UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 8, 2021
TRACON Pharmaceuticals, Inc. | ||
(Exact name of registrant as specified in its charter) | ||
Delaware | 001-36818 | 34-2037594 |
(State or other jurisdiction | (Commission File Number) | (IRS Employer Identification No.) |
of incorporation) |
|
|
|
| |
4350 La Jolla Village Drive, Suite 800 San Diego, California |
| |
(Address of principal executive offices) | (Zip Code) | |
| ||
Registrant’s telephone number, including area code: (858) 550-0780 ____________________________________________________________________________ | ||
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Securities Act:
Title of each class | Trading symbol(s) | Name of each exchange on which registered |
Common Stock, par value $0.001 per share | TCON | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 | Entry into a Material Definitive Agreement. |
On October 8, 2021, TRACON Pharmaceuticals, Inc. (the “Company”), Eucure (Beijing) Biopharma Co., Ltd. (“Eucure”) and Biocytogen Pharmaceuticals (Beijing) Co., Ltd., Eucure’s controlling affiliate, entered into a collaborative development and commercialization agreement (the “Collaboration Agreement”) for the development of YH001, a monospecific investigational anti-CTLA-4 antibody.
Pursuant to the Collaboration Agreement, the Company was granted an exclusive (including with respect to Eucure and its affiliates), nontransferable, license to develop and commercialize YH001 in North America for the treatment, through administration of YH001 by intravenous or subcutaneous means, of multiple human indications, including sarcoma, microsatellite stable colorectal cancer, renal cell carcinoma (“RCC”), and K-ras positive non-small cell lung cancer (collectively the “Initial Indications”) or one or more of bladder cancer, endometrial cancer, and melanoma as substitute indications, which may be substituted for Initial Indications at the Company’s discretion (each upon such substitution, a “Substitute Indication”). The Company is responsible for, and will bear the costs of, preparing and filing all regulatory submissions and conducting any Phase 1, Phase 2, Phase 3, or post-approval clinical trials in North America for YH001 in the Initial Indications and potentially the Substitute Indications, while Eucure is responsible for conducting, and will bear the costs of, the preparation of chemistry, manufacturing and controls activities for YH001. Eucure has agreed to manufacture and supply, or to arrange for a third party manufacturer to manufacture and supply, YH001 to the Company for clinical trials pursuant to the terms of a clinical supply and quality agreement to be separately negotiated.
Eucure may pursue clinical trials for YH001 in North America outside of the Initial Indications or Substitute Indications, and also within the Initial Indications or Substitute Indications as part of a combination therapy of YH001 and an additional Eucure product. During a specified period, the Company has the option, subject to Eucure’s prior written approval, to expand the license to include the development and commercialization of YH001 for the treatment, through administration by intravenous or subcutaneous means, of all human and veterinary therapeutic indications in North America for a payment to Eucure in the low single digit millions (the “Company Option”).
Pursuant to the Collaboration Agreement, the Company granted Eucure an irrevocable, perpetual, royalty-free, exclusive license, with the right to grant sublicenses to develop, register, sell, offer to sell, have sold, market and distribute YH001 in all territories outside of North America as well as within North America for all indications other than the Initial Indications and the Substitute Indications.
The Company will be responsible for commercializing YH001 in North America, including booking of sales revenue in the Initial and Substitute Indications. The Company will owe Eucure escalating double digit royalties on net sales of YH001 in North America ranging from the mid-twenties to mid-double digits; provided that until the end of the first full calendar year following the first commercial sale of YH001, royalties will range from the low double digits to the mid-double digits. If sales of YH001 exceed a pre-determined sales threshold in the first full year of sales following first commercial sale, the Company will owe a milestone to Eucure in the high single digit millions. Payment obligations under the Collaboration Agreement continue on a country-by-country basis until the latest of (i) expiration of the last to expire licensed patent covering YH001, (ii) expiration of marketing or regulatory exclusivity covering YH001 and (iii) 10 years from the first commercial sale of YH001 in such country in North America. Eucure has agreed to manufacture and supply, or to arrange for a third party manufacturer to manufacture and supply, YH001 to the Company at cost plus a low double digit markup for commercial sales pursuant to the terms of a commercial supply and quality agreement to be separately negotiated.
Pursuant to the Collaboration Agreement, each party agreed that during the term of the Collaboration Agreement, it would not develop, manufacture, commercialize or license from any third party a monospecific inhibitor to CTLA-4 administered by intravenous or subcutaneous means in the Initial and Substitute Indications in North America.
The term of the Collaboration Agreement continues until the earlier of (i) the date that the parties cease further development and commercialization of YH001 in North America or (ii) on a country-by-county basis, the expiration of the royalty obligations in such country. The Collaboration Agreement may be terminated earlier by a party in the event of an uncured material breach by the other party or bankruptcy of the other party, or for safety
reasons related to YH001. In the event of a termination of the Collaboration Agreement, other than by the Company as a result of Eucure’s material uncured breach or bankruptcy, (i) the Company’s license shall terminate and (ii) the Company is obligated to grant Eucure an irrevocable, perpetual, royalty-free, non-exclusive license with the right to grant sublicenses under its rights in all development data and intellectual property to develop, register, sell, offer to sell, have sold, market and distribute YH001 in North America. In the event of a termination of the Collaboration Agreement by the Company as a result of Eucure’s material uncured breach or bankruptcy, the license shall continue in the Initial Indications in North America, provided that (i) such license shall remain exclusive during the royalty term and non-exclusive thereafter; (ii) the Company shall have the right to have YH001 manufactured for its development and commercialization requirements in the Initial Indications in North America; and (iii) the license shall terminate in the event of an uncured material breach by the Company of any provision (including payment obligations) that survives termination of the Collaboration Agreement. In the event that the Collaboration Agreement terminates for safety reasons related to YH001, by mutual agreement of the parties or by Eucure in the event of an uncured material breach or bankruptcy by the Company, then the Company’s rights and obligations under the Collaboration Agreement will revert to Eucure. In the event that Eucure does not approve the Company Option, the Company may terminate the Collaboration Agreement for convenience with a 30-day notice to Eucure, provided that such termination is given within 12 months of the effective date of the Collaboration Agreement (the “Company Option Termination”). In the event of a Company Option Termination, Eucure shall reimburse the Company for all costs and expenses that it incurred in performing the development activities.
The description of the Collaboration Agreement above is qualified in its entirety by reference to the text of the Collaboration Agreement, a copy of which the Company intends to file, with certain confidential terms redacted, with the Securities and Exchange Commission (the “SEC”) as an exhibit to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2021.
On October 11, 2021, the Company issued a press release with respect to entering into the Collaboration Agreement. A copy of the press release is attached hereto as Exhibit 99.1.
Item 8.01 | Other Events |
YH001 is an investigational humanized anti-CTLA-4 IgG1 monoclonal antibody. YH001 is being developed by Eucure for the treatment of various cancer indications.
Cytotoxic T-lymphocyte-associated protein 4, or CTLA-4, is a protein expressed on all T-cells but which is expressed at the highest level on regulatory T-cells (“Treg”) and contributes to the suppressor function of Treg and acts as an off-switch to T-cell immune response to cancer cells. A CTLA-4 inhibitor has been approved as a single agent in melanoma and approved in combination with other therapies in multiple indications including non-small cell lung cancer, RCC and microsatellite instability high colorectal cancer.
Clinical Development of YH001
As of August 9, 2021, YH001 had been dosed to more than 34 patients in China and Australia.
Phase I Dose Escalation Clinical Trial in Australia
An open-label, single-arm Phase 1 dose escalation clinical trial of YH001 in combination with the PD-1 antibody, toripalimab, is ongoing in Australia. The safety and efficacy data from this trial were presented at the 2021 Chinese Society of Clinical Oncology (“CSCO”) Annual Meeting in September 2021. Based on the data presented in the CSCO Annual Meeting (“CSCO Presentation”), 21 subjects were enrolled in this trial as of August 9, 2021.
Study purpose. The primary objectives of the Phase 1 dose escalation clinical trial were to assess the safety and tolerability profile and maximum tolerated dose of YH001 in combination with the PD-1 inhibitor toripalimab in subjects with advanced solid tumors. The secondary objectives were to evaluate the PK profile and anti-tumor activity.
Study design. This trial adopted a modified “3+3” design. Subjects receive YH001 in six cohorts at 0.05 mg/kg, 0.1 mg/kg, 0.3 mg/kg, 1.0 mg/kg, 2.0 mg/kg, 4.0 mg/kg, and 6.0 mg/kg by IV administration during a three
week run-in period, after which subjects receive YH001 in combination with 240mg of the PD-1 antibody toripalimab every three weeks for four doses.
Safety. At the August 9, 2021 data cutoff, no dose limiting toxicities had occurred and a single serious adverse event of grade 3 colitis was reported, which led to treatment discontinuation. Thirty two YH001 drug-related adverse events (“AEs”) were reported, including 11 cases of grade 2 AEs and 20 cases of grade 1 AEs.
Efficacy. Among 16 patients that had image tumor assessments available at the August 9, 2021 data cut-off, two achieved partial response by RECIST, including in one patient with urothelial cancer who had failed prior treatment with a PD-1 antibody, and seven had stable disease. The figure below illustrates the patients with tumor assessments available as of the August 9, 2021 data cutoff.
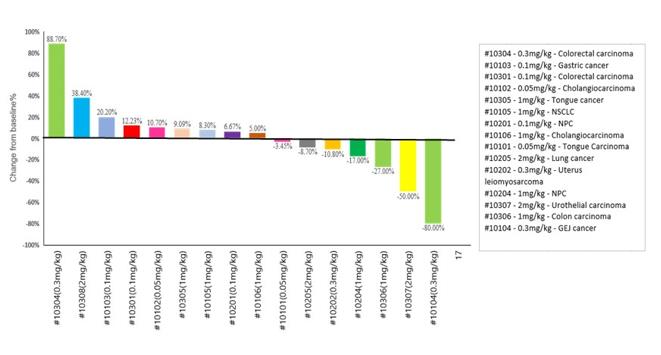
Conclusion. The authors concluded that YH001 was well tolerated up to 2 mg/kg when combined with toripalimab and demonstrated activity in patients with advanced solid tumors. Dose level cohorts above 2 mg/kg did not have tumor assessments available as of data cutoff at August 9, 2021.
Phase I Dose Escalation Clinical Trial in China
An open-label, single-arm Phase 1 dose escalation clinical trial of YH001 is ongoing in China.
Preclinical Studies
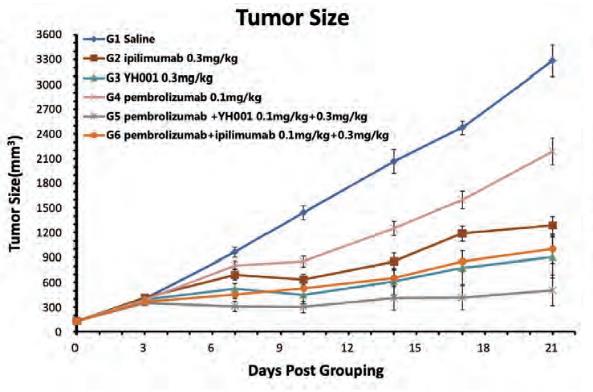
In pre-clinical studies in mice, YH001 was compared with ipilimumab, an approved CTLA-4 inhibitor marketed by Bristol Myers Squibb, and YH001 showed the following potential advantages:
| • | Superior in vivo activity than ipilimumab as a single agent and when combined with pembrolizumab, a PD-1 antibody marketed by Merck. The following graphs illustrate the tumor size growth and body weight changes in mice. |

| • | More potent and active than ipilimumab. YH001 was more potent and active than ipilimumab in blocking hCTLA-4 inhibition of CD80/86 activity. The following graph illustrates an in vitro reporter assay demonstrating the ability of YH001 or ipilimumab to induce T-cell proliferation by inhibiting the interaction of hCTLA-4 with CD80/86. |

Clinical Development in North America
The Company intends to initiate a Phase 1/2 clinical trial of YH001 in combination with envafolimab in sarcoma with expanded cohorts in the sarcoma subtypes of angiosarcoma, liposarcoma and alveolar soft part sarcoma in 2022. Additionally, the Company plans to initiate a Phase 1 trial of YH001 in combination with envafolimab and doxorubicin in 2023 in first line sarcoma in addition to multiple other trials.
Manufacturing
YH001 is manufactured by an experienced contract manufacturer in China.
Competition
There is no CTLA-4 therapy approved by the U.S. Food and Drug Administration (“FDA”) for the treatment of soft tissue sarcoma. If YH001 is approved, it may nevertheless compete with the currently marketed CTLA-4 inhibitor ipilimumab (Yervoy, marketed by Bristol Myers Squibb), which is approved by the FDA in multiple indications other than soft tissue sarcoma. Other antibodies to CTLA-4 are being studied in clinical trials of cancer patients.
Intellectual Property
Eucure has filed patent applications on the composition of matter and methods of use of YH001 in China, the European Union and the United States, including international application PCT/CN2017/102816 that was published March 28, 2019. The terms of the patents, if issued, would expire in 2037 or later.
Forward-Looking Statements
Certain statements contained in this report are forward-looking statements that involve a number of risks and uncertainties. Such forward-looking statements include, without limitation, statements regarding, among other things, the efficacy, safety and therapeutic potential of YH001, the results, conduct, progress and timing of clinical trials of YH001, plans regarding future clinical trials and regulatory actions, and potential future payments and activities under the Collaboration Agreement. Words such as “will”, “expect”, “may,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from our expectations. Factors that could cause actual results to differ materially from the forward-looking statements include, but are not limited to, risks and uncertainties that are inherent in clinical trials, including delays in our ability to acquire sufficient supply of clinical trial materials, delays in reaching agreement on acceptable terms with prospective clinical trial sites and disruptions to or delays in ongoing clinical trials caused by the COVID-19 global pandemic, and the possibility that the Collaboration Agreement is terminated early. Additional factors that could cause actual results to differ materially from those stated or implied by our forward-looking statements are disclosed in our filings with the SEC. These forward-looking statements represent our judgment as of the time of this report. We disclaim any intent or obligation to update these forward-looking statements, other than as we may be required under applicable law.
Item 9.01 | Financial Statements and Exhibits. |
(d) | Exhibits. |
Exhibit No. |
| Description |
99.1 |
| Press release issued by TRACON Pharmaceuticals, Inc. on October 11, 2021.
|
104 |
| Cover page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Date: October 11, 2021 |
|
|
| TRACON Pharmaceuticals, Inc. | ||
|
|
|
| |||
|
|
|
| By: |
| /s/ Charles P. Theuer, M.D., Ph.D. |
| Name: |
| Charles P. Theuer, M.D., Ph.D. | |||
|
|
| President and Chief Executive Officer
| |||