UNITED STATES SECURITIES AND EXCHANGE COMMISSION |
Washington, D.C. 20549 |
|
SCHEDULE TO |
Tender Offer Statement under Section 14(d)(1) or 13(e)(1) of the Securities Exchange Act of 1934 |
| |
| |
| |
MEDIMMUNE, INC. |
| (Name of Subject Company) |
| |
ASTRAZENECA BIOPHARMACEUTICALS INC. ASTRAZENECA PLC |
| (Names of Filing Persons – Offeror) |
| |
Common Stock, Par Value $0.01 Per Share |
| (Title of Class of Securities) |
|
584699102 |
| (Cusip Number of Class of Securities) |
| |
Graeme Musker AstraZeneca PLC 15 Stanhope Gate London, England, W1K 1LN Telephone: +44 20 7304 5000 |
(Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications on Behalf of Filing Persons) |
| |
Copies to: |
Paul R. Kingsley Davis Polk & Wardwell 450 Lexington Avenue New York, New York 10017 Telephone: (212) 450-4000 |
[NOT FOR RELEASE, PUBLICATION OR DISTRIBUTION IN WHOLE OR IN PART, IN, INTO OR FROM AUSTRALIA, CANADA OR JAPAN OR ANY OTHER JURISDICTION WHERE TO DO SO WOULD CONSTITUTE A VIOLATION OF THE RELEVANT LAWS OF SUCH JURISDICTION]
AstraZeneca to acquire MedImmune for $58 per share in a fully recommended, all-cash transaction with a total enterprise value of $15.2 billion
Acquisition delivers AstraZeneca biologics ambition faster
Creates a leading, fully-integrated biotechnology business within AstraZeneca with critical mass in research, development, regulatory and manufacturing with global sales reach
Significantly expands product pipeline by adding 45 projects including 2 late-stage products and a blockbuster marketed monoclonal antibody, Synagis
Summary
AstraZeneca PLC (“AstraZeneca”) today announced that it has entered into a definitive agreement to acquire MedImmune, Inc. (“MedImmune”), in an all-cash transaction. Under the terms of the agreement, which has unanimous MedImmune Board support, AstraZeneca will acquire all of the fully diluted shares of MedImmune common stock at a price of $58 per share, for a total consideration of approximately $15.6 billion (including approximately $340m net cash).
The acquisition of MedImmune significantly accelerates AstraZeneca’s biologics strategy. The combination of MedImmune with AstraZeneca’s wholly-owned subsidiary Cambridge Antibody Technology (“CAT”) will create a world-class, fully integrated biologics and vaccines business within the AstraZeneca Group with critical mass in research, development, regulatory, manufacturing and global sales and marketing reach.
MedImmune is a world-leading, profitable, biotechnology company with a record of proven success with revenue in 2006 of $1.3bn, profit before tax of $75m and gross assets of $3.0bn.
The acquisition extends AstraZeneca’s R&D science base to allow it to address novel drug targets through 3 key technological approaches: small molecules, biologics and, for the first time, vaccines.
Overall, the combination of MedImmune with AstraZeneca’s existing capabilities will be capable of delivering a greater number of new biologic products to bring benefit to patients in AstraZeneca’s prioritised disease areas.
The deal is expected to close in June 2007.
Highlights and acquisition benefits
R&D capability
| · | Expands and diversifies AstraZeneca’s science base by establishing an international platform capable of delivering a greater flow of new medicines in AstraZeneca’s prioritised disease areas, embracing small molecules, monoclonal antibodies, next generation biologics and vaccines |
| · | Natural fit between CAT and MedImmune |
| · | Complementary with existing AstraZeneca therapeutic area strengths in Oncology, Infection and Respiratory & Inflammation |
| · | Provides entry into vaccines; through proprietary live attenuated vaccines capability |
| · | Brings significant regulatory experience in making Biologics License Applications |
| · | Enhanced biologics capability positions AstraZeneca as a more compelling licensing partner, improving AstraZeneca’s externalisation position |
Manufacturing
| · | MedImmune is a leader in protein engineering and biologics manufacturing, with a production capacity of over 30,000L planned by 2010 and world leading cell line productivity levels. Through further modest investment, capacity could be increased to over 60,000L. This would secure production requirements for the long-term and avoid the need for major near-term ‘green-field’ manufacturing investment by AstraZeneca to support its biologics strategy |
Pipeline
| · | Adds 2 late-stage assets: the next generation follow-on to ‘Synagis’, ‘Numax’ and refrigerated formulation ‘FluMist’ with an anticipated US launch for 2007-2008 influenza season |
| · | Increases the proportion of biologics in AstraZeneca’s pipeline from 7 percent to 27 percent and enlarges the total pipeline by 45 projects to 163 projects |
| · | Diversifies and expands R&D capability to deliver a greater flow of new biologic products |
Financial benefits
| · | Synergies from the acquisition of MedImmune and from related AstraZeneca activities are expected to be towards $500m per annum by 2009 |
| · | The acquisition is expected to be cash earnings enhancing in 2009 |
| · | The acquisition will be fully funded in cash, bringing improved financial efficiency through balance sheet leverage. Previously announced $4bn share buyback programme for 2007 unchanged |
| · | Addition of attractive marketed products including ‘Synagis’ and ‘FluMist’ to AstraZeneca’s portfolio adds $1.2bn in sales. Consensus sales growth for this portfolio is forecast at 12% CAGR to 2010 |
| · | Provides AstraZeneca with several other substantial assets, including a royalty stream on the sales of the HPV vaccines with estimated consensus peak sales of $5.5bn, potential milestones and royalties on MedImmune’s other licensed products and $1.5bn cash, including $89.4m relating to MedImmune Ventures investments at book value |
People
| · | Strong desire to retain employees and maintain culture, with emphasis on retaining key talent and critical skills |
| · | One-time retention grant for employees |
| · | David M. Mott, the Chief Executive Officer and President of MedImmune, and James F. Young, Ph.D., the President, Research and Development of MedImmune, have committed to remain with MedImmune and it is expected that other members of MedImmune’s senior management will stay with the company following the closing |
| · | David M. Mott will take a leadership role within AstraZeneca |
Commenting on the Offer, David Brennan, Chief Executive Officer of AstraZeneca, said:
“This acquisition represents a transformational step to deliver our biologics strategy sooner than anticipated. It creates a leading fully integrated biologics and vaccines business with critical mass and enhances AstraZeneca’s R&D science base through which we will deliver a stronger product pipeline.
MedImmune adds an exciting existing pipeline, including 2 late-stage products, great expertise in biologic drug development and state of the art manufacturing facilities.
We look forward to welcoming MedImmune’s employees into AstraZeneca and are excited by the potential to create significant value for all our shareholders, employees and patients that this acquisition brings.”
David M. Mott, CEO and President of MedImmune, said:
"After conducting a full and open process, whereby we evaluated potential interest in the value we have built over our 19 year history, we are very pleased to become a part of AstraZeneca. We believe that this transaction is in the best interest of all parties, including shareholders, employees and ultimately patients. The potential to harness the combined skills and capabilities of MedImmune, AstraZeneca and CAT and take our combined world class biologics capabilities to the next level, is very exciting and a challenge to which I am personally committed."
The Transaction
The acquisition is structured as an all cash tender offer for all outstanding shares of MedImmune common stock followed by a merger in which each remaining untendered share of MedImmune would be converted into the same $58 cash per share price paid in the tender offer. The acquisition is subject to the satisfaction of customary conditions, including the tender of a majority of the outstanding MedImmune shares on a fully-diluted basis and the expiration or earlier termination of the Hart-Scott-Rodino waiting period and other regulatory approvals. The tender offer will be commenced within 10 working days and is expected to close in June 2007, unless extended. The tender offer is not subject to a financing contingency.
The acquisition price represents a premium of approximately 53.3% to MedImmune’s closing share price of $37.84 on 11th April, 2007, this being the last business day prior to MedImmune’s announcement to explore strategic alternatives.
The transaction has been unanimously recommended by the Board of Directors of MedImmune.
The acquisition will be effected pursuant to a merger agreement. The merger agreement contains certain termination rights for each of AstraZeneca and MedImmune and further provides that, upon termination of the merger agreement under specified circumstances, MedImmune may be required to pay AstraZeneca a termination fee of $450 million.
Financing
The total consideration for the acquisition of MedImmune amounts to approximately $15 billion in cash. AstraZeneca will draw from a committed banking facility in the amount of $15 billion to provide the initial financing for the acquisition.
Additional Information
The tender offer described in this press release has not yet commenced, and this press release is neither an offer to purchase nor a solicitation of an offer to sell MedImmune common stock. Investors and security holders are urged to read both the tender offer statement and the solicitation/recommendation statement regarding the tender offer described in this press release when they become available because they will contain important information. The tender offer statement will be filed by AstraZeneca and a subsidiary of AstraZeneca with the Securities and Exchange Commission (“SEC”), and the solicitation/recommendation statement will be filed by MedImmune with the SEC. Investors and security holders may obtain a free copy of these statements (when available) and other documents filed by AstraZeneca or MedImmune with the SEC at the website maintained by the SEC at www.sec.gov. The tender offer statement and related materials may be obtained for free by directing such requests to AstraZeneca (Investor Relations) at +44 (0) 207 304 5000. The solicitation/recommendation statement and such other documents may be obtained by directing such requests to MedImmune (Investor Relations) at 301-398-4358.
Enquiries:
AstraZeneca | |
Media Enquiries: | |
| Steve Brown / Edel McCaffrey (London) | (020) 7304 5033/5034 |
| Staffan Ternby (Sweden) | (8) 553 26107 |
| Emily Denney (Wilmington) | (302) 885 3451 |
Analyst/Investor Enquiries: | |
| Jonathan Hunt / Mina Blair / Karl Hård (London) | (020) 7304 5087/5084/5322 |
| Staffan Ternby (Sweden) | (8) 553 26107 |
| Ed Seage / Jorgen Winroth (US) | (302) 886 4065/(212) 579 0506 |
Merrill Lynch (Financial Adviser to AstraZeneca) | +44 (0) 20 7628 1000 |
| Richard Girling | |
Deutsche Bank (Joint Corporate Broker to AstraZeneca) | +44 (0) 20 7545 8000 |
| Charlie Foreman | |
Goldman Sachs (Joint Corporate Broker to AstraZeneca) | +44 (0) 20 7774 1000 |
| Phil Raper | |
| | |
MedImmune | |
Media Enquiries: | |
| Lori Weiman | 240-372-4829 |
| Jamie Lacey | 301-398-4035 |
Analyst/Investor Enquiries: | |
| Pete Vozzo | 301-398-4358 |
AstraZeneca will be holding an analyst presentation by webcast and teleconference as follows:
Presentation
The presentation will be available 15 minutes prior to the start of the analysts’ teleconference/webcast.
Audio webcast
The webcast will start at 11:30 BST.
Teleconference details
11:30 BST, 12:30 CEST, 06:30 EDT
There will be an interactive Q&A session
UK freephone | 0800 559 3272 |
US freephone | +1 886 239 0753 |
Sweden freephone | 0200 887 737 |
International | +44 (0)207 138 0815 |
Journalists are invited to listen only on | +44 (0)207 138 0810 |
A replay facility will be available from 15.30 BST on 23rd April 2007
UK freephone | 0800 559 3271 |
US freephone | +1 866 239 0765 |
Sweden freephone | 0200 887 740 |
International | +44 (0)207 806 1970 |
Replay passcode | 1880494# |
Not for release, publication or distribution, in whole or in part, in, into or from Australia, Canada or Japan
AstraZeneca acquisition of
MedImmune and 1Q results
Analyst call
| | |
Delivers biologics ambition faster
o Creates leading, fully-integrated biologics & vaccines business
o Critical mass in discovery, development, regulatory, manufacturing
and sales
o Natural fit with CAT
o Significantly expands pipeline
o Improves long term product flow
o Diversifies and expands R&D (small molecules, biologics,
vaccines)
o Enhances future growth prospects
o Profitable, high quality business entering growth phase
o Cash earnings enhancement in 2009
Accelerates delivery of AZ biologics strategy at lower execution risk
Transforms science base
2
| | |
The Deal
o Fully recommended all cash offer of $58 per share;
total enterprise value of $15.2bn
o Transaction has been unanimously recommended by the
boards of both companies.
o Expected to close in June 2007
3
| | |
MedImmune
o One of the World's Leading Biologics Players
o Top 10 biopharmaceutical company by equity value
o A leader in protein engineering, biologics development &
manufacturing
o 2006 revenues of $1.3bn
o 3 marketed products, including 'blockbuster'; Synagis
o Development pipeline adds 45 projects
MedImmune is a scarce asset - fully integrated biologics capability,
with world-class development and manufacturing,
profitable and entering growth phase
4
| | |
Key strengths
o Discovery 'Search'
o Target identification and access
o Highly externally focused
o Well 'networked' internal venture fund (MedImmune Ventures)
o Extensive and effective partnering machine
o Development
o Antibody generation - "create, engineer, refine"
o Vaccines - proprietary live attenuated vaccine platform
o Clinical - innovative, paediatrics
o Regulatory - significant FDA experience (Biologic License
Application)
5
| | |
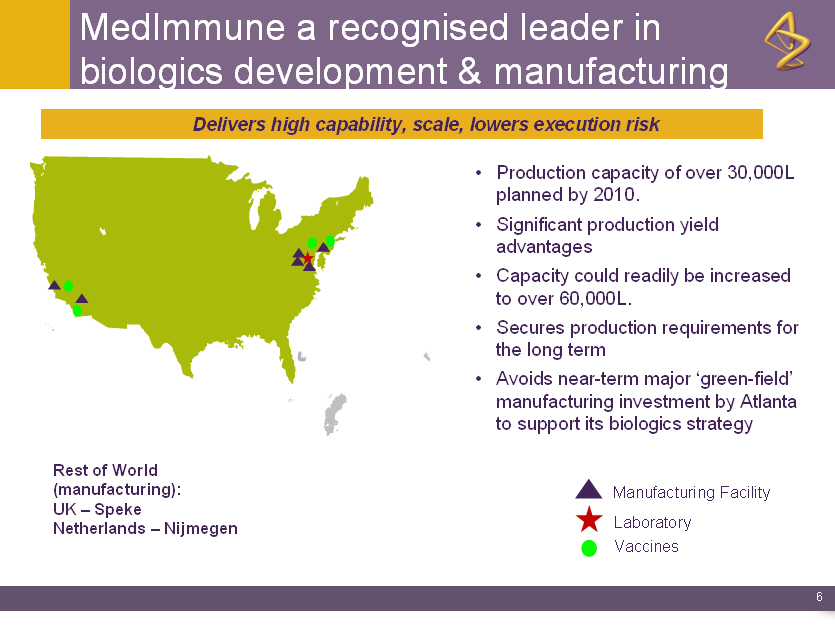
MedImmune a recognised leader in
biologics development & manufacturing
Delivers high capability, scale, lowers execution risk
o Production capacity of over 30,000L
planned by 2010.
o Significant production yield
advantages
o Capacity could readily be increased
to over 60,000L.
o Secures production requirements for
the long term
o Avoids near-term major `green-field'
manufacturing investment by Atlanta
to support its biologics strategy
Rest of World Manufacturing Facility
(manufacturing): Laboratory
UK - Speke Vaccines
Netherlands - Nijmegen
6
| | |
R&D capability
o Expands and diversifies AstraZeneca/CAT technology base
o small molecules,
o monoclonal antibodies & next generation biologics
o provides entry into vaccines
o Externalisation/ partnering
o Strengthened product pipeline
o particularly in AstraZeneca's core areas of Oncology, Infection
and Respiratory & Inflammation
7
| | |
MedImmune brings a significant pipeline
o 45 projects in R&D
o 7 antibody and 5 vaccine projects in clinical development
o RSV next generation follow-on, Numax (2H 07 filing)
o Superior potency giving possible wider utility and refreshed
patent estate
o Refrigerated FluMist (CAIV-T) with anticipated US launch for 2007-
2008 influenza season
o Extended paediatric label under review
Significant boost to the AstraZeneca pipeline, with vaccines, oncology and
inflammation-targeting biologics
8
| | |
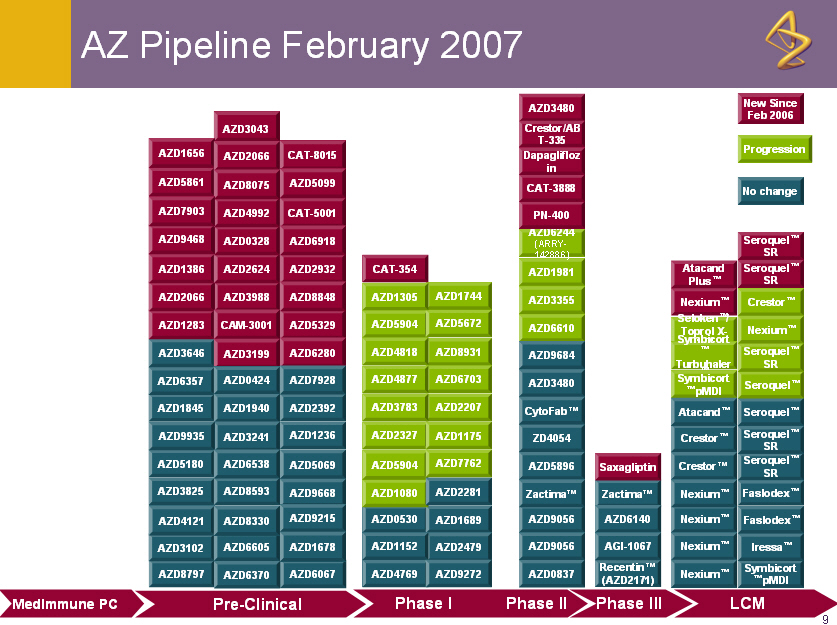
AZ Pipeline February 2007
AZD0530
AZD1845
AZD2327
AZD1689
AZD1080
AZD5896
AZD5904
AZD4769
AZD9056
Zactima(TM)
AZD6140
Recentin(TM)
(AZD2171)
AZD5672
AZD1981
AZD3355
AZD2479
AZD6610
AZD6244
(ARRY-
142886)
AZD1152
AZD5904
AZD0837
AZD3480
ZD4054
AZD9056
Zactima(TM)
Nexium(TM)
AZD2281
AZD3480
CytoFab(TM)
AGI-1067
Atacand
Plus(TM)
AZD8797 AZD6067 AZD6370
AZD3102
AZD6357
AZD2392
AZD1744
AZD3825
AZD8931
AZD6703
AZD4121
AZD1175
AZD2207
AZD1305
AZD3783 AZD1940
AZD1236
AZD4818
AZD5069
AZD9668
AZD9215
AZD1678 AZD6605
AZD5180
AZD8330
AZD3241
AZD4877
AZD7762
AZD3646
AZD9935
AZD1283
AZD3043
AZD5861
AZD5329
Crestor/AB
T-335
AZD1656
AZD3988
AZD2066
AZD2066
AZD6280
PN-400
Seroquel(TM)
SR
AZD1386 AZD2624
AZD0328 AZD9468
AZD2932
AZD4992
CAT-3888
CAT-8015
CAT-5001
AZD5099
AZD6918
AZD8848
AZD8075
CAT-354
CAM-3001
Seroquel(TM)
SR
AZD8593
Saxagliptin
Dapaglifloz
in
AZD9272
New Since
Feb 2006
No change
AZD3199
AZD7903
Progression
AZD0424
AZD9684
AZD6538
AZD7928
Nexium(TM)
Seloken(TM)/
Toprol XL
(TM)
Crestor(TM)
Crestor(TM)
Crestor(TM)
Nexium(TM)
Nexium(TM)
Seroquel(TM)
Symbicort
(TM) pMDI
Symbicort
(TM)
Turbuhaler (TM)
Symbicort
(TM) pMDI
Iressa(TM)
Seroquel(TM)
Seroquel(TM)
SR
Atacand(TM)
Nexium(TM)
Seroquel(TM)
SR
Seroquel(TM)
SR
Nexium(TM)
Faslodex(TM)
Faslodex(TM)
Pre-Clinical Phase I Phase II LCM Phase III MedImmune PC
9
| | |
AZD0530
AZD1845
AZD2327
AZD1080
AZD5896
AZD5904
AZD4769
AZD9056 Zactima(TM)
Recentin(TM)
(AZD2171)
AZD5672
AZD1981
AZD3355
AZD6610
AZD6244
(ARRY-
142886)
AZD1152
AZD5904
AZD0837
AZD3480
ZD4054
AZD9056
Zactima(TM)
Nexium(TM)
AZ Pipeline incorporating MedImmune
AZD2281
AZD3480
CytoFab(TM)
AZD6140
Atacand
Plus(TM)
AZD8797 AZD6067 AZD6370
AZD3102
AZD6357
AZD2392
AZD1744 AZD3825
AZD8931
AZD6703
AZD4121
AZD1175
AZD2207
AZD1305
AZD3783 AZD1940
AZD1236
AZD4818
AZD5069
AZD9668
AZD9215
AZD1678 AZD6605
AZD5180
AZD8330
AZD3241
AZD4877
AZD7762
AZD3646
AZD9935
AZD1283
AZD3043
AZD5861
AZD5329
Crestor/AB
T-335
AZD1656
AZD3988
AZD2066
AZD2066
AZD6280
PN-400
Seroquel(TM)
SR
AZD1386 AZD2624
AZD0328 AZD9468
AZD2932
AZD4992
CAT-3888
CAT-8015
CAT-5001
AZD5099
AZD6918
AZD8848
AZD8075
CAT-354
CAM-3001
Seroquel(TM)
SR
AZD8593 Saxagliptin
Dapaglifloz
in
AZD9272
New Since
Feb 2006
No change
AZD3199
AZD7903
Progression
AZD0424
AZD6538
AZD7928
Nexium(TM)
Seloken(TM)/
Toprol XL
(TM)
Crestor(TM)
Crestor(TM)
Crestor(TM)
Nexium(TM)
Nexium(TM)
Seroquel(TM)
Symbicort
(TM) pMDI
Symbicort
(TM)
Turbuhaler (TM)
Symbicort
(TM) pMDI
Iressa(TM)
Seroquel(TM)
Seroquel(TM)
SR
Atacand(TM)
Nexium(TM)
Seroquel(TM)
SR
Seroquel(TM)
SR
Nexium(TM)
Faslodex(TM)
Faslodex(TM)
AZ Pre-Clinical Phase I Phase II LCM Phase III MedImmune PC
Numax
Anti-IL9
MAb
Anti-IFNa
MAb
Anti-IL5R
MAb
Anti-
HMGB-1
MAb
Anti-CD22
MAb
Anti-CD20
MAb
Anti-CD19
MAb
Anti-IFNaR
MAb
Siplizumab
Anti-CD19
BiTE
Hsp 90
inhibitor
Anti-cMet
Avimers
Anti-ALK
MAb
Anti-CD22
MAb
Anti-CD20
MAb
Anti-CD19
MAb
Anti-EphA4
MAb
Anti-Ephrin
B2 MAb
Anti-EphB4
MAb
Anti-
EphA2
BiTE
Hedgehog
inhibitor
Anti-EphA2
conjugate
ZD4054
EBV
vaccine
Pneumoco
ccal vac
PIV-3
vaccine
RSV /PIV-
3 vaccine
hMPV/
PIV-3 vac
H5N1
vaccine
3rd gen
RSV MAb
Anti-RSV
drug
hMPV
vaccine
Anti-staph
HP MAb
FluMist
(CAIV-T)
Medimmune
Med
Partnered
Synagis
Anti-
Chitinase
MAb
AZD2836
hMPV MAb
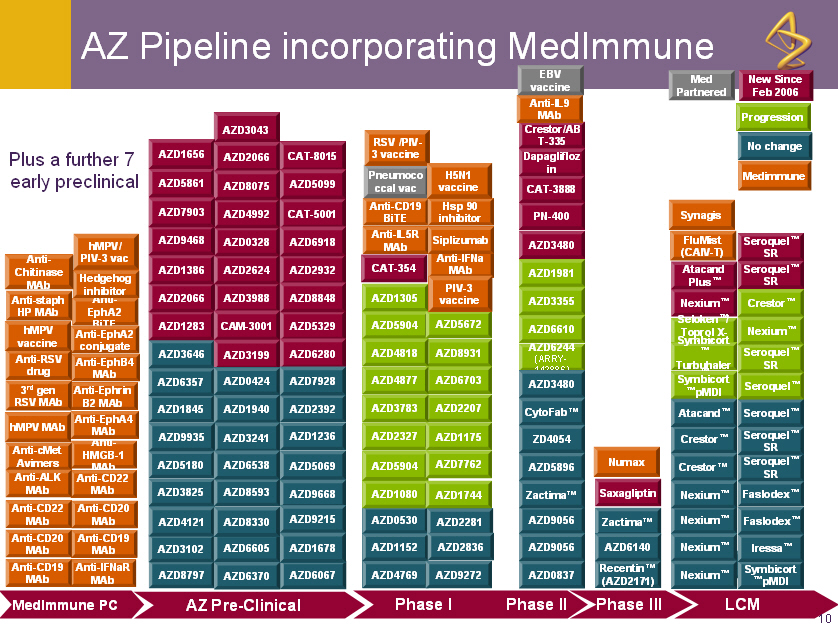
Plus a further 7
early preclinical
10
| | |
AZD0530
AZD1845
AZD2327
AZD2836
AZD1080
AZD5896
AZD5904
AZD4769
AZD9056 Zactima(TM)
AZD6140
Recentin(TM)
(AZD2171)
AZD5672
AZD1981
AZD3355
AZD6610
AZD6244
(ARRY-
142886)
AZD1152
AZD5904
AZD0837
AZD3480
ZD4054
AZD9056
Zactima(TM)
Nexium(TM)
AZ Pipeline incorporating MedImmune
AZD2281
AZD3480
CytoFab(TM)
Atacand
Plus(TM)
AZD8797 AZD6067 AZD6370
AZD3102
AZD6357
AZD2392
AZD1744
AZD3825
AZD8931
AZD6703
AZD4121
AZD1175
AZD2207
AZD1305
AZD3783 AZD1940
AZD1236
AZD4818
AZD5069
AZD9668
AZD9215
AZD1678 AZD6605
AZD5180
AZD8330
AZD3241
AZD4877
AZD7762
AZD3646
AZD9935
AZD1283
AZD3043
AZD5861
AZD5329
Crestor/AB
T-335
AZD1656
AZD3988
AZD2066
AZD2066
AZD6280
PN-400
Seroquel(TM)
SR
AZD1386 AZD2624
AZD0328 AZD9468
AZD2932
AZD4992
CAT-3888
CAT-8015
CAT-5001
AZD5099
AZD6918
AZD8848
AZD8075
CAT-354
CAM-3001
Seroquel(TM)
SR
AZD8593 Saxagliptin
Dapaglifloz
in
AZD9272
Biologicals
AZD3199
AZD7903
Small
Molecules
AZD0424
AZD6538
AZD7928
Nexium(TM)
Seloken(TM)/
Toprol XL
(TM)
Crestor(TM)
Crestor(TM)
Crestor(TM)
Nexium(TM)
Nexium(TM)
Seroquel(TM)
Symbicort
(TM) pMDI
Symbicort
(TM)
Turbuhaler (TM)
Symbicort
(TM) pMDI
Iressa(TM)
Seroquel(TM)
Seroquel(TM)
SR
Atacand(TM)
Nexium(TM)
Seroquel(TM)
SR
Seroquel(TM)
SR
Nexium(TM)
Faslodex(TM)
Faslodex(TM)
AZ Pre-Clinical Phase I Phase II LCM Phase III MedImmune PC
Numax
Anti-IL9
MAb
Anti-IFNa
MAb
Anti-IL5R
MAb
Anti-
HMGB-1
MAb
Anti-CD22
MAb
Anti-CD20
MAb
Anti-CD19
MAb
Anti-IFNaR
MAb
Siplizumab
Anti-CD19
BiTE
Hsp 90
inhibitor
Anti-cMet
Avimers
Anti-ALK
MAb
Anti-CD22
MAb
Anti-CD20
MAb
Anti-CD19
MAb
Anti-EphA4
MAb
Anti-Ephrin
B2 MAb
Anti-EphB4
MAb
Anti-
EphA2
BiTE
Hedgehog
inhibitor
Anti-EphA2
conjugate
ZD4054
EBV
vaccine
Pneumoco
ccal vac
PIV-3
vaccine
RSV /PIV-
3 vaccine
hMPV/ PIV-
3 vac
H5N1
vaccine
3rd gen
RSV MAb
Anti-RSV
drug
hMPV MAb
hMPV
vaccine
Anti-staph
HP MAb
FluMist
(CAIV-T)
Synagis
Anti-
Chitinase
MAb
Plus a further 7
early preclinical
11
| | |
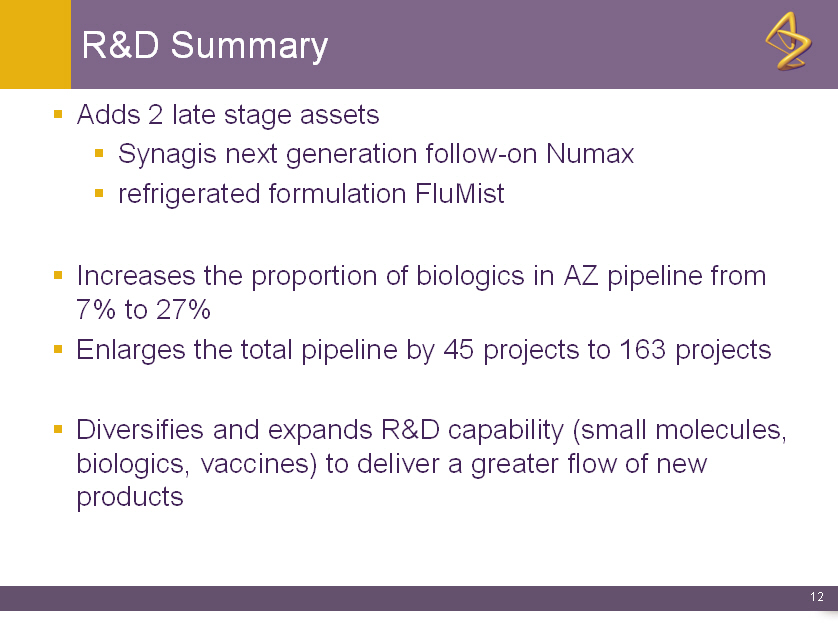
R&D Summary
o Adds 2 late stage assets
o Synagis next generation follow-on Numax
o refrigerated formulation FluMist
o Increases the proportion of biologics in AZ pipeline from
7% to 27%
o Enlarges the total pipeline by 45 projects to 163 projects
o Diversifies and expands R&D capability (small molecules,
biologics, vaccines) to deliver a greater flow of new
products
12
| | |
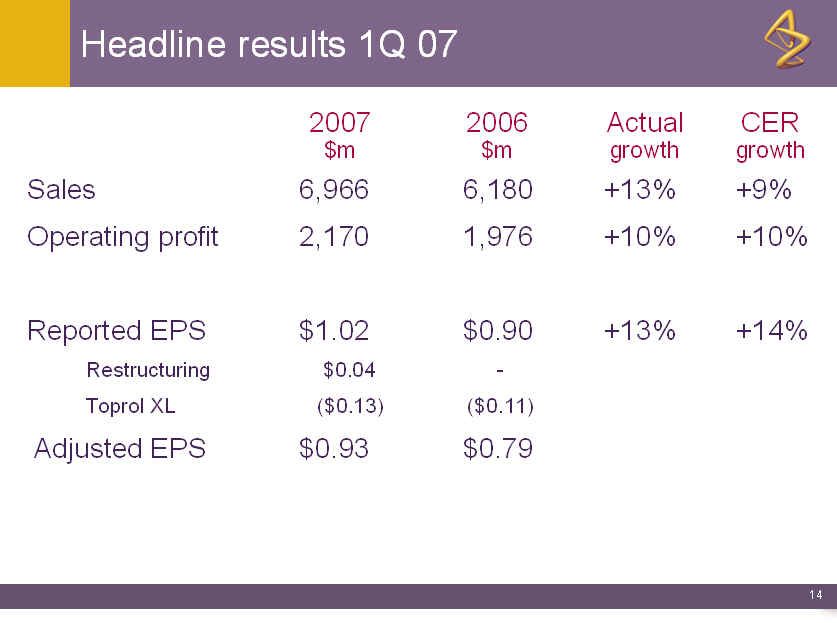
Headline results 1Q 07
2007 2006 Actual CER
$m $m growth growth
Sales 6,966 6,180 +13% +9%
Operating profit 2,170 1,976 +10% +10%
Reported EPS $1.02 $0.90 +13% +14%
Restructuring $0.04 -
Toprol XL ($0.13) ($0.11)
Adjusted EPS $0.93 $0.79
14
| | |
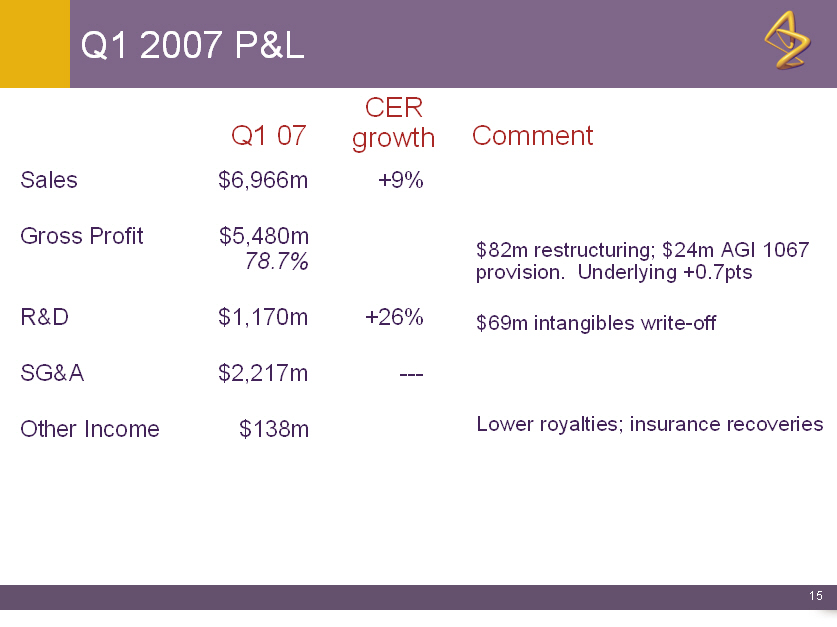
Q1 2007 P&L
CER
Q1 07 growth Comment
Sales $6,966m +9%
Gross Profit $5,480m
78.7% $82m restructuring; $24m AGI 1067
provision. Underlying +0.7pts
R&D $1,170m +26% $69m intangibles write-off
SG&A $2,217m ---
Other Income $138m Lower royalties; insurance recoveries
15
| | |
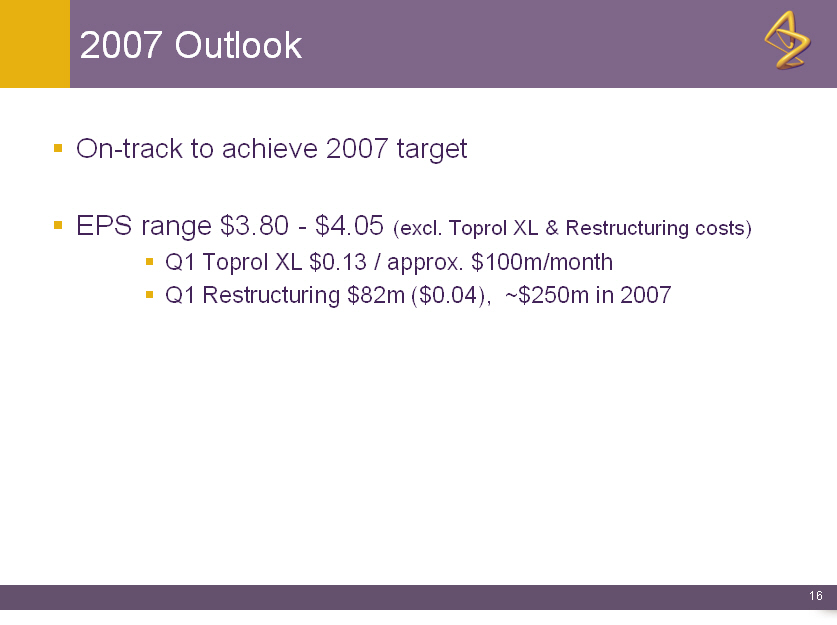
2007 Outlook
o On-track to achieve 2007 target
o EPS range $3.80 - $4.05 (excl. Toprol XL & Restructuring costs)
o Q1 Toprol XL $0.13 / approx. $100m/month
o Q1 Restructuring $82m ($0.04), ~$250m in 2007
16
| | |
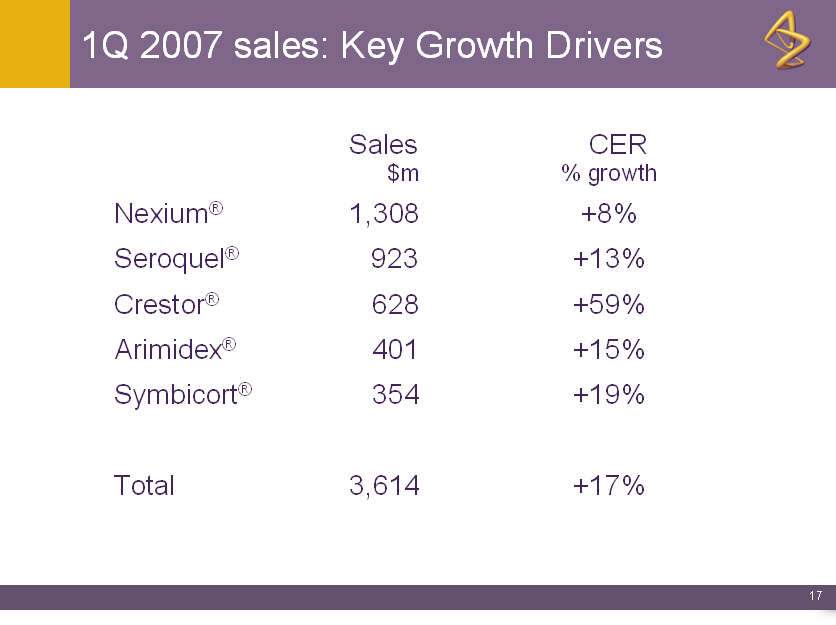
1Q 2007 sales: Key Growth Drivers
Sales CER
$m % growth
Nexium(R) 1,308 +8%
Seroquel(R) 923 +13%
Crestor(R) 628 +59%
Arimidex(R) 401 +15%
Symbicort(R) 354 +19%
Total 3,614 +17%
17
| | |
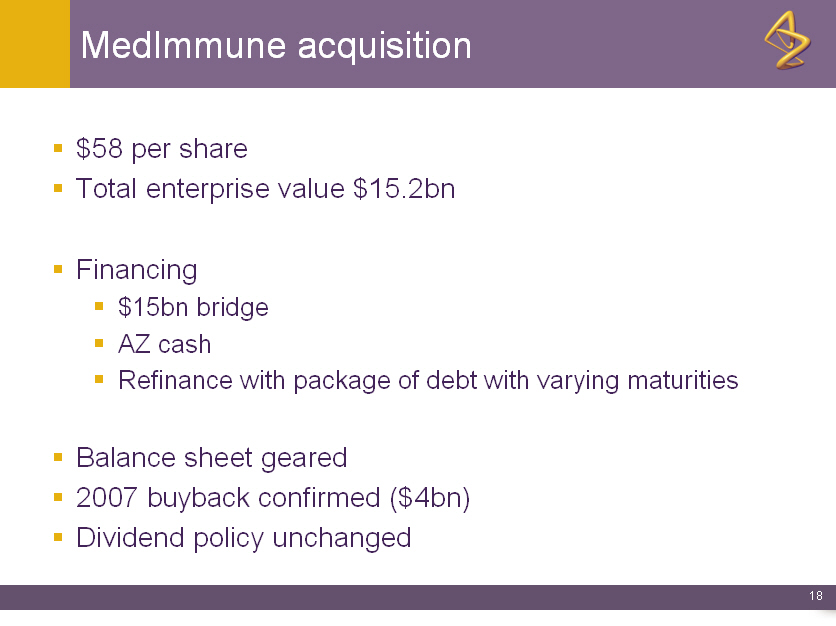
MedImmune acquisition
o $58 per share
o Total enterprise value $15.2bn
o Financing
o $15bn bridge
o AZ cash
o Refinance with package of debt with varying maturities
o Balance sheet geared
o 2007 buyback confirmed ($4bn)
o Dividend policy unchanged
18
| | |
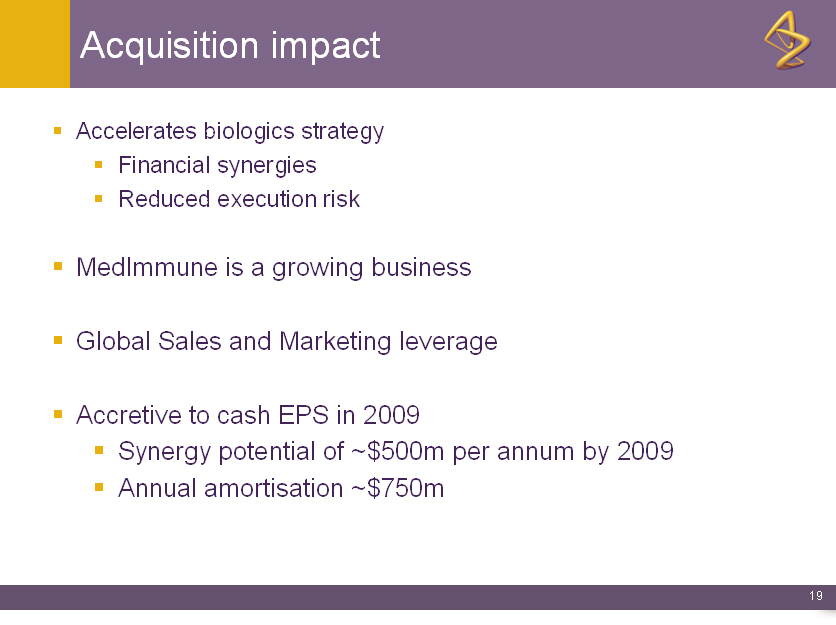
Acquisition impact
o Accelerates biologics strategy
o Financial synergies
o Reduced execution risk
o MedImmune is a growing business
o Global Sales and Marketing leverage
o Accretive to cash EPS in 2009
o Synergy potential of ~$500m per annum by 2009
o Annual amortisation ~$750m
19
| | |
People
o Strong desire to retain employees and maintain culture, with
emphasis on retaining key talent and critical skills
o One-time retention grant for employees
o Significantly increases AstraZeneca's skills and capabilities in
development and manufacture of biologics & vaccines
o Strong external collaboration/partnering skills
o David M Mott to take leadership role in AstraZeneca
o James F Young committed to remain in current role
High quality people; who combine strong science skills with
strong business skills
20
| | |
Delivers biologics ambition faster
o Creates leading, fully-integrated biologics
and vaccines business
o Significantly expands pipeline
o Enhances future growth prospects
Accelerates delivery of AZ biologics strategy at lower execution risk
Transforms science base
21
| | |
Additional Information
PRESENTATION DISCLAIMER
o The tender offer described in this presentation has not yet commenced, and
this presentation is neither an offer to purchase nor a solicitation of an
offer to sell MedImmune common stock. Investors and security holders are
urged to read both the tender offer statement and the
solicitation/recommendation statement regarding the tender offer described
in this presentation when they become available because they will contain
important information. The tender offer statement will be filed by
AstraZeneca and a subsidiary of AstraZeneca with the Securities and
Exchange Commission (SEC), and the solicitation/recommendation statement
will be filed by MedImmune with the SEC. Investors and security holders may
obtain a free copy of these statements (when available) and other documents
filed by AstraZeneca or MedImmune with the SEC at the website maintained by
the SEC at www.sec.gov. The tender offer statement and related materials
may be obtained for free by directing such requests to AstraZeneca
(Investor Relations) at +44 (0) 207 304 5000. The
solicitation/recommendation statement and such other documents may be
obtained by directing such requests to MedImmune (Investor Relations) at
301 398 4358.
o Copies of this presentation and any documentation relating to the Offer are
not being, and must not be, directly or indirectly, mailed or otherwise
forwarded, distributed or sent in or into or from Canada, Japan or
Australia or any other jurisdiction where to do so would be unlawful.
o This presentation may contain certain forward-looking statements about
AstraZeneca and MedImmune which are subject to risks and uncertainties and
may be influenced by factors that could cause actual outcomes and results
to be materially different from those predicted. We have identified the
forward-looking statements by using the words `anticipates', `believes',
`expects', `intends' and similar expressions in such statements. Important
factors that could cause actual results to differ materially from those
contained in forwardlooking statements, certain of which are beyond our
control, include, among other things: difficulties in integrating MedImmune
leading to greater costs and lower integration benefits than we anticipate;
regulatory conditions being imposed on the acquisition of MedImmune; patent
litigation and early loss or expiration of patents, marketing exclusivity
or trade marks; substantial adverse outcomes of litigation and government
litigation; exchange rate fluctuations; the risk that R&D will not yield
new products that achieve commercial success; the impact of competition,
price controls and price reductions; taxation risks; the risk of
substantial product liability claims; the impact of any failure by third
parties to supply materials or services; the risk of delay to new product
launches; the difficulties of obtaining and maintaining governmental
approvals for products; the risk of failure to observe ongoing regulatory
oversight; the risk that new products do not perform as we expect; and the
risk of environmental liabilities. For further discussion of factors that
may cause actual results to differ from expectations, you should read
AstraZeneca's most recent UK Annual Report and MedImmune's most recent
regulatory filings and submissions, including the Annual Reports on Form
20-F and Form 10-K of AstraZeneca and MedImmune, respectively. You are
cautioned not to place undue reliance on any forward-looking statements,
which speak only as of the date of this presentation. Except as required by
applicable law or regulation, AstraZeneca and MedImmune do not undertake
any obligation to publicly release any update or revisions to these
forward-looking statements.
22
| | |