Exhibit 99.01
30 April 2008
Dear Shareholder
It has been an extremely busy and productive few months at HeartWare and in the Ventricular Assist Device (VAD) space in general so I thought it an appropriate juncture to provide you with both internal and external updates.
The key event for HeartWare over the past quarter was the first-ever public presentation of our initial clinical results at the annual meeting of the International Society for Heart and Lung Transplantation (ISHLT) in early April. We also took advantage of the ISHLT conference to hold a series of meetings with our Medical Advisory Board, our current clinical investigators and our prospective US surgeons. Last week we also announced our decision to relocate our manufacturing operations to a far larger, high-quality facility, and just this past Monday, confirmed the appointment of Dr David Hathaway as Chief Medical Officer. We have further progressed discussions with the FDA regarding the start of our US clinical trial and continue to make encouraging advances in our product pipeline development efforts, including both our MVADTMminiature pump platform as well as our Transcutaneous Energy Transfer (TET) System.
Much of this is detailed in our 2007 Annual Report, which you will have received in recent weeks. The aim of this letter is to provide further detail regarding these developments and to share, in addition, a personal perspective of the VAD industry and the prospects for HeartWare as we move closer to market.
Presentation of HeartWare’s Clinical Results
Our initial clinical results were presented during the ISHLT meeting in Boston on 12 April by Dr. Georg Wieselthaler, cardiothoracic surgeon at Vienna General Hospital and a principle investigator in the international clinical trial for the HeartWare® LVAD System.
The data presented by Dr. Wieselthaler show a 6-month survival rate of 91% among the first 23 patients implanted with the HeartWare device. Of the 23 patients, 21 patients met the primary endpoint of the trial, defined as survival to 180 days or transplantation. These included 19 patients who were supported by the HeartWare system at 180 days and 2 patients who received transplants, after 157 days and 176 days respectively. In addition, Dr Wieselthaler presented an overview of the adverse clinical events experienced among this initial 23 patient group.
We are the first to acknowledge that 23 patients represent too small a sample on which to base any sweeping claims concerning the performance of our device. However, on the basis of these early data, our 180-day survival rate is extremely promising, our rate of adverse events is comfortably within acceptable limits and our extraordinarily low rate of heart transplantation appears to confirm that our patients continue to do well on our pump, even after fairly lengthy periods of mechanical support.
During his presentation, Dr Wieselthaler emphasized the benefit of the small size of the HeartWare device, noting in particular the advantages of pericardial placement and avoidance of abdominal surgery. Aside from our encouraging early clinical data, it is this surgical placement benefit — unique among centrifugal pumps — that will likely continue to positively differentiate the HeartWare device from all competing systems.
While this study only reported on the first 23 of our 32 patients, the fact that our initial positive experience has been maintained over subsequent implants has led us to extend our study at the request of our investigators. We have expanded to 50 the number of patients we can enroll in our international clinical trial so as to enable our 5 investigating centres in Europe and Australia to continue implanting our device in advance of our receipt of CE Mark, anticipated later this year. The average duration of support across our entire patient group exceeds 230 days. The cumulative period of support exceeds 7,350 days or approximately 20 years. Eight of our patients have been supported by the HeartWare® System for periods exceeding 12 months, including one patient who has been supported for more than 525 days. As this clinical experience builds, so too does our confidence in the competitive advantage inherent in our pump design and in the long term commercial prospects underpinned by this device.
Medical Advisory Board and Clinical Investigator Meetings
In attendance at the ISHLT meeting were the members of HeartWare’s Medical Advisory Board (MAB) as well as all current investigators in our international clinical trial. We took the opportunity to hold formal MAB and Investigator meetings to review our clinical data in advance of Dr Wieselthaler’s presentation and to seek independent perspective on the results. Our MAB members were encouraged by the results and very supportive of the transparent format with which we’re choosing to present all of our data.
Based on feedback from our MAB discussions and based on multiple surgeon meetings following the formal presentation of our results, it is apparent that the advantages of a small, pericardially implanted, centrifugal pump are resonating strongly amongst cardiac surgeons. The physicians were intrigued by our early data and a number of surgeons not previously exposed to our device have since made enquiries regarding the possibility of joining our US clinical study. Clearly this reaction bodes well for HeartWare as we expand our presence in Europe and initiate US implants in the near term.
An Industry Perspective
The ISHLT has for many years been dedicated to the discussion of heart and lung transplantation. The subject of mechanical circulatory support was a peripheral topic, of interest only to a relatively small, specialized group. However, discussion of mechanical support has come to represent a core area of focus during recent conferences — this year it occupied a significant portion of the meeting and each session was very well attended. There is no longer any question about the important role that mechanical support systems play in the management of heart failure and there is a clear consensus view that the use of these systems will become increasingly widespread. The number of heart transplants worldwide continues to fall each year as the availability of suitable donor organs declines. At the same time, the incidence of heart failure continues to rise. As the long term durability of the current generation of LVADs becomes evident, their use both as a bridge-to-transplant and as a long term alternative to transplantation is almost certain to increase. This trend is already evident and a majority of surgeons active in this area believe it is going to accelerate.
-2-
Growth in the use of LVADs will depend on LVAD developers and clinicians continuing to demonstrate acceptable patient survival data and long term device reliability as well as a continued reduction in the incidence of adverse events, particularly infection, bleeding and stroke. Clinical results presented at the ISHLT meeting relating to a number of 2nd and 3rd generation continuous-flow devices indicate strongly that results are trending in the right direction. It will be far easier to convince patients to consider VAD therapy if the results from multiple devices are positive since this validates that the therapy itself is beneficial. We have little doubt on this basis that, as a greater depth of long term data becomes available, the use of continuous flow LVADs for long term support will increase many fold over coming years.
FDA Approval of the HeartMate II
Last week the US FDA approved the use of the HeartMate II device as a bridge-to-transplant. The approval follows a successful US clinical trial involving more than 450 patients (including those enrolled under Continuous Access Protocols.)
The HeartMate II is the first continuous flow device to be approved in the US. Its approval provides US clinicians access for the first time to a relatively small, reliable pump. Clinical results for the HeartMate II have been very good — the US BTT study reported an 80% survival rate at 6 months and 77% at one year — and its reliability profile is far better than that of its predecessor, the HeartMate XVE, which until last week was the only approved device available in the US. With the approval of the HeartMate II, Thoratec can now expand the number of centres that have historically had access to the device from the 40 hospitals participating in the trial to the approximately 100 transplant centres in the US. There is a high level of enthusiasm among the heart failure community concerning the potential of this device and a broad expectation that its availability will lead to a sustained increase in US implant numbers over time.
We are very pleased by the positive momentum in our industry and are optimistic that Thoratec’s market development activities will help drive a shift in referral patterns and a consequent increase in implant numbers. We are encouraged by these efforts and wish Thoratec every success with the HeartMate II. The greater the success that Thoratec enjoys and the broader the adoption of the HeartMate II, the greater the market opportunity will be when HeartWare becomes commercially available in the US in 2 to 3 years from now. In the interim, US clinicians participating in our US clinical trial will be able to see first-hand the meaningful clinical advantages presented by the HeartWare® System.
US Clinical Trial Update
We are still awaiting approval from the US FDA to begin our US clinical trial. Dialogue with the FDA concerning our IDE submission has been ongoing since late last year and we have progressively addressed questions that the agency has raised concerning our submission. We are cautiously optimistic that all substantive questions have been adequately addressed and that any that remain will be easily resolved. On this basis, while we cannot predict the timing of the FDA decision, we remain hopeful that we will receive the IDE approval and start our US implants before mid year.
-3-
Our initial US clinical trial will be for a bridge-to-transplant indication, involving up to 150 patients at up to 28 participating centres. We look forward to sharing details of our trial design, which incorporates several novel elements, once we gain the approval.
In anticipation of our IDE, HeartWare has selected the initial group of clinical investigators. A series of surgeon training sessions have been scheduled during May, during which several of our US investigators will gain first-hand experience with the HeartWare systems and implant procedure. Following this training and subject only to final administrative sign-off at several of our proposed centres, we will be in a position to initiate US implants very soon after we receive our IDE.
New Manufacturing Facility
We announced two weeks ago that we had signed a lease on a new manufacturing facility in Miami Lakes, approximately 6 miles from our current facility in Miramar, FL. The move is already well underway as we have been planning for the relocation since February. We have taken possession of the site as of late last week and are currently working through a phased transition of our manufacturing infrastructure with a view to completing the move by the end of May.
As detailed in our announcement of 18 April, the new facility, previously occupied by Cordis Neurovascular, Inc., includes three clean rooms (a total area exceeding 10,000 square feet) all of which are ISO Class 100,000 compliant and fully operational. This is a purpose built, state of the art medical device manufacturing facility and represents a substantial upgrade from our current premises. The new facility has ample manufacturing capacity for us to expand our current production activities many-fold and should serve us well for the foreseeable future. We have taken a 3 year lease with the option to extend for two consecutive periods of 5 years each.
While the relocation will have no effect on the timing of our US clinical trial, it has modestly impacted our European regulatory timeline (as previously advised) due to the need to have the facility audited and ISO certified as a component of the CE Mark submission. We plan to schedule a regulatory audit of the facility as soon as practical following the completion of the move. This is expected to lead to receipt of CE Mark during the second half of 2008.
Appointment of Chief Medical Officer
We announced recently that we had appointed Dr David Hathaway to the position of Chief Medical Officer at HeartWare.
A trained cardiologist, David has enjoyed a stellar career both as a practicing clinician for 14 years and, subsequently, in a range of corporate roles with Bristol-Myers Squibb, Knoll Pharmaceutical Company and others. Details of his background were included in our ASX announcement of 29 April.
We are delighted that David will be joining the HeartWare team. His clinical trial management experience, his broad strategic perspective and his intimate knowledge of the management of heart failure patients will be of tremendous value as we seek to establish a leading position in the heart failure arena.
-4-
New Product Development
During the quarter we completed a further series of animal studies with the MVADTM miniaturized ventricular assist device. As previously advised, the key objective of these studies is to evaluate a number of alternative, less invasive implant techniques so as to determine which configuration of the device to advance towards GLP studies next year. We are evaluating several less invasive implant techniques, each of which eliminates the requirement for a sternotomy.
We continue to allocate resources carefully to the MVAD TM device program so as to avoid diluting our efforts on our first platform. It is evident from physician reaction to our current pump that we have a system in the clinic today that will expand the market significantly when it becomes widely available. We believe the MVAD TM device is a transformational technology that will make VAD therapy available and attractive to tens of thousands of patients who are unwilling or unable to undergo a sternotomy. Fortunately, it appears that our challenge will be deciding which of our current implant options to select since each approach has worked as well or better than we had expected.
Similarly, development of our Transcutaneous Energy Transfer (TET) System and of our fully implantable peripherals has progressed well. We expect in time that the entire industry will move towards fully implanted systems. While there remains much work to be done, we have a clear development plan in place and feel we are well positioned relative to others developing similar systems.
The prospects for the ventricular assist device sector are very bright. VAD’s in general are working well and patients are seeing the kind of survival benefit long hoped for in this market. The prospects for HeartWare, in particular, are better than they have ever been. We are on the cusp of starting our US trial and launching internationally with a device that uniquely addresses the needs of the surgeon, cardiologist and patient. We are within a few months of generating our first revenues and we have a pipeline that will lead to a many-fold increase in market size over time. We and our advisors feel that we are on the verge of a transformational 24 months. We are appreciative of your continued support and will continue to keep you abreast of progress.
If you’re able to, please join us at our upcoming Annual General Meeting in Sydney on 9th May.
Yours sincerely
Doug Godshall
Chief Executive Officer
-5-
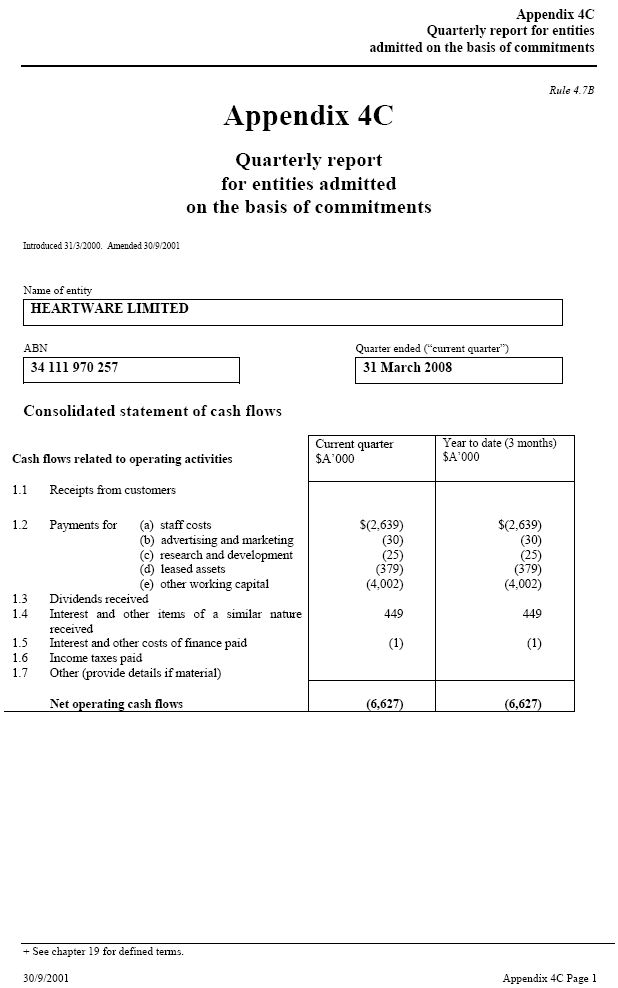
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Rule 4.7B Appendix 4C Quarterly report for entities admitted on the basis of commitments Introduced 31/3/2000. Amended 30/9/2001 Name of entity HEARTWARE LIMITED ABN _____ Quarter ended (“current quarter”) 34 111 970 257 31 March 2008 Consolidated statement of cash flows Current quarter _____ Year to date (3 months) Cash flows related to operating activities $A’000 $A’000 1.1 Receipts from customers 1.2 Payments for (a) staff costs $(2,639) $(2,639) (b) advertising and marketing (30) (30) (c) research and development (25) (25) (d) leased assets (379) (379) (e) other working capital (4,002) (4,002) 1.3 Dividends received 1.4 Interest and other items of a similar nature 449 449 received 1.5 Interest and other costs of finance paid (1) (1) 1.6 Income taxes paid 1.7 Other (provide details if material) Net operating cash flows (6,627) (6,627) + See chapter 19 for defined terms. 30/9/2001 Appendix 4C Page 1 |
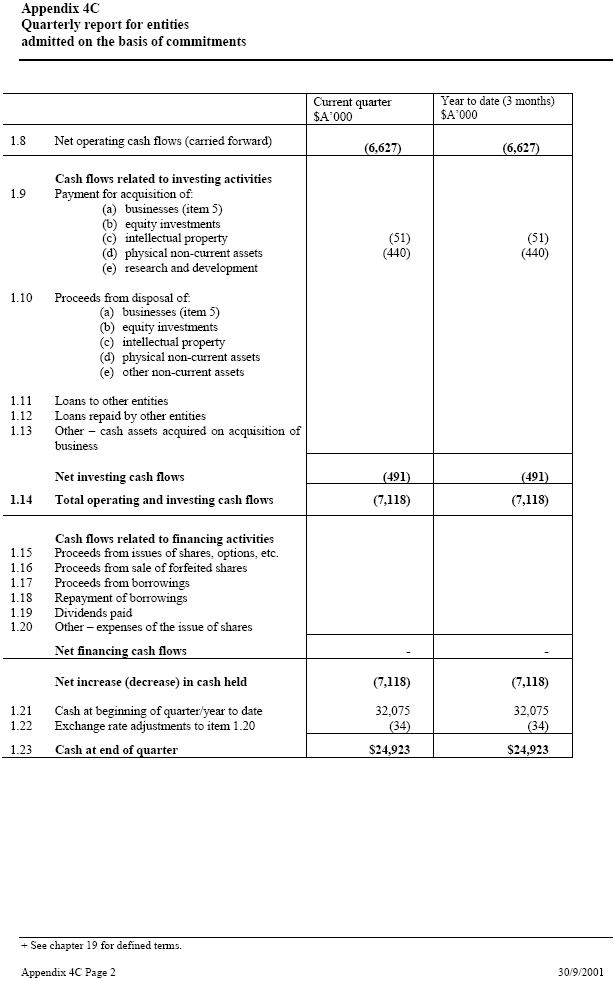
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Current quarter _____ Year to date (3 months) $A’000 $A’000 1.8 Net operating cash flows (carried forward) (6,627) (6,627) Cash flows related to investing activities 1.9 Payment for acquisition of: (a) businesses (item 5) (b) equity investments (c) intellectual property (51) (51) (d) physical non-current assets (440) (440) (e) research and development 1.10 Proceeds from disposal of: (a) businesses (item 5) (b) equity investments (c) intellectual property (d) physical non-current assets (e) other non-current assets 1.11 Loans to other entities 1.12 Loans repaid by other entities 1.13 Other – cash assets acquired on acquisition of _____ business Net investing cash flows (491) (491) 1.14 Total operating and investing cash flows (7,118) (7,118) Cash flows related to financing activities 1.15 Proceeds from issues of shares, options, etc. 1.16 Proceeds from sale of forfeited shares 1.17 Proceeds from borrowings 1.18 Repayment of borrowings 1.19 Dividends paid 1.20 Other – expenses of the issue of shares Net financing cash flows — — Net increase (decrease) in cash held (7,118) (7,118) 1.21 Cash at beginning of quarter/year to date 32,075 32,075 1.22 Exchange rate adjustments to item 1.20 (34) (34) 1.23 Cash at end of quarter $24,923 $24,923 + See chapter 19 for defined terms. Appendix 4C Page 2 30/9/2001 |
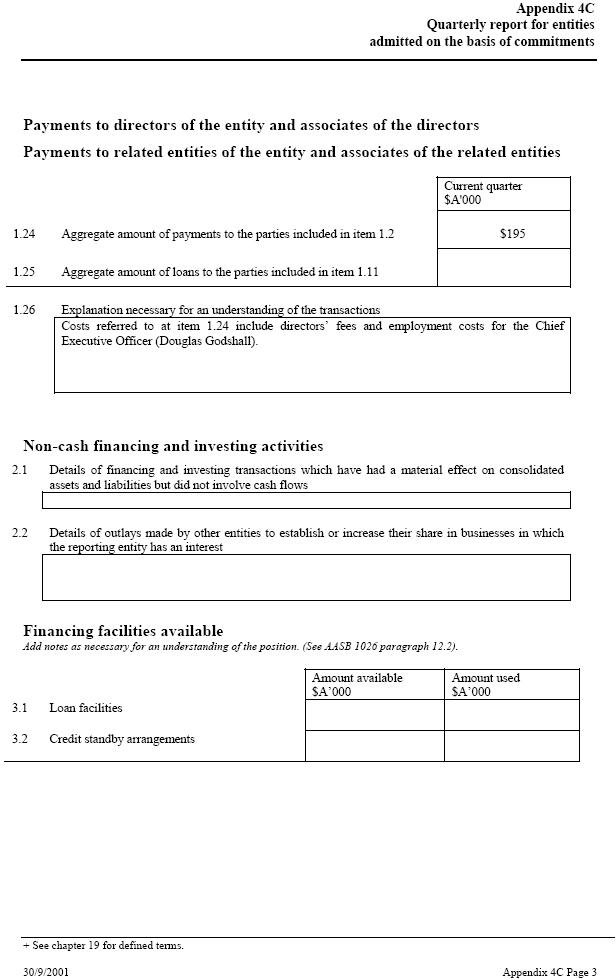
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Payments to directors of the entity and associates of the directors Payments to related entities of the entity and associates of the related entities Current quarter $A’000 1.24 Aggregate amount of payments to the parties included in item 1.2 $195 1.25 Aggregate amount of loans to the parties included in item 1.11 1.26 Explanation necessary for an understanding of the transactions Costs referred to at item 1.24 include directors’ fees and employment costs for the Chief _____ Executive Officer (Douglas Godshall). Non-cash financing and investing activities 2.1 Details of financing and investing transactions which have had a material effect on consolidated assets and liabilities but did not involve cash flows 2.2 Details of outlays made by other entities to establish or increase their share in businesses in which the reporting entity has an interest Financing facilities available Add notes as necessary for an understanding of the position. (See AASB 1026 paragraph 12.2). Amount available _____ Amount used $A’000 $A’000 3.1 Loan facilities 3.2 Credit standby arrangements + See chapter 19 for defined terms. 30/9/2001 Appendix 4C Page 3 |
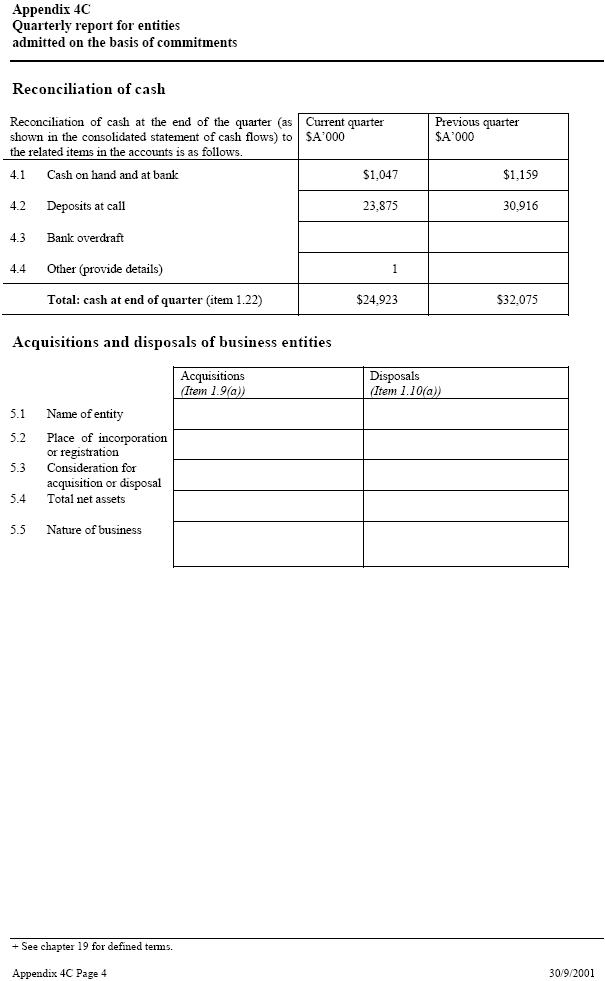
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Reconciliation of cash Reconciliation of cash at the end of the quarter (as Current quarter _____ Previous quarter shown in the consolidated statement of cash flows) to $A’000 $A’000 the related items in the accounts is as follows. 4.1 Cash on hand and at bank $1,047 $1,159 4.2 Deposits at call 23,875 30,916 4.3 Bank overdraft 4.4 Other (provide details) 1 Total: cash at end of quarter (item 1.22) $24,923 $32,075 Acquisitions and disposals of business entities Acquisitions _____ Disposals (Item 1.9(a)) (Item 1.10(a)) 5.1 Name of entity 5.2 Place of incorporation _____ or registration 5.3 Consideration for _____ acquisition or disposal 5.4 Total net assets 5.5 Nature of business + See chapter 19 for defined terms. Appendix 4C Page 4 30/9/2001 |
| Appendix 4C Quarterly report for entities admitted on the basis of commitments Compliance statement 1 This statement has been prepared under accounting policies which comply with accounting standards as defined in the Corporations Act. 2 This statement does give a true and fair view of the matters disclosed. Sign here: .... Date: ..30 April 2008 (Director) Print name: Douglas Godshall........................... Notes 1. The quarterly report provides a basis for informing the market how the entity’s activities have been financed for the past quarter and the effect on its cash position. An entity wanting to disclose additional information is encouraged to do so, in a note or notes attached to this report. 2. The definitions in, and provisions of, AASB 1026: Statement of Cash Flows apply to this report except for the paragraphs of the Standard set out below.• 6.2 — reconciliation of cash flows arising from operating activities to _____ operating profit or loss• 9.2 — itemised disclosure relating to acquisitions• 9.4 — itemised disclosure relating to disposals• 12.1(a) — policy for classification of cash items• 12.3 — disclosure of restrictions on use of cash• 13.1 — comparative information + See chapter 19 for defined terms. 30/9/2001 Appendix 4C Page 5 |