Exhibit 99.3

1

Dave Happel President & CEO • Cognoa: President & CEO Chrono Therapeutics: President & CEO Senior E xecutive and C ommercial roles at Horizon, Raptor, Dynavax, Chiron • M.B.A. – Indiana State University; B.A. C hemistry – Indiana University George Kemble Executive Chairman • AstraZeneca (formerly MedImmune, Aviron): SVP R esearch for B iologics & G eneral M anager of California operations, VP V accine R esearch & D evelopment for V accines • Ph.D. – Stanford University, D ept of M icrobiology & I mmunology Eduardo Martins CMO • Abbvie (formerly Allergan), Eiger BioPharmaceuticals, Gilead, Genentech, Dynavax, Intermune, SciClone • D.Phil. – University of Oxford M.D. – Federal University of Rio de Janeiro, Brazil Anthony Rimac CFO • Cognoa, ESCAPE Bio, Chrono Therapeutics, Aldea Pharmaceuticals, Adamas Pharmaceuticals, Aerovance • M.B.A. – Santa Clara University; B.A. – University of California Santa Barbara Elizabeth Rozek General Counsel • Cognoa, Basilea Pharmaceutica, US Department of Justice • J.D. – University of California Berkeley M.A. – University of California San Diego B.A. – Brown University Proven Team with Development and Commercialization Experience Across Hepatology, Metabolic Disease and Oncology 2

Forward Looking Statements 3 This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, the presentation of data from clinical trials, Sagimet’s clinical development plans and related anticipated clinical development milestones, market opportunity, competitive position and potential growth opportunities are forward - looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “would,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “believe,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other similar expressions. The forward - looking statements in this presentation are only predictions. These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others: the clinical development and therapeutic potential of denifanstat or any other drug candidates we may develop; our ability to advance drug candidates into and successfully complete clinical trials , the risk the topline clinical trials may not be predictive of, and may differ from final clinical data and later - stage clinical trials; that unfavorable new clinical trial data may emerge in other clinical trials of denifanstat , including Phase 3 clinical trials; that clinical trial data are subject to differing interpretations and assessments, including by regulatory authorities; our relationship with Ascletis , and the success of its development efforts for denifanstat ; the accuracy of our estimates regarding our capital requirements; and our ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and available at www.sec.gov . You should not rely on these forward - looking statements as predictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

Denifanstat: Differentiated Mechanism Believed to Target Key Drivers of NASH Adapted from Wegermann et al, Clinical Liver Disease, Vol 11, No 4, April 2018, DOI: 10.1002/cld.709 Denifanstat has independent mechanisms designed to: ඹ Block steatosis via inhibiting de novo lipogenesis in hepatocytes ය Reduce inflammation via preventing immune cell activation ර Blunt fibrosis via inhibiting stellate cell activation 4
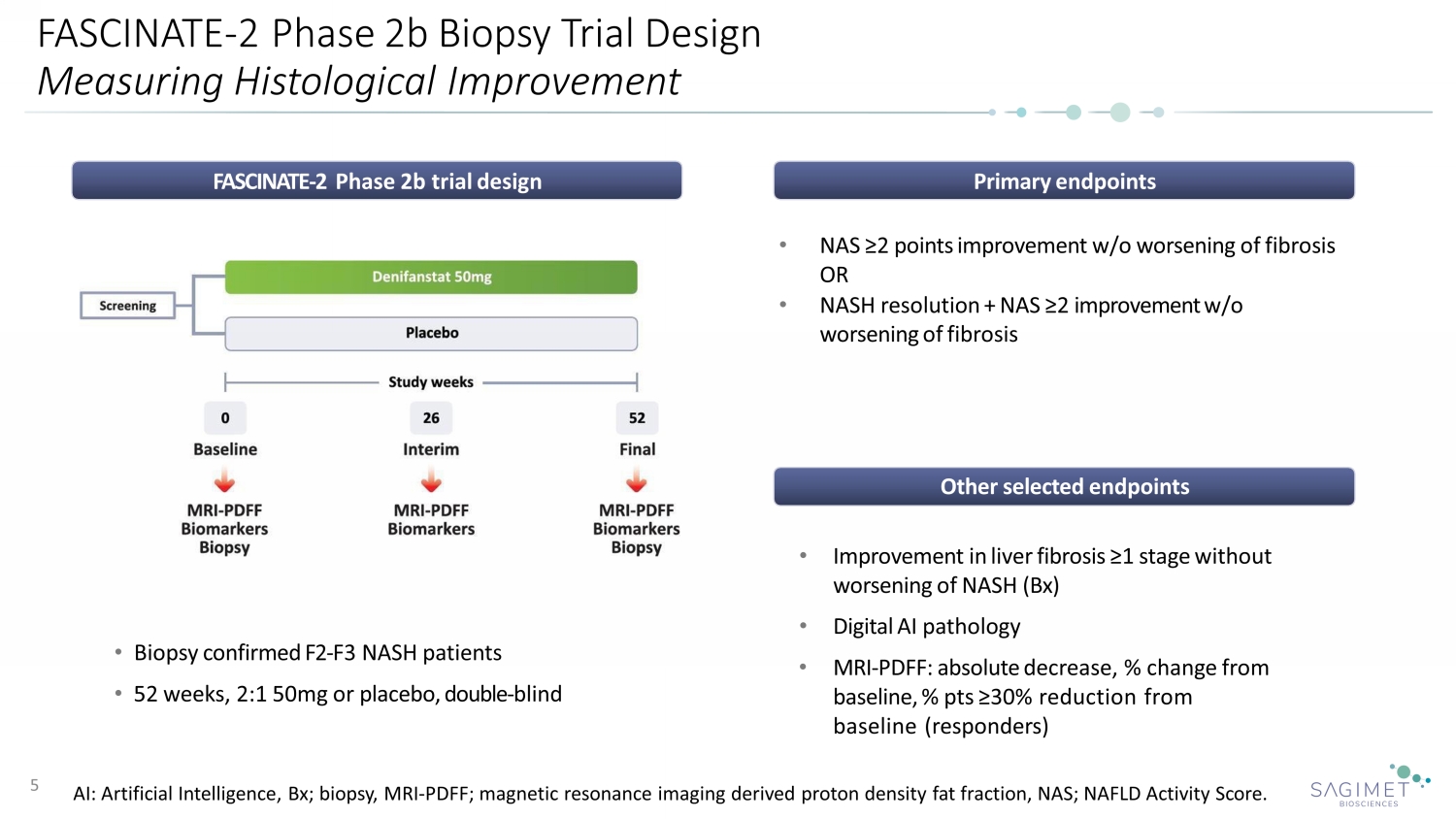
FASCINATE - 2 Phase 2b Biopsy Trial Design Measuring Histological Improvement 5 FASCINATE - 2 Phase 2b trial design • Biopsy confirmed F2 - F3 NASH patients • 52 weeks, 2:1 50mg or placebo, double - blind Primary endpoints Other selected endpoints • NAS ≥2 points improvement w/o worsening of fibrosis OR • NASH resolution + NAS ≥2 improvement w/o worsening of fibrosis • Improvement in liver fibrosis ≥1 stage without worsening of NASH (Bx) • Digital AI pathology • MRI - PDFF: absolute decrease, % change from baseline, % pts ≥30% reduction from baseline (responders) AI: Artificial Intelligence, Bx; biopsy, MRI - PDFF; magnetic resonance imaging derived proton density fat fraction, NAS; NAFLD Ac tivity Score.
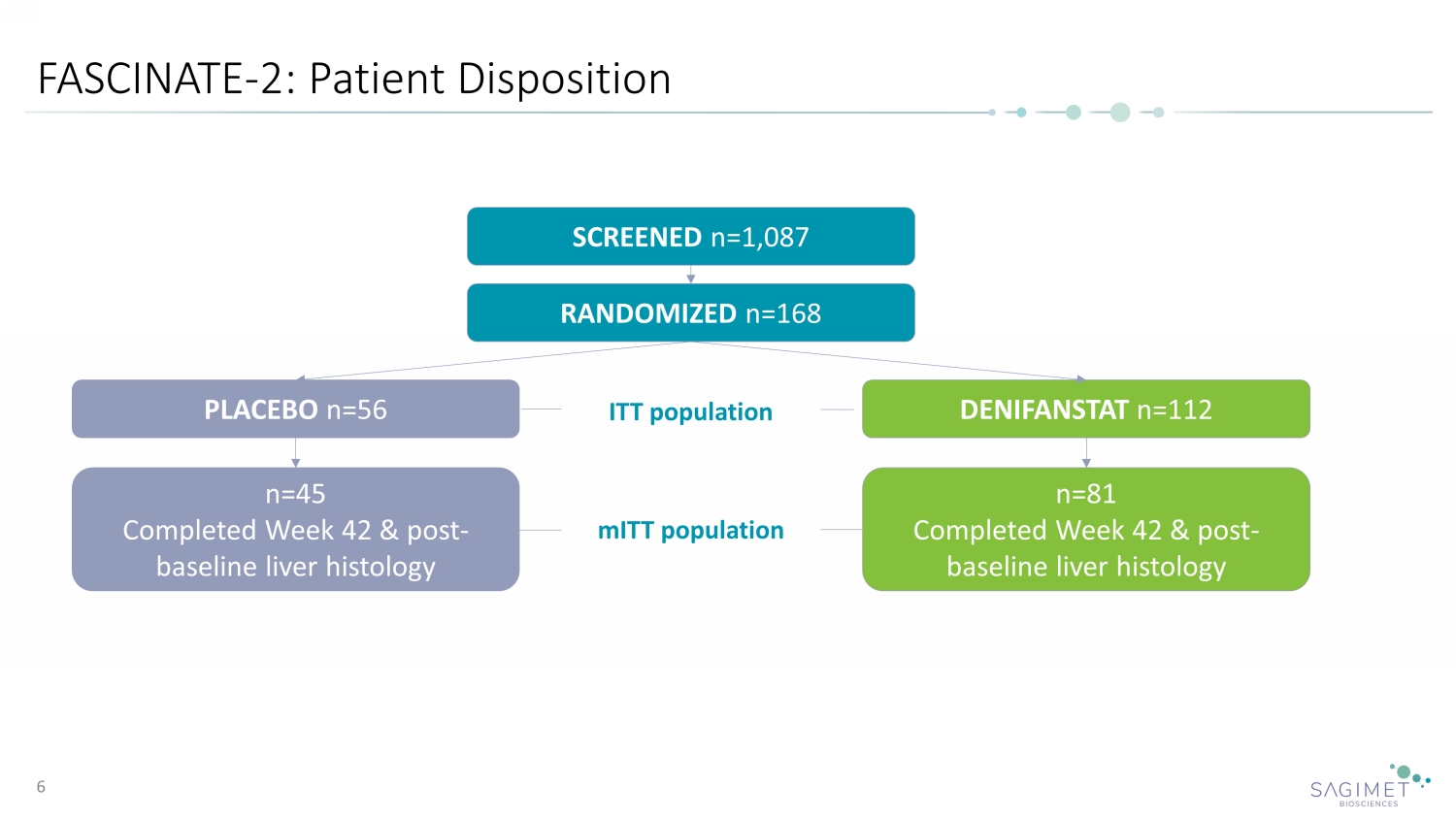
FASCINATE - 2: Patient Disposition 6 SCREENED n=1,087 RANDOMIZED n=168 PLACEBO n=56 DENIFANSTAT n=112 n=45 Completed Week 42 & post - baseline liver histology n=81 Completed Week 42 & post - baseline liver histology ITT population mITT population
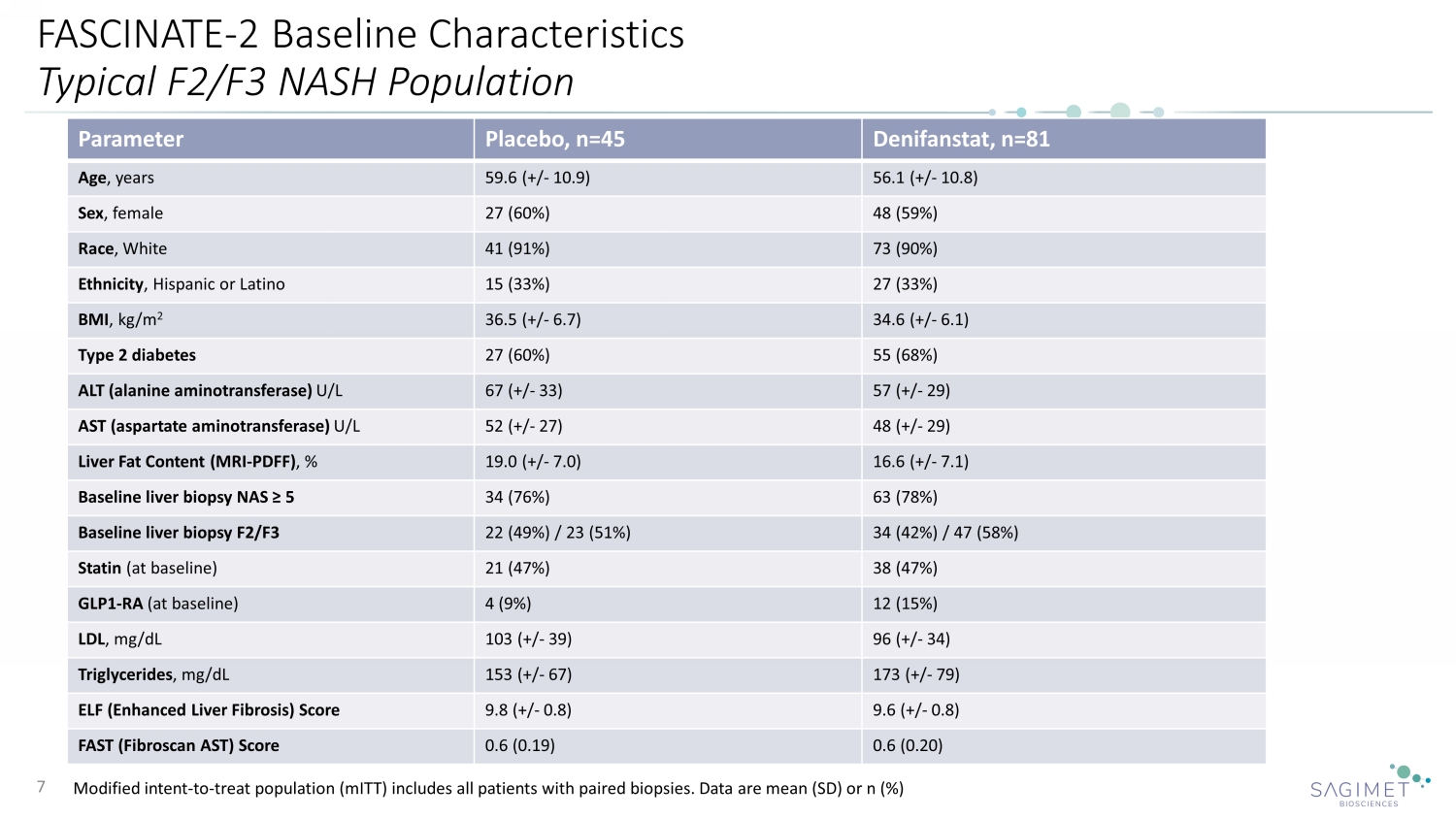
FASCINATE - 2 Baseline Characteristics Typical F2/F3 NASH Population Modified intent - to - treat population ( mITT ) includes all patients with paired biopsies. Data are mean (SD) or n (%) 7 Parameter Placebo, n=45 Denifanstat, n=81 Age , years 59.6 (+/ - 10.9) 56.1 (+/ - 10.8) Sex , female 27 (60%) 48 (59%) Race , White 41 (91%) 73 (90%) Ethnicity , Hispanic or Latino 15 (33%) 27 (33%) BMI , kg/m 2 36.5 (+/ - 6.7) 34.6 (+/ - 6.1) Type 2 diabetes 27 (60%) 55 (68%) ALT (alanine aminotransferase) U/L 67 (+/ - 33) 57 (+/ - 29) AST (aspartate aminotransferase) U/L 52 (+/ - 27) 48 (+/ - 29) Liver Fat Content (MRI - PDFF) , % 19.0 (+/ - 7.0) 16.6 (+/ - 7.1) Baseline liver biopsy NAS ≥ 5 34 (76%) 63 (78%) Baseline liver biopsy F2/F3 22 (49%) / 23 (51%) 34 (42%) / 47 (58%) Statin (at baseline) 21 (47%) 38 (47%) GLP1 - RA (at baseline) 4 (9%) 12 (15%) LDL , mg/dL 103 ( +/ - 39) 96 ( +/ - 34) Triglycerides , mg/dL 153 ( +/ - 67) 173 ( +/ - 79) ELF (Enhanced Liver Fibrosis) Score 9.8 ( +/ - 0.8) 9.6 ( +/ - 0.8) FAST (Fibroscan AST) Score 0.6 (0.19) 0.6 (0.20)
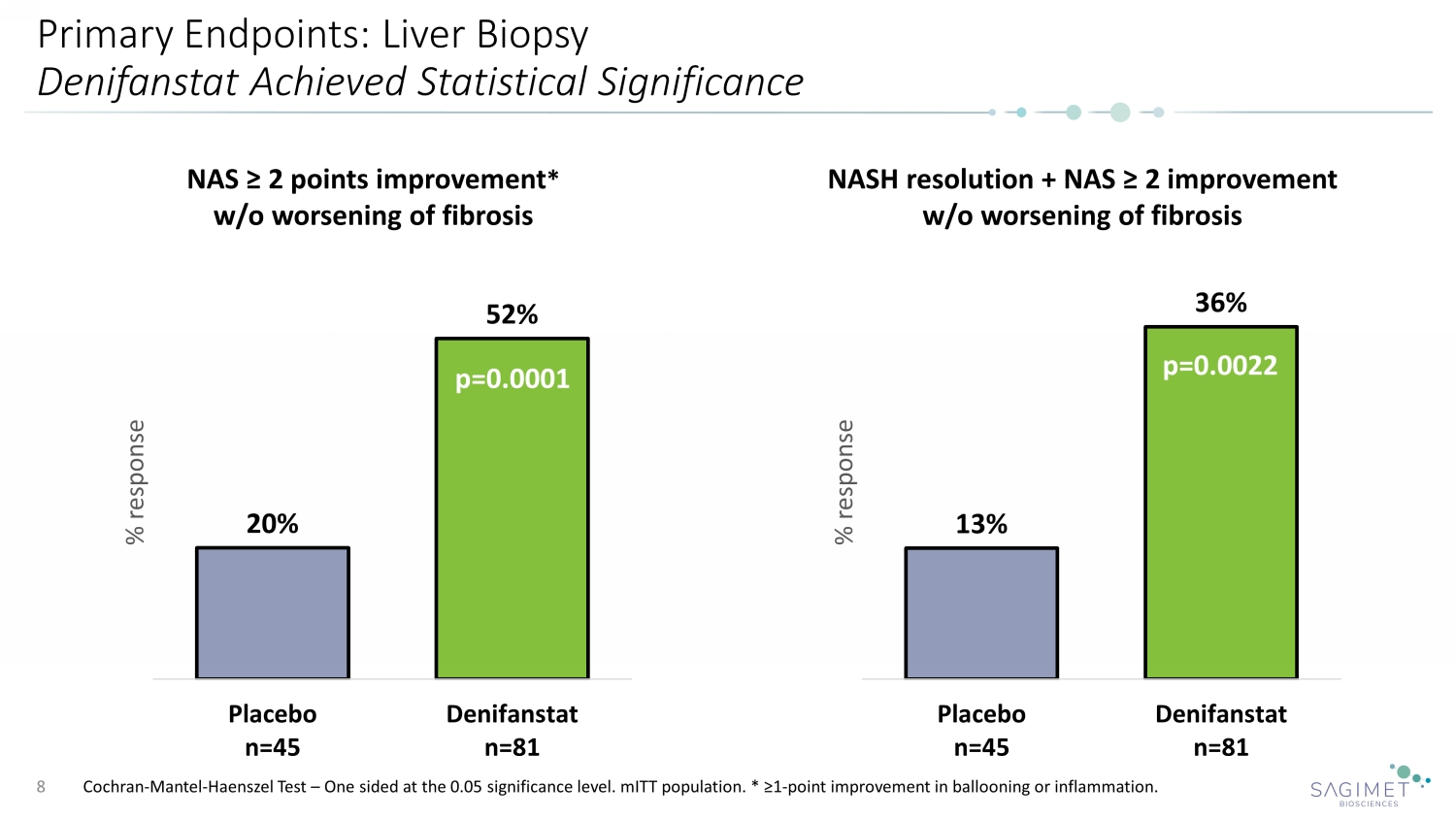
Primary Endpoints: Liver Biopsy Denifanstat Achieved Statistical Significance Cochran - Mantel - Haenszel Test – One sided at the 0.05 significance level. mITT population. * ≥1 - point improvement in ballooning or inflammation. 8 20% 52% Placebo n=45 Denifanstat n=81 % response NAS ≥ 2 points improvement * w/o worsening of fibrosis p=0.0001 13% 36% Placebo n=45 Denifanstat n=81 % response NASH resolution + NAS ≥ 2 improvement w/o worsening of fibrosis p=0.0022
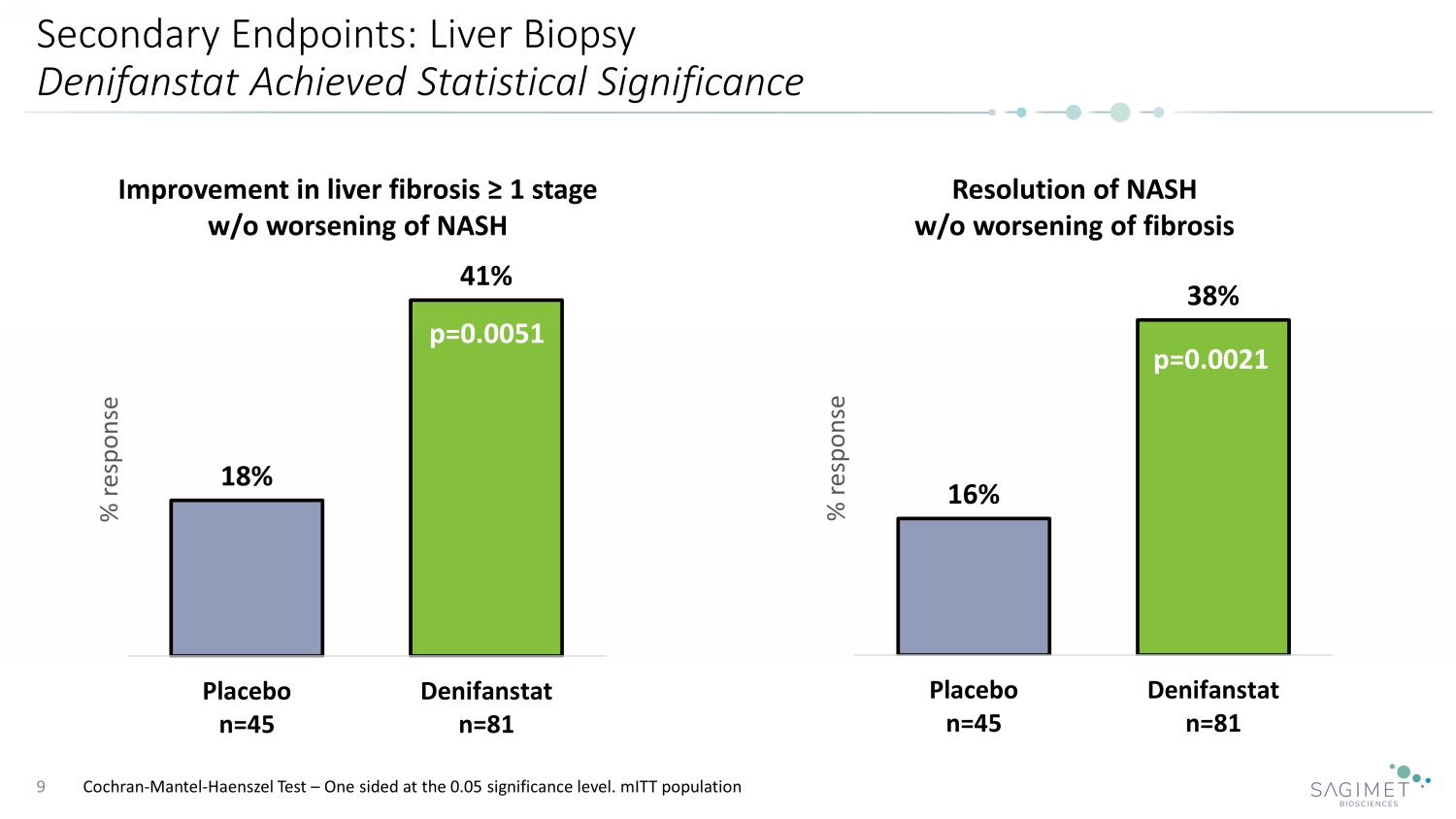
Secondary Endpoints: Liver Biopsy Denifanstat Achieved Statistical Significance 9 Resolution of NASH w/o worsening of fibrosis p= 16% 38% Placebo n=45 Denifanstat n=81 % response p=0.0021 Cochran - Mantel - Haenszel Test – One sided at the 0.05 significance level. mITT population 18% 41% Placebo n=45 Denifanstat n=81 % response Improvement in liver fibrosis ≥ 1 stage w/o worsening of NASH p=0.0051
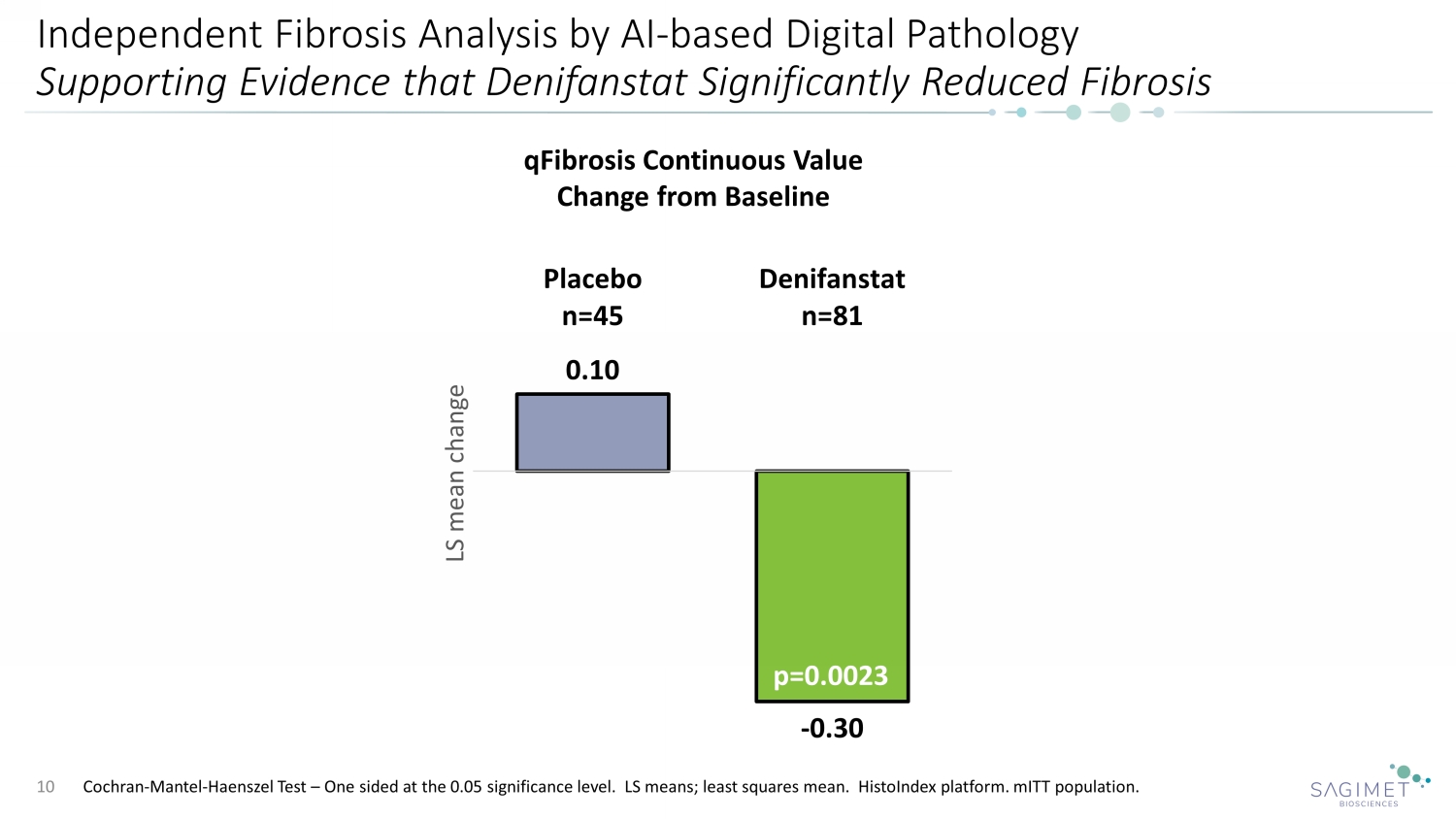
Independent Fibrosis Analysis by AI - based Digital Pathology Supporting Evidence that Denifanstat Significantly Reduced Fibrosis 10 0.10 - 0.30 Placebo n=45 Denifanstat n=81 LS mean change qFibrosis Continuous Value Change from Baseline p=0.0023 Cochran - Mantel - Haenszel Test – One sided at the 0.05 significance level. LS means; least squares mean. HistoIndex platform. mITT population.
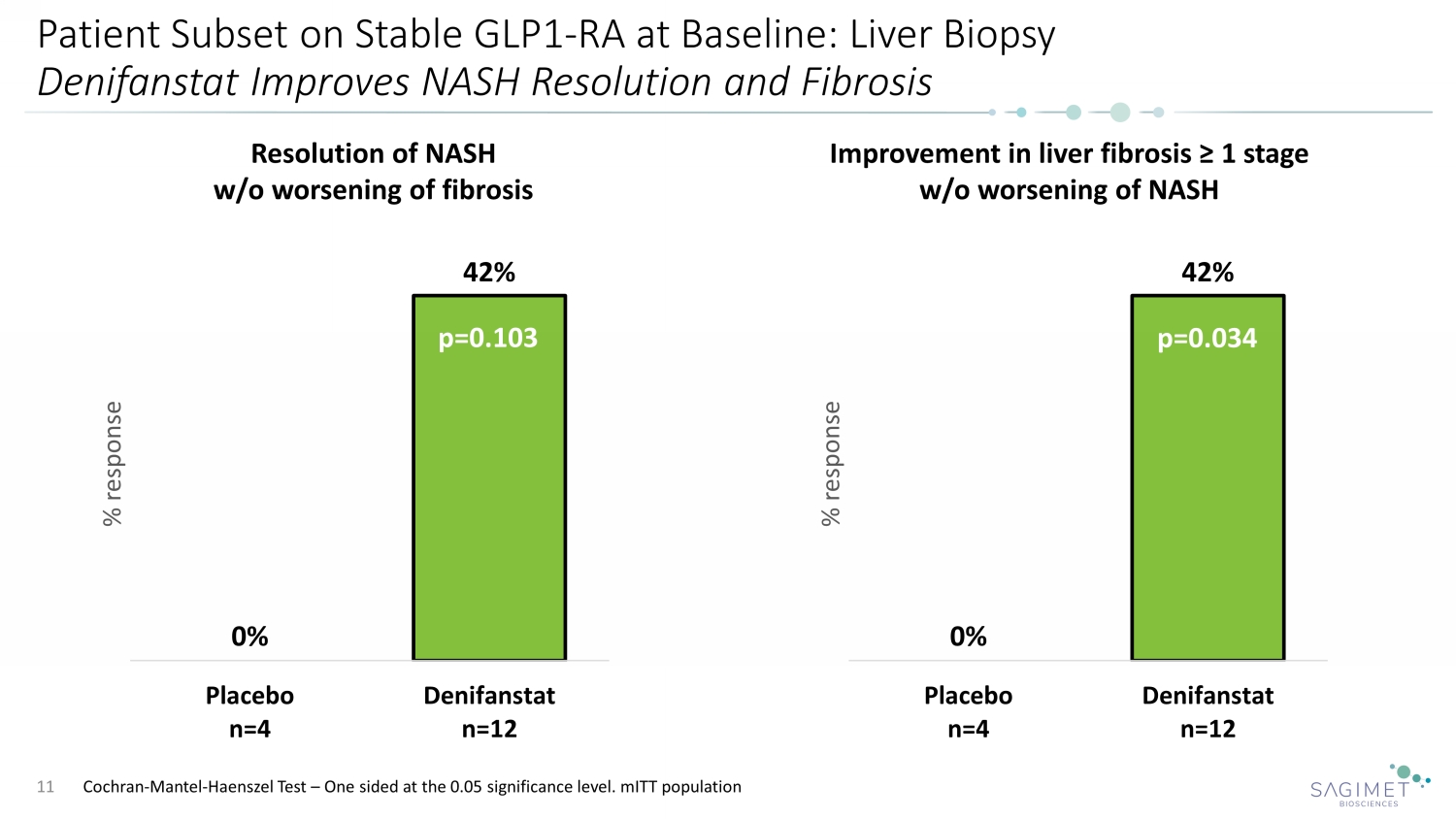
Patient Subset on Stable GLP1 - RA at Baseline: Liver Biopsy Denifanstat Improves NASH Resolution and Fibrosis 11 Resolution of NASH w/o worsening of fibrosis Improvement in liver fibrosis ≥ 1 stage w/o worsening of NASH 0% 42% Placebo n=4 Denifanstat n=12 % response 0% 42% Placebo n=4 Denifanstat n=12 % response p=0.034 p=0.103 Cochran - Mantel - Haenszel Test – One sided at the 0.05 significance level. mITT population
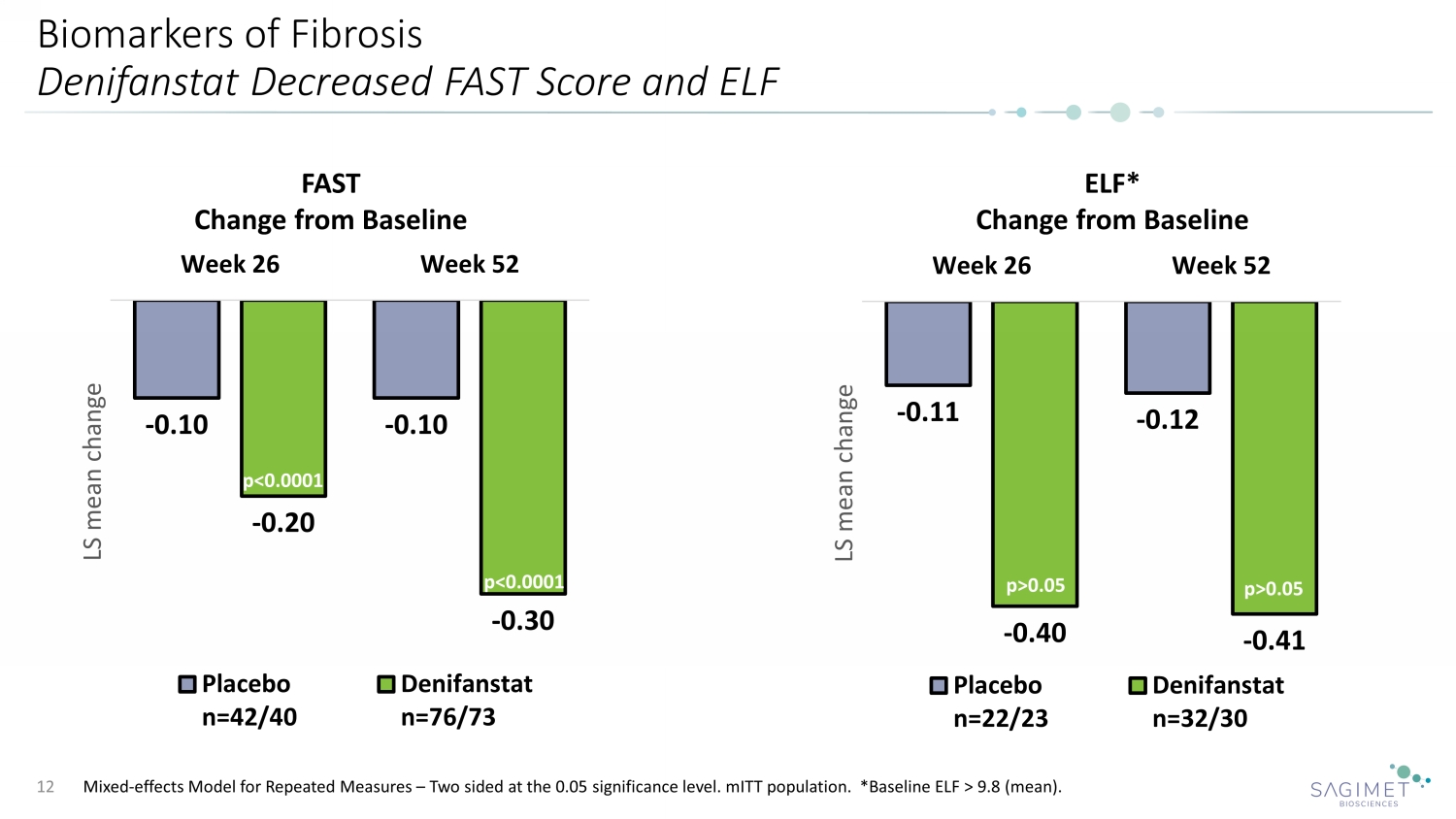
Biomarkers of Fibrosis Denifanstat Decreased FAST Score and ELF Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population. *Baseline ELF > 9.8 (mean). 12 p= p= - 0.11 - 0.12 - 0.40 - 0.41 Week 26 Week 52 LS mean change Placebo n=22/23 Denifanstat n=32/30 ELF* Change from Baseline - 0.10 - 0.10 - 0.20 - 0.30 Week 26 Week 52 LS mean change Placebo n=42/40 Denifanstat n=76/73 FAST Change from Baseline p<0.0001 p<0.0001 p>0.05 p>0.05
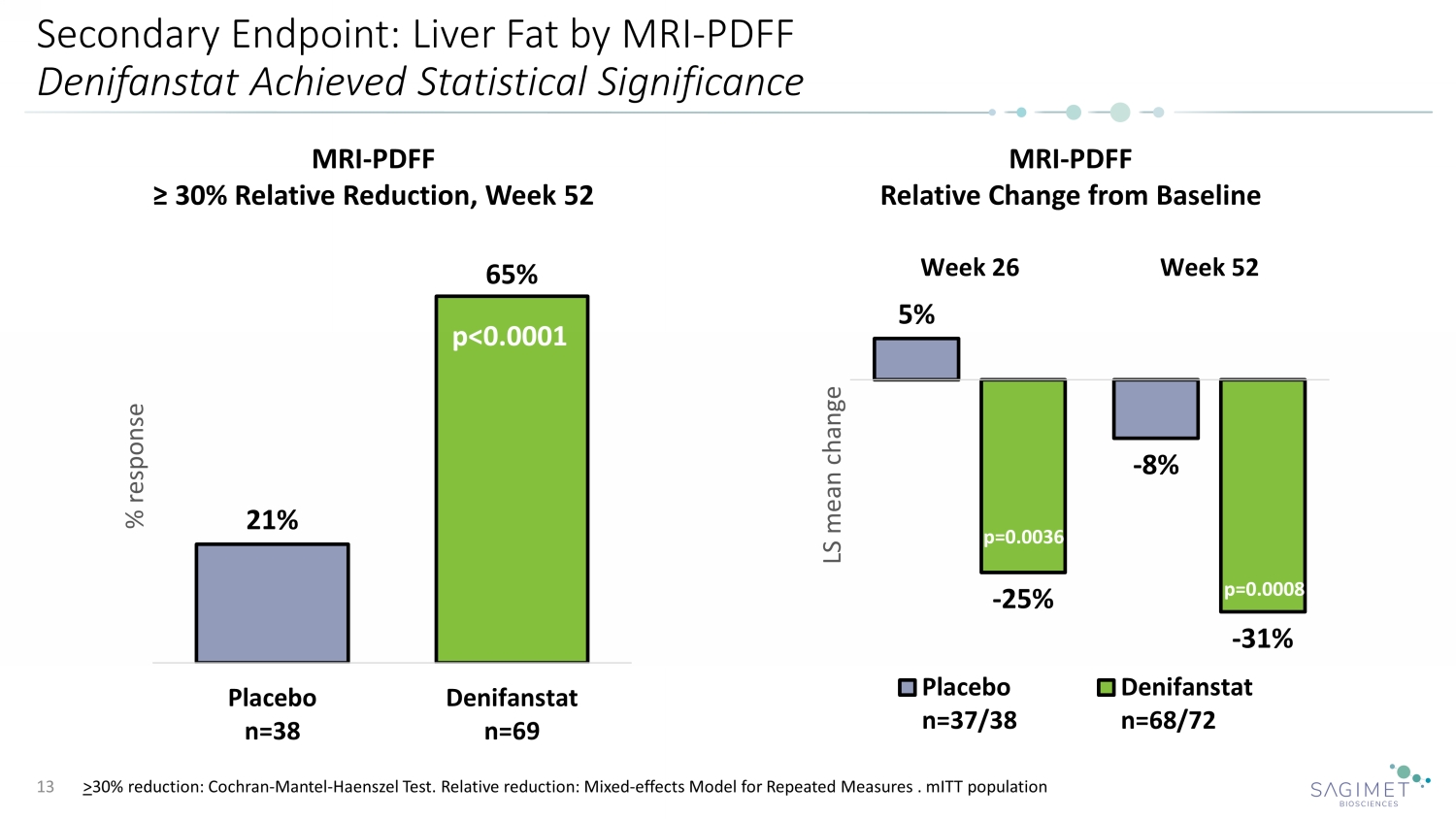
Secondary Endpoint: Liver Fat by MRI - PDFF Denifanstat Achieved Statistical Significance 13 21% 65% Placebo n=38 Denifanstat n=69 % response MRI - PDFF ≥ 30% Relative Reduction, Week 52 p<0.0001 MRI - PDFF Relative Change from Baseline 5% - 8% - 25% - 31% Week 26 Week 52 LS mean change Placebo n=37/38 Denifanstat n=68/72 p=0.0008 p=0.0036 > 30% reduction: Cochran - Mantel - Haenszel Test. Relative reduction: Mixed - effects Model for Repeated Measures . mITT population
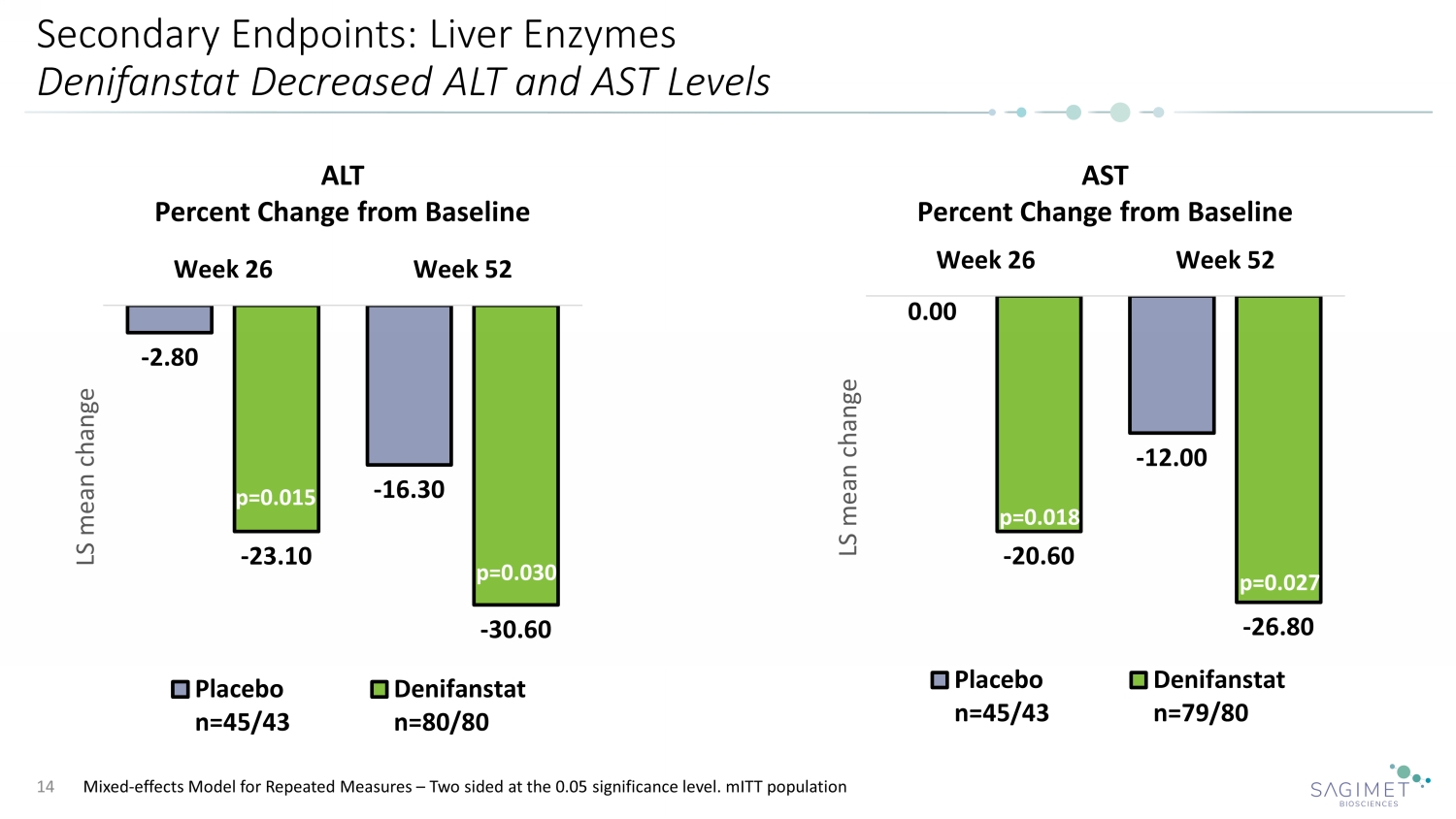
Secondary Endpoints: Liver Enzymes Denifanstat Decreased ALT and AST Levels 14 ALT Percent Change from Baseline AST Percent Change from Baseline Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population - 2.80 - 16.30 - 23.10 - 30.60 Week 26 Week 52 LS mean change Placebo n=45/43 Denifanstat n=80/80 p=0.015 p=0.030 0.00 - 12.00 - 20.60 - 26.80 Week 26 Week 52 LS mean change Placebo n=45/43 Denifanstat n=79/80 p=0.018 p=0.027
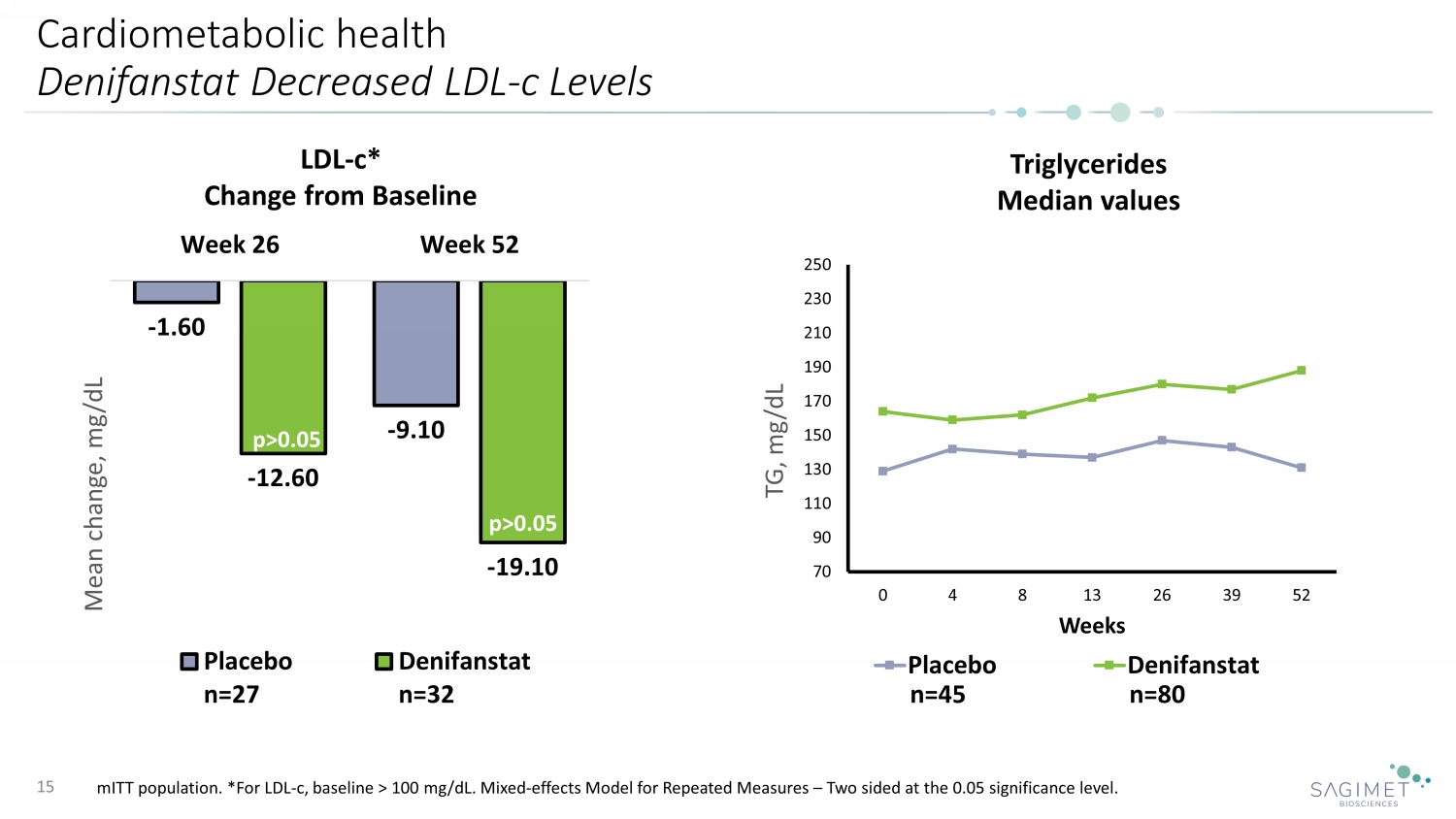
Cardiometabolic health Denifanstat Decreased LDL - c Levels 15 LDL - c* Change from Baseline p>0.05 p>0.05 Triglycerides Median values 70 90 110 130 150 170 190 210 230 250 0 4 8 13 26 39 52 TG, mg/dL Weeks Placebo Denifanstat - 1.60 - 9.10 - 12.60 - 19.10 Week 26 Week 52 Mean change, mg/dL Placebo n=27 Denifanstat n=32 mITT population. *For LDL - c, baseline > 100 mg/dL. Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance le vel. n=45 n=80 p>0.05 p>0.05
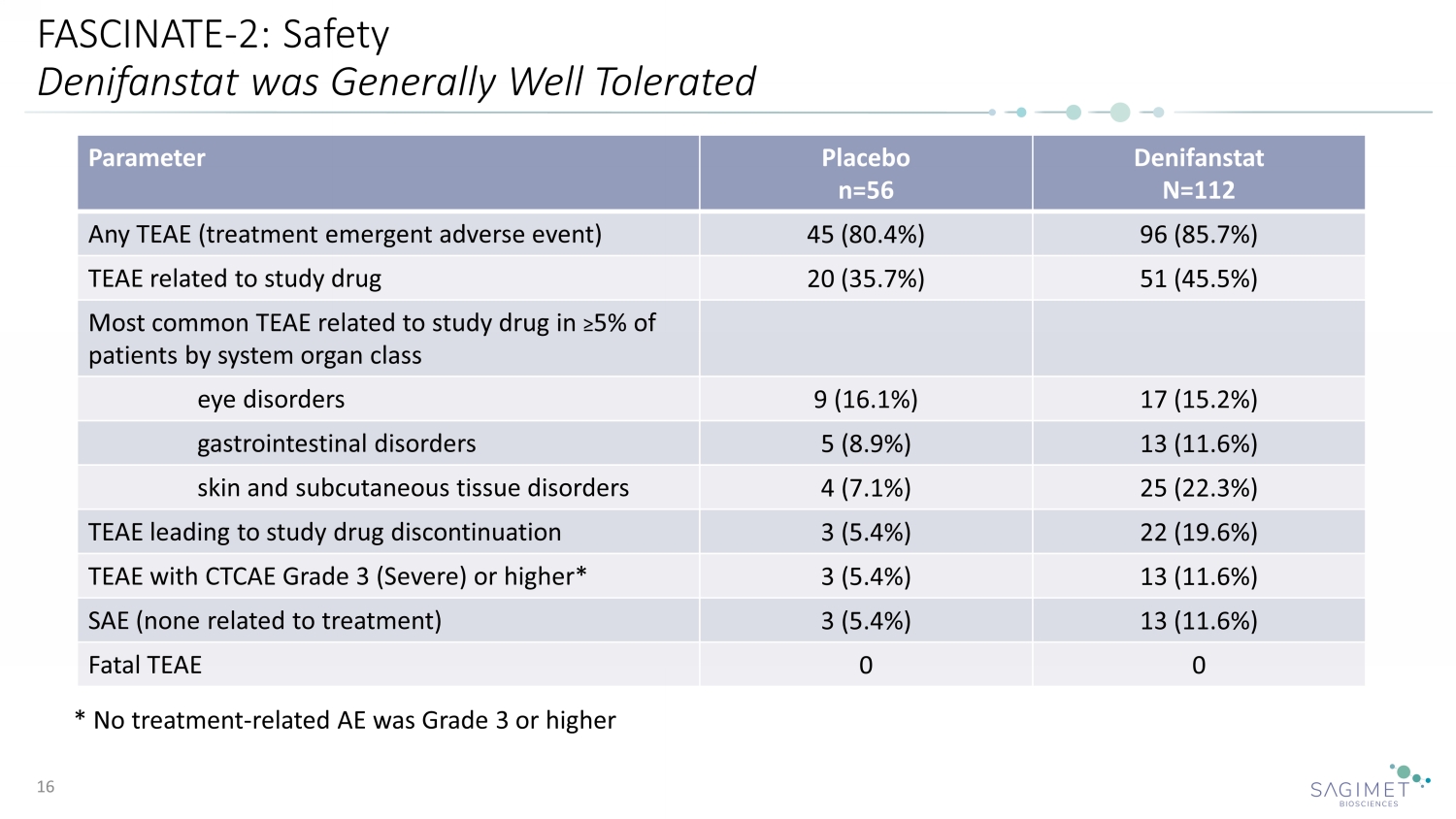
FASCINATE - 2: Safety Denifanstat was Generally Well Tolerated 16 Parameter Placebo n=56 Denifanstat N=112 Any TEAE (treatment emergent adverse event) 45 (80.4%) 96 (85.7%) TEAE related to study drug 20 (35.7%) 51 (45.5%) Most common TEAE related to study drug in ≥ 5% of patients by system organ class eye disorders 9 (16.1%) 17 (15.2%) gastrointestinal disorders 5 (8.9%) 13 (11.6%) skin and subcutaneous tissue disorders 4 (7.1%) 25 (22.3%) TEAE leading to study drug discontinuation 3 (5.4%) 22 (19.6%) TEAE with CTCAE Grade 3 (Severe) or higher* 3 (5.4%) 13 (11.6%) SAE (none related to treatment) 3 (5.4%) 13 (11.6%) Fatal TEAE 0 0 * No treatment - related AE was Grade 3 or higher
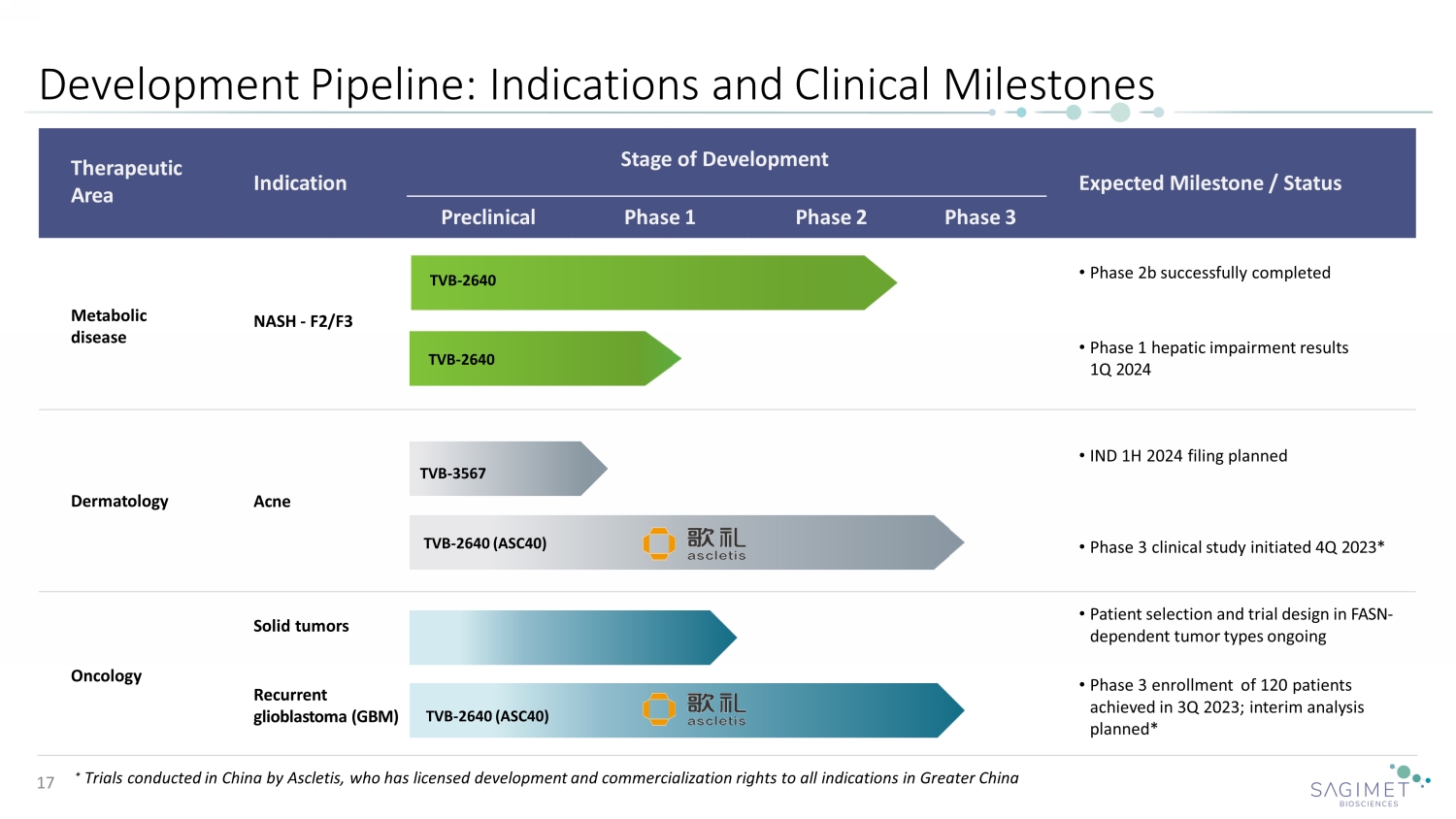
Therapeutic Area Stage of Development Indication Expected Milestone / Status Preclinical Phase 1 Phase 2 Phase 3 Metabolic disease • Phase 2b successfully completed NASH - F2/F3 • Phase 1 hepatic impairment results 1Q 2024 Dermatology Acne • IND 1H 2024 filing planned • Phase 3 clinical study initiated 4 Q 2023 * Oncology Solid tumors • Patient selection and trial design in FASN - dependent tumor types ongoing Recurrent glioblastoma ( GBM ) • Phase 3 enrollment of 120 patients achieved in 3Q 2023; interim analysis planned * Development Pipeline : Indications and Clinical Milestones 17 * Trials conducted in China by Ascletis, who has licensed development and commercialization rights to all indications in Greater China TVB - 2640 TVB - 2640 TVB - 2640 (ASC40) TVB - 2640 (ASC40) TVB - 3567

End 18

















