Advancing medicines. Solving problems. Improving lives. ® Disclaimer This presentation contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995 which are based on the beliefs and assumptions and on information currently available to the management of Aquestive Therapeutics, Inc. (the “Company”, “we” or “our”). Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. All statements other than statements of historical fact contained in this presentation are forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement and related timing of Anaphylm (trade name for AQST-109 epinephrine sublingual film product candidate) through the regulatory and development pipeline; statements regarding the clinical trial timing and plans for Anaphylm, including the ability to address the concerns of the United States Food And Drug Administration (FDA) provided in the End-of-Phase 2 (EOP2) meeting with the FDA; statements regarding the ability to receive FDA approval of Libervant™ for U.S. market access and overcome the orphan drug market exclusivity of a competing FDA approved nasal spray product extending to January 2027; statements regarding potential outlicensing of our product pipeline in the U.S. and abroad, including with respect to Anaphylm and Libervant; statements regarding our estimated financial position for the first quarter 2023 and financial outlook for 2023; statements regarding the Company’s estimated forecasts to pay down its current debt; and business strategies, market opportunities and other statements that are not historical facts. These forward-looking statements are also subject to the uncertain impact of the COVID-19 global pandemic on our business including with respect to our clinical trials including site initiation, patient enrollment and timing and adequacy of clinical trials; on regulatory submissions and regulatory reviews and approvals of our product candidates; pharmaceutical ingredients and other raw materials supply chain, manufacture, distribution; and sale of, and demand for, our products; our liquidity and availability of capital resources, customer demand for our products and services; customers' ability to pay for goods and services; and ongoing availability of an appropriate labor force and skilled professionals. Given these uncertainties, we are unable to provide assurance that operations can be maintained as planned prior to the COVID-19 pandemic. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the Company’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These risks, uncertainties and other factors include, but are not limited to, risks associated with the Company’s development work, including any delays or changes to the timing, cost and success of the Company’s product development activities and clinical trials for Anaphylm; risk of the Company’s failure to generate sufficient data in support of its New Drug Application (NDA) submission for FDA approval of Anaphylm; risk of the Company’s failure to address the concerns identified in the FDA EOP2 meeting for Anaphylm; risk of delays in or the failure to receive FDA approval of Anaphylm; risk of the failure to receive FDA approval for U.S. market access for Libervant; risk of our ability to out-license our proprietary products in the U.S. or abroad and risks that such product candidates will receive regulatory approval in those licensed territories; risk to growing our manufacturing revenues and generate cash and capabilities to support demand for current and future licensed products; risk of eroding market share for Suboxone® and risk of a sunsetting product, which accounts for the substantial part of our current operating revenue; risk regarding the Company’s future financial and operating results and financial position; risk of insufficient capital and cash resources, including insufficient access to available debt and equity financing and revenues from operations, to satisfy all of the Company’s short-term and longer term liquidity and cash requirements and other cash needs, at the times and in the amounts needed; risk of failure to satisfy all financial and other debt covenants and of any default; uncertainties related to general economic, political, business, industry, regulatory, financial and market conditions and other unusual items; and other risks and uncertainties affecting the Company described under “Risk Factors” in the Company’s annual report on Form 10-K for the year ended December 31, 2022, quarterly reports on Form 10-Q, current reports on Form 8-K and our other filings with the Securities and Exchange Commission. Forward-looking statements represent the Company’s beliefs and assumptions only as of the date of this presentation. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, the Company assumes no obligation to publicly update any forward-looking statements for any reason after the date of this presentation, or to conform any of the forward-looking statements to actual results or to changes in its expectations. Financial information contained in this presentation relating to the three months ended March 31, 2023 are preliminary and unaudited and remain subject to change. As such, the Company’s independent auditors have not audited, studied, reviewed or performed any procedures with respect to such preliminary information and, accordingly, they did not express an opinion or provide any other form of assurance with respect thereto for the purpose of this presentation. Our financial closing procedures for the three months ended March 31, 2023 have not been completed, and as such there can be no assurance that such preliminary results are indicative of the future performance of the Company and actual results may differ materially. PharmFilm® and the Aquestive logo are registered trademarks of Aquestive Therapeutics, Inc. The trade name for AQST-109 “Anaphylm” has been conditionally approved by the FDA. Final approval of the Anaphylm™ proprietary name is conditioned on FDA approval of the product candidate, AQST-109. All other registered trademarks referenced herein are the property of their respective owners. © 2023 Property of Aquestive Therapeutics, Inc. 2

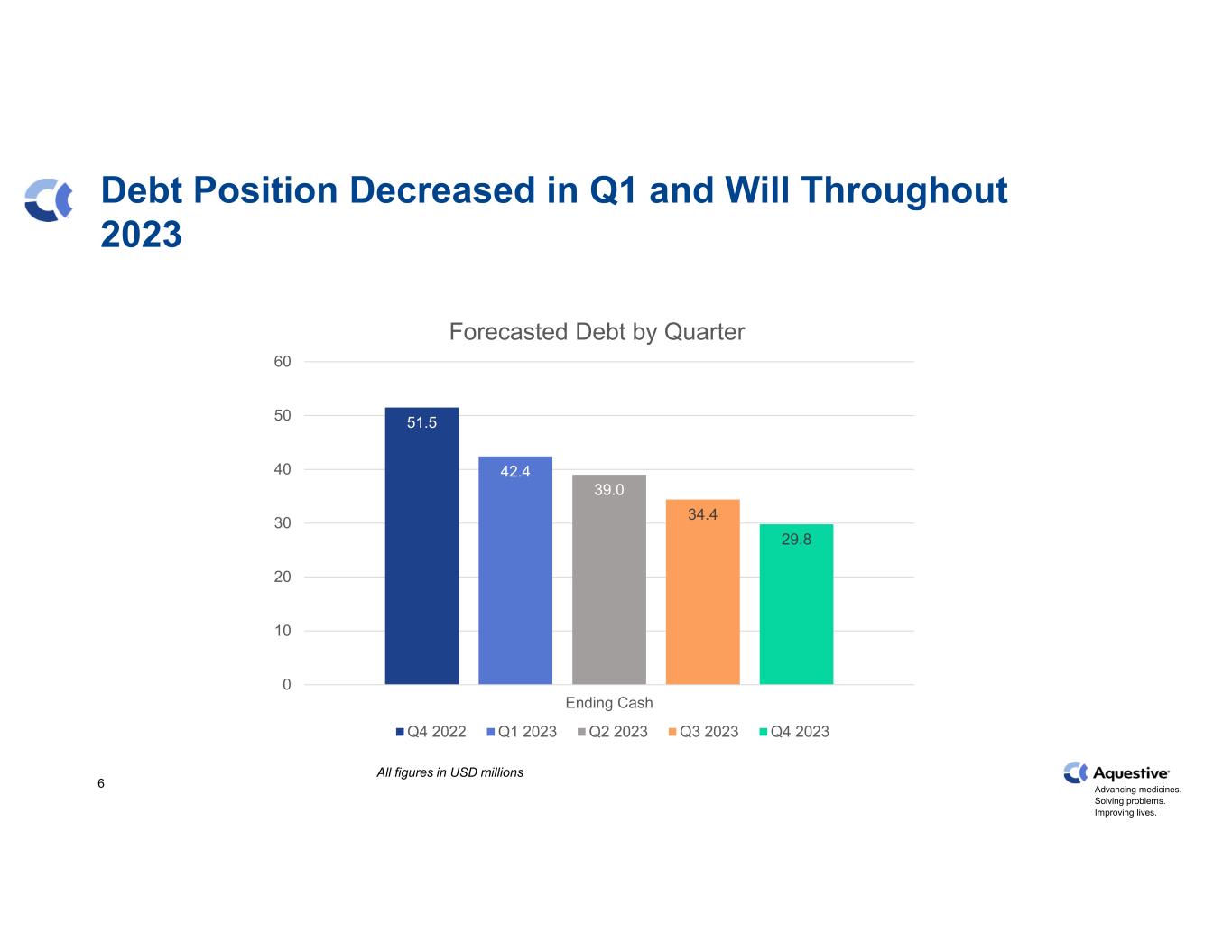
Advancing medicines. Solving problems. Improving lives. Q1 2023 Earnings: Key Messages AnaphylmTM (AQST-109 - Epinephrine Sublingual Film) Proprietary trade name Anaphylm conditionally approved by the FDA Completed additional studies to identify the appropriate autoinjector RLDs Continuing to work on the optimal administration parameters of Anaphylm Expects to submit the pivotal trial protocol to the FDA for review following alignment with the FDA Actively pursuing ex-US licensing opportunities for Anaphylm Financial Performance Generated net income of $8.1 million in Q1 2023 Generated $47 million of non-dilutive capital in the last twelve months Ended Q1 2023 with $26.9 million in cash and cash equivalents, in line with Q4 2022 Reduced debt by 18%, or $9.1 million, to $42.4 million LIBERVANTTM (diazepam) buccal film Continued engagement with the FDA on U.S. market entry before 2027 Expanded the ex-U.S. licensing agreement with Pharmanovia to include all countries except the U.S., Canada, and China Continuing to pursue out-licensing agreements for the US, Canada, and China 3

Advancing medicines. Solving problems. Improving lives. Manufacturing Operations Continues to Meet Expectations and Generate Cash Flow 8 30,916 49,182 47,505 36,413 42,182 47,041 41,097 36,863 33,458 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 PHARMFILM® VOLUME (MILLIONS)