Form 6-K
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the month of January 2008
Commission File Number: 001-33623
WuXi PharmaTech (Cayman) Inc.
288 Fute Zhong Road, Waigaoqiao Free Trade Zone
Shanghai 200131
People’s Republic of China
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F þ Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No þ
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b):82-N/A
WuXi PharmaTech (Cayman) Inc.
Form 6-K
TABLE OF CONTENTS
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| WuXi PharmaTech (Cayman) Inc. |
| |
| By: | | /s/ Benson Tsang |
| Name: | | Benson Tsang |
| Title: | | Chief Financial Officer |
Date: January 4, 2008
3
Exhibit 99.1

WUXI PHARMATECH TO ACQUIRE
APPTEC LABORATORY SERVICES, INC.
SHANGHAI, China and ST. PAUL, Minnesota, USA – January 3, 2008 – WuXi PharmaTech (NYSE: WX), China’s premier provider of pharmaceutical R&D outsourcing services has signed a definitive agreement to acquire US-based AppTec Laboratory Services, Inc. (AppTec). The acquisition of AppTec allows WuXi PharmaTech to immediately obtain biologics capabilities and expertise, gain a significant U.S. operational footprint, and expand its customer base and addressable market size. The combined business operations of WuXi PharmaTech and AppTec in both the U.S. and China will enable WuXi PharmaTech to provide a full service suite of outsourced chemistry and biology services to global pharmaceutical, biotechnology and medical device clients. The transaction consideration totals approximately $151 million with the assumption of AppTec debt totaling approximately $11.7 million.
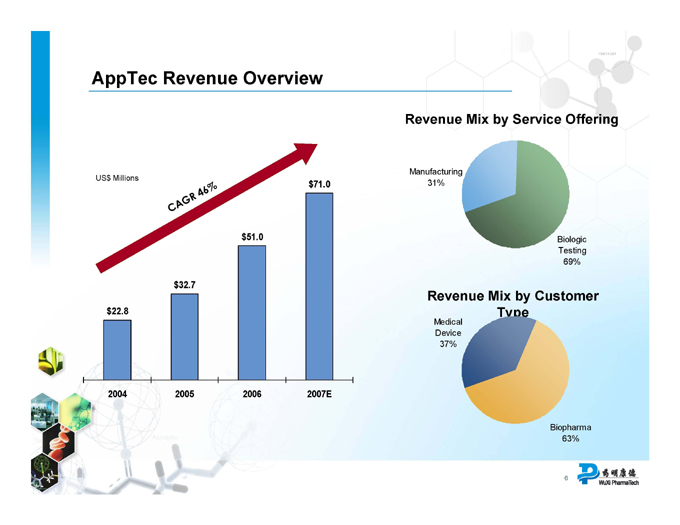
AppTec is a trusted quality service provider for the biopharmaceutical and medical device industries, offering testing, contract research and development, and biologics cGMP manufacturing services from a highly differentiated, fully integrated service platform. Along with state-of-the-art FDA registered facilities located in Philadelphia, St. Paul and Atlanta, AppTec brings established customer relationships with many leading pharmaceutical, biotechnology and medical device companies in the U.S. and around the world. AppTec’s full year 2007 revenue is expected to be in the range of $70 million to $72 million. Since 2004 and including the expected revenue for 2007, AppTec will have achieved a compounded annual revenue growth rate of approximately 46%.
Dr. Ge Li, Chairman and Chief Executive Officer of WuXi PharmaTech, commented, “We are very excited to announce the combination of WuXi PharmaTech and AppTec Laboratory Services, an important milestone in realizing our vision of becoming the global R&D outsourcing leader. WuXi’s chemistry services will be complemented by AppTec’s biologics testing and manufacturing capabilities to create a fully integrated service platform, from which we will be able to provide more value-added services to our customers. With an expanded geographic footprint, and a broader and deeper scope of services, we are well positioned to drive growth and continue to increase shareholder value.”
Dr. Bonita L. Baskin, Chief Executive Officer of AppTec, said, “Combining these two companies creates a unique single source platform that has the ability to transform the outsourced R&D model globally. I, along with the AppTec team, share with Dr. Li our excitement over the prospects of this combination.”
The transaction is expected to be immediately accretive to WuXi PharmaTech’s earnings per share, excluding the amortization of acquired intangible assets and one-time charges related to the acquisition.
The transaction has been approved by the AppTec and WuXi PharmaTech boards of directors and AppTec’s shareholders, and is expected to close, subject to regulatory and other customary closing conditions, in the first quarter of 2008. No approval by WuXi PharmaTech’s shareholders is required.
Conference Call
WuXi PharmaTech will host a conference call and Q&A session at 8:00 am (Eastern) / 5:00 am (Pacific) / 9:00 pm (Beijing/Hong Kong) on January 4, 2008 to provide details of the transaction.
4

The conference call may be accessed by calling (US) +1-888-262-8797 / (UK) +0-800-051-7166 / (HK) +800-965-503 / (China, Northern Region) +86-10-800-714-0970 / (China, Southern Region) +86-10-800-140-0945, Passcode: 1227949.
A live webcast of the conference call and replay will be available on the investor relations page of WuXi PharmaTech’s website at http://www.wuxipharmatech.com.
About WuXi PharmaTech
Founded in 2000, Shanghai-based WuXi PharmaTech is the leading China-based pharmaceutical and biotechnology R&D outsourcing company. As a research-driven and customer-focused company, WuXi PharmaTech provides pharmaceutical and biotechnology companies a broad and integrated portfolio of laboratory and research manufacturing services throughout the drug discovery and development process. WuXi PharmaTech’s services are designed to assist its global partners in shortening the cycle and lowering the cost of drug discovery and development by providing cost-effective and efficient outsourcing solutions that save its customers both time and money. Its operations are grouped into two segments: laboratory services, consisting of discovery chemistry, service biology, analytical, pharmaceutical development and process development services, and research manufacturing, focusing on manufacturing of advanced intermediates and active pharmaceutical ingredients for R&D use. In 2007, its 80 customers included nine of the world’s top ten pharmaceutical companies by revenue. For more information, please visit:http://www.wuxipharmatech.com.
About AppTec
With over 20 years experience, AppTec is a trusted leader in providing high-value testing, contract R&D, and cGMP manufacturing services for the biopharmaceutical and medical device industries. AppTec offers a fully integrated approach for the development of highly regulated products such as biopharmaceuticals, traditional pharmaceuticals, cellular therapeutics, medical devices, and combination and tissue-based products. Possessing the full set of competencies, facilities, and key personnel necessary, AppTec helps clients take their products efficiently and cost-effectively through the product development process. The company has three state-of-the art facilities, which are located in St. Paul, MN; Philadelphia, PA; and Atlanta, GA. All AppTec facilities are FDA registered and GLP/GMP compliant. Additional qualifications include ISO certification, AAALAC accreditation, FDA registration for HCT/Ps, and accreditation by the American Association of Tissue Banks.
Cautionary Note Regarding Forward-Looking Statements
Statements in this release contain “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and as defined in the Private Securities Litigation Reform Act of 1995. These forward-looking statements related to the proposed AppTec transaction include the anticipated timing and closing of the transaction, related benefits of the proposed transaction and the anticipated financial impact of the proposed transaction on our operating results. These statements are not historical facts but instead represent only our belief regarding future events, many of which, by their nature, are inherently uncertain and outside of our control. The AppTec transaction may not close when anticipated or at all, we may not realize the anticipated benefits of the transaction and the transaction may not be accretive. In addition to risks related to the integration of our and the AppTec business, our business is subject to a number of risks and uncertainties that could impact our business and operating results including our limited operating history; our reliance on a limited number of customers to
5

continue to account for a high percentage of our revenues; risk of payment failure by any of its large customers, which could significantly harm our cash flows and profitability; dependency upon the continued service of our senior management and key scientific personnel and ability to retain our existing customers or expand our customer base. For additional information on these and other important factors that could adversely affect our business, financial condition, results of operations and prospects, see “Risk Factors” beginning on page 11 of our prospectus filed with the Securities and Exchange Commission on August 9, 2007, and available on the Securities and Exchange Commission’s website at http://www.sec.gov. Any projections in this release are based on limited information currently available to us, which is subject to change. Although such projections and the factors influencing them will likely change, WuXi PharmaTech undertakes no obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release. Such information speaks only as of the date of this release.
For more information, please contact:
Investor Contact:
Dr. Hai Mi
Vice President, Corporate Communications
WuXi PharmaTech (Cayman) Inc.
Tel: +86-21-5046-3726
Email: ir@pharmatechs.com
Investor Relations (US):
Mahmoud Siddig
Director
Taylor Rafferty
Tel: +1-212-889-4350
Email: Pharmatechs@taylor-rafferty.com
Investor Relations (HK):
Ruby Yim
Managing Director
Taylor Rafferty
Tel: +852-3196-3712
Email: Pharmatechs@taylor-rafferty.com
Media Contact:
John Dooley
Taylor Rafferty
Tel: +1-212-889-4350
Email: Pharmatechs@taylor-rafferty.com
6
Exhibit 99.2

to acquire
Investor And Analyst Call Presentation January 4, 2008
7

154111-001
Safe Harbor Statement
The following information contains, or may be deemed to contain, “forward looking statements” (as defined in the U.S. Private Securities Litigation Reform Act of 1995). By their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future.
The forward-looking statements related to the proposed AppTec transaction include the anticipated timing and closing of the transaction, related benefits of the proposed transaction. These statements are not historical facts but instead represent only our belief regarding future events, many of which, by their nature, are inherently uncertain and outside our control. The AppTec transaction may not close when anticipated or at all, we may not realize the anticipated benefits of the transaction and the transaction may not be accretive. In addition to risks related to the integration of our and the AppTec business, our business is subject to a number of risks and uncertainties that could impact our business and operating results including our limited operating history; our reliance on a limited number of customers to continue to account for a high percentage of our revenues; risk of payment failure by any of its large customers, which could significantly harm our cash flows and profitability; dependency upon the continued service of our senior management and key scientific personnel and ability to retain our existing customers or expand our customer base. The financial information contained in this release should be read in conjunction with the consolidated financial statements and notes thereto included in our prospectus filed with the Securities and Exchange Commission on August 9, 2007, and is available on the Securities and Exchange Commission’s website at http://www.sec.gov. For additional information on these and other important factors that could adversely affect our business, financial condition, results of operations and prospects, see “Risk Factors” beginning on page 11 of our prospectus.
1
8
154111-001
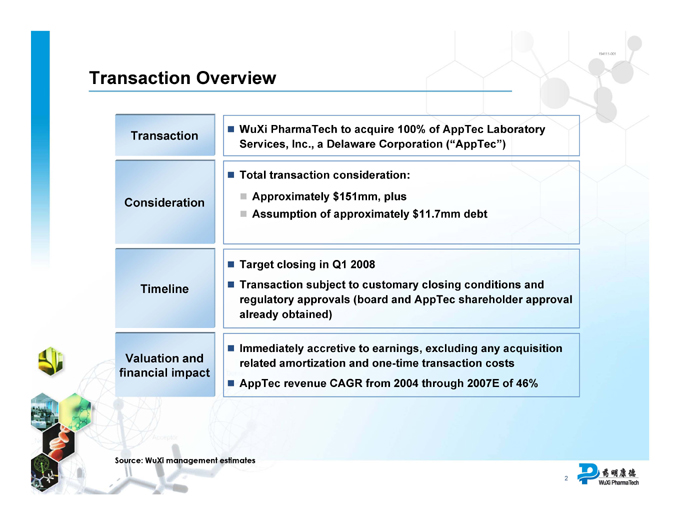
Transaction Overview
Transaction
WuXi PharmaTech to acquire 100% of AppTec Laboratory
Services, Inc., a Delaware Corporation (“AppTec”)
Consideration
Total transaction consideration:
Approximately $151mm, plus
Assumption of approximately $11.7mm debt
Timeline
Target closing in Q1 2008
Transaction subject to customary closing conditions and regulatory approvals (board and AppTec shareholder approval
already obtained)
Valuation and financial impact
Immediately accretive to earnings, excluding any acquisition related amortization and one-time transaction costs
AppTec revenue CAGR from 2004 through 2007E of 46%
Source: WuXi management estimates
2
9
154111-001
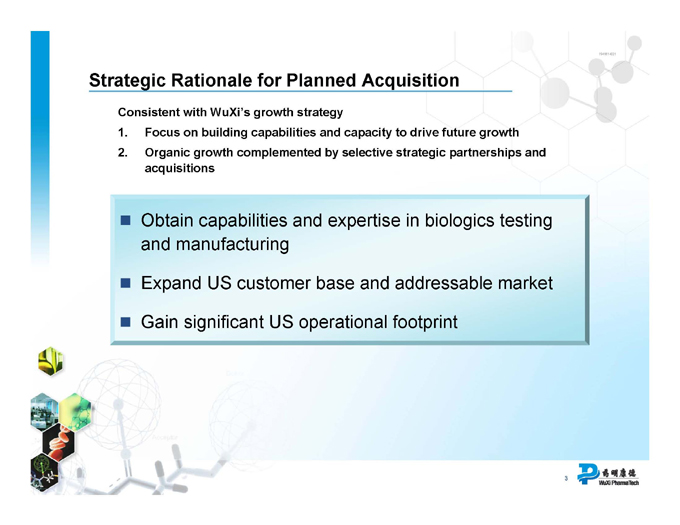
Strategic Rationale for Planned Acquisition
Consistent with WuXi’s growth strategy
1. Focus on building capabilities and capacity to drive future growth
2. Organic growth complemented by selective strategic partnerships and acquisitions
Obtain capabilities and expertise in biologics testing and manufacturing
Expand US customer base and addressable market
Gain significant US operational footprint
3
10
154111-001
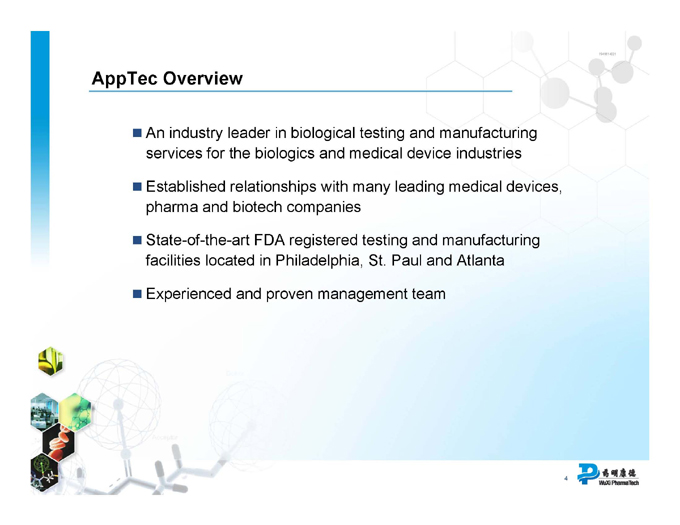
AppTec Overview
An industry leader in biological testing and manufacturing services for the biologics and medical device industries
Established relationships with many leading medical devices, pharma and biotech companies
State-of-the-art FDA registered testing and manufacturing facilities located in Philadelphia, St. Paul and Atlanta
Experienced and proven management team
4
11
154111-001
AppTec Overview (cont’d)
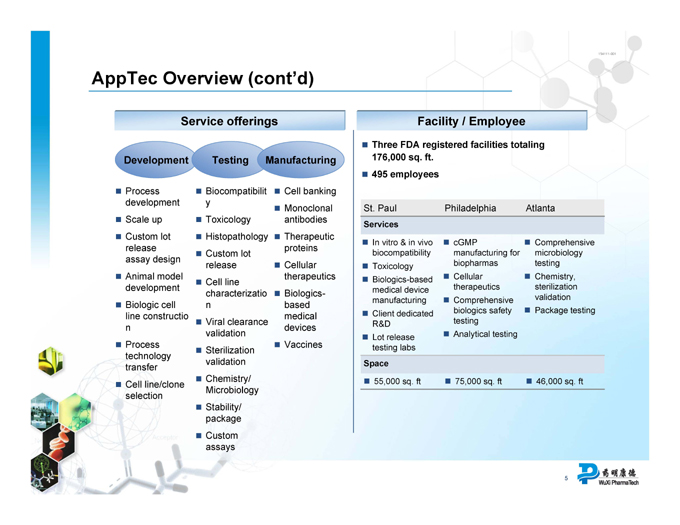
Service offerings
Development Testing Manufacturing
Process development Biocompatibility Cell banking
Toxicology Monoclonal antibodies
Scale up Histopathology
Custom lot release assay design Custom lot release Therapeutic proteins
Animal model development Cell line characterization Cellular therapeutics
Biologic cell line construction Viral clearance validation Biologics-based medical devices
Process technology transfer Sterilization validation Vaccines
Chemistry/Microbiology
Cell line/clone selection Stability/package Custom assays
Facility/Employee
Three FDA registered facilities totaling 176,000 sq. ft.
495 employees
St. Paul Philadelphia Atlanta
Services
In vitro & in vivo biocompatibility cGMP manufacturing for biopharmas
Comprehensive microbiology testing
Toxicology
Biologics-based medical device manufacturing
Cellular therapeutics
Chemistry, sterilization validation
Client dedicated R&D Comprehensive biologics safety testing Package testing
Lot release testing labs Analytical testing
Space
55,000 sq. ft 75,000 sq. ft 46,000 sq. ft
5
12
154111-001
AppTec Revenue Overview
US$ Millions
CAGR 46%
$71.0 $51.0 $32.7 $22.8
2004 2005 2006 2007E
Revenue Mix by Service Offering
Manufacturing 31%
Biologic Testing 69%
Revenue Mix by Customer Type
Medical Device 37%
Biopharma 63%
6
13
154111-001
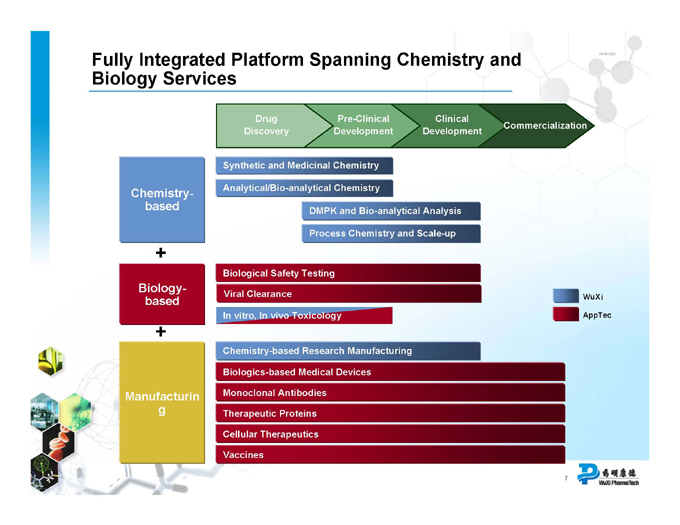
Fully Integrated Platform Spanning Chemistry and Biology Services
Drug Pre-Clinical Clinical
Commercialization Discovery Development Development
Synthetic and Medicinal Chemistry
Chemistry- based Analytical/Bio-analytical Chemistry DMPK and Bio-analytical Analysis Process Chemistry and Scale-up
+
Biology-based
Biological Safety Testing
Viral Clearance WuXi
In vitro, In vivo Toxicology AppTec
+
Manufacturing
Chemistry-based Research Manufacturing
Biologics-based Medical Devices
Monoclonal Antibodies
Therapeutic Proteins Cellular Therapeutics Vaccines
7
14
154111-001
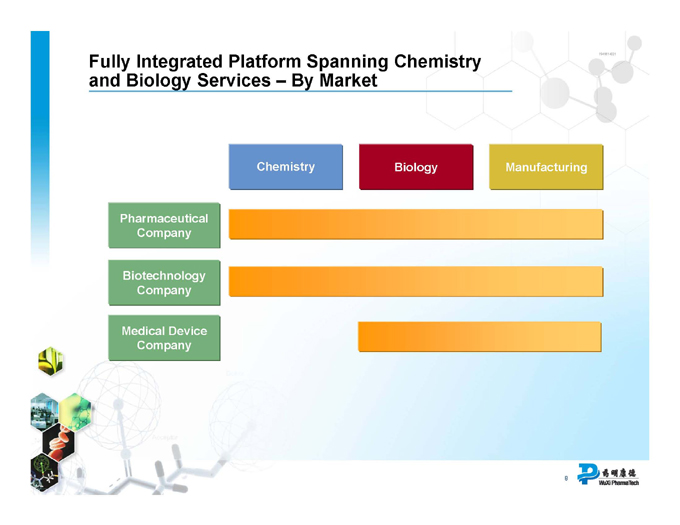
Fully Integrated Platform Spanning Chemistry and Biology Services – By Market
Chemistry Biology Manufacturing
Pharmaceutical Company
Biotechnology Company
Medical Device Company
8
15
154111-001
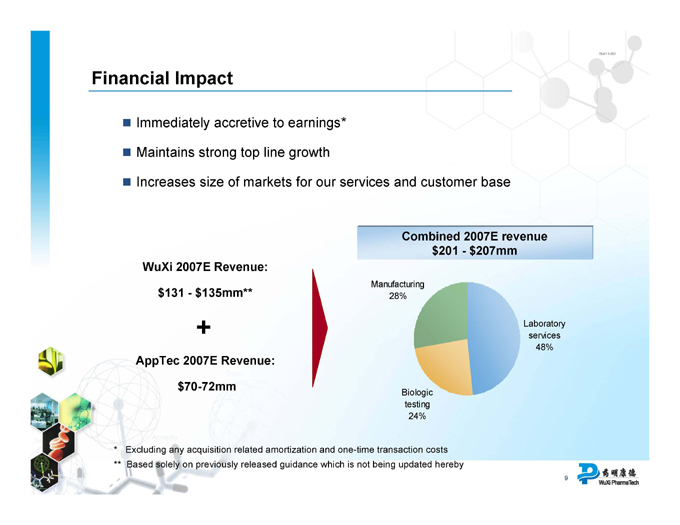
Financial Impact
Immediately accretive to earnings*
Maintains strong top line growth
Increases size of markets for our services and customer base
Combined 2007E revenue $201 - $207mm WuXi 2007E Revenue:
Manufacturing
$131 - $135mm** 28%
+ Laboratory services 48%
AppTec 2007E Revenue:
$70-72mm Biologic testing 24%
* Excluding any acquisition related amortization and one-time transaction costs
** Based solely on previously released guidance which is not being updated hereby
9
16
154111-001
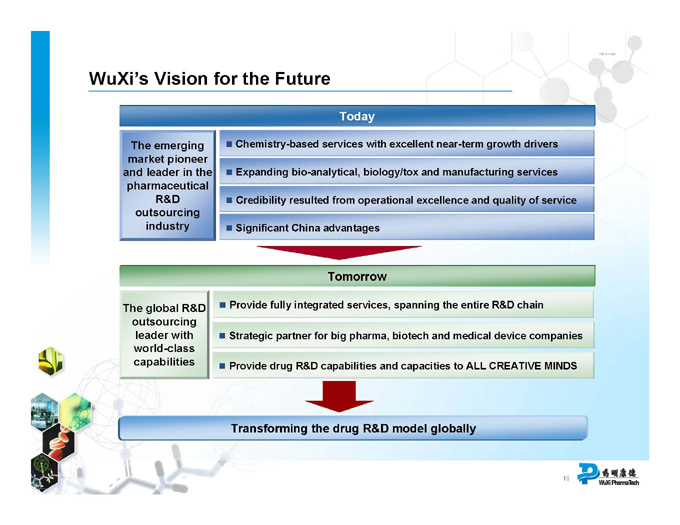
WuXi’s Vision for the Future
Today
Chemistry-based services with excellent near-term growth drivers
The emerging market pioneer
Expanding bio-analytical, biology/tox and manufacturing services and leader in the pharmaceutical
Credibility resulted from operational excellence and quality of service
R&D outsourcing industry
Significant China advantages
Tomorrow
The global R&D Provide fully integrated services, spanning the entire R&D chain outsourcing leader with Strategic partner for big pharma, biotech and medical device companies world-class capabilities Provide drug R&D capabilities and capacities to ALL CREATIVE MINDS
Transforming the drug R&D model globally
10
17

154111-001
Q&A
Questions & Answers
11
18
Exhibit 99.3
WuXi PharmaTech (NYSE: WX) Promotes Edward Hu to COO
January 4, 2008 - Shanghai, China - WuXi PharmaTech (NYSE: WX), China’s premier provider of pharmaceutical R&D outsourcing services is pleased to announce today that it has promoted Mr. Edward Hu from Executive Vice President of Operations to Chief Operating Officer.
Prior to joining WuXi PharmaTech in August 2007, Mr. Hu was a senior vice president and Chief Operating Officer at Tanox, Inc., a NASDAQ listed company later acquired by Genentech, Inc. He also worked for Biogen Idec as a business planning manager and for Merck as a senior financial analyst. In the last few months as Executive Vice President of Operations at WuXi PharmaTech, Mr. Hu took many initiatives to strengthen the company’s operations and corporate development; he is also instrumental in leading the AppTec acquisition.
“We are very happy to promote Ed to give him more responsibilities, as Ed has quickly demonstrated his leadership capabilities and ability to contribute to the company. Ed will be based in US to lead the integration of AppTec after the deal is closed,” commented Dr. Ge Li, Chairman and Chief Executive Officer of WuXi PharmaTech.
Mr. Hu received his MBA and MS in Chemistry from Carnegie Mellon University, Pittsburgh, Pennsylvania.
About WuXi PharmaTech
Founded in 2000, Shanghai-based WuXi PharmaTech is the leading China-based pharmaceutical and biotechnology R&D outsourcing company. As a research- driven and customer-focused company, WuXi PharmaTech provides pharmaceutical and biotechnology companies a broad and integrated portfolio of laboratory and research manufacturing services throughout the drug discovery and development process. WuXi PharmaTech’s services are designed to assist its global partners in shortening the cycle and lowering the cost of drug discovery and development by providing cost-effective and efficient outsourcing solutions that save its customers both time and money. Its operations are grouped into two segments: laboratory services, consisting of discovery chemistry, service biology, analytical, toxicology, pharmaceutical development and process development services, and research manufacturing, focusing on manufacturing of advanced intermediates and active pharmaceutical ingredients for R&D use. In 2007, its 80 customers included nine of the world’s top ten pharmaceutical companies by revenue. For more information, please visit:www.wuxipharmatech.com.
For more information, please contact:
Sherry Shao
Tel: +86-21-50464002
Email:pr@pharmatechs.com
19
Exhibit 99.4
WuXi PharmaTech (NYSE: WX) Mourns the Tragic Loss of Its Independent Director Shawn Wang
December 30, 2007 – Shanghai, China - WuXi PharmaTech (NYSE: WX), China’s premier provider of pharmaceutical R&D outsourcing services, sadly announced today one of its independent directors, Mr. Shawn Wang, Chief Financial Officer of Baidu, passed away in an accident during his vacation on December 27, 2007.
Mr. Shawn Wang was appointed as an independent director on WuXi PharmaTech’s Board of Directors in July, 2007 when the company was preparing its public listing on the New York Stock Exchange. During his brief tenure and as the Chairman of the Audit Committee, Shawn advised the company’s management in particular areas of financial reporting, Sarbanes-Oxley Act compliance and investor relations.
“We are all deeply saddened by the tragic and untimely loss of Shawn. He was not only a remarkable leader but also a very close personal friend of mine,” said Dr. Ge Li, Chairman and Chief Executive Officer of WuXi PharmaTech. “We are honored and grateful for having known and worked with Shawn. I will miss him very much and my thoughts and prayers go out to Shawn’s family.”
About WuXi PharmaTech
Founded in 2000, Shanghai-based WuXi PharmaTech is the leading China-based pharmaceutical and biotechnology R&D outsourcing company. As a research- driven and customer-focused company, WuXi PharmaTech provides pharmaceutical and biotechnology companies a broad and integrated portfolio of laboratory and research manufacturing services throughout the drug discovery and development process. WuXi PharmaTech’s services are designed to assist its global partners in shortening the cycle and lowering the cost of drug discovery and development by providing cost-effective and efficient outsourcing solutions that save its customers both time and money. Its operations are grouped into two segments: laboratory services, consisting of discovery chemistry, service biology, analytical, toxicology, pharmaceutical development and process development services, and research manufacturing, focusing on manufacturing of advanced intermediates and active pharmaceutical ingredients for R&D use. In 2007, its 80 customers included nine of the world’s top ten pharmaceutical companies by revenue. For more information, please visit:www.wuxipharmatech.com.
For more information, please contact:
Sherry Shao
Tel: +86-21-50464002
Email:pr@pharmatechs.com
20












