Exhibit 99.2
MANAGEMENT’S DISCUSSION AND ANALYSIS
The following information should be read in conjunction with the Company’s audited consolidated financial statements for the year ended June 30, 2007 and the related notes, which are prepared in accordance with Canadian generally accepted accounting principles. This Management’s Discussion and Analysis (“MD&A”) provides a review of the performance of the Company for the year ended June 30, 2007 as compared to the revised year ended June 30, 2006. Material differences between Canadian and U.S generally accepted accounting principles are described in note 26 to the financial statements for the year ended June 30, 2007. This MD&A includes financial information derived from the annual audited consolidated financial statements and from the unaudited interim consolidated financial statements. This review was performed by management with information available as of September 12, 2007.
Where “we”, “us”, “our”, “Transition” or the “Company” is used, it is referring to Transition Therapeutics Inc. and its wholly-owned subsidiaries, unless otherwise indicated. All amounts are in Canadian dollars, unless otherwise indicated.
In July 2007, the Company completed the consolidation of its issued and outstanding common shares on the basis of one (1) post-consolidation common share for every nine (9) pre-consolidation common shares. As a result of this consolidation, the number of common shares, warrants and options, related exercise prices and basic and diluted loss per common share have been retroactively adjusted to reflect the consolidation. Unless otherwise indicated all share prices have been multiplied by a factor of 9 and all common shares outstanding have been divided by a factor of 9 to give effect to the share consolidation.
FORWARD-LOOKING STATEMENTS
To the extent any statements made in this MD&A contain information that is not historical, these statements are forward-looking statements. Forward-looking statements are identified by words such as “expect”, “believe”, “intend”, “anticipate”, “will”, “may”, or other similar expressions. These forward-looking statements by their nature are not guarantees of the Company’s future performance and involve risks and uncertainties that could cause the actual results to differ materially from those discussed in, or implied by, these forward-looking statements. The Company considers the assumptions on which these forward-looking statements are based to be reasonable at the time this MD&A was prepared, but cautions the reader that these assumptions may ultimately prove to be incorrect due to certain risks and uncertainties including, but not limited to, the difficulty of predicting regulatory approvals, acceptance and demand for new pharmaceutical products, the impact of competitive products and pricing, the Company’s ability to finance, manufacture and commercialize its products, the protection of intellectual property and any other similar or related risks and uncertainties. The Company disclaims
1
any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. Given these uncertainties, the reader should not place undue reliance on these forward-looking statements.
OVERVIEW
Transition is a product-focused biopharmaceutical company, developing novel therapeutics for disease indications with large markets. The Company has three lead products: ELND-005/AZD-103 for the treatment of Alzheimer’s disease, GLP1-I.N.T.Ô and E1-I.N.T.Ô for the treatment of diabetes. Transition also has an emerging pipeline of pre-clinical drug candidates developed using its proprietary drug discovery engine.
General Risk Factors for the Biotechnology Industry
Prospects for companies in the biopharmaceutical industry generally may be regarded as uncertain given the nature of the industry and, accordingly, investments in such companies should be regarded as highly speculative. It is not possible to predict, based upon studies in animals and early clinical data, whether a new therapeutic or device will prove to be safe and effective in humans or whether it will ultimately receive regulatory approval. In addition, there is also no assurance that adequate funds or relationships required to continue product development such as those with employees, collaborators, or other third parties will be available and sustained.
If a product is ultimately approved for sale, there is also no assurance that it will ever result in significant revenues or profitable operations. There are many factors such as competition, patent protection and the regulatory environment that can influence a product’s profitability potential.
In addition, due to the speculative nature of this industry, market prices for securities of biotechnology companies may be highly volatile and subject to significant fluctuation and may not necessarily be related to the operating or other performances of such companies.
Recent Achievements
During fiscal 2007 and up to the date of this MD&A, the Company achieved the following significant milestones:
ELND-005/AZD-103 — Alzheimer’s Disease:
| • | | Clinical Data Results: On August 30, 2007, the Company Announced Completion of Multiple Phase I Clinical Studies with Alzheimer’s Disease Drug Candidate ELND-005/AZD-103. ELND-005/AZD-103 was safe and well-tolerated at all doses and dosing regimens examined.There were no severe or serious adverse events observed. ELND-005/AZD-103 was also shown to be orally bio-available, cross the |
2
| | | blood-brain barrier and achieve levels in the human brain and CSF that were shown to be effective in animal models for Alzheimer’s disease; |
| |
| • | | FDA Granted Fast Track Designation for Alzheimer’s Disease Drug Candidate ELND-005/AZD-103which is being developed in collaboration with Elan Pharma International Limited (“Elan”) for the treatment of Alzheimer’s disease; |
| |
| • | | Transition and Elan signed a US$200 million global collaboration agreement to develop and commercialize the Alzheimer’s disease product, ELND-005/AZD-103.Under the terms of the agreement, Transition has received an upfront payment of US$7.5 million and will receive an additional upfront payment of US$7.5 million in calendar 2007. Dependent upon the successful development, regulatory and commercial launch of ELND-005/AZD-103, Transition will be eligible to receive milestone payments of up to US$185 million and will share the costs of development and profits from commercialization. |
I.N.T.Ô — Diabetes:
| • | | Clinical Data Results: |
| |
| | | On June 28, 2007, the Company Announced Final Results from the Exploratory Phase IIa E1-I.N.T.™ Clinical Trial. A 4-Week Therapy with E1-I.N.T.™ Lead to Sustained Reductions in Blood Glucose Levels for 6 Months Post-treatment in Type 2 Diabetes Patients.In the E1-I.N.T.™ treated group of patients, the mean HbA1c level was reduced by 0.94% to 1.21% vs. baseline levels in months 2 to 6 post-treatment. In addition to the HbA1c reductions, the data demonstrated decreases in fasting blood glucose levels as well as improvements in glucose tolerance over a six month period following treatment with E1-I.N.T.™. These clinical improvements, including HbA1c reductions greater than 1% in patients six month post-treatment, highlight the potential that E1-I.N.T.™ therapy could provide patients significant clinical benefit in excess of 6 months; |
| |
| | | On March 5, 2007, Transition Released Positive Interim Data from E1-I.N.T.Ô Clinical Trials in Type 1 and Type 2 Diabetes.Data from the trial in type 2 diabetes patients demonstrated that E1-I.N.T.Ô significantly lowered blood glucose levels for patients using metformin with/without thiazolidinediones (TZD). In the type 1 diabetes study, 6 of 11 (54%) patients responded to E1-I.N.T.Ô therapy, either by decreasing their average daily insulin usage by more than 20% or reducing their HbA1c levels by 1.2 to 2%. There were no responders among the placebo group; |
| |
| • | | Transition received the remaining US$750,000 of the US$1 million relating to the amended I.N.T.™ license agreement between the Company and Novo Nordisk A/S (“Novo Nordisk”) which restated the rights and responsibilities of the parties. Novo Nordisk retains exclusive, worldwide rights to the E1-I.N.T. ™ program and the Company regains exclusive ownership and rights to all other I.N.T.™ programs, including GLP1-I.N.T.™; |
3
| • | | The Company and the Juvenile Diabetes Research Foundation International (“JDRF”), located in the United States, entered into an agreement in which the JDRF will provide milestone driven funding of up to US$4 millionto assist in the expedited development of GLP1-I.N.T.™ over a two year period. |
Corporate Development
| • | | On August 20, 2007 the Company’s common shares began trading on the NASDAQ Capital Market under the symbol “TTHI”.The Company’s common shares will continue to trade on the Toronto Stock Exchange in addition to the NASDAQ; |
| • | | On July 9, 2007 the Company completed a consolidation of its issued and outstanding common shares on the basis of one (1) post-consolidation common share for every nine (9) pre-consolidation common shares.The share consolidation was effected to satisfy the NASDAQ’s listing criteria regarding minimum bid price; |
| • | | On July 11, 2007 the Company completed a private placement financing issuing 1,736,107 common shares at a price of $14.40 per common share, raising gross proceeds of approximately $25,000,000 from a number of funds managed by Oracle Investment Management Inc., The Invus Group LLC, and a large Boston based investment management company.The Company has incurred total share issuance costs to date of $1,023,596, resulting in net cash proceeds of $23,976,404; |
| • | | On November 8, 2006 the Company completed a private placement financing issuing 2,986,867 common shares at a price of $8.37 per common share, raising gross proceeds of $25,000,000 from two funds managed by Great Point Partners, LLC. The Company incurred total share issuance costs of $1,035,249, resulting in net cash proceeds of $23,964,751; |
| • | | Received the second anniversary payment of $400,000from the sale of its subsidiary, Stem Cell Therapeutics Inc (“SCT”); |
| • | | Extinguished the indebtednessassumed related to the November 2005 Protana asset purchase. |
Strategic Acquisition
| • | | On June 1, 2007, the Company completed the acquisition of 100% of the outstanding common shares of NeuroMedix Inc. (“NeuroMedix”),a central nervous system (“CNS”) focused biotechnology company. NeuroMedix’s lead compound, Minozac, has been shown to prevent neuronal dysfunction in animal models of Alzheimer’s disease and traumatic brain injury. |
The Company’s cash, cash equivalents, and short term investments were $34,368,142 at June 30, 2007, and the net working capital position, excluding deferred revenue and advances was $32,624,693. The Company currently believes that it has adequate financial resources for anticipated expenditures until early fiscal 2011.
4
STRATEGIC COLLABORATION
In March 2006, Transition completed the acquisition of Ellipsis Neurotherapeutics Inc. (“ENI”). The key asset in the acquisition was the Alzheimer’s disease compound ELND-005/AZD-103, a disease modifying agent with the potential to both reduce disease progression and improve symptoms including cognitive function.
In September 2006, Transition announced a global collaboration with Elan to develop and commercialize ELND-005/AZD-103. Under the terms of the agreement, Transition has received an upfront payment of US$7.5 million and will receive an additional upfront payment of US$7.5 million in calendar 2007. Dependent upon the successful development, regulatory and commercial launch of ELND-005/AZD-103, Transition will be eligible to receive milestone payments of up to US$185 million. Transition and Elan will share the costs of development and profits from commercialization. Each party’s cost share and ownership interest may vary throughout the term of the Agreement dependant on certain elections that may be made during the development of ELND-005/AZD-103.
The upfront payment received of $8,420,250 (US$7,500,000) from Elan has been recorded as deferred revenue and advances.
STRATEGIC ACQUISITIONS
On May 9, 2007, the Company completed a tender offer for 94% of the outstanding common shares of NeuroMedix, a CNS focused biotechnology company. As the offer was accepted by holders of more than 90% of the common shares of NeuroMedix not held by Transition or its affiliates, the Company exercised its right under the compulsory acquisition provisions of section 206 of the Canada Business Corporations Act and acquired the remaining outstanding common shares of NeuroMedix not owned by Transition. Following the completion of the compulsory acquisition on June 1, 2007, NeuroMedix became a wholly-owned subsidiary of Transition. The NeuroMedix common shares were delisted from the TSX Venture Exchange effective May 15, 2007.
NeuroMedix’s lead compound, Minozac, has been shown to prevent neuronal dysfunction in animal models of Alzheimer’s disease and traumatic brain injury.
As a result of the NeuroMedix acquisition, the Company acquired net assets of $10,180,985 for total share consideration of $9,858,143 and acquisition costs of $322,842. Transition issued a total of 685,951 common shares as consideration for 100% of the NeuroMedix common shares received.
PROGRAMS
Transition is focused on developing innovative therapies in several distinct areas of opportunity. Transition’s vision is to build a company that has a strong foundation for
5
growth based on multiple technologies and product opportunities, which reduces risk and enhances return. The Company’s lead technologies are as follows:
ELND-005/AZD-103 for Alzheimer’s Disease
Alzheimer’s disease is a progressive brain disorder that gradually destroys a person’s memory and ability to learn, reason, make judgments, communicate and carry out daily activities. As Alzheimer’s disease progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. In late stages of the disease, individuals need help with dressing, personal hygiene, eating and other basic functions. People with Alzheimer’s disease die an average of eight years after first experiencing symptoms, but the duration of the disease can vary from three to 20 years.
The disease mainly affects individuals over the age 65 and it is estimated over 18 million people are suffering from Alzheimer’s disease worldwide. The likelihood of developing late-onset Alzheimer’s approximately doubles every five years after age 65. By age 85, the risk reaches nearly 50 percent. In the U.S., Alzheimer’s disease is the fourth leading cause of death and current direct/indirect costs of caring for an estimated 4.5 million Alzheimer’s disease patients are at least US$100 billion annually.
Current FDA approved Alzheimer’s disease medications may temporarily delay memory decline for some individuals, but none of the currently approved drugs is known to stop the underlying degeneration of brain cells. Certain drugs approved to treat other illnesses may sometimes help with the emotional and behavioral symptoms of Alzheimer’s disease. With an aging population, there is a great need for disease-modifying compounds that can slow or reverse disease progression.
In March 2006, the Company announced the acquisition of all the remaining outstanding shares of Alzheimer’s focused ENI that the Company did not already own. The key asset in the acquisition is the Alzheimer’s disease compound ELND-005/AZD-103, a disease modifying agent with the potential to both prevent and reduce disease progression, and improve symptoms such as cognitive function.
In April 2006, the Company received clearance from the Therapeutic Products Directorate of Health Canada to commence a Phase I clinical trial to evaluate the pharmacokinetics, safety and efficacy of escalating doses of ELND-005/AZD-103 in healthy volunteers. The study demonstrated that ELND-005/AZD-103 was well tolerated and no safety concerns or significant adverse events were observed in the study. In August 2006, the Company also received clearance from the FDA to commence a subsequent Phase I clinical trial evaluating higher doses of ELND-005/AZD-103.
In September 2006, Transition announced a global collaboration with Elan to develop and commercialize ELND-005/AZD-103.
6
In April 2007, Transition announced that the FDA granted Fast Track designation to the investigational drug candidate ELND-005/AZD-103 which is being developed in collaboration with Elan. Under the FDA Modernization Act of 1997, Fast Track designation is intended to facilitate the development and expedite the review of a drug or biologic if it is intended for the treatment of a serious or life-threatening condition, and it demonstrates the potential to address unmet medical needs for such a condition.
On August 30, 2007, the Company announced the completion of Phase I Clinical Studies with ELND-005/AZD-103. Transition and its development partner Elan have performed multiple Phase I studies evaluating the safety, tolerability and pharmacokinetic profile of ELND-005/AZD-103 in healthy volunteers. Approximately 110 subjects have been exposed to ELND-005/AZD-103 in multiple Phase I studies, including single and multiple ascending dosing; pharmacokinetic evaluation of levels in the brain; and CSF and plasma studies. ELND-005/AZD-103 was safe and well-tolerated at all doses and dosing regimens examined.There were no severe or serious adverse events observed. ELND-005/AZD-103 was also shown to be orally bio-available, cross the blood-brain barrier and achieve levels in the human brain and CSF that were shown to be effective in animal models for Alzheimer’s disease. The next steps in the development of ELND-005/AZD-103 will be submission of data supporting Phase II studies to the FDA. Transition and Elan anticipate starting Phase II by the end of calendar 2007 or early 2008.
Expenditures for the ELND-005/AZD-103 Program
During the year ended June 30, 2007, the Company incurred direct research and development costs for this program as follows:
| | | | | | | | | |
| ELND-005/AZD-103 | | | | | | |
| Program(1) | | F2007 | | | F2006 | |
| Pre-clinical studies | | $ | 1,051,401 | | | $ | 1,029,876 | |
| Clinical studies | | | 1,327,796 | | | | 0 | |
| Manufacturing | | | 1,371,296 | | | | 376,791 | |
| Other direct research | | | 228,581 | | | | 201,827 | |
| | | | | | | |
| TOTAL | | $ | 3,979,074 | | | $ | 1,608,494 | |
| | | | | | | |
| | |
| (1) | | These costs are direct research and development costs only and do not include patent costs, investment tax credits, salaries and benefits or an allocation of Company overhead. The costs are presented as gross amounts expensed by the Company, prior to the reimbursement of development costs from Elan which have been netted against R&D expense ($1,013,561 for the year ended June 30, 2007). |
I.N.T.TMfor Diabetes
General
Diabetes is a disease in which the body does not produce or properly use insulin. Insulin is a hormone released from islet cells located in the pancreas that is needed to convert sugar, starches and other food into energy needed for daily life. There are two primary forms of diabetes; type 1 diabetes and type 2 diabetes.
7
Type 1 diabetes develops when the body’s immune system destroys pancreatic islet beta cells, the only cells in the body that make the hormone insulin that regulates blood glucose. To survive, people with type 1 diabetes must have insulin delivered by injection or pump. Type 1 diabetes accounts for 5-10% of all diagnosed cases of diabetes.
Type 2 diabetes usually begins as insulin resistance, a disorder in which the cells do not use insulin properly. As the need for insulin increases, the pancreas gradually loses its ability to produce it. Current treatments for type 2 diabetes include lifestyle changes, oral medications, incretin therapy and insulin therapy. Type 2 diabetes accounts for about 90-95% of all diagnosed cases of diabetes.
Transition has developed a patented diabetes therapy, which offers a new paradigm in the treatment of diabetes. Transition’s Islet Neogenesis Therapy is based on the discovery that a short course of naturally occurring peptides can regenerate insulin-producing cells in the body. Transition is currently actively developing two I.N.T.TM products, E1-I.N.T.TM and GLP1-I.N.T.TM. In March and June 2007, the Company released positive data from its E1-I.N.T.Ô exploratory Phase IIa clinical trials in type 1 and type 2 diabetes.
Licensing Agreement
In August 2004, the Company signed a licensing agreement (the “Licensing Agreement”) with Novo Nordisk to develop I.N.T.TM for the treatment of diabetes. Under the terms of the Licensing Agreement, Novo Nordisk received exclusive worldwide rights to the Company’s I.N.T.TM technology except for I.N.T.TM for transplantation. In exchange for this license, Novo Nordisk agreed to make up-front and milestone payments which, assuming all development milestones are achieved, will total US$48 million, an equity investment in the Company of $6 million, commercial milestone payments and royalty payments on future net sales and to also assume all costs for the development of the licensed GLP1-I.N.T.™technology.
On July 17, 2006, the Company and Novo Nordisk amended the Licensing Agreement to restate the rights and responsibilities of the parties. Novo Nordisk retains exclusive, worldwide rights to the E1-I.N.T. ™ program and the Company regains exclusive ownership and rights to all other I.N.T.™ programs, including GLP1-I.N.T.™. Novo Nordisk has in association with the execution of the amendment, paid the Company $552,650 [US$500,000] for the achievement of the first developmental milestone, which has been recognized as milestone revenue in the three-month period ended September 30, 2006. Additionally, the Company has received from Novo Nordisk $570,300 [US$500,000] in research and development funding in calendar 2006, of which the final payment of $279,050 [US$250,000] was received during the three-month period ended September 30, 2006.
The other financial terms of the amended agreement remain the same, where the Company will receive future E1-I.N.T.™ developmental milestone payments potentially
8
totaling US$46 million plus commercial milestones and royalties on sales of E1-I.N.T.™ products.
The Company is currently advancing the clinical development of E1-I.N.T.™ for type 1 and type 2 diabetes. Novo Nordisk is in receipt of the final data from the exploratory Phase IIa clinical trials, and subsequent to their review of the data, Novo Nordisk shall decide whether to finalize development and commercialization of E1-I.N.T.™. Following such a decision the Company will be entitled to additional milestone payments and reimbursement of all E1-I.N.T.™ clinical development costs since August 2004.
To date, under the Licensing Agreement, the Company received $1,968,580 [US$1,500,000] in up-front payments that have been recorded as deferred revenue and are being recorded as licensing fee revenue over the term of the Licensing Agreement, which has been estimated as 15 years. Licensing fee revenue of $131,244 was recognized during fiscal 2007 [fiscal 2006 — $131,244].
In addition, the Company has received $1,191,025 [US$1,000,000] from Novo Nordisk in research and development funding as of June 30, 2007. Under the terms of the initial agreement, $385,671 [US$317,130] was spent on a joint research project in fiscals 2005 and 2006. As a result of the July 17, 2006 amendment to the Agreement, the Company has applied the remaining $805,354 [US$682,870] against patent costs incurred prior to the date of amendment and research and development costs.
E1- I.N.T.TM
Transition’s first Islet Neogenesis Therapy product, E1-I.N.T.TM, a combination of Transition’s epidermal growth factor analogue (“E1”) and gastrin analogue (“G1”), has completed two Phase I clinical trials, in which it was shown that E1-I.N.T.™ is safe to administer. Transition received FDA clearance to initiate exploratory Phase IIa clinical trials for E1-I.N.T.™in both type 1 and type 2 diabetics. These two clinical trials evaluated the efficacy, safety and tolerability of a 28-day course of daily E1-I.N.T.Ô treatments with a six-month follow-up.
In March, 2007, the Company announced positive unblinded interim safety, tolerability and efficacy data from these exploratory Phase IIa trials for type 1 and type 2 diabetes patients. In the type 1 diabetes study, 6 of 11 (54%) patients responded to E1-I.N.T.Ô therapy, either by decreasing their average daily insulin usage by more than 20% or reducing their HbA1c levels by 1.2 to 2%. There were no responders among the placebo group.
On June 28, 2007, the Company announced final results from the exploratory phase IIa E1-I.N.T.™ clinical trial. A 4-week therapy with E1-I.N.T.™ lead to sustained reductions in blood glucose levels for 6 months post-treatment in type 2 diabetes patients. In the E1-I.N.T.™ treated group of patients, the mean HbA1c level was reduced by 0.94% to 1.21% vs. baseline levels in months 2 to 6 post-treatment. More specifically, the mean HbA1c level among treated patients was reduced 0.43%, 0.94% (p<0.05), 1.09%
9
(p<0.05), 1.12% (p<0.05), 1.21% (p<0.05), and 1.14% in months 1, 2, 3, 4, 5, and 6 post-treatment, respectively. In contrast, the mean HbA1c levels of the placebo group ranged from a reduction of 0.1% to an increase of 1.0% over the same period. In addition to the HbA1c reductions, the data demonstrated decreases in fasting blood glucose levels as well as improvements in glucose tolerance over a six month period following treatment with E1-I.N.T.™. Trends in increased insulin levels as measured with an oral glucose tolerance test were also observed, particularly in patients where the HbA1c levels decreased over 1% with E1-I.N.T.™ therapy. These data are consistent with the increased glucose control observed in diabetes animal models where a short treatment with E1-I.N.T.™ resulted in a sustained increase in beta cell mass and function. These clinical improvements, including HbA1c reductions greater than 1% in patients six month post-treatment, highlight the potential that E1-I.N.T.™ therapy could provide patients significant clinical benefit in excess of 6 months.
These clinical data support the potential of gastrin as a therapeutic in combination with other diabetes therapies. Transition holds the exclusive rights to a series of proprietary gastrin based combination therapies including GLP1-I.N.T.Ô (a combination of gastrin analogue, G1, and a GLP-1 analogue) and combination therapies of gastrins and DPP-IV inhibitors. Transition will continue the development of these combination therapies into clinical trials with type 1 and type 2 diabetes patients.
GLP1- I.N.T.TM
Transition’s second Islet Neogenesis Therapy product, GLP1- I.N.T.Ô is a combination of one of the leading diabetes drug candidates, Glucagon-Like-Peptide-1 (“GLP-1”), with G1. The Company will perform additional safety and tolerability studies in humans in preparation for Phase II clinical development. The Company has entered into an agreement with the JDRF to support the clinical development of GLP1- I.N.T.Ô over the next two years.
Expenditures for the I.N.T.TMProgram
During the year ended June 30, 2007, the Company incurred direct research and development costs for this program as follows:
| | | | | | | | | |
| I.N.T.TMProgram(1) | | F2007 | | | F2006 | |
| Pre-clinical studies | | $ | 1,691,070 | | | $ | 0 | |
| Clinical studies | | | 1,571,336 | | | | 2,213,216 | |
| Manufacturing | | | 504,703 | | | | 989,792 | |
| Other direct research | | | 95,862 | | | | 128,817 | |
| | | | | | | |
| TOTAL | | $ | 3,862,971 | | | $ | 3,331,825 | |
| | | | | | | |
| | |
| (1) | | These costs are direct research and development costs only and do not include patent costs, investment tax credits, salaries and benefits or an allocation of Company overhead. The costs are presented as gross amounts expensed by the Company, prior to the reimbursement of development costs from Novo Nordisk and the JDRF which have been netted against R&D expense ($1,370,153 for the year ended June 30, 2007 and $385,672 for the year ended June 30, 2006 ). |
10
I.E.T. Technology
The goal of the Company’s I.E.T. technology was to provide therapeutic benefit for those patients with MS or hepatitis C that did not respond to their current interferon therapy. This technology combines interferon with the Company’s proprietary enabling technology. The Company is currently deploying its financial and human resources to advance its leading programs for Alzheimer’s disease and diabetes. The Company will only pursue further development of the I.E.T. program through a partnership.
Expenditures for the I.E.T. Program
During the year ended June 30, 2007, the Company incurred direct research and development costs for this program as follows:
| | | | | | | | | |
| I.E.T. Program(1) | | F2007 | | | F2006 | |
| Clinical studies | | $ | 294,274 | | | $ | 695,860 | |
| Manufacturing | | | 109,664 | | | | 384,121 | |
| Other direct research | | | 30,915 | | | | 25,105 | |
| | | | | | | |
| TOTAL | | $ | 434,853 | | | $ | 1,105,086 | |
| | | | | | | |
| | |
| (1) | | These costs are direct research and development costs only and do not include patent costs, investment tax credits, salaries and benefits or an allocation of Company overhead. |
Drug Discovery Initiatives
Transition has prioritized its drug discovery activities to accelerate the identification and optimization of novel lead molecules. The Company is pursuing a number of discovery programs to advance novel lead molecules into pre-clinical development.
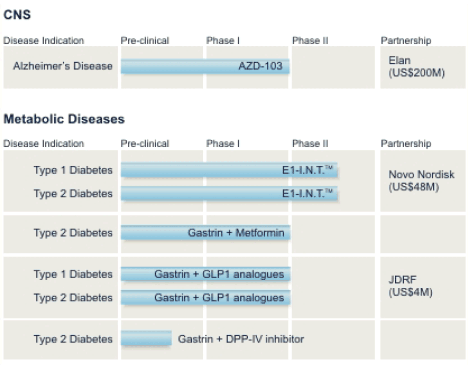
The Next Steps
Transition’s goal for its programs is to achieve product approval and ultimately significant revenues or royalties. To achieve product approval, the Company must successfully complete clinical trials and achieve regulatory approval. The stages of development of the Company’s technologies are illustrated below:
11
Note: The Company is pursuing a Gastrin program with the intent to enter into multiple Phase II clinical trials in diabetics currently taking Metformin/TZD’s or Byetta®.
OVERALL PERFORMANCE
During fiscal 2007, the Company continued to advance its lead products through the clinic. The Company is collaborating with Elan to develop the Alzheimer’s disease drug candidate ELND-005/AZD-103 which has been granted fast track status by the FDA. Phase I trials have been successfully completed and the next steps in the development of ELND-005/AZD-103 will be submission of data supporting Phase II studies to the FDA. Transition and Elan anticipate starting Phase II by the end of calendar 2007 or early 2008. The Company has also completed its E1-I.N.T.™ exploratory Phase IIa clinical trials in type 1 and type 2 diabetic patients and reported final results from the type 2 diabetes trial.
During the year ended June 30, 2007, the Company strengthened its cash position by completing an offering for 2,986,867 common shares for net cash proceeds of $23,964,751. Subsequent to the end of the year, the Company further strengthened its cash position by completing another private placement, issuing 1,736,107 common shares resulting in net cash proceeds of $23,976,404. The Company’s cash and cash equivalents and short-term investments were $34,368,142 at June 30, 2007. The Company currently believes that it has adequate financial resources for anticipated expenditures until early fiscal 2011.
The Company’s net loss for the year ended June 30, 2007 decreased by $6,056,300 or 26% to $16,961,790 from a loss of $23,018,090 reported in fiscal 2006. The decrease in
12
net loss is due to, amongst other items, decreases in research and development expenses, resulting from expense reimbursements from Novo Nordisk and the JDRF, decreases in amortization expense, an increase in interest income due to increased cash balances, combined with an increase in recovery of future income taxes, partially offset by an increase in general and administration expenses.
In upcoming periods, the Company’s losses are expected to increase primarily as a result of increased clinical expenditures as the Company continues the clinical development of multiple products. These losses will be partially off-set by an increase in interest income resulting from the significantly higher cash and short-term investment balances and partnership revenues.
SELECTED ANNUAL INFORMATION
The following table is a summary of selected audited consolidated financial information of the Company for each of the three most recently completed financial years:
| | | | | | | | | | | | | |
| | | June 30, | | June 30, | | June 30, |
| | | 2007 | | 2006 | | 2005 |
| Revenue | | $ | 683,894 | | | $ | 371,174 | | | $ | 109,370 | |
Net loss(1) | | $ | 16,961,790 | | | $ | 23,018,090 | | | $ | 14,223,108 | |
Basic and diluted net loss per common share and Class B share(2) | | $ | 0.87 | | | $ | 1.53 | | | $ | 1.08 | |
| Total assets | | $ | 63,995,728 | | | $ | 44,128,024 | | | $ | 40,429,192 | |
Total long-term liabilities(3) | | $ | 91,456 | | | $ | 2,862,711 | | | $ | 869,691 | |
| Cash dividends declared per share | | $ | — | | | $ | — | | | $ | — | |
| | |
| Notes: | | |
| |
| (1) | | Net loss before discontinued operations and extraordinary items was equivalent to the net loss for such periods. |
| |
| (2) | | Class B shares were removed from the Company’s authorized share capital in December 2004. |
| |
| (3) | | Total long-term liabilities exclude deferred revenue, a non-financial liability. |
ANNUAL RESULTS — YEAR ENDED JUNE 30, 2007 COMPARED TO YEAR ENDED JUNE 30, 2006
Results of Operations
For the fiscal year ended June 30, 2007, the Company recorded a net loss of $16,961,790 ($0.87 per common share) compared to a net loss of $23,018,090 ($1.53 per common share) for the fiscal year ended June 30, 2006. This decrease in net loss of $6,056,300 or 26% is due to, amongst other items, decreases in research and development expenses, resulting from expense reimbursements from Novo Nordisk and the JDRF, decreases in amortization expense, an increase in interest income due to increased cash balances,
13
combined with an increase in recovery of future income taxes, partially offset by an increase in general and administration expenses.
Revenue
The Company recorded licensing fees of $131,244 for both fiscal 2007 and fiscal 2006. Licensing fees represent the recognition of revenue from the Licensing Agreement with Novo Nordisk, as described above under the heading, “Licensing Agreement”. Based on the current recognition term of 15 years, licensing fees are expected to be $131,244 in the next fiscal year.
In connection with the amendment to the Novo Nordisk Licensing Agreement, the Company received $552,650 [US$500,000] for the achievement of the first developmental milestone, which has been recognized as milestone revenue in fiscal 2007.
Research and Development
Research and development expenses excluding amortization of intangibles decreased to $9,839,170 for the fiscal year ended June 30, 2007 from $11,060,455 for the same period in 2006. The decrease of $1,221,285 or 11% was primarily the result of a reduction in research and development expense resulting from expense reimbursements from Elan, Novo Nordisk and the JDRF, as well as a decrease in clinical program expenses relating to the Company’s E1-I.N.T.™ and I.E.T. clinical trials which were both completed during fiscal 2007. The decrease is partially offset by costs incurred to advance ELND-005/AZD-103 through Phase I clinical trials and for pre-clinical research studies supporting the GLP1-I.N.T.™ program.
The Company anticipates that research and development expenses in fiscal 2008 will increase significantly compared to fiscal 2007 as the Company will incur research and development costs relating to ELND-005/AZD-103 Phase II trials, costs associated with advancing the GLP1-I.N.T.Ô program, and costs relating to advancing pre-clinical compounds, as well as, the on-going costs of the drug discovery platform.
General and Administrative
General and administrative expenses increased to $5,317,524 for the fiscal year ended June 30, 2007 from $3,140,800 for the fiscal year ended June 30, 2006. This increase of $2,176,724 or 69% primarily resulted from increased professional fees associated with the Nasdaq listing, the Elan co-development agreement, expenses relating to the amalgamation of various subsidiaries, increased corporate development costs, option expense, and an increase in salaries incurred to strengthen the finance and management teams. The Company anticipates that general and administrative expenses will increase during fiscal 2008 as the Company incurs additional compliance, corporate development, and investor relations costs, in line with the Company’s strategy for its next stage of growth.
14
Amortization
Amortization for the year ended June 30, 2007 decreased by $2,740,015 or 29% to $6,823,259 as compared to $9,563,274 for the year ended June 30, 2006. The decrease in amortization expense is primarily due to the Waratah technology being fully amortized early in the third quarter of fiscal 2007. This decrease was partially off set by the full year impact of the amortization relating to the products, patents and technologies acquired from ENI as well as the amortization of the NeuroMedix technology acquired May 9, 2007.
The Company anticipates that amortization expense will decrease in the next fiscal year as the Waratah technology is now fully amortized. The decrease will be partially offset by a full year impact of the amortization relating to the NeuroMedix technology.
Recovery of Future Income taxes
Recovery of future income taxes for the year ended June 30, 2007 increased by $1,631,901 or 149% to a recovery of $2,729,422 as compared to a recovery of $1,097,521 for the year ended June 30, 2006.
The majority of the increase in recovery of future income taxes for fiscal 2007 is due to the recognition of future income tax assets resulting from the amalgamation of Ellipsis Neurotherapeutics Inc., 1255205 Ontario Inc., 1255206 Ontario Inc. and Waratah Pharmaceuticals Inc. As a result of the amalgamation, the Company has adjusted the valuation allowance on future income tax assets and has recognized a future income tax asset to the extent of offsetting future income tax liabilities of the amalgamated entity. Additional future income tax recovery also arose from changes in temporary differences. In the absence of additional acquisitions, the Company does not anticipate recording a future income tax recovery in fiscal 2008.
In connection with the NeuroMedix acquisition, the Company recognized a future tax liability of $3,514,857 which has been offset by future tax assets of the Company.
Interest Income, net
Interest income, net for the fiscal year ended June 30, 2007, was $1,226,099 as compared to $350,380 for the fiscal year ended June 30, 2006. The increase in interest income, net of $875,719 primarily resulted from increased cash balances due to the November 2006 private placement and the upfront payment received from Elan.
Interest income is expected to increase in fiscal 2008 due to the increased cash balances resulting from the July 2007 private placement where the Company raised net cash proceeds of $23,976,404. Also, an increase in cash balances is anticipated in the next
15
fiscal year as the remaining portion of the upfront payment is to be received from Elan, as well as, milestone payments under the terms of the collaboration agreement assuming certain events are achieved.
Capital Expenditures
During the fiscal year ended June 30, 2007, the Company’s capital expenditures were $49,526 as compared to $234,919 for the fiscal year ended June 30, 2006. The expenditures during fiscal 2007 were primarily for leasehold improvements and computer equipment and software. The Company does not presently anticipate any significant increase in capital expenditures during fiscal 2008.
SCT ANNIVERSARY PAYMENT
On October 4, 2004, the Company signed an agreement to sell one of its wholly-owned subsidiaries, SCT, whose only significant asset is technology. SCT is developing a series of regenerative therapies for the treatment of neurological diseases including stroke and Parkinson’s disease. The agreement includes an upfront cash payment of $325,000 and anniversary payments totaling $3.175 million that may be settled in either cash or shares at the option of the purchaser, and royalties on sales and other income.
This transaction was not recorded as a sale for accounting purposes as the risks and rewards of the ownership of SCT did not transfer to the purchaser under the terms of the share purchase agreement. Therefore, the Company classified the assets and liabilities of SCT as assets transferred under a contractual arrangement. Using the cost recovery method, the carrying value of the assets transferred under contractual arrangement were reduced by [i] proceeds upon receipt, [ii] losses of SCT and [iii] amortization of the technology, resulting in a carrying value at June 30, 2006 of nil.
During the three month period ending September 30, 2006, the Company received the second anniversary payment of $400,000 in cash which has been recorded as a gain in the consolidated statement of loss and deficit. Total payments received to date amount to $1,200,000 with $2,300,000 in anniversary payments remaining to be paid over the next two fiscal years.
16
SUMMARY OF QUARTERLY RESULTS
The following table is a summary of selected quarterly consolidated financial information of the Company for each of the eight most recently completed quarters ending at June 30, 2007.
| | | | | | | | | | | | | | | | | | | | | |
| | | First | | Second | | Third | | Fourth | | |
| | | Quarter | | Quarter | | Quarter | | Quarter | | Year |
2007 | | | | | | | | | | | | | | | | | | | | |
| Revenue | | $ | 585,461 | | | $ | 32,811 | | | $ | 32,811 | | | $ | 32,811 | | | $ | 683,894 | |
| | | | | | | | | | | | | | | | | | | | | |
Net loss(1) | | $ | 1,866,755 | | | $ | 4,965,881 | | | $ | 3,137,289 | | | $ | 6,991,865 | | | $ | 16,961,790 | |
| Basic and diluted net loss per Common Share | | $ | 0.09 | | | $ | 0.27 | | | $ | 0.18 | | | $ | 0.33 | | | $ | 0.87 | |
2006 | | | | | | | | | | | | | | | | | | | | |
| Revenue | | $ | 114,901 | | | $ | 190,651 | | | $ | 32,811 | | | $ | 32,811 | | | $ | 371,174 | |
| | | | | | | | | | | | | | | | | | | | | |
Net loss(1) | | $ | 4,322,288 | | | $ | 5,307,972 | | | $ | 6,536,992 | | | $ | 6,850,838 | | | $ | 23,018,090 | |
| Basic and diluted net loss per Common Share | | $ | 0.36 | | | $ | 0.36 | | | $ | 0.45 | | | $ | 0.36 | | | $ | 1.53 | |
| | |
| Notes: | | |
| |
| (1) | | Net loss before discontinued operations and extraordinary items was equivalent to the net loss for such periods. |
The fluctuations of Transition’s quarterly results are primarily due to changes in activity levels of the clinical trials being performed by the Company, amortization of the technology relating to the assets acquired from Protana and ENI, recognition of equity losses resulting from ENI and SCT, changes in the recovery of future income taxes and the growth of the Company’s management team.
17
FOURTH QUARTER RESULTS
The following table is a summary of selected information for the three month periods ended June 30, 2007 and June 30, 2006:
| | | | | | | | | |
| | | 2007 | | 2006 |
| Revenue — Licensing fees | | $ | 32,811 | | | $ | 32,811 | |
| Research and development, net | | $ | 4,805,661 | | | $ | 3,997,421 | |
| General and administrative | | $ | 2,024,065 | | | $ | 876,601 | |
| Amortization | | $ | 586,062 | | | $ | 3,017,877 | |
| Interest income, net | | $ | 305,782 | | | $ | 68,750 | |
| Losses of company transferred under contractual arrangement | | | — | | | $ | 83,181 | |
| Recovery of future income taxes | | | — | | | $ | 1,051,449 | |
| Net loss | | $ | 6,991,865 | | | $ | 6,850,838 | |
Review of Operations
For the three month period ended June 30, 2007, the Company’s net loss increased by $141,027 or 2% to $6,991,865 compared to $6,850,838 for the same period in fiscal 2006.
Research and development expenses increased by $808,240 or 20% to $4,805,661 compared to $3,997,421 for the same period in fiscal 2006. This increase was primarily due to increased ELND-005/AZD-103 and GLP1- I.N.TTMdevelopment costs incurred. The increase in research and development has been partially offset by a decrease in clinical program expenses relating to the Company’s E1-I.N.TTMand I.E.T. clinical trials.
General and administrative expenses increased by $1,147,464 or 131% to $2,024,065 from $876,601 for the same period in fiscal 2006. This significant increase was primarily due to option expense, increased professional fees relating to the Nasdaq listing and amalgamation of various subsidiaries, corporate development costs, and an increase in salaries incurred to strengthen the finance and management teams.
Amortization expense decreased $2,431,815 or 81% to $586,062 from $3,017,877 for the same period in fiscal 2006. This significant decrease is primarily due to the Waratah technology being fully amortized early in the third quarter of fiscal 2007.
Interest income, net, increased $174,035 or 345% to $305,782 from $68,750 for the same period in fiscal 2006. This increase primarily resulted from increased cash balances due to the November 2006 private placement and the upfront payment received from Elan.
18
Recovery of future income taxes decreased $1,051,449 to nil compared to $1,051,449 for the same period in fiscal 2006. The Company did not record a future income tax recovery in the fourth quarter of fiscal 2007. The Company only recognizes future income tax assets to the extent they offset the future income tax liability or there is reasonable assurance that the future income tax assets will be realized.
STRATEGIC ACQUISITIONS
Acquisition of NeuroMedix in the Fourth Quarter of Fiscal 2007
During the fourth quarter of fiscal 2007 the Company acquired 100% of the common shares of NeuroMedix as previously discussed herein.
Financing Activities
There were no financing activities during the fourth quarter of fiscal 2007.
CRITICAL ACCOUNTING ESTIMATES
The preparation of financial statements in accordance with Canadian generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results can differ from those estimates. We have identified the following areas which we believe require management’s most subjective judgments, often requiring the need to make estimates about the effects of matters that are inherently uncertain and may change in subsequent periods.
Valuation and Amortization of Intangible Assets
The Company’s intangible assets are comprised of purchased or licensed pharmaceutical technology, patents and workforce. The cost of the Company’s intangible assets are amortized over the estimated useful life ranging from 5 to 15 years. Factors considered in estimating the useful life of the intangible asset include the expected use of the asset by the Company, legal, regulatory and contractual provisions that may limit the useful life, the effects of competition and other economic factors, and the level of expenditures required to obtain the expected future cash flows from the intangible asset. The Company assesses its intangible assets for recoverability whenever indicators of impairment exist. When the carrying value of an asset is greater than its net recoverable value as determined on an undiscounted basis, an impairment loss is recognized to the extent that its fair value is below the asset’s carrying value.
19
Refundable Investment Tax Credits
The Company incurs research and development expenditures which are eligible for refundable investment tax credits from the provinces of Ontario and Quebec. The investment tax credits recorded are based on our best estimates of amounts expected to be recovered. Actual investment tax credits received are based on the ultimate determination of the taxation authorities and, accordingly these amounts may vary from the amounts recorded.
Valuation Allowance for Future Tax Assets
The Company has recorded a valuation allowance on certain future tax assets primarily related to the carryforward of operating losses and qualifying research and development expenses. The Company has determined that it is more likely than not that some of these carryforward amounts will not be realized based on historical results and estimated future taxable income. The generation of future taxable income or the implementation of tax planning strategies could result in the realization of some or all of the carryforward amounts, which could result in a material change in our net income (loss) through the recovery of future income taxes. However, there is no assurance that the Company will be able to record future income tax recoveries in the future.
Equity Based Valuations
When the Company issues equity based instruments (i.e. stock options), an estimate of fair value is derived for the equity instrument using the Black-Scholes pricing model. The application of this pricing model requires management to make assumptions regarding several variables, including the period for which the instrument will be outstanding, the price volatility of the Company’s stock over a relevant timeframe, the determination of a relevant risk free interest rate and an assumption regarding the Company’s dividend policy in the future. If other assumptions are used, the value derived for the equity instruments could be significantly impacted.
Recognition of Deferred Revenue and Advances
As a result of the Licensing Agreement, the Company has recorded deferred revenue which will be taken into income over the term of the Licensing Agreement. As the term of the Licensing Agreement is based on the life of the underlying patents, which varies among the patents, management has used its judgment to determine an appropriate period over which to recognize the deferred revenue. Actual results could differ materially from the estimates made by management. The first up-front payment received from Elan has been recorded as deferred revenue and advances and will be recognized as income on a systematic basis once the profitability of the collaboration arrangement can be reasonably estimated.
20
ADOPTION OF NEW ACCOUNTING POLICY
In fiscal 2007, the Company has adopted a new research inventory accounting policy as follows:
During the fourth quarter of the current year, the Company changed its accounting policy related to inventories to adopt CICA Handbook section 3031- Inventories, effective July 1, 2006. As a result of the adoption, the net realizable value of the inventory is now measured at the estimated selling price of the inventory less estimated costs of completion and estimated costs to make the sale. Previously the Company measured net realizable value at the inventory’s replacement cost. For the year ended June 30, 2007, the change resulted in an increase in net loss of $525,810. The adoption of the new accounting policy resulted in a $0.03 increase in the loss per share. The change in accounting policy has been applied in accordance with the transitional provisions which permitted the Company to charge the difference in the measurement of opening inventory to the opening deficit for the year without restatement of prior years.
RECENT CANADIAN ACCOUNTING PRONOUNCEMENTS
In January 2006, the Canadian Institute of Chartered Accountants (“CICA”) released new Handbook Section 3855, Financial Instruments, Recognition and Measurement, effective for annual and interim periods beginning on or after October 1, 2006. This new section establishes standards for the recognition and measurement of all financial instruments, provides a characteristics-based definition of a derivative financial instrument, and provides criteria to be used to determine when a financial instrument should be recognized and when a financial instrument is to be derecognized. The Company’s most significant financial instruments are its investments in cash equivalents and short-term investments. The Company will classify its short-term investments as held to maturity which will not result in any significant changes to the balance sheet or accounting for finance income. The Company has not concluded as to how it will classify its cash equivalents and its assessment of whether there are any embedded derivatives.
In January 2006, the CICA released new Handbook Section 1530, Comprehensive Income, and Section 3251, Equity, effective for annual and interim periods beginning on or after October 1, 2006. Section 1530 establishes standards for reporting comprehensive income. The section does not address issues of recognition or measurement for comprehensive income and its components. Section 3251 establishes standards for the presentation of equity and changes in equity during the reporting period. The requirements in Section 3251 are in addition to Section 1530. The Company has not yet assessed the impact the adoption of this new standard is expected to have on its consolidated financial position or results of operations.
In January 2006, the CICA released new Handbook Section 3865, Hedges, effective for annual and interim periods beginning on or after October 1, 2006. This new section
21
establishes standards for when and how hedge accounting may be applied. Hedge accounting is optional. The Company does not enter into any hedges or owns any derivative accounting instruments. The adoption of this new accounting standard is not expected to have any impact on the Company’s consolidated financial position or results of operations.
RECENT U.S ACCOUNTING PRONOUNCEMENTS
In June 2006, the FASB issued SFAS No. 154, Accounting Changes and Error Corrections (“SFAS 154”), a replacement of APB Opinion No. 20, Accounting Changes (“Opinion 20”) and FASB Statement No. 3, Reporting Accounting Changes in Interim Financial Statements. The Statement applies to all voluntary changes in accounting principle, and changes the requirements for accounting for and reporting of a change in accounting principle. SFAS 154 requires retrospective application to prior periods’ financial statements of a voluntary change in accounting principle unless it is impracticable. SFAS 154 requires that a change in method of depreciation, amortization, or depletion for long-lived, non-financial assets be accounted for as change in accounting estimate that is affected by a change in accounting principle. Opinion 20 previously required that such a change be reported as a change in accounting principle. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2006. The Company has not yet assessed the impact the adoption of this new standard is expected to have on its consolidated financial position or results of operations.
In June 2006, the FASB issued Interpretation No. 48 “Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement 109” (“FIN 48”). FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with FASB Statement No. 109, “Accounting for Income Taxes”, FIN 48 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition requirements. The new statement is effective for financial statements issued for fiscal years beginning after July 1, 2007. The Company has not yet assessed the impact the adoption of this new standard is expected to have on its consolidated financial position or results of operations.
The Emerging Issues Task Force issued draft abstract: Issue 07-3, Accounting for Non-refundable Advance Payments for Goods or Services to Be Used in Future Research and Development Activities, on April 3, 2007. The draft abstract may impact the treatment of non-refundable advance payments for goods or services that will be used or rendered for research and development activities. The draft abstract is expected to be effective for years beginning on or after December 15, 2007. The Company has not yet assessed the impact the adoption of this new abstract is expected to have on its consolidated financial position or results of operations.
22
In September 2006, the FASB issued FASB Statement No. 157 (“SFAS 157”), Fair Value Measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require or permit fair value measurements, the FASB having previously concluded in those accounting pronouncements that fair value is the relevant measurement attribute. Accordingly, SFAS 157 does not require any new fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007. The Company is currently evaluating the impact, if any, the adoption of SFAS 157 will have on its consolidated financial position, results of operations and cash flows.
On June 19, 2007, the Emerging Issues Task Force issued draft abstract: Issue 07-1, Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property. The draft abstract may impact the presentation of revenues and costs generated in a collaborative arrangement. The Task Force is expected to discuss this issue further at a future meeting. Management will assess the impact of the abstract when the Committee reaches a consensus.
DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROLS
As at June 30, 2007, Transition’s management evaluated the effectiveness of the design and operation of its disclosure controls. Based on their evaluation, the Chief Executive Officer and the Chief Financial Officer have concluded that Transition’s disclosure controls and procedures are effective.
There have been no significant changes in Transition’s internal control over financial reporting, other than a change of accounting information software, during the year ended June 30, 2007, that have materially affected, or are reasonably likely to materially affect Transition’s internal control over financial reporting.
LIQUIDITY AND CAPITAL RESOURCES
Overview
The Company commenced operations in July 1998, and has devoted its resources primarily to fund its research and development programs. All revenue to date has been generated from interest income on surplus funds, management fees, milestone payments, and licensing fees. The Company has incurred a cumulative deficit to June 30, 2007 of $89,691,569. Losses are expected to continue for the next several years as the Company invests in research and development, pre-clinical studies, clinical trials, manufacturing and regulatory compliance.
Since inception, the Company has been financed primarily from public and private sales of equity, the exercise of warrants and stock options, interest earned on cash deposits,
23
short-term investments and investment tax credits, revenues and reimbursements from partners, and proceeds from the sale of assets transferred under contractual arrangement.
The Company’s cash, cash equivalents and short-term investments and the Company’s working capital position were $34,368,142 and $32,624,693, respectively, at June 30, 2007, increased significantly from June 30, 2006 balances of $15,005,437 and $14,286,044, respectively. The increase is the net result of the net proceeds from the November private placement in the amount of $23,964,751, the $8,420,250 (US$7,500,000) upfront payment received from Elan, the milestone payment received from Novo Nordisk in the amount of $552,650, as well as, the second anniversary payment from the sale of SCT of $400,000, partially offset by expenditures incurred during fiscal 2007. In light of the July 11, 2007 financing in which the Company raised net proceeds of $23,976,404, the Company now believes that it has adequate financial resources for anticipated expenditures until early fiscal 2011.
Financial instruments of the Company consist mainly of cash and cash equivalents, short-term investments, receivables, accounts payable and accrued liabilities and amounts due to Elan. Financial instruments are initially recorded at historical cost. Management’s primary investment objective is to maintain safety of principal and provide adequate liquidity to meet all current payment obligations and future planned expenditures.
The Company is exposed to market risks related to volatility in interest rates for the Company’s investment portfolio and foreign currency exchange rates related to purchases of supplies and services made in US dollars.
The success of the Company is dependent on its ability to bring its products to market, obtain the necessary regulatory approvals and achieve future profitable operations. The continuation of the research and development activities and the commercialization of its products are dependent on the Company’s ability to successfully complete these activities and to obtain adequate financing through a combination of financing activities, operations, and partnerships. It is not possible to predict either the outcome of future research and development programs or the Company’s ability to fund these programs going forward.
Financing Activities
The Company extinguished the indebtedness assumed relating to the November 2005 Protana asset purchase through final payments disbursed in the three-month period ended September 30, 2006.
During the three-month period ended December 31, 2006 the Company closed on a private placement financing issuing 2,986,867 common shares at a price of $8.37 per common share, raising gross proceeds of $25,000,000 from two funds managed by Great Point Partners, LLC. The Company incurred total share issuance costs of $1,035,249, resulting in net cash proceeds of $23,964,751.
24
On July 11, 2007 the Company completed a private placement financing issuing 1,736,107 common shares at a price of $14.40 per common share, raising gross proceeds of approximately $25,000,000 from a number of funds managed by Oracle Investment Management Inc., The Invus Group LLC, and a large Boston based investment management company. The Company has incurred total share issuance costs to date of $1,023,596, resulting in net cash proceeds of $23,976,404.
The proceeds from these offerings are planned to be used to fund Transition’s clinical studies, research and product development, working capital and for general corporate purposes.
Contractual Obligations
Minimum payments under our contractual obligations as of June 30, 2007 are as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Less than 1 | | | 1 - 3 | | | 4 - 5 | | | After 5 | | | | |
| | | Year | | | Years | | | Years | | | Years | | | Total | |
| Operating leases | | $ | 188,440 | | | $ | 376,880 | | | $ | 324,247 | | | $ | — | | | $ | 889,567 | |
| Collaboration agreements | | $ | 155,327 | | | $ | — | | | $ | — | | | $ | — | | | $ | 155,327 | |
| Clinical and toxicity study agreements | | $ | 1,572,562 | | | $ | — | | | $ | — | | | $ | — | | | $ | 1,572,562 | |
| Manufacturing agreements | | $ | 154,211 | | | $ | — | | | $ | — | | | $ | — | | | $ | 154,211 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
TOTAL | | $ | 2,070,540 | | | $ | 376,880 | | | $ | 324,247 | | | $ | — | | | $ | 2,771,667 | |
| | | | | | | | | | | | | | | | |
Of these commitments, approximately $189,688 of the clinical and toxicity studies obligation and $51,741 of the manufacturing obligation relate to Elan’s share of the committed ELND-005/AZD-103 development cost. In addition, the Company has also licensed various technologies for its programs. The material licensing agreements are disclosed in the Company’s financial statements.
RELATED PARTY TRANSACTIONS
During fiscal 2007, the Company paid legal fees to a law firm where the Company’s Secretary is a partner and to a company controlled by the Company’s Secretary. Total fees and disbursements charged to the Company by these companies during the year ended June 30, 2007 were $2,700 and are included in general and administrative expenses. The balance owing at June 30, 2007 is Nil. These transactions occurred in the normal course of operations and were measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties.
25
OUTSTANDING SHARE DATA
Authorized
The authorized share capital of the Company consists of an unlimited number of common shares.
Issued and Outstanding
The following details the issued and outstanding equity securities of the Company:
Common Shares
As at September 12, 2007, the Company has 22,974,364 common shares outstanding.
Stock Options
As at September 12, 2007, the Company has 772,547 stock options outstanding (on an after exchanged basis for Waratah options) with exercise prices ranging from $2.52 to $18.00 and expiry dates ranging from November 1, 2007 to July 9, 2012. At September 12, 2007, on an if-converted basis, these stock options would result in the issuance of 772,547 common shares at an aggregate exercise price of $7,134,845.
RISKS AND UNCERTAINTIES
Prospects for companies in the biopharmaceutical industry generally may be regarded as uncertain given the nature of the industry and, accordingly, investments in such companies should be regarded as highly speculative. The Company’s technologies are currently in either the research and development stage or early in the clinical development stage, which are both risky stages for a company in the biopharmaceutical industry. It is not possible to predict, based upon studies in animals and early clinical data, whether a new therapeutic or device will prove to be safe and effective in humans.
The Company’s products will require additional development and testing, including extensive toxicity and other clinical testing, before the Company will be able to apply to obtain regulatory approval to market the product commercially. To date, the Company has not introduced a product into the market and there is no assurance that research and development programs conducted by the Company will result in any commercially viable products. If a product is approved for sale, there is no assurance that the Company will generate adequate funds to continue development or will ever achieve profitable operations. There are many factors such as financial and human resources, competition, patent protection, and the regulatory environment that can influence the Company’s ability to be profitable.
26
Financial and Human Resources
As of June 30, 2007, the Company had cash, cash equivalents and short-term investments of $34,368,142 and working capital of $32,624,693. Subsequent to the end of fiscal 2007, the Company further strengthened its cash position by completing another private placement, issuing 1,736,107 common shares resulting in net cash proceeds of $23,976,404. The Company anticipates that it will need additional financing in the future to fund its ongoing research and development programs and general corporate requirements. We may choose to seek additional funding through public or private offerings, corporate collaborations or partnership arrangements. The amount of financing required will depend on many factors including the financial requirements for the Company to fund its research and the ability of the Company to secure partnerships and achieve partnership milestones as well as to fund other working capital requirements. The Company’s ability to access the capital markets or to enlist partners is mainly dependent on the progress of its research and development and regulatory approval of its products. There is no assurance that additional funding will be available on acceptable terms, if at all.
To continue the Company’s research and development programs and to conduct future clinical trials, the Company will rely upon employees, collaborators and other third party relationships. There is no assurance that the Company will be able to maintain or establish these relationships as required.
History of Operating Losses
Since our inception, we have incurred significant losses each year. We expect to incur significant operating losses as we continue our product research and development and continue our clinical trials. We will need to generate significant revenues in order to achieve and maintain profitability. We cannot assure you that we will ever successfully commercialize or achieve revenues from sales of our therapeutic products if they are successfully developed or that we will ever achieve or maintain profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability.
Competition
The pharmaceutical industry is very competitive and there is frequent introduction of new products and technologies. Even if the Company develops a product, there is no assurance that it will be accepted in the marketplace which may result in insufficient product revenue to become profitable. The Company’s success will depend, in part, on its ability to continue to enhance its existing technologies as well as develop new technologies that address the changing needs of the market.
27
Patent Protection
The success of the Company will be, in part, dependent on obtaining and maintaining patent protection for our products. Our ability to compete effectively and to achieve partnerships will depend on our ability to develop and maintain proprietary aspects of our technology and to operate without infringing on the proprietary rights of others. There is no assurance that our patent applications will be approved on the basis submitted, if at all. In addition, any patents issued to the Company may be challenged, invalidated or circumvented.
Pre-Clinical and Clinical Testing
The Company is not able to predict the results of pre-clinical and clinical testing of drug products, including the products of the Company. It is not possible to accurately predict, based on studies or testing in laboratory conditions or in animals, whether a product will prove to be safe or effective in humans. In addition, success in one stage of human testing is not necessarily an indication that the particular product will succeed in later stages of testing and development. There can be no assurance that the pre-clinical or clinical testing of the Company’s products will yield satisfactory results that will enable the Company to progress toward commercialization of such products. Unsatisfactory results may cause the Company to reduce or abandon future testing or commercialization of particular products, and this may have a material adverse effect on the Company.
Regulatory Environment
Although we are in the process of developing several products, these products are subject to regulation in Canada, the US and other countries. There is no assurance that regulatory approval will be granted for any of the Company’s Products. The regulatory process could cause several problems for the Company including, but not limited to, delays in receipt of approvals which could result in time delays in the Company’s programs, limitations on intended use which could result in smaller markets for the Company’s products and failure to obtain necessary approvals, which could force the Company to cease development of one or more of its products.
Potential Product Liability
The Company may be subject to product liability claims in connection with the use of its products, and there can be no assurance that product liability insurance will be available at commercially reasonable terms.
Product liability claims might also exceed the amounts or fall outside of such coverage. Claims against the Company, regardless of their merit or potential outcome, may also have a material adverse effect on the Company’s ability to obtain physician endorsement of its products or expand its business.
In addition, certain drug retailers require minimum product liability insurance coverage as a condition of purchasing or accepting products for retail distribution. Failure to satisfy
28
such insurance requirements could impede the ability of the Company or potential distributors of the Company’s products to achieve broad retail distribution of its proposed products, which would have a material adverse effect on the Company.
Dependence on Third Parties
The Company is or may in the future be dependent on third parties for certain raw materials, product manufacture, marketing and distribution and, like other biotechnology and pharmaceutical companies, upon medical institutions to conduct clinical testing of its potential products. Although the Company does not anticipate any difficulty in obtaining any such materials and services, no assurance can be given that the Company can obtain such materials and services.
Other Risks
The Company is exposed to market risks related to volatility in interest rates for the Company’s investment portfolio and foreign currency exchange rates related to purchases of supplies and services made in U.S. dollars. In addition, the Company’s share price is subject to equity market risk, which may result in significant speculation and volatility of trading due to the uncertainty inherent in the Company’s business and in the biotechnology industry in general. The expectations of the Company made by securities analysts could also have a significant impact on the trading price of the Company’s shares.
OTHER
Additional information relating to the Company, including the Company’s most recently filed Annual Information Form, can be found on SEDAR at www.sedar.com.
29