
TRACON PHARMACEUTICALS Investor Presentation July 2018 NASDAQ: TCON Exhibit 99.1

This presentation contains statements that are, or may be deemed to be, "forward-looking statements." In some cases these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” "expects,” “plans,” "intends,” “may,” “could,” “might,” “will,” “should,” “approximately,” “potential,” or, in each case, their negatives or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. These statements relate to future events or our future financial performance or condition, business strategy, current and prospective product candidates, planned clinical trials and preclinical activities, product approvals, research and development costs, current and prospective collaborations, timing and likelihood of success of development activities and business strategies, plans and objectives of management for future operations, and future results of anticipated product development efforts, including potential benefits derived therefrom. These statements involve substantial known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, but are not limited to, risks associated with conducting clinical trials, whether any of our product candidates will be shown to be safe and effective, our ability to finance continued operations, our reliance on third parties for various aspects of our business, competition in our target markets, our ability to protect our intellectual property, our ability to execute our business development strategy and in-license rights to additional pipeline assets, and other risks and uncertainties described in our filings with the Securities and Exchange Commission, including under the heading “Risk Factors”. In light of the significant uncertainties in our forward-looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward-looking statements contained in this presentation represent our estimates and assumptions only as of the date of this presentation and, except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this presentation. This presentation also contains estimates, projections and other information concerning our industry, our business, and the markets for our drug candidates, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Forward-Looking Statements
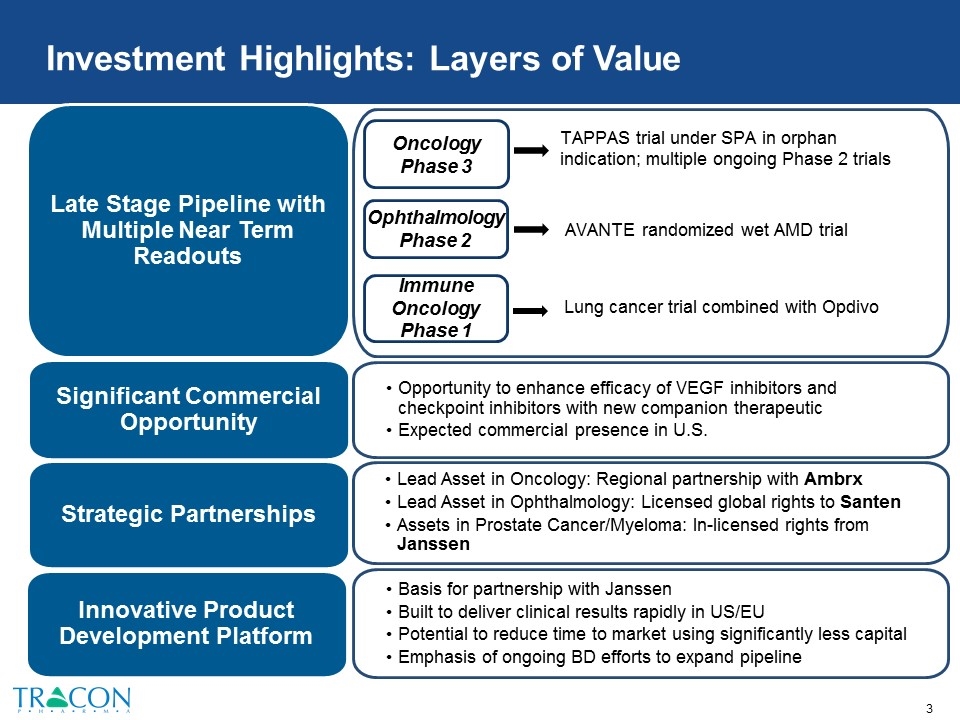
Investment Highlights: Layers of Value Basis for partnership with Janssen Built to deliver clinical results rapidly in US/EU Potential to reduce time to market using significantly less capital Emphasis of ongoing BD efforts to expand pipeline Innovative Product Development Platform Late Stage Pipeline with Multiple Near Term Readouts Opportunity to enhance efficacy of VEGF inhibitors and checkpoint inhibitors with new companion therapeutic Expected commercial presence in U.S. Significant Commercial Opportunity Strategic Partnerships Lead Asset in Oncology: Regional partnership with Ambrx Lead Asset in Ophthalmology: Licensed global rights to Santen Assets in Prostate Cancer/Myeloma: In-licensed rights from Janssen TAPPAS trial under SPA in orphan indication; multiple ongoing Phase 2 trials Oncology Phase 3 Ophthalmology Phase 2 AVANTE randomized wet AMD trial Lung cancer trial combined with Opdivo Immune Oncology Phase 1
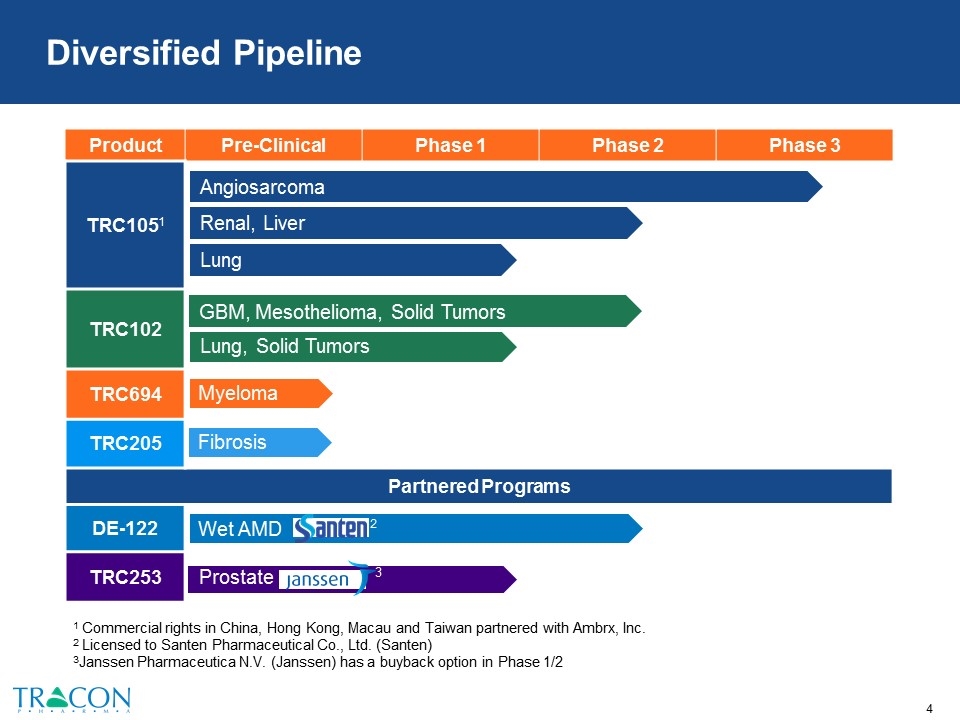
Diversified Pipeline 1 Commercial rights in China, Hong Kong, Macau and Taiwan partnered with Ambrx, Inc. 2 Licensed to Santen Pharmaceutical Co., Ltd. (Santen) 3Janssen Pharmaceutica N.V. (Janssen) has a buyback option in Phase 1/2 Product Pre-Clinical Phase 1 Phase 2 Phase 3 TRC1051 TRC102 TRC694 TRC205 Partnered Programs DE-122 TRC253 Renal, Liver Angiosarcoma Lung GBM, Mesothelioma, Solid Tumors Lung, Solid Tumors Myeloma Fibrosis 3 Prostate Wet AMD 2
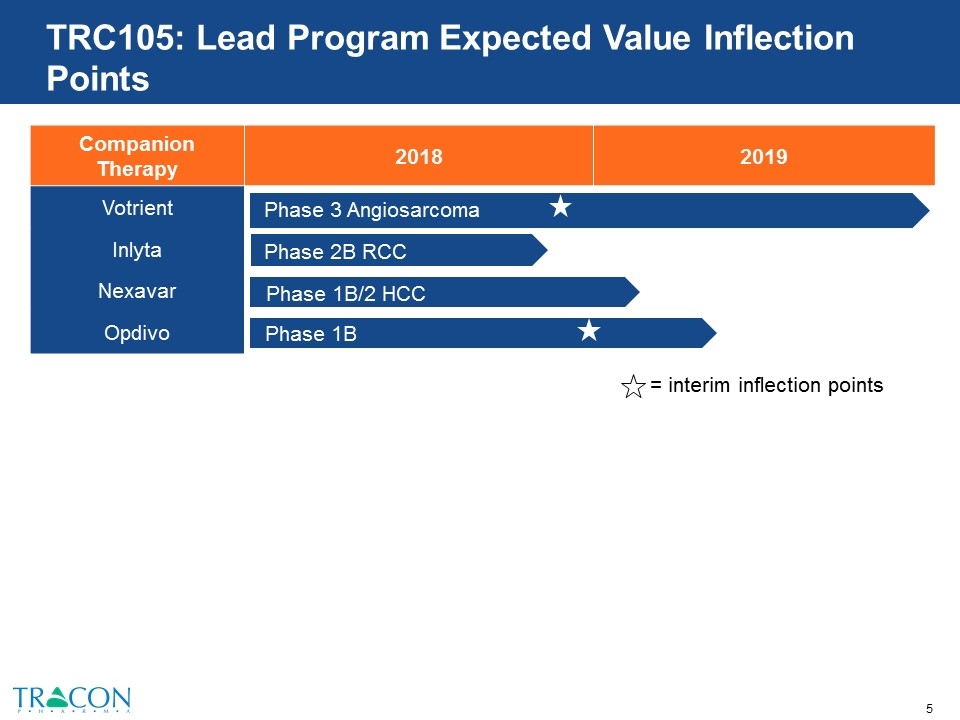
TRC105: Lead Program Expected Value Inflection Points Companion Therapy 2018 2019 Votrient Inlyta Nexavar Opdivo Phase 3 Angiosarcoma Phase 2B RCC Phase 1B/2 HCC Phase 1B NSCLC = interim inflection points
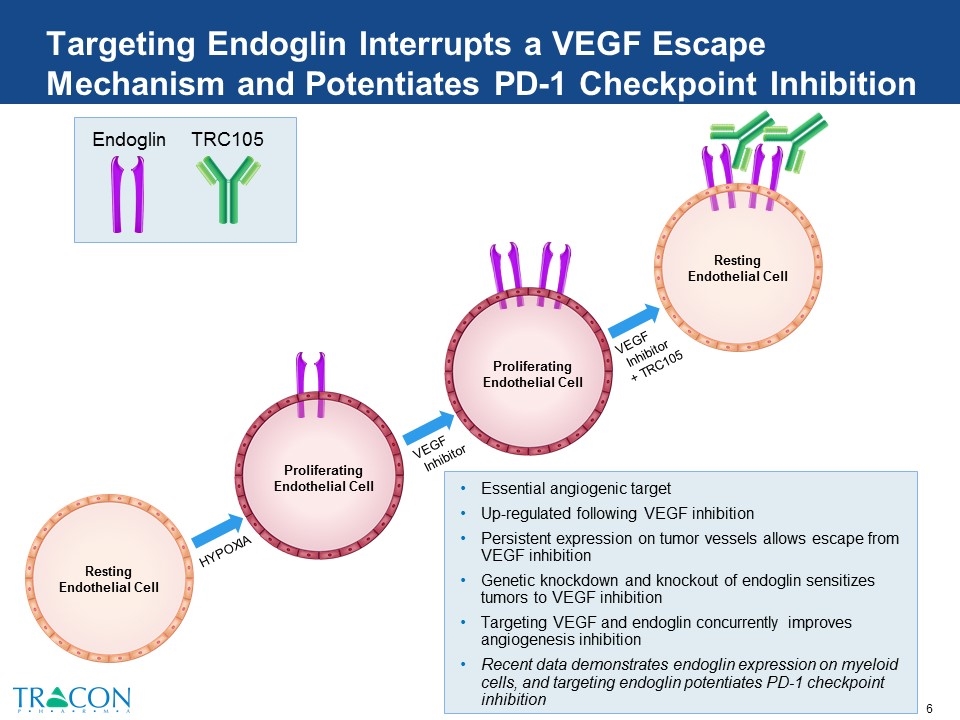
Targeting Endoglin Interrupts a VEGF Escape Mechanism and Potentiates PD-1 Checkpoint Inhibition Resting Endothelial Cell Essential angiogenic target Up-regulated following VEGF inhibition Persistent expression on tumor vessels allows escape from VEGF inhibition Genetic knockdown and knockout of endoglin sensitizes tumors to VEGF inhibition Targeting VEGF and endoglin concurrently improves angiogenesis inhibition Recent data demonstrates endoglin expression on myeloid cells, and targeting endoglin potentiates PD-1 checkpoint inhibition Resting Endothelial Cell TRC105 Endoglin Proliferating Endothelial Cell HYPOXIA VEGF Inhibitor VEGF Inhibitor + TRC105 Proliferating Endothelial Cell
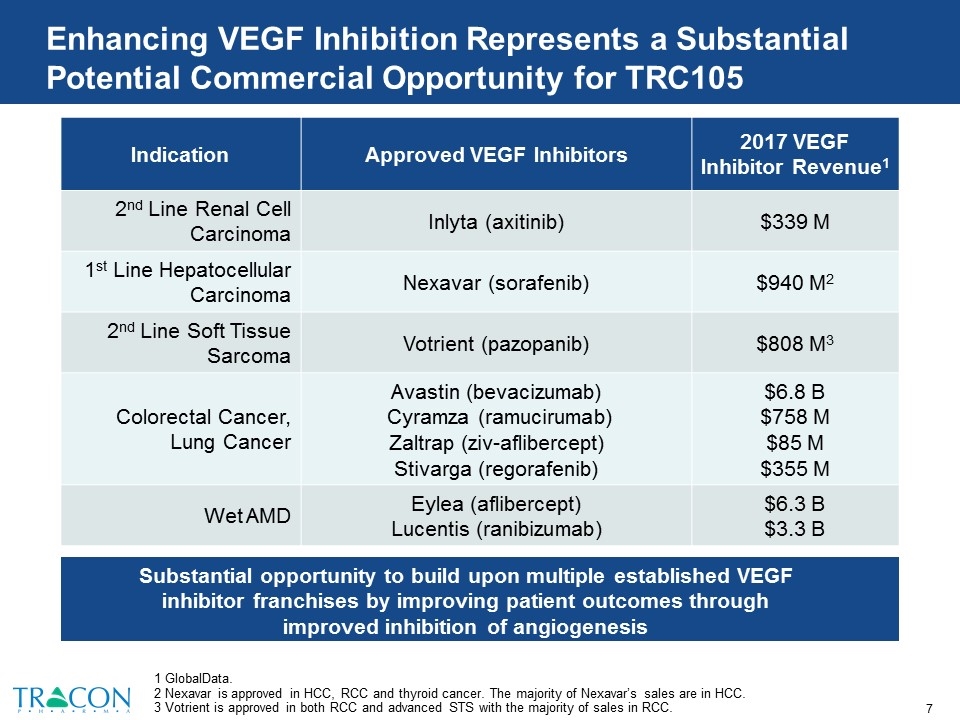
Enhancing VEGF Inhibition Represents a Substantial Potential Commercial Opportunity for TRC105 Substantial opportunity to build upon multiple established VEGF inhibitor franchises by improving patient outcomes through improved inhibition of angiogenesis Indication Approved VEGF Inhibitors 2017 VEGF Inhibitor Revenue1 2nd Line Renal Cell Carcinoma Inlyta (axitinib) $339 M 1st Line Hepatocellular Carcinoma Nexavar (sorafenib) $940 M2 2nd Line Soft Tissue Sarcoma Votrient (pazopanib) $808 M3 Colorectal Cancer, Lung Cancer Avastin (bevacizumab) Cyramza (ramucirumab) Zaltrap (ziv-aflibercept) Stivarga (regorafenib) $6.8 B $758 M $85 M $355 M Wet AMD Eylea (aflibercept) Lucentis (ranibizumab) $6.3 B $3.3 B 1 GlobalData. 2 Nexavar is approved in HCC, RCC and thyroid cancer. The majority of Nexavar’s sales are in HCC. 3 Votrient is approved in both RCC and advanced STS with the majority of sales in RCC.
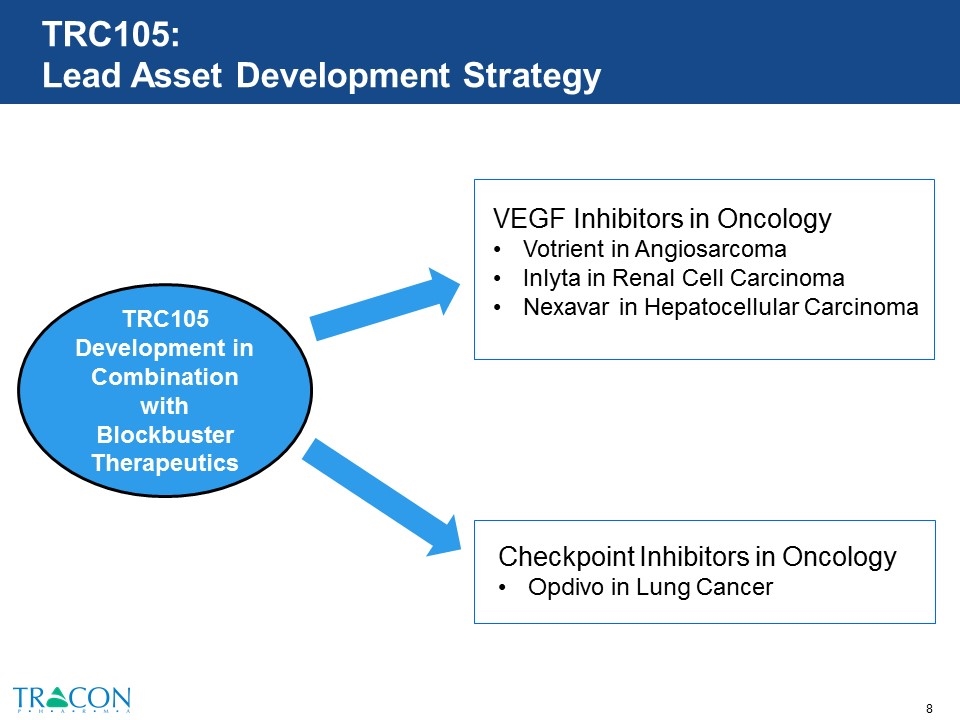
TRC105: Lead Asset Development Strategy TRC105 Development in Combination with Blockbuster Therapeutics VEGF Inhibitors in Oncology Votrient in Angiosarcoma Inlyta in Renal Cell Carcinoma Nexavar in Hepatocellular Carcinoma Checkpoint Inhibitors in Oncology Opdivo in Lung Cancer
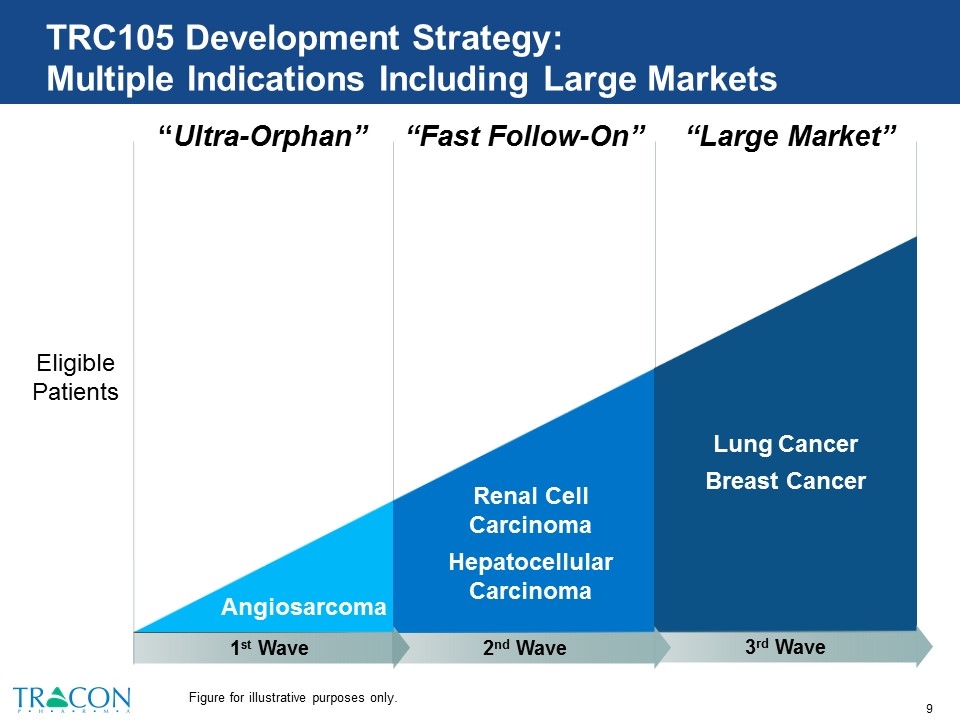
TRC105 Development Strategy: Multiple Indications Including Large Markets “Ultra-Orphan” “Fast Follow-On” “Large Market” Eligible Patients Angiosarcoma Renal Cell Carcinoma Hepatocellular Carcinoma Lung Cancer Breast Cancer 3rd Wave 2nd Wave 1st Wave Figure for illustrative purposes only.
Lead Indication: Angiosarcoma Ultra Orphan indication: ~ 600 cases annually in the US and 1,200 in Europe; greater incidence in Asia1 High Unmet Need: 5-year survival rate < 12% compared to 5-year survival rate of ~ 56% for all soft tissue sarcoma2 Treatment with chemotherapy (taxanes or doxorubicin) in the front line setting is associated with PFS of ~ 5 months and OS < 1 year3 Treatment with VEGF inhibitors in the second line setting is associated with PFS of 1.8 - 3.8 months and OS < 1 year Two subtypes: About 50% of patients present with a primary cutaneous lesion Market size: Estimated at $100M+ in US/EU assuming pricing similar to that of oncology therapeutics approved in other orphan indications4 1Suveillance, Epidemiology, and End Results Program, NCI, www.seer.cancer.gov; RARECARE database, www.rarecare.eu 2www.cancerresearchuk.org 3Penel et al, JCO 2008; Italiano et al, Cancer 2012 4TRACON estimate
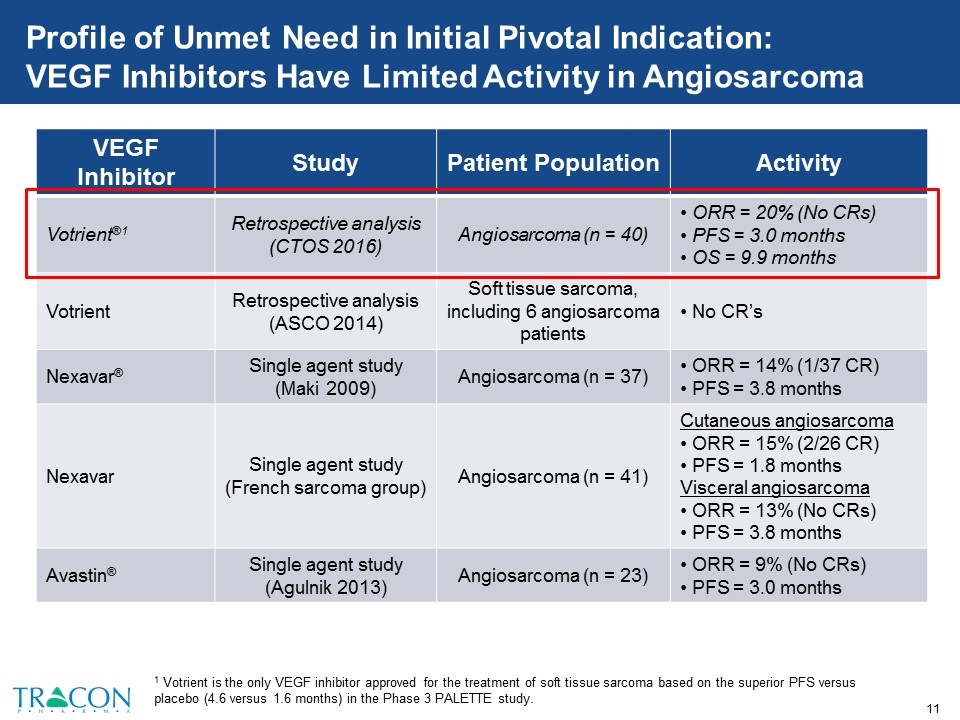
Profile of Unmet Need in Initial Pivotal Indication: VEGF Inhibitors Have Limited Activity in Angiosarcoma VEGF Inhibitor Study Patient Population Activity Votrient®1 Retrospective analysis (CTOS 2016) Angiosarcoma (n = 40) ORR = 20% (No CRs) PFS = 3.0 months OS = 9.9 months Votrient Retrospective analysis (ASCO 2014) Soft tissue sarcoma, including 6 angiosarcoma patients No CR’s Nexavar® Single agent study (Maki 2009) Angiosarcoma (n = 37) ORR = 14% (1/37 CR) PFS = 3.8 months Nexavar Single agent study (French sarcoma group) Angiosarcoma (n = 41) Cutaneous angiosarcoma ORR = 15% (2/26 CR) PFS = 1.8 months Visceral angiosarcoma ORR = 13% (No CRs) PFS = 3.8 months Avastin® Single agent study (Agulnik 2013) Angiosarcoma (n = 23) ORR = 9% (No CRs) PFS = 3.0 months 1 Votrient is the only VEGF inhibitor approved for the treatment of soft tissue sarcoma based on the superior PFS versus placebo (4.6 versus 1.6 months) in the Phase 3 PALETTE study.
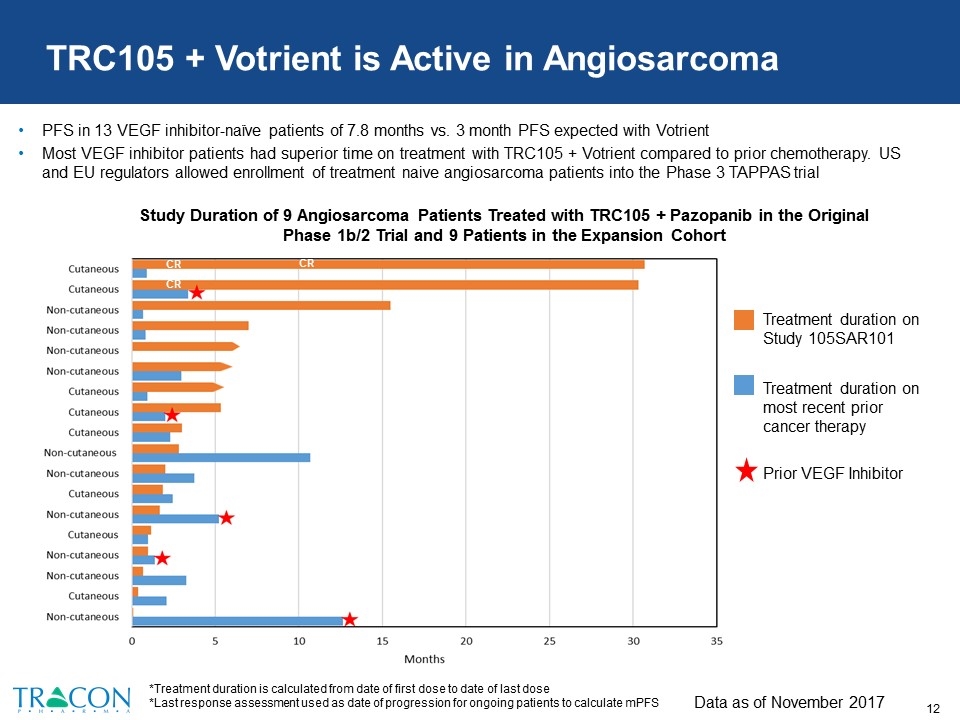
CR CR TRC105 + Votrient is Active in Angiosarcoma PFS in 13 VEGF inhibitor-naïve patients of 7.8 months vs. 3 month PFS expected with Votrient Most VEGF inhibitor patients had superior time on treatment with TRC105 + Votrient compared to prior chemotherapy. US and EU regulators allowed enrollment of treatment naive angiosarcoma patients into the Phase 3 TAPPAS trial Data as of November 2017 CR *Treatment duration is calculated from date of first dose to date of last dose *Last response assessment used as date of progression for ongoing patients to calculate mPFS Study Duration of 9 Angiosarcoma Patients Treated with TRC105 + Pazopanib in the Original Phase 1b/2 Trial and 9 Patients in the Expansion Cohort Treatment duration on Study 105SAR101 Treatment duration on most recent prior cancer therapy Prior VEGF Inhibitor
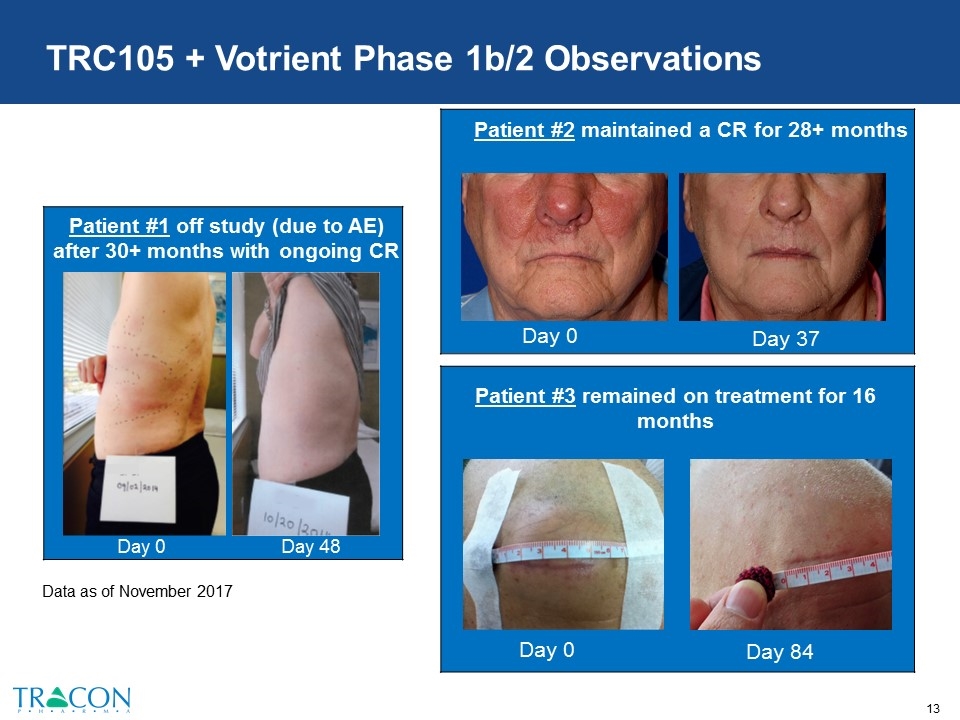
TRC105 + Votrient Phase 1b/2 Observations Complete Response Day 48 Day 0 Day 48 Patient #2 maintained a CR for 28+ months Day 0 Day 37 Patient #1 off study (due to AE) after 30+ months with ongoing CR Patient #3 remained on treatment for 16 months Day 0 Day 84 Data as of November 2017
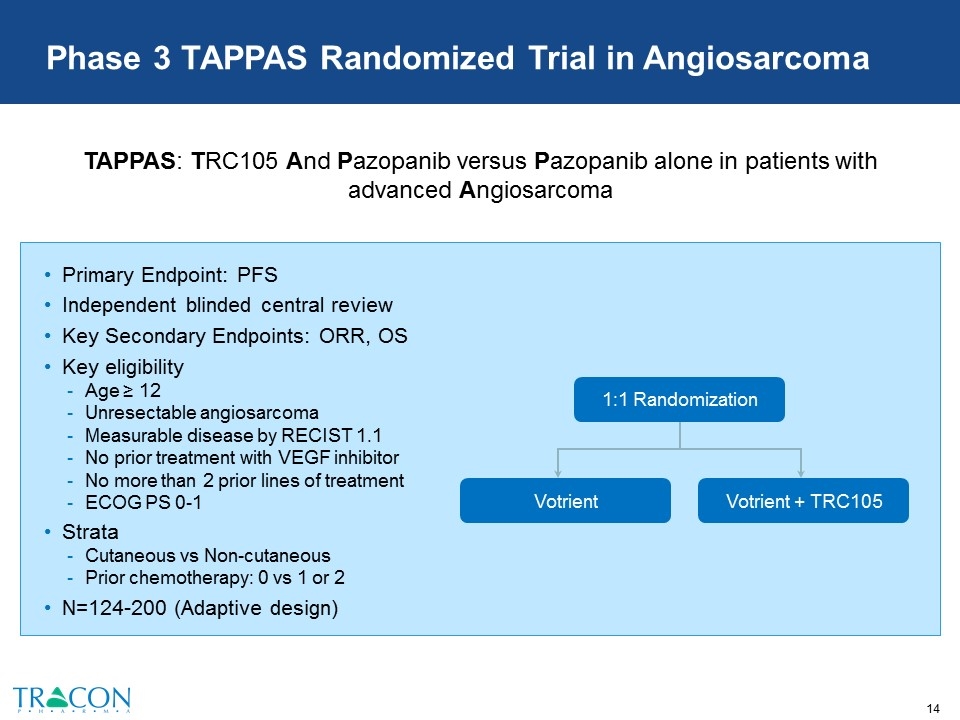
Phase 3 TAPPAS Randomized Trial in Angiosarcoma Primary Endpoint: PFS Independent blinded central review Key Secondary Endpoints: ORR, OS Key eligibility Age ≥ 12 Unresectable angiosarcoma Measurable disease by RECIST 1.1 No prior treatment with VEGF inhibitor No more than 2 prior lines of treatment ECOG PS 0-1 Strata Cutaneous vs Non-cutaneous Prior chemotherapy: 0 vs 1 or 2 N=124-200 (Adaptive design) Votrient + TRC105 Votrient 1:1 Randomization TAPPAS: TRC105 And Pazopanib versus Pazopanib alone in patients with advanced Angiosarcoma
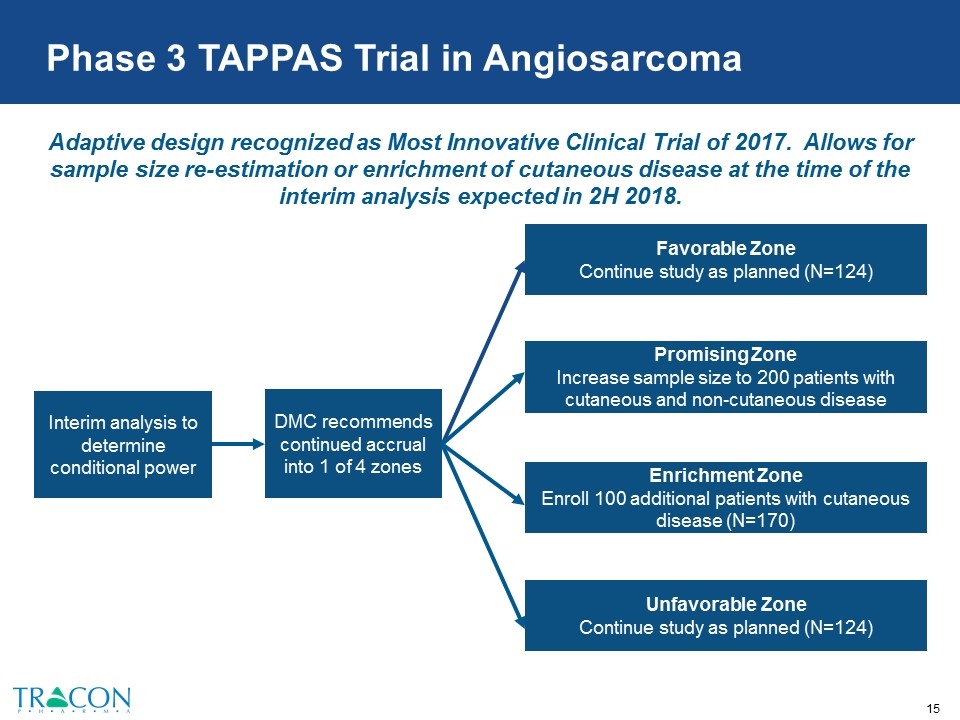
Adaptive design recognized as Most Innovative Clinical Trial of 2017. Allows for sample size re-estimation or enrichment of cutaneous disease at the time of the interim analysis expected in 2H 2018. Interim analysis to determine conditional power DMC recommends continued accrual into 1 of 4 zones Favorable Zone Continue study as planned (N=124) Promising Zone Increase sample size to 200 patients with cutaneous and non-cutaneous disease Enrichment Zone Enroll 100 additional patients with cutaneous disease (N=170) Unfavorable Zone Continue study as planned (N=124) Phase 3 TAPPAS Trial in Angiosarcoma
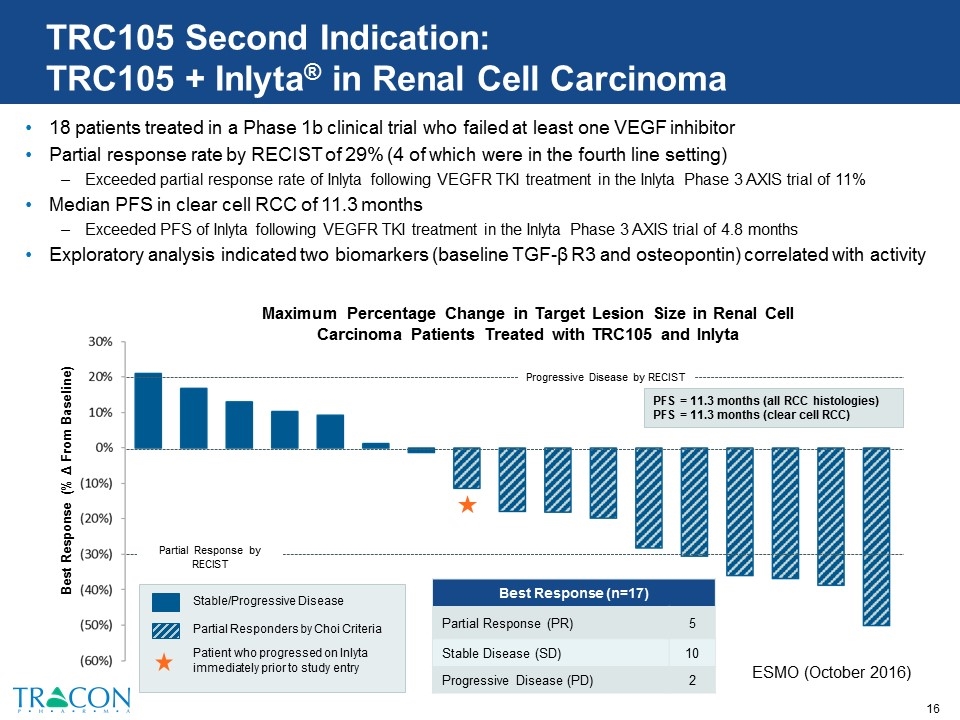
TRC105 Second Indication: TRC105 + Inlyta® in Renal Cell Carcinoma 18 patients treated in a Phase 1b clinical trial who failed at least one VEGF inhibitor Partial response rate by RECIST of 29% (4 of which were in the fourth line setting) Exceeded partial response rate of Inlyta following VEGFR TKI treatment in the Inlyta Phase 3 AXIS trial of 11% Median PFS in clear cell RCC of 11.3 months Exceeded PFS of Inlyta following VEGFR TKI treatment in the Inlyta Phase 3 AXIS trial of 4.8 months Exploratory analysis indicated two biomarkers (baseline TGF-β R3 and osteopontin) correlated with activity Maximum Percentage Change in Target Lesion Size in Renal Cell Carcinoma Patients Treated with TRC105 and Inlyta Best Response (% Δ From Baseline) Stable/Progressive Disease Partial Responders by Choi Criteria Patient who progressed on Inlyta immediately prior to study entry Progressive Disease by RECIST Partial Response by RECIST PFS = 11.3 months (all RCC histologies) PFS = 11.3 months (clear cell RCC) Best Response (n=17) Partial Response (PR) 5 Stable Disease (SD) 10 Progressive Disease (PD) 2 ESMO (October 2016)
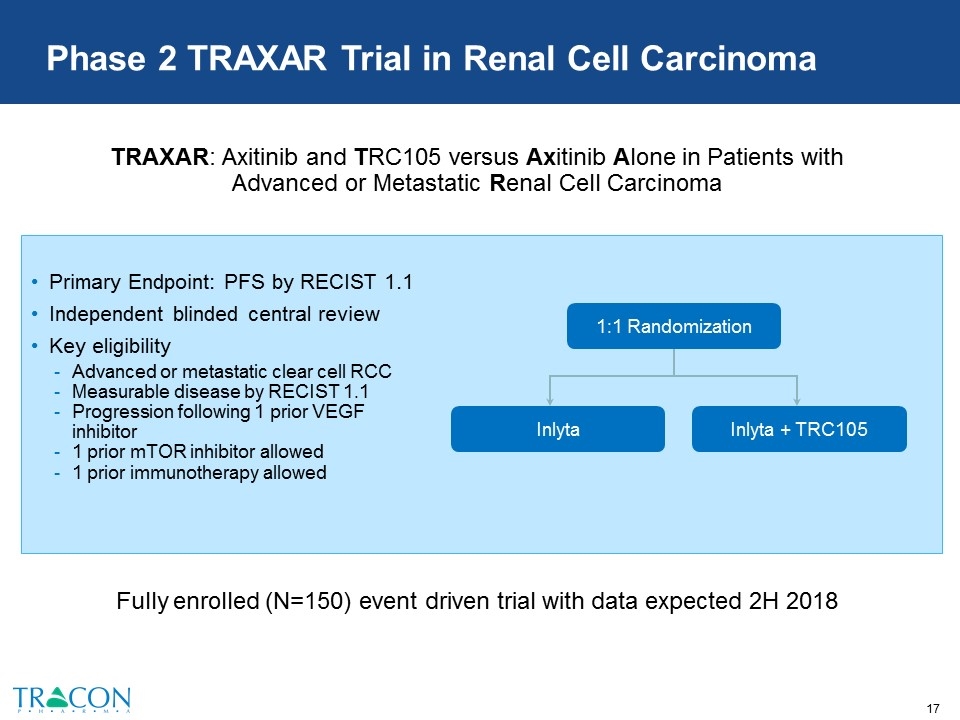
Phase 2 TRAXAR Trial in Renal Cell Carcinoma Primary Endpoint: PFS by RECIST 1.1 Independent blinded central review Key eligibility Advanced or metastatic clear cell RCC Measurable disease by RECIST 1.1 Progression following 1 prior VEGF inhibitor 1 prior mTOR inhibitor allowed 1 prior immunotherapy allowed Inlyta + TRC105 Inlyta 1:1 Randomization Fully enrolled (N=150) event driven trial with data expected 2H 2018 TRAXAR: Axitinib and TRC105 versus Axitinib Alone in Patients with Advanced or Metastatic Renal Cell Carcinoma
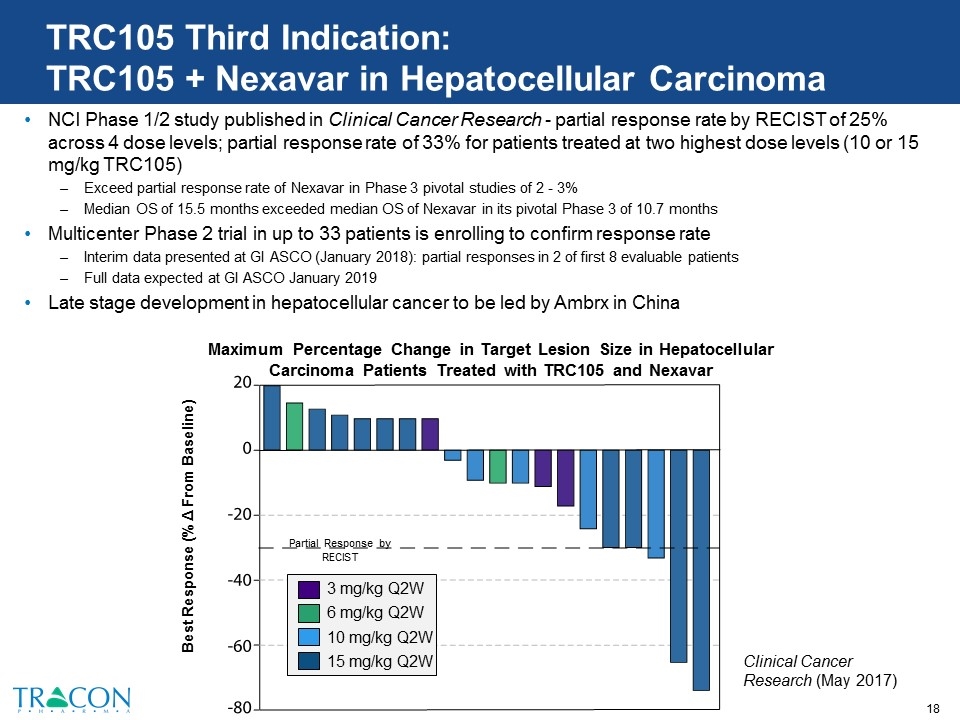
TRC105 Third Indication: TRC105 + Nexavar in Hepatocellular Carcinoma NCI Phase 1/2 study published in Clinical Cancer Research - partial response rate by RECIST of 25% across 4 dose levels; partial response rate of 33% for patients treated at two highest dose levels (10 or 15 mg/kg TRC105) Exceed partial response rate of Nexavar in Phase 3 pivotal studies of 2 - 3% Median OS of 15.5 months exceeded median OS of Nexavar in its pivotal Phase 3 of 10.7 months Multicenter Phase 2 trial in up to 33 patients is enrolling to confirm response rate Interim data presented at GI ASCO (January 2018): partial responses in 2 of first 8 evaluable patients Full data expected at GI ASCO January 2019 Late stage development in hepatocellular cancer to be led by Ambrx in China Maximum Percentage Change in Target Lesion Size in Hepatocellular Carcinoma Patients Treated with TRC105 and Nexavar Partial Response by RECIST Best Response (% Δ From Baseline) Clinical Cancer Research (May 2017) 3 mg/kg Q2W 6 mg/kg Q2W 10 mg/kg Q2W 15 mg/kg Q2W
TRC105 Large Indication: TRC105 + Opdivo® in Lung Cancer Endoglin is a TGF-β co-receptor expressed on fibroblasts and myeloid derived suppressor cells (MDSCs), cell types not addressed by checkpoint inhibition TGF-β signaling implicated as a primary means of tumor immune evasion that complements checkpoint inhibition and tumor mutational burden TRC105 potentiates the activity of PD-1 inhibition in syngeneic mouse tumor models Oral presentations from Leiden University researchers announced at International Microenvironment Cancer Society meeting in June 2018 TRC105 is being studied with Opdivo in second line non-small cell lung cancer in a Phase 1 trial Opdivo single agent response rate in this setting is 20%1 Correlation between response and MDSC tumor content will be assessed 1Opdivo® package insert
Santen License for DE-122 Global ophthalmology company with $1.8 billion in annual revenue leads global development and commercialization for DE-122 (ophthalmic formulation of TRC105) in wet AMD and other eye diseases Deal terms $20 million received thus far Santen pays all development costs Up to $145 million in additional milestone payments Royalties in the high single digits to low teens Failed Phase 2 and 3 studies from Ophthotech and Regeneron place DE-122 in lead for companion drug to build on $9B VEGF inhibitor market in wet AMD. Regulatory path is well defined. Companion Therapy 2018 2019 Lucentis Phase 2 Wet AMD
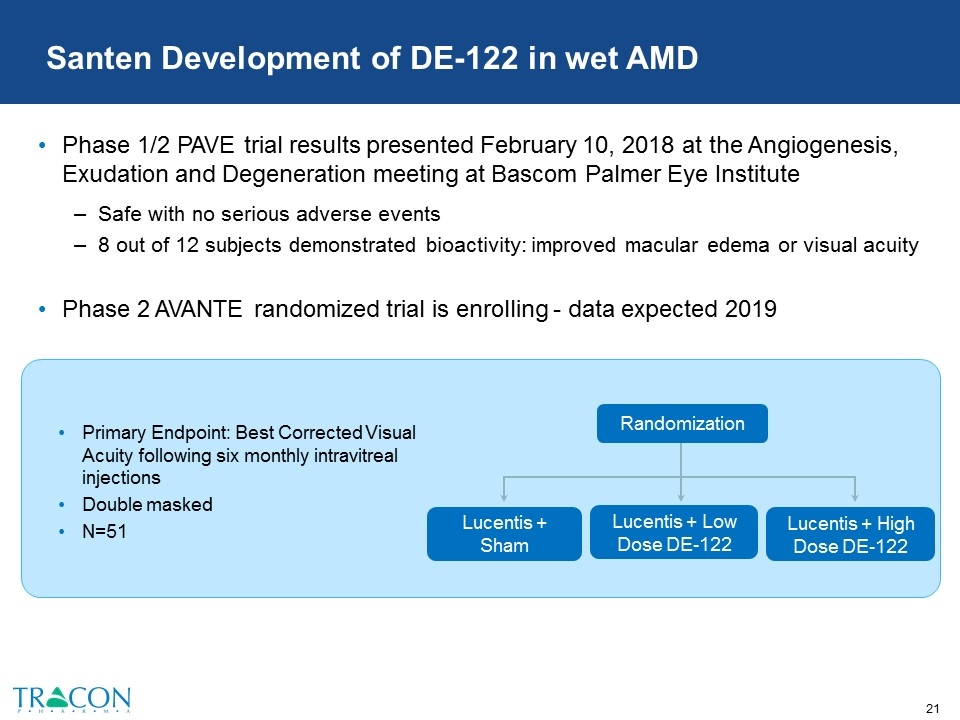
Santen Development of DE-122 in wet AMD Phase 1/2 PAVE trial results presented February 10, 2018 at the Angiogenesis, Exudation and Degeneration meeting at Bascom Palmer Eye Institute Safe with no serious adverse events 8 out of 12 subjects demonstrated bioactivity: improved macular edema or visual acuity Phase 2 AVANTE randomized trial is enrolling - data expected 2019 Primary Endpoint: Best Corrected Visual Acuity following six monthly intravitreal injections Double masked N=51 Lucentis + Low Dose DE-122 Lucentis + Sham Randomization Lucentis + High Dose DE-122
TRC102: Expected Value Inflection Points Companion Therapy 2018 2019 Alimta Temodar Temodar 1 Phase 1B/2 HCC Phase 1B NSCLC Phase 2 Mesothelioma Phase 2 GBM Phase 1B/2 Multiple Solid Tumors Phase 2 wet AMD 3 Prostate Small molecule designed to reverse resistance to chemotherapy and complement PARP inhibitors Inhibits base excision repair, a dominant pathway of DNA repair that allows for resistance to alkylating chemotherapy (e.g., Temodar®) and antimetabolite chemotherapy (e.g., Alimta®) Current clinical development funded by National Cancer Institute
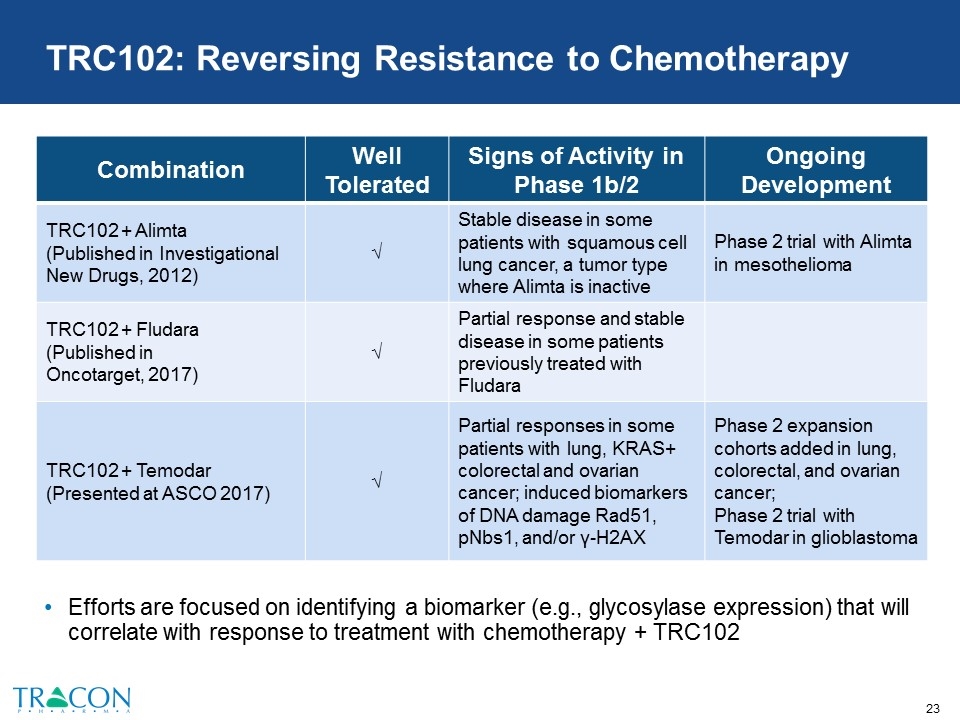
Efforts are focused on identifying a biomarker (e.g., glycosylase expression) that will correlate with response to treatment with chemotherapy + TRC102 TRC102: Reversing Resistance to Chemotherapy Combination Well Tolerated Signs of Activity in Phase 1b/2 Ongoing Development TRC102 + Alimta (Published in Investigational New Drugs, 2012) √ Stable disease in some patients with squamous cell lung cancer, a tumor type where Alimta is inactive Phase 2 trial with Alimta in mesothelioma TRC102 + Fludara (Published in Oncotarget, 2017) √ Partial response and stable disease in some patients previously treated with Fludara TRC102 + Temodar (Presented at ASCO 2017) √ Partial responses in some patients with lung, KRAS+ colorectal and ovarian cancer; induced biomarkers of DNA damage Rad51, pNbs1, and/or γ-H2AX Phase 2 expansion cohorts added in lung, colorectal, and ovarian cancer; Phase 2 trial with Temodar in glioblastoma
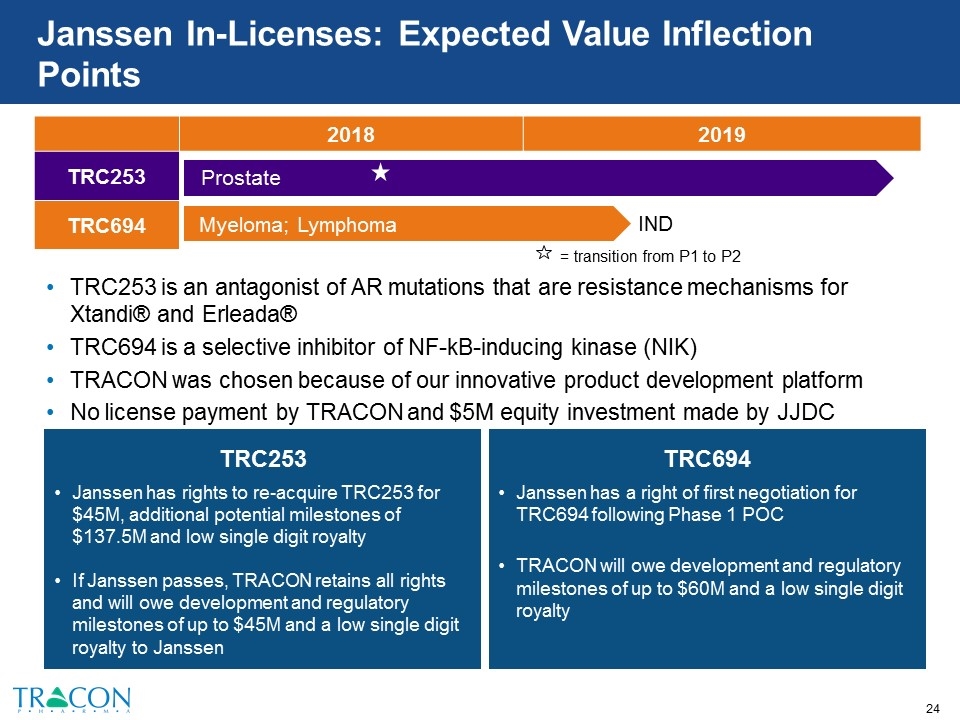
Janssen In-Licenses: Expected Value Inflection Points TRC253 is an antagonist of AR mutations that are resistance mechanisms for Xtandi® and Erleada® TRC694 is a selective inhibitor of NF-kB-inducing kinase (NIK) TRACON was chosen because of our innovative product development platform No license payment by TRACON and $5M equity investment made by JJDC TRC253 Janssen has rights to re-acquire TRC253 for $45M, additional potential milestones of $137.5M and low single digit royalty If Janssen passes, TRACON retains all rights and will owe development and regulatory milestones of up to $45M and a low single digit royalty to Janssen TRC694 Janssen has a right of first negotiation for TRC694 following Phase 1 POC TRACON will owe development and regulatory milestones of up to $60M and a low single digit royalty 2018 2019 TRC253 TRC694 Myeloma; Lymphoma IND Prostate = transition from P1 to P2
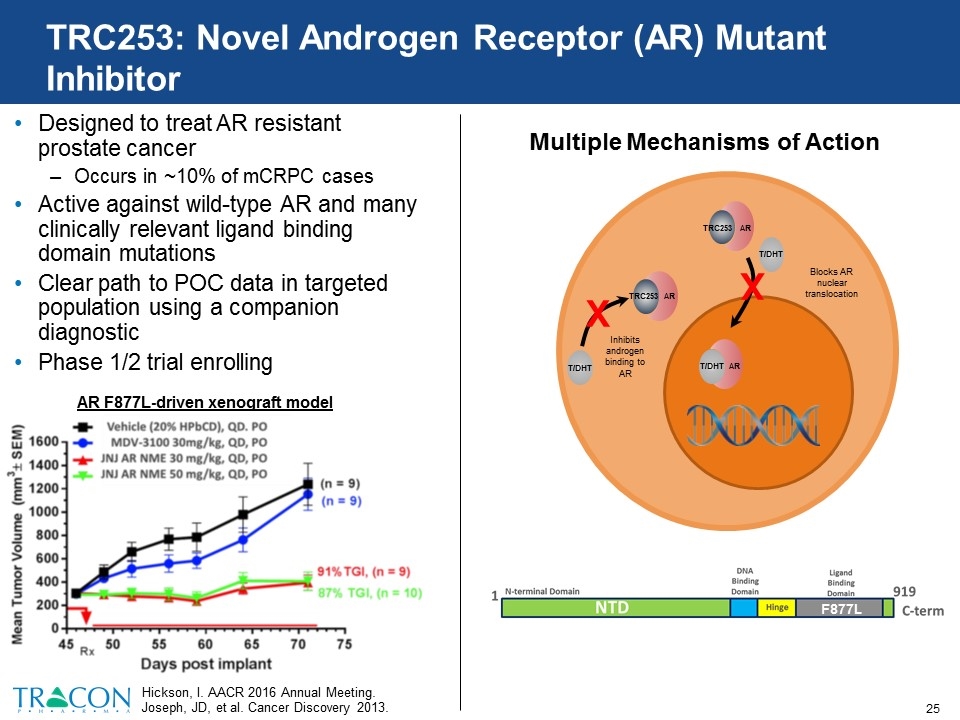
TRC253: Novel Androgen Receptor (AR) Mutant Inhibitor Designed to treat AR resistant prostate cancer Occurs in ~10% of mCRPC cases Active against wild-type AR and many clinically relevant ligand binding domain mutations Clear path to POC data in targeted population using a companion diagnostic Phase 1/2 trial enrolling Hickson, I. AACR 2016 Annual Meeting. Joseph, JD, et al. Cancer Discovery 2013. AR F877L-driven xenograft model Multiple Mechanisms of Action F877L T/DHT X Inhibits androgen binding to AR AR TRC253 AR TRC253 X Blocks AR nuclear translocation AR T/DHT T/DHT
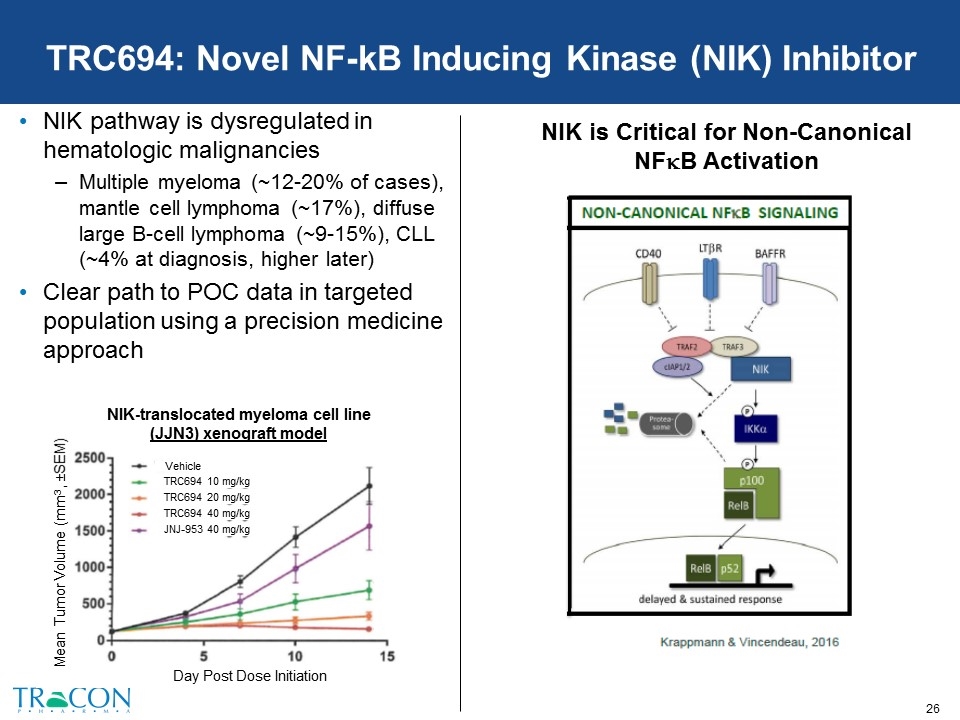
TRC694: Novel NF-kB Inducing Kinase (NIK) Inhibitor NIK pathway is dysregulated in hematologic malignancies Multiple myeloma (~12-20% of cases), mantle cell lymphoma (~17%), diffuse large B-cell lymphoma (~9-15%), CLL (~4% at diagnosis, higher later) Clear path to POC data in targeted population using a precision medicine approach NIK is Critical for Non-Canonical NFkB Activation Vehicle TRC694 10 mg/kg TRC694 20 mg/kg TRC694 40 mg/kg JNJ-953 40 mg/kg Day Post Dose Initiation Mean Tumor Volume (mm3, ±SEM) NIK-translocated myeloma cell line (JJN3) xenograft model
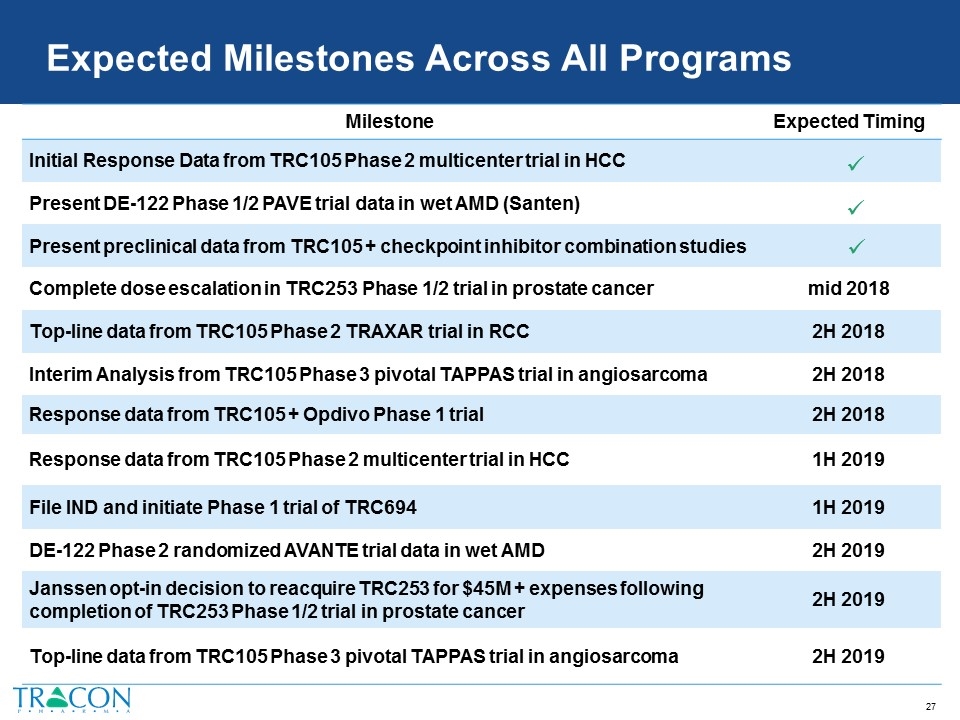
Expected Milestones Across All Programs Milestone Expected Timing Initial Response Data from TRC105 Phase 2 multicenter trial in HCC Present DE-122 Phase 1/2 PAVE trial data in wet AMD (Santen) Present preclinical data from TRC105 + checkpoint inhibitor combination studies Complete dose escalation in TRC253 Phase 1/2 trial in prostate cancer mid 2018 Top-line data from TRC105 Phase 2 TRAXAR trial in RCC 2H 2018 Interim Analysis from TRC105 Phase 3 pivotal TAPPAS trial in angiosarcoma 2H 2018 Response data from TRC105 + Opdivo Phase 1 trial 2H 2018 Response data from TRC105 Phase 2 multicenter trial in HCC 1H 2019 File IND and initiate Phase 1 trial of TRC694 1H 2019 DE-122 Phase 2 randomized AVANTE trial data in wet AMD 2H 2019 Janssen opt-in decision to reacquire TRC253 for $45M + expenses following completion of TRC253 Phase 1/2 trial in prostate cancer 2H 2019 Top-line data from TRC105 Phase 3 pivotal TAPPAS trial in angiosarcoma 2H 2019
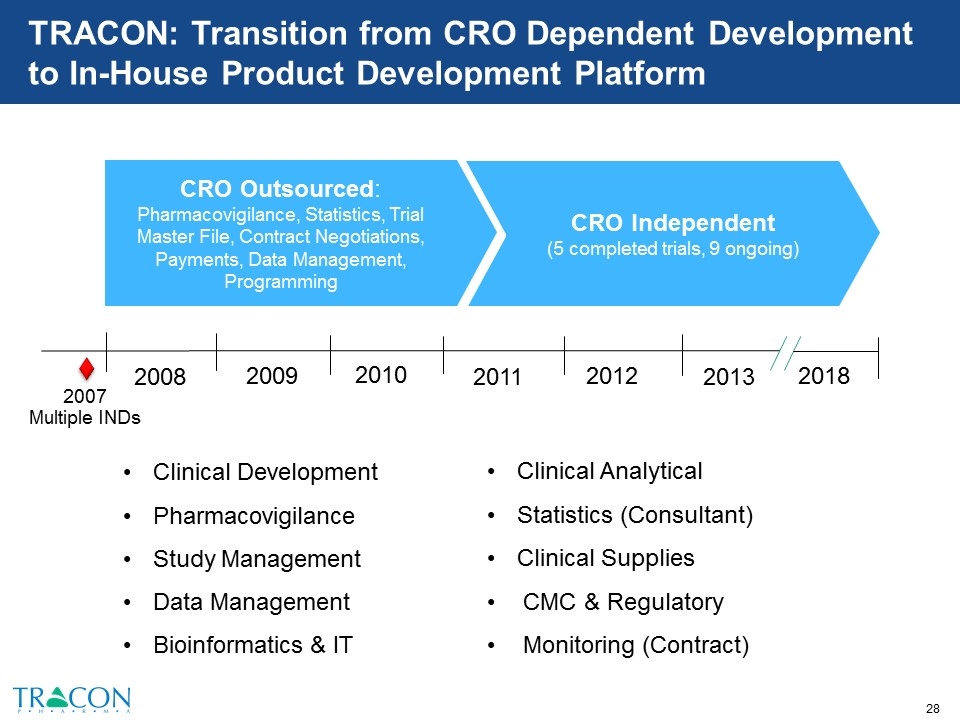
TRACON: Transition from CRO Dependent Development to In-House Product Development Platform CRO Independent (5 completed trials, 9 ongoing) CRO Outsourced: Pharmacovigilance, Statistics, Trial Master File, Contract Negotiations, Payments, Data Management, Programming 2008 2009 2010 2011 2012 2013 2018 2007 Multiple INDs Clinical Development Pharmacovigilance Study Management Data Management Bioinformatics & IT Clinical Analytical Statistics (Consultant) Clinical Supplies CMC & Regulatory Monitoring (Contract)

Expand Pipeline through Innovative Product Development Platform Partnerships: Trust it to TRACON Leverage a team of industry experts with previous leadership roles in project teams that contributed to multiple product approvals. In-house development platform built to deliver clinical results rapidly and reduce time to market, while using significantly less capital than competitors or CROs. Platform can develop first-in-class, best-in-class or fast-follower oncology and other physician specialist treated products. Rapid development and open communication results in an efficient and effective culture of collaboration.
Expand Pipeline through Innovative Product Development Platform (cont.) Cost, risk and profit share of partnered assets produces goal alignment FDA regulatory filings up to and including NDA/BLA can be leveraged for regulatory filings in all major territories Commercial presence in U.S. expected in 2021 (through TRC105 approval) to preserve maximum value of product between corporate partners Collaboration with Janssen, including equity investment from JJDC on TRC253/TRC694, validates TRACON’s product development platform

Business Development Strategy Leverage our Innovative Product Development Platform in New Corporate Partnerships to access promising specialty product assets Transactions similar to the Janssen transaction where TRACON licenses asset(s) for no license fee and develops asset(s) to certain value inflection points in return for substantial economics and/or downstream commercial rights For companies with little or no development infrastructure in the US, conduct clinical trials in exchange for substantial economics and/or product rights in the US
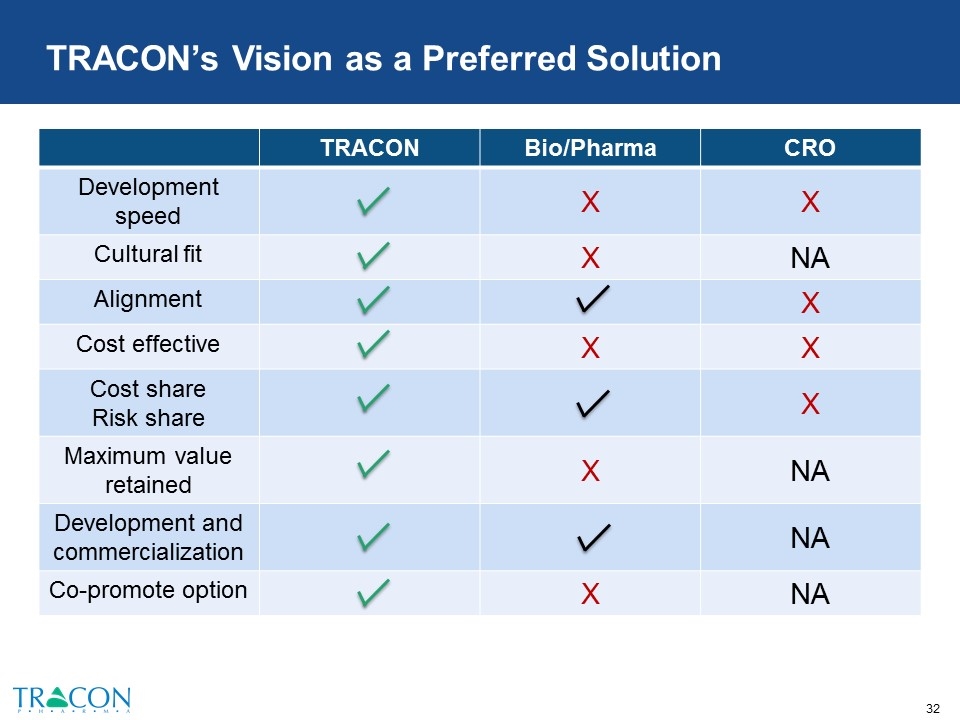
TRACON’s Vision as a Preferred Solution TRACON Bio/Pharma CRO Development speed X X Cultural fit X NA Alignment X Cost effective X X Cost share Risk share X Maximum value retained X NA Development and commercialization NA Co-promote option X NA
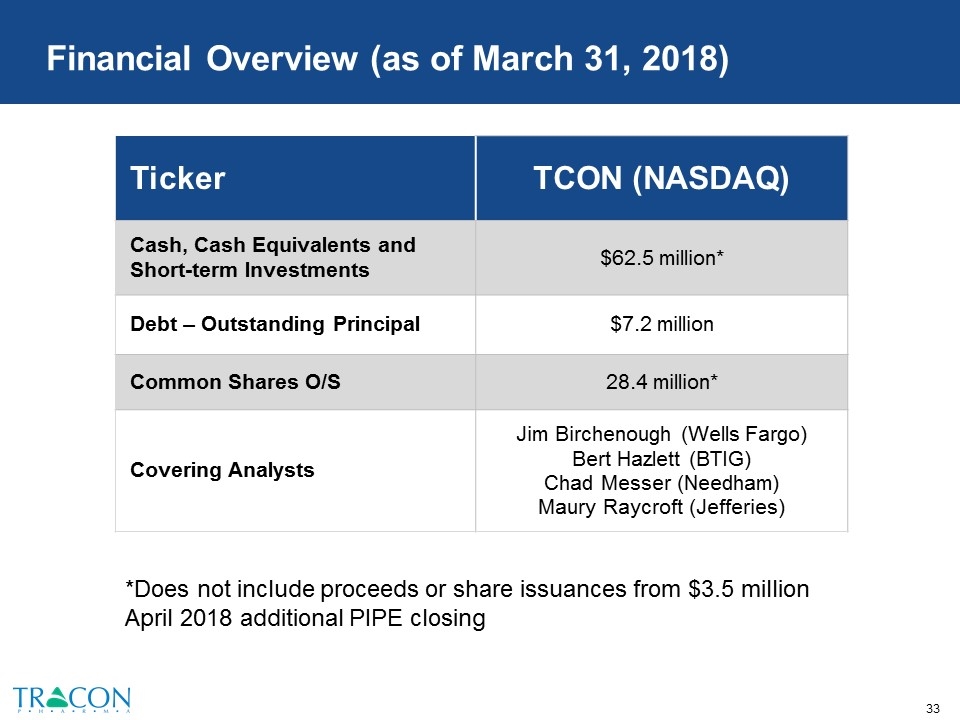
Financial Overview (as of March 31, 2018) Ticker TCON (NASDAQ) Cash, Cash Equivalents and Short-term Investments $62.5 million* Debt – Outstanding Principal $7.2 million Common Shares O/S 28.4 million* Covering Analysts Jim Birchenough (Wells Fargo) Bert Hazlett (BTIG) Chad Messer (Needham) Maury Raycroft (Jefferies) *Does not include proceeds or share issuances from $3.5 million April 2018 additional PIPE closing
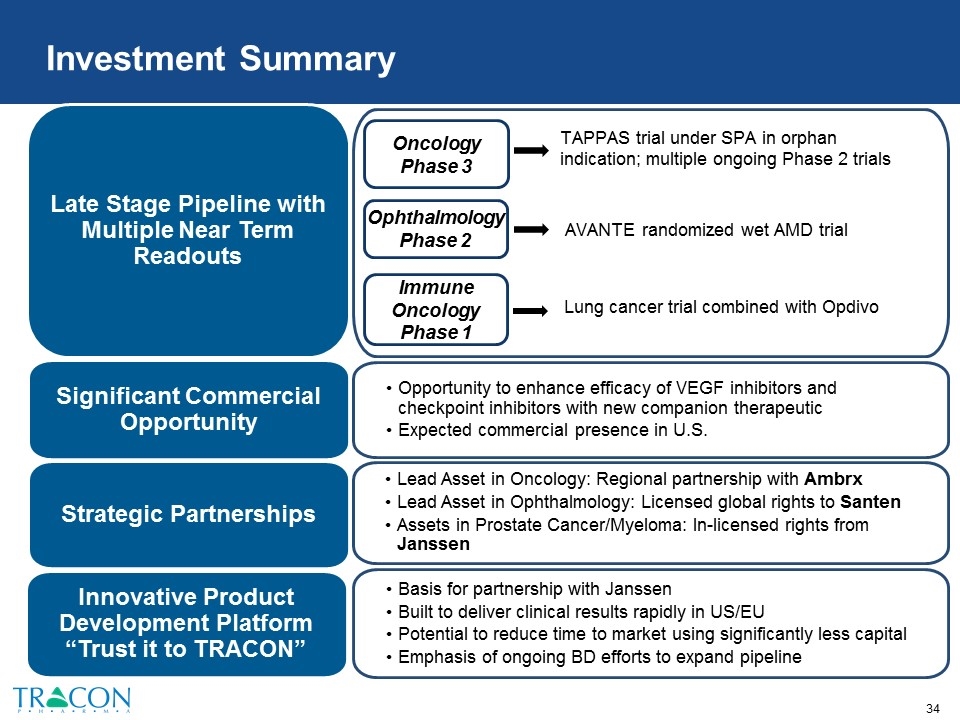
Investment Summary Basis for partnership with Janssen Built to deliver clinical results rapidly in US/EU Potential to reduce time to market using significantly less capital Emphasis of ongoing BD efforts to expand pipeline Late Stage Pipeline with Multiple Near Term Readouts Opportunity to enhance efficacy of VEGF inhibitors and checkpoint inhibitors with new companion therapeutic Expected commercial presence in U.S. Significant Commercial Opportunity Strategic Partnerships Lead Asset in Oncology: Regional partnership with Ambrx Lead Asset in Ophthalmology: Licensed global rights to Santen Assets in Prostate Cancer/Myeloma: In-licensed rights from Janssen TAPPAS trial under SPA in orphan indication; multiple ongoing Phase 2 trials Oncology Phase 3 Ophthalmology Phase 2 AVANTE randomized wet AMD trial Lung cancer trial combined with Opdivo Innovative Product Development Platform “Trust it to TRACON” Immune Oncology Phase 1

Backup
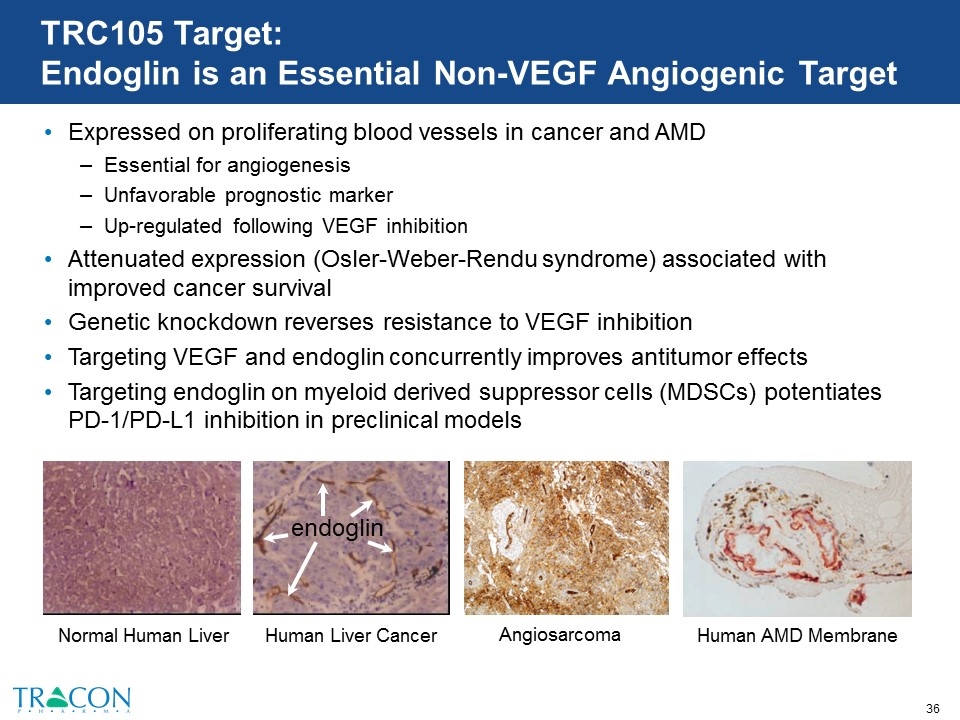
TRC105 Target: Endoglin is an Essential Non-VEGF Angiogenic Target Expressed on proliferating blood vessels in cancer and AMD Essential for angiogenesis Unfavorable prognostic marker Up-regulated following VEGF inhibition Attenuated expression (Osler-Weber-Rendu syndrome) associated with improved cancer survival Genetic knockdown reverses resistance to VEGF inhibition Targeting VEGF and endoglin concurrently improves antitumor effects Targeting endoglin on myeloid derived suppressor cells (MDSCs) potentiates PD-1/PD-L1 inhibition in preclinical models endoglin Normal Human Liver Human Liver Cancer Human AMD Membrane Angiosarcoma
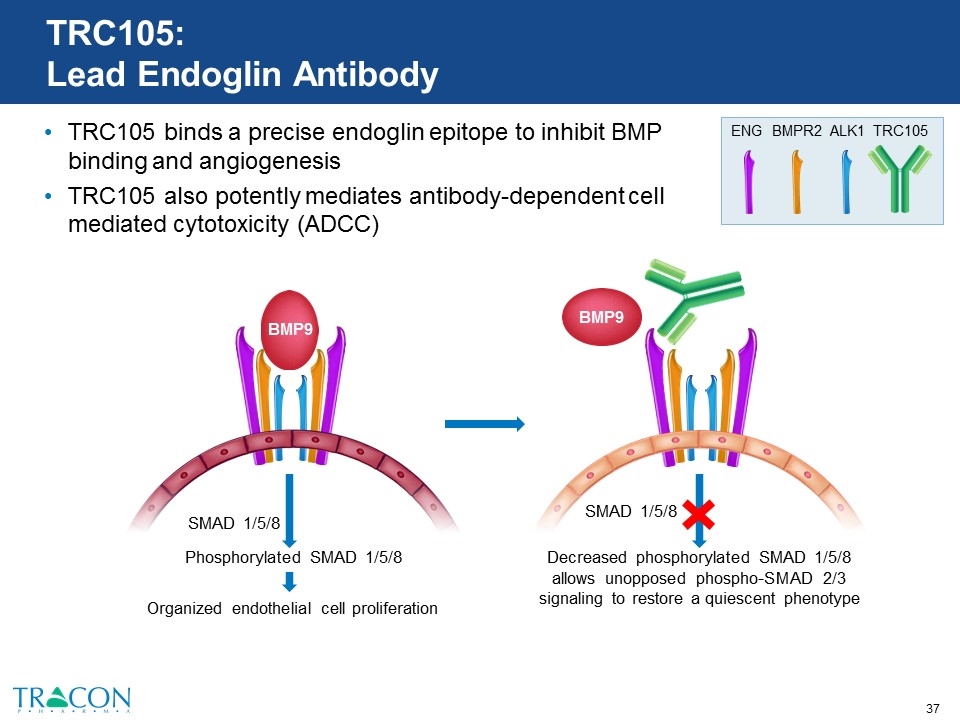
TRC105: Lead Endoglin Antibody TRC105 binds a precise endoglin epitope to inhibit BMP binding and angiogenesis TRC105 also potently mediates antibody-dependent cell mediated cytotoxicity (ADCC) Decreased phosphorylated SMAD 1/5/8 allows unopposed phospho-SMAD 2/3 signaling to restore a quiescent phenotype SMAD 1/5/8 BMP9 Phosphorylated SMAD 1/5/8 Organized endothelial cell proliferation SMAD 1/5/8 BMP9 TRC105 BMPR2 ALK1 ENG
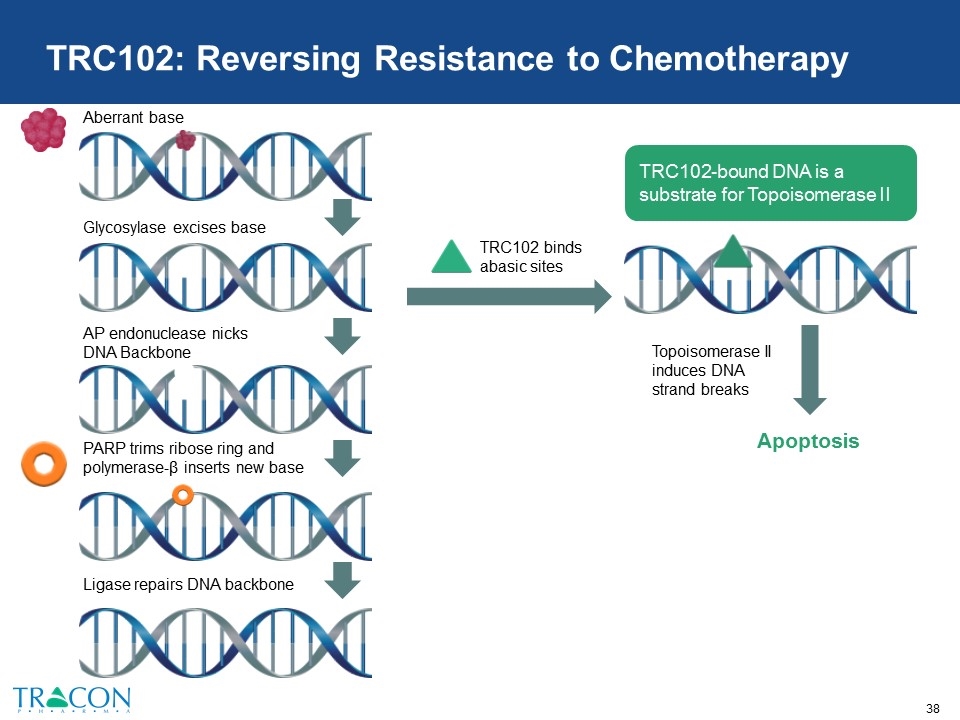
TRC102: Reversing Resistance to Chemotherapy TRC102 binds abasic sites Apoptosis Topoisomerase II induces DNA strand breaks TRC102-bound DNA is a substrate for Topoisomerase II Aberrant base Glycosylase excises base AP endonuclease nicks DNA Backbone PARP trims ribose ring and polymerase-β inserts new base Ligase repairs DNA backbone
Team of Industry Experts Charles Theuer MD PhD, President and CEO 23 years of experience in drug discovery and development Sutent, Rituxan, Zevalin 16 years of experience in drug discovery and development Sutent, Rituxan, Zevalin Suzy Benedict, VP Regulatory Affairs 15 years of regulatory affairs experience Viracept, Macugen Jennifer Ellis, VP Quality Assurance 25 years of drug development experience Sivextro, Inlyta, Viracept Sharon Real PhD, SVP Product Development 23 years of experience in drug discovery and development Sutent, Macugen, Viracept, Targretin Mark Wiggins MBA, Chief Business Officer 30 years of drug development experience Commercialization of Rituxan and Zevalin Bonne Adams MBA, SVP Clinical Operations
SAB and Board Bring Deep Industry Experience Scientific Advisory Board Charles Sawyers, MD Memorial Sloan Kettering Cancer Center William Kaelin, MD Harvard Medical School Jeff Hager, PhD Former CSO, Aragon Stanton Gerson, MD Case Cancer Center Brian Daniels, MD 5AM Ventures, former SVP, BMS Board of Directors William LaRue Former CFO, Cadence Pharmaceuticals Martin Mattingly, PharmD Former CEO, Trimeris and Ambrx Rainer Twiford, JD, PhD President, Brookline Investments Paul Walker Partner, NEA Ted Wang, PhD CEO and CIO, Puissance Capital Stephen Worland, PhD CEO, Effector Therapeutics Charles Theuer, MD, PhD President and CEO







































