QuickLinks -- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
October 31, 2007
Date of Report (Date of earliest event reported)
REABLE THERAPEUTICS FINANCE LLC
(Exact Name of Registrant as Specified in its Charter)
Delaware
(State or Other Jurisdiction
of Incorporation) | | 333-142188
(Commission
File Number) | | 20-5653965
(IRS Employer
Identification No.) |
| | | | | |
9800 Metric Blvd., Austin, Texas
(Address of Principal Executive Offices) | | 78758
(Zip Code) |
(512) 832-9500
Registrant's telephone number, including area code
N/A
(Former Name or Former Address, if Changed Since Last Report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
On July 15, 2007, ReAble Therapeutics Finance LLC, a Delaware limited liability company ("RTFL"), and Reaction Acquisition Merger Sub, a newly formed Delaware corporation and a wholly owned subsidiary of RTFL ("Reaction Merger Sub"), entered into an agreement and plan of merger (the "Merger Agreement") with DJO Incorporated, a Delaware corporation ("DJO"), pursuant to which Reaction Merger Sub will merge with and into DJO, with DJO continuing as the surviving corporation and a wholly owned subsidiary of RTFL (the "Merger").
On November 3, 2006, ReAble Therapeutics, Inc. ("ReAble") was acquired by Grand Slam Holdings, LLC ("BCP Holdings") (the "Blackstone Acquisition"). BCP Holdings is controlled by investment funds affiliated with Blackstone Capital Partners V L.P. ("Blackstone"). The Blackstone Acquisition, the equity contribution by affiliates of Blackstone, the initial borrowings under our existing senior secured credit facilities, the offering of our existing senior subordinated notes by RTFL and ReAble Therapeutics Finance Corporation ("Finco"), the repayment of ReAble's prior senior secured credit facilities, the repurchase of 9.75% senior subordinated notes due 2012 issued by a subsidiary of ReAble, Encore Medical IHC, Inc., the repayment of certain other indebtedness of ReAble and the payment of the related fees and expenses are collectively referred to as the "Prior Transactions."
Unless otherwise noted or the context otherwise requires, (1) references to "we," "our," "us," and "the company" prior to the Merger are to RTFL and its consolidated subsidiaries (which includes all of ReAble's operations), and following the Merger and the other transactions described herein are to RTFL and its consolidated subsidiaries (including DJO Incorporated and its subsidiaries); (2) references to "ReAble" are to ReAble Therapeutics, Inc. and its subsidiaries prior to the Merger and (3) references to "DJO" are to DJO Incorporated and its subsidiaries prior to the Merger.
In connection with the consummation of the Merger and the other transactions described herein: (1) ReAble, the ultimate parent company for our business, is expected to change its name to "DJO Incorporated"; (2) ReAble Therapeutics Holdings LLC ("Holdings") is expected to change its name to "DJO Holdings LLC"; (3) RTFL is expected to change its name to "DJO Finance LLC"; (4) ReAble Therapeutics Finance Corporation ("Finco") is expected to change its name to "DJO Finance Corporation"; and (5) DJO, the ultimate parent company for the DJO business prior to the Merger, is expected to change its name to "DJO Opco Holdings, Inc."
"Safe Harbor" Statement under Private Securities Litigation Reform Act of 1995
This Current Report on Form 8-K contains forward-looking statements. Generally, you can identify these statements because they contain words like "anticipates," "believes," "estimates," "expects," "forecasts," "future," "intends," "plans" and similar terms. These statements reflect only our current expectations. Forward-looking statements include statements concerning our plans, objectives, goals, strategies, future events, capital expenditures, future results, our competitive strengths, our business strategy, the trends in our industry, and the benefits of our recent acquisitions.
Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy, and actual results may differ materially from those we anticipated due to a number of uncertainties, including, among others, the risks we face as described under the "Risk Factors" section below and elsewhere in this Current Report. You should not place undue reliance on these forward-looking statements. These forward-looking statements are within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and are intended to be covered by the safe harbors created thereby. To the extent that such statements are not recitations of historical fact, such statements constitute forward-looking statements that, by definition, involve risks and uncertainties. In any forward-looking statement where we express an expectation or belief as to future results or events, such expectation or belief is expressed in good faith and is believed to have a reasonable basis, but there can
2
be no assurance that such future results or events expressed by the statement of expectation or belief will be achieved or accomplished. Our actual results, performance or achievements could differ materially from those expressed in, or implied by, forward-looking statements. We can give you no assurance that any of the events anticipated by forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations and financial condition.
Some of the important factors that we believe could affect our results include the risks more fully disclosed below under the section headed "Risk Factors" and elsewhere in this Current Report, as well as in our most recent Quarterly Reports on Form 10-Q, including, without limitation, in conjunction with the forward-looking statements included in this Current Report. We caution you that in light of these risks and uncertainties, the matters referred to in the forward-looking statements contained in this Current Report may not in fact occur. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events or otherwise, except as otherwise required by law.
As provided in General Instruction B.2 of Form 8-K, the information contained in this Current Report on Form 8-K shall not be deemed to be "filed" for purposes of Section 18 of the Exchange Act, nor shall it be deemed to be incorporated by reference in any filing under the Securities Act, except as shall be expressly set forth by specific reference in such a filing. By furnishing this information, we make no admission as to the materiality of any information in this report that is required to be disclosed solely by reason of Regulation FD.
The Company hereby furnishes the following information regarding its business that was prepared in connection with the financing activities related to the Merger:
3
Our Company
We are a leading global provider of high-quality non-surgical orthopedic devices, with a broad range of products used for rehabilitation, pain management and physical therapy. After giving effect to the Merger, we will be the largest non-surgical orthopedic rehabilitation device company in the United States, and among the largest globally, as measured by revenues. Many of our products have leading market positions. We derived over 60% of our pro forma net sales for the twelve months ended September 29, 2007 from products for which we estimate we have leading market positions. We believe that our strong brand names, comprehensive range of products, focus on quality, innovation and customer service, extensive distribution network, and our strong relationships with orthopedic and physical therapy professionals have contributed to our leading market positions. We believe that we are one of only a few orthopedic device companies that offer healthcare professionals and patients a diverse range of orthopedic rehabilitation products addressing the complete spectrum of preventative, pre-operative, post-operative, clinical and home rehabilitation care. In addition, we develop, manufacture and distribute a broad range of surgical reconstructive implant products.
The Merger will combine ReAble's rehabilitation and musculoskeletal pain management platforms with DJO's orthopedic bracing, cold therapy, bone growth stimulation and vascular systems platforms. The Merger increases our product, geographic and payor diversification and creates an extensive and diverse distribution network. We believe each company's products are currently under-penetrated in the other's core distribution channels, providing us with opportunities to generate incremental revenue through cross-selling and category expansion. We also expect to realize significant cost savings by consolidating facilities, eliminating duplicate operations, improving supply chain management and achieving other efficiencies. For the twelve months ended September 29, 2007, we generated pro forma net sales of $873.6 million and pro forma Adjusted EBITDA of $253.8 million. For the definition of Adjusted EBITDA and a reconciliation of pro forma net loss to pro forma Adjusted EBITDA, see footnote (1) under "—Summary Unaudited Pro Forma Condensed Consolidated Financial Data."
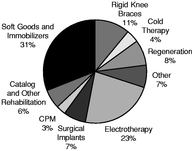
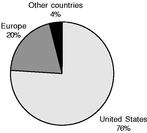
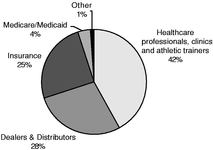
The following charts provide a breakdown of our pro forma net sales by product, geography and payor for the nine months ended September 29, 2007.
| Revenue by Product Category | | Revenue by Geography | | Revenue by Payor |
 |
|
 |
|
 |
4
Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals to treat patients with musculoskeletal conditions resulting from degenerative diseases, deformities, traumatic events and sports-related injuries. In addition, many of our non-surgical medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment.
Business Segments
After the completion of the Merger, we anticipate having three reportable segments: Domestic Rehabilitation, International Rehabilitation and Surgical Implant.
We market our domestic rehabilitation products through the three divisions described below. Our Domestic Rehabilitation Segment accounted for approximately 71% of our pro forma net sales for the twelve months ended September 29, 2007.
DonJoy, ProCare and Aircast. Our DonJoy, ProCare and Aircast division is the leader, based on revenues, in the U.S. orthopedic bracing and soft goods market, offering over 750 products in the following categories:
- •
- rigid knee bracing, which includes functional bracing for prevention and rehabilitation of ligament injuries, load shifting braces to relieve osteoarthritis pain and post-operative braces for protecting surgical repair;
- •
- orthopedic soft goods, which include over 500 products that offer immobilization and support from head to toe;
- •
- cold therapy products, which assist in the reduction of swelling and pain; and
- •
- vascular systems, which include products intended to prevent deep vein thrombosis following surgery.
This division also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at over 1,200 healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. As of September 29, 2007, the DonJoy, ProCare and Aircast division employed 392 independent commissioned sales representatives. These representatives are primarily dedicated to selling our products to healthcare providers. In addition, as of September 29, 2007, this division had over 100 direct and independent representatives managing our relationships with buying groups and national contracts and over 380 distributors focused on primary and acute care facilities.
Empi. Our Empi division is the leader, based on revenues, in the U.S. electrotherapy market, offering over 20 products in the following categories:
- •
- home electrotherapy, which includes transcutaneous electrical nerve stimulation ("TENS") for pain management and neuromuscular electrical nerve stimulation ("NMES") devices for rehabilitation;
- •
- bone growth stimulation products, which promote the healing of bone tissue through combined magnetic field ("CMF") technology;
- •
- iontophoresis, which includes devices and accessories to deliver medication transdermally; and
- •
- home traction, which includes cervical and lumbar traction devices designed to provide decompression to the spine.
5
As of September 29, 2007, this division employed over 200 direct and independent sales representatives and managers, targeting physicians, physical therapists and other healthcare providers that prescribe home electrotherapy devices, and approximately 300 support representatives that call on payors and patients. This division also includes our regeneration, or bone growth stimulation, business which has 155 employed and independent sales representatives and several independent spine distributors targeting healthcare providers that prescribe bone growth stimulation products. To facilitate reimbursement, our Empi division utilizes a proprietary third-party billing system, which is designed to improve billing accuracy, shorten payment cycles, improve collection rates and strengthen relationships with payors.
This division also includes our Rehab Med + Equip ("RME") business. RME sells a wide range of proprietary and third-party rehabilitation products to physical therapists and chiropractors through a printed catalog and through an on-line e-commerce site.
Chattanooga. Our Chattanooga division offers over 1,000 products in the clinical rehabilitation market in the following categories:
- •
- clinical electrotherapy devices (such as TENS, NMES, laser, ultrasound and light therapy);
- •
- clinical traction devices; and
- •
- other clinical products and supplies such as treatment tables, continuous passive motion ("CPM") devices and dry heat therapy.
The Chattanooga division markets its products globally primarily through a network of independent distributors. As of September 29, 2007, the Chattanooga division had over 4,500 independent distributors, serviced by 16 dedicated business-to-business sales employees.
Our International Rehabilitation Segment, which generates most of its revenues in the European and Canadian markets, is divided into three main businesses:
- •
- the international sales of our DonJoy, ProCare and Aircast products, including rigid knee bracing products, orthopedic soft goods, cold therapy products and vascular systems;
- •
- our Ormed business, which provides bracing, CPM, electrotherapy and other products in Germany; and
- •
- our Cefar/Compex business, which provides electrotherapy products for medical and consumer markets and other physical therapy and rehabilitation products primarily in Europe.
We market and sell our International Rehabilitation Segment products to orthopedic surgeons, pharmacies, orthopedic shops, hospitals, physical therapy and rehabilitation clinics and consumer retail outlets. As of September 29, 2007, this division had over 250 employed and independent representatives and a large network of independent distributors. The International Rehabilitation Segment accounted for approximately 22% of our pro forma net sales for the twelve months ended September 29, 2007.
Our Surgical Implant Segment develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market. We currently market and sell our Surgical Implant Segment products to hospitals and orthopedic surgeons through over 200 independent commissioned sales representatives in the United States and through independent distributors outside the United States. Our Surgical Implant Segment accounted for approximately 7% of our pro forma net sales for the twelve months ended September 29, 2007.
6
Market Opportunities
We participate globally in the rehabilitation, pain management, bone growth stimulation and reconstruction segments of the orthopedic device market. In the United States, we estimate these segments accounted for approximately $6.7 billion of total industry sales in 2006. We believe that several factors are driving growth in the orthopedic products industry, including the following:
- •
- Favorable demographics. An aging population is driving growth in the orthopedic products market. Many conditions that result in rehabilitation, physical therapy or orthopedic surgery are more likely to affect people in middle age or later in life. According to a 2005 United States Census Bureau projection, the aging baby boomer generation will result in the percentage of the U.S. population aged 65 and over growing from 12.4% in 2005 to 13.0% in 2010 and to 16.3% by 2020. In addition, according to the National Center for Health Statistics of the Centers for Disease Control, the average life expectancy in the United States increased from 75.4 years in 1990 to 77.5 years in 2003. According to a 2004 Frost & Sullivan report, these demographic trends in Europe are even more favorable as Europe has had the highest proportion of population age 65 and over among major world regions for many decades. As life expectancy increases, we believe people will remain active longer, causing the number of injuries requiring orthopedic rehabilitation, bone growth stimulation and reconstructive implants to increase.
- •
- Shift toward non-surgical rehabilitation devices and at-home physical therapy. We believe the growing awareness and clinical acceptance by healthcare professionals of the benefits of non-surgical, non-pharmaceutical treatment and rehabilitation products, combined with the increasing interest by patients in rehabilitation solutions that minimize risk and recuperation time and provide greater convenience, will continue to drive demand for these products. For example, TENS and NMES devices are increasingly being recognized as effective solutions for pain management and rehabilitation therapy, respectively. In addition, we believe that orthopedic surgeons are increasingly utilizing braces that assist in rehabilitation and bone growth stimulation devices that enable in-home treatment as viable alternatives to surgery. Many of our orthopedic rehabilitation products are designed for at-home use, which we believe should allow us to benefit from the market shift toward these treatment alternatives.
- •
- Lower cost alternatives appeal to third-party payors. With the cost of healthcare rising in the United States and internationally, third-party payors are seeking more cost-effective therapies without reducing quality of care. For example, third-party payors seek to reduce clinic visits and accommodate patients' preference for therapies that can be conveniently administered at home. We believe that many of our orthopedic rehabilitation products offer cost-effective alternatives to surgery, pharmaceutical and other traditional forms of physical therapy and pain management.
- •
- Increased need for rehabilitation due to increased orthopedic surgical volume. The combination of increased prevalence of degenerative joint disease (such as osteoarthritis), an increased number of sports-related injuries, an aging population and improvements in orthopedic surgical technique (such as arthroscopy) has contributed to an increase in the number of orthopedic surgeries. We believe that orthopedic surgical volume will continue to increase, which should result in an increase in the need for our products.
Competitive Strengths
We believe that we have a number of competitive strengths that will enable us to further enhance our position in the orthopedic rehabilitation device market:
- •
- Leading market positions. We derived over 60% of our pro forma net sales for the twelve months ended September 29, 2007 from products for which we estimate we have leading market positions, including soft goods, ankle braces, walking braces, rigid knee braces, clinical
7
electrotherapy, TENS, NMES, iontophoresis, cold therapy, home and clinical traction and CPM devices. We believe our orthopedic and physical therapy rehabilitation products marketed under the DonJoy, Aircast, ProCare, Chattanooga, Empi, Cefar/Compex and Ormed brands have a reputation for quality, durability and reliability among healthcare professionals. We believe the strength of our brands and our focus on customer service have allowed us to establish market leading positions in the highly fragmented and growing orthopedic rehabilitation market.
- •
- Comprehensive range of orthopedic rehabilitation products. We offer a diverse range of orthopedic devices, including orthopedic rehabilitation, pain management and physical therapy products, bone growth stimulation and surgical reconstructive implant products, to orthopedic specialists and patients for hospital, clinical and at-home therapies. Our broad product offering meets many of the needs of orthopedic rehabilitation professionals and patients and enables us to leverage our brand loyalty with our customer and distributor base. Our products are available across various stages of the orthopedic patient's continuum of care.
- •
- Extensive and diverse distribution network. We use multiple channels to distribute our products to our customers. As a result of the Merger, we will use the combined strength of over 5,500 distributors, our direct sales force of over 500 employed representatives and over 800 independent sales representatives to supply our products to physical therapy clinics, orthopedic surgeons and practices, orthotic and prosthetic clinics, hospitals, surgery centers, athletic trainers, chiropractors and retail outlets. We believe that our distribution network provides us with a significant competitive advantage in selling our existing products and in introducing new products.
- •
- Strong relationships with managed care organizations and rehabilitation healthcare providers. Our leading market positions in many of our orthopedic rehabilitation product lines and the breadth of our product offerings have enabled us to secure important preferred provider and managed care contracts. Our proprietary third-party billing system is designed to reduce our reimbursement cycles, improve relationships with managed care organizations and physicians and track patients to improve quality of care and create subsequent selling opportunities. Further, our OfficeCare business maintains inventory at over 1,200 healthcare facilities, primarily orthopedic practices, which further strengthens our relationships with these healthcare providers.
- •
- National contracts with group purchasing organizations. We enjoy strong relationships with a meaningful number of group purchasing organizations ("GPOs") due to our significant scale. We believe that our broad range of products is well suited to the goals of these buying groups. Under these national contracts, we provide favorable pricing to the buying group and are designated a preferred purchasing source for the members of the buying group for specified products. As we have made acquisitions and expanded our product range, we have been able to add incremental products to our national contracts. During the twelve months ended September 29, 2007, we signed or renewed five significant national contracts, and also added products from our recent acquisitions to a number of our national contracts.
- •
- Low cost, high quality manufacturing capabilities. Our principal manufacturing facility is located in Tijuana, Mexico and has been recognized for operational excellence. Our low cost manufacturing principles drive manufacturing efficiencies by employing lean manufacturing, Six Sigma concepts and continuous improvement processes. Following the Merger, we plan to move portions of ReAble's manufacturing to Mexico and expect to achieve savings from lower labor costs and implementation of more efficient processes. Further, we intend to extend lean manufacturing concepts to our manufacturing facilities in Clear Lake, South Dakota and Chattanooga, Tennessee.
- •
- Ability to generate significant cash flow. Historically, our strong competitive position, brand awareness and high quality products and service as well as our low cost manufacturing have
8
allowed us to generate attractive operating margins. These operating margins, together with limited capital expenditures and modest working capital requirements, significantly benefit our ability to generate free cash flow. Our combined capital expenditures of $21.3 million represented less than 8.4% of pro forma Adjusted EBITDA for the twelve months ended September 29, 2007.
- •
- Experienced and committed management team. The members of our combined management team have an average of over 24 years of relevant experience. This team has successfully integrated a number of acquisitions, including DJO's acquisition of Newmed SAS, a French orthopedics company ("Axmed") and Aircast Incorporated ("Aircast") in 2006 and ReAble's acquisition of Compex Technologies, Inc. ("Compex") and Cefar AB ("Cefar") in 2006. Certain members of our management are expected to make a contribution of new equity through the rollover of existing equity in connection with the Merger.
Strategy
Our strategy is to increase revenues and profitability and to enhance cash flow. Our key initiatives to implement this strategy include the following:
- •
- Increase our leading market positions. We believe we are the market leader in many of the markets in which we compete. We intend to continue to increase our market share by leveraging the cross-selling and other opportunities created by the Merger and by implementing the initiatives described below. The Merger will allow us to offer customers a more comprehensive range of products to better meet their evolving needs. We believe our size, scale, brand recognition, comprehensive and integrated product offerings and leading market positions will enable us to capitalize on the growth in the orthopedic product industry.
- •
- Increase sales force productivity. The distribution channels of DJO and ReAble are complementary and we believe that the Merger provides an opportunity to increase the productivity of our sales force. Following the Merger, our sales representatives will generally have a targeted customer base with a broader product offering for those customers. We plan to encourage cross-selling and increase the productivity of the entire sales force by focusing our sales organization and implementing a sales compensation plan to incentivize our sales representatives to sell a broader range of our products. For example, the Merger will combine DJO's strong relationship with orthopedic surgeons and ReAble's strong relationship with physical therapists. In addition, we intend to market DJO's bone growth stimulation products through Empi's extensive distribution network.
- •
- Maximize existing and secure additional national accounts. We plan to capitalize on the growing practice in healthcare in which hospitals and other large healthcare providers seek to consolidate their purchasing activities to national buying groups. Contracts with these national accounts represent a significant opportunity for revenue growth. We believe that our existing relationships with national buying groups and our broad range of products position us well not only to pursue additional national contracts, but also to expand the scope of our existing contracts.
- •
- Continue to develop and launch new products and product enhancements. Both DJO and ReAble have a history of developing and introducing innovative products into the marketplace, and we expect to continue future product launches by leveraging our internal research and development platforms. We believe our ability to develop new technology and to advance existing technology to create new products will position us to further diversify our revenues and to expand our target markets by providing viable alternatives to surgery or medication. We believe that product innovation though effective and focused research and development, as well as our relationships with a number of widely recognized orthopedic surgeons and professionals who assist us in product research, development and marketing, will provide a significant competitive advantage.
9
During the twelve months ended September 29, 2007, we launched 52 new products, which generated over $50 million in pro forma revenues.
- •
- Expand international sales. In recent years, we have successfully established direct distribution capabilities in several major international markets. We believe that sales to European and other markets outside the United States continue to represent a significant growth opportunity, and we intend to continue to expand our direct and independent distribution capabilities in attractive foreign markets. The Aircast, Axmed, Cefar and Compex acquisitions in 2006 have substantially increased our international revenues and operating infrastructure and have provided us with opportunities to expand our international product offerings. For the nine months ended September 29, 2007, international revenues increased to 23.0% of our pro forma revenues, compared to 18.9% for the year ended December 31, 2006, which included revenues of our International Rehabilitation Segment, as well as the international revenues of our Domestic Rehabilitation and Surgical Implant Segments.
- •
- Maximize cost savings opportunities. We expect the Merger to create significant opportunities to reduce manufacturing and other operating costs. We expect to achieve cost savings by leveraging DJO's low-cost manufacturing capabilities, rationalizing our combined manufacturing and distribution footprints, increasing procurement savings and eliminating duplicative overhead functions. We also intend to eliminate overlapping operating expenses and expect to reduce expenses through improved leveraging of our combined benefits of scale.
10
The Transactions
On July 15, 2007, RTFL and Reaction Merger Sub entered into the Merger Agreement with DJO pursuant to which Reaction Merger Sub will be merged with and into DJO, with DJO continuing as the surviving corporation and becoming a wholly owned subsidiary of RTFL. RTFL is controlled by affiliates of Blackstone Capital Partners V L.P. ("Blackstone"). As a result of the Merger:
- •
- each issued and outstanding share of common stock of DJO will generally be cancelled and converted into the right to receive $50.25 in cash, without interest and less any applicable withholding taxes; and
- •
- each outstanding option to purchase common stock under DJO's stock option plans, whether vested or unvested, except for options held by certain members of DJO management which will be rolled over in the Transactions, will be cancelled and entitle the holder to receive a cash payment equal to the excess, if any, of the per share merger consideration over the per share exercise price of the applicable stock option, multiplied by the number of shares subject to the stock option, less any applicable taxes required to be withheld.
The Merger is subject to a number of conditions and the completion of this offering is conditioned on the completion of the Merger.
In connection with the Merger, investment funds affiliated with Blackstone will provide equity financing in the aggregate amount of up to $421.9 million, which is expected to include the rollover of stock options by certain members of DJO management. In addition, in connection with the Merger, we intend to:
- •
- enter into new senior secured credit facilities, consisting of a $1,055.0 million six and a half-year term loan facility and a $100.0 million six-year revolving credit facility;
- •
- issue $575.0 million aggregate principal amount of new senior unsecured notes;
- •
- repay $428.3 million of ReAble's existing indebtedness, including accrued interest;
- •
- repay $288.6 million of DJO's existing indebtedness, including accrued interest; and
- •
- pay an estimated $84.0 million of fees and expenses.
11
Sources and Uses
The expected estimated sources and uses of the funds for the Transactions (assuming the Transactions had closed on September 29, 2007) are shown in the table below. Actual amounts may vary from estimated amounts depending on several factors, including differences from our estimate of the cost of repaying our and DJO's existing indebtedness, differences from our estimate of fees and expenses, fluctuations in cash on hand between September 29, 2007 and the actual closing date of the Transactions and any changes in the number of outstanding shares of DJO common stock or options to acquire DJO common stock since September 29, 2007, and any changes in these amounts may affect the amount of the final cash equity contribution.
(in millions)
Sources
| |
|
|---|
| New senior secured credit facilities: | | | |
| | Revolving credit facility(1) | | $ | — |
| | Term loan facility | | | 1,055.0 |
| New senior unsecured notes | | | 575.0 |
| Equity contribution(2) | | | 421.9 |
| Rollover equity(3) | | | 368.6 |
| Cash on hand | | | 43.8 |
| | |
|
| | Total sources | | $ | 2,464.3 |
| | |
|
Uses
| |
|
|---|
| DJO equity consideration(4) | | $ | 1,246.8 |
| Repayment of existing DJO indebtedness(5) | | | 288.6 |
| Repayment of existing ReAble indebtedness(6) | | | 428.3 |
| Cash on hand: | | | |
| | Prefunded integration costs | | | 33.0 |
| | Cash for operations | | | 15.0 |
| Rollover equity(3) | | | 368.6 |
| Transaction fees and expenses(7) | | | 84.0 |
| | |
|
| | Total uses | | $ | 2,464.3 |
| | |
|
- (1)
- We do not expect to have any outstanding borrowings under the revolving credit facility on the closing date of the Transactions.
- (2)
- Represents the approximate cash equity contribution to be made by investment funds affiliated with Blackstone and the expected contribution of new equity by certain members of DJO management through the rollover of existing stock options.
- (3)
- Represents (i) cash equity that investment funds affiliated with Blackstone contributed in connection with the Prior Transactions (as defined below) in November 2006, (ii) equity rollover in connection with a prior acquisition and (iii) the value of ReAble management stock options that were rolled over in the Prior Transactions, net of $1.4 million of rolled-over ReAble management stock options that will be cashed out in the Transactions. As of September 29, 2007, the total membership equity reflected on ReAble's balance sheet was $313.5 million.
- (4)
- Reflects the total consideration to be paid to holders of outstanding shares of DJO's common stock and holders of outstanding options to acquire shares of DJO common stock in the Merger, assuming that all stock options will be settled in cash. Assumes (i) 23.7 million shares outstanding, (ii) options to acquire DJO's common stock with a net option value of $57.7 million, which is calculated based on outstanding options to acquire 2.8 million shares of DJO common stock with an average exercise price of $29.69 per share, and (iii) the estimated number of shares issuable under the DJO employee stock purchase plan, with a net value of $0.3 million, in each case calculated as of September 29, 2007.
- (5)
- Consists of $285.5 million of outstanding borrowings and $3.1 million of accrued interest under DJO's existing senior secured credit facilities.
- (6)
- Consists of $423.4 million of outstanding borrowings and $4.9 million of accrued interest under ReAble's existing senior secured credit facilities.
- (7)
- Reflects our estimate of fees and expenses associated with the Transactions, including financing fees, advisory fees, other transaction costs and $4.4 million related to terminating DJO's and ReAble's existing interest rate swaps.
12
RISK FACTORS
Risks Related To Our Indebtedness
Our substantial leverage could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or our industry and expose us to interest rate risk.
After completing the Transactions, we will be highly leveraged. As of September 29, 2007, on a pro forma basis, our total indebtedness would have been $1,835.8 million. We also would have had an additional $100.0 million available for borrowing under our revolving credit facility.
Our high degree of leverage could have important consequences for you, including:
- •
- making it more difficult for us to make payments on the notes and our other debt;
- •
- increasing our vulnerability to general economic and industry conditions;
- •
- requiring a substantial portion of cash flow from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities;
- •
- exposing us to the risk of increased interest rates as certain of our borrowings, including borrowings under our new senior secured credit facilities, will be at variable rates of interest;
- •
- limiting our ability to make strategic acquisitions or causing us to make non-strategic divestitures;
- •
- limiting our ability to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions and general corporate or other purposes; and
- •
- limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors who are less highly leveraged.
We and our subsidiaries will be able to incur substantial additional indebtedness in the future. Although our new senior secured credit facilities, the indenture governing the new senior unsecured notes and the indenture governing our existing senior subordinated notes contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of significant qualifications and exceptions, and under certain circumstances, the amount of indebtedness that could be incurred in compliance with these restrictions could be substantial. If new indebtedness is added to our current debt levels, the related risks that we now face could intensify. In addition, the indenture governing the new senior unsecured notes will not prevent us from incurring obligations that do not constitute indebtedness under the indenture.
Our pro forma cash interest expense and fees for the unused portion of our new revolving credit facility for the twelve months ended September 29, 2007 would have been $171.5 million, compared to ReAble and DJO combined historical cash interest expense of $70.5 million for the twelve months ended September 29, 2007. At September 29, 2007, on a pro forma basis, we would have had $1,055.0 million of debt subject to floating interest rates under our new senior secured credit facilities. A 100 basis point increase in these floating interest rates would increase our cash annual interest expense by approximately $10.6 million. Any additional borrowings we make under our new senior secured credit facilities will also be subject to floating interest rates.
Our debt agreements will contain restrictions that limit our flexibility in operating our business.
Our new senior secured credit facilities and the indenture governing the new senior unsecured notes will contain and the indenture governing our existing senior subordinated notes already contains various covenants that will limit our ability to engage in specified types of transactions. These covenants will limit our and our restricted subsidiaries' ability to, among other things:
- •
- incur additional indebtedness or issue certain preferred shares;
- •
- pay dividends on, repurchase or make distributions in respect of our capital stock or make other restricted payments;
13
- •
- make certain investments;
- •
- sell certain assets;
- •
- create liens;
- •
- consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; and
- •
- enter into certain transactions with our affiliates.
In addition, we will be required to satisfy and maintain a specified senior secured leverage ratio, which becomes more restrictive over time. This covenant could materially and adversely affect our ability to finance our future operations or capital needs. Furthermore, it may restrict our ability to conduct and expand our business and pursue our business strategies. Our ability to meet this senior secured leverage ratio can be affected by events beyond our control, including changes in general economic and business conditions, and we cannot assure you that we will meet the senior secured leverage ratio in the future or at all.
A breach of any of these covenants could result in a default under one or more of these agreements, including as a result of cross default provisions. Upon the occurrence of an event of default under the new senior secured credit facilities, the lenders could elect to declare all amounts outstanding under the new senior secured credit facilities to be immediately due and payable and terminate all commitments to extend further credit. Such actions by those lenders could cause cross defaults under our other indebtedness. If we were unable to repay those amounts, the lenders under the new senior secured credit facilities could proceed against the collateral granted to them to secure that indebtedness. We have pledged a significant portion of our assets as collateral under the new senior secured credit facilities.
We may not be able to generate sufficient cash to service all of our indebtedness, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We cannot assure you that we will maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness. These alternative measures could affect the operation and growth of our business and may not be successful and may not permit us to meet our scheduled debt service obligations. If our operating results and available cash are insufficient to meet our debt service obligations, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. In that case, we may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them, and the proceeds from those dispositions may not be adequate to meet any debt service obligations then due. Additionally, our new senior secured credit facilities and the indenture governing our new senior unsecured notes will limit the use of the proceeds from dispositions of assets; as a result, we may not be permitted, under our new senior secured credit facilities and the indenture governing the notes, to use the proceeds from such dispositions to satisfy all current debt service obligations.
14
Risks Related To Our Business
We may not be able to successfully integrate ReAble and DJO, as well as other businesses ReAble and DJO have recently acquired, or businesses we acquire in the future, and we may not be able to realize the anticipated cost savings, revenue enhancements or other synergies from such acquisitions.
Our ability to successfully implement our business plan and achieve targeted financial results is highly dependent on our ability to successfully integrate ReAble and DJO, businesses ReAble and DJO have recently acquired and other businesses we acquire in the future. The process of integrating ReAble and DJO and such other acquired businesses involves risks. These risks include, but are not limited to:
- •
- demands on management related to the significant increase in the size of our business;
- •
- diversion of management's attention from the management of daily operations to the integration of newly acquired operations;
- •
- difficulties in the assimilation of different corporate cultures, practices and sales and distribution methodologies;
- •
- difficulties in conforming the acquired company's accounting policies to ours;
- •
- increased exposure to risks relating to business operations outside the United States;
- •
- retaining the loyalty and business of the customers of acquired businesses;
- •
- retaining employees that may be vital to the integration of the acquired business or to the future prospects of the combined businesses;
- •
- difficulties and unanticipated expenses related to the integration of departments, information technology systems, including accounting systems, technologies, books and records and procedures, and maintaining uniform standards, such as internal accounting controls, procedures and policies; and
- •
- costs and expenses associated with any undisclosed or potential liabilities.
Failure to successfully integrate ReAble and DJO, as well as Cefar, Compex, Saunders and IOMED or any other acquired businesses, may result in reduced levels of revenue, earnings or operating efficiency than we have achieved or might have achieved if we had not acquired such businesses, loss of customers of the acquired business.
In addition, an important part of our strategy is to generate revenue growth by cross selling the products of ReAble and DJO through the other's distribution network. Our ability to successfully cross sell our products to the extent we anticipate or at all is subject to considerable uncertainty. To the extent we are not successful in our cross selling efforts, our revenues and income will be adversely affected.
Furthermore, even if we are able to integrate successfully the operations of ReAble and DJO, as well as other businesses ReAble and DJO have recently acquired, or any other acquired business, we may not be able to realize the potential cost savings, synergies and revenue enhancements that were anticipated from the acquisition, either in the amount or within the time frame that we expect, and the costs of achieving these benefits may be higher than, and the timing may differ from, what we expect. Our ability to realize anticipated cost savings, synergies and revenue enhancements may be affected by a number of factors, including, but not limited to, the following:
- •
- the use of more cash or other financial resources on integration and implementation activities than we expect;
15
- •
- increases in other expenses unrelated to the acquisition, which may offset the cost savings and other synergies from the acquisition;
- •
- our ability to eliminate effectively duplicative back office overhead and overlapping and redundant selling, general and administrative functions, rationalize manufacturing capacity and shift production to more economical facilities; and
- •
- our ability to avoid labor disruptions in connection with any integration, particularly in connection with any headcount reduction.
Specifically, we expect the acquisition of DJO to create significant opportunities to reduce our manufacturing and other costs. We expect to achieve cost savings by leveraging DJO's low-cost manufacturing capabilities, rationalizing our combined manufacturing and distribution footprints, increasing procurement savings and eliminating duplicative overhead functions. We also expect to receive cost savings from initiatives we have undertaken in connection with a number of our previously completed acquisitions. However, while these anticipated cost savings reflect estimates and assumptions made by our management as to the benefits and associated expenses with respect to our cost savings initiatives, it is possible that these estimates and assumptions may not ultimately reflect actual results. In addition, these estimated cost savings are only estimates and may not actually be achieved in the timeframe anticipated or at all.
If we fail to realize anticipated cost savings, synergies or revenue enhancements, our financial results will be adversely affected, and we may not generate the cash flow from operations that we anticipated, or that is sufficient to repay our indebtedness.
As a result of the Merger, our concentration of manufacturing operations in Mexico increases our business and competitive risks.
Following the Merger, our most significant manufacturing facility will be DJO's facility in Tijuana, Mexico, and we will also have a relatively small manufacturing operation in Tunisia. In addition, we plan to move portions of ReAble's manufacturing to Mexico. Our current and future foreign operations are subject to risks of political and economic instability inherent in activities conducted in foreign countries. Because there are no readily accessible alternatives to these facilities, any event that disrupts manufacturing at or distribution or transportation from these facilities would materially adversely affect our operations. In addition, as a result of this concentration of manufacturing activities, our sales in foreign markets may be at a competitive disadvantage to products manufactured locally due to freight costs, custom and import duties and favorable tax rates for local businesses.
We may experience substantial fluctuations in our quarterly operating results and you should not rely on them as an indication of our future results.
Our quarterly operating results may vary significantly due to a combination of factors, many of which are beyond our control. These factors include:
- •
- demand for many of our products, which historically has been higher in the fourth quarter when scholastic sports and ski injuries are more frequent;
- •
- our ability to meet the demand for our products;
- •
- the direct distribution of our products in foreign countries that have seasonal variations;
- •
- the number, timing and significance of new products and product introductions and enhancements by us and our competitors, including delays in obtaining government review and clearance of medical devices;
16
- •
- our ability to develop, introduce and market new and enhanced versions of our products on a timely basis;
- •
- the impact of any acquisitions that occur in a quarter;
- •
- the impact of any changes in generally accepted accounting principles;
- •
- changes in pricing policies by us and our competitors and reimbursement rates by third-party payors, including government healthcare agencies and private insurers;
- •
- the loss of any of our significant distributors;
- •
- changes in the treatment practices of orthopedic and spine surgeons, primary care physicians, and pain-management specialists, and their allied healthcare professionals; and
- •
- the timing of significant orders and shipments.
Accordingly, our quarterly sales and operating results may vary significantly in the future and period-to-period comparisons of our results of operations may not be meaningful and should not be relied upon as indications of future performance. We cannot assure you that our sales will increase or be sustained in future periods or that we will be profitable in any future period.
We operate in a highly competitive business environment, and our inability to compete effectively could adversely affect our business prospects and results of operations.
We operate in highly competitive and fragmented markets. Our Domestic Rehabilitation Segment and International Rehabilitation Segment compete with both large and small companies, including several large, diversified companies with significant market share and numerous smaller niche companies, particularly in the physical therapy products market. Our Surgical Implant Segment competes with a small number of very large companies that dominate the market, as well as other companies similar to our size. We may not be able to offer products similar to, or more desirable than, those of our competitors or at a price comparable to that of our competitors. Compared to us, many of our competitors have:
- •
- greater financial, marketing and other resources;
- •
- more widely accepted products;
- •
- a larger number of endorsements from healthcare professionals;
- •
- a larger product portfolio;
- •
- superior ability to maintain new product flow;
- •
- greater research and development and technical capabilities;
- •
- patent portfolios that may present an obstacle to the conduct of our business;
- •
- stronger name recognition;
- •
- larger sales and distribution networks; and/or
- •
- international manufacturing facilities that enable them to avoid the transportation costs and foreign import duties associated with shipping our products manufactured in the United States to international customers.
Accordingly, we may be at a competitive disadvantage with respect to our competitors. These factors may materially impair our ability to develop and sell our products.
17
If we are unable to develop or license new products or product enhancements or find new applications for our existing products, we will not remain competitive.
The markets for our products are characterized by continued new product development and the obsolescence of existing products. Our future success and our ability to increase revenues and make payments on our indebtedness will depend, in part, on our ability to develop, license, acquire and distribute new and innovative products, enhance our existing products with new technology and find new applications for our existing products. However, we may not be successful in developing, licensing or introducing new products, enhancing existing products or finding new applications for our existing products. We also may not be successful in manufacturing, marketing and distributing products in a cost-effective manner, establishing relationships with marketing partners, obtaining coverage of and satisfactory reimbursement for our future products or product enhancements or obtaining required regulatory clearances and approvals in a timely fashion or at all. If we fail to keep pace with continued new product innovation or enhancement or fail to successfully commercialize our new or enhanced products, our competitive position, financial condition and results of operations could be materially and adversely affected.
In addition, if any of our new or enhanced products contain undetected errors or design defects, especially when first introduced, or if new applications that we develop for existing products do not work as planned, our ability to market these and other products could be substantially delayed, and we could ultimately become subject to product liability litigation, resulting in lost revenues, potential damage to our reputation and/or delays in regulatory clearance. In addition, approval of our products or obtaining acceptance of our products by physicians, physical therapists and other healthcare professionals that recommend and prescribe our products could be adversely affected.
The success of our surgical implant products depends on our relationships with leading surgeons who assist with the development and testing of our products.
A key aspect of the development and sale of our surgical implant products is the use of designing and consulting arrangements with orthopedic surgeons who are well recognized in the healthcare community. These surgeons assist in the development and clinical testing of new surgical implant products. They also participate in symposia and seminars introducing new surgical implant products and assist in the training of healthcare professionals in using our new products. We may not be successful in maintaining or renewing our current designing and consulting arrangements with these surgeons or in developing similar arrangements with new surgeons. In that event, our ability to develop, test and market new surgical implant products could be adversely affected.
Proposed laws that would limit the types of orthopedic professionals who can fit, sell or seek reimbursement for our products could, if adopted, adversely affect our business.
In response to pressure from certain groups (mostly orthotists), the United States Congress and state legislatures have periodically considered proposals that limit the types of orthopedic professionals who can fit or sell our orthotic device products or who can seek reimbursement for them. Several states have adopted legislation which imposes certification or licensing requirements on the measuring, fitting and adjusting of certain orthotic devices. Although some of these state laws exempt manufacturers' representatives, other states' laws subject the activities of such representatives to certification or licensing requirements. Additional states may be considering similar legislation. Such laws could reduce the number of potential customers by restricting the activities of our sales representatives in those jurisdictions where such legislation or regulations are enacted. Furthermore, because the sales of orthotic devices are driven in part by the number of professionals who fit and sell them, laws that limit these activities could reduce demand for these products. We may not be successful in opposing the adoption of such legislation or regulations and, therefore, such laws could have a material adverse effect on our business.
18
In addition, efforts have been made to establish similar requirements at the federal level for the Medicare program. For example, in 2000, Congress passed the Medicare, Medicaid and SCHIP Benefits Improvement and Protection Act of 2000 ("BIPA"). BIPA contained a provision requiring, as a condition for payment by the Medicare program, that certain certification or licensing requirements be met for individuals and suppliers furnishing certain, but not all, custom-fabricated orthotic devices. Although the Centers for Medicare and Medicaid Services have not implemented this requirement, we cannot predict the effect of implementation of BIPA or implementation of other such laws will have on our business.
If we fail to establish new sales and distribution relationships or maintain our existing relationships, or if our third-party distributors and independent sales representatives fail to commit sufficient time and effort or are otherwise ineffective in selling our products, our results of operations and future growth could be adversely impacted.
The sales and distribution of certain of our orthopedic rehabilitation products, regeneration products and our surgical implant products depend, in part, on our relationships with a network of third-party distributors and independent commissioned sales representatives. These third-party distributors and independent sales representatives maintain the relationships with the hospitals, orthopedic surgeons, physical therapists and other healthcare professionals that purchase, use and recommend the use of our products. Although our internal sales staff trains and manages these third-party distributors and independent sales representatives, we do not directly monitor the efforts that they make to sell our products. In addition, some of the independent sales representatives that we use to sell our surgical implant products also sell products that directly compete with our core product offerings. These sales representatives may not dedicate the necessary effort to market and sell our products. If we fail to attract and maintain relationships with third-party distributors and skilled independent sales representatives or fail to adequately train and monitor the efforts of the third-party distributors and sales representatives that market and sell our products, or if our existing third-party distributors and independent sales representatives choose not to carry our products, our results of operations and future growth could be adversely affected.
We rely on our own direct sales force for certain of our products, which may result in higher fixed costs than our competitors and may slow our ability to reduce costs in the face of a sudden decline in demand for our products.
We rely on our own direct sales force of over 340 representatives in the United States and over 190 representatives in Europe to market and sell certain of the orthopedic rehabilitation products which are intended for use in the home and in rehabilitation clinics. Some of our competitors rely predominantly on independent sales agents and third-party distributors. A direct sales force may subject us to higher fixed costs than those of companies that market competing products through independent third parties, due to the costs that we will bear associated with employee benefits, training and managing sales personnel. As a result, we could be at a competitive disadvantage. Additionally, these fixed costs may slow our ability to reduce costs in the face of a sudden decline in demand for our products, which could have a material adverse effect on our results of operations.
The success of all of our products depends heavily on our relationships with healthcare professionals who prescribe and recommend our products, and our failure to maintain or develop these relationships could adversely affect our business.
We have developed and maintain close relationships with a number of orthopedic surgeons, primary care physicians, other specialist physicians, physical therapists, athletic trainers, chiropractors and other healthcare professionals. We believe that sales of our products depend significantly on their recommendations of our products. Acceptance of our products depends on educating the healthcare
19
community as to the distinctive characteristics, perceived benefits, clinical efficacy and cost-effectiveness of our products compared to the products offered by our competitors and on training healthcare professionals in the proper use and application of our products. Failure to maintain these relationships and develop similar relationships with other leading healthcare professionals could result in a less frequent recommendation of our products, which may adversely affect our sales and profitability.
Our international operations expose us to risks related to conducting business in multiple jurisdictions outside the United States.
The international scope of our operations exposes us to economic, regulatory and other risks in the countries in which we operate. On a pro forma basis, we would have generated approximately 18% and 22% of our net revenues from customers outside the United States for the year ended December 31, 2006 and the nine months ended September 29, 2007, respectively. Doing business in foreign countries exposes us to a number of risks, including the following:
- •
- fluctuations in currency exchange rates;
- •
- imposition of investment, currency repatriation and other restrictions by foreign governments;
- •
- potential adverse tax consequences, including the imposition or increase of withholding and other taxes on remittances and other payments by foreign subsidiaries, which, among other things, may preclude payments or dividends from foreign subsidiaries from being used for our debt service, and exposure to adverse tax regimes;
- •
- difficulty in collecting accounts receivable and longer collection periods;
- •
- the imposition of additional foreign governmental controls or regulations on the sale of our products;
- •
- intellectual property protection difficulties;
- •
- changes in political and economic conditions;
- •
- difficulties in attracting high-quality management, sales and marketing personnel to staff our foreign operations;
- •
- labor disputes;
- •
- import and export restrictions and controls, tariffs and other trade barriers;
- •
- increased costs of transportation or shipping;
- •
- exposure to different approaches to treating injuries;
- •
- exposure to different legal, regulatory and political standards; and
- •
- difficulties of local governments in responding to severe weather emergencies, natural disasters or other such similar events.
In addition, as we grow our operations internationally, we will become increasingly dependent on foreign distributors and sales agents for our compliance and adherence to foreign laws and regulations that we may not be familiar with, and we cannot assure you that these distributors and sales agents will adhere to such laws and regulations or adhere to our own business practices and policies. Any violation of laws and regulations by foreign distributors or sales agents or a failure of foreign distributors or sales agents to comply with our business practices and policies could result in legal or regulatory sanctions against us or potentially damage our reputation in that international market. If we fail to manage these risks effectively, we may not be able to grow our international operations, and our business and results of operations may be materially adversely affected.
20
To date, we have not used international currency derivatives to hedge against our investment in our European subsidiaries or their operating results, which are converted into U.S. Dollars at period-end and average rates, respectively.
As a result of the Merger, our concentration of manufacturing operations in Mexico increases our business and competitive risks.
Following the Merger, our most significant manufacturing facility will be DJO's facility in Tijuana, Mexico, and we will also have a relatively small manufacturing operation in Tunisia. In addition, we plan to move portions of ReAble's manufacturing to Mexico. Our current and future foreign operations are subject to risks of political and economic instability inherent in activities conducted in foreign countries. Because there are no readily accessible alternatives to these facilities, any event that disrupts manufacturing at or distribution or transportation from these facilities would materially adversely affect our operations. In addition, as a result of this concentration of manufacturing activities, our sales in foreign markets may be at a competitive disadvantage to products manufactured locally due to freight costs, custom and import duties and favorable tax rates for local businesses.
Fluctuations in foreign exchange rates may adversely affect our financial condition and results of operations and may affect the comparability of our results between financial periods.
Our foreign operations expose us to currency fluctuations and exchange rate risks, and our acquisition of DJO will increase that exposure. We are exposed to the risk of currency fluctuations between the U.S. Dollar and the Euro, Pound Sterling, Canadian Dollar, Mexican Peso, Swiss Franc, Norwegian Krona, Danish Krona and Swedish Krona. On a pro forma basis after giving effect to the Transactions, sales denominated in foreign currencies accounted for approximately 18% of our consolidated net sales for the year ended December 31, 2006, of which 15% is related to the Euro, and approximately 22% of our consolidated net sales for the nine months ended September 29, 2007, of which 17% is related to the Euro. Our exposure to fluctuations in foreign currencies arises because certain of our subsidiaries' results are recorded in these currencies and then translated into U.S. Dollars for inclusion in our consolidated financial statements and certain of our subsidiaries enter into purchase or sale transactions using a currency other than our functional currency. Therefore, changes in currency exchange rates may adversely affect our financial condition and results of operations and may affect the comparability of our results between reporting periods.
We may not be able to effectively manage our currency translation risks, and volatility in currency exchange rates may adversely affect our financial condition and results of operations.
If adequate levels of reimbursement coverage from third-party payors for our products are not obtained, healthcare providers and patients may be reluctant to use our products, and our sales may decline.
Our sales depend largely on whether there is adequate reimbursement coverage by government healthcare programs, such as Medicare and Medicaid, and by private payors. We believe that surgeons, hospitals, physical therapists and other healthcare providers may not use, purchase or prescribe our products and patients may not purchase our products if these third-party payors do not provide satisfactory reimbursement for the costs of our products or the procedures involving the use of our products. Consequently, we may be unable to sell our products on a profitable basis if third-party payors deny coverage, reduce their current levels of reimbursement or fail to cause their levels of reimbursement to rise quickly enough to cover cost increases.
Changes in the coverage of, and reimbursement for, our products by these third-party payors could have a material adverse effect on our results of operations. Third-party payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, decide not to reimburse for treatments that include the use of our products. They may attempt to control costs by
21
(i) authorizing fewer elective surgical procedures, including joint reconstructive surgeries, (ii) requiring the use of the least expensive product available or (iii) reducing the reimbursement for or limiting the number of authorized visits for rehabilitation procedures. For example, in the United States, Medicare frequently engages in efforts to contain costs, which may result in a reduction of coverage of, and reimbursement for, our products. Because many private payors model their coverage and reimbursement policies on Medicare policies, third-party payors' coverage of, and reimbursement for, our products could be negatively impacted by legislative, regulatory or other measures that reduce Medicare coverage and reimbursement generally.
Our international sales also depend in part upon the coverage and eligibility for reimbursement of our products through government-sponsored healthcare payment systems and third-party payors, the amount of reimbursement and the cost allocation of payments between the patient and government-sponsored healthcare payment systems and third-party payors. Coverage and reimbursement practices vary significantly by country, with certain countries requiring products to undergo a lengthy regulatory review in order to be eligible for third-party coverage and reimbursement. In addition, healthcare cost containment efforts similar to those we face in the United States are prevalent in many of the foreign countries in which our products are sold, and these efforts are expected to continue in the future, possibly resulting in the adoption of more stringent reimbursement standards. For example, in Germany, our largest foreign country market, new regulations generally require adult patients to pay a portion of the cost of each medical technical device purchased. This may adversely affect our sales and profitability by making it more difficult for patients in Germany to pay for our products.
Any developments in the United States or our foreign markets that eliminate, reduce or materially modify coverage of, and reimbursement rates for, our products could have an adverse effect on our ability to sell our products.
22
Recent changes in coverage and reimbursement policies for our products by Medicare or reductions in reimbursement rates for our products could adversely affect our business and results of operations.
The Medicare Prescription Drug, Improvement and Modernization Act of 2003 ("Medicare Modernization Act") mandates a number of changes in the Medicare payment methodology and conditions for coverage of orthotic devices and durable medical equipment, including many of our products. These changes include a temporary freeze in annual increases in payments for durable medical equipment from 2004 through 2008, competitive bidding requirements, new clinical conditions for payment and quality standards. Although these changes affect our products generally, specific products may be affected by some but not all of the Medicare Modernization Act's provisions.
Prefabricated orthotic devices and certain durable medical equipment, including many of our products, may be subject to a competitive bidding process established under the Medicare Modernization Act. In April 2007, the Centers for Medicare and Medicaid Services, the agency responsible for administering the Medicare program, issued final regulations in connection with the competitive bidding program. Under competitive bidding, Medicare will no longer reimburse for certain products and services based on the Medicare fee schedule amount in designated competitive bidding areas. Instead, the Centers for Medicare and Medicaid Services will provide reimbursement for these items and services based on a competitive bidding process. Only those suppliers selected as a result of the competitive bidding process within each designated region will be eligible to have their products reimbursed through Medicare. Competitive bidding will go into effect September 30, 2008 initially in ten metropolitan statistical areas and will apply to ten product categories. In 2009, the program will be expanded to 80 metropolitan statistical areas (and additional areas thereafter), and additional product categories may be selected. The competitive bidding process may reduce the number of suppliers providing certain items and services to Medicare beneficiaries and the amounts paid for such items and services within a given geographic area. None of our products were included in the initial round of items subject to bidding; however, there is no assurance they will not be included in the future. Inclusion of any of our products in Medicare competitive bidding or other Medicare reimbursement reductions could result in such products being sold in lesser quantities or for a lower price, or such products being discontinued altogether. Any of these developments could have a material adverse effect on our results of operations. In addition, if we are not selected to participate in the competitive bidding program in a particular region, it could have a material adverse effect on our sales and profitability.
Our success depends on receiving regulatory approval for our products, and failure to do so could adversely affect our growth and operating results.
Our products are subject to extensive regulation in the United States by the Food and Drug Administration (the "FDA") and by similar governmental authorities in the foreign countries where we do business. The FDA regulates virtually all aspects of a medical device's development, testing, manufacturing, labeling, promotion, distribution and marketing. In general, unless an exemption applies, a medical device must receive either pre-market approval or pre-market clearance from the FDA before it can be marketed in the United States. While in the past we have received such approvals, we may not be successful in the future in receiving such approvals in a timely manner or at all. If we begin to have significant difficulty obtaining such FDA approvals or clearances in a timely manner or at all, we could suffer a material adverse effect on our revenues and growth.
If we fail to obtain regulatory approval for the modification of or new uses for our products, our growth and operating results could suffer.
In order to market modifications to our existing products or market our existing products for new indications, we may be required to obtain pre-market approvals, pre-market approval supplements or pre-market clearances. The FDA requires device manufacturers themselves to make and document a determination of whether or not a modification requires a new approval, supplement or clearance; however, the FDA can review and disagree with a manufacturer's decision. We may not be successful in
23
receiving such approvals or the FDA may not agree with our decisions not to seek approvals, supplements or clearances for any particular device modification. The FDA may require an approval or clearance for any past or future modification or a new indication for our existing products. Such submissions may require the submission of additional clinical or preclinical data and may be time consuming and costly, and may not ultimately be cleared or approved by the FDA. If the FDA requires us to obtain pre-market approvals, pre-market approval supplements or pre-market clearances for any modification to a previously cleared or approved device, we may be required to cease manufacturing and marketing the modified device or to recall such modified device until we obtain FDA clearance or approval, and we may be subject to significant regulatory fines or penalties. In addition, the FDA may not clear or approve such submissions in a timely manner, if at all. Because a significant portion of our revenues is generated by products that are modified or used for new treatments, delays or failures in obtaining such approvals could reduce our revenue and adversely affect our operating results.
We may fail to receive positive clinical results for our products in development that require clinical trials, and even if we receive positive clinical results, we may still fail to receive the necessary clearance or approvals to market our products.
In the development of new products or new indications for, or modifications to, existing products, we may conduct or sponsor clinical trials. Clinical trials are expensive and require significant investment of time and resources and may not generate the data we need to support a submission to the FDA. Clinical trials are subject to regulation by the FDA and, if federal funds are involved or if an investigator or site has signed a federal assurance, are subject to further regulation by the Office for Human Research Protections and the National Institutes of Health. Failure to comply with such regulation, including, but not limited to, failure to obtain adequate consent of subjects, failure to adequately disclose financial conflicts or failure to report data or adverse events accurately, could result in fines, penalties, suspension of trials, and the inability to use the data to support an FDA submission. In the international market, we are subject to regulations for clinical studies in each respective country.
If we fail to comply with the various regulatory regimes for the foreign markets in which we operate, our operational results could be adversely affected.
In many of the foreign countries in which we market our products, we are subject to extensive regulations that are similar to those of the FDA, including those in Europe. The regulation of our products in Europe falls primarily within the European Economic Area, which consists of the twenty-seven member states of the European Union, as well as Iceland, Liechtenstein and Norway. Only medical devices that comply with certain conformity requirements are allowed to be marketed within the European Economic Area. In addition, the national health or social security organizations of certain foreign countries, including those outside Europe, require our products to be qualified before they can be marketed in those countries. Failure to receive, or delays in the receipt of, relevant foreign qualifications in the European Economic Area or other foreign countries could have a material adverse effect on our business.
The FDA regulates the export of medical devices to foreign countries and certain foreign countries may require FDA certification that our products are in compliance with U.S. law. If we fail to obtain or maintain export certificates required for the export of our products, we could suffer a material adverse effect on our revenues and growth.
If we fail to comply with the FDA's Quality System Regulation, our manufacturing could be delayed, and our product sales and profitability could suffer.
Our manufacturing processes are required to comply with the FDA's Quality System Regulation, which covers the procedures concerning (and documentation of) the design, testing, production processes, controls, quality assurance, labeling, packaging, storage and shipping of our devices. We also are subject to state requirements and licenses applicable to manufacturers of medical devices. In
24
addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facilities and records for periodic unscheduled inspections by governmental agencies, including the FDA, state authorities and comparable agencies in other countries. Moreover, if we fail to pass a Quality System Regulation inspection or to comply with these and other applicable regulatory requirements, we may receive a warning letter or could otherwise be required to take corrective action and, in severe cases, suffer a disruption of our operations and manufacturing delays. If we fail to take adequate corrective action we could be subject to certain enforcement actions, including, among other things, significant fines, suspension of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions. We cannot assure you that the FDA or other governmental authorities would agree with our interpretation of applicable regulatory requirements or that we have in all instances fully complied with all applicable requirements. Any failure to comply with applicable requirements could adversely affect our product sales and profitability.
We have received FDA warning letters in the past. Most recently, ReAble received a warning letter in January 2007 in which the FDA investigator concluded that ReAble had failed to establish and maintain adequate procedures for handling and investigating complaints, failed to establish and maintain procedures for changes to specifications and processes, including verification or validation of such changes, and found that these failures caused certain products to be misbranded. In the warning letter, the FDA informed ReAble that it would not receive premarket approval for Class III devices to which these issues are reasonably related until the issues have been corrected. ReAble submitted a response and met with FDA representatives, and believes it has instituted the necessary changes and improvements in its policies and procedures to correct these issues. It is possible, however, that the FDA will find that ReAble has not corrected these issues, in which case, ReAble could be subject to the limit on premarket approvals and the other enforcement actions described above.
If the Office of Inspector General ("OIG"), the FDA or another regulatory agency determines that we have promoted off-label use of our products, we may be subject to various penalties, including civil or criminal penalties, and the off-label use of our products may result in injuries that lead to product liability suits, which could be costly to our business.
The OIG, the FDA and other regulatory agencies actively enforce regulations prohibiting the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as "off-label" use. Physicians may use our products for off-label uses, as the FDA does not restrict or regulate a physician's choice of treatment within the practice of medicine. However, if the OIG or FDA or another regulatory agency determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management's attention and result in substantial damage awards against us.
Our compensation, marketing and sales practices may contain certain risks with respect to the manner in which these practices were historically conducted that could have a material adverse effect on us.
We have entered into written agreements for designing and consulting services with surgeons for surgical implant products, and we compensate them under our designing surgeon agreements for services in developing products sold by us. The form of compensation for such services has historically been a royalty on the sale of our surgical implant products. We compensate the surgeons who help us in our product development and clinical efforts with cash payments. We believe that in each instance remuneration paid to surgeons represents fair market value for the services provided and is otherwise
25
in compliance with applicable laws. We also use an independent sales force for our surgical implant products for which we provide compliance-related training. The sales force has generally been compensated on commission based on a percentage of revenues generated by products sold as are typical in our industry. We also pay physicians certain rental and office support fees under our OfficeCare and EmpiCare programs. Under applicable federal and state healthcare fraud and abuse, anti-kickback, false claims and self-referral laws, it could be determined that our designing and consulting arrangements with surgeons, our marketing and sales practices, and our OfficeCare and EmpiCare programs fall outside permitted arrangements, thereby subjecting us to possible civil and/or criminal sanctions (including exclusion from the Medicare and Medicaid programs), which could have a material adverse effect on our surgical implant business and possibly on our other lines of business. The federal government has significantly increased investigations of medical device manufacturers with regards to alleged kickbacks and other forms of remuneration to physicians who use and prescribe their products. Such investigations often arise based on allegations of violations of the federal Anti-Kickback Statute. Although we believe we maintain a satisfactory compliance program, it may not be adequate in the detection or prevention of violations. The form and effectiveness of our compliance program may be taken into account by the government in assessing sanctions, if any, should it be determined that violations of laws have occurred.
Audits or denials of our claims by government agencies could reduce our revenues or profits.
As part of our business operations, we submit claims on behalf of patients directly to, and receive payments directly from, the Medicare and Medicaid programs and private payors. Therefore, we are subject to extensive government regulation, including requirements for submitting reimbursement claims under appropriate codes and maintaining certain documentation to support our claims. Medicare contractors and Medicaid agencies periodically conduct pre- and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize healthcare claims and supporting documentation. We have historically been subject to pre-payment and post-payment reviews as well as audits of claims and may experience such reviews and audits of claims in the future. Such reviews and/or similar audits of our claims could result in material delays in payment, as well as material recoupments or denials, which would reduce our net sales and profitability, or in exclusion from participation in the Medicare or Medicaid programs. Private payors may from time to time conduct similar reviews and audits.
Additionally, we participate in the government's Federal Supply Schedule program for medical equipment, whereby we contract with the government to supply certain of our products. Participation in this program requires us to follow certain pricing practices and other contract requirements. Failure to comply with such pricing practices and/or other contract requirements could result in delays in payment or fines or penalties, which could reduce our revenues or profits.
We could be subject to governmental investigations under various healthcare "fraud and abuse" laws with respect to our business arrangements with prescribing physicians and other health care professionals.
We are directly, or indirectly through our clients, subject to various federal and state laws pertaining to health care fraud and abuse. These laws, which directly or indirectly affect our ability to operate our business include, but are not limited to, the following:
- •
- the federal Anti-Kickback Statute, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs, such as Medicare and Medicaid, Veterans Administration health programs, and TRICARE;
- •
- several federal False Claims statutes, which imposes civil and criminal liability on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government;
26
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits executing a scheme to defraud any healthcare benefit program, and also prohibits false statements, defined as knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
- •
- the federal physician self-referral prohibition, commonly known as the Stark Law, which, in the absence of a statutory or regulatory exception, prohibits the referral of Medicare and Medicaid patients by a physician to an entity for the provision of certain designated healthcare services, if the physician or a member of the physician's immediate family has a direct or indirect financial relationship, including an ownership interest in, or a compensation arrangement with, the entity and also prohibits that entity from submitting a bill to a federal payor for services rendered pursuant to a prohibited referral; and
- •
- state law equivalents to the Anti-Kickback Law and the Stark Law, some of which may apply even more broadly than their federal counterparts because they are not limited to government reimbursed items and include items or services reimbursed by any payor.
The federal government has significantly increased investigations of medical device manufacturers with regard to alleged kickbacks and other forms of remuneration to physicians who use and prescribe their products. Such investigations often arise based on allegations of violations of the federal Anti-Kickback Statute.
These laws and regulations are complex and even minor, inadvertent irregularities in submissions can potentially give rise to claims that the law has been violated. Any violations of these laws or regulations could result in a material adverse effect on our business, financial condition and results of operations. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change one or more of our business practices to be in compliance with these laws. Required changes could be costly and time consuming. Any failure to make required changes could result in our losing business or our existing business practices being challenged as unlawful.
Healthcare reform, managed care and buying groups have put downward pressure on the prices of our products.
Healthcare reform and the healthcare industry's response to rising healthcare costs have resulted in a significant expansion of managed care organizations and buying groups. This growth of managed care and the advent of buying groups in the United States have caused a shift toward coverage and payments based on more cost-effective treatment alternatives. Buying groups enter into preferred supplier arrangements with one or more manufacturers of medical products in return for price discounts to members of these buying groups. Our failure to obtain new preferred supplier commitments from major group purchasing organizations or our failure to retain our existing preferred supplier commitments could adversely affect our sales and profitability. In Germany and other international markets where we sell our products, we have historically experienced downward pressure on product pricing and other effects of healthcare reform similar to that which we have experienced in the United States. We expect healthcare reform and managed care to continue to develop in the United States and these international markets and put further downward pressure on product pricing, which may adversely affect our sales and profitability.
Our products are subject to recalls even after receiving FDA or foreign regulatory clearance or approval. Product recalls could harm our reputation and business.
We are subject to ongoing medical device reporting regulations that require us to report to the FDA or similar governmental authorities in other countries if our products cause, or contribute to, death or serious injury, or malfunction in a way that would be likely to cause, or contribute to, death or serious injury if the malfunction were to recur. The FDA and similar governmental authorities in other
27
countries have the authority to require us to recall our products in the event of material deficiencies or defects in design or manufacturing, and we have been subject to product recalls in the past. In addition, in light of a material deficiency, defect in design or manufacturing or defect in labeling, we may voluntarily elect to recall our products. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall would divert managerial and financial resources and could harm our reputation with our customers and with the healthcare professionals that use, prescribe and recommend our products. We could have product recalls that result in significant costs to us in the future, and such recalls could have a material adverse effect on our business.
Product liability claims may harm our business, particularly if the number of claims increases significantly or our product liability insurance proves inadequate.
The manufacture and sale of orthopedic devices and related products exposes us to a significant risk of product liability claims. From time to time, we have been, and we are currently, subject to a number of product liability claims alleging that the use of our products resulted in adverse effects. Even if we are successful in defending against any liability claims, such claims could nevertheless distract our management, result in substantial costs, harm our reputation, adversely affect the sales of all our products and otherwise harm our business. If there is a significant increase in the number of product liability claims, our business could be adversely affected.
ReAble and DJO currently each carry product liability insurance up to a limit of $20 million, subject to an aggregate self-insurance retention of $750,000 for ReAble and a deductible of $100,000 on the primary layer of $10 million and another $100,000 on the excess layer of $10 million for DJO. We are currently evaluating our product liability insurance policies that we will have after the Merger. Our insurance policy is subject to annual renewal. Product liability claims made against us may exceed the coverage limit of our policy or such insurance coverage may not continue to be available on commercially reasonable terms or at all. Additionally, we are also subject to the risks that our insurers will exclude from coverage claims made against us or that our insurers may become insolvent. If we do not or cannot maintain adequate product liability insurance, our business and results of operations may be adversely affected.
As of September 29, 2007, ReAble exceeded, and expects in the future to continue to exceed, the coverage limits for certain product liability claims; however, the current and expected future excess has been accrued in ReAble's consolidated balance sheet as of that date.
If we lose one of our key suppliers or one of our contract manufacturers stops making the raw materials and components used in our products, we may be unable to meet customer orders for our products in a timely manner or within our budget.
We rely on a limited number of foreign and domestic suppliers for the raw materials and components used in our products. One or more of our suppliers may decide to cease supplying us with raw materials and components for reasons beyond our control. FDA regulations may require additional testing of any raw materials or components from new suppliers prior to our use of those materials or components. In addition, in the case of a device which is the subject of a pre-market approval, we may be required to obtain prior FDA permission (which may or may not be given), which could delay or prevent our access or use of such raw materials or components. If we are unable to obtain materials we need from our suppliers or our agreements with our suppliers are terminated, and we cannot obtain these materials from other sources, we may be unable to manufacture our products to meet customer orders in a timely manner or within our manufacturing budget. In that event, our business and results of operations could be adversely affected.
In addition, we rely on third parties to manufacture some of our products. For example, Medireha GmbH ("Medireha"), which is 50% owned by us, has been a supplier for a significant portion of our CPM devices. CPM devices represented approximately 3% of our pro forma net sales for the nine
28
months ended September 29, 2007. If we encounter a cessation, interruption or delay in the supply of the products purchased from Medireha, we may be unable to obtain such products through other sources on acceptable terms, within a reasonable amount of time or at all. We also use a single source for many of the devices Cefar and Compex distribute. In addition, if our agreements with the manufacturing companies were terminated, we may not be able to find suitable replacement within a reasonable amount of time or at all. Any such cessation, interruption or delay may impair our ability to meet scheduled deliveries of our products to our customers and may cause our customers to cancel orders. In that event, our reputation and results of operations may be adversely affected.
Some of our important suppliers are in China and other parts of Asia and provide predominately finished soft goods products. In fiscal year 2006, DJO obtained approximately 15% of its total purchased materials from suppliers in China and other parts of Asia. Political and economic instability and changes in government regulations in these areas could affect our ability to continue to receive materials from suppliers there. The loss of suppliers in China and other parts of Asia, any other interruption or delay in the supply of required materials or our inability to obtain these materials at acceptable prices and within a reasonable amount of time could impair our ability to meet scheduled product deliveries to our customers and could hurt our reputation and cause customers to cancel orders.
In addition, we purchase the microprocessor used in the OL1000 and SpinaLogic devices from a single manufacturer. Although there are feasible alternate microprocessors that might be used immediately, all are produced by a single supplier. In addition, there are single suppliers for other components used in the OL1000 and SpinaLogic devices and only two suppliers for the magnetic field sensor employed in them. Establishment of additional or replacement suppliers for these components cannot be accomplished quickly.
If our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors and may not be able to operate our business profitably.
We rely on a combination of patents, trade secrets, copyrights, trademarks, license agreements and contractual provisions to establish and protect our intellectual property rights in our products and the processes for the development, manufacture and marketing of our products.
We use non-patented, proprietary know-how, trade secrets, processes and other proprietary information and currently employ various methods to protect this proprietary information, including confidentiality agreements, invention assignment agreements and proprietary information agreements with vendors, employees, independent sales agents, distributors, consultants, and others. However, these agreements may be breached. The FDA or another governmental agency may require the disclosure of such information in order for us to have the right to market a product. Trade secrets, know-how and other unpatented proprietary technology may also otherwise become known to or independently developed by our competitors.
In addition, we also hold U.S. and foreign patents relating to a number of our components and products and have patent applications pending with respect to other components and products. We also apply for additional patents in the ordinary course of our business, as we deem appropriate. However, these precautions offer only limited protection, and our proprietary information may become known to, or be independently developed by, competitors, or our proprietary rights in intellectual property may be challenged, any of which could have a material adverse effect on our business, financial condition and results of operations. Additionally, we cannot assure you that our existing or future patents, if any, will afford us adequate protection or any competitive advantage, that any future patent applications will result in issued patents or that our patents will not be circumvented, invalidated or declared unenforceable. In addition, certain of our subsidiaries have not always taken commercially reasonable measures to protect their ownership of some of their patents. While such measures are currently employed and have been employed by us in the past, disputes may arise as to the ownership, or
29
co-ownership, of certain of our patents. We do not consider patent protection to be a significant competitive advantage in the marketplace for electrotherapy devices. However, patent protection may be of significance with respect to our orthopedic technology.
Any proceedings before the U.S. Patent and Trademark Office could result in adverse decisions as to the priority of our inventions and the narrowing or invalidation of claims in issued patents. We could also incur substantial costs in any such proceedings. In addition, the laws of some of the countries in which our products are or may be sold may not protect our products and intellectual property to the same extent as U.S. laws, if at all. We may also be unable to protect our rights in trade secrets and unpatented proprietary technology in these countries.
In addition, we hold patent and other intellectual property licenses from third parties for some of our products and on technologies that are necessary in the design and manufacture of some of our products. The loss of such licenses would prevent us from manufacturing, marketing and selling these products, which in turn could harm our business.
Our operating results and financial condition could be adversely affected if we become involved in litigation regarding our patents or other intellectual property rights.
Litigation involving patents and other intellectual property rights is common in our industry, and companies in our industry have used intellectual property litigation in an attempt to gain a competitive advantage. We currently are, and in the future may become, a party to lawsuits involving patents or other intellectual property. Such litigation is costly and time consuming. If we lose any of these proceedings, a court or a similar foreign governing body could invalidate or render unenforceable our owned or licensed patents, require us to pay significant damages, seek licenses and/or pay ongoing royalties to third parties, require us to redesign our products, or prevent us from manufacturing, using or selling our products, any of which would have an adverse effect on our results of operations and financial condition. While we have indemnification rights against third parties for damages arising from some current patent infringement litigation we have been involved with, the indemnifying parties may contest their indemnification obligations or may be unable to financially satisfy those obligations.
We have brought, and may in the future also bring, actions against third parties for an infringement of our intellectual property rights. We may not succeed in such actions. The defense and prosecution of intellectual property suits, proceedings before the U.S. Patent and Trademark Office or its foreign equivalents and related legal and administrative proceedings are both costly and time consuming. Protracted litigation to defend or enforce our intellectual property rights could seriously detract from the time our management would otherwise devote to running our business. Intellectual property litigation relating to our products could cause our customers or potential customers to defer or limit their purchase or use of the affected products until resolution of the litigation.
Our business strategy relies on certain assumptions concerning demographic and other trends that impact the market for our products. If these assumptions prove to be incorrect, demand for our products may be lower than we currently expect.
Our ability to achieve our business objectives is subject to a variety of factors, including the relative increase in the aging of the general population and an increase in participation in exercise and sports and more active lifestyles. In addition, our business strategy relies on an increasing awareness and clinical acceptance of non-invasive, non-systemic treatment and rehabilitation products, such as electrotherapy. We believe that these trends will increase the need for our orthopedic, physical therapy, regenerative and surgical implant products. The projected demand for our products could materially differ from actual demand if our assumptions regarding these trends and acceptance of our products by healthcare professionals and patients prove to be incorrect or do not materialize. If our assumptions regarding these factors prove to be incorrect, we may not be able to successfully implement our business strategy, which could adversely affect our results of operations. In addition, the perceived
30
benefits of these trends may be offset by competitive or business factors, such as the introduction of new products by our competitors or the emergence of other countervailing trends.
We may expand into new markets through the development of new products and our expansion may not be successful.
We may attempt to expand into new markets through the development of new product applications based on our existing specialized technology and design capabilities. These efforts could require us to make substantial investments, including significant research, development, engineering and capital expenditures for new, expanded or improved manufacturing facilities which would divert resources from other aspects of our business. Expansion into new markets may be costly and may not result in any benefit to us. Specific risks in connection with expanding into new markets include the inability to transfer our quality standards into new products, the failure of customers in new markets to accept our products and price competition in new markets. Such expansion efforts into new markets could be unsuccessful.
Consolidation in the healthcare industry could have an adverse effect on our revenues and results of operations.
Many healthcare industry companies, including medical device, orthopedic and physical therapy products companies, are consolidating to create larger companies. As the healthcare industry consolidates, competition to provide products and services to industry participants may become more intense. In addition, many of our customers are also consolidating, and our customers and other industry participants may try to use their purchasing power to negotiate price concessions or reductions for the products that we manufacture and market. If we are forced to reduce our prices because of consolidation in the healthcare industry, our revenues could decrease, and our business, financial condition and results of operations could be adversely affected.
We could incur significant costs complying with environmental and health and safety requirements, or as a result of liability for contamination or other harm caused by hazardous materials that we use.
Our research and development and manufacturing processes involve the use of hazardous materials. We are subject to federal, state, local and foreign environmental requirements, including regulations governing the use, manufacture, handling, storage and disposal of hazardous materials, discharges to air and water, the clean up of contamination and occupational health and safety matters. We cannot eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur liability as a result of any contamination or injury. Under some environmental laws and regulations, we could also be held responsible for costs relating to any contamination at our past or present facilities and at third-party waste disposal sites where we have sent workers. These could include costs relating to contamination that did not result from any violation of law, and in some circumstances, contamination that we did not cause. DJO became the owner of a New Jersey factory as a result of its Aircast acquisition and that factory is the site of an environmental clean-up project that is currently being performed by a prior owner. DJO sold that facility in August 2007. We may incur significant expenses in the future relating to any failure to comply with environmental laws. Any such future expenses or liability could have a significant negative impact on our financial condition. The enactment of stricter laws or regulations, the stricter interpretation of existing laws and regulations or the requirement to undertake the investigation or remediation of currently unknown environmental contamination at our own or third party sites may require us to make additional expenditures, which could be material.
Our reported results may be adversely affected by increases in reserves for sales allowances, product returns, rental credits, uncollectible accounts receivable and inventory.
Our net sales and profitability are affected by changes in reserves to account for sales allowances, product returns, rental credits, uncollectible accounts receivable and inventory. Any increase in our
31
reserves for sales allowances, product returns, rental credits, uncollectible accounts receivable or inventory could adversely affect our reported financial results by reducing our net revenues and/or profitability for the reporting period.
We have established a reserve for sales allowances which account for sales of our products below the invoice price. Such sales generally result from agreements that we enter into with certain customers that permit them to pay us for our products in amounts that are below the invoice price of the product. We estimate the amount of the reduction based on historical experience and invoices generated in the period.
The reserve for product returns accounts for customer returns of our products after purchase. These returns are mainly attributable to a third-party payor's refusal to provide a patient release or reimbursement for the product or the inability of the product to adequately address the patient's condition. If we increase the percentage of our sales made pursuant to agreements providing for reimbursement below invoice price or if customers return products at a higher than estimated rate, we may be required to increase our sales allowance and product return reserves beyond their current levels.
Our reserve for rental credits recognizes a timing difference between billing of a purchase and processing of a rental credit associated with some of our electrotherapy and traction devices. Many insurance providers require patients to rent our rehabilitation devices for a period of one to three months prior to purchase. If the patient has a long-term need for the device, these insurance companies may authorize purchase of the device after such time period. When the device is purchased, most providers require that rental payments previously made on the device be credited toward the purchase price. These credits are processed at the time the payment is received for the purchase of the device, which creates a time lag between billing of the purchase and processing of the rental credit. Our rental credit reserve accounts for unprocessed rental credits based on the number of devices converted to purchase. If the frequency of rental to purchase conversion increases, we may be required to increase our rental credit reserve beyond its current level.
We have a large balance of accounts receivable and have established a reserve for the portion of such accounts receivable that we estimate will not be collected because of our customers' and patients' non-payment. The reserve is based on historical trends and current relationships with our customers and providers. Changes in our collection rates can result from a number of factors, including turnover in personnel, changes in the payment policies or practices of payors or changes in industry rates or pace of reimbursement. Our reserve for uncollectible receivables has fluctuated in the past and will continue to fluctuate in the future. Changes in rates of collection or fluctuations, even if they are small in absolute terms, could require us to increase our reserve for uncollectible receivables beyond its current level.
The nature of our business requires us to maintain sufficient inventory on hand at all times to meet the requirements of our customers. We maintain inventory reserves for such issues as slow moving or excess inventory, product obsolescence and declines in valuation. In each division, we use a specific identification methodology, adjustment to which can occur whenever there is a change in strategy. In addition, we review sales performance on at least a quarterly basis to determine the amounts that we should add to the existing reserves. We monitor reserves on a quarterly basis and make changes when necessary. To determine the adequacy of our reserves at each reporting period, we analyze the following, among other factors: current inventory quantities on hand, product acceptance in the marketplace, customer demand, historical sales, forecasted sales, product obsolescence and technological innovations. If there is a material change due to these factors, the current level of the reserve for inventory may not be adequate and would result in our increasing the reserve level in the future. Any modifications to our estimates of our reserves are reflected in cost of sales within the statement of operations during the period in which such modifications are determined necessary by management.
32
Certain administrative functions relating to the OfficeCare and Insurance channels have been outsourced to a third-party contractor and this arrangement may not prove successful.
The OfficeCare sales channel maintains a range of products (mostly soft goods) on hand at over 1,200 healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. In the Insurance channel, products are provided, principally custom-made rigid knee braces, directly to patients. In both situations, patients or their third-party payors are billed after the product is provided to the patient. The revenue cycle of this program is outsourced, from billing to collections, to an independent third-party contractor. The outsource contractor that DJO used has undergone significant changes in their business operations in the last two years, including relocating some administrative functions overseas, in order to improve performance from order entry to collections. The contractor is working to upgrade the software system used in these revenue cycle processes. The inability of this provider to upgrade its processes and demonstrate improved billing and collection results could have an adverse effect on our operations and financial results in the OfficeCare and Insurance channels. In addition, we expect to transition these administrative functions to internal departments after the completion of the Merger.
If a natural or man-made disaster strikes our manufacturing facilities, we will be unable to manufacture our products for a substantial amount of time and our sales will decline.
A significant portion of our rehabilitation products are manufactured in a facility in Tijuana, Mexico, with a number of products for the European market manufactured in a Tunisian facility. In Vista, California we manufacture our custom rigid bracing products, which remain in the United States to facilitate quick turn-around on custom orders, vascular products, and our Regeneration product line. Our clinical electrotherapy devices, patient care products, physical therapy and chiropractic treatment tables and certain CPM devices are manufactured in our facilities located in Chattanooga, Tennessee. Our home electrotherapy devices sold in the United States as well as some components and related accessories are manufactured at our facility in Clear Lake, South Dakota. In our Surgical Implant Division, we manufacture our products in our manufacturing facility at Austin, Texas. These facilities and the manufacturing equipment we use to produce our products would be difficult to repair or replace. Our facilities may be affected by natural or man-made disasters. If one of our facilities were affected by a disaster, we would be forced to rely on third-party manufacturers or shift production to another manufacturing facility. In such an event, we would face significant delays in manufacturing which would prevent us from being able to sell our products. In addition, our insurance may not be sufficient to cover all of the potential losses and may not continue to be available to us on acceptable terms, or at all.
We intend to pursue, but may not be able to identify, finance or successfully complete, other strategic acquisitions.
Our growth strategy will continue to include the pursuit of acquisitions, both domestically and, in particular, internationally. However, we may not be able to identify acceptable opportunities or complete acquisitions of targets in a timely manner or on acceptable terms. In addition, acquisitions involve a number of risks, including those set forth under the risk factor captioned "We may not be able to successfully integrate ReAble and DJO, as well as other businesses ReAble and DJO have recently acquired or businesses we may acquire in the future, and we may not be able to realize the anticipated cost savings, revenue enhancement, or other synergies from such acquisitions." To the extent we are unable to consummate acquisitions, we will experience slower than expected growth.
In addition, we may require additional debt or equity financing for future acquisitions, and such financing may not be available on favorable terms, if available at all. We may not be able to successfully integrate or operate profitably any new business we acquire, and we cannot assure you that any such acquisition will meet our expectations. Finally, in the event we decide to discontinue pursuit
33
of a potential acquisition, we will be required to immediately expense all costs incurred in pursuit of the possible acquisition that could have an adverse effect on our results of operations in the period in which the expense is recognized. If we complete acquisitions, or obtain financing for them, on unfavorable terms, or if we fail to properly integrate an acquired business, our financial condition and result of operations would be adversely affected.
If we do not effectively manage our growth, our existing infrastructure may become strained, and we may be unable to increase sales of our products or generate revenue growth.
The growth that we have experienced, and in the future may experience, including due to acquisitions may provide challenges to our organization, requiring us to expand our personnel, manufacturing and distribution operations. Future growth may strain our infrastructure, operations, product development and other managerial and operating resources. If our business resources become strained, we may be unable to increase sales of our products or generate revenue growth.
The loss of the services of our key management and personnel could adversely affect our ability to operate our business.
Our future success will depend, in part, upon the continued service of key managerial, research and development staff and sales and technical personnel. In addition, our future success will depend on our ability to continue to attract and retain other highly qualified personnel. These executive officers have substantial experience and expertise in our industry. Our future success depends, to a significant extent, on the abilities and efforts of our executive officers and other members of our management team. We compete for such personnel with other companies, academic institutions, government entities and other organizations. We may not be successful in retaining our current personnel or in hiring or retaining qualified personnel in the future. Our failure to do so could have a material adverse effect on our business.
Affiliates of Blackstone own substantially all of the equity interest in us and may have conflicts of interest with us or you in the future.
Following the Merger, investment funds affiliated with Blackstone will collectively beneficially own 97.42% of our capital stock, and Blackstone designees will hold a majority of the seats on our board of directors. As a result, affiliates of Blackstone have control over our decisions to enter into any corporate transaction and have the ability to prevent any transaction that requires the approval of stockholders regardless of whether holders of our notes believe that any such transactions are in their own best interests. For example, affiliates of Blackstone could collectively cause us to make acquisitions that increase the amount of indebtedness or to sell assets, or could cause us to issue additional capital stock or declare dividends. So long as investment funds affiliated with Blackstone continue to indirectly own a significant amount of the outstanding shares of our common stock, affiliates of Blackstone will continue to be able to strongly influence or effectively control our decisions. In addition, Blackstone has no obligation to provide us with any additional debt or equity financing.
Additionally, Blackstone is in the business of making investments in companies and may from time to time acquire and hold interests in businesses that compete directly or indirectly with us. Blackstone may also pursue acquisition opportunities that may be complementary to our business and, as a result, those acquisition opportunities may not be available to us.
If we do not achieve and maintain effective internal controls over financial reporting, we could fail to accurately report our financial results.
During the course of the preparation of our financial statements, we evaluate our internal controls to identify and correct deficiencies in our internal controls over financial reporting. In the event we are
34
unable to identify and correct deficiencies in our internal controls in a timely manner, we may not record, process, summarize and report financial information accurately and within the time periods required for our financial reporting under the terms of the agreements governing our indebtedness.
We have completed a significant number of acquisitions in the past several years, and plan to continue to pursue growth through strategic acquisitions. Among the risks associated with acquisitions are the risks of control deficiencies that result from the integration of the acquired business. For example, on April 7, 2006, DJO acquired Aircast. During the course of the most recent audit of DJO's financial statements, which occurred in connection with the year ended December 31, 2006, it was determined that there was a material weakness in DJO's internal control over financial reporting regarding inventory that was in-transit from Aircast facilities in New Jersey to DJO's facilities in Indianapolis and Mexico as a part of the integration of the Aircast business into DJO's business operations. DJO did not perform adequate detailed procedures with respect to such inventory and failed to adequately reconcile the related intercompany transactions. Subsequent to the year ended December 31, 2006, DJO management remediated this control deficiency by implementing controls requiring the preparation and review of detailed analyses and reconciliations of in-transit inventory and related intercompany accounts. These analyses and reconciliations were completed for the transfer of the Aircast inventory in early February 2007. In addition, in connection with the integration of its recent acquisitions and its continuous assessment of its internal controls, including with respect to acquired foreign operations, ReAble has identified certain internal control deficiencies that they have remedied or for which they have undertaken steps to remediate.
It is possible that control deficiencies could be identified by our management or independent registered public accounting firm in the future or may occur without being identified. Such a failure could negatively impact the market price and liquidity of the notes, cause investors to lose confidence in our reported financial condition, lead to a default under our new senior secured credit facilities and otherwise materially adversely affect our business and financial condition.
35
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL INFORMATION
We have derived the following unaudited pro forma condensed consolidated financial information by applying pro forma adjustments to the historical audited and unaudited financial statements of ReAble and DJO. The unaudited pro forma condensed consolidated balance sheet data give effect to the Transactions, which include the following, as if they had occurred on September 29, 2007:
- •
- the Merger, including the payment of an estimated $1,246.8 million in consideration to the existing equity holders of DJO;
- •
- the contribution of an estimated $421.9 million in new equity by affiliates of Blackstone, which is expected to include the rollover of stock options by certain members of DJO management;
- •
- our borrowing of $1,055.0 million under the term loan under our new senior secured credit facilities;
- •
- our issuance of $575.0 million aggregate principal amount of new senior unsecured notes;
- •
- the repayment of $428.3 million outstanding, including accrued interest, under ReAble's existing senior secured credit facilities;
- •
- the repayment of $288.6 million outstanding, including accrued interest, under DJO's existing senior secured credit facilities;
- •
- the termination of interest rate swaps related to ReAble's existing senior secured credit facilities and DJO's existing senior secured credit facilities; and
- •
- the payment of an estimated $79.5 million in related transaction fees and expenses.
The unaudited pro forma condensed consolidated statements of operations data give effect to the Transactions, as well as the following transactions, as if they had occurred on January 1, 2006.
- •
- ReAble's acquisition of Compex on February 24, 2006, the incurrence of indebtedness to finance the acquisition, and the related divestiture of Slendertone;
- •
- the Prior Transactions, pursuant to which Blackstone acquired ReAble on November 3, 2006; and
- •
- DJO's acquisition of Aircast on April 7, 2006, including the incurrence of indebtedness to finance the acquisition.
We derived the unaudited pro forma condensed consolidated statement of operations data for the twelve months ended September 29, 2007, by adding the unaudited pro forma condensed consolidated statement of operations data for the year ended December 31, 2006 to the unaudited pro forma condensed consolidated statement of operations data for the nine months ended September 29, 2007 and subtracting the unaudited pro forma condensed consolidated statement of operations data for the nine months ended September 30, 2006.
We describe the assumptions underlying the pro forma adjustments in the accompanying notes, which you should read in conjunction with these unaudited pro forma condensed consolidated financial statements. We have based the unaudited pro forma adjustments upon available information and certain assumptions that we believe are reasonable under the circumstances. We present the unaudited pro forma condensed consolidated financial information for informational purposes only. The unaudited pro forma condensed consolidated statements of operations do not purport to represent what our results of operations would have been had the transactions described above actually occurred on the dates indicated and they do not purport to project our results of operations for any future period. The unaudited pro forma condensed consolidated balance sheet does not purport to reflect
36
what our financial condition would have been had the transactions described above closed on the date indicated or for any future or historical period.
The Merger will be accounted for under the purchase method of accounting described in Statement of Financial Accounting Standards (SFAS) No. 141, "Business Combinations," with intangible assets recorded in accordance with SFAS No. 142, "Goodwill and Other Intangible Assets." As part of the preparation of this unaudited pro forma condensed consolidated financial information, we have performed a preliminary review of the tangible and intangible assets to be acquired in the Merger, and we have based certain assumptions upon that preliminary review. A formal valuation will be completed following the consummation of the Merger to assist us in identifying and valuing all tangible and identifiable intangible assets and their respective lives. Thus, the actual allocation of the final purchase price will differ from the amounts we present herein, and those differences may be material. The actual adjustments and amounts will differ from those we present herein, and those differences may be material.
37
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED BALANCE SHEET
AS OF SEPTEMBER 29, 2007
| | ReAble
| | DJO
| | Adjustments for the Transactions
| | Pro Forma Total
|
|---|
| |
| | (in thousands)
| |
|
|---|
| Assets | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | |
| | Cash and cash equivalents | | $ | 31,805 | | $ | 11,956 | | $ | 4,239 | (a) | $ | 48,000 |
| | Accounts receivable, net | | | 87,680 | | | 96,348 | | | — | | | 184,028 |
| | Inventories, net | | | 66,729 | | | 43,422 | | | 6,578 | (b) | | 116,729 |
| | Deferred tax assets | | | 22,662 | | | 10,813 | | | 7,126 | (b) | | 40,601 |
| | Prepaid expenses and other current assets | | | 7,161 | | | 10,730 | | | — | | | 17,891 |
| | |
| |
| |
| |
|
| | | Total current assets | | | 216,037 | | | 173,269 | | | 17,943 | | | 407,249 |
| Property and equipment, net | | | 44,453 | | | 30,226 | | | 7,574 | (b) | | 82,253 |
| Goodwill | | | 517,070 | | | 281,176 | | | 513,269 | (b) | | 1,311,515 |
| Intangible assets, net | | | 331,392 | | | 144,764 | | | 743,236 | (b) | | 1,219,392 |
| Deferred tax assets, non-current | | | — | | | 10,288 | | | 3,746 | (c) | | 14,034 |
| Other non-current assets | | | 19,888 | | | 10,034 | | | 27,886 | (c) | | 57,808 |
| | |
| |
| |
| |
|
| | | Total assets | | $ | 1,128,840 | | $ | 649,757 | | $ | 1,313,654 | | $ | 3,092,251 |
| | |
| |
| |
| |
|
Liabilities, Minority Interests, Membership Equity and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
|
|
|
| Current liabilities: | | | | | | | | | | | | |
| | Long-term debt and capital leases, current portion | | $ | 7,965 | | $ | — | | $ | 6,500 | (d) | $ | 14,465 |
| | Accounts payable | | | 25,134 | | | 13,675 | | | — | | | 38,809 |
| | Accrued expenses | | | 48,734 | | | 41,472 | | | (8,031 | )(d) | | 82,175 |
| | |
| |
| |
| |
|
| | | Total current liabilities | | | 81,833 | | | 55,147 | | | (1,531 | ) | | 135,449 |
| Long-term debt and capital leases, net of current portion | | | 621,187 | | | 285,500 | | | 914,637 | (d) | | 1,821,324 |
| Deferred tax liabilities | | | 102,555 | | | — | | | 298,364 | (b) | | 400,919 |
| Other non-current liabilities | | | 8,721 | | | 5,354 | | | (4,446 | )(a) | | 9,629 |
| | |
| |
| |
| |
|
| | | Total liabilities | | | 814,296 | | | 346,001 | | | 1,207,024 | | | 2,367,321 |
| | |
| |
| |
| |
|
| Minority interests | | | 1,021 | | | — | | | — | | | 1,021 |
Membership equity and stockholders' equity |
|
|
313,523 |
|
|
303,756 |
|
|
106,630 |
(e) |
|
723,909 |
| | |
| |
| |
| |
|
| | Total liabilities, minority interests, membership equity and stockholders' equity | | $ | 1,128,840 | | $ | 649,757 | | $ | 1,313,654 | | $ | 3,092,251 |
| | |
| |
| |
| |
|
See accompanying notes to unaudited pro forma condensed consolidated balance sheet.
38
NOTES TO UNAUDITED PRO FORMA CONDENSED CONSOLIDATED BALANCE SHEET
- (a)
- Reflects the estimated sources and uses of cash for the Transaction as follows (in thousands):
| Sources: | | | |
| New term loan facility(1) | | $ | 1,055,000 |
| New senior unsecured notes | | | 575,000 |
| Equity contribution(2) | | | 421,880 |
| | |
|
| | Total sources | | $ | 2,051,880 |
| | |
|
| Uses: | | | |
| Purchase of equity(3) | | $ | 1,246,776 |
| Repayment of ReAble's existing indebtedness(4) | | | 428,278 |
| Repayment of DJO's existing indebtedness(4) | | | 288,616 |
| Cash paid for the termination of interest rate swaps(5) | | | 4,446 |
| Estimated transaction fees and expenses(6) | | | 79,525 |
| Net increase in cash and cash equivalents(7) | | | 4,239 |
| | |
|
| | Total uses | | $ | 2,051,880 |
| | |
|
- (1)
- Upon the closing of the Transactions, we will enter into new senior secured credit facilities, consisting of a revolving credit facility and a new term loan facility. We will incur $1,055.0 million of term loan borrowings at the closing of the Transactions. We do not expect to have any outstanding borrowings under the revolving credit facility on the closing date of the Transactions.
- (2)
- Represents the cash equity to be contributed by investment funds affiliated with Blackstone and the expected contribution of new equity by certain members of DJO management through the rollover of existing stock options.
- (3)
- Reflects the amount of total consideration to be paid to holders of outstanding shares of DJO common stock and holders of outstanding options to acquire shares of DJO common stock in the Merger, assuming that all stock options will be settled in cash. Assumes 23.7 million shares of DJO common stock outstanding as of September 29, 2007 and options to acquire DJO common stock with a net option value of $57.7 million, which is calculated based on outstanding options to acquire 2.8 million shares of DJO common stock with an average exercise price of $29.69 per share. Also included are an estimated maximum number of shares issuable under the DJO employee stock purchase plan as of September 29, 2007 with a net value of $0.3 million.
- (4)
- Reflects the amount of ReAble's and DJO's existing indebtedness to be repaid and consists of $423.4 million of outstanding borrowings under ReAble's existing senior secured credit facilities and $285.5 million aggregate principal amount of DJO's existing term loan, and $4.9 million and $3.1 million of accrued and unpaid interest relating to ReAble's existing senior secured credit facilities and DJO's existing term loan, respectively. We expect the Transactions to be consummated on November 20, 2007, in which case the aggregate accrued and unpaid interest under the indebtedness to be repaid would be approximately $1.9 million.
- (5)
- Represents use of cash from terminating interest rate swaps due to the repayment of ReAble's existing senior secured credit facilities and DJO's existing term loan. ReAble has two interest rate swap agreements in place for a notional amount of $100.0 million and $75.0 million, expiring in 2010 and 2012, respectively, relating to its existing senior secured credit facilities. DJO has one interest rate swap agreement in place for a notional amount of $250.0 million, expiring in 2011, relating to its existing term loan. These interest rate swaps were used to
39
manage the unfavorable movements in interest rates by hedging a portion of the outstanding borrowings under the existing debt and qualify for hedge accounting. The estimated cash used to terminate the interest rate swaps will increase the amount to be repaid under ReAble's and DJO's existing senior secured credit facilities and existing term loan, respectively, and are reflected as other non-current liabilities.
- (6)
- Reflects the estimated fees and expenses associated with the Transactions, as described in the table below (in thousands):
| Deferred financing costs: | | | |
| | Financing fees(i) | | $ | 33,150 |
| | Other financing costs(ii) | | | 9,000 |
| | |
|
| | | Total deferred financing costs | | | 42,150 |
| Other capitalized transaction costs(ii) | | | 34,500 |
| Transaction costs to be expensed by ReAble(iii) | | | 2,875 |
| | |
|
| | Total estimated transaction costs(iv) | | $ | 79,525 |
| | |
|
- (i)
- Reflects estimated financing fees we will incur in connection with the new senior secured credit facilities and the new senior unsecured notes, which will be capitalized and amortized over the terms of the applicable indebtedness.
- (ii)
- Represents transaction costs, other than those included in (i) above, including fees attributable to professional advisors and other fees associated with the completion of the Transactions, which will be allocated between deferred financing costs and costs associated with the Transactions. Also included is a $15.0 million transaction fee payable to Blackstone Management Partners L.L.C. V ("BMP"), an affiliate of Blackstone, at the closing of the Transactions pursuant to an amended and restated transaction and monitoring fee agreement that ReAble and BMP intend to enter into in connection with the Transactions, of which a portion has been allocated to deferred financing.
- (iii)
- Represents the bridge financing fees that will be expensed upon the completion of the Transactions.
- (iv)
- Excludes approximately $2.5 million of transaction costs incurred and paid by DJO prior to September 29, 2007.
- (7)
- Upon the completion of the Transactions, we expect to have approximately $48.0 million of cash on hand, which will consist of $33.0 million of cash retained to fund integration costs and $15.0 million of additional cash on hand. The adjustments to cash reflect anticipated cash balances prior to and at closing as follows:
| | September 29,
2007
|
|---|
| | (in thousands)
|
|---|
| ReAble cash and cash equivalents | | $ | 31,805 |
| DJO cash and cash equivalents | | | 11,956 |
| | |
|
| | Total pre-transaction cash and cash equivalents | | | 43,761 |
| Net increase in cash and cash equivalents | | | 4,239 |
| | |
|
| | Total pro forma cash and cash equivalents at closing | | $ | 48,000 |
| | |
|
- (b)
- Reflects the preliminary purchase price allocation and step-up adjustments based on management estimates and preliminary valuation studies. The final purchase price allocation will be based on
40
appraisals and the final valuation studies that are not yet available and may materially differ from the preliminary purchase price allocation shown below (in thousands):
| Total purchase cost: | | | | |
| Purchase of equity | | $ | 1,246,776 | |
| Capitalized transaction fees and expenses | | | 34,500 | |
| | |
| |
| | Total purchase cost | | $ | 1,281,276 | |
| | |
| |
| Net book value: | | | | |
| Current assets excluding deferred taxes(1) | | $ | 169,034 | |
| Tangible and other non-current assets excluding deferred taxes(2) | | | 42,935 | |
| Liabilities assumed | | | (346,001 | ) |
| Identifiable intangible assets(3) | | | 888,000 | |
| In-process research and development(4) | | | 3,000 | |
| Deferred taxes — current(5) | | | 17,939 | |
| Deferred taxes — non-current(5) | | | (288,076 | ) |
| Goodwill(6) | | | 794,445 | |
| | |
| |
| | Total beginning book value | | $ | 1,281,276 | |
| | |
| |
| (1) | | Current assets excluding deferred taxes | | $ | 162,456 | |
| | | Write-up of inventory to fair market value | | | 6,578 | |
| | | | |
| |
| | | Adjusted net book value of current assets | | $ | 169,034 | |
| | | | |
| |
(2) |
|
Tangible and other non-current assets excluding deferred taxes |
|
$ |
40,260 |
|
| | | Write-up of fixed assets to fair market value | | | 7,574 | |
| | | Write-off of DJO deferred financing costs | | | (4,899 | ) |
| | | | |
| |
| | | Adjusted net book value of tangible and other non-current assets | | $ | 42,935 | |
| | | | |
| |
| (3) | | The preliminary estimated fair values and lives of certain categories of intangible assets are set forth below (in thousands): | | | | |
| | Fair Market
Value at
September 29,
2007
| | Life
| | Annual
Amortization
Expense
|
|---|
| Contracts and customer relationships | | $ | 322,000 | | 11-15 years | | $ | 24,769 |
| Developed technology | | | 310,000 | | 8-20 years | | | 20,348 |
| Tradenames | | | 256,000 | | 20 years | | | 12,800 |
| | |
| | | |
|
| Total identifiable intangible assets | | | 888,000 | | | | $ | 57,917 |
| | | | | | | |
|
| Write-off of existing DJO intangibles | | | (144,764 | ) | | | | |
| | |
| | | | | |
| | Net pro forma adjustment to intangibles | | $ | 743,236 | | | | | |
| | |
| | | | | |
- (4)
- In connection with the purchase price allocation, we estimate that we will allocate approximately $3.0 million of the purchase cost to in-process research and development ("IPR&D"). In accordance with generally accepted accounting principles, the amount allocated to IPR&D will immediately be written-off to expense following the consummation of the Transactions. The impact of this write-off has been reflected as an adjustment to equity in the unaudited pro forma condensed consolidated balance sheet.
41
- (5)
- Adjustments to deferred tax assets and liabilities were the result of the settlement of DJO's stock options, the acquisition of intangible assets, the write-off of DJO's existing deferred financing costs relating to its existing term loan that will be repaid upon the completion of the Transactions, and adjustments for the write-up of fixed assets and inventory to fair market value as set forth below (in thousands):
| | Deferred Tax
Assets/(Liabilities)
| |
|---|
| | Current
| | Non-current
| |
|---|
| Settlement of DJO's stock options(i) | | $ | 24,394 | | $ | — | |
| Pro forma adjustment to identifiable intangible assets (excluding IPR&D) | | | — | | | (743,236 | ) |
| Write-off of DJO's deferred financing costs | | | — | | | 4,899 | |
| Pro forma adjustment for the write-up of fixed assets to fair market value | | | — | | | (7,574 | ) |
| Pro forma adjustment for the write-up of inventory to fair market value | | | (6,578 | ) | | — | |
| | |
| |
| |
| | Total | | | 17,816 | | | (745,911 | ) |
| Assumed tax rate | | | 40 | % | | 40 | % |
| | |
| |
| |
| | Pro forma adjustment to DJO's deferred tax assets (liabilities) | | | 7,126 | | | (298,364 | ) |
| DJO's historical deferred tax assets | | | 10,813 | | | 10,288 | |
| | |
| |
| |
| | Pro forma deferred tax assets (liabilities) | | $ | 17,939 | | $ | (288,076 | ) |
| | |
| |
| |
- (i)
- Represents the impact on deferred taxes resulting from the vesting and settlement of DJO stock options as a result of the Transactions, assuming that all DJO stock options will be settled in cash.
- (6)
- The adjustment to goodwill represents the write-off of DJO's existing goodwill prior to the Transactions and the recording of goodwill as the excess of purchase cost over the net tangible and identifiable intangible assets as set forth below (in thousands):
| Goodwill | | $ | 794,445 | |
| Write-off of existing DJO goodwill | | | (281,176 | ) |
| | |
| |
| | Net adjustment to goodwill | | $ | 513,269 | |
| | |
| |
42
- (c)
- Reflects the capitalization of estimated financing costs in connection with the indebtedness we will incur in the Transactions consisting of the new senior secured credit facilities and the new senior unsecured notes, which will be amortized over an average of six and one half years for the new senior secured credit facility and for the new senior unsecured notes, in each case using the interest method (in thousands):
| Deferred financing costs | | $ | 42,150 | |
| Write-off of ReAble's existing deferred financing costs | | | (9,365 | ) |
| Write-off of DJO's existing deferred financing costs | | | (4,899 | ) |
| | |
| |
| | Net pro forma adjustment to deferred financing costs | | $ | 27,886 | |
| | |
| |
In connection with the Transactions, ReAble will refinance its existing senior credit facility. Accordingly, ReAble will write-off all of its existing deferred financing costs relating to this debt. The pro forma adjustment below represents the tax impact of writing off existing deferred financing cost balances (in thousands):
| | Deferred Tax
Assets
| |
|---|
| | Non-Current
| |
|---|
| Write-off of ReAble's deferred financing costs | | $ | 9,365 | |
| Assumed tax rate | | | 40 | % |
| | |
| |
| | Pro forma adjustment to ReAble's deferred tax assets | | $ | 3,746 | |
| | |
| |
- (d)
- The adjustment to liabilities represents the net difference between the incurrence of new indebtedness and the repayment of existing indebtedness in connection with the Transactions as set forth below (in thousands):
| | ReAble
Existing
Indebtedness
| | DJO
Existing
Indebtedness
| | Repayment
| | New
Indebtedness
| | Adjustment
| | Pro Forma
|
|---|
| Current: | | | | | | | | | | | | | | | | | | |
| Capital leases | | $ | 287 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 287 |
| Existing ReAble senior secured credit facilities(1) | | | 4,050 | | | — | | | (4,050 | ) | | — | | | (4,050 | ) | | — |
| Existing ReAble European revolver | | | 2,783 | | | — | | | — | | | — | | | — | | | 2,783 |
| Existing ReAble European term loan | | | 376 | | | — | | | — | | | — | | | — | | | 376 |
| New term loan facility(2) | | | — | | | — | | | — | | | 10,550 | | | 10,550 | | | 10,550 |
| Note payable(3) | | | 469 | | | — | | | — | | | — | | | — | | | 469 |
| | |
| |
| |
| |
| |
| |
|
| | Total current | | $ | 7,965 | | $ | — | | $ | (4,050 | ) | $ | 10,550 | | $ | 6,500 | | $ | 14,465 |
| | |
| |
| |
| |
| |
| |
|
| | Accrued interest | | $ | 13,735 | | $ | 3,116 | | $ | (8,031 | ) | $ | — | | $ | (8,031 | ) | $ | 8,820 |
| | |
| |
| |
| |
| |
| |
|
| | | | | | | | | | | | | | | | | | | |
43
Non-current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Capital leases | | $ | 362 | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 362 |
| Existing ReAble senior secured credit facilities(1) | | | 419,313 | | | — | | | (419,313 | ) | | — | | | (419,313 | ) | | — |
| Existing senior subordinated notes | | | 200,000 | | | | | | — | | | — | | | — | | | 200,000 |
| Existing DJO senior secured credit facilities(1) | | | — | | | 285,500 | | | (285,500 | ) | | — | | | (285,500 | ) | | — |
| Existing ReAble European term loan | | | 1,512 | | | — | | | — | | | — | | | — | | | 1,512 |
| New senior unsecured notes | | | — | | | — | | | — | | | 575,000 | | | 575,000 | | | 575,000 |
| New senior secured credit facilities(2) | | | — | | | — | | | — | | | 1,044,450 | | | 1,044,450 | | | 1,044,450 |
| | |
| |
| |
| |
| |
| |
|
| | Total non-current | | | 621,187 | | | 285,500 | | | (704,813 | ) | | 1,619,450 | | | 914,637 | | | 1,821,324 |
| | |
| |
| |
| |
| |
| |
|
| | | Total indebtedness | | $ | 642,887 | | $ | 288,616 | | $ | (716,894 | ) | $ | 1,630,000 | | $ | 913,106 | | $ | 1,844,609 |
| | |
| |
| |
| |
| |
| |
|
- (1)
- The balance includes the principal amount of $423.4 million and $285.5 million related to ReAble's existing senior secured credit facilities and DJO's existing senior secured credit facilities, respectively, which will be repaid upon the completion of the Transactions.
- (2)
- Upon the closing of the Transactions, we will enter into a $1,055.0 million new term loan facility under our new senior secured credit facility, all of which will be drawn on the closing date of the Transactions. The new term loan facility includes amortization payments of 1% per year.
- (3)
- The balance represents a note payable acquired in connection with ReAble's acquisition of Empi on October 4, 2004.
- (e)
- Adjustment to total stockholders' equity was calculated as follows (in thousands):
| New equity contributed in Transactions | | $ | 421,880 | |
| Less: Write-off of in-process research and development | | | (3,000 | ) |
| Less: Write-off of debt issuance costs related to ReAble's existing senior credit facilities, net of taxes | | | (5,619 | ) |
| Bridge fees | | | (2,875 | ) |
| Less: Historical DJO equity | | | (303,756 | ) |
| | |
| |
| Net adjustment to membership equity and stockholders' equity | | $ | 106,630 | |
| | |
| |
44
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE TWELVE MONTHS ENDED DECEMBER 31, 2006
| | ReAble(1)
| | Adjustments
for the
Compex
Acquisition(a)
| | Adjustments
for the
Prior
Transactions
| | ReAble
Pro Forma
| | DJO
| | Adjustments
for the
Aircast
Acquisition(f)
| | DJO Pro
Forma
| | Adjustments
for the
Transactions
| | Total Pro
Forma
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 362,285 | | $ | 12,994 | | $ | — | | $ | 375,279 | | $ | 413,058 | | $ | 25,103 | | $ | 438,161 | | $ | — | | $ | 813,440 | |
| Cost of sales | | | 153,478 | | | 5,252 | | | — | | | 158,730 | | | 166,106 | | | 9,289 | | | 175,395 | | | — | | | 334,125 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | Gross margin | | | 208,807 | | | 7,742 | | | — | | | 216,549 | | | 246,952 | | | 15,814 | | | 262,766 | | | — | | | 479,315 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Selling, general and administrative | | | 230,852 | | | 10,434 | | | 14,979 | (b) | | 256,265 | | | 192,423 | | | 13,656 | | | 206,079 | | | 39,892 | (g) | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | 5,000
1,515 | (h)
(i) | |
508,751 | |
| | Research and development | | | 42,900 | | | 705 | | | — | | | 43,605 | | | 8,988 | | | 520 | | | 9,508 | | | — | | | 53,113 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Operating income (loss) | | | (64,945 | ) | | (3,397 | ) | | (14,979 | ) | | (83,321 | ) | | 45,541 | | | 1,638 | | | 47,179 | | | (46,407 | ) | | (82,549 | ) |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Interest expense | | | (34,619 | ) | | (381 | ) | | (18,386 | )(d) | | (53,386 | ) | | (20,181 | ) | | (8,653 | ) | | (28,834 | ) | | (95,761 | )(j) | | (177,981 | ) |
| | Interest income | | | 839 | | | — | | | — | | | 839 | | | 802 | | | 19 | | | 821 | | | — | | | 1,660 | |
| | Other income, net | | | 110 | | | 5 | | | — | | | 115 | | | 300 | | | 52 | | | 352 | | | — | | | 467 | |
| | Loss on early extinguishment of debt | | | (9,154 | ) | | — | | | — | | | (9,154 | ) | | (2,347 | ) | | — | | | (2,347 | ) | | — | | | (11,501 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations before income taxes and minority interests | | | (107,769 | ) | | (3,773 | ) | | (33,365 | ) | | (144,907 | ) | | 24,115 | | | (6,944 | ) | | 17,171 | | | (142,168 | ) | | (269,904 | ) |
| Provision (benefit) for income taxes | | | (20,208 | ) | | (1,397 | ) | | (13,346 | )(e) | | (34,951 | ) | | 11,474 | | | (2,078 | ) | | 9,396 | | | (56,867 | )(k) | | (82,422 | ) |
| Minority interests | | | 197 | | | — | | | — | | | 197 | | | — | | | — | | | — | | | — | | | 197 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations | | $ | (87,758 | ) | $ | (2,376 | ) | $ | (20,019 | ) | $ | (110,153 | ) | $ | 12,641 | | $ | (4,866 | ) | $ | 7,775 | | $ | (85,301 | ) | $ | (187,679 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
- (1)
- The unaudited combined results for the twelve months ended December 31, 2006 represent the combination of the Predecessor period from January 1, 2006 to November 3, 2006 and the Successor period from November 4, 2006 through December 31, 2006. This combination does not comply with GAAP or with the rules for unaudited pro forma presentation, but is presented because we believe it provides the most meaningful comparison of our results.
See accompanying notes to unaudited pro forma condensed consolidated statements of operations.
45
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2006
| | ReAble
| | Adjustments
for the
Compex
Acquisition(a)
| | Adjustments
for the
Prior
Transactions
| | ReAble
Pro
Forma
| | DJO
| | Adjustments
for the
Aircast
Acquisition(f)
| | DJO Pro
Forma
| | Adjustments
for the
Transactions
| | Total Pro
Forma
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 275,738 | | $ | 12,994 | | $ | — | | $ | 288,732 | | $ | 302,293 | | $ | 25,103 | | $ | 327,396 | | $ | — | | $ | 616,128 | |
| Cost of sales | | | 108,295 | | | 5,252 | | | — | | | 113,547 | | | 119,961 | | | 9,289 | | | 129,250 | | | — | | | 242,797 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | Gross margin | | | 167,443 | | | 7,742 | | | — | | | 175,185 | | | 182,332 | | | 15,814 | | | 198,146 | | | — | | | 373,331 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Selling, general and administrative | | | 129,915 | | | 10,434 | | | 13,340 | (b) | | | | | 138,735 | | | 13,656 | | | 152,391 | | | 29,928 | (g) | | | |
| | | | | | | | | | 2,250 | (c) | | 155,939 | | | | | | | | | | | | 3,750 | (h) | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | 1,136 | (i) | | 343,144 | |
| | Research and development | | | 13,042 | | | 705 | | | — | | | 13,747 | | | 6,745 | | | 520 | | | 7,265 | | | — | | | 21,012 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Operating income | | | 24,486 | | | (3,397 | ) | | (15,590 | ) | | 5,499 | | | 36,852 | | | 1,638 | | | 38,490 | | | (34,814 | ) | | 9,175 | |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Interest expense | | | (23,508 | ) | | (381 | ) | | (16,177 | )(d) | | (40,066 | ) | | (13,902 | ) | | (8,653 | ) | | (22,555 | ) | | (70,863 | )(j) | | (133,484 | ) |
| | Interest income | | | 400 | | | — | | | — | | | 400 | | | 565 | | | 19 | | | 584 | | | — | | | 984 | |
| | Other income (expense),
net | | | (15 | ) | | 5 | | | — | | | (10 | ) | | 254 | | | 52 | | | 306 | | | — | | | 296 | |
| | Loss on early extinguishment of debt | | | — | | | — | | | — | | | — | | | (2,347 | ) | | — | | | (2,347 | ) | | — | | | (2,347 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations before income taxes and minority interests | | | 1,363 | | | (3,773 | ) | | (31,767 | ) | | (34,177 | ) | | 21,422 | | | (6,944 | ) | | 14,478 | | | (105,677 | ) | | (125,376 | ) |
| Provision (benefit) for income taxes | | | 3,174 | | | (1,397 | ) | | (12,706 | )(e) | | (10,929 | ) | | 9,790 | | | (2,078 | ) | | 7,712 | | | (42,271 | )(k) | | (45,488 | ) |
| Minority interests | | | 137 | | | — | | | — | | | 137 | | | — | | | — | | | — | | | — | | | 137 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations | | $ | (1,948 | ) | $ | (2,376 | ) | $ | (19,061 | ) | $ | (23,385 | ) | $ | 11,632 | | $ | (4,866 | ) | $ | 6,766 | | $ | (63,406 | ) | $ | (80,025 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes to unaudited pro forma condensed consolidated statements of operations.
46
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE NINE MONTHS ENDED SEPTEMBER 29, 2007
| | ReAble
| | DJO
| | Adjustments
for the
Transactions
| | Total Pro
Forma
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 321,391 | | $ | 354,869 | | $ | — | | $ | 676,260 | |
| Cost of sales | | | 131,031 | | | 140,841 | | | — | | | 271,872 | |
| | |
| |
| |
| |
| |
| | Gross margin | | | 190,360 | | | 214,028 | | | — | | | 404,388 | |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Selling, general and administrative | | | 174,804 | | | 163,053 | | | 29,948 | (g) | | | |
| | | | | | | | | | 3,750 | (h) | | | |
| | | | | | | | | | 1,136 | (i) | | 372,691 | |
| | Research and development | | | 12,113 | | | 6,185 | | | — | | | 18,298 | |
| | |
| |
| |
| |
| |
| Operating income | | | 3,443 | | | 44,790 | | | (34,834 | ) | | 13,399 | |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Interest expense | | | (43,125 | ) | | (17,807 | ) | | (72,552 | )(j) | | (133,484 | ) |
| | Interest income | | | 671 | | | 875 | | | — | | | 1,546 | |
| | Other income, net | | | 234 | | | 1,227 | | | — | | | 1,461 | |
| | |
| |
| |
| |
| |
| Income (loss) from continuing operations before income taxes and minority interests | | | (38,777 | ) | | 29,085 | | | (107,386 | ) | | (117,078 | ) |
| Provision (benefit) for income taxes | | | (13,923 | ) | | 12,974 | | | (42,954 | )(k) | | (43,903 | ) |
| Minority interests | | | 278 | | | — | | | — | | | 278 | |
| | |
| |
| |
| |
| |
| Income (loss) from continuing operations | | $ | (25,132 | ) | $ | 16,111 | | $ | (64,432 | ) | $ | (73,453 | ) |
| | |
| |
| |
| |
| |
See accompanying notes to unaudited pro forma condensed consolidated statements of operations.
47
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE TWELVE MONTHS ENDED SEPTEMBER 29, 2007
| | ReAble(1)
| | Adjustments
for the Prior
Transactions
| | ReAble
Pro Forma
| | DJO
| | Adjustments
for the
Transactions
| | Total
Pro Forma
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 407,938 | | $ | — | | $ | 407,938 | | $ | 465,634 | | $ | — | | $ | 873,572 | |
| Cost of sales | | | 176,214 | | | — | | | 176,214 | | | 186,986 | | | — | | | 363,200 | |
| | |
| |
| |
| |
| |
| |
| |
| | Gross margin | | | 231,724 | | | — | | | 231,724 | | | 278,648 | | | — | | | 510,372 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | |
| | Selling, general and administrative | | | 275,741 | | | 1,639 | (b) | | 277,380 | | | 216,741 | | | 39,912 | (g) | | | |
| | | | | | | | | | | | | | | | 2,750 | (h) | | | |
| | | | | | | | | | | | | | | | 1,515 | (i) | | 538,298 | |
| | Research and development | | | 41,971 | | | — | | | 41,971 | | | 8,428 | | | — | | | 50,399 | |
| | |
| |
| |
| |
| |
| |
| |
| Operating income (loss) | | | (85,988 | ) | | (1,639 | ) | | (87,627 | ) | | 53,479 | | | (44,177 | ) | | (78,325 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Other income (expense): | | | | | | | | | | | | | | | | | | | |
| | Interest expense | | | (54,236 | ) | | 850 | (d) | | (53,386 | ) | | (24,086 | ) | | (100,509 | )(j) | | (177,981 | ) |
| | Interest income | | | 1,110 | | | — | | | 1,110 | | | 1,112 | | | — | | | 2,222 | |
| | Other income, net | | | 359 | | | — | | | 359 | | | 1,273 | | | — | | | 1,632 | |
| | Loss on early extinguishment of debt | | | (9,154 | ) | | — | | | (9,154 | ) | | | | | — | | | (9,154 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations before income taxes and minority interests | | | (147,909 | ) | | (789 | ) | | (148,698 | ) | | 31,778 | | | (144,686 | ) | | (261,606 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Provision (benefit) for income taxes | | | (37,305 | ) | | (315 | )(e) | | (37,620 | ) | | 14,658 | | | (57,874 | )(k) | | (80,836 | ) |
| Minority interests | | | 338 | | | — | | | 338 | | | — | | | — | | | 338 | |
| | |
| |
| |
| |
| |
| |
| |
| Income (loss) from continuing operations | | $ | (110,942 | ) | $ | (474 | ) | $ | (111,416 | ) | $ | 17,120 | | $ | (86,812 | ) | $ | (181,108 | ) |
| | |
| |
| |
| |
| |
| |
| |
- (1)
- The unaudited combined results for the twelve months ended September 29, 2007 represents the combination of the Predecessor period from September 30, 2006 to November 3, 2006 and the Successor periods from November 4, 2006 through December 31, 2006 and from January 1, 2007 through September 29, 2007. This combination does not comply with GAAP or with the rules for unaudited pro forma presentation, but is presented because we believe it provides the most meaningful comparison of our results.
See accompanying notes to unaudited pro forma condensed consolidated statements of operations.
48
NOTES TO THE UNAUDITED PRO FORMA
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Compex Acquisition Adjustments
- (a)
- Reflects adjustments for the Compex Acquisition on February 24, 2006, including the related divestiture of Slendertone on June 30, 2006 and the incurrence of indebtedness to finance the acquisition.
Unaudited Pro Forma Condensed Consolidated Statement of Operations for the Nine Months
Ended September 30, 2006 and for the Twelve Months Ended December 31, 2006
| | Historical
Compex(1)(2)
| | Divestiture of
Slendertone(3)
| | Other
Adjustments
| | Adjustments
for the Compex
Acquisition
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 17,698 | | $ | (4,704 | ) | $ | — | | $ | 12,994 | |
| Cost of sales | | | 8,043 | | | (2,791 | ) | | — | | | 5,252 | |
| | |
| |
| |
| |
| |
| Gross margin | | | 9,655 | | | (1,913 | ) | | — | | | 7,742 | |
| Operating expenses: | | | | | | | | | | | | | |
| Selling, general and administrative | | | 11,970 | | | (1,838 | ) | | 302 | (4) | | 10,434 | |
| Research and development | | | 705 | | | — | | | — | | | 705 | |
| | |
| |
| |
| |
| |
| Operating loss | | | (3,020 | ) | | (75 | ) | | (302 | ) | | (3,397 | ) |
| Other income (expense): | | | | | | | | | | | | | |
| Interest income | | | — | | | — | | | — | | | — | |
| Interest expense | | | (228 | ) | | — | | | (153 | )(5) | | (381 | ) |
| Other income (expense), net | | | 5 | | | — | | | — | | | 5 | |
| | |
| |
| |
| |
| |
| Loss from continuing operations | | | (3,243 | ) | | (75 | ) | | (455 | ) | | (3,773 | ) |
| Benefit for income taxes | | | (1,183 | ) | | (32 | ) | | (182 | )(6) | | (1,397 | ) |
| | |
| |
| |
| |
| |
| Loss from continuing operations | | $ | (2,060 | ) | $ | (43 | ) | $ | (273 | ) | $ | (2,376 | ) |
| | |
| |
| |
| |
| |
- (1)
- Reflects results of Compex only from January 1, 2006 to February 23, 2006, the date immediately prior to the date of the Compex Acquisition.
- (2)
- Compex results for the period from January 1, 2006 to February 23, 2006 have been adjusted to exclude approximately $2.5 million of charges related to compensation expense resulting from the acceleration of vesting of stock options associated with the Compex Acquisition and the severance payments and incentive bonuses earned as a result of the Compex Acquisition.
- (3)
- Subsequent to the acquisition of Compex, the operations of Slendertone were classified as discontinued operations. Accordingly, this column reflects results of operations of Slendertone only from January 1, 2006 to February 23, 2006.
- (4)
- Other adjustments to selling, general and administrative expenses are as follows (in thousands):
| Pro forma amortization expense | | $ | 396 | |
| Elimination of amortization on intangible assets of Compex | | | (94 | ) |
| | |
| |
| | Total adjustments to selling, general and administrative expenses | | $ | 302 | |
| | |
| |
49
- (5)
- Reflects changes to interest expense resulting from the Compex Acquisition and related finance charges. This interest expense was eliminated as part of the adjustments to interest expense relating to the Prior Transactions as set forth below (in thousands):
| Elimination of interest on Compex debt | | $ | (174 | ) |
| Estimated interest on additional borrowings under the existing revolving credit facility used to finance the Compex Acquisition | | | 313 | |
| Amount of debt issuance amortization costs | | | 14 | |
| | |
| |
| | Total adjustments to interest expense | | $ | 153 | |
| | |
| |
- (6)
- Other adjustments to benefit for income taxes reflect the pro forma tax effect of the adjustments for the Compex Acquisition at an estimated statutory tax rate of 40% as set forth below (in thousands):
| Total Compex Acquisition adjustments | | $ | (455 | ) |
| Tax rate | | | 40 | % |
| | |
| |
| | Tax effect of Compex Acquisition adjustments | | $ | (182 | ) |
| | |
| |
Prior Transactions Adjustments
The following reflects adjustments for the Prior Transactions, including the related financing and repayment of indebtedness.
- (b)
- Reflects the amortization expense of intangible assets as set forth below (in thousands):
| | Twelve Months
Ended
December 31,
2006
| | Nine Months
Ended
September 30,
2006
| | Twelve Months
Ended
September 29,
2007(1)
| |
|---|
| Estimated pro forma amortization expense | | $ | 25,375 | | $ | 19,031 | | $ | 26,664 | |
| Less: ReAble historical amortization expense | | | (10,000 | ) | | (5,295 | ) | | (25,025 | ) |
| Less: Amortization expense included in adjustments for the Compex Acquisition | | | (396 | ) | | (396 | ) | | — | |
| | |
| |
| |
| |
| | Total pro forma adjustment | | $ | 14,979 | | $ | 13,340 | | $ | 1,639 | |
| | |
| |
| |
| |
- (1)
- Estimated pro forma amortization expense for the twelve months ended September 29, 2007 includes approximately $1.3 million of amortization relating to acquisitions subsequent to the Prior Transactions.
- (c)
- Reflects an annual monitoring fee of $3.0 million that was payable for services to be rendered in 2007 by BMP under the existing transaction and monitoring fee agreement by and between ReAble and BMP that was entered into in connection with the Prior Transactions. This fee was paid on March 6, 2007. The pro forma adjustment reflects amortization for the nine month period ended September 30, 2006. The fee due under this agreement for 2006 was paid in a lump sum at the closing of the Blackstone Acquisition on November 3, 2006 and therefore an adjustment for the twelve months ended December 31, 2006 is not required.
50
- (d)
- Reflects interest expense resulting from our capital structure associated with the Prior Transactions (using current applicable LIBOR rates) as set forth below (in thousands):
| | Twelve Months Ended
December 31, 2006
| | Nine Months Ended
September 30, 2006
| | Twelve Months Ended
September 29, 2007
| |
|---|
| | Balance
| | Rate(1)
| | Interest
Expense
| | Balance
| | Rate(1)
| | Interest
Expense
| | Balance
| | Rate(1)
| | Interest
Expense
| |
|---|
| Revolving credit facility: | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Drawn | | $ | — | | 7.6 | % | $ | — | | $ | — | | 7.6 | % | $ | — | | $ | — | | 7.6 | % | $ | — | |
| | Undrawn | | | 50,000 | | 0.5 | % | | 250 | | | 50,000 | | 0.5 | % | | 188 | | | 50,000 | | 0.5 | % | | 250 | |
| Term loan facility | | | 340,000 | | 7.6 | % | | 25,840 | | | 340,000 | | 7.6 | % | | 19,380 | | | 340,000 | | 7.6 | % | | 25,840 | |
| Senior subordinated notes | | | 200,000 | | 11.75 | % | | 23,500 | | | 200,000 | | 11.75 | % | | 17,625 | | | 200,000 | | 11.75 | % | | 23,500 | |
| | | | | | | |
| | | | | | |
| | | | | | |
| |
| Total cash interest expense | | | | | | | | 49,590 | | | | | | | | 37,193 | | | | | | | | 49,590 | |
| Less: ReAble existing interest expense | | | | | | | | (34,619 | ) | | | | | | | (23,508 | ) | | | | | | | (54,236 | ) |
| Less: Interest expense included in adjustments for the Compex Acquisition | | | | | | | | (381 | ) | | | | | | | (381 | ) | | | | | | | — | |
| Amortization of capitalized debt issuance costs | | | 20,818 | | 8 years | | | 3,796 | | | 20,818 | | 8 years | | | 2,873 | | | 20,818 | | 8 years | | | 3,796 | |
| | | | | | | |
| | | | | | |
| | | | | | |
| |
| Total pro forma interest expense adjustment | | | | | | | $ | 18,386 | | | | | | | $ | 16,177 | | | | | | | $ | (850 | ) |
| | | | | | | |
| | | | | | |
| | | | | | |
| |
- (1)
- The interest rate on the existing term loan facility is variable and was determined using a three-month LIBOR rate of 5.1% plus an applicable margin of 2.5%. Upon closing of the Transactions, the existing senior secured credit facilities will be repaid and the existing senior subordinated notes will continue to remain outstanding.
- (e)
- Reflects the estimated tax impact relating to the adjustments for the Prior Transactions, calculated at an estimated 40% statutory rate as set forth below (in thousands):
| | Twelve Months
December 31,
2006
| | Nine Months
September 30,
2006
| | Twelve Months
September 29,
2007
| |
|---|
| Total adjustments for the Prior Transactions | | $ | (33,365 | ) | $ | (31,767 | ) | $ | (789 | ) |
| Statutory tax rate | | | 40 | % | | 40 | % | | 40 | % |
| | |
| |
| |
| |
| | Tax effect of adjustments for the Prior Transactions | | $ | (13,346 | ) | $ | (12,706 | ) | $ | (315 | ) |
| | |
| |
| |
| |
51
Aircast Acquisition Adjustments
- (f)
- Reflects adjustments for the Aircast Acquisition on April 7, 2006, and the incurrence of indebtedness to finance the Aircast Acquisition.
Unaudited Pro Forma Condensed Consolidated Statement of Operations for the Nine Months Ended September 30, 2006 and for the Twelve Months ended December 31, 2006
| | Historical
Aircast(1)
| | Other
Adjustments
| | Adjustments
for the Aircast
Acquisition
| |
|---|
| | (in thousands)
| |
|---|
| Net sales | | $ | 25,103 | | $ | — | | $ | 25,103 | |
| Cost of sales | | | 9,289 | | | — | | | 9,289 | |
| | |
| |
| |
| |
| Gross margin | | | 15,814 | | | — | | | 15,814 | |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
| Selling, general and administrative | | | 13,578 | | | 78 | (2) | | 13,656 | |
| Research and development | | | 520 | | | — | | | 520 | |
| | |
| |
| |
| |
| Operating income | | | 1,716 | | | (78 | ) | | 1,638 | |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
| Interest expense | | | (2,441 | ) | | (6,212 | )(3) | | (8,653 | ) |
| Interest income | | | 19 | | | — | | | 19 | |
| Other income (expense), net | | | (350 | ) | | 402 | (3) | | 52 | |
| | |
| |
| |
| |
| Income (loss) from operations | | | (1,056 | ) | | (5,888 | ) | | (6,944 | ) |
| Provision for income taxes | | | 277 | | | (2,355 | )(4) | | (2,078 | ) |
| | |
| |
| |
| |
| Income (loss) before extraordinary items | | $ | (1,333 | ) | $ | (3,533 | ) | $ | (4,866 | ) |
| | |
| |
| |
| |
- (1)
- Reflects results of Aircast only from January 1, 2006 to April 6, 2006, the date immediately prior to the date of the Aircast Acquisition.
- (2)
- Other adjustments to selling, general and administrative expenses are as follows (in thousands):
| Pro forma amortization expense | | $ | 2,859 | |
| Elimination of amortization on intangible assets of Aircast | | | (2,781 | ) |
| | |
| |
| | Total adjustments to selling, general and administrative expenses | | $ | 78 | |
| | |
| |
- (3)
- Reflects adjustments to eliminate interest expense on historical Aircast debt and interest expense on borrowings to fund the Aircast Acquisition as set forth below. Interest expense relating to the Aircast Acquisition is eliminated as part of the adjustments to interest expense relating to the Transactions (in thousands).
| Elimination of interest on Aircast debt | | $ | (2,441 | ) |
| Estimated interest on additional borrowings under DJO's existing term loan used to finance the Aircast Acquisition | | | 8,411 | |
| Amount of debt issuance amortization costs | | | 242 | |
| | |
| |
| | Total adjustments to interest expense | | $ | 6,212 | |
| | |
| |
| Elimination of interest relating to hedging instruments on Aircast debt | | $ | (402 | ) |
| | |
| |
52
- (4)
- Other adjustments to the provision for income taxes reflect the pro forma tax effect of the adjustments for the Aircast Acquisition at an estimated statutory tax rate of 40% as set forth below (in thousands):
| Total Aircast Acquisition adjustments | | $ | (5,888 | ) |
| Tax rate | | | 40 | % |
| | |
| |
| | Tax effect of Aircast Acquisition adjustments | | $ | (2,355 | ) |
| | |
| |
The Transactions
The following reflect adjustments for the Transactions.
- (g)
- Reflects the amortization expense of intangible assets as set forth below (in thousands):
| | Twelve Months
Ended
December 31,
2006
| | Nine Months
Ended
September 30,
2006
| | Nine Months
Ended
September 29,
2007
| | Twelve Months
Ended
September 29,
2007
| |
|---|
| Estimated pro forma amortization expense | | $ | 57,917 | | $ | 43,438 | | $ | 43,438 | | $ | 57,917 | |
| Less: DJO historical amortization expense | | | (15,166 | ) | | (10,651 | ) | | (13,490 | ) | | (18,005 | ) |
| Less: Amortization expense included in adjustments for the Aircast Acquisition | | | (2,859 | ) | | (2,859 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| | Total pro forma adjustment | | $ | 39,892 | | $ | 29,928 | | $ | 29,948 | | $ | 39,912 | |
| | |
| |
| |
| |
| |
- (h)
- Reflects an increased minimum annual monitoring fee of $8.0 million payable to BMP under an amended and restated transaction and monitoring fee agreement that BMP and ReAble intend to enter into in connection with the Transactions (in thousands).
| | Twelve Months
December 31, 2006
| | Nine Months Ended
September 30, 2006
| | Nine Months Ended
September 29, 2007
| | Twelve Months
Ended
September 29,
2007
| |
|---|
| Estimated pro forma monitoring fee | | $ | 8,000 | | $ | 6,000 | | $ | 6,000 | | $ | 8,000 | |
| Less: Existing monitoring fee included in ReAble's selling, general and administrative | | | (3,000 | ) | | — | | | (2,250 | ) | | (5,250 | ) |
| Less: Monitoring fee included in adjustment for the Prior Transactions | | | — | | | (2,250 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| | Total pro forma adjustment | | $ | 5,000 | | $ | 3,750 | | $ | 3,750 | | $ | 2,750 | |
| | |
| |
| |
| |
| |
- (i)
- Reflects the amortization of the $7.6 million write-up to fair market value of fixed assets using a weighted average life of five years as set forth below. Currently, we do not have sufficient information to determine the proper allocation between selling, general and administrative
53
and cost of sales, therefore, the entire pro forma adjustment has been included in selling, general and administrative expense (in thousands):
| | Twelve Months Ended
December 31, 2006
| | Nine Months Ended
September 30, 2006
| | Nine Months Ended
September 29, 2007
| | Twelve Months
Ended
September 29,
2007
|
|---|
| Total pro forma adjustment | | $ | 1,515 | | $ | 1,136 | | $ | 1,136 | | $ | 1,515 |
- (j)
- Reflects interest expense resulting from our new capital structure associated with the Transactions (using current applicable LIBOR rates) as set forth below (in thousands):
| | Twelve Months Ended December 31, 2006
| | Nine Months Ended September 30, 2006
| | Nine Months Ended September 29, 2007
| | Twelve Months Ended September 29, 2007
| |
|---|
| | Balance
(1)
| | Rate
(2)
| | Interest
Expense
(2)
| | Balance
(1)
| | Rate
(2)
| | Interest
Expense
(2)
| | Balance (1)
| | Rate
(2)
| | Interest
Expense
(2)
| | Balance (1)
| | Rate
(2)
| | Interest
Expense
(2)
| |
|---|
| New debt | | $ | 1,730,000 | | 8.5% | | $ | 147,728 | | $ | 1,730,000 | | 8.5% | | $ | 110,796 | | $ | 1,730,000 | | 8.5% | | $ | 110,796 | | $ | 1,730,000 | | 8.5% | | $ | 147,728 | |
| Existing senior subordinated notes | | | 200,000 | | 11.75% | | | 23,500 | | | 200,000 | | 11.75% | | | 17,625 | | | 200,000 | | 11.75% | | | 17,625 | | | 200,000 | | 11.75% | | | 23,500 | |
| Existing ReAble European revolver | | | 2,781 | | 4.0% | | | 111 | | | 2,781 | | 4.0% | | | 83 | | | 2,781 | | 4.0% | | | 83 | | | 2,781 | | 4.0% | | | 111 | |
| Existing ReAble European term loan | | | 1,890 | | 4.2% | | | 79 | | | 1,890 | | 4.2% | | | 59 | | | 1,890 | | 4.2% | | | 59 | | | 1,890 | | 4.2% | | | 79 | |
| Note payable | | | 469 | | 8.3% | | | 39 | | | 469 | | 8.3% | | | 29 | | | 469 | | 8.3% | | | 29 | | | 469 | | 8.3% | | | 39 | |
| Capital leases | | | 648 | | 6.0% | | | 39 | | | 648 | | 6.0% | | | 29 | | | 648 | | 6.0% | | | 29 | | | 648 | | 6.0% | | | 39 | |
| | | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| |
| Total cash interest expense before interest income | | | | | | | | 171,496 | | | | | | | | 128,621 | | | | | | | | 128,621 | | | | | | | $ | 171,496 | |
| Amortization of capitalized debt issuance costs | | $ | 42,150 | | 6.5 years | | | 6,485 | | $ | 42,150 | | 6.5 years | | | 4,863 | | $ | 42,150 | | 6.5 years | | | 4,863 | | $ | 42,150 | | 6.5 years | | | 6,485 | |
| | | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| |
| Total Pro forma interest expense | | | | | | | | 177,981 | | | | | | | | 133,484 | | | | | | | | 133,484 | | | | | | | | 177,981 | |
| Less: historical ReAble interest expense(3) | | | | | | | | (53,386 | ) | | | | | | | (40,066 | ) | | | | | | | (43,125 | ) | | | | | | | (53,386 | ) |
| Less historical DJO interest expense(3) | | | | | | | | (28,834 | ) | | | | | | | (22,555 | ) | | | | | | | (17,807 | ) | | | | | | | (24,086 | ) |
| | | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| |
| Pro forma interest expense adjustment | | | | | | | $ | 95,761 | | | | | | | $ | 70,863 | | | | | | | $ | 72,552 | | | | | | | $ | 100,509 | |
| | | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| |
- (1)
- The new debt balance consists of $1,055.0 million of term loan borrowings under our new senior secured credit facility, the undrawn portion of the revolving credit facility under our new senior secured credit facility in the amount of $100.0 million, and $575.0 million related to the new senior unsecured notes.
- (2)
- The terms of the new senior secured credit facilities are expected to require a fee of 0.5% for all undrawn amounts under the revolving credit facility. The interest expense figures for "new debt" above reflect an amount of $0.5 million for the twelve month periods and $0.4 million for the nine month periods with respect to the annual fee on undrawn amounts under the revolving credit facility. No amounts are expected to be drawn on the revolving credit facility at closing. The interest rate on the new debt used to compute pro forma interest expense is a blended rate for the undrawn portion of the revolving credit facility, the new term loan facility and the new senior unsecured notes. In the event of a1/8 of 1% change in the assumed blended interest rate, the amount of pre-tax interest expense on the $1,630 million of new indebtedness drawn would increase or decrease by $2.0 million for the year ended December 31, 2006 and the twelve months ended September 29, 2007 and $1.5 million for each of the nine months ended September 29, 2007 and September 30, 2006.
- (3)
- Represents pro forma interest expense for ReAble and DJO which reflect pro forma adjustments for the Compex Acquisition, the Prior Transactions and the Aircast Acquisition, but prior to pro forma adjustments for the Transactions.
54
- (k)
- Reflects the estimated tax impact relating to the adjustments for the Transactions, calculated at an estimated 40% statutory rate as set forth below (in thousands):
| | Twelve Months
December 31, 2006
| | Nine Months
September 30, 2006
| | Nine Months
September 29,
2007
| | Twelve Months
September 29,
2007
| |
|---|
| Total adjustments for the Transactions | | $ | (142,168 | ) | $ | (105,677 | ) | $ | (107,386 | ) | $ | (144,686 | ) |
| Statutory tax rate | | | 40 | % | | 40 | % | | 40 | % | | 40 | % |
| | |
| |
| |
| |
| |
| Tax effect of adjustments for the Transactions | | $ | (56,867 | ) | $ | (42,271 | ) | $ | (42,954 | ) | $ | (57,874 | ) |
| | |
| |
| |
| |
| |
- (l)
- As a result of the Transactions, we may record certain expenses that have not been included in the pro forma condensed consolidated statements of operations for any period. The items noted below have been excluded from the pro forma condensed consolidated statements of operations as these items are not expected to have a recurring impact (in thousands):
| | As of
September 29, 2007
| |
|---|
| Amortization of the inventory write-up | | $ | 6,578 | |
| Write-off of ReAble's existing deferred financing costs | | | 9,365 | |
| Write-off of in-process research and development | | | 3,000 | |
| | |
| |
| Total pre-tax expenses | | | 18,943 | |
| Tax rate | | | 40 | % |
| Estimated tax benefit (1) | | | 6,377 | |
| | |
| |
| | Total after-tax expenses | | $ | 12,566 | |
| | |
| |
- (1)
- Excludes write-off of IPR&D as this item will be expensed on a pre-tax basis.
55
ADDITIONAL UNAUDITED PRO FORMA FINANCIAL DATA
| | Pro Forma
Twelve Months Ended
September 29, 2007
(unaudited)
| |
|---|
| | (in thousands)
| |
|---|
| EBITDA(1) | | $ | 25,072 | |
| Depreciation and amortization(2) | | | 111,274 | |
| Capital expenditures(3) | | | 21,286 | |
| Net cash interest expense(4) | | | 168,774 | |
| Adjusted EBITDA(1) | | | 253,833 | |
| Adjusted EBITDA Margin(5) | | | 29.1 | % |
| Ratio of Adjusted EBITDA to net cash interest expense(1)(4) | | | 1.50 | x |
- (1)
- EBITDA, a measure used by our management to measure operating performance, is defined as net income (loss) plus interest expense, net, provision (benefit) for income taxes and depreciation and amortization. EBITDA is not a recognized term under GAAP and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity. Additionally, EBITDA is not intended to be a measure of free cash flow available for management's discretionary use, as it does not consider certain cash requirements such as interest payments, tax payments and debt service requirements. We believe EBITDA is helpful in highlighting trends because EBITDA provides additional information regarding our cash earnings from ongoing operations but can differ significantly from company to company. In addition, EBITDA provides more comparability between the historical results of ReAble and results that reflect purchase accounting relating to the Transactions and the new capital structure resulting from the Transactions. Management compensates for the limitations of using non-GAAP financial measures by presenting them together with GAAP results to provide a more complete understanding of the factors and trends affecting the business than GAAP results alone. This presentation of EBITDA may not be comparable to other similarly titled measures of other companies.
- Adjusted EBITDA is defined as EBITDA further adjusted to exclude certain of the non-cash items, non-recurring items and other adjustment items permitted in calculating covenant compliance under (i) the indenture governing the new senior unsecured notes, (ii) our existing and our new senior secured credit facilities and (iii) the existing senior subordinated notes. We believe that the inclusion of supplementary adjustments to EBITDA applied in presenting Adjusted EBITDA is appropriate to provide additional information to investors about certain material non-cash items, non-recurring items and other adjustment items. We describe these adjustments to EBITDA in the table below. Such supplementary adjustments to EBITDA may not be in accordance with current SEC practice or with regulations adopted by the SEC that apply to registration statements filed under the Securities Act and periodic reports presented under the Exchange Act.
56
- The following table provides a reconciliation from our net loss to EBITDA and pro forma Adjusted EBITDA:
| | Pro Forma
Twelve Months
Ended
September 29,
2007
(unaudited)
| |
|---|
| | (in thousands)
| |
|---|
| Net loss | | $ | (181,125 | ) |
| Interest expense, net(a) | | | 175,759 | |
| Income tax benefit | | | (80,836 | ) |
| Depreciation and amortization | | | 111,274 | |
| | |
| |
| | EBITDA | | | 25,072 | |
| Non cash items(b) | | | 76,337 | |
| Non recurring items(c) | | | 27,558 | |
| Other adjustment items(d) | | | 124,866 | |
| | |
| |
| | Adjusted EBITDA | | $ | 253,833 | |
| | |
| |
- (a)
- Reflects $178.0 million of interest expense, net of $2.2 million of interest income.
- (b)
- Non-cash items are comprised of the following:
| | Pro Forma
Twelve Months
Ended
September 29,
2007
(unaudited)
|
|---|
| | (in thousands)
|
|---|
| Stock compensation expense(i) | | $ | 20,186 |
| Loss on disposition of assets(ii) | | | 416 |
| Loss on early extinguishment of debt(iii) | | | 9,154 |
| Purchase accounting adjustments(iv) | | | 36,380 |
| Loss on asset impairment(v) | | | 1,066 |
| Physical inventory adjustments(vi) | | | 2,268 |
| Accounts receivable reserve adjustments(vii) | | | 6,867 |
| | |
|
| | Total non-cash items | | $ | 76,337 |
| | |
|
- (i)
- Represents non-cash stock based compensation expense of $10.9 million incurred by DJO and $9.3 million by ReAble.
- (ii)
- Represents losses on dispositions of assets by ReAble, which we believe are not indicative of results from continuing operations.
- (iii)
- Represents a write-off of deferred debt issuance costs of $9.2 million by ReAble in connection with the refinancing of ReAble's indebtedness in the Prior Transactions.
- (iv)
- Represents $25.2 million of expense incurred by ReAble related to the write-off of in-process research and development ("IPR&D") and $11.2 million of expense related to the write-up to fair market value of inventory acquired in connection with the Compex acquisition, the Cefar acquisition and the Prior Transactions during the twelve months ended September 29, 2007.
- (v)
- Represents $1.1 million of expense incurred by ReAble related to the impairment of certain application software that will not be placed in service.
- (vi)
- Represents adjustments related to DJO's complete physical inventory of raw materials subsequent to the integration of the Aircast acquisition.
- (vii)
- Represents a non-cash charge by DJO to increase estimates of uncollectible accounts related to receivables aged due to transition issues related to off-shoring a portion of the billing and collection process used by DJO's third-party insurance billing service provider.
57
- (c)
- Non-recurring items are comprised of the following:
| | Pro Forma
Twelve Months
Ended
September 29,
2007
(unaudited)
|
|---|
| | (in thousands)
|
|---|
| Employee severance(i) | | $ | 1,729 |
| Restructuring expense(ii) | | | 13,039 |
| Other(iii) | | | 12,790 |
| | |
|
| | Total non-recurring adjustments | | $ | 27,558 |
| | |
|
- (i)
- Employee severance expenses for the twelve month period ended September 29, 2007 include approximately $1.6 million incurred by ReAble in connection with the Compex acquisition, the Saunders acquisition, the IOMED acquisition and the Prior Transactions and approximately $0.1 million of DJO-related severance expenses.
- (ii)
- Restructuring expense for the twelve months ended September 29, 2007 represents (A) $5.3 million of expense related to field selling, information technology, operations and product line consolidation incurred in connection with the integration of Compex, (B) $1.5 million of inventory reserves related to the discontinuation of certain products acquired from Osteoimplant Technology, Inc., (C) $2.8 million of integration expenses related to the Cefar acquisition, (D) $2.3 million for restructuring advisory fees related to the Prior Transactions, and (E) $1.1 million of integration expenses related to the Performance Modalities, Inc. ("PMI") and Saunders acquisitions.
- (iii)
- Other expenses for the twelve months ended September 29, 2007 includes $3.4 million incurred by ReAble for other non-recurring expenses, including provisions made for litigation settlements and additional product liability claims. Other expenses incurred by DJO for the twelve month period ended September 29, 2007 includes $0.4 million related to the relocation of its headquarters facility, $8.4 million for non-recurring expenses related to the Aircast and Axmed acquisitions and $0.6 million in legal fees related to a legal settlement.
- (d)
- Other adjustment items are comprised of the following:
| | Pro Forma
Twelve Months
Ended
September 29,
2007
(unaudited)
| |
|---|
| | (in thousands)
| |
|---|
| Transaction expenses(i) | | $ | 22,746 | |
| Compex acquisition adjustments(ii) | | | 20,986 | |
| Minority interest(iii) | | | 338 | |
| Pre-acquisition EBITDA(iv) | | | 4,488 | |
| Discontinued operations(v) | | | 17 | |
| Other income(vi) | | | (973 | ) |
| Franchise taxes(vii) | | | 167 | |
| Monitoring fee(viii) | | | 8,000 | |
| Cost savings: | | | | |
| | Cost savings related to acquisition of DJO(ix) | | | 50,600 | |
| | Other cost savings(x) | | | 18,497 | |
| | |
| |
| | | Total other adjustment items | | $ | 124,866 | |
| | |
| |
- (i)
- Transaction expenses for the twelve months ended September 29, 2007 include ReAble expenses of $14.3 million for legal, advisory, consulting and related expenses incurred in connection with the closing of the Prior Transactions, $5.5 million of management retention bonuses paid at the closing of the Prior Transactions and $0.3 million of merger and acquisition related expenses. Transaction expenses for the twelve month period ended September 29, 2007 also include $2.6 million for fees and expenses incurred by DJO in connection with the Transactions.
58
- (ii)
- Represents ReAble adjustments and additional reserves related to the Compex acquisition and operations. The amounts principally include additional accounts receivable reserves. During December 2006, the decision was made to outsource the Compex accounts receivable collection effort to a third party. Based on collection estimates provided by the third party collection company, in the fourth quarter of 2006, ReAble recorded an additional adjustment to the carrying value of accounts receivable in the amount of $19.8 million.
- (iii)
- Represents ReAble's minority interest.
- (iv)
- Represents pre-acquisition EBITDA of (A) $0.1 million from September 30, 2006 to February 16, 2007 related to the Performance Modalities, Inc. ("PMI") acquisition, (B) $0.1 million from September 30, 2006 to April 13, 2007 for the Endeavor Medical Inc. ("Endeavor") acquisition, (C) $0.2 million of EBITDA from September 30, 2006 to November 7, 2006 primarily related to the Cefar acquisition, (D) $3.5 million of EBITDA from September 30, 2006 to July 2, 2007 for the Saunders acquisition, and (E) $0.5 million of EBITDA from September 30, 2006 to August 9, 2007 for the IOMED acquisition, which are included in the calculation of Adjusted EBITDA for the twelve months ended September 29, 2007 pursuant to the definition of Adjusted EBITDA contained in the credit agreement relating to our new senior secured credit facilities, the indenture governing the new senior unsecured notes and the indenture governing our existing senior subordinated notes.
- (v)
- Represents additional costs associated with the discontinuation of the Slendertone U.S. consumer product line.
- (vi)
- Represents $0.3 million and $0.6 million of net foreign exchange gains of ReAble and DJO, respectively, and $0.1 million of royalty income of ReAble.
- (vii)
- Represents state franchise taxes paid by ReAble.
- (viii)
- Represents an annual monitoring fee payable to an affiliate of Blackstone.
- (ix)
- Represents the estimated net cost savings associated with the acquisition of DJO in the Transactions. We expect that all actions necessary to begin realizing these estimated cost savings will have been taken by the end of 2008 and will be fully realized by 2009. The estimated cost savings are reflected as an adjustment to pro forma Adjusted EBITDA as follows:
| | (unaudited)
|
|---|
| | (in thousands)
|
|---|
| Savings in cost of goods sold: | | | |
| | Manufacturing(a) | | $ | 10,000 |
| | Freight(b) | | | 3,900 |
| | Procurement(c) | | | 11,200 |
| | |
|
| | | Subtotal | | | 25,100 |
Savings in operating expenses: |
|
|
|
| | General and administrative(d) | | | 5,900 |
| | Reimbursement/IT(e) | | | 6,300 |
| | Procurement and public company costs(f) | | | 5,500 |
| | International and other(g) | | | 7,800 |
| | |
|
| | | Subtotal | | | 25,500 |
| | |
|
| | | | Total | | $ | 50,600 |
| | |
|
- (a)
- Represents estimated savings of $7.5 million associated with relocating certain ReAble manufacturing functions to DJO's lower cost manufacturing facility in Mexico, as well as implementing DJO lean manufacturing principles in certain ReAble manufacturing locations. Also includes an estimated $2.5 million in savings related to manufacturing of certain outsourced ReAble products by DJO at reduced cost.
- (b)
- Represents estimated freight savings of $2.1 million expected to be achieved by applying our lower contracted rates to DJO freight usage and $1.8 million of distribution savings expected to be achieved by utilizing DJO's distribution model for certain combined distribution activities.
- (c)
- Represents estimated cost reductions of $4.1 million expected to be achieved by substituting DJO manufactured products for products ReAble currently purchases from DJO competitors,
59
$2.5 million expected to be achieved by insourcing to DJO manufacturing of certain components currently purchased by ReAble from third parties, $2.5 million expected to be achieved by shifting to the lower cost suppliers for common materials purchased by both ReAble or DJO and $2.1 million expected to be achieved by consolidating purchases to new and/or existing suppliers.
- (d)
- Represents estimated savings of $5.9 million expected to be achieved by consolidating corporate and certain other general and administrative functions.
- (e)
- Represents estimated savings of $6.3 million expected to be achieved through consolidation of certain information technology infrastructure and insourcing to ReAble reimbursement functions which are currently outsourced by DJO.
- (f)
- Represents estimated savings of $4.5 million expected to be achieved through reduced audit and insurance costs and through applying our lower contracted purchasing rates to other indirect procurement categories and $1.0 million expected to be achieved by eliminating DJO public company costs.
- (g)
- Represents estimated savings of $7.8 million expected to be achieved by consolidating certain international functions and certain non-customer facing sales and marketing functions.
- We have also identified specific initiatives for additional cost savings to be achieved as a result of the acquisition of DJO, which we have not reflected in the forgoing adjustments.
- We cannot assure you that we will achieve the estimated cost savings from the Merger in the time frame anticipated, or at all.
- (x)
- Reflects estimated forward cost savings of ReAble and DJO as a result of initiatives currently being implemented and not related to the Transactions, which are reflected as an adjustment to pro forma Adjusted EBITDA as follows:
| | (unaudited)
|
|---|
| | (in thousands)
|
|---|
| Estimated future cost savings—ReAble(a) | | $ | 11,797 |
| Estimated future cost savings—DJO(b) | | | 6,700 |
| | |
|
| | Total other cost savings | | $ | 18,497 |
| | |
|
- (a)
- Represents estimated future cost savings of (i) $4.4 million related to actions initiated in connection with the Cefar acquisition, principally related to facility and general and administrative consolidation, (ii) $7.0 million related to headcount reductions and production efficiencies in relation to the IOMED and Saunders acquisitions and (iii) $0.4 million principally from actions initiated in connection with the Endeavor acquisition and procurement savings related to purchases of certain product components.
- (b)
- Represents estimated future cost savings that will be realized upon the completion of certain cost improvement projects already initiated related to integrating the acquired Aircast business.
- We cannot assure you that we will achieve the estimated cost savings from these initiatives in the time frame anticipated, or at all.
- (2)
- Reflects depreciation of $26.7 million and amortization of $84.6 million.
- (3)
- Reflects capital expenditures for the twelve months ended September 29, 2007 of $12.3 million for ReAble and $9.0 million for DJO, respectively.
- (4)
- Net cash interest expense represents pro forma interest expense of $178.0 million for the twelve months ended September 29, 2007, less interest income of $2.2 million, commitment fees of $0.5 million on the unused portion of our new revolving credit facility and $6.5 million of amortization of deferred financing fees.
- (5)
- EBITDA margin presents EBITDA as a percentage of net sales, and pro forma Adjusted EBITDA margin presents pro forma Adjusted EBITDA as a percentage of pro forma net sales. The following table provides a reconciliation from net income (loss) margin to EBITDA margin and pro forma net sales margin to pro forma
60
Adjusted EBITDA margin, with each line item below presented as a percentage of net sales. See note (1) for an explanation of EBITDA and pro forma Adjusted EBITDA and the related adjustments.
| | Pro Forma
Twelve Months
Ended
September 29,
2007
(unaudited)
| |
|---|
| Net loss margin | | (20.7 | )% |
| Interest expense, net margin | | 20.1 | |
| Income tax provision (benefit) margin | | (9.2 | ) |
| Depreciation and amortization margin | | 12.7 | |
| | |
| |
| | EBITDA margin | | 2.9 | |
| Non cash items margin | | 8.7 | |
| Non recurring items margin | | 3.2 | |
| Other adjustment items margin | | 14.3 | |
| | |
| |
| | Adjusted EBITDA margin | | 29.1 | % |
| | |
| |
61
Impact of Significant Acquisitions on Revenue and Adjusted EBITDA
ReAble's and DJO's acquisitions over the past several years have contributed significantly to their growth. However, we estimate that the combined revenues of ReAble and DJO from 2004 to 2006, calculated on an aggregate basis with the pre-acquisition revenues of some of our most significant acquisitions during this period (Empi and Compex for ReAble, and Aircast for DJO, which we collectively refer to as the "Specified Acquisitions") would have generated a compound annual growth rate over this period of approximately 8%. In addition, we estimate that the combined Adjusted EBITDA of ReAble and DJO from 2004 to 2006, calculated on an aggregate basis during this period with the pre-acquisition Adjusted EBITDA of the companies that are the subject of the Specified Acquisitions, would have generated a compound annual growth rate over this period of approximately 10%.
Investors should be aware that the foregoing compound annual growth rate information represents estimates by our management and is presented for informational purposes in light of the significant growth in ReAble's and DJO's operating results during that period as a result of acquisitions, and does not represent what our compound annual growth rate would have been had the combination of ReAble and DJO and the Specified Acquisitions actually had been consummated at the beginning of 2004. In addition, the foregoing information was not prepared in accordance with the rules and regulations of the SEC with respect to the preparation of pro forma financial information, and were derived from management estimates and not from any pro forma financial statements prepared in accordance with GAAP or the appropriate rules and regulations of the SEC. Further, the financial information we have for the companies that are the subject of the Specified Acquisitions was derived from a variety of sources, including statements, accounts and other balances of the predecessor management of those companies, and in many cases was not audited or reviewed by any independent accounting firm. Accordingly, absent any such audit or review, we cannot assure you that such financial information was prepared in accordance with GAAP or that if such financial information were to have been audited or reviewed by an independent auditing firm, there would have been adjustments made to such financial information that would have a material and adverse impact on the information set forth above. The foregoing information also reflects only the combination of ReAble and DJO and the Specified Acquisitions, and does not include pre-acquisition information for other acquisitions. As a result, the combined revenue and Adjusted EBITDA we have estimated for purposes of calculating the compound annual growth rate information set forth above is lower in the periods for which we are making pre-acquisition assumptions than what we would have shown for combined revenue and Adjusted EBITDA if all acquisitions had been included and, consequently, the compound annual growth rates for revenue and Adjusted EBITDA presented above are higher than what they would have been had we included all acquisitions in the estimates set forth above. Additionally, investors should be aware that while we calculated "Adjusted EBITDA" for each of ReAble and DJO pursuant to the methodology for calculating Adjusted EBITDA as set forth in note (1) under the caption "—Summary Unaudited Condensed Consolidated Financial Information" in this offering circular, we do not have sufficient information regarding the companies that are the subject of the Specified Acquisitions to make all (or in some cases any) of the adjustments set forth in our calculation of Adjusted EBITDA with respect to each of those companies and, as a result, in many cases our calculation of Adjusted EBITDA for those companies was done in a manner that is different from the manner in which we calculated Adjusted EBITDA for ReAble and DJO (or for any of the other companies). Accordingly, our calculation of the combined Adjusted EBITDA for ReAble, DJO and the companies that are the subject of the Specified Acquisitions for any period may be lower than would have resulted if all appropriate adjustments were to have been made and, as a result, the actual compound annual growth rate in Adjusted EBITDA set forth above may be higher than would have resulted in all appropriate adjustments were to have been made.
62
In light of the foregoing considerations, investors should not place undue reliance on the compound annual growth rate information provided above. In particular, investors are cautioned that such information is presented solely to illustrate that the growth in ReAble's and DJO's operating results have been significantly impacted by acquisitions, and therefore that year-over-year comparisons of ReAble's or DJO's historical operating results should be made in light of those circumstances.
63
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
REABLE THERAPEUTICS FINANCE LLC |
Date: October 31, 2007 |
|
|
|
| | | By: | /s/ HARRY L. ZIMMERMAN
Name: Harry L. Zimmerman
Title: Executive Vice President—General Counsel |
62
QuickLinks
"Safe Harbor" Statement under Private Securities Litigation Reform Act of 1995The TransactionsSources and Uses(in millions)RISK FACTORSRisks Related To Our IndebtednessRisks Related To Our BusinessUNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL INFORMATIONUNAUDITED PRO FORMA CONDENSED CONSOLIDATED BALANCE SHEET AS OF SEPTEMBER 29, 2007NOTES TO UNAUDITED PRO FORMA CONDENSED CONSOLIDATED BALANCE SHEETUNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS FOR THE TWELVE MONTHS ENDED DECEMBER 31, 2006UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2006UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS FOR THE NINE MONTHS ENDED SEPTEMBER 29, 2007UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS FOR THE TWELVE MONTHS ENDED SEPTEMBER 29, 2007NOTES TO THE UNAUDITED PRO FORMA CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONSUnaudited Pro Forma Condensed Consolidated Statement of Operations for the Nine Months Ended September 30, 2006 and for the Twelve Months Ended December 31, 2006Unaudited Pro Forma Condensed Consolidated Statement of Operations for the Nine Months Ended September 30, 2006 and for the Twelve Months ended December 31, 2006SIGNATURES


