In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expects,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative or plural of those terms, and similar expressions intended to identify statements about the future, although not all forward-looking statements contain these words. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
You should refer to the risks and uncertainties described in the “Risk Factors” section contained in our SEC Reports for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Given these risks, uncertainties and other factors, many of which are beyond our control, we cannot assure you that the forward-looking statements in this document will prove to be accurate, and you should not place undue reliance on these forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements to reflect events or developments occurring after the date of this report, even if new information becomes available in the future.
Company Overview
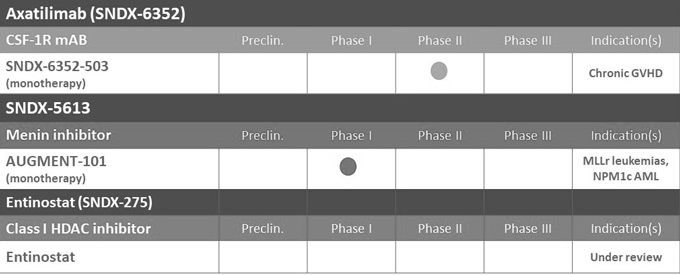
We are a clinical-stage biopharmaceutical company developing an innovative pipeline of cancer therapies. Our two lead product candidates are, SNDX-5613 and SNDX-6352, or axatilimab. We are developing SNDX-5613, targeting the binding interaction of menin with the mixed lineage leukemia 1 (MLL1) protein for the treatment of MLL-rearranged, or MLLr, acute leukemias and nucleophosmin 1, or NPM1, mutant acute myeloid leukemia (AML), as well as axatilimab, a monoclonal antibody that blocks the colony stimulating factor 1, or CSF-1 receptor. We have deprioritized the development of entinostat, our once-weekly, oral, small molecule, Class I HDAC inhibitor, to focus resources on advancing the remainder of our pipeline. We plan to continue to leverage the technical and business expertise of our management team and scientific collaborators to license, acquire and develop additional cancer therapies to expand our pipeline.
SNDX-5613
Our first clinical-stage product candidate, SNDX-5613, is a potent, orally active inhibitor of the high affinity interaction site on menin with the protein MLL1. This specific interaction is a key driver for two genetically defined acute leukemias: (i) MLLr and (ii) NPM1c AML. Both diseases have a poor prognosis. In preclinical testing, SNDX-5613 has demonstrated complete tumor regression and profound, dose-dependent and long-lasting survival benefit in leukemic models of disease. Initial clinical evidence with SNDX-5613 also supports the hypothesis that disruption of the Menin-MLL interaction can lead to responses in acute leukemias.
We are developing SNDX-5613 as a targeted therapy to potentially treat two genetically defined acute leukemias: (i) a genetically defined subset of acute leukemias with chromosomal rearrangements in the mixed lineage leukemia (MLL) gene, known as MLLr; and (ii) acute myeloid leukemia, or AML, with a somatic mutation in the nucleophosmin 1, or NPM1, gene, also known as NPM1c. Our near-term focus is to rapidly establish proof of concept that SNDX-5613 is a targeted therapy that can potentially provide meaningful clinical benefit to adult and pediatric leukemia patients having relapsed or refractory MLLr or NPM1c acute leukemias. Our IND application for SNDX-