SUMMARY
The following summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this prospectus, including our consolidated financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Unless the context indicates otherwise, references in this prospectus to “Syndax,” “the Company,” “we,” “us,” “our” and similar references refer to Syndax Pharmaceuticals, Inc. and its wholly owned subsidiaries.
Company Overview
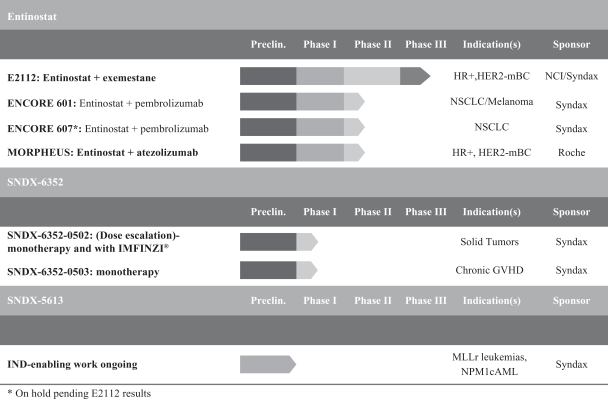
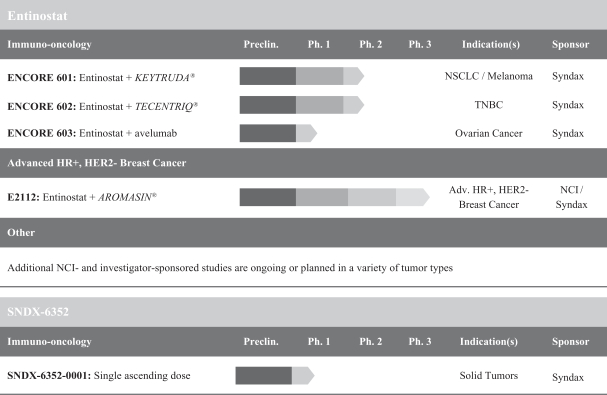
We are a clinical stage biopharmaceutical company developing an innovative pipeline of combination therapies in multiple cancer indications. Our lead product candidate, entinostat, is currently being evaluated in a Phase 3 clinical trial for advanced hormone receptor positive, or HR+, human epidermal growth factor receptor 2 negative, or HER2-, breast cancer. Entinostat was granted Breakthrough Therapy designation by the U.S. Food and Drug Administration, or the FDA, following positive results from our Phase 2b clinical trial, ENCORE 301. We are developing entinostat, which has direct effects on both cancer cells and immune regulatory cells, andSNDX-6352, a monoclonal antibody that targets the colony stimulating factor-1 receptor, or CSF-1R, to enhance the body’s immune response on tumors that have shown sensitivity to immunotherapy. We acquired the exclusive rights to SNDX-6352 in July 2016 and are evaluating entinostat as a combination therapeutic in Phase 1b/2 clinical trials with Merck & Co., Inc., or Merck, for non-small cell lung cancer and melanoma; with Genentech, Inc., or Genentech, for triple negative breast cancer, or TNBC; and with Merck KGaA, Darmstadt, Germany, or Merck KGaA, and Pfizer Inc., or Pfizer, for ovarian cancer. We are evaluating SNDX-6352 in a single ascending dose Phase 1 clinical trial. We plan to continue to leverage the technical and business expertise of our management team and scientific collaborators to license, acquire and develop additional cancer therapies to expand our pipeline.
Entinostat
Entinostat is our oral, small molecule product candidate that has direct effects on both cancer cells and immune regulatory cells, potentially enhancing the body’s immune response to tumors. The favorable safety profile of entinostat has been demonstrated in clinical trials in more than 1,000 cancer patients. We believe that, based on its mechanism of action, entinostat may have broad applications in additional tumor types, including head and neck, bladder and renal cell, which are immuno-responsive, or sensitive to immunotherapy.
Immuno-oncology is an emerging field of cancer medicine that has focused on the development of therapeutic approaches designed to activate the immune system to find and destroy cancer cells. Many tumors have the ability to evade the immune system through direct cellular interactions and recruitment of immuno-suppressive cells to the area surrounding the tumor. One such evasion mechanism is through the expression of proteins known as checkpoint proteins, such as programmed cell death protein ligand 1, or PD-L1, on the cancer cell surface. These checkpoint proteins bind to a corresponding receptor known as programmed cell death protein 1, or PD-1, which is expressed on particular immune cells known as cytotoxic T cells. Through this binding process, cytotoxic T cells are blocked from killing cancer cells. Antibodies known as immune checkpoint inhibitors block the interaction between PD-1 and PD-L1 to restore the ability of cytotoxic T cells to kill cancer cells and have shown significant clinical benefit in treating certain cancers. We believe that entinostat acts on a different tumor-evasion mechanism than is targeted by most other immunotherapies in development. Instead of focusing on the interaction between the T cell and the tumor, entinostat has been observed to decrease
1