UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) October 24, 2008
GREEN PLANET BIOENGINEERING CO. LIMITED
(Exact name of registrant as specified in its charter)
| Delaware | | 000-52622 | | 37-1532842 |
(State or other jurisdiction of incorporation) | | (Commission File Number) | | (IRS Employer Identification No.) |
18851 NE 29 th Avenue, Suite 700, Aventura, FL 33180 | | 33180 |
| (Address of principal executive offices) | | (Zip Code) |
1(877) 544-2288 or (601) 786 9171
Registrant’s telephone number, including area code
61 Broadway, 32 nd Floor,
New York, New York 10006
(Former name or address, if changed from last report)
o Written communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17CFR 240.13e-4(c))
TABLE OF CONTENTS
Item No. | | Description of Item | | Page No. |
| | | | | |
| Item 1.01 | | Entry Into a Material Definitive Agreement | | 4 |
| | | | | |
| Item 2.01 | | Completion of Acquisition or Disposition of Assets | | 4 |
| | | | | |
| Item 3.02 | | Unregistered Sale of Securities | | 42 |
| | | | | |
| Item 5.01 | | Change in Control of Registrant | | 42 |
| | | | | |
| Item 5.02 | | Departure of Directors or Principal Officers; Election of Directors; Appointment of Directors | | 43 |
| | | | | |
| Item 5.06 | | Change in Shell Company Status | | 43 |
| | | | | |
| Item 9.01 | | Financial Statements and Exhibits | | 43 |
EXPLANATORY NOTE
This Amendment on Form 8-K/A (the “Amendment”) amends the Current Report for Green Planet Bioengineering Co. Limited on Form 8-K, as initially filed with the Securities and Exchange Commission on October 29, 2008 (the “Original Report”). The purpose of this Amendment is to include as an Exhibit Amendment No. 1 to the Share Exchange Agreement by and among Elevated Throne Overseas Ltd., Green Planet Bioengineering Co. Ltd and all of the Shareholders of Elevated Throne Overseas Ltd. This Amendment is an amendment and restatement of the Original Report in its entirety in order to provide a complete presentation.
CAUTIONARY NOTE REGARDING PREDICTIVE STATEMENTS
Our disclosure and analysis in this Current Report on Form 8-K contains statements that depend upon or refer to future events or conditions or that include words such as “expects”, “anticipates”, “intends”, “plans”, “believes”, “estimates” and similar expressions. Although we believe that these statements are based upon reasonable assumptions, including projections of operating margins, earnings, cash flow, working capital, capital expenditures and other projections, they are subject to several risks and uncertainties, and therefore, we can give no assurance that such projections will be achieved.
Investors are cautioned that our statements are not guarantees of future performance and the actual results or developments may differ materially from the expectations expressed.
As for the statements that relate to future financial results and other projections, actual results will be different due to the inherent uncertainty of estimates, forecasts and projections and may be better or worse than projected. Given these uncertainties, you should not place any reliance on these statements. These statements also represent our estimates and assumptions only as of the date that they were made and we expressly disclaim any duty to provide updates to them or the estimates and assumptions associated with them after the date of this filing to reflect events or changes in circumstances or changes in expectations or the occurrence of anticipated events.
We undertake no obligation to publicly update any predictive statement in this Current Report, whether as a result of new information, future events or otherwise. You are advised, however, to consult any additional disclosures we make in our Form 10-K, Form 10-Q and Form 8-K reports to the SEC.
Unless otherwise noted, all currency figures in this filing are in U.S. dollars. References to “Yuan” or “RMB” are to the Chinese Yuan (also known as “renminbi”). According to Oanda.com, as of October 23, 2008 $1.00 = 6.85 RMB.
Item 1.01. Entry into a Material Definitive Agreement
Background
As more fully described below, on October 24, 2008, we consummated a number of related transactions through which we acquired control of Sanming Huajian Bio-Engineering Co., Ltd., a PRC-based company. Sanming Huajian Bio-Engineering Co., Ltd. is engaged in the research, development, production and sale of extracts from tobacco leaves to be used in various pharmaceutical and health products.
The Reverse Merger Transaction
On October 24, 2008, (“Closing Date”), Green Planet Bioengineering Co. Limited executed and consummated a Share Exchange Agreement (the “Closing”) by and among (i) Elevated Throne Overseas Ltd., a British Virgin Islands limited liability company; (ii) the stockholders of 100% of Elevated Throne Overseas Ltd.’s common stock (the “Elevated Throne Overseas Ltd., Shareholders”); and (iii) Green Planet Bioengineering Co. Limited ’s then-controlling stockholder, Cris Neely.
At closing, we acquired control of Elevated Throne Overseas Ltd., which is the parent company of FuJian Green Planet Bioengineering Co., LTD . , a wholly foreign-owned enterprise (“WFOE”) organized under the laws of the People’s Republic of China (“PRC”), by issuing to the Elevated Throne Overseas Ltd.’s Shareholders 14,141,667 shares of our Common Stock in exchange for all of the outstanding capital stock of Elevated Throne Overseas Ltd. (the “Reverse Merger Transaction”). Immediately after the Closing, we had a total of 15,141,667 shares of common stock outstanding, with the Elevated Throne Overseas Ltd.’s Shareholders (and their assignees) owning approximately 93.40% of our outstanding common stock, and the balance held by those who held our common stock prior to the Closing.
Our board of directors (the “ Board”) as well as the directors and the shareholders of Elevated Throne Overseas Ltd. each approved the Reverse Merger Transaction, including the transactions contemplated there under. Following the Closing Date, Elevated Throne Overseas Ltd. became our wholly-owned subsidiary.
Elevated Throne Overseas Ltd. owns 100% of FuJian Green Planet Bioengineering Co., LTD. , which is a WFOE under the laws of the PRC. WFOE has entered into a series of contractual arrangements with Sanming Huajian Bio-Engineering Co., Ltd., a limited liability company headquartered in, and organized under the laws of, the PRC. The contractual arrangements are discussed below in Item 2.01 under the section titled “Description of Business.”
As a result of the Reverse Merger Transaction, we acquired 100% of the capital stock of Elevated Throne Overseas Ltd. and consequently, control of the business and operations of Elevated Throne Overseas Ltd., Fujian Green Planet Bioengineering Co., Ltd., and Sanming Huajian Bio-Engineering Co., Ltd. Prior to the Reverse Merger Transaction, we were a public reporting blind pool company in the development stage. From and after the Closing Date of the Share Exchange Agreement, our primary operations consist of the business and operations of Sanming Huajian Bio-Engineering Co., Ltd., which are conducted in China.
Item 2.01. Completion of Acquisition or Disposition of Assets
Our Corporate Structure
We were incorporated in Delaware under the name Mondo Acquisition II, Inc. on October 30, 2006. Our initial business plan was to serve as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business. We are, based on proposed business activities, a “blank check” company. On October 2, 2008, we changed our name to Green Planet Bioengineering Co. Limited.
Our Board approved the Share Exchange Agreement on October 23, 2008, and we entered into the Share Exchange Agreement with Elevated Throne Overseas Ltd. and its Shareholders on October 24, 2008.
Elevated Throne Overseas Ltd. was incorporated under the laws of the British Virgin Islands on May 8, 2008, and Elevated Throne Overseas Ltd. formed FuJian Green Planet Bioengineering Co., Ltd. as a wholly foreign-owned enterprise under the laws of the PRC on July 25, 2008.
Sanming Huajian Bio-Engineering Co., Ltd. was organized under the laws of the PRC in April of 2004 under the name Sanming Zhonjian Biological Technology Industry Co., Ltd.
Under the laws of the PRC, certain restrictions are placed on round trip investments, which are defined under PRC law as an acquisition of a PRC entity by an offshore special purpose vehicle owned by one or more PRC residents. As a result, FuJian Green Planet Bioengineering Co., LTD. entered into a series of agreements with Sanming Huajian Bio-Engineering Co., Ltd., which we believe give us effective control over the business of Sanming Huajian Bio-Engineering Co., Ltd. These agreements are described below in the section entitled “Contractual Agreements with Sanming Huajian Bio-Engineering Co., Ltd.”
Our executive offices are located at No.126 Mingdu Building, Gongye Road, Sanming City, Fujian, China, and our telephone number is +86 598 8523617. Our website is www.greenplanetbio.com.cn . Information on our website or any other website is not a part of this report.
Contractual Agreements with Sanming Huajian Bio-Engineering Co., Ltd.
Prior to the reverse merger, our business was conducted through Sanming Huajian Bio-Engineering Co., Ltd., its largest shareholders being Mr. Min Zhao and Mr. MinYan Zhen with a 35.07% and 35.97% interest respectively. Sanming Huajian Bio-Engineering Co., Ltd. has the licenses and approvals necessary to operate its business in the PRC.
PRC law places certain restrictions on roundtrip investments through the acquisition of a PRC entity by PRC residents. To comply with these restrictions, in conjunction with the reverse acquisition, we (via our wholly-owned subsidiary, FuJian Green Planet Bioengineering Co., Ltd.) entered into and consummated certain contractual arrangements with Sanming Huajian Bio-Engineering Co., Ltd. and their respective stockholders pursuant to which we provide these companies with technology consulting and management services. Through these contractual arrangements, we have the ability to substantially influence these companies’ daily operations and financial affairs, appoint their senior executives and approve all matters requiring stockholder approval. As a result of these contractual arrangements, which enable us to control Sanming Huajian Bio-Engineering Co., Ltd. and operate our business in the PRC through Sanming Huajian Bio-Engineering Co., Ltd., we are considered the primary beneficiary of Sanming Huajian Bio-Engineering Co., Ltd. Accordingly, we consolidate the results, assets and liabilities of the Sanming Huajian Bio-Engineering Co., Ltd. in our financial statements.
On July 25, 2008, we entered into the following contractual arrangements, each of which are enforceable and valid in accordance with the laws of the PRC:
Entrusted Management Agreement . Pursuant to this entrusted management agreement among Fujian Green Planet Bioengineering Co., Ltd., Sanming Huajian, and the Sanming Huajian Shareholders (the "Entrusted Management Agreement"), Sanming Huajian and its shareholders agreed to entrust the business operations of Sanming Huajian and its management to Fujian Green Planet Bioengineering Co., Ltd. until Fujian Green Planet Bioengineering Co., Ltd. acquires all of the assets or equity of Sanming Huajian (as more fully described in the Exclusive Option Agreement below). Prior to the occurrence of such event, Sanming Huajian will only own those certain assets that are not sold to Fujian Green Planet Bioengineering Co., Ltd. We anticipate that Sanming Huajian will continue to be the contracting party under its customer contracts, banks loans and certain other assets until such time as those may be transferred to Fujian Green Planet Bioengineering Co., Ltd. Under the Entrusted Management Agreement, Fujian Green Planet Bioengineering Co., Ltd. will manage Sanming Huajian‘s operations and assets, and control all of Sanming Huajian’s cash flow through an entrusted bank account. In turn, it will be entitled to any of Sanming Huajian’s net profits as a management fee, and will be obligated to pay all Sanming Huajian payables and loan payments. The Entrusted Management Agreement will remain in effect until the acquisition of all assets or equity of Sanming Huajian by Fujian Green Planet Bioengineering Co., Ltd. is completed.
Shareholders’ Voting Proxy Agreement. Under the shareholders' voting proxy agreement among Fujian Green Planet Bioengineering Co., Ltd. and the Sanming Huajian Shareholders, the Sanming Huajian Shareholders irrevocably and exclusively appointed the members of the board of directors of Fujian Green Planet Bioengineering Co., Ltd. as their proxy to vote on all matters that require Sanming Huajian shareholder approval. The members of the board of directors of Fujian Green Planet Bioengineering Co., Ltd. are identical to those of the Company.
Share Pledge Agreement. Under this share pledge agreement among Fujian Green Planet Bioengineering Co., Ltd. and the Sanming Huajian Shareholders (the "Share Pledge Agreement"), the Sanming Huajian Shareholders pledged all of their equity interests in Sanming Huajian, including the proceeds thereof, to guarantee all of Fujian Green Planet Bioengineering Co., Ltd.’s rights and benefits under the Restructuring Agreements. Prior to termination of this Share Pledge Agreement, the pledged equity interests cannot be transferred without Fujian Green Planet Bioengineering Co., Ltd.’s prior consent.
Completion of the PRC Restructuring
The PRC restructuring transaction closed on the Closing Date. However, Fujian Green Planet Bioengineering Co., Ltd. is required under the agreements to complete additional post-closing steps required in order to maintain its good standing under PRC law. These steps include Fujian Green Planet Bioengineering Co., Ltd. making required regulatory filings and giving proof to regulatory authorities that it has received the required portion of its registered capital as of the deadline required under PRC law. Specifically, Fujian Green Planet Bioengineering Co., Ltd. must receive 15% of its total registered capital of $2.0MM by 3 months of effectiveness of business license, and the remaining $1.7MM by two years from effectiveness of business license, in order to maintain the validity of its business license and its certificate of approval to exist as a wholly foreign-owned entity in the PRC issued by the Fujian Provincial Municipal Government and the Sanming Administration for Industry and Commerce, respectively. This license and approval would become invalid and be immediately cancelled if Fujian Green Planet Bioengineering Co., Ltd. were to fail to make timely payment of the first installment of its registered capital, in which case we could cease to have any claim to control Sanming Huajian Bio-Engineering Co., Ltd. under PRC law. We anticipate that all required post-closing steps, including the payment and verification of the first installment of Fujian Green Planet Bioengineering Co., Ltd.’s registered capital, will be completed within approximately 30 days after the date of this report .
Upon consummation of the PRC Restructuring Agreements above, the contributions of Sanming Huajian Bio-Engineering Co., Ltd.’s registered capital, and therefore the ownership of Sanming Huajian Bio-Engineering Co., Ltd., took their current form, which is represented in the table below:
Name of Shareholder | | Amount of Contribution (RMB) ‘000 | | Percent of Capital Contribution | |
| Min Zhao | | | 1332.82 | | | 35.07 | % |
| Min Yan Zhen | | | 1366.86 | | | 35.97 | % |
| Jiangle Jianlong Mineral industry Co. | | | 1100.32 | | | 28.96 | % |
| | | | | | | | |
| Total | | | RMB 3800.00 | | | 100 | % |
Subsidiaries
As a result of the Reverse Merger Transaction, Elevated Throne Overseas Ltd. and FuJian Green Planet Bioengineering Co., Ltd. are our wholly-owned subsidiaries. Sanming Huajian Bio-Engineering Co., Ltd., the entity through which we operate our business, has no subsidiaries.
BUSINESS
Our History
Mondo Acquisition II, Inc. was incorporated in the State of Delaware on October 30, 2006. Since inception, we have been engaged in organizational efforts to obtain initial financing. We were formed as a vehicle to pursue a business combination through the acquisition of, or merger with, an operating business. We filed a registration statement on Form 10-SB with the U.S. Securities and Exchange Commission (the “SEC”) on May 2, 2007, and since its effectiveness, we have focused our efforts to identify a possible business combination. On October 2, 2008, we changed our name to Green Planet Bioengineering Co. Limited.
We are, based on proposed business activities, a "blank check" company. The U.S. Securities and Exchange Commission (the “SEC”) defines those companies as "any development stage company that is issuing a penny stock, within the meaning of Section 3 (a)(51) of the Exchange Act of 1934, as amended, (the “Exchange Act”) and that has no specific business plan or purpose, or has indicated that its business plan is to merge with an unidentified company or companies." Under SEC Rule 12b-2 under the Securities Act of 1933, as amended (the “Securities Act”), we also qualify as a “shell company,” because we have no or nominal assets (other than cash) and no or nominal operations. Many states have enacted statutes, rules and regulations limiting the sale of securities of "blank check" companies in their respective jurisdictions.
Sanming Huajian Bio-Engineering Co., Ltd’s Organization History
Sanming Huajian Bio-Engineering Co., Ltd was originally incorporated in April 2004 in the People’s Republic of China as Sanming Zhongjian Biological Technology Industry Co., Ltd. Its original registered capital was RMB 6 million and its original shareholders were Ou Shanyan (80%), Zhao Yime (10%) and Zheng Yingyue (10%). The company’s original business scope included planning to produce and sell environmentally conscious food, health products, chemical products, and biological products.
On August 17, 2004, the company changed its name from Sanming Zhongjian Biological Technology Industry Co., Ltd. to Sanming Huajian Bio-Engineering Co., Ltd. and its shareholders changed from Ou Shanyan, Zhao Yime, and Zheng Yingyue to Min Zhao and Zheng Jianrong with Min Zhao holding a 60% equity interest and Zheng Jianrong holding a 40% equity interest.
On May 22, 2006, the company changed its operation plan to focus on natural plant extractions, the production of bio-fertilizer and the sale of chemical and agricultural products and by-products as well as the development of biological engineering technology.
On July 8, 2006, the company’s registered capital increased to RMB 33,500,000 and its shareholders changed from Min Zhao and Zheng Jianrong to Min Zhao, with a 35.07% equity interest, Minyan Zhen with a 35.97% interest and Jiangle Jianlong Mineral Industry Co., Ltd., with a 28.96% equity interest.
On April 15, 2008, the company’s registered capital increased to RMB 38,000,000.
Sanming Huajian Bio-Engineering Co., Ltd’s Business
Sanming Huajian Bio-Engineering Co., Ltd is a research and development company with a focus on improving human health through the development, manufacture and commercialization of bio-ecological products and over-the-counter products utilizing the extractions of tobacco leaves.
Products
Since 2007, Sanming Huajian Bio Engineering Co., Ltd has developed a variety of natural organic products using tobacco leaves. These products are:
Solanesol
Solanesol is extracted from abandoned tobacco leaves and is a pharmaceutical intermediate. Not only can it produce raw Q10 and Vitamin K2 through the synthesis method, but it can also be used as a synthesized raw material in anti-allergic drugs, anti-ulcer drugs, hypolipidemic drugs and anti-cancer drugs.
Nicotine Sulphate
Nicotine sulfate is extracted from abandoned tobacco leaves and it is an important raw material for pesticide and can be used in the processing of insecticides. The nicotine content in pesticides is 40%.
Organic Fertilizers
“ Ji Mai” trademark fertilizer is made by using abandoned tobacco leaves as the main raw material, adding the appropriate accessories and fermented with microbial, which is applicable to all kinds of fruit trees, and is also used as a soil conditioner.
Botanical Pesticides
Pesticides consist of 40% Nicotine Sulphate and other insecticide raw materials and is regarded as organic or botanical because Nicotine Sulphate is derived from abandoned tobacco leaves.
Organic Green Barley Supplements (Paiqianshu)
“ Paiqianshu” mung bean vitamin oral liquid has the ability to eliminate lead from the human body without any side effects. It contains many nutrient elements such as Calcium gluconate, zinc gluconate, and vitamin C. This nutrient is extracted from green barley or ‘mung bean’.
Raw CoQ10
Coenzyme Q10 is also commonly referred to as ubiquinone, ubidecarenone, and coenzyme Q. It is a vitamin-like substance that is naturally present in most human cells except red blood cells and eye lens cells and is responsible for the production of the body’s own energy. In each human cell, food energy is converted into energy in the mitochondria with the aid of Coenzyme Q10.
Because disfunctional energy metabolism has been cited as a contributing factor for a number of medical conditions, coenzyme Q10 has been used in the treatment of cardiac, neurologic, oncologic, and immunologic disorders. Although the Dietary Supplement Health and Education Act of 1994 does not allow claims for treatment of specific diseases in the United States, coenzyme Q10 has been cleared for treatment indications in other countries, such as for congestive heart failure (CHF) in Japan since 1974. 3
1 Ernster L, Dallner G: Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271: 195-204, 1995
2 Dutton PL, Ohnishi T, Darrouzet E, Leonard, MA, Sharp RE, Cibney BR, Daldal F and Moser CC. 4 Coenzyme Q oxidation reduction reactions in mitochondrial electron transport (pp 65-82) in Coenzyme Q: Molecular mechanisms in health and disease edited by Kagan VE and Quinn PJ, CRC Press (2000), Boca Raton
3 Tran MT, Mitchell TM, Kennedy DT, Giles JT. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy 2001;21:797-806.
Natural Q10 Supplements
Because of its ability to transfer electrons and therefore act as an antioxidant, Coenzyme Q is also used as a dietary supplement. Supplements of Coenzyme Q10 is a treatment for some of the rare and serious mitochondrial disorders and other metabolic disorders where patients are not capable of producing enough Coenzyme 10Q because of their disorder. Supplements of Coenzyme Q10 has been found to have a beneficial effect on migraine headache symptoms. 4 Recent studies have also found Coenzyme 10Q to have beneficial effects on brain health and neurodegenerative diseases in animal models 5
Q10 supplements is one of many downstream products that is produced from raw Q10 (which initially derives from extracts of abandoned tobacco leaves- Solanesol)
Manufacturing Process
Extraction from Abandoned Tobacco Leaves .
Abandoned tobacco leaves are leaves that are left behind as waste by tobacco manufacturers in their production of tobacco. We have obtained an exclusive license to purchase the waste and use it to produce bio chemical materials, fertilizers and health products. The license is supported by the provincial government that certain suppliers can only sell their tobacco waste to Sanming Huajian Bio-Engineering Co.
We produce Solanesol by utilizing the Solid Phase Synthesis method from Fudan University, which significantly lowers the production cost of highly purified Solanesol. Using this process, the cost of producing Solanesol is RMB 300,000-400,000 per ton.
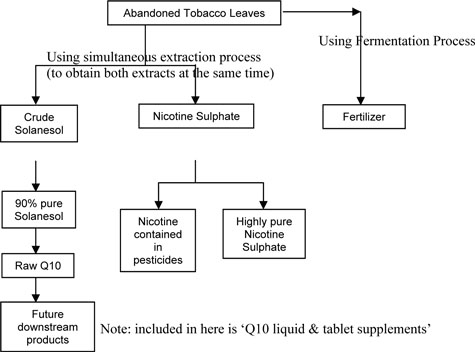
The following chart illustrates the process of extraction & its relevant products:
In addition to the above, company produces a nutrient product called “Paiqianshu” (green barley nutrient), it is an antioxidant that will help clean the major organs and digestive system of the human body. The manufacturing process for this is similar to the above but only extraction is using Green Barley or Mung bean and not abandoned tobacco leaves.
4 Rozen T, Oshinsky M, Gebeline C, Bradley K, Young W, Shechter A, Silberstein S (2002). " Open label trial of coenzyme Q10 as a migraine preventive ". Cephalalgia 22 (2): 137-41
Matthews, R. T.; et al. (July 1998). "Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects". PNAS 95 : 8892-8897.
Production Licenses
In accordance with the Preparation Registration List of Foreign Trade we have independent import and export rights to conduct business under registration number 00395610 and enterprise code 3500759394367.
Our production of Solanesol meets the quality standards of the Sanming Technical Supervision Bureau (Record Number Min QB/0400-1044-2008) and our production of bio-fertilizer meets standard NY 525-2002.
We have received a certificate of approval for domestic health care products from the State and Drug Administration, serial number 2007B0441, for the product Huajian Mung Bean Vitamin Liquid (approval code- Guo Shi Jian G20070216, valid until 4/25/12) .
Our management team received a registration certificate (Certificate # 603917) from and has been assessed by GIC and certified to comply with ISO9001:2000 for the production, sales and service of nicotine sulfate, Solanesol and bio-fertilizer. The Certificate was issued on April 3, 2008 and is valid until April 2, 2011.
Suppliers
| | | | | 2006 | | 2007 | |
| Name of suppliers | | Address | | RMB amount | | % | | RMB amount | | % | |
| Sanming Jinye Fukao Co., Ltd. | | | No.7 Shazhou Road,Sanyuan District,Sanming City | | | 1356444.21 | | | 15 | % | | 1807424.39 | | | 10 | % |
| Xiamen Jixiang Shishi Industry Co., Ltd. | | | No.1 Xinyan Road,Xinyan Industrial Zone,Haicang District,Xiamen | | | 92134.00 | | | 1 | % | | 275850.39 | | | 2 | % |
| Fujian Longyan Jinye Fukao Co., Ltd. | | | Kanshi Zhen,Yongding County,Fujian | | | 498981.60 | | | 5 | % | | 531933.96 | | | 3 | % |
| Fujian Wuyi Tobacco Leaf Co., Ltd. | | | No.400 Xichun East Road,Shaowu City,Fujian | | | 642105.60 | | | 7 | % | | 766268.46 | | | 5 | % |
| Fujian Tobacco Co, Ltd .Nanping Branch | | | No.389 Binjiang Central Road,Nanping City | | | 773307.46 | | | 8 | % | | 832603.76 | | | 5 | % |
| Fujian Tobacco Co, Ltd .Sming Branch | | | No.100 Chonggui Xincun,Meilie District,Sanming City | | | 438643.76 | | | 5 | % | | 429950.26 | | | 3 | % |
| Pucheng Zhengda Biochemistry Co., Ltd | | | No.305 Zhengda Road,Pucheng County,Fujian | | | 508559.25 | | | 5 | % | | 965469.23 | | | 6 | % |
| Wuhan Yanpu Chemical Raw Material Co. Ltd. | | | Cihui Farm,Dongxihu District,Wuhan,HubeiProvince | | | 3319897.25 | | | 36 | % | | 7641159.63 | | | 45 | % |
| Sanming Sanyuan Chemical Reagent Co., Ltd. | | | No.22 Beishan Road,Sanming City | | | 1162592.35 | | | 13 | % | | 2808888.22 | | | 16 | % |
| Sanming Baishida Starch Co., Ltd. | | | No.15 Ziling Road,Xuefeng Zhen,Mingxi County,Fujian | | | 493443.60 | | | 5 | % | | 922560.60 | | | 5 | % |
| Total | | 9286109.08 | | | | | | 16982108.90 | | | | |
Honors and Awards
The following table provides information regarding the honors and awards we have received.
| Award/Year | | Bestower | | Description |
| 2005-6-24 - Ming Ji Wai [2005] No.14 – awarded 300K RMB | | Company | | Project of industrializing Green Barley |
| 2006–6-21 – Ming Jing Mao Tou Zi Ji Shu [2006] No.9 | | Company | | Industrialized demonstration project of integrated use of abandoned tobacco leaves with high efficiency |
| 2006-7-12 – Ming Ke Cheng [2006] No. 27 – awarded 450K RMB | | Company | | Achieved highly purified Solanesol and nicotine extraction in high efficiency and clean process |
| 2006-9-5 – Ming Zheng Wen [2006] No. 95 | | Company | | Cooperation in the three rural economy – named the key enterprise of agricultural industrialization of the year 06-07 |
| 2006-9-25 – Ming Fa Gai Wai Jing [2006] No 254 – awarded 300K RMB | | Company | | 2 nd supporting award for highly purified Solanesol and nicotine extraction from tobacco wastes by clean process |
| 2006-11-20 – Ming Ke Cheng [2006] No. 29 – listed in the SPARKS program 2006 | | company | | Industrialized demonstration project of integrated use of abandoned tobacco leaves with high efficiency |
| 2006-10-26 – Ming Cai Jian Zhi [2006] No. 31-awarded 250K RMB | | company | | Highly purified Solanesol and by–products from tobacco wastes by clean process – this was supported by the drainage fee |
| 2007-3-12 – honored certificate from sanming administration of industry and commerce bureau | | company | | Title granted ‘the enterprise exempted from inspection in 2006’ |
| 2007-7-10 –Yuan Cai [2007] No. 14 – awarded 250K RMB | | company | | 3 rd supporting award for highly purified Solanesol and nicotine extraction from tobacco wastes by clean process |
| 2007-12-17 – Zu Zi[2007] No.2 | | company | | In the Fujian China Projects and Production Fair (the 618 Fair) – was listed the excellent transformed projects of the 618 fair in 2007 |
In 2006, we were awarded the SPARKS program certification, a major health and environment awareness project of China.
We obtained the "national-level Spark Program" title awarded by Chinese Ministry of Science and Technology in 2006. "Spark plan" refers to an industry in which we have played a leading role in certain economic areas, and relevant to science and technology’s large-scale access to rural areas by relying on scientific and technological progress, and close integration with the rural economy, and that we have developed leading products with regional resource advantages (i.e. getting rid of tobacco wastes, contribution to its environment) to form scale and promoted the development of enterprises and related industries to realize intensified, large-scale, industrialized operations ,which will account for considerable proportion in regional economy.
Environment Protection
We have carried out certain initiatives in order to comply with environmental regulations.
In January of 2008, Sanming City Environmental Monitoring Station began to conduct the monitoring of environmental protection acceptance on the completed production lines of our products. In April of 2008, the Station consummated all monitoring of boiler exhaust, factory sector noise, waste water and environmental air sensitive points and made a conclusion that we conducted clean production as we have taken and implemented various environmental indicators, and, therefore, Min Yan Jian Zi (2008) No.015 "The Monitoring Report of Environmental Protection Acceptance on completion of Construction projects" was successfully issued to the company.
Sales of Products
We mainly base our business on the wholesale sales of bio-ecological products. Our distribution process is established through contracted distributors and sales agreements. The company is continuously evaluating its sales and distribution strategy and assesses the performance of its business partners and execution of its business plan.
Delivery Methods
The method of delivery for our product throughout the PRC is ground delivery. The company does not have its own fleet of trucks. The company outsources the delivery of its products to various trucking companies.
Marketing and Sales
For raw chemical material products such as Solanesol, CoQ10 or Nicotine Sulphate, we utilize the following strategies:
| · | First, we have established a strong referral programs with major universities where most distributors look for new products and new technologies today. |
| · | Second, we have used the following channels to get the name and brands out to potential distributors: |
| | - | Internal web optimization through Search engines and Sponsored links |
| · | Third, we network through the local government in some provinces to introduce and refer us to established distributors. |
Raw materials
Our primary raw material is abandoned tobacco leaves. We purchase abandoned tobacco leaves from the below major suppliers during 2007:
Sanming Jinye Fukao Co., Ltd.
Xiamen Jixiang Shishi Industry Co., Ltd.
Longyan Jinye Fukao Co., Ltd.
Fujian Wuyi Tobacco Leaf Co., Ltd.
Fujian Tobacco Co, Ltd. Nanping Branch
Fujian Tobacco Co, Ltd. Sming Branch
The following is a list of raw materials used by Sanming Huajian Bioengineering Co., Ltd. in its manufacturing process:
| | | Name | | Quantity (Tons) |
| Raw materials | | abandoned tobacco leaves | | 782.4552 |
| | | #6 organic solvents | | 5.5638 |
| | | Slice alkali | | 0.0314 |
| | | Methanol | | 1.7057 |
| | | Acetone | | 0.2262 |
| | | Petroleum aether | | 13.0245 |
| | | Ethyl acetate | | 1.7127 |
| | | Silicon dioxide | | 5.0241 |
| | | Sweet potato residue | | 78.65 |
| | | Medicines residue | | 82.93 |
| | | Calcium, Magnesium and Phosphor | | 35.693 |
Market Analysis
Our product, Q10 has two distinct market segments: Raw Q10 and Retail Q10 products. The following chart outlines the global estimated demand of raw Q10 by 2010 in tonnage:
Country | | Demand (in tons) |
| US | | 200-220 |
| JAPAN | | 160-180 |
| ASIA (EXCLUDING JAPAN) | | 100-150 |
| EUROPE | | 80-100 |
| OTHER COUNTRIES | | 60 |
| TOTAL | | Approx. 750 |
Raw Q10 is supplied to companies or institutions that 1) use it for research and development purposes or 2) use it to produce its downstream retail products. Today, we produce and market raw Q10 and we are in the process of patenting our over-the-counter retail brand of Q10 supplement in both liquid and tablet form which we’ve fully developed in 2007. Our nutrient and supplement brand called “Green Planet” is now going through the trademark application process. Our current raw Q10 production capacity is 20 tons per year and with our growth plan realized we will be able to produce and supply a big part of China’s demand.
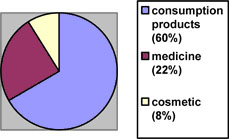
Retail Q10 products can be further classified into 3 sub-categories: Food, Nutrients, Supplements, and Cosmetics. The market share of each is displayed by the following pie chart:
Note: consumption products include nutrients, vitamins and supplements
Competition
We face competition from domestic and international producers, some of which are direct manufacturers that specialize only in specific products and do not offer a full range of bio-ecological product or retail consumer products.
Domestic Competitors
We face competition within the domestic market. In this area our primary competitors are the following:
· Xi'An Haotian Bio-Engineering Technology Co., Ltd
· Shaanxi Sciphar Biotechnology Co. Ltd.
· Tongfa Group
· Shanghai Seebio Biotech Inc.
The above stated wholesalers in all cases sell only the raw chemical materials. In addition, these wholesalers do not produce 100% of their products but will act as a middleman to buy & sell.
Our “one-stop shop” concept in promoting under one roof offers a variety of raw chemical material products as well as over-the-counter health products. This concept drastically differs from conventional manufacturers as wholesalers or distributors are given a broad spectrum of products to increase their desire to build a solid long term relationship with us, and, furthermore, we can closely listen to the needs and expectations of their overall customers.
International Competitors
We also compete against large surface manufacturers across the world, however, they only produce and promote raw chemical materials and some intermediate products. Some of the largest international competitors are:
· Kaneka Corporation (Japan)
· Hanseo Chemical Co Ltd (Korea)
· Federal Laboratories Corporation (Buffalo, NY)
We intend to expand to international markets. Currently, we have all the necessary licenses to export to international markets; however before we expand sales abroad we want to dominate the domestic market as a priority since the demand is rising and the supply is short.
Major Customers
Our current customers are primarily in China. We sell substantially all of our products to customers in domestic China. Our major customers for fiscal years 2006 and 2007 are as follows:
| Name of Customers | | Address |
| Fuzhou Yishun Imp.& Exp. Co. Ltd. | | No.B3,7F Office Building,Hongli Massion,Hudong Road,Gulou District,Fuzhou City |
| Sanming Wangnong Agricultural Materials Co. Ltd. | | No.5-6 of Building 143,Xinshi South Road,Sanyuan District,Sanming City |
| Shanghai Zhaoxiang Biologic Technology Co.Ltd. | | No.7018 Beisonggong Road,Songjiang District,Shanghai |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co.Ltd. | | Biomedical City of Qingyuan High-tech Industrial Development Zone,Qingyuan City,Guangdong |
| Fujian Wuyishang City Lukang Bioengineering Co. Ltd. | | Xiandian Industrial Park Area,Wuyishang City,Fujian |
| Jiangxi Lufeng Organic Agricultural Technology Development Co.Ltd. | | Jiulong Reclamation Farm,Wanzai County,Jiangxi Province |
| Total |
| | | 2006 | | 2007 | |
| Name of Customers | | RMB amount | | % | | RMB amount | | % | |
| Fuzhou Yishun Imp.& Exp. Co. Ltd. | | | 7649959.8 | | | 23 | % | | 12483069 | | | 20 | % |
| Sanming Wangnong Agricultural Materials Co. Ltd. | | | 3889980.3 | | | 12 | % | | 9551305.1 | | | 16 | % |
| Shanghai Zhaoxiang Biologic Technology Co.Ltd. | | | 9578568.3 | | | 29 | % | | 11589110 | | | 19 | % |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co.Ltd. | | | 6446753.4 | | | 20 | % | | 10639833 | | | 17 | % |
| Fujian Wuyishang City Lukang Bioengineering Co. Ltd. | | | 1097503.9 | | | 3 | % | | 6407661.3 | | | 11 | % |
| Jiangxi Lufeng Organic Agricultural Technology Development Co.Ltd. | | | 4337874.4 | | | 13 | % | | 10535859 | | | 17 | % |
| Total | | | 33000640 | | | | | | 61206836 | | | | |
None of the sales to any one customer comprises more than 20% of our sales revenue. Accordingly, the loss of any one customer would not have a material adverse effect on us. Furthermore, the company is currently broadening its product portfolio and as such working on minimizing its customer concentration risk.
Intellectual Property/Patents
The following table is a list of our current Patents issued by the People’s Republic of China:
| Patent Name | | Application No. | | Designer | | Application date | | Valid until | | Owner of patent |
Synchronization and high efficiency process of Solanesol and Nicotine Sulfate | | 200610069846.6 | | Min Zhao , Chen Yanmei, Liu Caiqing | | 2006.8.11 | | 2026.8.11 | | Sanming Huajian Bioengineering Co., Ltd |
A Method of Eliminating Plum bum Products with Basic liquid of zymogene mung bean | | 200710009735.0 | | Lin Xuanxian, Chen Jianmin, Chen Yanmei | | 2007.11.01 | | 2027.11.01 | | Sanming Huajian Bioengineering Co., Ltd |
Note- The patent of “Solensol-clean extraction method” is exclusively owned by Fudan University. However, we have obtained the right to use this technology patent until July 27, 2010, according to the statements of Article 3, Section 1 in “Technology Development Contract ”which was entered into on July 28,2005 between Fudan University and Sanming Huajian Bioengineering Co., Ltd and which is attached hereto as an exhibit. Since August 11, 2006, we have been designing the “ synchronization and high efficiency process of Solanesol and Nicotine Sulphate” and applied for the patent ownership and have used it in the production process.
Trademarks
The following table is a list of our current trademarks issued by the People’s Republic of China:
Trademark | | Certificate No. | | Category | | Registrant | | Valid Term |
| Paiqianshu | | 4322405 | | No.30 Refined food from plants, etc. | | Sanming Huajian bio | | from2007-4-20 to 2017-4-20 |
| Jimai QQ | | 4322404 (Application No. here. It will be the Certificate No. later.) | | No.30 Refined food from plants, etc. | | Sanming Huajian bio | | 10 years since the date of certificate issuing |
| Jimai | | 5425649 (Application No. here. It will be the Certificate No. later.) | | No.1 Fertilizer, chemical products, etc. | | Sanming Huajian bio | | 10 years since the date of certificate issuing |
| Jinliang | | 4538612 (Application No. here. It will be the Certificate No. later.) | | No.3 Cosmetic, household and personal care chemicals, etc. | | Sanming Huajian bio | | 10 years since the date of certificate issuing |
| PURESOLAN | | 6869795 (Application No. here. It will be the Certificate No. later.) | | No.5 Medical products, etc | | Fujian Green Planet | | 10 years since the date of certificate issuing |
| GREENPLANET | | 6871472 (Application No. here. It will be the Certificate No. later.) | | No.5 Medical products, etc | | Fujian Green Planet | | 10 years since the date of certificate issuing |
Sanming Huajian Bioengineering Co., Ltd. pays a license fee of RMB 500 per year for each trademark for the period of ownership from October 22, 2004 to October 22, 2014.
Insurance
Sanming Huajian Bio-Engineering Co., Ltd currently has property insurance with Ping which covers buildings and structures, machines, equipment, raw materials and manufactured products.
Sanming Huajian Bio-Engineering Co., Ltd does not have key man insurance, business liability or disruption insurance coverage for its operations in the People’s Republic of China.
Research and Development Activities
We have a research and development (R&D) division comprised of 30 people and we have invested approximately 2.5% of our sales in research and development activities during 2007. We anticipate to spend approximately 6% to 7% in the coming years in R&D of our sales, which we believe ensures that we stay ahead of the curve by constantly innovating and improving our technology.
We anticipate expanding our product line and our research and development activities are focused on improving our technology and machinery to deliver those goals. We anticipate spending approximately, $950,000 on research and development for fiscal year 2008. The company spent approximately $200,000 and approximately $112,659 for the years ended December 31, 2007 and 2006, respectively.
Employees
Sanming Huajian Bio-Engineering Co., Ltd currently has approximately 153 full-time employees broken down into:
Management (16)
Research and Development (30)
Supporting staff (7)
Manufacturing staff (100)
Employee benefits include:
The company provides benefits according to the laws of PRC when applicable. Benefits packages are not recognized in the PRC as in the United States.
RISK FACTORS
You should consider carefully each of the following business and investment risk factors and all of the other information in this report. If any of the following risks and uncertainties develops into actual events, the business, financial condition or results of our operations could be materially and adversely affected. If that happens, the trading price of our shares of common stock could decline significantly. The risk factors below contain forward-looking statements regarding our business. Actual results could differ materially from those set forth in the forward-looking statements. See "Special Note Regarding Forward-Looking Information."
Risks related to doing business in the People’s Republic of China
Our business operations take place primarily in the People’s Republic of China. Because Chinese laws, regulations and policies are continually changing, our Chinese operations will face several risks summarized below.
Our ability to operate in the People’s Republic of China may be harmed by changes in its laws and regulations
Our offices and manufacturing plants are located in the People’s Republic of China and the production, sale and distribution of our products are subject to Chinese rules and regulations . Currently, China does not have rules and regulations on raw material p roducts. However, health foods and the Q10 raw material sales must obtain government written instructions to a subordinate, therefore, we are obtaining GMP authentication as described herein.
The People’s Republic of China only recently has permitted provincial and local economic autonomy and private economic activities. The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership.
Our ability to operate in the People’s Republic of China may be harmed by changes in its laws and regulations, including those relating to taxation, import and export tariffs, environmental regulations, land use rights, property and other matters.
Also, we are a state-licensed corporation and production and manufacturing facility and are subject to Chinese regulation and environmental laws. The Chinese government has been active in regulating our industry. Our business and products are subject to government regulations mandating the use of good manufacturing practices. Changes in such laws or regulations in the People’s Republic of China that govern or apply to our operations could have a materially adverse effect on our business. For example, the law could change so as to inhibit our purchases from suppliers of tobacco leaves because of trade tariffs. Our manufacturing costs may be increased and consequently affect our profit margins and revenue.
If we were to lose our state-licensed status, we would no longer be able to manufacture our products in the People’s Republic of China, which is our sole operation.
There is no assurance that the People’s Republic of China’s economic reforms will not adversely affect our operations in the future
Although the Chinese government owns the majority of productive assets in the People’s Republic of China, in the past several years the government has implemented economic reform measures that emphasize decentralization and encourage private economic activity.
Because these economic reform measures may be inconsistent or ineffectual, there are no assurances that:
| | · | We will be able to capitalize on economic reforms; |
| | · | The Chinese government will continue its pursuit of economic reform policies; |
| | · | The economic policies, even if pursued, will be successful; |
| | · | Economic policies will not be significantly altered from time to time; and |
| | · | Business operations in the PRC will not become subject to the risk of nationalization. |
Since 1979, the Chinese government has reformed its economic systems. Because many reforms are unprecedented or experimental, they are expected to be refined and improved. Other political, economic and social factors, such as political changes, changes in the rates of economic growth, unemployment or inflation, or in the disparities in per capita wealth between regions within the People’s Republic of China, could lead to further readjustment of the reform measures. This refining and readjustment process may negatively affect our operations.
Over the last few years, the People’s Republic of China's economy has registered a high growth rate. During the past ten years, the rate of inflation in the People’s Republic of China has been as high as 20.7% and as low as -2.2%. Recently, there have been indications that rates of inflation have increased. In response, the Chinese government recently has taken measures to curb this excessively expansive economy. These corrective measures were designed to restrict the availability of credit or regulate growth and contain inflation. These measures have included devaluations of the Chinese currency, the Renminbi (RMB), restrictions on the availability of domestic credit, reducing the purchasing capability of certain of its customers, and limited re-centralization of the approval process for purchases of some foreign products. These austerity measures alone may not succeed in slowing down the economy's excessive expansion or control inflation, and may result in severe dislocations in the Chinese economy. The Chinese government may adopt additional measures to further combat inflation, including the establishment of freezes or restraints on certain projects or markets.
While inflation has been more moderate since 1995, high inflation may in the future cause Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in the People’s Republic of China, and thereby harm the market for our products. Future inflation in the PRC may inhibit our activity to conduct business in the People’s Republic of China.
To date, reforms to the People’s Republic of China's economic system have not adversely impacted our operations and are not expected to adversely impact operations in the foreseeable future; however, there can be no assurance that the reforms to the People’s Republic of China's economic system will continue or that we will not be adversely affected by changes in the People’s Republic of China's political, economic, and social conditions and by changes in policies of the Chinese government, such as changes in laws and regulations, measures which may be introduced to control inflation, changes in the rate or method of taxation, imposition of additional restrictions on currency conversion and remittance abroad, and reduction in tariff protection and other import restrictions.
Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in the People’s Republic of China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties or businesses.
For example, changes in policy could result in imposition of restrictions on currency conversion, imports or the source of suppliers, as well as new laws affecting joint ventures and foreign-owned enterprises doing business in the People’s Republic of China. Although the People’s Republic of China has been pursuing economic reforms for the past two decades, events such as a change in leadership or social disruptions that may occur upon the proposed privatization of certain state-owned industries could significantly affect the government's ability to continue with its reform.
We face economic risks in doing business in the People’s Republic of China. As a developing nation, the People’s Republic of China's economy is more volatile than that of developed Western industrial economies. It differs significantly from that of the U.S. or a Western European Country in such respects as structure, level of development, capital reinvestment, resource allocation and self-sufficiency. Only in recent years has the Chinese economy moved from what had been a command economy through the 1970s to one that during the 1990s encouraged substantial private economic activity. In 1993, the Constitution of the People’s Republic of China was amended to reinforce such economic reforms. The trends of the 1990s indicate that future policies of the Chinese government will emphasize greater utilization of market forces. The People’s Republic of China government has confirmed that economic development will follow the model of a market economy. For example, in 1999 the Government announced plans to amend the Chinese Constitution to recognize private property, although private business will officially remain subordinated to the state-owned companies, which are the mainstay of the Chinese economy. However, there can be no assurance that, under some circumstances, the government's pursuit of economic reforms will not be restrained or curtailed. Actions by the central government of the People’s Republic of China could have a significant adverse effect on economic conditions in the country as a whole and on the economic prospects for our Chinese operations. Economic reforms could either benefit or damage our operations and profitability. Some of the things that could have this effect are: i) level of government involvement in the economy; ii) control of foreign exchange; methods of allocating resources; iii) international trade restrictions; and iv) international conflict.
Under the present direction, we believe that the People’s Republic of China will continue to strengthen its economic and trading relationships with foreign countries and business development in the People’s Republic of China will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case. A change in policies by the People’s Republic of China government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the Chinese government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the People’s Republic of China's political, economic and social life.
The People’s Republic of China legal and judicial system may not adequately protect foreign investors and enforce their rights
The Chinese legal and judicial system may negatively impact foreign investors. In 1982, the National People's Congress amended the Constitution of China to authorize foreign investment and guarantee the "lawful rights and interests" of foreign investors in the People’s Republic of China. However, the People’s Republic of China's system of laws is not yet comprehensive. The legal and judicial systems in the People’s Republic of China are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the People’s Republic of China lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the Chinese judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The People’s Republic of China's legal system is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in Chinese enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the People’s Republic of China's political, economic or social life, will not affect the Chinese government's ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on our business and prospects.
The practical effect of the People's Republic of China legal system on our business operations in the People’s Republic of China can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the Foreign Invested Enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate Articles and contracts to Foreign Invested Enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are not qualitatively different from the general corporation laws of the several states, similarly, the People’s Republic of China accounting laws mandate accounting practices, which are not consistent with the U.S. (GAAP) Generally Accepted Accounting Principles. The People’s Republic of China's accounting laws require that an annual "statutory audit" be performed in accordance with People’s Republic of China accounting standards and that the books of account of Foreign Invested Enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People's Republic of China Wholly Foreign-Owned Enterprise Law requires a Wholly Foreign-Owned Enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. Second, while the enforcement of substantive rights may appear less clear than United States procedures, the Foreign Invested Enterprises and Wholly Foreign- Owned Enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution.
Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the Chinese legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of Foreign Invested Enterprises.
The Chinese legal system is a civil law system based on written statutes. Unlike common law systems, it is a system in which precedents set in earlier legal cases are not generally used. The overall effect of legislation enacted over the past 20 years has been to enhance the protections afforded to foreign invested enterprises in the PRC. However, these laws, regulations and legal requirements are relatively recent and are evolving rapidly, and their interpretation and enforcement involve uncertainties. These uncertainties could limit the legal protections available to foreign investors, such as the right of foreign invested enterprises to hold licenses and permits such as requisite business licenses.
In addition, some of our present and future executive officers and our directors may be residents of the People’s Republic of China and not of the United States, and substantially all the assets of these persons are located outside the United States. As a result, it could be difficult for investors to affect service of process in the United States, or to enforce a judgment obtained in the United States against us or any of these persons.
The People’s Republic of China laws and regulations governing our current business operations are sometimes vague and uncertain. There are substantial uncertainties regarding the interpretation and application of People’s Republic of China laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. We and any future subsidiaries are considered foreign persons or foreign funded enterprises under People’s Republic of China laws, and as a result, we are required to comply with People’s Republic of China laws and regulations. These laws and regulations are sometimes vague and may be subject to future changes, and their official interpretation and enforcement may involve substantial uncertainty. The effectiveness of newly enacted laws, regulations or amendments may be delayed, resulting in detrimental reliance by foreign investors. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We cannot predict what effect the interpretation of existing or new People’s Republic of China laws or regulations may have on our business.
Governmental control of currency conversion may affect the value of your investment .
The majority of our revenue will be settled in Renminbi, and any future restrictions on currency exchanges may limit our ability to use revenue generated in Renminbi to fund any future business activities outside the People’s Republic of China or to make dividend or other payments in U.S. dollars. Although the Chinese government introduced regulations in 1996 to allow greater convertibility of the Renminbi for current account transactions, significant restrictions still remain, including, primarily, the restriction that foreign-invested enterprises may only buy, sell or remit foreign currencies after providing valid commercial documents, at those banks in the People’s Republic of China authorized to conduct foreign exchange business.
In addition, conversion of Renminbi for capital account items, including direct investment and loans, is subject to governmental approval in the PRC, and companies are required to open and maintain separate foreign exchange accounts for capital account items. We cannot be certain that the Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of the Renminbi.
The value of our securities will be affected by the foreign exchange rate between U.S. dollars and Renminbi.
The value of our common stock will be affected by the foreign exchange rate between U.S. dollars and Renminbi, and between those currencies and other currencies in which our sales may be denominated. For example, to the extent that we need to convert U.S. dollars into Renminbi for our operational needs and should the Renminbi appreciate against the U.S. dollar at that time, our financial position, our business and the price of our common stock may be harmed. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of declaring dividends on our common stock or for other business purposes and the U.S. dollar appreciates against the Renminbi, the U.S. dollar equivalent of our earnings from our subsidiary in the People’s Republic of China would be reduced.
The People’s Republic of China government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the People’s Republic of China. We receive substantially all of our revenues in Renminbi, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing People’s Republic of China foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of the People’s Republic of China to pay capital expenses, such as the repayment of bank loans denominated in foreign currencies.
The People’s Republic of China government may also, at its discretion, restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain expenses as they come due.
The fluctuation of the Renminbi may materially and adversely affect your investment.
The value of the Renminbi against the U.S. Dollar and other currencies may fluctuate and is affected by, among other things, changes in the People’s Republic of China's political and economic conditions. As we rely almost entirely on revenues earned in the People’s Republic of China, any significant revaluation of the Renminbi may materially and adversely affect our cash flow, revenue and financial condition. For example, to the extent that we need to convert U.S. Dollars we receive from an offering of our securities into Renminbi for our operations, appreciation of the Renminbi against the U.S. Dollar could have a material adverse effect on our business, financial condition and results of operations. Conversely, if we decide to convert our Renminbi into U.S. Dollars for the purpose of making payments for dividends on our common shares or for other business purposes and the U.S. Dollar appreciates against the Renminbi, the U.S. Dollar equivalent of the Renminbi we convert would be reduced. In addition, the depreciation of significant U.S. Dollar denominated assets could result in a charge to our income statement and a reduction in the value of these assets.
On July 21, 2005, the People’s Republic of China government changed its decade-old policy of pegging the value of the Renminbi to the U.S. Dollar. Under the new policy, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. On July 21, 2005 the RMB was 8.28 for 1 USD and as of October 23, 2008, the RMB was 6.85 for 1 USD, which is approximately 20% appreciation of the RMB against the USD. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the People’s Republic of China government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. Dollar.
Recent PRC State Administration of Foreign Exchange Regulations regarding offshore financing activities by People’s Republic of China residents have undergone a number of changes which may increase the administrative burden we face. The failure by our shareholders who are People’s Republic of China residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our People’s Republic of China resident shareholders to liability under People’s Republic of China law.
State Administration of Foreign Exchange issued a public notice ("October Notice") effective from November 1, 2005, which requires registration with the State Administration of Foreign Exchange by the People’s Republic of China resident shareholders of any foreign holding company of a People’s Republic of China entity. Without registration, the People’s Republic of China entity cannot remit any of its profits out of the People’s Republic of China as dividends or otherwise; however, it is uncertain how the October Notice will be interpreted or implemented. In the event that the proper procedures are not followed under the October Notice, we could lose the ability to remit monies outside of the People’s Republic of China and would therefore be unable to pay dividends or make other distributions. Our People’s Republic of China resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the People’s Republic of China Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
Risk Related to the Company's Business and Industry
We give no assurances that any plans for future expansion will be implemented and if we do not secure adequate financing, our profitability may be adversely affected.
Our ability to implement our Three Rural Plan and ultimately generate enough revenue to be profitable is directly influenced by our ability to secure adequate financing. The company is currently profitable. We have recently received 6.3 million RMB (approximately $919,700) and have received a total of 31.7 million RMB (approximately $4,627,000) from prior investors. However, if we do not receive significant funding from future investors, we will experience delays in our growth strategies and, ultimately, in our profitability.
We have a limited operating history and limited historical financial information upon which you may evaluate our performance.
We are in our early stages of development and face risks associated with a new company in a growth industry. We may not successfully address these risks and uncertainties or successfully implement our operating strategies. If we fail to do so, it could materially harm our business to the point of having to cease operations and could impair the value of our common stock to the point investors may lose their entire investment. Even if we accomplish these objectives, we may not generate positive cash flows or the profits we anticipate in the future.
Although our revenues have grown rapidly since our inception from the increasing demand for our products, we cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties frequently experienced by companies at a similar stage of development, including our potential failure to:
| | · | expand our product offerings and maintain the high quality of our products; |
| | · | manage our expanding operations, including the integration of any future acquisitions; |
| | · | obtain sufficient working capital to support our expansion and to fill customers' orders in time; |
| | · | maintain adequate control of our expenses; |
| | · | implement our product development, marketing, sales, and acquisition strategies and adapt and modify them as needed; |
| | · | anticipate and adapt to changing conditions in the containerboard and paper products markets in which we operate as well as the impact of any changes in government regulation, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics. |
We will face a lot of competition, some of which may be better capitalized and more experienced than us.
We face competition in the bio-ecological products industries, both domestically and internationally. Although we view ourselves in a favorable position vis-à-vis our competition, some of the other companies that sell into our market may be more successful than us and/or have more experience and money that we do. This additional experience and money may enable our competitors to produce more cost-effective products and market their products with more success than we are able to, which would decrease our sales. We expect that we will be required to continue to invest in product development and productivity improvements to compete effectively in our markets. However, we cannot give you assurance that we can successfully remain competitive. If our competitors could develop a more efficient product or undertake more aggressive and costly marketing campaigns than us, which may adversely affect our marketing strategies and could have a material adverse effect on our business, results of operations or financial condition.
The People’s Republic of China legal and judicial system may not adequately protect foreign investors and enforce their rights
Our business is largely subject to the uncertain legal environment in the People’s Republic of China and your legal protection could be limited. As our present and possibly, future executive officers and directors are residents of the PRC, and our operating entity, Sanming Huajian Bioengineering Co., Ltd, is incorporated and situated in the People’s Republic of China, legal recourse against any of them could be limited or inadequate. The legal and judicial systems in the People’s Republic of China are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the People’s Republic of China lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the Chinese judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The People’s Republic of China legal system is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
A slowdown in the People’s Republic of China economy may adversely affect our operations.
A slowdown or other adverse developments in the People’s Republic of China economy may materially and adversely affect our customers, demand for our services and our business. Because our customers are primarily wholesalers, a drop in their customer base would naturally spell a drop of demand for our products.
All of our operations are conducted in the People’s Republic of China and most of all of our revenue is generated from sales in the People’s Republic of China
Although the People’s Republic of China economy has grown significantly in recent years, we cannot assure you that such growth will continue. Also, while we believe the demand for our products are independent of the health of the economy; we do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the People’s Republic of China economy. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in the People’s Republic of China may materially reduce the demand for our products and materially and adversely affect our business.
Conversely, our major competitors may be better able than us to successfully endure downturns in our sector. In periods of reduced demand for our products, we can either choose to maintain market share by reducing our selling prices to meet competition or maintain selling prices, which would likely sacrifice market share. Sales and overall profitability would be reduced under either scenario. In addition, we cannot assure you that additional competitors will not enter our existing markets, or that we will be able to compete successfully against existing or new competition.
Inflation in the People’s Republic of China could negatively affect our profitability and growth.
While the People’s Republic of China economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may have an adverse effect on profitability. In order to control inflation in the past, the People’s Republic of China government has imposed controls on bank credits, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In October 2004, the People's Bank of China, the People’s Republic of China’s central bank, raised interest rates for the first time in nearly a decade and indicated in a statement that the measure was prompted by inflationary concerns in the Chinese economy. Repeated rises in interest rates by the central bank would likely slow economic activity in People’s Republic of China which could, in turn, materially increase our costs and also reduce demand for our products.
A widespread health problem in the People’s Republic of China could negatively affect our operations.
A renewed outbreak of SARS, bird flu or another widespread public health problem in the People’s Republic of China, where all of our revenue is derived, could have an adverse effect on our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some offices that would adversely disrupt our operations.
Any of the foregoing events or other unforeseen consequences of public health problems could adversely affect our operations.
A widespread national disaster or Act of God in the People’s Republic of China could negatively affect our operations.
A widespread national disaster such as flooding, hurricane, or other weather conditions that may adversely impact the growing of tobacco leaves may have an adverse effect on our business. Any such disaster could have the effect of inhibiting the growing of tobacco leaves which could cause us to curtail our operations. Further, a scarcity of tobacco leaves could also have the effect of increasing the cost of our purchasing the extracts, which could have the effect of depleting our assets or curtailing our operations.
Enforcement against us or our directors/officers may be difficult
Because our principal assets are located outside of the United States and all of our directors and nearly all our officers reside outside of the United States, it may be difficult for you to enforce your rights based on United States Federal Securities Laws against us and our officers and directors in the United States or to enforce a United States court judgment against us or them in the People’s Republic of China.
Nearly all of our directors and officers reside outside of the United States . In addition, our operating subsidiary is located in the People’s Republic of China and substantially all of our assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the United States Federal securities laws against us in the courts of either the United States or the People’s Republic of China and, even if civil judgments are obtained in United States courts, to enforce such judgments in People’s Republic of China courts. Further, it is unclear if extradition treaties now in effect between the United States and the People’s Republic of China would permit effective enforcement against us or our officers and directors of criminal penalties under the U.S. Federal securities laws or otherwise.
We may have difficulty establishing adequate management, legal and financial controls in the People’s Republic of China.
The People’s Republic of China historically has not adopted a western style of management and financial reporting concepts and practices, as well as in modern banking, and other control systems. We may have difficulty in hiring and retaining a sufficient number of qualified employees to work in the People’s Republic of China. As a result of these factors, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet Western standards.
Inadequate funding for our capital expenditure may affect our growth and profitability
Our inability to fund our capital expenditure requirements may adversely affect our growth and profitability. Our continued growth is dependent upon our ability to raise capital from outside sources. Our ability to obtain financing will depend upon a number of factors, including:
| | · | our financial condition and results of operations, |
| | · | the condition of the People’s Republic of China economy and the containerboard sector in the PRC, |
| | · | conditions in relevant financial markets; and |
| | · | relevant People’s Republic of China laws regulating the same. |
If we are unable to obtain financing, as needed, on a timely basis and on acceptable terms to our investors or lenders, our financial position, competitive position, growth and profitability may be adversely affected.
We may not be able to effectively control and manage our growth.
If our business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. In addition, we may face challenges in managing expanding product offerings and in integrating acquired businesses with our own. Such eventualities will increase demands on our existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect our operations and cause production backlogs, longer product development time frames and administrative inefficiencies.
Significant fluctuations in raw material prices may have a material adverse effect on us
Although we have exclusive contracts with our raw materials suppliers, any significant fluctuation in price of our raw materials may have a material adverse effect on the manufacturing cost of our products. We are subject to market conditions and although these raw materials are generally available and we have not experienced any raw material shortage in the past, we cannot assure you that the necessary materials will continue to be available to us at prices currently in effect or acceptable to us.
We depend on a concentration of customers.
Our revenue is dependent, in large part, on significant orders from wholesale customers. We believe that revenue derived from such customers will continue to represent a significant portion of our total revenue although we plan to diversify our customer base by, among other things, expanding our sales. Our inability to continue to secure and maintain a sufficient number of large customers or increase our customer base would have a material adverse effect on our business, operating results and financial condition. Moreover, our success will depend in part upon our ability to obtain orders from new customers, as well as the financial condition and success of our customers and general economic conditions.
We may be exposed to intellectual property infringement and other claims by third parties, which, if successful, could cause us to pay significant damage awards and incur other costs.
Our success also depends in large part on our ability to use and develop our technology and know-how without infringing the intellectual property rights of third parties. As litigation becomes more common in the People’s Republic of China in resolving commercial disputes, we face a higher risk of being the subject of intellectual property infringement claims. The validity and scope of claims relating to the manufacturing of our products involve complex technical, legal and factual questions and analysis and, therefore, may be highly uncertain. The defense and prosecution of intellectual property suits, patent opposition proceedings and related legal and administrative proceedings can be both costly and time consuming and may significantly divert the efforts and resources of our technical and management personnel. An adverse determination in any such litigation or proceedings to which we may become a party could subject us to significant liability, including damage awards, to third parties or require us to seek licenses from third parties, to pay ongoing royalties, or to redesign our products or subject us to injunctions preventing the manufacture and sale of our products. Protracted litigation could also result in our customers or potential customers deferring or limiting their purchase or use of our products until resolution of such litigation. Further, we do not have adequate product liability insurance coverage against defective products. There is no guarantee that we will not be involved in any legal proceedings regarding our products.
We rely on Mr. Min Zhao, our chairman, chief executive officer and chief financial officer, for the management of our business, and the loss of his services may significantly harm our business and prospects.
We depend, to a large extent, on the abilities and participation of our current management team, but have a particular reliance upon Mr. Min Zhao for the direction of our business. The loss of the services of Mr. Zhao, for any reason, may have a material adverse effect on our business and prospects. We cannot assure you that the services of Mr. Zhao will continue to be available to us, or that we will be able to find a suitable replacement for Mr. Zhao.
We do not have key man insurance on Mr. Zhao, our chairman, chief executive officer and chief financial officer, upon whom we rely primarily for the direction of our business. If Mr. Zhao dies and we are unable to replace Mr. Zhao for a prolonged period of time, we may be unable to carry out our long term business plan and our future prospect for growth, and our business, may be harmed.
We may not be able to hire and retain qualified personnel to support our growth and if we are unable to retain or hire such personnel in the future, our ability to improve our products and implement our business objectives could be adversely affected.
Our future success depends heavily upon the continuing services of the members of our senior management team, in particular our chairman and president, Mr. Min Zhao. If one or more of our senior executives or other key personnel are unable or unwilling to continue in their present positions, we may not be able to replace them easily or at all, and our business may be disrupted and our financial condition and results of operations may be materially and adversely affected. Competition for senior management and personnel is intense, the pool of qualified candidates is very limited, and we may not be able to retain the services of our senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. Such failure could materially and adversely affect our future growth and financial condition.
We may not be successful in retaining a qualified Chief Financial Officer
We may not be successful in retaining an experienced Chief Financial Officer (“CFO”) who is conversant with U.S. Generally Accepted Accounting Principles and knowledgeable in our industry. We employ very experienced outside consultants to assist us in US GAAP and compliance matters. If we are unable to find a suitable CFO we could increase our reliance on outside consultants to comply with our continuing financial reporting obligations. This could potentially increase our operating costs.
Our management is comprised almost entirely of individuals residing in the People’s Republic of China with limited English skills
Our management is comprised almost entirely of individuals born and raised in the People’s Republic of China. As a result of differences in culture, educational background and business experiences, our management may analyze, evaluate and present business opportunities and results of operations differently from the way they are analyzed, evaluated and presented by management teams of public companies in Europe and the United States. In addition, our management has very limited skills in English. Consequently, it is possible that our management team will emphasize or fail to emphasize aspects of our business that might customarily be emphasized in a different manner by comparable public companies from different geographical and political areas.
Our management is not familiar with the United States securities laws.
Our management and the former owners of the businesses we acquire are generally unfamiliar with the requirements of the United States securities laws and may not appreciate the need to devote the resources necessary to comply with such laws. A failure to adequately respond to applicable securities laws could lead to investigations by the Securities and Exchange Commission and other regulatory authorities that could be costly, divert management's attention and disrupt our business.
We will continue to incur significant costs as a result of operating as a public company, and management will be required to devote substantial time to new compliance requirements.
As a public company, we incur significant legal, accounting and other expenses under the Sarbanes-Oxley Act of 2002, together with rules implemented by the Securities and Exchange Commission and applicable market regulators. These rules impose various requirements on public companies, including requiring certain corporate governance practices. Management and other personnel will need to devote a substantial amount of time to these new compliance requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time consuming and costlier.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, commencing in 2007, we must perform system and process evaluations and testing of our internal controls over financial reporting to allow management and our registered independent public accounting firm to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our registered independent public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Compliance with Section 404 may require that we incur substantial accounting expenses and expend significant management efforts. If we are not able to comply with the requirements of Section 404 in a timely manner, or if our registered independent accountants later identify deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other applicable regulatory authorities.
Risks Related to the Common Stock
Outstanding shares of our common stock cannot be offered, sold, pledged or otherwise transferred unless subsequently registered pursuant to, or exempt from registration under, the Securities Act and any other applicable federal or state securities laws or regulations. These restrictions will limit the ability of our stockholders to liquidate their investment.
We have not paid and do not anticipate paying any dividends on our common stock; therefore, our securities could face devaluation in the market.
We have paid no dividends on our common stock to date and it is not anticipated that any dividends will be paid to holders of our common stock in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, it is anticipated that any earnings will be retained to finance our future expansion and for the implementation of our new business plan. Lack of a dividend can further affect the market value of our common stock, and could significantly affect the value of any investment in us.
Penny Stock Regulations
The SEC has adopted regulations which generally define "penny stock" to be an equity security that has a market price of less than $5.00 per share. Our common stock, when and if a trading market develops, may fall within the definition of penny stock and subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the Securities and Exchange Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the "penny stock" rules may restrict the ability of broker-dealers to sell our common stock and may affect the ability of investors to sell their common stock in the secondary market.
FINANCIAL INFORMATION
Management’s Discussion and Analysis of Financial Conditions and Results of Operations
Overview
We are in the business of researching, developing, producing and selling extracts from abandoned tobacco leaves. We have received loans in the past which are outlined below in order to fund our operations:
Lender | | Amount (USD) | | Period | | Rate | |
| Sanming Wanxing Trade Co., Ltd. | | $ | 411,300 | | | 2006.12.10-2008.12.9 | | | 7.5 | % |
| Jiangle Xinfeng Mineral Industry Co., Ltd. | | $ | 383,880 | | | 2006.12.10-2008.12.9 | | | 7.5 | % |
| Jiangle Wanan Mineral Production Sales Office | | $ | 342,750 | | | 2007.1.1-2008.12.31 | | | 7.5 | % |
| Jiangle Xiangkun Mineral Industry Co., Ltd. | | $ | 205,650 | | | 2007.1.1-2008.12.31 | | | 7.5 | % |
| Sanming Mingdu Hotel Co., Ltd. | | $ | 479,850 | | | 2007.1.1-2008.12.31 | | | 7.5 | % |
Our operating results, in any given time period, are driven by several key factors such as order volume, product production, cost of raw materials, and industry competition.
The following discussion and analysis summarizes the significant factors affecting our operational results for the six months ended June 30, 2008 compared to the same period in 2007, the fiscal year 2007 compared to the fiscal year 2006. This discussion and analysis should be read in conjunction with the financial statements and notes included with this report.
SIX MONTHS ENDED JUNE 30, 2008 COMPARED WITH SIX MONTHS ENDED JUNE 30, 2007
The following table represents the statement of operations for the six months ended June, 2008 as compared to the period ended June 30, 2007. The discussion following the table is based on these results. In addition, the below financial information pertains to the operating company Sanming since the BVI and WFOE companies have no operations.
| Description | | Six Months ended June 30, 2008 (unaudited) ($) | | Six Months ended June 30, 2007 (unaudited) ($) | |
| Sales | | $ | 4,866,876 | | $ | 3,088,635 | |
| Cost of Sales | | $ | 1,843,353 | | $ | 1,211,737 | |
| Gross Profit | | $ | 3,023,523 | | $ | 1,876,898 | |
| Operating Expenses | | $ | 574,541 | | $ | 512,902 | |
| Operating Income | | $ | 2,448,982 | | $ | 1,363,996 | |
| Net Income | | $ | 1,817,130 | | $ | 922,247 | |
Net Sales
Net sales were $4,866,876 for the period ended June 2008 compared to $3,088,635 for the same period last year, which is an increase of 58%. The main reason for the increase in sales is the increasing demand for our product 90% Solanesol. We also experienced an increasing demand for our p articalate organic fertilizers products which also added to the increased sales year over year.
Cost of Sales
Cost of sales was $1,843,353 for the period ended June 2008 compared to $1,211,737 for the same period last year. The company experienced a stable raw material pricing during the first six months of 2008.
Gross Profit
The gross profit amounted to $3,023,523 for the first six months of 2008 compared to $1,876,898 for the same period last year. The gross profit margin was 62% compared to 61% for the periods ended June 2008 and June 2007, respectively.
Operating Income
The operating income was $2,448,982 for year to date June 2008 compared to $1,363,996 for the same period last year. This is approximately an increase of 80% year over year June. The increase is attributable to a stable gross profit margin % while the company has maintained expenses almost flat year to date June 2008 compared to year to date June 2007.
| Description | | Six Months ended June 30, 2008 (unaudited) ($) | | Six Months ended June 30, 2007 (unaudited) ($) | |
| Selling Expenses | | $ | 117,928 | | $ | 98,880 | |
| Administrative Expenses | | $ | 456,613 | | $ | 414,022 | |
| Total Operating Expense | | $ | 574,541 | | $ | 512,902 | |
| Operating Income | | $ | 2,448,982 | | $ | 1,363,996 | |
Selling Expenses
Our selling expenses amounted to $117,928 for the period ended June 2008 compared to $98,880 for the period ended June 2007. The small increase in expenses is due to increased sales personnel in order to capitalize on the sales opportunities in the growing market place
Administrative Expenses
The Administrative expenses were $456,613 for the six months ended June 2008 compared to $414,022 for the six months ended June 2007. The 10% increase in expenses is mainly due to non recurring expenses related to the company’s efforts to go public in the US.
Total Operating Expenses
The total operating expenses in relation to net sales were 12% and 17% for the periods ended June 2008 and June 2007, respectively. We are working on strict cost controls and have started to experience economies of scale in our business model.
Income Taxes
Income tax is accounted for using the tax effect accounting method, whereby the income tax expense of the current period is determined based on the total amount of the income tax payable for the year and the amount of the tax effect of timing differences. The liability method is used in determining the tax effect of the timing differences. We record our income taxes based on the requirements of SFAS No. 109, “Accounting for Income Taxes,” which includes an estimate of taxes payable or refundable for the current year and deferred tax liabilities and assets for the future tax consequences of events that have been recognized in our financial statements or tax returns.
Deferred tax assets and liabilities reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The management periodically assess the realizability of deferred tax assets and the adequacy of deferred tax liabilities, including the results of local, state, federal tax audits or estimates and judgments used
We operate in the People’s Republic of China and are subject to its tax laws. In accordance with the relevant tax laws and regulations of the People’s Republic of China, the corporation income tax rate has been revised to 25% across the board for all enterprises, whether domestic or foreign-owned from 33% with effect from January 1, 2008.
| | | Six Months ended June 30, 2008 | | Six Months ended June 30, 2007 | |
| Earnings before Tax | | $ | 2,418,151 | | $ | 1,358,848 | |
| PRC Income Tax Rate | | | 25 | % | | 33 | % |
| Provision for Income Tax | | $ | 601,021 | | $ | 436,601 | |
Net Income
The net income for the Company was $1,817,130 and $922,247 for the six months periods ended June 2008 and June 2007, respectively. The net profit margin was 37% and 30% for the same periods, respectively. The higher net profit margin is mainly due to a sales focus on products with high gross profit margins and a more effective manufacturing facility keeping start up costs and stand still expenses under control. The governments change in tax rate has also increased the net income.
Liquidity and Capital Resources
Net cash sourced in operating activities for the six months ended June 30, 2008 was $ 1,420,421 compared to $1,335,755 for the same period last year.
Working capital
Net accounts receivable was $ 2,847,810 as of June 30, 2008. Our inventory and accounts payable were $406,091 and $527,523 as of June 30, 2008, respectively.
Accounts Receivable
| | | 6/30/2008 | | 12/31/2007 | |
| Accounts Receivable – Trade | | $ | 2,847,810 | | $ | 2,113,989 | |
Less : Allowance for Doubtful Accounts | | $ | 0 | | $ | 0 | |
| Net Accounts Receivable | | $ | 2,847,810 | | $ | 2,113,989 | |
Inventory consists of the following:
| | | 6/30/2008 | | 12/31/2007 | |
| Raw Materials | | $ | 93,964 | | $ | 333,945 | |
| Work in progress | | $ | 288,913 | | $ | 301,417 | |
| Finished Goods | | $ | 23,214 | | $ | 32,370 | |
| Total | | $ | 406,091 | | $ | 667,732 | |
Accounts Receivable Aging Summary From Invoice Date
| Total | | 0-30 days | | 31-60 days | | 61-90 days | | 91-120 days | | > 120 days | |
| $ | 2,847,810 | | $ | 1,645,154 | | $ | 747,885 | | $ | 454,771 | | | 0 | | | 0 | |
Foreign Currency Translation
We maintain our financial statements in the functional currency of the People’s Republic of China, which is the “Renminbi” (RMB). Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
For financial reporting purposes, our financial statements are prepared using the functional currency and have been translated into United States dollars. Assets and liabilities are translated at the exchange rates at the balance sheet dates, revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustment to other comprehensive income, a component of stockholders’ equity.
Exchange Rates | | 6/30/2008 | | 6/30/2007 | |
| RMB : US$ exchange rate at: | | | 6.85 | | | 7.61 | |
The average exchange rate for the 6 months ended June 30, 2008 and 2007 were US$/RMB 7.05 and 7.77, respectively.
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at the rates used in translation.
THE FISCAL YEAR ENDED DECEMBER 31, 2007 AND THE FISCAL YEAR ENDED DECEMBER 31, 2006.
The below financial information pertains to the operating company Sanming since the BVI and WFOE companies have no operations and were not established at the end of the reporting periods.
| Description | | Fiscal Year ended December 31, 2007 (audited) ($) | | Fiscal Year ended December 31, 2006 (audited) ($) | |
| Sales | | $ | 8,059,104 | | $ | 4,143,890 | |
| Cost of Sales | | $ | 2,999,328 | | $ | 1,194,013 | |
| Gross Profit | | $ | 5,059,776 | | $ | 2,949,877 | |
| Operating Expenses | | $ | 1,009,289 | | $ | 560,082 | |
| Operating Income | | $ | 4,050,487 | | $ | 2,389,795 | |
| Net Income | | $ | 2,689,658 | | $ | 1,626,362 | |
Net Sales
Net sales for the fiscal year 2007 were 8,059,104 compared to $4,143,890, which is an increase of approximately 94%. The main reason for this increase is due to an increasing demand for our products and a broader product portfolio catering to a higher number of customers.
Cost of Sales
Cost of sales was $2,999,328 for the fiscal year 2007 compared to $1,194,013 for the year ended 2006. We experienced a stable raw material pricing during the two years.
Gross Profit
The gross profit for fiscal year 2007 was $5,059,776 compared to $2,949,877, which is 63% for 2007 and 72% for 2006. The lower gross profit % is mainly due to a shift in product portfolio in order to penetrate a broader market segment and experience less customer concentration. Various start up costs for new products brought down the gross profit as well.
Operating Income
The operating income amounted to$4,050,487 for 2007 compared to $2,389,795 for 2006.
| Description | | Fiscal Year 2007 (audited) ($) | | Fiscal Year 2006 (audited) ($) | |
| Selling Expenses | | | 227,535 | | | 105,347 | |
| Administrative Expenses | | | 781,754 | | | 454,735 | |
| Total Operating Expense | | | 1,009,289 | | | 560,082 | |
| Operating Income | | | 4,050,487 | | | 2,389,795 | |
Selling expenses totaled $227,535 and $105,347 for the years ended 2007 and 2006, respectively. The main cost drivers were personnel costs, travel and costs related to various marketing campaigns.
Administrative expenses amounted to $781,754 and $454,735 for the years ended 2007 and 2006 respectively. The main expenses were attributable to management and staff, accounting, audit fees and facilities expenses.
The total operating expenses in relation to net sales were 12% and 14% for the years ended 2007 and 2006, respectively. We began to experience economies of scale in our business model.
Income Taxes
Income tax is accounted for using the tax effect accounting method, whereby the income tax expense of the current period is determined based on the total amount of the income tax payable for the year and the amount of the tax effect of timing differences. The liability method is used in determining the tax effect of the timing differences. The Company records its income taxes based on the requirements of SFAS No. 109, “Accounting for Income Taxes,” which includes an estimate of taxes payable or refundable for the current year and deferred tax liabilities and assets for the future tax consequences of events that have been recognized in our financial statements or tax returns.
Deferred tax assets and liabilities reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The management periodically assess the realizability of deferred tax assets and the adequacy of deferred tax liabilities, including the results of local, state, federal tax audits or estimates and judgments used
We operate in the People’s Republic of China and are subject to its tax laws. In accordance with the relevant tax laws and regulations of the People’s Republic of China, the corporation income tax rate has been revised to 25% across the board for all enterprises, whether domestic or foreign-owned from 33% with effect from January 1, 2008.
Net Income
The net income for the Company was $2,689,658 and $1,626,362 for the years ended 2007 and 2006, respectively. The net profit margin was 33% and 39% for the same periods, respectively. The slightly lower net profit margin is mainly due to a shift in the product portfolio and various start-up costs for new product lines resulting in a temporarily lower gross profit margin.
Liquidity and Capital Resources
Our working capital and long-term funding primarily comes from operating cash flow and loans, while our financial resources are used in capital expenditure, operating activities and repayment of loans.
Working capital
Inventory consisted of the following:
| | | December 31, 2007 ($) | | December 31, 2006 ($) | |
| Raw Materials | | | 333,945 | | | 266,485 | |
| Work in progress | | | 301,417 | | | 166,270 | |
| Finished Goods | | | 32,370 | | | 33,670 | |
| Total | | | 667,732 | | | 466,425 | |
Accounts Receivable as of:
| | | December 31 , 2007 ($) | | December 31 , 2006 ($) | |
| Accounts Receivable – Trade | | | 2,113,989 | | | 1,587,223 | |
Less : Allowance for Doubtful Accounts | | | 0 | | | 0 | |
| Net Accounts Receivable | | | 2,113,989 | | | 1,587,223 | |
Foreign Currency Translation
We maintain our financial statements in the functional currency of the People’s Republic of China, which is the “Renminbi” (RMB). Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
For financial reporting purposes, our financial statements are prepared using the functional currency Renminbi, which have been translated into United States dollars. Assets and liabilities are translated at the exchange rates at the balance sheet dates, revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustment to other comprehensive income, a component of stockholders’ equity.
Exchange Rates | | 12/31/2007 | | 12/31/2006 | |
| | | | | | |
| Fiscal year end RMB : US$ exchange rate | | | 7.29 | | | 7.80 | |
| | | | | | | | |
| Average yearly RMB : US$ exchange rate | | | 7.60 | | | 7.96 | |
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into U.S. dollars at the rates used in translation.
Contractual Obligations
Contractual Obligations As of 12/31/07 | | Total | | Less Than 1 Year | | 1-3 Years | | 3-5 Years | | More Than 5 Years | |
Loan from third parties | | $ | 1,343,580 | | $ | 1,343,580 | | | - | | | - | | | - | |
Loan from government | | $ | 137,110 | | $ | 137,110 | | | - | | | - | | | - | |
Capital commitment PP&E | | $ | 50,042 | | | - | | $ | 50,042 | | | - | | | - | |
Office Lease arrangement | | $ | 32,184 | | $ | 10,865 | | $ | 21,319 | | | - | | | - | |
Loan from related party | | $ | 479,850 | | | On demand | | | - | | | - | | | - | |
| | | | | | | | | | | | | | | | | |
TOTAL | | $ | 2,042,756 | | $ | 1,491,545 | | $ | 71,361 | | | - | | | - | |
PROPERTIES
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants or allocates landholders a “land use right.”
We currently rent 150 square meters of office space from the Sanming Mindu Hotel at a rate of RMB 2000 per month. Our lease period is from September 30, 2005 to September 30, 2010.
The charts below describe our current land use rights.
| Land No. | 01-01-101 |
| Land Use Right Certificate No. | Ming Guo Yong (2005) No. 6238 |
| User of the Land | Sanming Huajian Bio-Engineering Co., Ltd |
| Location | Jikou Farm, Sanming City |
| Usage | Commercial Services |
| Area | 54319.4 |
| Form of Acquisition | Assignment |
| Expiration Date | 2054-07-08 |
| Encumbrances | None |
| Land No. | To be issued |
| Land Use Right Certificate No. | To be issued |
| User of the Land | Sanming Huajian Bio-Engineering Co., Ltd |
| Location | Jikou Farm, Sanming City |
| Usage | Commercial Services |
| Area | 9969.92 |
| Form of Acquisition | Assignment |
| Expiration Date | 50 years after the date of acquirement |
| Encumbrances | None |
| Land No. | To be issued |
| Land Use Right Certificate No. | To be issued |
| User of the Land | Sanming Huajian Bio-Engineering Co., Ltd |
| Location | Sanyuan District, Jingdong Industrial Zone, Sanming City |
| Usage | Commercial Services |
| Area | 153846 |
| Form of Acquisition | Assignment |
| Expiration Date | 50 years after the date of acquirement |
| Encumbrances | None |
In accordance with the written guarantee issued by Sanming Bureau of Land Resources on June 20, 2008, the certificates of two plots in the list are to be issued. The relevant contracts of land use right transference legally exist.
Buildings Owned by Sanming Huajian Bioengineering Co., Ltd.
Houses | | Certificate No. | | Area Square Meters |
| Main factory | | The certificates are to be issued. | | 3,483.938 |
| Transformer room | | | | 154.678 |
| Boiler room | | | | 136.318 |
| Cosmetic factory | | | | 924.706 |
| Synthetic building | | | | 3136.669 |
| Extracting factory | | | | 1,827.102 |
| Total: | | | | 9,663.441 |
In accordance with the written guarantee issued by Sanming Housing Administration Office of Sanyuan District on June 24, 2008, the Housing Ownership Certificates of the main factory, extracting factory, integration building and accessorial buildings are to be issued. The layout, construction, start and completion, check and acceptance are all in accordance with regulations.
Building Leases
No. | | Lessor | | Location | | Term | | Rent per Year
(USD) | |
| 1 | | Sanming Huajian Bioengineering Co., Ltd | | | #1402 Unit 1, Longfa Mansion (Bright of City), Hudong Road, Gulou District, Fuzhou City, Fujian Province | | | October 15, 2007 to
October 14, 2010 | | $ | 656.93 | |
| 2 | | Sanming Huajian Bioengineering Co., Ltd | | | #126, Gong Ye Nan Road, Sanming City, Fujian Province (Sanming Mingdu Hotel) | | | September 30, 2005 to
September 30, 2010 | | $ | 291.97 | |
Buildings Under Construction
| | 1. | Integration Building: Construction began in March 2005 with engineering costs of $503,649.63. Construction is 80% complete and is expected to cost an addition $145,985.40 to complete. |
| | 2. | Purifying Project: Construction began in January 2006 with engineering costs of $202,189.78. Construction is 70% complete and is expected to cost an additional $102,189.78 to complete. |
SECURITIES OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our voting securities following the closing of the Share Exchange Agreement and the Share Purchase Agreement by (i) any person or group owning more than 5% of each class of voting securities, (ii) each director, (iii) our chief executive officer and our top three most highly compensated officers and (iv) all executive officers and directors as a group.
Name and Address of Beneficial Owner (2) | | Amount and Nature of Beneficial Ownership (1) | | Percentage of Class (%) | |
| Min Zhao | | | 8,060,750 | | | 53.24 | |
| Min Yan Zhen | | | 6,080,917 | | | 40.16 | % |
| All Directors and Executive Officers (2 persons) | | | 14,141,667 | | | 93.40 | % |
| | (1) | In determining beneficial ownership of our common stock as of a given date, the number of shares shown includes shares of common stock which may be acquired on exercise of warrants or options or conversion of convertible securities within 60 days of that date. In determining the percent of common stock owned by a person or entity on October 24, 2008, (a) the numerator is the number of shares of the class beneficially owned by such person or entity, including shares which may be acquired within 60 days on exercise of warrants or options and conversion of convertible securities, and (b) the denominator is the sum of (i) the total shares of common stock outstanding on October 24, 2008 (15,141,667), and (ii) the total number of shares that the beneficial owner may acquire upon conversion of the preferred and on exercise of the warrants and options. Unless otherwise stated, each beneficial owner has sole power to vote and dispose of its shares. |
| | (2) | Unless otherwise indicated, the address of all beneficial owners is No. 126 Mingdu Building, Gongye road, Sanming City, Fujian, China. |
DIRECTORS AND EXECUTIVE OFFICERS, PROMOTERS
AND CONTROL PERSONS
In connection with the change of control of the Company described in Item 5.01 of this Current Report, Cris Neely, has resigned as President and we have appointed the following individuals as officers of the Company.
Name | | Age | | Position | | Service from |
| Zhao, Min | | 40 | | Chief Executive Officer , Chief Financial Officer | | 8/2004 |
| Chan, Jian Min | | 44 | | Chief Scientist | | 4/2006 |
| Ou, Shanyan | | 32 | | VP of Sales | | 6/2006 |
Eleven days after the Company files a Schedule 14-f with the Security and Exchange Commission pertaining to the appointment of new directors, the following individuals shall become members of the Company’s board of directors and Mr. Neely will resign from the board.
Name | | Age | | Position | | Service from |
| Zhao, Min | | 40 | | Chairman of the Board | | 8/2004 |
| Zhen, Min Yan | | 28 | | Director | | 4/2005 |
| Chan, Jian Min | | 44 | | Director | | 4/2006 |
| Ou, Shanyan | | 32 | | Director | | 6/2006 |
| Zheng, Jianrong | | 55 | | Director | | 8/2004 |
| Neely, Cris | | 51 | | Outgoing Director | | 9/2008 |
The following is a summary of the biographical information of our directors and officers:
Min Zhao - Chairman, CEO, & CFO, Director. Mr. Zhao has over 10 years of experience in the industry. In 2004, Mr. Zhao became the principal shareholder of Sanming Huajian Bioengineering Co., Ltd and was responsible for the daily operations of the company. In 2000, Mr. Zhao formed the Sanming Mingdu Hotel Co., Ltd. a three star hotel. Mr. Zhao graduated from the Chinese People’s Liberation Army University in 1986.
Min Yan Zhen - Director, Ms. Zhen is a graduate from the Fujian Province Medical College in 2005. She studied in Australia and obtained a degree with honors in human resources management.
Dr. Jian Min Chan - Chief scientist, Director. Professor Chan obtained his doctorate degree in 1993 at Fudan University. Since 2000, Mr. Chan has been the Chairman of the Department of Environmental Science & Engineering at Fudan University. From 1997 to 2000, Mr. Chan was an Associate Professor in the Department of Environmental Science & Engineering at Fudan University. In 1999, Dr. Chan was named the Distinguished Youth Professor of Shanghai, and thereafter, Professor Chan has earned many honors and awards from various committees, universities and the government of China.
Shanyan Ou – Vice President of Sales, Director. Ms. Ou is an active executive member of Sanming Youth Entrepreneur Association, Deputy to the National People’s Congress of Sanyuan District and a youth federation member of Sanyuan District Youth League. In 1999, Ms. Ou graduated from Beijing University major in English as a foreign language, and a business management certification from Capital Economical Trade University of China in 2003. In 2005, Ms. Ou obtained her MBA degree in Hong Kong Business Management Institute. Ms. Ou has over 10 years of sales and marketing experience and has held various senior positions with focus in biological drugs manufacturing and chemical industry.
Zheng Jianrong: Director, Mr. Zheng is the Chairman of Jiangle Jianlong Mineral Industry Limited. Mr. Zheng is a member of Sanming Political Consultative Conference and Chairman of the Jiangle fungus grass ganoderma lucidum bio-engineering. Mr. Zheng is the Fujian Province non-ferrous metal Leading enterprise representative. His domestic profession is mainly geared to investments in the mining industry and, biological medicine in mainland China.
Cris Neely: Outgoing President/Director, Mr. Neely has served as the Chief Financial Officer and as a Director for Teleplus World, Corp. since April 2007. Previous to Teleplus, Mr. Neely worked as a consultant for small/medium organizations focusing on Sarbanes-Oxley compliance, revenue recognition and financial/operational business assessments. Mr. Neely was previously the CFO of Siemens Enterprise Networks located in Boca Raton, Florida from 1999 through 2004. He also held various executive positions with Siemens Enterprise Networks including Senior Vice President Business Transformation, Director Internal Audit, Director of Finance for Wireless Terminals and Area Financial Manager. He has also held management positions with ROLM, IBM and Cisco during his career. Mr. Neely holds a Bachelor of Business Administration – Finance degree from the University of Texas at Arlington and an MBA from Amberton University.
All of our directors hold offices until the next annual meeting of the shareholders of the Company, and until their successors have been qualified after being elected or appointed. Officers serve at the discretion of the board of directors.
Family Relationships
Ms. Ou is the wife of Mr.Zhao.
There is no arrangement or understanding between or among our officers and directors pursuant to which any director or officer was or is to be selected as a director or officer, and there is no arrangement, plan or understanding as to whether non-management shareholders will exercise their voting rights to continue to elect the current Board of Directors.
Our directors and executive officers have not, during the past five years:
| | · | had any bankruptcy petition foiled by or against any business of which was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time, |
| | · | been convicted in a criminal proceeding and is not subject to a pending criminal proceeding, |
| | · | been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; or |
| | · | been found by a court of competent jurisdiction (in a civil action), the Securities Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated. |
AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors currently acts as our audit committee. Because we only recently consummated the Share Exchange Agreement, our board of directors is still in the process of finding an “audit committee financial expert” as defined in Regulation S-K and is “independent” as that term is used in Item 7(d)(3)(iv) of Schedule 14A under the Securities Exchange Act.
AUDIT COMMITTEE
We have not yet appointed an audit committee. At the present time, we believe that the members of board of directors are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting. We, however, recognize the importance of good corporate governance and intend to appoint an audit committee comprised entirely of independent directors, including at least one financial expert, during our 2008 fiscal year.
COMPENSATION COMMITTEE
We do not presently have a Compensation Committee. Our board of directors presently acts as the Compensation Committee.
EXECUTIVE COMPENSATION
The following is a summary of the compensation paid by Sanming Huajian Bioengineering Co., Ltd to its Chief Executive Officer for the two years ended December 31, 2007 and 2006 respectively. Sanming Huajian Bioengineering Co., Ltd. has no other executive officers that received compensation in excess of $100,000 for any of these two years. 1USD = 6.85 RMB.
Bioengineering Co., Ltd. has no other executive officers that received compensation in excess of $100,000 for any of these two years. 1USD = 6.85 RMB.
Name and Principal Position | | Fiscal Year | | Salary ($) | | Bonus ($) | | Stock Awards ($) | | Option Awards ($) | | Non-equity Incentive Plan Compensation ($) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($) | | Total ($) | |
| Mr. Min Zhao – CEO | | | 2007
2006 | | | 21,898
21,898 | | | 1,825
1,825 | | | 0
0 | | | 0
0 | | | 0
0 | | | 0
0 | | | 0
0 | | | 23,723
23,723 | |
Director Compensation
We do not currently nor have we ever compensated our directors.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
On January 1, 2007, we borrowed RMB 3,500,000 ($510,948.90) from the Sanming Mingdu Hotel Co. Ltd., which is a company controlled by Mr. Zhao, Sanming’s CEO, at an interest rate of 7.5% per year, for a period of two years .
We currently rent 150 square meters of office space from the Sanming Mindu Hotel at a rate of RMB 2000 ($291.97) per month. Our lease period is from September 30, 2005 to September 30, 2010.
Jiangle Jianlong Mineral Industry Co., Ltd is a shareholder of Sanming Huajian Bio Co. Ltd. with a 28.96% ownership. Zheng Jianrong is a shareholder of Jiangle Jianlong Mineral Industry Co., Ltd with a 75% ownership.
LEGAL PROCEEDINGS
We know of no material, active or pending legal proceedings against our company, nor are we involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of our directors, officers or affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to our interest.
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANTS
COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
The Company's Common Stock is not trading on any stock exchange. The Company is not aware of any market activity in its stock since its inception and through the date of this filing.
Warrants
The Company does not currently have any outstanding warrants for the purchase of shares of its Common Stock.
Securities authorized for issuance under equity compensation plans
As of the date of this Current Report, we do not have any securities authorized for issuance under any equity compensation plans and we do not have any equity compensation plans.
Dividends
There are no present material restrictions that limit our ability to pay dividends on common stock or that are likely to do so in the future. We have not paid any dividends with respect to our common stock, and do not intend to pay dividends in the foreseeable future.
Penny Stock Regulations
The SEC has adopted regulations which generally define "penny stock" to be an equity security that has a market price of less than $5.00 per share. Our common stock, when and if a trading market develops, may fall within the definition of penny stock and subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the "penny stock" rules may restrict the ability of broker-dealers to sell our common stock and may affect the ability of investors to sell their common stock in the secondary market.
RECENT SALES OF UNREGISTERED SECURITIES
Please refer to Item 1.01 – “ Entry into a Material Definitive Agreement .”
On October 24, , 2008, we entered into a Share Exchange Agreement, as described in Item 1.01, “Entry into a Material Definitive Agreement” and Item 3.02, “Unregistered Sales of Equity Securities”.
In connection with the foregoing, we relied upon the exemption from securities registration afforded by Rule 506 of Regulation D and/or Section 4(2) of the Securities Act, and transfers of such shares were restricted by Mondo Acquisition II, Inc. in accordance with the requirements of the Securities Act. All of the above-referenced persons were provided with access to our Securities and Exchange Commission filings.
DESCRIPTION OF SECURITIES
The following is a summary description of our capital stock and certain provisions of our certificate of incorporation and by-laws, copies of which have been filed as exhibits to this Form 8-K. The following discussion is qualified in its entirety by reference to such exhibits.
a) Common or Preferred Stock.
The Company is authorized by its Certificate of Incorporation to issue an aggregate of 260,000,000 shares of capital stock, of which 250,000,000 are shares of common stock, par value $.001 per share (the "Common Stock") and 10,000,000 are shares of preferred stock, par value $.001 per share (the “Preferred Stock”). Following the consummation of the Share Exchange Agreement, as of October 24, 2008, 15,141,667 shares of Common Stock were issued and outstanding.
In October, the company approved 50,000 shares of its common stock to a service provider for services rendered. In addition, the Company has issued, as compensation to a consultant, warrants to purchase an aggregate of 20,000,000 shares of its common stock (subject to adjustment upon certain events) at exercise prices ranging from $0.10 to $1.00 per share, however in no event shall the consultant be entitled to exercise these warrants for a number of warrant shares in excess of that number of warrant shares which, upon giving effect to such exercise, would cause the aggregate number of shares of common stock beneficially owned by consultant and its affiliates to exceed 4.99% of the outstanding shares of the common stock following such exercise. The Company has also granted to said consultant the right to convert any portion of a $1,000,000 consulting fee payable to said consultant into shares of the Company’s common stock (at a conversion price based upon the market price for the Company’s common stock on the day it first trades). The Company has also issued warrants to purchase 4,718,333 shares of its common stock (subject to adjustment upon certain events) at an exercise price of $0.001 per share as compensation to other consultants and service providers.
All outstanding shares of Common Stock are of the same class and have equal rights and attributes. The holders of Common Stock are entitled to one vote per share on all matters submitted to a vote of stockholders of the Company. All stockholders are entitled to share equally in dividends, if any, as may be declared from time to time by the Board of Directors out of funds legally available. In the event of liquidation, the holders of Common Stock are entitled to share ratably in all assets remaining after payment of all liabilities. The stockholders do not have cumulative or preemptive rights.
The description of certain matters relating to the securities of the Company is a summary and is qualified in its entirety by the provisions of the Company's Certificate of Incorporation and By-Laws.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Sanming Huajian Bioengineering Co., Ltd.
Financial Statements
For the years ended December 31, 2007 and 2006
(Stated in US dollars)
Sanming Huajian Bioengineering Co., Ltd.
Financial Statements
For the years ended December 31, 2007 and 2006
Index to Financial Statements
| | PAGES |
| | |
| Report of Independent Registered Public Accounting Firm | F-3 |
| | |
| Statements of Income and Comprehensive Income | F-4 |
| | |
| Balance Sheets | F-5 |
| | |
| Statements of Cash Flows | F-6 |
| | |
| Statements of Stockholders’ Equity | F-7 |
| | |
| Notes to Financial Statements | F-8 - F-25 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Sanming Huajian Bioengineering Co., Ltd
We have audited the accompanying balance sheets of Sanming Huajian Bioengineering Co., Ltd. (the “Company”) as of December 31, 2007 and 2006, and the related statements of income and comprehensive income, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2007 and 2006, and the results of their operations and cash flows for each of the two years in the period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America.
PKF
Certified Public Accountants
Hong Kong
September 26, 2008
Sanming Huajian Bioengineering Co., Ltd.
Statements of Income and Comprehensive Income
(Stated in US dollars)
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
| Revenue | | | | | |
| Sales | | $ | 8,059,104 | | $ | 4,143,890 | |
| Cost of sales | | | (2,999,328 | ) | | (1,194,013 | ) |
| | | | | | | | |
| Gross profit | | | 5,059,776 | | | 2,949,877 | |
| | | | | | | | |
| Operating expenses | | | | | | | |
| Administrative expenses | | | 781,754 | | | 454,735 | |
| Selling expenses | | | 227,535 | | | 105,347 | |
| | | | | | | | |
| | | | 1,009,289 | | | 560,082 | |
| | | | | | | | |
| Income from operations | | | 4,050,487 | | | 2,389,795 | |
| Interest income | | | 4,667 | | | 2,992 | |
| Subsidy income | | | 76,369 | | | 69,064 | |
| Other income | | | 1,317 | | | - | |
| Finance costs - Note 3 | | | (140,356 | ) | | (52,242 | ) |
| | | | | | | | |
| Income before income taxes | | | 3,992,484 | | | 2,409,609 | |
| Income taxes - Note 4 | | | (1,302,826 | ) | | (783,247 | ) |
| | | | | | | | |
| Net income | | $ | 2,689,658 | | $ | 1,626,362 | |
| | | | | | | | |
| Other comprehensive income | | | | | | | |
| Foreign currency translation adjustments | | $ | 529,165 | | $ | 179,976 | |
| | | | | | | | |
| Total comprehensive income | | $ | 3,218,823 | | $ | 1,806,338 | |
See Notes to Financial Statements
Sanming Huajian Bioengineering Co., Ltd.
Balance Sheets
(Stated in US dollars)
| | | As of December 31, | |
| | | | 2007 | | | 2006 | |
ASSETS | | | | | | | |
| Current assets | | | | | | | |
| Cash and cash equivalents | | $ | 333,081 | | $ | 304,519 | |
| Trade receivables (net of allowance of | | | | | | | |
| doubtful accounts of $Nil in 2007 and 2006) | | | 2,113,989 | | | 1,587,223 | |
| Deferred taxes - Note 4 | | | 22,580 | | | 17,447 | |
| Other receivables | | | 2,742 | | | 2,564 | |
| Inventories - Note 6 | | | 667,732 | | | 466,425 | |
| | | | | | | | |
| Total current assets | | | 3,140,124 | | | 2,378,178 | |
| Intangible assets - Note 7 | | | 104,096 | | | 130,586 | |
| Property, plant and equipment, net - Note 8 | | | 3,133,301 | | | 3,088,591 | |
| Land use rights - Note 9 | | | 7,349,936 | | | 6,893,236 | |
| Deferred taxes - Note 4 | | | 30,795 | | | 35,248 | |
| | | | | | | | |
TOTAL ASSETS | | $ | 13,758,252 | | $ | 12,525,839 | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | |
| | | | | | | | |
LIABILITIES | | | | | | | |
| Current liabilities | | | | | | | |
| Trade payables | | $ | 612,085 | | $ | 311,097 | |
| Other payables and accrued expenses - Note 10 | | | 499,812 | | | 1,107,888 | |
| Amount due to a related party - Note 11 | | | 43,392 | | | 3,846 | |
| Amount due to a stockholder - Note 11 | | | 27,420 | | | - | |
| Loan from a related party - Note 12 | | | 479,850 | | | - | |
| Unsecured short-term bank loans - Note 13 | | | - | | | 1,282,000 | |
| Short-term other loans - Note14 | | | 1,343,580 | | | - | |
| Loan from government - Note 15 | | | 137,100 | | | 57,690 | |
| Income tax payable | | | 414,572 | | | 606,732 | |
| Deferred revenue | | | 72,663 | | | 48,716 | |
| | | | | | | | |
| Total current liabilities | | | 3,630,474 | | | 3,417,969 | |
| Long-term other loans - Note 14 | | | - | | | 743,560 | |
| Other payable - Note 10 | | | 884,295 | | | 2,339,650 | |
| | | | | | | | |
TOTAL LIABILITIES | | | 4,514,769 | | | 6,501,179 | |
| | | | | | | | |
COMMITMENTS AND CONTINGENCIES - Note 19 | | | | | | | |
| | | | | | | | |
STOCKHOLDERS’ EQUITY | | | | | | | |
| Common stock - Note 16 | | | 4,133,068 | | | 4,133,068 | |
| Statutory reserve - Note 17 | | | 481,912 | | | 203,902 | |
| Accumulated other comprehensive income | | | 728,816 | | | 199,651 | |
| Retained earnings | | | 3,899,687 | | | 1,488,039 | |
| | | | | | | | |
TOTAL STOCKHOLDERS’ EQUITY | | | 9,243,483 | | | 6,024,660 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 13,758,252 | | $ | 12,525,839 | |
See Notes to Financial Statements
Sanming Huajian Bioengineering Co., Ltd.
Statements of Cash Flows
(Stated in US dollars)
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
Cash flows from operating activities | | | | | |
| Net income | | $ | 2,689,658 | | $ | 1,626,362 | |
| Adjustments to reconcile net income to net cash provided by operating activities : | | | | | | | |
| Depreciation | | | 187,719 | | | 104,503 | |
| Amortization for intangible assets | | | 34,147 | | | 31,781 | |
| Amortization for land use rights | | | 20,982 | | | 11,673 | |
| Deferred taxes | | | 2,860 | | | (30,256 | ) |
| | | | | | | | |
| Changes in operating assets and liabilities : | | | | | | | |
| Trade receivables | | | (400,077 | ) | | (1,554,662 | ) |
| Inventories | | | (162,235 | ) | | (456,857 | ) |
| Income tax payable | | | (225,002 | ) | | 594,285 | |
| Trade payables | | | 268,325 | | | 302,204 | |
| Other payables and accrued expenses | | | 191,410 | | | 225,007 | |
| Amount due to a related party | | | 37,723 | | | 3,014 | |
| Deferred revenue | | | 19,751 | | | 47,717 | |
| | | | | | | | |
| Net cash flows provided by operating activities | | | 2,665,261 | | | 904,771 | |
| | | | | | | | |
Cash flows from investing activities | | | | | | | |
| Payments to acquire intangible asset | | | - | | | ( 2,725 | ) |
| Payments to acquire property, plant and equipment | | | (24,732 | ) | | ( 667,552 | ) |
| Deposits paid for acquisition of land use rights | | | (2,402,978 | ) | | ( 3,089,022 | ) |
| Addition s for construction in progress | | | - | | | ( 1,212,127 | ) |
| | | | | | | | |
| Net cash flows used in investing activities | | | (2,427,710 | ) | | ( 4,971,426 | ) |
| | | | | | | | |
Cash flows from financing activities | | | | | | | |
| Proceeds of bank loans | | | - | | | 1,255,700 | |
| Repayment of bank loans | | | (1,316,700 | ) | | - | |
| New other loans | | | 526,680 | | | 728,306 | |
| Proceeds of loan from government | | | 131,670 | | | 56,507 | |
| Repayment of loan from government | | | (59,252 | ) | | - | |
| Advance from a related party | | | 460,845 | | | - | |
| Proceeds of capital received | | | - | | | 1,965,171 | |
| Advance from a stockholder | | | 26,334 | | | - | |
| | | | | | | | |
| Net cash flows (used in) provided by financing activities | | | (230,423 | ) | | 4,005,684 | |
| | | | | | | | |
| Effect of foreign currency translation on cash and cash equivalents | | | 21,434 | | | 10,738 | |
| | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | 28,562 | | | (50,233 | ) |
| | | | | | | | |
| Cash and cash equivalents - beginning of year | | | 304,519 | | | 354,752 | |
| | | | | | | | |
| Cash and cash equivalents - end of year | | $ | 333,081 | | $ | 304,519 | |
| | | | | | | | |
| Supplemental disclosures for cash flow information: | | | | | | | |
| Cash paid for : | | | | | | | |
| Interest | | $ | 140,248 | | $ | 52,123 | |
| Income taxes | | $ | 1,524,968 | | $ | 1,745,781 | |
| | | | | | | | |
| Non-cash investing and financing activities : | | | | | | | |
| Capital contribution settled by current accounts with the stockholders | | $ | - | | $ | 1,441,897 | |
See Notes to Financial Statements
Sanming Huajian Bioengineering Co., Ltd. Statements of Stockholders’ Equity
(Stated in US dollars)
| | | Common stock | | Statutory reserve | | Accumulated other Comprehensive | | Retained | | | |
| | | (Note 16) | | (Note 17) | | income | | earnings | | Total | |
| | | | | | | | | | | | |
| Balance, January 1, 2006 | | $ | 726,000 | | $ | - | | $ | 19,675 | | $ | 65,579 | | $ | 811,254 | |
| Increase of capital | | | 3,407,068 | | | - | | | - | | | - | | | 3,407,068 | |
| Net income | | | - | | | - | | | - | | | 1,626,362 | | | 1,626,362 | |
| Foreign currency translation adjustments | | | - | | | - | | | 179,976 | | | - | | | 179,976 | |
| Appropriation to statutory reserve | | | - | | | 203,902 | | | - | | | (203,902 | ) | | - | |
| | | | | | | | | | | | | | | | | |
| Balance, December 31, 2006 | | | 4,133,068 | | | 203,902 | | | 199,651 | | | 1,488,039 | | | 6,024,660 | |
| Net income | | | - | | | - | | | - | | | 2,689,658 | | | 2,689,658 | |
| Foreign currency translation adjustments | | | - | | | - | | | 529,165 | | | - | | | 529,165 | |
| Appropriation to statutory reserve | | | - | | | 278,010 | | | - | | | (278,010 | ) | | - | |
| | | | | | | | | | | | | | | | | |
| Balance, December 31, 2007 | | $ | 4,133,068 | | $ | 481,912 | | $ | 728,816 | | $ | 3,899,687 | | $ | 9,243,483 | |
See Notes to Financial Statements
Sanming Huajian Bioengineering Co., Ltd. Notes to Financial Statements
(Stated in US dollars)
| 1. | Description of business |
The Company is a high-technology private company established in Fujian Province, the People’s Republic of China (the “PRC”) on April 16, 2004 f or the production and sales of extracts from tobacco leaves residues . Over the years, the Company has developed a reputation of promoting natural health and for being environmentally responsible in China. In 2006, the Company was awarded the SPARKS program certification, a major health and environment awareness project of China. The Company's products include Solanesol, Nicotine Sulphate, organic pesticides, organic fertilizers, CoQ10 (raw format) and a patented organic health supplement called “Paiqianshu”, Paiqianshu comes in both liquid and pill forms and it’s made from natural green barley shoot extraction.
The Company is primarily engaged in manufacture, marketing and sale of extracts from tobacco leaves residues. The Company operates manufacturing and distribution primarily in the PRC.
| 2. | Summary of significant accounting policies |
Basis of presentation
The accompanying financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America.
These financial statements have been prepared on a going concern basis notwithstanding net current liabilities at the balance sheet date as the stockholders have undertaken to provide continuing financial support to the Company to meet its liabilities when they fall due.
Should the Company be unable to continue in business as a going concern, adjustment would have to be made to reduce the value of assets to their recoverable amounts, to reclassify non-current asset as current asset and to provide for any further liabilities which might arise.
Use of estimates
In preparing financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of accounts receivable, inventories, deferred taxes and the estimation on useful lives and realizability of intangible assets and property, plant and equipment. Actual results could differ from those estimates.
Concentrations of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents and trade receivables. As of December 31, 2007 and 2006, all of the Company’s cash and cash equivalents were held by major financial institutions located in the PRC, which management believes are of high credit quality. With respect to trade receivables, in order to reduce its credit risk, the Company has adopted credit policies, which include the analysis of the financial position of its customers and a regular review of their credit limits.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Concentrations of credit risk (cont’d)
During the reporting periods, customers represented 10% or more of the Company’s sales are as follows :-
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| F uzhou Yishun Imp. & Exp. Co., Ltd. | | $ | 1,643,646 | | $ | 960,605 | |
| Shanghai Zhaoxiang Biologic Technology Co., Ltd. | | | 1,525,938 | | | 1,202,781 | |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co., Ltd. | | | 1,400,947 | | | 809,519 | |
| Jiangxi Lufeng Organic Agricultural Technology Development Co., Ltd. | | | 1,387,256 | | | 544,707 | |
| Sanming Wangnong Agricultural Materials Co., Ltd. | | | 1,257,620 | | | 488,465 | |
| Fujian Wuyishang City Lukang Bioengineering Co., Ltd. | | | 843,697 | | | 137,813 | |
| | | | | | | | |
| | | $ | 8,059,104 | | $ | 4,143,890 | |
As of December 31, 2007 and 2006, percentage of the gross receivables due from these customers were as follows :-
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| F uzhou Yishun Imp. & Exp. Co., Ltd. | | | 19 | % | | 25 | % |
| Shanghai Zhaoxiang Biologic Technology Co., Ltd. | | | 11 | % | | 23 | % |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co., Ltd. | | | 14 | % | | 13 | % |
| Jiangxi Lufeng Organic Agricultural Technology Development Co., Ltd. | | | 21 | % | | 16 | % |
| Sanming Wangnong Agricultural Materials Co., Ltd. | | | 24 | % | | 23 | % |
| Fujian Wuyishang City Lukang Bioengineering Co., Ltd. | | | 11 | % | | - | |
Cash and cash equivalents
Cash and cash equivalents include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less to be cash equivalents. As of December 31, 2007 and 2006, almost all the cash and cash equivalents were denominated in Renminbi (“RMB”) and were placed with banks in the PRC. They are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Allowance of doubtful accounts
The Company establishes an allowance for doubtful accounts based on management’s assessment of the collectibility of trade and other receivables. A considerable amount of judgment is required in assessing the amount of the allowance, the Company considers the historical level of credit losses and applies percentages to aged receivable categories. The Company makes judgments about the creditworthiness of each customer based on ongoing credit evaluations, and monitors current economic trends that might impact the level of credit losses in the future. If the financial condition of the customers were to deteriorate, resulting in their inability to make payments, a larger allowance may be required.
Based on the above assessment, during the reporting periods, the management establishes the following rates of general provision provided on gross amount of trade and other receivables :-
| | | Rate | |
| | | | |
| Aged within half year | | | 0 | % |
| Aged over half year but within 1 year | | | 5 | % |
| Aged over 1 year but within 3 years | | | 20 | % |
| More than 3 years | | | 100 | % |
Additional specific provision is made against trade and other receivables aged less than 1 year to the extent which they are considered to be doubtful.
Bad debts are written off when identified. The Company extends unsecured credit to customers ranging from three to six months in the normal course of business. The Company does not accrue interest on trade accounts receivable.
Inventories
Inventories are stated at the lower of cost or market value. Cost is determined on a weighted-average basis and includes all expenditures incurred in bringing the goods to the point of sale and putting them in a saleable condition. In assessing the ultimate realization of inventories, the management makes judgments as to future demand requirements compared to current or committed inventory levels. The Company’s reserve requirements generally increase with its projected demand requirements; decrease due to market conditions, product life cycle changes. During the reporting periods, all of the Company’s products are saleable with high profit margins, the Company did not make any allowance for slow-moving or defective inventories.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Intangible assets
Intangible assets are stated at cost less accumulated amortization. Amortization is provided on a straight-line basis over their estimated useful lives. The principal amortization periods are as follows :-
| | | Amortization period | |
| | | | | |
| Technologies | | | 5 to 10 years | |
| Software | | | 5 years | |
The Company periodically reviews the original estimated useful lives of long-lived assets and makes adjustments when appropriate. Intangible assets with finite useful lives are tested for impairment whenever events or changes in circumstances indicate that an asset’s carrying amount may not be recoverable. The Company evaluates its intangible assets for impairment by comparing the future undiscounted cash flows of the underlying assets to their respective carrying amounts.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
Depreciation is provided on straight-line basis over their estimated useful lives. The principal depreciable periods are as follows :-
| | | Depreciable period | |
| | | | |
| | | 20 years | |
| Plant and machinery | | | 10 years | |
| Office equipment | | | 5 years | |
| Motor vehicles | | | 5 years | |
Construction in progress represents buildings and machinery under construction, which is stated at cost less any impairment losses, and is not depreciated. Cost comprises the direct costs of construction. Construction in progress is reclassified to the appropriate category of property, plant and equipment when completed and ready for use.
Maintenance or repairs are charged to expense as incurred. Upon sale or disposition, the applicable amounts of asset cost and accumulated depreciation are removed from the accounts and the net amount less proceeds from disposal is charged or credited to income.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Impairment of long-lived assets
Long-lived assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets. During the reporting periods, the Company has not identified any indicators that would require testing for impairment.
Revenue recognition
Revenue from sales of the Company’s products is recognized when the significant risks and rewards of ownership have been transferred to the buyer at the time of delivery, the sales price is fixed or determinable and collection is reasonably assured.
Deferred revenue
Deferred revenue represents subsidy income received from government. It mainly consisted of receipt of grant funds to subsidize the research and development activities. It is recognized as income when the relevant criteria are met.
Cost of sales
Cost of sales consists primarily of material costs, freight charges, purchasing and receiving costs, inspection costs, wages, employee compensation, depreciation and related costs, which are directly attributable to the production of products.
Selling expenses
Selling expenses are mainly consists of advertising, commission, entertainment, salaries, and traveling expense which are incurred during the selling activities.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Advertising and research and development expenses
Advertising and research and development costs are charged to expense as incurred.
Advertising expenses amounting to $527 and $2,336 for two years ended December 31, 2007 and 2006 respectively are included in selling expenses.
Research and development expenses amounting to $200,204 and $112,659 for two years ended December 31, 2007 and 2006 respectively are included in administrative expenses.
Stock-based compensation
During 2007 and 2006, the Company did not make any stock-based compensation payments.
Income taxes
The Company uses the asset and liability method of accounting for income taxes pursuant to SFAS No. 109 “Accounting for Income Taxes”. Under the asset and liability method of SFAS 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carryforwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Off-balance sheet arrangements
The Company does not have any off-balance sheet arrangements.
Comprehensive income
The Company has adopted SFAS 130, “Reporting Comprehensive Income”, which establishes standards for reporting and display of comprehensive income, its components and accumulated balances. Components of comprehensive income include net income and foreign currency translation adjustments.
Foreign currency translation
The functional currency of the Company is Renminbi (“RMB”) and RMB is not freely convertible into foreign currencies. The Company maintains its financial statements in the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transactions. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Foreign currency translation (cont’d)
For financial reporting purposes, the financial statements of the Company which are prepared using the functional currency have been translated into United States dollars (“$”). Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rate. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustments to other comprehensive income, a component of stockholders’ equity. The exchange rates in effect at December 31, 2007 and 2006 were $1 for RMB7.2940 and RMB7.8003 respectively. There is no significant fluctuation in exchange rate for the conversion of RMB to $ after the balance sheet date.
Fair value of financial instruments
All of the carrying values of the Company’s financial assets and liabilities approximate their fair values due to the short-term maturity of these instruments. The carrying amount of bank loan approximates its fair value because the applicable interest rate approximate current market rate.
It is management’s opinion that the Company is not exposed to significant interest or credit risks arising from these financial instruments.
In respect of foreign currency risk, the Company does not have significant foreign exchange risk arising from future commercial transactions and recognized financial assets and liabilities since almost all of them are denominated in RMB.
Recently issued accounting pronouncements
SAB 108 “Considering the Effects of Prior Year Misstatements when quantifying Misstatements in Current Year Financial Statements”
In September 2006, the Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin (“SAB”) No. 108, which provides guidance on the process of quantifying financial statement misstatements. In SAB No. 108, the SEC staff establishes an approach that requires quantification of financial statement errors, under both the iron-curtain and the roll-over methods, based on the effects of the error on each of our financial statements and the related financial statement disclosures. SAB No. 108 is generally effective for annual financial statements in the first fiscal year ended after November 15, 2006. The transition provisions of SAB No. 108 permits existing public companies to record the cumulative effect in the first year ended after November 15, 2006 by recording correcting adjustments to the carrying values of assets and liabilities as of the beginning of that year with the offsetting adjustment recorded to the opening balance of retained earnings. The adoption of SAB No. 108 had no material impact on the Company’s financial statements.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Recently issued accounting pronouncements (cont’d)
FASB Interpretation No. 48 “Accounting for Uncertainty in Income Taxes-an Interpretation of FASB Statement No. 109”
In June 2006, the FASB issued FIN 48 “Accounting for Uncertainty in Income Taxes-an Interpretation of FASB Statement No. 109” which clarifies the accounting for uncertainty in tax positions. This Interpretation requires that the Company recognizes in its financial statements the impact of a tax position if that position is more likely than not of being sustained on audit, based on the technical merits of the position. The provisions of FIN 48 become effective for the Company on January 1, 2007, with the cumulative effect of the change in accounting principle, if any, recorded as an adjustment to opening retained earnings. The adoption of FIN 48 had no material impact on the Company’s financial statements.
SFAS 157 “Fair Value Measurements”
In September 2006, the FASB issued SFAS No.157 “Fair Value Measurements”. This statement defines fair value, establishes a framework for measuring fair valueand expands disclosures of fair value measurements. SFAS No.157 applies under other accounting pronouncements that require or permit fair value measurements, and accordingly, does not require any new fair value measurements. SFAS No.157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, which is the Company’s fiscal year which began on January 1, 2008. In February 2008, the FASB issued FASB Staff Position No.157-2, “Effective Date of FASB Statement No.157”. FSP No.157-2 permits a one-year deferral in applying the measurement provisions of SFAS157 to non-financial assets and non-financial liabilities that are not recognized or disclosed at fair value in an entity’s financial statements on a recurring basis (at least annually). The adoption of SFAS No.157 and FSP 157-2 is not expected to have a material impact on the Company’s financial statements.
SFAS 159 “The Fair Value Option for Financial Assets and Financial Liabilities-Including an Amendment of FASB Statement No. 115”
In February 2007, the FASB issued SFAS No. 159 “The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115”. SFAS 159 permits entities to choose to measure many financial instruments and certain other items at fair value. Entities that elect the fair value option will report unrealized gains and losses in earnings at each subsequent reporting date. The fair value option may be elected on an instrument-by-instrument basis, with a few exceptions. SFAS 159 also establishes presentation and disclosure requirements to facilitate comparisons between entities that choose different measurement attributes for similar assets and liabilities. The requirements of SFAS 159 are effective for the fiscal year beginning on January 1, 2008. The management is in the process of evaluating the impact SFAS 159 will have on the Company’s financial statements upon adoption.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
2. | Summary of significant accounting policies (Cont’d) |
Recently issued accounting pronouncements (cont’d)
SFAS 160 “Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51”
In December 2007, the FASB issued SFAS No. 160 “Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51”. SFAS 160 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. The guidance will become effective for the fiscal year beginning after December 15, 2008. The management is in the process of evaluating the impact SFAS 160 will have on the Company’s financial statements upon adoption.
SFAS 141(Revised) “Business Combinations”
In December 2007, the FASB issued SFAS No. 141 (Revised) “Business Combinations”. SFAS 141 (Revised) establishes principles and requirements for how the acquirer of a business recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree. The statement also provides guidance for recognizing and measuring the goodwill acquired in the business combination and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The guidance will become effective for the fiscal year beginning after December 15, 2008. The management is in the process of evaluating the impact SFAS 141 (Revised) will have on the Company’s financial statements upon adoption.
SFAS 161 "Disclosures about Derivative Instruments and Hedging Activities - an amendment to FASB Statement No. 133"
In March 2008, FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities - an amendment to FASB Statement No. 133". SFAS 161 is intended to improve financial standards for derivative instruments and hedging activities by requiring enhanced disclosures to enable investors to better understand their effects on an entity's financial position, financial performance, and cash flows. Entities are required to provide enhanced disclosures about: (a) how and why an entity uses derivative instruments; (b) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations; and (c) how derivative instruments and related hedged items affect an entity's financial position, financial performance, and cash flows. It is effective for financial statements issued for fiscal year beginning after November 15, 2008, with early adoption encouraged. The management is in the process of evaluating the impact that SFAS 161 will have on the Company’s financial statements upon adoption.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
2. | Summary of significant accounting policies (Cont’d) |
Recently issued accounting pronouncements (cont’d)
SFAS 162 " The Hierarchy of Generally Accepted Accounting Principles"
In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles”. SFAS 162 identifies, within the accounting literature established by the FASB, the sources and hierarchy of the accounting principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with GAAP. SFAS 162 is effective 60 days following the Securities and Exchange Commission’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. The management is in the process of evaluating the impact that SFAS 162 will have on the Company’s financial statements upon adoption.
| | | Year ended December 31, | |
| | | | 2007 | | | 2006 | |
| | | | | | | | |
| Bank loans interest | | $ | 8,907 | | $ | 52,123 | |
| Other loans interest | | | 131,341 | | | - | |
| Bank charges | | | 108 | | | 119 | |
| | | | | | | | |
| | | $ | 140,356 | | $ | 52,242 | |
During the years ended December 31, 2007 and 2006, loans interest expenses to a related company were $34,563 and $- respectively.
The Company is subject to enterprise income tax at 33% of the assessable profits as reported in the statutory financial statements prepared under China Accounting regulations in the PRC.
The PRC’s legislative body, the National People’s Congress, adopted the unified Corporate Income Tax Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rates is set at 25% for both domestic enterprises and foreign-invested enterprises.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
The components of the provision for income taxes are :-
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Current taxes - PRC | | $ | 1,299,966 | | $ | 813,503 | |
| Deferred taxes - PRC | | | 2,860 | | | (30,256 | ) |
| | | | | | | | |
| | | $ | 1,302,826 | | $ | 783,247 | |
The effective income tax expense differs from the PRC statutory income tax rate of 33% in the PRC as follows :-
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Income before income taxes | | $ | 3,992,484 | | $ | 2,409,609 | |
| Provision for income taxes at statutory income tax rate of 33% | | | 1,317,520 | | | 795,171 | |
| Non-deductible (taxable) items for tax purpose | | | (6,081 | ) | | (21,233 | ) |
| Tax rate change | | | (8,613 | ) | | 9,309 | |
| | | | | | | | |
| | | $ | 1,302,826 | | $ | 783,247 | |
Deferred tax assets as of December 31, 2007 and 2006 are composed of the following :-
| | | As of December 31, | |
| | | 2007 | | 200 6 | |
| The PRC | | | | | |
| Current deferred tax assets : | | | | | | | |
| Accelerated amortization of land use rights | | $ | 1,494 | | $ | 679 | |
| Accelerated amortization of intangible assets | | | 4,776 | | | 4,466 | |
| Preliminary expenses | | | 9,188 | | | 11,341 | |
| Accrued expenses | | | 7,122 | | | 961 | |
| | | | | | | | |
| | | $ | 22,580 | | $ | 17,447 | |
| | | | | | | | |
| Non-current deferred tax assets : | | | | | | | |
| Accelerated amortization of intangible assets | | $ | 8,591 | | $ | 4,188 | |
| Preliminary expenses | | | 22,204 | | | 29,354 | |
| Accrued expenses | | | - | | | 1,706 | |
| | | | | | | | |
| | | $ | 30,795 | | $ | 35,248 | |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
In June 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”). This interpretation requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The Company adopted FIN 48 on January 1, 2007. The management evaluated the Company’s tax positions and considered that no additional provision for uncertainty in income taxes is necessary as of December 31, 2007.
The basic and diluted earnings per share are not presented because the Company’s common stock represents the paid up registered capital of the Company which are not divided into number of shares.
| | | As of December 31, | |
| | 2007 | | 2006 | |
| | | | | | |
| Raw materials | | $ | 333,945 | | $ | 266,485 | |
| Work-in-progress | | | 301,417 | | | 166,270 | |
| Finished goods | | | 32,370 | | | 33,670 | |
| | | | | | | | |
| | | $ | 667,732 | | $ | 466,425 | |
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Technologies - Note (a) | | $ | 185,085 | | $ | 173,070 | |
| Software | | | 2,975 | | | 2,782 | |
| | | | | | | | |
| | | | 188,060 | | | 175,852 | |
| Accumulated amortization | | | (83,964 | ) | | (45,266 | ) |
| | | | | | | | |
| Intangible assets, net | | $ | 104,096 | | $ | 130,586 | |
| | Note: | (a) | The technologies were purchased from third parties for producing an existing product - Solanesol and a new product - Organic Green Barley Supplements (Paiqianshu). The application for patent is in process and has been initially accepted by the relevant government department. |
| | | (b) | During the years ended December 31, 2007 and 2006, amortization charge was $34,147 and $31,781 respectively. |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
7. | Intangible assets (Cont’d) |
The estimated aggregate amortization expenses for intangible assets for the five succeeding years is as follows :-
| Year | | | |
| | | | |
| 2008 | | $ | 34,147 | |
| 2009 | | | 34,147 | |
| 2010 | | | 20,980 | |
| 2011 | | | 1,975 | |
| 2012 | | | 1,975 | |
| | | | | |
| | | $ | 93,224 | |
8. | Property, plant and equipment |
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| Cost : | | | | | | | |
| Buildings - Note (a) | | $ | 1,802,666 | | $ | 1,685,644 | |
| Plant and machinery | | | 804,102 | | | 749,980 | |
| Office equipment | | | 91,132 | | | 63,059 | |
| Motor vehicles | | | 86,776 | | | 81,143 | |
| | | | | | | | |
| | | | 2,784,676 | | | 2,579,826 | |
| Accumulated depreciation | | | (314,254 | ) | | (111,082 | ) |
| | | | | | | | |
| | | | 2,470,422 | | | 2,468,744 | |
| Construction in progress - Note (b) | | | 662,879 | | | | |
| | | | | | | | |
| Net | | $ | 3,133,301 | | $ | 3,088,591 | |
During the reporting periods, depreciation is included in :-
| | | Year ended December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Cost of sales and inventory overheads | | $ | 106,052 | | $ | 55,417 | |
| Administrative expenses | | | 81,667 | | | 49,086 | |
| | | | | | | | |
| Net | | $ | 187,719 | | $ | 104,503 | |
| | Note: | (a) | Property certificates of buildings with carrying amount of $1,672,874 as of December 31, 2007 are yet to be obtained. |
| | | (b) | Construction in progress mainly comprises capital expenditure for construction of the Company’s new office and machinery. |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Land use rights | | $ | 7,384,528 | | $ | 6,905,153 | |
| Accumulated amortization | | | (34,592 | ) ) | | (11,917 | ) |
| | | | | | | | |
| | | $ | 7,349,936 | | $ | 6,893,236 | |
The carrying amount of land use rights as of December 31, 2007 comprises three land use rights, which were acquired for building factories and offices, with amounts of $923,106, $92,810 and $6,334,020 respectively. The legal title of the second and third land use rights with carrying amount of $6,426,830 has not yet been transferred to the Company. The application of legal title is in process and the management expects there will be no legal hindrance in obtaining the legal titles and no extra cost will be incurred.
The land use right with carrying amount of $6,334,020 which was acquired on November 14, 2006 has not been used and developed, accordingly no amortization was provided.
During the two years ended December 31, 2007 and 2006, amortization amounted to $20,982 and $11,673 respectively.
The estimated aggregate amortization expenses for land use rights for the five succeeding years is as follows :-
| Year | | | |
| | | | |
| 2008 | | $ | 20,982 | |
| 2009 | | | 73,514 | |
| 2010 | | | 147,058 | |
| 2011 | | | 147,058 | |
| 2012 | | | 147,058 | |
| | | | | |
| | | $ | 535,670 | |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
10. | Other payables and accrued expenses |
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Rental payable | | $ | 1,542 | | $ | - | |
| Salaries payable | | | 60,219 | | | 57,418 | |
| Other accrued expenses | | | 201,394 | | | 37,153 | |
| Value-added tax payable | | | 178,956 | | | 134,361 | |
| Land use rights payable - note | | | 939,135 | | | 3,217,820 | |
| Other payables | | | 2,861 | | | 786 | |
| | | | | | | | |
| Total | | $ | 1,384,107 | | $ | 3,447,538 | |
| | | | | | | | |
| Current portion | | $ | 499,812 | | $ | 1,107,888 | |
| Non current portion - Land use rights payable - note | | | 884,295 | | | 2,339,650 | |
| | | | | | | | |
| Total | | $ | 1,384,107 | | $ | 3,447,538 | |
| | Note : | It is interest-free and repayable by instalments with last payment due by December 31, 2009. |
11. | Amounts due to a related party / a stockholder |
The amounts are interest-free, unsecured and repayable on demand.
12. | Loan from a related party |
The loan from a related company bears interest rate at 7.5% per annum, is unsecured and repayable on demand.
13. | Unsecured short-term bank loans |
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Bank loans repayable within 1 year | | $ | - | | $ | 1,282,000 | |
| | (a) | Bank loans were guaranteed by a shareholder of the Company and by a third party. |
| | (b) | Bank loans bore interest at fixed rate of 7.605% per annum. |
| | (c) | During the reporting periods, there was no covenant requirement under the banking facilities granted to the Company. |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| | | As of December 31, | |
| | | 2007 | | 2006 | |
| | | | | | |
| Repayable within 1year | | $ | 1,343,580 | | $ | - | |
| Repayable after 1 year | | | - | | | 743,560 | |
| | | | | | | | |
| | | $ | 1,343,580 | | $ | 743,560 | |
These other loans are borrowed from third parties, unsecured and bear interest at fixed rate of 7.5% per annum.
The government loan was designated for a research project. It is interest-free, unsecured and repayable within one year.
| | | As of December 31, | |
| | | 200 7 | | 200 6 | |
| | | | | | |
| Registered and paid up | | $ | 4,133,068 | | $ | 4,133,068 | |
The Company was established in Fujian province, the PRC in April 2004 as a domestic corporation. In its constitutional document, the percentage of equity interest of each stockholder of the Company was stated therein and each stockholder has paid up their respective capital before June 28, 2006.
The Company is classified as a non-joint capital stock corporation and therefore the capital stock, consistent with most of the PRC corporations, are not divided into a specific number of shares having a stated nominal amount.
In accordance with the relevant laws and regulations of the PRC, it is required that not less than 10% of its net income (the percentage is upon approval from the board of directors’ meeting), after offsetting any prior years’ losses, for PRC tax reporting purpose being transferred to the statutory reserve.
When the balance of such reserve reaches 50% of the registered capital, any further appropriation is optional. Upon approval from the board of directors of the Company, the statutory reserve can mainly be used to offset accumulated losses and increase capital.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
18. | Defined contribution plan |
The Company has a defined contribution plan for all qualified employees in the PRC. The employer and its employees are each required to make contributions to the plan at the rates specified in the plan. The only obligation of the Company with respect to retirement scheme is to make the required contributions under the plan. No forfeited contribution is available to reduce the contribution payable in the future years. The defined contribution plan contributions were charged to the statements of income. The Company contributed $41,144 and $21,792 for the two years ended December 31, 2007 and 2006 respectively.
19. | Commitments and contingencies |
As of December 31, 2007, the Company had capital commitments of $50,042 contracted but not provided for in the financial statements in respect of acquisition of plant and machinery.
| | (b) | Operating lease arrangements |
As of December 31, 2007, the Company had two non-cancelable operating leases for its office. The lease will expire in 2010 and the expected payments are as follows :-
| Year | | | |
| | | | |
| 2008 | | $ | 10,865 | |
| 2009 | | | 11,688 | |
| 2010 | | | 9,631 | |
| | | | | |
| | | $ | 32,184 | |
The rental expense relating to the operating leases was $4,641 and $3,013 for the two years ended December 31, 2007 and 2006 respectively.
As of December 31, 2007, the Company had no contingencies.
The Company is solely engaged in the manufacture, marketing, sale and distribution of extracts from tobacco leaves residues. Since the nature of the products, their production processes, the type of their customers and their distribution methods are substantially similar, they are considered as a single reportable segment under FAS 131, “Disclosures about Segments of an Enterprise and Related Information”.
All of the Company’s long-lived assets and revenues classified based on the customers are located in the PRC.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
21. | Related party transactions |
| | Apart from the transactions as disclosed elsewhere in the financial statements, during the two years ended December 31, 2007 and 2006 , the Company has entered into the following transactions with related parties :- |
| Related party relationship | | Type of transaction | | Year ended December 31, | |
| | | | | 2007 | | 2006 | |
| | | | | | | | |
| A related company (common | | Loans interest expenses | | $ | 34,563 | | $ | - | |
| stockholder and director) | | Rental expenses | | $ | 3,160 | | $ | 3,013 | |
22. | Post balance sheet event |
| | (1) | Pursuant to the resolution passed in the stockholders’ meeting on December 7, 2007, the Company’s registered share capital was increased from $4,133,068 to $4,758,358. All increased registered share capital has been paid up in January 2008. |
| | (2) | On July 25, 2008, the Company entered into the following contractual arrangements :- |
| | | Entrusted Management Agreement. Pursuant to this entrusted management agreement among Fujian Green Planet Bioengineering Co., Ltd. (“Green Planet”), the Company, and the Company’s Stockholders, the Company and the Company’s Stockholders agreed to entrust the business operations of the Company and its management to Green Planet until Green Planet acquires all of the assets or equity of the Company (as more fully described in the Exclusive Purchase Option Agreement below). Prior to the occurrence of such event, the Company will only own those certain assets that are not sold to Green Planet. The Company anticipates that it will continue to be the contracting party under its customer contracts, banks loans and certain other assets until such time as those may be transferred to Green Planet. Under the entrusted management agreement, Green Planet will manage the Company‘s operations and assets, and control all of the Company’s cash flow through an entrusted bank account. In turn, it will be entitled to any of the Company’s net profits as a management fee, and will be obligated to pay all the Company’s payables and loan payments. The entrusted management agreement will remain in effect until the acquisition of all assets or equity of the Company by Green Planet is completed. |
Exclusive Purchase Option Agreement. Under the exclusive option agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders granted Green Planet an irrevocable and exclusive purchase option to acquire the Company’s equity and/or remaining assets, but only to the extent that such purchase does not violate limitations imposed by PRC law. The option is exercisable when PRC law specifically allows foreign equity to be used as consideration to acquire a PRC entity's equity interests and/or assets, and when Green Planet has sufficient funds to purchase the Company’s equity or remaining assets. The consideration for the exercise of the option is the shares of common stock received by the Company’s Stockholders under the Share Exchange Agreement.
Sanming Huajian Bioengineering Co., Ltd.
Condensed Financial Statements
For the three and six months ended June 30, 2008
and 2007
(Stated in US dollars)
Sanming Huajian Bioengineering Co., Ltd.
Condensed Financial Statements
For the three and six months ended June 30, 2008 and 2007
Index to Condensed Financial Statements
| | | Pages |
| | | |
| Condensed Statements of Income and Comprehensive Income | | F-28 |
| | | |
| Condensed Balance Sheets | | F-29 |
| | | |
| Condensed Statements of Cash Flows | | F-30 |
| | | |
| Notes to Condensed Financial Statements | | F-31 - F-42 |
Sanming Huajian Bioengineering Co., Ltd.
Condensed Statements of Income and Comprehensive Income
(Unaudited)
(Stated in US Dollars)
| | | Three months ended June 30, | | Six months ended June 30, | |
| | | 2008 | | 2007 | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | | (Unaudited) | | (Unaudited) | |
| Revenue | | | | | | | | | | | | | |
| Sales revenue - Note 2 | | $ | 2,680,637 | | $ | 1,465,478 | | $ | 4,866,876 | | $ | 3,088,635 | |
| Cost of sales | | | (994,445 | ) | | (641,625 | ) | | (1,843,353 | ) | | (1,211,737 | ) |
| | | | | | | | | | | | | | |
| Gross profit | | | 1,686,192 | | | 823,853 | | | 3,023,523 | | | 1,876,898 | |
| | | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | | |
| Administrative expenses | | | 288,682 | | | 185,678 | | | 456,613 | | | 414,022 | |
| Selling expenses | | | 61,647 | | | 47,190 | | | 117,928 | | | 98,880 | |
| | | | | | | | | | | | | | |
| | | | 350,329 | | | 232,868 | | | 574,541 | | | 512,902 | |
| | | | | | | | | | | | | | |
| Income from operations | | | 1,335,863 | | | 590,985 | | | 2,448,982 | | | 1,363,996 | |
| Interest income | | | 3,027 | | | 1,172 | | | 4,890 | | | 2,213 | |
| Subsidy income | | | - | | | - | | | 42,552 | | | 64,855 | |
| Other income | | | - | | | 1,304 | | | - | | | 1,297 | |
| Finance costs - Note 3 | | | (40,084 | ) | | (32,538 | ) | | (78,273 | ) | | (73,513 | ) |
| | | | | | | | | | | | | | |
| Income before income taxes | | | 1,298,806 | | | 560,923 | | | 2,418,151 | | | 1,358,848 | |
| Income taxes - Note 4 | | | (342,255 | ) | | (147,536 | ) | | (601,021 | ) | | (436,601 | ) |
| | | | | | | | | | | | | | |
| Net income | | $ | 956,551 | | $ | 413,387 | | $ | 1,817,130 | | $ | 922,247 | |
| | | | | | | | | | | | | | |
| Other comprehensive income | | | | | | | | | | | | | |
| Foreign currency translation adjustments | | $ | 255,508 | | $ | 103,769 | | $ | 676,580 | | $ | 167,808 | |
| | | | | | | | | | | | | | |
| Total comprehensive income | | $ | 1,212,059 | | $ | 517,156 | | $ | 2,493,710 | | $ | 1,090,055 | |
See Notes to Condensed Financial Statements
Sanming Huajian Bioengineering Co., Ltd.
Condensed Balance Sheets
(Unaudited)
(Stated in US Dollars)
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
ASSETS | | | | | | | |
| Current assets | | | | | | | |
| Cash and cash equivalents | | $ | 2,470,483 | | $ | 333,081 | |
| Trade receivables | | | 2,847,810 | | | 2,113,989 | |
| Deferred taxes | | | 31,802 | | | 22,580 | |
| Other receivables | | | 4,377 | | | 2,742 | |
| Inventories - Note 6 | | | 406,091 | | | 667,732 | |
| | | | | | | | |
| Total current assets | | | 5,760,563 | | | 3,140,124 | |
| Intangible assets - Note 7 | | | 92,175 | | | 104,096 | |
| Property, plant and equipment, net - Note 8 | | | 3,231,471 | | | 3,133,301 | |
| Land use rights - Note 9 | | | 7,810,079 | | | 7,349,936 | |
| Deferred taxes | | | 30,230 | | | 30,795 | |
| | | | | | | | |
TOTAL ASSETS | | $ | 16,924,518 | | $ | 13,758,252 | |
| | | | | | | | |
| | | | | | | |
| | | | | | | | |
LIABILITIES | | | | | | | |
| Current liabilities | | | | | | | |
| Trade payables | | $ | 527,523 | | $ | 612,085 | |
| Other payables and accrued expenses - Note 10 | | | 536,154 | | | 499,812 | |
| Amounts due to a related party - Note 11 | | | 67,078 | | | 43,392 | |
| Amount due to a stockholder - Note 11 | | | - | | | 27,420 | |
| Loan from a related party - Note 12 | | | 510,650 | | | 479,850 | |
| Other loans - Note13 | | | 1,429,820 | | | 1,343,580 | |
| Loan from government - Note 14 | | | 145,900 | | | 137,100 | |
| Income tax payable | | | 355,708 | | | 414,572 | |
| Deferred revenue | | | 48,147 | | | 72,663 | |
| | | | | | | | |
| Total current liabilities | | | 3,620,980 | | | 3,630,474 | |
| Other payable - Note 10 | | | 941,055 | | | 884,295 | |
| | | | | | | | |
TOTAL LIABILITIES | | | 4,562,035 | | | 4,514,769 | |
| | | | | | | | |
COMMITMENTS AND CONTINGENCIES - Note 17 | | | | | | | |
| | | | | | | | |
STOCKHOLDERS’ EQUITY | | | | | | | |
| Common stock - Note 15 | | | 4,758,358 | | | 4,133,068 | |
| Statutory reserve | | | 481,912 | | | 481,912 | |
| Accumulated other comprehensive income | | | 1,405,396 | | | 728,816 | |
| Retained earnings | | | 5,716,817 | | | 3,899,687 | |
| | | | | | | | |
TOTAL STOCKHOLDERS’ EQUITY | | | 12,362,483 | | | 9,243,483 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 16,924,518 | | $ | 13,758,252 | |
See Notes to Condensed Financial Statements
Sanming Huajian Bioengineering Co., Ltd.
Condensed Statements of Cash Flows
(Unaudited)
(Stated in US Dollars)
| | | Six months ended June 30, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
Cash flows from operating activities | | | | | | | |
| Net income | | $ | 1,817,130 | | $ | 922,247 | |
| Adjustments to reconcile net income to net cash provided by operating activities : | | | | | | | |
| Depreciation | | | 101,641 | | | 92,278 | |
| Amortization for intangible assets | | | 18,085 | | | 16,538 | |
| Amortization for land use rights | | | 11,302 | | | 10,335 | |
| Deferred taxes | | | (5,086 | ) | | (14,096 | ) |
| | | | | | | | |
| Changes in operating assets and liabilities : | | | | | | | |
| Trade receivables | | | (581,486 | ) | | 651,280 | |
| Other receivables | | | (1,418 | ) | | - | |
| Inventories | | | 296,027 | | | (6,438 | ) |
| Income tax payable | | | (83,096 | ) | | (453,026 | ) |
| Trade payables | | | (120,404 | ) | | 131,912 | |
| Other payables and accrued expenses | | | 4,143 | | | (33,856 | ) |
| Amount due to a related party | | | 20,319 | | | 18,581 | |
| Amount due to a stockholder | | | (28,368 | ) | | - | |
| Deferred revenue | | | (28,368 | ) | | - | |
| | | | | | | | |
| Net cash flows provided by operating activities | | | 1,420,421 | | | 1,335,755 | |
| | | | | | | | |
Cash flows from investing activities | | | | | | | |
| Payments to acquire property, plant and equipment | | | (1,560 | ) | | (20,754 | ) |
| Payment for acquisition of land use rights | | | - | | | (486,413 | ) |
| | | | | | | | |
| Net cash flows used in investing activities | | | (1,560 | ) | | (507,167 | ) |
| | | | | | | | |
Cash flows from financing activities | | | | | | | |
| Repayment of bank loans | | | - | | | (1,297,100 | ) |
| New other loans | | | - | | | 518,840 | |
| Repayment of loan from government | | | - | | | (58,370 | ) |
| Loan from a related party | | | - | | | 453,985 | |
| Proceeds of capital received | | | 625,290 | | | - | |
| | | | | | | | |
| Net cash flows provided by (used in) financing activities | | | 625,290 | | | (382,645 | ) |
| | | | | | | | |
| Effect of foreign currency translation on cash and cash equivalents | | | 93,251 | | | 13,994 | |
| | | | | | | | |
| Net increase in cash and cash equivalents | | | 2,137,402 | | | 459,937 | |
| | | | | | | | |
| Cash and cash equivalents - beginning of period | | | 333,081 | | | 304,519 | |
| | | | | | | | |
| Cash and cash equivalents - end of period | | $ | 2,470,483 | | $ | 764,456 | |
| | | | | | | | |
| Supplemental disclosures for cash flow information : | | | | | | | |
| Cash paid for : | | | | | | | |
| Interest | | $ | 78,213 | | $ | 73,467 | |
| Income taxes | | $ | 732,733 | | $ | 903,723 | |
See Notes to Condensed Financial Statements
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 1. | Description of business |
The Company is a high-technology private company established in Fujian Province, the People’s Republic of China (the “PRC”) on April 16, 2004 f or the production and sales of extracts from tobacco leaves residues . Over the years, the Company has developed a reputation of promoting natural health and for being environmentally responsible in China. In 2006, the Company was awarded the SPARKS program certification, a major health and environment awareness project of China. The Company's products include Solanesol, Nicotine Sulphate, organic pesticides, organic fertilizers, CoQ10 (raw format) and a patented organic health supplement called “Paiqianshu”, Paiqianshu comes in both liquid and pill forms and it’s made from natural green barley shoot extraction.
The Company is primarily engaged in manufacture, marketing and sale of extracts from tobacco leaves residues. The Company operates manufacturing and distribution primarily in the PRC.
| 2. | Summary of significant accounting policies |
Basis of presentation
The accompanying condensed financial statements of the Company have been prepared in accordance with generally accepted accounting principles in the United States of America for interim financial information. Accordingly, they do not include all the information and notes necessary for comprehensive financial statements.
In the opinion of the management of the Company, all adjustments, which are of a normal recurring nature, and necessary for a fair statement of the results for the six-month periods have been made. Results for the interim periods presented are not necessarily indicative of the results that might be expected for the entire fiscal year. These condensed financial statements should be read in conjunction with the financial statements and the notes for the years ended December 31, 2007 and 2006.
Use of estimates
In preparing condensed financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of accounts receivable, inventories, deferred taxes and the estimation on useful lives and realizability of intangible assets and property, plant and equipment. Actual results could differ from those estimates.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Inventories
Inventories are stated at the lower of cost or market value. Cost is determined on a weighted-average basis and includes all expenditures incurred in bringing the goods to the point of sale and putting them in a saleable condition. In assessing the ultimate realization of inventories, the management makes judgments as to future demand requirements compared to current or committed inventory levels. The Company’s reserve requirements generally increase with its projected demand requirements; decrease due to market conditions, product life cycle changes. During the reporting periods, all of the Company’s products are saleable with high profit margins, the Company did not make any allowance for slow-moving or defective inventories.
Concentrations of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents and trade receivables. As of June 30, 2008 and December 31, 2007, all of the Company’s cash and cash equivalents were held by major financial institutions located in the PRC, which management believes are of high credit quality. With respect to trade receivables, in order to reduce its credit risk, the Company has adopted credit policies, which include the analysis of the financial position of its customers and a regular review of their credit limits.
During the reporting periods, customers representing 10% or more of the Company’s sales are :-
| | | Six months ended June 30, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| | | | | | |
| Fuzhou Yishun Imp. & Exp. Co., Ltd. | | $ | 876,602 | | $ | 727,032 | |
| Fujian Wuyishang City Lukang Bioengineering Co., Ltd. | | | 780,396 | | | 364,666 | |
| Shanghai Zhaoxiang Biologic Technology Co., Ltd. | | | 775,054 | | | 752,768 | |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co., Ltd. | | | 759,531 | | | 669,697 | |
| | | | | | | | |
| | | $ | 3,191,583 | | $ | 2,514,163 | |
As of June 30, 2008 and 2007, percentage of the gross receivables due from these customers were as follows :-
| | | As of June 30, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | |
| | | | | | |
| Fuzhou Yishun Imp. & Exp. Co., Ltd. | | | 20 | % | | 38 | % |
| Fujian Wuyishang City Lukang Bioengineering Co., Ltd. | | | 14 | % | | 13 | % |
| Shanghai Zhaoxiang Biologic Technology Co., Ltd. | | | 18 | % | | 23 | % |
| Guangdong Qingyuan Shengzhitang Modern Chinese Medicine Co., Ltd. | | | 13 | % | | 22 | % |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Recently issued accounting pronouncements
SFAS 159 “The Fair Value Option for Financial Assets and Financial Liabilities - Including an Amendment of FASB Statement No. 115”
In February 2007, the FASB issued SFAS No. 159 “The Fair Value Option for Financial Assets and Financial Liabilities - Including an Amendment of FASB Statement No. 115”. SFAS 159 permits entities to choose to measure many financial instruments and certain other items at fair value. Entities that elect the fair value option will report unrealized gains and losses in earnings at each subsequent reporting date. The fair value option may be elected on an instrument-by-instrument basis, with a few exceptions. SFAS 159 also establishes presentation and disclosure requirements to facilitate comparisons between entities that choose different measurement attributes for similar assets and liabilities. The requirements of SFAS 159 are effective for the fiscal year beginning on January 1, 2008. The adoption of SFAS 159 did not have a material impact on the Company’s financial statements.
SFAS 160 “Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51”
In December 2007, the FASB issued SFAS No. 160 “Noncontrolling Interests in Consolidated Financial Statements-an amendment of ARB No. 51”. SFAS 160 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. The guidance will become effective for the fiscal year beginning after December 15, 2008. The management is in the process of evaluating the impact SFAS 160 will have on the Company’s financial statements upon adoption.
SFAS 141 (Revised) “Business Combinations”
In December 2007, the FASB issued SFAS No. 141 (Revised) “Business Combinations”. SFAS 141 (Revised) establishes principles and requirements for how the acquirer of a business recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree. The statement also provides guidance for recognizing and measuring the goodwill acquired in the business combination and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. The guidance will become effective for the fiscal year beginning after December 15, 2008. The management is in the process of evaluating the impact SFAS 141 (Revised) will have on the Company’s financial statements upon adoption.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 2. | Summary of significant accounting policies (Cont’d) |
Recently issued accounting pronouncements (Cont’d)
SFAS 161 "Disclosures about Derivative Instruments and Hedging Activities - an amendment to FASB Statement No. 133"
In March 2008, FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities - an amendment to FASB Statement No. 133". SFAS No. 161 is intended to improve financial standards for derivative instruments and hedging activities by requiring enhanced disclosures to enable investors to better understand their effects on an entity's financial position, financial performance, and cash flows. Entities are required to provide enhanced disclosures about: (a) how and why an entity uses derivative instruments; (b) how derivative instruments and related hedged items are accounted for under Statement 133 and its related interpretations; and (c) how derivative instruments and related hedged items affect an entity's financial position, financial performance, and cash flows. It is effective for financial statements issued for fiscal year beginning after November 15, 2008, with early adoption encouraged. The management is in the process of evaluating the impact that SFAS 161 will have on the Company’s financial statements upon adoption.
SFAS 162 " The Hierarchy of Generally Accepted Accounting Principles"
In May 2008, the FASB issued SFAS No. 162, “The Hierarchy of Generally Accepted Accounting Principles”. SFAS 162 identifies, within the accounting literature established by the FASB, the sources and hierarchy of the accounting principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with GAAP. SFAS 162 is effective 60 days following the Securities and Exchange Commission’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411, The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. The management is in the process of evaluating the impact that SFAS 162 will have on the Company’s financial statements upon adoption.
| | | Three months ended June 30, | | Six months ended June 30, | |
| | | 2008 | | 2007 | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | | (Unaudited) | | (Unaudited) | |
| | | | | | | | | | |
| Bank loans interest | | $ | 4,166 | | $ | - | | $ | 7,471 | | $ | 8,775 | |
| Other loans interest | | | 35,887 | | | 32,519 | | | 70,742 | | | 64,693 | |
| Bank charges | | | 31 | | | 19 | | | 60 | | | 45 | |
| | | | | | | | | | | | | | |
| | | $ | 40,084 | | $ | 32,538 | | $ | 78,273 | | $ | 73,513 | |
During the periods ended June 30, 2008 and 2007, loans interest expenses to a related company were $18,617 and $17,024 respectively.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
The Company is subject to enterprise income tax at 33% of the assessable profits as reported in the statutory financial statements prepared under China Accounting regulations in the PRC
The PRC’s legislative body, the National People’s Congress, adopted the unified Corporate Income Tax Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rates is set at 25% for both domestic enterprises and foreign-invested enterprises.
In June 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”). This interpretation requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The Company adopted FIN 48 on January 1, 2007. The management evaluated the Company’s tax positions and considered that no additional provision for uncertainty in income taxes is necessary as of June 30, 2008.
The basic and diluted earnings per share are not presented because the Company’s common stock represents the outstanding registered capital of the Company which are not divided into number of shares.
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| | | | | | |
| Raw materials | | $ | 93,964 | | $ | 333,945 | |
| Work-in-progress | | | 288,913 | | | 301,417 | |
| Finished goods | | | 23,214 | | | 32,370 | |
| | | | | | | | |
| | | $ | 406,091 | | $ | 667,732 | |
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited ) | | (Audited ) | |
| | | | | | |
| Technologies - Note (a) | | $ | 196,965 | | $ | 185,085 | |
| Software | | | 3,166 | | | 2,975 | |
| | | | | | | | |
| | | | 200,131 | | | 188,060 | |
| Accumulated amortization | | | (107,956 | ) | | (83,964 | ) |
| | | | | | | | |
| Intangible assets, net | | $ | 92,175 | | $ | 104,096 | |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 7. | Intangible assets (Cont’d) |
| Note: | (a) | The technologies were purchased from third parties for producing an existing product - Solanesol and a new product - Oragic Green Barley Supplements (Paiqianshu) , the application for patent is in process and has been initially accepted by the relevant government department. |
| | | |
| | (b) | During the periods ended June 30, 2008 and 2007, amortization charge was $18,085 and $16,538 respectively. |
| | | |
| | (c) | The estimated aggregate amortization expenses for intangible assets for the five succeeding periods is as follows :- |
| Year | | | |
| | | | |
| 2008 | | $ | 18,392 | |
| 2009 | | | 36,785 | |
| 2010 | | | 22,601 | |
| 2011 | | | 2,128 | |
| 2012 | | | 2,128 | |
| | | | |
| | $ | 82,034 | |
| 8. | Property, plant and equipment |
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| Costs: | | | | | |
| Buildings - Note (a) | | $ | 1,918,374 | | $ | 1,802,666 | |
| Plant and machinery | | | 855,714 | | | 804,102 | |
| Office equipment | | | 96,982 | | | 91,132 | |
| Motor vehicles | | | 92,345 | | | 86,776 | |
| | | | | | | | |
| | | | 2,963,415 | | | 2,784,676 | |
| Accumulated depreciation | | | (438,975 | ) | | (314,254 | ) |
| | | | | | | | |
| | | | 2,524,440 | | | 2,470,422 | |
| Construction in progress - Note (b) | | | 707,031 | | | 662,879 | |
| | | | | | | | |
| Net | | $ | 3,231,471 | | $ | 3,133,301 | |
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 8. | Property, plant and equipment (Cont’d) |
During the reporting periods, depreciation is included in :-
| | | Three months ended June 30, | | Six months ended June 30, | |
| | | 2008 | | 2007 | | 2008 | | 2007 | |
| | | (Unaudited) | | (Unaudited) | | (Unaudited) | | (Unaudited) | |
| | | | | | | | | | |
| Cost of sales and inventory overheads | | $ | 28,986 | | $ | 26,249 | | $ | 57,138 | | $ | 52,221 | |
| | | | | | | | | | | | | | |
| Administrative expenses | | | 22,576 | | | 20,135 | | | 44,503 | | | 40,057 | |
| | | | | | | | | | | | | | |
| Net | | $ | 51,562 | | $ | 46,384 | | $ | 101,641 | | $ | 92,278 | |
| Note : | (a) | Property certificates of buildings with carrying amounts of $1,734,210 as of June 30, 2008 are yet to be obtained. |
| | | |
| | (b) | Construction in progress mainly comprises capital expenditure for construction of the Company’s new office and machinery. |
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| | | | | | |
| Land use rights | | $ | 7,858,516 | | $ | 7,384,528 | |
| Accumulated amortization | | | (48,437 | ) | | (34,592) | ) |
| | | | | | | | |
| | | $ | 7,810,079 | | $ | 7,349,936 | |
The carrying amount of land use rights as of June 30, 2008 comprises three land use rights, which were required for building factories and offices, with amounts of $971,794, $97,705 and $6,740,580 respectively. The legal title of the second and third land use rights with carrying amount of $6,838,285 has not yet been transferred to the Company. The application of legal title is in the process and the management expects there will be no legal hindrance in obtaining the legal titles and no extra costs will be incurred.
The land use right with carrying amount of $6,740,580 which was acquired on November 14, 2006 has not been used and developed, accordingly no amortization was provided.
During the periods ended June 30, 2008 and 2007, amortization change was $11,302 and $10,335 respectively.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 9. | Land use rights (Cont’d) |
The estimated aggregate amortization expenses for land use rights for the five succeeding years is as follows :-
| Year | | | |
| | | | |
| 2008 | | $ | 11,302 | |
| 2009 | | | 79,192 | |
| 2010 | | | 158,417 | |
| 2011 | | | 158,417 | |
| 2012 | | | 158,417 | |
| | | | | |
| | | $ | 565,745 | |
| 10. | Other payables and accrued expenses |
| | | June30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| | | | | | |
| Rental payable | | $ | 5,581 | | $ | 1,542 | |
| Salaries payable | | | 59,343 | | | 60,219 | |
| Other accrued expenses | | | 176,057 | | | 201,394 | |
| Value-added tax payable | | | 192,297 | | | 178,956 | |
| Land use rights payable - Note | | | 999,415 | | | 939,135 | |
| Other payables | | | 44,516 | | | 2,861 | |
| | | | | | | | |
| Total | | $ | 1,477,209 | | $ | 1,384,107 | |
| | | | | | | | |
| Current portion | | $ | 536,154 | | $ | 499,812 | |
| Non current portion - Land use rights payable - Note | | | 941,055 | | | 884,295 | |
| | | | | | | | |
| Total | | $ | 1,477,209 | | $ | 1,384,107 | |
Note : It is interest-free, and repayable by instalments with last payment due by December 31, 2009.
| 11. | Amounts due to a related party / a stockholder |
The amounts are interest-free, unsecured and repayable on demand.
| 12. | Loan from a related party |
The loan from a related company bears interest rate at 7.5% per annum, is unsecured and repayable on demand.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| | | | | | |
| Repayable within 1year | | $ | 1,429,820 | | $ | 1,343,580 | |
These other loans are borrowed from third parties, unsecured and bear interest at fixed rate of 7.5% per annum.
The government loan was designated for a research project. It is interest-free, unsecured and repayable within one year.
| | | June 30, | | December 31, | |
| | | 2008 | | 2007 | |
| | | (Unaudited) | | (Audited) | |
| | | | | | |
| Registered and paid up | | $ | 4,758,358 | | $ | 4,133,068 | |
The Company was established in Fujian province, the PRC in April 2004 as a domestic corporation. In its constitutional document, the percentage of equity interest of each stockholder of the Company was stated therein and each stockholder has paid up their respective capital before January 2008.
Pursant to the resolution passed in the stockholders’ meeting on December 7, 2007, the Company’s registered share capital was increased from $4,133,068 to $4,758,358. All increased registered share capital was paid up in January 2008.
The Company is classified as a non-joint capital stock corporation and therefore the capital stock, consistent with most of the PRC corporations, are not divided into a specific number of shares having a stated nominal amount.
| 16. | Defined contribution plan |
The Company has a defined contribution plan for all qualified employees in the PRC. The employer and its employees are each required to make contributions to the plan at the rates specified in the plan. The only obligation of the Company with respect to retirement scheme is to make the required contributions under the plan. No forfeited contribution is available to reduce the contribution payable in the future years. The defined contribution plan contributions were charged to the statements of income. The Company contributed $22,237 and $20,173 for the six months ended June 30, 2008 and 2007 respectively.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 17. | Commitments and contingencies |
As of June 30, 2008, the Company had capital commitments amounting to $53,254 in respect of the acquisition of property, plant and equipment which were contracted for but not provided in the financial statements.
| (b) | Operating lease arrangements |
As of June 30, 2008, the Company had two non-cancelable operating leases for its office. The lease will expire in 2010 and the expected payments are as follows :-
| Year | | | |
| | | | |
| 2008 | | $ | 7,623 | |
| 2009 | | | 12,438 | |
| 2010 | | | 8,499 | |
| | | | | |
| | | $ | 28,560 | |
The rental expenses relating to the operating leases was $5,532 and $1,557 for periods ended June 30, 2008 and 2007 respectively.
As of June 30, 2008, the Company had no contingencies.
The Company is solely engaged in the manufacture, marketing, sale and distribution of extracts from tobacco leaves residues. Since the nature of the products, their production processes, the type of their customers and their distribution methods are substantially similar, they are considered as a single reportable segment under FAS 131, “Disclosures about Segments of an Enterprise and Related Information”.
All of the Company’s long-lived assets and revenues classified based on the customers are located in the PRC.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 19. | Related party transactions |
Apart from the transactions as disclosed in elsewhere in the financial statements, the Company has entered into the following transactions with related parties :-
| | Type of transaction | | Three months ended June 30, | | Six months ended June 30, | |
| | | | | 2008 | | 2007 | | 2008 | | 2007 | |
| | | | | (Unaudited) | | (Unaudited) | | (Unaudited) | | (Unaudited) | |
| | | | | | | | | | | | |
| A related company (common | | Loans interest expenses | | $ | 9,444 | | $ | 8,558 | | $ | 18,617 | | $ | 17,024 | |
| stockholder and director) | | Rental expenses | | $ | 863 | | $ | 782 | | $ | 1,702 | | $ | 1,557 | |
| 20. | Post balance sheet events |
On July 25, 2008, the Company entered into the following contractual arrangements :-
Entrusted Management Agreement. Pursuant to this entrusted management agreement among Fujian Green Planet Bioengineering Co., Ltd. (“Green Planet”), the Company, and the Company’s Stockholders, the Company and the Company’s Stockholders agreed to entrust the business operations of the Company and its management to Green Planet until Green Planet acquires all of the assets or equity of the Company (as more fully described in the Exclusive Purchase Option Agreement below). Prior to the occurrence of such event, the Company will only own those certain assets that are not sold to Green Planet. The Company anticipates that it will continue to be the contracting party under its customer contracts, banks loans and certain other assets until such time as those may be transferred to Green Planet. Under the entrusted management agreement, Green Planet will manage the Company‘s operations and assets, and control all of the Company’s cash flow through an entrusted bank account. In turn, it will be entitled to any of the Company’s net profits as a management fee, and will be obligated to pay all the Company’s payables and loan payments. The entrusted management agreement will remain in effect until the acquisition of all assets or equity of the Company by Green Planet is completed.
Exclusive Purchase Option Agreement. Under the exclusive option agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders granted Green Planet an irrevocable and exclusive purchase option to acquire the Company’s equity and/or remaining assets, but only to the extent that such purchase does not violate limitations imposed by PRC law. The option is exercisable when PRC law specifically allows foreign equity to be used as consideration to acquire a PRC entity's equity interests and/or assets, and when Green Planet has sufficient funds to purchase the Company’s equity or remaining assets. The consideration for the exercise of the option is the shares of common stock received by the Company’s Stockholders under the Share Exchange Agreement.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Condensed Financial Statements
(Unaudited)
(Stated in US Dollars)
| 20. | Post balance sheet events (Cont’d) |
Share Pledge Agreement. Under this share pledge agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders pledged all of their equity interests in the Company, including the proceeds thereof, to guarantee all of Green Planet’s rights and benefits under the Restructuring Agreements. Prior to termination of this share pledge agreement, the pledged equity interests cannot be transferred without Green Planet’s prior consent.
Stockholders’ Voting Proxy Agreement. Under the stockholders' voting proxy agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders irrevocably and exclusively appointed the members of the board of directors of Green Planet as their proxy to vote on all matters that require the Company’s Stockholders’ approval. The members of the board of directors of Green Planet are identical to those of the Company.
Sanming Huajian Bioengineering Co., Ltd.
Notes to Financial Statements
(Stated in US dollars)
| 22. | Post balance sheet event (Cont’d) |
Share Pledge Agreement. Under this share pledge agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders pledged all of their equity interests in the Company, including the proceeds thereof, to guarantee all of Green Planet’s rights and benefits under the Restructuring Agreements. Prior to termination of this share pledge agreement, the pledged equity interests cannot be transferred without Green Planet’s prior consent.
Stockholders’ Voting Proxy Agreement. Under the stockholders' voting proxy agreement between Green Planet and the Company’s Stockholders, the Company’s Stockholders irrevocably and exclusively appointed the members of the board of directors of Green Planet as their proxy to vote on all matters that require the Company’s Stockholders’ approval. The members of the board of directors of Green Planet are identical to those of the Company.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission (the “SEC”) reports, statements and other information as required under the Securities Exchange Act of 1934. These reports, statements and other information may be read and copied at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room at 1-800-SEC-0330.
The SEC maintains a web site (http//:www.sec.gov) that contains the registration statements, reports, proxy and information statements and other information regarding registrants, like us, that file electronically with the SEC. You may access our SEC filings electronically at this SEC website. These SEC filings are also available to the public from commercial document retrieval services.
Item 3.02. Unregistered Sales of Registered Securities
Please refer to Item 1.01 – “ Entry into a Material Definitive Agreement .”
On October 24, 2008, we entered into a Share Exchange Agreement, as described in Item 1.01, “Entry into a Material Definitive Agreement” and Item 3.02, “Unregistered Sales of Equity Securities”.
We are under no obligation to register the shares issued in this transaction. The securities issued in this transaction were issued in connection with a private placement exempt from the registration pursuant to Section 4(2) of the Securities Act based upon our compliance with Regulation D as promulgated by the SEC under the Securities Act of 1933, as amended (the “Securities Act”).
In connection with the foregoing, we relied upon the exemption from securities registration afforded by Rule 506 of Regulation D and/or Section 4(2) of the Securities Act, and transfers of such shares were restricted by Green Planet Bioengineering Co. Ltd., Inc. in accordance with the requirements of the Securities Act. All of the above-referenced persons were provided with access to our Securities and Exchange Commission filings.
Item 5.01. Changes in Control of Registrant.
As more fully described in Items 1.01 and 2.01 above, on October 24, 2008, we consummated the Reverse Merger Transaction with the Elevated Throne Overseas Ltd. (“ETOL”) Shareholders (“ETOL Shareholders”), through which the ETOL Shareholders delivered to us all the issued and outstanding shares of stock of ETOL. In exchange for the 50,000 shares, we delivered to them 14,141,667 shares of our common stock. ETOL became a holder of 14,141,667 shares of our common stock,
Prior to Closing of the Reverse Merger Transaction, we were authorized to issue 250,000,000 shares of Common Stock, of which 1,000,000 shares of Common Stock were issued and outstanding, and 10,000,000 shares of preferred stock, of which none was issued and outstanding.
As a result of these transactions, ETOL became our majority shareholder. Min Zhao and Min Yan Zhen are the controlling stockholders of ETOL.
In connection with this change in control, and as explained more fully in Item 2.01 above under the section titled “Management” and in Item 5.02 below, upon the effectiveness of the Schedule 14f-1 Information Statement, which shall occur 11 days following the delivery and/or mailing of the Schedule 14f-1 to our stockholders in compliance with the provisions of Section 14(f) of the Act, and Rule 14(f)-1 thereunder, Cris Neely will resign as our sole director. Concurrently, the appointment of Min Zhao Min Yan Zhen, Jian Min Chen, Shanyan Ou and Zheng Jianrong as new members of our Board of Directors, will also become effective.
Please refer to Item 2.01 - “Completion of Acquisition or Disposition of Assets “- “Our Directors and Executive Officers” and Item 5.01 - “Changes in Control of Registrant” above, which description is in its entirety incorporated by reference to this Item 5.02 of this report.
Item 5.06. Change in Shell Company Status
As explained more fully in Item 2.01 above, we were a “shell company” (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended) immediately before the Closing of the Exchange. As a result of the Exchange, Sanming Huajian Bio-Engineering Co. became our wholly owned subsidiary and main operating business. Consequently, upon the Closing of the Exchange we ceased to be a shell company. For information about the Exchange, please see the information set forth above under Item 2.01 of this Current Report on Form 8-K above, which information is incorporated herein by reference.
Item 9.01. Financial Statements and Exhibits .
| 3.1 | Certificate of Incorporation of Mondo Acquisition II, Inc. (Incorporated by reference to the Form 10-SB as filed with the Securities and Exchange Commission on May 2, 2007). |
| 3.2 | Certificate of Amendment to Certificate of Incorporation (Incorporated by reference to the Current Report on Form 8-K, filed with the Securities and Exchange Commission on October 6, 2008) |
| 3.3 | Bylaws of Mondo Acquisition II, Inc. (Incorporated by reference to the Form 10SB as filed with the Securities and Exchange Commission on May 2, 2007). |
| 10.1 | Share Exchange Agreement by and among Elevated Throne Overseas Ltd., Green Planet Bioengineering Co., Ltd. and the shareholders of Elevated Throne Overseas Ltd., dated October 24, 2008.* |
| 10.2 | Management Entrustment Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Fujian Green Planet Bioengineering Co., Ltd., dated July 25, 2008 |
| 10.3 | Shareholder’s Voting Proxy Agreement between Fujian Green Planet Bioengineering Co., Ltd. and Min Zhao, Minyan Zheng and Jiangle Jianlong Mineral Industry Co., Ltd., dated July 25, 2008.* |
| 10.4 | Exclusive Option Agreement by and among Fujian Green Planet Bioengineering Co., Ltd.; Min Zhao, Minyan Zheng, Jiangle Jianlong Mineral Industry Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated July 25, 2008.* |
| 10.5 | Share Pledge Agreement between Min Zhao, Minyan Zheng and Jiangle Jianlong Mineral Industry Co., Ltd. and Fujian Green Planet Bioengineering Co., Ltd., dated July 25, 2008.* |
| 10.6 | State-Owned Land use right Transfer Agreement between Fujian Sanming Buearu of Land and Resources and Sanming Huajian Bio-Engineering Co., Ltd., dated April 8, 2008.* |
| 10.7 | State-Owned Land Use Right Transfer Agreement between Fujian Sanming Buearo of Land and Resources and Sanming Huajian Bio-Engineering Co., Ltd., dated January 6, 2005.* |
| 10.8 | State-Owned Land Use Right Transfer Agreement between Fujian Sanming Jinyuan Development Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated November 13, 2006.* |
| 10.9 | Construction Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Fujian Hongsheng Construction Group Co., Ltd, dated February 15, 2005.* |
| 10.10 | House Lease Agreement between Sasnming Mingdu Hotel Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated September 20, 2005.*+ |
| 10.11 | Office Lease Agreement between Liu Yongde, Wu Xinhuai and Sanming Huajian Bio-Engineering Co., Ltd., dated October 15, 2007.* |
| 10.12 | Trademark License Contract between Min Zhao and Sanming Huajian Bio-Engineering Co., Ltd., dated October 22, 2004.* |
| 10.13 | Trademark License Contract between Min Zhao and Sanming Huajian Bio-Engineering Co., Ltd., dated May 25, 2005.* |
| 10.14 | Technology Transfer Agreement between Lin Xuanxian and Sanming Huajian Bio-Engineering Co., Ltd., dated November 16, 2005.* |
| 10.15 | Trademark License Contract between Min Zhao and Sanming Huajian Bio-Engineering Co., Ltd., dated June 19, 2006.* |
| 10.16 | Trademark License Contract between Min Zhao and Sanming Huajian Bio-Engineering Co., Ltd., dated May 1, 2007.* |
| 10.17 | Technology Development Contract between Sanming Huajian Bio-Engineering Co., Ltd. and Fudan University.* |
| 10.18 | Young Corp Compensation and Resettlement Compensation Agreement between Huang Jialong and Sanming Huajian Bio-Engineering Co., Ltd., dated April 28, 2004. |
| 10.19 | Loan Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Agricultural Bank of China, Sanming City, Sanyuan Branch, dated May 16, 2006.*+ |
| 10.20 | Loan Agreement between Sanming Sanming Huajian Bio-Engineering Co., Ltd. and Agricultural Bank of China, Sanming City, Sanyuan Branch, dated June 22, 2006.* |
| 10.21 | Loan Agreement between Sanming Wanxing Economic and Trade Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated December 10, 2006.* |
| 10.22 | Loan Agreement between Jiangle Xinfeng Mining Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated December 10, 2006.* |
| 10.23 | Loan Agreement between Jiangle Wan’an Minerals Operation Department and Sanming Huajian Bio-Engineering Co., Ltd., dated December 31, 2006.* |
| 10.24 | Loan Agreement between Jiangle Xiangkun Mining Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated January 3, 2007.* |
| 10.25 | Loan Agreement between Sasnming Mingdu Hotel Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated January 5, 2007.* |
| 10.26 | Guarantee Contract between Industrial Bank Co., Ltd., Sanming Liedong Branch and Sanming Huajian Bio-Engineering Co., Ltd., dated May 30, 2007.* |
| 10.27 | Guarantee Contract between Industrial Bank Co., Ltd, Sanming Liedong Branch and Sanming Huajian Bio-Engineering Co., Ltd., dated June 12, 2007.*+ |
| 10.28 | Guarantee Contract between Industrial Bank Co., Ltd, Sanming Liedong Branch and Sanming Huajian Bio-Engineering Co., Ltd., dated June 12, 2007.* |
| 10.29 | Guarantee Contract between Industrial Bank Co., Ltd, Sanming Liedong Branch and Sanming Huajian Bio-Engineering Co., Ltd., dated June 21, 2007.* |
| 10.30 | Sale and Purchase Agreement between Wuhan Yanpu Industrial Chemicals Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd.* |
| 10.31 | Sale and Purchase Agreement between Sanming Watts Valve Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated January 11, 2006.* |
| 10.32 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Fujian Yili Boiler Co., Ltd., dated January 23, 2006.* |
| 10.33 | Sale and Purchase Agreement between Changshu Pharmaceutical & Chemical Machinery Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated February 17, 2006.*+ |
| 10.34 | Sale and Purchase agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Shanghai Zhongya Packing Co., Ltd., dated May 18, 2006.* |
| 10.35 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Sanming Sanyuan Reagent Co., Ltd., dated May 30, 2006.* |
| 10.36 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Jiangxi Lufeng Organic Agriculture Technology Development Co., Ltd., dated December 26, 2006.* |
| 10.37 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Qingyuan Sheng Zhi Tang Modern Traditional Chinese Medicine Co., Ltd., dated December 5, 2007.* |
| 10.38 | Sale and Purchase Agreement between Fujian Yi Shunda Import and Export Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated December 18, 2007.* |
| 10.39 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Jiangxi Lufeng Organic Agriculture Technology Development Co., Ltd., dated December 19, 2007.* |
| 10.40 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Sanming Wangnong Agricultural Limited, dated December 28, 2007.* |
| 10.41 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Shanghai Zhaoxiang Biotechnology Co., Ltd., dated January 8, 2008.* |
| 10.42 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Fujian Wuyi Mountain Lukang Biotechnology Co., Ltd., dated January 15, 2008.* |
| 10.43 | Sale and Purchase Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Hangzhou Xneyu Biotechnology Co., Ltd., dated February 26, 2008. |
| 10.44 | Product Purchase Order between Sanming Huajian Bio-Engineering Co., Ltd. and Shaxian Panling Bags Factory, dated May 22, 2006.* |
| 10.45 | Product Purchase Order between Sanming Huajian Bio-Engineering Co., Ltd. and Pucheng Chai Tai Biochemistry Co., Ltd., dated May 27, 2006.* |
| 10.46 | Product Purchase Order between Sanming Huajian Bio-Engineering Co., Ltd. and Sanming Best Starch Industry Co., Ltd., dated May 29, 2006.* |
| 10.47 | Product Purchase Order from Shanghai Zhaoxiang Biotechnology Co., Ltd., dated June 1, 2006.* |
| 10.48 | Product Purchase Order from Qingyuan Sheng Zhi Tang Modern Traditional Chinese Medicines Co., Ltd., dated July 3, 2006.* |
| 10.49 | Product Purchase Order from Fujian Wuyi Mountain Lukang Biotechnology Co., Ltd., dated August 1, 2006.* |
| 10.50 | Product Purchase Order from Jiangxi Lufeng Organic Agriculture Technology Development Co., Ltd., dated September 27, 2006.* |
| 10.51 | Product Purchase Order from Sanming Wanghong Agricultural Limited, dated October 9, 2006.* |
| 10.52 | Product Purchase Order from Hangzhou Xuiyu Biotechnology Co., Ltd., dated March 6, 2008.* |
| 10.53 | Sale and Purchase Agreement of Abandoned Tobacco Waste between Fujian Longyan Jihye Fukao Co., Ltd and Sanming Huajian Bio-Engineering Co., Ltd., dated May 16, 2006.* |
| 10.54 | Sale and Purchase Agreement of Abandoned Tobacco between Fujian Wayi Tobacco Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated May 23, 2006.* |
| 10.55 | Sale and Purchase Agreement of Abandoned Tobacco Waste between Fujian Tobacco Co., Ltd. Nanping Branch and Sanming Huajian Bio-Engineering Co., Ltd., dated June 2, 2006.* |
| 10.56 | Purchase and Sale of Scrap Material Agreement between Sanming Huajian Bio-Engineering Co., Ltd. and Xiamen Jixiang Shishi Industrial Co., Ltd., dated June 8, 2006.* |
| 10.57 | Destruction Agreement of Abandoned Tobacco Waste between Fujian Sanming Jinye Fukao Co., Ltd. and Sanming Huajian Bio-Engineering Co., Ltd., dated June 5, 2006.* |
| 10.58 | Implementation Agreement of the Sales of Tobacco Waste between Sanming Tobacco Monopoly Bureau and Sanming Huajian Bio-Engineering Co., Ltd., dated June 5, 2006.* |
| 10.59 | Amendment No 1 to the Share Exchange Agreement by and among Elevated Throne Overseas Ltd., Green Planet Bioengineering Co. Ltd. and all of the Shareholders of Elevated Throne Overseas Ltd.** |
* Incorporated by reference to the Current Report on Form 8-K filed with the Securities and Exchange Commission on October 29, 2008
** Filed Herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: November 24, 2008 |
| | |
| By: | /s/ Min Zhao |
| | Min Zhao Chief Executive Officer and Chief Financial Officer (Principal Executive Officer and Principal Accounting Officer) |