
A Multi-Center, Randomized, Double-Masked, Vehicle Controlled Phase 3 Study Evaluating the Efficacy and Safety of OTX-DP for the Treatment of Chronic Allergic Conjunctivitis Using a Modified Conjunctival Allergen Challenge Model (Ora-CAC®) Exhibit 99.2

Forward-Looking Statements This presentation contains forward-looking statements about future expectations, plans and prospects for the Company, including statements about the development and regulatory status of the Company’s product candidates, such as the Company’s expectations and plans regarding regulatory submissions for and the timing and conduct of clinical trials of DEXTENZA for post-surgical inflammation and pain, DEXTENZA for allergic conjunctivitis, DEXTENZA for dry eye disease and OTX-TP for glaucoma and ocular hypertension, the ongoing development of the Company’s sustained released hydrogel depot technology, pre-commercial activities, the potential commercialization of DEXTENZA, the potential utility of any of the Company’s product candidates, the advancement of the Company's other product candidates and earlier stage pipeline, future sales of ReSure® Sealant, the sufficiency of the Company’s cash resources and other statements containing the words "anticipate," "believe," "estimate," "expect," "intend", "goal," "may," "might," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Such forward-looking statements involve substantial risks and uncertainties that could cause Ocular Therapeutix’s clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, those related to the timing and costs involved in commercializing ReSure® Sealant and DEXTENZA, the initiation and conduct of clinical trials, availability of data from clinical trials and expectations for regulatory submissions and approvals, the Company’s scientific approach and general development progress, the availability or commercial potential of the Company’s product candidates, the sufficiency of cash resources and need for additional financing or other actions and other factors discussed in the “Risk Factors” section of the Company’s filings with the Securities and Exchange Commission, including the Company’s most recent Quarterly Report on Form 10-Q. In addition, the forward-looking statements included in this presentation represent the Company’s views as of the date of this presentation. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation.
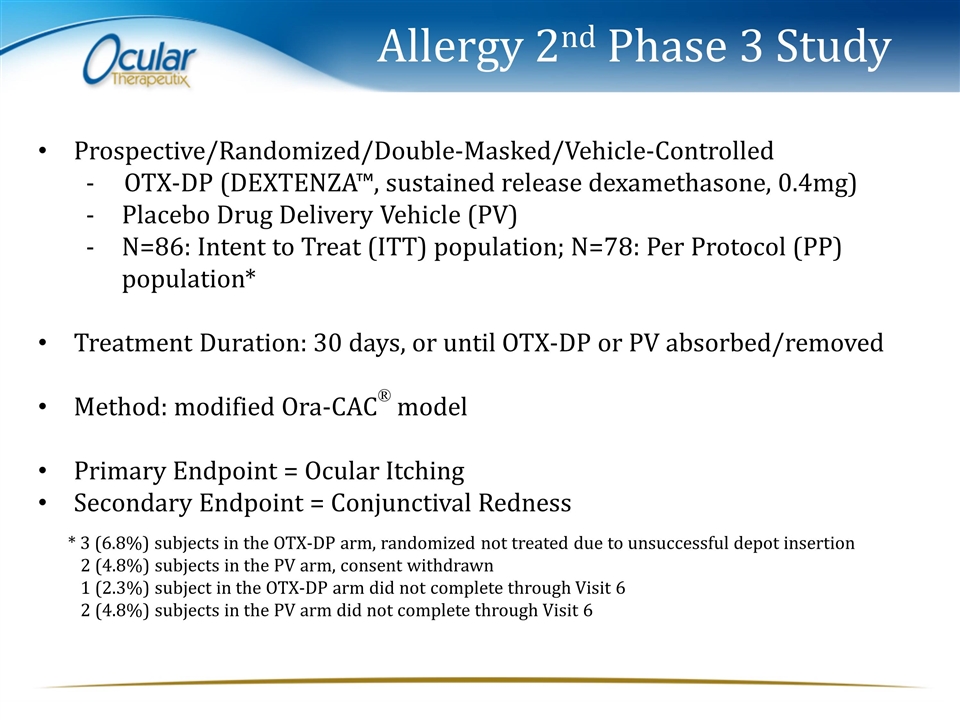
Allergy 2nd Phase 3 Study Prospective/Randomized/Double-Masked/Vehicle-Controlled - OTX-DP (DEXTENZA™, sustained release dexamethasone, 0.4mg) Placebo Drug Delivery Vehicle (PV) N=86: Intent to Treat (ITT) population; N=78: Per Protocol (PP) population* Treatment Duration: 30 days, or until OTX-DP or PV absorbed/removed Method: modified Ora-CAC® model Primary Endpoint = Ocular Itching Secondary Endpoint = Conjunctival Redness * 3 (6.8%) subjects in the OTX-DP arm, randomized not treated due to unsuccessful depot insertion 2 (4.8%) subjects in the PV arm, consent withdrawn 1 (2.3%) subject in the OTX-DP arm did not complete through Visit 6 2 (4.8%) subjects in the PV arm did not complete through Visit 6
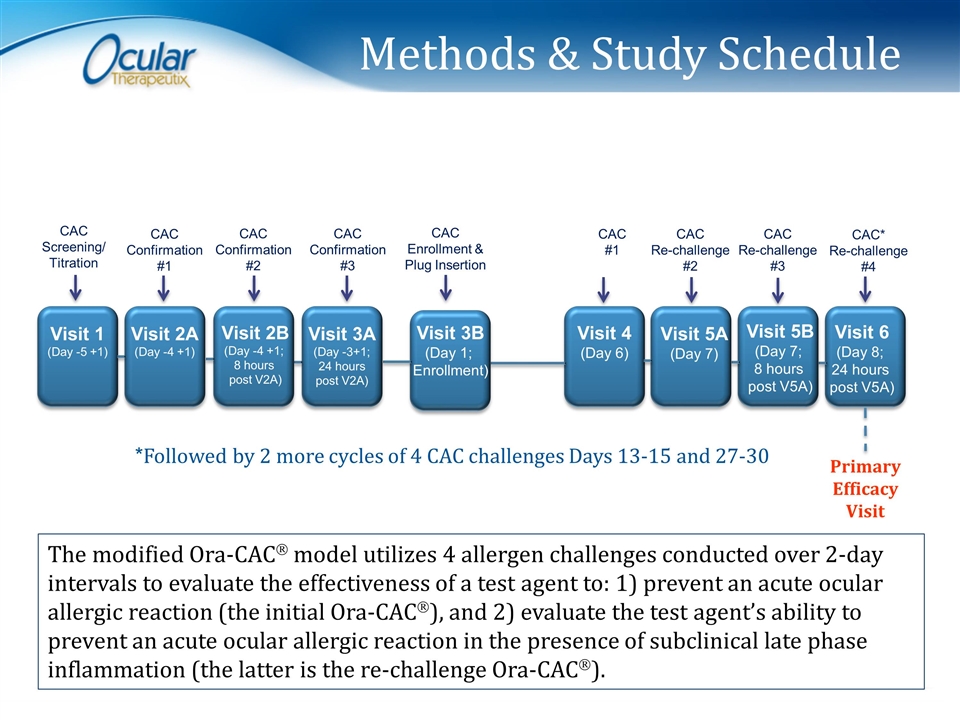
Methods & Study Schedule Visit 1 (Day -5 +1) Visit 2A (Day -4 +1) Visit 2B (Day -4 +1; 8 hours post V2A) Visit 3A (Day -3+1; 24 hours post V2A) Visit 3B (Day 1; Enrollment) Visit 4 (Day 6) Visit 5A (Day 7) Visit 5B (Day 7; 8 hours post V5A) Visit 6 (Day 8; 24 hours post V5A) CAC Confirmation #1 CAC Confirmation #2 CAC Confirmation #3 CAC Enrollment & Plug Insertion CAC #1 CAC Re-challenge #2 CAC Re-challenge #3 CAC* Re-challenge #4 Primary Efficacy Visit CAC Screening/ Titration The modified Ora-CACÒ model utilizes 4 allergen challenges conducted over 2-day intervals to evaluate the effectiveness of a test agent to: 1) prevent an acute ocular allergic reaction (the initial Ora-CACÒ), and 2) evaluate the test agent’s ability to prevent an acute ocular allergic reaction in the presence of subclinical late phase inflammation (the latter is the re-challenge Ora-CACÒ). *Followed by 2 more cycles of 4 CAC challenges Days 13-15 and 27-30
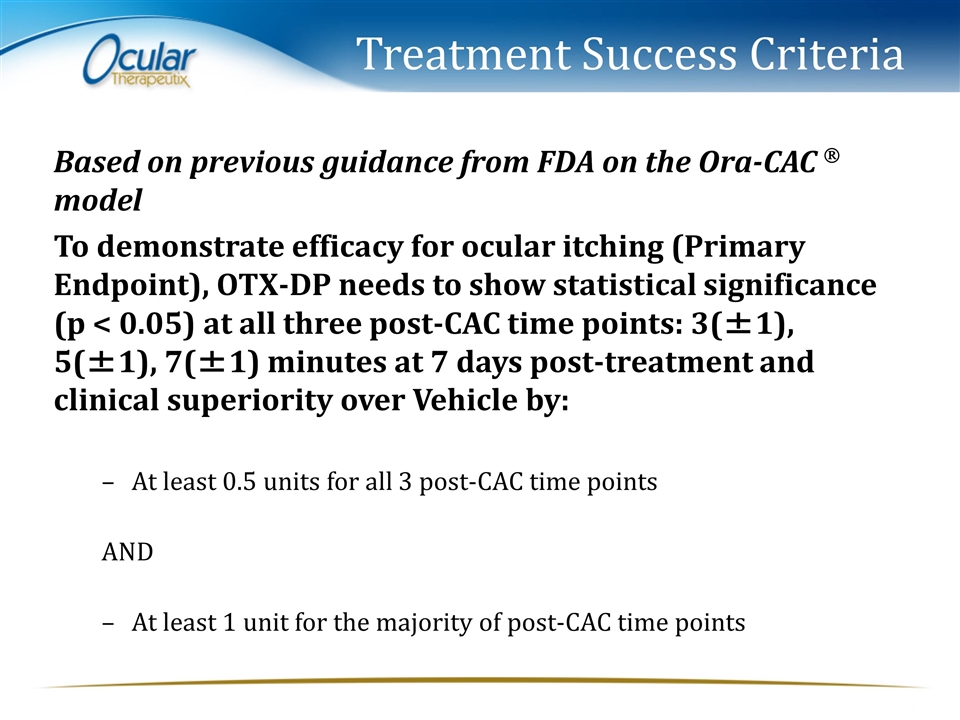
Treatment Success Criteria Based on previous guidance from FDA on the Ora-CAC Ò model To demonstrate efficacy for ocular itching (Primary Endpoint), OTX-DP needs to show statistical significance (p < 0.05) at all three post-CAC time points: 3(±1), 5(±1), 7(±1) minutes at 7 days post-treatment and clinical superiority over Vehicle by: At least 0.5 units for all 3 post-CAC time points AND At least 1 unit for the majority of post-CAC time points
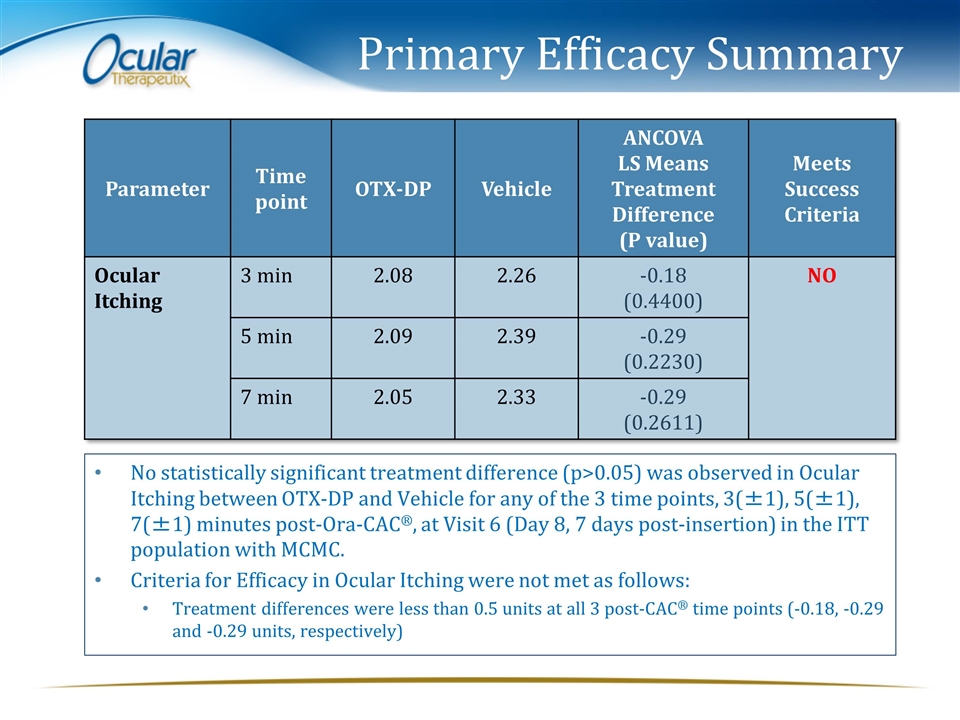
Primary Efficacy Summary Parameter Time point OTX-DP Vehicle ANCOVA LS Means Treatment Difference (P value) Meets Success Criteria Ocular Itching 3 min 2.08 2.26 -0.18 (0.4400) NO 5 min 2.09 2.39 -0.29 (0.2230) 7 min 2.05 2.33 -0.29 (0.2611) No statistically significant treatment difference (p>0.05) was observed in Ocular Itching between OTX-DP and Vehicle for any of the 3 time points, 3(±1), 5(±1), 7(±1) minutes post-Ora-CAC®, at Visit 6 (Day 8, 7 days post-insertion) in the ITT population with MCMC. Criteria for Efficacy in Ocular Itching were not met as follows: Treatment differences were less than 0.5 units at all 3 post-CAC® time points (-0.18, -0.29 and -0.29 units, respectively)
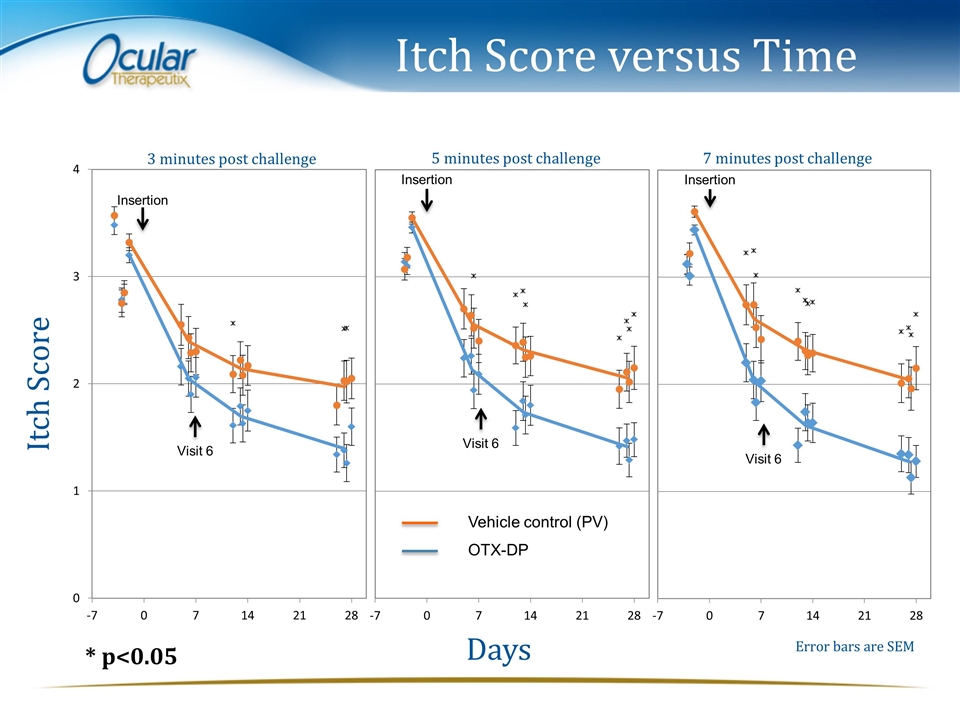
Itch Score versus Time Itch Score Days 3 minutes post challenge 5 minutes post challenge 7 minutes post challenge Error bars are SEM * p<0.05 Visit 6 Visit 6 Visit 6 Vehicle control (PV) OTX-DP Insertion Insertion Insertion
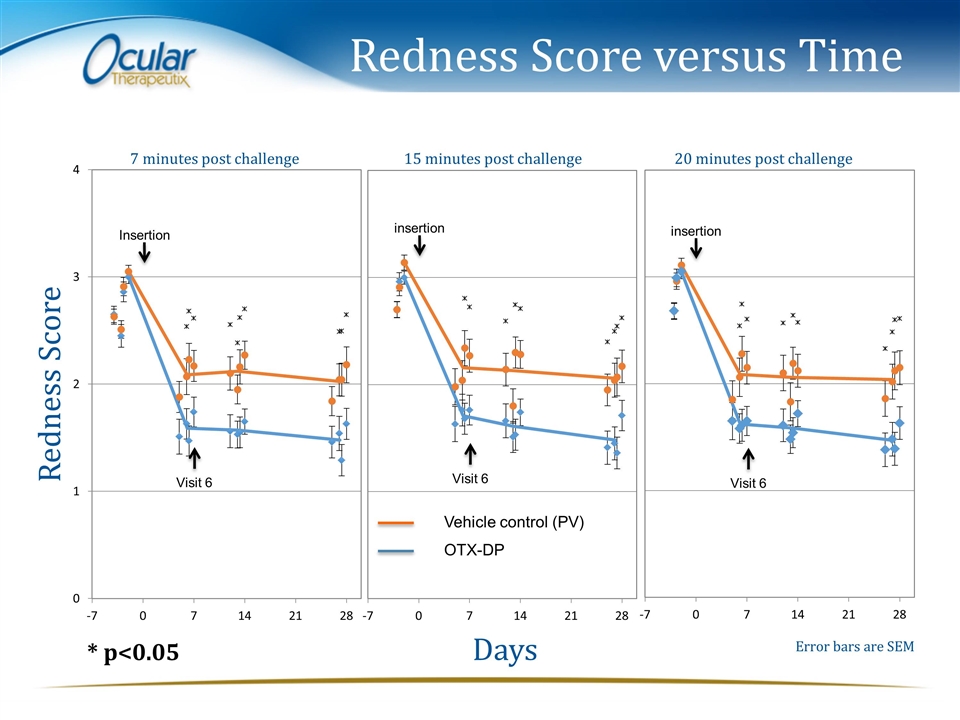
Redness Score versus Time Redness Score Days 7 minutes post challenge 15 minutes post challenge 20 minutes post challenge Error bars are SEM * p<0.05 Visit 6 Visit 6 Visit 6 Vehicle control (PV) OTX-DP insertion insertion Insertion
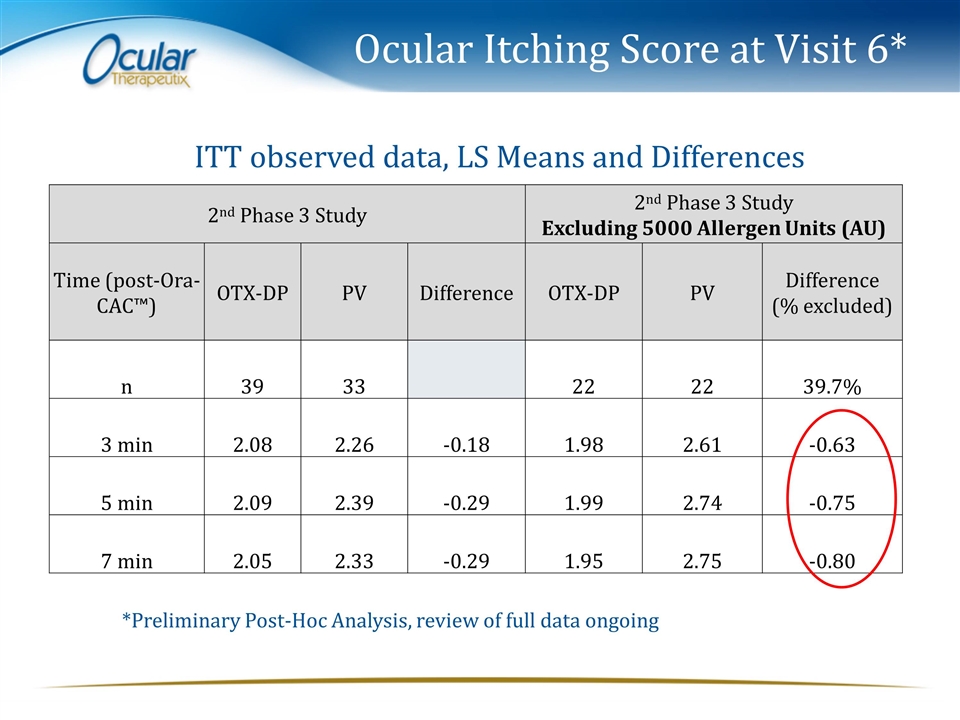
Ocular Itching Score at Visit 6* 2nd Phase 3 Study 2nd Phase 3 Study Excluding 5000 Allergen Units (AU) Time (post-Ora-CAC™) OTX-DP PV Difference OTX-DP PV Difference (% excluded) n 39 33 22 22 39.7% 3 min 2.08 2.26 -0.18 1.98 2.61 -0.63 5 min 2.09 2.39 -0.29 1.99 2.74 -0.75 7 min 2.05 2.33 -0.29 1.95 2.75 -0.80 ITT observed data, LS Means and Differences *Preliminary Post-Hoc Analysis, review of full data ongoing
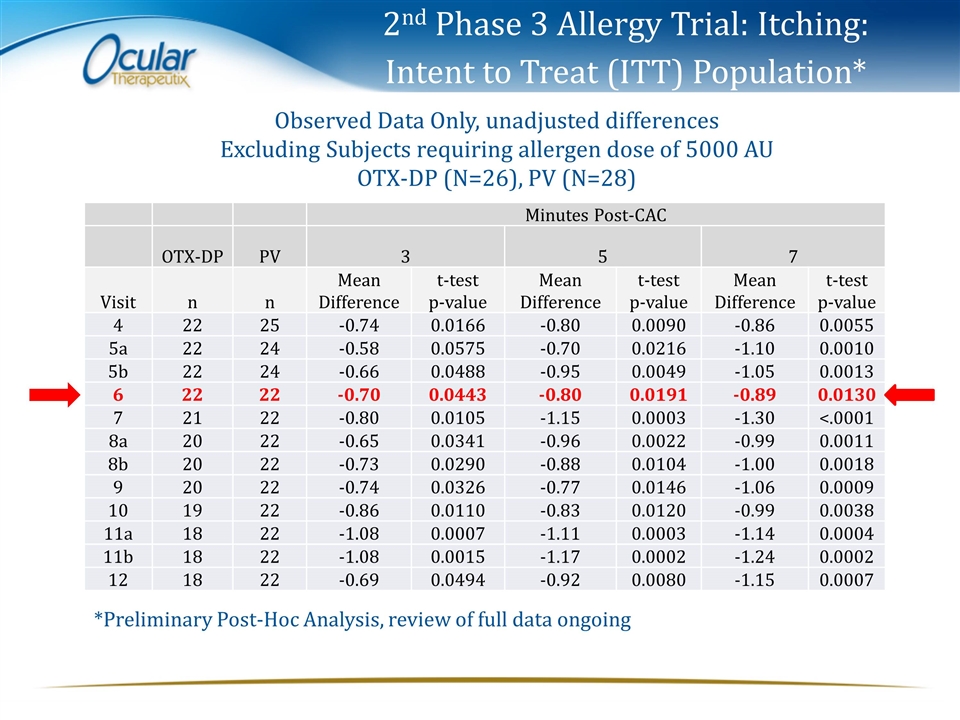
2nd Phase 3 Allergy Trial: Itching: Intent to Treat (ITT) Population* Minutes Post-CAC OTX-DP PV 3 5 7 Visit n n Mean Difference t-test p-value Mean Difference t-test p-value Mean Difference t-test p-value 4 22 25 -0.74 0.0166 -0.80 0.0090 -0.86 0.0055 5a 22 24 -0.58 0.0575 -0.70 0.0216 -1.10 0.0010 5b 22 24 -0.66 0.0488 -0.95 0.0049 -1.05 0.0013 6 22 22 -0.70 0.0443 -0.80 0.0191 -0.89 0.0130 7 21 22 -0.80 0.0105 -1.15 0.0003 -1.30 <.0001 8a 20 22 -0.65 0.0341 -0.96 0.0022 -0.99 0.0011 8b 20 22 -0.73 0.0290 -0.88 0.0104 -1.00 0.0018 9 20 22 -0.74 0.0326 -0.77 0.0146 -1.06 0.0009 10 19 22 -0.86 0.0110 -0.83 0.0120 -0.99 0.0038 11a 18 22 -1.08 0.0007 -1.11 0.0003 -1.14 0.0004 11b 18 22 -1.08 0.0015 -1.17 0.0002 -1.24 0.0002 12 18 22 -0.69 0.0494 -0.92 0.0080 -1.15 0.0007 Observed Data Only, unadjusted differences Excluding Subjects requiring allergen dose of 5000 AU OTX-DP (N=26), PV (N=28) *Preliminary Post-Hoc Analysis, review of full data ongoing
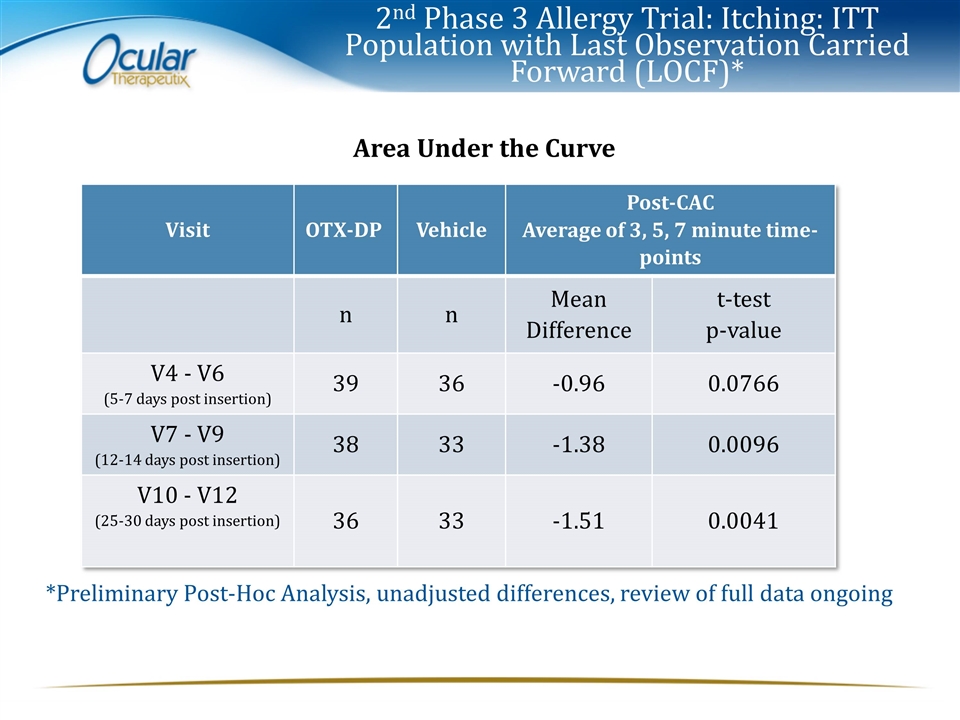
2nd Phase 3 Allergy Trial: Itching: ITT Population with Last Observation Carried Forward (LOCF)* Area Under the Curve Visit OTX-DP Vehicle Post-CAC Average of 3, 5, 7 minute time-points n n Mean Difference t-test p-value V4 - V6 (5-7 days post insertion) 39 36 -0.96 0.0766 V7 - V9 (12-14 days post insertion) 38 33 -1.38 0.0096 V10 - V12 (25-30 days post insertion) 36 33 -1.51 0.0041 *Preliminary Post-Hoc Analysis, unadjusted differences, review of full data ongoing
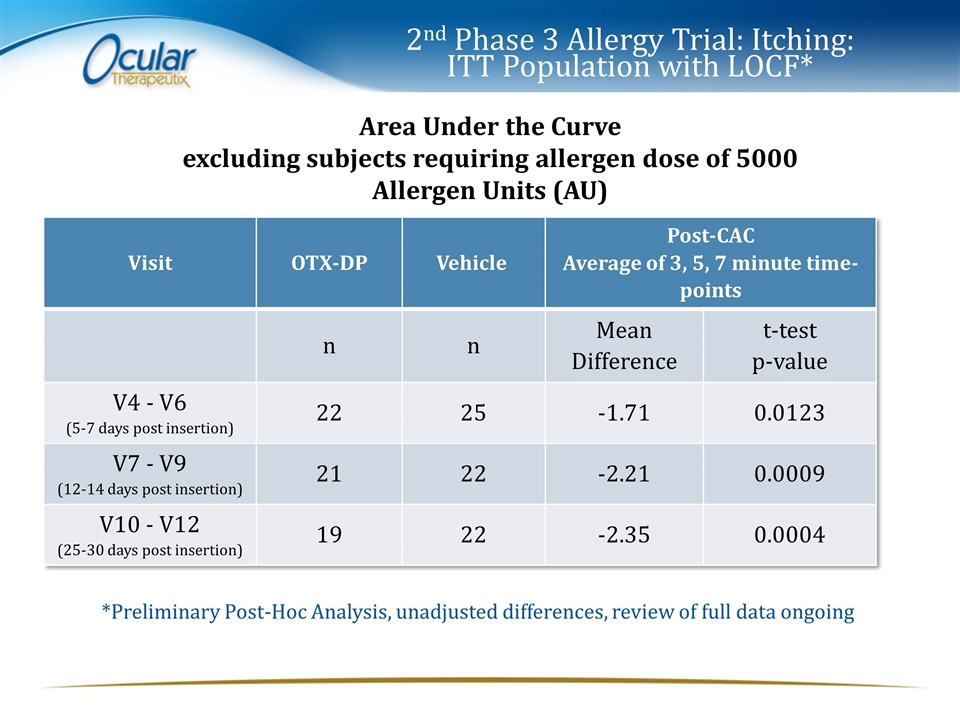
2nd Phase 3 Allergy Trial: Itching: ITT Population with LOCF* Area Under the Curve excluding subjects requiring allergen dose of 5000 Allergen Units (AU) Visit OTX-DP Vehicle Post-CAC Average of 3, 5, 7 minute time-points n n Mean Difference t-test p-value V4 - V6 (5-7 days post insertion) 22 25 -1.71 0.0123 V7 - V9 (12-14 days post insertion) 21 22 -2.21 0.0009 V10 - V12 (25-30 days post insertion) 19 22 -2.35 0.0004 *Preliminary Post-Hoc Analysis, unadjusted differences, review of full data ongoing
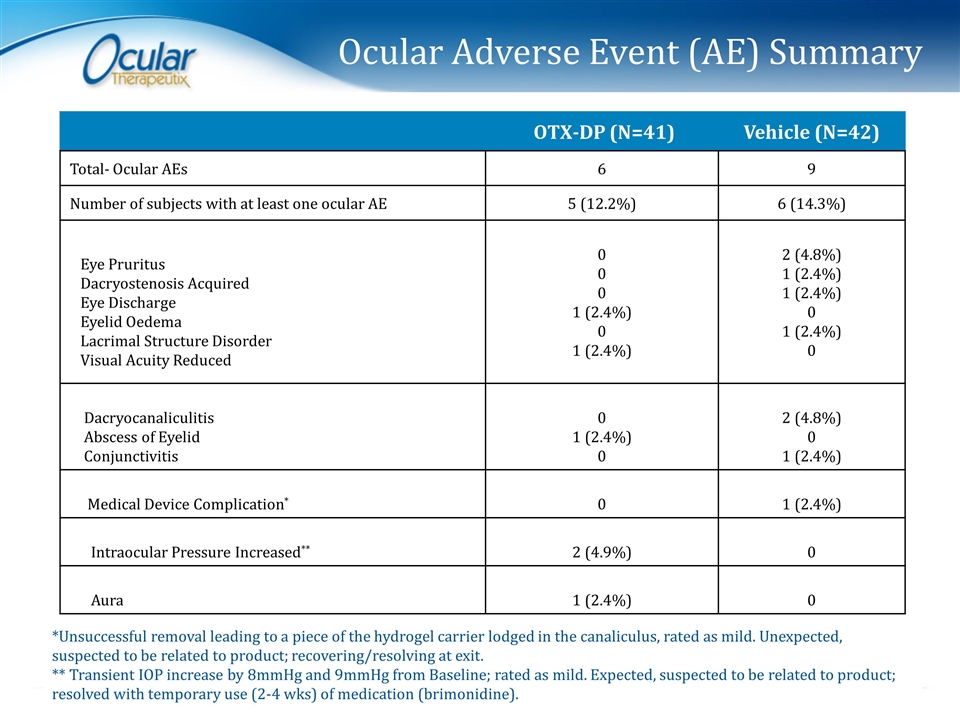
Ocular Adverse Event (AE) Summary OTX-DP (N=41) Vehicle (N=42) Total- Ocular AEs 6 9 Number of subjects with at least one ocular AE 5 (12.2%) 6 (14.3%) Eye Pruritus Dacryostenosis Acquired Eye Discharge Eyelid Oedema Lacrimal Structure Disorder Visual Acuity Reduced 0 0 0 1 (2.4%) 0 1 (2.4%) 2 (4.8%) 1 (2.4%) 1 (2.4%) 0 1 (2.4%) 0 Dacryocanaliculitis Abscess of Eyelid Conjunctivitis 0 1 (2.4%) 0 2 (4.8%) 0 1 (2.4%) Medical Device Complication* 0 1 (2.4%) Intraocular Pressure Increased** 2 (4.9%) 0 Aura 1 (2.4%) 0 *Unsuccessful removal leading to a piece of the hydrogel carrier lodged in the canaliculus, rated as mild. Unexpected, suspected to be related to product; recovering/resolving at exit. ** Transient IOP increase by 8mmHg and 9mmHg from Baseline; rated as mild. Expected, suspected to be related to product; resolved with temporary use (2-4 wks) of medication (brimonidine).
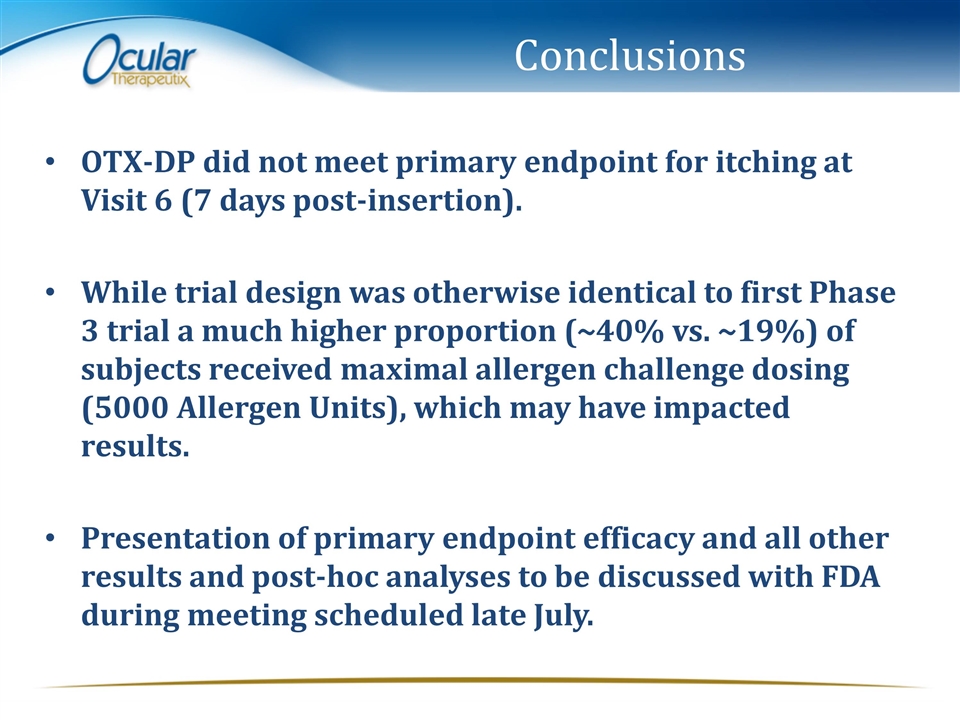
Conclusions OTX-DP did not meet primary endpoint for itching at Visit 6 (7 days post-insertion). While trial design was otherwise identical to first Phase 3 trial a much higher proportion (~40% vs. ~19%) of subjects received maximal allergen challenge dosing (5000 Allergen Units), which may have impacted results. Presentation of primary endpoint efficacy and all other results and post-hoc analyses to be discussed with FDA during meeting scheduled late July.













