
Sellas LIFE SCIENCES Group, inc. Company OVERVIEW JANUARY 2018 Exhibit 99.3

FORWARD LOOKING STATEMENTS This presentation may include forward-looking statements about strategy, future operations, future financial position, prospects, plans and objectives of SELLAS Life Sciences Group, Inc. (“SELLAS”). Examples of such statements include, but are not limited to, SELLAS’ ability to successfully initiate and complete clinical trials; the nature, strategy and focus of SELLAS; and the development, efficacy and commercial potential of any of SELLAS’ product candidates. SELLAS may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Actual results and performance could differ materially from those projected in the forward-looking statements, which are based on management’s current expectations and involve risks and uncertainties, such as our ability to obtain necessary funding to further the development of our product candidate galinpepimut-S, the success of any of our ongoing and planned clinical trials for our product candidates, as well as whether or not we will achieve any of the intended benefits of the business combination between Galena Biopharma, Inc. and SELLAS Life Sciences Group Ltd. (the “Merger”), as well as those identified under “Risk Factors,” including, but not limited to, those under the headings “Risk Related to SELLAS’ Financial Results and Capital Needs,” “Risks Related to the Development of SELLAS’ Product Candidates” and “Risks Related to SELLAS’ Business Operations,” in our proxy statement/prospectus/consent solicitation statement dated November 6, 2017 and filed with the Securities and Exchange Commission (the “SEC”) pursuant to Rule 424 on November 8, 2017, as supplemented, as well is in our in our Annual Report on Form 10-K filed with the SEC on March 15, 2017, and Quarterly Reports on Form 10-Q filed with the SEC on May 10, 2017, August 14, 2017 and November 9, 2017 (each filed under our prior name, Galena Biopharma, Inc.) and in other documents filed with the SEC and available on our website regarding the Merger, as such may be amended and supplemented from time to time. We undertake no obligation to amend or revise any such forward-looking statements, which speak only as of the date hereof.

EXECUTIVE SUMMARY

investment Highlights: Late-stage immunotherapy company Lead asset, galinpepimut-S (GPS): numerous potential indications in a multibillion dollar cancer immunotherapy market Targets an NCI top ranked antigen, WT1 Highly differentiated, innovative technology, licensed from Memorial Sloan Kettering Cancer Center Robust clinical development pipeline Planned next clinical trials (pending financing): Pivotal Phase 3 Acute Myeloid Leukemia Combination trial GPS + pembrolizumab (Keytruda) Phase 2a study Partnerships with key industry leaders Experienced Management Team, Board of Directors and Scientific Advisory Board Reverse Merger Closed 12/29/2017 (NASDAQ: SLS)

CLINICAL OVERVIEW GPS - an immunotherapy targeting the Wilms Tumor 1 (WT1) protein WT1 is a top-ranked cancer antigen target recognized by the National Cancer Institute Multivalent, heteroclitic technology targeting WT1 Exhibits strong immune response; ongoing development in monotherapy and combination IO settings Well tolerated with compelling efficacy profile in completed Phase 2 trials Acute myeloid leukemia: In older population (>60 years), 35.3 months median overall survival, longer than expected with SOC (~ 1 year); 67.6 median overall survival across all ages Malignant pleural mesothelioma: Blinded, randomized-controlled Phase 2 resulted in 22.8 months median overall survival compared with 18.3 months with control EMA & FDA Orphan Drug Designation and FDA Fast Track status for both AML and MPM Ongoing Phase 1/2 studies in Multiple Myeloma and Ovarian Cancer Multiple myeloma: 23.6 months median progression-free survival; median overall survival not yet reached Ovarian cancer: combination study with nivolumab (Opdivo®); enrollment completed Additional checkpoint combination study planned for 2018 Phase 1/2 trial GPS to be administered in combination with Merck’s anti-PD-1 therapy, pembrolizumab (Keytruda®), in a variety of cancer indications including: colorectal, ovarian, small cell lung, triple-negative breast, and AML
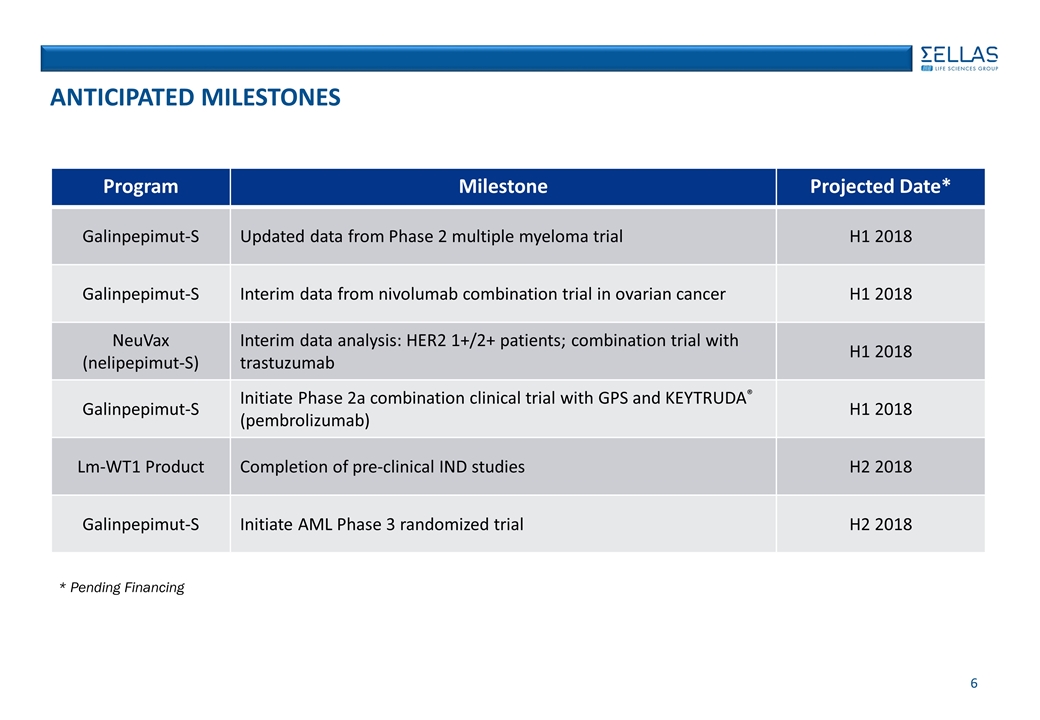
ANTICIPATED MILESTONES Program Milestone Projected Date* Galinpepimut-S Updated data from Phase 2 multiple myeloma trial H1 2018 Galinpepimut-S Interim data from nivolumab combination trial in ovarian cancer H1 2018 NeuVax (nelipepimut-S) Interim data analysis: HER2 1+/2+ patients; combination trial with trastuzumab H1 2018 Galinpepimut-S Initiate Phase 2a combination clinical trial with GPS and KEYTRUDA® (pembrolizumab) H1 2018 Lm-WT1 Product Completion of pre-clinical IND studies H2 2018 Galinpepimut-S Initiate AML Phase 3 randomized trial H2 2018 * Pending Financing

PARTNERSHIPS & collaborations

GALINPEPIMUT-S SCIENTIFIC OVERVIEW
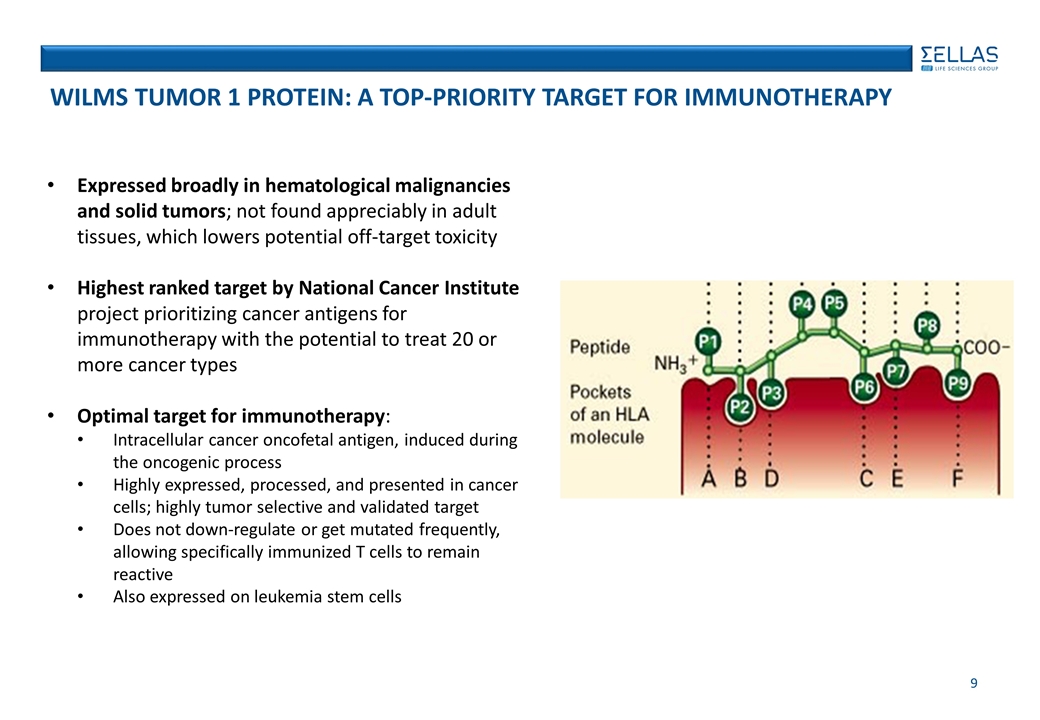
Expressed broadly in hematological malignancies and solid tumors; not found appreciably in adult tissues, which lowers potential off-target toxicity Highest ranked target by National Cancer Institute project prioritizing cancer antigens for immunotherapy with the potential to treat 20 or more cancer types Optimal target for immunotherapy: Intracellular cancer oncofetal antigen, induced during the oncogenic process Highly expressed, processed, and presented in cancer cells; highly tumor selective and validated target Does not down-regulate or get mutated frequently, allowing specifically immunized T cells to remain reactive Also expressed on leukemia stem cells Wilms Tumor 1 Protein: A top-Priority Target for Immunotherapy
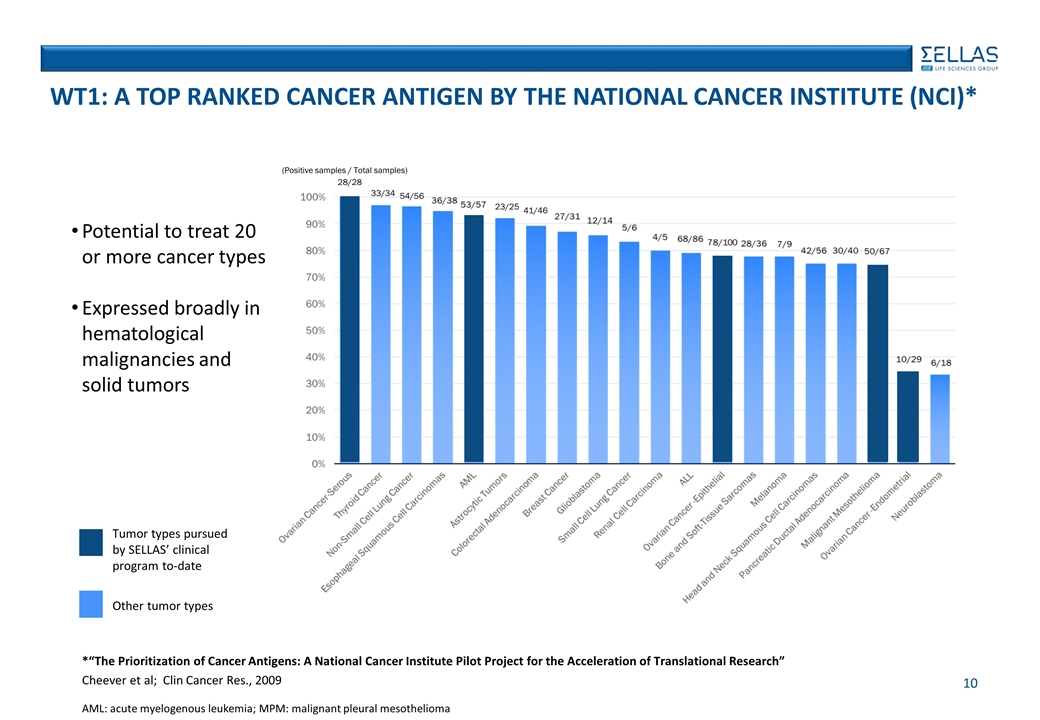
AML: acute myelogenous leukemia; MPM: malignant pleural mesothelioma Tumor types pursued by SELLAS’ clinical program to-date Other tumor types Potential to treat 20 or more cancer types Expressed broadly in hematological malignancies and solid tumors 28/28 33/34 54/56 23/25 41/46 (Positive samples / Total samples) WT1: A TOP RANKED CANCER ANTIGEN BY THE NATIONAL CANCER INSTITUTE (NCI)* *“The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research” Cheever et al; Clin Cancer Res., 2009
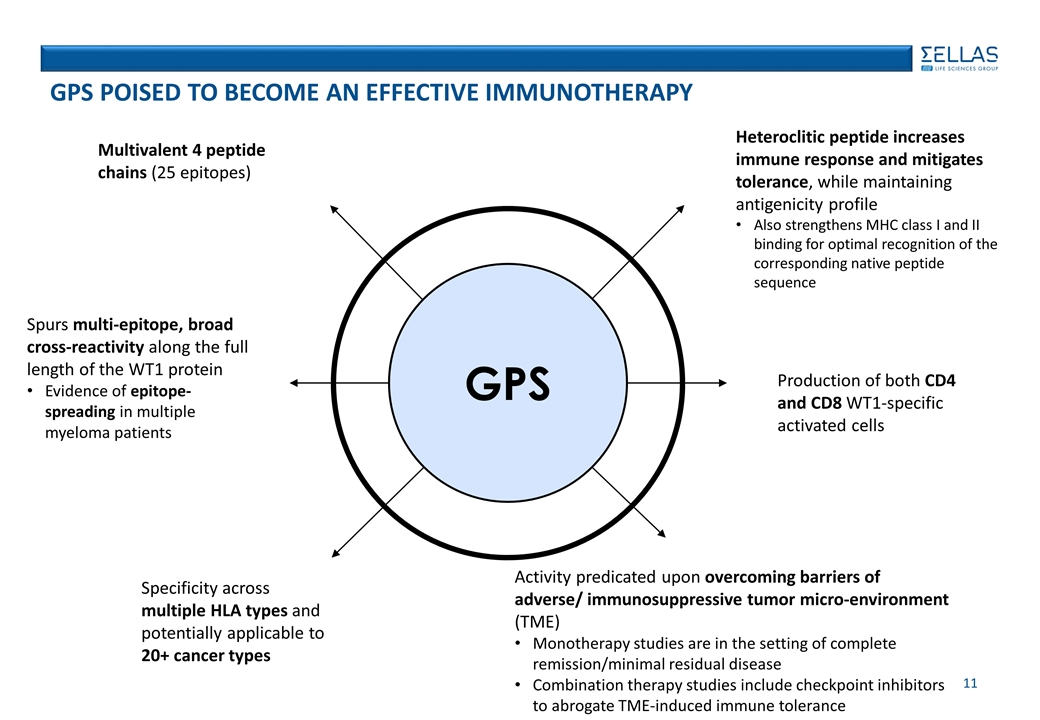
GPS POISED TO BECOME AN EFFECTIVE immunotherapy Specificity across multiple HLA types and potentially applicable to 20+ cancer types Heteroclitic peptide increases immune response and mitigates tolerance, while maintaining antigenicity profile Also strengthens MHC class I and II binding for optimal recognition of the corresponding native peptide sequence Production of both CD4 and CD8 WT1-specific activated cells Multivalent 4 peptide chains (25 epitopes) Spurs multi-epitope, broad cross-reactivity along the full length of the WT1 protein Evidence of epitope-spreading in multiple myeloma patients Activity predicated upon overcoming barriers of adverse/ immunosuppressive tumor micro-environment (TME) Monotherapy studies are in the setting of complete remission/minimal residual disease Combination therapy studies include checkpoint inhibitors to abrogate TME-induced immune tolerance GPS
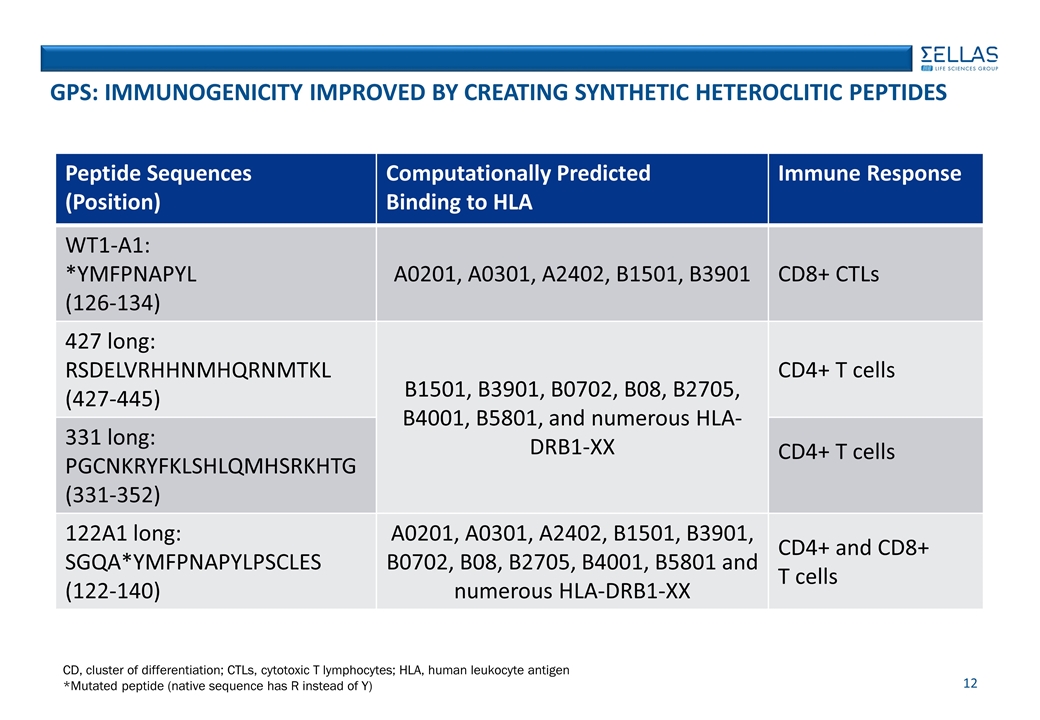
GPS: IMMUNOGENICITY IMPROVED BY CREATING SYNTHETIC HETEROCLITIC PEPTIDES Peptide Sequences (Position) Computationally Predicted Binding to HLA Immune Response WT1-A1: *YMFPNAPYL (126-134) A0201, A0301, A2402, B1501, B3901 CD8+ CTLs 427 long: RSDELVRHHNMHQRNMTKL (427-445) B1501, B3901, B0702, B08, B2705, B4001, B5801, and numerous HLA-DRB1-XX CD4+ T cells 331 long: PGCNKRYFKLSHLQMHSRKHTG (331-352) CD4+ T cells 122A1 long: SGQA*YMFPNAPYLPSCLES (122-140) A0201, A0301, A2402, B1501, B3901, B0702, B08, B2705, B4001, B5801 and numerous HLA-DRB1-XX CD4+ and CD8+ T cells CD, cluster of differentiation; CTLs, cytotoxic T lymphocytes; HLA, human leukocyte antigen *Mutated peptide (native sequence has R instead of Y)

SELLAS CLINICAL PROGRAMS
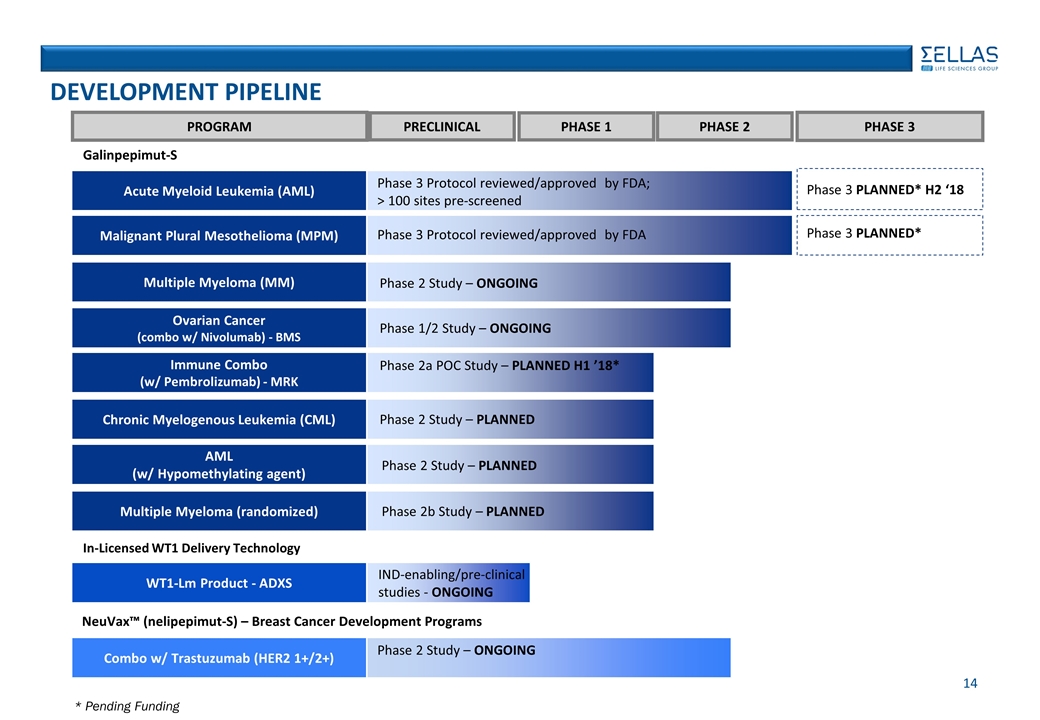
PROGRAM PRECLINICAL PHASE 1 PHASE 2 PHASE 3 In-Licensed WT1 Delivery Technology NeuVax™ (nelipepimut-S) – Breast Cancer Development Programs Acute Myeloid Leukemia (AML) Phase 3 PLANNED* H2 ‘18 Phase 3 Protocol reviewed/approved by FDA; > 100 sites pre-screened DEVELOPMENT PIPELINE 14 Galinpepimut-S Malignant Plural Mesothelioma (MPM) Phase 3 PLANNED* Phase 3 Protocol reviewed/approved by FDA Multiple Myeloma (MM) Phase 2 Study – ONGOING Ovarian Cancer (combo w/ Nivolumab) - BMS Immune Combo (w/ Pembrolizumab) - MRK Chronic Myelogenous Leukemia (CML) AML (w/ Hypomethylating agent) Multiple Myeloma (randomized) WT1-Lm Product - ADXS IND-enabling/pre-clinical studies - ONGOING Combo w/ Trastuzumab (HER2 1+/2+) Phase 2 Study – ONGOING Phase 1/2 Study – ONGOING Phase 2a POC Study – PLANNED H1 ’18* Phase 2 Study – PLANNED Phase 2 Study – PLANNED Phase 2b Study – PLANNED * Pending Funding
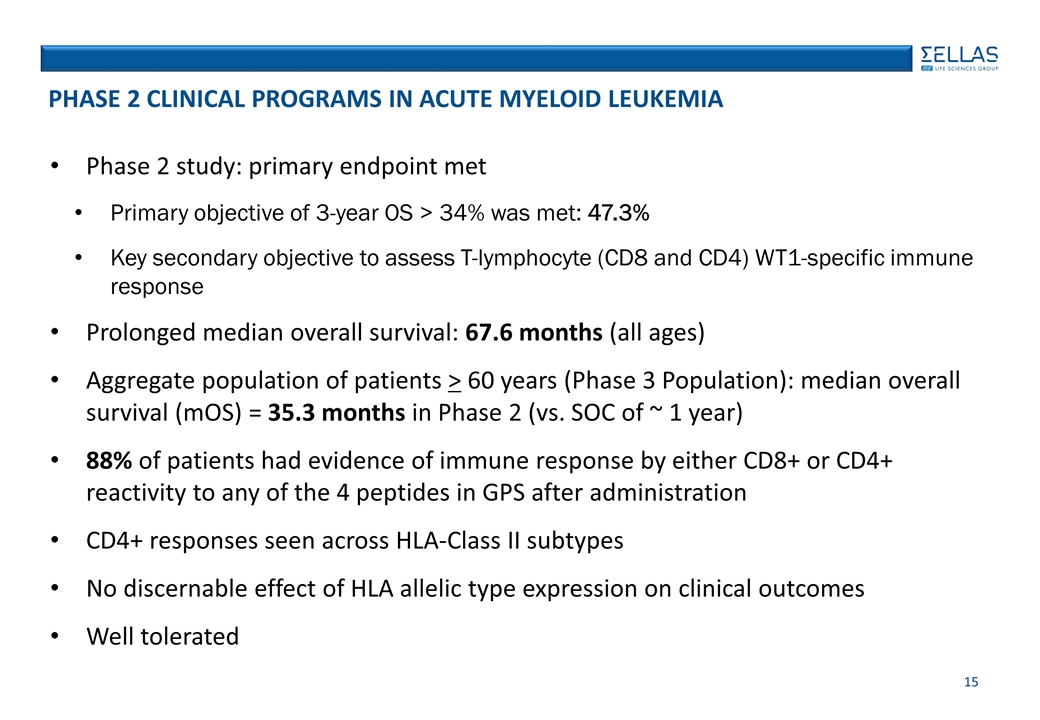
Phase 2 study: primary endpoint met Primary objective of 3-year OS > 34% was met: 47.3% Key secondary objective to assess T-lymphocyte (CD8 and CD4) WT1-specific immune response Prolonged median overall survival: 67.6 months (all ages) Aggregate population of patients > 60 years (Phase 3 Population): median overall survival (mOS) = 35.3 months in Phase 2 (vs. SOC of ~ 1 year) 88% of patients had evidence of immune response by either CD8+ or CD4+ reactivity to any of the 4 peptides in GPS after administration CD4+ responses seen across HLA-Class II subtypes No discernable effect of HLA allelic type expression on clinical outcomes Well tolerated Phase 2 Clinical Programs in Acute myeloid leukemia
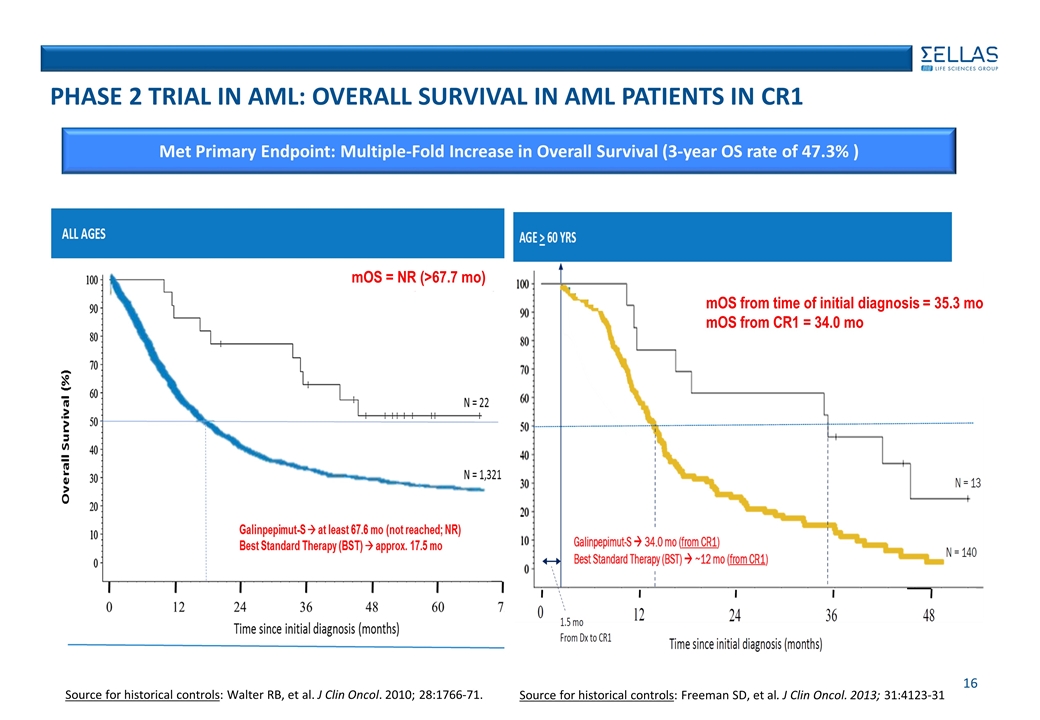
Source for historical controls: Walter RB, et al. J Clin Oncol. 2010; 28:1766-71. Source for historical controls: Freeman SD, et al. J Clin Oncol. 2013; 31:4123-31 phase 2 Trial in AML: Overall Survival in AML Patients in CR1 mOS from time of initial diagnosis = 35.3 mo mOS from CR1 = 34.0 mo mOS = NR (>67.7 mo) Met Primary Endpoint: Multiple-Fold Increase in Overall Survival (3-year OS rate of 47.3% )
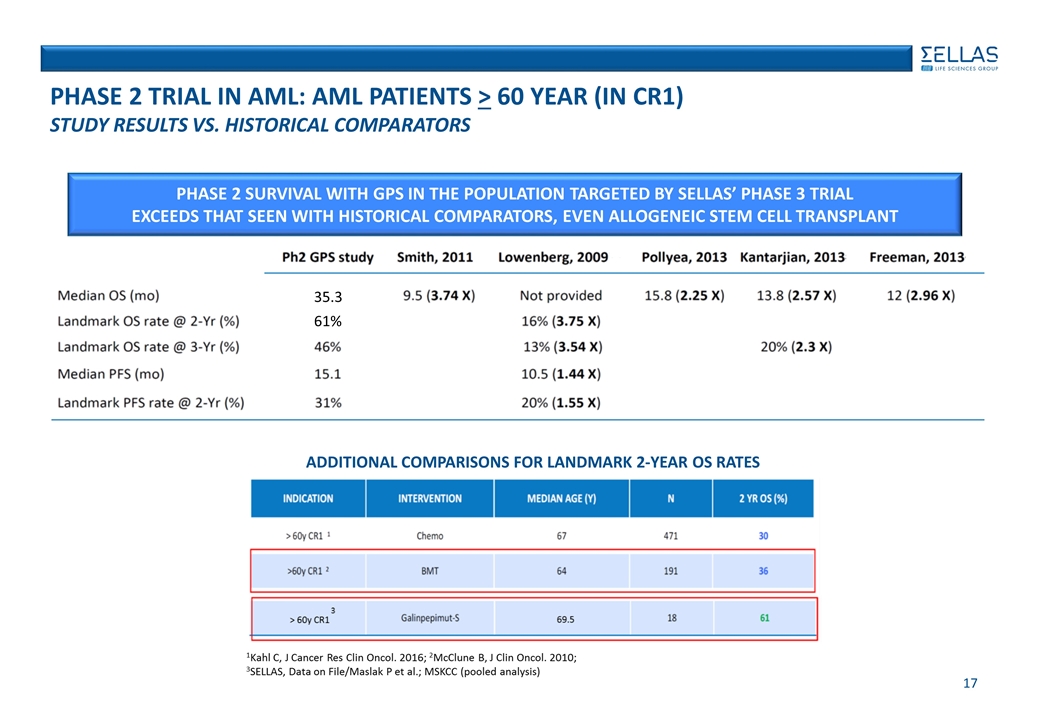
phase 2 Trial in AML: AML Patients > 60 year (in CR1) Study Results vs. Historical comparators Additional Comparisons for Landmark 2-year OS Rates 61% PHASE 2 SURVIVAL WITH GPS IN THE POPULATION TARGETED BY SELLAS’ PHASE 3 TRIAL EXCEEDS THAT SEEN WITH HISTORICAL COMPARATORS, EVEN ALLOGENEIC STEM CELL TRANSPLANT 35.3
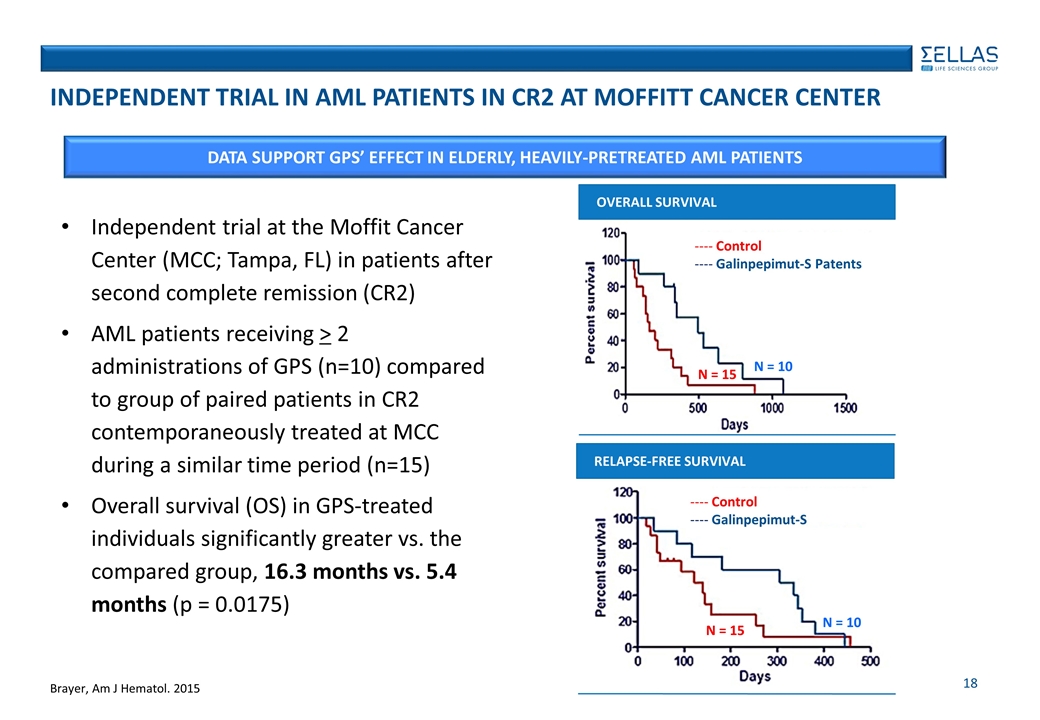
Brayer, Am J Hematol. 2015 Independent trial at the Moffit Cancer Center (MCC; Tampa, FL) in patients after second complete remission (CR2) AML patients receiving > 2 administrations of GPS (n=10) compared to group of paired patients in CR2 contemporaneously treated at MCC during a similar time period (n=15) Overall survival (OS) in GPS-treated individuals significantly greater vs. the compared group, 16.3 months vs. 5.4 months (p = 0.0175) DATA SUPPORT GPS’ EFFECT IN ELDERLY, HEAVILY-PRETREATED AML PATIENTS Independent trial in AML Patients in CR2 AT MOFFITT CANCER CENTER Relapse-Free Survival ---- Control ---- Galinpepimut-S N = 10 N = 15 OVERALL SURVIVAL ---- Control ---- Galinpepimut-S Patents N = 10 N = 15
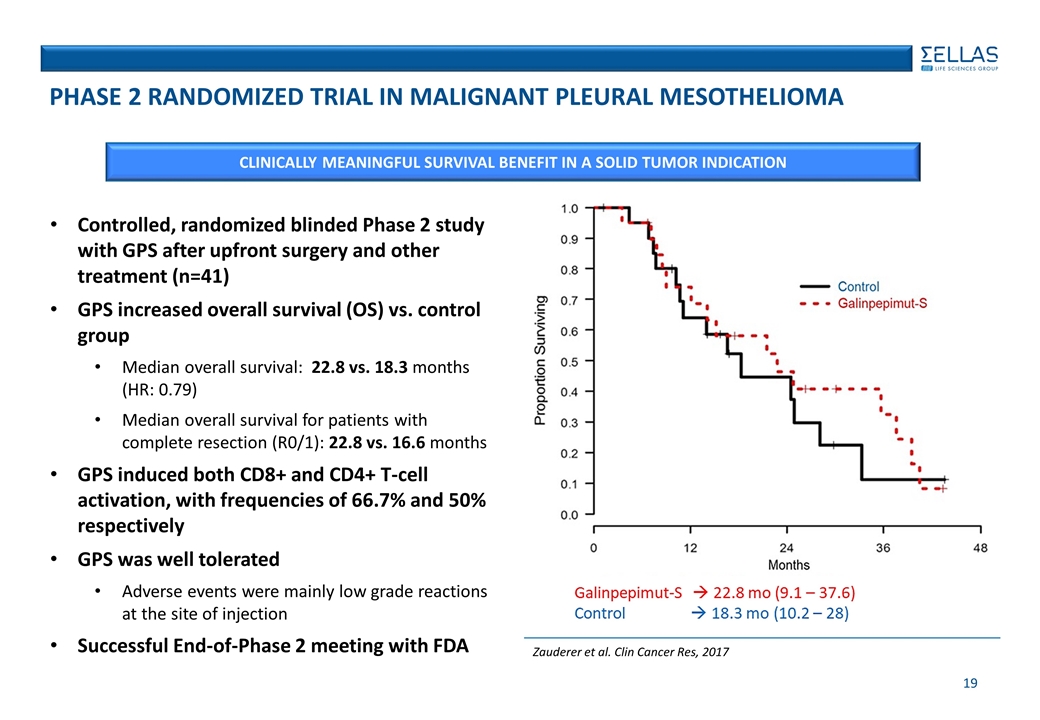
Controlled, randomized blinded Phase 2 study with GPS after upfront surgery and other treatment (n=41) GPS increased overall survival (OS) vs. control group Median overall survival: 22.8 vs. 18.3 months (HR: 0.79) Median overall survival for patients with complete resection (R0/1): 22.8 vs. 16.6 months GPS induced both CD8+ and CD4+ T-cell activation, with frequencies of 66.7% and 50% respectively GPS was well tolerated Adverse events were mainly low grade reactions at the site of injection Successful End-of-Phase 2 meeting with FDA CLINICALLY MEANINGFUL SURVIVAL BENEFIT IN A SOLID TUMOR INDICATION Phase 2 RANDOMIZED Trial in malignant pleural mesothelioma Zauderer et al. Clin Cancer Res, 2017
Phase 2, open-label study of GPS in myeloma patients following autologous stem cell transplant (SCT) Patients were treated for up to 9 months (12 GPS administrations) 15/18 patients had high-risk cytogenetics at baseline; all patients remained at least MRD(+) after SCT This type of MM patients typically has much poorer prognosis than lower risk types Clinical results were meaningfully superior to those from previous trials in this patient group Median PFS: GPS: 23.6 months as of June 2017 PFS at 12 months: 81% PFS at 18 months: 62% Overall survival at 18 months: 88%; median OS not yet reached High Frequency of WT1-specific immune responses (IR) by either CD4 or CD8 (91%) Positive relationship between IR and clinical response provides strong indication of immunobiological basis for effect seen on PFS Patients with CR/VGPR exhibited frequency of CD8+ and CD4+ IR of 82-100% Patients with CD8+ and CD4+ IR exhibited frequency of CR/VGPR of 57-63% Evidence of Epitope spreading High frequency (83.3%) of T-cell responses to an ’all-pool’ mixture of WT1-derived antigens (~240 epitopes) – against which patients were not specifically vaccinated ONGOING Phase 2 TRIAL in multiple myeloma (MM)
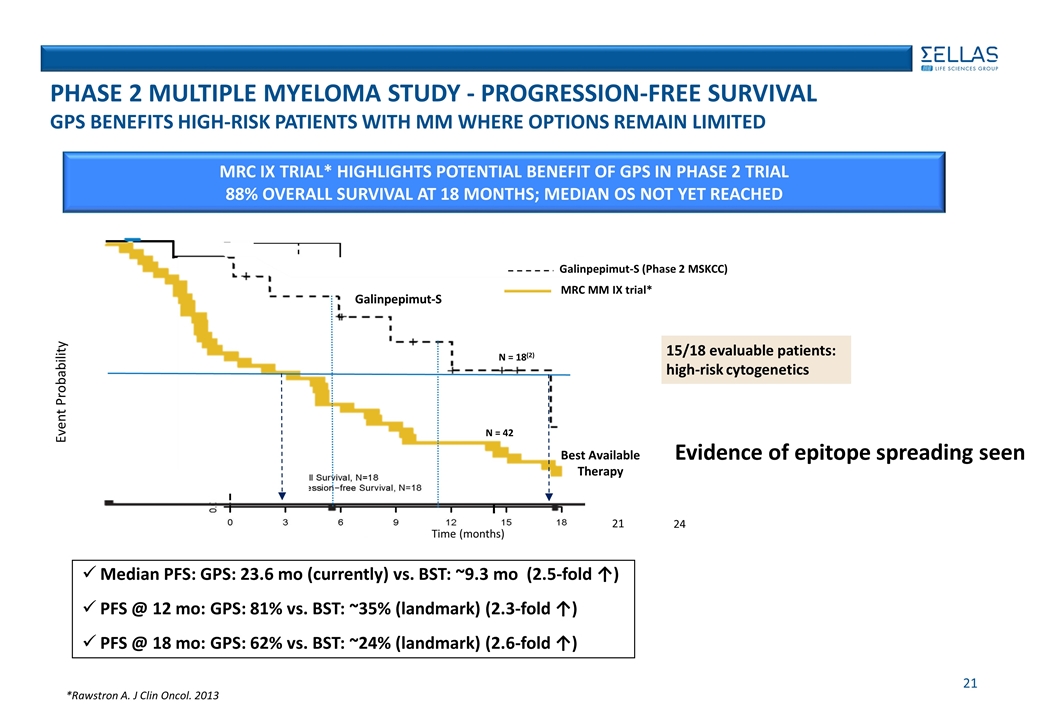
*Rawstron A. J Clin Oncol. 2013 15/18 evaluable patients: high-risk cytogenetics MRC IX Trial* Highlights potential benefit of gps in phase 2 trial 88% overall survival at 18 months; Median OS not yet reached Event Probability Time (months) 21 24 N = 18(2) Best Available Therapy N = 42 Galinpepimut-S Median PFS: GPS: 23.6 mo (currently) vs. BST: ~9.3 mo (2.5-fold ↑) PFS @ 12 mo: GPS: 81% vs. BST: ~35% (landmark) (2.3-fold ↑) PFS @ 18 mo: GPS: 62% vs. BST: ~24% (landmark) (2.6-fold ↑) MRC MM IX trial* Galinpepimut-S (Phase 2 MSKCC) PHASE 2 MULTIPLE MYELOMA STUDY - PROGRESSION-FREE SURVIVAL GPS Benefits High-Risk PATIENTS with MM Where Options Remain Limited Evidence of epitope spreading seen
Successful End-of-Phase 2 Meeting with FDA Agreed upon development, study design, endpoints, statistical analysis and CMC Primary endpoint is overall survival; secondary endpoints include LFS, safety/MRD, immune response 180 sites in North America, Europe, Australia/New Zealand, Asia; > 100 sites already pre-screened Trial population: N = 390; evaluable patients > 60 year Newly diagnosed AML in CR1, unable to undergo allotransplant following physician’s choice chemotherapy Trial design: Double-blind; 2:1 randomization, GPS to control Up to 16 doses in ~ 2 years post-remission therapy 90% power to detect a 45% survival difference (9 vs. 13 months) Three pre-planned interim analyses by DSMB Planned Phase 3 Study in AML
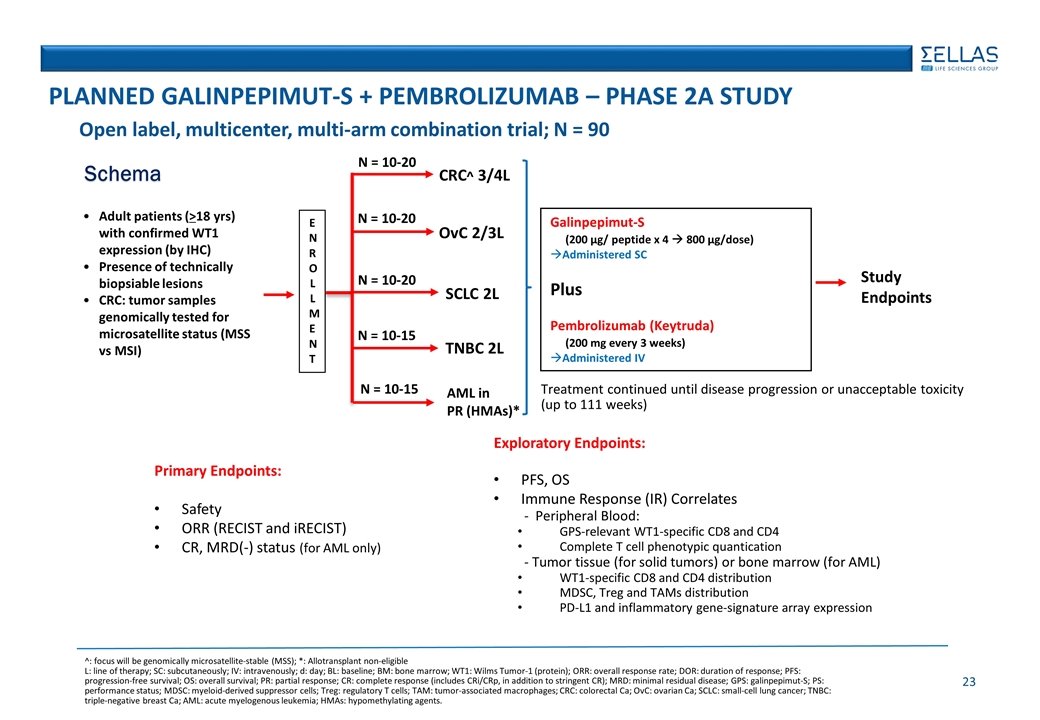
Open label, multicenter, multi-arm combination trial; N = 90 Schema Adult patients (>18 yrs) with confirmed WT1 expression (by IHC) Presence of technically biopsiable lesions CRC: tumor samples genomically tested for microsatellite status (MSS vs MSI) ENROLLMENT Galinpepimut-S (200 µg/ peptide x 4 à 800 µg/dose) Administered SC Plus Pembrolizumab (Keytruda) (200 mg every 3 weeks) Administered IV N = 10-20 N = 10-20 N = 10-20 N = 10-15 N = 10-15 Primary Endpoints: Safety ORR (RECIST and iRECIST) CR, MRD(-) status (for AML only) PLANNED Galinpepimut-S + PEMBROLIZUMAB – Phase 2A STUDY Exploratory Endpoints: PFS, OS Immune Response (IR) Correlates - Peripheral Blood: GPS-relevant WT1-specific CD8 and CD4 Complete T cell phenotypic quantication - Tumor tissue (for solid tumors) or bone marrow (for AML) WT1-specific CD8 and CD4 distribution MDSC, Treg and TAMs distribution PD-L1 and inflammatory gene-signature array expression ^: focus will be genomically microsatellite-stable (MSS); *: Allotransplant non-eligible L: line of therapy; SC: subcutaneously; IV: intravenously; d: day; BL: baseline; BM: bone marrow; WT1: Wilms Tumor-1 (protein); ORR: overall response rate; DOR: duration of response; PFS: progression-free survival; OS: overall survival; PR: partial response; CR: complete response (includes CRi/CRp, in addition to stringent CR); MRD: minimal residual disease; GPS: galinpepimut-S; PS: performance status; MDSC: myeloid-derived suppressor cells; Treg: regulatory T cells; TAM: tumor-associated macrophages; CRC: colorectal Ca; OvC: ovarian Ca; SCLC: small-cell lung cancer; TNBC: triple-negative breast Ca; AML: acute myelogenous leukemia; HMAs: hypomethylating agents. Study Endpoints CRC^ 3/4L SCLC 2L TNBC 2L AML in PR (HMAs)* OvC 2/3L Treatment continued until disease progression or unacceptable toxicity (up to 111 weeks)

CORPORATE

MANAGEMENT TEAM NAME POSITION PRIOR EXPERIENCE / AFFILIATIONS Angelos Stergiou, M.D., ScD h.c. Chief Executive Officer Nicholas J. Sarlis, M.D., Ph.D., FACP Chief Medical Officer Aleksey Krylov, CFA Chief Financial Officer (Interim) Gregory M. Torre, Ph.D., J.D. Chief Regulatory Officer SVP, Technical Operations David Moser, J.D. VP, Corporate Legal Affairs Laura Katz, MPH VP, Clinical Operations and Biostatistics
BOARD OF DIRECTORS NAME POSITION PRIOR EXPERIENCE / AFFILIATIONS Jane Wasman Board Chair, Nominating and Governance Committee Chair Angelos Stergiou, MD, ScD h.c. Chief Executive Officer John Varian Audit Committee Chair Robert Van Nostrand Compensation Committee Chair Dr. David Scheinberg Science Committee Chair Stephen Ghiglieri Board Member, Audit Committee Fabio Lopez Ceron Board Member
SCIENTIFIC ADVISORY BOARD NAME POSITION Jedd D. Wolchok, M.D., Ph.D. Chief, Melanoma & Immunotherapeutics Service at Memorial Sloan Kettering Cancer Center (MSKCC) Jeffrey Weber, M.D., Ph.D. Deputy Director of the Perlmutter Cancer Center, Co-director of the Melanoma Research Program at the New York University (NYU)-Langone Cancer Center Alexander M.M. Eggermont, M.D. Director General of Institut Gustave Roussy Cancer Campus Grand Paris, Villejuif, France Larry W. Kwak, M.D., Ph.D. Associate Director Cancer Center, Translational Research & Developmental Therapeutics for the City of Hope National Medical Center Javier Pinilla-Ibarz, M.D. Director of Immunotherapy for Malignant Hematology at the H. Lee Moffitt Cancer Center Sattva Neelapu, M.D., Ph.D. Associate Professor, Department of Lymphoma/Myeloma, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center Guenther Koehne, M.D., Ph.D. Chief, Bone Marrow Transplantation and Hematologic Oncology, Miami Cancer Institute

FINANCIAL SUMMARY (1) As of 1/3/2018, market close (2) As of 9/30/2017, pro-forma combined Reverse Merger Closed 12/29/2017 NASDAQ: SLS – Trading Commenced 1/2/2018 Common Shares Outstanding: 5.8 M (1) Warrants/Options/RSUs: 0.9 M (1) Cash: $15.3 M (2)

Sellas LIFE SCIENCES group, inc. Company OVERVIEW JANUARY 2018




























