PROSPECTUS SUPPLEMENT SUMMARY
The following summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference into this prospectus supplement. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our securities. For a more complete understanding of our company and this offering, you should read and consider carefully the more detailed information included or incorporated by reference in this prospectus supplement and the accompanying prospectus, including our historical financial statements and notes to those financial statements. Please read “Risk Factors” in this prospectus supplement, in our Annual Report on Form 10-K for the year ended December 31, 2021, in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2022 and in the other documents incorporated by reference, including other documents that we subsequently file with the SEC for more information about important risks that you should consider before investing in our securities.
Company Overview
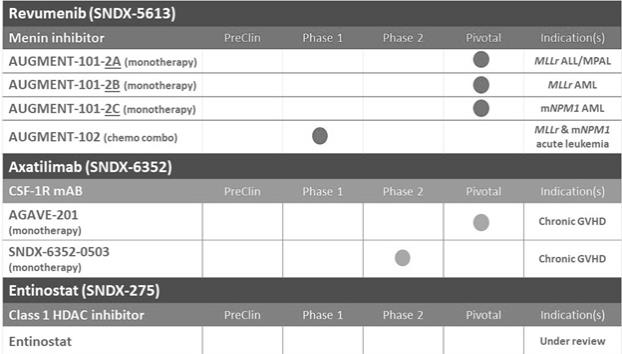
We are a clinical-stage biopharmaceutical company developing an innovative pipeline of cancer therapies. Our two lead product candidates are SNDX-5613, or revumenib, and SNDX-6352, or axatilimab. We are developing revumenib, a small molecule inhibitor of the menin-mixed lineage leukemia 1, or menin-MLL1, binding interaction for the treatment of MLL-rearranged, or MLLr, acute leukemias and nucleophosmin 1, or NPM1, mutant acute myeloid leukemia, or AML. We are also developing axatilimab, a monoclonal antibody that blocks the colony stimulating factor 1, or CSF-1 receptor, in chronic graft-versus-host disease, or cGVHD, as well as idiopathic pulmonary fibrosis, or IPF. We plan to continue to leverage the technical and business expertise of our management team and scientific collaborators to license, acquire and develop additional therapeutics to expand our pipeline.
Revumenib
Revumenib, is a potent, orally active inhibitor of the interaction between menin and the protein MLL1 (or KMT2A). This specific interaction is a key driver for two genetically defined acute leukemias: (i) MLLr (also referred to as KMT2A rearrangement, or KMT2Ar) and (ii) NPM1c AML. Both diseases have a poor prognosis. In preclinical testing, revumenib has demonstrated benefit in leukemic models of disease. Initial clinical evidence with revumenib also supports the hypothesis that disruption of the menin-MLL interaction can lead to responses in both acute leukemias subsets.
Our near-term focus is to rapidly establish proof-of-concept that revumenib is a targeted therapy that can potentially provide meaningful clinical benefit to adult and pediatric patients with relapsed or refractory MLLr or NPM1c acute leukemias. Our investigational new drug, or IND, application for revumenib took effect with the U.S. Food and Drug Administration, or FDA, in the second quarter of 2019 and we commenced AUGMENT-101, a clinical trial consisting initially of a Phase 1 dose escalation portion to determine the maximum tolerated dose, or MTD, and recommended Phase 2 dose of revumenib in patients with relapsed or refractory MLLr or NPM1c acute leukemia. We are conducting the trial at multiple centers in the United States and Europe. As discussed below, we have completed the Phase 1 portion of the trial, and the pivotal Phase 2 portion of the trial is ongoing, enrolling patients across each of three distinct trial populations: patients with NPM1 AML, patients with MLLr AML, and patients with MLLr ALL to determine the efficacy, safety, and tolerability of revumenib. Based on discussions with the FDA, AUGMENT-101 may serve as the basis for regulatory filings in each of the three populations. We expect enrollment to continue into 2023 and that we will report topline data from at least one of the trials starting in the third quarter of 2023. If the trial is successful, we expect to submit our first New Drug Application for revumenib by the end of 2023.
On November 3, 2022, we announced updated positive data from the Phase 1 portion of the ongoing AUGMENT-101 trial of revumenib in patients with R/R NPM1 mutant or MLLr acute leukemia. As of the March 2022 data cutoff date, sixty patients with R/R mNPM1 or MLLr (KMT2Ar) acute leukemia were evaluable for efficacy, an increase of 9 patients from the 51 evaluable for efficacy at the 2021 ASH Annual Meeting. These data highlighted an overall response rate 53% (32/60), which we define as CR, plus CRh, plus CRp, plus MLFS, an improved 30% (18/60) CR/CRh rate and a median duration of CR/CRh response of 9.1 months. Additionally, of the 12 patients who achieved a CR/CRh on revumenib treatment and then went on to receive a stem cell transplant, nine (75%) remained in remission as of the data cutoff date, with a median follow-up of 12.3 months. Three of 12 patients with MLLr AML were treated with revumenib maintenance in the compassionate use setting following stem cell transplant or non-myeloablative stem cell boost, two of whom (67%) remained in remission for over one year (as of the data cutoff date). To date, there have been no discontinuations due to treatment-related adverse events. This data will be featured in two oral sessions at the ASH Annual Meeting, on Saturday, December 10, 2022.