Exhibit 99.01
31 July 2009
Dear Shareholders,
As you will be aware, the US Federal Trade Commission (FTC) confirmed yesterday that it has authorized a lawsuit to block Thoratec Corporation’s proposed $282 million acquisition of HeartWare on the basis that the transaction would substantially reduce competition in the U.S. market for left ventricular devices (LVADs).
Both HeartWare and Thoratec, together with their advisers, are reviewing this development and are working hard with a view to mutually assessing the appropriate next steps. A joint release in this regard is expected shortly from both companies and we will obviously continue to update the market on a timely basis.
In this update I will touch on both some of the headline topics as well as some of the less visible, but perhaps more important, achievements of recent months.
Operations
I would like to start with the “behind-the-scenes” work done by our team over the last couple of months, as I have been particularly pleased by many of these achievements.
Our long term shareholders are keenly aware of the manufacturing capacity challenges that bedeviled us from 2006 into late 2008. As you know, we have applied significant focus since then with a view to establishing a robust and scaleable manufacturing capability. I am delighted to report that these efforts have all but eliminated pump supply from our list of concerns. We will never stop seeking ways to improve our efficiency, quality and cost position but we are now able routinely to ship implant kits within 24 hours. At present, we have approximately 50 pumps in inventory at a time when we have had an increase in pump usage through higher implant volumes and clinical site expansion. More importantly, we now have the ability to reliably and consistently produce 10 pumps per week and have little concern about our ability to increase that level significantly once higher output becomes necessary.
HeartWare International, Inc. | ARBN 132 897 762
Suite 101, 205 Newbury Street, Framingham MA 01701 | Ph: (508) 739 0950 | Fax: (508) 739 0948 | www.heartware.com

Another area that had been cumbersome and frustrating for us was our international logistics capabilities. It was not uncommon that when one of our European sites scheduled an urgent implant, we would arrange for one of our clinical support team to fly to Europe, hand-carry the system to the site and attend the implant in support. We received rave reviews for our dedication to our patients and customers but it was clear that this level of “service” was costly and would not be sustainable in a commercial phase. In anticipation of increasing volumes and in recognition of our need to improve our logistic capabilities, we have partnered with a specialist medical device warehousing and customer service provider in the Netherlands. They are able to interface with our sites and deal with customer calls in multiple languages, bill in local currencies and ship product anywhere in the EU within 24 hours. This has all but eliminated the logistics risks associated with shipping product from the US and the associated export and customs-related complexities without requiring HeartWare to establish any additional infrastructure. We and our customers are delighted with the high caliber, cost effective results we have seen to date.
The final “behind-the-scenes” achievement I wish to discuss occurred earlier in July when BSI, our notified body, returned to refresh the ISO audit that they conducted last year. These audits will occur annually and are important measuring sticks to confirm that our quality system is healthy and that we are complying with the requirements of ISO 13485:2003. As with last year, there were no major findings from this audit. While “on paper” the results were similar to those from last year, subjectively it is clear that we have taken a decided step forward. Our quality systems are now much more mature and are truly embedded across all aspects of what we do. While we work very hard to maintain a culture of innovation and small-company agility, our in-house processes, systems and disciplines are beginning to resemble those of a more established medical device enterprise, providing a robust platform from which to expand activities and minimize risk.
Europe Operations
As you will recall, we received CE Mark in January this year. Our priority for the first three months post approval was to convert our five former international clinical trial sites into commercial “customers”. We have always felt that it was critical both for our own confidence and for broad marketability of the system that those sites which used the device in the trial subsequently purchased the system for use in the course of normal clinical practice. We are delighted to see that the enthusiasm for our system at these sites appears to be even higher now than it was during the trial.
The second phase of our EU rollout plan, which commenced in May, was to selectively expand beyond those five trial centers. We have now trained five additional sites. Of these, two started implanting this month with the first new site having already done four implants and the second site doing its first implant yesterday. An additional site has devices on the shelf and is screening actively for patients. The remaining two are completing the logistical and legal requirements of pricing and contracting in order to begin implanting. We anticipate that at least one if not both of these remaining sites will have devices on the shelf and be ready to implant within the next 30 days.
- 2 -
This expansion of centers and implant numbers will continue through the end of the year and beyond. To date, we have been able to accomplish this with consultants and by leveraging our US staff. This was in part by plan but in part due to the reluctance of potential employees in Europe to join a company that was in the middle of a merger process. The job uncertainty made hiring nearly impossible in the EU until very recently. We anticipate having a small but fully capable team in place in the fourth quarter. This additional infrastructure will enable us to expand our customer base without eroding clinical results at new sites.
US Clinical Trial
While our US trial officially started in August of 2008, it didn’t begin in earnest until this January. At the start of the year, we had 4 implants and we now have 46. At the start of the year, we had 2 centers that had implanted and we now have 12. We had 3 centers with inventory and we now have 16 with a further 5 sites that are within 60 days of starting up. Our trial is capped at 28 sites and we have approximately 10 additional centers competing for the remaining 7 available openings.
Our internal forecasts suggested that we should be close to 50 or 55 implants by now but, at 46 implants to date, we are within close range of our target. We have a high degree of confidence that we remain on track to complete enrollment by April 2010 as initially planned. The swing factor will be the speed with which new sites are able to come on board and make an impact. We are working diligently to help sites complete the logistical procedures such as IRB approval and contract negotiation in order to accelerate these processes as best we can.
Cash Flows
As anticipated, since the start of our US clinical trial and receipt of CE Mark, we have seen a steady growth in revenues. Our revenue for Q1 2009 was US$1.4 million. Our revenue for Q2 2009 exceeded US$3.0 million. It is pleasing to report that July 2009 was our first “million dollar month”, with revenue exceeding US$1.6 million.
This early revenue is helping to reduce our burn rate as planned. Provided that enrollment and site expansion continue on plan, our revenue and bottom line should both start to improve steadily. Granted we will be making some additional resource commitments in order to achieve this ramp, but we plan to expand spending at a much lower rate than we expand revenue.
The last 3 months has seen significant expense incurred in non-recurring costs (e.g. professional fees relating to the Thoratec merger) but we are generally pleased with our ability to control our “core” costs. As revenues continue to build, we expect to see a steady improvement in our cash flows moving forward.
An ASX Appendix 4C quarterly report of cash flows is attached to this letter.
- 3 -
Closing Comments
HeartWare is in excellent shape. Perhaps the most telling pointer to our future prospects is the rate at which clinicians are choosing to implant our system. In 2007 we implanted 20 pumps. In 2008 we implanted 27 pumps. In the first 7 months of 2009 we have implanted over 60 pumps, taking our total implants to over 110 patients. As our implant rate accelerates, so too does our global base of clinical experience which, in turn, helps to further drive implant volumes.
Our clinical results have been encouraging, with a 90% survival rate at 6 months and an 86% survival rate at 12 months in our 50-patient international trial. It remains too early to speak of clinical results in our US experience but there is a growing level of enthusiasm among the expanding group of US clinicians who have experienced our system.
In addition to the above, despite my not making it a focus of this letter, work on our next-generation MVAD platform has generated very exciting progress. We have two variants of the MVAD device currently in pre-clinical studies and we have been very impressed by the results to date. We expect to “pick a winner” between these two by early next year and to progress the MVAD to GLP studies in the first half of 2010.
As always, we thank you for your continued support.
Yours sincerely
Doug Godshall
President and CEO
- 4 -
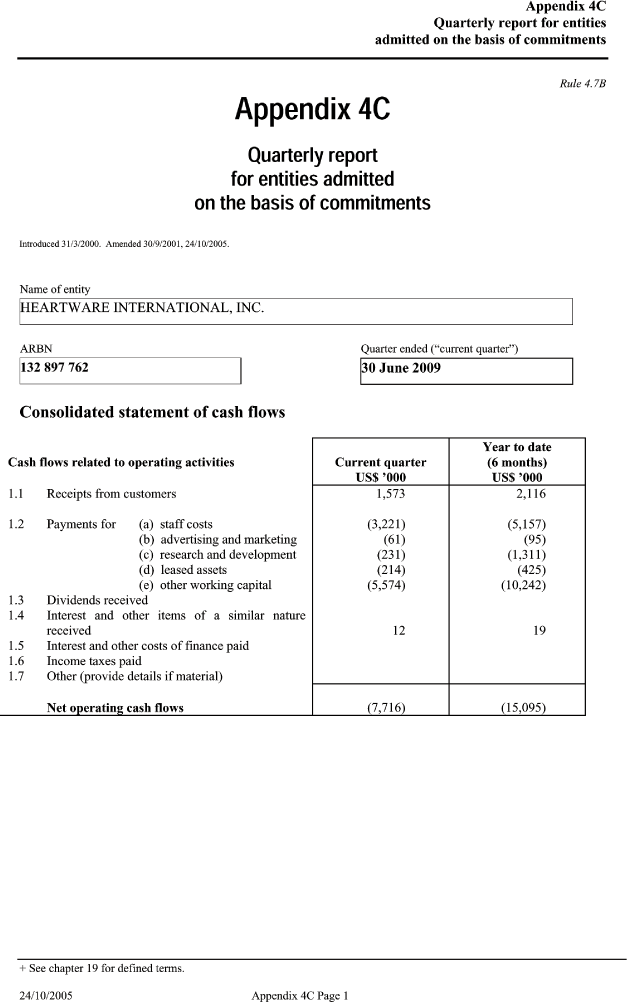
| Appendix 4C Quarterly report for entities admitted on the basis of commitmentsRule 4.7BAppendix 4C Quarterly report for entities admitted on the basis of commitments Introduced 31/3/2000. Amended 30/9/2001, 24/10/2005. Name of entity HEARTWARE INTERNATIONAL, INC. ARBN _____ Quarter ended (“current quarter”) 132 897 762 30 June 2009 Consolidated statement of cash flows _____ Year to date Cash flows related to operating activities Current quarter (6 months) US$ ’000 US$ ’000 1.1 Receipts from customers 1,573 2,116 1.2 Payments for (a) staff costs (3,221) (5,157) (b) advertising and marketing (61) (95) (c) research and development (231) (1,311) (d) leased assets (214) (425) (e) other working capital (5,574) (10,242) 1.3 Dividends received 1.4 Interest and other items of a similar nature _____ received 12 19 1.5 Interest and other costs of finance paid 1.6 Income taxes paid 1.7 Other (provide details if material) Net operating cash flows (7,716) (15,095) + See chapter 19 for defined terms. |
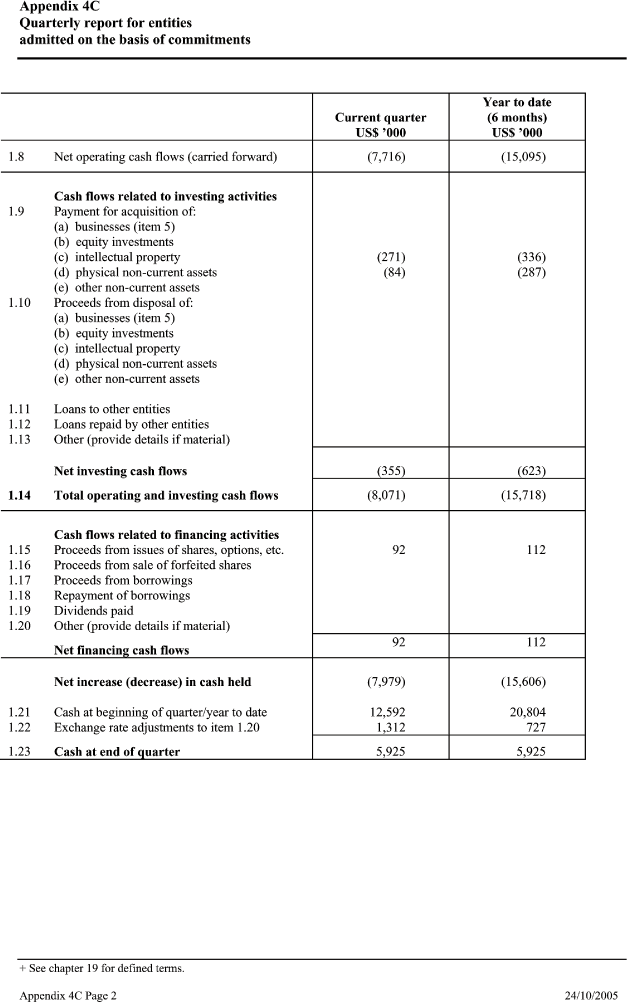
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Year to date Current quarter (6 months) US$ ’000 US$ ’000 1.8 Net operating cash flows (carried forward) (7,716) (15,095) Cash flows related to investing activities 1.9 Payment for acquisition of: (a) businesses (item 5) (b) equity investments (c) intellectual property (271) (336) (d) physical non-current assets (84) (287) (e) other non-current assets 1.10 Proceeds from disposal of: (a) businesses (item 5) (b) equity investments (c) intellectual property (d) physical non-current assets (e) other non-current assets 1.11 Loans to other entities 1.12 Loans repaid by other entities 1.13 Other (provide details if material) Net investing cash flows (355) (623) 1.14 Total operating and investing cash flows (8,071) (15,718) Cash flows related to financing activities 1.15 Proceeds from issues of shares, options, etc. 92 112 1.16 Proceeds from sale of forfeited shares 1.17 Proceeds from borrowings 1.18 Repayment of borrowings 1.19 Dividends paid 1.20 Other (provide details if material) 92 112 Net financing cash flows Net increase (decrease) in cash held (7,979) (15,606) 1.21 Cash at beginning of quarter/year to date 12,592 20,804 1.22 Exchange rate adjustments to item 1.20 1,312 727 1.23 Cash at end of quarter 5,925 5,925 |
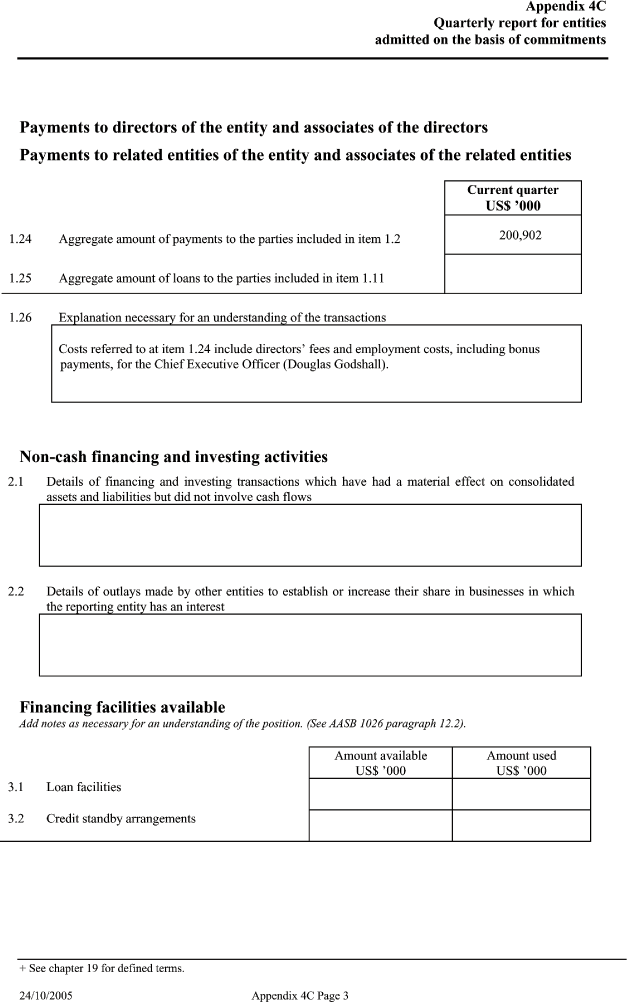
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Payments to directors of the entity and associates of the directors Payments to related entities of the entity and associates of the related entities _____ Current quarter _____ US$ ’000 1.24 Aggregate amount of payments to the parties included in item 1.2 200,902 1.25 Aggregate amount of loans to the parties included in item 1.11 1.26 Explanation necessary for an understanding of the transactions _____ Costs referred to at item 1.24 include directors’ fees and employment costs, including bonus payments, for the Chief Executive Officer (Douglas Godshall). Non-cash financing and investing activities 2.1 Details of financing and investing transactions which have had a material effect on consolidated assets and liabilities but did not involve cash flows 2.2 Details of outlays made by other entities to establish or increase their share in businesses in which the reporting entity has an interest Financing facilities availableAdd notes as necessary for an understanding of the position. (See AASB 1026 paragraph 12.2).Amount available Amount used US$ ’000 US$ ’000 3.1 Loan facilities 3.2 Credit standby arrangements |

| Appendix 4C Quarterly report for entities admitted on the basis of commitments Reconciliation of cash Reconciliation of cash at the end of the quarter (as shown in the consolidated statement of cash flows) to Current quarter Previous quarter the related items in the accounts is as follows. US$ ’000 US$ ’000 4.1 Cash on hand and at bank 1,607 808 4.2 Deposits at call 4,318 11,784 4.3 Bank overdraft 4.4 Other (provide details) Total: cash at end of quarter (item 1.23) 5,925 12,592 Acquisitions and disposals of business entities _____ Acquisitions Disposals(Item 1.9(a)) (Item 1.10(a))5.1 Name of entity 5.2 Place of incorporation _____ or registration 5.3 Consideration for _____ acquisition or disposal 5.4 Total net assets 5.5 Nature of business _____ Compliance statement 1 HeartWare International, Inc. has received financial reporting relief under ASIC class order 98/1418 and as such this statement has been prepared in accordance with United States Generally Accepted Accounting Principles. 2 This statement does give a true and fair view of the matters disclosed. Sign here: Date: 31 July 2009 (Director) Print name: Douglas Godshall |

| Appendix 4C Quarterly report for entities admitted on the basis of commitments Notes 1. The quarterly report provides a basis for informing the market how the entity’s activities have been financed for the past quarter and the effect on its cash position. An entity wanting to disclose additional information is encouraged to do so, in a note or notes attached to this report. 2. The definitions in, and provisions of,AASB 1026: Statement of Cash Flowsapply to this report except for the paragraphs of the Standard set out below. 6.2 — reconciliation of cash flows arising from operating activities to operating profit or loss 9.2 — itemised disclosure relating to acquisitions 9.4 — itemised disclosure relating to disposals 12.1(a) — policy for classification of cash items 12.3 — disclosure of restrictions on use of cash 13.1 — comparative information 3. Accounting Standards. ASX will accept, for example, the use of International Accounting Standards for foreign entities. If the standards used do not address a topic, the Australian standard on that topic (if any) must be complied with. |