Exhibit 99.1

30 January 2009
Dear Shareholder,
2008 was a year that none of us will forget. It has been our good fortune that our shareholders have believed enough in the HeartWare mission to stand by us despite the myriad economic challenges over the past 12 months. As a result of your support and the extraordinary efforts of our team, HeartWare had its best year to date. In retrospect, it is quite remarkable what was achieved through 2008:
| • | we moved to world-class clean room facilities | ||
| • | we received ISO certification | ||
| • | we transitioned from an Australian to a U.S. headquartered company | ||
| • | we commenced our U.S. IDE clinical trial | ||
| • | we registered our first revenue | ||
| • | we completed enrolment of our 50-patient international clinical trial | ||
| • | we refined 2 new MVAD concepts and conducted preclinical studies on both | ||
| • | we improved pump production from approximately 6 units per month to a steady state of 20 per month | ||
| • | presented clinical data for the first time by Dr. Georg Wieselthaler at the ISHLT Meeting | ||
| • | we attracted one of the top surgeons in the world to be our Principal Investigator in the U.S. | ||
| • | we applied to list on NASDAQ and await confirmation of our listing date |
We announced earlier today that we have received CE Mark for our system. Receipt of CE Mark represents the culmination of much of the work that was carried out over the past 3 years and marks an important transition for the company. HeartWare’s device will now be available for commercial sale throughout Europe. Of course, we have anticipated the receipt of CE Mark for some time and have already begun to establish the necessary infrastructure in Europe to enable us to initiate commercial activities. Nonetheless, actual receipt of this first key regulatory approval presents a tangible acknowledgement of the extraordinary efforts of our team. We look forward to our first European sales and will move quickly towards establishing our presence in a number of targeted institutions across the continent. We anticipate our first sales in Europe will occur in the second half of February.
The other near-term milestone which many of our shareholders are anticipating is our listing on the NASDAQ Exchange. The listing process is confidential and it has been made clear to us that we must not discuss the specifics of the process. We are, however, able to confirm that the process is continuing in a positive direction and that we will announce a listing date in the near future.
As we look forward we anticipate another exceptional year for HeartWare. While we have set ourselves a range of objectives for 2009, our three highest level priorities are as follows:
| 1. | Commercial launch of the HeartWare® Ventricular Assist System in Europe and Australia | ||
| 2. | Enrolment of our U.S. Bridge to Transplant trial | ||
| 3. | Completion of a successful listing on NASDAQ and establishment of a liquid U.S. market for HeartWare shares |
HeartWare International, Inc. | ARBN 132 897 762
Suite 101, 205 Newbury Street, Framingham MA 01701 | Ph: (508) 739 0950 | Fax: (508) 739 0948 | www.heartware.com
Suite 101, 205 Newbury Street, Framingham MA 01701 | Ph: (508) 739 0950 | Fax: (508) 739 0948 | www.heartware.com

Commercial Launch in Europe
Having now received CE Mark we will move quickly towards a commercial launch of the system in February. While we have established our core team in Europe, we have deliberately held off expanding our staff numbers in advance of receiving regulatory approval. Fortunately, we have been approached over recent months by numerous highly qualified individuals wishing to join the company, so finding additional high calibre talent does not appear to be a challenge for us. As we expand our European presence, we will focus primarily on building our clinical support team since they are the ones who do the most to optimize outcomes for patients.
We are also fortunate to have a list of physicians in Europe who are keenly waiting to have access to the HeartWare® System. It is very pleasing that we are now in a position to begin shipping product shortly to meet this demand.
U.S. Clinical Trial
To date we have two centers that have commenced implants in the U.S. clinical trial — Washington Hospital Center and Jewish Hospital in Louisville. Between these two centers, 7 patients have been implanted with the HeartWare® System.
We are approximately six weeks off our initial schedule, which anticipated 5 U.S. centers implanting by the end of December 2008. However, we currently have 9 centers that have been initiated. These centers are either already screening patients or will begin screening patients in coming days. Beyond Washington Hospital Center and Jewish Hospital, Louisville, the additional 7 centers are:
| • | University of Michigan | ||
| • | Northwestern University | ||
| • | Texas Heart Institute | ||
| • | Ohio State University | ||
| • | The Cleveland Clinic | ||
| • | Johns Hopkins University; and | ||
| • | Penn State/Hershey Medical Center |
In addition to these, there are a number of centers that are close to completing all the pre-requisites and they should be initiated within the next few weeks.
Shareholders may recall that the FDA initially restricted us to an initial group of only 10 hospitals. After 10 patients had been implanted for 90 days and the FDA had reviewed the data from those patients, then we would be permitted to expand beyond 10 to a maximum of 28 centers. As we announced in a recent presentation at the JP Morgan conference, the FDA removed this restriction at the end of December which allows us to expand our implanting centers to 28 at whatever pace we choose.
This was excellent news as it enables us to stay on our aggressive schedule which would have us complete enrolment of this 150 patient trial by April 2010 though we will be striving to complete enrolment much sooner. We had not expected to be adding sites 11 through 28 until after June so our site establishment process was not as advanced with those sites as with our initial 10 but we are now accelerating the process across multiple additional target centers.
NASDAQ Listing
Adding NASDAQ as a second exchange will enable many more investors to gain access to our shares. There are many funds in the U.S. that cannot or are unwilling to purchase shares on the ASX. A majority of the world’s specialty medical device investors are located in the U.S. and we believe that making HeartWare more accessible to them via our listing will be a tremendous boost to our long-term financial well being.
- 2 -

As previously advised, nothing will change for shareholders who elect to hold their HeartWare interest on the ASX. Following the NASDAQ listing, HeartWare International will trade on two exchanges, with HeartWare common shares trading on NASDAQ under the symbol HTWR and HeartWare Chess Depository Interests (CDI’s) continuing to trade on ASX under the symbol HIN. Since shareholders can easily convert their holdings between the two forms of security, we would expect the two markets to broadly track one another in terms of price, after allowing for the USD/AUD exchange rate and the 1:35 ratio between common shares and CDI’s.
Closing Comments
Our MVAD and TETS pipelines will continue to progress this year and their true value will start to be realized in 2010 and beyond. We certainly anticipate that a great deal will be accomplished this year and that our pipeline will come into even clearer resolution.
As you will notice in our cash flow statements, our spending in the fourth quarter was a little higher than in prior quarters. This is largely due to the last remnants of redomiciliation expense, expanded clinical activity and, lastly, a substantial depreciation of the Australian dollar which has exaggerated the actual amount of our reported US dollar denominated expenditure. With revenue starting to pick up, and most of the non-recurring expenses behind us, we anticipate monthly spend to start coming down rather significantly through the balance of 2009.
As we move into a new phase of growth for HeartWare in 2009, we will undoubtedly face a new set of challenges. In particular, we will be pressure-testing different parts of the organization than were tested in 2008. The VAD market continues to grow at a handsome rate. We are encouraged by the rave reviews we hear from our physicians and are confident that we will overcome the challenges before us and continue our steady advance towards our goals.
Thank you for your continued support.
Yours sincerely
Doug Godshall
Chief Executive Officer
Chief Executive Officer
- 3 -
| Appendix 4C Quarterly report for entities admitted on the basis of commitments |
| Rule 4.7B |
| Appendix 4C |
| Quarterly report for entities admitted on the basis of commitments |
| Introduced 31/3/2000. Amended 30/9/2001 |
| Name of entity |
| HEARTWARE INTERNATIONAL, INC. |
| ABN _____ Quarter ended (“current quarter”) |
| 98 008 624 691 31 December 2008 |
| Consolidated statement of cash flows |
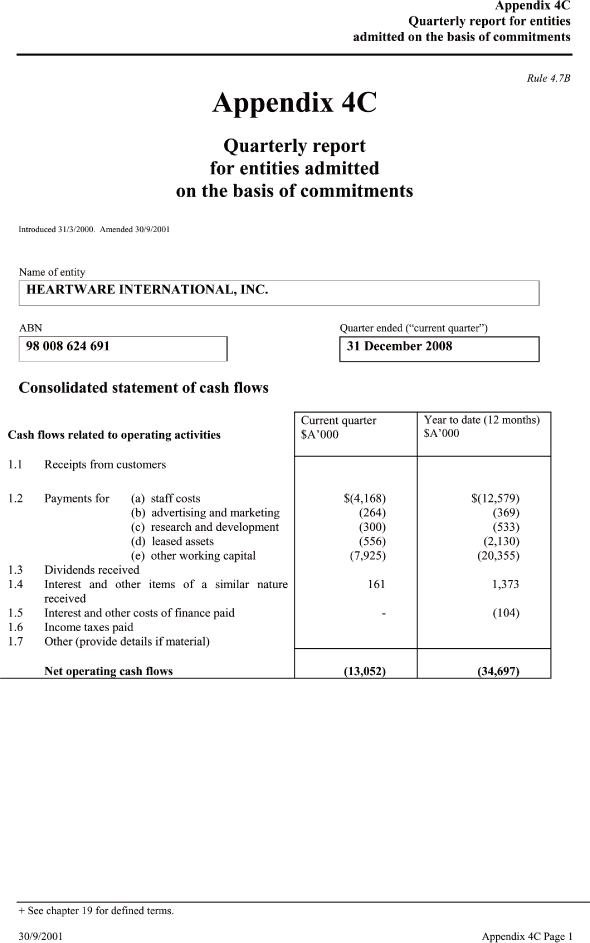
| Cash flows related to operating activitiesCurrent quarter _____ Year to date (12 months) $A’000 $A’000 |
| 1.1 Receipts from customers |
| 1.2 Payments for |
| (a) staff costs $ (4,168) $(12,579) |
| (b) advertising and marketing (264) (369) |
| (c) research and development (300) (533) |
| (d) leased assets (556) (2,130) |
| (e) other working capital (7,925) (20,355) |
| 1.3 Dividends received |
| 1.4 Interest and other items of a similar nature received 161 1,373 |
| 1.5 Interest and other costs of finance paid — (104) |
| 1.6 Income taxes paid |
| 1.7 Other (provide details if material) |
| — |
| Net operating cash flows (13,052) (34,697) — |
| + See chapter 19 for defined terms. |
| Appendix 4C Page 1 |
| Appendix 4C Quarterly report for entities admitted on the basis of commitments |
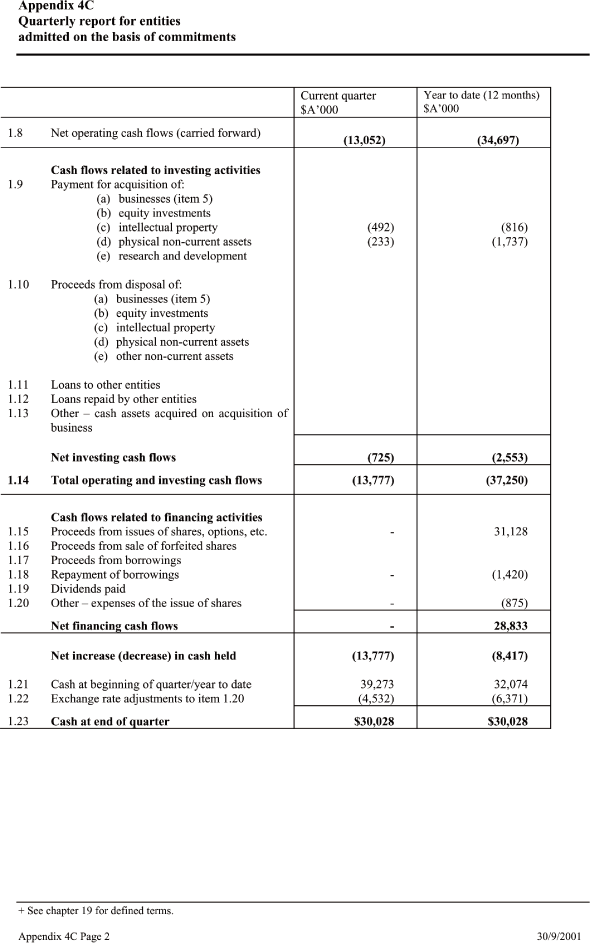
| Current quarter _____ Year to date (12 months) $A’000 $A’000 |
| 1.8 Net operating cash flows (carried forward)(13,052) (34,697) |
| Cash flows related to investing activities |
| 1.9 Payment for acquisition of: (492) (816) |
| (a) businesses (item 5) (233) (1,737) |
| (b) equity investments |
| (c) intellectual property |
| (d) physical non-current assets |
| (e) research and development |
| 1.10 Proceeds from disposal of: |
| (a) businesses (item 5) |
| (b) equity investments |
| (c) intellectual property |
| (d) physical non-current assets |
| (e) other non-current assets |
| 1.11 Loans to other entities |
| 1.12 Loans repaid by other entities |
| 1.13 Other – cash assets acquired on acquisition of business |
| — |
| Net investing cash flows (725) (2,553) — |
| 1.14 Total operating and investing cash flows (13,777) (37,250) — |
| Cash flows related to financing activities |
| 1.15 Proceeds from issues of shares, options, etc. — 31,128 |
| 1.16 Proceeds from sale of forfeited shares |
| 1.17 Proceeds from borrowings |
| 1.18 Repayment of borrowings — (1,420) |
| 1.19 Dividends paid |
| 1.20 Other – expenses of the issue of shares — (875) |
| —Net financing cash flows — 28,833 |
| — |
| Net increase (decrease) in cash held (13,777) (8,417) |
| 1.21 Cash at beginning of quarter/year to date 39,273 32,074 |
| 1.22 Exchange rate adjustments to item 1.20 (4,532) (6,371) — |
| 1.23Cash at end of quarter $ 30,028 $ 30,028 |
| — |
| + See chapter 19 for defined terms. |
| Appendix 4C Page 2 |
| Appendix 4C Quarterly report for entities admitted on the basis of commitments |
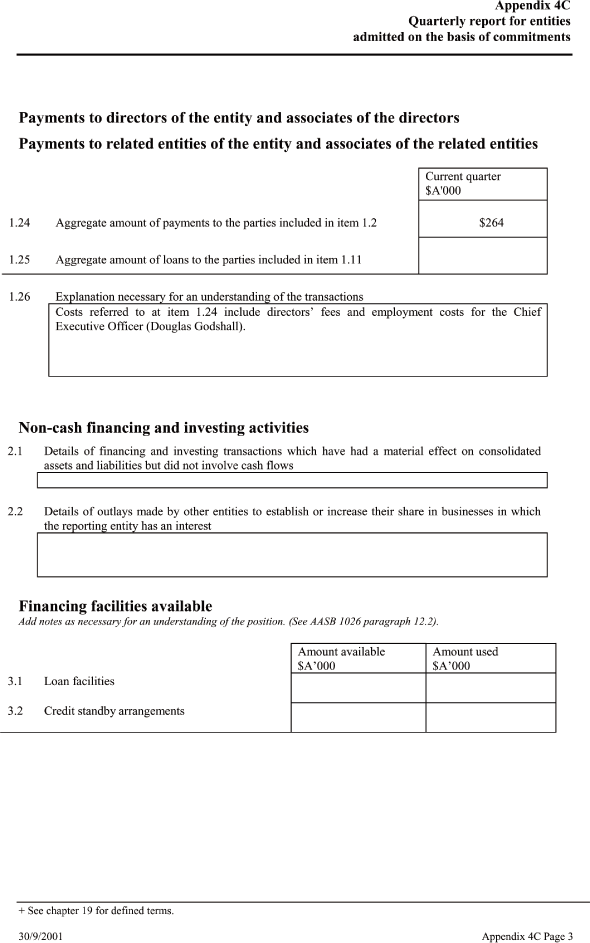
| Payments to directors of the entity and associates of the directors |
| Payments to related entities of the entity and associates of the related entities |
| Current quarter $A’000 |
| 1.24 Aggregate amount of payments to the parties included in item 1.2 $264 |
| 1.25 Aggregate amount of loans to the parties included in item 1.11 |
| 1.26 Explanation necessary for an understanding of the transactions Costs referred to at item 1.24 include directors’ fees and employment costs for the Chief Executive Officer (Douglas Godshall). |
| Non-cash financing and investing activities |
| 2.1 Details of financing and investing transactions which have had a material effect on consolidated assets and liabilities but did not involve cash flows |
| 2.2 Details of outlays made by other entities to establish or increase their share in businesses in which the reporting entity has an interest |
| Financing facilities available |
| Add notes as necessary for an understanding of the position. (See AASB 1026 paragraph 12.2). |
| Amount available _____ Amount used $A’000 $A’000 |
| 3.1 Loan facilities |
| 3.2 Credit standby arrangements |
| + See chapter 19 for defined terms. |
| Appendix 4C Page 3 |
| Appendix 4C Quarterly report for entities admitted on the basis of commitments |
| Reconciliation of cash |
| Reconciliation of cash at the end of the quarter (as shown in the consolidated statement of cash flows) to the related items in the accounts is as follows. |
| Current quarter _____ Previous quarter $A’000 $A’000 |
| | | |
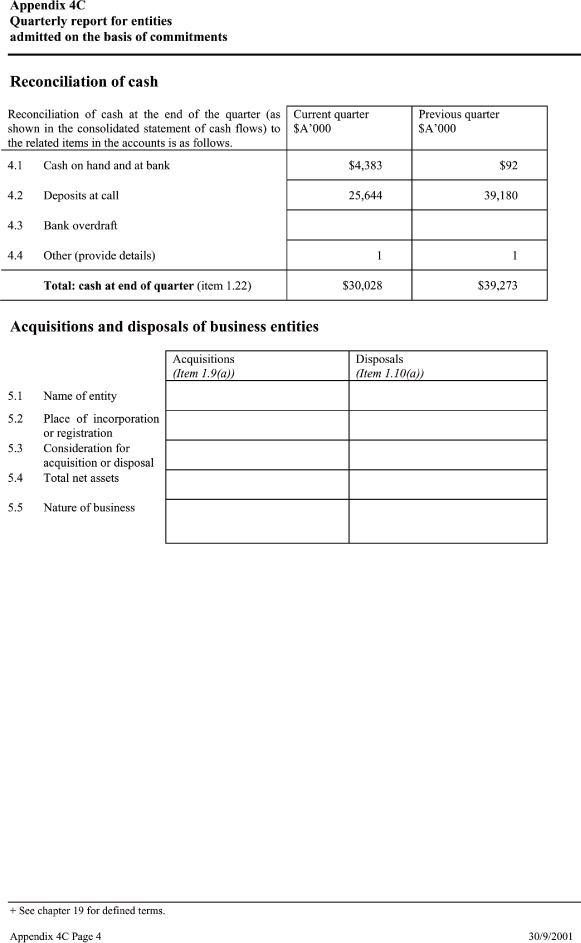
| 4.1 Cash on hand and at bank $ 4,383 $ 92 4.2 Deposits at call 25,644 39,180 4.3 Bank overdraft 4.4 Other (provide details) 1 1 — |
| Total: cash at end of quarter(item 1.22) $30,028 $39,273 ===== |
| Acquisitions and disposals of business entities |
| Acquisitions _____ Disposals(Item 1.9(a)) (Item 1.10(a)) |
| 5.1 Name of entity |
| 5.2 Place of incorporation or registration |
| 5.3 Consideration for acquisition or disposal |
| 5.4 Total net assets |
| 5.5 Nature of business |
| + See chapter 19 for defined terms. |
| Appendix 4C Page 4 30/9/2001 |

| Appendix 4C Quarterly report for entities admitted on the basis of commitments |
| Compliance statement |
| 1 This statement has been prepared under accounting policies which comply with accounting standards as defined in the Corporations Act. |
| 2This statement does give a true and fair view of the matters disclosed. |
| Sign here: Date: ..30 January 2009 |
| (Director) |
| Print name: Douglas Godshall................................ |
| Notes |
| 1. The quarterly report provides a basis for informing the market how the entity’s activities have been financed for the past quarter and the effect on its cash position. An entity wanting to disclose additional information is encouraged to do so, in a note or notes attached to this report. |
| 2. The definitions in, and provisions of,AASB 1026: Statement of Cash Flowsapply to this report except for the paragraphs of the Standard set out below. |
| · 6.2 — reconciliation of cash flows arising from operating activities to operating profit or loss |
| · 9.2 — itemised disclosure relating to acquisitions |
| · 9.4 — itemised disclosure relating to disposals |
| · 12.1(a) — policy for classification of cash items |
| · 12.3 — disclosure of restrictions on use of cash |
| · 13.1 — comparative information |
| + See chapter 19 for defined terms. |
| 30/9/2001 Appendix 4C Page 5 |