
Letter to Shareholders 1 May 2023 01 Crediting the Power of Our People Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Appendix 02 12 03 13 14 15 16 17 05 07 09 10 Introduction Examining the Macro Environment Advocating for a Patient-Centric Health Care System Reform Advancing Our Pipeline to Deliver Life- Transforming Treatments to Patients Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Business Momentum to Sustain Long-Term Growth Contents 1 In this letter, the words “we”, “us” and “our” are also used to refer to subsidiaries of Takeda in general or to those who work for them. These expressions are also used where no useful purpose is served by identifying the particular company or companies. In addition, this letter may contain forward-looking statements, beliefs or opinions regarding Takeda’s future business, future position and results of operations, including estimates, forecasts, targets and plans for Takeda. The cautionary language regarding forward-looking statements included on page 46 of the Notice of Convocation of the 147th Ordinary General Meeting of Shareholders distributed with this letter is hereby incorporated by reference. Christophe Weber President & CEO Takeda Pharmaceutical Company Limited

Dear Takeda Shareholder, I hope you and your family are well, safe and healthy. On behalf of the Takeda Board of Directors, I am delighted to invite you to our Annual Shareholders Meeting on June 28, 2023. This is a very exciting time for Takeda. After several years of business transformation, integration and deleveraging, we have built competitive global scale with a strong financial foundation and are now firmly focused on long-term growth and shareholder returns. This is why in fiscal year 2023 (FY2023) we are proposing to increase our dividend from JPY 180 to JPY 188 per share, reflecting our improved debt profile and strong cash flow, and underscoring our confidence in our future growth. Our Total Shareholder Return (TSR) of approximately 30% in FY2022 is recognition that market sentiment has evolved and is taking note of the strength of the business and our potential. Everything we do is guided by our values, which are fundamental to our strategy, our identity and our culture. Takeda’s core values are Integrity, Fairness, Honesty and Perseverance. They are brought to life through actions based on putting the Patient at the center, building Trust with society, reinforcing our Reputation and developing our Business, in that order. Our values are shown in our annual employee survey to be the highest differentiator of what makes our 50,000 colleagues so proud to work at Takeda, as they are for me. Our values are shaped by our more than 240-year heritage and we focus on company performance over the long term (10 years and beyond), medium-term (three to 10 years) and short term (one to three years). How we chose to price our recently approved vaccine for dengue fever, QDENGA®, is an excellent example of our values in action. The price per dose of QDENGA in Indonesia is USD 26 ex-factory (our price to distributor), which will ultimately result in a recommended price to consumers of USD 40 in the private market. In Germany, meanwhile, the ex-factory price will be USD 85, which translates to a recommended retail price of USD 119 per dose. This reflects not only our tiered-pricing strategy, which takes into consideration each country’s economic development and health system maturity, but also our willingness to ensure broader access to this innovative vaccine. We hope that it will lead to broader long-term adoption by endemic countries facing this serious public health challenge. We are determined and proud to roll out such an innovative pricing model – a first in vaccine pricing – and to continue to deliver highly innovative, life-transforming medicines for patients with high unmet medical needs. 02 Introduction Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Examining the Macro Environment Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix

Examining the Macro Environment As a global company, our business is impacted by political, economic, social and technological developments around the world. We continuously monitor, assess and make predictions about the external environment and adapt our strategy to position Takeda for success. In this letter, I highlight some of the major trends in the global macro environment, including what I believe is the most urgent challenge facing the health care sector today, and discuss our strategy for resilience and growth within this environment. Global Tensions and Challenges While some impacts of the global COVID-19 pandemic have now eased, economic pressures and geopolitical risks have intensified over the past year, creating an uncertain outlook and a tough environment for the global economy. First, it’s clear that the devastating war in Ukraine is far from over. The war is a humanitarian crisis for the people of Ukraine and continues to put stress on the global economy. Consistent with our values and ethical responsibility, Takeda provided support and humanitarian relief to our patients and colleagues in Ukraine. In Russia, we discontinued activities that are not essential to maintaining supply of medicines to patients, many of which are life-saving. Second, the lingering impacts of the pandemic, coupled with geopolitical factors, have driven supply disruptions for some major industries, energy price increases and labor-market pressures. The average inflation rate worldwide remains high despite concerted efforts by central banks to tamp down inflation by raising interest rates, resulting in an increased risk of a global recession. Crucially, Takeda had the foresight to negotiate 100% of our debt at fixed interest rates averaging approximately 2%, giving us resilience against high interest rates, and our continued financial discipline and strong free cash flow put us in a position of strength in a challenging macro environment. During economic recession, the pharmaceutical industry faces significant price pressure as many governments struggle to fund their health care systems. Our intent is to constantly improve our effectiveness to offset this effect. Third, continued tensions between China and the U.S., EU and other countries further add to geopolitical and economic uncertainties and pose risks for global companies. Nevertheless, the provision of quality health care to its citizens appears to be a strategic imperative for the Chinese government and we see encouraging signs of a more open, pro-innovation posture in the country. Takeda is experiencing rapid growth in China thanks to the launch of 10 new products in the past three years – the highest among non-China headquartered companies. We will continue to invest in providing patients in China with innovative medicines and treatments while actively managing any risk of economic decoupling that 03 Examining the Macro Environment Introduction Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix

may impact health care. Meanwhile, we continue to reinforce our position in the U.S., where we now generate nearly 50% of our total revenue, driven by our Growth & Launch Products2. Despite the global uncertainties, we as an organization have shown continued resilience and growth through innovation to deliver life-transforming treatments to patients. We remain confident that our headquarters and global hubs, research centers and global supply chains, located in Japan, the United States, Europe and Singapore will help ensure our resilience in a more complex globalized world. A More Sustainable Approach to Pandemic Preparedness In my letter last year, I reflected on the impact of the pandemic on our global health care systems and communities and advocated for significant reforms. I acknowledge that while the global health community has made some progress in certain areas, including funding research to better understand these diseases and how to prevent and treat them, more work is needed in the realm of health equity. The world missed the opportunity to provide vaccine equity globally during the COVID-19 pandemic. If well prepared, we can have far greater success the next time a pandemic occurs. Despite well-intentioned initiatives by the World Health Organization and other health authorities, the discussion of waivers for intellectual property rights and some other provisions is concerning. This approach positions intellectual property rights as a deterrent to equity, when we know that there are far more pertinent factors, such as pricing, supply allocation and adherence to a first come first served principle as seen during the COVID-19 pandemic. Weakening intellectual property rights would undermine the necessary economic incentive to conduct long-term, costly, research and development (R&D) investment. Takeda is committed to providing the broadest possible access to our innovative medicines and vaccines, and we believe that intellectual property should not be viewed as a barrier to access. In fact, expanding access and reducing health inequities remains a key focus for us and is something that’s ingrained in the work we do every day. At the World Economic Forum, Takeda was among the few pharmaceutical companies to sign the Zero Health Gaps Equity Pledge. The Takeda Center for Health Equity and Patient Affairs has collaborated with a diverse global network of health equity partners since 2020 to empower communities to identify and address health inequities sustainably, leading to improved care and expanded access at every stage of the patient journey. It is our promise to continue applying our resources and capabilities to make sure everyone has access to proper health care. The Zero Health Gaps Equity Pledge is a reminder that there’s much work to be done and I look forward to seeing what we can achieve during this fiscal year. Examining the Macro Environment Introduction Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix 04 2 Please refer to slide 20 of the FY2022 Earnings Announcement (available at https://www.takeda.com/investors/financial-results/quarterly-results/) for definition of Growth & Launch Products.
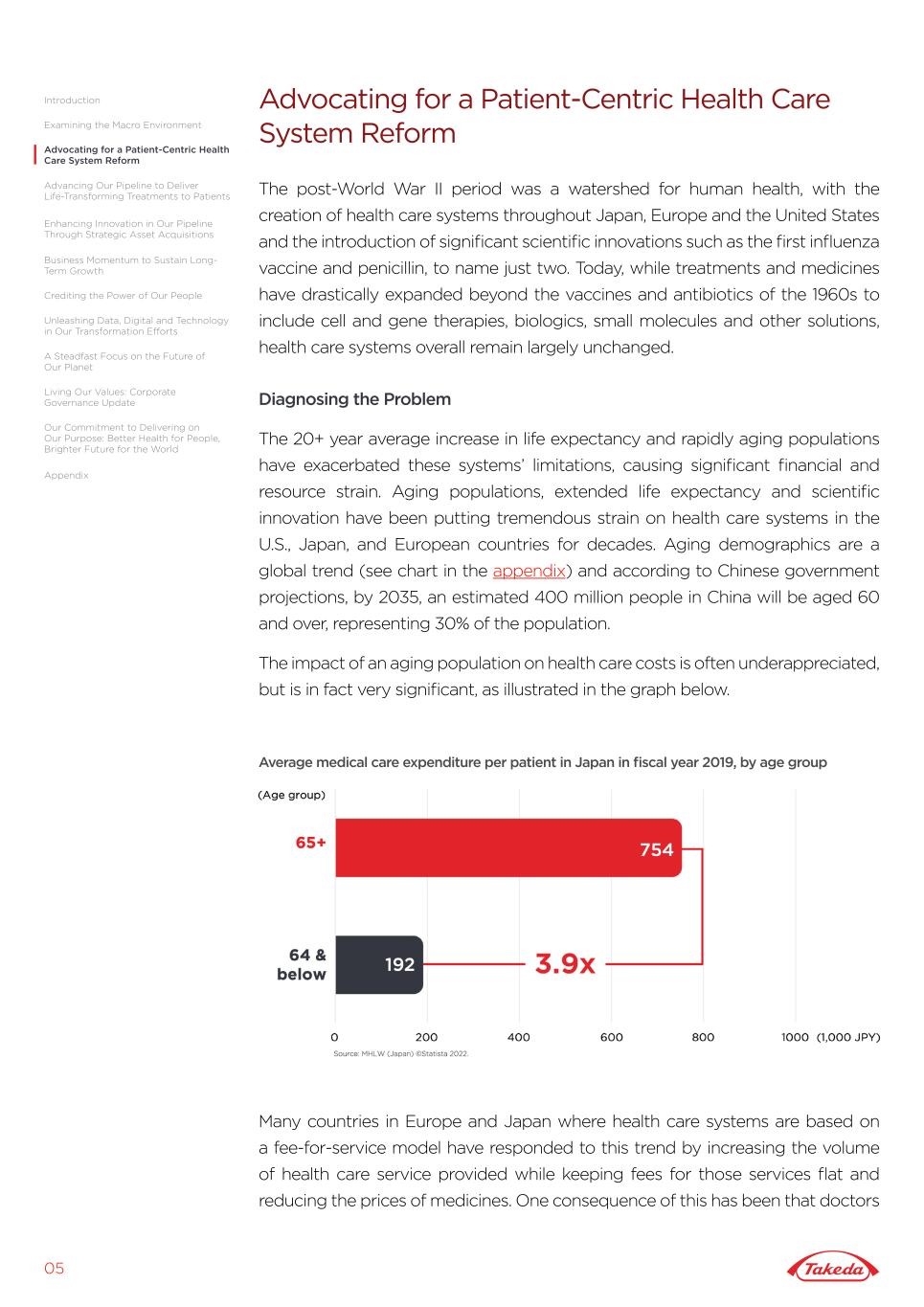
Advocating for a Patient-Centric Health Care System Reform The post-World War II period was a watershed for human health, with the creation of health care systems throughout Japan, Europe and the United States and the introduction of significant scientific innovations such as the first influenza vaccine and penicillin, to name just two. Today, while treatments and medicines have drastically expanded beyond the vaccines and antibiotics of the 1960s to include cell and gene therapies, biologics, small molecules and other solutions, health care systems overall remain largely unchanged. Diagnosing the Problem The 20+ year average increase in life expectancy and rapidly aging populations have exacerbated these systems’ limitations, causing significant financial and resource strain. Aging populations, extended life expectancy and scientific innovation have been putting tremendous strain on health care systems in the U.S., Japan, and European countries for decades. Aging demographics are a global trend (see chart in the appendix) and according to Chinese government projections, by 2035, an estimated 400 million people in China will be aged 60 and over, representing 30% of the population. The impact of an aging population on health care costs is often underappreciated, but is in fact very significant, as illustrated in the graph below. 05 Advocating for a Patient-Centric Health Care System Reform Average medical care expenditure per patient in Japan in fiscal year 2019, by age group Many countries in Europe and Japan where health care systems are based on a fee-for-service model have responded to this trend by increasing the volume of health care service provided while keeping fees for those services flat and reducing the prices of medicines. One consequence of this has been that doctors Introduction Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix Examining the Macro Environment

and nurses have to deal with significantly more patients, while their compensation has only modestly improved. This in turn is having a direct impact on the ability to attract and retain health care practitioners. The COVID-19 pandemic accelerated this progressive crisis and brought many European health care systems to the brink of collapse while significantly increasing health inequity in public-private systems such as in the U.S. Moving Toward a Sustainable, Pro-Innovation, Universal Health Care System We believe that health care systems need to urgently move away from fee-for- service and toward value-based health care, an approach that pays for outcomes and care quality. The advantages of such a system are twofold: patients benefit from a system that is set up around delivering results for them – the true purpose of any health care system – and efficiency improves as a result of linking the compensation of health care providers to patient outcomes. This model can be used to expand coverage, improve equity and provide an attractive and sustainable health care profession that is rewarded for outcomes and quality care. A value-based system also encourages the continued funding, innovation and research that is necessary to achieve the next scientific breakthrough. Health care costs will likely continue to outpace GDP growth due to the impact of aging populations, extended life expectancy and new scientific innovations. That said, if governments can demonstrate improved efficiency and effectiveness in health care systems, the investment is more readily justifiable. The restrictive drug pricing and reimbursement policies adopted in the EU and the U.K., coupled with persistent price reductions in Japan, raise concerns about the impact on innovation. Unpredictable and escalating payment rates in the U.K. mean that participating pharmaceutical companies will be paying 26.5% of their U.K. sales to the government in 2023 – a considerable and unsustainable increase from approximately 5% in 2019. When innovation is not rewarded, R&D investment is reduced, as has been seen in the U.K., or the introduction of new drugs significantly declines, as the drug lag in Japan illustrates. In the U.S., which has long rewarded innovation in the industry, policy changes put future innovation at risk and jeopardize access to some medicines. While the Inflation Reduction Act offers some positives for Medicare patients such as greater predictability in their out-of-pocket prescription expenses, it also establishes an unprecedented government price-setting system for medicines that could potentially result in declines in R&D investment in the country. At Takeda, the patients we serve – and achieving outcomes for them and their health – are at the heart of everything that we do. That’s why we advocate for the worthwhile transition to value-based health care. At the same time, we should be realistic about the scale of the challenge. The dynamics of a private health care market, such as in the U.S., may allow for greater 06 Advocating for a Patient-Centric Health Care System Reform Introduction Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix Examining the Macro Environment

agility, but in publicly funded markets a successful transition to this system is unlikely in the near-term. Furthermore, a shift to a value-based system alone will not be enough to sustain it without an accompanying increase in government funding. That is why Takeda is doubling down on driving growth and protecting our margin even without a change in the health care status quo. We are doing this through a focus on innovative medicines and vaccines that move swiftly and safely through the approval process and qualify for reimbursement, and through investment in our in-house R&D engine and strategic targeted asset acquisitions to complement our pipeline. We are also building resilience by managing our exposure to external risks, while investing in data, digital and technology (DD&T) to upskill our people and drive value creation. Advancing Our Pipeline to Deliver Life- Transforming Treatments to Patients Our innovative R&D strategy is delivering, as evidenced by our approximately 40 molecules in clinical development – the majority of which represent best-in-class or first-in-class potential – and a steady stream of early research programs across a range of modalities. Our pipeline continues to mature, with 10 new molecular entities (NME) in late-stage development, thanks to organic additions as well as in-licensing and acquisitions. In December 2022, we achieved a significant milestone as QDENGA was approved for use in the EU against any serotype in individuals four years of age and older regardless of previous dengue exposure. This followed a positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in October, recommending approval in the EU and in dengue-endemic countries participating in the parallel EU-Medicines- for-all procedure. QDENGA has now also been approved for use in Indonesia, Brazil, Argentina, the U.K. and Thailand, with broad labels, for use regardless of serostatus. Dengue-endemic countries need innovative prevention solutions, beyond what is currently available, to help reduce the number of infections and manage the threat that dengue poses to patients and their families, communities and the country’s economic health. We are proud to be part of the solution. We reached another critical milestone late last calendar year when the U.S. Food & Drug Administration (FDA) accepted our dengue vaccine candidate for priority review. In March 2023, the Japanese Ministry of Health, Labour and Welfare approved the use of ENTYVIO® subcutaneous injection for the maintenance treatment of moderate to severe ulcerative colitis. We believe the subcutaneous formulation will provide a wider range of dosing options for ulcerative colitis patients, meeting 07 Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix Examining the Macro Environment

their individual lifestyle and health care needs and improving their quality of life. We have also filed for regulatory approval for ENTYVIO subcutaneous injection for ulcerative colitis in the U.S. and are targeting further filings of this formulation for Crohn’s disease. FDA approval of the subcutaneous formulation of our largest selling product has the potential to be one of Takeda’s largest catalysts for growth this year. Also in the U.S., we are targeting filing of another of our highly innovative treatments, ALOFISEL®, for perianal fistulas, and we anticipate a regulatory approval decision for HYQVIA® for the maintenance treatment of chronic inflammatory demyelinating polyradiculoneuropathy within this fiscal year. In April, we received FDA approval to expand the use of HYQVIA to treat primary immunodeficiency in children. We have achieved numerous milestones along our journey to discover and deliver critical treatments to underserved patient populations. LIVTENCITY® received approval from the European Commission (EC), making it the first and only treatment approved by the EC for adults with post- transplant cytomegalovirus (CMV) infection and/or disease that are refractory (with or without resistance) to one or more prior therapies. CMV is one of the most common and serious post-transplant infections and can lead to loss of a transplanted organ and failure of graft. Following launch in the U.S., 98% of transplant centers have initiated therapy for at least one patient. People undergoing transplants have a lengthy and complex health care journey and we’re encouraged by the positive uptake and look forward to making LIVTENCITY available to even more patients worldwide. EXKIVITY® received approval from China’s National Medical Products Administration (NMPA). It is now the first and only treatment available in China for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor Exon20 insertion mutations, whose disease has progressed on or after platinum-based chemotherapy. Importantly, this approval was granted as part of the NMPA’s Breakthrough Therapy program. Among our late-stage development programs, we received favorable safety and efficacy results for TAK-755 (apadamtase alfa /cinaxadamtase alfa) from the first and only Phase 3 trial in congenital thrombotic thrombocytopenic purpura (cTTP), an ultra-rare disease with limited treatment options. Regulatory filing for TAK-755 in the U.S. was completed in May 2023, with the potential for approval this fiscal year, and we are targeting further filings for cTTP in the EU and Japan this fiscal year followed by China in FY2024. In January 2023, we announced positive Phase 2 data for TAK-999 (fazirsiran) in alpha-1 antitrypsin deficiency-associated liver disease together with our partner, 08 Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Appendix Examining the Macro Environment
Arrowhead Pharmaceuticals. Takeda is responsible for taking this program into Phase 3 development, which we initiated this spring. In our mid-stage pipeline, our oral orexin agonist for narcolepsy, TAK-861, met our pre-specified criteria to advance into Phase 2b studies in narcolepsy type 1 and 2, which we also initiated this year. As we advance our exciting and maturing pipeline and navigate a dynamic global regulatory and access environment, we must regularly make important decisions on where to allocate our resources. As part of our continual prioritization process, we have decided to discontinue our discovery and pre-clinical efforts in adeno- associated virus (AAV) gene therapy. This modality faces significant challenges from clinical, regulatory, access and commercial perspectives. This reprioritization will allow us to simplify our development organization, focus our resources and provide a sustainable and continuous flow of innovation to patients where we feel that we can have the most impact. We are proud of the progress that Takeda and the scientific community have made in treating rare diseases with a range of therapies including genetic treatments, and while we will not continue in AAV gene therapy, we remain committed to patients with rare diseases and to leveraging other genetic therapies to treat and potentially cure diseases within our therapeutic area strategies. Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Complementing our in-house R&D engine, in FY2022, we reinforced our long- term growth potential through business development to bring three highly innovative programs into our mid-to-late stage pipeline. From Nimbus Therapeutics, we acquired a potentially best-in-class oral, selective allosteric tyrosine kinase 2 (TYK2) inhibitor that has possible application across a broad range of diseases, including psoriasis, psoriatic arthritis, inflammatory bowel disease (IBD) and systemic lupus erythematosus. Takeda has a long-standing legacy in developing transformative therapies for both gastrointestinal and inflammatory diseases. This TYK2 inhibitor – now named TAK-279 – represents a significant potential opportunity with psoriasis and IBD, each representing USD 30 billion markets and could offer the convenience of an oral therapy while addressing unmet medical needs. In March, we presented data at the American Academy of Dermatology (AAD) Annual Meeting from our Phase 2b study of TAK-279 and the results showed a strong overall clinical benefit in patients with moderate to severe plaque psoriasis, an autoimmune disease afflicting 125 million people around the world. In FY2023, we plan to initiate a Phase 3 study of TAK-279 in psoriasis and 09 Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Business Momentum to Sustain Long- Term Growth Crediting the Power of Our People Appendix Examining the Macro Environment

expect topline results for a Phase 2b study in psoriatic arthritis. We also plan to evaluate TAK-279 in additional immune-mediated diseases, including systemic lupus erythematosus and IBD, and will explore further potential indications in the future. We are aiming to file a regulatory submission in psoriasis between FY2025 and FY2027, which could result in this product reaching a significant patient population prior to the loss-of-exclusivity (LOE) of ENTYVIO. We would also expect continued momentum from indication expansions and market growth by the time ENTYVIO ultimately faces biosimilar erosion, potentially in 2032 – further reinforcing our efforts to deliver growth into the next decade. We also secured an exclusive licensing agreement with HUTCHMED for fruquintinib, to offer a potential new treatment option for patients with refractory metastatic colorectal cancer, regardless of biomarker status. This agreement will give us exclusive rights to further develop and bring this therapy to patients worldwide – excluding Mainland China, Hong Kong and Macau. And finally, we obtained development and commercialization rights in the U.S. and other territories for TAK-227, a first-in-class therapy for celiac disease, from Zedira and Dr. Falk Pharma. Currently there are no approved therapies for the treatment of celiac disease, and with three programs now in clinical development, Takeda is at the forefront of developing transformative therapies for patients with this challenging lifelong autoimmune condition. Business Momentum to Sustain Long-Term Growth The continued strengthening of our pipeline is a direct reflection of our financial discipline and success in deleveraging, driven by our strong free cash flow. We will maintain our strong financial discipline as we continue to evaluate future opportunities and enhance our pipeline with mid-to-late-stage assets. As I previewed above, amid an uncertain and challenging global environment, our financial performance reflects sustained momentum as we enter a new phase for our company. With the Shire integration now behind us, our successful deleveraging and margin improvement enable us to look ahead. In fiscal year 2022, we delivered core revenue growth3 at constant exchange rate4 (CER) of +3.5%, driven by sales of our Growth & Launch Products, which grew 19% to JPY 1,594.8 billion (USD 12.0 billion5) and now account for 40% of the business. On a reported basis, total company revenue was JPY 4,027.5 billion (USD 30.3 billion5), with a year-over-year increase of +12.8%. And we delivered or exceeded our management guidance for core revenue, core operating profit and Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Crediting the Power of Our People Appendix Examining the Macro Environment 10 3 Core revenue, core operating profit, core operating profit margin, core EPS, CER % Change, net debt, adjusted EBITDA, and free cash flow are non-IFRS measures, i.e., measures not calculated and presented in accordance with IFRS. See the financial appendix at the end of Takeda’s FY2022 Earnings Announcement for further information about Takeda’s Non-IFRS Measures and reconciliations to the most directly comparable measures calculated and presented in accordance with IFRS. 4 CER (Constant Exchange Rate) change eliminates the effect of foreign exchange rates from year-over-year comparisons by translating reported or core results for the current period using corresponding exchange rates in the same period of the previous fiscal year. 5 USD calculated for reference at 132.75 JPY/USD.

core EPS growth at CER. A weaker yen is typically a tailwind for Takeda as more than 80% of our revenue is generated outside Japan. Performance was notable across several key business areas, including Gastroenterology thanks to strong ENTYVIO sales growth. In FY2022 Q2, we raised the peak sales outlook range for ENTYVIO to USD 7.5-9.0 billion based on our expectation for sustained global sales growth and our updated assumption for the timing of biosimilar competition. We are proud of the strength of our Plasma-Derived Therapies (PDT) business, which grew 15% on a CER basis, driven by demand for immunoglobulin (IG) and albumin in China. Plasma collection in the U.S. now exceeds pre-pandemic levels. In March, we announced a JPY 100 billion investment to build a new PDT manufacturing facility in Osaka, Japan, representing Takeda’s largest ever investment in expanding our manufacturing capacity in Japan. We expect the new state-of-the-art facility to be operational by 2030 and that it will further strengthen our capabilities to meet growing patient need for PDTs in Japan and globally, with improved margins through better capacity utilization. Takeda delivered core operating profit growth of +9.1% at CER and a robust margin of 29.5%. This strong performance was driven by a combination of momentum in our Growth & Launch Products, solid commercial execution across the portfolio and disciplined cost control. We also continued to deliver important cash flow (JPY 446.2 billion free cash flow in FY2022, or JPY 837.3 billion excluding the upfront payment for TAK-279 from Nimbus), allowing us to invest in strategic business development activities and returning value to shareholders, while also paying down debt. Our success in rapid deleveraging resulted in net debt to adjusted EBITDA of 2.6x at the end of March 2023. Excluding the upfront cash payment for TAK-279, this ratio would have been 2.3x. Looking ahead, FY2023 presents temporary challenges with loss-of-exclusivity (LOE) headwinds impacting revenue growth. While we believe the continued expansion of Growth & Launch products will largely offset this topline LOE impact (predominantly of VYVANSE® in the U.S. and AZILVA® in Japan), core revenue is expected to decline by low-single-digit percentage at CER due to the combination of generic competition and lower expectations for coronavirus vaccines revenue. In FY2023, our management guidance is for a low-10s% decline in our core operating profit at CER, but with continued expansion of our Growth & Launch products, and further launches from our late-stage pipeline, we expect to return to sales, profit and margin growth in the near-term. We will continue to invest in R&D and data and technology to innovate and secure long- term competitiveness to grow over the long-term. 11 Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Crediting the Power of Our People Appendix Examining the Macro Environment
Crediting the Power of Our People Ultimately, it is our people who drive and implement our strategy and are pivotal to our success. I continue to be inspired by our approximately 50,000 dedicated Takeda employees who live our values every day. Results from our annual global Employee Experience Survey confirm that our employees overwhelmingly understand how their work impacts patients and that they are able to make decisions guided by our values. Together as one Takeda, we are steadfast in our commitment to creating a culture that fosters collaboration, well-being and resilience to meet the challenges of a continuously changing world. We invest in our people, both financially and developmentally, and are proud to provide our global team with a variety of flexible ways of working that enable their success, including, for some, a hybrid working environment. This includes talent development initiatives, innovative data, digital and technology (DD&T) tools and our recently launched initiative to reimagine our work experience and enable the flexible working models of the future. As part of these initiatives, we are transforming Takeda offices into ‘Takeda Community Spaces’ centered around collaborative innovation, employee well-being and learning. These spaces are designed for maximizing in-person interactions, where people can focus, collaborate and connect more closely. Our approach is in line with our guiding principles around ways of working, with a focus on embracing flexibility, fostering inclusion with regular face-to- face interactions and leveraging data and insights. People leaders are at the forefront and they are empowered to implement the best ways of working for their teams. We also announced last year that we will create a ‘One Takeda Cambridge Campus’ in Boston that will enable us to bring our colleagues and partners together to brainstorm, inspire and create innovations for patients. These components help us to build an exceptional people experience that promotes well-being and performance. We are convinced that executing this transformation well will be a competitive advantage. As part of this, we are upskilling and insourcing employees, building in-house capabilities or ‘muscle within’ and maintaining our focus on our DD&T learning programs to create an agile, resilient and future-proof organization – one that prepares Takeda for long-term sustainability. By nurturing a culture of lifelong learning, our people, regardless of role, can reach their highest potential, allowing them to thrive in both their work and personal lives. I’m proud, too, of the speak-up culture that Takeda has fostered over the years. This is about empowering our employees to grow our diverse, equitable, inclusive, safe, open and collaborative working environments, where everyone can contribute, perform and thrive as individuals. As a values-based and patient- centric company, we strive to have a workforce as diverse as the communities 12 Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Unleashing Data, Digital and Technology in Our Transformation Efforts A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Appendix Examining the Macro Environment
and patients we serve. Our people policies and practices have been recognized again by the Top Employers Institute, which named Takeda as a Global Top Employer for the sixth consecutive year in 2023, one of only 15 companies to achieve this recognition. Unleashing Data, Digital and Technology in Our Transformation Efforts Our unwavering commitment to putting patients first always guides our discovery and excellence, and helps us as we strive to address unmet medical needs. The digital age is revolutionizing how we serve patients, support our employees and work towards a better, healthier and brighter world. Takeda is striding towards a reimagined future where data, digital and technology transform the way we work across our entire value chain and enable us to become the most trusted digital biopharmaceutical company. To-date, we’ve delivered on these efforts by providing personalized digital experiences to patients across the care pathway. We’re harnessing data as a digital enabler to generate sustainable value, democratizing technology and developing a digital- first mindset within our talent to speed innovation and improve outcomes and experiences for patients around the globe. How we discover, develop and deliver products faster is being fueled by our organizational agility and creating new digital capabilities. We strive to harness the full potential of data and digital, while staying true to our core values and ethics. Digital technology is now enabling us to design and conduct decentralized clinical trials to bring our trials to patients rather than having them travel to our sites. This approach offers a range of benefits for patients, including increased convenience, reduced travel burden and greater access to clinical trials. In manufacturing, we’re building highly advanced plants with automated visual inspection, using digital technologies to help improve efficiency and product quality. In Singen, Germany, for example, we’ve built a vaccine facility with state-of- the-art process equipment to enhance vaccine production. In addition, across all plasma sites that manufacture immunoglobulins (IG) we are applying predictive analytics to improve process efficiency and increase process robustness and understanding. We are expanding our Innovation Capability Center (ICC) model, which launched in 2021. The center is focused on delivering innovative solutions to internal customers primarily using in-house resources. It serves as an example of how we are leveraging DD&T to bring our insourcing strategy to life. 13 Unleashing Data, Digital and Technology in Our Transformation Efforts Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction A Steadfast Focus on the Future of Our Planet Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Appendix Examining the Macro Environment

We are also incorporating virtual reality into employee training programs for aseptic practices in clean rooms and using artificial intelligence (AI) to streamline the onboarding experience for new colleagues. Our goal is to create more personalized experiences for all – fostering inclusion and collaboration and fueling innovation. Through Takeda ID, an app that provides a unified identity for patients, partners and caregivers to easily access the full suite of Takeda’s life-saving care and resources, we have established a digital trust leadership position and created a more streamlined, personalized and relevant experience when digitally interacting with Takeda. Internally, the app will save time and resources by removing the need to create separate authentication service for every new digital resource. Use of the app will also help to manage our exposure to digital threats and strengthen adherence to compliance and data privacy standards across our online channels. Our significant investment in DD&T innovation across Takeda, including the addition of new roles dedicated to this work, is just one sign of our commitment to improving the patient experience, revolutionizing our approach to science and enhancing value creation and our resilience in a challenging environment. A Steadfast Focus on the Future of Our Planet We know that taking care of patients goes beyond developing life-transforming treatments. It’s also about being responsible, ethical and protecting our planet. There’s no doubt that pressure on environmental health continues to be an increasing threat to human health. Degrading air quality, increasing scarcity of clean water and other natural resources, waning biodiversity and the impacts of climate change are all threats to our well-being. These are issues Takeda is keenly aware of and considers in assessing how we can lessen our own impact. The cost of energy remains high worldwide. Takeda’s virtual power purchase agreement with Enel North America continues to guide our renewable energy production in the U.S. through the prioritization of clean energy solutions, progress towards net-zero targets and the creation of 350,000 megawatt hours (MWh) of renewable energy credits per year, for 12 years. We continue to lead the way and partner with others to find new and innovative ways to protect our planet. We are committed to decarbonizing our operations and working with suppliers to eliminate greenhouse gas emissions from our entire value chain. We are taking action to address our environmental impact on many important fronts, including reducing our impacts on critical natural resources such as water and biodiversity, and minimizing our impacts throughout the full life cycle of our products by applying the principles of a circular economy. Operationally, our environmental sustainability efforts focus on achieving net- 14 A Steadfast Focus on the Future of Our Planet Unleashing Data, Digital and Technology in Our Transformation Efforts Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Living Our Values: Corporate Governance Update Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Appendix Examining the Macro Environment

zero by 2035 for our own value chain and by 2040 for our entire value chain, including our suppliers and customers, in accordance with the Science Based Targets initiative’s (SBTi’s) Corporate Net-Zero Standard. Our initiatives include conserving natural resources and designing our products with sustainability principles in mind. As we put our values into practice each and every day, we continue to empower our employees to play their part to help conserve the world’s natural resources. I’m proud of the work we’re able to accomplish together. Living Our Values: Corporate Governance Update Our robust corporate governance model has been, and will continue to be, critical to Takeda’s success. Takeda recently announced that the Board of Directors will propose a new candidate for independent external director at the 147th Ordinary Meeting of Shareholders on June 28, 2023. If approved by shareholders, the new candidate, Miki Tsusaka, will join the board effective June 28. We are confident that Ms. Tsusaka will bring her valuable experience, including in digital transformation, as well as new thinking and innovation rooted in our patient-focused values. We are consistently working to advance the diversity of the Board, including in gender and nationalities, skills and experiences, while ensuring good representation of our global stakeholders without expanding the Board size further. After nearly 40 years of service, Masato Iwasaki, representative director, Japan General Affairs, will retire from Takeda. As head of Japan General Affairs, overseeing overall business activities in Japan, a member of the Takeda Executive Team and a Takeda Board member, Masato’s contributions during his long tenure at the company have been significant. I would like to thank Masato for successfully leading our business in Japan. Masato has been a strong proponent of Takeda’s globalization efforts and a key supporter of Takeda’s commitment to diversity, equity and inclusion. With Masato’s retirement, Milano Furuta, president of Japan Pharma Business Unit, will be named Japan country head in addition to his current role. I am proud to work with a talented Takeda Executive Team (TET), which represents nine nationalities across four generations (30-60s). This diverse leadership team sets the tone and helps create an environment where leaders can focus on delivering results while prioritizing the well-being and development of their teams in line with Takeda’s values, a concept we refer to as Caring Leadership. Under their leadership, I am confident that each of our 15 Living Our Values: Corporate Governance Update A Steadfast Focus on the Future of Our Planet Unleashing Data, Digital and Technology in Our Transformation Efforts Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Appendix Examining the Macro Environment

employees around the world will continue to make a meaningful impact on the lives of patients. Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World With all that we have achieved this past fiscal year, I’m encouraged by what’s to come. This is an exciting time for Takeda and despite the macro challenges our industry is facing, we are equipped with the right values, resilience and strategy to grow. Our vision to deliver life-transforming treatments to patients worldwide is not possible without our valued shareholders, so I thank you all sincerely for believing in Takeda. I’m proud of our progress, but I know there’s more to be done. As we look ahead, we aspire to build a sustainable future for patients, our people and the planet. On behalf of the Takeda Board of Directors, I am very grateful for your trust and support and look forward to welcoming you to our Annual Shareholders Meeting on June 28, 2023. 16 CHRISTOPHE WEBER President & CEO Takeda Pharmaceutical Company Limited Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Living Our Values: Corporate Governance Update A Steadfast Focus on the Future of Our Planet Unleashing Data, Digital and Technology in Our Transformation Efforts Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Appendix Examining the Macro Environment

17 Appendix Chart: Percentage of the population aged over 65, 2019 and 2050 Appendix Our Commitment to Delivering on Our Purpose: Better Health for People, Brighter Future for the World Living Our Values: Corporate Governance Update A Steadfast Focus on the Future of Our Planet Unleashing Data, Digital and Technology in Our Transformation Efforts Crediting the Power of Our People Business Momentum to Sustain Long- Term Growth Enhancing Innovation in Our Pipeline Through Strategic Asset Acquisitions Advancing Our Pipeline to Deliver Life-Transforming Treatments to Patients Advocating for a Patient-Centric Health Care System Reform Introduction Examining the Macro Environment
















