Exhibit 99.01
| | | |
HEARTWARE LIMITED
ABN 34 111 970 257 | |  |
| | | |
| | | Level 57, MLC Centre |
| | | 19-29 Martin Place |
| | | Sydney NSW 2000 |
| | | Ph: (+61) 2 9238 2064 |
| | | Fax: (+61) 2) 92382063 |
| | | www.heartware.com.au |
31 October 2007
Dear Shareholder
It is now a little over 12 months since I joined HeartWare. We have made remarkable progress over that time and I am today more convinced than ever of the opportunity before us. The team has continued to work tirelessly over the past three months to advance our clinical and regulatory programs and we remain on target to achieve our key milestones early in 2008. This letter aims to provide an overview of our activities during the quarter and a summary of our current position.
Key developments include:
| | • | | Completion of patient enrolment in our international clinical trial |
| |
| | • | | IDE submission imminent |
| |
| | • | | Confirmation by US centers of desire to participate in HeartWare’s US clinical trial |
| |
| | • | | Hiring James Schuermann to lead HeartWare Sales and Marketing activities |
| |
| | • | | Presentations of HeartWare interim clinical data by Dr George Wieselthaler and Dr Martin Streuber |
International Clinical Trial Update
We announced last month completion of enrolment of our 20 patient international clinical trial, marking an important milestone for HeartWare. We advised further that it was our intention to seek approval from our participating hospitals to amend the protocol so as to allow the continuation of implants under the trial, to a maximum of 30 patients.
I am pleased to report that we have now received approval for the study extension from the Ethics Committees of four out of our five centers, including St Vincents Hospital (Sydney), Royal Perth Hospital, Vienna General Hospital and Hannover Medical Center. All these centers have product inventory on site and we expect to recommence implants at these centers over coming weeks. Following two recent implants at Hannover, our total patient enrolment now stands at 22.
| | | | |
| | | -2- | |  |
The objective of this trial is to achieve 70% of patients meeting the primary endpoint — heart transplantation or survival to 180 days supported by the device. Of the 22 patients enrolled in the international trial so far, 12 have now passed the primary endpoint. These 12 patients include 3 who received transplants (at 425, 348 and 157 days respectively) and one whose device was successfully explanted after 268 days of support (our first “Bridge to Recovery” case, as reported on 24th July 2007). Two patients have died within the first 180 days and neither patient was deemed to have died of pump related complications. The cumulative period of support on the device is 3,900 days, or approximately 10.5 years. The average duration of support is 180 days per patient.
We remain on track to file our Investigational Device Exemption (“IDE”) and for a CE mark this quarter, as previously advised. Subject to the time required for the regulatory processes, we expect to receive authorization to commence our US study and to receive our CE mark for the device early next year, allowing the commencement of commercial sales in Europe.
IDE Submission
I am pleased to confirm that we have completed our IDE documentation and will be submitting our application to the US FDA in the next day or so. The ASX will be formally advised once this submission has been shipped to the FDA.
The submission, which follows a long period of dialogue with the FDA, brings together everything we have learned about the HeartWare® LVAD System, including all preclinical, clinical and technical data. The submission also incorporates a proposed protocol for our US clinical trial. The protocol, while based broadly on previous Bridge-to-Transplant clinical protocols, introduces several new elements which may help expedite our clinical program.
Following the submission of the IDE, we expect a period of dialogue with the FDA, during which clarification might be sought and additional information requested. This dialogue process occurs over a series of 30-day cycles. It is always difficult to estimate how many such cycles might be required but at this stage we hope to receive IDE approval early in 2008.
In preparation for the start of our US clinical trial in 2008, HeartWare has met with many US transplant centers to gauge their level of interest in participating in its US clinical trial. We are extremely pleased by the level of enthusiasm shown by our targeted centers and are now commencing the formal process of signing up sites for our study. Once contract negotiations are completed, we will provide details of the lead hospitals in our study.
Appointment of New Senior Executives
I am very pleased to announce the recent appointment of Mr. James Schuermann to the position of Vice President, Sales and Marketing, reporting directly to me. Sales and Marketing is a critical role at this stage of our growth. In the next several months we expect the commercial launch of the HeartWare® LVAD System in Europe and the start of US clinical activities.
Prior to joining HeartWare, James spent nine years in sales and marketing at Boston Scientific Corporation. Over this time he progressed from sales through product management until being appointed Director of Marketing in 2005. With a broad matrixed team of over 200 product managers and salespeople, Jim led marketing activities for a US$280M worldwide business that emerged as one of the strongest in the company. He is an exceptionally well-qualified medical device executive and has already begun to make a significant impact on the business.
| | | | |
| | | -3- | |  |
Ms. Jane Reedy has been with HeartWare for almost three years and until recently held the role of Vice President, Sales & Marketing. Jane, who has made an enormous contribution, will cease full time employment with the Company at the end of the year.
I am also pleased to advise that Mr. Ramon Paz has been promoted to the position Vice President, Quality Assurance, reporting directly to me. This promotion reflects not only the outstanding work Ramon has done over the past 12 months, but also the critical nature of the Quality Assurance function. As we move into a US clinical trial environment, ongoing improvement of our quality systems is one of our highest corporate priorities. With over 20 years experience in the medical device industry, much of it spent developing and implementing Quality systems, Ramon continues to play a vital and important role at HeartWare.
Presentations of Interim Clinical Data
At the Heart Failure Meeting held earlier this month at the Cleveland Clinic, Dr George Wieselthaler presented an overview of the HeartWare® LVAD System clinical experience. Dr Wieselthaler was the first surgeon to implant the HeartWare® LVAD and has conducted 7 implants to date. Dr. Weiselthaler is also presenting on HeartWare’s clinical experience this week at the Japanese Society for Artificial Organs.
Dr Martin Strueber, Principal Investigator for the HeartWare trial at Hannover Medical Center, will be delivering a presentation on the HeartWare clinical experience at the 5th Berlin Symposium — Mechanical Circulatory Support on 11 November 2007.
It is gratifying that HeartWare is gaining increased prominence at high-profile cardiac surgery conferences. We are also well-established in the heart failure clinical community. We have begun working with our investigators and advisers to plan and implement a comprehensive publication strategy to ensure clear communication of our unique advantages as we seek to continue to build our clinical credentials.
Manufacturing Update
We now have sufficient pumps in the field to reach our new threshold of 30 implants, including the requisite back-up inventory. We have been able to build this strong inventory position while running our manufacturing line at a significantly reduced rate. We did this so that we could re-allocate a majority of our resources to a number of specific projects aimed at further improving the efficiency of our production processes. These projects relate primarily to the automation of several of the more labor-intensive steps of the process. In the past 2 months, 100% of our pumps have passed the final screening test on our manufacturing line, reflecting the impressive upgrades to our manufacturing capability implemented over recent months.
| | | | |
| | | -4- | |  |
With our existing processes and our plan to expand the footprint of our manufacturing floor during 2008, we are comfortable of being able to supply the demands of both our clinical trial and international sales through 2008. Additionally, with the efficiency gains that we anticipate from the manufacturing improvement projects now underway, we expect a significant boost to our production capability over the coming 12 months.
MVAD™ Device Update
One of HeartWare’s competitive strengths lies in our miniaturization platform and our ability to generate a pipeline of further miniaturized devices. The Company’s Advanced Product Development (“APD”) team has further advanced the MVADTM device, which continues to demonstrate extraordinary promise in ongoing animal studies. The MVADTM device, at approximately one-third the size of the HeartWare® LVAD, is designed for implant by less invasive surgery, which we believe will lead to important clinical advantages.
The first public presentation of HeartWare’s MVADTM device technology will occur at the annual meeting of the International Society of Rotary Blood Pumps (“ISRBP”) in Sydney, which will take place on 2 November 2007.
Quarterly Cash Flows
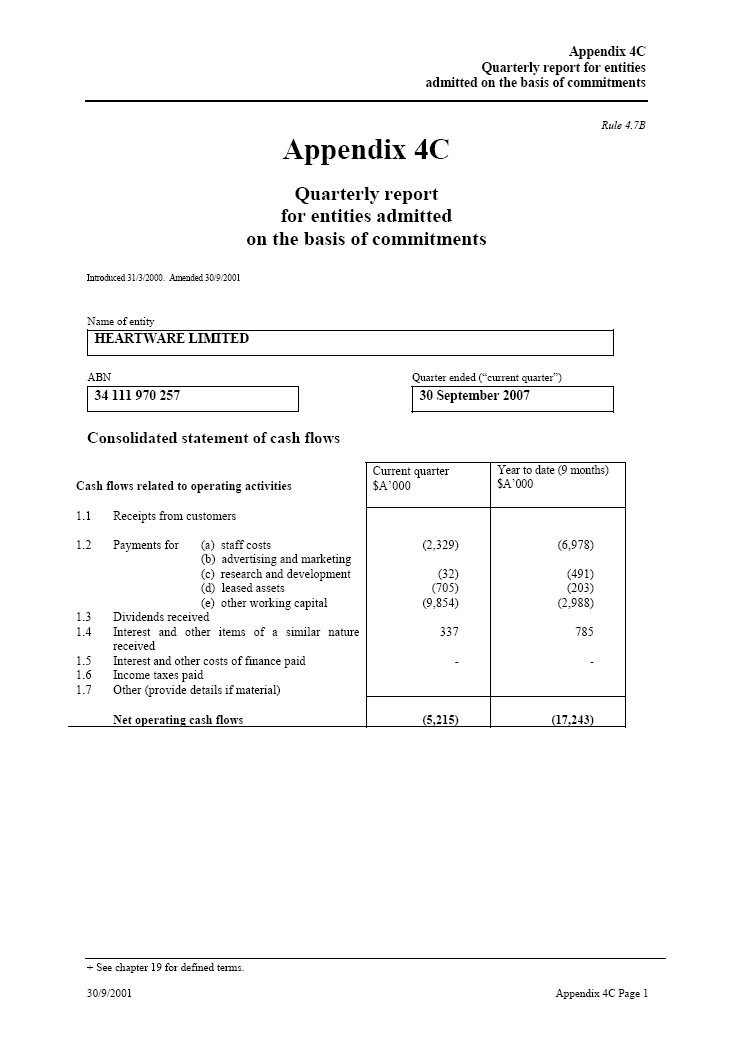
In accordance with the ASX Listing Rules, I attach the Cash Flow Statement for the 3 and 9 months ended 30 September 2007 (see attached ASX Appendix 4C).
Summary
HeartWare is moving quickly towards achieving two critical milestones in the company’s development — namely the start of our US clinical trial and the receipt of CE mark to enable sales in Europe. I continue to be encouraged by the enthusiasm of surgeons around the world for our device. I am hopeful that our clinical results will continue to demonstrate the significant benefits we believe to be inherent in our pump design. While there remains much work to be done, the Company’s future looks more promising than ever.
Thank you once again for your continued support.
Yours sincerely
 Doug Godshall
Doug GodshallChief Executive Officer
Attachment: ASX Appendix 4C
| Rule 4.7BAppendix 4C Quarterly report for entities admitted on the basis of commitments Introduced 31/3/2000. Amended 30/9/2001 Name of entityHEARTWARE LIMITED ABN _____ Quarter ended (“current quarter”)34 111 970 257 30 September 2007 Consolidated statement of cash flows Cash flows related to operating activitiesCurrent quarter _____ Year to date (9 months) $A’000 $A’000 1.1 Receipts from customers 1.2 Payments for (a) staff costs (2,329) (6,978) (b) advertising and marketing (32) (491) (c) research and development (705) (203) (d) leased assets (9,854) (2,988) (e) other working capital 1.3 Dividends received 1.4 Interest and other items of a similar nature received 337 785 1.5 Interest and other costs of finance paid — — 1.6 Income taxes paid 1.7 Other (provide details if material)Net operating cash flows (5,215) (17,243) |
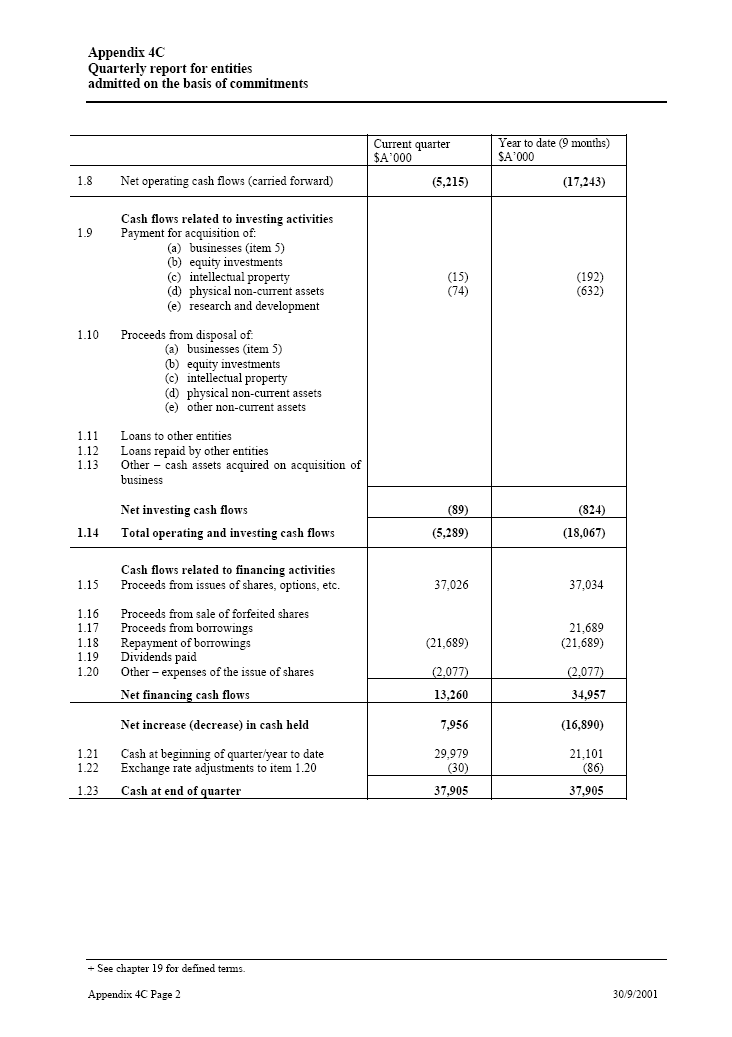
| Current quarter _____ Year to date (9 months) $A’000 $A’000 1.8 Net operating cash flows (carried forward)(5,215) (17,243) Cash flows related to investing activities 1.9 Payment for acquisition of: (15) (192) (a) businesses (item 5) (74) (632) (b) equity investments (c) intellectual property (d) physical non-current assets (e) research and development 1.10 Proceeds from disposal of: (a) businesses (item 5) (b) equity investments (c) intellectual property (d) physical non-current assets (e) other non-current assets 1.11 Loans to other entities 1.12 Loans repaid by other entities 1.13 Other — cash assets acquired on acquisition of businessNet investing cash flows (89) (824) 1.14 Total operating and investing cash flows (5,289) (18,067) Cash flows related to financing activities 1.15 Proceeds from issues of shares, options, etc. 37,026 37,034 1.16 Proceeds from sale of forfeited shares 1.17 Proceeds from borrowings 21,689 1.18 Repayment of borrowings (21,689) (21,689) 1.19 Dividends paid 1.20 Other — expenses of the issue of shares (2,077) (2,077)Net financing cash flows 13,260 34,957 Net increase (decrease) in cash held 7,956 (16,890) 1.21 Cash at beginning of quarter/year to date 29,979 21,101 1.22 Exchange rate adjustments to item 1.20 (30) (86) 1.23Cash at end of quarter 37,905 37,905 |
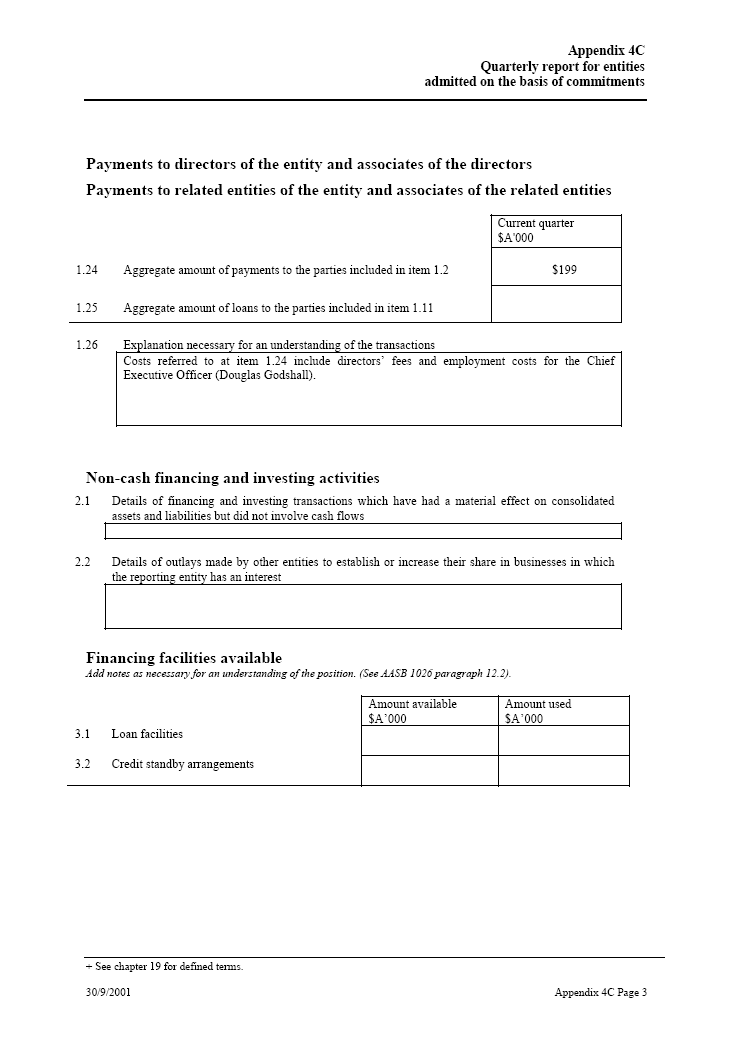
| Payments to directors of the entity and associates of the directors Payments to related entities of the entity and associates of the related entities Current quarter $A’000 1.24 Aggregate amount of payments to the parties included in item 1.2 $199 1.25 Aggregate amount of loans to the parties included in item 1.11 1.26 Explanation necessary for an understanding of the transactions Costs referred to at item 1.24 include directors’ fees and employment costs for the Chief Executive Officer (Douglas Godshall).Non-cash financing and investing activities 2.1 Details of financing and investing transactions which have had a material effect on consolidated assets and liabilities but did not involve cash flows 2.2 Details of outlays made by other entities to establish or increase their share in businesses in which the reporting entity has an interestFinancing facilities availableAdd notes as necessary for an understanding of the position. (See AASB 1026 paragraph 12.2). Amount available _____ Amount used $A’000 $A’000 3.1 Loan facilities 3.2 Credit standby _____ arrangements |
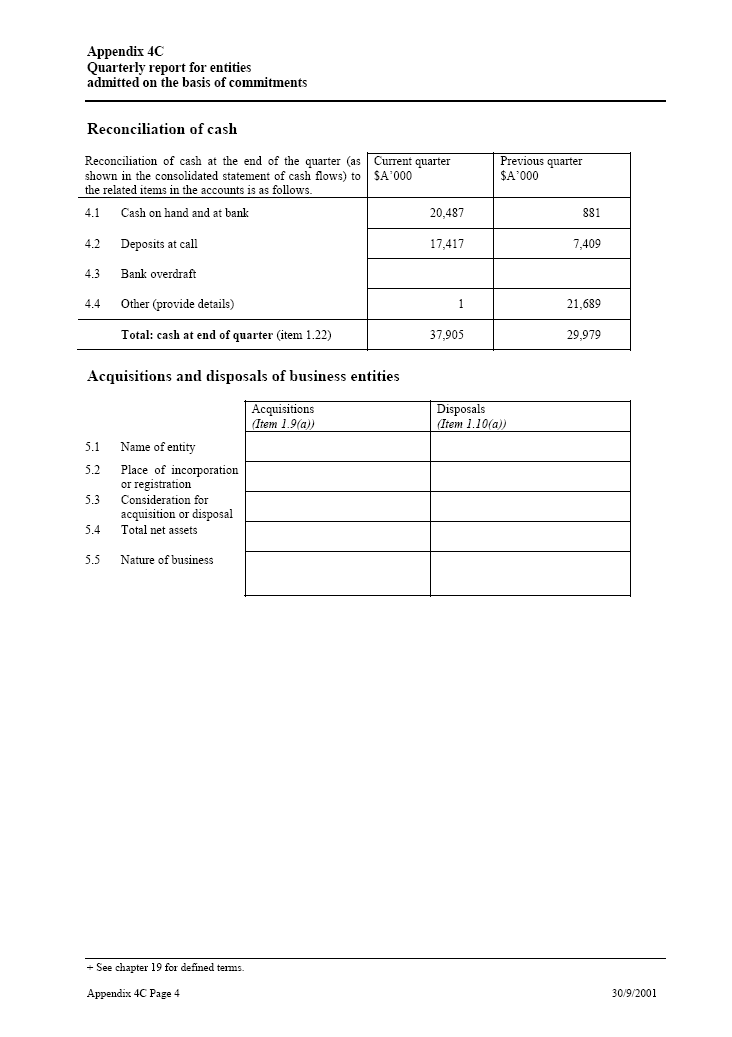
| Reconciliation of cash Reconciliation of cash at the end of the quarter _____ Current quarter _____ Previous quarter (as shown in the consolidated statement of cash flows) $A’000 $A’000 to the related items in the accounts is as follows. 4.1 Cash on hand and at bank 20,487 881 4.2 Deposits at call 17,417 7,409 4.3 Bank overdraft 4.4 Other (provide details) 1 21,689Total: cash at end of quarter(item 1.22) 37,905 29,979Acquisitions and disposals of business entities Acquisitions _____ Disposals(Item 1.9(a)) (Item 1.10(a)) 5.1 Name of entity 5.2 Place of incorporation or registration 5.3 Consideration for acquisition or disposal 5.4 Total net assets 5.5 Nature of business |

| Compliance statement 1 This statement has been prepared under accounting policies which comply with accounting standards as defined in the Corporations Act. 2This statement does give a true and fair view of the matters disclosed. Sign here: Date: ..31 October 2007 (Director) Print name: Douglas Godshall...Notes 1. The quarterly report provides a basis for informing the market how the entity’s activities have been financed for the past quarter and the effect on its cash position. An entity wanting to disclose additional information is encouraged to do so, in a note or notes attached to this report. 2. The definitions in, and provisions of,AASB 1026: Statement of Cash Flowsapply to this report except for the paragraphs of the Standard set out below. · 6.2 — reconciliation of cash flows arising from operating activities to operating profit or loss · 9.2 — itemised disclosure relating to acquisitions · 9.4 — itemised disclosure relating to disposals · 12.1(a) — policy for classification of cash items · 12.3 — disclosure of restrictions on use of cash · 13.1 — comparative information |