UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | | |
| x | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
Or
| ¨ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34468
VITACOST.COM, INC.
(Exact name of registrant as specified in its charter)
| Delaware | | 37-1333024 |
| (State or other jurisdiction of | | (I.R.S. Employer |
| incorporation or organization) | | Identification Number) |
5400 Broken Sound Blvd., NW, Suite 500 Boca Raton, Florida | | 33487 |
| (Address of principal executive offices) | | (Zip Code)) |
Registrant’s telephone number, including area code:(561) 982-4180
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: | | Name of Each Exchange on Which Registered |
| Common Stock, $0.00001 par value | | The NASDAQ Stock Market LLC (NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes¨ Nox
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes¨ Nox
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yesx No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer¨ | | Accelerated Filerx | | Non-Accelerated Filer¨ | | Smaller Reporting Company¨ |
Indicate by check mark whether the registrant is a shell company as defined in Rule 12-b-2 of Exchange Act. Yes¨ Nox
As of June 30, 2012, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of voting and non-voting equity held by non-affiliates of the registrant was approximately $128.4 million. Shares of the registrant’s common stock held by each executive officer and director have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 27, 2013, the registrant has 33,505,521 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement to be delivered to stockholders in connection with the registrant’s 2013 Annual Meeting of Stockholders are incorporated by reference into Part III of this Form 10-K.
VITACOST.COM, INC. FORM 10-K
TABLE OF CONTENTS
| | | | Page |
| PART I |
| Item 1. | Business | | 1 |
| Item 1A. | Risk Factors | | 11 |
| Item 1B. | Unresolved Staff Comments | | 20 |
| Item 2. | Properties | | 20 |
| Item 3. | Legal Proceedings | | 20 |
| Item 4. | Mine Safety Disclosures | | 20 |
| PART II |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities | | 21 |
| Item 6. | Selected Financial Data | | 23 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | | 24 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | | 30 |
| Item 8. | Financial Statements and Supplementary Data | | 31 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | | 48 |
| Item 9A | Controls and Procedures | | 48 |
| Item 9B. | Other Information | | 48 |
| PART III |
| Item 10. | Directors, Executive Officers, and Corporate Governance | | 49 |
| Item 11. | Executive Compensation | | 49 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | | 49 |
| Item 13. | Certain Relationships, Related Transactions and Director Independence | | 49 |
| Item 14. | Principal Accountant Fees and Services | | 49 |
| PART IV |
| Item 15. | Exhibits and Financial Statement Schedules | | 50 |
| Signatures | | | 51 |
| | Exhibit 31.1 Certification of CEO Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | | |
| | Exhibit 31.2 Certification of CFO Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | | |
| | Exhibit 32.1 Certification of CEO and CFO Pursuant to 18 U.S.C. Section 1350, as Adopted | | |
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K contains trends, analyses and other forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements concerning our plans, objectives, goals, strategies, future events, future revenue or performance, capital expenditures, financing needs and other information that is not historical information. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “continue,” “seek” or the negative of these terms or other comparable terminology or by discussions of strategy.
All forward-looking statements are based upon our current expectations and various assumptions and we do not assume any obligation to update any of these statements. We believe there is a reasonable basis for our expectations and beliefs, but they are inherently uncertain. We may not realize our expectations and our beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements and are subject to change due to the inherent risks and uncertainties, such as those disclosed or incorporated by reference in our filings with the SEC. Important factors that could cause our actual results, performance and achievements, or industry results to differ materially from the forward-looking statements are set forth in this Annual Report on Form 10-K under Part I, Item 1 — Business, Item 1A — Risk Factors and Part II, Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operation and include, among others:
| • | significant competition in our industry; |
| • | unfavorable publicity or consumer perception of our products on the Internet; |
| • | the incurrence of material product liability and product recall costs; |
| • | costs of compliance and our failure to comply with government regulations; |
| • | our inability to successfully defend intellectual property claims; |
| • | our failure to keep pace with the changing demands and preferences of our customers for new products; |
| • | the current global economic climate; |
| • | disruptions in our information technology systems; and |
| • | the lack of long-term experience with human consumption of some of our products with innovative ingredients. |
Forward-looking statements in this Annual Report on Form 10-K speak only as of the date hereof, and forward-looking statements in documents attached that are incorporated by reference speak only as of the date of those documents. We do not undertake any obligations to update or release any revisions to any forward-looking statement or to report any events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law.
PART I
Item 1. Business.
Vitacost.com, Inc.
We are a leading online retailer of health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals (which we refer to as “vitamins and dietary supplements”), as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. We sell these products directly to consumers primarily through our website,www.vitacost.com. We strive to offer our customers the broadest selection of healthy living products at extremely competitive prices, while providing superior customer service.
We offer our customers a broad selection of approximately 40,000 Stock Keeping Units (“SKUs”), from over 2,000 third-party brands, such as New Chapter, Nature’s Way, Twinlab, Source Naturals, Jarrow Formulas, Jason, Desert Essence, Atkins, Bob’s Red Mill, BSN, Optimum Nutrition, USP Labs and MuscleTech in addition to our own proprietary brands: Vitacost, Cosmeceutical Sciences Institute (“CSI”), Best of All, and Smart Basics. We support our operations through our customer service center and our two distribution centers, delivering what we believe are industry-leading customer satisfaction results. Our website allows customers to easily browse and purchase products at prices typically significantly lower than manufacturers’ suggested retail prices. Our website also serves as an educational resource for consumers seeking information on healthy living and the attributes of health and wellness supplements.
Our growth is driven primarily by our ability to expand our customer base and grow our product offerings. Our customers are typically individuals seeking value in their purchases of health and wellness products. Our active customer base, which we define as customers who have purchased from us within the last twelve months, has steadily increased from approximately 270,000 at the end of 2005 to approximately 2.1 million as of December 31, 2012. On average, our customers make purchases from us two to three times a year and during 2012, approximately 72% of our orders were placed by repeat customers.
Corporate Information
We were incorporated in Delaware in May 1994 and began operations as a catalog retailer of third-party vitamins and supplements under the name Nature’s Wealth Company. In 1999, we launched Vitacost.com and introduced our proprietary vitamins and supplements. In 2000, we began operating under the name Vitacost.com, Inc. (the “Company”, “Vitacost”, or “Vitacost.com”). In September 2009, the Company completed its Initial Public Offering. On September 28, 2011, Vitacost.com, Inc. completed a restructuring whereby it merged with and into Vitacost Merger Corporation, a wholly owned subsidiary of Vitacost.com, Inc., with Vitacost Merger Corporation surviving the merger. The surviving company continues to operate the business under the name Vitacost.com, Inc.
Industry Overview
The expansion of the Internet has benefited online retailers by improving methods of communication, delivery of content and ease of commerce. At the same time, consumers are leveraging online resources to make informed healthcare, dietary and nutritional choices and related purchasing.
U.S. Nutrition Industry. According to the Nutrition Business Journal’s Direct to Consumer Selling in the Nutrition Industry Report 2012 (“NBJ”), total U.S. sales for the nutrition industry (including natural & organic food, functional foods, supplements and natural/organic personal care & household products) grew 8% to $126 billion in 2011. NBJ is forecasting U.S. sales for the total nutrition industry to grow at an 8% rate per year for the next nine years, reaching $244 billion in 2020. Steady growth reflects an overall health and wellness trend in the U.S. with an increased focus by consumers on their diet, exercise and overall health. Products targeting weight management, sports nutrition, chemical-free cleaners, and specialty diets such as gluten-free are becoming mainstay purchases made by today’s consumers. U.S. sales for the total nutrition industry through the Internet grew significantly faster than the overall category, increasing approximately 14% in 2011 to $3 billion, and accounted for an estimated 3% of total U.S. nutrition sales. NBJ is forecasting the internet channel to remain strong with sales expected to grow at a 13% compound annual growth rate (“CAGR”) over the next nine years, reaching $10 billion by 2020.
U.S. Dietary Supplement Market. According to the NBJ’s Direct to Consumer Selling in the Nutrition Industry Report 2012, U.S. sales of dietary supplements (including vitamins, herbs, meal supplements and sports nutrition and specialty supplements) grew 7% to $30 billion in 2011. NBJ is forecasting U.S. sales of dietary supplements to grow at a 7% rate per year for the next nine years reaching $53 billion in 2020. Steady growth reflects customers’ purchases of these natural products to protect their health and ward off more expensive medical visits and prescription drugs. The dietary supplement industry is highly fragmented with products sold through multiple channels including retailers such as mass merchants, grocery stores, drug stores and specialty retailers, as well as through direct mail, catalogs, multi-level marketers and the Internet. U.S. sales of dietary supplements through the Internet grew significantly faster than the overall category increasing approximately 15% in 2011 to $1.5 billion and accounted for an estimated 5% of the total U.S. dietary supplement category. According to NBJ, internet sales of dietary supplements are expected to grow at a 13% CAGR over the next nine years, reaching $4.5 billion by 2020.
Our Value Proposition
We strive to offer our customers the broadest product selection of healthy living products at the best value, while providing superior customer service.
Broad Third-Party and Proprietary Product Selection. We offer approximately 40,000 SKUs representing over 2,000 brands, including nationally-recognized third-party brands and our proprietary brands. Our product selection is designed to appeal to a variety of demographic groups, including those seeking health maintenance and general well-being, individuals making household purchasing decisions for the family, baby boomers, the elderly and those with specific health concerns or goals. Our product selection regularly evolves as consumer preferences change.
Consistently Superior Value. We offer products at savings to our customers with prices typically significantly lower than manufacturers’ suggested retail prices. We provide even greater savings to our customers through our proprietary product lines.
Superior Customer Services.Our website is designed to attract natural search traffic while providing a convenient, educational, secure and efficient shopping experience. Products are cross-indexed to allow consumers to easily locate and compare products when searching by brand, ingredient or keyword. In addition, we maintain a customer service center which provides customers with answers to product and technical questions as well as processes customer orders. Finally, customer orders are quickly and accurately processed in our fulfillment centers.
Growth Initiatives
Our growth strategy is based on the following key initiatives:
| · | Increase Brand and Company Awareness: We are increasing customer awareness of Vitacost.com and our vast healthy living and proprietary brand offerings. In January 2012, we unveiled a fresh new brand identity, complete with a new logo and corporate slogan, “take the cost out of healthy living”. In addition, we re-launched our website with a cleaner look and feel and improved usability features. We expanded our online content through a broader initiative to develop a deeper connection to our customers. |
| · | Expand Customer Base: We are focused on acquiring new customers in an effort to expand our customer base. Historically, our customers have had high repeat order rates and lifetime values, as we primarily sell consumable products. We believe future top-line growth will stem from an expanded customer base and increasing our touch points on the internet to target customers directly where and how they shop. Our marketing activities are primarily focused on online initiatives such as search engine marketing, search engine optimization and developing a network of affiliates. In addition, we are leveraging our existing customer base to attract new customers through our “Refer a Friend” program, which rewards existing customers for signing up new customers. |
| · | Expand Product Offerings: We continue to increase our product offerings of both third party and proprietary products to drive traffic to our site and increase basket size as we strive to become the leading online destination for health and wellness products. We continue to add brands and line extensions in our core Vitamins, Minerals, Herbs and Supplements (“VMHS”) category as well as in faster growing healthy living categories such as food, beauty, and sports nutrition. During 2012, we added over 5,000 net new SKUs, ending the year with approximately 40,000 SKUs live on our website. |
| · | Increase Lifetime Value by Increasing Frequency of Purchases and Improving Customer Retention: As we continue to diversify our product offerings in the healthy living space, there is a renewed effort to educate customers on the breadth of our product offerings to increase total basket size and the frequency of purchases in order to drive greater lifetime value. We are also focused on increasing lifetime value per customer by improving customer retention through the use of automatic reordering programs along with targeted, personalized emails and promotional offers as well as offering a positive overall customer experience. |
| · | International Expansion: We currently ship products internationally to numerous countries including Canada, Hong Kong, Japan, Taiwan and the United Kingdom, despite limited marketing efforts outside of the United States. We are now beginning to focus on creating an enhanced in-country experience for customers by increasing awareness of our international shipping destinations and improving the overall web and shipping experience for international customers. International represents a large, growing long-term opportunity for us with international accounting for 6% of total revenue in 2012. |
| · | Expand and Optimize Distribution: We believe that processing customer orders on a timely basis is a key component of customer satisfaction. Our long-term initiatives are to reduce processing time, while increasing our fulfillment capacity and driving efficiency in the cost of processing orders. During 2012, we increased the storage capacity of our two existing fulfillment centers to hold up to 50,000 SKUs. We are currently in the process of upgrading our warehouse management systems and modifying our order processing methodology in an effort to achieve these objectives. |
| · | Improve Operating Efficiencies: We are focused on improving operating efficiencies across our organization by reducing costs at our existing fulfillment centers primarily through improved labor productivity as well as gaining efficiency in our sales and marketing spend. We also seek to gain sales leverage on our fixed cost structure as we expand our overall sales. |
Products
We provide online shoppers with one of the broadest selections of high-quality health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals, as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. We offer products in a wide range of potency levels and dosage forms for our dietary supplements such as tablets, capsules, vegi-capsules, softgels, gelcaps, liquids and powders. Our focus on providing a broad selection enables our customers to purchase products from preferred, trusted brands through a single, comprehensive source.
We offer products that encompass four main categories: Vitamins, Minerals, Herbs and Supplements; Sports Nutrition; Beauty; and Natural and Organic Food.
Vitamins, Minerals, Herbs and Supplements. VMHS products are generally taken to maintain or improve health and address specific health conditions. The Food and Drug Administration (“FDA”) classifies these products under the term “dietary supplements.” In this category, we offer our Vitacost branded products as well as third-party brands such as Nature’s Way, Twinlab, Jarrow, Carlson and Rainbow Light. Vitamin and mineral products include multi-vitamins, lettered vitamins, such as Vitamin A, C, D, E and B-complex, along with minerals such as calcium, magnesium, chromium and zinc. These products help prevent deficiencies that can occur when diet alone does not provide all of the necessary vitamins and minerals. Herbal products include whole herbs, standardized extracts, herb combination formulas and teas. Herbs offer a natural solution to address specific health concerns. Supplements include essential fatty acids, probiotics, anti-oxidants, phytonutrients and condition-specific formulas.
Sports Nutrition. Sports nutrition products are used in conjunction with cardiovascular conditioning, weight training and sports activities. Major categories in sports nutrition include protein and weight gain powders, meal replacements, nutrition bars, sports drinks and pre- and post-workout supplements to either increase energy or enhance recovery after exercise. We offer bodybuilding and sports products from third parties such as Optimum Nutrition, CytoSport and BSN as well as our Vitacost branded sports nutrition products.
Beauty. Our beauty category consists of a variety of natural products for skin, body, hair and oral health. We offer hundreds of natural personal care products from category leaders such as JASON and Kiss My Face, as well as our proprietary CSI branded products. These products appeal to allergen conscious, environmentally conscious, or socially conscious consumers seeking products that are made without harsh chemicals and additives or are not tested on animals.
Natural and Organic Food. Natural and organic food consists of organic and specialty products such as organic peanut butter, gluten free foods and low mercury tuna and salmon. We offer third-party brands such as Kashi, Eden Foods and Amy’s Organic, as well as our Best of All natural food products.
In 2012 and 2011, our proprietary brands accounted for approximately 22% and 23% of our net sales, respectively. Our proprietary brands include:
| • | Vitacost. Our Vitacost brand is our largest proprietary brand. Under the Vitacost brand, we offer over 900 products including multivitamins, minerals, herbs, amino acids, anti-oxidants and others. |
| • | Cosmeceutical Sciences Institute. Under our CSI brand, we market and sell health and beauty products such as facial cleanser, facial and body moisturizing creams and lotions, and other skincare products. |
| • | Best of All. Under our Best of All brand, we market and sell organic food products such as banana chips, trail mix, almonds, cashews and more. |
| • | Smart Basics. Under our Smart Basics brand, we market and sell organic fruit juices and extracts and related dietary supplements. |
| • | Walker Diet. Under our Walker Diet brand, we market and sell low carb powders used to assist in weight loss and management. |
Merchandising & New Product Development
We believe we carry most major domestic brands of VMHS products as well as many smaller specialty brands. We sell most of our suppliers’ most popular product lines. We also offer our proprietary brands based on our own formulations. We currently stock approximately 40,000 SKUs at both of our fulfillment centers. Currently, no single SKU represents more than 2% of our net sales. In developing new proprietary products, we review our sales and cost data and customer feedback along with evaluating new industry trends.
Marketing
Our marketing strategy is designed to increase brand awareness and drive highly targeted new and repeat customers to our website. We use a multi-channel approach which includes search engine marketing, search engine optimization, email campaigns, partnerships, broad reach advertising, customer referral and affiliate programs to acquire and retain our customer base. We are also actively embracing mobile technology and upgrading our mobile platform as we believe mobile will be a key source of new customers going forward given the rapid growth and increased acceptance of mobile shopping.
Online Marketing. We make our website available via keywords and shopping feeds on internet search engines including but not limited to: Google, Bing, Nextag and Shopzilla. Banner advertisements on display networks are also used to drive traffic to our website. In addition, we sell our products through Amazon.com while operating an affiliate program aimed at creating brand awareness through websites that participate in the LinkShare network.
Email Campaigns. Our email marketing campaigns distribute information on new arrivals, promotional discounts and product information to customers.
Direct Mail and Promotional Inserts. Direct mail is used on a limited basis to increase awareness of our proprietary brands and to target market to certain customer segments.
Customer referrals. We operate a ‘Refer-A-Friend’ program to further drive customers to our site. Any existing customer can ‘refer’ a new customer to Vitacost and both are rewarded with a $10 credit to apply to an order of $30 or more on our Vitacost.com website.
Partnerships. We have entered into select partnerships, and continuously evaluate new partnerships, with organizations that have access to large customer bases that may serve as potential Vitacost.com customers or that will improve the brand image and awareness of Vitacost.com.
Manufacturing
Proprietary Manufacturing. On August 28, 2012, we entered into an agreement to outsource all of our manufacturing operations and lease our manufacturing facilities to a third party provider, effective September 1, 2012. Prior to this transaction, we manufactured approximately sixty percent of our proprietary products.
Contract Manufacturing. As of December 31, 2012, all of our manufacturing is provided by third party manufacturers. Each of our contract manufacturers is required to maintain high standards of quality control consistent with federal regulatory guidelines and manufacture our products according to our strict specifications. We have implemented vendor qualification programs for all of our suppliers and manufacturers, including full analytical testing of the products we purchase.
Customer Service
We strive to offer outstanding customer service with each customer’s complete satisfaction as our goal. To achieve this goal, we maintain a fully staffed customer service center to respond to customers via incoming calls, e-mails and live-chat while providing accurate and timely shipping, all driven by our 5-Star Guarantee. We believe our customer service initiatives allow us to establish and maintain long-term customer relationships and facilitate repeat visits and purchases.
Customer Service Center.Our service center serves as the primary contact between our customers and the company. Customer service agents are available to answer questions and to accept customer orders. Our customer service specialists receive regular training so that they can effectively and efficiently field questions from current and prospective consumers. Our specialists are also trained not to answer questions that should be directed to a customer’s physician, such as questions relating to drug interactions.
Our 5-Star Guarantee. Our 5-Star Guarantee makes it easy, convenient and safe for customers to purchase our products. Under the Guarantee we:
| · | Offer vitamins, supplements, organic products, body care and natural health products at everyday low prices, providing savings off retail 365 days a year, with no minimums, no memberships and no hidden charges. |
| · | Provide a 30-day unconditional money-back guarantee for all our products. |
| · | Provide the highest quality supplements and other natural health products. |
| · | Maintain one of the largest selections of healthy living products available anywhere, with approximately 40,000 items and more than 2,000 brands from which to choose. |
| · | Guarantee a safe, secure online shopping experience through the use of state-of-the-art Secure Socket Layer 128-bit encryption on our website. |
During 2012, we were named in the top 10 online retailers by ForeSee in the ForeSee E-Retail Satisfaction Index for Spring 2012, maintaining our top 10 status for the third year in a row. ForeSee’s customer satisfaction ratings are done using the American Customer Satisfaction Index methodology, a sophisticated scientific formula developed at the University of Michigan that predicts consumer spending behavior. In December 2012, we ranked #3 in customer satisfaction in the ForeSee E-Retail Satisfaction Index (U.S. Holiday Edition 2012) which measured customer satisfaction with the top 100 e-retailers during the holiday shopping season.
In December 2012, we also received Google Trusted Store Status, an independent certification designating that the company offers excellent customer service and reliable shipping. In addition, we also hold an ‘Elite’ rating for superior customer service from STELLAService, an independent, third-party company that rates the customer service performance of online retailers. STELLAService ratings are performed using 300 unique customer service metrics and features for each online retailer across all areas of the shopping experience evaluating usability of the site, shipping, delivery and returns, and overall customer support.
Technology and Operations
Our website is supported by a technology infrastructure that is designed to provide a superior customer experience, including speed, ease of use and security. Our systems and tools allow us to monitor our website and services in real time, scale to size as required and balance traffic geographically across multiple sites. We also track and manage our inventory, order fulfillment, customer service and marketing through technologies that allow us to condense and distribute customer and sales data as part of our business intelligence management. From this data, our finance, marketing, operations and product development teams are able to optimize decision-making processes to analyze and project growth drivers and forecast demand.
We maintain strategic partnerships with vendors in the U.S. and abroad, a combination of in-house and external development, to ensure that we can rapidly deploy information technology solutions that we believe are key to our success—covering fulfillment, merchandising, supply chain management and back-office compliance. Our efforts are prioritized by the management team to map available capacity to have a positive strategic and tactical impact on the business.
Our technology infrastructure uses highly scalable, fault tolerant enterprise-standard technologies. We use a combination of internal and Tier 1 third party data centers that support product development, quality control, Ecommerce, CRM and customer experience. Coupled with the use of leading network technologies, virtualization and cloud-based solutions, we manage redundant coverage that significantly mitigates the risk of downtime.
We follow rigorous industry standards to protect our internal operations and the personal information we collect from our customers. We do not sell or disclose the personal information of our customers. We continue to maintain and upgrade our technology framework to support high levels of security while meeting the compliance requirements of Payment Card Industry (“PCI”) security standards. We are considered a “sender” under the CAN-SPAM Act and comply with the applicable aspects thereof.
We have installed technologically advanced finished goods inventory control systems to track our finished goods from receipt through shipment. All items are barcoded to facilitate electronic tracking allowing us to tie each SKU number back to our inventory control, shipping and sales departments. Our inventory control system analyzes and automatically recommends reorders for a majority of the products we sell, managing and mitigating out-of-stock situations. We consistently evaluate low volume items in order to minimize losses due to product expiration or obsolescence and to efficiently manage our warehouse space.
Competition
The nutrition and dietary supplement market is large, growing, competitive and highly fragmented. Our competition includes multi-level marketers, online VMHS specialty and mass retailers, and extends offline to brick and mortar stores including but not limited to grocery, membership clubs, specialty and mass retailers. We believe the following are the principal competitive factors in our market:
| • | Selection and availability of product |
| • | Reliability and speed of delivery |
| • | Customer service and support |
While we believe we compete favorably, the nature and extent to which our competitors implement various pricing and promotional activities in response to increasing competition and our response to these competitive actions, could adversely affect our profitability.
Trademark and Other Intellectual Property
We rely on a combination of patent, copyright and trademark and trade secret laws, confidentiality procedures and contractual provisions to protect our proprietary rights with respect to our technology and proprietary information. We have applied for or registered all relevant trademarks with the U.S. Patent and Trademark Office (USPTO), including our Vitacost, Nutraceutical Sciences Institute, Cosmeceutical Sciences Institute, Best of All, Walker Diet, Smart Basics, Take the Cost out of Healthy Living, Momonomics and Wellness Times trademarks, among others. We believe our trademarks to be valuable and are identified strongly with our brands. The issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all of the areas in which it is used. We have also applied for foreign protection of certain of our trademarks in the European and Asian markets in which we operate and have registered Vitacost, NSI and Nutraceutical Sciences Institute in certain countries in these regions.
Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage.
We have obtained a number of U.S. patents on some product formulations and have applications pending for others. In designing our product formulations, we have attempted to blend an optimal combination of nutrients which appear to have beneficial impact based upon scientific literature. However, because formal clinical studies have in most instances not been conducted by us to validate the intended health benefits of the nutrients, we are generally prohibited by the FDA from making disease treatment and prevention claims in the promotion of products using these formulations. While we seek broad coverage for our patents, there is always a risk that an alteration to the formulation may provide sufficient basis for a competitor to avoid infringement claims by us. In addition, our issued patents expire over the next several years and we cannot provide any assurance that any patents will be issued from pending applications or that any issued patents will adequately protect our intellectual property. We intend to reevaluate each of our registered U.S. patents for strength and value as renewal dates approach. We believe our patents and trademarks are valuable and provide us certain benefits in marketing our products. We intend to actively protect our patents, trademarks, trade secrets and other intellectual property.
Government Regulation
We are subject to federal and state consumer protection laws, including laws protecting the privacy of consumer non-public information and regulations prohibiting unfair and deceptive acts and trade practices. In particular, under federal and state financial privacy laws and regulations, we must provide:
| • | notice to consumers of our policies on sharing non-public information with third parties; |
| • | advance notice of any changes to our policies; and |
| • | with limited exceptions, provide consumers the right to prevent sharing of their non-public personal information with unaffiliated third parties. |
Furthermore, the growth and demand for online commerce could result in more stringent consumer protection laws that impose additional compliance burdens on online retailers. These consumer protection laws could result in substantial compliance costs and could interfere with the conduct of our business.
There is currently great uncertainty in many states whether or how existing laws governing issues such as property ownership, sales and other taxes, and libel and personal privacy applies to the Internet and commercial online retailers. These issues may take years to resolve. For example, tax authorities in a number of states, as well as a Congressional advisory commission, are currently reviewing the appropriate tax treatment of companies engaged in online commerce, and new state tax regulations may subject us to additional state sales and income taxes. New legislation or regulation, the application of laws and regulations from jurisdictions whose laws do not currently apply to our business, or a change in application of existing laws and regulations to the Internet and commercial online services could result in significant additional taxes on our business. These taxes could have a material adverse effect on our results of operations.
Our products are subject to extensive regulation in the U.S. and abroad. As applied to products Vitacost sells, FDA enforces the Federal Food, Drug and Cosmetic Act (“FDCA”), and related regulations, which govern the identity, purity, quality, strength, and composition of dietary supplements and regulate the formulation, manufacture, packaging, labeling, holding, sale, and distribution of dietary supplements, foods, cosmetics, medical devices, animal foods and drugs, and over-the-counter (“OTC”) allopathic and homeopathic human drugs (collectively “FDA regulated products”). FDA prohibits the distribution and/or sale of misbranded and/or adulterated FDA regulated products. In particular, FDA evaluates dietary supplements to determine the intention of the manufacturer or distributor through promotion, label or labeling claims which cause the products to be unapproved new drugs.
The Federal Trade Commission, or FTC, enforces the Federal Trade Commission Act, or FTCA, and related regulations, which govern the advertising associated with the promotion and sale of our products to prevent misleading or deceptive claims.
The U.S. Postal Inspection Service enforces federal laws governing fraudulent use of the mail. Regulation of certain aspects of the dietary supplement business at the federal level is also governed by the Consumer Product Safety Commission (“CPSC”) (e.g., concerning the presence of adulterated substances, such as toxic levels of lead or iron, that render products unsafe for consumption and require a CPSC ordered recall), the Department of Agriculture (e.g., for products that are intended for ingestion as dietary supplements for animals) and the Environmental Protection Agency (e.g., in the methods of disposal used for certain dietary ingredients, such as colloidal silver).
The manufacture, packaging, labeling, holding, sale, and distribution of dietary supplements are also subject to extensive local, state, and foreign government regulation. For example, under the European Union Directive, only dietary supplements listed in Annex II to that directive or otherwise ruled saleable in Europe by the European Union may be sold in Europe subject to EU restrictions on dosage amounts, forms, label claims and advertising. The Bureau of Customs and Border Patrol (“CBP”), a division of the Department of Homeland Security, also regulates shipments containing dietary ingredients, dietary supplements, cosmetics, drugs, biologics, and medical devices and engages in enforcement activity in concert with the FDA to block the import or export of articles deemed adulterated or otherwise unlawful for sale in the United States (imports) or in the non-U.S. country to which articles are addressed. CBP holds on articles or demands for recall can interfere with the timely delivery of products to market and can result in regulatory fines and penalties.
The FDCA has been amended several times affecting provisions that concern dietary ingredients and dietary supplements, including by the Dietary Supplement Health and Education Act of 1994 (“DSHEA”). DSHEA formally defined what may be sold as a dietary supplement, defined statements of nutritional support and the conditions under which they may lawfully be used, and included provisions that permit the FDA to regulate manufacturing practices and labeling claims peculiar to dietary supplements. “Dietary supplements” are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances that are used to supplement the diet, as well as concentrates, constituents, extracts, metabolites, or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market before October 15, 1994 may be used in dietary supplements without notifying the FDA. However, a “new” dietary ingredient (i.e., a dietary ingredient that was not marketed in the U.S. before October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without having been “chemically altered.” A new dietary ingredient notification must provide the FDA with evidence of a “history of use or other evidence of safety” which establishes that use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the new dietary ingredient can be marketed. There can be no assurance that the FDA will accept evidence purporting to establish the safety of any new dietary ingredients that we may want to market, and the FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients.
Increased FDA enforcement could lead the FDA to challenge dietary ingredients already on the market as “illegal” under the FDCA because of the failure to file a new dietary ingredient notification or because the substance may be one found to be the subject of an investigational new drug application for which clinical trials have commenced and been publicized.
The FDA generally prohibits labeling a dietary supplement with any “health claim” (i.e., any statement associating a nutrient with risk-reduction, but not treatment, of a disease or health-related condition), unless the claim is pre-approved by the FDA. The FDA prohibits entirely disease diagnosis, prevention and treatment claims when made for a dietary supplement. However, “statements of nutritional support,” including so-called “structure/function claims,” are permitted to be included in labeling for dietary supplements without FDA pre-approval. Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect the structure, function or well-being of the body, but such statements may not state that a dietary supplement will reduce the risk or incidence of a disease unless such claim has been reviewed and approved by the FDA. A company that uses a statement of nutritional support in labeling must possess evidence substantiating that the statement is truthful and not misleading. Such statements must be submitted to the FDA no later than thirty days after first marketing the product with the certification that they possess the necessary evidence and must be accompanied by an FDA mandated label disclaimer that “This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” There can be no assurance; however, that the FDA will not determine that a particular statement of nutritional support that we want to use is an unacceptable disease claim or an unauthorized nutrient-disease relationship claim otherwise permitted with FDA approval as a “health claim.” Such a determination might prevent the use of such a claim.
In addition, DSHEA provides that certain “third-party literature,” such as a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may “in connection with the sale of a dietary supplement to consumers” be exempt from labeling regulation. However, the FDA has adopted an “intent to use” doctrine whereby such literature even if exempt from labeling may nonetheless form the basis for an agency determination that the literature in context reveals a company intent to sell a dietary ingredient or dietary supplement as a drug, thereby rendering the supplement an unlawful, unapproved new drug. Because the “intent to use” doctrine is predicated on a subjective assessment of all facts and circumstances associated with the promotion and sale of a dietary supplement, we cannot know whether any particular piece of literature otherwise exempt from labeling will be deemed by the FDA unlawful for use in association with the sale of the dietary ingredient or dietary supplement.
As authorized by the FDCA, the FDA has adopted and implemented Good Manufacturing Practices (“GMPs”), specifically for dietary supplements. These GMPs impose extensive process controls on the manufacture, holding, labeling, packaging, and distribution of dietary supplements and the components of dietary supplements. They require that every dietary supplement be made in accordance with a master manufacturing record with all dietary ingredients verified by identity testing before use, that each step in manufacture, holding, labeling, packaging, and distribution be defined with written standard operating procedures, monitored, and documented, and that any deviation in manufacture, holding, labeling, packaging, or distribution be contemporaneously documented, assessed by a quality control expert, and corrected through documented corrective action steps (whether through an intervention that restores the product to the specifications in the master manufacturing record or to document destruction of the non-conforming product). The GMPs are designed to ensure documentation, including testing results that confirm the identity, purity, quality, strength, and composition of finished dietary supplements. In addition, GMPs require a company to make and keep written records of every product complaint that is related to GMPs. The written record of the product complaint must include the following: the name and description of the dietary supplement; the batch, lot, or control number of the dietary supplement, if available; the date the complaint was received and the name, address, or telephone number of the person making the complaint, if available; the nature of the complaint, including, if known, how the product was used; the reply to the complainant, if any; and findings of the company’s quality control investigation and follow-up action taken when an investigation is performed. The regulations directly affect all who manufacture the dietary supplements we sell and our distribution of dietary supplements. The FDA may deem any dietary supplement adulterated, whether presenting a risk of illness or injury or not, based on a failure to comply with any one or more process controls in the GMP regulations. If deemed adulterated, a dietary supplement may not be lawfully sold and may have to be recalled from the market. It is possible that the FDA will find one or more of the process controls implemented by our contract manufacturers, or by those whose dietary supplements we sell to be inadequate and, thus, requiring corrective action, requiring any one or more of the dietary supplements we sell to be unlawful for sale, or resulting in a judicial order that may impair our ability to market, and sell dietary supplements.
The FDA also requires adverse event notices on labels and serious adverse event reporting for all supplements and OTC drugs. An “adverse event” is defined by statute to include “any health-related event associated with the use of a dietary supplement that is adverse.” While all adverse event complaints received must be recorded in accordance with the GMPs discussed above, only serious adverse events must be reported to FDA. A “serious adverse event” is an adverse event that: results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, or a congenital anomaly or birth defect; or requires, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above. When a manufacturer, packer, or distributor whose name appears on the product label of a dietary supplement receives any report of a serious adverse event associated with the use of the dietary supplement in the United States, the company must submit a “serious adverse event report” on MedWatch Form 3500A. The report must be filed within 15 business days of receipt of information regarding the adverse event. All adverse event reports, whether serious or not, must be recorded and kept in company records under the GMP rules. A company must maintain records of each report of any adverse event (both serious and non-serious) for a minimum of 6 years. These records should include any documents related to the report, including: the company’s serious adverse event report to the FDA with attachments; any new medical information about the serious adverse event received; all reports to the FDA of new medical information related to the serious adverse event; and any communications between the company and any other person(s) who provided information related to the adverse event.
The regulation of dietary supplements may increase or become more restrictive in the future. There can be no assurance that, if more stringent statutes are enacted for dietary supplements, or if more stringent regulations are promulgated, we will be able to comply with such statutes or regulations without incurring substantial expense.
The FDA regulates the formulation, manufacturing, packaging, labeling and distribution of all OTC drugs. It allows OTC allopathic drug products to be sold without premarket approval so long as the products comply with FDA’s “monograph” system that specifies active drug ingredients that are generally recognized as safe and effective for particular uses. If an OTC drug is not in compliance with the applicable FDA monograph, the product generally cannot be sold without first obtaining FDA approval of a new drug application, which can be a long and expensive procedure.
The homeopathic OTC drugs that we sell are regulated by FDA as non-prescription, over-the-counter drugs. These products must generally meet the manufacturing standards set forth in the Homeopathic Pharmacopeia of the United States (HPUS) and the limitations for OTC ingredients. Claims made for them must not deviate from those contained in specific homeopathic treatises recognized by the FDA as appropriate for use. The products must be labeled in compliance with FDA regulations for all OTC drugs. If these requirements are not met, the FDA can consider the products unapproved new drugs and prohibit their sale.
Cosmetics are not subject to pre-market approval by the FDA, but the products, their ingredients and their label and labeling content, are regulated by the FDA, and it is the burden of those who sell cosmetics to ensure that they are safe for use as directed. The FDA prohibits certain ingredients from being contained in cosmetic products that are authorized only for drug use or are deemed adulterated. In addition, the labeling of cosmetic products is subject to the requirements of the FDCA, the Fair Packaging Labeling Act and other FDA regulations. The FDA limits cosmetic product claims to those of beautification and enhancement to the external appearance of the skin. Structure/function claims are generally prohibited for cosmetic products as are disease prevention and treatment claims. It is possible that cosmetic product ingredients now commonly in use that are derived from nanotechnology may be restricted or prohibited in future. It is also possible that claims now commonly in use concerning cosmetic reduction in the external appearance of aging, the effect of cosmetic ingredients on fine lines and wrinkles, or on other aspects of appearance may in the future be deemed prohibited, implied disease treatment claims.
Animal foods are also regulated by FDA and each state’s department of agriculture. Those regulators generally defer to a product’s compliance with the “American Association of Feed Control Officials” annual manual that sets out labeling requirements, approved ingredients, and nutrient needs for particular animal types including companion animals. FDA generally exercises enforcement discretion and allows some animal foods to make structure function claims. However, there is no legal authority in the FDCA for animal foods to make those types of claims. It is possible in the future that FDA may consider all structure/function claims prohibited.
Medical devices that we sell are OTC and not subject to pre-market approval but may be subject to a notification requirement (called a 510k) or a premarket compliance requirement, like an OTC monograph. Manufacturers of medical devices must register and list their products with FDA annually whether they are located domestically or overseas. All medical devices must be manufactured under good manufacturing standards called “quality assurance” standards set by FDA. Medical devices must be labeled in accordance with FDA’s general device labeling requirements and whatever particular label requirements FDA may designate for that type of device. When a company receives a positive response to a 510k notification, if the company promotes the product with any deviation from the labeling and claims that were submitted to the agency, the agency may consider the product misbranded. Similarly, if the company changes the design or performance of the device in any way from the notification then FDA may consider the product adulterated.
Conventional food manufacturing, labeling and distribution is also under FDA jurisdiction. Manufacturers must ensure their products are produced in a clean environment to prevent adulteration by contamination and must manufacture their products with either approved food additives or ingredients that are “generally recognized as safe” (GRAS). Conventional foods must meet FDA’s labeling requirements including a Nutrition Facts statement. Conventional foods may make structure/function statements, like dietary supplements but unlike dietary supplements are not required to file notice of those statements with FDA. Like dietary supplements, infant formulas are regulated by FDA as a type of food with their own requirements for manufacturing and labeling. Infant formulas, however, are required to go through a preapproval process, manufacturing inspection prior to product launch, and are held to a nutritional sufficiency standard unlike other foods due to the nature of this type of product.
Conventional foods and dietary supplements must also comply with the Organic Act (for the designation of organic ingredients) enforced by the US Department of Agriculture and the Food Allergen Labeling and Consumer Protection Act of 2004 (“FALCPA”) enforced by the FDA. Under the Organic Act there are specific requirements for the certification of ingredients as “organic” and requirements on how to use the term “organic” in labeling, whether for an ingredient or a complete product. USDA has also issued an enforcement policy applying the use of the term “organic” to cosmetics and their ingredients even though there is no law authorizing that use. Under FALCPA, all packaged foods (including supplements) containing any of the eight identified major food allergens (milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, and soybeans) must declare such allergens, at least once, by their common or usual name in the ingredient list or in a “CONTAINS” statement immediately following the ingredient list in bold. A packaged food “contains” an allergen for the purposes of the law when any intentionally added ingredient contains an allergen. Thus, even if an allergen is present only in a coloring or flavoring, FALCPA applies. Likewise, the law applies even if an allergen is present only in a small amount of an “incidental” (but intentionally added, non-cross-contact) additive, like a releasing agent. Disclosure in this case is required even though such an ingredient usually could be omitted altogether from the ingredients list. If a manufacturer believes that no allergen is present there is a preapproval process to seek an exception. Failure to comply with the FALCPA may lead to civil sanctions, criminal penalties, or both under the FDCA. In addition, the FDA is authorized to seize non-conforming products controlled by a company and issue a recall of products already on the market.
The FDA has broad authority to enforce the provisions of the FDCA concerning all of the products it regulates, including powers to issue a public “warning letter” to a company to quarantine and prohibit the sale of products deemed adulterated or misbranded, to publicize information about illegal products, to request a voluntary recall of illegal products from the market, to request that the Department of Justice initiate a seizure action, an injunction action or a criminal prosecution in U.S. courts, and to seek disgorgement from a federal court of all proceeds received from the sale of products deemed misbranded or adulterated.
The FTC exercises jurisdiction over the advertising of dietary supplements, OTC drugs, medical devices, and cosmetics. In recent years, the FTC has instituted numerous enforcement actions against dietary supplement companies for making false or misleading advertising claims and for failing to adequately substantiate claims made in advertising. These enforcement actions have often resulted in consent decrees and the payment of civil penalties and/or restitution by the companies involved. The FTC also regulates other aspects of consumer purchases including, but not limited to, promotional offers of savings compared policies, telemarketing, continuity plans, and “free” offers.
We are also subject to regulation under various state, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements, animal foods, medical devices, conventional foods, and OTC drugs. For example, under Proposition 65 in the State of California, a list of substances are deemed to pose a risk of carcinogenicity or birth defects at or above certain levels. If any such ingredient exceeds the permissible levels in a dietary supplement, cosmetic, or drug, the product may be lawfully sold in California only if accompanied by a prominent warning label alerting consumers that the product contains an ingredient linked to cancer or birth defect risk. Private attorney general actions as well as California attorney general actions may be brought against non-compliant parties and can result in substantial costs and fines. In additional California has a law called the “Consumers Legal Remedies Act” (Cal. Civ. Code § 1750 et seq) that allows private parties to assert a class action claim for false or deceptive advertising. It is typically asserted in combination with claims for false advertising and unfair competition under the California Business and Professions Code. California law firms specializing in these type of consumer class action claims have recently been targeting dietary supplement and OTC homeopathic drug makers and sellers of products sold in California, claiming injury based on the products’ failure to deliver results as claimed in product labeling and promotion.
Government regulations in foreign countries may prevent or delay the introduction, or require the reformulation, of certain of our products. Compliance with such foreign governmental regulations is generally the responsibility of our distributors in those countries. These distributors are independent contractors whom we do not control.
In addition, from time to time in the future, we may become subject to additional laws or regulations administered by the FDA, the FTC, or by other federal, state, local or foreign regulatory authorities, to the repeal of laws or regulations that we generally consider favorable, such as DSHEA, or to more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations, and we cannot predict what effect additional governmental regulation, if and when it occurs, would have on our business in the future. Such developments could, however, require reformulation of certain products to meet new standards, recalls or discontinuance of certain products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, additional personnel or other new requirements. Any such developments could have a material adverse effect on our business.
Europe. The European Union (“EU”) is responsible for the development of legislation governing foods, nutritional supplements, and medicines sold in Europe. Member States of the EU (“Member States”) are authorized to develop local legislation governing these products, provided such legislation is not more restrictive than the legislation promulgated by the EU. Member States are responsible for enforcement of the applicable legislation. In 2002, the EU established a process for Member States to bring this regulating legislation in line with a published directive of the EU, which addressed the labeling and marketing of vitamins and minerals, what nutrients are permitted or not permitted and other packaging requirements. In 2004, the EU established standards for the manufacture and marketing of herbal medicines with the Traditional Herbal Medicinal Products Directive. This requires, among other things, manufacturers of herbal medicinal products to comply with Pharmaceutical Group Standards, and only requires proof of safety, not efficacy.
In 2006, the EU adopted its Commission Directive 2006/37/EC, amending its Directive 2002/46/EC. Under the amended directive, only nutrients listed in Annex II, or approved by subsequent order of the EU, may be lawfully sold in Member States. The EU also regulates labels, labeling, and advertising associated with the promotion and sale of dietary supplements in Europe. These regulations may make it unlawful for us to sell in Europe certain products lawfully labeled and sold in the United States, adversely affecting the finances of the business.
In the United Kingdom, the principal governing legislation is the Food Safety Act of 1990 (governing safety of food products) and the Medicines Act of 1968 (governing licensing and sale of medicine). Further guidance is provided by numerous Statutory Instruments addressing the formulation, purity, packaging, advertising and labeling of such products. Medicinal products are regulated and enforced by the Medicines and Healthcare Products Regulatory Agency (MHRA), an agency of the Department of Health. The MHRA determines if an herbal remedy is medicinal by virtue of its “presentation” or “function.” Food products are regulated by the Food Standard Agency (FSA), which reports to the Department of Health and to the Department of Environment, Food and Rural Affairs. Vitamin and mineral supplements and soup products with herbal ingredients are generally considered food supplements and are subject to the purview of the FSA. Additional legislative standards have been adopted in the other EU countries, typically similar in scope to the UK. The regulatory scheme in Canada is similar but not identical to that of the U.S. concerning medicines and healthcare products or material health products and is regulated by Health Canada.
Employees
As of December 31, 2012, we had 671 full-time employees, 78 of which work for a third-party manufacturer based on a manufacturing outsourcing agreement that we entered into effective September 1, 2012. Additionally, we maintain temporary employees primarily in our fulfillment centers to meet changing demands of that function. None of our employees are covered by a collective bargaining agreement and we are unaware of any union organizing efforts. We consider our relationship with our employees to be good.
Geographic Information
During our last three years, substantially all of our revenue was generated within North America, and all of our long-lived assets are located within the United States. However, we believe International represents a large long-term opportunity for us with international revenue accounting for 6%, 5%, and 3% of total revenue in 2012, 2011 and 2010, respectively.
Available Information
Our Web site iswww.vitacost.com . The information posted on our website is not incorporated into this Annual Report on Form 10-K. We have made available through our Web site, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports, as soon as reasonably practicable after we electronically file such materials with, or furnish them to, the Securities and Exchange Commission. In addition, they are available directly on the website for the Securities and Exchange Commission.
Item 1A. Risk Factors
We operate in a rapidly changing environment that involves significant risks, a number of which are beyond our control. In addition to the other information contained in this Annual Report on Form 10-K, the following discussion highlights some of these risks and the possible impact of these factors on our business, financial condition and future results of operations. If any of the following risks actually occur, our business, financial condition or results of operations may be adversely impacted. In addition, these risks and uncertainties may impact the forward-looking statements described elsewhere in this Form 10-K and in the documents incorporated herein by reference. They could affect our actual results of operations, causing them to differ materially from those expressed in forward-looking statements.
Risks Relating to Our Business
If we are unable to effectively manage our growth plan, our business strategy, financial condition, results of operations, or cash flows could be materially adversely affected.
Our growth plan requires significant management time and operational and financial resources. There is no assurance that we have the operational and financial resources to successfully manage our growth. In addition, rapid growth in our headcount and operations may place a significant strain on our management and our administrative, operational and financial infrastructure. Failure to adequately manage our growth could have a material adverse effect on our business, financial condition, results of operations, or cash flows.
We operate in a highly competitive industry, and our failure to compete effectively could materially adversely affect our market share, financial condition and growth prospects.
The U.S. health products industry is a large and highly fragmented industry. Our competitors include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, online merchants, mail-order companies and a variety of other participants in the industry. The principal elements of competition in the industry are price, selection and distribution channel offerings. We believe that the market is also highly sensitive to the introduction of new products, including various prescription drugs, which may rapidly capture a significant share of the market. In the United States, we also compete for sales with heavily advertised national brands manufactured by large pharmaceutical and food companies, as well as other retailers. In addition, as some products gain market acceptance, we experience increased competition for those products as more participants enter the market. Certain of our competitors are larger than us and have longer operating histories, larger customer bases, greater brand recognition and greater resources for marketing, advertising and product promotion. They may be able to secure inventory from vendors on more favorable terms, operate with a lower cost structure or adopt more aggressive pricing policies. In addition, our competitors may be more effective and efficient in introducing new products. We may not be able to compete effectively, and our attempt to do so may require us to increase marketing and/or reduce our prices, which may result in lower margins. Failure to effectively compete could materially adversely affect our market share, financial condition and growth prospects.
If we are unable to sufficiently increase our revenue to offset increased costs as we expand our business, we may experience operating losses, net losses or negative cash flows.
We expect operating expenses and working capital requirements to increase substantially as we expand our business. We expect our costs of product development, sales and marketing, research and development and general and administrative expenses to increase substantially as a result of our growth. If we are unable to continue to sufficiently increase our revenue to offset these increased costs, we will not maintain profitability and may experience operating losses, net losses or negative cash flows.
If we are unable to retain key personnel, our business, financial condition or results of operations could be materially adversely affected.
Our success depends to a significant degree upon the continued contributions of our executive officers and other key personnel. Although we have employment agreements with our executive officers, we cannot guarantee that such persons will remain affiliated with us. If any of our key personnel were to cease their affiliation with us, our operating results could suffer. Further, we do not maintain key person life insurance on any of our executive officers. If we are unable to retain the services of key personnel, our business, financial condition or results of operations could be materially and adversely affected.
Our network and communications systems are vulnerable to system interruption and damage, which could limit our ability to operate our business and could have a material adverse effect on our business, financial condition or results of operations.
Our ability to receive and fulfill orders promptly and accurately is critical to our success and largely depends on the efficient and uninterrupted operation of our computer and communications hardware and software systems. We experience periodic system interruptions that impair the performance of our transaction systems or make our website inaccessible to our customers. These system interruptions may prevent us from efficiently accepting and fulfilling orders, sending out promotional emails and other customer communications in a timely manner, introducing new products and features on our website, promptly responding to customers, or providing services to third parties. Frequent or persistent interruptions in our services could cause current or potential customers to believe that our systems are unreliable, which could cause them to avoid our website, drive them to our competitors, and harm our reputation. To minimize future system interruptions, we must continue to improve our systems and network infrastructure to accommodate increases in website traffic and sales volume and to replace aging hardware and software. We may be unable to promptly and effectively upgrade and expand our systems and integrate additional functionality into our existing systems. In addition, upgrades to our systems may cause existing systems to fail or operate incorrectly. Any unscheduled interruption in our services could result in fewer orders, additional operating expenses, or reduced customer satisfaction, any of which would harm our business, financial condition and operating results. In addition, the timing and cost of upgrades to our systems and infrastructure may substantially affect our ability to maintain profitability.
Our systems and operations and those of our suppliers and Internet service providers are vulnerable to damage or interruption from fire, flood, earthquakes, power loss, server failure, telecommunications and Internet service failure, acts of war or terrorism, computer viruses and denial-of-service attacks, physical or electronic break-ins, sabotage, human error and similar events. Any of these events could lead to system interruptions, order fulfillment delays, and loss of critical data for us, our suppliers, or our Internet service providers, and could prevent us from accepting and fulfilling customer orders. Any significant interruption in the availability or functionality of our website or our customer processing, distribution, or communications systems, for any reason, could seriously harm our business, financial condition, and operating results.
We depend primarily upon search engines and other online sources to increase traffic to our website, and need to convert this traffic into customers in a cost-effective manner; our failure to do so could have a material adverse effect on our business, financial condition or results of operations.
Our success depends on our ability to attract visitors to our website and convert them into customers in a cost-effective manner. We utilize search engines and other online sources as a means to direct traffic to our website. Our website is included in search results as a result of both paid search listings, where we purchase specific search terms that result in the inclusion of our website in the search result, and algorithmic searches that depend upon the searchable content in our website. Search engines and other online sources revise their algorithms from time to time in an attempt to optimize their search results.
If one or more of the search engines or other online sources which we use to direct traffic to our website were to modify its general methodology for how it displays our website, fewer visitors may visit our website, which could have a material adverse effect on our business and results of operations. Further, if any free search engine which we use to direct traffic to our website begins charging fees for listing or placement, or if one or more of the search engines or other online sources on which we rely for purchased listings, modifies or terminates its relationship with us, the traffic to our website could decrease and our expenses could increase which could have a material adverse effect on our business, financial condition or results of operations.
Taxation risks could subject us to liability for past sales, increase our costs and cause our future sales to decrease.
One or more states or foreign countries may seek to impose sales or other tax collection obligations on out-of-jurisdiction eCommerce companies. Certain states have imposed such a sales tax obligation requirement on online retailers that use residents of that state to directly or indirectly refer potential customers, via a link on an Internet website or otherwise, to the online retailer. A successful assertion by one or more states or foreign countries that we should collect sales or other taxes on the sale of merchandise or services could result in substantial tax liabilities for past sales, decrease our ability to compete with traditional retailers and otherwise harm our business, financial condition or results of operations.
We do not collect sales or other taxes on shipments into most states in the U.S. Currently, U.S. Supreme Court decisions restrict the imposition of obligations to collect state and local sales and use taxes with respect to sales made over the Internet. However, a number of states, as well as the U.S. Congress, have been considering initiatives that could limit or supersede the Supreme Court’s position regarding sales and use taxes on Internet sales. If any of these initiatives were successful, we could be required to collect sales and use taxes in additional states. The imposition by state and local governments of various taxes upon Internet commerce could create administrative burdens for us, reduce our competitive advantage over traditional retailers and decrease our future sales. Our warehousing centers, and any future expansion of them, along with other aspects of our evolving business, may result in additional sales and other tax obligations.
We rely on third-party carriers as part of our inventory fulfillment and order delivery processing, and these third parties may fail to meet shipping schedules or requirements, which could damage our reputation and reduce our sales and our margins.
We cannot control all of the factors that might affect our timely and cost-effective procurement of products from our vendors and delivery of our products to our customers. We rely on third-party carriers both for the delivery of inventory and for the shipment of our products to our customers. Consequently, we are subject to risks associated with these carriers, including increased fuel costs, security concerns, labor disputes, union organizing activity and inclement weather. Any disruption in the ability of these carriers to timely deliver products to us and to our customers could damage our reputation and brand and result in customer dissatisfaction. This could, in turn, materially and adversely affect our business, financial condition or results of operations.
We could be harmed by data loss or other security breaches.
Our servers are vulnerable to computer viruses, physical or electronic break-ins and similar disruptions, which could lead to interruptions and delays in our service and operations as well as loss, misuse or theft of data. Any attempts by hackers to disrupt our service or our internal systems, if successful, could harm our business, be expensive to remedy and damage our reputation. Although we have developed systems and processes that are designed to protect customer information and prevent data loss and other security breaches, such measures cannot provide absolute security. In addition, we rely on third party technology and systems in certain aspects of our businesses, including encryption and authentication technology to securely transmit confidential information. Any significant disruption to our service or internal computer systems could adversely affect our business and results of operations.
Complying with new and existing government regulation, both in the U.S. and abroad, could significantly increase our costs.
The processing, formulation, packaging, labeling, advertising, distribution and sale of our products are subject to regulation by several U.S. federal agencies, including the FDA, the FTC, the Postal Service, the Consumer Product Safety Commission, the Department of Agriculture and the Environmental Protection Agency, as well as various state, local and international laws and agencies of the localities in which our products are sold. Government regulations may prevent or delay the introduction or require the reformulation of our products.
The FDA regulates, among other things, the manufacture, composition, safety, labeling, marketing and distribution of dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for human use), OTC drugs (allopathic and homeopathic), conventional foods, infant formulas, animal foods, medical devices, and cosmetics. The FDA may not accept the evidence of safety we present for new dietary supplements we wish to market, or they may determine that a particular dietary supplement or ingredient that we currently market presents an unacceptable health risk. If that occurs, we could be required to cease distribution of and/or recall supplements or products containing that ingredient. In July 2011 the FDA introduced a draft Guidance Document called“Guidance for Industry: Dietary Supplements: New Dietary Ingredient Notifications and Related Issues ” relating to pre-market safety notifications. In it FDA identified standards of proof for safety and ‘grandfathering’ that would impose new burdens on the dietary supplement industry and may impact the legality of some of our existing products. FDA has accepted industry comments on the guidance but has not yet published its response or otherwise finalized the draft.
A draft guidance is not considered a final rule by the FDA. In June 2012, the FDA stated that it planned to issue a revised draft guidance to clarify controversial points in the original version. Nevertheless, in enforcement actions taken by the agency, it has adhered to the basic interpretation expressed in the draft guidance even before the draft guidance has been finalized. Consequently, ingredients previously considered lawfully saleable without submission of a notice containing proof of safety may now need to be considered adulterated and unsaleable unless and until FDA fails to object on grounds of safety to a New Dietary Ingredient notice.
The FDA and the Federal Trade Commission (FTC) may also determine that certain labeling, advertising and promotional claims, statements or activities are not in compliance with applicable laws and regulations and may determine that a particular statement is an unapproved health claim, an unacceptable drug claim, a false or misleading label claim, or a deceptive advertising claim. Failure to comply with FDA or other regulatory requirements could prevent us from marketing particular dietary supplement products or subject us to administrative, civil or criminal penalties.
Legislation has been introduced in the U.S. Senate seeking to provide the FDA with increased authority to regulate dietary supplements and increase labeling requirements with respect to dietary supplements. The Food Safety Modernization Act (FSMA) now requires dietary supplement companies to register with the agency and renew those registrations biannually. The FSMA permits summary revocation of registrations (and elimination of the right to sell products in interstate commerce) based on findings by the FDA that a product might present an unreasonable risk of serious illness, injury, or death. Legislation introduced but not passed by Congress would require the FDA Commissioner to obtain a list of all ingredients and claims for dietary supplements and distinguish from among them which products are potentially unsafe and which claims are misleading. FDA and FTC are cooperating in joint enforcement projects, including the issuance of warning and enforcement letters by both agencies. The FTC staff has demanded two clinical trials as a condition precedent for the making of certain categories of claims, such as those concerning weight loss and those concerning immune support, which position, while struck down by an FTC Administrative Law Judge, is pending on appeal before the Federal Trade Commission.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted numerous enforcement actions against dietary supplement companies for failing to have adequate substantiation for claims made in advertising or for using false or misleading advertising claims. The FTC routinely polices the market for deceptive dietary supplement advertising and accepts and reviews complaints from the public concerning such advertising.
The FTC also regulates deceptive advertising claims and promotional offers of savings compared to “regular” prices. The National Advertising Division (“NAD”), of the Council of Better Business Bureaus oversees an industry-sponsored self-regulatory system that permits competitors to resolve disputes over advertising claims, including promotions for savings off of regular prices. The NAD has no enforcement authority of its own but may refer promotions to the FTC that the NAD views as violating FTC guidelines or rules. Violations of these orders could result in substantial monetary penalties.
In Europe, non-compliance by us or others of relevant legislation can result in regulators bringing administrative or, in some cases, criminal proceedings. For example, in the U.K., it is common for regulators, including the Medicines and Healthcare Products Regulatory Agency Enforcement & Intelligence Group, to prosecute retailers and manufacturers for non-compliance with legislation governing foodstuffs and medicines. European Union (EU) regulations and directives are implemented and enforced by individual member states and, so, enforcement priorities and applicable law can occur in multiple countries at one time. Failure by us, the manufacturers or suppliers to comply with applicable legislation could result in prosecution and have a material adverse effect on our business, financial condition and results of operations.
Europe has adopted broad regulations and directives on health and nutrition claims. These regulations cover claims that can be made for foods (including supplements). Certain claims, such as those regarding general well-being, behavioral functions and weight-loss, may be prohibited or require prior approval. Unless subject to derogation, products that include certain claims cannot be lawfully marketed in EU member states absent preapproval. Applicable derogations under EU directives can enlarge the period within which we may seek approval for products containing claims up through January 19, 2014. An approval must proceed through the European Food Safety Authority (EFSA), and the process includes the submission of a detailed dossier in support of the product claims. Lengthy delays within the new EU framework have been reported. This may severely impact our European marketing and expansion efforts. As of December 14, 2012, the transitional period ends for products which were on the market when Regulation 1924/2006 entered into force in 2007, but which did not comply with its provisions. The currently adopted list of claims includes 222 approved general function claims for use in the European Union. Claims considered “on hold” pending a final decision may continue to remain in the market under the responsibility of the food business operator in question, provided those claims comply with applicable national provisions. Presently, the assessments of health claims for plant and herbal substances—the so-called “botanical” substances—are “on hold.”
We are also anticipating the implementation of an EU directive relating to food supplements, which could significantly impact the formulation of our products. The implementation of this directive is expected to include dosage restrictions for certain vitamin and mineral supplements and may lead to some of our products being recalled or discontinued. Thus far, the maximum and minimum levels of vitamins and minerals present in food supplements per daily portion consumed have not been harmonized and, so, national legislation of Member States remains significant.
In addition, an EU Directive governing product safety requires manufacturers to notify regulators about unsafe products and gives regulators in each member state the power to order product recalls. As a result, the number of product recalls in Europe has increased substantially. A product recall in Europe could have a material adverse effect on our business, financial condition and results of operations.
Our ability to access capital markets may be adversely affected which could limit our ability to fund our operating costs if we do not generate sufficient cash from operations.
From time to time, we may need to access debt or equity capital markets in order to execute on our growth strategy. Any restriction on our ability to access capital markets could limit our ability to pursue our growth strategy and could materially adversely affect our business, financial condition or results of operations.
The current global economic climate could adversely affect our industry and, therefore, restrict our future growth.
The current global economic climate could negatively affect our sales because many consumers consider the purchase of our products discretionary. If the markets for our products significantly deteriorate due to the economic climate, our business, financial condition or results of operations could be materially and adversely affected.
Our failure to efficiently respond to changing consumer preferences and demand for new products and services could significantly harm our product sales, inventory management and customer relationships and our business, results of operations and financial condition could be materially adversely affected.
Our continued success depends, in part, on our ability to anticipate and respond to changing consumer trends and preferences. We may not be able to respond in a timely or commercially appropriate manner to these changes. Our failure to accurately predict these trends could negatively impact our inventory levels, sales and consumer opinion of us as a source for the latest products. The success of our new product offerings depends upon a number of factors, including our ability to:
| • | accurately anticipate customer needs, |
| • | innovate and develop new products, |
| • | successfully commercialize new products in a timely manner, |
| • | competitively price our products, |
| • | procure and maintain products in sufficient volumes and in a timely manner; and |
| • | differentiate our product offerings from those of our competitors. |
If we do not introduce new products, make enhancements to existing products or maintain the appropriate inventory levels to meet customers’ demand in a timely manner, our business, results of operations and financial condition could be materially adversely affected.
Unfavorable changes to government regulations or adverse consumer attitudes about online commerce could have a material adverse effect on our business, financial condition or results of operation by impeding the growth and use of the Internet and thereby decreasing revenue.
As the role and importance of online commerce has grown in the U.S., there have been continuing efforts to increase the legal and regulatory obligations and restrictions on companies conducting commerce through the Internet, primarily in the areas of taxation, consumer privacy, restrictions on imports and exports, customs, tariffs, user privacy, data protection, pricing, content, copyrights, distribution, electronic contracts and other communications, consumer protection, the provision of online payment services, broadband residential Internet access and the characteristics and quality of products and services, which could increase the cost of conducting business over the Internet. In addition, consumer unwillingness or inability to use the Internet to conduct business, due to adverse regulation, security concerns, service interruptions or otherwise, could materially reduce our growth. Governmental laws and regulations or adverse attitudes about online commerce could increase the costs and liabilities associated with our online commerce activities, increase the price of our product to consumers, or reduce traffic to our website. Unfavorable resolution of these issues could have a material adverse effect on our business, financial condition or results of operations.
Laws or regulations relating to privacy and data protection may materially adversely affect the growth of our business or our marketing efforts, which could reduce our sales and cause us to incur significant costs.
We are subject to increasing regulation at the federal, state and international levels relating to privacy and the use of personal user information. For example, we are subject to various telemarketing laws that regulate the manner in which we may solicit future suppliers and customers. Such regulations, along with increased governmental or private enforcement, may increase the cost of growing our business. In addition, many jurisdictions have laws that limit the use of personal information gathered online or offline or require companies to establish privacy policies. The FTC has adopted regulations regarding the collection and use of personal identifying information obtained from children under thirteen years of age. Proposed legislation in this country and existing laws in foreign countries require companies to establish procedures to notify users of privacy and security policies, obtain consent from users for the collection and use of personal information, and/or provide users with the ability to access, correct and delete personal information stored by us. From time to time, Congress has proposed legislation regarding data security and privacy protection. Any enacted data protection regulations may restrict our ability to collect demographic and personal information, which could be costly or harm our marketing efforts, and could require us to implement new and potentially costly processes, procedures and/or protective measures.
Third parties could use our trademarks as keywords in Internet search engine advertising programs, which may direct potential customers to competitors’ websites, which, in turn, could result in decreased sales and could harm our reputation.
Competitors and other third parties could infringe on our trademarks and confusingly similar terms as keywords in Internet search engine advertising programs and in the resulting sponsored link advertisements which may divert potential customers to their websites. Preventing such unauthorized use is difficult. Further, the legal precedent on whether such activity infringes on our intellectual property varies significantly within the United States and in other countries. If we are unable to protect our trademarks or confusingly similar terms from such unauthorized use, competitors and other third parties could drive potential online customers away from our website, which could result in a loss of sales and have a material adverse effect on our business, financial condition or results of operations.
We are subject to a number of risks related to credit card payments we accept which, if we fail to be in compliance with applicable credit card rules and regulations, will result in additional fees, fines and ultimately the revocation of the right to use the credit card company, which could have a material adverse effect on our business, financial condition or results of operations.
For credit and debit card payments, we pay interchange and other fees, which may increase over time and raise our operating costs and lower our profit margins. We are also subject to payment card association operating rules, certification requirements and rules governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our customers. In addition, we have and may continue to suffer losses as a result of orders placed with fraudulent credit and debit card data. We do not carry insurance against the risk of credit card fraud, so the failure to adequately control fraudulent credit card transactions could reduce our net revenue and our gross profit percentage. We have implemented technology to help us detect the fraudulent use of credit card information. Under current practices, a merchant is liable for fraudulent credit card transactions when the merchant does not obtain a cardholder’s signature. A failure to adequately control fraudulent credit card transactions would result in significantly higher credit card-related costs and could have a material adverse effect on our business, financial condition or results of operations.
The content of our website and direct mailing pieces could expose us to significant liability which could reduce our profits.
Because we post product information and other content on our website and in our direct mailing pieces, we face potential liability for, among other things, copyright infringement, patent infringement, trademark infringement, defamation, unauthorized practice of medicine, false or misleading advertising and other claims based on the nature and content of the materials we post. Although we maintain general liability insurance, our insurance may not cover potential claims of this type or may not be adequate to cover us for all liability that may be imposed. Any imposition of liability that is not covered by insurance, or is in excess of insurance coverage, could materially adversely affect our business, financial condition or results of operations.
Our failure to protect our intellectual property rights could reduce our sales and increase our costs.
We have applied for or registered all relevant trademarks with the U.S. Patent and Trademark Office, including our Vitacost, Nutraceutical Sciences Institute, Cosmeceutical Sciences Institute, Best of All, Walker Diet, Smart Basics, Take the Cost out of Healthy Living, Momonomics and Wellness Times trademarks, among others. We believe our trademarks to be valuable and are identified strongly with our brands. Issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all of the areas in which it is used. We have also applied for foreign protection of certain of our trademarks in the European and Asian markets in which we operate and have registered Vitacost, NSI and Nutraceutical Sciences Institute in certain countries in these regions.
Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We believe our trademarks are valuable and provide us certain benefits in marketing our products. We intend to actively protect our trademarks, trade secrets and other intellectual property.
We may in the future be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses, be involved in protracted litigation or prevent us from selling or using some aspect of our products. Claims of intellectual property infringement may also require us to enter into costly royalty or license agreements. Alternatively, we may be unable to obtain necessary royalty or license agreements on terms acceptable to us, if at all. Claims that our technology or products infringe on intellectual property rights of others could be costly and would divert the attention of our management and key personnel, which in turn could materially adversely affect our business, financial condition or results of operations.
Our inventory is concentrated in two warehouse locations, which exposes us to the risk of natural disasters or other force majeure events. Losses at either location could materially adversely affect our product distributions, sales and consumer satisfaction.
Our two distribution warehouses are located in Lexington, North Carolina and Las Vegas, Nevada. Any significant disruption in either of these locations for any reason, such as a power failure, equipment breakdown, workforce disruption, or natural or similar disasters, could materially adversely affect our product distributions, sales and consumer satisfaction.
Insurance coverage, even where available, may not be sufficient to cover losses we may incur, which could increase our costs and lower our profits.
Our business exposes us to the risk of liabilities arising out of our products and operations. For example, we may be liable for claims brought by users of our products or by employees, customers or other third parties for personal injury or property damage occurring in the course of our operations. Our operations are subject to closure and loss due to power outages, Internet and telephone line failures, work stoppages and acts of nature. We seek to minimize these risks through various insurance policies from third-party insurance carriers. However, our insurance coverage is subject to large individual claim deductibles, individual claim and aggregate policy limits and other terms and conditions. Our estimate of retained-insurance liabilities is subject to change as new events or circumstances develop that might materially impact the ultimate cost to settle these losses. We cannot assure you that our insurance will be sufficient to cover our losses. We do not view insurance, by itself, as a material mitigant to these business risks. Any losses that are not completely covered by our insurance could have a material adverse effect on our business, financial condition or results of operations.
The insurance industry has become more selective in offering certain types of liability insurance coverage, and we may not be able to maintain our existing coverage or obtain increased coverage in the future, which could increase our costs and reduce our profits.
The insurance industry has become more selective in offering certain types of insurance, including product liability, product recall and property casualty insurance. While we believe our current insurance policies provide us adequate coverage for our current business operations, there can be no assurance that we will be able to maintain such coverage or obtain comparable coverage on terms and conditions favorable to us, if at all. Further, as we expand our business, we expect to correspondingly increase our insurance coverage, and there can be no assurance that we will be able to obtain such increased coverage if and when needed.
We may not be able to maintain the uniqueness of our domain name, which may result in confusion to existing and new customers and lost sales and, therefore, could have a material adverse effect on our business, financial condition or results of operations.
Maintaining our Internet domain name is critical to our success. Under current domain name registration practices, no other entity may obtain an identical domain name but can obtain a similar or identical name with a different suffix, such as “.net” or “.org,” or with a different country designation, such as “.jp” for Japan.
We have not registered our domain name with each of the suffixes or jurisdictions available. As a result, third parties may use domain names that are similar to our domain name, which may result in confusion to existing and new customers and lost sales. Failure to maintain our domain name’s uniqueness could have a material adverse effect on our business, financial condition or results of operations.
Our quarterly results of operations may fluctuate in the future. As a result, we may fail to meet the expectations of investors or securities analysts, which could cause our stock price to decline.
Our quarterly revenue and results of operations may fluctuate as a result of a variety of factors, many of which are outside of our control. If our quarterly revenue or results of operations fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Fluctuations in our results of operations may be due to a number of factors, including, but not limited to, those listed below and identified throughout this “Risk Factors” section in this Annual Report:
| • | our ability to retain and increase sales to existing customers and attract new customers; |
| • | changes in the volume and mix of dietary supplements and health and wellness products sold in a particular quarter; |
| • | the timing and success of new dietary supplement introductions or reformulations by us or our competitors; |
| • | changes in our pricing policies or those of our competitors; |
| • | competition, including entry into the market by new competitors including traditional brick and mortar retailers and new product offerings by existing competitors; |
| • | the amount and timing of expenditures related to expanding our operations, research and development or introducing new products; |
| • | changes in the payment terms for our products and services; and |
| • | the purchasing cycles of our customers. |
Most of our expenses are relatively fixed in the short-term, and our expense levels are based in part on our expectations regarding future revenue levels. As a result, if revenue for a particular quarter is below our expectations, we may not be able to proportionally reduce operating expenses for that quarter, causing a disproportionate effect on our expected results of operations for that quarter.
Healthcare reform measures and related changes in applicable federal and state laws could materially adversely affect our business.
The efforts of governmental and third-party payers to contain or reduce the costs of healthcare may adversely affect our business, operating results, and financial condition. We expect that there will continue to be a number of legislative and regulatory proposals aimed at changing the healthcare system, which could include restructuring the tax benefits available through flexible spending accounts and/or health savings accounts. If the laws or regulations are changed to limit further the tax benefits through these accounts, such a change could have a material adverse effect on our business by reducing revenues and eroding our margins.
The Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003 imposes certain obligations on the senders of commercial emails, which could minimize the effectiveness of our email marketing campaign, and establishes financial penalties for non-compliance, which could increase the costs of our business and, could have a material adverse effect on our business, financial condition or results of operations.
In December 2003, the Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003, or the CAN-SPAM Act, was enacted. The CAN-SPAM Act establishes certain requirements for commercial email messages and penalizes commercial email message transmissions that are intended to deceive the recipient as to source or content. The CAN-SPAM Act, among other things, requires that senders of commercial emails allow recipients to opt out of receiving future emails from the sender. The ability of our customers to opt out of receiving commercial emails may minimize the effectiveness of our email marketing campaign. Moreover, non-compliance with the CAN-SPAM Act carries significant financial penalties. If we were found to be in violation of the CAN-SPAM Act, applicable state laws not preempted by the CAN-SPAM Act, or foreign laws regulating the distribution of commercial email, we could be required to pay penalties, which could have a material adverse effect on our business, result of operations, financial condition and cash flows.
Certain stockholders own a significant amount of our common stock, which could discourage an acquisition of Vitacost.com or make removal of incumbent management more difficult.
Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Equity Partners IV, L.P. (collectively, “GHP”) beneficially own approximately 19.16% of our outstanding stock. Because GHP owns a significant percentage of our capital stock, it could significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions.
Risks Relating to Our Products
We may incur product liability claims, which could increase our costs and/or materially adversely affect our business, reputation, financial condition or results of operations.
As a retailer and formulator of products designed for human consumption or use on or in the body, we are subject to product liability claims if the use of our products is alleged to have resulted in illness or injury or if our products include inadequate instructions or warnings. Our products generally consist of vitamins, minerals, herbs and other ingredients that are classified as foods, OTC drugs, dietary supplements, medical devices, and cosmetics and generally are not subject to pre-market regulatory approval or clearance in the U.S. by the FDA or other governmental authorities. Our products could contain spoiled or contaminated substances, and some of our products contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur. Furthermore, as a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products that we do not manufacture. We could be subject to product liability claims, including among others, that our products include insufficient instructions for use or inadequate warnings concerning possible side effects or interactions with other substances. Any product liability claim against us could result in increased costs and adversely affect our reputation with our customers, which in turn could materially adversely affect our business, financial condition or results of operations.
We depend upon certain suppliers and manufacturers; if these suppliers or manufacturers do not provide us materials or products when and as needed and we are unable to efficiently obtain alternative supply sources, we could be unable to meet the demand for our products, and our business, financial condition or results of operations may be materially adversely affected.
We rely upon suppliers for certain of our products. Furthermore, we engage manufacturers to produce our proprietary products. Disruption in the operations of any such supplier or manufacturer can occur for a number of reasons, many of which are beyond our control, such as regulatory requirements, import restrictions, loss of certifications, power interruptions, fires, hurricanes, drought or other climate-related events, war or other events. If any of our suppliers or manufacturers become unable or unwilling to continue to provide us supplies or products in the required volumes and quality levels or in a timely manner, we would be required to identify and obtain acceptable replacement supply or product sources. If we are unable to efficiently obtain alternative sources, our business, financial condition or results of operations may be materially adversely affected.
Unfavorable publicity or consumer acceptance of our products or of nutritional supplements generally could reduce our sales.
We are highly dependent upon consumer acceptance of the safety, efficacy and quality of our products, as well as similar products distributed by other companies. Consumer acceptance of products can be significantly influenced by scientific research or findings, national media attention and other publicity about product use. A product may initially be received favorably, resulting in high sales associated with that product that may not be sustainable as consumer preferences change. In addition, recent studies have challenged the safety or benefit of certain nutritional supplements and dietary ingredients. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. Any research or publicity that is perceived by our consumers as less than favorable or that questions earlier favorable research or publicity could have a material adverse effect on our ability to generate revenue. Adverse publicity in the form of published scientific research, statements by regulatory authorities or otherwise, whether or not accurate, that associates consumption of our products or any other similar products with illness or other adverse effects, or that questions the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our business, reputation, financial condition or results of operations.
An unexpected interruption or shortage in the supply or significant increase in the cost of raw materials could limit our ability to secure a sufficient supply of products, which could reduce our sales and our margins.
An unexpected interruption of supply or a significant increase in the cost of raw materials for any reason, such as regulatory requirements, import restrictions, loss of certifications, disruption of distribution channels as a result of weather, terrorism or acts of war, or other events, could result in significant cost increases and/or shortages of our products. Our inability to obtain a sufficient amount of products or to pass through higher cost of products we offer could have a material adverse effect on our business, financial condition or results of operations.
If we experience product recalls, we may incur significant and unexpected costs and damage to our reputation which in turn could have a material adverse effect on our business, financial condition or results of operations.
We may be subject to product recalls, withdrawals or seizures if any of the products we formulate or sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the labeling, promotion, sale or distribution of our products. A recall, withdrawal or seizure of any of our products could materially and adversely affect consumer confidence in our brands and lead to decreased demand for our products. In addition, a recall, withdrawal or seizure of any of our products would require significant management attention, would likely result in substantial and unexpected expenditures and could materially adversely affect our business, financial condition or results of operations.
Our inability to comply with health and safety regulations could materially and adversely affect our business, financial condition or results of operations.
Our operations are subject to environmental and health and safety laws and regulations, and some of our operations require environmental permits and controls to prevent and limit pollution of the environment. We could incur significant costs as a result of violations of, or liabilities under, environmental laws and regulations, or to maintain compliance with such environmental laws, regulations, or permit requirements.
Risks Related to Our Common Stock
If we are unable to comply with rules and regulations of the NASDAQ Stock Market, our common stock could be delisted from NASDAQ.
On December 7, 2010, trading in our common stock was temporarily suspended by NASDAQ for failure to comply with rules and regulations relating to the timely filing of SEC reports. On December 21, 2010, we received a letter from The NASDAQ Stock Market indicating that based on its review of the Company, the NASDAQ staff had determined that continued listing of our securities on NASDAQ was no longer warranted. In accordance with the procedures set forth in the NASDAQ Listing Rules, we timely appealed the staff determination, and requested a hearing before a NASDAQ Hearings Panel (the “Panel”). The hearing was held on February 3, 2011. On February 28, 2011, we received a written notice from NASDAQ indicating that the Panel had determined to grant our request to remain listed on NASDAQ, subject to certain conditions, including that (1) on or before June 20, 2011, we become current in our filing obligations, and demonstrated compliance with all quantitative requirements for continued listing, and (2) on or before July 5, 2011, we have solicited proxies and held our annual meeting. On June 20, 2011, trading of our securities on NASDAQ resumed. On July 6, 2011, we received a letter from NASDAQ indicating that we had met the requirements of the Panel’s decision and the applicable listing requirements and that accordingly the Panel had determined to continue listing our securities on the NASDAQ Stock Market. If we are unable to comply with the NASDAQ requirements in the future, our common stock could again be suspended from trading or be delisted from NASDAQ. A trading suspension or delisting of our common stock could negatively impact us by reducing the liquidity and market price of our common stock and the number of investors willing to hold or acquire our common stock, which could negatively impact our stock price and ability to raise equity financing.
Volatility of our stock price could materially adversely affect an investment in our common stock.
The market price of our common stock could fluctuate significantly as a result of, among other things:
| • | quarterly and annual variations in our operating results, |
| • | the level and quality of research analyst coverage for our common stock, changes in financial estimates or investment recommendations by securities analysts following our business or failure to meet such estimates, |
| • | the financial disclosure we may provide to the public, any changes in such disclosure or our failure to meet such disclosure, |
| • | the public’s response to our press releases, our other public announcements and our filings with the Securities and Exchange Commission, |
| • | various market factors or perceived market factors, including rumors, whether or not correct, involving us, our customers, our suppliers or our competitors, |
| • | changes in accounting standards, policies, guidance, interpretations or principles, |
| • | sales of common stock by our directors, officers or significant stockholders, |
| • | introductions of new products or new pricing policies by us or by our competitors, |
| • | recruitment or departure of key personnel, |
| • | developments with respect to intellectual property rights, |
| • | acquisitions or strategic alliances by us or our competitors, |
| • | changes in shipping costs, |
| • | short sales, hedging and other derivative transactions in shares of our common stock, |
| • | the operating and stock price performance of other companies that investors may deem comparable to us, |
| • | broad market conditions and trends in the eCommerce industry and the economy as a whole; and, |
| • | other events or factors, including those resulting from war, incidents of terrorism or responses to such events. |
Fluctuations in the price of our common stock could contribute to the loss of all or part of a stockholder’s investment. Any of the factors listed above could have a material adverse effect on an investment in our common stock. In addition, stocks of Internet-related and eCommerce companies have historically experienced significant price and volume fluctuations that may have been unrelated or disproportionate to these companies’ operating performance. Public announcements by us or other such companies concerning, among other things, performance, accounting practices or legal problems could cause the market price of our common stock to decline regardless of our actual operating performance. We could be the subject of securities class action litigation due to future stock price volatility, which could divert management’s attention and materially adversely affect our results of operations.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties.
We conduct our operations through three facilities:
| • | We lease 35,600 square feet of office space in Boca Raton, Florida which we use as our corporate headquarters. |
| • | We own a 227,000 square foot distribution facility located on 27 acres in Lexington, North Carolina that serves as our call center and east coast distribution center. This facility includes approximately 13,000 square feet of office space. This facility also includes 25,000 square feet of space that we lease to a third party manufacturer of certain of our proprietary products. |
| • | We lease approximately 155,000 square feet in Las Vegas, Nevada that serves as our west coast distribution facility. |
See Note 7 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for more information about our lease commitments.
Item 3. Legal Proceedings.
Refer to Note 11, Contingencies, of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for a discussion on the nature of the legal proceedings against us, which is incorporated herein by reference
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities.
Our common stock, par value $0.00001 per share, is listed on The NASDAQ Global Market under the symbol VITC and has been included for listing thereon since September 24, 2009. As previously reported, the NASDAQ halted trading of our stock from December 7, 2010 through June 17, 2011. Accordingly, the sales price per share of our common stock during that period reflects the closing price on December 7, 2010 of $5.70 a share.
The following table sets forth for the indicated periods the high and low sales price per share for our common stock on the NASDAQ Global Market:
| | | High | | | Low | |
| Year Ended December 31, 2012: | | | | | | | | |
| Fourth quarter | | $ | 7.25 | | | $ | 5.89 | |
| Third quarter | | $ | 6.90 | | | $ | 5.05 | |
| Second quarter | | $ | 8.38 | | | $ | 5.86 | |
| First quarter | | $ | 8.59 | | | $ | 6.44 | |
| Year Ended December 31, 2011: | | | | | | | | |
| Fourth quarter | | $ | 6.60 | | | $ | 4.66 | |
| Third quarter | | $ | 5.72 | | | $ | 4.33 | |
| Second quarter | | $ | 5.70 | | | $ | 3.41 | |
| First quarter | | $ | 5.70 | | | $ | 5.70 | |
Holders of Record
At February 27, 2013, our common stock was held by approximately 48 holders of record.
Dividends
We have never declared or paid any dividends on our common stock, and our Board of Directors does not contemplate doing so in the near future. Any decisions as to future payment of dividends will depend on our earnings and financial position and such other factors, as our Board of Directors deems relevant.
Equity Compensation Plan Information
The following is a summary of our equity compensation plans as of December 31, 2012:
| | | Number of Securities
to Be Issued Upon
Exercise of
Outstanding Options,
Warrants, and Rights | | | Weighted Average
Exercise Price of
Outstanding Options,
Warrants, and Rights | | | Number of Securities
Remaining Available
for Future Issuance
Under Equity
Compensation Plans | |
| Equity compensation plans approved by security holders | | | 5,209,250 | | | $ | 6.40 | | | | 4,337,678 | |
| Equity compensation plans not approved by security holders | | | - | | | $ | - | | | | - | |
Additional information about our equity compensation plans is incorporated by reference from the Notes to our Consolidated Financial Statements included in this Form 10-K.
Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock between September 24, 2009 and December 31, 2012, with the cumulative total return of the (i) Russell 2000 Index, (ii) the NASDAQ Internet Index and (iii) a custom selected Peer Group Index. This graph assumes the investment of $100 on September 24, 2009 in our common stock, the Russell 2000 Index, the NASDAQ Internet Index, and a custom selected Peer Group Index. The custom Peer Group Index consists of the following companies: Blue Nile, Shutterfly, Vitamin Shoppe, Overstock.com, Herbalife Ltd., 1-800-FLOWERS.COM, Nu Skin Enterprises, Whole Foods Market, US Auto Parts Network, GNC Holdings, Stamps.com, USANA Health Sciences, PetMed Express and NutriSystem. As previously reported, the NASDAQ halted trading of our stock from December 7, 2010 through June 17, 2011. The historical stock price performance is not necessarily indicative of future stock price performance.
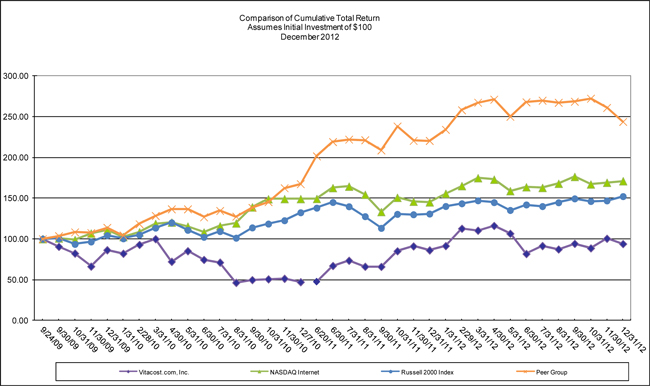
Item 6. Selected Financial Data
The selected consolidated financial data presented below has been derived from our consolidated financial statements. The statement of operations data for each of the fiscal years ended December 31, 2012, 2011 and 2010 and the balance sheet data at December 31, 2012 and 2011 are derived from our audited financial statements that are included in this annual report. The statement of operations data for the years ended December 31, 2009 and 2008 and the balance sheet data at December 31, 2010, 2009 and 2008 are derived from audited 2010, 2009 and 2008 financial statements that are not included in this annual report. The historical results presented below are not necessarily indicative of the results to be expected in any future period.
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | | | 2009 | | | 2008 | |
| | | (in thousands, except per share data) | |
| Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Net sales | | $ | 330,680 | | | $ | 260,523 | | | $ | 220,681 | | | $ | 191,807 | | | $ | 143,602 | |
| Cost of goods sold | | | 254,527 | | | | 200,596 | | | | 164,206 | | | | 130,605 | | | | 105,529 | |
| Gross profit | | | 76,153 | | | | 59,927 | | | | 56,475 | | | | 61,202 | | | | 38,073 | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | |
| Fulfillment | | | 33,505 | | | | 22,290 | | | | 17,354 | | | | 8,954 | | | | 8,393 | |
| Sales and marketing | | | 32,306 | | | | 22,842 | | | | 18,728 | | | | 14,284 | | | | 13,147 | |
| General and administrative | | | 29,614 | | | | 29,620 | | | | 33,989 | | | | 29,083 | | | | 14,871 | |
| Total operating expenses | | | 95,425 | | | | 74,752 | | | | 70,070 | | | | 52,321 | | | | 36,411 | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating (loss) income | | | (19,272 | ) | | | (14,825 | ) | | | (13,595 | ) | | | 8,881 | | | | 1,662 | |
| | | | | | | | | | | | | | | | | | | | | |
| Other income (expense), net | | | 154 | | | | 47 | | | | (175 | ) | | | (151 | ) | | | (1,163 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Income tax expense | | | (53 | ) | | | (53 | ) | | | (1,421 | ) | | | (2,836 | ) | | | (482 | ) |
| Net (loss) income | | $ | (19,171 | ) | | $ | (14,831 | ) | | $ | (15,191 | ) | | $ | 5,894 | | | $ | 17 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) income per share: | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.59 | ) | | $ | (0.53 | ) | | $ | (0.55 | ) | | $ | 0.24 | | | $ | - | |
| Diluted | | $ | (0.59 | ) | | $ | (0.53 | ) | | $ | (0.55 | ) | | $ | 0.24 | | | $ | - | |
| Weighted average number of shares outstanding | | | | | | | | | | | | | | | | | | | | |
| Basic | | | 32,675 | | | | 27,829 | | | | 27,704 | | | | 24,217 | | | | 23,188 | |
| Diluted | | | 32,675 | | | | 27,829 | | | | 27,704 | | | | 24,674 | | | | 23,975 | |
| | | | | | | | | | | | | | | | | | | | | |
| Consolidated Balance Sheets Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 32,152 | | | $ | 12,939 | | | $ | 11,952 | | | $ | 8,658 | | | $ | 61 | |
| Inventory | | | 33,319 | | | | 34,822 | | | | 29,828 | | | | 28,097 | | | | 21,663 | |
| Working capital (deficit) | | | 30,846 | | | | 13,201 | | | | 22,377 | | | | 49,599 | | | | (5,990 | ) |
| Total assets | | | 107,663 | | | | 90,629 | | | | 99,464 | | | | 107,679 | | | | 46,869 | |
| Total debt | | | - | | | | - | | | | 59 | | | | 9,405 | | | | 18,232 | |
| Stockholders' equity | | | 66,658 | | | | 48,807 | | | | 62,186 | | | | 74,500 | | | | 6,965 | |
This information should be read in connection with Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operations and our financial statements and the related notes included elsewhere in this annual report.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of the Company’s financial condition and results of operations should be read in connection with Item 6,Selected Financial Data and Item 8,Financial Statements and Supplementary Data of this Annual Report on Form 10-K. The Company does not believe that its historical operating results will be indicative of future operating results. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The Company’s actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those under the headings “Risk Factors” and “Forward-Looking Information” of this Annual Report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Overview
We are a leading online retailer of health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals (which we refer to as “vitamins and dietary supplements”), as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. We sell these products directly to consumers primarily through our website,www.vitacost.com . We strive to offer our customers the broadest selection of healthy living products at extremely competitive prices, while providing superior customer service.
We offer our customers a broad selection of approximately 40,000 Stock Keeping Units (“SKUs”), from over 2,000 third-party brands, such as New Chapter, Nature’s Way, Twinlab, Source Naturals, Jarrow Formulas, Jason, Desert Essence, Atkins, Bob’s Red Mill, BSN, Optimum Nutrition, USP Labs and MuscleTech in addition to our own proprietary brands: Vitacost, Cosmeceutical Sciences Institute (“CSI”), Best of All, and Smart Basics. We support our operations through our customer service center and our two distribution centers, delivering what we believe are industry-leading customer satisfaction results. Our website allows customers to easily browse and purchase products at prices typically significantly lower than manufacturers’ suggested retail prices. Our website also serves as an educational resource for consumers seeking information on healthy living and the attributes of health and wellness supplements.
Our growth is driven primarily by our ability to expand our customer base and grow our product offerings. Our customers are typically individuals seeking value in their purchases of health and wellness products. Our active customer base, which we define as customers who have purchased from us within the last twelve months, has steadily increased from approximately 270,000 at the end of 2005 to approximately 2.1 million as of December 31, 2012. On average, our customers make purchases from us two to three times a year and during 2012, approximately 72% of our orders were placed by repeat customers.
We were incorporated in Delaware in May 1994 and began operations as a catalog retailer of third-party vitamins and supplements under the name Nature’s Wealth Company. In 1999, we launched Vitacost.com and introduced our proprietary vitamins and supplements. In 2000, we began operating under the name Vitacost.com, Inc. In September 2009 we completed our Initial Public Offering. On September 28, 2011, Vitacost.com, Inc. completed a restructuring whereby it merged with and into Vitacost Merger Corporation, a wholly owned subsidiary of Vitacost.com, Inc., with Vitacost Merger Corporation surviving the merger. The surviving company continues to operate the business under the name Vitacost.com, Inc.
Recent Transactions
On August 28, 2012, we entered into an agreement to outsource all of our remaining manufacturing operations and lease our manufacturing facilities to a third party provider effective September 1, 2012. Prior to this transaction, approximately forty percent of our proprietary products were supplied by contract manufacturers. We do not expect the transaction to have a material impact on our results from operations.
On February 16, 2012, the Company entered into a Purchase Agreement (the “Purchase Agreement”) by and among Vitacost, JHH Capital, LLC, an entity affiliated with Jeffrey Horowitz, our Chief Executive Officer (“JHH”), Great Hill Equity Partners III, L.P. (“Great Hill III”), Great Hill Equity Partners IV, L.P. (“Great Hill IV”), Great Hill Investors, LLC (“Great Hill Investors”), Freshford Partners, LP (“Freshford Partners”), Freshford Master Fund, Ltd (“Freshford Master Fund”) and Baron Small Cap Fund (“Baron” and, together with JHH, Great Hill III, Great Hill IV, Great Hill Investors, Freshford Partners, Freshford Master Fund, collectively, the “Investors”) pursuant to which the Investors purchased, and Vitacost sold, an aggregate of 4.9 million shares of the Company’s common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1.7 million shares of the Company’s common stock for an aggregate purchase price of $34.8 million. The warrants have an exercise price of $7.04 per share and a term of four years. The net proceeds of $33.6 million, after the deduction of fees of $1.2 million incurred in connection with the transaction, were allocated between common stock and warrants based on their relative fair values as of the purchase date. A portion of the proceeds from the financing are being used to expand and optimize our distribution network. We spent $4.5 million on the expansion and upgrade of our fulfillment centers in 2012 and expect additional investments in 2013. In addition, an estimated $15.0 million may be used to invest in and stock a third distribution center to support our future sales growth. We are evaluating an investment in a new facility in late 2013 or beyond. We intend to use the remaining proceeds from the financing for general operations.
Trends and Other Factors Affecting Our Business
We continue to experience intense competition as the vitamin and supplement industry shifts towards a greater internet presence. This competitive environment continues to drive margin pressure as deep discounting results from aggressive customer acquisition and retention actions. In order to differentiate ourselves from our competitors, we are expanding our product offerings through the introduction of new and diverse products, improving our customer service and support, improving our reliability and speed of delivery and by adjusting our product mix and pricing.
The health and wellness industry continues to benefit from positive demographic trends, with an aging population, trends affecting health and lifestyle preferences, and an increasingly health conscious consumer. In addition, the increasing cost of traditional healthcare has helped bolster demand for natural products to help ward off more expensive medical visits and prescription drugs. Changes in these trends and other factors that we may not foresee may also impact our business, including potential regulatory actions by the Food and Drug Administration (“FDA”) and the Federal Trade Commission (“FTC”) that may affect the viability of certain products that we offer.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles of the U.S. (“GAAP”).
The preparation of these financial statements requires estimates and assumptions that affect the reported amounts of assets and liabilities, revenue and expenses and related disclosures of contingent assets and liabilities in the consolidated financial statements and accompanying notes. Critical accounting policies are those that are the most important to the portrayal of our financial condition and results of operations and require our most difficult, subjective and complex judgments as a result of the need to make estimates about the effect of matters that are inherently uncertain. While our significant accounting policies are described in more detail in the notes to our consolidated financial statements, our most critical accounting policies, discussed below, pertain to revenue recognition, income taxes, stock-based compensation, contingencies, inventories and goodwill. In applying such policies, we exercise our best judgment and best estimates. Actual results may differ from these estimates under different assumptions or conditions. For a further discussion of these Critical Accounting Policies and Estimates, as well as a description of our other significant accounting policies, see Note 1 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K.
Revenue Recognition. Revenue from the sale of vitamins, nutritional supplements and other health and wellness products is recognized when all the following criteria are met: a customer executes an order, the sales price and the shipping and handling fee have been determined, credit card authorization has occurred and collection is reasonably assured, and the product has been received by the customer. Our internet sales are made to customers permitting certain limited rights of return for a 30-day period. It has been our experience that such returns have been insignificant.
Income Taxes. Income taxes are computed under an asset and liability approach whereby deferred tax assets and liabilities are recognized based on the difference between the financial statement amounts and the tax basis of assets and liabilities using tax rates in effect for the year in which the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. We evaluate our deferred tax assets on a regular basis to determine if valuation allowances are required. In our evaluation, we consider taxable loss carryback availability, expectations of sufficient future taxable income, trends in earnings, existence of taxable income in recent years, the future reversal of temporary differences and the available tax planning strategies that could be implemented, if required. Valuation allowances are established based on the consideration of all available evidence using a more likely than not standard. Our deferred tax assets for which a valuation allowance has not been established relate to amounts that can be realized through future reversals of existing taxable temporary differences. Our deferred tax asset valuation allowance will be reversed if and when it becomes more likely than not that we can generate sufficient taxable income in the future to utilize the tax benefits of the related deferred tax assets.
Stock-based Compensation. We use a fair value-based method to recognize non-cash compensation expense for share-based transactions. Under the fair value recognition provisions, we recognize stock-based compensation net of an estimated forfeiture rate and only recognize compensation cost for those shares expected to vest on a straight line basis over the requisite service period of the award.
Contingencies. On an ongoing basis, we assess potential liabilities related to lawsuits or claims brought against us. While it is typically very difficult to determine the timing and ultimate outcome of such actions, we use our best judgment to determine if it is probable that we will incur an expense related to the settlement or final adjudication of such matters and whether a reasonable estimation of such probable loss, if any, can be made. In assessing probable losses, we take into consideration estimates of the amount of insurance recoveries, if any. We accrue a liability when we believe a loss is probable and the amount of loss can be reasonably estimated. Due to the inherent uncertainties related to the eventual outcome of litigation and potential insurance recoveries, it is possible that certain matters may be resolved for amounts materially different from any provisions or disclosures that we have previously made. For a further discussion of contingencies, see Note 11 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K.
Inventory. Inventory, consisting principally of health and wellness products, is primarily stated at the lower of cost or market using the first in, first out (“FIFO”) method. Management determines the inventory reserve by regularly reviewing and evaluating individual inventory items and movement history. Future factors, such as changes in consumer demand and reports in the media deemed negative to certain products we market and sell, could impact management’s inventory.
Goodwill. The excess of purchase price over fair value of assets acquired and liabilities assumed in business combinations is classified as goodwill. We review goodwill for impairment at the reporting unit level annually or, when events or circumstances dictate, more frequently. We assess qualitative factors to determine whether it is more likely than not that the fair value of our reporting unit is less than its carrying amount. In performing the qualitative assessment, we assess relevant events and circumstances that may impact the fair value of our reporting unit, including the following: (i) macroeconomic conditions, (ii) industry and market considerations, (iii) overall financial performance, (iv) events affecting the reporting unit, (v) share price and (vi) recent fair value calculation for our reporting unit, if available. We performed this qualitative assessment as of December 31, 2012 as allowable per the authoritative guidance.
After assessing the above described events and circumstances, if we determine that it is more likely than not that the fair value of our reporting unit is greater than its carrying value, then no further testing is required. Otherwise, we would perform a two-step impairment test to evaluate goodwill. Under the first step, we compare the estimated fair value of the reporting unit with its carrying value. If the estimated fair value of the reporting unit is less than its carrying value, we will complete a second step to determine the amount of the goodwill impairment that we should record.
Based on our qualitative assessment, we concluded that it was more likely than not that the estimated fair value of our reporting unit exceeded its carrying value as of December 31, 2012 and thus, did not proceed to the two-step goodwill impairment test. No indicators of impairment exist primarily because our reporting unit’s fair value has consistently exceeded its carrying value by a significant margin.
Sources of Revenue
We derive our revenue principally through the sale of products and freight billed to customers associated with the shipment of products. For 2012, net product sales accounted for approximately 96% of our total net sales, with freight comprising the remainder.
Cost of Goods Sold and Operating Expenses
Cost of Goods Sold. Cost of goods sold consists primarily of the cost of the product sold and the cost of shipping the products to the customers.
Fulfillment. Fulfillment expenses include the costs of warehousing and shipping supplies, machinery and equipment, maintenance, employees, professional services and rent.
Sales and Marketing. Sales and marketing expenses include online advertising and promotional expenditures, including third-party content license fees, affiliates and partners’ commissions, traditional media advertising, print expenses and payroll related expenses for personnel engaged in marketing, sales, customer service, and website development and maintenance. We expense advertising costs as incurred.
General and Administrative. General and administrative expenses consist of executive compensation, information technology expenses, credit card processing fees, legal fees, professional services, employee expenses and general corporate expenses.
Results of Operations
The following table sets forth certain consolidated statement of operations data as a percentage of net sales for the periods indicated:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| Net sales | | | 100.0 | % | | | 100.0 | % | | | 100.0 | % |
| Cost of goods sold | | | 77.0 | | | | 77.0 | | | | 74.4 | |
| Gross profit | | | 23.0 | | | | 23.0 | | | | 25.6 | |
| Operating expenses: | | | | | | | | | | | | |
| Fulfillment | | | 10.1 | | | | 8.5 | | | | 7.9 | |
| Sales and marketing | | | 9.8 | | | | 8.8 | | | | 8.5 | |
| General and administrative | | | 9.0 | | | | 11.4 | | | | 15.4 | |
| Total operating expense | | | 28.9 | | | | 28.7 | | | | 31.8 | |
| Operating loss | | | (5.9 | ) | | | (5.7 | ) | | | (6.2 | ) |
| Net loss | | | (5.9 | )% | | | (5.7 | )% | | | (6.9 | )% |
Comparison of Year Ended December 31, 2012 to Year Ended December 31, 2011
| | | Year Ended | | | $ | | | % | |
| | | December 31, | | | Increase | | | Increase | |
| | | 2012 | | | 2011 | | | (Decrease) | | | (Decrease) | |
| | | (In thousands) | | | | |
| Third-party products | | $ | 245,800 | | | $ | 190,541 | | | $ | 55,259 | | | | 29.0 | % |
| Proprietary products | | | 72,335 | | | | 60,973 | | | | 11,362 | | | | 18.6 | |
| Freight | | | 12,545 | | | | 9,009 | | | | 3,536 | | | | 39.3 | |
| Net sales | | | 330,680 | | | | 260,523 | | | | 70,157 | | | | 26.9 | |
| Cost of goods sold | | | 254,527 | | | | 200,596 | | | | 53,931 | | | | 26.9 | |
| Gross profit | | $ | 76,153 | | | $ | 59,927 | | | $ | 16,226 | | | | 27.1 | % |
Net Sales. Net sales increased by $70.2 million, or 26.9%, to $330.7 million for 2012 compared to 2011. Net sales of third-party products increased $55.3 million or 29.0%, to $245.8 million for 2012 compared to 2011. Net sales of our proprietary products increased $11.4 million, or 18.6%, to $72.3 million, and freight increased $3.5 million, or 39.3%, to $12.5 million for 2012 compared to 2011.
The increase in net sales was primarily the result of the increase in our customer base and number of shipped orders compared to the 2011. As of December 31, 2012, we had 2.1 million active customers, an increase of 51.9% from December 31, 2011. In 2012, we shipped 4.9 million orders, which represents a 36.6% increase over 2011. The growth in active customers and the number of shipped orders was due to a greater emphasis on new customer acquisition, as we expanded our marketing activities, promotional offers and grew our network of affiliates and partners during the period. In the second half of 2011 we began distributing our products on Amazon.com, which was a significant driver of growth in 2012.
Cost of Goods Sold. Cost of goods sold increased $53.9 million, or 26.9%, to $254.5 million for 2012 compared to 2011. As a percentage of net sales, cost of goods sold remained at 77.0% for 2012 and 2011.
Gross Profit. Gross profit increased $16.2 million, or 27.1%, to $76.2 million for 2012 compared to 2011 and gross profit as a percentage of net sales remained at 23.0% for 2012 and 2011. Gross margin was positively impacted by the reduction in our shipping costs per order, as we implemented a program to reduce shipping expenses in the latter part of 2011. This was offset by a reduction in product margin, primarily due to increased promotions and a shift in product mix with an increased percentage of sales from third-party products which typically carry a lower gross margin.
Fulfillment. Fulfillment expense increased $11.2 million, or 50.3%, to $33.5 million for 2012 compared to 2011. As a percentage of net sales, fulfillment expense increased to 10.1% for 2012 from 8.5% for 2011. The increase in fulfillment expense was primarily due to a 36.6% increase in the number of shipped orders in 2012 compared to 2011. The remaining increase was primarily the result of fees associated with our freight savings program. We are investing in and aggressively working to improve our fulfillment efficiency over the long-term.
Sales and Marketing. Sales and marketing expense increased approximately $9.5 million, or 41.4%, to $32.3 million for 2012 when compared to 2011. As a percentage of net sales, expenses increased to 9.8% for 2012 from 8.8% for 2011. Included in the 2011 amount was $0.9 million in severance and executive recruiting expenses. Excluding these items, sales and marketing expenses increased $10.4 million period-over-period. The increase in sales and marketing expense was primarily due to a $5.4 million increase in fees paid to affiliates and partners associated with the expanded distribution of our products, increased employee expenses of $2.4 million, increased online and broadcast advertising expense of $2.0 million, and increased other sales and marketing costs of $0.6 million. We believe sales and marketing expense may continue to increase in the future as we continue to invest in marketing initiatives to drive additional growth in our customer base.
General and Administrative. General and administrative expense remained flat at $29.6 million in 2012 when compared to 2011. As a percentage of net sales, expenses decreased to 9.0% in 2012 from 11.4% for 2011. Included in the 2012 amount were $0.5 million in expenses consisting primarily of the following: $0.4 million in severance, executive recruiting and relocation expenses, $0.2 million in fees associated with the financing in the first quarter of 2012, $0.1 million in additional legal expenses associated with the Class Action lawsuit, partially offset by $0.3 million related to the release of a litigation accrual. Included in the 2011 amount were $2.9 million in expenses consisting of the following: $2.1 million in expenses primarily related to our equity capitalization issue and strategic review, $0.5 million in severance and executive recruiting expenses, and $0.3 million related to a litigation accrual. Excluding the aforementioned items, general and administrative expense increased $2.4 million period-over-period. The increase was primarily attributable to increased credit card fees of $1.0 million due to higher sales, increased employee compensation costs of $0.6 million due to headcount growth in critical departments, and increased other administrative costs of $0.8 million. We believe general and administrative expense may increase in the future as we continue to invest in our information technology infrastructure to support our future growth.
Income Tax Expense. Income tax expense remained at $0.1 million for 2012 and 2011. This was a result of reducing our net deferred tax assets to zero at December 31, 2010 and maintaining a valuation allowance on our deferred tax assets through 2012.
Comparison of Year Ended December 31, 2011 to Year Ended December 31, 2010
| | | Year Ended | | | $ | | | % | |
| | | December 31, | | | Increase | | | Increase | |
| | | 2011 | | | 2010 | | | (Decrease) | | | (Decrease) | |
| | | (In thousands) | | | | |
| Third-party products | | $ | 190,541 | | | $ | 147,065 | | | $ | 43,476 | | | | 29.6 | % |
| Proprietary products | | | 60,973 | | | | 59,159 | | | | 1,814 | | | | 3.1 | |
| Freight | | | 9,009 | | | | 14,457 | | | | (5,448 | ) | | | (37.7 | ) |
| Net sales | | | 260,523 | | | | 220,681 | | | | 39,842 | | | | 18.1 | |
| Cost of goods sold | | | 200,596 | | | | 164,206 | | | | 36,390 | | | | 22.2 | |
| Gross profit | | $ | 59,927 | | | $ | 56,475 | | | $ | 3,452 | | | | 6.1 | % |
Net Sales. Net sales increased by $39.8 million, or 18.1%, to $260.5 million for 2011 compared to 2010. Net sales of third-party products increased $43.5 million, or 29.6%, to $190.5 million for 2011. Third-party products include advertising and other fees earned from affiliate programs of an insignificant amount for 2011 and $0.8 million for 2010. Net sales of our proprietary products increased $1.8 million, or 3.1%, to $61.0 million, and freight decreased $5.4 million, or 37.7%, to $9.0 million for 2011 compared to 2010.
The increase in net sales was primarily the result of an increase in our customer base and number of shipped orders compared to the year ended December 31, 2010. We also expanded shipping and promotional offers in 2011, along with growing our network of affiliates. In July 2011, we began distributing our products on Amazon.com. In February 2011, we extended our “free shipping” policy to include all orders above $49, prior to that only orders above $100 included “free shipping”. The change in our “free shipping” policy accounted for additional product sales, although it reduced freight revenue in 2011.
Cost of Goods Sold. Cost of goods sold increased $36.4 million, or 22.2%, to $200.6 million for 2011 compared to 2010. As a percentage of net sales, cost of goods sold increased to 77.0% for 2011 from 74.4% for 2010. The increase in costs of goods sold is primarily due to a shift in product mix to higher cost third-party products partially offset by a reduction in our shipping costs per order, as we implemented a program to reduce shipping expenses in the fourth quarter of 2011.
Gross Profit. As a result of the changes discussed in net sales and cost of goods sold, gross profit increased $3.5 million, or 6.1%, to $59.9 million for 2011 compared to 2010 and gross profit as a percentage of net sales decreased to 23.0% for 2011 from 25.6% for 2010.
Fulfillment. Fulfillment expense increased $4.9 million, or 28.4%, to $22.3 million for 2011 compared to 2010. As a percentage of net sales, fulfillment expense increased to 8.5% for 2011 from 7.9% for 2010. The increase in fulfillment expense was primarily attributable to an increase in shipped orders in 2011 and expenses associated with a program to reduce our freight costs per shipment. Additionally, there were higher operating costs at our distribution centers as a result of certain operating inefficiencies, primarily attributable to difficulties related to optimizing our warehouse systems and a significant unanticipated shift to products requiring special handling (e.g. liquids and glass packaging) resulting in higher labor costs. These inefficiencies began in 2010 and continued through 2011.
Sales and Marketing. Sales and marketing expense increased $4.1 million, or 22.0%, to $22.8 million for 2011. As a percentage of net sales, sales and marketing expense increased to 8.8% for 2011 from 8.5% for 2010. Included in the 2011 amount were $0.9 million in expenses consisting of the $0.7 million severance payment to our former Chief Marketing Officer and $0.2 million in recruiting fees related to the hiring of our new Chief Marketing officer. The remaining increase in sales and marketing expense of $3.2 million was primarily related to increases in online advertising, including fees paid to affiliates and Amazon.com associated with the expanded distribution of our products, employee compensation costs, and depreciation partially offset by decreases in print advertising and co-op advertising.
General and Administrative. General and administrative expenses decreased $4.4 million, or 12.9%, to $29.6 million for 2011. As a percentage of net sales, general and administrative expenses decreased to 11.4% for 2011 from 15.4% for 2010. Included in the 2011 amount were $2.9 million in expenses consisting of the following: $2.1 million in expenses primarily related to our equity capitalization issue and strategic review, $0.5 million in severance and executive recruiting expenses, and $0.3 million related to a litigation accrual. Included in the 2010 amount were an estimated $10.6 million in expenses consisting of the following: $3.6 million in fees associated with our class action lawsuit, conclusion of the proxy solicitation and other matters, $3.5 million accrual for the settlement of our derivative lawsuit, $1.2 million in accrued severance to our former Chief Executive Officer, $1.1 million in accrued severance to our former Chief Financial Officer, $0.7 million in proxy reimbursement fees to Great Hill Partners and $0.5 million in professional services fees to our independent accountants. Excluding the aforementioned items in both periods, general and administrative expenses increased $3.3 million period-over-period. The increase period-over-period was primarily attributable to increased employee compensation costs of $2.9 million due to headcount growth in critical departments, increased credit card fees of $0.6 million due to higher sales and increased depreciation of $0.2 million; partially offset by a $0.4 million decrease in professional services fees.
Interest Expense. Interest expense decreased $0.4 million, or 99.2%, to an insignificant amount for 2011. The decrease was primarily attributable to the pay off of our notes payable and line of credit balances in 2010.
Income Tax Expense. Income tax expense decreased $1.4 million, or 96.3%, to $0.1 million for 2011. This was a result of reducing our net deferred tax assets to zero at December 31, 2010. Maintaining a valuation allowance on our deferred tax assets throughout 2011 resulted in an insignificant income tax expense for the year.
Historical Cash Flows
Comparison of Year Ended December 31, 2012 to Year Ended December 31, 2011
Net Cash Used in Operating Activities. For the year ended December 31, 2012, net cash used in operations was $9.4 million compared to $7.0 million for the year ended December 31, 2011. The increase in 2012 of $2.4 million was primarily due to our increased net loss.
Net Cash (Used in) Provided By Investing Activities. For the year ended December 31, 2012, net cash used in investing activities was $6.4 million compared to net cash provided by investing activities of $7.6 million for the year ended December 31, 2011. The $14.0 million change in cash flows from investing activities was primarily due to a decrease in proceeds from maturities of securities available for sale as we liquidated all our securities available-for-sale during 2011 and an increase in our capital expenditures.
Net Cash Provided by Financing Activities. For the year ended December 31, 2012, net cash provided by financing activities was $35.0 million compared to $0.4 million for the year ended December 31, 2011. The change in cash flows from financing activities was primarily due to the net proceeds of $33.6 million from the financing in the first quarter of 2012 and the increase in proceeds from the exercise of stock options of $1.0 million.
Liquidity and Capital Resources
Liquidity. The significant components of our working capital are cash and cash equivalents, inventory and accounts receivable, primarily from credit card processors, reduced by accounts payable and accrued expenses. Cash and cash equivalents consist of cash and money market accounts. The working capital characteristics of our business allow us to collect cash from sales to customers within a few business days of the related sale. At December 31, 2012, we had a working capital surplus of $30.8 million.
Amounts deposited with third party financial institutions exceed the Federal Deposit Insurance Corporation, or FDIC, and Securities Investor Protection Corporation, or SIPC, insurance limits, as applicable. These cash and cash equivalent balances could be impacted if the underlying financial institutions fail or are subjected to other adverse conditions in the financial markets. To date we have experienced no loss or lack of access to our cash and cash equivalents; however, we can provide no assurances that access to our invested cash and cash equivalents will not be impacted by adverse conditions in the financial markets.
Our future capital requirements will depend on many factors, including:
| • | the rate of our revenue growth, |
| • | the timing and extent of expenditures to enhance our website, network infrastructure, and transaction processing systems, |
| • | the extent of our advertising and marketing programs, |
| • | the efficiency of our fulfillment process and systems, |
| • | the levels of inventory we maintain, and |
| • | other factors relating to our business. |
On February 16, 2012, we entered into the Purchase Agreement pursuant to which the Investors purchased, and we sold, an aggregate of 4.9 million shares of our common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1.7 million shares of common stock for an aggregate purchase price of $34.8 million. The warrants have an exercise price of $7.04 per share and a term of four years. A portion of the proceeds from the financing are being used to expand and optimize our distribution network. We spent $4.5 million on the expansion and upgrade of our fulfillment centers in 2012 and expect additional investments in 2013. In addition, an estimated $15.0 million may be used to invest in and stock a third distribution center to support our future sales growth. We are evaluating an investment in a new facility in late 2013 or beyond. We intend to use the remaining proceeds from the financing for general operations.
At December 31, 2012, we had $32.2 million in cash and cash equivalents. In 2012, our net cash used in operating activities was $9.4 million which was primarily due to our net loss. We are investing in our growth strategy, which includes increasing our brand and company awareness, expanding our customer base, growing our product assortment and expanding and optimizing our fulfillment centers. As a result, we are working to increase our customers’ lifetime value by increasing the frequency of purchases and improving customer retention, while also improving our operating efficiencies. We believe the implementation of our growth strategy will reduce our net loss and our net cash used in operations in the future.
We believe that our cash and cash equivalents currently on hand and our net cash flows from operations will be sufficient to continue our operations for the next twelve months, although we may require additional financing in the future in order to execute our operating plan. We cannot predict whether future financing, if any, will be in the form of equity, debt, or a combination of both. We may not be able to obtain additional funds on a timely basis, on acceptable terms or at all.
Aggregate Contractual Obligations. A summary of contractual cash obligations as of December 31, 2012 is as follows:
| | | Payments Due By Period | |
| | | | | | Less Than | | | | | | | | | More Than | |
| | | Total | | | 1 Year | | | 1 to 3 Years | | | 3 to 5 Years | | | 5 Years | |
| | | (In thousands) | |
| Operating Leases | | $ | 5,929 | | | $ | 1,700 | | | $ | 2,248 | | | $ | 1,230 | | | $ | 751 | |
| Total Contractual Obligations | | $ | 5,929 | | | $ | 1,700 | | | $ | 2,248 | | | $ | 1,230 | | | $ | 751 | |
We have various non-cancelable operating leases that expire in various years through August 2019 for the rental of office space. Rent expense totaled $2.0 million and $2.1 million for the years ended December 31, 2012 and 2011, respectively.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Recent Accounting Pronouncements
Refer to Note 1 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for a discussion of recently issued accounting standards applicable to us, which is incorporated herein by reference.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows. However, we do not believe that a change in market interest rates would have a material effect on our results of operations or financial condition. Although we derive a minor portion of our sales outside of the U.S., all of our sales are denominated in U.S. dollars. We have limited exposure to financial market risks, including changes in interest rates and foreign currency exchange rates. Inflation generally affects us by increasing costs of inventory, labor and equipment. We do not believe that inflation had any material effect on our results of operations in the periods presented in our financial statements.
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Report of Independent Registered Certified Public Accounting Firm | | 32 |
| Consolidated Balance Sheets as of December 31, 2012 and 2011 | | 33 |
| Consolidated Statements of Comprehensive Loss for the Years Ended December 31, 2012, 2011 and 2010 | | 34 |
| Consolidated Statement of Stockholders’ Equity for the Years Ended December 31, 2012, 2011 and 2010 | | 35 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2012, 2011 and 2010 | | 36 |
| Notes to Consolidated Financial Statements | | 37 |
REPORT OF INDEPENDENT REGISTERED CERTIFIED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
of Vitacost.com, Inc.:
In our opinion, the consolidated balance sheets and the related consolidated statements of comprehensive loss, stockholders’ equity and cash flows present fairly, in all material respects, the financial position of Vitacost.com, Inc. and its subsidiary (the “Company”) at December 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2012, based on criteria established inInternal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Miami, Florida
March 4, 2013
VITACOST.COM, INC.
CONSOLIDATED BALANCE SHEETS
| | | As of December 31, | |
| | | 2012 | | | 2011 | |
| | | (In thousands, except par value) | |
| Assets | | | | | | | | |
| Current Assets | | | | | | | | |
| Cash and cash equivalents | | $ | 32,152 | | | $ | 12,939 | |
| Accounts receivable, net | | | 2,613 | | | | 2,169 | |
| Inventory | | | 33,319 | | | | 34,822 | |
| Prepaid expenses | | | 1,270 | | | | 1,912 | |
| Other receivables | | | 2,054 | | | | 264 | |
| Other assets | | | 93 | | | | 2,344 | |
| Total current assets | | | 71,501 | | | | 54,450 | |
| | | | | | | | | |
| Property and equipment, net | | | 33,491 | | | | 33,629 | |
| Restricted cash | | | 225 | | | | 225 | |
| Deposits | | | 246 | | | | 125 | |
| Goodwill | | | 2,200 | | | | 2,200 | |
| | | | | | | | | |
| Total assets | | $ | 107,663 | | | $ | 90,629 | |
| | | | | | | | | |
| Liabilities and Stockholders' Equity | | | | | | | | |
| Current Liabilities | | | | | | | | |
| Accounts payable | | $ | 28,696 | | | $ | 30,250 | |
| Deferred revenue | | | 5,414 | | | | 4,573 | |
| Accrued expenses | | | 6,545 | | | | 6,425 | |
| Total current liabilities | | | 40,655 | | | | 41,248 | |
| | | | | | | | | |
| Deferred tax liability | | | 350 | | | | 574 | |
| Total liabilities | | | 41,005 | | | | 41,822 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders' Equity | | | | | | | | |
| Preferred stock, par value $.00001 per share; 25,000 shares authorized; no shares issued and outstanding | | | - | | | | - | |
| Common stock, par value $.00001 per share; 100,000 shares authorized; 33,500 and 27,975 shares issued and outstanding at December 31, 2012 and December 31, 2011, respectively | | | - | | | | - | |
| Additional paid-in capital | | | 109,022 | | | | 76,262 | |
| Warrants | | | 4,262 | | | | - | |
| Accumulated deficit | | | (46,626 | ) | | | (27,455 | ) |
| Total stockholders' equity | | | 66,658 | | | | 48,807 | |
| Total liabilities and stockholders' equity | | $ | 107,663 | | | $ | 90,629 | |
The accompanying notes are an integral part of these consolidated financial statements.
VITACOST.COM, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| | | (In thousands, except per share) | |
| Net sales | | $ | 330,680 | | | $ | 260,523 | | | $ | 220,681 | |
| Cost of goods sold | | | 254,527 | | | | 200,596 | | | | 164,206 | |
| Gross profit | | | 76,153 | | | | 59,927 | | | | 56,475 | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Fulfillment | | | 33,505 | | | | 22,290 | | | | 17,354 | |
| Sales and marketing | | | 32,306 | | | | 22,842 | | | | 18,727 | |
| General and administrative | | | 29,614 | | | | 29,620 | | | | 33,989 | |
| | | | 95,425 | | | | 74,752 | | | | 70,070 | |
| | | | | | | | | | | | | |
| Operating loss | | | (19,272 | ) | | | (14,825 | ) | | | (13,595 | ) |
| | | | | | | | | | | | | |
| Other income (expense) | | | 154 | | | | 47 | | | | (175 | ) |
| | | | | | | | | | | | | |
| Loss before income taxes | | | (19,118 | ) | | | (14,778 | ) | | | (13,770 | ) |
| Income tax expense | | | (53 | ) | | | (53 | ) | | | (1,421 | ) |
| Net loss | | $ | (19,171 | ) | | $ | (14,831 | ) | | $ | (15,191 | ) |
| | | | | | | | | | | | | |
| Basic and diluted per share information: | | | | | | | | | | | | |
| Net loss available to common stockholders | | $ | (0.59 | ) | | $ | (0.53 | ) | | $ | (0.55 | ) |
| Weighted average shares outstanding | | | 32,675 | | | | 27,829 | | | | 27,704 | |
| | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | |
| Net loss | | $ | (19,171 | ) | | $ | (14,831 | ) | | $ | (15,191 | ) |
| Other comprehensive income (loss), net of tax: | | | | | | | | | | | | |
| Unrealized gain (loss) on available-for-sale securities | | | - | | | | 20 | | | | (20 | ) |
| Other comprehensive income (loss) | | | - | | | | 20 | | | | (20 | ) |
| Comprehensive loss | | $ | (19,171 | ) | | $ | (14,811 | ) | | $ | (15,211 | ) |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
VITACOST.COM, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | Accumulated | | | (Accumulated | | | | |
| | | | | | Additional | | | | | | Other | | | Deficit) | | | | |
| | | Common Stock | | | Paid-In | | | | | | Comprehensive | | | Retained | | | | |
| | | Shares | | | Amount | | | Capital | | | Warrants | | | (Loss) Income | | | Earnings | | | Total | |
| | | (In thousands) | |
| Balance at December 31, 2009 | | | 27,489 | | | $ | - | | | $ | 71,933 | | | $ | - | | | $ | - | | | $ | 2,567 | | | $ | 74,500 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (15,191 | ) | | | (15,191 | ) |
| Other compehensive loss | | | - | | | | - | | | | - | | | | - | | | | (20 | ) | | | - | | | | (20 | ) |
| Stock options exercised | | | 292 | | | | - | | | | 851 | | | | - | | | | - | | | | - | | | | 851 | |
| Stock-based compensation expense | | | - | | | | - | | | | 1,463 | | | | - | | | | - | | | | - | | | | 1,463 | |
| Income tax benefit from stock options exercised | | | - | | | | - | | | | 583 | | | | - | | | | - | | | | - | | | | 583 | |
| Balance at December 31, 2010 | | | 27,781 | | | | - | | | | 74,830 | | | | - | | | | (20 | ) | | | (12,624 | ) | | | 62,186 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (14,831 | ) | | | (14,831 | ) |
| Other compehensive income | | | - | | | | - | | | | - | | | | - | | | | 20 | | | | - | | | | 20 | |
| Stock options exercised | | | 194 | | | | - | | | | 424 | | | | - | | | | - | | | | - | | | | 424 | |
| Stock-based compensation expense | | | - | | | | - | | | | 1,008 | | | | - | | | | - | | | | - | | | | 1,008 | |
| Balance at December 31, 2011 | | | 27,975 | | | | - | | | | 76,262 | | | | - | | | | - | | | | (27,455 | ) | | | 48,807 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (19,171 | ) | | | (19,171 | ) |
| Stock options exercised | | | 605 | | | | - | | | | 1,384 | | | | - | | | | - | | | | - | | | | 1,384 | |
| Stock-based compensation expense | | | - | | | | - | | | | 1,999 | | | | - | | | | - | | | | - | | | | 1,999 | |
| Stock issued in private placement, net | | | 4,920 | | | | - | | | | 29,377 | | | | - | | | | - | | | | - | | | | 29,377 | |
| Warrants issued in private placement, net | | | - | | | | - | | | | - | | | | 4,262 | | | | - | | | | - | | | | 4,262 | |
| Balance at December 31, 2012 | | | 33,500 | | | $ | - | | | $ | 109,022 | | | $ | 4,262 | | | $ | - | | | $ | (46,626 | ) | | $ | 66,658 | |
The accompanying notes are an integral part of these consolidated financial statements.
VITACOST.COM, INC.
CONSOLIDATED STATEMENT OF CASH FLOWS
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| | | (In thousands) | |
| Cash Flows From Operating Activities | | | | | | | | | | | | |
| Net loss | | $ | (19,171 | ) | | $ | (14,831 | ) | | $ | (15,191 | ) |
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 6,474 | | | | 6,174 | | | | 5,138 | |
| Amortization of premium on debt securities available-for-sale | | | - | | | | 72 | | | | 685 | |
| Change in fair value of interest rate swap | | | - | | | | - | | | | (9 | ) |
| Realized gain on the sale of securities available-for-sale | | | - | | | | (1 | ) | | | (7 | ) |
| Stock-based compensation expense | | | 1,999 | | | | 1,008 | | | | 1,463 | |
| Deferred taxes | | | (224 | ) | | | 53 | | | | 3,051 | |
| Loss (gain) on disposition of property and equipment and other assets | | | 47 | | | | 41 | | | | (69 | ) |
| Changes in assets and liabilities: | | | | | | | | | | | | |
| (Increase) decrease in: | | | | | | | | | | | | |
| Accounts receivable | | | (444 | ) | | | (1,729 | ) | | | 295 | |
| Other receivables | | | (1,790 | ) | | | 823 | | | | (32 | ) |
| Inventory | | | 1,503 | | | | (4,994 | ) | | | (1,731 | ) |
| Prepaid expenses | | | 642 | | | | (551 | ) | | | 627 | |
| Deposits | | | (121 | ) | | | (11 | ) | | | 161 | |
| Other assets | | | 2,251 | | | | 1,210 | | | | (3,553 | ) |
| Increase (decrease) in: | | | | | | | | | | | | |
| Accounts payable | | | (1,554 | ) | | | 7,570 | | | | 5,840 | |
| Deferred revenue | | | 841 | | | | 2,438 | | | | 215 | |
| Accrued expenses | | | 120 | | | | (4,246 | ) | | | 7,389 | |
| Income taxes payable | | | - | | | | - | | | | (51 | ) |
| Net cash (used in) provided by operating activities | | | (9,427 | ) | | | (6,974 | ) | | | 4,221 | |
| Cash Flows From Investing Activities | | | | | | | | | | | | |
| Proceeds from disposition of property, equipment | | | 74 | | | | - | | | | 232 | |
| Settlement of interest rate swap | | | - | | | | - | | | | (460 | ) |
| Payments for the purchase of property and equipment | | | (6,457 | ) | | | (3,040 | ) | | | (16,965 | ) |
| Increase in restricted cash | | | - | | | | (225 | ) | | | - | |
| Purchases of securities available-for-sale | | | - | | | | - | | | | (27,457 | ) |
| Proceeds from maturities of securities available-for-sale | | | - | | | | 10,861 | | | | 51,634 | |
| Net cash (used in) provided by investing activities | | | (6,383 | ) | | | 7,596 | | | | 6,984 | |
| Cash Flows From Financing Activities | | | | | | | | | | | | |
| Proceeds from private placement, net | | | 33,639 | | | | - | | | | - | |
| Principal payments on notes payable | | | - | | | | (59 | ) | | | (5,852 | ) |
| Net borrowings on line of credit | | | - | | | | - | | | | (3,458 | ) |
| Repayments on capital lease obligation | | | - | | | | - | | | | (35 | ) |
| Proceeds from the exercise of stock options | | | 1,384 | | | | 424 | | | | 851 | |
| Tax benefit from stock based compensation | | | - | | | | - | | | | 583 | |
| Net cash provided by (used in) financing activities | | | 35,023 | | | | 365 | | | | (7,911 | ) |
| Net increase in cash and cash equivalents | | | 19,213 | | | | 987 | | | | 3,294 | |
| Cash and cash equivalents: | | | | | | | | | | | | |
| Beginning of year | | | 12,939 | | | | 11,952 | | | | 8,658 | |
| Ending of year | | $ | 32,152 | | | $ | 12,939 | | | $ | 11,952 | |
| | | | | | | | | | | | | |
| Supplemental Disclosures of Cash Flow Information | | | | | | | | | | | | |
| Cash payments for: | | | | | | | | | | | | |
| Interest | | $ | - | | | $ | - | | | $ | 350 | |
| Income taxes | | $ | - | | | $ | - | | | $ | 1,461 | |
| | | | | | | | | | | | | |
| Supplemental Schedule of Noncash Investing Activities | | | | | | | | | | | | |
| Equipment purchased not yet paid (Note 4) | | $ | - | | | $ | (1,212 | ) | | $ | 2,203 | |
| Application of deposits towards purchases of equipment | | $ | - | | | $ | - | | | $ | 4,381 | |
The accompanying notes are an integral part of these consolidated financial statements.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Business, Recent Developments, Significant Accounting Policies and Recent Accounting Guidance
Nature of Business
Vitacost.com, Inc. (“Vitacost” or the “Company”) is a leading online retailer of health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals, as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. Vitacost was incorporated in 1994 and began its online retail activity in 1999. Vitacost sells an internally developed proprietary line of nutraceuticals as well as a wide selection of other manufacturers’ brand-name health and wellness products. The Company ships products from two distribution centers located in Lexington, North Carolina and Las Vegas, Nevada.
Significant Accounting Policies
Principles of Consolidation:
The consolidated financial statements include the accounts of Vitacost and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated in consolidation.
Reclassifications:
A reclassification on the 2011 consolidated balance sheets has been made to conform to the 2012 presentation. Reclassifications on the 2011 and 2010 consolidated statements of cash flows has been made to conform to the 2012 presentation.
Accounting Estimates:
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates inherent in the preparation of the accompanying consolidated financial statements include management’s forecast of future cash flows used as a basis to assess recoverability of long-lived assets, including intangible assets and goodwill, the reserve for inventory obsolescence, deferred revenue and assumptions used in the valuation of the Company’s stock-based compensation.
Cash and Cash Equivalents:
The Company considers all highly-liquid investments purchased with original maturities of three months or less at the date of purchase to be cash equivalents. The Company maintains its cash and cash equivalents in various bank deposit accounts which, at times, may exceed the federally-insured limits. The Company has not experienced any losses in such accounts. The Company believes it is not exposed to any significant credit risk on such accounts.
Restricted Cash:
Restricted cash consists of cash pledged as collateral to secure a vendor obligation.
Accounts Receivable:
Accounts receivable consist primarily of amounts in transit from banks for customer credit card, debit card and electronic benefit transfer transactions that are generally processed by the banks within seven days of authorization. Based on the Company’s historical collection experience and a review of the status of accounts receivable, an allowance for doubtful accounts of $0.2 million was recorded as of December 31, 2012 and 2011.
Inventory:
Inventory, consisting principally of health and wellness products, is primarily stated at the lower of cost or market using the first in, first out (“FIFO”) method.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Property and Equipment:
Property and equipment is stated at cost less accumulated depreciation. Leasehold improvements are amortized using the straight-line method over the shorter of the estimated useful life of the assets or the lease term. Depreciation is computed using the straight-line method over the following estimated useful lives:
| | | Years |
| Furniture, fixtures and equipment | | 3 – 10 |
| Computers | | 3 – 5 |
| Software | | 3 – 5 |
| Leasehold and building improvements | | 3 – 39 |
| Buildings | | 39 |
Upon retirement or sale, the cost and accumulated depreciation are eliminated from the accounts and the gain or loss, if any, is included in the consolidated statements of operations.
Goodwill:
The excess of purchase price over fair value of assets acquired and liabilities assumed in business combinations is classified as goodwill. The Company reviews goodwill for impairment at the reporting unit level annually or, when events or circumstances dictate, more frequently. The Company assesses qualitative factors to determine whether it is more likely than not that the fair value of its reporting unit is less than its carrying amount. In performing the qualitative assessment, the Company assesses relevant events and circumstances that may impact the fair value of its reporting unit, including the following: (i) macroeconomic conditions, (ii) industry and market considerations, (iii) overall financial performance, (iv) events affecting the reporting unit, (v) share price and (vi) recent fair value calculation for the reporting unit, if available. The Company performed this qualitative assessment as of December 31, 2012 as allowable per the applicable authoritative guidance.
After assessing the above described events and circumstances, if the Company determines that it is more likely than not that the fair value of its reporting unit is greater than its carrying value, then no further testing is required. Otherwise, the Company would perform a two-step impairment test to evaluate goodwill. Under the first step, the Company compares the estimated fair value of the reporting unit with its carrying value. If the estimated fair value of the reporting unit is less than its carrying value, the Company will complete a second step to determine the amount of the goodwill impairment that should be recorded.
Based on the Company’s qualitative assessment, it has concluded that it was more likely than not that the estimated fair value of its reporting unit exceeded its carrying value as of December 31, 2012 and thus, did not proceed to the two-step goodwill impairment test. No indicators of impairment exist primarily because the Company’s reporting unit’s fair value has consistently exceeded its carrying value by a significant margin.
Long-Lived Assets:
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company would evaluate potential impairment by comparing the carrying amount of the asset with the estimated undiscounted future cash flows associated with the use of the asset and its eventual disposition. Should the review indicate the asset is not recoverable, the Company’s carrying value of the asset would be reduced to its estimated fair value, which is measured by future discounted cash flows.
Revenue Recognition and Freight:
Revenue from the sale of vitamins, nutritional supplements and other health and wellness products is recognized when all the following criteria are met: a customer executes an order, the sales price and the shipping and handling fee have been determined, credit card authorization has occurred, collection is reasonably assured and the product has been received by the customer. The Company establishes a deferred revenue liability which represents orders that have been shipped but not yet received by the customer at the end of a given period. The Company’s sales terms grant customers certain limited rights of return for a 30-day period. It has been the Company’s experience that such returns have been insignificant. The Company recorded a reserve for returns of $0.2 million at December 31, 2012 and 2011.
Freight billed to customers is classified as revenue and totaled approximately $12.5 million, $9.0 million and $14.5 million for the years ended December 31, 2012, 2011, and 2010, respectively. Freight costs are expensed as incurred and recorded as a component of cost of goods sold. Freight expense totaled approximately $31.7 million, $26.0 million, and $23.0 million for the years ended December 31, 2012, 2011, and 2010, respectively.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Operating Expenses:
The Company’s operating expenses are grouped into three categories: fulfillment, sales and marketing, and general and administrative. The following is a brief synopsis of each category:
Fulfillment Expenses:
Fulfillment expenses include the costs of warehousing and shipping supplies, machinery and equipment, maintenance, employees, professional services and rent.
Sales and Marketing Expenses:
Sales and marketing expenses include online advertising and promotional expenditures, including third-party content license fees, affiliates and partners’ commissions, traditional media advertising, print expenses and payroll related expenses for personnel engaged in marketing, sales, customer service, and website development and maintenance. We expense advertising costs as incurred.
General and Administrative Expenses:
General and administrative expenses consist of executive compensation, information technology expenses, credit card processing fees, legal fees, professional services, employee expenses and general corporate expenses.
Earnings per Share
The Company computed earnings per share by dividing net income by the weighted-average number of common shares outstanding during the period. Diluted earnings per share is computed by giving effect to all potentially dilutive common shares, including stock options and warrants. The following table reconciles basic weighted-average shares outstanding to diluted weighted-average shares outstanding for the years ended December 31, 2012, 2011, and 2010:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| | | (In thousands) | |
| Weighted-average shares outstanding - basic | | | 32,675 | | | | 27,829 | | | | 27,704 | |
| Effect of dilutive securities | | | - | | | | - | | | | - | |
| Weighted-average shares outstanding - diluted | | | 32,675 | | | | 27,829 | | | | 27,704 | |
For the periods where the Company reported losses, all common stock equivalents are excluded from the computation of diluted earnings per share, since the result would be antidilutive. Securities that were not included in the calculation of diluted earnings per share because to do so would have been antidilutive for the periods presented, are as follows:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| | | (In thousands) | |
| Stock options | | | 2,391 | | | | 2,582 | | | | 2,341 | |
| Warrants | | | 1,681 | | | | - | | | | - | |
| Total antidilutive common stock equivalents excluded from diluted earnings per share | | | 4,072 | | | | 2,582 | | | | 2,341 | |
Stock-Based Compensation:
In September 2011, the Company obtained shareholder approval of the 2011 Incentive Compensation Plan (the “Plan”) which replaced the 2007 Stock Award Plan (the “2007 Plan”). The 2007 Plan will continue to govern awards previously granted under it. The Plan’s share reserve includes the sum of 6.0 million shares of the Company’s common stock, plus (i) any shares of its common stock which have been reserved but not issued pursuant to any awards granted under the 2007 Plan, and (ii) the number of shares subject to outstanding awards under the 2007 Plan that expire or otherwise terminate without having been exercised in full, or are forfeited to or repurchased by the Company. Under the terms of the Plan, options to purchase stock are granted at an exercise price that approximates the fair market value of the underlying shares at the time of the grant. Nonqualified options generally become exercisable on the date of grant and expire in ten years. Incentive stock options generally become exercisable over a five-year period and the maximum term of the option may not exceed ten years. Compensation expense related to stock options recognized in earnings in 2012, 2011, and 2010, was approximately $2.0 million, $1.0 million, and $1.5 million on a pretax basis, respectively.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company recognizes compensation expense for stock awards based on their fair value and related tax effects over the requisite service period. The fair value of each option award is estimated on the date of grant using an option valuation method that uses the assumptions noted in the below table. The valuation technique incorporates assumptions for the expected volatility of the stock price, the expected term of the option, expected dividends, forfeitures and risk-free interest rate for the expected term of the option. As a result of its limited trading history, the Company uses expected volatilities based on the historical volatility of a sample of companies in a similar industry and of a comparable size as the Company. The expected term of the option is calculated using simplified method, which analyzes the vesting terms of an option along with the contractual term, setting the expected term at a midpoint in between. The risk-free interest rate takes into account the time-value of money. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at time of grant. The Company estimates forfeitures based on historical pre-vesting forfeitures and revises those estimates in subsequent periods if actual forfeitures differ from those estimates.
The fair value of each option granted was estimated using the following assumptions:
| | | | 2012 | | | | 2011 | | | | 2010 | |
| Expected life - employees | | | 6-7 years | | | | N/A | | | | 7 years | |
| Expected life - executives | | | 6-7 years | | | | 5.5 - 6.5 years | | | | 5.75 - 7 years | |
| Volatility percentage | | | 54.0%-55.2 | % | | | 62.6% - 63.9 | % | | | 67.9% - 68.8 | % |
| Risk free interest rate | | | 0.83%-1.22 | % | | | 1.13% - 1.94 | % | | | 1.55% - 3.16 | % |
| Dividends | | | None | | | | None | | | | None | |
Fair Value of Financial Instruments:
Existing accounting guidance defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and requires disclosures about fair value measurements. The guidance applies to all assets and liabilities that are being measured and reported on a fair value basis. It requires disclosure that establishes a framework for measuring fair value in GAAP and about fair value measurements. This guidance enables the reader of the financial statements to assess the inputs used to develop those measurements by establishing a hierarchy for ranking the quality and reliability of the information used to determine fair values. Assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market data.
The carrying amounts of other financial instruments, including cash and cash equivalents, accounts receivable, other receivables and accounts payable approximate fair value due to the short maturity of these instruments. Cash and cash equivalents are a Level 1 instrument within the fair value hierarchy.
Concentration of Credit Risk:
The Company’s cash and cash equivalents were held by one major financial institution and for certain accounts exceed federally insured limits. These cash and cash equivalent balances could be impacted if this financial institution fails or is subjected to other adverse conditions in the financial markets. To date, the Company has experienced no loss or lack of access to its cash and cash equivalents.
Income Taxes:
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income in the period that includes the enactment date.
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The Company has determined that there were no material uncertain tax positions and accordingly no associated interest and penalties were required to be accrued at December 31, 2012 and 2011, respectively.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Contingencies:
On an ongoing basis, the Company assesses potential liabilities related to lawsuits or claims brought against it. While it is typically very difficult to determine the timing and ultimate outcome of such actions, the Company’s management uses their best judgment to determine if it is probable that it will incur an expense related to the settlement or final adjudication of such matters and whether a reasonable estimation of such probable loss, if any, can be made. In assessing probable losses, the Company takes into consideration estimates of the amount of insurance recoveries, if any. The Company accrues a liability when it believes a loss is probable and the amount of loss can be reasonably estimated. Due to the inherent uncertainties related to the eventual outcome of litigation and potential insurance recoveries, it is possible that certain matters may be resolved for amounts materially different from any provisions or disclosures that the Company has previously made.
Recent Accounting Guidance
Recently Adopted Accounting Guidance:
On January 1, 2012, the Company adopted provisions of the authoritative guidance related to changes to fair value measurement and disclosure. Specifically, the guidance includes clarification about when the concept of highest and best use is applicable to fair value measurements, requires quantitative disclosures about inputs used and qualitative disclosures about the sensitivity of recurring Level 3 measurements, and requires the classification of all assets and liabilities measured at fair value in the fair value hierarchy, including those assets and liabilities which are not recorded at fair value but for which fair value is disclosed. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
On January 1, 2012, the Company adopted provisions of the authoritative guidance related to the changes to the presentation of comprehensive income. Specifically, the guidance gives an entity the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The Company elected to present components of net income and other comprehensive income in a single continuous statement. The components of other comprehensive income are presented net of the related tax effects. Other than the changes in presentation, the adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
2. Private Placement
On February 16, 2012, the Company entered into a Purchase Agreement (the “Purchase Agreement”) by and among Vitacost, JHH Capital, LLC, an entity affiliated with Jeffrey Horowitz, our Chief Executive Officer (“JHH”), Great Hill Equity Partners III, L.P. (“Great Hill III”), Great Hill Equity Partners IV, L.P. (“Great Hill IV”), Great Hill Investors, LLC (“Great Hill Investors”), Freshford Partners, LP (“Freshford Partners”), Freshford Master Fund, Ltd (“Freshford Master Fund”) and Baron Small Cap Fund (“Baron” and, together with JHH, Great Hill III, Great Hill IV, Great Hill Investors, Freshford Partners, Freshford Master Fund, collectively, the “Investors”) pursuant to which the Investors purchased, and Vitacost sold, an aggregate of 4.9 million shares of the Company’s common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1.7 million shares of the Company’s common stock for an aggregate purchase price of $34.8 million. The warrants have an exercise price of $7.04 per share and a term of four years. The net proceeds of $33.6 million, after the deduction of fees of $1.2 million incurred in connection with the transaction, were allocated between common stock and warrants based on their relative fair values as of the purchase date.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
3. Inventory
Inventory consists of the following as of December 31, 2012 and 2011:
| | | December 31, | |
| | | 2012 | | | 2011 | |
| | | (In thousands) | |
| Raw materials | | $ | - | | | $ | 2,959 | |
| Work in process and bulk | | | 761 | | | | 2,591 | |
| Finished goods | | | 32,558 | | | | 29,272 | |
| | | $ | 33,319 | | | $ | 34,822 | |
On August 28, 2012, the Company entered into an agreement to outsource its manufacturing operations and lease its manufacturing facilities to a third party provider effective September 1, 2012. In connection with the outsourcing of its manufacturing operations, the Company sold certain inventory to the third party provider which will be used to manufacture products for the Company. The total selling price was $2.2 million, which will be paid in six monthly payments of $0.4 million with the first payment due on October 1, 2012. The Company incurred a loss of $0.1 million on the sale of the inventory.
4. Property and Equipment
Property and equipment consists of the following as of December 31, 2012 and 2011:
| | | December 31, | |
| | | 2012 | | | 2011 | |
| | | (In thousands) | |
| Buildings and building improvements | | $ | 12,759 | | | $ | 12,428 | |
| Furniture, fixtures and equipment | | | 24,157 | | | | 21,594 | |
| Computers | | | 3,387 | | | | 2,709 | |
| Software | | | 7,778 | | | | 7,235 | |
| Leasehold improvements | | | 2,670 | | | | 2,620 | |
| Land | | | 460 | | | | 460 | |
| | | | 51,211 | | | | 47,046 | |
| Less accumulated depreciation | | | (21,045 | ) | | | (14,995 | ) |
| | | | 30,166 | | | | 32,051 | |
| Construction-in-progress | | | 3,325 | | | | 1,578 | |
| | | $ | 33,491 | | | $ | 33,629 | |
Construction-in-progress was primarily related to the upgrade of the Company’s Lexington, North Carolina distribution facility. During 2011, the Company was released from its obligation to purchase $1.2 million of equipment which was previously recorded in construction-in-progress.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
5. Accrued Expenses
Accrued expenses consist of the following at December 31, 2012 and 2011:
| | | December 31, | |
| | | 2012 | | | 2011 | |
| | | (In thousands) | |
| Accrued salaries and related expenses | | $ | 1,693 | | | $ | 2,322 | |
| Advertising and related expenses | | | 1,110 | | | | 487 | |
| Other accrued expenses | | | 3,742 | | | | 3,616 | |
| | | $ | 6,545 | | | $ | 6,425 | |
6. Stock Option Plan
A summary of the Company’s stock option activity for common stock for the years ended December 31, 2012, 2011 and 2010 is as follows:
| | | Options | |
| | | Shares | | | Weighted
Average
Exercise
Price | | | Weighted
Average Grant
Date Fair Value | | | Weighted Average
Remaining
Contractual Life
(Years) | | | Aggregate
Intrinsic Value | |
| | | ( In thousands, except per share) | |
| Outstanding, January 1, 2010 | | | 2,746 | | | $ | 5.67 | | | $ | 2.16 | | | | 6.67 | | | $ | 11,990 | |
| Granted | | | 447 | | | | 9.36 | | | | 5.60 | | | | 9.21 | | | | | |
| Exercised | | | (292 | ) | | | 2.91 | | | | 1.14 | | | | 3.96 | | | | 2,400 | |
| Cancelled | | | (47 | ) | | | 7.25 | | | | 2.02 | | | | 9.80 | | | | | |
| Forfeited | | | (136 | ) | | | 8.08 | | | | 3.14 | | | | 9.44 | | | | | |
| Outstanding, December 31, 2010 | | | 2,718 | | | $ | 6.42 | | | $ | 2.79 | | | | 7.01 | | | $ | 1,897 | |
| Granted | | | 1,840 | | | | 4.42 | | | | 2.77 | | | | 10.19 | | | | | |
| Exercised | | | (194 | ) | | | 2.19 | | | | 0.46 | | | | 2.68 | | | | 733 | |
| Cancelled | | | (25 | ) | | | 7.76 | | | | 2.88 | | | | 8.14 | | | | | |
| Forfeited | | | (77 | ) | | | 9.46 | | | | 5.99 | | | | 7.19 | | | | | |
| Outstanding, December 31, 2011 | | | 4,262 | | | $ | 5.69 | | | $ | 2.83 | | | | 7.59 | | | $ | 6,945 | |
| Granted | | | 2,197 | | | | 6.73 | | | | 3.42 | | | | 8.13 | | | | | |
| Exercised | | | (605 | ) | | | 2.29 | | | | 0.85 | | | | 2.01 | | | | 2,573 | |
| Cancelled | | | (28 | ) | | | 7.89 | | | | 3.33 | | | | 0.06 | | | | | |
| Forfeited | | | (617 | ) | | | 6.61 | | | | 3.62 | | | | - | | | | | |
| Outstanding, December 31, 2012 | | | 5,209 | | | $ | 6.40 | | | $ | 3.21 | | | | 7.79 | | | $ | 6,238 | |
| | | | | | | | | | | | | | | | | | | | | |
| Exercisable at December 31, 2011 | | | 2,582 | | | $ | 6.12 | | | $ | 2.68 | | | | 6.09 | | | $ | 4,373 | |
| Exercisable at December 31, 2012 | | | 2,391 | | | $ | 6.84 | | | $ | 4.18 | | | | 6.81 | | | $ | 3,341 | |
As of December 31, 2012, there was approximately $7.2 million of total unrecognized compensation cost, net of estimated forfeitures related to stock options granted under the Company’s stock incentive plan, which is expected to be recognized over a weighted average period of 3.64 years.
7. Leases
Certain office space is leased under noncancelable operating leases expiring in various years through 2019. Future minimum lease payments, by year, and in the aggregate, under operating leases are due as follows as of December 31, 2012:
| | | Operating | |
| Year Ending December 31, | | Leases | |
| | | | (In thousands) | |
| 2013 | | $ | 1,700 | |
| 2014 | | | 1,660 | |
| 2015 | | | 588 | |
| 2016 | | | 606 | |
| 2017 | | | 624 | |
| Thereafter | | | 751 | |
| Total minimum lease payments | | $ | 5,929 | |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The total rental expense included in the statements of comprehensive loss for the years ended December 31, 2012, 2011, and 2010 was $2.0 million, $2.1 million, and $2.1 million, respectively
8. Income Taxes
The Company files income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions. Pursuant to applicable statutes of limitation, returns open for adjustment by the Internal Revenue Service (“IRS”) are the consolidated federal income tax returns for tax years ended December 31, 2011 and 2012. Pursuant to applicable statutes of limitation, state income tax returns open for adjustment by the state tax authorities are for tax years ended December 31, 2004 through December 31, 2012. The IRS previously examined the Company’s 2008 federal income tax return, which resulted in no change to the Company’s tax return. The Company has also recently concluded an examination of its income tax returns for 2009 and 2010 by the IRS. The examination resulted in no material changes.
A number of years may elapse before an uncertain tax position is audited and finally resolved. Settlement of any particular position would usually require the use of cash. The resolution of a matter would be recognized as an adjustment to the provision for income taxes and the effective tax rate in the period of resolution. The Company has determined that there are no material uncertain tax positions and accordingly no associated interest and penalties are required to be accrued at December 31, 2012.
Reconciliation of the federal statutory income tax rate to the Company’s effective tax rate is as follows:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| Taxes computed at federal tax rate | | $ | (6,500 | ) | | $ | (5,025 | ) | | $ | (4,682 | ) |
| State income taxes, net of federal income tax benefit, excluding change in valuation allowance | | | (281 | ) | | | (277 | ) | | | (236 | ) |
| Non-taxable income | | | - | | | | (6 | ) | | | (27 | ) |
| Meals and entertainment | | | 7 | | | | 6 | | | | 6 | |
| Incentive stock options | | | 268 | | | | (34 | ) | | | 83 | |
| Other tax assets | | | 916 | | | | 556 | | | | (75 | ) |
| Change in valuation allowance – state | | | 437 | | | | 404 | | | | 944 | |
| Change in valuation allowance - federal | | | 5,206 | | | | 4,429 | | | | 5,408 | |
| Income tax expense | | $ | 53 | | | $ | 53 | | | $ | 1,421 | |
The provision for income taxes charged to operations for the years ended December 31, 2012, 2011 and 2010, consists of the following:
| | | Year Ended December 31, | |
| | | 2012 | | | 2011 | | | 2010 | |
| Current tax expense (benefit) | | $ | 277 | | | $ | - | | | $ | (1,630 | ) |
| Deferred tax (benefit) expense | | | (224 | ) | | | 53 | | | | 3,051 | |
| Income tax expense | | $ | 53 | | | $ | 53 | | | $ | 1,421 | |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The tax effects of temporary differences that give rise to significant components of deferred tax assets at December 31, 2012 and 2011 are presented below:
| | | December 31, | |
| | | 2012 | | | 2011 | |
| Deferred tax assets: | | | | | | | | |
| Net operating loss and credits carryforward | | $ | 15,758 | | | $ | 8,930 | |
| Inventory | | | 366 | | | | 375 | |
| Accrued compensation | | | 29 | | | | 393 | |
| Stock-based compensation | | | 3,258 | | | | 4,153 | |
| Accrued expenses | | | - | | | | 174 | |
| Deferred revenue and reserves | | | 678 | | | | 791 | |
| | | | 20,089 | | | | 14,816 | |
| Deferred tax liability: | | | | | | | | |
| Depreciation and amortization | | | (3,611 | ) | | | (4,205 | ) |
| | | | - | | | | - | |
| Valuation allowance - state | | | (1,785 | ) | | | (1,348 | ) |
| Valuation allowance - federal | | | (15,043 | ) | | | (9,837 | ) |
| Deferred tax liability, net | | $ | (350 | ) | | $ | (574 | ) |
The deferred tax amounts mentioned above have been classified on the accompanying consolidated balance sheets as follows:
| | | December 31, | |
| | | 2012 | | | 2011 | |
| Deferred tax liability, net | | | | | | | | |
| Noncurrent liability | | $ | 350 | | | $ | 574 | |
The Company evaluates its deferred tax assets on a regular basis to determine if valuation allowances are required. In its evaluation, the Company considers taxable loss carryback availability, expectations of sufficient future taxable income, trends in earnings, existence of taxable income in recent years, the future reversal of temporary differences and the available tax planning strategies that could be implemented, if required. Valuation allowances are established based on the consideration of all available evidence using a more likely than not standard. Based on the Company’s evaluation, a valuation allowance of $16.8 million was established against its net deferred tax assets as of December 31, 2012. The Company’s deferred tax assets for which it has not established a valuation allowance relate to amounts than can be realized through future reversals of existing taxable temporary differences. The Company’s valuation allowance will be reversed if and when it becomes more likely than not that the Company can generate sufficient taxable income in the future to utilize the tax benefits of the related deferred tax assets. The noncurrent deferred tax liability of $0.4 million relates to the excess book basis over tax basis of our goodwill, net of a tax credit carryforward, which has an indefinite life. Because goodwill has an indefinite life, the reversal of the book basis over tax basis is not considered a source of taxable income to support the realization of the Company’s deferred tax assets. Subsequent revisions to the estimated net realizable value of the deferred tax asset or deferred tax liability could cause the provision for income taxes to vary significantly from period to period.
As of December 31, 2012, the Company had unused federal net operating loss carryforwards of approximately $42.7 million, which were generated during the year ended December 31, 2012, 2011 and 2010. As of December 31, 2012, the Company had Florida and North Carolina net operating loss carryforwards of approximately $12.4 million and $11.0 million, respectively. The state net operating loss carryforwards, if not utilized, expire beginning in December 2023.
During the years ended December 31, 2012, 2011 and 2010 employees exercised incentive stock options and sold the stock in disqualifying dispositions. For the year ended December 31, 2010 a portion of the tax benefit associated with the sale of the stock has been recognized as an income tax benefit. Generally, incentive stock options provide employees with significant tax benefits by allowing the employee to exercise stock options without being taxed on the intrinsic value on the exercise date provided the employee owns the stock for specified periods of time. Since the employer will not receive a tax benefit upon the exercise of incentive stock options, unless there is a disqualifying disposition, the compensation expense associated with incentive stock options is treated as a permanent difference between book and tax accounting and consequently affects the effective tax rate of the Company. For the year ended December 31, 2010, the disqualifying disposition of incentive stock options resulted in a related tax benefit of $0.6 million, which has been recorded as a reduction in income taxes payable and an increase in additional paid-in capital in the accompanying consolidated balance sheet; there were no tax benefits recognized during the years ended December 31, 2012 and 2011.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9. Related Party Transactions
In October 2010, the Company entered into a stockholder agreement with Great Hill Investors, LLC, Great Hill Equity Partners III, L.P., and Great Hill Equity Partners IV, L.P. (collectively, “GHP”), whereby GHP agreed to various restrictions with respect to the voting, transfer and sale of shares of the Company’s common stock which it currently owns and with respect to any shares it may own in the future in excess of 30% of the Company’s outstanding common stock. In addition, the Company and Great Hill entered into a registration rights agreement, which provides Great Hill with certain demand, incidental and shelf registration rights subject to various exceptions and qualifications. Lastly, the Company agreed to reimburse Great Hill $0.7 million for its reasonable and documented out-of-pocket expenses incurred in connection with a consent solicitation that occurred in 2010. The Company reimbursed $0.4 million and $0.3 million to Great Hill in 2011 and 2010, respectively.
The Company paid Board members a total of $0.1 million in consulting fees during the year ended December 31, 2010. Such amounts have been included in general and administrative expenses in the statements of comprehensive loss. No consulting fee payments were made to Board members in 2012 and 2011.
As discussed in Note 2, on February 16, 2012, the Company entered into the Purchase Agreement with the Investors for the purchase of shares of common stock and warrants to purchase shares of common stock of the Company for an aggregate purchase price of $34.8 million. Concurrently with entering into the Purchase Agreement, the Company entered into a registration rights agreement with Jeffrey Horowitz and JHH which provides Jeffrey Horowitz and JHH with demand, shelf and piggyback registration rights subject to various exceptions and qualifications. The registration rights agreement further provides that if the Company enters into a registration rights agreement (or similar agreement) with any person or entity on more favorable terms than those contained in the registration rights agreement, the registration rights agreement will be amended to include such more favorable terms. Pursuant to the registration rights agreement, the Company has agreed to pay all of the registration costs and expenses incurred in connection with such demand, shelf and piggyback registrations.
10. Retirement Plan
The Company has a defined contribution plan which covers substantially all of the Company’s employees. Under the plan, the Company matches 100% of the first 3% of contributions and 50% of the next 2% of contributions. The Company’s contribution is based on a maximum of 5% of the employee’s contributions. The amount contributed to the plan by the Company amounted to $0.7 million, $0.6 million, and $0.3 million for the years ended December 31, 2012, 2011, and 2010, respectively.
11. Contingencies
Securities Class Action:
On May 24, 2010, a punitive class action complaint was filed in the United States District Court for the Southern District of Florida against the Company and certain current and former officers and directors by a stockholder on behalf of herself and other stockholders who purchased Vitacost common stock between September 24, 2009 and April 20, 2010, captioned Miyahira v. Vitacost.com, Inc., Ira P. Kerker, Richard P. Smith, Stewart Gitler, Allen S. Josephs, David N. Ilfeld, Lawrence A. Pabst, Eran Ezra, and Robert G. Trapp, Case 9:10-cv-80644-KLR. After being appointed to represent the purported class of shareholders, the lead plaintiffs filed an amended complaint asserting claims under Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 and Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder against Vitacost, its current and former officers and directors, and the underwriters of its initial public offering (“IPO”). On December 12, 2011, the Court granted defendants’ motion to dismiss the complaint, and granted plaintiffs leave to amend.
On January 11, 2012, lead plaintiff filed its second amended complaint asserting claims under Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 against Vitacost, its current and former officers and directors, and its underwriters. Lead plaintiff purports to bring its action on behalf of investors who purchased stock in connection with or traceable to the Company’s IPO between September 24, 2009 and April 20, 2010. The complaint alleges that the defendants violated the federal securities laws during the period by, among other things, disseminating false and misleading statements and/or concealing material facts concerning the Company’s current and prospective business and financial results. The complaint also alleges that as a result of these actions the Company’s stock price was artificially inflated during the class period. The complaint seeks unspecified compensatory damages, costs, and expenses.
On June 25, 2012, the Southern District of Florida entered its order granting defendants’ motion to dismiss in full and dismissing the second amended complaint with prejudice. On July 23, 2012, lead plaintiff filed a notice of appeal to the Eleventh Circuit of the order granting defendants’ motion to dismiss. Plaintiff-Appellant filed its opening appellate brief on September 17, 2012, Defendant-Appellees filed their responding brief on October 29, 2012, and Plaintiff Appellant filed its reply in support of its opening brief on December 3, 2012. Oral argument before the Eleventh Circuit is currently scheduled for the week of April 8, 2013.
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company records provisions in its consolidated financial statements for pending litigation when it determines that an unfavorable outcome is probable and the amount of loss can be reasonably estimated. As of December 31, 2012, the Company has concluded that it is not probable that a loss has been incurred and is unable to estimate the possible loss or range of loss that could result from an unfavorable verdict. Therefore, the Company has not provided any amounts in the consolidated financial statements for an unfavorable outcome. The Company believes that it has meritorious arguments for affirmation of the Southern District of Florida’s order that it will raise in the appeal. It is possible that the Company’s consolidated financial statements could be materially adversely affected by an unfavorable outcome.
Sales or Other Taxes:
A number of states have sought to impose sales or other tax collection obligations on online retailers. Certain states have imposed such a sales tax obligation requirement on remote online retailers that use residents of that state to directly or indirectly refer potential customers, via a link on an internet website or other means, to the online retailer for a commission-based fee. There is still significant uncertainty as to whether or how existing laws governing these matters apply to Vitacost and its relationships with its sales affiliates and how these laws will be interpreted for the Company and other online retailers. As a result, it is currently not possible to determine the ultimate outcome as to whether these obligations apply to the Company under its specific facts and circumstances. Because the Company does not believe that it is probable that these obligations are applicable to its specific facts and circumstances, it has not accrued for these obligations as of December 31, 2012. The Company is also currently unable to estimate the amount of the loss, if any, should these obligations apply to it. The eventual outcome of a successful assertion by one or more states that the Company should collect sales or other taxes may be materially different from any provisions or disclosures the Company has previously made and could have a material adverse effect on the Company’s financial position, results of operations and cash flows.
Other matters:
In addition to the matter described above, the Company is involved in litigation and administrative proceedings primarily arising in the normal course of its business. In the opinion of the Company, except as set forth above, its liability, if any, under any other pending litigation or administrative proceedings would not materially affect its financial condition, results of operations or cash flows. Furthermore, the Company has not been the subject of any product liability litigation.
12. Quarterly Selected Financial Data (Unaudited)
| | | First Quarter | | | Second Quarter | | | Third Quarter | | | Fourth Quarter | |
| (In Thousands, Except per | | | | | | | | | | | | | | | | | | | | | | | | |
| Share Data) | | 2012 | | | 2011 | | | 2012 | | | 2011 | | | 2012 | | | 2011 | | | 2012 | | | 2011 | |
| Net sales | | $ | 83,592 | | | $ | 63,762 | | | $ | 79,877 | | | $ | 65,891 | | | $ | 82,218 | | | $ | 63,456 | | | $ | 84,992 | | | $ | 67,414 | |
| Operating loss | | | (6,573 | ) | | | (2,216 | ) | | | (4,310 | ) | | | (3,684 | ) | | | (5,206 | ) | | | (5,359 | ) | | | (3,184 | ) | | | (3,566 | ) |
| Net loss | | | (6,554 | ) | | | (2,222 | ) | | | (4,302 | ) | | | (3,700 | ) | | | (5,141 | ) | | | (5,341 | ) | | | (3,173 | ) | | | (3,568 | ) |
| Loss per share | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | | (0.21 | ) | | | (0.08 | ) | | | (0.13 | ) | | | (0.13 | ) | | | (0.15 | ) | | | (0.19 | ) | | | (0.09 | ) | | | (0.13 | ) |
| Diluted | | $ | (0.21 | ) | | $ | (0.08 | ) | | $ | (0.13 | ) | | $ | (0.13 | ) | | $ | (0.15 | ) | | $ | (0.19 | ) | | $ | (0.09 | ) | | $ | (0.13 | ) |
During the quarter ended December 31, 2012, the Company recorded an adjustment to reduce bonus expense by $1.1 million dollars.
During the quarter ended September 30, 2011, the Company recorded an out of period adjustment to increase stock-based compensation expense by $0.4 million related to a stock-based option grant that occurred in June 2011 under the Company’s 2007 Stock Award Plan, the terms of which provided for the immediate vesting of certain options under the grant. The adjustment was to expense the portion of the award which vested immediately at the grant date. The Company concluded that the adjustment was not material to the previously reported consolidated financial statements.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, conducted an evaluation of the effectiveness of our disclosure controls and procedures, as such term is defined in Exchange Act Rule 13a-15(e), as of the end of the period covered by this report. Based upon such evaluation, our Chief Executive Officer and Chief Financial Officer concluded that those controls and procedures are effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure and are effective to provide reasonable assurance that such information is recorded, processed, summarized and reported within the time periods specified by the SEC’s rules and forms.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2012.
The effectiveness of our internal control over financial reporting as of December 31, 2012 has been audited by PricewaterhouseCoopers LLP, an independent registered certified public accounting firm, as stated in their report, which is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 during the quarter ended December 31, 2012 that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including the Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls or internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within Vitacost have been detected.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2012.
Item 11. Executive Compensation
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2012.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2012.
Item 13. Certain Relationships, Related Transactions and Director Independence
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2012.
Item 14. Principal Accounting Fees and Services
The information required under this item is incorporated herein by reference to the Company’s definitive proxy statement pursuant to Regulation 14A, which proxy statement will be filed with the Securities and Exchange Commission not later than 120 days after the close of the Company’s fiscal year ended December 31, 2012.
PART IV
Item 15. Exhibits and Financial Statement Schedules
| (a) | The following documents are filed as part of this Report: |
| 1. | Financial Statements: The information concerning Vitacost’s financial statements, and Report of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm required by this Item is incorporated by reference herein to the section of this Report in Item 8, entitled “Financial Statements and Supplementary Data.” |
| 2. | Financial Statement Schedule: The following financial statement schedule of Vitacost.com, Inc., for the fiscal years ended December 31, 2012, 2011 and 2010, is filed as part of this Report and should be read in conjunction with the Consolidated Financial Statements of Vitacost.com, Inc. |
Schedule II Valuation and Qualifying Accounts
Schedules not listed above have been omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Consolidated Financial Statements or Notes thereto.
| 3. | Exhibits: See Item 15(b) below. We have filed, or incorporated into this Report by reference, the exhibits listed on the accompanying Index to Exhibits immediately following the signature page of this Form 10-K. |
We have filed, or incorporated into the Report by reference, the exhibits listed on the accompanying Index to Exhibits immediately following the signature page of this Form 10-K.
| (c) | Financial Statement Schedules: See Item 15(a), above. |
Item 15(A)(2) Financial Statement Schedule II
All schedules have been omitted as the requested information is inapplicable or the information is presented in the financial statements or related notes included as part of this Report.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | VITACOST.COM, INC. |
| | | | |
| Dated: March 4, 2013 | | By: | /s/ Jeffrey J. Horowitz |
| | | | Jeffrey J. Horowitz, |
| | | | Chief Executive Officer |
| | | | |
| | | | /s/ Brian D. Helman |
| | | | Brian D. Helman |
| | | | Chief Financial Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS , that each person whose signature appears below constitutes and appoints Jeffrey J. Horowitz and Brian D. Helman each as his attorney-in-fact, each with the power of substitution, for him in any and all capacities, to sign any amendments to this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Signature | | Title | | Date |
| /s/ Jeffrey J. Horowitz | | Chief Executive Officer (Principal Executive Officer) | | March 4, 2013 |
| Jeffrey J. Horowitz | | | | |
| | | | | |
| /s/ Brian D. Helman | | Chief Financial Officer (Principal Financial Officer) | | March 4, 2013 |
| Brian D. Helman | | | | |
| | | | | |
| /s/ Michael Kumin | | Director (Interim Chairman of the Board) | | March 4, 2013 |
| Michael Kumin | | | | |
| | | | | |
| /s/ Christopher Gaffney | | Director | | March 4, 2013 |
| Christopher Gaffney | | | | |
| | | | | |
| /s/ Stuart Goldfarb | | Director | | March 4, 2013 |
| Stuart Goldfarb | | | | |
| | | | | |
| /s/ Edwin J. Kozlowski | | Director | | March 4, 2013 |
| Edwin J. Kozlowski | | | | |
| | | | | |
| /s/ Robert G. Trapp | | Director | | March 4, 2013 |
| Robert G. Trapp | | | | |
EXHIBIT INDEX
Exhibit Number | | Exhibits |
| 3.1(1) | | Certificate of Incorporation of the Registrant |
| 3.2(1) | | Bylaws of the Registrant |
| 4.1(2) | | Specimen of Common Stock Certificate |
| 4.2†(3) | | Form of Nonstatutory Stock Option Agreement |
| 4.3†(4) | | Form of Incentive Stock Option Agreement |
| 4.4(5) | | Stockholder Agreement dated October 8, 2010 by and between Vitacost.com, Inc. and Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Partners IV, L.P. |
| 4.5(5) | | Registration Rights Agreement dated October 8, 2010 by and between Vitacost.com, Inc. and Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Partners IV, L.P. |
| 4.6(14) | | Form of Warrant to Purchase Common Stock |
| 10.1†(3) | | 2000 Stock Option Plan |
| 10.2†(6) | | 2007 Stock Award Plan |
| 10.3†(7) | | 2011 Incentive Compensation Plan |
| 10.4†(8) | | Employment Agreement dated October 19, 2010 between Vitacost.com, Inc. and Stephen E. Markert, Jr. |
| 10.5†(9) | | Employment Agreement dated February 16, 2011 between Vitacost.com, Inc. and Jeffery Horowitz |
| 10.6†(10) | | Employment Agreement dated August 4, 2011 between Vitacost.com, Inc. and Robert Wegner |
| 10.7†(11) | | Employment Agreement dated August 18, 2011 between Vitacost.com, Inc. and David Zucker |
| 10.8†(12) | | Employment Agreement dated November 28, 2011 between Vitacost.com, Inc. and Nachiket Desai |
| 10.9†(13) | | Employment Agreement dated January 25, 2012 between Vitacost.com, Inc. and Brian Helman |
| 10.10†(2) | | Form of Indemnification Agreement between Vitacost.com, Inc. and each officer and director |
| 10.11(14) | | Purchase Agreement among Vitacost.com, Inc. and the Purchasers identified on Exhibit A thereto, dated February 16, 2012 |
| 10.12(14) | | Registration Rights Agreement among Vitacost.com, Inc., Jeffrey Horowitz and JHH Capital, LLC, dated February 16, 2012 |
| 10.13†(15) | | Employment Termination and General Release Agreement between Vitacost.com, Inc. and Stephen E. Markert, Jr., dated February 24, 2012 |
| 10.14†(16) | | Employment Agreement between Vitacost.com, Inc. and Joseph R. Topper, Jr., dated August 6, 2012 |
| 10.15(17) | | Manufacturing Agreement between Vitacost.com, Inc. and Nature’s Value Inc., dated August 28, 2012 |
| 10.16(17) | | Facilities Lease Agreement among Vitacost.com, Inc., Nutra-Pharma Manufacturing Corp. and Nature’s Value, Inc., dated August 28, 2012 |
| 10.17(17) | | Equipment Rental Agreement among Vitacost.com, Inc., Nutra-Pharma Manufacturing Corp. and Nature’s Value, Inc., dated August 28, 2012 |
| 10.18(17) | | Services Agreement among Vitacost.com, Inc. and Nutra-Pharma Manufacturing Corp., dated August 28, 2012 |
| 21.1 | | List of Subsidiaries of Registrant |
| 23.1 | | Consent of Independent Registered Certified Public Accounting Firm |
| 31.1 | | Certification of Chief Executive Officer Pursuant to Rule 13-14(a) of the Securities and Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | | Certification of Chief Financial Officer Pursuant to Rule 13-14(a) of the Securities and Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 101.INS | | XBRL Instance Document |
| 101.SCH | | XBRL Taxonomy Extension Schema Document |
| 101.CAL | | XBRL Taxonomy Calculation Linkbase Document |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document |
| † | Denotes a management contract or compensatory plan or arrangement. |
| (1) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K12B filed with the Securities and Exchange Commission on September 28, 2011. |
| (2) | Filed as an Exhibit to Amendment No. 7 of Registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on September 21, 2009. |
| (3) | Filed as an Exhibit to Registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 20, 2007. |
| (4) | Filed as an Exhibit to Registrant’s Amendment No. 4 of Registration Statement on Form S-1 filed with the Securities and Exchange Commission on July 28, 2009. |
| (5) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 13, 2010. |
| (6) | Filed as an Exhibit to Registrant’s Amendment No. 3 of Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 12, 2009. |
| (7) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on September 8, 2011. |
| (8) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2010. |
| (9) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 22, 2011. |
| (10) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 9, 2011. |
| (11) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 22, 2011. |
| (12) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 28, 2011. |
| (13) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 31, 2012. |
| (14) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 21, 2012. |
| (15) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 29, 2012. |
| (16) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 6, 2012. |
| (17) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 28, 2012. |
