Exhibit 99.1
Agenda
Thursday, November 5, 2015
| | |
| |
| 8:30 a.m. – 9:00 a.m. | | Interactive Product Display Open (available all day) |
| |
| 9:00 a.m. – 9:15 a.m. | | Introduction: HeartWare Today and Tomorrow |
| |
| | | Doug Godshall, CEO, HeartWare International, Inc. |
| |
| | | Amir Gross, CEO, Valtech Cardio, Ltd. |
| |
| 9:15 a.m. – 9:35 a.m. | | HeartWare Market View and the Strategic Combination |
| |
| | | Doug Godshall |
| |
| 9:35 a.m. – 9:50 a.m. | | Mitral and Tricuspid Regurgitation Overview: The Need, Anatomy, |
| |
| | | Treatment Today and Challenges |
| |
| | | Michael Mack, M.D., Baylor Health Care System |
| |
| 9:50 a.m. – 10:05 a.m. | | Valtech Portfolio Overview |
| |
| | | Prof. Francesco Maisano, M.D., University Hospital of Zurich |
| |
| 10:05 a.m. – 10:25 a.m. | | Repair and Replacement |
| |
| | | Prof. Francesco Maisano, M.D. |
| |
| 10:25 a.m. – 10:35 a.m. | | Q&A |
| |
| 10:35 a.m. – 10:45 a.m. | | Break |
| |
| 10:45 a.m. – 11:00 a.m. | | Cardioband® Deep Dive |
| |
| | | Prof. Karl-Heinz Kuck, M.D., Ph.D., Asklepios Klinik St. Georg |
| |
| 11:00 a.m. – 11:15 a.m. | | Cardioband® Deeper Dive: Core Lab Insights |
| |
| | | Paul Grayburn, M.D., Baylor University Medical Center |
| |
| 11:15 a.m. – 11:30 a.m. | | MVAD® Overview and User Experience |
| |
| | | Paul Jansz, M.D., St. Vincent’s Hospital |
| |
| 11:30 a.m. – 11:45 a.m. | | The HeartWare-Valtech Combination: The Physician’s View |
| |
| | | Steven Boyce, M.D., MedStar Washington Hospital |
| |
| 11:45 a.m. – 12:05 p.m. | | Physician Panel – Moderated by Dr. Michael Mack |
| |
| 12:05 p.m. – 12:15 p.m. | | Q&A |
| |
| 12:15 p.m. – 12:35 p.m. | | Lunch Break |
| |
| 12:35 p.m. – 12:50 p.m. | | The Combined Company Overview |
| |
| | | Peter McAree, CFO, HeartWare International, Inc. |
| |
| 12:50 p.m. – 1:00 p.m. | | Closing Remarks |
| |
| | | Doug Godshall |
Doug Godshall
President and Chief Executive Officer, HeartWare

Mr. Godshall has been President and Chief Executive Officer of HeartWare since September 2006 and became a director in October 2006. Prior to joining HeartWare, Mr. Godshall served in various executive and managerial positions at Boston Scientific Corporation, where he had been employed since 1990, including as a member of Boston Scientific’s Operating Committee and as President, Vascular Surgery since January 2005. Prior to that, he spent five years as Vice President, Business Development at Boston Scientific, where he focused on acquisition strategies for the cardiology, electrophysiology, neuroradiology and vascular surgery divisions. Mr. Godshall has a Bachelor of Arts degree in business from Lafayette College and an M.B.A. from Northeastern University.
Amir Gross
Founder and Chief Executive Officer, Valtech Cardio
| | |
 | | Mr. Gross founded Valtech Cardio in March 2005 to design and develop solutions for percutaneous mitral valve repair technology. Prior to founding Valtech, Mr. Gross gained broad experience in the medical device space as part of Rainbow Medical, and held various positions in a series of Israeli medical device startups, including EarlySense, BetaStim, GluSense and BioControl. He holds an LLB degree in medicine and ethics from the University of Manchester. At Valtech, Mr. Gross has overall responsibility for the company’s strategy and vision and is responsible for building the Valtech culture and overseeing all company functions, including operations, marketing, finance, human resources, compliance with safety regulations and public relations. |
1
Michael Mack, M.D., FACC
Dr. Mack has practiced cardiothoracic surgery in Dallas, Texas since 1982. He is board-certified in internal medicine, general surgery, and thoracic surgery and is currently the Director of Cardiovascular Surgery for Baylor Scott & White Health, Chair of the Baylor Scott & White Health Cardiovascular Governance Council and Director of Cardiovascular Research at The Heart Hospital Baylor Plano. He also co-founded and is Chair of the Board of Cardiopulmonary Research Science and Technology Institute (CRSTI). He has more than 450 peer-reviewed medical publications.

Dr. Mack was President of the Society of Thoracic Surgeons (STS) in 2011 and is Past President of the Thoracic Surgery Foundation for Research and Education (TSFRE) 2009-2011, the Southern Thoracic Surgical Association (STSA) 2009, and the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) 2000.
He has served on the Board of Directors of the STS and is currently on the Board of Directors of CTSNet, and is a member of the American College of Cardiology Foundation (ACCF) Board of Trustees and the ACC Interventional Scientific Council, the ACC National Cardiovascular Data Registry (NCDR) Management Board and the ACC Governance Task Force. He is an honorary Fellow of the German Society for Thoracic and Cardiovascular Surgery and the Indian Association of Cardiothoracic Surgery. He was the first Chair of the STS/ACC National Transcatheter Valve Therapy (TVT) Registry Steering Committee and is the liaison to the TVT Registry from the ACCF NCDR Management Board and is on the Steering Committee of the Cardiothoracic Surgery Network (CTSN) of the NIH. He is a member of the FDA MDEpiNet Advisory and Research and Publications Committees, and the Brookings Institute National Medical Device Postmarket Surveillance System Planning Board.
Francesco Maisano, M.D., FESC
Professor Maisano is the Chair of Cardiovascular Surgery at the University Hospital of Zurich. Earlier in his career, Professor Maisano served as Senior Staff Heart Surgeon at San Raffaele University Hospital in Milan, where he developed significant expertise in the surgical and endovascular treatment of cardiovascular diseases. He has held several international academic roles, has served as a scientific advisor, and serves on professional, review and editorial boards.

Professor Maisano’s main area of focus is on heart valve treatment and innovative technologies, which he was able to extend from the surgical to the catheter-based pathway, training on interventional approaches. He was instrumental in initiating and advancing the Transcatheter Valve Treatment program at San Raffaele Hospital.
In 2013, he held the same position at the Division of Cardiovascular Surgery of the University Hospital in Zurich, where, in 2014, he was appointed Full Professor and Chair of Cardiovascular Surgery. Professor Maisano has extended his vision of the Heart Team approach into clinical practice, leading the full spectrum of multidisciplinary cardiovascular therapies and research programs, from heart failure to valvular therapies. From 2008 to 2013, Dr. Maisano was Chief Medical Officer of Valtech Cardio, and helped pioneer the Cardioband® procedure.
Professor Maisano qualified in medicine at the University La Sapienza in Rome in 1990, completed a postgraduate fellowship at the University of Alabama at Birmingham in 1994, and earned a master’s specialization in cardiac surgery in 1995 at Catholic University in Rome.
Karl-Heinz Kuck, M.D., Ph.D.

Professor Kuck is the current President of the European Heart Rhythm Association (EHRA) and Head of the Second Medical Department (Cardiology) at St. Georg Hospital in Hamburg, Germany. He has built a global reputation as a scientist, cardiologist and electrophysiologist, authoring more than 450 scientific papers – many related to his specialization in catheter ablation. He also leads cardiology at one of Europe’s largest hospital groups in Hamburg, Germany. Professor Kuck serves as a reviewer for multiple medical journals, includingEuropean Heart Journal,Europace, Heart Rhythm, Journal of Clinical Electrophysiology, JACC andCirculation Arrhythmia and Electrophysiology. He received his Doctor of Medicine degree from the University of Cologne.
Paul Grayburn, M.D., FACC
Dr. Grayburn is the Director of Cardiology Research at Baylor Heart and Vascular Institute in Dallas, Texas. Dr. Grayburn also holds faculty positions at Johns Hopkins, Mayo Clinic, Vanderbilt, UT Southwestern and the University of Maryland. Earlier in his career, Dr. Grayburn served as Assistant and Associate Professor of Medicine at UT Southwestern Medical School.
| | |
 | | Dr. Grayburn has served on multiple editorial boards and boards of directors of publications and professional medical associations. He currently serves on the editorial boards ofCirculation, American Journal of Cardiology, Heart andJACC: Cardiovascular Imaging. He is also Associate Editor of theAmerican Journal of Cardiology. Dr. Grayburn has participated as an investigator in multiple clinical trials and has authored numerous peer-reviewed publications. Dr. Grayburn earned his Bachelor of Arts degree from Texas A&M University and his degree in medicine from the University of Texas Medical Branch. He received further training in internal medicine, cardiology and interventional cardiology from St. Paul Medical Center in Dallas and from the University of Kentucky. |
4
Paul Jansz, BMed, FRACS, Ph.D.

Dr. Jansz is Consultant Cardiothoracic and Transplant Surgeon at St. Vincent’s Hospital in Sydney, Australia and previously held a similar position at Papworth Hospital in the United Kingdom. Dr. Jansz’s clinical research experience encompasses multiple trials within advanced heart failure research. Among these, he served as a lead investigator in HeartWare’s HVAD® System clinical trial and was also a sub-investigator in a clinical trial of an investigational mitral valve implant and for the MitraClip® System. He is also an investigator in HeartWare’s clinical trial of the MVAD® System. Dr. Jansz received a Bachelor of Medicine degree from the University of Newcastle, is a Fellow of the Royal Australasian College of Surgeons and earned his Doctor of Philosophy degree from the University of New South Wales.
Steven Boyce, M.D.
Dr. Boyce, surgical director of the advanced heart failure program at MedStar Washington Hospital Center, is an internationally known cardiothoracic surgeon and one of the busiest in the country, performing over 425 heart surgeries per year.

| Dr. Boyce received his undergraduate degree at Johns Hopkins, his medical degree from the University of Maryland, and completed his internship and residency in surgery at the University of California, San Francisco. He completed his chief residency in cardiothoracic surgery and fellowship in cardiac transplantation at UCLA. Following a faculty appointment at Harvard Medical School, he moved to Washington, D.C. |
Dr. Boyce has been instrumental in the development of the field of mechanical circulatory assist devices, in particular mini left ventricular assist devices, also known as LVADs. He has been an investigator in more than 60 clinical trials, including HeartWare’s ADVANCE and ENDURANCE trials. He has authored over 100 peer-reviewed papers. He is also the Founder of MyLVAD.com. For many years, Dr. Boyce has been named one of the “Best Doctors in America,” “America’s Top Surgeons” and a Washingtonian “Top Doctor.”
Peter McAree
Senior Vice President, Chief Financial Officer and Treasurer, HeartWare

Mr. McAree joined HeartWare in July 2012 as Chief Financial Officer with overall responsibility for the strategy and operations of the company’s accounting and finance functions, as well as oversight of the Information Technology department. Mr. McAree has more than 25 years of financial management and public accounting experience, serving most recently as Senior Vice President and Chief Financial Officer of Caliper Life Sciences prior to its acquisition by PerkinElmer in November 2011. Before that, he served as Chief Financial Officer of Zymark Corporation prior to the company’s merger with Caliper in 2003. Mr. McAree is a Certified Public Accountant in Massachusetts and holds a degree from Bentley University.
HeartWare ®
November 5, 2015
A MEETING WITH HEARTWARE AND VALTECH MANAGEMENT
2
Safe Harbor Statement
Forward-Looking Statements
This announcement contains forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation our expectations with respect to the: commercialization of the HeartWare HVAD System and introduction of the MVAD System; timing, progress and outcomes of clinical trials; regulatory and quality compliance; research and development activities; consummation of our proposed acquisition of Valtech and our ability to take advantage of acquired and pipeline technology. Management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue reliance on forward-looking statements because they speak only as of the date when made. HeartWare does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by federal securities laws and the rules and regulations of the Securities and Exchange Commission. HeartWare may not actually achieve the plans, projections or expectations disclosed in forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation those described in Part I, Item 1A. “Risk Factors” in HeartWare’s Annual Report on Form 10-K filed with the Securities and Exchange Commission. HeartWare may update risk factors from time to time in Part II, Item 1A. “Risk Factors” in Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, or other filings with the Securities and Exchange Commission.
3
Additional Shareholder Information
Participants in the Solicitation
HeartWare, Valtech and their respective directors, executive officers, certain members of management and certain employees may be deemed to be participants in the solicitation of proxies in connection with the proposed acquisition of Valtech Cardio, Ltd. A description of the interests in HeartWare of its directors and executive officers is set forth in HeartWare’s proxy statement for its 2015 Annual Meeting of Shareholders, which was filed with the Securities and Exchange Commission (the “SEC”) on April 30, 2015. Additional information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of proxies in connection with the proposed transaction, and a description of their direct and indirect interests in the proposed transaction, which may differ from the interests of HeartWare stockholders or Valtech shareholders generally, are set forth in a preliminary proxy statement/prospectus filed with the SEC on October 16, 2015.
Additional Information and Where To Find It
In connection with the proposed transactions, HW Global, Inc. (“Holdco”), has filed a Registration Statement on Form S-4 that contains a preliminary proxy statement/prospectus, which is not yet final and will be amended. Holdco intends to file a final prospectus and other relevant materials and HeartWare intends to file a definitive proxy statement and other relevant materials with the SEC in connection with the proposed transactions. Investors and security holders of HeartWare and Valtech are urged to read these materials (when they become available) before making any voting or investment decision with respect to the transactions because they will contain important information about HeartWare, Valtech and the transactions. The proxy statement/prospectus and other relevant materials, and any other documents filed by Holdco or HeartWare with the SEC, may be obtained free of charge at the SEC website at www.sec.gov. In addition, investors and security holders may obtain free copies of these documents by directing a written request to HeartWare’s investor relations department at HeartWare International, Inc., 500 Old Connecticut Path, Framingham, MA 01701, Attention: Investor Relations.
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended (the “Securities Act”).
4
HeartWare Today and Tomorrow
Doug Godshall
President and CEO, HeartWare International
5
Agenda
Time Topic Presenter(s)
8:30 Interactive Product Display Open (available all day)
Doug Godshall
9:00 Introduction: HeartWare Today and Tomorrow Amir Gross
9:15 HeartWare Market View and the Strategic Combination Doug Godshall
Mitral and Tricuspid Regurgitation Overview: The Need,
9:35 Dr. Michael Mack
Anatomy, Treatment Today and Challenges
9:50 Valtech Portfolio Overview Prof. Francesco Maisano
10:05 Repair and Replacement Prof. Francesco Maisano
10:25 Q&A
10:35 Break
10:45 Cardioband® Deep Dive Prof. Karl-Heinz Kuck
11:00 Cardioband® Deeper Dive: Core Lab Insights Dr. Paul Grayburn
11:15 MVAD® Overview and User Experience Dr. Paul Jansz
11:30 The HeartWare-Valtech Combination: The Physician’s View Dr. Steven Boyce
11:45 Physician Panel Guest Physicians
12:05 Q&A
12:15 Lunch Break
12:35 The Combined Company Overview Peter McAree
12:50 Closing Remarks Doug Godshall
6
Mission
We create revolutionary technology for the treatment of heart failure to allow patients to get back to life.
9,000+ ~125
Patients U.S.
Implanted Centers
Globally
47 ~180
Intl.
Countries Centers
2009: CE Mark
2012: FDA Approval
HVAD® System—A Proven Track Record
Note: Data as of November 2, 2015.
7
Commercial Execution Excellence: Strong Commercial and Clinical Team Accustomed to Breaking Into Competitive Markets
Broad Global Presence
HVAD® is the most-implanted LVAD internationally for the past 3 years Industry-leading sales and marketing serving top global heart centers 25% of sales team, including both North American and International Senior Directors of Sales, has deep valve experience Highly skilled clinical specialists, many with advanced technical or clinical degrees Best-in-class distributor partners with backgrounds in interventional cardiology and structural heart
– Over 90% of partners have valve experience
8
Ventricular Assist Devices (VADs) Meet a Significant Unmet Need for the Large Advanced Heart Failure Population
U.S. Heart Failure Prevalence
By NYHA Functional Classification
4 3.8 3.6
3.1
3
Valves 3.2M VADs today:
(Millions) FMR potential 6K patients of
2 500K potential
Patients 1 0.8 0.4
0
I II III Early IV Late IV
Heart Failure Class(shock)
VAD devices target treatment of Class III & Class IV patients Valve devices target treatment of Class I through IV patients
Note: New York Heart Association (NYHA)
9
MCS Portfolio and Pipeline: Maintaining and Enhancing Competitiveness
Pumps
HVAD
Longer sintering
DT indication
MVAD
Next-gen, more
versatile pump
CircuLite
Class III, partial-
assist device
Longhorn
Eliminates
outflow graft
Electronics
Lavare
HVAD pulsatility
algorithm
HVAD Controller
Upgrade
Improved
patient
management
Pal
Versatile,
simple and
smart controller
TET
Eliminates
driveline
Remote
Monitoring
Connected
patient care
Tools
MVAD Gimbal
Angle and
depth
adjustment
Thoracotomy
Tools
HVAD and
MVAD
10
MVAD System Commentary
Controller assembly fix complete, software patch progressing towards submission efficiently Investigation continues with no anticipated design modifications Narrowing our focus to specific areas within our manufacturing process, which we may elect to further tighten up Working with investigators to review status and develop restart plan Finalizing decision tree for return to the clinic
11
HeartWare Leadership Team Here Today
Doug Godshall Peter McAree Mark Strong Jeff LaRose
President & CEO SVP, CFO SVP, Research & Executive VP, Chief
Development and Scientific Officer
Quality
Jim Schuermann Larry Knopf Chris Taylor Stuart Logan
SVP, Sales and SVP, General VP, Corporate Director, New Business
Marketing Counsel and Communications and Development and
Secretary Investor Relations Strategic Planning
12
Why Mitral and Why Valtech? Because We Can Win BIG
Heart Failure Cardioband is a leading interventional MR technology, and
Leadership Valtech offers technology leadership throughout its pipeline
Significant Market Mitral market likely > TAVR
Only 1 significant mitral repair competitor today, replacement
Opportunity years away
Better Meet Our Mitral regurgitation is either secondary to HF or leads to HF
Patients’ Needs Tricuspid and mitral regurgitation are common in VAD patients
Concentrated customer base with significant VAD center overlap
Service Our Heart Increasing involvement of Heart Team in VAD and with HTWR
Team Customer HTWR becomes a more sophisticated clinical partner with
meaningful cross-selling and market development synergies
Attractive Growth Better gross margin and accelerated growth
Goal is to build a $1B+ revenue company – Valtech a key
Platform contributor
13
Valtech Overview
Larry Best
Executive Chairman, Valtech Cardio
Amir Gross
Founder and CEO, Valtech Cardio
14
Why HeartWare?
Strategic fit
Size and stage
Management, track record and culture
Stock vs. cash
Risk / reward
Valtech commitment
15
Valtech: Valve Expertise Has Yielded Best-in-Class Repair and Replacement Portfolio for Mitral and Tricuspid Valves
Products
Leveraging proven surgical solutions
– Annuloplasty
– Chord repair
– Valve replacement
Near-term revenue generation
– Multiple commercial-stage products
over next few years
Serving multiple large, untapped markets
– Portfolio potentially serving – and
expanding – current mitral and tricuspid
markets
Team
Experienced
– 62 deep; broad functional experience
– Exceptional R&D; significant valve and
transcatheter delivery experience
Proven execution
– Since 2010: 4 FIM (>120 patients
treated), 2 CE Mark products
16
Valtech Cardio Leadership Team Here Today
Amir Gross Nitza Shoham, Ph.D. Tal Sheps Ilia Hariton, Ph.D.
Founder & CEO VP, Clinical & VP, Cardioband VP, Research &
Regulatory Affairs Development
17
Valtech Offers a Comprehensive Set of Interventional and Surgical Valve Solutions
Late-Stage
Cardinal Cardinal V-Chordal Cardioband Cardioband Cardiovalve
Mitral Tricuspid Mitral Mitral Tricuspid Mitral
d
CE Marked, Adjustable, Surgical and CE Marked Transcatheter, Transcatheter,
adjustable, semi-rigid, transcatheter transcatheter, transfemoral- transfemoral-
semi-rigid, surgical mitral transfemoral- transseptal transseptal
surgical tricuspid chordae transseptal tricuspid valve and
mitral valve valve repair system mitral valve reconstruction transapical
annuloplasty annuloplasty reconstruction system mitral valve
ring system ring system system replacement
Surgical Therapy Interventional (Transcatheter) Therapy
18
Valtech Management’s Perspective
Early HeartWare investment enabled creation of a strong partnership
Appreciation for HeartWare’s achievements with VADs and its proven commercial execution
Strong alignment of cultures and shared vision; we have watched HeartWare for 8 years
We want to develop technologies and evaluate them clinically; we want an aggressive partner to commercialize them
With HeartWare, we will be able to continue our mission; we chose the best partner
Stock transaction further aligns interests and allows for employees to participate in long-term success; structure is highly motivating to the team
19
HeartWare Market View and the Strategic Combination
Doug Godshall
President and CEO, HeartWare International
20
Intensive 3+ Year Valve Diligence Process
Activity
HeartWare
Strategy
Market Research
Market Tracking
Diligence
Valtech
Investment
2013
2014
2015
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Strategic Valve Vision Set
Initial Valve IC
Comprehensive qualitative and
and CT
quantitative market analysis by
2015 update to
Surgeon
independent consulting firm
market analysis
Interviews
Discussions with VAD Customers
Major Valve Conference Attendance
In-Person Valve KOL Interviews
Valtech
Other Companies
$5M Bridge Loan
$10M Investment
in Valtech (~3% equity)
Business Combination Announced
21
HeartWare Customer Feedback
Valtech Deal
“Good acquisition as this reflects
Heartware’s dedication to heart failure”
“Makes sense from a portfolio perspective”
“Great products and the next step forward for mitral therapy”
“You guys hit a home run”
Cardinal Surgical Device
“Easier to implant”
“Great results”
“Sizing is better and easier then with the other rings”
“Better positioning than rigid rings”
“No issue with higher pricing as results are good”
Cardioband Transcatheter Repair Device
“Incredible technology! I’m a fan of MitraClip but there is a bigger application for Valtech, I can’t wait to get it”
“Cardioband is the best approach to mitral repair that we have seen so far”
“Ingenious design. When are you looking for clinical trial sites?”
VADs and Valves
“I would repair the tricuspid with
Cardioband before implanting a VAD”
“As a VAD surgeon, I think about mitral regurgitation every day of the week”
22
Structural Heart Market: High Unmet Need in Mitral and Tricuspid Regurgitation
Structural Heart Disease
Estimated U.S. Prevalence
Other Aortic
~1M Stenosis
1.0M
Tricuspid
Regurgitation
1.6M
Mitral
Regurgitation
4.2M
Primary Disease Available Currently
Cause Treatments Treated
Aortic Age-related Surgical
replacement 80,000
Stenosis calcification TAVR
Surgical repair &
Mitral Left heart replacement
Regurgitation failure Transcatheter 60,000
repair: MitraClip®
Tricuspid Right and left Surgical repair
and 8,000
Regurgitation heart failure replacement
Surgical atrial
Other Varies by and ventricular Varies by
indication septal defect indication
repair
U.S. mitral and tricuspid opportunities in total estimated to be ~6x the aortic stenosis market
Note: Tricuspid prevalence numbers estimate population of moderate-severe tricuspid regurgitation patients only. Source: AHA Heart Disease & Stroke Statistics 2015, Nkomo 2006 Lancet, Stuge 2006 JTCS, US Census Data.
MitraClip is a registered trademark of Abbott Laboratories.
23
Uniquely Complex Anatomy of the Mitral Apparatus Makes Transcatheter Mitral Valve Replacement Design More Challenging
Atrial Septal Defect
Valve size critical to prevent atrial wall damage from transseptal delivery
LVOT Obstruction
Blocks blood path causing increased after-load on the left ventricle
Leaflet Control
Prevent systolic anterior motion (SAM) LVOT obstruction
Chordae Interference
Transapical delivery may damage chordae
High Pressure System
Risk of valve dislodgement, requires secure fixation
Irregular & Variable Mitral Annulus Shape
Inadequate fit may result in paravalvular leak
Valve Positioning and Placement
Non-planar annulus, optimal placement can be challenging
Anchoring Without Radial Force
Establish secure fixation in non-distinct annulus, prevent conduction system interference
Left Ventricle Trauma
Transapical access damages diseased left ventricle tissue
24
Our Mitral and Tricuspid Market Investment Thesis Consistent With Valtech’s Portfolio Approach
Repair’s Value Surgical mitral repair long understood to be the preferred approach
Understood From
Surgery Annuloplasty the most frequently performed surgical mitral repair
Mitral Anatomy Multiple tools required to serve the spectrum of MR patients
Complex A leading player in the mitral market needs repair and replacement
Transcatheter mitral valve replacement (TMVR) will be much more
Physicians Will Prefer challenging than TAVR
to Repair First Repair will be a safer treatment and therefore the cardiologist’s first
choice when, possible
Transseptal Required The majority of inoperable mitral and tricuspid regurgitation patients
to Meet TMVR are sick heart failure patients; left ventricular trauma a watch-out
Potential Transseptal delivery necessary to achieve large market opportunity
Tricuspid regurgitation is drastically undertreated today, and there is a
Tricuspid Next growing body of evidence supporting intervention
Horizon Tricuspid is a repair story; annuloplasty is preferred surgical approach
25
Physicians Prefer a Transseptal Approach for its Better Safety Profile
Transapical
First-generation transcatheter replacement valve access method, requires surgical thoracotomy and left ventricle puncture
“Transapical is relatively invasive. We often need to crack ribs and collapse a lung to gain adequate access.” –U.S. CT
Surgeon
“Transapical is relatively a straight shot to the mitral valve but you risk chordal interference and left ventricle damage.”
–U.S. CT Surgeon
Transseptal
Transseptal is expected to be a next- generation alternative to transapical and is perceived to be less invasive
“If transseptal was feasible, I think you would have a winner there in terms of the access.” –U.S. CT Surgeon
“If I had an equivalent transseptal device, I would consider a patient for a transseptal over transapical.” –U.S. CT
Surgeon
Source: Robin Cooper Interviews 2015 (n=15).
26
Replacement Will Eventually Have Strong Place in the Market; Repair Represents Much Stronger Near- and Mid-Term Opportunity as Valve Technology Matures
Repair
Transapical Replacement Transseptal Replacement
Phase I
Phase II
Phase III
Repair + Early TA Replacement
Repair + Mature TA and Early TS Replacement
Repair + Mature TS Replacement
Mature repair market
Repair proven Repair safer and more disease Broader stages use in earlier and Younger FMR patients
Growth in DMR
lower-risk patients segment
Mostly reserved for
high-risk severe FMR
Replacement Early transapical patients Mature transseptal
replacement replacement valves
Mature transapical,
outcomes varied Older FMR patients
early transseptal
replacement Select DMR etiologies
Note: Transapical (TA), Transseptal (TS).
27
Covering the Spectrum of Heart Failure
Heart Failure Continuum
Today
Cardioband Mitral
Tomorrow
Cardinal Mitral and Cardioband Mitral MVAD HVAD
Tricuspid and Tricuspid
Future
Cardinal Mitral and Cardioband Mitral Cardiovalve Circulite MVAD HVAD Longhorn
Tricuspid and Tricuspid
28
Powerful Investment Thesis: Consistent, Meaningful Milestone Cadence
2016 2017 2018 2019
MVAD CE MVAD long-
MVAD restart MVAD short- term submission
HVAD DT term indication
MVAD IDE CircuLite IDE
CircuLite FIM TET FIM Longhorn FIM
Cardioband
Launch Cardioband
Cardiovalve PMA
Cardioband FIM Cardioband TR Cardiovalve
IDE CE
Cardioband TR CE Mark
Cardinal IDE Cardinal PMA Cardioband TR
Launch EU PMA
Cardioband TR Cardinal IDE Cardiovalve
FIM IDE
VAD Milestones Valve Milestones
29
HeartWare + Valtech: Creating the Technology Leader in Advanced Heart Failure
MVAD System will lead a transformation of MCS portfolio and
VAD market, picking up where HVAD leaves off
Synergies with disease, customer, referral channel and
delivery system will create compounding benefits over next
decade
Adjustable repair of MR and TR expected to emerge as first-
line option, whether surgical or interventional
Nearer-term commercial opportunity in established surgical
and interventional mitral repair market with >$450M in sales
Sophisticated Cardiovalve design and delivery system to
enable HeartWare leadership in mitral replacement
Combined pipeline creates unique opportunity for
differentiated value creation
30
Welcome to Our Guest Clinicians
Michael Mack, M.D., FACC Prof. Francesco Maisano, M.D., FESC
Director of Cardiovascular Surgery Chair of Cardiovascular Surgery
Baylor Scott & White Health University Hospital of Zurich
Prof. Karl-Heinz Kuck, M.D., Ph.D. Paul Grayburn, M.D., FACC
Head of Cardiology Director of Cardiology Research
St. Georg Hospital Hamburg Baylor Heart and Vascular Institute
Paul Jansz, BMed, FRACS, Ph.D. Steven Boyce, M.D.
Deputy Director of Heart & Lung Director of the Advanced Heart
Transplant Program, Surgical Director MCS Failure Program
St. Vincent’s Hospital MedStar Washington Hospital
31
Mitral and Tricuspid Regurgitation Overview:
The Need, Anatomy, Treatment Today and Challenges
Michael Mack, M.D., FACC
Baylor Scott & White Health
32
Conflict of Interest Disclosure
Co-PI- COAPT Trial- Abbott Vascular Sponsor
– Uncompensated
33
Mitral Regurgitation (MR)
Mitral
Regurgitation
Primary
(Degenerative)
34
Secondary MR: Disease of the Left Ventricle Not the Mitral Valve
Normal LV Dilated LV tethering one or both leaflets
35
Why Do We Care About Secondary MR?
It is Associated with Advanced CHF !
36
Heart Failure: A Growing Global Concern
Prevalence and Incidence Mortality
Overall 2.1% prevalence: For AHA/ACC stage C/D
5.1M heart failure patients in patients diagnosed with HF:
2010 – 30% will die in the first
825,000 people ? 45 years of year. 3-5
age are newly diagnosed – 60% will die within 5
each year with HF1 years.5
15 M heart failure patients in
the ESC 51-member
countries2
– Overall 2-3% prevalence2
HF prevalence in the US is projected to increase 46% from 2012 to 2030, resulting in > 8M people ? 18 years of age with HF.6
37
Frequency of MR in a CHF Population = 90% Heart Failure Clinic, EF<35%, Class III-IV
Severe
Retrospective Mod-Sev 4.3
12.5 0-Trace
1996-2001 10.4
Echo reports
Quantitative MR
Mild
Moderate 31.9
21.9
Mild-Mod N = 558
11.8
Patel JB- J Cardiac Failure 10:285, 2004
38
FMR: A Vicious Cycle
MR
Annular-Ventricular Volume overload
dilatation
39
100 Ischemic or Nonischemic Cardiomyopathy
(%)
80
Survival 60 No FMR
51%
Free 40 Mild-Mod FMR
-
20 p<0.0001 24%
Hospital Severe FMR 11%
0 0 1 2 3 4 5 6 7
Time (years)
Rossi et al. Heart 2011;97:1675-1680
40
100 Ischemic or Nonischemic Cardiomyopathy
(%)
80
Survival 60 No FMR
51%
Free 40 Mild-Mod FMR
-
20 p<0.0001 ERO ?20 24%
Hospital Severe FMR 11%
0 0 1 2 3 4 5 6 7
Time (years)
Rossi et al. Heart 2011;97:1675-1680
41
100 Ischemic or Nonischemic Cardiomyopathy
(%) EF 34%
80 LVSD 51mm
Survival 60 EF 33% No FMR
LVSD 53mm 51%
Free 40 EF 29% Mild-Mod FMR
—LVSD 57mm
20 p<0.0001 24%
Hospital Severe FMR 11%
0 0 1 2 3 4 5 6 7
1 2 3 4 5 6 7 8
Time (years)
Rossi et al. Heart 2011;97:1675-1680
42
Degree of MR Predicts Survival Post MI
Bursi, F. et al. Circulation 2005;111:295-301
43
Degree of MR Predicts Survival in CHF (Ischemic and Dilated Cardiomyopathy)
Trichon, AJC 2003;91:538-543
44
Heart Failure is Associated with High Hospitalization and Readmission Rates
In 2010, there were 1 million hospitalizations in the US with HF as the principal diagnosis
Hospitalization rate did not change significantly from 2000
Average length of hospital stay
Approximately 5 days (US)
11 days (Europe)
HF is also associated with high readmission rates:
~25% all-cause readmission within 30 days and ~50% within 6 months
45
Medically Managed Patients With Severe MR Outcomes
100%
Mortality
90%
90%
Proportion of Surviving Patients Hospitalized for Heart
80% Failure
70% 68%
Patients 60% 58%
50% 50%
of 50% 46%
41%
40% 37%
Percentage 30% 29%
20%
20%
10%
0%
Year 1 Year 2 Year 3 Year 4 Year 5
46
Chronic Severe Secondary Mitral Regurgitation: Intervention
Recommendations COR LOE
MV surgery is reasonable for patients with chronic severe
secondary MR (stages C and D) who are undergoing CABG or IIa C
AVR
MV repair may be considered for patients with chronic
moderate secondary MR (stage B) who are undergoing other IIb C
cardiac surgery
Repair or Replacement not stipulated
47
Secondary MR – Undersized Annuloplasty
Disease of the left ventricle NOT of the mitral valve
MR caused by apical lateral distraction of the papillary muscles tethering the leaflets
Annular dilation is secondary and occurs greatest in the septal-lateral (anterior-posterior) dimension
Surgical repair based on over correction of the annular dilation
48
Braun J, et al., Leiden Ann Thorac Surg 2008;85:430 –437
49
Braun J, et al., Leiden Ann Thorac Surg 2008;85:430 –437
50
Secondary MR Before and After Mitral Valve Annuloplasty
51
Surgical Options to Correct Secondary MR
After GDMT and Resynchronization When Appropriate
52
251 Patients Randomized November 18, Primary endpoint- LVESVI at One Year 2013, at NEJM.org.
53
Treatment of Patients with 3-4+ FMR, LVEF>20%, no CABG from 2000-10 (n=1,538)
Conservative Management Isolated MV Surgery 100%
6% 8%
11% 12%
90% 18% 80%
Patients 70% of
60% 50%
% %
% %
40% %
Percentage 30% 20% 10% 0%
All Patients 20%-30% 30-40% 40%-50% 50-60%
Duke Left Ventricular Ejection Fraction (LVEF)
Database
54
Medical Therapy/ Resynchronization
Surgical Intervention
Advanced HF Transplantation
Bridge To
LVAD
Recovery
Destination Therapy
55
â-Blockade therapy in chronic heart failure: Diastolic function and mitral regurgitation improvement by carvedilol
Soccorso Capomolla, MD, Oreste Febo, MD, Marco Gnemmi, mD, Giorgio Riccardi, MD, Cristina Opasich, MD, Angelo Caporotondi, MD, Andrea Mortara, MD, GianDomenico Pinna, BME, and Franco Cobelli, MD, Pavia, Italy
Am Heart J 2000;139:596 Jet Area
9
88 p<0.05
) 2 7 (cm 66 Area 5 Jet 44
3
MR 22 1
00 Baseline 6 Months Baseline 6 Months Carvedilol Control
Capomolla et al. Am Heart J 2000;139:596-608
56
CARE HF
100
CRT
880 72%
(percent) 660 No CRT
56%
440
Survival p<0.002
220
00
0 200 400 600 800 1000 1200
0 1 2 3 4 5 6 7 8
Time (days)
from Cleland et al. N Engl J Med 2005;352:1539-1549
57
Adult and Pediatric Heart Transplants Number of Transplants by Year and Location
5000
Other 4500 Europe 4000 North America 3500 transplants 3000 of 2500
Number 2000 1500
1000 500 0
JHLT. 2013 Oct; 32(10): 951-964 58
Medical Therapy and Resynchronization
Transcatheter Mitral and Tricuspid Intervention
Surgical Intervention
Advanced HF Transplantation
Bridge To Recovery
Destination Therapy
59
Transcatheter Mitral Valve Repair MitraClip System
60
Commercial MitraClip Experience: Worldwide
– Treating Centers:
Patients:
Implant Rate:
Etiology:
Functional MR
Etiology
463
25,508 Mixed 96% 13%
65% DMR 22%
Degenerative MR 22% FMR 65%
Mixed 13%
Data as of 01/31/2015. Source: Abbott Vascular.
61
COAPT: Trial design
~420 patients enrolled at up to 75 US sites
Significant FMR (?3+ by core lab)
Not appropriate for mitral valve surgery (local heart team) Specific anatomical criteria
November, 2015
Randomized oup
M care
300 Patients at
84 Sites
PIs: Michael Mack and Gregg W. Stone
Sponsor: Abbott Vascular 62
Transcatheter Mitral Valve Replacement -TMVR
63
64
Transcatheter Mitral Valve Replacement -TMVR
>$1 Billion spent in last 3 months for 5 companies with a combined total of ~50 cases !
65
Increases in Pressure Start the Cycle of Worsening Heart Failure
Pulmonary Artery Pressure
Left Heart Failure
Right Heart Failure
Left Atrial Pressure Cardiac Output Right Atrial Pressure
Dyspnea Fatigue Heptic Insufficiency
Orthopnea Confusion Renal Insufficiency
Pulmonary Edema Renal Insufficiency Peripheral Edema
Peripheral Edema
66
Pathophysiology of Functional Tricuspid Regurgitation
Left-sided heart disease
Atrial fibrillation
RV afterload increase (+/- PH)
RV remodeling
Altered RV function
Tricuspid annular dilation
Abnormalities in tricuspid anatomy and function lead to FTR
Source: Dreyfus 2015 JACC.
67
68
Majority of TR Surgical Procedures are Repairs Performed in Combination with Mitral Valve Surgery
STS Database Tricuspid Valve Procedures
6000
Repair Replacement
5000
Operations 4000
of 3000
Number 2000
1000
0
2004 2005 2006 2007
Bicuspidizatio De Vega Annuloplasty Clover
n Cinch Repair Prosthetic Technique
posterior Suture around ring Leaflet suture
leaflet the annulus
Source: Kilic 2013 Annals of Thoracic Surgery, Rogers 2009 Circulation, Taramasso 2012 JACC.
69
FMR / FTR Summary
Advanced CHF is a growing problem with significant mortality and repeat hospitalization
Most patients with CHF have FMR and in very advanced stages FTR
GDMT and resynchronization therapy work
Surgery is seldom used
Transcatheter therapy and MCS are major opportunities
A vertically integrated device company capable of managing different stages of CHF disease state makes total sense
70
Valtech Portfolio Overview
Prof. Francesco Maisano, M.D., FESC
University Hospital of Zurich
71
Valtech Cardio Portfolio:
A Journey from Valve Surgery to Interventions
FRANCESCO MAISANO, MD
72
73
Increasing Prevalence of Valvular Heart Disease in the Elderly
Population-based Studies Olmsted County, MN
All valve disease Mitral valve disease Aortic valve disease
14 14
severe Almost 1 in 10 people or 12 >75 years old have 12
(%)
10 mod-severe MR! 10
moderate disease 8 8
6 6 of valve 4 4
2 2
Prevalence 0 0
<45 45-54 55-64 65-74 >75 <45 45-54 55-64 65-74 >75
Source: Nkomo VT at al. Lancet 2006;368:1005-1011
74
Management of 3+/4+ MR with HF (CCF)
1,095 pts* with 3+/4+ MR and HF between 2000 and 2008 (74% FMR, 21% DMR). Rx before 10/2011:
DMR pts (n=226): 84% MV surgery, 16% medical Rx
FMR pts (n=814): 36% MV surgery (77% w/CABG), 64% med Rx.
Un-operated pts had lower LVEF (mean 27% vs. 42%, p<0.0001 and higher STS score (median 5.8 vs. 4.0, p<0.001) compared with operated pts.
100
Prognosis of Mortality 90
Surviving pts hospitalized for HF 171 of 474 (36%)
un-operated 80
68 un-operated pts pts with 58 60 with FMR and 3+/4+ MR 50 50 Patients 46 good echos
41
and HF 40 37 of would have been
29
% eligible for
20 20
MitraClip based 0 on published
1 2 3 4 5 criteria
Years
Source: Goel SS et al. JACC 2014;63,:185–90
75
Isolated MV Surgery: STS Database 2002-2010
n = 77,836 cases; 58.4% repair, 41.6% replacement
2 eras: 2002-2006, 2007-2010 Overall op mortality
MV repair rate ’d from 54.8% to 61.8% ?’d from 3.2% to 2.9% (P=0.03),
(p=0.002); seen in every risk strata. and RR of observed vs. expected
MV repair pts were much lower risk; STS <1% mortality ?’d from 1.13 (1.06–1.19) to
in 67% repair vs. 18% replacement. 1.02 (0.97–1.08), P=0.02
Source: Chatterjee S et al. Ann Thorac Surg 2013;96:1587–95
76
Isolated MV Surgery: STS database 2002-2010
Proportion Treated with MV Repair vs. Replacement According to Predicted Risk
N = 77,836 cases; 58.4% repair, 41.6% replacement
2002—2006 2007—2010
Repair Replacement Repair Replacement
100% 100% 80% 80% 60% 60%
40% 40%
70.0
62.4
20% 20%
24.5 29.6 22.7
17.8 12.8 17.7
0% 0%
Low risk Int risk (4- High risk Extreme Low risk Int risk (4- High risk Extreme (<4%) <8%) (8-<12%) risk (?12%) (<4%) <8%) (8-<12%) risk (?12%)
Source: Chatterjee S et al. Ann Thorac Surg 2013;96:1587–95
77
Transcatheter Replacement Valves
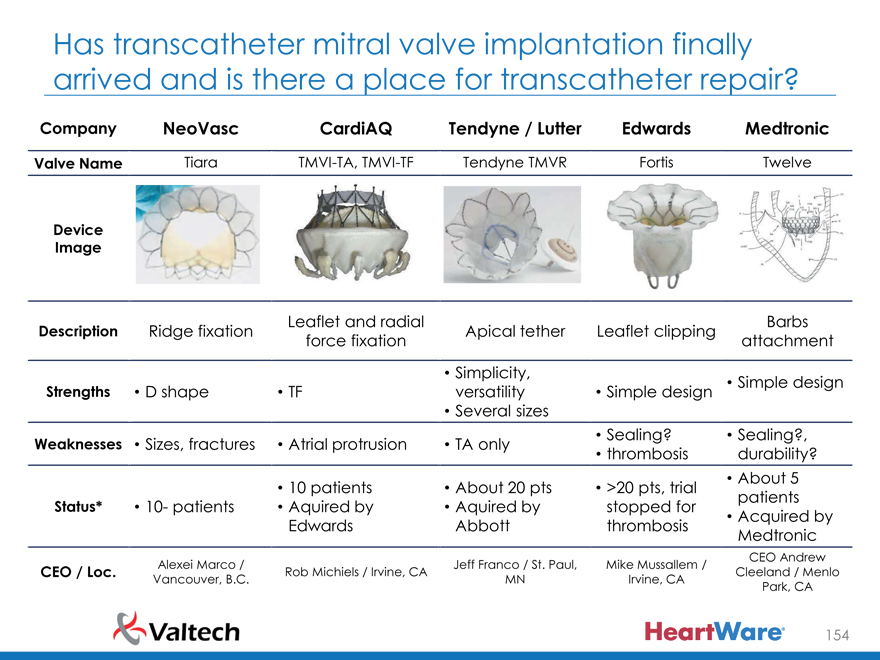
Company NeoVasc CardiAQ Tendyne / Lutter Edwards Medtronic
Valve Name Tiara TMVI-TA, TMVI-TF Tendyne TMVR Fortis Twelve
Device
Image
Leaflet and radial Barbs
Description Ridge fixation Apical tether Leaflet clipping
force fixation attachment
Simplicity,
Strengths D shape TF versatility Simple design Simple design
Several sizes
Sealing? Sealing?
Weaknesses Sizes, fractures Atrial protrusion TA only thrombosis Durability?
10 patients About 20 pts >20 pts, trial About 5
Status* 10- patients Acquired by Acquired by stopped for patients
Edwards Abbott thrombosis Acquired by
Medtronic
Alexei Marco / Jeff Franco / St. Paul, Mike Mussallem / CEO Andrew
CEO / Loc. Rob Michiels / Irvine, CA Cleeland / Menlo
Vancouver, B.C. MN Irvine, CA Park, CA
78
Mitral Regurgitation Disease Process
Mitral
Regurgitation
Annular- Volume
Ventricular Overload
Dilatation
79
Rationale for Interventional Treatment of FMR in HF
To reduce symptoms To prevent acute HF
by reducing LA by reducing the
pressure under resting chance of flash
conditions pulmonary edema
To improve To initiate reverse
compliance to remodeling by
therapy by increasing reducing volume
cardiac output overload
80
LA pressure acute reduction in a HF patient undergoing adjustable transcatheter mitral annuloplasty
81
Significant Clinical Benefits Have Been Reported in Severe Heart Failure Despite Optimal Medical Therapy
94% successful implant rate (cohort of 50 patients) THERAPY OUTCOMES
Improved short-term survival related to NYHA
functional class
Significant improvements in 6-minute walk test, 94% IMPLANT
MLHFQ score, and NT-proBNP plasma levels, as well RATE*
as EF, LV ESV and LVEDV which supports reverse
remodeling of the LV Success
CLINICAL IMPROVEMENTS at 6 months
MR GRADE NYTHA CLASS MLHFQ SCORE
87% less than 72% in class I or II Mean improvement
or equal to 2+ of -22 points
6 MINUTE WALK TEST
Median of 311 meters 81 meters Median of 230 meters(P <0.0005)
at 6 months at baseline 19 patients
Source: Franzen et al. MitraClip Therapy In Patients With End-Stage Systolic Heart Failure. Eur J Heart Failure. 2011; 13: 569-576.
82
Hospitalizations for Heart Failure
1.6 % reduction 0.0001
1.4 1.52 Rate 1.2 Year
1.0
Patient 0.8 Hospitalization Per 0.6
0.4 0.47 HF
0.2 0.0
6 Months Prior to MitraClip 6 Months Post-Discharge
N=135 N=128†
† Patients who died before discharge are excluded from the post-discharge analysis.
Source: ACCESS-EU Phase II – PCR London Valves 2014.
83
Timing
NYHA Treatment Stage Setting
Stage I
Outpatient Clinic Stage II
Stage III
Hospitalization
Stage IV
Inotrope Use
Time From Diagnosis
84
Timing
NYHA EARLY MID LATE Treatment Stage Setting
Stage I
Outpatient Clinic Stage II
Medical + CRT LVAD Stage III Therapy Mitral HTx
Hospitalization
Stage IV
Inotrope Use
Time From Diagnosis
85
Timing
NYHA Treatment Stage Setting
Stage I
Outpatient Clinic Stage II
Stage III
Hospitalization
Stage IV
Inotrope Use
Time From Diagnosis
86
TAVI vs. Transcatheter Valve Interventions
Variability of mitral valve
disease
– Multiple devices
– Multiple techniques
Anatomical complexity
– Most techniques are different
from a “stent-based”
approach
Image guidance is more
sophisticated
– Echocardiography is the
leading image guidance
method
87
The Landscape of Valve Surgery and Interventions
Conventional Minimally
Robotic Transcatheter Surgery invasive
88
Overlapping Targets
SURGERY INTERVENTIONAL CARDIOLOGY
Less invasive
89
TEAMWORK
90
CardioBand is an example of the natural evolution of a surgical technique into a percutaneous intervention
91
History of Mitral and Tricuspid Interventions
Annuloplasty from Evolution to Revolution
1960 2015
First steps Classic Physio Etiologix Cardinal Cardioband
1968 1982 2006 2010
Carpentier A. Carpentier- First Adjustable
La Edwards asymmetric Semi-Rigid
valvuloplastie CLASSIC ring designed Annuloplasty
reconstitutive annuloplast to treat Ring System
ring asymmetric
dilatation
92
Unhappy FMR Repair Surgeon
93
Etiology Specific Annuloplasty Rings
US2015230920 (A1)—METHODS FOR REPAIR OF ABNORMAL MITRAL VALVES
94
Maisano et al. J Am Coll Cardiol 2011;58: 2174–82
95
Analysis of Overall Outcomes With a Specific Technique (1998)
96
Endovascular Evolution Preclinical Testing (2007)
97
Structure vs. Function
Structure
Function
Lesion
Dysfunction
98
Mechanism of Regurgitation Functional Classification
« Surgeons are not basically concerned with lesions. We care more about function. Therefore one may define the aim of a valve reconstuction as restoring normal leaflet function rather than normal valve anatomy »
—A. Carpentier, the French Correction 1984
99
The Future of Valve Interventions
Cross-fertilization between surgical and interventional devices, will offer a better individualized treatment
Innovative solutions for the treatment of mitral valve disease should be simple, easy to use, reproducible, effective in the long term
To simplify minimally invasive approach, more sutureless devices should be developed
To optimize outcomes, fine tuning of devices under physiologic conditions are needed
100
Sutureless/knot-less Technologies
Cor-Knot
SAPIEN in MAC Open Access
101
Annuloplasty
Annuloplasty is the most commonly performed surgical repair procedure
As a stand-alone in patients with FMR
In combination with leaflet repair in DMR patients
Source: http://my.clevelandclinic.org/services/heart/services/valve-treatment/mitral-valve-mitral-valve-repair
102
History of Mitral and Tricuspid Interventions
Annuloplasty from Evolution to Revolution
1960
2015
First steps
1968
Carpentier A. La valvuloplastie reconstitutive
Classic
1982
Carpentier-Edwards CLASSIC annuloplasty ring
Physio
1995
Phisio II Global Shape optimisation
Etiologix
dilatation
Cardinal
2010
Adjustable Semi-Rigid Annuloplasty Ring System
Cardioband
103
Cardinal Adjustable Annuloplasty Ring
104
Anterior section (Rigid)
Posterior section (Flexible)
Cardinal System
Adjustment Handle
9 Sizers
(24-40mm)
Sizer Handle
Ring (3 sizes)
Cardinal Ring Sizes Corresponding Sizers
Small 24-32
Medium 28-36
Large 32-40
105
Standard Implant
106
Weaning from CPB
107
10.00
8.00
4.00
2.00 p<0,0001
0.00
before adjustment after adjustment
108
History of Mitral and Tricuspid Interventions
Annuloplasty from Evolution to Revolution
1960 2015
First steps
1968
Carpentier A. La valvuloplastie reconstitutive
Classic
1982
Carpentier-Edwards CLASSIC annuloplasty ring
Physio
1995
Phisio II Global Shape optimisation
Etiologix
2006
First asymmetric ring designed to treat asymmetric dilatation
Cardinal
Cardioband
109
Surgical Annuloplasty Delivered With a Catheter
110
Cardioband
111
Benefits of Percutaneous Direct Annuloplasty by Cardioband
Trans-femoral venous access (transseptal) –best for safety
Supraannular fixation like in surgery
Significant Reduction of Annular dimensions –device enables reduction of up to size 28 surgical ring Preserves the native anatomy –keeps future options open
112
Direct Annuloplasty
Cardioband implantation
Implantation completed
Annular reduction
113
Fluoro and Echo Guidance
114
Baseline
Final Size
115
95% patients with MR2+ At 12 Months By Core Lab
Cardioband MR Reduction
Core Lab; Dr. Paul Grayburn, Baylor University
100%
Patients 80% of
60%
Percentage 40% 20%
0%
+
+
Baseline n=45
88% MR 2+ at Discharge
+
+
0-1+
Discharge n=42
83% MR 2+ at 1 Month
+
+
0-1+
87% MR 2+ at 6 Months
+
+
0-1+
95% MR 2+ at 12 Months
+
+
0-1+
116
Septo-lateral Diameter Reduction (Intraop TEE)
A-P Diameter [mm]
15 20 25 30 35 40 45
P<0.01
36±5 mm
20% average reduction
Baseline
29±6mm
Post procedure
117
Surgical MVR
Interventional MV Rep
Interventional MVR
Surgical MV Rep
118
Cardioband Evolution (Pipeline)
2006
2017
Cardinal
2010
Adjustable Semi-Rigid Annuloplasty Ring System
Cardioband
2013
Transfemoral “Surgical-like” annuloplasty for Mitral Valve Repair
Cardioband TR
2016
Transfemoral “Surgical-like” annuloplasty for Tricuspid Valve Repair
Cardioband Leaflet
2017
Transfemoral “Surgical-like” leaflet augmentation for Mitral and Tricuspid Valve Repair
More to Come…
Cardioband is a Platform
119
120
Cardioband Tricuspid
Tricuspid
121
Steve Bolling TVT 2015
122
Steve Bolling TVT 2015
123
Steve Bolling TVT 2015
124
Cardioband TR
125
Feasibility
P S
P S
A
P S
A
S A
FTR Surgical Annuloplasty
127
Mirror the Cardioband delivery system
MR TR
128
Considerations When Evaluating Design Approaches For a Interventional TR Solution
Navia, JTCVS 2010
129
130
Transcatheter Treatment of Mitral Regurgitation
Repair / Replacement
Atrial fibrillation ablation, left appendage closure, tricuspid valve treatment, (left ventricle)
131
Cardiovalve
132
Cardiovalve’s Mission
To provide a repeatable and reproducible mitral valve replacement solution via a fully controlled trans-femoral access
Trans-femoral = Safety
(And…
The Interventionalist prefers not to need a surgeon in the room) Cardiovalve is uniquely designed for Trans femoral/trans septal delivery By novel delivery system/implant integrated design a safe and robust procedure is made possible
133
Cardiovalve Transfemoral Procedure and Delivery System
Femoral vein, transseptal access to LA, fully-percutaneous procedure Echo and fluoroscopy based navigation No AV loop required The delivery system:
Multiple degrees of control for accurate positioning High control plus gradual deployment for safety Low profile (<28) to be safely accommodated through the venous system
134
The Implant
Low ventricular presence Cylindrical, robust and classic valve design Ventricular and Atrial anchoring mechanism Highly effective sealing
135
Preclinical Validation
TF procedures in challenging porcine anatomy Chronic proof of concept and clinical effect Pre-Clinical safety demonstrated Technology tested and established
Crossing the Fossa Lower legs deployed Grasping native leaflets Implant is fully deployed
136
Cardiovalve Pre-Clinical
>50 acute, sub-chronic and chronic successful pigs Chronic animals show favorable tissue coverage and good anatomical response
137
Case In a Box Movie
138
Summary and Next Steps
Chronic trials on going
Procedural standardization in full animal set up
Expected FIM within 18 months
139
TMV Replacement AND Repair Valtech’s Vision
Cardioband TF for early and intermediate (late) stage FMR patients Cardiovalve TF for DMR and (late) end stage FMR If Cardiovalve is denied a combined repair of Cardioband and a Clip may be considered Eliminating risk from the equation by using the venous TF approach enables to cover all population in a manner that best fits the patients and their specific disease
There is a place for both!
140
Mitral
Tricuspid
Cardiovalve
Mitral
Tricuspid
Mitral
Tricuspid
Allen et al, Circulation 2012
141
Treating Valve Disease in the Future Today
Patient-optimized care
Transcatheter Interventions
Minimally Invasive Surgery
Open Heart Procedures
Tailored approach – the best option for the patient
142
Complementary Role of Surgery and Interventions
Minimal invasiveness Maximal safety Tailored approach
Unbiased choice
143
144
Device Performance Procedural standards Imaging guidance
Heartware
Preprocedural Planning of TIARA MV implant
(Courtesy of C. Ruiz)
145
Coregistration
146
Repair and Replacement
Prof. Francesco Maisano, M.D., FESC
University Hospital of Zurich
147
The Challenges for Transcatheter Mitral Interventions
Anatomical complexity
– Most technologies are different from a “stent-based” approach
Image guidance is sophisticated
– Echocardiography is the leading image guidance method
Variability of mitral valve disease
– Multiple devices
– Multiple techniques
– Associated procedures
Surgical standards are high
148
FIH transcatheter Mitral Valve Implantation: CardiaQ in 2012
149
8 years before…not even on the radar
150
2003, FIH MitraClip implant in DMR
151
MitraClip Therapy is Routine in Most High-Volume Centers
More than 25,000 patients have been treated with the MitraClip device worldwide
– Nearly 2,000 patients have been enrolled in prospective clinical trials
– A majority are considered high risk for mitral valve (MV) surgery
In 2008, MitraClip received CE Mark for reconstruction of the insufficient MV through tissue approximation
In 2013, FDA approved MitraClip for treating significant symptomatic degenerative mitral regurgitation (DMR) in patients who have been determined to be at prohibitive risk for MV surgery by a Heart Team
152
Surgery for Mitral Regurgitation
Repair and Replacement
+
Atrial fibrillation ablation, left appendage closure, tricuspid valve treatment, (left ventricle)
153
Has transcatheter mitral valve implantation finally arrived and is there a place for transcatheter repair?
Company NeoVasc CardiAQ Tendyne / Lutter Edwards Medtronic
Valve Name Tiara TMVI-TA, TMVI-TF Tendyne TMVR Fortis Twelve
Device Image
Leaflet and radial Barbs Description Ridge fixation Apical tether Leaflet clipping force fixation attachment
Simplicity,
Simple design Strengths D shape TF versatility Simple design
Several sizes
Sealing? Sealing?, Weaknesses Sizes, fractures Atrial protrusion TA only thrombosis durability?
About 5
10 patients About 20 pts >20 pts, trial patients Status* 10- patients Aquired by Aquired by stopped for
Acquired by Edwards Abbott thrombosis Medtronic
CEO Andrew Alexei Marco / Jeff Franco / St. Paul, Mike Mussallem / CEO / Loc. Rob Michiels / Irvine, CA Cleeland / Menlo Vancouver, B.C. MN Irvine, CA
Park, CA
154
Patient Device Procedura Something Bad luck? selection? issues? issues? else?
Higher early mortality compared to early experience with transcatheter repair
155
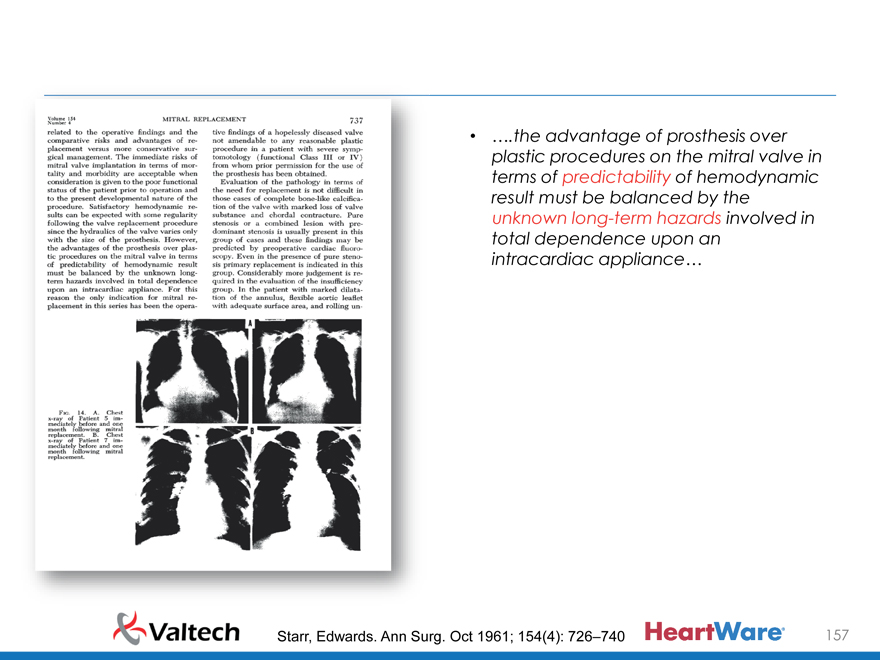
Considerable work has been performed in the develpment of a total mitral prosthesis…
Problems of fixation, valve function and thrombotic occlusion of the prosthesis have prevented long-term survival in most instances
While early satisfactory results were obtained in some patients, survival beyond three months has not been reported to now
Source: Starr, Edwards. Ann Surg. Oct 1961; 154(4): 726–740
156
….the advantage of prosthesis over plastic procedures on the mitral valve in terms of predictability of hemodynamic result must be balanced by the unknown long-term hazards involved in total dependence upon an intracardiac appliance…
Starr, Edwards. Ann Surg. Oct 1961; 154(4): 726–740 157
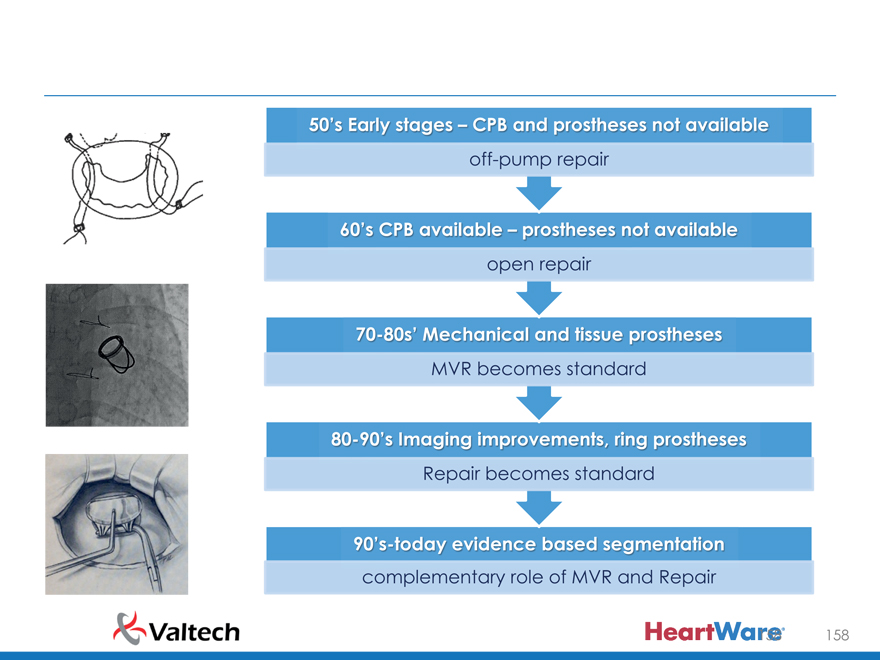
50’s Early stages CPB and prostheses not available off-pump repair
60’s CPB available prostheses not available open repair
70-80’s Mechanica an tissue prostheses
MVR becomes standard
80-90’s Imaging improvement , ring prostheses
Repair becomes standard
90’s today evidence based segmentation complementary role of MVR and Repair 158
Repair and Replacement for FMR
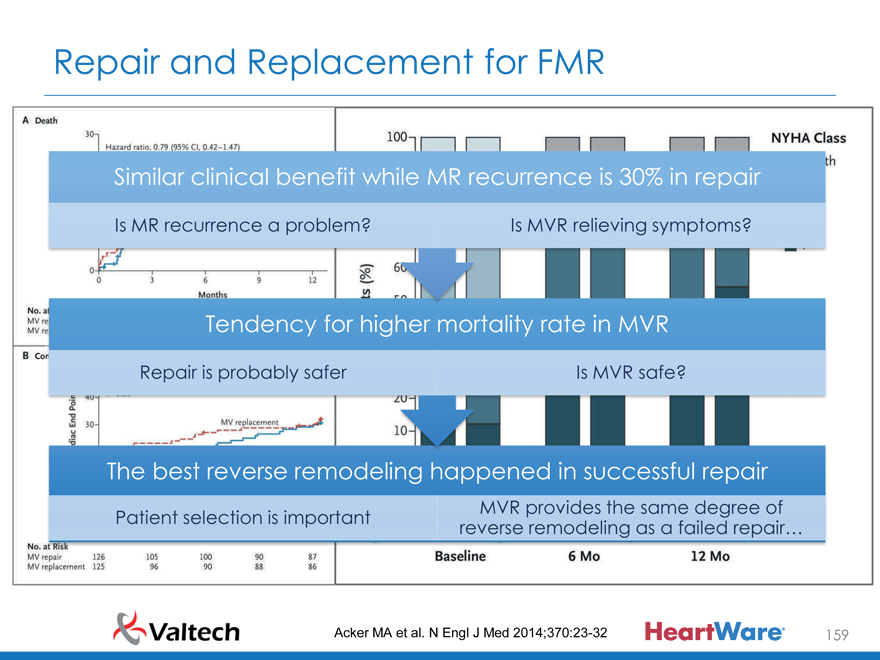
Similar clinical benefit while MR recurrence is 30% in repair
Is MR recurrence a problem? Is MVR relieving symptoms?
Tendency for higher mortality rate in MVR
Repair is probably safer Is MVR safe?
The best reverse remodeling happened in successful repair
MVR provides the same degree of Patient selection is important reverse remodeling as a failed repair…
Acker MA et al. N Engl J Med 2014;370:23-32 159
Is It Better To Survive With Recurrent Regurgitation Or Die With A Competent Mitral Valve?
160
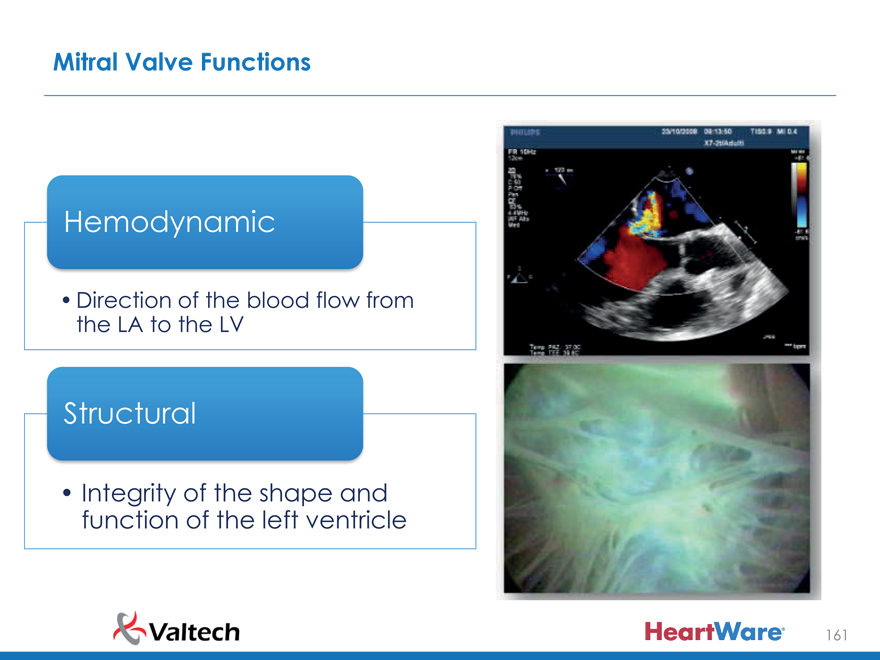
Mitral Valve Functions
Hemodynamic
Direction of the blood flow from the LA to the LV
Structural
Integrity of the shape and function of the left ventricle
161
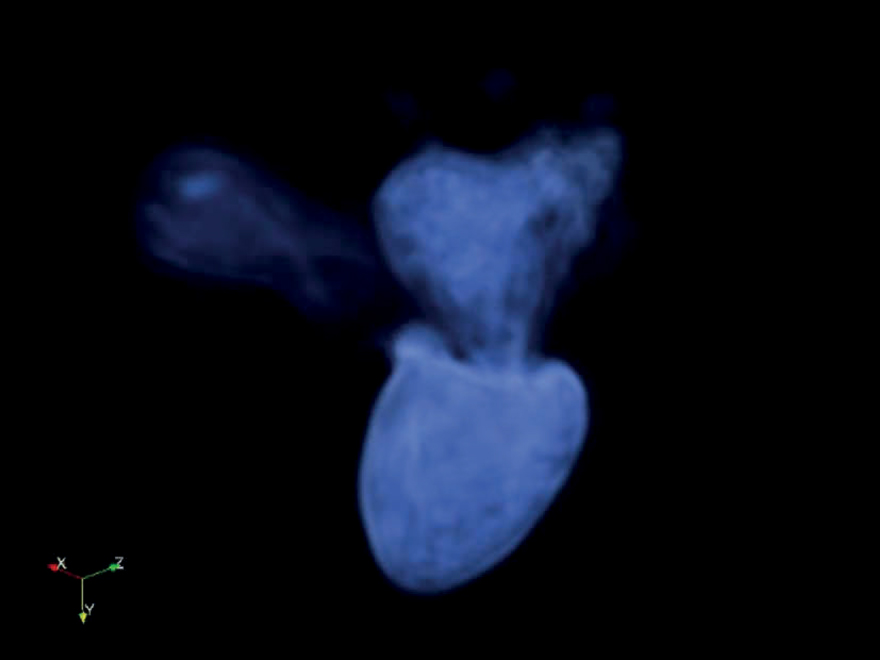
The Mitro-Ventricular Architecture
162
163
Can We Reproduce This?
Any technique will impact physiologic behaviour of the mitral valve apparatus
– Repair is probably better than Replacement and should be first line option in case of early intervention
164
The Challenges
Fixation Sealing Delivery Imaging Indications Durability
Complex and
No More than Patient TMVI vs. Stent dynamic calcifications just a stent selection repair durability structure
Large High pressure Need for Leaflet
Planning Timing anatomy environament orientation degeneration
Non circular Risk of LVOT Additional Fixation fixation and and dynamic Guidance Anticoagulation obstruction sealing elements anatomy features
165
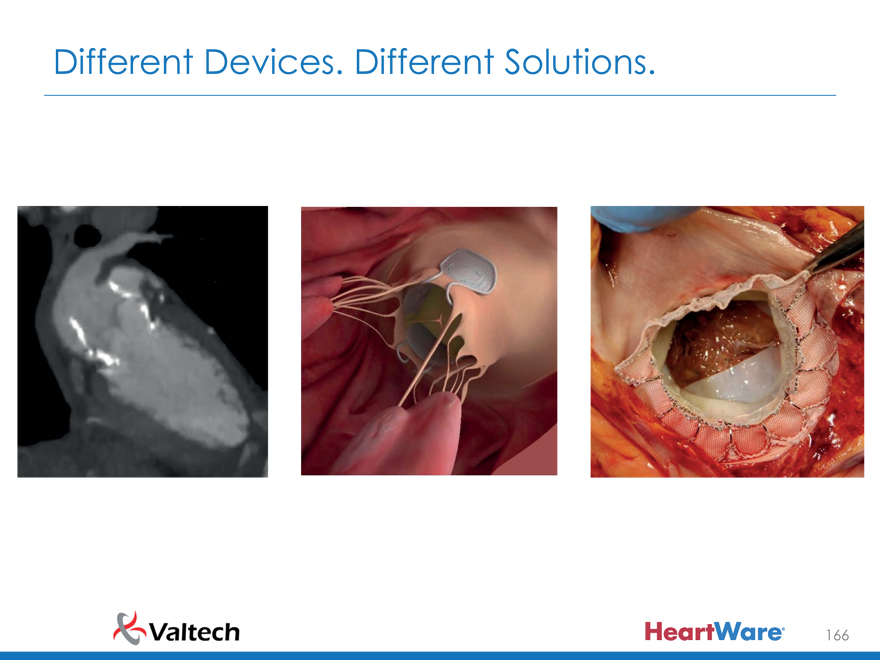
Different Devices. Different Solutions.
166
The Complementary Roles of Transcatheter Techniques
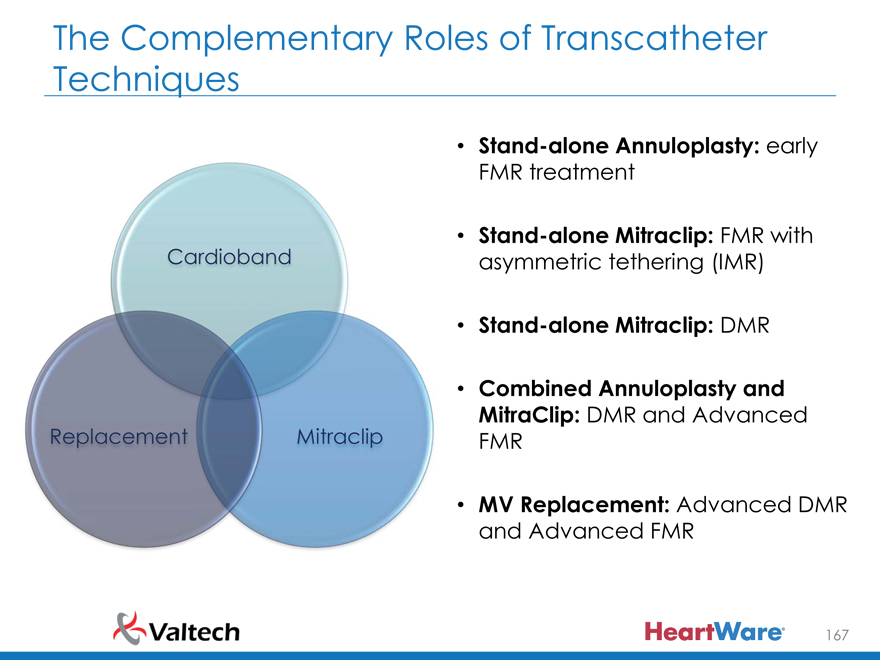
Stand-alone Annuloplasty: early FMR treatment
Cardioband • Stand-alone Mitraclip: FMR with asymmetric tethering (IMR)
Stand-alone Mitraclip: DMR
Combined Annuloplasty and
Replacement Mitraclip MitraClip: DMR and Advanced
FMR
MV Replacement: Advanced DMR and Advanced FMR
167
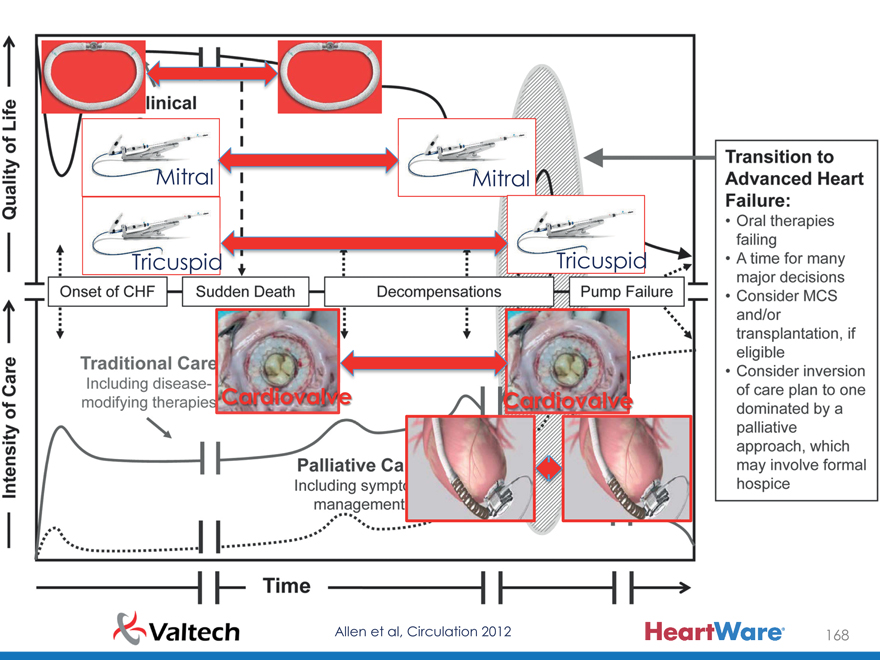
Mitral Mitral
Tricuspid Tricuspid
Cardiovalve Cardiovalve
Allen et al, Circulation 2012 168
Transapical vs. Transfemoral
169
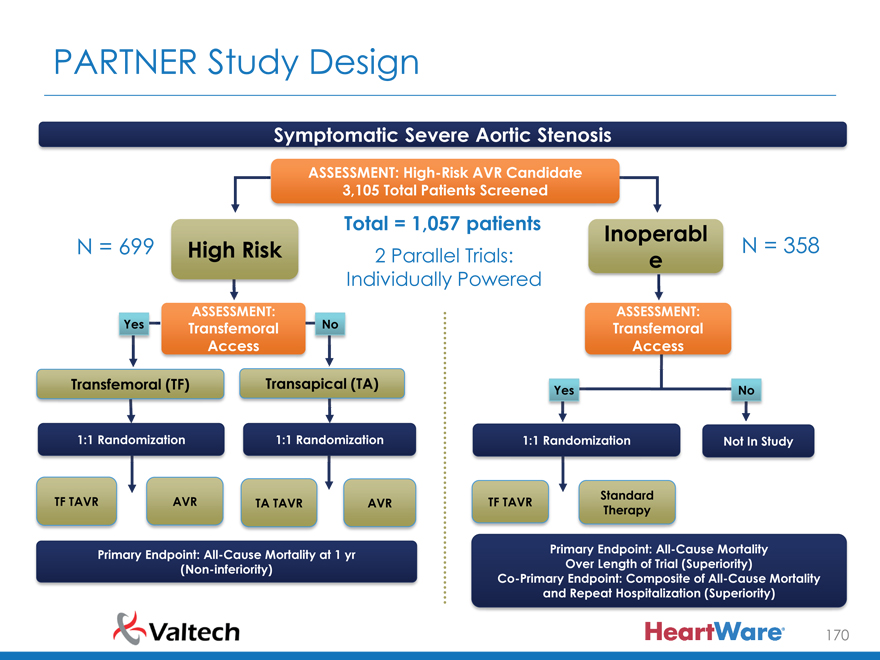
PARTNER Study Design
Symptomatic Severe Aortic Stenosis
ASSESSMENT: High-Risk AVR Candidate 3,105 Total Patients Screened
Total = 1,057 patients Inoperabl
N = 699 High Risk 2 Parallel Trials: N = 358
e
Individually Powered
Yes ASSESSMENT: No ASSESSMENT:
Transfemoral Transfemoral Access Access
Transfemoral (TF) Transapical (TA) Yes No
1:1 Randomization 1:1 Randomization 1:1 Randomization Not In Study
N = 244 N = 248 N = 104 N = 103 N = 179 N = 179
Standard TF TAVR AVR TA TAVR AVR TF TAVR
Therapy
VS VS VS
Primary Endpoint: All-Cause Mortality at 1 yr Primary Endpoint: All-Cause Mortality (Non-inferiority) Over Length of Trial (Superiority) Co-Primary Endpoint: Composite of All-Cause Mortality and Repeat Hospitalization (Superiority)
170
Al Cause Mortality Transfemoral (N 492)
HR [95% CI] =
0.83 [0.60, 1.15] P (log rank) = 0.25
26.4
22.2
No. at Risk Months
TAVR 244 215 188 119 59 AVR 248 180 168 109 56
171
All-Cause Mortality Transapical (N 20 )
HR [95% CI] =
1.22 [0.75, 1.98] P (log rank) = 0.41
29.0
27.9
No. at Risk Months
TAVR 104 83 72 28 8 AVR 103 72 68 30 9
172
All-Cause Mortality
Transfemoral v TA vs Surgery
Months
TA approach is more invasive than TF, does not reduce the operative risk Long term results are suboptimal
selection bias?
long term effect of the apical scar?
173
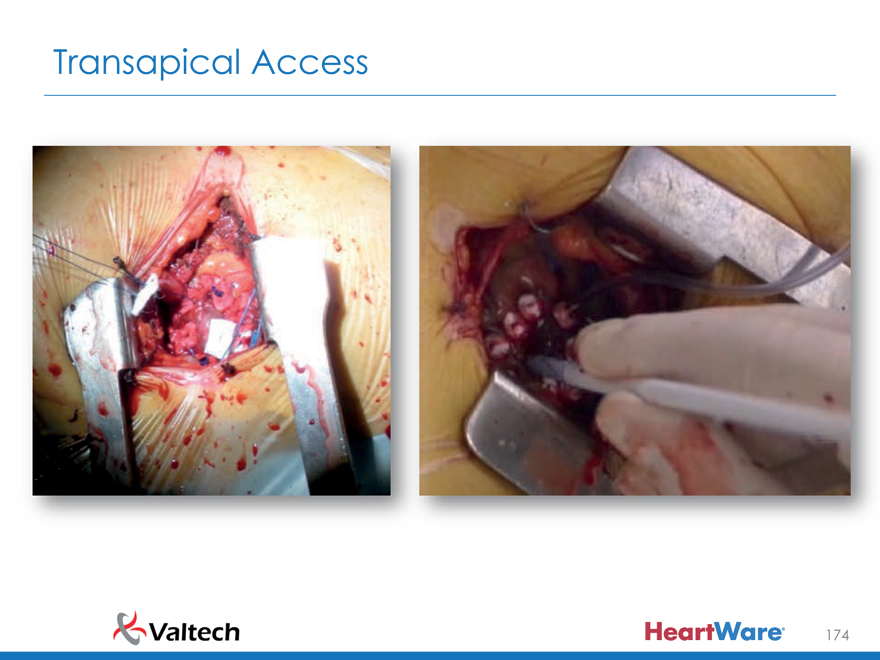
Transapical Access
174
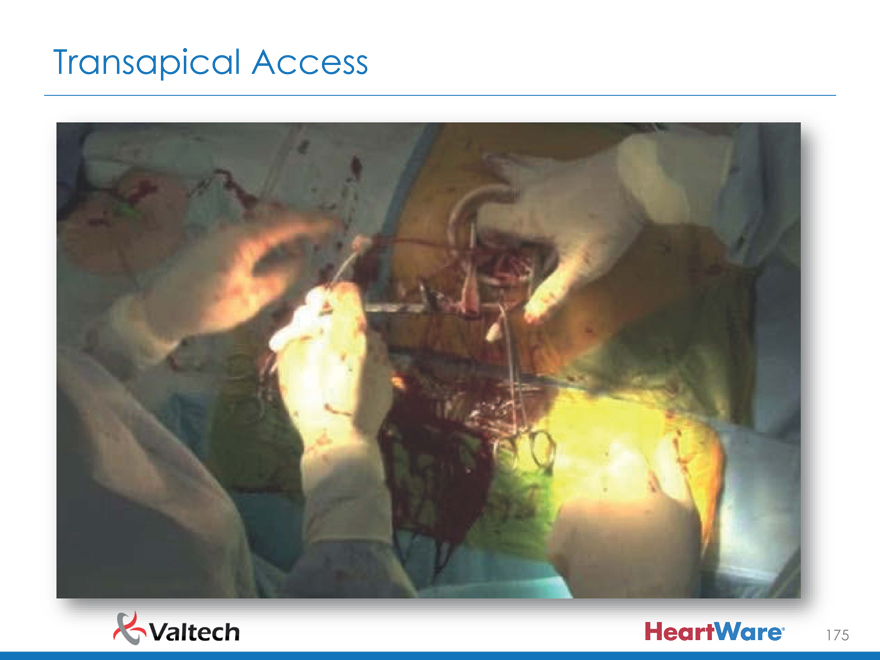
Transapical Access
175
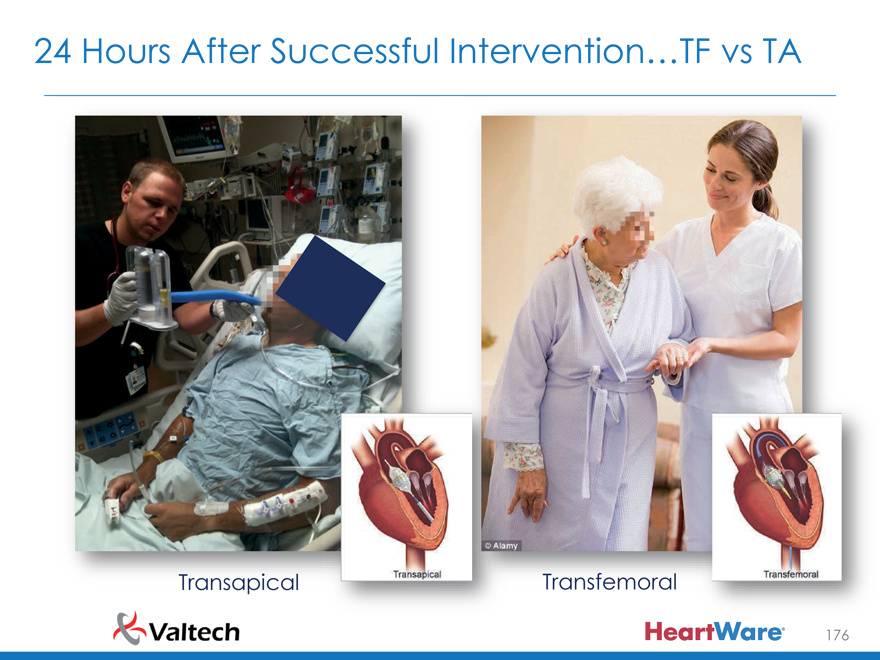
24 Hours After Successful Intervention…TF vs TA
Transapical Transfemoral
176
Future of Mitral and Tricuspid Transcatheter Interventions
Repair will be first line
– Leaves the options open
– Lower risks
– Lower physiologic impact
– Early indication feasible
Mitral replacement will be done in patients with suboptimal anatomy or end-stage FMR
Transfemoral approach will be the only successful method
– Safer
– Faster recovery
– Real competition with surgery
177
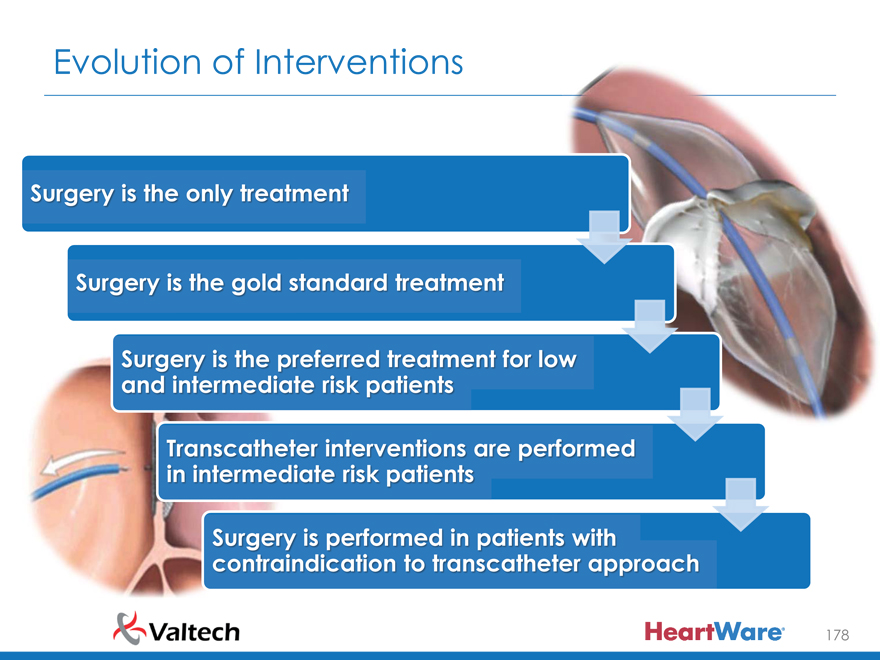
Evolution of Interventions
Surger i th onl treatment
Surger i th gol standard treatment
Surger i th preferred treatment fo low and intermediat risk patients
Transcathete interventions ar performed in intermediat risk patients
Surger i performed i patient with contraindicatio to transcathete approach
178
Cardioband Deep Dive
Prof. Karl-Heinz Kuck, M.D., Ph.D.
Asklepios Klinik St. Georg
179
CARDIOBAND PERCUTANEOUS
DIRECT MITRAL ANNULOPLASTY
180
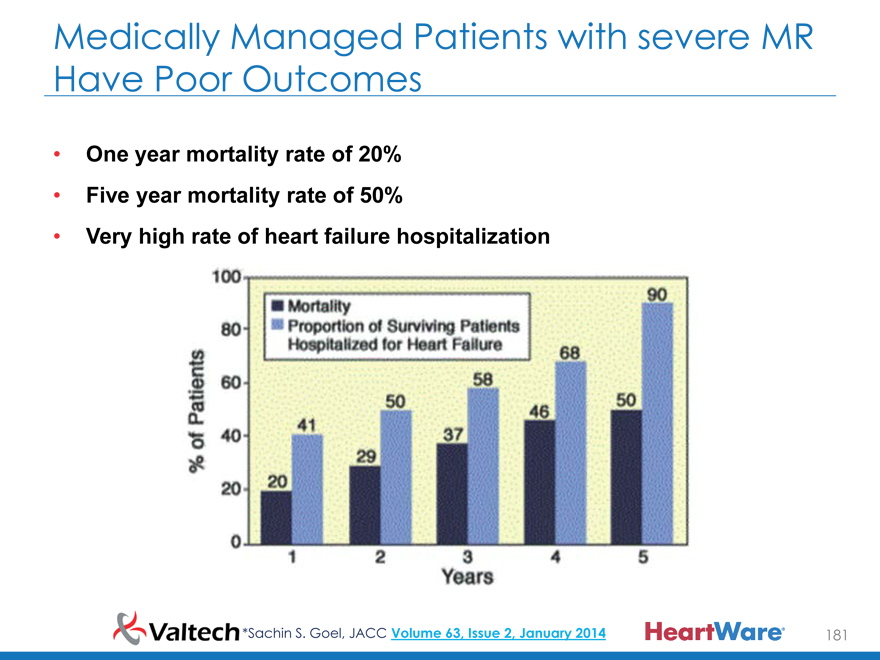
Medically Managed Patients with severe MR
Have Poor Outcomes
One year mortality rate of 20%
Five year mortality rate of 50%
Very high rate of heart failure hospitalization
*Sachin S. Goel, JACC Volume 63, Issue 2, January 2014 181
How are Patients with Isolated FMR
Treated?
Duke Databank: 1,538 pts with echocardiographic 3+ to 4+ FMR
and LVEF ?20% between 2000 and 2010 not undergoing CABG
* Courtesy of Dr. Michael Mack 182
Cardioband: Surgical-Like Annuloplasty
Surgery Cardioband
Which is Cardioband?
183
Surgery Cardioband
But Via a Catheter.
184
Benefits of Percutaneous Direct Annuloplasty
by Cardioband
Trans-femoral venous access
(transseptal) – best for safety
Supraannular fixation like in
surgery
Significant Reduction of
Annular dimensions – device
enables reduction of up to size
28 surgical ring
Preserves the native anatomy –
keeps future options open
185
CT Based—Automatic Planning
Pre-procedural CT Analysis providing:
–Sizing of the Annulus
–Expected Fluoroscopy Projections
–Transseptal Puncture location
–3D preview of system position
1
2
4
8
9
7
6
3
5
10
186
Cardioband Procedure—Step by Step
1 Transseptal
Puncture 2 System
Insertion 3 Implant
Deployment 4 Implant Size
Adjustment
187
Reduction of Septo-Lateral (A-P) Diameter by
Cinching Intra Procedure
On Cadaveric Heart (TVT 2012)
Annular Cinching by 10% Annular Cinching by 20% Annular Cinching by 40%
188
And Reduction of MR
Baseline
Final size Post
Adjustment
189
UPDATE FROM EUROPEAN TRIAL -
45 PATIENTS RESULTS
190
The Study
A single arm, multi-center, prospective
study with intra-subject comparisons to
evaluate the performance and safety of
the Cardioband for repair of functional
mitral regurgitation.
191
Participating Sites
192
Highlights of Early Outcomes (N=45)
193
Study Demographics (N=45)
Variable No. (%) or Mean
Age (years) 71 (49-81) Male 34
Gender
Female 11
Euroscore II (%) 7 Baseline NYHA Class of III or IV (%) 87 Ischemic 28 Non Ischemic 17 LVEDD (mm) Avg (min-max) 61 (43-75) EF (%) Avg (min-max) 32 (15-59) Prev CABG 14 (31%)
COPD 9 (20%)
Moderate to Severe Renal Failure 31 (69%) Severe Pulmonary Hypertension 8 (18%)
Afib 36 (80%)
194
Reported Safety Events at 30 Days
Patients Experiencing Event, # (%)
30 Day Events*
All Patients N=45
-Death 2 (4.4%) Hemorrhagic Stroke** 1 (2.2%) Need for elective Mitral Operation** 1 (2.2%) -Bleeding Complications 1 (2.2%) -Renal Failure 2 (4.4%) -Myocardial Infarction 0 (0.0%) -Respiratory Failure 0 (0.0%) -Cardiac Tamponade 0 (0.0%)
* VARC Guidelines (European Heart Journal, 2012, 33:2403-2414)
** Part of the Death cases
One additional death case per ITT—compassionate
195
Primary Endpoints
Safety Performance
Overall rate of Major Serious Technical success rate of the Adverse Events (SAEs) and implantation of the Serious Adverse Device Cardioband Effects (SADE) until hospital Technical feasibility of discharge and at post- Cardioband adjustment operative 30 days Cardioband ability to reduce mitral valve regurgitation
Major SAEs: Death, (MR) Intra-procedure, at myocardial infarction, hospital discharge and at 30 cardiac tamponade, device days related cardiac surgery, stroke
*Events will be defined according to VARC II guidelines
196
Secondary Endpoints
Safety Performance
Overall rate of Major Serious MR Severity at 6, 12 and 24 Adverse Events (SAEs) and months Serious Adverse Device Change in 6 MWT in 6, 12 Effects (SADE) until 24 and 24 months months Change in quality of life (MLWHFQ) at 6, 12 and 24 months
197
Major Inclusions
Age > 18 years
Moderate to severe functional MR
Symptomatic patients (NYHA Class II-IV) despite optimal medical therapy , including CRT if indicated
LVEF ? 25%, LVEDD ? 70mm
Subject is high risk to undergo MV surgery (as assessed by a cardiac surgeon and a cardiologist, at the site and according to ESC/EACTS guidelines on the management of valvular heart disease)
198
Major Exclusions
Heavily calcified annulus or leaflets
Untreated clinically significant CAD requiring revascularization
Any recent cardiovascular intervention
CVA or TIA within 6 months or severe carotid stenosis (>70% by Ultra sound)
Renal insufficiency requiring dialysis
Pulmonary hypertension >70mmHg at rest
Mitral valve anatomy which may preclude proper device treatment
Right-sided congestive heart failure with echocardiographic evidence of severe right ventricular dysfunction and severe tricuspid regurgitation
199
95% patients with MR?2+ At 12 Months By Core Lab
Cardioband MR Reduction
Core Lab; Dr. Paul Grayburn, Baylor University
88% MR ? 2+ 83% MR ? 2+ 87% MR ? 2+ 95% MR ? 2+ at Discharge at 1 Month at 6 Months at 12 Months
100% +
+ + +
80% + + Patients + + of + 60%
40%
0-1+ 0-1+ 0-1+ Percentage 0-1+
20%
+
0%
Baseline Discharge 1 Month 6 Months 1 Year n=45 n=42 n=41 n=24 n=19
200
Annular Remodeling by Reduction in Septo Lateral (A-P) Dimension (N=45) before procedure and at discharge 20% average reduction in A-P Baseline Discharge
201
Functional Improvement at 6 Months
202
Functional Improvement at 12 Months
Six Min Walk MLHFQ Score
P<0.05 P<0.05
450 40
400 37.94
35
385.79
350
30
(mean) 300
25
250 Score
255.86 20 200
Walked 18.72 150 MLHFQ 15
100 10
Meters 50
5 0 0
Baseline 12 Months
Baseline 12 Months
N= 18 N= 18
203
Summary
Transcatheter surgical annuloplasty is feasible
Safety profile similar to equivilant transcatheter procedures
Significant and consistent reduction in SL dimension
Significant and consistent reduction in MR
Options remain open for future interventions
Cardioband—Delivering Surgical Annuloplasty Through A Catheter
204
Thank you
205
Cardioband Deeper Dive:
Core Lab Insights
Paul Grayburn, M.D., FACC
Baylor University Medical Center
206
Disclosure Statement of Financial Interest
Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below.
Affiliation/Financial Relationship
Grant/Research Support (to BRI):
– Abbott Vascular, Medtronic, Edwards, Tendyne, Valtech
Consulting Fees/Honoraria:
– Abbot, Tendyne
Echo Core Lab:
– Valtech
Major Stock Shareholder/Equity:
– Baylor Heart and Vascular Hospital and The Heart Hospital Baylor Plano
207
Participating Sites
Zurich University Hospital (1)
Asklepios Klinik, St. Georg (8) Bic Hos Medizinische Klinik Universitätsklinikum Bonn (11)
Heart Center HSR, University of Köln Milan (7) (3)
Echo Core Laboratory Baylor University Medical Center, Dallas TX
208
Implant Technique
A B
C D
209
Insertion of the First Anchor
210
Anchor Implantation and Cinching
A B
C D
211
3D TEE Showing First Anchor
212
3D TEE Showing Final Anchor
213
3D TEE of Cardioband After Cinching
214
3D TEE Images: Pre-, During, Post-
Baseline (pre-device) Device Pre-Cinching Device After Cinching
A B C
215
3D Images (top) & Reconstruction (bottom)
216
Structural Reconstruction Seen Post-Cinch
Baseline 3D Dimensions Post-Cinch 3D Dimensions
AP = 3.63cm AP = 3.16cm
AL-PM = 4.01cm AL-PM = 3.15cm
217
Aortic-Mitral Angle Pre- and Post-Cardioband Device
Baseline Post-Cinching
A B
Ao-MV Angle = 133.1° Ao-MV Angle = 125.6°
218
Results of 3D Measurements
219
Conclusions
Acute intraoperative change in annulus geometry as measured by 3D TEE
– AP diameter and IC diameter
– Annulus circumference
– Aortomitral angle
Translates into clinical benefits
– MR reduction
– LV and LA remodeling
– Quality of life measures
220
Perspectives on the MVAD® System
Paul Jansz, BMed. FRACS, Ph.D
St. Vincent’s Hospital
221
Perspectives on the MVAD® System
No Financial Interests/Conflicts.
Investigational Devices will not be discussed in detail.
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 222
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
St. Vincent’s Experience
Adult Hospital
1000 -1200 Cardiothoracic Cases per year
80 – 100 Transplants
– Heart
– Lung
– Heart Lung
Large ECMO Program
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 223
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
St. Vincent’s Experience
Mechanical Assist
More than 200 VADs implanted
Experience using many long-term, durable MCS device
– BVS 5000: 13
– C-Pulse: 2
– TAH: 7
– Heartmate 1 (IP,VE,XVE): 21
– VentraAssist: 33
HVAD® System
Clinical trial site in HVAD CE Mark trial
– HVAD implants: 119
– HVAD BiVADs: 13
MVAD System
Investigator
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 224
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
St. Vincent’s Experience
Surgical Valve
Aortic Program
– Homografts, Repair, Replacement
– Sutureless Valnes
– Minimally Invasive
Hemisternotomy
Right Anterior Thorocotomy
Mitral Program
– Robotic Repairs: 109
– Minimally Invasive Repairs: 304
Structural Heart
Aortic (TAVI)
– CorValve, Sapien
Trans-Aortic, Trans-Apical, Femoral
Mitral
– MitraClip: 55
– Replacement (Tendyne): 4
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 225
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
MVAD Advantage™ CE Mark Trial
Multi-center, prospective, non-randomized, single-arm trial
70 patients at 11 sites in Australia, Austria, France, Germany & the UK
Primary Endpoint: Survival at 6 months
Implantation via thoracotomy or sternotomy
First patient enrolled July 2015
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use.
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
The MVAD System Has Breakthrough Potential
Small size enables less invasive implant and improved anatomical fit
MVAD® Pump
Advanced impeller technology for advanced hemocompatibility
Lighter and thoughtfully designed peripherals to enhance patient quality of life
PAL? Patient Peripherals
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 227
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
MVAD Pump in situ
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 228
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
Advanced Impeller Technology
>50% reduction in shear stress* that may lead to fewer VAD complications
- Optimized flow paths to guide 99% of blood through ultra-low stress blood channels
- Smooth impeller edges to decrease forces applied to the blood
- Small priming volume & reduced surface contact to limit blood time in the pump
*Compared to the HVAD® Pump, HeartWare data on file
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use.
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
Smaller Pump Allows for Less Invasive Surgery
Globally, 1 in 4 HVAD System patients implanted via thoracotomy
Potential advantages:
– Reduced incision size
– Reduced bleeding
– Reduced Right Heart Failure
– Preservation of the sternum for heart transplant
– Easier procedure for re-do patients
– Shorter hospital length of stay
HVAD Pump MVAD Pump
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 230
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
Highlights of the Implant Procedure
2015 EACTS Techno College Video, Presented by J. Schmitto
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use.
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
Gimbal Adjustment Via ECHO Guidance
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 232
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
qPulseTM Cycle
Customizable speed adjustments (3 settings)
Designed to promote aortic valve opening, enable ventricular washing, and reduce bleeding complications
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use.
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
qPulse Setting Adjustments Under ECHO Guidance
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use. 234
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
PAL Patient Peripherals
Thoughtfully designed peripherals to provide improvements in patient quality of life
Simplified patient experience with streamlined design
– Integrated controller and battery system
– Snap-on, cable-free battery attachment
– Time-based (hours and minutes) battery run-time display
Increased patient safety and confidence
– Internal battery for back-up power
– Clear and actionable text-based alerts
– Enhanced data access on controller for improved TM remote troubleshooting Peripherals for an Active Lifestyle
Exclusively for Clinical Investigation.
Investigational device to be used by Qualified Investigators Only.
CAUTION: Investigational Device. Limited by Federal (or United States) law to investigational use.
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
THANK YOU
236
HEARTWARE, HVAD, MVAD and the HEARTWARE logo are registered trademarks of HeartWare.
The HeartWare—Valtech Combination:
A Physician’s View
Dr. Steven W. Boyce
MedStar Heart and Vascular Institute MedStar Washington Hospital Center
237
In Many Cases the FMR Valve Patient May Also Be a VAD Patient (Now or in the Future)
Clinical • Functional Mitral Regurgitation Progression Device Therapy (FMR) is a ventricular problem Myocardial insult • Valve dysfunction exacerbates ventricular dysfunction
ICD
Left Ventricular • The same Heart Failure and Surgical (LV) Remodeling team that manages a FMR patient, manages the VAD patient
Mitral Apparatus Remodeling
In fact, the FMR patient may CRT becomes a VAD patient—for many Mitral Valve severe FMR patients the Heart Team Dysfunction weighs valve intervention against Mitral Repair / VAD intervention
Further LV Replacement
Remodeling / HF • A differentiated Mitral therapy is a Progression logical complement to a VAD business…
VAD
238
Valve and VAD Patient Profiles Converging Due to Improved Technology and Patient Identification
Heart Failure Team
Convergence
Valve Patients of VAD and VAD Patients
Valv Patients
Advent of Transcatheter Improving Outcomes, Solutions Technology
? Less invasive? Improved survival and adverse ? Better QoL event profile? Potential reverse remodeling ? Enhanced QoL with smaller peripherals and non-wired systems (FIS)
239
The Heart Team Manages NYHA III and IV Patients
High-risk patients with significant MR and TR—currently treated with medication alone
With a non-invasive approach, physicians will correct the valvular pathology to slow disease progression
FUTURE: Many INTERMACS 3 and 4 patients referred for a VAD may undergo percutaneous repair prior to minimally invasive VAD (e.g. MVAD)
240
Valve Interventions and VADS
MR and VADS
MR is not a contraindications to LVAD implantation
Mitral valve repair might be indicated if lower speeds are necessary to preserve AV integrity or maintain pulsatility within the peripheral circulation
TR and VADS
Concomitant TVR provides salient benefits in patients with moderate-to-severe TR receiving LVAD support
Concomitant tricuspid valve repair in patients undergoing LVAD placement reduces incidence of acute right heart failure
Despite being contraindicated in the HeartWare ADVANCE study, 16% of those patients receive a concomitant mitral or tricuspid intervention
Source: Wang 2014 Circulation, Rodriguez 2013 JHLT, Deo 2012 J Card Surg.
241
Combined Portfolio Offers Solutions for Broader Heart Failure Population Managed by the Heart Team
Heart Failure
Primary Valve HFrEF HFpEF Right Heart Disease
MCS Valve MCS MCS Valve Valve
(mitral) (tricuspid) (mitral & tricuspid)
Note: Heart Failure Reduced Ejection Fraction (HFrEF), Heart Failure Preserved Ejection Fraction (HFpEF).
242
A Focused Provider of Therapies and Support for the Advanced Heart Failure Continuum Makes Sense
Combined Heart Failure Treatment Paradigm
Company Offering
Class I Class II Class III Class IV
Early Stage Mid Stage Late Stage End Stage
Portfolio Valtech
Focus HeartWare
Medical Surgical Transcatheter Tx/CRT Valve Tx Valve Repair
Treatment Transcatheter Options Valve Replace
Mechanical Heart Circulatory Support Transplant
Patients may receive multiple valve interventions over the course of their disease
VAD patients often receive concomitant mitral or tricuspid valve procedures
VADs remain the treatment option for progressed patients
243
Logical Combination From A Physician’s Perspective
These therapies will increasingly target a similar patient profile
Valve “tune-up” would serve many VAD patients well
Common Patient • Many who are anatomically eligible but contraindicated for a VAD would benefit from valve therapy Common Clinical • Increasing role of Heart Team in HF patient care paradigm Decision • Heart Team evaluates VAD-eligible patients; cardiologists Pathway becoming increasingly involved
Common Referral
Same Cardiology referrer driving patient flow
Channel
Common Clinical • VAD clinical support specialists are some of the most Knowledge Base knowledgeable on heart failure
244
Thank You
245
Physician Panel Patient Profiles
Michael Mack, M.D., FACC
Baylor Scott & White Health
246
Patient 1
Clinical background: Echo parameters:
81 year old patient MR: severe
Male LVEDD: 67mm
NYHA class: III EF: 40%
Ischemic RV general condition: moderate
EUROSCORE II: 5.2% LV general condition: moderate-severe
Risk Factors and comorbidities: • Pulmonary pressure: 63mm/hg
Age > 80Y Tethering: 11mm
LV dysfunction
High Pulmonary pressure
Myocardial infarction
Bradycardias with pacemaker
Chronic kidney disease
Suspect for Cold (chest X-Ray)
247
Patient 1
Severe TR RV larger than LV
248
Patient 1
Echo evaluation and suggested therapy:
The patient has severe MR and severe TR. RV larger than the LV.
As the RV dysfunction is moderate and the MR is not the primary disease, the suggested treatment will be LVAD with tricuspid annuloplasty ring.
249
Patient 2
Clinical background: Echo parameters:
78 year old patient MR: moderate-severe
Female LVEDD: 55mm
NYHA class: III EF: 40%
Ischemic RV general condition: moderate
EUROSCORE II: 8.29 LV general condition: moderate-
STS score: 4.098 severe
Pulmonary pressure: 65mm/hg
Risk Factors and comorbidities: • Tethering: 7mm
STEMI
Atrial fibrillation
Stent ACI right side
Diabetes mellitus
Hypertonia
250
Patient 2
Severe MR LV
251
Patient 2
Echo evaluation and suggested therapy:
The patient has severe MR with moderate RV dysfunction and severe LV dysfunction. The LVEDD is not extremely large (55mm) and with no TR.
The patient can benefit from mitral valve repair by Cardioband with reduction of MR and improvement of the right side.
252
Patient 3
Clinical background: Echo parameters:
22 your old patient MR: severe
Female LVEDD: 78.5mm
NYHA class: III EF: 25%
None- ischemic RV general condition: severe
EUROSCORE II: 3.14 LV general condition: moderate
STS score: 0.437 Pulmonary pressure: 65mm/hg
Tethering: 9mm
Risk Factors and comorbidities:
Dilated cardiomyopathy
Asthma bronchial
253
Patient 3
Severe MR Tethering
254
Patient 3
Echo evaluation and suggested therapy:
The patient have severe MR with extremely large LV (78.5mm) and tethering. The right side dysfunction is only moderate and the EF is in low end.
Due to theses reasons, the patient will not benefit from Cardioband as the EF might decrease after implantation. The best suggested treatment will be a VAD.
255
Patient 4
Clinical background: Echo parameters:
81 year old patient MR: moderate-severe
Male LVEDD: 67mm
NYHA class: III EF: 30%
Ischemic RV general condition: moderate
EUROSCORE II: 22.2% LV general condition: severe
Pulmonary pressure: elevated
Risk Factors and comorbidities: • Tethering: 7mm
Previous CABG
PAOD
Renal disease
Polyarthritis
256
Patient 4
Severe MR Left side pressure
257
Patient 4
Echo evaluation and suggested therapy:
The patient has severe MR with moderate RV dysfunction and severe LV dysfunction, demonstrated by high left side pressure.
In this case, it is clear that the MR is the main issue, this patient can benefit from mitral valve repair by Cardioband
258
Patient 5
Clinical background: Echo parameters:
75 year old patient MR: moderate-severe
Male LVEDD: 51mm
NYHA class: IV EF: 30%
Ischemic RV general condition: severe
EUROSCORE II: 10.21 (calculated LV general condition: moderate for no mechanical ventilation at Pulmonary pressure: 52mm/hg procedure date) Tethering: 11mm
STS score: 5.374
Risk Factors and comorbidities:
ACVB (LIMA ad LAD)
Biologic aortic valve prosthesis
Persistent atrial fibrillation
Renal failure
None insulin dependent Diabetes
Bradycardias with pacemaker
259
Patient 5
260
Patient 5
Echo evaluation and suggested therapy:
The patient has severe MR, severe TR and severe right side insufficiency, well demonstrated by enlarged IVC.
In addition, the RV is larger than the LV.
As the MR is not the main issue, the suggested treatment will be double VAD (both sides) or LVAD with temporary RVAD for pressure balance.
261
Discussion
262
The Combined Company Overview
Peter McAree
Senior Vice President and CFO HeartWare International
263
Our Story: HeartWare has achieved exceptional growth fueled by HVAD
WW HeartWare Revenue
2009-2015 $300 Year CAGR $250 2012-2014 58% M $ 2009-2014 63% U.S. BTT $200
approval $150 (late ‘12)
278
CE Mark
Revenue, $100 approval 208 209
(early ‘09)
$50 111 83 55 24 $0
2009 2010 2011 2012 2013 2014 2015 YTD*
Cash: $249 million NOLs: $450 million
Convertible debt: $245 million Outstanding Shares: 17.3 M shares
* 10% constant currency growth, adjusted for $16M FX impact Note: Data as of November 3, 2015.
264
Growth Objectives
Near-term Headwinds Long-term Vision
Conclusion of ENDURANCE2 $700M Total VAD+Valve
Revenue by 2020
HVAD U.S. clinical unit sales
HeartMate III U.S. trial and launch in Europe
Growing > 20%
Unrest in Middle Eastern and Eastern European markets
MVAD trial impact on HVAD GM% > 70% sales in 5 participating regions
Foreign exchange Leadership in heart failure therapy
265
Future VAD Outlook: DT Approval and MVAD Expected to Enhance Growth Potential Through 2020
$600 5,566 6,000
$500 5,000 $ M $400 4,000 2,751 $300 3,000 Units 2,079 524 Revenue, $200 2,000 1,217
278 $100 208 1,000
111
$0 -2012 2013 2014 2020
Region 2014 Revenue 2015-2020 Key Drivers 2014-2020 Unit CAGR
MVAD Clinical
DT approval
U.S. $151 ~15%
MVAD Commercial
Circulite Clinical
MVAD Commercial
International $127 • Japan Commercial ~10%
FIS
Note: Fully implantable system (FIS). Source: HTWR financials and estimates.
266
Valtech Investment: Combined VAD + Valve Portfolio
Heart Failure Growth • Combined portfolio provides leadership position in two of Platform the highest-growth areas in heart failure
Expect the transcatheter mitral market to be an even greater market opportunity than TAVR
Differentiated Value • Appreciably scales and accelerates long-term revenue Creation growth potential
Multiple opportunities for revenue growth
Significant margin expansion opportunity
Ri Mitigating • Deal structure designed to align focus on value creation Structure • Contingent structure provides attractive risk/reward
Capital Efficient • Structure preserves cash to fund operations of the combined business
267
Combined Portfolio Provides Leadership Position in Two of the Highest-Growth Areas in Heart Failure
VAD
2015 2030
Class IV Class III
$2.2B $800M
+7% CAGR
Valve
$7.5B
Mitral Tricuspid
$600M
+18% CAGR
Source: HTWR estimates.
268
The Transcatheter Mitral Market to Likely Be An Even Greater Market Opportunity than TAVR
WW TAVR Market
$1,600 2007-2014 CAGR $1,476 $1,400 Total 104% Other NA $1,200 Medtronic 83% $1,079 $ M
Edwards 125% $1,000 $848 $800
Revenue, $585 $600 $372 $400 $201 $200 $99
$0 $10
2007 2008 2009 2010 2011 2012 2013 2014
Note: Other includes Boston Scientific, St. Jude. and other. Average of Edward’s daily close price for cited year. Source: J.P. Morgan published estimates. Google Finance.
269
Valtech Appreciably Scales and Accelerates Long-Term Revenue Growth Potential
WW Valtech Revenue Projections
Case 2 $1,200 Mitral Tricuspid $1,146
CAGR $173 $1,000 $957 2016-2020 110%
$149
2020-2025 44% $765 $800 2016-2025 70%
$129
M $600 $536 $
$87 $972
$400 $323 $807
$636 $55
$186 $449 $200 $119
$71 $269
$10 $27 $163
$0 $66 $107
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025
Source: HTWR estimates.
270
Building Off of Improved VAD Margins Into Even Higher Gross Margin Valve Business
Historical HeartWare VAD Gross Margins vs. Projected Valve Franchise Gross Margins
100% 90% 80% 70%
Margin 60% Gross Margin 50% Potential Gross 40% VAD 70-73%
%
30%
Valve 75-80%
20% 10% 0%
2012 2013 2014 2025
HeartWare VAD Business Combined HF
Source: HTWR estimates. Franchise
271
Combined Portfolio Provides Leadership Position in VADs and Valves, Two of the Highest-Growth Areas in Heart Failure
Pro Forma Revenue Projections
VAD and Valve Franchises $2,500 CAGR
$2,341
VAD Valve Combined
‘14-’20 11% NA 17%
$2,000 Replace
‘20-’25 18% 44% 27%
43% 1,145
$1,500 $ M $1,000
$710 Repair 57% 186 $1,196
$500
$208 $278 $111 $524
$-
2012A 2013A 2014A 2020E 2025E 2025E Valve Revenue
Note: Valve franchise includes mitral and tricuspid repair and replacement portfolios. Case 2 valve revenues. Split Source: HTWR estimates.
272
Milestone-Based Transaction Aligned With Value Creation, Preserves Cash to Operate Combined Company
Valtech WW Revenue and Transaction Terms
$1,400 2015-2025
$1,200 Repair Replacement $1,000 $ M $800 $600 $400 $200 $0
2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025
Event and 4.4M
Payments? Close, ? Cardioband ? Warrants ? $375M stock or shares Tricuspid FIM exercisable at cash at $450M LTM or CardioValve $75M LTM net net Valtech ? Cardioband FIM, 700K Valtech product product sales CE Mark, shares sales 800K shares
Portfolio Mitral and Tricuspid Surgical Comprehensive Portfolio of Breadth and Transcatheter Repair Market Transcatheter Replacement and Repair Products
Source: HTWR estimates.
273
Cardioband Snapshot
WW Revenue and EBITDA Projections Mitral Tricuspid
Case 2, Cardioband Mitral and Tricuspid Functional, moderate and severe
Patients mitral regurgitation, moderate and $700 high surgical risk Revenue Expected EU: 2015 EU: 2020 $600 Commercial Launch US: 2018 US: 2022
EBITDA
Customer Interventional Cardiologists $500 Regulatory PMA
Clinical Moderate-large study $400 Transcatheter Transcatheter
Market
M Mitral Repair Tricuspid Repair
$ $300
2020 2025 2030
WW Transcatheter $200 Repair Procedures 25 60 75
(in thousands) $100 Cardioband’s WW
Mitral Transcatheter 20% 35% 40%
$0 Repair Market Share
50% of MR patients have moderate or severe TR
-$100 Tricuspid
Patients receiving Mitral
Opportunity
Cardioband eligible for Tricuspid
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 Cardioband
Source: HTWR estimates.
274
CardioValve Snapshot
WW Revenue and EBITDA Projections
Case 2, CardioValve Mitral Transseptal Transapical
Functional and degenerative, moderate and severe mitral
700 Patients regurgitation, moderate and high Revenue surgical risk 600 EBITDA Expected EU: 2020 EU: 2021
Commercial Launch US: 2022 US: 2024
500 Customer Interventional Cardiologists
Regulatory PMA
400 Clinical Moderate-large study
M
$ 300 Market Transcatheter Mitral Replacement
200 2020 2025 2030
WW Transcatheter 100 Replacement
10 70 125
Procedures
(in thousands)
0
Cardiovalve’s WW Mitral Transcatheter
-100 10% 25% 25%
Replacement
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 Market Share
Source: HTWR estimates.
275
Cardinal Snapshot
WW Revenue and EBITDA Projections Mitral Tricuspid
Case 2, Cardinal Mitral and Tricuspid
Functional moderate-severe, low-
Patients moderate surgical risk
$700
Revenue Expected EU: 2017 EU: 2018 $600 Commercial Launch US: 2018 US: 2020
EBITDA
Customer Cardiothoracic Surgeons $500 Regulatory 510K
Clinical If required, small study
$400
M Market Surgical Mitral Surgical Tricuspid
$ $300
2020 2025 2030
WW Surgical Repair $200 Procedures 100 160 180
(in thousands) $100 Cardinal’s WW
Mitral Surgical 10% 10% 10%
$0 Market Share
50% of MR patients have moderate-severe TR
-$100 Tricuspid
90% tricuspid surgeries are repairs
Opportunity
Patients receiving Mitral Cardinal
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 eligible for Tricuspid Cardinal
Source: HTWR estimates.
276
Multiple Opportunities for Differentiated Revenue Growth
$700 Revenue $600 EBITDA $500 $400 M $300 $ $200 $100 $0
-$100
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025
CE Mark Pre-clinical CE Mark
Transcatheter mitral and Transcatheter mitral Surgical mitral and tricuspid tricuspid repair replacement repair
FMR + FTR moderate and FMR + DMR moderate and FMR + FTR moderate and severe regurgitation, moderate severe regurgitation, moderate severe regurgitation, low and and high surgical risk patients and high surgical risk patients moderate surgical risk patients
~60,000 TMVr patients in 2025; ~70,000 TMVR patients in 2025; ~160,000 treated surgical MR estimated market share of 35% estimated market share of 25% patients in 2025; estimated market share of 10%
Note: Functional Mitral Regurgitation (FMR), Functional Tricuspid Regurgitation (FTR), Transcatheter mitral valve repair (TMVr) Transcatheter mitral valve replacement (TMVR). Source: HTWR estimates.
277
Cardioband Valve Repair Platform Alone Offers A Substantial Value Creation Opportunity
WW Valtech Revenue Projections
Case 2, No CardioValve $1,200
Valtech Revenue, Potential
Upside if Valves Do Not Meet • Transcatheter repair likely $1,000 Potential plays a larger role in treatment paradigm if transcatheter valves do not $800 Valtech Revenue, no achieve full potential
Cardiovalve $652
M $566 • Cardiovalve mitigates $ $600 replacement therapy class
$487
risk with safer, differentiated $369 design $400
$260
Achieves >10% IRR $200 $158
$119 $71
$10 $27
$0
2016 2017 2018 2019 2020 2021 2022 2023 2024 2025
Source: HTWR estimates.
278
Cash Management: Steady Cash Improvement Since 2012
$80 CapEx Operating Cash $ M
$70 $65 $60
Usage $50
Cash $40 Current Cash
$30 $25 $24 Position: $249M $20
Historic $10 $4 $0 2012 2013 2014 2015 LTM*
September 2015 through December 2020 Cash Implications ($M)
FCF generation (full year basis) beginning in 2017 Core VAD $82
Ability to flex Portfolio Investment timing as needed
2016-2017 are peak investment periods Valve $(75)
EBITDA positive in 2019, FCF generation starting in 2020 Convertible Debt $(43) Potential to refinance (e.g. bank debt) (‘17)
*Data as of September 30, 2015 Source: HTWR financials.
279
Valtech Transaction Creates A Stronger Better HeartWare
Creating the Most Exciting, High-Growth Player in Cardiovascular Devices
Advances our technology leadership in heart failure
Broad combined platform scales and accelerates long-term revenue growth
Significant margin expansion opportunity
Transaction structure aligns focus on value creation and generates returns in excess of cost of capital
280
HeartWare Today and Tomorrow
Closing
Doug Godshall
President and CEO, HeartWare International
281
HeartWare + Valtech: Creating the Technology Leader in Heart Failure
?MVADSystem will lead a transformation of MCS portfolio and VAD market, picking up where HVAD leaves off?Synergies with disease, customer, referral channel and delivery system will create compounding benefits over next decade?Adjustable repair of MR and TR expected to emerge as first-line option, whether surgical or interventional?Nearer-term commercial opportunity in established surgical and interventional mitral repair market with >$450M in sales?Sophisticated CardioValve design and delivery system to enable HeartWare leadership in mitral replacement?Combined pipeline creates unique opportunity for differentiated value creation
282
Thank You
283
HeartWare® Valtech