|
Exhibit 99.7 
|
HeartWere

HeartWare® Valtech
Advancing Our Technology Leadership in Advanced Heart Failure
Investor and Analyst Conference Call and Webcast
September 1, 2015
HeartWare®
2
Safe Harbor Statement
Forward-Looking Statements
This announcement contains forward-looking statements that are based on management’s beliefs, assumptions
and expectations and on information currently available to management. All statements that address operating
performance, events or developments that we expect or anticipate will occur in the future are forward-looking
statements, including without limitation our expectations with respect to the: commercialization of HeartWare and
Valtech products; timing, progress and outcomes of clinical trials and regulatory approvals; research and
development activities; our ability to integrate, manage and take advantage of acquired and pipeline
technology; and the costs and benefits to be achieved from the Valtech transaction. Management believes that
these forward-looking statements are reasonable as and when made. However, you should not place undue
reliance on forward-looking statements because they speak only as of the date when made. HeartWare does not
assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new
information, future events or otherwise, except as may be required by federal securities laws and the rules and
regulations of the Securities and Exchange Commission. HeartWare may not actually achieve the plans, projections
or expectations disclosed in forward-looking statements, and actual results, developments or events could differ
materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a
number of risks and uncertainties, including without limitation those described in Part I, Item 1A “Risk Factors” in
HeartWare’s Annual Report on Form 10-K filed with the SEC. HeartWare may update risk factors from time to time in
Part II, Item 1A “Risk Factors” in Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, or other filings with the
SEC.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 3
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Additional Shareholder Information
Participants in the Solicitation
HeartWare, Valtech and their respective directors, executive officers, certain members of management and certain employees may be deemed to be participants in the solicitation of proxies in connection with the proposed transaction. A description of the interests in HeartWare of its directors and executive officers is set forth in HeartWare’s proxy statement for its 2015 Annual Meeting of Shareholders, which was filed with the SEC on April 30, 2015. This document is available free of charge at the SEC’s website at www.sec.gov or by going to HeartWare’s Investors page on its corporate website at www.heartware.com. Additional information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of proxies in connection with the proposed transaction, and a description of their direct and indirect interests in the proposed transaction, which may differ from the interests of HeartWare stockholders or Valtech shareholders generally, will be set forth in a proxy statement/prospectus when it is filed with the SEC.
Additional Information and Where You Can Find It
A new holding company will file a Registration Statement on Form S-4 containing a joint proxy statement/prospectus and other documents concerning the proposed transaction with the SEC. Investors are urged to read the joint proxy statement/prospectus when it becomes available and other relevant documents filed with the SEC because they will contain important information. Security holders may obtain a free copy of the proxy statement/prospectus (when it is available) and other documents filed by HeartWare and the new holding company with the SEC on the SEC’s website at www.sec.gov. The joint proxy statement/prospectus and other documents may also be obtained for free by contacting HeartWare Investor Relations by e-mail at investors@heartware.com or by telephone at +1 (508) 739-0864.
List of Participants
Doug Godshall President and CEO, HeartWare International
Peter McAree Senior Vice President and CFO, HeartWare International
Amir Gross Founder and CEO, Valtech Cardio
Prof. Francesco Chair of Cardiovascular Surgery, University Hospital of
Maisano, M.D. Zurich
Prof. Karl-Heinz Head of Cardiology Department, Asklepios Klinik St. Kuck, M.D., Ph.D. Georg, Hamburg, Germany
President and CEO, HeartWare International
DOUG GODSHALL
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 6 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
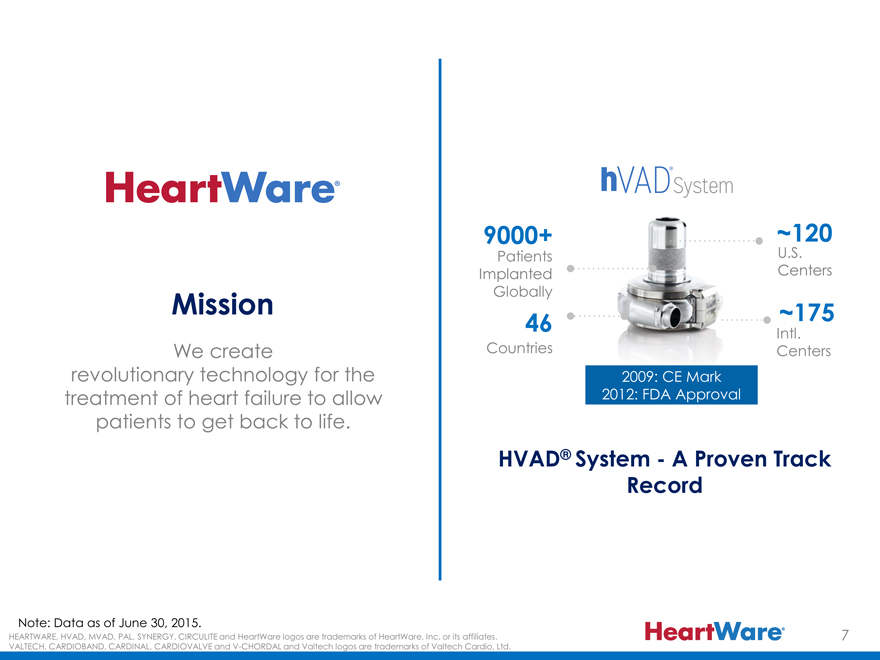
9000+ ~120
Patients U.S.
Implanted Centers
Mission Globally ~175
46 Intl.
We create Countries Centers
revolutionary technology for the 2009: CE Mark
treatment of heart failure to allow 2012: FDA Approval
patients to get back to life.
HVAD® System—A Proven Track
Record
Note: Data as of June 30, 2015.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 7
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare + Valtech: Creating the Technology Leader in
Advanced Heart Failure
Advance Our Mission: Accelerates goal of revolutionizing the treatment of
advanced heart failure patients
Leverage Competitive Differentiation: Adds uniquely comprehensive portfolio
with potential to serve patients across the spectrum of mitral and tricuspid
valve disease
Expand Our Market Opportunity: Opens access to the multi-billion-dollar-
potential mitral and tricuspid valve repair and replacement market
Accelerate Our Growth: Adds near-term revenue opportunity in existing
$250M transcatheter mitral repair market
Deepen Our Team: Combined team of ~700 with 6 first-in-man (FIM)
experiences to execute on mechanical circulatory support and mitral and
tricuspid valve pipeline
Drive Shareholder Value: Provides exceptional opportunity for significant
value creation with risk-mitigating and cash-preserving transaction structure
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 8
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
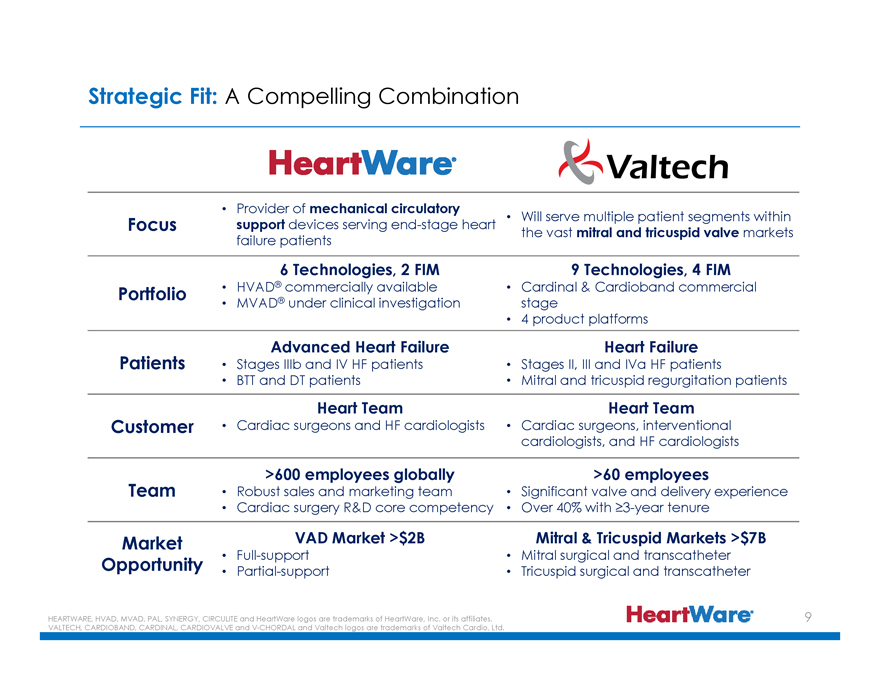
Strategic Fit: A Compelling Combination
HeartWare® Valtech
Focus
Portfolio
Patients
Customer
Team
Market Opportunity
Provider of mechanical circulatory support devices serving end-stage heart failure patients
6 Technologies, 2 FIM
HVAD® commercially available
MVAD® under clinical investigation
Advanced Heart Failure
Stages IIIb and IV HF patients
BTT and DT patients
Heart Team
Cardiac surgeons and HF cardiologists
>600 employees globally
Robust sales and marketing team
Cardiac surgery R&D core competency
VAD Market >$2B
Full-support
Partial-support
Will serve multiple patient segments within the vast mitral and tricuspid valve markets
9 Technologies, 4 FIM
Cardinal & Cardioband commercial stage
4 product platforms
Heart Failure
Stages II, III and IVa HF patients
Mitral and tricuspid regurgitation patients
Heart Team
Cardiac surgeons, interventional cardiologists, and HF cardiologists
>60 employees
Significant valve and delivery experience
Over 40% with 3-year tenure
Mitral & Tricuspid Markets >$7B
Mitral surgical and transcatheter
Tricuspid surgical and transcatheter
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates.
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
9
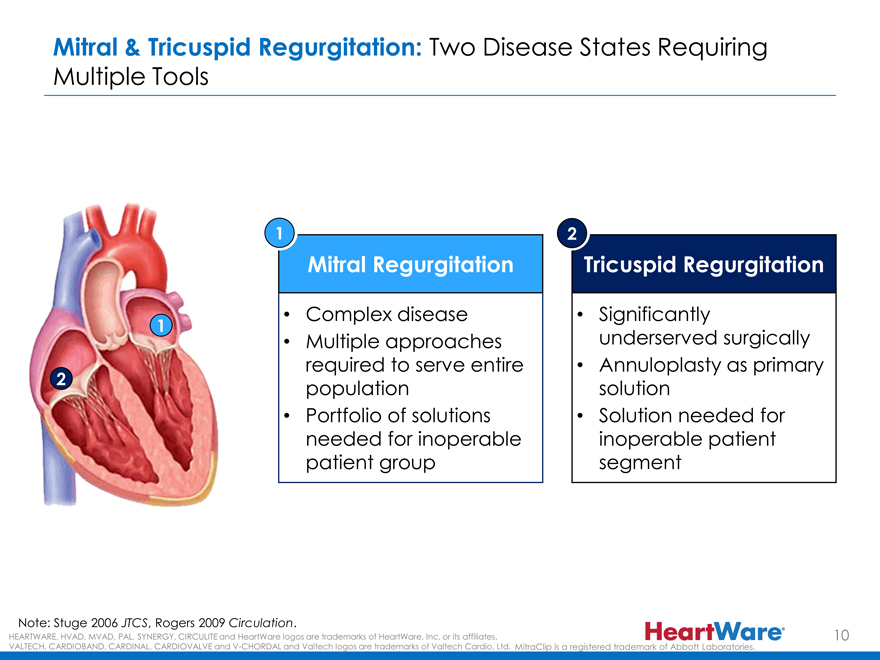
Mitral & Tricuspid Regurgitation: Two Disease States Requiring
Multiple Tools
1 2
Mitral Regurgitation Tricuspid Regurgitation
Complex disease Significantly
1
Multiple approaches underserved surgically required to serve entire Annuloplasty as primary
2 population solution
Portfolio of solutions Solution needed for needed for inoperable inoperable patient patient group segment
Note: Stuge 2006 JTCS, Rogers 2009 Circulation.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 10 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd. MitraClip is a registered trademark of Abbott Laboratories.
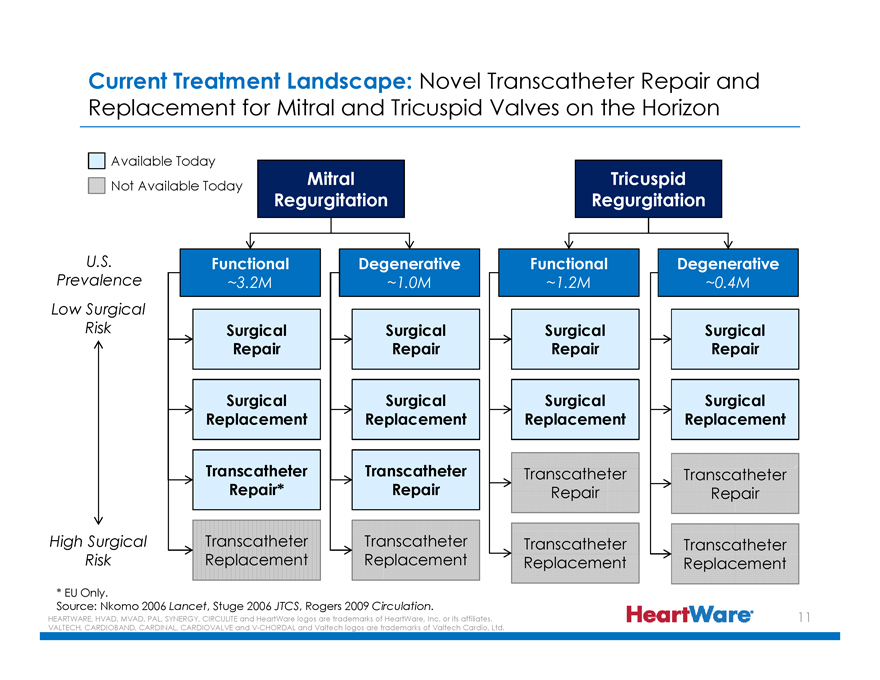
Current Treatment Landscape: Novel Transcatheter Repair and
Replacement for Mitral and Tricuspid Valves on the Horizon
Available Today
Not Available Today
Mitral Regurgitation
Tricuspid Regurgitation
U.S. Prevalence Functional ~3.2M Degenerative ~1.0M Functional ~1.2M Degenerative ~0.4M
Low Surgical Risk Surgical Surgical Surgical Surgical
Repair Repair Repair Repair
Surgical Surgical Surgical Surgical
Replacement Replacement Replacement Replacement
Transcatheter Transcatheter Transcatheter Transcatheter
Repair* Repair Repair Repair
High Surgical Risk Transcatheter Transcatheter Transcatheter Transcatheter
Replacement Replacement Replacement Replacement
* EU Only.
Source: Nkomo 2006 Lancet, Stuge 2006 JTCS, Rogers 2009 Circulation.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates.
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
11
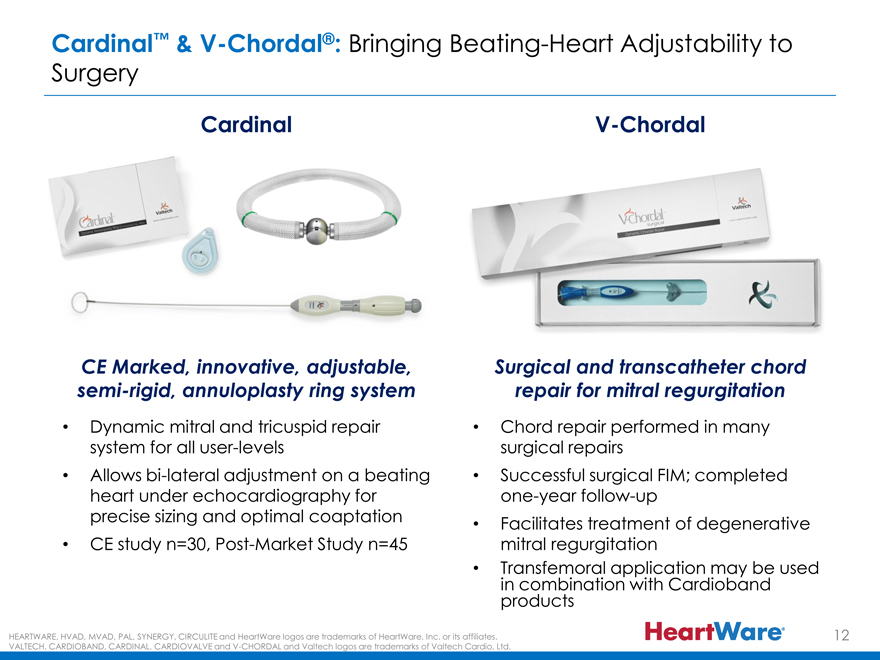
Cardinal™ & V-Chordal®: Bringing Beating-Heart Adjustability to
Surgery
Cardinal V-Chordal
CE Marked, innovative, adjustable, Surgical and transcatheter chord
semi-rigid, annuloplasty ring system repair for mitral regurgitation
Dynamic mitral and tricuspid repair Chord repair performed in many
system for all user-levels surgical repairs
Allows bi-lateral adjustment on a beating Successful surgical FIM; completed
heart under echocardiography for one-year follow-up
precise sizing and optimal coaptation • Facilitates treatment of degenerative
CE study n=30, Post-Market Study n=45 mitral regurgitation
Transfemoral in combination application with Cardioband may be used
products
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 12
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
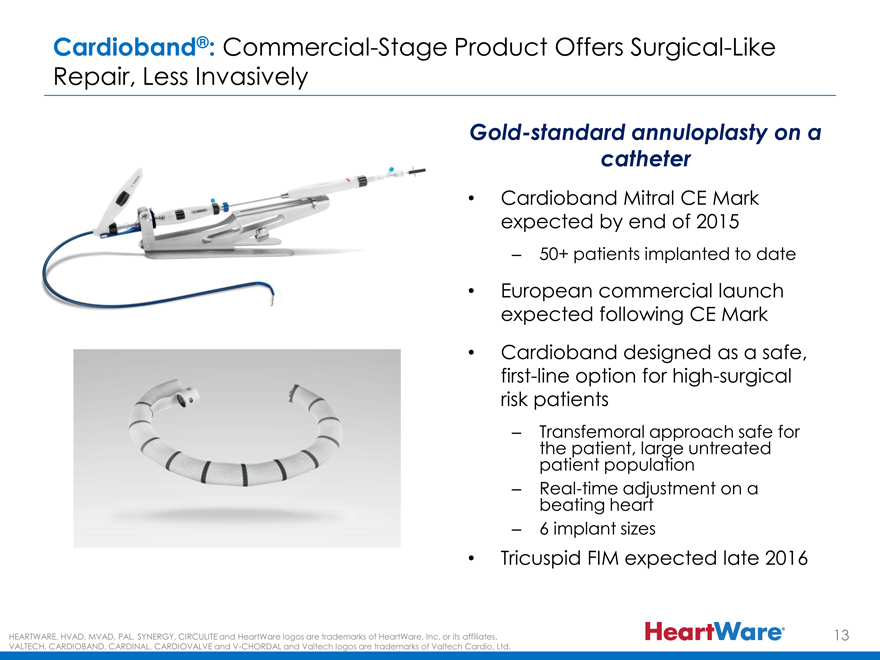
Cardioband®: Commercial-Stage Product Offers Surgical-Like Repair, Less Invasively
Gold-standard annuloplasty on a catheter
Cardioband Mitral CE Mark expected by end of 2015
– 50+ patients implanted to date
European commercial launch expected following CE Mark
Cardioband designed as a safe, first-line option for high-surgical risk patients
– the Transfemoral patient, large approach untreated safe for patient population
– Real beating -time heart adjustment on a
– 6 implant sizes
Tricuspid FIM expected late 2016
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 13 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
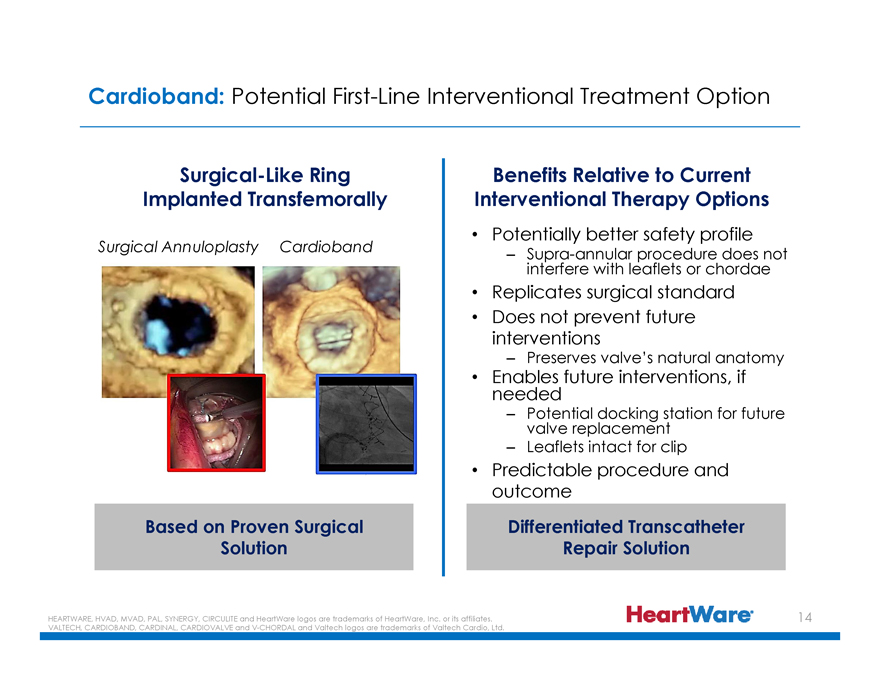
Cardioband: Potential First-Line Interventional Treatment Option
Surgical-Like Ring Implanted Transfemorally
Surgical Annuloplasty Cardioband
Based on Proven Surgical Solution
Benefits Relative to Current Interventional Therapy Options
Potentially better safety profile
– Supra-annular procedure does not interfere with leaflets or chordae
Replicates surgical standard
Does not prevent future interventions
– Preserves valve’s natural anatomy
Enables future interventions, if needed
– Potential docking station for future valve replacement
– Leaflets intact for clip
Predictable procedure and outcome
Differentiated Transcatheter Repair Solution
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
14
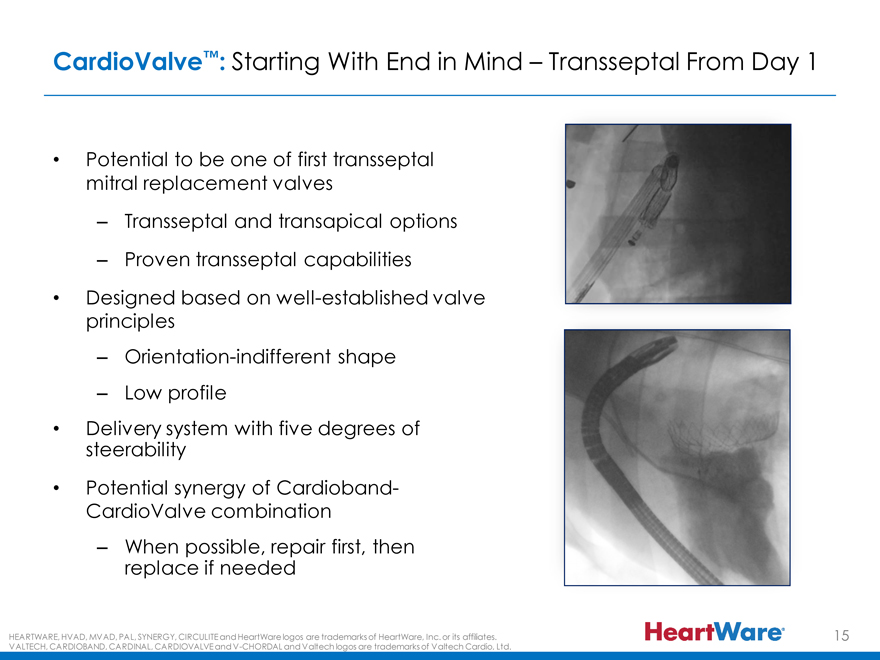
CardioValve™: Starting With End in Mind – Transseptal From Day 1
Potential to be one of first transseptal mitral replacement valves
Transseptal and transapical options
Proven transseptal capabilities
Designed based on well-established valve principles
Orientation-indifferent shape
Low profile
steerability Delivery system with five degrees of
Potential synergy of Cardioband-CardioValve combination – replace When possible, if needed repair first, then
Strategic Rationale: A New Portfolio of Technologies to Expand Our Treatment Footprint in Advanced Heart Failure
Create the Leading • Valtech portfolio enables a move upstream in Advanced Heart Failure heart failure care paradigm Company • VADs provide option for end-stage patients
Strengthen Ability to • Surgical and interventional heart failure portfolio Serve Heart Team meets the needs of hospital heart failure Customer treatment teams
HeartWare field team possesses substantial Leverage Commercial technical knowledge in treatment of heart failure
Extensive MCS and valve center relationships
Reach
Outreach to cardiologists more compelling with combined portfolio
Accelerate Expansion • Valtech product suite advances HeartWare’s customer reach to support interventional
Into Interventional cardiologists
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 16 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Founder and CEO, Valtech Cardio, Ltd.
AMIR GROSS
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 17 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Valtech’s Chosen Path: HeartWare Team, Culture and Deal Structure Provide Platform for Next Stage
Early HeartWare investment enabled creation of a strong partnership
Appreciation for HeartWare’s achievements with VADs and its proven commercial execution
Strong alignment of entrepreneurial cultures and shared vision; belief in HeartWare team and VAD business ? Participation in future value creation and remaining a separate business unit is attractive to our team
Stock transaction further aligns interests and allows for participation in long-term success
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 18
Valtech: Valve Expertise Has Yielded Best-in-Class Repair and Replacement Portfolio for Mitral and Tricuspid Valves
Products
• Leveraging proven surgical solutions
– Annuloplasty
– Chord repair
– Valve replacement
• Near-term revenue generation
– Multiple commercial-stage products over next few years
• Serving multiple large, untapped markets
– Portfolio potentially serving – and expanding – current mitral and tricuspid markets
Team
• Experienced
– 62 deep; broad functional experience
– Exceptional R&D; significant valve and transcatheter delivery experience
• Proven execution
– Since 2010: 4 FIM (>120 patients treated), 1 CE Mark (1 more imminent)
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 19 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
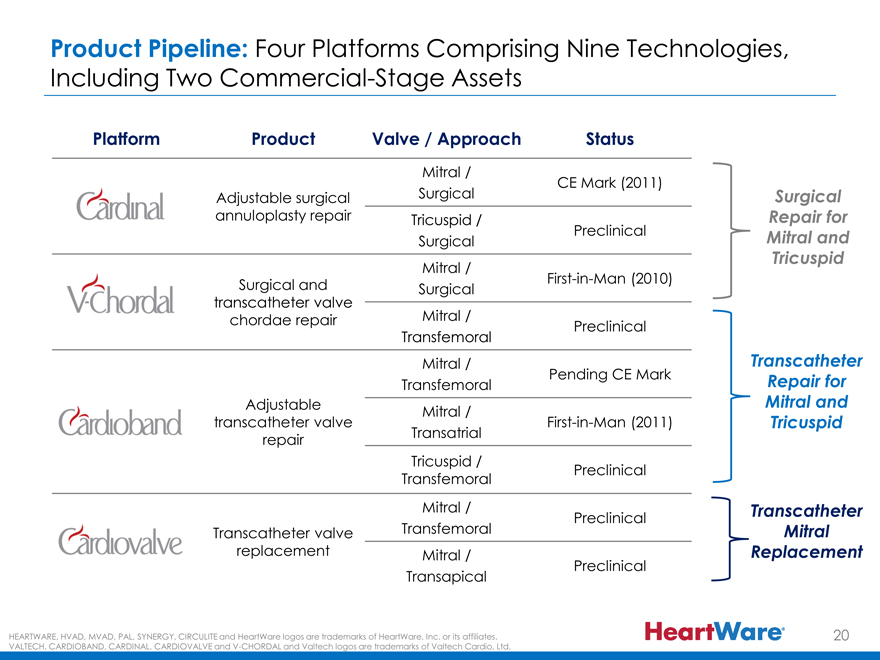
Product Pipeline: Four Platforms Comprising Nine Technologies,
Including Two Commercial-Stage Assets
Platform Product Valve / Approach Status
Mitral /
CE Mark (2011)
Adjustable surgical Surgical Surgical
annuloplasty repair Tricuspid / Repair for
Preclinical Mitral and
Surgical
Tricuspid
Mitral /
Surgical and First-in-Man (2010)
Surgical
transcatheter valve
chordae repair Mitral /
Preclinical
Transfemoral
Mitral / Transcatheter
Pending CE Mark Repair for
Transfemoral Mitral and
Adjustable Mitral /
transcatheter valve First-in-Man (2011) Tricuspid
repair Transatrial
Tricuspid /
Preclinical
Transfemoral
Mitral / Transcatheter
Preclinical
Transcatheter valve Transfemoral Mitral
replacement Mitral / Replacement
Preclinical
Transapical
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 20
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
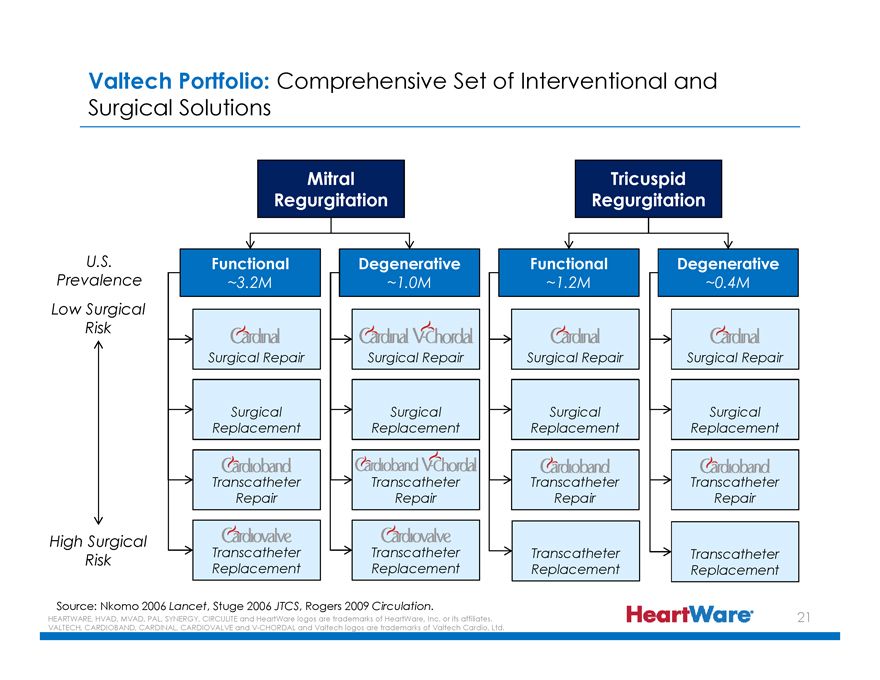
Valtech Portfolio: Comprehensive Set of Interventional and
Surgical Solutions
Mitral Regurgitation
Tricuspid Regurgitation
U.S. Prevalence
Functional Degenerative Functional Degenerative
~3.2M ~1.0M ~1.2M ~0.4M
Low Surgical
Risk
Cardinal Surgical Repair Cardinal V-Chordal Surgical Repair Cardinal Surgical Repair Cardinal Surgical Repair
Surgical Surgical Surgical Surgical
Replacement Replacement Replacement Replacement
Cardioband Transcatheter Repair
Cardioband V-Chordal Transcatheter Repair
Cardioband Transcatheter Repair
Cardioband Transcatheter Repair
High Surgical Risk
Cardiovalve Transcatheter Replacement
Cardiovalve Transcatheter Replacement
Transcatheter Repair
Transcatheter Repair
Source: Nkomo 2006 Lancet, Stuge 2006 JTCS, Rogers 2009 Circulation.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates.
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
21
Chair of Cardiovascular Surgery, University Hospital of Zurich
PROF. FRANCESCO MAISANO, M.D.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 22 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
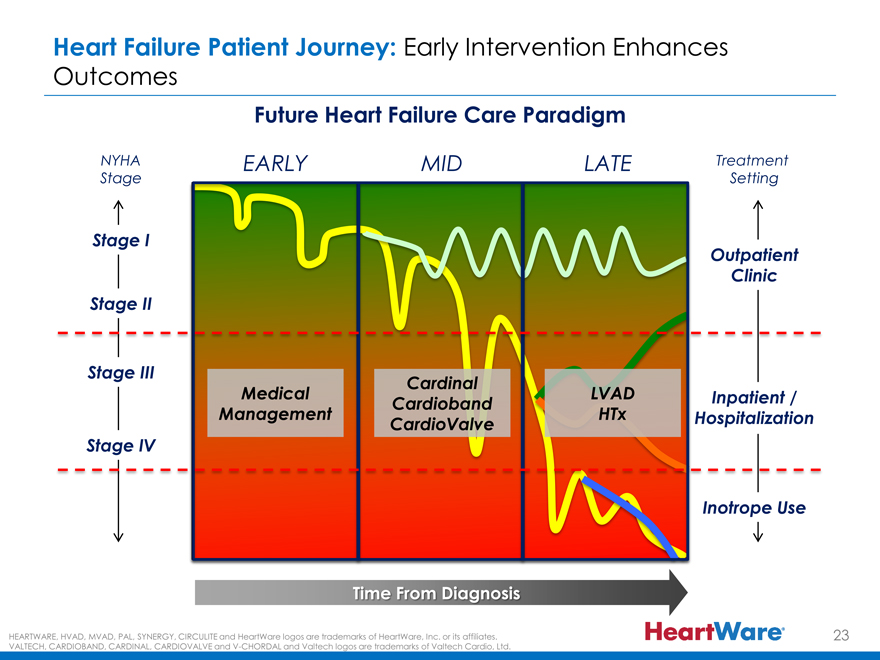
Heart Failure Patient Journey: Early Intervention Enhances
Outcomes
Future Heart Failure Care Paradigm
NYHA EARLY MID LATE Treatment
Stage Setting
Stage I
Outpatient
Clinic
Stage II
Stage III
Cardinal
Medical LVAD Inpatient /
Cardioband
Management HTx Hospitalization
CardioValve
Stage IV
Inotrope Use
Time From Diagnosis
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 23
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
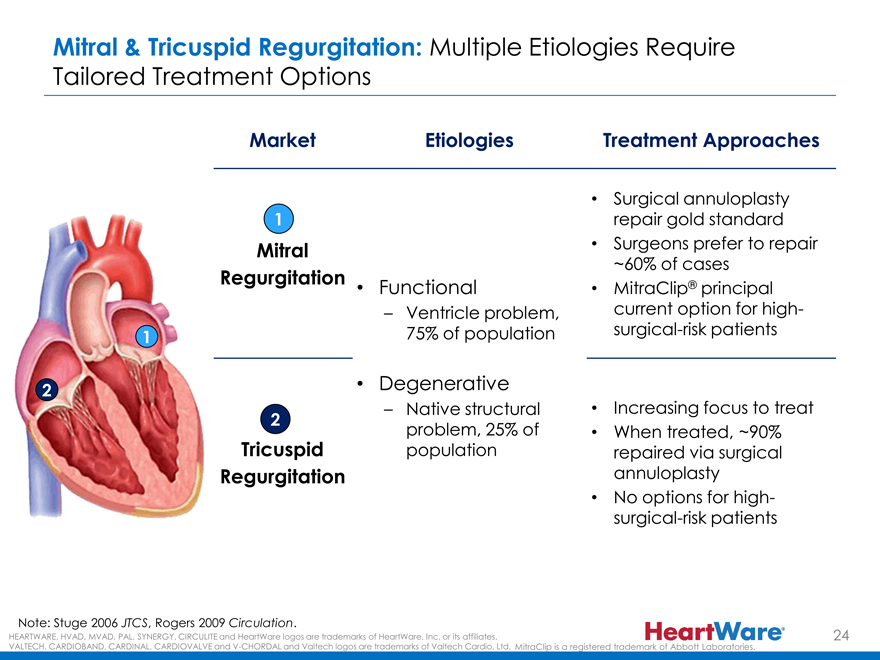
Mitral & Tricuspid Regurgitation: Multiple Etiologies Require
Tailored Treatment Options
Market Etiologies Treatment Approaches
Surgical annuloplasty
1 repair gold standard
Mitral • Surgeons prefer to repair
~60% of cases
Regurgitation ®
Functional MitraClip principal
– Ventricle problem, current option for high-
1 75% of population surgical-risk patients
2 • Degenerative
– Native structural • Increasing focus to treat
2
problem, 25% of • When treated, ~90%
Tricuspid population repaired via surgical
Regurgitation annuloplasty
No options for high-
surgical-risk patients
Note: Stuge 2006 JTCS, Rogers 2009 Circulation.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 24
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd. MitraClip is a registered trademark of Abbott Laboratories.
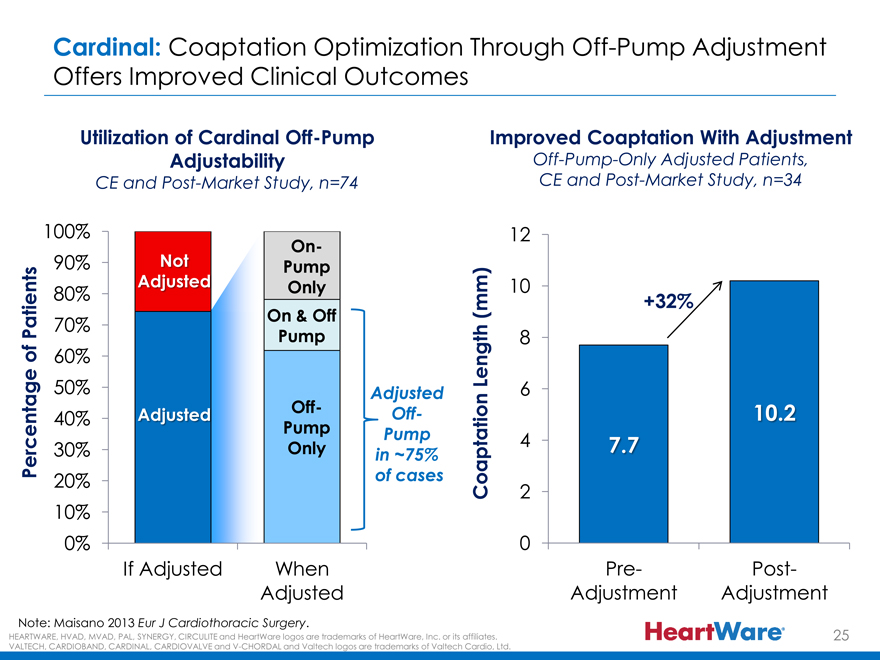
Cardinal: Coaptation Optimization Through Off-Pump Adjustment Offers Improved Clinical Outcomes
Utilization of Cardinal Off-Pump Improved Coaptation With Adjustment
Adjustability Off-Pump-Only Adjusted Patients, CE and Post-Market Study, n=74 CE and Post-Market Study, n=34
100% 12
On-
90% Not Pump Adjusted Only 10
80% (mm) +32% Patients 70% On & Off Pump 8 of 60% Length
50% Adjusted 6
Adjusted Off- Off- 10.2 40% Pump
Pump 4 7.7
Percentage 30% Only in ~75%
20% of cases
Coaptation 2
10%
0% 0
If Adjusted When Pre- Post- Adjusted Adjustment Adjustment
Note: Maisano 2013 Eur J Cardiothoracic Surgery.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 25 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
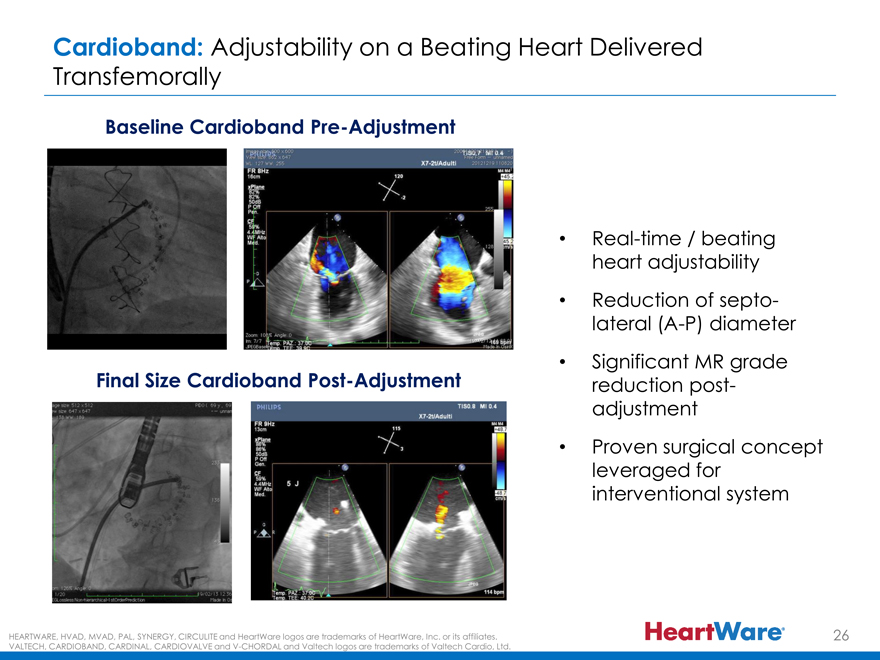
Cardioband: Adjustability on a Beating Heart Delivered
Transfemorally
Baseline Cardioband Pre-Adjustment
Real-time / beating
heart adjustability
Reduction of septo-
lateral (A-P) diameter
Final Size Cardioband Post-Adjustment • Significant MR grade
reduction post-
adjustment
Proven surgical concept
leveraged for
interventional system
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 26
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Head of Cardiology Department, Asklepios Klinik St. Georg, Hamburg, Germany
PROF. KARL-HEINZ KUCK, M.D., PH.D.
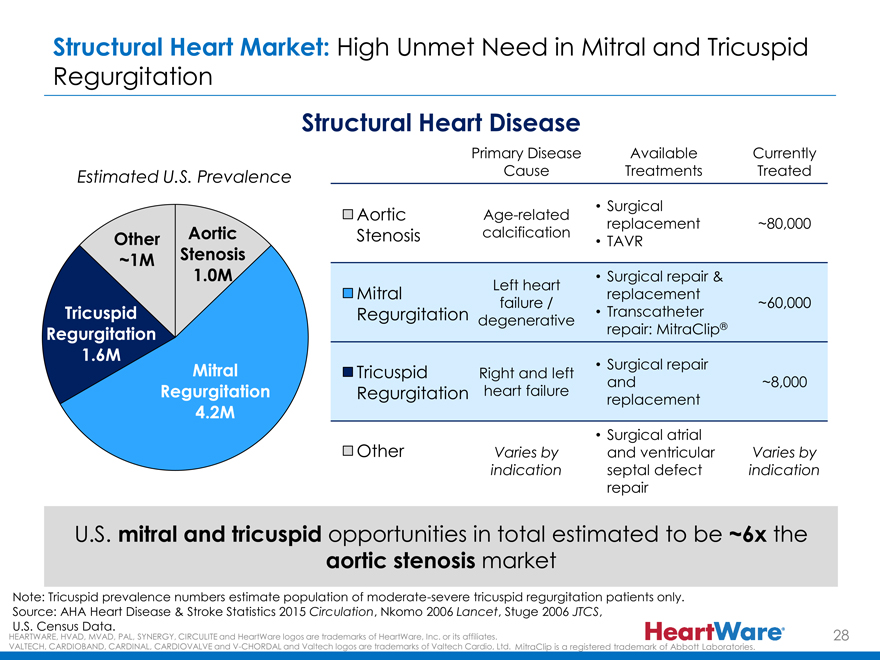
Structural Heart Market: High Unmet Need in Mitral and Tricuspid
Regurgitation
Structural Heart Disease
Primary Disease Available Currently
Estimated U.S. Prevalence Cause Treatments Treated
Surgical
Aortic Age-related
replacement ~80,000
Other Aortic Stenosis calcification
TAVR
~1M Stenosis
1.0M • Surgical repair &
Left heart
Mitral replacement
failure / ~60,000
Tricuspid Regurgitation • Transcatheter
degenerative ®
Regurgitation repair: MitraClip
1.6M
Mitral Tricuspid • Surgical repair
Right and left
and ~8,000
Regurgitation Regurgitation heart failure
replacement
4.2M
Other • Surgical atrial
Varies by and ventricular Varies by
indication septal defect indication
repair
U.S. mitral and tricuspid opportunities in total estimated to be ~6x the
aortic stenosis market
Note: Tricuspid prevalence numbers estimate population of moderate-severe tricuspid regurgitation patients only.
Source: AHA Heart Disease & Stroke Statistics 2015 Circulation, Nkomo 2006 Lancet, Stuge 2006 JTCS,
U.S. Census Data.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 28
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd. MitraClip is a registered trademark of Abbott Laboratories.
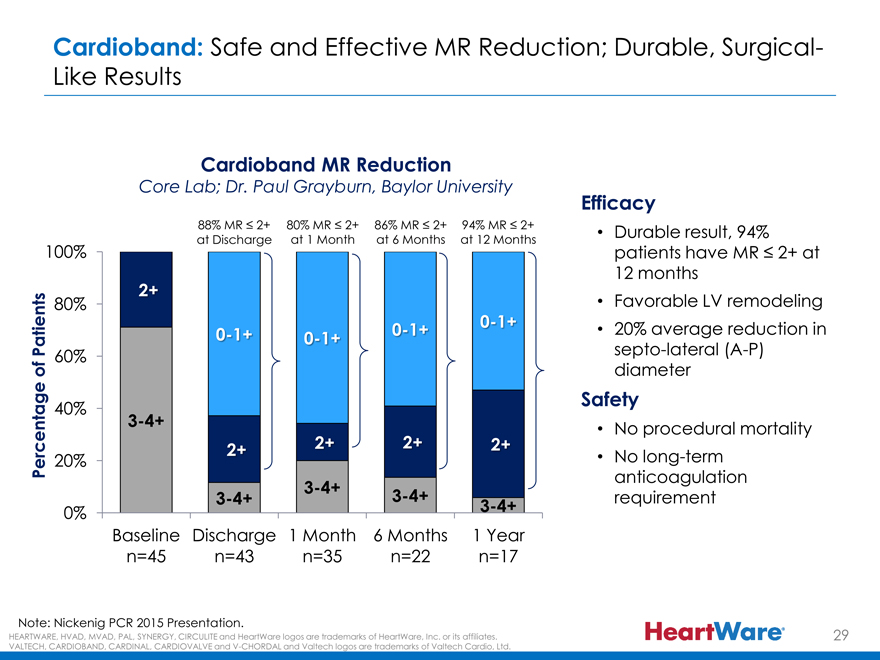
Cardioband: Safe and Effective MR Reduction; Durable, Surgical-Like Results
Cardioband MR Reduction
Core Lab; Dr. Paul Grayburn, Baylor University
Efficacy
88% MR 2+ 80% MR 2+ 86% MR 2+ 94% MR 2+ • Durable result, 94% at Discharge at 1 Month at 6 Months at 12 Months
100% patients have MR 2+ at
12 months
2+
80% • Favorable LV remodeling
0-1+ 0-1+ • 20% average reduction in
0-1+ 0-1+
Patients septo-lateral (A-P) 60% of diameter
40% Safety
3-4+ No
procedural mortality
2+ 2+ 2+ 2+
Percentage 20% • No long-term anticoagulation
3-4+ 3-4+
3-4+ 3-4+ requirement 0% Baseline Discharge 1 Month 6 Months 1 Year n=45 n=43 n=35 n=22 n=17
Note: Nickenig PCR 2015 Presentation.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 29 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Transcatheter Valve Therapies: Complementary Role of Repair and Replacement
Mitral valve is not just a valve, it is a complex apparatus integrated in the left ventricle
Durability, safety and disruption of adjacent cardiac structures are important concerns
Safety, including avoidance of permanent implant-related complications, will drive transcatheter repair as first-line therapy
Transcatheter replacement may be an option for patients with more advanced disease who have severe anatomical and functional abnormalities and are not eligible for repair
Source: Maisano 2015 European Heart Journal.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 30 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
Senior Vice President and CFO, HeartWare International
PETER MCAREE
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 31 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
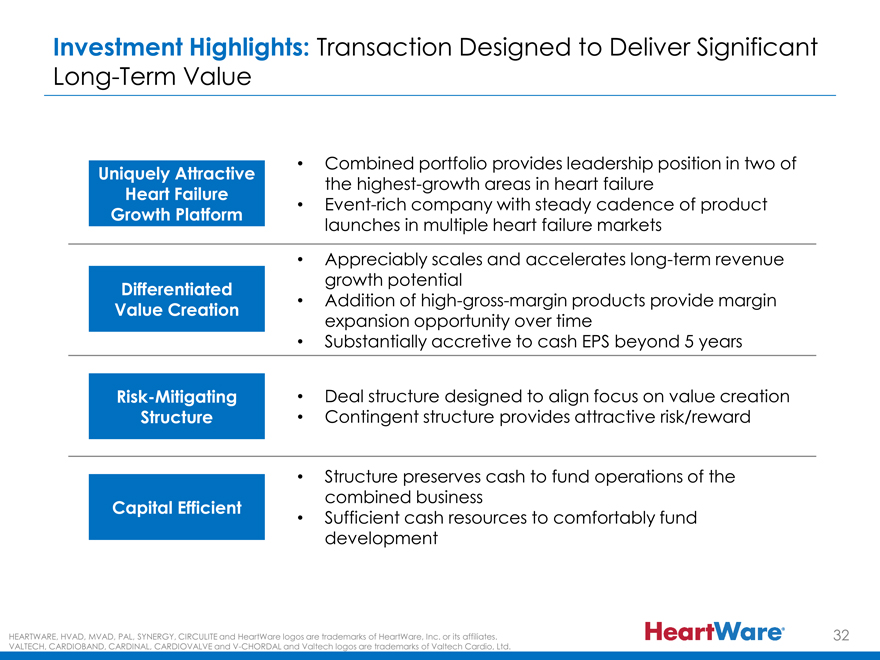
Investment Highlights: Transaction Designed to Deliver Significant Long-Term Value
Combined portfolio provides leadership position in two of
Uniquely Attractive the highest-growth areas in heart failure
Heart Failure
Event-rich company with steady cadence of product
Growth Platform launches in multiple heart failure markets
Appreciably scales and accelerates long-term revenue growth potential
Differentiated
Addition of high-gross-margin products provide margin
Value Creation expansion opportunity over time
Substantially accretive to cash EPS beyond 5 years
Risk-Mitigating • Deal structure designed to align focus on value creation Structure • Contingent structure provides attractive risk/reward
Structure preserves cash to fund operations of the combined business
Capital Efficient
Sufficient cash resources to comfortably fund development
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 32 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
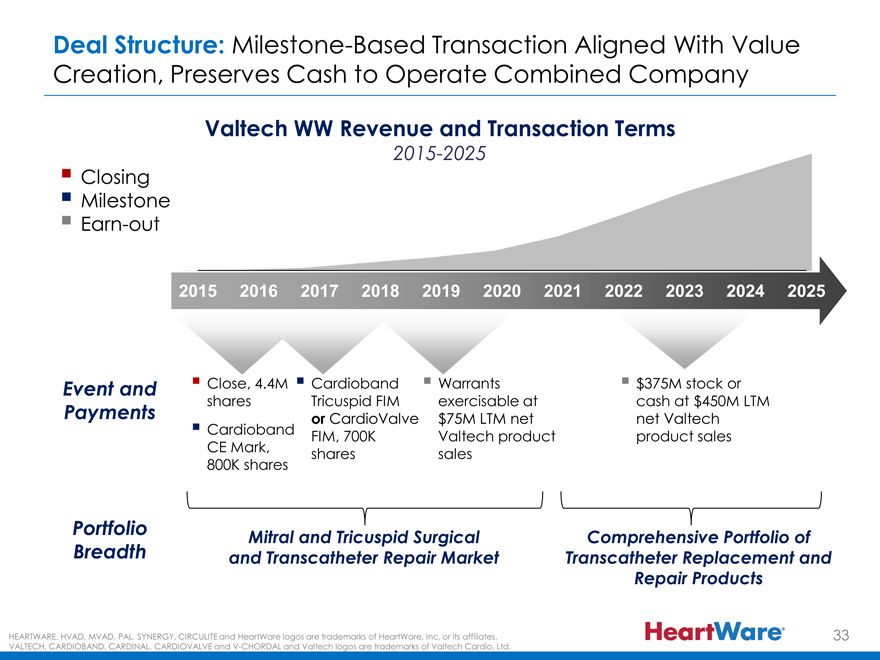
Deal Structure: Milestone-Based Transaction Aligned With Value
Creation, Preserves Cash to Operate Combined Company
Valtech WW Revenue and Transaction Terms
2015-2025
? Closing
? Milestone
? Earn-out
2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025
Event and ? Close, 4.4M ? Cardioband ? Warrants ? $375M stock or
shares Tricuspid FIM exercisable at cash at $450M LTM
Payments or CardioValve $75M LTM net net Valtech
? Cardioband FIM, 700K Valtech product product sales
CE Mark, shares sales
800K shares
Portfolio Mitral and Tricuspid Surgical Comprehensive Portfolio of
Breadth and Transcatheter Repair Market Transcatheter Replacement and
Repair Products
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 33
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
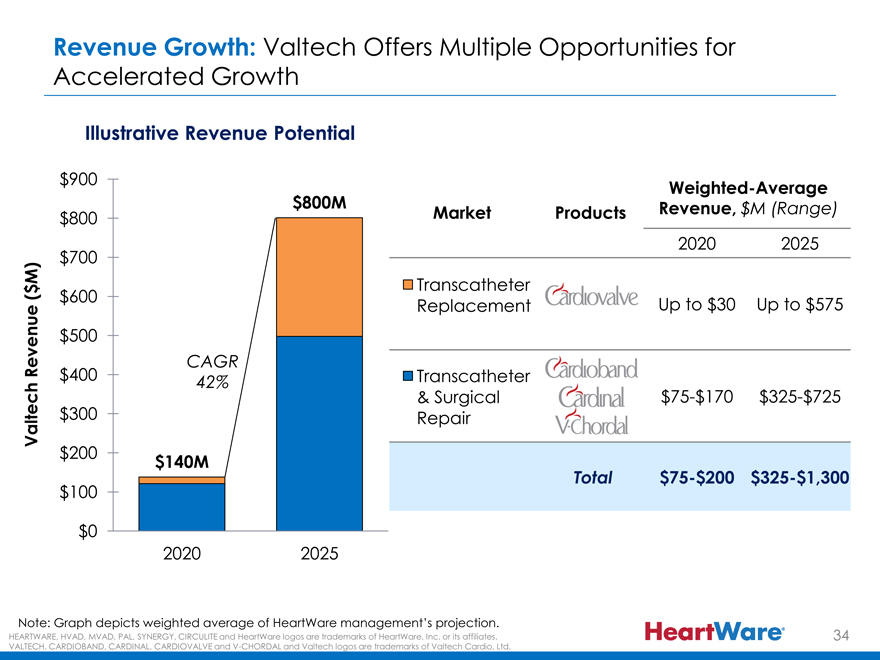
Revenue Growth: Valtech Offers Multiple Opportunities for
Accelerated Growth
Illustrative Revenue Potential
$900
Weighted-Average
$800M Revenue, $M (Range)
$800 Market Products
2020 2025
M) $700
$ Transcatheter
( $600 Replacement Up to $30 Up to $575
$500
Revenue CAGR
$400 42% Transcatheter
$300 & Surgical $75-$170 $325-$725
Valtech Repair
$200
$140M
Total $75-$200 $325-$1,300
$100
$0
2020 2025
Note: Graph depicts weighted average of HeartWare management’s projection.
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 34
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
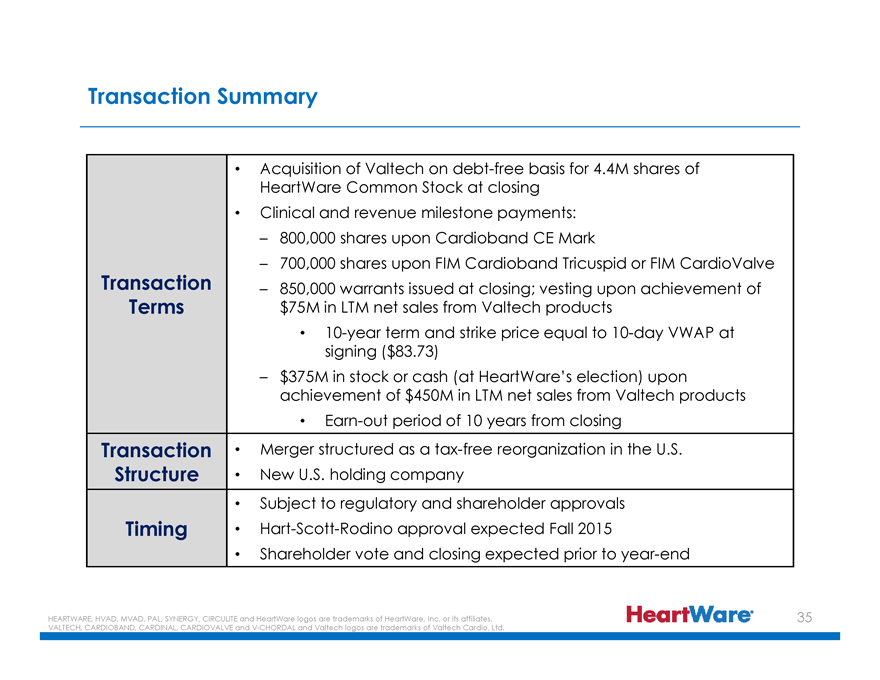
Transaction Summary
Transaction Terms
Acquisition of Valtech on debt-free basis for 4.4M shares of HeartWare Common Stock at closing
Clinical and revenue milestone payments:
– 800,000 shares upon Cardioband CE Mark
– 700,000 shares upon FIM Cardioband Tricuspid or FIM CardioValve
– 850,000 warrants issued at closing; vesting upon achievement of $75M in LTM net sales from Valtech products
10-year term and strike price equal to 10-day VWAP at signing ($83.73)
– $375M in stock or cash (at HeartWare’s election) upon achievement of $450M in LTM net sales from Valtech products
Earn-out period of 10 years from closing
Transaction Structure
Merger structured as a tax-free reorganization in the U.S.
New U.S. holding company
Timing
Subject to regulatory and shareholder approvals
Hart-Scott-Rodino approval expected Fall 2015
Shareholder vote and closing expected prior to year-end
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates.
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
35
President and CEO, HeartWare International
DOUG GODSHALL
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 36 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
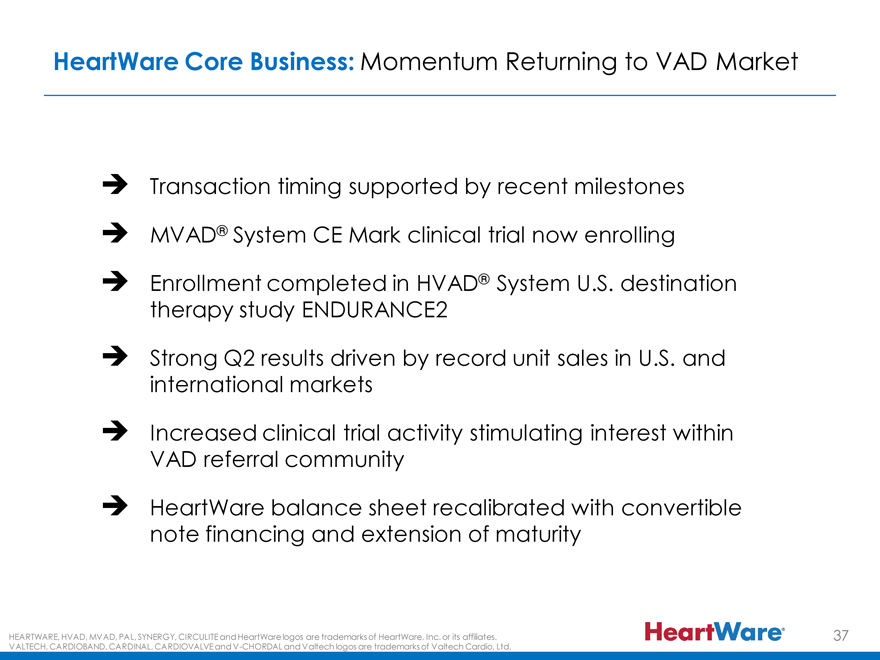
HeartWare Core Business: Momentum Returning to VAD Market
Transaction timing supported by recent milestones MVAD® System CE Mark clinical trial now enrolling Enrollment completed in HVAD® System U.S. destination therapy study ENDURANCE2 Strong Q2 results driven by record unit sales in U.S. and international markets Increased clinical trial activity stimulating interest within VAD referral community HeartWare balance sheet recalibrated with convertible note financing and extension of maturity
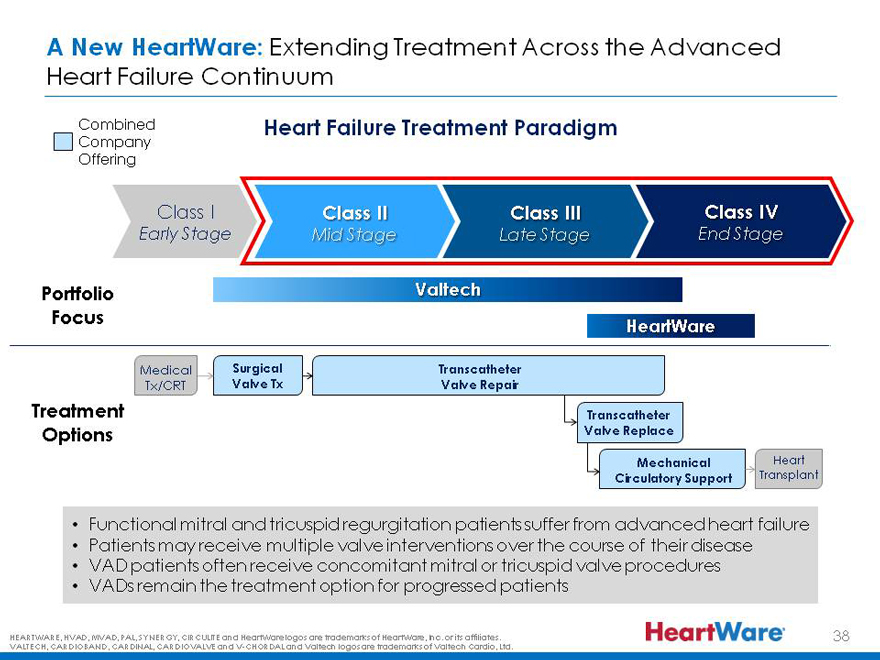
A New HeartWare: Extending Treatment Across the Advanced Heart Failure Continuum
Combined Company Offering
Heart Failure Treatment Paradigm
Class I Class II Class III Class IV
Early Stage Mid Stage Late Stage End Stage
Portfolio Focus
Valtech
HeartWare
Medical Surgical Transcatheter
Tx/CRT Valve Tx Valve Repair
Treatment Options
Transcatheter Valve Replace
Mechanical Circulatory Support
Heart Transplant
Functional mitral and tricuspid regurgitation patients suffer from advanced heart failure
Patients may receive multiple valve interventions over the course of their disease
VAD patients often receive concomitant mitral or tricuspid valve procedures
VADs remain the treatment option for progressed patients
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
38
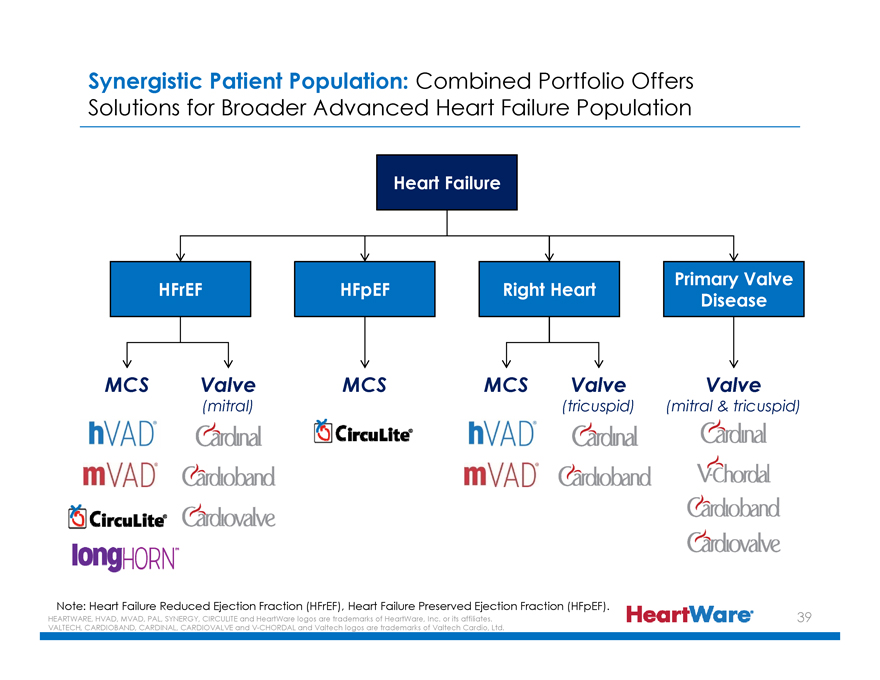
Synergistic Patient Population: Combined Portfolio Offers Solutions for Broader Advanced Heart Failure Population
Heart Failure
HFrEF HFpEF Right Heart Primary Valve Disease
MCS Valve MCS MCS Valve Valve
(mitral) (tricuspid) (mitral & tricuspid)
hVAD®
mVAD®
CircuLite®
longHORNTM
Cardinal
Cardioband
Cardiovalve
CircuLite®
hVAD®
mVAD®
Cardinal
Cardioband
Cardinal
V-Chordal
Cardioband
Cardiovalve
Note: Heart Failure Reduced Ejection Fraction (HFrEF), Heart Failure Preserved Ejection Fraction (HFpEF).
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates.
VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare®
39
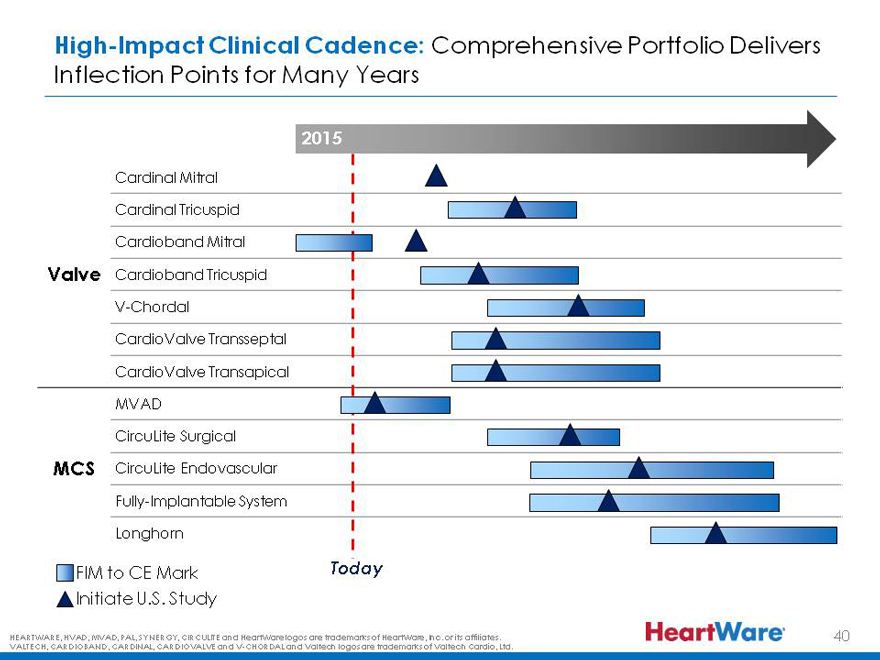
High-Impact Clinical Cadence: Comprehensive Portfolio Delivers Inflection Points for Many Years
2015
Cardinal Mitral
Cardinal Tricuspid
Cardioband Mitral
Valve Cardioband Tricuspid
V-Chordal
CardioValve Transseptal
CardioValve Transapical
MVAD
CircuLite Surgical
MCS CircuLite Endovascular
Fully-Implantable System
Longhorn
FIM to CE Mark Today
Initiate U.S. Study
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
40
Integration Plan and Opportunities: Valtech to Operate as a Strategic Business Unit, Enabling Seamless Integration
Valtech to operate as a separate, strategic business unit located and operating in Israel
– delivering Seamless integration on valve product allows strong pipeline Valtech team to remain focused on – business Integration officer to oversee successful transition into a HeartWare
– Cardinal Recruit 20 and -25 field Cardioband team members in 2016 to support commercialization of
HeartWare core team remains focused on advancing MCS business
Anticipate complementary market development, clinical and back-office capabilities between MCS and Valve businesses
– Leverage heart team customer base
– Use experienced clinical team to execute clinical development strategy
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 41 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.
HeartWare + Valtech: Creating the Technology Leader in Advanced Heart Failure
MVAD® System leading a transformation of MCS portfolio and VAD market Synergies with disease, customer, referral channel and delivery system will create compounding benefits over next decade Adjustable repair of MR and TR expected to emerge as first-line option, whether surgical or interventional Nearer-term commercial opportunity in established interventional mitral repair market with >$250M in sales
Sophisticated CardioValve design and delivery system to enable HeartWare leadership in mitral replacement Combined pipeline creates unique opportunity for differentiated value creation
HEARTWARE, HVAD, MVAD, PAL, SYNERGY, CIRCULITE and HeartWare logos are trademarks of HeartWare, Inc. or its affiliates. 42 VALTECH, CARDIOBAND, CARDINAL, CARDIOVALVE and V-CHORDAL and Valtech logos are trademarks of Valtech Cardio, Ltd.