
January 2020 Corporate Overview Exhibit 99.1

This presentation has been prepared by Dicerna Pharmaceuticals, Inc. (“we,” “us,” “our,” “Dicerna,” or the “Company”) and includes forward-looking statements. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Examples of forward-looking statements include, among others, statements we make regarding: (i) the therapeutic and commercial potential of nedosiran (DCR-PHXC), DCR-HBVS, DCR-A1AT and the GalXC™ platform; (ii) research and development plans and timelines, as well as regulatory pathways and plans, related to nedosiran, DCR-HBVS, DCR-A1AT and GalXC; (iii) the potential of Dicerna’s technology and drug candidates in the Company’s research and development pipeline; (iv) the Company’s collaborations with Novo Nordisk A/S; Roche; Eli Lilly and Company; Alexion Pharmaceuticals, Inc.; Boehringer Ingelheim International GmbH; and (v) the Company’s strategy, business plans and focus. The process by which an early-stage investigational therapy such as nedosiran and an early-stage platform such as GalXC could potentially lead to an approved product is long and subject to significant risks. Applicable risks and uncertainties include those relating to Dicerna’s clinical research and other risks identified under the heading "Risk Factors" included in the Company’s most recent Form 10-K filing and in other subsequent filings with the Securities and Exchange Commission. These risks and uncertainties include, among others, the cost, timing and results of preclinical studies and clinical trials and other development activities; the likelihood of Dicerna’s clinical programs being executed within timelines provided and reliance on the Company’s contract research organizations and predictability of timely enrollment of subjects and patients to advance Dicerna’s clinical trials; the potential for future data to alter initial and preliminary results of early-stage clinical trials; the unpredictability of the duration and results of the regulatory review of Investigational New Drug (IND) applications and Clinical Trial Applications that are necessary to continue to advance and progress the Company’s clinical programs and the regulatory review of submissions relevant to regulatory agencies for marketing approvals, including New Drug Applications (NDAs); market acceptance for approved products and innovative therapeutic treatments; competition; the possible impairment of, inability to obtain and costs of obtaining needed intellectual property rights; possible safety or efficacy concerns that could emerge as new data are generated in R&D; that the Company may not realize the intended benefits of its collaborations; general business, financial and accounting risks; and the risks and potential outcomes from litigation. Dicerna is providing this information as of this date and does not undertake any obligation to update or revise it, whether as a result of new information, future events or circumstances or otherwise. Additional information concerning Dicerna and its business may be available in press releases or other public announcements and public filings made after the date of this information. Dicerna™, GalXC™, and PHYOX™ are trademarks of Dicerna Pharmaceuticals, Inc. Forward-looking Statements
Dicerna Vision and Strategy VISION Develop and commercialize our core high-probability-of-success programs either alone or in collaboration with partners Broadly enable the use of our GalXC™ technology by collaborating with therapeutic area leaders on non-core opportunities STRATEGY Maximize the impact of RNAi on medicine
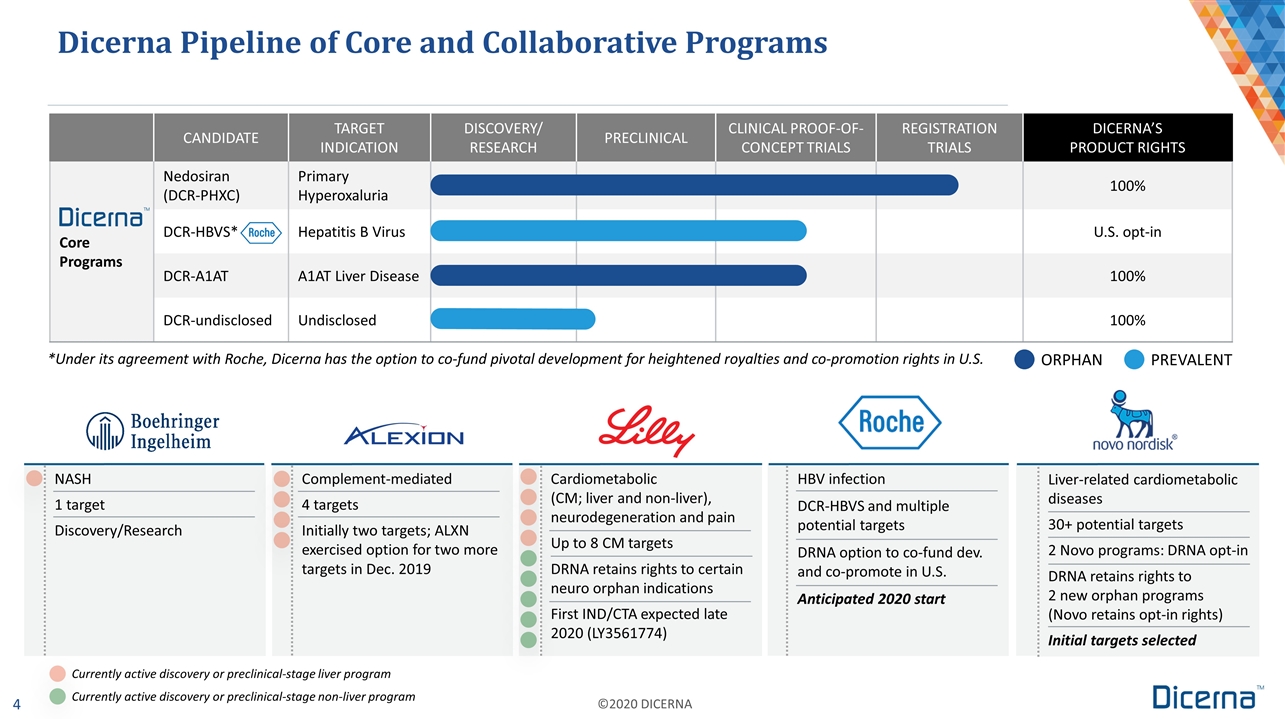
Dicerna Pipeline of Core and Collaborative Programs *Under its agreement with Roche, Dicerna has the option to co-fund pivotal development for heightened royalties and co-promotion rights in U.S. ORPHAN PREVALENT CANDIDATE TARGET INDICATION DISCOVERY/ RESEARCH PRECLINICAL CLINICAL PROOF-OF-CONCEPT TRIALS REGISTRATION TRIALS DICERNA’S PRODUCT RIGHTS Core Programs Nedosiran (DCR-PHXC) Primary Hyperoxaluria 100% DCR-HBVS* Hepatitis B Virus U.S. opt-in DCR-A1AT A1AT Liver Disease 100% DCR-undisclosed Undisclosed 100% NASH 1 target Discovery/Research Cardiometabolic (CM; liver and non-liver), neurodegeneration and pain Up to 8 CM targets DRNA retains rights to certain neuro orphan indications First IND/CTA expected late 2020 (LY3561774) HBV infection DCR-HBVS and multiple potential targets DRNA option to co-fund dev. and co-promote in U.S. Anticipated 2020 start Complement-mediated 4 targets Initially two targets; ALXN exercised option for two more targets in Dec. 2019 Liver-related cardiometabolic diseases 30+ potential targets 2 Novo programs: DRNA opt-in DRNA retains rights to 2 new orphan programs (Novo retains opt-in rights) Initial targets selected Currently active discovery or preclinical-stage liver program Currently active discovery or preclinical-stage non-liver program
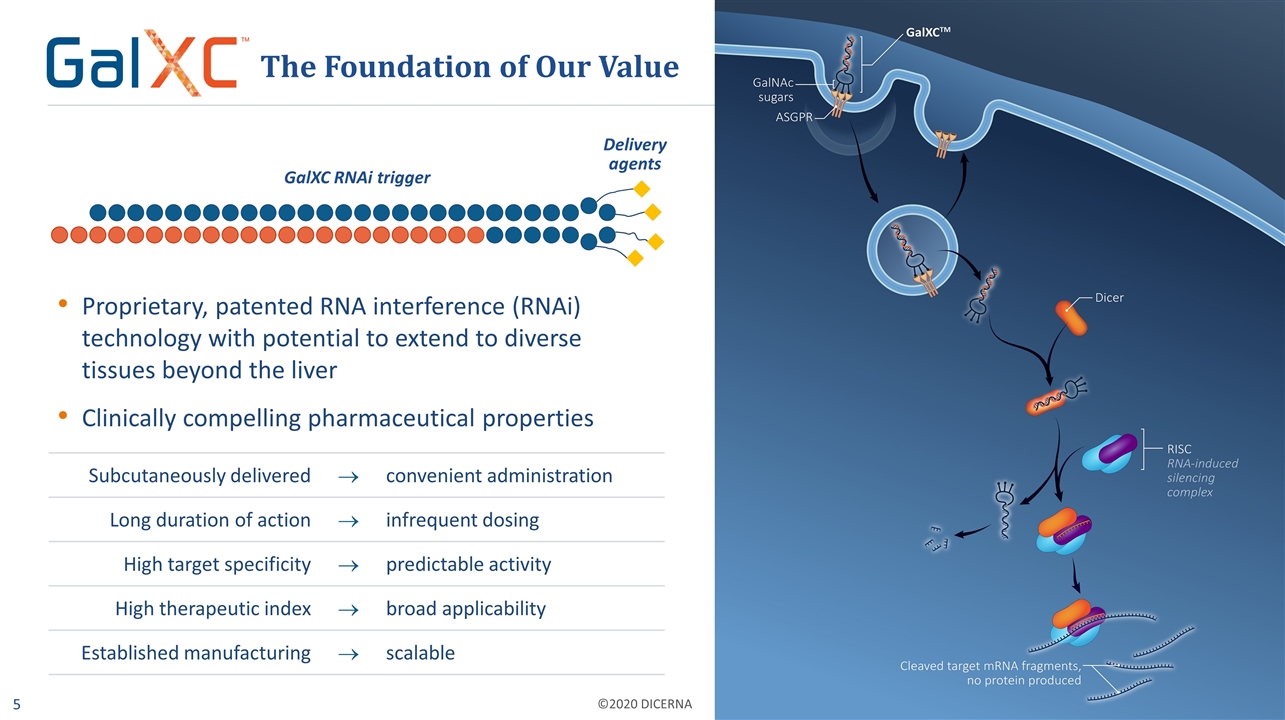
Delivery agents Proprietary, patented RNA interference (RNAi) technology with potential to extend to diverse tissues beyond the liver Clinically compelling pharmaceutical properties The Foundation of Our Value GalXC RNAi trigger Subcutaneously delivered ® convenient administration Long duration of action ® infrequent dosing High target specificity ® predictable activity High therapeutic index ® broad applicability Established manufacturing ® scalable

Multiple Milestones Throughout 2020 Nedosiran: Multi-dose data from PHYOX™3 open-label clinical trial – 1H 2020 Nedosiran: PHYOX2 pivotal clinical trial enrollment completion – by the end of 1H 2020 DCR-HBVS: Phase 1 proof-of-concept data from all planned cohorts – mid-2020 DCR-A1AT: First patient dosing in Phase 1/2 trial – 2H 2020 Collaborative Program: IND or CTA filing for LY3561774 – late 2020 Nedosiran: PHYOX2 last patient out – by YE 2020 GalXC: Present data for extending GalXC technology to additional tissues – 2020

Primary Hyperoxaluria

A family of ultra-rare, life-threatening genetic disorders resulting in renal complications Primary Hyperoxaluria (PH) Oxalate Calcium oxalate crystallization Kidney stones Nedosiran Kidney function preservation Known Types of Primary Hyperoxaluria PH1 PH2 PH3 Genetic mutation AGXT Genetic mutation GRHPR Genetic mutation HOGA1 LDHA GO GO = glycolate oxidase LDHA = lactate dehydrogenase A Three known types of PH, each resulting from a mutation in one of three different genes, cause enzyme deficiencies manifesting in overproduction of oxalate Abnormal production and accumulation of oxalate leads to: Recurrent kidney stones Nephrocalcinosis (deposition of calcium in the kidney) Chronic kidney disease that may progress to end-stage renal disease, requiring regular dialysis and transplant (dual liver-kidney or kidney) Systemic oxalosis, which also impacts the heart, skin, eyes, bones Dicerna’s nedosiran silences LDHA, the ultimate step in the oxalate production pathway Impacted by systemic oxalosis
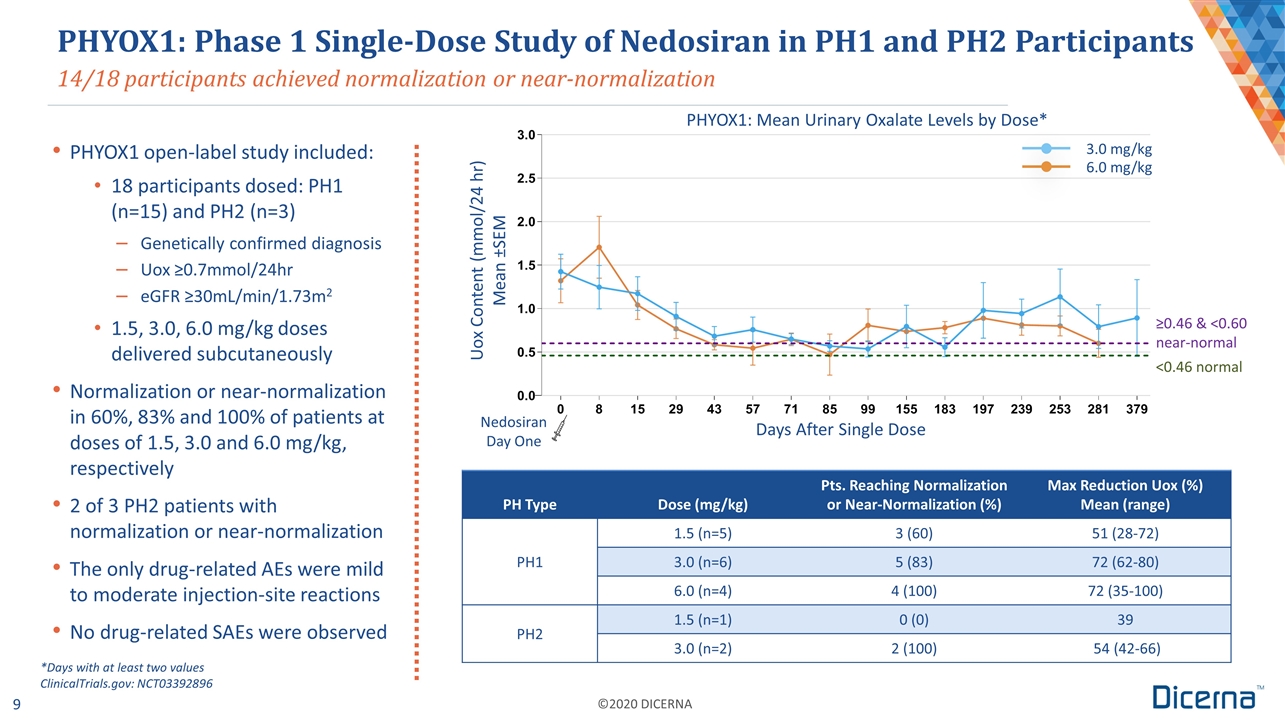
14/18 participants achieved normalization or near-normalization PHYOX1: Phase 1 Single-Dose Study of Nedosiran in PH1 and PH2 Participants Days After Single Dose Nedosiran Day One 3.0 mg/kg 6.0 mg/kg ≥0.46 & <0.60 near-normal <0.46 normal PHYOX1: Mean Urinary Oxalate Levels by Dose* PHYOX1 open-label study included: 18 participants dosed: PH1 (n=15) and PH2 (n=3) Genetically confirmed diagnosis Uox ≥0.7mmol/24hr eGFR ≥30mL/min/1.73m2 1.5, 3.0, 6.0 mg/kg doses delivered subcutaneously Normalization or near-normalization in 60%, 83% and 100% of patients at doses of 1.5, 3.0 and 6.0 mg/kg, respectively 2 of 3 PH2 patients with normalization or near-normalization The only drug-related AEs were mild to moderate injection-site reactions No drug-related SAEs were observed PH Type Dose (mg/kg) Pts. Reaching Normalization or Near-Normalization (%) Max Reduction Uox (%) Mean (range) PH1 1.5 (n=5) 3 (60) 51 (28-72) 3.0 (n=6) 5 (83) 72 (62-80) 6.0 (n=4) 4 (100) 72 (35-100) PH2 1.5 (n=1) 0 (0) 39 3.0 (n=2) 2 (100) 54 (42-66) *Days with at least two values ClinicalTrials.gov: NCT03392896 Uox Content (mmol/24 hr) Mean ±SEM
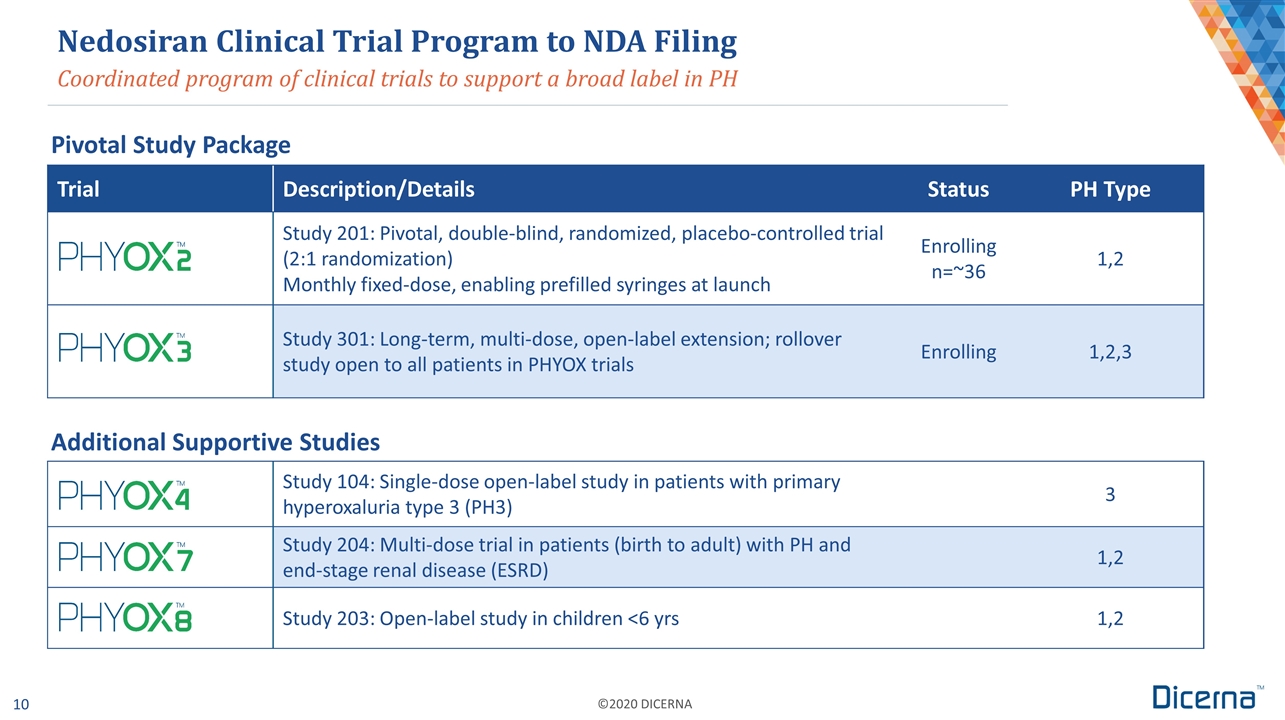
Coordinated program of clinical trials to support a broad label in PH Trial Description/Details Status PH Type Study 201: Pivotal, double-blind, randomized, placebo-controlled trial (2:1 randomization) Monthly fixed-dose, enabling prefilled syringes at launch Enrolling n=~36 1,2 Study 301: Long-term, multi-dose, open-label extension; rollover study open to all patients in PHYOX trials Enrolling 1,2,3 Study 104: Single-dose open-label study in patients with primary hyperoxaluria type 3 (PH3) 3 Study 204: Multi-dose trial in patients (birth to adult) with PH and end-stage renal disease (ESRD) 1,2 Study 203: Open-label study in children <6 yrs 1,2 Nedosiran Clinical Trial Program to NDA Filing Pivotal Study Package Additional Supportive Studies
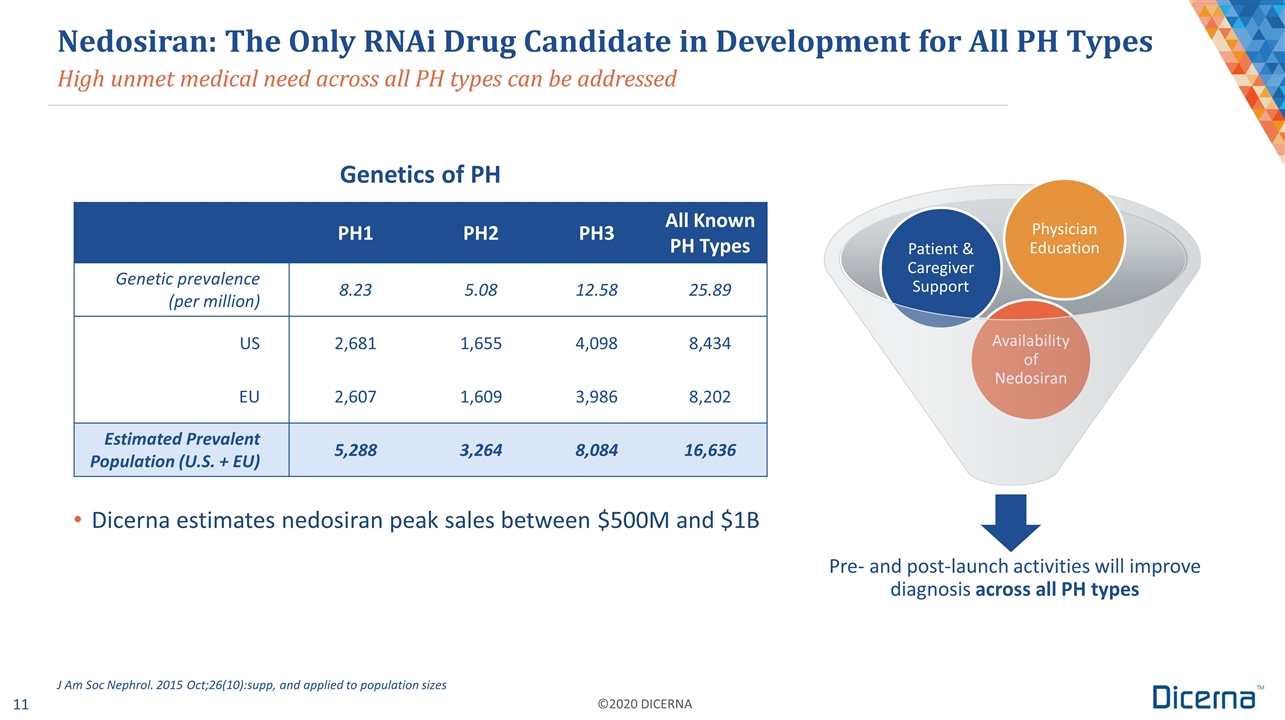
High unmet medical need across all PH types can be addressed Nedosiran: The Only RNAi Drug Candidate in Development for All PH Types Genetics of PH PH1 PH2 PH3 All Known PH Types Genetic prevalence (per million) 8.23 5.08 12.58 25.89 US 2,681 1,655 4,098 8,434 EU 2,607 1,609 3,986 8,202 Estimated Prevalent Population (U.S. + EU) 5,288 3,264 8,084 16,636 J Am Soc Nephrol. 2015 Oct;26(10):supp, and applied to population sizes Dicerna estimates nedosiran peak sales between $500M and $1B Pre- and post-launch activities will improve diagnosis across all PH types Patient & Caregiver Support Availability of Nedosiran Physician Education

Chronic Hepatitis B Virus Infection
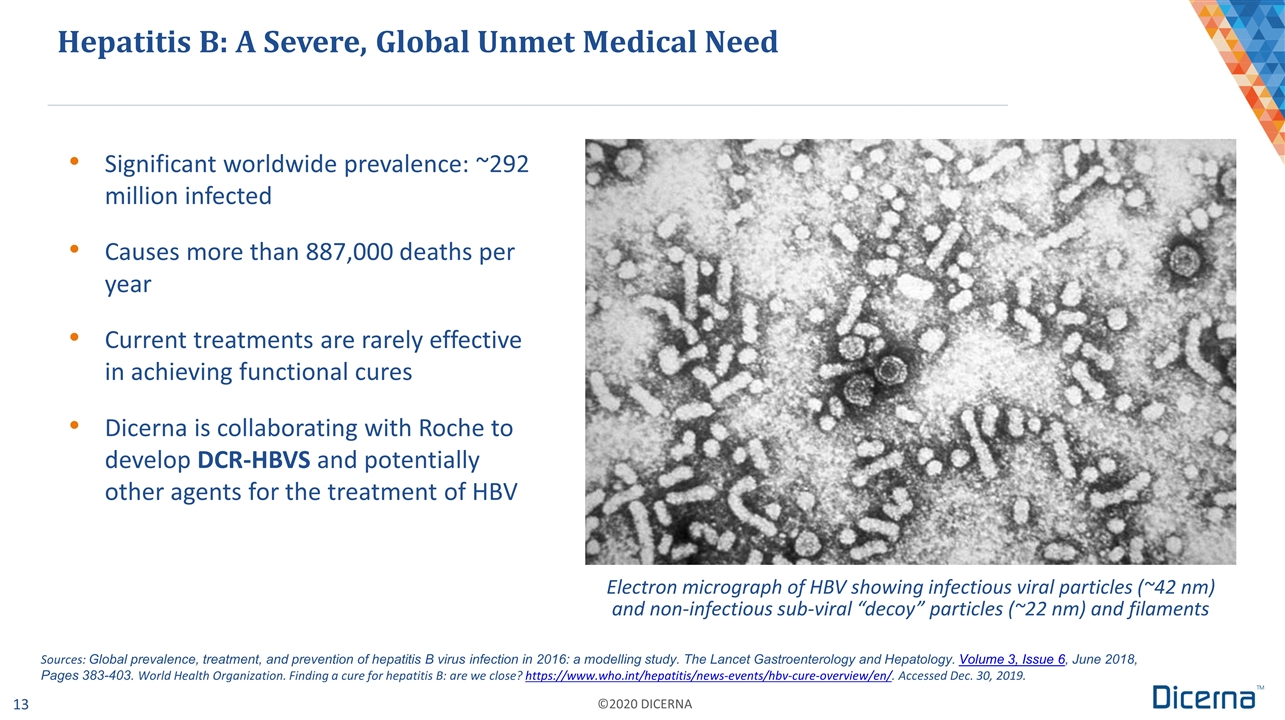
Significant worldwide prevalence: ~292 million infected Causes more than 887,000 deaths per year Current treatments are rarely effective in achieving functional cures Dicerna is collaborating with Roche to develop DCR-HBVS and potentially other agents for the treatment of HBV the treatment Hepatitis B: A Severe, Global Unmet Medical Need Electron micrograph of HBV showing infectious viral particles (~42 nm) and non-infectious sub-viral “decoy” particles (~22 nm) and filaments Sources: Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. The Lancet Gastroenterology and Hepatology. Volume 3, Issue 6, June 2018, Pages 383-403. World Health Organization. Finding a cure for hepatitis B: are we close? https://www.who.int/hepatitis/news-events/hbv-cure-overview/en/. Accessed Dec. 30, 2019.
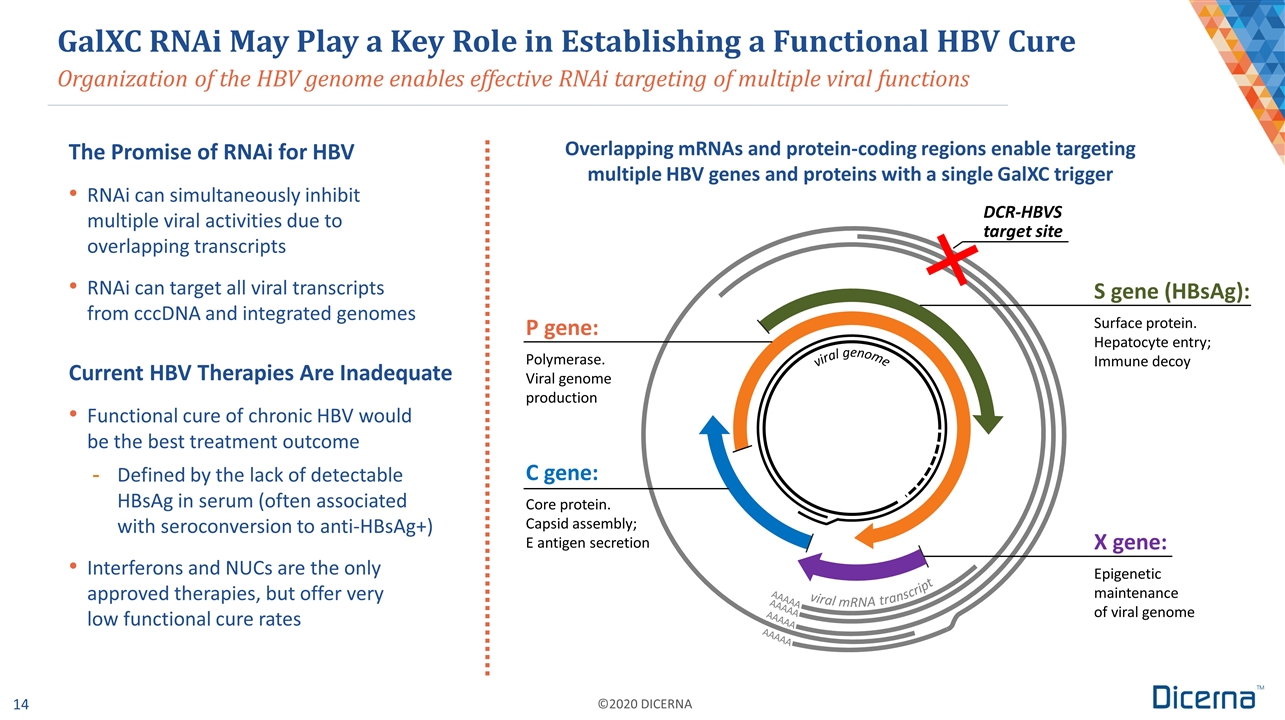
Organization of the HBV genome enables effective RNAi targeting of multiple viral functions GalXC RNAi May Play a Key Role in Establishing a Functional HBV Cure The Promise of RNAi for HBV RNAi can simultaneously inhibit multiple viral activities due to overlapping transcripts RNAi can target all viral transcripts from cccDNA and integrated genomes Current HBV Therapies Are Inadequate Functional cure of chronic HBV would be the best treatment outcome Defined by the lack of detectable HBsAg in serum (often associated with seroconversion to anti-HBsAg+) Interferons and NUCs are the only approved therapies, but offer very low functional cure rates AAAAA P gene: Polymerase. Viral genome production S gene (HBsAg): Surface protein. Hepatocyte entry; Immune decoy X gene: Epigenetic maintenance of viral genome C gene: Core protein. Capsid assembly; E antigen secretion DCR-HBVS target site Overlapping mRNAs and protein-coding regions enable targeting multiple HBV genes and proteins with a single GalXC trigger AAAAA AAAAA AAAAA viral genome viral mRNA transcript
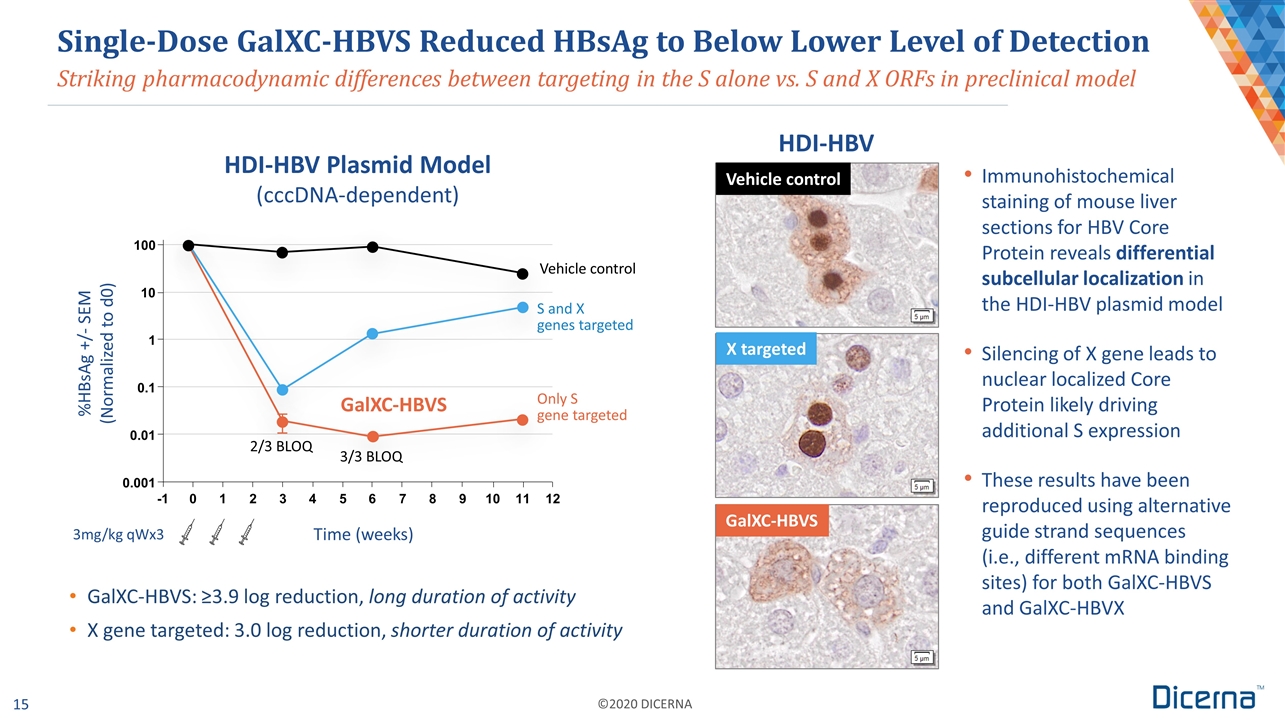
Time (weeks) 3mg/kg qWx3 Striking pharmacodynamic differences between targeting in the S alone vs. S and X ORFs in preclinical model Single-Dose GalXC-HBVS Reduced HBsAg to Below Lower Level of Detection GalXC-HBVS: ≥3.9 log reduction, long duration of activity X gene targeted: 3.0 log reduction, shorter duration of activity Immunohistochemical staining of mouse liver sections for HBV Core Protein reveals differential subcellular localization in the HDI-HBV plasmid model Silencing of X gene leads to nuclear localized Core Protein likely driving additional S expression These results have been reproduced using alternative guide strand sequences (i.e., different mRNA binding sites) for both GalXC-HBVS and GalXC-HBVX 2/3 BLOQ 3/3 BLOQ Vehicle control GalXC-HBVS HDI-HBV Plasmid Model (cccDNA-dependent) HDI-HBV Vehicle control GalXC-HBVS X targeted Only S gene targeted -1 0 1 2 3 4 5 6 7 8 9 0.001 0.01 0.1 1 10 100 10 11 12 %HBsAg +/- SEM (Normalized to d0) S and X genes targeted
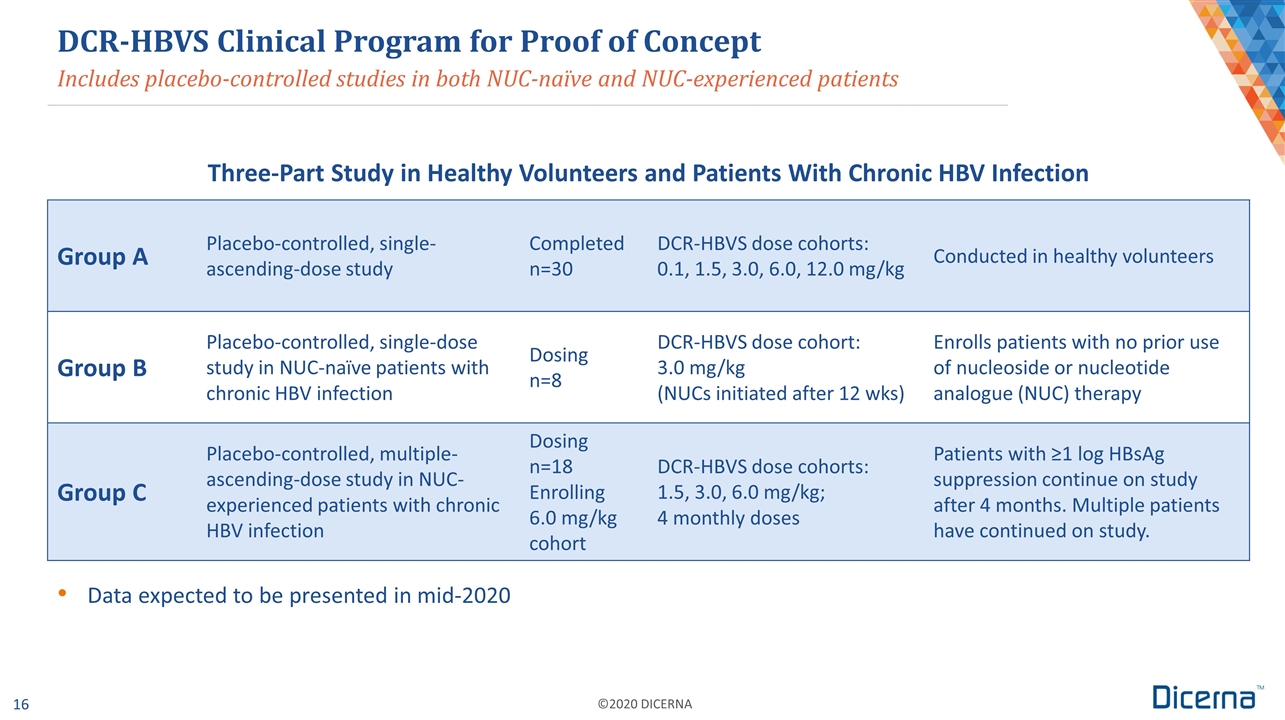
Includes placebo-controlled studies in both NUC-naïve and NUC-experienced patients Group A Placebo-controlled, single- ascending-dose study Completed n=30 DCR-HBVS dose cohorts: 0.1, 1.5, 3.0, 6.0, 12.0 mg/kg Conducted in healthy volunteers Group B Placebo-controlled, single-dose study in NUC-naïve patients with chronic HBV infection Dosing n=8 DCR-HBVS dose cohort: 3.0 mg/kg (NUCs initiated after 12 wks) Enrolls patients with no prior use of nucleoside or nucleotide analogue (NUC) therapy Group C Placebo-controlled, multiple- ascending-dose study in NUC-experienced patients with chronic HBV infection Dosing n=18 Enrolling 6.0 mg/kg cohort DCR-HBVS dose cohorts: 1.5, 3.0, 6.0 mg/kg; 4 monthly doses Patients with ≥1 log HBsAg suppression continue on study after 4 months. Multiple patients have continued on study. DCR-HBVS Clinical Program for Proof of Concept Three-Part Study in Healthy Volunteers and Patients With Chronic HBV Infection Data expected to be presented in mid-2020

Alpha-1 Antitrypsin Deficiency-Associated Liver Disease
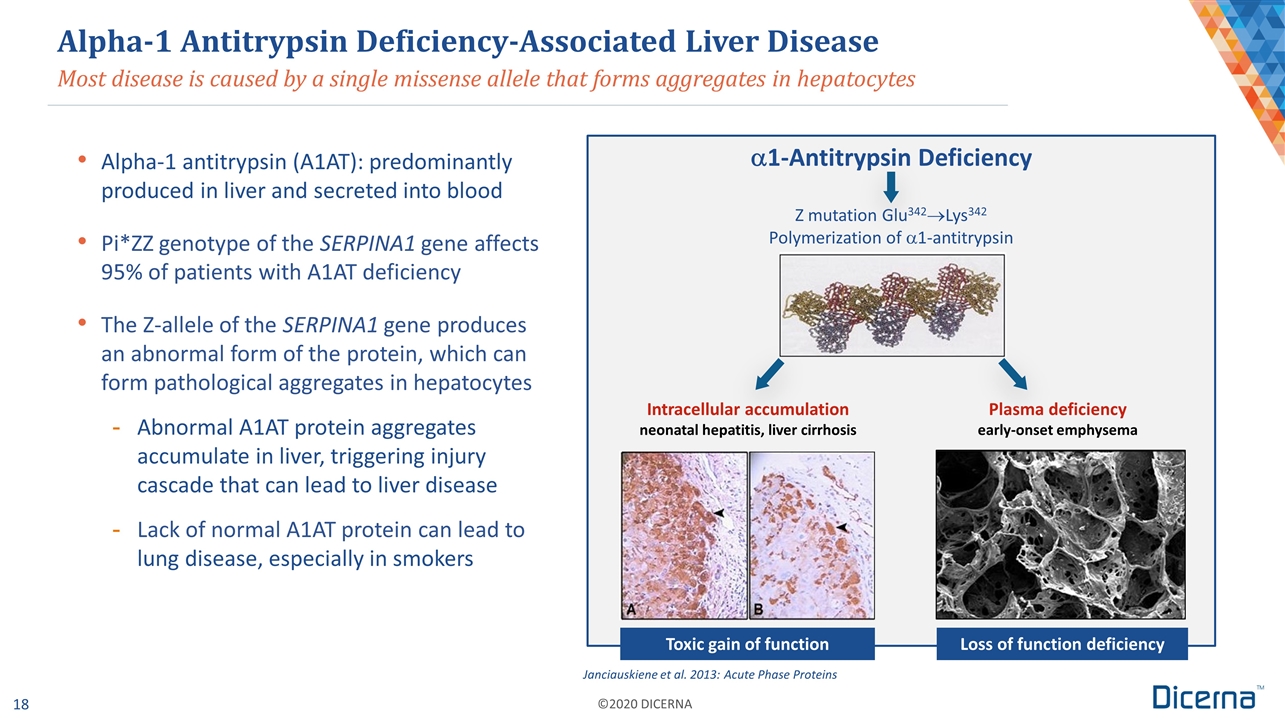
Most disease is caused by a single missense allele that forms aggregates in hepatocytes Alpha-1 Antitrypsin Deficiency-Associated Liver Disease Alpha-1 antitrypsin (A1AT): predominantly produced in liver and secreted into blood Pi*ZZ genotype of the SERPINA1 gene affects 95% of patients with A1AT deficiency The Z-allele of the SERPINA1 gene produces an abnormal form of the protein, which can form pathological aggregates in hepatocytes Abnormal A1AT protein aggregates accumulate in liver, triggering injury cascade that can lead to liver disease Lack of normal A1AT protein can lead to lung disease, especially in smokers Janciauskiene et al. 2013: Acute Phase Proteins Loss of function deficiency Toxic gain of function a1-Antitrypsin Deficiency Z mutation Glu342®Lys342 Polymerization of a1-antitrypsin Intracellular accumulation neonatal hepatitis, liver cirrhosis Plasma deficiency early-onset emphysema
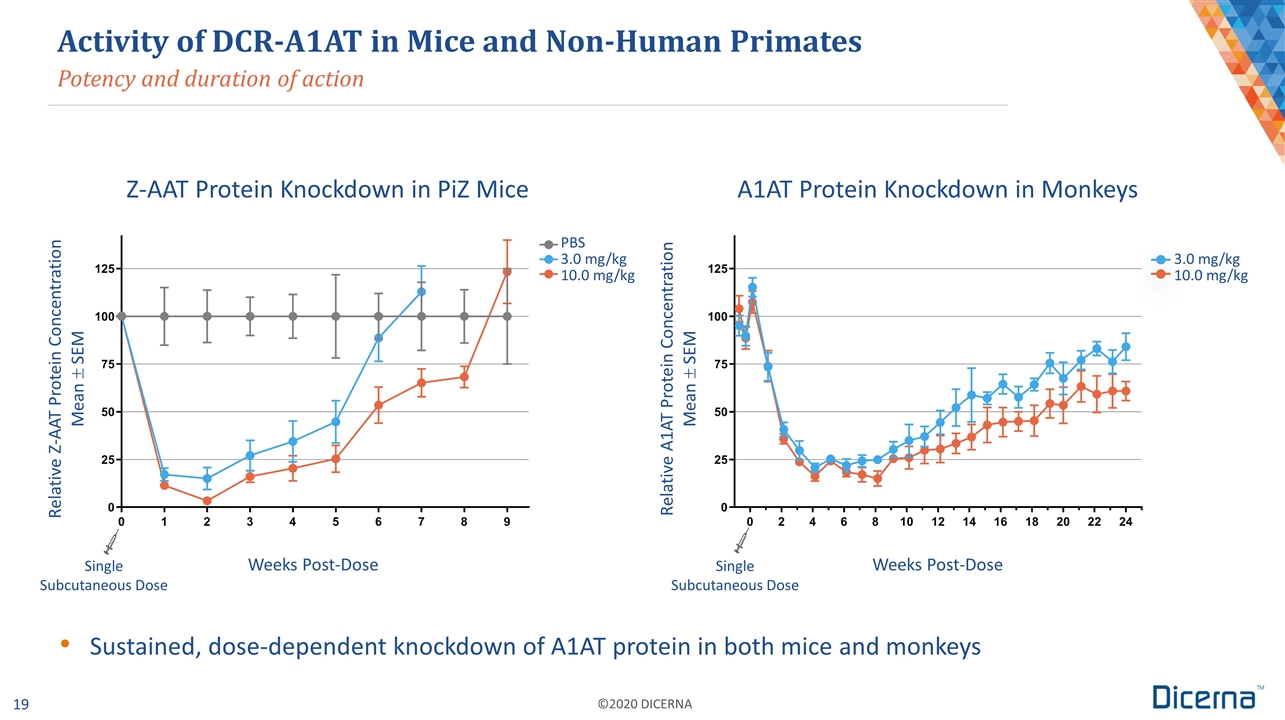
Activity of DCR-A1AT in Mice and Non-Human Primates Potency and duration of action Relative Z-AAT Protein Concentration Mean ± SEM Relative A1AT Protein Concentration Mean ± SEM Weeks Post-Dose Single Subcutaneous Dose PBS 3.0 mg/kg 10.0 mg/kg Weeks Post-Dose 3.0 mg/kg 10.0 mg/kg Z-AAT Protein Knockdown in PiZ Mice A1AT Protein Knockdown in Monkeys Sustained, dose-dependent knockdown of A1AT protein in both mice and monkeys Single Subcutaneous Dose

Part of larger clinical plan to achieve a rapid path to approval Two-part study: SAD in healthy volunteers (HVs) and MAD in patients with A1AT deficiency-associated liver disease HVs: Single-ascending-dose study, placebo-controlled, up to 36 patients 5 dose cohorts: 0.1, 1.0, 3.0, 6.0, 12.0 mg/kg, with a potential additional cohort Overlapping cohorts PK/PD data will be used to determine MAD regimen Patients with A1AT deficiency-associated liver disease: Multiple-ascending-dose study, up to 24 patients 2-3 dose cohorts: dose levels dependent on PK/PD data from HVs 2-4 doses to be administered within a 13-week period Currently dosing healthy volunteers Expect to dose first patient in 2H 2020 We believe the Phase 1/2 program will enable a pivotal trial without additional studies DCR-A1AT Clinical Program for Proof of Concept

Corporate Collaborations
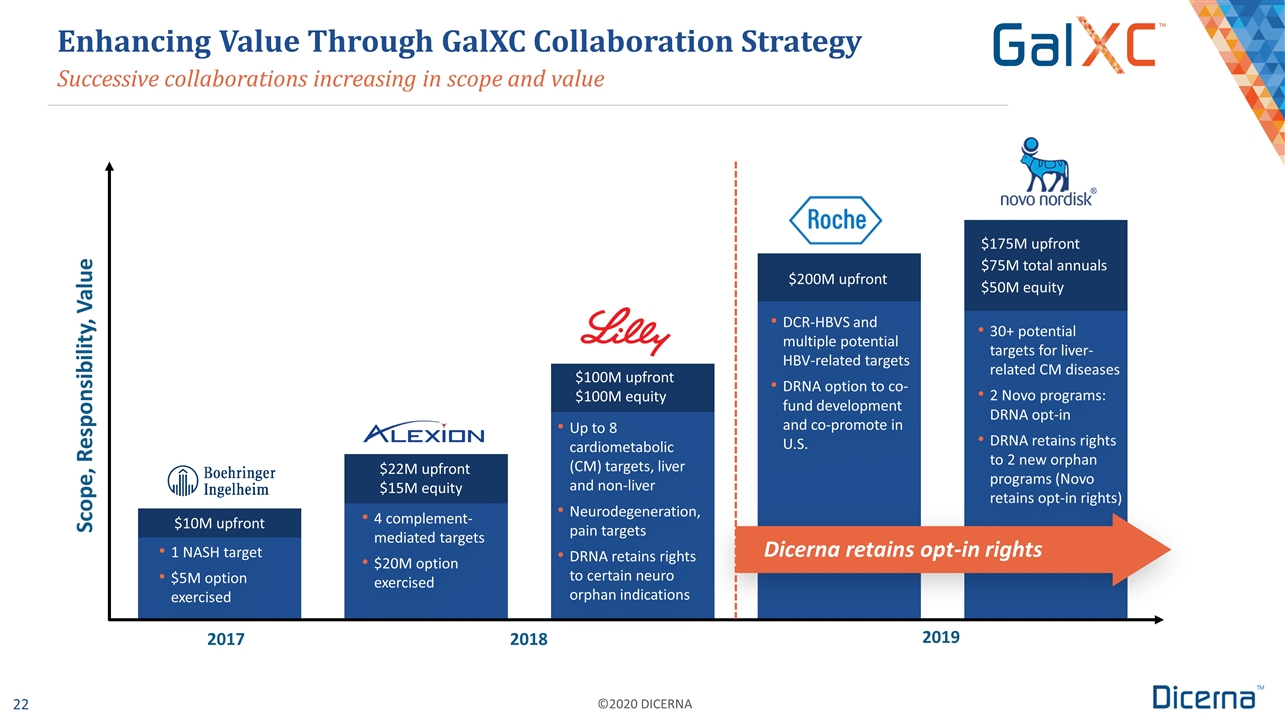
Up to 8 cardiometabolic (CM) targets, liver and non-liver Neurodegeneration, pain targets DRNA retains rights to certain neuro orphan indications Successive collaborations increasing in scope and value Enhancing Value Through GalXC Collaboration Strategy Scope, Responsibility, Value 1 NASH target $5M option exercised 4 complement-mediated targets $20M option exercised $100M upfront $100M equity $200M upfront $175M upfront $75M total annuals $50M equity DCR-HBVS and multiple potential HBV-related targets DRNA option to co-fund development and co-promote in U.S. 30+ potential targets for liver-related CM diseases 2 Novo programs: DRNA opt-in DRNA retains rights to 2 new orphan programs (Novo retains opt-in rights) $10M upfront $22M upfront $15M equity Dicerna retains opt-in rights 2017 2018 2019

Summary
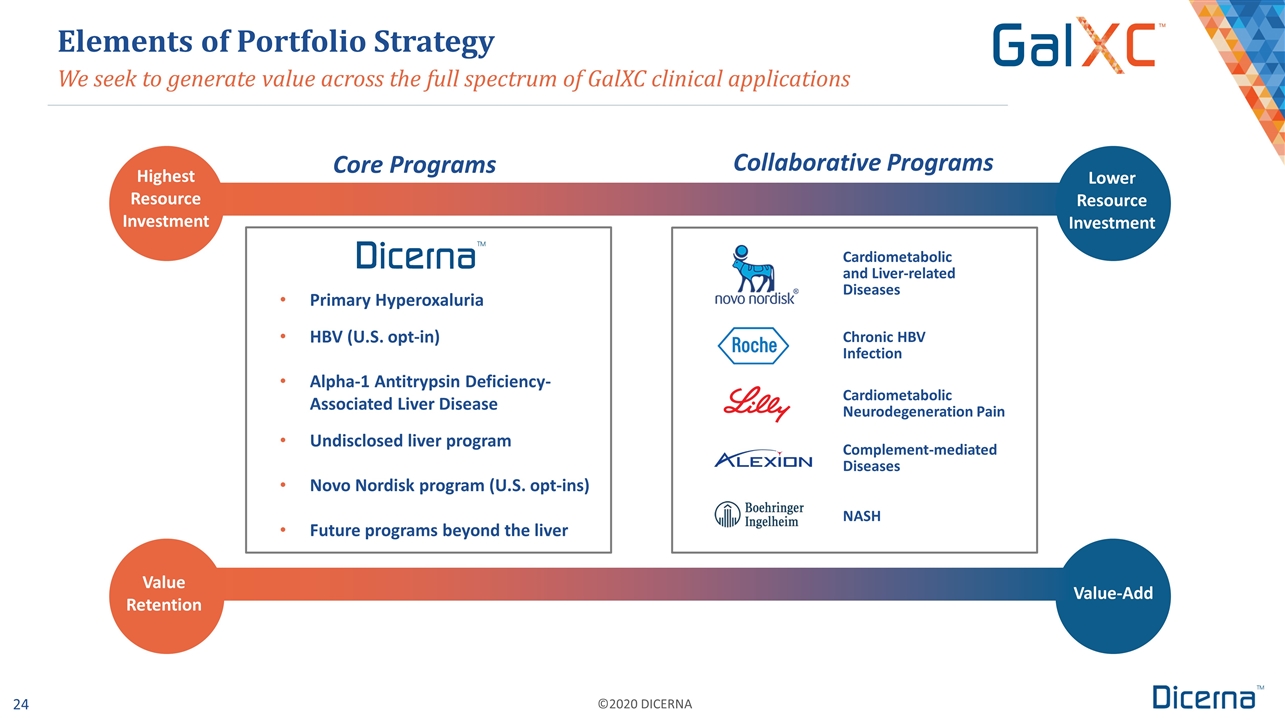
Primary Hyperoxaluria HBV (U.S. opt-in) Alpha-1 Antitrypsin Deficiency-Associated Liver Disease Undisclosed liver program Novo Nordisk program (U.S. opt-ins) Future programs beyond the liver We seek to generate value across the full spectrum of GalXC clinical applications Elements of Portfolio Strategy Highest Resource Investment Lower Resource Investment Complement-mediated Diseases Cardiometabolic Neurodegeneration Pain Value Retention Value-Add Core Programs Collaborative Programs Cardiometabolic and Liver-related Diseases Chronic HBV Infection NASH

Strong Cash Position Provides Runway Through Commercialization Company well capitalized with approximately $350 million in cash (and short-term investments) as of January 1, 2020* including the following amounts received in December 2019: $20 million option exercise payment from Alexion $50 equity investment from Novo Nordisk Pro forma cash of approximately $725 million as of January 1, 2020, which also includes: $200 million upfront payment from Roche, received on January 7, 2020 $175 million upfront payment from Novo Nordisk expected in early Q1 2020 We expect our current cash and estimated future proceeds from existing collaborations will fund operations beyond 2022 *Unaudited estimated amount of cash, cash equivalents and marketable securities. Dicerna expects to announce audited full-year 2019 financial results, including year-end cash, cash equivalents and marketable securities, during Q1 2020. Additional cash upside expected from collaboration milestones and potential royalties on product sales

Multiple Milestones Throughout 2020 Nedosiran: Multi-dose data from PHYOX™3 open-label clinical trial – 1H 2020 Nedosiran: PHYOX2 pivotal clinical trial enrollment completion – by the end of 1H 2020 DCR-HBVS: Phase 1 proof-of-concept data from all planned cohorts – mid-2020 DCR-A1AT: First patient dosing in Phase 1/2 trial – 2H 2020 Collaborative Program: IND or CTA filing for LY3561774 – late 2020 Nedosiran: PHYOX2 last patient out – by YE 2020 GalXC: Present data for extending GalXC technology to additional tissues – 2020
