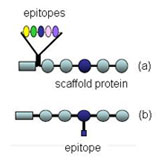
Parallax’s innovative process for the development of new antigens, which is patent pending, will also be used in the identification of test markers for its Target System platform, adding a dimension that further distinguishes Parallax from its competition. Parallax believes that the innovative antigen development process will allow it to create new barriers to entry on certain antigens that it identifies by subsequent patent application filings for use in conjunction the Parallax Hand-Held Analyzer.
The main barriers and constraints to the use of rapid diagnostic tests can be put into three main categories:
Parallax also plans to develop and utilize its patented antibody development process in order to produce and deliver antibody test markers to its spin-off companies. Parallax will also develop relationships with antibody test marker producers to accelerate the delivery of new tests to its spin-off companies. Parallax will look to identify, negotiate and acquire markers from third-party producers. In this case, Parallax will test and approve the antibody test marker to be used in its Target System.
Intellectual property protection will be sought for all of Parallax’s primary and secondary products. Moreover, all products and supporting products such as novel biomarker candidates, antibodies, proteins, and diagnostics tests surrounding Parallax’s core indication areas will be IP-protected to create a barrier to entry for competitors.
U.S. Patent Application Nos. 11/856,925 “Method for Determining the Immune Status of a Subject”
U.S. Patent Application Nos. 11/924,033 “Portable Apparatus for Improved Sample Analysis”
By protecting these elements of Parallax’s testing technology platform as well as the individual tests and/ or test elements, Parallax efficiently builds another level of entry deterrence for potential competitors in its market segment. Parallax will preserve its IP by achieving an appropriate balance between trade secrets and patents.
Parallax will also utilize trademark applications to protect its IP that may not be suitable for patent protection. Unlike patent applications, which in many cases must be filed in advance of a particular date, there is no specific date by which a trademark application must be filed. Instead, the time constraint is in a different direction. In the United States an ordinary so-called "use" trademark application can only be filed after the goods or services have been in interstate commerce.
Parallax holds exclusive rights to all Montecito Bio Sciences, Ltd. (“Montecito”) patents, clearances and products in the area of Infectious Diseases. It is expected that after successful re-introduction of the Parallax Target System and the introduction of its novel CD4 immune status test, additional tests will be developed and protected under the full responsibility of Parallax. Generally, Parallax and Montecito will own improvements to the basic technology platform exclusively.
Overview of Medical Devices and Their Regulatory Pathways: 510(k) FDA Cleared Tests
Medical Devices: The Basics
The definition has several components. A medical device:
| | · | diagnoses, cures, lessens, treats, or prevents disease |
| | · | affects the function or structure of the body |
| | · | does not achieve primary intended purposes through chemical action |
FDA's Center for Devices and Radiological Health regulates companies that design, manufacture, repackage, relabeling, and/or import medical devices into the United States. The agency does not regulate the practice of medicine – how and which physicians can use a device. The only exception is FDA's regulation of mammography facilities under the Mammography Quality Standards Act.
What is a combination product?
Combination products are therapeutic and diagnostic products that combine drugs, devices, and/or biological products. The term acknowledges the role technological advancements have made in merging medical product types. Examples of combination products include a drug-eluting stent, a nicotine patch, and surgical mesh with antibiotic coating, prefilled syringes, and a steroid-eluting pacing lead.
Combination products raise regulatory challenges because they involve components that were normally regulated under different types of authorities and often by different FDA Centers. Differences in regulatory pathways for each component can affect the regulatory processes for all aspects of product development and management, including preclinical testing, clinical investigation, marketing applications, manufacturing and quality control, adverse event reporting, promotion and advertising, and post-approval modifications.
Government Regulations
The long legal journey toward medical device regulation began with the Pure Food and Drugs Act of 1906. Medical devices were not included as no one envisioned how technology would grow increasingly complex and need to be regulated. The Medical Device Amendments of 1976 gave FDA authority to ensure the safety and effectiveness of a range of life-saving medical devices while also protecting the public from fraudulent devices. The Amendments:
| | · | defined a medical device, |
| | · | established three device classes (I, II, and III), |
| | · | identified pathways to market, |
| | · | established Advisory Panels, and |
| | · | set clinical investigation requirements. |
Subsequent legislation strengthened the FDA’s regulatory authority:
Table 1: Major Medical Device Legislation
| Legislation | | Significance |
| Safe Medical Devices Act of 1990 | · | established Quality System requirements |
| · | supported post market surveillance |
| · | allowed FDA discretion for PMAs brought to panel |
| FDA Modernization Act of 1997 | · | supported for early collaboration, expanded Class I and Class II exemptions |
| · | set the “least burdensome provision”* |
| · | supported dispute resolution |
| · | established evaluation of automatic Class III designation (giving the sponsor the opportunity to request lower classification due to a minimal risk device, known as “de novo” review) |
| · | mandated free and open participation by all interested persons |
| Medical Device User Fee and Modernization Act (MDUFMA) of 2002 | · | established a fee schedule for most types of device submissions to achieve shorter review times |
| · | requires FDA to include pediatric experts on the panel for a product intended for pediatric use |
| FDA Modernization Act of 2007 | · | reauthorized and expanded MDUFMA |
The least burdensome provision allows industry and FDA to consider the least burdensome appropriate means of evaluating a device’s effectiveness when there’s a reasonable likelihood of its approval. The intent is to help expedite the availability of new device technologies without compromising scientific integrity in the decision-making process or FDA's ability to protect the public health. This provision does not lower the standard for premarket clearance and approval.
Three classes of regulatory control
The three device classes are based on the degree of regulatory control necessary to ensure their safety and effectiveness:
Class I: | devices present a low risk of harm to the user and are subject to general controls that are sufficient to protect the user. Most are exempt from the regulatory process. |
| | |
| | Examples: non-powered breast pumps, elastic bandages, tongue depressors, examination gloves, most hearing aids, arm slings, microbial analyzers, keratoscopes |
| | |
| Class II: | devices are more complicated and require special controls for labeling, guidance, tracking, design, performance standards, and post market monitoring. Most require Premarket Notification 510(k). |
| | |
| | Examples: powered wheelchairs, CT scanners, and contact lens care products, endolymphatic shunts |
| | |
| Class III: | devices usually sustain or support life, are implanted, or present potential unreasonable risk of illness or injury. They have the toughest regulatory controls. Most of these devices require Premarket Approval because general and special controls alone cannot reasonably assure their safety and effectiveness. |
| | |
| | Examples: pacemakers, implanted weight loss devices, non-invasive glucose testing devices, medical imaging analyzers, cochlear implants, breast implants |
How FDA Reviews Medical Devices
Investigational Device Exemptions (IDE)
An IDE allows an investigational device to be used in a clinical study to collect the safety and effectiveness data required for a Premarket Approval (PMA) application or a Premarket Notification (510(k)) submission to FDA. Both FDA and an Institutional Review Board (IRB) must approve clinical studies with devices of significant risk before the study can begin. Studies with devices posing non-significant risk must be approved by an IRB before the study can begin.
FDA observes a 30-day review period for IDE applications. The agency focuses its review on the data provided to demonstrate the safety and anticipated benefits of the device for use in humans, as well as the scientific validity of the proposed clinical trial protocol.
Following clinical studies, a device’s journey to market can take one of four major pathways:
| | 1. | Investigational Device Exemptions (IDE) |
| | 2. | Premarket Notification (510(k)) |
| | 3. | Premarket Approval Application (PMA) |
| | 4. | Humanitarian Device Exemption (HDE) |
Premarket Notification (510(k))
510(k) is required when demonstrating substantial equivalence to a legally marketed device, when making significant modifications to a marketed device, and when a person required to register with FDA introduces a device for the first time. If a device requires the submission of a 510(k), it cannot be commercially distributed until the FDA authorizes it.
Substantial Equivalence
A device is substantially equivalent (SE) if it has the same intended use and same technological characteristics as a legally marketed device, known as the predicate. A legally marketed device:
| | 1. | was legally marketed prior to May 28, 1976 (“pre-amendments device”), for which a PMA is not required, |
| | | or |
| | 2. | was reclassified from Class III to Class II or Class I, |
| | | or |
| | 3. | was found SE through the 510(k) process. |
Applicants must compare their device to one or more similar legally marketed devices and make and support their SE claims. If the device is SE to a predicate, it is placed in the same class. If it is not SE, it becomes non-SE and is placed into Class III.
Examples of 510(k)s include x-ray machines, dialysis machines, fetal monitors, lithotripsy machines, and muscle stimulators.
Premarket Approval (PMA)
PMA refers to the scientific and regulatory review necessary to evaluate the safety and effectiveness of Class III devices or devices that were found not substantially equivalent to a Class I or II predicate through the 510(k) process.
PMA is the most involved process. To reasonably assure that a device is safe and effective, PMA requires valid scientific evidence that the probable benefits to health from the intended use of a device outweigh the probable risks, and that the device will significantly help a large portion of the target population. Sources of valid scientific evidence may include well controlled investigations, partially controlled studies, historical controls, well documented case histories by qualified experts, and robust human experience.
Independence is an important concept for PMAs, meaning that each PMA should establish the safety and effectiveness of the device under review, and that data about one device cannot be used to support another. Examples of PMAs include digital mammography, minimally invasive and non-invasive glucose testing devices, implanted defibrillators, and implantable middle ear devices.
Table 2: Summary Comparison of 510(k) and PMA
| 510(k) Submissions | | PMA Submissions |
| · | primarily for Class II devices | | · | primarily for Class III devices |
| · | a Class I or II pre-amendment or legally marketed device (predicate) exists | | · | a Class I or II pre-amendment or legally marketed device (predicate) does not exist |
| · | third party review option is available for devices not requiring clinical data | | · | device is life supporting and/or has potential risk to patient |
| · | documented proof of Substantial Equivalence to a predicate is required | | · | documented safety and effectiveness data for the device is required |
Humanitarian Device Exemption (HDE)
An HDE is a device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 4,000 individuals in the United States per year. HDEs are exempt from requirements to demonstrate effectiveness. Still, they must pose no unreasonable risks, or at least the probable benefits should outweigh the risks. And the device must be used at a facility with an Institutional Review Board.
HDEs provide a powerful incentive for device manufacturers to develop devices that help diagnose or treat patients with rare conditions. Otherwise, a company’s research and development costs would likely exceed the market returns for serving such small patient populations.
Examples of HDEs include a fetal bladder stent, iris replacement, radioactive microspheres for cancer treatment, and semi-constructed finger joints.
Post-Approval Studies
FDA can impose requirements at the time of approval of a PMA or HDE, or by regulation afterwards. One requirement may be the need for post-approval studies. The CDRH Post-Approval Studies Program helps ensure that well designed post-approval studies are conducted effectively and efficiently and in the least burdensome manner. Post-approval studies should not be used to evaluate unresolved premarket issues that are important to the initial establishment of device safety and effectiveness.
With post-approval studies, FDA can evaluate device performance and potential problems when the device is used more widely than in clinical trials and over a longer period of time. This allows FDA to build in accountability and gather essential post market information, including:
| | · | longer-term performance of the device (for example, effects of re-treatments and product changes) |
| | · | community performance (clinicians and patients) |
| | · | effectiveness of training programs |
| | · | sub-group performance |
| | · | outcomes of concern – real and potential |
Employees
Prior to the Merger, the Company had one employee, Mr. Gardner Williams, the Company’s President and CEO. On October 31, 2012, in connection with the Merger, Mr. Williams resigned from all positions held, and the board of directors appointed Mr. J. Michael Redmond as President, Chief Executive Officer and Director, Ms. Calli Bucci as Chief Financial Officer and Treasurer, Dr. Roger Morris as Chief Science Officer, and Mr. Michael Contarino as Vice President. Mr. Redmond is currently the Company’s only full time employee. Ms. Bucci, Dr. Morris and Mr. Contarino provide services to the Company on an as-needed basis.
In addition, the Company appointed Mr. Edward W. Withrow III, Dr. Jorn Gorlach, Mr. E. William Withrow Jr., Mr. David Engert and Mr. Anand Kumar as its board of directors.
Reports to Security Holders
The Company is a reporting company and complies with the requirements of the Exchange Act. The Company files quarterly and annual reports and other information with the SEC.
The public may read and copy any materials the Company files with the SEC at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, which can be found at http://www.sec.gov.
Contractual Obligations
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
PROPERTIES
Subsequent to the Merger, the Company’s principal executive offices are located at 2 Canal Park 5th Floor Cambridge, MA 02141. The Company currently rents this space for approximately $300 a month. Currently, this space is sufficient to meet the Company’s needs. However, once the Company expands its business to a significant degree, it will require additional space. The Company does not currently own any real estate.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Security Ownership of Management
The following table sets forth certain information concerning the number of shares of the Company’s common stock owned beneficially as of October 30, 2012, by: (i) each of its directors; (ii) each of its named executive officers; and (iii) each person or group known by the Company Parallaxto beneficially own more than 5% of its outstanding shares of common stock. Unless otherwise indicated, the shareholders listed below possess sole voting and investment power with respect to the shares they own.
Name and Address of Beneficial Owner | | Title of Class | | Amount and Nature of Beneficial Ownership (1) | | Percent of Class (2) |
| Montecito Bio Sciences, Ltd. | | Common | | 38,156,227 | | | 25.2 | % |
| Withrow, Sinclair & Co | | Common | | 5,569,725 | | | 3.68 | % |
| Edward W. Withrow III | | Common | | 7,631,245 | | | 5.05 | % |
| M. Katsuka Sandoval | | Common | | 5,000,000 | | | 3.30 | % |
| Edward W. Withrow III | | Preferred/Common | | 1,453,570 | | | .009 | % |
| Calli Bucci | | Common | | 381,562 | | | .002 | % |
| J. Michael Redmond | | Common | | 454,240 | | | .003 | % |
| J. Michael Redmond | | ESOP | | 916,666 | | | .006 | % |
| Jorn & Jennifer Gorlach | | Common | | 7,000,000 | | | 4.60 | % |
| Avantegarde, LLC | | Common | | 3,250,000 | | | 2.15 | % |
| Jorn Gorlach | | Preferred/Common | | 726,785 | | | .004 | % |
| David Engert | | Preferred/Common | | 726,785 | | | .004 | % |
| E. William Withrow Jr. | | Common | | 152,625 | | | .001 | % |
| All Officers and Directors as a Group | | | | 71,419,430 | | | 47.27 | % |
| (1) | The number and percentage of shares beneficially owned is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has sole or shared voting power or investment power and also any shares, which the individual has the right to acquire within 60 days through the exercise of any stock option or other right. The persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to community property laws where applicable and the information contained in the footnotes to this table. |
| (2) | Based on 151,063,898 issued and outstanding shares of common stock as of November 12, 2012 |
DIRECTORS AND EXECUTIVE OFFICERS
Identification of Directors and Executive Officers
The following table sets forth the names and ages of the Company’s current directors and executive officers:
| Name | Age | Position with the Company | Date of Appointment |
| J. Michael Redmond | 52 | President, Chief Executive Officer, Director | November 1, 2012 |
| Calli Bucci | 47 | Treasurer, Chief Financial Officer | November 1, 2012 |
| Kyle W. Withrow | 38 | Secretary | November 1, 2012 |
| Edward W. Withrow III | 48 | Executive Chairman | November 1, 2012 |
| Jorn Gorlach, MD | 50 | Director | November 1, 2012 |
| David Engert, MD | 59 | Director | November 1, 2012 |
| Anand Kumar | 69 | Director | November 1, 2012 |
| E. William Withrow, Jr. | 74 | Director | November 1, 2012 |
Term of Office
Each director of the Company serves for a term of one year and until his successor is elected at the Company’s Annual Shareholders’ Meeting and is qualified, subject to removal by the Company’s shareholders. Each officer serves for a term of one year and until his successor is elected at a meeting of the Board of Directors and is qualified.
On July 23, 2012, Mr. Tom Mackay resigned from all positions with the Company. The Board of Directors of the Company accepted the resignation of Mr. Mackay, and accepted the appointment of Mr. Gardner Williams as the Company’s President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director.
On November 1, 2012, Mr. Williams resigned from all positions with the Company. The Board of Directors of the Company accepted the resignation of Mr. Williams, and accepted the appointment of Mr. J. Michael Redmond, President and CEO of Parallax Diagnostics, Inc., as the Company’s President, Chief Executive Officer and Director.
Concurrently with the assignment of Mr. Redmond, the Board of Directors assigned Ms. Calli Bucci as Chief Financial Officer and Treasurer of the Company, and Mr. Kyle W. Withrow as Secretary of the Company.
Background and Business Experience
J. Michael Redmond. President, Chief Executive Officer, and Director
Mr. J. Michael Redmond, age 52, has over twenty-five years of experience in the medical device and biotech markets. In May of 2009, Mr. Redmond founded JMR, Inc. and has served as its President since that time. JMR, Inc. provides business development and marketing services to diagnostic and biotech companies. As President, Mr. Redmond is responsible for developing and implementing the business plan of the company.
From May 2007 to June 2009, Mr. Redmond served as the Vice President of Marketing and Business Development for DxTech, Inc., a startup company focused on a disruptive model for point of care diagnostic testing. As the Vice President of Marketing and Business Development, Mr. Redmond was responsible for creating and implementing the company’s business plan, raising capital and forming strategic alliances with industry partners.
From 1996 to 2007, Mr. Redmond worked in various titles and capacities for Bioject, Inc., an early stage drug delivery company. From 1996 to 1997, Mr. Redmond served as the company’s Vice President of Sales and Marketing. From 1998 to 2002, Mr. Redmond served as the company’s Vice President of Business Development, and from 2003 to 2007, Mr. Redmond served as the company’s Senior Vice President of Business Development, Sales and Marketing. In these positions, Mr. Redmond’s responsibilities included negotiating corporate partnerships with major pharmaceutical and biotech companies, launching new products, securing distribution channels, P&L responsibility and raising capital.
From 1989 to 1996, Mr. Redmond was employed with KMC Systems, a private label developer and manufacturer of medical devices and instruments. At KMC Systems, Mr. Redmond served as the Director of Sales and Marketing and the Director of Business Development, Sales and Marketing. Mr. Redmond was responsible for developing new business in the U.S. and Europe as well as negotiating long-term product development and production contracts. Additionally, from 1983 to 1989, Mr. Redmond was employed with Abbott Laboratories in the diagnostic division. While at Abbott Laboratories, Mr. Redmond served as Product Manager, Account Executive, and Diagnostics Systems Sales Specialist.
Mr. Redmond earned a Bachelor of Arts degree from Denison University in 1983. He is qualified to be the, President, CEO and Director of the Company because of his extensive experience in a multitude of different capacities in the medical device and biotech markets.
Calli Bucci, Chief Financial Officer, Treasurer
Ms. Bucci has over 25 years experience in the field of finance and business management. Prior to holding the position of interim Chief Financial Officer at Ecologic Transportation, Ms. Bucci has been Controller of the Company since January 2010, and was responsible for general ledger, quarterly certified reviews, annual audits, preparation for SEC filings, customer billing and invoicing, multi-state payroll, licenses and consolidated corporate income taxes.
Before joining the Company, Ms. Bucci held the position of Chief Financial Officer at InstaSave, Inc., a Internet based promotional incentive company, from December 2007, where she was responsible for financial reporting, capital structure strategy and modeling, financial transactions with consumers, consumer product goods companies and retailers, investor relations, audits, payroll and corporate income taxes.
Ms. Bucci held the position of Senior Accountant at Gelfand, Rennert & Feldman, a division of PriceWaterhouse Coopers, from 1993-1999, working on the accounts of high net worth individuals and entertainment corporations. At Gelfand, Rennert & Feldman, Ms. Bucci was responsible for financial transactions, contract administration, audits, general ledger reviews and annual tax preparation for her clients.
From 1989-1993 Ms. Bucci held the position of Chief Financial Officer and Director of Contract Administration at Intercontinental Releasing Corporation (“IRC”), a Los Angeles, California based Motion Picture Distribution Company. Ms. Bucci was responsible for all functions within the company’s accounting department, from financial statements and forecasting, to annual audits and corporate taxes. During her tenure with IRC, Ms. Bucci also designed and implemented a custom computerized availabilities system for the film rights of the Company’s film library consisting of 35 films and distributed to over 30 foreign territories throughout the world. She was also responsible for the administration and facilitation of all client contracts, involving multiple foreign currencies and international import regulations.
Ms. Bucci holds a BS from the University of California Berkley, majoring in Accounting. Ms. Bucci lives with her family in West Los Angeles, California.
Kyle W. Withrow, Secretary
Mr. Withrow, age 37, currently serves as corporate Secretary, and has 10 years experience working with public companies. Mr. Withrow began his career working in the public markets dealing with Marketing and Branding for a point-of-care diagnostics company. He has also assisted in overseeing the public filings for three separate bulletin board companies.
After traveling extensively thru the US, Europe and Asia, Mr. Withrow attended San Diego State University and holds a degree in Bachelors of Sciences with an emphasis in Psychology. Mr. Withrow used his knowledge first in the opening and operating of boutique restaurants and night clubs throughout the West Coast of the US. Working hands on with both large and small Marketing and Branding companies.
Mr. Withrow has held a position with both Parallax Diagnostics, Inc. and Montecito Bio Sciences, Ltd. since their inception, His responsibilities included marketing and development, as well as management of aspects of the day to day operations, from assisting in mergers documentation to public filings for the companies.
Edward W. Withrow III, Executive Chairman
Mr. Edward W. Withrow III, age 48, currently serves as the Chairman of the Board for Ecologic Transportation, Inc., a company he founded in 2009 that is dedicated to providing environmentally friendly transportation services. Mr. Withrow III also currently serves as the President and CEO of Montecito Bio Sciences, Ltd., a bio-medical diagnostics company. As President and CEO, Mr. Withrow III is responsible for creating and implementing the Company’s business plan, raising capital and forming strategic alliances with industry partners.
From 2002 to 2005, Mr. Withrow III served as the CEO for Addison-Davis Diagnostics, Inc. Addison Davis Diagnostics, Inc. offers point-of-care screening tests to the global health care market. As CEO, Mr. Withrow III was responsible for corporate governance, strategic planning, capitalization, and business development. From 2002 to 2004, Mr. Withrow III served as the CEO for Reward Enterprises, Inc., a public company and early adopter of VoIP telecommunications in the international market with operations in North Africa and India.
Mr. Withrow III graduated from Alameda High School in Alameda California in 1982 and attended Santa Barbara City College and University of California Santa Barbara from 1982 to 1985 where he studied Law and Society and economics. He lives with his wife and son in Malibu, California.
Mr. Withrow III is qualified to be a director because of his knowledge of and prior experience working in the diagnostics products market.
Dr. Jorn Gorlach, age 50, has over twenty years of experience in the bio-medical field. In 2001, Dr. Gorlach co-founded AAvantgarde, a management consulting firm focused on the development and support of start-up companies. Since the inception of AAvantgarde in 2001, Dr. Gorlach has also served as one of its directors. As a co-founder and director of AAvantgarde, Dr. Gorlach is responsible for management consulting, licensing, and general operations. Since 2006, Dr. Gorlach has also served as a co-founder and director of Montecito Bio Sciences, Ltd., a diagnostics and testing company with proprietary technology for point of care diagnostics, testing, and data communication. Dr. Gorlach, in his role as co-founder and director, is responsible for developing and implementing the business plan of the company.
In 2002, Dr. Gorlach co-founded AAvantgarde Laboratories AG and has served as its CEO since that time. AAvantgarde Laboratories AG is a research, development, and licensing company of biotechnology products, particularly in the field of diagnostics, biological prognostics, and diseases. As CEO, Dr. Gorlach is responsible for developing the company’s business plan, developing outlines for product concept, research, and development, and leading financing activities and investor relations. In 2001, Dr. Gorlach co-founded Arcanum Discovery, Inc., a proteomics and drug discovery company focusing on novel drug target identifiers and validation. Additionally, from 2001 to 2002, Dr. Gorlach served as head of business development and finances for Arcanum Discovery, Inc. where he developed the company’s product concept, research and development, and business plan as well as managed financing activities and investor relations. In 2001, Dr. Gorlach co-founded Ercole Biotech, Inc., a research stage biopharmaceutical company involved in the creation of oligonucleotide drugs. Since its inception until 2003, Dr. Gorlach served as a director of the company where he was responsible for developing business strategy, financial planning, and contract negotiation strategy.
In 1997, Dr. Gorlach co-founded Paradigm Genetics, Inc., a bio-technology research company. From 1997 to 1999, Dr. Gorlach served as the company’s Director of Research where he was responsible for developing concepts regarding novel functional genomics platform, focusing on high throughput, industrialization, systematization, and biology/IT integration. From 1999 to 2000, Dr. Gorlach served as the Director of Project Management for Paradigm Genetics, Inc. As Director of Project Management, Dr. Gorlach managed customer projects and research progress. From 2000 to 2001, Dr. Gorlach served as the company’s vice president of business development. As a member of the company’s executive team, Dr. Gorlach was responsible for new projects and the development of plans in future key business fields. Beginning in 2001 and continuing through 2002, Dr. Gorlach served as a consultant for Paradigm Genetics, Inc., where he supported the company’s agricultural project initiatives and customer negotiations.
From 1996 to 1997, Dr. Gorlach served as the Group Leader of Combinatorial Biochemistry for Novartis, Inc., a healthcare and scientific research company. As Group Leader of Combinatorial Biochemistry, Dr. Gorlach led team efforts in developing pharmaceutically active macrolide and cloning multiple polyketides genes.
From 1994 to 1996, Dr. Gorlach was a research scientist for Ciba-Geigy, Inc., a chemical company. As a research scientist, Dr. Gorlach focused on acquired immunity and chemical regulation in wheat.
From 1991 to 1994, Dr. Gorlach was a research fellow for the Swiss Federal Institute in Zurich, Switzerland. As a research fellow, Dr. Gorlach focused his attention on gene regulation of amino acid biosynthetic pathways.
Dr. Gorlach has a Bachelor of Science Degree in Chemistry and Biology as well as a Bachelor of Science Degree in Biochemistry from the University of Hannover. In 1991, Dr. Gorlach obtained a Master in Science from the University of Hannover in Biochemistry. In 1994, Dr. Gorlach received a Ph.D. in Molecular Biology from ETH Zurich, and in 2000, received a MBA from the Kenan-Flagler Business School at the University of North Carolina-Chapel Hill.
Dr. Gorlach is qualified to be a director of the Company because of his extensive experience in business development, project management, strategic planning, and business management in a multitude of different capacities in the bio-technology field.
Mr. Engert, age 59, has served as the President and Chief Executive Officer of NightHawk Radiology Holdings, Inc. since November 2008 and as a member of its board of directors since April 30, 2008. He also sits on the Board of Directors of Healthation, Inc., a healthcare information technology company. Mr. Engert was the founder and owner of ES3, a strategic consulting and investment company since 2007. From 2002 to 2006, he served as the president, chief executive officer and director of Quality Care Solutions, Inc., one of the nation’s leading providers of advanced healthcare payer enterprise application solutions, which was acquired by Trizetto, Inc. in January 2007. Prior to 2002, Mr. Engert held a number of senior level management positions in the healthcare industry over the previous 10 years, including senior vice president & general manager at McKesson Corporation's Managed Care Division.
Mr. Engert is qualified to be a director of the Company because of his extensive experience as a chief executive officer of companies both public and private, in the healthcare industry.
Mr. Kumar, age 69, has over twenty-five years of experience in international business development. In 1999, Mr. Kumar founded Global Telesolutions, a company responsible for creating partnerships and in-country relationships for various companies in Asia and the Indian subcontinent. From 1999 to 2010, Mr. Kumar served as the CEO for Global Telesolutions where, among other things, he developed presence and business in the Middle East and Indian, built global network partnerships for telecommunications and traffic, and oversaw international staff for operations.
From 1995 to 1999, Mr. Kumar served as the Executive Vice President for Facilicom International, a leading international telecommunications carrier. As Executive Vice President, Mr. Kumar developed multi-country business and network presence for operations, negotiated with vendors, regulators, and partners, and oversaw Europe and Asia managers and assisted in multi-national sales closings.
From 1986 to 1993, Mr. Kumar served as the President for Washington International Teleport. As President, Mr. Kumar built the first direct international earth station after U.S. de-regulation, obtained new national and international video and data clients, and created the satellite, fiber hybrid network video concept. From 1981 to 1986, Mr. Kumar served as the President of Communications Strategies Group, a company that delivers comprehensive public relations and strategic communications services to organizations. As President, Mr. Kumar investigated technology business opportunities for international clients and ran special training sessions in various areas of telecommunications practice.
Mr. Kumar earned a B.S.E.E. from Jadavpur University and a M.S.E.E. and PhD candidacy degree from the University of Connecticut.
Mr. Kumar is qualified to be a director of the Company because of his extensive experience as an executive officer of companies both public and private, in the telecommunications and telemedical industries.
Edward W. “Bill” Withrow Jr., Director
Mr. Withrow earned his Master’s in Business Administration from Harvard University, with a concentration in Investment Banking. He has a bachelor’s degree in Business, with a concentration in Finance and Accounting, from the University of Colorado. He served twenty-four years on active duty in the U.S. Navy as a professional logistician, retiring with the rank of Captain. He spent approximately 20 years as a financial professional with Drexel Burnham Lambert, Paine Webber, Merrill Lynch and Wells Fargo.
Mr. Withrow has been very active in civic leadership for the past 20 years serving in a number of elected and appointed positions, including Mayor of Alameda, California. He is currently serving as the regionally elected President of the Governing Board of The Peralta Colleges, an institution consisting of 2,000 faculty and staff and approximately 30,000 students.
Mr. Withrow is qualified to be a director of the Company because of his extensive experience as an executive officer of companies both public and private, strategic planning and finance.
Identification of Significant Employees/Consultants
Dr. Roger Morris, Chief Science Officer
Dr. Roger Morris has experience providing over all guidance for technology development, intellectual property and scientific communications strategies. He has twenty years management and technical experience in developing clinical diagnostic systems with Baxter Healthcare Corporation, bioMerieux and most recently XL TechGroup. During this time he has developed and launched a number of successful automated diagnostic system products.
Roger received his B.Sc. and PhD in biochemistry from the University of Salford, UK, he did his Post Doctorate work at Cornell and has published over 20 scientific articles and received 14 patents.
Michael Contarino, Vice President
Mike Contarino has extensive experience developing, integrating and driving complex programs to meet corporate goals. Additionally, Mike has directed Regulatory Affairs, Quality Assurance and Manufacturing. As a technical and operations professional, Mike brings over 30 years successful leadership in product development, commercialization of complex medical/diagnostic instrumentation, and operations management.
Mike was employed by Tecan-Boston, a subsidiary of Tecan AG, where he held the positions of Vice President of R&D, and led all aspects of its novel, automated micro fluidic system platform for Drug Discovery, including overall site management, R&D and Operations.
Previously, Mike held the position of Vice President of Systems Development for Instrumentation Laboratory (IL), a global leader in medical diagnostic systems. Mike accelerated product development cycles by introducing design control processes and procedures in R&D. At IL, Mike was a member of the Executive Committee and Scientific Advisory Boards for homeostasis and critical care, was technical lead of the Merger & Acquisition (M&A) team, and had responsibility for R&D sites in the US, Italy and Spain.
Prior to IL, Mike was the Director of Engineering at KMC Systems Inc. a recognized leader in systems development of diagnostic platforms. Mike successfully commercialized immunoassay, clinical chemistry and robotic pipetting systems.
Mike earned his Mechanical Engineering degree from the University of Lowell, completed the Management Development Program at Boston University and is a Member of the Society of Automotive Engineers (SAE).
Dr. David Stark, V.P. Regulatory Approval and Clinical Trial Manager
Dr. Stark has 18 years experience from the toxicology labs to the investigator site and has been essential to all aspects clinical and device research. Dr. Stark is the President and CEO of Stark-SMO, a Site Management Organization whose services go far beyond that of an ordinary SMO. Due to his extensive and broad experiences in the inner workings of the research and regulatory aspects of clinical trials, Dr. Stark brings a unique vision to the industry and the Company as a motivated designer of superior approaches to research challenges. Most importantly, Dr. Stark is highly qualified to manage the development opportunities of the Company.
Formerly the Director of the National Institute of Clinical Research (NICR), he has been responsible for the design, organization and implementation of clinical trials for pharmaceutical and device companies. He has a broad background in designing, conducting, and monitoring clinical trials of new pharmaceuticals and devices. He is one of the few that has worked in the manufacturing validation of pharmaceuticals, the clinical field, and the regulatory (IRB) arenas, and therefore possesses a big-picture understanding of pharmaceutical development.
Through Dr. Stark’s diverse and devoted networking within the industry, Stark-SMO has assembled a wide network of more than 5000 physicians throughout the United States, which extends to the international community. Currently, he is negotiating a unique DMF partnership with drug manufacturers in China.
In addition to his significant accomplishments on the industry side of clinical drug and device development, Dr. Stark has experience with the FDA (major focus on IND’s NDA’s and 510K applications). Prior to his employment at NICR, Dr. Stark was the President and Chief Executive Officer of Powder Ice, Inc a medical products company. Additionally, Dr. Stark is a California state licensed Qualified Medical Examiner and Certified Clinical Research Associate.
Ricky Richardson, Consultant
Mr. Richardson recently severed as Director of Operations for Stryker Orthopaedics. During his tenure, he was responsible for the Continuous Improvement and Global Sourcing Departments where he championed several successful initiatives to improve internal manufacturing efficiencies and streamline the supplier base. He also oversaw FDA remediation programs in Operations; resulting in the achievement of several critical compliance milestones for the manufacturing business units.
Prior to joining Stryker Orthopaedics, Mr. Richardson served as Vice President of Supplier Management/ Customer Relations at Bioject Medical Technologies. He also held the positions of VP Operations, VP Manufacturing and Product Development directing several successful R&D and manufacturing programs with leading Biotech Healthcare companies. He joined Bioject in 1994 as Senior Manufacturing Engineer. From May 1991 to October 1994, he was employed as a Product Manager, Quality Engineer and Production Supervisor with Baxter Healthcare. From 1987 to April 1991, Mr. Richardson was a Manufacturing Supervisor at Texas Instruments and championed one of the pilot cell teams that received recognition by winning the Malcolm Baldrige. From 1984 to 1987 he was a Lieutenant, Field Artillery, with the U.S. Army.
Mr. Richardson holds a Bachelor’s degree in engineering from the U.S. Military Academy, West Point, NY.
Other than as described below, there are no family relationships among our directors or executive officers.
Mr. Kyle W. Withrow, Secretary of the Company, is the brother of Mr. Edward W. Withrow, III, and the Company’s Executive Chairman of the board of directors. Mr. E. William Withrow Jr., Director, is the father of Mr. Kyle W. Withrow and Mr. Edward W. Withrow III.
Involvement in Certain Legal Proceedings
During the past ten years no director, executive officer, promoter or control person of the Company has been involved in the following:
| | (1) | A petition under the Federal bankruptcy laws or any state insolvency law which was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing; |
| | (2) | Such person was convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| | (3) | Such person was the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities: |
| | i. | Any Federal or State securities or commodities law or regulation; or |
| | ii. | Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or |
| | iii. | Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | (8) | Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Audit Committee and Audit Committee Financial Expert
The Company does not currently have an audit committee serving on its Board of Directors. However, the Company intends, in the coming months, to establish an audit committee of the Board of Directors that shall consist of independent directors. The audit committee’s duties will be to recommend to the Company’s board of directors the engagement of an independent registered public accounting firm to audit the Company’s financial statements and to review the Company’s accounting and auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations performed by the internal auditors and independent registered public accounting firm, including their recommendations to improve the system of accounting and internal controls. The audit committee shall at all times be composed exclusively of directors who are, in the opinion of the Company’s board of directors, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
EXECUTIVE COMPENSATION
The table below summarizes the compensation paid to the following persons:
| | (a) | our principal executive officer; |
| | (b) | each of our two most highly compensated executive officers who were serving as executive officers at the end of the fiscal years ended December 31, 2011 and 2010; and |
| | (c) | up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as our executive officer at the end of the fiscal years ended December 31, 2011 and 2010, |
who will collectively be referred to as the named executive officers of the Company, and are set out in the following summary compensation table, except that no disclosure is provided for any named executive officer, other than our principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:
| Summary Compensation Table |
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) |
J. Michael Redmond President, CEO | 2012 | $177,403 | Nil | $33 | Nil | Nil | Nil | Nil | $177,436 |
| 2011 | $203,365 | Nil | Nil | Nil | Nil | Nil | Nil | $203,365 |
| 2010 | Nil | Nil | $13 | $137,500 | Nil | Nil | Nil | $137,513 |
Calli Bucci CFO, Treasurer | 2012 | Nil | Nil | $38 | Nil | Nil | Nil | $10 [1] | $48 |
| 2011 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 2010 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Edward W. Withrow III Executive Chairman | 2012 | Nil | Nil | Nil | Nil | Nil | Nil | $137,500 [1] | $137,500 |
| 2011 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 2010 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Gardner Williams Former President, CEO, and Director | 2012 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 2011 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| 2010 | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
| [1] | Compensation earned but deferred. |
Narrative Disclosure to Summary Compensation Table
There are no compensatory plans or arrangements, including payments to be received from the Company with respect to any executive officer, that would result in payments to such person because of his or her resignation, retirement or other termination of employment with the Company, or its subsidiaries, any change in control, or a change in the person’s responsibilities following a change in control of the Company.
On October 1, 2010, the board of directors of Parallax adopted the Parallax Kline Stock Option Plan, a copy of which is attached herewith and included in this filing as Exhibit 4.5. Parallax reserved 3,000,000 shares of common stock for issuance upon exercise of options granted from time to time under the stock option plan. The stock option plan is intended to assist Parallax in securing and retaining key employees, directors and consultants by allowing them to participate in Parallax’s ownership and growth through the grant of incentive and non-qualified options. Under the stock option plan, Parallax may grant incentive stock options only to key employees and employee directors, or Parallax may grant non-qualified options to employees, officers, directors and consultants. Subject to the provisions of the stock option plan, the board of directors will determine who shall receive options, the number of shares of common stock that may be purchased under the options. Prior to the Merger Agreement, Parallax granted options to purchase a total of 1,950,000 shares. In connection with the options granted, a total of $281,250 was recorded as deferred compensation, and was amortized over a 12-18 month vesting period.
Parallax granted the following stock options to directors and officers through the Parallax Kline 2010 ESOP:
On October 31, 2010, J. Michael Redmond, the Company’s President and CEO, was granted an option to purchase 1,375,000 shares of Parallax’s common stock at a price of $0.10 per share.
On November 15, 2010, Dr. Roger Morris, Chief Science Officer, was granted an option to purchase 150,000 shares of Parallax’s common stock at a price of $0.25 per share.
On November 15, 2010, Michael Contarino, the Company’s Vice President, was granted an option to purchase 150,000 shares of Parallax’s common stock at a price of $0.25 per share.
On February 1, 2011, Norman Kunin, the Company’s former Chief Financial Officer, was granted an option to purchase 50,000 shares of Parallax’s common stock at a price of $0.25 per share.
The foregoing summary of the Stock Option Agreements are not complete and are qualified in their entirety by reference to the complete text, an example of which is attached hereto as Exhibit 14.6.
Aggregated Option Exercised in Last Fiscal Year and Fiscal Year-End Values
There were no options exercised during the year ended December 31, 2011 or December 31, 2010 by any officer or director of our company.
Outstanding Equity Awards of the Parallax Kline 2010 ESOP
On October 31, 2010, Parallax, under its 2010 ESOP, granted qualified stock options to its Chief Executive Officer to purchase 1,375,000 shares of its common stock for five years at $0.10 per share, which vest quarterly over a period of twelve months. As of the date of this filing, the options are fully vested, and were expensed by Parallax over the vesting period of 12 months, at an aggregate value of $137,500.
On November 15, 2010, Parallax, under its 2010 ESOP, granted qualified stock options to two of its consultants to purchase 300,000 shares of its common stock for five years at $0.25 per share, which vest quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by Parallax over the vesting period of 18 months, at an aggregate value of $75,000.
On January 10, 2011, Parallax, under its 2010 ESOP granted qualified stock options to one of its consultants to purchase 75,000 shares of Parallax’s common stock at a price of $0.25 per share, which vest quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by Parallax over the vesting period of 18 months, at an aggregate value of $18,750.
On January 28, 2011, Parallax, under its 2010 ESOP, granted qualified stock options to one if its consultants to purchase 150,000 shares of Parallax’s common stock at a price of $0.25 per share, which vest quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by Parallax over the vesting period of 18 months, at an aggregate value of $37,500.
On February 1, 2011, Parallax, under its 2010 ESOP, granted qualified stock options to its former Chief Financial Officer to purchase 50,000 shares of its common stock for five years at $0.25 per share, which vest quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by Parallax over the vesting period of 18 months, at an aggregate value of $12,500.
Compensation of Directors
The Company reimburses its directors for expenses incurred in connection with attending board meetings. The Company has not paid any director's fees or other cash compensation for services rendered as a director since our inception to the date of this filing.
The Company has no formal plan for compensating its directors for their service in their capacity as directors. However, certain directors and officers of the Company have received stock options to purchase common shares under the Company’s 2012 ESOP, and may receive additional stock options at the discretion of the Company’s board of directors.
Employment Agreements
On November 15, 2010, Parallax executed and entered into an employment agreement with its CEO, Mr. J. Michael Redmond (the “Employment Agreement”). The Employment Agreement has a term of three years from the effective date, November 15, 2010. Under the Employment Agreement, Mr. Redmond agreed to serve as the President, CEO, and Director of Parallax. Mr. Redmond shall have such authority, and Parallax’s board of directors may reasonably assign responsibility to him. Pursuant to the Employment Agreement, Mr. Redmond will have a base salary of $200,000 per annum per year. Additionally, as part of Parallax’s 2010 Employee Stock Option Plan, Mr. Redmond was granted one million three hundred seventy five thousand (1,375,000) options of Parallax’s common stock which vest on a quarterly basis over a three year period at an exercise price of ten cents ($0.10) per share. The foregoing summary of the Employment Agreement is not complete and is qualified in its entirety by reference to the complete text, which is attached hereto as Exhibit 10.23.
Consulting Agreements
On July 1, 2011, Parallax entered into a Development and Supply Agreement with Corder Engineering, LLC. The Statement of Work stipulates that Corder Engineering, LLC shall provide ten (10) Evaluation Units which replicate the functionality Target. Target 1000 firmware ver. 320 and add software for a C-reactive protein (CRP) quantitative assay. The total payment under the Agreement stipulates $35,000 over a twelve week period. As of June 30, 2012, payments totaling $22,500 have been made, and $12,500 has been accrued. A copy of the Development and Supply Agreement is attached herewith as Exhibit 10.24.
On July 1, 2011, Parallax entered into a Supply Agreement with Meyers Stevens Group, Inc. (“Meyers Stevens”). The Statement of Work stipulates that Meyers Stevens will manufacture assays and supply a Data Package for the Company and will yield approximately 100 to 200 fully functional assay test devices for internal investigational use. Estimated delivery of the assays is eight (8) weeks from the date of the Agreement for a total cost of $10,194. As of June 30, 2012, payments totaling $8,980 have been made, and $1,214 has been accrued. . A copy of the Supply Agreement is attached herewith as Exhibit 10.25.
On January 2, 2012, Parallax entered into a consulting agreement with Huntington Chase Financial Group LLC (“HCFG”), a Nevada corporation. The consulting agreement provides for HCFG to provide advisory services to the Company for a period of three years for a fee of $12,500 per month. A copy of the consulting agreement is attached herewith as Exhibit 10.26.
On July 11, 2012, the Company entered into a Consulting Agreement with Greg Suess (“Suess”) for advisory services provided to the Company. As compensation for services rendered, valued at $5,000, Suess was afforded the opportunity to purchase 75,000 restricted shares of the Company’s common stock at a price of $0.001 per share. Suess purchased the shares on July 24, 2012 for cash in the amount of $75.00. As a result, $5,000 was expensed in July, 2012. A copy of the consulting agreement is attached herewith as Exhibit 10.27.
Long-Term Incentive Plans
There are no arrangements or plans in which the Company provides pension, retirement or similar benefits for directors or executive officers.
Compensation Committee
The Company currently does not have a compensation committee of the Board of Directors. The Board of Directors as a whole determines executive compensation.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Related Party Transactions
None of the directors or executive officers of the Company, nor any person who owned of record or was known to own beneficially more than 5% of the Company’s outstanding shares of its Common Stock, nor any associate or affiliate of such persons or companies, has any material interest, direct or indirect, in any transaction that has occurred during the past fiscal year, or in any proposed transaction, which has materially affected or will affect the Company.
On June 17, 2011, Parallax entered into a Convertible Preferred Purchase Agreement with Hamburg Investment Company, LLC ("HIC"), a German company controlled by Mr. Jorn Gorlach, a member of the Company’s board of directors, whereby 100,000 shares of Preferred Stock would be issued to HIC for a purchase price of $1.00 per share, or $100,000. As a result, $99,990 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, Parallax issued a warrant to convert 100% of HIC’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if Parallax has reached certain financing levels. The foregoing summary of the Convertible Preferred Purchase Agreement is not complete and is qualified in its entirety by reference to the complete text, which is attached hereto as Exhibit 10.28.
On June 17, 2011, Parallax entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), a Nevada corporation controlled by Mr. Edward W. Withrow III, the Company’s Executive Chairman, whereby 100,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $1.00 per share, or $100,000. As a result, $99,990 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, Parallax issued a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if Parallax has reached certain financing levels. The foregoing summary of the Convertible Preferred Purchase Agreement is not complete and is qualified in its entirety by reference to the complete text, which is attached hereto as Exhibit 10.29.
On September 30, 2011, Parallax entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), a Nevada corporation controlled by Mr. Edward W. Withrow III, the Company’s Executive Chairman, whereby 10,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, Parallax issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if Parallax has reached certain financing levels. The foregoing summary of the Convertible Preferred Purchase Agreement is not complete and is qualified in its entirety by reference to the complete text, which is attached hereto as Exhibit 10.30.
On December 6, 2011, Parallax entered into a Convertible Preferred Purchase Agreement with David Engert, ("Engert"), an individual and a member of the Company’s board of directors, whereby 10,000 shares of Preferred Stock would be issued to Engert for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, Parallax issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of Engert’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if Parallax has reached certain financing levels.
On May 3, 2012, Parallax entered into a Convertible Preferred Purchase Agreement with Donald Wachelka, ("Wachelka"), an individual, whereby 10,000 shares of Preferred Stock would be issued to Wachelka for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, Parallax issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of Wachelka’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if Parallax has reached certain financing levels.
With regard to any future related party transaction, the Company plans to fully disclose any and all related party transactions in the following manner:
| | § | Disclosing such transactions in reports where required; |
| | § | Disclosing in any and all filings with the SEC, where required; |
| | § | Obtaining disinterested directors consent; and |
| | § | Obtaining shareholder consent where required. |
Director Independence
For purposes of determining director independence, The Company has applied the definitions set out in NASDAQ Rule 5605(a)(2). The OTCBB on which shares of Common Stock are quoted does not have any director independence requirements. The NASDAQ definition of “Independent Officer” means a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company's Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
According to the NASDAQ definition, Mr. Redmond is not an independent director because he is also an executive officer of the Company.
Review, Approval or Ratification of Transactions with Related Persons
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
LEGAL PROCEEDINGS
The Company knows of no material, existing or pending legal proceedings against the Company, nor is the Company involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of the Company’s directors, officers or any affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to its interest.
The public may read and copy any materials the Company files with the SEC at the SEC's Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, which can be found at http://www.sec.gov.
RISK FACTORS
An investment in our Company is highly speculative in nature and involves an extremely high degree of risk.
We are a development stage company with a limited operating history and may never be able to effectuate our business plan or achieve sufficient revenues or profitability; at this stage of our business, even with our good faith efforts, potential investors have a high probability of losing their entire investment.
We are subject to all of the risks inherent in a development stage company. In particular, potential investors should be aware that we have not proven that we can:
| | · | raise sufficient capital in the public and/or private markets; |
| | · | have access to a line of credit in the institutional lending marketplace for the expansion of our business; |
| | · | respond effectively to competitive pressures; or |
| | · | recruit and build a management team to accomplish our business plan. |
Accordingly, our prospects must be considered in light of the risks, expenses and difficulties frequently encountered in establishing a new business, and our Company is a highly speculative venture involving significant financial risk.
We have a limited track record that would provide a basis for assessing our ability to conduct successful business activities. We may not be successful in carrying out our business objectives.
The revenue and income potential of our proposed business and operations are unproven as a limited operating history makes it difficult to evaluate the future prospects of our business. There is limited information at this time on which to base an assumption that our business operations will prove to be successful or that we will ever be able to operate profitably. Accordingly, we have a limited track record of successful business activities, strategic decision making by management, fund-raising ability, and other factors that would allow an investor to assess the likelihood that we will be successful in marketing our services. As such, there is a substantial risk that we will not be successful in generating sufficient operating revenues or in achieving profitable operations, irrespective of competition.
The time needed to obtain regulatory approvals and respond to changes in regulatory requirements could adversely affect our business.
Many of our proposed and existing products are subject to regulation by the FDA and other governmental or public health agencies. In particular, we are subject to strict governmental controls on the development, manufacture, labeling, distribution and marketing of our products. In addition, we are often required to obtain approval or registration with foreign governments or regulatory bodies before we can import and sell our products in foreign countries.
The process of obtaining required approvals or clearances from governmental or public health agencies can involve lengthy and detailed laboratory testing, human clinical trials, sampling activities and other costly, time-consuming procedures. For example, we will be seeking FDA approval for the use of a CD4 rapid test. Approval of these claims will include the submission of clinical data and could require significant time to obtain. The submission of an application to the FDA or other regulatory authority for these or other claims does not guarantee that an approval or clearance to market the product will be received. Each authority may impose its own requirements and delay or refuse to grant approval or clearance, even though a product has been approved in another country.
Moreover, the approval or clearance process for a new product can be complex and lengthy. This time span increases our costs to develop new products and increases the risk that we will not succeed in introducing or selling them in the United States or other countries.
Newly promulgated or changed regulations could also require us to undergo additional trials or procedures, or could make it impractical or impossible for us to market our products for certain uses, in certain markets, or at all.
The regulations in some states may restrict our ability to sell products in those states. While we intend to work with state legislators and regulators to remove or modify any applicable restrictions, there is no guarantee we will be successful in these efforts.
In addition, all in vitro diagnostic products that are to be sold in the European Union (“EU”) must bear the CE mark indicating conformance with the essential requirements of the In Vitro Diagnostic Directive (“IVDD”). We will not be permitted to sell our products in the EU without a CE mark after this date. While we intend to CE mark certain existing and future products, and are not aware of any material reason why we will be unable to do so, there can be no assurance that compliance with all provisions of the IVDD will be demonstrated and the CE mark obtained prior to the deadline.
If we are unable to obtain additional funding, our business operations will be harmed.
We will require additional funds to operate our business and address all necessary infrastructure concerns. We anticipate that we will require a minimum of $1,500,000 to fund our continued operations for the next twelve months. The inability to raise the required capital will restrict our ability to grow and may reduce our ability to continue to conduct business operations. If we are unable to obtain necessary financing, we will likely be required to curtail our development plans which could cause the Company to become dormant. Any additional equity financing may involve substantial dilution to our then existing shareholders.
There is substantial doubt about our ability to continue as a going concern.
In their audit report with regard to our financial statements as of December 31, 2010, 2009 and 2008, our independent registered public accountants have expressed an opinion that substantial doubt exists as to whether we can continue as a going concern. Because we have limited cash resources, we believe that if we do not raise additional capital within the next 12 months in addition to the net proceeds from this offering, we may be required to suspend or cease the implementation of our business plan. As such we may have to cease operations and you could lose your entire investment. Accordingly, we may find it difficult or impossible to attract investors.
Adverse capital and credit market conditions may significantly affect our ability to meet liquidity needs, access to capital and cost of capital.
The capital and credit markets have been experiencing extreme volatility and disruption for more than twelve months. We have historically relied on credit to fund our business and we need liquidity to pay our operating expenses. Without sufficient liquidity, we will be forced to curtail our operations, and our business will suffer. Disruptions, uncertainty or volatility in the capital and credit markets may also limit our access to capital required to operate our business. Such market conditions may limit our ability to replace, in a timely manner, maturing liabilities and access the capital necessary to operate and grow our business. As such, we may be forced to delay raising capital or bear an unattractive cost of capital which could decrease our profitability and significantly reduce our financial flexibility. Our results of operations, financial condition, cash flows and capital position could be materially adversely affected by disruptions in the financial markets.
Our ability to sell products could be adversely affected by competition from new and existing diagnostic products and by treatment or other non-diagnostic products which may be developed.
The diagnostic industry is focused on the testing of biological specimens in a laboratory or at the point of care and is highly competitive and rapidly changing. Our principal competitors often have considerably greater financial, technical and marketing resources. As new products enter the market, our products may become obsolete or a competitor’s products may be more effective or more effectively marketed and sold than ours. If we fail to maintain and enhance our competitive position, our customers may decide to use products developed by competitors which could result in a loss of revenues.
In addition, the development and commercialization of products outside of the diagnostics industry could adversely affect sales of our product. For example, the development of a safe and effective vaccine to HIV or treatments for other diseases or conditions that our products are designed to detect, could reduce, or eventually eliminate the demand for our CD4 rapid test or other diagnostic products and thereby result in a loss of revenues.
Our research, development and commercialization efforts may not succeed or our competitors may develop and commercialize more effective or successful diagnostic products.
In order to remain competitive, we must regularly commit substantial resources to research and development and the commercialization of new products.
The research and development process generally takes a significant amount of time from inception to commercial product launch. This process is conducted in various stages. During each stage there is a substantial risk that we will not achieve our goals on a timely basis, if at all, and we may have to abandon a product in which we have invested substantial amounts.
Successful products require significant development and investment, including testing, to demonstrate their cost-effectiveness or other benefits prior to commercialization. In addition, regulatory approval must be obtained before most products may be sold. Additional development efforts on these products will be required before any regulatory authority will review them. Regulatory authorities may not approve these products for commercial sale. In addition, even if a product is developed and all applicable regulatory approvals are obtained, there may be little or no market for the product. Accordingly, if we fail to develop commercially successful products, or if competitors develop more effective products or a greater number of successful new products, customers may decide to use products developed by our competitors. This would result in a loss of revenues and adversely affect our results of operations, cash flows and business.
If we lose our key personnel or are unable to attract and retain qualified personnel as necessary, our business could be harmed.
Our success will depend to a large extent upon the contributions of our executive officers, management, and sales, marketing, operations and scientific staff. We may not be able to attract or retain qualified employees in the future due to the intense competition for qualified personnel among medical products businesses.
If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will adversely affect our ability to effectively manufacture, sell and market our products, to meet the demands of our strategic partners in a timely fashion, or to support internal research and development programs. Although we believe we will be successful in attracting and retaining qualified personnel, competition for experienced scientists and other personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms.
We may be held liable for injuries resulting from the use of our diagnostic products.
We may be held liable if any of our products, or any product which is made with the use or incorporation of any of our technologies, causes injury of any type or is found otherwise unsuitable during product testing, manufacturing, marketing, sale or usage. Although we intend to obtain product liability insurance prior to implementation of the commercialization of our products, this insurance may not fully cover potential liabilities. As we bring new products to market, we may need to increase our product liability coverage.
Efforts to consolidate or restructure could adversely affect our business.
We may from time to time restructure and consolidate various aspects of our operations in order to achieve cost savings and other efficiencies. We must obtain FDA approval to transfer certain operations to another location. This transfer and the need to obtain FDA approval could interfere with or delay our manufacturing processes and disrupt continued operations. Any delay in or disruption of operations, and in particular manufacturing operations, could result in increased costs or could delay or prevent us from selling certain products and thereby result in a loss of revenue.
Future acquisitions or investments could disrupt our ongoing business, distract our management, increase our expenses and adversely affect our business.
We may consider strategic acquisitions or investments as a way to expand our business in the future. These activities, and their impact on our business, are subject to the following risk factors:
| | • | Suitable acquisitions or investments may not be found or consummated on terms that are satisfactory to us; |
| | • | We may be unable to successfully integrate an acquired company’s personnel, assets, management systems and technology into our business; |
| | • | Acquisitions may require substantial expense and management time and could disrupt our business; |
| | • | An acquisition and subsequent integration activities may require greater capital resources than originally anticipated at the time of acquisition; |
| | • | An acquisition may result in the incurrence of unexpected expenses, the dilution of our earnings or our existing stockholders’ percentage ownership, or potential losses from undiscovered liabilities not covered by an indemnification from the seller(s) of the acquired business; |
| | • | An acquisition may result in the loss of existing key personnel or customers or the loss of the acquired company’s key personnel or customers; |
| | • | The benefits to be derived from an acquisition could be affected by other factors, such as regulatory developments, general economic conditions and increased competition; and |
| | • | An acquisition of a foreign business may involve additional risks, including not being able to successfully assimilate differences in foreign business practices or overcome language barriers. |
The incurrence of one or more of the above or other factors may prevent us from achieving all or a significant part of the benefits expected from an acquisition or investment. This may adversely affect our financial condition, results of operations and ability to grow our business.
Our failure to develop new distribution channels may result in lower revenues.
We intend to market many of our products by collaborating with laboratories, diagnostic companies and distributors. Our sales will depend to a substantial degree on our ability to sell products to these customers and develop new product distribution channels, and on the marketing abilities of the companies with which we collaborate.
In addition, some distributors have experienced, and may continue to experience, pressure from their customers to reduce the price of their products and testing services.
Although we will try to maintain the relationships that we hope to develop and expand our business with our distributors, there can be no assurance that such companies will continue to purchase or distribute our products or maintain order volumes, or that new distribution channels will be available on satisfactory terms.
The use of sole supply sources for critical components of our products could adversely affect our business.
If suppliers of certain antigens we utilize in our tests are unable or unwilling to supply the required component, we would need to find another source, and perform additional development work and obtain FDA approval for the use of the alternative component for our products. Completing that development and obtaining such FDA approval could require significant time to complete and may not occur at all. These events could either disrupt our ability to manufacture and sell certain of our products or completely prevent us from doing so. Either event would have a material adverse effect on our results of operations, cash flows and business.
We may depend upon strategic partners to assist in developing and commercializing some of our diagnostic products.
Although we intend to pursue some product opportunities independently, opportunities that require a significant level of investment for development and commercialization or a distribution network may necessitate involving one or more strategic partners. In particular, our strategy for development and commercialization of a Target System rapid CD4 test, rapid TB or Malaria test, and certain other products may entail entering into additional arrangements with distributors or other corporate partners, universities, research laboratories, licensees and others. We may be required to transfer material rights to such strategic partners, licensees and others. While we expect that our future partners, licensees and others have and will have an economic motivation to succeed in performing their contractual responsibilities, the amount and timing of resources to be devoted to these activities will be controlled by others. Consequently, there can be no assurance that any revenues or profits will be derived from such arrangements.
Our success depends on our ability to protect our proprietary technology.
The diagnostics industry places considerable importance on obtaining patent, trademark, and trade secret protection, as well as other intellectual property rights, for new technologies, products and processes. Our success depends, in part, on our ability to develop and maintain a strong intellectual property portfolio or obtain licenses to patents for products and technologies both in the United States and in other countries.
As appropriate, we intend to file patent applications and obtain patent protection for our proprietary technology. These patent applications and patents will cover, as applicable, compositions of matter for our products, methods of making those products, methods of using those products, and apparatus relating to the use or manufacture of those products. We will also rely on trade secrets, know-how, and continuing technological advancements to protect our proprietary technology.
We have entered, and will continue to enter, into confidentiality agreements with our employees, consultants, advisors and collaborators. However, these parties may not honor these agreements and we may not be able to successfully protect our rights to unpatented trade secrets and know-how. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets and know-how.
Many of our employees, including scientific and management personnel, were previously employed by competing companies. Although we encourage and expect all of our employees to abide by any confidentiality agreement with a prior employer, competing companies may allege trade secret violations and similar claims against us.
We may collaborate with universities and governmental research organizations which, as a result, may acquire part of the rights to any inventions or technical information derived from collaboration with them. To facilitate development and commercialization of a proprietary technology base, we may need to obtain licenses to patents or other proprietary rights from other parties. Obtaining and maintaining such licenses may require the payment of substantial costs. In addition, if we are unable to obtain these types of licenses, our product development and commercialization efforts may be delayed or precluded.
A market for our products may not develop.
Our future success will depend, in part, on the market acceptance, and the timing of such acceptance, of new products such as the Target System Rapid CD4 test and products currently under development or that we acquire and introduced in the future. To achieve market acceptance, we must make substantial marketing efforts and spend significant funds to inform potential customers and the public of the perceived benefits of these products. We currently have limited evidence on which to evaluate the market reaction to products that may be developed, and there can be no assurance that any products will meet with market acceptance and fill the market need that is perceived to exist.
Because new legislation, including the Sarbanes-Oxley Act of 2002, increases the cost of compliance with federal securities regulations as well as the risks of liability to officers and directors, we may find it more difficult for us to retain or attract officers and directors.
The Sarbanes-Oxley Act of 2002 was enacted in response to public concerns regarding corporate accountability in connection with recent accounting scandals. The stated goals of the Sarbanes-Oxley Act are to increase corporate responsibility, to provide for enhanced penalties for accounting and auditing improprieties at publicly traded companies, and to protect investors by improving the accuracy and reliability of corporate disclosures pursuant to the securities laws. The Sarbanes-Oxley Act generally applies to all companies that file or are required to file periodic reports with the SEC, under the Securities Exchange Act of 1934. Upon becoming a public company, we will be required to comply with the Sarbanes-Oxley Act and it is costly to remain in compliance with the federal securities regulations. Additionally, we may be unable to attract and retain qualified officers, directors and members of board committees required to provide for our effective management as a result of Sarbanes-Oxley Act of 2002. The enactment of the Sarbanes-Oxley Act of 2002 has resulted in a series of rules and regulations by the SEC that increase responsibilities and liabilities of directors and executive officers. The perceived increased personal risk associated with these recent changes may make it more costly or deter qualified individuals from accepting these roles. Significant costs incurred as a result of becoming a public company could divert the use of finances from our operations resulting in our inability to achieve profitability.
There is currently no trading market for our common stock, which will limit the ability of our stockholders to liquidate their investment.
Outstanding shares of our common stock cannot be offered, sold, pledged or otherwise transferred unless subsequently registered pursuant to, or exempt from registration under, the Securities Act of 1933, as amended (the “Securities Act”) and any other applicable federal or state securities laws or regulations. These restrictions will limit the ability of our stockholders to liquidate their investment.
We may, in the future, issue additional common shares, which would reduce investors’ percent of ownership and may dilute our share value.
Our Articles of Incorporation authorize the issuance of 400,000,000 shares of common stock. The future issuance of common stock may result in substantial dilution in the percentage of our common stock held by our then existing shareholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the value of the shares held by our investors, and might have an adverse effect on any trading market for our common stock.
The issuance of preferred stock could adversely affect the voting power or other rights of the holders of our common stock.
Our Articles of Incorporation authorizes the issuance of up to 100,000,000 shares of preferred stock with designations, rights and preferences determined from time to time by our directors. Accordingly, our directors are empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the common stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of the Company.
Our common shares may be subject to the “Penny Stock” Rules of the SEC and the trading market in our securities is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment in our stock.
The Securities and Exchange Commission has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| | · | that a broker or dealer approve a person’s account for transactions in penny stocks; and |
| | · | the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
| | · | obtain financial information and investment experience objectives of the person; and |
| | · | make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Commission relating to the penny stock market, which, in highlight form:
| | · | sets forth the basis on which the broker or dealer made the suitability determination; and |
| | · | that the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common shares and cause a decline in the market value of our stock. Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Because we do not presently intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
For the indefinite future, we intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them.
You should consider the United States federal income tax consequences of owning our securities.
There are risks associated with the United States federal income tax consequences of owning our common stock. Because the tax consequences of owning our common stock are complex and certain tax consequences may differ depending on the holder's particular tax circumstances, each potential investor should consult with and rely on its own tax advisor about the tax consequences. In addition, there can be no assurance that the United States federal income tax treatment currently applicable to owning our common stock will not be modified by legislative, administrative, or judicial action that may have a retroactive effect. No representation or warranty of any kind is made with respect to the acceptance by the Internal Revenue Service or any court of law regarding the treatment of any item of income, deduction, gain, loss or credit by an investor on its tax return.
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Common Stock
The Company’s common stock is currently quoted on the OTCQB. The Company’s common stock has been quoted on the OTCQB since May 3, 2007, under the symbol “EDVP.QB.” Because the Company is quoted on the OTCQB, its securities may be less liquid, receive less coverage by security analysts and news media, and generate lower prices than might otherwise be obtained if they were listed on a national securities exchange.
As of the date of this Report, an aggregate of 151,063,898 shares of its common stock were issued and outstanding and were owned by approximately 52 holders of record.
Re-Purchase of Equity Securities
None.
Dividends
The Company has not paid any cash dividends on its common stock since inception and presently anticipate that all earnings, if any, will be retained for development of its business and that no dividends on its common stock will be declared in the foreseeable future. Any future dividends will be subject to the discretion of its Board of Directors and will depend upon, among other things, future earnings, operating and financial condition, capital requirements, general business conditions and other pertinent facts. Therefore, there can be no assurance that any dividends on its common stock will be paid in the future.
DESCRIPTION OF THE REGISTRANT’S SECURITIES
Pursuant to the Company’s Articles of Incorporation and amendment(s) thereto, the aggregate number of common shares which this Company has authority to issue is two hundred fifty million (250,000,000) shares of Common Stock, par value $0.001 per share.
The Company refers you to its Articles of Incorporation, any amendments thereto, Bylaws, and the applicable provisions of the Nevada General Corporations Law for a more complete description of the rights and liabilities of holders of its securities.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Nevada Revised Statutes provide, in general, that a corporation incorporated under the laws of the State of Nevada, such as the Company, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Nevada corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the State of Nevada or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Regarding indemnification for liabilities arising under the Securities Act of 1933 which may be permitted for directors or officers pursuant to the foregoing provisions, the Company is informed that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy, as expressed in the Act and is therefore unenforceable.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Company’s financial statements and notes thereto are hereby incorporated by this reference to the Company’s most recent Quarterly Report for the quarterly period ended September 30, 2012, as filed with the Securities and Exchange Commission on November 8, 2012.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
On October 23, 2012, the Board of Directors of Endeavor Power Corp. (the “Company”) dismissed M&K CPA’s, PLLC (“M&K”), the Company’s former independent registered public accounting firm. On October 24, 2012, the Board of Directors of the Company selected Stan Jeong Ha Lee, CPA (the “New Accountant”) to serve as the Company’s auditor for the fiscal year ended December 31, 2011.
During the period of M&K’s engagement with the Company and through October 23, 2012, there have been no disagreements with the Former Accountant (as defined in Item 304(a)(1)(iv) of Regulation S-K) on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the Former Accountant, would have caused them to make reference thereto in their report on financial statements for any period.
During the period of the Former Accountant’s engagement and through October 23, 2012, there were no reportable events as defined in Item 304(a)(1)(iv) of Regulation S-K.
During the period of the Former Accountant’s engagement and through October 23, 2012, neither the Registrant nor anyone on its behalf has consulted with the New Accountant regarding either:
| | · | The application of accounting principles to specified transaction, either completed or proposed; or the type of audit opinion that might be rendered on the Registrant’s financial statements, and neither was a written report provided to the Registrant nor was oral advice provided that the New Accountant concluded was an important factor considered by the Registrant in reaching a decision as to an accounting, auditing, or financial reporting issue; or |
| | · | Any matter that was either the subject of a disagreement or a reportable event, as each term is defined in Items 304(a)(1)(iv) or (v) of Regulation S-K, respectively. |
The Company has provided M&K a copy of the foregoing disclosures, a copy of which was attached as Exhibit 16.6 as part of the Company’s Current Report filed on Form 8-K on October 25, 2012.
On October 24, 2012, with the prior approval of its Board of Directors, the Registrant engaged the New Accountant as its independent registered public accounting firm.
The Company has not consulted with the New Accountant regarding the application of accounting principles to a specified transaction or the type of audit opinion that might be rendered on the Company's financial statements during the two most recent fiscal years through present.
ITEM 9.01 FINANCIAL STATEMENTS AND EXHIBITS.
FINANCIAL INFORMATION
INDEX TO FINANCIAL STATEMENTS
| | | |
| | |
Unaudited Condensed Consolidated Pro Forma Financial Information of Endeavor Power Corporation | | |
| | |
| | |
| | |
| | |
| | |
| | | |
FINANCIAL INFORMATION OF ENDEAVOR POWER CORPORATION | | |
Interim Financial Statements of Endeavor Power Corporation: | | |
| | |
| | |
| | |
| | |
| | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | | |
FINANCIAL INFORMATION OF PARALLAX DIAGNOSTICS, INC. | | |
Interim Financial Statements of Parallax Diagnostics, Inc: | | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. (“Endeavor Holdings”) entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Parallax Diagnostics, Inc, a Nevada corporation (“Parallax”) and the shareholders of Parallax (the “Parallax Shareholders”), whereby Endeavor Holdings acquired 24,870,000 shares of common stock (100%) of Parallax (the “Parallax Stock”) from the Parallax Shareholders. In exchange for the Parallax Stock, the Company issued 90,375,750 shares of its common stock to the Parallax Shareholders. The 90,375,750 shares, issued at par value $0.001, represent approximately 60% of the Company’s total issued and outstanding shares. The Common Stock Purchase Agreement, and subsequent transaction closing, was completed on October 22, 2012. On October 27, 2012, the Common Stock Purchase Agreement was finalized, and a Change in Control of the Registrant took place.
The foregoing summary description of the terms of the Agreement and Plan of Merger may not contain all information that is of interest to the reader. For further information regarding specific terms and conditions of the Agreement and Plan of Merger, and is incorporated herein as Exhibit 2.1.
As a result of the transactions effected by the Merger Agreement, (i) the former business of Parallax is now our primary business and (ii) there is a change of control whereby the former shareholders of Parallax, will now own a controlling 60% ownership interest in the Company on a fully diluted basis.
Pro Forma Financial Information
September 30, 2012
The unaudited condensed consolidated pro forma financial information of Endeavor Power Corporation (“EDVP”) as of September 30, 2012, gives effect to the Share Exchange as if the transaction had occurred on September 30, 2012.
The unaudited condensed consolidated pro forma balance sheet gives effect to the transaction as of September 30, 2012. The unaudited condensed consolidated pro forma statement of operations for the nine month ended September 30, 2012, gives effect to the transaction as if it had occurred January 1, 2011.
December 31, 2011
The unaudited condensed consolidated pro forma financial information of EDVP as of December 31, 2011 gives effect to the Share Exchange as if the transaction had occurred on December 31, 2011.
The unaudited condensed consolidated pro forma balance sheet gives effect to the transaction as of December 31, 2011. The unaudited condensed consolidated pro forma statement of operations for the calendar year ended December 31, 2011, gives effect to the transaction as if it had occurred January 1, 2011.
The unaudited condensed consolidated pro forma financial information has been included as required by the rules of the Securities and Exchange Commission and is presented for illustrative purposes only. Such information is not necessarily indicative of the operating results or financial position that would have occurred had the transaction taken place or had occurred on the earliest date of January 1, 2011.
CONDENSED CONSOLIDATED PRO FORMA FINANCIAL INFORMATION
FOR ENDEAVOR POWER CORPORATION
AS OF AND FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2012
| ENDEAVOR POWER CORPORATION | |
| |
| As of September 30, 2012 ** | |
| | | | | | | | | | | | Endeavor | |
| | | Endeavor | | | Parallax | | | | | | Pro Forma | |
| | | Historical | | | Consolidation | | | Pro Forma | | | Consolidated | |
| | | 09/30/2012 | | | 9/30/2012 | | | Adjustments | | | 09/30/2012 | |
| | | (unaudited) | | | | | | | | | | |
| ASSETS | | | | | | | | | | | | |
| Current assets | | $ | — | | | $ | 26,792 | | | $ | — | | | $ | 26,792 | |
| Property and equipment, net | | | 4,972 | | | | 32,495 | | | | — | | | | 37,467 | |
| Intangible assets, net | | | — | | | | 1,299,984 | | | | — | | | | 1,299,984 | |
| Goodwill | | | — | | | | — | | | | 741,927 | [10] | | | 741,927 | |
| TOTAL ASSETS | | $ | 4,972 | | | $ | 1,359,271 | | | $ | 741,927 | | | $ | 2,106,170 | |
| | | | | | | | | | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' DEFICIT | | | | | | | | | | | | | |
| Current liabilities | | $ | 397,623 | | | $ | 601,195 | | | $ | — | | | $ | 998,818 | |
| Long-term liabilities | | | 417,438 | | | | 1,500,000 | | | | (417,438 | )[9] | | | 1,500,000 | |
| Total Liabilities | | | 815,061 | | | | 2,101,195 | | | | (417,438 | ) | | | 2,498,818 | |
| | | | | | | | | | | | | | | | | |
| Stockholders’ Deficit | | | | | | | | | | | | | | | | |
| Preferred stock | | | — | [1] | | | 23 | [3] | | | — | | | | 23 | |
| Common stock | | | 151,064 | [2] | | | 2,487 | [4] | | | (90,376 | )[5] | | | 151,064 | [8] |
| | | | | | | | | | | | 90,376 | [6] | | | | |
| | | | | | | | | | | | (2,487 | )[7][10] | | | | |
| Additional paid in capital - preferred | | | — | | | | 499,977 | | | | (499,977 | )[10] | | | — | |
| Additional paid in capital - common | | | 17,529,437 | | | | 88,772 | | | | 90,376 | [5] | | | 17,946,875 | |
| | | | | | | | | | | | (90,376 | )[6] | | | | |
| | | | | | | | | | | | 417,438 | [9] | | | | |
| | | | | | | | | | | | (88,772 | )[10] | | | | |
| Subscriptions receivable | | | | | | | (20 | ) | | | — | | | | (20 | ) |
| Accumulated deficit | | | (18,490,590 | ) | | | (1,333,163 | ) | | | 1,333,163 | [10] | | | (18,490,590 | ) |
| Total Stockholders’ Deficit | | | (810,089 | ) | | | (741,924 | ) | | | 1,159,365 | | | | (392,648 | ) |
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | | $ | 4,972 | | | $ | 1,359,271 | | | $ | 741,927 | | | $ | 2,106,170 | |
| [1] | 10,000,000 shares authorized, $.001 par, none issued |
| [2] | 250,000,000 shares authorized , $.001 par, 151,063,898 shares issued and outstanding |
| [3] | 100,000,000 shares authorized, $.001 par, 220,000 shares issued and outstanding |
| [4] | 400,000,000 shares authorized , $.001 par, 28,020,000 shares issued and outstanding |
| [5] | EDVP Cancellation of shares prior to Merger Agreement per Board Resolution dated October 25, 2012 = 90,375,750 shares @ $.001 par = $90,376 |
| [6] | Issuance of EDVP shares to PRLX Shareholders - 90,375,750 shares x $.001 par value = $90,376 |
| [7] | Issuance of PRLX shares to EDVP Shareholders - 24,870,000 shares x $.0001 par value = $2,487 |
| [8] | Total shares issued and outstanding, 151,063,898 x par $.001 = $151,064 |
| [9] | Cancellation of EDVP debt per Board Resolution dated October 12, 2012 |
| [10] | Elimination of subsidiary equity due to consolidation |
| | |
| ** | As if the transaction took place September 30, 2012 |
| ENDEAVOR POWER CORPORATION |
|
| As of and for the Nine Months Ended September 30, 2012 ** |
| (unaudited) |
| | | Endeavor | | | Parallax | | | | | | Endeavor | |
| | | Historical | | | Consolidation | | | Pro Forma | | | Proforma | |
| | | 09/30/2012 | | | 09/30/2012 | | | Adjustments | | | 09/30/2012 | |
| | | | | | | | | | | | | |
| Revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Cost of revenues | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Gross Profit | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| General and administrative expenses | | | 57,059 | | | | 121,484 | | | | | | | | 178,543 | |
| | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | (57,059 | ) | | | (121,484 | ) | | | — | | | | (178,543 | ) |
| | | | | | | | | | | | | | | | | |
| Other income (expense) | | | (36,361 | ) | | | (32,470 | ) | | | — | | | | (68,831 | ) |
| | | | | | | | | | | | | | | | | |
| Net (loss) | | $ | (93,420 | ) | | $ | (153,954 | ) | | $ | — | | | $ | (247,374 | ) |
| | | | | | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.00 | ) | | | | | | | | | | $ | (0.00 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 151,063,898 | | | | | | | | | | | | 151,063,898 | |
| ** | As if the transaction took place January 1, 2011 |
CONDENSED CONSOLIDATED PRO FORMA FINANCIAL INFORMATION
FOR ENDEAVOR POWER CORPORATION
AS OF AND FOR THE YEAR ENDED DECEMBER 31, 2011
| ENDEAVOR POWER CORPORATION |
|
| As of December 31, 2011** |
| | | | | | | | | | | | Endeavor | | | |
| | | Endeavor | | | Parallax | | | | | | Pro Forma | | | |
| | | Historical | | | Consolidation | | | Pro Forma | | | Consolidated | | | |
| | | 12/31/2011 | | | 12/31/2011 | | | Adjustments | | | 12/31/2011 | | | |
| | | (unaudited) | | | | | | | | | | | | |
| ASSETS | | | | | | | | | | | | | | |
| Current assets | | $ | — | | | $ | 136,066 | | | $ | — | | | $ | 136,066 | | | |
| Property and equipment, net | | | 8,347 | | | | 41,114 | | | | — | | | | 49,461 | | | |
| Intangible assets, net | | | — | | | | 1,370,490 | | | | — | | | | 1,370,490 | | | |
| Goodwill | | | — | | | | — | | | | 243,424 | [12] | | | 243,424 | | | |
| TOTAL ASSETS | | $ | 8,347 | | | $ | 1,547,670 | | | $ | 243,424 | | | $ | 1,799,441 | | | |
| | | | | | | | | | | | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' DEFICIT | | | | | | | | | | | | | | | |
| Current liabilities | | $ | 307,578 | | | $ | 290,776 | | | $ | — | | | $ | 598,354 | | | |
| Long-term liabilities | | | 417,438 | | | | 1,500,000 | | | | (417,438 | )[11] | | | 1,500,000 | | | |
| Total Liabilities | | | 725,016 | | | | 1,790,776 | | | | (417,438 | ) | | | 2,098,354 | | | |
| | | | | | | | | | | | | | | | | | | |
| Stockholders' Deficit | | | | | | | | | | | | | | | | | | |
| Preferred stock | | | — | [1] | | | 22 | [3] | | | — | | | | 22 | | | |
| Common stock | | | 151,064 | [2] | | | 2,802 | [4] | | | (90,376 | )[5] | | | 151,064 | [9] | | |
| | | | | | | | | | | | (90,376 | )[6] | | | | | | |
| | | | | | | | | | | | (335 | )[7] | | | | | | |
| | | | | | | | | | | | 20 | [8] | | | | | | |
| | | | | | | | | | | | (2,487 | )[9][12] | | | | | | |
| Additional paid in capital - preferred | | | — | | | | 399,978 | | | | (399,978 | )[12] | | | — | | | |
| Additional paid in capital - common | | | 17,529,437 | | | | 227,456 | | | | 90,376 | [5] | | | 17,947,190 | | | |
| | | | | | | | | | | | (90,376 | )[6] | | | | | | |
| | | | | | | | | | | | 335 | [7] | | | | | | |
| | | | | | | | | | | | (20 | )[8] | | | | | | |
| | | | | | | | | | | | 417,438 | [11] | | | | | | |
| | | | | | | | | | | | (227,456 | )[12] | | | | | | |
| Subscriptions receivable | | | | | | | (20 | ) | | | — | | | | (20 | ) | | |
| Accumulated deficit | | | (18,397,170 | ) | | | (873,345 | ) | | | 873,345 | [12] | | | (18,397,170 | ) | | |
| Total Stockholders’ Deficit | | | (716,669 | ) | | | (243,107 | ) | | | 660,862 | | | | (298,914 | ) | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | | $ | 8,347 | | | $ | 1,547,670 | | | $ | 243,424 | | | $ | 1,799,441 | | | |
| [1] | 10,000,000 shares authorized, $.001 par, none issued |
| [2] | 250,000,000 shares authorized , $.001 par, 151,063,898 shares issued and outstanding |
| [3] | 100,000,000 shares authorized, $.001 par, 220,000 shares issued and outstanding |
| [4] | 400,000,000 shares authorized , $.001 par, 28,020,000 shares issued and outstanding |
| [5] | EDVP Cancellation of shares prior to Merger Agreement per Board Resolution dated October 25, 2012 = 90,375,750 shares @ $.001 par = $90,376 |
| [6] | Issuance of EDVP shares to PRLX Shareholders - 90,375,750 shares x $.001 par value = $90,376 |
| [7] | PRLX Cancellation of shares prior to Merger Agreement = 3,350,000 shares @ $.0001 par = $335 |
| [8] | Issuance of additional shares prior to Merger Agreement = 200,000 shares @ $.0001 par = $20 |
| [9]\ | Issuance of PRLX shares to EDVP Shareholders - 24,870,000 shares x $.0001 par value = $2,487 |
| [10] | Total shares issued and outstanding, 151,063,898 x par $.001 = $151,064 |
| [11] | Cancellation of EDVP debt per Board Resolution dated October 12, 2012 |
| [12] | Elimination of subsidiary equity due to consolidation |
| | |
| ** | As if the transaction took place December 31, 2011 |
| ENDEAVOR POWER CORPORATION |
|
| As of and for the Year Ended December 31, 2011** |
| (unaudited) |
| | | | | | | | | | | | | |
| | | Endeavor | | | Parallax | | | | | | Endeavor | |
| | | Historical | | | Consolidation | | | Pro Forma | | | Proforma | |
| | | 12/31/2011 | | | 12/31/2011 | | | Adjustments | | | 12/31/2011 | |
| | | | | | | | | | | | | |
| Revenue | | $ | 192,246 | | | $ | — | | | $ | — | | | $ | 192,246 | |
| Cost of revenues | | | 90,091 | | | | — | | | | — | | | | 90,091 | |
| | | | | | | | | | | | | | | | | |
| Gross Profit | | $ | 102,155 | | | $ | — | | | $ | — | | | $ | 102,155 | |
| | | | | | | | | | | | | | | | | |
| General and administrative expenses | | | 2,020,129 | | | | 428,956 | | | | — | | | | 2,449,085 | |
| | | | | | | | | | | | | | | | | |
| Operating income (loss) | | | (1,917,974 | ) | | | (428,956 | ) | | | — | | | | (2,346,930 | ) |
| | | | | | | | | | | | | | | | | |
| Other income (expense) | | | (49,313 | ) | | | (374,267 | ) | | | — | | | | (423,580 | ) |
| | | | | | | | | | | | | | | | | |
| Net (loss) | | $ | (1,967,287 | ) | | $ | (803,223 | ) | | $ | — | | | $ | (2,770,510 | ) |
| | | | | | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.02 | ) | | | | | | | | | | $ | (0.02 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 102,202,088 | | | | | | | | | | | | 151,063,898 | |
| ** | As if the transaction took place January 1, 2011 |
UNAUDITED INTERIM FINANCIAL STATEMENT
FOR ENDEAVOR POWER CORPORATION
AS OF AND FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2012
| ENDEAVOR POWER CORPORATION |
| (A DEVELOPMENT STAGE COMPANY) |
|
| (Unaudited) |
| | | September 30, 2012 | | | December 31, 2011 | |
| ASSETS | | | | | | |
| | | | | | | |
| Current assets | | $ | — | | | $ | — | |
| | | | | | | | | |
| Property and equipment, net | | | 4,972 | | | | 8,347 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 4,972 | | | $ | 8,347 | |
| | | | | | | | | |
| | | | | | | | | |
| | |
| LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| LIABILITIES: | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 164,449 | | | $ | 115,154 | |
| Notes and loans payable | | | 84,075 | | | | 84,075 | |
| Due to related parties | | | 149,099 | | | | 108,349 | |
| Total Current Liabilities | | | 397,623 | | | | 307,578 | |
| | | | | | | | | |
| Related party loans | | | 417,438 | | | | 417,438 | |
| | | | | | | | | |
| Total Liabilities | | | 815,061 | | | | 725,016 | |
| | | | | | | | | |
| | | | | | | | | |
| STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| Preferred stock, no par value, 10,000,000 shares authorized, no shares issued or outstanding | | | — | | | | — | |
| Common stock, $0.001 par value, 250,000,000 shares authorized, 151,063,898 and 151,063,898 shares issued and outstanding at September 30, 2012 and December 31, 2011, respectively | | | 151,064 | | | | 151,064 | |
| Additional paid in capital | | | 17,529,437 | | | | 17,529,437 | |
| (Deficit) accumulated during the development stage | | | (18,490,590 | ) | | | (18,397,170 | ) |
| | | | | | | | | |
| Total Stockholders' (Deficit) | | | (810,089 | ) | | | (716,670 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | $ | 4,972 | | | $ | 8,347 | |
The accompanying notes are an integral part of these consolidated financial statements
ENDEAVOR POWER CORPORATION |
| (A DEVELOPMENT STAGE COMPANY) |
|
| (Unaudited) |
| | | | | | | | | Cumulative from | |
| | | For the three months ended | | | For the nine months ended | | | July 6, 2005 (inception) | |
| | | September 30, 2012 | | | September 30, 2011 | | | September 30, 2012 | | | September 30, 2011 | | | To September 30, 2012 | |
| Revenue | | $ | — | | | $ | — | | | $ | — | | | $ | 192,246 | | | $ | 212,643 | |
| | | | | | | | | | | | | | | | | | | | | |
| Cost of sales | | | — | | | | — | | | | — | | | | 90,091 | | | | 126,137 | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | — | | | | — | | | | — | | | | 102,155 | | | | 86,506 | |
| | | | | | | | | | | | | | | | | | | | | |
| General and administrative expenses | | | 16,843 | | | | 16,395 | | | | 57,059 | | | | 2,013,359 | | | | 7,682,520 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating (loss) | | | (16,843 | ) | | | (16,395 | ) | | | (57,059 | ) | | | (1,911,204 | ) | | | (7,596,014 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Other income (expenses) | | | | | | | | | | | | | | | | | | | | |
| Loss on settlement of debt | | | — | | | | — | | | | — | | | | — | | | | (3,292,149 | ) |
| Interest expense | | | (12,362 | ) | | | (12,362 | ) | | | (36,361 | ) | | | (36,952 | ) | | | (999,794 | ) |
| Interest income | | | — | | | | — | | | | — | | | | — | | | | 1,823 | |
| Total other income (expenses) | | | (12,362 | ) | | | (12,362 | ) | | | (36,361 | ) | | | (36,952 | ) | | | (4,302,481 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) – continuing operations | | $ | (29,205 | ) | | $ | (28,757 | ) | | $ | (93,420 | ) | | $ | (1,948,156 | ) | | $ | (11,898,495 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Discontinued operations | | | | | | | | | | | | | | | | | | | (6,592,095 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) | | | | | | | | | | | | | | | | | | $ | (18,490,590 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 151,063,898 | | | | 151,063,898 | | | | 151,063,898 | | | | 151,201,261 | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.00 | ) | | $ | (0.00 | ) | | $ | (0.00 | ) | | $ | (0.01 | ) | | | | |
The accompanying notes are an integral part of these consolidated financial statements
| ENDEAVOR POWER CORPORATION |
| (A DEVELOPMENT STAGE COMPANY) |
|
| (Unaudited) |
| | | | | | Cumulative From | |
| | | | | | July 6, 2005 | |
| | | Nine Months Ended | | | (Inception) to | |
| | | September 30,
2012 | | | September 30, 2011 | | | September 30,
2012 | |
| | | | | | | | | | |
| Cash Flow from operations: | | | | | | | | | |
| Net loss | | $ | (93,420 | ) | | $ | (1,948,156 | ) | | $ | (11,898,495 | ) |
| Adjustments to reconcile net (loss) to net cash (used in) operating activities: | | | | | | | | | | | | |
| Accretion expense | | | — | | | | — | | | | 826,541 | |
| Depreciation expense | | | 3,375 | | | | 3,375 | | | | 8,528 | |
| Common shares issued for services | | | — | | | | 1,800,000 | | | | 6,880,452 | |
| Common shares issued for incentives | | | — | | | | — | | | | 110,250 | |
| Loss on settlement of debt | | | — | | | | — | | | | 3,292,149 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Increase in accounts and accrued expenses | | | 49,295 | | | | 49,398 | | | | 221,689 | |
| Increase in related party payables | | | 40,750 | | | | 3,000 | | | | 74,905 | |
| Net cash (used in) operating activities | | | — | | | | (92,403 | ) | | | (483,981 | ) |
| | | | | | | | | | | | | |
| Cash Flow from investing activities: | | | | | | | | | | | | |
| Cash (used in) purchase of property and equipment | | | — | | | | — | | | | (13,500 | ) |
| Net cash provided by investing activities | | | — | | | | — | | | | (13,500 | ) |
| | | | | | | | | | | | | |
| Cash Flow from financing activities: | | | | | | | | | | | | |
| Net proceeds from related party loans | | | — | | | | — | | | | 1,061,561 | |
| Proceeds from notes payable | | | — | | | | 65,000 | | | | 84,075 | |
| Proceeds from shareholders | | | — | | | | — | | | | 264,949 | |
| Proceeds from Issuance of common shares | | | — | | | | — | | | | 83,991 | |
| Repayment on cancellation of common shares | | | — | | | | — | | | | (5,000 | ) |
| Net cash provided by financing activities | | | — | | | | 65,000 | | | | 1,489,576 | |
| | | | | | | | | | | | | |
| Cash flows from discontinued operations: | | | | | | | | | | | | |
| Net cash (used in) operating activities | | | — | | | | — | | | | (382,377 | ) |
| Net cash (used in) investing activities | | | — | | | | — | | | | (609,718 | ) |
| Net cash (used in) discontinued operations | | | — | | | | — | | | | (992,095 | ) |
| | | | | | | | | | | | | |
| Increase (decrease) in cash | | | — | | | | (27,403 | ) | | | — | |
| | | | | | | | | | | | | |
| Cash - beginning of period | | | — | | | | 27,802 | | | | — | |
| | | | | | | | | | | | | |
| Cash - end of period | | $ | — | | | $ | 399 | | | $ | — | |
| | | | | | | | | | | | | |
| NONCASH ACTIVITIES | | | — | | | | | | | | | |
| Common shares issued to acquire mineral properties | | $ | — | | | $ | — | | | $ | 5,600,000 | |
| Common shares issued to settle note payable | | $ | — | | | $ | — | | | $ | 500,000 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | | | | |
| Interest paid | | $ | — | | | $ | — | | | $ | — | |
| Income taxes paid | | $ | — | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements
ENDEAVOR POWER CORPORATION (A DVELOPMENT STAGE COMPANY)
SEPTEMBER 30, 2012
NOTE 1. OVERVIEW AND NATURE OF BUSINESS
Endeavor Power Corporation (the “Company”) was incorporated in the State of Nevada on July 6, 2005 under the name VB Biotech Laboratories, Inc. On September 21, 2007, the Company filed a Certificate of Amendment with the State of Nevada to change its operating name to VB Trade, Inc., with principal business operations to develop an online website that allowed web designers to sell their website designs in exchange for a commission on all products that were sold through the website. On September 21, 2007, the Company entered into a Plan of Merger (the “Merger”) with Endeavor Uranium, Inc., a mineral exploration company with mineral properties in the northwestern United States. Effectively, the Company changed its name to Endeavor Uranium, Inc. as part of the Merger transaction. On December 23, 2008, the Company entered into a Joint Venture Agreement (the “Agreement”) with Federated Energy Corporation, a Tennessee corporation, for working interests in prospective oil and gas wells located in Nowata County, Oklahoma. Effectively on December 23, 2008, the Company changed its operating name to Endeavor Power Corporation.
In November, 2010, Management assessed a potential business opportunity and determined that in an effort to create value for its Shareholders, the Company should change its business direction. On November 8, 2010, the Company discontinued its operations in its working interests in oil and gas exploration and changed its operating focus to the development of E-Waste processing services aimed at industrial and government clients. The Company’s new direction sought to limit the impact of discarded “E-Waste” on the environment. Discarded computers and electronic equipment pose environmental hazards.
On May 26, 2011, Mr. Alfonso Knoll resigned from all positions with the Company, including but not limited to, that of President, Chief Executive Officer, Chief Financial Officer, Treasurer and Secretary. The resignation did not involve any disagreement with the Company. On June 8, 2011, the Company entered into a Settlement Agreement and General Mutual Release (“Settlement Agreement”) to terminate Mr. Knoll’s Employment Agreement dated November 8, 2010, and to accept his resignation. Pursuant to the Settlement Agreement, Mr. Knoll immediately ceased all services to the Company and, on June 11, 2011, returned to the Company any and all shares of its common stock currently held by him.
On June 2, 2011, Mr. Matthew Carley was appointed as the Company’s President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director. Mr. Carley accepted the appointment, but effectively resigned his positions on September 27, 2011. The Company’s Board of Directors accepted the resignation of Mr. Carley, as well as the resignation of Mr. Keith Kress as a member of the Board of Directors. Simultaneously, the Board of Directors appointed Tom Mackay as the President/Chief Executive Officer, Secretary, Treasurer/Chief Financial Officer and the sole member of the Board of Directors.
In accordance with a change in management effective September 27, 2011, the Company’s business operations changed. The Company intended to provide managerial services, and pursue potential funding opportunities for the Company. It retained consultants to perform the necessary due diligence on certain mining properties located in Venezuela, Brazil, Bolivia, Guyana and several other South American countries. Management, however, determined that the outcome of such due diligence did not provide the Company a viable opportunity, nor did it provide sufficient economic benefit for the Company. Management has therefore ceased its due diligence and exploration of mining property opportunities, and is pursuing other viable business opportunities to increase shareholder market value.
Going Concern
These financial statements have been prepared on a going concern basis, which implies that the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The Company has not generated significant revenues to date and has never paid any dividends and is unlikely to pay dividends or generate significant earnings in the immediate or foreseeable future. As at September 30, 2012, the Company had a working capital deficit of $397,623, and an accumulated deficit of $18,490,590. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders, the ability to raise equity or debt financing, and the attainment of profitable operations from the Company's future business. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States, and are expressed in US dollars. The Company’s fiscal year-end is December 31.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. As at September 30, 2012, the Company had no cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States and requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to the valuation of its mineral properties, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
Basic and Diluted Net Income (Loss) Per Share
The Company computes net income (loss) per share in accordance with ASC 260, Earning per Share. ASC 260 requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period using the treasury stock method and convertible preferred stock using the if-converted method. In computing Diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. Diluted EPS excludes all dilutive potential shares if their effect is anti dilutive.
Comprehensive Loss
ASC 220, Comprehensive Income, establishes standards for the reporting and display of comprehensive loss and its components in the financial statements. As at September 30, 2012, the Company has no items that represent comprehensive loss and, therefore, has not included a schedule of comprehensive loss in the financial statements.
Foreign Currency Translation
The Company’s functional and reporting currency is the United States dollar. Monetary assets and liabilities denominated in foreign currencies are translated in accordance with ASC 830 Foreign Currency Translation Matters, using the exchange rate prevailing at the balance sheet date. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income. Foreign currency transactions are primarily undertaken in Canadian dollars. The Company has not, to the date of these financial statements, entered into derivative instruments to offset the impact of foreign currency fluctuations.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 605, Revenue Recognition. Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service has been provided, and collectability is reasonably assured.
Property and Equipment
Property and equipment is comprised of vehicles and general equipment and are recorded at cost and is depreciated using the straight-line method over the estimated useful lives of three years. Maintenance and repairs are charged to expense as incurred. Significant renewals and betterments are capitalized.
Financial Instruments
Pursuant to ASC 820, Fair Value Measurements and Disclosures and ASC 825, Financial Instruments, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 and 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 and 825 prioritizes the inputs into three levels that may be used to measure fair value:
| | Level 1 | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. |
| | Level 2 | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. |
| | Level 3 | Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
The Company’s financial instruments consist principally of cash, accounts payable, and accrued liabilities. Pursuant to ASC 820 and 825, the fair value of cash is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The recorded values of all other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Share-Based Payments, using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
Recently Adopted Accounting Standards:
The Company evaluates the pronouncements of various authoritative accounting organizations, primarily the Financial Accounting Standards Board (“FASB”), the US Securities and Exchange Commission (“SEC”), and the Emerging Issues Task Force (“EITF”), to determine the impact of new pronouncements on US GAAP and the impact on the Company. The Company has adopted the following new accounting standards during 2012:
| | Trouble Debt Restructuring: Issued in April, 2011, ASU 2011-02 clarifies which loan modifications constitute troubled debt restructurings. It is intended to assist creditors in determining whether a modification of the terms of a receivable meets the criteria to be considered a troubled debt restructuring, both for purposes of recording an impairment loss and for disclosure of troubled debt restructurings. The new guidance is effective for interim and annual periods beginning on or after June 15, 2011, and applies retrospectively to restructurings occurring on or after the beginning of the fiscal year of adoption. Early adoption is permitted. | |
| | | |
| | Comprehensive Income: Issued in June, 2011, ASU 2011-05 eliminates the current option to present other comprehensive income and its components in the statement of changes in equity. It will require companies to report the total of comprehensive income including the components of net income and the components of other comprehensive income in either a single continuous statement of comprehensive income or in two separate but consecutive statements. The amendments in the ASU are effective for interim and annual periods beginning on or after December 15, 2011, and should be applied retrospectively. Early adoption is permitted. | |
| | | |
| | Intangibles: Issued in September, 2011, ASU 2011-08 permits entities to first perform a qualitative assessment to determine whether it is more likely than not (a likelihood of more than 50 percent) that the fair value of a reporting unit is less than its carrying amount. If the entity determines that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, it would then perform the first step of the goodwill impairment test; otherwise, no further impairment test would be required. The amendments will be effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted. | |
| | | |
| | Disclosures about Offsetting Assets and Liabilities: Issued in December, 2011, ASU 2011-11 requires disclosures to provide information to help reconcile differences in the offsetting requirements under U.S. GAAP and IFRS. The differences in the offsetting requirements account for a significant difference in the amounts presented in statements of financial position prepared in accordance with U.S. GAAP and IFRS for certain entities. An entity is required to apply the amendments for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. | |
Recently Issued Accounting Standards Updates:
There were various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries. None of the updates are expected to a have a material impact on the Company's consolidated financial position, results of operations or cash flows.
NOTE 3. PROPERTY AND EQUIPMENT
Property and Equipment consists of the following:
| | | September 30, 2012 | | | December 31, 2011 | |
| General Equipment | | $ | 2,500 | | | $ | 2,500 | |
| Automobiles | | | 11,000 | | | | 11,000 | |
| Sub-Total | | | 13,500 | | | | 13,500 | |
| Accumulated Deprecation | | | (8,528 | ) | | | (5,153 | ) |
| Property and Equipment, Net | | $ | 4,972 | | | $ | 8,347 | |
Depreciation expense totaled $3,375 and $4,500 for the nine months ended September 30, 2012 and the year ended December 31, 2011, respectively.
NOTE 4. NOTES AND LOANS PAYABLE
| In June, 2010, the Company issued a note payable in the principal amount of $9,075 to a non-related party. Under the terms of the note, the amount is unsecured, non-interest bearing, and due upon demand. |
| In June, 2010, the Company issued a note payable in the principal amount of $10,000 to a non-related party. Under the terms of the note, the amount is unsecured, accrues interest at a rate of 8% per annum, and is due upon demand. As of September 30, 2012, the Company has recorded $2,011 in interest as an accrued expense. |
| On April 21, 2011, the Company issued a note payable in the principal amount of $65,000 to a non-related party. The note is unsecured, bears interest at a rate of 10% per annum, and is due upon demand. As of September 30, 2012, the Company has recorded $10,721 in interest as an accrued expense. |
NOTE 5. RELATED PARTY PAYABLE
As at September 30, 2012, related party payable consists of $149.099 representing miscellaneous operating expenses accrued and/or paid on behalf of the Company by certain related parties. The amounts owing are unsecured, non-interest bearing, and due upon demand.
NOTE 6. NOTES PAYABLE – RELATED PARTY
On August 25, 2009, the Company issued a convertible promissory note (the “Note”) of $826,541 to a related party to settle outstanding debt obligations owing as of the issuance date. Under the terms of the Note, the amount owing accrued interest at a rate of 10% per annum, was due August 25, 2011, and contained certain provisions to convert the debt into common shares of the Company.
On June 17, 2010, the Company issued the related party 375,000 common shares to settle $75,000 of the outstanding Note, reducing the principal balance to $751,541.
In September, 2010, the Company issued the related party 140,000,000 common shares to settle $500,000 of the Note, reducing the principal balance to $251,541.
On September 17, 2010, the Company amended the Note with the related party to combine the remaining principal of $251,541 with the accrued interest to date of $65,897, for a revised principal balance of $317,438. The new principal balance continues to accrue interest at a rate of 10% per annum, but no longer contains a provision for conversion.
In November, 2010, the Company received an additional $100,000 from the related party, increasing the principal balance to $417,438.
As at September 30, 2012, the principal balance of the Note is $417,438. The Company has recorded $105,497 in interest as an accrued expense.
NOTE 7. COMMON STOCK
All common shares issued for services or settlements of debt are valued based on the end-of-day market prices on the date of issuance, unless otherwise specified.
The Company and its Board of Directors authorized a 1:100 reverse common stock split on August 16, 2010, The effects of the reverse stock split resulted in the number of issued and outstanding common stock to decrease from 106,388,200 common shares to 1,063,898 common shares, have been applied on a retroactive basis, and are reflected below where applicable.
The total number of authorized shares of common stock that may be issued by the Company is 250,000,000 with a par value of $0.001 per share.
| During the year ended December 31, 2006, the Company issued 325,000,000 founder shares for cash proceeds of $5,000. These shares were cancelled during the year ended December 31, 2007. |
| During the year ended December 31, 2006, the Company issued 513,448 (post-split adjusted) common shares for cash proceeds of $78,991. |
| In October 2007, the Company issued 140,000 (post-split adjusted) common shares at $0.40 per common share, with a fair value of $5,600,000, to acquire mineral properties. |
On July 22, 2009, the Company issued 460 (post-split adjusted) common shares of the Company with a fair value of $67,376 to settle debt obligations of $27,500, resulting in a loss on settlement of debt of $39,876.
On July 22, 2009, the Company issued 460 (post-split adjusted) common shares of the Company with a fair value of $67,376 to settle debt obligations of $27,500, resulting in a loss on settlement of debt of $39,876.
On July 22, 2009, the Company issued 1,667 (post-split adjusted) common shares of the Company with a fair value of $245,000 to settle debt obligations of $100,000, resulting in a loss on settlement of debt of $145,000.
On July 22, 2009, the Company issued 100 (post-split adjusted) common shares of the Company with a fair value of $14,700 to settle debt obligations of $12,000, resulting in a loss on settlement of debt of $2,700.
On July 22, 2009, the Company issued 750 (post-split adjusted) common shares of the Company with a fair value of $110,250 to a related party as incentive bonus shares for the conversion of amounts owing into a long-term convertible note payable.
On August 25, 2009, the Company issued 32,000 (post-split adjusted) common shares of the Company for consulting services with a fair value of $3,776,000.
On June 17, 2010, the Company issued 375,000 (post-split adjusted) common shares to a related party for the repayment of payable note payable of $75,000, resulting in a loss on settlement of debt of $180,000. Per the convertible note agreement the Company was to convert the debt at $0.006 per share (post split) for a total issuance of 12,500,000 shares. The additional 25,000,000 shares were issued at the closing price of the stock on the day of issuance resulting in the $180,000 loss.
.
On September 20, 2010, the Company issued 140,000,000 common shares to settle outstanding notes payable of $500,000 resulting in a loss on settlement of debt of $3,116,667. Per the convertible note agreement the Company was to convert the debt at $0.006 per share (post split) for a total issuance of 83,333,333 shares. The additional 56,666,667 shares were issued at the closing price of the stock on the day of issuance resulting in the $3,166,667 loss.
On November 8, 2010, the Company issued 3,500,000 common shares to the CEO of the Company for management services with a fair value of $910,000. As a result, $906,500 was recorded as paid in capital.
On February 23, 2011, the Company issued 10,000,000 shares of its common stock in exchange for services rendered to the Company, valued at $1,800,000. As a result, $1,790,000 was recorded as paid in capital.
On June 14, 2011, pursuant to the Settlement Agreement related to the resignation of the Company’s former CEO/President, the 3,500,000 shares of common stock previously issued on November 8, 2010, were returned to treasury. As a result, paid in capital was reduced by $3,500.
As of September 30, 2012, the Company had 151,063,898 common shares issued and outstanding.
NOTE 8. WARRANTS
As of September 30, 2012, the Company had the following share purchase warrants outstanding:
| Outstanding and Exercisable Warrants |
| | Number of | Remaining Contractual Life | Exercise Price times Number | Weighted Average |
| Exercise Price | Shares | (in years) | of Shares | Exercise Price |
| $0.900 | 500,000 | 0 | $ | 450,000 | $ | 0.900 |
| Warrants | | | | | Weighted Average Exercise Price | |
| Outstanding at December 31, 2011 | | | 500,000 | | | $ | 0.900 | |
| Issued | | | — | | | | — | |
| Exercised | | | — | | | | — | |
| Expired / Cancelled | | | (500,000 | ) | | | 0.900 | |
| Outstanding at September 30, 2012 | | | — | | | $ | — | |
The outstanding share purchase warrants expired on August 25, 2012.
NOTE 9. INCOME TAXES
The components of the net deferred tax asset at September 30, 2012 and December 31, 2011, the statutory tax rate, the effective tax rate and the amount of the valuation allowance are indicated below:
| | | September 30, 2012 | | | December 31, 2011 | |
| | | | | | | |
| Income (Loss) Before Taxes | | $ | (93,430 | ) | | $ | (1,967,287 | ) |
| Statutory rate | | | 34 | % | | | 34 | % |
| | | | | | | | | |
| Computed expected tax payable (recovery) | | $ | 31,763 | | | $ | 668,878 | |
| Non-deductible expenses | | | — | | | | (1,530 | ) |
| Change in valuation allowance | | | (31,763 | ) | | | (667,348 | ) |
| | | | | | | | | |
| Reported income taxes | | $ | – | | | $ | – | |
The significant components of deferred income tax assets and liabilities at September 30, 2012 and December 31, 2011 are as follows:
| | | September 30, 2012 | | | December 31, 2011 | |
| | | | | | | |
| Net operating loss carried forward | | $ | 5,983,712 | | | $ | 5,890,292 | |
| | | | | | | | | |
| Valuation allowance | | | (5,983,712 | ) | | | (5,890,292 | ) |
| | | | | | | | | |
| Net deferred income tax asset | | $ | – | | | $ | – | |
As at September 30, 2012, the Company had $5,983,712 of net operating losses which expire commencing in the year 2026.
NOTE 10. SUBSEQUENT EVENTS
The Company has evaluated events and transactions that occurred between September 30, 2012 and the date the consolidated financial statements were available for issue, for possible disclosure or recognition in the consolidated financial statements. The Company has determined that there were no such events or transactions that warrant disclosure or recognition in the consolidated financial statements except as noted below.
On October 27, 2012, Mr. Tom Mackay resigned from all positions with the Company. The Board of Directors of the Company accepted the resignation of Mr. Mackay, and accepted the appointment of Mr. Gardner Williams as the Company’s President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director.
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. (“Endeavor Holdings”) entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Parallax Diagnostics, Inc, a Nevada corporation (“PRLX”) and the shareholders of PRLX (the “PRLX Shareholders”), whereby Endeavor Holdings acquired 24,870,000 shares of common stock (100%) of PRLX (the “PRLX Stock”) from the PRLX Shareholders. In exchange for the PRLX Stock, the Company issued 90,375,750 shares of its common stock to the PRLX Shareholders at par value $.0001, representing approximately 60% of the Company’s total issued and outstanding shares.
FOR ENDEAVOR POWER CORPORATION
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2011 AND 2010
Stan J.H. Lee, CPA
2160 North Central Rd. Suite 209 *Fort Lee * NJ 07024-7547
P.O. Box 436402 * San Diego * CA 92143-6402
619-623-7799 * Fax 619-564-3408 * E-mail: stan2u@gmail.com
To the Management and Members of
Endeavor Power Corp.
(a development stage company)
We have audited the accompanying balance sheet of Endeavor Power Corp. (a development stage company) (the “Company”) as of December 31, 2011, the related consolidated statements of operations, stockholders’ deficit and cash flows for the year then ended, and cumulative from July 6, 2005 (inception) to December 31, 2011 for the statements of operations and cash flows. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. The financial statements of Endeavor Power Corp. (a development stage company) as of December 31, 2010 and the period from inception (July 6, 2005) to December 31, 2010, were audited by other auditors whose report dated April 12, 2011, expressed an unqualified opinion on those statements. Their report included an explanatory paragraph regarding going concern.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Endeavor Power Corp. (a development stage company) as of December 31, 2011, and the results of its operations and its cash flows for the year then ended, and cumulative from July 6, 2005 (inception) to December 31, 2011 for the statements of operations and cash flows, in conformity with accounting principles generally accepted in the United States of America.
The financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has not established any source of revenue to cover its operating costs and losses from operations, which raises substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ Stan J.H. Lee, CPA | |
Stan J.H. Lee, CPA | |
| |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Endeavor Power Corp.
(A Development Stage Company)
We have audited the accompanying balance sheets of Endeavor Power Corp. (A Development Stage Company) as of December 31, 2010, and the related statements of operations, stockholders' equity (deficit) and cash flows for the twelve month period ended December 31, 2010. The financial statements for the period from inception (July 6, 2005) to December 31, 2009 were audited by other auditors whose report expressed an unqualified opinion on those statements. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that the Company plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Endeavor Power Corp. as of December 31, 2010, and the results of its operations and cash flows for the period described above in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statement, the Company suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ M&K CPAS, PLLC | |
www.mkacpas.com Houston, Texas | |
| April 12, 2011 | |
|
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | December 31, 2011 | | | December 31, 2010 | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash & cash equivalents | | $ | — | | | $ | 27,802 | |
| Total Current Assets | | | — | | | | 27,802 | |
| | | | | | | | | |
| Property and equipment, net | | | 8,347 | | | | 12,847 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 8,347 | | | $ | 40,649 | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| LIABILITIES: | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 115,154 | | | $ | 53,069 | |
| Notes and loans payable | | | 84,075 | | | | 19,075 | |
| Due to related parties | | | 108,349 | | | | 120,116 | |
| Total Current Liabilities | | | 307,578 | | | | 192,260 | |
| | | | | | | | | |
| Long term Liabilities | | | | | | | | |
| Notes & loans payable | | | 417,438 | | | | 417,438 | |
| Total Long term Liabilities | | | 417,438 | | | | 417,438 | |
| | | | | | | | | |
| Total Liabilities | | | 725,016 | | | | 609,698 | |
| | | | | | | | | |
| STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| Preferred stock, no par value, 10,000,000 shares authorized, none issued | | | — | | | | — | |
| Common stock, $.001 par value, 250,000,000 shares authorized, 151,063,898 and 144,563,898 issued and outstanding at December 31, 2011 and December 31, 2010, respectively | | | 151,064 | | | | 144,564 | |
| Additional paid in capital | | | 17,529,437 | | | | 15,716,270 | |
| (Deficit) accumulated during the development stage | | | (18,397,170 | ) | | | (16,429,883 | ) |
| | | | | | | | | |
| Total Stockholders' (Deficit) | | | (716,669 | ) | | | (569,049 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | $ | 8,347 | | | $ | 40,649 | |
The accompanying notes are an integral part of these financial statements
|
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | For the years ended | | | From July 6, 2005 | |
| | | December 31, | | | (inception) to | |
| | | 2011 | | | 2010 | | | December 31, 2011 | |
| | | | | | | | | | |
| Revenue | | $ | 192,246 | | | $ | 20,397 | | | $ | 212,643 | |
| | | | | | | | | | | | | |
| Cost of sales | | | 90,091 | | | | 36,046 | | | | 126,137 | |
| | | | | | | | | | | | | |
| Gross profit | | | 102,155 | | | | (15,649 | ) | | | 86,506 | |
| | | | | | | | | | | | | |
| General and administrative expenses | | | 2,020,129 | | | | 1,062,752 | | | | 7,625,461 | |
| | | | | | | | | | | | | |
| Operating (loss) | | | (1,917,974 | ) | | | (1,078,401 | ) | | | (7,538,955 | ) |
| | | | | | | | | | | | | |
| Loss on settlement of debt | | | — | | | | (3,292,149 | ) | | | (3,292,149 | ) |
| Interest expense | | | (49,313 | ) | | | (759,387 | ) | | | (975,794 | ) |
| Interest income | | | — | | | | — | | | | 1,823 | |
| | | | | | | | | | | | | |
| Net (loss) – Continuing Operations | | | (1,967,287 | ) | | | (5,129,937 | ) | | | (11,805,075 | ) |
| Discontinued operations | | | — | | | | — | | | | (6,592,095 | ) |
| | | | | | | | | | | | | |
| Net (loss) | | $ | (1,967,287 | ) | | $ | (5,129,937 | ) | | $ | (11,805,075 | ) |
| | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 102,202,088 | | | | 41,102,254 | | | | | |
| | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.02 | ) | | $ | (0.12 | ) | | | | |
The accompanying notes are an integral part of these financial statements
| ENDEAVOR POWER CORP. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| PERIOD FROM JULY 6, 2005 (INCEPTION) TO DECEMBER 31, 2011 |
| | | | | | | | | | | | (DEFICIT) | | | | |
| | | | | | | | | | | | ACCUMULATED | | | | |
| | | | | | | | | | | | DURING THE | | | | |
| | | COMMON STOCK | | | PAID IN | | | EXPLORATION | | | | |
| | | SHARES | | | AMOUNT | | | CAPITAL | | | STAGE | | | TOTAL | |
| | | | | | | | | | | | | | | | |
| Balance, July 6, 2005 (date of inception) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of common shares for services | | | 1 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (750 | ) | | | (750 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2005 | | | 1 | | | | — | | | | — | | | | (750 | ) | | | (750 | ) |
| | | | | | | | | | | | | | | | | | | | | |
Issuance of founders shares for cash @ $.000015 per share | | | 3,250,000 | | | | 3,250 | | | | 1,750 | | | | — | | | | 5,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of common shares for cash @ $.0015 per share | | | 513,460 | | | | 513 | | | | 78,478 | | | | — | | | | 78,991 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (23,830 | ) | | | (23,830 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2006 | | | 3,763,461 | | | | 3,763 | | | | 80,228 | | | | (24,580 | ) | | | 59,411 | |
| | | | | | | | | | | | | | | | | | | | | |
| Cancellation of common shares | | | (3,250,000 | ) | | | (3,250 | ) | | | (1,750 | ) | | | — | | | | (5,000 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of common shares for mineral property @ $.40 per share | | | 140,000 | | | | 140 | | | | 5,599,860 | | | | — | | | | 5,600,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (73,915 | ) | | | (73,915 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2007 | | | 653,461 | | | | 653 | | | | 5,678,338 | | | | (98,495 | ) | | | 5,580,496 | |
| | | | | | | | | | | | | | | | | | | | | |
| Assumption Agreement | | | | | | | | | | | 255,050 | | | | — | | | | 255,050 | |
| | | | | | | | | | | | | | | | | | | | | |
| Settlement of related party debt | | | | | | | | | | | 37,883 | | | | — | | | | 37,883 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (6,107,944 | ) | | | (6,107,944 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2008 | | | 653,461 | | | | 653 | | | | 5,971,271 | | | | (6,206,439 | ) | | | (234,515 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of shares to settle debt | | | 3,437 | | | | 3 | | | | 504,698 | | | | — | | | | 504,701 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 32,000 | | | | 32 | | | | 3,775,968 | | | | — | | | | 3,776,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | 826,541 | | | | — | | | | 826,541 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | (5,093,507 | ) | | | (5,093,507 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2009 | | | 688,898 | | | | 689 | | | | 11,078,478 | | | | (11,299,946 | ) | | | (220,779 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of shares for repayment of loan | | | 375,000 | | | | 375 | | | | 254,625 | | | | — | | | | 255,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of shares to settle debt | | | 140,000,000 | | | | 140,000 | | | | 3,476,667 | | | | — | | | | 3,616,667 | |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of shares for services | | | 3,500,000 | | | | 3,500 | | | | 906,500 | | | | — | | | | 910,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (5,129,937 | ) | | | (5,129,937 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2010 | | | 144,563,898 | | | | 144,564 | | | | 15,716,270 | | | | (16,429,883 | ) | | | (569,049 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Issuance of shares for services | | | 10,000,000 | | | | 10,000 | | | | 1,790,000 | | | | — | | | | 1,800,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Cancellation of common shares | | | (3,500,000 | ) | | | (3,500 | ) | | | 3,500 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Write-off related party balance | | | | | | | | | | | 19,667 | | | | — | | | | 19,667 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | (1,967,287 | ) | | | (1,967,287 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2011 | | | 151,063,898 | | | $ | 151,064 | | | $ | 17,529,437 | | | $ | (18,397,170 | ) | | $ | (716,669 | ) |
The accompanying notes are an integral part of these financial statements
| ENDEAVOR POWER CORP. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | | Cumulative | |
| | | | | | | | | From 07/06/05 | |
| | | For the year ended December 31, | | | (Inception) to | |
| | | 2011 | | | 2010 | | | 12/31/2011 | |
| | | | | | | | | | |
| Cash Flows from operations: | | | | | | | | | |
| Net (loss) | | $ | (1,967,287 | ) | | $ | (5,129,937 | ) | | $ | (11,805,075 | ) |
| Adjustments to reconcile net (loss) to net cash (used in) operating activities: | | | | | | | | | | | | |
| Accretion expense | | | — | | | | 688,784 | | | | 826,541 | |
| Depreciation expense | | | 4,500 | | | | 653 | | | | 5,153 | |
| Common shares issued for services | | | 1,800,000 | | | | 910,000 | | | | 6,880,452 | |
| Common shares issued for incentives | | | — | | | | — | | | | 110,250 | |
| Loss on settlement of debt | | | — | | | | 3,292,149 | | | | 3,292,149 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Increase in accounts payable and accrued expenses | | | 62,085 | | | | 69,296 | | | | 172,394 | |
| Increase in related party payables | | | 7,900 | | | | 16,667 | | | | 34,155 | |
| Net cash (used in) operating activities | | | (92,802 | ) | | | (152,388 | ) | | | (483,981 | ) |
| | | | | | | | | | | | | |
| Cash Flows from investing activities: | | | | | | | | | | | | |
| Cash (used in) purchase of property and equipment | | | — | | | | (13,500 | ) | | | (13,500 | ) |
| Net cash (used in) investing activities | | | — | | | | (13,500 | ) | | | (13,500 | ) |
| | | | | | | | | | | | | |
| Cash Flows from financing activities: | | | | | | | | | | | | |
| Proceeds from related party loans | | | — | | | | 174,615 | | | | 1,061,561 | |
| Proceeds from notes payable | | | 65,000 | | | | 19,075 | | | | 84,075 | |
| proceeds from shareholders | | | — | | | | — | | | | 264,949 | |
| Proceeds from issuance of common shares | | | — | | | | — | | | | 83,991 | |
| Repayment on cancellation of common shares | | | — | | | | — | | | | (5,000 | ) |
| Net cash provided by financing activities | | | 65,000 | | | | 193,690 | | | | 1,489,576 | |
| | | | | | | | | | | | | |
| Cash Flows from discontinued operations: | | | | | | | | | | | | |
| Net cash (used in) operating activities | | | — | | | | — | | | | (382,377 | ) |
| Net cash (used in) investing activities | | | — | | | | — | | | | (609,718 | ) |
| Net cash (used in) discontinued operations | | | — | | | | — | | | | (992,095 | ) |
| | | | | | | | | | | | | |
| Increase in cash | | | (27,802 | ) | | | 27,802 | | | | — | |
| | | | | | | | | | | | | |
| Cash - beginning of period | | | 27,802 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Cash - end of period | | $ | — | | | $ | 27,802 | | | $ | — | |
| | | | | | | | | | | | | |
| NONCASH ACTIVITIES | | | | | | | | | | | | |
| Common shares issued to acquire mineral properties | | $ | — | | | $ | — | | | $ | 5,600,000 | |
| Common shares issued to settle note payable | | $ | — | | | $ | 500,000 | | | $ | 500,000 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | |
| Interest paid | | $ | — | | | $ | — | | | $ | — | |
| Income taxes paid | | $ | — | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these financial statements
ENDEAVOR POWER CORP.
(A DVELOPMENT STAGE COMPANY)
DECEMBER 31, 2011
NOTE 1. OVERVIEW AND NATURE OF BUSINESS
Endeavor Power Corp. (the “Company”) was incorporated in the State of Nevada on July 6, 2005 under the name VB Biotech Laboratories, Inc. On September 21, 2007, the Company filed a Certificate of Amendment with the State of Nevada to change its operating name to VB Trade, Inc., with principal business operations to develop an online website that allowed web designers to sell their website designs in exchange for a commission on all products that were sold through the website. On September 21, 2007, the Company entered into a Plan of Merger (the “Merger”) with Endeavor Uranium, Inc., a mineral exploration company with mineral properties in the northwestern United States. Effectively, the Company changed its name to Endeavor Uranium, Inc. as part of the Merger transaction. On December 23, 2008, the Company entered into a Joint Venture Agreement (the “Agreement”) with Federated Energy Corporation, a Tennessee corporation, for working interests in prospective oil and gas wells located in Nowata County, Oklahoma. Effectively on December 23, 2008, the Company changed its operating name to Endeavor Power Corp.
In November, 2010, Management assessed a potential business opportunity and determined that in an effort to create value for its Shareholders, the Company should change its business direction. On November 8, 2010, the Company discontinued its operations in its working interests in oil and gas exploration and changed its operating focus to the development of E-Waste processing services aimed at industrial and government clients. The Company’s new direction sought to limit the impact of discarded “E-Waste” on the environment. Discarded computers and electronic equipment pose environmental hazards.
On May 26, 2011, Mr. Alfonso Knoll resigned from all positions with the Company, including but not limited to, that of President, Chief Executive Officer, Chief Financial Officer, Treasurer and Secretary. The resignation did not involve any disagreement with the Company. On June 8, 2011, the Company entered into a Settlement Agreement and General Mutual Release (“Settlement Agreement”) to terminate Mr. Knoll’s Employment Agreement dated November 8, 2010, and to accept his resignation. Pursuant to the Settlement Agreement, Mr. Knoll immediately ceased all services to the Company and, on June 11, 2011, returned to the Company any and all shares of its common stock then held by him.
On June 2, 2011, Mr. Matthew Carley was appointed as the Company’s President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director. Mr. Carley accepted the appointment, but resigned his positions effective September 27, 2011. The Company’s Board of Directors accepted the resignation of Mr. Carley, as well as the resignation of Mr. Keith Kress as a member of the Board of Directors. Simultaneously, Tom Mackay was appointed as the President/Chief Executive Officer, Secretary, Treasurer/Chief Financial Officer and the sole member of the Board of Directors.
In accordance with a change in management effective September 27, 2011, the Company’s business operations changed. The Company intended to provide managerial services, and pursue potential funding opportunities for the Company. It retained consultants to perform the necessary due diligence on certain mining properties located in Venezuela, Brazil, Bolivia, Guyana and several other South American countries. Management, however, determined that the outcome of such due diligence did not provide the Company a viable opportunity, nor did it provide sufficient economic benefit for the Company. Management has therefore ceased its due diligence and exploration of mining property opportunities, and is pursuing other viable business opportunities to increase shareholder market value.
Going Concern
These financial statements have been prepared on a going concern basis, which implies that the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The Company has not generated significant revenues to date and has never paid any dividends and is unlikely to pay dividends or generate significant earnings in the immediate or foreseeable future. As at December 31, 2011, the Company had a working capital deficit of $307,578, and an accumulated deficit of $18,397,170. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders, the ability to raise equity or debt financing, and the attainment of profitable operations from the Company's future business. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States, and are expressed in US dollars. The Company’s fiscal year-end is December 31.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. As at December 31, 2011 and 2010, the Company had no cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States and requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to the valuation of its mineral properties, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
Basic and Diluted Net Income (Loss) Per Share
The Company computes net income (loss) per share in accordance with ASC 260, Earning per Share. ASC 260 requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period using the treasury stock method and convertible preferred stock using the if-converted method. In computing Diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. Diluted EPS excludes all dilutive potential shares if their effect is anti dilutive.
Comprehensive Loss
ASC 220, Comprehensive Income, establishes standards for the reporting and display of comprehensive loss and its components in the financial statements. As at December 31, 2011, the Company has no items that represent comprehensive loss and, therefore, has not included a schedule of comprehensive loss in the financial statements.
Foreign Currency Translation
The Company’s functional and reporting currency is the United States dollar. Monetary assets and liabilities denominated in foreign currencies are translated in accordance with ASC 830 Foreign Currency Translation Matters, using the exchange rate prevailing at the balance sheet date. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income. Foreign currency transactions are primarily undertaken in Canadian dollars. The Company has not, to the date of these financial statements, entered into derivative instruments to offset the impact of foreign currency fluctuations.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 605, Revenue Recognition. Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service has been provided, and collectability is reasonably assured.
Property and Equipment
Property and equipment is comprised of vehicles and general equipment and are recorded at cost and is depreciated using the straight-line method over the estimated useful lives of three years. Maintenance and repairs are charged to expense as incurred. Significant renewals and betterments are capitalized.
Financial Instruments
Pursuant to ASC 820, Fair Value Measurements and Disclosures and ASC 825, Financial Instruments, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 and 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 and 825 prioritizes the inputs into three levels that may be used to measure fair value:
| | Level 1 | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. |
| | Level 2 | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. |
| | Level 3 | Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
The Company’s financial instruments consist principally of cash, accounts payable, and accrued liabilities. Pursuant to ASC 820 and 825, the fair value of cash is determined based on “Level 1” inputs, which consist of quoted prices in active markets for identical assets. The recorded values of all other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Share-Based Payments, using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
Recently Adopted Accounting Standards: The Company evaluates the pronouncements of various authoritative accounting organizations, primarily the Financial Accounting Standards Board (“FASB”), the US Securities and Exchange Commission (“SEC”), and the Emerging Issues Task Force (“EITF”), to determine the impact of new pronouncements on US GAAP and the impact on the Company. The Company has adopted the following new accounting standards during 2011:
ASU No. 2010-13: Issued in April 2010, ASU No. 2010-13 clarifies the classification of an employee share based payment award with an exercise price denominated in the currency of a market in which the underlying security trades. This ASU will be effective for the first fiscal quarter beginning after December 15, 2010, with early adoption permitted.
ASU 2010-29: Issued in December 2010, ASU 2010-29 requires a public entity to disclose pro forma information for business combinations that occurred in the current reporting period. The disclosures include pro forma revenue and earnings of the combined entity for the current reporting period as though the acquisition date for all business combinations that occurred during the year had been as of the beginning of the annual reporting period. This ASU will be effective for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010, with early adoption permitted.
Recently Issued Accounting Standards Updates: The following accounting standards updates were recently issued and have not yet been adopted by the Company. These standards are currently under review to determine their impact on the Company’s consolidated financial position, results of operations, or cash flows.
| | Trouble Debt Restructuring: Issued in April, 2011, ASU 2011-02 clarifies which loan modifications constitute troubled debt restructurings. It is intended to assist creditors in determining whether a modification of the terms of a receivable meets the criteria to be considered a troubled debt restructuring, both for purposes of recording an impairment loss and for disclosure of troubled debt restructurings. The new guidance is effective for interim and annual periods beginning on or after June 15, 2011, and applies retrospectively to restructurings occurring on or after the beginning of the fiscal year of adoption. Early adoption is permitted. | |
| | | |
| | Comprehensive Income: Issued in June, 2011, ASU 2011-05 eliminates the current option to present other comprehensive income and its components in the statement of changes in equity. It will require companies to report the total of comprehensive income including the components of net income and the components of other comprehensive income in either a single continuous statement of comprehensive income or in two separate but consecutive statements. The amendments in the ASU are effective for interim and annual periods beginning on or after December 15, 2011, and should be applied retrospectively. Early adoption is permitted. | |
| | Intangibles: Issued in September, 2011, ASU 2011-08 permits entities to first perform a qualitative assessment to determine whether it is more likely than not (a likelihood of more than 50 percent) that the fair value of a reporting unit is less than its carrying amount. If the entity determines that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, it would then perform the first step of the goodwill impairment test; otherwise, no further impairment test would be required. The amendments will be effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted. | |
| | | |
| | Disclosures about Offsetting Assets and Liabilities: Issued in December, 2011, ASU 2011-11 requires disclosures to provide information to help reconcile differences in the offsetting requirements under U.S. GAAP and IFRS. The differences in the offsetting requirements account for a significant difference in the amounts presented in statements of financial position prepared in accordance with U.S. GAAP and IFRS for certain entities. An entity is required to apply the amendments for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods. | |
There were other various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries. None of the updates are expected to a have a material impact on the Company's consolidated financial position, results of operations or cash flows.
NOTE 3. PROPERTY AND EQUIPMENT
Property and Equipment consists of the following:
| | | December 31, 2011 | | | December 31, 2010 | |
| General Equipment | | $ | 2,500 | | | $ | 2,500 | |
| Automobiles | | | 11,000 | | | | 11,000 | |
| Sub-Total | | | 13,500 | | | | 13,500 | |
| Accumulated Deprecation | | | (5,153 | ) | | | (653 | ) |
| Property and Equipment, Net | | $ | 8,347 | | | $ | 12,487 | |
Depreciation expense totaled $4,500 and $653 for the years ended December 31, 2011 and 2010, respectively.
NOTE 5. NOTES AND LOANS PAYABLE
| In June, 2010, the Company issued a note payable in the principal amount of $9,075 to a non-related party. Under the terms of the note, the amount is unsecured, non-interest bearing, and due upon demand. |
| In June, 2010, the Company issued a note payable in the principal amount of $10,000 to a non-related party. Under the terms of the note, the amount is unsecured, accrues interest at a rate of 8% per annum, and is due upon demand. As of December 31, 2011, the Company has recorded $1,412 in interest as an accrued expense. |
| On April 21, 2011, the Company issued a note payable in the principal amount of $65,000 to a non-related party. The note is unsecured, bears interest at a rate of 10% per annum, and is due upon demand. As of December 31, 2011, the Company has recorded $6,179 in interest as an accrued expense. |
NOTE 6. RELATED PARTY PAYABLE
As at December 31, 2011, related party payable consists of $108,349 representing miscellaneous operating expenses paid on behalf of the Company by certain related parties. The amounts owing are unsecured, non-interest bearing, and due upon demand.
NOTE 7. NOTES PAYABLE – RELATED PARTY
On August 25, 2009, the Company issued a convertible promissory note (the “Note”) of $826,541 to a related party to settle outstanding debt obligations owing as of the issuance date. Under the terms of the Note, the amount owing accrues interest at a rate of 10% per annum, was due August 25, 2011, and contained certain provisions to convert the debt into common shares of the Company.
On June 17, 2010, the Company issued the related party 375,000 common shares to settle $75,000 of the outstanding Note, reducing the principal balance to $751,541.
In September, 2010, the Company issued the related party 140,000,000 common shares to settle $500,000 of the Note, reducing the principal balance to $251,541.
On September 17, 2010, the Company amended the Note with the related party to combine the remaining principal of $251,541 with the accrued interest to date of $65,897, for a revised principal balance of $317,438. The new principal balance continues to accrue interest at a rate of 10% per annum, but no longer contains a provision for conversion.
In November, 2010, the Company received an additional $100,000 from the related party, increasing the principal balance to $417,438.
As at December 31, 2011, the principal balance of the Note is $417,438. The Company has recorded $74,275 in interest as an accrued expense.
NOTE 8. COMMON STOCK
All common shares issued for services or settlements of debt are valued based on the end-of-day market prices on the date of issuance, unless otherwise specified.
The Company and its Board of Directors authorized a 1:100 reverse common stock split on August 16, 2010, The effects of the reverse stock split resulted in the number of issued and outstanding common stock to decrease from 106,388,200 common shares to 1,063,898 common shares, have been applied on a retroactive basis, and are reflected below where applicable.
The total number of authorized shares of common stock that may be issued by the Company is 250,000,000 with a par value of $0.001 per share.
| During the year ended December 31, 2006, the Company issued 325,000,000 founder shares for cash proceeds of $5,000. These shares were cancelled during the year ended December 31, 2007. |
| During the year ended December 31, 2006, the Company issued 513,448 (post-split adjusted) common shares for cash proceeds of $78,991. |
| In October 2007, the Company issued 140,000 (post-split adjusted) common shares at $0.40 per common share, with a fair value of $5,600,000, to acquire mineral properties. |
On July 22, 2009, the Company issued 460 (post-split adjusted) common shares of the Company with a fair value of $67,376 to settle debt obligations of $27,500, resulting in a loss on settlement of debt of $39,876.
On July 22, 2009, the Company issued 460 (post-split adjusted) common shares of the Company with a fair value of $67,376 to settle debt obligations of $27,500, resulting in a loss on settlement of debt of $39,876.
On July 22, 2009, the Company issued 1,667 (post-split adjusted) common shares of the Company with a fair value of $245,000 to settle debt obligations of $100,000, resulting in a loss on settlement of debt of $145,000.
On July 22, 2009, the Company issued 100 (post-split adjusted) common shares of the Company with a fair value of $14,700 to settle debt obligations of $12,000, resulting in a loss on settlement of debt of $2,700.
On July 22, 2009, the Company issued 750 (post-split adjusted) common shares of the Company with a fair value of $110,250 to a related party as incentive bonus shares for the conversion of amounts owing into a long-term convertible note payable.
On August 25, 2009, the Company issued 32,000 (post-split adjusted) common shares of the Company for consulting services with a fair value of $3,776,000.
On June 17, 2010, the Company issued 375,000 (post-split adjusted) common shares to a related party for the repayment of payable note payable of $75,000, resulting in a loss on settlement of debt of $180,000. Per the convertible note agreement the Company was to convert the debt at $0.006 per share (post split) for a total issuance of 12,500,000 shares. The additional 25,000,000 shares were issued at the closing price of the stock on the day of issuance resulting in the $180,000 loss.
On September 20, 2010, the Company issued 140,000,000 common shares to settle outstanding notes payable of $500,000 resulting in a loss on settlement of debt of $3,116,667. Per the convertible note agreement the Company was to convert the debt at $0.006 per share (post split) for a total issuance of 83,333,333 shares. The additional 56,666,667 shares were issued at the closing price of the stock on the day of issuance resulting in the $3,166,667 loss.
On November 8, 2010, the Company issued 3,500,000 common shares to the CEO of the Company for management services with a fair value of $910,000. As a result, $906,500 was recorded as paid in capital.
On February 23, 2011, the Company issued 10,000,000 shares of its common stock in exchange for services rendered to the Company, valued at $1,800,000. As a result, $1,790,000 was recorded as paid in capital.
On June 14, 2011, pursuant to the Settlement Agreement related to the resignation of the Company’s former CEO/President, the 3,500,000 shares of common stock previously issued on November 8, 2010, were returned to treasury. As a result, paid in capital was reduced by $3,500.
As of December 31, 2011, the Company had 151,063,898 common shares issued and outstanding.
NOTE 9. WARRANTS
During the year ended December 31, 2011, the Company had the following share purchase warrants outstanding:
| Outstanding and Exercisable Warrants |
| | Number of | Remaining Contractual Life | Exercise Price times Number | Weighted Average |
| Exercise Price | Shares | (in years) | of Shares | Exercise Price |
| $0.900 | 500,000 | 0.66 | $ | 450,000 | $ | 0.900 |
| | | | | | Weighted | |
| | | | | | Average | |
| | | Number | | | Exercise | |
| Warrants | | of Shares | | | Price | |
| | | | | | | |
| Outstanding at December 31, 2010 | | | 500,000 | | | $ | 0.900 | |
| Issued | | | — | | | | — | |
| Exercised | | | — | | | | — | |
| Expired / Cancelled | | | — | | | | — | |
| Outstanding at December 31, 2011 | | | 500,000 | | | $ | 0.900 | |
The outstanding share purchase warrants expire on August 25, 2012.
NOTE 10. DISCONTINUED OPERATIONS
On November 8, 2010, the Company discontinued all operations related to the former activities of exploring mineral properties. As at November 8, 2010, the Company had no assets or liabilities relating to mineral properties and exploration and did not incur any mineral property or exploration cost during the year 2010 or 2011.
The results of discontinued operations are summarized as follows:
| | | Accumulated from July 6, 2005 to December 31, 2009 | |
| Operating expenses: | | | |
| Impairment of joint venture costs | | $ | 609,718 | |
| Impairment of mineral property costs | | | 5,600,000 | |
| Mineral property expenditures | | | 382,377 | |
| Total expenses | | | 6,592,095 | |
| Net Loss | | $ | (6,592,095 | ) |
As at December 31, 2011 and 2010, there were no remaining assets and liabilities of the discontinued mineral properties exploration operation.
NOTE 11. INCOME TAXES
The components of the net deferred tax asset at December 31, 2011 and 2010, the statutory tax rate, the effective tax rate and the amount of the valuation allowance are indicated below:
| | | December 31, 2011 | | | December 31, 2010 | |
| | | | | | | |
| Income (Loss) Before Taxes | | $ | (1,967,287 | ) | | $ | (5,129,937 | ) |
| Statutory rate | | | 34 | % | | | 34 | % |
| | | | | | | | | |
| Computed expected tax payable (recovery) | | $ | 668,878 | | | $ | 1,744,179 | |
| Non-deductible expenses | | | (1,530 | ) | | | (1,377,620 | ) |
| Change in valuation allowance | | | (667,348 | ) | | | (366,559 | ) |
| | | | | | | | | |
| Reported income taxes | | $ | – | | | $ | – | |
The significant components of deferred income tax assets and liabilities at December 31, 2011 and 2010 are as follows:
| | | 2011 | | | 2010 | |
| | | | | | | |
| Net operating loss carried forward | | $ | 5,890,292 | | | $ | 3,927,505 | |
| | | | | | | | | |
| Valuation allowance | | | (5,890,292 | ) | | | (3,927,505 | ) |
| | | | | | | | | |
| Net deferred income tax asset | | $ | – | | | $ | – | |
As at December 31, 2011, the Company had $5,890,292 of net operating losses which expire commencing in the year 2026.
NOTE 12. SUBSEQUENT EVENTS
The Company has evaluated events and transactions that occurred between December 31, 2011 and the date the consolidated financial statements were available for issue, for possible disclosure or recognition in the consolidated financial statements. The Company has determined that there were no such events or transactions that warrant disclosure or recognition in the consolidated financial statements.
UNAUDITED INTERIM FINANCIAL STATEMENT
FOR PARALLAX DIAGNOSTICS, INC.
AS OF AND FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2012
| PARALLAX DIAGNOSTICS, INC. | |
| (A DEVELOPMENT STAGE COMPANY) | |
| |
| | | September 30, 2012 | | | December 31, 2011 | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash & cash equivalents | | $ | 26,792 | | | $ | 136,066 | |
| Total Current Assets | | | 26,792 | | | | 136,066 | |
| | | | | | | | | |
| Property, Plant & Equipment, net | | | 32,495 | | | | 41,114 | |
| | | | | | | | | |
| Intangible Assets, net | | | 1,299,984 | | | | 1,370,490 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 1,359,271 | | | $ | 1,547,670 | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 122,666 | | | $ | 104,415 | |
| Related party payables | | | 299,380 | | | | 152,361 | |
| Notes & loans payable | | | 165,149 | | | | 20,000 | |
| Total Current Liabilities | | | 587,195 | | | | 276,776 | |
| | | | | | | | | |
| Deferred Revenue | | | 1,500,000 | | | | 1,500,000 | |
| | | | | | | | | |
| Related party loans | | | 14,000 | | | | 14,000 | |
| Total Liabilities | | | 2,101,195 | | | | 1,790,776 | |
| | | | | | | | | |
| STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| Preferred Stock, $0.0001 par value, 100,000,000 shares authorized, 230,000 and 220,000 issued and outstanding as of September 30, 2012 and December 31, 2011, respectively | | | 23 | | | | 22 | |
| Common stock, $0.0001 par value, 400,000,000 shares authorized, 24,870,000 and 28,020,000 issued and outstanding as of September 30, 2012 and December 31, 2011, respectively | | | 2,487 | | | | 2,802 | |
| Additional paid in capital - Preferred | | | 499,977 | | | | 399,978 | |
| Additional paid in capital - Common | | | 88,772 | | | | 227,456 | |
| Subscriptions receivable | | | (20 | ) | | | (20 | ) |
| Accumulated Deficit | | | (1,333,163 | ) | | | (873,344 | ) |
| Total Stockholders' (Deficit) | | | (741,924 | ) | | | (243,106 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | $ | 1,359,271 | | | $ | 1,547,670 | |
The accompanying notes are an integral part of these financial statements
| PARALLAX DIAGNOSTICS, INC. | |
| (A DEVELOPMENT STAGE COMPANY) | |
| |
| | | | | | | | | | | | | | | Cumulative From | |
| | | | | | | | | | | | | | | April 12, 2010 | |
| | | For the three months ended | | | For the nine months ended | | | (Inception) to | |
| | | September 30, 2012 | | | September 30, 2011 | | | September 30, 2012 | | | September 30, 2011 | | | September 30, 2012 | |
| | | | | | | | | | | | | | | | |
| General and administrative expenses | | $ | 121,484 | | | $ | 161,635 | | | $ | 370,771 | | | $ | 313,020 | | | $ | 852.481 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating (loss) | | | (121,484 | ) | | | (161,635 | ) | | | (370,771 | ) | | | (313,020 | ) | | | (852.481 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Other Expenses | | | | | | | | | | | | | | | | | | | | |
| Depreciation & amortization | | | 29,331 | | | | 25,002 | | | | 87,995 | | | | 104,923 | | | | 198,282 | |
| Amortization of deferred compensation | | | 2,778 | | | | 58,331 | | | | 42,370 | | | | 214,925 | | | | 281,250 | |
| Interest expense | | | 361 | | | | — | | | | 1,051 | | | | — | | | | 1,149 | |
| Total Other Expenses | | | 32,470 | | | | 83,333 | | | | 131,416 | | | | 319,848 | | | | 480,681 | |
| | | | | | | | | | | | | | | | | | | | | |
| Provision for income taxes | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) | | $ | (153,954 | ) | | $ | (244,968 | ) | | $ | (502,187 | ) | | $ | (632,868 | ) | | $ | (1,333,162 | ) |
| | | | | | | | | | | �� | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.01 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) | | $ | (0.02 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 24,394,944 | | | | 28,000,000 | | | | 24,394,944 | | | | 28,000,000 | | | | | |
The accompanying notes are an integral part of these financial statements
| PARALLAX DIAGNOSTICS, INC. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | | Cumulative From | |
| | | | | | | | | April 12, 2010 | |
| | | For the nine months ended | | | (Inception) to | |
| | | September 30, 2012 | | | September 30, 2011 | | | September 30, 2012 | |
| | | | | | | | | | |
| Cash Flow from operations: | | | | | | | | | |
| Net loss | | $ | (502,187 | ) | | $ | (632,868 | ) | | $ | (1,333,162 | ) |
| Depreciation | | | 12,989 | | | | 4,917 | | | | 23,268 | |
| Amortization of intangible assets | | | 75,006 | | | | 100,008 | | | | 200,016 | |
| Amortization of deferred compensation | | | 42,370 | | | | 214,925 | | | | 281,250 | |
| Stock compensation | | | 5,000 | | | | — | | | | 5,000 | |
| Adjustments to reconcile net (loss) to net cash (used in) operating activities: | | | | | | | | | | | | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Increase in accounts payable and accrued expenses | | | 19,399 | | | | 101,391 | | | | 123,815 | |
| Increase in expenses payable to related parties | | | 147,019 | | | | 139,724 | | | | 299,380 | |
| Net cash provided by (used in) operating activities | | | (200,404 | ) | | | (71,903 | ) | | | (400,433 | ) |
| | | | | | | | | | | | | |
| Cash Flow from investing activities: | | | | | | | | | | | | |
| (Increase) in Property, Plant & Equipment | | | (4,370 | ) | | | (49,174 | ) | | | (55,763 | ) |
| (Increase) in Intangible Assets | | | — | | | | (1,500,000 | ) | | | (1,500,000 | ) |
| Net cash (used in) investing activities | | | (4,370 | ) | | | (1,500,000 | ) | | | (1,555,763 | ) |
| | | | | | | | | | | | | |
| Cash Flow from financing activities: | | | | | | | | | | | | |
| Proceeds from related party loans | | | — | | | | 14,000 | | | | 14,000 | |
| Proceeds from notes & loans payable | | | — | | | | — | | | | 24,500 | |
| Repayment of notes & loans payable | | | (4,500 | ) | | | — | | | | (4,500 | ) |
| Increase in Deferred Revenue | | | — | | | | 1,500,000 | | | | 1,500,000 | |
| (Increase) in Deferred Compensation | | | — | | | | (281,250 | ) | | | (281,250 | ) |
| Increase (Decrease) in Capital Stock due to Merger | | | (315 | ) | | | (700 | ) | | | 2,487 | |
| Increase in Paid In Capital due to Merger | | | 315 | | | | 227,439 | | | | 227,771 | |
| Issuance of Preferred Stock | | | 1 | | | | 21 | | | | 23 | |
| Issuance of Common Stock | | | — | | | | — | | | | (20 | ) |
| Subscriptions receivable | | | | | | | — | | | | — | |
| Increase in Preferred Paid In Capital due to stock issuance | | | 99,999 | | | | 199,980 | | | | 499,977 | |
| Net cash provided by financing activities | | | 95,500 | | | | 1,759,489 | | | | 1,982,988 | |
| | | | | | | | | | | | | |
| Increase in cash | | | (109.274 | ) | | | 138,412 | | | | 26,792 | |
| | | | | | | | | | | | | |
| Cash - beginning of period | | | 136,066 | | | | 3,600 | | | | — | |
| | | | | | | | | | | | | |
| Cash - end of period | | $ | 26,792 | | | $ | 142,012 | | | $ | 26,792 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| NONCASH ACTIVITIES | | | | | | | | | | | | |
| Reclassification of Note Payable from capital | | $ | 144,000 | | | $ | — | | | $ | 144,000 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | |
| | | | | | | | | | | | | |
| Interest paid | | $ | — | | | $ | — | | | $ | — | |
| Income taxes paid | | $ | — | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these financial statements
PARALLAX DIAGNOSTICS, INC.
(A Development Stage Company)
September 30, 2012
NOTE 1. OVERVIEW
The accompanying unaudited financial statements of Parallax Diagnostics, Inc. have been prepared in accordance with generally accepted accounting principles for interim financial information and with the instructions to Form 10-Q and Item 210 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. These financial statements should be read in conjunction with the audited financial statements and notes included thereto for the year ended December 31, 2011, on Form 10K, as filed with the Securities and Exchange Commission.
The financial statements reflect all adjustments consisting of normal recurring adjustments, which, in the opinion of management, are necessary for a fair presentation of the results for the periods shown.
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and that effect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Parallax Diagnostics, Inc. (the “Company”) was incorporated on April 12, 2010 in the state of Nevada. The Company was formed to serve as a vehicle to effect an asset acquisition, merger, exchange of capital stock, or other business combination with a domestic or foreign business.
On January 11, 2011 (the “Closing Date”), the Company entered into and closed a share exchange agreement (the “Share Exchange Agreement”) with Amersey Investments, LLC (“Amersey”), Parallax Diagnostics, Inc., a Delaware corporation (“Parallax”) and its sole shareholder, Montecito Bio Sciences, Ltd. (“Montecito”). On the Closing Date, pursuant to the terms and conditions of the Share Exchange Agreement, (i) the Company acquired 100% of the issued and outstanding shares of common stock of Parallax in exchange for the issuance of 21,000,000 shares of our common stock, par value $0.0001 and (ii) Parallax merged with and into the Company whereupon the Company continued as the surviving entity and the corporate existence of Parallax ceased (the “Merger”). Additionally, as further consideration for the share exchange and Merger and in accordance with the Shares Exchange Agreement, Amersey cancelled to treasury 28,000,000 shares of the Company’s common stock.
As a result of the transactions effected by the Share Exchange Agreement, (i) the former business of Parallax is now the Company’s sole business and (ii) there is a change of control whereby the former shareholder of Parallax, Montecito, will now own a controlling 75% ownership interest in the Company. In addition, the Company changed its name to “Parallax Diagnostics, Inc.”
As a further condition of the Share Exchange Agreement, the current officers and directors of the Company resigned and J. Michael Redmond was appointed to serve as a Director and also as the CEO and President of the Company. Additionally, Mr. Norman A. Kunin was appointed to serve as the Company’s CFO, Mr. Mike Contarino was appointed to serve as the Company’s Vice President and Dr. Roger Morris was appointed to serve as the Company’s Chief Science Officer. Mr. Edward W. Withrow III, Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
The Company is a development stage company as defined by ASC 915-10, “Accounting and Reporting by Development Stage Enterprises”. A development stage enterprise is one in which planned principal operations have not commenced or if its operations have commenced, there has been no significant revenues there from. At September 30, 2012 the Company has not yet commenced any operations. All activity from April 12, 2010 (date of inception) through September 30, 2012 relates to the Company’s formation and the pending registration statement described below.
The Company has incurred losses since inception resulting in an accumulated deficit of $1,333,162 since inception and further losses are anticipated in the development of its business. The Company’s ability to continue as a going concern is dependent upon its ability to generate profitable operations in the future and/or to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due.
The financial statements reflect all adjustments consisting of normal recurring adjustments, which, in the opinion of management, are necessary for a fair presentation of the results for the periods shown. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts of and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and that effect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
The Company’s fiscal year end is December 31.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies is presented to assist in understanding the Company’s financial statements. These accounting policies conform to accounting principles, generally accepted in the United States of America, and have been consistently applied in the preparation of the financial statements.
Development Stage Company
The Company is a development stage company as defined by ASC 915-10-05, “Development Stage Entity.” The Company is still devoting substantially all of its efforts on establishing the business and its planned principal operations have not commenced. All losses accumulated, since inception, have been considered as part of the Company’s development stage activities.
Use of Estimates
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States of America, requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, disclosures of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Fiscal Year End
The Company has a fiscal year ending on December 31.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents.
Property, Plant and Equipment
Property and equipment are carried at the cost of acquisition or construction and depreciated over the estimated useful lives of the assets. Costs associated with repairs and maintenance are expensed as incurred. Costs associated with improvements which extend the life, increase the capacity or improve the efficiency of our property and equipment are capitalized and depreciated over the remaining life of the related asset. Gains and losses on dispositions of equipment are reflected in operations. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which are 5 to 7 years.
Fair Value of Financial Instruments
The carrying amounts reported in the balance sheet for cash, accounts payable and accrued expenses approximate fair value based on the short-term maturity of these instruments.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC 740-10, “Accounting for Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year; and, (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of available positive and negative evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
ASC 740-10 prescribes a recognition threshold and measurement attribute for the financial statement recognition of a tax position taken or expected to be taken on a tax return. Under ASC 740-10, a tax benefit from an uncertain tax position taken or expected to be taken may be recognized only if it is “more likely than not” that the position is sustainable upon examination, based on its technical merits. The tax benefit of a qualifying position under ASC 740-10 would equal the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all the relevant information. A liability (including interest and penalties, if applicable) is established to the extent a current benefit has been recognized on a tax return for matters that are considered contingent upon the outcome of an uncertain tax position. Related interest and penalties, if any, are included as components of income tax expense and income taxes payable.
Earnings (Loss) per Share
Basic earnings (loss) per share is computed by dividing net income, or loss, by the weighted average number of shares of common stock outstanding for the period. Diluted earnings (loss) per share is computed by dividing net income, or loss, by the weighted average number of shares of both common and preferred stock outstanding for the period.
New Accounting Standards
Management does not believe that any recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on the accompanying financial statements.
NOTE 3. PROPERTY, PLANT AND EQUIPMENT
Property, Plant and Equipment consists of the following:
| | | September 30, 2012 | | | December 31, 2011 | |
| Office Equipment | | $ | 10,569 | | | $ | 6,199 | |
| | | | | | | | | |
| Medical Devices & Instruments | | | 45,194 | | | | 45,194 | |
| | | | | | | | | |
| Sub-Total | | | 55,763 | | | | 51,393 | |
| | | | | | | | | |
| Accumulated Deprecation | | | (23,268 | ) | | | (10,279 | ) |
| | | | | | | | | |
| Property Plant & Equipment, Net | | $ | 32,495 | | | $ | 41,114 | |
Depreciation expense totaled $4,329 and $10,279 for the three months ended September 30, 2012 and the year ended December 31, 2011, respectively.
NOTE 4. INTANGIBLE ASSETS
Intangible Assets consists of the following:
| | | September 30, 2012 | | | December 31, 2011 | |
| Products & Processes | | $ | 750,000 | | | $ | 750,000 | |
| | | | | | | | | |
| Trademarks & Patents | | | 750,000 | | | | 750,000 | |
| | | | | | | | | |
| Sub-Total | | | 1,500,000 | | | | 1,500,000 | |
| | | | | | | | | |
| Accumulated Amortization | | | (200,016 | ) | | | (125,010 | ) |
| | | | | | | | | |
| Intangible Assets, net | | $ | 1,299,984 | | | $ | 1,374,990 | |
Amortization expense totaled $25,002 and $125,010 for the three months ended September 30, 2012 and the year ended December 31, 2011, respectively.
NOTE 5. RELATED PARTY PAYABLE
As at September 30, 2012, affiliates and related parties are due a total of $313,380, which is comprised of $12,500 cash loans, $291,538 of accrued compensation to officers of the Company, and $9,342 due to related parties for reimbursable expenses.
NOTE 6. NOTES AND LOANS PAYABLE
Prior to the merger, the Company issued a Convertible Promissory Note (the “Note”) in the principal amount of $144,000 to Prominence Capital LLC. The Note bore interest at the rate of 6% per annum, the principal balance due on or before September 30, 2011, and included the option to convert the balance into common stock of the Company at the request of the Note Holder. At the date of the Merger, the Note was recorded the as a capital contribution, after discussions with the Note Holder of its intent to convert the Note into common stock. Subsequent to the merger, the Company was notified that the Note was purchased by the Kasper Group Ltd., a shareholder of the Company, (the “Kasper Note”). The Company has therefore reversed the amount previously recognized as a capital contribution, and has appropriately reflected the principal balance of the Kasper Note as a debt owed to a related party. The Kasper Note is interest free, is due upon demand, and contains a repayment provision to convert the debt into common stock of the Company, at the option of the Kasper Group, Ltd.
In addition, the Company entered into a Confidential Settlement Agreement with Grant Park Global, LLC (“Grant Park”), wherein the Company issued a Promissory Note to Grant Park for the principal amount of $20,000. The Note bears interest at a rate of 7% per annum, and is due and payable upon demand within 12 months.
Accrued interest as of September 30, 2012 is $1,149.
NOTE 7. DEFERRED REVENUE
On September 30, 2011, the Company entered into Modification Agreements with Montecito Bio Sciences, Ltd. (“Montecito”) regarding the Agreement of the Assignment of Intellectual Property (the “Assignment Agreement”) and the Assignment of the License of Intellectual Property (the “License Agreement”), both Agreements having been entered into on September 10, 2010. The nature of the Modifications to the Assignment Agreement and the License Agreement were to increase the royalty amounts (“Royalties”) due to Montecito from Four Percent (4%) to Five Percent (5%) and from Three and One Half Percent (3½%) to Four and One Half Percent (4½%), respectively. The Assignment Agreement Royalties shall revert back to Four Percent (4%) after the Company has paid Montecito Seven Hundred Fifty Thousand Dollars ($750,000) of royalty revenue and the License Agreement Royalties shall revert back to Three and One Half Percent (3½%) after the Company has paid Montecito Seven Hundred Fifty Thousand Dollars ($750,000) of royalty revenue. As of September 30, 2012, the Company has recorded Deferred Revenue in the amount of $1,500,000.
NOTE 8. COMMITMENTS AND CONTINGENCIES
On July 1, 2011, the Company entered into a Development and Supply Agreement with Corder Engineering, LLC. The Statement of Work stipulates that Corder Engineering, LLC shall provide ten (10) Evaluation Units which replicate the functionality Target. Target 1000 firmware ver. 320 and add software for a C-reactive protein (CRP) quantitative assay. The total payment under the Agreement stipulates $35,000 over a twelve week period. As of September 30, 2012, payments totaling $22,500 have been made, and $12,500 has been accrued.
On July 1, 2011, the Company entered into a Supply Agreement with Meyers Stevens Group, Inc. ("Meyers Stevens"). The Statement of Work stipulates that Meyers Stevens will manufacture assays and supply a Data Package for the Company and will yield approximately 100 to 200 fully functional assay test devices for internal investigational use. Estimated delivery of the assays is eight (8) weeks from the date of the Agreement for a total cost of $10,194. As of September 30, 2012, payments totaling $8,980 have been made, and $1,214 has been accrued.
On January 2, 2012, the Company entered into a consulting agreement with Huntington Chase Financial Group LLC (“HCFG”), a Nevada corporation. The consulting agreement provides for HCFG to provide advisory services to the Company for a period of three years for a fee of $12,500 per month. As of September 30, 2012, $112,500 has been recorded as accrued compensation.
On July 11, 2012, the Company entered into a Consulting Agreement with Greg Suess (“Suess”) for advisory services provided to the Company. As compensation for services rendered, valued at $5,000, Suess was afforded the opportunity to purchase 75,000 restricted shares of the Company’s common stock at a price of $0.001 per share. Suess purchased the shares on July 24, 2012 for cash in the amount of $75.00. As a result, $5,000 was expensed in July, 2012.
NOTE 9. PREFERRED STOCK
The total number of authorized shares of preferred stock that may be issued by the Company is 100,000,000 with a par value of $0.0001 per share.
On June 17, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Hamburg Investment Company, LLC ("HIC"), whereby 100,000 shares of Preferred Stock would be issued to HIC for a purchase price of $1.00 per share, or $100,000. As a result, $99,990 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, the Company issued a warrant to convert 100% of HIC’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On June 17, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), whereby 100,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $1.00 per share, or $100,000. As a result, $99,990 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, the Company issued a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On September 30, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), whereby 10,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, the Company issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On December 6, 2011, the Company entered into a Convertible Preferred Purchase Agreement with David Engert, ("Engert") whereby 10,000 shares of Preferred Stock would be issued to Engert for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, the Company issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of Engert’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On May 3, 2012, the Company entered into a Convertible Preferred Purchase Agreement with Donald Wachelka, ("Wachelka") whereby 10,000 shares of Preferred Stock would be issued to Wachelka for a purchase price of $10.00 per share, or $100,000. As a result, $99,999 has been recorded to Preferred paid in capital. In connection with the issuance of Preferred Stock, the Company issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of Wachelka’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
As at September 30, 2012, 230,000 shares of preferred stock are issued and outstanding.
NOTE 10. COMMON STOCK
The total number of authorized shares of common stock that may be issued by the Company is 400,000,000 with a par value of $0.0001 per share.
On April 15, 2010, the Company issued 35,000,000 shares of its common shares at $.0001 per share to Amersey Investments, LLC (“Amersey”) for $3,500 cash.
On January 7, 2011, of the 35,000,000 shares of the Company’s common stock held by Amersey, 7,000,000 shares were transferred to and distributed among 10 shareholders, for no cash.
On January 11, 2011, pursuant to the Share Exchange Agreement, Amersey cancelled to treasury 28,000,000 shares of the Company's common stock.
On January 11, 2011, the Company issued 21,000,000 shares of its common stock pursuant to the Share Exchange Agreement. As a result, $2,100 was recorded as paid in capital.
On January 15, 2011, the Company issued 125,000 shares of its common stock to its President, J. Michael Redmond, for cash in the amount of $125. As a result, $78 was recorded as paid in capital.
During December, 2011, in connection with the settlement agreement with Grant Park Global LLC, the Company issued 20,000 shares of its common stock for cash in the amount $20. As a result, $18 has been recorded as paid in capital.
On July 25, 2012, the Company issued 75,000 shares of its common stock to a consultant for services rendered in the amount of $5,000. As a result, $5,000 was recorded as stock compensation, and $4,992 was recorded as paid in capital.
In August, 2012, 3,350,000 shares of the Company’s common stock were canceled and returned to the treasury.
As of September 30, 2012 the Company has 24,870,000 common shares issued and outstanding.
NOTE 11. EQUITY COMPENSATION PLAN
On October 1, 2010, the board of directors adopted the Company's Stock Option Plan. The Company has reserved 3,000,000 shares of common stock for issuance upon exercise of options granted from time to time under the stock option plan. The stock option plan is intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. Under the stock option plan, the Company may grant incentive stock options only to key employees and employee directors, or the Company may grant non-qualified options to employees, officers, directors and consultants. The stock option plan is currently administered by the Company's board of directors. Subject to the provisions of the stock option plan, the board will determine who shall receive options, the number of shares of common stock that may be purchased under the options. The Company has granted options to purchase a total of 1,950,000 shares. In connection with the options granted, a total of $281,250 has been recorded as deferred compensation, and is being amortized over a 12-18 month vesting period. For the three month period ended September 30, 2012, the Company expensed $2,778 in deferred compensation. As of September 30, 2012 all deferred compensation in the amount of $281,250 has been expensed.
NOTE 12. INCOME TAX
Deferred tax assets consist of:
| | | September 30, 2012 | | | December 31, 2011 | |
| Net operating loss carry forward | | $ | 1,403,162 | | | $ | 830,974 | |
| | | | | | | | | |
| Start-up costs capitalized for tax purposes | | | — | | | | — | |
| | | | | | | | | |
| Gross deferred tax assets | | | 1,403,162 | | | | 830,974 | |
| | | | | | | | | |
| Valuation allowance | | | (1,403,162 | ) | | | (830,974 | ) |
| | | | | | | | | |
| Net deferred tax assets | | $ | — | | | $ | — | |
Realization of deferred tax assets is dependent upon sufficient future taxable income during the period that deductible temporary differences and net operating loss carry forwards are expected to be available to reduce taxable income. As the achievement of required future taxable income is uncertain, the Company recorded a full valuation allowance.
The difference between the statutory tax rate of 35% and the effective tax rate of 0% is due to the valuation allowance for deferred income tax assets.
NOTE 13. SUBSEQUENT EVENTS
In 2009, the FASB ASC Topic 865 (formerly FASB 165, Subsequent Events), which defines the period after the balance sheet date that subsequent events should be evaluated and provides guidance in determining if the event should be reflected in the current financial statements. This ASC Topic also requires disclosure regarding the date through which subsequent events have been evaluated.
The Company has evaluated events and transactions that occurred between September 30, 2012, and the date the financial statements were available for issue, for possible disclosure or recognition in the financial statements. The Company has determined that there were no such events or transactions that warrant disclosure or recognition in the financial statements except as noted below.
On November 1, 2012, the Company and the Company’s shareholders (the “PRLX Shareholders”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Endeavor Power Group, a Nevada Corporation (“EDVP”) and its wholly owned subsidiary Endeavor Holdings, Inc. (“Endeavor Holdings”) whereby Endeavor Holdings acquired 24,870,000 shares of the Company’s common stock (100%) (the “PRLX Stock”) from the PRLX Shareholders. In exchange for the PRLX Stock, EDVP issued 90,375,750 shares of its common stock to the PRLX Shareholders. The 90,375,750 shares, issued at par value $.0001, representing approximately 60% of EDVP’s total issued and outstanding shares. The Common Stock Purchase Agreement, and subsequent transaction closing, was completed on October 22, 2012. On October 27, 2012, the Common Stock Purchase Agreement was finalized, and a Change in Control of EDVP took place.
As a result of the transactions effected by the Merger Agreement, (i) the former business of Parallax is now EDVP’s primary business and (ii) there is a change of control whereby the former shareholders of Parallax, will own a controlling 60% ownership interest in EDVP on a fully diluted basis.
During the period October 1, 2012 through November 14, 2012, the Company increased its loans from related parties by $22,115, from a total of $313,380 at September 30, 2012 to $335,495 at November 14, 2012. The increase represents accrued compensation owed to related parties in the amount of $22,115.
FOR PARALLAX DIAGNOSTICS, INC.
AS OF AND FOR THE YEARS ENDED DECEMBER 31, 2011 AND 2010
Stan J.H. Lee, CPA
2160 North Central Rd. Suite 209 *Fort Lee * NJ 07024-7547
P.O. Box 436402 * San Diego * CA 92143-6402
619-623-7799 * Fax 619-564-3408 * E-mail) stan2u@gmail.com
To the Management and Members of
PARALLAX DIAGNOSTICS INC.
We have audited the accompanying balance sheets of PARALLAX DIAGNOSTICS, INC. (the “Company”) as of December 31, 2011 and 2010, the related statements of operations, stockholders’ deficit and cash flows for the years then ended, and the cash flows for period beginning April 12, 2010 (inception) to December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of PARALLAX DIAGNOSTICS INC. as of December 31, 2011 and 2010, and the results of its operations and its cash flows for the period aforementioned in conformity with accounting principles generally accepted in the United States of America.
The financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in the note to the financial statements, the Company has not established any source of revenue to cover its operating costs and losses from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ Stan J.H. Lee, CPA | |
| Stan J.H. Lee, CPA | |
| March 27, 2012 | |
| PARALLAX DIAGNOSTICS, INC. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| DECEMBER 31, 2011 AND 2010 |
| | | 2011 | | | 2010 | |
| ASSETS | | | | | | |
| Current Assets | | | | | | |
| Cash & cash equivalents | | $ | 136,066 | | | $ | 3,600 | |
| Total Current Assets | | | 136,066 | | | | 3,600 | |
| | | | | | | | | |
| Property, Plant & Equipment, net | | | 41,114 | | | | — | |
| | | | | | | | | |
| Intangible Assets, net of amortization | | | 1,370,490 | | | | — | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 1,547,670 | | | $ | 3,600 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 104,415 | | | $ | 26,402 | |
| Related party payables | | | 152,361 | | | | — | |
| Related party loans | | | 14,000 | | | | 1,450 | |
| Notes & loans payable | | | 20,000 | | | | — | |
| Total Current Liabilities | | | 290,776 | | | | 27,852 | |
| | | | | | | | | |
| Deferred Revenue | | | 1,500,000 | | | | — | |
| | | | | | | | | |
| Total Liabilities | | | 1,790,776 | | | | 27,852 | |
| | | | | | | | | |
| STOCKHOLDERS’ (DEFICIT) | | | | | | | | |
Preferred Stock, $0.0001 par value, 100,000,000 shares authorized, 220,000 and none issued and outstanding as of December 31, 2011 and December 31, 2010, respectively | | | 22 | | | | — | |
Common stock, $0.0001 par value, 400,000,000 shares authorized, 28,020,000 and 35,000,000 issued and outstanding as of December 31, 2011 and December 31, 2010, respectively | | | 2,802 | | | | 3,500 | |
| Additional paid in capital - Preferred | | | 399,978 | | | | — | |
| Additional paid in capital - Common | | | 227,456 | | | | | |
| Subscriptions receivable | | | (20 | ) | | | | |
| Accumulated Deficit | | | (873,345 | ) | | | (27,752 | ) |
| Total Stockholders’ (Deficit) | | | (243,106 | ) | | | (24,252 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | $ | 1,547,670 | | | $ | 3,600 | |
See Notes to Financial Statements
| PARALLAX DIAGNOSTICS, INC. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | | | | | | | Cumulative From | |
| | | For the 12 | | | For the 9 | | | April 12, 2010 | |
| | | Months ended | | | Months ended | | | (Inception) to | |
| | | December 31, 2011 | | | December 31, 2010 | | | December 31, 2011 | |
| | | | | | | | | | |
| General and administrative expenses | | $ | 428,956 | | | $ | 27,752 | | | $ | 456,708 | |
| | | | | | | | | | | | | |
| Operating (loss) | | | (428,956 | ) | | | (27,752 | ) | | | (456,708 | ) |
| | | | | | | | | | | | | |
| Other Expenses | | | | | | | | | | | | |
| Depreciation & amortization | | | 135,289 | | | | — | | | | 135,289 | |
| Amortization of deferred compensation | | | 238,880 | | | | — | | | | 238,880 | |
| Interest expense | | | 98 | | | | | | | | 98 | |
| Total Other Expenses | | | 374,267 | | | | — | | | | 374,267 | |
| | | | | | | | | | | | | |
| Provision for income taxes | | | — | | | | — | | | | | |
| | | | | | | | | | | | | |
| Net (loss) | | $ | (803,223 | ) | | $ | (27,752 | ) | | $ | (830,975 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Net (loss) per common share - basic and diluted | | $ | (0.03 | ) | | $ | (0.00 | ) | | $ | — | |
| | | | | | | | | | | | | |
| Weighted average common shares outstanding - basic and diluted | | | 31,018,462 | | | | 35,000,000 | | | | 35,000,000 | |
See Notes to the Financial Statements
| PARALLAX DIAGNOSTICS, INC. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| | | | Cumulative From | |
| | | | | | | | | April 12, 2010 | |
| | | For the 12 months ended | | | For the 9 months ended | | | (Inception) to | |
| | | December 31, 2011 | | | December 31, 2010 | | | December 31, 2011 | |
| | | | | | | | | | |
| Cash Flow from operations: | | $ | (803,223 | ) | | $ | (27,752 | ) | | $ | (830,975 | ) |
| Net loss | | | | | | | | | | | | |
| Depreciation | | | 10,279 | | | | — | | | | 10,279 | |
| Amortization of intangible assets | | | 125,010 | | | | — | | | | 125,010 | |
| Amortization of deferred compensation | | | 238,880 | | | | | | | | 238,880 | |
Adjustments to reconcile net (loss) to net cash (used in) operating activities: | | | | | | | | | | | | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Increase in accounts payable and accrued expenses | | | 78,013 | | | | 26,402 | | | | 104,415 | |
| Increase in expenses payable to related parties | | | 152,361 | | | | — | | | | 152,361 | |
| Net cash (used in) operating activities | | | (198,680 | ) | | | (1,350 | ) | | | (200,030 | ) |
| | | | | | | | | | | | | |
| Cash Flow from investing activities: | | | | | | | | | | | | |
| (Increase) in Property, Plant & Equipment | | | (51,393 | ) | | | — | | | | (51,393 | ) |
| (Increase) in Intangible Assets | | | (1,500,000 | ) | | | — | | | | (1,500,000 | ) |
| | | | (1,551,393 | ) | | | — | | | | (1,551,393 | ) |
| Cash Flow from financing activities: | | | | | | | | | | | | |
| Proceeds from related party loans | | | 14,000 | | | | 1,450 | | | | 15,450 | |
| (Repayment) of related party loans | | | (1,450 | ) | | | — | | | | (1,450 | ) |
| Proceeds from loans payable | | | 24,500 | | | | — | | | | 24,500 | |
| Increase in Deferred Revenue | | | 1,500,000 | | | | — | | | | 1,500,000 | |
| (Increase) in Deferred Compensation | | | (281,250 | ) | | | — | | | | (281,250 | ) |
| Increase (Decrease) in Capital Stock due to Merger | | | (700 | ) | | | 3,500 | | | | 2,800 | |
| Increase in Additional Paid In Capital due to Merger | | | 227,456 | | | | — | | | | 227,456 | |
| Issuance of Preferred Stock | | | 22 | | | | — | | | | 22 | |
| Issuance of Common Stock | | | 2 | | | | — | | | | 2 | |
| Increase in Preferred Paid In Capital due to stock issuance | | | 399,978 | | | | — | | | | 399,978 | |
| Net cash provided by financing activities | | | 1,882,539 | | | | 4,950 | | | | 1,887,489 | |
| | | | | | | | | | | | | |
| Increase in cash | | | 132,466 | | | | 3,600 | | | | 136,066 | |
| | | | | | | | | | | | | |
| Cash - beginning of period | | | 3,600 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Cash - end of period | | $ | 136,066 | | | $ | 3,600 | | | $ | 136,066 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| NONCASH ACTIVITIES | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | |
| | | | | | | | | | | | | |
| Interest paid | | $ | — | | | $ | — | | | $ | — | |
| Income taxes paid | | $ | — | | | $ | — | | | $ | — | |
See Notes to the Financial Statements
| PARALLAX DIAGNOSTICS, INC. |
| (A DEVELOPMENT STAGE COMPANY) |
|
| PERIOD FROM APRIL 12, 2010 (INCEPTION) TO DECEMBER 31,2011 |
| | | | | | | | | | | | | | | | | | | | | (DEFICIT) | | | | |
| | | | | | | | | | | | | | | | | | | | | ACCUMULATED | | | | |
| | | | | | | | | | | | | | | | | | | | | DURING THE | | | | |
| | | COMMON/PREFERRED STOCK | | | PAID IN CAPITAL | | | SUBSCRIPTIONS | | | DEFERRED | | | EXPLORATION | | | | |
| | | SHARES | | | AMOUNT | | | PREFERRED | | | COMMON | | | RECEIVABLE | | | COMP | | | STAGE | | | TOTAL | |
| Balance, April 12, 2010 (date of inception) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash , April 15, 2010 | | | 35,000,000 | | | | 3,500 | | | | | | | | | | | | | | | | | | | | — | | | | 3,500 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | | | | | | | | | | | | | | | | | (27,752 | ) | | | (27,752 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2010 | | | 35,000,000 | | | | 3,500 | | | | | | | | — | | | | | | | | | | | | (27,752 | ) | | | (24,252 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock, January 11, 2011 | | | 21,000,000 | | | | 2,100 | | | | | | | | (2,100 | ) | | | | | | | | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subscription Payment | | | | | | | | | | | | | | | 125 | | | | | | | | | | | | — | | | | 125 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock Cancellation | | | (28,000,000 | ) | | | (2,800 | ) | | | | | | | 2,800 | | | | | | | | | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accounting for Parallax / ABC Merger | | | | | | | | | | | | | | | (54,637 | ) | | | | | | | | | | | | | | | (54,637 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of Stock Options | | | | | | | | | | | | | | | 281,250 | | | | | | | | (182,985 | ) | | | (98,265 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (96,986 | ) | | | (96,986 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, March 31, 2011 | | | 28,000,000 | | | | 2,800 | | | | — | | | | 227,438 | | | | | | | | (182,985 | ) | | | (223,003 | ) | | | (175,750 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (159,322 | ) | | | (159,322 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of stock Options | | | | | | | | | | | | | | | | | | | | | | | 58,329 | | | | (58,329 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of preferred stock | | | 100,000 | | | | 10 | | | | 99,990 | | | | | | | | | | | | | | | | | | | | 100,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of preferred stock | | | 100,000 | | | | 10 | | | | 99,990 | | | | | | | | (50,000 | ) | | | | | | | | | | | 50,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2011 | | | 28,200,000 | | | | 2,820 | | | | 199,980 | | | | 227,438 | | | | (50,000 | ) | | | (124,656 | ) | | | (440,654 | ) | | | (185,072 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (161,635 | ) | | | (161,635 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of stock options | | | | | | | | | | | | | | | | | | | | | | | 58,331 | | | | (58,331 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of preferred stock | | | 10,000 | | | | 1 | | | | 99,999 | | | | | | | | | | | | | | | | | | | | 100,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subscriptions Received | | | | | | | | | | | | | | | | | | | 50,000 | | | | | | | | | | | | 50,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, September 30, 2011 | | | 28,210,000 | | | | 2,821 | | | | 299,979 | | | | (227,438 | ) | | | — | | | | (66,325 | ) | | | (660,620 | ) | | | (196,707 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (146,400 | ) | | | (146,399 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of stock options | | | | | | | | | | | | | | | | | | | | | | | 23,955 | | | | (23,955 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of preferred stock | | | 10,000 | | | | 1 | | | | 99,999 | | | | | | | | | | | | | | | | | | | | 100,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for cash | | | 20,000 | | | | 2 | | | | | | | | 18 | | | | (20 | ) | | | | | | | | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2011 | | | 28,240,000 | | | $ | 2,824 | | | $ | 399,978 | | | $ | (227,456 | ) | | $ | (20 | ) | | $ | (42,370 | ) | | $ | (830,975 | ) | | $ | (243,106 | ) |
See Notes to Financial Statements
PARALLAX DIAGNOSTICS, INC.
(A Development Stage Company)
December 31, 2010 and 2011
NOTE 1. OVERVIEW
Parallax Diagnostics, Inc.(the “Company”) was incorporated on April 12, 2010 in the state of Nevada. The Company was formed to serve as a vehicle to effect an asset acquisition, merger, exchange of capital stock or other business combination with a domestic or foreign On January 11, 2011 (the “Closing Date”), the Company entered into and closed a share exchange agreement (the “Share Exchange Agreement”) with Amersey Investments, LLC (“Amersey”), Parallax Diagnostics, Inc., a Delaware corporation (“Parallax”) and its sole shareholder, Montecito Bio Sciences, Ltd. (“Montecito”). On the Closing Date, pursuant to the terms and conditions of the Share Exchange Agreement, (i) the Company acquired 100% of the issued and outstanding shares of common stock of Parallax in exchange for the issuance of 21,000,000 shares of our common stock, par value $0.0001 and (ii) Parallax merged with and into the Company whereupon the Company continued as the surviving entity and the corporate existence of Parallax ceased (the “Merger”). Additionally, as further consideration for the share exchange and Merger and in accordance with the Shares Exchange Agreement, Amersey cancelled to treasury 28,000,000 shares of the Company’s common stock.
business.
As a result of the transactions effected by the Share Exchange Agreement, (i) the former business of Parallax is now the Company’s sole business and (ii) there is a change of control whereby the former shareholder of Parallax, Montecito, will now own a controlling 75% ownership interest in the Company. In addition, the Company changed its name to “Parallax Diagnostics, Inc.”
As a further condition of the Share Exchange Agreement, the current officers and directors of the Company resigned and J. Michael Redmond was appointed to serve as a Director and also as the CEO and President of the Company. Additionally, Mr. Norman A. Kunin was appointed to serve as the Company’s CFO, Mr. Mike Contarino was appointed to serve as the Company’s Vice President and Dr. Roger Morris was appointed to serve as the Company’s Chief Science Officer. Mr. Edward W. Withrow III, Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
At December 31, 2011 the Company has not yet commenced material operations. All activity from April 12, 2010 (date of inception) through December 31, 2011 relates to the Company’s formation and development of our business plan.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies is presented to assist in understanding the Company’s financial statements. These accounting policies conform to accounting principles, generally accepted in the United States of America, and have been consistently applied in the preparation of the financial statements.
Development Stage Company
The Company is a development stage company as defined by ASC 915-10-05, “Development Stage Entity”. The Company is still devoting substantially all of its efforts on establishing the business and its planned principal operations have not commenced. All losses accumulated, since inception, have been considered as part of the Company’s development stage activities.
Use of Estimates
The preparation of financial statements, in conformity with generally accepted accounting principles in the United States of America, requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, disclosures of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
Fiscal Year End
The Company has a fiscal year ending on December 31.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents.
Property, Plant and Equipment
Property and equipment are carried at the cost of acquisition or construction and depreciated over the estimated useful lives of the assets. Costs associated with repairs and maintenance are expensed as incurred. Costs associated with improvements which extend the life, increase the capacity or improve the efficiency of our property and equipment are capitalized and depreciated over the remaining life of the related asset. Gains and losses on dispositions of equipment are reflected in operations. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which are 5 to 7 years.
Fair Value of Financial Instruments
The carrying amounts reported in the balance sheet for cash, accounts payable and accrued expenses approximate fair value based on the short-term maturity of these instruments.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC 740-10, “Accounting for Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year; and, (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of available positive and negative evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
ASC 740-10 prescribes a recognition threshold and measurement attribute for the financial statement recognition of a tax position taken or expected to be taken on a tax return. Under ASC 740-10, a tax benefit from an uncertain tax position taken or expected to be taken may be recognized only if it is “more likely than not” that the position is sustainable upon examination, based on its technical merits. The tax benefit of a qualifying position under ASC 740-10 would equal the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all the relevant information. A liability (including interest and penalties, if applicable) is established to the extent a current benefit has been recognized on a tax return for matters that are considered contingent upon the outcome of an uncertain tax position. Related interest and penalties, if any, are included as components of income tax expense and income taxes payable.
Earnings (Loss) per Share
Basic earnings (loss) per share is computed by dividing net income, or loss, by the weighted average number of shares of common stock outstanding for the period. Diluted earnings (loss) per share is computed by dividing net income, or loss, by the weighted average number of shares of both common and preferred stock outstanding for the period.
New Accounting Standards
Management does not believe that any recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on the accompanying financial statements.
NOTE 3. PROPERTY, PLANT AND EQUIPMENT
Property, Plant and Equipment consists of the following:
| | | December 31, | |
| | | 2011 | | | 2010 | |
| Office Equipment | | $ | 6,199 | | | $ | — | |
| Medical Devices & Instruments | | | 45,194 | | | | — | |
| Sub-Total | | | 51,393 | | | | — | |
| Accumulated Deprecation | | | (10,279 | ) | | | — | |
| Property Plant & Equipment, Net | | $ | 41,114 | | | $ | — | |
Depreciation expense totaled $10,279 and none for the years ended December 31, 2011 and 2010, respectively.
NOTE 4. INTANGIBLE ASSETS
Intangible Assets consists of the following:
| | | December 31, | |
| | | 2011 | | | 2010 | |
| Products & Processes | | $ | 750,000 | | | $ | — | |
| Trademarks & Patents | | | 750,000 | | | | — | |
| Sub-Total | | | 1,500,000 | | | | — | |
| Accumulated Amortization | | | (125,010 | ) | | | — | |
| Intangible Assets, net | | $ | 1,374,990 | | | $ | — | |
Amortization expense totaled $125,010 and none for the years ended December 31, 2011 and 2010, respectively.
NOTE 5. RELATED PARTY PAYABLE
As at December 31, 2011, affiliates and related parties are due a total of $166,361 which is comprised of $14,000 cash loans, $143,019 of accrued compensation to officers of the Company and $9,342 due to related parties for reimbursable expenses.
NOTE 6. DEFERRED REVENUE
On September 30, 2011, the Company entered into Modification Agreements with Montecito Bio Sciences, Ltd. (“Montecito”) regarding the Agreement of the Assignment of Intellectual Property (the “Assignment Agreement”) and the Assignment of the License of Intellectual Property (the “License Agreement”), both Agreements having been entered into on September 10, 2010. The nature of the Modifications to the Assignment Agreement and the License Agreement were to increase the royalty amounts (“Royalties”) due to Montecito from Four Percent (4%) to Five Percent (5%) and from Three and One Half Percent (3 ½%) to Four and One Half Percent (4 1/2%), respectively. The Assignment Agreement Royalties shall revert back to Four Percent (4%) after the Company has paid Montecito Seven Hundred Fifty Thousand Dollars ($750,000) of royalty revenue and the License Agreement Royalties shall revert back to Three and One Half Percent (3 ½%) after the Company has paid Montecito Seven Hundred Fifty Thousand Dollars ($750,000) of royalty revenue. As of December 31, 2011, the Company has recorded Deferred Revenue in the amount of $1,500,000.
NOTE 7. COMMITMENTS AND CONTINGENCIES
On July 1, 2011 the Company entered into a Development and Supply Agreement with Corder Engineering, LLC. The Statement of Work stipulates that Corder Engineering, LLC shall provide ten (10) Evaluation Units which replicate the functionality Target. Target 1000 firmware ver. 320 and add software for a C-reactive protein (CRP) quantitative assay. The total payment under the Agreement stipulates $35,000 over a twelve week period. As of December 31, 2011, payments totaling $17,500 have been made, and $17,500 has been accrued.
On July 1, 2011 the Company entered into a Supply Agreement with Meyers Stevens Group, Inc. ("Meyers Stevens"). The Statement of Work stipulates that Meyers Stevens will manufacture assays and supply a Data Package for the Company and will yield approximately 100 to 200 fully functional assay test devices for internal investigational use. Estimated delivery of the assays is eight (8) weeks from the date of the Agreement for a total cost of $10,194. As of December 31, 2011, payments totaling $8,980 have been made, and $1,214 has been accrued.
NOTE 8. PREFERRED STOCK
The total number of authorized shares of preferred stock that may be issued by the Company is 100,000,000 with a par value of $0.0001 per share.
On June 17, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Hamburg Investment Company, LLC ("HIC"), whereby 100,000 shares of Preferred Stock would be issued to HIC for a purchase price of $1.00 per share, or $100,000.00. In connection with the issuance of Preferred Stock, the Company issued a warrant to convert 100% of HIC’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On June 17, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), whereby 100,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $1.00 per share, or $100,000.00. In connection with the issuance of Preferred Stock, the Company issued a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $1.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On September 30, 2011, the Company entered into a Convertible Preferred Purchase Agreement with Huntington Chase Financial Group LLC ("HCFG"), whereby 10,000 shares of Preferred Stock would be issued to HCFG for a purchase price of $10.00 per share, or $100,000.00. In connection with the issuance of Preferred Stock, the Company issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of HCFG’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
On December 6, 2011, the Company entered into a Convertible Preferred Purchase Agreement with David Engert, ("Engert") whereby 10,000 shares of Preferred Stock would be issued to Engert for a purchase price of $10.00 per share, or $100,000.00. In connection with the issuance of Preferred Stock, the Company issued 200,000 warrants to purchase Common Stock of the Company, with a strike price of $1.50 per share for 24 months, and a warrant to convert 100% of Engert’s shares of Preferred Stock to shares of Common Stock at an exercise price of $10.00 per share. The number of shares of common stock underlying the warrant and the exercise price are subject to adjustment within six months if the Company has reached certain financing levels.
As at December 31, 2011, 220,000 shares of preferred stock are issued and outstanding.
NOTE 9. COMMON STOCK
The total number of authorized shares of common stock that may be issued by the Company is 400,000,000 with a par value of $0.0001 per share.
On April 15, 2010, the Company issued 35,000,000 common shares for cash at a price of $.0001
On January 11, 2011, pursuant to the Share Exchange Agreement, Amersey Investments, LLC cancelled to treasury 28,000,000 shares of the Company's common stock
During January, 2011, the Company issued 21,000,000 common shares as a result of the merger.
.
During January, 2011 the Company issued 125,000 common shares for cash at a price of $.001 per share.
As at December 31, 2011 the Company has 28,000,000 common shares issued and outstanding.
NOTE 10. EQUITY COMPENSATION PLAN
On October 1, 2010, the board of directors adopted the Company's Stock Option Plan. The Company has reserved 3,000,000 shares of common stock for issuance upon exercise of options granted from time to time under the stock option plan. The stock option plan is intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. Under the stock option plan, the Company may grant incentive stock options only to key employees and employee directors, or the Company may grant non-qualified options to employees, officers, directors and consultants. The stock option plan is currently administered by the Company's board of directors. Subject to the provisions of the stock option plan, the board will determine who shall receive options, the number of shares of common stock that may be purchased under the options. As at December 31, 2011, the Company has granted options to purchase 1,950,000 shares. In connection with the options granted, a total of $281,250 has been recorded as deferred compensation, and is being amortized over a 12-18 month vesting period. During the year 2011, the Company recognized $238,880 in amortization.
NOTE 11. INCOME TAX
Deferred tax assets consist of:
| | | 2011 | | | 2010 | |
| Net operating loss carry forward | | $ | 830,975 | | | $ | 27,752 | |
| Start-up costs capitalized for tax purposes | | | — | | | | — | |
| Gross deferred tax assets | | | 830,975 | | | | 27,752 | |
| Valuation allowance | | | (830,975 | ) | | | (27,752 | ) |
| Net deferred tax assets | | $ | — | | | $ | — | |
Realization of deferred tax assets is dependent upon sufficient future taxable income during the period that deductible temporary differences and net operating loss carry forwards are expected to be available to reduce taxable income. As the achievement of required future taxable income is uncertain, the Company recorded a full valuation allowance
The difference between the statutory tax rate of 35% and the effective tax rate of 0% is due to the valuation allowance for deferred income tax assets.
NOTE 12. SUBSEQUENT EVENTS
In 2009, the FASB ASC Topic 865 (formerly FASB 165, Subsequent Events) , which defines the period after the balance sheet date that subsequent events should be evaluated and provides guidance in determining if the event should be reflected in the current financial statements. This ASC Topic also requires disclosure regarding the date through which subsequent events have been evaluated.
On January 2, 2012 the Company entered into a consulting agreement with Huntington Chase Financial Group LLC (“HCFG”), a Nevada corporation. The consulting agreement provides for HCFG to provide advisory services to the Company for a period of three years for a fee of $12,500 per month.
During the period January 1, 2012 through March 31, 2012, the Company increased its loans from related parties by $13,000, from a total of $166,361 at December 31, 2011 to $179,361 at March 8, 2012. The increase represents accrued compensation owed to related parties in the amount of $13,000.
* * *
EXHIBITS
Exhibit Number | | Description of Exhibit | | Filing Reference |
| (2) | | Plan of Purchase, Sale, Reorganization, Arrangement, Liquidation or Succession | | |
| 2.1* | | | | Filed herewith |
| 2.2* | | | | Filed herewith |
| (3) | | Articles of Incorporation and Bylaws | | |
| 3.1 | | Articles of Incorporation | | Filed with the SEC on March 5, 2007 as part of our Registration Statement on Form SB-2. |
| 3.1(a) | | Amended and Restated Articles of Incorporation | | Filed with the SEC on May 17, 2010 as part of our Annual Report on Form 10-K. |
| 3.2 | | Bylaws | | Filed with the SEC on March 5, 2007 as part of our Registration Statement on Form SB-2. |
| 3.2(a) | | Amended Bylaws | | Filed with the SEC on May 17, 2010 as part of our Annual Report on Form 10-K. |
| 3.3* | | | | Filed herewith |
| (4) | | Instruments Defining the Rights of Security Holders | | |
| | | | | |
| 4.1 | | 2011 Equity Incentive Plan dated March 26, 2011 | | Filed with the SEC on March 31, 2011 as part of our Current Report on Form 8-K. |
| 4.2 | | Sample Stock Option Agreement | | Filed with the SEC on March 31, 2011 as part of our Current Report on Form 8-K. |
| 4.3 | | Sample Stock Award Agreement for Stock Units | | Filed with the SEC on March 31, 2011 as part of our Current Report on Form 8-K. |
| 4.4 | | Sample Stock Award Agreement for Restricted Stock | | Filed with the SEC on March 31, 2011 as part of our Current Report on Form 8-K. |
| 4.5* | | | | Filed herewith |
| 4.6* | | Sample Stock Option Plan | | |
| (10) | | Material Contracts | | |
| | | | | |
| 10.1 | | Second Amendment to Joint Venture Agreement between the Company and Federated Energy Corporation dated September 15, 2009 | | Filed with the SEC on September 19, 2009 as part of our Current Report on Form 8-K. |
| 10.2 | | Farmount Agreement between the Company and Togs Energy, Inc. and M-C Production & Drilling Co, Inc. dated July 21, 2009 | | Filed with the SEC on July 23, 2009 as part of our Current Report on Form 8-K. |
| 10.3 | | Convertible Promissory Note to Regal Capital Development, Inc. dated August 25, 2009 | | Filed with the SEC on September 4, 2009 as part of our Current Report on Form 8-K. |
| 10.4 | | Common Stock Purchase Warrant to Regal Capital Development, Inc. dated August 25, 2009 | | Filed with the SEC on September 4, 2009 as part of our Current Report on Form 8-K. |
| 10.5 | | Settlement Agreement between the Company and Regal Capital Development, Inc. dated September 11, 2010 | | Filed with the SEC on July 12, 2010 as part of our Current Report on Form 8-K. |
| 10.6 | | Promissory Note to Regal Capital Development, Inc. dated September 11, 2010 | | Filed with the SEC on July 12, 2010 as part of our Current Report on Form 8-K. |
| 10.7 | | Amended Promissory Note to Regal Capital Development, Inc. dated September 11, 2010 | | Filed with the SEC on April 14, 2011 as part of our Annual Report on Form 10-K. |
| 10.8 | | Settlement Agreement between the Company and Andrew I. Telsey, P.C., dated August 3, 2010. | | Filed with the SEC on August 22, 2011 as part of our Quarterly Report on Form 10-Q. |
| 10.9 | | Settlement Agreement between the Company and Regal Capital Development, Inc. dated September 17, 2010 | | Filed with the SEC on October 21, 2010 as part of our Current Report on Form 8-K. |
| 10.10 | | Promissory Note to Regal Capital Development, Inc. dated September 17, 2010 | | Filed with the SEC on October 21, 2010 as part of our Current Report on Form 8-K. |
| 10.11 | | Employment Agreement between the Company and Alfonso Knoll dated November 8, 2010. | | Filed with the SEC on November 12, 2010 as part of our Current Report on Form 8-K. |
| 10.12 | | Promissory Note to Regal Capital Development, Inc. dated November 23, 2010. | | Filed with the SEC on November 30, 2010 as part of our Current Report on Form 8-K. |
| 10.13 | | Amendment to Employment Agreement between the Company and Alfonso Knoll dated November 17, 2010 | | Filed with the SEC on November 30, 2010 as part of our Current Report on Form 8-K. |
| 10.14 | | Consulting Agreement between the Company and The Musser Group, LLC dated February 21, 2011 | | Filed with the SEC on February 25, 2011 as part of our Current Report on Form 8-K. |
| 10.15 | | Promissory Note to Marans Invest & Finance S.A. dated April 8, 2011 | | Filed with the SEC on August 22, 2011 as part of our Quarterly Report on Form 10-Q. |
| 10.16 | | Promissory Note to Rast Trade Corp. dated April 21, 2011 | | Filed with the SEC on August 22, 2011 as part of our Quarterly Report on Form 10-Q. |
| 10.17 | | Settlement Agreement between the Company and Mr. Alfonso Knoll dated June 8, 2011 | | Filed with the SEC on June 16, 2011 as part of our Current Report on Form 8-K. |
| 10.18 | | Settlement Agreement between the Company and The Musser Group, LLC dated July 19, 2011 | | Filed with the SEC on August 22, 2011 as part of our Quarterly Report on Form 10-Q. |
| 10.19* | | | | Filed herewith |
| 10.20* | | | | Filed herewith |
| 10.21* | | | | Filed herewith |
| 10.22* | | | | Filed herewith |
| 10.23* | | | | Filed herewith |
| 10.24* | | | | Filed herewith |
| 10.25* | | | | Filed herewith |
| 10.26* | | | | Filed herewith |
| 10.27* | | | | Filed herewith |
| 10.28* | | | | Filed herewith |
| 10.29* | | | | Filed herewith |
| 10.30* | | | | Filed herewith |
| | | | | |
| (14) | | Code of Ethics | | |
| 14.1 | | Code of Ethics | | Filed with the SEC on April 14, 2011 as part of our Annual Report on Form 10-K. |
| (16) | | Letter Re Change in Certifying Accountant | | |
| 16.1 | | Letter from Moore and Associates, Chartered dated August 13, 2009 | | Filed with the SEC on August 13, 2009 as part of our Current Report on Form 8-K. |
| 16.2 | | Letter from Seale & Beers, CPAs dated August 26, 2009 | | Filed with the SEC on August 27, 2009 as part of our Current Report on Form 8-K. |
| 16.3 | | Letter from M&K CPAs, PLLC dated March 12, 2010 | | Filed with the SEC on March 12, 2010 as part of our Current Report on Form 8-K. |
| 16.4 | | Letter from Ron Chadwick, P.C. dated August 3, 2010 | | Filed with the SEC on August 4, 2010 as part of our Current Report on Form 8-K. |
| 16.5 | | Letter from Davis Accounting Group, P.C. dated November 29, 2010 | | Filed with the SEC on November 30, 2010 as part of our Current Report on Form 8-K. |
| 16.6 | | Letter from M&K CPAs, PLLC dated October 23, 2012 | | Filed with the SEC on October 25, 2012 as part of our Current Report on Form 8-K. |
| (23) | | Consent Letters | | |
| 23.1 | | Letter from Stan J.H. Lee dated October 31, 2012 | | Filed with the SEC on November 5, 2012 as part of our Annual Report on Form 10-K. |
| (99) | | Other Documents | | |
| 99.1* | | | | Filed herewith |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Company has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | Endeavor Power Corp. |
| | | |
| Date: November 14, 2012 | /s/ J. Michael Redmond | |
| | By: J. Michael Redmond |
| | Its: President and Chief Executive Officer |