Exhibit 99.1

ANNUAL INFORMATION FORM
Fiscal Year Ended February 28, 2014
May 29, 2014
TABLE OF CONTENTS
Basis of Presentation | 1 | |||
Cautionary Note Regarding Forward-Looking information | 1 | |||
Corporate Structure | 4 | |||
General Development of the Business | 6 | |||
Recent Developments | 13 | |||
Business of the Corporation | 20 | |||
Risk Factors | 42 | |||
Dividends | 58 | |||
Description of the Share Capital | 58 | |||
Market for Securities | 60 | |||
Directors and Officers | 60 | |||
Cease Trade Orders, Bankruptcies, Penalties or Sanctions | 63 | |||
Legal Proceedings and Regulatory Actions | 64 | |||
Interest of Management and Others in Material Transactions | 64 | |||
Transfer Agents and Registrars | 64 | |||
Material Contracts | 64 | |||
Interest of Experts | 64 | |||
Report on Audit Committee | 64 | |||
Additional Information | 66 | |||
Schedule “A” Charter of the Audit Committee of the Board of Directors | A-1 |
BASIS OF PRESENTATION
As used in this annual information form, or AIF, unless the context otherwise requires, references to “Neptune”, the “Corporation”, “we”, “us”, “our” or similar terms refer to Neptune Technologies & Bioressources Inc. and its subsidiaries, references to “Acasti” refer to Acasti Pharma Inc. and references to “NeuroBio” refer to NeuroBioPharm Inc.
Market data and certain industry data and forecasts included in this AIF were obtained from internal company surveys, market research, publicly available information, reports of governmental agencies and industry publications and surveys. Neptune has relied upon industry publications as its primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. Neptune has not independently verified any of the data from third-party sources, nor has Neptune ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which Neptune believes to be reliable based upon management’s knowledge of the industry, have not been independently verified. Forecasts are particularly likely to be inaccurate, especially over long periods of time. In addition, Neptune does not know what assumptions regarding general economic growth were used in preparing the forecasts cited in this AIF. While Neptune is not aware of any misstatements regarding Neptune’s industry data presented herein, Neptune’s estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under “Risk Factors” in this AIF. While Neptune believes its internal business research is reliable and market definitions are appropriate, neither such research nor definitions have been verified by any independent source. This AIF may only be used for the purpose for which it has been published.
Unless otherwise noted, in this annual information form, all information is presented as of February 28, 2014. All references in this annual information form to “dollars”, “CDN$” and “$” refer to Canadian dollars, and references to “US$” refer to United States dollars, unless otherwise expressly stated.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This AIF contains certain information that may constitute forward-looking information within the meaning of Canadian securities laws and forward-looking statements within the meaning of U.S. federal securities laws, both of which we refer to as forward-looking information. Forward-looking information can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts. Forward-looking information in this AIF includes, but is not limited to, information or statements about:
| • | Neptune’s ability to finalize reconstruction of its production facility, the timing and cost of completion of the reconstruction project, and the amount of production capacity for krill oil products at the new production facility; |
| • | Neptune’s ability to obtain all necessary operating permits from the Québec Ministry of Sustainable Development, Environment and the Fight Against Climate Change (the “Ministry of Environment”) and theCommission de la santé et de la sécurité du travail (the “CSST”) to start production at its new production facility; |
| • | Neptune’s ability to commission and complete the start-up and ramp-up of production at its new production facility; |
| • | Neptune’s ability to generate revenue through production at its new production facility; |
| • | Neptune’s ability to maintain and develop its existing third party supply and production agreements on terms favourable to Neptune; |
1
| • | Neptune’s ability to obtain financing, on terms favourable to Neptune to implement its operating and growth strategy; |
| • | Neptune’s ability to recover additional insurance proceeds relating to the incident at its production plant under its various insurance policies; |
| • | Neptune’s ability to regain lost customers and re-establish itself in the nutraceutical market; |
| • | Neptune’s ability to oppose or settle notices allegingnon-compliance by the Ministry of Environment and the CSST and any other proceedings brought by other parties relating to the November 2012 incident at its former operating facility; |
| • | Neptune’s ability, and the ability of its distribution partners, to continue to commercialize krill oil products, including Neptune Krill Oil (“NKO®”) and ECOKRILL Oil (“EKO™”) and to regain and maintain its market share position for krill oil products; |
| • | Neptune’s ability to continue to invest in product development and trials; |
| • | plans of Neptune’s subsidiaries, Acasti and NeuroBio, to conduct new clinical trials for product candidates, including the timing and results of these clinical trials; |
| • | Neptune’s ability to maintain and defend its intellectual property rights in NKO® and EKO™ and in its product candidates; |
| • | the ability of Neptune’s subsidiaries, Acasti and NeuroBio, to commercialize other product candidates in the United States, Canada and internationally; |
| • | the timing of the receipt of royalty payments under the terms of Neptune’s settlement agreements; |
| • | Neptune’s estimates of the size of the potential markets for NKO® and EKO™ and its product candidates and the rate and degree of market acceptance of EKO™ and NKO® and its product candidates; |
| • | Neptune’s abitlity to use the net proceeds from its latest public offering for the purposes identified in Neptune’s propsectus supplement dated February 28, 2014; |
| • | the health benefits of NKO® and EKO™ and Neptune’s product candidates as compared to other products in the nutraceutical and pharmaceutical markets; |
| • | Neptune’s expectations regarding its financial performance, including its revenues, expenses, gross margins, liquidity, capital resources and capital expenditures; and |
| • | Neptune’s expectations regarding its significant impairment losses and future write-downs, charge-offs or impairment losses. |
Although the forward-looking information is based upon what we believe are reasonable assumptions, no person should place undue reliance on such information since actual results may vary materially from the forward-looking information. Certain key assumptions made in providing the forward-looking information include the following:
| • | Neptune will obtain all required operating permits to resume operations at the new production facility by approximately early June 2014; |
| • | the start-up and ramp-up period and performance of the new production facility will be consistent with management’s expectations; |
| • | sales objectives for its krill oil products assume that Neptune will be able to maintain customer relationships and that demand for its products will continue; |
2
| • | customer demand for Neptune’s products, particularly NKO®, will be consistent with or stronger than pre-November 2012 levels; |
| • | Neptune’s business plan to focus on the production of its lead products, NKO® and EKO™, will not be substantially modified; |
| • | capital derived from future financings will be available to Neptune on terms that are favourable; |
| • | Neptune will be able to protect its intellectual property; and |
| • | Neptune will be able to continue to meet the continued listing requirements of the NASDAQ Stock Market and the Toronto Stock Exchange. |
In addition, the forward-looking information is subject to a number of known and unknown risks, uncertainties and other factors, including those described in this AIF under the heading “Risk Factors”, many of which are beyond our control, that could cause actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking information, including, without limitation:
| • | the heavy dependence of the Corporation’s future prospects on the timely and successful reconstruction of its production plant; |
| • | the need for the Corporation to obtain all required operating permits to resume its production; |
| • | the Corporation’s need for additional funding; |
| • | the Corporation’s potential inability to recover all of the insurance proceeds it has claimed; |
| • | possibility that new claims or lawsuits relating to the plant explosion may be brought against the Corporation; |
| • | the Corporation’s potential inability to restore or grow its customer base; |
| • | the Corporation’s reliance on a limited number of distributors and significant concentration of accounts receivables; |
| • | the fact that the Corporation has suffered significant impairment losses and its assets may be subject to future write-downs, charge-offs or impairment losses; |
| • | the Corporation may lose its control of Acasti; |
| • | the Corporation’s history of net losses and inability to achieve profitability to date; |
| • | NKO® and EKO™ may not be successfully commercialized; |
| • | changes in regulatory requirements and interpretations of regulatory requirements; |
| • | the Corporation’s reliance on third parties for the manufacture, supply and distribution of its products and for the supply of raw materials; |
| • | the Corporation’s ability to manage its growth efficiently; |
| • | the Corporation’s ability to further penetrate core or new markets; |
| • | the Corporation’s ability to attract and retain skilled labor; |
| • | the Corporation’s ability to attract, hire and retain key management and personnel; |
3
| • | the success of current and future clinical trials by the Corporation and its subsidiaries; |
| • | the Corporation’s ability to achieve its publicly announced milestones on time or at all; |
| • | product liability lawsuits could be brought against the Corporation and its subsidiaries; |
| • | intense competition from other companies in the pharmaceutical and nutraceutical industry; |
| • | the Corporation’s ability to secure and defend its intellectual property rights; and |
| • | the fact that the Corporation does not currently intend to pay any cash dividends on the Common Shares in the foreseeable future. |
Consequently, all the forward-looking information is qualified by this cautionary statement and there can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the expected consequences or effects on our business, financial condition or results of operations. Accordingly, you should not place undue reliance on the forward-looking information. Except as required by applicable law, Neptune does not undertake to update or amend any forward-looking information, whether as a result of new information, future events or otherwise. All forward-looking information is made as of the date of this AIF.
CORPORATE STRUCTURE
Corporation Overview
Neptune was incorporated on October 9, 1998 pursuant to a certificate of incorporation issued under Part 1A of theCompanies Act(Québec). On February 14, 2011, theBusiness Corporations Act(Québec) came into effect and replaced theCompanies Act(Québec). Neptune is now governed by theBusiness Corporations Act(Québec). On May 30, 2000, the articles of the Corporation were amended in order to proceed with the restructuring of the Corporation’s capital stock and to convert its then issued and outstanding shares into newly-created classes of shares. The Corporation’s articles were also amended on May 31, 2000 to create Series A Preferred Shares. On August 29, 2000, the Corporation converted all its issued and outstanding Class A shares into Class B subordinate shares. On September 25, 2000, the Corporation further amended its share capital to eliminate its Class A shares and converted its Class B subordinate shares into common shares. On May 11, 2001, the Corporation amended its articles of incorporation to repeal the restrictions with respect to closed companies. On November 1, 2013, the Corporation amended its articles of incorporation to reflect certain changes to items relating to board matters.
Neptune’s head office and registered office is located at 545, Promenade du Centropolis, Suite 100, Laval, Québec, Canada, H7T 0A3. The Corporation’s website address iswww.neptunebiotech.com. The Corporation is also the owner of the websiteswww.mynko.com andwww.neptunekrilloil.com.
Intercorporate Relationships
Neptune has two wholly-owned subsidiaries, Neptune Technologies & Bioressources USA Inc., or Neptune USA, and Neptune Technologies & Bioressources Hong Kong Limited, or Neptune Hong Kong, and two subsidiaries, Acasti and NeuroBio. As of the date of this AIF, Neptune owns 49.07% of the voting rights attached to the securities of Acasti and 95% of the voting rights attached to the securities of NeuroBio. See “Corporate Structure—Corporate Structure Diagram”.
Acasti was incorporated on February 1, 2002 pursuant to a certificate of incorporation issued under Part 1A of theCompanies Act(Québec) under the name 9113-0310 Québec Inc. and, prior to its partial spin-off in 2008, was a wholly-owned subsidiary of Neptune. The common shares of Acasti are listed and posted for trading on the TSX Venture Exchange, or TSXV, under the symbol “APO” and on the NASDAQ Stock Market, or NASDAQ, under the symbol “ACST”. Acasti is a company involved in the pharmaceutical industry.
4
NeuroBio was incorporated on October 15, 2008 pursuant to a certificate of incorporation issued under Part 1A of theCompanies Act(Québec) under the name Neurovimer Pharma Inc. NeuroBio is also a company involved in the pharmaceutical industry.
Neptune USA was incorporated on June 1, 2006 under the laws of the State of Delaware and Neptune Hong Kong was incorporated on May 3, 2012 under the laws of Hong Kong. Neptune USA and Neptune Hong Kong do not carry on an active business at this time.
Corporate Structure Diagram
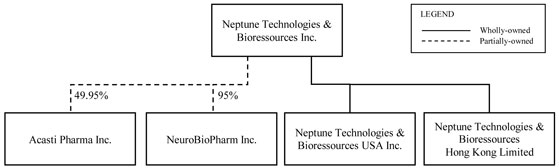
As of the date of this AIF, Neptune owns 51,942,183 Class A shares of Acasti, which are common shares, representing 49.07% of Class A shares issued and outstanding and 49.07% of the voting rights attached to the securities of Acasti. Acasti Class A shares (common shares) are voting, participating and with no par value. Neptune also owns 592,500 common share purchase warrants of Acasti. See “General Development of the Business—Fiscal Year Ended February 28, 2014”.
As of the date of this AIF, Neptune holds 95% of the voting rights attached to the securities of NeuroBio through the holding of 6,500,990 Class A subordinate voting shares of NeuroBio, representing approximately 76% of the Class A subordinate voting shares issued and outstanding, 2,475,000 Class B multiple voting shares of NeuroBio, representing 99% of Class B multiple voting shares issued and outstanding, 17,325,000 Class G non-voting shares of NeuroBio, representing 99% of Class G non-voting shares issued and outstanding, and 25,740,000 Class H subordinate voting shares of NeuroBio, representing 99% of Class H subordinate voting shares issued and outstanding. As of the date of this AIF, Neptune also holds warrants of NeuroBio, namely 1,940,000 Series 2011-1 warrants, 1,885,574 Series 2011-2 warrants and 46,246 Series 2011-3 warrants to purchase 3,871,820 Class A subordinate voting shares of NeuroBio. On October 31, 2012, 2,000,000 Class A subordinate voting shares and 4,000,000 Series 2011-1 warrants of NeuroBio held by Neptune were distributed to Neptune’s shareholders by way of a dividend-in-kind. See “General Development of the Business—Fiscal Year Ended February 28, 2013”.
Reorganization of the Share Capital of NeuroBio
On April 12, 2011, NeuroBio proceeded with the following transactions affecting its capital structure: (i) NeuroBio consolidated all classes of its capital stock on a 2:1 basis; (ii) NeuroBio exchanged the resulting 50 Class A shares for 1,000 new Class A subordinate voting shares, 26,000,000 Class H subordinate voting shares redeemable for $0.45 per share and 6,000,000 Series 2011-1 warrants; (iii) NeuroBio exchanged the resulting 17,500,000 Class C non-voting shares, 3,500,000 Series 4 warrants and 1,500,000 Series 5 warrants for 17,500,000 Class G non-voting shares redeemable for $0.20 per share, 3,450,075 Series 2011-2 warrants and 8,050,175 Series 2011-3 warrants; and (iv) NeuroBio converted its accounts payable to Neptune in the amount of approximately $850,000 into 8,500,000 Class A subordinate voting shares.
The purpose of the transaction was to establish and freeze the estimated fair value of NeuroBio for its shareholder. Following the transaction, the valuation of the Class A subordinate voting shares was determined by the last transaction of NeuroBio; which is the conversion of its account payable to Neptune into 8,500,000 Class A subordinate voting shares, at $0.10 per share.
5
On March 18, 2014, NeuroBrio extended the time of expiry of its Series 2011-1 warrants from April 12, 2014 to April 12, 2015, barring the earlier listing of Class A shares on a recognized stock exchange.
GENERAL DEVELOPMENT OF THE BUSINESS
Three Year History
Fiscal Year Ended February 29, 2012
During the fiscal year ended February 29, 2012, Neptune continued its investor relations efforts to increase Neptune’s visibility toward the investment community in Canada and the United States, with the objective of reaching higher trading volumes. Neptune presented at the 23rd annual Roth OC Growth Stock Conference in California. Over 400 companies selected by Roth Capital Partners were presenting at the conference and over 1,000 buy-side investors attended the conference. On the research and development front, Neptune presented at the 2011 Scientific Sessions of the American Heart Association its clinical results on the absorption of NKO® compared to competitive products. Neptune sustained its research initiatives by investing in product development, preclinical and clinical studies to validate the health benefits of its products.
On May 3, 2011, Neptune completed a non-brokered private placement of $12,438,000 through the offering of common shares at a price of $2.15 (US$2.25) plus 25% warrant coverage at $2.65 (US$2.75). In total, Neptune issued 5,787,057 common shares and 1,446,265 warrants. Following the end of the first quarter, officers and directors of Neptune exercised 550,000 options a strike price of $2.60, representing an amount of $1,430,000 in aggregate cash proceeds.
Also in May 2011, Neptune announced that it and its marine derived products successfully completed an extensive review of key environmental claims by NSF International. See “Business of the Corporation—Supply of Krill”.
In the second quarter, Neptune appointed Raj Nakra Associates as an agent for the Indian market. Neptune also finalized agreements with two major U.S. distributors to sell NKO® through their well-established network of U.S. national retailers and wholesalers.
On November 28, 2011, Neptune’s common shares started trading on the TSX following Neptune’s migration from the TSXV. In December 2011, Neptune announced the first phase of the currently underway expansion project of its Sherbrooke plant. See “Business of theCorporation - Manufacturing and Facilities”.
In September 2011, Neptune announced the conclusion of a memorandum of understanding, or MOU, with Shanghain KaiChuang Deep Sea Fisheries Co., Ltd., or SKFC, to form a 50/50 joint venture named Neptune-SKFC Biotechnology, to manufacture and commercialize Neptune’s krill products in Asia. The MOU is still subject to further negotiations and to approval by the boards of each party as well as by Chinese regulators. There has been no significant developtment since the conclusion of the MOU in September 2011 and there are no guaratees that the joint venture ever materialized.
On October 4, 2011, the Corporation filed Complaints against Aker Biomarine ASA, Aker Biomarine Antarctic USA Inc. and Schiff Nutrition International Inc. (collectively “Aker”) and against Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC and Azantis Inc (collectively “Enzymotec”). Both Complaints were for the infringement of the Corporation’s US patent 8,030,348 and for damages. See “Business of theCorporation - Economic Dependence/Litigation”.
On December 21, 2011, the Corporation received a motion filed by the University of Sherbrooke, asking the Court to order the transfer of certain intellectual property to Neptune. See “Business of the Corporation—Economic Dependence/Litigation”.
6
Fiscal Year Ended February 28, 2013
Prior to the incident that destroyed Neptune’s production plant located in Sherbrooke, Québec on November 8, 2012, the Corporation continued to expand its customer base worldwide and revenue growth was driven by repeat demand from existing customers and incoming demand from new customers from North America, Europe and Australia.
In the first quarter, from March 11 to 14, 2012, Neptune attended the 24th annual Roth OC Growth Stock Conference in California. Neptune also took that opportunity to make a presentation at The Ritz Carlton in Laguna Niguel, California on March 12, 2012 in front of a large number of buy-side investors.
The Corporation presented novel innovative product opportunities customized for dietary supplements, functional and medical foods and introduced a new pipeline of novel formulations containing its proprietary marine omega-3 phospholipids enhanced with validated bioactive ingredients targeted to specific health applications to its clientele in Engredea/Natural Products Expo West in Anaheim on March 9th-11th, 2012 and in Vitafoods Europe in Geneva on May 22nd-24th, 2012.
Also on March 27, 2012, Neptune entered into a multi-year partnership with former NFL (National Football League) Super Bowl Champion and Hall-of Fame quarterback, John Elway. John Elway retired in 1999 and statistically was the second most prolific passer in NFL history. He is currently Executive Vice President of Football Operations for the Denver Broncos in addition to being part owner of four successful Elway’s Restaurants and the same number of automobile dealerships bearing his name. The compensation package is a combination of cash payment as well as stock options over the contractual period.
On May 10, 2012, Mr. Elway along with Neptune’s team, attended the SupplySide MarketPlace Trade show at the Javits Center in New York City. Mr. Elway took this opportunity to meet with investors and partners and also stopped at Neptune’s booth to meet with participants at the show. This was the first of many public appearances of John Elway as Neptune’s ambassador.
On April 11, 2012, Neptune’s Board of Directors, as part of its annual review of direct and indirect remunerations, confirmed the grants of a total of 1,580,000 incentive stock options of Neptune and 730,000 rights on NeuroBio warrants held by Neptune to employees, executive officers and directors. Neptune incentive stock options have an exercise price of $3.15 and a 3 year maturity. Rights on NeuroBio warrants have an aggregate exercise price $0.75 and maturities of April 12, 2016, and were subject to shareholder approval, which was obtained on June 21, 2012. Insiders have been granted a total of 800,000 Neptune incentive stock options, and 435,000 rights on NeuroBio warrants.
On April 26, 2012, the Corporation granted one three-year warrant to purchase 1,000,002 common shares to a consultant under a financial consulting agreement. The warrants will be exercisable at a price of US$5.00 per share until June 15, 2015. The warrant shall be subject to vesting in six equal instalments of 166,667 warrant shares, the first vesting being on the date of issuance and the remaining vesting being respectively on the last day of each quarter. The financial consulting agreement came to term on April 26, 2013.
On May 22, 2012, Neptune filed for Reexamination the Aker Biomarine’s granted Australian patent (AU2008231570). Neptune also communicated its conclusion that Aker’s patent had no impact on its position as the leading krill oil provider to the Australian market. Neptune also reaffirmed that it firmly believes that Aker’s patent is invalid. Specifically, there are clear disclosures in prior printed publications and patents, some of which predate Aker’s application by almost twenty years, which teach exactly what Aker claims to have invented. Furthermore, and tellingly, it is noted that both the United States and European Patent Offices have rejected these claims, or narrower versions thereof, for lack of novelty and obviousness. Accordingly, in light of the prior printed publications and patents put forth in this Reexamination Request, Neptune believes the Australian Patent Office (the “APO”) will reconsider its grant of Aker’s patent and declare the recently-issued claims to be unpatentable.
Also on May 22, 2012, following an audit by an auditor recognized by Friend of the Sea, or FOS, Neptune became the first krill oil manufacturer entitled to use the “Friend of the Sea” environmental certification. See “Business of the Corporation—Supply of Krill”.
7
On May 23, 2012, Neptune announced that Dr. Harlan Waksal, Executive Vice-President, Business & Scientific Affairs of Acasti, was appointed to the Corporation’s Board of Director. Dr. Harlan Waksal is a retired physician, founder of Imclone System Inc. in which he has been involved as the President, Chief Executive Officer, Chief Operating Officer and Executive Vice-President from 1987 to 2003. Imclone System has developed and obtained approval for a new targeted biologic cancer therapy known as Erbitux and was later acquired by Eli Lily for $6.5 billion US in October 2008. Dr. Harlan Waksal currently sits on the Board of Directors of Oberlin College and Senesco Technologies, is the author of over 50 scientific publications and has been the author of multiple patents and patents applications.
On June 7, 2012, the Corporation announced that the U.S. Patent & Trademark Office, or USPTO, allowed one of its continuation patent applications, number 13/189,714, which claims the benefit of Neptune’s U.S. Patent No. 8,030,348. This continuation application contains claims to further embodiments of the inventions that were disclosed in the ‘348 Patent; specifically to krill extracts comprising a phospholipid suitable for human consumption. These claims cover a number of krill oil products presently sold in the U.S. market. The continuation application, which was filed less than a year ago, was allowed by the USPTO after a thorough examination. During prosecution, Neptune provided the USPTO with a substantial volume of prior art references and other materials, including the papers from re-examination requests filed by Aker Biomarine ASA directed to the ‘348 Patent and a related Neptune patent, and the oppositions being undertaken on related Neptune patents in Europe and Australia.
On August 28, 2012, the Corporation and its subsidiary Acasti announced the extension of the relationship with The Howard Group as the companies’ investor relations consultant. Since 1988, The Howard Group has provided comprehensive investor and financial relations, business development solutions and in-depth strategic planning to public companies. The Howard Group is associated with the Insight Limited Partnership II, which invests in micro and small cap companies. Traditional and new online initiatives will be directed at the investment community and investing public on behalf of Neptune and Acasti to increase the following and participation of the market in those two corporations. The term of the IR Agreement is for a period of 12 months. In addition to a fee of $6,000 per month, The Howard Group has been granted options to purchase an aggregate total of 50,000 common shares of Neptune at a price of $5.00 per share and 50,000 common shares of Acasti a price of $2.50. The options will vest in equal amounts over an 18 months term.
On September 7, 2012, Neptune announced that its board of directors had approved the distribution of 2,000,000 units of NeuroBio owned by Neptune pro rata to the holders of record of common shares of Neptune as at October 15, 2012 by way of a dividend-in-kind. The dividend was distributed on October 31, 2012 and each shareholder on the dividend record date received one unit for each lot of approximately 29.27 common shares of Neptune held. Each unit consisted of one class A subordinate voting share of NeuroBio and two series 2011-1 warrants and the estimated fair market value of the unit was approximately $0.10 per unit. Each full warrant entitles its holder to purchase one class A subordinate voting share of NeuroBio at a price of $0.40 plus a transfer premium of $0.35 payable to Neptune upon exercise with each warrant expiring on the occurrence of the earliest of the two following events: (i) fifteen days after the listing of the class A subordinate voting shares on a recognized stock exchange; or (ii) April 12, 2014. The terms applicable to the distribution of the dividend were described in the final prospectus filed by NeuroBio on September 5, 2012 with the securities commissions and other similar regulatory authorities in each of the provinces and territories of Canada. After the distribution of the dividend-in-kind, Neptune’s ownership interest in NeuroBio class A shares was reduced to 76% from 99%. At such time, Neptune still owned 96% of all voting rights in NeuroBio.
On October 2, 2012, Neptune announced that the U.S. Patent & Trademark Office granted its new patent, US 8,278,351. The continuation patent claims the benefit of another of Neptune’s U.S. Patents, No. 8,030,348, (the “348 Patent”) and contains claims to krill extracts comprising a phospholipid suitable for human consumption. These new claims cover all of Neptune’s products, including the NKO® brand, and a number of krill oil products currently sold in the U.S. market. This new issued patent was granted after a thorough examination by the USPTO, including consideration of the papers from the re-examination requests filed by Aker Biomarine ASA regarding Neptune patents related to the ‘351 patent. The continuation patent, filed about a year ago, was allowed by the USPTO after a thorough examination which included a review of a substantial volume of prior art references and other materials, including the papers from the re-examination requests filed by Aker Biomarine ASA directed to the Patent and a related Neptune patent in the U.S., as well as the oppositions being undertaken on related Neptune patents in Europe and Australia.
8
The same day, Neptune announced that it had filed a second patent infringement lawsuit in the United States District Court for the District of Delaware alleging infringement of its recently issued continuation patent against Aker. Neptune has also filed a separate infringement action against Enzymotec. In addition to seeking monetary damages for all of the above defendants infringement of the ’351 Patent, Neptune is also requesting injunctive relief to prevent the Defendants from continuing to infringe Neptune’s patent. Should Neptune prevail in securing the requested injunctions, it would prevent the Defendants from manufacturing, using, offering for sale, selling and/or importing into the United States infringing krill oils.
Also on October 2, 2012, Neptune announced the closing of its Public Offering of US$34.1 million of common shares pursuant to which Neptune issued 7,318,000 common shares at US$4.10 per share. Prior to the closing, the underwriters exercised their over-allotment option to purchase an additional 989,762 common shares, resulting in a total of 8,307,762 common shares being issued on the day of the closing for gross proceeds of approximately US$34.1 million. The common shares were issued in the United States pursuant to Neptune’s effective shelf registration statement filed with the U.S. Securities and Exchange Commission (the “SEC”) and in Canada pursuant to a final short form base shelf prospectus filed with the securities regulatory authorities in the Provinces of Québec, Ontario, Manitoba, Alberta and British Columbia.
On November 6, 2012, Neptune hosted its 1st Annual Charity Poker Game at the Venetian Hotel in Las Vegas, prior to the SupplySide West Tradeshow. The game featured guest of honor John Elway, former Denver Broncos quarterback and Hall of Famer. Proceeds for the event were for the benefit of Vitamin Angels, a non-profit organization dedicated to reducing child mortality worldwide by connecting children in need with micronutrients.
In the afternoon of November 8, 2012, an explosion and fire destroyed Neptune’s production plant located in Sherbrooke, Québec, Canada.
On December 4, 2012, Neptune announced that it had entered into a prepayment agreement with Acasti pursuant to which Acasti exercised its option under its exclusive technology license agreement dated August 7, 2008 entered into with Neptune to pay in advance all of the future royalties payable to Neptune under the license agreement. The prepayment had the effect of increasing Neptune’s equity participation in Acasti (from approximately 57% to approximately 61%), after Neptune obtained the required approvals, by the issuance of 6,750,000 Class A shares in the share capital of Acasti, issued at a price of $2.30 per share, upon the exercise of a warrant delivered to Neptune at the signature of the prepayment agreement. This reflected a prepayment value, determined with the assistance of outside valuation specialists, using the pre-established prepayment formula set forth in the license agreement, that amounts to approximately $15.5 million. The prepayment and the issuance of the shares to Neptune received the approval of the TSXV and of the disinterested shareholders of Acasti (excluding Neptune and non-arm’s length parties to Neptune) at the June 27, 2013 annual meeting of shareholders of Acasti. Acasti is no longer required to pay any royalties to Neptune under the License Agreement during its term for the use of Neptune’s intellectual property under license.
In January 2013, the Board of Directors approved an equity incentive plan for employees, directors and consultants subject to the approval of the Toronto Stock Exchange and the shareholders of the Corporation at their next annual meeting. The plan provides for the issuance of restricted share units, performance share units, restricted shares, deferred share units or other share-based awards, under restricted conditions as may be determined by the Board of Directors. Upon fulfillment of the restricted conditions, as the case may be, the plan provides for settlement of the award through shares. At February 28, 2013, no instruments were issued by the Corporation under this plan.
On January 18, 2013 Neptune received a first interim insurance payment of $6 million further to the explosion that destroyed Neptune’s production plant. Neptune has insurance coverage in place covering among other things property damage, business interruption and general liability up to specified amounts and subject to limited deductibles and certain exclusions. Neptune is pursuing the balance of its insurance claim and will record any additional recovery if and when received.
On January 24, 2013, Neptune announced that the USPTO had allowed a second continuation patent application, application number 13/545,830, which claims the benefit of Neptune 348 Patent and 351 Patent. This second continuation application contains only a single claim, which is directed to a capsule comprising an Antarctic krill oil extract comprising a phospholipid suitable for human consumption. This claim covers most, if not all, krill oil products presently sold in the U.S. market. This second continuation application was allowed by the USPTO
9
after a thorough examination. During prosecution, Neptune provided the USPTO with all prior art references and other materials, including all the documents referred to in all of the re-examination requests filed by Aker Biomarine ASA directed to the ‘348 and ‘351 Patents, as well as all the documents relating to the oppositions currently underway on related Neptune patents in Australia.
On January 24, 2013, Neptune also announced that, effective January 23, 2013, Henri Harland, President and Chief Executive Officer of Neptune, would assume for an interim period of time, during the implementation of Neptune’s Plan, the functions and responsibilities held previously by Michel Chartrand, as Chief Operating Officer, who would continue to hold office as member of the Board of Director. Neptune also confirmed that its directors, senior management and employees had accepted salary reductions of 20% for an interim period during the implementation of Neptune’s plan to resume production.
On January 30, 2013, Neptune announced that it had filed a complaint under Section 337 of the US Tariff Act of 1930 with the United States International Trade Commission (the “ITC”), alleging that Aker; Enzymotec; and Olympic Seafood AS, Olympic Biotec Ltd., Rimfrost USA, LLC, Bioriginal Food & Science Corp. and Avoca, Inc., a division of Pharmachem Laboratories Inc. (collectively “Rimfrost”) are engaging in unfair trade practices by, at least, the importation, sale for importation, and sale after importation of certain krill-based products, namely krill paste and krill oils, that directly or indirectly infringe one or more claims of Neptune’s 351 Patent. On April 15, 2013, the ITC voted to institute an investigation of alleged patent infringements by Aker; Enzymotec and Rimfrost.
On February 26, 2013, Neptune announced that the USPTO had granted to Neptune a new continuation patent (the 675 Patent). This new patent claims the benefit of Neptune 348 Patent and 351 Patent. The 675 Patent contains a single claim directed to a capsule comprising an Antarctic krill oil extract comprising a phospholipid suitable for human consumption. This claim covers most, if not all, krill oil products presently sold in the U.S. market, as well as the pharmaceutical concentrates of Neptune’s subsidiaries Acasti and NeuroBio. Following this decision, Neptune filed an amended complaint in the ITC to add allegations of infringement of the 675 Patent against all of the proposed respondents, including Aker, Enzymotec and Rimfrost. Accordingly, Neptune had requested and was granted by the ITC a postponement of the deadline by which the ITC will decide whether to institute an investigation.
Fiscal Year Ended February 28, 2014
On March 14, 2013, Neptune announced that Dr. Harlan Waksal, member of the Board and executive VP, Business and Scientific Operations at Neptune’s subsidiary Acasti, would be presenting at the 25th Annual ROTH Conference on March 19, 2013.
On April 10, 2013, Neptune announced that the dismissal of Neptune’s appeal related to its European patent EP 1417211, by the European Patent Office’s (the “EPO”) Technical Appeal Board had no impact on its international patent strategy. The EPO’s Technical Appeal Board was solely concerned with the issue of flavanoids in krill extracts and did not address phospholipid compositions, which form a large part of Neptune’s extensive international patent portfolio. The decision of the EPO’s Technical Appeal Board did not affect ongoing disputes, including the filing with the ITC as the patent in question concerned flavonoids rather than phospholipids. The impact of the decision was also limited by the fact that Europe is the only jurisdiction where Neptune’s patent portfolio includes flavonoids.
On April 15, 2013. Neptune’s subsidiary, Acasti, announced that the ITC had decided to institute an investigation of alleged patent infringements by Aker, Enzymotec and Rimfrost. The investigation was instituted on the basis of the complaint filed with the ITC earlier in 2013 alleging violations by the respondents regarding the importation and sale of certain omega-3 extracts in the United States. Neptune and Acasti requested that the ITC issue an exclusion order and cease and desist order to ban the importation and sale of infringing extracts and products.
On May 15, 2013, Neptune announced that the Class Action Lawsuit alleging violations of the Securities Act of 1934 brought by Robbins Geller Rudman & Dowd LLP was voluntarily dismissed by the plaintiffs without prejudice. See “Business of the Corporation—Economic Dependence/Litigation—Class Action Suit”.
10
On May 22, 2013, Neptune announced initiatives to ensure effective management through a difficult period and remained focussed on three key priorities for restoring and ramping up its long-term supply chain. These priorities included rebuilding its production facility, establishing third party manufacturing partnerships and securing the supply of raw materials. The necessary permits to commence the reconstruction of the plant were received and Neptune announced that it was going to make use of the adjacent expansion facility to reconstruct an operational plant. In conjunction with the reconstruction of the production facility, Neptune began taking steps to secure and increase its long-term supply chain through third party manufacturing agreements in order to safeguard future operations. Three confirmed options for suppliers had been identified by Neptune at this point and Neptune continued to explore partnerships to allow it to provide supply to customers in the interim.
On May 27, 2013, reconstruction of Neptune’s production facility began and Neptune announced that it had hired an engineering firm, an architect and a plant manager.
On July 12, 2013, Neptune announced that it had acquired, through the exercise of a previously issued warrant, 6,750,000 Class A common shares in the capital of Acasti. The shares were acquired at a price of CDN$2.30 per share which reflected a total exercise price of CDN$15.5 million. The warrant was delivered to Neptune pursuant to a royalty prepayment agreement between Neptune and Acasti dated December 4, 2012 under which Acasti had been granted the option to pay in advance all of the future royalties payable under its exclusive technology license. As a result of Acasti exercising this option, it was relieved of its obligation to pay royalties to Neptune under the license agreement in question. The exercise of the warrant meant an increase in Neptune’s equity participation in Acasti from approximately 57% to approximately 60%. Both the prepayment agreement and the issuance of shares to Neptune were approved by the TSX Venture Exchange and the disinterested shareholders of Acasti at the annual shareholders meeting of Acasti held on June 27, 2013.
On July 16, 2013, Neptune announced that the Canadian Intellectual Property Office granted Neptune a composition patent, number CA 2,493,888, covering omega-3 phospholipids comprising polyunsaturated fatty acids.(“PUFAs”) The patent was granted for the Canadian market and will remain valid until 2022. This patent covers novel omega-3 phospholipid compositions which are suitable for human consumption, both synthetic and natural. The patent protects Neptune’s krill oil, namely NKO®, and also covers oils and powders extracted from krill and any marine or aquatic biomass containing marine phospholipids bonded to EPA or DHA.
On August 26, 2013, Neptune announced that it received an additional $5 million in insurance recoveries related to the November 2012 incident which destroyed the production facility bringing the total insurance recoveries to approximately $12 million. This additional sum further solidified Neptune’s ability to continue to implement its action plan to resume operations.
On September 2, 2013, Neptune announced that it had reached a settlement with respondents Rimfrost, resolving the ITC investigation related to infringement of Neptune’s composition of matter patents. As part of the settlement, Neptune granted a world-wide, non-exclusive, royalty-bearing licence to these settling respondents, allowing them to market and sell nutraceutical products containing components extracted from krill. The respondents in question also agreed to pay Neptune an additional royalty amount for the manufacture and sale of krill products prior to the effective license commencement date. Neptune also agreed to dismiss a related patent infringement case against Rimfrost filed in March of 2013. Moreover, on October 2, 2013, Neptune signed a strategic non-exclusive krill oil manufacturing and supply agreement with Rimfrost giving Neptune the right to purchase, at a preferred price, up to 800,000 kg of commodity grade krill oil during the first three-year term of the renewable agreement. Under the agreement, Neptune has agreed to purchase certain minimum quantities of commodity grade krill oil from Rimfrost in 2013 and 2014, which purchases may be deferred to the following calendar years.
On November 4, 2013, Neptune finalized a secured financing of $12.5 million with IQ, a government sponsored corporation whose mission is to contribute to Québec’s economic development in accordance with the Government of Québec’s economic policy, to partially fund the reconstruction of its production facility (which includes a security interest over all assets, including the Corporation’s intellectual property). The IQ secured loan has an annual interest rate of 7.0% and a two-year grace period for the start of principal repayment from the first disbursement date, following which the loan will be payable in equal monthly instalments over a four year period. The loan is repayable at any time without penalty. IQ disbursed the loan to reimburse Neptune’s reconstruction expenses. To date, Neptune has received approximately $8.5 million from IQ and expects to receive an additional $4.0 million which will be used to pay expenses incurred in connection with the reconstruction of the new production facility.
11
As part of the IQ loan, Neptune issued to IQ 750,000 common share purchase warrants at an exercise price of $3.37 per warrant. The number of warrants will vest on a prorata basis according to the amount disbursed by IQ on each disbursement date. At February 28, 2014, 511,995 warrants had vested.
On November 5, 2013, Neptune announced the appointment Reed V. Tuckson, M.D, the Managing Director of Tuckson Health Connections LLC, to its Board of Directors which increased the number of independent members to 4 out of a total of 6 board members.
On November 8, 2013, Neptune announced its intention to oppose a statement of offense issued by the CSST, the province of Québec’s commission charged with overseeing health and safety in the workplace. The CSST issued a statement seeking payment of approximately $64,500 before the completion of its investigation into the cause the explosion at Neptune’s production facility. Neptune maintained that it had adhered to best practices and procedures regarding workplace safety at all times and offered its continued cooperation with the CSST as their investigation continued. On November 12, 2013, Neptune entered a not guilty plea with respect to the statement of offense from the CSST.
On November 12, 2013, Neptune announced the inter partes request made by Aker BioMarine AS for the review of one of Neptune’s patents by the USPTO. The patent in question, US Patent No. 8,383,675, was one of the two patents being defended by Neptune against Aker and Enzymotec before the ITC. Despite this request for review, Neptune continued to focus on preparing to try its case before the ITC.
On November 13, 2013, Neptune hosted its Second Annual Charity Poker Game at the Venetian Hotel in Las Vegas, prior to the SupplySide West Tradeshow. The game featured guest of honor John Elway, former Denver Broncos quarterback and Hall of Famer. Proceeds of the event benefitted Vitamin Angels, a non-profit organization dedicated to reducing child mortality worldwide by connecting children in need with micronutrients.
On November 28, 2013, Neptune signed a legally binding term sheet with Aker with a view of finalizing the dismissal of all Aker respondents from the ITC investigation brought by Neptune and Acasti, as well as the dismissal of all lawsuits brought by Neptune against Aker and companies in its value chain.
On December 3, 2013, Neptune announced that it had acquired for investment purposes securities of Acasti in connection with the closing of Acasti’s public offering. Neptune acquired 592,500 units at a price of US$1.25 per unit for total consideration of US$740,625. Each unit consists of one class A common share and one common share purchase warrant of Acasti. Each warrant entitles its holder to purchase one class A common share at an exercise price of US$1.50 per share, subject to adjustment at any time until December 3, 2018. Following the closing, Neptune was the beneficial owner and controlled 51,942,183 class A common shares and 592,500 common share purchase warrants of Acasti.
On December 16, 2013, Neptune announced that the administrative law judge presiding over the pending ITC investigation involving Neptune, Acasti and Enzymotec granted the parties’ joint motion to stay the proceedings for thirty days. The motion to stay was filed because the parties had agreed to a settlement term sheet with the hope of concluding a binding settlement agreement before the expiration of the stay. Neptune has entered into a settlement agreement with all the other respondents named in the ITC investigation and motions to terminate the investigation as to those respondents have been submitted.
On December 17, 2013, Neptune announced that it had concluded a settlement and license agreement with Aker. As part of the settlement, Neptune granted a world-wide, non-exclusive, royalty-bearing license to Aker to market and sell nutraceutical products in the licensed countries. Pursuant to the terms of the settlement, royalty levels depend on the outcome of the review proceedings being conducted before the USPTO regarding Neptune’s 351 Patent. Aker also agreed to pay a non-refundable one-time payment to Neptune for the manufacture and sale of krill products prior to the effective USPTO decision date.
12
On December 19, 2013, Neptune announced the appointment of Jerald J. Wenker, President and COO of Dermalogica, as a special advisor to the Board of Directors as well as his acceptance of the nomination for election to serve on the Board of Directors of Neptune.
On January 9, 2014, Neptune announced that it had received New Food Raw Material certification in China after no quality or safety concerns were found by China’s National Health and Family Planning Commission allowing Neptune to sell its krill oil nutraceutical products in China.
On February 14, 2014, Neptune announced that it had not been able to arrive at a final settlement agreement with Enzymotec that would resolve the ITC investigation into the infringement of Neptune’s composition of matter patents, and related federal court matters. Despite the presiding administrative law judge granting an extended stay through February 5, 2014, no settlement could be achieved as the parties reached an impasse on certain fundamental settlement terms, including terms that had already been agreed to in the term sheet. Neptune and Enzymotec agreed to participate in the ITC’s mediation program in a final attempt to reach a mutually satisfactory agreement. See “Business of the Corporation—Economic Dependence/Litigation—Enzymotec Limited and others”.
On February 18, 2014, Neptune announced the appointment of John Moretz, President and CEO of Moretz Marketing LLC and managing director of Kathy Ireland, LLC, as special advisor to the Board of Directors as well as his acceptance of the nomination for election to serve on Board of Directors of Neptune.
On February 27, 2014, Neptune announced that it had began an underwritten public offering of its common shares in the United States and Canada pursuant to the effective shelf registration statement filed with the SEC and a final short form base shelf prospectus filed the securities regulators in the provinces of Québec, Ontario, Manitoba, Alberta and British Columbia. Roth Capital Partners and Euro Pacific Canada Inc. acted as joint book-running managers and National Securities Corporation acted as lead manager for the offering. The offering of 10,000,000 newly issued common shares were priced at US$2.50 per share on February 28, 2014. See “Recent Developments—Financing of the New Production Facility Reconstruction and Insurance Proceeds”.
RECENT DEVELOPMENTS
New Production Facility Reconstruction and Operations
Neptune is in the process of completing the reconstruction of its sole manufacturing facility, located in Sherbrooke, Quebec, Canada. When completed, and operating at full capacity, the new production facility is expected to produce approximately 150,000 kilograms of krill oil products annually, with production of NKO® being prioritized to meet customer demand.
Prior to commencing operations at the new production facility, Neptune is required to obtain the following two permits:
| • | a certificate of authorization required under theEnvironment Quality Act (Québec) from the Ministry of Environment relating to environmental matters at the new production facility; and |
| • | alevée d’interdiction de démarrer, or permit to lift the prohibition to begin operations, from the CSST relating to safety in the workplace requirements. |
Neptune is working closely with the Ministry of Environment and the CSST to finalize the securing of the operating permits. Neptune expects to begin production once the remaining two permits are obtained. Based on the current status of its exchanges with the Ministry of Environment and the CSST, Neptune expects that the required permits will be obtained and production will commence by approximately early June 2014.
Neptune has received the authorization of its Emergency Response Plan (ERP) from the City of Sherbrooke Fire and Rescue Service, relating to the new production facility’s fire safety and emergency evacuation plan and on-site fire security equipment. No further approvals are required from the City of Sherbrooke Fire and Rescue Service for production to resume.
13
At the time of the November 2012 plant explosion, Neptune was in the process of constructing an expansion facility for its plant. The expansion facility sustained limited damage in the explosion and the plant reconstruction has resulted in the expansion facility becoming the new base for the Corporation’s main production facility. As the initial intended use of the expansion facility has changed, plant modifications and additional purchases to replace equipment lost in the incident were required. As a result, the initial $21 million estimated cost of the expansion project has been revised to approximately $48.3 million, up from the amount of approximately $45 million that was previously disclosed. To date, Neptune has funded approximately $43.3 million of the total estimated cost through:
| • | insurance recoveries (approximately $17.5 million received to date), see “Recent Developments—Financing of the New Production Facility Reconstruction and Insurance Proceeds”; |
| • | a loan of $12.5 million from IQ (approximately $8.5 million disbursed to date with the balance of the loan expected to be received following the submission by the Corporation of its audited report on the admissible expenses), see “General Development of the Business—Fiscal Year Ended February 28, 2014”; |
| • | an interest free loan of $3.5 million from Canada Economic Development (“CED”) (approximately $3.0 million disbursed to date with the balance of the loan expected to be received following the submission by the Corporation of the reports required by CED), |
| • | certain amounts received from settlement agreements relating to intellectual property matters, and |
| • | Neptune’s working capital. |
New Production Facility Ramp-Up Period
Neptune expects that upon the commissioning of the new production facility, a start-up and ramp-up period will be required before full production capacity will be achievable. The ramp-up period is expected to be completed in three phases over a period of three months, with each phase lasting one month. During this ramp-up period, Neptune expects to progressively increase production in each of the three phases to an annual production capacity of 50,000, 100,000 and 150,000 kilograms of krill oil products respectively, until the new production facility’s full commercial annual production capacity of krill oil is reached.
Human Resources
Neptune is currently employing 117 employees. Most key employees have been retained and a few management and production employees remain to be hired by the Corporation. Neptune does not anticipate any problems in hiring the remaining employees in a timely manner. See “Business of the Corporation—Employees”.
On April 28, 2014, Neptune announced the resignation of Mr. Henri Harland as President and Chief Executive Officer of Neptune. Neptune has begun the search for a new President and Chief Executive Officer. During the interim period, Neptune continues to be managed by a management and operations committee under the leadership of Neptune’s Chief Financial Officer, Mr. André Godin.
On May 29, 2014, Henri Harland, the former President and Chief Executive Officer of the Corporation filed a lawsuit against the Corporation, Neptune and NeuroBioPharm in connection with his departure as President and Chief Executive Officer of each of Neptune, Acasti and NeuroBioPharm. Among other things, Mr. Harland alleged that his resignation occurred as a result of a constructive dismissal and is seeking approximately $8.5 million in damages, interest and costs. In addition, Mr. Harland is seeking from Neptune, Acasti and NeuroBioPharm, as applicable, the issuance of 500,000 shares of each of Neptune, Acasti and NeuroBioPharm as well as two blocks of 1,000,000 call options each on the shares held by Neptune in Acasti and NeuroBioPharm. As a result of the lawsuit, Mr. Harland was requested to resign as Director of the Corporation. The following day, Neptune and its subsidiaries jointly announced that they believed the claim as formulated was without merit or cause, they will vigorously defend the lawsuit and will take any steps necessary to protect their interests.
Financing of the New Production Facility Reconstruction and Insurance Proceeds
On November 4, 2013, Neptune finalized a secured financing of $12.5 million with IQ, a government sponsored corporation whose mission is to contribute to Québec’s economic development in accordance with the Government of Québec’s economic policy, to partially fund the reconstruction of its production facility (which includes a security interest over all assets, including the Corporation’s intellectual property). The IQ secured loan has an annual interest rate of 7.0% and a two-year grace period for the start of principal repayment from the first disbursement date, following which the loan will be payable in equal monthly instalments over a four year period. The loan is repayable at any time without penalty. IQ disbursed the loan to reimburse Neptune’s reconstruction expenses. To date, Neptune has received approximately $8.5 million from IQ and expects to receive an additional $4.0 million which will be used to pay expenses incurred in connection with the reconstruction of the new production facility. As part of the IQ loan, the Corporation granted warrants to purchase 750,000 common shares of the Corporation to IQ. The warrants will be exercisable at an exercise price of $3.37 per warrant. The warrants will vest on a project driven basis concurrently with each loan disbursement date prorated according to the amount disbursed by IQ. At February 28, 2014, 511,995 warrants had vested.
14
On March 6, 2014, Neptune announced the closing of a public offering for gross proceeds of approximately US$28.75 million. Neptune intends to allocate the net proceeds from the offering for sales, marketing and distribution of its krill oil products, to support NeuroBio, in the development and validation of its product candidates, to finance the ramp-up of its production facility, to maintain, manage and develop its intellectual property portfolio and to protect it against infringement by third parties and for general corporate and other working capital purposes.
On April 4, 2014, Neptune announced the closing of a private placement of CAD$2,503,320 of common shares of Neptune at a price of CAD$2.76 per share resulting in a total issuance of 907,000 shares. The shares were all qualified under Quebec Stock Savings Plan II (“QSSP II”) and were issued to the Fiera Capital QSSP II Investment Fund Inc. and Cote 100 Inc., that acquired 725,000 and 182,000 shares respectively. The shares could not be qualified under the QSSP II and subscribed for by the Funds under the Neptune’s public offering completed on March 5, 2014, because of the particular requirements of the QSSP II. Other than the qualification of the shares, the terms of the shares issued are the same as those of the common shares of Neptune issued as part of the public offering.
Since November 2012, Neptune has received insurance recoveries totalling $17.5 million. Although its new production facility is operational, Neptune is still pursuing the balance of its insurance claim and will record any additional recovery if and when received.
Since the November 2012 plant explosion, management has periodically reevaluated the need to recognize impairment losses as information becomes available. An additional impairment loss related to property, plant and equipment of approximately $1.2 million was identified during the ongoing process of the reconstruction of the Corporation’s plant, related financing and insurance recoveries, and was recorded in the Corporation’s financial statements. See “Risk Factors—The Corporation suffered significant impairment losses and its assets may be subject to future write-downs, charge-offs or impairment losses.”
Incident Investigation, Environmental Matters and Site Clean-Up
On May 8, 2014, the CSST released its report in connection with its ongoing investigation to determine the cause of the November 2012 explosion at Neptune’s production plant. Although the CSST’s report highlights that the exact cause of the incident could not be identified, the CSST identified as potential causes that could explain the incident the following principal factors: deficiencies in the design and control of the production process, the classification of the old production facility and deficiencies in the management of health and safety issues. The CSST’s report makes no mention of additional fines or penalties against Neptune beyond the November 5, 2013 statement of offence described below. Following the November 2012 incident, Neptune offered its full cooperation to the CSST and continues to work with the CSST, including by implementing recommendations and corrective measures sought by the CSST, towards completing its new state of the art production facility and making operations at its new production facility as safe as possible.
On November 5, 2013, Neptune received a statement of offense issued by the CSST seeking payment of a fine of approximately $64,500 in connection with the incident. On November 12, 2013, Neptune entered a not guilty plea with respect to the statement of offense from the CSST.
On November 16, 2012, following the incident at the plant, Neptune received from the Ministry of Environment a notice alleging non-compliance by Neptune with environmental regulations relating to equipment specifications. The Ministry of Environment’s notice alleged that Neptune had modified certain of its equipment without notifying the Ministry of Environment and that its plant production capacity was above the permitted limit in the certificate of authorization issued by the Ministry of Environment. Neptune is cooperating with the Ministry of Environment with the view to settling the notice alleging the non-compliance. The Ministry of Environment’s investigation is ongoing and representatives of Neptune have met with inspectors from the Ministry of Environment.
15
Neptune also provided to the Ministry of Environment a dismantling and clean-up plan for the destroyed plant, accompanied by an environmental monitoring program for soil, surface water and groundwater quality. To date, the destroyed plant has been dismantled and the required clean-up of the premises in accordance with Ministry of Environment standards, which includes the removal of 130 metric tons of contaminated soil from the site further to environmental studies performed by independent environmental consultants retained by Neptune, is in its advanced stages and the Corporation anticipates it will be completed by mid-2014.
Activities of Neptune’s Subsidiaries—Acasti and NeuroBio
Acasti Pharma Inc.
Acasti initiated two Phase II clinical trials in Canada (the COLT trial and the TRIFECTA trial) designed to evaluate the safety and efficacy of CaPre® for the management of mild to moderate hypertriglyceridemia (high triglycerides with levels ranging from 200 to 499 mg/dL) and severe hypertriglyceridemia (high triglycerides with levels over 500 mg/dL). Due to a recent decision of the U.S. Food and Drug Administration’s (the “FDA”) not to grant authorization to commercialize a competitor’s drug in the mild to moderate patient population before the demonstration of clinical outcome benefits, Acasti is reassessing its clinical strategy and may put a primary and first focus on the severe hypertriglyceridemia population.
COLT Trial
On August 13, 2013, Acasti announced the completion and results of its open-label Phase II COLT trial (clinical trial.gov identifier NCT01516151). The final results of the COLT trial indicated that CaPre® was safe and effective in reducing triglycerides in patients with mild to severe hypertriglyceridemia with significant mean (average) triglyceride reductions above 20% after 8 weeks of treatment with daily doses of both 4.0g and 2.0g. Demographics and baseline characteristics of the patient population were balanced in terms of age, race and gender. A total of 288 patients were enrolled and randomized and 270 patients completed the study, which exceeded the targeted number of evaluable patients. From this patient population, approximately 90% had mild to moderate hypertriglyceridemia. CaPre® was safe and well tolerated. The proportion of patients treated with CaPre® that experienced one or more adverse events in the COLT trial was similar to that of the standard of care group (30.0% versus 34.5%, respectively). A substantial majority of adverse events were mild (82.3%) and no severe treatment-related adverse effects have been reported.
The COLT trial met its primary objective showing CaPre® to be safe and effective in reducing triglycerides in patients with mild to severe hypertriglyceridemia. After only a 4-week treatment, CaPre® achieved a statistically significant triglyceride reduction as compared to standard of care alone. Patients treated with 4.0g of CaPre® per day over 4 weeks reached a mean triglyceride decrease of 15.4% from baseline and a mean improvement of 18.0% over the standard of care. Results also showed increased benefits after 8 weeks of treatment, with patients on a daily dose of 4.0g of CaPre® registering a mean triglyceride decrease of 21.6% from baseline and a statistically significant mean improvement of 14.4% over the standard of care. It is noteworthy that a mean triglyceride reduction of 7.1% was observed for the standard of care group at week 8, which may be explained by lipid lowering medication adjustments during the study, which was allowed to be administered in the standard of care group alone.
Moreover, after 8 weeks of treatment, patients treated with 1.0g for the first 4 weeks of treatment and 2.0g for the following 4 weeks showed a statistically significant triglycerides mean improvement of 16.2% over the standard of care, corresponding to a 23.3% reduction for the 1.0-2.0g daily dose as compared to a 7.1% reduction for the standard of care. After 8 weeks of treatment, patients treated with 2.0g of CaPre® for the entire 8 weeks showed statistically significant triglycerides mean improvements of -14.8% over the standard of care, corresponding to a 22.0% reduction for the 2.0g as compared to a 7.1% reduction for the standard of care. Also, after 8 weeks of treatment, patients treated with 4.0g for the entire 8 weeks showed statistically significant triglycerides, non-HDL-C (non-high density lipoprotein, which includes all cholesterol contained in the bloodstream except HDL-C (high density lipoprotein (good cholesterol)) and HbA1C (haemoglobin A1C) mean improvements of, respectively, 14.4% and 9.8% and 15.0% as compared to standard of care. The 4.0g group mean improvements in (i) triglycerides of 14.4% corresponds to a reduction of 21.6% as compared to a reduction of a 7.1% for the standard of care group, (ii) non-HDL-C of 9.8% corresponds to a reduction of 12.0% as compared to a reduction of 2.3% for the standard of care group, and (iii) HbA1C of 15.0% corresponds to a reduction of 3.5% as compared to an increase of 11.5% for the standard of care group. In addition, all combined doses of CaPre® showed a statistically significant treatment effect on HDL-C levels, with an increase of 7.4% as compared to standard of care. Trends (p-value < 0.1) were also noted on patients treated with 4.0g of CaPre® for the entire 8-week treatment period with mean reduction of total
16
cholesterol of 7.0% and increase of HDL-C levels of 7.7% as compared to the standard of care. Furthermore, after doubling the daily dosage of CaPre® after an initial period of 4 weeks, the results indicate a dose response relationship corresponding to a maintained and improved efficacy of CaPre® after an 8-week period. The efficacy of CaPre® at all doses in reducing triglyceride levels and increased effect with dose escalation suggests that CaPre® may be titrable, allowing physicians to adjust dosage in order to better manage patients’ medical needs. In addition, the results of the COLT trial indicate that CaPre® has no significant deleterious effect on LDL-C (bad cholesterol) levels.
Further details on Acasti’s COLT Trial are available in Acasti’s prospectus dated October 25, 2013, which can be accessed online atwww.sedar.com, under the heading “Acasti’s Business – Clinical and Nonclinical Research – Clinical – COLT Trial”.
TRIFECTA Trial
The TRIFECTA trial (clinical trial.gov identifier NCT01455844), a 12-week, randomized, double-blind, placebo-controlled study, is designed to assess the effect of CaPre®, at a dose of 1.0 or 2.0g, on fasting plasma triglycerides as compared to a placebo in patients with mild to severe hypertriglyceridemia. A total of 366 patients have been randomized over the 429 planned protocol (342 evaluable patients).
Similar to the COLT trial, the primary objective of the TRIFECTA trial is to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 10.0 mmol/L (200-877 mg/dL) and to assess the tolerability and safety of CaPre®. The secondary objectives of the TRIFECTA trial are to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 5.69 mmol/L (200-499 mg/dL); to evaluate the dose dependent effect on fasting plasma triglycerides in patients with triglycerides > 5.7 and <10 mmol/L (500-877 mg/dL); to evaluate the effect of CaPre® in patients with mild to moderate hypertriglyceridemia and severe hypertriglyceridemia on fasting plasma levels of LDL-C (direct measurement), and on fasting plasma levels of HDL-C, non-HDL-C, hs-CRP and omega-3 index.
On December 20, 2012, the TRIFECTA trial completed an interim analysis. The review committee made up of medical physicians assembled to evaluate the progress of the TRIFECTA trial reviewed the interim analysis relative to drug safety and efficacy and unanimously agreed that the study should continue as planned. All committee members agreed that there were no toxicity issues related to the intake of CaPre® and that the signals of a possible therapeutic effect, noted as reduction of triglycerides in the groups evaluated, were reassuring and sufficiently clinically significant to allow the further continuation of the TRIFECTA trial. The data was provided to the committee members blind, meaning that the identity of the three groups was not revealed. Since the data revealed a possible therapeutic effect without any safety concerns, the committee decided that it was not necessary to unblind the data. The number of targeted patients evaluable as per protocol has been reached. Acasti is currently evaluating efficacy and safety of CaPre® for the treatment of patients with mild to severe hypertriglyceridemia, which is the primary objective of the study. A secondary objective of the study was to assess the efficacy of CaPre® in two distinct patient populations: those with mild to moderate hypertriglyceridemia and those with severe hypertriglyceridemia. Based on patient information currently available, the Corporation does not expect the sample size to be large enough to conclude on the efficacy of CaPre® on severe hypertriglyceridemia as part of the TRIFECTA trial. Acasti does not expect the FDA to request efficacy data on patients with severe hypertriglyceridemia before granting permission to conduct a phase III trial. Acasti believes the trial will be completed by the end of the second quarter of calendar 2014 and results will be available at a future date yet to be determined.
PK Trial
On November 11, 2013, Acasti announced that it submitted an investigational new drug application to the FDA to initiate a PK (pharmacokinetic) trial of CaPre® in the United States. The proposed PK trial is an open-label, randomized, multiple-dose, single-center, parallel-design study to evaluate blood profiles and bioavailability of omega-3 phospholipids on healthy volunteers taking single and multiple daily oral doses of 1.0g, 2.0g and 4.0g of CaPre®. Acasti expects that the duration of the PK trial would likely be over a 30-day period and involve the enrollment of approximately 42 healthy subjects.
17
On January 9, 2014, Acasti announced that the FDA granted Acasti approval to conduct its PK trial, having found no objections with the proposed PK trial design, protocol or safety profile of CaPre®. Acasti also announced that Quintiles, the world’s largest provider of biopharmaceutical development and commercial outsourcing services, has been hired to conduct the PK trial. Acasti expects results of its PK trial to be available by mid to late 2014.
Next Steps
Acasti is corresponding with the FDA and has responded to the FDA’s recommendations regarding its upcoming IND filing for its phase III clinical trial of CaPre® in the United States. The FDA has invited Acasti to formally request an end of phase II/pre phase III meeting to allow them to provide feedback on the submission and to address specific questions for which Acasti is seeking approval and final response from the FDA. Acasti intends to seek such meeting as soon as TRIFECTA trial results are available.
Acasti intends to conduct a phase III clinical trial in the United States, with potentially a few Canadian clinical trial sites, in a patient population with very high triglycerides (>500 mg/dL). This study would constitute the primary basis of an efficacy claim for CaPre® in an NDA submission for severe hypertriglyceridemia. Acasti is also evaluating the possibility of submitting a Special Protocol Assessment (“SPA”) to the FDA in order to form the basis for the design of its intended Phase III clinical trial. An SPA is a declaration from the FDA that an uncompleted Phase III trial’s design, clinical endpoints, and statistical analyses are acceptable for FDA approval. A request would be submitted for the protocol at least 90 days prior to the anticipated start of the Phase III clinical trial.
In addition to conducting and completing the TRIFECTA, PK and a Phase III clinical trial, Acasti expects that additional time and capital will be required to complete the filing of a NDA to obtain FDA approval for CaPre® in the United States before reaching commercialization, which may initially be only for the treatment of severe hypertriglyceridemia. The FDA may require Acasti to conduct additional clinical studies to obtain FDA approval for the treatment of mild to moderate hypertriglyceridemia, which may include a cardiovascular outcomes study.
Public Offering and Private Placement of Units of Acasti
On December 3, 2013, Acasti completed an underwritten public offering of 18,400,000 units of Acasti at a price of US$1.25 per unit for total gross proceeds of approximately US$23 million, each unit consisting of one Class A share (“Acasti Common Share”) and one Acasti Common Share purchase warrant of Acasti. Each warrant entitles the holder to purchase one Acasti Common Share at an exercise price of US$1.50 per share, subject to adjustment, at any time until December 3, 2018. Neptune acquired US$741,000 of units in the offering. Acasti intends to use the proceeds from the offering to continue the clinical trial program for CaPre®, including for the initiation and completion of a phase III clinical trial to investigate the safety and efficacy profile of CaPre® in a patient population with very high triglycerides (>500 mg/dL). The phase III clinical trial is expected to take at least 18 to 24 months.
On February 7, 2014, Acasti announced the closing of a private placement in Québec of units of Acasti at a price of $1.33 per unit for total gross proceeds of $2.15 million, each unit consisting of one Class A Common Share of Acasti and one Acasti Common Share purchase warrant. Each warrant entitles the holder to purchase one Acasti Common Share at an exercise price of $1.60 per share, subject to adjustment, at any time until December 3, 2018. The terms of the units issued under the private placement are substantially the same as those of the units issued under the public offering described in the above paragraph. Acasti intends to use the proceeds from the private placement for general corporate and working capital purposes.
Following the offerings, Neptune owns 51,942,183 Acasti Common Shares, which currently represents approximately 49.07% of the Acasti Common Shares issued and outstanding. Neptune does not expect to provide material capital to Acasti in the short term.
NeuroBioPharm Inc.
NeuroBio is in the early stages of developing omega-3 phospholipids medical foods, over-the-counter products and prescription drugs. NeuroBio is dedicated to the research, development and commercialization of active pharmaceutical ingredients (“APIs”) for the management of neurodevelopmental, memory, concentration, learning and neurological disorders, from prevention to treatment. NeuroBio’s product candidates are at different development and/or validation stages and are expected to require the approval of the FDA and/or Health Canada before commercialization. Approvals from similar regulatory organizations are also expected to be required before sales are authorized.
18
The development of NeuroBio’s product candidates was delayed by the November 2012 incident at Neptune’s production facility. The preclinical and clinical studies that were planned to start in late 2012 and early 2013 were postponed. Preclinical studies that were in progress, however, were not interrupted. NeuroBio is dependent on the support of Neptune as its controlling shareholder.
The NeuroBio product portfolio includes highly concentrated phospholipids extracted and purified from different marine species, including krill, which functionalize EPA and DHA most often stabilized by potent antioxidant esters. NeuroBio’s potential medical food and over the counter drug product portfolio consists of MPL VI, MPL VII, MPL VIII. NeuroBio’s potential prescription drug candidate is MPL IX. NeuroBio’s product candidates are at different development and/or validation stages and are expected to require the approval of the FDA and/or Health Canada before commercialization. Approvals from similar regulatory organizations are also expected to be required before sales are authorized. See “Business of the Corporation—Regulatory Environment”.
NeuroBio is currently preparing a randomized placebo-controlled double-blind study to evaluate the effect of MPLIX on Mild Cognitive Impairment (“MCI”) in an elderly population between the ages of 65 and 80 years old. This phase II study will help establish the sensitivity and precision of the assessment tools, determine the effect of the product candidate on cognitive functions, depression, anxiety and quality of life in a MCI population, and will examine the placebo effect. In addition, the data collected will be used to determine the appropriate statistical parameters to design a pivotal clinical study.
NeuroBio also intends to conduct a prospective study in children, between the ages of 6 and 15 years old, with attention-deficit hyperactivity disorder (“ADHD”) symptoms. This prospective study aims to determine the benefits of MPLIX as an add-on to ADHD pharmacotherapy as compared to a stand-alone Omega-3 phospholipids therapy and the possibility of decreasing the side effects related to the ADHD pharmacotherapy.
NeuroBio also expects to continue its nonclinical studies investigating the potential therapeutic effects of its product candidates, including non-clinical toxicology studies to assess the safety of its product candidates.
Approvals of applicable regulatory authorities, including the Natural Health Products Directorate (Canada), are required before the studies of NeuroBio may begin. See “Business of the Corporation—Regulatory Environment”.
Intellectual Property
On April 24, 2014, Neptune announced that the USPTO had granted Neptune a new continuation patent (U.S. Patent No. 8,680,080) relating to the treatment of Alzheimer’s. The patent, which is the Corporation’s first specifically targeting neurological conditions, is granted for the US market and is valid until 2022. The claims focus on treating Alzheimer’s disease by administering an effective amount of a phospholipid composition, wherein the phospholipid composition comprises DHA and EPA.
On April 27, 2014, Acasti and Neptune announced that a patent infringement settlement and license agreement had been signed with Enzymotec that resolves the ITC’s investigation of infringement of Neptune’s composition of matter patents, related federal court actions initiated by Neptune against Enzymotec and its distributors and various patent review proceedings requested by Enzymotec. As part of the settlement agreement, Neptune granted a world-wide, non-exclusive, royalty-bearing license to Enzymotec, allowing it to market and sell its nutraceutical products under Neptune’s ‘348 family of patents (US Patent No. 8,030,348 and all the continuations). Under the terms of the settlement, royalty levels in the United States are dependent on the outcome of pending inter partes review proceedings before the USPTO regarding certain claims of Neptune’s ‘351 composition of matter patent (US Patent No. 8,278,351). Furthermore, royalty levels in Australia are dependent on a potential request by Enzymotec to the APO for a post-grant review of certain claims of Neptune’s allowed composition of matter patent application (AU2002322233). Enzymotec also agreed to pay Neptune a non-refundable one-time upfront settlement payment.
19
For additional information relating to the Neptune’s intellectual property, please refer to “Business of the Corporation—Intellectual Property” and for information relating to the settlement agreements entered into by Neptune, please refer to “Business of the Corporation—Economic Dependence/Litigation.”
BUSINESS OF THE CORPORATION
Overview
Neptune is a biotechnology company engaged primarily in the development, manufacture and commercialization of marine-derived omega-3 PUFAs. Neptune produces omega-3 PUFAs through its patented process of extracting oils from Antarctic krill, which omega-3 PUFAs are then principally sold as bulk oil to Neptune’s distributors who commercialize them under their private labels primarily in the U.S., European and Australian nutraceutical markets. Neptune’s lead products, Neptune Krill Oil (NKO®) and ECOKRILL Oil (EKO™), generally come in capsule form, serve as a dietary supplement to consumers and are available at several leading major retailers under distributors’ private labels.
Neptune pioneered the commercialization of omega-3 PUFAs extracted from krill for human health maintenance in 2002 and is continuing its product development based on its proprietary technology. The Corporation believes that its ability to provide a safe and effective product is a key factor in building and sustaining its credibility with its distribution partners.
Through Neptune’s subsidiaries, Acasti and NeuroBio, in which Neptune respectively holds approximately 49.07% and 95% of the voting rights, Neptune is pursuing opportunities in the medical food and prescription drug markets. Neptune has granted licensing rights to both Acasti and NeuroBio that allow them to leverage the intellectual property, clinical data and know-how developed by Neptune to focus on, respectively, the research and development of safe and therapeutically effective compounds for highly prevalent atherosclerotic conditions, such as cardiometabolic disorders and cardiovascular diseases, and for neurodegenerative and inflammation related conditions.
The Krill Industry
Krill, which resembles shrimp, is a generic term designating approximately 85 species of deep and cold water pelagic marine planktonic animals (zooplankton) that make up part of the global marine biomass. According to the Australian government’s Department of Sustainability, Environment, Water, Population and Communities (Australian Antarctic Division), krill is the most abundant animal biomass on the planet and is found in schools that can sometimes cover several square kilometers of ocean.
Because krill feeds on phytoplankton, namely diatoms and dinoflagellates, its lipid content is a major source of PUFAs, mainly docosahexaenoic acid, or DHA, and eicosapentaenoic acid, or EPA, two types of marine omega-3 fatty acids beneficial for health maintenance. Krill contains proteins offering a range of amino acids and effective digestive enzymes. In addition, it contains powerful antioxidants, including astaxanthin. Krill also contains phospholipids, amino acids and minerals providing clinically proven benefits in the absorption and digestion of nutrients for humans and animals.
Neptune’s patented krill oil extraction process produces a compound substance that contains enhanced levels of EPA and DHA, phospholipids and antioxidants, making it highly bioavailable (capable of absorption) and resistant to oxidation. Based on our internal research, we believe Neptune’s krill oil has a lower level of oxidation than fish oil due to its high natural content of antioxidants, which also results in a longer shelf life of Neptune’s krill oil products.
Despite the higher price per kilogram of krill oil compared to fish oil, the krill oil market had global revenues of US$51.1 million in 2011, and is projected to grow at a compound annual growth rate, or CAGR, of 16.4% between 2011 and 2016, according to a Frost & Sullivan industry report entitled the2012 Global Overview of the EPA and DHA Omega 3 Ingredients Markets, or the Frost & Sullivan July 2012 Report.
20
NKO® and EKO™ – Our Lead Products
Neptune Krill Oil (NKO®) and ECOKRILL Oil (EKO™)
NKO®, which was first commercialized in 2003, is a marine oil extracted from Antarctic krill (Euphasia superba) that contains the two essential omega-3 PUFAs, EPA and DHA, and provides a blend of nutritional elements. NKO®’s elevated content of phospholipids rich in omega-3 and omega-9 fatty acids and antioxidants such as astaxanthin, vitamin A and vitamin E offers a safe and effective product free of preservatives with clinically proven health benefits.
The Corporation believes NKO® has a biomolecular profile of phospholipids, omega-3 fatty acids and important antioxidants that surpasses corresponding profile of fish oils. This combination of phospholipids and omega-3 fatty acids facilitates the passage of fatty acids molecules through the body’s intestinal wall, increasing the bioavailability of omega-3 fatty acids. Independent research has shown that astaxanthin has a stronger antioxidant level than vitamin A and vitamin E as well as other antioxidants such as lycopene and lutein. Neptune believes that NKO® contains higher amounts of astaxanthin than all other krill oil products on the market.
EKO™, Neptune’s other lead product, which was first commercialized in 2010 is similar to NKO® in that it undergoes the same krill oil extraction process. The difference between EKO™ and NKO® is that EKO™ has lower specifications of PUFAs, phospholipids and antioxidants and, as a result, EKO™ has a lower price point than NKO®.
Neptune believes that NKO® is the first and only krill oil product providing clinically proven health benefits in the areas of cardiovascular, joint, cognitive and women’s health. In 2004, the Alternative Medicine Review published the results of a 12-week, double-blind, randomized trial that demonstrated that daily doses of 1-3g NKO® are significantly more effective than 3g EPA/DHA fish oil in the management of abnormal cholesterol levels (hyperlipidemia). Daily doses of 1-3g NKO® were proven effective in that trial to decrease low density lipoprotein (“LDL” or “bad cholesterol”) by 33.9%, triglycerides by 11.5% and increase high density lipoprotein (“HDL” or “good cholesterol”) by 43.3%.
The results of a double blind clinical study performed in May 2003 by Fotini Sampalis M.D., Ph.D., et. al., which were published in the Alternative Medicine Review, support the proposition that NKO® can reduce certain physical and emotional symptoms of premenstrual syndrome, such as stress, irritability and abdominal pain, and that NKO® is more effective than omega-3 fish oils for the management of such premenstrual symptoms.
An analysis of the Framingham Risk Score (which is used to estimate the 10-year cardiovascular risk of an individual based on data obtained from the Framingham Heart Study, a long-term, ongoing cardiovascular study on residents of the town of Framingham, Massachusetts) data completed in 2003 suggests that the use of NKO® alone or in combination with a statin provides a safe and cost effective treatment option for the management of hyperlipidemia that can significantly increase HDL (“good cholesterol”) and reduce overall risk for cardiovascular disease by 53%.
A double-blind clinical study performed in 2007 found that NKO® at a daily dose of 300 mg may within a short time to reaction (7-14 days) significantly inhibit inflammation by reducing C-reactive protein as well as significantly alleviate symptoms caused by osteoarthritis and rheumatoid arthritis.
A double-blind clinical trial undertaken by BioTeSys GmbH in February 2009 supports the benefits of NKO® versus a range of other omega-3 products for improving the EPA to arachidonic acid ratio and the omega-3 index. The main objective of the trial was to show the bioavailability of a physiological dosage of omega-3 fatty acids. Within the clinical trial, different sources of EPA and DHA, including different chemical bounds of EPA and DHA, were compared to each other. The obtained data reflects that uptake of EPA and DHA out of NKO® was most prominent and showed significant higher bioavailability in comparison to fish oil and a blend of lecithin, astaxanthin and fish oil. The study stated that, overall, the NKO® product showed clear superiority followed by ethyl esters, fish oil and the blend of lecithin, astaxanthin and fish oil.
21
Following the explosion that destroyed Neptune’s sole production plant on November 8, 2012, Neptune temporarily ceased to produce and commercialize NKO® and EKO™ and has not resumed production or commercialization. Neptune is in the process of completing the reconstruction of its sole production plant that, when completed, is expected to enable the Corporation to produce approximately 150,000 kilograms of krill oil products annually. See “Recent Developments—New Production Facility Reconstruction and Operations”. Once operations resume, Neptune intends to prioritize the production of NKO®, which will be produced exclusively at the new production facility, to meet customer demand for this product.
During the period following the plant explosion, Neptune has been supplying the market with commodity grade krill oil acquired from third-party manufacturers to maintain key customer relationships and part of its market share. Once production has resumed at the new production facility, based on market demand, Neptune may supply and/or further refine to meet EKO™ standards commodity grade krill oil produced by third-party manufacturers as a strategy to diversify its sources and means of production and product offering.
Following the 2012 incident, sales by Neptune of commodity grade krill oil have yielded sales margins of approximately 11%, which is significantly lower than Neptune’s historical sales margins for NKO® and EKO™ prior to the incident, which ranged between 45% and 50%. Neptune expects to see a progressive increase in its sales margins as production is ramped up at its new production facility and the new production facility operates at full capacity.
After production has resumed at its new production facility, the feasibility in the mid to long-term of an expansion of capacity may be considered in order to increase production at the new facility to 300,000 kilograms of krill oil annually. The cost of such an expansion project has not been determined by Neptune and would require additional financing. The feasibility and timing of such an expansion project will depend, among other factors, on the demand for the Corporation’s products and the ability to obtain additional financing on favorable terms or at all.
As part of its growth strategy, and with the objective of further increasing sales to existing customers and developing new customer relationships across new geographies, Neptune also intends to evaluate potential arrangements with third parties, which may include strategic alliances, joint venture investments, acquisitions or licensing or distribution arrangements.
Other Nutraceutical Products
New Formulation Derived from NKO®
In addition to NKO® and EKO™, Neptune is also working on new formulations derived from NKO® that target more specific conditions, including NKO Beat™, which targets heart and circulation issues, NKO Flex™, which targets bone and joint issues, and NKO Focus™, which targets brain and vision issues. The Corporation expects to launch these new formulations during its fiscal year ending on February 28, 2015.
Neptune Krill Aquatein™( NKA™)
Neptune Krill Aquatein (krill protein concentrate), or NKA™, is a product that features a range of marine amino acids, including the eight essential amino acids. NKA™ contains pre-digested proteins that are an important source of short-chain amino acids in the form of peptides that facilitate digestion by more effective assimilation.
More complete analyses of the composition of NKA™ were performed and different methods for improving quality and efficiency of production have been investigated. NKA™ is being positioned to be sold for both human and animal nutrition. For the fiscal year ended February 28, 2014, NKA™ did not account for any revenues and Neptune believes NKA™ will not generate meaningful revenues during the current fiscal year.
Pharmaceutical Products and Product Candidates—Acasti
Our subsidiary, Acasti, focuses on the research and development of safe and therapeutically effective compounds for highly prevalent atherosclerotic conditions, such as cardiometabolic disorders and cardiovascular diseases.
22
CaPre®
Acasti’s lead prescription drug candidate is CaPre®, which is a highly purified omega-3 phospholipid concentrate derived from krill oil and is being developed to help prevent and treat hypertriglyceridemia, which is a condition characterized by abnormally high levels of triglycerides in the bloodstream. The active ingredient of CaPre® is a mixture of concentrated omega-3 fatty acids purified from crude krill oil and developed as an oral formulation. CaPre® contains EPA and DHA bound to phospholipids as well as free EPA and DHA for a total concentration of approximately two-thirds phospholipids and approximately 30% EPA and DHA.
Acasti’s near term strategy is to develop and commercialize CaPre® in the United States as a prescription drug with a claim for the treatment of severe hypertriglyceridemia (triglycerides with levels over 500mg/dl, or severe hypertriglyceridemia) and, as a next step, the treatment of hypertriglyceridemia (triglycerides with levels ranging from 200 to 500 mg/dl, or hypertriglyceridemia).
CaPre® is designed to be used as a therapy in conjunction with positive lifestyle changes and administered either alone or in conjunction with other treatment regiments such as statins (a class of drug used to reduce cholesterol levels) and potentially for use by statin-intolerant or statin-resistant patients. In addition to targeting the reduction of hypertriglyceridemia and severe hypertriglyceridemia, nonclinical and preliminary clinical data collected by Acasti to date has indicated that CaPre® may also normalize blood lipids by reducing LDL (bad cholesterol) and very low density lipoprotein while increasing HDL (good cholesterol).
Acasti initiated two Phase II clinical trials in Canada (the TRIFECTA trial and the COLT trial), one of which, the COLT trial, has now been completed. Both trials were designed to evaluate the safety and efficacy of CaPre® for the management of hypertriglyceridemia and severe hypertriglyceridemia and aim to evaluate the effect of different daily doses of CaPre® on patients with hypertriglyceridemia to severe hypertriglyceridemia. A total of approximately 600 patients were enrolled in the two trials. Obtaining regulatory approval for CaPre® requires that safety is confirmed and it is effective at reducing triglycerides at a level that would medically benefit the patient. Acasti’s longer-term objective is to demonstrate that CaPre® can also reduce LDL and raise HDL. Acasti believes there are no drugs currently on the market that have been proven effective to a clinically relevant extent for all three indications, although based on nonclinical studies Acasti believes CaPre® may provide significant benefits in all three areas. In parallel with the ongoing Phase II TRIFECTA trial, in Canada, on January 9, 2014, Acasti announced that the FDA granted Acasti approval to conduct its PK trial, having found no objections with the proposed PK trial design, protocol or safety profile of CaPre®. Acasti also announced that Quintiles, the world’s largest provider of biopharmaceutical development and commercial outsourcing services, has been hired to conduct the PK trial. Acasti expects results of its PK trial to be available by mid to late 2014. See “Business of the Corporation- Studies & Trials for Pharmaceutical Product Candidates—Acasti’s Product Candidate: CaPre®” and “Business of the Corporation- Regulatory Environment”.
ONEMIA®
ONEMIA®, a medical food and currently Acasti’s only commercialized product to date, is a purified omega-3 phospholipids concentrate derived from krill oil with lower levels of phopholipids, EPA and DHA content than CaPre®. The term “medical food” is defined in the United States Orphan Drug Act as a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. Nonclinical studies conducted by Acasti, supported by clinical testing conducted on NKO®, have shown ONEMIA® to be safe and effective for the dietary management of omega-3 phospholipids deficiency and the related abnormal lipid profiles and cardiometabolic disorders. Phospholipid deficiency and abnormal lipid profiles can lead to a number of conditions, including hyperlipidemia (which generally manifests as high LDL and high triglycerides), atherosclerosis (the build-up of plaque on the inside of blood vessels), diabetes, rheumatoid arthritis, certain gastroenterology disorders and metabolic syndrome.
ONEMIA® was introduced in the U.S. market in 2011. In 2012, Acasti made its first sales of ONEMIA® to a medical food distributor in the United States, which began distribution of ONEMIA® through its network of dispensing physicians under its own brand name. ONEMIA® is also available behind-the-counter in pharmacies. Acasti expects continued sales of ONEMIA® in the short-term to provide revenues that will contribute, in part, to finance Acasti’s research and development projects while continuing to generate awareness of ONEMIA® throughout the medical community in an effort to build a market foundation for CaPre® to further advance. During the fiscal year ended on February 28, 2014, Acasti generated revenues of approximately $500,875 from sales of ONEMIA®.
23
In 2012, Acasti interviewed and collected data on a voluntary basis from physicians either buying, using, or testing ONEMIA® on some of their patients. The 20 physicians (consisting of five primary care physicians and 15 cardiologists or endocrinologists) that participated are also prescribers of Lovaza and recommended ONEMIA® to 348 patients without controlling their diet, exercise or monitoring compliance with the recommended dosage. Most physicians were willing to try ONEMIA® as a potentially more cost efficient option relative to Lovaza without side effects such as reflux and other gastrointestinal disorders, and having a once per day dosing convenience making it easier to use than Lovaza with its dosage requirements of four 1g capsules per day. This survey also showed that primary care physicians responded favorably to features of ONEMIA® such as once-a-day dosing, bioavailability due to the element of marine phospholipids in ONEMIA® and the ability to take ONEMIA® with or without a meal.
Acasti continues to explore the benefit of combining ONEMIA® with a statin treatment. Non-clinical activities have been undertaken in order to determine whether or not ONEMIA® should be added to a statin treatment. The accumulated non-clinical data showed that it would be beneficial to explore in humans testing the positive results which were observed in animal testing to the effect that ONEMIA® may benefit patients taking statins dealing with complex and hard to manage lipid profiles.
Pharmaceutical Products and Product Candidates—NeuroBio
Our subsidiary, NeuroBio, is in the early stages of developing omega-3 phospholipids medical foods, over-the-counter products and prescription drugs. NeuroBio is dedicated to the research, development and commercialization of active pharmaceutical ingredients, or APIs, for the management of neurodevelopmental, memory, concentration, learning and neurological disorders, from prevention to treatment. NeuroBio addresses mental and neurological conditions, specifically mood disorders such as depression, attention-deficit hyperactivity disorder, or ADHD, and cognitive decline associated with aging.
MPL VI, MPL VII and MPL VIII – Medical Food
MPL VI is intended for the dietary management of cognitive decline associated with neurodegenerative conditions.
We believe MPL VII is well-positioned to exhibit an intrinsic biological activity, because of its distinctive DHA-bounded phosphatidylcholine content, for dietary management of memory, concentration and learning disorders, allowing a variety of applications. For this specific product, NeuroBio believes it has an innovative clinical approach to quantify cognitive improvement and reach rapidly the market with conclusive results.
MPL VIII was designed and intended to supplement nutrition intake by children and adults suffering from ADHD for which phospholipid deficiency may represent a key risk factor. MPL VIII is an original and a proprietary formulation that contains a specific API having a high concentration in selected phospholipids and with a specific omega-3 profile.
Currently, none of MPL VI, MPL VII or MPL VIII have been approved for sale in any jurisdiction.
MPL IX – Prescription Drug
MPL IX is under preclinical evaluation for neurological disorders and will be tested in several preclinical models, at various daily doses and durations of treatment, the product will be administered orally, to assess the safety and efficacy of given compositions and to determine the pharmacokinetic profile.
Data is intended to demonstrate that MPL IX can, based on dosage, significantly reduce important neurological disorders and improve cognitive functions in these animal models. Most importantly, these effects will need to be achieved without the common side-effect of other traditional treatments.
24
NeuroBio’s product candidates are at different development and/or validation stages and are expected to require the approval of the FDA and/or Health Canada before commercialization. Approvals from similar regulatory organizations are also expected to be required before sales are authorized. See “Business of the Corporation—Studies & Trials for Pharmaceutical Product Candidates—NeuroBio’s Product Candidates” and “Business of the Corporation—Regulatory Environment”.
Our Market
Neptune’s Market: The Nutraceutical Market
The nutraceutical market encompasses functional foods and dietary supplements, which include a wide range of nutrients such as vitamins, minerals, fatty acids, amino acids and herbal supplements. Neptune focuses on dietary supplements. According to Agriculture and Agri-Food Canada, a government organization that provides statistics on the nutraceutical market, the nutraceutical market is growing rapidly, in part driven by the health demands of an aging population. According to a report published by RNCOS Industry Research Solutions in May 2012 entitled US Nutraceuticals Market Analysis, the nutraceutical market has become one of the fastest growing industries in the United States. In 2008, the U.S. Census Bureau, using data from the 2000 U.S. Census, projected that by 2030, the number of Americans 65 years old and older will increase from 40.3 million to just over 72.0 million, then representing over 19% of the population in the United States.
The Corporation believes that health issues such as high (and in some cases low) cholesterol, heart disorders, cognitive function and brain performance disorders and joint issues (including inflammation) are driving the nutraceutical market expansion. We believe the following factors, among others, favor the growth of the nutraceutical market:
| • | improved understanding and scientific knowledge of the contribution of diet in health maintenance and disease prevention; |
| • | increased consumer demand for dietary supplements that help to maintain vitality and promote health; and |
| • | increased health care costs and the trend towards self-treatment with a focus on natural products. |
Neptune primarily sells omega-3 PUFAs into the nutraceutical market. The most predominant omega-3 fatty acids are DHA and EPA derived from plant and marine sources.
The omega-3 fatty acids contained in Neptune’s products are sourced from krill, a zooplankton, with the advantage that omega-3 fatty acids from krill are carried by phospholipids and not triglycerides such as in fish oil. Phospholipids, a major component of biological membranes, are more easily absorbed by the body than triglycerides, resulting in a higher bioavailability of omega-3 fatty acids contained in krill oil.
The FDA announced in 2004 the availability of a qualified health claim for reduced risk of coronary heart disease for conventional foods that contain EPA and DHA omega-3 fatty acids. In 2000, the FDA announced a similar qualified health claim for dietary supplements containing EPA and DHA omega-3 fatty acids and the reduced risk of coronary heart disease.
In addition, extensive research, including Neptune’s clinical trial work, has further demonstrated certain clinical benefits of omega-3. Omega-3 fatty acids reduce inflammation and prevent risk factors associated with chronic diseases, such as heart disease and arthritis, and appear to be particularly important for cognitive (memory and concentration) and behavioural functions. Many forms of arthritis, such as osteoarthritis and rheumatoid arthritis, are inflammatory disorders and lead to pain, stiffness, swelling and functional impairment. Osteoarthritis is the most common form of arthritis and affects approximately 27 million people in the United States, according to a January 2008 publication of the medical journal Arthritis Rheum. It is caused by the breakdown and eventual loss of the cartilage between the bones of the joints. Non-surgical treatment options for osteoarthritis include analgesic and anti-inflammatory pain medications, nutritional supplementation, physical therapy, exercise and weight loss.
25
The PUFAs ingredient market and, more specifically, sales of omega-3 ingredients, are experiencing sustained growth, driven by the world retail market for dietary supplements and functional food. Based on the trends reported in the Frost & Sullivan July 2012 Report, the worldwide omega-3 market is expected to exceed US$3.1 billion in annual ingredient sales by 2016 and general market data indicates that sales of higher quality and higher performance omega-3’s are generating increasing revenues.
According to the Frost & Sullivan July 2012 Report, the global market revenue for marine and algae EPA/DHA omega-3 ingredients was US$1.8 billion in 2011, and is projected to grow at a CAGR of 11.8% from 2012 to 2016. Global consumption was measured at 103,284 metric tons in 2011, and is projected to grow at a CAGR of 9.4% from 2012 to 2016.
The world retail market for dietary supplements is highly fragmented, and is comprised of a large number of products and many small manufacturers. According to the Frost & Sullivan July 2012 Report, dietary supplements continued to be the largest market for marine omega-3 oils in the global market in 2011 with a 46.2% share and a total of US$834.6 million in revenue. The Frost & Sullivan July 2012 Report also estimates that pharmaceuticals, infant formulas and foods and beverages were the next largest consumers of marine oil omega-3, with 19.8%, 14.3% and 13.4% shares, respectively, in 2011.
Neptune has conducted clinical trials for functional food applications of NKO® with the multinational corporations Nestlé and Yoplait. However, the parties have decided not to pursue the development of these functional food applications. Neptune is instead currently focusing on the dietary supplement market, particularly in light of the limits on Neptune’s current maximum production capacity.
Acasti’s and NeuroBio’s Market: The Pharmaceutical Market
Cardiometabolic Disorder Treatments—Acasti
Heart attacks, strokes and other cardiovascular events represent the leading cause of death and disability among men and women in the United States. According to the 2011 At-A-Glance Report from the U.S. Center for Disease Control, more than 1 out of every 3 adults in the United States (approximately 83 million) currently lives with one or more types of cardiovascular disease; an estimated 935,000 heart attacks and 795,000 strokes occur in the United States each year; and an estimated 71 million adults in the United States have high cholesterol (i.e., high levels of LDL). Having abnormally high levels of lipids or lipoproteins, such as cholesterol and triglycerides, which are fats carried in the blood, is an important risk factor for cardiovascular disease.
The prevalence of hypertriglyceridemia is quickly increasing in the United States and globally, correlating to the increasing incidence of obesity and diabetes. Market participants estimate that one-third of the population in the United States has elevated levels of triglycerides, including over 40 million people diagnosed with hypertriglyceridemia and over 4 million people diagnosed with severe hypertriglyceridemia. According to The American Heart Association Scientific Statement on Triglycerides and Cardiovascular Disease (2011), triglyceride levels provide important information as a marker associated with the risk for heart disease and stroke, especially when an individual also has low HDL and elevated levels of LDL. Lowering triglyceride levels is one of the primary goals to reduce a patient’s risk of atherosclerotic cardiovascular disease. Hypertriglyceridemia is due to both genetic and environmental factors, including obesity, sedentary lifestyle and high-calorie diets. Hypertriglyceridemia is also associated with comorbid conditions such as diabetes, chronic renal failure, pancreatitis and nephrotic syndrome.
The National Cholesterol Education Program, or NCEP, Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol recommends that the first priority for the management of hypertriglyceridemia is triglyceride reduction to decrease the risk of pancreatitis. In addition, severe hypertriglyceridemia is also associated with a markedly increased risk for cardiovascular disease and recent studies by market participants have demonstrated that elevated triglyceride levels can be regarded as an independent risk factor for cardiovascular disease-related events such as myocardial infarction, ischemic heart disease and ischemic stroke.
The rise in obesity over the last 20 years has led to a parallel increase in triglyceride levels among the population and awareness of medical and health practitioners about the critical role that high triglyceride levels, particularly together with abnormal levels of LDL, HDL and non HDL (which is collectively referred to as dyslipidemia), have as a predictor of cardiovascular events. Accordingly, the introduction of new prescription drugs and drug therapies to lower the risk of cardiovascular events by addressing dyslipidemia has become a priority. The initial treatment recommendation for patients with dyslipidemia is typically a lifestyle change (diet and increased
26
exercise). Dyslipidemia is also treated with statins, which account for a large portion of prescriptions for dyslipidemia. However, statins alone are primarily used for reducing LDL only and appear to have only modest effects on triglyceride levels. Recognizing that statins alone are not effective triglyceride lowering drugs, the NCEP panel recommends the use of more focused therapies to lower triglyceride levels in patients with severe hypertriglyceridemia. The first-line drug therapy in patients with severe hypertriglyceridemia is often a prescription omega-3 fatty acid or fibrates, but clinical tests have shown that fibrates may also induce side effects.
According to an investigation published by the American Medical Association in 2009, fewer than 4% of adults in the United States with hypertriglyceridemia receive prescription medication to lower trigylceride levels, representing a significant unmet medical need. Many available treatment options have limitations in the treatment of hypertriglyceridemia which Acasti believes CaPre® can address. The use of fibrates, for example, has been shown to raise the risk of abnormal increases in liver enzymes and creatinine (a marker of kidney function) and, when combined with a statin, rhabdomyolysis (muscle breakdown). Acasti does not believe that CaPre® produces such side effects. Furthermore, Acasti believes that CaPre® in combination with statins could become a standard of care in patients with mixed dyslipidemia because of its once per day dosing convenience.
There are several marketed prescription omega-3 fatty acids currently approved for treatment of dyslipidemia in the United States and elsewhere. According to the Frost Sullivan 2012 Global Overview of the EPA and DHA Omega-3 Ingredients Markets, the global market revenue for marine and algae EPA/DHA omega-3 ingredients market in 2011 was approximately $1.8 billion. Lovaza and Omacor, which are sold in the United States and Europe, respectively, are omega-3 ethyl-esters derived from fish oil comprised of EPA and DHA and are indicated for the treatment of severe hypertriglyceridemia in twice-daily doses of two 1-gram capsules or once-a-day dose of four 1-gram capsules. In addition, Vascepa and Epadel are two approved omega-3 ethyl-esters derived from fish oil comprised of EPA that are sold in the United States and Japan, respectively. Market participants have estimated that the total prescription omega-3 market generated over $2 billion in sales worldwide in 2012. Acasti believes that there will be increased growth in the prescription omega-3 market based on the expected introduction, and resulting increased promotion and awareness, of new prescription omega-3 products, as well as the emergence of new clinical data regarding the efficacy of omega-3s in the treatment and prevention of cardiometabolic disorders.
Neurodegenerative and inflammation related conditions—NeuroBio
NeuroBio focuses on mental and neurological conditions, specifically mood disorders such as depression, ADHD and cognitive decline associated with aging. The prevalence of these disorders in North America is summarized in the following table:
Disorder | Market | Prevalence | Source | |||
| Memory, learning, and concentration and neurological disorders | Medical Food/Prescription Drug | Affecting at some point during their lifespan the majority of people during the educational and professional stage and later 19% of adults aged >65 years | Alzheimer’s Association, 2010 Alzheimer’s Disease Facts and Figures,Alzheimer’s & Dementia,Volume 6 | |||
| ADHD (attention-deficit hyperactivity disorder) | Medical food/Prescription Drug | 9.0% of children 13-18 yrs (lifetime prevalence) | Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J.; Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Study-Adolescent Supplement (NCS-A).J Am Acad Child Adolesc Psychiatry.2010 Oct;49(10):980-989. | |||
27
Studies & Trials for Pharmaceutical Product Candidates
General
Neptune is continuously investing in medical research and development aimed at demonstrating the benefits of its products on human health. From time to time, Neptune enters into clinical research programs with strategic partners for the completion of clinical trial.
Acasti’s Product Candidate: CaPre®
Acasti has initiated the Phase II TRIFECTA and COLT clinical trials under Canada’s Natural Health Product Directorate, or NHPD, guidelines. The COLT trial has been completed and its results were announced on August 13, 2013. Acasti believes the trial will be completed by the end of the second quarter of calendar 2014 and results will be available at a future date yet to be determined. Due to a recent decision of the FDA not to grant authorization to commercialize a competitor’s drug in the mild to moderate patient population before the demonstration of clinical outcome benefits, Acasti is reassessing its clinical strategy and may put a primary and first focus on the severe hypertriglyceridemia population.
Colt Trial
The COLT trial, a randomized open-label dose-ranging, multi-center trial, was designed to assess the safety and efficacy of CaPre® in the treatment of hypertriglyceridemia and severe hypertriglyceridemia (clinical trial.gov identifier NCT01516151). The primary objectives of the COLT trial were to evaluate the efficacy of 0.5, 1.0, 2.0 and 4.0g of CaPre® per day in reducing fasting plasma triglycerides over four and eight weeks in 276 randomized enrolled patients (230 evaluable patients) with hypertriglyceridemia and severe hypertriglyceridemia as compared to the standard of care alone.
The primary objective of the COLT trial was to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 10.0 mmol/L (200-877mg/dL). The secondary objectives of the COLT trial were to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 5.69 mmol/L (200-499mg/dL); to evaluate the dose dependent effect on fasting plasma triglycerides in patients with triglycerides > 5.7 and <10 mmol/L (500-877 mg/dL); to evaluate the effect of Capre® in patients with hypertriglyceridemia and severe hypertriglyceridemia on fasting plasma levels of LDL-C (direct measurement), fasting plasma levels of HDL-C, non-HDL-C, hs-CRP, omega-3 index; and to assess the tolerability and safety of Capre®.
On August 13, 2013, Acasti announced the completion and results of its open-label Phase II COLT trial (clinical trial.gov identifier NCT01516151). The final results of the COLT trial indicated that CaPre® was safe and effective in reducing triglycerides in patients with mild to severe hypertriglyceridemia with significant mean (average) triglyceride reductions above 20% after 8 weeks of treatment with daily doses of both 4.0g and 2.0g. Demographics and baseline characteristics of the patient population were balanced in terms of age, race and gender. A total of 288 patients were enrolled and randomized and 270 patients completed the study, which exceeded the targeted number of evaluable patients. From this patient population, approximately 90% had mild to moderate hypertriglyceridemia. CaPre® was safe and well tolerated. The proportion of patients treated with CaPre® that experienced one or more adverse events in the COLT trial was similar to that of the standard of care group (30.0% versus 34.5%, respectively). A substantial majority of adverse events were mild (82.3%) and no severe treatment-related adverse effects have been reported.
The COLT trial met its primary objective showing CaPre® to be safe and effective in reducing triglycerides in patients with mild to severe hypertriglyceridemia. After only a 4-week treatment, CaPre® achieved a statistically significant triglyceride reduction as compared to standard of care alone. Patients treated with 4.0g of CaPre® per day over 4 weeks reached a mean triglyceride decrease of 15.4% from baseline and a mean improvement of 18.0% over the standard of care. Results also showed increased benefits after 8 weeks of treatment, with patients on a daily dose of 4.0g of CaPre® registering a mean triglyceride decrease of 21.6% from baseline and a statistically significant mean improvement of 14.4% over the standard of care. It is noteworthy that a mean triglyceride reduction of 7.1% was observed for the standard of care group at week 8, which may be explained by lipid lowering medication adjustments during the study, which was allowed to be administered in the standard of care group alone.
28
Moreover, after 8 weeks of treatment, patients treated with 1.0g for the first 4 weeks of treatment and 2.0g for the following 4 weeks showed a statistically significant triglycerides mean improvement of 16.2% over the standard of care, corresponding to a 23.3% reduction for the 1.0-2.0g daily dose as compared to a 7.1% reduction for the standard of care. After 8 weeks of treatment, patients treated with 2.0g of CaPre® for the entire 8 weeks showed statistically significant triglycerides mean improvements of -14.8% over the standard of care, corresponding to a 22.0% reduction for the 2.0g as compared to a 7.1% reduction for the standard of care. Also, after 8 weeks of treatment, patients treated with 4.0g for the entire 8 weeks showed statistically significant triglycerides, non-HDL-C (non-high density lipoprotein, which includes all cholesterol contained in the bloodstream except HDL-C (high density lipoprotein (good cholesterol)) and HbA1C (haemoglobin A1C) mean improvements of, respectively, 14.4% and 9.8% and 15.0% as compared to standard of care. The 4.0g group mean improvements in (i) triglycerides of 14.4% corresponds to a reduction of 21.6% as compared to a reduction of a 7.1% for the standard of care group, (ii) non-HDL-C of 9.8% corresponds to a reduction of 12.0% as compared to a reduction of 2.3% for the standard of care group, and (iii) HbA1C of 15.0% corresponds to a reduction of 3.5% as compared to an increase of 11.5% for the standard of care group. In addition, all combined doses of CaPre® showed a statistically significant treatment effect on HDL-C levels, with an increase of 7.4% as compared to standard of care. Trends (p-value < 0.1) were also noted on patients treated with 4.0g of CaPre® for the entire 8-week treatment period with mean reduction of total cholesterol of 7.0% and increase of HDL-C levels of 7.7% as compared to the standard of care. Furthermore, after doubling the daily dosage of CaPre® after an initial period of 4 weeks, the results indicate a dose response relationship corresponding to a maintained and improved efficacy of CaPre® after an 8-week period. The efficacy of CaPre® at all doses in reducing triglyceride levels and increased effect with dose escalation suggests that CaPre® may be titrable, allowing physicians to adjust dosage in order to better manage patients’ medical needs. In addition, the results of the COLT trial indicate that CaPre® has no significant deleterious effect on LDL-C (bad cholesterol) levels.
TRIFECTA Trial
Acasti’s TRIFECTA trial (clinical trial.gov identifier NCT01455844), a 12-week, randomized, double-blind, placebo-controlled study, is designed to assess the effect of CaPre®, at a dose of 1.0 or 2.0g, on fasting plasma triglycerides as compared to a placebo in patients with mild to severe hypertriglyceridemia. A total of 366 patients have been randomized over the 429 planned protocol (342 evaluable patients).
Similar to the COLT trial, the primary objective of the TRIFECTA trial is to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 10.0 mmol/L (200-877 mg/dL) and to assess the tolerability and safety of CaPre®. The secondary objectives of the TRIFECTA trial are to evaluate the effect of CaPre® on fasting plasma triglycerides in patients with triglycerides between 2.28 and 5.69 mmol/L (200-499 mg/dL); to evaluate the dose dependent effect on fasting plasma triglycerides in patients with triglycerides > 5.7 and <10 mmol/L (500-877 mg/dL); to evaluate the effect of CaPre® in patients with mild to moderate hypertriglyceridemia and severe hypertriglyceridemia on fasting plasma levels of LDL-C (direct measurement), and on fasting plasma levels of HDL-C, non-HDL-C, hs-CRP and omega-3 index.
On December 20, 2012, the TRIFECTA trial completed an interim analysis. The review committee made up of medical physicians assembled to evaluate the progress of the TRIFECTA trial reviewed the interim analysis relative to drug safety and efficacy and unanimously agreed that the study should continue as planned. All committee members agreed that there were no toxicity issues related to the intake of CaPre® and that the signals of a possible therapeutic effect, noted as reduction of triglycerides in the groups evaluated, were reassuring and sufficiently clinically significant to allow the further continuation of the TRIFECTA trial. The data was provided to the committee members blind, meaning that the identity of the three groups was not revealed. Since the data revealed a possible therapeutic effect without any safety concerns, the committee decided that it was not necessary to unblind the data. The number of targeted patients evaluable as per protocol has been reached. Acasti is currently evaluating efficacy and safety of CaPre® for the treatment of patients with mild to severe hypertriglyceridemia, which is the primary objective of the study. A secondary objective of the study was to assess the efficacy of CaPre® in two distinct patient populations: those with mild to moderate hypertriglyceridemia and those with severe hypertriglyceridemia. Based on patient information currently available, the Corporation does not expect the sample size to be large enough to conclude on the efficacy of CaPre® on severe hypertriglyceridemia as part of the TRIFECTA trial. Acasti does not expect the FDA to request efficacy data on patients with severe hypertriglyceridemia before granting permission to conduct a phase III trial. Acasti believes the trial will be completed by the end of the second quarter of calendar 2014 and results will be available at a future date yet to be determined.
29
PK Trial
On November 11, 2013, Acasti announced that it submitted an investigational new drug application to the FDA to initiate a PK (pharmacokinetic) trial of CaPre® in the United States. The proposed PK trial is an open-label, randomized, multiple-dose, single-center, parallel-design study to evaluate blood profiles and bioavailability of omega-3 phospholipids on healthy volunteers taking single and multiple daily oral doses of 1.0g, 2.0g and 4.0g of CaPre®. Acasti expects that the duration of the PK trial would likely be over a 30-day period and involve the enrollment of approximately 42 healthy subjects.
On January 9, 2014, Acasti announced that the FDA granted Acasti approval to conduct its PK trial, having found no objections with the proposed PK trial design, protocol or safety profile of CaPre®. Acasti also announced that Quintiles, the world’s largest provider of biopharmaceutical development and commercial outsourcing services, has been hired to conduct the PK trial. Acasti expects results of its PK trial to be available by mid to late 2014.
Next Steps
Acasti is corresponding with the FDA and has responded to the FDA’s recommendations regarding its upcoming IND filing for its phase III clinical trial of CaPre® in the United States. The FDA has invited Acasti to formally request an end of phase II/pre phase III meeting to allow them to provide feedback on the submission and to address specific questions for which Acasti is seeking approval and final response from the FDA. Acasti intends to seek such meeting as soon as TRIFECTA trial results are available.
Acasti intends to conduct a phase III clinical trial in the United States, with potentially a few Canadian clinical trial sites, in a patient population with very high triglycerides (>500 mg/dL). This study would constitute the primary basis of an efficacy claim for CaPre® in an NDA submission for severe hypertriglyceridemia. Acasti is also evaluating the possibility of submitting an SPA to the FDA in order to form the basis for the design of its intended Phase III clinical trial. An SPA is a declaration from the FDA that an uncompleted Phase III trial’s design, clinical endpoints, and statistical analyses are acceptable for FDA approval. A request would be submitted for the protocol at least 90 days prior to the anticipated start of the Phase III clinical trial.
In addition to conducting and completing the TRIFECTA, PK and a Phase III clinical trial, Acasti expects that additional time and capital will be required to complete the filing of a NDA to obtain FDA approval for CaPre® in the United States before reaching commercialization, which may initially be only for the treatment of severe hypertriglyceridemia. The FDA may require Acasti to conduct additional clinical studies to obtain FDA approval for the treatment of mild to moderate hypertriglyceridemia, which may include a cardiovascular outcomes study.
NeuroBio’s Product Candidates
The development of NeuroBio’s product candidates was delayed by the November 2012 incident at Neptune’s production facility. The preclinical and clinical studies that were planned to start in late 2012 and early 2013 were postponed. Preclinical studies that were in progress, however, were not interrupted.
Certain preclinical results have indicated the safety and efficacy of NeuroBio’s APIs portfolio in either nutritional intervention or therapeutic management of memory, concentration and learning disorders, ADHD and cognitive decline associated with aging.
Neuro Bio is currently preparing a randomized placebo-controlled double-blind study to evaluate the effect of MPLIX on Mild Cognitive Impairment (“MCI”) in an elderly population between the ages of 65 and 80 years old. This phase II study will help establish the sensitivity and precision of the assessment tools, determine the effect of the product candidate on cognitive functions, depression, anxiety and quality of life in a MCI population, and will examine the placebo effect. In addition, the data collected will be used to determine the appropriate statistical parameters to design a pivotal clinical study.
30
NeuroBio also intends to conduct a prospective study in children, between the ages of 6 and 15 years old, with attention-deficit hyperactivity disorder (“ADHD”) symptoms. This prospective study aims to determine the benefits of MPLIX as an add-on to ADHD pharmacotherapy as compared to a stand-alone Omega-3 phospholipids therapy and the possibility of decreasing the side effects related to the ADHD pharmacotherapy.
NeuroBio also expects to continue its nonclinical studies investigating the potential therapeutic effects of its product candidates, including non-clinical toxicology studies to assess the safety of its product candidates.
Approvals of applicable regulatory authorities, including the Natural Health Products Directorate (Canada), are required before the studies of NeuroBio may begin.
The NeuroBio product portfolio includes highly concentrated phospholipids extracted and purified from different marine species, including krill, which functionalize EPA and DHA most often stabilized by potent antioxidant esters. NeuroBio’s potential medical food and over the counter drug product portfolio consists of MPL VI, MPL VII, MPL VIII. NeuroBio’s potential prescription drug candidate is MPL IX. NeuroBio’s product candidates are at different development and/or validation stages and are expected to require the approval of the FDA and/or Health Canada before commercialization. Approvals from similar regulatory organizations are also expected to be required before sales are authorized. See “Business of theCorporation - Regulatory Environment” and “Risk Factors- Risks Related to the Corporation’s Industry - The Corporation is subject to significant government regulations.”
Product | Channel | Indication | Stage of development | |||||
MPL VI | Medical Food | Prevention of cognitive decline | Preclinical | |||||
MPL VII | Medical Food | Memory, concentration and learning disorders | Preclinical | |||||
MPL VIII | Medical Food | ADHD | Preclinical | |||||
MPL IX | Prescription Drug | Neurological disorders | Preclinical | |||||
Supply of Krill
Neptune sources the krill used in the manufacturing of its products generally from three suppliers. Neptune considers its relationship with its suppliers to be good and believes it is not dependent upon any of these suppliers since alternative sources of krill supply are readily available.
There are two primary ocean regions where krill is harvested: the Southern Ocean (Antarctic krill) and the North Pacific Ocean (Pacific krill, mainly off the coasts of Japan and Canada). The total quantity of the krill species in these two oceans is estimated to be at least 500,000,000 metric tons. The World Health Organization estimates that approximately 271,000 metric tons of both krill species are harvested annually from these two oceans. From 2002 to 2011, between 105,000 to 212,000 metric tons originated from the Southern Ocean (Antarctic krill Euphausia superba) and, on average 60,000 metric tons originated from the Northern Pacific Ocean (Pacific krill Euphausia pacifica) each year. The annual Antarctic krill catches represent an estimated 0.05% of the existing resource. Neptune uses Antarctic krill. According to the Commission for the Conservation of Antarctic Marine Living Resources, or CCAMLR, from 2008 to 2011 annual quotas for Antarctic krill have increased by 33%. 11. As a result, the Corporation believes that krill is an abundant and accessible resource with potential for long-term sustainable exploitation with adequate traceability measures. The average market price for whole frozen krill is around US$900 per metric ton.
Krill harvested for the krill oil products manufactured by Neptune represents less than 0.0006% of the total-estimated biomass and less than 0.03% of the precautionary catch limit. Neptune commits 100% of its krill capture for human health benefits. Worldwide, approximately 88% of total catches are used by fisheries for low valued products such as fishing baits (45%) and krill meal for aquaculture (43%). Approximately 12% of the total krill catch is used for direct human consumption as food (whole or processed).
31
In May 2011, NSF International, an independent, not-for-profit organization that provides standards development, product certification, auditing, education and risk management for public health and the environment, completed a review of key environmental claims for Neptune and the marine derived products manufactured by Neptune. The audit performed by NSF International was conducted to ensure clarity and conformance with the strict criteria of the International Organization for Standardization (ISO) 14021: Environmental labels and declaration, as well as U.S. Federal Trade Commission (16 CFR PART 260): Guides for the Use of Environmental Marketing Claims. NSF certification granted to Neptune in May 2011 expired during FY2014 and was not renewed by the Corporation after it became entitled to use the “Friend of the Sea” environmental certification described below.
In May 2012, following an audit by an auditor recognized by FOS, Neptune became entitled to use the “Friend of the Sea” environmental certification. Neptune can use the “Friend of the Sea” logo on the krill oil products that it manufactures. FOS is an internationally recognized organization which verifies the sustainable origin of marine products. The logo provides an effective way to communicate environmental performance customers and Neptune successfully obtained FOS certification by complying with strict krill sustainability criteria. The “Friend of the Sea” certification can be granted when, among other things, the audit confirms that stocks are not overfished, endangered species are not caught, fishing does not impact the seabed, and the company gradually reduces its carbon footprint. The “Friend of the Sea” certification can also be extended to distributors that can prove that Neptune is their sole krill oil provider. Once audited, they can include the “Friend of the Sea” logo on their packaging and marketing material.
Manufacturing and Facilities
Before the incident that destroyed Neptune’s sole manufacturing facility, located on Pepin Street in Sherbrooke, Quebec, Canada, on November 8, 2012, Neptune produced all of its products at such plant. Neptune is currently in the process of rebuilding a state of the art production facility. Construction on Neptune’s new state of the art production facility commenced on May 28, 2013 by overhauling the expansion facility that was under construction adjacent to the former plant. See “Recent Developments—New Production Facility Reconstruction and Operations”. The commodity krill oil currently sold by Neptune is produced in collaboration with third parties.
Upon completion of the new production facility, a start-up and ramp-up period will be required before full production capacity will be achievable. The ramp-up period is expected to be completed in three phases over a period of three months, with each phase lasting one month. During this ramp-up period, Neptune expects to progressively increase production in each of the three phases to an annual production capacity of 50,000, 100,000 and 150,000 kilograms of krill oil products respectively, until the new production facility’s full commercial annual production capacity of krill oil is reached. See “Recent Developments—New Production Facility Ramp-Up Period”.
Neptune also leases office space in facilities located at 545, Promenade du Centropolis, in Laval, Quebec since October 1, 2012.
Sales/Distribution
Neptune sells its krill oil products in bulk oil or in capsules to multiple distributors, who commercialize these products under their private label in different market segments, including health food stores, mass (food and drug), direct sales (multi-level marketing, internet, catalogue, radio) and via healthcare professional recommendation. The encapsulation process is subcontracted to third parties in Canada, the United States, Asia and Europe. While the Corporation may have purchase orders in place with approximately 40 to 50 different distributors at any one time, the majority of the Corporation’s sales are concentrated with a relatively small group of distributors. As at February 28, 2014, five customers represented 68% (2013 – 88%) of total trade accounts receivable of the Corporation. Agreements with these distribution partners may be terminated or altered by them unilaterally in certain circumstances. See “Risk Factors—Risks Related to the Corporation’s Business—The Corporation derives its revenue from a limited number of distributors and has a significant concentration of its accounts receivable.” In addition, the agreements between Neptune and its distributors contain certain customary indemnification provisions with respect to liability incurred from claims resulting from items that are the responsibility of the distributor, such as encapsulation or packaging.
ONEMIA® is being distributed in the United States by Acasti to physicians, who then can either provide it to their patients directly or via a website by using a dedicated medical food access code. Acasti also makes ONEMIA® available via distributors and behind-the-counter in pharmacies. In 2012, Acasti made its first sales of ONEMIA® to a medical food distributor in the United States, which has begun distribution through its network of dispensing
32
physicians under its own brand name. Acasti intends to make ONEMIA® available via additional distributors and behind-the-counter in more pharmacies in the United States and to secure distribution partners to commercialize ONEMIA® outside of the United States. Revenues of Acasti for the fiscal years ended February 28, 2014, February 28, 2013 and February 29, 2012 were all derived from the sale of ONEMIA® and amounted to approximately $500,875, $724,000 and $10,000, respectively. During its fiscal year ended February 28, 2013, more than 90% of sales of ONEMIA® were made through Acasti’s distribution partner in the United States and the remaining 10% came from direct sales by Acasti. See “Risk Factors—Risks Related to the Corporation’s Business—The Corporation may not be able to further penetrate core or new markets.”
During the 2014 fiscal year, approximately 50% (2013 – 34%) of Neptune’s sales were made to customers in the United States, 11% to customers in Europe, 25% to customers in Australia, 4% to customers in Canada and 4% to customers in other countries. Sales of Neptune products for the fiscal year ended February 28, 2014 amounted to $19,334,719, a decrease from $25,863,612 for the fiscal year ended February 28, 2013. Neptune’s sales are not cyclical or seasonal.
Intellectual Property
It is an important part of our business to obtain intellectual property protection for our technology, products, applications and processes and/or to maintain trade secrets. Our success depends, in part, on our ability to obtain, license and enforce patents, protect our proprietary information and maintain trade secret protection without infringing the proprietary rights of third parties. Our strategic approach is to file and/or license patent applications whenever possible to obtain patent protection. We also rely on trade secrets, proprietary unpatented information and trademarks to protect our technology and enhance our competitive position. We have confidence in our patents and will continue to take all appropriate actions needed to protect our intellectual property rights in the United States and elsewhere in the world as required.
The Corporation has a firm policy to protect its intellectual property rights, including its patents, trademarks and trade secrets, through legal action.Certain of Neptune’s competitors have been marketing, advertising and selling finished krill-based products which we believe infringe on patents owned by Neptune or for which Neptune has exclusive rights. Neptune is taking legal actions against those companies in order to protect its intellectual property and its business. See “Risk Factors—Risks Related to the Corporation’s Intellectual Property—A failure by the Corporation to protect its intellectual property may have a material adverse effect on its ability to develop and commercialize its products.”
Brand Names and Trademarks
Neptune has filed and registered the trademarks OPA 3™ and NKO® in over thirty countries and has filed numerous trademark applications in various jurisdictions. Neptune Krill Oil™, EKO™ and NKA™ are other trademarks of Neptune.
NKO® distributors use private labels with the NKO® logo displayed on them and with names and trademarks pre-approved by Neptune.
Acasti has applied in many countries of the world for trademark protection of CaPre®, and has filed for U.S. trademark protection of ONEMIA®. Acasti also is the owner of the trademark BREAKING DOWN THE WALLS OF CHOLESTEROL™ in Canada and the United States. The trademark CaPre® is now registered in Canada, the United States, the European Union, Australia and China.
33
Patents
Neptune owns the following portfolio of patents, which are grouped in three main patent families and filed in various jurisdictions worldwide, including the United States, Canada, Japan, Australia and Europe:
Patent Family Description | Description | WO (PCT) Application Number & U.S. Patent Number(s) | Expiration Date of the Patent Family | Number of Patents Worldwide | ||||
Novel Phospholipid/Flavonoid | Composition ofMatter | WO2003/011873 US8,030,348; US8,278,351; US8,383,675 | WO 2003/011873 Family – 2022 US8,030,348 term adjusted to 2024 | 5 | ||||
Cardiovascular Neurological Health | Method of Use | WO2002/102394 US8,057,825 | WO 2002/102394 Family - 2022 US8,057,825 term adjusted to 2025 | 22 | ||||
Extraction Process | Process | WO2000/023546 US6,800,299 | 2019 | 32 |
| * | Neptune’s European patent EP1,417,211 was revoked on April 9, 2013 |
On July 16, 2013, Neptune announced that the Canadian Intellectual Property Office granted Neptune a composition patent (CA2,493,888) covering omega-3 phospholipids comprising PUFAs, the main bioactive ingredients in all recognized krill oils. The patent, which was granted for the Canadian market and is valid until 2022, covers novel omega-3 phospholipid compositions, synthetic and/or natural, regardless of the extraction process, suitable for human consumption. The patent protects Neptune’s krill oils, namely NKO®, and also covers amongst others, oils and powders extracted from krill and any marine or aquatic biomasses containing marine phospholipids bonded to EPA and/or DHA, distributed and/or sold in the Canadian market.
Canadian patent 2,493,888 is part of a patent family that has faced third party challenges in other jurisdictions. The corresponding European Patent (Patent No. EP1417211) was revoked by the EPO due to a third party opposition. Neptune appealed the decision to revoke the patent to the EPO’s Boards of Appeal and this appeal was dismissed. Neptune has petitioned the Enlarged Board of Appeal to review the Boards of Appeal’s decision. This review is currently pending. In addition, corresponding Australian Patent Application No. 2002322233 is currently in an opposition proceeding; however, Aker BioMarine AS has withdrawn its opposition. Similarly, US patent 8,278,351 is currently being challenged pursuant to ex parte re-examination proceedings before the USPTO. Additionally, an inter partes review of US patent 8,278,351 has been requested in the USPTO but the USPTO has not yet decided on institution of this proceeding.
Furthermore, all claims in U.S. patent 8,057,825 for “Krill Extracts for the Treatment of Cardiovascular Diseases” were deemed to be invalid pursuant to a third party re-examination process before the USPTO. Neptune is currently appealing this decision to USPTO’s Patent Trial and Appeal Board. Corresponding European Patent No. EP1997498 is also currently being opposed.
On April 18, 2014, the USPTO issued a final decision in the inter partes reexamination of Neptune’s 8,030,348 patent rejecting all of Neptune’s claims. Despite the USPTO’s decision, the ‘348 Patent is still valid as Neptune has the right to appeal, which appeal was filed by Neptune on May 19, 2014.
In Canada, the United States and Europe, a patent is generally valid for 20 years from the date of first filing. Patent terms can vary slightly for other jurisdictions, with 20 years from filing being the norm. In certain jurisdictions exclusivity can be formally extended beyond the normal patent term to compensate for regulatory delays during the pre-market approval process. Certain of Neptune’s issued patents face challenges by third parties, such as reexamination in the United States and opposition proceedings before the EPO and APO. See “Legal Proceedings and Regulatory Actions”.
Licensing Arrangements
The Corporation uses, for its production, its own process technology. Additionally, the Corporation has undertaken to pay an annual commission to a Corporation controlled by Mr. Henri Harland for services rendered as well as for the transfer in February 2001 to the Corporation of the license rights with the University, including the right of first refusal and of the option to purchase the intellectual property rights. This royalty of 1% on any sales and on other income of the Corporation is for an indeterminate period of time and it shall be paid semi-annually and disbursement of such royalty payment per year will not be superior to the Corporation’s net earnings before interest, taxes, depreciation and amortization (EBITDA).
34
Terms of the License Granted to Acasti
In August 2008, Neptune granted to Acasti a license to rights on its intellectual property portfolio related to cardiovascular pharmaceutical applications. This license allows Acasti to exploit the subject intellectual property rights in order to develop novel APIs into commercial products for the medical food and the prescription drug markets. Acasti is responsible for carrying out the research and development of the API, as well as required regulatory submissions and approvals and intellectual property filings relating to the cardiovascular applications. The following table summarizes the patent applications related to Acasti’s license from Neptune.
Patent description | US Patent # | Expiration Date of the Patent | Holder | |||||||
Composition of Matter (NATURALPHOSPHOLIPIDSOFMARINEORIGINCONTAININGFLAVONOIDSANDPOLYUNSATURATEDPHOSPHOLIPIDSANDTHEIRUSES) | US8,030,348 | (1) | 2022 | Neptune | ||||||
Method of Use for Dyslipidemia (KRILLAND/ORMARINEEXTRACTSFORPREVENTIONAND/ORTREATMENTOFCARDIOVASCULARDISEASES,ARTHRITIS,SKINCANCER,PREMENSTRUALSYNDROME,DIABETESANDTRANSDERMALTRANSPORT) | US8,057,825 | 2022 | Neptune | |||||||
Method of Extraction (METHODOFEXTRACTINGLIPIDSFROMMARINEANDAQUATICANIMALTISSUES) | US6,800,299 | 2020 | Neptune | |||||||
| Note: |
| (1) | Two continuations also stem from U.S. Pat. 8,030,348 (U.S. Pat. 8,278,351 and 8,383,675). |
The license agreement provides that the products developed by Acasti must comply with the ranges specified in the license agreement pertaining to the concentration of phospholipids.
Acasti is obligated under the license agreement to pay Neptune, until the expiration of Neptune’s licensed patents, a royalty equal to the sum of (a) in relation to sales of products in the licensed field, the greater of: (i) 7.5% of Acasti’s net sales and (ii) 15% of Acasti’s gross margin; and (b) 20% of revenues from sub-licenses granted by Acasti to third parties. The license will expire on the date of expiration of the last-to-expire of the licensed patent claims and/or continuation in part and/or divisional of the licensed patent claims. After the last-to expire of the licensed patents, which is currently expected to occur in 2022, the license agreement will automatically renew for an additional period of 15 years, during which period royalties will equal half of those calculated according to the above formula. Notwithstanding the above, the license agreement provides for minimum royalty payments as follows: year 1 - nil; year 2 - $50,000; year 3 - $200,000; year 4 - $225,000 (initially $300,000, but reduced to $225,000 following Acasti’s abandonment of its option right to develop products for the over-the-counter market pursuant to the license); year 5 - $700,000; and year 6 and thereafter - $750,000. Minimum royalties are based on contract years based on the effective date of the license agreement, which is August 7, 2008.
On December 4, 2012, Acasti announced that it entered into a prepayment agreement with Neptune pursuant to which Acasti exercised its option under the license agreement to pay in advance all of the future royalties payable under the license. The value of the prepayment, determined with the assistance of outside valuations specialists, using the pre-established formula set forth in the license agreement, amounts to approximately $15.5 million, which Acasti intends to pay through the issuance of 6,750,000 Class A Shares of Acasti, issuable at a price of $2.30 per share, upon the exercise of a warrant issued to Neptune.
The prepayment agreement and the issuance of the Class A shares to Neptune upon the exercise of the warrant was approved by the TSXV and the disinterested shareholders of Acasti at Acasti’s annual meeting of shareholders, which occurred on June 27, 2013.
35
Pursuant to the terms and conditions of the license agreement, Acasti is required, at Neptune’s option, to have its products, if any, manufactured by Neptune at prices determined according to different cost-plus rates for each of the product categories under the license. A copy of the license agreement is available on SEDAR atwww.sedar.com.
Terms of the License Granted to NeuroBio
In 2008, Neptune also entered into a license agreement that provides NeuroBio the same rights and obligations as provided to Acasti. See “Business of theCorporation - Intellectual Property—Licensing Arrangements—Terms of the License Granted to Acasti”. Pursuant to the license agreement, NeuroBio is permitted to use the licensed intellectual property rights solely for the development, distribution and sale of products for use in the human neurological field (all conditions, abnormalities and/or diseases related to cognitive function and/or affective and/or neurological systems).
The patents subject to the license with NeuroBio are the following:
Patent description | International Patent Publication # | Exclusivity | ||
Composition of Matter | WO 2003/011873 | 2024 | ||
Method of Use | WO 2002/102394 | 2024 | ||
Method of Extraction | WO 2000/023546 | 2020 |
NeuroBio is obligated under the license to pay Neptune until the expiration of the licensed patents on licensed intellectual property a royalty equal to the sum of (a) in relation to sales of products in the licensed field, if any, the greater of: (i) 7.5% of net sales, and (ii) 15% of NeuroBio’s gross margin; and (b) 20% of revenues from sub-licenses granted by NeuroBio to third parties, if any. The license will expire on the date of expiration of the last-to-expire of the licensed patent claims and/or continuation in part and/or divisional of the licensed patent claims. After the last to expire of the licensed patents on licensed intellectual property, which is currently expected to occur in 2022, the license agreement will automatically renew for an additional period of 15 years, during which period royalties will equal half of those calculated according to the above formula. In addition, the license provides for minimum royalty payments notwithstanding the above of: years 1 and 2 - nil; year 3 - $50,000; year 4 - $200,000; year 5 - $300,000; year 6 - $900,000 and year 7 and thereafter - $1,000,000. Minimum royalties are based on contract years based on the effective date of the license, October 15, 2008.
NeuroBio has the option to pay future royalties in advance, in cash or through the issuance of shares, in whole or in part, based on an established economic model contained in the license. NeuroBio can also abandon its rights under all or part of the license agreement and consequently remove itself from the obligation to pay all or part of the minimum royalties by paying a penalty equal to half of the next year’s minimum royalties. In addition, at Neptune’s option, NeuroBio is required to have its products, if any, manufactured by Neptune at prices determined according to different cost-plus rates for each of the product categories under the license.
On March 18, 2014, NeuroBio announced that it will exercise its option to pay all future royalties in advance. An outside party will conduct an independent valuation to determine the value of royalty prepayment.
A copy of the NeuroBio license agreement is available on SEDAR atwww.sedar.com.
Regulatory Environment
Commercial products developed or under development by Neptune, directly or through its subsidiaries, can be categorized as ingredients to be used in foods, dietary supplements, medical foods, natural health products or as APIs to be used in drug products.
Those ingredients may qualify as “novel foods” or “new dietary ingredients”, depending on final applications and countries where they are or will be marketed. Generally speaking, novel foods are defined as food substances that do not have a prior history of safe use or result from a process previously not used for foods. Similarly, a new dietary ingredient refers to a substance not previously used as a dietary supplement in humans prior to October 15, 1994. In the United States, the FDA (Center for Food Safety and Applied Nutrition) regulates matters associated with the safety of ingredients for use in food and dietary supplements. Any substance intentionally added to food is a
36
food additive, thus requiring approval by the FDA, unless the substance is “Generally Recognized As Safe”, or GRAS, under the conditions of its intended use, or unless the use of the substance is otherwise excluded from the definition of a food additive. GRAS status may be achieved through a voluntary notification procedure. A mandatory notification process for a “new dietary ingredient” is also in place according to the U.S. Food, Drug, and Cosmetic Act which requires that manufacturers and distributors who wish to market dietary supplements that contain new dietary ingredients notify the FDA.
In Canada, novel foods are regulated by the Novel Foods Regulation (under theFood and Drugs Act) which requires that a notification be made to the Health Products and Food Branch prior to the marketing or advertising of a novel food in the Canadian marketplace. Natural health products (equivalent to dietary or food supplements) sold in Canada are subject to theNatural Health Products Regulations, which came into force on January 1, 2004. All natural health products must have a product license before they can be sold in Canada, which requires applicants to gather and provide detailed information about the quality, safety and efficacy of ingredients to be used for assessment and pre-market approval. Manufacturing facilities located in Canada and producing omega-3 supplements are subject to regulation by the Canadian Food Inspection Agency.
In Europe, the legislation governing nutritional supplements is enacted and enforced by each individual country’s governmental authorities. In an effort to harmonize the often differing regulations of its member states, the European Union adopted in 2002 the Food Supplements Directive. This directive seeks to harmonize the rules governing the composition, labelling and marketing of nutritional supplements throughout the European Union. The Food Supplements Directive outlines a specific process and timetable for the member states to bring their domestic legislation in line with the directive’s provisions. The directive, upon recommendation by the European Food Safety Authority, or EFSA, specifies what nutrients and nutrient sources may be used, identifies the levels at which these nutrients may be found in a supplement and the labelling and other information which must be provided on packaging.
APIs developed or under development by Acasti and NeuroBio are regulated through different procedures and requirements. In Canada, biopharmaceutical product candidates are regulated by theFood and Drugs Act and the rules and regulations promulgated thereunder, which are enforced by the Therapeutic Products Directorate of Health Canada. In the United States, drugs and biological product candidates are subject to regulation and premarket approval by the FDA (Center for Drug Evaluation and Research or Center for Biologies Evaluation and Research). It is also possible that such products would be regulated in Canada as natural health products pursuant to theNatural Health Products Regulations.
In Europe, the European Medicines Agency, or EMA, is the regulatory agency which controls all aspects of the development, manufacture and commercialization of drug products for the countries of the European Union. Each country of the European Union also has its own national regulatory agency which works under the umbrella of the EMA.
These laws and regulations in Canada, the United States and Europe require the licensing of manufacturing and contract research facilities, carefully controlled research and testing of product candidates and governmental review and approval of results prior to marketing therapeutic product candidates. Additionally, they require adherence to good laboratory practices for pre-clinical safety testing in animals, good clinical practices during clinical testing and good manufacturing practices during production. The systems of new drug approvals in Canada, the United States and the European Union are generally considered to be among the most rigorous in the world.
In general, the steps required for approval of a new drug in Canada, the United States and Europe are:
1.Research
Prior to preclinical studies, a research phase takes place which involves characterization of the physical chemical properties and biological activity of the product. This is often followed by evaluation of efficacy in animal models.
37
2.Preclinical Studies
Preclinical studies involve evaluations of animal pharmacology and toxicity, pharmacokinetics and metabolism of a drug in animals to provide evidence of the safety, bioavailability and activity of the drug in animals. The results of these studies as well as the comprehensive descriptions of proposed human clinical studies are then submitted as part of the IND application to the FDA, its Canadian equivalent, a Clinical Trial Application, to Health Canada, or its European equivalent, an Investigational Medicinal Product Dossier, to the EMA.
3.Clinical Trials
Phase I Clinical Trials: Phase I clinical trials are usually first-in-man trials and take from a few months to two years to complete. They are generally conducted on a small number of healthy human subjects to evaluate the drug’s safety, schedule and dose, pharmacokinetics and pharmacodynamics.
Phase II Clinical Trials: Phase II clinical trials usually take approximately one to three years to complete and are carried out on a relatively small to moderate number of patients (compared to Phase III) suffering from the targeted condition or disease to determine the drug’s efficacy, optimal doses, treatment regimens, pharmacokinetics, pharmacodynamics and dose response relationships. This phase also provides additional safety data and serves to identify possible common short-term side effects and risks in a larger group of patients. Phase II clinical trials often include randomization of patients as well as a placebo arm.
Phase III Clinical Trials: Phase III clinical trials usually take approximately two to five years to complete and involve tests on a much larger population of patients (several hundred to several thousand patients) suffering from the targeted condition or disease. These studies usually include randomization of patients, a placebo arm and blinding of both patients and investigators at geographically dispersed test sites (multi-centre trials) to establish clinical safety effectiveness.
New Drug Application: Upon completion of the Phase III clinical studies, the Corporation sponsoring the new drug then assembles all the pre-clinical, clinical and manufacturing data and submits it to the FDA, Health Canada or the EMA as part of a New Drug Application in the United States, a New Drug Submission in Canada or a Market Authorization Application in Europe, respectively. The submission or application is then reviewed by the regulatory body for approval to market the product candidate. This process usually takes six months to two years to complete. However, there is no assurance of approval.
Obtained regulatory approvals, permits and authorizations:
Neptune has obtained the following regulatory approvals, permits and authorizations:
| • | European Food Safety Authority (EFSA) has approved NKO® as food for particular nutritional use (PARNUTS) for commercialization in the European Union. |
| • | European Food Safety Authority (EFSA) has approved NKO® as a Novel Food for commercialization in the European Union. |
| • | NKO® was the subject of a Generally Recognized as Safe (GRAS) notification to the FDA as a food ingredient in the United States to which the FDA did not object. |
| • | NKO® has obtained approval as a Complementary Medicine from the Therapeutic Good Administration (TGA) in Australia. |
| • | NKO® has a natural product number (NPN) issued by Health Canada. |
| • | Health claims in Canada—Multiple claims for health benefits of NKO® approved by NHPD (7 claims). |
38
Competition
General
The nutraceutical and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to our products. It is probable that the number of companies seeking to develop products and therapies similar to our products will increase. Many of these and other existing or potential competitors have substantially greater financial, technical and human resources than we do and may be better equipped to develop, manufacture and market products.
For instance, Aker BioMarine ASA, a Norway-based corporation that is in the business of harvesting and commercializing marine ingredients, launched a krill oil product under the brand name SuperbaTM in 2009. Enzymotec Ltd., an Israel-based biotechnology corporation, is also a krill oil supplier. These companies and others may develop and introduce products and processes competitive with ours.
Acasti’s potential competitors in the United States, Europe and Asia include large, well-established pharmaceutical companies as well as specialty pharmaceutical sales and marketing companies and specialized cardiovascular biopharmaceutical companies. These companies include GlaxoSmithKline plc, which currently markets Lovaza®, a prescription only omega-3 fatty acid indicated for patients with very high triglycerides, and Abbott Laboratories, which currently markets Tricor and Trilipix, prescription drugs indicated for the treatment of very high triglycerides and mixed dyslipidemia. In addition, in July 2012, the FDA approved Vascepa™, a prescription drug developed by Amarin Corporation plc, as an adjunct to diet to reduce triglyceride levels in adult patients with severe (triglycerides greater than or equal to 500mg/dL) hypertriglyceridemia (very high triglycerides). The active ingredient in Vascepa™ is an ester form of EPA.
Also, we are aware of other pharmaceutical companies that are developing products that, if approved, would compete with CaPre®. These include a free fatty acid form of omega-3 (comprised of 55% EPA and 20% DHA) being developed by AstraZeneca PLC, which announced on May 6, 2014 that the FDA had approved its product as an adjunct to diet to reduce triglyceride levels in adults with severe hypertriglyceridaemia, and an omega-3 based drug candidate for hypertriglyceridemia being developed by Trygg Pharma, a joint venture 50% owned by the Aker BioMarine Group. We also believe that certain other pharmaceutical companies are developing potential treatments for inflammatory and metabolic diseases based on omega-3 fatty acids. See “Risk Factors—Risks Related to the Corporation’s Business—The Corporation’s industry is subject to rapid technological change and competition.”
Employees
As at the date of this AIF, Neptune, along with Acasti and NeuroBio, has approximately 114 employees (111 full-time and three part-time) working at its business offices in Laval and at its production facility in Sherbrooke. We believe that Neptune employees possess specialized skills and knowledge in the following fields, which are valuable assets of the Corporation: (i) marine biomasses, (ii) marine oil extraction processes, (iii) scientific issues, (iv) commercialization and business development, (v) intellectual property protection, (vi) legal matters, (vii) clinical validation of biological therapeutic properties, (viii) quality assurance/quality control, (ix) regulatory compliance related to the Corporation’s operations, and (x) industrialization. Neptune is not a party to any collective bargaining agreement. Neptune considers its relations with its employees to be good and its operations have never been interrupted as the result of a labor dispute.
Economic Dependence/Litigation
Economic Dependence
Neptune has recovered certain inventory of krill and has made purchases of commodity krill oil on the market. In the normal course, when its production plant is operational, Neptune sources the krill used in the manufacturing of its products generally from three suppliers. Neptune considers its relationship with its suppliers to be good and believes it is not dependent upon any of these suppliers since alternative sources of krill supply are readily available. See “Business of the Corporation—Supply of Krill”.
39
Litigation
University of Sherbrooke
On December 22, 2011, the Corporation received a motion filed by the University of Sherbrooke, the then worldwide registered owner of patents relating to the extraction process (the “Patents”) licensed to the Corporation, asking the Court to order the transfer and force the Corporation to take ownership of the Patents. The motion was filed in connection with the Court appeal matter that was settled in 2010 between the parties. On June 26, 2013, the University and Neptune reached an agreement whereininter alia,the parties agreed to the dismissal of the proceedings between and confirmed the assignment of the patents at issue to Neptune.
US Nutraceuticals LLC
On or around January 27, 2010, the Corporation and Acasti filed a Motion for the Issuance of a Permanent Injunction before the Quebec Superior Court against US Nutraceuticals LLC (d.b.a. Valensa), a US based Corporation. Neptune and Acasti are seeking inter alia an injunction ordering Valensa to amend some patent applications filed by Valensa to add Neptune as co-owner, or in the alternative to have Valensa assign these patent applications to Neptune, as well as punitive damages, loss of profit and loss of business opportunity for an amount currently established at $3,000,000. On or around February 3, 2014, Neptune and Valensa filed dismissals with the Court and the case was closed. There are no pending litigation in Quebec or anywhere else in the world between the Corporation and Valensa.
Aker Biomarine ASA and others
On November 13, 2009, Neptune filed a patent infringement lawsuit against Aker BioMarine ASA, Jedwards International, Inc and Virgin Antartic LLC, asserting its U.S. patent relating to a method of extraction of total lipids fractions from Krill. Neptune alleges that the Defendants have used solvents for the extraction of their krill oil, which are covered by the patent (US6,800,299) licensed to Neptune. The Complaint (case 1:09-cv-11946-MLW) filed in 2009 by Neptune et al. against Aker et al. in the District of Massachusetts for the infringement of the Université of Sherbrooke’s US patent licensed to Neptune (US. Pat. 6,800,299) was also dismissed on or around February 11, 2014, as per the terms of the Settlement agreement reached between Aker and the Corporation on November 28, 2013.
On October 4, 2011, the Corporation filed a Complaint in the US District Court for the District of Delaware against Aker Biomarine ASA, Aker Biomarine Antarctic USA Inc., and Schiff Nutrition International Inc. (Aker et al.) for the infringement of the Corporation’s US patent 8,030,348 and for damages. On December 19, 2011, Aker et al. filed Counterclaims denying any infringement, seeking the invalidity of the Corporation’s patent, and seeking an award for costs and damages. This Complaint against Aker et al. will be dismissed in accordance with the Settlement agreement reached between Aker and the Corporation on November 28, 2013.
On October 2, 2012, the Corporation filed a Complaint in the US District Court for the District of Delaware against Aker Biomarine ASA, Aker Biomarine Antartic USA Inc., Aker Biomarine Antartic AS, Schiff Nutrition Group Inc., and Schiff Nutrition International Inc. (Aker et al.) for the infringement of the Corporation’s US patent 8,278,351 and for damages. This Complaint against Aker et al. was dismissed on or around April 10, 2104 in accordance with the Settlement agreement reached between Aker and the Corporation on November 28, 2013.
On March 6, 2013, the Corporation filed a Complaint in the US District Court for the District of Delaware against Aker Biomarine ASA, Aker Biomarine Antartic USA Inc., Aker Biomarine Antartic AS, Schiff Nutrition Group Inc., and Schiff Nutrition International Inc. (Aker et al.) for the infringement of the Corporation’s US patent 8,383,675 and for damages.This Complaint against Aker et al. was dismissed on or around April 10, 2104 in accordance with the Settlement agreement reached between Aker and the Corporation on November 28, 2013.
On December 17, 2013, Neptune and Acasti announced a settlement and license agreement with Aker which resulted in the dismissal of all Aker respondents from the then on-going claims brought by Neptune against Aker at the ITC, as well as the dismissal of all current lawsuits brought by Neptune against Aker and certain other associated companies. As part of the settlement, Neptune granted aworld-wide,non-exclusive,royalty-bearing license to Aker and each of their respective affiliates, allowing such licensees to market and sell its nutraceutical products. Under the terms of the settlement agreement, royalty levels are dependent on the outcome of the inter partes review proceedings requested by Aker before the USPTO regarding Neptune’s ‘351 composition of matter patent (No. 8,278,351). Aker also agreed to pay Neptune an additional non-refundable payment for the manufacture and sale of krill products prior to the effective USPTO decision date. The USPTO’s decision whether to hold a ‘351 inter partes review has not been made.
40
On December 20, 2012, the Corporation filed a claim for the revocation of Aker Biomarine ASA’s standard patent (2008231570) and four innovation patents before the Australian Federal Court. The Corporation is seeking a declaration that all the claims in Aker’s patents, are, and at all materials times have been, invalid. The Aker patents claim a krill oil composition and methods of extraction that lack novelty and are, in the Corporation’s opinion, not patentable inventions since the Corporation marketed its NKO® krill oil product many years before Aker filed its patents in Australia. A Notice of Discontinuance was filed by the parties on or around December 17, 2013. The case was dismissed in accordance with the Settlement agreement reached between Aker and the Corporation.
Enzymotec Limited and others
On October 4, 2011, the Corporation filed a Complaint in the US District Court for the District of Delaware against Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC, and Azantis Inc. for the infringement of the Corporation’s US patent 8,030,348 and for damages. In addition, on October 2, 2012, the Corporation filed a Complaint in the US District Court for the District of Delaware against Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC for the infringement of the Corporation’s US patent 8,278,351 and for damages. On January 14, 2013, Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC filed a Counterclaim denying any infringement, seeking the invalidity of the Corporation’s patent, and seeking an award for costs and damages. On March 6, 2013, the Corporation filed a Complaint in the US District Court for the District of Delaware against Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC for the infringement of the Corporation’s US patent 8,383,675 and for damages.
On April 27, 2014, Acasti and Neptune announced that a final and binding patent infringement settlement and license agreement has been signed with Enzymotec that resolves the ITC’s investigation of infringement of Neptune’s composition of matter patents, related federal court actions initiated by Neptune against Enzymotec and its distributors and various patent review proceedings requested by Enzymotec.
As part of the settlement, Neptune granted a world-wide, non-exclusive, royalty-bearing license to Enzymotec, allowing it to market and sell its nutraceutical products under Neptune’s ‘348 family of patents (US Patent No. 8,030,348 and all the continuations). Under the terms of the settlement, royalty levels in the United States are dependent on the outcome of pending inter partes review proceedings before the USPTO regarding certain claims of Neptune’s ‘351 composition of matter patent (US Patent No. 8,278,351). Furthermore, royalty levels in Australia are dependent on a potential request by Enzymotec to the APO for a post-grant review of certain claims of Neptune’s allowed composition of matter patent application (AU2002322233). Enzymotec also agreed to pay Neptune a non-refundable one-time upfront settlement payment.
All the Complaints against Enzymotec Limited, Enzymotec USA Inc., Mercola.com Health Resources, LLC, and Azantis Inc. will be dismissed in accordance with the Settlement agreements reached on April 27, 2014 between Enzymotec and the Corporation.
Rimfrost USA and others
On March 6, 2013, the Corporation filed a Complaint in the US District Court for the District of Delaware against Rimforst for the infringement of the Corporation’s US patents 8,030,348, 8,287,351 and 8,383,675, and for damages.
On September 26, 2013, Neptune and Acasti reached a settlement with Rimfrost resolving the then on-going claims brought by Neptune against Rimfrost at the ITC and resulting in the dismissal of all current lawsuits brought by Neptune against Rimfrost. As part of the settlement, the Corporation granted a world-wide, non-exclusive, royalty-bearing license to Rimfrost, allowing it to market and sell within the nutraceutical market krill products and/or products containing components extracted from krill. Rimfrost also agreed to pay Neptune an additional settlement amount for the manufacture and sale of krill products prior to the effective license commencement date, which will be applied against the price for krill oil purchased by Neptune from Rimfrost under the manufacturing and supply agreement. See “General Development of the Business—Fiscal Year Ended February 28, 2014.”
41
European Patent #1,417,211
On March 9, 2010, Neptune filed an appeal with the EPO’s Board of Appeal contesting a 2009 decision of the EPO regarding the European composition of phospholipids and use patent #1417211. On April 9, 2013, the European Opposition Board dismissed Neptune’s appeal and the European patent EP1,417,211 was revoked.
ITC Complaint
On January 29, 2013, the Corporation filed a Complaint under Section 337 of the US Tariff Act of 1930 with the ITC alleging that Aker, Enzymotec Limited, Enzymotec USA, Inc., Rimfrost are engaging in unfair trade practices by, at least, the importation, sale for importation, and sale after importation of certain krill-based products, namely krill paste and krill oils, that directly or indirectly infringe one or more claims of Neptune’s U.S. Patents No. 8,278,351 and 8,383,675. The investigation was officially instituted on April 11, 2013. On May 13, 2014, the Administrative Law Judge granted the parties’ motion and issued the Initial Determination to terminate the investigation against the last remaining Respondents Enzymotec Ltd. and Enzymotec USA, Inc. The ITC investigation is therefore over.
Class Action Suit
On December 20, 2012, the Corporation was informed that it and certain of its officers had been named as defendants in a purported class action lawsuit filed by a US law firm on December 19, 2012 in the United States District Court for the Southern District of New York. The complaint charged the Corporation and certain of its officers with alleged violations of the Securities Exchange Act of 1934. The complaint was served on the Corporation and the Defendants on February 15, 2013 and filed on behalf of all persons and entities who purchased the common stock of Neptune during a specified period. In May 2013, the case was voluntarily dismissed by the plaintiffs without prejudice.
Sub-contractor suit
On April 2, 2013, the Corporation received a motion filed by G.S.C. Communication Inc. against the Corporation and Enterprises Laliberté Division Électricité Inc. The motion was filed as a result of the November 8, 2012 incident and the plaintiff is seeking monetary relief, for an amount of approximately $38,000, for the costs of the plaintiff’s tools destroyed during the fire. The case is currently pending and is currently handled by the Corporation’s insurers. No trial dates have been set.
Henri Harland
On May 29, 2014, Henri Harland, the former President and Chief Executive Officer of the Corporation filed a lawsuit against the Corporation, Neptune and NeuroBioPharm in connection with his departure as President and Chief Executive Officer of each of Neptune, Acasti and NeuroBioPharm. Among other things, Mr. Harland alleged that his resignation occurred as a result of a constructive dismissal and is seeking approximately $8.5 million in damages, interest and costs. In addition, Mr. Harland is seeking from Neptune, Acasti and NeuroBioPharm, as applicable, the issuance of 500,000 shares of each of Neptune, Acasti and NeuroBioPharm as well as two blocks of 1,000,000 call options each on the shares held by Neptune in Acasti and NeuroBioPharm. As a result of the lawsuit, Mr. Harland was requested to resign as Director of the Corporation. The following day, Neptune and its subsidiaries jointly announced that they believed the claim as formulated was without merit or cause, they will vigorously defend the lawsuit and will take any steps necessary to protect their interests.
RISK FACTORS
Investing in securities of the Corporation involves a high degree of risk. Prospective investors should carefully consider the following risks, as well as the other information contained in this AIF and the other information in our publicly filed documents before investing in securities of the Corporation. If any of the following risks actually occurs, the Corporation’s business, financial condition, liquidity, results of operation and prospects could be materially harmed. Additional risks and uncertainties, including those of which the Corporation is currently unaware or that it deems immaterial, may also adversely affect the Corporation’s business, financial condition, liquidity, results of operation and prospects.
Risks Related to the Corporation’s Business
Neptune’s future prospects are heavily dependent on the timely and successful reconstruction of its production plant.
On November 8, 2012, an explosion and fire destroyed Neptune’s krill oil production plant located in Sherbrooke, Québec, Canada. Currently, Neptune anticipates that the reconstruction of its new production facility will be completed by approximately late March 2014. Since the destruction of its plant and pending the commencement of production and successful ramp-up of the new production facility, Neptune has not commercialized its leading products, NKO® and EKO™, and has met customer demand on an interim basis by sourcing and supplying commodity grade krill oil at significantly lower profit margins than the profit margins for NKO® and EKO™. As such, the start-up of Neptune’s new production facility and the resumption of the production of NKO®, in particular, is a key component to the success of Neptune’s future operations and financial performance. There are, however, a number of risks that may delay, prevent and/or increase the cost of the completion and start-up of the new production facility and the resumption of production:
| • | Neptune may encounter delays, unforeseen challenges or cost overruns in obtaining the last remaining pieces of needed equipment or the installation of equipment; |
42
| • | Neptune requires permits from applicable worker safety and environmental authorities in order to commence operations at the new production facility and these permits may be withheld, or delayed, or only provided on terms that are not favorable to Neptune; and |
| • | upon start-up and during the ramp-up period, the new plant may not operate as anticipated and Neptune may not be able to produce its krill oil products at its target specifications or volume levels. |
Additionally, following the reconstruction of the new production facility, the Corporation will again rely on a single manufacturing, processing and packaging facility for the production of the Corporation’s krill oil products. Accordingly, Neptune will be highly dependent on the uninterrupted and efficient operation of the new production facility, and any disruption to the operations of the new production facility, as a result of equipment failures, natural disasters, fires, accidents, work stoppages, power outages or other reasons, would materially adversely affect the business, financial condition or results of operations of the Corporation.
The Corporation requires certain permits to resume its production and the receipt of such permits could be delayed, denied or subject to conditions that could result in additional costs and delays.
Neptune is required to obtain the following two operating permits before production can resume at its new production facility:
| • | a certificate of authorization required under theEnvironment Quality Act (Québec) from the Ministry of Environment relating to environmental matters relating to the new production facility’s operations; and |
| • | alevée d’interdiction de démarrer, or permit to lift the prohibition to begin operations, from the CSST relating to safety in the workplace requirements. |
Neptune is currently working closely with the Ministry of Environment and the CSST to obtain the operating permits. While Neptune currently expects the required permits to be issued in time for its target resumption of production by approximately early June 2014, it is possible that the issuance of these permits could be delayed, denied or subject to additional conditions that require Neptune to make modifications to its new production facility or otherwise put procedures in place that result in a delay and increased cost of the resumption of its operations. Any further delay in the operation of the production facility would likely have a material adverse effect on Neptune’s business, financial condition and results of operations.
After completion of the construction of its production plant, Neptune may not be able to maintain its operations and research and development without additional funding.
Neptune estimates that the cost of rebuilding its production plant will total approximately $48.3 million. The construction of the Corporation’s new production facility has been financed through a combination of insurance proceeds, loans from IQ and Canada Economic Development, royalty payments from settlement agreements and working capital.
The sale by the Corporation of commodity grade krill oil during the reconstruction period generated significantly lower profit margins than the sale of NKO® and EKO™ and Neptune is currently incurring an operating loss. As of February 28, 2014, Neptune had approximately $6.5 million of cash and a monthly negative cash flow from operations. Following the ramp-up phase of production at the new production facility, Neptune will require substantial additional funds for further research and development, clinical testing, regulatory approval and commercialization of its products and product candidates. The Corporation may seek additional funding for these purposes through public or private equity or debt financing, joint venture arrangements, and collaborative arrangements with other pharmaceutical companies, and/or from other sources. There can be no assurance that additional funding will be available on acceptable terms or at all to enable Neptune to continue and complete the
43
research and development of the Corporation’s product candidates and their successful commercialization. Should the Corporation fail to obtain the necessary capital, it may be unable to resume production or may be required to delay, reduce or eliminate one or more of its various research and development programs or seek financial support from one of its strategic partners or from third-parties who may require that the Corporation waive significant rights regarding protection of its proprietary technologies or offer it financial support on less favourable terms than those normally acceptable to the Corporation. The failure to obtain additional financing on favourable terms, or at all, could have a material adverse effect on Neptune’s business, financial condition and results of operations.
The Corporation may not recover all of the insurance proceeds it has claimed.
Neptune maintains insurance coverage to help protect it against, among other things, property damage, business interruption and general liabilities. After the November 2012 plant explosion, Neptune submitted recovery claims to its insurers and currently anticipates a maximum compensation of $19 million, of which the Corporation has received $17.5 million in recovered proceeds to date. There can be no assurance that the remaining additional insurance proceeds will be received. Additionally, premiums payable for insurance coverage of the new production facility may be significantly higher than coverage of the facility prior to the November 2012 incident.
The Corporation may be subject to other claims against it relating to the plant explosion.
The Corporation is currently subject to the statement of offense that it received on November 5, 2013 from the CSST alleging violations of workplace safety regulations and seeking a fine of approximately $64,500 and the November 16, 2012 notice from the Ministry of Environment alleging non-compliance by Neptune with environmental regulations and permits relating to its equipment specifications and plant production capacity, each relating to the November 2012 production facility explosion. In addition, further to the publication of the CSST report on May 8, 2014, Neptune may be subject to additional administrative proceedings or criminal, civil or other legal actions. Further, the CSST report, may result in negative publicity and media coverage for the Corporation in connection with the incident. Negative publicity about Neptune may have an adverse impact on the Corporation’s reputation with its customers and the morale of its employees.
In addition to any proceedings that could result from the CSST report, Neptune could also become subject to other civil, penal, criminal or administrative proceedings related to the incident, and if any damages or other remedial measures are imposed against Neptune pursuant to such proceedings, they could be significant and have a material adverse effect on the business, results and financial condition of the Corporation. Addressing any negative publicity and any resulting litigation may distract management, increase costs and divert resources, which could also have a material adverse effect on our business, financial condition or results of operations.
The Corporation has a history of net losses and the Corporation may never achieve profitability.
The Corporation has been reporting losses since the Corporation’s inception and, as at February 28, 2014, the Corporation has an accumulated deficit of $62,097,779. It is expected that the Corporation will continue to generate losses until income from product sales generate sufficient revenues to fund Neptune’s and its subsidiaries’ continuing operations, including research and product development, which the Corporation cannot assure you will occur in the near term or at all.
The Corporation relies on third parties for the supply of raw materials and the distribution and commercialization of its products and such reliance may adversely affect the Corporation if the third parties are unable or unwilling to fulfill their obligations.
Part of the Corporation’s strategy is to enter into and maintain arrangements with third parties related to the development, clinical testing, marketing, distribution and commercialization of its products. The Corporation’s revenues are dependent on the successful efforts of these third parties, including the efforts of the Corporation’s distribution partners. Entering into strategic relationships can be a complex process and the interests of the Corporation’s distribution partners may not be or remain aligned with the Corporation’s interests. Some of the Corporation’s current and future distribution partners may decide to compete with the Corporation, refuse or be unable to fulfill or honour their contractual obligations to the Corporation, or change their plans to reduce their commitment to, or even abandon, their relationships with the Corporation. There can be no assurance that the Corporation’s distribution partners will market the Corporation’s products successfully or that any such third-party
44
collaboration will be on favourable terms. The Corporation may not be able to control the amount and timing of resources the Corporation’s distribution partners devote to the Corporation’s products. In addition, the Corporation may incur liabilities relating to the distribution and commercialization by its distributors of its krill oil products. While the agreements with such distributors generally include customary indemnification provisions indemnifying the Corporation for liabilities relating to the encapsulation or packaging of its krill oil products, there can be no assurance that these indemnification rights will be sufficient in amount, scope or duration to fully offset the potential liabilities associated with the Corporation’s distributors handling and use of our products. Any such liabilities, individually or in the aggregate, could have a material adverse effect on our business, financial condition or results of operations.
The Corporation may not be able to fully restore and grow its customer base.
Since the destruction of its production facility in November 2012, Neptune has not produced and commercialized its lead products, NKO® and EKO™. During that time, the Corporation has sought to preserve its customer base by sourcing and supplying commodity grade krill oil. Also during that time, competition in Neptune’s industry has continued to intensify. As a result, Neptune has experienced the loss of a portion of its pre-incident customer base. Even if Neptune recommences the production of its higher margin NKO® and EKO™, not all of its current and former customers may restore their demand for those products and Neptune might not be able to find new customers for NKO® and EKO™.
Neptune may be unable to restore its customer base to levels prior to the loss of its production facility or thereafter to increase its customer base to expected levels prior to the incident. Prior to the destruction of its production facility, Neptune was producing approximately 150,000 kilograms of krill oil annually, which was the Corporation’s maximum annual production capacity. While the new production facility, once complete, is expected to accommodate the production of approximately 150,000 kilograms of krill oil products annually, Neptune’s ability to supply krill oil products to its customer base in excess of this expected amount will require the expansion of Neptune’s production capacity and/or the entering into of third-party manufacturing or supply arrangements. There is no assurance that Neptune will be able to obtain accomplish either. The inability to restore and grow its customer base could have a material adverse effect on Neptune’s business and results of operations.
The Corporation’s success depends largely on the commercialization of krill oil products, including NKO® and EKO™.
The Corporation’s ability to generate revenues from production of NKO® and EKO™ is expected to be primarily based on the commercialization success of NKO® and EKO™. The overall commercialization success of Neptune’s krill oil products, including NKO® and EKO™, depends on several factors, including:
| • | ability to commence production of Neptune krill oil products at the new production facility; |
| • | continued market acceptance of Neptune’s krill oil products by the nutraceutical market and medical community; |
| • | the amount of resources devoted by the Corporation’s distribution partners to continue the commercialization efforts of Neptune’s krill oil products in our core geographic markets; |
| • | maintaining supply agreements to ensure the availability of krill in order to produce sufficient krill oil to meet the order demands of the Corporation’s distribution partners for Neptune’s krill oil products; |
| • | receipt of regulatory approvals for Neptune’s krill oil products from regulatory agencies in certain territories in which the Corporation wishes to expand its commercialization efforts; |
| • | the number of competitors in the Corporation’s market; and |
| • | protecting and enforcing the Corporation’s intellectual property and avoiding patent infringement claims. |
45
Although the Corporation is developing other products that contain krill, all of them are at earlier stages of development and none of them may reach the clinical trial phase, obtain regulatory approval or, even if approved, be successfully commercialized.
The Corporation derives its revenue from a limited number of distributors and has a significant concentration of its accounts receivable.
As at February 28, 2014, the Corporation realized sales from the neutraceutical segment totaling $10,125,523 from three distributors, representing 24.7%, 17.5% and 11.6% of the Corporation’s consolidated sales, respectively. As at February 28, 2014, five distributors represented 68% of total trade accounts receivable of the Corporation, with the largest amount to one distributer representing 44% of total trade accounts receivable. The percentage aging of trade receivable balances as of February 28, 2014 is 16% current, 7% past due 0 – 30 days, 10% past due 31-120 days, 0% past due 121-180 days, and 67% past due more than 180 days. During the year ended February 28, 2014, the Corporation recorded a bad debt expense of $2,193,000 related to one significant customer, for which total trade receivable due at February 28, 2014 is $4,365,000. Adverse changes in a customer’s financial position could cause the Corporation to assume more credit risk relating to that customer’s future purchases or result in uncollectable accounts receivable from that customer. Agreements with these or other significant distribution partners may be terminated or altered by them unilaterally in certain circumstances. Any adverse change in the relationship with the Corporation’s principal distributors, including non-payment of amounts owing from a distributer, could have a material adverse effect on the Corporation’s business, consolidated results of operations, financial condition and cash flows.
The Corporation may be unable to manage its growth efficiently.
The Corporation’s future financial performance and its ability to commercialize its products and to compete effectively will depend, in part, on its ability to manage any future growth effectively. To that end, the Corporation must be able to continue and increase its production capabilities, hire, train and integrate additional management, and potentially administer internal sales and marketing personnel on an effective and efficient basis. Although completion of the reconstruction of Neptune’s production facility has been completed, there can be no guarantee that the Corporation will be able to meet the product order demands of its distributors.
The Corporation may not be able to accomplish any of the above actions, and its failure to do so could prevent it from successfully growing. Any increase in resources devoted to manufacturing, research, product development and sales, marketing and distribution efforts without a corresponding increase in the Corporation’s operational, financial and management information systems could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation suffered significant impairment losses and its assets may be subject to future write-downs, charge-offs or impairment losses.
The Corporation suffered impairment losses and costs related to the plant explosion in November 2012 amounting to approximately $10.1 million, which was recorded in the third and fourth quarters of fiscal year 2013. During fiscal year 2014, management reevaluated certain of its estimates based on information that became available. An additional impairment loss related to property, plant and equipment of approximately $1.2 million was identified during the reconstruction of the Corporation’s plant. This loss was recorded in the fourth quarter of fiscal 2014. Management will continue to assess the value of its assets on an ongoing basis and may conclude additional impairment losses are required. Any increase in the Corporation’s previously assessed charge-offs, write-downs or impairment losses or any new impairment losses may have a material adverse effect on Neptune’s financial condition and results of operations.
Neptune could lose its control of Acasti.
Neptune currently owns approximately 49.07% of Acasti’s outstanding common shares, five members of Neptune’s Board of Directors are also members of Acasti’s Board of Directors, and Neptune’s Chief Executive Officer is also the Chief Executive Officer of Acasti as of February 28, 2014. As a result, Neptune exercises control over Acasti. However, if all outstanding warrants, call options and restrictive share units of Acasti were to be exercised, Neptune’s ownership interest in Acasti’s common shares would fall to approximately 34.34%. If Neptune’s ownership of Acasti’s common shares declines, Neptune may lose its ability to elect members of its Board of Directors to
46
Acasti’s Board of Directors and to otherwise exercise control over Acasti. A loss of Neptune’s control over Acasti, could, among other things result in:
| • | investors and analysts placing a different, and possibly lower, value on the Common Shares to reflect a lower degree of exposure by Neptune to Acasti’s krill oil-based pharmaceutical business; |
| • | Acasti making decisions in connection with the development and commercialization of Acasti’s products with less or no involvement and approval from Neptune; and |
| • | a different presentation of Neptune’s financial statements as relates to Acasti, including assets and any future revenues generated by Acasti which would not be directly included in Neptune’s consolidated financial statements. |
Neptune does not expect to provide material capital to Acasti in the short term and therefore, its ownership interest in Acasti may continue to decline.
The Corporation may not be able to further penetrate core or new markets.
If the Corporation fails to further penetrate its core markets and existing geographic markets or expand its business into new markets, the growth in sales of the Corporation’s products, along with the Corporation’s operating results, could be negatively impacted. The Corporation’s ability to further penetrate its core markets and existing geographic markets or to expand its business into additional countries in Europe, Asia or elsewhere, to the extent the Corporation believes that it has identified attractive geographic expansion opportunities in the future, is subject to numerous factors, many of which are beyond the Corporation’s control. The Corporation cannot assure that its efforts to increase market penetration in its core markets and existing geographic markets will be successful. The Corporation’s failure to do so could have a material adverse effect on the Corporation’s operating results.
The Corporation must attract and retain skilled labor in order to maintain and increase its business.
The Corporation’s ability to resume its production operations and sustain and expand its business depends in part on its ability to attract and retain skilled manufacturing workers, equipment operators, engineers and other technical personnel. Demand for these workers is currently high and the supply is limited, particularly in the case of skilled and experienced machinists and engineers. Further, the Corporation may be faced with increased training costs and reduced productivity as it trains new employees hired to meet the Corporation’s krill oil production needs. Additionally, a significant increase in the wages paid by competing employers could result in a reduction in the Corporation’s skilled labor force, increases in the rates of wages it must pay or both. If the Corporation’s compensation costs increase or it cannot attract and retain skilled labor, including engineers and machinists, the Corporation’s earnings could be reduced, and production capacity and growth potential could be impaired.
The Corporation may not be able to attract, hire and retain key management and personnel.
We depend substantially on our ability to hire, train, motivate and retain high quality personnel, especially our scientists and management team. Particularly, in light of the limited number of employees that cover our numerous programs and key functions, if we are unable to retain existing personnel or identify or hire additional personnel, we may not be able to research, develop, commercialize or market our products and product candidates as expected or on a timely basis and we may not be able to adequately support current and future alliances with strategic partners.
Furthermore, if we were to lose key management personnel, we would lose a portion of our institutional knowledge and technical know-how, potentially causing a substantial delay in one or more of our development programs until adequate replacement personnel could be hired and trained. Other than our stock option plan, we have not adopted any policies or entered into any agreements specifically designed to motivate officers or other employees to remain with us. We do not have key man life insurance policies on the lives of most of our key personnel.
47
The Corporation’s current and future clinical trials may prove unsuccessful or be delayed by certain factors.
The Corporation is not able to predict the results of pre-clinical and clinical testing of its product candidates. It is not possible to predict, based on studies or testing in laboratory conditions or in animals, whether a product candidate will prove to be safe or effective in humans. Further, preclinical and clinical data may not be sufficient to support approval to commercialize a product. Pre-clinical and clinical data must be developed under strict regulatory standards and may be found, on review by health regulatory authorities, to be of insufficient quality to support an application for commercialization of a product. In addition, success in one stage of testing is not necessarily an indication that the particular product will succeed in later stages of testing and development. Further, clinical trials require the enrollment of patients and the Corporation may experience difficulties identifying and enrolling suitable human subjects for ongoing and future trials of its products. This could be as a result of a number of factors including, but not limited to, design protocol, the size of the available patient population, the eligibility criteria for participation in the clinical trials, and the availability of clinical trial sites.
The Corporation’s ability to commercialize any of its products, including CaPre®, is dependent upon the success of product development efforts and the success of clinical studies. If these clinical trials and product development efforts fail to produce satisfactory results, or if the Corporation is unable to maintain the financial and operational capability to complete these development efforts, it may be unable to generate revenues for this and other product candidates.
A number of companies in the life sciences industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Share prices of biotechnology companies have declined significantly in certain instances where clinical results were not favourable, were perceived negatively or otherwise did not meet expectations. Unfavourable results or negative perceptions regarding the results of pre-clinical or clinical trials for any of the Corporation’s product candidates currently under development could cause the Corporation’s share price to decline significantly.
The Corporation may not achieve its publicly announced milestones on time.
From time to time, the Corporation publicly announces the timing of certain events it expects to occur. These statements are forward-looking and are based on the best estimate of management at the time relating to the occurrence of such events. However, the actual timing of such events may differ from what has been publicly disclosed. The timing of events such as completion of a non-clinical or clinical program, discovery of a new product candidate, filing of an application to obtain regulatory approval, beginning of commercialization of certain products or product candidates, or announcement of additional clinical programs for a product candidate may ultimately vary from what is publicly disclosed. For example, CaPre®, Acasti’s leading drug candidate, is currently being evaluated in a Phase I pharmacokinetic clinical trial and in a Phase II clinical trial. Non-clinical safety studies are also planned. The Corporation cannot assure that the clinical trials or non-clinical safety studies for CaPre® or any other of the Corporation’s or its subsidiaries’ product candidates will be completed, that it will make regulatory submissions or receive regulatory approvals as planned, or that it will be able to adhere to its current schedule for the manufacturing and launch of any of its products. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a supplier or a distribution partner or any other event having the effect of delaying the publicly announced timeline. The Corporation undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, after the distribution of this AIF, except as otherwise required by law. Any variation in the timing of certain events having the effect of postponing such events could have a material adverse effect on the Corporation’s business plan, financial condition or operating results.
The Corporation’s subsidiaries are subject to risks affecting emerging biopharmaceutical companies.
The Corporation’s subsidiaries are subject to risks affecting emerging biopharmaceutical companies. For example, Acasti’s prospects depend entirely on the success of CaPre®, which is still in clinical development, and Acasti may not be able to obtain required regulatory approvals for CaPre® or to generate revenues from CaPre®. Acasti may be unable to develop alternative product candidates and even if Acasti receives regulatory approval for CaPre®, Acasti still may not be able to successfully commercialize it and the revenue that Acasti generates from its sales, if any, may be limited. Termination or suspension of, or delays in the commencement or completion of, any necessary future studies of CaPre® for any indications could occur. Clinical drug development involves a lengthy
48
and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Acasti relies on third parties to conduct its clinical trials for CaPre®, for the manufacturing, production and supply of CaPre® and ONEMIA® and may be adversely affected if those third parties are unable or unwilling to fulfill their obligations. For a complete description of such risks, see the “Risk Factors” section in Acasti’s annual information form dated May 29, 2014, available on SEDAR atwww.sedar.com.
If product liability lawsuits are brought against the Corporation, they could result in costly and time-consuming litigation and significant liabilities.
The development of human therapeutic products involves an inherent risk of product liability claims and associated adverse publicity. The Corporation’s products may be found to be, or to contain substances that are, harmful to the health of its consumers. This sort of finding may expose the Corporation to substantial risk of litigation and liability and/or for the Corporation to discontinue production of certain products.
The Corporation has a product liability insurance, renewable on an annual basis, to cover civil liability claims relating to its products in an amount equal to $5,000,000 per year for all such claims. The Corporation also maintains a quality-assurance process that is QMP (Quality Management Program) certified by the Canadian Food Inspection Agency. However, this coverage may not insure against all claims made.
Product liability insurance is costly, often limited in scope, and could be unavailable or only available on terms unfavourable to the Corporation. There can be no assurance that the Corporation will be able to obtain or maintain insurance on reasonable terms or to otherwise protect itself against potential product liability claims that could impede or prevent commercialization of the Corporation’s future products and product candidates. Furthermore, a product liability claim could tarnish the Corporation’s reputation, whether or not such claims are covered by insurance or are with or without merit. A product liability claim against the Corporation or the withdrawal of a product from the market could have a materially adverse effect on the Corporation’s business or its financial condition.
The Corporation may be adversely affected by environmental and safety regulations or concerns.
The Corporation’s krill oil extraction process involves the use of certain hazardous materials, including acetone. The Corporation is subject to Canadian federal, provincial and municipal laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. In the event of an accident that involves hazardous materials, the Corporation could be held liable for damages, which could exceed the resources of the Corporation. There can be no assurance that the Corporation will not be required to incur significant costs to comply with regulatory requirements in the future, or that the operations, business or assets of the Corporation will not be materially adversely affected by current or future legislative or regulatory requirements. The Corporation currently has no immediate plans for major capital expenditures in respect of environmental protection installations.
Once production has resumed at the new production faciltity, should the Corporation want to increase its production capacity, it will be required to obtain a permit from the Ministry of Environment that will allow it to produce in excess of the 150,000 kilograms which the Corporation expects to be entitled to produce upon the receipt of the relevant permits from the CSST and the Ministry of Environment. The Corporation may not be successful in obtaining such permit on favourable terms or at all, or in a timely manner. Any of the foregoing could have a material adverse effect on our business, operations and financial condition.
The Corporation is dependent on third parties to obtain certain raw materials necessary to develop and produce its products.
The Corporation depends on third parties to obtain certain raw materials necessary to develop and produce its products. If the Corporation is no longer able to obtain raw materials, including krill or commodity krill oil, from one or more of its suppliers on terms reasonable to the Corporation or at all, the Corporation’s revenues could suffer. This could also have a significant impact on the Corporation’s capacity to complete certain of its current research and development projects and, accordingly, would negatively affect its projected commercial and financial growth. In addition, a significant increase in the price of raw materials that cannot be passed on to the Corporation’s
49
distributors could have a material adverse effect on the Corporation’s results of operations and financial condition. While potential alternative suppliers of raw materials may be identified, they must first pass intensive validation tests to ensure their compliance with product specifications. No assurance can be given regarding the successful outcomes of such tests or the Corporation’s ability to secure alternate sources of supply at competitive pricing and upon fair and reasonable contractual terms and conditions.
The Corporation’s industry is subject to rapid technological change and competition.
The Corporation operates in a sector that is subject to rapid and substantial change. There can be no assurance that products developed by others will not render the Corporation’s products, product candidates or technologies non-competitive or that the Corporation will be able to keep pace with technological developments. Competitors may have developed or may be in the process of developing technologies that could be the basis for competitive products. Some of these products may prove more effective and less costly than products developed by the Corporation or its product candidates. Scientific and technological developments and regulatory requirements may, within a relatively short timeframe, render the products and processes developed or planned by the Corporation obsolete.
Competition in the health and nutrition industry and in the pharmaceutical sector is extremely intense. Many companies, as well as research organizations, currently engage in, or have in the past engaged in, efforts related to the development of products similar to the Corporation’s products and product candidates. The Corporation competes with companies that produce similar or identical products or that proposes different approaches to the separation or purification of components of krill.
For instance, Aker BioMarine ASA, a Norway-based corporation that is in the business of harvesting and commercializing marine ingredients, launched a krill oil product under the brand name SuperbaTM in 2009. Enzymotec Ltd., an Israel-based biotechnology corporation, is also a krill oil supplier. These companies and others may develop and introduce products and processes competitive with ours. Acasti’s potential competitors in the United States, Europe and Asia include large, well-established pharmaceutical companies as well as specialty pharmaceutical sales and marketing companies and specialized cardiovascular biopharmaceutical companies. These companies include GlaxoSmithKline plc, which currently markets Lovaza, a prescription omega-3 for patients with severe hypertriglyceridemia, Abbott Laboratories, which currently markets Tricor and Trilipix (both fibrates) and Niaspan (niacin) for treatment of severe hypertriglyceridemia, and Amarin Corporation, which currently markets Vascepa, an ethyl-ester form of EPA, for the treatment of patients with severe hypertriglyceridemia. In March 2011, Pronova BioPharma Norge AS, which owns the patents for Lovaza, entered into an agreement with Apotex Corp. and Apotex Inc. to settle their patent litigation in the United States related to Lovaza. Pursuant to the terms of the settlement agreement, Pronova granted Apotex a license to enter the U.S. market with a generic version of Lovaza in the first quarter of 2015, or earlier, depending on circumstances. As a result, Acasti expects Apotex to compete against it as well. Other companies are also seeking to introduce generic versions of Lovaza. In addition, Acasti is aware of other pharmaceutical companies that are developing products that, if approved, would compete with CaPre®. These include AstraZeneca which announced on May 6, 2014 that the FDA had approved EPANOVA (omega-3-carboxylic acids) as an adjunct to diet to reduce triglyceride levels in adults with severe hypertriglyceridaemia. Acasti believes other emerging biopharmaceutical companies are also developing potential treatments for hypertriglyceridemia based on omega-3 fatty acids, but Acasti is unaware of the development stage of their product candidates. CaPre® may also face competition from omega-3 dietary supplements that are available without a prescription.
These and other competitors may have greater resources than the Corporation. Accordingly, no assurance can be given that products developed by these other companies or their technology will not affect the Corporation’s ability to compete in the nutraceutical market. There is a risk that one or more of the Corporation’s competitors may develop more effective or more affordable products than the Corporation, or may achieve earlier patent protection or product commercialization that the Corporation, or that such competitors will commercialize products that will render the Corporation’s product candidates obsolete, possibly before the Corporation is able to commercialize them.
50
The Corporation is subject to foreign currency fluctuations.
The Corporation is exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Currency risk relates to the portion of the Corporation’s business transactions denominated in currencies other than the Canadian dollar. During the fiscal year ended February 28, 2014, approximately 86% of the Corporation’s revenues were in United States dollars and 12% were in Euros, while the vast majority of its costs were in Canadian dollars. If the values of foreign currencies including the United States dollar and Euro fluctuate significantly more than expected in the foreign exchange markets, the Corporation’s operating results and financial condition may be adversely affected.
The Corporation uses hedging strategies to a limited extent by entering into currency forwards to purchase or sell amounts of foreign currency in the future at predetermined exchange rates. The purpose of these currency forwards is to fix the risk of fluctuations in future exchange rates. Significant fluctuations in the rate of exchange could adversely affect the Corporation’s financial performance. There is a risk of loss arising from an eventual weakening of the United States dollar or Canadian dollar.
The Corporation may be negatively impacted by the value of its intangible assets.
The Corporation is required to review the carrying value of its intangible assets for impairment annually or when events change. Intangible assets include net book value of product rights, trademarks and process know-how covered by certain patented and non-patented information. Management reviews the carrying value based on projected future results. If events such as generic competition or inability to manufacture or obtain supply of product occur that may cause sales of the related products to decline, the Corporation adjusts the projected results accordingly. Any impairment in the carrying value results in a write-down of the intangible asset that is charged to income during the period in which the impairment is determined. Any write-down of intangible assets may have a material adverse effect on the Corporation’s results of operations in the period in which the write-down occurs.
The Corporation may be subject to Product Liability Claims and Recalls of its Products.
Drug development involves the testing of approved and experimental drugs on human subjects. Such studies create a risk of liability for personal injury or death to participants as a result of an unexpected adverse reaction to the tested drug or as a result of negligence or misconduct. Furthermore, the administration of drugs to humans after marketing clearance is obtained can result in product liability claims. Such liability might result from claims made directly by consumers or by regulatory agencies, pharmaceutical companies or others. Although the Corporation carries insurance that it believes is adequate for the types of clinical studies it conducts, there can be no assurance that insurance will be adequate or will continue to be available on terms acceptable to the Corporation. Insurance will generally not protect the Corporation against certain of its own actions such as negligence.
The obligation to pay any product liability claim in excess of whatever insurance the Corporation is able to acquire, or the recall of any of its products, could have a material adverse effect on the business, financial condition and future prospects of the Corporation.
Risks Related to the Corporation’s Intellectual Property
The Corporation’s commercial success depends, in part, on its intellectual property rights.
The Corporation’s success depends in part on its ability to develop products, obtain patents, protect its trade secrets and operate without infringing third-party exclusive rights or without others infringing the Corporation’s exclusive rights or those granted to it under license. The Corporation has filed and is actively pursuing patent applications in Canada, the United States, Europe and elsewhere. The patent position of pharmaceutical firms is generally uncertain and involves complex legal, factual and scientific issues, several of which remain unresolved. The Corporation does not know whether all of its pending patent applications will be granted and whether the Corporation will be able to develop other patentable proprietary technology and/or products. Furthermore, the Corporation cannot be completely certain that its existing or future patents provide a definitive and competitive advantage or afford protection against competitors with similar technology. Furthermore, the Corporation cannot give any assurance that such patents will not be challenged or circumvented by others using alternative technology or whether existing third-party patents will prevent the Corporation from marketing its products. In addition, competitors or potential competitors may independently develop, or have independently developed products as effective as those of the Corporation or invent or have invented other products based on the Corporation’s patented products.
51
If third-party licenses are required, the Corporation may not be able to obtain them, or if obtainable, they may not be available on reasonable terms. Furthermore, the Corporation could develop or obtain alternative technologies related to third-party patents that may inadvertently cover its products. Inability to obtain such licenses or alternative technologies could delay the market launch of certain Neptune products, or even prevent the Corporation from developing, manufacturing or selling certain products. In addition, the Corporation could incur significant costs in defending itself in patent infringement proceedings initiated against it or in bringing infringement proceedings against others.
In some cases, the Corporation cannot determine with any certainty whether it has priority of invention in relation to any new product or new process covered by a patent application or if it was the first to file a patent application for any such new invention. Furthermore, in the event of patent litigation there can be no assurance that the Corporation’s patents would be held valid or enforceable by a court of competent jurisdiction or that a court would rule that the competitor’s products or technologies constitute patent infringement.
Moreover, a significant part of the Corporation’s technological know-how constitutes trade secrets. The Corporation requires that its employees, consultants, advisers and collaborators sign confidentiality agreements. However, these agreements may not provide adequate protection in the event of unauthorized use or disclosure of the Corporation’s trade secrets, know-how or other proprietary information.
Claims that the Corporation’s technology or products infringe on intellectual property rights of others could be costly to defend or settle, could cause reputational injury and would divert the attention of management and key personnel, which in turn could have a material adverse effect on the Corporation’s business, results of operations, financial condition and cash flows.
A failure by the Corporation to protect its intellectual property may have a material adverse effect on its ability to develop and commercialize its products.
The Corporation will be able to protect its intellectual property rights from unauthorized use by third parties only to the extent that its intellectual property rights are covered and protected by valid and enforceable patents or are effectively maintained as trade secrets. The Corporation tries to protect its intellectual property position by, among other things, filing patent applications related to its proprietary technologies, inventions and improvements that are important to the development of its business.
Because the patent position of pharmaceutical companies involves complex legal and factual questions, the issuance, scope, validity, and enforceability of patents cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. If the Corporation’s patents are invalidated or found to be unenforceable, it would lose the ability to exclude others from making, using or selling the inventions claimed. Moreover, an issued patent does not guarantee the Corporation the right to use the patented technology or commercialize a product using that technology. Third parties may have blocking patents that could be used to prevent the Corporation from developing its product candidates, selling its products or commercializing its patented technology. As a result, patents that the Corporation owns may not allow it to exploit the rights conferred by its intellectual property protection.
The Corporation also relies on trade secrets, know-how and technology, which are not protected by patents, to maintain its competitive position. The Corporation tries to protect this information by entering into confidentiality agreements with parties who have access to such confidential information, such as its current and prospective suppliers, distributors, manufacturers, commercial partners, employees and consultants. Any of these parties may breach the agreements and disclose confidential information to the Corporation’s competitors. It is possible that a competitor will make unauthorized use of such information, and that the Corporation’s competitive position could be disadvantaged.
52
Enforcing a claim that a third party infringes on, has illegally obtained or is using an intellectual property right, including a trade secret or know-how, is expensive and time-consuming and the outcome is unpredictable. In addition, enforcing such a claim could divert management’s attention from the Corporation’s business. If any intellectual property right were to be infringed by, disclosed to or independently developed by a competitor, the Corporation’s competitive position could be harmed. Any adverse outcome of such litigation or settlement of such a dispute could subject the Corporation to significant liabilities, could put one or more of its patents at risk of being invalidated or interpreted narrowly, could put one or more of its pending patent applications at risk of not issuing, or could facilitate the entry of generic products. Any such litigation could also divert the Corporation’s research, technical and management personnel from their normal responsibilities.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of the Corporation’s confidential information could be compromised by disclosure during this type of litigation. For example, confidential information may be disclosed, inadvertently or as ordered by the court, in the form of documents or testimony in connection with discovery requests, depositions or trial testimony. This disclosure would provide the Corporation’s competitors with access to its proprietary information and may harm its competitive position.
Risks Related to the Corporation’s Industry
The Corporation is subject to significant government regulations.
The research, development, production and commercialization of the Corporation’s products is generally subject to comprehensive regulations under legislation and regulations enforced by Health Canada and other regulatory bodies in Canada and various regional, national and local regulatory bodies, including the FDA in the United States. See “Business of the Corporation—Regulatory Environment”. These regulations may require the (i) approval of manufacturing facilities, including adhering to GMPs during the production, storage, controlled research and quality testing of products, (ii) review and approval of applications to establish the safety and efficacy of the product for each marketing claim sought, and (iii) the control of marketing activities. The process of obtaining required approvals (such as from the FDA and Health Canada) can be costly, time consuming and without guaranteed certainty of approval. Regulatory authorities may change processes, laws, regulations and policies related to product development or commercialization and business operations and require the Corporation to make changes to the product, its claims or its operations. The Corporation could encounter difficulties or incur excessive costs in obtaining the necessary approvals or permits, which could delay or prevent the commercialization and production of its new products.
In December 2006, the U.S. Congress passed legislation requiring companies that manufacture or distribute dietary supplements to report serious adverse events allegedly associated with their products to the FDA and institute recordkeeping procedures for all alleged adverse events (serious and non-serious). The legislation requires manufacturers and distributors of dietary supplements to report to the FDA any serious adverse event reports received, even if the party making the report provides no medical or other information to the manufacturer or distributor. There is a risk that consumers, the press or government regulators could misinterpret adverse event reports as evidence of causation by the ingredient or product complained of, which could lead to consumer confusion, damage to our reputation, banned or recalled ingredients or products, increased insurance costs, class action litigation and a potential increase in product liability litigation, among other things. Distribution of the Corporation’s products outside Canada and the United States is also subject to comprehensive government regulation. Regulations, specifically requirements in respect of product releases on the market and the time involved in respect of regulatory assessment and the sanctions imposed in the event of infringement vary from country to country. No assurance can be given that the Corporation will obtain the requisite approvals in the relevant countries or that it will not incur significant expense in obtaining regulatory approvals or maintaining them in effect.
Failure to obtain the necessary regulatory approvals, the suspension or revocation of current approvals or any failure to comply with regulatory requirements may have a material adverse effect on the Corporation’s operations, its financial situation and its operating results.
Neptune’s subsidiaries, Acasti and NeuroBio, are developing products and product candidates for the pharmaceutical market. Products intended for therapeutic use for humans are governed by a wide array of regulatory agencies. For most of these products, applicable regulations require testing and government review and approval prior to marketing the product. See “Business of the Corporation—Regulatory Environment”. This procedure can take a number of years and involves the expenditure of substantial resources. Any failure or delay by the
53
Corporation to obtain regulatory approvals or clearances could adversely affect the marketing of any products it developed and its ability to generate product revenue. There can be no assurance that any of the Corporation’s pharmaceutical product candidates will be approved by any regulatory agency on a timely basis, or at all. Regulatory approval in Canada, Europe and the United States does not assure approval by other national regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country.
In the event that a regulatory authority revokes any clearances or approvals granted in respect of the Corporation’s pharmaceutical products, the Corporation’s business and financial condition could be adversely affected. Numerous statutes and regulations govern the manufacture and sale of pharmaceutical products in Canada, the United States and other countries where the Corporation markets or intends to market its products. Such laws and regulations govern, among other things, the approval of manufacturing facilities, testing procedures and controlled research, non-clinical and clinical data required prior to and after marketing approval, compliance with GMP affecting production and storage, the advertising and labelling of products and the reporting of adverse events. Failure to comply with statutes and regulations could result in warning letters, fines and other civil penalties, unanticipated expenditures, withdrawal of regulatory approval, delays in approving or refusing to approve a product, product recall or seizure, interruption of production, operating restrictions, injunctions or criminal sanctions. The Corporation and its manufacturers and suppliers are also subject to numerous federal, state, provincial and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances.
The global regulatory environment continues to evolve with changes to regulations, rules, standards and guidelines and the establishment of new health authorities and/or mergers of divisions within them. The Corporation’s existing or future regulatory clearances or approvals may be negatively affected as a result of such changes or reorganization.
The Corporation is heavily dependent on the export of products to the United States. The FDA is able to block the import entry of any product that “appears” to violate U.S. law, which represents a low evidentiary standard for the FDA. Future changes in U.S. requirements and interpretations of those requirements, coupled with the “appears” to violate the law standard for refusing entry of imported products, increases the possibility that the Corporation’s products may not have full access to the U.S. market and poses additional risks to the Corporation’s business.
The market for the Corporation’s products has not been fully defined.
The Corporation believes that products based on its core technology will have numerous applications and that there is a growing market for the products that it has developed. However, there can be no assurance that these assumptions will prove justified, particularly considering competition from existing or new products and considering the uncertain commercial viability of the Corporation’s products. Therefore, there can be no assurance that any of the Corporation’s products in development or products recently launched will achieve market acceptance.
The degree of market acceptance for the Corporation’s products and those of its customers will depend upon a number of factors, including competitive pricing, the extent to which the products fulfill customer expectations and demands, the receipt of regulatory approvals, the establishment and demonstration in the medical community of the clinical efficacy and safety of the products, the establishment and demonstration of the potential advantages over competing products and, in the case of pharmaceuticals, the establishment and demonstration of the potential advantages over existing and new treatment methods and the reimbursement policies of government and third-party payers, and in the case of the Corporation’s nutraceuticals, the acceptance of the listing of the product and appropriate distribution with large retailers. There can be no assurance that consumers, physicians, patients, payers, the medical community in general, distributors or retailers will accept and utilize any existing or new products that may be developed by the Corporation.
Legislative or regulatory reform of the health care system may adversely affect the Corporation’s business and financial condition.
The Corporation’s revenues from sales of pharmaceutical products will depend in part on reimbursement policies and regulations of government health administration authorities, private health insurers and other organizations. The business and financial condition of pharmaceutical companies will continue to be affected by the efforts of governments and third-party payers to contain or reduce the costs of health care through various means.
54
For example, in certain markets, including Canada, pricing or profitability of prescription pharmaceuticals is subject to government control. In the United States there have been, and the Corporation expects that there will continue to be, a number of federal and state proposals to implement similar government controls. In addition, an increasing emphasis on managed health care in the United States has increased and will continue to increase the pressure on pharmaceutical pricing. In Canada, the United States and elsewhere, sales of prescription pharmaceutical products are dependent, in part, on the availability of reimbursement to the consumer from third-party payers, such as government and private insurance plans. Third-party payers are increasingly challenging the prices charged for medical products and services. To the extent the Corporation succeeds in bringing new products to market, there can be no assurance that these products will be considered cost-effective and reimbursement to consumers will be available or will be sufficient to allow the sale of these products on a competitive basis. The Corporation may not be able to obtain prices for its products under development that will make them commercially viable.
Risks Related to the Corporation’s Securities
The following risk factors apply with respect to the securities of the Corporation.
The price of the Corporation’s shares may fluctuate.
Market prices for securities in general, and that of pharmaceutical and nutraceutical companies in particular, tend to fluctuate. Factors such as the announcement to the public or in various scientific or industry forums of technological innovations, new commercial products, patents, patent infringement claims (whether brought by the Corporation against third parties or claimed against the Corporation), exclusive rights obtained by the Corporation or others, results of pre-clinical and clinical studies by the Corporation or others, a change of regulations, publications, financial results, public concerns over the risks of pharmaceutical products and dietary supplements, future sales of securities by the Corporation or its shareholders and many other factors could have considerable effects on the price of the Corporation’s securities. There can be no assurance that the market price of the Common Shares will not experience significant fluctuations in the future.
The market price of the Corporation’s shares could decline as a result of future issuances or actual or potential sales.
The market price of the common shares could decline as a result of future issuances by the Corporation or sales by its existing holders of common shares, or the perception that these sales could occur. Sales by shareholders might also make it more difficult for Neptune to sell equity securities at a time and price that Neptune deems appropriate, which could reduce its ability to raise capital and have an adverse effect on its business.
The market price of the Corporation’s shares could decline as a result of operating results falling below the expectations of investors or fluctuations in operating results each quarter.
The Corporation’s revenues and expenses may fluctuate significantly and any failure to meet financial expectations may disappoint securities analysts or investors and result in a decline in the price of the Corporation’s common shares. The Corporation’s revenues and expenses have fluctuated in the past and are likely to do so in the future. These fluctuations could cause the Corporation’s share price to decline. Some of the factors that could cause revenues and expenses to fluctuate include the following:
| • | the inability to complete product development in a timely manner that results in a failure or delay in receiving the required regulatory approvals or allowances to commercialize product candidates; |
| • | the timing of regulatory submissions and approvals; |
| • | the timing and willingness of any current or future collaborators to invest the resources necessary to commercialize the Corporation’s products; |
| • | the outcome of any litigation; |
| • | changes in foreign currency fluctuations; |
55
| • | the timing of achievement and the receipt of milestone payments from current or future third parties; |
| • | failure to enter into new or the expiration or termination of current agreements with third parties; and |
| • | failure to introduce the Corporation’s products to the market in a manner that generates anticipated revenues. |
If the Corporation’s quarterly operating results fall below the expectations of investors or securities analysts, the price of the Corporation’s common shares could decline substantially. Furthermore, any quarterly fluctuations in the Corporation’s operating results may, in turn, cause the price of its stock to fluctuate substantially.
The Corporation does not currently intend to pay any cash dividends on its common shares in the foreseeable future.
The Corporation has never paid any cash dividends on its common shares. The Corporation does not anticipate paying any cash dividends on its common shares in the foreseeable future because, among other reasons, the Corporation currently intends to retain any future earnings to finance its business. The future payment of cash dividends will be dependent on factors such as cash on hand and achieving profitability, the financial requirements to fund growth, the Corporation’s general financial condition and other factors the board of directors of the Corporation may consider appropriate in the circumstances. Until the Corporation pays cash dividends, which it may never do, its shareholders will not be able to receive a return on their common shares unless they sell them.
There can be no assurance that an active market for the Corporation’s securities will be sustained.
There can be no assurance that an active market for Neptune’s securities will be sustained. Holders of securities of the Corporation may be unable to sell their investments on satisfactory terms. As a result of any risk factor discussed herein, the market price of the securities of the Corporation at any given point in time may not accurately reflect the long-term value of the Corporation. Furthermore, responding to these risk factors could result in substantial costs and divert management’s attention and resources. Substantial and potentially permanent declines in the value of the securities may result and adversely affect the liquidity of the market for the securities of the Corporation.
Other factors unrelated to the performance of the Corporation that may have an effect on the price and liquidity of its securities include: extent of analytical coverage; lessening in trading volume and general market interest in the securities; the size of the Corporation’s public float; and any event resulting in a delisting of securities.
The Corporation’s shareholder rights plan and certain Canadian laws could delay or deter a change of control.
The Corporation’s shareholder rights plan entitles a rights holder, other than a person or group holding 20% or more of our common shares, to subscribe for our common shares at a discount of 50% to the market price at that time, subject to certain exceptions. See “Description of the Share Capital—Shareholder Rights Plan”.
TheInvestment Canada Act(Canada) subjects an acquisition of control of a Corporation by a non-Canadian to government review if the value of the assets as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be a net benefit to Canada.
Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for our shareholders to sell their shares.
The Corporation may pursue opportunities or transactions that may adversely affect its business and financial condition.
Management of Neptune, in the ordinary course of Neptune’s business, regularly explores potential strategic opportunities and transactions. These opportunities and transactions may include strategic joint venture relationships, significant debt or equity investments in Neptune by third parties, the acquisition or disposition of material assets, the licensing, acquisition or disposition of material intellectual property, the development of new product lines or new applications for its existing products, significant distribution arrangements, the sale of all of the
56
shares of Neptune and other similar opportunities and transactions. The public announcement of any of these or similar strategic opportunities or transactions might have a significant effect on the price of the securities of the Corporation. Neptune’s policy is to not publicly disclose the pursuit of a potential strategic opportunity or transaction unless it is required to do so by applicable law, including applicable securities laws relating to continuous disclosure obligations. There can be no assurance that investors who buy or sell securities of Neptune are doing so at a time when Neptune is not pursuing a particular strategic opportunity or transaction that, when announced, would have a significant effect on the price of the securities of the Corporation.
In addition, any such future corporate development may be accompanied by certain risks, including exposure to unknown liabilities of the strategic opportunities and transactions, higher than anticipated transaction costs and expenses, the difficulty and expense of integrating operations and personnel of any acquired companies, disruption of the Corporation’s ongoing business, diversion of management’s time and attention, and possible dilution to shareholders. The Corporation may not be able to successfully overcome these risks and other problems associated with any future acquisitions and this may adversely affect the Corporation’s business and financial condition.
Risks Related to the Corporation’s Status as a Foreign Private Issuer
As a foreign private issuer, the Corporation is subject to different U.S. Securities laws and regulations than a domestic U.S. issuer, which may limit the information publicly available to the Corporation’s U.S. shareholders.
The Corporation is a foreign private issuer under applicable U.S. federal securities laws, and therefore, it is not required to comply with all the periodic disclosure and current reporting requirements of the U.S. Exchange Act. As a result, the Corporation does not file the same reports that a U.S. domestic issuer would file with the SEC, although the Corporation is required to file with or furnish to the SEC the continuous disclosure documents that it is required to file in Canada under Canadian securities laws. In addition, the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the U.S. Exchange Act. Therefore, the Corporation’s shareholders may not know on as timely a basis when the Corporation’s officers, directors and principal shareholders purchase or sell common shares as the reporting periods under the corresponding Canadian insider reporting requirements are longer. In addition, as a foreign private issuer, the Corporation is exempt from the proxy rules under the U.S. Exchange Act.
The Corporation may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses to the Corporation.
In order to maintain its current status as a foreign private issuer, a majority of the Corporation’s common shares must be either directly or indirectly owned by non-residents of the United States unless the Corporation also satisfies one of the additional requirements necessary to preserve this status. The Corporation may in the future lose its foreign private issuer status if a majority of the Corporation’s common shares are held in the United States and it fails to meet the additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to the Corporation under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs it incurs as a Canadian foreign private issuer eligible to use MJDS. If the Corporation is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Corporation may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
U.S. investors may be unable to enforce certain judgments.
Neptune is a Corporation existing under theBusiness Corporations Act (Québec). A number of the Corporation’s directors and officers are residents of Canada or other jurisdictions outside of the United States, and substantially all of the Corporation’s assets are located outside the United States. As a result, it may be difficult to effect service within the United States upon the Corporation or upon its directors and officers. Execution by United States courts of any judgment obtained against the Corporation or any of the Corporation’s directors or officers in United States courts may be limited to the assets of such companies or such persons, as the case may be, located in the United States. It may also be difficult for holders of securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon civil liability and the civil liability of
57
the Corporation’s directors and executive officers under the United States federal securities laws. The Corporation has been advised that a judgment of a U.S. court predicated solely upon civil liability under U.S. federal securities laws or the securities or “blue sky” laws of any state within the United States, would likely be enforceable in Canada if the United States court in which the judgment was obtained has a basis for jurisdiction in the matter that would be recognized by a Canadian court for the same purposes. However, there may be doubt as to the enforceability in Canada against these non-U.S. entities or their controlling persons, directors and officers who are not residents of the United States, in original actions or in actions for enforcement of judgments of courts of the United States, of liabilities predicated solely upon U.S. federal or state securities laws.
DIVIDENDS
The Corporation does not anticipate paying any dividend on its common shares in the foreseeable future. We presently intend to retain future earnings to finance the expansion and growth of our business. Any future determination to pay dividends will be at the discretion of our Board of Directors and will depend on our financial condition, results of operations, capital requirements and other factors the Board of Directors deems relevant. In addition, the terms of any future debt or credit facility may preclude the Corporation from paying dividends.
On September 5, 2012, a prospectus qualifying the distribution of 2,000,000 Class A subordinate voting shares and 4,000,000 Series 2011-1 warrants of NeuroBio held by Neptune by way of a dividend-in-kind was filed with Canadian securities regulatory authorities. Payment of the dividend occurred on October 31, 2012. See “General Development of the Business—Fiscal Year Ended February 28, 2013”.
DESCRIPTION OF THE SHARE CAPITAL
The authorized share capital of the Corporation is comprised of an unlimited number of common shares, or Common Shares, and an unlimited number of preferred shares, or Preferred Shares, issuable in one or more series. By way of by-law, in accordance with its articles of incorporation, the Corporation created the “Series A Preferred Shares”, which are non-voting shares.
As at May 21, 2014, there were a total of (i) 74,386,448 Common Shares and no Preferred Shares issued and outstanding, (ii) 750,000 warrants to purchase Common Shares issued and outstanding, and (iii) 7,334,168 options to purchase Common Shares issued outstanding and (iv) 739,918 restricted share units issued and outstanding.
Common Shares
Voting Rights
Each Common Share entitles its holder to receive notice of, and to attend and vote at, all annual or special meetings of the shareholders of the Corporation. Each Common Share entitles its holder to one vote at any meeting of the shareholders, other than meetings at which only the holders of a particular class or series of shares are entitled to vote due to statutory provisions or the specific attributes of this class or series.
Dividends
Subject to the prior rights of the holders of Preferred Shares ranking before the Common Shares as to dividends, the holders of Common Shares are entitled to receive dividends as declared by the board of directors of the Corporation from the Corporation’s funds that are duly available for the payment of dividends.
Winding-up and Dissolution
In the event of the Corporation’s voluntary or involuntary winding-up or dissolution, or any other distribution of the Corporation’s assets among its shareholders for the purposes of winding up its affairs, the holders of Common Shares shall be entitled to receive, after payment by the Corporation to the holders of Preferred Shares ranking prior to Common Shares regarding the distribution of the Corporation’s assets in the case of winding-up or dissolution, share for share, the remainder of the property of the Corporation, with neither preference nor distinction.
58
Preferred Shares
The Preferred Shares carry no voting rights. Preferred Shares may be issued at any time, in one or more series. The Corporation’s board of directors has the power to set the number of Preferred Shares and the consideration per share, as well as to determine the provisions attaching to each series of Preferred Shares (including dividends, redemption rights and conversion rights, where applicable). The shares in each series of Preferred Shares rank prior to the Common Shares of the Corporation with regard to payment of dividends, reimbursement of capital and division of assets in the event of the Corporation’s winding-up or dissolution. The holders of Preferred Shares shall not be entitled to receive notice of, or to attend or vote at the meetings of the shareholders, except: (i) in the event of a separate meeting or vote by class or by series as specified by law, (ii) where entitled to vote by class or series on amendments to the attributes attaching to the class or series, or (iii) where applicable, in the event of the Corporation’s omission to pay the number of periodical dividends, whether consecutive or not, as applicable to any series.
The board of directors of the Corporation has passed a by-law creating the Series A Preferred Shares. Series A Preferred Shares may be issued only as part of an acquisition by the Corporation of other companies or material assets. Series A Preferred Shares are non-voting, and entitle holders thereof to a fixed, preferential and non-cumulative annual dividend of 5% of the amount paid for the said shares.
Shareholder Rights Plan
On May 26, 2010, the Corporation entered into a shareholder rights plan agreement, or “Rights Plan”. The Rights Plan entitles a holder of rights (other than the Acquiring Person, as defined below, or any affiliate or associate of an Acquiring Person or any person acting jointly or in concert with an Acquiring Person or any affiliate or associate of an Acquiring Person) to purchase our Common Shares at a discount of 50% to the market price upon a person becoming an “Acquiring Person”, subject to certain exceptions and the terms and conditions set out in the Rights Plan. An “Acquiring Person” is defined in the Rights Plan as a beneficial owner of 20% or more of our Common Shares. The Rights Plan is subject to shareholders’ approval every three years in order to remain in effect. On May 9, 2013, the board of directors of the Corporation approved to reconfirm the Rights Plan. On June 27, 2013, the shareholders passed a resolution to ratify, confirm and approve the adoption of the Rights Plan and all rights issuable pursuant to the Rights Plan. In order to implement the Rights Plan, Neptune issued one right in respect of each Common Share outstanding as of 5:01 p.m. (Montreal time) on May 26, 2010, the “Effective Date”. One right will also be issued and attached to each subsequently issued Common Share. The rights will separate and trade separately from the Common Shares to which they are attached and will become exercisable after the “Separation Time”. The “Separation Time” is the close of business on the tenth business day following the earliest of:
| (a) | the date of the first public announcement or disclosure made by us or an Acquiring Person that a person has become an Acquiring Person; |
| (b) | the date of the commencement of, or first public announcement of the intent of any person to commence, a take-over bid (other than a Permitted Bid (as defined in the Rights Plan) or a Competing Permitted Bid (as defined in the Rights Plan) by any person for our Common Shares; |
| (c) | the date upon which a Permitted Bid or Competing Permitted Bid ceases to be such; or |
| (d) | such later date as may be determined by the board of directors. |
After the time at which a person becomes an Acquiring Person, and subject to the terms and conditions set out in the Rights Plan, each right would, upon exercise, entitle a rights holder, other than the Acquiring Person and related parties, to purchase Common Shares at a 50% discount to the market price at the time.
Under the Rights Plan, a “Permitted Bid” is a bid made to all holders of the Common Shares and which is open for acceptance for not less than 60 days. If at the end of 60 days at least 50% of the outstanding Common Shares, other than those owned by the offeror and certain related parties, have been tendered, the offeror may take up and pay for the Common Shares but must extend the bid for a further 10 days to allow other shareholders to tender.
A copy of the Rights Plan is available on SEDAR atwww.sedar.com.
59
MARKET FOR SECURITIES
The Corporation’s Common Shares are listed and posted for trading on (i) the Toronto Stock Exchange, or TSX, under the symbol “NTB”, and (ii) The NASDAQ Stock Market, or NASDAQ, under the symbol “NEPT”.
Trading Prices and Volumes for Neptune
The price ranges and trading volume of Corporation’s Common Shares for the most recently completed financial year on the TSX and the NASDAQ was as follows:
Period | TSX (CDN$) | NASDAQ (US$) | ||||||||||||||||||||||
| High | Low | Volume (daily average) | High | Low | Volume (daily average) | |||||||||||||||||||
February 2014 | 3.83 | 2.77 | 49,208 | 3.46 | 2.49 | 489,621 | ||||||||||||||||||
January 2014 | 4.00 | 3.02 | 60,220 | 3.61 | 2.85 | 222,341 | ||||||||||||||||||
December 2013 | 3.45 | 2.61 | 34,421 | 3.24 | 2.44 | 173,061 | ||||||||||||||||||
November 2013 | 3.29 | 2.49 | 32,854 | 3.15 | 2.36 | 142,798 | ||||||||||||||||||
October 2013 | 4.08 | 3.10 | 58,121 | 3.96 | 2.97 | 239,710 | ||||||||||||||||||
September 2013 | 3.75 | 3.18 | 30,427 | 3.59 | 3.01 | 144,262 | ||||||||||||||||||
August 2013 | 4.35 | 3.07 | 49,328 | 4.21 | 2.94 | 284,788 | ||||||||||||||||||
July 2013 | 4.34 | 3.00 | 80,501 | 4.24 | 3.08 | 296,332 | ||||||||||||||||||
June 2013 | 3.45 | 2.90 | 38,206 | 3.30 | 2.80 | 178,913 | ||||||||||||||||||
May 2013 | 3.20 | 2.58 | 109,587 | 3.13 | 2.48 | 283,848 | ||||||||||||||||||
April 2013 | 2.75 | 2.34 | 26,676 | 2.70 | 2.33 | 218,145 | ||||||||||||||||||
March 2013 | 2.76 | 2.42 | 23,343 | 2.70 | 2.37 | 248,211 | ||||||||||||||||||
DIRECTORS AND OFFICERS
Name, Occupation and Security Holding of Directors
The following table sets forth each director and executive officer’s name, province and country of residence, his/her principal occupation, including the committees of the Board, the year in which he or she first became a director, as at the date of this annual information form. All members of the Board of Directors herein below will hold their positions until the next annual meeting of shareholders of the Corporation.
Name and Province and Country of Residence | Principal Occupation | Position Within the Corporation | Year of Nomination as a Director of the Corporation | |||
Henri Harland(3,4) Québec, Canada | Director of the Corporation | Director of the Corporation(4) | 1998 | |||
Ronald Denis(1,2,3) Québec, Canada | Chief of Surgery at Hôpital du Sacré-Coeur, Montréal | Director and Chairman of the Board of the Corporation | 2000 | |||
Valier Boivin(1,2,3) Québec, Canada | President of VMCAP Inc. | Director | 2013 | |||
Daniel Perry(1,2,3) France | President/Manager Société ADG 7 Tours | Director | 2000 | |||
Harlan W. Waksal(3) New York, United States | Vice-President, Business and Scientific Affairs at Acasti | Director | 2012 | |||
Reed V. Tuckson Washington, United States | Managing Director, Tuckson Health Connections, LLC | Director | 2013 | |||
Tina Sampalis, M.D., Ph.D. Québec, Canada | Chief Global Strategy Officer of the Corporation | Chief Global Strategy Officer of the Corporation | — | |||
60
Name and Province and Country of Residence | Principal Occupation | Position Within the Corporation | Year of Nomination as a Director of the Corporation | |||
André Godin Québec, Canada | Chief Financial Officer of the Corporation(5) | Chief Financial Officer of the Corporation(5) | — | |||
Michel Timperio Québec, Canada | Senior Vice-President, Global Sales | Senior Vice-President, Global Sales | — | |||
Waël Massrieh Québec, Canada | Vice-President, Scientific Affairs | Vice-President, Scientific Affairs | — | |||
| (1) | Member of the Audit Committee of the Corporation |
| (2) | Member of the Compensation Committee of the Corporation |
| (3) | Member of the Governance Committee of the Corporation |
| (4) | Mr. Harland resigned from his role as President and Chief Executve Officer of the Corporation on April 28, 2014 |
| (5) | Following Mr. Harland’s resignation as President and Chief Executve Officer of the Corporation on April 28, 2014 and until such time that a replacement is found, Neptune continues to be managed by a management and operations committee under the leadership of Mr. Godin, the interim Chief Executve Officer of the Corporation |
As of February 28, 2014, the directors and executive officers of the Corporation, as a group, beneficially owned or exercised control or direction over approximately 3,832,209 (5.1%) of the outstanding Common Shares of Neptune.
The information as to outstanding Common Shares beneficially owned or over which the above-named individuals exercise control or direction and the foregoing information is not within the knowledge of the Corporation and has been furnished by the respective persons. The following are brief biographies of Neptune’s directors and executive officers:
Mr. Henri Harland – Director
Mr. Henri Harland is an Actuary and holds a MBA (Finance) from Laval University. Mr. Harland has been a director, President and Chief Executive Officer of the Corporation since its incorporation on October 9, 1998. He is the founder of the Corporation and has been involved in the krill research project since 1991. For more than ten years he has held the position of President and Chief Executive Officer of Gestion Harland Inc., a financial engineering group. Previously, he acted as an independent financial consultant for companies in different industrial sectors in both North America and Europe guiding them through recapitalization, financing and business development. Mr. Harland resigned from his role as President and Chief Executve Officer of the Corporation on April 28, 2014.
Dr. Ronald Denis – Chairman of the Board and Director
Dr. Ronald Denis has been Chief of Surgery and director of the Trauma Program at Hôpital du Sacré-Coeur in Montréal since 1997. Also, since 1987, Dr. Denis has occupied the position of medical co-director of the Canadian Formula 1 Grand Prix. Dr. Denis sits on several scientific boards and management committees.
Mr. Valier Boivin – Director
Mr. Valier Boivin holds a bachelor’s degree in Economic and Administrative Sciences (UQAC-1973), a master’s degree in Taxation (Université de Sherbrooke, 1978) and a law degree (Université de Montréal, 1985). Furthermore, he is a member of the “Barreau du Québec” since 1986 and the “Ordre des comptables agréés du Québec” since 1974. He held the position of Professor at the Université du Québec à Chicoutimi until 1978 and then joined the master’s degree in taxation program as Professor, at the Université de Sherbrooke until 1987. Founder (in 1987) of Boivin O’Neil, s.e.n.c., he practices business law. Specialized in Mergers & Acquisitions and corporate financing, he acts as legal and strategic counsel to many private and public companies. Since January 2009, he is President of the regional economic intervention fund, FIER Ville-Marie L.P. Mr. Boivin is also socially involved with various professional associations, non-profit organizations and charitable foundations.
61
Mr. Daniel Perry – Director
From 1993 to 2013, Mr. Daniel Perry was General Manager of companies operating recreational/tourism complexes in France. Mr. Perry is also a specialist and consultant for different corporations involved in the marketing of new products in Europe.
Dr. Harlan W. Waksal – Director
Dr. Harlan W. Waksal is a retired physician. Dr. Waksal is the Vice-President, Business and Scientific Affairs at Acasti, the Corporation’s subsidiary. He received his B.A. from Oberlin College and M.D. from Tufts University School of Medicine, and his post graduate training in Internal Medicine and in Pathology. In addition, he did research in immunology at the Weizmann Institute of Science. Dr. Waksal was a founder of Imclone Systems Incorporated; a New York based pharmaceutical company specializing in developing new treatment for various forms of cancer. He served as the Chief Operating Officer and member of the Board of Directors from 1986 until 2001 and as President/CEO from 2001 until 2002. During his tenure, he was responsible for building the scientific and operation infrastructure of the company. Dr. Waksal is the author of over 50 scientific publications and has been the author of multiple patents and patent applications. His current activities are focused on managing various real estate developments and serving on select Board of Directors. Dr. Waksal currently serves on the Boards of the Oberlin College, Senesco Technologies, Inc. He also serves on the Advisory Board of Northern Rivers Funds.
Reed V. Tuckson, M.D. – Director
Dr. Tuckson is a graduate of Howard University, Georgetown University School of Medicine, and the Hospital of the University of Pennsylvania’s General Internal Medicine Residency and Fellowship Programs, where he was also a Robert Wood Johnson Foundation Clinical Scholar studying at the Wharton School of Business. Dr. Tuckson is currently the Managing Director of Tuckson Health Connections, LLC, a health and medical care consulting business. Previously, he served a long tenure as Executive Vice President and Chief of Medical Affairs for UnitedHealth Group, a Fortune 25 health and well-being company. Dr. Tuckson is member of the Advisory Committee to the Director of the National Institutes of Health and is also an active member of the Institute of Medicine of the National Academy of Sciences. He also serves on the Boards of the American Telemedicine Association, Howard University and Cell Therapeutics Inc., a public corporation.
Dr. Tina Sampalis M.D., Ph.D. – Chief Global Strategy Officer
Dr. Tina Sampalis is an Oncology Surgeon, trained in Physiology at McGill University, Medicine at the University of Patras (Greece), Dermatology at Göttingen University (Germany) and Marselisborg University (Denmark), Pediatric, General and Oncology Surgery at the University of Athens (Greece), graduate training (PhD) in Surgical Research at the University of Athens and a second PhD in Epidemiology and Experimental Surgery at McGill University. She has received several international scholarships and awards for her work on the clinical implementation of retinols skin and breast cancer and for her work on scintimammography. U.S. and Canadian patent applications have been filed for the development and implementation of innovative micro-invasive and stereotactic robotic surgical techniques for breast cancer. Between May 2000 and June 2007, she has held the position of Vice-President of Research and Business Development and since June 2007 the position of Chief Scientific Officer of the Corporation. She has ceased to occupy this position following her nomination as Chief Global Strategy Officer of the Corporation, which was announced on May 25, 2012.
Mr. André Godin – Chief Financial Officer
Mr. André Godin, C.A., has a Bachelor in Administration and has been a Member of the Canadian Institute of Chartered Accountants since 1988. He has more than 10 years experience in the Biotech/Pharma industry as former President of a Dietary Supplement Corporation and as a Corporate Controller for a pharmaceutical Corporation in OTC products. Mr. Godin has been Vice-President, Administration and Finance for Neptune since 2003 before his nomination as Chief Financial Officer in 2012.
62
Mr. Michel Timperio – Senior Vice-President, Global Sales
Mr. Michel Timperio obtained a Bachelor degree from Concordia University in 1980 and attended additionnal courses at l’ENAP (National Institute of Public Administration). Mr. Timperio was elected as Chairman of the Board of Neptune in 2000 until 2008. He joined Neptune as a full time employee in September 2010. Prior to joining the Neptune team, Mr. Timperio started his career with Amstrong World Industries as sales representative for Eastern Canada and subsequently with Reicchold Chemicals as regional sales manager in 1982. After earning a young entrepreur loan and scholarship, he started his own distribution business, Specgraphix/Unic in 1985 specializing in the distribution of printing raw material products. During his business endeavours he was the recipient of many sales recognition awards as top sales executives and was also elected as alderman seating on the executive council of one of the largest Montreal suburb, in the city of Longueuil. In 1987, He hosted the first socio-economic summit of one of the largest administrative region in the province of Québec.
Mr. Waël Massrieh – Vice President, Scientific Affairs
Mr. Wael Massrieh completed his Bachelors of Science in Microbiology and Immunology at McGill University where he then pursued his Doctorate in Medicine, in the division of Experimental. His research on pro-inflammatory cytokines and regulation of transcription factors during premature labor research is published multiple times in peer reviewed publication. In addition to completing a “Mini-MBA” program, certified by the International Pharmaceutical Academy in September 2011, he is a member of the Project Management Institute and is completing the Project Management Professional (PMP®) Certification. Prior to joining Neptune, he held the position of Director of R&D at BIOTONIX INC, where he steered and advanced research on body posture and psychology. Mr. Wael Massrieh joined Neptune in 2007 and held the position of Director of R&D and is currently the Vice President of Scientific affairs.
CEASE TRADE ORDERS, BANKRUPTCIES, PENALTIES OR SANCTIONS
To the knowledge of Neptune, none of the directors or executive officers of the Corporation:
| (a) | is, or has been, within the last ten years, a director, chief executive officer or chief financial officer of any Corporation that: |
| (i) | was subject to a cease trade order, an order similar to a cease trade order, or an order that denied the relevant Corporation access to any exemption under applicable securities legislation, that was in effect for a period of more than 30 consecutive days (an “Order”), which Order was issued while the director or executive officer was acting in the capacity as director, chief executive officer or chief financial officer; or |
| (ii) | was subject to an Order that was issued after the director or executive officer ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer; |
To the knowledge of Neptune, no director or executive officer of the Corporation, or shareholder holding a sufficient number of securities of the Corporation to affect materially the control of the Corporation:
| (a) | is, or has been, within the last ten years, a director or executive officer of any Corporation that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver manager or trustee appointed to hold its assets; or |
| (b) | has, within the last ten years, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold his or its assets of the proposed director. |
63
To the knowledge of Neptune, no director, executive officer or shareholder holding a sufficient number of securities of the Corporation to affect materially the control of the Corporation has been subject to:
| (a) | any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or |
| (b) | any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable security holder in deciding whether to vote for a proposed director. |
LEGAL PROCEEDINGS AND REGULATORY ACTIONS
The Corporation is not aware of any legal proceedings or regulatory actions in which it is involved and no such proceedings or regulatory actions are known by the Corporation to be contemplated, except in regards of what is mentioned in the section “Business of the Corporation—Economic Dependence/Litigation”.
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
Except for what is stated below, none of the insiders of the Corporation, the Directors, or any of their respective associates or affiliates, has or has had any material interest, direct or indirect, in any material transaction whether proposed or concluded, since the beginning of the Corporation’s most recently completed financial year and for the three (3) last completed financial years.
The Corporation entered into an agreement with a company controlled by Mr. Henri Harland, as of February 23, 2001, calling for royalties to be paid in semi-annual instalments equal to 1% of the Corporation’s annual revenues, for an unlimited period. For the financial year ended February 28, 2014, $436,589 in royalties on sales is payable in cash by the Corporation. See “Business of the Corporation—Intellectual Property—Licensing Arrangements”.
TRANSFER AGENTS AND REGISTRARS
Computershare Trust Company of Canada, at its offices in Montreal, is the transfer agent and registrar for our Common Shares.
MATERIAL CONTRACTS
The Corporation has not entered into any material contract, other than those entered into in the normal course of business, within the most recently completed financial year, or before the most recently completed financial year, which is still in effect except for the Technology License Agreement entered into with Acasti on August 7, 2008 and the prepayment agreement entered into with Neptune on December 4, 2012, and the Technology License Agreement entered into with NeuroBio on October 15, 2008. See “Business of the Corporation—Intellectual Property”.
INTEREST OF EXPERTS
KPMG LLP (“KPMG”) has audited our consolidated financial statements for the years ended February 28, 2014 and February 28, 2013. KPMG is independent with respect to Neptune Technologies & Bioressources Inc., Acasti Pharma Inc. and NeuroBioPharm Inc. within the meaning of the relevant rules and related interpretation prescribed by the releveant professional bodies in Canada.
REPORT ON AUDIT COMMITTEE
Audit Committee’s Charter
The Charter of the Audit Committee is annexed to this circular as Schedule A. The Charter was adopted by the Board of Directors on June 6, 2007.
64
Composition of the Audit Committee
The Audit Committee is composed of 3 members of the Board of Directors. Dr. Ronald Denis, Mr. Daniel Perry, and Mr. Valier Boivin are the directors that seat on the Audit Committee.
Under National Instrument 52-110Audit Committees (“NI 52-110”), a director of an Audit Committee is “independent” if he or she has no direct or indirect material relationship with the issuer, that is, a relationship which could, in the view of the Board of Directors, reasonably interfere with the exercise of the member’s independent judgment. All current members are independent. All members of the Audit Committee are considered to be “financially literate” within the meaning of applicable Canadian securities regulations in that they each have the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raided by the Corporation financial statements.
From the experience set forth below, the Corporation believes that these persons have sufficient knowledge and background to actively participate on the Audit Committee.
Relevant Education and Experience
The following describes the relevant education and experience of each member of the Audit Committee of the Corporation that provides him or her with (a) an understanding of the accounting principles used by the Corporation to prepare its financial statements, (b) the ability to assess the general application of such accounting principles, (c) experience preparing, auditing, analyzing or evaluating financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to those that can reasonably be expected to be raised by the Corporation’s financial statements or experience actively supervising one or more persons engaged in such activities and (d) an understanding of internal controls and procedures for financial reporting.
Dr. Ronald Denis– Dr. Denis has been Chief of Surgery and Director of the Trauma Program at Hôpital Sacré-Coeur since 1997. In his duties, Mr. Denis has to manage Sacré-Coeur Hospital Trauma Program budget and staff, also he has had to regularly review and analyze financial statements. Dr. Denis’ experience required and contributed to the development of his ability to analyze financial statements and understand IFRS.
Valier Boivin – Mr. Boivin holds a bachelor’s degree in Economic and Administrative Sciences (UQAC-1973), a master’s degree in Taxation (Université de Sherbrooke, 1978) and a law degree (Université de Montréal, 1985). Furthermore, he is a member of the “Barreau du Québec” since 1986 and the “Ordre des comptables agréés du Québec” since 1974. He held the position of Professor at the Université du Québec à Chicoutimi until 1978 and then joined the master’s degree in taxation program as Professor, at the Université de Sherbrooke until 1987. Founder (in 1987) of Boivin O’Neil, s.e.n.c., he practices business law. Specialized in Mergers & Acquisitions and corporate financing, he acts as legal and strategic counsel to many private and public companies. Since January 2009, he is President of the regional economic intervention fund, FIER Ville-Marie L.P. Mr. Boivin is also socially involved with various professional associations, non-profit organizations and charitable foundations. Mr. Boivin’s experience required and contributed to the development of his ability to analyze financial statements and understand IFRS.
Daniel Perry– Since March 1993, Mr. Perry is General Manager of a corporation operating a recreational/tourism complex in France. Also, Mr. Perry is a specialist and consultant in the marketing of new products on the European continent. Mr. Perry’s experience required and contributed to the development of his ability to analyze financial statements and understand IFRS.
External Auditor Fees
Audit Fees
“Audit fees” consist of fees for professional services for the audit of the Corporation’s annual financial statements, interim reviews and limited procedures on interim financial statements, securities filings, Sarbanes Oxley Act Section 404 opinions and consultations on accounting or disclosure issues. During the fiscal year ended February 28, 2014, KPMG LLP, the Corporation’s external auditors, billed $714,000 to the Corporation, respectively $460,000 for the Corporation, $214,500 for Acasti and $39,500 for NeuroBio, for audit fees. During the fiscal year ended February 28, 2013, these fees were $546,000 to the Corporation, respectively $347,000 for the Corporation, $68,500 for Acasti and $130,500 for NeuroBio (of which $30,000 for the year ended February 29, 2012).
65
Audit-Related Fees
“Audit-related fees” consist of fees for professional services that are reasonably related to the performance of the audit or review of the Corporation’s financial statements and which are not reported under “Audit Fees” above. For the fiscal year ended February 28, 2014, KPMG LLP, the Corporation’s external auditors, billed $20,000 to the Corporation, respectively $6,000 for the Corporation and $14,000 for Acasti. Audit-related fees include, but are not limited to, services provided for other types of audit engagements and French translation services.
For the fiscal year ended February 28, 2013, KPMG LLP, the Corporation’s external auditors, billed $6,000 to the Corporation for audit related services provided to the Corporation. No fees as to this matter were billed to Acasti or NeuroBio for the fiscal year ended February 28, 2013.
Tax Fees
“Tax fees” consist of fees for professional services for tax compliance, tax advice and tax planning. KPMG LLP, the Corporation’s external auditors, billed a total of $120,080 to the Corporation, respectively $85,580 for the Corporation, $25,500 for Acasti and $9,000 for NeuroBio, for tax fees for fiscal year ended February 28, 2014 and a total of $52,500 to the Corporation, respectively $40,000 for the Corporation, $7,500 for Acasti and $5,000 for NeuroBio, for tax fees for fiscal year ended February 28, 2013. Tax fees include, but are not limited to, preparation of tax returns and R&D tax credit claims.
All Other Fees
The “other fees” include all other fees billed for professional services other than those mentioned hereinabove. KPMG LLP, the Corporation’s external auditors, billed no fees as to this matter the fiscal years ended February 28, 2014 and February 28, 2013.
ADDITIONAL INFORMATION
Additional information relating to the Corporation may also be found on the SEDAR website atwww.sedar.com, and on EDGAR atwww.sec.gov/edgar.shtml.
Additional information, including directors’ and officers’ remuneration and indebtedness, principal holders of our securities, options to purchase securities and interests of informed persons in material transactions, if applicable, is contained in Neptune’s Management Proxy Circular dated May 22, 2014 and available on SEDAR. Additional financial information is also provided in our financial statements and MD&A for the most recently completed financial year.
66
SCHEDULE “A”
CHARTER OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORS
The Audit Committee of the Board of Directors assists the Board in fulfilling its oversight responsibilities relating to the quality and integrity of the accounting, auditing and reporting practices of the Corporation and such other duties as directed by the Board of Directors or imposed by legislative authorities or stock exchanges.
Structure and Organization
| 1. | The membership of the Committee will consist of at least three independent members of the Board of Directors, the majority of whom will not be employees, controlling shareholders or executives of the Corporation or of any associates or affiliates of the Corporation. Committee members and the Committee Chairman shall be designated by and serve at the pleasure of the Board of Directors. All members must be financially literate and at least one member must have accounting or related financial management expertise, in each case in the judgment of the Board of Directors. |
| 2. | The Committee shall meet at least four times per year or more frequently as circumstances require. The Committee may ask members of management or others to attend meetings and provide pertinent information as necessary. The required quorum for the Committee will be the majority of the members forming the Committee. |
| 3. | The Committee is expected to maintain free and open communication with management and the external auditors. |
| 4. | The Committee has the authority to investigate any matter brought to its attention and to retain outside counsel for this purpose if, in its judgment, that is appropriate. |
General Responsibilities
The Committee shall:
| 1. | Meet periodically with representatives of the external auditors, the internal audit manager (if any) and management in separate sessions, if considered necessary, to discuss any matters that the Committee or these groups believe should be discussed privately with the Committee. Provide sufficient opportunity for the external auditors to meet with the Audit Committee as appropriate without members of management being present. |
| 2. | Prepare the minutes of all Committee meetings and report of such meetings to the Board of Directors. |
| 3. | Review and reassess the adequacy of this Charter annually. |
Responsibilities for Engaging External Auditors
The Committee shall:
| 1. | Recommend for approval by the Board of Directors and ratification by the shareholders the selection and retention of an independent firm of chartered professional accountants as external auditors, approve compensation of the external auditors, and review and approve in advance the discharge of the external auditors. |
| 2. | Review the independence of the external auditors. In considering the independence of the external auditors, the Committee will review the nature of the services provided by the external auditors and the fees charged, and such other matters as the Committee deems appropriate. |
| 3. | Ensure that the external auditors are in good standing with the Canadian Public Accountability Board (CPAB) and that the CPAB has not imposed any sanction on them. The Audit Committee is also responsible for ensuring that the external auditors comply with the rotation requirements with respect to partners involved in the audit of the Corporation. |
| 4. | Arrange for the external auditors to be available to the Board of Directors at least annually to help provide a basis for the Board’s approval of the external auditors’ appointment. |
A-1
| 5. | Approve all allowable non-audit related services to be provided to the Corporation or one of its subsidiaries by the Corporation’s external auditors if applicable. |
| 6. | Non-audit services of minimal amount satisfy the pre-approval requirements on the following conditions: |
| (a) | that the aggregate amount of all non-audit services that were not pre-approved is reasonably expected to constitute no more than five per cent of the total amount of fees paid by the Corporation and its subsidiaries to the Corporation’s external auditors during the fiscal year in which the services are provided; |
| (b) | that the Corporation or its subsidiaries, as the case may be, did not recognize the services as non-audit services at the time of the engagement; and |
| (c) | that the services are promptly brought to the attention of the Audit Committee and approved, prior to the completion of the audit, by the Audit Committee or by one or more of its members to whom authority to grant such approvals had been delegated by the Audit Committee. |
Responsibilities for Oversight of the Quality and Integrity of Accounting, Auditing and Reporting Practices of the Corporation
The Committee shall:
| 1. | Directly review the work of the external auditors engaged for the purpose of preparing or issuing an auditor’s report or performing other audit, review or attestation services for the Corporation. The Committee shall be directly responsible of the resolution of disagreements between management and the external auditors regarding financial reporting. |
| 2. | Review the Corporation’s financial statements, management’s discussion and analysis (MD&A) and annual and interim earnings press releases together with management and the external auditors, if applicable, before the Corporation publicly discloses this information. This review should cover the quality of the financial reporting and such other matters as the Committee deems appropriate. |
| 3. | Review with the external auditors and management the audit plan of the external auditors for the current year and the following year. |
| 4. | Review with financial and accounting personnel, the adequacy and effectiveness of the accounting, financial, and computerized information systems controls of the Corporation, and the results of any external audit procedures, if applicable. |
| 5. | Establish procedures for the receipt, retention and treatment of complaints received regarding accounting, internal accounting controls or auditing matters. Such complaints are to be treated confidentially and anonymously. |
| 6. | Review and approve all related party transactions undertaken by the Corporation. |
Periodic Responsibilities
The Committee shall:
| 1. | Review periodically with management any legal and regulatory matters that may have a material impact on the Corporation’s financial statements, compliance policies and compliance programs. |
| 2. | Review with management and approve transactions involving management and/or members of the Board of Directors, which would require disclosure under Toronto Stock Exchange rules. |
| 3. | Supervise the corporate compliance program and periodically review whether any improvements should be made thereto and make appropriate recommendations to management. |
A-2
| 4. | Perform such other functions assigned by law, the Corporation’s Articles or bylaws, or by the Board of Directors. |
| 5. | Review services and related fees for work done by the external auditors as well as an updated projection of the total costs for the fiscal year. |
| 6. | Review and approve the engagement policy of the Corporation with respect to partners, employees, former partners and employees of the current and previous external auditors of the Corporation. |
| 7. | Implement a process for the identification of the principal business risks and monitor the implementation of appropriate methods of risk management. This process will require consultation with management in order to determine how risks are handled and to solicit the opinion of the internal audit department with respect to the effectiveness of the risk limitation strategies. |
Authority of the Audit Committee
The Committee shall have the authority to:
| 1. | Engage independent counsel and other advisors as it determines necessary to carry out its duties. |
| 2. | Pay the compensation for any advisors employed by the Committee. The Committee shall notify the Board of Directors on the extent of the financing required to pay for the compensation of the independent expert advisors retained to advise the Committee. |
| 3. | Communicate directly with the internal and external auditors. |
A-3