UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-Q
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2018
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36281
DICERNA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
Delaware |
| 20-5993609 |
(State or other jurisdiction of incorporation or organization) |
| (IRS Employer Identification No.) |
87 Cambridgepark Drive
Cambridge, MA 02140
(Address of principal executive offices and zip code)
(617) 621-8097
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days) Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ☐ |
| Accelerated filer | ☐ |
|
|
|
|
|
Non-accelerated filer | ☐ |
| Smaller reporting company | ☒ |
|
|
|
|
|
|
|
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒
As of November 2, 2018, there were 62,731,942 shares of the registrant’s common stock, par value $0.0001 per share, outstanding.
INDEX TO FORM 10-Q
|
|
| Page
|
| |||
|
|
| |
PART I |
| ||
| Item 1. | 5 | |
|
| Condensed Consolidated Balance Sheets as of September 30, 2018 and December 31, 2017 | 5 |
|
| 6 | |
|
| 7 | |
|
| 8 | |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 20 |
| Item 3. | 33 | |
| Item 4. | 33 | |
|
|
| |
PART II |
| ||
| Item 1. | 34 | |
| Item 1A. | 34 | |
| Item 2. | 64 | |
| Item 3. | 64 | |
| Item 4. | 64 | |
| Item 5. | 64 | |
| Item 6. | 65 | |
2
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical fact are “forward-looking statements” for purposes of this Quarterly Report on Form 10-Q. In some cases, you can identify forward-looking statements by terminology such as “may,” “could,” “will,” “would,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “intend,” “predict,” “seek,” “contemplate,” “project,” “continue,” “potential,” “ongoing,” “goal,” or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
| • | how long we expect to maintain liquidity to fund our planned level of operations and our ability to obtain additional funds for our operations; |
| • | the initiation, timing, progress, and results of our research and development programs, preclinical studies, any clinical trials, and Investigational New Drug application, Clinical Trial Application, New Drug Application, and other regulatory submissions; |
| • | our ability to identify and develop product candidates for treatment of additional disease indications; |
| • | our or a collaborator’s ability to obtain and maintain regulatory approval of any of our product candidates; |
| • | the rate and degree of market acceptance of any approved product candidates; |
| • | the commercialization of any approved product candidates; |
| • | our ability to establish and maintain additional collaborations and retain commercial rights for our product candidates in the collaborations; |
| • | the implementation of our business model and strategic plans for our business, technologies, and product candidates; |
| • | our estimates of our expenses, ongoing losses, future revenue, and capital requirements; |
| • | our ability to obtain and maintain intellectual property protection for our technologies and product candidates and our ability to operate our business without infringing the intellectual property rights of others; |
| • | our reliance on third parties to conduct our preclinical studies or any clinical trials; |
| • | our reliance on third-party suppliers and manufacturers to supply the materials and components for, manufacture, and research and develop our preclinical and clinical trial drug supplies; |
| • | our ability to attract and retain qualified key management and technical personnel; |
| • | our dependence on our existing collaborators, Boehringer Ingelheim International GmbH, Alexion Pharmaceuticals, Inc., and Eli Lilly and Company, for developing, obtaining regulatory approval for, and commercializing product candidates in the collaborations; |
| • | our receipt and timing of any potential milestone payments or royalties under our existing research collaboration and license agreements or any future arrangements with our existing collaboration partners or any other collaborators; |
| • | our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business Startups Act; |
| • | our financial performance; and |
| • | developments relating to our competitors or our industry. |
These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those set forth in Part II, Item 1A – “Risk Factors” below and for the reasons described elsewhere in this Quarterly Report on Form 10-Q. Any forward-looking statement in this Quarterly Report on Form 10-Q reflects our current view with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to our operations, results of operations, industry, and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
3
This Quarterly Report on Form 10-Q also contains estimates, projections, and other information concerning our industry, our business, and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates, and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections, or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by third parties, industry, medical and general publications, government data, and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
Except where the context otherwise requires, in this Quarterly Report on Form 10-Q, “we,” “us,” “our,” “Dicerna,” and the “Company” refer to Dicerna Pharmaceuticals, Inc. and, where appropriate, its consolidated subsidiaries.
Trademarks
This Quarterly Report on Form 10-Q includes trademarks, service marks, and trade names owned by us or other companies. All trademarks, service marks, and trade names included in this Quarterly Report on Form 10-Q are the property of their respective owners.
4
DICERNA PHARMACEUTICALS, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
(in thousands, except share data)
|
| September 30, 2018 |
|
| December 31, 2017 |
| ||
ASSETS |
|
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
| $ | 46,399 |
|
| $ | 68,789 |
|
Held-to-maturity investments |
|
| 133,980 |
|
|
| 44,889 |
|
Withholding tax receivable |
|
| — |
|
|
| 1,583 |
|
Prepaid expenses and other current assets |
|
| 3,107 |
|
|
| 3,415 |
|
Total current assets |
|
| 183,486 |
|
|
| 118,676 |
|
NONCURRENT ASSETS: |
|
|
|
|
|
|
|
|
Property and equipment, net |
|
| 1,212 |
|
|
| 1,512 |
|
Restricted cash equivalents |
|
| 744 |
|
|
| 744 |
|
Other noncurrent assets |
|
| 66 |
|
|
| 70 |
|
Total noncurrent assets |
|
| 2,022 |
|
|
| 2,326 |
|
TOTAL ASSETS |
| $ | 185,508 |
|
| $ | 121,002 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 3,595 |
|
| $ | 4,920 |
|
Accrued expenses and other current liabilities |
|
| 8,101 |
|
|
| 5,726 |
|
Litigation settlement payable |
|
| 12,797 |
|
|
| — |
|
Current portion of deferred revenue |
|
| 4,635 |
|
|
| 6,180 |
|
Total current liabilities |
|
| 29,128 |
|
|
| 16,826 |
|
NONCURRENT LIABILITIES: |
|
|
|
|
|
|
|
|
Deferred revenue, net of current portion |
|
| — |
|
|
| 3,090 |
|
Total noncurrent liabilities |
|
| — |
|
|
| 3,090 |
|
TOTAL LIABILITIES |
|
| 29,128 |
|
|
| 19,916 |
|
COMMITMENTS AND CONTINGENCIES |
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY: |
|
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value – 5,000,000 shares authorized; no shares issued or outstanding at September 30, 2018 or December 31, 2017 |
|
| — |
|
|
| — |
|
Common stock, $0.0001 par value – 150,000,000 shares authorized; 61,889,206 and 51,644,841 shares issued and outstanding at September 30, 2018 and December 31, 2017, respectively |
| 6 |
|
|
| 5 |
| |
Additional paid-in capital |
|
| 542,572 |
|
|
| 417,037 |
|
Accumulated deficit |
|
| (386,198 | ) |
|
| (315,956 | ) |
Total stockholders’ equity |
|
| 156,380 |
|
|
| 101,086 |
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
| $ | 185,508 |
|
| $ | 121,002 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
5
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
(in thousands, except share and per share data)
|
| Three Months Ended September 30, |
|
| Nine Months Ended September 30, |
| ||||||||||
|
| 2018 |
|
| 2017 |
|
| 2018 |
|
| 2017 |
| ||||
Revenue from collaborative arrangements |
| $ | 1,545 |
|
| $ | — |
|
| $ | 4,635 |
|
| $ | — |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 11,695 |
|
|
| 8,527 |
|
|
| 31,927 |
|
|
| 26,338 |
|
General and administrative |
|
| 5,354 |
|
|
| 4,137 |
|
|
| 14,449 |
|
|
| 12,324 |
|
Litigation expense |
|
| 3,694 |
|
|
| 2,548 |
|
|
| 29,122 |
|
|
| 6,157 |
|
Total operating expenses |
|
| 20,743 |
|
|
| 15,212 |
|
|
| 75,498 |
|
|
| 44,819 |
|
Loss from operations |
|
| (19,198 | ) |
|
| (15,212 | ) |
|
| (70,863 | ) |
|
| (44,819 | ) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
| 401 |
|
|
| 179 |
|
|
| 1,020 |
|
|
| 360 |
|
Interest expense |
|
| (223 | ) |
|
| — |
|
|
| (399 | ) |
|
| — |
|
Total other income, net |
|
| 178 |
|
|
| 179 |
|
|
| 621 |
|
|
| 360 |
|
Net loss |
|
| (19,020 | ) |
|
| (15,033 | ) |
|
| (70,242 | ) |
|
| (44,459 | ) |
Dividends on redeemable convertible preferred stock |
|
| — |
|
|
| (4,111 | ) |
|
| — |
|
|
| (6,733 | ) |
Deemed dividend related to beneficial conversion feature of redeemable convertible preferred stock |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (6,144 | ) |
Net loss attributable to common stockholders |
| $ | (19,020 | ) |
| $ | (19,144 | ) |
| $ | (70,242 | ) |
| $ | (57,336 | ) |
Net loss per share attributable to common stockholders – basic and diluted |
| $ | (0.35 | ) |
| $ | (0.92 | ) |
| $ | (1.32 | ) |
| $ | (2.76 | ) |
Weighted average common shares outstanding – basic and diluted |
|
| 54,799,644 |
|
|
| 20,841,728 |
|
|
| 53,037,516 |
|
|
| 20,809,372 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
6
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
(in thousands)
|
| Nine Months Ended September 30, |
| |||||
|
| 2018 |
|
| 2017 |
| ||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
Net loss |
| $ | (70,242 | ) |
| $ | (44,459 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Non-cash litigation expense |
|
| 10,315 |
|
|
| — |
|
Stock-based compensation expense |
|
| 5,673 |
|
|
| 6,003 |
|
Depreciation expense |
|
| 580 |
|
|
| 571 |
|
Loss on disposal of property and equipment |
|
| 9 |
|
|
| 51 |
|
Amortization of premium on investments |
|
| (390 | ) |
|
| (106 | ) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Litigation settlement payable |
|
| 12,797 |
|
|
| — |
|
Deferred revenue |
|
| (4,635 | ) |
|
| — |
|
Prepaid expenses and other assets |
|
| 312 |
|
|
| (1,963 | ) |
Accounts payable |
|
| (1,389 | ) |
|
| 1,114 |
|
Withholding tax receivable |
|
| 1,583 |
|
|
| — |
|
Accrued expenses and other liabilities |
|
| 2,045 |
|
|
| (584 | ) |
Net cash used in operating activities |
|
| (43,342 | ) |
|
| (39,373 | ) |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
| (245 | ) |
|
| (133 | ) |
Maturities of held-to-maturity investments |
|
| 50,000 |
|
|
| 45,000 |
|
Purchases of held-to-maturity investments |
|
| (138,699 | ) |
|
| (64,853 | ) |
Net cash used in investing activities |
|
| (88,944 | ) |
|
| (19,986 | ) |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock, net of underwriters' commissions |
|
| 108,099 |
|
|
| — |
|
Proceeds from issuance of redeemable convertible preferred stock |
|
| — |
|
|
| 70,000 |
|
Redeemable convertible preferred stock issuance costs |
|
| — |
|
|
| (750 | ) |
Proceeds from stock option exercises and issuances under Employee Stock Purchase Plan |
|
| 1,832 |
|
|
| 215 |
|
Settlement of restricted stock for tax withholding |
|
| (35 | ) |
|
| (11 | ) |
Net cash provided by financing activities |
|
| 109,896 |
|
|
| 69,454 |
|
NET (DECREASE) INCREASE IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH EQUIVALENTS |
|
| (22,390 | ) |
|
| 10,095 |
|
CASH, CASH EQUIVALENTS, AND RESTRICTED CASH EQUIVALENTS — Beginning of period |
|
| 69,533 |
|
|
| 21,981 |
|
CASH, CASH EQUIVALENTS, AND RESTRICTED CASH EQUIVALENTS — End of period |
| $ | 47,143 |
|
| $ | 32,076 |
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
NONCASH FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Dividends on redeemable convertible preferred stock |
| $ | — |
|
| $ | 6,733 |
|
Deemed dividend related to beneficial conversion feature of redeemable preferred stock |
| $ | — |
|
| $ | 6,144 |
|
Common stock issuance costs included in accounts payable and accrued expenses |
| $ | 349 |
|
| $ | — |
|
NONCASH INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
Property and equipment purchases included in accounts payable |
| $ | 44 |
|
| $ | — |
|
Reconciliation of cash, cash equivalents and restricted cash equivalents within the Company’s condensed consolidated balance sheets:
| Nine Months Ended September 30, |
| ||||||
|
| 2018 |
|
| 2017 |
| ||
Cash and cash equivalents |
|
| 46,399 |
|
|
| 30,960 |
|
Restricted cash equivalents |
|
| 744 |
|
|
| 1,116 |
|
Cash, cash equivalents and restricted cash equivalents presented above |
| $ | 47,143 |
|
| $ | 32,076 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
7
DICERNA PHARMACEUTICALS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
(tabular amounts in thousands, except share and per share data and where otherwise noted)
1. | DESCRIPTION OF BUSINESS AND BASIS OF PRESENTATION |
Business
Dicerna Pharmaceuticals, Inc. (“Dicerna” or the “Company”) is a biopharmaceutical company focused on the discovery and development of innovative subcutaneously delivered ribonucleic acid (“RNA”) interference (“RNAi”)-based pharmaceuticals using its GalXCTM RNAi platform for the treatment of diseases involving the liver, including rare diseases, viral infectious diseases, chronic liver diseases, and cardiovascular diseases. Within these therapeutic areas, the Company believes its GalXC RNAi platform will allow the Company to build a broad pipeline of therapeutics with commercially attractive pharmaceutical properties, including a subcutaneous route of administration, infrequent dosing (e.g., dosing that is monthly or quarterly, and potentially even less frequent), high therapeutic index, and specificity to a single target gene.
Basis of presentation
These condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and in accordance with the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial information. The year-end condensed balance sheet data was derived from audited financial statements, but does not include all disclosures required by GAAP to constitute a complete set of financial statements. These condensed consolidated financial statements have been prepared on the same basis as the Company’s annual consolidated financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the Company’s financial position at September 30, 2018 and results of operations and cash flows for the interim periods ended September 30, 2018 and 2017. These unaudited condensed consolidated interim financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2017. The results of the three and nine months ended September 30, 2018 are not necessarily indicative of the results to be expected for the year ending December 31, 2018 or for any other interim period or for any other future year.
Changes in stockholders’ equity for the nine months ended September 30, 2018 are as follows:
|
| Common Stock |
|
|
|
|
|
|
|
|
|
| Total |
| ||||||
Description |
| Number of shares |
|
| Amount |
|
| Additional Paid-in Capital |
|
| Accumulated Deficit |
|
| Stockholders' Equity |
| |||||
BALANCE – December 31, 2017 |
|
| 51,644,841 |
|
| $ | 5 |
|
| $ | 417,037 |
|
| $ | (315,956 | ) |
| $ | 101,086 |
|
Proceeds from issuance of common stock from public offering, net of underwriting fees and issuance costs |
|
| 8,832,565 |
|
|
| 1 |
|
|
| 107,749 |
|
|
| — |
|
|
| 107,750 |
|
Issuance of common stock to Alnylam Pharmaceuticals, Inc. |
|
| 983,208 |
|
|
| — |
|
|
| 10,315 |
|
|
| — |
|
|
| 10,315 |
|
Exercise of common stock options |
|
| 258,417 |
|
|
| — |
|
|
| 1,444 |
|
|
| — |
|
|
| 1,444 |
|
Sale of common stock related to Employee Stock Purchase Plan |
|
| 118,239 |
|
|
| — |
|
|
| 309 |
|
|
| — |
|
|
| 309 |
|
Exercise of warrants to common stock |
|
| 45,710 |
|
|
| — |
|
|
| 45 |
|
|
| — |
|
|
| 45 |
|
Settlement of restricted stock for stock withholding |
|
| 6,226 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| 5,673 |
|
|
| — |
|
|
| 5,673 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (70,242 | ) |
|
| (70,242 | ) |
BALANCE – September 30, 2018 |
|
| 61,889,206 |
|
| $ | 6 |
|
| $ | 542,572 |
|
| $ | (386,198 | ) |
| $ | 156,380 |
|
8
Significant judgments and estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the Company’s condensed consolidated financial statements, as well as the revenues and expenses incurred during the reporting periods. On an ongoing basis, the Company evaluates judgments and estimates, including those related to revenue recognition and accrued expenses. The Company bases its estimates on historical experience and on various other factors that the Company believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results could differ materially from those estimates.
Grants
The Company records income from various grants as an offset to research and development expenses.
Liquidity
Based on the Company’s current operating plan and liquidity, management believes that, subject to the closing of the transactions contemplated by the Collaboration and License Agreement (the “Lilly Collaboration Agreement”) between the Company and Eli Lilly and Company (“Lilly”) and the share issuance agreement (the “Lilly Share Issuance Agreement”) between the Company and Lilly, available cash, cash equivalents, and held-to-maturity investments will be sufficient to fund the Company’s planned level of operations beyond the year ended December 31, 2020. Subsequent to that period, if the Company is unable to generate funding from one or more sources within a reasonable timeframe, it may have to delay, reduce, or terminate its research and development programs, pre-clinical or clinical trials, limit strategic opportunities, or undergo reductions in its workforce or other corporate restructuring activities.
Summary of Significant Accounting Policies – There have been no changes to the significant accounting policies disclosed in the Company’s most recent Annual Report on Form 10-K, except as a result of adopting the Financial Accounting Standards Board (“FASB”)’s Accounting Standards Update (“ASU”) No. 2014-09, Revenue from Contracts with Customers (Topic 606), as discussed below.
Revenue recognition
The Company generates revenue from research collaboration and license agreements with third-party customers. Goods and services in the agreements typically include (i) the grant of licenses for the use of the Company’s technology and (ii) the provision of services associated with the research and development of customer product candidates. Such agreements may provide for consideration to the Company in the form of upfront payments, research and development services, option payments, milestone payments, and royalty payments on licensed products.
The Company accounts for a contract when the Company has approval and commitment from both parties, when the rights of the parties are identified, when payment terms are identified, when the contract has commercial substance, and when collectability of consideration is probable.
In determining the appropriate amount of revenue to be recognized as the Company fulfills its obligations under each of its agreements, management completes the following steps: (i) identifies the promised goods or services in the contract; (ii) determines whether the promised goods or services are performance obligations; (iii) measures the transaction price, including whether there are any constraints on variable consideration; (iv) allocates the transaction price to the performance obligations; and (v) recognizes revenue when (or as) the Company satisfies each performance obligation.
9
In order to account for its contracts with customers, the Company identifies the promised goods or services in the contract and evaluates whether such promised goods or services represent performance obligations. The Company accounts for those components as separate performance obligations when the following criteria are met:
| • | the customer can benefit from the good or service either on its own or together with other resources that are readily available to the customer, and |
| • | the Company’s promise to transfer the good or service to the customer is separately identifiable from other promises in the contract. |
This evaluation requires subjective determinations and requires the Company to make judgments about the promised goods and services and whether such goods and services are separable from the other aspects of the contractual relationship. In determining the performance obligations, the Company evaluates certain criteria, including whether the promised good or service is capable of being distinct and whether such good or service is distinct within the context of the contract, based on consideration of the relevant facts and circumstances for each arrangement. Factors considered in this determination include the research, manufacturing, and commercialization capabilities of the partner; the availability of research and manufacturing expertise in the general marketplace; and the level of integration, interrelation, and interdependence among the promises to transfer goods or services.
As part of the accounting for the relevant arrangements, the Company makes key assumptions that require judgment to determine the stand-alone selling price for each performance obligation identified in the underlying contract, which may include, as applicable, relevant market data, forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates, and probabilities of technical and regulatory success. The transaction price is allocated among the performance obligations using the relative selling price method, and the applicable revenue recognition criteria are applied to each of the separate performance obligations. At contract inception, the Company determines the standalone selling price for each performance obligation identified in the contract. If an observable price of the promised good or service sold separately is not readily available, the Company utilizes assumptions that require judgment to estimate the standalone selling price, which may include development timelines, probabilities of technical and regulatory success, reimbursement rates for personnel costs, forecasted revenues, potential limitations to the selling price of the product, expected technological life of the product, and discount rates.
Licenses of intellectual property: If a license granted to a customer to use the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from consideration allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company applies judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, to conclude upon the appropriate method of measuring progress for purposes of recognizing revenue related to consideration allocated to the performance obligation. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Milestone payments: At the inception of each contract with a customer that includes development or regulatory milestone payments, the Company evaluates whether the milestones are considered probable of being reached and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of the Company or of the licensee, such as regulatory approvals, are assessed as to the probability of achieving the related milestones. At the end of each subsequent reporting period, the Company re-evaluates the probability of achievement of all milestones and any related constraint, and, if necessary, adjusts the estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis and are recorded as revenue and through earnings in the period of adjustment.
Options: Customer options, such as options granted to allow a licensee to choose to research and develop product candidates against target genes to be identified in the future, generally do not provide a material right to the customer and therefore do not give rise to a separate performance obligation. As such, the additional consideration that would result from a customer exercising an option in the future is not included in the transaction price for the current contract. Instead, additional option fee payments are recognized or begin being recognized as revenue when the licensee exercises the options, and the exercise of the option would be treated as a separate contract for accounting purposes.
10
Research and development services: Arrangements that include a promise to provide research or development services at the licensee’s discretion are assessed to determine whether the services provide a material right to the licensee and are capable of being distinct, are not highly interdependent or do not significantly modify one another, and if so, the services are accounted for as separate performance obligations as the services are provided to the customer. Otherwise, when research or development services are determined not to be capable of being distinct or distinct within the context of the contract, those services are added to the performance obligation that includes the underlying license.
Royalties: For arrangements that include sales-based royalties, including commercial milestone payments based on the level of sales, and when the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any royalty revenue resulting from any out-licensing arrangement.
The Company receives payments from its licensees as established in each contract. Upfront payments and fees are recorded as deferred revenue upon receipt or when due and may require deferral of revenue recognition to a future period until the Company performs its obligations under these arrangements. Where applicable, amounts are recorded as contracts receivable when the Company’s right to consideration is unconditional. The Company does not assess whether a contract with a customer has a significant financing component if the expectation at contract inception is such that the period between payment by the licensees and the transfer of the promised goods or services to the licensees will be one year or less.
Recent Accounting Pronouncements
Adopted in 2018
Revenue recognition
In May 2014, the FASB issued Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”), which amends the guidance for accounting for revenue from contracts with customers, superseding the revenue recognition requirements in ASC 605, Revenue Recognition. ASC 606 is effective for annual reporting periods beginning after December 15, 2017. Under ASC 606, two adoption methods were allowed: retrospectively to all prior reporting periods presented, with certain practical expedients permitted, or retrospectively with the cumulative effect of initially adopting ASC 606 recognized at the date of initial application. The Company elected to apply ASC 606 retrospectively to all prior periods presented. Adoption of ASC 606 did not have a significant quantitative impact on the Company’s condensed consolidated financial statements; however, adoption of ASC 606 has resulted in additional revenue-related disclosures in the notes to the Company’s condensed consolidated financial statements, as discussed above and in Note 6 – Collaborative Research and License Agreement.
Statement of cash flows
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash (“ASU 2016-18”), a consensus of the FASB’s EITF. ASU 2016-18 requires that the statement of cash flows explain the change during the period in the total of cash and cash equivalents, including amounts generally described as restricted cash or restricted cash equivalents. Entities are required to reconcile such total to amounts on the balance sheet and disclose the nature of the restrictions. By requiring that the statement of cash flows explain the change during the period in the total of cash, cash equivalents and restricted cash, the new guidance eliminates current diversity in practice. The Company adopted ASU 2016-18 on January 1, 2018 and has applied this new guidance retrospectively to all periods presented. Consequently, transfers between restricted and unrestricted cash equivalents accounts are no longer reported as a cash flow in the Company’s condensed consolidated statement of cash flows.
Not yet adopted
Leases
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) (“ASU 2016-02”), which amends the existing accounting standards for lease accounting, including requiring lessees to recognize most leases on their balance sheets and making targeted changes to lessor accounting. ASU 2016-02 will be effective for the Company beginning in the first quarter of 2019. ASU 2016-02 requires a modified retrospective transition approach for all leases existing at, or entered into after, the date of initial application, with an option to use certain transition relief. In July 2018, the FASB issued ASU 2018-11, Leases (Topic 842): Targeted Improvements (“ASU 2018-11”), which allows entities to initially apply the new lease guidance at the adoption date and recognize a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. The Company is in the process of gathering a complete inventory of its lease contracts and evaluating the impact of the new guidance on its condensed consolidated financial statements and related disclosures; however, management expects that the adoption of ASU 2016-02 will result in the recognition of a
11
right of use asset and related liability associated with the Company’s non-cancelable operating lease arrangement for office and laboratory space that was executed in 2014 (see Note 7 – Commitments and Contingencies). The Company is in the process of determining whether it will utilize the optional transition method presented in ASU 2018-11.
2. | NET LOSS PER SHARE |
The Company computes basic net loss per common share by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding. In periods of net income, the Company’s accounting policy includes allocating a proportional share of net income to participating securities, as determined by dividing total weighted average participating securities by the sum of the total weighted average common shares and participating securities (the “two-class method”). The Company’s vested restricted shares participated in any dividends declared by the Company and were therefore considered to be participating securities. Participating securities have the effect of diluting both basic and diluted earnings per share during periods of income. During periods when the Company incurred a net loss, the Company did not allocate a loss to participating securities because they had no contractual obligation to share in the losses of the Company. The outstanding securities presented below were excluded from the calculation of net loss per share, because such securities would have been anti-dilutive due to the Company’s net loss per share during the periods ending on the dates presented.
|
| September 30, |
| |||||
| 2018 |
|
| 2017 |
| |||
Options to purchase common stock |
|
| 7,573,698 |
|
|
| 6,134,301 |
|
Warrants to purchase common stock |
|
| 2,198 |
|
|
| 87,901 |
|
Unvested restricted common stock |
|
| — |
|
|
| 10,000 |
|
Redeemable convertible preferred stock |
|
| — |
|
|
| 740,126 |
|
Total |
|
| 7,575,896 |
|
|
| 6,972,328 |
|
3. | HELD-TO-MATURITY INVESTMENTS |
The following tables provide information regarding the Company’s held-to-maturity investments.
|
| Amortized Cost |
|
| Gross Unrealized Gains |
|
| Gross Unrealized Losses |
|
| Fair Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Held-to-maturity investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. treasury securities maturing in one year or less |
| $ | 133,980 |
|
| $ | — |
|
| $ | (57 | ) |
| $ | 133,923 |
|
|
| Amortized Cost |
|
| Gross Unrealized Gains |
|
| Gross Unrealized Losses |
|
| Fair Value |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Held-to-maturity investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. treasury securities maturing in one year or less |
| $ | 44,889 |
|
| $ | — |
|
| $ | (30 | ) |
| $ | 44,859 |
|
The Company’s investment policy mandates that, at the time of purchase, the maturity of each investment within its portfolio shall not exceed 24 months. In addition, the weighted average maturity of the investment portfolio must not exceed 12 months.
12
During the three and nine months ended September 30, 2018, the Company granted stock options to purchase 175,500 and 1,936,850 shares, respectively, of common stock with aggregate grant date fair values of $1.7 million and $14.9 million, respectively, compared to stock options to purchase 304,500 and 1,635,497 shares of common stock granted with aggregate grant date fair values of $0.7 million and $3.5 million, for the three and nine months ended September 30, 2017, respectively.
The assumptions used to estimate the grant date fair value using the Black-Scholes option pricing model were as follows:
|
| Three Months Ended |
|
| Nine Months Ended |
|
|
| September 30, 2018 |
| |||
Common stock price |
| $12.74 - $15.74 |
|
| $9.14 - $15.74 |
|
Expected option term (in years) |
| 6.00 - 6.25 |
|
| 5.50 - 6.25 |
|
Expected volatility |
| 77.0% - 77.9% |
|
| 75.9% - 90.9% |
|
Risk-free interest rate |
| 2.78% - 2.90% |
|
| 2.32% - 2.90% |
|
Expected dividend yield |
| 0.00% |
|
| 0.00% |
|
|
| Three Months Ended |
|
| Nine Months Ended |
|
|
| September 30, 2017 |
| |||
Common stock price |
| $3.24 - $3.99 |
|
| $2.49 - $3.99 |
|
Expected option term (in years) |
| 5.50 - 6.25 |
|
| 5.50 - 6.25 |
|
Expected volatility |
| 79.6% - 90.8% |
|
| 79.4% - 90.8% |
|
Risk-free interest rate |
| 1.87% - 2.04% |
|
| 1.86% - 2.07% |
|
Expected dividend yield |
| 0.00% |
|
| 0.00% |
|
The Company has classified stock-based compensation in its condensed consolidated statements of operations as follows:
| Three Months Ended |
|
| Nine Months Ended |
|
| Three Months Ended |
|
| Nine Months Ended |
| |||||
|
| September 30, 2018 |
|
| September 30, 2017 |
| ||||||||||
Research and development expense |
| $ | 718 |
|
| $ | 2,275 |
|
| $ | 899 |
|
| $ | 2,816 |
|
General and administrative expenses |
|
| 1,430 |
|
|
| 3,398 |
|
|
| 1,071 |
|
|
| 3,187 |
|
Total |
| $ | 2,148 |
|
| $ | 5,673 |
|
| $ | 1,970 |
|
| $ | 6,003 |
|
5. | FAIR VALUE MEASUREMENTS |
A summary of the Company’s financial assets that are measured or disclosed at fair value on a recurring basis as of September 30, 2018 and December 31, 2017 are presented below.
| September 30, 2018 |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| |||||
Cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market fund |
| $ | 29,459 |
|
| $ | 29,459 |
|
| $ | — |
|
| $ | — |
|
Held-to-maturity investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. treasury securities |
|
| 133,923 |
|
|
| — |
|
|
| 133,923 |
|
|
| — |
|
Restricted cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market fund |
|
| 744 |
|
|
| — |
|
|
| 744 |
|
|
| — |
|
Total |
| $ | 164,126 |
|
| $ | 29,459 |
|
| $ | 134,667 |
|
| $ | — |
|
13
| December 31, 2017 |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| |||||
Cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market fund |
| $ | 51,441 |
|
| $ | 51,441 |
|
| $ | — |
|
| $ | — |
|
Held-to-maturity investments |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. treasury securities |
|
| 44,859 |
|
|
| — |
|
|
| 44,859 |
|
|
| — |
|
Restricted cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market fund |
|
| 744 |
|
|
| — |
|
|
| 744 |
|
|
| — |
|
Total |
| $ | 97,044 |
|
| $ | 51,441 |
|
| $ | 45,603 |
|
| $ | — |
|
The Company’s cash equivalents, which are in money market funds, are classified within Level 1 of the fair value hierarchy because they are valued using quoted prices as of September 30, 2018 and December 31, 2017.
The Company’s held-to-maturity investments and restricted cash equivalents bore interest at the prevailing market rates for instruments with similar characteristics and therefore approximated fair value. These financial instruments were classified within Level 2 of the fair value hierarchy because the inputs to the fair value measurement are valued using observable inputs as of September 30, 2018 and December 31, 2017.
As of September 30, 2018 and December 31, 2017, the Company’s accounts payable and accrued expenses approximated their estimated fair values because of the short-term nature of these financial instruments. As of December 31, 2017, the carrying amount of the withholding tax receivable also approximated its estimated fair value due to the short-term nature of the instrument.
The Company has a cash obligation of $13.0 million payable to Alnylam Pharmaceuticals, Inc. (“Alnylam”). Upon receipt of certain upfront cash payments owed to the Company resulting from signing the Alexion Pharmaceuticals, Inc. (“Alexion”) and Lilly agreements in October 2018, the Company anticipates that the cash obligation will be payable in the fourth quarter of 2018, and has therefore adjusted the liability equal to the estimated present value of the obligation of $12.8 million and included the obligation in current liabilities. As the present value was determined using market rates based on the nature of the obligation and the Company’s creditworthiness, the carrying value approximates the fair value. There was no liability recorded related to the settlement as of December 31, 2017.
The Company’s policy is to recognize transfers between levels of the fair value hierarchy, if any, at the end of the reporting period; however, there have been no such transfers during any of the periods presented.
6. | COLLABORATIVE RESEARCH AND LICENSE AGREEMENT |
On October 27, 2017, the Company entered into a collaborative research and license agreement (the “BI Agreement”) with Boehringer Ingelheim International GmbH (“BI”), pursuant to which the Company and BI jointly research and develop product candidates for the treatment of chronic liver disease using the GalXC platform, Dicerna’s proprietary RNAi-based technology. The BI Agreement is for the development of product candidates against one target gene with an option for BI to add the development of product candidates that target a second gene. Pursuant to the BI Agreement, Dicerna granted BI a worldwide license in connection with the research and development of such product candidates and has transferred to BI certain intellectual property rights of the product candidates selected by BI for clinical development and commercialization. Dicerna also may provide assistance to BI in order to help BI further develop selected product candidates. Under the terms of the BI Agreement, BI agreed to pay Dicerna a non-refundable upfront payment of $10.0 million for the first target, less a refundable withholding tax in Germany of $1.6 million. The German withholding tax was withheld by BI and remitted to the German tax authorities in accordance with local tax law; the Company received reimbursement of this tax in July 2018.
During the term of the research program, BI will reimburse Dicerna the cost of certain materials and third-party expenses that have been included in the preclinical studies. The Company is eligible to receive up to $191.0 million in potential development and commercial milestones related to the initial target. Dicerna is also eligible to receive royalty payments on potential global net sales, subject to certain adjustments, tiered from high single digits up to low double digits. BI’s option to add a second target would provide for an option fee payment and success-based development and commercialization milestones and royalty payments to Dicerna.
14
Milestone payments that are contingent upon the Company’s performance under the BI Agreement include potential developmental milestones totaling $99.0 million, including milestones for the first commercial sale. The Company has excluded the amounts from allocable consideration at the outset of the arrangement, as described below. All potential net sales milestones, totaling $95.0 million, will be accounted for in the same manner as royalties and recorded as revenue at the later of the achievement of the milestone or the satisfaction of the performance obligation.
The Company assessed the BI Agreement in accordance with ASC 606 and concluded that BI is a customer. The Company identified the following performance obligations under the contract: the license of intellectual property and conducting agreed-upon research program services. The Company has concluded that the license and research and development services do not have standalone value and are not capable of being distinct; therefore, the Company considers these to be one performance obligation. The Company concluded the option underlying the transfer of future licenses and potential associated research for any not-yet-known target gene is not a performance obligation of the contract at inception because the option fee reflects the standalone selling price of the option, and therefore, the option is not considered to be a material right. The Company considered the level of BI’s therapeutic expertise specifically related to RNAi, as well as BI’s know-how vis-à-vis the Company’s GalXC conjugates, and concluded that BI cannot currently benefit from the granted license on its own or together with other resources that are readily available to BI, including relationships with oligonucleotide vendors who synthesize GalXC conjugates under contract with the Company. As a result, the combination of the license of intellectual property together with the provision of research and development support services together represent the highest level of goods and services that can be deemed distinct.
Based on management’s evaluation, the non-refundable upfront fee and the agreed-upon reimbursable third-party expenses constituted the amount of the consideration to be included in the transaction price and has been allocated to the performance obligation identified based on the Company’s best estimate of the relative standalone selling price via application of a market assessment approach. None of the development milestones have been included in the transaction price, since none of such milestone amounts are within the control of the Company and are not considered probable to occur until confirmed by BI, at BI’s sole discretion. Any consideration related to commercial sales-based milestones (including royalties) will be recognized when the related sales occur, since these amounts have been determined to relate predominantly to the license granted to BI and therefore are recognized at the later of when the performance obligation is satisfied or the related sales occur. The Company will re-evaluate the transaction price in each reporting period and as uncertain events are resolved or other changes in circumstances occur.
The $10.3 million transaction price is being recognized over the current research term, which is estimated to extend through June 30, 2019, which represents the Company’s best estimate of the period of the obligation to provide research support services to BI, and is the expected period over which the Company estimates the deferred revenue balance will be recognized in revenue. Related revenue is being recognized on a straight-line basis, which is in management’s judgment an appropriate measure of progress toward satisfying the performance obligation, largely in absence of evidence that obligations are fulfilled in a specific pattern.
The following table presents changes in the Company’s deferred revenue accounts during the nine months ended September 30, 2018.
| Balance at beginning of period |
|
| Additions |
|
| Deductions |
|
| Balance at end of period |
| |||||
Current portion of deferred revenue |
| $ | 6,180 |
|
| $ | — |
|
| $ | (1,545 | ) |
| $ | 4,635 |
|
Deferred revenue, net of current portion |
| $ | 3,090 |
|
| $ | — |
|
| $ | (3,090 | ) |
| $ | — |
|
The Company recognized revenues of $1.5 million and $4.6 million for the three and nine months ended September 30, 2018, as a result of changes in the deferred revenue balances. There was no activity related to the Company’s deferred revenue accounts during the three and nine months ended September 30, 2017.
15
Facility lease
Future minimum lease payments on the Company’s non-cancelable operating lease for office and laboratory space are as follows:
* | The end of the lease term is November 30, 2020. |
Litigation
On June 10, 2015, Alnylam filed a complaint against the Company in the Superior Court of Middlesex County, Massachusetts (the “Court”). The complaint alleged misappropriation of confidential, proprietary, and trade secret information, as well as other related claims, in connection with the Company’s hiring of a number of former employees of Merck & Co., Inc. (“Merck”) and its discussions with Merck regarding the acquisition of its subsidiary, Sirna Therapeutics, Inc., which was subsequently acquired by Alnylam.
On April 18, 2018, the Company and Alnylam entered into a Confidential Settlement Agreement and General Release (the “Settlement Agreement”), resolving all ongoing litigation between the Company and Alnylam. The terms of the Settlement Agreement include mutual releases and dismissals with prejudice of all claims and counterclaims in the following litigation between the parties: (i) Alnylam Pharmaceuticals, Inc. v. Dicerna Pharmaceuticals, Inc., No. 15-4126 pending in the Massachusetts Superior Court for Middlesex County and (ii) Dicerna Pharmaceuticals, Inc., v. Alnylam Pharmaceuticals, Inc. No.1:17-cv-11466 pending in the United States District Court for the District of Massachusetts. Pursuant to the terms of the Settlement Agreement, the Company has agreed to make the following payments to Alnylam: (i) a $2.0 million upfront payment in cash, which the Company made in May 2018; (ii) an additional $13.0 million in cash to be paid as 10% of any upfront or first year cash consideration that the Company receives pursuant to future collaborations related to Ga1NAc-conjugated RNAi research and development (excluding any amounts received or to be received by the Company from its existing collaboration with BI), provided that the $13.0 million must be paid by no later than April 28, 2022; and (iii) issuance of shares of the Company’s common stock (the “Shares”) pursuant to a share issuance agreement between the parties (the “Share Issuance Agreement”).
Under the Settlement Agreement, for periods ranging from 18 months up to four years, the Company will be restricted in its development and other activities relating to oligonucleotide-based therapeutics directed toward a defined set of eight Alnylam targets (the “Oligo Restrictions”). The Oligo Restrictions pertain to targets where Dicerna does not have, or does not currently intend to have, a therapeutic program, or are expected to be consistent with Dicerna’s execution on programs in the normal course of business. The Settlement Agreement does not include any admission of liability or wrongdoing by either party or any licenses to any intellectual property from either party.
On April 20, 2018, the Company and Alnylam entered into the Share Issuance Agreement, pursuant to which the Company agreed to issue to Alnylam 983,208 Shares in satisfaction of the Company’s obligation under the Settlement Agreement to deliver Shares to Alnylam. The Share Issuance Agreement contains customary representations and warranties of each party. Pursuant to the terms of the Share Issuance Agreement, Alnylam may not, without the prior approval of the Company, dispose of any of the Shares for a six-month period commencing on the closing date of the Share issuance. Thereafter, through the fifth anniversary of the closing date of the Share issuance, Alnylam will only dispose of the maximum number of Shares that it would be permitted to dispose if the Shares were subject to the volume restrictions set forth in Rule 144(e) of the Securities Act of 1933, as amended.
16
The Company paid the upfront payment of $2.0 million dollars in May 2018 and recorded the cash obligation of $13.0 million as a liability discounted to the estimated present value of $8.7 million at an effective interest rate of 10.0%. Ten percent of any first year or upfront consideration or proceeds paid to the Company arising from any new collaboration agreements are payable to Alnylam until the cash obligation is satisfied. If the entire obligation is not paid by the fourth-year anniversary of the agreement, the balance is payable to Alnylam in cash at that time. Upon signing the agreement, the Company recorded a liability equal to the estimated present value of the obligation at that time. The Company applies the effective interest method, as the present value is accreted through maturity. As of September 30, 2018, $0.4 million of interest expense had been recorded on this liability. Upon receipt of certain upfront cash payments owed to the Company resulting from signing the Alexion and Lilly agreements in October 2018, the Company anticipates that the $13.0 million cash obligation will become payable in the fourth quarter of 2018. As a result, the estimated present value of $12.8 million, which was revised based on the expected timing of the remaining payments, is recorded in current liabilities. The impact of revising the expected timing of repayment is recorded as a component of litigation expense in the condensed consolidated statement of operations.
The 983,208 shares issued pursuant to the Share Issuance Agreement was recorded at fair market value of $10.3 million based on the Company’s closing share price on April 18, 2018, the date the Settlement Agreement was executed. The Company did not assign any value to the Oligo Restrictions as the Company did not incur additional losses or give up any value as a result of the restrictions.
Total litigation expense was $3.7 million and $29.1 million for the three and nine months ended September 30, 2018, respectively, all of which related to the litigation and settlement agreement with Alnylam. The litigation expense for the nine months ended September 30, 2018 includes $24.7 million related to the Settlement Agreement. The Company recorded litigation expenses, also related to the Alnylam litigation, of $2.5 million and $6.2 million for three and nine months ended September 30, 2017, respectively, which was previously included as a component of general and administrative expenses and recast to litigation expense for comparative purposes.
From time to time, the Company may be subject to various claims and legal proceedings. If the potential loss from any claim, asserted or unasserted, or legal proceeding is considered probable and the amount is reasonably estimable, the Company will accrue a liability for the estimated loss. There were no contingent liabilities recorded as of September 30, 2018 and December 31, 2017.
8. | SUBSEQUENT EVENTS |
Alexion
Alexion Collaborative Research and License Agreement
On October 22, 2018, the Company and Alexion Pharma Holding Unlimited Company (“Alexion Pharma Holding”), an affiliate of Alexion Pharmaceuticals, Inc. (“Alexion Pharmaceuticals” and together with Alexion Pharma Holding, “Alexion”) entered into a Collaborative Research and License Agreement (the “Alexion Collaboration Agreement”). The Alexion Collaboration Agreement is for the joint discovery and development of RNAi therapies for complement-mediated diseases. Under the terms of the Alexion Collaboration Agreement, the Company and Alexion will collaborate on the discovery and development of subcutaneously delivered GalXC candidates, currently in pre-clinical development, for the treatment of complement-mediated diseases with potential global commercialization by Alexion. The Company will lead the joint discovery and research efforts through the pre-clinical stage, and Alexion will lead development efforts beginning with Phase 1 studies. The Company will be responsible for manufacturing of the GalXC candidates through the completion of Phase 1, the costs of which will be paid by Alexion. Alexion will be solely responsible for the manufacturing of any product candidate subsequent to the completion of Phase 1. The Alexion Collaboration Agreement provides Alexion with exclusive worldwide licenses as well as development and commercial rights for two of the Company’s pre-clinical, subcutaneously delivered GalXC RNAi candidates and an exclusive option for the discovery and development of GalXC RNAi candidates against two additional complement pathway targets.
Under the terms of the Alexion Collaboration Agreement, Alexion will pay the Company a non-refundable, non-reimbursable, and non-creditable upfront payment of $22.0 million, with Alexion Pharmaceuticals making a concurrent $15.0 million equity investment at a premium in Dicerna pursuant to a share issuance agreement between the Company and Alexion Pharmaceuticals (the “Alexion Share Issuance Agreement”). The Alexion Collaboration Agreement also provides for potential additional payments to the Company of up to $600.0 million, which is comprised of option exercise fees of up to $20.0 million, representing $10.0 million for each of the candidates selected; development milestones of up to $105.0 million for each product; and aggregate sales milestones of up to $160.0 million. Under the agreement, Alexion will also pay to the Company mid-single to low-double digit royalties on potential product sales on a country-by-country, product-by-product basis until the later of the expiration of patent rights in a country, the expiration of market or regulatory exclusivity in such country, or 10 years after the first product sale in such country, subject to certain royalty step-down provisions set forth in the agreement.
17
The Alexion Collaboration Agreement includes various representations, warranties, covenants, indemnities, and other customary provisions. Alexion may terminate the Alexion Collaboration Agreement at any time without cause following a 90-day notice period.
Alexion Share Issuance Agreement
In connection with the Alexion Collaboration Agreement, the Company and Alexion entered into the Alexion Share Issuance Agreement on October 22, 2018, pursuant to which the Company agreed to issue to Alexion 835,834 shares of the Company’s common stock at a purchase price of $17.95 per share for an aggregate purchase price of approximately $15.0 million.
Pursuant to the terms of the Alexion Share Issuance Agreement, Alexion may not, without the prior approval of the Company, dispose of any of the Alexion Shares for a six-month period of time commencing on the closing date of the Alexion Share issuance.
Lilly
Lilly Collaboration and License Agreement
On October 25, 2018, the Company and Lilly entered into a Collaboration and License Agreement the Lilly Collaboration Agreement. The Lilly Collaboration Agreement is for the discovery, development, and commercialization of potential new medicines in the areas of cardio-metabolic disease, neurodegeneration, and pain. Under the terms of the Lilly Collaboration Agreement, the Company and Lilly will seek to use the Company’s proprietary GalXC RNAi technology platform to progress new drug targets toward clinical development and commercialization. In addition, the Company and Lilly will collaborate to extend the GalXC RNAi platform technology to non-liver tissues, including neural tissues.
The Lilly Collaboration Agreement provides that the Company will work exclusively with Lilly in the neurodegeneration and pain fields with the exception of mutually agreed upon orphan indications. Additionally, the Company will work exclusively with Lilly on select targets in the cardio-metabolic field. Under the Lilly Collaboration Agreement, the Company will provide Lilly with exclusive and non-exclusive licenses to support the companies’ activities and to enable Lilly to commercialize products derived from or containing compounds developed pursuant to such agreement. The Lilly Collaboration Agreement contemplates in excess of 10 targets.
Under the terms of the Lilly Collaboration Agreement, Lilly will pay the Company a non-refundable, non-creditable upfront payment of $100.0 million, with Lilly making a concurrent $100.0 million equity investment in the Company pursuant to the Lilly Share Issuance Agreement. Under the Lilly Collaboration Agreement, the Company is also eligible to potentially receive up to approximately $350.0 million per target in development and commercialization milestones, in addition to a $5.0 million payment due when the first non-hepatocyte target achieves proof of principle. In addition, the Lilly Collaboration Agreement also provides that Lilly will pay to the Company mid-single to low-double digit royalties on potential product sales on a country-by-country and product-by-product basis until the later of expiration of patent rights in a country, the expiration of data or regulatory exclusivity in such country, or 10 years after the first product sale in such country, subject to certain royalty step-down provisions set forth in the agreement.
The Lilly Collaboration Agreement includes various representations, warranties, covenants, indemnities, and other customary provisions. Lilly may terminate the Lilly Collaboration Agreement at any time without cause following a 90-day notice period. The Lilly Collaboration Agreement is subject to clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”), and other customary closing conditions.
18
Lilly Share Issuance Agreement
In connection with the Lilly Collaboration Agreement, the Company and Lilly entered into the Lilly Share Issuance Agreement on October 25, 2018, pursuant to which the Company agreed to issue to Lilly 5,414,185 shares of the Company’s common stock at a purchase price of $18.47 per share, for an aggregate purchase price of approximately $100.0 million. The issuance of shares of common stock under the Lilly Share Issuance Agreement is subject to clearance under the HSR Act and other customary closing conditions. The Lilly Share Issuance Agreement contains customary representations, warranties, and covenants of each party. The Lilly Share Issuance Agreement will automatically terminate if the Lilly Collaboration Agreement is terminated prior to the closing of the transactions contemplated by the Lilly Share Issuance Agreement.
Pursuant to the terms of the Lilly Share Issuance Agreement, Lilly may not, without the prior approval of the Company or except in the case of a third party tender offer, dispose of any of the Lilly Shares for a nine-month period of time commencing on the closing date of the Lilly Share issuance.
Alnylam
As a result of the recently executed partnership agreements with Alexion and Lilly, the Company believes that its cash obligation of $13.0 million payable to Alnylam will become due and that it will make this payment during the fourth quarter of 2018. As a result, the estimated present value of $12.8 million, which was revised based on the expected timing of the remaining payments, is recorded in current liabilities. The impact of revising the expected timing of repayment was recorded as a $3.7 million charge to litigation expense in the condensed consolidated statement of operations for three and nine months ended September 30, 2018.
19
The following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed here. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in this section as well as factors described in Part II, Item 1A – “Risk Factors.”
Overview
Dicerna Pharmaceuticals, Inc. (“we”, “the Company,” or “Dicerna”) is a biopharmaceutical company focused on the discovery and development of innovative subcutaneously delivered ribonucleic acid (“RNA”) interference (“RNAi”)-based pharmaceuticals using our GalXCTM RNAi platform for the treatment of diseases involving the liver, including rare diseases, viral infectious diseases, chronic liver diseases, and cardiovascular diseases. Within these therapeutic areas, we believe our GalXC RNAi platform will allow us to build a broad pipeline of therapeutics with commercially attractive pharmaceutical properties, including a subcutaneous route of administration, infrequent dosing (e.g., dosing that is monthly or quarterly, and potentially even less frequent), high therapeutic index, and specificity to a single target gene.
All of our GalXC drug discovery and development efforts are based on the therapeutic modality of RNAi, a highly potent and specific mechanism for silencing the activity of a targeted gene. In this naturally occurring biological process, double-stranded RNA molecules induce the enzymatic destruction of the messenger ribonucleic acid (“mRNA”) of a target gene that contains sequences that are complementary to one strand of the therapeutic double-stranded RNA molecule. The Company’s approach is to design proprietary double-stranded RNA molecules that have the potential to engage the enzyme Dicer and initiate an RNAi process to silence a specific target gene. Our GalXC RNAi platform utilizes a particular structure of double-stranded RNA molecules configured for subcutaneous delivery to the liver. Due to the enzymatic nature of RNAi, a single GalXC molecule incorporated into the RNAi machinery can destroy hundreds or thousands of mRNAs from the targeted gene.
The GalXC RNAi platform supports Dicerna’s long-term strategy to retain, subject to the evaluation of potential licensing opportunities as they may arise, a full or substantial ownership stake and to invest internally in diseases with focused patient populations, such as certain rare diseases. We see such diseases as representing opportunities that carry a relatively higher probability of success, with genetically and molecularly defined disease markers, high unmet need, a limited number of Centers of Excellence to facilitate reaching these patients, and the potential for more rapid clinical development programs. For more complex diseases with multiple gene dysfunctions and larger patient populations, we plan to pursue collaborations that can provide the enhanced scale, resources, and commercial infrastructure required to maximize these prospects, such as our existing collaborative research and license agreements, as discussed below.
Development Programs
In choosing which development programs to internally advance, we apply scientific, clinical, and commercial criteria that we believe allow us to best leverage our GalXC RNAi platform and maximize value. The Company is focusing its efforts on three priority therapeutic programs that currently have a Clinical Trial Application (“CTA”) filed, Investigational New Drug application (“IND”) filed, or are in enabling studies in preparation to submit additional regulatory applications that will be necessary to initiate clinical trials. The Company is also focusing its efforts on a series of potential programs in the clinical candidate selection stage, or for which a provisional clinical candidate has been selected, that may be elevated into IND/CTA enabling studies in the future, either on our own or in collaboration with larger pharmaceutical companies. Our three priority programs are: DCR-PHXC for the treatment of primary hyperoxaluria (“PH”); DCR-HBVS for the treatment of chronic hepatitis B virus (“HBV”) infection; and a program for an undisclosed rare disease. Our potential programs include additional rare disease programs, a program for the treatment of hypercholesterolemia, and multiple programs targeting undisclosed targets in chronic liver diseases and cardiovascular diseases. In May 2018, the Company dosed the first PH patient with DCR-PHXC in the Group B portion of the Phase 1 clinical trial and received notice from the United States (“U.S.”) Food and Drug Administration (“FDA”) granting Orphan Drug Designation to DCR-PHXC for treatment of PH. In August 2018, the European Medicines Agency’s Committee for Orphan Medicinal Products (“COMP”) designated DCR-PHXC as an orphan medicinal product for the treatment of PH in the European Union (“EU”). We have received regulatory and ethical approvals for the PHYOX clinical trial in the United Kingdom, France, Netherlands, and Germany. We expect to submit requests for additional regulatory clearances necessary to commence clinical trials for our programs in 2018 and 2019.
20
The table below sets forth the state of development of our various GalXC RNAi platform product candidates as of November 5, 2018.
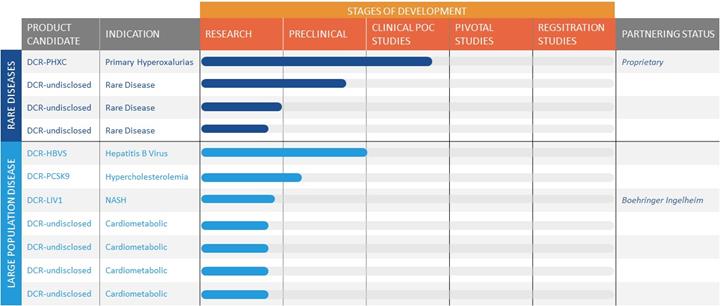
Our current GalXC RNAi platform development programs are as follows:
| • | Primary Hyperoxaluria. We are developing DCR-PHXC for the treatment of all types of PH. PH is a family of rare inborn errors of metabolism in which the liver produces excessive levels of oxalate, which in turn causes damage to the kidneys and other tissues in the body. DCR-PHXC is currently being investigated in a Phase 1 clinical trial called PHYOX. In non-clinical models of PH, DCR-PHXC reduces oxalate production to near-normal levels, ameliorating the disease condition. |
PHYOX is a Phase 1 single ascending-dose study of DCR-PHXC in normal healthy volunteers (“NHVs”) and study participants with PH. The study is divided into two groups: Group A is a placebo-controlled, single-blind, single center study, which has enrolled 25 NHVs; Group B is an open-label, multi-center study enrolling up to 18 participants with PH type 1 (“PH1”) or PH type 2 (“PH2”). The primary objective of the study is to evaluate the safety and tolerability of single doses of DCR-PHXC in both groups. The secondary objectives are to evaluate the pharmacodynamic effect of single doses of DCR-PHXC on biochemical markers, and to characterize the pharmacokinetics of single doses of DCR-PHXC in NHVs and study participants with PH. We have submitted CTAs for the PHYOX trial in the UK, France, Netherlands, and Germany and have received the required regulatory and ethical approvals. The FDA has accepted the Company’s IND and issued a “study may proceed” letter for the PHYOX trial. We have completed the Group A portion of the study in NHVs and started the Group B portion of the study. Group B consists of three cohorts of participants with PH1 dosed at 1.5, 3, and 6 mg/kg. An additional fourth cohort consists of participants with PH2 dosed at a flexible dosing level. We have enrolled 12 participants out of 18 (four PH1 participants in Cohort 1, four PH1 participants in Cohort 2, three PH1 participants in Cohort 3, and one PH2 participant in Cohort 4). We reported interim results from the PHYOX trial on September 5, 2018 and presented updated results (as of October 1, 2018) at Kidney Week in San Diego on October 25, 2018.
As of October 1, 2018, no severe or serious adverse events have occurred thus far in the PHYOX trial, and there have been no clinically significant changes in electrocardiography (“ECG”), vital signs, laboratory, or hematology values. The investigators have observed in a total of 27 participants dosed (Group A and B together) mild-to-moderate injection site reactions in 5 participants (19%), all of which were transient and resolved without intervention within 24 to 72 hours.
21
Investigators reported that a single 3.0-mg/kg dose of DCR-PHXC brought urinary oxalate levels into the normal range (defined as 24-hour excretion ≤0.46 mmol) at one or more post-dose time points in three out of four PH participants dosed at this level, including a mean maximal reduction in 24-hour urinary oxalate of 65% for the cohort, and a single 1.5-mg/kg dose led to near-normalization (defined as 24-hour excretion <0.6 and >0.46 mmol) in three out of four PH1 participants dosed at this level, and led to a mean maximal reduction in urinary oxalate of 50% in the five PH participants dosed at that level, including one PH2 participant.
Additionally, we intend to initiate a multi-dose study, which we hope will serve as a registration trial in the first quarter of 2019, pending regulatory feedback.
| • | Chronic Hepatitis B Virus infection. We have declared a GalXC RNAi platform-based product candidate for the treatment of HBV, DCR-HBVS, and are conducting formal non-clinical development studies. We filed a clinical trial application (“CTA”) with the New Zealand Medicines and Medical Devices Safety Authority and the Health and Disability Ethics Committee to initiate a phase 1 clinical trial in healthy volunteers and patients with chronic HBV. Pending CTA approval, we intend to initiate the clinical study in early 2019. We expect to file for regulatory clearance in Australia during the fourth quarter of 2018. Current therapies for HBV rarely lead to a long-term immunological cure as measured by the clearance of HBV surface antigen (“HBsAg”) and sustained HBV deoxyribonucleic acid (“DNA”) suppression in patient plasma or blood. DCR-HBVS targets HBV messenger RNA and leads to greater than 99% reduction in circulated HBsAg in mouse models of HBV infection. DCR-HBVS is comprised of a single GalXC molecule that targets HBV mRNAs within the HBsAg gene sequence region. In preclinical studies with a standard mouse model of HBV infection, we have found that targeting this region leads to superior HBsAg suppression, both in magnitude of suppression and duration of suppression, compared to targeting within the X gene sequence region. We believe that this difference in suppression derives from the role of the X gene product in indirectly regulating viral gene transcription such that the lack of X gene product leads to higher levels of viral gene transcription. Based on our preclinical studies, and only if we receive appropriate regulatory approval to begin human clinical trials, we hope to determine the potential of DCR-HBVS to reduce HBsAg and HBV DNA levels in the blood of HBV patients in a commercially attractive subcutaneous dosing paradigm. |
| • | An undisclosed rare disease involving the liver. We are developing a GalXC-based therapeutic, targeting a liver-expressed gene involved in a serious rare disease. For competitive reasons, we have not yet publicly disclosed the target gene or disease. We have selected this target gene and disease based on criteria that include having a strong therapeutic hypothesis, a readily-identifiable patient population, the availability of a potentially predictive biomarker, high unmet medical need, favorable competitive positioning, and what we believe is a rapid projected path to approval. The disease is a genetic disorder where mutations in the disease gene lead to the production of an abnormal protein. The protein causes progressive liver damage and fibrosis, in some cases leading to cirrhosis and liver failure, and we believe that silencing of the disease gene will prevent production of the abnormal protein and thereby slow or stop progression of the liver fibrosis. Greater than 100,000 people in the U.S. are believed to be homozygous (i.e., having identical pairs of genes for any given pair of hereditary characteristics) for the mutation that causes the liver disease, and at least 10% of those people, and potentially a significantly higher fraction, are believed to have liver-associated disease as a consequence. As our corporate resources have expanded, we are no longer seeking a risk-sharing collaborator for this program prior to entry into the clinic and have terminated collaboration discussions regarding the program. We intend to continue developing this program as a wholly-owned program and expect to submit regulatory filings to initiate clinical trials in the first half of 2019. |
22
| enabling the development of next generation GalXC molecules. Improvements to our GalXC compound include modification of the tetraloop end of the molecule, which can be applied to any target gene, resulting in a substantially longer duration of action and higher potency of target gene silencing in animal models across multiple targets. Modification of the tetraloop only impacts the passenger strand and does not impact the guide strand. These modifications are unique to our GalXC molecules and, we believe, provide a competitive advantage for the Company. |
In addition to the GalXC development programs outlined above, we are party to an agreement (the “BI Agreement”) with Boehringer Ingelheim International GmbH (“BI”), pursuant to which the Company and BI jointly research and develop product candidates for the treatment of chronic liver diseases, with an initial focus on nonalcoholic steatohepatitis (“NASH”) using our GalXC platform. NASH is caused by the buildup of fat in the liver, potentially leading to liver fibrosis and cirrhosis. NASH has an especially high prevalence among obese and diabetic patients and is an area of high unmet medical need. The BI Agreement is for the development of product candidates against one target gene with an option for BI to add the development of product candidates that target a second gene. We are working exclusively with BI to develop the product candidates against the undisclosed target gene. We are responsible for the discovery and initial profiling of the product candidates, including primary pre-clinical studies, synthesis, and delivery. BI is responsible for evaluating and selecting the product candidates for further development. If BI selects one or more product candidates, it will be responsible for further pre-clinical development, clinical development, manufacturing, and commercialization of those products. Also pursuant to the BI Agreement, we granted BI a worldwide license in connection with the research and development of the product candidates and have transferred to BI certain intellectual property rights of the product candidates selected by BI for clinical development and commercialization. We also may provide assistance to BI in order to help BI further develop selected product candidates. Pursuant to the BI Agreement, BI agreed to pay us a non-refundable upfront payment of $10.0 million for the first target. During the term of the research program, BI will reimburse us the cost of materials and third-party expenses that have been included in the preclinical studies up to an agreed-upon limit. We are eligible to receive up to $191.0 million in potential development and commercial milestones related to the initial target. We are also eligible to receive royalty payments on potential global net sales, subject to certain adjustments, tiered from high single digits up to low double digits. BI’s exercise of the option to add a second target, upon completion of a work plan, budget, and other activities, would provide for a $5.0 million option fee payment and provides for success-based development and commercialization milestones and royalty payments to us.
We have also developed a wholly-owned clinical candidate, DCR-BCAT, targeting the β-catenin oncogene. DCR-BCAT is based on an extended version of our earlier generation non-GalXC Dicer Substrate RNAi technology and is delivered by our lipid nanoparticle tumor delivery system, EnCoreTM. We plan to out-license, spin out, or seek external funding to advance the DCR-BCAT opportunity, given our focus on our GalXC platform-based programs.
Corporate Developments
On April 18, 2018, we entered into a Confidential Settlement Agreement and General Release (the “Settlement Agreement”) with Alnylam Pharmaceuticals, Inc. (“Alnylam”), resolving all ongoing litigation between the Company and Alnylam. The terms of the Settlement Agreement include mutual releases and dismissals with prejudice of all claims and counterclaims in the following litigation between the parties: (i) Alnylam Pharmaceuticals, Inc. v. Dicerna Pharmaceuticals, Inc., No. 15-4126 pending in the Massachusetts Superior Court for Middlesex County and (ii) Dicerna Pharmaceuticals, Inc., v. Alnylam Pharmaceuticals, Inc. No.1:17-cv-11466 pending in the United States District Court for the District of Massachusetts. Pursuant to the terms of the Settlement Agreement, we have agreed to make the following payments to Alnylam: (i) a $2.0 million upfront payment in cash, which we made in May 2018; (ii) an additional $13.0 million in cash, to be paid as 10% of any upfront or first year cash consideration that we receive pursuant to future collaborations related to Ga1NAc-conjugated RNAi research and development (excluding any amounts received or to be received by the Company from its existing collaboration with BI), provided that the $13.0 million must be paid by no later than April 28, 2022; and (iii) issuance of shares of our common stock (the “Shares”) pursuant to a share issuance agreement between the parties (the “Share Issuance Agreement”).
Under the Settlement Agreement, for periods ranging from 18 months up to four years, we will be restricted in our development and other activities relating to oligonucleotide-based therapeutics directed toward a defined set of eight Alnylam targets (the “Oligo Restrictions”). The Oligo Restrictions pertain to targets where Dicerna does not have, or does not currently intend to have, a therapeutic program, or are expected to be consistent with our execution on programs in the normal course of business. The Settlement Agreement does not include any admission of liability or wrongdoing by either party or any licenses to any other intellectual property from either party.
23
On April 20, 2018, we entered into the Share Issuance Agreement, pursuant to which we agreed to issue to Alnylam 983,208 Shares in satisfaction of our obligation under the Settlement Agreement to deliver Shares to Alnylam. The Share Issuance Agreement contains customary representations and warranties of each party. Pursuant to the terms of the Share Issuance Agreement, Alnylam may not, without our prior approval, dispose of any of the Shares for a six-month period commencing on the closing date of the Share issuance. Thereafter, through the fifth anniversary of the closing date of the Share issuance, Alnylam will only dispose of the maximum number of Shares that it would be permitted to dispose if the Shares were subject to the volume restrictions set forth in Rule 144(e) of the Securities Act of 1933, as amended.
On September 6, 2018, we entered into an underwriting agreement with Citigroup Global Markets Inc. and Leerink Partners LLC as representatives of the underwriters relating to the underwritten public offering of 7,680,492 shares of our common stock, par value, and the grant to the underwriters of a 30-day option to purchase up to an additional 1,152,073 shares of our common stock. We completed the sale of 8,832,565 shares to the underwriters on September 11, 2018, which resulted in net proceeds to us of approximately $107.7 million, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of our condensed consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements, as well as the revenue and expenses incurred during the reported periods. On an ongoing basis, we evaluate these estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ from these estimates under different assumptions or conditions.
The critical accounting policies that we believe impact significant judgments and estimates used in the preparation of our financial statements presented in this report are described in our Management’s Discussion and Analysis of Financial Condition and Results of Operations in our Annual Report on Form 10-K filed with the SEC on March 8, 2018. There have been no changes to our critical accounting policies during the three or nine months ended September 30, 2018 from those discussed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Critical Accounting Policies and Significant Judgments and Estimates” in our Annual Report on Form 10-K filed with the SEC on March 8, 2018, except as discussed below.
Recent Accounting Pronouncements
A summary of recent accounting pronouncements that have been adopted or are expected to be adopted by the Company is included in Note 1 – Description of Business and Basis of Presentation to our condensed consolidated financial statements (see Part I, Item 1 – “Financial Statements” of this Quarterly Report on Form 10-Q). Additional information regarding relevant accounting pronouncements is provided below.
Adopted in 2018
Revenue recognition
In May 2014, the accounting guidance related to revenue recognition was amended to replace current guidance with a single, comprehensive standard for accounting for revenue from contracts with customers. The new guidance became effective for us on January 1, 2018. The new revenue standard applies to all contracts with customers, and only contracts with customers are in the scope of the new revenue standard. Once a contractual arrangement is scoped into the new guidance, revenue is recognized based on a model that includes identifying performance obligations and determining and allocating the transaction price to the performance obligations identified in the contract. Revenue is recognized as those performance obligations are satisfied. We elected to apply this new guidance retrospectively to all prior periods presented, and adoption of this new guidance did not have a significant quantitative impact on our condensed consolidated financial statements; however, adoption of this guidance has resulted in additional revenue-related disclosures in the notes to our condensed consolidated financial statements.
24
In November 2016, new accounting guidance was issued related to the statement of cash flows implications related to restricted cash and cash equivalents. The guidance requires that the statement of cash flows explain the change during the period in the total of cash and cash equivalents, including amounts generally described as restricted cash or restricted cash equivalents. Entities are also required to reconcile such total to amounts on the balance sheet and disclose the nature of the restrictions. We applied this new guidance on January 1, 2018 and have made current and retrospective presentation adjustments such that transfers between restricted and unrestricted cash accounts are no longer reported as a cash flow in our condensed consolidated statement of cash flows.
Not yet adopted
Leases
In February 2016, accounting guidance related to leases was issued that will require an entity to recognize leased assets and the rights and obligations created by those leased assets on the balance sheet and to disclose key information about an entity’s leasing arrangements. This guidance will become effective for us on January 1, 2019, with early adoption permitted. We expect that the adoption of this guidance will impact our condensed consolidated financial statements and notes thereto, resulting, among other factors, from the recognition of a right of use asset and related liability related to our non-cancelable operating lease arrangement for our office and laboratory space in Cambridge, Massachusetts. In July 2018, additional guidance intending to provide some transition relief to the guidance issued in February 2016 was issued. As of September 30, 2018, and as presented in Note 7 – Commitments and Contingencies to our condensed consolidated financial statements (see Part 1, Item 1 – “Financial Statements” of this Quarterly Report on Form 10-Q), our total future minimum lease obligation associated with this lease was $3.7 million, and a substantial portion of this commitment will remain outstanding at the time we adopt the new guidance. Our evaluation of the new lease guidance and its full impact on our condensed consolidated financial statements will continue throughout 2018.
On October 25, 2018, we announced the presentation of late-breaking data from our ongoing PHYOX Phase 1 trial, in which single-dose administration of DCR-PHXC, our lead GalXCTM product candidate, was associated with normalization or near-normalization of urinary oxalate levels in a majority of adult patients with PH1 and PH2. In a poster presented at the American Society of Nephrology (“ASN”) Annual Kidney Week 2018 in San Diego, California, investigators reported that a single 3.0-mg/kg dose of DCR-PHXC brought urinary oxalate levels into the normal range (defined as 24-hour excretion ≤0.46 mmol) at one or more post-dose time points in three out of four PH participants dosed at this level, including a mean maximal reduction in 24-hour urinary oxalate of 65% for the cohort. Investigators also reported that a single 1.5-mg/kg dose led to near-normalization (defined as 24-hour excretion <0.6 and >0.46 mmol) in three out of four PH1 participants dosed at this level and led to a mean maximal reduction in urinary oxalate of 50% in the five patients dosed at that level, including one PH2 patient. All patients demonstrated a clinically significant reduction in urinary oxalate (defined as a >30% reduction compared to baseline).
In their poster presentation, which is based on data as of October 1, 2018, the PHYOX investigators also reported that DCR-PHXC is safe and well-tolerated in this ongoing study, based on data from 12 participants with PH1 (n=11) and PH2 (n=1) and 25 adult NHVs. The ASN presentation follows our announcement in September 2018 of preliminary proof-of-concept for DCR-PHXC, based on interim PHYOX data demonstrating substantial and clinically significant reductions in urinary oxalate in all assessed patients with PH. We plan to initiate a Phase 2/3 registration trial for DCR-PHXC in the first quarter of 2019, pending regulatory feedback.
We are investigating DCR-PHXC for the treatment of all forms of PH, a family of severe, rare, inherited disorders of the liver that often result in kidney failure. The Company initiated the PHYOX trial in NHVs in the fourth quarter of 2017 and dosed the first PH patient in May 2018.
The primary objective of the PHYOX trial (ClinicalTrials.gov: NCT03392896) is to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single-ascending doses of DCR-PHXC. Secondary endpoints include the change in 24-hour urinary oxalate excretion from baseline, defined as the mean of two 24-hour collections during screening. The trial is divided into two groups:
| • | Group A is a placebo-controlled, single-blind Phase 1 trial in 25 NHVs enrolled at a single site in the United Kingdom. |
| • | Group B is an open-label, multi-center trial of DCR-PHXC in 16 patients with PH, including three cohorts of patients with PH1 dosed at 1.5, 3.0, and 6.0-mg/kg, and a fourth PH2-only cohort with flexible dosing. Group B patients are enrolled at five sites in the EU and one site in the United States. |
25
In terms of safety, no severe or serious adverse events have occurred as of October 1, 2018, and there have been no clinically significant changes in ECG, vital signs, laboratory, or hematology values. Among the 27 participants dosed with DCR-PHXC in both Groups A and B, the investigators observed a total of five participants with mild-to-moderate injection site reactions (19%), all of which were transient and resolved without intervention within 24 to 72 hours.
Alexion
Alexion Collaborative Research and License Agreement
On October 22, 2018, we and Alexion Pharma Holding Unlimited Company (“Alexion Pharma Holding”), an affiliate of Alexion Pharmaceuticals, Inc. (“Alexion Pharmaceuticals” and together with Alexion Pharma Holding, “Alexion”) entered into a Collaborative Research and License Agreement (the “Alexion Collaboration Agreement”). The Alexion Collaboration Agreement is for the joint discovery and development of RNAi therapies for complement-mediated diseases. Under the terms of the Alexion Collaboration Agreement, we will collaborate with Alexion on the discovery and development of subcutaneously delivered GalXC candidates, currently in pre-clinical development, for the treatment of complement-mediated diseases with potential global commercialization by Alexion. We will lead the joint discovery and research efforts through the pre-clinical stage, and Alexion will lead development efforts beginning with Phase 1 studies. We will be responsible for manufacturing of the GalXC candidates through the completion of Phase 1, the costs of which will be paid by Alexion. Alexion will be solely responsible for the manufacturing of any product candidate subsequent to the completion of Phase 1. The Alexion Collaboration Agreement provides Alexion with exclusive worldwide licenses as well as development and commercial rights for two of the Company’s pre-clinical, subcutaneously delivered GalXC RNAi candidates and an exclusive option for the discovery and development of GalXC RNAi candidates against two additional complement pathway targets.
Under the terms of the Alexion Collaboration Agreement, Alexion will pay us a non-refundable, non-reimbursable, and non-creditable upfront payment of $22.0 million, with Alexion Pharmaceuticals making a concurrent $15.0 million equity investment of at a premium in Dicerna pursuant to a share issuance agreement between us and Alexion Pharmaceuticals (the “Alexion Share Issuance Agreement”). The Alexion Collaboration Agreement also provides for potential additional payments to us of up to $600.0 million, which is comprised of option exercise fees of up to $20.0 million, representing $10.0 million for each of the candidates selected; development milestones of up to $105.0 million for each product; and aggregate sales milestones of up to $160.0 million. Under the agreement, Alexion will also pay us mid-single to low-double digit royalties on potential product sales on a country-by-country, product-by-product basis until the later of the expiration of patent rights in a country, the expiration of market or regulatory exclusivity in such country, or 10 years after the first product sale in such country, subject to certain royalty step-down provisions set forth in the agreement.
Alexion Share Issuance Agreement
In connection with the Alexion Collaboration Agreement, we and Alexion entered into the Alexion Share Issuance Agreement on October 22, 2018, pursuant to which we agreed to issue to Alexion 835,834 shares of our common stock at a purchase price of $17.95 per share for an aggregate purchase price of approximately $15.0 million.
Lilly
Lilly Collaboration and License Agreement
On October 25, 2018, we and Eli Lilly and Company (“Lilly”) entered into a Collaboration and License Agreement (the “Lilly Collaboration Agreement”). The Lilly Collaboration Agreement is for the discovery, development, and commercialization of potential new medicines in the areas of cardio-metabolic disease, neurodegeneration, and pain. Under the terms of the Lilly Collaboration Agreement, we and Lilly will seek to use our proprietary GalXC RNAi technology platform to progress new drug targets toward clinical development and commercialization. In addition, we will collaborate with Lilly to extend the GalXC RNAi platform technology to non-liver tissues, including neural tissues.
The Lilly Collaboration Agreement provides that we will work exclusively with Lilly in the neurodegeneration and pain fields with the exception of mutually agreed upon orphan indications. Additionally, we will work exclusively with Lilly on select targets in the cardio-metabolic field. Under the Lilly Collaboration Agreement, we will provide Lilly with exclusive and non-exclusive licenses to support the companies’ activities and to enable Lilly to commercialize products derived from or containing compounds developed pursuant to such agreement. The Lilly Collaboration Agreement contemplates in excess of 10 targets.
26
Under the terms of the Lilly Collaboration Agreement, Lilly will pay us a non-refundable, non-creditable upfront payment of $100.0 million, with Lilly making a concurrent $100.0 million equity investment in us pursuant to a share issuance agreement between the parties (the “Lilly Share Issuance Agreement”). Under the Lilly Collaboration Agreement, we are also eligible to potentially receive up to approximately $350.0 million per target in development and commercialization milestones, in addition to a $5.0 million payment due when the first non-hepatocyte target achieves proof of principle. In addition, the Lilly Collaboration Agreement also provides that Lilly will pay us mid-single to low-double digit royalties on product sales on a country-by-country and product-by-product basis until the later of expiration of patent rights in a country, the expiration of data or regulatory exclusivity in such country, or 10 years after the first product sale in such country, subject to certain royalty step-down provisions set forth in the agreement.
The Lilly Collaboration Agreement includes various representations, warranties, covenants, indemnities, and other customary provisions. Lilly may terminate the Lilly Collaboration Agreement at any time without cause following a 90-day notice period. The Lilly Collaboration Agreement is subject to clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”), and other customary closing conditions.
Lilly Share Issuance Agreement
In connection with the Lilly Collaboration Agreement, the parties entered into the Lilly Share Issuance Agreement on October 25, 2018, pursuant to which we agreed to issue to Lilly 5,414,185 shares of our common stock at a purchase price of $18.47 per share, for an aggregate purchase price of approximately $100.0 million. The issuance of shares of common stock under the Lilly Share Issuance Agreement is subject to clearance under the HSR Act and other customary closing conditions.
Alnylam
As a result of the recently executed partnership agreements with Alexion and Lilly, we expect that our $13.0 million cash obligation payable to Alnylam will become due; we anticipate that we will make this payment during the fourth quarter of 2018. As a result, we recalculated the cash obligation to an estimated present value of $12.8 million, which was revised based on the expected timing of the remaining payments, and recorded it in current liabilities as of September 30, 2018. The impact of revising the expected timing of repayment is recorded as a component of litigation expense in the condensed consolidated statement of operations.
Financial Operations Overview
Comparison of the Three and Nine Months Ended September 30, 2018 and 2017
The following table summarizes the results of our operations for the periods indicated (amounts in thousands, except percentages).
|
| Three Months Ended September 30, |
|
| Increase/ |
|
| Nine Months Ended September 30, |
|
| Increase/ |
| ||||||||||||
|
| 2018 |
|
| 2017 |
|
| (Decrease) |
|
| 2018 |
|
| 2017 |
|
| (Decrease) |
| ||||||
Revenue from collaborative arrangements |
| $ | 1,545 |
|
|
| — |
|
| $ | 1,545 |
|
| $ | 4,635 |
|
|
| — |
|
| $ | 4,635 |
|
Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 11,695 |
|
|
| 8,527 |
|
|
| 3,168 |
|
|
| 31,927 |
|
|
| 26,338 |
|
|
| 5,589 |
|
General and administrative |
|
| 5,354 |
|
|
| 4,137 |
|
|
| 1,217 |
|
|
| 14,449 |
|
|
| 12,324 |
|
|
| 2,125 |
|
Litigation expense |
|
| 3,694 |
|
|
| 2,548 |
|
|
| 1,146 |
|
|
| 29,122 |
|
|
| 6,157 |
|
|
| 22,965 |
|
Total operating expenses |
|
| 20,743 |
|
|
| 15,212 |
|
|
| 5,531 |
|
|
| 75,498 |
|
|
| 44,819 |
|
|
| 30,679 |
|
Loss from operations |
|
| (19,198 | ) |
|
| (15,212 | ) |
|
| (3,986 | ) |
|
| (70,863 | ) |
|
| (44,819 | ) |
|
| (26,044 | ) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
| 401 |
|
|
| 179 |
|
|
| 222 |
|
|
| 1,020 |
|
|
| 360 |
|
|
| 660 |
| |
Interest expense |
|
| (223 | ) |
|
| — |
|
|
| (223 | ) |
|
| (399 | ) |
|
| (399 | ) |
|
| — |
|
Total other income, net |
| 178 |
|
|
| 179 |
|
|
| (1 | ) |
| 621 |
|
|
| (39 | ) |
|
| 660 |
| ||
Net loss |
|
| (19,020 | ) |
|
| (15,033 | ) |
|
| (3,987 | ) |
|
| (70,242 | ) |
|
| (44,858 | ) |
|
| (25,384 | ) |
Dividends on redeemable convertible preferred stock |
|
| — |
|
|
| (4,111 | ) |
|
| 4,111 |
|
|
| 4,111 |
|
|
| (6,733 | ) |
|
| 10,844 |
|
Deemed dividend related to beneficial conversion feature of redeemable convertible preferred stock |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (6,144 | ) |
|
| 6,144 |
|
Net loss attributable to common stockholders |
| $ | (19,020 | ) |
| $ | (19,144 | ) |
| $ | 124 |
|
| $ | (66,131 | ) |
| $ | (57,735 | ) |
| $ | (8,396 | ) |
27
Revenue from collaborative arrangements
Revenue recognized during the three and nine months ended September 30, 2018 represents the periodic amortization of a non-refundable upfront payment of $10.0 million for the first target and $0.3 million in reimbursable costs of certain materials and third-party expenses that have been included in pre-clinical studies pursuant to the BI Agreement, which was signed in the fourth quarter of 2017. We do not expect to generate any product revenue for the foreseeable future.
Research and development expenses
The following table summarizes our research and development expenses incurred during the periods indicated (amounts in thousands):
| Three Months Ended September 30, |
| ||||||||||
|
| 2018 |
|
| 2017 |
|
| Increase (Decrease) |
| |||
Direct research and development expenses |
| $ | 6,207 |
|
| $ | 3,463 |
|
| $ | 2,744 |
|
Platform-related expenses |
|
| 1,636 |
|
|
| 1,883 |
|
|
| (247 | ) |
Employee-related expenses |
|
| 3,018 |
|
|
| 2,439 |
|
|
| 579 |
|
Facilities, depreciation, and other expenses |
|
| 834 |
|
|
| 742 |
|
|
| 92 |
|
Total |
| $ | 11,695 |
|
| $ | 8,527 |
|
| $ | 3,168 |
|
| Nine Months Ended September 30, |
| ||||||||||
|
| 2018 |
|
| 2017 |
|
| Increase (Decrease) |
| |||
Direct research and development expenses |
| $ | 16,037 |
|
| $ | 10,775 |
|
| $ | 5,262 |
|
Platform-related expenses |
|
| 4,174 |
|
|
| 5,425 |
|
|
| (1,251 | ) |
Employee-related expenses |
|
| 9,274 |
|
|
| 7,788 |
|
|
| 1,486 |
|
Facilities, depreciation, and other expenses |
|
| 2,442 |
|
|
| 2,350 |
|
|
| 92 |
|
Total |
| $ | 31,927 |
|
| $ | 26,338 |
|
| $ | 5,589 |
|
Research and development expenses increased primarily due to direct research and development expenses for both the three and nine months ended September 30, 2018 compared to the three and nine months ended September 30, 2017. The $2.7 million increase in direct research and development expenses in the three months ended September 30, 2018 included a $2.1 million increase in clinical study costs, reflecting increased activities associated with our PHXC program, and a $0.4 million increase in consulting costs associated with various clinical development activities. The increase in research and development expenses for the three months ended September 30, 2018 includes a $0.6 million increase in employee-related expenses associated with increased headcount necessary to support our growth. Finally, the increase in research and development activities was partially offset by $0.2 million lower platform-related expenses.
The $5.3 million increase in direct research and development expenses in the nine months ended September 30, 2018 included a $2.9 million increase in clinical study costs due to increased activities associated with our PHXC program and a $1.4 million increase in toxicology studies for other product candidates. In addition, research and development expenses increased $1.5 million in the nine months ended September 30, 2018 as a result of employee-related expenses associated with increased headcount necessary to support our growth. Finally, the increase in research and development expenses in the nine months ended September 30, 2018 was partially offset by $1.3 million lower platform-related expenses.
Total research and development expenses for the three and nine months ended September 30, 2018 were additionally offset by $0.2 million and $0.6 million of grant income, respectively, while total research and development expenses for the three and nine months ended September 30, 2017 were offset by $0.5 million and $0.9 million of grant income, respectively.
We expect our overall research and development expenses to continue to increase during the fourth quarter of 2018 and for the foreseeable future, primarily as we complete clinical manufacturing activities, advance pre-clinical toxicology studies, continue clinical activities associated with our lead product candidates, and initiate activities under the recently signed Alexion and Lilly agreements.
28
General and administrative expenses
General and administrative expenses increased for the three and nine months ended September 30, 2018 compared to the three and nine months ended September 30, 2017. The increase in the three months ended September 30, 2018 is primarily related to increases in consulting costs of $0.4 million, board of directors’ compensation of $0.4 million, and $0.1 million of corporate legal expenses. The increase in the nine months ended September 30, 2018 is primarily related to increases in consulting costs of $0.7 million, board of directors’ compensation of $0.6 million, and $0.4 million of corporate legal expenses.
Our use of consultants increased largely due to accounting support for the implementation of new accounting standards and preparation for our planned compliance with Sarbanes-Oxley Section 404(b) in 2019, as well as to support new product and business development initiatives. The increase in board of directors’ compensation is largely related to stock-based compensation. The increase in corporate legal expenses is due to general corporate matters.
Litigation expenses
Litigation expenses are comprised solely of litigation and settlement expenses associated with the litigation with Alnylam. Litigation expenses increased predominantly due to $3.7 million and $24.7 million of settlement expenses recorded in the three and nine months ended September 30, 2018, respectively. We incurred $3.7 million of litigation settlement expense in the three months ended September 30, 2018 due to the acceleration of the previously disclosed $13.0 million payable to Alnylam resulting from the signing of the Lilly and Alexion collaboration agreements (see Note 8 – Subsequent Events). We also recorded $21.0 million of litigation settlement expense in the three months ended June 30, 2018 upon signing of the settlement agreement with Alnylam.
Interest income
Interest income primarily represents interest earned from our money market accounts and held-to-maturity investments. Interest income for the three and nine months ended September 30, 2018 increased to $0.4 million and $1.0 million, respectively, compared to $0.2 million and $0.4 million, respectively, for the three and nine months ended September 30, 2017. The increases were primarily due to higher held-to-maturity investments balances during the three and nine months ended September 30, 2018 resulting from our follow-on public offerings completed in December 2017 and September 2018.
Interest expense
Interest expense of $0.2 million and $0.4 million during the three and nine months ended September 30, 2018, respectively, represents interest expense incurred on our liability with Alnylam.
Dividends
There were no dividends recorded related to redeemable convertible preferred stock for the three and nine months ended September 30, 2018, as all shares of the redeemable convertible preferred stock were converted into shares of our common stock on December 18, 2017.
Net loss attributable to common stockholders
Net loss attributable to common stockholders for the three and nine months ended September 30, 2018 decreased to $19.0 million and increased to $70.2 million, respectively, compared to $19.1 million and $57.3 million for the three and nine months ended September 30, 2017, respectively. The decrease for the three months ended September 30, 2018 was driven by the $4.1 million dividend on the redeemable convertible preferred stock recorded in the three months ended September 30, 2017, offset by a $3.2 million increase in research and development and a $1.2 million increase in general and administrative expense. The increase in net loss attributable to common stockholders for the nine months ended September 30, 2018 primarily relates to a one-time increase of $23.0 million in litigation expense associated with the settlement of the Alnylam litigation, partially offset by the $12.9 million dividend on the redeemable convertible preferred stock in the nine months ended September 30, 2017.
29
Liquidity and Capital Resources
As of September 30, 2018, we had cash and cash equivalents and held-to-maturity investments of $180.4 million and $0.7 million in cash equivalents held in restriction.
On October 31, 2016, a universal shelf registration statement on Form S-3 permitting the sale of up to $150.0 million of our common stock and other securities was declared effective by the U.S. Securities and Exchange Commission (“SEC”). In December 2017, we sold an aggregate of 24,206,663 shares of our common stock, for gross proceeds of $46.0 million, pursuant to this registration statement.
On May 31, 2018, a universal shelf registration statement on Form S-3 permitting the sale of up to $250.0 million of our common stock and other securities was declared effective by the SEC. In September 2018, we sold an aggregate of 8,832,565 shares of our common stock for gross proceeds of $115.0 million pursuant to this registration statement. We currently intend to use the net proceeds from the offering for preclinical studies and clinical trials, and to use the remainder of any net proceeds for continued technology platform development, working capital, and general corporate purposes.
In October 2018, we entered into collaboration agreements with both Alexion and Lilly. Subject to the closing of the transaction contemplated by the Lilly Collaboration Agreement and the Lilly Share Issuance Agreement, the amount of cash generated from upfront payments and equity investments in both collaborations provides us with sufficient resources to continue our planned operations and clinical activities beyond the year ended December 31, 2020. As a result of the recently executed partnership agreements with Alexion and Lilly, we expect that our $13.0 million cash obligation payable pursuant to the Alnylam Settlement Agreement will become due; we will make this payment during the fourth quarter of 2018.
Cash flows
The following table shows a summary of our cash flows for the periods indicated (amounts in thousands):
|
| Nine Months Ended September 30, |
| |||||
| 2018 |
|
| 2017 |
| |||
Net cash used in operating activities |
| $ | (43,342 | ) |
| $ | (39,373 | ) |
Net cash used in investing activities |
|
| (88,944 | ) |
|
| (19,986 | ) |
Net cash provided by financing activities |
|
| 109,896 |
|
|
| 69,454 |
|
Increase in cash, cash equivalents and restricted cash equivalents |
| $ | (22,390 | ) |
| $ | 10,095 |
|
Operating activities
Net cash used in operating activities increased $3.6 million in the nine months ended September 30, 2018 compared to the nine months ended September 30, 2017 primarily due to an increase in operating expenses.
Investing activities
Net cash used in investing activities for the nine months ended September 30, 2018 increased compared to the nine months ended September 30, 2017. The increase of $69.0 million was due to a $73.8 million increase in purchases of held-to-maturity investments subsequent to our follow-on public offering in September 2018.
Financing activities
Net cash provided by financing activities for the nine months ended September 30, 2018 increased compared to the nine months ended September 30, 2017. The increase of $40.0 million was primarily due to receipt of $107.7 million in net proceeds in September 2018 from a follow-on public offering of our common stock compared with the proceeds from the redeemable convertible preferred stock financing of $69.3 million that occurred in the nine months ended September 30, 2017.
30
We expect that our primary uses of capital will continue to be third-party clinical research and development services and manufacturing costs, compensation and related expenses, laboratory and related supplies, legal and other regulatory expenses, and general overhead costs. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates and the extent to which we may enter into additional collaborations with third parties to participate in their development and commercialization, we are unable to estimate the amounts of capital outlays and operating expenditures associated with our anticipated development activities. However, based on our current operating plan, we believe that, subject to the closing of the transactions contemplated by the Lilly Collaboration Agreement and the Lilly Share Issuance Agreement, available cash, cash equivalents, and held-to-maturity investments, in addition to upfront cash and equity investments from the Alexion and Lilly agreements, will be sufficient to fund the execution of our current clinical and operating plans beyond the year ending December 31, 2020. We have based this estimate on assumptions that may prove to be incorrect, and we could utilize our available capital resources sooner than we currently expect.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially as a result of a number of factors. Our future capital requirements are difficult to forecast and will depend on many factors, including:
| • | the potential receipt of any milestone payments under the BI Agreement, the Alexion Collaboration Agreement, or the Lilly Collaboration Agreement; |
| • | the terms and timing of any other collaboration, licensing, and other arrangements that we may establish; |
| • | the initiation, progress, timing, and completion of pre-clinical studies and clinical trials for our potential product candidates; |
| • | the number and characteristics of product candidates that we pursue; |
| • | the outcome, timing, and cost of regulatory approvals; |
| • | delays that may be caused by changing regulatory requirements; |
| • | the cost and timing of hiring new employees to support our continued growth; |
| • | the costs involved in filing and prosecuting patent applications and enforcing and defending patent claims; |
| • | the costs of filing and prosecuting intellectual property rights and enforcing and defending any intellectual property-related claims; |
| • | the costs of responding to and defending ourselves against complaints and potential litigation; |
| • | the costs and timing of procuring clinical and commercial supplies for our product candidates; |
| • | the extent to which we acquire or in-license other product candidates and technologies; and |
| • | the extent to which we acquire or invest in other businesses, product candidates, or technologies. |
Until such time, if ever, that we generate product revenue, we expect to finance our future cash needs through a combination of public or private equity offerings, debt financings, and research collaboration and license agreements. We may be unable to raise capital or enter into such other arrangements when needed or on favorable terms, or at all. Our failure to raise capital or enter into such other arrangements in a reasonable timeframe would have a negative impact on our financial condition, and we may have to delay, reduce, or terminate our research and development programs, pre-clinical or clinical trials, limit strategic opportunities, or undergo reductions in our workforce or other corporate restructuring activities.
Please see the risk factors set forth in Part II, Item 1A – “Risk Factors” in this Quarterly Report on Form 10-Q for additional risks associated with our substantial capital requirements.
31
Contractual Obligations and Commitments
The following is a summary of our significant contractual obligation as of September 30, 2018 (amounts in thousands):
|
| Payments Due By Period |
| |||||||||||||||||
| Total |
|
| Less Than 1 Year |
|
| More Than 1 Year and Less Than 3 Years |
|
| More Than 3 Years and Less Than 5 Years |
|
| More Than 5 Years |
| ||||||
Operating lease obligation* |
| $ | 3,668 |
|
| $ | 1,666 |
|
| $ | 2,002 |
|
| $ | — |
|
| $ | — |
|
* | Represents future minimum lease payments under our existing non-cancelable operating lease for our office and laboratory space in Cambridge, Massachusetts. The end of the lease term is November 30, 2020. |
We also have obligations to make future payments to licensors that become due and payable on the achievement of certain development, regulatory, and commercial milestones. We have not included any such potential obligations on our condensed consolidated balance sheet or in the table above since the achievement and timing of these milestones were not probable or estimable as of September 30, 2018.
We recorded $12.8 million for the litigation settlement liability related to Alnylam on our condensed consolidated balance sheet as of September 30, 2018.
Off-Balance Sheet Arrangements
As of September 30, 2018 and December 31, 2017, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as “special purpose” entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. Other than our operating lease for our Company headquarters, we do not engage in off-balance sheet arrangements. Upon adoption of the new lease accounting standard on January 1, 2019, we anticipate that the requirement to capitalize all long-term leases will result in our existing operating lease being recorded on our condensed consolidated balance sheet.
32
The primary objectives of our investment activities are to ensure liquidity and to preserve principal while at the same time maximizing the income we receive from our marketable securities without significantly increasing risk. Some of the securities that we invest in may have market risk related to changes in interest rates. As of September 30, 2018, we had cash and cash equivalents and held-to-maturity investments of $180.4 million. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash and cash equivalents and held-to-maturity investments and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash and cash equivalents or held-to-maturity investments. To minimize the risk in the future, we intend to maintain our portfolio of cash and cash equivalents and held-to-maturity investments in a variety of securities, including commercial paper, money market funds, and government securities.
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file under the Securities Exchange Act of 1934, as amended, with the SEC is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
As of the end of the period covered by this Quarterly Report on Form 10-Q, we carried out an evaluation, under the supervision and with the participation of our management, including the chief executive officer and the chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15. Based upon, and as of the date of, this evaluation, the chief executive officer and the chief financial officer concluded that our disclosure controls and procedures were effective. Accordingly, management believes that the financial statements included in this report fairly present in all material respects our financial condition, results of operations, and cash flows for the periods presented.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting during the quarter ended September 30, 2018, which was identified in connection with management’s evaluation required by Exchange Act Rules 13a-15 and 15d-15 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on the Effectiveness of Controls
Our management, including the chief executive officer and chief financial officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of one person, by collusion of two or more people, or by management override of the control. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
33
From time to time we may be subject to legal proceedings, claims, and litigation arising in the ordinary course of business. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition, or results of operations.
We are providing the following cautionary discussion of risk factors, uncertainties, and assumptions that we believe are relevant to our business. These are factors that, individually or in the aggregate, we think could cause our actual results to differ materially from expected and historical results and our forward-looking statements. We note these factors for investors as permitted by Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider this section to be a complete discussion of all potential risks or uncertainties that may substantially impact our business. Moreover, we operate in a competitive and rapidly changing environment. New factors emerge from time to time and it is not possible to predict the impact of all of these factors on our business, financial condition, or results of operations.
Risks Related to Our Business
We will need to raise substantial additional funds to advance development of our product candidates, and we cannot guarantee that we will have sufficient funds available in the future to develop and commercialize our current or future product candidates.
We will need to raise substantial additional funds to expand our development, regulatory, manufacturing, marketing, and sales capabilities, whether internally or through other organizations. We have used substantial funds to develop our product candidates and delivery technologies and will require significant funds to conduct further research and development and preclinical testing and clinical trials of our product candidates, to seek regulatory approvals for our product candidates, and to manufacture and market products, if any are approved for commercial sale. As of September 30, 2018, we had $180.4 million in cash and cash equivalents and held-to-maturity investments. Based on our current operating plan and liquidity, we believe that, subject to the closing of the transactions contemplated by the Lilly Collaboration Agreement and the Lilly Share Issuance Agreement, our available cash, cash equivalents, and held-to-maturity investments will be sufficient to fund our planned level of operations beyond the year ended December 31, 2020. Our future capital requirements and the period for which we expect our existing resources to support our operations may vary significantly from what we expect. Our monthly spending levels vary based on new and ongoing development and corporate activities. Because the length of time and activities associated with successful development of our product candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. To execute our business plan, we will need, among other things:
| • | to obtain the human and financial resources necessary to develop, test, obtain regulatory approval for, manufacture, and market our product candidates; |
| • | to build and maintain a strong intellectual property portfolio and avoid infringing intellectual property of third parties; |
| • | to establish and maintain successful licenses, collaborations, and alliances; |
| • | to satisfy the requirements of clinical trial protocols, including patient enrollment; |
| • | to establish and demonstrate the clinical efficacy and safety of our product candidates; |
| • | to manage our spending as costs and expenses increase due to preclinical studies and clinical trials, regulatory approvals, manufacturing scale-up, and commercialization; |
| • | to obtain additional capital to support and expand our operations; and |
| • | to market our products to achieve acceptance and use by the medical community. |
If we are unable to obtain funding on a timely basis or on acceptable terms, we may have to delay, reduce, or terminate our research and development programs and preclinical studies or clinical trials, if any, limit strategic opportunities, or undergo reductions in our workforce or other corporate restructuring activities. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies or product candidates that we would otherwise pursue on our own. We do not expect to realize revenue from product sales or royalties in the foreseeable future, if at all, and milestone payments, if any, are based on third party determinations and/or events outside our control. Our revenue sources are, and will remain,
34
extremely limited unless and until our product candidates are clinically tested, approved for commercialization, and successfully marketed. To date, we have financed our operations primarily through the sale of securities, debt financings, and credit and loan facilities. We will be required to seek additional funding in the future and intend to do so through a combination of public or private equity offerings, debt financings, and research collaborations and license agreements. Our ability to raise additional funds will depend on financial, economic, and other factors, many of which are beyond our control. For example, a number of factors, including the timing and outcomes of our clinical activities, our status as a smaller reporting company under SEC regulations, as well as conditions in the global financial markets, may present significant challenges to accessing the capital markets at a time when we would like or require, and at an increased cost of capital. Additional funds may not be available to us on acceptable terms or at all. If we raise additional funds by issuing equity securities, our stockholders will suffer dilution, and the terms of any financing may adversely affect the rights of our stockholders. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. Debt financing, if available, may involve restrictive covenants limiting our flexibility in conducting future business activities, and, in the event of insolvency, debt holders would be repaid before holders of equity securities receive any distribution of corporate assets.
We are a biopharmaceutical company with a history of losses, expect to continue to incur significant losses for the foreseeable future and may never achieve or maintain profitability, which could result in a decline in the market value of our common stock.
We are a biopharmaceutical company with a limited operating history focused on the discovery and development of treatments based on the emerging therapeutic modality RNAi, a biological process in which RNA molecules inhibit gene expression. Since our inception in October 2006, we have devoted our resources to the development of RNAi molecules and delivery technologies. We have had significant operating losses since our inception. As of September 30, 2018, we had an accumulated deficit of $386.2 million. For the nine months ended September 30, 2018 and for the years ended December 31, 2017, 2016, and 2015, our net loss attributable to common stockholders was $70.2 million, $80.1 million, $59.5 million, and $62.8 million, respectively. Substantially all of our operating losses have resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations and litigation expenses associated with the Alnylam litigation settled in April 2018. Our technologies and product candidates are in early stages of development, and we are subject to the risks of failure inherent in the development of product candidates based on novel technologies.
We have not generated, and do not expect to generate, any revenue from product sales for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials, and the regulatory approval process for product candidates. The amount of future losses is uncertain. Our ability to achieve profitability, if ever, will depend on, among other things, us or our existing collaborators, or any future collaborators, successfully developing product candidates, obtaining regulatory approvals to market and commercialize product candidates, manufacturing any approved products on commercially reasonable terms, establishing a sales and marketing organization or suitable third-party alternatives for any approved product, and raising sufficient funds to finance business activities. If we or our existing collaborators, or any future collaborators, are unable to develop and commercialize one or more of our product candidates or if sales revenue from any product candidate that receives approval is insufficient, we will not achieve profitability, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
| • | variations in the level of expense related to our product candidates or future development programs; |
| • | results of clinical trials, or the addition or termination of clinical trials or funding support by us, our existing collaborators, or any future collaborator or licensor; |
| • | the timing of the release of results from any clinical trials conducted by us or our collaborator BI; |
| • | our execution of any collaboration, licensing, or similar arrangement, and the timing of payments we may make or receive under such existing or future arrangements or the termination or modification of any such existing or future arrangements; |
| • | any intellectual property infringement or misappropriation lawsuit or opposition, interference, re-examination, post-grant review, inter partes review, nullification, derivation action, or cancellation proceeding in which we may become involved; |
| • | additions and departures of key personnel; |
35
| • | strategic decisions by us and our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments, or changes in business strategy; |
| • | if any of our product candidates receive regulatory approval, market acceptance and demand for such product candidates; |
| • | if any of our third-party manufacturers fail to execute on our manufacturing requirements; |
| • | regulatory developments affecting our product candidates or those of our competitors; |
| • | disputes concerning patents, proprietary rights, or license and collaboration agreements that negatively impact our receipt of milestone payments or royalties or require us to make significant payments arising from licenses, settlements, adverse judgments, or ongoing royalties; |
| • | changes in general market and economic conditions; and |
| • | changes in tax laws. |
If our quarterly operating results fluctuate or fall below the expectations of investors or securities analysts, the price of our common stock could fluctuate or decline substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Our approach to the discovery and development of innovative therapeutic treatments based on novel technologies is unproven and may not result in marketable products.
We plan to develop subcutaneously delivered RNAi-based pharmaceuticals using our GalXC RNAi platform for the treatment of rare diseases involving the liver and for other therapeutic areas involving the liver such as chronic liver diseases, as well as cardiovascular diseases and viral infectious diseases. We believe that product candidates identified with our drug discovery and delivery platform may offer an improved therapeutic approach to small molecules and monoclonal antibodies, as well as several advantages over earlier generation RNAi molecules. However, the scientific research that forms the basis of our efforts to develop product candidates is relatively new. The scientific evidence to support the feasibility of developing therapeutic treatments based on RNAi and GalXC is both preliminary and limited.
Relatively few product candidates based on RNAi have been tested in animals or humans, and a number of clinical trials conducted by other companies using RNAi technologies have not been successful. We may discover that GalXC does not possess certain properties required for a drug to be safe and effective, such as the ability to remain stable in the human body for the period of time required for the drug to reach the target tissue or the ability to cross the cell wall and enter into cells within the target tissue for effective delivery. We currently have only limited data, and no conclusive evidence, to suggest that we can introduce these necessary drug-like properties into GalXC. We may spend substantial funds attempting to introduce these properties and may never succeed in doing so. In addition, product candidates based on GalXC may demonstrate different chemical and pharmacological properties in patients than they do in laboratory studies. Even if product candidates, such as DCR-PHXC, have successful results in animal studies, they may not demonstrate the same chemical and pharmacological properties in humans and may interact with human biological systems in unforeseen, ineffective, or harmful ways. As a result, we may never succeed in developing a marketable product, we may not become profitable, and the value of our common stock will decline.
Further, the FDA has relatively limited experience with RNAi or GalXC based therapeutics. We and our current collaborators, or any future collaborators, may never receive approval to market and commercialize any product candidate. Even if we or a collaborator obtain regulatory approval, the approval may be for disease indications or patient populations that are not as broad as we intended or desired or may require labeling that includes significant use or distribution restrictions or safety warnings. We or a collaborator may be required to perform additional or unanticipated clinical trials to obtain approval or be subject to post-marketing testing requirements to maintain regulatory approval. If our technologies based on GalXC prove to be ineffective, unsafe, or commercially unviable, our entire platform and pipeline would have little, if any, value, which would have a material adverse effect on our business, financial condition, results of operations, and prospects.
36
The market may not be receptive to our product candidates based on a novel therapeutic modality, and we may not generate any future revenue from the sale or licensing of product candidates.
Even if approval is obtained for a product candidate, we may not generate or sustain revenue from sales of the product due to numerous factors, including whether the product can be sold at a competitive price and otherwise is accepted in the market. The product candidates that we are developing are based on new technologies and therapeutic approaches. Market participants with significant influence over acceptance of new treatments, such as physicians and third-party payors, may not adopt a treatment based on GalXC technology, and we may not be able to convince the medical community and third-party payors, including health insurers, to accept and use, or to provide favorable coverage or reimbursement for, any product candidates developed by us or our existing collaborator or any future collaborators. Market acceptance of our product candidates will depend on, among other factors:
| • | the timing of our receipt of any marketing and commercialization approvals; |
| • | the terms of any approvals and the countries in which approvals are obtained; |
| • | the safety and efficacy of our product candidates; |
| • | the prevalence and severity of any adverse side effects associated with our product candidates; |
| • | limitations or warnings contained in any labeling approved by the FDA or other regulatory authority; |
| • | relative convenience and ease of administration of our product candidates; |
| • | the willingness of physicians and patients to accept any new methods of administration; |
| • | the success of our physician education programs; |
| • | the availability of adequate government and third-party payor coverage and reimbursement; |
| • | the pricing of our products, particularly as compared to alternative treatments; |
| • | our ability to compliantly market and sell our products; and |
| • | availability of alternative effective treatments for the disease indications our product candidates are intended to treat and the relative risks, benefits and costs of those treatments. |
With our focus on the emerging therapeutic modality RNAi, these risks may increase to the extent the market becomes more competitive or less favorable to this approach. Additional risks apply to any disease indications we pursue which are for rare diseases. Because of the small patient population for a rare disease, if pricing is not approved or accepted in the market at an appropriate level for an approved rare disease product, such drug may not generate enough revenue to offset costs of development, manufacturing, marketing, and commercialization, despite any benefits received from our efforts to obtain orphan drug designation by regulatory agencies in major commercial markets, such as the U.S., the European Union (“EU”), and Japan. These benefits may include market exclusivity, assistance in clinical trial design, or a reduction in user fees or tax credits related to development expense. Market size is also a variable in disease indications that are not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist if we ever get to the point of product commercialization, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations, and prospects.
If a product candidate that has orphan drug designation subsequently receives the first FDA approval for the indication for that designation, the product candidate is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same product candidate for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, could also block the approval of one of our product candidates for seven years if a competitor obtains approval of the same product candidate as defined by the FDA.
Even if we, or any future collaborators, obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective, makes a major contribution to patient care, or meets certain other criteria.
37
On August 3, 2017, the Congress passed the FDA Reauthorization Act of 2017, (“FDARA”). FDARA, among other things, codified the FDA’s preexisting regulatory interpretation, to require that a drug sponsor demonstrate the clinical superiority of an orphan drug that is otherwise the same as a previously approved drug for the same rare disease in order to receive orphan drug exclusivity. The law reverses prior precedent holding that the Orphan Drug Act unambiguously requires that the FDA recognize the orphan exclusivity period regardless of a showing of clinical superiority. The FDA may further reevaluate the Orphan Drug Act and its regulations and policies. We do not know if, when, or how the FDA may change the orphan drug regulations and policies in the future, and it is uncertain how any changes might affect our business. Depending on what changes the FDA may make to its orphan drug regulations and policies, our business could be adversely impacted.
As in the U.S., we may apply for designation of a product candidate as an orphan drug for the treatment of a specific indication in the EU before the application for marketing authorization is made. For example, in August 2018, the European Medicines Agency’s Committee for Orphan Medicinal Products designated DCR-PHXC as an orphan medicinal product for the treatment of PH in the EU. Sponsors of orphan drugs in the EU can enjoy economic and marketing benefits, including up to 10 years of market exclusivity for the approved indication. During such period, marketing authorization applications for a “similar medicinal product” will not be accepted, unless another applicant can show that its product is safer, more effective, or otherwise clinically superior to the orphan-designated product. In the EU, a “similar medicinal product” is a medicinal product containing a similar active substance or substances as contained in a currently authorized orphan medicinal product, and which is intended for the same therapeutic indication. The respective orphan designation and exclusivity frameworks in the U.S. and in the EU are subject to change, and any such changes may affect our ability to obtain U.S. or EU orphan designations in the future.
Our product candidates are in early stages of development and may fail or suffer delays that materially and adversely affect their commercial viability.
We have no products on the market and all of our product candidates are in early stages of development. Our ability to achieve and sustain profitability depends on obtaining regulatory approvals, including ethics committee approval to conduct clinical trials at particular sites, and successfully commercializing our product candidates, either alone or with third parties, such as our collaborator, BI. Before obtaining regulatory approval for the commercial distribution of our product candidates, we or a collaborator must conduct extensive preclinical and other non-clinical tests and clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Non-clinical testing and clinical trials are expensive, difficult to design and implement, can take many years to complete, and are uncertain as to outcome. The start or end of a clinical study is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparative drug or required prior therapy, clinical outcomes, and financial constraints. For instance, delays or difficulties in patient enrollment or difficulties in retaining trial participants can result in increased costs, longer development times, or termination of a clinical trial. Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the eligibility criteria for the clinical trial, the age and condition of the patients, the stage and severity of disease, the nature of the protocol, the proximity of patients to clinical sites, and the availability of effective treatments for the relevant disease.
A product candidate can unexpectedly fail at any stage of preclinical and clinical development. The historical failure rate for product candidates is high due to many factors, including scientific feasibility, safety, efficacy, and changing standards of medical care. The results from preclinical testing or early clinical trials of a product candidate may not predict the results that will be obtained in later phase clinical trials of the product candidate. We, the FDA, or other applicable regulatory authorities, an individual Institutional Review Board (“IRB”) with respect to its institution, or an independent ethics committee may suspend clinical trials of a product candidate at any time for various reasons, including a belief that individuals participating in such trials are being exposed to unacceptable health risks or adverse side effects. We may not have the financial resources to continue development of, or to enter into collaborations for, a product candidate if we experience any problems or other unforeseen events that delay or prevent regulatory approval of, or our ability to commercialize, product candidates, including:
| • | negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours, leading to a decision or requirement to conduct additional preclinical testing or clinical trials or abandon a program; |
| • | serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates; |
| • | delays in submitting INDs or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or IRBs to commence a clinical trial, or a suspension or termination of a clinical trial once commenced; |
| • | conditions imposed by the FDA or comparable foreign authorities, such as the European Medicines Agency (“EMA”), regarding the scope or design of our clinical trials; |
38
| • | high drop-out rates of study participants; |
| • | inadequate supply or quality of drug product or product candidate components or materials or other supplies necessary for the conduct of our clinical trials; |
| • | greater than anticipated clinical trial costs; |
| • | poor effectiveness of our product candidates during clinical trials; |
| • | unfavorable FDA or other regulatory agency inspection and review of a clinical trial site; |
| • | failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual obligations in a timely manner, or at all; |
| • | delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; and |
| • | varying interpretations of data by the FDA and foreign regulatory agencies. |
We are dependent on our collaboration partners for the successful development of product candidates in the collaboration.
We have entered into collaboration agreements with BI, Alexion, and Lilly providing for the joint development of certain RNAi therapies. The success of our collaborations with BI, Alexion, and Lilly and the realization of the milestone and royalty payments under the collaboration agreements depends upon the efforts of BI, Alexion, and Lilly, any of which may not be successful in obtaining approvals for the product candidates developed under the collaboration or in marketing, or arranging for necessary supply, manufacturing or distribution relationships for, any approved products. Our collaboration partners may change their strategic focus or pursue alternative technologies in a manner that results in reduced, delayed, or no additional payments to us under the collaboration agreements. BI, Alexion, and Lilly have a variety of marketed products and product candidates under collaboration with other companies, possibly including some of our competitors, and BI’s own corporate objectives may not be consistent with our interests. If BI, Alexion, or Lilly fails to develop, obtain regulatory approval for, or ultimately commercialize any product candidate under our collaborations, or if BI, Alexion, or Lilly terminates the applicable collaboration, our business, financial condition, results of operations, and prospects could be materially and adversely affected. Each of our collaboration agreements is terminable by the applicable collaboration partner any time at will, subject to compliance with applicable notice periods. In addition, if we have a dispute or enter into litigation with any of our collaboration partners in the future, it could delay development programs, create uncertainty as to ownership of intellectual property rights, distract management from other business activities, and generate substantial expense.
If third parties on which we depend to conduct our preclinical studies, or any future clinical trials, do not perform as contractually required, fail to satisfy regulatory or legal requirements, or miss expected deadlines, our development program could be delayed with materially adverse effects on our business, financial condition, results of operations, and prospects.
We rely on third-party clinical investigators, contract research organizations (“CROs”), clinical data management organizations, and consultants to design, conduct, supervise, and monitor preclinical studies of our product candidates and will do the same for any clinical trials. Because we rely on third parties and do not have the ability to conduct preclinical studies or clinical trials independently, we have less control over the timing, quality, compliance, and other aspects of preclinical studies and clinical trials than we would if we conducted them on our own. These investigators, CROs, and consultants are not our employees and we have limited control over the amount of time and resources that they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw time and resources from our programs. The third parties with which we contract might not be diligent, careful, compliant, or timely in conducting our preclinical studies or clinical trials, resulting in the preclinical studies or clinical trials being delayed or unsuccessful.
If we cannot contract with acceptable third parties on commercially reasonable terms, or at all, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials, or meet expected deadlines, our clinical development programs could be delayed and otherwise adversely affected. In all events, we are responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the general investigational plan and protocols for the trial as well as applicable laws and regulations. The FDA and certain foreign regulatory authorities, such as the EMA, require preclinical studies to be conducted in accordance with applicable good laboratory practices and clinical trials to be conducted in accordance with applicable FDA regulations and applicable good clinical practices, including requirements for conducting, recording, and reporting the results of preclinical studies and clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Any such event could have a material adverse effect on our business, financial condition, results of operations, and prospects.
39
Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical studies, and clinical trial materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third-party supply and manufacturing companies and organizations to supply the materials, components, and manufacturing services for our research and development, preclinical study, and clinical trial drug supplies.
We do not own or lease manufacturing facilities or supply sources for such components and materials. Our manufacturing requirements include oligonucleotides and custom amidites, some of which we procure from a single source supplier on a purchase order basis. In addition, for each product candidate we typically contract with only one manufacturer for the formulation and filling of drug product. There can be no assurance that our supply of research and development, preclinical study, and clinical trial drugs and other materials will not be limited, interrupted, restricted in certain geographic regions, or of satisfactory quality, or continue to be available at acceptable prices. In particular, any replacement of our drug substance manufacturer could require significant effort and expertise because there may be a limited number of qualified replacements.
If we are at any time unable to provide an uninterrupted supply of our product candidates or, following regulatory approval, any products to patients, we may lose patients, physicians may elect to utilize competing therapeutics instead of our products, and our clinical trials may be adversely affected, which could materially and adversely affect our clinical trial outcomes.
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as current good manufacturing practices (“cGMP”). In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations regarding quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may experience shortages resulting in delayed shipments, supply constraints and/or stock-outs of our products, be forced to manufacture the materials ourselves, for which we currently do not have the capabilities or resources, or enter into an agreement with another third party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget.
We expect to continue to rely on third-party manufacturers if we receive regulatory approval for any product candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| • | an inability to initiate or continue preclinical studies or clinical trials of product candidates under development; |
| • | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
| • | lack of or loss of the cooperation of a collaborator; |
| • | subjecting manufacturing facilities of our product candidates to additional inspections by regulatory authorities; |
| • | requirements to cease distribution or to recall batches of our product candidates; and |
| • | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products. |
40
We may not successfully engage in strategic transactions, including any additional collaborations we seek, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expense, and present significant distractions to our management.
From time to time, we may consider strategic transactions, such as collaborations, acquisitions of companies, asset purchases, and out- or in-licensing of product candidates or technologies. In particular, in addition to our current collaboration with BI, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biopharmaceutical, biotechnology, or pharmaceutical companies. The competition for collaborators is intense, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may be unable to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product do not meet expectations, or the collaborator terminates the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non-recurring or other charges, increase our near- and long-term expenditures, and pose significant integration or implementation challenges or disrupt our management or business. These transactions entail numerous operational and financial risks, including exposure to unknown liabilities, disruption of our business, and diversion of our management’s time and attention in order to obtain and manage a collaboration or develop acquired products, product candidates or technologies, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition, or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, deterioration of relationships with key suppliers, manufacturers, or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business. Accordingly, although there can be no assurance that we will undertake or successfully complete any transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition, and prospects. Conversely, failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies or product candidates more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to develop and successfully commercialize product candidates may be adversely affected.
The development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing, or may develop product candidates and processes competitive with our product candidates, some of which may become commercially available before any of our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that enter the market. We are aware of many companies that are working in the field of RNAi therapeutics, including major pharmaceutical companies and a number of biopharmaceutical companies including Alnylam, Arrowhead Pharmaceuticals, Inc. (“Arrowhead”), and Arbutus Biopharma Corporation. We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we may try to develop product candidates.
We also compete with companies working to develop antisense and other RNA-based drugs. Like RNAi therapeutics, antisense drugs target mRNA with the objective of suppressing the activity of specific genes. The development of antisense drugs is more advanced than that of RNAi therapeutics, and antisense technology may become the preferred technology for products that target mRNAs. Significant competition also exists from companies such as Alnylam and Arrowhead to discover and develop safe and effective means to deliver therapeutic RNAi molecules to the relevant cell and tissue types.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources, or experience than we have. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including safety and effectiveness, ease with which our products can be administered and the extent to which patients and physicians accept relatively new routes of administration, timing and scope of regulatory approvals, availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage, and patent position of our products. Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our product candidates. Competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
41
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including: Douglas M. Fambrough, III, Ph.D., our chief executive officer; Bob D. Brown, Ph.D., our chief scientific officer; Ralf Rosskamp, M.D., our chief medical officer; John B. Green, our chief financial officer; and James B. Weissman, our chief business officer. The loss of one or more members of our management team or other key employees or advisors could delay our research and development programs and materially harm our business, financial condition, results of operations, and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly complex nature of our product candidates and technologies and the specialized nature of the regulatory approval process. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical, and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities, and other organizations.
If our product candidates advance into clinical trials, we may experience difficulties in managing our growth and expanding our operations.
We have limited experience in drug development and very limited experience with clinical trials of product candidates. As our product candidates enter and advance through preclinical studies and any clinical trials, we will need to expand our development, regulatory, and manufacturing capabilities or contract with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators, suppliers, and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial, and management controls; reporting systems, and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
If any of our product candidates are approved for marketing and commercialization and we are unable to develop sales, marketing, and distribution capabilities on our own or enter into agreements with third parties to perform these functions on acceptable terms, we will be unable to successfully commercialize any such future products.
We currently have no sales, marketing, or distribution capabilities or experience. If any of our product candidates are approved, we will need to develop internal sales, marketing, and distribution capabilities to commercialize such products, which would be expensive and time-consuming, or enter into collaborations with third parties to perform these services. If we decide to market our products directly, we will need to commit significant financial, legal, and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration, and compliance capabilities. If we rely on third parties with such capabilities to market our approved products or decide to co-promote products with collaborators, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable, compliant terms, or at all. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of the third parties and there can be no assurance that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance of any approved product. If we are not successful in commercializing any product approved in the future, either on our own or through third parties, our business, financial condition, results of operations, and prospects could be materially and adversely affected.
If we fail to comply with U.S. and foreign regulatory requirements, regulatory authorities could limit or withdraw any marketing or commercialization approvals we may receive and subject us to other penalties that could materially harm our business.
The Company, our product candidates, our suppliers, and our contract manufacturers, distributors, and contract testing laboratories are subject to extensive regulation by governmental authorities in the EU, the U.S., and other countries, with the regulations differing from country to country.
Even if we receive marketing and commercialization approval of a product candidate, we and our third-party services providers will be subject to continuing regulatory requirements, including a broad array of regulations related to establishment registration and product listing, manufacturing processes, risk management measures, quality and pharmacovigilance systems, post-approval clinical studies, labeling, advertising and promotional activities, record keeping, distribution, adverse event reporting, import and export of pharmaceutical products, pricing, sales and marketing, and fraud and abuse requirements. We are required to submit safety and other post market information and reports and are subject to continuing regulatory review, including in relation to adverse patient experiences with the product and clinical results that are reported after a product is made commercially available, both in the U.S. and any foreign jurisdiction in which we seek regulatory approval. The FDA and certain foreign regulatory authorities, such as the EMA,
42
have significant post-market authority, including the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate safety risks related to the use of a product or to require withdrawal of the product from the market. The FDA also has the authority to require a risk evaluation and mitigation strategy (“REMS”) plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug. The EMA now routinely requires risk management plans (“RMPs”) as part of the marketing authorization application process, and such plans must be continually modified and updated throughout the lifetime of the product as new information becomes available. In addition, for nationally authorized medicinal products, the relevant governmental authority of any EU member state can request an RMP whenever there is a concern about a risk affecting the benefit risk balance of the product. The manufacturer and manufacturing facilities we use to make a future product, if any, will also be subject to periodic review and inspection by the FDA and other regulatory agencies, including for continued compliance with cGMP requirements. The discovery of any new or previously unknown problems with our third-party manufacturers, manufacturing processes, or facilities may result in restrictions on the product, manufacturer, or facility, including withdrawal of the product from the market. If we rely on third-party manufacturers, we will not have control over compliance with applicable rules and regulations by such manufacturers. Any product promotion and advertising will also be subject to regulatory requirements and continuing regulatory review. If we or our collaborators, manufacturers, or service providers fail to comply with applicable continuing regulatory requirements in the U.S. or foreign jurisdictions in which we seek to market our products, we or they may be subject to, among other things, fines, warning and untitled letters, clinical holds, delay or refusal by the FDA or foreign regulatory authorities to approve pending applications or supplements to approved applications, suspension, refusal to renew or withdrawal of regulatory approval, product recalls, seizures or administrative detention of products, refusal to permit the import or export of products, operating restrictions, inability to participate in government programs including Medicare and Medicaid, and total or partial suspension of production or distribution, injunction, restitution, disgorgement, debarment, civil penalties, and criminal prosecution.
We have a subsidiary located in the United Kingdom (“UK”), which we established in order to allow us to conduct clinical trials in EU member states. On June 23, 2016, the UK held a referendum in which voters approved an exit from the EU, commonly referred to as “Brexit.” The withdrawal of the UK from the EU will take effect either on the effective date of the withdrawal agreement or, in the absence of agreement, two years after the UK provides a notice of withdrawal pursuant to the EU Treaty. On March 29, 2017, the Prime Minister of the UK delivered a formal notice of withdrawal to the EU. On May 22, 2017, the Council of the EU (the “Council”), adopted a decision authorizing the opening of Brexit negotiations with the UK and formally nominated the European Commission as EU negotiator. The Council also adopted negotiating directives for the talks, which began on April 18, 2018. It appears likely that the UK’s withdrawal from the EU will involve a process of lengthy negotiations between the UK and EU member states to determine the future terms of the UK’s relationship with the EU. Because this could lead to a period of uncertainty, we have begun the process of establishing a subsidiary in Ireland for ongoing regulatory initiatives in the EU.
Price controls imposed in foreign markets and downward pricing pressure in the U.S. may adversely affect our future profitability.
In some countries, particularly member states of the EU, the pricing of prescription drugs may be subject to governmental control, at national as well as at regional levels. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, in the U.S. and elsewhere, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing and reimbursement negotiations, and pricing negotiations may continue after coverage or reimbursement has been obtained. Reference pricing used by various EU member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our RNAi therapeutic candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of any product candidate approved for marketing is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, financial condition, results of operations, or prospects could be adversely affected.
Our business entails a significant risk of product liability and our ability to obtain sufficient insurance coverage could harm our business, financial condition, results of operations, or prospects.
Our business exposes us to significant product liability risks inherent in the development, testing, manufacturing, and marketing of therapeutic treatments. Product liability claims could delay or prevent completion of our development programs. If we succeed in marketing products, such claims could result in an investigation by certain regulatory authorities, such as the FDA or foreign regulatory authorities, of the safety and effectiveness of our products, our manufacturing processes and facilities, or our marketing programs and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used, or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, injury to our reputation, costs to defend related litigation, a diversion of
43
management’s time and our resources, substantial monetary awards to clinical trial participants or patients, and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development and may need to obtain higher levels prior to marketing any of our product candidates. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include, but is not limited to, intentional failures to comply with FDA or U.S. healthcare laws and regulations or applicable laws, regulations, guidance, or codes of conduct set by foreign governmental authorities or self-regulatory industry organizations, provide accurate information to any governmental authorities such as the FDA, comply with manufacturing standards we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws, regulations, guidance, and codes of conduct intended to prevent fraud, kickbacks, self-dealing, and other abusive practices. These laws, regulations, guidance, and codes of conduct may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, business or conduct involving healthcare professionals, and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions, including debarment or disqualification of those employees from participation in FDA-regulated activities, and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, regulations, guidance, or codes of conduct. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines, exclusion from government programs, or other sanctions.
Our internal computer systems, or those of third parties with which we do business, including our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs or the theft of Company or patient confidential information.
Despite the implementation of security measures, our internal computer systems and those of third parties with which we do business, including our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failures. Such events could cause interruptions of our operations. For instance, the loss of preclinical data or data from any future clinical trial involving our product candidates could result in delays in our development and regulatory filing efforts and significantly increase our costs. Certain data breaches must also be reported to affected individuals and the government, and in some cases to the media, under provisions of the U.S. federal Health Insurance Portability and Accountability Act (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), other U.S. federal and state law, and requirements of non-U.S. jurisdictions, including the European Union Data Protection Directive, and financial penalties may also apply. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data, or inappropriate disclosure of confidential or proprietary information of the Company or patients, we could incur liability and the development of our product candidates could be delayed.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development, and manufacturing involve the use of hazardous materials and various chemicals. We maintain quantities of various flammable and toxic chemicals in our facilities in Cambridge, Massachusetts, that are required for our research, development, and manufacturing activities. We are subject to federal, state, and local laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. We believe our procedures for storing, handling, and disposing these materials in our Cambridge facilities comply with the relevant guidelines of Cambridge, the Commonwealth of Massachusetts, and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health, and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens, and the handling of animals and biohazardous materials. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against
44
potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials. Additional federal, state, and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate any of these laws or regulations.
Our information technology systems could face serious disruptions that could adversely affect our business.
Despite the use of off-site (cloud-based) information storage systems for certain key corporate information, our internal information technology and other infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt our operations. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause interruptions in our collaborations and delays in our research and development work.
Our current operations are largely concentrated in one location and any events affecting this location may have material adverse consequences.
Our current operations are carried out primarily in our facilities located in Cambridge, Massachusetts. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure, or other natural or manmade accidents, or incidents that prevent us from fully utilizing the facilities, may have a material adverse effect on our ability to operate our business, particularly on a daily basis, and have significant negative consequences on our financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our product candidates, or interruption of our business operations. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at these facilities, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facilities are unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption may have a material adverse effect on our business, financial position, results of operations, and prospects.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses during our history, do not expect to become profitable for the foreseeable future, and may never achieve profitability. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire. We may be unable to use these losses to offset income before such unused losses expire. Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50 percentage point change by value in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be further limited. We are in the process of performing an analysis on whether we have experienced any ownership changes in the past. It is possible that we have experienced an ownership change, including pursuant to the initial public offering of our common stock, which closed on February 4, 2014, our follow-on offering of common stock in 2015, our underwritten follow-on public offerings in December 2017 and September 2018, and the issuance of common stock in connection with the conversion in December 2017, of our redeemable convertible preferred stock, which we had issued in April 2017 (“Redeemable Convertible Preferred”), and that our net operating losses are subject to such limitation. As of December 31, 2017, we had significant U.S. federal and Massachusetts net operating loss carryforwards that could be reduced or lost if we have or do experience an ownership change, which could have an adverse effect on our business, financial position, results of operations, and prospects.
The investment of our cash and cash equivalents and held-to-maturity investments is subject to risks which may cause losses and affect the liquidity of these investments.
As of September 30, 2018, we had $180.4 million in cash and cash equivalents and held-to-maturity investments. We historically have invested substantially all of our available cash and cash equivalents in corporate bonds, commercial paper, securities issued by the U.S. government, certificates of deposit, and money market funds meeting the criteria of our investment policy, which is focused on the preservation of our capital. These investments are subject to general credit, liquidity, market, and interest rate risks. For example, the impact of U.S. sub-prime mortgage defaults in recent years affected various sectors of the financial markets and caused credit and liquidity issues. We may realize losses in the fair value of these investments or a complete loss of these investments, which would have a negative effect on our consolidated financial statements.
45
In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity, and financial condition.
Changes in accounting rules and regulations, or interpretations thereof, could result in unfavorable accounting charges or require us to change our compensation policies.
Accounting methods and policies for public companies and biopharmaceutical companies, including policies governing revenue recognition, research and development and related expenses, and accounting for stock-based compensation, are subject to review, interpretation, and guidance from our auditors and relevant accounting authorities, including the SEC. Changes to accounting methods or policies, or interpretations thereof, may require us to reclassify, restate, or otherwise change or revise our consolidated financial statements, including those contained in our Annual Reports on Form 10-K.
Risks Related to Intellectual Property
If we are not able to obtain and enforce patent protection for our technologies or product candidates, development and commercialization of our product candidates may be adversely affected.
Our success depends in part on our ability to obtain and maintain patents and other forms of intellectual property rights, including in-licenses of intellectual property rights of others, for our product candidates, methods used to manufacture our product candidates and methods for treating patients using our product candidates, as well as our ability to preserve our trade secrets, to prevent third parties from infringing upon our proprietary rights, and to operate without infringing upon the proprietary rights of others. There can be no assurance that an issued patent will remain valid and enforceable in a court of law through the entire patent term. Should the validity of a patent be challenged, the legal process associated with defending the patent may be costly and time consuming. Issued patents can be subject to oppositions, interferences, post-grant proceedings, and other third-party challenges that can result in the revocation of the patent or limit patent claims such that patent coverage lacks sufficient breadth to protect subject matter that is commercially relevant. Competitors may be able to circumvent our patents. Development and commercialization of pharmaceutical products can be subject to substantial delays and it is possible that at the time of commercialization any patent covering the product will have expired or will be in force for only a short period of time thereafter.
As of November 5, 2018, our worldwide patent estate, not including the patents and patent applications that we have licensed from third parties, included over 50 issued patents or allowed patent applications and at least 85 pending patent applications supporting commercial development of our RNAi molecules and delivery technologies. We may not be able to apply for patents on certain aspects of our product candidates or delivery technologies in a timely fashion or at all. Our existing issued and granted patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing products and technology. There is no guarantee that any of our pending patent applications will result in issued or granted patents, that any of our issued or granted patents will not later be found to be invalid or unenforceable, or that any issued or granted patents will include claims that are sufficiently broad to cover our product candidates or delivery technologies or to provide meaningful protection from our competitors. Moreover, the patent position of biotechnology and pharmaceutical companies can be highly uncertain because it involves complex legal and factual questions. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our current and future proprietary technology and product candidates are covered by valid and enforceable patents or are effectively maintained as trade secrets. If third parties disclose or misappropriate our proprietary rights, it may materially and adversely impact our position in the market.
The U.S. Patent and Trademark Office (“USPTO”) and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case. The standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology and pharmaceutical patents. As such, we do not know the degree of future protection that we will have on our proprietary products and technology. While we will endeavor to protect our product candidates with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time-consuming, expensive, and sometimes unpredictable.
46
In addition, there are numerous recent changes to the patent laws and proposed changes to the rules of the USPTO which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. The U.S. Supreme Court has ruled on several patent cases in recent years, some of which cases either narrow the scope of patent protection available in certain circumstances or weaken the rights of patent owners in certain situations. The 2013 decision by the U.S. Supreme Court in Association for Molecular Pathology v. Myriad Genetics, Inc. precludes a claim to a nucleic acid having a stated nucleotide sequence which is identical to a sequence found in nature and unmodified. We currently are not aware of an immediate impact of this decision on our patents or patent applications because we are developing nucleic acid products that are not found in nature. However, this decision has yet to be clearly interpreted by courts and by the USPTO. We cannot assure you that the interpretations of this decision or subsequent rulings will not adversely impact our patents or patent applications. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing U.S. patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings for a given period before or after allowance or grant, during which time third parties can raise objections against such initial grant. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether. Our patent risks include that:
| • | others may, or may be able to, make, use, or sell compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or license; |
| • | we or our licensors, collaborators, or any future collaborators may not be the first to file patent applications covering certain aspects of our inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| • | a third party may challenge our patents, and, if challenged, a court may not hold that our patents are valid, enforceable, and infringed; |
| • | a third party may challenge our patents in various patent offices, and, if challenged, we may be compelled to limit the scope of our allowed or granted claims or lose the allowed or granted claims altogether; |
| • | any issued patents that we own or have licensed from others may not provide us with any competitive advantages, or may be challenged by third parties; |
| • | we may not develop additional proprietary technologies that are patentable; |
| • | the patents of others could harm our business; and |
| • | our competitors could conduct research and development activities in countries where we will not have enforceable patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation could be costly and licenses may be unavailable on commercially reasonable terms.
Research and development of RNAi-based therapeutics and other oligonucleotide-based therapeutics has resulted in many patents and patent applications from organizations and individuals seeking to obtain patent protection in the field. Our efforts are based on RNAi technology that we have licensed and that we have developed internally and own or co-own. We have chosen this approach to increase our likelihood of technical success and our freedom to operate. We have obtained grants and issuances of RNAi-based patents and have licensed other patents from third parties on exclusive and non-exclusive bases. The issued patents and pending patent applications in the U.S. and in key markets around the world that we own, co-own, or license claim many different methods, compositions, and processes relating to the discovery, development, manufacture, and commercialization of RNAi therapeutics. Specifically, we own, co-own, or have licensed a portfolio of patents, patent applications, and other intellectual property covering: (1) certain aspects of the structure and uses of RNAi molecules, including their manufacture and use as therapeutics, and RNAi-related mechanisms, (2) chemical modifications to RNAi molecules that improve their properties and suitability for therapeutic uses, (3) RNAi molecules directed to specific gene sequences and drug targets as treatments for particular diseases, and (4) delivery technologies, such as in the field of lipid nanoparticles and lipid nanoparticle formulation, and chemical modifications such as conjugation to targeting moieties.
47
The RNAi-related intellectual property landscape, including patent applications in prosecution where no definitive claims have yet issued, is still evolving, and it is difficult to conclusively assess our freedom to operate. Other companies are pursuing patent applications and possess issued patents broadly directed to RNAi compositions, methods of making and using RNAi, and to RNAi-related delivery and modification technologies. Our competitive position may suffer if patents issued to third parties cover our products, or our manufacture or uses relevant to our commercialization plans. In such cases, we may not be in a position to commercialize products unless we enter into a license agreement with the intellectual property right holder, if available, on commercially reasonable terms or successfully pursue litigation, opposition, interference, re-examination, post-grant review, inter partes review, nullification, derivation action, or cancellation proceeding to limit, nullify, or invalidate the third-party intellectual property right concerned. Even if we are successful in limiting, nullifying, or invalidating third-party intellectual property rights through such proceedings, we may incur substantial costs and could require significant time and attention of our personnel.
While we believe our intellectual property allows us to pursue our current development programs, the biological process of RNAi is a natural process and cannot be patented. Several companies in the space are pursuing alternate methods to exploit this phenomenon and have built their intellectual property around these methods. For example, Alnylam controls three patent families containing both pending patent applications and issued patents (e.g., U.S. Patent Numbers 8,853,384 and 9,074,213, and European Patent EP 1 352 061 B1) that pertain to RNAi. These are referred to in their corporate literature as the “Tuschl family” (e.g. patents and applications claiming priority to WO2002/044321, filed November 29, 2001, and their priority filings) and the “Kreutzer-Limmer family” (e.g. patents and applications claiming priority to WO 2002/044895, filed January 29, 2000, WO 2002/055693, filed January 9, 2002, and their priority filings). Both families contain patent applications still in prosecution, with the applicants actively seeking to extend the reach of this intellectual property in ways that might strategically impact our business. Additional areas of intellectual property pursued by Alnylam and others include oligonucleotide delivery-related technologies (such as conjugation to targeting moieties) and oligonucleotides directed to specific gene targets. In addition, Silence Therapeutics owns patents directed to certain chemical modifications of RNAi molecules, including U.S. Patent Number 9,222,092, with a priority date of August 5, 2002.
Patent applications in the U.S. and elsewhere are generally published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our product candidates or platform technology could have been filed by others without our knowledge. Additionally, pending claims in patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our product candidates, or the use of our product candidates. Third-party intellectual property right holders may also bring patent infringement claims against us. No such patent infringement actions have been brought against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve any future infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable, and time-consuming litigation, and may be prevented from or experience substantial delays in marketing our products. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might also be forced to redesign product candidates so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
As the field of RNAi therapeutics matures, patent applications are being processed by national patent offices around the world. There is uncertainty about which patents they will issue, and, if they do, as to when, to whom, and with what claims. It is likely that there will be significant litigation in the courts and other proceedings, such as interference, re-examination, opposition, post-grant review, inter partes review, nullification, derivation action, or cancellation proceedings, in various patent offices relating to patent rights in the RNAi therapeutics field. In many cases, the possibility of appeal or opposition exists for either us or our opponents, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. The timing and outcome of these and other proceedings is uncertain and may adversely affect our business if we are not successful in defending the patentability and scope of our pending and issued patent claims or if third parties are successful in obtaining claims that cover our RNAi technology or any of our product candidates. In addition, third parties may attempt to invalidate our intellectual property rights. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management, and could have a material adverse effect on our business and our ability to successfully compete in the field of RNAi therapeutics.
There are many issued and pending patents that claim aspects of oligonucleotide chemistry and modifications that we may need to apply to our therapeutic candidates. There are also many issued patents that claim targeting genes or portions of genes that may be relevant for drugs we wish to develop. Thus, it is possible that one or more organizations will hold patent rights to which we will need a license. If those organizations refuse to grant us a license to such patent rights on reasonable terms, we may be unable to market products or perform research and development or other activities covered by these patents.
48
We may license patent rights from third-party owners or licensees. If such owners or licensees do not properly or successfully obtain, maintain, or enforce the patents underlying such licenses, or if they retain or license to others any competing rights, our competitive position and business prospects may be adversely affected.
We may, in the future, rely on intellectual property rights licensed from third parties to protect our technology, including licenses that give us rights to third-party intellectual property that is necessary or useful for our business. We also may license additional third-party intellectual property in the future. Our success may depend in part on the ability of our licensors to obtain, maintain, and enforce patent protection for our licensed intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications licensed to us. Even if patents issue or are granted, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue litigation less aggressively than we would. Further, we may not obtain exclusive rights, which would allow for third parties to develop competing products. Without protection for, or exclusive right to, the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects. In addition, we sublicense certain of our rights under our third-party licenses to BI and may sublicense such rights to current or future collaborators. Any impairment of these sublicensed rights could result in reduced revenue under our collaboration agreement with BI or result in termination of an agreement by one or more of our existing or any other future collaborators.
We may be unable to protect our intellectual property rights throughout the world.
Obtaining a valid and enforceable issued or granted patent covering our technology in the U.S. and worldwide can be extremely costly. In jurisdictions where we have not obtained patent protection, competitors may use our technology to develop their own products, and further, may export otherwise infringing products to territories where we have patent protection, but where it is more difficult to enforce a patent as compared to the U.S. We also may face competition in jurisdictions where we do not have issued or granted patents or where our issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly that relating to biopharmaceuticals. This could make it difficult for us to prevent the infringement of our patents or marketing of competing products in violation of our proprietary rights generally in certain jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We generally file a provisional patent application first (a priority filing) at the USPTO. A U.S. utility application and/or international application under the Patent Cooperation Treaty (“PCT”) are usually filed within 12 months after the priority filing. Based on the PCT filing, national and regional patent applications may be filed in the EU, Japan, Australia, and Canada and, depending on the individual case, also in any or all of, inter alia, China, India, South Korea, Singapore, Taiwan, and South Africa. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant. Finally, the grant proceeding of each national or regional patent is an independent proceeding which may lead to situations in which applications might be refused in some jurisdictions, while granted by others. Depending on the country, various scopes of patent protection may be granted on the same product candidate or technology.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the U.S., and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business and results of operations may be adversely affected.
49
We, our licensors, or existing or future collaborators may become subject to third-party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other proprietary rights, all of which could be costly, time consuming, delay, or prevent the development and commercialization of our product candidates, or put our patents and other proprietary rights at risk.
We, our licensors, or existing or future collaborators may be subject to third-party claims for infringement or misappropriation of patent or other proprietary rights. We are generally obligated under our license or collaboration agreements to indemnify and hold harmless our licensors or collaborators for damages arising from intellectual property infringement by us. If we, our licensors or existing or future collaborators are found to infringe a third-party patent or other intellectual property rights, we could be required to pay damages, potentially including treble damages, if we are found to have willfully infringed. In addition, we, our licensors, or existing or future collaborators may choose to seek, or be required to seek, a license from a third party, which may not be available on acceptable terms, if at all. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we, our licensors, or existing or future collaborators may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products or our technology, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during patent prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during patent prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
If we fail to comply with our obligations under any license, collaboration, or other agreements, we may be required to pay damages and could lose intellectual property rights that are necessary for developing and protecting our product candidates and delivery technologies, or we could lose certain rights to grant sublicenses.
Any future licenses we enter into are likely to impose various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages, and the licensor may have the right to terminate the license, which could result in us being unable to develop, manufacture, and sell products that are covered by the licensed technology, or enable a competitor to gain access to the licensed technology. Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we would be required to pay on sales of future products, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in products that we successfully develop and commercialize, if any. Therefore, even if we successfully develop and commercialize products, we may be unable to achieve or maintain profitability.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for certain aspects of our product candidates and delivery technologies, we also consider trade secrets, including confidential and unpatented know-how, important to the maintenance of our competitive position. We protect trade secrets and confidential and unpatented know-how, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to such knowledge, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that obligate them to maintain confidentiality and assign their inventions to us.
50
Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, some courts in the U.S. and certain foreign jurisdictions are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We are also subject both in the U.S. and outside the U.S. to various regulatory schemes regarding requests for the information we provide to regulatory authorities, which may include, in whole or in part, trade secrets or confidential commercial information. While we are likely to be notified in advance of any disclosure of such information and would likely object to such disclosure, there can be no assurance that our challenge to the request would be successful.
We may be, in the future, subject to claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of our employees’ or consultants’ former employers or their clients. These claims may be costly to defend and if we do not successfully do so, we may be required to pay monetary damages, may be prohibited from using some of our research and development work, and may lose valuable intellectual property rights or personnel.
Many of our employees were previously employed at universities or biotechnology or pharmaceutical companies, including our competitors or potential competitors. From time to time, we have received correspondence from other companies alleging the improper use or disclosure, or inquiring regarding the use or disclosure, by certain of our employees who have previously been employed elsewhere in our industry, including with our competitors, of their former employer’s trade secrets or other proprietary information. Responding to these allegations can be costly and disruptive to our business, even when the allegations are without merit, and can be a distraction to management.
We may be subject to additional claims in the future that these or other employees of the Company have, or we have, inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending current or future claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, personnel, or the ability to use some of our research and development work. A loss of intellectual property, key research personnel, or their work product could hamper our ability to commercialize, or prevent us from commercializing, our product candidates, which could severely harm our business.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. Any trademark litigation could be expensive. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential collaborators or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
Risks Related to Government Regulation
We may be unable to obtain U.S. or foreign regulatory approval and, as a result, may be unable to commercialize our product candidates.
Our product candidates are subject to extensive governmental regulations relating to, among other things, research, development, testing, manufacture, quality control, approval, labeling, packaging, promotion, storage, record-keeping, advertising, distribution, sampling, pricing, sales and marketing, safety, post-approval monitoring and reporting, and export and import of drugs. Rigorous preclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain, and subject to unanticipated delays. It is possible that none of the product candidates we may develop will obtain the regulatory approvals necessary for us or our collaborators to begin selling them.
51
We have very limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA as well as foreign regulatory authorities, such as the EMA. The time required to obtain FDA and foreign regulatory approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity, and novelty of the product candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictably or uniformly and can change. Any analysis we perform of data from preclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit, or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in the policy of the FDA or foreign regulatory authorities during the period of product development, clinical trials, and regulatory review by the FDA or foreign regulatory authorities. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign laws, regulations, guidance, or interpretations will be changed, or what the impact of such changes, if any, may be.
Because the drugs we are developing may represent a new class of drug, the FDA and its foreign counterparts have not yet established any definitive policies, practices, or guidelines in relation to these drugs. While we believe the product candidates that we are currently developing are regulated as new drugs under the Federal Food, Drug, and Cosmetic Act, the FDA could decide to reclassify them, namely to regulate them or other products we may develop as biologics under the Public Health Service Act. The lack of policies, practices, or guidelines may hinder or slow review by the FDA or foreign regulatory authorities of any regulatory filings that we may submit. Moreover, the FDA or foreign regulatory authorities may respond to these submissions by defining requirements we may not have anticipated. Such responses could lead to significant delays in the clinical development of our product candidates. In addition, because there may be approved treatments for some of the diseases for which we may seek approval, in order to receive regulatory approval, we may need to demonstrate through clinical trials that the product candidates we develop to treat these diseases, if any, are not only safe and effective, but safer or more effective than existing products.
Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular product candidate for which we are seeking approval. Furthermore, any regulatory approval to market a product may be subject to limitations on the approved uses for which we may market the product or the labeling or other restrictions. Regulatory authorities also may impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product. In addition, the FDA has the authority to require a REMS plan as part of an NDA or biologics license application or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug or biologic, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria, and requiring treated patients to enroll in a registry. These limitations and restrictions may limit the size of the market for the product and affect coverage and reimbursement by third-party payors.
We are also subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing, and third-party reimbursement. The foreign regulatory approval process varies among countries and may include all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities outside the U.S. and vice versa.
If we or current or future collaborators, manufacturers, or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions and substantial penalties, which could affect our ability to develop, market, and sell our products, and may harm our reputation.
Although we do not currently have any products on the market, once our therapeutic candidates or clinical trials are covered by federal healthcare programs, we will be subject to additional healthcare statutory and regulatory requirements and enforcement by the federal, state and foreign governments of the jurisdictions in which we conduct our business. Healthcare providers, physicians, and third-party payors play a primary role in the recommendation and prescription of any therapeutic candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse, transparency, and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and distribute our therapeutic candidates for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include, but are not limited to, the following:
| • | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering, or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare or Medicaid; |
52
| • | HIPAA includes a fraud and abuse provision referred to as the HIPAA All-Payor Fraud Law, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items, or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | HIPAA, as amended by HITECH, and its implementing regulations, which impose obligations on certain covered entity healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| • | federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| • | the federal Physician Payment Sunshine Act and the implementing regulations, also referred to as “Open Payments,” issued under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, collectively referred to as the “ACA,” which require that manufacturers of pharmaceutical and biological drugs reimbursable under Medicare, Medicaid, and Children’s Health Insurance Programs report to the Department of Health and Human Services all consulting fees, travel reimbursements, research grants, and other payments, transfers of value, or gifts made to physicians and teaching hospitals with limited exceptions; |
| • | analogous state laws and regulations, such as state anti-kickback and false claims laws potentially applicable to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers; and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and |
| • | the EU General Data Protection Regulation, which was officially adopted in April 2016 and went into effect in May 2018, introduces new data protection requirements in the European Union, as well as substantial fines for breaches of the data protection rules. The EU General Data Protection Regulation will increase our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional mechanisms to ensure compliance with the new EU data protection rules. |
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions, and settlements in the healthcare industry. Responding to investigations can be time- and resource-consuming and can divert management’s attention from the business. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business.
Ensuring that our business arrangements with third parties comply with applicable healthcare laws and regulations could involve substantial costs. If our operations are found to be in violation of any such requirements, we may be subject to penalties, including civil or criminal penalties, monetary damages, the curtailment or restructuring of our operations, or exclusion from participation in government contracting, healthcare reimbursement, or other government programs, including Medicare and Medicaid, any of which could adversely affect our financial results. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time, and resources.
53
If we or current or future collaborators, manufacturers, or service providers fail to comply with applicable federal, state, or foreign laws or regulations, we could be subject to enforcement actions, which could affect our ability to develop, market, and sell our products successfully and could harm our reputation and lead to reduced acceptance of our products by the market. These enforcement actions include, among others:
| • | adverse regulatory inspection findings; |
| • | warning or untitled letters; |
| • | voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals; |
| • | restrictions on, or prohibitions against, marketing our products; |
| • | restrictions on, or prohibitions against, importation or exportation of our products; |
| • | suspension of review or refusal to approve pending applications or supplements to approved applications; |
| • | exclusion from participation in government-funded healthcare programs; |
| • | exclusion from eligibility for the award of government contracts for our products; |
| • | a corporate integrity agreement; |
| • | FDA debarment of individuals at our Company; |
| • | suspension or withdrawal of product approvals; |
| • | seizure or administrative detention of products; |
| • | injunctions; and |
| • | civil and criminal penalties and fines. |
Any drugs we develop may become subject to unfavorable pricing regulations, third-party coverage, and reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing, coverage, and reimbursement for new drugs vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. Although we intend to monitor these regulations, our programs are currently in the early stages of development and we will not be able to assess the impact of price regulations for a number of years. As a result, we might obtain regulatory approval for a product in a particular country but then be subject to price regulations that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country.
Our ability to commercialize any products successfully will also depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers, and other organizations. However, there may be significant delays in obtaining coverage for newly-approved drugs. Moreover, eligibility for coverage does not necessarily signify that a drug will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale, and distribution costs. Also, interim payments for new drugs, if applicable, may be insufficient to cover our costs and may not be made permanent. Thus, even if we succeed in bringing one or more products to the market, these products may not be considered medically necessary or cost-effective, and the amount reimbursed for any products may be insufficient to allow us to sell our products on a competitive basis. Because our programs are in the early stages of development, we are unable at this time to determine their cost effectiveness, or the likely level or method of reimbursement. In addition, obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical, and cost-effectiveness data for the use of our product on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any product candidate that we successfully develop.
54
Increasingly, the third-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are seeking greater upfront discounts, additional rebates, and other concessions to reduce the prices for pharmaceutical products. If the price we are able to charge for any products we develop, or the reimbursement provided for such products, is inadequate in light of our development and other costs, our return on investment could be adversely affected.
We currently expect that certain drugs we develop may need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable drugs) may be eligible for coverage under Medicare through Medicare Part B. Specifically, Medicare Part B coverage may be available for eligible beneficiaries when the following, among other requirements have been satisfied:
| • | the product is reasonable and necessary for the diagnosis or treatment of the illness or injury for which the product is administered according to accepted standards of medical practice; |
| • | the product is typically furnished incident to a physician’s services; |
| • | the indication for which the product will be used is included or approved for inclusion in certain Medicare-designated pharmaceutical compendia (when used for an off-label use); and |
| • | the product has been approved by the FDA. |
Average prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Reimbursement rates under Medicare Part B would depend in part on whether the newly approved product would be eligible for a unique billing code. Self-administered, outpatient drugs are typically reimbursed under Medicare Part D, and drugs that are administered in an inpatient hospital setting are typically reimbursed under Medicare Part A under a bundled payment. It is difficult for us to predict how Medicare coverage and reimbursement policies will be applied to our products in the future and coverage and reimbursement under different federal healthcare programs are not always consistent. Medicare reimbursement rates may also reflect budgetary constraints placed on the Medicare program.
Third-party payors often rely upon Medicare coverage policies and payment limitations in setting their own reimbursement rates. These coverage policies and limitations may rely, in part, on compendia listings for approved therapeutics. Our inability to promptly obtain relevant compendia listings, coverage, and adequate reimbursement from both government-funded and private payors for new drugs that we develop and for which we obtain regulatory approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our financial condition.
We expect that these and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement, and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs once marketing approval is obtained.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. A number of legislative and regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed, and such efforts have expanded substantially in recent years. These developments could, directly or indirectly, affect our ability to sell our products, if approved, at a favorable price.
For example, in the U.S., in 2010, the U.S. Congress passed the ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry, and impose additional policy reforms. Both the U.S. Congress and President Trump have expressed an intention to repeal or replace the ACA, and as a result, certain sections of the ACA have not been fully implemented or have been effectively repealed. The uncertainty around the future of the ACA, and in particular, the impact to reimbursement levels, may lead to uncertainty or delay in the purchasing decisions of our customers, which may in turn negatively impact our product sales. If there are not adequate reimbursement levels, our business and results of operations could be adversely affected.
55
Among the provisions of the ACA addressing coverage and reimbursement of pharmaceutical products, of importance to our potential therapeutic candidates are the following
| • | increases to pharmaceutical manufacturer rebate liability under the Medicaid Drug Rebate Program due to an increase in the minimum basic Medicaid rebate on most branded prescription drugs and the application of Medicaid rebate liability to drugs used in risk-based Medicaid managed care plans; |
| • | the expansion of the 340B Drug Pricing Program to require discounts for “covered outpatient drugs” sold to certain children’s hospitals, critical access hospitals, freestanding cancer hospitals, rural referral centers, and sole community hospitals; |
| • | requirements imposed on pharmaceutical companies to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “Donut Hole”; |
| • | requirements imposed on pharmaceutical companies to pay an annual non-tax-deductible fee to the federal government based on each company’s market share of prior year total sales of branded drugs to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs, and Department of Defense. Since we currently expect our branded pharmaceutical sales to constitute a small portion of the total federal healthcare program pharmaceutical market, we do not currently expect this annual assessment to have a material impact on our financial condition; and |
| • | For products classified as biologics, marketing approval for a follow-on biologic product may not become effective until 12 years after the date on which the reference innovator biologic product was first licensed by the FDA, with a possible six-month extension for pediatric products. After this exclusivity ends, it may be possible for biosimilar manufacturers to enter the market, which is likely to reduce the pricing for the innovator product and could affect our profitability if our products are classified as biologics. |
Separately, pursuant to the health reform legislation and related initiatives, the Centers for Medicare and Medicaid Services (“CMS”) is working with various healthcare providers to develop, refine, and implement Accountable Care Organizations (“ACOs”), and other innovative models of care for Medicare and Medicaid beneficiaries, including the Bundled Payments for Care Improvement Initiative, the Comprehensive Primary Care Initiative, the Duals Demonstration, and other models. The continued development and expansion of ACOs and other innovative models of care will have an uncertain impact on any future reimbursement we may receive for approved therapeutics administered by such organizations.
From time to time, legislation is drafted, introduced, and passed in the U.S. Congress that could significantly change the statutory provisions governing coverage, reimbursement, and marketing of products regulated by CMS or other government agencies. In addition to new legislation, CMS coverage and reimbursement policies are often revised or interpreted in ways that may significantly affect our business and our products.
The healthcare industry is heavily regulated in the U.S. at the federal, state, and local levels, and our failure to comply with applicable requirements may subject us to penalties and negatively affect our financial condition.
As a healthcare company, our operations, clinical trial activities, and interactions with healthcare providers may be subject to extensive regulation in the U.S., particularly if we receive FDA approval for any of our products in the future. For example, if we receive FDA approval for a product for which reimbursement is available under a federal healthcare program (e.g., Medicare, Medicaid), it would be subject to a variety of federal laws and regulations, including those that prohibit the filing of false or improper claims for payment by federal healthcare programs (e.g., the federal FCA), prohibit unlawful inducements for the referral of business reimbursable by federal healthcare programs (e.g., the federal Anti-Kickback Statute), and require disclosure of certain payments or other transfers of value made to U.S.-licensed physicians and teaching hospitals or Open Payments. We are not able to predict how third parties will interpret these laws and apply applicable governmental guidance and may challenge our practices and activities under one or more of these laws. If our past or present operations are found to be in violation of any of these laws, we could be subject to civil and criminal penalties which could hurt our business, our operations, and financial condition.
56
The federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting, or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for, purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any item or service reimbursable under Medicare, Medicaid, or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly, and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing, or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Our practices may not in all cases meet all of the criteria for protection under a statutory exception or regulatory safe harbor.
Additionally, the intent standard under the Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal FCA (discussed below).
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Federal false claims and false statement laws, including the federal FCA, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to, or approval by, the federal healthcare programs, including Medicare and Medicaid, or knowingly making, using, or causing to be made or used, a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. For instance, historically, pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, off-label, and thus generally non-reimbursable, uses.
HIPAA prohibits, among other offenses, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors, or falsifying, concealing, or covering up a material fact, or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for items or services under a healthcare benefit program. To the extent that we act as a business associate to a healthcare provider engaging in electronic transactions, we may also be subject to the privacy and security provisions of HIPAA, as amended by HITECH, which restricts the use and disclosure of patient-identifiable health information, mandates the adoption of standards relating to the privacy and security of patient-identifiable health information, and requires the reporting of certain security breaches to healthcare provider customers with respect to such information. Additionally, many states have enacted similar laws that may impose more stringent requirements on entities like ours. Failure to comply with applicable laws and regulations could result in substantial penalties and adversely affect our financial condition and results of operations.
Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Additionally, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
Certain Dicerna products, if approved, may be eligible for coverage under Medicare and Medicaid, among other government healthcare programs. Accordingly, Dicerna may be subject to a number of obligations based on its participation in these programs, such as a requirement to calculate and report certain price reporting metrics to the government, such as average sales price and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. Further, these prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. It is difficult to predict how Medicare coverage and reimbursement policies will be applied to Dicerna’s products in the future, and coverage and reimbursement under different federal healthcare programs are not always consistent. Medicare reimbursement rates may also reflect budgetary constraints placed on the Medicare program.
57
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical and biotechnology companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical and biotechnology companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
If our operations are found to be in violation of any of the federal and state healthcare laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil, criminal, and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Our ability to obtain reimbursement or funding from the federal government may be impacted by possible reductions in federal spending.
U.S. federal government agencies currently face potentially significant spending reductions. The Budget Control Act of 2011 (the “BCA”) established a Joint Select Committee on Deficit Reduction, which was tasked with achieving a reduction in the federal debt level of at least $1.2 trillion. That committee did not draft a proposal by the BCA’s deadline. As a result, automatic cuts, referred to as sequestration, in various federal programs were scheduled to take place, beginning in January 2013, although the American Taxpayer Relief Act of 2012 delayed the BCA’s automatic cuts until March 1, 2013. While the Medicare program’s eligibility and scope of benefits are generally exempt from these cuts, Medicare payments to providers and Part D health plans are not exempt. The BCA did, however, provide that the Medicare cuts to providers and Part D health plans would not exceed two percent. President Obama issued the sequestration order on March 1, 2013, and cuts went into effect on April 1, 2013. Additionally, the Bipartisan Budget Act of 2015 extended sequestration for Medicare through fiscal year 2027.
The U.S. federal budget remains in flux, which could, among other things, cut Medicare payments to providers. The Medicare program is frequently mentioned as a target for spending cuts. The full impact on our business of any future cuts in Medicare or other programs is uncertain. In addition, we cannot predict any impact President Trump’s administration and the U.S. Congress may have on the federal budget. If federal spending is reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or the National Institutes of Health, to continue to function at current levels. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market, and sell any products we may develop.
If any of our product candidates receive marketing approval and we or others later identify undesirable side effects caused by the product candidate, our ability to market and derive revenue from the product candidates could be compromised.
In the event that any of our product candidates receive regulatory approval and we or others identify undesirable side effects, adverse events, or other problems caused by one of our products, any of the following adverse events could occur, which could result in the loss of significant revenue to us and materially and adversely affect our results of operations and business:
| • | regulatory authorities may withdraw their approval of the product or seize the product; |
| • | we may need to recall the product or change the way the product is administered to patients; |
| • | additional restrictions may be imposed on the marketing of the particular product or the manufacturing processes for the product or any component thereof; |
| • | we may not be able to secure or maintain adequate coverage and reimbursement for our proprietary product candidates from government (including U.S. federal healthcare programs) and private payors; |
58
| • | we may be subject to fines, restitution or disgorgement of profits or revenues, injunctions, or the imposition of civil penalties, or criminal prosecution; |
| • | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| • | regulatory authorities may require us to implement a REMS, or to conduct post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product; we may be required to create a Medication Guide outlining the risks of such side effects for distribution to patients; |
| • | we could be sued and held liable for harm caused to patients; |
| • | the product may become less competitive; and |
| • | our reputation may suffer. |
Risks Related to Our Common Stock
We are an “emerging growth company” and a “smaller reporting company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies and smaller reporting companies will make our common stock less attractive to investors.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act (“JOBS Act”). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley Act”), (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700.0 million as of the prior June 30 or if we have total annual gross revenue of $1.07 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31, or if we issue more than $1.07 billion in non-convertible debt during any three-year period before that time, in which case we would no longer be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may, under certain circumstances, still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Our stock price is volatile and purchasers of our common stock could incur substantial losses.
Our stock price is volatile. From January 30, 2014, the first day of trading of our common stock, through November 2, 2018, the closing sale price of our common stock has ranged between a high of $46.00 per share and a low of $2.45 per share. The market price for our common stock may be influenced by many factors, including the other risks described in this “Risk Factors” section, and the following:
| • | the success or failure of competitive products or technologies; |
| • | results of preclinical studies and clinical trials of our product candidates, or those of our competitors, our existing collaborator, or any future collaborators; |
| • | regulatory or legal developments in the U.S. and other countries, especially changes in laws or regulations applicable to our product candidates; |
| • | introductions and announcements of new products by us, our commercialization collaborators, or our competitors, and the timing of these introductions or announcements; |
| • | actions taken by regulatory agencies with respect to our or our competitors’ product candidates, products, clinical studies, manufacturing process, or sales and marketing terms; |
| • | actual or anticipated variations in our financial results or those of companies that are perceived to be similar to us; |
59
| • | the success of our or our competitors’ efforts to acquire or in-license additional technologies, products, or product candidates; |
| • | developments concerning our or our competitors’ collaborations, including but not limited to, those with sources of manufacturing supply and commercialization partners; |
| • | announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures, or capital commitments; |
| • | our ability or inability to raise additional capital and the terms on which we raise it; |
| • | the recruitment or departure of key personnel; |
| • | changes in the structure of healthcare payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors; |
| • | actual or anticipated changes in earnings estimates or changes in stock market analyst recommendations regarding our common stock, other comparable companies, or our industry generally; |
| • | our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | announcement and expectation of additional financing efforts; |
| • | speculation in the press or investment community; |
| • | trading volume of our common stock; |
| • | sales of our common stock by us or our stockholders; |
| • | the absence of lock-up agreements with the holders of substantially all of our outstanding shares in connection with follow-on public offerings of our common stock; |
| • | the concentrated ownership of our common stock; |
| • | changes in accounting principles; |
| • | terrorist acts, acts of war, or periods of widespread civil unrest; |
| • | natural disasters and other calamities; |
| • | general economic, industry, and market conditions; and |
| • | developments concerning complaints or litigation against us. |
In addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical, and biotechnology stocks in particular have experienced extreme volatility that has often been unrelated to the operating performance of the issuer. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance.
The future issuance of equity or of debt securities that are convertible into equity will dilute our share capital.
We may choose to raise additional capital in the future depending on market conditions, strategic considerations, and operational requirements. To the extent that additional capital is raised through the issuance of shares or other securities convertible into shares, our stockholders will be diluted. Future issuances of our common stock or other equity securities, or the perception that such sales may occur, could adversely affect the trading price of our common stock and impair our ability to raise capital through future offerings of shares or equity securities. We cannot predict the effect, if any, that future sales of common stock or the availability of common stock for future sales will have on the trading price of our common stock.
60
The employment agreements with our executive officers may require us to pay severance benefits to officers who are terminated in connection with a change of control of the Company, which could harm our financial condition.
Our executive officers are parties to employment agreements providing, in the event of a termination of employment in connection with a change of control of the Company, for significant cash payments for severance and other benefits and acceleration of vesting of up to all outstanding stock options. The accelerated vesting of options could result in dilution to our existing stockholders and reduce the market price of our common stock. The payment of these severance benefits could harm our financial condition. In addition, these potential severance payments may discourage or prevent third parties from seeking a business combination with us.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property, or our stock performance, or if our target studies and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of September 30, 2018, our executive officers and directors, together with holders of five percent or more of our outstanding common stock and their respective affiliates, beneficially owned, in the aggregate, approximately 58% of our outstanding common stock, including shares subject to outstanding options and warrants that are exercisable within 60 days after such date, based on the Forms 3 and 4 and Schedules 13D and 13G filed by them with the SEC. As a result, these stockholders, if acting together, will continue to have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation, or sale of all or substantially all of our assets, and any other significant corporate transaction. The interests of these stockholders may not be the same as, or may even conflict with, the interests of our other stockholders. For example, these stockholders could delay or prevent a change of control of our Company, even if such a change of control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our Company or our assets and might affect the prevailing market price of our common stock. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Substantial future sales of our common stock in the public market, or the perception that these sales could occur, could cause the price of the common stock to decline.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market believes that our stockholders intend to sell, substantial amounts of our common stock in the public market, the market price of our common stock could decline significantly. Our officers and directors have agreed with the underwriters not to dispose of or hedge any shares of our common stock or securities that are convertible into or exchangeable for shares of our common stock during the period from September 6, 2018, the date of our follow-on offering, continuing through the date 90 days hereafter, except with the prior written consent of Citigroup and Leerink Partners LLC. However, these restrictions are subject to certain exceptions, including, among others, to sales made pursuant to a trading plan that complies with Rule 10b5-1 under the Exchange Act, or a 10b5-1 Trading Plan, which sales may include (a) up to 115,023 shares by Dennis Langer, a member of our Board of Directors, (b) up to 250,000 shares of our common stock by Douglas M. Fambrough, III, Ph.D., our chief executive officer, (c) up to 106,839 shares by Bob D. Brown, Ph.D., our chief scientific officer, (d) up to 40,000 shares by James B. Weissman, our chief business officer, and (e) up to 25,000 shares by John B. Green, our chief financial officer.
61
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and our amended and restated bylaws may delay or prevent an acquisition of us or a change in our management. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. These provisions include:
| • | a prohibition on actions by our stockholders by written consent; |
| • | a requirement that special meetings of stockholders, which the Company is not obligated to call more than once per calendar year, be called only by the chairman of our board of directors, our chief executive officer, our board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors, or, subject to certain conditions, by our secretary at the request of the stockholders holding of record, in the aggregate, shares entitled to cast not less than ten percent of the votes at a meeting of the stockholders (assuming all shares entitled to vote at such meeting were present and voted); |
| • | advance notice requirements for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings; and |
| • | the authority of the board of directors to issue preferred stock, such as the Redeemable Convertible Preferred, with such terms as the board of directors may determine. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, as amended, which prohibits a person who owns in excess of 15 percent of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15 percent of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. These provisions would apply even if the proposed merger or acquisition could be considered beneficial by some stockholders.
We incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, we incur, and particularly after we are no longer an emerging growth company and when we cease to be a smaller reporting company, we will continue to incur, significant legal, accounting, and other expenses that we did not incur as an emerging growth company or smaller reporting company.
The Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the Nasdaq, and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. For example, we expect that these rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our board of directors. However, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
We are not currently required to comply with the rules of the SEC that implement Section 404(b) of the Sarbanes-Oxley Act. Pursuant to Section 404 of the Sarbanes-Oxley Act (“Section 404”), we are required to furnish a report by our management on our internal control over financial reporting. However, while we remain an emerging growth company and a smaller reporting company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements. In addition, if we are not able to continue to meet these requirements, we may not be able to remain listed on The Nasdaq Global Select Market.
62
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. As a result, capital appreciation, if any, of our common stock will be sole source of gain of our common stockholders for the foreseeable future.
We may incur significant costs from class action litigation due to our historical or expected stock volatility.
Our stock price has fluctuated and may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development efforts or the development efforts of our collaborators or competitors, the addition or departure of our key personnel, variations in our quarterly operating results, and changes in market valuations of pharmaceutical and biotechnology companies. This risk is especially relevant to us because pharmaceutical and biotechnology companies have experienced significant stock price volatility in recent years. When the market price of a stock has been volatile as our stock price has been and may be, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit of this type against us, even if the lawsuit is without merit, we could incur substantial costs defending the lawsuit. The lawsuit could also divert the time and attention of our management.
Our amended and restated bylaws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our amended and restated bylaws provide that, subject to limited exceptions, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, or other employees to us or our stockholders, any action asserting a claim against us arising pursuant to any provision of the Delaware General Corporation Law, as amended, our amended and restated certificate of incorporation or our amended and restated bylaws, any action to interpret, apply, enforce, or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws, any action to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws, or any other action asserting a claim against us that is governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and to have consented to the provisions of our amended and restated certificate of incorporation described above. This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers, and employees. Alternatively, if a court were to find these provisions of our amended and restated certificate of incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business and financial condition.
Our stockholders may experience significant dilution as a result of future equity offerings and exercise of outstanding options.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock, as we did with the Redeemable Convertible Preferred, which was converted into common stock in December 2017 and with the follow-on offerings of our common stock in December 2017 and September 2018. We cannot assure you that we will be able to sell shares or other securities in any offering at a price per share that is equal to or greater than the price paid by our existing shareholders, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share paid by our existing stockholders.
In addition, we have a significant number of securities allowing the purchase of our common stock. As of November 2, 2018, we also had 1,230,487 shares of common stock reserved for future issuance under our stock incentive plans. As of that date, there were also stock options and awards to purchase 7,778,491 shares of our common stock outstanding and warrants to purchase 2,198 shares of our common stock outstanding. The exercise of outstanding options and warrants having an exercise price per share that is less than the offering price per share paid by our existing stockholders will increase dilution to such stockholders.
63
Future sales of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. As of November 2, 2018, we had 62,731,942 shares of common stock outstanding, all of which shares, other than shares held by our directors and certain officers, were eligible for sale in the public market, subject in some cases to compliance with the requirements of Rule 144, including the volume limitations and manner of sale requirements. In addition, shares of common stock issuable upon exercise of outstanding options and shares reserved for future issuances under our stock incentive plans will become eligible for sale in the public market to the extent permitted by applicable vesting requirements and subject in some cases to compliance with the requirements of Rule 144.
Sales of shares issued in private placements may cause the market price of our shares to decline.
In April 2017, we issued 700,000 shares of the Redeemable Convertible Preferred in a private placement, which were convertible into shares of our common stock at an agreed conversion rate. In December 2017, all shares of Redeemable Convertible Preferred were converted into shares of our common stock. We granted the holders of Redeemable Convertible Preferred certain demand, shelf, and “piggyback” registration rights with respect to the shares of common stock issued upon conversion of the Redeemable Convertible Preferred. Such registration rights continue subsequent to the conversion and repurchase of the Redeemable Convertible Preferred with respect to the shares of common stock issued in such conversion. In accordance with such registration rights, we filed a shelf registration statement on Form S-3 covering the resale of 24,491,663 shares of our common stock by the former holders of Redeemable Convertible Preferred. The registration statement was declared effective on May 9, 2018, and all shares of common stock issued upon conversion of the Redeemable Convertible Preferred may now be freely sold in the open market. Additionally, we issued 983,208 shares of our common stock to Alnylam in April 2018 and 835,834 shares of our common stock to Alexion in October 2018. We have also entered into an agreement providing for the issuance of 5,414,185 shares of our common stock to Lilly, subject to certain closing conditions. The shares issued to Alnylam are freely tradeable in the open market, subject to certain volume limitations and compliance with applicable securities laws. The shares issued to Alexion are, and any shares issued to Lilly will be, subject to a lock-up period, but following the expiration of such lock-up periods such shares of our common stock may be freely sold in the open market, subject to compliance with applicable securities laws. The sale of a significant amount of these shares in the open market or the perception that these sales may occur could cause the market price of our common stock to decline or become highly volatile.
(a) Unregistered Sales of Equity Securities
Not applicable.
(b) Use of Proceeds
Not applicable.
(c) Issuer Purchases of Equity Securities
We did not repurchase any of our equity securities during the period covered by this report.
Not applicable.
Not applicable.
None
64
Exhibit Number
| Description of Documents
|
3.1(1) | Amended and Restated Certificate of Incorporation of the Company. |
|
|
3.2(1) | |
|
|
31.1(2) | |
|
|
31.2(2) | |
|
|
32.1* | |
|
|
101.INS(2) | XBRL Report Instance Document |
|
|
101.SCH(2) | XBRL Taxonomy Extension Schema Document |
|
|
101.CAL(2) | XBRL Taxonomy Calculation Linkbase Document |
|
|
101.LAB(2) | XBRL Taxonomy Label Linkbase Document |
|
|
101.PRE(2) | XBRL Taxonomy Presentation Linkbase Document |
|
|
101.DEF(2) | XBRL Taxonomy Extension Definition Linkbase Document |
+ | Confidential treatment with respect to specific portions of this Exhibit has been requested, and such portions are omitted and have been filed separately with the Securities and Exchange Commission. |
* | Exhibit 32.1 is being furnished and shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, nor shall such exhibit be deemed to be incorporated by reference in any registration statement or other document filed under the Securities Act, or the Exchange Act, except as otherwise stated in such filing. |
(1) | Incorporated by reference to the indicated exhibit in the Company’s Current Report on Form 8-K filed on February 5, 2014. |
(2) | Filed herewith. |
65
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| DICERNA PHARMACEUTICALS, INC. | |
|
|
|
|
Date: November 5, 2018 |
| By: | /s/ John B. Green |
|
|
| John B. Green |
|
|
| Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
66