,659 (189) Cash and cash equivalents at beginning of year 19,950 291 480 Cash and cash equivalents at end of year $26,501 $19,950 $291
24 Summary PRWT Cash Flow Statement (US$ in 000's) Year Ended December 31, 2008 2007 2006 Cash flows from operating activities Net (loss) income ($15,583) ($1,861) $118 Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activties: Depreciation and amortization 3,094 234 241 Bad debt expense 68 60 182 Deferred income taxes (16) 763 (54) Share-based compensation 2,868 - - Minority interest expense 467 511 225 Unrealized loss on warrants - 457 - Non-cash interest expense 3,653 - - Environmental obligation and recovery 222 76 - Short term investments (9,055) - - Changes in assets and liabilities 3,430 27,146 2,118 Net cash (used in) provided by operating activities ($10,851) $27,389 $2,831 Cash flows from investing activities Acquisition of assets - (4,660) - Due from affiliates (139) (51) - (Issuance) repayment of shareholder loans (181) (468) 26 Purchase of property and equipment and construction in process (12,820) (457) (151) Net cash used in investing activities ($13,140) ($5,636) ($124) Cash flows from financing activities Repayments of long-term debt (231) (504) (837) Borrowings of long-term debt 41,514 - - Proceeds (repayments) of line of credit 126 1,174 (1,942) Deposited relating to letters of credit (947) - - Dividends paid to minority interest (260) - - Redemption of outstanding warrants (4,000) - - Redemption of preferred stock - (2,000) - Purchase of treasury stock (5,660) (763) (116) Net cash provided by (used in) financing activities $30,542 ($2,093) ($2,895) Net increase (decrease) in cash and cash equivalents 6,551 19,659 (189) Cash and cash equivalents at beginning of year 19,950 291 480 Cash and cash equivalents at end of year $26,501 $19,950 $291
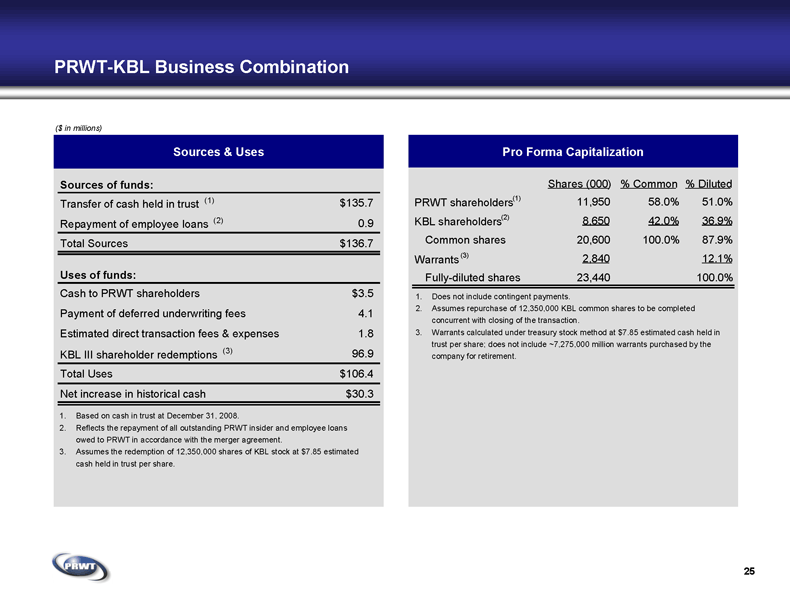 25 PRWT-KBL Business Combination ($ in millions) Sources & Uses Pro Forma Capitalization 1. Based on cash in trust at December 31, 2008. 2. Reflects the repayment of all outstanding PRWT insider and employee loans
25 PRWT-KBL Business Combination ($ in millions) Sources & Uses Pro Forma Capitalization 1. Based on cash in trust at December 31, 2008. 2. Reflects the repayment of all outstanding PRWT insider and employee loans
owed to PRWT in accordance with the merger agreement. 3. Assumes the redemption of 12,350,000 shares of KBL stock at $7.85 estimated
cash held in trust per share. 1. Does not include contingent payments. 2. Assumes repurchase of 12,350,000 KBL common shares to be completed
concurrent with closing of the transaction. 3. Warrants calculated under treasury stock method at $7.85 estimated cash held in
trust per share; does not include ~7,275,000 million warrants purchased by the
company for retirement. Sources of funds: Transfer of cash held in trust (1) $135.7 Repayment of employee loans (2) 0.9 Total Sources $136.7 Uses of funds: Cash to PRWT shareholders $3.5 Payment of deferred underwriting fees 4.1 Estimated direct transaction fees & expenses 1.8 KBL III shareholder redemptions (3) 96.9 Total Uses $106.4 Net increase in historical cash $30.3 Shares (000) % Common % Diluted PRWT shareholders (1) 11,950 58.0% 51.0% KBL shareholders (2) 8,650 42.0% 36.9% Common shares 20,600 100.0% 87.9% Warrants (3) 2,840 12.1% Fully-diluted shares 23,440 100.0%
 26 U.S. Facilities: A Facilities Management Services Provider since 1967 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Access additional potential customers Expand into commercial markets with focus on life
26 U.S. Facilities: A Facilities Management Services Provider since 1967 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Access additional potential customers Expand into commercial markets with focus on life
sciences – recently won first contract in partnership with
Jones Lang Lasalle Target additional federal government customers – recently
placed on GSA schedules for facilities management
services Continue geographic expansion beyond core Philadelphia
and Virginia markets Maximize cross-selling opportunities with Cherokee and
PRWT Services Boiler & Chiller Plants Bridges & Navigation
Systems Building Alteration &
Construction Custodial & Related Services Equipment Systems Integrated Pest Management Mailroom & Messenger
Services Physical & Electronic
Security Project Management Trash Removal &
Recycling Warehouse & Logistics
Facilities City of Philadelphia
TRIPLEX Curran-Fromhold/
Riverside
Correctional
Facilities U.S. Army Corps of
Engineers Atlantic
Intracoastal
Waterway Virginia Department
of Transportation Social Security
Administration
Mid-Atlantic Service
Center Thurgood Marshall
Federal Judiciary
Building D.C. Unified
Communication
Center
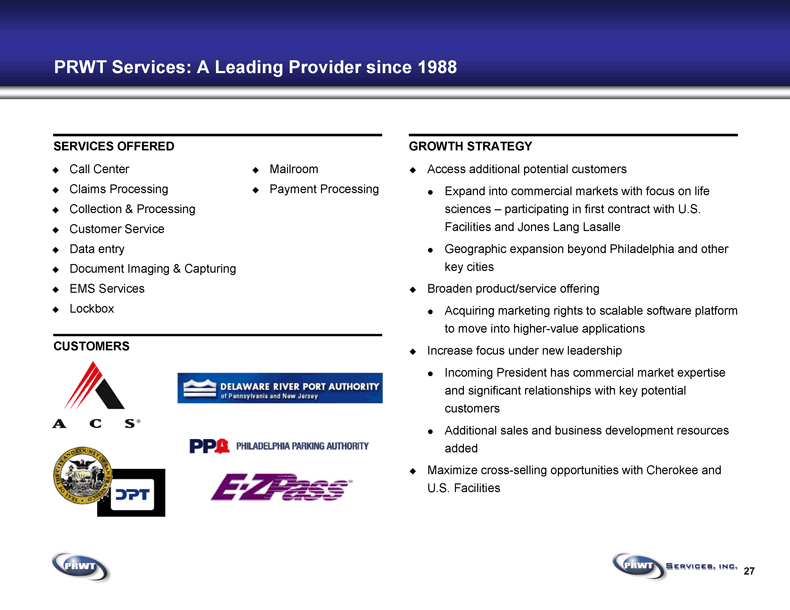 27 PRWT Services: A Leading Provider since 1988 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Call Center Claims Processing Collection & Processing Customer Service Data entry Document Imaging & Capturing EMS Services Lockbox Mailroom Payment Processing Access additional potential customers Expand into commercial markets with focus on life
27 PRWT Services: A Leading Provider since 1988 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Call Center Claims Processing Collection & Processing Customer Service Data entry Document Imaging & Capturing EMS Services Lockbox Mailroom Payment Processing Access additional potential customers Expand into commercial markets with focus on life
sciences – participating in first contract with U.S.
Facilities and Jones Lang Lasalle Geographic expansion beyond Philadelphia and other
key cities Broaden product/service offering Acquiring marketing rights to scalable software platform
to move into higher-value applications Increase focus under new leadership Incoming President has commercial market expertise
and significant relationships with key potential
customers Additional sales and business development resources
added Maximize cross-selling opportunities with Cherokee and
U.S. Facilities
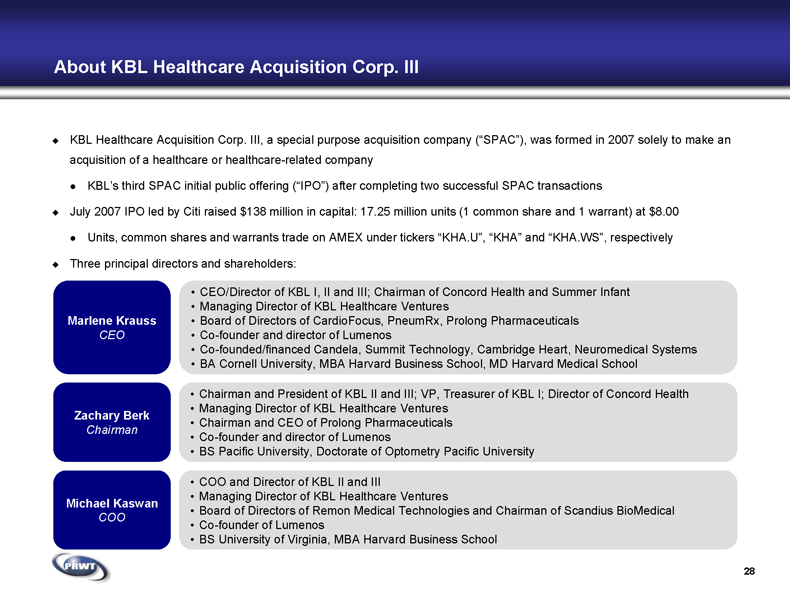 28 About KBL Healthcare Acquisition Corp. III KBL Healthcare Acquisition Corp. III, a special purpose acquisition company (“SPAC”), was formed in 2007 solely to make an
28 About KBL Healthcare Acquisition Corp. III KBL Healthcare Acquisition Corp. III, a special purpose acquisition company (“SPAC”), was formed in 2007 solely to make an
acquisition of a healthcare or healthcare-related company KBL’s third SPAC initial public offering (“IPO”) after completing two successful SPAC transactions July 2007 IPO led by Citi raised $138 million in capital: 17.25 million units (1 common share and 1 warrant) at $8.00 Units, common shares and warrants trade on AMEX under tickers “ KHA.U”, “KHA” and “KHA.WS”, respectively Three principal directors and shareholders: Marlene Krauss
CEO Zachary Berk
Chairman COO and Director of KBL II and III Managing Director of KBL Healthcare Ventures Board of Directors of Remon Medical Technologies and Chairman of Scandius BioMedical Co-founder of Lumenos BS University of Virginia, MBA Harvard Business School Michael Kaswan
COO Chairman and President of KBL II and III; VP, Treasurer of KBL I; Director of Concord Health Managing Director of KBL Healthcare Ventures Chairman and CEO of Prolong Pharmaceuticals Co-founder and director of Lumenos BS Pacific University, Doctorate of Optometry Pacific University CEO/Director of KBL I, II and III; Chairman of Concord Health and Summer Infant Managing Director of KBL Healthcare Ventures Board of Directors of CardioFocus, PneumRx, Prolong Pharmaceuticals Co-founder and director of Lumenos Co-founded/financed Candela, Summit Technology, Cambridge Heart, Neuromedical Systems BA Cornell University, MBA Harvard Business School, MD Harvard Medical School
 To Engage in Business Combination with
To Engage in Business Combination with  2 Interests of the Parties in the Merger KBL HEALTHCARE ACQUISTION CORP. III (“KBL”) AND PRWT SERVICES, INC. (“PRWT”) ARE HOLDING PRESENTATIONS FOR CERTAIN OF KBL’S STOCKHOLDERS, AS WELL AS
2 Interests of the Parties in the Merger KBL HEALTHCARE ACQUISTION CORP. III (“KBL”) AND PRWT SERVICES, INC. (“PRWT”) ARE HOLDING PRESENTATIONS FOR CERTAIN OF KBL’S STOCKHOLDERS, AS WELL AS 3 Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS REGARDING KBL’S
3 Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS INCLUDE, BUT ARE NOT LIMITED TO, STATEMENTS REGARDING KBL’S Executive Summary Transaction Consideration Management
Executive Summary Transaction Consideration Management  Investment Highlights 5 $1 billion potential opportunity based on existing pharmaceutical platform State-of-the-art pharmaceutical facility with significant under-utilized capacity, strong operating leverage and
Investment Highlights 5 $1 billion potential opportunity based on existing pharmaceutical platform State-of-the-art pharmaceutical facility with significant under-utilized capacity, strong operating leverage and Company History Certified by National
Company History Certified by National 7 Cherokee Pharmaceuticals Cherokee Pharmaceuticals facility in Riverside, PA. 340 Acres 105 Buildings 400 Employees
7 Cherokee Pharmaceuticals Cherokee Pharmaceuticals facility in Riverside, PA. 340 Acres 105 Buildings 400 Employees 8 Cherokee Merck Transaction TRANSACTION OVERVIEW TRANSACTION RATIONALE Acquired Cherokee pharmaceutical facility
8 Cherokee Merck Transaction TRANSACTION OVERVIEW TRANSACTION RATIONALE Acquired Cherokee pharmaceutical facility 9 Cherokee Business Model Substantial revenue growth opportunity Estimated current capacity >$600 million per year, only ~25% utilized Total potential opportunity >$1 billion Significant near-term growth locked in via existing and pending contracts Target 20%+ EBITDA margins Base business and initial supply agreement cover most fixed costs and provide reasonable margin New business expected to be highly profitable due to minimal incremental staff and fixed costs required
9 Cherokee Business Model Substantial revenue growth opportunity Estimated current capacity >$600 million per year, only ~25% utilized Total potential opportunity >$1 billion Significant near-term growth locked in via existing and pending contracts Target 20%+ EBITDA margins Base business and initial supply agreement cover most fixed costs and provide reasonable margin New business expected to be highly profitable due to minimal incremental staff and fixed costs required  10 Cherokee Competitive Positioning Customers under long-term contract and have
10 Cherokee Competitive Positioning Customers under long-term contract and have 11 Cherokee Active Pharmaceutical Ingredient (API) Manufacturing PRODUCTS Multiple APIs used in injectable antibiotics for
11 Cherokee Active Pharmaceutical Ingredient (API) Manufacturing PRODUCTS Multiple APIs used in injectable antibiotics for 12 Cherokee Additional Offerings Specialty Chemical
12 Cherokee Additional Offerings Specialty Chemical 13 Cherokee Future Possibilities Expand Current Facility Ample land, infrastructure,
13 Cherokee Future Possibilities Expand Current Facility Ample land, infrastructure, 14 Cherokee Pharmaceuticals = >$1 Billion Potential ~ $155mm TOTAL Antibiotics
14 Cherokee Pharmaceuticals = >$1 Billion Potential ~ $155mm TOTAL Antibiotics  15 Legacy Businesses Provides professional facilities management and maintenance
15 Legacy Businesses Provides professional facilities management and maintenance 16 Diversity Matters BIG PHARMA WITH SUPPLIER DIVERSITY PROGRAMS NMSDC links 3,500 corporate members with 15,000
16 Diversity Matters BIG PHARMA WITH SUPPLIER DIVERSITY PROGRAMS NMSDC links 3,500 corporate members with 15,000 Diversity Matters RATIONALE FOR CORPORATE DIVERSITY PROGRAMS PRWT POISED TO CAPTURE MINORITY SPEND Successful track record with Merck and other customers Attractive minority supplier of scale for large pharma -
Diversity Matters RATIONALE FOR CORPORATE DIVERSITY PROGRAMS PRWT POISED TO CAPTURE MINORITY SPEND Successful track record with Merck and other customers Attractive minority supplier of scale for large pharma - 18 Management Willie Johnson, Founder/Chairman 18 years Cmmr, PA Office of Social
18 Management Willie Johnson, Founder/Chairman 18 years Cmmr, PA Office of Social Financial Overview REVENUE Cherokee Pharmaceuticals U.S. Facilities PRWT Services EBITDA ($ in millions) ($ in millions) Contingent EBITDA
Financial Overview REVENUE Cherokee Pharmaceuticals U.S. Facilities PRWT Services EBITDA ($ in millions) ($ in millions) Contingent EBITDA  Conclusion 20 State-of-the-Art
Conclusion 20 State-of-the-Art 21 Appendix PRWT Services, Inc.
21 Appendix PRWT Services, Inc. 22 Summary Unaudited Pro Forma Combined Balance Sheet (1) (2) (US$ in 000’s) 1. The summary unaudited pro forma combined balance sheet combines the historical condensed balance sheet of PRWT and the historical balance sheet of KBL as of December 31, 2008
22 Summary Unaudited Pro Forma Combined Balance Sheet (1) (2) (US$ in 000’s) 1. The summary unaudited pro forma combined balance sheet combines the historical condensed balance sheet of PRWT and the historical balance sheet of KBL as of December 31, 2008 23 Summary PRWT Income Statement 1. We believe that EBITDA is useful to stockholders as a measure of comparative operating performance, as it is less susceptible to variances in actual performance resulting from
23 Summary PRWT Income Statement 1. We believe that EBITDA is useful to stockholders as a measure of comparative operating performance, as it is less susceptible to variances in actual performance resulting from 24 Summary PRWT Cash Flow Statement (US$ in 000's) Year Ended December 31, 2008 2007 2006 Cash flows from operating activities Net (loss) income ($15,583) ($1,861) $118 Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activties: Depreciation and amortization 3,094 234 241 Bad debt expense 68 60 182 Deferred income taxes (16) 763 (54) Share-based compensation 2,868 - - Minority interest expense 467 511 225 Unrealized loss on warrants - 457 - Non-cash interest expense 3,653 - - Environmental obligation and recovery 222 76 - Short term investments (9,055) - - Changes in assets and liabilities 3,430 27,146 2,118 Net cash (used in) provided by operating activities ($10,851) $27,389 $2,831 Cash flows from investing activities Acquisition of assets - (4,660) - Due from affiliates (139) (51) - (Issuance) repayment of shareholder loans (181) (468) 26 Purchase of property and equipment and construction in process (12,820) (457) (151) Net cash used in investing activities ($13,140) ($5,636) ($124) Cash flows from financing activities Repayments of long-term debt (231) (504) (837) Borrowings of long-term debt 41,514 - - Proceeds (repayments) of line of credit 126 1,174 (1,942) Deposited relating to letters of credit (947) - - Dividends paid to minority interest (260) - - Redemption of outstanding warrants (4,000) - - Redemption of preferred stock - (2,000) - Purchase of treasury stock (5,660) (763) (116) Net cash provided by (used in) financing activities $30,542 ($2,093) ($2,895) Net increase (decrease) in cash and cash equivalents 6,551 19,659 (189) Cash and cash equivalents at beginning of year 19,950 291 480 Cash and cash equivalents at end of year $26,501 $19,950 $291
24 Summary PRWT Cash Flow Statement (US$ in 000's) Year Ended December 31, 2008 2007 2006 Cash flows from operating activities Net (loss) income ($15,583) ($1,861) $118 Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activties: Depreciation and amortization 3,094 234 241 Bad debt expense 68 60 182 Deferred income taxes (16) 763 (54) Share-based compensation 2,868 - - Minority interest expense 467 511 225 Unrealized loss on warrants - 457 - Non-cash interest expense 3,653 - - Environmental obligation and recovery 222 76 - Short term investments (9,055) - - Changes in assets and liabilities 3,430 27,146 2,118 Net cash (used in) provided by operating activities ($10,851) $27,389 $2,831 Cash flows from investing activities Acquisition of assets - (4,660) - Due from affiliates (139) (51) - (Issuance) repayment of shareholder loans (181) (468) 26 Purchase of property and equipment and construction in process (12,820) (457) (151) Net cash used in investing activities ($13,140) ($5,636) ($124) Cash flows from financing activities Repayments of long-term debt (231) (504) (837) Borrowings of long-term debt 41,514 - - Proceeds (repayments) of line of credit 126 1,174 (1,942) Deposited relating to letters of credit (947) - - Dividends paid to minority interest (260) - - Redemption of outstanding warrants (4,000) - - Redemption of preferred stock - (2,000) - Purchase of treasury stock (5,660) (763) (116) Net cash provided by (used in) financing activities $30,542 ($2,093) ($2,895) Net increase (decrease) in cash and cash equivalents 6,551 19,659 (189) Cash and cash equivalents at beginning of year 19,950 291 480 Cash and cash equivalents at end of year $26,501 $19,950 $291 25 PRWT-KBL Business Combination ($ in millions) Sources & Uses Pro Forma Capitalization 1. Based on cash in trust at December 31, 2008. 2. Reflects the repayment of all outstanding PRWT insider and employee loans
25 PRWT-KBL Business Combination ($ in millions) Sources & Uses Pro Forma Capitalization 1. Based on cash in trust at December 31, 2008. 2. Reflects the repayment of all outstanding PRWT insider and employee loans 26 U.S. Facilities: A Facilities Management Services Provider since 1967 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Access additional potential customers Expand into commercial markets with focus on life
26 U.S. Facilities: A Facilities Management Services Provider since 1967 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Access additional potential customers Expand into commercial markets with focus on life 27 PRWT Services: A Leading Provider since 1988 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Call Center Claims Processing Collection & Processing Customer Service Data entry Document Imaging & Capturing EMS Services Lockbox Mailroom Payment Processing Access additional potential customers Expand into commercial markets with focus on life
27 PRWT Services: A Leading Provider since 1988 SERVICES OFFERED GROWTH STRATEGY CUSTOMERS Call Center Claims Processing Collection & Processing Customer Service Data entry Document Imaging & Capturing EMS Services Lockbox Mailroom Payment Processing Access additional potential customers Expand into commercial markets with focus on life 28 About KBL Healthcare Acquisition Corp. III KBL Healthcare Acquisition Corp. III, a special purpose acquisition company (“SPAC”), was formed in 2007 solely to make an
28 About KBL Healthcare Acquisition Corp. III KBL Healthcare Acquisition Corp. III, a special purpose acquisition company (“SPAC”), was formed in 2007 solely to make an